User login
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
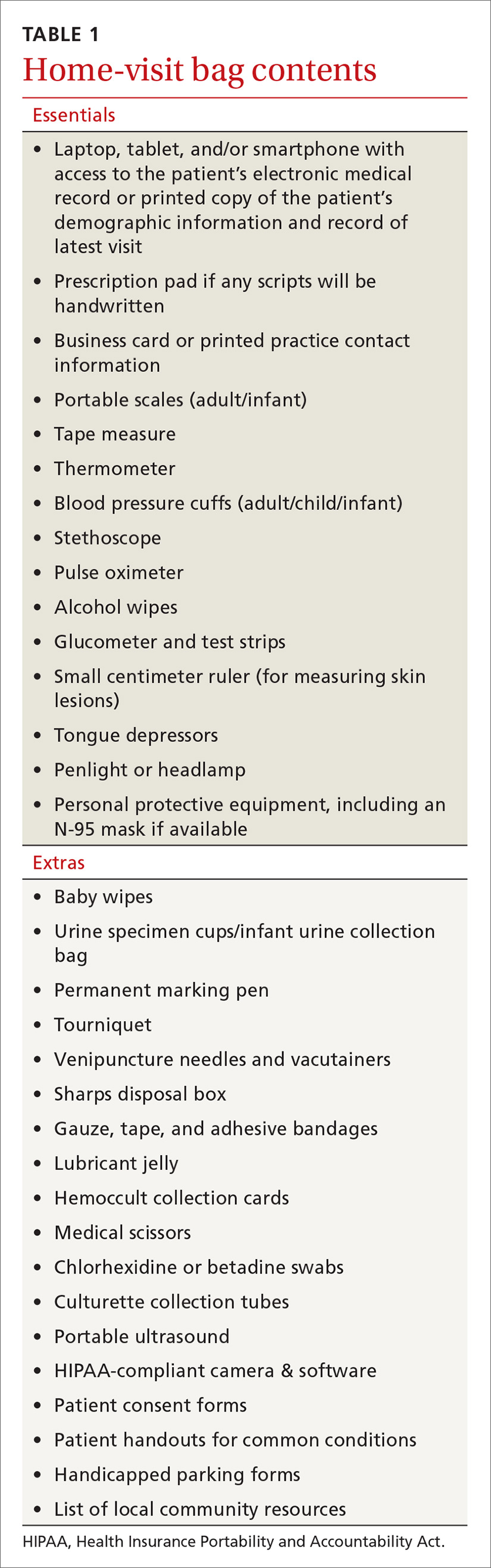
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
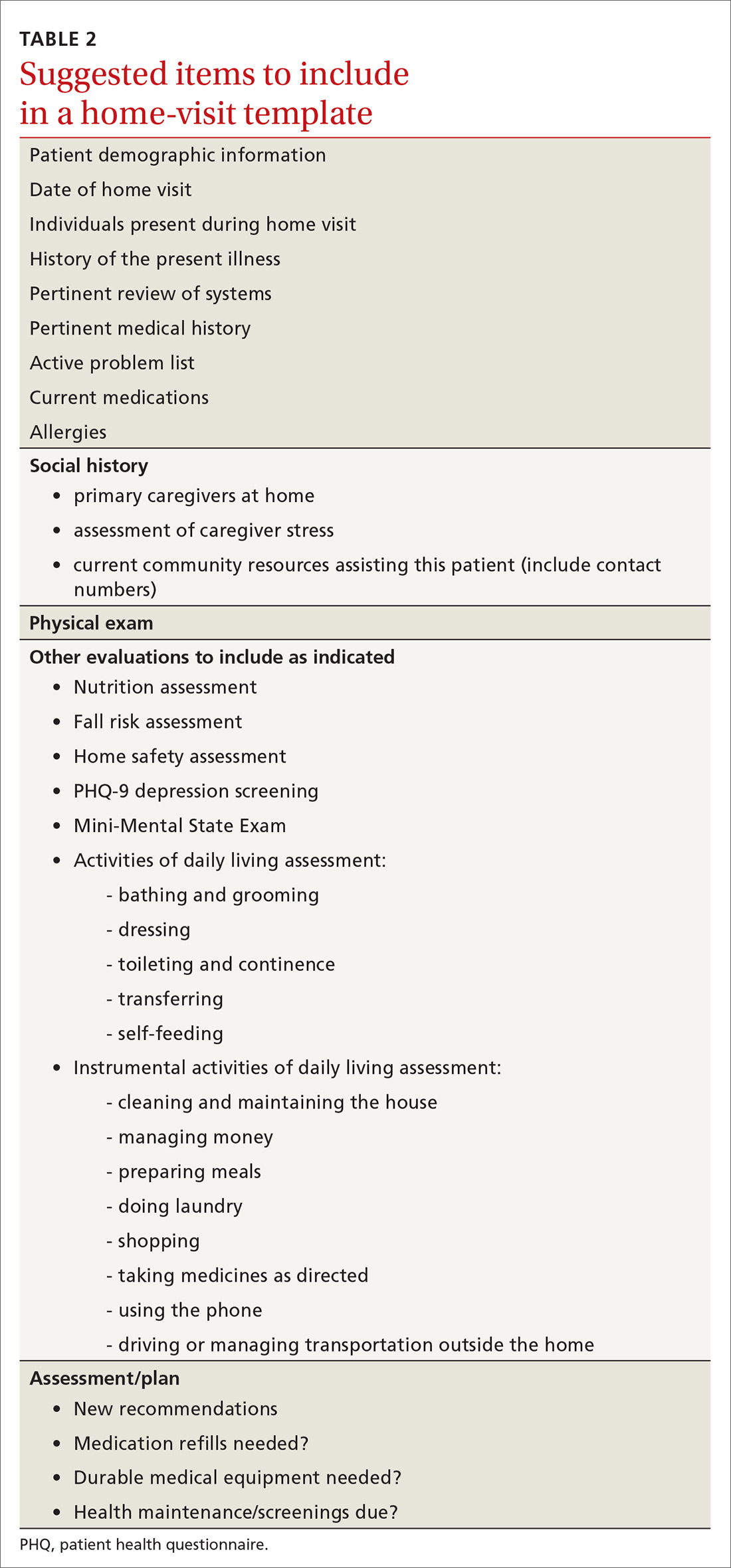
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
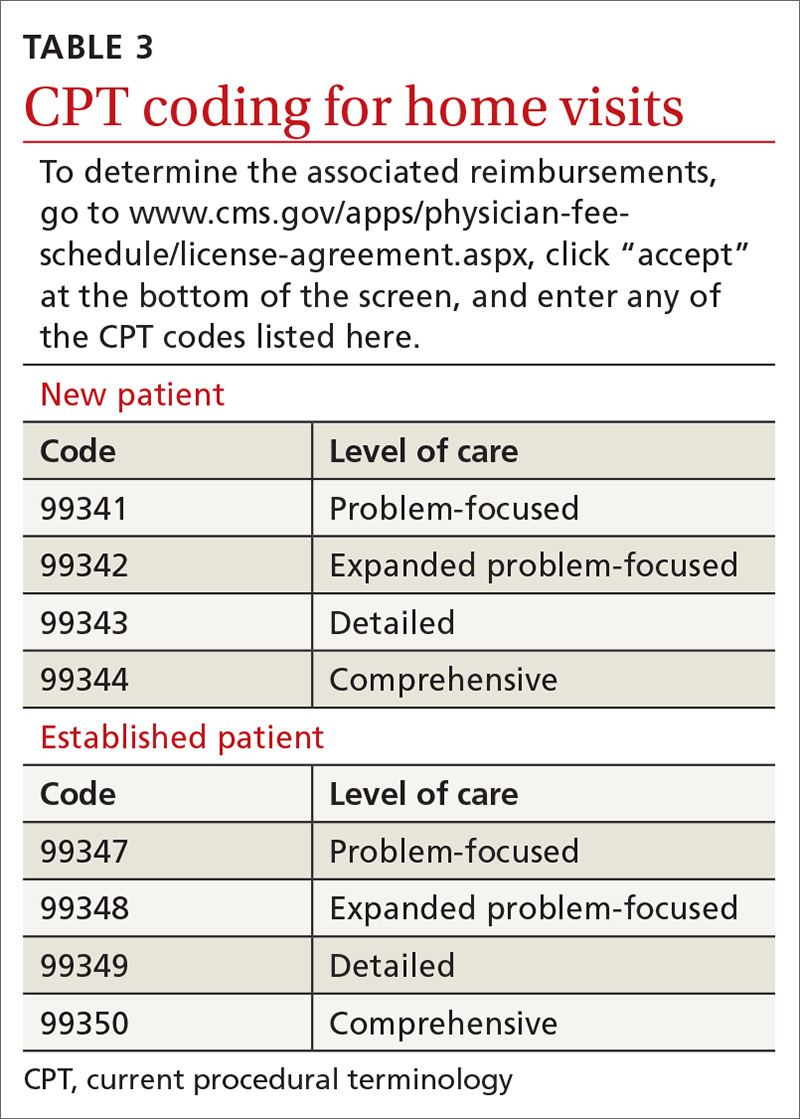
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The evidence for noncosmetic uses of botulinum toxin
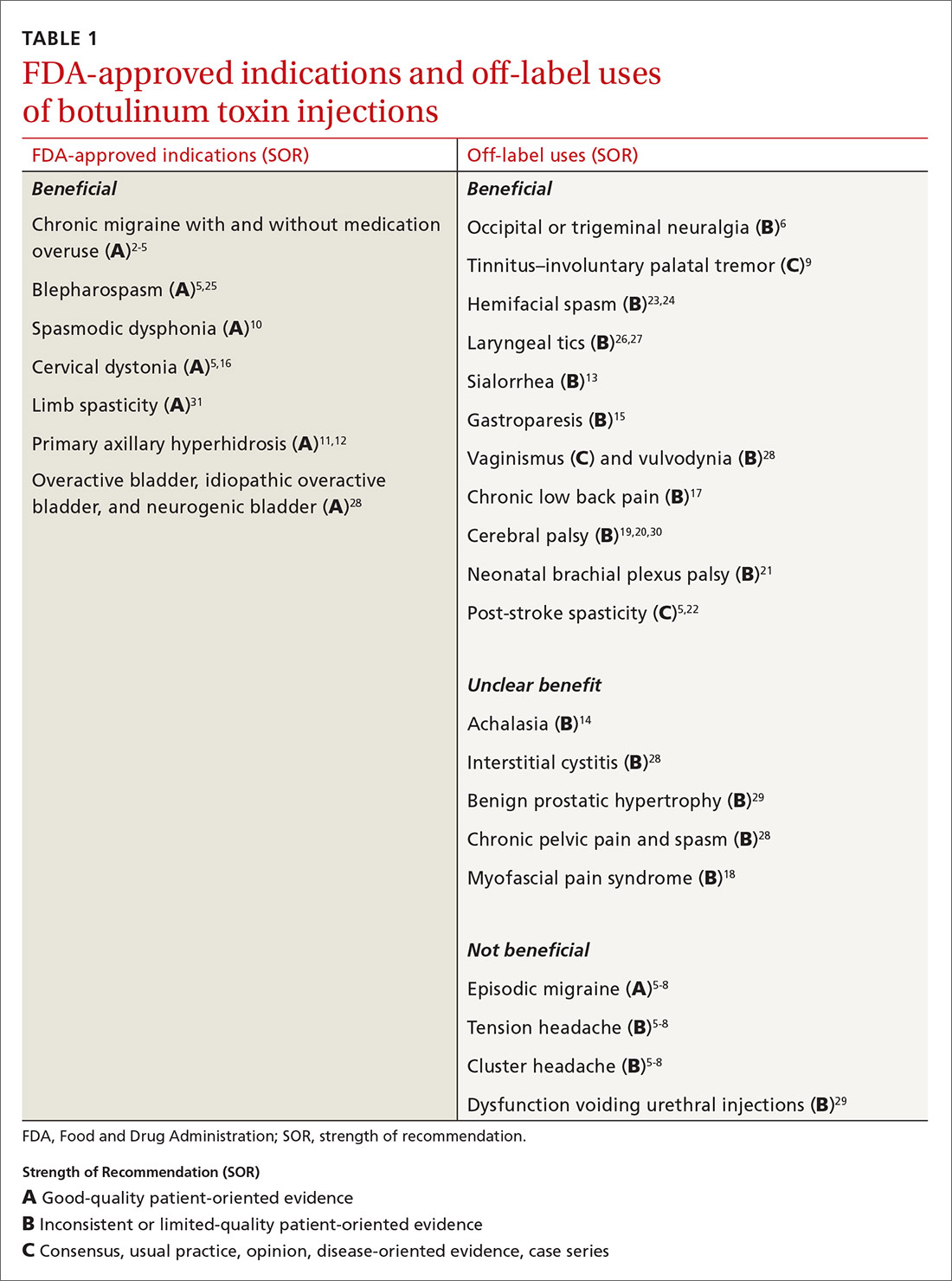
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.
But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
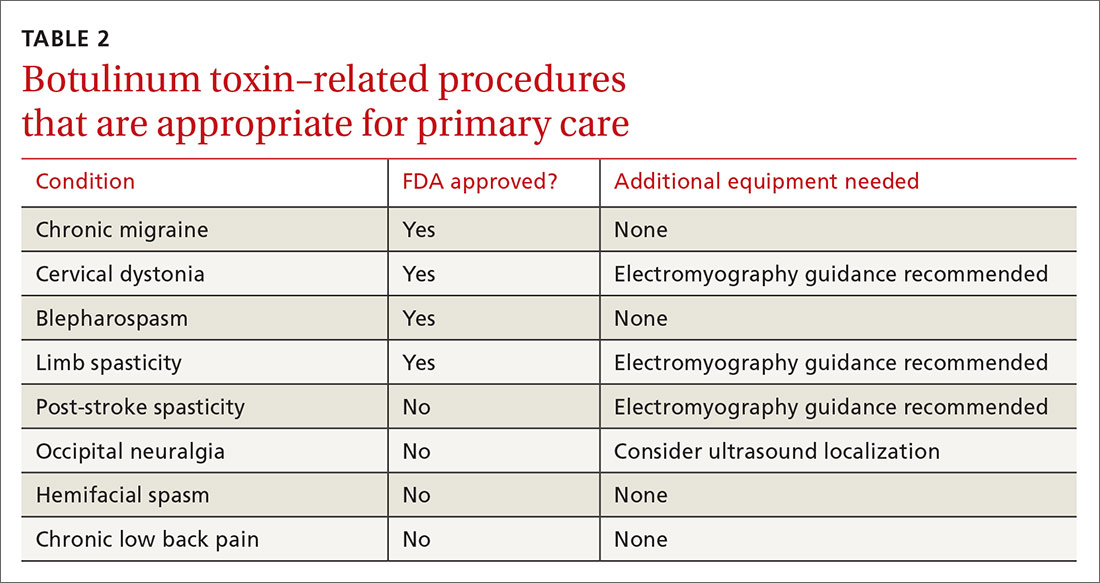
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).
Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches
Episodic migraine, tension headache, and cluster headaches. There is no significant BoNT-A-related pain reduction in episodic migraine (n = 1838; 0.05 headaches/mo; 95% CI, –0.26 to –0.36) or tension headaches (n = 675; –1.43 headaches/mo; 95% CI, –3.13 to –0.27).5,6 For cluster headaches, a single prospective study with low enrollment showed no consistent benefit,7 while a pilot study showed some improvement, with reduction of attacks by 50% in half of subjects.8
Occipital neuralgia and trigeminal neuralgia entail paroxysmal, brief, shock-like pain without associated deficits affecting the respective nerve distributions. Multiple prospective and double-blind placebo-controlled studies with relatively low enrollment show consistent improvement in pain intensity, number of pain-free days, analgesic consumption, and headache frequency with BoNT-A added to nerve blocks.6
ENT disorders
Tinnitus by involuntary palatal tremor causes a discontinuous clicking noise. Palatal tremor can be treated with BoNT-A 15 U to tensor veli palatini and levator veli muscles to provide temporary relief for 2 to 6 months.9
Spasmodic dysphonia and voice tremor are the result of laryngeal hyperkinesis, and BoNT-A has been deemed the gold standard of treatment. BoNT-A is administered via bilateral injection of the thyroarytenoid muscles for patients with adductor-type spasmodic dysphonia and of the posterior cricoarytenoid muscles for those with the abductor type. A series of 1300 patients (predominantly with the adductor type) treated with BoNT-A showed a 100% improvement in symptoms for 6 to 15 weeks. Patients with abductor-type spasmodic dysphonia were found to have 89% improvement in Voice Related Quality of Life Index score.10
Secretory disorders
Primary axillary hyperhidrosis (PAH) is an idiopathic excessive production of sweat occurring for at least 6 months, typically with onset before age 25 years. PAH can cause significant psychosocial and physical impairment. Current treatments include topical aluminum chloride, systemic anticholinergics, and thoracic sympathectomy, which can provide temporary relief but are not well tolerated.
Continue to: BoNT-A treatment is efficacious...
BoNT-A treatment is efficacious, safe, and improves quality of life for PAH patients. A 52-week, multicenter, double-blind, randomized, placebo-controlled study showed significant reductions in symptom severity, decreased sweating at rest by gravimetric testing, and improvements in self-reported quality of life.11 A 10-year retrospective study in patients ages 12 years and older showed a 75% to 100% improvement in hyperhidrosis, with a median treatment effect duration of 7 months.12
Sialorrhea, or hypersalivation, is typically associated with neurological conditions such as cerebral palsy, amyotrophic lateral sclerosis, Parkinson disease, and posttraumatic brain injuries. It typically is treated with anticholinergic drugs, surgery, and irradiation of salivary glands, which can have significant adverse effects and complications. In a randomized blinded study, BoNT-A injections in the parotid and submandibular glands resulted in a dramatic reduction of sialorrhea and were safe and well tolerated.13
Gastric disorders
Achalasia is a syndrome of aperistalsis and incomplete lower esophageal sphincter (LES) relaxation with a “bird beak” appearance on barium swallow. Patients who meet diagnostic criteria are treated with pneumatic dilation or myotomy; however, some patients demonstrate symptoms of achalasia but don’t meet the diagnostic criteria. In these patients, BoNT-A injection in the LES provides symptomatic relief. In a case series, LES BoNT-A injections 20 U were used as a decision tool in whether to proceed with definitive treatment.14
Gastroparesis is a disorder of impaired gastric motility without mechanical obstruction. Pyloric sphincter BoNT-A injections are useful in refractory patients. Multiple prospective, noncontrolled (4), retrospective (3), and randomized placebo-controlled (2), studies with limited enrollment showed benefit for 37.5% to 100% of patients receiving BoNT-A injections of 80 to 200 U.15
Musculoskeletal disorders
Cervical dystonia (CD) entails involuntary contractions of the neck and upper shoulder musculature, causing abnormal neck, shoulder, and head posturing. BoNT-A is first-line treatment for CD.5 BoNT-A is more efficacious than trihexyphenidyl based on multiple large, high-quality studies.16
Continue to: Chronic low back pain
Chronic low back pain (CLBP) is defined as back pain persisting ≥ 12 weeks. More than 80% of adults have had at least 1 episode of back pain in their lifetime.
Myofascial pain syndrome (MPS) consists of myofascial trigger points (palpable, tender nodules that produce pain) with multiple pathophysiological etiologies that include dysfunctional acetylcholine activity, which releases nociceptive neurotransmitters. Studies have yielded inconsistent effects of BoNT-A on MPS.18
Spastic disorders
Cerebral palsy (CP) involves altered muscle tone, posture, and movement secondary to central motor dysfunction with spasticity. Evaluation of BoNT-A as an adjunctive therapy in CP has been extensive and conflicting. A prospective cohort study evaluating gastrocsoleus BoNT-A injections along with gait analysis in 37 children with CP showed no significant improvements.30 In 60 children with CP who received BoNT-A injections, there was improvement in muscle tone and range of motion, while gait improved in patients up to (but not after) age 7 years.19 A multicenter Dutch study of 65 children compared BoNT-A injections in addition to a comprehensive rehabilitation program vs rehabilitation alone, with no difference identified.20
Neonatal brachial plexus palsy (NBPP) is damage to the brachial plexus as a result of trauma during the perinatal period. It is typically self-resolving but can cause residual functional impairment. Surgery is recommended for serious injuries or if functional recovery is not achieved within 9 months. Off-label use of BoNT-A has been shown to be effective in relieving muscle contractures and imbalance, but data are limited and there have only been small studies performed.21 A retrospective cohort study of 59 patients with NBPP who received BoNT-A injections showed improved range of motion and function of the affected extremity. Moreover, surgical intervention was deferred, modified, or averted in patients who were under consideration for more invasive treatment.21
Post-stroke spasticity can be temporarily relieved with the use of BoNT-A injections. Several studies have examined the effect of BoNT-A coupled with rehabilitation programs vs injections alone in the treatment of post-stroke spasticity. Devier et al found that improvements in spasticity scores did not differ between groups; however, implementing rehabilitation after BoNT-A injections was associated with improved function compared to injection alone.31 A 2018 randomized, double-blind, placebo-controlled trial demonstrated improvements in both treatment groups: those who received BoNT-A plus targeted rehab regimen and those who received saline injection plus rehab.22 In this case, it appears BoNT-A acts as more of an adjunct to physical therapy in the treatment of post-stroke spasticity.5
Continue to: Hemifacial spasm
Hemifacial spasm is an involuntary, brief, irregular unilateral (sometimes bilateral) spasm of the face in the distribution of the facial nerve. Injections with BoNT-A have been deemed effective by the American Academy of Neurology.23 A 16-year retrospective study examined the efficacy and adverse effects of BoNT-A in the treatment of hemifacial spasm in 113 patients with a mean age of 63.1 years; it demonstrated high efficacy and mild temporary adverse effects.24 The duration of improvement averaged 16 weeks; pretarsal injections had better results than preseptal injections; and there were no differences between the commercial brands.
