User login
Can skin care aid use of diabetes devices?
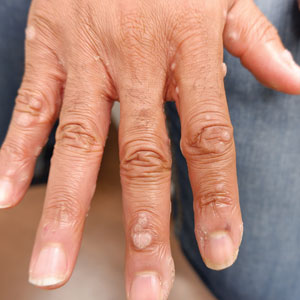
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Evidence Behind Topical Hair Loss Remedies on TikTok
Hair loss is an exceedingly common chief concern in outpatient dermatology clinics. An estimated 50% of males and females will experience androgenetic alopecia.1 Approximately 2% of new dermatology outpatient visits in the United States and the United Kingdom are for alopecia areata, the second most common type of hair loss.2 As access to dermatology appointments remains an issue with some studies citing wait times ranging from 2 to 25 days for a dermatologic consultation, the ease of accessibility of medical information on social media continues to grow,3 which leaves many of our patients turning to social media as a first-line source of information. As dermatology resident physicians, it is essential to be aware of popular dermatologic therapies on social media so that we may provide evidence-based opinions to our patients.
Remedies for Hair Loss on Social Media
Many trends on hair loss therapies found on TikTok focus on natural remedies that are produced by ingredients accessible to patients at home and over the counter, which may increase the appeal due to ease of treatment.
Rosemary Oil—The top trends in hair loss remedies I have come across are rosemary oil and rosemary water. Rosemary (Rosmarinus officinalis) has been known to possess antimicrobial and antioxidant properties but also has shown enhancement of microcapillary perfusion, which could explain its role in the prevention of hair loss and aiding hair growth in a similar mechanism to minoxidil.4,5 Unlike many other natural hair loss remedies, there are randomized controlled trials that assess the efficacy of rosemary oil for the treatment of hair loss. In a 2015 study of 100 patients with androgenetic alopecia, there was no statistically significant difference in mean hair count measured by microphotographic assessment after 6 months of treatment in 2 groups treated with either minoxidil solution 2% or rosemary oil, and both groups experienced a significant increase in hair count at 6 months (P<.05) compared with baseline and 3 months.6 Additionally, essential oils, including a mixture of thyme, rosemary, lavender, and cedarwood oils for alopecia were superior to placebo carrier oils in a posttreatment photographic assessment of their efficacy.7
Rice Water—The use of rice water and rice bran extract is a common hair care practice in Asia. Rice bran extract preparations have been shown in vivo to increase the number of anagen hair follicles as well as the number of anagen-related molecules in the dermal papillae.8,9 However, there are limited clinical data to support the use of rice water for hair growth.10
Onion Juice—Sharquie and Al-Obaidi11 conducted a study comparing crude onion juice to tap water in 38 patients with alopecia areata. They found that onion juice produced hair regrowth in significantly more patients than tap water (P<.0001).11 The mechanism of crude onion juice in hair growth is unknown; however, the induction of irritant or allergic contact dermatitis to components in crude onion juice may stimulate antigenic competition.12
Garlic Gel—Garlic gel, which is in the genus Allium, produces organosulfur compounds that provide antimicrobial and anti-inflammatory benefits.12 Additionally, in a double-blind randomized controlled trial, garlic powder was shown to increase cutaneous capillary perfusion.5 One study in 40 patients with alopecia areata demonstrated garlic gel 5% added to betamethasone valerate cream 0.1% was statistically superior to betamethasone alone in stimulating terminal hair growth (P=.001).13
Limitations and Downsides to Hair Loss Remedies on Social Media
Social media continues to be a prominent source of medical information for our patients, but most sources of hair content on social media are not board-certified dermatologists. A recent review of alopecia-related content found only 4% and 10% of posts were created by medical professionals on Instagram and TikTok, respectively, making misinformation extremely likely.14 Natural hair loss remedies contrived by TikTok have little clinical evidence to support their claims. Few data are available that compare these treatments to gold-standard hair loss therapies. Additionally, while some of these agents may be beneficial, the lack of standardized dosing may counteract these benefits. For example, videos on rosemary water advise the viewer to boil fresh rosemary sprigs in water and apply the solution to the hair daily with a spray bottle or apply cloves of garlic directly to the scalp, as opposed to a measured and standardized percentage. Some preparations may even induce harm to patients. Over-the-counter oils with added fragrances and natural compounds in onion and garlic may cause contact dermatitis. Finally, by using these products, patients may delay consultation with a board-certified dermatologist, leading to delays in applying evidence-based therapies targeted to specific hair loss subtypes while also incurring unnecessary expenses for these preparations.
Final Thoughts
Hair loss affects a notable portion of the population and is a common chief concern in dermatology clinics. Misinformation on social media continues to grow in prevalence. It is important to be aware of the hair loss remedies that are commonly touted to patients online and the evidence behind them.
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. StatPearls. StatPearls Publishing; 2022.
- McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 suppl):S49-S51.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188. doi:10.1001/jamadermatol.2020.5173
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523. doi:10.3390/ijms21020523
- Ezekwe N, King M, Hollinger JC. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: a systematic review [published online August 1, 2020]. J Clin Aesthet Dermatol. 2020;13:23-27.
- Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352. doi:10.1001/archderm.134.11.1349
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44-53. doi:10.1248/bpb.b13-00528
- Kim YM, Kwon SJ, Jang HJ, et al. Rice bran mineral extract increases the expression of anagen-related molecules in human dermal papilla through wnt/catenin pathway. Food Nutr Res. 2017;61:1412792. doi:10.1080/16546628.2017.1412792
- Hashemi K, Pham C, Sung C, et al. A systematic review: application of rice products for hair growth. J Drugs Dermatol. 2022;21:177-185. doi:10.36849/jdd.6345
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346. doi:10.1111/j.1346-8138.2002.tb00277.x
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89. doi:10.1159/000492035
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32. doi:10.4103/0378-6323.30648
- Laughter M, Anderson J, Kolla A, et al. An analysis of alopecia related content on Instagram and TikTok. J Drugs Dermatol. 2022;21:1316-1321. doi:10.36849/JDD.6707
Hair loss is an exceedingly common chief concern in outpatient dermatology clinics. An estimated 50% of males and females will experience androgenetic alopecia.1 Approximately 2% of new dermatology outpatient visits in the United States and the United Kingdom are for alopecia areata, the second most common type of hair loss.2 As access to dermatology appointments remains an issue with some studies citing wait times ranging from 2 to 25 days for a dermatologic consultation, the ease of accessibility of medical information on social media continues to grow,3 which leaves many of our patients turning to social media as a first-line source of information. As dermatology resident physicians, it is essential to be aware of popular dermatologic therapies on social media so that we may provide evidence-based opinions to our patients.
Remedies for Hair Loss on Social Media
Many trends on hair loss therapies found on TikTok focus on natural remedies that are produced by ingredients accessible to patients at home and over the counter, which may increase the appeal due to ease of treatment.
