User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
First reported U.S. case of COVID-19 linked to Guillain-Barré syndrome
further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case of COVID-19 that initially presented as acute GBS. The patient was a 61-year-old woman returning home from Wuhan during the pandemic.
Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
The first U.S. case is described in the June issue of the Journal of Clinical Neuromuscular Disease.
Like cases from China and Italy, the U.S. patient’s symptoms of GBS reportedly occurred within days of being infected with SARS-CoV-2. “This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted.
The 54-year-old man was transferred to Allegheny General Hospital after developing ascending limb weakness and numbness that followed symptoms of a respiratory infection. Two weeks earlier, he initially developed rhinorrhea, odynophagia, fevers, chills, and night sweats. The man reported that his wife had tested positive for COVID-19 and that his symptoms started soon after her illness. The man also tested positive for COVID-19.
His deficits were characterized by quadriparesis and areflexia, burning dysesthesias, mild ophthalmoparesis, and dysautonomia. He did not have the loss of smell and taste documented in other COVID-19 patients. He briefly required mechanical ventilation and was successfully weaned after receiving a course of intravenous immunoglobulin.
Compared with other cases reported in the literature, the unique clinical features in the U.S. case are urinary retention secondary to dysautonomia and ocular symptoms of diplopia. These highlight the variability in the clinical presentation of GBS associated with COVID-19, the researchers noted.
They added that, with the Pittsburgh patient, electrophysiological findings were typical of demyelinating polyneuropathy seen in patients with GBS. The case series from Italy suggests that axonal variants could be as common in COVID-19–associated GBS.
“Although the number of documented cases internationally is notably small to date, it’s not completely surprising that a COVID-19 diagnosis may lead to a patient developing GBS. The increase of inflammation and inflammatory cells caused by the infection may trigger an irregular immune response that leads to the hallmark symptoms of this neurological disorder,” Dr. Rana said in a news release.
“Since GBS can significantly affect the respiratory system and other vital organs being pushed into overdrive during a COVID-19 immune response, it will be critically important to further investigate and understand this potential connection,” he added.
A version of this article originally appeared on Medscape.com.
further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case of COVID-19 that initially presented as acute GBS. The patient was a 61-year-old woman returning home from Wuhan during the pandemic.
Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
The first U.S. case is described in the June issue of the Journal of Clinical Neuromuscular Disease.
Like cases from China and Italy, the U.S. patient’s symptoms of GBS reportedly occurred within days of being infected with SARS-CoV-2. “This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted.
The 54-year-old man was transferred to Allegheny General Hospital after developing ascending limb weakness and numbness that followed symptoms of a respiratory infection. Two weeks earlier, he initially developed rhinorrhea, odynophagia, fevers, chills, and night sweats. The man reported that his wife had tested positive for COVID-19 and that his symptoms started soon after her illness. The man also tested positive for COVID-19.
His deficits were characterized by quadriparesis and areflexia, burning dysesthesias, mild ophthalmoparesis, and dysautonomia. He did not have the loss of smell and taste documented in other COVID-19 patients. He briefly required mechanical ventilation and was successfully weaned after receiving a course of intravenous immunoglobulin.
Compared with other cases reported in the literature, the unique clinical features in the U.S. case are urinary retention secondary to dysautonomia and ocular symptoms of diplopia. These highlight the variability in the clinical presentation of GBS associated with COVID-19, the researchers noted.
They added that, with the Pittsburgh patient, electrophysiological findings were typical of demyelinating polyneuropathy seen in patients with GBS. The case series from Italy suggests that axonal variants could be as common in COVID-19–associated GBS.
“Although the number of documented cases internationally is notably small to date, it’s not completely surprising that a COVID-19 diagnosis may lead to a patient developing GBS. The increase of inflammation and inflammatory cells caused by the infection may trigger an irregular immune response that leads to the hallmark symptoms of this neurological disorder,” Dr. Rana said in a news release.
“Since GBS can significantly affect the respiratory system and other vital organs being pushed into overdrive during a COVID-19 immune response, it will be critically important to further investigate and understand this potential connection,” he added.
A version of this article originally appeared on Medscape.com.
further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case of COVID-19 that initially presented as acute GBS. The patient was a 61-year-old woman returning home from Wuhan during the pandemic.
Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
The first U.S. case is described in the June issue of the Journal of Clinical Neuromuscular Disease.
Like cases from China and Italy, the U.S. patient’s symptoms of GBS reportedly occurred within days of being infected with SARS-CoV-2. “This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted.
The 54-year-old man was transferred to Allegheny General Hospital after developing ascending limb weakness and numbness that followed symptoms of a respiratory infection. Two weeks earlier, he initially developed rhinorrhea, odynophagia, fevers, chills, and night sweats. The man reported that his wife had tested positive for COVID-19 and that his symptoms started soon after her illness. The man also tested positive for COVID-19.
His deficits were characterized by quadriparesis and areflexia, burning dysesthesias, mild ophthalmoparesis, and dysautonomia. He did not have the loss of smell and taste documented in other COVID-19 patients. He briefly required mechanical ventilation and was successfully weaned after receiving a course of intravenous immunoglobulin.
Compared with other cases reported in the literature, the unique clinical features in the U.S. case are urinary retention secondary to dysautonomia and ocular symptoms of diplopia. These highlight the variability in the clinical presentation of GBS associated with COVID-19, the researchers noted.
They added that, with the Pittsburgh patient, electrophysiological findings were typical of demyelinating polyneuropathy seen in patients with GBS. The case series from Italy suggests that axonal variants could be as common in COVID-19–associated GBS.
“Although the number of documented cases internationally is notably small to date, it’s not completely surprising that a COVID-19 diagnosis may lead to a patient developing GBS. The increase of inflammation and inflammatory cells caused by the infection may trigger an irregular immune response that leads to the hallmark symptoms of this neurological disorder,” Dr. Rana said in a news release.
“Since GBS can significantly affect the respiratory system and other vital organs being pushed into overdrive during a COVID-19 immune response, it will be critically important to further investigate and understand this potential connection,” he added.
A version of this article originally appeared on Medscape.com.
What COVID-19 has taught us about senior care
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
COVID-19: Medicare data show long hospital stays, disparities
according to a new analysis by the Centers for Medicare & Medicaid Services.
