User login
June 2018: Click for Credit
Here are 4 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Dermatology Practice Gaps: Missed Diagnoses
To take the posttest, go to: https://bit.ly/2IhaxSm
Expires February 11, 2019
2. When to Worry About Congenital Melanocytic Nevi
To take the posttest, go to: https://bit.ly/2IhMOBC
Expires March 2, 2019
3. Unscheduled Visits for Pain After Hernia Surgery Common, Costly
To take the posttest, go to: https://bit.ly/2GbfWIV
Expires February 15, 2019
4. Melanoma Incidence Increased in Older Non-Hispanic Whites
To take the posttest, go to: https://bit.ly/2wOkVzZ
Expires February 9, 2019
Here are 4 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Dermatology Practice Gaps: Missed Diagnoses
To take the posttest, go to: https://bit.ly/2IhaxSm
Expires February 11, 2019
2. When to Worry About Congenital Melanocytic Nevi
To take the posttest, go to: https://bit.ly/2IhMOBC
Expires March 2, 2019
3. Unscheduled Visits for Pain After Hernia Surgery Common, Costly
To take the posttest, go to: https://bit.ly/2GbfWIV
Expires February 15, 2019
4. Melanoma Incidence Increased in Older Non-Hispanic Whites
To take the posttest, go to: https://bit.ly/2wOkVzZ
Expires February 9, 2019
Here are 4 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Dermatology Practice Gaps: Missed Diagnoses
To take the posttest, go to: https://bit.ly/2IhaxSm
Expires February 11, 2019
2. When to Worry About Congenital Melanocytic Nevi
To take the posttest, go to: https://bit.ly/2IhMOBC
Expires March 2, 2019
3. Unscheduled Visits for Pain After Hernia Surgery Common, Costly
To take the posttest, go to: https://bit.ly/2GbfWIV
Expires February 15, 2019
4. Melanoma Incidence Increased in Older Non-Hispanic Whites
To take the posttest, go to: https://bit.ly/2wOkVzZ
Expires February 9, 2019
Heart Failure: A Dynamic Approach to Classification and Management
CE/CME No: CR-1805
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Develop an understanding of the classification of heart failure and its associated signs and symptoms.
• Describe clinical findings of heart failure from the physical exam.
• Understand the utility of laboratory and imaging studies for diagnosis of heart failure and its underlying causes.
• Demonstrate knowledge of nonpharmacotherapeutic and pharmacotherapeutic options for managing heart failure.
FACULTY
Michael Roscoe is Chair/Program Director and Associate Professor, Andrew Lampkins is Associate Professor, Sean Harper is Assistant Professor, and Gina Niemeier is Associate Program Director and Assistant Professor, in the PA Department at the University of Evansville in Indiana.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through April 30, 2019.
Article begins on next page >>
Heart failure is a complex syndrome with a spectrum of signs and symptoms that range from asymptomatic to terminal. This variability of presentation, paired with the irreversibility of the process, make it both difficult and critical to identify this syndrome early to prevent progression. Here is an overview of the classification and common presentations of heart failure, as well as a guide to diagnostic modalities and treatment options.
Heart failure (HF) is a complex syndrome, not a specific disease; it is always associated with an underlying cause. Hypertension and coronary artery disease are the two most common causes in all age groups, but a number of other conditions—valvular disease, unrecognized obstructive sleep apnea, obesity, chronic kidney disease, anemia, hyperlipidemia, diabetes, and atrial fibrillation—have been identified as secondary causes.1-3
Often, however, clinicians do not identify HF until the syndrome reaches an advanced stage—at which point, the damage is irreversible and pharmacotherapeutic management is limited to control of signs and symptoms. The ramifications are concerning, since HF has achieved near-epidemic scope in the United States. An estimated 5.7 million Americans ages 20 and older have HF; prevalence is projected to increase by 46% between 2012 and 2030—resulting in more than 8 million affected individuals (ages 18 and older).4,5 More than 1 million patients are discharged from the hospital annually with a primary diagnosis of HF.4 And in 2013, one in nine death certificates in the US mentioned HF.4
Most cases of HF are managed in primary care. Established, evidence-based therapies should be implemented in the outpatient setting when possible, as early in the course as possible. Referral to a cardiologist is needed when the underlying cause of HF remains undetermined, or when specialized treatment is required.