Rosemary Oil—The top trends in hair loss remedies I have come across are rosemary oil and rosemary water. Rosemary (Rosmarinus officinalis) has been known to possess antimicrobial and antioxidant properties but also has shown enhancement of microcapillary perfusion, which could explain its role in the prevention of hair loss and aiding hair growth in a similar mechanism to minoxidil.4,5 Unlike many other natural hair loss remedies, there are randomized controlled trials that assess the efficacy of rosemary oil for the treatment of hair loss. In a 2015 study of 100 patients with androgenetic alopecia, there was no statistically significant difference in mean hair count measured by microphotographic assessment after 6 months of treatment in 2 groups treated with either minoxidil solution 2% or rosemary oil, and both groups experienced a significant increase in hair count at 6 months (P<.05) compared with baseline and 3 months.6 Additionally, essential oils, including a mixture of thyme, rosemary, lavender, and cedarwood oils for alopecia were superior to placebo carrier oils in a posttreatment photographic assessment of their efficacy.7
Rice Water—The use of rice water and rice bran extract is a common hair care practice in Asia. Rice bran extract preparations have been shown in vivo to increase the number of anagen hair follicles as well as the number of anagen-related molecules in the dermal papillae.8,9 However, there are limited clinical data to support the use of rice water for hair growth.10
Onion Juice—Sharquie and Al-Obaidi11 conducted a study comparing crude onion juice to tap water in 38 patients with alopecia areata. They found that onion juice produced hair regrowth in significantly more patients than tap water (P<.0001).11 The mechanism of crude onion juice in hair growth is unknown; however, the induction of irritant or allergic contact dermatitis to components in crude onion juice may stimulate antigenic competition.12
Garlic Gel—Garlic gel, which is in the genus Allium, produces organosulfur compounds that provide antimicrobial and anti-inflammatory benefits.12 Additionally, in a double-blind randomized controlled trial, garlic powder was shown to increase cutaneous capillary perfusion.5 One study in 40 patients with alopecia areata demonstrated garlic gel 5% added to betamethasone valerate cream 0.1% was statistically superior to betamethasone alone in stimulating terminal hair growth (P=.001).13
Limitations and Downsides to Hair Loss Remedies on Social Media
Social media continues to be a prominent source of medical information for our patients, but most sources of hair content on social media are not board-certified dermatologists. A recent review of alopecia-related content found only 4% and 10% of posts were created by medical professionals on Instagram and TikTok, respectively, making misinformation extremely likely.14 Natural hair loss remedies contrived by TikTok have little clinical evidence to support their claims. Few data are available that compare these treatments to gold-standard hair loss therapies. Additionally, while some of these agents may be beneficial, the lack of standardized dosing may counteract these benefits. For example, videos on rosemary water advise the viewer to boil fresh rosemary sprigs in water and apply the solution to the hair daily with a spray bottle or apply cloves of garlic directly to the scalp, as opposed to a measured and standardized percentage. Some preparations may even induce harm to patients. Over-the-counter oils with added fragrances and natural compounds in onion and garlic may cause contact dermatitis. Finally, by using these products, patients may delay consultation with a board-certified dermatologist, leading to delays in applying evidence-based therapies targeted to specific hair loss subtypes while also incurring unnecessary expenses for these preparations.
Final Thoughts
Hair loss affects a notable portion of the population and is a common chief concern in dermatology clinics. Misinformation on social media continues to grow in prevalence. It is important to be aware of the hair loss remedies that are commonly touted to patients online and the evidence behind them.
Hair loss is an exceedingly common chief concern in outpatient dermatology clinics. An estimated 50% of males and females will experience androgenetic alopecia.1 Approximately 2% of new dermatology outpatient visits in the United States and the United Kingdom are for alopecia areata, the second most common type of hair loss.2 As access to dermatology appointments remains an issue with some studies citing wait times ranging from 2 to 25 days for a dermatologic consultation, the ease of accessibility of medical information on social media continues to grow,3 which leaves many of our patients turning to social media as a first-line source of information. As dermatology resident physicians, it is essential to be aware of popular dermatologic therapies on social media so that we may provide evidence-based opinions to our patients.
Remedies for Hair Loss on Social Media
Many trends on hair loss therapies found on TikTok focus on natural remedies that are produced by ingredients accessible to patients at home and over the counter, which may increase the appeal due to ease of treatment.
Rosemary Oil—The top trends in hair loss remedies I have come across are rosemary oil and rosemary water. Rosemary (Rosmarinus officinalis) has been known to possess antimicrobial and antioxidant properties but also has shown enhancement of microcapillary perfusion, which could explain its role in the prevention of hair loss and aiding hair growth in a similar mechanism to minoxidil.4,5 Unlike many other natural hair loss remedies, there are randomized controlled trials that assess the efficacy of rosemary oil for the treatment of hair loss. In a 2015 study of 100 patients with androgenetic alopecia, there was no statistically significant difference in mean hair count measured by microphotographic assessment after 6 months of treatment in 2 groups treated with either minoxidil solution 2% or rosemary oil, and both groups experienced a significant increase in hair count at 6 months (P<.05) compared with baseline and 3 months.6 Additionally, essential oils, including a mixture of thyme, rosemary, lavender, and cedarwood oils for alopecia were superior to placebo carrier oils in a posttreatment photographic assessment of their efficacy.7
Rice Water—The use of rice water and rice bran extract is a common hair care practice in Asia. Rice bran extract preparations have been shown in vivo to increase the number of anagen hair follicles as well as the number of anagen-related molecules in the dermal papillae.8,9 However, there are limited clinical data to support the use of rice water for hair growth.10
Onion Juice—Sharquie and Al-Obaidi11 conducted a study comparing crude onion juice to tap water in 38 patients with alopecia areata. They found that onion juice produced hair regrowth in significantly more patients than tap water (P<.0001).11 The mechanism of crude onion juice in hair growth is unknown; however, the induction of irritant or allergic contact dermatitis to components in crude onion juice may stimulate antigenic competition.12
Garlic Gel—Garlic gel, which is in the genus Allium, produces organosulfur compounds that provide antimicrobial and anti-inflammatory benefits.12 Additionally, in a double-blind randomized controlled trial, garlic powder was shown to increase cutaneous capillary perfusion.5 One study in 40 patients with alopecia areata demonstrated garlic gel 5% added to betamethasone valerate cream 0.1% was statistically superior to betamethasone alone in stimulating terminal hair growth (P=.001).13
Limitations and Downsides to Hair Loss Remedies on Social Media
Social media continues to be a prominent source of medical information for our patients, but most sources of hair content on social media are not board-certified dermatologists. A recent review of alopecia-related content found only 4% and 10% of posts were created by medical professionals on Instagram and TikTok, respectively, making misinformation extremely likely.14 Natural hair loss remedies contrived by TikTok have little clinical evidence to support their claims. Few data are available that compare these treatments to gold-standard hair loss therapies. Additionally, while some of these agents may be beneficial, the lack of standardized dosing may counteract these benefits. For example, videos on rosemary water advise the viewer to boil fresh rosemary sprigs in water and apply the solution to the hair daily with a spray bottle or apply cloves of garlic directly to the scalp, as opposed to a measured and standardized percentage. Some preparations may even induce harm to patients. Over-the-counter oils with added fragrances and natural compounds in onion and garlic may cause contact dermatitis. Finally, by using these products, patients may delay consultation with a board-certified dermatologist, leading to delays in applying evidence-based therapies targeted to specific hair loss subtypes while also incurring unnecessary expenses for these preparations.
Final Thoughts
Hair loss affects a notable portion of the population and is a common chief concern in dermatology clinics. Misinformation on social media continues to grow in prevalence. It is important to be aware of the hair loss remedies that are commonly touted to patients online and the evidence behind them.
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. StatPearls. StatPearls Publishing; 2022.
- McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 suppl):S49-S51.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188. doi:10.1001/jamadermatol.2020.5173
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523. doi:10.3390/ijms21020523
- Ezekwe N, King M, Hollinger JC. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: a systematic review [published online August 1, 2020]. J Clin Aesthet Dermatol. 2020;13:23-27.
- Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352. doi:10.1001/archderm.134.11.1349
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44-53. doi:10.1248/bpb.b13-00528
- Kim YM, Kwon SJ, Jang HJ, et al. Rice bran mineral extract increases the expression of anagen-related molecules in human dermal papilla through wnt/catenin pathway. Food Nutr Res. 2017;61:1412792. doi:10.1080/16546628.2017.1412792
- Hashemi K, Pham C, Sung C, et al. A systematic review: application of rice products for hair growth. J Drugs Dermatol. 2022;21:177-185. doi:10.36849/jdd.6345
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346. doi:10.1111/j.1346-8138.2002.tb00277.x
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89. doi:10.1159/000492035
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32. doi:10.4103/0378-6323.30648
- Laughter M, Anderson J, Kolla A, et al. An analysis of alopecia related content on Instagram and TikTok. J Drugs Dermatol. 2022;21:1316-1321. doi:10.36849/JDD.6707
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. StatPearls. StatPearls Publishing; 2022.
- McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 suppl):S49-S51.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188. doi:10.1001/jamadermatol.2020.5173
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523. doi:10.3390/ijms21020523
- Ezekwe N, King M, Hollinger JC. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: a systematic review [published online August 1, 2020]. J Clin Aesthet Dermatol. 2020;13:23-27.
- Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352. doi:10.1001/archderm.134.11.1349
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44-53. doi:10.1248/bpb.b13-00528
- Kim YM, Kwon SJ, Jang HJ, et al. Rice bran mineral extract increases the expression of anagen-related molecules in human dermal papilla through wnt/catenin pathway. Food Nutr Res. 2017;61:1412792. doi:10.1080/16546628.2017.1412792
- Hashemi K, Pham C, Sung C, et al. A systematic review: application of rice products for hair growth. J Drugs Dermatol. 2022;21:177-185. doi:10.36849/jdd.6345
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346. doi:10.1111/j.1346-8138.2002.tb00277.x
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89. doi:10.1159/000492035
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32. doi:10.4103/0378-6323.30648
- Laughter M, Anderson J, Kolla A, et al. An analysis of alopecia related content on Instagram and TikTok. J Drugs Dermatol. 2022;21:1316-1321. doi:10.36849/JDD.6707
Resident Pearl
- With terabytes of information at their fingertips, patients often turn to social media for hair loss advice. Many recommended therapies lack evidence-based research, and some may even be harmful to patients or delay time to efficacious treatments.
Which nonopioid meds are best for easing acute low back pain?
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ORTHOPAEDIC RESEARCH
Any level of physical activity tied to better later-life memory
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY
Swallow this: Tiny tech tracks your gut in real time
From heartburn to hemorrhoids and everything in between, gastrointestinal troubles affect 60 million to 70 million Americans. Part of what makes them so frustrating – besides the frequent flights to the bathroom – are the invasive and uncomfortable tests one must endure for diagnosis, such as endoscopy (feeding a flexible tube into a person’s digestive tract) or x-rays that can involve higher radiation exposure.
But a revolutionary new option promising greater comfort and convenience could become available within the next few years.
The technology is described in Nature Electronics, along with the results of in vitro and animal testing of how well it works.
“You can think of this like a GPS that you can see on your phone as your Lyft or Uber driver is moving around,” says study author Azita Emami, PhD, a professor of electrical engineering and medical engineering at the California Institute of Technology, Pasadena. “You can see the driver coming through the streets, and you can track it in real time, but imagine you can do that with much higher precision for a much smaller device inside the body.”
It’s not the first option for GI testing that can be swallowed. A “capsule endoscopy” camera can take pictures of the digestive tract. And a “wireless motility capsule” uses sensors to measure pH, temperature, and pressure. But these technologies may not work for the entire time it takes to pass through the gut, usually about 1-3 days. And while they gather information, you can’t track their location in the GI tract in real time. Your doctor can learn a lot from this level of detail.
“If a patient has motility problems in their GI tract, it can actually tell the [doctor] where the motility problem is happening, where the slowdown is happening, which is much more informative,” says Dr. Emami. Such slowdowns are common in notoriously frustrating GI issues like irritable bowel syndrome, or IBS, and inflammatory bowel disease, or IBD.
To develop this technology, the research team drew inspiration from magnetic resonance imaging, or MRI. Magnetic fields transmit data from the Bluetooth-enabled device to a smartphone. An external component, a magnetic field generator that looks like a flat mat, powers the device and is small enough to be carried in a backpack – or placed under a bed, attached to a jacket, or mounted to a toilet seat. The part that can be swallowed has tiny chips embedded in a capsulelike package.
Before this technology can go to market, more testing is needed, including clinical trials in humans, says Dr. Emami. That will likely take a few years.
The team also aims to make the device even smaller (it now measures about 1 cm wide and 2 cm long) and less expensive, and they want it to do more things, such as sending medicines to the GI tract. Those innovations could take a few more years.
A version of this article first appeared on WebMD.com.
From heartburn to hemorrhoids and everything in between, gastrointestinal troubles affect 60 million to 70 million Americans. Part of what makes them so frustrating – besides the frequent flights to the bathroom – are the invasive and uncomfortable tests one must endure for diagnosis, such as endoscopy (feeding a flexible tube into a person’s digestive tract) or x-rays that can involve higher radiation exposure.
But a revolutionary new option promising greater comfort and convenience could become available within the next few years.
The technology is described in Nature Electronics, along with the results of in vitro and animal testing of how well it works.
“You can think of this like a GPS that you can see on your phone as your Lyft or Uber driver is moving around,” says study author Azita Emami, PhD, a professor of electrical engineering and medical engineering at the California Institute of Technology, Pasadena. “You can see the driver coming through the streets, and you can track it in real time, but imagine you can do that with much higher precision for a much smaller device inside the body.”
It’s not the first option for GI testing that can be swallowed. A “capsule endoscopy” camera can take pictures of the digestive tract. And a “wireless motility capsule” uses sensors to measure pH, temperature, and pressure. But these technologies may not work for the entire time it takes to pass through the gut, usually about 1-3 days. And while they gather information, you can’t track their location in the GI tract in real time. Your doctor can learn a lot from this level of detail.
“If a patient has motility problems in their GI tract, it can actually tell the [doctor] where the motility problem is happening, where the slowdown is happening, which is much more informative,” says Dr. Emami. Such slowdowns are common in notoriously frustrating GI issues like irritable bowel syndrome, or IBS, and inflammatory bowel disease, or IBD.
To develop this technology, the research team drew inspiration from magnetic resonance imaging, or MRI. Magnetic fields transmit data from the Bluetooth-enabled device to a smartphone. An external component, a magnetic field generator that looks like a flat mat, powers the device and is small enough to be carried in a backpack – or placed under a bed, attached to a jacket, or mounted to a toilet seat. The part that can be swallowed has tiny chips embedded in a capsulelike package.
Before this technology can go to market, more testing is needed, including clinical trials in humans, says Dr. Emami. That will likely take a few years.
The team also aims to make the device even smaller (it now measures about 1 cm wide and 2 cm long) and less expensive, and they want it to do more things, such as sending medicines to the GI tract. Those innovations could take a few more years.
A version of this article first appeared on WebMD.com.
From heartburn to hemorrhoids and everything in between, gastrointestinal troubles affect 60 million to 70 million Americans. Part of what makes them so frustrating – besides the frequent flights to the bathroom – are the invasive and uncomfortable tests one must endure for diagnosis, such as endoscopy (feeding a flexible tube into a person’s digestive tract) or x-rays that can involve higher radiation exposure.
But a revolutionary new option promising greater comfort and convenience could become available within the next few years.
The technology is described in Nature Electronics, along with the results of in vitro and animal testing of how well it works.
“You can think of this like a GPS that you can see on your phone as your Lyft or Uber driver is moving around,” says study author Azita Emami, PhD, a professor of electrical engineering and medical engineering at the California Institute of Technology, Pasadena. “You can see the driver coming through the streets, and you can track it in real time, but imagine you can do that with much higher precision for a much smaller device inside the body.”
