User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
ADHD rates holding steady in U.S. children
TOPLINE:
While the prevalence of attention-deficit/hyperactivity disorder in U.S. children increased from the late 1990s to 2016,
METHODOLOGY:
- Based on prior data, the prevalence of ADHD in children rose from 6.1% in 1997-1998 to 10.2% in 2015-2016, with a 42.0% increase from 2003 to 2011. The new report provides updated prevalence data for 2017-2022.
- The cross-sectional analysis used data from the National Health Interview Survey (NHIS) from 2017 to 2022 for more than 37,609 U.S. children and adolescents 4-17 years old (52% male, 53% non-Hispanic White, 24% Hispanic, 11% non-Hispanic Black, and 12% non-Hispanic other race).
- Information on health care provider–diagnosed ADHD was reported by a parent or guardian.
TAKEAWAY:
- A total of 4,098 children and adolescents (10.9%) were reported to have an ADHD diagnosis during the study period.
- The weighted prevalence of ADHD ranged from 10.08% to 10.47% from 2017 to 2022, which is similar to the prevalence in 2015-2016 (10.20%).
- There was no significant change on an annual basis or in all subgroups evaluated. Notably, the estimated prevalence of ADHD among U.S. children and adolescents was higher than worldwide estimates (5.3%) in earlier years (1978-2005).
- The prevalence of ADHD in U.S. children differed significantly by age, sex, race/ethnicity, and family income, in line with previous findings, with higher rates in those 12-17 years (vs. 4-11 years), males, non-Hispanic populations, and those with higher family income.
IN PRACTICE:
The estimated ADHD prevalence remains “high” and “further investigation is warranted to assess potentially modifiable risk factors and provide adequate resources for treatment of individuals with ADHD in the future,” the authors write.
SOURCE:
The study, with first author Yanmei Li, Guangdong Pharmaceutical University, Guangzhou, China, was published online in JAMA Network Open.
LIMITATIONS:
Information on ADHD relied on parent-reported diagnosis, which may lead to misreporting and recall bias. The NHIS underwent a major redesign in 2019, which may affect comparability with prior years, and the COVID-19 pandemic affected collection in 2020.
DISCLOSURES:
The study had no specific funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
While the prevalence of attention-deficit/hyperactivity disorder in U.S. children increased from the late 1990s to 2016,
METHODOLOGY:
- Based on prior data, the prevalence of ADHD in children rose from 6.1% in 1997-1998 to 10.2% in 2015-2016, with a 42.0% increase from 2003 to 2011. The new report provides updated prevalence data for 2017-2022.
- The cross-sectional analysis used data from the National Health Interview Survey (NHIS) from 2017 to 2022 for more than 37,609 U.S. children and adolescents 4-17 years old (52% male, 53% non-Hispanic White, 24% Hispanic, 11% non-Hispanic Black, and 12% non-Hispanic other race).
- Information on health care provider–diagnosed ADHD was reported by a parent or guardian.
TAKEAWAY:
- A total of 4,098 children and adolescents (10.9%) were reported to have an ADHD diagnosis during the study period.
- The weighted prevalence of ADHD ranged from 10.08% to 10.47% from 2017 to 2022, which is similar to the prevalence in 2015-2016 (10.20%).
- There was no significant change on an annual basis or in all subgroups evaluated. Notably, the estimated prevalence of ADHD among U.S. children and adolescents was higher than worldwide estimates (5.3%) in earlier years (1978-2005).
- The prevalence of ADHD in U.S. children differed significantly by age, sex, race/ethnicity, and family income, in line with previous findings, with higher rates in those 12-17 years (vs. 4-11 years), males, non-Hispanic populations, and those with higher family income.
IN PRACTICE:
The estimated ADHD prevalence remains “high” and “further investigation is warranted to assess potentially modifiable risk factors and provide adequate resources for treatment of individuals with ADHD in the future,” the authors write.
SOURCE:
The study, with first author Yanmei Li, Guangdong Pharmaceutical University, Guangzhou, China, was published online in JAMA Network Open.
LIMITATIONS:
Information on ADHD relied on parent-reported diagnosis, which may lead to misreporting and recall bias. The NHIS underwent a major redesign in 2019, which may affect comparability with prior years, and the COVID-19 pandemic affected collection in 2020.
DISCLOSURES:
The study had no specific funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
While the prevalence of attention-deficit/hyperactivity disorder in U.S. children increased from the late 1990s to 2016,
METHODOLOGY:
- Based on prior data, the prevalence of ADHD in children rose from 6.1% in 1997-1998 to 10.2% in 2015-2016, with a 42.0% increase from 2003 to 2011. The new report provides updated prevalence data for 2017-2022.
- The cross-sectional analysis used data from the National Health Interview Survey (NHIS) from 2017 to 2022 for more than 37,609 U.S. children and adolescents 4-17 years old (52% male, 53% non-Hispanic White, 24% Hispanic, 11% non-Hispanic Black, and 12% non-Hispanic other race).
- Information on health care provider–diagnosed ADHD was reported by a parent or guardian.
TAKEAWAY:
- A total of 4,098 children and adolescents (10.9%) were reported to have an ADHD diagnosis during the study period.
- The weighted prevalence of ADHD ranged from 10.08% to 10.47% from 2017 to 2022, which is similar to the prevalence in 2015-2016 (10.20%).
- There was no significant change on an annual basis or in all subgroups evaluated. Notably, the estimated prevalence of ADHD among U.S. children and adolescents was higher than worldwide estimates (5.3%) in earlier years (1978-2005).
- The prevalence of ADHD in U.S. children differed significantly by age, sex, race/ethnicity, and family income, in line with previous findings, with higher rates in those 12-17 years (vs. 4-11 years), males, non-Hispanic populations, and those with higher family income.
IN PRACTICE:
The estimated ADHD prevalence remains “high” and “further investigation is warranted to assess potentially modifiable risk factors and provide adequate resources for treatment of individuals with ADHD in the future,” the authors write.
SOURCE:
The study, with first author Yanmei Li, Guangdong Pharmaceutical University, Guangzhou, China, was published online in JAMA Network Open.
