User login
Is an underlying cardiac condition causing your patient’s palpitations?
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
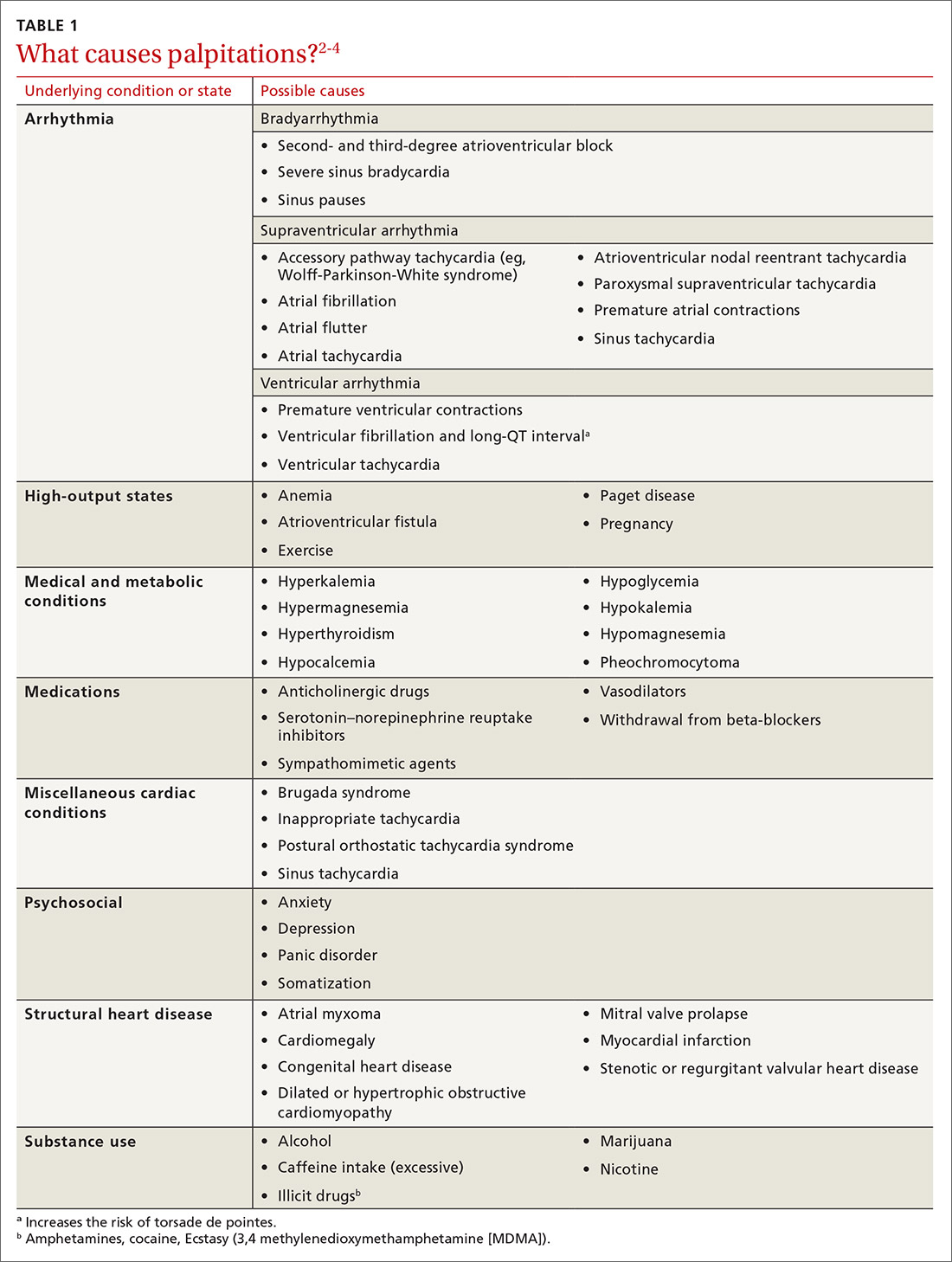
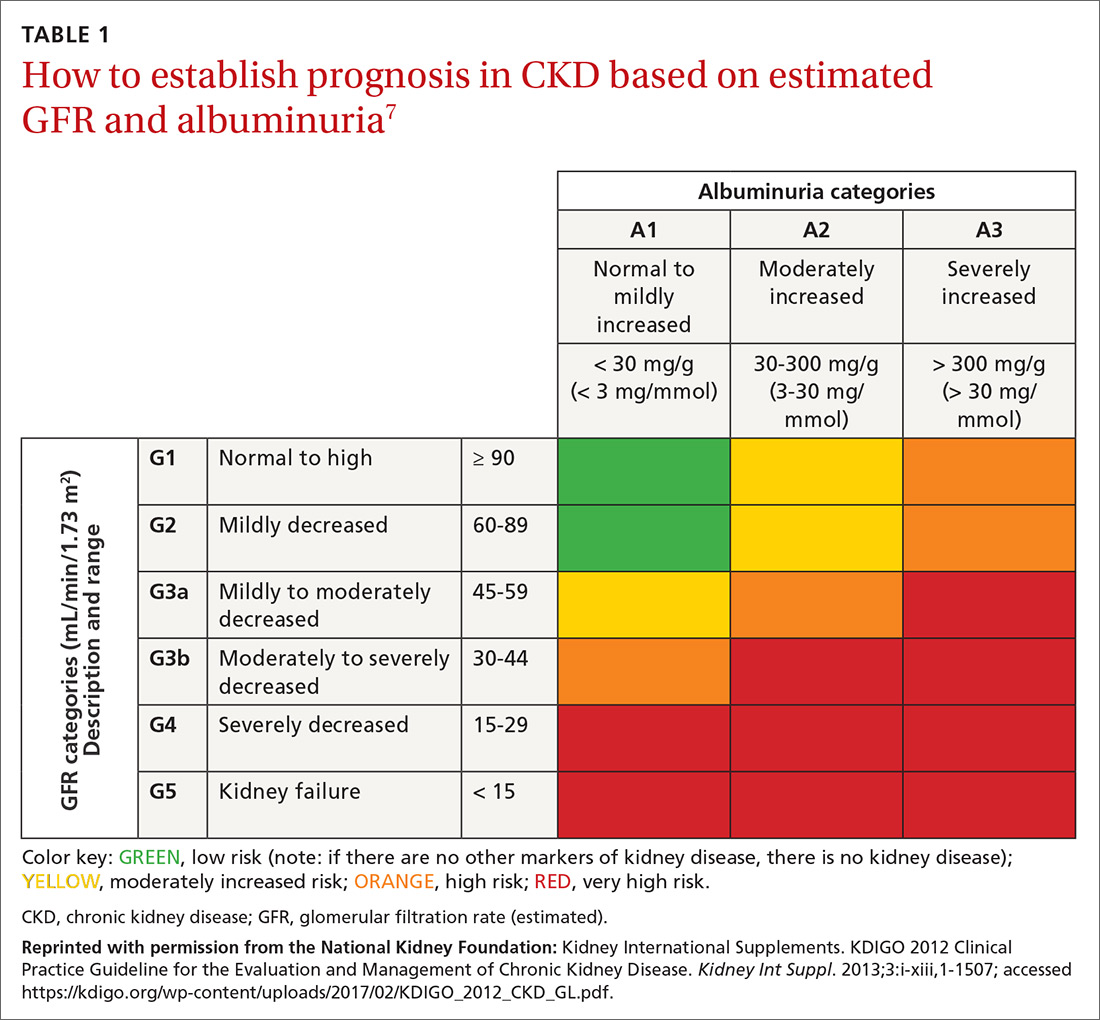
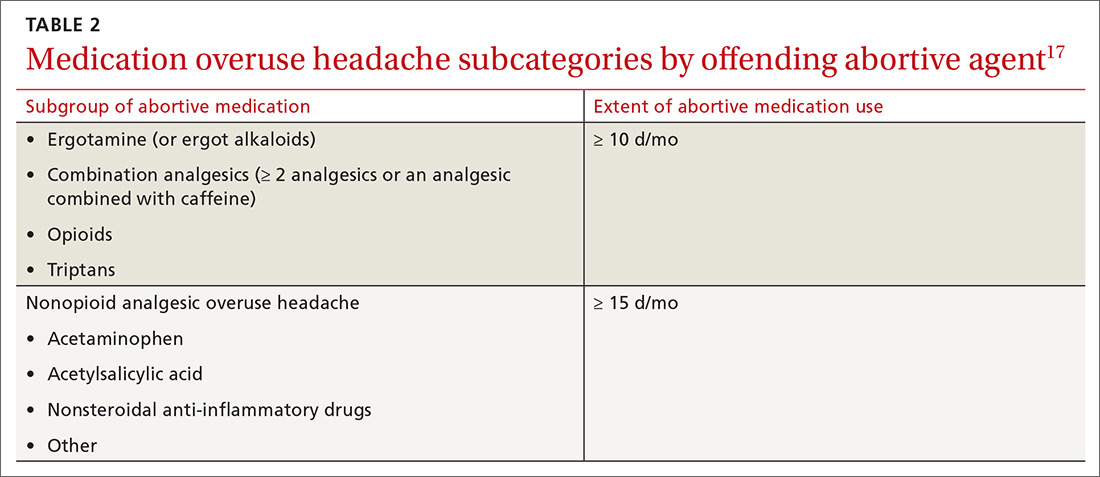
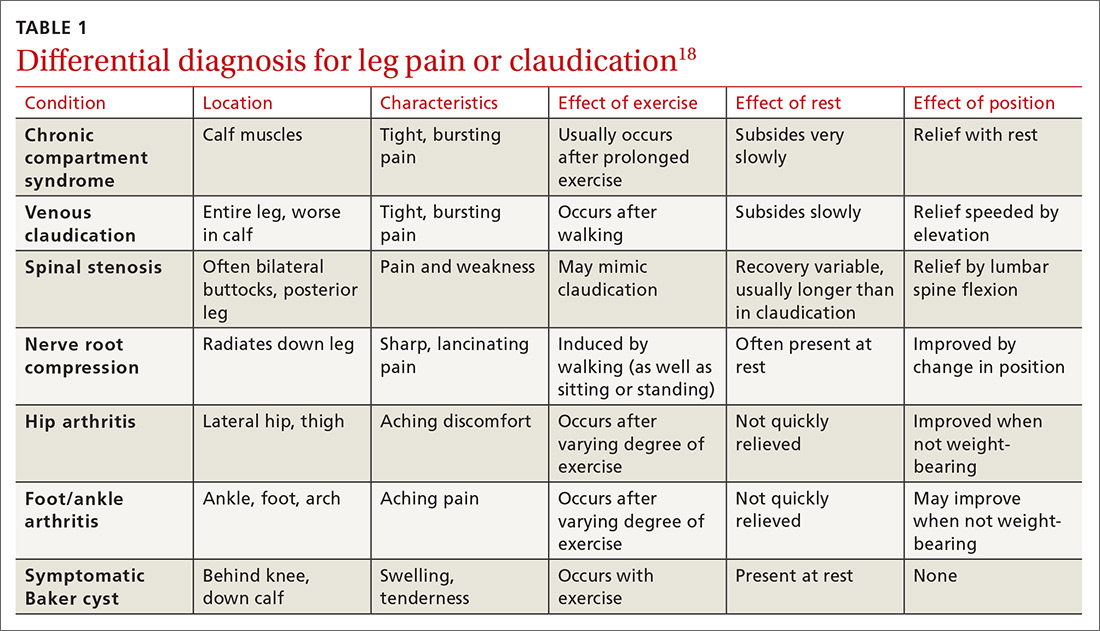
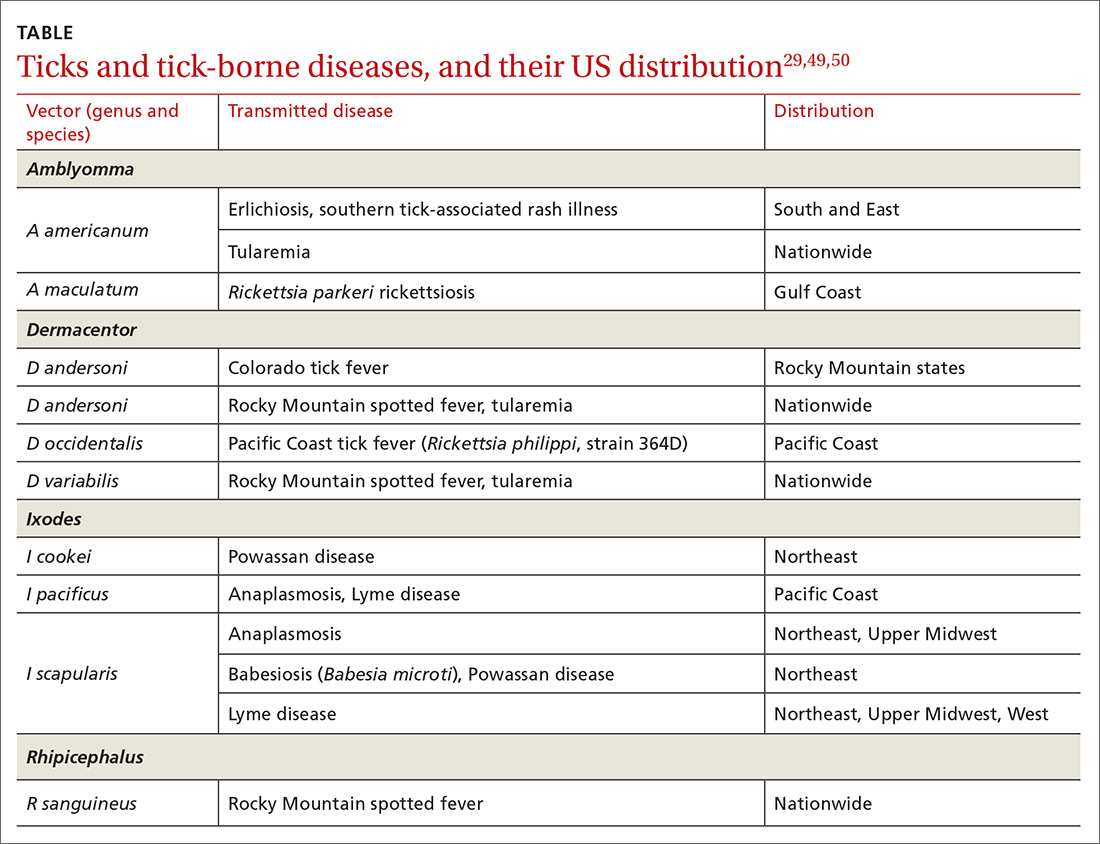
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
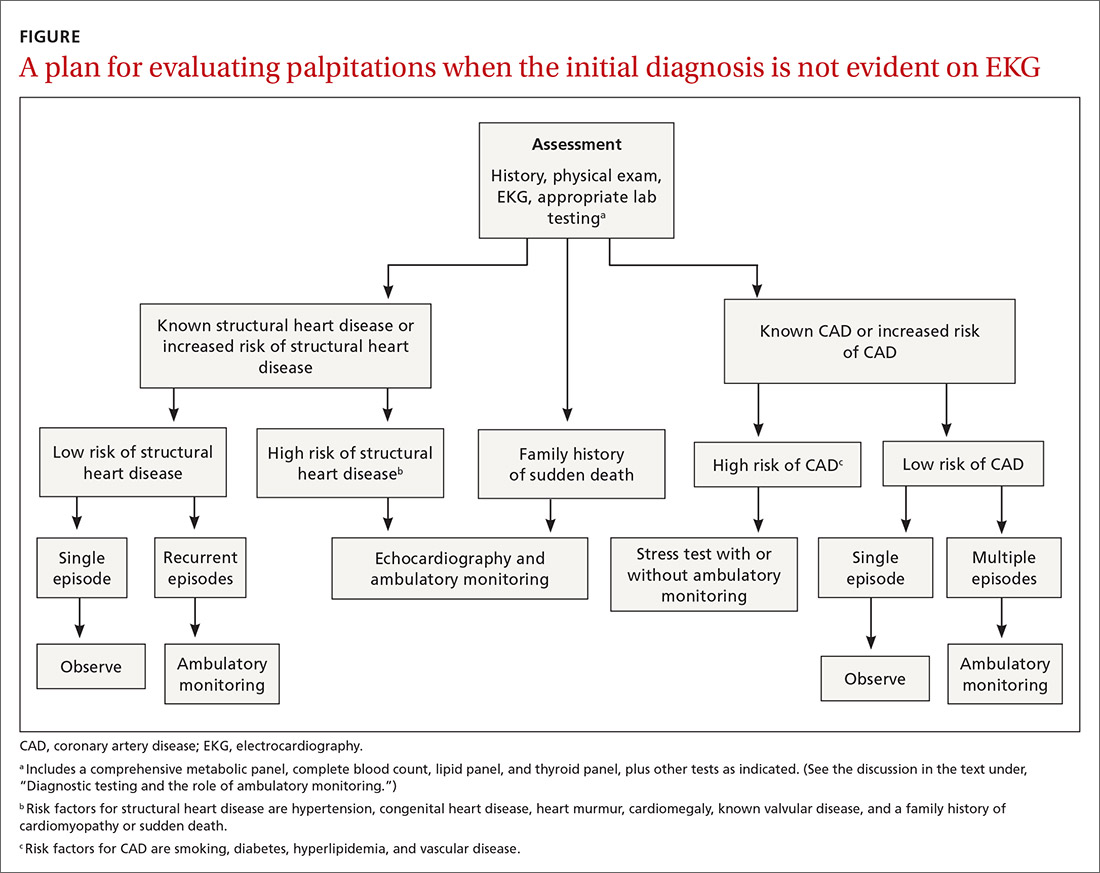
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
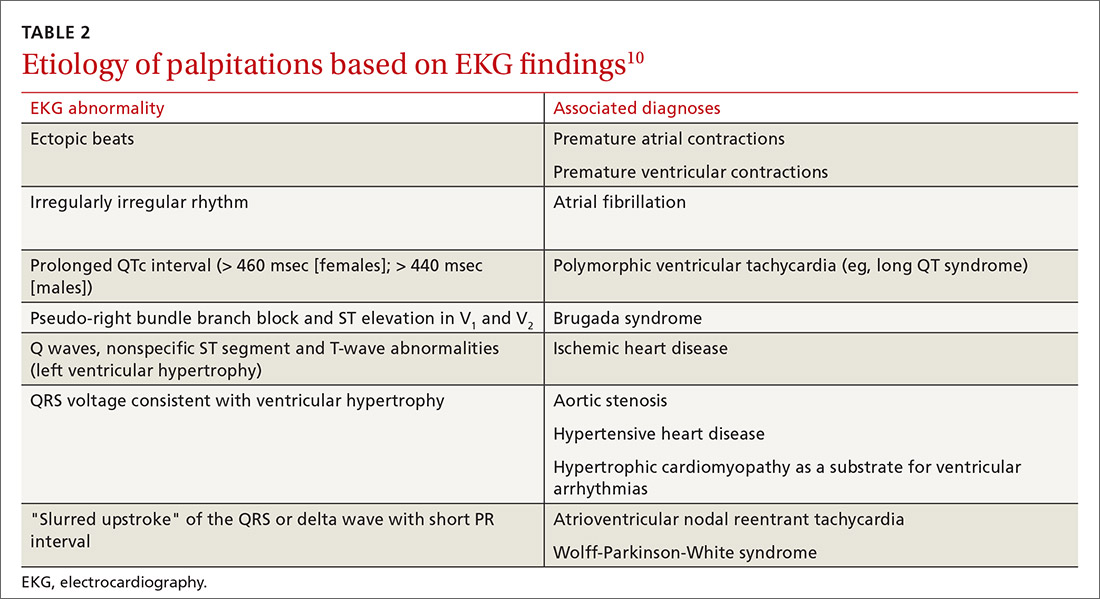
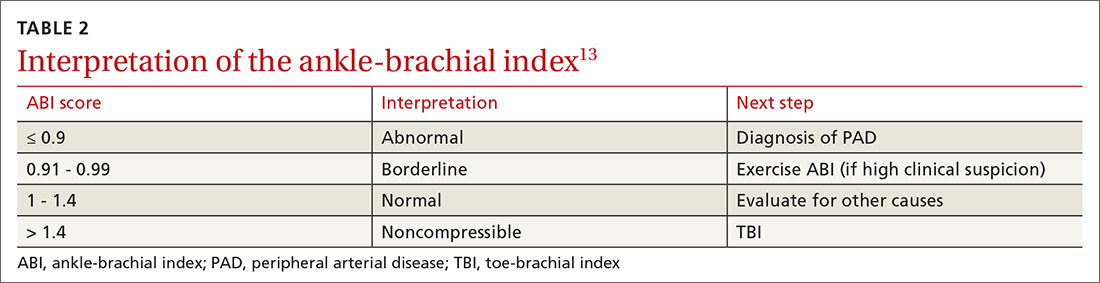
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
PRACTICE RECOMMENDATIONS
› Order echocardiography for patients who have palpitations and risk factors for structural heart disease. C
› Order stress testing for patients who have exertional symptoms or multiple risk factors for coronary artery disease. C
› Evaluate all patients who have syncope associated with their palpitations for a cardiac cause. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Atopic dermatitis: More than just a rash
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin condition that is well known for its relapsing, pruritic rash in children and adults. Less recognized are its associated conditions—allergic rhinitis, asthma, food allergies, attention-deficit/hyperactivity disorder (ADHD), depression, and anxiety—and its burden on patients and their families. In fact, families that have children with AD report lower overall quality of life than those with otherwise healthy children.1 Given AD’s prevalence across age groups and its effect on the family, family physicians are uniquely positioned to diagnose, care for, and counsel patients with AD and its associated maladies.
The prevalence and pathogenesis of AD
AD affects up to 20% of children and 5% of adults in the United States.2 AD typically manifests before a child reaches age 5 (often in the first 6 months of life), and it is slightly more common in females (1.3:1). A family history of atopy (eczema, asthma, allergic rhinitis) is common. In fact, children with one atopic parent have a 2- to 3-fold increased risk of atopic dermatitis; those with 2 atopic parents have a 3- to 5-fold increased risk.3
The pathophysiology of AD is complex, culminating in impaired barrier function of the skin and transepidermal water loss resulting in dry and inflamed skin. Additionally, alterations in a cell-mediated immune response leading to an immunoglobulin (Ig) E-mediated hypersensitivity is also theorized to play a role in the development of AD.
