User login
Practical Neuroscience for Primary Care Physicians: Winter 2007
A supplement to Internal Medicine News.
TOPIC HIGHLIGHTS/FACULTY
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Applying Cultural Flexibility to Depression in Minority Populations
William B. Lawson, MD, PhD, DFAPA
Professor and Chair
Department of Psychiatry and Behavioral Sciences
Director, Mood Research Program
Howard University College of Medicine and Hospital
Washington, D.C.
Dr Lawson has disclosed that he has received clinical grants from AstraZeneca, the National Institute of Mental Health, and Pfizer Inc., and is a consultant to AstraZeneca and Pfizer.
Resources in the Spotlight
Practical Bits: Quick and Practical Diagnostic Tools
Case File on Seasonal Affective Disorder
David L. Dunner, MD, FACPsychDirector, Center for Anxiety and Depression
Professor Emeritus, Department of Psychiatry and Behavioral Sciences
University of Washington School of Medicine
Seattle, Wash.
Dr Dunner has disclosed that he has received grant support from, is on the advisory board of, and/or on the speaker's bureau of Bristol-Myers Squibb Company, Corcept Therapeutics, Cyberonics, Inc., Cypress Bioscience Inc., Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Healthcare Technology Systems, Janssen, L.P., Novartis Pharmaceuticals Corporation, Organon, Otsuka America Pharmaceuticals, Pfizer Inc., Roche Diagnostics, Shire Pharmaceuticals Group plc, Somerset Pharmaceuticals, Inc., and Wyeth Pharmaceuticals.
Case File on Depression in Minorities
Peggy L. Johnson, MD
Assistant Professor
Vice Chair for Clinical Services
Department of Psychiatry
Boston University School of Medicine
Boston, Mass.
Dr Johnson has nothing to disclose.
Clinical Approaches to Recognizing and Managing Bipolar Disorder
Andrew J. Cutler, MD
Courtesy Assistant Professor, Department of Psychiatry
University of Florida
President and Medical Director
Florida Clinical Research Center, LLC
Maitland, Fla.
Dr Cutler has disclosed that he has received research grants from Abbott Laboratories Pharmaceutical Product Division, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Cephalon, Inc., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Janssen, L.P., JDS Pharmaceuticals LLC, Johnson & Johnson PRD, Memory Pharmaceuticals, Novartis Pharmaceuticals Corporation, Organon, Otsuka America Pharmaceuticals, Pfizer, Sanofi, Sepracor Inc., Shire Pharmaceuticals Group plc, Solvay Pharmaceuticals, Vanda Pharmaceuticals, and Wyeth Pharmaceuticals. He is a consultant and on the speaker's bureau for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline,Janssen, Otsuka, Pfizer, Sepracor, Shire, and Vanda. He is also a consultant to Supernus Pharmaceuticals, Inc.
Adults With ADHD Need to Know Treatment Options
Carl C. Bell, MD
Chief, Executive Officer and President
Community Mental Health Council, Inc.
Director, Public and Community Psychiatry
University of Illinois, Chicago, Ill.
A supplement to Internal Medicine News.
TOPIC HIGHLIGHTS/FACULTY
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Applying Cultural Flexibility to Depression in Minority Populations
William B. Lawson, MD, PhD, DFAPA
Professor and Chair
Department of Psychiatry and Behavioral Sciences
Director, Mood Research Program
Howard University College of Medicine and Hospital
Washington, D.C.
Dr Lawson has disclosed that he has received clinical grants from AstraZeneca, the National Institute of Mental Health, and Pfizer Inc., and is a consultant to AstraZeneca and Pfizer.
Resources in the Spotlight
Practical Bits: Quick and Practical Diagnostic Tools
Case File on Seasonal Affective Disorder
David L. Dunner, MD, FACPsychDirector, Center for Anxiety and Depression
Professor Emeritus, Department of Psychiatry and Behavioral Sciences
University of Washington School of Medicine
Seattle, Wash.
Dr Dunner has disclosed that he has received grant support from, is on the advisory board of, and/or on the speaker's bureau of Bristol-Myers Squibb Company, Corcept Therapeutics, Cyberonics, Inc., Cypress Bioscience Inc., Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Healthcare Technology Systems, Janssen, L.P., Novartis Pharmaceuticals Corporation, Organon, Otsuka America Pharmaceuticals, Pfizer Inc., Roche Diagnostics, Shire Pharmaceuticals Group plc, Somerset Pharmaceuticals, Inc., and Wyeth Pharmaceuticals.
Case File on Depression in Minorities
Peggy L. Johnson, MD
Assistant Professor
Vice Chair for Clinical Services
Department of Psychiatry
Boston University School of Medicine
Boston, Mass.
Dr Johnson has nothing to disclose.
Clinical Approaches to Recognizing and Managing Bipolar Disorder
Andrew J. Cutler, MD
Courtesy Assistant Professor, Department of Psychiatry
University of Florida
President and Medical Director
Florida Clinical Research Center, LLC
Maitland, Fla.
Dr Cutler has disclosed that he has received research grants from Abbott Laboratories Pharmaceutical Product Division, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Cephalon, Inc., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Janssen, L.P., JDS Pharmaceuticals LLC, Johnson & Johnson PRD, Memory Pharmaceuticals, Novartis Pharmaceuticals Corporation, Organon, Otsuka America Pharmaceuticals, Pfizer, Sanofi, Sepracor Inc., Shire Pharmaceuticals Group plc, Solvay Pharmaceuticals, Vanda Pharmaceuticals, and Wyeth Pharmaceuticals. He is a consultant and on the speaker's bureau for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline,Janssen, Otsuka, Pfizer, Sepracor, Shire, and Vanda. He is also a consultant to Supernus Pharmaceuticals, Inc.
Adults With ADHD Need to Know Treatment Options
Carl C. Bell, MD
Chief, Executive Officer and President
Community Mental Health Council, Inc.
Director, Public and Community Psychiatry
University of Illinois, Chicago, Ill.
A supplement to Internal Medicine News.
TOPIC HIGHLIGHTS/FACULTY
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Applying Cultural Flexibility to Depression in Minority Populations
William B. Lawson, MD, PhD, DFAPA
Professor and Chair
Department of Psychiatry and Behavioral Sciences
Director, Mood Research Program
Howard University College of Medicine and Hospital
Washington, D.C.
Dr Lawson has disclosed that he has received clinical grants from AstraZeneca, the National Institute of Mental Health, and Pfizer Inc., and is a consultant to AstraZeneca and Pfizer.
Resources in the Spotlight
Practical Bits: Quick and Practical Diagnostic Tools
Case File on Seasonal Affective Disorder
David L. Dunner, MD, FACPsychDirector, Center for Anxiety and Depression
Professor Emeritus, Department of Psychiatry and Behavioral Sciences
University of Washington School of Medicine
Seattle, Wash.
Dr Dunner has disclosed that he has received grant support from, is on the advisory board of, and/or on the speaker's bureau of Bristol-Myers Squibb Company, Corcept Therapeutics, Cyberonics, Inc., Cypress Bioscience Inc., Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Healthcare Technology Systems, Janssen, L.P., Novartis Pharmaceuticals Corporation, Organon, Otsuka America Pharmaceuticals, Pfizer Inc., Roche Diagnostics, Shire Pharmaceuticals Group plc, Somerset Pharmaceuticals, Inc., and Wyeth Pharmaceuticals.
Case File on Depression in Minorities
Peggy L. Johnson, MD
Assistant Professor
Vice Chair for Clinical Services
Department of Psychiatry
Boston University School of Medicine
Boston, Mass.
Dr Johnson has nothing to disclose.
Clinical Approaches to Recognizing and Managing Bipolar Disorder
Andrew J. Cutler, MD
Courtesy Assistant Professor, Department of Psychiatry
University of Florida
President and Medical Director
Florida Clinical Research Center, LLC
Maitland, Fla.
Dr Cutler has disclosed that he has received research grants from Abbott Laboratories Pharmaceutical Product Division, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Cephalon, Inc., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Janssen, L.P., JDS Pharmaceuticals LLC, Johnson & Johnson PRD, Memory Pharmaceuticals, Novartis Pharmaceuticals Corporation, Organon, Otsuka America Pharmaceuticals, Pfizer, Sanofi, Sepracor Inc., Shire Pharmaceuticals Group plc, Solvay Pharmaceuticals, Vanda Pharmaceuticals, and Wyeth Pharmaceuticals. He is a consultant and on the speaker's bureau for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline,Janssen, Otsuka, Pfizer, Sepracor, Shire, and Vanda. He is also a consultant to Supernus Pharmaceuticals, Inc.
Adults With ADHD Need to Know Treatment Options
Carl C. Bell, MD
Chief, Executive Officer and President
Community Mental Health Council, Inc.
Director, Public and Community Psychiatry
University of Illinois, Chicago, Ill.
Practical Neuroscience for Primary Care Physicians: Fall 2007
A supplement to Internal Medicine News.
TOPIC HIGHLIGHTS/FACULTY
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Effective Approaches to Depression in Men
Michael E. Thase, MD
Professor, Department of Psychiatry
University of Pennsylvania School of Medicine and Philadelphia Veterans Affairs Medical Center
Philadelphia, Penn.
University of Pittsburgh School of Medicine
Pittsburgh, Penn.
Dr Thase has disclosed that he is a consultant to AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Cyberonics, Inc, Eli Lilly & Company, GlaxoSmithKline, Janssen L.P., MedAvante, Inc., Neuronetics, Novartis Pharmaceuticals Corporation, Organon, Sepracor Inc., Shire US Inc., Supernus Pharmaceuticals, Inc., and Wyeth. He is on the speakers bureau of AstraZeneca, Bristol-Myers Squibb, Cyberonics, Lilly, GlaxoSmithKline, Organon, sanofi-aventis, and Wyeth.
Practical Bits
Quick and Practical Diagnostic Tools
Resources in the Spotlight
Case Files on Smoking/Myocardial Infarction and Smoking/Gastric Bypass Surgery
Ellen A. Dornelas, PhD
Director of Behavioral Health Programs
Preventive Cardiology
Hartford Hospital
University of Connecticut School of Medicine
Farmington, Conn.
Dr Dornelas has disclosed that she has received clinical grants from Pfizer Inc. Dr Miller has nothing to disclose.
Strategies for Managing Anxiety Disorders
Thomas L. Schwartz, MD
Associate Professor of Psychiatry
Director of Adult Outpatient Services
Director of the Depression and Anxiety Disorders Research Program
Assistant Director of Residency Training
State University of New York (SUNY) Upstate Medical University
Syracuse, N.Y.
Dr Schwartz has disclosed that he has received clinical grants from Wyeth and Forest Laboratories, Inc., and is a consultant to Wyeth.
Cast a Wide Net With Chronic Pain
Carl C. Bell, MD
Chief, Executive Officer and President
Community Mental Health Council, Inc.
Director, Public and Community Psychiatry
University of Illinois
Chicago, Ill.
A supplement to Internal Medicine News.
TOPIC HIGHLIGHTS/FACULTY
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Effective Approaches to Depression in Men
Michael E. Thase, MD
Professor, Department of Psychiatry
University of Pennsylvania School of Medicine and Philadelphia Veterans Affairs Medical Center
Philadelphia, Penn.
University of Pittsburgh School of Medicine
Pittsburgh, Penn.
Dr Thase has disclosed that he is a consultant to AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Cyberonics, Inc, Eli Lilly & Company, GlaxoSmithKline, Janssen L.P., MedAvante, Inc., Neuronetics, Novartis Pharmaceuticals Corporation, Organon, Sepracor Inc., Shire US Inc., Supernus Pharmaceuticals, Inc., and Wyeth. He is on the speakers bureau of AstraZeneca, Bristol-Myers Squibb, Cyberonics, Lilly, GlaxoSmithKline, Organon, sanofi-aventis, and Wyeth.
Practical Bits
Quick and Practical Diagnostic Tools
Resources in the Spotlight
Case Files on Smoking/Myocardial Infarction and Smoking/Gastric Bypass Surgery
Ellen A. Dornelas, PhD
Director of Behavioral Health Programs
Preventive Cardiology
Hartford Hospital
University of Connecticut School of Medicine
Farmington, Conn.
Dr Dornelas has disclosed that she has received clinical grants from Pfizer Inc. Dr Miller has nothing to disclose.
Strategies for Managing Anxiety Disorders
Thomas L. Schwartz, MD
Associate Professor of Psychiatry
Director of Adult Outpatient Services
Director of the Depression and Anxiety Disorders Research Program
Assistant Director of Residency Training
State University of New York (SUNY) Upstate Medical University
Syracuse, N.Y.
Dr Schwartz has disclosed that he has received clinical grants from Wyeth and Forest Laboratories, Inc., and is a consultant to Wyeth.
Cast a Wide Net With Chronic Pain
Carl C. Bell, MD
Chief, Executive Officer and President
Community Mental Health Council, Inc.
Director, Public and Community Psychiatry
University of Illinois
Chicago, Ill.
A supplement to Internal Medicine News.
TOPIC HIGHLIGHTS/FACULTY
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Effective Approaches to Depression in Men
Michael E. Thase, MD
Professor, Department of Psychiatry
University of Pennsylvania School of Medicine and Philadelphia Veterans Affairs Medical Center
Philadelphia, Penn.
University of Pittsburgh School of Medicine
Pittsburgh, Penn.
Dr Thase has disclosed that he is a consultant to AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Cyberonics, Inc, Eli Lilly & Company, GlaxoSmithKline, Janssen L.P., MedAvante, Inc., Neuronetics, Novartis Pharmaceuticals Corporation, Organon, Sepracor Inc., Shire US Inc., Supernus Pharmaceuticals, Inc., and Wyeth. He is on the speakers bureau of AstraZeneca, Bristol-Myers Squibb, Cyberonics, Lilly, GlaxoSmithKline, Organon, sanofi-aventis, and Wyeth.
Practical Bits
Quick and Practical Diagnostic Tools
Resources in the Spotlight
Case Files on Smoking/Myocardial Infarction and Smoking/Gastric Bypass Surgery
Ellen A. Dornelas, PhD
Director of Behavioral Health Programs
Preventive Cardiology
Hartford Hospital
University of Connecticut School of Medicine
Farmington, Conn.
Dr Dornelas has disclosed that she has received clinical grants from Pfizer Inc. Dr Miller has nothing to disclose.
Strategies for Managing Anxiety Disorders
Thomas L. Schwartz, MD
Associate Professor of Psychiatry
Director of Adult Outpatient Services
Director of the Depression and Anxiety Disorders Research Program
Assistant Director of Residency Training
State University of New York (SUNY) Upstate Medical University
Syracuse, N.Y.
Dr Schwartz has disclosed that he has received clinical grants from Wyeth and Forest Laboratories, Inc., and is a consultant to Wyeth.
Cast a Wide Net With Chronic Pain
Carl C. Bell, MD
Chief, Executive Officer and President
Community Mental Health Council, Inc.
Director, Public and Community Psychiatry
University of Illinois
Chicago, Ill.
Practical Neuroscience for Primary Care Physicians: Summer 2007
A supplement to Internal Medicine News.
[[{"attributes":{},"fields":{}}]]
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Practical Approaches to Depression in Seniors
William Clay Jackson, MD, DipTh
Family Medicine and Palliative Medicine
Memphis, Tenn.
Dr Jackson has received funding from Eli Lilly and Company. He is a consultant to Eli Lilly and AstraZeneca.
Advances in Assessing and Managing Insomnia
Ellen H. Miller, MD
Clinical Associate Professor of Medicine
Albert Einstein College of Medicine
New York, N.Y.
Private Practice in Internal Medicine and Endocrinology
Hewlett, N.Y.
Dr Miller has nothing to disclose.
Case File: Elderly Man With Insomnia and Depression
Joseph A. Lieberman III, MD, MPH
Associate Editor, Delaware Medical Journal
Professor of Family Medicine
Jefferson Medical College of Philadelphia
Hockessin, Del.
A Multidisciplinary Approach to the Management of Chronic Pain
Rollin M. Gallagher, MD, MPH, DABPM
Director of Pain Management, Department of Anesthesiology
Philadelphia VA Medical Center
Clinical Professor of Psychiatry and Anesthesiology
Director, Center for Pain Medicine, Research and Policy
University of Pennsylvania School of Medicine
Philadelphia, Penn.
Dr Gallagher has nothing to disclose.
Resources in the Spotlight
Practical Bits
Quick and Practical Diagnostic Tools
A supplement to Internal Medicine News.
[[{"attributes":{},"fields":{}}]]
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Practical Approaches to Depression in Seniors
William Clay Jackson, MD, DipTh
Family Medicine and Palliative Medicine
Memphis, Tenn.
Dr Jackson has received funding from Eli Lilly and Company. He is a consultant to Eli Lilly and AstraZeneca.
Advances in Assessing and Managing Insomnia
Ellen H. Miller, MD
Clinical Associate Professor of Medicine
Albert Einstein College of Medicine
New York, N.Y.
Private Practice in Internal Medicine and Endocrinology
Hewlett, N.Y.
Dr Miller has nothing to disclose.
Case File: Elderly Man With Insomnia and Depression
Joseph A. Lieberman III, MD, MPH
Associate Editor, Delaware Medical Journal
Professor of Family Medicine
Jefferson Medical College of Philadelphia
Hockessin, Del.
A Multidisciplinary Approach to the Management of Chronic Pain
Rollin M. Gallagher, MD, MPH, DABPM
Director of Pain Management, Department of Anesthesiology
Philadelphia VA Medical Center
Clinical Professor of Psychiatry and Anesthesiology
Director, Center for Pain Medicine, Research and Policy
University of Pennsylvania School of Medicine
Philadelphia, Penn.
Dr Gallagher has nothing to disclose.
Resources in the Spotlight
Practical Bits
Quick and Practical Diagnostic Tools
A supplement to Internal Medicine News.
[[{"attributes":{},"fields":{}}]]
Letter From Guest Editor
Larry Culpepper, MD, MPH
Chief of Family Medicine
Boston Medical Center
Professor and Chairman of Family Medicine
Boston University School of Medicine
Boston, Mass.
Special Populations in Depression: Practical Approaches to Depression in Seniors
William Clay Jackson, MD, DipTh
Family Medicine and Palliative Medicine
Memphis, Tenn.
Dr Jackson has received funding from Eli Lilly and Company. He is a consultant to Eli Lilly and AstraZeneca.
Advances in Assessing and Managing Insomnia
Ellen H. Miller, MD
Clinical Associate Professor of Medicine
Albert Einstein College of Medicine
New York, N.Y.
Private Practice in Internal Medicine and Endocrinology
Hewlett, N.Y.
Dr Miller has nothing to disclose.
Case File: Elderly Man With Insomnia and Depression
Joseph A. Lieberman III, MD, MPH
Associate Editor, Delaware Medical Journal
Professor of Family Medicine
Jefferson Medical College of Philadelphia
Hockessin, Del.
A Multidisciplinary Approach to the Management of Chronic Pain
Rollin M. Gallagher, MD, MPH, DABPM
Director of Pain Management, Department of Anesthesiology
Philadelphia VA Medical Center
Clinical Professor of Psychiatry and Anesthesiology
Director, Center for Pain Medicine, Research and Policy
University of Pennsylvania School of Medicine
Philadelphia, Penn.
Dr Gallagher has nothing to disclose.