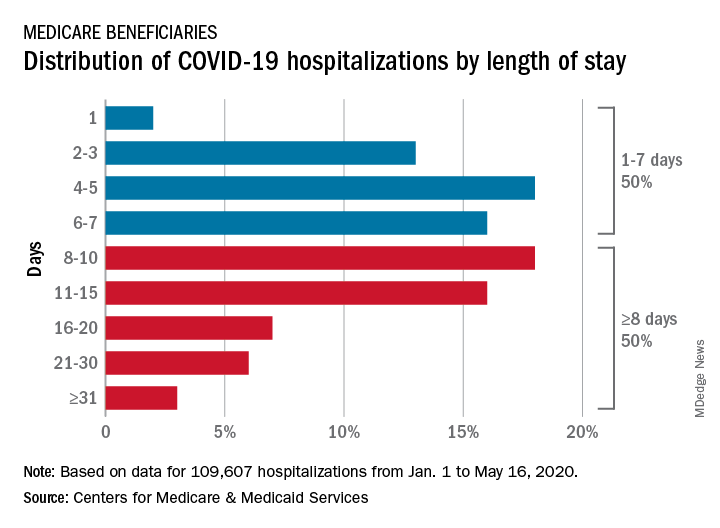
CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
Guidance on infection prevention for health care personnel
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
Cortisol levels on COVID-19 admission may be a marker of severity
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Experts publish imaging recommendations for pediatric COVID-19
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
FROM PEDIATRIC PULMONOLOGY
ED visits for life-threatening conditions declined early in COVID-19 pandemic
ED visits for myocardial infarction, stroke, and hyperglycemic crisis dropped substantially in the 10 weeks after COVID-19 was declared a national emergency on March 13, according to the Centers for Disease Control and Prevention.

Compared with the 10-week period from Jan. 5 to March 14, ED visits were down by 23% for MI, 20% for stroke, and 10% for hyperglycemic crisis from March 15 to May 23, Samantha J. Lange, MPH, and associates at the CDC reported June 22 in the Morbidity and Mortality Weekly Report.
“A short-term decline of this magnitude … is biologically implausible for MI and stroke, especially for older adults, and unlikely for hyperglycemic crisis, and the finding suggests that patients with these conditions either could not access care or were delaying or avoiding seeking care during the early pandemic period,” they wrote.
The largest decreases in the actual number of visits for MI occurred among both men (down by 2,114, –24%) and women (down by 1,459, –25%) aged 65-74 years. For stroke, men aged 65-74 years had 1,406 (–19%) fewer visits to the ED and women 75-84 years had 1,642 (–23%) fewer visits, the CDC researchers said.
For hypoglycemic crisis, the largest declines during the early pandemic period occurred among younger adults: ED visits for men and women aged 18-44 years were down, respectively, by 419 (–8%) and 775 (–16%), they reported based on data from the National Syndromic Surveillance Program.
“Decreases in ED visits for hyperglycemic crisis might be less striking because patient recognition of this crisis is typically augmented by home glucose monitoring and not reliant upon symptoms alone, as is the case for MI and stroke,” Ms. Lange and her associates noted.
Charting weekly visit numbers showed that the drop for all three conditions actually started the week before the emergency was declared and reached its nadir the week after (March 22) for MI and 2 weeks later (March 29) for stroke and hypoglycemic crisis.
Visits for hypoglycemic crisis have largely returned to normal since those low points, but MI and stroke visits “remain below prepandemic levels” despite gradual increases through April and May, they said.
It has been reported that “deaths not associated with confirmed or probable COVID-19 might have been directly or indirectly attributed to the pandemic. The striking decline in ED visits for acute life-threatening conditions might partially explain observed excess mortality not associated with COVID-19,” the investigators wrote.
ED visits for myocardial infarction, stroke, and hyperglycemic crisis dropped substantially in the 10 weeks after COVID-19 was declared a national emergency on March 13, according to the Centers for Disease Control and Prevention.

Compared with the 10-week period from Jan. 5 to March 14, ED visits were down by 23% for MI, 20% for stroke, and 10% for hyperglycemic crisis from March 15 to May 23, Samantha J. Lange, MPH, and associates at the CDC reported June 22 in the Morbidity and Mortality Weekly Report.
“A short-term decline of this magnitude … is biologically implausible for MI and stroke, especially for older adults, and unlikely for hyperglycemic crisis, and the finding suggests that patients with these conditions either could not access care or were delaying or avoiding seeking care during the early pandemic period,” they wrote.
The largest decreases in the actual number of visits for MI occurred among both men (down by 2,114, –24%) and women (down by 1,459, –25%) aged 65-74 years. For stroke, men aged 65-74 years had 1,406 (–19%) fewer visits to the ED and women 75-84 years had 1,642 (–23%) fewer visits, the CDC researchers said.
For hypoglycemic crisis, the largest declines during the early pandemic period occurred among younger adults: ED visits for men and women aged 18-44 years were down, respectively, by 419 (–8%) and 775 (–16%), they reported based on data from the National Syndromic Surveillance Program.
“Decreases in ED visits for hyperglycemic crisis might be less striking because patient recognition of this crisis is typically augmented by home glucose monitoring and not reliant upon symptoms alone, as is the case for MI and stroke,” Ms. Lange and her associates noted.
Charting weekly visit numbers showed that the drop for all three conditions actually started the week before the emergency was declared and reached its nadir the week after (March 22) for MI and 2 weeks later (March 29) for stroke and hypoglycemic crisis.
Visits for hypoglycemic crisis have largely returned to normal since those low points, but MI and stroke visits “remain below prepandemic levels” despite gradual increases through April and May, they said.
It has been reported that “deaths not associated with confirmed or probable COVID-19 might have been directly or indirectly attributed to the pandemic. The striking decline in ED visits for acute life-threatening conditions might partially explain observed excess mortality not associated with COVID-19,” the investigators wrote.
ED visits for myocardial infarction, stroke, and hyperglycemic crisis dropped substantially in the 10 weeks after COVID-19 was declared a national emergency on March 13, according to the Centers for Disease Control and Prevention.

Compared with the 10-week period from Jan. 5 to March 14, ED visits were down by 23% for MI, 20% for stroke, and 10% for hyperglycemic crisis from March 15 to May 23, Samantha J. Lange, MPH, and associates at the CDC reported June 22 in the Morbidity and Mortality Weekly Report.
“A short-term decline of this magnitude … is biologically implausible for MI and stroke, especially for older adults, and unlikely for hyperglycemic crisis, and the finding suggests that patients with these conditions either could not access care or were delaying or avoiding seeking care during the early pandemic period,” they wrote.