CLASSIFICATION OF HF
The two most widely recognized classification systems for HF are those of the American College of Cardiology and the American Heart Association (ACC/AHA) and of the New York Heart Association (NYHA). Both focus on one-year mortality. The stages of the ACC/AHA system (A to D) are based on worsening of both structural heart disease and clinical symptoms of HF. The NYHA designations (Class I to IV) are based on the functional capability associated with physical activity. Both systems are outlined in Table 1.3,6,7

While these systems are used to “stage” HF, there are several ways the syndrome is classified in the medical literature. For example, HF can be described by
- Anatomy (left- or right-sided)
- Physiology (dilated and hypertrophic)
- Course (chronic or acute heart failure [cardiogenic shock])
- Output (high- or low-output failure)
- Ejection fraction (reduced or preserved)
- Pressure phase (systolic and diastolic).
All these classifications have merit; however, this article will attempt to simplify the approach to patients with HF and focus on systolic HF, defined as a reduced left ventricular ejection fraction (LVEF), and diastolic HF, defined as a preserved LVEF. Although the common perception of HF among clinicians—and thus the traditional diagnostic focus—is reduced LVEF (systolic HF), preserved LVEF (diastolic HF) in fact represents approximately 50% of cases.1 Diastolic HF is estimated to be increasing in prevalence and is expected to become the more common phenotype.8
Continue to: SIGNS AND SYMPTOMS
SIGNS AND SYMPTOMS
Heart failure is characterized by a constellation of signs and symptoms of pulmonary and/or systemic venous congestion caused by impaired ability of the heart to fill with or eject blood in proportion to the metabolic needs of the body.1 Manifestations include fatigue, dyspnea, fluid retention, and cachexia, and patients can present anywhere on the spectrum—from asymptomatic at rest to severely symptomatic.
Symptoms
In both reduced LVEF and preserved LVEF HF, common early symptoms include dyspnea and fatigue on exertion, with or without some degree of lower-extremity swelling.2 Lack of treatment and disease progression increase symptom severity—to the extent that dyspnea and fatigue start to occur at rest. Reviewing the anatomic classifications/findings of HF facilitates understanding of the clinical symptoms.
Right HF. Right ventricular dysfunction is rarely found in isolation; when symptoms are present, further evaluation of the left ventricle and the pulmonary system (to look for cor pulmonale) is warranted. Right HF is associated with an inability to manage venous return and move volume into the pulmonary circuit. This produces the predominant symptom of fluid retention. Peripheral edema is a cardinal symptom of right HF; edema can also present systemically, mostly as hepatic congestion or general gastrointestinal complaints resulting from impaired gastrointestinal perfusion.9
Left HF. Left ventricular dysfunction is the more common anatomic finding in HF and is often generalized to represent all cases. The dysfunction can be found in isolation and is actually the leading cause of right HF. In left HF (regardless of etiology), the heart does not produce enough “forward” pressure (ie, cannot pump or fill with enough blood) for the cardiovascular system to remain in balance. Thus, “back” pressure into the pulmonary circuit develops.
The combination of insufficient systemic perfusion capability with a dysfunctional left pump means that the most common symptom in all cases of left HF is shortness of breath—specifically, exertional dyspnea. Dyspnea can progress to orthopnea, paroxysmal nocturnal dyspnea, and, finally, dyspnea at rest. Patients often present with a cough that is worse while lying down and that can be nonproductive or productive, depending on volume status.9,10
Signs
Vital signs range from normal to indicative of shock. Increased sympathetic nervous system activity is common; this may manifest as coldness of the extremities and diaphoresis. Keep in mind that HF is a clinical diagnosis: The physical examination, focusing on peripheral signs, is key in all HF patients for both diagnosis and management.
PHYSICAL EXAMINATION
The physical exam includes the heart, neck, lungs, abdomen, and extremities. The cardiac exam includes evaluation for hypertrophy, by palpating for the point of maximum impulse to assess for lifts, heaves, valvular disease, and S3 and S4 sounds. Respiratory, abdominal, and extremity exams all focus on the evaluation of fluid status and edema.
A key, often underutilized measurement is jugular venous pressure (JVP). Elevated JVP has been identified as the most specific sign of fluid overload in HF and the most important physical finding in the initial and subsequent examinations of a patient with HF.11 (For good reason, clinicians who treat patients with HF must be able to recognize volume overload and hypovolemia; euvolemia allows patients to remain symptom-free and makes it possible to initiate life-prolonging therapy, which will be discussed in the Treatment section.2) JVP is an indirect measure of pressure within the right side of the heart (central venous pressure).