Resources in the Spotlight
Practical Bits
Quick and Practical Diagnostic Tools
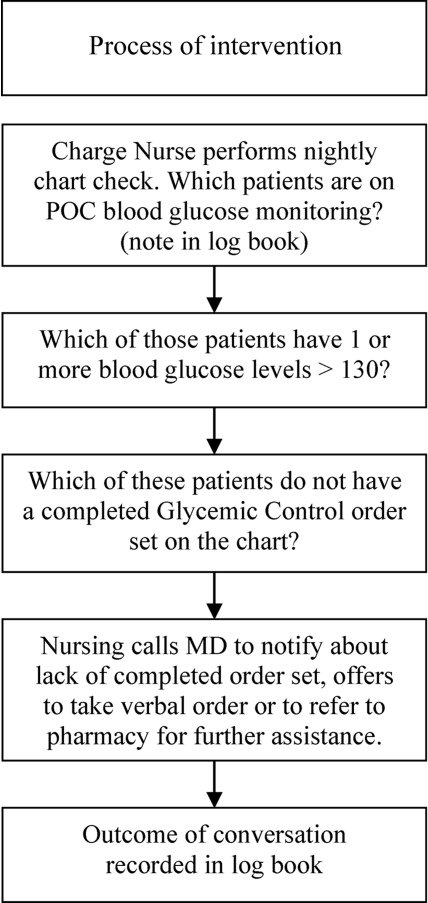
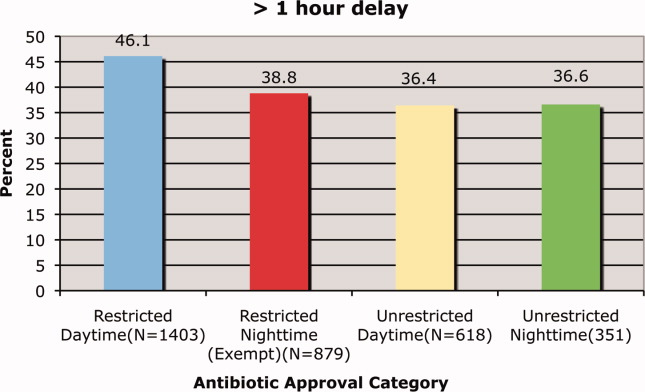
Letter to the Editor
Prado et al.'s1 insightful analysis on a rapid response system failure draws attention to afferent limb system failures of medical emergency teams (METs). The article also serves to highlight several key quality improvement (QI) educational points. The authors demonstrate a thorough grasp of the literature concerning METs. The case description reveals a detailed investigation that is thorough enough to create a timeline of events. I applaud the literature review and construction of a timeline, as these represent the first several steps of a root‐cause analysisbut they are somewhat insufficient. More work can be done here.
Extending their line of inquiry may uncover specific system factors involved in the afferent limb failure. To further the analysis, careful interviews of all involved personnel (including patients, family members, and nurses) may help identify the factors that compromise afferent limbs of METs and thereby make necessary improvements, as in the innovative Josie King Safety Program at Johns Hopkins Hospital (Baltimore, MD). Prado et al.1 are extremely fortunate in that their institution has a monitoring system in place to track MET activations. A more ambitious, though potentially more fruitful project, would be to, examine previous afferent limb failures in an effort to identify systems factors that are more generalizable to other institutions.
The difficulties in obtaining data are 2‐fold: first in gathering the data, and second in extending the data beyond one's own institution. The very nature of QI data, eg, data that are locally obtained and relevant to a particular institution, hinders its generalizability. However, afferent limb failures are real and perhaps ubiquitous.2, 3 The challenge then, is to develop strategies that can improve the functioning of METs (both afferent and efferent limbs) regardless of the institution.
As afferent limbs of METs have been identified as a priority for future attention for the greatest benefit,2, 4 the process of analyzing root‐causes of systems failures seems to be analogous to identifying risk factors for a novel disease. Once identified, the appropriate risk‐factor modifications can be undertaken. Only by careful examination of the data can true, relevant factors be identified. For this reason, I feel that Prado et al.'s1 excellent work should be expanded upon and replicated in other institutions.
Should these types of QI projects become more amenable to extrapolation to other institutions, a predominant reporting format may be needed. The Standards for Quality Improvement Reporting Excellence (SQUIRE) guideline
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,,, et al.Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards.Cochrane Database Syst Rev2007(3):CD005529.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365(9477):2091–2097.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- SGIM. Quality Portfolio Introduction. Available at: http://www.sgim.org/index.cfm?pageId=846. Accessed September2009.
Prado et al.'s1 insightful analysis on a rapid response system failure draws attention to afferent limb system failures of medical emergency teams (METs). The article also serves to highlight several key quality improvement (QI) educational points. The authors demonstrate a thorough grasp of the literature concerning METs. The case description reveals a detailed investigation that is thorough enough to create a timeline of events. I applaud the literature review and construction of a timeline, as these represent the first several steps of a root‐cause analysisbut they are somewhat insufficient. More work can be done here.
Extending their line of inquiry may uncover specific system factors involved in the afferent limb failure. To further the analysis, careful interviews of all involved personnel (including patients, family members, and nurses) may help identify the factors that compromise afferent limbs of METs and thereby make necessary improvements, as in the innovative Josie King Safety Program at Johns Hopkins Hospital (Baltimore, MD). Prado et al.1 are extremely fortunate in that their institution has a monitoring system in place to track MET activations. A more ambitious, though potentially more fruitful project, would be to, examine previous afferent limb failures in an effort to identify systems factors that are more generalizable to other institutions.
The difficulties in obtaining data are 2‐fold: first in gathering the data, and second in extending the data beyond one's own institution. The very nature of QI data, eg, data that are locally obtained and relevant to a particular institution, hinders its generalizability. However, afferent limb failures are real and perhaps ubiquitous.2, 3 The challenge then, is to develop strategies that can improve the functioning of METs (both afferent and efferent limbs) regardless of the institution.
As afferent limbs of METs have been identified as a priority for future attention for the greatest benefit,2, 4 the process of analyzing root‐causes of systems failures seems to be analogous to identifying risk factors for a novel disease. Once identified, the appropriate risk‐factor modifications can be undertaken. Only by careful examination of the data can true, relevant factors be identified. For this reason, I feel that Prado et al.'s1 excellent work should be expanded upon and replicated in other institutions.
Should these types of QI projects become more amenable to extrapolation to other institutions, a predominant reporting format may be needed. The Standards for Quality Improvement Reporting Excellence (SQUIRE) guideline
Prado et al.'s1 insightful analysis on a rapid response system failure draws attention to afferent limb system failures of medical emergency teams (METs). The article also serves to highlight several key quality improvement (QI) educational points. The authors demonstrate a thorough grasp of the literature concerning METs. The case description reveals a detailed investigation that is thorough enough to create a timeline of events. I applaud the literature review and construction of a timeline, as these represent the first several steps of a root‐cause analysisbut they are somewhat insufficient. More work can be done here.
Extending their line of inquiry may uncover specific system factors involved in the afferent limb failure. To further the analysis, careful interviews of all involved personnel (including patients, family members, and nurses) may help identify the factors that compromise afferent limbs of METs and thereby make necessary improvements, as in the innovative Josie King Safety Program at Johns Hopkins Hospital (Baltimore, MD). Prado et al.1 are extremely fortunate in that their institution has a monitoring system in place to track MET activations. A more ambitious, though potentially more fruitful project, would be to, examine previous afferent limb failures in an effort to identify systems factors that are more generalizable to other institutions.
The difficulties in obtaining data are 2‐fold: first in gathering the data, and second in extending the data beyond one's own institution. The very nature of QI data, eg, data that are locally obtained and relevant to a particular institution, hinders its generalizability. However, afferent limb failures are real and perhaps ubiquitous.2, 3 The challenge then, is to develop strategies that can improve the functioning of METs (both afferent and efferent limbs) regardless of the institution.
As afferent limbs of METs have been identified as a priority for future attention for the greatest benefit,2, 4 the process of analyzing root‐causes of systems failures seems to be analogous to identifying risk factors for a novel disease. Once identified, the appropriate risk‐factor modifications can be undertaken. Only by careful examination of the data can true, relevant factors be identified. For this reason, I feel that Prado et al.'s1 excellent work should be expanded upon and replicated in other institutions.
Should these types of QI projects become more amenable to extrapolation to other institutions, a predominant reporting format may be needed. The Standards for Quality Improvement Reporting Excellence (SQUIRE) guideline
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,,, et al.Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards.Cochrane Database Syst Rev2007(3):CD005529.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365(9477):2091–2097.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- SGIM. Quality Portfolio Introduction. Available at: http://www.sgim.org/index.cfm?pageId=846. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,,, et al.Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards.Cochrane Database Syst Rev2007(3):CD005529.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365(9477):2091–2097.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- SGIM. Quality Portfolio Introduction. Available at: http://www.sgim.org/index.cfm?pageId=846. Accessed September2009.
Aching for a Diagnosis
A 23‐year‐old Caucasian man presented to an outpatient clinic with a sore throat and associated subjective fevers. His evaluation included a negative rapid streptococcus test; nevertheless, he was empirically treated with amoxicillin. The following day, he experienced increasing sore throat and presented to the emergency department (ED). He was treated with prednisone and morphine sulfate and discharged home with azithromycin.
Initial considerations in a healthy young man who presents with fever and pharyngitis should focus on common infectious etiologies. Viral illnesses are the most frequent causes of sore throat and fever. These often manifest as mononucleosis‐like illnesses and include Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). In this age group, it is also critical to consider sexually transmitted diseases (STDs) such as gonorrhea, human immunodeficiency virus (HIV), herpes simplex virus, and syphilis. Consideration of streptococcal pharyngitis is important. Since the rapid streptococcal antigen test is neither sensitive nor specific, confirmation of infection should be based on clinical findings and a culture of the pharynx for group A Streptococcus. Other common etiologies of fever and pharyngitis include acute or chronic sinusitis with postnasal drainage. Due to the progressive nature of the sore throat, there should be an evaluation for difficulty swallowing, problems phonating, or neck discomfort, any of which would be concerning for a retropharyngeal abscess. Additional history should be obtained with focus on sexual history, previous STDs, recent sick contacts, and other supporting signs and symptoms of viral illnesses.
Eight days after the initial onset of symptoms, the patient developed acute low back pain. The back pain was midline, severe, and constant around the lumbar spine. There was no saddle anesthesia, bowel or bladder dysfunction, or weakness or numbness in the extremities. He also noted swelling of the left fourth metacarpophalangeal joint and an erythematous rash on his right knee and anterior tibial region of the right leg. He continued to experience subjective fevers, sore throat, and swollen neck glands. Due to the severity and discomfort of symptoms, the patient returned to the ED.
With no history of trauma, the subsequent development of acute low back pain may be related to the patient's sore throat and fever. Monoarticular arthritis with contralateral skin lesions should raise suspicion for a systemic process, particularly infection or a rheumatologic syndrome. Infectious etiologies would include rheumatic fever, endocarditis with septic emboli, and osteomyelitis. Rheumatologic causes, such as ankylosing spondylitis and juvenile rheumatoid arthritis (RA), are also possibilities. The infectious evaluation should include an assessment of a history of intravenous drug use (IVDU) and underlying valvular disorders, which will increase the risk for endocarditis and therefore septic emboli. Acute HIV infection can be seen as early as 1 to 2 weeks postexposure and should be considered as well. Appropriate testing would include both conventional HIV antibody tests and HIV viral load assay. Lastly, in considering the patient's symptoms, obtaining his travel history to identify risk for Lyme disease would also be appropriate.
The patient did not report any further positive findings on review of systems. He did not have any significant past medical history and did not take any chronic medications. He had no sick contacts. He rarely drank alcohol and denied IVDU and sexual activity over the past year. He was previously involved in monogamous relationships with women. His last HIV test, 1 year prior, was negative. He did not have any history of STDs. He was a graduate student in computer science and lived in southern California. He had recently traveled to central California and France for 2 weeks, staying in larger cities. He had not been hiking during that time. His family history was significant for hypertension.
The travel history is provocative for 3 diseases of the reticuloendothelial system with possible systemic manifestations. First, toxoplasmosis, which is endemic in France where rare or raw beef and lamb are frequently consumed. It may present as a mononucleosis‐like illness and rarely as atypical pneumonia. Second, tuberculosis, which is also endemic in France, especially in major cities. Although most commonly a self‐limited respiratory disease, it may disseminate with systemic symptoms. Third, primary coccidioidomycosis, which is prevalent in the central valleys of California. The climate and wind patterns lead to aerosolization of the spores and make this a common respiratory pathogen.
The physical exam should include a detailed evaluation of the eyes for uveitis and iritis, seen in some rheumatologic disorders. A pharyngeal exam with assessment for exudate can support streptococcal pharyngitis or diphtheria. Evaluation for lymphadenopathy, while nonspecific, would be important for streptococcal pharyngitis, rheumatic fever, and juvenile RA. Further characterizing the rash is essential in distinguishing viral exanthems from the fleeting salmon‐colored maculopapular rash of juvenile RA. Assessment for peripheral stigmata of endocarditis should be done. A thorough joint exam should evaluate evidence of inflammatory or infectious joint disease.
On physical exam, he was a thin man who appeared anxious but in no acute distress. His temperature was 36.7C, blood pressure 111/68 mm Hg, heart rate 83 beats/minute, respiratory rate 16 breaths/minute, and oxygen saturation 99% on room air. Erythema was noted in the posterior oropharynx with no tonsillar exudate. There were several subcentimeter, nontender, and mobile lymph nodes in the anterior cervical chain bilaterally. The cardiovascular exam revealed normal sinus rhythm with a 2/6 systolic murmur at the apex, without radiation. His lungs were clear to auscultation. Skin exam revealed 2 blanching erythematous, indurated, and tender lesions on the right pretibial region, 2‐cm and 4‐cm in diameter. Two other similar, but smaller, lesions were noted on the left upper extremity and left ankle. His lumbar spine was slightly tender to touch. A complete joint exam was normal, including the left fourth metacarpophalangeal joint. Neurological exam, including bilateral strength, sensation, reflexes, and gait, was unremarkable.
Younger patients are subject to social‐acceptance bias and can deny sexual activity on initial inquiry. An objective evaluation for STDs with serologic workup should still be pursued. The cervical lymphadenopathy and tonsillar erythema continue to suggest a viral illness. While the systolic murmur may be physiologic, subjective fevers, disseminated cutaneous lesions, and arthritis warrant evaluation for bacterial endocarditis with blood cultures and an echocardiogram.
On exam, there is no evidence of true joint involvement and this decreases the likelihood of rheumatologic conditions, such as ankylosing spondylitis and juvenile RA. However, the skin lesions are suspicious for erythema nodosum (EN), which should prompt a biopsy and an evaluation for infectious etiologies. Serologies should include evaluation of Chlamydia, Mycoplasma, Coccidioides, and Histoplasma. I would also examine the feet carefully for potential transcutaneous inoculation by microorganisms that can produce a rash similar to EN. For instance, penetrating skin trauma can lead to pseudomonal infection. Brucella (from ingesting unpasteurized milk or milk products), Bartonella (from the scratches of feline animals), and Francisella tularensis (from rabbit exposure) can also produce skin lesions that mimic EN. These are best distinguished through a detailed history, concomitant serologic workup, and biopsy. Other noninfectious etiologies of EN can include inflammatory bowel disease, Behcet's, and sarcoidosis; however, the patient does not currently report any symptoms supporting these diagnoses. In addition to the above evaluation, complete blood count with differential, liver function tests, creatinine, and urinalysis should be obtained.
The patient's white blood cell (WBC) count was 12,100/L with 73% neutrophils, 14% lymphocytes, and 12% monocytes. Hemoglobin was 11.8 g/dL and platelet count 292,000/L. Chemistry panel and liver function tests were unremarkable. Erythrocyte sedimentation rate (ESR) was 71 mm/hour (range, 010). Urinalysis was negative for protein and red blood cells. Chest x‐ray did not illustrate any abnormalities. Computed tomography (CT) of the lumbar spine revealed a small posterior disc bulge at L4‐5 and L5‐S1.
The moderate leukocytosis with neutrophilic predominance and monocytosis raises concern for a systemic inflammatory process; the elevated ESR further supports this. Monocytosis can be seen in a number of infectious, autoimmune, and malignant conditions. Tuberculosis, brucellosis, bacterial endocarditis, syphilis, infectious mononucleosis, and viral illnesses are among the infections typically characterized by monocytosis. Autoimmune illnesses, such as systemic lupus erythematosus and RA can also have similar presentations. The patient does not have any features of an underlying malignancy, such as weight loss or night sweats; however, if the autoimmune and infectious evaluations are negative, Hodgkin's disease and certain leukemias should be considered. There is no evidence of osteomyelitis on the spine CT, which decreases the possibility of (but does not exclude) infectious or rheumatologic conditions of the spine. I would suggest a comprehensive laboratory evaluation for the discussed infectious and rheumatologic disorders.
The patient's back pain was controlled with antiinflammatory medications overnight. Due to the patient's stable condition and lack of a diagnosis, empiric antibiotics were not initiated. An extensive workup was sent, including antistreptolysin O, polymerase chain reaction for Chlamydia, Neisseria gonorrhoeae, EBV, and parvovirus B19 DNA, serologies for Coccidioides immunoglobulin G (IgG) and IgM, urinary antigen for Histoplasma, HIV enzyme‐linked immunosorbent assay (ELISA) and Western blot, serum angiotensin‐converting enzyme level, C‐reactive protein, rheumatoid factor, antinuclear antibody, and antidouble‐stranded DNA antibodies.
Without a clear diagnosis, I would recommend against treatment with empiric antibiotics. At this point, I agree with waiting for the results of the pending workup.
On hospital day 1, the patient developed severe acute left ankle pain. On examination, the joint was exquisitely tender with decreased range of motion. Arthrocentesis was promptly performed. The synovial fluid WBC count was 1370/L with a differential of 82% neutrophils and 18% monocytes. No crystals were identified and the bacterial Gram stain was negative. He was treated with antiinflammatory medications. Bacterial blood cultures, obtained from the day of admission, were negative.
The arthrocentesis reveals a polymorphonuclear‐predominant fluid; however, the WBC count in the fluid is only mildly elevated. While the elevated monocyte count could again be consistent with viral arthropathies or juvenile RA, there is currently no systemic evidence of either illness. It is important to await the results of the final cultures, but the low WBC count and negative Gram stain decrease the probability of a septic joint. Empiric antibiotics to cover Gram‐positive organisms and gonococci would not be unreasonable, pending joint fluid culture results. The monocytosis could also be consistent with a fungal arthritis.
On hospital day 2, the results of the rheumatologic and infectious evaluation were negative with the exception of C‐reactive protein, which was 11.8 mg/dL (normal, 0.8), antinuclear antibody titer of 1:160 (normal, 1:40), Coccidioides IgM enzyme immunoassay (EIA) 0.710 (negative, 0.150), and Coccidioides tube‐precipitin (TP) immunodiffusion (ID) antibody‐positive. Coccidioides IgG EIA was negative.
The serologic tests are consistent with primary coccidioidomycosis. This is often a challenging diagnosis due to the nonspecific signs and symptoms, such as cough, fever, myalgias, and fatigue. Since screening EIAs are sensitive but not specific, concern for coccidioidomycosis or abnormal EIA results should prompt confirmatory testing with complement fixation titers (CF) and TP ID. Treatment with fluconazole should be initiated. Since the patient does not have central nervous system (CNS) symptoms, I would not recommend lumbar puncture at this point. However, a bone scan should be done for assessment of the back pain.
The patient was diagnosed with primary coccidioidomycosis infection with immune‐complexmediated arthritis and EN. A bone scan was negative. The patient was treated with fluconazole and discharged with 3 months of therapy. At follow‐up clinic visits after completion of therapy, his symptoms had resolved and his titers had normalized.
