User login
Medial Oblique Meniscomeniscal Ligament of Knee
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
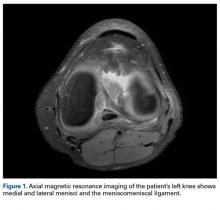
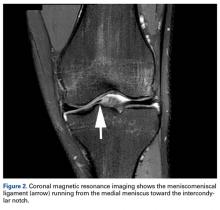
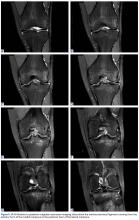
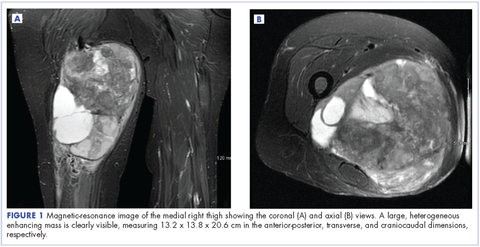
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
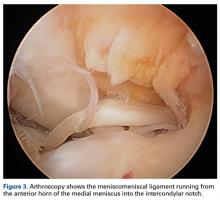
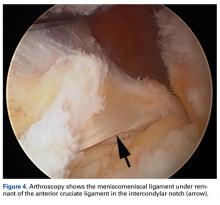
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Imipramine-Induced Hyperpigmentation
Imipramine is a tricyclic medication uncommonly used to treat depression, anxiety, and other psychiatric illnesses. Although relatively rare, it has been associated with hyperpigmentation of the skin including slate gray discoloration of sun-exposed areas.
We present the case of a 63-year-old woman who had been taking imipramine for more than 20 years when she developed bluish gray discoloration on the face and neck. Histopathology of biopsy specimens showed numerous perivascular and interstitial brown globules in the dermis that were composed of melanin only, as evidenced by positive Fontana-Masson staining and negative Perls Prussian blue staining. A diagnosis of imipramine-induced hyperpigmentation was made based on histopathology and clinical history.
In addition to the case presentation, we provide a review of drugs that commonly cause hyperpigmentation as well as their associated histopathologic staining characteristics.
Case Report
A 63-year-old woman presented with blue-gray discoloration on the face and neck. She first noted the discoloration on the left side of the forehead 3 years prior; it then spread to the right side of the forehead, cheeks, and neck. She denied pruritus, pain, redness, and scaling of the involved areas; any recent changes in medications; or the use of any topical products on the affected areas. Her medical history was remarkable for hypertension, which was inconsistently controlled with lisinopril and hydrochlorothiazide, and depression, which had been managed with oral imipramine.
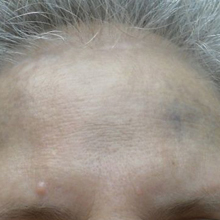
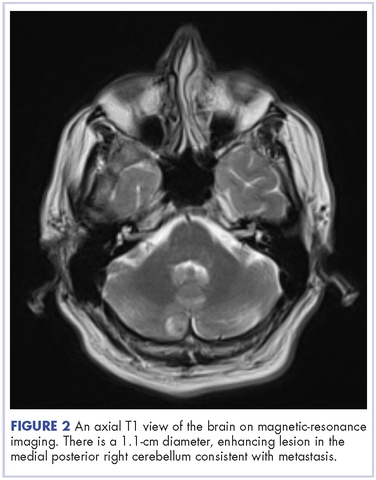
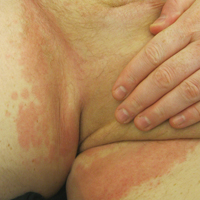
Physical examination disclosed blue-gray hyperpigmented patches with irregular borders on the bilateral forehead, temples, and periorbital skin (Figure 1). Reticulated brown patches were noted on the bilateral cheeks, and the neck displayed diffuse muddy brown patches with sparing of the submental areas.
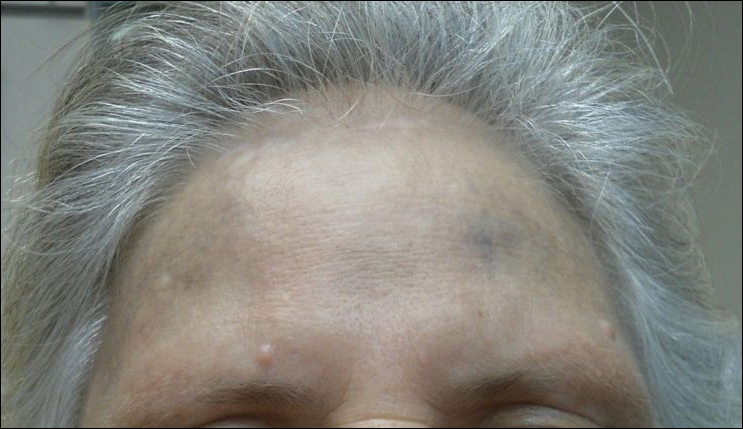
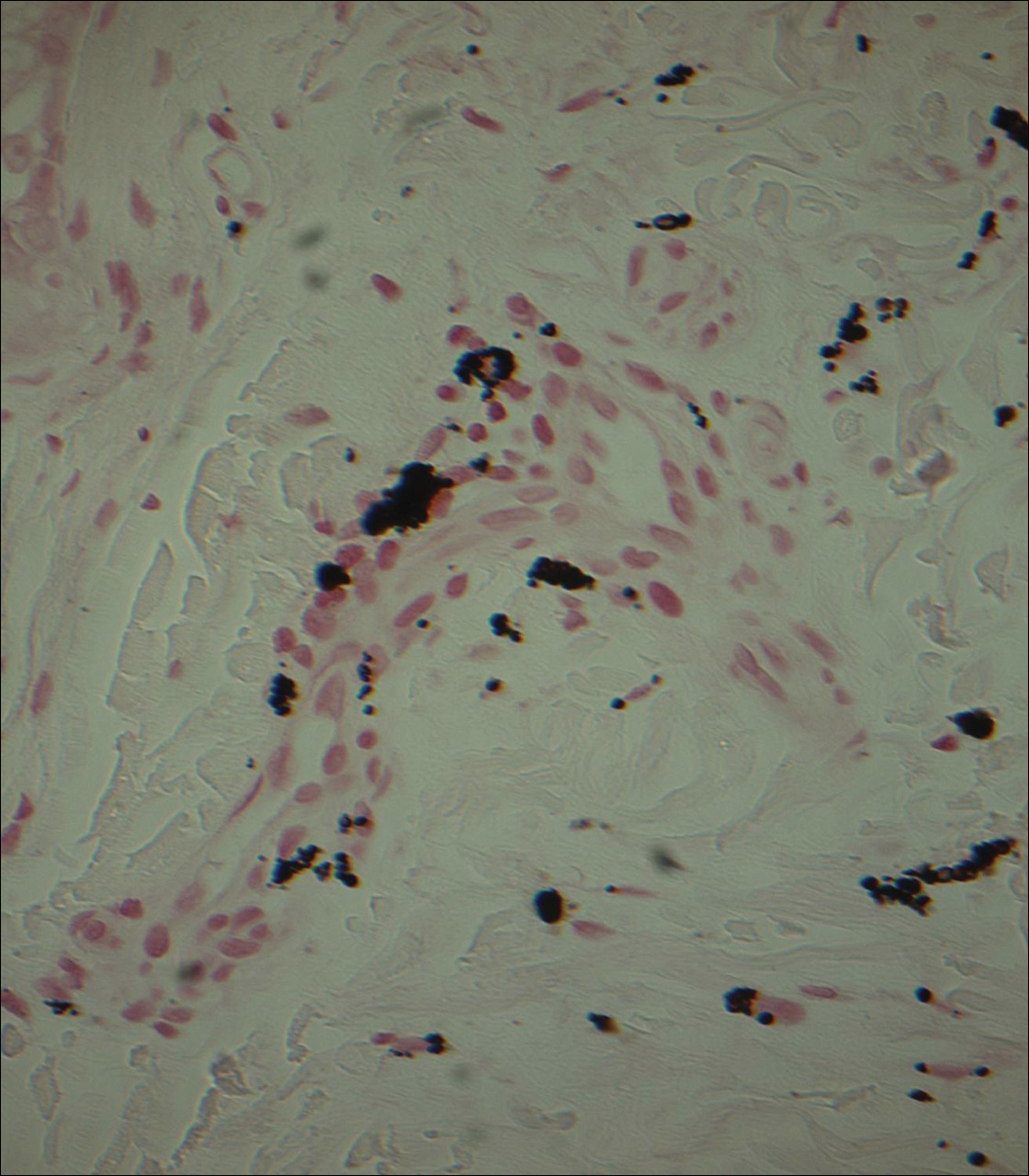
Punch biopsies obtained from the lateral forehead showed an unremarkable epidermis with deposition of numerous golden brown granules in the upper and mid dermis and in perivascular macrophages (Figure 2). The pigmented granules showed positive staining with Fontana-Masson (Figure 3), and a Perls Prussian blue stain for hemosiderin was negative. Based on the clinical history, a diagnosis of imipramine-induced hyperpigmentation was made.
The patient revealed that she had taken imipramine for more than 20 years for depression as prescribed by her mental health professional. She had tried several other antidepressants but none were as effective as imipramine. Therefore, she was not willing to discontinue it despite the likelihood that the hyperpigmentation would persist and could worsen with continued use of the medication. Diligent photoprotection was advised. Additionally, she started taking lisinopril some time after the appearance of the hyperpigmentation presented and had not taken hydrochlorothiazide consistently for several years. Although these drugs are known to cause various cutaneous reactions, it was not considered likely in this case.
Comment
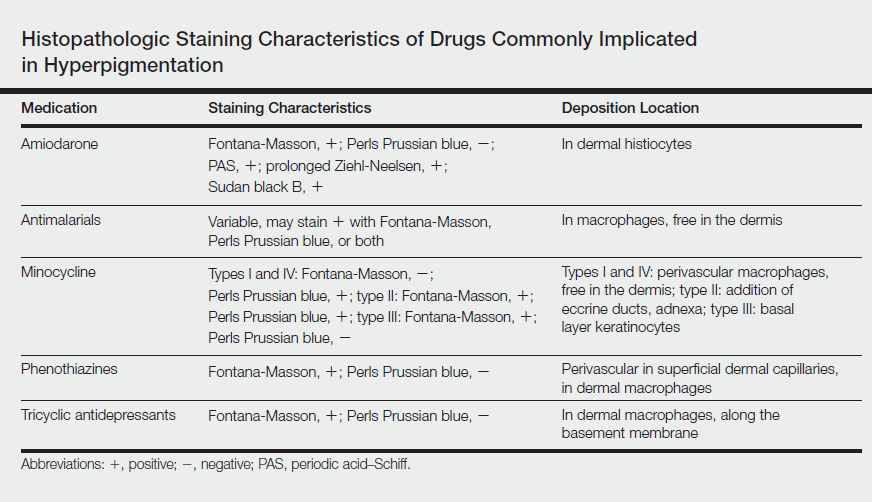
Drug-induced hyperpigmentation accounts for 10% to 20% of all cases of acquired hyperpigmentation.1 Common causative drugs include amiodarone, antimalarials, minocycline, and rarely psychotropics including phenothiazines and tricyclic antidepressants such as imipramine.1-4 Although amiodarone-induced hyperpigmentation is associated with lipofuscin in addition to melanin, most other medications, including imipramine, induce cutaneous effects through deposition of melanin and/or hemosiderin. A review of the histopathologic staining characteristics in pigment anomalies caused by these drugs is summarized in the Table.
Imipramine-induced hyperpigmentation presents as slate gray discrete macules and patches on sun-exposed skin that may appear anywhere from 2 to 22 years after initiating the medication.1-4 Affected areas include the malar cheeks, temples, periorbital areas, hands, forearms, and seldom the iris and sclera.2-4 Although the blue to slate gray coloring is classic, other colors have been described including brown, golden brown, and purple.2
Histopathology of imipramine-induced hyperpigmentation shows golden brown, round to oval granules in the superficial dermis and within dermal macrophages.1,3 Generally, Fontana-Masson staining is positive for melanin and Perls Prussian blue staining is negative for iron.1,2,4
Imipramine-induced hyperpigmentation likely results from photoexcitation of imipramine or one of its metabolites. These compounds activate tyrosinase, increasing melanogenesis and leading to formation of melanin-imipramine or melanin-metabolite complexes.1-3 Complexes are deposited in the dermis and basal layer or are engulfed by dermal macrophages and darkened on sun exposure due to their high melanin content.1 Other possible mechanisms of hyperpigmentation include nonspecific inflammation caused by the drug in the skin, hemosiderin deposition from vessel damage and subsequent erythrocyte extravasation, or deposition of newly formed pigments related to the drug.1
Most patients report satisfactory resolution of imipramine-induced discoloration within 1 year of stopping imipramine or switching to a different antidepressant.1,4 Patients who are unwilling to discontinue imipramine may achieve resolution with alexandrite or Q-switched ruby laser therapy.1,4 Strict sun protective measures are necessary, both to prevent new deposition of melanin and to prevent darkening of existing pigment.
Despite the advent of new psychotropic medications, imipramine remains the antidepressant of choice for many patients. Although rare, it is important to be able to recognize imipramine-induced hyperpigmentation and to encourage patient-psychiatrist communication to determine an antidepressant regimen that avoids unnecessary cutaneous side effects.
- D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
- Ming ME, Bhawan J, Stefanato CM, et al. Imipramine-induced hyperpigmentation: four cases and a review of the literature. J Am Acad Dermatol. 1999;40(2, pt 1):159-166.
- Sicari MC, Lebwohl M, Baral J, et al. Photoinduced dermal pigmentation in patients taking tricyclic antidepressants: histology, electron microscopy, and energy dispersive spectroscopy. J Am Acad Dermatol.1999;40(2, pt 2):290-293.
- Atkin DH, Fitzpatrick RE. Laser treatment of imipramine-induced hyperpigmentation. J Am Acad Dermatol. 2000;43(1, pt 1):77-80.
Imipramine is a tricyclic medication uncommonly used to treat depression, anxiety, and other psychiatric illnesses. Although relatively rare, it has been associated with hyperpigmentation of the skin including slate gray discoloration of sun-exposed areas.
We present the case of a 63-year-old woman who had been taking imipramine for more than 20 years when she developed bluish gray discoloration on the face and neck. Histopathology of biopsy specimens showed numerous perivascular and interstitial brown globules in the dermis that were composed of melanin only, as evidenced by positive Fontana-Masson staining and negative Perls Prussian blue staining. A diagnosis of imipramine-induced hyperpigmentation was made based on histopathology and clinical history.
In addition to the case presentation, we provide a review of drugs that commonly cause hyperpigmentation as well as their associated histopathologic staining characteristics.
Case Report
A 63-year-old woman presented with blue-gray discoloration on the face and neck. She first noted the discoloration on the left side of the forehead 3 years prior; it then spread to the right side of the forehead, cheeks, and neck. She denied pruritus, pain, redness, and scaling of the involved areas; any recent changes in medications; or the use of any topical products on the affected areas. Her medical history was remarkable for hypertension, which was inconsistently controlled with lisinopril and hydrochlorothiazide, and depression, which had been managed with oral imipramine.
Physical examination disclosed blue-gray hyperpigmented patches with irregular borders on the bilateral forehead, temples, and periorbital skin (Figure 1). Reticulated brown patches were noted on the bilateral cheeks, and the neck displayed diffuse muddy brown patches with sparing of the submental areas.

Punch biopsies obtained from the lateral forehead showed an unremarkable epidermis with deposition of numerous golden brown granules in the upper and mid dermis and in perivascular macrophages (Figure 2). The pigmented granules showed positive staining with Fontana-Masson (Figure 3), and a Perls Prussian blue stain for hemosiderin was negative. Based on the clinical history, a diagnosis of imipramine-induced hyperpigmentation was made.


The patient revealed that she had taken imipramine for more than 20 years for depression as prescribed by her mental health professional. She had tried several other antidepressants but none were as effective as imipramine. Therefore, she was not willing to discontinue it despite the likelihood that the hyperpigmentation would persist and could worsen with continued use of the medication. Diligent photoprotection was advised. Additionally, she started taking lisinopril some time after the appearance of the hyperpigmentation presented and had not taken hydrochlorothiazide consistently for several years. Although these drugs are known to cause various cutaneous reactions, it was not considered likely in this case.
Comment
Drug-induced hyperpigmentation accounts for 10% to 20% of all cases of acquired hyperpigmentation.1 Common causative drugs include amiodarone, antimalarials, minocycline, and rarely psychotropics including phenothiazines and tricyclic antidepressants such as imipramine.1-4 Although amiodarone-induced hyperpigmentation is associated with lipofuscin in addition to melanin, most other medications, including imipramine, induce cutaneous effects through deposition of melanin and/or hemosiderin. A review of the histopathologic staining characteristics in pigment anomalies caused by these drugs is summarized in the Table.