It’s not the first option for GI testing that can be swallowed. A “capsule endoscopy” camera can take pictures of the digestive tract. And a “wireless motility capsule” uses sensors to measure pH, temperature, and pressure. But these technologies may not work for the entire time it takes to pass through the gut, usually about 1-3 days. And while they gather information, you can’t track their location in the GI tract in real time. Your doctor can learn a lot from this level of detail.
“If a patient has motility problems in their GI tract, it can actually tell the [doctor] where the motility problem is happening, where the slowdown is happening, which is much more informative,” says Dr. Emami. Such slowdowns are common in notoriously frustrating GI issues like irritable bowel syndrome, or IBS, and inflammatory bowel disease, or IBD.
To develop this technology, the research team drew inspiration from magnetic resonance imaging, or MRI. Magnetic fields transmit data from the Bluetooth-enabled device to a smartphone. An external component, a magnetic field generator that looks like a flat mat, powers the device and is small enough to be carried in a backpack – or placed under a bed, attached to a jacket, or mounted to a toilet seat. The part that can be swallowed has tiny chips embedded in a capsulelike package.
Before this technology can go to market, more testing is needed, including clinical trials in humans, says Dr. Emami. That will likely take a few years.
The team also aims to make the device even smaller (it now measures about 1 cm wide and 2 cm long) and less expensive, and they want it to do more things, such as sending medicines to the GI tract. Those innovations could take a few more years.
A version of this article first appeared on WebMD.com.
FROM NATURE ELECTRONICS
Colorectal cancer incidence doubled in younger adults
according to a new report from the American Cancer Society.
Diagnoses in people younger than 55 years doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019.
In addition, more advanced disease is being diagnosed; the proportion of individuals of all ages presenting with advanced-stage CRC increased from 52% in the mid-2000s to 60% in 2019.
“We know rates are increasing in young people, but it’s alarming to see how rapidly the whole patient population is shifting younger, despite shrinking numbers in the overall population,” said Rebecca Siegel, MPH, senior scientific director of surveillance research at the American Cancer Society and lead author of the report.
“The trend toward more advanced disease in people of all ages is also surprising and should motivate everyone 45 and older to get screened,” she added.
The report was published online in CA: A Cancer Journal for Clinicians.
CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death of both men and women in the United States. It is estimated that there will be 153,020 new cases of CRC in the U.S. in 2023, including 106,970 tumors in the colon and 46,050 in the rectum.
Overall, in 2023, an estimated 153,020 people will be diagnosed with CRC in the U.S., and of those, 52,550 people will die from the disease.
The incidence of CRC rapidly decreased during the 2000s among people aged 50 and older, largely because of an increase in cancer screening with colonoscopy. But progress slowed during the past decade, and now the trends toward declining incidence is largely confined to those aged 65 and older.
The authors point out that more than half of all cases and deaths are associated with modifiable risk factors, including smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body weight. A large proportion of CRC incidence and mortality is preventable through recommended screening, surveillance, and high-quality treatment.
But it remains unclear why rates are rising among younger adults and why there is a trend toward the disase being initially diagnosed at more advanced stages.
“We have to address why the rates in young adults continue to trend in the wrong direction,” said Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society and senior author of the study. “We need to invest more in research to uncover the causes of the rising trends and to discover new treatment for advanced-stage diseases to reduce the morbidity and mortality associated with this disease in this young population, who are raising families and supporting other family members.”
For their report, Ms. Siegel and colleagues used incidence data from 1995 to 2019 from 50 states and the District of Columbia. The data came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and the National Program of Cancer Registries of the U.S. Centers for Disease Control and Prevention, as provided by the North American Association of Central Cancer Registries. National mortality data through 2020 were provided by the National Center for Health Statistics.
The authors note that while overall, deaths from CRC are continuing to fall, “this progress is tempered by a rapidly changing landscape of disease that foreshadows less favorable trends ahead.”
The incidence rates have increased by 2% per year among people younger than 50 years as well as among those aged 50-54 years while, for the past decade, the rates have declined among those aged 65 and older. Incidence rates have stabilized among persons aged 50-64 years.
Although the majority of diagnoses continue to occur among people aged 65 years and older, 19,550 cases (13%) will occur in those younger than age 50 years, and one-third will be diagnosed in those aged 50-64 years.
Other key findings include the following.
- Declines in incidence and mortality have slowed, from 3% to 4% per year during the 2000s to 1% per year for incidence and 2% per year for mortality during the past decade.
- The incidence rate was 33% higher among men than women from 2015 to 2019, which may reflect differences in risk factors, such as excess body weight, processed meat consumption, and a history of smoking.
- The percentage of patients who present with advanced-stage disease has increased from a low of 52% in the mid-2000s to 60% in 2019 despite an increase in the use of screening.
- Death rates from CRC have risen since around 2005 by 1% annually among those younger than 50 years and by 0.6% in people aged 50-54.
- The report also identified racial/ethnic differences in incidence and mortality: Incidence was highest among Alaska Natives (88.5 per 100,000), American Indians (46.0 per 100,000), and Black persons, compared with White persons (41.7 per 100,000 vs. 35.7 per 100,000). Mortality followed a similar pattern; the highest rates were observed among Alaska Natives (50.5 per 100,000), American Indians (17.5 per 100,000), and Black persons, compared with White persons (17.6 per 100,000 vs. 13.1 per 100,000).
- There was also a shift to left-sided tumors, despite greater efficacy of screening for preventing left-sided lesions. The proportion of CRCs occurring in the rectum has steadily risen from 27% in 1995 to 31% in 2019.
“These highly concerning data illustrate the urgent need to invest in targeted cancer research studies dedicated to understanding and preventing early-onset colorectal cancer,” said Karen E. Knudsen, MBA, PhD, and CEO of the American Cancer Society. “The shift to diagnosis of more advanced disease also underscores the importance of screening and early detection, which saves lives.”
The study was supported by the American Cancer Society.
A version of this article first appeared on Medscape.com.
according to a new report from the American Cancer Society.
Diagnoses in people younger than 55 years doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019.
In addition, more advanced disease is being diagnosed; the proportion of individuals of all ages presenting with advanced-stage CRC increased from 52% in the mid-2000s to 60% in 2019.
“We know rates are increasing in young people, but it’s alarming to see how rapidly the whole patient population is shifting younger, despite shrinking numbers in the overall population,” said Rebecca Siegel, MPH, senior scientific director of surveillance research at the American Cancer Society and lead author of the report.
“The trend toward more advanced disease in people of all ages is also surprising and should motivate everyone 45 and older to get screened,” she added.
The report was published online in CA: A Cancer Journal for Clinicians.
CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death of both men and women in the United States. It is estimated that there will be 153,020 new cases of CRC in the U.S. in 2023, including 106,970 tumors in the colon and 46,050 in the rectum.
Overall, in 2023, an estimated 153,020 people will be diagnosed with CRC in the U.S., and of those, 52,550 people will die from the disease.
The incidence of CRC rapidly decreased during the 2000s among people aged 50 and older, largely because of an increase in cancer screening with colonoscopy. But progress slowed during the past decade, and now the trends toward declining incidence is largely confined to those aged 65 and older.
The authors point out that more than half of all cases and deaths are associated with modifiable risk factors, including smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body weight. A large proportion of CRC incidence and mortality is preventable through recommended screening, surveillance, and high-quality treatment.
But it remains unclear why rates are rising among younger adults and why there is a trend toward the disase being initially diagnosed at more advanced stages.