Signs and symptoms
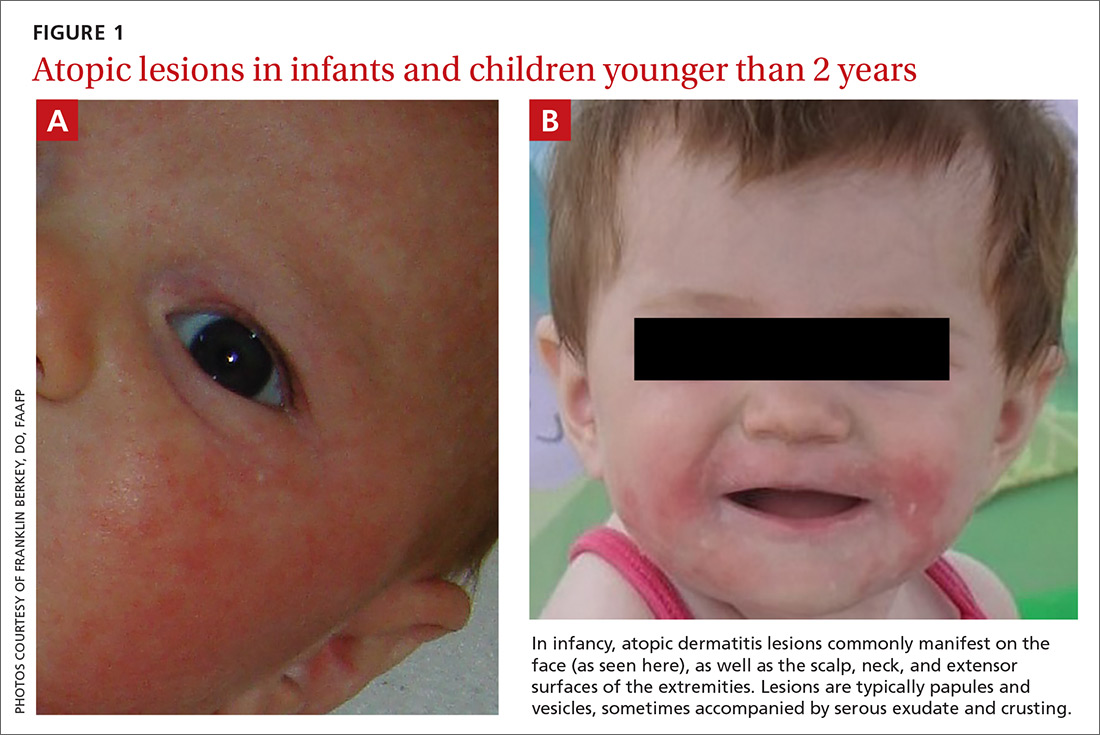
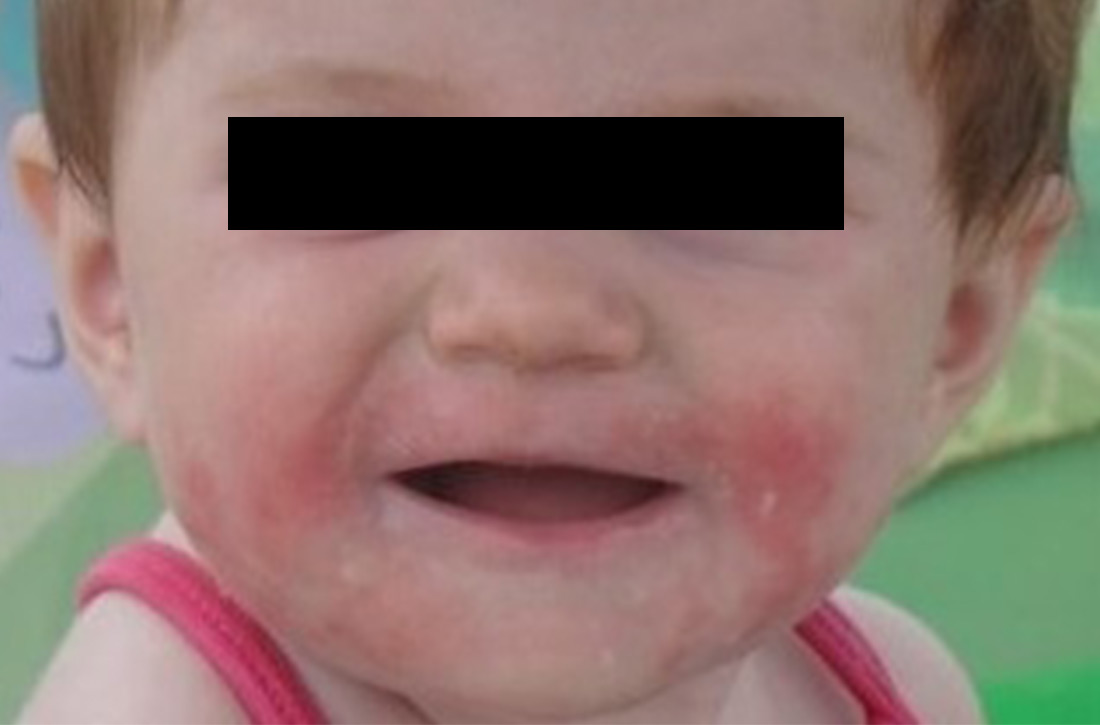
Signs at birth. Physical signs of atopic dermatitis typically appear between birth and 6 months. In infancy, lesions generally occur on the scalp, face (FIGURES 1A and 1B), neck, and extensor surfaces of the extremities. Lesions are typically papules and vesicles, sometimes accompanied by serous exudate and crusting. Eczematous lesions typically spare the groin and diaper area, and their presence in this area should raise suspicion for an alternative diagnosis.
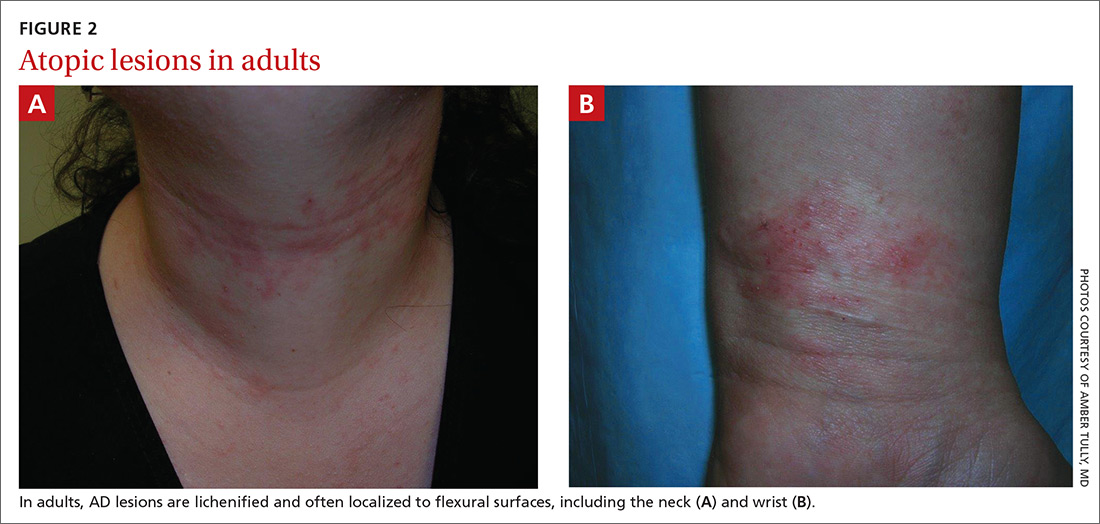
Beginning at age 2 years, eczematous lesions are more commonly limited to the folds of the flexor surfaces. Instead of the weeping and crusting lesions seen in infancy, eczema in older children manifests as dry, lichenified papules and plaques in areas that are typically affected in adults: the wrist, hands, ankles, and popliteal and antecubital fossa.2
Although lesions in adults are similar to those of childhood, they may manifest in a more localized area (hand or eyelid, for example). As is the case in childhood, the lesions are dry, sometimes lichenified, and found on the flexural surfaces (FIGURES 2A and 2B).2
Symptom triggers are unproven
While anecdotal reports cite various triggers for AD flares, a systematic review found little scientific evidence to substantiate identifiable triggers.4 Triggers often cited and studied are foods, dust mite exposure, airborne allergens, detergents, sunlight, fabrics, bacterial infections, and stress. While as many as one-third of people with AD who also have confirmed dust mite allergy report worsening of symptoms when exposed to dust, a Cochrane review of 7 randomized controlled trials totaling 324 adults and children with eczema found that efforts at dust mite mitigation (laundering of bed covers, increased vacuuming, spraying for mites) were not effective in reducing symptoms.5
Continue to: How quality of life diminishes with AD
How quality of life diminishes with AD
AD substantially lessens quality of life. For children, the most distressing physical symptoms include itching that inhibits sleep and provokes scratching, pain, and bleeding. Emotional distress can cause irritability, crying, and uncooperativeness with treatments. Parents also report that they frequently restrict their children from activities, such as playing in the heat or swimming, that may lead to worsening of their eczema.6
The loss of sleep associated with AD is not completely understood but is likely multifactorial. Pruritus and scratching leading to sleeplessness is the most obvious culprit, but an altered circadian rhythm, immune system response, and changes in skin physiology are also likely factors.7 Whatever the cause, sleep disturbance is reported in as many as 60% of patients with AD, and the degree of sleep disturbance is proportional to increases in disease severity and worsening of quality-of-life scores.8 Lost sleep is not limited to patients; parents of children with AD also report significant loss of sleep and subsequent decreased work productivity and quality of life.9
Children with AD are often the target of bullying.10 A 2015 survey by the National Eczema Association indicates that 1 in 5 children reported being bullied due to their AD.11
Associated conditions and comorbidities
AD increases patients’ risks for other illnesses, due either to their underlying atopy or to the effects of
Atopic march
Atopic march—the clinical succession of AD, allergic rhinitis, and asthma—is a well-established clinical progression. The presence of all 3 conditions appears to be more common in children diagnosed with AD before 2 years of age.12 Typically, allergic rhinitis manifests at around age 4, and asthma develops between ages 6 and 8. The severity of AD predicts progression. Compared with an 8% chance of asthma developing among the general population, children with mild AD have a 20% to 30% chance of developing asthma, and those with severe AD have about a 70% chance.12
Continue to: Food allergies
Food allergies
Patients with AD are at higher risk for food-induced anaphylaxis, with up to one-third of AD patients having an IgE-mediated food allergy.13 While it is theorized that the impaired skin barrier of an atopic child may allow for early sensitization and allergy development, a landmark 2015 study demonstrated that early allergen introduction (specifically, peanuts) may serve as a preventive strategy in those at high risk of food allergies.14 Current guidelines recommend that physicians be aware of the increased possibility of food allergies in those with AD, and consider evaluating a child for milk, egg, peanut, wheat, and soy allergy if the child is younger than 5 years and has eczema that does not resolve with treatment, or has eczema and a history of an allergic reaction to a specific food.15
Interestingly, despite the strong association between AD and food allergies, it is not clear that food allergies trigger atopic flares; as such, elimination diets are not universally recommended in those without a proven food allergy.
Psychiatric diagnoses
Children with AD have an increased prevalence of several psychiatric conditions, including ADHD, depression, anxiety, conduct disorder, and autism when compared with peers who do not have AD, and the probability correlates with the severity of AD
What we do know is that one of the strongest associations between AD and a psychiatric condition is with ADHD, with a recent pooled meta-analysis showing a 46% increase in risk.17 The incidence of depression among children with AD appears to correlate with the severity of AD symptoms: estimated at 5% with mild AD, 7% with moderate disease, and 14% with severe disease (compared with 3% without AD). Similar incremental increases are seen when correlating AD and anxiety.16
Nonpharmacologic care
Bathing
Bathing habits are critical to controlling AD. While bathing serves to both hydrate the skin and remove allergens, the water’s evaporation off the skin surface can lead to increased transepidermal water loss. Combining bathing and immediate application of a moisturizer improves skin hydration in patients with AD vs bathing alone.18 Thus, consensus guidelines recommend once-daily bathing (bath or shower) to remove scale and crust, followed by immediate application of a moisturizing emollient.19
Continue to: Emollients
Emollients
Application of moisturizing emollients is the mainstay of nonpharmacologic care of AD, and there is strong evidence that their regimented use reduces disease burden and the need for prescription treatment.19 Emollient creams and ointments help retain moisture and improve the skin’s barrier. While ointments may provide a better barrier, patients tend to prefer creams as they are less greasy than ointments.
Emollient therapy may also help prevent development of AD, especially in those infants thought to be at high risk with a family history of atopy. In a multinational randomized controlled trial, infants who received daily full-body application of emollient beginning at 3 weeks of life were significantly less likely than controls to develop AD by 6 months.20 While the mechanism of action is not clearly understood, it is believed that early emollient use prevents skin dehydration and maintains the skin’s barrier integrity, thus decreasing allergen epidermal penetration and subsequent inflammation.
Bleach bath
A bleach bath, prepared by adding 1/2 cup of unconcentrated bleach (5.25% sodium hypochlorite) to a standard 40-gallon bathtub, produces a chlorine mixture equivalent to an average swimming pool. Soaking in a bleach bath for 10 minutes once or twice weekly is thought to reduce inflammation and bacteria on the skin, but studies of its efficacy in improving atopic symptoms are mixed.
In a pooled analysis of 5 studies evaluating bleach baths vs standard baths, there was no significant difference in disease severity at 4 weeks.21 Thus, while bleach baths were effective in decreasing disease severity, they appeared to be no more effective than a standard water bath.21 Bleach baths may be helpful, however, in cases of moderate-to-severe disease with frequent bacterial infections.19
Pharmacologic therapy
Steroids
For symptoms refractory to nonpharmacologic skin care, topical steroids are the initial pharmacologic treatment for AD.19 Choose steroid potency based on symptom severity and disease location. Low- to medium-potency is appropriate for mild disease, and medium- to high-potency is useful for moderate-to-severe symptoms. High-potency steroids are generally avoided on the face and skin folds; however, they can be used for short periods in these areas to induce remission. They must then be quickly tapered and discontinued.
Continue to: Frequency
Frequency. Topical corticosteroids are typically applied twice daily, although recent studies indicate that once-daily application is just as efficacious.22 In addition to treatment of an acute flare, topical steroids are useful as maintenance therapy for patients with recurrent outbreaks in the same anatomical site. Guidelines suggest once- or twice-weekly application of a medium-potency steroid to prolong time between flares.19
For children, a practical guide is for caregivers to apply the amount of steroid covering 1 adult fingertip to an area of the child’s skin equal to that of 2 adult palms.23 Topical steroids are generally well tolerated and have a good safety profile. Adverse effects are proportional to the amount and duration of use and include purpura, telangiectasias, striae, and skin atrophy. The risk of skin atrophy increases with higher potency steroids, occlusion (covering affected area after steroid application), use on thin-skinned areas, and older patient age.24
Reassure patients/parents about the safety of topical steroids, as fears regarding the potential adverse effects can limit compliance. In one study of 200 patients with AD, 72.5% of respondents expressed fear of using steroids on their own skin or that of their child, and 24% admitted being noncompliant with therapy based on these concerns.25
Treating flares. Oral steroids are sometimes needed to abort or control an AD flare in older children and adults. A tapering course of prednisone over 5 to 7 days, transitioning to medium- to high-dose topical steroids, may be needed to achieve symptom control.
Topical calcineurin inhibitors
Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, are generally second-line therapy to topical corticosteroids. However, as nonsteroidal agents, topical calcineurin inhibitors do not cause skin atrophy and can be a first-line option in areas where atrophy is more common (face, eyelids, neck, and skin folds).26
Continue to: A Cochrane review found...
A Cochrane review found tacrolimus 0.1% to be better than low‐potency topical corticosteroids on the face and neck areas, while results were equivocal when compared with moderate‐potency topical corticosteroids on the trunk and extremities (no difference based on physician assessment, but marginal benefit favoring tacrolimus based on participant scoring).27 When compared head-to-head, tacrolimus was more effective than pimecrolimus, although tacrolimus has a higher rate of local irritation. The most common adverse effects are stinging and burning at the application site, although these adverse effects generally improve with repeated application.
There have been long-term safety concerns with topical calcineurin inhibitors—chiefly a 2006 Food and Drug Administration (FDA) black box warning regarding a possible link between topical calcineurin inhibitors and cancer. However, while there may be a slight increased risk of lymphoma in AD patients, a recent meta-analysis did not find an association between topical calcineurin inhibitors use and lymphoma.28 Given the initial concern—and pending additional data—the FDA currently recommends reserving topical calcineurin inhibitors for second-line therapy and only for the minimum amount of time to induce improvement. It also recommends avoiding their use in patients younger than 2 years and in those with compromised immune systems.
Cisaborole
Cisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor, received FDA approval in 2016 for mild-to-moderate AD. By inhibiting PDE4, the drug limits inflammation. In a multicenter randomized trial, patients applying cisaborole 2% twice a day noted reductions in pruritus, inflammation, excoriation, and lichenification.29 Adverse effects are minimal and limited to application site irritation.
Systemic treatments
While beyond the care of a family physician, symptoms refractory to conservative, nonpharmacologic measures and combinations of topical pharmaceuticals can be treated with systemic immunomodulators such as cyclosporine, azathioprine, and methotrexate. Phototherapy is also effective in patients with more widespread skin involvement. Dupilumab, an injectable monoclonal antibody that binds to interleukin-4 receptor and inhibits inflammation, is approved to treat moderate-to-severe AD in adults.30
Ineffective therapies: Oral montelukast and probiotics
While oral antihistamines are frequently prescribed and used, there are no studies evaluating the use of antihistamines (H1) as monotherapy for AD.31 Nonetheless, while not altering the disease process, the sedative effect of antihistamines may palliate the nocturnal pruritus frequently associated with AD. Although nonsedating antihistamines may still have a role for atopic patients with concurrent seasonal and environmental allergies, there is no evidence to support their use in the treatment of AD.
Continue to: Data are limited...
Data are limited on the effectiveness of leukotriene receptor antagonists for AD, and all studies meeting inclusion for a Cochrane review assessed oral montelukast. The review found no benefit with the use of montelukast 10 mg in terms of severity of disease, pruritus, or need for topical steroids.32
A systematic review investigating the benefit of probiotics for the treatment of AD found no improvement in patient-rated eczema scores for quality of life.33 Additionally, a review of 11 randomized controlled trials including 596 participants found no evidence to suggest efficacy of fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine, sea buckthorn oil, hempseed oil, or sunflower oil in the treatment of AD.34
Education can reduce AD severity
Family physicians can be a source of education and support for patients and families of patients with AD. Support programs for adults with AD—including education, relaxation techniques, and cognitive behavioral therapy—have been shown to decrease disease severity.35 Comparable improvement in disease severity has been demonstrated in children with AD when similar education is provided to them and their families.
CORRESPONDENCE
Franklin Berkey, DO, Penn State Health, 1850 East Park Avenue, Suite 207, State College, PA 16803; fberkey@ pennstatehealth.psu.edu.
1. Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
2. Ahn C, Huang W. Clinical presentation of atopic dermatitis. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:38-46.
3. Eichenfield LF, Tom WL, Chamblin SL, et al. Guidelines of care for the management of atopic dermatitis. Part 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
4. Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol. 2006;155:504-514.
5. Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015:CD008426.
6. Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
7. Chang Y-S, Chiang B-L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17:462.
8. Camfferman D, Kennedy JD, Gold M, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359-369.
9. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
10. Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:P26-P30.
11. National Eczema Association. Tools for school: addressing school bullying for kids with eczema. Accessed January 5, 2021. https://nationaleczema.org/children-with-eczema-experience-bullying/
12. Bantz SK, Zhu Z, Zhen T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202
13. Laird M, Sicco KL. Defining and measuring the scope of atopic dermatitis. Adv Exp Med Biol. 2017;1027:93-104.
14. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
15. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58.
16. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
17. Strom MA, Fishbein AB, Paller AS, et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920-929.
18. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
19. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
20. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
21. Chopra R, Vakharia PP, Sacotte R, et al. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-440.
22. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ. 2007;334:1272.
23. Long CC, Mills CM, Finlay AY. A practical guide to topical therapy in children. Br J Dermatol. 1998;138:293-296.
24. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
25. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931-936.
26. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: a meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
27. Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015:CD009864.
28. Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
29. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.
30. Dupilumab [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2017.
31. van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25.
32. Ferguson L, Futamura M, Vakirlis E, et al. Leukotriene receptor antagonists for eczema. Cochrane Database Syst Rev. 2018:CD011224.
33. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018:CD006135.
34. Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
35. Sy W, Lamb AJ. Atopic dermatitis disease education. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:179-184.
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin condition that is well known for its relapsing, pruritic rash in children and adults. Less recognized are its associated conditions—allergic rhinitis, asthma, food allergies, attention-deficit/hyperactivity disorder (ADHD), depression, and anxiety—and its burden on patients and their families. In fact, families that have children with AD report lower overall quality of life than those with otherwise healthy children.1 Given AD’s prevalence across age groups and its effect on the family, family physicians are uniquely positioned to diagnose, care for, and counsel patients with AD and its associated maladies.
The prevalence and pathogenesis of AD
AD affects up to 20% of children and 5% of adults in the United States.2 AD typically manifests before a child reaches age 5 (often in the first 6 months of life), and it is slightly more common in females (1.3:1). A family history of atopy (eczema, asthma, allergic rhinitis) is common. In fact, children with one atopic parent have a 2- to 3-fold increased risk of atopic dermatitis; those with 2 atopic parents have a 3- to 5-fold increased risk.3
The pathophysiology of AD is complex, culminating in impaired barrier function of the skin and transepidermal water loss resulting in dry and inflamed skin. Additionally, alterations in a cell-mediated immune response leading to an immunoglobulin (Ig) E-mediated hypersensitivity is also theorized to play a role in the development of AD.
Signs and symptoms
Signs at birth. Physical signs of atopic dermatitis typically appear between birth and 6 months. In infancy, lesions generally occur on the scalp, face (FIGURES 1A and 1B), neck, and extensor surfaces of the extremities. Lesions are typically papules and vesicles, sometimes accompanied by serous exudate and crusting. Eczematous lesions typically spare the groin and diaper area, and their presence in this area should raise suspicion for an alternative diagnosis.
Beginning at age 2 years, eczematous lesions are more commonly limited to the folds of the flexor surfaces. Instead of the weeping and crusting lesions seen in infancy, eczema in older children manifests as dry, lichenified papules and plaques in areas that are typically affected in adults: the wrist, hands, ankles, and popliteal and antecubital fossa.2
Although lesions in adults are similar to those of childhood, they may manifest in a more localized area (hand or eyelid, for example). As is the case in childhood, the lesions are dry, sometimes lichenified, and found on the flexural surfaces (FIGURES 2A and 2B).2
Symptom triggers are unproven
While anecdotal reports cite various triggers for AD flares, a systematic review found little scientific evidence to substantiate identifiable triggers.4 Triggers often cited and studied are foods, dust mite exposure, airborne allergens, detergents, sunlight, fabrics, bacterial infections, and stress. While as many as one-third of people with AD who also have confirmed dust mite allergy report worsening of symptoms when exposed to dust, a Cochrane review of 7 randomized controlled trials totaling 324 adults and children with eczema found that efforts at dust mite mitigation (laundering of bed covers, increased vacuuming, spraying for mites) were not effective in reducing symptoms.5
Continue to: How quality of life diminishes with AD
How quality of life diminishes with AD
AD substantially lessens quality of life. For children, the most distressing physical symptoms include itching that inhibits sleep and provokes scratching, pain, and bleeding. Emotional distress can cause irritability, crying, and uncooperativeness with treatments. Parents also report that they frequently restrict their children from activities, such as playing in the heat or swimming, that may lead to worsening of their eczema.6
The loss of sleep associated with AD is not completely understood but is likely multifactorial. Pruritus and scratching leading to sleeplessness is the most obvious culprit, but an altered circadian rhythm, immune system response, and changes in skin physiology are also likely factors.7 Whatever the cause, sleep disturbance is reported in as many as 60% of patients with AD, and the degree of sleep disturbance is proportional to increases in disease severity and worsening of quality-of-life scores.8 Lost sleep is not limited to patients; parents of children with AD also report significant loss of sleep and subsequent decreased work productivity and quality of life.9
Children with AD are often the target of bullying.10 A 2015 survey by the National Eczema Association indicates that 1 in 5 children reported being bullied due to their AD.11
Associated conditions and comorbidities
AD increases patients’ risks for other illnesses, due either to their underlying atopy or to the effects of
Atopic march
Atopic march—the clinical succession of AD, allergic rhinitis, and asthma—is a well-established clinical progression. The presence of all 3 conditions appears to be more common in children diagnosed with AD before 2 years of age.12 Typically, allergic rhinitis manifests at around age 4, and asthma develops between ages 6 and 8. The severity of AD predicts progression. Compared with an 8% chance of asthma developing among the general population, children with mild AD have a 20% to 30% chance of developing asthma, and those with severe AD have about a 70% chance.12
Continue to: Food allergies
Food allergies
Patients with AD are at higher risk for food-induced anaphylaxis, with up to one-third of AD patients having an IgE-mediated food allergy.13 While it is theorized that the impaired skin barrier of an atopic child may allow for early sensitization and allergy development, a landmark 2015 study demonstrated that early allergen introduction (specifically, peanuts) may serve as a preventive strategy in those at high risk of food allergies.14 Current guidelines recommend that physicians be aware of the increased possibility of food allergies in those with AD, and consider evaluating a child for milk, egg, peanut, wheat, and soy allergy if the child is younger than 5 years and has eczema that does not resolve with treatment, or has eczema and a history of an allergic reaction to a specific food.15
Interestingly, despite the strong association between AD and food allergies, it is not clear that food allergies trigger atopic flares; as such, elimination diets are not universally recommended in those without a proven food allergy.