Blepharospasm is a focal dystonia marked by excessive blinking and involuntary eye closures due to overexcitability of orbicularis oculi and periocular muscles, and BoNT-A is the treatment of choice.5,25 A retrospective review of 19 patients with blepharospasm who were treated with BoNT-A for more than 5 years found that BoNT-A is a stable and effective treatment with an adverse event rate of 4%. Additionally, there were no differences found in clinical efficacy between the 4 BoNT-A brands on the market.25
Laryngeal tics can cause significant psychosocial distress for patients. This condition is characterized by involuntary, recurrent rhythmic sounds that are often preceded by premonitory urges that are relieved by the behavior. An open-label, uncontrolled, confirmatory study with 30 subjects showed that bilateral vocal cord BoNT-A injections resulted in 93% improvement in vocal tics.26 A subsequent study highlighted case histories of 2 patients with laryngeal tics who received thyroarytenoid muscle BoNT-A injections and had marked reduction in symptoms and premonitory sensations.27 Although these small studies have suggested possible effectiveness of BoNT-A for laryngeal tics, there is no high-quality evidence.
Urologic disorders
Overactive, idiopathic overactive, or neurogenic bladder causes increased urinary frequency, urgency, and nocturia without infectious etiology; they can be a result of neurologic dysregulation, detrusor overactivity, or idiopathic causes. Intravesical BoNT-A injection of 100 to 300 U has been found effective for symptoms refractory to anticholinergic and lifestyle therapy, with increased cystometric capacity (229.1 to 427 mL, P < .00001), decreased maximum detrusor pressure (60.7 to 26.1 cm H2O, P < .00001), and resolution of urgency in 87% of patients (P < .001).28
Interstitial cystitis, also known as painful bladder syndrome, is characterized by reduced bladder emptying, urethral pressure, and residual urine pressure, with symptoms of increased urinary frequency without infection. Intravesicular BoNT-A injections have not consistently been effective in treatment of this condition.28
Continue to: Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome
Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome involve urethral sphincter spasticity with difficulty passing urine and possibly retention. Urethral sphincter injections of 100 U BoNT-A improved flow rates and decreased residual volume. A randomized, double-blinded, placebo-controlled study showed a significantly improved International Prostate Symptom Score (IPSS), quality of life index, maximum flow rate, voided volume, and decreased detrusor voiding pressure at 1 month.29
Benign prostatic hypertrophy (BPH) is a very common condition leading to outlet obstruction. The mainstays of treatment are 5-α-reductase inhibitors, α-adrenergic blockers, and surgical removal.
Gynecologic disorders
Vaginismus is the involuntary, recurrent, or persistent contraction of the perineal muscles surrounding the outer third of the vagina; it is classified by 4 progressively more severe degrees of intensity. Levator ani, bulbospongiosus, bulbocavernosus, pubococcygeus, and/or puborectalis muscle BoNT-A injections have shown benefits in decreasing resistance to vaginal exams (95.8%) and the ability to achieve satisfactory sexual intercourse after first injection (75%-100%). Effects were transient for up to 15.4% of patients requiring repeat injections.28
Vulvodynia is vulvar pain and orgasmic difficulties and has been treated with bulbospongiosus muscle BoNT-A injections in retrospective studies. A single randomized, double-blinded, placebo-controlled study showed significantly improved pain scores after 1 to 2 injection series.28
Chronic pelvic pain is a syndrome of somatic functional or regional pain, which can be caused by the spasm of the pelvic musculature with or without trigger points. Patients with pain refractory to treatment have been treated with levator ani injections. A retrospective cohort study found 79.3% of patients experienced pain relief and 20.7% reported improved symptoms. In a double-blind, randomized, placebo-controlled trial, pelvic floor muscles were injected with 80 U BoNT or saline, and symptoms were evaluated along with vaginal manometry. BoNT was associated with a reduction in some pain but not as much as placebo, while vaginal pressures decreased more with BoNT than with placebo.28
Blake Busey, DO, FAAFP, Texas Tech University of Health Sciences El Paso–Transmountain, 2000B Transmountain Road, Suite B400, El Paso, TX 79911; [email protected]
1. American Society of Plastic Surgeons. New statistics reveal the shape of plastic surgery [news release]. March 1, 2018. www.plasticsurgery.org/news/press-releases/new-statistics-reveal-the-shape-of-plastic-surgery. Accessed October 23, 2020.
2. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalgia. 2010;30:804-814.
3. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616.
4. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826.
6. Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818-3844.
7. Sostak P, Krause P, Förderreuther S, et al. Botulinum toxin type-A therapy in cluster headache: an open study. J Headache Pain. 2007;8:236-241.
8. Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503-509.
9. Mandavia R, Dessouky O, Dhar V, et al. The use of botulinum toxin in otorhinolaryngology: an updated review. Clin Otolaryngol. 2014;39:203-209.
10. Klein AM, Stong BC, Wise J, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:421-423.
11. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596-599.
12. Rosen R, Stewart T. Results of a 10-year follow-up study of botulinum toxin A therapy for primary axillary hyperhidrosis in Australia. Intern Med J. 2018;48:343-347.
13. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins (Basel). 2018;10:55.
14. Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
15. Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc. 2015;7:790-798.
16. Zakin E, Simpson D. Evidence on botulinum toxin in selected disorders. Toxicon. 2018;147:134-140.
17. Jabbari B, Ney J, Sichani A, et al. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264.
18. Climent JM, Kuan TS, Fenollosa P, et al. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459.
19. Mirska A, Cybula K, Okurowska-Zawada B, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517-522.
20. Schasfoort F, Pangalila R, Sneekes EM, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732-742.
21. Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6:1107-1119.
22. Prazeres A, Lira M, Aguiar P, et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol Int. 2018;10:7385.
23. Simpson DM, Blitzer A, Brashear A, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
24. Sorgun MH, Yilmaz R, Akin YA, et al. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22:1319-1325.
25. Lee S, Park S, Lew H. Long-term efficacy of botulinum neurotoxin-A treatment for essential blepharospasm. Korean J Ophthalmol. 2018;32:1-7.
26. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
27. Vincent DA Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22:251-256.
28. Moga MA, Dimienescu OG, Balan A, et al. Therapeutic approaches of botulinum toxin in gynecology. Toxins (Basel) 2018;10:169.
29. Jhang J-F, Kuo H-C. Novel applications of onabotulinumtoxinA in lower urinary tract dysfunction. Toxins (Basel). 2018;10:260.
30. Hastings-Ison T, Sangeux M, Thomason P, et al. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: a prospective kinematic study. J Child Orthop. 2018;12:390-397.
31. Devier D, Harnar J, Lopez L, et al. Rehabilitation plus onabotulinumtoxina improves motor function over onabotulinumtoxina alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins. 2017;9:216.
32. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24:1-6.
33. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes—an evidence based review. Toxicon. 2018;147:120-128.
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.