“We have to address why the rates in young adults continue to trend in the wrong direction,” said Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society and senior author of the study. “We need to invest more in research to uncover the causes of the rising trends and to discover new treatment for advanced-stage diseases to reduce the morbidity and mortality associated with this disease in this young population, who are raising families and supporting other family members.”
For their report, Ms. Siegel and colleagues used incidence data from 1995 to 2019 from 50 states and the District of Columbia. The data came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and the National Program of Cancer Registries of the U.S. Centers for Disease Control and Prevention, as provided by the North American Association of Central Cancer Registries. National mortality data through 2020 were provided by the National Center for Health Statistics.
The authors note that while overall, deaths from CRC are continuing to fall, “this progress is tempered by a rapidly changing landscape of disease that foreshadows less favorable trends ahead.”
The incidence rates have increased by 2% per year among people younger than 50 years as well as among those aged 50-54 years while, for the past decade, the rates have declined among those aged 65 and older. Incidence rates have stabilized among persons aged 50-64 years.
Although the majority of diagnoses continue to occur among people aged 65 years and older, 19,550 cases (13%) will occur in those younger than age 50 years, and one-third will be diagnosed in those aged 50-64 years.
Other key findings include the following.
- Declines in incidence and mortality have slowed, from 3% to 4% per year during the 2000s to 1% per year for incidence and 2% per year for mortality during the past decade.
- The incidence rate was 33% higher among men than women from 2015 to 2019, which may reflect differences in risk factors, such as excess body weight, processed meat consumption, and a history of smoking.
- The percentage of patients who present with advanced-stage disease has increased from a low of 52% in the mid-2000s to 60% in 2019 despite an increase in the use of screening.
- Death rates from CRC have risen since around 2005 by 1% annually among those younger than 50 years and by 0.6% in people aged 50-54.
- The report also identified racial/ethnic differences in incidence and mortality: Incidence was highest among Alaska Natives (88.5 per 100,000), American Indians (46.0 per 100,000), and Black persons, compared with White persons (41.7 per 100,000 vs. 35.7 per 100,000). Mortality followed a similar pattern; the highest rates were observed among Alaska Natives (50.5 per 100,000), American Indians (17.5 per 100,000), and Black persons, compared with White persons (17.6 per 100,000 vs. 13.1 per 100,000).
- There was also a shift to left-sided tumors, despite greater efficacy of screening for preventing left-sided lesions. The proportion of CRCs occurring in the rectum has steadily risen from 27% in 1995 to 31% in 2019.
“These highly concerning data illustrate the urgent need to invest in targeted cancer research studies dedicated to understanding and preventing early-onset colorectal cancer,” said Karen E. Knudsen, MBA, PhD, and CEO of the American Cancer Society. “The shift to diagnosis of more advanced disease also underscores the importance of screening and early detection, which saves lives.”
The study was supported by the American Cancer Society.
A version of this article first appeared on Medscape.com.
according to a new report from the American Cancer Society.
Diagnoses in people younger than 55 years doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019.
In addition, more advanced disease is being diagnosed; the proportion of individuals of all ages presenting with advanced-stage CRC increased from 52% in the mid-2000s to 60% in 2019.
“We know rates are increasing in young people, but it’s alarming to see how rapidly the whole patient population is shifting younger, despite shrinking numbers in the overall population,” said Rebecca Siegel, MPH, senior scientific director of surveillance research at the American Cancer Society and lead author of the report.
“The trend toward more advanced disease in people of all ages is also surprising and should motivate everyone 45 and older to get screened,” she added.
The report was published online in CA: A Cancer Journal for Clinicians.
CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death of both men and women in the United States. It is estimated that there will be 153,020 new cases of CRC in the U.S. in 2023, including 106,970 tumors in the colon and 46,050 in the rectum.
Overall, in 2023, an estimated 153,020 people will be diagnosed with CRC in the U.S., and of those, 52,550 people will die from the disease.
The incidence of CRC rapidly decreased during the 2000s among people aged 50 and older, largely because of an increase in cancer screening with colonoscopy. But progress slowed during the past decade, and now the trends toward declining incidence is largely confined to those aged 65 and older.
The authors point out that more than half of all cases and deaths are associated with modifiable risk factors, including smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body weight. A large proportion of CRC incidence and mortality is preventable through recommended screening, surveillance, and high-quality treatment.
But it remains unclear why rates are rising among younger adults and why there is a trend toward the disase being initially diagnosed at more advanced stages.
“We have to address why the rates in young adults continue to trend in the wrong direction,” said Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society and senior author of the study. “We need to invest more in research to uncover the causes of the rising trends and to discover new treatment for advanced-stage diseases to reduce the morbidity and mortality associated with this disease in this young population, who are raising families and supporting other family members.”
For their report, Ms. Siegel and colleagues used incidence data from 1995 to 2019 from 50 states and the District of Columbia. The data came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and the National Program of Cancer Registries of the U.S. Centers for Disease Control and Prevention, as provided by the North American Association of Central Cancer Registries. National mortality data through 2020 were provided by the National Center for Health Statistics.
The authors note that while overall, deaths from CRC are continuing to fall, “this progress is tempered by a rapidly changing landscape of disease that foreshadows less favorable trends ahead.”
The incidence rates have increased by 2% per year among people younger than 50 years as well as among those aged 50-54 years while, for the past decade, the rates have declined among those aged 65 and older. Incidence rates have stabilized among persons aged 50-64 years.
Although the majority of diagnoses continue to occur among people aged 65 years and older, 19,550 cases (13%) will occur in those younger than age 50 years, and one-third will be diagnosed in those aged 50-64 years.
Other key findings include the following.
- Declines in incidence and mortality have slowed, from 3% to 4% per year during the 2000s to 1% per year for incidence and 2% per year for mortality during the past decade.
- The incidence rate was 33% higher among men than women from 2015 to 2019, which may reflect differences in risk factors, such as excess body weight, processed meat consumption, and a history of smoking.
- The percentage of patients who present with advanced-stage disease has increased from a low of 52% in the mid-2000s to 60% in 2019 despite an increase in the use of screening.
- Death rates from CRC have risen since around 2005 by 1% annually among those younger than 50 years and by 0.6% in people aged 50-54.
- The report also identified racial/ethnic differences in incidence and mortality: Incidence was highest among Alaska Natives (88.5 per 100,000), American Indians (46.0 per 100,000), and Black persons, compared with White persons (41.7 per 100,000 vs. 35.7 per 100,000). Mortality followed a similar pattern; the highest rates were observed among Alaska Natives (50.5 per 100,000), American Indians (17.5 per 100,000), and Black persons, compared with White persons (17.6 per 100,000 vs. 13.1 per 100,000).
- There was also a shift to left-sided tumors, despite greater efficacy of screening for preventing left-sided lesions. The proportion of CRCs occurring in the rectum has steadily risen from 27% in 1995 to 31% in 2019.
“These highly concerning data illustrate the urgent need to invest in targeted cancer research studies dedicated to understanding and preventing early-onset colorectal cancer,” said Karen E. Knudsen, MBA, PhD, and CEO of the American Cancer Society. “The shift to diagnosis of more advanced disease also underscores the importance of screening and early detection, which saves lives.”
The study was supported by the American Cancer Society.
A version of this article first appeared on Medscape.com.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Multimodal Treatment of Epidermodysplasia Verruciformis in an HIV-Positive Man
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.