Discussion
The diagnosis of coccidioidomycosis is often challenging due to its protean manifestations. Four clinical syndromes are commonly seen: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease involving the skin, lymph nodes, bones, joints, and meninges. The most common clinical manifestation, acute pneumonia, may be indistinguishable from other causes of community‐acquired pneumonia (CAP). In a study of CAP in Arizona, 29% of cases were positive for coccidioidal infection through serologic evaluation.1 Features suggestive of coccidioidal infection include fatigue, severe headache, and pleuritic chest pain. Adenopathy in the hilar or paratracheal regions can be seen in 25% of infections.2 Chronic progressive pneumonia refers to infections in which symptoms, including cough, hemoptysis, and weight loss, persist for longer than 3 months. Pulmonary nodules and cavities are residual manifestations of primary pulmonary infection and occur in 2% to 8% of cases. Extrapulmonary disease develops in less than 5% of immunocompetent patients with primary pulmonary infection, with higher prevalence in patients of African American and Filipino decent. Immunocompromised patients are at increased risk for extrapulmonary infection. The most serious site of extrapulmonary disease is the meninges. Coccidioidal meningitis carries nearly 100% mortality rate if left untreated. The presentation is variable with up to 75% of cases reporting headache. While coccidioidal pneumonia also frequently presents with headache, symptoms including altered mental status, focal neurological deficits, and persistent or progressive headache are more suggestive of meningeal disease.3
Patients with any presentation of coccidioidomycosis can display immune‐mediated manifestations such as EN, arthralgias (desert rheumatism), and in some cases mild conjunctivitis.4 It is hypothesized that these findings occur due to a hypersensitivity reaction to coccidioidomycosis.4 EN is an inflammatory process of the subcutaneous fat, which presents as tender and erythematous nodules typically on the lower extremities. EN is not a disease entity or site of metastatic infection, but a response to underlying illness. Its recognition should trigger a search for the primary etiology, as guided by the patient's history and clinical presentation. The differential diagnosis for EN is broad and includes rheumatologic, infectious, medication‐related, inflammatory, and idiopathic processes (Table 1). Coccidioidomycosis should be strongly considered based on geographical location, with the vast majority of cases seen in southern California, Arizona, Nevada, New Mexico, and Texas. While the pathophysiology of EN has not been completely elucidated, the lesions may reflect a vigorous immune response conferring a protective advantage. Interestingly, a study of pregnant women with coccidioidomycosis revealed a decreased incidence of disseminated disease in patients with EN.5, 6
| Rheumatologic/autoimmune |
| Systemic lupus erythematosus |
| Wegener's granulomatosis |
| Sarcoidosis |
| Infectious |
| Streptococcus pyogenes causing pharyngitis (most common) |
| Borrelia burgdorferi |
| Mycoplasma pneumoniae |
| Bartonella henselae |
| Shigella |
| Campylobacter jejuni |
| Salmonella |
| Yersinia enterocolitica |
| Chlamydia |
| Brucella |
| Escherichia coli |
| Treponema pallidum |
| Mycobacterium leprae |
| Neisseria gonorrhoeae |
| Mycobacterium tuberculosis |
| Human immunodeficiency virus |
| Epstein‐Barr virus |
| Cytomegalovirus |
| Influenza |
| Varicella Zoster virus |
| Coccidioides immitis |
| Histoplasma capsulatum |
| Blastomyces dermatitidis |
| Dermatophytic fungal infections (rare) |
| Gastrointestinal |
| Ulcerative colitis |
| Crohn's disease |
| Celiac disease |
| Behcet's disease |
| Medications |
| Oral contraceptives |
| Proton pump inhibitors |
| Sulfonamides |
| Leukotriene modifiers (montelukast) |
| Hepatitis B vaccine |
| Isoretinoin |
| Miscellaneous |
| Hodgkins lymphoma |
| Sweet's syndrome |
Coccidioidomycosis is also associated with immune‐mediated arthralgias and arthritis. These manifestations occur in up to one‐third of patients with concomitant EN. Arthritis may be monoarticular or polyarticular, often affecting large joints such as the knees or ankles. It is important to note that septic arthritis can also occur and should be differentiated from rheumatism by joint aspiration.
The diagnosis of coccidioidomycosis can be made by serologic testing, direct isolation of the organism on culture, or visualization on tissue biopsy. Of these methods, serologic testing is most commonly utilized. The 2007 Infectious Disease Society of America (IDSA) and American Thoracic Society guidelines recommend diagnostic testing in hospitalized patients with CAP who reside in or have recently traveled (within 2 weeks) to endemic areas.7 There are multiple approaches to serologic diagnosis based on identification of IgM or IgG antibodies to various coccidioidal antigens. During the early phase of infection, TP ID and EIA can be utilized to detect IgM antibodies. While EIA testing has 92% sensitivity, it has high rates of false‐positive results, and therefore confirmatory testing with ID is recommended. ID has variable sensitivity, but 90% of patients will test positive by 3 weeks of infection.8 During the later phase of the infection, IgG antibodies are detected either quantitatively by CF or qualitatively by ID and EIA. CF can provide information on the severity of illness and prognosis based on titer levels, as well as serving as a marker for response to treatment.2 Positive titers greater than 1:32 suggest disseminated disease. In addition, CF titer in the cerebrospinal fluid is the test of choice in diagnosis of coccidioidal meningitis. An evaluation for disseminated disease should be initiated if the patient has any risk factors or clinically concerning symptoms for bone or CNS involvement. This evaluation includes a bone scan and lumbar puncture. All patients should be assessed for immunocompromised status.
The management of coccidioidomycosis is based on the extent of infection, the severity of illness, and the immune status of the patient. In 95% of cases of uncomplicated pulmonary disease in an immunocompetent host, the symptoms will resolve without treatment with antifungal agents.9 The decision to treat uncomplicated pulmonary disease is based on severity of illness. While there is no consensus recommendation, commonly used indicators for treatment include persistent fever, age >55 years, symptoms greater than 2 months, hilar adenopathy, diffuse pulmonary infiltrates, weight loss, and inability to work.9 In patients with chronic progressive pneumonia or extrapulmonary involvement, treatment with antifungal medications should be initiated. While fluconazole remains the preferred treatment in coccidioidal pneumonia and meningitis, amphotericin B preparations should be considered for diffuse coccidioidal pneumonia and disseminated disease, including refractory meningitis.9 The use of newer azoles, particularly posaconazole, has been studied in a limited number of patients with refractory coccidioidomycosis with improvement in symptoms.10 Frequent follow‐up visits are recommended to detect progression of disease or to document resolution, with improving symptoms and decreasing titers. Duration of therapy in uncomplicated cases should be at least 3 months. Treatment of extrapulmonary disease can span years, and in the case of meningitis lifetime treatment is recommended given the high rate of relapse.
While the patient and the clinicians were aching for a diagnosis after the initial negative evaluation, recognition of the immunologic manifestations of coccidioidomycosis was essential in this case. Coccidioidomycosis should be considered in patients presenting with EN, regardless of presence of concurrent pulmonary symptoms; particularly in patients living in or with recent travel to endemic areas. Furthermore, the severity of symptoms can guide the decision and duration of treatment.
Teaching Points
-
Coccidioidomycosis has 4 main clinical presentations: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease.
-
Independent of pulmonary symptoms, coccidioidomycosis can present with immune‐mediated manifestations, such as EN and arthritis.
-
The diagnosis of coccidioidomycosis often relies on serologic testing for early and late infection.
-
Treatment of coccidioidomycosis is based on risk factors and severity of symptoms. High‐risk and symptomatic patients can be treated with fluconazole or amphotericin B.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
- ,,, et al.Coccidioidomycosis as a common cause of community‐acquired pneumonia.Emerg Infect Dis.2006;12:958–962.
- ,.Coccidioidomycosis.Mayo Clin Proc.2008;83:343–349.
- ,.Coccidioidal meningitis.Clin Infect Dis.2006;42:103–107.
- ,.Coccidioidomycosis: host response and vaccine development.Clin Microbiol Rev.2004;17:804–839.
- ,,.Erythema nodosum in pregnant patients with coccidioidomycosis.Clin Infect Dis.1998;27:1201–1203.
- .Protective effects of erythema nodosum in coccidioidomycosis.Lancet.1999;353:168.
- ,,, et al.Infectious Disease Society of America/American Thoracic Society consensus guidelines on management of community acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72
- .Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci.2007;1111:301–314.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
A 23‐year‐old Caucasian man presented to an outpatient clinic with a sore throat and associated subjective fevers. His evaluation included a negative rapid streptococcus test; nevertheless, he was empirically treated with amoxicillin. The following day, he experienced increasing sore throat and presented to the emergency department (ED). He was treated with prednisone and morphine sulfate and discharged home with azithromycin.
Initial considerations in a healthy young man who presents with fever and pharyngitis should focus on common infectious etiologies. Viral illnesses are the most frequent causes of sore throat and fever. These often manifest as mononucleosis‐like illnesses and include Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). In this age group, it is also critical to consider sexually transmitted diseases (STDs) such as gonorrhea, human immunodeficiency virus (HIV), herpes simplex virus, and syphilis. Consideration of streptococcal pharyngitis is important. Since the rapid streptococcal antigen test is neither sensitive nor specific, confirmation of infection should be based on clinical findings and a culture of the pharynx for group A Streptococcus. Other common etiologies of fever and pharyngitis include acute or chronic sinusitis with postnasal drainage. Due to the progressive nature of the sore throat, there should be an evaluation for difficulty swallowing, problems phonating, or neck discomfort, any of which would be concerning for a retropharyngeal abscess. Additional history should be obtained with focus on sexual history, previous STDs, recent sick contacts, and other supporting signs and symptoms of viral illnesses.
Eight days after the initial onset of symptoms, the patient developed acute low back pain. The back pain was midline, severe, and constant around the lumbar spine. There was no saddle anesthesia, bowel or bladder dysfunction, or weakness or numbness in the extremities. He also noted swelling of the left fourth metacarpophalangeal joint and an erythematous rash on his right knee and anterior tibial region of the right leg. He continued to experience subjective fevers, sore throat, and swollen neck glands. Due to the severity and discomfort of symptoms, the patient returned to the ED.
With no history of trauma, the subsequent development of acute low back pain may be related to the patient's sore throat and fever. Monoarticular arthritis with contralateral skin lesions should raise suspicion for a systemic process, particularly infection or a rheumatologic syndrome. Infectious etiologies would include rheumatic fever, endocarditis with septic emboli, and osteomyelitis. Rheumatologic causes, such as ankylosing spondylitis and juvenile rheumatoid arthritis (RA), are also possibilities. The infectious evaluation should include an assessment of a history of intravenous drug use (IVDU) and underlying valvular disorders, which will increase the risk for endocarditis and therefore septic emboli. Acute HIV infection can be seen as early as 1 to 2 weeks postexposure and should be considered as well. Appropriate testing would include both conventional HIV antibody tests and HIV viral load assay. Lastly, in considering the patient's symptoms, obtaining his travel history to identify risk for Lyme disease would also be appropriate.
The patient did not report any further positive findings on review of systems. He did not have any significant past medical history and did not take any chronic medications. He had no sick contacts. He rarely drank alcohol and denied IVDU and sexual activity over the past year. He was previously involved in monogamous relationships with women. His last HIV test, 1 year prior, was negative. He did not have any history of STDs. He was a graduate student in computer science and lived in southern California. He had recently traveled to central California and France for 2 weeks, staying in larger cities. He had not been hiking during that time. His family history was significant for hypertension.
The travel history is provocative for 3 diseases of the reticuloendothelial system with possible systemic manifestations. First, toxoplasmosis, which is endemic in France where rare or raw beef and lamb are frequently consumed. It may present as a mononucleosis‐like illness and rarely as atypical pneumonia. Second, tuberculosis, which is also endemic in France, especially in major cities. Although most commonly a self‐limited respiratory disease, it may disseminate with systemic symptoms. Third, primary coccidioidomycosis, which is prevalent in the central valleys of California. The climate and wind patterns lead to aerosolization of the spores and make this a common respiratory pathogen.
The physical exam should include a detailed evaluation of the eyes for uveitis and iritis, seen in some rheumatologic disorders. A pharyngeal exam with assessment for exudate can support streptococcal pharyngitis or diphtheria. Evaluation for lymphadenopathy, while nonspecific, would be important for streptococcal pharyngitis, rheumatic fever, and juvenile RA. Further characterizing the rash is essential in distinguishing viral exanthems from the fleeting salmon‐colored maculopapular rash of juvenile RA. Assessment for peripheral stigmata of endocarditis should be done. A thorough joint exam should evaluate evidence of inflammatory or infectious joint disease.
On physical exam, he was a thin man who appeared anxious but in no acute distress. His temperature was 36.7C, blood pressure 111/68 mm Hg, heart rate 83 beats/minute, respiratory rate 16 breaths/minute, and oxygen saturation 99% on room air. Erythema was noted in the posterior oropharynx with no tonsillar exudate. There were several subcentimeter, nontender, and mobile lymph nodes in the anterior cervical chain bilaterally. The cardiovascular exam revealed normal sinus rhythm with a 2/6 systolic murmur at the apex, without radiation. His lungs were clear to auscultation. Skin exam revealed 2 blanching erythematous, indurated, and tender lesions on the right pretibial region, 2‐cm and 4‐cm in diameter. Two other similar, but smaller, lesions were noted on the left upper extremity and left ankle. His lumbar spine was slightly tender to touch. A complete joint exam was normal, including the left fourth metacarpophalangeal joint. Neurological exam, including bilateral strength, sensation, reflexes, and gait, was unremarkable.
Younger patients are subject to social‐acceptance bias and can deny sexual activity on initial inquiry. An objective evaluation for STDs with serologic workup should still be pursued. The cervical lymphadenopathy and tonsillar erythema continue to suggest a viral illness. While the systolic murmur may be physiologic, subjective fevers, disseminated cutaneous lesions, and arthritis warrant evaluation for bacterial endocarditis with blood cultures and an echocardiogram.
On exam, there is no evidence of true joint involvement and this decreases the likelihood of rheumatologic conditions, such as ankylosing spondylitis and juvenile RA. However, the skin lesions are suspicious for erythema nodosum (EN), which should prompt a biopsy and an evaluation for infectious etiologies. Serologies should include evaluation of Chlamydia, Mycoplasma, Coccidioides, and Histoplasma. I would also examine the feet carefully for potential transcutaneous inoculation by microorganisms that can produce a rash similar to EN. For instance, penetrating skin trauma can lead to pseudomonal infection. Brucella (from ingesting unpasteurized milk or milk products), Bartonella (from the scratches of feline animals), and Francisella tularensis (from rabbit exposure) can also produce skin lesions that mimic EN. These are best distinguished through a detailed history, concomitant serologic workup, and biopsy. Other noninfectious etiologies of EN can include inflammatory bowel disease, Behcet's, and sarcoidosis; however, the patient does not currently report any symptoms supporting these diagnoses. In addition to the above evaluation, complete blood count with differential, liver function tests, creatinine, and urinalysis should be obtained.
The patient's white blood cell (WBC) count was 12,100/L with 73% neutrophils, 14% lymphocytes, and 12% monocytes. Hemoglobin was 11.8 g/dL and platelet count 292,000/L. Chemistry panel and liver function tests were unremarkable. Erythrocyte sedimentation rate (ESR) was 71 mm/hour (range, 010). Urinalysis was negative for protein and red blood cells. Chest x‐ray did not illustrate any abnormalities. Computed tomography (CT) of the lumbar spine revealed a small posterior disc bulge at L4‐5 and L5‐S1.
The moderate leukocytosis with neutrophilic predominance and monocytosis raises concern for a systemic inflammatory process; the elevated ESR further supports this. Monocytosis can be seen in a number of infectious, autoimmune, and malignant conditions. Tuberculosis, brucellosis, bacterial endocarditis, syphilis, infectious mononucleosis, and viral illnesses are among the infections typically characterized by monocytosis. Autoimmune illnesses, such as systemic lupus erythematosus and RA can also have similar presentations. The patient does not have any features of an underlying malignancy, such as weight loss or night sweats; however, if the autoimmune and infectious evaluations are negative, Hodgkin's disease and certain leukemias should be considered. There is no evidence of osteomyelitis on the spine CT, which decreases the possibility of (but does not exclude) infectious or rheumatologic conditions of the spine. I would suggest a comprehensive laboratory evaluation for the discussed infectious and rheumatologic disorders.
The patient's back pain was controlled with antiinflammatory medications overnight. Due to the patient's stable condition and lack of a diagnosis, empiric antibiotics were not initiated. An extensive workup was sent, including antistreptolysin O, polymerase chain reaction for Chlamydia, Neisseria gonorrhoeae, EBV, and parvovirus B19 DNA, serologies for Coccidioides immunoglobulin G (IgG) and IgM, urinary antigen for Histoplasma, HIV enzyme‐linked immunosorbent assay (ELISA) and Western blot, serum angiotensin‐converting enzyme level, C‐reactive protein, rheumatoid factor, antinuclear antibody, and antidouble‐stranded DNA antibodies.
Without a clear diagnosis, I would recommend against treatment with empiric antibiotics. At this point, I agree with waiting for the results of the pending workup.
On hospital day 1, the patient developed severe acute left ankle pain. On examination, the joint was exquisitely tender with decreased range of motion. Arthrocentesis was promptly performed. The synovial fluid WBC count was 1370/L with a differential of 82% neutrophils and 18% monocytes. No crystals were identified and the bacterial Gram stain was negative. He was treated with antiinflammatory medications. Bacterial blood cultures, obtained from the day of admission, were negative.
The arthrocentesis reveals a polymorphonuclear‐predominant fluid; however, the WBC count in the fluid is only mildly elevated. While the elevated monocyte count could again be consistent with viral arthropathies or juvenile RA, there is currently no systemic evidence of either illness. It is important to await the results of the final cultures, but the low WBC count and negative Gram stain decrease the probability of a septic joint. Empiric antibiotics to cover Gram‐positive organisms and gonococci would not be unreasonable, pending joint fluid culture results. The monocytosis could also be consistent with a fungal arthritis.
On hospital day 2, the results of the rheumatologic and infectious evaluation were negative with the exception of C‐reactive protein, which was 11.8 mg/dL (normal, 0.8), antinuclear antibody titer of 1:160 (normal, 1:40), Coccidioides IgM enzyme immunoassay (EIA) 0.710 (negative, 0.150), and Coccidioides tube‐precipitin (TP) immunodiffusion (ID) antibody‐positive. Coccidioides IgG EIA was negative.
The serologic tests are consistent with primary coccidioidomycosis. This is often a challenging diagnosis due to the nonspecific signs and symptoms, such as cough, fever, myalgias, and fatigue. Since screening EIAs are sensitive but not specific, concern for coccidioidomycosis or abnormal EIA results should prompt confirmatory testing with complement fixation titers (CF) and TP ID. Treatment with fluconazole should be initiated. Since the patient does not have central nervous system (CNS) symptoms, I would not recommend lumbar puncture at this point. However, a bone scan should be done for assessment of the back pain.
The patient was diagnosed with primary coccidioidomycosis infection with immune‐complexmediated arthritis and EN. A bone scan was negative. The patient was treated with fluconazole and discharged with 3 months of therapy. At follow‐up clinic visits after completion of therapy, his symptoms had resolved and his titers had normalized.
Discussion
The diagnosis of coccidioidomycosis is often challenging due to its protean manifestations. Four clinical syndromes are commonly seen: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease involving the skin, lymph nodes, bones, joints, and meninges. The most common clinical manifestation, acute pneumonia, may be indistinguishable from other causes of community‐acquired pneumonia (CAP). In a study of CAP in Arizona, 29% of cases were positive for coccidioidal infection through serologic evaluation.1 Features suggestive of coccidioidal infection include fatigue, severe headache, and pleuritic chest pain. Adenopathy in the hilar or paratracheal regions can be seen in 25% of infections.2 Chronic progressive pneumonia refers to infections in which symptoms, including cough, hemoptysis, and weight loss, persist for longer than 3 months. Pulmonary nodules and cavities are residual manifestations of primary pulmonary infection and occur in 2% to 8% of cases. Extrapulmonary disease develops in less than 5% of immunocompetent patients with primary pulmonary infection, with higher prevalence in patients of African American and Filipino decent. Immunocompromised patients are at increased risk for extrapulmonary infection. The most serious site of extrapulmonary disease is the meninges. Coccidioidal meningitis carries nearly 100% mortality rate if left untreated. The presentation is variable with up to 75% of cases reporting headache. While coccidioidal pneumonia also frequently presents with headache, symptoms including altered mental status, focal neurological deficits, and persistent or progressive headache are more suggestive of meningeal disease.3
Patients with any presentation of coccidioidomycosis can display immune‐mediated manifestations such as EN, arthralgias (desert rheumatism), and in some cases mild conjunctivitis.4 It is hypothesized that these findings occur due to a hypersensitivity reaction to coccidioidomycosis.4 EN is an inflammatory process of the subcutaneous fat, which presents as tender and erythematous nodules typically on the lower extremities. EN is not a disease entity or site of metastatic infection, but a response to underlying illness. Its recognition should trigger a search for the primary etiology, as guided by the patient's history and clinical presentation. The differential diagnosis for EN is broad and includes rheumatologic, infectious, medication‐related, inflammatory, and idiopathic processes (Table 1). Coccidioidomycosis should be strongly considered based on geographical location, with the vast majority of cases seen in southern California, Arizona, Nevada, New Mexico, and Texas. While the pathophysiology of EN has not been completely elucidated, the lesions may reflect a vigorous immune response conferring a protective advantage. Interestingly, a study of pregnant women with coccidioidomycosis revealed a decreased incidence of disseminated disease in patients with EN.5, 6
| Rheumatologic/autoimmune |
| Systemic lupus erythematosus |
| Wegener's granulomatosis |
| Sarcoidosis |
| Infectious |
| Streptococcus pyogenes causing pharyngitis (most common) |
| Borrelia burgdorferi |
| Mycoplasma pneumoniae |
| Bartonella henselae |
| Shigella |
| Campylobacter jejuni |
| Salmonella |
| Yersinia enterocolitica |
| Chlamydia |
| Brucella |
| Escherichia coli |
| Treponema pallidum |
| Mycobacterium leprae |
| Neisseria gonorrhoeae |
| Mycobacterium tuberculosis |
| Human immunodeficiency virus |
| Epstein‐Barr virus |
| Cytomegalovirus |
| Influenza |
| Varicella Zoster virus |
| Coccidioides immitis |
| Histoplasma capsulatum |
| Blastomyces dermatitidis |
| Dermatophytic fungal infections (rare) |
| Gastrointestinal |
| Ulcerative colitis |
| Crohn's disease |
| Celiac disease |
| Behcet's disease |
| Medications |
| Oral contraceptives |
| Proton pump inhibitors |
| Sulfonamides |
| Leukotriene modifiers (montelukast) |
| Hepatitis B vaccine |
| Isoretinoin |
| Miscellaneous |
| Hodgkins lymphoma |
| Sweet's syndrome |
Coccidioidomycosis is also associated with immune‐mediated arthralgias and arthritis. These manifestations occur in up to one‐third of patients with concomitant EN. Arthritis may be monoarticular or polyarticular, often affecting large joints such as the knees or ankles. It is important to note that septic arthritis can also occur and should be differentiated from rheumatism by joint aspiration.
