User login
A “Solution” for Patients Unable to Swallow a Pill: Crushed Terbinafine Mixed With Syrup
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Portable MRI has potential for MS
, suggesting that it could have potential for use in screening high-risk patients.
Although previous studies had shown that the approach could hold up to high-field MRI, the new study was a blind comparison in which raters did not have access to the high-field images.
In addition to portability, the device has potential advantages over high-field MRI, including low cost and no need for high-field physical shielding. It could be used for point-of-care testing, especially in remote or low-resource areas. It does not produce ionizing radiation, and has been used in intensive care units and pediatric facilities.
Advantages and limitations
The device isn’t ready for general use in MS. It performed well in periventricular lesions but less well in other areas. Ongoing research could improve its performance, including multiplanar imaging and image analysis.
“I think it still needs some work, but to me if it’s less expensive it will be particularly better for third-world countries and that sort of place, or possibly for use in the field in the United States or in North America. If something is detected, you can then bring the person in for a better scan, but I don’t know how sensitive it is – how much pathology you might miss. But in countries where there are no MRIs, it’s certainly better than nothing,” said Anne Cross, MD, who comoderated the session at the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study was presented.
She also noted that the device is potentially safer than high-field MRI. “I don’t think it would be something insurance companies or patients would want to pay $1,000 for when they could get a better scan somewhere, but it’ll get better,” said Dr. Cross, who is a professor of neurology and chair of neuroimmunology at Washington University in St. Louis.
How reliable are low-field images?
In previous work, in which evaluators compared the two scans side by side, the researchers showed in 36 patients that the device performed well, compared with a 64mT scanner. “When we look at tandem evaluations, we can identify dissemination in space in 80%. When a patient has at least one lesion that is larger than 4 millimeters in its largest diameter, we are able to detect it in the ultralow field MRI with 100% sensitivity. The open question here is, what is the diagnostic utility of these scanners when we don’t have any information about the high-field images?” said Serhat Okar, MD, during his presentation of the study. Dr. Okar is a neurologist and postdoctoral researcher at the National Institutes of Health.
To answer that question, the researchers asked two raters to examine scans from the low-field MRI, but only an independent party evaluator had access to both scans.
The study included 55 MS patients who were seen for either clinical or research purposes. The average age was 41 years, and 43 patients were female. Two neuroradiologists served as scan raters. Rater 1 had 17 years of experience, and rater 2 had 9 years of experience. They each conducted assessments for periventricular, juxtacortical, infratentorial, deep white matter, and deep gray matter lesions, as well as dissemination in space. They marked the scan and filled out an online form with number of observed lesions and whether they observed dissemination in space, with responses checked against a high-field image by an independent neuroradiologist for true positive and false positive findings.
There was significant discordance between raters for observation of dissemination in space, with rater 1 reporting 81% positivity and reader 2, 49%. False positive analyses revealed a difference in their approaches: Rater 1 was more conservative in marking lesions, which led to fewer true positive and fewer false positive findings. Both raters had good performance in the periventricular lesions with similar, low rates of false positives.
Other areas were a different story. Both raters found a greater number of true positive and false positive areas in the juxtacortical, deep white matter, and deep gray matter areas.
The study was funded by Hyperfine. Dr. Okar and Dr. Cross have no relevant financial disclosures.
, suggesting that it could have potential for use in screening high-risk patients.
Although previous studies had shown that the approach could hold up to high-field MRI, the new study was a blind comparison in which raters did not have access to the high-field images.
In addition to portability, the device has potential advantages over high-field MRI, including low cost and no need for high-field physical shielding. It could be used for point-of-care testing, especially in remote or low-resource areas. It does not produce ionizing radiation, and has been used in intensive care units and pediatric facilities.
Advantages and limitations
The device isn’t ready for general use in MS. It performed well in periventricular lesions but less well in other areas. Ongoing research could improve its performance, including multiplanar imaging and image analysis.
“I think it still needs some work, but to me if it’s less expensive it will be particularly better for third-world countries and that sort of place, or possibly for use in the field in the United States or in North America. If something is detected, you can then bring the person in for a better scan, but I don’t know how sensitive it is – how much pathology you might miss. But in countries where there are no MRIs, it’s certainly better than nothing,” said Anne Cross, MD, who comoderated the session at the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study was presented.
She also noted that the device is potentially safer than high-field MRI. “I don’t think it would be something insurance companies or patients would want to pay $1,000 for when they could get a better scan somewhere, but it’ll get better,” said Dr. Cross, who is a professor of neurology and chair of neuroimmunology at Washington University in St. Louis.
How reliable are low-field images?
In previous work, in which evaluators compared the two scans side by side, the researchers showed in 36 patients that the device performed well, compared with a 64mT scanner. “When we look at tandem evaluations, we can identify dissemination in space in 80%. When a patient has at least one lesion that is larger than 4 millimeters in its largest diameter, we are able to detect it in the ultralow field MRI with 100% sensitivity. The open question here is, what is the diagnostic utility of these scanners when we don’t have any information about the high-field images?” said Serhat Okar, MD, during his presentation of the study. Dr. Okar is a neurologist and postdoctoral researcher at the National Institutes of Health.
To answer that question, the researchers asked two raters to examine scans from the low-field MRI, but only an independent party evaluator had access to both scans.
The study included 55 MS patients who were seen for either clinical or research purposes. The average age was 41 years, and 43 patients were female. Two neuroradiologists served as scan raters. Rater 1 had 17 years of experience, and rater 2 had 9 years of experience. They each conducted assessments for periventricular, juxtacortical, infratentorial, deep white matter, and deep gray matter lesions, as well as dissemination in space. They marked the scan and filled out an online form with number of observed lesions and whether they observed dissemination in space, with responses checked against a high-field image by an independent neuroradiologist for true positive and false positive findings.
There was significant discordance between raters for observation of dissemination in space, with rater 1 reporting 81% positivity and reader 2, 49%. False positive analyses revealed a difference in their approaches: Rater 1 was more conservative in marking lesions, which led to fewer true positive and fewer false positive findings. Both raters had good performance in the periventricular lesions with similar, low rates of false positives.
Other areas were a different story. Both raters found a greater number of true positive and false positive areas in the juxtacortical, deep white matter, and deep gray matter areas.
The study was funded by Hyperfine. Dr. Okar and Dr. Cross have no relevant financial disclosures.
, suggesting that it could have potential for use in screening high-risk patients.
Although previous studies had shown that the approach could hold up to high-field MRI, the new study was a blind comparison in which raters did not have access to the high-field images.
In addition to portability, the device has potential advantages over high-field MRI, including low cost and no need for high-field physical shielding. It could be used for point-of-care testing, especially in remote or low-resource areas. It does not produce ionizing radiation, and has been used in intensive care units and pediatric facilities.
Advantages and limitations
The device isn’t ready for general use in MS. It performed well in periventricular lesions but less well in other areas. Ongoing research could improve its performance, including multiplanar imaging and image analysis.
“I think it still needs some work, but to me if it’s less expensive it will be particularly better for third-world countries and that sort of place, or possibly for use in the field in the United States or in North America. If something is detected, you can then bring the person in for a better scan, but I don’t know how sensitive it is – how much pathology you might miss. But in countries where there are no MRIs, it’s certainly better than nothing,” said Anne Cross, MD, who comoderated the session at the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study was presented.
She also noted that the device is potentially safer than high-field MRI. “I don’t think it would be something insurance companies or patients would want to pay $1,000 for when they could get a better scan somewhere, but it’ll get better,” said Dr. Cross, who is a professor of neurology and chair of neuroimmunology at Washington University in St. Louis.
How reliable are low-field images?
In previous work, in which evaluators compared the two scans side by side, the researchers showed in 36 patients that the device performed well, compared with a 64mT scanner. “When we look at tandem evaluations, we can identify dissemination in space in 80%. When a patient has at least one lesion that is larger than 4 millimeters in its largest diameter, we are able to detect it in the ultralow field MRI with 100% sensitivity. The open question here is, what is the diagnostic utility of these scanners when we don’t have any information about the high-field images?” said Serhat Okar, MD, during his presentation of the study. Dr. Okar is a neurologist and postdoctoral researcher at the National Institutes of Health.
To answer that question, the researchers asked two raters to examine scans from the low-field MRI, but only an independent party evaluator had access to both scans.
The study included 55 MS patients who were seen for either clinical or research purposes. The average age was 41 years, and 43 patients were female. Two neuroradiologists served as scan raters. Rater 1 had 17 years of experience, and rater 2 had 9 years of experience. They each conducted assessments for periventricular, juxtacortical, infratentorial, deep white matter, and deep gray matter lesions, as well as dissemination in space. They marked the scan and filled out an online form with number of observed lesions and whether they observed dissemination in space, with responses checked against a high-field image by an independent neuroradiologist for true positive and false positive findings.
There was significant discordance between raters for observation of dissemination in space, with rater 1 reporting 81% positivity and reader 2, 49%. False positive analyses revealed a difference in their approaches: Rater 1 was more conservative in marking lesions, which led to fewer true positive and fewer false positive findings. Both raters had good performance in the periventricular lesions with similar, low rates of false positives.