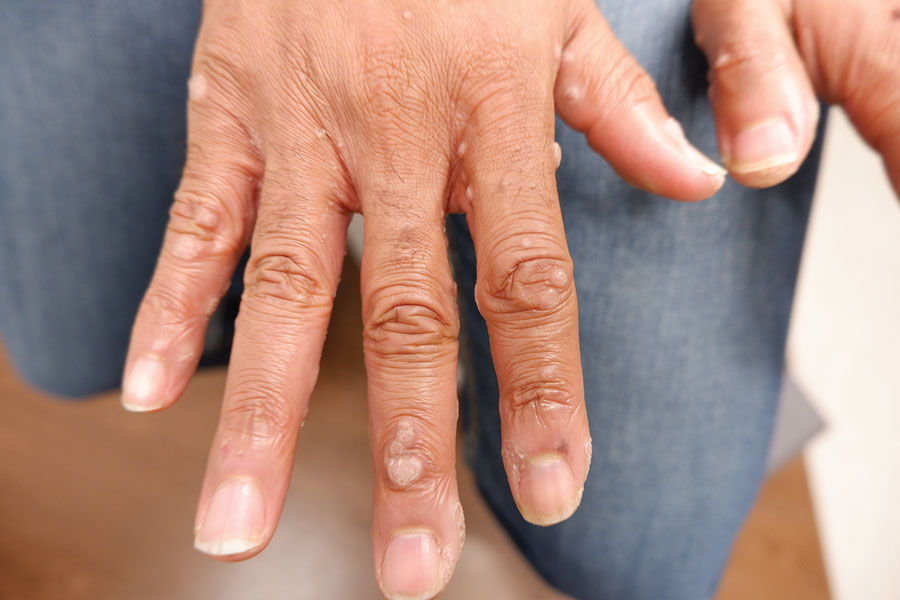
A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.
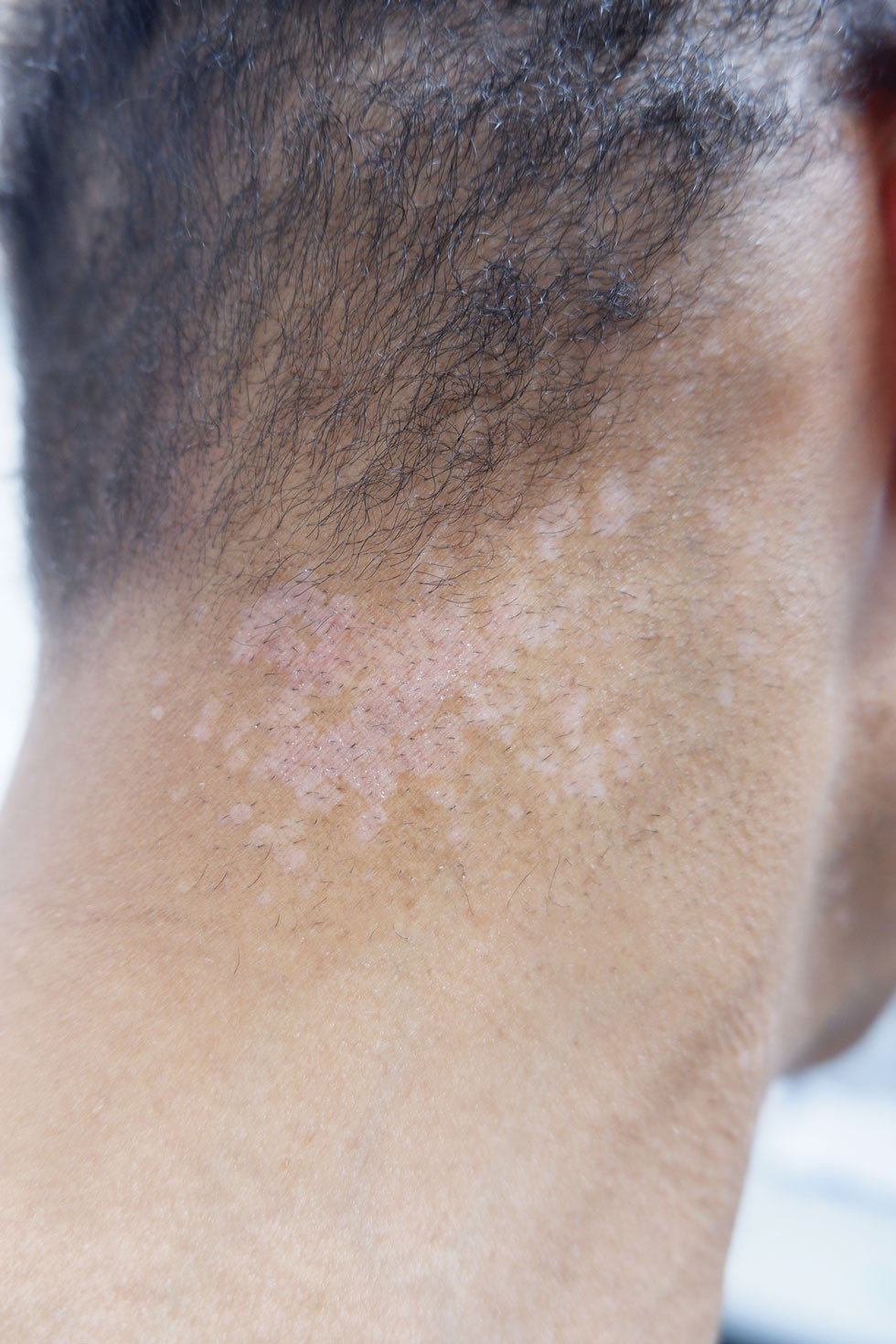
Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.
Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is associated with immunocompromised patients with conditions such as HIV.
- Multimodal treatment of HIV-associated acquired EDV with acitretin, intralesional Candida albicans antigen, and cryotherapy may be efficacious for patients with recalcitrant disease.
500 more steps a day tied to 14% lower CVD risk in older adults
Older adults who added a quarter mile of steps to their day showed a reduction in risk of cardiovascular events by 14% within 4 years, according to a study in more than 400 individuals.
“Aging is such a dynamic process, but most studies of daily steps and step goals are conducted on younger populations,” lead author Erin E. Dooley, PhD, an epidemiologist at the University of Alabama at Birmingham, said in an interview.
The impact of more modest step goals in older adults has not been well studied, Dr. Dooley said.
The population in the current study ranged from 71 to 92 years, with an average age of 78 years. The older age and relatively short follow-up period show the importance of steps and physical activity in older adults, she said.
Dr. Dooley presented the study at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
She and her colleagues analyzed a subsample of participants in Atherosclerosis Risk in Communities (ARIC) study, an ongoing study conducted by the National Heart, Lung, and Blood Institute. The study population included 452 adults for whom step data were available at visit 6 of the ARIC study between 2016 and 2017. Participants wore an accelerometer on the waist for at least 10 hours a day for at least 3 days. The mean age of the participants was 78.4 years, 59% were women, and 20% were Black.
Outcomes were measured through December 2019 and included fatal and nonfatal cardiovascular disease (CVD) events of coronary heart disease, stroke, and heart failure.
Overall, each additional 500 steps per day was linked to a 14% reduction in risk of a CVD event (hazard ratio, 0.86; 95% confidence interval, 0.76-0.98). The mean step count was 3,447 steps per day, and 34 participants (7.5%) experienced a CVD event over 1,269 person-years of follow-up.
The cumulative risk of CVD was significantly higher (11.5%) in the quartile of adults with the lowest step count (defined as fewer than 2,077 steps per day), compared with 3.5% in those with the highest step count (defined as at least 4,453 steps per day).
In addition, adults in the highest quartile of steps had a 77% reduced risk of a proximal CVD (within 3.5 years) event over the study period (HR, 0.23).
Additional research is needed to explore whether increased steps prevent or delay CVD and whether low step counts may be a biomarker for underlying disease, the researchers noted in their abstract.
However, the results support the value of even a modest increase in activity to reduce CVD risk in older adults.