Psychiatric diagnoses
Children with AD have an increased prevalence of several psychiatric conditions, including ADHD, depression, anxiety, conduct disorder, and autism when compared with peers who do not have AD, and the probability correlates with the severity of AD
What we do know is that one of the strongest associations between AD and a psychiatric condition is with ADHD, with a recent pooled meta-analysis showing a 46% increase in risk.17 The incidence of depression among children with AD appears to correlate with the severity of AD symptoms: estimated at 5% with mild AD, 7% with moderate disease, and 14% with severe disease (compared with 3% without AD). Similar incremental increases are seen when correlating AD and anxiety.16
Nonpharmacologic care
Bathing
Bathing habits are critical to controlling AD. While bathing serves to both hydrate the skin and remove allergens, the water’s evaporation off the skin surface can lead to increased transepidermal water loss. Combining bathing and immediate application of a moisturizer improves skin hydration in patients with AD vs bathing alone.18 Thus, consensus guidelines recommend once-daily bathing (bath or shower) to remove scale and crust, followed by immediate application of a moisturizing emollient.19
Continue to: Emollients
Emollients
Application of moisturizing emollients is the mainstay of nonpharmacologic care of AD, and there is strong evidence that their regimented use reduces disease burden and the need for prescription treatment.19 Emollient creams and ointments help retain moisture and improve the skin’s barrier. While ointments may provide a better barrier, patients tend to prefer creams as they are less greasy than ointments.
Emollient therapy may also help prevent development of AD, especially in those infants thought to be at high risk with a family history of atopy. In a multinational randomized controlled trial, infants who received daily full-body application of emollient beginning at 3 weeks of life were significantly less likely than controls to develop AD by 6 months.20 While the mechanism of action is not clearly understood, it is believed that early emollient use prevents skin dehydration and maintains the skin’s barrier integrity, thus decreasing allergen epidermal penetration and subsequent inflammation.
Bleach bath
A bleach bath, prepared by adding 1/2 cup of unconcentrated bleach (5.25% sodium hypochlorite) to a standard 40-gallon bathtub, produces a chlorine mixture equivalent to an average swimming pool. Soaking in a bleach bath for 10 minutes once or twice weekly is thought to reduce inflammation and bacteria on the skin, but studies of its efficacy in improving atopic symptoms are mixed.
In a pooled analysis of 5 studies evaluating bleach baths vs standard baths, there was no significant difference in disease severity at 4 weeks.21 Thus, while bleach baths were effective in decreasing disease severity, they appeared to be no more effective than a standard water bath.21 Bleach baths may be helpful, however, in cases of moderate-to-severe disease with frequent bacterial infections.19
Pharmacologic therapy
Steroids
For symptoms refractory to nonpharmacologic skin care, topical steroids are the initial pharmacologic treatment for AD.19 Choose steroid potency based on symptom severity and disease location. Low- to medium-potency is appropriate for mild disease, and medium- to high-potency is useful for moderate-to-severe symptoms. High-potency steroids are generally avoided on the face and skin folds; however, they can be used for short periods in these areas to induce remission. They must then be quickly tapered and discontinued.
Continue to: Frequency
Frequency. Topical corticosteroids are typically applied twice daily, although recent studies indicate that once-daily application is just as efficacious.22 In addition to treatment of an acute flare, topical steroids are useful as maintenance therapy for patients with recurrent outbreaks in the same anatomical site. Guidelines suggest once- or twice-weekly application of a medium-potency steroid to prolong time between flares.19
For children, a practical guide is for caregivers to apply the amount of steroid covering 1 adult fingertip to an area of the child’s skin equal to that of 2 adult palms.23 Topical steroids are generally well tolerated and have a good safety profile. Adverse effects are proportional to the amount and duration of use and include purpura, telangiectasias, striae, and skin atrophy. The risk of skin atrophy increases with higher potency steroids, occlusion (covering affected area after steroid application), use on thin-skinned areas, and older patient age.24
Reassure patients/parents about the safety of topical steroids, as fears regarding the potential adverse effects can limit compliance. In one study of 200 patients with AD, 72.5% of respondents expressed fear of using steroids on their own skin or that of their child, and 24% admitted being noncompliant with therapy based on these concerns.25
Treating flares. Oral steroids are sometimes needed to abort or control an AD flare in older children and adults. A tapering course of prednisone over 5 to 7 days, transitioning to medium- to high-dose topical steroids, may be needed to achieve symptom control.
Topical calcineurin inhibitors
Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, are generally second-line therapy to topical corticosteroids. However, as nonsteroidal agents, topical calcineurin inhibitors do not cause skin atrophy and can be a first-line option in areas where atrophy is more common (face, eyelids, neck, and skin folds).26
Continue to: A Cochrane review found...
A Cochrane review found tacrolimus 0.1% to be better than low‐potency topical corticosteroids on the face and neck areas, while results were equivocal when compared with moderate‐potency topical corticosteroids on the trunk and extremities (no difference based on physician assessment, but marginal benefit favoring tacrolimus based on participant scoring).27 When compared head-to-head, tacrolimus was more effective than pimecrolimus, although tacrolimus has a higher rate of local irritation. The most common adverse effects are stinging and burning at the application site, although these adverse effects generally improve with repeated application.
There have been long-term safety concerns with topical calcineurin inhibitors—chiefly a 2006 Food and Drug Administration (FDA) black box warning regarding a possible link between topical calcineurin inhibitors and cancer. However, while there may be a slight increased risk of lymphoma in AD patients, a recent meta-analysis did not find an association between topical calcineurin inhibitors use and lymphoma.28 Given the initial concern—and pending additional data—the FDA currently recommends reserving topical calcineurin inhibitors for second-line therapy and only for the minimum amount of time to induce improvement. It also recommends avoiding their use in patients younger than 2 years and in those with compromised immune systems.
Cisaborole
Cisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor, received FDA approval in 2016 for mild-to-moderate AD. By inhibiting PDE4, the drug limits inflammation. In a multicenter randomized trial, patients applying cisaborole 2% twice a day noted reductions in pruritus, inflammation, excoriation, and lichenification.29 Adverse effects are minimal and limited to application site irritation.
Systemic treatments
While beyond the care of a family physician, symptoms refractory to conservative, nonpharmacologic measures and combinations of topical pharmaceuticals can be treated with systemic immunomodulators such as cyclosporine, azathioprine, and methotrexate. Phototherapy is also effective in patients with more widespread skin involvement. Dupilumab, an injectable monoclonal antibody that binds to interleukin-4 receptor and inhibits inflammation, is approved to treat moderate-to-severe AD in adults.30
Ineffective therapies: Oral montelukast and probiotics
While oral antihistamines are frequently prescribed and used, there are no studies evaluating the use of antihistamines (H1) as monotherapy for AD.31 Nonetheless, while not altering the disease process, the sedative effect of antihistamines may palliate the nocturnal pruritus frequently associated with AD. Although nonsedating antihistamines may still have a role for atopic patients with concurrent seasonal and environmental allergies, there is no evidence to support their use in the treatment of AD.
Continue to: Data are limited...
Data are limited on the effectiveness of leukotriene receptor antagonists for AD, and all studies meeting inclusion for a Cochrane review assessed oral montelukast. The review found no benefit with the use of montelukast 10 mg in terms of severity of disease, pruritus, or need for topical steroids.32
A systematic review investigating the benefit of probiotics for the treatment of AD found no improvement in patient-rated eczema scores for quality of life.33 Additionally, a review of 11 randomized controlled trials including 596 participants found no evidence to suggest efficacy of fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine, sea buckthorn oil, hempseed oil, or sunflower oil in the treatment of AD.34
Education can reduce AD severity
Family physicians can be a source of education and support for patients and families of patients with AD. Support programs for adults with AD—including education, relaxation techniques, and cognitive behavioral therapy—have been shown to decrease disease severity.35 Comparable improvement in disease severity has been demonstrated in children with AD when similar education is provided to them and their families.
CORRESPONDENCE
Franklin Berkey, DO, Penn State Health, 1850 East Park Avenue, Suite 207, State College, PA 16803; fberkey@ pennstatehealth.psu.edu.
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin condition that is well known for its relapsing, pruritic rash in children and adults. Less recognized are its associated conditions—allergic rhinitis, asthma, food allergies, attention-deficit/hyperactivity disorder (ADHD), depression, and anxiety—and its burden on patients and their families. In fact, families that have children with AD report lower overall quality of life than those with otherwise healthy children.1 Given AD’s prevalence across age groups and its effect on the family, family physicians are uniquely positioned to diagnose, care for, and counsel patients with AD and its associated maladies.
The prevalence and pathogenesis of AD
AD affects up to 20% of children and 5% of adults in the United States.2 AD typically manifests before a child reaches age 5 (often in the first 6 months of life), and it is slightly more common in females (1.3:1). A family history of atopy (eczema, asthma, allergic rhinitis) is common. In fact, children with one atopic parent have a 2- to 3-fold increased risk of atopic dermatitis; those with 2 atopic parents have a 3- to 5-fold increased risk.3
The pathophysiology of AD is complex, culminating in impaired barrier function of the skin and transepidermal water loss resulting in dry and inflamed skin. Additionally, alterations in a cell-mediated immune response leading to an immunoglobulin (Ig) E-mediated hypersensitivity is also theorized to play a role in the development of AD.
Signs and symptoms
Signs at birth. Physical signs of atopic dermatitis typically appear between birth and 6 months. In infancy, lesions generally occur on the scalp, face (FIGURES 1A and 1B), neck, and extensor surfaces of the extremities. Lesions are typically papules and vesicles, sometimes accompanied by serous exudate and crusting. Eczematous lesions typically spare the groin and diaper area, and their presence in this area should raise suspicion for an alternative diagnosis.
Beginning at age 2 years, eczematous lesions are more commonly limited to the folds of the flexor surfaces. Instead of the weeping and crusting lesions seen in infancy, eczema in older children manifests as dry, lichenified papules and plaques in areas that are typically affected in adults: the wrist, hands, ankles, and popliteal and antecubital fossa.2
Although lesions in adults are similar to those of childhood, they may manifest in a more localized area (hand or eyelid, for example). As is the case in childhood, the lesions are dry, sometimes lichenified, and found on the flexural surfaces (FIGURES 2A and 2B).2
Symptom triggers are unproven
While anecdotal reports cite various triggers for AD flares, a systematic review found little scientific evidence to substantiate identifiable triggers.4 Triggers often cited and studied are foods, dust mite exposure, airborne allergens, detergents, sunlight, fabrics, bacterial infections, and stress. While as many as one-third of people with AD who also have confirmed dust mite allergy report worsening of symptoms when exposed to dust, a Cochrane review of 7 randomized controlled trials totaling 324 adults and children with eczema found that efforts at dust mite mitigation (laundering of bed covers, increased vacuuming, spraying for mites) were not effective in reducing symptoms.5
Continue to: How quality of life diminishes with AD
How quality of life diminishes with AD
AD substantially lessens quality of life. For children, the most distressing physical symptoms include itching that inhibits sleep and provokes scratching, pain, and bleeding. Emotional distress can cause irritability, crying, and uncooperativeness with treatments. Parents also report that they frequently restrict their children from activities, such as playing in the heat or swimming, that may lead to worsening of their eczema.6
The loss of sleep associated with AD is not completely understood but is likely multifactorial. Pruritus and scratching leading to sleeplessness is the most obvious culprit, but an altered circadian rhythm, immune system response, and changes in skin physiology are also likely factors.7 Whatever the cause, sleep disturbance is reported in as many as 60% of patients with AD, and the degree of sleep disturbance is proportional to increases in disease severity and worsening of quality-of-life scores.8 Lost sleep is not limited to patients; parents of children with AD also report significant loss of sleep and subsequent decreased work productivity and quality of life.9
Children with AD are often the target of bullying.10 A 2015 survey by the National Eczema Association indicates that 1 in 5 children reported being bullied due to their AD.11
Associated conditions and comorbidities
AD increases patients’ risks for other illnesses, due either to their underlying atopy or to the effects of
Atopic march
Atopic march—the clinical succession of AD, allergic rhinitis, and asthma—is a well-established clinical progression. The presence of all 3 conditions appears to be more common in children diagnosed with AD before 2 years of age.12 Typically, allergic rhinitis manifests at around age 4, and asthma develops between ages 6 and 8. The severity of AD predicts progression. Compared with an 8% chance of asthma developing among the general population, children with mild AD have a 20% to 30% chance of developing asthma, and those with severe AD have about a 70% chance.12
Continue to: Food allergies
Food allergies
Patients with AD are at higher risk for food-induced anaphylaxis, with up to one-third of AD patients having an IgE-mediated food allergy.13 While it is theorized that the impaired skin barrier of an atopic child may allow for early sensitization and allergy development, a landmark 2015 study demonstrated that early allergen introduction (specifically, peanuts) may serve as a preventive strategy in those at high risk of food allergies.14 Current guidelines recommend that physicians be aware of the increased possibility of food allergies in those with AD, and consider evaluating a child for milk, egg, peanut, wheat, and soy allergy if the child is younger than 5 years and has eczema that does not resolve with treatment, or has eczema and a history of an allergic reaction to a specific food.15
Interestingly, despite the strong association between AD and food allergies, it is not clear that food allergies trigger atopic flares; as such, elimination diets are not universally recommended in those without a proven food allergy.
Psychiatric diagnoses
Children with AD have an increased prevalence of several psychiatric conditions, including ADHD, depression, anxiety, conduct disorder, and autism when compared with peers who do not have AD, and the probability correlates with the severity of AD
What we do know is that one of the strongest associations between AD and a psychiatric condition is with ADHD, with a recent pooled meta-analysis showing a 46% increase in risk.17 The incidence of depression among children with AD appears to correlate with the severity of AD symptoms: estimated at 5% with mild AD, 7% with moderate disease, and 14% with severe disease (compared with 3% without AD). Similar incremental increases are seen when correlating AD and anxiety.16
Nonpharmacologic care
Bathing
Bathing habits are critical to controlling AD. While bathing serves to both hydrate the skin and remove allergens, the water’s evaporation off the skin surface can lead to increased transepidermal water loss. Combining bathing and immediate application of a moisturizer improves skin hydration in patients with AD vs bathing alone.18 Thus, consensus guidelines recommend once-daily bathing (bath or shower) to remove scale and crust, followed by immediate application of a moisturizing emollient.19
Continue to: Emollients
Emollients
Application of moisturizing emollients is the mainstay of nonpharmacologic care of AD, and there is strong evidence that their regimented use reduces disease burden and the need for prescription treatment.19 Emollient creams and ointments help retain moisture and improve the skin’s barrier. While ointments may provide a better barrier, patients tend to prefer creams as they are less greasy than ointments.
Emollient therapy may also help prevent development of AD, especially in those infants thought to be at high risk with a family history of atopy. In a multinational randomized controlled trial, infants who received daily full-body application of emollient beginning at 3 weeks of life were significantly less likely than controls to develop AD by 6 months.20 While the mechanism of action is not clearly understood, it is believed that early emollient use prevents skin dehydration and maintains the skin’s barrier integrity, thus decreasing allergen epidermal penetration and subsequent inflammation.
Bleach bath
A bleach bath, prepared by adding 1/2 cup of unconcentrated bleach (5.25% sodium hypochlorite) to a standard 40-gallon bathtub, produces a chlorine mixture equivalent to an average swimming pool. Soaking in a bleach bath for 10 minutes once or twice weekly is thought to reduce inflammation and bacteria on the skin, but studies of its efficacy in improving atopic symptoms are mixed.
In a pooled analysis of 5 studies evaluating bleach baths vs standard baths, there was no significant difference in disease severity at 4 weeks.21 Thus, while bleach baths were effective in decreasing disease severity, they appeared to be no more effective than a standard water bath.21 Bleach baths may be helpful, however, in cases of moderate-to-severe disease with frequent bacterial infections.19
Pharmacologic therapy
Steroids
For symptoms refractory to nonpharmacologic skin care, topical steroids are the initial pharmacologic treatment for AD.19 Choose steroid potency based on symptom severity and disease location. Low- to medium-potency is appropriate for mild disease, and medium- to high-potency is useful for moderate-to-severe symptoms. High-potency steroids are generally avoided on the face and skin folds; however, they can be used for short periods in these areas to induce remission. They must then be quickly tapered and discontinued.
Continue to: Frequency
Frequency. Topical corticosteroids are typically applied twice daily, although recent studies indicate that once-daily application is just as efficacious.22 In addition to treatment of an acute flare, topical steroids are useful as maintenance therapy for patients with recurrent outbreaks in the same anatomical site. Guidelines suggest once- or twice-weekly application of a medium-potency steroid to prolong time between flares.19
For children, a practical guide is for caregivers to apply the amount of steroid covering 1 adult fingertip to an area of the child’s skin equal to that of 2 adult palms.23 Topical steroids are generally well tolerated and have a good safety profile. Adverse effects are proportional to the amount and duration of use and include purpura, telangiectasias, striae, and skin atrophy. The risk of skin atrophy increases with higher potency steroids, occlusion (covering affected area after steroid application), use on thin-skinned areas, and older patient age.24
Reassure patients/parents about the safety of topical steroids, as fears regarding the potential adverse effects can limit compliance. In one study of 200 patients with AD, 72.5% of respondents expressed fear of using steroids on their own skin or that of their child, and 24% admitted being noncompliant with therapy based on these concerns.25
Treating flares. Oral steroids are sometimes needed to abort or control an AD flare in older children and adults. A tapering course of prednisone over 5 to 7 days, transitioning to medium- to high-dose topical steroids, may be needed to achieve symptom control.
Topical calcineurin inhibitors
Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, are generally second-line therapy to topical corticosteroids. However, as nonsteroidal agents, topical calcineurin inhibitors do not cause skin atrophy and can be a first-line option in areas where atrophy is more common (face, eyelids, neck, and skin folds).26
Continue to: A Cochrane review found...
A Cochrane review found tacrolimus 0.1% to be better than low‐potency topical corticosteroids on the face and neck areas, while results were equivocal when compared with moderate‐potency topical corticosteroids on the trunk and extremities (no difference based on physician assessment, but marginal benefit favoring tacrolimus based on participant scoring).27 When compared head-to-head, tacrolimus was more effective than pimecrolimus, although tacrolimus has a higher rate of local irritation. The most common adverse effects are stinging and burning at the application site, although these adverse effects generally improve with repeated application.
There have been long-term safety concerns with topical calcineurin inhibitors—chiefly a 2006 Food and Drug Administration (FDA) black box warning regarding a possible link between topical calcineurin inhibitors and cancer. However, while there may be a slight increased risk of lymphoma in AD patients, a recent meta-analysis did not find an association between topical calcineurin inhibitors use and lymphoma.28 Given the initial concern—and pending additional data—the FDA currently recommends reserving topical calcineurin inhibitors for second-line therapy and only for the minimum amount of time to induce improvement. It also recommends avoiding their use in patients younger than 2 years and in those with compromised immune systems.
Cisaborole
Cisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor, received FDA approval in 2016 for mild-to-moderate AD. By inhibiting PDE4, the drug limits inflammation. In a multicenter randomized trial, patients applying cisaborole 2% twice a day noted reductions in pruritus, inflammation, excoriation, and lichenification.29 Adverse effects are minimal and limited to application site irritation.
Systemic treatments
While beyond the care of a family physician, symptoms refractory to conservative, nonpharmacologic measures and combinations of topical pharmaceuticals can be treated with systemic immunomodulators such as cyclosporine, azathioprine, and methotrexate. Phototherapy is also effective in patients with more widespread skin involvement. Dupilumab, an injectable monoclonal antibody that binds to interleukin-4 receptor and inhibits inflammation, is approved to treat moderate-to-severe AD in adults.30
Ineffective therapies: Oral montelukast and probiotics
While oral antihistamines are frequently prescribed and used, there are no studies evaluating the use of antihistamines (H1) as monotherapy for AD.31 Nonetheless, while not altering the disease process, the sedative effect of antihistamines may palliate the nocturnal pruritus frequently associated with AD. Although nonsedating antihistamines may still have a role for atopic patients with concurrent seasonal and environmental allergies, there is no evidence to support their use in the treatment of AD.
Continue to: Data are limited...
Data are limited on the effectiveness of leukotriene receptor antagonists for AD, and all studies meeting inclusion for a Cochrane review assessed oral montelukast. The review found no benefit with the use of montelukast 10 mg in terms of severity of disease, pruritus, or need for topical steroids.32
A systematic review investigating the benefit of probiotics for the treatment of AD found no improvement in patient-rated eczema scores for quality of life.33 Additionally, a review of 11 randomized controlled trials including 596 participants found no evidence to suggest efficacy of fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine, sea buckthorn oil, hempseed oil, or sunflower oil in the treatment of AD.34
Education can reduce AD severity
Family physicians can be a source of education and support for patients and families of patients with AD. Support programs for adults with AD—including education, relaxation techniques, and cognitive behavioral therapy—have been shown to decrease disease severity.35 Comparable improvement in disease severity has been demonstrated in children with AD when similar education is provided to them and their families.
CORRESPONDENCE
Franklin Berkey, DO, Penn State Health, 1850 East Park Avenue, Suite 207, State College, PA 16803; fberkey@ pennstatehealth.psu.edu.
1. Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
2. Ahn C, Huang W. Clinical presentation of atopic dermatitis. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:38-46.
3. Eichenfield LF, Tom WL, Chamblin SL, et al. Guidelines of care for the management of atopic dermatitis. Part 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
4. Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol. 2006;155:504-514.
5. Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015:CD008426.
6. Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
7. Chang Y-S, Chiang B-L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17:462.
8. Camfferman D, Kennedy JD, Gold M, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359-369.
9. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
10. Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:P26-P30.
11. National Eczema Association. Tools for school: addressing school bullying for kids with eczema. Accessed January 5, 2021. https://nationaleczema.org/children-with-eczema-experience-bullying/
12. Bantz SK, Zhu Z, Zhen T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202
13. Laird M, Sicco KL. Defining and measuring the scope of atopic dermatitis. Adv Exp Med Biol. 2017;1027:93-104.
14. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
15. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58.
16. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
17. Strom MA, Fishbein AB, Paller AS, et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920-929.
18. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
19. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
20. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
21. Chopra R, Vakharia PP, Sacotte R, et al. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-440.
22. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ. 2007;334:1272.