Most texts on performing the physical exam recommend measuring JVP using the right internal jugular vein. However, use of the internal jugular vein is limiting in HF patients, because it is covered by the sternocleidomastoid muscle for most of its course in the neck and visible only in a small triangle between the two heads in the root of the neck (see Figure). Conversely, the external jugular veins are subcutaneous along their entire course and pulsations are easily visible—but superficial and prone to external pressure and internal occlusion. A nonpulsatile, distended jugular vein should not be used to estimate venous pressure.2
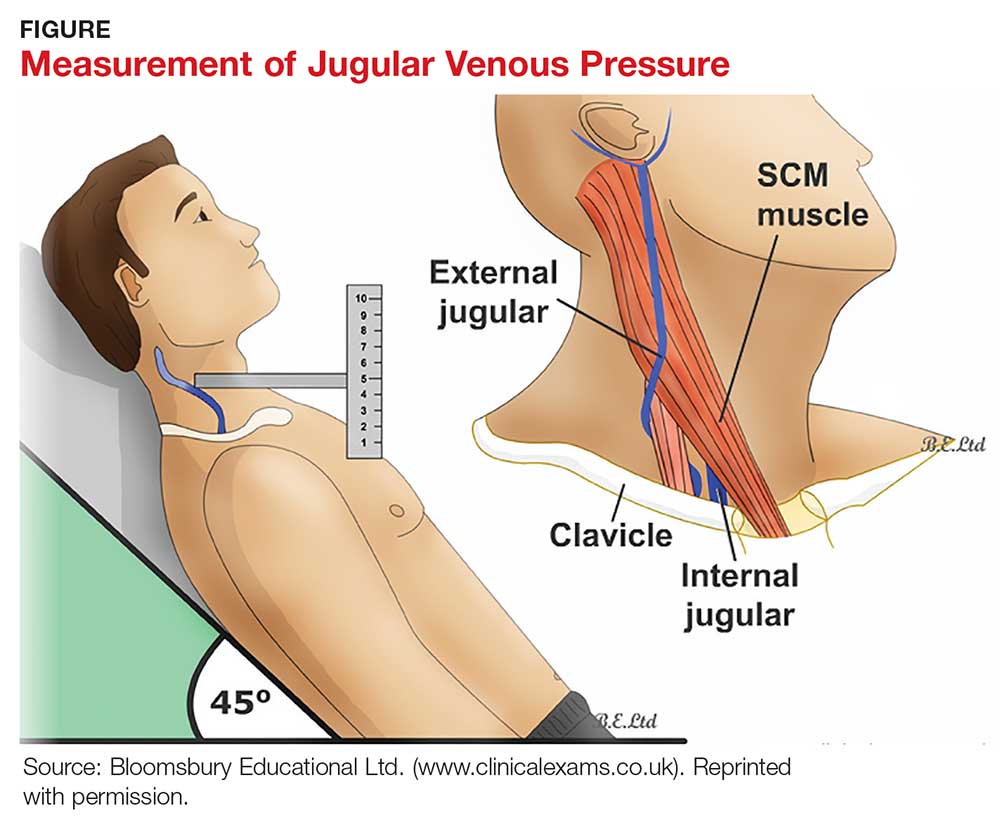
What, then, is the best method? Vinayak et al evaluated the comparative effectiveness of the internal and external jugular veins for detection of central venous pressure. They found that the external jugular vein is easier to visualize and has excellent reliability for determining low and high venous pressures.12
The process for estimating JVP is the same regardless of which jugular vein (internal or external) is used. The technique is described by Bickley in Bates’ Guide to Physical Examination and History Taking:
The patient lies supine and at an angle between 30° and 45°. Turn the head slightly, and with tangential lighting, identify the external and internal jugular veins. Identify the highest point of pulsation in the right jugular vein and extend a long rectangular object or card horizontally from this point and a centimeter ruler vertically from the sternal angle, making an exact right angle. Measure the vertical distance (in centimeters) above the sternal angle where the horizontal object crosses the ruler and add this distance to 4 cm. Measurements of > 3-4 cm above the sternal angle or > 8 cm in total distance are considered elevated.13
Continue to: DIAGNOSTIC AND SCREENING TESTS
DIAGNOSTIC AND SCREENING TESTS
Several diagnostic studies can identify HF or elucidate underlying causes. However, not all tests yield sufficient information for diagnosis—and commonly held “maxims” about findings that definitively rule in or out the diagnosis have been confounded by the available evidence.