The diagnosis of coccidioidomycosis can be made by serologic testing, direct isolation of the organism on culture, or visualization on tissue biopsy. Of these methods, serologic testing is most commonly utilized. The 2007 Infectious Disease Society of America (IDSA) and American Thoracic Society guidelines recommend diagnostic testing in hospitalized patients with CAP who reside in or have recently traveled (within 2 weeks) to endemic areas.7 There are multiple approaches to serologic diagnosis based on identification of IgM or IgG antibodies to various coccidioidal antigens. During the early phase of infection, TP ID and EIA can be utilized to detect IgM antibodies. While EIA testing has 92% sensitivity, it has high rates of false‐positive results, and therefore confirmatory testing with ID is recommended. ID has variable sensitivity, but 90% of patients will test positive by 3 weeks of infection.8 During the later phase of the infection, IgG antibodies are detected either quantitatively by CF or qualitatively by ID and EIA. CF can provide information on the severity of illness and prognosis based on titer levels, as well as serving as a marker for response to treatment.2 Positive titers greater than 1:32 suggest disseminated disease. In addition, CF titer in the cerebrospinal fluid is the test of choice in diagnosis of coccidioidal meningitis. An evaluation for disseminated disease should be initiated if the patient has any risk factors or clinically concerning symptoms for bone or CNS involvement. This evaluation includes a bone scan and lumbar puncture. All patients should be assessed for immunocompromised status.
The management of coccidioidomycosis is based on the extent of infection, the severity of illness, and the immune status of the patient. In 95% of cases of uncomplicated pulmonary disease in an immunocompetent host, the symptoms will resolve without treatment with antifungal agents.9 The decision to treat uncomplicated pulmonary disease is based on severity of illness. While there is no consensus recommendation, commonly used indicators for treatment include persistent fever, age >55 years, symptoms greater than 2 months, hilar adenopathy, diffuse pulmonary infiltrates, weight loss, and inability to work.9 In patients with chronic progressive pneumonia or extrapulmonary involvement, treatment with antifungal medications should be initiated. While fluconazole remains the preferred treatment in coccidioidal pneumonia and meningitis, amphotericin B preparations should be considered for diffuse coccidioidal pneumonia and disseminated disease, including refractory meningitis.9 The use of newer azoles, particularly posaconazole, has been studied in a limited number of patients with refractory coccidioidomycosis with improvement in symptoms.10 Frequent follow‐up visits are recommended to detect progression of disease or to document resolution, with improving symptoms and decreasing titers. Duration of therapy in uncomplicated cases should be at least 3 months. Treatment of extrapulmonary disease can span years, and in the case of meningitis lifetime treatment is recommended given the high rate of relapse.
While the patient and the clinicians were aching for a diagnosis after the initial negative evaluation, recognition of the immunologic manifestations of coccidioidomycosis was essential in this case. Coccidioidomycosis should be considered in patients presenting with EN, regardless of presence of concurrent pulmonary symptoms; particularly in patients living in or with recent travel to endemic areas. Furthermore, the severity of symptoms can guide the decision and duration of treatment.
Teaching Points
-
Coccidioidomycosis has 4 main clinical presentations: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease.
-
Independent of pulmonary symptoms, coccidioidomycosis can present with immune‐mediated manifestations, such as EN and arthritis.
-
The diagnosis of coccidioidomycosis often relies on serologic testing for early and late infection.
-
Treatment of coccidioidomycosis is based on risk factors and severity of symptoms. High‐risk and symptomatic patients can be treated with fluconazole or amphotericin B.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
A 23‐year‐old Caucasian man presented to an outpatient clinic with a sore throat and associated subjective fevers. His evaluation included a negative rapid streptococcus test; nevertheless, he was empirically treated with amoxicillin. The following day, he experienced increasing sore throat and presented to the emergency department (ED). He was treated with prednisone and morphine sulfate and discharged home with azithromycin.
Initial considerations in a healthy young man who presents with fever and pharyngitis should focus on common infectious etiologies. Viral illnesses are the most frequent causes of sore throat and fever. These often manifest as mononucleosis‐like illnesses and include Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). In this age group, it is also critical to consider sexually transmitted diseases (STDs) such as gonorrhea, human immunodeficiency virus (HIV), herpes simplex virus, and syphilis. Consideration of streptococcal pharyngitis is important. Since the rapid streptococcal antigen test is neither sensitive nor specific, confirmation of infection should be based on clinical findings and a culture of the pharynx for group A Streptococcus. Other common etiologies of fever and pharyngitis include acute or chronic sinusitis with postnasal drainage. Due to the progressive nature of the sore throat, there should be an evaluation for difficulty swallowing, problems phonating, or neck discomfort, any of which would be concerning for a retropharyngeal abscess. Additional history should be obtained with focus on sexual history, previous STDs, recent sick contacts, and other supporting signs and symptoms of viral illnesses.
Eight days after the initial onset of symptoms, the patient developed acute low back pain. The back pain was midline, severe, and constant around the lumbar spine. There was no saddle anesthesia, bowel or bladder dysfunction, or weakness or numbness in the extremities. He also noted swelling of the left fourth metacarpophalangeal joint and an erythematous rash on his right knee and anterior tibial region of the right leg. He continued to experience subjective fevers, sore throat, and swollen neck glands. Due to the severity and discomfort of symptoms, the patient returned to the ED.
With no history of trauma, the subsequent development of acute low back pain may be related to the patient's sore throat and fever. Monoarticular arthritis with contralateral skin lesions should raise suspicion for a systemic process, particularly infection or a rheumatologic syndrome. Infectious etiologies would include rheumatic fever, endocarditis with septic emboli, and osteomyelitis. Rheumatologic causes, such as ankylosing spondylitis and juvenile rheumatoid arthritis (RA), are also possibilities. The infectious evaluation should include an assessment of a history of intravenous drug use (IVDU) and underlying valvular disorders, which will increase the risk for endocarditis and therefore septic emboli. Acute HIV infection can be seen as early as 1 to 2 weeks postexposure and should be considered as well. Appropriate testing would include both conventional HIV antibody tests and HIV viral load assay. Lastly, in considering the patient's symptoms, obtaining his travel history to identify risk for Lyme disease would also be appropriate.
The patient did not report any further positive findings on review of systems. He did not have any significant past medical history and did not take any chronic medications. He had no sick contacts. He rarely drank alcohol and denied IVDU and sexual activity over the past year. He was previously involved in monogamous relationships with women. His last HIV test, 1 year prior, was negative. He did not have any history of STDs. He was a graduate student in computer science and lived in southern California. He had recently traveled to central California and France for 2 weeks, staying in larger cities. He had not been hiking during that time. His family history was significant for hypertension.
The travel history is provocative for 3 diseases of the reticuloendothelial system with possible systemic manifestations. First, toxoplasmosis, which is endemic in France where rare or raw beef and lamb are frequently consumed. It may present as a mononucleosis‐like illness and rarely as atypical pneumonia. Second, tuberculosis, which is also endemic in France, especially in major cities. Although most commonly a self‐limited respiratory disease, it may disseminate with systemic symptoms. Third, primary coccidioidomycosis, which is prevalent in the central valleys of California. The climate and wind patterns lead to aerosolization of the spores and make this a common respiratory pathogen.
The physical exam should include a detailed evaluation of the eyes for uveitis and iritis, seen in some rheumatologic disorders. A pharyngeal exam with assessment for exudate can support streptococcal pharyngitis or diphtheria. Evaluation for lymphadenopathy, while nonspecific, would be important for streptococcal pharyngitis, rheumatic fever, and juvenile RA. Further characterizing the rash is essential in distinguishing viral exanthems from the fleeting salmon‐colored maculopapular rash of juvenile RA. Assessment for peripheral stigmata of endocarditis should be done. A thorough joint exam should evaluate evidence of inflammatory or infectious joint disease.
On physical exam, he was a thin man who appeared anxious but in no acute distress. His temperature was 36.7C, blood pressure 111/68 mm Hg, heart rate 83 beats/minute, respiratory rate 16 breaths/minute, and oxygen saturation 99% on room air. Erythema was noted in the posterior oropharynx with no tonsillar exudate. There were several subcentimeter, nontender, and mobile lymph nodes in the anterior cervical chain bilaterally. The cardiovascular exam revealed normal sinus rhythm with a 2/6 systolic murmur at the apex, without radiation. His lungs were clear to auscultation. Skin exam revealed 2 blanching erythematous, indurated, and tender lesions on the right pretibial region, 2‐cm and 4‐cm in diameter. Two other similar, but smaller, lesions were noted on the left upper extremity and left ankle. His lumbar spine was slightly tender to touch. A complete joint exam was normal, including the left fourth metacarpophalangeal joint. Neurological exam, including bilateral strength, sensation, reflexes, and gait, was unremarkable.
Younger patients are subject to social‐acceptance bias and can deny sexual activity on initial inquiry. An objective evaluation for STDs with serologic workup should still be pursued. The cervical lymphadenopathy and tonsillar erythema continue to suggest a viral illness. While the systolic murmur may be physiologic, subjective fevers, disseminated cutaneous lesions, and arthritis warrant evaluation for bacterial endocarditis with blood cultures and an echocardiogram.
On exam, there is no evidence of true joint involvement and this decreases the likelihood of rheumatologic conditions, such as ankylosing spondylitis and juvenile RA. However, the skin lesions are suspicious for erythema nodosum (EN), which should prompt a biopsy and an evaluation for infectious etiologies. Serologies should include evaluation of Chlamydia, Mycoplasma, Coccidioides, and Histoplasma. I would also examine the feet carefully for potential transcutaneous inoculation by microorganisms that can produce a rash similar to EN. For instance, penetrating skin trauma can lead to pseudomonal infection. Brucella (from ingesting unpasteurized milk or milk products), Bartonella (from the scratches of feline animals), and Francisella tularensis (from rabbit exposure) can also produce skin lesions that mimic EN. These are best distinguished through a detailed history, concomitant serologic workup, and biopsy. Other noninfectious etiologies of EN can include inflammatory bowel disease, Behcet's, and sarcoidosis; however, the patient does not currently report any symptoms supporting these diagnoses. In addition to the above evaluation, complete blood count with differential, liver function tests, creatinine, and urinalysis should be obtained.
The patient's white blood cell (WBC) count was 12,100/L with 73% neutrophils, 14% lymphocytes, and 12% monocytes. Hemoglobin was 11.8 g/dL and platelet count 292,000/L. Chemistry panel and liver function tests were unremarkable. Erythrocyte sedimentation rate (ESR) was 71 mm/hour (range, 010). Urinalysis was negative for protein and red blood cells. Chest x‐ray did not illustrate any abnormalities. Computed tomography (CT) of the lumbar spine revealed a small posterior disc bulge at L4‐5 and L5‐S1.
The moderate leukocytosis with neutrophilic predominance and monocytosis raises concern for a systemic inflammatory process; the elevated ESR further supports this. Monocytosis can be seen in a number of infectious, autoimmune, and malignant conditions. Tuberculosis, brucellosis, bacterial endocarditis, syphilis, infectious mononucleosis, and viral illnesses are among the infections typically characterized by monocytosis. Autoimmune illnesses, such as systemic lupus erythematosus and RA can also have similar presentations. The patient does not have any features of an underlying malignancy, such as weight loss or night sweats; however, if the autoimmune and infectious evaluations are negative, Hodgkin's disease and certain leukemias should be considered. There is no evidence of osteomyelitis on the spine CT, which decreases the possibility of (but does not exclude) infectious or rheumatologic conditions of the spine. I would suggest a comprehensive laboratory evaluation for the discussed infectious and rheumatologic disorders.
The patient's back pain was controlled with antiinflammatory medications overnight. Due to the patient's stable condition and lack of a diagnosis, empiric antibiotics were not initiated. An extensive workup was sent, including antistreptolysin O, polymerase chain reaction for Chlamydia, Neisseria gonorrhoeae, EBV, and parvovirus B19 DNA, serologies for Coccidioides immunoglobulin G (IgG) and IgM, urinary antigen for Histoplasma, HIV enzyme‐linked immunosorbent assay (ELISA) and Western blot, serum angiotensin‐converting enzyme level, C‐reactive protein, rheumatoid factor, antinuclear antibody, and antidouble‐stranded DNA antibodies.
Without a clear diagnosis, I would recommend against treatment with empiric antibiotics. At this point, I agree with waiting for the results of the pending workup.
On hospital day 1, the patient developed severe acute left ankle pain. On examination, the joint was exquisitely tender with decreased range of motion. Arthrocentesis was promptly performed. The synovial fluid WBC count was 1370/L with a differential of 82% neutrophils and 18% monocytes. No crystals were identified and the bacterial Gram stain was negative. He was treated with antiinflammatory medications. Bacterial blood cultures, obtained from the day of admission, were negative.
The arthrocentesis reveals a polymorphonuclear‐predominant fluid; however, the WBC count in the fluid is only mildly elevated. While the elevated monocyte count could again be consistent with viral arthropathies or juvenile RA, there is currently no systemic evidence of either illness. It is important to await the results of the final cultures, but the low WBC count and negative Gram stain decrease the probability of a septic joint. Empiric antibiotics to cover Gram‐positive organisms and gonococci would not be unreasonable, pending joint fluid culture results. The monocytosis could also be consistent with a fungal arthritis.
On hospital day 2, the results of the rheumatologic and infectious evaluation were negative with the exception of C‐reactive protein, which was 11.8 mg/dL (normal, 0.8), antinuclear antibody titer of 1:160 (normal, 1:40), Coccidioides IgM enzyme immunoassay (EIA) 0.710 (negative, 0.150), and Coccidioides tube‐precipitin (TP) immunodiffusion (ID) antibody‐positive. Coccidioides IgG EIA was negative.
The serologic tests are consistent with primary coccidioidomycosis. This is often a challenging diagnosis due to the nonspecific signs and symptoms, such as cough, fever, myalgias, and fatigue. Since screening EIAs are sensitive but not specific, concern for coccidioidomycosis or abnormal EIA results should prompt confirmatory testing with complement fixation titers (CF) and TP ID. Treatment with fluconazole should be initiated. Since the patient does not have central nervous system (CNS) symptoms, I would not recommend lumbar puncture at this point. However, a bone scan should be done for assessment of the back pain.
The patient was diagnosed with primary coccidioidomycosis infection with immune‐complexmediated arthritis and EN. A bone scan was negative. The patient was treated with fluconazole and discharged with 3 months of therapy. At follow‐up clinic visits after completion of therapy, his symptoms had resolved and his titers had normalized.
Discussion
The diagnosis of coccidioidomycosis is often challenging due to its protean manifestations. Four clinical syndromes are commonly seen: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease involving the skin, lymph nodes, bones, joints, and meninges. The most common clinical manifestation, acute pneumonia, may be indistinguishable from other causes of community‐acquired pneumonia (CAP). In a study of CAP in Arizona, 29% of cases were positive for coccidioidal infection through serologic evaluation.1 Features suggestive of coccidioidal infection include fatigue, severe headache, and pleuritic chest pain. Adenopathy in the hilar or paratracheal regions can be seen in 25% of infections.2 Chronic progressive pneumonia refers to infections in which symptoms, including cough, hemoptysis, and weight loss, persist for longer than 3 months. Pulmonary nodules and cavities are residual manifestations of primary pulmonary infection and occur in 2% to 8% of cases. Extrapulmonary disease develops in less than 5% of immunocompetent patients with primary pulmonary infection, with higher prevalence in patients of African American and Filipino decent. Immunocompromised patients are at increased risk for extrapulmonary infection. The most serious site of extrapulmonary disease is the meninges. Coccidioidal meningitis carries nearly 100% mortality rate if left untreated. The presentation is variable with up to 75% of cases reporting headache. While coccidioidal pneumonia also frequently presents with headache, symptoms including altered mental status, focal neurological deficits, and persistent or progressive headache are more suggestive of meningeal disease.3
Patients with any presentation of coccidioidomycosis can display immune‐mediated manifestations such as EN, arthralgias (desert rheumatism), and in some cases mild conjunctivitis.4 It is hypothesized that these findings occur due to a hypersensitivity reaction to coccidioidomycosis.4 EN is an inflammatory process of the subcutaneous fat, which presents as tender and erythematous nodules typically on the lower extremities. EN is not a disease entity or site of metastatic infection, but a response to underlying illness. Its recognition should trigger a search for the primary etiology, as guided by the patient's history and clinical presentation. The differential diagnosis for EN is broad and includes rheumatologic, infectious, medication‐related, inflammatory, and idiopathic processes (Table 1). Coccidioidomycosis should be strongly considered based on geographical location, with the vast majority of cases seen in southern California, Arizona, Nevada, New Mexico, and Texas. While the pathophysiology of EN has not been completely elucidated, the lesions may reflect a vigorous immune response conferring a protective advantage. Interestingly, a study of pregnant women with coccidioidomycosis revealed a decreased incidence of disseminated disease in patients with EN.5, 6
| Rheumatologic/autoimmune |
| Systemic lupus erythematosus |
| Wegener's granulomatosis |
| Sarcoidosis |
| Infectious |
| Streptococcus pyogenes causing pharyngitis (most common) |
| Borrelia burgdorferi |
| Mycoplasma pneumoniae |
| Bartonella henselae |
| Shigella |
| Campylobacter jejuni |
| Salmonella |
| Yersinia enterocolitica |
| Chlamydia |
| Brucella |
| Escherichia coli |
| Treponema pallidum |
| Mycobacterium leprae |
| Neisseria gonorrhoeae |
| Mycobacterium tuberculosis |
| Human immunodeficiency virus |
| Epstein‐Barr virus |
| Cytomegalovirus |
| Influenza |
| Varicella Zoster virus |
| Coccidioides immitis |
| Histoplasma capsulatum |
| Blastomyces dermatitidis |
| Dermatophytic fungal infections (rare) |
| Gastrointestinal |
| Ulcerative colitis |
| Crohn's disease |
| Celiac disease |
| Behcet's disease |
| Medications |
| Oral contraceptives |
| Proton pump inhibitors |
| Sulfonamides |
| Leukotriene modifiers (montelukast) |
| Hepatitis B vaccine |
| Isoretinoin |
| Miscellaneous |
| Hodgkins lymphoma |
| Sweet's syndrome |
Coccidioidomycosis is also associated with immune‐mediated arthralgias and arthritis. These manifestations occur in up to one‐third of patients with concomitant EN. Arthritis may be monoarticular or polyarticular, often affecting large joints such as the knees or ankles. It is important to note that septic arthritis can also occur and should be differentiated from rheumatism by joint aspiration.