23. Long CC, Mills CM, Finlay AY. A practical guide to topical therapy in children. Br J Dermatol. 1998;138:293-296.
24. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
25. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931-936.
26. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: a meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
27. Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015:CD009864.
28. Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
29. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.
30. Dupilumab [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2017.
31. van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25.
32. Ferguson L, Futamura M, Vakirlis E, et al. Leukotriene receptor antagonists for eczema. Cochrane Database Syst Rev. 2018:CD011224.
33. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018:CD006135.
34. Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
35. Sy W, Lamb AJ. Atopic dermatitis disease education. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:179-184.
1. Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
2. Ahn C, Huang W. Clinical presentation of atopic dermatitis. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:38-46.
3. Eichenfield LF, Tom WL, Chamblin SL, et al. Guidelines of care for the management of atopic dermatitis. Part 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
4. Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol. 2006;155:504-514.
5. Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015:CD008426.
6. Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
7. Chang Y-S, Chiang B-L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17:462.
8. Camfferman D, Kennedy JD, Gold M, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359-369.
9. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
10. Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:P26-P30.
11. National Eczema Association. Tools for school: addressing school bullying for kids with eczema. Accessed January 5, 2021. https://nationaleczema.org/children-with-eczema-experience-bullying/
12. Bantz SK, Zhu Z, Zhen T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202
13. Laird M, Sicco KL. Defining and measuring the scope of atopic dermatitis. Adv Exp Med Biol. 2017;1027:93-104.
14. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
15. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58.
16. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
17. Strom MA, Fishbein AB, Paller AS, et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920-929.
18. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
19. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
20. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
21. Chopra R, Vakharia PP, Sacotte R, et al. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-440.
22. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ. 2007;334:1272.
23. Long CC, Mills CM, Finlay AY. A practical guide to topical therapy in children. Br J Dermatol. 1998;138:293-296.
24. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
25. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931-936.
26. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: a meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
27. Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015:CD009864.
28. Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
29. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.
30. Dupilumab [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2017.
31. van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25.
32. Ferguson L, Futamura M, Vakirlis E, et al. Leukotriene receptor antagonists for eczema. Cochrane Database Syst Rev. 2018:CD011224.
33. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018:CD006135.
34. Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
35. Sy W, Lamb AJ. Atopic dermatitis disease education. In: Fortson E, Feldman SR, Stroud LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:179-184.
PRACTICE RECOMMENDATIONS
› Advise patients to regularly apply moisturizers, which reduces atopic dermatitis (AD) severity and may avert the need for pharmacologic intervention. A
› Assure patients that a topical corticosteroid is safe and effective as first-line treatment for AD symptoms refractory to nonpharmacologic recommendations. A
› Consider topical calcineurin inhibitors for both acute and chronic AD in adults and children, especially in areas more prone to topical corticosteroid adverse effects. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tactics to prevent or slow progression of CKD in patients with diabetes
Chronic kidney disease (CKD) is a significant comorbidity of diabetes mellitus. The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as the presence of kidney damage or decreased kidney function for ≥ 3 months. CKD caused by diabetes is called diabetic kidney disease (DKD), which is 1 of 3 principal microvascular complications of diabetes. DKD can progress to end-stage renal disease (ESRD), requiring kidney replacement therapy, and is the leading cause of CKD and ESRD in the United States.1-3 Studies have also shown that, particularly in patients with diabetes, CKD considerably increases the risk of cardiovascular events, which often occur prior to ESRD.1,4
This article provides the latest recommendations for evaluating and managing DKD to help you prevent or slow its progression.
Defining and categorizing diabetic kidney disease
CKD is defined as persistently elevated excretion of urinary albumin (albuminuria) and decreased estimated glomerular filtration rate (eGFR), or as the presence of signs of progressive kidney damage.5,6 DKD, also known as diabetic nephropathy, is CKD attributed to long-term diabetes. A patient’s eGFR is the established basis for assignment to a stage (1, 2, 3a, 3b, 4, or 5) of CKD (TABLE 17) and, along with the category of albuminuria (A1, A2, or A3), can indicate prognosis.
Taking its toll in diabetes
As many as 40% of patients with diabetes develop DKD.8-10 Most studies of DKD have been conducted in patients with type 1 diabetes (T1D), because the time of clinical onset is typically known.
Type 1 diabetes. DKD usually occurs 10 to 15 years, or later, after the onset of diabetes.6 As many as 30% of people with T1D have albuminuria approximately 15 years after onset of diabetes; almost one-half of those develop DKD.5,11 After approximately 22.5 years without albuminuria, patients with T1D have approximately a 1% annual risk of DKD.12
Type 2 diabetes (T2D). DKD is often present at diagnosis, likely due to a delay in diagnosis and briefer clinical exposure, compared to T1D. Albuminuria has been reported in as many as 40% of patients with T2D approximately 10 years after onset of diabetes.12,13
Multiple risk factors with no standout “predictor”
Genetic susceptibility, ethnicity, glycemic control, smoking, blood pressure (BP), and the eGFR have been identified as risk factors for renal involvement in diabetes; obesity, oral contraceptives, and age can also contribute. Although each risk factor increases the risk of DKD, no single factor is adequately predictive. Moderately increased albuminuria, the earliest sign of DKD, is associated with progressive nephropathy.12
Continue to: How great is the risk?
How great is the risk? From disease onset to proteinuria and from proteinuria to ESRD, the risk of DKD in T1D and T2D is similar. With appropriate treatment, albuminuria can regress, and the risk of ESRD can be < 20% at 10 years in T1D.12 As in T1D, good glycemic control might result in regression of albuminuria in T2D.14
For unknown reasons, the degree of albuminuria can exist independent of the progression of DKD. Factors responsible for a progressive decline in eGFR in DKD without albuminuria are unknown.12,15
Patient evaluation with an eye toward comorbidities
A comprehensive initial medical evaluation for DKD includes a review of microvascular complications; visits to specialists; lifestyle and behavior patterns (eg, diet, sleep, substance use, and social support); and medication adherence, adverse drug effects, and alternative medicines. Although DKD is often a clinical diagnosis, it can be ruled in by persistent albuminuria or decreased eGFR, or both, in established diabetes or diabetic retinopathy when other causes are unlikely (see “Recommended DKD screening protocol,” below).
Screening for mental health conditions and barriers to self-management is also key.6
Comorbidities, of course, can complicate disease management in patients with diabetes.16-20 Providers and patients therefore need to be aware of potential diabetic comorbidities. For example, DKD and even moderately increased albuminuria significantly increase the risk of cardiovascular disease (CVD).12 Other possible comorbidities include (but are not limited to) nonalcoholic steatohepatitis, fracture, hearing impairment, cancer (eg, liver, pancreas, endometrium, colon, rectum, breast, and bladder), pancreatitis, hypogonadism, obstructive sleep apnea, periodontal disease, anxiety, depression, and eating disorders.6
Continue to: Recommended DKD screening protocol
Recommended DKD screening protocol
In all cases of T2D, in cases of T1D of ≥ 5 years’ duration, and in patients with diabetes and comorbid hypertension, perform annual screening for albuminuria, an elevated creatinine level, and a decline in eGFR.
To confirm the diagnosis of DKD, at least 2 of 3 urine specimens must demonstrate an elevated urinary albumin:creatinine ratio (UACR) over a 3- to 6-month period.21 Apart from renal damage, exercise within 24 hours before specimen collection, infection, fever, congestive heart failure, hyperglycemia, menstruation, and hypertension can elevate the UACR.6
Levels of the UACR are established as follows22:
- Normal UACR is defined as < 30 milligrams of albumin per gram of creatinine (expressed as “mg/g”).
- Increased urinary albumin excretion is defined as ≥ 30 mg/g.
- Moderately increased albuminuria, a predictor of potential nephropathy, is the excretion of 30 to 300 mg/g.
- Severely increased albuminuria is excretion > 300 mg/g; it is often followed by a gradual decline in eGFR that, without treatment, eventually leads to ESRD.
The rate of decline in eGFR once albuminuria is severely increased is equivalent in T1D and T2D.12 Without intervention, the time from severely increased albuminuria to ESRD in T1D and T2D averages approximately 6 or 7 years.
Clinical features
DKD is typically a clinical diagnosis seen in patients with longstanding diabetes, albuminuria, retinopathy, or a reduced eGFR in the absence of another primary cause of kidney damage. In patients with T1D and DKD, signs of retinopathy and neuropathy are almost always present at diagnosis, unless a diagnosis is made early in the course of diabetes.12 Therefore, the presence of retinopathy suggests that diabetes is the likely cause of CKD.
Continue to: The presence of microvascular disease...
The presence of microvascular disease in patients with T2D and DKD is less predictable.12 In T2D patients who do not have retinopathy, consider causes of CKD other than DKD. Features suggesting that the cause of CKD is an underlying condition other than diabetes are rapidly increasing albuminuria or decreasing eGFR; urinary sediment comprising red blood cells or white blood cells; and nephrotic syndrome.6
As the prevalence of diabetes increases, it has become more common to diagnose DKD by eGFR without albuminuria—underscoring the importance of routine monitoring of eGFR in patients with diabetes.6
Sources of expert guidance. The Chronic Kidney Disease Epidemiology Collaboration equation23 is preferred for calculating eGFR from serum creatinine: An eGFR < 60 mL/min/1.73 m2 is considered abnormal.3,12 At these rates, the prevalence of complications related to CKD rises and screening for complications becomes necessary.
A more comprehensive classification of the stages of CKD, incorporating albuminuria and progression of CKD, has been recommended by Kidney Disease: Improving Global Outcomes (KDIGO).7 Because eGFR and excretion of albumin vary, abnormal test results need to be verified over time to stage the degree of CKD.3,12 Kidney damage often manifests as albuminuria, but also as hematuria, other types of abnormal urinary sediment, radiographic abnormalities, and other abnormal presentations.
Management
Nutritional factors
Excessive protein intake has been shown to increase albuminuria, worsen renal function, and increase CVD mortality in DKD.24-26 Therefore, daily dietary protein intake of 0.8 g/kg body weight is recommended for patients who are not on dialysis.3 Patients on dialysis might require higher protein intake to preserve muscle mass caused by protein-energy wasting, which is common in dialysis patients.6
Continue to: Low sodium intake
Low sodium intake in CKD patients has been shown to decrease BP and thus slow the progression of renal disease and lower the risk of CVD. The recommended dietary sodium intake in CKD patients is 1500-3000 mg/d.3
Low potassium intake. Hyperkalemia is a serious complication of CKD. A low-potassium diet is recommended in ESRD patients who have a potassium level > 5.5 mEq/L.6
Blood pressure
Preventing and treating hypertension is critical to slowing the progression of CKD and reducing cardiovascular risk. BP should be measured at every clinic visit. Aside from lifestyle changes, medication might be needed to reach target BP.
The American Diabetes Association recommends a BP goal of ≤ 140/90 mm Hg for hypertensive patients with diabetes, although they do state that a lower BP target (≤ 130/80 mm Hg) might be more appropriate for patients with DKD.27
The American College of Cardiology recommends that hypertensive patients with CKD have a BP target of ≤ 130/80 mm Hg.28
Continue to: ACE inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have renoprotective benefits. These agents are recommended as first-line medications for patients with diabetes, hypertension, and an eGFR < 60 mL/min/1.73 m2 and a UACR > 300 mg/g.29-31 Evidence also supports their use when the UACR is 30 to 299 mg/g.
Studies have shown that, in patients with DKD, ACE inhibitors and ARBs can slow the progression of renal disease.29,30,32 There is no difference between ACE inhibitors and ARBs in their effectiveness for preventing progression of DKD.6 There is no added benefit in combining an ACE inhibitor and an ARB33; notably, combination ACE inhibitor and ARB therapy can increase the risk of adverse events, such as hyperkalemia and acute kidney injury, especially in patients with DKD.33
There is no evidence for starting an ACE inhibitor or ARB to prevent CKD in patients with diabetes who are not hypertensive.5
ACE inhibitors and ARBs should be used with caution in women of childbearing age, who should use a reliable form of contraception if taking one of these drugs.
Diuretics. Thiazide-type and loop diuretics might potentiate the positive effects of ACE inhibitors and ARBs. KDOQI guidelines recommend that, in patients who require a second agent to control BP, a diuretic should be considered in combination with an ACE inhibitor or an ARB.20 A loop diuretic is preferred if the eGFR is < 30 mL/min/1.73 m2.
Continue to: Nondihydropyridine calcium-channel blockers
Nondihydropyridine calcium-channel blockers (CCBs), such as diltiazem and verapamil, have been shown to be more effective then dihydrophyridine CCBs, such as amlodipine and nifedipine, in slowing the progression of renal disease because of their antiproteinuric effects. However, the antiproteinuric effects of nondihydropyridine CCBs are not as strong as those of ACE inhibitors or ARBs, and these drugs do not appear to potentiate the effects of an ACE inhibitor or ARB when used in combination.20
Nondihydropyridine CCBs might be a reasonable alternative in patients who cannot tolerate an ACE inhibitor or an ARB.
Mineralocorticoid receptor antagonists in combination with an ACE inhibitor or ARB have been demonstrated to reduce albuminuria in short-term studies.34,35
Glycemic levels
Studies conducted in patients with T1D, and others in patients with T2D, have shown that tight glycemic control can delay the onset and slow the progression of albuminuria and a decline in the eGFR.10,36-39 The target glycated hemoglobin (A1C) should be < 7% to prevent or slow progression of DKD.40 However, patients with DKD have an increased risk of hypoglycemic events and increased mortality with more intensive glycemic control.40,41 Given those findings, some patients with DKD and significant comorbidities, ESRD, or limited life expectancy might need to have an A1C target set at 8%.6,42
Adjustments to antidiabetes medications in DKD
In patients with stages 3 to 5 DKD, several common antidiabetic medications might need to be adjusted or discontinued because they decrease creatinine clearance.
Continue to: First-generation sulfonylureas
First-generation sulfonylureas should be avoided in DKD. Glipizide and gliclazide are preferred among second-generation sulfonylureas because they do not increase the risk of hypoglycemia in DKD patients, although patients taking these medications still require close monitoring of their blood glucose level.20
Metformin. In 2016, recommendations changed for the use of metformin in patients with DKD: The eGFR, not the serum creatinine level, should guide treatment.43 Metformin can be used safely in patients with (1) an eGFR of < 60 mL/min/1.73 m2 and (2) an eGFR of 30 mL/min/1.73 m2 with close monitoring. Metformin should not be initiated if the eGFR is < 45 mL/min/1.73 m2.43
Antidiabetes medications with direct effect on the kidney
Several antidiabetes medications have a direct effect on the kidney apart from their effect on the blood glucose level.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to reduce albuminuria and slow the decrease of eGFR independent of glycemic control. In addition, SGLT2 inhibitors have also been shown to have cardiovascular benefits in patients with DKD.44,45
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to delay and decrease the progression of DKD.46-48 Also, similar to what is seen with SGLT2 inhibitors, GLP-1 agonists have demonstrable cardiovascular benefit in patients with DKD.46,48
Continue to: Dyslipidemia and DKD
Dyslipidemia and DKD
Because the risk of CVD is increased in patients with DKD, addressing other modifiable risk factors, including dyslipidemia, is recommended in these patients. Patients with diabetes and stages 1 to 4 DKD should be treated with a high-intensity statin or a combination of a statin and ezetimibe.49,50
If a patient is taking a statin and starting dialysis, it’s important to discuss with him or her whether to continue the statin, based on perceived benefits and risks. It is not recommended that statins be initiated in patients on dialysis unless there is a specific cardiovascular indication for doing so. Risk reduction with a statin has been shown to be significantly less in dialysis patients than in patients who are not being treated with dialysis.49
Complications of CKD
Anemia is a common complication of CKD. KDIGO recommends measuring the hemoglobin concentration annually in DKD stage 3 patients without anemia; at least every 6 months in stage 4 patients; and at least every 3 months in stage 5. DKD patients with anemia should have additional laboratory testing: the absolute reticulocyte count, serum ferritin, serum transferrin saturation, vitamin B12, and folate.51
Mineral and bone disorder should be screened for in patients with DKD. TABLE 252 outlines when clinical laboratory tests should be ordered to assess for mineral bone disease.
When to refer to a nephrologist
Refer patients with stage 4 or 5 CKD (eGFR, ≤ 30 mL/min/1.73 m2) to a nephrologist for discussion of kidney replacement therapy.6 Patients with stage 3a CKD and severely increased albuminuria or with stage 3b CKD and moderately or severely increased albuminuria should also be referred to a nephrologist for intervention to delay disease progression.
Continue to: Identifying the need for early referral...
Identifying the need for early referral to a nephrologist has been shown to reduce the cost, and improve the quality, of care.53 Other indications for earlier referral include uncertainty about the etiology of renal disease, persistent or severe albuminuria, persistent hematuria, a rapid decline in eGFR, and acute kidney injury. Additionally, referral at an earlier stage of DKD might be needed to assist with complications associated with DKD, such as anemia, secondary hyperparathyroidism, mineral and bone disorder, resistant hypertension, fluid overload, and electrolyte disturbances.6
ACKNOWLEDGEMENT
The authors thank Colleen Colbert, PhD, and Iqbal Ahmad, PhD, for their review and critique of the manuscript of this article. They also thank Christopher Babiuch, MD, for his guidance in the preparation of the manuscript.
CORRESPONDENCE
Faraz Ahmad, MD, MPH, Care Point East Family Medicine, 543 Taylor Avenue, 2nd floor, Columbus, OH 43203; faraz. [email protected].
1. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83:1373-1381.
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7.
3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533.
4. Fox CS, Matsushita K, Woodward M, et al; . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673.
5. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124.
6. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159. Accessed January 5, 2021. https://care.diabetesjournals.org/content/41/Supplement_1
7. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. Accessed January 5, 2021. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
8. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-610.
9. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539.
10. de Boer IH; DCCT/EDIC Research Group. Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:24-30.
11. Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S3-S21.
12. Mottl AK, Tuttle KR. Diabetic kidney disease: Pathogenesis and epidemiology. UpToDate. Updated August 19, 2019. Accessed January 5, 2021. www.uptodate.com/contents/diabetic-kidney-disease-pathogenesis-and-epidemiology
13. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 2 diabetes mellitus. UpToDate. Updated November 3, 2020. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-2-diabetes-mellitus
14. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428.
15. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7-A8.
16. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277-284.
17. de Boer IH, Gao X, Cleary PA, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: The DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969-1977.
18. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941-1949.
19. Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patient with insulin dependent diabetes? BMJ. 1993;306:1722-1725.
20. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12-154.
21. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus. UpToDate. Updated December 3, 2019. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-1-diabetes-mellitus
22. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17-26.
23. Levey AS, Stevens LA, Schmid CH, et al; , A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
24. Wrone EM, Carnethon MR, Palaniappan L, et al; . Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580-587.
25. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467.
26. Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876-883.
27. de Boer, IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284.
28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
29. Brenner BM, Cooper ME, de Zeeuw D, et al; Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
30. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462.
31. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355;253-259.
32. Lewis EJ, Hunsicker LG, Clarke WR, et al; . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
33. Fried LF, Emanuele N, Zhang JH, et al; . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
34. Bakris GL, Agarwal R, Chan JC, et al; . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884-894.
35. Filippatos G, Anker SD, M, et al. Randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114.
36. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.N Engl J Med. 2008;358:2560-2572.
37. Ismail-Beigi F, Craven T, Banerji MA, et al; . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
38. Zoungas S, Chalmers J, Neal B, et al; . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392-1406.
39. Zoungas S, Arima H, Gerstein HC, et al; . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
40. Miller ME, Bonds DE, Gerstein HC, et al; . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340;b5444.
41. Papademetriou V, Lovato L, Doumas M, et al; . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659.
42. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886.
43. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J. 2017;10:301-304.
44. Wanner C, Inzucchi SE, Lachin JM, et al; Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
45. Neal B, Perkovic V, Mahaffey KW, et al; . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
46. Marso SP, Daniels GH, Brown-Frandsen K, et al; . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
47. Mann JFE, DD, Brown-Frandsen K, et al; . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
48. Marso SP, Bain SC, Consoli A, et al; . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
49. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309.
50. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143.
51. National Kidney Foundation KDOQI. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335. Accessed January 5, 2021. www.sciencedirect.com/journal/kidney-international-supplements/vol/2/issue/4
52. National Kidney Foundation KDOQI. Evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). 2010. Accessed January 5, 2021. www.kidney.org/sites/default/files/02-10-390B_LBA_KDOQI_BoneGuide.pdf
53. Smart MA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
Chronic kidney disease (CKD) is a significant comorbidity of diabetes mellitus. The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as the presence of kidney damage or decreased kidney function for ≥ 3 months. CKD caused by diabetes is called diabetic kidney disease (DKD), which is 1 of 3 principal microvascular complications of diabetes. DKD can progress to end-stage renal disease (ESRD), requiring kidney replacement therapy, and is the leading cause of CKD and ESRD in the United States.1-3 Studies have also shown that, particularly in patients with diabetes, CKD considerably increases the risk of cardiovascular events, which often occur prior to ESRD.1,4
This article provides the latest recommendations for evaluating and managing DKD to help you prevent or slow its progression.
Defining and categorizing diabetic kidney disease
CKD is defined as persistently elevated excretion of urinary albumin (albuminuria) and decreased estimated glomerular filtration rate (eGFR), or as the presence of signs of progressive kidney damage.5,6 DKD, also known as diabetic nephropathy, is CKD attributed to long-term diabetes. A patient’s eGFR is the established basis for assignment to a stage (1, 2, 3a, 3b, 4, or 5) of CKD (TABLE 17) and, along with the category of albuminuria (A1, A2, or A3), can indicate prognosis.
Taking its toll in diabetes
As many as 40% of patients with diabetes develop DKD.8-10 Most studies of DKD have been conducted in patients with type 1 diabetes (T1D), because the time of clinical onset is typically known.
Type 1 diabetes. DKD usually occurs 10 to 15 years, or later, after the onset of diabetes.6 As many as 30% of people with T1D have albuminuria approximately 15 years after onset of diabetes; almost one-half of those develop DKD.5,11 After approximately 22.5 years without albuminuria, patients with T1D have approximately a 1% annual risk of DKD.12
Type 2 diabetes (T2D). DKD is often present at diagnosis, likely due to a delay in diagnosis and briefer clinical exposure, compared to T1D. Albuminuria has been reported in as many as 40% of patients with T2D approximately 10 years after onset of diabetes.12,13
Multiple risk factors with no standout “predictor”
Genetic susceptibility, ethnicity, glycemic control, smoking, blood pressure (BP), and the eGFR have been identified as risk factors for renal involvement in diabetes; obesity, oral contraceptives, and age can also contribute. Although each risk factor increases the risk of DKD, no single factor is adequately predictive. Moderately increased albuminuria, the earliest sign of DKD, is associated with progressive nephropathy.12
Continue to: How great is the risk?
How great is the risk? From disease onset to proteinuria and from proteinuria to ESRD, the risk of DKD in T1D and T2D is similar. With appropriate treatment, albuminuria can regress, and the risk of ESRD can be < 20% at 10 years in T1D.12 As in T1D, good glycemic control might result in regression of albuminuria in T2D.14
For unknown reasons, the degree of albuminuria can exist independent of the progression of DKD. Factors responsible for a progressive decline in eGFR in DKD without albuminuria are unknown.12,15
Patient evaluation with an eye toward comorbidities
A comprehensive initial medical evaluation for DKD includes a review of microvascular complications; visits to specialists; lifestyle and behavior patterns (eg, diet, sleep, substance use, and social support); and medication adherence, adverse drug effects, and alternative medicines. Although DKD is often a clinical diagnosis, it can be ruled in by persistent albuminuria or decreased eGFR, or both, in established diabetes or diabetic retinopathy when other causes are unlikely (see “Recommended DKD screening protocol,” below).