Laboratory tests. The lab parameter most associated with HF is brain natriuretic peptide (BNP). Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension.14 In contrast to atrial natriuretic peptide (ANP), BNP is secreted not only from the atria but also from the ventricles, especially in patients with HF.15
Circulating concentrations of several cardiac natriuretic peptides—including ANP, BNP, and the two peptides’ N-terminal pro-hormones (N-terminal pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide, respectively) are elevated in both symptomatic and asymptomatic patients with left ventricular dysfunction.16 These levels are generally elevated for both systolic and diastolic HF. However, in one study, as many as 30% of diastolic HF patients had a low BNP level, despite signs and symptoms of HF and significantly elevated left ventricular filling pressures, as determined by invasive hemodynamic monitoring.17 Comorbid obesity is associated with low levels of natriuretic peptides.18
Additional lab tests can provide information about underlying causes of HF or reveal contraindications to certain treatment options (to be discussed in the Treatment section). A complete blood count (CBC) might reveal anemia, which can cause or aggravate HF, and which is an important consideration in management because of its association with decreased renal function, hemodilution, and proinflammatory cytokines. Leukocytosis can signal underlying infection. A troponin assay is helpful for ruling out acute MI as a cause of worsening HF in acute cases. Thyroid function tests and iron studies can be considered to rule out secondary causes of HF.
A serum electrolyte screen should be ordered; results are usually within normal ranges. Hyponatremia is an indicator of activation of the renin–angiotensin–aldosterone system (RAAS) and may be seen in the context of prolonged salt restriction and diuretic therapy. Hyperkalemia and hypokalemia are also prevalent in HF; both are limiting factors for some treatment options. A low sodium level is often the result of increased congestion and release of vasopressin; a level of ≤ 135 mEq/L predicts a poorer outcome.
Kidney function tests can determine whether HF is associated with impaired kidney function, secondary to poor renal perfusion. Poor renal function may limit treatment options. Patients with severe HF, particularly those receiving a high dosage of a diuretic for a long period, may have elevated levels of blood urea nitrogen and creatinine, indicating renal insufficiency.
Electrocardiography and chest radiography. ECGs and chest radiographs are noninvasive tests that have been used widely in the diagnosis of HF. They might indicate an underlying cause (eg, acute MI, ischemia, secondary arrhythmia) but are often nonspecific—and thus may be unhelpful in the diagnosis and treatment of HF.
The most common ECG findings in HF are nonspecific ST-T wave abnormalities.19 Other findings often consist of low-voltage left ventricular hypertrophy, conduction defects, and repolarization changes. With chest radiography, primary findings in HF include pulmonary edema—seen as perivascular edema, peribronchial cuffing, perihilar haze, interstitial edema (Kerley B, or septal, lines), and alveolar fluid—and pleural effusion.19,20
For both these modalities, however, there are commonly held conceptions that particular findings rule out HF—which has been disproven by Fonseca and colleagues.19 Because most patients with HF have an abnormal ECG, some studies have proposed that a normal ECG virtually rules out left ventricular systolic dysfunction.20 Evaluating the value of ECG in HF diagnosis, Fonseca et al found that about 85% of patients with an abnormal ECG had HF—but so did 30% of patients with a normal ECG. They concluded that, if used alone, ECG could have missed as many as 25% of patients with HF.19
Likewise, it has been suggested that a patient cannot have HF if heart size is normal on a chest radiograph.20 Fonseca et al found that cardiac enlargement, while the most informative radiologic measurement in HF, was present in only half of patients with HF.19 About 57% of patients who had an abnormal chest radiograph had HF, but so did about 40% of those who had a normal chest radiograph.19 Therefore, abnormal chest radiograph for identification of HF had an estimated sensitivity of 57%, a specificity of 78%, positive predictive value of 50%, and negative predictive value of 83%.19 The conclusion: Caution should be taken regarding the use of chest radiography in isolation to make a diagnosis of HF.
Echocardiography. The most useful test in evaluating HF is the echocardiogram, because it can distinguish between HF with and without preserved left ventricular systolic function. This is critical: The most clinically relevant classification of HF differentiates systolic and diastolic HF, based on LVEF.2 This determination has both prognostic and therapeutic implications.21
Echocardiography is widely available, safe, and noninvasive. The “echo” can identify the size of the atria and ventricles, valve function and dysfunction, and any associated shunting. Pericardial effusions and heart wall-motion abnormalities (for example, an old MI) are also easily identified.9
A normal ejection fraction does not rule out HF. Therefore, assessment of LVEF should not be considered until after a clinical diagnosis has been made, because more than half of HF patients have a normal LVEF—evidence that can confound the diagnostic process.2
Continue to: TREATMENT OF HEART FAILURE WITH REDUCED LVEF
TREATMENT OF HEART FAILURE WITH REDUCED LVEF
The body’s neurohormonal system, including the RAAS and the sympathetic nervous system, is activated to compensate for the insufficient cardiac performance found in HF. However, activation of these systems contributes to worsening HF, deterioration of quality of life, and poor outcomes.22 Therefore, therapies that suppress these responses can reduce the progression of HF.