Other areas were a different story. Both raters found a greater number of true positive and false positive areas in the juxtacortical, deep white matter, and deep gray matter areas.
The study was funded by Hyperfine. Dr. Okar and Dr. Cross have no relevant financial disclosures.
AT ACTRIMS FORUM 2023
Mini-invasive MV repair as safe, effective as sternotomy surgery but has advantages: UK Mini-Mitral Trial
Patients with degenerative mitral valve (MV) regurgitation that calls for surgery may, for the most part, safely choose either a standard procedure requiring a midline sternotomy or one performed through a minithoracotomy, suggests a randomized comparison of the two techniques.
Still, the minimally invasive approach showed some advantages in the study. Patients’ quality of recovery was about the same with both procedures at 12 weeks, but those who had the minimally invasive thoracoscopy-guided surgery had shown greater improvement 6 weeks earlier.
Also in the UK Mini Mitral Trial, hospital length of stay (LOS) was significantly shorter for patients who underwent the mini-thoracotomy procedure, and that group spent fewer days in the hospital over the following months.
But neither procedure had an edge in terms of postoperative clinical risk in the study. Rates of clinical events, such as death or hospitalization for heart failure (HHF), were about the same over 1 year.
Patients in this trial had been deemed suitable for either of the two surgeries, which were always performed by surgeons specially chosen by the steering committee for their experience and expertise.
This first randomized head-to-head comparison of the two approaches in such patients should make both patients and clinicians more confident about choosing the minimally invasive surgery for degenerative MV disease, said Enoch Akowuah, MD, Newcastle (England) University, United Kingdom.
Dr. Akowuah presented the UK Mini-Mitral Trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
A “main takeaway” for clinical practice from the trial would be that minithoracotomy MV repair “is as safe and effective as conventional sternotomy for degenerative mitral regurgitation,” said discussant Amy E. Simone, PA-C, following Dr. Akowuah’s presentation.
“I think this study is unique in that its focus is on delivering high-quality, cost-efficient care for mitral regurgitation, but also with an emphasis on patients’ goals and wishes,” said Ms. Simone, who directs the Marcus Heart Valve Center of the Piedmont Heart Institute, Atlanta.
Cardiac surgeon Thomas MacGillivray, MD, another discussant, agreed that the data presented from at least this study suggest neither the minithoracotomy nor sternotomy approach is better than the other. But he questioned whether that would hold true if applied to broader clinical practice.
Dr. MacGillivray, of MedStar Washington Hospital Center, Washington, observed that only 330 patients were randomly assigned among a total of 1,167 candidates for candidates for MV repair surgery.
Indeed, he noted, more than 200 declined and about 600 were declared ineligible for the study, “even though it had seemed as if all were appropriate for mitral valve repair. That could be viewed as a significant limitation in terms of scalability in the real world.”
Some of those patients weren’t randomly assigned because they ultimately were not considered appropriate for both procedures, and some expressed a preference for one or the other approach, Dr. Akowuah replied. Those were the most common reasons. Many others did not enter the study, he said, because their mitral regurgitation was functional, not degenerative.
The two randomization groups fared similarly for the primary endpoint reflecting recovery from surgery, so the trial was actually “negative,” Dr. Akowuah said in an interview. However, “I see it as very much a win for minithoracotomy. The outstanding questions for clinicians and patients have been about the clinical efficacy and safety of the technique. And we’ve shown in this trial that minithoracotomy is safe and effective.”
If the minithoracotomy procedure is available, he continued, “and it’s just as clinically effective and safe – and we weren’t sure that was the case until we did this trial – and the repair is almost as durable, then why have a sternotomy?”
The researchers assigned 330 patients with degenerative MV disease who were deemed suitable for either type of surgery to undergo the standard operation via sternotomy or the minithoracotomy procedure at 10 centers in the United Kingdom. The steering committee had hand-selected its 28 experienced surgeons, each of whom performed only one of the two surgeries consistently for the trial’s patients.
The technically more demanding minithoracotomy procedure took longer to perform by a mean of 44 minutes, it prolonged cross-clamp time by 11 minutes, and it required 30 minutes more cardiopulmonary bypass support, Dr. Akowuah reported.
The two patient groups showed no significant differences in the primary endpoint of physical function and ability to return to usual activity levels at 12 weeks, as assessed by scores on the 36-Item Short Form Survey and wrist-worn accelerometer monitoring. At 6 weeks, however, the mini-thoracotomy patients had shown a significant early but temporary advantage for those recovery measures.
The minithoracotomy group clearly fared better, however, on some secondary endpoints. For example, their median hospital LOS was 5 days, compared with 6 days for the sternotomy group (P = .003), and 33.1% of the mini-thoracotomy patients were discharged within 4 days of the surgery, compared with only 15.3% of patients who had the standard procedure (P < .001).
The minithoracotomy group also had marginally more days alive out of the hospital at both 30 days (23.6 days vs. 22.4 days in the sternotomy group) and 90 days (82.7 days and 80.5 days, respectively) after the surgery (P = .03 for both differences).
Safety outcomes at 12 weeks were similar, with no significant differences in rate of death, strokes, MI, or renal impairment, or in ICU length of stay or need for more than 48 hours of mechanical ventilation, Dr. Akowuah reported.
Safety outcomes at 1 year were also similar. Mortality by then was 2.4% for the minithoracotomy patients and 2.5% for the sternotomy group, nor were there significant differences in HHF rates or need for repeat MV surgical repair.
Dr. Akowuah said the patients will be followed for up to 5 years for the primary outcomes, echocardiographic changes, and clinical events.
The minithoracotomy surgery’s longer operative times and specialized equipment make it more a expensive procedure than the standard surgery, he said. “So we need to work out in a cost-effectiveness analysis whether that is offset by the benefits,” such as shorter hospital stays or perhaps fewer transfusions or readmissions.
The study was funded by the United Kingdom’s National Institute for Health and Care Research. Dr. Akowuah reported no relevant financial relationships with industry.
A version of this article first appeared on Medscape.com.
Patients with degenerative mitral valve (MV) regurgitation that calls for surgery may, for the most part, safely choose either a standard procedure requiring a midline sternotomy or one performed through a minithoracotomy, suggests a randomized comparison of the two techniques.
Still, the minimally invasive approach showed some advantages in the study. Patients’ quality of recovery was about the same with both procedures at 12 weeks, but those who had the minimally invasive thoracoscopy-guided surgery had shown greater improvement 6 weeks earlier.
Also in the UK Mini Mitral Trial, hospital length of stay (LOS) was significantly shorter for patients who underwent the mini-thoracotomy procedure, and that group spent fewer days in the hospital over the following months.
But neither procedure had an edge in terms of postoperative clinical risk in the study. Rates of clinical events, such as death or hospitalization for heart failure (HHF), were about the same over 1 year.
Patients in this trial had been deemed suitable for either of the two surgeries, which were always performed by surgeons specially chosen by the steering committee for their experience and expertise.
This first randomized head-to-head comparison of the two approaches in such patients should make both patients and clinicians more confident about choosing the minimally invasive surgery for degenerative MV disease, said Enoch Akowuah, MD, Newcastle (England) University, United Kingdom.
Dr. Akowuah presented the UK Mini-Mitral Trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
A “main takeaway” for clinical practice from the trial would be that minithoracotomy MV repair “is as safe and effective as conventional sternotomy for degenerative mitral regurgitation,” said discussant Amy E. Simone, PA-C, following Dr. Akowuah’s presentation.
“I think this study is unique in that its focus is on delivering high-quality, cost-efficient care for mitral regurgitation, but also with an emphasis on patients’ goals and wishes,” said Ms. Simone, who directs the Marcus Heart Valve Center of the Piedmont Heart Institute, Atlanta.
Cardiac surgeon Thomas MacGillivray, MD, another discussant, agreed that the data presented from at least this study suggest neither the minithoracotomy nor sternotomy approach is better than the other. But he questioned whether that would hold true if applied to broader clinical practice.
Dr. MacGillivray, of MedStar Washington Hospital Center, Washington, observed that only 330 patients were randomly assigned among a total of 1,167 candidates for candidates for MV repair surgery.
Indeed, he noted, more than 200 declined and about 600 were declared ineligible for the study, “even though it had seemed as if all were appropriate for mitral valve repair. That could be viewed as a significant limitation in terms of scalability in the real world.”
Some of those patients weren’t randomly assigned because they ultimately were not considered appropriate for both procedures, and some expressed a preference for one or the other approach, Dr. Akowuah replied. Those were the most common reasons. Many others did not enter the study, he said, because their mitral regurgitation was functional, not degenerative.
The two randomization groups fared similarly for the primary endpoint reflecting recovery from surgery, so the trial was actually “negative,” Dr. Akowuah said in an interview. However, “I see it as very much a win for minithoracotomy. The outstanding questions for clinicians and patients have been about the clinical efficacy and safety of the technique. And we’ve shown in this trial that minithoracotomy is safe and effective.”
If the minithoracotomy procedure is available, he continued, “and it’s just as clinically effective and safe – and we weren’t sure that was the case until we did this trial – and the repair is almost as durable, then why have a sternotomy?”
