User login
Sweet Syndrome With Aseptic Splenic Abscesses and Multiple Myeloma
To the Editor:
An 84-year-old man was admitted to the hospital with 5 erythematous cutaneous nodules of several days’ duration on the legs ranging in size from 1.0 to 1.5 cm. Upon admission, the patient also had a chest radiograph suspicious for pneumonia. The patient had received sulfamethoxazole/trimethoprim for a urinary tract infection as an outpatient 5 days prior to presentation, but he stopped the medication due to the appearance of the cutaneous nodules. Of note, the patient also reported unintentional weight loss of 15 pounds over the last few months.
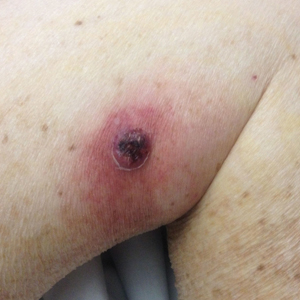
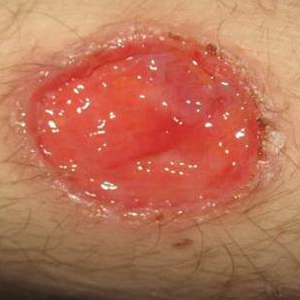
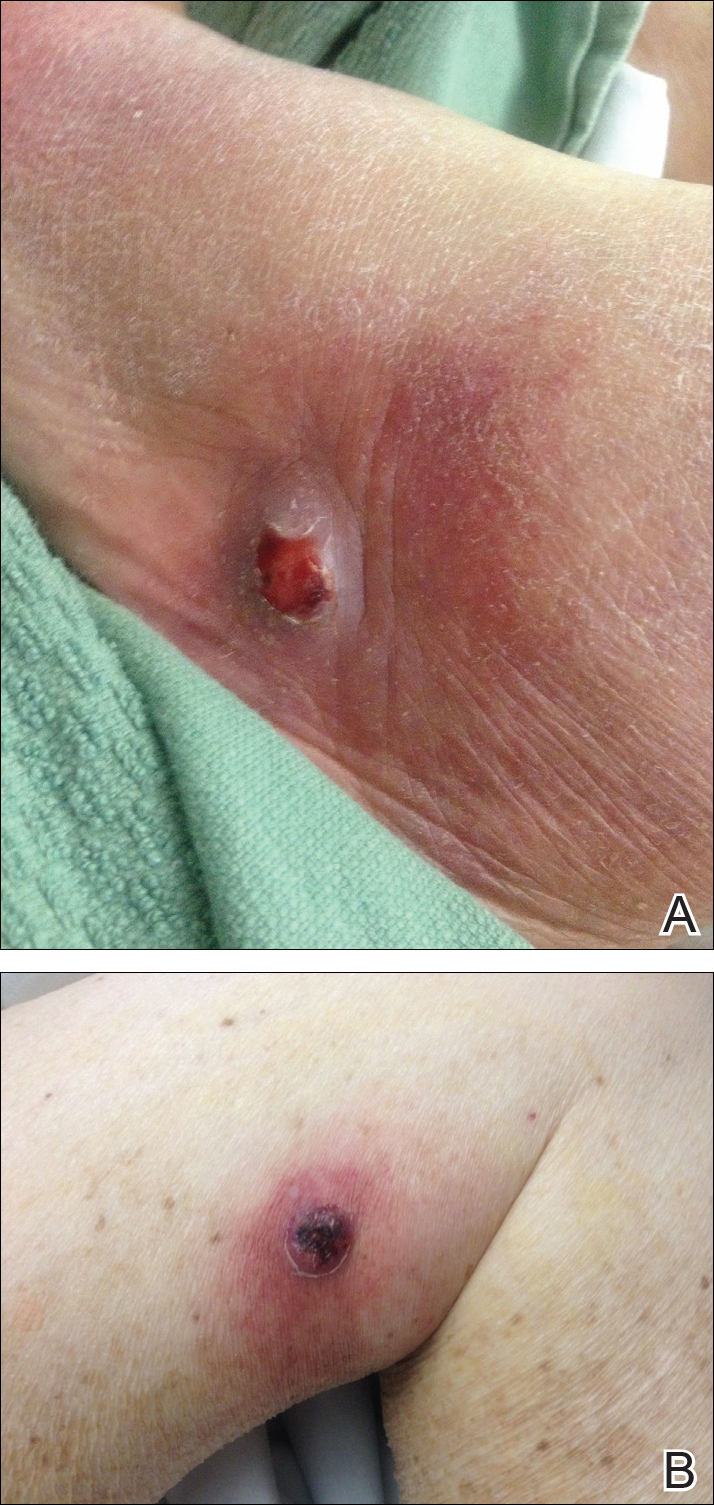
New nodules had developed at a rate of 1 to 2 lesions daily in the 3 days prior to presentation and continued to develop after admission to the hospital. The nodules appeared as tender, erythematous lesions that evolved to form pustules and developed overlying crusts in later stages (Figure 1). They were limited to the arms and legs, primarily involving the lower legs. There was no evidence of oral or ocular involvement. A hemoglobin count of 10.9 g/dL (reference range, 14.0–17.5 g/dL), white blood cell count of 8.8×109/L (reference range, 4.5–11.0×109/L), and erythrocyte sedimentation rate of 69 mm/h (reference range, 0–20 mm/h) were noted on admission.
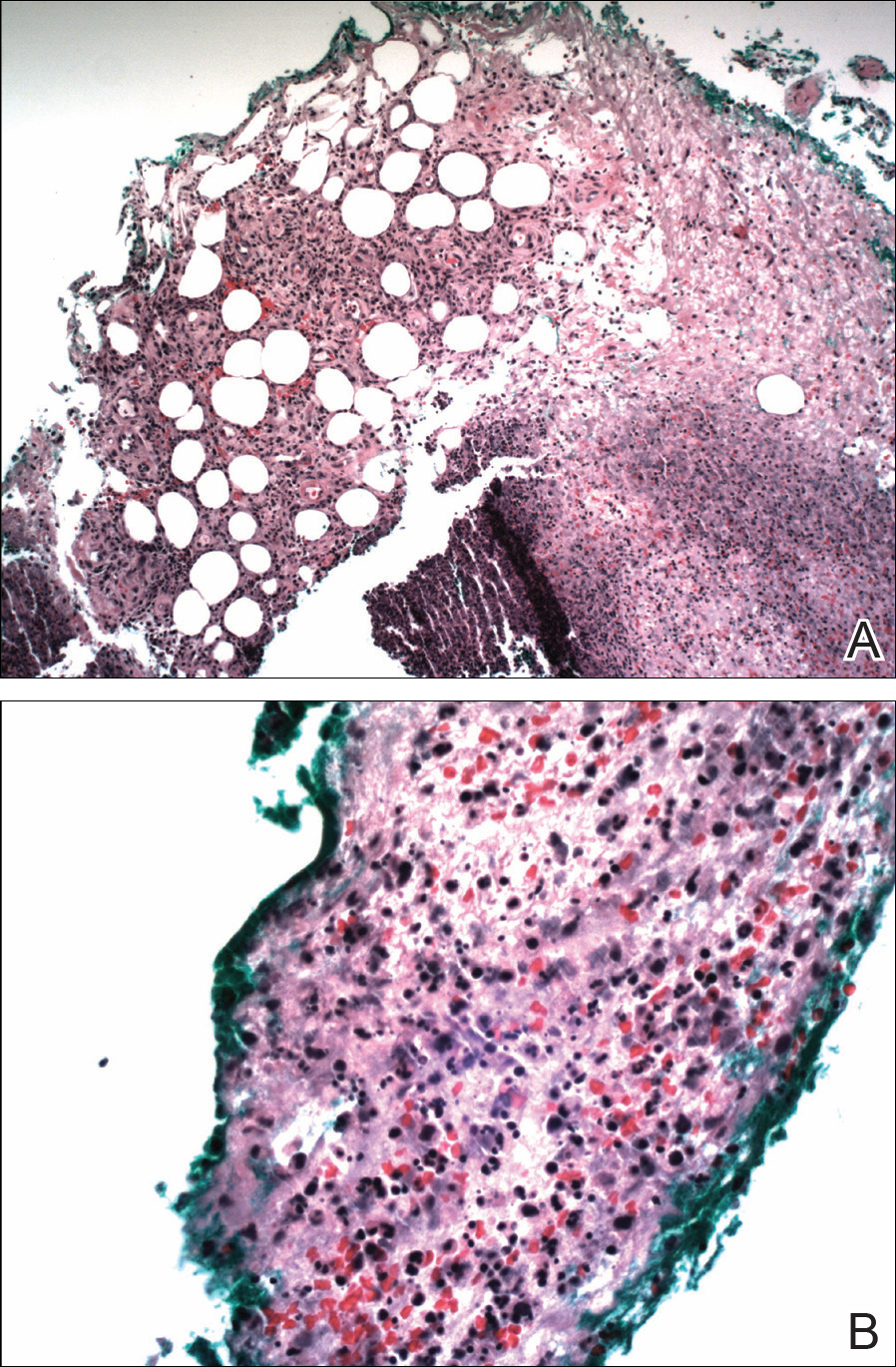
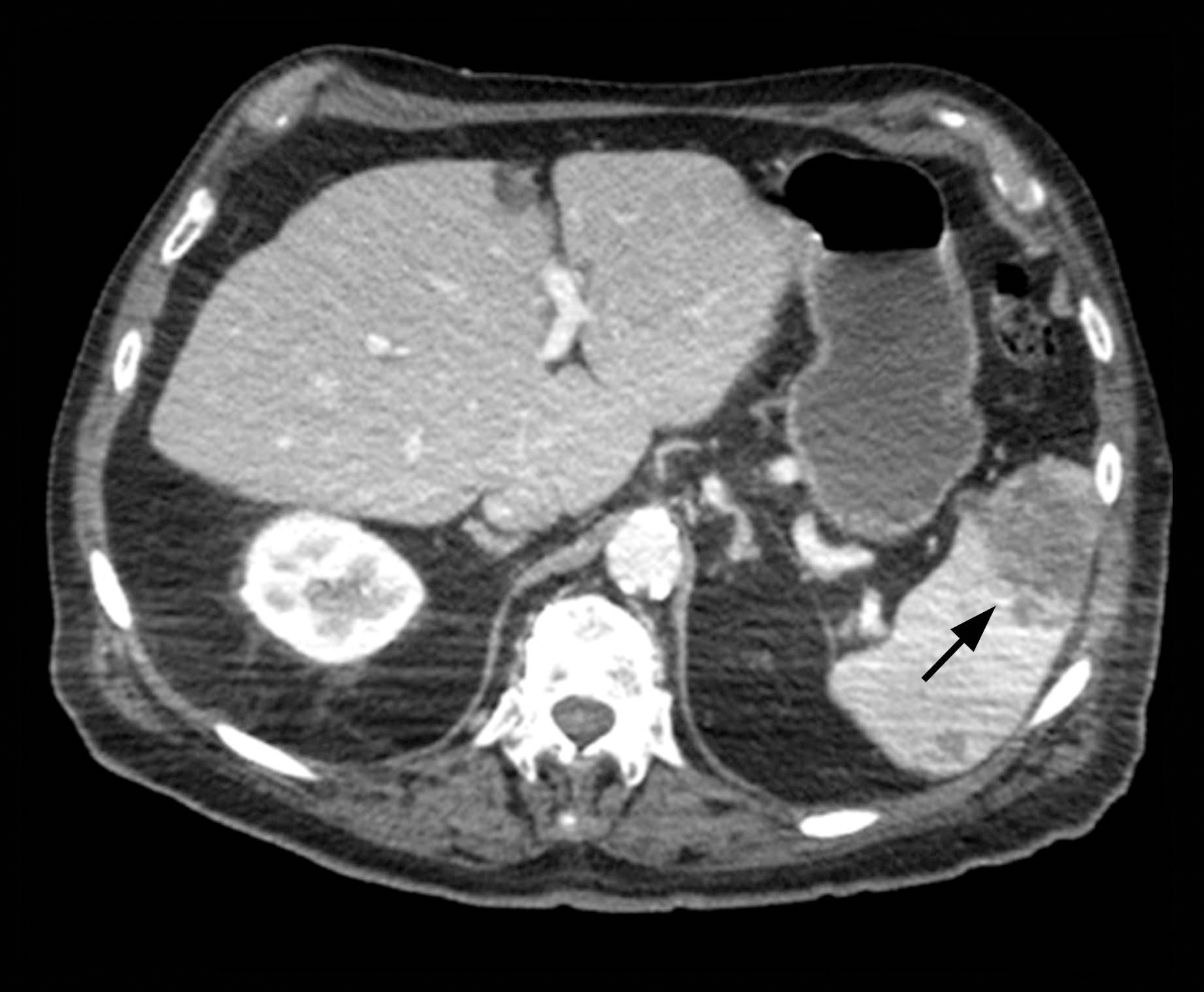
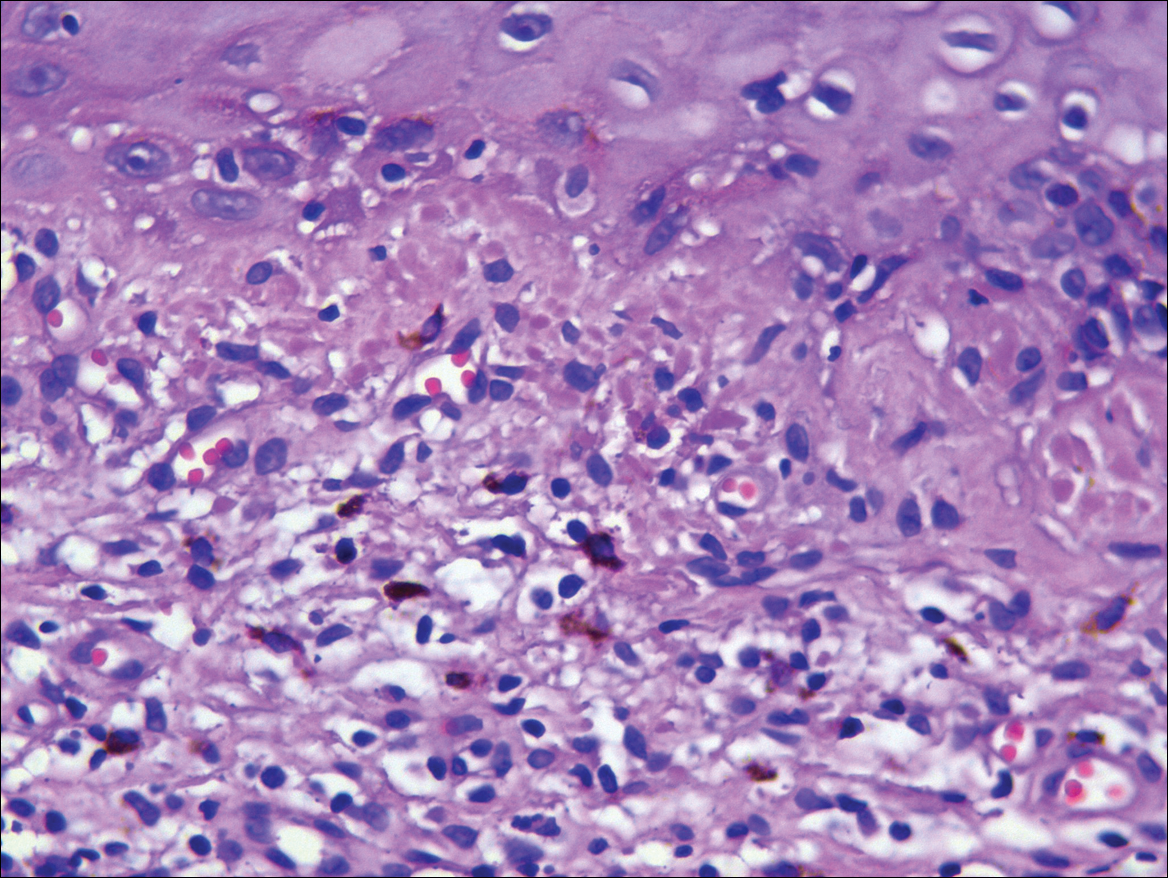
The patient was started on ceftriaxone and azithromycin for suspected pneumonia. The differential diagnosis for the cutaneous nodules included lymphoma, acid-fast bacilli (AFB) infection, deep fungal infection, pyoderma gangrenosum, Sweet syndrome (SS), panniculitis, erythema elevatum diutinum, and polyarteritis nodosa. A punch biopsy of a nodule on the left foot was performed. Histopathology demonstrated a neutrophilic panniculitis (Figure 2) with an epidermal abscess. No vasculitis was identified, and periodic acid–Schiff and AFB staining of the skin biopsy were negative. These findings were consistent with SS. Computed tomography scans of the chest, abdomen, and pelvis, which were completed early in the course of hospitalization due to concern for underlying malignancy, revealed pericardial and pleural effusions as well as cystic lesions in the lungs, spleen, kidneys, and prostate, with the largest lesion on the spleen measuring 5.6×4.8 cm (Figure 3). Computed tomography scanning was negative for areas of consolidation in the lungs. A splenic biopsy was performed by an interventional radiologist during the patient's hospitalization that identified an aseptic, neutrophilic process. Fungal, bacterial, and AFB cultures of the splenic tissue and cystic contents were negative. Bilateral pleural effusions also were identified, and a thoracentesis was performed. The pleural fluid indicated rare mesothelial cells in the background of acute inflammation with no growth of the bacterial, fungal, or AFB cultures.
Due to the association of hematologic malignances with SS, a bone marrow biopsy was performed, which revealed multiple myeloma. Serum protein electrophoresis demonstrated monoclonal gammopathy of κ light chains. During the course of his hospitalization, new skin lesions continued to develop on the hands, face, and trunk. The patient was discharged from the hospital shortly after diagnosis to receive outpatient treatment for multiple myeloma with lenalidomide and dexamethasone. Upon follow-up with the patient’s family via telephone 3 weeks into treatment, his son confirmed that the nodules were resolving.
Our case could be consistent with either drug-induced or malignancy-associated SS. Sweet syndrome initially was described in 1964 in 8 female patients with leukocytosis and cutaneous plaques infiltrated by neutrophils.1 The skin lesions typically are red and painful, ranging in size from 0.5 cm to 12.0 cm, and can last weeks to years if not treated.2 Variations of skin lesions include bullous and pustular morphologies.3
Diagnostic criteria for SS have been established.4 Both of the major criteria must be met as well as 2 of 4 minor criteria. Major criteria include abrupt onset of tender erythematous plaques and nodules; secondly, a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis must be seen on histopathology. Minor criteria include pyrexia, association with underlying condition (malignancy, pregnancy, drug exposure, inflammatory disorder), responsiveness to systemic steroids, and abnormal laboratory values (erythrocyte sedimentation rate, white blood cell count, C-reactive protein, neutrophilia).4
Sweet syndrome can be divided into 3 classifications: classical or idiopathic, drug-induced, or malignancy-associated.4 Classical SS most commonly is seen in middle-aged women after an upper respiratory or gastrointestinal infection. Drug-induced SS most often is associated with granulocyte-stimulating factor colony therapy4; however, it has been associated with use of trimethoprim/sulfamethoxazole.5 Malignancy-associated SS most commonly is seen in individuals with hematologic malignancy, specifically acute myeloid leukemia. Although its association with multiple myeloma is not as frequent, cases of malignancy-associated SS identifying this association have been reported.6,7 Mucosal involvement in the form of aphthouslike lesions more frequently is seen in malignancy-associated SS.8 Differing from classical SS, which has a female predilection of around 4:1, the malignancy-associated disorder has a 1:1 female-to-male ratio.4
In the majority of cases of SS, the neutrophilic infiltrate is in the papillary and upper reticular dermis; however, if the neutrophilic infiltrate is predominately in the subcutaneous tissue (known as subcutaneous SS), there is a strong association with malignancy.9 The histopathology in our case demonstrated a neutrophilic infiltrate in the subcutaneous tissue.
Fever is the most common systemic manifestation of SS and is present in 54% to 65% of patients.8,10 Besides the skin, the most common site affected is the eye, with 13% to 75% of patients reporting ocular involvement, usually conjunctivitis.4,10 Although infrequent, extracutaneous SS has been identified in the bones, central nervous system, kidneys, heart, liver, spleen, lungs, ears, eyes, and intestines.4 A case of SS with splenic involvement in the form of sterile abscesses also was reported.11 This case was related to parvovirus B19.
Sweet syndrome is a condition characterized by tender, erythematous cutaneous lesions with histopathology demonstrating neutrophilic infiltrate in the absence of vasculitis. We report a case of suspected extracutaneous SS in the form of splenic cysts in a patient whose SS was associated with malignancy and/or drug ingestion.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome and malignancy. Am J Med. 1987;82:1220-1226.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2002;41:182-184.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Belhadjali H, Chaabane S, Njim L, et al. Sweet’s syndrome associated with multiple myeloma. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:31-33.
- Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol. 2003;30:261-264.
- Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc. 1995;70:234-240.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560.
- Neoh CY, Tan AW, Ng SK. Sweet’s syndrome: a spectrum of unusual clinical presentation and associations. Br J Dermatol. 2007;156:480-485.
- Fortna RR, Toporcer M, Elder DE, et al. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and review of the literature on extracutaneous Sweet syndrome. Am J Dermatopathol. 2010;32:621-627.
To the Editor:
An 84-year-old man was admitted to the hospital with 5 erythematous cutaneous nodules of several days’ duration on the legs ranging in size from 1.0 to 1.5 cm. Upon admission, the patient also had a chest radiograph suspicious for pneumonia. The patient had received sulfamethoxazole/trimethoprim for a urinary tract infection as an outpatient 5 days prior to presentation, but he stopped the medication due to the appearance of the cutaneous nodules. Of note, the patient also reported unintentional weight loss of 15 pounds over the last few months.
New nodules had developed at a rate of 1 to 2 lesions daily in the 3 days prior to presentation and continued to develop after admission to the hospital. The nodules appeared as tender, erythematous lesions that evolved to form pustules and developed overlying crusts in later stages (Figure 1). They were limited to the arms and legs, primarily involving the lower legs. There was no evidence of oral or ocular involvement. A hemoglobin count of 10.9 g/dL (reference range, 14.0–17.5 g/dL), white blood cell count of 8.8×109/L (reference range, 4.5–11.0×109/L), and erythrocyte sedimentation rate of 69 mm/h (reference range, 0–20 mm/h) were noted on admission.