Imipramine-induced hyperpigmentation presents as slate gray discrete macules and patches on sun-exposed skin that may appear anywhere from 2 to 22 years after initiating the medication.1-4 Affected areas include the malar cheeks, temples, periorbital areas, hands, forearms, and seldom the iris and sclera.2-4 Although the blue to slate gray coloring is classic, other colors have been described including brown, golden brown, and purple.2
Histopathology of imipramine-induced hyperpigmentation shows golden brown, round to oval granules in the superficial dermis and within dermal macrophages.1,3 Generally, Fontana-Masson staining is positive for melanin and Perls Prussian blue staining is negative for iron.1,2,4
Imipramine-induced hyperpigmentation likely results from photoexcitation of imipramine or one of its metabolites. These compounds activate tyrosinase, increasing melanogenesis and leading to formation of melanin-imipramine or melanin-metabolite complexes.1-3 Complexes are deposited in the dermis and basal layer or are engulfed by dermal macrophages and darkened on sun exposure due to their high melanin content.1 Other possible mechanisms of hyperpigmentation include nonspecific inflammation caused by the drug in the skin, hemosiderin deposition from vessel damage and subsequent erythrocyte extravasation, or deposition of newly formed pigments related to the drug.1
Most patients report satisfactory resolution of imipramine-induced discoloration within 1 year of stopping imipramine or switching to a different antidepressant.1,4 Patients who are unwilling to discontinue imipramine may achieve resolution with alexandrite or Q-switched ruby laser therapy.1,4 Strict sun protective measures are necessary, both to prevent new deposition of melanin and to prevent darkening of existing pigment.
Despite the advent of new psychotropic medications, imipramine remains the antidepressant of choice for many patients. Although rare, it is important to be able to recognize imipramine-induced hyperpigmentation and to encourage patient-psychiatrist communication to determine an antidepressant regimen that avoids unnecessary cutaneous side effects.
Imipramine is a tricyclic medication uncommonly used to treat depression, anxiety, and other psychiatric illnesses. Although relatively rare, it has been associated with hyperpigmentation of the skin including slate gray discoloration of sun-exposed areas.
We present the case of a 63-year-old woman who had been taking imipramine for more than 20 years when she developed bluish gray discoloration on the face and neck. Histopathology of biopsy specimens showed numerous perivascular and interstitial brown globules in the dermis that were composed of melanin only, as evidenced by positive Fontana-Masson staining and negative Perls Prussian blue staining. A diagnosis of imipramine-induced hyperpigmentation was made based on histopathology and clinical history.
In addition to the case presentation, we provide a review of drugs that commonly cause hyperpigmentation as well as their associated histopathologic staining characteristics.
Case Report
A 63-year-old woman presented with blue-gray discoloration on the face and neck. She first noted the discoloration on the left side of the forehead 3 years prior; it then spread to the right side of the forehead, cheeks, and neck. She denied pruritus, pain, redness, and scaling of the involved areas; any recent changes in medications; or the use of any topical products on the affected areas. Her medical history was remarkable for hypertension, which was inconsistently controlled with lisinopril and hydrochlorothiazide, and depression, which had been managed with oral imipramine.
Physical examination disclosed blue-gray hyperpigmented patches with irregular borders on the bilateral forehead, temples, and periorbital skin (Figure 1). Reticulated brown patches were noted on the bilateral cheeks, and the neck displayed diffuse muddy brown patches with sparing of the submental areas.

Punch biopsies obtained from the lateral forehead showed an unremarkable epidermis with deposition of numerous golden brown granules in the upper and mid dermis and in perivascular macrophages (Figure 2). The pigmented granules showed positive staining with Fontana-Masson (Figure 3), and a Perls Prussian blue stain for hemosiderin was negative. Based on the clinical history, a diagnosis of imipramine-induced hyperpigmentation was made.


The patient revealed that she had taken imipramine for more than 20 years for depression as prescribed by her mental health professional. She had tried several other antidepressants but none were as effective as imipramine. Therefore, she was not willing to discontinue it despite the likelihood that the hyperpigmentation would persist and could worsen with continued use of the medication. Diligent photoprotection was advised. Additionally, she started taking lisinopril some time after the appearance of the hyperpigmentation presented and had not taken hydrochlorothiazide consistently for several years. Although these drugs are known to cause various cutaneous reactions, it was not considered likely in this case.
Comment
Drug-induced hyperpigmentation accounts for 10% to 20% of all cases of acquired hyperpigmentation.1 Common causative drugs include amiodarone, antimalarials, minocycline, and rarely psychotropics including phenothiazines and tricyclic antidepressants such as imipramine.1-4 Although amiodarone-induced hyperpigmentation is associated with lipofuscin in addition to melanin, most other medications, including imipramine, induce cutaneous effects through deposition of melanin and/or hemosiderin. A review of the histopathologic staining characteristics in pigment anomalies caused by these drugs is summarized in the Table.

Imipramine-induced hyperpigmentation presents as slate gray discrete macules and patches on sun-exposed skin that may appear anywhere from 2 to 22 years after initiating the medication.1-4 Affected areas include the malar cheeks, temples, periorbital areas, hands, forearms, and seldom the iris and sclera.2-4 Although the blue to slate gray coloring is classic, other colors have been described including brown, golden brown, and purple.2
Histopathology of imipramine-induced hyperpigmentation shows golden brown, round to oval granules in the superficial dermis and within dermal macrophages.1,3 Generally, Fontana-Masson staining is positive for melanin and Perls Prussian blue staining is negative for iron.1,2,4
Imipramine-induced hyperpigmentation likely results from photoexcitation of imipramine or one of its metabolites. These compounds activate tyrosinase, increasing melanogenesis and leading to formation of melanin-imipramine or melanin-metabolite complexes.1-3 Complexes are deposited in the dermis and basal layer or are engulfed by dermal macrophages and darkened on sun exposure due to their high melanin content.1 Other possible mechanisms of hyperpigmentation include nonspecific inflammation caused by the drug in the skin, hemosiderin deposition from vessel damage and subsequent erythrocyte extravasation, or deposition of newly formed pigments related to the drug.1
Most patients report satisfactory resolution of imipramine-induced discoloration within 1 year of stopping imipramine or switching to a different antidepressant.1,4 Patients who are unwilling to discontinue imipramine may achieve resolution with alexandrite or Q-switched ruby laser therapy.1,4 Strict sun protective measures are necessary, both to prevent new deposition of melanin and to prevent darkening of existing pigment.
Despite the advent of new psychotropic medications, imipramine remains the antidepressant of choice for many patients. Although rare, it is important to be able to recognize imipramine-induced hyperpigmentation and to encourage patient-psychiatrist communication to determine an antidepressant regimen that avoids unnecessary cutaneous side effects.
- D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
- Ming ME, Bhawan J, Stefanato CM, et al. Imipramine-induced hyperpigmentation: four cases and a review of the literature. J Am Acad Dermatol. 1999;40(2, pt 1):159-166.
- Sicari MC, Lebwohl M, Baral J, et al. Photoinduced dermal pigmentation in patients taking tricyclic antidepressants: histology, electron microscopy, and energy dispersive spectroscopy. J Am Acad Dermatol.1999;40(2, pt 2):290-293.
- Atkin DH, Fitzpatrick RE. Laser treatment of imipramine-induced hyperpigmentation. J Am Acad Dermatol. 2000;43(1, pt 1):77-80.
- D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
- Ming ME, Bhawan J, Stefanato CM, et al. Imipramine-induced hyperpigmentation: four cases and a review of the literature. J Am Acad Dermatol. 1999;40(2, pt 1):159-166.
- Sicari MC, Lebwohl M, Baral J, et al. Photoinduced dermal pigmentation in patients taking tricyclic antidepressants: histology, electron microscopy, and energy dispersive spectroscopy. J Am Acad Dermatol.1999;40(2, pt 2):290-293.
- Atkin DH, Fitzpatrick RE. Laser treatment of imipramine-induced hyperpigmentation. J Am Acad Dermatol. 2000;43(1, pt 1):77-80.
Practice Points
- Imipramine is a tricyclic medication used for the treatment of depression and mood disorders.
- A rare side effect of treatment with imipramine is a blue-gray discoloration of the skin.
- Thorough medication review is important in patients who present with skin discoloration.
Thyroid Cartilage Fracture in Context of Noncompetitive "Horseplay" Wrestling
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
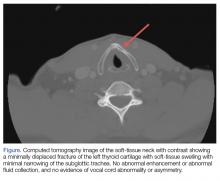
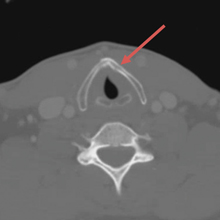
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
Retropharyngeal Hematoma in a 90-Year-Old Woman
Case
A 90-year-old woman with chronic obstructive pulmonary disease; hypertension; chronic kidney disease; diastolic dysfunction; severe tricuspid regurgitation; and atrial fibrillation (AF), for which she was taking rivaroxaban, presented to the ED for evaluation of injuries she sustained during a fall. The patient’s family stated that she fell while walking with the assistance of a walker and landed on her face. There was no reported loss of consciousness. Upon arrival at the ED, the patient’s vital signs were: blood pressure, 188/105 mm Hg; heart rate, 91 beats/min; respiratory rate, 20 breaths/min; and temperature, 97.88°F (36.6°C). Oxygen (O2) saturation was 90% on room air, but increased to 98% after the patient received 10 L/min of O2 through a non-rebreather mask.
On physical examination, the patient was awake, alert, and oriented to person, place, and time, with a Glasgow Coma Scale score of 15. She was able to move all four extremities and had 4/5 motor strength in the upper extremities bilaterally, and 3/5 motor strength in the bilateral lower limbs, which her family reported was the same as her baseline. On pulmonary examination, the lungs were clear to auscultation bilaterally and had no stridor. On auscultation she had a regular rate, with no murmurs or rubs.
The patient had nasal bone tenderness with epistaxis that resolved spontaneously and did not require packing; she had no other facial tenderness. The oropharynx was clear. There was mild posterior midline tenderness over C5 and C6, but no skin ecchymosis or neck swelling. Along with the non-rebreather mask, the patient was placed in a neck collar while she awaited transport to radiology for computed tomography (CT) studies.
The CT scan of the cervical spine demonstrated a minimally displaced fracture of the right anterior arch, both sides of the posterior arch of C1, and a comminuted minimally displaced fracture involving the posterior arch and spinous process of C5, with mild retrolisthesis of C5 over C6.
Based on the CT findings, the patient was taken to the operating room (OR) where she underwent awake fiberoptic laryngoscopy. During transfer to the OR, the patient’s O2 dropped to 87%; however, after successful intubation without complication, O2 saturation improved to 95%. After intubation, the patient was admitted to the intensive care unit for observation, and rivaroxaban therapy was discontinued.
A CT scan of the neck postintubation showed a mild interval decrease in the retropharyngeal hematoma, but an increase in the anterior disc space at C5-C6 with mild retrolisthesis, which raised suspicion for an anterior longitudinal ligamentous injury. A repeat CT scan on hospital day 4 revealed a new bleed within the old retropharyngeal hematoma, with no increase in thickness or size of the initial hematoma. The head and neck surgical team kept the patient intubated while awaiting resolution of the hematoma, with no plan of surgery.
On hospital day 6, the patient was transferred to another facility for continued long-term care. She was transitioned to a tracheostomy 4 days later. Follow-up approximately 2 weeks after presentation confirmed complete resolution of the hematoma, and no surgical intervention was required.
Discussion
Overview
Retropharyngeal hematomas are infrequent, but potentially life-threatening complications of cervical fractures, foreign body trauma, infection, violent coughing, and anticoagulation therapy.1 Although retropharyngeal hematomas associated with warfarin have been well described, to our knowledge, there are no reported cases associated with a direct oral anticoagulant (DOAC).2
Though multiple studies have supported the effectiveness and safety of DOACs for prevention of stroke and systemic embolism in patients with AF, the risk of hemorrhage still exists.3 Postmarketing surveillance studies of DOACs report an overall risk of bleeding comparable to warfarin. Gastrointestinal bleeding was found to be slightly higher in patients taking a DOAC compared to those on warfarin, but the risk of intracranial bleeding from DOACs was notably lower.3 With limited effective reversal agents, DOACs present a tremendous challenge in managing acute life-threatening hemorrhage.4
Signs and Symptoms
Patients with retropharyngeal hematomas can present with dyspnea, sore throat, dysphagia, or odynophagia. Neck tenderness and swelling can suggest a retropharyngeal hematoma.5 The diagnosis of a retropharyngeal hemorrhage is of clinical importance because of the possible threat of airway obstruction—which may not be initially detectable clinically, and depends on how quickly the blood fills the retropharyngeal space.1,6
Diagnosis
Computed tomography with intravenous contrast is the imaging study of choice for diagnosing retropharyngeal hematomas in the emergent care setting, and can detect the presence of any associated vertebral facture.5,7,8 Lateral neck X-ray imaging can detect prevertebral swelling, but is not as sensitive as CT and may underestimate the extent of spinal injury; moreover, lesions or early bleeding may be missed.9 In the absence of vertebral fracture on CT imaging, magnetic resonance imaging should be considered to evaluate for possible associated ligamentous injury.9
Treatment and Management
Airway Management. Given the risk of progression to complete airway obstruction, the first step in managing retropharyngeal hematomas is to secure the patient’s airway. Even though the published literature recommends either endotracheal intubation or tracheostomy, the latter should only be considered as a last resort for patients on DOACs because of the increased risk of bleeding.
The fiberoptic approach to endotracheal intubation minimizes the risk of further trauma and rupture of the hematoma.1,10 Once the patient’s airway is secure, the hematoma can be managed conservatively with spinal immobilization and observation for resolution, which may take 2 to 3 weeks.6,11
Surgical Intervention. Some clinicians believe early surgical intervention leads to early recovery and a shorter hospitalization.12 Surgical intervention using a transoral or anterior cervical approach is recommended for large hematomas that fail to regress.6 Surgical intervention may be considered for patients taking warfarin after successful anticoagulation reversal is achieved using fresh frozen plasma (FFP) and vitamin K. However, due to the increased bleeding potential and limited reversal options, there is an increased risk of surgical complications in patients on DOACs.5
Direct Oral Anticoagulation Reversal
The anticoagulation effect of DOACs resolves after five half-lives from the last administered dose, which in the case of rivaroxaban, is between 1 to 2 days.13 Therefore, when emergent surgical intervention is required for a retropharyngeal hematoma, understanding the options and limitations of reversal agents is necessary.
Idarucizumab. Currently the only DOAC anticoagulation reversal agent approved by the US Food and Drug Administration, idarucizumab is only effective for reversing the anticoagulation effects of dabigatran.4,14
Prothrombin Complex Concentrate. Also referred to as factor IX complex, prothrombin complex concentrate (PCC) has been shown to correct prolonged prothrombin time in experimental models of bleeding. Although there is no clinical evidence for its use in DOAC-associated bleeding, PCC should be considered in life-threatening cases, including large or expanding prevertebral hematoma, or other cases in which the potential benefit outweighs the potential risk of thrombosis associated with PCC.4
Fresh Frozen Plasma. In the absence of PCC, FFP may be considered, though there are no data supporting its use as a reversal agent for rivaroxaban.15
Conclusion
Although a rare entity, retropharyngeal hematoma should be suspected in patients with cervical fractures or trauma, especially in the setting of anticoagulation. Early airway management should be considered in a patient with a retropharyngeal hematoma, as symptoms of airway obstruction may be insidious. In patients on DOACs, the potential benefit of earlier resolution with surgical intervention must be strongly weighed against the increased risk of bleeding.
1. Duvillard C, Ballester M, Romanet P. Traumatic retropharyngeal hematoma: a rare and critical pathology needed for early diagnosis. Eur Arch Otorhinolaryngol. 2005;262(9):713-715. doi:10.1007/s00405-004-0767-3.
2. Karmacharya P, Pathak R, Ghimire S, et al. Upper airway hematoma secondary to warfarin therapy: a systematic review of reported cases. N Am J Med Sci. 2015;7(11):494-502. doi:10.4103/1947-2714.170606.
3. Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Emerg Med. 2016;34(11S):9-13. doi:10.1016/j.ajem.2016.09.047.
4. Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20:249. doi:10.1186/s13054-016-1413-3.
5. Toker I, Duman Atilla O, Yesilaras M, Ursavas B. Retropharyngeal hematoma due to oral warfarin usage. Turk J Emerg Med. 2014;14(4):182-184. doi:10.5505/1304.7361.2014.25594.
6. Senel AC, Gunduz AK. Retropharyngeal hematoma secondary to minor blunt neck trauma: case report. Rev Bras Anestesiol. 2012;62(5):731-735. doi:10.1016/S0034-7094(12)70171-X.
7. Koulouris G, Pianta M, Stuckey S. The ‘sentinel clot’ sign in spontaneous retropharyngeal hematoma secondary to parathyroid apoplexy. Ear Nose Throat J. 2006;85(9):606-608.
8. Ryan MF, Meurer D, Tyndall JA. Expanding prevertebral soft tissue swelling subsequent to a motor vehicle collision. Case Rep Emerg Med. 2014;2014:870580. doi:10.1155/2014/870580.
9. Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J. 2010;19(suppl 1):S8-S17. doi:10.1007/s00586-009-1123-5.
10. Shaw CB, Bawa R, Snider G, Wax MK. Traumatic retropharyngeal hematoma: a case report. Otolaryngol Head Neck Surg. 1995;113(4):485-488. doi:10.1016/S0194-59989570091-9.
11. Mackenzie JW, Jellicoe JA. Acute upper airway obstruction. Spontaneous retropharyngeal haematoma in a patient with polycythaemia rubra vera. Anaesthesia. 1986;41(1):57-60.
12. Park JH, Jeong EK, Kang DH, Jeon SR. Surgical treatment of a life-threatening large retropharyngeal hematoma after minor trauma: two case reports and a literature review. J Korean Neurosurg Soc. 2015;58(3):304-307. doi:10.3340/jkns.2015.58.3.304.
13. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52(2):69-82. doi:10.1007/s40262-012-0030-9.
14. Christos S, Naples R. Anticoagulation reversal and treatment strategies in major bleeding: update 2016. West J Emerg Med. 2016;17(3):264-270. doi:10.5811/westjem.2016.3.29294. Erratum in: West J Emerg Med. 2016;17(5):669-670.
15. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790-1798. doi:10.1111/jth.13117.
Case
A 90-year-old woman with chronic obstructive pulmonary disease; hypertension; chronic kidney disease; diastolic dysfunction; severe tricuspid regurgitation; and atrial fibrillation (AF), for which she was taking rivaroxaban, presented to the ED for evaluation of injuries she sustained during a fall. The patient’s family stated that she fell while walking with the assistance of a walker and landed on her face. There was no reported loss of consciousness. Upon arrival at the ED, the patient’s vital signs were: blood pressure, 188/105 mm Hg; heart rate, 91 beats/min; respiratory rate, 20 breaths/min; and temperature, 97.88°F (36.6°C). Oxygen (O2) saturation was 90% on room air, but increased to 98% after the patient received 10 L/min of O2 through a non-rebreather mask.
On physical examination, the patient was awake, alert, and oriented to person, place, and time, with a Glasgow Coma Scale score of 15. She was able to move all four extremities and had 4/5 motor strength in the upper extremities bilaterally, and 3/5 motor strength in the bilateral lower limbs, which her family reported was the same as her baseline. On pulmonary examination, the lungs were clear to auscultation bilaterally and had no stridor. On auscultation she had a regular rate, with no murmurs or rubs.
The patient had nasal bone tenderness with epistaxis that resolved spontaneously and did not require packing; she had no other facial tenderness. The oropharynx was clear. There was mild posterior midline tenderness over C5 and C6, but no skin ecchymosis or neck swelling. Along with the non-rebreather mask, the patient was placed in a neck collar while she awaited transport to radiology for computed tomography (CT) studies.
The CT scan of the cervical spine demonstrated a minimally displaced fracture of the right anterior arch, both sides of the posterior arch of C1, and a comminuted minimally displaced fracture involving the posterior arch and spinous process of C5, with mild retrolisthesis of C5 over C6.
Based on the CT findings, the patient was taken to the operating room (OR) where she underwent awake fiberoptic laryngoscopy. During transfer to the OR, the patient’s O2 dropped to 87%; however, after successful intubation without complication, O2 saturation improved to 95%. After intubation, the patient was admitted to the intensive care unit for observation, and rivaroxaban therapy was discontinued.
A CT scan of the neck postintubation showed a mild interval decrease in the retropharyngeal hematoma, but an increase in the anterior disc space at C5-C6 with mild retrolisthesis, which raised suspicion for an anterior longitudinal ligamentous injury. A repeat CT scan on hospital day 4 revealed a new bleed within the old retropharyngeal hematoma, with no increase in thickness or size of the initial hematoma. The head and neck surgical team kept the patient intubated while awaiting resolution of the hematoma, with no plan of surgery.
On hospital day 6, the patient was transferred to another facility for continued long-term care. She was transitioned to a tracheostomy 4 days later. Follow-up approximately 2 weeks after presentation confirmed complete resolution of the hematoma, and no surgical intervention was required.
Discussion
Overview
Retropharyngeal hematomas are infrequent, but potentially life-threatening complications of cervical fractures, foreign body trauma, infection, violent coughing, and anticoagulation therapy.1 Although retropharyngeal hematomas associated with warfarin have been well described, to our knowledge, there are no reported cases associated with a direct oral anticoagulant (DOAC).2
Though multiple studies have supported the effectiveness and safety of DOACs for prevention of stroke and systemic embolism in patients with AF, the risk of hemorrhage still exists.3 Postmarketing surveillance studies of DOACs report an overall risk of bleeding comparable to warfarin. Gastrointestinal bleeding was found to be slightly higher in patients taking a DOAC compared to those on warfarin, but the risk of intracranial bleeding from DOACs was notably lower.3 With limited effective reversal agents, DOACs present a tremendous challenge in managing acute life-threatening hemorrhage.4
Signs and Symptoms
Patients with retropharyngeal hematomas can present with dyspnea, sore throat, dysphagia, or odynophagia. Neck tenderness and swelling can suggest a retropharyngeal hematoma.5 The diagnosis of a retropharyngeal hemorrhage is of clinical importance because of the possible threat of airway obstruction—which may not be initially detectable clinically, and depends on how quickly the blood fills the retropharyngeal space.1,6
Diagnosis
Computed tomography with intravenous contrast is the imaging study of choice for diagnosing retropharyngeal hematomas in the emergent care setting, and can detect the presence of any associated vertebral facture.5,7,8 Lateral neck X-ray imaging can detect prevertebral swelling, but is not as sensitive as CT and may underestimate the extent of spinal injury; moreover, lesions or early bleeding may be missed.9 In the absence of vertebral fracture on CT imaging, magnetic resonance imaging should be considered to evaluate for possible associated ligamentous injury.9
Treatment and Management
Airway Management. Given the risk of progression to complete airway obstruction, the first step in managing retropharyngeal hematomas is to secure the patient’s airway. Even though the published literature recommends either endotracheal intubation or tracheostomy, the latter should only be considered as a last resort for patients on DOACs because of the increased risk of bleeding.
The fiberoptic approach to endotracheal intubation minimizes the risk of further trauma and rupture of the hematoma.1,10 Once the patient’s airway is secure, the hematoma can be managed conservatively with spinal immobilization and observation for resolution, which may take 2 to 3 weeks.6,11
Surgical Intervention. Some clinicians believe early surgical intervention leads to early recovery and a shorter hospitalization.12 Surgical intervention using a transoral or anterior cervical approach is recommended for large hematomas that fail to regress.6 Surgical intervention may be considered for patients taking warfarin after successful anticoagulation reversal is achieved using fresh frozen plasma (FFP) and vitamin K. However, due to the increased bleeding potential and limited reversal options, there is an increased risk of surgical complications in patients on DOACs.5
Direct Oral Anticoagulation Reversal
The anticoagulation effect of DOACs resolves after five half-lives from the last administered dose, which in the case of rivaroxaban, is between 1 to 2 days.13 Therefore, when emergent surgical intervention is required for a retropharyngeal hematoma, understanding the options and limitations of reversal agents is necessary.
Idarucizumab. Currently the only DOAC anticoagulation reversal agent approved by the US Food and Drug Administration, idarucizumab is only effective for reversing the anticoagulation effects of dabigatran.4,14
Prothrombin Complex Concentrate. Also referred to as factor IX complex, prothrombin complex concentrate (PCC) has been shown to correct prolonged prothrombin time in experimental models of bleeding. Although there is no clinical evidence for its use in DOAC-associated bleeding, PCC should be considered in life-threatening cases, including large or expanding prevertebral hematoma, or other cases in which the potential benefit outweighs the potential risk of thrombosis associated with PCC.4
Fresh Frozen Plasma. In the absence of PCC, FFP may be considered, though there are no data supporting its use as a reversal agent for rivaroxaban.15
Conclusion
Although a rare entity, retropharyngeal hematoma should be suspected in patients with cervical fractures or trauma, especially in the setting of anticoagulation. Early airway management should be considered in a patient with a retropharyngeal hematoma, as symptoms of airway obstruction may be insidious. In patients on DOACs, the potential benefit of earlier resolution with surgical intervention must be strongly weighed against the increased risk of bleeding.
Case
A 90-year-old woman with chronic obstructive pulmonary disease; hypertension; chronic kidney disease; diastolic dysfunction; severe tricuspid regurgitation; and atrial fibrillation (AF), for which she was taking rivaroxaban, presented to the ED for evaluation of injuries she sustained during a fall. The patient’s family stated that she fell while walking with the assistance of a walker and landed on her face. There was no reported loss of consciousness. Upon arrival at the ED, the patient’s vital signs were: blood pressure, 188/105 mm Hg; heart rate, 91 beats/min; respiratory rate, 20 breaths/min; and temperature, 97.88°F (36.6°C). Oxygen (O2) saturation was 90% on room air, but increased to 98% after the patient received 10 L/min of O2 through a non-rebreather mask.
On physical examination, the patient was awake, alert, and oriented to person, place, and time, with a Glasgow Coma Scale score of 15. She was able to move all four extremities and had 4/5 motor strength in the upper extremities bilaterally, and 3/5 motor strength in the bilateral lower limbs, which her family reported was the same as her baseline. On pulmonary examination, the lungs were clear to auscultation bilaterally and had no stridor. On auscultation she had a regular rate, with no murmurs or rubs.
The patient had nasal bone tenderness with epistaxis that resolved spontaneously and did not require packing; she had no other facial tenderness. The oropharynx was clear. There was mild posterior midline tenderness over C5 and C6, but no skin ecchymosis or neck swelling. Along with the non-rebreather mask, the patient was placed in a neck collar while she awaited transport to radiology for computed tomography (CT) studies.
The CT scan of the cervical spine demonstrated a minimally displaced fracture of the right anterior arch, both sides of the posterior arch of C1, and a comminuted minimally displaced fracture involving the posterior arch and spinous process of C5, with mild retrolisthesis of C5 over C6.
Based on the CT findings, the patient was taken to the operating room (OR) where she underwent awake fiberoptic laryngoscopy. During transfer to the OR, the patient’s O2 dropped to 87%; however, after successful intubation without complication, O2 saturation improved to 95%. After intubation, the patient was admitted to the intensive care unit for observation, and rivaroxaban therapy was discontinued.
A CT scan of the neck postintubation showed a mild interval decrease in the retropharyngeal hematoma, but an increase in the anterior disc space at C5-C6 with mild retrolisthesis, which raised suspicion for an anterior longitudinal ligamentous injury. A repeat CT scan on hospital day 4 revealed a new bleed within the old retropharyngeal hematoma, with no increase in thickness or size of the initial hematoma. The head and neck surgical team kept the patient intubated while awaiting resolution of the hematoma, with no plan of surgery.
On hospital day 6, the patient was transferred to another facility for continued long-term care. She was transitioned to a tracheostomy 4 days later. Follow-up approximately 2 weeks after presentation confirmed complete resolution of the hematoma, and no surgical intervention was required.
Discussion
Overview
Retropharyngeal hematomas are infrequent, but potentially life-threatening complications of cervical fractures, foreign body trauma, infection, violent coughing, and anticoagulation therapy.1 Although retropharyngeal hematomas associated with warfarin have been well described, to our knowledge, there are no reported cases associated with a direct oral anticoagulant (DOAC).2
Though multiple studies have supported the effectiveness and safety of DOACs for prevention of stroke and systemic embolism in patients with AF, the risk of hemorrhage still exists.3 Postmarketing surveillance studies of DOACs report an overall risk of bleeding comparable to warfarin. Gastrointestinal bleeding was found to be slightly higher in patients taking a DOAC compared to those on warfarin, but the risk of intracranial bleeding from DOACs was notably lower.3 With limited effective reversal agents, DOACs present a tremendous challenge in managing acute life-threatening hemorrhage.4
Signs and Symptoms
Patients with retropharyngeal hematomas can present with dyspnea, sore throat, dysphagia, or odynophagia. Neck tenderness and swelling can suggest a retropharyngeal hematoma.5 The diagnosis of a retropharyngeal hemorrhage is of clinical importance because of the possible threat of airway obstruction—which may not be initially detectable clinically, and depends on how quickly the blood fills the retropharyngeal space.1,6
Diagnosis
Computed tomography with intravenous contrast is the imaging study of choice for diagnosing retropharyngeal hematomas in the emergent care setting, and can detect the presence of any associated vertebral facture.5,7,8 Lateral neck X-ray imaging can detect prevertebral swelling, but is not as sensitive as CT and may underestimate the extent of spinal injury; moreover, lesions or early bleeding may be missed.9 In the absence of vertebral fracture on CT imaging, magnetic resonance imaging should be considered to evaluate for possible associated ligamentous injury.9
Treatment and Management
Airway Management. Given the risk of progression to complete airway obstruction, the first step in managing retropharyngeal hematomas is to secure the patient’s airway. Even though the published literature recommends either endotracheal intubation or tracheostomy, the latter should only be considered as a last resort for patients on DOACs because of the increased risk of bleeding.
The fiberoptic approach to endotracheal intubation minimizes the risk of further trauma and rupture of the hematoma.1,10 Once the patient’s airway is secure, the hematoma can be managed conservatively with spinal immobilization and observation for resolution, which may take 2 to 3 weeks.6,11
Surgical Intervention. Some clinicians believe early surgical intervention leads to early recovery and a shorter hospitalization.12 Surgical intervention using a transoral or anterior cervical approach is recommended for large hematomas that fail to regress.6 Surgical intervention may be considered for patients taking warfarin after successful anticoagulation reversal is achieved using fresh frozen plasma (FFP) and vitamin K. However, due to the increased bleeding potential and limited reversal options, there is an increased risk of surgical complications in patients on DOACs.5
Direct Oral Anticoagulation Reversal
The anticoagulation effect of DOACs resolves after five half-lives from the last administered dose, which in the case of rivaroxaban, is between 1 to 2 days.13 Therefore, when emergent surgical intervention is required for a retropharyngeal hematoma, understanding the options and limitations of reversal agents is necessary.
Idarucizumab. Currently the only DOAC anticoagulation reversal agent approved by the US Food and Drug Administration, idarucizumab is only effective for reversing the anticoagulation effects of dabigatran.4,14
Prothrombin Complex Concentrate. Also referred to as factor IX complex, prothrombin complex concentrate (PCC) has been shown to correct prolonged prothrombin time in experimental models of bleeding. Although there is no clinical evidence for its use in DOAC-associated bleeding, PCC should be considered in life-threatening cases, including large or expanding prevertebral hematoma, or other cases in which the potential benefit outweighs the potential risk of thrombosis associated with PCC.4
Fresh Frozen Plasma. In the absence of PCC, FFP may be considered, though there are no data supporting its use as a reversal agent for rivaroxaban.15
Conclusion
Although a rare entity, retropharyngeal hematoma should be suspected in patients with cervical fractures or trauma, especially in the setting of anticoagulation. Early airway management should be considered in a patient with a retropharyngeal hematoma, as symptoms of airway obstruction may be insidious. In patients on DOACs, the potential benefit of earlier resolution with surgical intervention must be strongly weighed against the increased risk of bleeding.
1. Duvillard C, Ballester M, Romanet P. Traumatic retropharyngeal hematoma: a rare and critical pathology needed for early diagnosis. Eur Arch Otorhinolaryngol. 2005;262(9):713-715. doi:10.1007/s00405-004-0767-3.
2. Karmacharya P, Pathak R, Ghimire S, et al. Upper airway hematoma secondary to warfarin therapy: a systematic review of reported cases. N Am J Med Sci. 2015;7(11):494-502. doi:10.4103/1947-2714.170606.
3. Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Emerg Med. 2016;34(11S):9-13. doi:10.1016/j.ajem.2016.09.047.
4. Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20:249. doi:10.1186/s13054-016-1413-3.
5. Toker I, Duman Atilla O, Yesilaras M, Ursavas B. Retropharyngeal hematoma due to oral warfarin usage. Turk J Emerg Med. 2014;14(4):182-184. doi:10.5505/1304.7361.2014.25594.
6. Senel AC, Gunduz AK. Retropharyngeal hematoma secondary to minor blunt neck trauma: case report. Rev Bras Anestesiol. 2012;62(5):731-735. doi:10.1016/S0034-7094(12)70171-X.
7. Koulouris G, Pianta M, Stuckey S. The ‘sentinel clot’ sign in spontaneous retropharyngeal hematoma secondary to parathyroid apoplexy. Ear Nose Throat J. 2006;85(9):606-608.
8. Ryan MF, Meurer D, Tyndall JA. Expanding prevertebral soft tissue swelling subsequent to a motor vehicle collision. Case Rep Emerg Med. 2014;2014:870580. doi:10.1155/2014/870580.
9. Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J. 2010;19(suppl 1):S8-S17. doi:10.1007/s00586-009-1123-5.
10. Shaw CB, Bawa R, Snider G, Wax MK. Traumatic retropharyngeal hematoma: a case report. Otolaryngol Head Neck Surg. 1995;113(4):485-488. doi:10.1016/S0194-59989570091-9.
11. Mackenzie JW, Jellicoe JA. Acute upper airway obstruction. Spontaneous retropharyngeal haematoma in a patient with polycythaemia rubra vera. Anaesthesia. 1986;41(1):57-60.
12. Park JH, Jeong EK, Kang DH, Jeon SR. Surgical treatment of a life-threatening large retropharyngeal hematoma after minor trauma: two case reports and a literature review. J Korean Neurosurg Soc. 2015;58(3):304-307. doi:10.3340/jkns.2015.58.3.304.
13. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52(2):69-82. doi:10.1007/s40262-012-0030-9.
14. Christos S, Naples R. Anticoagulation reversal and treatment strategies in major bleeding: update 2016. West J Emerg Med. 2016;17(3):264-270. doi:10.5811/westjem.2016.3.29294. Erratum in: West J Emerg Med. 2016;17(5):669-670.
15. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790-1798. doi:10.1111/jth.13117.
1. Duvillard C, Ballester M, Romanet P. Traumatic retropharyngeal hematoma: a rare and critical pathology needed for early diagnosis. Eur Arch Otorhinolaryngol. 2005;262(9):713-715. doi:10.1007/s00405-004-0767-3.
2. Karmacharya P, Pathak R, Ghimire S, et al. Upper airway hematoma secondary to warfarin therapy: a systematic review of reported cases. N Am J Med Sci. 2015;7(11):494-502. doi:10.4103/1947-2714.170606.
3. Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Emerg Med. 2016;34(11S):9-13. doi:10.1016/j.ajem.2016.09.047.
4. Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20:249. doi:10.1186/s13054-016-1413-3.
5. Toker I, Duman Atilla O, Yesilaras M, Ursavas B. Retropharyngeal hematoma due to oral warfarin usage. Turk J Emerg Med. 2014;14(4):182-184. doi:10.5505/1304.7361.2014.25594.