Small steps may get patients started
Dr. Dooley said she was surprised at the degree of benefits on heart health from 500 steps, and noted that the findings have clinical implications.
“Steps may be a more understandable metric for physical activity for patients than talking about moderate to vigorous intensity physical activity,” she said in an interview. “While we do not want to diminish the importance of higher intensity physical activity, encouraging small increases in the number of daily steps can also have great benefits for heart health.
“Steps are counted using a variety of devices and phones, so it may be helpful for patients to show clinicians their activity during well visits,” Dr. Dooley said. “Walking may also be more manageable for people as it is low impact. Achievable goals are also important. This study suggests that, for older adults, around 3,000 steps or more was associated with reduced CVD risk,” although the greatest benefits were seen with the most active group who averaged 4,500 or more steps per day.
More research is needed to show how steps may change over time, and how this relates to CVD and heart health,” she said. “At this time, we only had a single measure of physical activity.”
Study fills research gap for older adults
“Currently, the majority of the literature exploring a relationship between physical activity and the risk for developing cardiovascular disease has evaluated all adults together, not only those who are 70 year of age and older,” Monica C. Serra, PhD, of the University of Texas, San Antonio, said in an interview. “This study allows us to start to target specific cardiovascular recommendations for older adults.”.
“It is always exciting to see results from physical activity studies that continue to support prior evidence that even small amounts of physical activity are beneficial to cardiovascular health,” said Dr. Serra, who is also vice chair of the program committee for the meeting. “These results suggest that even if only small additions in physical activity are achievable, they may have cumulative benefits in reducing cardiovascular disease risk.” For clinicians, the results also provide targets that are easy for patients to understand, said Dr. Serra. Daily step counts allow clinicians to provide specific and measurable goals to help their older patients increase physical activity.
“Small additions in total daily step counts may have clinically meaningful benefits to heart health, so promoting their patients to make any slight changes that are able to be consistently incorporated into their schedule should be encouraged. This may be best monitored by encouraging the use of an activity tracker,” she said.
Although the current study adds to the literature with objective measures of physical activity utilizing accelerometers, these devices are not as sensitive at picking up activities such as bicycling or swimming, which may be more appropriate for some older adults with mobility limitations and chronic conditions, Dr. Serra said. Additional research is needed to assess the impact of other activities on CVD in the older population.
The meeting was sponsored by the American Heart Association. The study received no outside funding. Dr. Dooley and Dr. Serra had no financial conflicts to disclose.
Older adults who added a quarter mile of steps to their day showed a reduction in risk of cardiovascular events by 14% within 4 years, according to a study in more than 400 individuals.
“Aging is such a dynamic process, but most studies of daily steps and step goals are conducted on younger populations,” lead author Erin E. Dooley, PhD, an epidemiologist at the University of Alabama at Birmingham, said in an interview.
The impact of more modest step goals in older adults has not been well studied, Dr. Dooley said.
The population in the current study ranged from 71 to 92 years, with an average age of 78 years. The older age and relatively short follow-up period show the importance of steps and physical activity in older adults, she said.
Dr. Dooley presented the study at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
She and her colleagues analyzed a subsample of participants in Atherosclerosis Risk in Communities (ARIC) study, an ongoing study conducted by the National Heart, Lung, and Blood Institute. The study population included 452 adults for whom step data were available at visit 6 of the ARIC study between 2016 and 2017. Participants wore an accelerometer on the waist for at least 10 hours a day for at least 3 days. The mean age of the participants was 78.4 years, 59% were women, and 20% were Black.
Outcomes were measured through December 2019 and included fatal and nonfatal cardiovascular disease (CVD) events of coronary heart disease, stroke, and heart failure.
Overall, each additional 500 steps per day was linked to a 14% reduction in risk of a CVD event (hazard ratio, 0.86; 95% confidence interval, 0.76-0.98). The mean step count was 3,447 steps per day, and 34 participants (7.5%) experienced a CVD event over 1,269 person-years of follow-up.
The cumulative risk of CVD was significantly higher (11.5%) in the quartile of adults with the lowest step count (defined as fewer than 2,077 steps per day), compared with 3.5% in those with the highest step count (defined as at least 4,453 steps per day).
In addition, adults in the highest quartile of steps had a 77% reduced risk of a proximal CVD (within 3.5 years) event over the study period (HR, 0.23).
Additional research is needed to explore whether increased steps prevent or delay CVD and whether low step counts may be a biomarker for underlying disease, the researchers noted in their abstract.
However, the results support the value of even a modest increase in activity to reduce CVD risk in older adults.
Small steps may get patients started
Dr. Dooley said she was surprised at the degree of benefits on heart health from 500 steps, and noted that the findings have clinical implications.
“Steps may be a more understandable metric for physical activity for patients than talking about moderate to vigorous intensity physical activity,” she said in an interview. “While we do not want to diminish the importance of higher intensity physical activity, encouraging small increases in the number of daily steps can also have great benefits for heart health.
“Steps are counted using a variety of devices and phones, so it may be helpful for patients to show clinicians their activity during well visits,” Dr. Dooley said. “Walking may also be more manageable for people as it is low impact. Achievable goals are also important. This study suggests that, for older adults, around 3,000 steps or more was associated with reduced CVD risk,” although the greatest benefits were seen with the most active group who averaged 4,500 or more steps per day.
More research is needed to show how steps may change over time, and how this relates to CVD and heart health,” she said. “At this time, we only had a single measure of physical activity.”
Study fills research gap for older adults
“Currently, the majority of the literature exploring a relationship between physical activity and the risk for developing cardiovascular disease has evaluated all adults together, not only those who are 70 year of age and older,” Monica C. Serra, PhD, of the University of Texas, San Antonio, said in an interview. “This study allows us to start to target specific cardiovascular recommendations for older adults.”.
“It is always exciting to see results from physical activity studies that continue to support prior evidence that even small amounts of physical activity are beneficial to cardiovascular health,” said Dr. Serra, who is also vice chair of the program committee for the meeting. “These results suggest that even if only small additions in physical activity are achievable, they may have cumulative benefits in reducing cardiovascular disease risk.” For clinicians, the results also provide targets that are easy for patients to understand, said Dr. Serra. Daily step counts allow clinicians to provide specific and measurable goals to help their older patients increase physical activity.
“Small additions in total daily step counts may have clinically meaningful benefits to heart health, so promoting their patients to make any slight changes that are able to be consistently incorporated into their schedule should be encouraged. This may be best monitored by encouraging the use of an activity tracker,” she said.
Although the current study adds to the literature with objective measures of physical activity utilizing accelerometers, these devices are not as sensitive at picking up activities such as bicycling or swimming, which may be more appropriate for some older adults with mobility limitations and chronic conditions, Dr. Serra said. Additional research is needed to assess the impact of other activities on CVD in the older population.
The meeting was sponsored by the American Heart Association. The study received no outside funding. Dr. Dooley and Dr. Serra had no financial conflicts to disclose.
Older adults who added a quarter mile of steps to their day showed a reduction in risk of cardiovascular events by 14% within 4 years, according to a study in more than 400 individuals.
“Aging is such a dynamic process, but most studies of daily steps and step goals are conducted on younger populations,” lead author Erin E. Dooley, PhD, an epidemiologist at the University of Alabama at Birmingham, said in an interview.
The impact of more modest step goals in older adults has not been well studied, Dr. Dooley said.
The population in the current study ranged from 71 to 92 years, with an average age of 78 years. The older age and relatively short follow-up period show the importance of steps and physical activity in older adults, she said.