Treatment of HF is generally divided into symptom-relieving treatment and disease-modifying/life-prolonging treatment.1 Symptom relief is similar in both systolic and diastolic HF. However, most evidence-based, disease-modifying treatment focuses on systolic HF; guidelines for disease-modifying treatment of diastolic HF are minimal.1
Treatment of reversible causes
Since HF is caused by something else, the primary focus of management is addressing underlying causes. The primary goal is to relieve symptoms while improving functional status—which should lead to a decrease in hospitalization and premature death.
The first step is to evaluate patients’ use of medications that can contribute to a worsening of HF.9 The most common offending medications are calcium-channel blockers with negative inotropy (non-dihydropyridine calcium-channel blockers, eg, verapamil and diltiazem); some antiarrhythmic drugs (eg, amiodarone); thiazolidinediones (glitazones); and NSAIDs.9 If identified as a possible contributor to HF symptoms, these agents should be stopped (if possible) or replaced.
Nonpharmacotherapy
Effective counseling and education of patients with HF may help with long-term adherence to treatment plans. Patients can be taught to monitor their weight at home and to adjust the dosage of diuretics as advised: A sudden increase in weight (> 2 kg in one to three d), for example, should alert a patient to alter treatment or seek advice.23
Diet modification is a multifactorial recommendation. Proper nutrition is critical because HF patients are at increased risk for malnutrition due to poor appetite, malabsorption, and increased nutritional requirements.23 Weight reduction in obese patients helps reduce cardiac workload. Patients should be placed on salt restriction (2 to 2.6 g/d of sodium).9,23
Exercise has been shown to relieve symptoms, provide a greater sense of well-being, and improve functional capacity. It does not, however, result in obvious improvement in cardiac function.22
Alcohol consumption should be restricted because of the myocardial depressant properties of alcohol and its direct toxic effect on the myocardium.22 Smoking should be discouraged because it has a direct effect on coronary artery disease.
Influenza and pneumococcal vaccination should be considered in all patients with HF.23 Heart failure predisposes patients to, and can be exacerbated by, pulmonary infection and exacerbation of chronic obstructive pulmonary disease.
Evaluation and management of obstructive sleep apnea should be performed. Sleep-disordered breathing, an umbrella term that covers obstructive and central sleep apneas, has been found to increase the risk for poor prognosis in HF.24 All patients with HF should be tested for obstructive sleep apnea because, often, only the patient’s bed partner is aware of disordered sleep. For unknown reasons, patients with HF do not report subjective sleepiness.25
Continue to: Pharmacotherapy
Pharmacotherapy
Diuretics have not been found to have benefit for reducing early mortality but are the most common agents used for symptomatic relief of sodium and water retention.26 In fact, few patients with signs and symptoms of fluid retention can be managed without a diuretic.9 Caution must be observed, however, because excessive diuresis can lead to electrolyte imbalance and neurohormonal activation.
Treating mild fluid retention with a thiazide diuretic (hydrochlorothiazide, metolazone, or chlorthalidone) is often sufficient (see Table 2 for dosing and other information on these and other drugs for treating HF).9 Thiazide diuretics are dependent on the glomerular filtration rate and are ineffective when it falls below 30-40 mL/min. Adverse reactions to diuretics include hypokalemia, dehydration (intravascular volume depletion) with prerenal azotemia, skin rash, neutropenia, thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.