The largest decreases in the actual number of visits for MI occurred among both men (down by 2,114, –24%) and women (down by 1,459, –25%) aged 65-74 years. For stroke, men aged 65-74 years had 1,406 (–19%) fewer visits to the ED and women 75-84 years had 1,642 (–23%) fewer visits, the CDC researchers said.
For hypoglycemic crisis, the largest declines during the early pandemic period occurred among younger adults: ED visits for men and women aged 18-44 years were down, respectively, by 419 (–8%) and 775 (–16%), they reported based on data from the National Syndromic Surveillance Program.
“Decreases in ED visits for hyperglycemic crisis might be less striking because patient recognition of this crisis is typically augmented by home glucose monitoring and not reliant upon symptoms alone, as is the case for MI and stroke,” Ms. Lange and her associates noted.
Charting weekly visit numbers showed that the drop for all three conditions actually started the week before the emergency was declared and reached its nadir the week after (March 22) for MI and 2 weeks later (March 29) for stroke and hypoglycemic crisis.
Visits for hypoglycemic crisis have largely returned to normal since those low points, but MI and stroke visits “remain below prepandemic levels” despite gradual increases through April and May, they said.
It has been reported that “deaths not associated with confirmed or probable COVID-19 might have been directly or indirectly attributed to the pandemic. The striking decline in ED visits for acute life-threatening conditions might partially explain observed excess mortality not associated with COVID-19,” the investigators wrote.
FROM MMWR
‘Collateral damage’: COVID-19 threatens patients with COPD
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
FROM CHEST
Where does dexamethasone fit in with diabetic ketoacidosis in COVID-19?
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
After the ICU: A ‘fraternity of people who are struggling’
By the time she was discharged from a suburban New Jersey hospital on April 10, Kathleen Ronan thought the worst was behind her. For a week before her husband rushed her to the emergency department (ED), incoherent and struggling to breathe, the novel coronavirus had ravaged her body. She tried to treat her fevers with acetaminophen and ice packs. Despite taking enough Tylenol to risk liver damage and packing herself on ice like the catch of the day, Ronan’s fever continued to rise. By the time her temperature reached 104.5° F, Ronan knew the time had come for more drastic measures.
A team of masked and gowned nurses greeted her at a triage tent outside the ED, and from there, everything becomes hazy for Ronan. She was immediately rushed to the hospital’s special COVID-19 intensive care unit (ICU), where she spent 5 days. But she has few distinct memories from this time. What she does remember is the exhaustion, the pain, the loneliness, and the fear. Her family couldn’t visit, and though Ronan works as a home health nurse, her brain was so addled with fever that she couldn’t make sense of what was happening. After a week in the hospital, 5 days of which were spent in the ICU, 51-year-old Ronan was discharged.
Her years of working as a home health nurse told her that the return home wouldn’t be easy, but nothing prepared her for just how much she would struggle. The once-active Ronan, who had supplemented long days on her feet caring for others as a nurse with regular trips to the gym, now needed a walker to traverse the few steps from her bed to the toilet, an effort that left her gasping for air. Her brain couldn’t even focus on an audiobook, let alone a short magazine article.
“It just completely knocked the stuffing out of me,” Ronan said.
Ronan’s lingering symptoms aren’t unique to COVID-19 patients. In as many as 80% of patients leaving the ICU, . Although underlying illness plays a role in these symptoms, the amount of time spent in critical care is a major factor.
Nor is PICS simply a set of side effects that will go away on their own. It includes ongoing cognitive difficulties and physical weakness, both of which can lead to employment problems. Beyond that, depression and anxiety can exacerbate – and be exacerbated by – these challenges. Psychologist Jim Jackson, PsyD, assistant director of the ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tennessee, recently spoke with a former ICU patient who has struggled since her discharge 30 years ago.
“Her life essentially stopped with her critical care stay. She hasn’t been able to move forward,” he said. “She’s part of a whole fraternity of people who are struggling.”
The good news is that over the past decade, researchers have made important strides in understanding what makes PICS symptoms worse and how critical care physicians can tweak ICU protocols to reduce PICS severity. Practitioners will need to draw on this knowledge to help Ronan and the thousands of COVID-19 ICU patients like her.
Surviving the ICU
Although the new coronavirus has pushed the world’s critical care system to its limits, it was an outbreak in 1952 that inspired the creation of intensive care units. That summer, a wave of paralytic polio swept over Copenhagen, Denmark, and anesthesiologist Bjørn Ibsen, MD, PhD, used mechanical ventilation — physically operated by medical and dental students – to help 316 children breathe for weeks at a time while their small bodies worked to fight off the virus. The effort halved the mortality rate from polio that affected breathing, from 80% to 40%.
In these wards, dedicated to the very sickest, each patient was assigned his or her own nurse. Over the next decade, hospitals in the United Kingdom and the United States established their own ICUs to treat patients with a variety of conditions. Although it helped improve survival, mortality rates in critical care units remained stubbornly high, owing to the patients’ severe underlying illnesses.
“We thought we were doing a good job if the patient survived, but we had no idea what happened after discharge,” said Carla Sevin, MD, medical director of Vanderbilt’s ICU Recovery Center. Nor did their efforts to find out always bring answers. “We struggled to get people to come in for support — they were debilitated, physically burdened, and weak.”
Through further advances in life support, by the early 2000s, the average mortality rates in American ICUs had dropped to 8% to 19%. As the number of critical care survivors began to climb, clinical researchers noticed that the lives of these patients and their families were profoundly altered by their severe illness.
As Dale Needham, MD, PhD, began his pulmonology and critical care residency in Toronto, Canada, in 2005, a group of physicians there began a 5-year longitudinal study to assess long-term outcomes of patients who developed acute respiratory distress syndrome (ARDS). Although ARDS is an acute condition, the investigators found that patients felt effects for years. Younger patients recovered better than older ones, but none of the patients› physical functioning was equivalent to that of age-matched control persons. Even 5 years later, former ICU patients only reached 76% of expected physical functioning, according to results published in the New England Journal of Medicine. The study was a wake-up call.
At a meeting in Chicago in 2010, Needham, now an intensivist at Johns Hopkins Hospital in Baltimore, Maryland, gathered an interdisciplinary group of colleagues, including patients and caregivers, to clarify the phenomena they were seeing. What emerged from that meeting, published in 2012 in Critical Care Medicine, were the diagnostic criteria for PICS: According to the new definition, PICS is characterized by new or worsening physical and neuropsychiatric deficits that range from forgetfulness and loss of motivation to physical weakness and insomnia.