Dr. Dooley presented the study at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
She and her colleagues analyzed a subsample of participants in Atherosclerosis Risk in Communities (ARIC) study, an ongoing study conducted by the National Heart, Lung, and Blood Institute. The study population included 452 adults for whom step data were available at visit 6 of the ARIC study between 2016 and 2017. Participants wore an accelerometer on the waist for at least 10 hours a day for at least 3 days. The mean age of the participants was 78.4 years, 59% were women, and 20% were Black.
Outcomes were measured through December 2019 and included fatal and nonfatal cardiovascular disease (CVD) events of coronary heart disease, stroke, and heart failure.
Overall, each additional 500 steps per day was linked to a 14% reduction in risk of a CVD event (hazard ratio, 0.86; 95% confidence interval, 0.76-0.98). The mean step count was 3,447 steps per day, and 34 participants (7.5%) experienced a CVD event over 1,269 person-years of follow-up.
The cumulative risk of CVD was significantly higher (11.5%) in the quartile of adults with the lowest step count (defined as fewer than 2,077 steps per day), compared with 3.5% in those with the highest step count (defined as at least 4,453 steps per day).
In addition, adults in the highest quartile of steps had a 77% reduced risk of a proximal CVD (within 3.5 years) event over the study period (HR, 0.23).
Additional research is needed to explore whether increased steps prevent or delay CVD and whether low step counts may be a biomarker for underlying disease, the researchers noted in their abstract.
However, the results support the value of even a modest increase in activity to reduce CVD risk in older adults.
Small steps may get patients started
Dr. Dooley said she was surprised at the degree of benefits on heart health from 500 steps, and noted that the findings have clinical implications.
“Steps may be a more understandable metric for physical activity for patients than talking about moderate to vigorous intensity physical activity,” she said in an interview. “While we do not want to diminish the importance of higher intensity physical activity, encouraging small increases in the number of daily steps can also have great benefits for heart health.
“Steps are counted using a variety of devices and phones, so it may be helpful for patients to show clinicians their activity during well visits,” Dr. Dooley said. “Walking may also be more manageable for people as it is low impact. Achievable goals are also important. This study suggests that, for older adults, around 3,000 steps or more was associated with reduced CVD risk,” although the greatest benefits were seen with the most active group who averaged 4,500 or more steps per day.
More research is needed to show how steps may change over time, and how this relates to CVD and heart health,” she said. “At this time, we only had a single measure of physical activity.”
Study fills research gap for older adults
“Currently, the majority of the literature exploring a relationship between physical activity and the risk for developing cardiovascular disease has evaluated all adults together, not only those who are 70 year of age and older,” Monica C. Serra, PhD, of the University of Texas, San Antonio, said in an interview. “This study allows us to start to target specific cardiovascular recommendations for older adults.”.
“It is always exciting to see results from physical activity studies that continue to support prior evidence that even small amounts of physical activity are beneficial to cardiovascular health,” said Dr. Serra, who is also vice chair of the program committee for the meeting. “These results suggest that even if only small additions in physical activity are achievable, they may have cumulative benefits in reducing cardiovascular disease risk.” For clinicians, the results also provide targets that are easy for patients to understand, said Dr. Serra. Daily step counts allow clinicians to provide specific and measurable goals to help their older patients increase physical activity.
“Small additions in total daily step counts may have clinically meaningful benefits to heart health, so promoting their patients to make any slight changes that are able to be consistently incorporated into their schedule should be encouraged. This may be best monitored by encouraging the use of an activity tracker,” she said.
Although the current study adds to the literature with objective measures of physical activity utilizing accelerometers, these devices are not as sensitive at picking up activities such as bicycling or swimming, which may be more appropriate for some older adults with mobility limitations and chronic conditions, Dr. Serra said. Additional research is needed to assess the impact of other activities on CVD in the older population.
The meeting was sponsored by the American Heart Association. The study received no outside funding. Dr. Dooley and Dr. Serra had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2023
Lilly cuts insulin price by 70%, caps out-of-pocket cost
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
FDA declines approval for omecamtiv mecarbil in HFrEF
The Food and Drug Administration has declined to approve omecamtiv mecarbil (Cytokinetics) for treatment of adults with chronic heart failure with reduced ejection fraction (HFrEF), citing a lack of evidence on efficacy.
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
Last December, a panel of FDA advisers recommended against approval of omecamtiv mecarbil for chronic HFrEF, as reported by this news organization.
The FDA Cardiovascular and Renal Drugs Advisory Committee voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF. The drug had a PDUFA date of February 28.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
In a complete response letter, the FDA said GALACTIC-HF is “not sufficiently persuasive to establish substantial evidence of effectiveness for reducing the risk of heart failure events and cardiovascular death” in adults with HFrEF, Cytokinetics shared in a news release.
Further, the FDA said results from an additional clinical trial of omecamtiv mecarbil are required to establish substantial evidence of effectiveness for the treatment of HFrEF, with benefits that outweigh the risks, Cytokinetics said.
The company said it will request a meeting with the FDA to gain a better understanding of what may be required to support potential approval of omecamtiv mecarbil. However, the company also said it has “no plans” to conduct an additional clinical trial of omecamtiv mecarbil.
Instead, the company said its focus remains on the development of aficamten, the next-in-class cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, a phase 3 clinical trial in patients with obstructive hypertrophic cardiomyopathy.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has declined to approve omecamtiv mecarbil (Cytokinetics) for treatment of adults with chronic heart failure with reduced ejection fraction (HFrEF), citing a lack of evidence on efficacy.
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
Last December, a panel of FDA advisers recommended against approval of omecamtiv mecarbil for chronic HFrEF, as reported by this news organization.
The FDA Cardiovascular and Renal Drugs Advisory Committee voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF. The drug had a PDUFA date of February 28.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
In a complete response letter, the FDA said GALACTIC-HF is “not sufficiently persuasive to establish substantial evidence of effectiveness for reducing the risk of heart failure events and cardiovascular death” in adults with HFrEF, Cytokinetics shared in a news release.
Further, the FDA said results from an additional clinical trial of omecamtiv mecarbil are required to establish substantial evidence of effectiveness for the treatment of HFrEF, with benefits that outweigh the risks, Cytokinetics said.
The company said it will request a meeting with the FDA to gain a better understanding of what may be required to support potential approval of omecamtiv mecarbil. However, the company also said it has “no plans” to conduct an additional clinical trial of omecamtiv mecarbil.
Instead, the company said its focus remains on the development of aficamten, the next-in-class cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, a phase 3 clinical trial in patients with obstructive hypertrophic cardiomyopathy.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has declined to approve omecamtiv mecarbil (Cytokinetics) for treatment of adults with chronic heart failure with reduced ejection fraction (HFrEF), citing a lack of evidence on efficacy.
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
Last December, a panel of FDA advisers recommended against approval of omecamtiv mecarbil for chronic HFrEF, as reported by this news organization.
The FDA Cardiovascular and Renal Drugs Advisory Committee voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF. The drug had a PDUFA date of February 28.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
In a complete response letter, the FDA said GALACTIC-HF is “not sufficiently persuasive to establish substantial evidence of effectiveness for reducing the risk of heart failure events and cardiovascular death” in adults with HFrEF, Cytokinetics shared in a news release.
Further, the FDA said results from an additional clinical trial of omecamtiv mecarbil are required to establish substantial evidence of effectiveness for the treatment of HFrEF, with benefits that outweigh the risks, Cytokinetics said.
The company said it will request a meeting with the FDA to gain a better understanding of what may be required to support potential approval of omecamtiv mecarbil. However, the company also said it has “no plans” to conduct an additional clinical trial of omecamtiv mecarbil.
Instead, the company said its focus remains on the development of aficamten, the next-in-class cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, a phase 3 clinical trial in patients with obstructive hypertrophic cardiomyopathy.
A version of this article first appeared on Medscape.com.