LIMITATIONS:
Information on ADHD relied on parent-reported diagnosis, which may lead to misreporting and recall bias. The NHIS underwent a major redesign in 2019, which may affect comparability with prior years, and the COVID-19 pandemic affected collection in 2020.
DISCLOSURES:
The study had no specific funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Illicit steroids: If MDs don’t ask, patients won’t tell
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Every click you make, the EHR is watching you
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.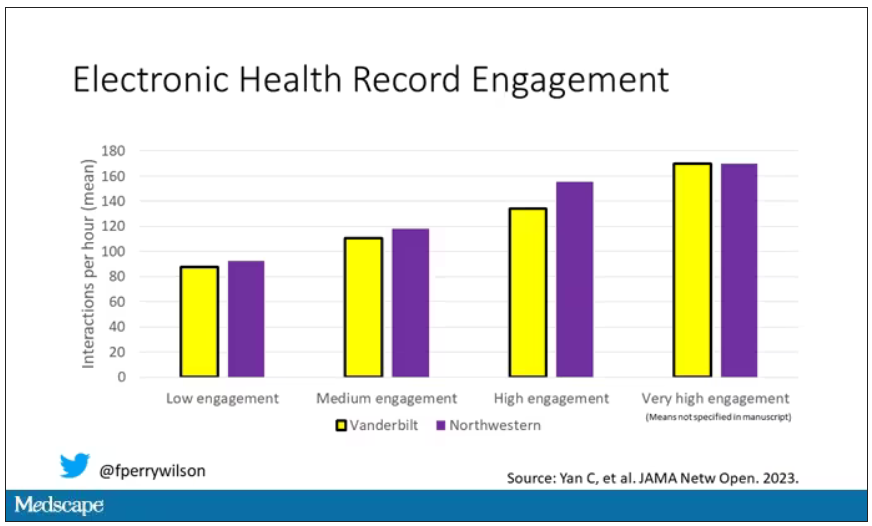
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.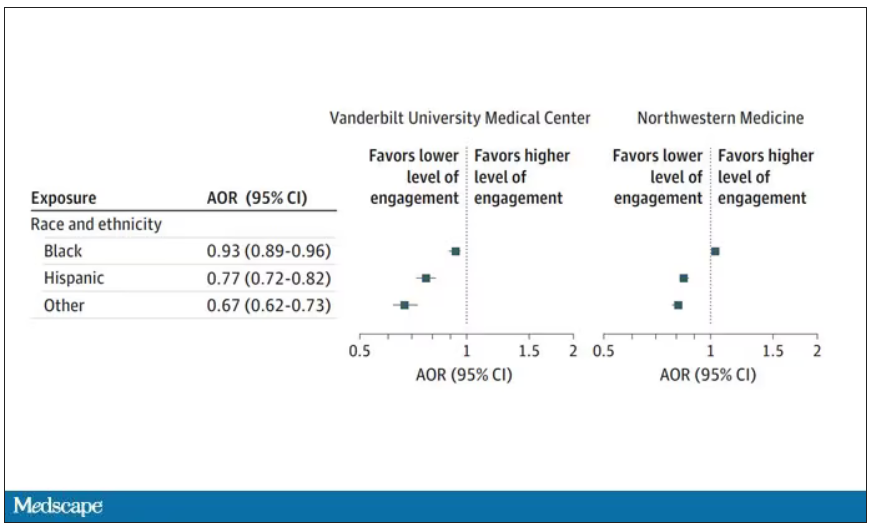
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.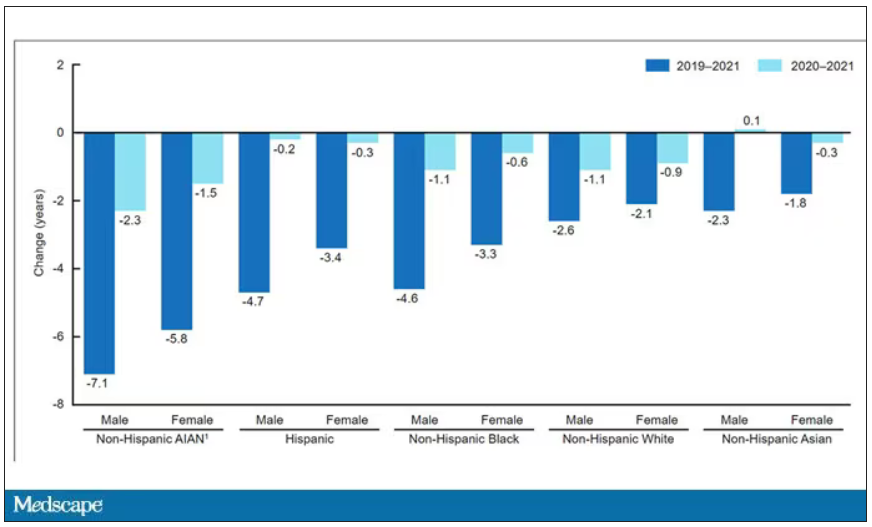
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Sleep irregularity
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Burnout in medical profession higher among women, younger clinicians
The poster child for a burned-out physician is a young woman practicing in primary care, according to a new study of more than 1,300 clinicians.
The study, published in JAMA Network Open. investigated patterns in physician burnout among 1,373 physicians at Massachusetts General Physicians Organization, a hospital-owned group practice. It assessed burnout in 3 years: 2017, 2019, and 2021.
Respondents were queried about their satisfaction with their career and compensation, as well as their well-being, administrative workload, and leadership and diversity.
Female physicians exhibited a higher burnout rate than male physicians (odds ratio, 1.47; 95% confidence interval, 1.02-2.12), while among primary care physicians (PCPs), the burnout rate was almost three times higher than among those in internal medicine (OR, 2.82; 95% CI, 1.76-4.50). Among physicians with 30 or more years of experience, the burnout rate was lower than among those with 10 years of experience or less (OR, 0.21; 95% CI, 0.13-0.35).
The fact that burnout disproportionately affects female physicians could reflect the additional household and family obligations women are often expected to handle, as well as their desire to form relationships with their patients, according to Timothy Hoff, PhD, a professor of management, healthcare systems, and health policy at Northeastern University, Boston.