Screening for mental health conditions and barriers to self-management is also key.6
Comorbidities, of course, can complicate disease management in patients with diabetes.16-20 Providers and patients therefore need to be aware of potential diabetic comorbidities. For example, DKD and even moderately increased albuminuria significantly increase the risk of cardiovascular disease (CVD).12 Other possible comorbidities include (but are not limited to) nonalcoholic steatohepatitis, fracture, hearing impairment, cancer (eg, liver, pancreas, endometrium, colon, rectum, breast, and bladder), pancreatitis, hypogonadism, obstructive sleep apnea, periodontal disease, anxiety, depression, and eating disorders.6
Continue to: Recommended DKD screening protocol
Recommended DKD screening protocol
In all cases of T2D, in cases of T1D of ≥ 5 years’ duration, and in patients with diabetes and comorbid hypertension, perform annual screening for albuminuria, an elevated creatinine level, and a decline in eGFR.
To confirm the diagnosis of DKD, at least 2 of 3 urine specimens must demonstrate an elevated urinary albumin:creatinine ratio (UACR) over a 3- to 6-month period.21 Apart from renal damage, exercise within 24 hours before specimen collection, infection, fever, congestive heart failure, hyperglycemia, menstruation, and hypertension can elevate the UACR.6
Levels of the UACR are established as follows22:
- Normal UACR is defined as < 30 milligrams of albumin per gram of creatinine (expressed as “mg/g”).
- Increased urinary albumin excretion is defined as ≥ 30 mg/g.
- Moderately increased albuminuria, a predictor of potential nephropathy, is the excretion of 30 to 300 mg/g.
- Severely increased albuminuria is excretion > 300 mg/g; it is often followed by a gradual decline in eGFR that, without treatment, eventually leads to ESRD.
The rate of decline in eGFR once albuminuria is severely increased is equivalent in T1D and T2D.12 Without intervention, the time from severely increased albuminuria to ESRD in T1D and T2D averages approximately 6 or 7 years.
Clinical features
DKD is typically a clinical diagnosis seen in patients with longstanding diabetes, albuminuria, retinopathy, or a reduced eGFR in the absence of another primary cause of kidney damage. In patients with T1D and DKD, signs of retinopathy and neuropathy are almost always present at diagnosis, unless a diagnosis is made early in the course of diabetes.12 Therefore, the presence of retinopathy suggests that diabetes is the likely cause of CKD.
Continue to: The presence of microvascular disease...
The presence of microvascular disease in patients with T2D and DKD is less predictable.12 In T2D patients who do not have retinopathy, consider causes of CKD other than DKD. Features suggesting that the cause of CKD is an underlying condition other than diabetes are rapidly increasing albuminuria or decreasing eGFR; urinary sediment comprising red blood cells or white blood cells; and nephrotic syndrome.6
As the prevalence of diabetes increases, it has become more common to diagnose DKD by eGFR without albuminuria—underscoring the importance of routine monitoring of eGFR in patients with diabetes.6
Sources of expert guidance. The Chronic Kidney Disease Epidemiology Collaboration equation23 is preferred for calculating eGFR from serum creatinine: An eGFR < 60 mL/min/1.73 m2 is considered abnormal.3,12 At these rates, the prevalence of complications related to CKD rises and screening for complications becomes necessary.
A more comprehensive classification of the stages of CKD, incorporating albuminuria and progression of CKD, has been recommended by Kidney Disease: Improving Global Outcomes (KDIGO).7 Because eGFR and excretion of albumin vary, abnormal test results need to be verified over time to stage the degree of CKD.3,12 Kidney damage often manifests as albuminuria, but also as hematuria, other types of abnormal urinary sediment, radiographic abnormalities, and other abnormal presentations.
Management
Nutritional factors
Excessive protein intake has been shown to increase albuminuria, worsen renal function, and increase CVD mortality in DKD.24-26 Therefore, daily dietary protein intake of 0.8 g/kg body weight is recommended for patients who are not on dialysis.3 Patients on dialysis might require higher protein intake to preserve muscle mass caused by protein-energy wasting, which is common in dialysis patients.6
Continue to: Low sodium intake
Low sodium intake in CKD patients has been shown to decrease BP and thus slow the progression of renal disease and lower the risk of CVD. The recommended dietary sodium intake in CKD patients is 1500-3000 mg/d.3
Low potassium intake. Hyperkalemia is a serious complication of CKD. A low-potassium diet is recommended in ESRD patients who have a potassium level > 5.5 mEq/L.6
Blood pressure
Preventing and treating hypertension is critical to slowing the progression of CKD and reducing cardiovascular risk. BP should be measured at every clinic visit. Aside from lifestyle changes, medication might be needed to reach target BP.
The American Diabetes Association recommends a BP goal of ≤ 140/90 mm Hg for hypertensive patients with diabetes, although they do state that a lower BP target (≤ 130/80 mm Hg) might be more appropriate for patients with DKD.27
The American College of Cardiology recommends that hypertensive patients with CKD have a BP target of ≤ 130/80 mm Hg.28
Continue to: ACE inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have renoprotective benefits. These agents are recommended as first-line medications for patients with diabetes, hypertension, and an eGFR < 60 mL/min/1.73 m2 and a UACR > 300 mg/g.29-31 Evidence also supports their use when the UACR is 30 to 299 mg/g.
Studies have shown that, in patients with DKD, ACE inhibitors and ARBs can slow the progression of renal disease.29,30,32 There is no difference between ACE inhibitors and ARBs in their effectiveness for preventing progression of DKD.6 There is no added benefit in combining an ACE inhibitor and an ARB33; notably, combination ACE inhibitor and ARB therapy can increase the risk of adverse events, such as hyperkalemia and acute kidney injury, especially in patients with DKD.33
There is no evidence for starting an ACE inhibitor or ARB to prevent CKD in patients with diabetes who are not hypertensive.5
ACE inhibitors and ARBs should be used with caution in women of childbearing age, who should use a reliable form of contraception if taking one of these drugs.
Diuretics. Thiazide-type and loop diuretics might potentiate the positive effects of ACE inhibitors and ARBs. KDOQI guidelines recommend that, in patients who require a second agent to control BP, a diuretic should be considered in combination with an ACE inhibitor or an ARB.20 A loop diuretic is preferred if the eGFR is < 30 mL/min/1.73 m2.
Continue to: Nondihydropyridine calcium-channel blockers
Nondihydropyridine calcium-channel blockers (CCBs), such as diltiazem and verapamil, have been shown to be more effective then dihydrophyridine CCBs, such as amlodipine and nifedipine, in slowing the progression of renal disease because of their antiproteinuric effects. However, the antiproteinuric effects of nondihydropyridine CCBs are not as strong as those of ACE inhibitors or ARBs, and these drugs do not appear to potentiate the effects of an ACE inhibitor or ARB when used in combination.20
Nondihydropyridine CCBs might be a reasonable alternative in patients who cannot tolerate an ACE inhibitor or an ARB.
Mineralocorticoid receptor antagonists in combination with an ACE inhibitor or ARB have been demonstrated to reduce albuminuria in short-term studies.34,35
Glycemic levels
Studies conducted in patients with T1D, and others in patients with T2D, have shown that tight glycemic control can delay the onset and slow the progression of albuminuria and a decline in the eGFR.10,36-39 The target glycated hemoglobin (A1C) should be < 7% to prevent or slow progression of DKD.40 However, patients with DKD have an increased risk of hypoglycemic events and increased mortality with more intensive glycemic control.40,41 Given those findings, some patients with DKD and significant comorbidities, ESRD, or limited life expectancy might need to have an A1C target set at 8%.6,42
Adjustments to antidiabetes medications in DKD
In patients with stages 3 to 5 DKD, several common antidiabetic medications might need to be adjusted or discontinued because they decrease creatinine clearance.
Continue to: First-generation sulfonylureas
First-generation sulfonylureas should be avoided in DKD. Glipizide and gliclazide are preferred among second-generation sulfonylureas because they do not increase the risk of hypoglycemia in DKD patients, although patients taking these medications still require close monitoring of their blood glucose level.20
Metformin. In 2016, recommendations changed for the use of metformin in patients with DKD: The eGFR, not the serum creatinine level, should guide treatment.43 Metformin can be used safely in patients with (1) an eGFR of < 60 mL/min/1.73 m2 and (2) an eGFR of 30 mL/min/1.73 m2 with close monitoring. Metformin should not be initiated if the eGFR is < 45 mL/min/1.73 m2.43
Antidiabetes medications with direct effect on the kidney
Several antidiabetes medications have a direct effect on the kidney apart from their effect on the blood glucose level.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to reduce albuminuria and slow the decrease of eGFR independent of glycemic control. In addition, SGLT2 inhibitors have also been shown to have cardiovascular benefits in patients with DKD.44,45
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to delay and decrease the progression of DKD.46-48 Also, similar to what is seen with SGLT2 inhibitors, GLP-1 agonists have demonstrable cardiovascular benefit in patients with DKD.46,48
Continue to: Dyslipidemia and DKD
Dyslipidemia and DKD
Because the risk of CVD is increased in patients with DKD, addressing other modifiable risk factors, including dyslipidemia, is recommended in these patients. Patients with diabetes and stages 1 to 4 DKD should be treated with a high-intensity statin or a combination of a statin and ezetimibe.49,50
If a patient is taking a statin and starting dialysis, it’s important to discuss with him or her whether to continue the statin, based on perceived benefits and risks. It is not recommended that statins be initiated in patients on dialysis unless there is a specific cardiovascular indication for doing so. Risk reduction with a statin has been shown to be significantly less in dialysis patients than in patients who are not being treated with dialysis.49
Complications of CKD
Anemia is a common complication of CKD. KDIGO recommends measuring the hemoglobin concentration annually in DKD stage 3 patients without anemia; at least every 6 months in stage 4 patients; and at least every 3 months in stage 5. DKD patients with anemia should have additional laboratory testing: the absolute reticulocyte count, serum ferritin, serum transferrin saturation, vitamin B12, and folate.51
Mineral and bone disorder should be screened for in patients with DKD. TABLE 252 outlines when clinical laboratory tests should be ordered to assess for mineral bone disease.
When to refer to a nephrologist
Refer patients with stage 4 or 5 CKD (eGFR, ≤ 30 mL/min/1.73 m2) to a nephrologist for discussion of kidney replacement therapy.6 Patients with stage 3a CKD and severely increased albuminuria or with stage 3b CKD and moderately or severely increased albuminuria should also be referred to a nephrologist for intervention to delay disease progression.
Continue to: Identifying the need for early referral...
Identifying the need for early referral to a nephrologist has been shown to reduce the cost, and improve the quality, of care.53 Other indications for earlier referral include uncertainty about the etiology of renal disease, persistent or severe albuminuria, persistent hematuria, a rapid decline in eGFR, and acute kidney injury. Additionally, referral at an earlier stage of DKD might be needed to assist with complications associated with DKD, such as anemia, secondary hyperparathyroidism, mineral and bone disorder, resistant hypertension, fluid overload, and electrolyte disturbances.6
ACKNOWLEDGEMENT
The authors thank Colleen Colbert, PhD, and Iqbal Ahmad, PhD, for their review and critique of the manuscript of this article. They also thank Christopher Babiuch, MD, for his guidance in the preparation of the manuscript.
CORRESPONDENCE
Faraz Ahmad, MD, MPH, Care Point East Family Medicine, 543 Taylor Avenue, 2nd floor, Columbus, OH 43203; faraz. [email protected].
Chronic kidney disease (CKD) is a significant comorbidity of diabetes mellitus. The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as the presence of kidney damage or decreased kidney function for ≥ 3 months. CKD caused by diabetes is called diabetic kidney disease (DKD), which is 1 of 3 principal microvascular complications of diabetes. DKD can progress to end-stage renal disease (ESRD), requiring kidney replacement therapy, and is the leading cause of CKD and ESRD in the United States.1-3 Studies have also shown that, particularly in patients with diabetes, CKD considerably increases the risk of cardiovascular events, which often occur prior to ESRD.1,4
This article provides the latest recommendations for evaluating and managing DKD to help you prevent or slow its progression.
Defining and categorizing diabetic kidney disease
CKD is defined as persistently elevated excretion of urinary albumin (albuminuria) and decreased estimated glomerular filtration rate (eGFR), or as the presence of signs of progressive kidney damage.5,6 DKD, also known as diabetic nephropathy, is CKD attributed to long-term diabetes. A patient’s eGFR is the established basis for assignment to a stage (1, 2, 3a, 3b, 4, or 5) of CKD (TABLE 17) and, along with the category of albuminuria (A1, A2, or A3), can indicate prognosis.
Taking its toll in diabetes
As many as 40% of patients with diabetes develop DKD.8-10 Most studies of DKD have been conducted in patients with type 1 diabetes (T1D), because the time of clinical onset is typically known.
Type 1 diabetes. DKD usually occurs 10 to 15 years, or later, after the onset of diabetes.6 As many as 30% of people with T1D have albuminuria approximately 15 years after onset of diabetes; almost one-half of those develop DKD.5,11 After approximately 22.5 years without albuminuria, patients with T1D have approximately a 1% annual risk of DKD.12
Type 2 diabetes (T2D). DKD is often present at diagnosis, likely due to a delay in diagnosis and briefer clinical exposure, compared to T1D. Albuminuria has been reported in as many as 40% of patients with T2D approximately 10 years after onset of diabetes.12,13
Multiple risk factors with no standout “predictor”
Genetic susceptibility, ethnicity, glycemic control, smoking, blood pressure (BP), and the eGFR have been identified as risk factors for renal involvement in diabetes; obesity, oral contraceptives, and age can also contribute. Although each risk factor increases the risk of DKD, no single factor is adequately predictive. Moderately increased albuminuria, the earliest sign of DKD, is associated with progressive nephropathy.12
Continue to: How great is the risk?
How great is the risk? From disease onset to proteinuria and from proteinuria to ESRD, the risk of DKD in T1D and T2D is similar. With appropriate treatment, albuminuria can regress, and the risk of ESRD can be < 20% at 10 years in T1D.12 As in T1D, good glycemic control might result in regression of albuminuria in T2D.14
For unknown reasons, the degree of albuminuria can exist independent of the progression of DKD. Factors responsible for a progressive decline in eGFR in DKD without albuminuria are unknown.12,15
Patient evaluation with an eye toward comorbidities
A comprehensive initial medical evaluation for DKD includes a review of microvascular complications; visits to specialists; lifestyle and behavior patterns (eg, diet, sleep, substance use, and social support); and medication adherence, adverse drug effects, and alternative medicines. Although DKD is often a clinical diagnosis, it can be ruled in by persistent albuminuria or decreased eGFR, or both, in established diabetes or diabetic retinopathy when other causes are unlikely (see “Recommended DKD screening protocol,” below).
Screening for mental health conditions and barriers to self-management is also key.6
Comorbidities, of course, can complicate disease management in patients with diabetes.16-20 Providers and patients therefore need to be aware of potential diabetic comorbidities. For example, DKD and even moderately increased albuminuria significantly increase the risk of cardiovascular disease (CVD).12 Other possible comorbidities include (but are not limited to) nonalcoholic steatohepatitis, fracture, hearing impairment, cancer (eg, liver, pancreas, endometrium, colon, rectum, breast, and bladder), pancreatitis, hypogonadism, obstructive sleep apnea, periodontal disease, anxiety, depression, and eating disorders.6
Continue to: Recommended DKD screening protocol
Recommended DKD screening protocol
In all cases of T2D, in cases of T1D of ≥ 5 years’ duration, and in patients with diabetes and comorbid hypertension, perform annual screening for albuminuria, an elevated creatinine level, and a decline in eGFR.
To confirm the diagnosis of DKD, at least 2 of 3 urine specimens must demonstrate an elevated urinary albumin:creatinine ratio (UACR) over a 3- to 6-month period.21 Apart from renal damage, exercise within 24 hours before specimen collection, infection, fever, congestive heart failure, hyperglycemia, menstruation, and hypertension can elevate the UACR.6
Levels of the UACR are established as follows22:
- Normal UACR is defined as < 30 milligrams of albumin per gram of creatinine (expressed as “mg/g”).
- Increased urinary albumin excretion is defined as ≥ 30 mg/g.
- Moderately increased albuminuria, a predictor of potential nephropathy, is the excretion of 30 to 300 mg/g.
- Severely increased albuminuria is excretion > 300 mg/g; it is often followed by a gradual decline in eGFR that, without treatment, eventually leads to ESRD.
The rate of decline in eGFR once albuminuria is severely increased is equivalent in T1D and T2D.12 Without intervention, the time from severely increased albuminuria to ESRD in T1D and T2D averages approximately 6 or 7 years.
Clinical features
DKD is typically a clinical diagnosis seen in patients with longstanding diabetes, albuminuria, retinopathy, or a reduced eGFR in the absence of another primary cause of kidney damage. In patients with T1D and DKD, signs of retinopathy and neuropathy are almost always present at diagnosis, unless a diagnosis is made early in the course of diabetes.12 Therefore, the presence of retinopathy suggests that diabetes is the likely cause of CKD.
Continue to: The presence of microvascular disease...
The presence of microvascular disease in patients with T2D and DKD is less predictable.12 In T2D patients who do not have retinopathy, consider causes of CKD other than DKD. Features suggesting that the cause of CKD is an underlying condition other than diabetes are rapidly increasing albuminuria or decreasing eGFR; urinary sediment comprising red blood cells or white blood cells; and nephrotic syndrome.6
As the prevalence of diabetes increases, it has become more common to diagnose DKD by eGFR without albuminuria—underscoring the importance of routine monitoring of eGFR in patients with diabetes.6
Sources of expert guidance. The Chronic Kidney Disease Epidemiology Collaboration equation23 is preferred for calculating eGFR from serum creatinine: An eGFR < 60 mL/min/1.73 m2 is considered abnormal.3,12 At these rates, the prevalence of complications related to CKD rises and screening for complications becomes necessary.
A more comprehensive classification of the stages of CKD, incorporating albuminuria and progression of CKD, has been recommended by Kidney Disease: Improving Global Outcomes (KDIGO).7 Because eGFR and excretion of albumin vary, abnormal test results need to be verified over time to stage the degree of CKD.3,12 Kidney damage often manifests as albuminuria, but also as hematuria, other types of abnormal urinary sediment, radiographic abnormalities, and other abnormal presentations.
Management
Nutritional factors
Excessive protein intake has been shown to increase albuminuria, worsen renal function, and increase CVD mortality in DKD.24-26 Therefore, daily dietary protein intake of 0.8 g/kg body weight is recommended for patients who are not on dialysis.3 Patients on dialysis might require higher protein intake to preserve muscle mass caused by protein-energy wasting, which is common in dialysis patients.6
Continue to: Low sodium intake
Low sodium intake in CKD patients has been shown to decrease BP and thus slow the progression of renal disease and lower the risk of CVD. The recommended dietary sodium intake in CKD patients is 1500-3000 mg/d.3
Low potassium intake. Hyperkalemia is a serious complication of CKD. A low-potassium diet is recommended in ESRD patients who have a potassium level > 5.5 mEq/L.6
Blood pressure
Preventing and treating hypertension is critical to slowing the progression of CKD and reducing cardiovascular risk. BP should be measured at every clinic visit. Aside from lifestyle changes, medication might be needed to reach target BP.
The American Diabetes Association recommends a BP goal of ≤ 140/90 mm Hg for hypertensive patients with diabetes, although they do state that a lower BP target (≤ 130/80 mm Hg) might be more appropriate for patients with DKD.27
The American College of Cardiology recommends that hypertensive patients with CKD have a BP target of ≤ 130/80 mm Hg.28
Continue to: ACE inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have renoprotective benefits. These agents are recommended as first-line medications for patients with diabetes, hypertension, and an eGFR < 60 mL/min/1.73 m2 and a UACR > 300 mg/g.29-31 Evidence also supports their use when the UACR is 30 to 299 mg/g.
Studies have shown that, in patients with DKD, ACE inhibitors and ARBs can slow the progression of renal disease.29,30,32 There is no difference between ACE inhibitors and ARBs in their effectiveness for preventing progression of DKD.6 There is no added benefit in combining an ACE inhibitor and an ARB33; notably, combination ACE inhibitor and ARB therapy can increase the risk of adverse events, such as hyperkalemia and acute kidney injury, especially in patients with DKD.33
There is no evidence for starting an ACE inhibitor or ARB to prevent CKD in patients with diabetes who are not hypertensive.5
ACE inhibitors and ARBs should be used with caution in women of childbearing age, who should use a reliable form of contraception if taking one of these drugs.
Diuretics. Thiazide-type and loop diuretics might potentiate the positive effects of ACE inhibitors and ARBs. KDOQI guidelines recommend that, in patients who require a second agent to control BP, a diuretic should be considered in combination with an ACE inhibitor or an ARB.20 A loop diuretic is preferred if the eGFR is < 30 mL/min/1.73 m2.
Continue to: Nondihydropyridine calcium-channel blockers
Nondihydropyridine calcium-channel blockers (CCBs), such as diltiazem and verapamil, have been shown to be more effective then dihydrophyridine CCBs, such as amlodipine and nifedipine, in slowing the progression of renal disease because of their antiproteinuric effects. However, the antiproteinuric effects of nondihydropyridine CCBs are not as strong as those of ACE inhibitors or ARBs, and these drugs do not appear to potentiate the effects of an ACE inhibitor or ARB when used in combination.20
Nondihydropyridine CCBs might be a reasonable alternative in patients who cannot tolerate an ACE inhibitor or an ARB.
Mineralocorticoid receptor antagonists in combination with an ACE inhibitor or ARB have been demonstrated to reduce albuminuria in short-term studies.34,35
Glycemic levels
Studies conducted in patients with T1D, and others in patients with T2D, have shown that tight glycemic control can delay the onset and slow the progression of albuminuria and a decline in the eGFR.10,36-39 The target glycated hemoglobin (A1C) should be < 7% to prevent or slow progression of DKD.40 However, patients with DKD have an increased risk of hypoglycemic events and increased mortality with more intensive glycemic control.40,41 Given those findings, some patients with DKD and significant comorbidities, ESRD, or limited life expectancy might need to have an A1C target set at 8%.6,42
Adjustments to antidiabetes medications in DKD
In patients with stages 3 to 5 DKD, several common antidiabetic medications might need to be adjusted or discontinued because they decrease creatinine clearance.