The researchers assigned 330 patients with degenerative MV disease who were deemed suitable for either type of surgery to undergo the standard operation via sternotomy or the minithoracotomy procedure at 10 centers in the United Kingdom. The steering committee had hand-selected its 28 experienced surgeons, each of whom performed only one of the two surgeries consistently for the trial’s patients.
The technically more demanding minithoracotomy procedure took longer to perform by a mean of 44 minutes, it prolonged cross-clamp time by 11 minutes, and it required 30 minutes more cardiopulmonary bypass support, Dr. Akowuah reported.
The two patient groups showed no significant differences in the primary endpoint of physical function and ability to return to usual activity levels at 12 weeks, as assessed by scores on the 36-Item Short Form Survey and wrist-worn accelerometer monitoring. At 6 weeks, however, the mini-thoracotomy patients had shown a significant early but temporary advantage for those recovery measures.
The minithoracotomy group clearly fared better, however, on some secondary endpoints. For example, their median hospital LOS was 5 days, compared with 6 days for the sternotomy group (P = .003), and 33.1% of the mini-thoracotomy patients were discharged within 4 days of the surgery, compared with only 15.3% of patients who had the standard procedure (P < .001).
The minithoracotomy group also had marginally more days alive out of the hospital at both 30 days (23.6 days vs. 22.4 days in the sternotomy group) and 90 days (82.7 days and 80.5 days, respectively) after the surgery (P = .03 for both differences).
Safety outcomes at 12 weeks were similar, with no significant differences in rate of death, strokes, MI, or renal impairment, or in ICU length of stay or need for more than 48 hours of mechanical ventilation, Dr. Akowuah reported.
Safety outcomes at 1 year were also similar. Mortality by then was 2.4% for the minithoracotomy patients and 2.5% for the sternotomy group, nor were there significant differences in HHF rates or need for repeat MV surgical repair.
Dr. Akowuah said the patients will be followed for up to 5 years for the primary outcomes, echocardiographic changes, and clinical events.
The minithoracotomy surgery’s longer operative times and specialized equipment make it more a expensive procedure than the standard surgery, he said. “So we need to work out in a cost-effectiveness analysis whether that is offset by the benefits,” such as shorter hospital stays or perhaps fewer transfusions or readmissions.
The study was funded by the United Kingdom’s National Institute for Health and Care Research. Dr. Akowuah reported no relevant financial relationships with industry.
A version of this article first appeared on Medscape.com.
Patients with degenerative mitral valve (MV) regurgitation that calls for surgery may, for the most part, safely choose either a standard procedure requiring a midline sternotomy or one performed through a minithoracotomy, suggests a randomized comparison of the two techniques.
Still, the minimally invasive approach showed some advantages in the study. Patients’ quality of recovery was about the same with both procedures at 12 weeks, but those who had the minimally invasive thoracoscopy-guided surgery had shown greater improvement 6 weeks earlier.
Also in the UK Mini Mitral Trial, hospital length of stay (LOS) was significantly shorter for patients who underwent the mini-thoracotomy procedure, and that group spent fewer days in the hospital over the following months.
But neither procedure had an edge in terms of postoperative clinical risk in the study. Rates of clinical events, such as death or hospitalization for heart failure (HHF), were about the same over 1 year.
Patients in this trial had been deemed suitable for either of the two surgeries, which were always performed by surgeons specially chosen by the steering committee for their experience and expertise.
This first randomized head-to-head comparison of the two approaches in such patients should make both patients and clinicians more confident about choosing the minimally invasive surgery for degenerative MV disease, said Enoch Akowuah, MD, Newcastle (England) University, United Kingdom.
Dr. Akowuah presented the UK Mini-Mitral Trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
A “main takeaway” for clinical practice from the trial would be that minithoracotomy MV repair “is as safe and effective as conventional sternotomy for degenerative mitral regurgitation,” said discussant Amy E. Simone, PA-C, following Dr. Akowuah’s presentation.
“I think this study is unique in that its focus is on delivering high-quality, cost-efficient care for mitral regurgitation, but also with an emphasis on patients’ goals and wishes,” said Ms. Simone, who directs the Marcus Heart Valve Center of the Piedmont Heart Institute, Atlanta.
Cardiac surgeon Thomas MacGillivray, MD, another discussant, agreed that the data presented from at least this study suggest neither the minithoracotomy nor sternotomy approach is better than the other. But he questioned whether that would hold true if applied to broader clinical practice.
Dr. MacGillivray, of MedStar Washington Hospital Center, Washington, observed that only 330 patients were randomly assigned among a total of 1,167 candidates for candidates for MV repair surgery.
Indeed, he noted, more than 200 declined and about 600 were declared ineligible for the study, “even though it had seemed as if all were appropriate for mitral valve repair. That could be viewed as a significant limitation in terms of scalability in the real world.”
Some of those patients weren’t randomly assigned because they ultimately were not considered appropriate for both procedures, and some expressed a preference for one or the other approach, Dr. Akowuah replied. Those were the most common reasons. Many others did not enter the study, he said, because their mitral regurgitation was functional, not degenerative.
The two randomization groups fared similarly for the primary endpoint reflecting recovery from surgery, so the trial was actually “negative,” Dr. Akowuah said in an interview. However, “I see it as very much a win for minithoracotomy. The outstanding questions for clinicians and patients have been about the clinical efficacy and safety of the technique. And we’ve shown in this trial that minithoracotomy is safe and effective.”
If the minithoracotomy procedure is available, he continued, “and it’s just as clinically effective and safe – and we weren’t sure that was the case until we did this trial – and the repair is almost as durable, then why have a sternotomy?”
The researchers assigned 330 patients with degenerative MV disease who were deemed suitable for either type of surgery to undergo the standard operation via sternotomy or the minithoracotomy procedure at 10 centers in the United Kingdom. The steering committee had hand-selected its 28 experienced surgeons, each of whom performed only one of the two surgeries consistently for the trial’s patients.
The technically more demanding minithoracotomy procedure took longer to perform by a mean of 44 minutes, it prolonged cross-clamp time by 11 minutes, and it required 30 minutes more cardiopulmonary bypass support, Dr. Akowuah reported.
The two patient groups showed no significant differences in the primary endpoint of physical function and ability to return to usual activity levels at 12 weeks, as assessed by scores on the 36-Item Short Form Survey and wrist-worn accelerometer monitoring. At 6 weeks, however, the mini-thoracotomy patients had shown a significant early but temporary advantage for those recovery measures.
The minithoracotomy group clearly fared better, however, on some secondary endpoints. For example, their median hospital LOS was 5 days, compared with 6 days for the sternotomy group (P = .003), and 33.1% of the mini-thoracotomy patients were discharged within 4 days of the surgery, compared with only 15.3% of patients who had the standard procedure (P < .001).
The minithoracotomy group also had marginally more days alive out of the hospital at both 30 days (23.6 days vs. 22.4 days in the sternotomy group) and 90 days (82.7 days and 80.5 days, respectively) after the surgery (P = .03 for both differences).
Safety outcomes at 12 weeks were similar, with no significant differences in rate of death, strokes, MI, or renal impairment, or in ICU length of stay or need for more than 48 hours of mechanical ventilation, Dr. Akowuah reported.
Safety outcomes at 1 year were also similar. Mortality by then was 2.4% for the minithoracotomy patients and 2.5% for the sternotomy group, nor were there significant differences in HHF rates or need for repeat MV surgical repair.
Dr. Akowuah said the patients will be followed for up to 5 years for the primary outcomes, echocardiographic changes, and clinical events.
The minithoracotomy surgery’s longer operative times and specialized equipment make it more a expensive procedure than the standard surgery, he said. “So we need to work out in a cost-effectiveness analysis whether that is offset by the benefits,” such as shorter hospital stays or perhaps fewer transfusions or readmissions.