But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).

Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches
Episodic migraine, tension headache, and cluster headaches. There is no significant BoNT-A-related pain reduction in episodic migraine (n = 1838; 0.05 headaches/mo; 95% CI, –0.26 to –0.36) or tension headaches (n = 675; –1.43 headaches/mo; 95% CI, –3.13 to –0.27).5,6 For cluster headaches, a single prospective study with low enrollment showed no consistent benefit,7 while a pilot study showed some improvement, with reduction of attacks by 50% in half of subjects.8
Occipital neuralgia and trigeminal neuralgia entail paroxysmal, brief, shock-like pain without associated deficits affecting the respective nerve distributions. Multiple prospective and double-blind placebo-controlled studies with relatively low enrollment show consistent improvement in pain intensity, number of pain-free days, analgesic consumption, and headache frequency with BoNT-A added to nerve blocks.6
ENT disorders
Tinnitus by involuntary palatal tremor causes a discontinuous clicking noise. Palatal tremor can be treated with BoNT-A 15 U to tensor veli palatini and levator veli muscles to provide temporary relief for 2 to 6 months.9
Spasmodic dysphonia and voice tremor are the result of laryngeal hyperkinesis, and BoNT-A has been deemed the gold standard of treatment. BoNT-A is administered via bilateral injection of the thyroarytenoid muscles for patients with adductor-type spasmodic dysphonia and of the posterior cricoarytenoid muscles for those with the abductor type. A series of 1300 patients (predominantly with the adductor type) treated with BoNT-A showed a 100% improvement in symptoms for 6 to 15 weeks. Patients with abductor-type spasmodic dysphonia were found to have 89% improvement in Voice Related Quality of Life Index score.10
Secretory disorders
Primary axillary hyperhidrosis (PAH) is an idiopathic excessive production of sweat occurring for at least 6 months, typically with onset before age 25 years. PAH can cause significant psychosocial and physical impairment. Current treatments include topical aluminum chloride, systemic anticholinergics, and thoracic sympathectomy, which can provide temporary relief but are not well tolerated.
Continue to: BoNT-A treatment is efficacious...
BoNT-A treatment is efficacious, safe, and improves quality of life for PAH patients. A 52-week, multicenter, double-blind, randomized, placebo-controlled study showed significant reductions in symptom severity, decreased sweating at rest by gravimetric testing, and improvements in self-reported quality of life.11 A 10-year retrospective study in patients ages 12 years and older showed a 75% to 100% improvement in hyperhidrosis, with a median treatment effect duration of 7 months.12
Sialorrhea, or hypersalivation, is typically associated with neurological conditions such as cerebral palsy, amyotrophic lateral sclerosis, Parkinson disease, and posttraumatic brain injuries. It typically is treated with anticholinergic drugs, surgery, and irradiation of salivary glands, which can have significant adverse effects and complications. In a randomized blinded study, BoNT-A injections in the parotid and submandibular glands resulted in a dramatic reduction of sialorrhea and were safe and well tolerated.13
Gastric disorders
Achalasia is a syndrome of aperistalsis and incomplete lower esophageal sphincter (LES) relaxation with a “bird beak” appearance on barium swallow. Patients who meet diagnostic criteria are treated with pneumatic dilation or myotomy; however, some patients demonstrate symptoms of achalasia but don’t meet the diagnostic criteria. In these patients, BoNT-A injection in the LES provides symptomatic relief. In a case series, LES BoNT-A injections 20 U were used as a decision tool in whether to proceed with definitive treatment.14
Gastroparesis is a disorder of impaired gastric motility without mechanical obstruction. Pyloric sphincter BoNT-A injections are useful in refractory patients. Multiple prospective, noncontrolled (4), retrospective (3), and randomized placebo-controlled (2), studies with limited enrollment showed benefit for 37.5% to 100% of patients receiving BoNT-A injections of 80 to 200 U.15
Musculoskeletal disorders
Cervical dystonia (CD) entails involuntary contractions of the neck and upper shoulder musculature, causing abnormal neck, shoulder, and head posturing. BoNT-A is first-line treatment for CD.5 BoNT-A is more efficacious than trihexyphenidyl based on multiple large, high-quality studies.16
Continue to: Chronic low back pain
Chronic low back pain (CLBP) is defined as back pain persisting ≥ 12 weeks. More than 80% of adults have had at least 1 episode of back pain in their lifetime.
Myofascial pain syndrome (MPS) consists of myofascial trigger points (palpable, tender nodules that produce pain) with multiple pathophysiological etiologies that include dysfunctional acetylcholine activity, which releases nociceptive neurotransmitters. Studies have yielded inconsistent effects of BoNT-A on MPS.18
Spastic disorders
Cerebral palsy (CP) involves altered muscle tone, posture, and movement secondary to central motor dysfunction with spasticity. Evaluation of BoNT-A as an adjunctive therapy in CP has been extensive and conflicting. A prospective cohort study evaluating gastrocsoleus BoNT-A injections along with gait analysis in 37 children with CP showed no significant improvements.30 In 60 children with CP who received BoNT-A injections, there was improvement in muscle tone and range of motion, while gait improved in patients up to (but not after) age 7 years.19 A multicenter Dutch study of 65 children compared BoNT-A injections in addition to a comprehensive rehabilitation program vs rehabilitation alone, with no difference identified.20
Neonatal brachial plexus palsy (NBPP) is damage to the brachial plexus as a result of trauma during the perinatal period. It is typically self-resolving but can cause residual functional impairment. Surgery is recommended for serious injuries or if functional recovery is not achieved within 9 months. Off-label use of BoNT-A has been shown to be effective in relieving muscle contractures and imbalance, but data are limited and there have only been small studies performed.21 A retrospective cohort study of 59 patients with NBPP who received BoNT-A injections showed improved range of motion and function of the affected extremity. Moreover, surgical intervention was deferred, modified, or averted in patients who were under consideration for more invasive treatment.21
Post-stroke spasticity can be temporarily relieved with the use of BoNT-A injections. Several studies have examined the effect of BoNT-A coupled with rehabilitation programs vs injections alone in the treatment of post-stroke spasticity. Devier et al found that improvements in spasticity scores did not differ between groups; however, implementing rehabilitation after BoNT-A injections was associated with improved function compared to injection alone.31 A 2018 randomized, double-blind, placebo-controlled trial demonstrated improvements in both treatment groups: those who received BoNT-A plus targeted rehab regimen and those who received saline injection plus rehab.22 In this case, it appears BoNT-A acts as more of an adjunct to physical therapy in the treatment of post-stroke spasticity.5
Continue to: Hemifacial spasm
Hemifacial spasm is an involuntary, brief, irregular unilateral (sometimes bilateral) spasm of the face in the distribution of the facial nerve. Injections with BoNT-A have been deemed effective by the American Academy of Neurology.23 A 16-year retrospective study examined the efficacy and adverse effects of BoNT-A in the treatment of hemifacial spasm in 113 patients with a mean age of 63.1 years; it demonstrated high efficacy and mild temporary adverse effects.24 The duration of improvement averaged 16 weeks; pretarsal injections had better results than preseptal injections; and there were no differences between the commercial brands.
Blepharospasm is a focal dystonia marked by excessive blinking and involuntary eye closures due to overexcitability of orbicularis oculi and periocular muscles, and BoNT-A is the treatment of choice.5,25 A retrospective review of 19 patients with blepharospasm who were treated with BoNT-A for more than 5 years found that BoNT-A is a stable and effective treatment with an adverse event rate of 4%. Additionally, there were no differences found in clinical efficacy between the 4 BoNT-A brands on the market.25
Laryngeal tics can cause significant psychosocial distress for patients. This condition is characterized by involuntary, recurrent rhythmic sounds that are often preceded by premonitory urges that are relieved by the behavior. An open-label, uncontrolled, confirmatory study with 30 subjects showed that bilateral vocal cord BoNT-A injections resulted in 93% improvement in vocal tics.26 A subsequent study highlighted case histories of 2 patients with laryngeal tics who received thyroarytenoid muscle BoNT-A injections and had marked reduction in symptoms and premonitory sensations.27 Although these small studies have suggested possible effectiveness of BoNT-A for laryngeal tics, there is no high-quality evidence.
Urologic disorders
Overactive, idiopathic overactive, or neurogenic bladder causes increased urinary frequency, urgency, and nocturia without infectious etiology; they can be a result of neurologic dysregulation, detrusor overactivity, or idiopathic causes. Intravesical BoNT-A injection of 100 to 300 U has been found effective for symptoms refractory to anticholinergic and lifestyle therapy, with increased cystometric capacity (229.1 to 427 mL, P < .00001), decreased maximum detrusor pressure (60.7 to 26.1 cm H2O, P < .00001), and resolution of urgency in 87% of patients (P < .001).28
Interstitial cystitis, also known as painful bladder syndrome, is characterized by reduced bladder emptying, urethral pressure, and residual urine pressure, with symptoms of increased urinary frequency without infection. Intravesicular BoNT-A injections have not consistently been effective in treatment of this condition.28
Continue to: Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome
Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome involve urethral sphincter spasticity with difficulty passing urine and possibly retention. Urethral sphincter injections of 100 U BoNT-A improved flow rates and decreased residual volume. A randomized, double-blinded, placebo-controlled study showed a significantly improved International Prostate Symptom Score (IPSS), quality of life index, maximum flow rate, voided volume, and decreased detrusor voiding pressure at 1 month.29
Benign prostatic hypertrophy (BPH) is a very common condition leading to outlet obstruction. The mainstays of treatment are 5-α-reductase inhibitors, α-adrenergic blockers, and surgical removal.
Gynecologic disorders
Vaginismus is the involuntary, recurrent, or persistent contraction of the perineal muscles surrounding the outer third of the vagina; it is classified by 4 progressively more severe degrees of intensity. Levator ani, bulbospongiosus, bulbocavernosus, pubococcygeus, and/or puborectalis muscle BoNT-A injections have shown benefits in decreasing resistance to vaginal exams (95.8%) and the ability to achieve satisfactory sexual intercourse after first injection (75%-100%). Effects were transient for up to 15.4% of patients requiring repeat injections.28
Vulvodynia is vulvar pain and orgasmic difficulties and has been treated with bulbospongiosus muscle BoNT-A injections in retrospective studies. A single randomized, double-blinded, placebo-controlled study showed significantly improved pain scores after 1 to 2 injection series.28
Chronic pelvic pain is a syndrome of somatic functional or regional pain, which can be caused by the spasm of the pelvic musculature with or without trigger points. Patients with pain refractory to treatment have been treated with levator ani injections. A retrospective cohort study found 79.3% of patients experienced pain relief and 20.7% reported improved symptoms. In a double-blind, randomized, placebo-controlled trial, pelvic floor muscles were injected with 80 U BoNT or saline, and symptoms were evaluated along with vaginal manometry. BoNT was associated with a reduction in some pain but not as much as placebo, while vaginal pressures decreased more with BoNT than with placebo.28
Blake Busey, DO, FAAFP, Texas Tech University of Health Sciences El Paso–Transmountain, 2000B Transmountain Road, Suite B400, El Paso, TX 79911; [email protected]
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.

But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).

Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches
Episodic migraine, tension headache, and cluster headaches. There is no significant BoNT-A-related pain reduction in episodic migraine (n = 1838; 0.05 headaches/mo; 95% CI, –0.26 to –0.36) or tension headaches (n = 675; –1.43 headaches/mo; 95% CI, –3.13 to –0.27).5,6 For cluster headaches, a single prospective study with low enrollment showed no consistent benefit,7 while a pilot study showed some improvement, with reduction of attacks by 50% in half of subjects.8
Occipital neuralgia and trigeminal neuralgia entail paroxysmal, brief, shock-like pain without associated deficits affecting the respective nerve distributions. Multiple prospective and double-blind placebo-controlled studies with relatively low enrollment show consistent improvement in pain intensity, number of pain-free days, analgesic consumption, and headache frequency with BoNT-A added to nerve blocks.6
ENT disorders
Tinnitus by involuntary palatal tremor causes a discontinuous clicking noise. Palatal tremor can be treated with BoNT-A 15 U to tensor veli palatini and levator veli muscles to provide temporary relief for 2 to 6 months.9
Spasmodic dysphonia and voice tremor are the result of laryngeal hyperkinesis, and BoNT-A has been deemed the gold standard of treatment. BoNT-A is administered via bilateral injection of the thyroarytenoid muscles for patients with adductor-type spasmodic dysphonia and of the posterior cricoarytenoid muscles for those with the abductor type. A series of 1300 patients (predominantly with the adductor type) treated with BoNT-A showed a 100% improvement in symptoms for 6 to 15 weeks. Patients with abductor-type spasmodic dysphonia were found to have 89% improvement in Voice Related Quality of Life Index score.10
Secretory disorders
Primary axillary hyperhidrosis (PAH) is an idiopathic excessive production of sweat occurring for at least 6 months, typically with onset before age 25 years. PAH can cause significant psychosocial and physical impairment. Current treatments include topical aluminum chloride, systemic anticholinergics, and thoracic sympathectomy, which can provide temporary relief but are not well tolerated.
Continue to: BoNT-A treatment is efficacious...
BoNT-A treatment is efficacious, safe, and improves quality of life for PAH patients. A 52-week, multicenter, double-blind, randomized, placebo-controlled study showed significant reductions in symptom severity, decreased sweating at rest by gravimetric testing, and improvements in self-reported quality of life.11 A 10-year retrospective study in patients ages 12 years and older showed a 75% to 100% improvement in hyperhidrosis, with a median treatment effect duration of 7 months.12
Sialorrhea, or hypersalivation, is typically associated with neurological conditions such as cerebral palsy, amyotrophic lateral sclerosis, Parkinson disease, and posttraumatic brain injuries. It typically is treated with anticholinergic drugs, surgery, and irradiation of salivary glands, which can have significant adverse effects and complications. In a randomized blinded study, BoNT-A injections in the parotid and submandibular glands resulted in a dramatic reduction of sialorrhea and were safe and well tolerated.13
Gastric disorders
Achalasia is a syndrome of aperistalsis and incomplete lower esophageal sphincter (LES) relaxation with a “bird beak” appearance on barium swallow. Patients who meet diagnostic criteria are treated with pneumatic dilation or myotomy; however, some patients demonstrate symptoms of achalasia but don’t meet the diagnostic criteria. In these patients, BoNT-A injection in the LES provides symptomatic relief. In a case series, LES BoNT-A injections 20 U were used as a decision tool in whether to proceed with definitive treatment.14