The issue, Needham says, is that although the trouble starts in the ICU, it only becomes clear once patients leave. “ICU doctors aren’t the ones dealing with this,” Needham said. “We need to build stronger bridges between critical care and other professions.” That’s where PICS comes in, a definition that exists explicitly to alert healthcare providers about the constellation of challenges many of these individuals face as they try to reenter “normal” life.
Defining the problem
As an ICU nurse at the Mayo Clinic in Rochester, Minnesota, Annie Johnson, ACNP-BC, knew lots about helping hospitalized patients, but she says she didn’t know anything about what to do after discharge – at least not until her own mother became a patient.
On the first day of retirement in October 2014, Johnson’s mother flatlined. Quick-thinking paramedics resuscitated her, and after several days in critical care, she was discharged. Since then, her heart has remained healthy. Johnson’s sister, who spent time worrying over her mother at the hospital, also had lingering effects. Both have since struggled, plagued by nightmares, flashbacks, and insomnia.
Johnson initially believed her mom’s and sister’s neuropsychiatric, post-ICU struggles were unique to her family. It was only a year later, at a seminar she was attending, that she first heard the words “post–intensive care syndrome.” Suddenly, Johnson had a name for her family’s experiences, and she began to create support groups and resources to help other families like hers.
“I thought of all the patients I had treated over the years who had been on ventilators for days and days and days. And if this happened to my mom after 48 hours, what must they be going through?” she asked.
Once physicians formally defined PICS, the Society for Critical Care Medicine helped create programs to educate ICU staff, patients, and families about potential post-discharge challenges. Researchers also began to investigate factors affecting post-ICU functioning. Follow-up studies of patients with delirium (ranging from general confusion about time and place to extreme agitation and violence) showed they had striking cognitive deficits. Problems with short-term memory, flexible thinking, and motivation plagued patients for years after their critical illness, similar to the physical deficiencies seen after ARDS. Delirium was one of the strongest risk factors for neuropsychiatric problems.
“Delirium is basically a stress test for the brain,” said Babar Khan, MD, a critical care specialist at Indiana University’s Regenstrief Institute, in Bloomington. But whether delirium accentuates preexisting cognitive difficulties or creates them afresh isn’t yet clear.
Sophia Wang, MD, a geriatric psychiatrist at Indiana University who works with many critical care patients, says patients who had experienced delirium in the ICU showed significant defects in memory and executive functioning long after their hospital stay. She points to a 2015 study that followed 47 ICU patients for a year post discharge. Among those who experienced delirium, brain volumes, as measured by MRI, were smaller at 3 months, something associated with cognitive problems at 1 year. Many struggled at work, and unemployment was common. Depression and posttraumatic stress compounded these difficulties. Among those with acute respiratory distress, ICU patients who are young, female, and unemployed are most likely to suffer from posttraumatic stress disorder after they are discharge.
Critical care medicine may have given these patients a second chance at life, Wang says, but the life they return to often looks nothing like the one they had before their illness.
Prolonged mechanical ventilation and the heavy sedation that often accompanies it are predictors of PICS severity. Some of these links could be explained by the gravity of the illness that landed someone in critical care, but others are more likely to be iatrogenic, says Gerald Weinhouse, MD, a pulmonology and critical care physician and co-director of the Critical Illness Recovery Program at the Brigham and Women’s Hospital in Boston. The involvement of loved ones at the patient’s bedside, however, improved the entire family’s outcome.
When Weinhouse saw those data, he and his colleagues founded a peer support program for ICU survivors. In a study published in 2019 in Critical Care Medicine, they identified six different models for peer support for those with PICS and their families, including both online and in-person approaches. An ongoing challenge for physicians, Weinhouse says, is getting patients to engage with these programs, given that their calendars are crowded with medical appointments and that they suffer from increased physical and mental disability.
Studies such as these led critical care physicians to form the ICU Liberation Collaborative to rethink critical care medicine. At Vanderbilt, Sevin and Jackson headed up one of the world’s first post-ICU clinics, which uses an interdisciplinary team to help patients maximize their functioning. They redesigned their critical care unit in a way that allows families to spend the night and that encourages patient mobility. Both Needham and Weinhouse continue tracking patient outcomes.
Even before the novel coronavirus struck, the United States — and the world — had begun to realize that graduating from the ICU was only the start of what was often an extensive recovery.
The long road back
When COVID-19 patients began flooding intensive care wards around the world, physicians scrambled to meet their complex and desperate acute medical needs. Over the past few months, physicians have focused on keeping these patients alive. “We’ve never seen anything like it ― not even during polio — with the sheer number of patients, all with respiratory distress,” Needham said.
But he and his colleagues know this is only the beginning.
“We’re aware that survivorship issues are coming. There’s going to be a wave of sick people who survived the coronavirus but are going to need more help,” Weinhouse said.
Intensivists have been drawing on PICS research in their fight to help COVID-19 patients. Work from the past few years has shown that although sedation is required during intubation itself, not everyone needs it while on a ventilator. Titrating down sedating medication helps reduce delirium, Wang says. Such medication has been shown to contribute to later cognitive problems. Needham’s studies showing that prolonged bedrest by ICU patients causes muscular atrophy has led him to encourage patients to move as much as possible. With the help of physical therapists, many patients on ventilators can be awake, alert, and moving around the ward.
One of the biggest challenges critical-care coronavirus patients face is prolonged isolation. The constant presence of a familiar face helps orient confused and delirious patients and provides emotional support during a frightening time. But because the immediate need for infection control outweighs these benefits, few hospitals allow visitors, especially for COVID-19 patients.
To address this, some units have been using video technology to allow loved ones to call in. At Johns Hopkins, physicians have also been relying on the expertise of occupational therapists (OTs). Needham says that one OT found that rubbing the hand and back of an agitated, delirious patient helped soothe and calm him better than many medications.
Ronan, who spent 5 days in intensive care, echoes that problem. She says she found the relative lack of human contact to be one of the most challenging parts of being in a bed on a COVID-19 ward. Separated from her husband and daughter, suffering from high fever and severe illness, she lost all track of time.
Her return home was difficult, too. Although her job as a home health nurse had prepared her on some level for the challenges she would face after discharge, Ronan says the hospital provided little practical help.
“Everything is so much harder at home, even little things like going to the bathroom,” she said. “I feel like I’m trying to bail out a sinking ship with a teacup.”