The study was funded by the United Kingdom’s National Institute for Health and Care Research. Dr. Akowuah reported no relevant financial relationships with industry.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Spreading Painful Lesions on the Legs
The Diagnosis: Cutaneous Leishmaniasis
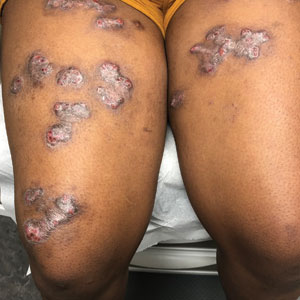
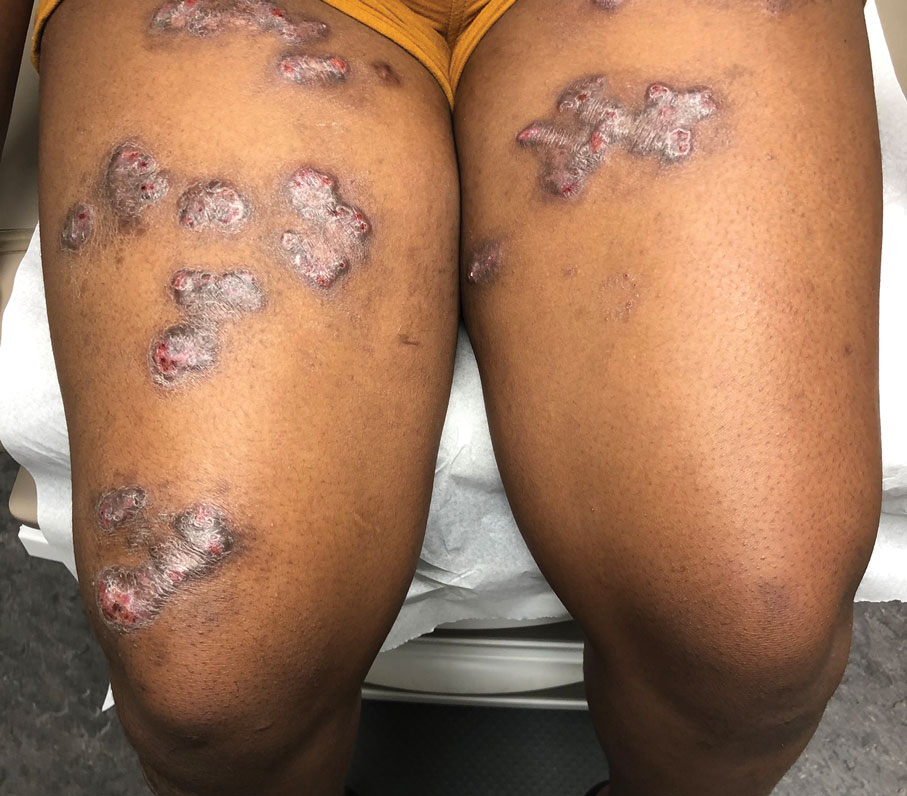
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
A 14-year-old adolescent girl presented with spreading painful lesions on the legs and left forearm of 2 years’ duration. Her travel history included several countries in South and Central America, traversing the Colombian jungle on foot. Near the end of the jungle trip, she noted a skin lesion on the left forearm around the site of an insect bite. Within 1 month, the lesions spread to the legs. She was treated with topical corticosteroids without improvement. Physical examination revealed verrucous, reddish-brown plaques on the legs and left forearm. Intranasal examination revealed a red rounded lesion inside the left nostril.
How to help pediatricians apply peanut allergy guidelines
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Distinct suicidal thought patterns flag those at highest risk
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.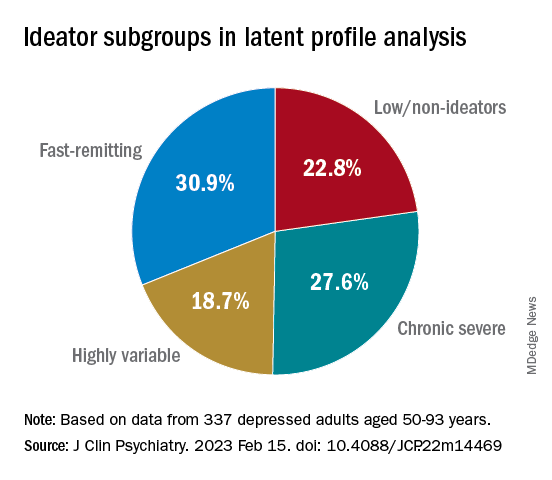
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.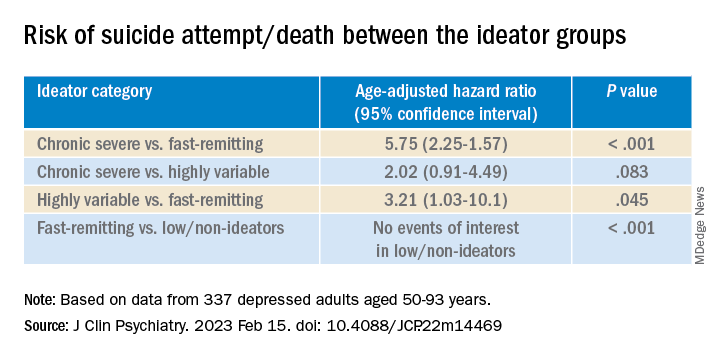
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Causal AI quantifies CV risk, providing patient-specific goals
NEW ORLEANS – Causal artificial intelligence (AI) can translate polygenic scores (PGS) and other genetic information into risk reduction strategies for coronary artery disease (CAD) that is tailored for each individual patient, according to an analysis presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Tested for LDL cholesterol (LDL-C) and systolic blood pressure (SBP), causal AI explained how much each of these risk factors must improve at the level of each individual patient “to overcome overall inherited risk,” reported Brian Ference, MD, MPhil, director of translational therapeutics, University of Cambridge (England).
Unlike the “black box” risk assessments common to machine learning, which relies on disparate forms of information of often unknown relative significance, causal AI explains cause and effect. In the case of CAD, its ability to encode the biological causes means that it can “both predict outcomes and prescribe specific actions to change those outcomes,” Dr. Ference explained.
The concept is testable against observed biology using randomized evidence, which was the objective of the study Dr. Ference presented in the late-breaker session.
Causal AI trained on nearly 2 million patients
This study employed a causal AI platform trained on roughly 1.3 million participants in Mendelian randomization studies, as well as more than 500,000 participants in randomized clinical trials. The PGS estimate of inherited risk was constructed from almost 4.1 million variants from genomewide association studies.
To test the ability of causal AI to reveal how much LDL-C or SBP had to be reduced to overcome the inherited risk of CAD based on PGS, it was applied to 445,765 participants of European ancestry in the UK Biobank. The goal was to determine how much those with greater than average risk would need to lower their LDL-C or SBP to achieve average CAD risk.
When validated against observed rates of events, causal AI accurately characterized risk before estimating what reductions in LDL-C, SBP, or both would attenuate that risk.
Providing examples, Dr. Ference explained that a PGS in the 80th percentile can be overcome by lowering LDL-C by 14 mg/dL. Alternatively, the 80th percentile risk could also be overcome by simultaneously lowering LDL-C and SBP by 7 mg/dL and 2.5 mm Hg, respectively.
Required risk factor reductions increase with age because of the increased risk of the events. For example, while a 14.8 mg/dL reduction in LDL-C would be adequate to overcome risk defined by a PGS in the 80th percentile at age 35, reductions of 18.2 mg/dL, 28.9 mg/dL, and 42.6 mg/dL would be required, respectively, at ages 45, 55, and 65 years. The values climb similarly for SBP.
Family history of CAD adds an independent variable that further contributes to the ability of causal AI to estimate risk and the degree of risk factor attenuation to overcome the risk.
Even though family history is equivalent to having PGS above the 95th percentile, it is an independent and additive variable, according to Dr. Ference. As a result, inherited risk of CAD depends on both.
Still when family history is factored into the analysis, “causal AI accurately estimated the magnitude of lower LDL-C, SBP, or both needed to overcome overall inherited risk at all levels of higher or lower PGS,” he reported.
According to Dr. Ference, the value of causal AI is that it can generate very specific goals for each patient regarding modifiable risk factors. Causal effects of risk factors encoded in time units of exposure allow the patient and the clinician to understand the biology and the basis of the disease burden.
Treatments become understandable to patients
“Encoding biology creates algorithms that are deeply explainable because they reveal why a person is at risk, how to reduce that risk, and how much each person will benefit from specific actions to reduce risk,” Dr. Ference said.
A real-world, randomized trial to confirm that the information from causal AI can reduce the risk of CAD is expected to start in 2023, but Dr. Ference thinks that causal AI for managing CAD risk, independent of this planned trial, is essentially inevitable. PGS, which he thinks will be performed routinely in all individuals within 10 years, is only likely to improve. He foresees large advantages of this form of personalized medicine.
Ami Bhatt, MD, chief innovation officer for the American College of Cardiology, Washington, agreed, seeing a direct relationship between precision health as the pathway to improvements in population health.
By explaining risk factors in terms of mechanisms and specific goals to ameliorate these risks, it “engages our patients with agency,” said Dr. Bhatt. She suggested that the information provided by causal AI has the potential to empower patients while creating a collaborative approach with clinicians to CAD prevention.
With patient-specific information provided in the context of the disease biology, “you increase the sense of transparency,” Dr. Bhatt said.
She suggested this direction of research is wholly consistent with initiatives such as those from the World Health Organization to improve precision medicine as a step toward equipping patients to manage their own health.
Dr. Ference reported financial relationships with Amgen, AstraZeneca, CiVi Pharma, Daiichi Sankyo, DalCOR, Esperion, Eli Lilly, Ionis Pharmaceuticals, KrKA, Medicines Company, Merck, Mylan, Novo Nordisk, Novartis, and Sanofi, and Viatris. Dr. Bhatt reported no potential conflicts of interest.
NEW ORLEANS – Causal artificial intelligence (AI) can translate polygenic scores (PGS) and other genetic information into risk reduction strategies for coronary artery disease (CAD) that is tailored for each individual patient, according to an analysis presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Tested for LDL cholesterol (LDL-C) and systolic blood pressure (SBP), causal AI explained how much each of these risk factors must improve at the level of each individual patient “to overcome overall inherited risk,” reported Brian Ference, MD, MPhil, director of translational therapeutics, University of Cambridge (England).