Continue to: First-generation sulfonylureas
First-generation sulfonylureas should be avoided in DKD. Glipizide and gliclazide are preferred among second-generation sulfonylureas because they do not increase the risk of hypoglycemia in DKD patients, although patients taking these medications still require close monitoring of their blood glucose level.20
Metformin. In 2016, recommendations changed for the use of metformin in patients with DKD: The eGFR, not the serum creatinine level, should guide treatment.43 Metformin can be used safely in patients with (1) an eGFR of < 60 mL/min/1.73 m2 and (2) an eGFR of 30 mL/min/1.73 m2 with close monitoring. Metformin should not be initiated if the eGFR is < 45 mL/min/1.73 m2.43
Antidiabetes medications with direct effect on the kidney
Several antidiabetes medications have a direct effect on the kidney apart from their effect on the blood glucose level.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to reduce albuminuria and slow the decrease of eGFR independent of glycemic control. In addition, SGLT2 inhibitors have also been shown to have cardiovascular benefits in patients with DKD.44,45
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to delay and decrease the progression of DKD.46-48 Also, similar to what is seen with SGLT2 inhibitors, GLP-1 agonists have demonstrable cardiovascular benefit in patients with DKD.46,48
Continue to: Dyslipidemia and DKD
Dyslipidemia and DKD
Because the risk of CVD is increased in patients with DKD, addressing other modifiable risk factors, including dyslipidemia, is recommended in these patients. Patients with diabetes and stages 1 to 4 DKD should be treated with a high-intensity statin or a combination of a statin and ezetimibe.49,50
If a patient is taking a statin and starting dialysis, it’s important to discuss with him or her whether to continue the statin, based on perceived benefits and risks. It is not recommended that statins be initiated in patients on dialysis unless there is a specific cardiovascular indication for doing so. Risk reduction with a statin has been shown to be significantly less in dialysis patients than in patients who are not being treated with dialysis.49
Complications of CKD
Anemia is a common complication of CKD. KDIGO recommends measuring the hemoglobin concentration annually in DKD stage 3 patients without anemia; at least every 6 months in stage 4 patients; and at least every 3 months in stage 5. DKD patients with anemia should have additional laboratory testing: the absolute reticulocyte count, serum ferritin, serum transferrin saturation, vitamin B12, and folate.51
Mineral and bone disorder should be screened for in patients with DKD. TABLE 252 outlines when clinical laboratory tests should be ordered to assess for mineral bone disease.
When to refer to a nephrologist
Refer patients with stage 4 or 5 CKD (eGFR, ≤ 30 mL/min/1.73 m2) to a nephrologist for discussion of kidney replacement therapy.6 Patients with stage 3a CKD and severely increased albuminuria or with stage 3b CKD and moderately or severely increased albuminuria should also be referred to a nephrologist for intervention to delay disease progression.
Continue to: Identifying the need for early referral...
Identifying the need for early referral to a nephrologist has been shown to reduce the cost, and improve the quality, of care.53 Other indications for earlier referral include uncertainty about the etiology of renal disease, persistent or severe albuminuria, persistent hematuria, a rapid decline in eGFR, and acute kidney injury. Additionally, referral at an earlier stage of DKD might be needed to assist with complications associated with DKD, such as anemia, secondary hyperparathyroidism, mineral and bone disorder, resistant hypertension, fluid overload, and electrolyte disturbances.6
ACKNOWLEDGEMENT
The authors thank Colleen Colbert, PhD, and Iqbal Ahmad, PhD, for their review and critique of the manuscript of this article. They also thank Christopher Babiuch, MD, for his guidance in the preparation of the manuscript.
CORRESPONDENCE
Faraz Ahmad, MD, MPH, Care Point East Family Medicine, 543 Taylor Avenue, 2nd floor, Columbus, OH 43203; faraz. [email protected].
1. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83:1373-1381.
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7.
3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533.
4. Fox CS, Matsushita K, Woodward M, et al; . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673.
5. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124.
6. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159. Accessed January 5, 2021. https://care.diabetesjournals.org/content/41/Supplement_1
7. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. Accessed January 5, 2021. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
8. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-610.
9. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539.
10. de Boer IH; DCCT/EDIC Research Group. Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:24-30.
11. Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S3-S21.
12. Mottl AK, Tuttle KR. Diabetic kidney disease: Pathogenesis and epidemiology. UpToDate. Updated August 19, 2019. Accessed January 5, 2021. www.uptodate.com/contents/diabetic-kidney-disease-pathogenesis-and-epidemiology
13. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 2 diabetes mellitus. UpToDate. Updated November 3, 2020. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-2-diabetes-mellitus
14. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428.
15. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7-A8.
16. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277-284.
17. de Boer IH, Gao X, Cleary PA, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: The DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969-1977.
18. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941-1949.
19. Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patient with insulin dependent diabetes? BMJ. 1993;306:1722-1725.
20. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12-154.
21. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus. UpToDate. Updated December 3, 2019. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-1-diabetes-mellitus
22. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17-26.
23. Levey AS, Stevens LA, Schmid CH, et al; , A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
24. Wrone EM, Carnethon MR, Palaniappan L, et al; . Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580-587.
25. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467.
26. Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876-883.
27. de Boer, IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284.
28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
29. Brenner BM, Cooper ME, de Zeeuw D, et al; Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
30. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462.
31. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355;253-259.
32. Lewis EJ, Hunsicker LG, Clarke WR, et al; . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
33. Fried LF, Emanuele N, Zhang JH, et al; . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
34. Bakris GL, Agarwal R, Chan JC, et al; . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884-894.
35. Filippatos G, Anker SD, M, et al. Randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114.
36. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.N Engl J Med. 2008;358:2560-2572.
37. Ismail-Beigi F, Craven T, Banerji MA, et al; . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
38. Zoungas S, Chalmers J, Neal B, et al; . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392-1406.
39. Zoungas S, Arima H, Gerstein HC, et al; . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
40. Miller ME, Bonds DE, Gerstein HC, et al; . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340;b5444.
41. Papademetriou V, Lovato L, Doumas M, et al; . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659.
42. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886.
43. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J. 2017;10:301-304.
44. Wanner C, Inzucchi SE, Lachin JM, et al; Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
45. Neal B, Perkovic V, Mahaffey KW, et al; . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
46. Marso SP, Daniels GH, Brown-Frandsen K, et al; . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
47. Mann JFE, DD, Brown-Frandsen K, et al; . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
48. Marso SP, Bain SC, Consoli A, et al; . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
49. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309.
50. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143.
51. National Kidney Foundation KDOQI. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335. Accessed January 5, 2021. www.sciencedirect.com/journal/kidney-international-supplements/vol/2/issue/4
52. National Kidney Foundation KDOQI. Evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). 2010. Accessed January 5, 2021. www.kidney.org/sites/default/files/02-10-390B_LBA_KDOQI_BoneGuide.pdf
53. Smart MA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
1. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83:1373-1381.
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7.
3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533.
4. Fox CS, Matsushita K, Woodward M, et al; . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673.
5. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124.
6. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159. Accessed January 5, 2021. https://care.diabetesjournals.org/content/41/Supplement_1
7. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. Accessed January 5, 2021. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
8. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-610.
9. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539.
10. de Boer IH; DCCT/EDIC Research Group. Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:24-30.
11. Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S3-S21.
12. Mottl AK, Tuttle KR. Diabetic kidney disease: Pathogenesis and epidemiology. UpToDate. Updated August 19, 2019. Accessed January 5, 2021. www.uptodate.com/contents/diabetic-kidney-disease-pathogenesis-and-epidemiology
13. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 2 diabetes mellitus. UpToDate. Updated November 3, 2020. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-2-diabetes-mellitus
14. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428.
15. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7-A8.
16. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277-284.
17. de Boer IH, Gao X, Cleary PA, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: The DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969-1977.
18. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941-1949.
19. Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patient with insulin dependent diabetes? BMJ. 1993;306:1722-1725.
20. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12-154.
21. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus. UpToDate. Updated December 3, 2019. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-1-diabetes-mellitus
22. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17-26.
23. Levey AS, Stevens LA, Schmid CH, et al; , A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
24. Wrone EM, Carnethon MR, Palaniappan L, et al; . Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580-587.
25. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467.
26. Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876-883.
27. de Boer, IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284.
28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
29. Brenner BM, Cooper ME, de Zeeuw D, et al; Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
30. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462.
31. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355;253-259.
32. Lewis EJ, Hunsicker LG, Clarke WR, et al; . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
33. Fried LF, Emanuele N, Zhang JH, et al; . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
34. Bakris GL, Agarwal R, Chan JC, et al; . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884-894.
35. Filippatos G, Anker SD, M, et al. Randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114.
36. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.N Engl J Med. 2008;358:2560-2572.
37. Ismail-Beigi F, Craven T, Banerji MA, et al; . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
38. Zoungas S, Chalmers J, Neal B, et al; . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392-1406.
39. Zoungas S, Arima H, Gerstein HC, et al; . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
40. Miller ME, Bonds DE, Gerstein HC, et al; . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340;b5444.
41. Papademetriou V, Lovato L, Doumas M, et al; . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659.
42. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886.
43. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J. 2017;10:301-304.
44. Wanner C, Inzucchi SE, Lachin JM, et al; Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
45. Neal B, Perkovic V, Mahaffey KW, et al; . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
46. Marso SP, Daniels GH, Brown-Frandsen K, et al; . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
47. Mann JFE, DD, Brown-Frandsen K, et al; . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
48. Marso SP, Bain SC, Consoli A, et al; . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
49. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309.
50. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143.
51. National Kidney Foundation KDOQI. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335. Accessed January 5, 2021. www.sciencedirect.com/journal/kidney-international-supplements/vol/2/issue/4
52. National Kidney Foundation KDOQI. Evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). 2010. Accessed January 5, 2021. www.kidney.org/sites/default/files/02-10-390B_LBA_KDOQI_BoneGuide.pdf
53. Smart MA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
PRACTICE RECOMMENDATIONS
› Screen patients with diabetes annually for diabetic kidney disease with measurement of urinary albumin and the estimated glomerular filtration rate. B
› Optimize blood glucose and blood pressure control in patients with diabetes to prevent or delay progression to diabetic kidney disease. A
› Treat hypertensive patients with diabetes and stages 1 to 4 chronic kidney disease with an angiotensin-converting enzyme inhibitor or angiotensin II-receptor blocker as a first-line antihypertensive, absent contraindications. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Breaking the cycle of medication overuse headache
Medication overuse headache (MOH), a secondary headache diagnosis, is a prevalent phenomenon that complicates headache diagnosis and treatment, increases the cost of care, and reduces quality of life. Effective abortive medication is essential for the headache sufferer; when an abortive is used too frequently, however, headache frequency increases—potentially beginning a cycle in which the patient then takes more medication to abort the headache. Over time, the patient suffers from an ever-increasing number of headaches, takes even more abortive medication, and so on. In the presence of MOH, there is a reduction in pain response to preventive and abortive treatments; when medication overuse is eliminated, pain response improves.1
Although MOH is well recognized among headache specialists, the condition is often overlooked in primary care. Since headache is a top complaint in primary care, however, and prevention is a major goal in family medicine, the opportunity for you to recognize, treat, and prevent MOH is significant. In fact, a randomized controlled trial showed that brief patient education about headache care and MOH provided by a primary care physician can lead to a significant reduction in headache frequency among patients with MOH.2
This article reviews the recognition and diagnosis of MOH, based on historical features and current criteria; addresses risk factors for abortive medication overuse and how to withdraw an offending agent; and explores the value of bridging and preventive therapies to reduce the overall frequency of headache.
What defines MOH?
Typically, MOH is a chronification of a primary headache disorder. However, in patients with a history of migraine who are undergoing treatment for another chronic pain condition with an opioid or other analgesic, MOH can be induced.3 An increase in the frequency of headache raises the specter of a concomitant increase in the level of disability4; psychiatric comorbidity5; and more headache days, with time lost from school and work.
The Migraine Disability Assessment (MIDAS) questionnaire, a validated instrument that helps the provider (1) measure the impact that headache has on a patient’s life and (2) follow treatment progress, also provides information to employers and insurance companies on treatment coverage and the need for work modification. The MIDAS score is 3 times higher in patients with MOH than in patients with episodic migraine.6,7
The annual associated cost per person of MOH has been estimated at $4000, resulting in billions of dollars in associated costs8; most of these costs are related to absenteeism and disability. After detoxification for MOH, annual outpatient medication costs are reduced by approximately 24%.9
Efforts to solve a common problem create another
Headache affects nearly 50% of the general population worldwide,10 accounting for about 4% of primary care visits11 and approximately 20% of outpatient neurology consultations.12 Although inpatient stays for headache are approximately half the duration of the overall average hospital stay, headache accounts for 3% of admissions.13 According to the Global Burden of Disease study, tension-type headache, migraine, and MOH are the 3 most common headache disorders.10 Headache is the second leading cause of disability among people 15 to 49 years of age.10
Continue to: The prevalence of MOH...
The prevalence of MOH in the general population is 2%.7,14,15 A population-based study showed that the rate of progression from episodic headache (< 15 d/mo) to chronic headache (≥ 15 d/mo) in the general population is 2.5% per year16; however, progression to chronic headache is 14% per year in patients with medication overuse. One-third of the general population with chronic migraine overuses symptomatic medication; in US headache clinics, roughly one-half of patients with chronic headache overuse acute medication.6
Definitions and diagnosis
MOH is a secondary headache diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3) (TABLE 1),17 which lists diagnostic criteria for recognized headache disorders.
Terminology. MOH has also been called rebound headache, drug-induced headache, and transformed migraine, but these terms are outdated and are not formal diagnoses. Patients sometimes refer to substance-withdrawal headaches (not discussed in this article) as rebound headaches, so clarity is important when discussing headache with patients: namely, that MOH is an exacerbation of an existing headache condition caused by overuse of abortive headache medications, including analgesics, combination analgesics, triptans, barbiturates, and opioids.
MOH was recognized in the early 1950s and fully differentiated as a diagnosis in 2005 in the second edition of the ICHD. The disorder is subcategorized by offending abortive agent (TABLE 217) because the frequency of analgesic use required to develop MOH differs by agent.
Risk factors for MOH and chronification of a primary headache disorder. There are several risk factors for developing MOH, and others that contribute to increasing headache frequency in general (TABLE 35,14,18-23). Some risk factors are common to each. All are important to address because some are modifiable.
Continue to: Pathophysiology
Pathophysiology. The pathophysiology and psychology behind MOH are largely unknown. Physiologic changes in pain processing and functional imaging changes have been demonstrated in patients with MOH, both of which are reversible upon withdrawal of medication.23 Genetic factors and changes in hormone and neurotransmitter levels are found in MOH patients; this is not the case in patients who have an episodic headache pattern only.24
Presentation. Diagnostic criteria for MOH do not include clinical characteristics. Typically, the phenotype of MOH in a given patient is similar to the underlying primary headache25—although this principle can be complicated to tease out because these medications can suppress some symptoms. Diagnosis of a primary headache disorder should be documented along with the diagnosis of MOH.
Medication overuse can exist without MOH: Not every patient who frequently uses an abortive medication develops MOH.
Treatment is multifaceted—and can become complex
Mainstays of treatment of MOH are education about the disorder and detoxification from the overused agent, although specific treatments can differ depending on the agent involved, the frequency and duration of its use, and a patient’s behavioral patterns and psychiatric comorbidities. Often, a daily medication to prevent headache is considered upon, or after, withdrawal of the offending agent. The timing of introducing a preventive might impact its effectiveness. Some refractory cases require more intensive therapy, including hospitalization at a specialized tertiary center.
But before we look at detoxification from an overused agent, it’s important to review one of the best strategies of all in combatting MOH.
Continue to: First and best strategy
First and best strategy: Avoid onset of MOH
Select an appropriate abortive to reduce the risk of MOH. With regard to specific acute headache medications, some nuances other than type of headache should be considered. Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as abortive therapy by the American Headache Society for their efficacy, favorable adverse effect profile, and low cost. NSAIDs are protective against development of MOH if a patient’s baseline headache frequency is < 10/mo; at a frequency of 10 to 14 d/mo, however, the risk of MOH increases when using an NSAID.6 A similar effect has been seen with triptans.16 Longer-acting NSAIDs, such as nabumetone and naproxen, have been proposed as less likely to cause MOH, and are even used as bridging therapy sometimes (as long as neither of these was the overused medication).26
The time it takes to develop MOH is shortest with triptans, followed by ergots, then analgesics.27
Prospective cohort studies6,16 have shown that barbiturates and opioids are more likely to induce MOH; for that reason, agents in these analgesic classes are almost universally avoided unless no other medically acceptable options exist. Using barbiturate-containing compounds or opioids > 4 d/mo exponentially increases the likelihood of MOH.
Promising preclinical data demonstrate that the gepant, or small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist, class of medications used as abortive therapy does not induce medication overuse cutaneous allodynia.28
Provide education. Primary prevention of MOH involves (1) increasing patients’ awareness of how to take medications appropriately and (2) restricting intake of over-the-counter abortive medications. Often, the expert recommendation is to limit abortives to approximately 2 d/wk because more frequent use places patients at risk of further increased use and subsequent MOH.
Continue to: A randomized controlled trial in Norway...
A randomized controlled trial in Norway compared outcomes in 2 groups of patients with MOH: One group was given advice on the disorder by a physician; the other group was not provided with advice. In the “business-as-usual” group, there was no significant improvement; however, when general practitioners provided simple advice (lasting roughly 9 minutes) about reducing abortive medication use to a safe level and cautioned patients that they would be “feeling worse before feeling better,” headache days were reduced by approximately 8 per month and medication days, by 16 per month.2
A subsequent, long-term follow-up study29 of patients from the Norway trial2 who had been given advice and education showed a relapse rate (ie, into overuse of headache medication) of only 8% and sustained reduction of headache days and medication use at 16 months.
Offer support and other nondrug interventions. A recent review of 3 studies23 recommended that extra support for patients from a headache nurse, close follow-up, keeping an electronic diary that provides feedback, and undertaking a short course of psychotherapy can reduce medication overuse and prevent relapse.
If MOH develops, initiate withdrawal, introduce a preventive
Withdraw overused medication. Most current evidence suggests that withdrawal of the offending agent is the most effective factor in reducing headache days and improving quality of life. A randomized controlled trial compared the effects of (1) complete and immediate withdrawal of an abortive medication with (2) reducing its use (ie, limiting intake to 2 d/wk), on headache frequency, disability, and quality of life.30 There was a reduction of headache days in both groups; however, reduction was much greater at 2 months in the complete withdrawal group than in the restricted intake group (respectively, a 41% and a 26% reduction in headache days per month). This effect was sustained at 6 and 12 months in both groups. The study confirmed the results of earlier research2,15: Abrupt withdrawal leads to reversion to an episodic pattern at 2 to 6 months in approximately 40% to 60% of patients.
More studies are needed to determine the most appropriate treatment course for MOH; however, complete withdrawal of the causative drug is the most important intervention.
Continue to: Consider withdrawal plus preventive treatment
Consider withdrawal plus preventive treatment. Use of sodium valproate, in addition to medication overuse detoxification, led to a significant reduction in headache days and improvement in quality of life at 12 weeks but no difference after 24 weeks, compared with detoxification alone in a randomized, double-blind, placebo-controlled study.31
A study of 61 patients showed a larger reduction (by 7.2 d/mo) in headache frequency with any preventive medication in addition to medication withdrawal, compared to withdrawal alone (by 4.1 d/mo) after 3 months; however, the relative benefit was gone at 6 months.32
A study of 98 patients compared immediate and delayed initiation of preventive medication upon withdrawal of overused abortive medication.33 Response was defined as a > 50% reduction in headache frequency and was similar in both groups; results showed a 28% response with immediate initiation of a preventive; a 23% response with delayed (ie, 2 months after withdrawal) initiation; and a 48% response in both groups at 12 months.
Collectively, these studies suggest that adding a preventive medication at the time of withdrawal has the potential to reduce headache frequency more quickly than withdrawal alone. However, after 3 to 6 months, the outcome of reduced headache frequency is the same whether or not a preventive medication is used—as long as the offending agent has been withdrawn.
Do preventives work without withdrawing overused medication? Patients with MOH often show little or no improvement with addition of a preventive medication only; their response to a preventive improves after withdrawal of the overused medication. Patients without previous headache improvement after addition of a preventive, who also did not improve 2 months after withdrawal, then demonstrated an overall reduction in headache by 26% when a preventive was reintroduced after withdrawal.2
Continue to: The research evidence for preventives
The research evidence for preventives. Medications for headache prevention have not been extensively evaluated specifically for treating MOH. Here is what’s known:
- Flunarizine, amitriptyline, and beta-blockers usually are ineffective for MOH.24
- Results for topiramate are mixed: A small, double-blind, placebo-controlled chronic migraine study in Europe showed that, in a subgroup of patients with MOH, topiramate led to a small but significant reduction (3.5 d/mo) in headache frequency, compared to placebo.27 A similar study done in the United States did not show a significant difference between the active-treatment and placebo groups.34
- Findings regarding onabotulinumtoxinA are intriguing: In a posthoc analysis of onabotulinumtoxinA to treat chronic migraine, patients with MOH who did not undergo detoxification had an 8 d/mo greater reduction in headache, compared to placebo.35 However, when compared to placebo in conjunction with detoxification, onabotulinumtoxinA demonstrated no benefit.36
- Newer CGRP antagonist and CGRP receptor antagonist monoclonal antibodies are successful preventive medications that have demonstrated a reduction in acute medication use days per month and headache days per month37; these compounds have not been compared to withdrawal alone.
Reducing the severity and duration of withdrawal symptoms
Withdrawal from overused abortive headache medications can lead to worsening headache, nausea, vomiting, hypotension, tachycardia, sleep disturbances, restlessness, anxiety, and nervousness. Symptoms usually last 2 to 10 days but can persist for as long as 4 weeks; duration of withdrawal symptoms varies with the medication that is being overused. In patients who have used a triptan, for example, mean duration of withdrawal is 4.1 days; ergotamine, 6.7 days; and NSAIDs, 9.5 days.23 Tapered withdrawal is sometimes recommended with opioids and barbiturates to reduce withdrawal symptoms. It is unclear whether starting a preventive medication during withdrawal assists in reducing withdrawal symptoms.38
Bridging therapy to reduce symptoms of withdrawal is often provided despite debatable utility. Available evidence does not favor one agent or method but suggests some strategies that could be helpful:
- A prednisone taper has a potential role during the first 6 days of withdrawal by reducing rebound headache and withdrawal symptoms39; however, oral prednisolone has been shown to have no benefit.40
- Alone, IV methylprednisolone seems not to be of benefit; however, in a retrospective study of 94 patients, IV methylprednisolone plus diazepam for 5 days led to a significant reduction in headache frequency and drug consumption that was sustained after 3 months.41
- Celecoxib was compared to prednisone over a 20-day course: a celecoxib dosage of 400 mg/d for the first 5 days, tapered by 100 mg every 5 days, and an oral prednisone dosage of 75 mg/d for the first 5 days, then tapered every 5 days. Patients taking celecoxib had lower headache intensity but there was no difference in headache frequency and acute medication intake between the groups.42
Other strategies. Using antiemetics and NSAIDs to reduce withdrawal symptoms is widely practiced, but no placebo-controlled trials have been conducted to support this strategy.
Patients in withdrawal might be more likely to benefit from inpatient care if they have a severe comorbidity, such as opioid or barbiturate use; failure to respond to, tolerate, or adhere to treatment; or relapse after withdrawal.38
Continue to: Cognitive behavioral therapy...
Cognitive behavioral therapy, exercise, a headache diary, and biofeedback should be considered in every patient’s treatment strategy because a multidisciplinary approach increases adherence and leads to improvement in headache frequency and a decrease in disability and medication use.43
Predictors of Tx success
A prospective cohort study determined that the rate of MOH relapse is 31% at 6 months, 41% at 1 year, and 45% at 4 years, with the highest risk of relapse during the first year.44 Looking at the correlation between type of medication overused and relapse rate, the research indicates that
- triptans have the lowest risk of relapse,44
- simple analgesics have a higher risk of relapse than triptans,22,44 and
- opioids have the highest risk of relapse.22
Where the data don’t agree. Data on combination analgesics and on ergots are conflicting.22 In addition, data on whether the primary type of headache predicts relapse rate conflict; however, migraine might predict a better outcome than tension-type headache.22
To recap and expand: Management pearls
The major goals of headache management generally are to rule out secondary headache, reach a correct diagnosis, reduce overall headache frequency, and provide effective abortive medication. A large component of reducing headache frequency is addressing and treating medication overuse.