Gastroparesis is a disorder of impaired gastric motility without mechanical obstruction. Pyloric sphincter BoNT-A injections are useful in refractory patients. Multiple prospective, noncontrolled (4), retrospective (3), and randomized placebo-controlled (2), studies with limited enrollment showed benefit for 37.5% to 100% of patients receiving BoNT-A injections of 80 to 200 U.15
Musculoskeletal disorders
Cervical dystonia (CD) entails involuntary contractions of the neck and upper shoulder musculature, causing abnormal neck, shoulder, and head posturing. BoNT-A is first-line treatment for CD.5 BoNT-A is more efficacious than trihexyphenidyl based on multiple large, high-quality studies.16
Continue to: Chronic low back pain
Chronic low back pain (CLBP) is defined as back pain persisting ≥ 12 weeks. More than 80% of adults have had at least 1 episode of back pain in their lifetime.
Myofascial pain syndrome (MPS) consists of myofascial trigger points (palpable, tender nodules that produce pain) with multiple pathophysiological etiologies that include dysfunctional acetylcholine activity, which releases nociceptive neurotransmitters. Studies have yielded inconsistent effects of BoNT-A on MPS.18
Spastic disorders
Cerebral palsy (CP) involves altered muscle tone, posture, and movement secondary to central motor dysfunction with spasticity. Evaluation of BoNT-A as an adjunctive therapy in CP has been extensive and conflicting. A prospective cohort study evaluating gastrocsoleus BoNT-A injections along with gait analysis in 37 children with CP showed no significant improvements.30 In 60 children with CP who received BoNT-A injections, there was improvement in muscle tone and range of motion, while gait improved in patients up to (but not after) age 7 years.19 A multicenter Dutch study of 65 children compared BoNT-A injections in addition to a comprehensive rehabilitation program vs rehabilitation alone, with no difference identified.20
Neonatal brachial plexus palsy (NBPP) is damage to the brachial plexus as a result of trauma during the perinatal period. It is typically self-resolving but can cause residual functional impairment. Surgery is recommended for serious injuries or if functional recovery is not achieved within 9 months. Off-label use of BoNT-A has been shown to be effective in relieving muscle contractures and imbalance, but data are limited and there have only been small studies performed.21 A retrospective cohort study of 59 patients with NBPP who received BoNT-A injections showed improved range of motion and function of the affected extremity. Moreover, surgical intervention was deferred, modified, or averted in patients who were under consideration for more invasive treatment.21
Post-stroke spasticity can be temporarily relieved with the use of BoNT-A injections. Several studies have examined the effect of BoNT-A coupled with rehabilitation programs vs injections alone in the treatment of post-stroke spasticity. Devier et al found that improvements in spasticity scores did not differ between groups; however, implementing rehabilitation after BoNT-A injections was associated with improved function compared to injection alone.31 A 2018 randomized, double-blind, placebo-controlled trial demonstrated improvements in both treatment groups: those who received BoNT-A plus targeted rehab regimen and those who received saline injection plus rehab.22 In this case, it appears BoNT-A acts as more of an adjunct to physical therapy in the treatment of post-stroke spasticity.5
Continue to: Hemifacial spasm
Hemifacial spasm is an involuntary, brief, irregular unilateral (sometimes bilateral) spasm of the face in the distribution of the facial nerve. Injections with BoNT-A have been deemed effective by the American Academy of Neurology.23 A 16-year retrospective study examined the efficacy and adverse effects of BoNT-A in the treatment of hemifacial spasm in 113 patients with a mean age of 63.1 years; it demonstrated high efficacy and mild temporary adverse effects.24 The duration of improvement averaged 16 weeks; pretarsal injections had better results than preseptal injections; and there were no differences between the commercial brands.
Blepharospasm is a focal dystonia marked by excessive blinking and involuntary eye closures due to overexcitability of orbicularis oculi and periocular muscles, and BoNT-A is the treatment of choice.5,25 A retrospective review of 19 patients with blepharospasm who were treated with BoNT-A for more than 5 years found that BoNT-A is a stable and effective treatment with an adverse event rate of 4%. Additionally, there were no differences found in clinical efficacy between the 4 BoNT-A brands on the market.25
Laryngeal tics can cause significant psychosocial distress for patients. This condition is characterized by involuntary, recurrent rhythmic sounds that are often preceded by premonitory urges that are relieved by the behavior. An open-label, uncontrolled, confirmatory study with 30 subjects showed that bilateral vocal cord BoNT-A injections resulted in 93% improvement in vocal tics.26 A subsequent study highlighted case histories of 2 patients with laryngeal tics who received thyroarytenoid muscle BoNT-A injections and had marked reduction in symptoms and premonitory sensations.27 Although these small studies have suggested possible effectiveness of BoNT-A for laryngeal tics, there is no high-quality evidence.
Urologic disorders
Overactive, idiopathic overactive, or neurogenic bladder causes increased urinary frequency, urgency, and nocturia without infectious etiology; they can be a result of neurologic dysregulation, detrusor overactivity, or idiopathic causes. Intravesical BoNT-A injection of 100 to 300 U has been found effective for symptoms refractory to anticholinergic and lifestyle therapy, with increased cystometric capacity (229.1 to 427 mL, P < .00001), decreased maximum detrusor pressure (60.7 to 26.1 cm H2O, P < .00001), and resolution of urgency in 87% of patients (P < .001).28
Interstitial cystitis, also known as painful bladder syndrome, is characterized by reduced bladder emptying, urethral pressure, and residual urine pressure, with symptoms of increased urinary frequency without infection. Intravesicular BoNT-A injections have not consistently been effective in treatment of this condition.28
Continue to: Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome
Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome involve urethral sphincter spasticity with difficulty passing urine and possibly retention. Urethral sphincter injections of 100 U BoNT-A improved flow rates and decreased residual volume. A randomized, double-blinded, placebo-controlled study showed a significantly improved International Prostate Symptom Score (IPSS), quality of life index, maximum flow rate, voided volume, and decreased detrusor voiding pressure at 1 month.29
Benign prostatic hypertrophy (BPH) is a very common condition leading to outlet obstruction. The mainstays of treatment are 5-α-reductase inhibitors, α-adrenergic blockers, and surgical removal.
Gynecologic disorders
Vaginismus is the involuntary, recurrent, or persistent contraction of the perineal muscles surrounding the outer third of the vagina; it is classified by 4 progressively more severe degrees of intensity. Levator ani, bulbospongiosus, bulbocavernosus, pubococcygeus, and/or puborectalis muscle BoNT-A injections have shown benefits in decreasing resistance to vaginal exams (95.8%) and the ability to achieve satisfactory sexual intercourse after first injection (75%-100%). Effects were transient for up to 15.4% of patients requiring repeat injections.28
Vulvodynia is vulvar pain and orgasmic difficulties and has been treated with bulbospongiosus muscle BoNT-A injections in retrospective studies. A single randomized, double-blinded, placebo-controlled study showed significantly improved pain scores after 1 to 2 injection series.28
Chronic pelvic pain is a syndrome of somatic functional or regional pain, which can be caused by the spasm of the pelvic musculature with or without trigger points. Patients with pain refractory to treatment have been treated with levator ani injections. A retrospective cohort study found 79.3% of patients experienced pain relief and 20.7% reported improved symptoms. In a double-blind, randomized, placebo-controlled trial, pelvic floor muscles were injected with 80 U BoNT or saline, and symptoms were evaluated along with vaginal manometry. BoNT was associated with a reduction in some pain but not as much as placebo, while vaginal pressures decreased more with BoNT than with placebo.28
Blake Busey, DO, FAAFP, Texas Tech University of Health Sciences El Paso–Transmountain, 2000B Transmountain Road, Suite B400, El Paso, TX 79911; [email protected]
1. American Society of Plastic Surgeons. New statistics reveal the shape of plastic surgery [news release]. March 1, 2018. www.plasticsurgery.org/news/press-releases/new-statistics-reveal-the-shape-of-plastic-surgery. Accessed October 23, 2020.
2. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalgia. 2010;30:804-814.
3. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616.
4. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826.
6. Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818-3844.
7. Sostak P, Krause P, Förderreuther S, et al. Botulinum toxin type-A therapy in cluster headache: an open study. J Headache Pain. 2007;8:236-241.
8. Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503-509.
9. Mandavia R, Dessouky O, Dhar V, et al. The use of botulinum toxin in otorhinolaryngology: an updated review. Clin Otolaryngol. 2014;39:203-209.
10. Klein AM, Stong BC, Wise J, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:421-423.
11. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596-599.
12. Rosen R, Stewart T. Results of a 10-year follow-up study of botulinum toxin A therapy for primary axillary hyperhidrosis in Australia. Intern Med J. 2018;48:343-347.
13. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins (Basel). 2018;10:55.
14. Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
15. Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc. 2015;7:790-798.
16. Zakin E, Simpson D. Evidence on botulinum toxin in selected disorders. Toxicon. 2018;147:134-140.
17. Jabbari B, Ney J, Sichani A, et al. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264.
18. Climent JM, Kuan TS, Fenollosa P, et al. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459.
19. Mirska A, Cybula K, Okurowska-Zawada B, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517-522.
20. Schasfoort F, Pangalila R, Sneekes EM, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732-742.
21. Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6:1107-1119.
22. Prazeres A, Lira M, Aguiar P, et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol Int. 2018;10:7385.
23. Simpson DM, Blitzer A, Brashear A, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
24. Sorgun MH, Yilmaz R, Akin YA, et al. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22:1319-1325.
25. Lee S, Park S, Lew H. Long-term efficacy of botulinum neurotoxin-A treatment for essential blepharospasm. Korean J Ophthalmol. 2018;32:1-7.
26. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
27. Vincent DA Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22:251-256.
28. Moga MA, Dimienescu OG, Balan A, et al. Therapeutic approaches of botulinum toxin in gynecology. Toxins (Basel) 2018;10:169.
29. Jhang J-F, Kuo H-C. Novel applications of onabotulinumtoxinA in lower urinary tract dysfunction. Toxins (Basel). 2018;10:260.
30. Hastings-Ison T, Sangeux M, Thomason P, et al. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: a prospective kinematic study. J Child Orthop. 2018;12:390-397.
31. Devier D, Harnar J, Lopez L, et al. Rehabilitation plus onabotulinumtoxina improves motor function over onabotulinumtoxina alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins. 2017;9:216.
32. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24:1-6.
33. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes—an evidence based review. Toxicon. 2018;147:120-128.
1. American Society of Plastic Surgeons. New statistics reveal the shape of plastic surgery [news release]. March 1, 2018. www.plasticsurgery.org/news/press-releases/new-statistics-reveal-the-shape-of-plastic-surgery. Accessed October 23, 2020.
2. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalgia. 2010;30:804-814.
3. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616.
4. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826.
6. Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818-3844.
7. Sostak P, Krause P, Förderreuther S, et al. Botulinum toxin type-A therapy in cluster headache: an open study. J Headache Pain. 2007;8:236-241.
8. Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503-509.
9. Mandavia R, Dessouky O, Dhar V, et al. The use of botulinum toxin in otorhinolaryngology: an updated review. Clin Otolaryngol. 2014;39:203-209.
10. Klein AM, Stong BC, Wise J, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:421-423.
11. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596-599.
12. Rosen R, Stewart T. Results of a 10-year follow-up study of botulinum toxin A therapy for primary axillary hyperhidrosis in Australia. Intern Med J. 2018;48:343-347.
13. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins (Basel). 2018;10:55.
14. Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
15. Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc. 2015;7:790-798.
16. Zakin E, Simpson D. Evidence on botulinum toxin in selected disorders. Toxicon. 2018;147:134-140.
17. Jabbari B, Ney J, Sichani A, et al. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264.
18. Climent JM, Kuan TS, Fenollosa P, et al. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459.
19. Mirska A, Cybula K, Okurowska-Zawada B, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517-522.
20. Schasfoort F, Pangalila R, Sneekes EM, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732-742.
21. Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6:1107-1119.
22. Prazeres A, Lira M, Aguiar P, et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol Int. 2018;10:7385.
23. Simpson DM, Blitzer A, Brashear A, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
24. Sorgun MH, Yilmaz R, Akin YA, et al. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22:1319-1325.
25. Lee S, Park S, Lew H. Long-term efficacy of botulinum neurotoxin-A treatment for essential blepharospasm. Korean J Ophthalmol. 2018;32:1-7.
26. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
27. Vincent DA Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22:251-256.
28. Moga MA, Dimienescu OG, Balan A, et al. Therapeutic approaches of botulinum toxin in gynecology. Toxins (Basel) 2018;10:169.
29. Jhang J-F, Kuo H-C. Novel applications of onabotulinumtoxinA in lower urinary tract dysfunction. Toxins (Basel). 2018;10:260.
30. Hastings-Ison T, Sangeux M, Thomason P, et al. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: a prospective kinematic study. J Child Orthop. 2018;12:390-397.
31. Devier D, Harnar J, Lopez L, et al. Rehabilitation plus onabotulinumtoxina improves motor function over onabotulinumtoxina alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins. 2017;9:216.
32. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24:1-6.
33. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes—an evidence based review. Toxicon. 2018;147:120-128.
PRACTICE RECOMMENDATIONS
› Do not use botulinum toxin for episodic migraine, tension headache, or cluster headaches. B
› Consider off-label use of botulinum toxin for select patients with occipital and trigeminal neuralgia, gastroparesis, vaginismus, benign prostatic hypertrophy, neonatal brachial plexus palsy, post-stroke spasticity, and hemifacial spasm. B
› Consider the use of botulinum toxin as an adjunct in chronic low back pain management. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What imaging can disclose about suspected stroke and its Tx
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3
NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25
Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3

NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25

Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3

NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25

Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
How to assess and relieve that perplexing rashless itch
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.
An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2
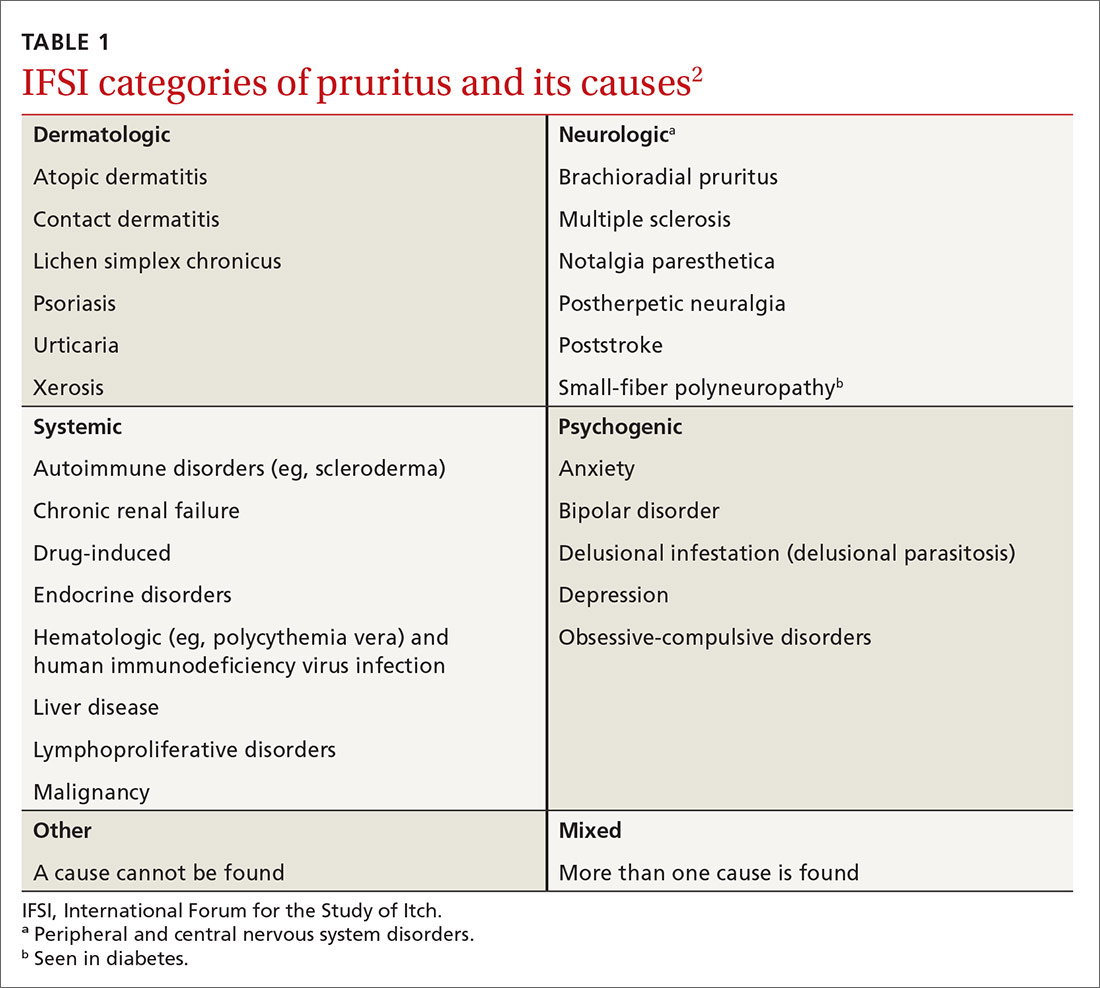
A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2
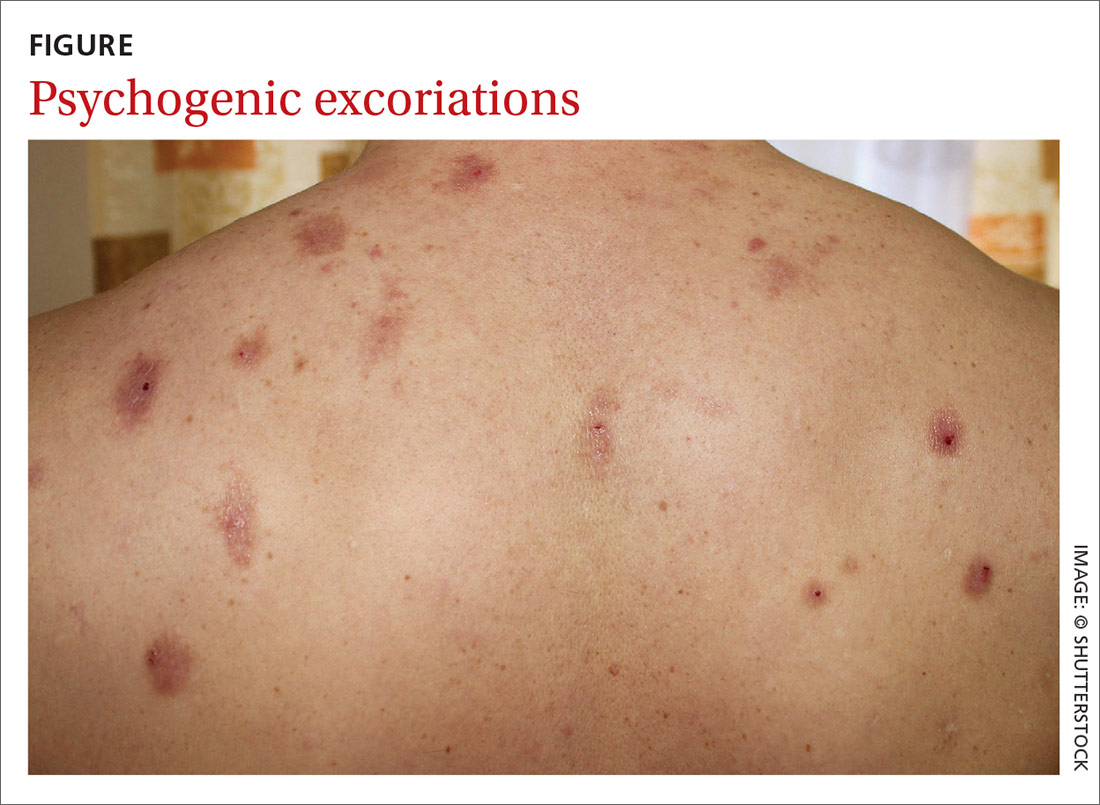
Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17
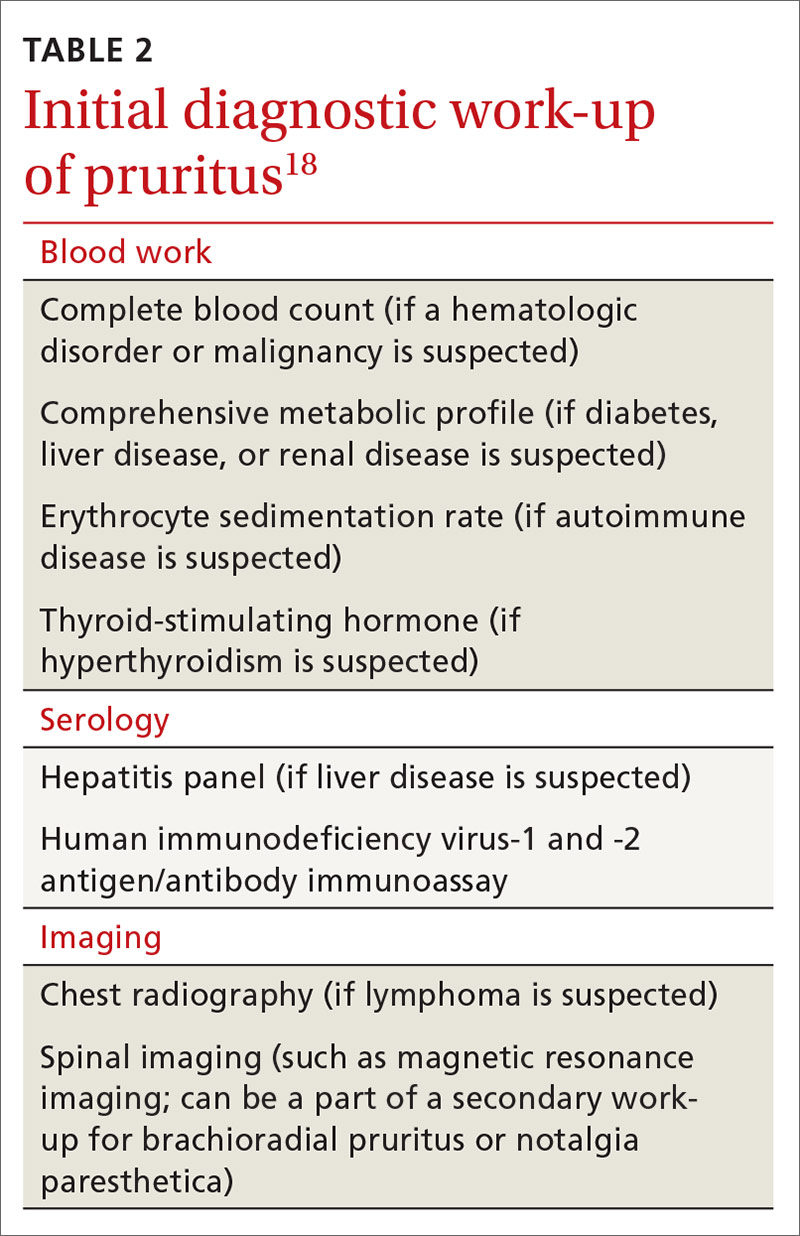
Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7
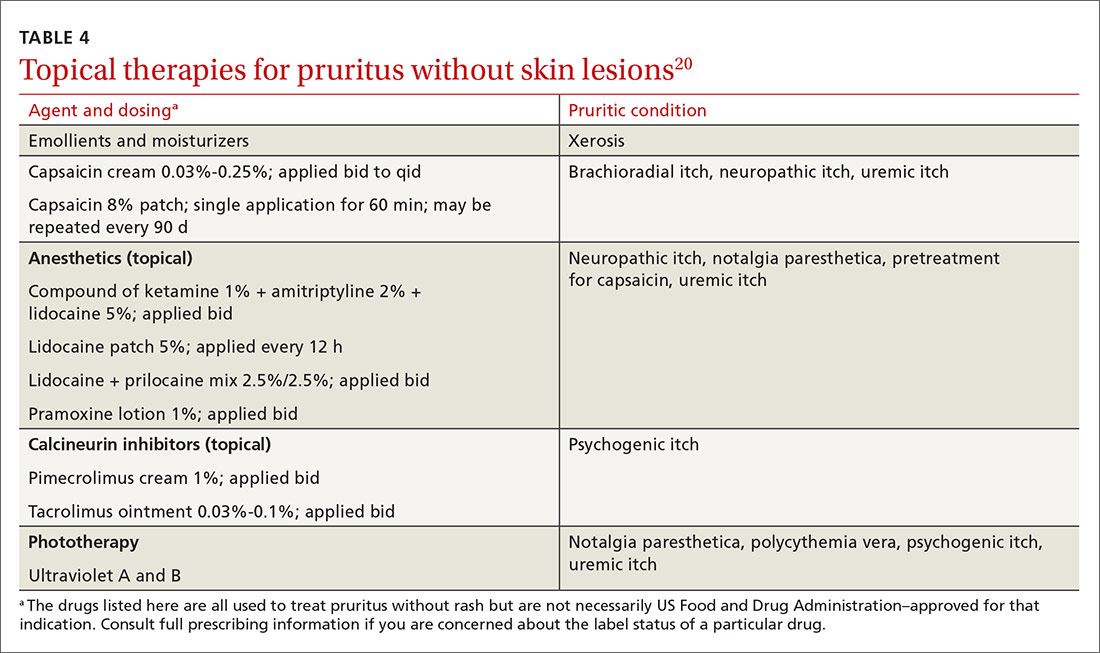
Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
PRACTICE RECOMMENDATIONS
› Undertake a diagnostic work-up for systemic causes of pruritus in patients who have a chronic, generalized itch and abnormal findings on physical examination. C
› Prescribe gabapentin for its effectiveness in treating pruritus caused by uremic and neurologic itch. B
› Consider prescribing one of the bile-acid sequestrants in patients with cholestatic pruritus because these agents can provide moderate relief of the symptom. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Low back pain in youth: Recognizing red flags
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
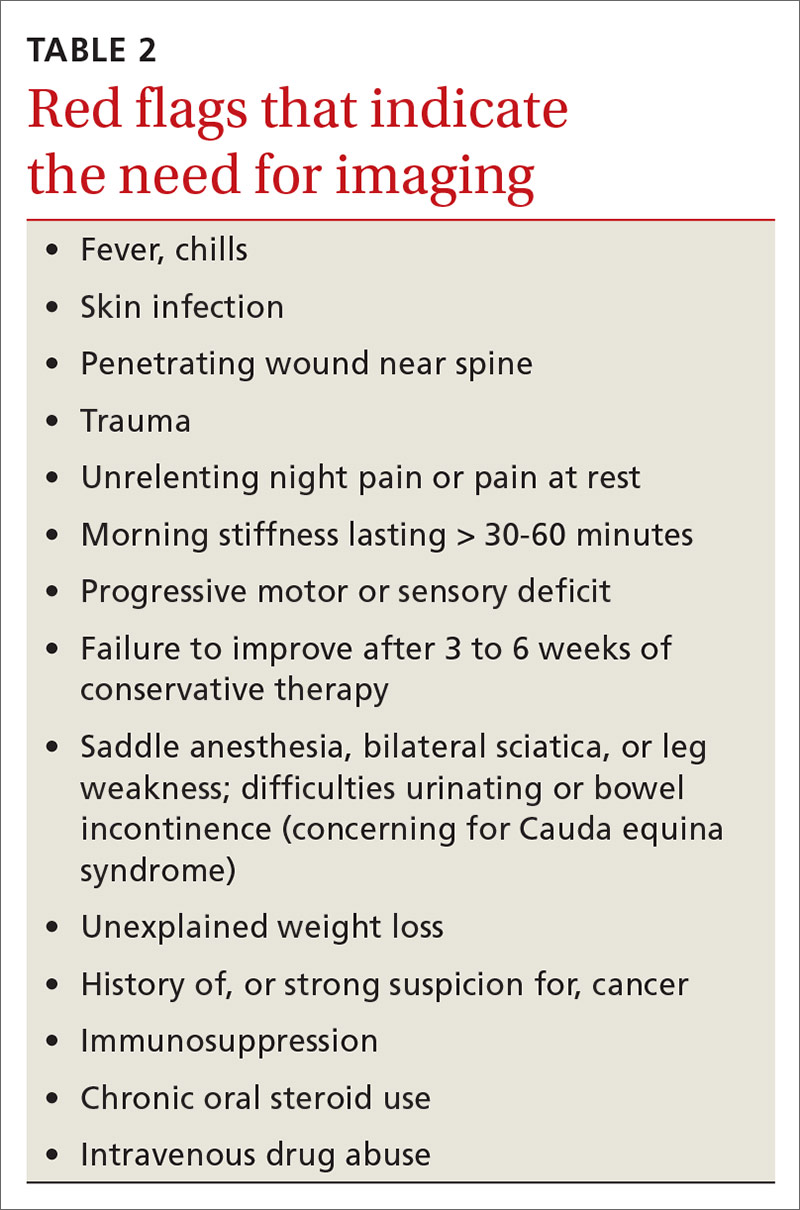
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
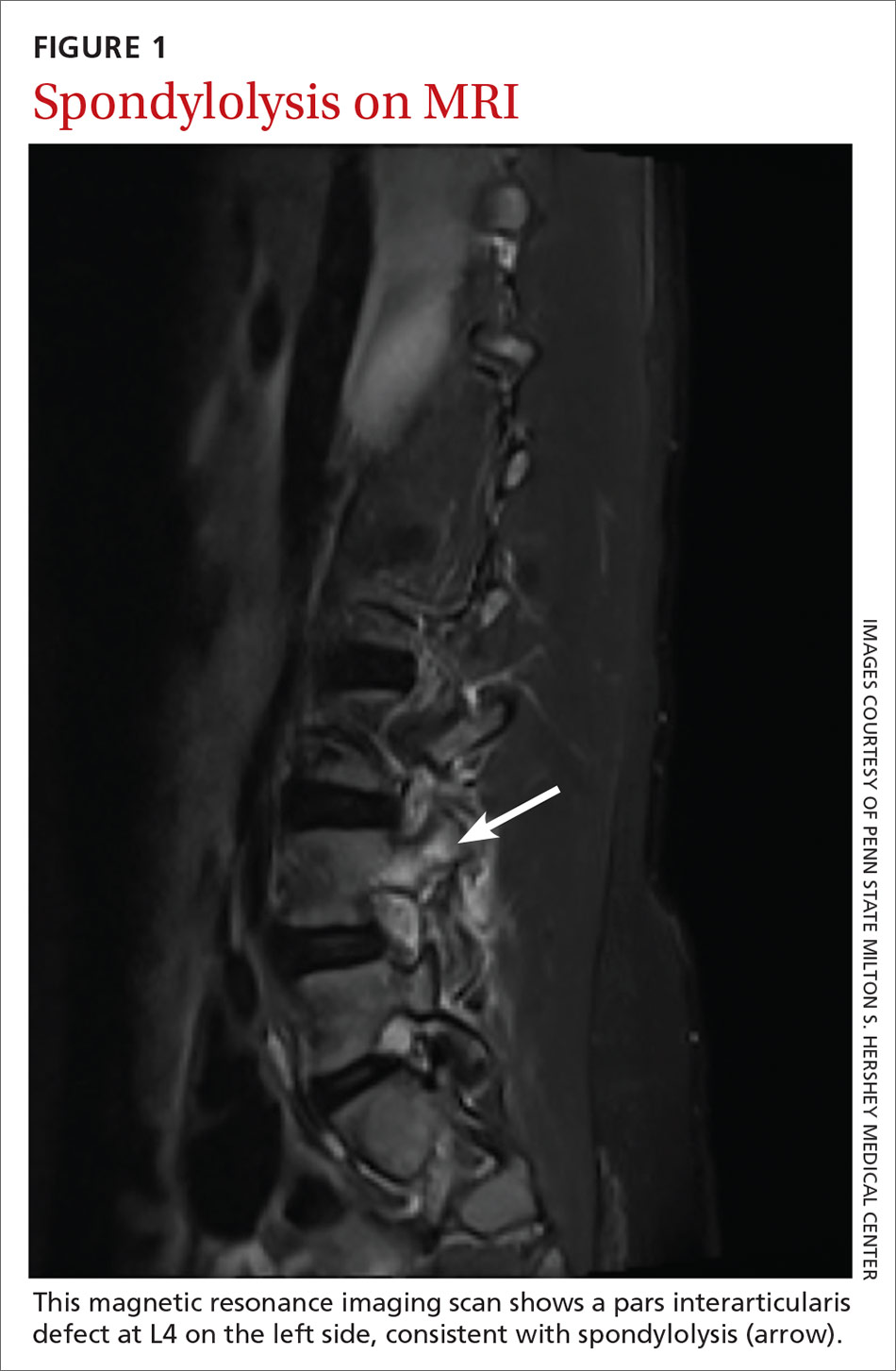
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
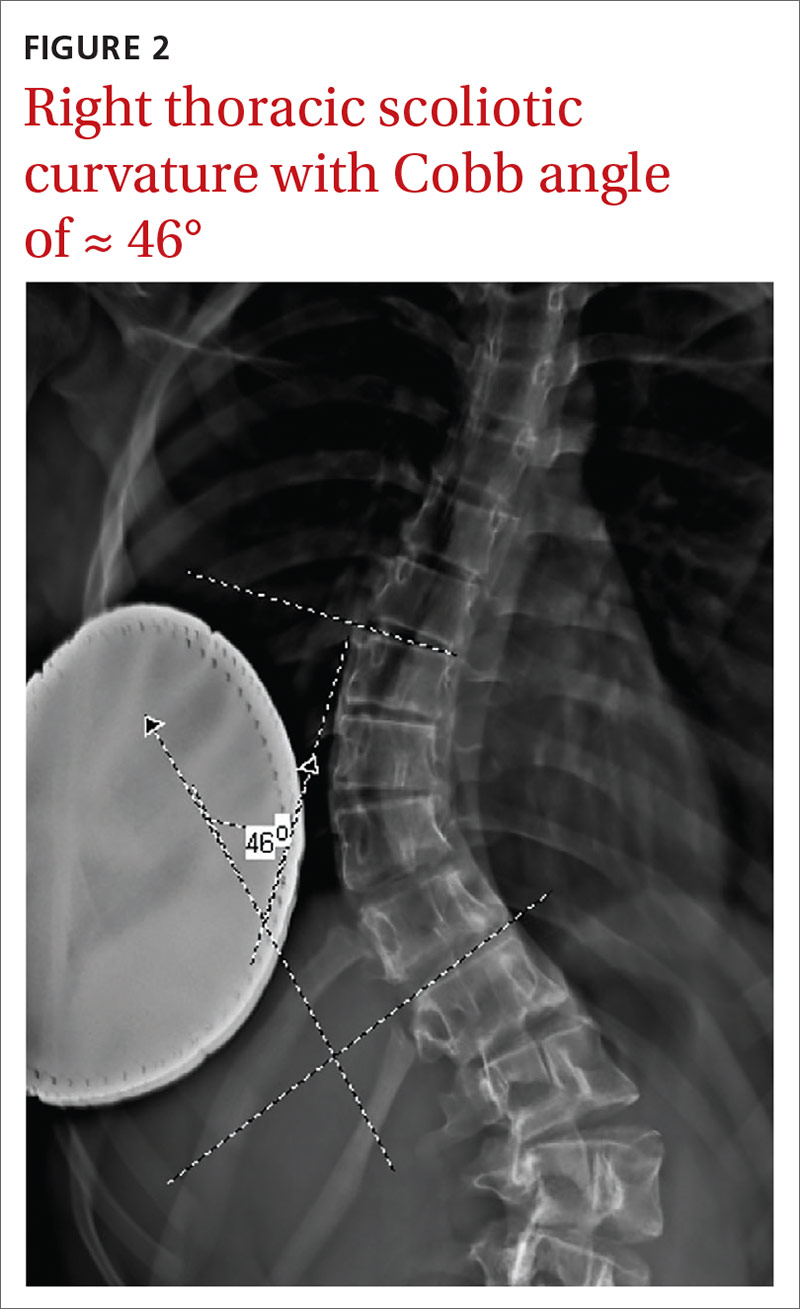
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
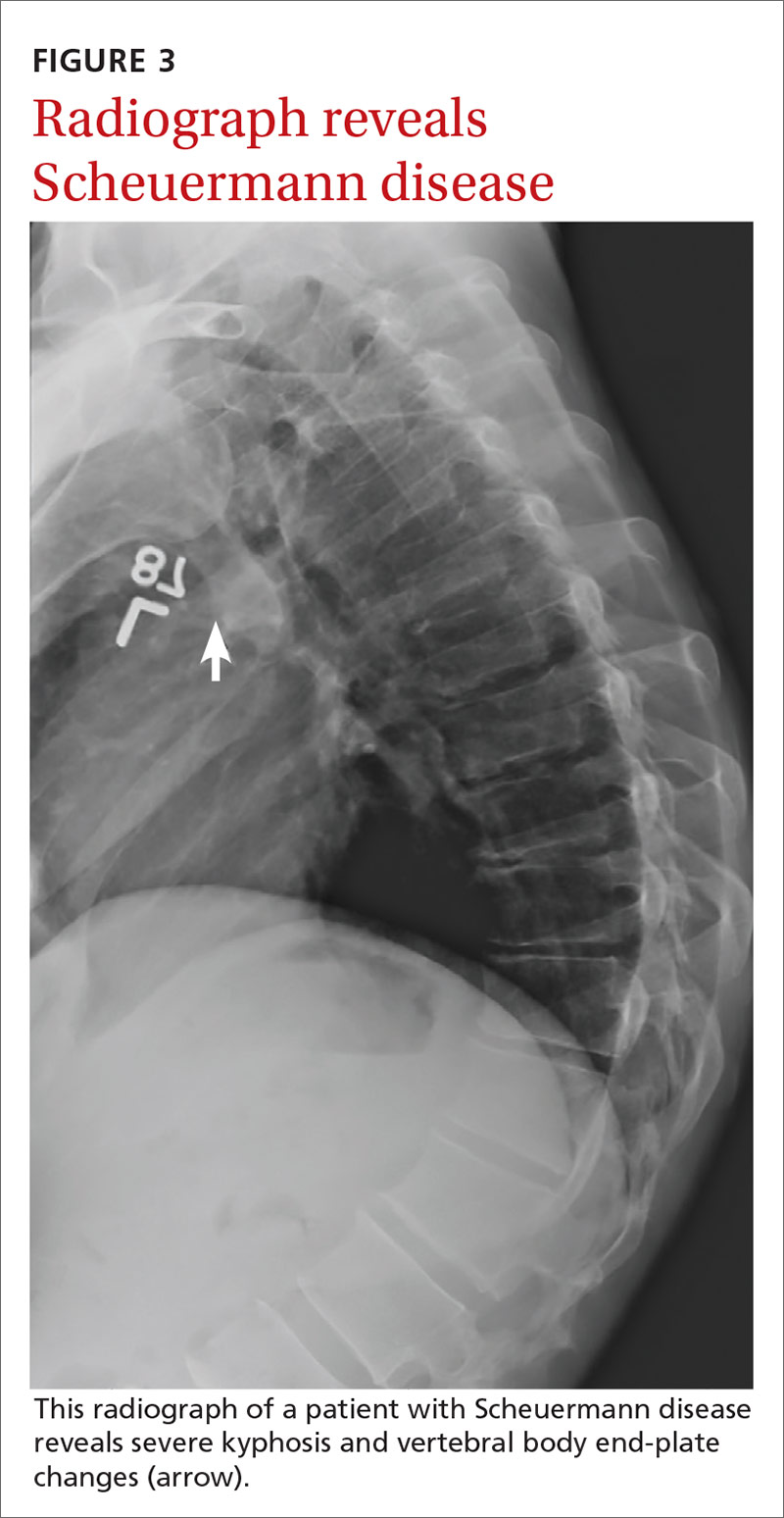
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.