Unlike the “black box” risk assessments common to machine learning, which relies on disparate forms of information of often unknown relative significance, causal AI explains cause and effect. In the case of CAD, its ability to encode the biological causes means that it can “both predict outcomes and prescribe specific actions to change those outcomes,” Dr. Ference explained.
The concept is testable against observed biology using randomized evidence, which was the objective of the study Dr. Ference presented in the late-breaker session.
Causal AI trained on nearly 2 million patients
This study employed a causal AI platform trained on roughly 1.3 million participants in Mendelian randomization studies, as well as more than 500,000 participants in randomized clinical trials. The PGS estimate of inherited risk was constructed from almost 4.1 million variants from genomewide association studies.
To test the ability of causal AI to reveal how much LDL-C or SBP had to be reduced to overcome the inherited risk of CAD based on PGS, it was applied to 445,765 participants of European ancestry in the UK Biobank. The goal was to determine how much those with greater than average risk would need to lower their LDL-C or SBP to achieve average CAD risk.
When validated against observed rates of events, causal AI accurately characterized risk before estimating what reductions in LDL-C, SBP, or both would attenuate that risk.
Providing examples, Dr. Ference explained that a PGS in the 80th percentile can be overcome by lowering LDL-C by 14 mg/dL. Alternatively, the 80th percentile risk could also be overcome by simultaneously lowering LDL-C and SBP by 7 mg/dL and 2.5 mm Hg, respectively.
Required risk factor reductions increase with age because of the increased risk of the events. For example, while a 14.8 mg/dL reduction in LDL-C would be adequate to overcome risk defined by a PGS in the 80th percentile at age 35, reductions of 18.2 mg/dL, 28.9 mg/dL, and 42.6 mg/dL would be required, respectively, at ages 45, 55, and 65 years. The values climb similarly for SBP.
Family history of CAD adds an independent variable that further contributes to the ability of causal AI to estimate risk and the degree of risk factor attenuation to overcome the risk.
Even though family history is equivalent to having PGS above the 95th percentile, it is an independent and additive variable, according to Dr. Ference. As a result, inherited risk of CAD depends on both.
Still when family history is factored into the analysis, “causal AI accurately estimated the magnitude of lower LDL-C, SBP, or both needed to overcome overall inherited risk at all levels of higher or lower PGS,” he reported.
According to Dr. Ference, the value of causal AI is that it can generate very specific goals for each patient regarding modifiable risk factors. Causal effects of risk factors encoded in time units of exposure allow the patient and the clinician to understand the biology and the basis of the disease burden.
Treatments become understandable to patients
“Encoding biology creates algorithms that are deeply explainable because they reveal why a person is at risk, how to reduce that risk, and how much each person will benefit from specific actions to reduce risk,” Dr. Ference said.
A real-world, randomized trial to confirm that the information from causal AI can reduce the risk of CAD is expected to start in 2023, but Dr. Ference thinks that causal AI for managing CAD risk, independent of this planned trial, is essentially inevitable. PGS, which he thinks will be performed routinely in all individuals within 10 years, is only likely to improve. He foresees large advantages of this form of personalized medicine.
Ami Bhatt, MD, chief innovation officer for the American College of Cardiology, Washington, agreed, seeing a direct relationship between precision health as the pathway to improvements in population health.
By explaining risk factors in terms of mechanisms and specific goals to ameliorate these risks, it “engages our patients with agency,” said Dr. Bhatt. She suggested that the information provided by causal AI has the potential to empower patients while creating a collaborative approach with clinicians to CAD prevention.
With patient-specific information provided in the context of the disease biology, “you increase the sense of transparency,” Dr. Bhatt said.
She suggested this direction of research is wholly consistent with initiatives such as those from the World Health Organization to improve precision medicine as a step toward equipping patients to manage their own health.
Dr. Ference reported financial relationships with Amgen, AstraZeneca, CiVi Pharma, Daiichi Sankyo, DalCOR, Esperion, Eli Lilly, Ionis Pharmaceuticals, KrKA, Medicines Company, Merck, Mylan, Novo Nordisk, Novartis, and Sanofi, and Viatris. Dr. Bhatt reported no potential conflicts of interest.
NEW ORLEANS – Causal artificial intelligence (AI) can translate polygenic scores (PGS) and other genetic information into risk reduction strategies for coronary artery disease (CAD) that is tailored for each individual patient, according to an analysis presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Tested for LDL cholesterol (LDL-C) and systolic blood pressure (SBP), causal AI explained how much each of these risk factors must improve at the level of each individual patient “to overcome overall inherited risk,” reported Brian Ference, MD, MPhil, director of translational therapeutics, University of Cambridge (England).
Unlike the “black box” risk assessments common to machine learning, which relies on disparate forms of information of often unknown relative significance, causal AI explains cause and effect. In the case of CAD, its ability to encode the biological causes means that it can “both predict outcomes and prescribe specific actions to change those outcomes,” Dr. Ference explained.
The concept is testable against observed biology using randomized evidence, which was the objective of the study Dr. Ference presented in the late-breaker session.
Causal AI trained on nearly 2 million patients
This study employed a causal AI platform trained on roughly 1.3 million participants in Mendelian randomization studies, as well as more than 500,000 participants in randomized clinical trials. The PGS estimate of inherited risk was constructed from almost 4.1 million variants from genomewide association studies.
To test the ability of causal AI to reveal how much LDL-C or SBP had to be reduced to overcome the inherited risk of CAD based on PGS, it was applied to 445,765 participants of European ancestry in the UK Biobank. The goal was to determine how much those with greater than average risk would need to lower their LDL-C or SBP to achieve average CAD risk.
When validated against observed rates of events, causal AI accurately characterized risk before estimating what reductions in LDL-C, SBP, or both would attenuate that risk.
Providing examples, Dr. Ference explained that a PGS in the 80th percentile can be overcome by lowering LDL-C by 14 mg/dL. Alternatively, the 80th percentile risk could also be overcome by simultaneously lowering LDL-C and SBP by 7 mg/dL and 2.5 mm Hg, respectively.
Required risk factor reductions increase with age because of the increased risk of the events. For example, while a 14.8 mg/dL reduction in LDL-C would be adequate to overcome risk defined by a PGS in the 80th percentile at age 35, reductions of 18.2 mg/dL, 28.9 mg/dL, and 42.6 mg/dL would be required, respectively, at ages 45, 55, and 65 years. The values climb similarly for SBP.
Family history of CAD adds an independent variable that further contributes to the ability of causal AI to estimate risk and the degree of risk factor attenuation to overcome the risk.
Even though family history is equivalent to having PGS above the 95th percentile, it is an independent and additive variable, according to Dr. Ference. As a result, inherited risk of CAD depends on both.
Still when family history is factored into the analysis, “causal AI accurately estimated the magnitude of lower LDL-C, SBP, or both needed to overcome overall inherited risk at all levels of higher or lower PGS,” he reported.
According to Dr. Ference, the value of causal AI is that it can generate very specific goals for each patient regarding modifiable risk factors. Causal effects of risk factors encoded in time units of exposure allow the patient and the clinician to understand the biology and the basis of the disease burden.
Treatments become understandable to patients
“Encoding biology creates algorithms that are deeply explainable because they reveal why a person is at risk, how to reduce that risk, and how much each person will benefit from specific actions to reduce risk,” Dr. Ference said.
A real-world, randomized trial to confirm that the information from causal AI can reduce the risk of CAD is expected to start in 2023, but Dr. Ference thinks that causal AI for managing CAD risk, independent of this planned trial, is essentially inevitable. PGS, which he thinks will be performed routinely in all individuals within 10 years, is only likely to improve. He foresees large advantages of this form of personalized medicine.
Ami Bhatt, MD, chief innovation officer for the American College of Cardiology, Washington, agreed, seeing a direct relationship between precision health as the pathway to improvements in population health.
By explaining risk factors in terms of mechanisms and specific goals to ameliorate these risks, it “engages our patients with agency,” said Dr. Bhatt. She suggested that the information provided by causal AI has the potential to empower patients while creating a collaborative approach with clinicians to CAD prevention.
With patient-specific information provided in the context of the disease biology, “you increase the sense of transparency,” Dr. Bhatt said.
She suggested this direction of research is wholly consistent with initiatives such as those from the World Health Organization to improve precision medicine as a step toward equipping patients to manage their own health.
Dr. Ference reported financial relationships with Amgen, AstraZeneca, CiVi Pharma, Daiichi Sankyo, DalCOR, Esperion, Eli Lilly, Ionis Pharmaceuticals, KrKA, Medicines Company, Merck, Mylan, Novo Nordisk, Novartis, and Sanofi, and Viatris. Dr. Bhatt reported no potential conflicts of interest.
AT ACC 2023
Biomarkers linked to elevated T2D MACE risk in DECLARE-TIMI 58
The researchers found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hsTnT) levels helped identify a subset of T2D patients at higher risk of major adverse cardiovascular events who would benefit most from dapagliflozin.
“We’ve shown previously that these two biomarkers are very robust risk indicators for cardiovascular death and heart failure events,” senior study author David A. Morrow, MD, of Harvard University, Boston, said in an interview. “In this study, we now show that the two biomarkers also yield important prognostic information for MACE [major adverse cardiovascular events].”