Khan and other physicians, aware of the challenges Ronan and others face once home, aim to create post-ICU clinics specifically for COVID-19 patients. They want to build what Khan calls a “one-stop shop” for all the support patients need to recover. Some of that can be provided via telehealth, which may also help ease the physical burden.
Because there’s so much physicians don’t know about the coronavirus, Johnson says, such clinics are not only a chance to help the sickest COVID-19 patients, they will also help researchers learn more about the virus and improve critical care for other illnesses.
Today, nearly 2 months after discharge, Ronan is back on the job but struggles with a persistent cough — likely due to the lung damage she sustained while ill. She has constant fatigue, as well as ongoing upset stomach from all the medications she took to reduce fever and body aches. When she dons a mask for work, the tangible reminder of her hospital stay sends her into a panic attack. Physically, she’s weaker than before.
Researchers are still trying to understand everything that Ronan and other COVID-19 patients need to move on with their lives after being in the ICU. Mysteries abound, but the ground laid by Sevin, Needham, Weinhouse, and others has provided a solid foundation on which to build.
This article first appeared on Medscape.com.
By the time she was discharged from a suburban New Jersey hospital on April 10, Kathleen Ronan thought the worst was behind her. For a week before her husband rushed her to the emergency department (ED), incoherent and struggling to breathe, the novel coronavirus had ravaged her body. She tried to treat her fevers with acetaminophen and ice packs. Despite taking enough Tylenol to risk liver damage and packing herself on ice like the catch of the day, Ronan’s fever continued to rise. By the time her temperature reached 104.5° F, Ronan knew the time had come for more drastic measures.
A team of masked and gowned nurses greeted her at a triage tent outside the ED, and from there, everything becomes hazy for Ronan. She was immediately rushed to the hospital’s special COVID-19 intensive care unit (ICU), where she spent 5 days. But she has few distinct memories from this time. What she does remember is the exhaustion, the pain, the loneliness, and the fear. Her family couldn’t visit, and though Ronan works as a home health nurse, her brain was so addled with fever that she couldn’t make sense of what was happening. After a week in the hospital, 5 days of which were spent in the ICU, 51-year-old Ronan was discharged.
Her years of working as a home health nurse told her that the return home wouldn’t be easy, but nothing prepared her for just how much she would struggle. The once-active Ronan, who had supplemented long days on her feet caring for others as a nurse with regular trips to the gym, now needed a walker to traverse the few steps from her bed to the toilet, an effort that left her gasping for air. Her brain couldn’t even focus on an audiobook, let alone a short magazine article.
“It just completely knocked the stuffing out of me,” Ronan said.
Ronan’s lingering symptoms aren’t unique to COVID-19 patients. In as many as 80% of patients leaving the ICU, . Although underlying illness plays a role in these symptoms, the amount of time spent in critical care is a major factor.
Nor is PICS simply a set of side effects that will go away on their own. It includes ongoing cognitive difficulties and physical weakness, both of which can lead to employment problems. Beyond that, depression and anxiety can exacerbate – and be exacerbated by – these challenges. Psychologist Jim Jackson, PsyD, assistant director of the ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tennessee, recently spoke with a former ICU patient who has struggled since her discharge 30 years ago.
“Her life essentially stopped with her critical care stay. She hasn’t been able to move forward,” he said. “She’s part of a whole fraternity of people who are struggling.”
The good news is that over the past decade, researchers have made important strides in understanding what makes PICS symptoms worse and how critical care physicians can tweak ICU protocols to reduce PICS severity. Practitioners will need to draw on this knowledge to help Ronan and the thousands of COVID-19 ICU patients like her.
Surviving the ICU
Although the new coronavirus has pushed the world’s critical care system to its limits, it was an outbreak in 1952 that inspired the creation of intensive care units. That summer, a wave of paralytic polio swept over Copenhagen, Denmark, and anesthesiologist Bjørn Ibsen, MD, PhD, used mechanical ventilation — physically operated by medical and dental students – to help 316 children breathe for weeks at a time while their small bodies worked to fight off the virus. The effort halved the mortality rate from polio that affected breathing, from 80% to 40%.
In these wards, dedicated to the very sickest, each patient was assigned his or her own nurse. Over the next decade, hospitals in the United Kingdom and the United States established their own ICUs to treat patients with a variety of conditions. Although it helped improve survival, mortality rates in critical care units remained stubbornly high, owing to the patients’ severe underlying illnesses.
“We thought we were doing a good job if the patient survived, but we had no idea what happened after discharge,” said Carla Sevin, MD, medical director of Vanderbilt’s ICU Recovery Center. Nor did their efforts to find out always bring answers. “We struggled to get people to come in for support — they were debilitated, physically burdened, and weak.”
Through further advances in life support, by the early 2000s, the average mortality rates in American ICUs had dropped to 8% to 19%. As the number of critical care survivors began to climb, clinical researchers noticed that the lives of these patients and their families were profoundly altered by their severe illness.
As Dale Needham, MD, PhD, began his pulmonology and critical care residency in Toronto, Canada, in 2005, a group of physicians there began a 5-year longitudinal study to assess long-term outcomes of patients who developed acute respiratory distress syndrome (ARDS). Although ARDS is an acute condition, the investigators found that patients felt effects for years. Younger patients recovered better than older ones, but none of the patients› physical functioning was equivalent to that of age-matched control persons. Even 5 years later, former ICU patients only reached 76% of expected physical functioning, according to results published in the New England Journal of Medicine. The study was a wake-up call.
At a meeting in Chicago in 2010, Needham, now an intensivist at Johns Hopkins Hospital in Baltimore, Maryland, gathered an interdisciplinary group of colleagues, including patients and caregivers, to clarify the phenomena they were seeing. What emerged from that meeting, published in 2012 in Critical Care Medicine, were the diagnostic criteria for PICS: According to the new definition, PICS is characterized by new or worsening physical and neuropsychiatric deficits that range from forgetfulness and loss of motivation to physical weakness and insomnia.
The issue, Needham says, is that although the trouble starts in the ICU, it only becomes clear once patients leave. “ICU doctors aren’t the ones dealing with this,” Needham said. “We need to build stronger bridges between critical care and other professions.” That’s where PICS comes in, a definition that exists explicitly to alert healthcare providers about the constellation of challenges many of these individuals face as they try to reenter “normal” life.