6. Senel AC, Gunduz AK. Retropharyngeal hematoma secondary to minor blunt neck trauma: case report. Rev Bras Anestesiol. 2012;62(5):731-735. doi:10.1016/S0034-7094(12)70171-X.
7. Koulouris G, Pianta M, Stuckey S. The ‘sentinel clot’ sign in spontaneous retropharyngeal hematoma secondary to parathyroid apoplexy. Ear Nose Throat J. 2006;85(9):606-608.
8. Ryan MF, Meurer D, Tyndall JA. Expanding prevertebral soft tissue swelling subsequent to a motor vehicle collision. Case Rep Emerg Med. 2014;2014:870580. doi:10.1155/2014/870580.
9. Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J. 2010;19(suppl 1):S8-S17. doi:10.1007/s00586-009-1123-5.
10. Shaw CB, Bawa R, Snider G, Wax MK. Traumatic retropharyngeal hematoma: a case report. Otolaryngol Head Neck Surg. 1995;113(4):485-488. doi:10.1016/S0194-59989570091-9.
11. Mackenzie JW, Jellicoe JA. Acute upper airway obstruction. Spontaneous retropharyngeal haematoma in a patient with polycythaemia rubra vera. Anaesthesia. 1986;41(1):57-60.
12. Park JH, Jeong EK, Kang DH, Jeon SR. Surgical treatment of a life-threatening large retropharyngeal hematoma after minor trauma: two case reports and a literature review. J Korean Neurosurg Soc. 2015;58(3):304-307. doi:10.3340/jkns.2015.58.3.304.
13. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52(2):69-82. doi:10.1007/s40262-012-0030-9.
14. Christos S, Naples R. Anticoagulation reversal and treatment strategies in major bleeding: update 2016. West J Emerg Med. 2016;17(3):264-270. doi:10.5811/westjem.2016.3.29294. Erratum in: West J Emerg Med. 2016;17(5):669-670.
15. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790-1798. doi:10.1111/jth.13117.
Nontraumatic Disc Herniation as a Cause of Unusual Cervical Spondylotic Myelopathy
Case
A 55-year-old previously healthy woman with an insignificant medical history presented to the ED for evaluation of right-sided numbness, tingling, and inability to sense temperature. The patient stated the numbness and tingling first began in her right leg and thigh 2 months earlier, and had progressively worsened to her entire right-side. She said she first experienced the thermoanesthesia while taking a shower the morning of presentation. While showering, the patient noted that she could not feel any hot or cold sensation on the right side of her body, including her right leg and arm. She also reported decreased sensation to her extremities on the right side.
She denied any new weakness, headache, chest pain, shortness of breath, fever, chills, nausea, vomiting, back pain, neck pain, or any other symptoms. In addition, she denied any difficulty swallowing, speaking, blurry vision, or double vision. Regarding her social history, the patient denied a history of sexually transmitted diseases, including syphilis; or any tobacco, alcohol, or illicit drug use. The patient confirmed that she had never experienced any of the presenting symptoms prior to 2 months ago. There was no history of trauma or falls. A review of systems was otherwise negative.
Vital signs at presentation were: blood pressure, 129/88 mm Hg; heart rate, 99 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.5°F. Oxygen saturation was 98% on room air. Physical examination revealed a middle-aged woman who was awake, alert, and oriented. Her head was normocephalic and atraumatic, and her pupils were 5 mm, equal, round, and reactive to light bilaterally. Her cranial nerves II through XII were intact. She had normal 5/5 strength in both her upper and lower extremities bilaterally, and had 2+ and equal bilateral patella and Achilles deep tendon reflexes. The patient had no truncal ataxia, and she had a normal gait on ambulation. She was unable to sense temperature (assessed by touching a cold metal tray with her right hand). There was no neck or back midline tenderness to palpation of her spine.
Initial laboratory studies included a complete blood count (CBC); basic metabolic panel (BMP), including blood urea nitrogen; and urine drug screen (UDS). The CBC and BMP were within normal limits, except for an elevated creatine kinase of 249 U/L. The UDS was positive for cocaine. A head computed tomography (CT) scan without contrast was unremarkable.
The patient was admitted to the hospital for further evaluation. Additional laboratory workup during the inpatient stay included nonreactive treponemal immunoglobulin G/immunoglobulin M; nonreactive HIV antigen antibody assay; normal thyroid stimulating hormone; normal free thyroxine, folate, and vitamin B12 levels; normal erythrocyte sedimentation rate, and C-reactive protein levels. The patient’s hemoglobin A1C was also within normal range.
Other imaging studies, which included MR angiography (MRA) of the head and neck, and MRI of the thoracic and lumbar spine, were unremarkable, with the exception of some chronic spondylitic changes.
Due to the significant C3-C4 stenosis, orthopedic surgery services were consulted for a spinal surgery workup. The orthopedic examination identified a few beats of clonus, intact proprioception, and no dysmetria. The patient had decreased sensation to fine touch in the distribution of C7 at the level of the triceps, midphalangeal joints to distal fingertips on the right, fourth, and fifth fingers on the left and right lateral lower extremity. A Hoffmann sign was positive bilaterally. A CT scan of the cervical spine showed severe canal stenosis at the C3-C4 level secondary to a large C3-C4 left paracentral disc protrusion with AP dimensions of the canal measured at 4 to 5 mm. There was no evidence of acute cervical spine fracture or subluxation.
The patient was offered operative and nonoperative management options, including anterior cervical discectomy and fusion vs conservative management with corticosteroid therapy. She agreed to conservative management and received intravenous (IV) dexamethasone with subjective improvement in her symptoms. The patient was discharged home on hospital day 3, with instructions to follow-up with a spine surgeon in 2 weeks. She was also counseled on abstaining from further cocaine or other illicit drug use.
The patient eventually returned for an elective anterior cervical discectomy and fusion 2 months later, after several outpatient visits and progression of symptoms. She was discharged home on postoperative day 1 with pain well-controlled and was able to ambulate without assistance. On follow-up, she reported 15% improvement in her symptoms.
Discussion
Cervical spondylotic myelopathy (CSM) is the most common myelopathy in patients aged 55 years and older. Immediate neuroimaging studies followed by spinal surgery consultation are recommended for patients presenting with acute symptoms suggestive of cord compression.1,2
Diagnosis and Differential Diagnosis
Diagnosis of CSM can be made with a thorough patient history, neurological examination, and MRI/MRA. However, because cases of cervical disc herniation (CDH) are often atraumatic, the patient history may not always be contributory to the diagnosis and severity of the offending cause.
During our patient’s hospital course, there was initially a concern for Brown-Séquard syndrome (BSS) due to the lateralizing symptoms and radiographic findings. This is a rare condition that can occur in the setting of spinal trauma, unilateral disc herniation, tumors, epidural hematomas, and spinal cord ischemia.3,4 In one retrospective case review by Sayer et al,4 the incidence of CDH causing BSS was only 0.21% (5 per 2,350 patients), and 67% of the cases involved C5-C6 or C6-C7.
Although disc herniation usually presents with symptoms on the ipsilateral side in patients with BSS, there are rare case reports of patients with contralateral symptoms in the form of complete or incomplete BSS manifesting as ipsilateral motor deficit and/or loss of contralateral pain and temperature due to an incomplete spinal cord compression.5-7 We were able to rule-out BSS in our patient due to the absence of motor symptoms.
Treatment
Corticosteroid Therapy. High-dose IV corticosteroids should be given to all patients with CSM prior to surgery to reduce cord edema caused by spinal cord injury. In one randomized control trial by Bracken et al,8 patients given methylprednisolone within 8 hours of spinal cord injury had improvement in motor function, sensation to pinprick, and touch at 6 months when compared to placebo. When the aforementioned steps are taken in the emergent care setting, they may significantly improve patient outcomes.
Surgical Intervention. All cases of CSM in the review literature were treated surgically with laminectomy or hemilaminectomy, anterior discectomy with or without fusion, or corpectomy followed by interbody fusion, with the goal of achieving cord decompression. A large majority of patients underwent anterior discectomy with interbody fusion, and all of the cases recommend early surgical intervention in severe CSM to prevent rapidly worsening symptoms, including permanent hemiparesis.
Early surgical intervention is positively correlated with better outcomes, most often resulting in significant improvement of symptoms to full recovery.3,4,6,7,9-12 In one case report, surgical intervention did not result in a significant improvement, and the patient had been suffering from progressive symptoms for 7 years prior to diagnosis and treatment.11
Conservative Management. Conservative management of CSM includes immobilization, activity modification, pain management, and/or corticosteroids therapy.13 However, for patients undergoing surgical decompression, 50% to 80% reported symptom improvement.14,15 This evidence strongly supports management of CSM with early diagnosis and surgical intervention. Despite delays in diagnosis and treatment, surgical intervention can still offer significant relief of weakness and sensory deficits associated with severe CSM.11
Conclusion
Cervical spondylotic myelopathy is the most common myelopathy in patients aged 55 years and older. Common symptoms involve upper extremity sensation, gait disturbances, and deterioration of hand use16; however, there is a large differential for patients presenting to the ED with these symptoms, including mass effect, infection, vascular conditions, metabolic disorders, inflammatory conditions, and trauma.
Our patient with CSM presented with signs of an incomplete cord syndrome with lateralizing features caused by asymmetric disc herniation. This case is unique in that though our patient had some symptom resolution with corticosteroids therapy alone, she ultimately returned for definitive surgical decompression after symptom progression.
1. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23(22):2398-2403.
2. Ishida Y, Tominaga T. Predictors of neurologic recovery in acute central cervical cord injury with only upper extremity impairment. Spine (Phila Pa 1976). 2002;27(15):1652-1658. discussion 1658.
3. Porto GB, Tan LA, Kasliwal MK, Traynelis VC. Progressive Brown-Séquard syndrome: a rare manifestation of cervical disc herniation. J Clin Neurosci. 2016;29:196-198. doi:10.1016/j.jocn.2015.12.021
4. Sayer FT, Vitali AM, Low HL, Paquette S, Honey CR. Brown-Sèquard syndrome produced by C3-C4 cervical disc herniation: a case report and review of the literature. Spine (Phila Pa 1976). 2008;33(9):E279-E282. doi:10.1097/BRS.0b013e31816c835d.
5. Urrutia J, Fadic R. Cervical disc herniation producing acute Brown-Sequard syndrome: dynamic changes documented by intraoperative neuromonitoring. Eur Spine J. 2012;21 Suppl 4:S418-S421. doi:10.1007/s00586-011-1881-8.
6. Choi KB, Lee CD, Chung DJ, Lee SH. Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc. 2009;46(5):505-510. doi:10.3340/jkns.2009.46.5.505. doi:10.3340/jkns.2009.46.5.505.
7. Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sèquard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3(6):530-533.
8. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405-1411. doi:10.1056/NEJM199005173222001.
9. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
10. Antich PA, Sanjuan AC, Girvent FM, Simó JD. High cervical disc herniation and Brown-Sequard syndrome. A case report and review of the literature. J Bone Joint Surg Br. 1999;81(3):462-463.
11. Guan D, Wang G, Claire M, Kuang Z. Brown-Sequard syndrome produced by calcified herniated cervical disc and posterior vertebral osteophyte: case report.
J Orthop. 2015;12(Suppl 2):S260-S263. doi:10.1016/j.jor.2015.10.007.
12. Abouhashem S, Ammar M, Barakat M, Abdelhameed E. Management of Brown-Sequard syndrome in cervical disc diseases. Turk Neurosurg. 2013;23(4):470-475. doi:10.5137/1019-5149.JTN.7433-12.0.
13. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Suppl 1):S43-S50. doi:10.1227/01.NEU.0000215386.05760.6D.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):S1:30-S1:35; discussion S1:35-S1:36. doi:10.1016/j.surneu.2005.02.016.
15. Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-378. doi:10.1007/s00264-006-0315-4.
16. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769; discussion 769-770
Case
A 55-year-old previously healthy woman with an insignificant medical history presented to the ED for evaluation of right-sided numbness, tingling, and inability to sense temperature. The patient stated the numbness and tingling first began in her right leg and thigh 2 months earlier, and had progressively worsened to her entire right-side. She said she first experienced the thermoanesthesia while taking a shower the morning of presentation. While showering, the patient noted that she could not feel any hot or cold sensation on the right side of her body, including her right leg and arm. She also reported decreased sensation to her extremities on the right side.
She denied any new weakness, headache, chest pain, shortness of breath, fever, chills, nausea, vomiting, back pain, neck pain, or any other symptoms. In addition, she denied any difficulty swallowing, speaking, blurry vision, or double vision. Regarding her social history, the patient denied a history of sexually transmitted diseases, including syphilis; or any tobacco, alcohol, or illicit drug use. The patient confirmed that she had never experienced any of the presenting symptoms prior to 2 months ago. There was no history of trauma or falls. A review of systems was otherwise negative.
Vital signs at presentation were: blood pressure, 129/88 mm Hg; heart rate, 99 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.5°F. Oxygen saturation was 98% on room air. Physical examination revealed a middle-aged woman who was awake, alert, and oriented. Her head was normocephalic and atraumatic, and her pupils were 5 mm, equal, round, and reactive to light bilaterally. Her cranial nerves II through XII were intact. She had normal 5/5 strength in both her upper and lower extremities bilaterally, and had 2+ and equal bilateral patella and Achilles deep tendon reflexes. The patient had no truncal ataxia, and she had a normal gait on ambulation. She was unable to sense temperature (assessed by touching a cold metal tray with her right hand). There was no neck or back midline tenderness to palpation of her spine.
Initial laboratory studies included a complete blood count (CBC); basic metabolic panel (BMP), including blood urea nitrogen; and urine drug screen (UDS). The CBC and BMP were within normal limits, except for an elevated creatine kinase of 249 U/L. The UDS was positive for cocaine. A head computed tomography (CT) scan without contrast was unremarkable.
The patient was admitted to the hospital for further evaluation. Additional laboratory workup during the inpatient stay included nonreactive treponemal immunoglobulin G/immunoglobulin M; nonreactive HIV antigen antibody assay; normal thyroid stimulating hormone; normal free thyroxine, folate, and vitamin B12 levels; normal erythrocyte sedimentation rate, and C-reactive protein levels. The patient’s hemoglobin A1C was also within normal range.
Other imaging studies, which included MR angiography (MRA) of the head and neck, and MRI of the thoracic and lumbar spine, were unremarkable, with the exception of some chronic spondylitic changes.
Due to the significant C3-C4 stenosis, orthopedic surgery services were consulted for a spinal surgery workup. The orthopedic examination identified a few beats of clonus, intact proprioception, and no dysmetria. The patient had decreased sensation to fine touch in the distribution of C7 at the level of the triceps, midphalangeal joints to distal fingertips on the right, fourth, and fifth fingers on the left and right lateral lower extremity. A Hoffmann sign was positive bilaterally. A CT scan of the cervical spine showed severe canal stenosis at the C3-C4 level secondary to a large C3-C4 left paracentral disc protrusion with AP dimensions of the canal measured at 4 to 5 mm. There was no evidence of acute cervical spine fracture or subluxation.
The patient was offered operative and nonoperative management options, including anterior cervical discectomy and fusion vs conservative management with corticosteroid therapy. She agreed to conservative management and received intravenous (IV) dexamethasone with subjective improvement in her symptoms. The patient was discharged home on hospital day 3, with instructions to follow-up with a spine surgeon in 2 weeks. She was also counseled on abstaining from further cocaine or other illicit drug use.
The patient eventually returned for an elective anterior cervical discectomy and fusion 2 months later, after several outpatient visits and progression of symptoms. She was discharged home on postoperative day 1 with pain well-controlled and was able to ambulate without assistance. On follow-up, she reported 15% improvement in her symptoms.
Discussion
Cervical spondylotic myelopathy (CSM) is the most common myelopathy in patients aged 55 years and older. Immediate neuroimaging studies followed by spinal surgery consultation are recommended for patients presenting with acute symptoms suggestive of cord compression.1,2
Diagnosis and Differential Diagnosis
Diagnosis of CSM can be made with a thorough patient history, neurological examination, and MRI/MRA. However, because cases of cervical disc herniation (CDH) are often atraumatic, the patient history may not always be contributory to the diagnosis and severity of the offending cause.
During our patient’s hospital course, there was initially a concern for Brown-Séquard syndrome (BSS) due to the lateralizing symptoms and radiographic findings. This is a rare condition that can occur in the setting of spinal trauma, unilateral disc herniation, tumors, epidural hematomas, and spinal cord ischemia.3,4 In one retrospective case review by Sayer et al,4 the incidence of CDH causing BSS was only 0.21% (5 per 2,350 patients), and 67% of the cases involved C5-C6 or C6-C7.
Although disc herniation usually presents with symptoms on the ipsilateral side in patients with BSS, there are rare case reports of patients with contralateral symptoms in the form of complete or incomplete BSS manifesting as ipsilateral motor deficit and/or loss of contralateral pain and temperature due to an incomplete spinal cord compression.5-7 We were able to rule-out BSS in our patient due to the absence of motor symptoms.
Treatment
Corticosteroid Therapy. High-dose IV corticosteroids should be given to all patients with CSM prior to surgery to reduce cord edema caused by spinal cord injury. In one randomized control trial by Bracken et al,8 patients given methylprednisolone within 8 hours of spinal cord injury had improvement in motor function, sensation to pinprick, and touch at 6 months when compared to placebo. When the aforementioned steps are taken in the emergent care setting, they may significantly improve patient outcomes.