The patient was started on ceftriaxone and azithromycin for suspected pneumonia. The differential diagnosis for the cutaneous nodules included lymphoma, acid-fast bacilli (AFB) infection, deep fungal infection, pyoderma gangrenosum, Sweet syndrome (SS), panniculitis, erythema elevatum diutinum, and polyarteritis nodosa. A punch biopsy of a nodule on the left foot was performed. Histopathology demonstrated a neutrophilic panniculitis (Figure 2) with an epidermal abscess. No vasculitis was identified, and periodic acid–Schiff and AFB staining of the skin biopsy were negative. These findings were consistent with SS. Computed tomography scans of the chest, abdomen, and pelvis, which were completed early in the course of hospitalization due to concern for underlying malignancy, revealed pericardial and pleural effusions as well as cystic lesions in the lungs, spleen, kidneys, and prostate, with the largest lesion on the spleen measuring 5.6×4.8 cm (Figure 3). Computed tomography scanning was negative for areas of consolidation in the lungs. A splenic biopsy was performed by an interventional radiologist during the patient's hospitalization that identified an aseptic, neutrophilic process. Fungal, bacterial, and AFB cultures of the splenic tissue and cystic contents were negative. Bilateral pleural effusions also were identified, and a thoracentesis was performed. The pleural fluid indicated rare mesothelial cells in the background of acute inflammation with no growth of the bacterial, fungal, or AFB cultures.


Due to the association of hematologic malignances with SS, a bone marrow biopsy was performed, which revealed multiple myeloma. Serum protein electrophoresis demonstrated monoclonal gammopathy of κ light chains. During the course of his hospitalization, new skin lesions continued to develop on the hands, face, and trunk. The patient was discharged from the hospital shortly after diagnosis to receive outpatient treatment for multiple myeloma with lenalidomide and dexamethasone. Upon follow-up with the patient’s family via telephone 3 weeks into treatment, his son confirmed that the nodules were resolving.
Our case could be consistent with either drug-induced or malignancy-associated SS. Sweet syndrome initially was described in 1964 in 8 female patients with leukocytosis and cutaneous plaques infiltrated by neutrophils.1 The skin lesions typically are red and painful, ranging in size from 0.5 cm to 12.0 cm, and can last weeks to years if not treated.2 Variations of skin lesions include bullous and pustular morphologies.3
Diagnostic criteria for SS have been established.4 Both of the major criteria must be met as well as 2 of 4 minor criteria. Major criteria include abrupt onset of tender erythematous plaques and nodules; secondly, a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis must be seen on histopathology. Minor criteria include pyrexia, association with underlying condition (malignancy, pregnancy, drug exposure, inflammatory disorder), responsiveness to systemic steroids, and abnormal laboratory values (erythrocyte sedimentation rate, white blood cell count, C-reactive protein, neutrophilia).4
Sweet syndrome can be divided into 3 classifications: classical or idiopathic, drug-induced, or malignancy-associated.4 Classical SS most commonly is seen in middle-aged women after an upper respiratory or gastrointestinal infection. Drug-induced SS most often is associated with granulocyte-stimulating factor colony therapy4; however, it has been associated with use of trimethoprim/sulfamethoxazole.5 Malignancy-associated SS most commonly is seen in individuals with hematologic malignancy, specifically acute myeloid leukemia. Although its association with multiple myeloma is not as frequent, cases of malignancy-associated SS identifying this association have been reported.6,7 Mucosal involvement in the form of aphthouslike lesions more frequently is seen in malignancy-associated SS.8 Differing from classical SS, which has a female predilection of around 4:1, the malignancy-associated disorder has a 1:1 female-to-male ratio.4
In the majority of cases of SS, the neutrophilic infiltrate is in the papillary and upper reticular dermis; however, if the neutrophilic infiltrate is predominately in the subcutaneous tissue (known as subcutaneous SS), there is a strong association with malignancy.9 The histopathology in our case demonstrated a neutrophilic infiltrate in the subcutaneous tissue.
Fever is the most common systemic manifestation of SS and is present in 54% to 65% of patients.8,10 Besides the skin, the most common site affected is the eye, with 13% to 75% of patients reporting ocular involvement, usually conjunctivitis.4,10 Although infrequent, extracutaneous SS has been identified in the bones, central nervous system, kidneys, heart, liver, spleen, lungs, ears, eyes, and intestines.4 A case of SS with splenic involvement in the form of sterile abscesses also was reported.11 This case was related to parvovirus B19.
Sweet syndrome is a condition characterized by tender, erythematous cutaneous lesions with histopathology demonstrating neutrophilic infiltrate in the absence of vasculitis. We report a case of suspected extracutaneous SS in the form of splenic cysts in a patient whose SS was associated with malignancy and/or drug ingestion.
To the Editor:
An 84-year-old man was admitted to the hospital with 5 erythematous cutaneous nodules of several days’ duration on the legs ranging in size from 1.0 to 1.5 cm. Upon admission, the patient also had a chest radiograph suspicious for pneumonia. The patient had received sulfamethoxazole/trimethoprim for a urinary tract infection as an outpatient 5 days prior to presentation, but he stopped the medication due to the appearance of the cutaneous nodules. Of note, the patient also reported unintentional weight loss of 15 pounds over the last few months.
New nodules had developed at a rate of 1 to 2 lesions daily in the 3 days prior to presentation and continued to develop after admission to the hospital. The nodules appeared as tender, erythematous lesions that evolved to form pustules and developed overlying crusts in later stages (Figure 1). They were limited to the arms and legs, primarily involving the lower legs. There was no evidence of oral or ocular involvement. A hemoglobin count of 10.9 g/dL (reference range, 14.0–17.5 g/dL), white blood cell count of 8.8×109/L (reference range, 4.5–11.0×109/L), and erythrocyte sedimentation rate of 69 mm/h (reference range, 0–20 mm/h) were noted on admission.

The patient was started on ceftriaxone and azithromycin for suspected pneumonia. The differential diagnosis for the cutaneous nodules included lymphoma, acid-fast bacilli (AFB) infection, deep fungal infection, pyoderma gangrenosum, Sweet syndrome (SS), panniculitis, erythema elevatum diutinum, and polyarteritis nodosa. A punch biopsy of a nodule on the left foot was performed. Histopathology demonstrated a neutrophilic panniculitis (Figure 2) with an epidermal abscess. No vasculitis was identified, and periodic acid–Schiff and AFB staining of the skin biopsy were negative. These findings were consistent with SS. Computed tomography scans of the chest, abdomen, and pelvis, which were completed early in the course of hospitalization due to concern for underlying malignancy, revealed pericardial and pleural effusions as well as cystic lesions in the lungs, spleen, kidneys, and prostate, with the largest lesion on the spleen measuring 5.6×4.8 cm (Figure 3). Computed tomography scanning was negative for areas of consolidation in the lungs. A splenic biopsy was performed by an interventional radiologist during the patient's hospitalization that identified an aseptic, neutrophilic process. Fungal, bacterial, and AFB cultures of the splenic tissue and cystic contents were negative. Bilateral pleural effusions also were identified, and a thoracentesis was performed. The pleural fluid indicated rare mesothelial cells in the background of acute inflammation with no growth of the bacterial, fungal, or AFB cultures.


Due to the association of hematologic malignances with SS, a bone marrow biopsy was performed, which revealed multiple myeloma. Serum protein electrophoresis demonstrated monoclonal gammopathy of κ light chains. During the course of his hospitalization, new skin lesions continued to develop on the hands, face, and trunk. The patient was discharged from the hospital shortly after diagnosis to receive outpatient treatment for multiple myeloma with lenalidomide and dexamethasone. Upon follow-up with the patient’s family via telephone 3 weeks into treatment, his son confirmed that the nodules were resolving.
Our case could be consistent with either drug-induced or malignancy-associated SS. Sweet syndrome initially was described in 1964 in 8 female patients with leukocytosis and cutaneous plaques infiltrated by neutrophils.1 The skin lesions typically are red and painful, ranging in size from 0.5 cm to 12.0 cm, and can last weeks to years if not treated.2 Variations of skin lesions include bullous and pustular morphologies.3
Diagnostic criteria for SS have been established.4 Both of the major criteria must be met as well as 2 of 4 minor criteria. Major criteria include abrupt onset of tender erythematous plaques and nodules; secondly, a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis must be seen on histopathology. Minor criteria include pyrexia, association with underlying condition (malignancy, pregnancy, drug exposure, inflammatory disorder), responsiveness to systemic steroids, and abnormal laboratory values (erythrocyte sedimentation rate, white blood cell count, C-reactive protein, neutrophilia).4
Sweet syndrome can be divided into 3 classifications: classical or idiopathic, drug-induced, or malignancy-associated.4 Classical SS most commonly is seen in middle-aged women after an upper respiratory or gastrointestinal infection. Drug-induced SS most often is associated with granulocyte-stimulating factor colony therapy4; however, it has been associated with use of trimethoprim/sulfamethoxazole.5 Malignancy-associated SS most commonly is seen in individuals with hematologic malignancy, specifically acute myeloid leukemia. Although its association with multiple myeloma is not as frequent, cases of malignancy-associated SS identifying this association have been reported.6,7 Mucosal involvement in the form of aphthouslike lesions more frequently is seen in malignancy-associated SS.8 Differing from classical SS, which has a female predilection of around 4:1, the malignancy-associated disorder has a 1:1 female-to-male ratio.4
In the majority of cases of SS, the neutrophilic infiltrate is in the papillary and upper reticular dermis; however, if the neutrophilic infiltrate is predominately in the subcutaneous tissue (known as subcutaneous SS), there is a strong association with malignancy.9 The histopathology in our case demonstrated a neutrophilic infiltrate in the subcutaneous tissue.
Fever is the most common systemic manifestation of SS and is present in 54% to 65% of patients.8,10 Besides the skin, the most common site affected is the eye, with 13% to 75% of patients reporting ocular involvement, usually conjunctivitis.4,10 Although infrequent, extracutaneous SS has been identified in the bones, central nervous system, kidneys, heart, liver, spleen, lungs, ears, eyes, and intestines.4 A case of SS with splenic involvement in the form of sterile abscesses also was reported.11 This case was related to parvovirus B19.
Sweet syndrome is a condition characterized by tender, erythematous cutaneous lesions with histopathology demonstrating neutrophilic infiltrate in the absence of vasculitis. We report a case of suspected extracutaneous SS in the form of splenic cysts in a patient whose SS was associated with malignancy and/or drug ingestion.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome and malignancy. Am J Med. 1987;82:1220-1226.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2002;41:182-184.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Belhadjali H, Chaabane S, Njim L, et al. Sweet’s syndrome associated with multiple myeloma. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:31-33.
- Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol. 2003;30:261-264.
- Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc. 1995;70:234-240.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560.
- Neoh CY, Tan AW, Ng SK. Sweet’s syndrome: a spectrum of unusual clinical presentation and associations. Br J Dermatol. 2007;156:480-485.
- Fortna RR, Toporcer M, Elder DE, et al. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and review of the literature on extracutaneous Sweet syndrome. Am J Dermatopathol. 2010;32:621-627.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome and malignancy. Am J Med. 1987;82:1220-1226.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2002;41:182-184.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Belhadjali H, Chaabane S, Njim L, et al. Sweet’s syndrome associated with multiple myeloma. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:31-33.
- Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol. 2003;30:261-264.
- Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc. 1995;70:234-240.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560.
- Neoh CY, Tan AW, Ng SK. Sweet’s syndrome: a spectrum of unusual clinical presentation and associations. Br J Dermatol. 2007;156:480-485.
- Fortna RR, Toporcer M, Elder DE, et al. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and review of the literature on extracutaneous Sweet syndrome. Am J Dermatopathol. 2010;32:621-627.
Practice Points
- Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an inflammatory process characterized by a diffuse dermal neutrophilic infiltrate in the absence of vasculitis.
- A diagnosis of SS warrants further investigation due to its association with malignancy, especially hematologic malignancy.
- Other organs in SS also may have aseptic involvement.
Beltlike Lichen Planus Pigmentosus Complicated With Focal Amyloidosis
To the Editor:
A 68-year-old man presented with slightly itchy macules on the waist and abdomen of approximately 2 years’ duration. He reported that the initial lesions were dark red and subsequently coalesced to form a beltlike pigmentation on the abdomen. He denied any prior treatment, and the lesions did not spontaneously resolve. The patient was taking escitalopram oxalate, telmisartan, and aspirin for depression and cardiovascular disease that was diagnosed 3 years prior. He reported no exposure to UV radiation or a heat source. He denied use of any cosmetics on the body as well as a family history of similar symptoms.
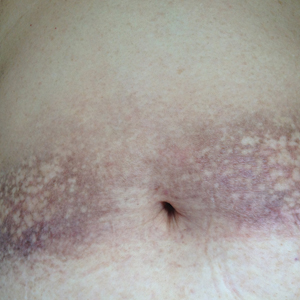
Physical examination showed reticulate brown-purple macules with slight scale on the surface that had become confluent, forming a beltlike pigmentation on the waist and abdomen (Figure 1). Wickham striae were not seen. The oral mucosa and nails were not affected. Microscopic examination for fungal infections was negative.

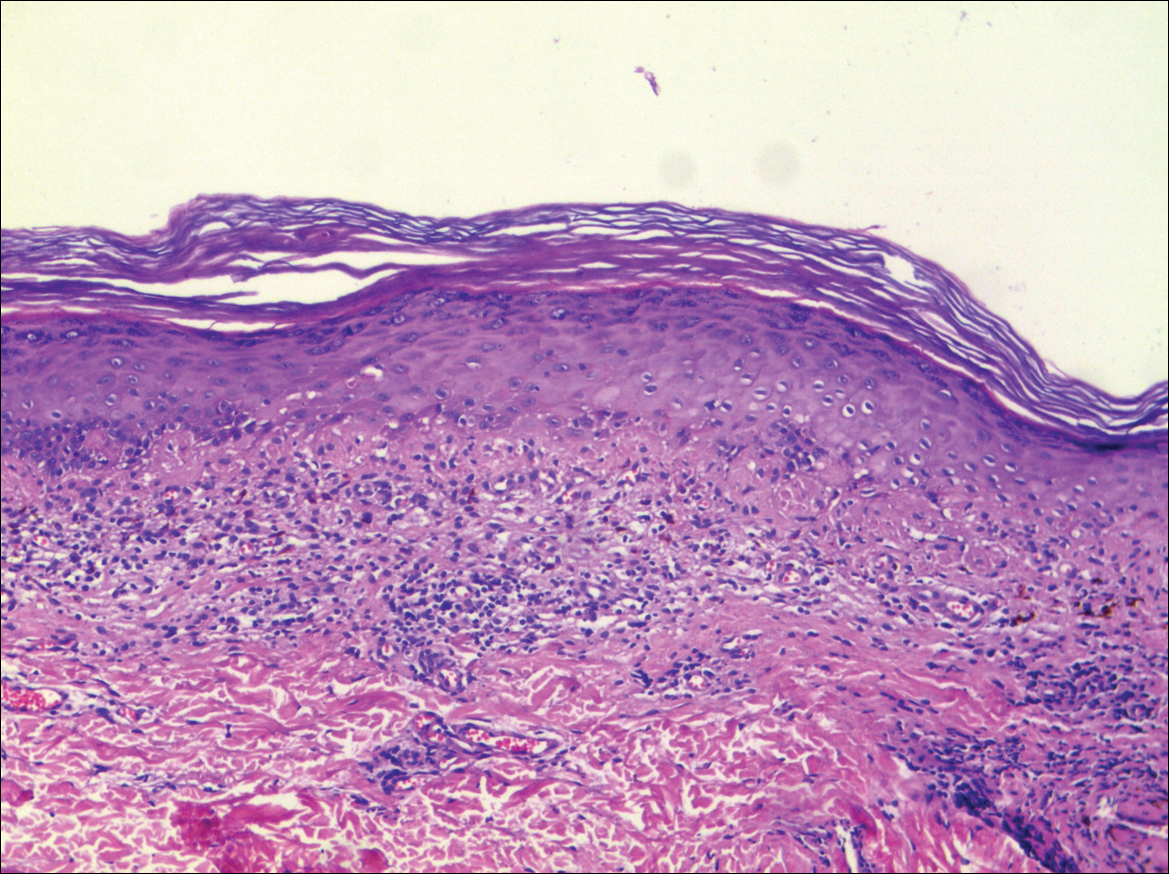
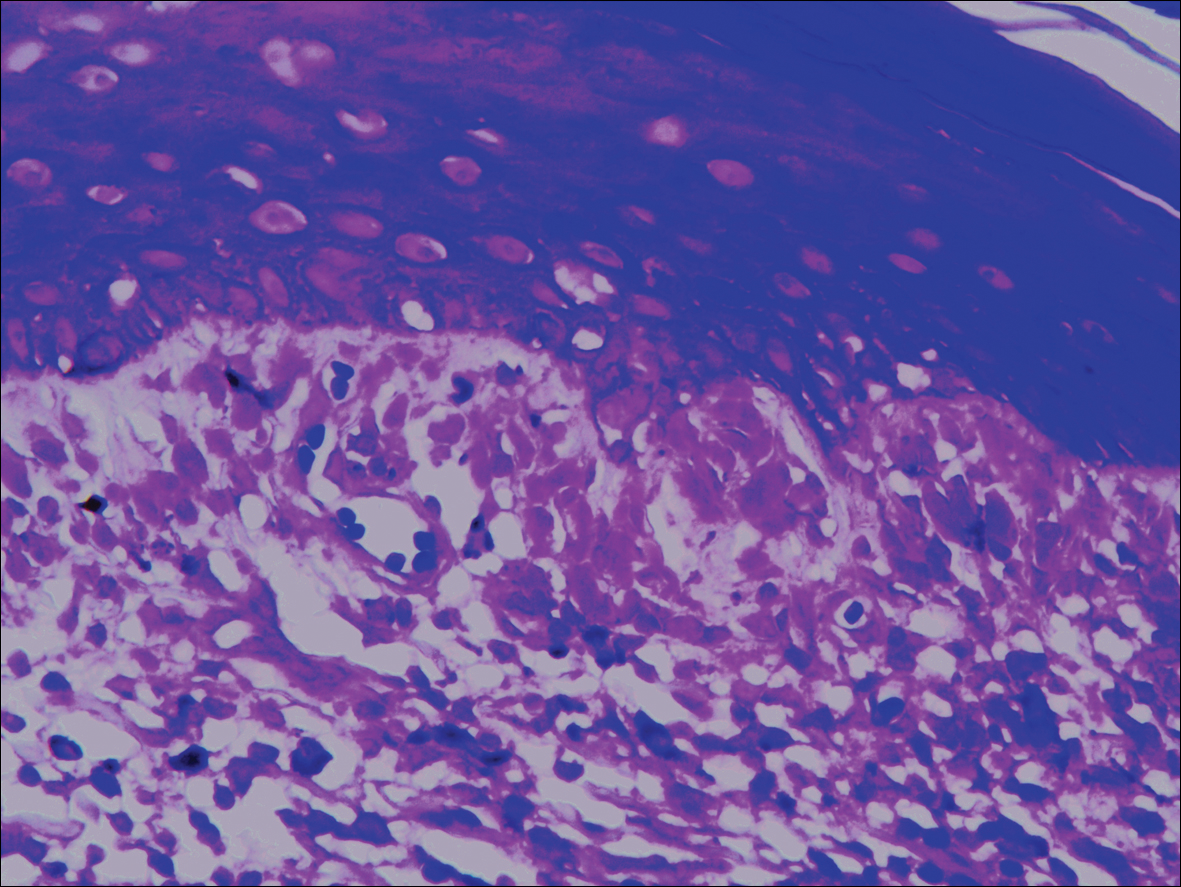
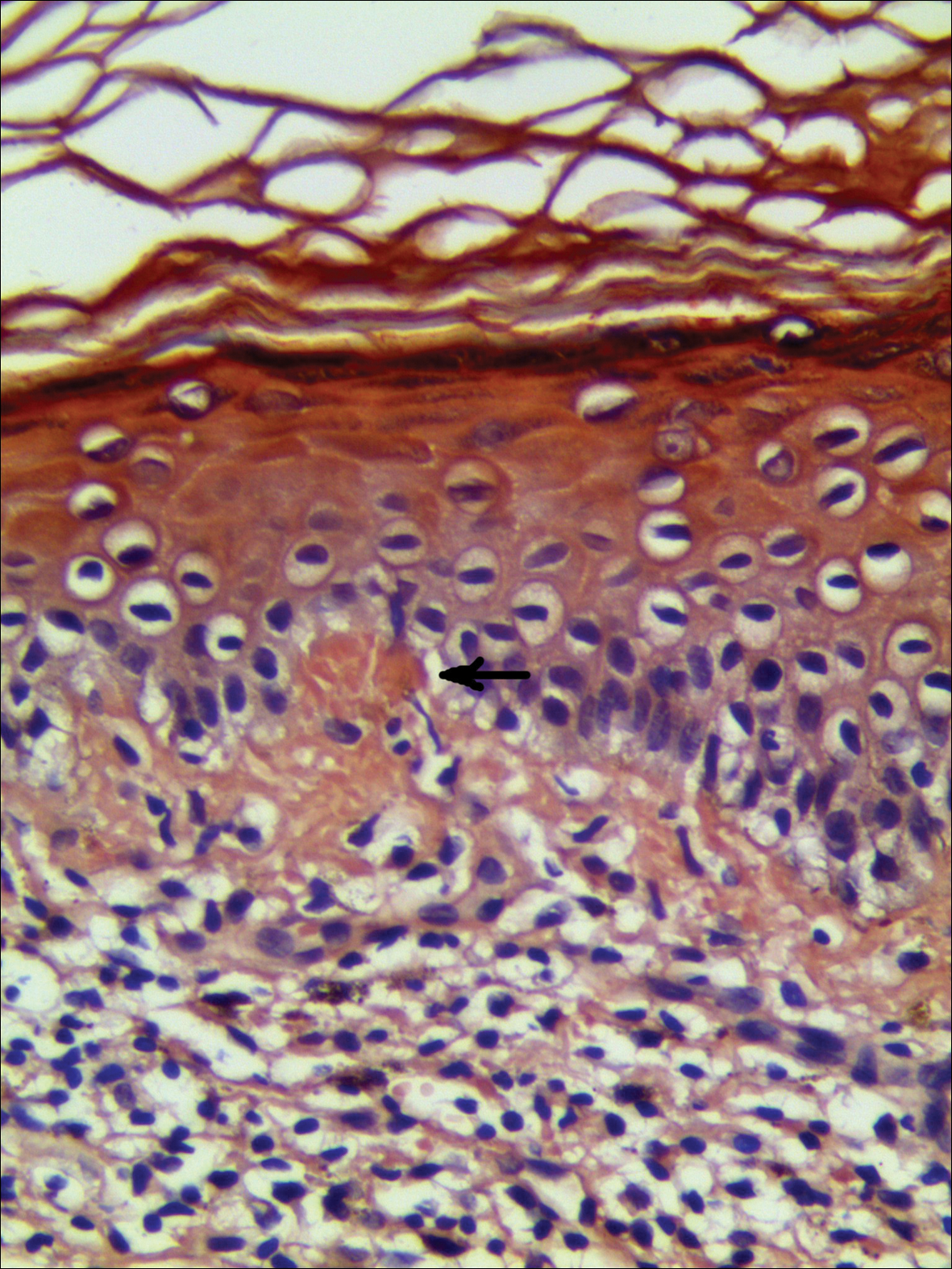
Systematic physical and laboratory examinations revealed no abnormalities. A skin biopsy from a macule on the abdomen showed hyperkeratosis, thinned out stratum spinosum with flattening of rete ridges, hypergranulosis with vacuolar alteration of the basal cell layer, and bandlike infiltration of lymphocytes and melanophages with incontinence of pigment (Figure 2). Focalized purplish homogeneous deposits were observed in the upper dermis (Figure 3), of which positive crystal violet staining indicated amyloidosis (Figure 4). Congo red stain revealed amyloid deposition (Figure 5). Thus, the diagnosis of lichen planus pigmentosus (LPP) complicated with focal amyloidosis was made. The patient was treated with topical corticosteroids and tretinoin, and no notable therapeutic effects were observed at 3-month follow-up.
Lichen planus pigmentosus, a variant of lichen planus, is a condition of unknown etiology exhibiting dark brown macules and/or papules and a long clinical course. The face, neck, trunk, arms, and legs are the most common areas of presentation, whereas involvement of the scalp, nails, or oral mucosa is relatively rare.
The first clinicohistopathological study with a large sample size was documented by Bhutani et al1 in 1974 who termed the currently recognized entity lichen planus pigmentosus. Lichen planus pigmentosus is a frequently encountered hyperpigmentation disorder in Indians, whereas sporadic cases also are reported in other regions and ethnicities.2 In cases of LPP, the pigmentation is symmetrical, and its pattern most often is diffuse, then reticular, blotchy, and perifollicular.3 Two unique patterns of LPP have been documented, including linear/blaschkoid LPP and zosteriform LPP.4,5 Our patient showed a unique beltlike distribution pattern.
The pathogenesis of LPP still is unclear, and several inciting factors such as mustard oil, gold therapy,6 and hepatitis C virus infection have been cited.7 Mancuso and Berdondini8 reported a case of LPP flaring immediately after relapse of nephrotic syndrome. It also has been considered as a paraneoplastic phenomen.9 No exact cause was found in our patient after a series of relative examinations.
The histopathologic changes associated with LPP consist of atrophic epidermis; bandlike lymphocytic infiltrate with vacuolar degeneration of the basal layer in the epidermis; and prominent melanin incontinence in the upper dermis, which can be diverse depending on different sites of skin biopsy and the phase of LPP. Histopathologic findings in our patient were consistent with LPP. The differential diagnosis for the reticulate pattern of pigmentation seen in our patient included confluent and reticulated papillomatosis and poikilodermalike cutaneous amyloidosis, both easily excluded with histopathologic confirmation.
Local amyloidosis also was confirmed by crystal violet staining in our case and its etiology was uncertain. Generalized and local amyloidosis has been reported in association with lichen planus. The diagnosis of lichen planus was followed by the diagnosis of amyloidosis, and the typical skin lesions of these 2 conditions were able to be differentiated in these reported cases.10,11 However, beltlike pigmentation was the only manifestation for our patient and we could not separate the 2 conditions with the naked eye.
Chronic irritation to the skin resulting in excessive production of degenerate keratins and their subsequent conversion into amyloid deposits has been proposed to be an etiologic factor of amyloidosis.11 Because of the distribution pattern in our case, we believe focal amyloidosis could be attributed to chronic friction and scratching.
- Bhutani LK, Bedi TR, Pandhi RK, et al. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Kanwar AJ, Kaur S. Lichen planus pigmentosus. J Am Acad Dermatol. 1989;21(4, pt 1):815.
- Kanwar AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Akarsu S, Ilknur T, Özer E, et al. Lichen planus pigmentosus distributed along the lines of Blaschko. Int J Dermatol. 2013;52:253-254.
- Cho S, Whang KK. Lichen planus pigmentosus presenting in zosteriform pattern. J Dermatol. 1997;24:193-197.
- Ingber A, Weissmann-Katzenelson V, David M, et al. Lichen planus and lichen planus pigmentosus following gold therapy—case reports and review of the literature [in German]. Z Hautkr. 1986;61:315-319.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Mancuso G, Berdondini RM. Coexistence of lichen planus pigmentosus and minimal change nephrotic syndrome. Eur J Dermatol. 2009;19:389-390.
- Sassolas B, Zagnoli A, Leroy JP, et al. Lichen planus pigmentosus associated with acrokeratosis of Bazex. Clin Exp Dermatol. 1994;19:70-73.
- Maeda H, Ohta S, Saito Y, et al. Epidermal origin of the amyloid in localized cutaneous amyloidosis. Br J Dermatol. 1982;106:345-351.
- Hongcharu W, Baldassano M, Gonzalez E. Generalized lichen amyloidosis associated with chronic lichen planus. J Am Acad Dermatol. 2000;43:346-348.
To the Editor:
A 68-year-old man presented with slightly itchy macules on the waist and abdomen of approximately 2 years’ duration. He reported that the initial lesions were dark red and subsequently coalesced to form a beltlike pigmentation on the abdomen. He denied any prior treatment, and the lesions did not spontaneously resolve. The patient was taking escitalopram oxalate, telmisartan, and aspirin for depression and cardiovascular disease that was diagnosed 3 years prior. He reported no exposure to UV radiation or a heat source. He denied use of any cosmetics on the body as well as a family history of similar symptoms.
Physical examination showed reticulate brown-purple macules with slight scale on the surface that had become confluent, forming a beltlike pigmentation on the waist and abdomen (Figure 1). Wickham striae were not seen. The oral mucosa and nails were not affected. Microscopic examination for fungal infections was negative.