“Female physicians tend to practice differently than their male counterparts,” said Dr. Hoff, who studies primary care. “They may focus more on the relational aspects of care, and that could lead to a higher rate of burnout.”
The study used the Maslach Burnout Inventory and three burnout subscales: exhaustion, cynicism, and reduced personal efficacy. The cohort was composed of 50% men, 67% White respondents, and 87% non-Hispanic respondents. A little over two-thirds of physicians had from 11 to 20 years of experience.
About 93% of those surveyed responded; by comparison, response rates were between 27% and 32% in previous analyses of physician burnout, the study authors say. They attribute this high participation rate to the fact that they compensated each participant with $850, more than is usually offered.
Hilton Gomes, MD, a partner at a concierge primary care practice in Miami – who has been practicing medicine for more than 15 years – said the increased rates of burnout among his younger colleagues are partly the result of a recent shift in what is considered the ideal work-life balance.
“Younger generations of doctors enter the profession with a strong desire for a better work-life balance. Unfortunately, medicine does not typically lend itself to achieving this balance,” he said.
Dr. Gomes recalled a time in medical school when he tried to visit his former pediatrician, who couldn’t be found at home.
“His wife informed me that he was tending to an urgent sick visit at the hospital, while his wife had to deal with their own grandson’s fracture being treated at urgent care,” Dr. Gomes said. “This illustrates, in my experience, how older generations of physicians accepted the demands of the profession as part of their commitment, and this often involved putting our own families second.”
Dr. Gomes, like many other PCPs who have converted to concierge medicine, previously worked at a practice where he saw nearly two dozen patients a day for a maximum of 15 minutes each.
“The structure of managed care often results in primary care physicians spending less time with patients and more time on paperwork, which is not the reason why physicians enter the field of medicine,” Dr. Gomes said.
Physicians are not alone in their feelings of physical and mental exhaustion. In the Medscape Physician Assistant Burnout Report 2023, 16% of respondents said the burnout they experienced was so severe that they were thinking of leaving medicine.
In 2022, PCP burnout cost the United States $260 million in excess health care expenditures. Burnout has also increased rates of physician suicide over the past 50 years and has led to a rise in medical errors.
Physicians say that programs that teach them to perform yoga and take deep breaths – which are offered by their employers – are not the solution.
“We sort of know what the realities of physician burnout are now; the imperative is to address it,” Dr. Hoff said. “We need studies that focus on the concepts of sustainability.”
The study was funded by the Massachusetts General Physicians Organization. A coauthor reports receiving a grant from the American Heart Association. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The poster child for a burned-out physician is a young woman practicing in primary care, according to a new study of more than 1,300 clinicians.
The study, published in JAMA Network Open. investigated patterns in physician burnout among 1,373 physicians at Massachusetts General Physicians Organization, a hospital-owned group practice. It assessed burnout in 3 years: 2017, 2019, and 2021.
Respondents were queried about their satisfaction with their career and compensation, as well as their well-being, administrative workload, and leadership and diversity.
Female physicians exhibited a higher burnout rate than male physicians (odds ratio, 1.47; 95% confidence interval, 1.02-2.12), while among primary care physicians (PCPs), the burnout rate was almost three times higher than among those in internal medicine (OR, 2.82; 95% CI, 1.76-4.50). Among physicians with 30 or more years of experience, the burnout rate was lower than among those with 10 years of experience or less (OR, 0.21; 95% CI, 0.13-0.35).
The fact that burnout disproportionately affects female physicians could reflect the additional household and family obligations women are often expected to handle, as well as their desire to form relationships with their patients, according to Timothy Hoff, PhD, a professor of management, healthcare systems, and health policy at Northeastern University, Boston.
“Female physicians tend to practice differently than their male counterparts,” said Dr. Hoff, who studies primary care. “They may focus more on the relational aspects of care, and that could lead to a higher rate of burnout.”
The study used the Maslach Burnout Inventory and three burnout subscales: exhaustion, cynicism, and reduced personal efficacy. The cohort was composed of 50% men, 67% White respondents, and 87% non-Hispanic respondents. A little over two-thirds of physicians had from 11 to 20 years of experience.
About 93% of those surveyed responded; by comparison, response rates were between 27% and 32% in previous analyses of physician burnout, the study authors say. They attribute this high participation rate to the fact that they compensated each participant with $850, more than is usually offered.
Hilton Gomes, MD, a partner at a concierge primary care practice in Miami – who has been practicing medicine for more than 15 years – said the increased rates of burnout among his younger colleagues are partly the result of a recent shift in what is considered the ideal work-life balance.
“Younger generations of doctors enter the profession with a strong desire for a better work-life balance. Unfortunately, medicine does not typically lend itself to achieving this balance,” he said.
Dr. Gomes recalled a time in medical school when he tried to visit his former pediatrician, who couldn’t be found at home.
“His wife informed me that he was tending to an urgent sick visit at the hospital, while his wife had to deal with their own grandson’s fracture being treated at urgent care,” Dr. Gomes said. “This illustrates, in my experience, how older generations of physicians accepted the demands of the profession as part of their commitment, and this often involved putting our own families second.”
Dr. Gomes, like many other PCPs who have converted to concierge medicine, previously worked at a practice where he saw nearly two dozen patients a day for a maximum of 15 minutes each.
“The structure of managed care often results in primary care physicians spending less time with patients and more time on paperwork, which is not the reason why physicians enter the field of medicine,” Dr. Gomes said.
Physicians are not alone in their feelings of physical and mental exhaustion. In the Medscape Physician Assistant Burnout Report 2023, 16% of respondents said the burnout they experienced was so severe that they were thinking of leaving medicine.
In 2022, PCP burnout cost the United States $260 million in excess health care expenditures. Burnout has also increased rates of physician suicide over the past 50 years and has led to a rise in medical errors.
Physicians say that programs that teach them to perform yoga and take deep breaths – which are offered by their employers – are not the solution.
“We sort of know what the realities of physician burnout are now; the imperative is to address it,” Dr. Hoff said. “We need studies that focus on the concepts of sustainability.”
The study was funded by the Massachusetts General Physicians Organization. A coauthor reports receiving a grant from the American Heart Association. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The poster child for a burned-out physician is a young woman practicing in primary care, according to a new study of more than 1,300 clinicians.