Surgical Intervention. All cases of CSM in the review literature were treated surgically with laminectomy or hemilaminectomy, anterior discectomy with or without fusion, or corpectomy followed by interbody fusion, with the goal of achieving cord decompression. A large majority of patients underwent anterior discectomy with interbody fusion, and all of the cases recommend early surgical intervention in severe CSM to prevent rapidly worsening symptoms, including permanent hemiparesis.
Early surgical intervention is positively correlated with better outcomes, most often resulting in significant improvement of symptoms to full recovery.3,4,6,7,9-12 In one case report, surgical intervention did not result in a significant improvement, and the patient had been suffering from progressive symptoms for 7 years prior to diagnosis and treatment.11
Conservative Management. Conservative management of CSM includes immobilization, activity modification, pain management, and/or corticosteroids therapy.13 However, for patients undergoing surgical decompression, 50% to 80% reported symptom improvement.14,15 This evidence strongly supports management of CSM with early diagnosis and surgical intervention. Despite delays in diagnosis and treatment, surgical intervention can still offer significant relief of weakness and sensory deficits associated with severe CSM.11
Conclusion
Cervical spondylotic myelopathy is the most common myelopathy in patients aged 55 years and older. Common symptoms involve upper extremity sensation, gait disturbances, and deterioration of hand use16; however, there is a large differential for patients presenting to the ED with these symptoms, including mass effect, infection, vascular conditions, metabolic disorders, inflammatory conditions, and trauma.
Our patient with CSM presented with signs of an incomplete cord syndrome with lateralizing features caused by asymmetric disc herniation. This case is unique in that though our patient had some symptom resolution with corticosteroids therapy alone, she ultimately returned for definitive surgical decompression after symptom progression.
Case
A 55-year-old previously healthy woman with an insignificant medical history presented to the ED for evaluation of right-sided numbness, tingling, and inability to sense temperature. The patient stated the numbness and tingling first began in her right leg and thigh 2 months earlier, and had progressively worsened to her entire right-side. She said she first experienced the thermoanesthesia while taking a shower the morning of presentation. While showering, the patient noted that she could not feel any hot or cold sensation on the right side of her body, including her right leg and arm. She also reported decreased sensation to her extremities on the right side.
She denied any new weakness, headache, chest pain, shortness of breath, fever, chills, nausea, vomiting, back pain, neck pain, or any other symptoms. In addition, she denied any difficulty swallowing, speaking, blurry vision, or double vision. Regarding her social history, the patient denied a history of sexually transmitted diseases, including syphilis; or any tobacco, alcohol, or illicit drug use. The patient confirmed that she had never experienced any of the presenting symptoms prior to 2 months ago. There was no history of trauma or falls. A review of systems was otherwise negative.
Vital signs at presentation were: blood pressure, 129/88 mm Hg; heart rate, 99 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.5°F. Oxygen saturation was 98% on room air. Physical examination revealed a middle-aged woman who was awake, alert, and oriented. Her head was normocephalic and atraumatic, and her pupils were 5 mm, equal, round, and reactive to light bilaterally. Her cranial nerves II through XII were intact. She had normal 5/5 strength in both her upper and lower extremities bilaterally, and had 2+ and equal bilateral patella and Achilles deep tendon reflexes. The patient had no truncal ataxia, and she had a normal gait on ambulation. She was unable to sense temperature (assessed by touching a cold metal tray with her right hand). There was no neck or back midline tenderness to palpation of her spine.
Initial laboratory studies included a complete blood count (CBC); basic metabolic panel (BMP), including blood urea nitrogen; and urine drug screen (UDS). The CBC and BMP were within normal limits, except for an elevated creatine kinase of 249 U/L. The UDS was positive for cocaine. A head computed tomography (CT) scan without contrast was unremarkable.
The patient was admitted to the hospital for further evaluation. Additional laboratory workup during the inpatient stay included nonreactive treponemal immunoglobulin G/immunoglobulin M; nonreactive HIV antigen antibody assay; normal thyroid stimulating hormone; normal free thyroxine, folate, and vitamin B12 levels; normal erythrocyte sedimentation rate, and C-reactive protein levels. The patient’s hemoglobin A1C was also within normal range.
Other imaging studies, which included MR angiography (MRA) of the head and neck, and MRI of the thoracic and lumbar spine, were unremarkable, with the exception of some chronic spondylitic changes.
Due to the significant C3-C4 stenosis, orthopedic surgery services were consulted for a spinal surgery workup. The orthopedic examination identified a few beats of clonus, intact proprioception, and no dysmetria. The patient had decreased sensation to fine touch in the distribution of C7 at the level of the triceps, midphalangeal joints to distal fingertips on the right, fourth, and fifth fingers on the left and right lateral lower extremity. A Hoffmann sign was positive bilaterally. A CT scan of the cervical spine showed severe canal stenosis at the C3-C4 level secondary to a large C3-C4 left paracentral disc protrusion with AP dimensions of the canal measured at 4 to 5 mm. There was no evidence of acute cervical spine fracture or subluxation.
The patient was offered operative and nonoperative management options, including anterior cervical discectomy and fusion vs conservative management with corticosteroid therapy. She agreed to conservative management and received intravenous (IV) dexamethasone with subjective improvement in her symptoms. The patient was discharged home on hospital day 3, with instructions to follow-up with a spine surgeon in 2 weeks. She was also counseled on abstaining from further cocaine or other illicit drug use.
The patient eventually returned for an elective anterior cervical discectomy and fusion 2 months later, after several outpatient visits and progression of symptoms. She was discharged home on postoperative day 1 with pain well-controlled and was able to ambulate without assistance. On follow-up, she reported 15% improvement in her symptoms.
Discussion
Cervical spondylotic myelopathy (CSM) is the most common myelopathy in patients aged 55 years and older. Immediate neuroimaging studies followed by spinal surgery consultation are recommended for patients presenting with acute symptoms suggestive of cord compression.1,2
Diagnosis and Differential Diagnosis
Diagnosis of CSM can be made with a thorough patient history, neurological examination, and MRI/MRA. However, because cases of cervical disc herniation (CDH) are often atraumatic, the patient history may not always be contributory to the diagnosis and severity of the offending cause.
During our patient’s hospital course, there was initially a concern for Brown-Séquard syndrome (BSS) due to the lateralizing symptoms and radiographic findings. This is a rare condition that can occur in the setting of spinal trauma, unilateral disc herniation, tumors, epidural hematomas, and spinal cord ischemia.3,4 In one retrospective case review by Sayer et al,4 the incidence of CDH causing BSS was only 0.21% (5 per 2,350 patients), and 67% of the cases involved C5-C6 or C6-C7.
Although disc herniation usually presents with symptoms on the ipsilateral side in patients with BSS, there are rare case reports of patients with contralateral symptoms in the form of complete or incomplete BSS manifesting as ipsilateral motor deficit and/or loss of contralateral pain and temperature due to an incomplete spinal cord compression.5-7 We were able to rule-out BSS in our patient due to the absence of motor symptoms.
Treatment
Corticosteroid Therapy. High-dose IV corticosteroids should be given to all patients with CSM prior to surgery to reduce cord edema caused by spinal cord injury. In one randomized control trial by Bracken et al,8 patients given methylprednisolone within 8 hours of spinal cord injury had improvement in motor function, sensation to pinprick, and touch at 6 months when compared to placebo. When the aforementioned steps are taken in the emergent care setting, they may significantly improve patient outcomes.
Surgical Intervention. All cases of CSM in the review literature were treated surgically with laminectomy or hemilaminectomy, anterior discectomy with or without fusion, or corpectomy followed by interbody fusion, with the goal of achieving cord decompression. A large majority of patients underwent anterior discectomy with interbody fusion, and all of the cases recommend early surgical intervention in severe CSM to prevent rapidly worsening symptoms, including permanent hemiparesis.
Early surgical intervention is positively correlated with better outcomes, most often resulting in significant improvement of symptoms to full recovery.3,4,6,7,9-12 In one case report, surgical intervention did not result in a significant improvement, and the patient had been suffering from progressive symptoms for 7 years prior to diagnosis and treatment.11
Conservative Management. Conservative management of CSM includes immobilization, activity modification, pain management, and/or corticosteroids therapy.13 However, for patients undergoing surgical decompression, 50% to 80% reported symptom improvement.14,15 This evidence strongly supports management of CSM with early diagnosis and surgical intervention. Despite delays in diagnosis and treatment, surgical intervention can still offer significant relief of weakness and sensory deficits associated with severe CSM.11
Conclusion
Cervical spondylotic myelopathy is the most common myelopathy in patients aged 55 years and older. Common symptoms involve upper extremity sensation, gait disturbances, and deterioration of hand use16; however, there is a large differential for patients presenting to the ED with these symptoms, including mass effect, infection, vascular conditions, metabolic disorders, inflammatory conditions, and trauma.
Our patient with CSM presented with signs of an incomplete cord syndrome with lateralizing features caused by asymmetric disc herniation. This case is unique in that though our patient had some symptom resolution with corticosteroids therapy alone, she ultimately returned for definitive surgical decompression after symptom progression.
1. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23(22):2398-2403.
2. Ishida Y, Tominaga T. Predictors of neurologic recovery in acute central cervical cord injury with only upper extremity impairment. Spine (Phila Pa 1976). 2002;27(15):1652-1658. discussion 1658.
3. Porto GB, Tan LA, Kasliwal MK, Traynelis VC. Progressive Brown-Séquard syndrome: a rare manifestation of cervical disc herniation. J Clin Neurosci. 2016;29:196-198. doi:10.1016/j.jocn.2015.12.021
4. Sayer FT, Vitali AM, Low HL, Paquette S, Honey CR. Brown-Sèquard syndrome produced by C3-C4 cervical disc herniation: a case report and review of the literature. Spine (Phila Pa 1976). 2008;33(9):E279-E282. doi:10.1097/BRS.0b013e31816c835d.
5. Urrutia J, Fadic R. Cervical disc herniation producing acute Brown-Sequard syndrome: dynamic changes documented by intraoperative neuromonitoring. Eur Spine J. 2012;21 Suppl 4:S418-S421. doi:10.1007/s00586-011-1881-8.
6. Choi KB, Lee CD, Chung DJ, Lee SH. Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc. 2009;46(5):505-510. doi:10.3340/jkns.2009.46.5.505. doi:10.3340/jkns.2009.46.5.505.
7. Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sèquard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3(6):530-533.
8. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405-1411. doi:10.1056/NEJM199005173222001.
9. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
10. Antich PA, Sanjuan AC, Girvent FM, Simó JD. High cervical disc herniation and Brown-Sequard syndrome. A case report and review of the literature. J Bone Joint Surg Br. 1999;81(3):462-463.
11. Guan D, Wang G, Claire M, Kuang Z. Brown-Sequard syndrome produced by calcified herniated cervical disc and posterior vertebral osteophyte: case report.
J Orthop. 2015;12(Suppl 2):S260-S263. doi:10.1016/j.jor.2015.10.007.
12. Abouhashem S, Ammar M, Barakat M, Abdelhameed E. Management of Brown-Sequard syndrome in cervical disc diseases. Turk Neurosurg. 2013;23(4):470-475. doi:10.5137/1019-5149.JTN.7433-12.0.
13. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Suppl 1):S43-S50. doi:10.1227/01.NEU.0000215386.05760.6D.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):S1:30-S1:35; discussion S1:35-S1:36. doi:10.1016/j.surneu.2005.02.016.
15. Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-378. doi:10.1007/s00264-006-0315-4.
16. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769; discussion 769-770
1. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23(22):2398-2403.
2. Ishida Y, Tominaga T. Predictors of neurologic recovery in acute central cervical cord injury with only upper extremity impairment. Spine (Phila Pa 1976). 2002;27(15):1652-1658. discussion 1658.
3. Porto GB, Tan LA, Kasliwal MK, Traynelis VC. Progressive Brown-Séquard syndrome: a rare manifestation of cervical disc herniation. J Clin Neurosci. 2016;29:196-198. doi:10.1016/j.jocn.2015.12.021
4. Sayer FT, Vitali AM, Low HL, Paquette S, Honey CR. Brown-Sèquard syndrome produced by C3-C4 cervical disc herniation: a case report and review of the literature. Spine (Phila Pa 1976). 2008;33(9):E279-E282. doi:10.1097/BRS.0b013e31816c835d.
5. Urrutia J, Fadic R. Cervical disc herniation producing acute Brown-Sequard syndrome: dynamic changes documented by intraoperative neuromonitoring. Eur Spine J. 2012;21 Suppl 4:S418-S421. doi:10.1007/s00586-011-1881-8.
6. Choi KB, Lee CD, Chung DJ, Lee SH. Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc. 2009;46(5):505-510. doi:10.3340/jkns.2009.46.5.505. doi:10.3340/jkns.2009.46.5.505.
7. Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sèquard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3(6):530-533.
8. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405-1411. doi:10.1056/NEJM199005173222001.
9. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
10. Antich PA, Sanjuan AC, Girvent FM, Simó JD. High cervical disc herniation and Brown-Sequard syndrome. A case report and review of the literature. J Bone Joint Surg Br. 1999;81(3):462-463.
11. Guan D, Wang G, Claire M, Kuang Z. Brown-Sequard syndrome produced by calcified herniated cervical disc and posterior vertebral osteophyte: case report.
J Orthop. 2015;12(Suppl 2):S260-S263. doi:10.1016/j.jor.2015.10.007.
12. Abouhashem S, Ammar M, Barakat M, Abdelhameed E. Management of Brown-Sequard syndrome in cervical disc diseases. Turk Neurosurg. 2013;23(4):470-475. doi:10.5137/1019-5149.JTN.7433-12.0.
13. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Suppl 1):S43-S50. doi:10.1227/01.NEU.0000215386.05760.6D.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):S1:30-S1:35; discussion S1:35-S1:36. doi:10.1016/j.surneu.2005.02.016.
15. Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-378. doi:10.1007/s00264-006-0315-4.
16. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769; discussion 769-770
Don’t Always Rush to Rally Renal
Case
An otherwise healthy 20-month-old boy presented to the ED for evaluation after his father witnessed the child ingest a model race car fuel additive. According to the patient’s father, the boy was playing with several closed bottles that were stored in the garage, when he witnessed the boy open up and take a sip of a pink-colored fuel additive, which the father believed to contain 100% methanol. The patient’s father further noted that immediately after drinking the fluid, the patient spat and drooled, and had one episode of nonbloody emesis prior to arrival at the ED.
Initial vital signs at presentation were: blood pressure, 84/54 mm Hg; heart rate, 97 beats/min; respiratory rate, 24 breaths/min; and temperature 98°F. Oxygen saturation was 99% on room air. Physical examination was notable for mild erythema in the posterior oropharynx. Otherwise, the patient was acting appropriately for his age and in no acute distress. Laboratory studies were within normal limits, except for the following: serum anion gap, 18 mEq/L (reference range for children < 3 years old, 10-14 mEq/L); serum bicarbonate, 19 mmol/L (reference range for children 12-24 months, 17-25 mmol/L); and serum creatinine, 2.8 mg/dL (reference range for children 12 to 24 months, 0.2-0.5 mg/dL). A repeat creatinine test taken after bolus of fluid administration was 2.4 mg/dL. A renal ultrasound, performed to investigate the cause of the renal failure, was unremarkable.
What toxic exposures are of concern based on the clinical history?
The history of exposure to a liquid stored in a garage raises the likelihood of exposure to an automobile-related item such as diethylene glycol, ethylene glycol (EG), and methanol.
Diethylene Glycol. Diethylene glycol is an ingredient in brake and power steering fluids, and has toxic properties qualitatively similar to EG.
Ethylene Glycol. A clear, colorless, odorless fluid with a sweet taste, EG is an ingredient in radiator antifreeze, refrigerant fluid, coolants, and pesticides. Like methylene, EG reaches peak plasma concentration within 1 to 4 hours, but toxic clinical findings do not occur for 3 to 6 hours.1
Methanol. Methanol is a clear, colorless, alcohol found in antifreeze, windshield washer fluid, and race car fuel.2 Although methanol reaches peak plasma concentration in about 30 to 60 minutes, signs of systemic toxicity (ie, metabolic acidosis) typically take 6 to 12 hours to manifest.1
In both EG and methanol, there is a delay in toxic clinical findings because the parent compounds are not toxic in their initial form; rather, major toxicity is derived from their metabolites: formic acid and oxalic acid, respectively.
Other Toxins. Many other potentially toxic liquids are associated with a homeowner’s occupation or avocational interests. These include painting supplies (eg, industrial paints containing lead), gardening materials (eg, pesticides containing organophosphates), fuels (eg, gasoline, polychlorinated biphenyls in coolant, and lubricants), and cleaning supplies (eg, caustics, detergents, and air freshener).
Case Continuation
Since the patient’s elevated anion gap raised concerns for methanol or EG exposure, he was given fomepizole and transferred to a tertiary care children’s hospital for further management and possible hemodialysis. Upon arrival at the receiving hospital, the patient’s vital signs and physical examination remained unchanged. Repeat laboratory studies were notable for a creatinine level of 0.3 mg/dL. The patient’s father was instructed to retrieve the implicated bottle from home. An inspection of the bottle’s ingredients was notable for nitromethane, castor oil, and methanol.
What is nitromethane and what are its uses?
Nitromethane, the simplest nitro compound, is a colorless, viscous, lipid-soluble fluid.3 The polarity of nitromethane permits its use as a stabilizer in a number of chemical solvents, such as dry cleaning fluid, degreasers, and "super glue."4,5 Nitromethane is also commonly added to model-engine and drag-race fuels, which also contain methanol and castor oil.3 In this capacity, nitromethane functions as an oxygen carrier, allowing more efficient fuel use in combustion cylinders (compared to gasoline), thereby increasing the horsepower of the vehicle.6 It is therefore commonly added to fuel for drag racers, radio-controlled cars, and model aircrafts.4 In the small concentrations typically inadvertently ingested, the clinical effects of nitromethane itself are inconsequential.