Although NT-proBNP is typically measured to diagnose heart failure, and hsTnT to diagnose acute MI, Dr. Morrow pointed out that this analysis demonstrated the potential for using the two tests to evaluate risks in T2D patients.
Study results
The secondary analysis included 14,565 patients in the DECLARE-TIMI 58 trial. The patients had T2D and multiple risk factors for atherosclerotic cardiovascular disease (about 60%) or established ASCVD (about 40%). All patients had available blood samples and the data were collected from May 2013 to September 2018. The primary outcome was MACE, a composite of MI, ischemic stroke, and cardiovascular death. The results were reported online in JAMA Cardiology.
The analysis found that higher baseline concentrations of NT-proBNP increased MACE risks by 62% (95% confidence interval, 1.49-1.76) and hsTnT elevated those risks by 59% (95% CI, 1.46-1.74).
Among placebo patients, when divided into risk quartiles, those in the highest quartile had significantly higher risk with both elevated NT-proBNP and hsTnT, compared with those with low concentrations. For example, patients with established ASCVD had a 22.9% risk vs. 9.5% with elevated NT-proBNP (P < .001) and a 24.2% vs. 7.2% risk with elevated hsTnT (P < .001). The gap was similar for patients with multiple risk factors.
Dr. Morrow noted that the main DECLARE-TIMI 58 trial showed that dapagliflozin reduced the rates of cardiovascular death or hospitalization for heart failure in patients with T2D, when compared to placebo, but didn’t reach statistical significance for MACE (N Engl J Med. 2019;380:347-57).
“We have previously shown that among patients with T2D who have high risk indicators, such as prior MI or long-standing diabetes, dapagliflozin also appeared to reduce MACE,” Dr. Morrow said. “In this study, we find that these two widely available biomarkers also identify a high-risk group who may have even more potential benefits from treatment with an SGLT2i.”
Dr. Morrow noted that the study design – a nested prospective biomarker study within a randomized, double-blind, placebo-controlled clinical trial – “is a particular strength.”
Results clarify which patients will benefit
This secondary analysis of DECLARE-TIMI 58 brings more clarity to the types of T2D patients who will get the most cardiovascular benefits from dapagliflozin, said Matthew J. Budoff, MD, professor of medicine at University of California, Los Angeles, and Endowed Chair of Preventive Cardiology at the Lundquist Institute in Torrance, Calif.
“The big picture is, we’ve known for some time from epidemiologic studies that these biomarkers, when they’re elevated, mean that the patient is at higher risk of having a cardiovascular event,” he said, “but I think what it helps us with is in knowing in whom to use dapagliflozin for prevention of ASCVD. The effect in the DECLARE-TIMI 58 trial was quite modest, but if you can subgroup it, in these high-risk people there’s a more profound effect. It helps in risk stratification because the absolute benefit is larger.”
The specific biomarkers, NT-proBNP and hsTnT, “haven’t been explored very much in clinical trials,” Dr. Budoff said, “so I do think that it’s nice that in a randomized trial it plays out the way we might expect.”
He added that “for many clinicians this is novel, because I don’t think they were aware of the biomarker data, so I think that this does add some clinical benefit in that context.” The findings also strengthen the case to get T2D patients with higher ASCVD risk onto SGLT2 inhibitors if they’re not already, he said.
Dr. Morrow disclosed relationships with AstraZeneca, Roche Diagnostics, Abbott Laboratories, Anthos Therapeutics, ARCA Biopharma, Merck, Novartis, Pfizer, Regeneron, Siemens, and InCarda outside the reported work.
Dr. Budoff has no relevant disclosures.
The researchers found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hsTnT) levels helped identify a subset of T2D patients at higher risk of major adverse cardiovascular events who would benefit most from dapagliflozin.
“We’ve shown previously that these two biomarkers are very robust risk indicators for cardiovascular death and heart failure events,” senior study author David A. Morrow, MD, of Harvard University, Boston, said in an interview. “In this study, we now show that the two biomarkers also yield important prognostic information for MACE [major adverse cardiovascular events].”
Although NT-proBNP is typically measured to diagnose heart failure, and hsTnT to diagnose acute MI, Dr. Morrow pointed out that this analysis demonstrated the potential for using the two tests to evaluate risks in T2D patients.
Study results
The secondary analysis included 14,565 patients in the DECLARE-TIMI 58 trial. The patients had T2D and multiple risk factors for atherosclerotic cardiovascular disease (about 60%) or established ASCVD (about 40%). All patients had available blood samples and the data were collected from May 2013 to September 2018. The primary outcome was MACE, a composite of MI, ischemic stroke, and cardiovascular death. The results were reported online in JAMA Cardiology.
The analysis found that higher baseline concentrations of NT-proBNP increased MACE risks by 62% (95% confidence interval, 1.49-1.76) and hsTnT elevated those risks by 59% (95% CI, 1.46-1.74).
Among placebo patients, when divided into risk quartiles, those in the highest quartile had significantly higher risk with both elevated NT-proBNP and hsTnT, compared with those with low concentrations. For example, patients with established ASCVD had a 22.9% risk vs. 9.5% with elevated NT-proBNP (P < .001) and a 24.2% vs. 7.2% risk with elevated hsTnT (P < .001). The gap was similar for patients with multiple risk factors.
Dr. Morrow noted that the main DECLARE-TIMI 58 trial showed that dapagliflozin reduced the rates of cardiovascular death or hospitalization for heart failure in patients with T2D, when compared to placebo, but didn’t reach statistical significance for MACE (N Engl J Med. 2019;380:347-57).
“We have previously shown that among patients with T2D who have high risk indicators, such as prior MI or long-standing diabetes, dapagliflozin also appeared to reduce MACE,” Dr. Morrow said. “In this study, we find that these two widely available biomarkers also identify a high-risk group who may have even more potential benefits from treatment with an SGLT2i.”
Dr. Morrow noted that the study design – a nested prospective biomarker study within a randomized, double-blind, placebo-controlled clinical trial – “is a particular strength.”
Results clarify which patients will benefit
This secondary analysis of DECLARE-TIMI 58 brings more clarity to the types of T2D patients who will get the most cardiovascular benefits from dapagliflozin, said Matthew J. Budoff, MD, professor of medicine at University of California, Los Angeles, and Endowed Chair of Preventive Cardiology at the Lundquist Institute in Torrance, Calif.
“The big picture is, we’ve known for some time from epidemiologic studies that these biomarkers, when they’re elevated, mean that the patient is at higher risk of having a cardiovascular event,” he said, “but I think what it helps us with is in knowing in whom to use dapagliflozin for prevention of ASCVD. The effect in the DECLARE-TIMI 58 trial was quite modest, but if you can subgroup it, in these high-risk people there’s a more profound effect. It helps in risk stratification because the absolute benefit is larger.”
The specific biomarkers, NT-proBNP and hsTnT, “haven’t been explored very much in clinical trials,” Dr. Budoff said, “so I do think that it’s nice that in a randomized trial it plays out the way we might expect.”
He added that “for many clinicians this is novel, because I don’t think they were aware of the biomarker data, so I think that this does add some clinical benefit in that context.” The findings also strengthen the case to get T2D patients with higher ASCVD risk onto SGLT2 inhibitors if they’re not already, he said.
Dr. Morrow disclosed relationships with AstraZeneca, Roche Diagnostics, Abbott Laboratories, Anthos Therapeutics, ARCA Biopharma, Merck, Novartis, Pfizer, Regeneron, Siemens, and InCarda outside the reported work.
Dr. Budoff has no relevant disclosures.
The researchers found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hsTnT) levels helped identify a subset of T2D patients at higher risk of major adverse cardiovascular events who would benefit most from dapagliflozin.
“We’ve shown previously that these two biomarkers are very robust risk indicators for cardiovascular death and heart failure events,” senior study author David A. Morrow, MD, of Harvard University, Boston, said in an interview. “In this study, we now show that the two biomarkers also yield important prognostic information for MACE [major adverse cardiovascular events].”
Although NT-proBNP is typically measured to diagnose heart failure, and hsTnT to diagnose acute MI, Dr. Morrow pointed out that this analysis demonstrated the potential for using the two tests to evaluate risks in T2D patients.
Study results
The secondary analysis included 14,565 patients in the DECLARE-TIMI 58 trial. The patients had T2D and multiple risk factors for atherosclerotic cardiovascular disease (about 60%) or established ASCVD (about 40%). All patients had available blood samples and the data were collected from May 2013 to September 2018. The primary outcome was MACE, a composite of MI, ischemic stroke, and cardiovascular death. The results were reported online in JAMA Cardiology.
The analysis found that higher baseline concentrations of NT-proBNP increased MACE risks by 62% (95% confidence interval, 1.49-1.76) and hsTnT elevated those risks by 59% (95% CI, 1.46-1.74).
Among placebo patients, when divided into risk quartiles, those in the highest quartile had significantly higher risk with both elevated NT-proBNP and hsTnT, compared with those with low concentrations. For example, patients with established ASCVD had a 22.9% risk vs. 9.5% with elevated NT-proBNP (P < .001) and a 24.2% vs. 7.2% risk with elevated hsTnT (P < .001). The gap was similar for patients with multiple risk factors.