The study, published in JAMA Network Open. investigated patterns in physician burnout among 1,373 physicians at Massachusetts General Physicians Organization, a hospital-owned group practice. It assessed burnout in 3 years: 2017, 2019, and 2021.
Respondents were queried about their satisfaction with their career and compensation, as well as their well-being, administrative workload, and leadership and diversity.
Female physicians exhibited a higher burnout rate than male physicians (odds ratio, 1.47; 95% confidence interval, 1.02-2.12), while among primary care physicians (PCPs), the burnout rate was almost three times higher than among those in internal medicine (OR, 2.82; 95% CI, 1.76-4.50). Among physicians with 30 or more years of experience, the burnout rate was lower than among those with 10 years of experience or less (OR, 0.21; 95% CI, 0.13-0.35).
The fact that burnout disproportionately affects female physicians could reflect the additional household and family obligations women are often expected to handle, as well as their desire to form relationships with their patients, according to Timothy Hoff, PhD, a professor of management, healthcare systems, and health policy at Northeastern University, Boston.
“Female physicians tend to practice differently than their male counterparts,” said Dr. Hoff, who studies primary care. “They may focus more on the relational aspects of care, and that could lead to a higher rate of burnout.”
The study used the Maslach Burnout Inventory and three burnout subscales: exhaustion, cynicism, and reduced personal efficacy. The cohort was composed of 50% men, 67% White respondents, and 87% non-Hispanic respondents. A little over two-thirds of physicians had from 11 to 20 years of experience.
About 93% of those surveyed responded; by comparison, response rates were between 27% and 32% in previous analyses of physician burnout, the study authors say. They attribute this high participation rate to the fact that they compensated each participant with $850, more than is usually offered.
Hilton Gomes, MD, a partner at a concierge primary care practice in Miami – who has been practicing medicine for more than 15 years – said the increased rates of burnout among his younger colleagues are partly the result of a recent shift in what is considered the ideal work-life balance.
“Younger generations of doctors enter the profession with a strong desire for a better work-life balance. Unfortunately, medicine does not typically lend itself to achieving this balance,” he said.
Dr. Gomes recalled a time in medical school when he tried to visit his former pediatrician, who couldn’t be found at home.
“His wife informed me that he was tending to an urgent sick visit at the hospital, while his wife had to deal with their own grandson’s fracture being treated at urgent care,” Dr. Gomes said. “This illustrates, in my experience, how older generations of physicians accepted the demands of the profession as part of their commitment, and this often involved putting our own families second.”
Dr. Gomes, like many other PCPs who have converted to concierge medicine, previously worked at a practice where he saw nearly two dozen patients a day for a maximum of 15 minutes each.
“The structure of managed care often results in primary care physicians spending less time with patients and more time on paperwork, which is not the reason why physicians enter the field of medicine,” Dr. Gomes said.
Physicians are not alone in their feelings of physical and mental exhaustion. In the Medscape Physician Assistant Burnout Report 2023, 16% of respondents said the burnout they experienced was so severe that they were thinking of leaving medicine.
In 2022, PCP burnout cost the United States $260 million in excess health care expenditures. Burnout has also increased rates of physician suicide over the past 50 years and has led to a rise in medical errors.
Physicians say that programs that teach them to perform yoga and take deep breaths – which are offered by their employers – are not the solution.
“We sort of know what the realities of physician burnout are now; the imperative is to address it,” Dr. Hoff said. “We need studies that focus on the concepts of sustainability.”
The study was funded by the Massachusetts General Physicians Organization. A coauthor reports receiving a grant from the American Heart Association. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Lead pollutants as harmful to health as particulate matter
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
FROM THE LANCET PLANETARY HEALTH
Maternal pertussis vax effective for infants in most vulnerable months
Maternal pertussis vaccinations, given during pregnancy, prevent an estimated 65% of pertussis infections in infants, new research indicates.
The study, led by Annette K. Regan, PhD, MPH, a perinatal and pediatric infectious disease epidemiologist at Curtin University, Perth, Australia, was published online in Pediatrics.
Dr. Regan – who is also with the University of San Francisco and the University of California, Los Angeles – and colleagues reviewed data on 279,418 infants born to 252,444 mothers in Australia.
There, about 52% of the women in this study received the Tdap vaccine through a maternal pertussis vaccination program.
Duration of effectiveness in infants was one of the main questions the study sought to answer.
The authors wrote that they assessed vaccine effectiveness through 18 months of age. “We observed significant protection against disease until at least 8 months of age, 2 months longer than reported in previous studies.” From 70% to 90% of all pertussis-attributable hospitalizations and death occur in infancy.
Answering the ‘blunting’ question
This study also set out to clarify an important clinical question regarding a potential “blunting” effect in infants. Previous work had suggested that maternal antibodies from the vaccination could interfere with the effectiveness of infants’ DtaP (the version of Tdap for infants) and other vaccines.
Dr. Regan and colleagues found that, “although we observed slightly lower VE [vaccine effectiveness] point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002), we did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% confidence interval, 0.61-3.39).
Best time to give mothers the vaccine
Another clinical debate has centered on when to give the mother the vaccine during pregnancy. The authors concluded: “Our findings support the infant health benefits of recommendations to administer a booster dose of pertussis vaccine near 28 weeks of gestational age.”
That 28-week mark was associated with lower risk of infection in infants through 8 months of age, they wrote.
Positive results in the United States
In an invited commentary, Kathryn M. Edwards, MD, with the division of infectious diseases, department of pediatrics, at Vanderbilt University Medical Center, Nashville, Tenn., highlighted similar positive findings for maternal pertussis vaccination in the United States.
The Centers for Disease Control and Prevention did an ecologic study of infant pertussis cases reported between Jan. 1, 2000, and Dec. 31, 2019. Rates were compared for the years before maternal Tdap vaccinations were recommended against the 7-year period after they were implemented.
That study found that in the period before maternal Tdap vaccination, annual pertussis incidence did not change among infants younger than 2 months and increased slightly in infants 6-12 months.
However, during the period after maternal Tdap vaccination had started (2012-2019), pertussis incidence significantly decreased in infants younger than 2 months and was unchanged in infants 6-12 months.