The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22

Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35

If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18

In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.

The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22

Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35

If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18

In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
PRACTICE RECOMMENDATIONS
› Be aware that low back pain is rare in children < 7 years but increases in incidence as children near adolescence. A
› Consider imaging in the setting of bony tenderness, pain that awakens the patient from sleep, or in the presence of other “red flag” symptoms. A
› Consider spondylolysis and spondylolisthesis in adolescent athletes with low back pain lasting longer than 3 to 6 weeks. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neurofibromatosis type 1: More than skin deep
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder that is estimated to occur in 1:2500 births and to have a prevalence of 1:2000 to 1:4000.1,2 It was first described in 1882 by Friedrich Daniel Von Recklinghausen, who identified patients and their relatives with signs of neuroectodermal abnormalities (café-au-lait macules [CALMs], axillary and inguinal freckling, and neurofibromas).
NF1 may begin insidiously in childhood and evolves as the patient ages. It is associated with intracranial, intraspinal, and intraorbital neoplasms, although other organs and tissues can also be involved.
The family physician might be the first one to recognize the signs of this condition during a well-child exam and is in a unique position to coordinate a multidisciplinary approach to care.
A mutated allele and early manifestations on the skin
NF1 has been attributed to genetic mosaicism and is classified as segmental, generalized, or (less frequently) gonadal. The disorder results from germline mutations in the NF1 tumor-suppressor gene on chromosome 17, known to codify the cytoplasmic protein called neurofibromin.3 The penetrance of NF1 is complete, which means that 100% of patients with the mutated allele will develop the disease.
Patients typically have symptoms by the third decade of life, although many will show signs of the disease in early childhood. CALMs are the earliest expression of NF1. They manifest in the first 2 years of life and are found in almost all affected patients. The lesions are well defined and measure 10 to 40 mm. They are typically light brown, although they may darken with sun exposure.
Histologically, the lesions will show macromelanosomes and high concentrations of melanin but do not represent an increased risk for malignancy.4 Not all isolated CALMs are a sign of NF1. While children younger than 29 months with 6 or more CALMs have a high risk for NF1 (80.4%; 95% confidence interval [CI], 74.6% to 86.2%), those who are older than 29 months with at least 1 atypical CALM or fewer than 6 CALMs have just a 0.9% (95% CI, 0% to 2.6%) risk for constitutional NF1.5
Freckles are also observed in 90% of patients with NF1; these tend to develop after the third year of life. The breast and trunk are the most commonly affected areas in adults. The pathophysiology is unknown, but this freckling is believed to be related to skin friction, high humidity, and ambient temperature.6
Continue to: Neurofibromas are benign...
Neurofibromas are benign subcutaneous palpable lesions that grow within peripheral nerve tissue, including spinal, subcutaneous, plexiform, or dermal encapsulated nerves. Originating in Schwann cells, they are composed of fibroblasts, mast cells, macrophages, endothelial cells, and other perineural cells. Some patients show disfiguration when hundreds of these masses are present (FIGURE). These tumors increase in number as the patient ages or during pregnancy, which is thought to be secondary to hormonal changes.7 They are sometimes painful and can be pruritic. Their appearance can also cause patient distress.

The diagnosis is a clinical one
Suspicion for NF1 should be high in patients presenting with the dermatologic findings described, although CALMs and freckling are not exclusive to NF1. Diagnostic criteria for NF1, which distinguish it from other conditions, were first outlined in a National Institutes of Health Consensus Development Conference Statement in 1987.8 The list of criteria has subsequently been expanded.
While the presence of at least 2 criteria is required for diagnosis,2 NF1 should be suspected in individuals who have any of the following findings8,9:
- the presence of at least 6 CALMs that are > 5 mm in prepubertal children and > 15 mm in adults
- 2 or more neurofibromas of any type, or at least one plexiform neurofibroma
- axillary or groin freckling
- optic pathway glioma
- 2 or more Lisch nodules (iris hamartomas seen on slit-lamp examination)
- bony dysplasia (sphenoid wing dysplasia, bowing of long bone ± pseudarthrosis)
- first-degree relative with NF1.
What you’ll see as the disease progresses
NF1 can affect a variety of systems, and potential complications of the disease are numerous and varied (see TABLE9). Here is some of what you may see as the patient’s disease progresses to various organ systems:
Learning disabilities and other cognitive and behavioral problems, such as attention-deficit/hyperactivity disorder, may affect up to 70% of children with NF1. Additionally, children with NF1 have visual/spatial problems, impaired visual motor integration, and language deficits.10 The etiology of cognitive impairment in NF1 is unknown.11
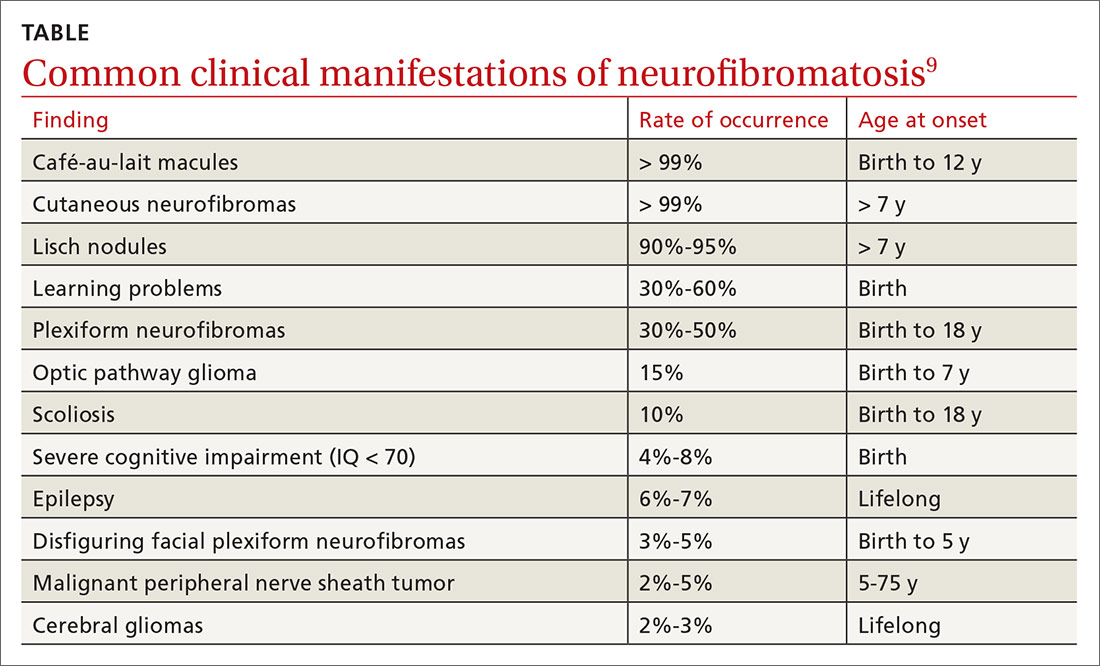
Continue to: Hypertension
Hypertension is common and may contribute to premature death in patients with NF1. Up to 27% of patients will have significant cardiovascular anomalies, including pulmonary valve stenosis, hypertrophic cardiomyopathy in patients with complete deletions of the NF1 gene, intracardiac neurofibromas, renal artery stenosis, coarctation of the aorta, and cerebral infarctions.12 Renal artery stenosis occurs in approximately 2% of the NF1 population, and the diagnosis should be considered in hypertensive children, young adults, pregnant women, older individuals with refractory hypertension, and those with an abdominal bruit.13
Psychological issues. The disfigurement caused by neurofibromas and the uncertainty of an unpredictable disease course can cause psychological manifestations for patients with NF1. Anxiety and depression are common. Not surprisingly, patients with more severe disease report more adverse psychological effects.
Orthopedic deformities. Spinal deformities are the most common skeletal manifestation of NF1, with an incidence estimated from 10% to 25% in various studies. Bone mineral density, as measured by age- and gender-adjusted Z-scores, is significantly lower in NF1 patients than in the general population.14 Children may develop bowing of the long bones, particularly the tibia, and pseudarthrosis, a false joint in a long bone. Children with NF1 need yearly assessment of the spine. Patients with clinical evidence of scoliosis should be referred to Orthopedics for further evaluation.
Eye issues. A majority of adult patients develop neurofibroma-like nodules in the iris known as Lisch nodules. The nodules are not thought to cause any ophthalmologic complications. Patients may also develop palpebral neurofibroma, which may become large and sporadically show malignant transformation. Optic nerve glioma may cause strabismus and proptosis, and a large number of patients will also develop glaucoma and globe enlargement.15
Gastrointestinal lesions and cancer. Neurofibromas can grow in the stomach, liver, mesentery, retroperitoneum, and bowel. Adenocarcinoma developed in 23% of patients.16 Gastrointestinal tract bleeding, pseudo-obstruction, and protein-losing enteropathy also may occur.17
Continue to: Central nervous system manifestations
Central nervous system manifestations. Neurological manifestations have been observed in 55% of patients with NF1.18 These include headache, hydrocephalus, epilepsy, lacunar stroke, white matter disease, intraspinal neurofibroma, facial palsy, radiculopathy, and polyneuropathy. Tumors include optic pathway tumors, meningioma, and cerebral glioma. Glioma is the predominant tumor type in NF1 and occurs in all parts of the nervous system, with a predilection for the optic pathways, brainstem, and cerebellum.18
Malignant peripheral nerve sheath tumors. There is an 8% to 13% lifetime risk for malignant peripheral nerve sheath tumors (MPNST), predominantly in individuals between the ages of 20 and 35.19,20 Any change in neurofibroma from soft to hard, or a rapid increase in the size, is suspicious for MPNST. Other symptoms include persistent pain lasting for longer than a month, pain that disturbs sleep, and new neurological deficits. These cancers can be hard to detect, leading to poor prognosis secondary to metastasis.19,20 The greatest risk factors for MPNST are pain associated with a mass and the presence of cutaneous and subcutaneous neurofibromas.21
Treatment is symptom based, but there is a new option
Treatment is individualized to the patient’s symptoms. Neurofibromas that are disfiguring, disruptive, or malignant may be surgically removed.
In April 2020, the US Food and Drug Administration (FDA) approved selumetinib (Koselugo) for the treatment of pediatric patients (ages ≥ 2 years) with NF1 who have symptomatic, inoperable plexiform neurofibromas (PNs).22 In a clinical trial, patients received selumetinib 25 mg/m2 orally twice a day until they demonstrated disease progression or experienced “unacceptable” adverse events.22,23 The overall response rate was 66%, defined as “the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3 to 6 months.”22
Of note, all patients had a partial, not complete, response. Common adverse effects included vomiting, rash, abdominal pain, diarrhea, and nausea.23 Selumetinib may also cause more serious adverse effects, including cardiomyopathy and ocular toxicity. Prior to treatment initiation and at regular intervals during treatment, patients should undergo cardiac and ophthalmic evaluation.22,23 Selumetinib was granted priority review and orphan drug status by the FDA.22
Continue to: You play a key role in ongoing monitoring
You play a key role in ongoing monitoring
In light of the condition’s heterogeneity, the goals of care include early recognition and treatment of complications, especially neoplasms; optimization of quality of life; and identification and treatment of comorbidities. Family physicians are well positioned to monitor patients with NF1 for age-specific disease manifestations and potential complications.9 All patients require:
- an annual physical examination by a physician who is familiar with the individual and with the disease
- annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- regular blood pressure monitoring
- other studies (eg, MRI) only as indicated on the basis of clinically apparent signs or symptoms
- monitoring by an appropriate specialist if there are abnormalities of the central nervous, skeletal, or cardiovascular systems
- referral to a neurologist for any unexplained neurological signs and symptoms. Referral should be urgent if there are acute symptoms of progressive sensory disturbance, motor deficit and incoordination, or sphincter disturbances since these might indicate an intracranial lesion or spinal cord compression. Headaches on waking, morning vomiting, and altered consciousness are suggestive of raised intracranial pressure.
Children with NF1 benefit from coordinated care between the FP and a pediatrician or other specialist familiar with the disease. In addition to providing usual well care, perform regular assessment of development and school performance. Pay careful attention to the cardiovascular system (particularly blood pressure) and evaluate for scoliosis.
Young adults should be continually monitored for all complications, especially hypertension. This population requires continued education about NF1 and its possible complications and may benefit from counseling about disease inheritance. Screen for anxiety and depression; offer psychological support.
Adults require monitoring based on patient preference and disease severity. For this population, blood pressure should be measured annually, or more frequently if the patient’s values indicate borderline hypertension. Provide education about complications, especially MPNSTs and spinal cord compression. Patients who have abnormalities of the central nervous, skeletal, or cardiovascular systems should be monitored by an appropriate specialist. If desired, the patient may be referred to a geneticist, especially if he or she expresses concern about inheritance. Cutaneous neurofibromas can be removed if they cause discomfort, although removal occasionally results in neurological deficit.
CORRESPONDENCE
T. Grant Phillips, MD, Associate Director, UPMC Altoona Family Physicians Residency, 501 Howard Avenue, Altoona, PA 16601-4899; [email protected]
1. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019;103:1035-1054.
2. Miller DT, Freedenberg D, Schorry E, et al; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143:e20190660.
3. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 200l;61:1-14.
4. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-844.
5. Ben-Shachar S, Dubov T, Toledano-Alhadef H, et al. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J Am Acad Dermatol. 2017;76:1077-1083.e3.
6. Friedman JM. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. www.ncbi.nlm.nih.gov/books/NBK1109. Accessed Septemeber 28, 2020.
7. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A:1624-1633.
8. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD, July 13-15, 1987. Neurofibromatosis. 1988;1:172-178. https://consensus.nih.gov/1987/1987Neurofibramatosis064html.htm. Accessed Septemeber 28, 2020.
9. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
10. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6:185-194.
11. North KN, Riccardi VM, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121-1127.
12. Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
13. Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: a report of the NF1 Cardiovascular Task Force. Genet Med. 2003;4:105-111.
14. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
15. Abdolrahimzadeh B, Piraino DC, Albanese G, et al. Neurofibromatosis: an update of ophthalmic characteristics and applications of optical coherence tomography. Clin Ophthalmol. 2016;10:851-860.
16. Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105.
17. Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J Gastroenterol. 2008;14:80-82.
18. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(pt 3):473-481.
19. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res. 2002;62:1573-1577.
20. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311-314.
21. King AA, Debaun MR, Riccardi VM, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392.
22. US Food and Drug Administration. FDA approves first therapy for children with debilitating and disfiguring rare disease [news release]. April 10, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed September 28, 2020.
23. Koselugo (selumetinib) [product information]. Wilmington, DC: AstraZeneca Pharmaceuticals LP; April 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed September 24, 2020.
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder that is estimated to occur in 1:2500 births and to have a prevalence of 1:2000 to 1:4000.1,2 It was first described in 1882 by Friedrich Daniel Von Recklinghausen, who identified patients and their relatives with signs of neuroectodermal abnormalities (café-au-lait macules [CALMs], axillary and inguinal freckling, and neurofibromas).
NF1 may begin insidiously in childhood and evolves as the patient ages. It is associated with intracranial, intraspinal, and intraorbital neoplasms, although other organs and tissues can also be involved.
The family physician might be the first one to recognize the signs of this condition during a well-child exam and is in a unique position to coordinate a multidisciplinary approach to care.
A mutated allele and early manifestations on the skin
NF1 has been attributed to genetic mosaicism and is classified as segmental, generalized, or (less frequently) gonadal. The disorder results from germline mutations in the NF1 tumor-suppressor gene on chromosome 17, known to codify the cytoplasmic protein called neurofibromin.3 The penetrance of NF1 is complete, which means that 100% of patients with the mutated allele will develop the disease.
Patients typically have symptoms by the third decade of life, although many will show signs of the disease in early childhood. CALMs are the earliest expression of NF1. They manifest in the first 2 years of life and are found in almost all affected patients. The lesions are well defined and measure 10 to 40 mm. They are typically light brown, although they may darken with sun exposure.
Histologically, the lesions will show macromelanosomes and high concentrations of melanin but do not represent an increased risk for malignancy.4 Not all isolated CALMs are a sign of NF1. While children younger than 29 months with 6 or more CALMs have a high risk for NF1 (80.4%; 95% confidence interval [CI], 74.6% to 86.2%), those who are older than 29 months with at least 1 atypical CALM or fewer than 6 CALMs have just a 0.9% (95% CI, 0% to 2.6%) risk for constitutional NF1.5
Freckles are also observed in 90% of patients with NF1; these tend to develop after the third year of life. The breast and trunk are the most commonly affected areas in adults. The pathophysiology is unknown, but this freckling is believed to be related to skin friction, high humidity, and ambient temperature.6
Continue to: Neurofibromas are benign...
Neurofibromas are benign subcutaneous palpable lesions that grow within peripheral nerve tissue, including spinal, subcutaneous, plexiform, or dermal encapsulated nerves. Originating in Schwann cells, they are composed of fibroblasts, mast cells, macrophages, endothelial cells, and other perineural cells. Some patients show disfiguration when hundreds of these masses are present (FIGURE). These tumors increase in number as the patient ages or during pregnancy, which is thought to be secondary to hormonal changes.7 They are sometimes painful and can be pruritic. Their appearance can also cause patient distress.