Dr. Morrow noted that the main DECLARE-TIMI 58 trial showed that dapagliflozin reduced the rates of cardiovascular death or hospitalization for heart failure in patients with T2D, when compared to placebo, but didn’t reach statistical significance for MACE (N Engl J Med. 2019;380:347-57).
“We have previously shown that among patients with T2D who have high risk indicators, such as prior MI or long-standing diabetes, dapagliflozin also appeared to reduce MACE,” Dr. Morrow said. “In this study, we find that these two widely available biomarkers also identify a high-risk group who may have even more potential benefits from treatment with an SGLT2i.”
Dr. Morrow noted that the study design – a nested prospective biomarker study within a randomized, double-blind, placebo-controlled clinical trial – “is a particular strength.”
Results clarify which patients will benefit
This secondary analysis of DECLARE-TIMI 58 brings more clarity to the types of T2D patients who will get the most cardiovascular benefits from dapagliflozin, said Matthew J. Budoff, MD, professor of medicine at University of California, Los Angeles, and Endowed Chair of Preventive Cardiology at the Lundquist Institute in Torrance, Calif.
“The big picture is, we’ve known for some time from epidemiologic studies that these biomarkers, when they’re elevated, mean that the patient is at higher risk of having a cardiovascular event,” he said, “but I think what it helps us with is in knowing in whom to use dapagliflozin for prevention of ASCVD. The effect in the DECLARE-TIMI 58 trial was quite modest, but if you can subgroup it, in these high-risk people there’s a more profound effect. It helps in risk stratification because the absolute benefit is larger.”
The specific biomarkers, NT-proBNP and hsTnT, “haven’t been explored very much in clinical trials,” Dr. Budoff said, “so I do think that it’s nice that in a randomized trial it plays out the way we might expect.”
He added that “for many clinicians this is novel, because I don’t think they were aware of the biomarker data, so I think that this does add some clinical benefit in that context.” The findings also strengthen the case to get T2D patients with higher ASCVD risk onto SGLT2 inhibitors if they’re not already, he said.
Dr. Morrow disclosed relationships with AstraZeneca, Roche Diagnostics, Abbott Laboratories, Anthos Therapeutics, ARCA Biopharma, Merck, Novartis, Pfizer, Regeneron, Siemens, and InCarda outside the reported work.
Dr. Budoff has no relevant disclosures.
FROM JAMA CARDIOLOGY
NUDGE-FLU: Electronic ‘nudges’ boost flu shot uptake in seniors
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
EHR alerts boosted MRA prescribing to patients with HFrEF
NEW ORLEANS – EHR-embedded alerts that a patient with heart failure with reduced ejection fraction (HFrEF) is a great candidate for treatment with a mineralocorticoid receptor antagonist (MRA) more than doubled prescribing of this “pillar” class for HFrEF, compared with control practices that used usual care and no alerts.
That’s according to results of BETTER CARE-HF, a single-center, randomized trial with more than 2,000 patients and involving 180 cardiologists.
“EHR-embedded tools cans be a rapid, low-cost, and high-impact method to increase prescription of life-saving therapies across large populations,” said Amrita Mukhopadhyay, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Her study targeted underprescribing of an MRA – spironolactone or eplerenone (Inspra) – because of its “vastly underprescribed” status in U.S. practice, where roughly two-thirds of patients with HFrEF do not receive an MRA despite clear recommendations from several medical groups that it is an essential part of treatment for most patients with HFrEF. Dr. Mukhopadhyay estimated that more comprehensive prescribing of MRAs to U.S. patients with HFrEF could prevent more than 20,000 deaths annually.
She also explained that the EHR-embedded alert was carefully devised, through interviews with cardiologists and pilot testing, to optimize the nudge so that it was less intrusive but effective for capturing attention and initiating action.
‘Clinically relevant, impressive results’
“This is a really important study, because despite overwhelming evidence for more than a decade favoring MRA use for patients with HFrEF there is an incredibly large treatment gap. MRAs can reduce all-cause death in people with HFrEF by 25%-30%, as well as reduce hospitalizations for heart failure, at a cost of less than $50 a year,” commented Gregg C. Fonarow, MD, interim chief of cardiology at the University of California, Los Angeles. The study showed “very clinically relevant, impressive results” for individualized, patient-specific alerts to prescribe an MRA and order the laboratory tests, particularly for serum potassium levels, needed to safely start the treatment, Dr. Fonarow said in an interview.
The BETTER CARE-HF study ran at more than 60 practices in the New York City region operated by the NYU Langone Health system, which sponsored the study. The trial randomized 180 cardiologists from these practices in a cluster format to one of three study arms: Sixty cardiologists received the EHR-embedded alerts for their relevant patients (755 patients) when the patient was in the physician’s office; another 60 cardiologists received a less tailored, monthly message that flagged all patients with HFrEF in a cardiologist’s practice who remained untreated candidates for MRA intervention (812 patients); and a third arm of 60 cardiologists and their HFrEF patients served as controls where the clinicians received no alert or message (644 patients).
The study included 2,211 patients with HFrEF and not on MRA treatment at baseline who were all identified as good candidates for starting treatment with the class, with no contraindications, no preexisting hyperkalemia, and no advanced-stage renal dysfunction.
The study’s primary outcome was the percentage of patients in each subgroup who received a new prescription for an MRA. This occurred in 29.6% of the patients whose physicians received an alert, in 15.6% of the patients whose physicians received a monthly message, and in 11.7% of patients in the control practices. Statistical analyses showed that the alerts led to a significant 2.53-fold increase in MRA prescribing, while the messages linked with a significant 67% increase in prescribing, compared with the control practices, reported Dr. Mukhopadhyay, a health services researcher at NYU Langone Health in New York. Simultaneously with her report, the results also appeared in the Journal of the American College of Cardiology.
The findings also showed that the alert and message had no significant impact on the prescribing of any other medication classes for HFrEF, compared with the controls. And the alert intervention had minimal adverse effects. While patients in the alert arm showed a significant, 45% relative increase in the incidence of hyperkalemia episodes, compared with control patients (because of a 4.5% absolute increase in hyperkalemia events), the rate of “significant” hyperkalemia with a value of at least 5.5 mmol/L, occurred in 5.0% of patients in the alert group and 5.1% of patients in the control arm.
Potassium testing poses another barrier
Even though the alerts substantially improved MRA prescribing, 70% of patients deemed MRA eligible in the alert subgroup still failed to receive a prescription. One additional barrier specific to MRA prescribing is the need it triggers for serial laboratory testing to monitor serum potassium levels. “Potassium testing generates additional work outside the index visit, which along with the risk for hyperkalemia exists as a barrier,” commented Lee R. Goldberg, MD, a heart failure specialist and professor at the University of Pennsylvania in Philadelphia. “This may be the next aspect to focus on to improve MRA uptake,” he said as a designated discussant for the report.
“It’s not enough to just prompt medication treatment. We also need to prompt appropriate laboratory testing,” noted Dr. Fonarow.
He also said that the approach tested by Dr. Mukhopadhyay could now be expanded to outpatient cardiologists. “The onus is on everyone involved in caring for patients with HFrEF failure to explain why maximum effort is not being made to deploy” all of the guideline-directed medical therapies for the disorder.
EHR alerts “are one way to bridge the prescribing gap, but we need multiple approaches so that all eligible patients receive guideline-directed medical therapy,” Dr. Fonarow said.
BETTER CARE-HF received no commercial funding, and Dr. Mukhopadhyay had no disclosures. Dr. Fonarow has been a consultant to AstraZeneca, Amgen, Cytokinetics, Lilly, Merck, Novartis, and Pfizer. Dr. Goldberg has received personal fees from Abbott, VisCardia, and Zoll/Respircardia.
NEW ORLEANS – EHR-embedded alerts that a patient with heart failure with reduced ejection fraction (HFrEF) is a great candidate for treatment with a mineralocorticoid receptor antagonist (MRA) more than doubled prescribing of this “pillar” class for HFrEF, compared with control practices that used usual care and no alerts.
That’s according to results of BETTER CARE-HF, a single-center, randomized trial with more than 2,000 patients and involving 180 cardiologists.
“EHR-embedded tools cans be a rapid, low-cost, and high-impact method to increase prescription of life-saving therapies across large populations,” said Amrita Mukhopadhyay, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Her study targeted underprescribing of an MRA – spironolactone or eplerenone (Inspra) – because of its “vastly underprescribed” status in U.S. practice, where roughly two-thirds of patients with HFrEF do not receive an MRA despite clear recommendations from several medical groups that it is an essential part of treatment for most patients with HFrEF. Dr. Mukhopadhyay estimated that more comprehensive prescribing of MRAs to U.S. patients with HFrEF could prevent more than 20,000 deaths annually.
She also explained that the EHR-embedded alert was carefully devised, through interviews with cardiologists and pilot testing, to optimize the nudge so that it was less intrusive but effective for capturing attention and initiating action.