“As with the Australian data, the U.S. data support the overall benefit of the maternal Tdap program and, as with the Australian data, do not suggest that blunting has led to an increase in cases within the first year of life,” Dr. Edwards wrote.
The CDC notes that pertussis cases are rising and outbreaks are happening across the United States.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” the CDC states.
Uptake low despite positive data
Dr. Edwards noted that, despite positive data supporting maternal vaccination to reduce pertussis, uptake rates are low – between 50% and 60% in Australia, the United Kingdom, and the United States. “Active engagement to increase these rates should be implemented.”
Maternal vaccination might also be implemented soon to protect against other diseases including respiratory syncytial virus and group B streptococcal disease after promising study data, she said.
As with pertussis, the potential “blunting” effect will need to be carefully monitored, she said, “as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics.”
One coauthor has received institutional honoraria for participation in advisory groups for Merck Sharpe & Dohme and Pfizer unrelated to this work. Another coauthor was supported by scholarships provided by the Wesfarmers Centre of Vaccines and Infectious Disease at the Telethon Kids Institute. Dr. Edwards reported receiving grants from the CDC and consulting for Bionet, Dynavax, and IBM. She is a member of the data safety and monitoring board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and Brighton Collaboration.
Maternal pertussis vaccinations, given during pregnancy, prevent an estimated 65% of pertussis infections in infants, new research indicates.
The study, led by Annette K. Regan, PhD, MPH, a perinatal and pediatric infectious disease epidemiologist at Curtin University, Perth, Australia, was published online in Pediatrics.
Dr. Regan – who is also with the University of San Francisco and the University of California, Los Angeles – and colleagues reviewed data on 279,418 infants born to 252,444 mothers in Australia.
There, about 52% of the women in this study received the Tdap vaccine through a maternal pertussis vaccination program.
Duration of effectiveness in infants was one of the main questions the study sought to answer.
The authors wrote that they assessed vaccine effectiveness through 18 months of age. “We observed significant protection against disease until at least 8 months of age, 2 months longer than reported in previous studies.” From 70% to 90% of all pertussis-attributable hospitalizations and death occur in infancy.
Answering the ‘blunting’ question
This study also set out to clarify an important clinical question regarding a potential “blunting” effect in infants. Previous work had suggested that maternal antibodies from the vaccination could interfere with the effectiveness of infants’ DtaP (the version of Tdap for infants) and other vaccines.
Dr. Regan and colleagues found that, “although we observed slightly lower VE [vaccine effectiveness] point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002), we did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% confidence interval, 0.61-3.39).
Best time to give mothers the vaccine
Another clinical debate has centered on when to give the mother the vaccine during pregnancy. The authors concluded: “Our findings support the infant health benefits of recommendations to administer a booster dose of pertussis vaccine near 28 weeks of gestational age.”
That 28-week mark was associated with lower risk of infection in infants through 8 months of age, they wrote.
Positive results in the United States
In an invited commentary, Kathryn M. Edwards, MD, with the division of infectious diseases, department of pediatrics, at Vanderbilt University Medical Center, Nashville, Tenn., highlighted similar positive findings for maternal pertussis vaccination in the United States.
The Centers for Disease Control and Prevention did an ecologic study of infant pertussis cases reported between Jan. 1, 2000, and Dec. 31, 2019. Rates were compared for the years before maternal Tdap vaccinations were recommended against the 7-year period after they were implemented.
That study found that in the period before maternal Tdap vaccination, annual pertussis incidence did not change among infants younger than 2 months and increased slightly in infants 6-12 months.
However, during the period after maternal Tdap vaccination had started (2012-2019), pertussis incidence significantly decreased in infants younger than 2 months and was unchanged in infants 6-12 months.
“As with the Australian data, the U.S. data support the overall benefit of the maternal Tdap program and, as with the Australian data, do not suggest that blunting has led to an increase in cases within the first year of life,” Dr. Edwards wrote.
The CDC notes that pertussis cases are rising and outbreaks are happening across the United States.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” the CDC states.
Uptake low despite positive data
Dr. Edwards noted that, despite positive data supporting maternal vaccination to reduce pertussis, uptake rates are low – between 50% and 60% in Australia, the United Kingdom, and the United States. “Active engagement to increase these rates should be implemented.”
Maternal vaccination might also be implemented soon to protect against other diseases including respiratory syncytial virus and group B streptococcal disease after promising study data, she said.
As with pertussis, the potential “blunting” effect will need to be carefully monitored, she said, “as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics.”
One coauthor has received institutional honoraria for participation in advisory groups for Merck Sharpe & Dohme and Pfizer unrelated to this work. Another coauthor was supported by scholarships provided by the Wesfarmers Centre of Vaccines and Infectious Disease at the Telethon Kids Institute. Dr. Edwards reported receiving grants from the CDC and consulting for Bionet, Dynavax, and IBM. She is a member of the data safety and monitoring board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and Brighton Collaboration.
Maternal pertussis vaccinations, given during pregnancy, prevent an estimated 65% of pertussis infections in infants, new research indicates.
The study, led by Annette K. Regan, PhD, MPH, a perinatal and pediatric infectious disease epidemiologist at Curtin University, Perth, Australia, was published online in Pediatrics.
Dr. Regan – who is also with the University of San Francisco and the University of California, Los Angeles – and colleagues reviewed data on 279,418 infants born to 252,444 mothers in Australia.
There, about 52% of the women in this study received the Tdap vaccine through a maternal pertussis vaccination program.
Duration of effectiveness in infants was one of the main questions the study sought to answer.
The authors wrote that they assessed vaccine effectiveness through 18 months of age. “We observed significant protection against disease until at least 8 months of age, 2 months longer than reported in previous studies.” From 70% to 90% of all pertussis-attributable hospitalizations and death occur in infancy.
Answering the ‘blunting’ question
This study also set out to clarify an important clinical question regarding a potential “blunting” effect in infants. Previous work had suggested that maternal antibodies from the vaccination could interfere with the effectiveness of infants’ DtaP (the version of Tdap for infants) and other vaccines.
Dr. Regan and colleagues found that, “although we observed slightly lower VE [vaccine effectiveness] point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002), we did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% confidence interval, 0.61-3.39).