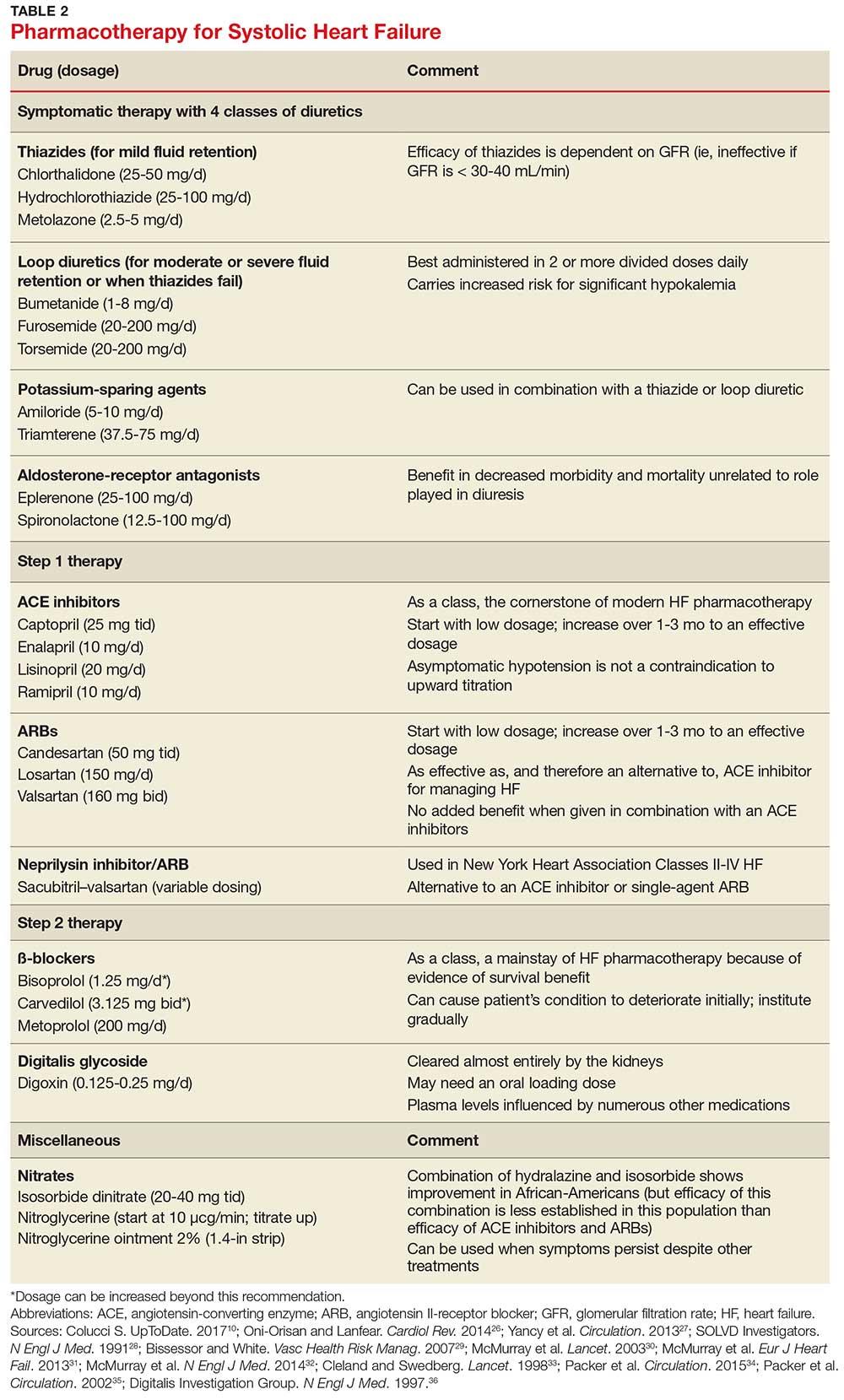
In cases of moderate or severe HF, or failure of thiazide diuretics to relieve mild symptoms, an oral loop diuretic (furosemide, bumetanide, or torsemide; see Table 2 for dosing) can be used if kidney function is preserved.9 These agents are best administered in two or more divided doses. Major adverse reactions are similar to those of thiazide diuretics, plus ototoxicity. Special caution must be used when a loop diuretic is co-administered with digitalis because the combination can cause significant hypokalemia.9
A potassium-sparing diuretic (triamterene or amiloride; see Table 2) can be used in combination with thiazide and loop diuretics.9 The location of their action is at the distal tubule, but diuretic potency is mild. Potassium-sparing agents can minimize hypokalemia induced by other diuretics. Adverse effects include hyperkalemia, kidney dysfunction, and gastrointestinal symptoms.
The aldosterone-receptor antagonists spironolactone and eplerenone are specific inhibitors of aldosterone, an effect that has been shown to improve clinical outcomes.9 Aldosterone-receptor antagonists are indicated for patients with NYHA class II-IV HF who have LVEF ≤ 35% or those with a history of acute MI, LVEF < 40%, and symptoms of HF.27 In these patients, a reduction in mortality and relative risk has been demonstrated with the use of aldosterone-receptor antagonists, unrelated to their role in diuresis. The adverse effect profile of spironolactone includes gynecomastia.