The diagnosis of coccidioidomycosis can be made by serologic testing, direct isolation of the organism on culture, or visualization on tissue biopsy. Of these methods, serologic testing is most commonly utilized. The 2007 Infectious Disease Society of America (IDSA) and American Thoracic Society guidelines recommend diagnostic testing in hospitalized patients with CAP who reside in or have recently traveled (within 2 weeks) to endemic areas.7 There are multiple approaches to serologic diagnosis based on identification of IgM or IgG antibodies to various coccidioidal antigens. During the early phase of infection, TP ID and EIA can be utilized to detect IgM antibodies. While EIA testing has 92% sensitivity, it has high rates of false‐positive results, and therefore confirmatory testing with ID is recommended. ID has variable sensitivity, but 90% of patients will test positive by 3 weeks of infection.8 During the later phase of the infection, IgG antibodies are detected either quantitatively by CF or qualitatively by ID and EIA. CF can provide information on the severity of illness and prognosis based on titer levels, as well as serving as a marker for response to treatment.2 Positive titers greater than 1:32 suggest disseminated disease. In addition, CF titer in the cerebrospinal fluid is the test of choice in diagnosis of coccidioidal meningitis. An evaluation for disseminated disease should be initiated if the patient has any risk factors or clinically concerning symptoms for bone or CNS involvement. This evaluation includes a bone scan and lumbar puncture. All patients should be assessed for immunocompromised status.
The management of coccidioidomycosis is based on the extent of infection, the severity of illness, and the immune status of the patient. In 95% of cases of uncomplicated pulmonary disease in an immunocompetent host, the symptoms will resolve without treatment with antifungal agents.9 The decision to treat uncomplicated pulmonary disease is based on severity of illness. While there is no consensus recommendation, commonly used indicators for treatment include persistent fever, age >55 years, symptoms greater than 2 months, hilar adenopathy, diffuse pulmonary infiltrates, weight loss, and inability to work.9 In patients with chronic progressive pneumonia or extrapulmonary involvement, treatment with antifungal medications should be initiated. While fluconazole remains the preferred treatment in coccidioidal pneumonia and meningitis, amphotericin B preparations should be considered for diffuse coccidioidal pneumonia and disseminated disease, including refractory meningitis.9 The use of newer azoles, particularly posaconazole, has been studied in a limited number of patients with refractory coccidioidomycosis with improvement in symptoms.10 Frequent follow‐up visits are recommended to detect progression of disease or to document resolution, with improving symptoms and decreasing titers. Duration of therapy in uncomplicated cases should be at least 3 months. Treatment of extrapulmonary disease can span years, and in the case of meningitis lifetime treatment is recommended given the high rate of relapse.
While the patient and the clinicians were aching for a diagnosis after the initial negative evaluation, recognition of the immunologic manifestations of coccidioidomycosis was essential in this case. Coccidioidomycosis should be considered in patients presenting with EN, regardless of presence of concurrent pulmonary symptoms; particularly in patients living in or with recent travel to endemic areas. Furthermore, the severity of symptoms can guide the decision and duration of treatment.
Teaching Points
-
Coccidioidomycosis has 4 main clinical presentations: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease.
-
Independent of pulmonary symptoms, coccidioidomycosis can present with immune‐mediated manifestations, such as EN and arthritis.
-
The diagnosis of coccidioidomycosis often relies on serologic testing for early and late infection.
-
Treatment of coccidioidomycosis is based on risk factors and severity of symptoms. High‐risk and symptomatic patients can be treated with fluconazole or amphotericin B.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
- ,,, et al.Coccidioidomycosis as a common cause of community‐acquired pneumonia.Emerg Infect Dis.2006;12:958–962.
- ,.Coccidioidomycosis.Mayo Clin Proc.2008;83:343–349.
- ,.Coccidioidal meningitis.Clin Infect Dis.2006;42:103–107.
- ,.Coccidioidomycosis: host response and vaccine development.Clin Microbiol Rev.2004;17:804–839.
- ,,.Erythema nodosum in pregnant patients with coccidioidomycosis.Clin Infect Dis.1998;27:1201–1203.
- .Protective effects of erythema nodosum in coccidioidomycosis.Lancet.1999;353:168.
- ,,, et al.Infectious Disease Society of America/American Thoracic Society consensus guidelines on management of community acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72
- .Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci.2007;1111:301–314.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
- ,,, et al.Coccidioidomycosis as a common cause of community‐acquired pneumonia.Emerg Infect Dis.2006;12:958–962.
- ,.Coccidioidomycosis.Mayo Clin Proc.2008;83:343–349.
- ,.Coccidioidal meningitis.Clin Infect Dis.2006;42:103–107.
- ,.Coccidioidomycosis: host response and vaccine development.Clin Microbiol Rev.2004;17:804–839.
- ,,.Erythema nodosum in pregnant patients with coccidioidomycosis.Clin Infect Dis.1998;27:1201–1203.
- .Protective effects of erythema nodosum in coccidioidomycosis.Lancet.1999;353:168.
- ,,, et al.Infectious Disease Society of America/American Thoracic Society consensus guidelines on management of community acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72
- .Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci.2007;1111:301–314.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www.blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www.blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www.blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
In reponse to: Optimization of antiviral prescribing for influenza
We thank Dr. Aldaja for the comments on our work. We agree that delays in laboratory diagnosis, and the inability to identify influenza viral subtype may further undermine the low rates of appropriate prescription of oseltamivir noted in our study. Additionally, we also suspect that improved diagnostic and treatment practices for patients with seasonal influenza are likely to benefit patients if an influenza pandemic were to arise.
We thank Dr. Aldaja for the comments on our work. We agree that delays in laboratory diagnosis, and the inability to identify influenza viral subtype may further undermine the low rates of appropriate prescription of oseltamivir noted in our study. Additionally, we also suspect that improved diagnostic and treatment practices for patients with seasonal influenza are likely to benefit patients if an influenza pandemic were to arise.
We thank Dr. Aldaja for the comments on our work. We agree that delays in laboratory diagnosis, and the inability to identify influenza viral subtype may further undermine the low rates of appropriate prescription of oseltamivir noted in our study. Additionally, we also suspect that improved diagnostic and treatment practices for patients with seasonal influenza are likely to benefit patients if an influenza pandemic were to arise.
RTNI to Safely Reduce Dysglycemia
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).
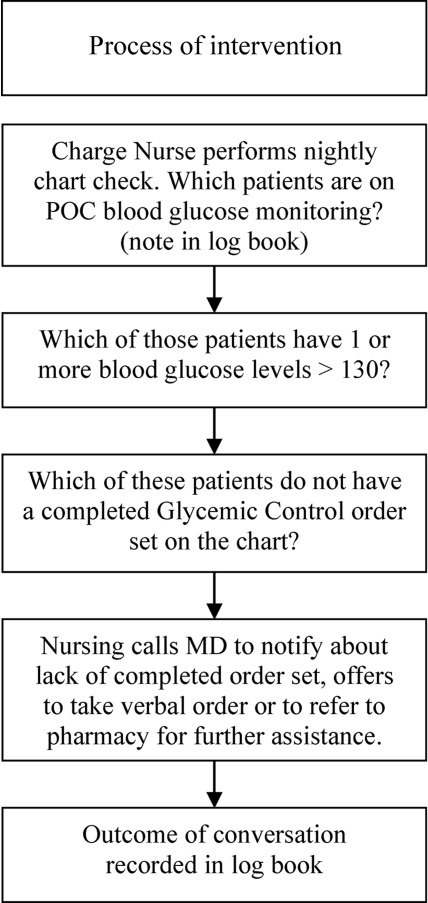
The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).
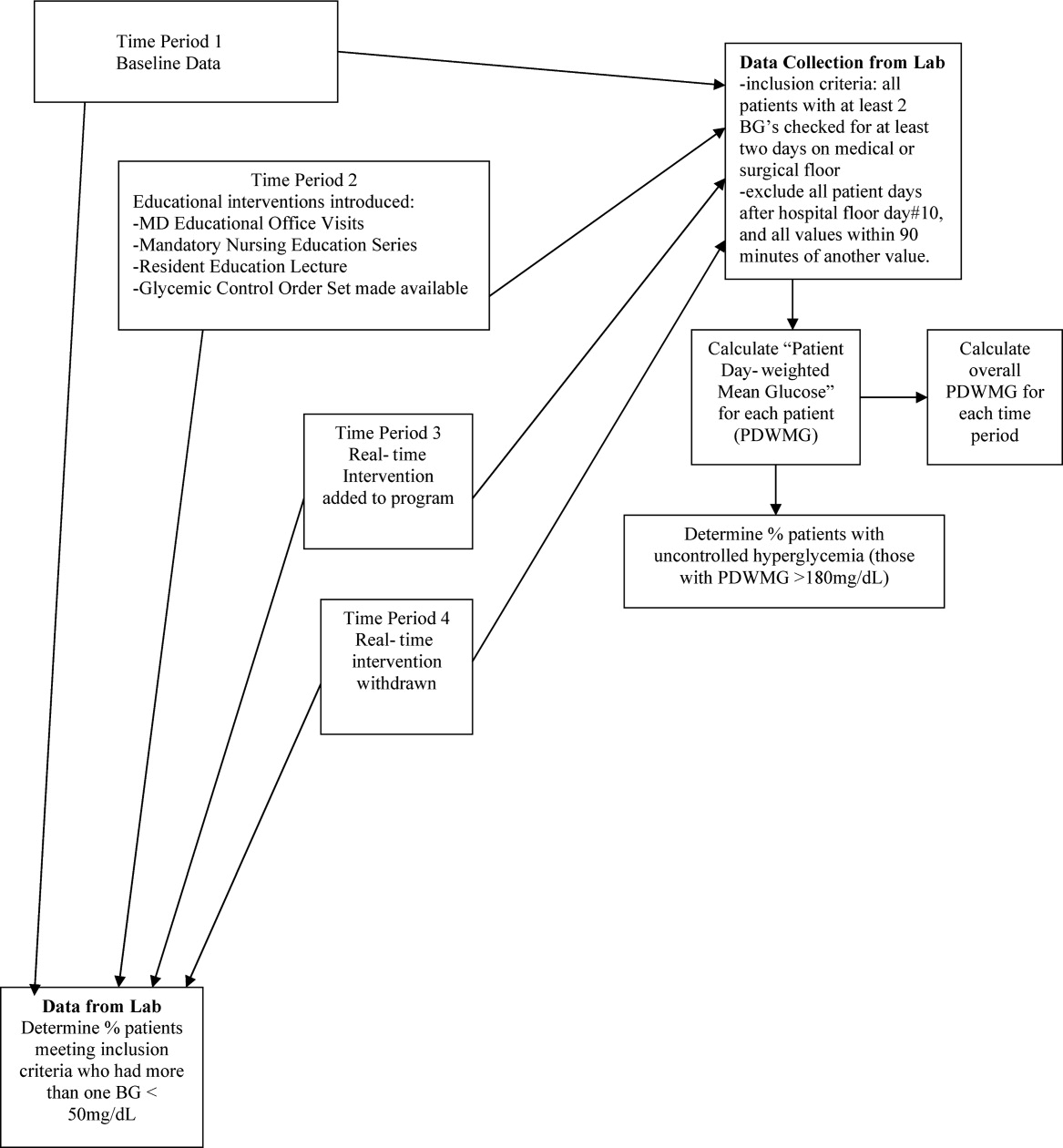
Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.
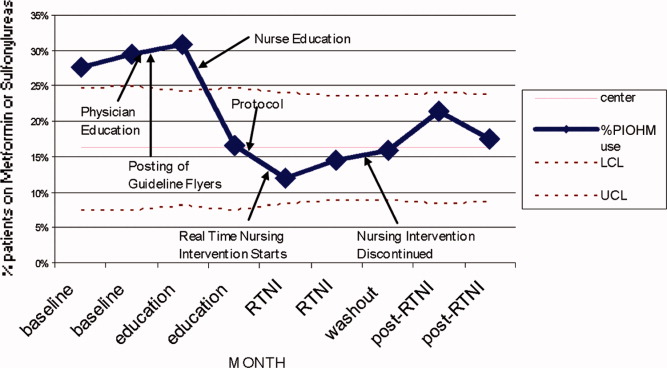

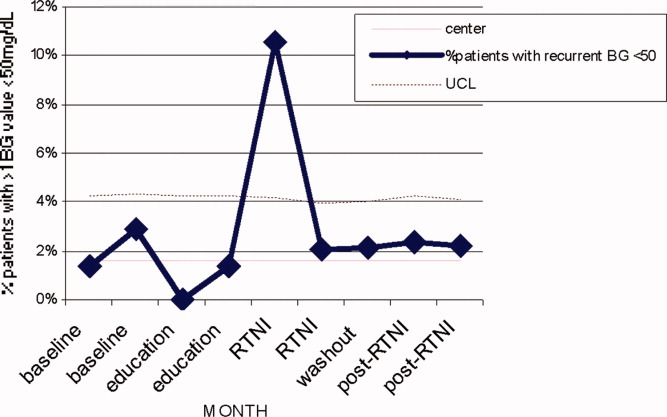
| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).

The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).

Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.



| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).

The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).

Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.



| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
Copyright © 2010 Society of Hospital Medicine
ICU Characteristics in Michigan
Organization of physician services in intensive care units (ICUs) varies widely and influences mortality, morbidity, and costs of care. Intensive care provided by intensivists in a high‐intensity physician staffing model, in which intensivists are the sole attending physicians or consult on all patients, has been associated with desirable outcomes such as decreased length of stay, resource utilization, and mortality.1‐4 As a result, higher intensity ICU models have been recommended by various healthcare agencies, including the National Quality Forum and the Leapfrog Group.5‐7
One national survey indicated that 47% of ICUs surveyed had some intensivist coverage and only 4% of intensive care units met Leapfrog high‐intensity model standards.8 However, only one‐third of ICUs responded to this survey, smaller ICUs were overrepresented, and the survey may not have reflected the influence of newer policy initiatives because it was conducted in 1997. Though the attributes by which intensivists improve patient outcomes is unknown, researchers have suggested it is by having a knowledgeable physician present in the ICU, having a physician communicate with other clinicians and families, and by having a physician who manages the ICU by writing policies and procedures and administrative activities.9
Results have been conflicting as patients managed by intensivists have also been found to have an increased mortality, particularly when managed on an elective consultation basis in an open ICU, where patient orders are written by several physician specialties.10, 11 Alternative ICU staffing models, such as the use of hospitalists, have been utilized to compensate for the intensivist workforce shortage. Hospitalists often provide ICU care, although they are seldom board‐certified in critical care. Hospitalist care has been shown to provide clinical and efficiency benefits such as decreased length of hospital stay.12‐14
Understanding the manner in which critical care is currently delivered, particularly the utilization of intensivist and nonintensivist care providers, can provide insights into subsequent allocation of a limited intensivist workforce as nonintensivist care providers such as hospitalists become more available. To understand how intensivists and other practitioners, such as hospitalists, deliver critical care in Michigan, we performed a cross‐sectional survey of Michigan hospitals participating in the Keystone ICU project, a statewide quality‐improvement initiative.
Methods
The hospitals involved and the methods of Keystone ICU have been published previously.15 The Keystone ICU project is a collaborative quality improvement initiative first organized in October 2003 by the Michigan Health and Hospitals Association (MHA) Keystone Center for Patient Safety and Quality. At its inception, 103 ICUs voluntarily agreed to participate in Keystone ICU and reported data representing 85% of ICU beds in Michigan. Nonparticipating hospitals (n = 37) were smaller, 79% having fewer than 100 beds, many of which did not have ICUs. All ICUs from the 72 hospitals participating in the Keystone ICU project as of July 2005 were asked to complete surveys as part of ongoing data collection.
Keystone ICU sought to improve safety culture, increase adherence to evidence‐based practices among patients receiving mechanical ventilation, and reduce central lineassociated bloodstream infections and ventilator‐associated pneumonia through a number of interventions. Keystone also encouraged teams to standardize their physician staffing, and presented teams with evidence regarding the benefits of ICU physician staffing. Because many of the ICUs were small and believed it was not practical to staff their ICUs with intensivists, Keystone encouraged ICUs to create as many of the attributes of intensivist staffing as possible: having someone present who is knowledgeable, able to manage at the unit level, and who communicates well with clinicians and families.9 As part of this project, we developed a survey to describe the physician staffing in Michigan ICUs. Additional elements of the survey sought to ascertain how medical decision‐making occurred, which decisions were made by what types of clinicians, and who performed various procedures in the ICU.
Survey Development
The survey for this study was developed based on expert opinion and on previous work by the research team (A.D.A., P.J.P., S.A.F.). The survey was pilot tested in a small group of non‐Michigan hospitals and found to be understandable and readable. The survey was then revised and disseminated to all hospitals participating in the Keystone ICU project. Construct validity was determined by review of literature and discussion with the research team (A.D.A., P.J.P., S.A.F., R.C.H.). Content validity was determined by the pilot test, which included interviews with the individuals who pilot‐tested the survey. The survey sought to describe the organization of ICU physician services (including both intensivist and nonintensivist). A copy of the survey is available upon request.
Survey Protocol
Surveys were sent by e‐mail to the official nurse and/or physician project leader at each site in July 2005 from contact information provided by MHA. Another copy of the survey was emailed to ICUs that did not respond to the initial survey after 3 months and, if needed, a third survey was sent at 6 months with a follow‐up telephone call by 1 of the investigators (R.C.H.). The completed surveys were returned to MHA for compilation and analysis. The research project was reviewed by the University of Michigan Institutional Review Board and determined to be exempt from ongoing IRB review per federal exemption category 45 CFR 46.101.(b). The funder was not involved in the design of the study, collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
Statistical Analysis
Survey respondents were first characterized using simple univariable and bivariable methods. When appropriate, groups were compared based on chi‐square, Mann‐Whitney U test, or t test. Additionally, a series of multivariable analyses was performed, which sought to understand structural factors associated with the presence of higher‐intensity models, as well as use of hospitalists or intensivists. Results of the multivariate analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). The critical region was defined as an alpha of 0.05. Statistical analysis was performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
Response Rate
Ninety‐seven responses were received, including at least 1 response from every Keystone ICU hospital located in Michigan. Because our goal was to describe the organization of ICU physician services in non‐Federal hospitals, 1 Michigan VA hospital was eliminated from further consideration. Four hospitals with more than 1 ICU, which delivered care identically in all of their ICUs, provided 1 response and were counted as 1 site. As a result, 96 survey responses representing 115 ICUs in 72 Michigan hospitals were each counted as 1 site in the analysis. This included responses from ICUs not included in earlier analyses, which joined Keystone ICU after earlier work had been underway.15
Baseline Demographics
The mean (standard deviation [SD]) hospital size represented in the survey was 280 (22) beds, with a median of 249 (range, 40‐1031) beds. The mean size (SD) of the ICU was 13.3 (7.0) beds, median 12 beds, range 4 to 42 beds. There were 16 ICUs dedicated exclusively to the care of medicine patients, 14 dedicated surgical units, 8 dedicated cardiac ICUs, and 3 dedicated Neuro ICUs. The remainder had a mixed patient population. Seventy‐one ICUs (74%) cared for medical patients, 69 (72%) cared for surgical patients, 64 (67%) cared for cardiac patients, and 52 (53%) cared for neurological patients.