The diagnosis is a clinical one
Suspicion for NF1 should be high in patients presenting with the dermatologic findings described, although CALMs and freckling are not exclusive to NF1. Diagnostic criteria for NF1, which distinguish it from other conditions, were first outlined in a National Institutes of Health Consensus Development Conference Statement in 1987.8 The list of criteria has subsequently been expanded.
While the presence of at least 2 criteria is required for diagnosis,2 NF1 should be suspected in individuals who have any of the following findings8,9:
- the presence of at least 6 CALMs that are > 5 mm in prepubertal children and > 15 mm in adults
- 2 or more neurofibromas of any type, or at least one plexiform neurofibroma
- axillary or groin freckling
- optic pathway glioma
- 2 or more Lisch nodules (iris hamartomas seen on slit-lamp examination)
- bony dysplasia (sphenoid wing dysplasia, bowing of long bone ± pseudarthrosis)
- first-degree relative with NF1.
What you’ll see as the disease progresses
NF1 can affect a variety of systems, and potential complications of the disease are numerous and varied (see TABLE9). Here is some of what you may see as the patient’s disease progresses to various organ systems:
Learning disabilities and other cognitive and behavioral problems, such as attention-deficit/hyperactivity disorder, may affect up to 70% of children with NF1. Additionally, children with NF1 have visual/spatial problems, impaired visual motor integration, and language deficits.10 The etiology of cognitive impairment in NF1 is unknown.11

Continue to: Hypertension
Hypertension is common and may contribute to premature death in patients with NF1. Up to 27% of patients will have significant cardiovascular anomalies, including pulmonary valve stenosis, hypertrophic cardiomyopathy in patients with complete deletions of the NF1 gene, intracardiac neurofibromas, renal artery stenosis, coarctation of the aorta, and cerebral infarctions.12 Renal artery stenosis occurs in approximately 2% of the NF1 population, and the diagnosis should be considered in hypertensive children, young adults, pregnant women, older individuals with refractory hypertension, and those with an abdominal bruit.13
Psychological issues. The disfigurement caused by neurofibromas and the uncertainty of an unpredictable disease course can cause psychological manifestations for patients with NF1. Anxiety and depression are common. Not surprisingly, patients with more severe disease report more adverse psychological effects.
Orthopedic deformities. Spinal deformities are the most common skeletal manifestation of NF1, with an incidence estimated from 10% to 25% in various studies. Bone mineral density, as measured by age- and gender-adjusted Z-scores, is significantly lower in NF1 patients than in the general population.14 Children may develop bowing of the long bones, particularly the tibia, and pseudarthrosis, a false joint in a long bone. Children with NF1 need yearly assessment of the spine. Patients with clinical evidence of scoliosis should be referred to Orthopedics for further evaluation.
Eye issues. A majority of adult patients develop neurofibroma-like nodules in the iris known as Lisch nodules. The nodules are not thought to cause any ophthalmologic complications. Patients may also develop palpebral neurofibroma, which may become large and sporadically show malignant transformation. Optic nerve glioma may cause strabismus and proptosis, and a large number of patients will also develop glaucoma and globe enlargement.15
Gastrointestinal lesions and cancer. Neurofibromas can grow in the stomach, liver, mesentery, retroperitoneum, and bowel. Adenocarcinoma developed in 23% of patients.16 Gastrointestinal tract bleeding, pseudo-obstruction, and protein-losing enteropathy also may occur.17
Continue to: Central nervous system manifestations
Central nervous system manifestations. Neurological manifestations have been observed in 55% of patients with NF1.18 These include headache, hydrocephalus, epilepsy, lacunar stroke, white matter disease, intraspinal neurofibroma, facial palsy, radiculopathy, and polyneuropathy. Tumors include optic pathway tumors, meningioma, and cerebral glioma. Glioma is the predominant tumor type in NF1 and occurs in all parts of the nervous system, with a predilection for the optic pathways, brainstem, and cerebellum.18
Malignant peripheral nerve sheath tumors. There is an 8% to 13% lifetime risk for malignant peripheral nerve sheath tumors (MPNST), predominantly in individuals between the ages of 20 and 35.19,20 Any change in neurofibroma from soft to hard, or a rapid increase in the size, is suspicious for MPNST. Other symptoms include persistent pain lasting for longer than a month, pain that disturbs sleep, and new neurological deficits. These cancers can be hard to detect, leading to poor prognosis secondary to metastasis.19,20 The greatest risk factors for MPNST are pain associated with a mass and the presence of cutaneous and subcutaneous neurofibromas.21
Treatment is symptom based, but there is a new option
Treatment is individualized to the patient’s symptoms. Neurofibromas that are disfiguring, disruptive, or malignant may be surgically removed.
In April 2020, the US Food and Drug Administration (FDA) approved selumetinib (Koselugo) for the treatment of pediatric patients (ages ≥ 2 years) with NF1 who have symptomatic, inoperable plexiform neurofibromas (PNs).22 In a clinical trial, patients received selumetinib 25 mg/m2 orally twice a day until they demonstrated disease progression or experienced “unacceptable” adverse events.22,23 The overall response rate was 66%, defined as “the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3 to 6 months.”22
Of note, all patients had a partial, not complete, response. Common adverse effects included vomiting, rash, abdominal pain, diarrhea, and nausea.23 Selumetinib may also cause more serious adverse effects, including cardiomyopathy and ocular toxicity. Prior to treatment initiation and at regular intervals during treatment, patients should undergo cardiac and ophthalmic evaluation.22,23 Selumetinib was granted priority review and orphan drug status by the FDA.22
Continue to: You play a key role in ongoing monitoring
You play a key role in ongoing monitoring
In light of the condition’s heterogeneity, the goals of care include early recognition and treatment of complications, especially neoplasms; optimization of quality of life; and identification and treatment of comorbidities. Family physicians are well positioned to monitor patients with NF1 for age-specific disease manifestations and potential complications.9 All patients require:
- an annual physical examination by a physician who is familiar with the individual and with the disease
- annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- regular blood pressure monitoring
- other studies (eg, MRI) only as indicated on the basis of clinically apparent signs or symptoms
- monitoring by an appropriate specialist if there are abnormalities of the central nervous, skeletal, or cardiovascular systems
- referral to a neurologist for any unexplained neurological signs and symptoms. Referral should be urgent if there are acute symptoms of progressive sensory disturbance, motor deficit and incoordination, or sphincter disturbances since these might indicate an intracranial lesion or spinal cord compression. Headaches on waking, morning vomiting, and altered consciousness are suggestive of raised intracranial pressure.
Children with NF1 benefit from coordinated care between the FP and a pediatrician or other specialist familiar with the disease. In addition to providing usual well care, perform regular assessment of development and school performance. Pay careful attention to the cardiovascular system (particularly blood pressure) and evaluate for scoliosis.
Young adults should be continually monitored for all complications, especially hypertension. This population requires continued education about NF1 and its possible complications and may benefit from counseling about disease inheritance. Screen for anxiety and depression; offer psychological support.
Adults require monitoring based on patient preference and disease severity. For this population, blood pressure should be measured annually, or more frequently if the patient’s values indicate borderline hypertension. Provide education about complications, especially MPNSTs and spinal cord compression. Patients who have abnormalities of the central nervous, skeletal, or cardiovascular systems should be monitored by an appropriate specialist. If desired, the patient may be referred to a geneticist, especially if he or she expresses concern about inheritance. Cutaneous neurofibromas can be removed if they cause discomfort, although removal occasionally results in neurological deficit.
CORRESPONDENCE
T. Grant Phillips, MD, Associate Director, UPMC Altoona Family Physicians Residency, 501 Howard Avenue, Altoona, PA 16601-4899; [email protected]
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder that is estimated to occur in 1:2500 births and to have a prevalence of 1:2000 to 1:4000.1,2 It was first described in 1882 by Friedrich Daniel Von Recklinghausen, who identified patients and their relatives with signs of neuroectodermal abnormalities (café-au-lait macules [CALMs], axillary and inguinal freckling, and neurofibromas).
NF1 may begin insidiously in childhood and evolves as the patient ages. It is associated with intracranial, intraspinal, and intraorbital neoplasms, although other organs and tissues can also be involved.
The family physician might be the first one to recognize the signs of this condition during a well-child exam and is in a unique position to coordinate a multidisciplinary approach to care.
A mutated allele and early manifestations on the skin
NF1 has been attributed to genetic mosaicism and is classified as segmental, generalized, or (less frequently) gonadal. The disorder results from germline mutations in the NF1 tumor-suppressor gene on chromosome 17, known to codify the cytoplasmic protein called neurofibromin.3 The penetrance of NF1 is complete, which means that 100% of patients with the mutated allele will develop the disease.
Patients typically have symptoms by the third decade of life, although many will show signs of the disease in early childhood. CALMs are the earliest expression of NF1. They manifest in the first 2 years of life and are found in almost all affected patients. The lesions are well defined and measure 10 to 40 mm. They are typically light brown, although they may darken with sun exposure.
Histologically, the lesions will show macromelanosomes and high concentrations of melanin but do not represent an increased risk for malignancy.4 Not all isolated CALMs are a sign of NF1. While children younger than 29 months with 6 or more CALMs have a high risk for NF1 (80.4%; 95% confidence interval [CI], 74.6% to 86.2%), those who are older than 29 months with at least 1 atypical CALM or fewer than 6 CALMs have just a 0.9% (95% CI, 0% to 2.6%) risk for constitutional NF1.5
Freckles are also observed in 90% of patients with NF1; these tend to develop after the third year of life. The breast and trunk are the most commonly affected areas in adults. The pathophysiology is unknown, but this freckling is believed to be related to skin friction, high humidity, and ambient temperature.6
Continue to: Neurofibromas are benign...
Neurofibromas are benign subcutaneous palpable lesions that grow within peripheral nerve tissue, including spinal, subcutaneous, plexiform, or dermal encapsulated nerves. Originating in Schwann cells, they are composed of fibroblasts, mast cells, macrophages, endothelial cells, and other perineural cells. Some patients show disfiguration when hundreds of these masses are present (FIGURE). These tumors increase in number as the patient ages or during pregnancy, which is thought to be secondary to hormonal changes.7 They are sometimes painful and can be pruritic. Their appearance can also cause patient distress.

The diagnosis is a clinical one
Suspicion for NF1 should be high in patients presenting with the dermatologic findings described, although CALMs and freckling are not exclusive to NF1. Diagnostic criteria for NF1, which distinguish it from other conditions, were first outlined in a National Institutes of Health Consensus Development Conference Statement in 1987.8 The list of criteria has subsequently been expanded.
While the presence of at least 2 criteria is required for diagnosis,2 NF1 should be suspected in individuals who have any of the following findings8,9:
- the presence of at least 6 CALMs that are > 5 mm in prepubertal children and > 15 mm in adults
- 2 or more neurofibromas of any type, or at least one plexiform neurofibroma
- axillary or groin freckling
- optic pathway glioma
- 2 or more Lisch nodules (iris hamartomas seen on slit-lamp examination)
- bony dysplasia (sphenoid wing dysplasia, bowing of long bone ± pseudarthrosis)
- first-degree relative with NF1.
What you’ll see as the disease progresses
NF1 can affect a variety of systems, and potential complications of the disease are numerous and varied (see TABLE9). Here is some of what you may see as the patient’s disease progresses to various organ systems:
Learning disabilities and other cognitive and behavioral problems, such as attention-deficit/hyperactivity disorder, may affect up to 70% of children with NF1. Additionally, children with NF1 have visual/spatial problems, impaired visual motor integration, and language deficits.10 The etiology of cognitive impairment in NF1 is unknown.11