Systematic physical and laboratory examinations revealed no abnormalities. A skin biopsy from a macule on the abdomen showed hyperkeratosis, thinned out stratum spinosum with flattening of rete ridges, hypergranulosis with vacuolar alteration of the basal cell layer, and bandlike infiltration of lymphocytes and melanophages with incontinence of pigment (Figure 2). Focalized purplish homogeneous deposits were observed in the upper dermis (Figure 3), of which positive crystal violet staining indicated amyloidosis (Figure 4). Congo red stain revealed amyloid deposition (Figure 5). Thus, the diagnosis of lichen planus pigmentosus (LPP) complicated with focal amyloidosis was made. The patient was treated with topical corticosteroids and tretinoin, and no notable therapeutic effects were observed at 3-month follow-up.




Lichen planus pigmentosus, a variant of lichen planus, is a condition of unknown etiology exhibiting dark brown macules and/or papules and a long clinical course. The face, neck, trunk, arms, and legs are the most common areas of presentation, whereas involvement of the scalp, nails, or oral mucosa is relatively rare.
The first clinicohistopathological study with a large sample size was documented by Bhutani et al1 in 1974 who termed the currently recognized entity lichen planus pigmentosus. Lichen planus pigmentosus is a frequently encountered hyperpigmentation disorder in Indians, whereas sporadic cases also are reported in other regions and ethnicities.2 In cases of LPP, the pigmentation is symmetrical, and its pattern most often is diffuse, then reticular, blotchy, and perifollicular.3 Two unique patterns of LPP have been documented, including linear/blaschkoid LPP and zosteriform LPP.4,5 Our patient showed a unique beltlike distribution pattern.
The pathogenesis of LPP still is unclear, and several inciting factors such as mustard oil, gold therapy,6 and hepatitis C virus infection have been cited.7 Mancuso and Berdondini8 reported a case of LPP flaring immediately after relapse of nephrotic syndrome. It also has been considered as a paraneoplastic phenomen.9 No exact cause was found in our patient after a series of relative examinations.
The histopathologic changes associated with LPP consist of atrophic epidermis; bandlike lymphocytic infiltrate with vacuolar degeneration of the basal layer in the epidermis; and prominent melanin incontinence in the upper dermis, which can be diverse depending on different sites of skin biopsy and the phase of LPP. Histopathologic findings in our patient were consistent with LPP. The differential diagnosis for the reticulate pattern of pigmentation seen in our patient included confluent and reticulated papillomatosis and poikilodermalike cutaneous amyloidosis, both easily excluded with histopathologic confirmation.
Local amyloidosis also was confirmed by crystal violet staining in our case and its etiology was uncertain. Generalized and local amyloidosis has been reported in association with lichen planus. The diagnosis of lichen planus was followed by the diagnosis of amyloidosis, and the typical skin lesions of these 2 conditions were able to be differentiated in these reported cases.10,11 However, beltlike pigmentation was the only manifestation for our patient and we could not separate the 2 conditions with the naked eye.
Chronic irritation to the skin resulting in excessive production of degenerate keratins and their subsequent conversion into amyloid deposits has been proposed to be an etiologic factor of amyloidosis.11 Because of the distribution pattern in our case, we believe focal amyloidosis could be attributed to chronic friction and scratching.
To the Editor:
A 68-year-old man presented with slightly itchy macules on the waist and abdomen of approximately 2 years’ duration. He reported that the initial lesions were dark red and subsequently coalesced to form a beltlike pigmentation on the abdomen. He denied any prior treatment, and the lesions did not spontaneously resolve. The patient was taking escitalopram oxalate, telmisartan, and aspirin for depression and cardiovascular disease that was diagnosed 3 years prior. He reported no exposure to UV radiation or a heat source. He denied use of any cosmetics on the body as well as a family history of similar symptoms.
Physical examination showed reticulate brown-purple macules with slight scale on the surface that had become confluent, forming a beltlike pigmentation on the waist and abdomen (Figure 1). Wickham striae were not seen. The oral mucosa and nails were not affected. Microscopic examination for fungal infections was negative.

Systematic physical and laboratory examinations revealed no abnormalities. A skin biopsy from a macule on the abdomen showed hyperkeratosis, thinned out stratum spinosum with flattening of rete ridges, hypergranulosis with vacuolar alteration of the basal cell layer, and bandlike infiltration of lymphocytes and melanophages with incontinence of pigment (Figure 2). Focalized purplish homogeneous deposits were observed in the upper dermis (Figure 3), of which positive crystal violet staining indicated amyloidosis (Figure 4). Congo red stain revealed amyloid deposition (Figure 5). Thus, the diagnosis of lichen planus pigmentosus (LPP) complicated with focal amyloidosis was made. The patient was treated with topical corticosteroids and tretinoin, and no notable therapeutic effects were observed at 3-month follow-up.




Lichen planus pigmentosus, a variant of lichen planus, is a condition of unknown etiology exhibiting dark brown macules and/or papules and a long clinical course. The face, neck, trunk, arms, and legs are the most common areas of presentation, whereas involvement of the scalp, nails, or oral mucosa is relatively rare.
The first clinicohistopathological study with a large sample size was documented by Bhutani et al1 in 1974 who termed the currently recognized entity lichen planus pigmentosus. Lichen planus pigmentosus is a frequently encountered hyperpigmentation disorder in Indians, whereas sporadic cases also are reported in other regions and ethnicities.2 In cases of LPP, the pigmentation is symmetrical, and its pattern most often is diffuse, then reticular, blotchy, and perifollicular.3 Two unique patterns of LPP have been documented, including linear/blaschkoid LPP and zosteriform LPP.4,5 Our patient showed a unique beltlike distribution pattern.
The pathogenesis of LPP still is unclear, and several inciting factors such as mustard oil, gold therapy,6 and hepatitis C virus infection have been cited.7 Mancuso and Berdondini8 reported a case of LPP flaring immediately after relapse of nephrotic syndrome. It also has been considered as a paraneoplastic phenomen.9 No exact cause was found in our patient after a series of relative examinations.
The histopathologic changes associated with LPP consist of atrophic epidermis; bandlike lymphocytic infiltrate with vacuolar degeneration of the basal layer in the epidermis; and prominent melanin incontinence in the upper dermis, which can be diverse depending on different sites of skin biopsy and the phase of LPP. Histopathologic findings in our patient were consistent with LPP. The differential diagnosis for the reticulate pattern of pigmentation seen in our patient included confluent and reticulated papillomatosis and poikilodermalike cutaneous amyloidosis, both easily excluded with histopathologic confirmation.
Local amyloidosis also was confirmed by crystal violet staining in our case and its etiology was uncertain. Generalized and local amyloidosis has been reported in association with lichen planus. The diagnosis of lichen planus was followed by the diagnosis of amyloidosis, and the typical skin lesions of these 2 conditions were able to be differentiated in these reported cases.10,11 However, beltlike pigmentation was the only manifestation for our patient and we could not separate the 2 conditions with the naked eye.
Chronic irritation to the skin resulting in excessive production of degenerate keratins and their subsequent conversion into amyloid deposits has been proposed to be an etiologic factor of amyloidosis.11 Because of the distribution pattern in our case, we believe focal amyloidosis could be attributed to chronic friction and scratching.
- Bhutani LK, Bedi TR, Pandhi RK, et al. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Kanwar AJ, Kaur S. Lichen planus pigmentosus. J Am Acad Dermatol. 1989;21(4, pt 1):815.
- Kanwar AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Akarsu S, Ilknur T, Özer E, et al. Lichen planus pigmentosus distributed along the lines of Blaschko. Int J Dermatol. 2013;52:253-254.
- Cho S, Whang KK. Lichen planus pigmentosus presenting in zosteriform pattern. J Dermatol. 1997;24:193-197.
- Ingber A, Weissmann-Katzenelson V, David M, et al. Lichen planus and lichen planus pigmentosus following gold therapy—case reports and review of the literature [in German]. Z Hautkr. 1986;61:315-319.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Mancuso G, Berdondini RM. Coexistence of lichen planus pigmentosus and minimal change nephrotic syndrome. Eur J Dermatol. 2009;19:389-390.
- Sassolas B, Zagnoli A, Leroy JP, et al. Lichen planus pigmentosus associated with acrokeratosis of Bazex. Clin Exp Dermatol. 1994;19:70-73.
- Maeda H, Ohta S, Saito Y, et al. Epidermal origin of the amyloid in localized cutaneous amyloidosis. Br J Dermatol. 1982;106:345-351.
- Hongcharu W, Baldassano M, Gonzalez E. Generalized lichen amyloidosis associated with chronic lichen planus. J Am Acad Dermatol. 2000;43:346-348.
- Bhutani LK, Bedi TR, Pandhi RK, et al. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Kanwar AJ, Kaur S. Lichen planus pigmentosus. J Am Acad Dermatol. 1989;21(4, pt 1):815.
- Kanwar AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Akarsu S, Ilknur T, Özer E, et al. Lichen planus pigmentosus distributed along the lines of Blaschko. Int J Dermatol. 2013;52:253-254.
- Cho S, Whang KK. Lichen planus pigmentosus presenting in zosteriform pattern. J Dermatol. 1997;24:193-197.
- Ingber A, Weissmann-Katzenelson V, David M, et al. Lichen planus and lichen planus pigmentosus following gold therapy—case reports and review of the literature [in German]. Z Hautkr. 1986;61:315-319.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Mancuso G, Berdondini RM. Coexistence of lichen planus pigmentosus and minimal change nephrotic syndrome. Eur J Dermatol. 2009;19:389-390.
- Sassolas B, Zagnoli A, Leroy JP, et al. Lichen planus pigmentosus associated with acrokeratosis of Bazex. Clin Exp Dermatol. 1994;19:70-73.
- Maeda H, Ohta S, Saito Y, et al. Epidermal origin of the amyloid in localized cutaneous amyloidosis. Br J Dermatol. 1982;106:345-351.
- Hongcharu W, Baldassano M, Gonzalez E. Generalized lichen amyloidosis associated with chronic lichen planus. J Am Acad Dermatol. 2000;43:346-348.
Practice Points
- Lichen planus pigmentosus can present in a unique beltlike distribution pattern.
- Focal amyloidosis due to chronic friction and scratching cannot be excluded from the differential diagnosis.
Acute Generalized Exanthematous Pustulosis Caused by Pantoprazole
To the Editor:
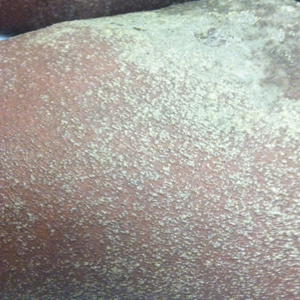
A 34-year-old woman presented with a generalized pustular eruption with subjective fevers, chills, night sweats, and light-headedness. Ten days prior to admission she developed a generalized erythematous and pruritic rash; she had started pantoprazole for reflux 4 days prior to the rash. On admission, skin examination revealed facial edema and diffuse erythema covering 80% of the total body surface area with multiple 1- to 4-mm pustules coalescing into lakes of pus on the trunk as well as bilateral upper and lower arms and legs sparing the palms and soles. Desquamation and serous drainage with crust were observed on the skin of the head, upper trunk, and thighs (Figure 1). Vital signs were notable for hypotension. Laboratory tests on admission were remarkable for leukocytosis (white blood cell count: 22.5×103/μL [reference range, 4.5–11×103/μL]) with absolute eosinophilia but no neutrophilia. C-reactive protein (CRP) was elevated (237.9 mg/L [reference range, 5.0–9.9 mg/L]). Renal and hepatic functions were normal. Blood cultures grew methicillin-sensitive Staphylococcus aureus (MSSA). Further infectious disease workup for viral and fungal pathogens was negative.
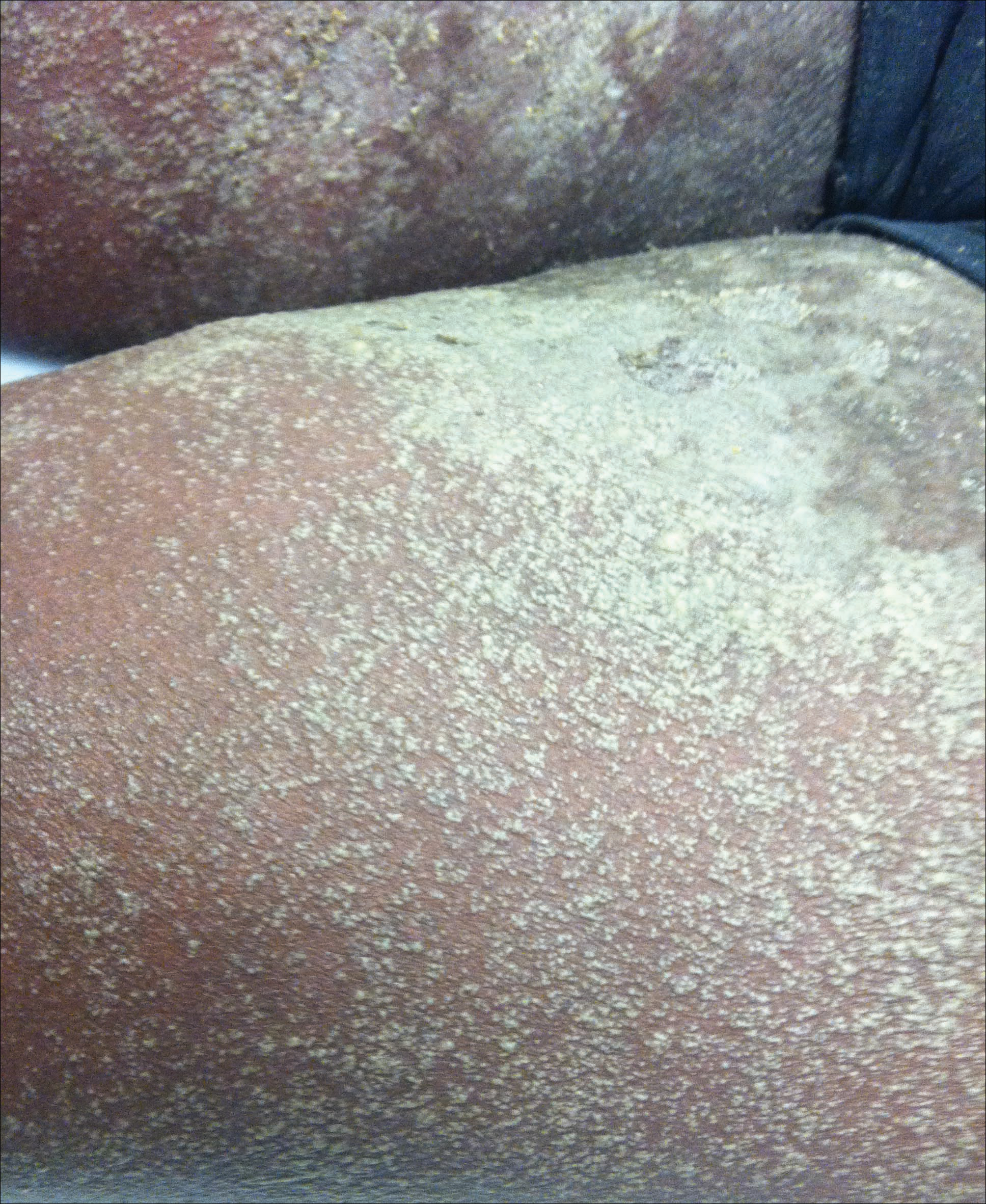
Skin biopsy from the left thigh revealed subcorneal, pustular, acute spongiotic dermatitis with marked intraepidermal spongiosis and papillary edema; exocytosis of eosinophils; and single cell necrosis of keratinocytes (Figure 2). These findings were consistent with acute generalized exanthematous pustulosis (AGEP). Pantoprazole was discontinued, and cardiovascular support and antibiotic therapy for MSSA bacteremia were initiated. Respiratory, kidney, and liver functions remained normal throughout the 11-day hospitalization, and the pustular dermatitis, MSSA bacteremia, and cardiovascular symptoms resolved within 10 days.