ICU Staffing Models
To better understand the role of intensivists in critical care delivery in Michigan, we examined differences in sites where patients are managed as closed sites exclusively by intensivists (closed ICU sites) in comparison to ICUs that had multiple attending specialties (open ICU sites). In addition, ICU sites where intensivists made most clinical decisionsa circumstance likely reflecting a high‐intensity staffing model of care5were compared with ICUs sites where decision‐making was made by nonintensivists or was shared (Table 1). Twenty‐four of 96 (25%) ICU sites were closed, and only intensivists served as the attending of record. Hospitals with closed ICUs or in which intensivists made most clinical decisions were larger and had larger ICUs than sites with open ICUs or with nonintensivist decision‐making (P < 0.05). These 24 closed sites represented 17 of 72 hospitals (24%), with the remainder of hospitals (76%) not having closed ICUs. Intensivists participated in rounds in 43 of 72 sites (60%) that were not closed. House officer participation in the care of ICU patients was not related to the presence or absence of intensivists (2 = 0.04; P = 0.847), although the average size of hospitals with house officers was larger than those without house officers (P < 0.0001).
| Closed ICUs (n = 24) [n (%)] | Open ICUs (n = 72) [n (%)] | Intensivist Decision‐making (n = 30) [n (%)] | Shared Decision‐making (n = 31) [n (%)] | Nonintensivist Decision‐making (n = 34) [n (%)] | |
|---|---|---|---|---|---|
| |||||
| ICU beds (mean SD) | 21.8 15.3* | 15.2 13.0* | 21.3 18.7* | 19.2 13.4 | 10.5 5.2* |
| Hospital beds (mean SD) | 489.8 295.3* | 326.3 222.6* | 460.8 222.3* | 408.6 259.7 | 247.8 230.0* |
| Nonintensivist attendings | |||||
| Hospitalist | 34 (47.2) | 9 (30) | 14 (45.1) | 13 (38.2) | |
| Primary care physician | 55 (76.4) | 11 (36.7) | 23 (74.2) | 27 (79.4) | |
| Cardiologist | 54 (75) | 10 (33.3) | 25 (80.6) | 23 (67.6) | |
| Pulmonologist | 34 (47.2) | 9 (30) | 15 (48.3) | 15 (44.1) | |
| Other IM specialist | 48 (66.7) | 11 (36.7) | 25 (80.6) | 17 (50) | |
| Surgeon | 59 (81.9) | 14 (46.7) | 25 (80.6) | 27 (79.4) | |
| Critical care board certification (% of attending physicians) | (n = 28) | (n = 31) | (n = 33) | ||
| 100 | 11 (45.8) | 7 (10.1) | 11 (39.3) | 6 (19.4) | 0 (0) |
| 75 | 3 (12.5) | 6 (8.7) | 7 (25.0) | 2 (6.5) | 0 (0) |
| 50 | 2 (8.3) | 4 (5.8) | 3 (10.7) | 2 (6.5) | 1 (3.0) |
| <50 | 8 (33.3) | 52 (75.4) | 7 (25.0) | 21 (67.7) | 32 (97.0) |
| ICU administration | |||||
| ICU director financial support | 18 (75.0) | 49 (68.1) | 25 (83.3) | 23 (74.2) | 18 (52.9) |
| Meeting with ICU team | 21 (87.5) | 56 (77.8) | 26 (86.7) | 27 (87.1) | 23 (67.7) |
| M&M sessions | 9 (37.5) | 33 (45.8) | 16 (53.3) | 12 (38.7) | 14 (41.2) |
Multivariate analysis determined that the presence of hospitalists serving as attending physicians was strongly associated with an open ICU (OR = 12.2; 95%CI = 2.5‐60.2), as was the absence of intensivists at the site (OR = 12.2; 95%CI = 1.4‐105.8), while ICU and hospital size were not associated. When the analyses were limited to hospitals with intensivists (n = 69), decision‐making by intensivists was not associated with ICU or hospital size (OR = 1.0; 95%CI = 1.0‐1.0); or whether hospitalists acted as attendings (OR = 0.7; 95%CI = 0.2‐2.0).
Board Certification and ICU Administration
Only 18 sites (20%) acknowledged that 100% of their ICU attending physicians were board‐certified in critical care, with nearly two‐thirds of sites having fewer than 50% critical‐care board‐certified attending physicians (Table 1). The medical director of the ICU met for an administrative meeting with the ICU team of nurses, respiratory therapists, and other personnel on a regular (ie, at least quarterly) basis at 77 sites (80%) and held regular morbidity and mortality sessions to discuss ICU care with other physicians who work in the ICU at 43 sites (45%). The majority of sites (n = 67; 70%) provided salary support for the ICU medical director.
Critical‐care board‐certification was more common at sites with closed ICUs and at sites where decision‐making was performed by intensivists (P < 0.001). However, board‐certification was not uniform in closed ICUs (100% certification = 46%, >50% certification = 67%) or in ICUs where intensivists made most decisions (100% certification = 39%, >50% certification = 75%).
Hospitals in which hospitalists served as attending physicians were less likely to have 50% or greater critical‐care board‐certification in their ICU (OR = 0.13; 95%CI = 0.03‐0.50). ICU size, hospital size, and years in practice were not associated with critical‐care board‐certification. Hospital size, ICU size, and the presence of intensivists or hospitalists were not associated with whether the medical director receives support from the hospital.
Physician Extenders
Nineteen sites (20%) reported the utilization of advanced practice nurses; 15 sites (16%) reported use of physician assistants; and 7 sites (7%) reported use of both advance practice nurses and physician assistants to provide intensive care. Physician extenders were not more likely to work in closed ICUs (10/24) than in open ICUs (14/72) (2 = 3.63; P = 0.57).
Of the 27 sites reporting use of advanced practice nurses or physician assistants, the role of physician extenders was described as being similar to physicians in 8 sites (30%), somewhat autonomous but with limitations in 18 (67%), and in a role closer to a ward clerk or assistant in 1 site (4%). The activities of physician extenders included writing orders at 24 of these 27 sites (89%); writing progress notes at 25 sites (92%); communicating with consultants at 24 (89%) and with primary care physicians at 22 sites (82%); and coordinating discharge plans at 20 sites (74%). Physician extenders rounded alone at 16 sites (33%).
Clinical Activities
Intensivists participated in daily rounds at most sites (n = 67; 70%). Nonintensivists served as attending of record in 72 (75%) sites. Nonintensivist physicians participating in daily patient rounds were: surgeons (n = 66; 68% of sites), primary care physicians (n = 61; 64%), nonpulmonary internal medicine specialists (n = 53; 55%), cardiologists (n = 58; 60%), non‐critical‐care pulmonologists (n = 39; 41%), and hospitalists (n = 36; 38%). Intensivists were the primary decision‐makers at 30 sites (31%), nonintensivists at 34 (35%), and decision making was shared at 31 (32%).
At more than one‐half of sites, decisions regarding mechanical ventilation, the use of sedatives or paralytics, and the choice of vasopressor agents were made by intensivists, with other decisionssuch as the decision to call consultants, choice of antibiotics, or family meetingsshared between intensivists and nonintensivists more than 40% of the time (Table 2). During regular working hours, invasive procedures were performed by multiple clinicians, including house officers, intensivists, surgeons, and anesthesiologists and were not the province of any particular type of clinician (Table 3).
| Decision‐making | |||
|---|---|---|---|
| Intensivist n (%) | Nonintensivist n (%) | Shared n (%) | |
| |||
| Ventilator management | 62 (66.7) | 24 (25.8) | 7 (7.5) |
| Choice of ventilator weaning strategies | 64 (68.8) | 24 (25.8) | 5 (5.4) |
| Decision to extubate | 63 (68.5) | 24 (26.1) | 5 (5.4) |
| Choice of sedation or paralytic agents | 56 (65.1) | 24 (27.9) | 6 (7.0) |
| Choice of vasopressor agents | 47 (51.1) | 25 (27.1) | 20 (21.7) |
| Decision to call other consultants (eg, cardiology, infectious diseases) | 19 (20.4) | 31 (33.3) | 43 (46.2) |
| Choices related to more general medical management (eg, antibiotics, diabetes management) | 30 (32.2) | 25 (26.9) | 38 (40.1) |
| Family meetings, code status discussions | 26 (28.6) | 26 (28.6) | 39 (42.8) |
| Procedure | Hospitalist n (%) | Intensivist n (%) | Surgeon n (%) | Anesthesiologist n (%) | House Officer or Other MD n (%) | Other non‐MD n (%) |
|---|---|---|---|---|---|---|
| Arterial line placement | 15 (15.6) | 50 (52.1) | 40 (41.7) | 31 (32.3) | 59 (61.4) | 7 (7.3) |
| Femoral venous line placement | 14 (14.6) | 54 (56.3) | 42 (43.8) | 17 (17.7) | 55 (57.3) | 4 (4.2) |
| Subclavian or internal jugular line placement | 14 (14.6) | 54 (56.2) | 47 (49.0) | 25 (26.0) | 62 (64.6) | 5 (5.2) |
| Pulmonary artery catheterization | 8 (8.3) | 56 (58.3) | 24 (25.0) | 21 (21.9) | 54 (56.2) | 2 (2.1) |
| Intubation | 14 (14.6) | 47 (49.0) | 14 (14.6) | 74 (77.1) | 42 (43.8) | 15 (15.6) |
| Bronchoscopy | 2 (2.1) | 67 (69.8) | 17 (17.7) | 5 (5.2) | 29 (30.2) | 0 (0) |
Regardless of the staffing model employed, the majority of sites (88%) provided care on a call‐based, rather than shift‐based system. Nighttime admissions and cross‐coverage issues were handled by house officers at more than one‐third of sites, with nonintensivist house physicians performing these tasks at 15% of sites (Table 4). Intensivists managed cross‐coverage issues by telephone at 29% of sites, and saw new admissions in person after hours at 8% of sites. Intensivists did not deliver care in scheduled shifts at any of these sites.
| Care Provider | Nighttime Admissions n (%) | Cross‐coverage n (%) |
|---|---|---|
| ||
| Emergency room physician | 13 (13.5) | 8 (8.3) |
| House physician | 15 (15.6) | 17 (17.7) |
| House officer | 42 (43.8) | 37 (38.5) |
| ICU nurse | 5 (5.2) | 10 (10.4) |
| PA or NP | 8 (8.3) | 5 (5.2) |
| Intensivist in person | 8 (8.3) | |
| Intensivist by telephone | 28 (29.2) | |
| Other | 9 (9.4) | 9 (9.4) |
Discussion
As all Keystone ICU participating sites responded to the questionnaire, we believe these results to be representative of critical care practice in the state of Michigan at the present time. Michigan ICU staffing structures are variable. Only a minority (25%) of Michigan Keystone ICU sites operated in an environment where intensivists are the only attending physicians of record. Although intensivists rounded in 60% of sites not utilizing a closed model, 75% of sites had nonintensivist attending physicians, with primary care physicians and hospitalists commonly providing ICU services. The utilization of hospitalists to provide critical care services was found in the absence of intensivists, regardless of hospital or ICU size.
Closed ICUs were seen in larger hospitals and in larger ICUs. This finding is similar to data obtained on a national level.8‐16 A high‐intensity model of care was also uncommon, although decision‐making was at least shared between intensivists and nonintensivists at two‐thirds of sites. These findings are in keeping with the observation that intensivist‐directed care advocated by the Leapfrog Group has not been widely implemented,17 including in Michigan, a regional rollout leader for the Leapfrog Group.
Fewer ICUs reported utilizing a nonintensivist model than was reported in the survey by Angus et al.,8 where approximately one‐half of ICUs delivered care in this manner. This survey was performed in 1997, prior to the launch of the Leapfrog Group effort, and may have reflected a relative over representation of smaller, general ICUs. Our study is the first statewide analysis of critical care practices in the postLeapfrog Group era. Our finding that an array of approaches to critical care delivery existed in Michigan, even when intensivists rounded on patients, is similar to that found among Leapfrog‐compliant hospitals sampled from several regions of the United States.18
Other than intensivists, surgeons, primary care, and hospitalist physicians provided care in Michigan ICUs. The hospitalist movement is relatively new.19 However, in our survey 37.5% of sites had hospitalists serving as attending physicians. Although the closed ICU model was more prevalent in larger ICUs and hospitals, the use of a hospitalist model to staff ICUs was not related to hospital size, but was instead a function of whether or not intensivists were present in a given setting. In lieu of a projected shortage of intensivists, we believe this confirms the crucial role that hospitalists will play in the provision of critical care services in the future.
The attributes of intensivist care that led to improved outcomes in previous studies1‐4 are unknown. To the extent that the involvement of intensivists on an elective rather than mandatory consultative basis may explain the higher mortality found in 1 recent study,1011 we hypothesize that having a knowledgeable physician present who communicates with clinicians and families and manages at the unit level is an important factor leading to improved outcomes. While hospitalists can have these attributes, their knowledge of specific critical care therapies and technologies may vary with the extent of their ICU training and experience. Further research should seek to quantify the attributes by which intensivists are associated with improved outcomes and seek ways to foster those attributes among hospitalists who participate in critical care delivery. Central to this will be ensuring that training programs ensure competency in critical care therapies and technologies among hospitalists and other non‐ICU physicians.
We recognize several limitations in this study. First, the validity of the survey may introduce misclassification of ICU staffing. However, the survey instrument was informed by previously‐validated instruments and experts in ICU physician staffing and hospitalist care. Second, we did not link variation in staffing to outcomes. While such analysis is important, it is beyond the scope of this survey. Third, our study was conducted in 1 state and the results may not be generalizable across the United States. Nevertheless, Michigan is a large state with a diverse array of hospitals, and as our study sample broadly represented this diversity, we believe our results are likely to be generalizable.
In conclusion, few ICUs in Michigan are closed and many utilize nonintensivist critical‐care providers such as hospitalists, primary care providers, and physician extenders to deliver clinical care. Our findings have significant implications for future efforts at a national level that involve the training of hospitalists and their acceptance as critical care practitioners. We suggest future research involving intensive care delivery focus on the feasibility of training sufficient hospitalists to satisfy a growing need for critical care that cannot be filled by intensivists, along with strategic planning to insure the model of care provided is commensurate with the complexity of illness. Although this approach appears to be occurring in Michigan on an ad hoc basis, we believe coordination between larger, intensivist‐run ICUs and smaller, nonintensivist‐run ICUs should be formalized in order to optimize the delivery of intensive care.25
- ,,, et al.;the members of the American College of Critical Care Medicine Task Force on Models for the Definition of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model.Crit Care Med.2001;29;2007–2019.
- ,,, et al.Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of “open” and “closed” formats.JAMA.1996;276:24–31.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- ,,, et al.Effects of an organized critical care service on outcomes and resource utilization: a cohort study.Crit Care Med.1999;27:270–274.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002;288:2151–2162.
- Leapfrog Group. Leapfrog Group Factsheet: ICU physician staffing (IPS). Available at: http://www.leapfroggroup.org/media/file/Leapfrog‐ICU_ Physician_Staffing_Fact_Sheet.pdf. Accessed June 2009.
- National Quality Forum. Safe Practices for Better Healthcare. Available at: http://www.qualityforum.org/pdf/reports/safe_practices.pdf. Accessed June 2009.
- ,,,,,; on behalf of the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS).Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34:1016–1024.
- ,,, et al.Team care: beyond open and closed intensive care units.Curr Opin Crit Care.2006;12:604–608.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–809.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Outcomes of care by hospitalists, general internists and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Descriptive analysis of critical care units in the United States.Crit Care Med.1992;20:846–863.
- .Leapfrog and critical care: evidence‐ and reality‐based intensive care for the 21st century.Am J Med.2004;116:188–193.
- ,,,,.The organization of intensive care unit physician services.Crit Care Med.2007;35:2256–2261.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2:327–330.
- ,,, et al.Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care.Crit Care Med.2003;31:2677–2683.
Organization of physician services in intensive care units (ICUs) varies widely and influences mortality, morbidity, and costs of care. Intensive care provided by intensivists in a high‐intensity physician staffing model, in which intensivists are the sole attending physicians or consult on all patients, has been associated with desirable outcomes such as decreased length of stay, resource utilization, and mortality.1‐4 As a result, higher intensity ICU models have been recommended by various healthcare agencies, including the National Quality Forum and the Leapfrog Group.5‐7
One national survey indicated that 47% of ICUs surveyed had some intensivist coverage and only 4% of intensive care units met Leapfrog high‐intensity model standards.8 However, only one‐third of ICUs responded to this survey, smaller ICUs were overrepresented, and the survey may not have reflected the influence of newer policy initiatives because it was conducted in 1997. Though the attributes by which intensivists improve patient outcomes is unknown, researchers have suggested it is by having a knowledgeable physician present in the ICU, having a physician communicate with other clinicians and families, and by having a physician who manages the ICU by writing policies and procedures and administrative activities.9
Results have been conflicting as patients managed by intensivists have also been found to have an increased mortality, particularly when managed on an elective consultation basis in an open ICU, where patient orders are written by several physician specialties.10, 11 Alternative ICU staffing models, such as the use of hospitalists, have been utilized to compensate for the intensivist workforce shortage. Hospitalists often provide ICU care, although they are seldom board‐certified in critical care. Hospitalist care has been shown to provide clinical and efficiency benefits such as decreased length of hospital stay.12‐14
Understanding the manner in which critical care is currently delivered, particularly the utilization of intensivist and nonintensivist care providers, can provide insights into subsequent allocation of a limited intensivist workforce as nonintensivist care providers such as hospitalists become more available. To understand how intensivists and other practitioners, such as hospitalists, deliver critical care in Michigan, we performed a cross‐sectional survey of Michigan hospitals participating in the Keystone ICU project, a statewide quality‐improvement initiative.
Methods
The hospitals involved and the methods of Keystone ICU have been published previously.15 The Keystone ICU project is a collaborative quality improvement initiative first organized in October 2003 by the Michigan Health and Hospitals Association (MHA) Keystone Center for Patient Safety and Quality. At its inception, 103 ICUs voluntarily agreed to participate in Keystone ICU and reported data representing 85% of ICU beds in Michigan. Nonparticipating hospitals (n = 37) were smaller, 79% having fewer than 100 beds, many of which did not have ICUs. All ICUs from the 72 hospitals participating in the Keystone ICU project as of July 2005 were asked to complete surveys as part of ongoing data collection.
Keystone ICU sought to improve safety culture, increase adherence to evidence‐based practices among patients receiving mechanical ventilation, and reduce central lineassociated bloodstream infections and ventilator‐associated pneumonia through a number of interventions. Keystone also encouraged teams to standardize their physician staffing, and presented teams with evidence regarding the benefits of ICU physician staffing. Because many of the ICUs were small and believed it was not practical to staff their ICUs with intensivists, Keystone encouraged ICUs to create as many of the attributes of intensivist staffing as possible: having someone present who is knowledgeable, able to manage at the unit level, and who communicates well with clinicians and families.9 As part of this project, we developed a survey to describe the physician staffing in Michigan ICUs. Additional elements of the survey sought to ascertain how medical decision‐making occurred, which decisions were made by what types of clinicians, and who performed various procedures in the ICU.
Survey Development
The survey for this study was developed based on expert opinion and on previous work by the research team (A.D.A., P.J.P., S.A.F.). The survey was pilot tested in a small group of non‐Michigan hospitals and found to be understandable and readable. The survey was then revised and disseminated to all hospitals participating in the Keystone ICU project. Construct validity was determined by review of literature and discussion with the research team (A.D.A., P.J.P., S.A.F., R.C.H.). Content validity was determined by the pilot test, which included interviews with the individuals who pilot‐tested the survey. The survey sought to describe the organization of ICU physician services (including both intensivist and nonintensivist). A copy of the survey is available upon request.
Survey Protocol
Surveys were sent by e‐mail to the official nurse and/or physician project leader at each site in July 2005 from contact information provided by MHA. Another copy of the survey was emailed to ICUs that did not respond to the initial survey after 3 months and, if needed, a third survey was sent at 6 months with a follow‐up telephone call by 1 of the investigators (R.C.H.). The completed surveys were returned to MHA for compilation and analysis. The research project was reviewed by the University of Michigan Institutional Review Board and determined to be exempt from ongoing IRB review per federal exemption category 45 CFR 46.101.(b). The funder was not involved in the design of the study, collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
Statistical Analysis
Survey respondents were first characterized using simple univariable and bivariable methods. When appropriate, groups were compared based on chi‐square, Mann‐Whitney U test, or t test. Additionally, a series of multivariable analyses was performed, which sought to understand structural factors associated with the presence of higher‐intensity models, as well as use of hospitalists or intensivists. Results of the multivariate analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). The critical region was defined as an alpha of 0.05. Statistical analysis was performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
Response Rate
Ninety‐seven responses were received, including at least 1 response from every Keystone ICU hospital located in Michigan. Because our goal was to describe the organization of ICU physician services in non‐Federal hospitals, 1 Michigan VA hospital was eliminated from further consideration. Four hospitals with more than 1 ICU, which delivered care identically in all of their ICUs, provided 1 response and were counted as 1 site. As a result, 96 survey responses representing 115 ICUs in 72 Michigan hospitals were each counted as 1 site in the analysis. This included responses from ICUs not included in earlier analyses, which joined Keystone ICU after earlier work had been underway.15
Baseline Demographics
The mean (standard deviation [SD]) hospital size represented in the survey was 280 (22) beds, with a median of 249 (range, 40‐1031) beds. The mean size (SD) of the ICU was 13.3 (7.0) beds, median 12 beds, range 4 to 42 beds. There were 16 ICUs dedicated exclusively to the care of medicine patients, 14 dedicated surgical units, 8 dedicated cardiac ICUs, and 3 dedicated Neuro ICUs. The remainder had a mixed patient population. Seventy‐one ICUs (74%) cared for medical patients, 69 (72%) cared for surgical patients, 64 (67%) cared for cardiac patients, and 52 (53%) cared for neurological patients.