Seek to understand the nature of the patient’s headache disorder. Components of the history are key in identifying the underlying headache diagnosis and ruling out other, more concerning secondary headache diagnoses. The ICHD-3 is an excellent resource for treating headache disorders because the classification lists specific diagnostic criteria for all recognized headache diagnoses.
Continue to: Medication withdrawal...
Medication withdrawal—with or without preventive medication—should reduce the frequency of MOH in 2 or 3 months. If headache does not become less frequent, however, the headache diagnosis might need to be reconsidered. Minimizing the use of abortive medication is generally recommended, but reduction or withdrawal of these medications does not guarantee that patients will revert to an episodic pattern of headache.
Treating withdrawal symptoms is a reasonable approach in some patients, but evidence does not support routinely providing bridging therapy.
Apply preventives carefully. Abortive medication withdrawal should generally be completed before initiating preventive medication; however, over the short term, starting preventive therapy while withdrawing the overused medication could assist in reducing headache frequency rapidly. This strategy can put patients at risk of medication adverse effects and using the medications longer than necessary, yet might be reasonable in certain patients, given their comorbidities, risk of relapse, and physician and patient preference. A preventive medication for an individual patient should generally be chosen in line with recommendations of the American Academy of Neurology45 and on the basis of the history and comorbidities.
Provide education, which is essential to lowering barriers to success. Patients with MOH must be counseled to understand that (1) a headache treatment that is supposed to be making them feel better is, in fact, making them feel worse and (2) they will get worse before they get better. Many patients are afraid to be without medication to use as needed. It is helpful to educate them on the different types of treatments (abortive, preventive); how MOH interferes with headache prophylaxis and medication efficacy; how MOH alters brain function (ie, aforementioned physiologic changes in pain processing and functional imaging changes23); and that such change is reversible when medication is withdrawn.
ACKNOWLEDGEMENT
The author thanks Jeffrey Curtis, MD, MPH, for his support and editing assistance with the manuscript.
CORRESPONDENCE
Allison Crain, MD, 2927 N 7th Avenue, Phoenix, AZ 85013; [email protected].
1. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
2. Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 2015;86:505-512.
3. Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179-190.
4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301-315.
5. Chu H-T, Liang C-S, Lee J-T, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. 2018;58:407-415.
6. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
7. Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
8. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703-711.
9. Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665-672.
10. . Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954-976.
11. Kernick D, Stapley S, Goadsby PJ, et al. What happens to new-onset headache presenting to primary care? A case–cohort study using electronic primary care records. Cephalalgia. 2008;28:1188-1195.
12. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
13. Munoz-Ceron J, Marin-Careaga V, L, et al. Headache at the emergency room: etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PloS One. 2019;14:e0208728.
14. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010;9:391-401.
15. Tassorelli C, Jensen R, Allena M, et al; the . A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014;34:645-655.
16. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
18. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65-72.
19. Hagen K, Linde M, Steiner TJ, et al. Risk factors for medication-overuse headache: an 11-year follow-up study. The Nord-Trøndelag Health Studies. Pain. 2012;153:56-61.
20. Katsarava Z, Schneewiess S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788-790.
21. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. 2019;93:e2224-e2236.
22. Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139:405-414.
23. Ferraro S, Grazzi L, Mandelli M, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. 2012;13:255-262.
24. Diener H-C, Holle D, Solbach K, et al. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12:575-583.
25. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011-1014.
26. Mauskop A, ed. Migraine and Headache. 2nd ed. Oxford University Press; 2013.
27. Diener H-C, Bussone G, Van Oene JC, et al; . Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
28. Navratilova E, Behravesh S, Oyarzo J, et al. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache Cephalalgia. 2020;40:892-902.
29. Kristoffersen ES, Straand J, Russell MB, et al. Lasting improvement of medication-overuse headache after brief intervention—a long-term follow-up in primary care. Eur J Neurol. 2017;24:883-891.
30. Carlsen LN, Munksgaard SB, Jensen RH, et al. Complete detoxification is the most effective treatment of medication-overuse headache: a randomized controlled open-label trial. Cephalalgia. 2018;38:225-236.
31. Sarchielli P, Messina P, Cupini LM, et al; SAMOHA Study Group. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1289-1297.
32. Hagen K, Stovner LJ. A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol Scand Suppl. 2011;124(suppl 191):38-43.
33. Munksgaard SB, Bendtsen L, Jensen RH. Detoxification of medication-overuse headache by a multidisciplinary treatment programme is highly effective: a comparison of two consecutive treatment methods in an open-label design. Cephalalgia. 2012;32:834-844.
34. Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153-1162.
35. Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48-56.
36. Sandrini G, Perrotta A, Tassorelli C, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427-433.
37. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(suppl 1):99-105.
38. Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention and treatment of medication overuse headache. Lancet Neurol. 2019;18:891-902.
39. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000;20:107-113.
40. Bøe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: a randomized, double-blind study. Neurology. 2007;69:26-31.
41. Paolucci M, Altamura C, Brunelli N, et al. Methylprednisolone plus diazepam i.v. as bridge therapy for medication overuse headache. Neurol Sci. 2017;38:2025-2029.
42. Taghdiri F, Togha M, Razeghi Jahromi S, et al. Celecoxib vs prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind clinical trial. Headache. 2015;55:128-135.
43. Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795-816.
44. Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005;25:12-15.
45. Silberstein SD, Holland S, Freitag F, et al; . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78:1137-1145.
Medication overuse headache (MOH), a secondary headache diagnosis, is a prevalent phenomenon that complicates headache diagnosis and treatment, increases the cost of care, and reduces quality of life. Effective abortive medication is essential for the headache sufferer; when an abortive is used too frequently, however, headache frequency increases—potentially beginning a cycle in which the patient then takes more medication to abort the headache. Over time, the patient suffers from an ever-increasing number of headaches, takes even more abortive medication, and so on. In the presence of MOH, there is a reduction in pain response to preventive and abortive treatments; when medication overuse is eliminated, pain response improves.1
Although MOH is well recognized among headache specialists, the condition is often overlooked in primary care. Since headache is a top complaint in primary care, however, and prevention is a major goal in family medicine, the opportunity for you to recognize, treat, and prevent MOH is significant. In fact, a randomized controlled trial showed that brief patient education about headache care and MOH provided by a primary care physician can lead to a significant reduction in headache frequency among patients with MOH.2
This article reviews the recognition and diagnosis of MOH, based on historical features and current criteria; addresses risk factors for abortive medication overuse and how to withdraw an offending agent; and explores the value of bridging and preventive therapies to reduce the overall frequency of headache.
What defines MOH?
Typically, MOH is a chronification of a primary headache disorder. However, in patients with a history of migraine who are undergoing treatment for another chronic pain condition with an opioid or other analgesic, MOH can be induced.3 An increase in the frequency of headache raises the specter of a concomitant increase in the level of disability4; psychiatric comorbidity5; and more headache days, with time lost from school and work.
The Migraine Disability Assessment (MIDAS) questionnaire, a validated instrument that helps the provider (1) measure the impact that headache has on a patient’s life and (2) follow treatment progress, also provides information to employers and insurance companies on treatment coverage and the need for work modification. The MIDAS score is 3 times higher in patients with MOH than in patients with episodic migraine.6,7
The annual associated cost per person of MOH has been estimated at $4000, resulting in billions of dollars in associated costs8; most of these costs are related to absenteeism and disability. After detoxification for MOH, annual outpatient medication costs are reduced by approximately 24%.9
Efforts to solve a common problem create another
Headache affects nearly 50% of the general population worldwide,10 accounting for about 4% of primary care visits11 and approximately 20% of outpatient neurology consultations.12 Although inpatient stays for headache are approximately half the duration of the overall average hospital stay, headache accounts for 3% of admissions.13 According to the Global Burden of Disease study, tension-type headache, migraine, and MOH are the 3 most common headache disorders.10 Headache is the second leading cause of disability among people 15 to 49 years of age.10
Continue to: The prevalence of MOH...
The prevalence of MOH in the general population is 2%.7,14,15 A population-based study showed that the rate of progression from episodic headache (< 15 d/mo) to chronic headache (≥ 15 d/mo) in the general population is 2.5% per year16; however, progression to chronic headache is 14% per year in patients with medication overuse. One-third of the general population with chronic migraine overuses symptomatic medication; in US headache clinics, roughly one-half of patients with chronic headache overuse acute medication.6
Definitions and diagnosis
MOH is a secondary headache diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3) (TABLE 1),17 which lists diagnostic criteria for recognized headache disorders.
Terminology. MOH has also been called rebound headache, drug-induced headache, and transformed migraine, but these terms are outdated and are not formal diagnoses. Patients sometimes refer to substance-withdrawal headaches (not discussed in this article) as rebound headaches, so clarity is important when discussing headache with patients: namely, that MOH is an exacerbation of an existing headache condition caused by overuse of abortive headache medications, including analgesics, combination analgesics, triptans, barbiturates, and opioids.
MOH was recognized in the early 1950s and fully differentiated as a diagnosis in 2005 in the second edition of the ICHD. The disorder is subcategorized by offending abortive agent (TABLE 217) because the frequency of analgesic use required to develop MOH differs by agent.
Risk factors for MOH and chronification of a primary headache disorder. There are several risk factors for developing MOH, and others that contribute to increasing headache frequency in general (TABLE 35,14,18-23). Some risk factors are common to each. All are important to address because some are modifiable.
Continue to: Pathophysiology
Pathophysiology. The pathophysiology and psychology behind MOH are largely unknown. Physiologic changes in pain processing and functional imaging changes have been demonstrated in patients with MOH, both of which are reversible upon withdrawal of medication.23 Genetic factors and changes in hormone and neurotransmitter levels are found in MOH patients; this is not the case in patients who have an episodic headache pattern only.24
Presentation. Diagnostic criteria for MOH do not include clinical characteristics. Typically, the phenotype of MOH in a given patient is similar to the underlying primary headache25—although this principle can be complicated to tease out because these medications can suppress some symptoms. Diagnosis of a primary headache disorder should be documented along with the diagnosis of MOH.
Medication overuse can exist without MOH: Not every patient who frequently uses an abortive medication develops MOH.
Treatment is multifaceted—and can become complex
Mainstays of treatment of MOH are education about the disorder and detoxification from the overused agent, although specific treatments can differ depending on the agent involved, the frequency and duration of its use, and a patient’s behavioral patterns and psychiatric comorbidities. Often, a daily medication to prevent headache is considered upon, or after, withdrawal of the offending agent. The timing of introducing a preventive might impact its effectiveness. Some refractory cases require more intensive therapy, including hospitalization at a specialized tertiary center.
But before we look at detoxification from an overused agent, it’s important to review one of the best strategies of all in combatting MOH.
Continue to: First and best strategy
First and best strategy: Avoid onset of MOH
Select an appropriate abortive to reduce the risk of MOH. With regard to specific acute headache medications, some nuances other than type of headache should be considered. Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as abortive therapy by the American Headache Society for their efficacy, favorable adverse effect profile, and low cost. NSAIDs are protective against development of MOH if a patient’s baseline headache frequency is < 10/mo; at a frequency of 10 to 14 d/mo, however, the risk of MOH increases when using an NSAID.6 A similar effect has been seen with triptans.16 Longer-acting NSAIDs, such as nabumetone and naproxen, have been proposed as less likely to cause MOH, and are even used as bridging therapy sometimes (as long as neither of these was the overused medication).26
The time it takes to develop MOH is shortest with triptans, followed by ergots, then analgesics.27
Prospective cohort studies6,16 have shown that barbiturates and opioids are more likely to induce MOH; for that reason, agents in these analgesic classes are almost universally avoided unless no other medically acceptable options exist. Using barbiturate-containing compounds or opioids > 4 d/mo exponentially increases the likelihood of MOH.
Promising preclinical data demonstrate that the gepant, or small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist, class of medications used as abortive therapy does not induce medication overuse cutaneous allodynia.28
Provide education. Primary prevention of MOH involves (1) increasing patients’ awareness of how to take medications appropriately and (2) restricting intake of over-the-counter abortive medications. Often, the expert recommendation is to limit abortives to approximately 2 d/wk because more frequent use places patients at risk of further increased use and subsequent MOH.
Continue to: A randomized controlled trial in Norway...
A randomized controlled trial in Norway compared outcomes in 2 groups of patients with MOH: One group was given advice on the disorder by a physician; the other group was not provided with advice. In the “business-as-usual” group, there was no significant improvement; however, when general practitioners provided simple advice (lasting roughly 9 minutes) about reducing abortive medication use to a safe level and cautioned patients that they would be “feeling worse before feeling better,” headache days were reduced by approximately 8 per month and medication days, by 16 per month.2
A subsequent, long-term follow-up study29 of patients from the Norway trial2 who had been given advice and education showed a relapse rate (ie, into overuse of headache medication) of only 8% and sustained reduction of headache days and medication use at 16 months.
Offer support and other nondrug interventions. A recent review of 3 studies23 recommended that extra support for patients from a headache nurse, close follow-up, keeping an electronic diary that provides feedback, and undertaking a short course of psychotherapy can reduce medication overuse and prevent relapse.
If MOH develops, initiate withdrawal, introduce a preventive
Withdraw overused medication. Most current evidence suggests that withdrawal of the offending agent is the most effective factor in reducing headache days and improving quality of life. A randomized controlled trial compared the effects of (1) complete and immediate withdrawal of an abortive medication with (2) reducing its use (ie, limiting intake to 2 d/wk), on headache frequency, disability, and quality of life.30 There was a reduction of headache days in both groups; however, reduction was much greater at 2 months in the complete withdrawal group than in the restricted intake group (respectively, a 41% and a 26% reduction in headache days per month). This effect was sustained at 6 and 12 months in both groups. The study confirmed the results of earlier research2,15: Abrupt withdrawal leads to reversion to an episodic pattern at 2 to 6 months in approximately 40% to 60% of patients.
More studies are needed to determine the most appropriate treatment course for MOH; however, complete withdrawal of the causative drug is the most important intervention.
Continue to: Consider withdrawal plus preventive treatment
Consider withdrawal plus preventive treatment. Use of sodium valproate, in addition to medication overuse detoxification, led to a significant reduction in headache days and improvement in quality of life at 12 weeks but no difference after 24 weeks, compared with detoxification alone in a randomized, double-blind, placebo-controlled study.31
A study of 61 patients showed a larger reduction (by 7.2 d/mo) in headache frequency with any preventive medication in addition to medication withdrawal, compared to withdrawal alone (by 4.1 d/mo) after 3 months; however, the relative benefit was gone at 6 months.32
A study of 98 patients compared immediate and delayed initiation of preventive medication upon withdrawal of overused abortive medication.33 Response was defined as a > 50% reduction in headache frequency and was similar in both groups; results showed a 28% response with immediate initiation of a preventive; a 23% response with delayed (ie, 2 months after withdrawal) initiation; and a 48% response in both groups at 12 months.
Collectively, these studies suggest that adding a preventive medication at the time of withdrawal has the potential to reduce headache frequency more quickly than withdrawal alone. However, after 3 to 6 months, the outcome of reduced headache frequency is the same whether or not a preventive medication is used—as long as the offending agent has been withdrawn.
Do preventives work without withdrawing overused medication? Patients with MOH often show little or no improvement with addition of a preventive medication only; their response to a preventive improves after withdrawal of the overused medication. Patients without previous headache improvement after addition of a preventive, who also did not improve 2 months after withdrawal, then demonstrated an overall reduction in headache by 26% when a preventive was reintroduced after withdrawal.2
Continue to: The research evidence for preventives
The research evidence for preventives. Medications for headache prevention have not been extensively evaluated specifically for treating MOH. Here is what’s known:
- Flunarizine, amitriptyline, and beta-blockers usually are ineffective for MOH.24
- Results for topiramate are mixed: A small, double-blind, placebo-controlled chronic migraine study in Europe showed that, in a subgroup of patients with MOH, topiramate led to a small but significant reduction (3.5 d/mo) in headache frequency, compared to placebo.27 A similar study done in the United States did not show a significant difference between the active-treatment and placebo groups.34
- Findings regarding onabotulinumtoxinA are intriguing: In a posthoc analysis of onabotulinumtoxinA to treat chronic migraine, patients with MOH who did not undergo detoxification had an 8 d/mo greater reduction in headache, compared to placebo.35 However, when compared to placebo in conjunction with detoxification, onabotulinumtoxinA demonstrated no benefit.36
- Newer CGRP antagonist and CGRP receptor antagonist monoclonal antibodies are successful preventive medications that have demonstrated a reduction in acute medication use days per month and headache days per month37; these compounds have not been compared to withdrawal alone.
Reducing the severity and duration of withdrawal symptoms
Withdrawal from overused abortive headache medications can lead to worsening headache, nausea, vomiting, hypotension, tachycardia, sleep disturbances, restlessness, anxiety, and nervousness. Symptoms usually last 2 to 10 days but can persist for as long as 4 weeks; duration of withdrawal symptoms varies with the medication that is being overused. In patients who have used a triptan, for example, mean duration of withdrawal is 4.1 days; ergotamine, 6.7 days; and NSAIDs, 9.5 days.23 Tapered withdrawal is sometimes recommended with opioids and barbiturates to reduce withdrawal symptoms. It is unclear whether starting a preventive medication during withdrawal assists in reducing withdrawal symptoms.38
Bridging therapy to reduce symptoms of withdrawal is often provided despite debatable utility. Available evidence does not favor one agent or method but suggests some strategies that could be helpful:
- A prednisone taper has a potential role during the first 6 days of withdrawal by reducing rebound headache and withdrawal symptoms39; however, oral prednisolone has been shown to have no benefit.40
- Alone, IV methylprednisolone seems not to be of benefit; however, in a retrospective study of 94 patients, IV methylprednisolone plus diazepam for 5 days led to a significant reduction in headache frequency and drug consumption that was sustained after 3 months.41
- Celecoxib was compared to prednisone over a 20-day course: a celecoxib dosage of 400 mg/d for the first 5 days, tapered by 100 mg every 5 days, and an oral prednisone dosage of 75 mg/d for the first 5 days, then tapered every 5 days. Patients taking celecoxib had lower headache intensity but there was no difference in headache frequency and acute medication intake between the groups.42
Other strategies. Using antiemetics and NSAIDs to reduce withdrawal symptoms is widely practiced, but no placebo-controlled trials have been conducted to support this strategy.
Patients in withdrawal might be more likely to benefit from inpatient care if they have a severe comorbidity, such as opioid or barbiturate use; failure to respond to, tolerate, or adhere to treatment; or relapse after withdrawal.38
Continue to: Cognitive behavioral therapy...
Cognitive behavioral therapy, exercise, a headache diary, and biofeedback should be considered in every patient’s treatment strategy because a multidisciplinary approach increases adherence and leads to improvement in headache frequency and a decrease in disability and medication use.43
Predictors of Tx success
A prospective cohort study determined that the rate of MOH relapse is 31% at 6 months, 41% at 1 year, and 45% at 4 years, with the highest risk of relapse during the first year.44 Looking at the correlation between type of medication overused and relapse rate, the research indicates that
- triptans have the lowest risk of relapse,44
- simple analgesics have a higher risk of relapse than triptans,22,44 and
- opioids have the highest risk of relapse.22
Where the data don’t agree. Data on combination analgesics and on ergots are conflicting.22 In addition, data on whether the primary type of headache predicts relapse rate conflict; however, migraine might predict a better outcome than tension-type headache.22
To recap and expand: Management pearls
The major goals of headache management generally are to rule out secondary headache, reach a correct diagnosis, reduce overall headache frequency, and provide effective abortive medication. A large component of reducing headache frequency is addressing and treating medication overuse.
Seek to understand the nature of the patient’s headache disorder. Components of the history are key in identifying the underlying headache diagnosis and ruling out other, more concerning secondary headache diagnoses. The ICHD-3 is an excellent resource for treating headache disorders because the classification lists specific diagnostic criteria for all recognized headache diagnoses.
Continue to: Medication withdrawal...
Medication withdrawal—with or without preventive medication—should reduce the frequency of MOH in 2 or 3 months. If headache does not become less frequent, however, the headache diagnosis might need to be reconsidered. Minimizing the use of abortive medication is generally recommended, but reduction or withdrawal of these medications does not guarantee that patients will revert to an episodic pattern of headache.
Treating withdrawal symptoms is a reasonable approach in some patients, but evidence does not support routinely providing bridging therapy.
Apply preventives carefully. Abortive medication withdrawal should generally be completed before initiating preventive medication; however, over the short term, starting preventive therapy while withdrawing the overused medication could assist in reducing headache frequency rapidly. This strategy can put patients at risk of medication adverse effects and using the medications longer than necessary, yet might be reasonable in certain patients, given their comorbidities, risk of relapse, and physician and patient preference. A preventive medication for an individual patient should generally be chosen in line with recommendations of the American Academy of Neurology45 and on the basis of the history and comorbidities.
Provide education, which is essential to lowering barriers to success. Patients with MOH must be counseled to understand that (1) a headache treatment that is supposed to be making them feel better is, in fact, making them feel worse and (2) they will get worse before they get better. Many patients are afraid to be without medication to use as needed. It is helpful to educate them on the different types of treatments (abortive, preventive); how MOH interferes with headache prophylaxis and medication efficacy; how MOH alters brain function (ie, aforementioned physiologic changes in pain processing and functional imaging changes23); and that such change is reversible when medication is withdrawn.
ACKNOWLEDGEMENT
The author thanks Jeffrey Curtis, MD, MPH, for his support and editing assistance with the manuscript.
CORRESPONDENCE
Allison Crain, MD, 2927 N 7th Avenue, Phoenix, AZ 85013; [email protected].
Medication overuse headache (MOH), a secondary headache diagnosis, is a prevalent phenomenon that complicates headache diagnosis and treatment, increases the cost of care, and reduces quality of life. Effective abortive medication is essential for the headache sufferer; when an abortive is used too frequently, however, headache frequency increases—potentially beginning a cycle in which the patient then takes more medication to abort the headache. Over time, the patient suffers from an ever-increasing number of headaches, takes even more abortive medication, and so on. In the presence of MOH, there is a reduction in pain response to preventive and abortive treatments; when medication overuse is eliminated, pain response improves.1
Although MOH is well recognized among headache specialists, the condition is often overlooked in primary care. Since headache is a top complaint in primary care, however, and prevention is a major goal in family medicine, the opportunity for you to recognize, treat, and prevent MOH is significant. In fact, a randomized controlled trial showed that brief patient education about headache care and MOH provided by a primary care physician can lead to a significant reduction in headache frequency among patients with MOH.2
This article reviews the recognition and diagnosis of MOH, based on historical features and current criteria; addresses risk factors for abortive medication overuse and how to withdraw an offending agent; and explores the value of bridging and preventive therapies to reduce the overall frequency of headache.
What defines MOH?
Typically, MOH is a chronification of a primary headache disorder. However, in patients with a history of migraine who are undergoing treatment for another chronic pain condition with an opioid or other analgesic, MOH can be induced.3 An increase in the frequency of headache raises the specter of a concomitant increase in the level of disability4; psychiatric comorbidity5; and more headache days, with time lost from school and work.