Acute generalized exanthematous pustulosis is an uncommon, self-limited, generalized sterile pustular eruption notable for the usual absence of systemic symptoms and extracutaneous organ involvement. Hotz et al1 found that mean peripheral neutrophil counts (mean, 21.5×103/μL) and CRP levels (mean, 241.6 mg/L) were notably elevated in patients with systemic (ie, hepatic, pulmonary, renal, bone marrow) involvement. In our patient, only the CRP approached the elevated value reported by Hotz et al.1 However, the patient exhibited only cardiovascular instability in the context of secondary bacteremia and no other systemic symptoms. The combination of highly elevated neutrophilia and CRP may be a better marker for AGEP-precipitated extracutaneous organ involvement.
Although infectious pathogens such as Epstein-Barr virus and cytomegalovirus have been implicated, the majority of AGEP cases are adverse reactions (ARs) to medications, such as β-lactam antibiotics. In our patient, the widely prescribed proton pump inhibitor (PPI) pantoprazole was the most likely cause. Acute generalized exanthematous pustulosis was reported in a patient taking another PPI, omeprazole.2 However, PPIs are recognized to cause many cutaneous and other organ ARs, though prevalence of ARs is still low. In Thailand, Chularojanamontri et al3 reported 13.8 per 100,000 individuals developed a cutaneous AR to PPIs, and the ARs most frequently were attributed to omeprazole. They found that drug exanthems were the most common cutaneous ARs.3 However, more severe hypersensitivity reactions have been reported, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and autoimmune eruptions such as cutaneous lupus erythematosus.3,4 Other systemic reactions to PPIs include increased risks for urticaria, pneumonia, Clostridium difficile infections, and acute interstitial nephritis.4,5
- Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
- Nantes Castillejo O, Zozaya Urmeneta JM, Valcayo Peñalba A, et al. Acute generalized exanthematous pustulosis induced by omeprazole [in Spanish]. Gastroenterol Hepatol. 2008;31:295-298.
- Chularojanamontri L, Jiamton S, Manapajon A, et al. Cutaneous reactions to proton pump inhibitors: a case-control study. J Drugs Dermatol. 2012;11:E43-E47.
- Chang YS. Hypersensitivity reactions to proton pump inhibitors. Curr Opin Allergy Clin Immunol. 2012;12:348-353.
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6:443-551.
To the Editor:
A 34-year-old woman presented with a generalized pustular eruption with subjective fevers, chills, night sweats, and light-headedness. Ten days prior to admission she developed a generalized erythematous and pruritic rash; she had started pantoprazole for reflux 4 days prior to the rash. On admission, skin examination revealed facial edema and diffuse erythema covering 80% of the total body surface area with multiple 1- to 4-mm pustules coalescing into lakes of pus on the trunk as well as bilateral upper and lower arms and legs sparing the palms and soles. Desquamation and serous drainage with crust were observed on the skin of the head, upper trunk, and thighs (Figure 1). Vital signs were notable for hypotension. Laboratory tests on admission were remarkable for leukocytosis (white blood cell count: 22.5×103/μL [reference range, 4.5–11×103/μL]) with absolute eosinophilia but no neutrophilia. C-reactive protein (CRP) was elevated (237.9 mg/L [reference range, 5.0–9.9 mg/L]). Renal and hepatic functions were normal. Blood cultures grew methicillin-sensitive Staphylococcus aureus (MSSA). Further infectious disease workup for viral and fungal pathogens was negative.

Skin biopsy from the left thigh revealed subcorneal, pustular, acute spongiotic dermatitis with marked intraepidermal spongiosis and papillary edema; exocytosis of eosinophils; and single cell necrosis of keratinocytes (Figure 2). These findings were consistent with acute generalized exanthematous pustulosis (AGEP). Pantoprazole was discontinued, and cardiovascular support and antibiotic therapy for MSSA bacteremia were initiated. Respiratory, kidney, and liver functions remained normal throughout the 11-day hospitalization, and the pustular dermatitis, MSSA bacteremia, and cardiovascular symptoms resolved within 10 days.

Acute generalized exanthematous pustulosis is an uncommon, self-limited, generalized sterile pustular eruption notable for the usual absence of systemic symptoms and extracutaneous organ involvement. Hotz et al1 found that mean peripheral neutrophil counts (mean, 21.5×103/μL) and CRP levels (mean, 241.6 mg/L) were notably elevated in patients with systemic (ie, hepatic, pulmonary, renal, bone marrow) involvement. In our patient, only the CRP approached the elevated value reported by Hotz et al.1 However, the patient exhibited only cardiovascular instability in the context of secondary bacteremia and no other systemic symptoms. The combination of highly elevated neutrophilia and CRP may be a better marker for AGEP-precipitated extracutaneous organ involvement.
Although infectious pathogens such as Epstein-Barr virus and cytomegalovirus have been implicated, the majority of AGEP cases are adverse reactions (ARs) to medications, such as β-lactam antibiotics. In our patient, the widely prescribed proton pump inhibitor (PPI) pantoprazole was the most likely cause. Acute generalized exanthematous pustulosis was reported in a patient taking another PPI, omeprazole.2 However, PPIs are recognized to cause many cutaneous and other organ ARs, though prevalence of ARs is still low. In Thailand, Chularojanamontri et al3 reported 13.8 per 100,000 individuals developed a cutaneous AR to PPIs, and the ARs most frequently were attributed to omeprazole. They found that drug exanthems were the most common cutaneous ARs.3 However, more severe hypersensitivity reactions have been reported, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and autoimmune eruptions such as cutaneous lupus erythematosus.3,4 Other systemic reactions to PPIs include increased risks for urticaria, pneumonia, Clostridium difficile infections, and acute interstitial nephritis.4,5
To the Editor:
A 34-year-old woman presented with a generalized pustular eruption with subjective fevers, chills, night sweats, and light-headedness. Ten days prior to admission she developed a generalized erythematous and pruritic rash; she had started pantoprazole for reflux 4 days prior to the rash. On admission, skin examination revealed facial edema and diffuse erythema covering 80% of the total body surface area with multiple 1- to 4-mm pustules coalescing into lakes of pus on the trunk as well as bilateral upper and lower arms and legs sparing the palms and soles. Desquamation and serous drainage with crust were observed on the skin of the head, upper trunk, and thighs (Figure 1). Vital signs were notable for hypotension. Laboratory tests on admission were remarkable for leukocytosis (white blood cell count: 22.5×103/μL [reference range, 4.5–11×103/μL]) with absolute eosinophilia but no neutrophilia. C-reactive protein (CRP) was elevated (237.9 mg/L [reference range, 5.0–9.9 mg/L]). Renal and hepatic functions were normal. Blood cultures grew methicillin-sensitive Staphylococcus aureus (MSSA). Further infectious disease workup for viral and fungal pathogens was negative.

Skin biopsy from the left thigh revealed subcorneal, pustular, acute spongiotic dermatitis with marked intraepidermal spongiosis and papillary edema; exocytosis of eosinophils; and single cell necrosis of keratinocytes (Figure 2). These findings were consistent with acute generalized exanthematous pustulosis (AGEP). Pantoprazole was discontinued, and cardiovascular support and antibiotic therapy for MSSA bacteremia were initiated. Respiratory, kidney, and liver functions remained normal throughout the 11-day hospitalization, and the pustular dermatitis, MSSA bacteremia, and cardiovascular symptoms resolved within 10 days.

Acute generalized exanthematous pustulosis is an uncommon, self-limited, generalized sterile pustular eruption notable for the usual absence of systemic symptoms and extracutaneous organ involvement. Hotz et al1 found that mean peripheral neutrophil counts (mean, 21.5×103/μL) and CRP levels (mean, 241.6 mg/L) were notably elevated in patients with systemic (ie, hepatic, pulmonary, renal, bone marrow) involvement. In our patient, only the CRP approached the elevated value reported by Hotz et al.1 However, the patient exhibited only cardiovascular instability in the context of secondary bacteremia and no other systemic symptoms. The combination of highly elevated neutrophilia and CRP may be a better marker for AGEP-precipitated extracutaneous organ involvement.
Although infectious pathogens such as Epstein-Barr virus and cytomegalovirus have been implicated, the majority of AGEP cases are adverse reactions (ARs) to medications, such as β-lactam antibiotics. In our patient, the widely prescribed proton pump inhibitor (PPI) pantoprazole was the most likely cause. Acute generalized exanthematous pustulosis was reported in a patient taking another PPI, omeprazole.2 However, PPIs are recognized to cause many cutaneous and other organ ARs, though prevalence of ARs is still low. In Thailand, Chularojanamontri et al3 reported 13.8 per 100,000 individuals developed a cutaneous AR to PPIs, and the ARs most frequently were attributed to omeprazole. They found that drug exanthems were the most common cutaneous ARs.3 However, more severe hypersensitivity reactions have been reported, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and autoimmune eruptions such as cutaneous lupus erythematosus.3,4 Other systemic reactions to PPIs include increased risks for urticaria, pneumonia, Clostridium difficile infections, and acute interstitial nephritis.4,5
- Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
- Nantes Castillejo O, Zozaya Urmeneta JM, Valcayo Peñalba A, et al. Acute generalized exanthematous pustulosis induced by omeprazole [in Spanish]. Gastroenterol Hepatol. 2008;31:295-298.
- Chularojanamontri L, Jiamton S, Manapajon A, et al. Cutaneous reactions to proton pump inhibitors: a case-control study. J Drugs Dermatol. 2012;11:E43-E47.
- Chang YS. Hypersensitivity reactions to proton pump inhibitors. Curr Opin Allergy Clin Immunol. 2012;12:348-353.
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6:443-551.
- Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
- Nantes Castillejo O, Zozaya Urmeneta JM, Valcayo Peñalba A, et al. Acute generalized exanthematous pustulosis induced by omeprazole [in Spanish]. Gastroenterol Hepatol. 2008;31:295-298.
- Chularojanamontri L, Jiamton S, Manapajon A, et al. Cutaneous reactions to proton pump inhibitors: a case-control study. J Drugs Dermatol. 2012;11:E43-E47.
- Chang YS. Hypersensitivity reactions to proton pump inhibitors. Curr Opin Allergy Clin Immunol. 2012;12:348-353.
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6:443-551.
Interstitial Granulomatous Dermatitis and Palisaded Neutrophilic Granulomatous Dermatitis
To the Editor:
Palisaded neutrophilic granulomatous dermatitis (PNGD) is a rare disorder that often is associated with systemic disease. It has been shown to manifest in the presence of systemic lupus erythematosus; rheumatoid arthritis; Wegener granulomatosis; and other diseases, mainly autoimmune conditions. Interstitial granulomatous dermatitis (IGD) associated with arthritis was first described by Ackerman et al1 in 1993. In 1994, IGD was placed among the spectrum of PNGD by Chu et al.2 The disease entities included in the spectrum of PNGD of the immune complex disease are Churg-Strauss granuloma, cutaneous extravascular necrotizing granuloma, rheumatoid papules, superficial ulcerating rheumatoid necrobiosis, and IGD with arthritis.2 It has been suggested that IGD has a distinct clinical presentation with associated histopathology, while others suggest it still is part of the PNGD spectrum.2,3 We present 2 cases of granulomatous dermatitis and their findings related to IGD and PNGD.
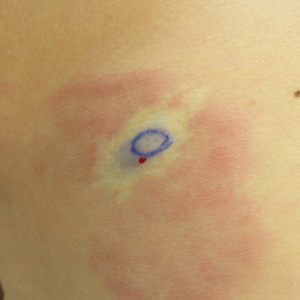
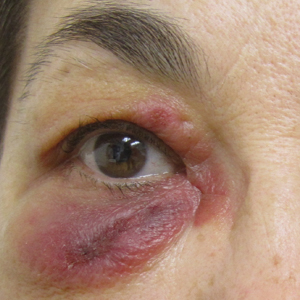
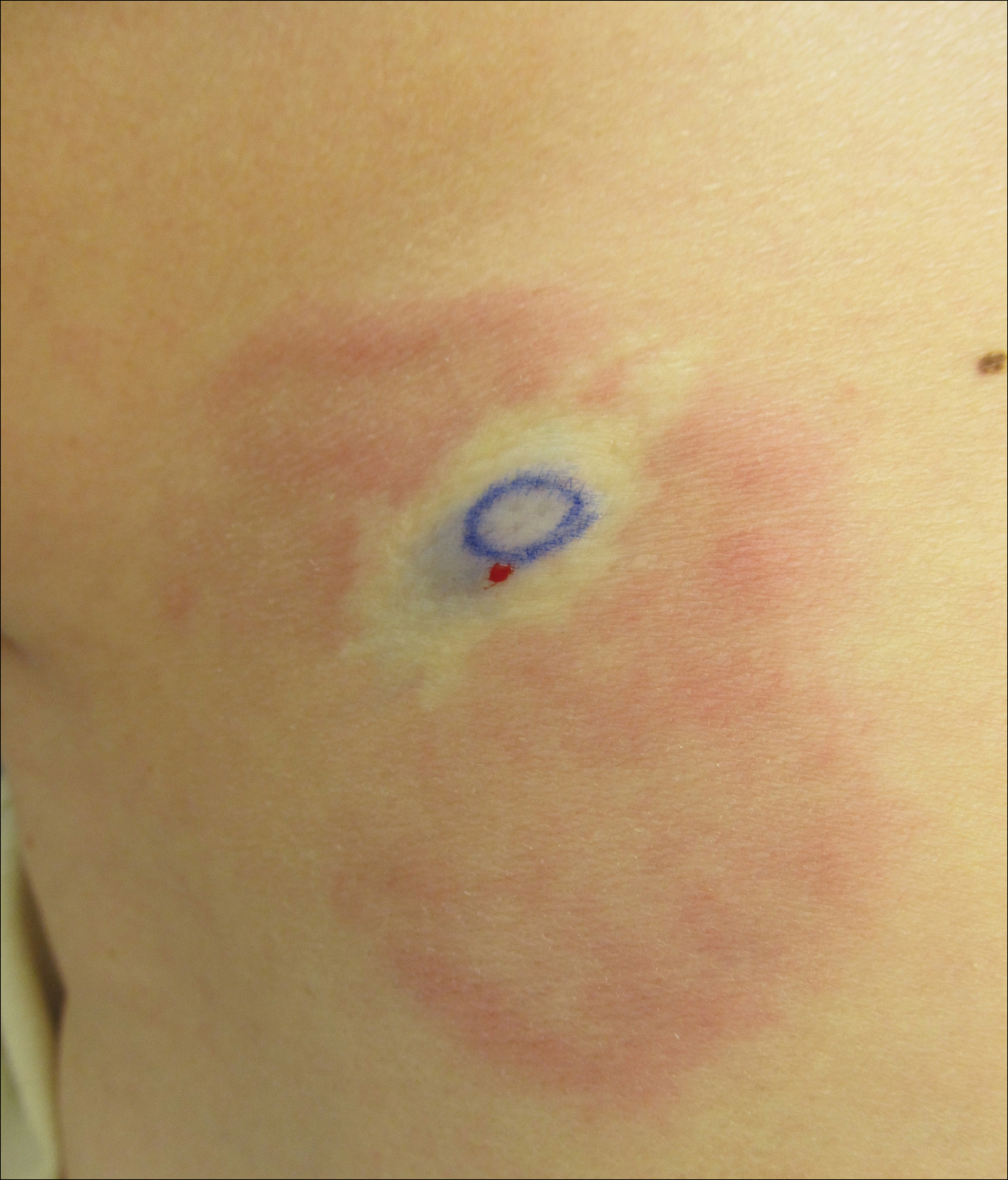
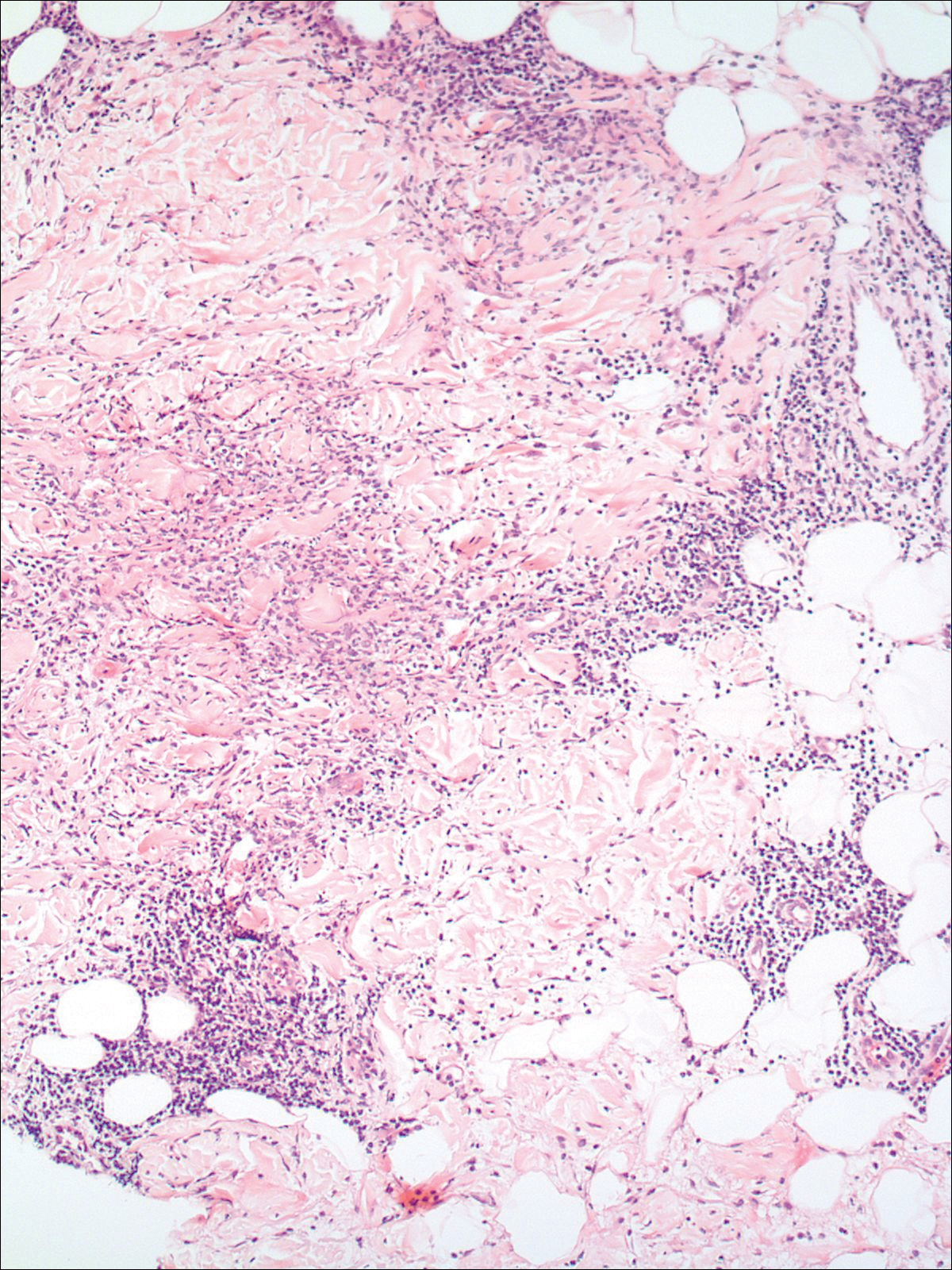
A 58-year-old woman presented with recurrent painful lesions on the trunk, arms, and legs of 2 years’ duration. The lesions spontaneously resolved without scarring or hyperpigmentation but would recur in different areas on the trunk. She was diagnosed with rheumatoid arthritis following a recent autoimmune workup. At presentation, physical examination revealed tender erythematous edematous plaques on the bilateral upper back (Figure 1) and erythematous nodules on the bilateral upper arms. The patient previously had an antinuclear antibody titer of 1:320 with a speckled pattern. A repeat antinuclear antibody titer taken 1 year later was negative. Her rheumatoid factor initially was positive and remained positive upon repeat testing. Punch biopsies were performed for histologic evaluation of the lesions and immunofluorescence. Biopsies examined with hematoxylin and eosin stain revealed perivascular and interstitial mixed (lymphocytic, neutrophilic, eosinophilic) bottom-heavy inflammation with nuclear dust and basophilic degeneration of collagen (Figure 2). Immunofluorescence studies were negative. The patient deferred treatment.
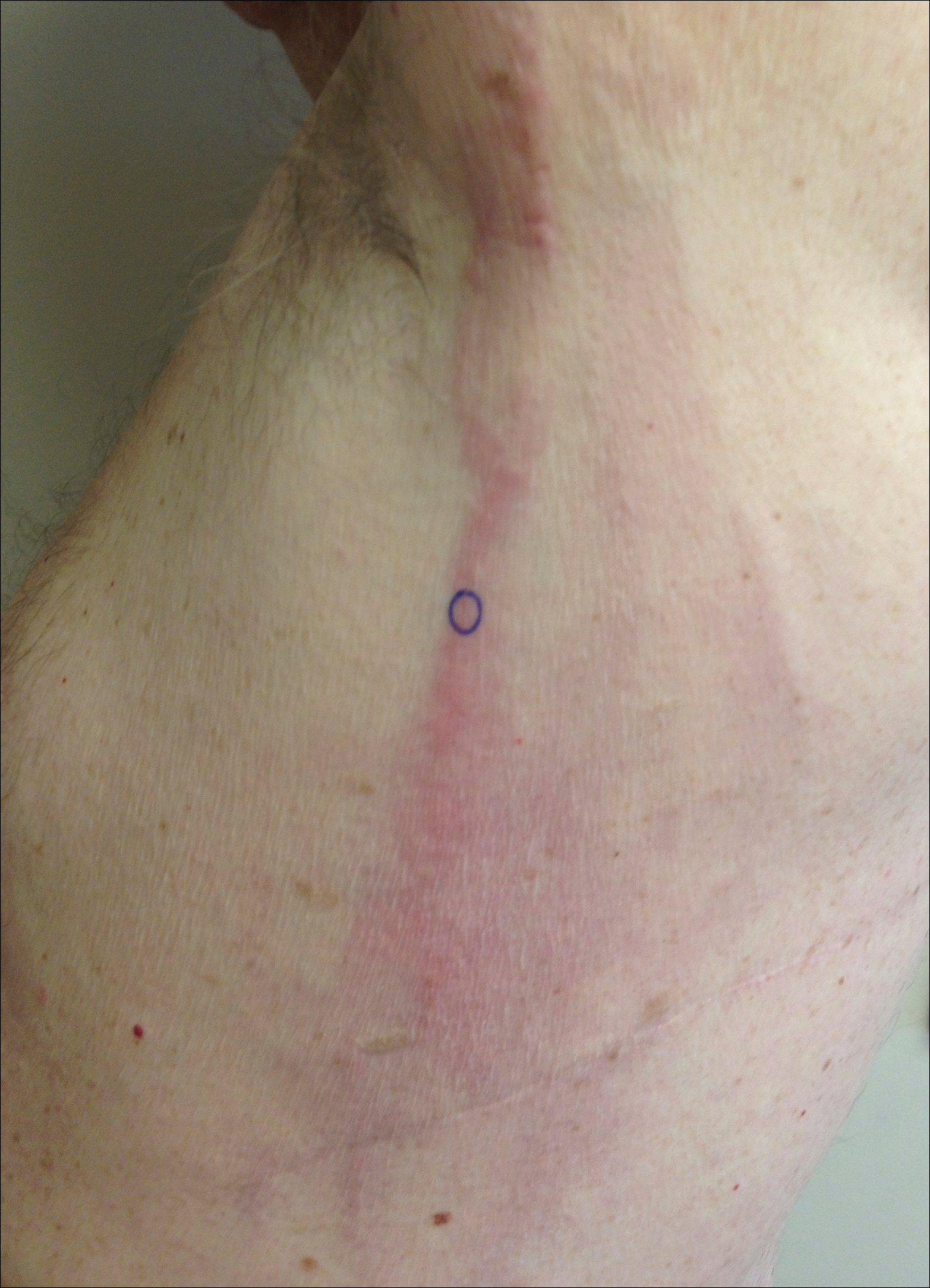
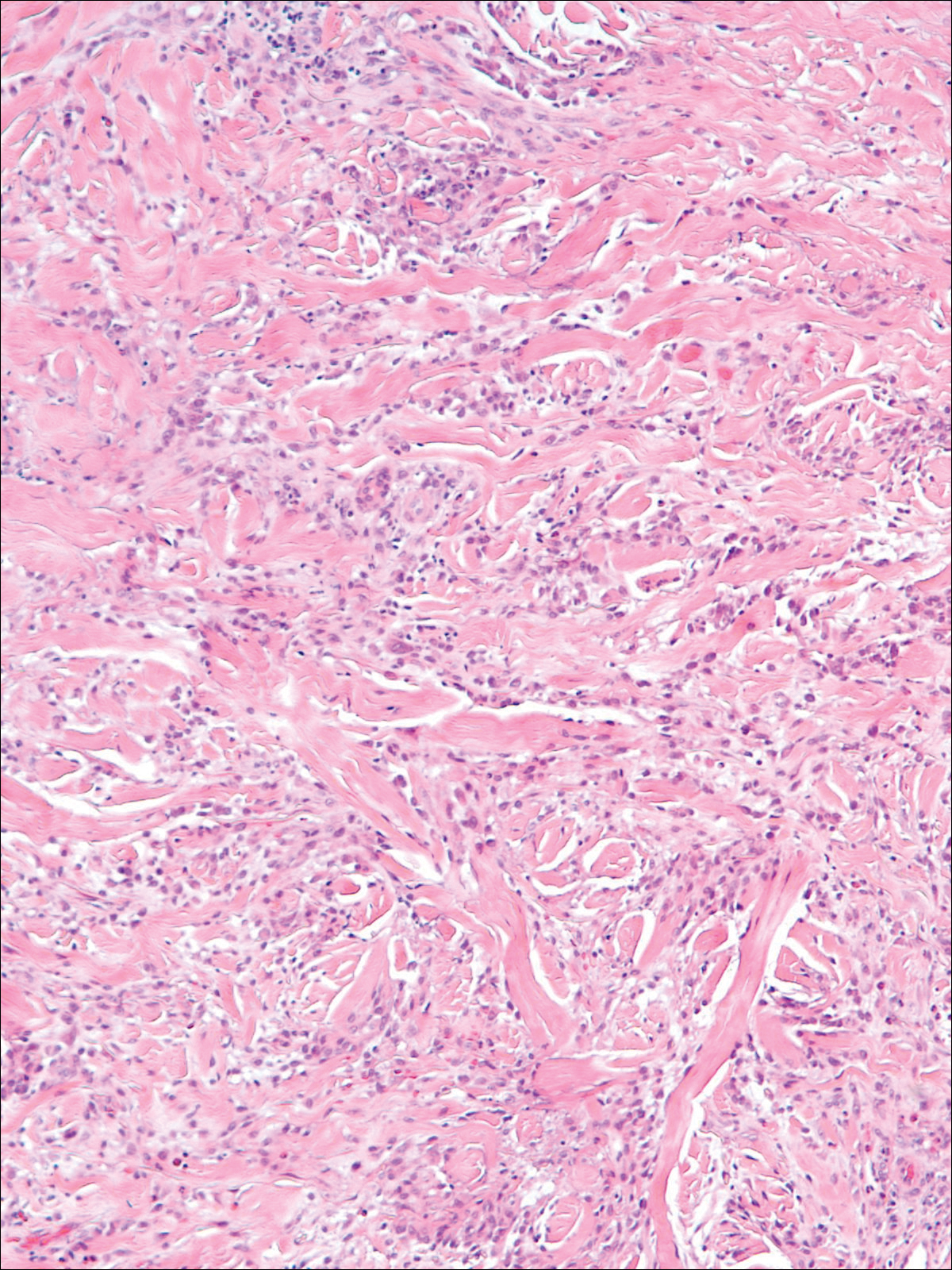
A 74-year-old man presented with a rash on the flank and back with associated pruritus and occasional pain of 2 months’ duration. His primary care physician prescribed a course of cephalexin, but the rash did not improve. Review of systems was positive for intermittent swelling of the hands, feet, and lips, and negative for arthritis. His medical history included 2 episodes of rheumatic fever, one complicated by pneumonia. His medications included finasteride, simvastatin, bisoprolol-hydrochlorothiazide, aspirin, tiotropium, vitamin D, and fish oil. At presentation, physical examination revealed tender violaceous plaques with induration and central clearing distributed on the left side of the back, left side of the flank, and left axilla. The lesion on the axilla measured 30.0×3.5 cm and the lesions on the left side of the back measured 30.0×9.0 cm. The rims of the lesions were elevated and consistent with the rope sign (Figure 3). A punch biopsy of the lesion on the left axilla showed perivascular and interstitial infiltrate of lymphocytes, neutrophils, histiocytes, and eosinophils. There was no evidence of fibrin deposition in the blood vessels. Small areas of necrobiotic collagen surrounded by multinucleated giant cells and lymphocytes were noted (Figure 4). The rash improved spontaneously at the time of suture removal. No treatment was initiated.
Granulomatous dermatitis in the presence of an autoimmune disorder can present as IGD or PNGD. Both forms of granulomatous dermatitis are rare conditions and considered to be part of the same clinicopathological spectrum. These conditions can be difficult to distinguish clinically but are histologically unique.
Interstitial granulomatous dermatitis and PNGD can have a variable clinical expression. Palisaded neutrophilic granulomatous dermatitis generally presents as flesh-colored to erythematous papules or plaques, most commonly located on the upper arms. The lesions may have a central umbilication with perforation and ulceration.4 Interstitial granulomatous dermatitis most commonly presents as erythematous plaques and papules. The lesions are symmetric and asymptomatic. They most commonly appear on the trunk, axillae, buttocks, thighs, and groin. Subcutaneous linear cords (the rope sign) is a characteristic associated with IGD.3,5 However, the rope sign also has been reported in a patient with PNGD with systemic lupus,6 which further demonstrates the overlapping spectrum of clinical expression seen in these 2 forms of granulomatous dermatitis. Therefore, a diagnosis cannot be made by clinical expression alone; histologic findings are needed for confirmation.
When differentiating IGD and PNGD histologically, it is important to keep in mind that these features exist on a spectrum and depend on the age of the lesion. Deposition of the immune complex around the dermal blood vessel initiates the pathogenesis. Early lesions of PNGD show a neutrophilic infiltrate, focal leukocytoclastic vasculitis, and dense nuclear dust. Developed lesions show zones of basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris.2 The histologic pattern of IGD features smaller areas of palisading histiocytes surrounding foci of degenerated collagen. Neutrophils and eosinophils are seen among the degenerated collagen. There is no evidence of vasculitis and dermal mucin usually is absent.7
Palisaded neutrophilic granulomatous dermatitis has been reported to improve with systemic steroids and dapsone.8 Th
Some authors have disputed the spectrum that Chu et al2 had determined in their study and proposed IGD is a separate entity from the PNGD spectrum. Verneuil et al9 stated that the clinical presentations in Chu et al’s2 study (symmetric papules of the extremities) had not been reported in a patient with IGD. However, in a study of IGD by Peroni et al,3 7 of 12 patients presented with symmetrical papules of the extremities. We believe that the spectrum proposed by Chu et al2 still holds true.
These 2 reports demonstrate the diverse presentation of IGD and PNGD. It is important for dermatologists to keep in mind the PNGD spectrum when a patient presents with granulomatous dermatitis in the presence of an autoimmune disorder.
- Ackerman AB, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Am Society Clin Pathol. 1993;3:309-312.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Hantash BM, Chiang D, Kohler S, et al. Palisaded neutrophilic and granulomatous dermatitis associated with limited systemic sclerosis. J Am Acad Dermatol. 2008;58:661-664.
- Garcia-Rabasco A, Esteve-Martinez A, Zaragoza-Ninet V, et al. Interstitial granulomatous dermatitis in a patient with lupus erythematosus. Am J Dermatopathol. 2011;33:871-872.
- Gulati A, Paige D, Yaqoob M, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J Am Acad Dermatol. 2009;61:711-714.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Fett N, Kovarik C, Bennett D. Palisaded neutrophilic granulomatous dermatitis without a definable underlying disorder treated with dapsone. J Am Acad Dermatol. 2011;65:E92-E93.
- Verneuil L, Dompmartin A, Comoz F, et al. Interstitial granulomatous dermatitis with cutaneous cords and arthritis: a disorder associated with autoantibodies. J Am Acad Dermatol. 2001;45:286-291.
To the Editor:
Palisaded neutrophilic granulomatous dermatitis (PNGD) is a rare disorder that often is associated with systemic disease. It has been shown to manifest in the presence of systemic lupus erythematosus; rheumatoid arthritis; Wegener granulomatosis; and other diseases, mainly autoimmune conditions. Interstitial granulomatous dermatitis (IGD) associated with arthritis was first described by Ackerman et al1 in 1993. In 1994, IGD was placed among the spectrum of PNGD by Chu et al.2 The disease entities included in the spectrum of PNGD of the immune complex disease are Churg-Strauss granuloma, cutaneous extravascular necrotizing granuloma, rheumatoid papules, superficial ulcerating rheumatoid necrobiosis, and IGD with arthritis.2 It has been suggested that IGD has a distinct clinical presentation with associated histopathology, while others suggest it still is part of the PNGD spectrum.2,3 We present 2 cases of granulomatous dermatitis and their findings related to IGD and PNGD.
A 58-year-old woman presented with recurrent painful lesions on the trunk, arms, and legs of 2 years’ duration. The lesions spontaneously resolved without scarring or hyperpigmentation but would recur in different areas on the trunk. She was diagnosed with rheumatoid arthritis following a recent autoimmune workup. At presentation, physical examination revealed tender erythematous edematous plaques on the bilateral upper back (Figure 1) and erythematous nodules on the bilateral upper arms. The patient previously had an antinuclear antibody titer of 1:320 with a speckled pattern. A repeat antinuclear antibody titer taken 1 year later was negative. Her rheumatoid factor initially was positive and remained positive upon repeat testing. Punch biopsies were performed for histologic evaluation of the lesions and immunofluorescence. Biopsies examined with hematoxylin and eosin stain revealed perivascular and interstitial mixed (lymphocytic, neutrophilic, eosinophilic) bottom-heavy inflammation with nuclear dust and basophilic degeneration of collagen (Figure 2). Immunofluorescence studies were negative. The patient deferred treatment.