Defining the problem
As an ICU nurse at the Mayo Clinic in Rochester, Minnesota, Annie Johnson, ACNP-BC, knew lots about helping hospitalized patients, but she says she didn’t know anything about what to do after discharge – at least not until her own mother became a patient.
On the first day of retirement in October 2014, Johnson’s mother flatlined. Quick-thinking paramedics resuscitated her, and after several days in critical care, she was discharged. Since then, her heart has remained healthy. Johnson’s sister, who spent time worrying over her mother at the hospital, also had lingering effects. Both have since struggled, plagued by nightmares, flashbacks, and insomnia.
Johnson initially believed her mom’s and sister’s neuropsychiatric, post-ICU struggles were unique to her family. It was only a year later, at a seminar she was attending, that she first heard the words “post–intensive care syndrome.” Suddenly, Johnson had a name for her family’s experiences, and she began to create support groups and resources to help other families like hers.
“I thought of all the patients I had treated over the years who had been on ventilators for days and days and days. And if this happened to my mom after 48 hours, what must they be going through?” she asked.
Once physicians formally defined PICS, the Society for Critical Care Medicine helped create programs to educate ICU staff, patients, and families about potential post-discharge challenges. Researchers also began to investigate factors affecting post-ICU functioning. Follow-up studies of patients with delirium (ranging from general confusion about time and place to extreme agitation and violence) showed they had striking cognitive deficits. Problems with short-term memory, flexible thinking, and motivation plagued patients for years after their critical illness, similar to the physical deficiencies seen after ARDS. Delirium was one of the strongest risk factors for neuropsychiatric problems.
“Delirium is basically a stress test for the brain,” said Babar Khan, MD, a critical care specialist at Indiana University’s Regenstrief Institute, in Bloomington. But whether delirium accentuates preexisting cognitive difficulties or creates them afresh isn’t yet clear.
Sophia Wang, MD, a geriatric psychiatrist at Indiana University who works with many critical care patients, says patients who had experienced delirium in the ICU showed significant defects in memory and executive functioning long after their hospital stay. She points to a 2015 study that followed 47 ICU patients for a year post discharge. Among those who experienced delirium, brain volumes, as measured by MRI, were smaller at 3 months, something associated with cognitive problems at 1 year. Many struggled at work, and unemployment was common. Depression and posttraumatic stress compounded these difficulties. Among those with acute respiratory distress, ICU patients who are young, female, and unemployed are most likely to suffer from posttraumatic stress disorder after they are discharge.
Critical care medicine may have given these patients a second chance at life, Wang says, but the life they return to often looks nothing like the one they had before their illness.
Prolonged mechanical ventilation and the heavy sedation that often accompanies it are predictors of PICS severity. Some of these links could be explained by the gravity of the illness that landed someone in critical care, but others are more likely to be iatrogenic, says Gerald Weinhouse, MD, a pulmonology and critical care physician and co-director of the Critical Illness Recovery Program at the Brigham and Women’s Hospital in Boston. The involvement of loved ones at the patient’s bedside, however, improved the entire family’s outcome.
When Weinhouse saw those data, he and his colleagues founded a peer support program for ICU survivors. In a study published in 2019 in Critical Care Medicine, they identified six different models for peer support for those with PICS and their families, including both online and in-person approaches. An ongoing challenge for physicians, Weinhouse says, is getting patients to engage with these programs, given that their calendars are crowded with medical appointments and that they suffer from increased physical and mental disability.
Studies such as these led critical care physicians to form the ICU Liberation Collaborative to rethink critical care medicine. At Vanderbilt, Sevin and Jackson headed up one of the world’s first post-ICU clinics, which uses an interdisciplinary team to help patients maximize their functioning. They redesigned their critical care unit in a way that allows families to spend the night and that encourages patient mobility. Both Needham and Weinhouse continue tracking patient outcomes.
Even before the novel coronavirus struck, the United States — and the world — had begun to realize that graduating from the ICU was only the start of what was often an extensive recovery.
The long road back
When COVID-19 patients began flooding intensive care wards around the world, physicians scrambled to meet their complex and desperate acute medical needs. Over the past few months, physicians have focused on keeping these patients alive. “We’ve never seen anything like it ― not even during polio — with the sheer number of patients, all with respiratory distress,” Needham said.
But he and his colleagues know this is only the beginning.
“We’re aware that survivorship issues are coming. There’s going to be a wave of sick people who survived the coronavirus but are going to need more help,” Weinhouse said.
Intensivists have been drawing on PICS research in their fight to help COVID-19 patients. Work from the past few years has shown that although sedation is required during intubation itself, not everyone needs it while on a ventilator. Titrating down sedating medication helps reduce delirium, Wang says. Such medication has been shown to contribute to later cognitive problems. Needham’s studies showing that prolonged bedrest by ICU patients causes muscular atrophy has led him to encourage patients to move as much as possible. With the help of physical therapists, many patients on ventilators can be awake, alert, and moving around the ward.
One of the biggest challenges critical-care coronavirus patients face is prolonged isolation. The constant presence of a familiar face helps orient confused and delirious patients and provides emotional support during a frightening time. But because the immediate need for infection control outweighs these benefits, few hospitals allow visitors, especially for COVID-19 patients.
To address this, some units have been using video technology to allow loved ones to call in. At Johns Hopkins, physicians have also been relying on the expertise of occupational therapists (OTs). Needham says that one OT found that rubbing the hand and back of an agitated, delirious patient helped soothe and calm him better than many medications.
Ronan, who spent 5 days in intensive care, echoes that problem. She says she found the relative lack of human contact to be one of the most challenging parts of being in a bed on a COVID-19 ward. Separated from her husband and daughter, suffering from high fever and severe illness, she lost all track of time.
Her return home was difficult, too. Although her job as a home health nurse had prepared her on some level for the challenges she would face after discharge, Ronan says the hospital provided little practical help.
“Everything is so much harder at home, even little things like going to the bathroom,” she said. “I feel like I’m trying to bail out a sinking ship with a teacup.”
Khan and other physicians, aware of the challenges Ronan and others face once home, aim to create post-ICU clinics specifically for COVID-19 patients. They want to build what Khan calls a “one-stop shop” for all the support patients need to recover. Some of that can be provided via telehealth, which may also help ease the physical burden.
Because there’s so much physicians don’t know about the coronavirus, Johnson says, such clinics are not only a chance to help the sickest COVID-19 patients, they will also help researchers learn more about the virus and improve critical care for other illnesses.