The Migraine Disability Assessment (MIDAS) questionnaire, a validated instrument that helps the provider (1) measure the impact that headache has on a patient’s life and (2) follow treatment progress, also provides information to employers and insurance companies on treatment coverage and the need for work modification. The MIDAS score is 3 times higher in patients with MOH than in patients with episodic migraine.6,7
The annual associated cost per person of MOH has been estimated at $4000, resulting in billions of dollars in associated costs8; most of these costs are related to absenteeism and disability. After detoxification for MOH, annual outpatient medication costs are reduced by approximately 24%.9
Efforts to solve a common problem create another
Headache affects nearly 50% of the general population worldwide,10 accounting for about 4% of primary care visits11 and approximately 20% of outpatient neurology consultations.12 Although inpatient stays for headache are approximately half the duration of the overall average hospital stay, headache accounts for 3% of admissions.13 According to the Global Burden of Disease study, tension-type headache, migraine, and MOH are the 3 most common headache disorders.10 Headache is the second leading cause of disability among people 15 to 49 years of age.10
Continue to: The prevalence of MOH...
The prevalence of MOH in the general population is 2%.7,14,15 A population-based study showed that the rate of progression from episodic headache (< 15 d/mo) to chronic headache (≥ 15 d/mo) in the general population is 2.5% per year16; however, progression to chronic headache is 14% per year in patients with medication overuse. One-third of the general population with chronic migraine overuses symptomatic medication; in US headache clinics, roughly one-half of patients with chronic headache overuse acute medication.6
Definitions and diagnosis
MOH is a secondary headache diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3) (TABLE 1),17 which lists diagnostic criteria for recognized headache disorders.
Terminology. MOH has also been called rebound headache, drug-induced headache, and transformed migraine, but these terms are outdated and are not formal diagnoses. Patients sometimes refer to substance-withdrawal headaches (not discussed in this article) as rebound headaches, so clarity is important when discussing headache with patients: namely, that MOH is an exacerbation of an existing headache condition caused by overuse of abortive headache medications, including analgesics, combination analgesics, triptans, barbiturates, and opioids.
MOH was recognized in the early 1950s and fully differentiated as a diagnosis in 2005 in the second edition of the ICHD. The disorder is subcategorized by offending abortive agent (TABLE 217) because the frequency of analgesic use required to develop MOH differs by agent.
Risk factors for MOH and chronification of a primary headache disorder. There are several risk factors for developing MOH, and others that contribute to increasing headache frequency in general (TABLE 35,14,18-23). Some risk factors are common to each. All are important to address because some are modifiable.
Continue to: Pathophysiology
Pathophysiology. The pathophysiology and psychology behind MOH are largely unknown. Physiologic changes in pain processing and functional imaging changes have been demonstrated in patients with MOH, both of which are reversible upon withdrawal of medication.23 Genetic factors and changes in hormone and neurotransmitter levels are found in MOH patients; this is not the case in patients who have an episodic headache pattern only.24
Presentation. Diagnostic criteria for MOH do not include clinical characteristics. Typically, the phenotype of MOH in a given patient is similar to the underlying primary headache25—although this principle can be complicated to tease out because these medications can suppress some symptoms. Diagnosis of a primary headache disorder should be documented along with the diagnosis of MOH.
Medication overuse can exist without MOH: Not every patient who frequently uses an abortive medication develops MOH.
Treatment is multifaceted—and can become complex
Mainstays of treatment of MOH are education about the disorder and detoxification from the overused agent, although specific treatments can differ depending on the agent involved, the frequency and duration of its use, and a patient’s behavioral patterns and psychiatric comorbidities. Often, a daily medication to prevent headache is considered upon, or after, withdrawal of the offending agent. The timing of introducing a preventive might impact its effectiveness. Some refractory cases require more intensive therapy, including hospitalization at a specialized tertiary center.
But before we look at detoxification from an overused agent, it’s important to review one of the best strategies of all in combatting MOH.
Continue to: First and best strategy
First and best strategy: Avoid onset of MOH
Select an appropriate abortive to reduce the risk of MOH. With regard to specific acute headache medications, some nuances other than type of headache should be considered. Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as abortive therapy by the American Headache Society for their efficacy, favorable adverse effect profile, and low cost. NSAIDs are protective against development of MOH if a patient’s baseline headache frequency is < 10/mo; at a frequency of 10 to 14 d/mo, however, the risk of MOH increases when using an NSAID.6 A similar effect has been seen with triptans.16 Longer-acting NSAIDs, such as nabumetone and naproxen, have been proposed as less likely to cause MOH, and are even used as bridging therapy sometimes (as long as neither of these was the overused medication).26
The time it takes to develop MOH is shortest with triptans, followed by ergots, then analgesics.27
Prospective cohort studies6,16 have shown that barbiturates and opioids are more likely to induce MOH; for that reason, agents in these analgesic classes are almost universally avoided unless no other medically acceptable options exist. Using barbiturate-containing compounds or opioids > 4 d/mo exponentially increases the likelihood of MOH.
Promising preclinical data demonstrate that the gepant, or small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist, class of medications used as abortive therapy does not induce medication overuse cutaneous allodynia.28
Provide education. Primary prevention of MOH involves (1) increasing patients’ awareness of how to take medications appropriately and (2) restricting intake of over-the-counter abortive medications. Often, the expert recommendation is to limit abortives to approximately 2 d/wk because more frequent use places patients at risk of further increased use and subsequent MOH.
Continue to: A randomized controlled trial in Norway...
A randomized controlled trial in Norway compared outcomes in 2 groups of patients with MOH: One group was given advice on the disorder by a physician; the other group was not provided with advice. In the “business-as-usual” group, there was no significant improvement; however, when general practitioners provided simple advice (lasting roughly 9 minutes) about reducing abortive medication use to a safe level and cautioned patients that they would be “feeling worse before feeling better,” headache days were reduced by approximately 8 per month and medication days, by 16 per month.2
A subsequent, long-term follow-up study29 of patients from the Norway trial2 who had been given advice and education showed a relapse rate (ie, into overuse of headache medication) of only 8% and sustained reduction of headache days and medication use at 16 months.
Offer support and other nondrug interventions. A recent review of 3 studies23 recommended that extra support for patients from a headache nurse, close follow-up, keeping an electronic diary that provides feedback, and undertaking a short course of psychotherapy can reduce medication overuse and prevent relapse.
If MOH develops, initiate withdrawal, introduce a preventive
Withdraw overused medication. Most current evidence suggests that withdrawal of the offending agent is the most effective factor in reducing headache days and improving quality of life. A randomized controlled trial compared the effects of (1) complete and immediate withdrawal of an abortive medication with (2) reducing its use (ie, limiting intake to 2 d/wk), on headache frequency, disability, and quality of life.30 There was a reduction of headache days in both groups; however, reduction was much greater at 2 months in the complete withdrawal group than in the restricted intake group (respectively, a 41% and a 26% reduction in headache days per month). This effect was sustained at 6 and 12 months in both groups. The study confirmed the results of earlier research2,15: Abrupt withdrawal leads to reversion to an episodic pattern at 2 to 6 months in approximately 40% to 60% of patients.
More studies are needed to determine the most appropriate treatment course for MOH; however, complete withdrawal of the causative drug is the most important intervention.
Continue to: Consider withdrawal plus preventive treatment
Consider withdrawal plus preventive treatment. Use of sodium valproate, in addition to medication overuse detoxification, led to a significant reduction in headache days and improvement in quality of life at 12 weeks but no difference after 24 weeks, compared with detoxification alone in a randomized, double-blind, placebo-controlled study.31
A study of 61 patients showed a larger reduction (by 7.2 d/mo) in headache frequency with any preventive medication in addition to medication withdrawal, compared to withdrawal alone (by 4.1 d/mo) after 3 months; however, the relative benefit was gone at 6 months.32
A study of 98 patients compared immediate and delayed initiation of preventive medication upon withdrawal of overused abortive medication.33 Response was defined as a > 50% reduction in headache frequency and was similar in both groups; results showed a 28% response with immediate initiation of a preventive; a 23% response with delayed (ie, 2 months after withdrawal) initiation; and a 48% response in both groups at 12 months.
Collectively, these studies suggest that adding a preventive medication at the time of withdrawal has the potential to reduce headache frequency more quickly than withdrawal alone. However, after 3 to 6 months, the outcome of reduced headache frequency is the same whether or not a preventive medication is used—as long as the offending agent has been withdrawn.
Do preventives work without withdrawing overused medication? Patients with MOH often show little or no improvement with addition of a preventive medication only; their response to a preventive improves after withdrawal of the overused medication. Patients without previous headache improvement after addition of a preventive, who also did not improve 2 months after withdrawal, then demonstrated an overall reduction in headache by 26% when a preventive was reintroduced after withdrawal.2
Continue to: The research evidence for preventives
The research evidence for preventives. Medications for headache prevention have not been extensively evaluated specifically for treating MOH. Here is what’s known:
- Flunarizine, amitriptyline, and beta-blockers usually are ineffective for MOH.24
- Results for topiramate are mixed: A small, double-blind, placebo-controlled chronic migraine study in Europe showed that, in a subgroup of patients with MOH, topiramate led to a small but significant reduction (3.5 d/mo) in headache frequency, compared to placebo.27 A similar study done in the United States did not show a significant difference between the active-treatment and placebo groups.34
- Findings regarding onabotulinumtoxinA are intriguing: In a posthoc analysis of onabotulinumtoxinA to treat chronic migraine, patients with MOH who did not undergo detoxification had an 8 d/mo greater reduction in headache, compared to placebo.35 However, when compared to placebo in conjunction with detoxification, onabotulinumtoxinA demonstrated no benefit.36
- Newer CGRP antagonist and CGRP receptor antagonist monoclonal antibodies are successful preventive medications that have demonstrated a reduction in acute medication use days per month and headache days per month37; these compounds have not been compared to withdrawal alone.
Reducing the severity and duration of withdrawal symptoms
Withdrawal from overused abortive headache medications can lead to worsening headache, nausea, vomiting, hypotension, tachycardia, sleep disturbances, restlessness, anxiety, and nervousness. Symptoms usually last 2 to 10 days but can persist for as long as 4 weeks; duration of withdrawal symptoms varies with the medication that is being overused. In patients who have used a triptan, for example, mean duration of withdrawal is 4.1 days; ergotamine, 6.7 days; and NSAIDs, 9.5 days.23 Tapered withdrawal is sometimes recommended with opioids and barbiturates to reduce withdrawal symptoms. It is unclear whether starting a preventive medication during withdrawal assists in reducing withdrawal symptoms.38
Bridging therapy to reduce symptoms of withdrawal is often provided despite debatable utility. Available evidence does not favor one agent or method but suggests some strategies that could be helpful:
- A prednisone taper has a potential role during the first 6 days of withdrawal by reducing rebound headache and withdrawal symptoms39; however, oral prednisolone has been shown to have no benefit.40
- Alone, IV methylprednisolone seems not to be of benefit; however, in a retrospective study of 94 patients, IV methylprednisolone plus diazepam for 5 days led to a significant reduction in headache frequency and drug consumption that was sustained after 3 months.41
- Celecoxib was compared to prednisone over a 20-day course: a celecoxib dosage of 400 mg/d for the first 5 days, tapered by 100 mg every 5 days, and an oral prednisone dosage of 75 mg/d for the first 5 days, then tapered every 5 days. Patients taking celecoxib had lower headache intensity but there was no difference in headache frequency and acute medication intake between the groups.42
Other strategies. Using antiemetics and NSAIDs to reduce withdrawal symptoms is widely practiced, but no placebo-controlled trials have been conducted to support this strategy.
Patients in withdrawal might be more likely to benefit from inpatient care if they have a severe comorbidity, such as opioid or barbiturate use; failure to respond to, tolerate, or adhere to treatment; or relapse after withdrawal.38
Continue to: Cognitive behavioral therapy...
Cognitive behavioral therapy, exercise, a headache diary, and biofeedback should be considered in every patient’s treatment strategy because a multidisciplinary approach increases adherence and leads to improvement in headache frequency and a decrease in disability and medication use.43
Predictors of Tx success
A prospective cohort study determined that the rate of MOH relapse is 31% at 6 months, 41% at 1 year, and 45% at 4 years, with the highest risk of relapse during the first year.44 Looking at the correlation between type of medication overused and relapse rate, the research indicates that
- triptans have the lowest risk of relapse,44
- simple analgesics have a higher risk of relapse than triptans,22,44 and
- opioids have the highest risk of relapse.22
Where the data don’t agree. Data on combination analgesics and on ergots are conflicting.22 In addition, data on whether the primary type of headache predicts relapse rate conflict; however, migraine might predict a better outcome than tension-type headache.22
To recap and expand: Management pearls
The major goals of headache management generally are to rule out secondary headache, reach a correct diagnosis, reduce overall headache frequency, and provide effective abortive medication. A large component of reducing headache frequency is addressing and treating medication overuse.
Seek to understand the nature of the patient’s headache disorder. Components of the history are key in identifying the underlying headache diagnosis and ruling out other, more concerning secondary headache diagnoses. The ICHD-3 is an excellent resource for treating headache disorders because the classification lists specific diagnostic criteria for all recognized headache diagnoses.
Continue to: Medication withdrawal...
Medication withdrawal—with or without preventive medication—should reduce the frequency of MOH in 2 or 3 months. If headache does not become less frequent, however, the headache diagnosis might need to be reconsidered. Minimizing the use of abortive medication is generally recommended, but reduction or withdrawal of these medications does not guarantee that patients will revert to an episodic pattern of headache.
Treating withdrawal symptoms is a reasonable approach in some patients, but evidence does not support routinely providing bridging therapy.
Apply preventives carefully. Abortive medication withdrawal should generally be completed before initiating preventive medication; however, over the short term, starting preventive therapy while withdrawing the overused medication could assist in reducing headache frequency rapidly. This strategy can put patients at risk of medication adverse effects and using the medications longer than necessary, yet might be reasonable in certain patients, given their comorbidities, risk of relapse, and physician and patient preference. A preventive medication for an individual patient should generally be chosen in line with recommendations of the American Academy of Neurology45 and on the basis of the history and comorbidities.
Provide education, which is essential to lowering barriers to success. Patients with MOH must be counseled to understand that (1) a headache treatment that is supposed to be making them feel better is, in fact, making them feel worse and (2) they will get worse before they get better. Many patients are afraid to be without medication to use as needed. It is helpful to educate them on the different types of treatments (abortive, preventive); how MOH interferes with headache prophylaxis and medication efficacy; how MOH alters brain function (ie, aforementioned physiologic changes in pain processing and functional imaging changes23); and that such change is reversible when medication is withdrawn.
ACKNOWLEDGEMENT
The author thanks Jeffrey Curtis, MD, MPH, for his support and editing assistance with the manuscript.
CORRESPONDENCE
Allison Crain, MD, 2927 N 7th Avenue, Phoenix, AZ 85013; [email protected].
1. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
2. Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 2015;86:505-512.
3. Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179-190.
4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301-315.
5. Chu H-T, Liang C-S, Lee J-T, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. 2018;58:407-415.
6. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
7. Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
8. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703-711.
9. Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665-672.
10. . Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954-976.
11. Kernick D, Stapley S, Goadsby PJ, et al. What happens to new-onset headache presenting to primary care? A case–cohort study using electronic primary care records. Cephalalgia. 2008;28:1188-1195.
12. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
13. Munoz-Ceron J, Marin-Careaga V, L, et al. Headache at the emergency room: etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PloS One. 2019;14:e0208728.
14. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010;9:391-401.
15. Tassorelli C, Jensen R, Allena M, et al; the . A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014;34:645-655.
16. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
18. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65-72.
19. Hagen K, Linde M, Steiner TJ, et al. Risk factors for medication-overuse headache: an 11-year follow-up study. The Nord-Trøndelag Health Studies. Pain. 2012;153:56-61.
20. Katsarava Z, Schneewiess S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788-790.
21. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. 2019;93:e2224-e2236.
22. Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139:405-414.
23. Ferraro S, Grazzi L, Mandelli M, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. 2012;13:255-262.
24. Diener H-C, Holle D, Solbach K, et al. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12:575-583.
25. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011-1014.
26. Mauskop A, ed. Migraine and Headache. 2nd ed. Oxford University Press; 2013.
27. Diener H-C, Bussone G, Van Oene JC, et al; . Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
28. Navratilova E, Behravesh S, Oyarzo J, et al. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache Cephalalgia. 2020;40:892-902.
29. Kristoffersen ES, Straand J, Russell MB, et al. Lasting improvement of medication-overuse headache after brief intervention—a long-term follow-up in primary care. Eur J Neurol. 2017;24:883-891.
30. Carlsen LN, Munksgaard SB, Jensen RH, et al. Complete detoxification is the most effective treatment of medication-overuse headache: a randomized controlled open-label trial. Cephalalgia. 2018;38:225-236.
31. Sarchielli P, Messina P, Cupini LM, et al; SAMOHA Study Group. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1289-1297.
32. Hagen K, Stovner LJ. A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol Scand Suppl. 2011;124(suppl 191):38-43.
33. Munksgaard SB, Bendtsen L, Jensen RH. Detoxification of medication-overuse headache by a multidisciplinary treatment programme is highly effective: a comparison of two consecutive treatment methods in an open-label design. Cephalalgia. 2012;32:834-844.
34. Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153-1162.
35. Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48-56.
36. Sandrini G, Perrotta A, Tassorelli C, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427-433.
37. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(suppl 1):99-105.
38. Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention and treatment of medication overuse headache. Lancet Neurol. 2019;18:891-902.
39. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000;20:107-113.
40. Bøe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: a randomized, double-blind study. Neurology. 2007;69:26-31.
41. Paolucci M, Altamura C, Brunelli N, et al. Methylprednisolone plus diazepam i.v. as bridge therapy for medication overuse headache. Neurol Sci. 2017;38:2025-2029.
42. Taghdiri F, Togha M, Razeghi Jahromi S, et al. Celecoxib vs prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind clinical trial. Headache. 2015;55:128-135.
43. Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795-816.
44. Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005;25:12-15.
45. Silberstein SD, Holland S, Freitag F, et al; . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78:1137-1145.
1. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
2. Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 2015;86:505-512.
3. Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179-190.
4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301-315.
5. Chu H-T, Liang C-S, Lee J-T, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. 2018;58:407-415.
6. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
7. Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
8. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703-711.
9. Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665-672.
10. . Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954-976.
11. Kernick D, Stapley S, Goadsby PJ, et al. What happens to new-onset headache presenting to primary care? A case–cohort study using electronic primary care records. Cephalalgia. 2008;28:1188-1195.
12. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
13. Munoz-Ceron J, Marin-Careaga V, L, et al. Headache at the emergency room: etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PloS One. 2019;14:e0208728.
14. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010;9:391-401.
15. Tassorelli C, Jensen R, Allena M, et al; the . A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014;34:645-655.
16. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
18. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65-72.
19. Hagen K, Linde M, Steiner TJ, et al. Risk factors for medication-overuse headache: an 11-year follow-up study. The Nord-Trøndelag Health Studies. Pain. 2012;153:56-61.
20. Katsarava Z, Schneewiess S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788-790.
21. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. 2019;93:e2224-e2236.
22. Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139:405-414.
23. Ferraro S, Grazzi L, Mandelli M, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. 2012;13:255-262.
24. Diener H-C, Holle D, Solbach K, et al. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12:575-583.
25. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011-1014.
26. Mauskop A, ed. Migraine and Headache. 2nd ed. Oxford University Press; 2013.
27. Diener H-C, Bussone G, Van Oene JC, et al; . Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
28. Navratilova E, Behravesh S, Oyarzo J, et al. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache Cephalalgia. 2020;40:892-902.
29. Kristoffersen ES, Straand J, Russell MB, et al. Lasting improvement of medication-overuse headache after brief intervention—a long-term follow-up in primary care. Eur J Neurol. 2017;24:883-891.
30. Carlsen LN, Munksgaard SB, Jensen RH, et al. Complete detoxification is the most effective treatment of medication-overuse headache: a randomized controlled open-label trial. Cephalalgia. 2018;38:225-236.
31. Sarchielli P, Messina P, Cupini LM, et al; SAMOHA Study Group. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1289-1297.
32. Hagen K, Stovner LJ. A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol Scand Suppl. 2011;124(suppl 191):38-43.
33. Munksgaard SB, Bendtsen L, Jensen RH. Detoxification of medication-overuse headache by a multidisciplinary treatment programme is highly effective: a comparison of two consecutive treatment methods in an open-label design. Cephalalgia. 2012;32:834-844.
34. Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153-1162.
35. Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48-56.
36. Sandrini G, Perrotta A, Tassorelli C, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427-433.
37. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(suppl 1):99-105.
38. Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention and treatment of medication overuse headache. Lancet Neurol. 2019;18:891-902.
39. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000;20:107-113.
40. Bøe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: a randomized, double-blind study. Neurology. 2007;69:26-31.
41. Paolucci M, Altamura C, Brunelli N, et al. Methylprednisolone plus diazepam i.v. as bridge therapy for medication overuse headache. Neurol Sci. 2017;38:2025-2029.
42. Taghdiri F, Togha M, Razeghi Jahromi S, et al. Celecoxib vs prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind clinical trial. Headache. 2015;55:128-135.
43. Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795-816.
44. Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005;25:12-15.
45. Silberstein SD, Holland S, Freitag F, et al; . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78:1137-1145.
PRACTICE RECOMMENDATIONS
› Avoid prescribing barbiturates or opioids for a headache disorder. A
› Limit use of a headache-abortive medication to twice a week when starting a patient on the drug. C
› Consider providing bridging therapy during detoxification of the overused medication. C
› Do not provide a preventive medication without withdrawing the overused agent. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
At-home exercises for 4 common musculoskeletal complaints
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.
Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; [email protected]
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.
Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; [email protected]
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.
Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; [email protected]
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
PRACTICE RECOMMENDATIONS
❯ Have patients apply ice to an acute injury for 15 to 20 minutes at a time to help control inflammation, and prescribe an anti-inflammatory medication, if indicated. A
❯ Reserve heat application for use following the acute phase of injury to decrease stiffness. A
❯ Instruct patients who have an acute lateral ankle sprain to begin “ankle ABCs” and other range-of-motion exercises once acute pain subsides. C
❯ Consider recommending an eccentric heel stretch to help alleviate plantar fasciitis symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to refine your approach to peripheral arterial disease
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1
Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.
Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.
An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.
Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.
Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1
Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.
Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.
An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.
Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.
Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1
Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.
Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.
An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.
Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.
Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
PRACTICE RECOMMENDATIONS
❯ Use the ankle-brachial index for diagnosis in patients with history/physical exam findings suggestive of peripheral arterial disease (PAD). A
❯ Strongly encourage smoking cessation in patients with PAD as doing so reduces 5-year mortality and amputation rates. B
❯ Use structured exercise programs for patients with intermittent claudication prior to consideration of revascularization; doing so offers similar benefit and lower risks. A
❯ Recommend revascularization for patients who have limb ischemia or lifestyle-limiting claudication despite medical and exercise therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A new model of care to return holism to family medicine
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
PRACTICE RECOMMENDATIONS
❯ Build care teams into your practice so that you integrate “what matters” into the center of the clinical encounter. C
❯ Add practice approaches that help patients engage in healthy lifestyles and that remove social and economic barriers for improving health and well-being. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to identify and treat common bites and stings
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19
Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2
Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30
Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37
Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43
Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47
Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49
Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.
Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19
Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2
Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30
Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37
Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43
Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47
Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49
Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.
Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19
Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2
Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30
Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37
Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43
Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47
Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49
Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.
Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
PRACTICE RECOMMENDATIONS
❯ Recommend that patients use an insect repellent, such as an over-the-counter formulation that contains DEET, picaridin, or PMD (a chemical constituent of Eucalyptus citriodora oil) to prevent flea bites. C
❯ Prescribe nonsedating oral antihistamines as first-line symptomatic treatment of mild-to-moderate pruritus secondary to an insect bite. C
❯ When indicated, refer patients for venom immunotherapy, which is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis and death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series