A 74-year-old man presented with a rash on the flank and back with associated pruritus and occasional pain of 2 months’ duration. His primary care physician prescribed a course of cephalexin, but the rash did not improve. Review of systems was positive for intermittent swelling of the hands, feet, and lips, and negative for arthritis. His medical history included 2 episodes of rheumatic fever, one complicated by pneumonia. His medications included finasteride, simvastatin, bisoprolol-hydrochlorothiazide, aspirin, tiotropium, vitamin D, and fish oil. At presentation, physical examination revealed tender violaceous plaques with induration and central clearing distributed on the left side of the back, left side of the flank, and left axilla. The lesion on the axilla measured 30.0×3.5 cm and the lesions on the left side of the back measured 30.0×9.0 cm. The rims of the lesions were elevated and consistent with the rope sign (Figure 3). A punch biopsy of the lesion on the left axilla showed perivascular and interstitial infiltrate of lymphocytes, neutrophils, histiocytes, and eosinophils. There was no evidence of fibrin deposition in the blood vessels. Small areas of necrobiotic collagen surrounded by multinucleated giant cells and lymphocytes were noted (Figure 4). The rash improved spontaneously at the time of suture removal. No treatment was initiated.


Granulomatous dermatitis in the presence of an autoimmune disorder can present as IGD or PNGD. Both forms of granulomatous dermatitis are rare conditions and considered to be part of the same clinicopathological spectrum. These conditions can be difficult to distinguish clinically but are histologically unique.
Interstitial granulomatous dermatitis and PNGD can have a variable clinical expression. Palisaded neutrophilic granulomatous dermatitis generally presents as flesh-colored to erythematous papules or plaques, most commonly located on the upper arms. The lesions may have a central umbilication with perforation and ulceration.4 Interstitial granulomatous dermatitis most commonly presents as erythematous plaques and papules. The lesions are symmetric and asymptomatic. They most commonly appear on the trunk, axillae, buttocks, thighs, and groin. Subcutaneous linear cords (the rope sign) is a characteristic associated with IGD.3,5 However, the rope sign also has been reported in a patient with PNGD with systemic lupus,6 which further demonstrates the overlapping spectrum of clinical expression seen in these 2 forms of granulomatous dermatitis. Therefore, a diagnosis cannot be made by clinical expression alone; histologic findings are needed for confirmation.
When differentiating IGD and PNGD histologically, it is important to keep in mind that these features exist on a spectrum and depend on the age of the lesion. Deposition of the immune complex around the dermal blood vessel initiates the pathogenesis. Early lesions of PNGD show a neutrophilic infiltrate, focal leukocytoclastic vasculitis, and dense nuclear dust. Developed lesions show zones of basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris.2 The histologic pattern of IGD features smaller areas of palisading histiocytes surrounding foci of degenerated collagen. Neutrophils and eosinophils are seen among the degenerated collagen. There is no evidence of vasculitis and dermal mucin usually is absent.7
Palisaded neutrophilic granulomatous dermatitis has been reported to improve with systemic steroids and dapsone.8 Th
Some authors have disputed the spectrum that Chu et al2 had determined in their study and proposed IGD is a separate entity from the PNGD spectrum. Verneuil et al9 stated that the clinical presentations in Chu et al’s2 study (symmetric papules of the extremities) had not been reported in a patient with IGD. However, in a study of IGD by Peroni et al,3 7 of 12 patients presented with symmetrical papules of the extremities. We believe that the spectrum proposed by Chu et al2 still holds true.
These 2 reports demonstrate the diverse presentation of IGD and PNGD. It is important for dermatologists to keep in mind the PNGD spectrum when a patient presents with granulomatous dermatitis in the presence of an autoimmune disorder.
To the Editor:
Palisaded neutrophilic granulomatous dermatitis (PNGD) is a rare disorder that often is associated with systemic disease. It has been shown to manifest in the presence of systemic lupus erythematosus; rheumatoid arthritis; Wegener granulomatosis; and other diseases, mainly autoimmune conditions. Interstitial granulomatous dermatitis (IGD) associated with arthritis was first described by Ackerman et al1 in 1993. In 1994, IGD was placed among the spectrum of PNGD by Chu et al.2 The disease entities included in the spectrum of PNGD of the immune complex disease are Churg-Strauss granuloma, cutaneous extravascular necrotizing granuloma, rheumatoid papules, superficial ulcerating rheumatoid necrobiosis, and IGD with arthritis.2 It has been suggested that IGD has a distinct clinical presentation with associated histopathology, while others suggest it still is part of the PNGD spectrum.2,3 We present 2 cases of granulomatous dermatitis and their findings related to IGD and PNGD.
A 58-year-old woman presented with recurrent painful lesions on the trunk, arms, and legs of 2 years’ duration. The lesions spontaneously resolved without scarring or hyperpigmentation but would recur in different areas on the trunk. She was diagnosed with rheumatoid arthritis following a recent autoimmune workup. At presentation, physical examination revealed tender erythematous edematous plaques on the bilateral upper back (Figure 1) and erythematous nodules on the bilateral upper arms. The patient previously had an antinuclear antibody titer of 1:320 with a speckled pattern. A repeat antinuclear antibody titer taken 1 year later was negative. Her rheumatoid factor initially was positive and remained positive upon repeat testing. Punch biopsies were performed for histologic evaluation of the lesions and immunofluorescence. Biopsies examined with hematoxylin and eosin stain revealed perivascular and interstitial mixed (lymphocytic, neutrophilic, eosinophilic) bottom-heavy inflammation with nuclear dust and basophilic degeneration of collagen (Figure 2). Immunofluorescence studies were negative. The patient deferred treatment.


A 74-year-old man presented with a rash on the flank and back with associated pruritus and occasional pain of 2 months’ duration. His primary care physician prescribed a course of cephalexin, but the rash did not improve. Review of systems was positive for intermittent swelling of the hands, feet, and lips, and negative for arthritis. His medical history included 2 episodes of rheumatic fever, one complicated by pneumonia. His medications included finasteride, simvastatin, bisoprolol-hydrochlorothiazide, aspirin, tiotropium, vitamin D, and fish oil. At presentation, physical examination revealed tender violaceous plaques with induration and central clearing distributed on the left side of the back, left side of the flank, and left axilla. The lesion on the axilla measured 30.0×3.5 cm and the lesions on the left side of the back measured 30.0×9.0 cm. The rims of the lesions were elevated and consistent with the rope sign (Figure 3). A punch biopsy of the lesion on the left axilla showed perivascular and interstitial infiltrate of lymphocytes, neutrophils, histiocytes, and eosinophils. There was no evidence of fibrin deposition in the blood vessels. Small areas of necrobiotic collagen surrounded by multinucleated giant cells and lymphocytes were noted (Figure 4). The rash improved spontaneously at the time of suture removal. No treatment was initiated.