What is the differential for creatinine elevation?
Creatinine itself is a normal breakdown product of muscle metabolism produced by spontaneous conversion from creatine and is found at a fairly constant serum level in proportion to muscle mass.7 Thus, as people age and muscle mass decreases, their baseline creatinine levels decrease proportionally.
Elimination. The majority of creatinine (85%-90%) is filtered and excreted by the kidneys, with the remaining 10% to 15% secreted by the tubules, allowing creatinine to be a surrogate measure of the glomerular filtration rate.7 Exogenous sources of creatine or creatinine include meat and creatine supplements, the latter of which are used as an "energy source" to enhance athletic performance.
Etiology. The etiology for an elevated serum creatinine concentration includes renal failure, both acute and chronic; volume depletion; hemorrhage (low blood volume); and medications, including diuretics, angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics. These etiologies can also be categorized as processes that increase creatinine production, decrease elimination (H2 antagonist and trimethoprim both inhibit the cation secretory pump in the tubules), or interfere with the creatinine assay (ketones, keto acids, lipemia, hemolysis, cephalosporins).7
Because creatinine is filtered so efficiently by the kidney, neither exogenous nor endogenous creatinine sources are expected to increase serum creatinine in the absence of renal dysfunction. However, transient elevation may occur in body builders who use extreme doses of creatine. Patients with rhabdomyolysis often develop elevated creatinine concentrations, but nearly always in the setting of myoglobinuric renal failure.
Jaffe Reaction and Enzymatic Methods. Serum creatinine can be measured using either the Jaffe reaction or the enzymatic method. In the Jaffe reaction, creatinine reacts with alkaline sodium picrate to form a red-orange chromophore, which absorbs light in the range of 470 to 550 nanometers on spectroscopy.6,8,9 The active methylene group on nitromethane also reacts with alkaline sodium picrate to form a chromophore which absorbs light in the same wavelength range.10 Thus, serum creatinine measurements via the Jaffe reaction are falsely elevated due to the cross-reactivity between nitromethane and alkaline sodium picrate. In some reported cases, there is a 20-fold increase in the measured serum creatinine in the presence of nitromethane; renal function, however, remains normal.5
This false reading seen in the Jaffe reaction can be avoided by utilizing the enzymatic method of creatinine measurement, a three-step process that ultimately produces hydrogen peroxide, which is measured and accurately correlates with serum creatinine—even in the presence of nitromethane.8 This distinction explains the dramatically different creatinine concentrations measured at the two institutions in this case.
Case Conclusion
The patient was monitored overnight at the children’s hospital. Repeat laboratory studies in the morning showed a normal creatinine level of 0.3 mg/dL and a negative methanol level. The patient was discharged home in the care of his father, who was instructed to follow-up with his son’s pediatrician. The father also received counseling on safe storage practices for dangerous chemicals.
1. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711. doi:10.1016/j.ccc.2012.07.002.
2. McMahon DM, Winstead S, Weant KA. Toxic alcohol ingestions: focus on ethylene glycol and methanol. Adv Emerg Nurs J. 2009;31(3):206-213. doi:10.1097/TME.0b013e3181ad8be8.
3. Cook MD, Clark RF. Creatinine elevation associated with nitromethane exposure: a marker of potential methanol toxicity. J Emerg Med. 2007;33(3):249-253. doi:10.1016/j.jemermed.2007.02.015.
4. Markofsky SB. Nitro compounds, aliphatic. In: Elvers B, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. doi:10.1002/14356007.a17_401. [digital]
5. Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be "fueled" by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):
315-320.
6. Ngo AS, Rowley F, Olson KR. Case files of the California poison control system, San Francisco division: blue thunder ingestion: methanol, nitromethane, and elevated creatinine. J Med Toxicol. 2010;6(1):67-71. doi:10.1007/s13181-010-0042-5.
7. Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51-52.
8. Booth C, Naidoo D, Rosenberg A, Kainer G. Elevated creatinine after ingestion of model aviation fuel: interference with the Jaffe reaction by nitromethane. J Paediatr Child Health. 1999;35(5):503-504.
9. de Lelis Medeiros de Morais C, Gomes de Lima KM. Determination and analytical validation of creatinine content in serum using image analysis by multivariate transfer calibration procedures. Anal Meth. 2015;7:6904-6910. doi:10.1039/C5AY01369K.
10. Killorn E, Lim RK, Rieder M. Apparent elevated creatinine after ingestion of nitromethane: interference with the Jaffe reaction. Ther Drug Monit. 2011;33(1):1-2. doi:10.1097/FTD.0b013e3181fe7e52.
Case
An otherwise healthy 20-month-old boy presented to the ED for evaluation after his father witnessed the child ingest a model race car fuel additive. According to the patient’s father, the boy was playing with several closed bottles that were stored in the garage, when he witnessed the boy open up and take a sip of a pink-colored fuel additive, which the father believed to contain 100% methanol. The patient’s father further noted that immediately after drinking the fluid, the patient spat and drooled, and had one episode of nonbloody emesis prior to arrival at the ED.
Initial vital signs at presentation were: blood pressure, 84/54 mm Hg; heart rate, 97 beats/min; respiratory rate, 24 breaths/min; and temperature 98°F. Oxygen saturation was 99% on room air. Physical examination was notable for mild erythema in the posterior oropharynx. Otherwise, the patient was acting appropriately for his age and in no acute distress. Laboratory studies were within normal limits, except for the following: serum anion gap, 18 mEq/L (reference range for children < 3 years old, 10-14 mEq/L); serum bicarbonate, 19 mmol/L (reference range for children 12-24 months, 17-25 mmol/L); and serum creatinine, 2.8 mg/dL (reference range for children 12 to 24 months, 0.2-0.5 mg/dL). A repeat creatinine test taken after bolus of fluid administration was 2.4 mg/dL. A renal ultrasound, performed to investigate the cause of the renal failure, was unremarkable.
What toxic exposures are of concern based on the clinical history?
The history of exposure to a liquid stored in a garage raises the likelihood of exposure to an automobile-related item such as diethylene glycol, ethylene glycol (EG), and methanol.
Diethylene Glycol. Diethylene glycol is an ingredient in brake and power steering fluids, and has toxic properties qualitatively similar to EG.
Ethylene Glycol. A clear, colorless, odorless fluid with a sweet taste, EG is an ingredient in radiator antifreeze, refrigerant fluid, coolants, and pesticides. Like methylene, EG reaches peak plasma concentration within 1 to 4 hours, but toxic clinical findings do not occur for 3 to 6 hours.1
Methanol. Methanol is a clear, colorless, alcohol found in antifreeze, windshield washer fluid, and race car fuel.2 Although methanol reaches peak plasma concentration in about 30 to 60 minutes, signs of systemic toxicity (ie, metabolic acidosis) typically take 6 to 12 hours to manifest.1
In both EG and methanol, there is a delay in toxic clinical findings because the parent compounds are not toxic in their initial form; rather, major toxicity is derived from their metabolites: formic acid and oxalic acid, respectively.
Other Toxins. Many other potentially toxic liquids are associated with a homeowner’s occupation or avocational interests. These include painting supplies (eg, industrial paints containing lead), gardening materials (eg, pesticides containing organophosphates), fuels (eg, gasoline, polychlorinated biphenyls in coolant, and lubricants), and cleaning supplies (eg, caustics, detergents, and air freshener).
Case Continuation
Since the patient’s elevated anion gap raised concerns for methanol or EG exposure, he was given fomepizole and transferred to a tertiary care children’s hospital for further management and possible hemodialysis. Upon arrival at the receiving hospital, the patient’s vital signs and physical examination remained unchanged. Repeat laboratory studies were notable for a creatinine level of 0.3 mg/dL. The patient’s father was instructed to retrieve the implicated bottle from home. An inspection of the bottle’s ingredients was notable for nitromethane, castor oil, and methanol.
What is nitromethane and what are its uses?
Nitromethane, the simplest nitro compound, is a colorless, viscous, lipid-soluble fluid.3 The polarity of nitromethane permits its use as a stabilizer in a number of chemical solvents, such as dry cleaning fluid, degreasers, and "super glue."4,5 Nitromethane is also commonly added to model-engine and drag-race fuels, which also contain methanol and castor oil.3 In this capacity, nitromethane functions as an oxygen carrier, allowing more efficient fuel use in combustion cylinders (compared to gasoline), thereby increasing the horsepower of the vehicle.6 It is therefore commonly added to fuel for drag racers, radio-controlled cars, and model aircrafts.4 In the small concentrations typically inadvertently ingested, the clinical effects of nitromethane itself are inconsequential.
What is the differential for creatinine elevation?
Creatinine itself is a normal breakdown product of muscle metabolism produced by spontaneous conversion from creatine and is found at a fairly constant serum level in proportion to muscle mass.7 Thus, as people age and muscle mass decreases, their baseline creatinine levels decrease proportionally.
Elimination. The majority of creatinine (85%-90%) is filtered and excreted by the kidneys, with the remaining 10% to 15% secreted by the tubules, allowing creatinine to be a surrogate measure of the glomerular filtration rate.7 Exogenous sources of creatine or creatinine include meat and creatine supplements, the latter of which are used as an "energy source" to enhance athletic performance.
Etiology. The etiology for an elevated serum creatinine concentration includes renal failure, both acute and chronic; volume depletion; hemorrhage (low blood volume); and medications, including diuretics, angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics. These etiologies can also be categorized as processes that increase creatinine production, decrease elimination (H2 antagonist and trimethoprim both inhibit the cation secretory pump in the tubules), or interfere with the creatinine assay (ketones, keto acids, lipemia, hemolysis, cephalosporins).7
Because creatinine is filtered so efficiently by the kidney, neither exogenous nor endogenous creatinine sources are expected to increase serum creatinine in the absence of renal dysfunction. However, transient elevation may occur in body builders who use extreme doses of creatine. Patients with rhabdomyolysis often develop elevated creatinine concentrations, but nearly always in the setting of myoglobinuric renal failure.
Jaffe Reaction and Enzymatic Methods. Serum creatinine can be measured using either the Jaffe reaction or the enzymatic method. In the Jaffe reaction, creatinine reacts with alkaline sodium picrate to form a red-orange chromophore, which absorbs light in the range of 470 to 550 nanometers on spectroscopy.6,8,9 The active methylene group on nitromethane also reacts with alkaline sodium picrate to form a chromophore which absorbs light in the same wavelength range.10 Thus, serum creatinine measurements via the Jaffe reaction are falsely elevated due to the cross-reactivity between nitromethane and alkaline sodium picrate. In some reported cases, there is a 20-fold increase in the measured serum creatinine in the presence of nitromethane; renal function, however, remains normal.5
This false reading seen in the Jaffe reaction can be avoided by utilizing the enzymatic method of creatinine measurement, a three-step process that ultimately produces hydrogen peroxide, which is measured and accurately correlates with serum creatinine—even in the presence of nitromethane.8 This distinction explains the dramatically different creatinine concentrations measured at the two institutions in this case.
Case Conclusion
The patient was monitored overnight at the children’s hospital. Repeat laboratory studies in the morning showed a normal creatinine level of 0.3 mg/dL and a negative methanol level. The patient was discharged home in the care of his father, who was instructed to follow-up with his son’s pediatrician. The father also received counseling on safe storage practices for dangerous chemicals.
Case
An otherwise healthy 20-month-old boy presented to the ED for evaluation after his father witnessed the child ingest a model race car fuel additive. According to the patient’s father, the boy was playing with several closed bottles that were stored in the garage, when he witnessed the boy open up and take a sip of a pink-colored fuel additive, which the father believed to contain 100% methanol. The patient’s father further noted that immediately after drinking the fluid, the patient spat and drooled, and had one episode of nonbloody emesis prior to arrival at the ED.
Initial vital signs at presentation were: blood pressure, 84/54 mm Hg; heart rate, 97 beats/min; respiratory rate, 24 breaths/min; and temperature 98°F. Oxygen saturation was 99% on room air. Physical examination was notable for mild erythema in the posterior oropharynx. Otherwise, the patient was acting appropriately for his age and in no acute distress. Laboratory studies were within normal limits, except for the following: serum anion gap, 18 mEq/L (reference range for children < 3 years old, 10-14 mEq/L); serum bicarbonate, 19 mmol/L (reference range for children 12-24 months, 17-25 mmol/L); and serum creatinine, 2.8 mg/dL (reference range for children 12 to 24 months, 0.2-0.5 mg/dL). A repeat creatinine test taken after bolus of fluid administration was 2.4 mg/dL. A renal ultrasound, performed to investigate the cause of the renal failure, was unremarkable.
What toxic exposures are of concern based on the clinical history?
The history of exposure to a liquid stored in a garage raises the likelihood of exposure to an automobile-related item such as diethylene glycol, ethylene glycol (EG), and methanol.
Diethylene Glycol. Diethylene glycol is an ingredient in brake and power steering fluids, and has toxic properties qualitatively similar to EG.
Ethylene Glycol. A clear, colorless, odorless fluid with a sweet taste, EG is an ingredient in radiator antifreeze, refrigerant fluid, coolants, and pesticides. Like methylene, EG reaches peak plasma concentration within 1 to 4 hours, but toxic clinical findings do not occur for 3 to 6 hours.1
Methanol. Methanol is a clear, colorless, alcohol found in antifreeze, windshield washer fluid, and race car fuel.2 Although methanol reaches peak plasma concentration in about 30 to 60 minutes, signs of systemic toxicity (ie, metabolic acidosis) typically take 6 to 12 hours to manifest.1
In both EG and methanol, there is a delay in toxic clinical findings because the parent compounds are not toxic in their initial form; rather, major toxicity is derived from their metabolites: formic acid and oxalic acid, respectively.
Other Toxins. Many other potentially toxic liquids are associated with a homeowner’s occupation or avocational interests. These include painting supplies (eg, industrial paints containing lead), gardening materials (eg, pesticides containing organophosphates), fuels (eg, gasoline, polychlorinated biphenyls in coolant, and lubricants), and cleaning supplies (eg, caustics, detergents, and air freshener).
Case Continuation
Since the patient’s elevated anion gap raised concerns for methanol or EG exposure, he was given fomepizole and transferred to a tertiary care children’s hospital for further management and possible hemodialysis. Upon arrival at the receiving hospital, the patient’s vital signs and physical examination remained unchanged. Repeat laboratory studies were notable for a creatinine level of 0.3 mg/dL. The patient’s father was instructed to retrieve the implicated bottle from home. An inspection of the bottle’s ingredients was notable for nitromethane, castor oil, and methanol.
What is nitromethane and what are its uses?
Nitromethane, the simplest nitro compound, is a colorless, viscous, lipid-soluble fluid.3 The polarity of nitromethane permits its use as a stabilizer in a number of chemical solvents, such as dry cleaning fluid, degreasers, and "super glue."4,5 Nitromethane is also commonly added to model-engine and drag-race fuels, which also contain methanol and castor oil.3 In this capacity, nitromethane functions as an oxygen carrier, allowing more efficient fuel use in combustion cylinders (compared to gasoline), thereby increasing the horsepower of the vehicle.6 It is therefore commonly added to fuel for drag racers, radio-controlled cars, and model aircrafts.4 In the small concentrations typically inadvertently ingested, the clinical effects of nitromethane itself are inconsequential.
What is the differential for creatinine elevation?
Creatinine itself is a normal breakdown product of muscle metabolism produced by spontaneous conversion from creatine and is found at a fairly constant serum level in proportion to muscle mass.7 Thus, as people age and muscle mass decreases, their baseline creatinine levels decrease proportionally.
Elimination. The majority of creatinine (85%-90%) is filtered and excreted by the kidneys, with the remaining 10% to 15% secreted by the tubules, allowing creatinine to be a surrogate measure of the glomerular filtration rate.7 Exogenous sources of creatine or creatinine include meat and creatine supplements, the latter of which are used as an "energy source" to enhance athletic performance.
Etiology. The etiology for an elevated serum creatinine concentration includes renal failure, both acute and chronic; volume depletion; hemorrhage (low blood volume); and medications, including diuretics, angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics. These etiologies can also be categorized as processes that increase creatinine production, decrease elimination (H2 antagonist and trimethoprim both inhibit the cation secretory pump in the tubules), or interfere with the creatinine assay (ketones, keto acids, lipemia, hemolysis, cephalosporins).7
Because creatinine is filtered so efficiently by the kidney, neither exogenous nor endogenous creatinine sources are expected to increase serum creatinine in the absence of renal dysfunction. However, transient elevation may occur in body builders who use extreme doses of creatine. Patients with rhabdomyolysis often develop elevated creatinine concentrations, but nearly always in the setting of myoglobinuric renal failure.
Jaffe Reaction and Enzymatic Methods. Serum creatinine can be measured using either the Jaffe reaction or the enzymatic method. In the Jaffe reaction, creatinine reacts with alkaline sodium picrate to form a red-orange chromophore, which absorbs light in the range of 470 to 550 nanometers on spectroscopy.6,8,9 The active methylene group on nitromethane also reacts with alkaline sodium picrate to form a chromophore which absorbs light in the same wavelength range.10 Thus, serum creatinine measurements via the Jaffe reaction are falsely elevated due to the cross-reactivity between nitromethane and alkaline sodium picrate. In some reported cases, there is a 20-fold increase in the measured serum creatinine in the presence of nitromethane; renal function, however, remains normal.5
This false reading seen in the Jaffe reaction can be avoided by utilizing the enzymatic method of creatinine measurement, a three-step process that ultimately produces hydrogen peroxide, which is measured and accurately correlates with serum creatinine—even in the presence of nitromethane.8 This distinction explains the dramatically different creatinine concentrations measured at the two institutions in this case.