ICU Staffing Models
To better understand the role of intensivists in critical care delivery in Michigan, we examined differences in sites where patients are managed as closed sites exclusively by intensivists (closed ICU sites) in comparison to ICUs that had multiple attending specialties (open ICU sites). In addition, ICU sites where intensivists made most clinical decisionsa circumstance likely reflecting a high‐intensity staffing model of care5were compared with ICUs sites where decision‐making was made by nonintensivists or was shared (Table 1). Twenty‐four of 96 (25%) ICU sites were closed, and only intensivists served as the attending of record. Hospitals with closed ICUs or in which intensivists made most clinical decisions were larger and had larger ICUs than sites with open ICUs or with nonintensivist decision‐making (P < 0.05). These 24 closed sites represented 17 of 72 hospitals (24%), with the remainder of hospitals (76%) not having closed ICUs. Intensivists participated in rounds in 43 of 72 sites (60%) that were not closed. House officer participation in the care of ICU patients was not related to the presence or absence of intensivists (2 = 0.04; P = 0.847), although the average size of hospitals with house officers was larger than those without house officers (P < 0.0001).
| Closed ICUs (n = 24) [n (%)] | Open ICUs (n = 72) [n (%)] | Intensivist Decision‐making (n = 30) [n (%)] | Shared Decision‐making (n = 31) [n (%)] | Nonintensivist Decision‐making (n = 34) [n (%)] | |
|---|---|---|---|---|---|
| |||||
| ICU beds (mean SD) | 21.8 15.3* | 15.2 13.0* | 21.3 18.7* | 19.2 13.4 | 10.5 5.2* |
| Hospital beds (mean SD) | 489.8 295.3* | 326.3 222.6* | 460.8 222.3* | 408.6 259.7 | 247.8 230.0* |
| Nonintensivist attendings | |||||
| Hospitalist | 34 (47.2) | 9 (30) | 14 (45.1) | 13 (38.2) | |
| Primary care physician | 55 (76.4) | 11 (36.7) | 23 (74.2) | 27 (79.4) | |
| Cardiologist | 54 (75) | 10 (33.3) | 25 (80.6) | 23 (67.6) | |
| Pulmonologist | 34 (47.2) | 9 (30) | 15 (48.3) | 15 (44.1) | |
| Other IM specialist | 48 (66.7) | 11 (36.7) | 25 (80.6) | 17 (50) | |
| Surgeon | 59 (81.9) | 14 (46.7) | 25 (80.6) | 27 (79.4) | |
| Critical care board certification (% of attending physicians) | (n = 28) | (n = 31) | (n = 33) | ||
| 100 | 11 (45.8) | 7 (10.1) | 11 (39.3) | 6 (19.4) | 0 (0) |
| 75 | 3 (12.5) | 6 (8.7) | 7 (25.0) | 2 (6.5) | 0 (0) |
| 50 | 2 (8.3) | 4 (5.8) | 3 (10.7) | 2 (6.5) | 1 (3.0) |
| <50 | 8 (33.3) | 52 (75.4) | 7 (25.0) | 21 (67.7) | 32 (97.0) |
| ICU administration | |||||
| ICU director financial support | 18 (75.0) | 49 (68.1) | 25 (83.3) | 23 (74.2) | 18 (52.9) |
| Meeting with ICU team | 21 (87.5) | 56 (77.8) | 26 (86.7) | 27 (87.1) | 23 (67.7) |
| M&M sessions | 9 (37.5) | 33 (45.8) | 16 (53.3) | 12 (38.7) | 14 (41.2) |
Multivariate analysis determined that the presence of hospitalists serving as attending physicians was strongly associated with an open ICU (OR = 12.2; 95%CI = 2.5‐60.2), as was the absence of intensivists at the site (OR = 12.2; 95%CI = 1.4‐105.8), while ICU and hospital size were not associated. When the analyses were limited to hospitals with intensivists (n = 69), decision‐making by intensivists was not associated with ICU or hospital size (OR = 1.0; 95%CI = 1.0‐1.0); or whether hospitalists acted as attendings (OR = 0.7; 95%CI = 0.2‐2.0).
Board Certification and ICU Administration
Only 18 sites (20%) acknowledged that 100% of their ICU attending physicians were board‐certified in critical care, with nearly two‐thirds of sites having fewer than 50% critical‐care board‐certified attending physicians (Table 1). The medical director of the ICU met for an administrative meeting with the ICU team of nurses, respiratory therapists, and other personnel on a regular (ie, at least quarterly) basis at 77 sites (80%) and held regular morbidity and mortality sessions to discuss ICU care with other physicians who work in the ICU at 43 sites (45%). The majority of sites (n = 67; 70%) provided salary support for the ICU medical director.
Critical‐care board‐certification was more common at sites with closed ICUs and at sites where decision‐making was performed by intensivists (P < 0.001). However, board‐certification was not uniform in closed ICUs (100% certification = 46%, >50% certification = 67%) or in ICUs where intensivists made most decisions (100% certification = 39%, >50% certification = 75%).
Hospitals in which hospitalists served as attending physicians were less likely to have 50% or greater critical‐care board‐certification in their ICU (OR = 0.13; 95%CI = 0.03‐0.50). ICU size, hospital size, and years in practice were not associated with critical‐care board‐certification. Hospital size, ICU size, and the presence of intensivists or hospitalists were not associated with whether the medical director receives support from the hospital.
Physician Extenders
Nineteen sites (20%) reported the utilization of advanced practice nurses; 15 sites (16%) reported use of physician assistants; and 7 sites (7%) reported use of both advance practice nurses and physician assistants to provide intensive care. Physician extenders were not more likely to work in closed ICUs (10/24) than in open ICUs (14/72) (2 = 3.63; P = 0.57).
Of the 27 sites reporting use of advanced practice nurses or physician assistants, the role of physician extenders was described as being similar to physicians in 8 sites (30%), somewhat autonomous but with limitations in 18 (67%), and in a role closer to a ward clerk or assistant in 1 site (4%). The activities of physician extenders included writing orders at 24 of these 27 sites (89%); writing progress notes at 25 sites (92%); communicating with consultants at 24 (89%) and with primary care physicians at 22 sites (82%); and coordinating discharge plans at 20 sites (74%). Physician extenders rounded alone at 16 sites (33%).
Clinical Activities
Intensivists participated in daily rounds at most sites (n = 67; 70%). Nonintensivists served as attending of record in 72 (75%) sites. Nonintensivist physicians participating in daily patient rounds were: surgeons (n = 66; 68% of sites), primary care physicians (n = 61; 64%), nonpulmonary internal medicine specialists (n = 53; 55%), cardiologists (n = 58; 60%), non‐critical‐care pulmonologists (n = 39; 41%), and hospitalists (n = 36; 38%). Intensivists were the primary decision‐makers at 30 sites (31%), nonintensivists at 34 (35%), and decision making was shared at 31 (32%).
At more than one‐half of sites, decisions regarding mechanical ventilation, the use of sedatives or paralytics, and the choice of vasopressor agents were made by intensivists, with other decisionssuch as the decision to call consultants, choice of antibiotics, or family meetingsshared between intensivists and nonintensivists more than 40% of the time (Table 2). During regular working hours, invasive procedures were performed by multiple clinicians, including house officers, intensivists, surgeons, and anesthesiologists and were not the province of any particular type of clinician (Table 3).
| Decision‐making | |||
|---|---|---|---|
| Intensivist n (%) | Nonintensivist n (%) | Shared n (%) | |
| |||
| Ventilator management | 62 (66.7) | 24 (25.8) | 7 (7.5) |
| Choice of ventilator weaning strategies | 64 (68.8) | 24 (25.8) | 5 (5.4) |
| Decision to extubate | 63 (68.5) | 24 (26.1) | 5 (5.4) |
| Choice of sedation or paralytic agents | 56 (65.1) | 24 (27.9) | 6 (7.0) |
| Choice of vasopressor agents | 47 (51.1) | 25 (27.1) | 20 (21.7) |
| Decision to call other consultants (eg, cardiology, infectious diseases) | 19 (20.4) | 31 (33.3) | 43 (46.2) |
| Choices related to more general medical management (eg, antibiotics, diabetes management) | 30 (32.2) | 25 (26.9) | 38 (40.1) |
| Family meetings, code status discussions | 26 (28.6) | 26 (28.6) | 39 (42.8) |
| Procedure | Hospitalist n (%) | Intensivist n (%) | Surgeon n (%) | Anesthesiologist n (%) | House Officer or Other MD n (%) | Other non‐MD n (%) |
|---|---|---|---|---|---|---|
| Arterial line placement | 15 (15.6) | 50 (52.1) | 40 (41.7) | 31 (32.3) | 59 (61.4) | 7 (7.3) |
| Femoral venous line placement | 14 (14.6) | 54 (56.3) | 42 (43.8) | 17 (17.7) | 55 (57.3) | 4 (4.2) |
| Subclavian or internal jugular line placement | 14 (14.6) | 54 (56.2) | 47 (49.0) | 25 (26.0) | 62 (64.6) | 5 (5.2) |
| Pulmonary artery catheterization | 8 (8.3) | 56 (58.3) | 24 (25.0) | 21 (21.9) | 54 (56.2) | 2 (2.1) |
| Intubation | 14 (14.6) | 47 (49.0) | 14 (14.6) | 74 (77.1) | 42 (43.8) | 15 (15.6) |
| Bronchoscopy | 2 (2.1) | 67 (69.8) | 17 (17.7) | 5 (5.2) | 29 (30.2) | 0 (0) |
Regardless of the staffing model employed, the majority of sites (88%) provided care on a call‐based, rather than shift‐based system. Nighttime admissions and cross‐coverage issues were handled by house officers at more than one‐third of sites, with nonintensivist house physicians performing these tasks at 15% of sites (Table 4). Intensivists managed cross‐coverage issues by telephone at 29% of sites, and saw new admissions in person after hours at 8% of sites. Intensivists did not deliver care in scheduled shifts at any of these sites.
| Care Provider | Nighttime Admissions n (%) | Cross‐coverage n (%) |
|---|---|---|
| ||
| Emergency room physician | 13 (13.5) | 8 (8.3) |
| House physician | 15 (15.6) | 17 (17.7) |
| House officer | 42 (43.8) | 37 (38.5) |
| ICU nurse | 5 (5.2) | 10 (10.4) |
| PA or NP | 8 (8.3) | 5 (5.2) |
| Intensivist in person | 8 (8.3) | |
| Intensivist by telephone | 28 (29.2) | |
| Other | 9 (9.4) | 9 (9.4) |
Discussion
As all Keystone ICU participating sites responded to the questionnaire, we believe these results to be representative of critical care practice in the state of Michigan at the present time. Michigan ICU staffing structures are variable. Only a minority (25%) of Michigan Keystone ICU sites operated in an environment where intensivists are the only attending physicians of record. Although intensivists rounded in 60% of sites not utilizing a closed model, 75% of sites had nonintensivist attending physicians, with primary care physicians and hospitalists commonly providing ICU services. The utilization of hospitalists to provide critical care services was found in the absence of intensivists, regardless of hospital or ICU size.
Closed ICUs were seen in larger hospitals and in larger ICUs. This finding is similar to data obtained on a national level.8‐16 A high‐intensity model of care was also uncommon, although decision‐making was at least shared between intensivists and nonintensivists at two‐thirds of sites. These findings are in keeping with the observation that intensivist‐directed care advocated by the Leapfrog Group has not been widely implemented,17 including in Michigan, a regional rollout leader for the Leapfrog Group.
Fewer ICUs reported utilizing a nonintensivist model than was reported in the survey by Angus et al.,8 where approximately one‐half of ICUs delivered care in this manner. This survey was performed in 1997, prior to the launch of the Leapfrog Group effort, and may have reflected a relative over representation of smaller, general ICUs. Our study is the first statewide analysis of critical care practices in the postLeapfrog Group era. Our finding that an array of approaches to critical care delivery existed in Michigan, even when intensivists rounded on patients, is similar to that found among Leapfrog‐compliant hospitals sampled from several regions of the United States.18
Other than intensivists, surgeons, primary care, and hospitalist physicians provided care in Michigan ICUs. The hospitalist movement is relatively new.19 However, in our survey 37.5% of sites had hospitalists serving as attending physicians. Although the closed ICU model was more prevalent in larger ICUs and hospitals, the use of a hospitalist model to staff ICUs was not related to hospital size, but was instead a function of whether or not intensivists were present in a given setting. In lieu of a projected shortage of intensivists, we believe this confirms the crucial role that hospitalists will play in the provision of critical care services in the future.
The attributes of intensivist care that led to improved outcomes in previous studies1‐4 are unknown. To the extent that the involvement of intensivists on an elective rather than mandatory consultative basis may explain the higher mortality found in 1 recent study,1011 we hypothesize that having a knowledgeable physician present who communicates with clinicians and families and manages at the unit level is an important factor leading to improved outcomes. While hospitalists can have these attributes, their knowledge of specific critical care therapies and technologies may vary with the extent of their ICU training and experience. Further research should seek to quantify the attributes by which intensivists are associated with improved outcomes and seek ways to foster those attributes among hospitalists who participate in critical care delivery. Central to this will be ensuring that training programs ensure competency in critical care therapies and technologies among hospitalists and other non‐ICU physicians.
We recognize several limitations in this study. First, the validity of the survey may introduce misclassification of ICU staffing. However, the survey instrument was informed by previously‐validated instruments and experts in ICU physician staffing and hospitalist care. Second, we did not link variation in staffing to outcomes. While such analysis is important, it is beyond the scope of this survey. Third, our study was conducted in 1 state and the results may not be generalizable across the United States. Nevertheless, Michigan is a large state with a diverse array of hospitals, and as our study sample broadly represented this diversity, we believe our results are likely to be generalizable.
In conclusion, few ICUs in Michigan are closed and many utilize nonintensivist critical‐care providers such as hospitalists, primary care providers, and physician extenders to deliver clinical care. Our findings have significant implications for future efforts at a national level that involve the training of hospitalists and their acceptance as critical care practitioners. We suggest future research involving intensive care delivery focus on the feasibility of training sufficient hospitalists to satisfy a growing need for critical care that cannot be filled by intensivists, along with strategic planning to insure the model of care provided is commensurate with the complexity of illness. Although this approach appears to be occurring in Michigan on an ad hoc basis, we believe coordination between larger, intensivist‐run ICUs and smaller, nonintensivist‐run ICUs should be formalized in order to optimize the delivery of intensive care.25
Organization of physician services in intensive care units (ICUs) varies widely and influences mortality, morbidity, and costs of care. Intensive care provided by intensivists in a high‐intensity physician staffing model, in which intensivists are the sole attending physicians or consult on all patients, has been associated with desirable outcomes such as decreased length of stay, resource utilization, and mortality.1‐4 As a result, higher intensity ICU models have been recommended by various healthcare agencies, including the National Quality Forum and the Leapfrog Group.5‐7
One national survey indicated that 47% of ICUs surveyed had some intensivist coverage and only 4% of intensive care units met Leapfrog high‐intensity model standards.8 However, only one‐third of ICUs responded to this survey, smaller ICUs were overrepresented, and the survey may not have reflected the influence of newer policy initiatives because it was conducted in 1997. Though the attributes by which intensivists improve patient outcomes is unknown, researchers have suggested it is by having a knowledgeable physician present in the ICU, having a physician communicate with other clinicians and families, and by having a physician who manages the ICU by writing policies and procedures and administrative activities.9
Results have been conflicting as patients managed by intensivists have also been found to have an increased mortality, particularly when managed on an elective consultation basis in an open ICU, where patient orders are written by several physician specialties.10, 11 Alternative ICU staffing models, such as the use of hospitalists, have been utilized to compensate for the intensivist workforce shortage. Hospitalists often provide ICU care, although they are seldom board‐certified in critical care. Hospitalist care has been shown to provide clinical and efficiency benefits such as decreased length of hospital stay.12‐14
Understanding the manner in which critical care is currently delivered, particularly the utilization of intensivist and nonintensivist care providers, can provide insights into subsequent allocation of a limited intensivist workforce as nonintensivist care providers such as hospitalists become more available. To understand how intensivists and other practitioners, such as hospitalists, deliver critical care in Michigan, we performed a cross‐sectional survey of Michigan hospitals participating in the Keystone ICU project, a statewide quality‐improvement initiative.
Methods
The hospitals involved and the methods of Keystone ICU have been published previously.15 The Keystone ICU project is a collaborative quality improvement initiative first organized in October 2003 by the Michigan Health and Hospitals Association (MHA) Keystone Center for Patient Safety and Quality. At its inception, 103 ICUs voluntarily agreed to participate in Keystone ICU and reported data representing 85% of ICU beds in Michigan. Nonparticipating hospitals (n = 37) were smaller, 79% having fewer than 100 beds, many of which did not have ICUs. All ICUs from the 72 hospitals participating in the Keystone ICU project as of July 2005 were asked to complete surveys as part of ongoing data collection.
Keystone ICU sought to improve safety culture, increase adherence to evidence‐based practices among patients receiving mechanical ventilation, and reduce central lineassociated bloodstream infections and ventilator‐associated pneumonia through a number of interventions. Keystone also encouraged teams to standardize their physician staffing, and presented teams with evidence regarding the benefits of ICU physician staffing. Because many of the ICUs were small and believed it was not practical to staff their ICUs with intensivists, Keystone encouraged ICUs to create as many of the attributes of intensivist staffing as possible: having someone present who is knowledgeable, able to manage at the unit level, and who communicates well with clinicians and families.9 As part of this project, we developed a survey to describe the physician staffing in Michigan ICUs. Additional elements of the survey sought to ascertain how medical decision‐making occurred, which decisions were made by what types of clinicians, and who performed various procedures in the ICU.
Survey Development
The survey for this study was developed based on expert opinion and on previous work by the research team (A.D.A., P.J.P., S.A.F.). The survey was pilot tested in a small group of non‐Michigan hospitals and found to be understandable and readable. The survey was then revised and disseminated to all hospitals participating in the Keystone ICU project. Construct validity was determined by review of literature and discussion with the research team (A.D.A., P.J.P., S.A.F., R.C.H.). Content validity was determined by the pilot test, which included interviews with the individuals who pilot‐tested the survey. The survey sought to describe the organization of ICU physician services (including both intensivist and nonintensivist). A copy of the survey is available upon request.