Today, nearly 2 months after discharge, Ronan is back on the job but struggles with a persistent cough — likely due to the lung damage she sustained while ill. She has constant fatigue, as well as ongoing upset stomach from all the medications she took to reduce fever and body aches. When she dons a mask for work, the tangible reminder of her hospital stay sends her into a panic attack. Physically, she’s weaker than before.
Researchers are still trying to understand everything that Ronan and other COVID-19 patients need to move on with their lives after being in the ICU. Mysteries abound, but the ground laid by Sevin, Needham, Weinhouse, and others has provided a solid foundation on which to build.
This article first appeared on Medscape.com.
By the time she was discharged from a suburban New Jersey hospital on April 10, Kathleen Ronan thought the worst was behind her. For a week before her husband rushed her to the emergency department (ED), incoherent and struggling to breathe, the novel coronavirus had ravaged her body. She tried to treat her fevers with acetaminophen and ice packs. Despite taking enough Tylenol to risk liver damage and packing herself on ice like the catch of the day, Ronan’s fever continued to rise. By the time her temperature reached 104.5° F, Ronan knew the time had come for more drastic measures.
A team of masked and gowned nurses greeted her at a triage tent outside the ED, and from there, everything becomes hazy for Ronan. She was immediately rushed to the hospital’s special COVID-19 intensive care unit (ICU), where she spent 5 days. But she has few distinct memories from this time. What she does remember is the exhaustion, the pain, the loneliness, and the fear. Her family couldn’t visit, and though Ronan works as a home health nurse, her brain was so addled with fever that she couldn’t make sense of what was happening. After a week in the hospital, 5 days of which were spent in the ICU, 51-year-old Ronan was discharged.
Her years of working as a home health nurse told her that the return home wouldn’t be easy, but nothing prepared her for just how much she would struggle. The once-active Ronan, who had supplemented long days on her feet caring for others as a nurse with regular trips to the gym, now needed a walker to traverse the few steps from her bed to the toilet, an effort that left her gasping for air. Her brain couldn’t even focus on an audiobook, let alone a short magazine article.
“It just completely knocked the stuffing out of me,” Ronan said.
Ronan’s lingering symptoms aren’t unique to COVID-19 patients. In as many as 80% of patients leaving the ICU, . Although underlying illness plays a role in these symptoms, the amount of time spent in critical care is a major factor.
Nor is PICS simply a set of side effects that will go away on their own. It includes ongoing cognitive difficulties and physical weakness, both of which can lead to employment problems. Beyond that, depression and anxiety can exacerbate – and be exacerbated by – these challenges. Psychologist Jim Jackson, PsyD, assistant director of the ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tennessee, recently spoke with a former ICU patient who has struggled since her discharge 30 years ago.
“Her life essentially stopped with her critical care stay. She hasn’t been able to move forward,” he said. “She’s part of a whole fraternity of people who are struggling.”
The good news is that over the past decade, researchers have made important strides in understanding what makes PICS symptoms worse and how critical care physicians can tweak ICU protocols to reduce PICS severity. Practitioners will need to draw on this knowledge to help Ronan and the thousands of COVID-19 ICU patients like her.
Surviving the ICU
Although the new coronavirus has pushed the world’s critical care system to its limits, it was an outbreak in 1952 that inspired the creation of intensive care units. That summer, a wave of paralytic polio swept over Copenhagen, Denmark, and anesthesiologist Bjørn Ibsen, MD, PhD, used mechanical ventilation — physically operated by medical and dental students – to help 316 children breathe for weeks at a time while their small bodies worked to fight off the virus. The effort halved the mortality rate from polio that affected breathing, from 80% to 40%.
In these wards, dedicated to the very sickest, each patient was assigned his or her own nurse. Over the next decade, hospitals in the United Kingdom and the United States established their own ICUs to treat patients with a variety of conditions. Although it helped improve survival, mortality rates in critical care units remained stubbornly high, owing to the patients’ severe underlying illnesses.
“We thought we were doing a good job if the patient survived, but we had no idea what happened after discharge,” said Carla Sevin, MD, medical director of Vanderbilt’s ICU Recovery Center. Nor did their efforts to find out always bring answers. “We struggled to get people to come in for support — they were debilitated, physically burdened, and weak.”
Through further advances in life support, by the early 2000s, the average mortality rates in American ICUs had dropped to 8% to 19%. As the number of critical care survivors began to climb, clinical researchers noticed that the lives of these patients and their families were profoundly altered by their severe illness.
As Dale Needham, MD, PhD, began his pulmonology and critical care residency in Toronto, Canada, in 2005, a group of physicians there began a 5-year longitudinal study to assess long-term outcomes of patients who developed acute respiratory distress syndrome (ARDS). Although ARDS is an acute condition, the investigators found that patients felt effects for years. Younger patients recovered better than older ones, but none of the patients› physical functioning was equivalent to that of age-matched control persons. Even 5 years later, former ICU patients only reached 76% of expected physical functioning, according to results published in the New England Journal of Medicine. The study was a wake-up call.
At a meeting in Chicago in 2010, Needham, now an intensivist at Johns Hopkins Hospital in Baltimore, Maryland, gathered an interdisciplinary group of colleagues, including patients and caregivers, to clarify the phenomena they were seeing. What emerged from that meeting, published in 2012 in Critical Care Medicine, were the diagnostic criteria for PICS: According to the new definition, PICS is characterized by new or worsening physical and neuropsychiatric deficits that range from forgetfulness and loss of motivation to physical weakness and insomnia.
The issue, Needham says, is that although the trouble starts in the ICU, it only becomes clear once patients leave. “ICU doctors aren’t the ones dealing with this,” Needham said. “We need to build stronger bridges between critical care and other professions.” That’s where PICS comes in, a definition that exists explicitly to alert healthcare providers about the constellation of challenges many of these individuals face as they try to reenter “normal” life.
Defining the problem
As an ICU nurse at the Mayo Clinic in Rochester, Minnesota, Annie Johnson, ACNP-BC, knew lots about helping hospitalized patients, but she says she didn’t know anything about what to do after discharge – at least not until her own mother became a patient.
On the first day of retirement in October 2014, Johnson’s mother flatlined. Quick-thinking paramedics resuscitated her, and after several days in critical care, she was discharged. Since then, her heart has remained healthy. Johnson’s sister, who spent time worrying over her mother at the hospital, also had lingering effects. Both have since struggled, plagued by nightmares, flashbacks, and insomnia.