Continue to: Inhibitors of the RAAS
Inhibitors of the RAAS. As noted, the RAAS plays a key role during the development and worsening of HF.22
Angiotensin-converting enzyme inhibitors (ACE inhibitors) constitute the cornerstone of modern HF pharmacotherapy and have compelling evidence of survival benefit.28 These agents (captopril, enalapril, lisinopril, and ramipril; see Table 2 for dosing) have been shown to be effective therapy for HF and post-MI left ventricular dysfunction.29 They reduce early mortality by approximately 20% in symptomatic HF and can prevent hospitalization, increase exercise tolerance, and reduce adverse events (symptoms).9
Because of these benefits, ACE inhibitors should be firstline therapy in all HF patients with left ventricular dysfunction. They are usually used in combination with a diuretic. In addition, ACE inhibitors should be used in patients with reduced LVEF, even if they are asymptomatic, because these agents prevent progression to clinical HF.
Since ACE inhibitors carry the risk for severe hypotension, caution must be used, especially during treatment initiation. Patients whose systolic blood pressure is < 100 mm Hg, or who are hypovolemic, should be started at a low dosage (captopril, 6.25 mg tid; enalapril, 2.5 mg/d; or other equivalent ACE inhibitor dose); for other patients, these dosages can be doubled at initiation.9 Within days of initiation, but no longer than two weeks later, patients should be screened for hypotension and have both kidney function and potassium levels monitored. The dosage of ACE inhibitors should be increased over one to three months to an effective dosage (eg, captopril, 25 mg tid; enalapril, 10 mg bid; ramipril, 10 mg/d; and lisinopril, 20 mg/d, or other equivalent ACE inhibitor dose).9
Asymptomatic hypotension is not a contraindication to continuation or uptitration of the dosage of ACE inhibitors. Patients may also experience an increase in the serum creatinine or potassium level; likewise, this should not prompt a change in dosage if the elevated level stabilizes. The most common adverse effects of ACE inhibitors are dizziness and cough.9
Angiotensin II-receptor blockers (ARBs) have been shown to be as effective as ACE inhibitors for the management of hypertension, congestive HF, and chronic renal failure.30 ARBs decrease the adverse effects of angiotensin II, but do not have the same effects that ACE inhibitors do on other pathways found in HF (specifically, on bradykinin, prostaglandins, and nitric oxide).29 Candesartan and valsartan have been shown to have benefits in HF and are an equivalent alternative to ACE inhibitors; they are often used when a patient cannot tolerate an ACE inhibitor. Because ARBs and ACE inhibitors affect the RAAS at different points in the pathway, there is a theoretical basis for ARB and ACE inhibitor combination therapy; in clinical studies to date, however, the combination has shown no beneficial effect and rather was associated with more adverse effects.29
Neprilysin inhibitor. Recently, the combination of the neprilysin inhibitor sacubitril and an ARB, valsartan, has surfaced as an alternative to ACE inhibitor or single-agent ARB therapy.31,32 Inhibition of the enzyme neprilysin results in an increase in levels of endogenous vasoactive peptides (eg, natriuretic peptides and bradykinin), which may benefit hemodynamics in patients with HF. However, use of an ARB in combination with sacubitril is necessary to counteract the increase in angiotensin II levels that also results from inhibition of neprilysin.33
Sacubitril–valsartan is typically reserved for patients with mild-to-moderate HF who have either an elevated BNP (≥ 50 pg/mL) or an HF-related hospitalization within the past year. Upon transitioning from an ACE inhibitor (or single-agent ARB), a 36-hour washout period must be observed before starting sacubitril–valsartan to minimize the risk for angioedema. Because therapy with sacubitril–valsartan leads directly to an elevation in the BNP level, using the level of N-terminal pro-brain natriuretic peptide—which is not degraded by neprilysin—to monitor disease progression is recommended.34
ß-
Initiation of ß-blockers in stable patients can cause general deterioration; initiation must therefore be done gradually:
- Carvedilol, initiated at 3.125 mg bid, can be increased to 6.25 mg, then 12.5 mg, and then 25 mg, all bid, at intervals of approximately two weeks
- Sustained-release metoprolol can be started at 12.5 mg/d or 25 mg/d and doubled at two weeks to a target of 200 mg/d
- Bisoprolol, initiated at 1.25 mg/d, can be increased incrementally to 2.5 mg/d, 3.75 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d at one-to-four-week intervals.9
Patients taking ß-blockers need to monitor their weight at home as an indicator of fluid retention. If HF becomes worse, an increase in the dosage of the accompanying diuretic, a delay in the increase of the ß-blocker, or downward adjustment of the ß-blocker is usually sufficient.