Granulomatous dermatitis in the presence of an autoimmune disorder can present as IGD or PNGD. Both forms of granulomatous dermatitis are rare conditions and considered to be part of the same clinicopathological spectrum. These conditions can be difficult to distinguish clinically but are histologically unique.
Interstitial granulomatous dermatitis and PNGD can have a variable clinical expression. Palisaded neutrophilic granulomatous dermatitis generally presents as flesh-colored to erythematous papules or plaques, most commonly located on the upper arms. The lesions may have a central umbilication with perforation and ulceration.4 Interstitial granulomatous dermatitis most commonly presents as erythematous plaques and papules. The lesions are symmetric and asymptomatic. They most commonly appear on the trunk, axillae, buttocks, thighs, and groin. Subcutaneous linear cords (the rope sign) is a characteristic associated with IGD.3,5 However, the rope sign also has been reported in a patient with PNGD with systemic lupus,6 which further demonstrates the overlapping spectrum of clinical expression seen in these 2 forms of granulomatous dermatitis. Therefore, a diagnosis cannot be made by clinical expression alone; histologic findings are needed for confirmation.
When differentiating IGD and PNGD histologically, it is important to keep in mind that these features exist on a spectrum and depend on the age of the lesion. Deposition of the immune complex around the dermal blood vessel initiates the pathogenesis. Early lesions of PNGD show a neutrophilic infiltrate, focal leukocytoclastic vasculitis, and dense nuclear dust. Developed lesions show zones of basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris.2 The histologic pattern of IGD features smaller areas of palisading histiocytes surrounding foci of degenerated collagen. Neutrophils and eosinophils are seen among the degenerated collagen. There is no evidence of vasculitis and dermal mucin usually is absent.7
Palisaded neutrophilic granulomatous dermatitis has been reported to improve with systemic steroids and dapsone.8 Th
Some authors have disputed the spectrum that Chu et al2 had determined in their study and proposed IGD is a separate entity from the PNGD spectrum. Verneuil et al9 stated that the clinical presentations in Chu et al’s2 study (symmetric papules of the extremities) had not been reported in a patient with IGD. However, in a study of IGD by Peroni et al,3 7 of 12 patients presented with symmetrical papules of the extremities. We believe that the spectrum proposed by Chu et al2 still holds true.
These 2 reports demonstrate the diverse presentation of IGD and PNGD. It is important for dermatologists to keep in mind the PNGD spectrum when a patient presents with granulomatous dermatitis in the presence of an autoimmune disorder.
- Ackerman AB, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Am Society Clin Pathol. 1993;3:309-312.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Hantash BM, Chiang D, Kohler S, et al. Palisaded neutrophilic and granulomatous dermatitis associated with limited systemic sclerosis. J Am Acad Dermatol. 2008;58:661-664.
- Garcia-Rabasco A, Esteve-Martinez A, Zaragoza-Ninet V, et al. Interstitial granulomatous dermatitis in a patient with lupus erythematosus. Am J Dermatopathol. 2011;33:871-872.
- Gulati A, Paige D, Yaqoob M, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J Am Acad Dermatol. 2009;61:711-714.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Fett N, Kovarik C, Bennett D. Palisaded neutrophilic granulomatous dermatitis without a definable underlying disorder treated with dapsone. J Am Acad Dermatol. 2011;65:E92-E93.
- Verneuil L, Dompmartin A, Comoz F, et al. Interstitial granulomatous dermatitis with cutaneous cords and arthritis: a disorder associated with autoantibodies. J Am Acad Dermatol. 2001;45:286-291.
- Ackerman AB, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Am Society Clin Pathol. 1993;3:309-312.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Hantash BM, Chiang D, Kohler S, et al. Palisaded neutrophilic and granulomatous dermatitis associated with limited systemic sclerosis. J Am Acad Dermatol. 2008;58:661-664.
- Garcia-Rabasco A, Esteve-Martinez A, Zaragoza-Ninet V, et al. Interstitial granulomatous dermatitis in a patient with lupus erythematosus. Am J Dermatopathol. 2011;33:871-872.
- Gulati A, Paige D, Yaqoob M, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J Am Acad Dermatol. 2009;61:711-714.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Fett N, Kovarik C, Bennett D. Palisaded neutrophilic granulomatous dermatitis without a definable underlying disorder treated with dapsone. J Am Acad Dermatol. 2011;65:E92-E93.
- Verneuil L, Dompmartin A, Comoz F, et al. Interstitial granulomatous dermatitis with cutaneous cords and arthritis: a disorder associated with autoantibodies. J Am Acad Dermatol. 2001;45:286-291.
Practice Points
- The clinical features of interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis exist on a spectrum, and these is considerable overlap between the features of these 2 clinicopathologic entities.
- Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis may respond to systemic steroids or treatment of the underlying systemic disease. Some cases spontaneously resolve.
Tinea Incognito in a Tattoo
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
Practice Points
- Health care providers should have a low threshold to perform a potassium hydroxide preparation when the possibility of a superficial fungal infection is considered.
- Tinea incognito occurs when a superficial fungal infection has unusual clinical features in the setting of local immune suppression.
Secondary Syphilis: An Atypical Presentation Complicated by a False Negative Rapid Plasma Reagin Test
To the Editor:
According to the Centers for Disease Control and Prevention, the number of syphilis cases in the United States decreased 95% from 1945 to 2000.1 Since 2000, the number of cases of syphilis in the United States has increased from 2.1 cases per 100,000 to 8.7 cases per 100,000.1 We report the case of an atypical presentation of secondary syphilis with a false negative rapid plasma reagin (RPR) test, which resulted in delayed diagnosis and treatment. The goal of this report is to raise awareness of the increasing prevalence of syphilis in the United States, draw attention to atypical presentations of syphilis, and inform physicians of some of the pitfalls in current syphilis screening and testing modalities.
A 37-year-old man presented with cutaneous ulcers on the forehead, thighs, and forearms of 3 months’ duration. The lesions started as a scarlet fever–like rash consisting of diffuse boils that would burst and become ulcerated. He reported arthralgias and drenching night sweats and had unintentionally lost 20 pounds over the last 3 months. He also had pharyngitis 8 months prior to presentation and sinusitis 4 months prior to presentation. These symptoms were present during his initial evaluation. One month prior to the current presentation, a nurse practitioner from an outside clinic had prescribed sulfamethoxazole/trimethoprim and ordered an RPR test, which was nonreactive. The lesions did not resolve, and the patient was referred to our dermatology department.
On physical examination, multiple 1- to 3-cm erythematous, well-defined papules were noted on the thighs and forearms. Some of the papules were covered with crusts, some were ulcerated with yellow discharge, and all were nontender. The differential diagnoses included dermatomyositis, polyarteritis nodosa, deep fungal infection, mycobacterial infection, leishmaniasis, and cutaneous anthrax. Secondary syphilis was a possible differential but was discounted due to the nonreactive RPR 1 month prior to presentation.
Punch biopsies were collected from lesions on the forehead, forearms, and thighs and sent to multiple institutions for pathology evaluation, which revealed dermal and pannicular necrosis and acute suppurative and granulomatous inflammation focally involving vessels (Figure 1). The biopsies were negative for acid-fast and fungal organisms, Mycobacterium tuberculosis, Leishmania, and anthrax. A work-up for Wegener granulomatosis was recommended by the pathology department.
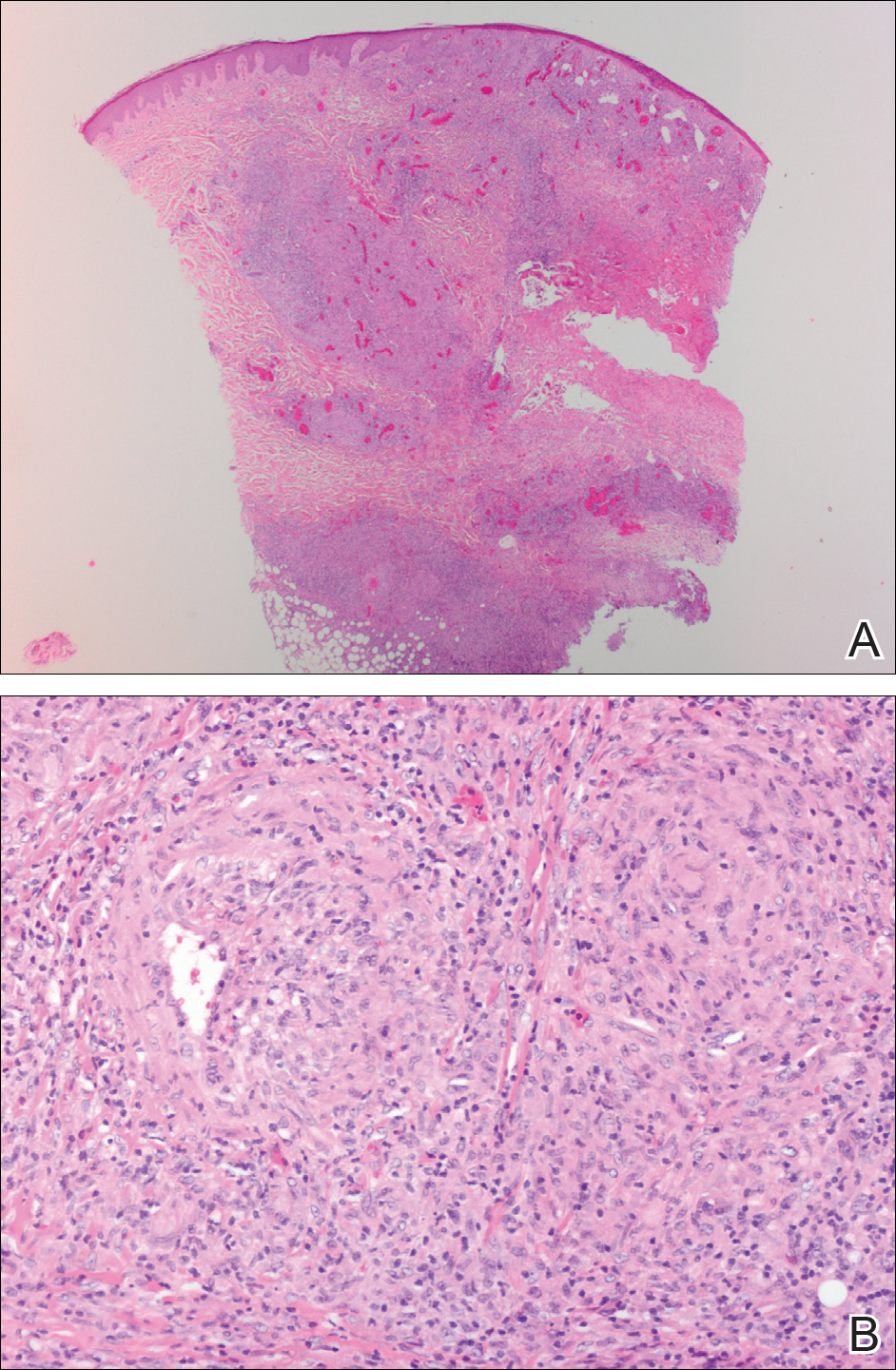
Three days later, the patient was admitted to the hospital for syncope. The hospitalist noted the cutaneous lesions and reordered the RPR test, which was now reactive. The ulcers had worsened since the original presentation (Figure 2). A fluorescent treponemal antibody absorption (FTA-ABS) test confirmed the reactive RPR, and a diagnosis of secondary syphilis was made. He was allergic to penicillin G, so the patient was prescribed doxycycline 100 mg twice daily for 28 days. His cutaneous ulcers have since healed with no recurrence of symptoms.
Secondary syphilis often is preceded by a prodrome of fever, malaise, sore throat, adenopathy, unintentional weight loss, myalgias, and headaches. It usually presents as a nonpruritic papulosquamous eruption with painless mucosal ulcers but rarely presents as cutaneous ulcers.2-4 Cutaneous ulcers are typical of lues maligna, which usually occurs in immunosuppressed patients.5,6 Our patient was human immunodeficiency virus–negative and was not otherwise immunocompromised.

Rapid plasma reagin is a common screening test for syphilis. In this case, it was initially negative, which may be attributed to the prozone phenomenon, a false negative result due to a high antibody titer that prevents the flocculation reaction from occurring. The prozone phenomenon can occur with a titer as low as 1:8.7 A 50% dilution of the negative sample should overcome the prozone phenomenon and yield a positive result7; unfortunately, this is not standard practice in all hospital laboratories.
The standard method of diagnosing syphilis in the United States is to screen with nontreponemal tests (eg, RPR) followed by treponemal tests (eg, FTA-ABS) to confirm a positive screen. According to the United States Preventive Services Task Force, the sensitivity of the RPR test is approximately 78% to 86%, while FTA-ABS has a sensitivity of 84% for detecting primary syphilis and 100% for secondary and tertiary syphilis.8 Seña et al4 suggest that FTA-ABS should be used as the screening test for syphilis. Fluorescent treponemal antibody absorption testing more accurately detects syphilis, while RPR testing is more useful in monitoring serum response once treatment has been initiated.
In conclusion, our patient could have benefited from earlier diagnosis and treatment if a treponemal test had been performed earlier or if the initial nonreactive RPR test was diluted and retested.
Acknowledgments
We would like to acknowledge Dr. Timothy Weiland (Pathology Department, Altru Health System, Grand Forks, North Dakota), and Dr. Mark Koponen (University of North Dakota, Grand Forks).
- Syphilis—CDC fact sheet. Centers for Disease Control and Prevention website. http://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Updated June 13, 2017. Accessed May 18, 2018.
- Stary A, Stary G. Sexually transmitted infections. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:1368-1426.
- Habif TP. Sexually transmitted bacterial infections. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:377-417.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Bayramgürler D, Bilen N, Yıldız K, et al. Lues maligna in a chronic alcoholic patient. J Dermatol. 2005;32:217-219.
- Bhate C, Tajirian AL, Kapila R, et al. Secondary syphilis resembling erythema multiforme. Int J Dermatol. 2010;49:1321-1324.
- Liu LL, Lin LR, Tong ML, et al. Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin Infect Dis. 2014;59:384-389.
- Archived final recommendation statement. syphilis infection: screening. US Preventive Services Task Force website. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/syphilis-infection-screening. Published December 30, 2013. Accessed May 22, 2018.
To the Editor:
According to the Centers for Disease Control and Prevention, the number of syphilis cases in the United States decreased 95% from 1945 to 2000.1 Since 2000, the number of cases of syphilis in the United States has increased from 2.1 cases per 100,000 to 8.7 cases per 100,000.1 We report the case of an atypical presentation of secondary syphilis with a false negative rapid plasma reagin (RPR) test, which resulted in delayed diagnosis and treatment. The goal of this report is to raise awareness of the increasing prevalence of syphilis in the United States, draw attention to atypical presentations of syphilis, and inform physicians of some of the pitfalls in current syphilis screening and testing modalities.
A 37-year-old man presented with cutaneous ulcers on the forehead, thighs, and forearms of 3 months’ duration. The lesions started as a scarlet fever–like rash consisting of diffuse boils that would burst and become ulcerated. He reported arthralgias and drenching night sweats and had unintentionally lost 20 pounds over the last 3 months. He also had pharyngitis 8 months prior to presentation and sinusitis 4 months prior to presentation. These symptoms were present during his initial evaluation. One month prior to the current presentation, a nurse practitioner from an outside clinic had prescribed sulfamethoxazole/trimethoprim and ordered an RPR test, which was nonreactive. The lesions did not resolve, and the patient was referred to our dermatology department.
On physical examination, multiple 1- to 3-cm erythematous, well-defined papules were noted on the thighs and forearms. Some of the papules were covered with crusts, some were ulcerated with yellow discharge, and all were nontender. The differential diagnoses included dermatomyositis, polyarteritis nodosa, deep fungal infection, mycobacterial infection, leishmaniasis, and cutaneous anthrax. Secondary syphilis was a possible differential but was discounted due to the nonreactive RPR 1 month prior to presentation.
Punch biopsies were collected from lesions on the forehead, forearms, and thighs and sent to multiple institutions for pathology evaluation, which revealed dermal and pannicular necrosis and acute suppurative and granulomatous inflammation focally involving vessels (Figure 1). The biopsies were negative for acid-fast and fungal organisms, Mycobacterium tuberculosis, Leishmania, and anthrax. A work-up for Wegener granulomatosis was recommended by the pathology department.

Three days later, the patient was admitted to the hospital for syncope. The hospitalist noted the cutaneous lesions and reordered the RPR test, which was now reactive. The ulcers had worsened since the original presentation (Figure 2). A fluorescent treponemal antibody absorption (FTA-ABS) test confirmed the reactive RPR, and a diagnosis of secondary syphilis was made. He was allergic to penicillin G, so the patient was prescribed doxycycline 100 mg twice daily for 28 days. His cutaneous ulcers have since healed with no recurrence of symptoms.
Secondary syphilis often is preceded by a prodrome of fever, malaise, sore throat, adenopathy, unintentional weight loss, myalgias, and headaches. It usually presents as a nonpruritic papulosquamous eruption with painless mucosal ulcers but rarely presents as cutaneous ulcers.2-4 Cutaneous ulcers are typical of lues maligna, which usually occurs in immunosuppressed patients.5,6 Our patient was human immunodeficiency virus–negative and was not otherwise immunocompromised.

Rapid plasma reagin is a common screening test for syphilis. In this case, it was initially negative, which may be attributed to the prozone phenomenon, a false negative result due to a high antibody titer that prevents the flocculation reaction from occurring. The prozone phenomenon can occur with a titer as low as 1:8.7 A 50% dilution of the negative sample should overcome the prozone phenomenon and yield a positive result7; unfortunately, this is not standard practice in all hospital laboratories.
The standard method of diagnosing syphilis in the United States is to screen with nontreponemal tests (eg, RPR) followed by treponemal tests (eg, FTA-ABS) to confirm a positive screen. According to the United States Preventive Services Task Force, the sensitivity of the RPR test is approximately 78% to 86%, while FTA-ABS has a sensitivity of 84% for detecting primary syphilis and 100% for secondary and tertiary syphilis.8 Seña et al4 suggest that FTA-ABS should be used as the screening test for syphilis. Fluorescent treponemal antibody absorption testing more accurately detects syphilis, while RPR testing is more useful in monitoring serum response once treatment has been initiated.
In conclusion, our patient could have benefited from earlier diagnosis and treatment if a treponemal test had been performed earlier or if the initial nonreactive RPR test was diluted and retested.
Acknowledgments
We would like to acknowledge Dr. Timothy Weiland (Pathology Department, Altru Health System, Grand Forks, North Dakota), and Dr. Mark Koponen (University of North Dakota, Grand Forks).
To the Editor:
According to the Centers for Disease Control and Prevention, the number of syphilis cases in the United States decreased 95% from 1945 to 2000.1 Since 2000, the number of cases of syphilis in the United States has increased from 2.1 cases per 100,000 to 8.7 cases per 100,000.1 We report the case of an atypical presentation of secondary syphilis with a false negative rapid plasma reagin (RPR) test, which resulted in delayed diagnosis and treatment. The goal of this report is to raise awareness of the increasing prevalence of syphilis in the United States, draw attention to atypical presentations of syphilis, and inform physicians of some of the pitfalls in current syphilis screening and testing modalities.
A 37-year-old man presented with cutaneous ulcers on the forehead, thighs, and forearms of 3 months’ duration. The lesions started as a scarlet fever–like rash consisting of diffuse boils that would burst and become ulcerated. He reported arthralgias and drenching night sweats and had unintentionally lost 20 pounds over the last 3 months. He also had pharyngitis 8 months prior to presentation and sinusitis 4 months prior to presentation. These symptoms were present during his initial evaluation. One month prior to the current presentation, a nurse practitioner from an outside clinic had prescribed sulfamethoxazole/trimethoprim and ordered an RPR test, which was nonreactive. The lesions did not resolve, and the patient was referred to our dermatology department.
On physical examination, multiple 1- to 3-cm erythematous, well-defined papules were noted on the thighs and forearms. Some of the papules were covered with crusts, some were ulcerated with yellow discharge, and all were nontender. The differential diagnoses included dermatomyositis, polyarteritis nodosa, deep fungal infection, mycobacterial infection, leishmaniasis, and cutaneous anthrax. Secondary syphilis was a possible differential but was discounted due to the nonreactive RPR 1 month prior to presentation.
Punch biopsies were collected from lesions on the forehead, forearms, and thighs and sent to multiple institutions for pathology evaluation, which revealed dermal and pannicular necrosis and acute suppurative and granulomatous inflammation focally involving vessels (Figure 1). The biopsies were negative for acid-fast and fungal organisms, Mycobacterium tuberculosis, Leishmania, and anthrax. A work-up for Wegener granulomatosis was recommended by the pathology department.

Three days later, the patient was admitted to the hospital for syncope. The hospitalist noted the cutaneous lesions and reordered the RPR test, which was now reactive. The ulcers had worsened since the original presentation (Figure 2). A fluorescent treponemal antibody absorption (FTA-ABS) test confirmed the reactive RPR, and a diagnosis of secondary syphilis was made. He was allergic to penicillin G, so the patient was prescribed doxycycline 100 mg twice daily for 28 days. His cutaneous ulcers have since healed with no recurrence of symptoms.
Secondary syphilis often is preceded by a prodrome of fever, malaise, sore throat, adenopathy, unintentional weight loss, myalgias, and headaches. It usually presents as a nonpruritic papulosquamous eruption with painless mucosal ulcers but rarely presents as cutaneous ulcers.2-4 Cutaneous ulcers are typical of lues maligna, which usually occurs in immunosuppressed patients.5,6 Our patient was human immunodeficiency virus–negative and was not otherwise immunocompromised.