Johnson initially believed her mom’s and sister’s neuropsychiatric, post-ICU struggles were unique to her family. It was only a year later, at a seminar she was attending, that she first heard the words “post–intensive care syndrome.” Suddenly, Johnson had a name for her family’s experiences, and she began to create support groups and resources to help other families like hers.
“I thought of all the patients I had treated over the years who had been on ventilators for days and days and days. And if this happened to my mom after 48 hours, what must they be going through?” she asked.
Once physicians formally defined PICS, the Society for Critical Care Medicine helped create programs to educate ICU staff, patients, and families about potential post-discharge challenges. Researchers also began to investigate factors affecting post-ICU functioning. Follow-up studies of patients with delirium (ranging from general confusion about time and place to extreme agitation and violence) showed they had striking cognitive deficits. Problems with short-term memory, flexible thinking, and motivation plagued patients for years after their critical illness, similar to the physical deficiencies seen after ARDS. Delirium was one of the strongest risk factors for neuropsychiatric problems.
“Delirium is basically a stress test for the brain,” said Babar Khan, MD, a critical care specialist at Indiana University’s Regenstrief Institute, in Bloomington. But whether delirium accentuates preexisting cognitive difficulties or creates them afresh isn’t yet clear.
Sophia Wang, MD, a geriatric psychiatrist at Indiana University who works with many critical care patients, says patients who had experienced delirium in the ICU showed significant defects in memory and executive functioning long after their hospital stay. She points to a 2015 study that followed 47 ICU patients for a year post discharge. Among those who experienced delirium, brain volumes, as measured by MRI, were smaller at 3 months, something associated with cognitive problems at 1 year. Many struggled at work, and unemployment was common. Depression and posttraumatic stress compounded these difficulties. Among those with acute respiratory distress, ICU patients who are young, female, and unemployed are most likely to suffer from posttraumatic stress disorder after they are discharge.
Critical care medicine may have given these patients a second chance at life, Wang says, but the life they return to often looks nothing like the one they had before their illness.
Prolonged mechanical ventilation and the heavy sedation that often accompanies it are predictors of PICS severity. Some of these links could be explained by the gravity of the illness that landed someone in critical care, but others are more likely to be iatrogenic, says Gerald Weinhouse, MD, a pulmonology and critical care physician and co-director of the Critical Illness Recovery Program at the Brigham and Women’s Hospital in Boston. The involvement of loved ones at the patient’s bedside, however, improved the entire family’s outcome.
When Weinhouse saw those data, he and his colleagues founded a peer support program for ICU survivors. In a study published in 2019 in Critical Care Medicine, they identified six different models for peer support for those with PICS and their families, including both online and in-person approaches. An ongoing challenge for physicians, Weinhouse says, is getting patients to engage with these programs, given that their calendars are crowded with medical appointments and that they suffer from increased physical and mental disability.
Studies such as these led critical care physicians to form the ICU Liberation Collaborative to rethink critical care medicine. At Vanderbilt, Sevin and Jackson headed up one of the world’s first post-ICU clinics, which uses an interdisciplinary team to help patients maximize their functioning. They redesigned their critical care unit in a way that allows families to spend the night and that encourages patient mobility. Both Needham and Weinhouse continue tracking patient outcomes.
Even before the novel coronavirus struck, the United States — and the world — had begun to realize that graduating from the ICU was only the start of what was often an extensive recovery.
The long road back
When COVID-19 patients began flooding intensive care wards around the world, physicians scrambled to meet their complex and desperate acute medical needs. Over the past few months, physicians have focused on keeping these patients alive. “We’ve never seen anything like it ― not even during polio — with the sheer number of patients, all with respiratory distress,” Needham said.
But he and his colleagues know this is only the beginning.
“We’re aware that survivorship issues are coming. There’s going to be a wave of sick people who survived the coronavirus but are going to need more help,” Weinhouse said.
Intensivists have been drawing on PICS research in their fight to help COVID-19 patients. Work from the past few years has shown that although sedation is required during intubation itself, not everyone needs it while on a ventilator. Titrating down sedating medication helps reduce delirium, Wang says. Such medication has been shown to contribute to later cognitive problems. Needham’s studies showing that prolonged bedrest by ICU patients causes muscular atrophy has led him to encourage patients to move as much as possible. With the help of physical therapists, many patients on ventilators can be awake, alert, and moving around the ward.
One of the biggest challenges critical-care coronavirus patients face is prolonged isolation. The constant presence of a familiar face helps orient confused and delirious patients and provides emotional support during a frightening time. But because the immediate need for infection control outweighs these benefits, few hospitals allow visitors, especially for COVID-19 patients.
To address this, some units have been using video technology to allow loved ones to call in. At Johns Hopkins, physicians have also been relying on the expertise of occupational therapists (OTs). Needham says that one OT found that rubbing the hand and back of an agitated, delirious patient helped soothe and calm him better than many medications.
Ronan, who spent 5 days in intensive care, echoes that problem. She says she found the relative lack of human contact to be one of the most challenging parts of being in a bed on a COVID-19 ward. Separated from her husband and daughter, suffering from high fever and severe illness, she lost all track of time.
Her return home was difficult, too. Although her job as a home health nurse had prepared her on some level for the challenges she would face after discharge, Ronan says the hospital provided little practical help.
“Everything is so much harder at home, even little things like going to the bathroom,” she said. “I feel like I’m trying to bail out a sinking ship with a teacup.”
Khan and other physicians, aware of the challenges Ronan and others face once home, aim to create post-ICU clinics specifically for COVID-19 patients. They want to build what Khan calls a “one-stop shop” for all the support patients need to recover. Some of that can be provided via telehealth, which may also help ease the physical burden.
Because there’s so much physicians don’t know about the coronavirus, Johnson says, such clinics are not only a chance to help the sickest COVID-19 patients, they will also help researchers learn more about the virus and improve critical care for other illnesses.
Today, nearly 2 months after discharge, Ronan is back on the job but struggles with a persistent cough — likely due to the lung damage she sustained while ill. She has constant fatigue, as well as ongoing upset stomach from all the medications she took to reduce fever and body aches. When she dons a mask for work, the tangible reminder of her hospital stay sends her into a panic attack. Physically, she’s weaker than before.
Researchers are still trying to understand everything that Ronan and other COVID-19 patients need to move on with their lives after being in the ICU. Mysteries abound, but the ground laid by Sevin, Needham, Weinhouse, and others has provided a solid foundation on which to build.
This article first appeared on Medscape.com.