‘Clinically relevant, impressive results’
“This is a really important study, because despite overwhelming evidence for more than a decade favoring MRA use for patients with HFrEF there is an incredibly large treatment gap. MRAs can reduce all-cause death in people with HFrEF by 25%-30%, as well as reduce hospitalizations for heart failure, at a cost of less than $50 a year,” commented Gregg C. Fonarow, MD, interim chief of cardiology at the University of California, Los Angeles. The study showed “very clinically relevant, impressive results” for individualized, patient-specific alerts to prescribe an MRA and order the laboratory tests, particularly for serum potassium levels, needed to safely start the treatment, Dr. Fonarow said in an interview.
The BETTER CARE-HF study ran at more than 60 practices in the New York City region operated by the NYU Langone Health system, which sponsored the study. The trial randomized 180 cardiologists from these practices in a cluster format to one of three study arms: Sixty cardiologists received the EHR-embedded alerts for their relevant patients (755 patients) when the patient was in the physician’s office; another 60 cardiologists received a less tailored, monthly message that flagged all patients with HFrEF in a cardiologist’s practice who remained untreated candidates for MRA intervention (812 patients); and a third arm of 60 cardiologists and their HFrEF patients served as controls where the clinicians received no alert or message (644 patients).
The study included 2,211 patients with HFrEF and not on MRA treatment at baseline who were all identified as good candidates for starting treatment with the class, with no contraindications, no preexisting hyperkalemia, and no advanced-stage renal dysfunction.
The study’s primary outcome was the percentage of patients in each subgroup who received a new prescription for an MRA. This occurred in 29.6% of the patients whose physicians received an alert, in 15.6% of the patients whose physicians received a monthly message, and in 11.7% of patients in the control practices. Statistical analyses showed that the alerts led to a significant 2.53-fold increase in MRA prescribing, while the messages linked with a significant 67% increase in prescribing, compared with the control practices, reported Dr. Mukhopadhyay, a health services researcher at NYU Langone Health in New York. Simultaneously with her report, the results also appeared in the Journal of the American College of Cardiology.
The findings also showed that the alert and message had no significant impact on the prescribing of any other medication classes for HFrEF, compared with the controls. And the alert intervention had minimal adverse effects. While patients in the alert arm showed a significant, 45% relative increase in the incidence of hyperkalemia episodes, compared with control patients (because of a 4.5% absolute increase in hyperkalemia events), the rate of “significant” hyperkalemia with a value of at least 5.5 mmol/L, occurred in 5.0% of patients in the alert group and 5.1% of patients in the control arm.
Potassium testing poses another barrier
Even though the alerts substantially improved MRA prescribing, 70% of patients deemed MRA eligible in the alert subgroup still failed to receive a prescription. One additional barrier specific to MRA prescribing is the need it triggers for serial laboratory testing to monitor serum potassium levels. “Potassium testing generates additional work outside the index visit, which along with the risk for hyperkalemia exists as a barrier,” commented Lee R. Goldberg, MD, a heart failure specialist and professor at the University of Pennsylvania in Philadelphia. “This may be the next aspect to focus on to improve MRA uptake,” he said as a designated discussant for the report.
“It’s not enough to just prompt medication treatment. We also need to prompt appropriate laboratory testing,” noted Dr. Fonarow.
He also said that the approach tested by Dr. Mukhopadhyay could now be expanded to outpatient cardiologists. “The onus is on everyone involved in caring for patients with HFrEF failure to explain why maximum effort is not being made to deploy” all of the guideline-directed medical therapies for the disorder.
EHR alerts “are one way to bridge the prescribing gap, but we need multiple approaches so that all eligible patients receive guideline-directed medical therapy,” Dr. Fonarow said.
BETTER CARE-HF received no commercial funding, and Dr. Mukhopadhyay had no disclosures. Dr. Fonarow has been a consultant to AstraZeneca, Amgen, Cytokinetics, Lilly, Merck, Novartis, and Pfizer. Dr. Goldberg has received personal fees from Abbott, VisCardia, and Zoll/Respircardia.
NEW ORLEANS – EHR-embedded alerts that a patient with heart failure with reduced ejection fraction (HFrEF) is a great candidate for treatment with a mineralocorticoid receptor antagonist (MRA) more than doubled prescribing of this “pillar” class for HFrEF, compared with control practices that used usual care and no alerts.
That’s according to results of BETTER CARE-HF, a single-center, randomized trial with more than 2,000 patients and involving 180 cardiologists.
“EHR-embedded tools cans be a rapid, low-cost, and high-impact method to increase prescription of life-saving therapies across large populations,” said Amrita Mukhopadhyay, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Her study targeted underprescribing of an MRA – spironolactone or eplerenone (Inspra) – because of its “vastly underprescribed” status in U.S. practice, where roughly two-thirds of patients with HFrEF do not receive an MRA despite clear recommendations from several medical groups that it is an essential part of treatment for most patients with HFrEF. Dr. Mukhopadhyay estimated that more comprehensive prescribing of MRAs to U.S. patients with HFrEF could prevent more than 20,000 deaths annually.
She also explained that the EHR-embedded alert was carefully devised, through interviews with cardiologists and pilot testing, to optimize the nudge so that it was less intrusive but effective for capturing attention and initiating action.
‘Clinically relevant, impressive results’
“This is a really important study, because despite overwhelming evidence for more than a decade favoring MRA use for patients with HFrEF there is an incredibly large treatment gap. MRAs can reduce all-cause death in people with HFrEF by 25%-30%, as well as reduce hospitalizations for heart failure, at a cost of less than $50 a year,” commented Gregg C. Fonarow, MD, interim chief of cardiology at the University of California, Los Angeles. The study showed “very clinically relevant, impressive results” for individualized, patient-specific alerts to prescribe an MRA and order the laboratory tests, particularly for serum potassium levels, needed to safely start the treatment, Dr. Fonarow said in an interview.
The BETTER CARE-HF study ran at more than 60 practices in the New York City region operated by the NYU Langone Health system, which sponsored the study. The trial randomized 180 cardiologists from these practices in a cluster format to one of three study arms: Sixty cardiologists received the EHR-embedded alerts for their relevant patients (755 patients) when the patient was in the physician’s office; another 60 cardiologists received a less tailored, monthly message that flagged all patients with HFrEF in a cardiologist’s practice who remained untreated candidates for MRA intervention (812 patients); and a third arm of 60 cardiologists and their HFrEF patients served as controls where the clinicians received no alert or message (644 patients).
The study included 2,211 patients with HFrEF and not on MRA treatment at baseline who were all identified as good candidates for starting treatment with the class, with no contraindications, no preexisting hyperkalemia, and no advanced-stage renal dysfunction.
The study’s primary outcome was the percentage of patients in each subgroup who received a new prescription for an MRA. This occurred in 29.6% of the patients whose physicians received an alert, in 15.6% of the patients whose physicians received a monthly message, and in 11.7% of patients in the control practices. Statistical analyses showed that the alerts led to a significant 2.53-fold increase in MRA prescribing, while the messages linked with a significant 67% increase in prescribing, compared with the control practices, reported Dr. Mukhopadhyay, a health services researcher at NYU Langone Health in New York. Simultaneously with her report, the results also appeared in the Journal of the American College of Cardiology.
The findings also showed that the alert and message had no significant impact on the prescribing of any other medication classes for HFrEF, compared with the controls. And the alert intervention had minimal adverse effects. While patients in the alert arm showed a significant, 45% relative increase in the incidence of hyperkalemia episodes, compared with control patients (because of a 4.5% absolute increase in hyperkalemia events), the rate of “significant” hyperkalemia with a value of at least 5.5 mmol/L, occurred in 5.0% of patients in the alert group and 5.1% of patients in the control arm.
Potassium testing poses another barrier
Even though the alerts substantially improved MRA prescribing, 70% of patients deemed MRA eligible in the alert subgroup still failed to receive a prescription. One additional barrier specific to MRA prescribing is the need it triggers for serial laboratory testing to monitor serum potassium levels. “Potassium testing generates additional work outside the index visit, which along with the risk for hyperkalemia exists as a barrier,” commented Lee R. Goldberg, MD, a heart failure specialist and professor at the University of Pennsylvania in Philadelphia. “This may be the next aspect to focus on to improve MRA uptake,” he said as a designated discussant for the report.
“It’s not enough to just prompt medication treatment. We also need to prompt appropriate laboratory testing,” noted Dr. Fonarow.
He also said that the approach tested by Dr. Mukhopadhyay could now be expanded to outpatient cardiologists. “The onus is on everyone involved in caring for patients with HFrEF failure to explain why maximum effort is not being made to deploy” all of the guideline-directed medical therapies for the disorder.
EHR alerts “are one way to bridge the prescribing gap, but we need multiple approaches so that all eligible patients receive guideline-directed medical therapy,” Dr. Fonarow said.
BETTER CARE-HF received no commercial funding, and Dr. Mukhopadhyay had no disclosures. Dr. Fonarow has been a consultant to AstraZeneca, Amgen, Cytokinetics, Lilly, Merck, Novartis, and Pfizer. Dr. Goldberg has received personal fees from Abbott, VisCardia, and Zoll/Respircardia.
AT ACC 2023