Rapid plasma reagin is a common screening test for syphilis. In this case, it was initially negative, which may be attributed to the prozone phenomenon, a false negative result due to a high antibody titer that prevents the flocculation reaction from occurring. The prozone phenomenon can occur with a titer as low as 1:8.7 A 50% dilution of the negative sample should overcome the prozone phenomenon and yield a positive result7; unfortunately, this is not standard practice in all hospital laboratories.
The standard method of diagnosing syphilis in the United States is to screen with nontreponemal tests (eg, RPR) followed by treponemal tests (eg, FTA-ABS) to confirm a positive screen. According to the United States Preventive Services Task Force, the sensitivity of the RPR test is approximately 78% to 86%, while FTA-ABS has a sensitivity of 84% for detecting primary syphilis and 100% for secondary and tertiary syphilis.8 Seña et al4 suggest that FTA-ABS should be used as the screening test for syphilis. Fluorescent treponemal antibody absorption testing more accurately detects syphilis, while RPR testing is more useful in monitoring serum response once treatment has been initiated.
In conclusion, our patient could have benefited from earlier diagnosis and treatment if a treponemal test had been performed earlier or if the initial nonreactive RPR test was diluted and retested.
Acknowledgments
We would like to acknowledge Dr. Timothy Weiland (Pathology Department, Altru Health System, Grand Forks, North Dakota), and Dr. Mark Koponen (University of North Dakota, Grand Forks).
- Syphilis—CDC fact sheet. Centers for Disease Control and Prevention website. http://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Updated June 13, 2017. Accessed May 18, 2018.
- Stary A, Stary G. Sexually transmitted infections. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:1368-1426.
- Habif TP. Sexually transmitted bacterial infections. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:377-417.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Bayramgürler D, Bilen N, Yıldız K, et al. Lues maligna in a chronic alcoholic patient. J Dermatol. 2005;32:217-219.
- Bhate C, Tajirian AL, Kapila R, et al. Secondary syphilis resembling erythema multiforme. Int J Dermatol. 2010;49:1321-1324.
- Liu LL, Lin LR, Tong ML, et al. Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin Infect Dis. 2014;59:384-389.
- Archived final recommendation statement. syphilis infection: screening. US Preventive Services Task Force website. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/syphilis-infection-screening. Published December 30, 2013. Accessed May 22, 2018.
- Syphilis—CDC fact sheet. Centers for Disease Control and Prevention website. http://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Updated June 13, 2017. Accessed May 18, 2018.
- Stary A, Stary G. Sexually transmitted infections. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:1368-1426.
- Habif TP. Sexually transmitted bacterial infections. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:377-417.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Bayramgürler D, Bilen N, Yıldız K, et al. Lues maligna in a chronic alcoholic patient. J Dermatol. 2005;32:217-219.
- Bhate C, Tajirian AL, Kapila R, et al. Secondary syphilis resembling erythema multiforme. Int J Dermatol. 2010;49:1321-1324.
- Liu LL, Lin LR, Tong ML, et al. Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin Infect Dis. 2014;59:384-389.
- Archived final recommendation statement. syphilis infection: screening. US Preventive Services Task Force website. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/syphilis-infection-screening. Published December 30, 2013. Accessed May 22, 2018.
Practice Points
- Fluorescent treponemal antibody absorption testing more accurately detects syphilis than rapid plasma reagin (RPR).
- Rapid plasma reagin testing is more useful in monitoring serum response once treatment has been initiated.
- If only RPR is being performed at your institution, ensure the laboratory is performing serial dilutions to negate the prozone phenomenon.
Solitary Angiokeratoma of the Vulva Mimicking Malignant Melanoma
To the Editor:
Angiokeratoma is a benign vascular tumor characterized by several dilated vessels in the superficial dermis accompanied by epidermal hyperplasia and hyperkeratosis.1 Angiokeratoma of the vulva is a rare clinical finding, usually involving multiple lesions as part of the Fordyce type.2 Solitary angiokeratoma occurs predominantly on the lower legs,3 and although other locations have been described, the presence of a solitary angiokeratoma on the vulva is rare.4 We report 2 cases of solitary angiokeratoma on the vulva that was misdiagnosed as malignant melanoma. Both patients were referred to our center for evaluation and excision.
A 65-year-old woman (patient 1) and a 67-year-old woman (patient 2) presented with a bluish black, growing, asymptomatic lesion on the right (Figure 1) and left labia majora, respectively. Both patients were referred by outside physicians for excision because of suspected malignant melanoma. Physical examinations revealed bluish black globular nodules that measured 0.5 and 0.3 cm in diameter, respectively. Dermoscopy (patient 1) revealed dark lacunae. Histopathologic examination of the vulvar lesion (patient 2) showed dilated, blood-filled, vascular spaces in the papillary dermis, accompanied by overlying acanthosis, hyperkeratosis, and papillomatosis that was consistent with angiokeratoma (Figure 2).
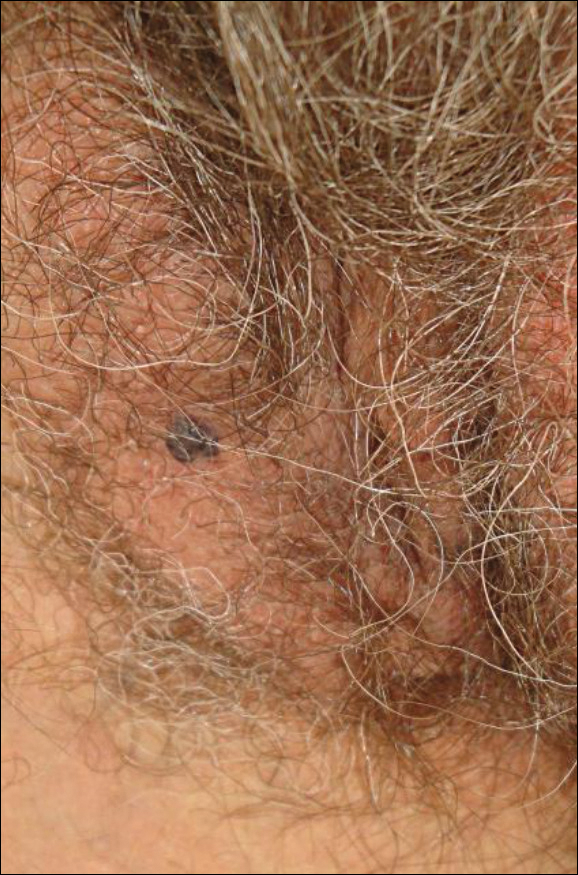
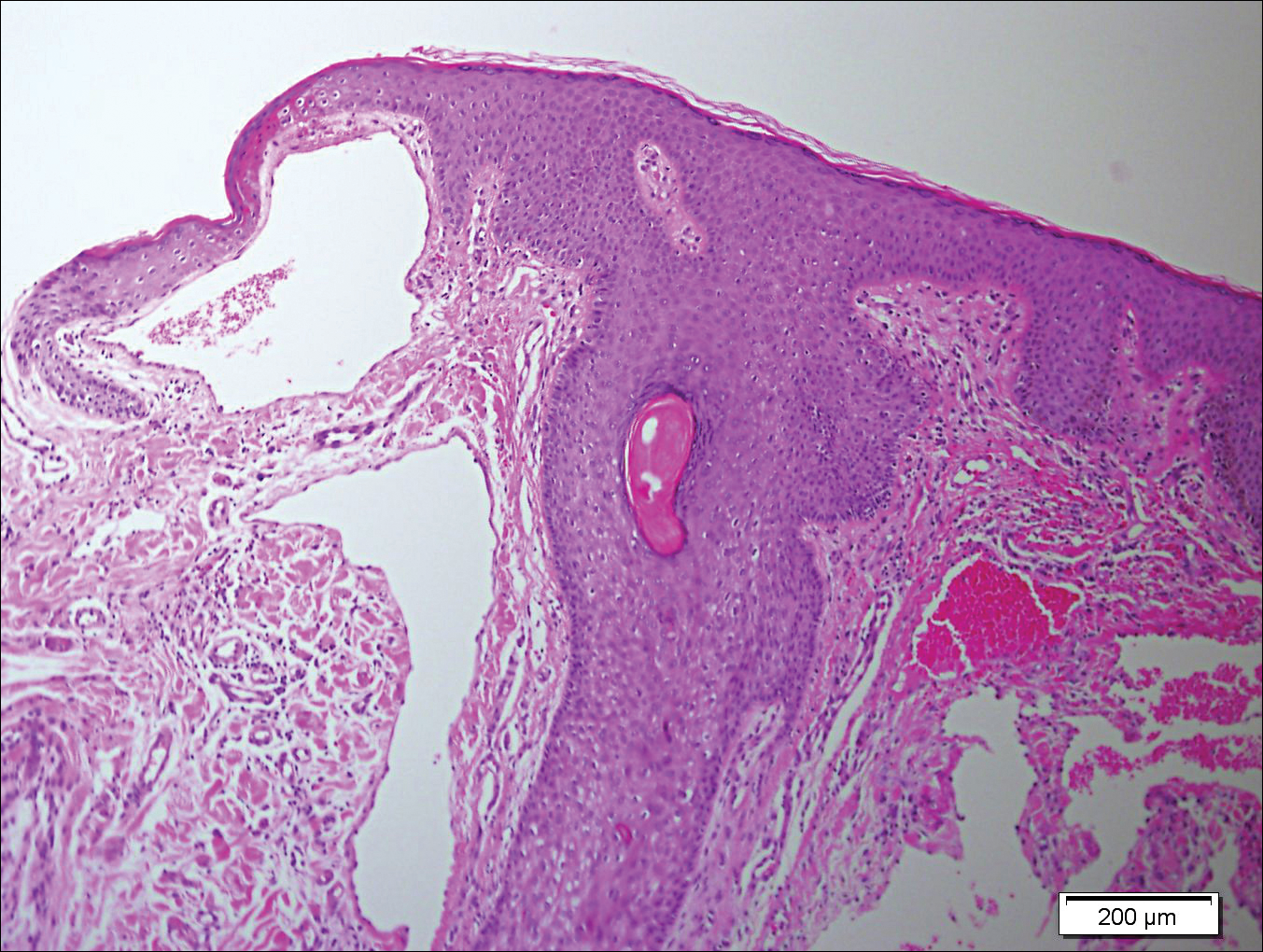
Angiokeratoma, particularly the solitary type, often is misdiagnosed. Clinical differential diagnoses may include a wide range of pathologic conditions, including condyloma acuminata, basal cell carcinoma, pyogenic granuloma, lymphangioma, nevi, condyloma lata, nodular prurigo, seborrheic keratosis, granuloma inguinale, and deep fungal infection.2,5 However, due to its quickly growing nature and its dark complexion, malignant melanoma often is initially diagnosed. Because patients affected by angiokeratoma of the vulva usually are aged 20 to 40 years,5 and vulvar melanoma is typical for middle-aged women (median age, 68 years),6 this misdiagnosis is more likely in older patients. It should be noted that a high index of suspicion for melanoma often is present when examining the vulva, considering that this area is difficult to monitor, and there is an especially poor prognosis of vulvar melanoma due to its late detection.6,7
In the past, biopsy was considered mandatory for confirming the diagnosis of vulvar angiokeratoma.5,8,9 However, dermoscopy has emerged as a valuable tool for diagnosis of angiokeratoma10 and also was helpful as a diagnostic aid in one of our patients (patient 1). Therefore, we believe that dermoscopy should be performed prior to a biopsy of angiokeratomas of the vulva.
- Requena L, Sangueza OP. Cutaneous vascular anomalies. part I. hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
- Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282.
- Gomi H, Eriyama Y, Horikawa E, et al. Solitary angiokeratoma. J Dermatol. 1988;15:349-350.
- Yamazaki M, Hiruma M, Irie H, et al. Angiokeratoma of the clitoris: a subtype of angiokeratoma vulvae. J Dermatol. 1992;19:553-555.
- Cohen PR, Young AW Jr, Tovell HM. Angiokeratoma of the vulva: diagnosis and review of the literature. Obstet Gynecol Surv. 1989;44:339-346.
- Sugiyama VE, Chan JK, Shin JY, et al. Vulvar melanoma: a multivariable analysis of 644 patients. Obstet Gynecol. 2007;110:296-301.
- De Simone P, Silipo V, Buccini P, et al. Vulvar melanoma: a report of 10 cases and review of the literature. Melanoma Res. 2008;18:127-133.
- Novick NL. Angiokeratoma vulvae. J Am Acad Dermatol. 1985;12:561-563.
- Yigiter M, Arda IS, Tosun E, et al. Angiokeratoma of clitoris: a rare lesion in an adolescent girl. Urology. 2008;71:604-606.
- Zaballos P, Daufi C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325.
To the Editor:
Angiokeratoma is a benign vascular tumor characterized by several dilated vessels in the superficial dermis accompanied by epidermal hyperplasia and hyperkeratosis.1 Angiokeratoma of the vulva is a rare clinical finding, usually involving multiple lesions as part of the Fordyce type.2 Solitary angiokeratoma occurs predominantly on the lower legs,3 and although other locations have been described, the presence of a solitary angiokeratoma on the vulva is rare.4 We report 2 cases of solitary angiokeratoma on the vulva that was misdiagnosed as malignant melanoma. Both patients were referred to our center for evaluation and excision.
A 65-year-old woman (patient 1) and a 67-year-old woman (patient 2) presented with a bluish black, growing, asymptomatic lesion on the right (Figure 1) and left labia majora, respectively. Both patients were referred by outside physicians for excision because of suspected malignant melanoma. Physical examinations revealed bluish black globular nodules that measured 0.5 and 0.3 cm in diameter, respectively. Dermoscopy (patient 1) revealed dark lacunae. Histopathologic examination of the vulvar lesion (patient 2) showed dilated, blood-filled, vascular spaces in the papillary dermis, accompanied by overlying acanthosis, hyperkeratosis, and papillomatosis that was consistent with angiokeratoma (Figure 2).


Angiokeratoma, particularly the solitary type, often is misdiagnosed. Clinical differential diagnoses may include a wide range of pathologic conditions, including condyloma acuminata, basal cell carcinoma, pyogenic granuloma, lymphangioma, nevi, condyloma lata, nodular prurigo, seborrheic keratosis, granuloma inguinale, and deep fungal infection.2,5 However, due to its quickly growing nature and its dark complexion, malignant melanoma often is initially diagnosed. Because patients affected by angiokeratoma of the vulva usually are aged 20 to 40 years,5 and vulvar melanoma is typical for middle-aged women (median age, 68 years),6 this misdiagnosis is more likely in older patients. It should be noted that a high index of suspicion for melanoma often is present when examining the vulva, considering that this area is difficult to monitor, and there is an especially poor prognosis of vulvar melanoma due to its late detection.6,7
In the past, biopsy was considered mandatory for confirming the diagnosis of vulvar angiokeratoma.5,8,9 However, dermoscopy has emerged as a valuable tool for diagnosis of angiokeratoma10 and also was helpful as a diagnostic aid in one of our patients (patient 1). Therefore, we believe that dermoscopy should be performed prior to a biopsy of angiokeratomas of the vulva.
To the Editor:
Angiokeratoma is a benign vascular tumor characterized by several dilated vessels in the superficial dermis accompanied by epidermal hyperplasia and hyperkeratosis.1 Angiokeratoma of the vulva is a rare clinical finding, usually involving multiple lesions as part of the Fordyce type.2 Solitary angiokeratoma occurs predominantly on the lower legs,3 and although other locations have been described, the presence of a solitary angiokeratoma on the vulva is rare.4 We report 2 cases of solitary angiokeratoma on the vulva that was misdiagnosed as malignant melanoma. Both patients were referred to our center for evaluation and excision.
A 65-year-old woman (patient 1) and a 67-year-old woman (patient 2) presented with a bluish black, growing, asymptomatic lesion on the right (Figure 1) and left labia majora, respectively. Both patients were referred by outside physicians for excision because of suspected malignant melanoma. Physical examinations revealed bluish black globular nodules that measured 0.5 and 0.3 cm in diameter, respectively. Dermoscopy (patient 1) revealed dark lacunae. Histopathologic examination of the vulvar lesion (patient 2) showed dilated, blood-filled, vascular spaces in the papillary dermis, accompanied by overlying acanthosis, hyperkeratosis, and papillomatosis that was consistent with angiokeratoma (Figure 2).


Angiokeratoma, particularly the solitary type, often is misdiagnosed. Clinical differential diagnoses may include a wide range of pathologic conditions, including condyloma acuminata, basal cell carcinoma, pyogenic granuloma, lymphangioma, nevi, condyloma lata, nodular prurigo, seborrheic keratosis, granuloma inguinale, and deep fungal infection.2,5 However, due to its quickly growing nature and its dark complexion, malignant melanoma often is initially diagnosed. Because patients affected by angiokeratoma of the vulva usually are aged 20 to 40 years,5 and vulvar melanoma is typical for middle-aged women (median age, 68 years),6 this misdiagnosis is more likely in older patients. It should be noted that a high index of suspicion for melanoma often is present when examining the vulva, considering that this area is difficult to monitor, and there is an especially poor prognosis of vulvar melanoma due to its late detection.6,7
In the past, biopsy was considered mandatory for confirming the diagnosis of vulvar angiokeratoma.5,8,9 However, dermoscopy has emerged as a valuable tool for diagnosis of angiokeratoma10 and also was helpful as a diagnostic aid in one of our patients (patient 1). Therefore, we believe that dermoscopy should be performed prior to a biopsy of angiokeratomas of the vulva.
- Requena L, Sangueza OP. Cutaneous vascular anomalies. part I. hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
- Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282.
- Gomi H, Eriyama Y, Horikawa E, et al. Solitary angiokeratoma. J Dermatol. 1988;15:349-350.
- Yamazaki M, Hiruma M, Irie H, et al. Angiokeratoma of the clitoris: a subtype of angiokeratoma vulvae. J Dermatol. 1992;19:553-555.
- Cohen PR, Young AW Jr, Tovell HM. Angiokeratoma of the vulva: diagnosis and review of the literature. Obstet Gynecol Surv. 1989;44:339-346.
- Sugiyama VE, Chan JK, Shin JY, et al. Vulvar melanoma: a multivariable analysis of 644 patients. Obstet Gynecol. 2007;110:296-301.
- De Simone P, Silipo V, Buccini P, et al. Vulvar melanoma: a report of 10 cases and review of the literature. Melanoma Res. 2008;18:127-133.
- Novick NL. Angiokeratoma vulvae. J Am Acad Dermatol. 1985;12:561-563.
- Yigiter M, Arda IS, Tosun E, et al. Angiokeratoma of clitoris: a rare lesion in an adolescent girl. Urology. 2008;71:604-606.
- Zaballos P, Daufi C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325.
- Requena L, Sangueza OP. Cutaneous vascular anomalies. part I. hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
- Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282.
- Gomi H, Eriyama Y, Horikawa E, et al. Solitary angiokeratoma. J Dermatol. 1988;15:349-350.
- Yamazaki M, Hiruma M, Irie H, et al. Angiokeratoma of the clitoris: a subtype of angiokeratoma vulvae. J Dermatol. 1992;19:553-555.
- Cohen PR, Young AW Jr, Tovell HM. Angiokeratoma of the vulva: diagnosis and review of the literature. Obstet Gynecol Surv. 1989;44:339-346.
- Sugiyama VE, Chan JK, Shin JY, et al. Vulvar melanoma: a multivariable analysis of 644 patients. Obstet Gynecol. 2007;110:296-301.
- De Simone P, Silipo V, Buccini P, et al. Vulvar melanoma: a report of 10 cases and review of the literature. Melanoma Res. 2008;18:127-133.
- Novick NL. Angiokeratoma vulvae. J Am Acad Dermatol. 1985;12:561-563.
- Yigiter M, Arda IS, Tosun E, et al. Angiokeratoma of clitoris: a rare lesion in an adolescent girl. Urology. 2008;71:604-606.
- Zaballos P, Daufi C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325.
Practice Points
- Solitary angiokeratoma of the vulva often is misdiagnosed as malignant melanoma due to its rapid growth and dark color.
- Dermoscopy is a valuable tool for diagnosing vulvar angiokeratoma to avoid unnecessary excisions.
Carotenoderma Associated With a Diet Rich in Red Palm Oil
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
Practice Points
- Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands.
- Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment.
Atypical Presentation of Acquired Angioedema
To the Editor:
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.

The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.
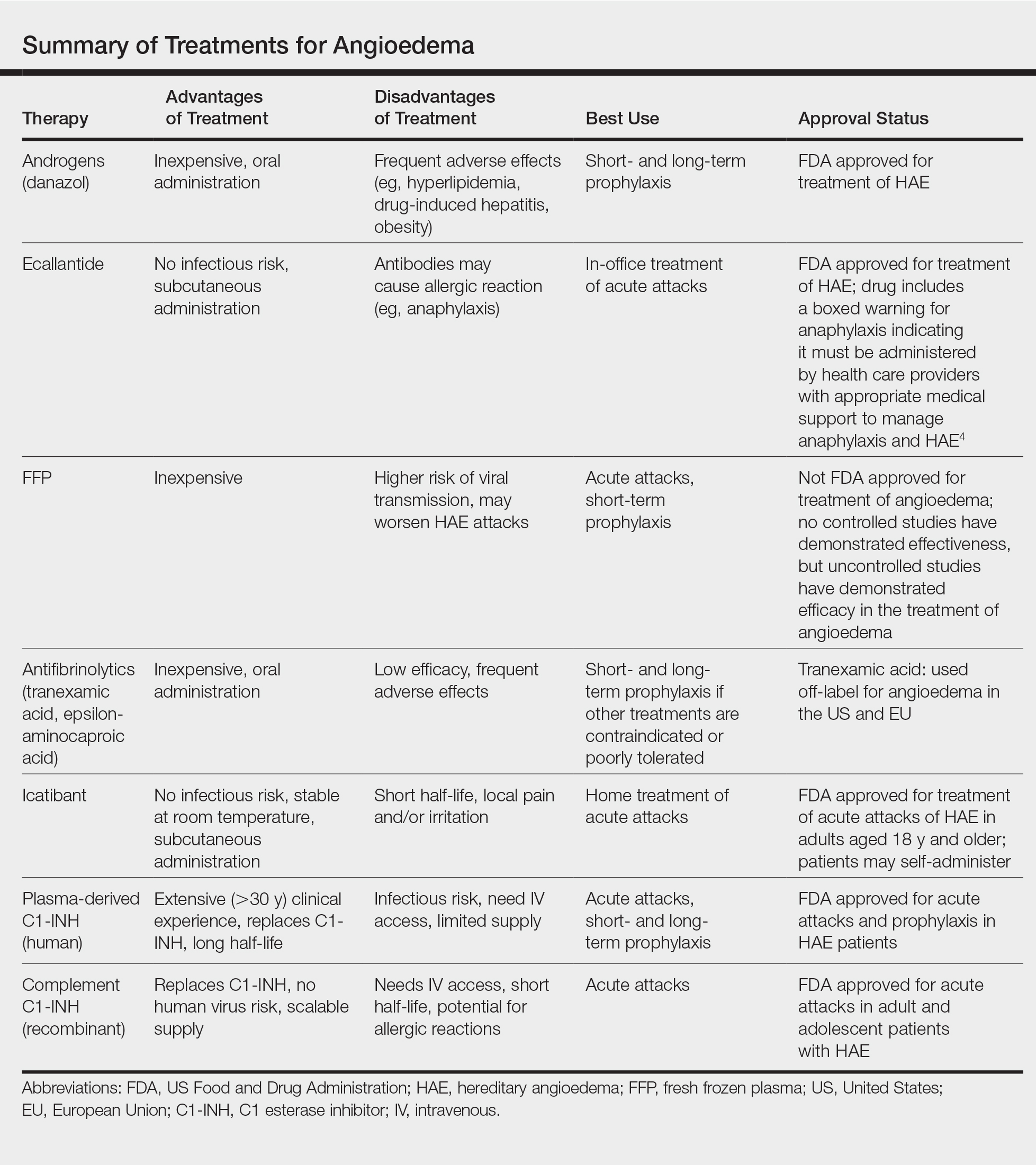
The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.
- Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009;102:366-372.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging connection between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-159.
- Buyantseva LV, Sardana N, Craig TJ. Update on treatment of hereditary angioedema. Asian Pac J Allergy Immunol. 2012;30:89-98.
- Kalbitor [package insert]. Burlington, MA: Dyax Corp; 2015.
To the Editor:
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.

The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.

The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.
To the Editor:
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.