Continue to: Hypertension
Hypertension is common and may contribute to premature death in patients with NF1. Up to 27% of patients will have significant cardiovascular anomalies, including pulmonary valve stenosis, hypertrophic cardiomyopathy in patients with complete deletions of the NF1 gene, intracardiac neurofibromas, renal artery stenosis, coarctation of the aorta, and cerebral infarctions.12 Renal artery stenosis occurs in approximately 2% of the NF1 population, and the diagnosis should be considered in hypertensive children, young adults, pregnant women, older individuals with refractory hypertension, and those with an abdominal bruit.13
Psychological issues. The disfigurement caused by neurofibromas and the uncertainty of an unpredictable disease course can cause psychological manifestations for patients with NF1. Anxiety and depression are common. Not surprisingly, patients with more severe disease report more adverse psychological effects.
Orthopedic deformities. Spinal deformities are the most common skeletal manifestation of NF1, with an incidence estimated from 10% to 25% in various studies. Bone mineral density, as measured by age- and gender-adjusted Z-scores, is significantly lower in NF1 patients than in the general population.14 Children may develop bowing of the long bones, particularly the tibia, and pseudarthrosis, a false joint in a long bone. Children with NF1 need yearly assessment of the spine. Patients with clinical evidence of scoliosis should be referred to Orthopedics for further evaluation.
Eye issues. A majority of adult patients develop neurofibroma-like nodules in the iris known as Lisch nodules. The nodules are not thought to cause any ophthalmologic complications. Patients may also develop palpebral neurofibroma, which may become large and sporadically show malignant transformation. Optic nerve glioma may cause strabismus and proptosis, and a large number of patients will also develop glaucoma and globe enlargement.15
Gastrointestinal lesions and cancer. Neurofibromas can grow in the stomach, liver, mesentery, retroperitoneum, and bowel. Adenocarcinoma developed in 23% of patients.16 Gastrointestinal tract bleeding, pseudo-obstruction, and protein-losing enteropathy also may occur.17
Continue to: Central nervous system manifestations
Central nervous system manifestations. Neurological manifestations have been observed in 55% of patients with NF1.18 These include headache, hydrocephalus, epilepsy, lacunar stroke, white matter disease, intraspinal neurofibroma, facial palsy, radiculopathy, and polyneuropathy. Tumors include optic pathway tumors, meningioma, and cerebral glioma. Glioma is the predominant tumor type in NF1 and occurs in all parts of the nervous system, with a predilection for the optic pathways, brainstem, and cerebellum.18
Malignant peripheral nerve sheath tumors. There is an 8% to 13% lifetime risk for malignant peripheral nerve sheath tumors (MPNST), predominantly in individuals between the ages of 20 and 35.19,20 Any change in neurofibroma from soft to hard, or a rapid increase in the size, is suspicious for MPNST. Other symptoms include persistent pain lasting for longer than a month, pain that disturbs sleep, and new neurological deficits. These cancers can be hard to detect, leading to poor prognosis secondary to metastasis.19,20 The greatest risk factors for MPNST are pain associated with a mass and the presence of cutaneous and subcutaneous neurofibromas.21
Treatment is symptom based, but there is a new option
Treatment is individualized to the patient’s symptoms. Neurofibromas that are disfiguring, disruptive, or malignant may be surgically removed.
In April 2020, the US Food and Drug Administration (FDA) approved selumetinib (Koselugo) for the treatment of pediatric patients (ages ≥ 2 years) with NF1 who have symptomatic, inoperable plexiform neurofibromas (PNs).22 In a clinical trial, patients received selumetinib 25 mg/m2 orally twice a day until they demonstrated disease progression or experienced “unacceptable” adverse events.22,23 The overall response rate was 66%, defined as “the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3 to 6 months.”22
Of note, all patients had a partial, not complete, response. Common adverse effects included vomiting, rash, abdominal pain, diarrhea, and nausea.23 Selumetinib may also cause more serious adverse effects, including cardiomyopathy and ocular toxicity. Prior to treatment initiation and at regular intervals during treatment, patients should undergo cardiac and ophthalmic evaluation.22,23 Selumetinib was granted priority review and orphan drug status by the FDA.22
Continue to: You play a key role in ongoing monitoring
You play a key role in ongoing monitoring
In light of the condition’s heterogeneity, the goals of care include early recognition and treatment of complications, especially neoplasms; optimization of quality of life; and identification and treatment of comorbidities. Family physicians are well positioned to monitor patients with NF1 for age-specific disease manifestations and potential complications.9 All patients require:
- an annual physical examination by a physician who is familiar with the individual and with the disease
- annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- regular blood pressure monitoring
- other studies (eg, MRI) only as indicated on the basis of clinically apparent signs or symptoms
- monitoring by an appropriate specialist if there are abnormalities of the central nervous, skeletal, or cardiovascular systems
- referral to a neurologist for any unexplained neurological signs and symptoms. Referral should be urgent if there are acute symptoms of progressive sensory disturbance, motor deficit and incoordination, or sphincter disturbances since these might indicate an intracranial lesion or spinal cord compression. Headaches on waking, morning vomiting, and altered consciousness are suggestive of raised intracranial pressure.
Children with NF1 benefit from coordinated care between the FP and a pediatrician or other specialist familiar with the disease. In addition to providing usual well care, perform regular assessment of development and school performance. Pay careful attention to the cardiovascular system (particularly blood pressure) and evaluate for scoliosis.
Young adults should be continually monitored for all complications, especially hypertension. This population requires continued education about NF1 and its possible complications and may benefit from counseling about disease inheritance. Screen for anxiety and depression; offer psychological support.
Adults require monitoring based on patient preference and disease severity. For this population, blood pressure should be measured annually, or more frequently if the patient’s values indicate borderline hypertension. Provide education about complications, especially MPNSTs and spinal cord compression. Patients who have abnormalities of the central nervous, skeletal, or cardiovascular systems should be monitored by an appropriate specialist. If desired, the patient may be referred to a geneticist, especially if he or she expresses concern about inheritance. Cutaneous neurofibromas can be removed if they cause discomfort, although removal occasionally results in neurological deficit.
CORRESPONDENCE
T. Grant Phillips, MD, Associate Director, UPMC Altoona Family Physicians Residency, 501 Howard Avenue, Altoona, PA 16601-4899; [email protected]
1. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019;103:1035-1054.
2. Miller DT, Freedenberg D, Schorry E, et al; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143:e20190660.
3. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 200l;61:1-14.
4. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-844.
5. Ben-Shachar S, Dubov T, Toledano-Alhadef H, et al. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J Am Acad Dermatol. 2017;76:1077-1083.e3.
6. Friedman JM. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. www.ncbi.nlm.nih.gov/books/NBK1109. Accessed Septemeber 28, 2020.
7. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A:1624-1633.
8. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD, July 13-15, 1987. Neurofibromatosis. 1988;1:172-178. https://consensus.nih.gov/1987/1987Neurofibramatosis064html.htm. Accessed Septemeber 28, 2020.
9. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
10. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6:185-194.
11. North KN, Riccardi VM, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121-1127.
12. Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
13. Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: a report of the NF1 Cardiovascular Task Force. Genet Med. 2003;4:105-111.
14. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
15. Abdolrahimzadeh B, Piraino DC, Albanese G, et al. Neurofibromatosis: an update of ophthalmic characteristics and applications of optical coherence tomography. Clin Ophthalmol. 2016;10:851-860.
16. Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105.
17. Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J Gastroenterol. 2008;14:80-82.
18. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(pt 3):473-481.
19. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res. 2002;62:1573-1577.
20. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311-314.
21. King AA, Debaun MR, Riccardi VM, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392.
22. US Food and Drug Administration. FDA approves first therapy for children with debilitating and disfiguring rare disease [news release]. April 10, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed September 28, 2020.
23. Koselugo (selumetinib) [product information]. Wilmington, DC: AstraZeneca Pharmaceuticals LP; April 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed September 24, 2020.
1. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019;103:1035-1054.
2. Miller DT, Freedenberg D, Schorry E, et al; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143:e20190660.
3. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 200l;61:1-14.
4. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-844.
5. Ben-Shachar S, Dubov T, Toledano-Alhadef H, et al. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J Am Acad Dermatol. 2017;76:1077-1083.e3.
6. Friedman JM. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. www.ncbi.nlm.nih.gov/books/NBK1109. Accessed Septemeber 28, 2020.
7. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A:1624-1633.
8. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD, July 13-15, 1987. Neurofibromatosis. 1988;1:172-178. https://consensus.nih.gov/1987/1987Neurofibramatosis064html.htm. Accessed Septemeber 28, 2020.
9. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
10. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6:185-194.
11. North KN, Riccardi VM, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121-1127.
12. Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
13. Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: a report of the NF1 Cardiovascular Task Force. Genet Med. 2003;4:105-111.
14. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
15. Abdolrahimzadeh B, Piraino DC, Albanese G, et al. Neurofibromatosis: an update of ophthalmic characteristics and applications of optical coherence tomography. Clin Ophthalmol. 2016;10:851-860.
16. Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105.
17. Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J Gastroenterol. 2008;14:80-82.
18. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(pt 3):473-481.
19. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res. 2002;62:1573-1577.
20. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311-314.
21. King AA, Debaun MR, Riccardi VM, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392.
22. US Food and Drug Administration. FDA approves first therapy for children with debilitating and disfiguring rare disease [news release]. April 10, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed September 28, 2020.
23. Koselugo (selumetinib) [product information]. Wilmington, DC: AstraZeneca Pharmaceuticals LP; April 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed September 24, 2020.
Primary prevention of VTE spans a spectrum
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
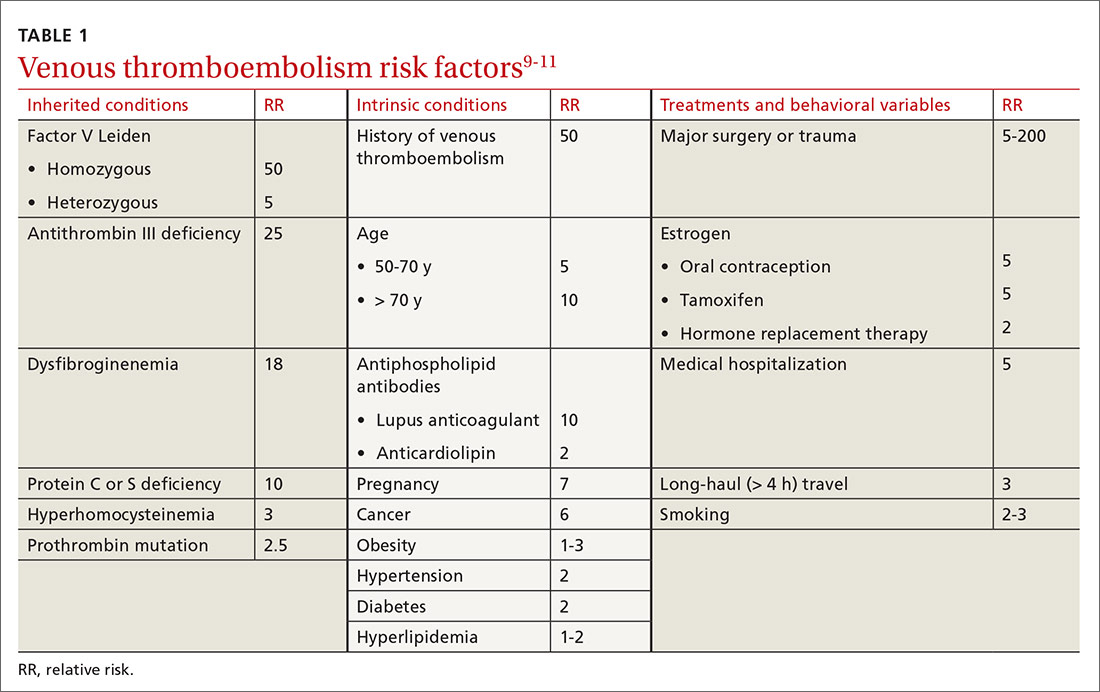
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
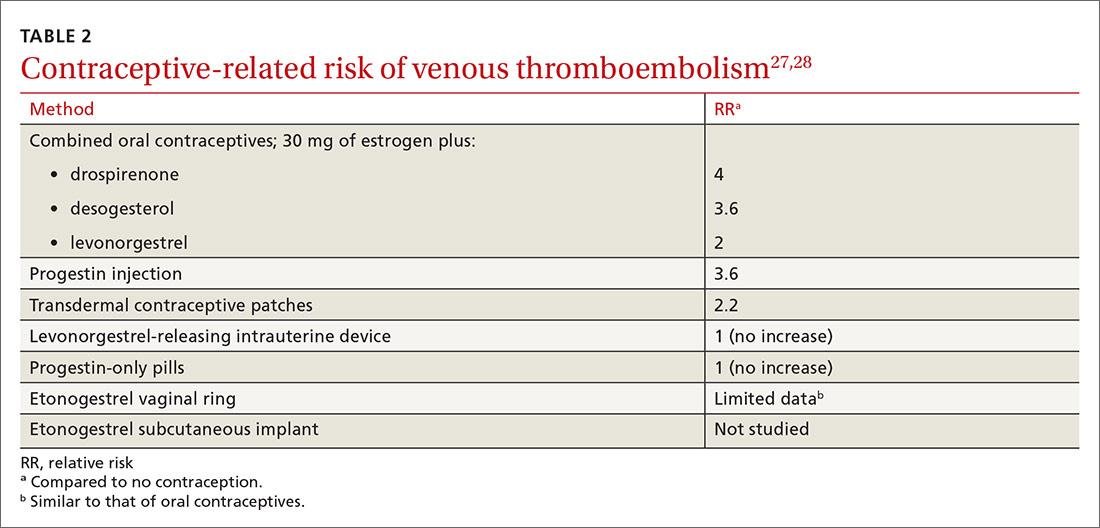
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
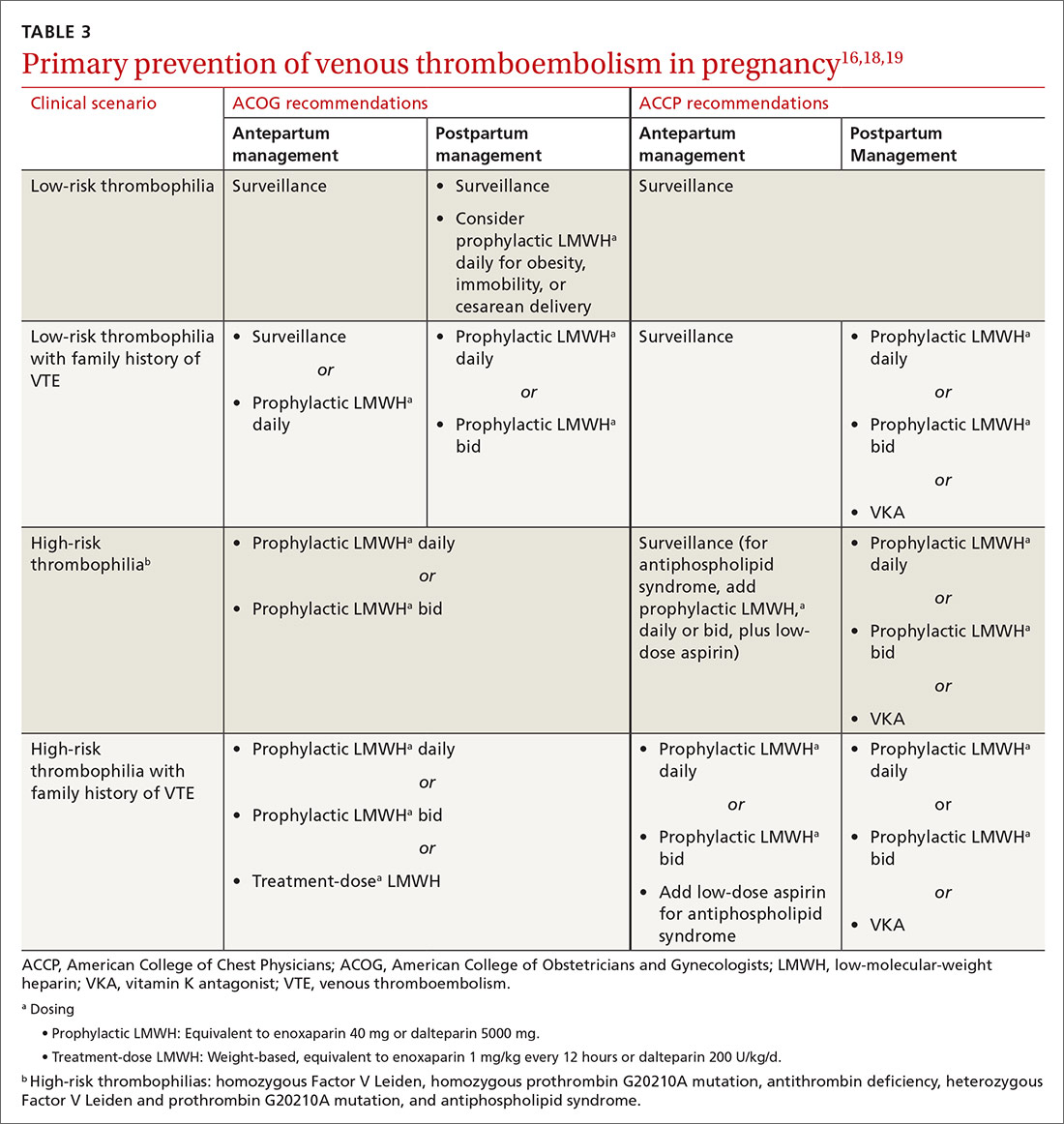
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment
Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; [email protected].
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.

Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27

Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).

Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38

Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; [email protected].
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.

Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27

Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).

Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38

Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; [email protected].
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
PRACTICE RECOMMENDATIONS
› Consider the mild reduction in the risk of venous thromboembolism (VTE) provided by statins when contemplating their use for cardiovascular disease prevention. B
› Avoid testing for thrombophilia to determine the risk of VTE, except in pregnant patients who meet criteria for antiphospholipid syndrome or have a family history of VTE. B
› Recommend an intrauterine device or progestin-only pill for contraception if the patient’s risk of VTE is high. B
› Stratify hospitalized medical and nonorthopedic surgical patients by risk score to determine the need for VTE prophylaxis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is your patient’s cannabis use problematic?
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.

Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).
CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27

Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).
Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; [email protected].
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.

Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).

CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27

Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).

Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; [email protected].
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.

Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).

CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27

Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).

Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; [email protected].
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
PRACTICE RECOMMENDATIONS
› Address underlying conditions for which patients use recreational cannabis to manage symptoms. B
› Consider discrete, in-office sessions of motivational interviewing and referral for cognitive behavioral therapy for patients with problematic cannabis use. B
› Provide counseling around harm reduction for all patients—especially those with problematic cannabis use. C
› Consider referral to an addiction specialist for patients with cannabis use disorder or other problematic cannabis use. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series