Survey Protocol
Surveys were sent by e‐mail to the official nurse and/or physician project leader at each site in July 2005 from contact information provided by MHA. Another copy of the survey was emailed to ICUs that did not respond to the initial survey after 3 months and, if needed, a third survey was sent at 6 months with a follow‐up telephone call by 1 of the investigators (R.C.H.). The completed surveys were returned to MHA for compilation and analysis. The research project was reviewed by the University of Michigan Institutional Review Board and determined to be exempt from ongoing IRB review per federal exemption category 45 CFR 46.101.(b). The funder was not involved in the design of the study, collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
Statistical Analysis
Survey respondents were first characterized using simple univariable and bivariable methods. When appropriate, groups were compared based on chi‐square, Mann‐Whitney U test, or t test. Additionally, a series of multivariable analyses was performed, which sought to understand structural factors associated with the presence of higher‐intensity models, as well as use of hospitalists or intensivists. Results of the multivariate analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). The critical region was defined as an alpha of 0.05. Statistical analysis was performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
Response Rate
Ninety‐seven responses were received, including at least 1 response from every Keystone ICU hospital located in Michigan. Because our goal was to describe the organization of ICU physician services in non‐Federal hospitals, 1 Michigan VA hospital was eliminated from further consideration. Four hospitals with more than 1 ICU, which delivered care identically in all of their ICUs, provided 1 response and were counted as 1 site. As a result, 96 survey responses representing 115 ICUs in 72 Michigan hospitals were each counted as 1 site in the analysis. This included responses from ICUs not included in earlier analyses, which joined Keystone ICU after earlier work had been underway.15
Baseline Demographics
The mean (standard deviation [SD]) hospital size represented in the survey was 280 (22) beds, with a median of 249 (range, 40‐1031) beds. The mean size (SD) of the ICU was 13.3 (7.0) beds, median 12 beds, range 4 to 42 beds. There were 16 ICUs dedicated exclusively to the care of medicine patients, 14 dedicated surgical units, 8 dedicated cardiac ICUs, and 3 dedicated Neuro ICUs. The remainder had a mixed patient population. Seventy‐one ICUs (74%) cared for medical patients, 69 (72%) cared for surgical patients, 64 (67%) cared for cardiac patients, and 52 (53%) cared for neurological patients.
ICU Staffing Models
To better understand the role of intensivists in critical care delivery in Michigan, we examined differences in sites where patients are managed as closed sites exclusively by intensivists (closed ICU sites) in comparison to ICUs that had multiple attending specialties (open ICU sites). In addition, ICU sites where intensivists made most clinical decisionsa circumstance likely reflecting a high‐intensity staffing model of care5were compared with ICUs sites where decision‐making was made by nonintensivists or was shared (Table 1). Twenty‐four of 96 (25%) ICU sites were closed, and only intensivists served as the attending of record. Hospitals with closed ICUs or in which intensivists made most clinical decisions were larger and had larger ICUs than sites with open ICUs or with nonintensivist decision‐making (P < 0.05). These 24 closed sites represented 17 of 72 hospitals (24%), with the remainder of hospitals (76%) not having closed ICUs. Intensivists participated in rounds in 43 of 72 sites (60%) that were not closed. House officer participation in the care of ICU patients was not related to the presence or absence of intensivists (2 = 0.04; P = 0.847), although the average size of hospitals with house officers was larger than those without house officers (P < 0.0001).
| Closed ICUs (n = 24) [n (%)] | Open ICUs (n = 72) [n (%)] | Intensivist Decision‐making (n = 30) [n (%)] | Shared Decision‐making (n = 31) [n (%)] | Nonintensivist Decision‐making (n = 34) [n (%)] | |
|---|---|---|---|---|---|
| |||||
| ICU beds (mean SD) | 21.8 15.3* | 15.2 13.0* | 21.3 18.7* | 19.2 13.4 | 10.5 5.2* |
| Hospital beds (mean SD) | 489.8 295.3* | 326.3 222.6* | 460.8 222.3* | 408.6 259.7 | 247.8 230.0* |
| Nonintensivist attendings | |||||
| Hospitalist | 34 (47.2) | 9 (30) | 14 (45.1) | 13 (38.2) | |
| Primary care physician | 55 (76.4) | 11 (36.7) | 23 (74.2) | 27 (79.4) | |
| Cardiologist | 54 (75) | 10 (33.3) | 25 (80.6) | 23 (67.6) | |
| Pulmonologist | 34 (47.2) | 9 (30) | 15 (48.3) | 15 (44.1) | |
| Other IM specialist | 48 (66.7) | 11 (36.7) | 25 (80.6) | 17 (50) | |
| Surgeon | 59 (81.9) | 14 (46.7) | 25 (80.6) | 27 (79.4) | |
| Critical care board certification (% of attending physicians) | (n = 28) | (n = 31) | (n = 33) | ||
| 100 | 11 (45.8) | 7 (10.1) | 11 (39.3) | 6 (19.4) | 0 (0) |
| 75 | 3 (12.5) | 6 (8.7) | 7 (25.0) | 2 (6.5) | 0 (0) |
| 50 | 2 (8.3) | 4 (5.8) | 3 (10.7) | 2 (6.5) | 1 (3.0) |
| <50 | 8 (33.3) | 52 (75.4) | 7 (25.0) | 21 (67.7) | 32 (97.0) |
| ICU administration | |||||
| ICU director financial support | 18 (75.0) | 49 (68.1) | 25 (83.3) | 23 (74.2) | 18 (52.9) |
| Meeting with ICU team | 21 (87.5) | 56 (77.8) | 26 (86.7) | 27 (87.1) | 23 (67.7) |
| M&M sessions | 9 (37.5) | 33 (45.8) | 16 (53.3) | 12 (38.7) | 14 (41.2) |
Multivariate analysis determined that the presence of hospitalists serving as attending physicians was strongly associated with an open ICU (OR = 12.2; 95%CI = 2.5‐60.2), as was the absence of intensivists at the site (OR = 12.2; 95%CI = 1.4‐105.8), while ICU and hospital size were not associated. When the analyses were limited to hospitals with intensivists (n = 69), decision‐making by intensivists was not associated with ICU or hospital size (OR = 1.0; 95%CI = 1.0‐1.0); or whether hospitalists acted as attendings (OR = 0.7; 95%CI = 0.2‐2.0).
Board Certification and ICU Administration
Only 18 sites (20%) acknowledged that 100% of their ICU attending physicians were board‐certified in critical care, with nearly two‐thirds of sites having fewer than 50% critical‐care board‐certified attending physicians (Table 1). The medical director of the ICU met for an administrative meeting with the ICU team of nurses, respiratory therapists, and other personnel on a regular (ie, at least quarterly) basis at 77 sites (80%) and held regular morbidity and mortality sessions to discuss ICU care with other physicians who work in the ICU at 43 sites (45%). The majority of sites (n = 67; 70%) provided salary support for the ICU medical director.
Critical‐care board‐certification was more common at sites with closed ICUs and at sites where decision‐making was performed by intensivists (P < 0.001). However, board‐certification was not uniform in closed ICUs (100% certification = 46%, >50% certification = 67%) or in ICUs where intensivists made most decisions (100% certification = 39%, >50% certification = 75%).
Hospitals in which hospitalists served as attending physicians were less likely to have 50% or greater critical‐care board‐certification in their ICU (OR = 0.13; 95%CI = 0.03‐0.50). ICU size, hospital size, and years in practice were not associated with critical‐care board‐certification. Hospital size, ICU size, and the presence of intensivists or hospitalists were not associated with whether the medical director receives support from the hospital.
Physician Extenders
Nineteen sites (20%) reported the utilization of advanced practice nurses; 15 sites (16%) reported use of physician assistants; and 7 sites (7%) reported use of both advance practice nurses and physician assistants to provide intensive care. Physician extenders were not more likely to work in closed ICUs (10/24) than in open ICUs (14/72) (2 = 3.63; P = 0.57).
Of the 27 sites reporting use of advanced practice nurses or physician assistants, the role of physician extenders was described as being similar to physicians in 8 sites (30%), somewhat autonomous but with limitations in 18 (67%), and in a role closer to a ward clerk or assistant in 1 site (4%). The activities of physician extenders included writing orders at 24 of these 27 sites (89%); writing progress notes at 25 sites (92%); communicating with consultants at 24 (89%) and with primary care physicians at 22 sites (82%); and coordinating discharge plans at 20 sites (74%). Physician extenders rounded alone at 16 sites (33%).
Clinical Activities
Intensivists participated in daily rounds at most sites (n = 67; 70%). Nonintensivists served as attending of record in 72 (75%) sites. Nonintensivist physicians participating in daily patient rounds were: surgeons (n = 66; 68% of sites), primary care physicians (n = 61; 64%), nonpulmonary internal medicine specialists (n = 53; 55%), cardiologists (n = 58; 60%), non‐critical‐care pulmonologists (n = 39; 41%), and hospitalists (n = 36; 38%). Intensivists were the primary decision‐makers at 30 sites (31%), nonintensivists at 34 (35%), and decision making was shared at 31 (32%).
At more than one‐half of sites, decisions regarding mechanical ventilation, the use of sedatives or paralytics, and the choice of vasopressor agents were made by intensivists, with other decisionssuch as the decision to call consultants, choice of antibiotics, or family meetingsshared between intensivists and nonintensivists more than 40% of the time (Table 2). During regular working hours, invasive procedures were performed by multiple clinicians, including house officers, intensivists, surgeons, and anesthesiologists and were not the province of any particular type of clinician (Table 3).
| Decision‐making | |||
|---|---|---|---|
| Intensivist n (%) | Nonintensivist n (%) | Shared n (%) | |
| |||
| Ventilator management | 62 (66.7) | 24 (25.8) | 7 (7.5) |
| Choice of ventilator weaning strategies | 64 (68.8) | 24 (25.8) | 5 (5.4) |
| Decision to extubate | 63 (68.5) | 24 (26.1) | 5 (5.4) |
| Choice of sedation or paralytic agents | 56 (65.1) | 24 (27.9) | 6 (7.0) |
| Choice of vasopressor agents | 47 (51.1) | 25 (27.1) | 20 (21.7) |
| Decision to call other consultants (eg, cardiology, infectious diseases) | 19 (20.4) | 31 (33.3) | 43 (46.2) |
| Choices related to more general medical management (eg, antibiotics, diabetes management) | 30 (32.2) | 25 (26.9) | 38 (40.1) |
| Family meetings, code status discussions | 26 (28.6) | 26 (28.6) | 39 (42.8) |
| Procedure | Hospitalist n (%) | Intensivist n (%) | Surgeon n (%) | Anesthesiologist n (%) | House Officer or Other MD n (%) | Other non‐MD n (%) |
|---|---|---|---|---|---|---|
| Arterial line placement | 15 (15.6) | 50 (52.1) | 40 (41.7) | 31 (32.3) | 59 (61.4) | 7 (7.3) |
| Femoral venous line placement | 14 (14.6) | 54 (56.3) | 42 (43.8) | 17 (17.7) | 55 (57.3) | 4 (4.2) |
| Subclavian or internal jugular line placement | 14 (14.6) | 54 (56.2) | 47 (49.0) | 25 (26.0) | 62 (64.6) | 5 (5.2) |
| Pulmonary artery catheterization | 8 (8.3) | 56 (58.3) | 24 (25.0) | 21 (21.9) | 54 (56.2) | 2 (2.1) |
| Intubation | 14 (14.6) | 47 (49.0) | 14 (14.6) | 74 (77.1) | 42 (43.8) | 15 (15.6) |
| Bronchoscopy | 2 (2.1) | 67 (69.8) | 17 (17.7) | 5 (5.2) | 29 (30.2) | 0 (0) |
Regardless of the staffing model employed, the majority of sites (88%) provided care on a call‐based, rather than shift‐based system. Nighttime admissions and cross‐coverage issues were handled by house officers at more than one‐third of sites, with nonintensivist house physicians performing these tasks at 15% of sites (Table 4). Intensivists managed cross‐coverage issues by telephone at 29% of sites, and saw new admissions in person after hours at 8% of sites. Intensivists did not deliver care in scheduled shifts at any of these sites.
| Care Provider | Nighttime Admissions n (%) | Cross‐coverage n (%) |
|---|---|---|
| ||
| Emergency room physician | 13 (13.5) | 8 (8.3) |
| House physician | 15 (15.6) | 17 (17.7) |
| House officer | 42 (43.8) | 37 (38.5) |
| ICU nurse | 5 (5.2) | 10 (10.4) |
| PA or NP | 8 (8.3) | 5 (5.2) |
| Intensivist in person | 8 (8.3) | |
| Intensivist by telephone | 28 (29.2) | |
| Other | 9 (9.4) | 9 (9.4) |
Discussion
As all Keystone ICU participating sites responded to the questionnaire, we believe these results to be representative of critical care practice in the state of Michigan at the present time. Michigan ICU staffing structures are variable. Only a minority (25%) of Michigan Keystone ICU sites operated in an environment where intensivists are the only attending physicians of record. Although intensivists rounded in 60% of sites not utilizing a closed model, 75% of sites had nonintensivist attending physicians, with primary care physicians and hospitalists commonly providing ICU services. The utilization of hospitalists to provide critical care services was found in the absence of intensivists, regardless of hospital or ICU size.
Closed ICUs were seen in larger hospitals and in larger ICUs. This finding is similar to data obtained on a national level.8‐16 A high‐intensity model of care was also uncommon, although decision‐making was at least shared between intensivists and nonintensivists at two‐thirds of sites. These findings are in keeping with the observation that intensivist‐directed care advocated by the Leapfrog Group has not been widely implemented,17 including in Michigan, a regional rollout leader for the Leapfrog Group.
Fewer ICUs reported utilizing a nonintensivist model than was reported in the survey by Angus et al.,8 where approximately one‐half of ICUs delivered care in this manner. This survey was performed in 1997, prior to the launch of the Leapfrog Group effort, and may have reflected a relative over representation of smaller, general ICUs. Our study is the first statewide analysis of critical care practices in the postLeapfrog Group era. Our finding that an array of approaches to critical care delivery existed in Michigan, even when intensivists rounded on patients, is similar to that found among Leapfrog‐compliant hospitals sampled from several regions of the United States.18
Other than intensivists, surgeons, primary care, and hospitalist physicians provided care in Michigan ICUs. The hospitalist movement is relatively new.19 However, in our survey 37.5% of sites had hospitalists serving as attending physicians. Although the closed ICU model was more prevalent in larger ICUs and hospitals, the use of a hospitalist model to staff ICUs was not related to hospital size, but was instead a function of whether or not intensivists were present in a given setting. In lieu of a projected shortage of intensivists, we believe this confirms the crucial role that hospitalists will play in the provision of critical care services in the future.
The attributes of intensivist care that led to improved outcomes in previous studies1‐4 are unknown. To the extent that the involvement of intensivists on an elective rather than mandatory consultative basis may explain the higher mortality found in 1 recent study,1011 we hypothesize that having a knowledgeable physician present who communicates with clinicians and families and manages at the unit level is an important factor leading to improved outcomes. While hospitalists can have these attributes, their knowledge of specific critical care therapies and technologies may vary with the extent of their ICU training and experience. Further research should seek to quantify the attributes by which intensivists are associated with improved outcomes and seek ways to foster those attributes among hospitalists who participate in critical care delivery. Central to this will be ensuring that training programs ensure competency in critical care therapies and technologies among hospitalists and other non‐ICU physicians.
We recognize several limitations in this study. First, the validity of the survey may introduce misclassification of ICU staffing. However, the survey instrument was informed by previously‐validated instruments and experts in ICU physician staffing and hospitalist care. Second, we did not link variation in staffing to outcomes. While such analysis is important, it is beyond the scope of this survey. Third, our study was conducted in 1 state and the results may not be generalizable across the United States. Nevertheless, Michigan is a large state with a diverse array of hospitals, and as our study sample broadly represented this diversity, we believe our results are likely to be generalizable.
In conclusion, few ICUs in Michigan are closed and many utilize nonintensivist critical‐care providers such as hospitalists, primary care providers, and physician extenders to deliver clinical care. Our findings have significant implications for future efforts at a national level that involve the training of hospitalists and their acceptance as critical care practitioners. We suggest future research involving intensive care delivery focus on the feasibility of training sufficient hospitalists to satisfy a growing need for critical care that cannot be filled by intensivists, along with strategic planning to insure the model of care provided is commensurate with the complexity of illness. Although this approach appears to be occurring in Michigan on an ad hoc basis, we believe coordination between larger, intensivist‐run ICUs and smaller, nonintensivist‐run ICUs should be formalized in order to optimize the delivery of intensive care.25
- ,,, et al.;the members of the American College of Critical Care Medicine Task Force on Models for the Definition of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model.Crit Care Med.2001;29;2007–2019.
- ,,, et al.Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of “open” and “closed” formats.JAMA.1996;276:24–31.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- ,,, et al.Effects of an organized critical care service on outcomes and resource utilization: a cohort study.Crit Care Med.1999;27:270–274.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002;288:2151–2162.
- Leapfrog Group. Leapfrog Group Factsheet: ICU physician staffing (IPS). Available at: http://www.leapfroggroup.org/media/file/Leapfrog‐ICU_ Physician_Staffing_Fact_Sheet.pdf. Accessed June 2009.
- National Quality Forum. Safe Practices for Better Healthcare. Available at: http://www.qualityforum.org/pdf/reports/safe_practices.pdf. Accessed June 2009.
- ,,,,,; on behalf of the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS).Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34:1016–1024.
- ,,, et al.Team care: beyond open and closed intensive care units.Curr Opin Crit Care.2006;12:604–608.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–809.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Outcomes of care by hospitalists, general internists and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Descriptive analysis of critical care units in the United States.Crit Care Med.1992;20:846–863.
- .Leapfrog and critical care: evidence‐ and reality‐based intensive care for the 21st century.Am J Med.2004;116:188–193.
- ,,,,.The organization of intensive care unit physician services.Crit Care Med.2007;35:2256–2261.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2:327–330.
- ,,, et al.Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care.Crit Care Med.2003;31:2677–2683.
- ,,, et al.;the members of the American College of Critical Care Medicine Task Force on Models for the Definition of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model.Crit Care Med.2001;29;2007–2019.
- ,,, et al.Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of “open” and “closed” formats.JAMA.1996;276:24–31.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- ,,, et al.Effects of an organized critical care service on outcomes and resource utilization: a cohort study.Crit Care Med.1999;27:270–274.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002;288:2151–2162.
- Leapfrog Group. Leapfrog Group Factsheet: ICU physician staffing (IPS). Available at: http://www.leapfroggroup.org/media/file/Leapfrog‐ICU_ Physician_Staffing_Fact_Sheet.pdf. Accessed June 2009.
- National Quality Forum. Safe Practices for Better Healthcare. Available at: http://www.qualityforum.org/pdf/reports/safe_practices.pdf. Accessed June 2009.
- ,,,,,; on behalf of the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS).Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34:1016–1024.
- ,,, et al.Team care: beyond open and closed intensive care units.Curr Opin Crit Care.2006;12:604–608.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–809.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Outcomes of care by hospitalists, general internists and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Descriptive analysis of critical care units in the United States.Crit Care Med.1992;20:846–863.
- .Leapfrog and critical care: evidence‐ and reality‐based intensive care for the 21st century.Am J Med.2004;116:188–193.
- ,,,,.The organization of intensive care unit physician services.Crit Care Med.2007;35:2256–2261.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2:327–330.
- ,,, et al.Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care.Crit Care Med.2003;31:2677–2683.
Copyright © 2010 Society of Hospital Medicine
Delayed Antimicrobial Administration
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).
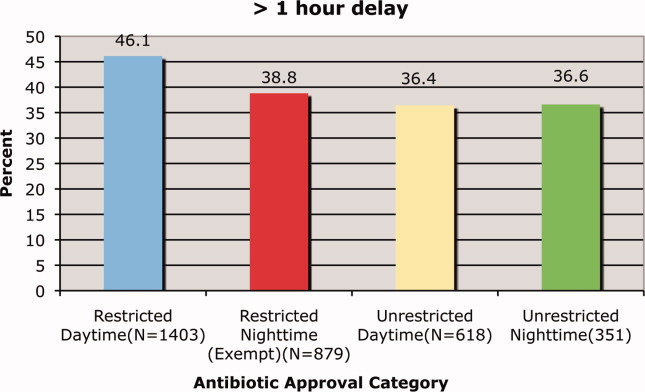
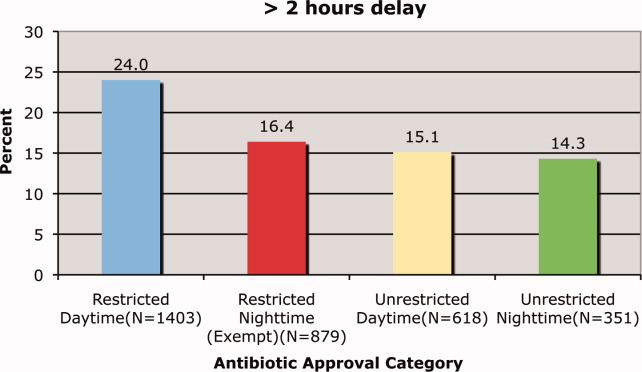
Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).


Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).


Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
Copyright © 2010 Society of Hospital Medicine