The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.

The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.
- Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009;102:366-372.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging connection between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-159.
- Buyantseva LV, Sardana N, Craig TJ. Update on treatment of hereditary angioedema. Asian Pac J Allergy Immunol. 2012;30:89-98.
- Kalbitor [package insert]. Burlington, MA: Dyax Corp; 2015.
- Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009;102:366-372.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging connection between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-159.
- Buyantseva LV, Sardana N, Craig TJ. Update on treatment of hereditary angioedema. Asian Pac J Allergy Immunol. 2012;30:89-98.
- Kalbitor [package insert]. Burlington, MA: Dyax Corp; 2015.
Practice Points
- Late-onset angioedema without urticaria can be secondary to acquired angioedema with C1 esterase inhibitor deficiency (C1-INH).
- Most patients with angioedema with C1-INH inhibitor deficiency will have either a monoclonal gammopathy or a lymphoma.
Carcinoma Erysipeloides of Papillary Serous Ovarian Cancer Mimicking Cellulitis of the Abdominal Wall
To the Editor:
A 40-year-old woman with a history of stage IIIC ovarian cancer presented with progressing abdominal erythema and pain of 1 month’s duration. She had been diagnosed 4 years prior with grade 3, poorly differentiated papillary serous carcinoma involving the bilateral ovaries, uterine tubes, uterus, and omentum with lymphovascular invasion. She underwent tumor resection and debulking followed by paclitaxel plus platinum-based chemotherapy. The cancer recurred 2 years later with carcinomatous ascites. She declined chemotherapy but underwent therapeutic paracentesis.
One month prior to presentation, the patient developed a small, tender, erythematous patch on the abdomen. Her primary physician started her on cephalexin for presumed cellulitis without improvement. The erythema continued to spread on the abdomen with worsening pain, which prompted her presentation to the emergency department. She was admitted and started on intravenous vancomycin.
On admission to the hospital, the patient was cachexic and afebrile with a white blood cell count of 10,400/µL (reference range, 4500–11,000/µL). Physical examination revealed a well-demarcated, 15×20-cm, erythematous, blanchable, indurated plaque in the periumbilical region (Figure 1). The plaque was tender to palpation with guarding but no increased warmth. Punch biopsies of the abdominal skin revealed carcinoma within the lymphatic channels in the deep dermis and dilated lymphatics throughout the overlying dermis (Figure 2). These findings were diagnostic for carcinoma erysipeloides. Tissue and blood cultures were negative for bacterial, fungal, or mycobacterial growth. Vancomycin was discontinued, and she was discharged with pain medication. She declined chemotherapy due to the potential side effects and elected to continue symptomatic management with palliative paracentesis. After she was discharged, she underwent a tunneled pleural catheterization for recurrent malignant pleural effusions.
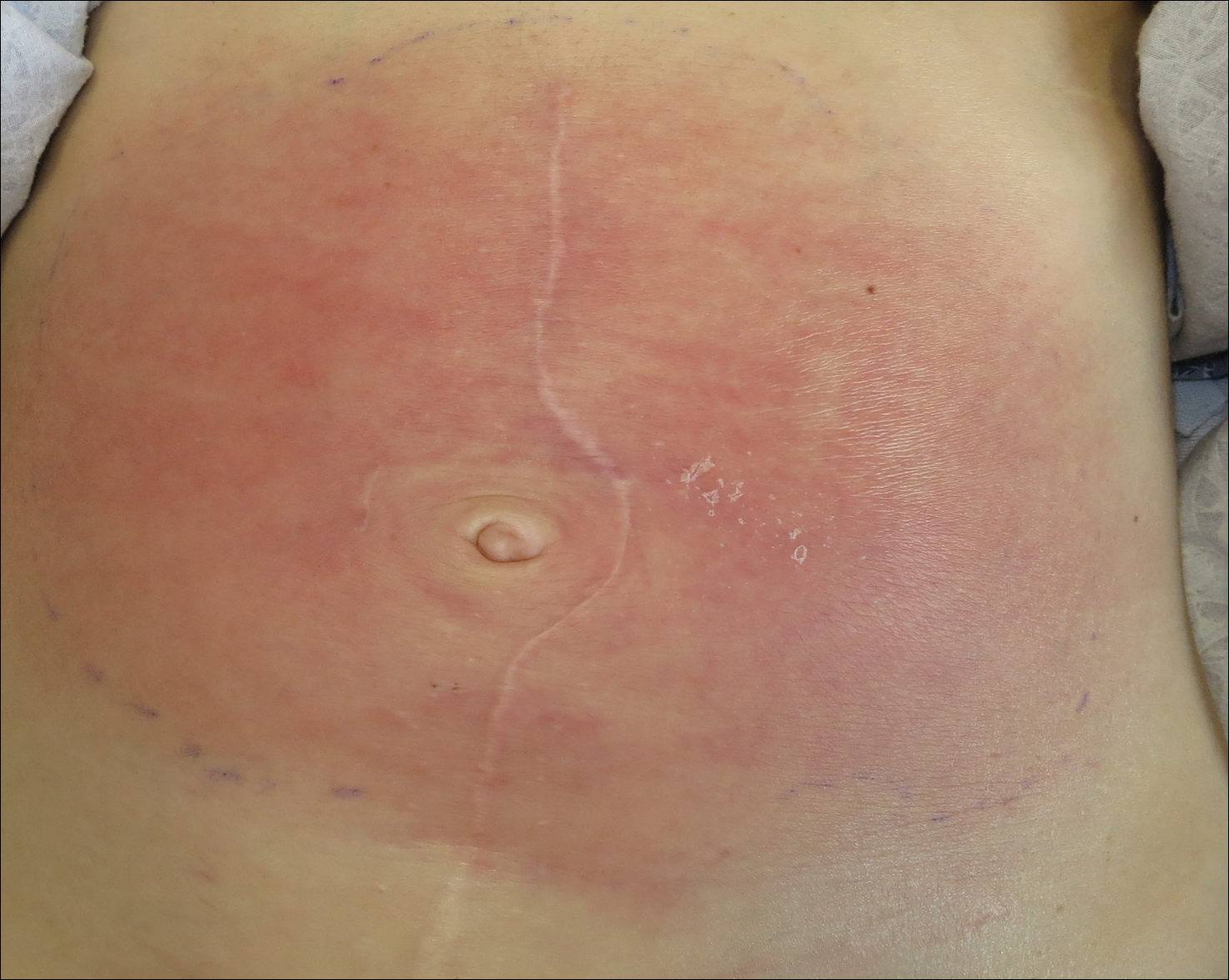
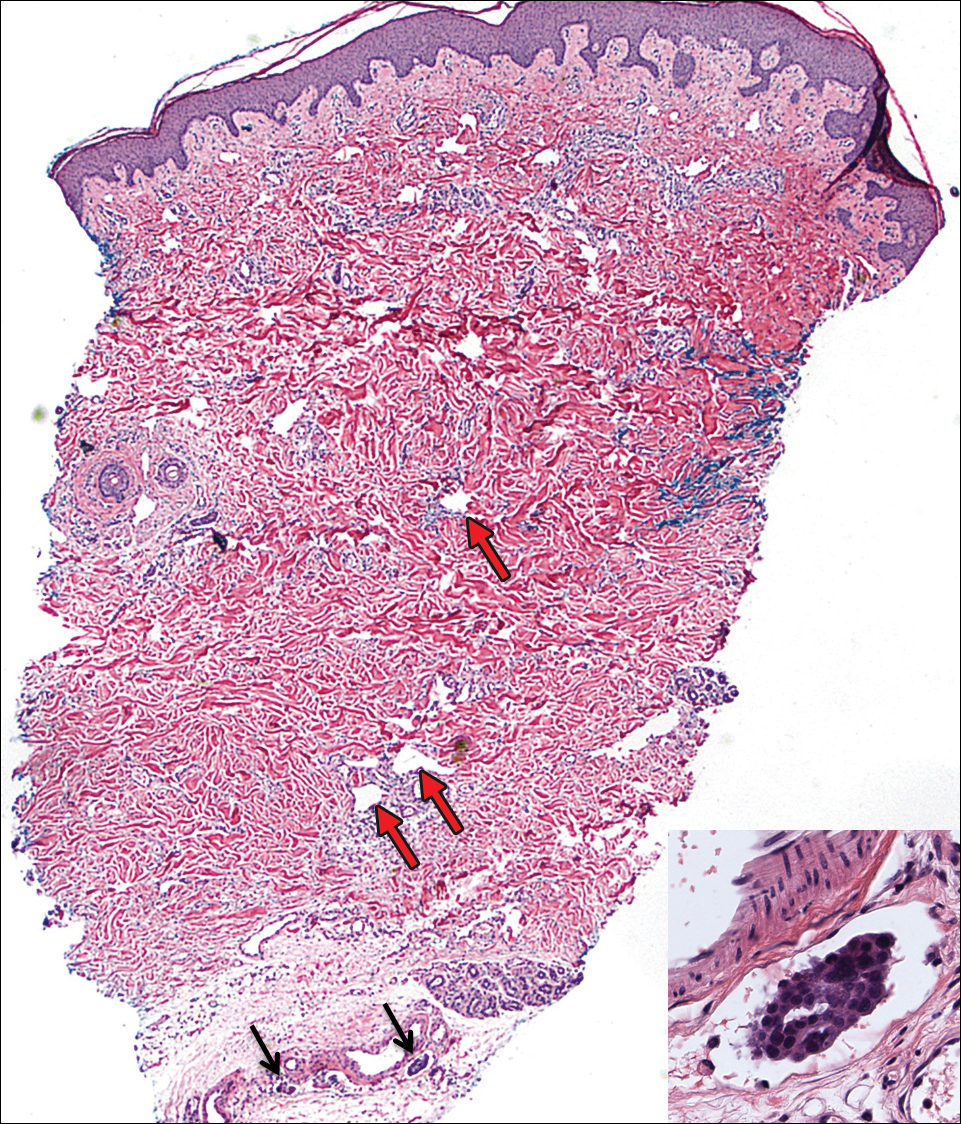
Carcinoma erysipeloides is a rare cutaneous metastasis secondary to internal malignancy that presents as well-demarcated areas of erythema and is sometimes misdiagnosed as cellulitis or erysipelas. Histology is notable for lymphovascular congestion without inflammation. Carcinoma erysipeloides most commonly is associated with breast cancer, but it also has been described in cancers of the prostate, larynx, stomach, lungs, thyroid, parotid gland, fallopian tubes, cervix, pancreas, and metastatic melanoma.1-5 While the pathogenesis of carcinoma erysipeloides is poorly understood, it is thought to occur by direct spread of tumor cells from the lymph nodes to the cutaneous lymphatics, causing obstruction and edema.
Ovarian cancer has the highest mortality of all gynecologic cancers and often is associated with delayed diagnosis. Cutaneous metastasis is a late manifestation often presenting as subcutaneous nodules.6,7 Carcinoma erysipeloides is an even rarer presentation of ovarian cancer, with a poor prognosis and a median survival of 18 months.8 A PubMed search of articles indexed for MEDLINE using the term carcinoma erysipeloides revealed 9 cases of carcinoma erysipeloides from ovarian cancer: 1 describing erythematous papules, plaques, and zosteriform vesicles on the upper thighs to the lower abdomen,9 and 8 describing erythematous plaques on the breasts.8,10 We report a case of carcinoma erysipeloides associated with stage IIIc ovarian cancer localized to the abdominal wall mimicking cellulitis. Our report reminds clinicians of this important diagnosis in ovarian cancer and of the importance of a skin biopsy to expedite a definitive diagnosis. Immunohistochemistry using ovarian tumor markers (eg, paired-box gene 8, cancer antigen 125) is an additional tool to accurately identify malignant cells in skin biopsy.8,10 Once diagnosed, primary treatment for carcinoma erysipeloides is treatment of the underlying malignancy.
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682-685.
- Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142-145.
- Karmali S, Rudmik L, Temple W, et al. Melanoma erysipeloides. Can J Surg. 2005;48:159-160.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Carcinoma erysipeloides of the breast in a patient with advanced ovarian carcinoma. Clin Infect Dis. 2012;54:575-576.
- Hazelrigg DE, Rudolph AH. Inflammatory metastic carcinoma. carcinoma erysipelatoides. Arch Dermatol. 1977;113:69-70.
- Cowan LJ, Roller JI, Connelly PJ, et al. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion—a case report and literature review. Gynecol Oncol. 1995;57:433-435.
- Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol. 2003;90:670-672.
- Klein RL, Brown AR, Gomez-Castro CM, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: a case report and literature review. J Cancer. 2010;1:27-31.
- Lee HC, Chu CY, Hsiao CH. Carcinoma erysipeloides from ovarian clear-cell carcinoma. J Clin Oncol. 2007;25:5828-5830.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Photo quiz. rash in a patient with ovarian cancer. Clin Infect Dis. 2012;54:538, 575-576.
To the Editor:
A 40-year-old woman with a history of stage IIIC ovarian cancer presented with progressing abdominal erythema and pain of 1 month’s duration. She had been diagnosed 4 years prior with grade 3, poorly differentiated papillary serous carcinoma involving the bilateral ovaries, uterine tubes, uterus, and omentum with lymphovascular invasion. She underwent tumor resection and debulking followed by paclitaxel plus platinum-based chemotherapy. The cancer recurred 2 years later with carcinomatous ascites. She declined chemotherapy but underwent therapeutic paracentesis.
One month prior to presentation, the patient developed a small, tender, erythematous patch on the abdomen. Her primary physician started her on cephalexin for presumed cellulitis without improvement. The erythema continued to spread on the abdomen with worsening pain, which prompted her presentation to the emergency department. She was admitted and started on intravenous vancomycin.
On admission to the hospital, the patient was cachexic and afebrile with a white blood cell count of 10,400/µL (reference range, 4500–11,000/µL). Physical examination revealed a well-demarcated, 15×20-cm, erythematous, blanchable, indurated plaque in the periumbilical region (Figure 1). The plaque was tender to palpation with guarding but no increased warmth. Punch biopsies of the abdominal skin revealed carcinoma within the lymphatic channels in the deep dermis and dilated lymphatics throughout the overlying dermis (Figure 2). These findings were diagnostic for carcinoma erysipeloides. Tissue and blood cultures were negative for bacterial, fungal, or mycobacterial growth. Vancomycin was discontinued, and she was discharged with pain medication. She declined chemotherapy due to the potential side effects and elected to continue symptomatic management with palliative paracentesis. After she was discharged, she underwent a tunneled pleural catheterization for recurrent malignant pleural effusions.


Carcinoma erysipeloides is a rare cutaneous metastasis secondary to internal malignancy that presents as well-demarcated areas of erythema and is sometimes misdiagnosed as cellulitis or erysipelas. Histology is notable for lymphovascular congestion without inflammation. Carcinoma erysipeloides most commonly is associated with breast cancer, but it also has been described in cancers of the prostate, larynx, stomach, lungs, thyroid, parotid gland, fallopian tubes, cervix, pancreas, and metastatic melanoma.1-5 While the pathogenesis of carcinoma erysipeloides is poorly understood, it is thought to occur by direct spread of tumor cells from the lymph nodes to the cutaneous lymphatics, causing obstruction and edema.
Ovarian cancer has the highest mortality of all gynecologic cancers and often is associated with delayed diagnosis. Cutaneous metastasis is a late manifestation often presenting as subcutaneous nodules.6,7 Carcinoma erysipeloides is an even rarer presentation of ovarian cancer, with a poor prognosis and a median survival of 18 months.8 A PubMed search of articles indexed for MEDLINE using the term carcinoma erysipeloides revealed 9 cases of carcinoma erysipeloides from ovarian cancer: 1 describing erythematous papules, plaques, and zosteriform vesicles on the upper thighs to the lower abdomen,9 and 8 describing erythematous plaques on the breasts.8,10 We report a case of carcinoma erysipeloides associated with stage IIIc ovarian cancer localized to the abdominal wall mimicking cellulitis. Our report reminds clinicians of this important diagnosis in ovarian cancer and of the importance of a skin biopsy to expedite a definitive diagnosis. Immunohistochemistry using ovarian tumor markers (eg, paired-box gene 8, cancer antigen 125) is an additional tool to accurately identify malignant cells in skin biopsy.8,10 Once diagnosed, primary treatment for carcinoma erysipeloides is treatment of the underlying malignancy.
To the Editor:
A 40-year-old woman with a history of stage IIIC ovarian cancer presented with progressing abdominal erythema and pain of 1 month’s duration. She had been diagnosed 4 years prior with grade 3, poorly differentiated papillary serous carcinoma involving the bilateral ovaries, uterine tubes, uterus, and omentum with lymphovascular invasion. She underwent tumor resection and debulking followed by paclitaxel plus platinum-based chemotherapy. The cancer recurred 2 years later with carcinomatous ascites. She declined chemotherapy but underwent therapeutic paracentesis.
One month prior to presentation, the patient developed a small, tender, erythematous patch on the abdomen. Her primary physician started her on cephalexin for presumed cellulitis without improvement. The erythema continued to spread on the abdomen with worsening pain, which prompted her presentation to the emergency department. She was admitted and started on intravenous vancomycin.
On admission to the hospital, the patient was cachexic and afebrile with a white blood cell count of 10,400/µL (reference range, 4500–11,000/µL). Physical examination revealed a well-demarcated, 15×20-cm, erythematous, blanchable, indurated plaque in the periumbilical region (Figure 1). The plaque was tender to palpation with guarding but no increased warmth. Punch biopsies of the abdominal skin revealed carcinoma within the lymphatic channels in the deep dermis and dilated lymphatics throughout the overlying dermis (Figure 2). These findings were diagnostic for carcinoma erysipeloides. Tissue and blood cultures were negative for bacterial, fungal, or mycobacterial growth. Vancomycin was discontinued, and she was discharged with pain medication. She declined chemotherapy due to the potential side effects and elected to continue symptomatic management with palliative paracentesis. After she was discharged, she underwent a tunneled pleural catheterization for recurrent malignant pleural effusions.


Carcinoma erysipeloides is a rare cutaneous metastasis secondary to internal malignancy that presents as well-demarcated areas of erythema and is sometimes misdiagnosed as cellulitis or erysipelas. Histology is notable for lymphovascular congestion without inflammation. Carcinoma erysipeloides most commonly is associated with breast cancer, but it also has been described in cancers of the prostate, larynx, stomach, lungs, thyroid, parotid gland, fallopian tubes, cervix, pancreas, and metastatic melanoma.1-5 While the pathogenesis of carcinoma erysipeloides is poorly understood, it is thought to occur by direct spread of tumor cells from the lymph nodes to the cutaneous lymphatics, causing obstruction and edema.
Ovarian cancer has the highest mortality of all gynecologic cancers and often is associated with delayed diagnosis. Cutaneous metastasis is a late manifestation often presenting as subcutaneous nodules.6,7 Carcinoma erysipeloides is an even rarer presentation of ovarian cancer, with a poor prognosis and a median survival of 18 months.8 A PubMed search of articles indexed for MEDLINE using the term carcinoma erysipeloides revealed 9 cases of carcinoma erysipeloides from ovarian cancer: 1 describing erythematous papules, plaques, and zosteriform vesicles on the upper thighs to the lower abdomen,9 and 8 describing erythematous plaques on the breasts.8,10 We report a case of carcinoma erysipeloides associated with stage IIIc ovarian cancer localized to the abdominal wall mimicking cellulitis. Our report reminds clinicians of this important diagnosis in ovarian cancer and of the importance of a skin biopsy to expedite a definitive diagnosis. Immunohistochemistry using ovarian tumor markers (eg, paired-box gene 8, cancer antigen 125) is an additional tool to accurately identify malignant cells in skin biopsy.8,10 Once diagnosed, primary treatment for carcinoma erysipeloides is treatment of the underlying malignancy.
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682-685.
- Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142-145.
- Karmali S, Rudmik L, Temple W, et al. Melanoma erysipeloides. Can J Surg. 2005;48:159-160.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Carcinoma erysipeloides of the breast in a patient with advanced ovarian carcinoma. Clin Infect Dis. 2012;54:575-576.
- Hazelrigg DE, Rudolph AH. Inflammatory metastic carcinoma. carcinoma erysipelatoides. Arch Dermatol. 1977;113:69-70.
- Cowan LJ, Roller JI, Connelly PJ, et al. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion—a case report and literature review. Gynecol Oncol. 1995;57:433-435.
- Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol. 2003;90:670-672.
- Klein RL, Brown AR, Gomez-Castro CM, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: a case report and literature review. J Cancer. 2010;1:27-31.
- Lee HC, Chu CY, Hsiao CH. Carcinoma erysipeloides from ovarian clear-cell carcinoma. J Clin Oncol. 2007;25:5828-5830.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Photo quiz. rash in a patient with ovarian cancer. Clin Infect Dis. 2012;54:538, 575-576.
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682-685.
- Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142-145.
- Karmali S, Rudmik L, Temple W, et al. Melanoma erysipeloides. Can J Surg. 2005;48:159-160.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Carcinoma erysipeloides of the breast in a patient with advanced ovarian carcinoma. Clin Infect Dis. 2012;54:575-576.
- Hazelrigg DE, Rudolph AH. Inflammatory metastic carcinoma. carcinoma erysipelatoides. Arch Dermatol. 1977;113:69-70.
- Cowan LJ, Roller JI, Connelly PJ, et al. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion—a case report and literature review. Gynecol Oncol. 1995;57:433-435.
- Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol. 2003;90:670-672.
- Klein RL, Brown AR, Gomez-Castro CM, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: a case report and literature review. J Cancer. 2010;1:27-31.
- Lee HC, Chu CY, Hsiao CH. Carcinoma erysipeloides from ovarian clear-cell carcinoma. J Clin Oncol. 2007;25:5828-5830.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Photo quiz. rash in a patient with ovarian cancer. Clin Infect Dis. 2012;54:538, 575-576.
Practice Points
- Carcinoma erysipeloides is a rare cutaneous marker of metastatic ovarian cancer.
- Clinicians should be aware of carcinoma erysipeloides in ovarian cancer and maintain a low threshold for biopsy for accurate diagnosis and management planning.