Best time to give mothers the vaccine
Another clinical debate has centered on when to give the mother the vaccine during pregnancy. The authors concluded: “Our findings support the infant health benefits of recommendations to administer a booster dose of pertussis vaccine near 28 weeks of gestational age.”
That 28-week mark was associated with lower risk of infection in infants through 8 months of age, they wrote.
Positive results in the United States
In an invited commentary, Kathryn M. Edwards, MD, with the division of infectious diseases, department of pediatrics, at Vanderbilt University Medical Center, Nashville, Tenn., highlighted similar positive findings for maternal pertussis vaccination in the United States.
The Centers for Disease Control and Prevention did an ecologic study of infant pertussis cases reported between Jan. 1, 2000, and Dec. 31, 2019. Rates were compared for the years before maternal Tdap vaccinations were recommended against the 7-year period after they were implemented.
That study found that in the period before maternal Tdap vaccination, annual pertussis incidence did not change among infants younger than 2 months and increased slightly in infants 6-12 months.
However, during the period after maternal Tdap vaccination had started (2012-2019), pertussis incidence significantly decreased in infants younger than 2 months and was unchanged in infants 6-12 months.
“As with the Australian data, the U.S. data support the overall benefit of the maternal Tdap program and, as with the Australian data, do not suggest that blunting has led to an increase in cases within the first year of life,” Dr. Edwards wrote.
The CDC notes that pertussis cases are rising and outbreaks are happening across the United States.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” the CDC states.
Uptake low despite positive data
Dr. Edwards noted that, despite positive data supporting maternal vaccination to reduce pertussis, uptake rates are low – between 50% and 60% in Australia, the United Kingdom, and the United States. “Active engagement to increase these rates should be implemented.”
Maternal vaccination might also be implemented soon to protect against other diseases including respiratory syncytial virus and group B streptococcal disease after promising study data, she said.
As with pertussis, the potential “blunting” effect will need to be carefully monitored, she said, “as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics.”
One coauthor has received institutional honoraria for participation in advisory groups for Merck Sharpe & Dohme and Pfizer unrelated to this work. Another coauthor was supported by scholarships provided by the Wesfarmers Centre of Vaccines and Infectious Disease at the Telethon Kids Institute. Dr. Edwards reported receiving grants from the CDC and consulting for Bionet, Dynavax, and IBM. She is a member of the data safety and monitoring board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and Brighton Collaboration.
FROM PEDIATRICS
FDA approves topical roflumilast for psoriasis in children aged 6-11
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
Precision medicine takes individual approach to diabetes
HAMBURG, GERMANY –
“Diabetes recommendations often focus on what works well for the average person. However, because diabetes is an incredibly heterogeneous disease, few people are Mr. or Mrs. ‘average’ and one-size-fits-all approaches fail many people in need. Precision medicine seeks to address this major problem,” said Precision Medicine in Diabetes Initiative (PDMI) cochair Paul Franks, PhD, MPhil, head of the department of translational medicine at the Novo Nordisk Foundation in Denmark.
The report is the second from the joint American Diabetes Association/European Association for the Study of Diabetes PDMI, a consortium organized in 2018 with the aim of addressing “the untenable health and economic burdens of diabetes prevention and care.”
Based on findings from 15 systematic reviews and expert opinions, the new statement covers the key precision medicine pillars of prevention, diagnosis, treatment, and prognosis for each of four major recognized forms of diabetes: monogenic, gestational, type 1, and type 2. It addresses clinical translation of precision medicine research, including near-term actionable measures. Working groups were tasked with defining the key research questions that need to be addressed for precision diabetes medicine to be implemented into clinical practice by 2030.
Dr. Franks noted that “precision medicine seeks to improve diabetes prevention and care by combining data about a person’s health or disease state and response to medications. The aim is to tailor the advice given about diabetes prevention or treatment to the person in question, rather than having them make do with generic advice. Precision medicine very much focuses on treating the person and not the disease.”
A 90-minute symposium summarizing the report was presented at the annual meeting of the European Association for the Study of Diabetes. An executive summary was simultaneously published in the journal Nature Medicine. Four additional complementary papers, covering cardiometabolic disease precision medicine, diabetes heterogeneity, precision medicine of obesity, and precision cardiometabolic medicine in low- and middle-income countries, were published separately in The Lancet Diabetes & Endocrinology.
In a comment, Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, England, called the new report “fantastic collaborative work.”
However, Dr. Khunti said, “I think at the moment we’re at the discovery stage of precision medicine. The clinical utility of that, we’ll have to see over the years.”
Dr. Khunti also pointed out: “A lot of the work done in precision medicine has been on specific diseases, like diabetes and cardiovascular disease. But, 30% of people don’t just have one disease, they have multiple long-term conditions. I think we need to start thinking about that now, rather than single conditions, because we want to look at drug targets that will hit multiple long-term conditions rather than one single condition.”
Currently, a dearth of data
Even just within diabetes, there is a dearth of quality data. In fact, Dr. Franks told this news organization, there has only been one precision medicine trial in diabetes, called TriMaster, comparing individual responses to three different second-line treatments for type 2 diabetes after metformin. “The problem with that trial is that the second-line medications it investigated aren’t widely prescribed now. The trial was designed back in 2014. It took a long time, then there was COVID, and by the time it was published too much time had elapsed and it was already out of date.”
Ideally, to make this effort current, Dr. Franks said, “is to get drug companies to implement these trials into their development pipelines. If you think about it, it’s far more efficient to implement precision medicine early in the drug development process than late, because when you do it late you end up having to do lots of comparisons of different possibilities. When you do it early you sort out those comparisons as part of the development process, so it really comes down to companies being willing to do that and regulators being willing to accept results from those trials. That’s another challenge, which is why we stress regulatory engagement as a key thing.”
In the future, he said, using the second-line type 2 diabetes drug as an example, when a person is diagnosed with type 2 diabetes they might automatically be given a companion diagnostic that’s more sophisticated and more precise than current ways of defining cardiovascular risk to better predict which individuals are more likely to experience a cardiovascular event.