Case Conclusion
The patient was monitored overnight at the children’s hospital. Repeat laboratory studies in the morning showed a normal creatinine level of 0.3 mg/dL and a negative methanol level. The patient was discharged home in the care of his father, who was instructed to follow-up with his son’s pediatrician. The father also received counseling on safe storage practices for dangerous chemicals.
1. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711. doi:10.1016/j.ccc.2012.07.002.
2. McMahon DM, Winstead S, Weant KA. Toxic alcohol ingestions: focus on ethylene glycol and methanol. Adv Emerg Nurs J. 2009;31(3):206-213. doi:10.1097/TME.0b013e3181ad8be8.
3. Cook MD, Clark RF. Creatinine elevation associated with nitromethane exposure: a marker of potential methanol toxicity. J Emerg Med. 2007;33(3):249-253. doi:10.1016/j.jemermed.2007.02.015.
4. Markofsky SB. Nitro compounds, aliphatic. In: Elvers B, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. doi:10.1002/14356007.a17_401. [digital]
5. Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be "fueled" by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):
315-320.
6. Ngo AS, Rowley F, Olson KR. Case files of the California poison control system, San Francisco division: blue thunder ingestion: methanol, nitromethane, and elevated creatinine. J Med Toxicol. 2010;6(1):67-71. doi:10.1007/s13181-010-0042-5.
7. Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51-52.
8. Booth C, Naidoo D, Rosenberg A, Kainer G. Elevated creatinine after ingestion of model aviation fuel: interference with the Jaffe reaction by nitromethane. J Paediatr Child Health. 1999;35(5):503-504.
9. de Lelis Medeiros de Morais C, Gomes de Lima KM. Determination and analytical validation of creatinine content in serum using image analysis by multivariate transfer calibration procedures. Anal Meth. 2015;7:6904-6910. doi:10.1039/C5AY01369K.
10. Killorn E, Lim RK, Rieder M. Apparent elevated creatinine after ingestion of nitromethane: interference with the Jaffe reaction. Ther Drug Monit. 2011;33(1):1-2. doi:10.1097/FTD.0b013e3181fe7e52.
1. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711. doi:10.1016/j.ccc.2012.07.002.
2. McMahon DM, Winstead S, Weant KA. Toxic alcohol ingestions: focus on ethylene glycol and methanol. Adv Emerg Nurs J. 2009;31(3):206-213. doi:10.1097/TME.0b013e3181ad8be8.
3. Cook MD, Clark RF. Creatinine elevation associated with nitromethane exposure: a marker of potential methanol toxicity. J Emerg Med. 2007;33(3):249-253. doi:10.1016/j.jemermed.2007.02.015.
4. Markofsky SB. Nitro compounds, aliphatic. In: Elvers B, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. doi:10.1002/14356007.a17_401. [digital]
5. Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be "fueled" by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):
315-320.
6. Ngo AS, Rowley F, Olson KR. Case files of the California poison control system, San Francisco division: blue thunder ingestion: methanol, nitromethane, and elevated creatinine. J Med Toxicol. 2010;6(1):67-71. doi:10.1007/s13181-010-0042-5.
7. Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51-52.
8. Booth C, Naidoo D, Rosenberg A, Kainer G. Elevated creatinine after ingestion of model aviation fuel: interference with the Jaffe reaction by nitromethane. J Paediatr Child Health. 1999;35(5):503-504.
9. de Lelis Medeiros de Morais C, Gomes de Lima KM. Determination and analytical validation of creatinine content in serum using image analysis by multivariate transfer calibration procedures. Anal Meth. 2015;7:6904-6910. doi:10.1039/C5AY01369K.
10. Killorn E, Lim RK, Rieder M. Apparent elevated creatinine after ingestion of nitromethane: interference with the Jaffe reaction. Ther Drug Monit. 2011;33(1):1-2. doi:10.1097/FTD.0b013e3181fe7e52.
Management of high-grade pleomorphic sarcoma with colon metastasis
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
Intramedullary spinal cord and leptomeningeal metastases presenting as cauda equina syndrome in a patient with melanoma
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
Presumed Serum Sickness Following Thymoglobulin Treatment of Acute Cellular Rejection of a Cardiac Allograft
Serum sickness was first described by von Pirquet and Schick1 as a constellation of signs and symptoms displayed in patients receiving equine serum as an antitoxin for the treatment of scarlet fever and diphtheria. Serum sickness is an immune complex–mediated hypersensitivity reaction that can be clinically diagnosed in patients who present with fever, rash, and polyarthralgia or polyarthritis following exposure to heterologous serum proteins.2,3 Symptom onset typically occurs within 1 to 2 weeks of first exposure to the serum, and resolution frequently occurs with discontinuation of the offending agent. Other symptoms may include malaise, gastrointestinal tract concerns, headache, blurred vision, or lymphadenopathy.4 Proteinuria, hematuria, and a transient decrease in creatinine clearance also have been reported in serum sickness.4
Serum sickness is caused by a type III immune complex–mediated hypersensitivity reaction to heterologous rabbit or equine serum proteins. Nonhuman proteins present in antithymocyte globulin (ATG) stimulate the production of IgG, IgM, IgA, and IgE antibodies.2-4 If the resultant immune complexes overwhelm the mononuclear phagocyte system, these complexes are deposited in blood vessels and tissues, which leads to complement activation and the production of complement fragments such as C3a and C5a.5 C3a is an anaphylatoxin that causes mast cell degranulation and the consequent formation of urticarial lesions. C5a is a neutrophil chemoattractant that promotes inflammation at the site of complement deposition.
Serum sickness–like reactions may occur days to weeks following administration of certain drugs, such as cefaclor or penicillin. Although the symptoms and timing of serum sickness–like reactions are similar to serum sickness, they are not caused by an immune complex–mediated mechanism and are believed to be secondary to an idiosyncratic delayed drug reaction.6
Thymoglobulin, a type of ATG, is a polyclonal antibody generated in rabbits that targets numerous human epitopes, including cell surface markers on T cells (CD2, CD3, CD4, CD8), B cells (CD21, CD19, CD40), and adhesion molecules (CD6, CD25, CD44, CD45, and the integrin LFA-1 [lymphocyte function-associated antigen-1]).7,8 Thymoglobulin has proven efficacy in the setting of cardiac transplantation.9-11 Although calcineurin inhibitors form the foundation in the armamentarium of immunosuppressive agents in cardiac transplantation, their nephrotoxicity has limited their unrestrained use in patients.9 By delaying the need for calcineurin inhibitors, thymoglobulin preserves greater renal function without increasing the risk for acute rejection.9,10 Akin to its use in the patient presented in this case report, thymoglobulin also is used in the treatment of acute cellular rejection in heart transplant recipients with signs of heart failure.11
Case Report
A 35-year-old man with a history of familial cardiomyopathy who underwent orthotopic heart transplantation presented with grade 3R acute cellular rejection. The patient’s immunosuppressive regimen consisted of thymoglobulin 150 mg once daily, tacrolimus 2.5 mg twice daily, hydrocortisone 100 mg once daily, and mycophenolate mofetil 1000 mg twice daily. On day 7 of thymoglobulin treatment, the dermatology department was consulted to evaluate a pruritic eruption. The patient reported that he noticed redness of the palms and soles, as well as redness accentuated in the axilla, groin, and other skin creases 2 days prior. The patient also reported symmetric bilateral hand pain that had started 1 day following rash onset. He denied fever and remained afebrile throughout his hospitalization.
On physical examination, the patient displayed a blanching, erythematous, edematous, evanescent macular rash with some areas of wheal formation symmetrically distributed in the bilateral axillae, inframammary folds, and groin (Figure, A and B). The palms and soles were tender with diffuse blanching erythema. The eruption was accentuated at the lateral and medial borders of both feet (Figure, C). There was concern that the patient may have a form of serum sickness with a blunted incomplete response due to his concomitant use of immunosuppressive agents. Shortly after evaluation, the patient left the hospital against medical advice before the recommended evaluation and systemic workup could be implemented.
The patient returned for an outpatient appointment approximately 1 week later. Medical records indicated that the patient’s skin eruption had resolved. Tests for antithymoglobulin antibodies at this visit were negative. The antithymoglobulin antibody enzyme-linked immunosorbent assay has a diagnostic sensitivity of 86%12 and large interlaboratory variability.13 Given the presence of other features of serum sickness, a false-negative result was considered by dermatology. Nonetheless, one must consider other differential diagnoses, including a simple cutaneous adverse drug eruption or viral exanthem that might have in fact been causative.
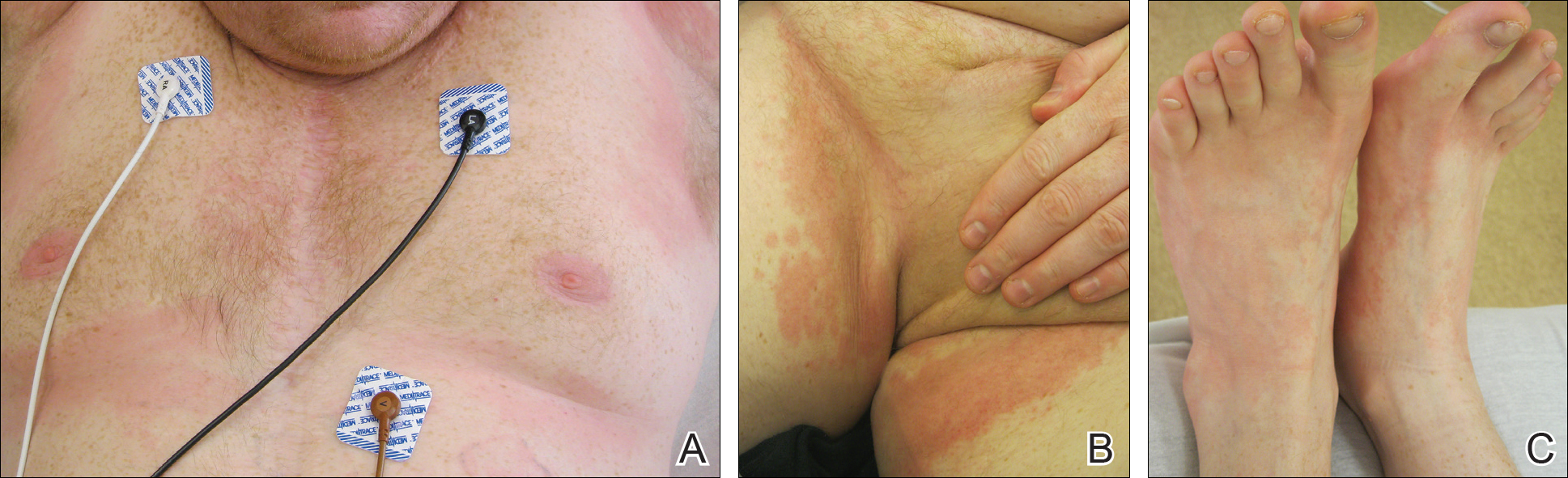
Comment
We present an atypical case of possible serum sickness in a heart transplant recipient following thymoglobulin treatment of acute cellular rejection of the cardiac allograft. Serum sickness is a clinical diagnosis supported by laboratory data. Some authors have suggested major and minor diagnostic criteria to aid with the diagnosis.7 Major diagnostic criteria include onset more than 7 days after the initial thymoglobulin administration, persistent high fevers (temperature, >38.4°C), persistent arthritis/arthralgia, and positive heterologous antibodies on enzyme-linked immunosorbent assay. Minor diagnostic criteria include rash, acute renal failure, trismus, and low serum complement (C3 and C4).
The variable cutaneous presentations of serum sickness are important to recognize in the process of making the correct diagnosis. Rash is frequently reported in serum sickness, with some studies displaying rates of up to 93%.4,14 The skin findings are most frequently described as urticarial or serpiginous macular lesions.3 Other variations of the eruption exist, and morbilliform eruptions or a combination of morbilliform and urticarial eruptions have been reported.3 It is important to judge cutaneous eruptions of serum sickness within the context of the potential cytopenia in a patient being treated with ATG. As such, purpuric eruptions have been attributed to serum sickness in thrombocytopenic patients receiving ATG for bone marrow failure.14
Usually, cutaneous eruptions of serum sickness initially are identified in the groin, axilla, and periumbilical region, and then they proceed to include the trunk and extremities. Erythema of the palms and soles frequently is described as well as a linear accentuation of the rash along the lateral and medial borders of the feet and hands at the margin of the plantar or palmar skin, respectively.14 The mucous membranes frequently are spared in serum sickness.
Despite the lack of evidence-based guidelines, case series and literature reviews have suggested a treatment regimen for serum sickness,7,15-18 calling for immediate withdrawal of the offending agent. Antihistamines may be added to control pruritus and rash. Patients with high fever, a progressive rash, or severe arthralgia have benefited from short courses of oral16,18 or intravenous7,17 glucocorticoids. The extent of the eruption in our patient was concerning, particularly because he was already receiving systemic corticosteroids in conjunction with other immunosuppressives, which may have explained his lack of fever.
Because our patient satisfied some diagnostic criteria for serum sickness and failed to satisfy others, our team was faced with the challenge of balancing the risks of possible serum sickness with the risks of the potential for progressive cardiac rejection from the withdrawal of thymoglobulin.7 There is some evidence in the literature for the use of therapeutic plasma exchange (TPE) for the treatment of serum sickness if the offending agent could not be discontinued. Tanriover et al19 presented a case series of 5 renal transplant recipients treated with thymoglobulin who developed serum sickness. The diagnosis of serum sickness was made clinically and augmented by the presence of antiheterologous antibodies. All 5 patients had persistent symptoms of serum sickness despite 2 days of glucocorticoid treatment. Interestingly, 3 patients had complete resolution of all symptoms after a single TPE treatment, and 2 patients achieved resolution of fever and arthritis after 2 consecutive days of TPE treatments.19 Because plasmapheresis is used to treat cardiac allograft rejection in patients showing signs of heart failure,11 the employment of TPE in these patients may have dual beneficial effects of concurrently treating serum sickness and allograft rejection.
Given the patient’s noncompliance and leaving the hospital against medical advice, a full workup was not able to be pursued in this case, though fortunately the eruption and his other symptoms had resolved by the time he was seen for outpatient follow-up 1 week later. Noncompliance with immunosuppressive therapy is a considerable risk factor for morbidity and mortality following heart transplantation. These patients have more transplant coronary artery disease and substantially shorter clinical event-free time.20 Our patient demonstrates the need for proactive compliance-enhancing interventions in heart transplant patients who experience allograft rejection.
- von Pirquet C, Schick B. Serum Sickness. Schick B, trans-ed. Baltimore, MD; Williams & Wilkins; 1951.
- Vincent C, Revillard JP. Antibody response to horse gamma-globulin in recipients of renal allografts: relationship with transplant crises and transplant survival. Transplantation. 1977;24:141-147.
- Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407-1413.
- Bielory L, Gascon P, Lawley TJ, et al. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988;67:40-57.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286.
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587-1591.
- Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650.
- Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194-1200.
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22:78-89.
- Cantarovich M, Giannetti N, Barkun J, et al. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779-781.
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2010;9:160-167.
- Tatum AH, Bollinger RR, Sanfilippo F. Rapid serologic diagnosis of serum sickness from antithymocyte globulin therapy using enzyme immunoassay. Transplantation. 1984;38:582-586.
- Kimball JA, Pescovitz MD, Book BK, et al. Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation. 1995;60:1379-1383.
- Bielory L, Yancey KB, Young NS, et al. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985;13:411-417.
- Joubert GI, Hadad K, Matsui D, et al. Selection of treatment of cefaclor-associated urticarial, serum sickness-like reactions and erythema multiforme by emergency pediatricians: lack of a uniform standard of care. Can J Clin Pharmacol. 1999;6:197-201.
- Clark BM, Kotti GH, Shah AD, et al. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26:705-708.
- Finger E, Scheinberg M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J Clin Rheumatol. 2007;13:94-95.
- Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86:330-334.
- Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody-induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279-281.
- Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. 2004;23:1245-1251.
Serum sickness was first described by von Pirquet and Schick1 as a constellation of signs and symptoms displayed in patients receiving equine serum as an antitoxin for the treatment of scarlet fever and diphtheria. Serum sickness is an immune complex–mediated hypersensitivity reaction that can be clinically diagnosed in patients who present with fever, rash, and polyarthralgia or polyarthritis following exposure to heterologous serum proteins.2,3 Symptom onset typically occurs within 1 to 2 weeks of first exposure to the serum, and resolution frequently occurs with discontinuation of the offending agent. Other symptoms may include malaise, gastrointestinal tract concerns, headache, blurred vision, or lymphadenopathy.4 Proteinuria, hematuria, and a transient decrease in creatinine clearance also have been reported in serum sickness.4
Serum sickness is caused by a type III immune complex–mediated hypersensitivity reaction to heterologous rabbit or equine serum proteins. Nonhuman proteins present in antithymocyte globulin (ATG) stimulate the production of IgG, IgM, IgA, and IgE antibodies.2-4 If the resultant immune complexes overwhelm the mononuclear phagocyte system, these complexes are deposited in blood vessels and tissues, which leads to complement activation and the production of complement fragments such as C3a and C5a.5 C3a is an anaphylatoxin that causes mast cell degranulation and the consequent formation of urticarial lesions. C5a is a neutrophil chemoattractant that promotes inflammation at the site of complement deposition.