This concept, referred to as “precision diagnostics,” is a “core driver of precision medicine,” Dr. Franks said. “If we can get a higher predictive accuracy on cardiovascular outcomes in people with diabetes, essentially treatment allocation is likely to be more precise too, because you’re not treating people you don’t need to treat and you’re not missing people you should have treated. I think that’s probably how it will work out.”
‘Studying diverse populations benefits everyone’
An important component emphasized in the report is the lack of “relevant, high-quality research in people of non-European ancestry, hindering the development and implementation of precision diabetes medicine in many of the most heavily burdened populations worldwide.”
That specific issue was addressed during the symposium by Shivani Misra, MBBS, PhD clinical senior lecturer in diabetes and endocrinology at Imperial College, London, and the lead author of the separate complementary paper on the topic.
Dr. Misra argued against the notion that precision medicine is only for wealthy countries, noting that diabetes and other noncommunicable diseases are becoming major health problems in low- and middle-income countries. “Resource-restricted settings may derive the greatest benefits from precision medicine,” she said. “Studying diverse populations benefits everyone.”
And worldwide, she noted, “the right drug for the right person will improve cost-effectiveness in the long-term.”
Dr. Franks is an employee of the Novo Nordisk Foundation, a “purely philanthropic enterprise-owning foundation” with a portfolio of 151 companies. He has received consultancy fees from Zoe Ltd., Eli Lilly, and Novo Nordisk, and research funding from multiple pharmaceutical companies. Dr. Khunti has acted as a consultant, speaker, or received grants for investigator-initiated studies from AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie/Menarini Group, Janssen, and Napp. Dr. Misra has received speaker fees from Sanofi and ABCD and an investigator-initiated research grant from Dexcom, and is a trustee for the Diabetes Research and Wellness Foundation.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY –
“Diabetes recommendations often focus on what works well for the average person. However, because diabetes is an incredibly heterogeneous disease, few people are Mr. or Mrs. ‘average’ and one-size-fits-all approaches fail many people in need. Precision medicine seeks to address this major problem,” said Precision Medicine in Diabetes Initiative (PDMI) cochair Paul Franks, PhD, MPhil, head of the department of translational medicine at the Novo Nordisk Foundation in Denmark.
The report is the second from the joint American Diabetes Association/European Association for the Study of Diabetes PDMI, a consortium organized in 2018 with the aim of addressing “the untenable health and economic burdens of diabetes prevention and care.”
Based on findings from 15 systematic reviews and expert opinions, the new statement covers the key precision medicine pillars of prevention, diagnosis, treatment, and prognosis for each of four major recognized forms of diabetes: monogenic, gestational, type 1, and type 2. It addresses clinical translation of precision medicine research, including near-term actionable measures. Working groups were tasked with defining the key research questions that need to be addressed for precision diabetes medicine to be implemented into clinical practice by 2030.
Dr. Franks noted that “precision medicine seeks to improve diabetes prevention and care by combining data about a person’s health or disease state and response to medications. The aim is to tailor the advice given about diabetes prevention or treatment to the person in question, rather than having them make do with generic advice. Precision medicine very much focuses on treating the person and not the disease.”
A 90-minute symposium summarizing the report was presented at the annual meeting of the European Association for the Study of Diabetes. An executive summary was simultaneously published in the journal Nature Medicine. Four additional complementary papers, covering cardiometabolic disease precision medicine, diabetes heterogeneity, precision medicine of obesity, and precision cardiometabolic medicine in low- and middle-income countries, were published separately in The Lancet Diabetes & Endocrinology.
In a comment, Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, England, called the new report “fantastic collaborative work.”
However, Dr. Khunti said, “I think at the moment we’re at the discovery stage of precision medicine. The clinical utility of that, we’ll have to see over the years.”
Dr. Khunti also pointed out: “A lot of the work done in precision medicine has been on specific diseases, like diabetes and cardiovascular disease. But, 30% of people don’t just have one disease, they have multiple long-term conditions. I think we need to start thinking about that now, rather than single conditions, because we want to look at drug targets that will hit multiple long-term conditions rather than one single condition.”
Currently, a dearth of data
Even just within diabetes, there is a dearth of quality data. In fact, Dr. Franks told this news organization, there has only been one precision medicine trial in diabetes, called TriMaster, comparing individual responses to three different second-line treatments for type 2 diabetes after metformin. “The problem with that trial is that the second-line medications it investigated aren’t widely prescribed now. The trial was designed back in 2014. It took a long time, then there was COVID, and by the time it was published too much time had elapsed and it was already out of date.”
Ideally, to make this effort current, Dr. Franks said, “is to get drug companies to implement these trials into their development pipelines. If you think about it, it’s far more efficient to implement precision medicine early in the drug development process than late, because when you do it late you end up having to do lots of comparisons of different possibilities. When you do it early you sort out those comparisons as part of the development process, so it really comes down to companies being willing to do that and regulators being willing to accept results from those trials. That’s another challenge, which is why we stress regulatory engagement as a key thing.”
In the future, he said, using the second-line type 2 diabetes drug as an example, when a person is diagnosed with type 2 diabetes they might automatically be given a companion diagnostic that’s more sophisticated and more precise than current ways of defining cardiovascular risk to better predict which individuals are more likely to experience a cardiovascular event.
This concept, referred to as “precision diagnostics,” is a “core driver of precision medicine,” Dr. Franks said. “If we can get a higher predictive accuracy on cardiovascular outcomes in people with diabetes, essentially treatment allocation is likely to be more precise too, because you’re not treating people you don’t need to treat and you’re not missing people you should have treated. I think that’s probably how it will work out.”
‘Studying diverse populations benefits everyone’
An important component emphasized in the report is the lack of “relevant, high-quality research in people of non-European ancestry, hindering the development and implementation of precision diabetes medicine in many of the most heavily burdened populations worldwide.”
That specific issue was addressed during the symposium by Shivani Misra, MBBS, PhD clinical senior lecturer in diabetes and endocrinology at Imperial College, London, and the lead author of the separate complementary paper on the topic.
Dr. Misra argued against the notion that precision medicine is only for wealthy countries, noting that diabetes and other noncommunicable diseases are becoming major health problems in low- and middle-income countries. “Resource-restricted settings may derive the greatest benefits from precision medicine,” she said. “Studying diverse populations benefits everyone.”
And worldwide, she noted, “the right drug for the right person will improve cost-effectiveness in the long-term.”
Dr. Franks is an employee of the Novo Nordisk Foundation, a “purely philanthropic enterprise-owning foundation” with a portfolio of 151 companies. He has received consultancy fees from Zoe Ltd., Eli Lilly, and Novo Nordisk, and research funding from multiple pharmaceutical companies. Dr. Khunti has acted as a consultant, speaker, or received grants for investigator-initiated studies from AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie/Menarini Group, Janssen, and Napp. Dr. Misra has received speaker fees from Sanofi and ABCD and an investigator-initiated research grant from Dexcom, and is a trustee for the Diabetes Research and Wellness Foundation.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY –
“Diabetes recommendations often focus on what works well for the average person. However, because diabetes is an incredibly heterogeneous disease, few people are Mr. or Mrs. ‘average’ and one-size-fits-all approaches fail many people in need. Precision medicine seeks to address this major problem,” said Precision Medicine in Diabetes Initiative (PDMI) cochair Paul Franks, PhD, MPhil, head of the department of translational medicine at the Novo Nordisk Foundation in Denmark.
The report is the second from the joint American Diabetes Association/European Association for the Study of Diabetes PDMI, a consortium organized in 2018 with the aim of addressing “the untenable health and economic burdens of diabetes prevention and care.”
Based on findings from 15 systematic reviews and expert opinions, the new statement covers the key precision medicine pillars of prevention, diagnosis, treatment, and prognosis for each of four major recognized forms of diabetes: monogenic, gestational, type 1, and type 2. It addresses clinical translation of precision medicine research, including near-term actionable measures. Working groups were tasked with defining the key research questions that need to be addressed for precision diabetes medicine to be implemented into clinical practice by 2030.
Dr. Franks noted that “precision medicine seeks to improve diabetes prevention and care by combining data about a person’s health or disease state and response to medications. The aim is to tailor the advice given about diabetes prevention or treatment to the person in question, rather than having them make do with generic advice. Precision medicine very much focuses on treating the person and not the disease.”
A 90-minute symposium summarizing the report was presented at the annual meeting of the European Association for the Study of Diabetes. An executive summary was simultaneously published in the journal Nature Medicine. Four additional complementary papers, covering cardiometabolic disease precision medicine, diabetes heterogeneity, precision medicine of obesity, and precision cardiometabolic medicine in low- and middle-income countries, were published separately in The Lancet Diabetes & Endocrinology.
In a comment, Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, England, called the new report “fantastic collaborative work.”
However, Dr. Khunti said, “I think at the moment we’re at the discovery stage of precision medicine. The clinical utility of that, we’ll have to see over the years.”
Dr. Khunti also pointed out: “A lot of the work done in precision medicine has been on specific diseases, like diabetes and cardiovascular disease. But, 30% of people don’t just have one disease, they have multiple long-term conditions. I think we need to start thinking about that now, rather than single conditions, because we want to look at drug targets that will hit multiple long-term conditions rather than one single condition.”
Currently, a dearth of data
Even just within diabetes, there is a dearth of quality data. In fact, Dr. Franks told this news organization, there has only been one precision medicine trial in diabetes, called TriMaster, comparing individual responses to three different second-line treatments for type 2 diabetes after metformin. “The problem with that trial is that the second-line medications it investigated aren’t widely prescribed now. The trial was designed back in 2014. It took a long time, then there was COVID, and by the time it was published too much time had elapsed and it was already out of date.”
Ideally, to make this effort current, Dr. Franks said, “is to get drug companies to implement these trials into their development pipelines. If you think about it, it’s far more efficient to implement precision medicine early in the drug development process than late, because when you do it late you end up having to do lots of comparisons of different possibilities. When you do it early you sort out those comparisons as part of the development process, so it really comes down to companies being willing to do that and regulators being willing to accept results from those trials. That’s another challenge, which is why we stress regulatory engagement as a key thing.”
In the future, he said, using the second-line type 2 diabetes drug as an example, when a person is diagnosed with type 2 diabetes they might automatically be given a companion diagnostic that’s more sophisticated and more precise than current ways of defining cardiovascular risk to better predict which individuals are more likely to experience a cardiovascular event.
This concept, referred to as “precision diagnostics,” is a “core driver of precision medicine,” Dr. Franks said. “If we can get a higher predictive accuracy on cardiovascular outcomes in people with diabetes, essentially treatment allocation is likely to be more precise too, because you’re not treating people you don’t need to treat and you’re not missing people you should have treated. I think that’s probably how it will work out.”
‘Studying diverse populations benefits everyone’
An important component emphasized in the report is the lack of “relevant, high-quality research in people of non-European ancestry, hindering the development and implementation of precision diabetes medicine in many of the most heavily burdened populations worldwide.”
That specific issue was addressed during the symposium by Shivani Misra, MBBS, PhD clinical senior lecturer in diabetes and endocrinology at Imperial College, London, and the lead author of the separate complementary paper on the topic.
Dr. Misra argued against the notion that precision medicine is only for wealthy countries, noting that diabetes and other noncommunicable diseases are becoming major health problems in low- and middle-income countries. “Resource-restricted settings may derive the greatest benefits from precision medicine,” she said. “Studying diverse populations benefits everyone.”
And worldwide, she noted, “the right drug for the right person will improve cost-effectiveness in the long-term.”
Dr. Franks is an employee of the Novo Nordisk Foundation, a “purely philanthropic enterprise-owning foundation” with a portfolio of 151 companies. He has received consultancy fees from Zoe Ltd., Eli Lilly, and Novo Nordisk, and research funding from multiple pharmaceutical companies. Dr. Khunti has acted as a consultant, speaker, or received grants for investigator-initiated studies from AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie/Menarini Group, Janssen, and Napp. Dr. Misra has received speaker fees from Sanofi and ABCD and an investigator-initiated research grant from Dexcom, and is a trustee for the Diabetes Research and Wellness Foundation.
A version of this article first appeared on Medscape.com.
FROM EASD 2023
FDA approves ninth Humira biosimilar, with interchangeability
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.