Serum sickness–like reactions may occur days to weeks following administration of certain drugs, such as cefaclor or penicillin. Although the symptoms and timing of serum sickness–like reactions are similar to serum sickness, they are not caused by an immune complex–mediated mechanism and are believed to be secondary to an idiosyncratic delayed drug reaction.6
Thymoglobulin, a type of ATG, is a polyclonal antibody generated in rabbits that targets numerous human epitopes, including cell surface markers on T cells (CD2, CD3, CD4, CD8), B cells (CD21, CD19, CD40), and adhesion molecules (CD6, CD25, CD44, CD45, and the integrin LFA-1 [lymphocyte function-associated antigen-1]).7,8 Thymoglobulin has proven efficacy in the setting of cardiac transplantation.9-11 Although calcineurin inhibitors form the foundation in the armamentarium of immunosuppressive agents in cardiac transplantation, their nephrotoxicity has limited their unrestrained use in patients.9 By delaying the need for calcineurin inhibitors, thymoglobulin preserves greater renal function without increasing the risk for acute rejection.9,10 Akin to its use in the patient presented in this case report, thymoglobulin also is used in the treatment of acute cellular rejection in heart transplant recipients with signs of heart failure.11
Case Report
A 35-year-old man with a history of familial cardiomyopathy who underwent orthotopic heart transplantation presented with grade 3R acute cellular rejection. The patient’s immunosuppressive regimen consisted of thymoglobulin 150 mg once daily, tacrolimus 2.5 mg twice daily, hydrocortisone 100 mg once daily, and mycophenolate mofetil 1000 mg twice daily. On day 7 of thymoglobulin treatment, the dermatology department was consulted to evaluate a pruritic eruption. The patient reported that he noticed redness of the palms and soles, as well as redness accentuated in the axilla, groin, and other skin creases 2 days prior. The patient also reported symmetric bilateral hand pain that had started 1 day following rash onset. He denied fever and remained afebrile throughout his hospitalization.
On physical examination, the patient displayed a blanching, erythematous, edematous, evanescent macular rash with some areas of wheal formation symmetrically distributed in the bilateral axillae, inframammary folds, and groin (Figure, A and B). The palms and soles were tender with diffuse blanching erythema. The eruption was accentuated at the lateral and medial borders of both feet (Figure, C). There was concern that the patient may have a form of serum sickness with a blunted incomplete response due to his concomitant use of immunosuppressive agents. Shortly after evaluation, the patient left the hospital against medical advice before the recommended evaluation and systemic workup could be implemented.
The patient returned for an outpatient appointment approximately 1 week later. Medical records indicated that the patient’s skin eruption had resolved. Tests for antithymoglobulin antibodies at this visit were negative. The antithymoglobulin antibody enzyme-linked immunosorbent assay has a diagnostic sensitivity of 86%12 and large interlaboratory variability.13 Given the presence of other features of serum sickness, a false-negative result was considered by dermatology. Nonetheless, one must consider other differential diagnoses, including a simple cutaneous adverse drug eruption or viral exanthem that might have in fact been causative.

Comment
We present an atypical case of possible serum sickness in a heart transplant recipient following thymoglobulin treatment of acute cellular rejection of the cardiac allograft. Serum sickness is a clinical diagnosis supported by laboratory data. Some authors have suggested major and minor diagnostic criteria to aid with the diagnosis.7 Major diagnostic criteria include onset more than 7 days after the initial thymoglobulin administration, persistent high fevers (temperature, >38.4°C), persistent arthritis/arthralgia, and positive heterologous antibodies on enzyme-linked immunosorbent assay. Minor diagnostic criteria include rash, acute renal failure, trismus, and low serum complement (C3 and C4).
The variable cutaneous presentations of serum sickness are important to recognize in the process of making the correct diagnosis. Rash is frequently reported in serum sickness, with some studies displaying rates of up to 93%.4,14 The skin findings are most frequently described as urticarial or serpiginous macular lesions.3 Other variations of the eruption exist, and morbilliform eruptions or a combination of morbilliform and urticarial eruptions have been reported.3 It is important to judge cutaneous eruptions of serum sickness within the context of the potential cytopenia in a patient being treated with ATG. As such, purpuric eruptions have been attributed to serum sickness in thrombocytopenic patients receiving ATG for bone marrow failure.14
Usually, cutaneous eruptions of serum sickness initially are identified in the groin, axilla, and periumbilical region, and then they proceed to include the trunk and extremities. Erythema of the palms and soles frequently is described as well as a linear accentuation of the rash along the lateral and medial borders of the feet and hands at the margin of the plantar or palmar skin, respectively.14 The mucous membranes frequently are spared in serum sickness.
Despite the lack of evidence-based guidelines, case series and literature reviews have suggested a treatment regimen for serum sickness,7,15-18 calling for immediate withdrawal of the offending agent. Antihistamines may be added to control pruritus and rash. Patients with high fever, a progressive rash, or severe arthralgia have benefited from short courses of oral16,18 or intravenous7,17 glucocorticoids. The extent of the eruption in our patient was concerning, particularly because he was already receiving systemic corticosteroids in conjunction with other immunosuppressives, which may have explained his lack of fever.
Because our patient satisfied some diagnostic criteria for serum sickness and failed to satisfy others, our team was faced with the challenge of balancing the risks of possible serum sickness with the risks of the potential for progressive cardiac rejection from the withdrawal of thymoglobulin.7 There is some evidence in the literature for the use of therapeutic plasma exchange (TPE) for the treatment of serum sickness if the offending agent could not be discontinued. Tanriover et al19 presented a case series of 5 renal transplant recipients treated with thymoglobulin who developed serum sickness. The diagnosis of serum sickness was made clinically and augmented by the presence of antiheterologous antibodies. All 5 patients had persistent symptoms of serum sickness despite 2 days of glucocorticoid treatment. Interestingly, 3 patients had complete resolution of all symptoms after a single TPE treatment, and 2 patients achieved resolution of fever and arthritis after 2 consecutive days of TPE treatments.19 Because plasmapheresis is used to treat cardiac allograft rejection in patients showing signs of heart failure,11 the employment of TPE in these patients may have dual beneficial effects of concurrently treating serum sickness and allograft rejection.
Given the patient’s noncompliance and leaving the hospital against medical advice, a full workup was not able to be pursued in this case, though fortunately the eruption and his other symptoms had resolved by the time he was seen for outpatient follow-up 1 week later. Noncompliance with immunosuppressive therapy is a considerable risk factor for morbidity and mortality following heart transplantation. These patients have more transplant coronary artery disease and substantially shorter clinical event-free time.20 Our patient demonstrates the need for proactive compliance-enhancing interventions in heart transplant patients who experience allograft rejection.
Serum sickness was first described by von Pirquet and Schick1 as a constellation of signs and symptoms displayed in patients receiving equine serum as an antitoxin for the treatment of scarlet fever and diphtheria. Serum sickness is an immune complex–mediated hypersensitivity reaction that can be clinically diagnosed in patients who present with fever, rash, and polyarthralgia or polyarthritis following exposure to heterologous serum proteins.2,3 Symptom onset typically occurs within 1 to 2 weeks of first exposure to the serum, and resolution frequently occurs with discontinuation of the offending agent. Other symptoms may include malaise, gastrointestinal tract concerns, headache, blurred vision, or lymphadenopathy.4 Proteinuria, hematuria, and a transient decrease in creatinine clearance also have been reported in serum sickness.4
Serum sickness is caused by a type III immune complex–mediated hypersensitivity reaction to heterologous rabbit or equine serum proteins. Nonhuman proteins present in antithymocyte globulin (ATG) stimulate the production of IgG, IgM, IgA, and IgE antibodies.2-4 If the resultant immune complexes overwhelm the mononuclear phagocyte system, these complexes are deposited in blood vessels and tissues, which leads to complement activation and the production of complement fragments such as C3a and C5a.5 C3a is an anaphylatoxin that causes mast cell degranulation and the consequent formation of urticarial lesions. C5a is a neutrophil chemoattractant that promotes inflammation at the site of complement deposition.
Serum sickness–like reactions may occur days to weeks following administration of certain drugs, such as cefaclor or penicillin. Although the symptoms and timing of serum sickness–like reactions are similar to serum sickness, they are not caused by an immune complex–mediated mechanism and are believed to be secondary to an idiosyncratic delayed drug reaction.6
Thymoglobulin, a type of ATG, is a polyclonal antibody generated in rabbits that targets numerous human epitopes, including cell surface markers on T cells (CD2, CD3, CD4, CD8), B cells (CD21, CD19, CD40), and adhesion molecules (CD6, CD25, CD44, CD45, and the integrin LFA-1 [lymphocyte function-associated antigen-1]).7,8 Thymoglobulin has proven efficacy in the setting of cardiac transplantation.9-11 Although calcineurin inhibitors form the foundation in the armamentarium of immunosuppressive agents in cardiac transplantation, their nephrotoxicity has limited their unrestrained use in patients.9 By delaying the need for calcineurin inhibitors, thymoglobulin preserves greater renal function without increasing the risk for acute rejection.9,10 Akin to its use in the patient presented in this case report, thymoglobulin also is used in the treatment of acute cellular rejection in heart transplant recipients with signs of heart failure.11
Case Report
A 35-year-old man with a history of familial cardiomyopathy who underwent orthotopic heart transplantation presented with grade 3R acute cellular rejection. The patient’s immunosuppressive regimen consisted of thymoglobulin 150 mg once daily, tacrolimus 2.5 mg twice daily, hydrocortisone 100 mg once daily, and mycophenolate mofetil 1000 mg twice daily. On day 7 of thymoglobulin treatment, the dermatology department was consulted to evaluate a pruritic eruption. The patient reported that he noticed redness of the palms and soles, as well as redness accentuated in the axilla, groin, and other skin creases 2 days prior. The patient also reported symmetric bilateral hand pain that had started 1 day following rash onset. He denied fever and remained afebrile throughout his hospitalization.
On physical examination, the patient displayed a blanching, erythematous, edematous, evanescent macular rash with some areas of wheal formation symmetrically distributed in the bilateral axillae, inframammary folds, and groin (Figure, A and B). The palms and soles were tender with diffuse blanching erythema. The eruption was accentuated at the lateral and medial borders of both feet (Figure, C). There was concern that the patient may have a form of serum sickness with a blunted incomplete response due to his concomitant use of immunosuppressive agents. Shortly after evaluation, the patient left the hospital against medical advice before the recommended evaluation and systemic workup could be implemented.
The patient returned for an outpatient appointment approximately 1 week later. Medical records indicated that the patient’s skin eruption had resolved. Tests for antithymoglobulin antibodies at this visit were negative. The antithymoglobulin antibody enzyme-linked immunosorbent assay has a diagnostic sensitivity of 86%12 and large interlaboratory variability.13 Given the presence of other features of serum sickness, a false-negative result was considered by dermatology. Nonetheless, one must consider other differential diagnoses, including a simple cutaneous adverse drug eruption or viral exanthem that might have in fact been causative.

Comment
We present an atypical case of possible serum sickness in a heart transplant recipient following thymoglobulin treatment of acute cellular rejection of the cardiac allograft. Serum sickness is a clinical diagnosis supported by laboratory data. Some authors have suggested major and minor diagnostic criteria to aid with the diagnosis.7 Major diagnostic criteria include onset more than 7 days after the initial thymoglobulin administration, persistent high fevers (temperature, >38.4°C), persistent arthritis/arthralgia, and positive heterologous antibodies on enzyme-linked immunosorbent assay. Minor diagnostic criteria include rash, acute renal failure, trismus, and low serum complement (C3 and C4).
The variable cutaneous presentations of serum sickness are important to recognize in the process of making the correct diagnosis. Rash is frequently reported in serum sickness, with some studies displaying rates of up to 93%.4,14 The skin findings are most frequently described as urticarial or serpiginous macular lesions.3 Other variations of the eruption exist, and morbilliform eruptions or a combination of morbilliform and urticarial eruptions have been reported.3 It is important to judge cutaneous eruptions of serum sickness within the context of the potential cytopenia in a patient being treated with ATG. As such, purpuric eruptions have been attributed to serum sickness in thrombocytopenic patients receiving ATG for bone marrow failure.14
Usually, cutaneous eruptions of serum sickness initially are identified in the groin, axilla, and periumbilical region, and then they proceed to include the trunk and extremities. Erythema of the palms and soles frequently is described as well as a linear accentuation of the rash along the lateral and medial borders of the feet and hands at the margin of the plantar or palmar skin, respectively.14 The mucous membranes frequently are spared in serum sickness.
Despite the lack of evidence-based guidelines, case series and literature reviews have suggested a treatment regimen for serum sickness,7,15-18 calling for immediate withdrawal of the offending agent. Antihistamines may be added to control pruritus and rash. Patients with high fever, a progressive rash, or severe arthralgia have benefited from short courses of oral16,18 or intravenous7,17 glucocorticoids. The extent of the eruption in our patient was concerning, particularly because he was already receiving systemic corticosteroids in conjunction with other immunosuppressives, which may have explained his lack of fever.
Because our patient satisfied some diagnostic criteria for serum sickness and failed to satisfy others, our team was faced with the challenge of balancing the risks of possible serum sickness with the risks of the potential for progressive cardiac rejection from the withdrawal of thymoglobulin.7 There is some evidence in the literature for the use of therapeutic plasma exchange (TPE) for the treatment of serum sickness if the offending agent could not be discontinued. Tanriover et al19 presented a case series of 5 renal transplant recipients treated with thymoglobulin who developed serum sickness. The diagnosis of serum sickness was made clinically and augmented by the presence of antiheterologous antibodies. All 5 patients had persistent symptoms of serum sickness despite 2 days of glucocorticoid treatment. Interestingly, 3 patients had complete resolution of all symptoms after a single TPE treatment, and 2 patients achieved resolution of fever and arthritis after 2 consecutive days of TPE treatments.19 Because plasmapheresis is used to treat cardiac allograft rejection in patients showing signs of heart failure,11 the employment of TPE in these patients may have dual beneficial effects of concurrently treating serum sickness and allograft rejection.
Given the patient’s noncompliance and leaving the hospital against medical advice, a full workup was not able to be pursued in this case, though fortunately the eruption and his other symptoms had resolved by the time he was seen for outpatient follow-up 1 week later. Noncompliance with immunosuppressive therapy is a considerable risk factor for morbidity and mortality following heart transplantation. These patients have more transplant coronary artery disease and substantially shorter clinical event-free time.20 Our patient demonstrates the need for proactive compliance-enhancing interventions in heart transplant patients who experience allograft rejection.
- von Pirquet C, Schick B. Serum Sickness. Schick B, trans-ed. Baltimore, MD; Williams & Wilkins; 1951.
- Vincent C, Revillard JP. Antibody response to horse gamma-globulin in recipients of renal allografts: relationship with transplant crises and transplant survival. Transplantation. 1977;24:141-147.
- Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407-1413.
- Bielory L, Gascon P, Lawley TJ, et al. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988;67:40-57.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286.
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587-1591.
- Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650.
- Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194-1200.
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22:78-89.
- Cantarovich M, Giannetti N, Barkun J, et al. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779-781.
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2010;9:160-167.
- Tatum AH, Bollinger RR, Sanfilippo F. Rapid serologic diagnosis of serum sickness from antithymocyte globulin therapy using enzyme immunoassay. Transplantation. 1984;38:582-586.
- Kimball JA, Pescovitz MD, Book BK, et al. Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation. 1995;60:1379-1383.
- Bielory L, Yancey KB, Young NS, et al. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985;13:411-417.
- Joubert GI, Hadad K, Matsui D, et al. Selection of treatment of cefaclor-associated urticarial, serum sickness-like reactions and erythema multiforme by emergency pediatricians: lack of a uniform standard of care. Can J Clin Pharmacol. 1999;6:197-201.
- Clark BM, Kotti GH, Shah AD, et al. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26:705-708.
- Finger E, Scheinberg M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J Clin Rheumatol. 2007;13:94-95.
- Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86:330-334.
- Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody-induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279-281.
- Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. 2004;23:1245-1251.
- von Pirquet C, Schick B. Serum Sickness. Schick B, trans-ed. Baltimore, MD; Williams & Wilkins; 1951.
- Vincent C, Revillard JP. Antibody response to horse gamma-globulin in recipients of renal allografts: relationship with transplant crises and transplant survival. Transplantation. 1977;24:141-147.
- Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407-1413.
- Bielory L, Gascon P, Lawley TJ, et al. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988;67:40-57.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286.
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587-1591.
- Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650.
- Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194-1200.
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22:78-89.
- Cantarovich M, Giannetti N, Barkun J, et al. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779-781.
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2010;9:160-167.
- Tatum AH, Bollinger RR, Sanfilippo F. Rapid serologic diagnosis of serum sickness from antithymocyte globulin therapy using enzyme immunoassay. Transplantation. 1984;38:582-586.
- Kimball JA, Pescovitz MD, Book BK, et al. Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation. 1995;60:1379-1383.
- Bielory L, Yancey KB, Young NS, et al. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985;13:411-417.
- Joubert GI, Hadad K, Matsui D, et al. Selection of treatment of cefaclor-associated urticarial, serum sickness-like reactions and erythema multiforme by emergency pediatricians: lack of a uniform standard of care. Can J Clin Pharmacol. 1999;6:197-201.
- Clark BM, Kotti GH, Shah AD, et al. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26:705-708.
- Finger E, Scheinberg M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J Clin Rheumatol. 2007;13:94-95.
- Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86:330-334.
- Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody-induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279-281.
- Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. 2004;23:1245-1251.
Practice Points
- Serum sickness can be seen in patients treated with thymoglobulin to prevent transplant rejection.
- Serum sickness can display multiple cutaneous manifestation, thus making it an important entity for dermatologists.