Continue to: Digitalis glycoside
Digitalis glycoside. Digoxin has been shown to relieve the symptoms of HF, decrease the risk for hospitalization, and increase exercise tolerance; however, it has not been shown to offer a mortality benefit.36 Digoxin should be considered for patients who remain symptomatic when taking a diuretic and an ACE inhibitor and for patients who are also in atrial fibrillation and need rate control.
Digoxin is cleared almost entirely by the kidneys and therefore must be used with care in patients with renal dysfunction. Patients usually can be started on the expected maintenance dosage (0.125-0.25 mg/d). An oral loading dose of 0.75-1.25 mg over 24 to 48 hours can be used if an early effect is needed.9
Digoxin can induce ventricular arrhythmias (especially in a setting of hypokalemia and ischemia) and is sensitive to other medications that can drastically increase its level—most notably, amiodarone, quinidine, propafenone, and verapamil.9 The digoxin blood level should be measured every seven to 14 days until a maintenance dosage is established, and again whenever there is a change in medication or kidney function. The optimum serum level is 0.7-1.2 ng/mL; toxicity is not usually seen at < 1.8 ng/mL.9
Hydralazine and nitrates. The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in African-American patients with HF, but efficacy is less well-established in this population than for ACE inhibitor and ARB therapy.9 This combination can be considered in patients who are unable to tolerate ACE inhibitor or ARB therapy. It can also be considered in those who have persistent symptoms despite treatment with a ß-blocker, ACE inhibitor, or aldosterone antagonist.9
Intravenous nitrates are used primarily for acute HF, especially when accompanied by hypertension or myocardial ischemia. The starting dosage for nitroglycerin is approximately 10 μcg/min, titrated upward by 10-20 μcg/min to a maximum of 200 μcg/min. Isosorbide dinitrate (20-40 mg tid) and nitroglycerine ointment 2% (1.4 in applied every six to eight hours [although generally reserved for inpatient therapy]) are equally effective.9 Adverse effects that may limit the use of these agents are headache and hypotension. Patients also develop tolerance for nitrates, which can be mitigated if a daily 8-to-12–hour nitrate-free period is instituted.9
TREATMENT OF HEART FAILURE WITH PRESERVED LVEF
Treatment options for patients with preserved LVEF (diastolic HF) are not as clear as those for patients with reduced LVEF (systolic HF). No traditional therapies (ACE inhibitors, ARBs, ß-blockers, digoxin) have been shown to improve survival in this population, although a recent study on the effects of spironolactone in diastolic HF did show some improvement of diastolic dysfunction without adverse effects.17,37 In the absense of clear evidence-based therapies, treatment focuses on managing comorbidities, addressing reversible causes, and alleviating fluid overload with a diuretic.9,17
Diuretic therapy is critical to control fluid overload; regimens are similar to those for HF with reduced LVEF. ACE inhibitors and ARBs have not been shown to improve outcomes in this population but can be used to manage comorbid hypertension. Spironolactone has also not been shown to improve outcomes in patients with diastolic HF.9
The principal conditions that can lead to HF with preserved LVEF are hypertension, pericardial disease, and atrial tachycardia. Tachycardia is associated with overall shorter diastolic filling time; controlling an accelerated heart rate, therefore, is theorized to be an important therapeutic goal.9 Other disease states, including diabetes mellitus, sleep-disordered breathing, obesity, and chronic kidney disease, can all lead to HF with preserved LVEF.
CONCLUSION
Managing HF today requires an “upstream” model of care, by which providers consider the diagnosis/syndrome of HF in the asymptomatic patient with risk factors (Stage A/Class I and Stage B/Class II). Perhaps a better way to state this is that providers must change their approach from ruling-in to ruling-out HF. Any person in whom a decrease in activity level, mild shortness of breath, or edema are observed, and who has known risk factors, should be considered to have HF until proven otherwise.
Because of the wide variability in the underlying causes of HF, providers must be dynamic in both their clinical approach and their treatment plans to optimize care for the individual patient. Finally, providers must also take note of the increasing prevalence of diastolic HF and, again, focus on early diagnosis.
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28.
2. Ahmed A. DEFEAT - Heart failure: a guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100(1):39-50.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001; 38(7):2101-2113.
4. Mozaffarian D, Benjamin EJ, Go AS, et al; Writing Group Members, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;26: 133(4):e38-e360.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
6. American Heart Association. Classes of heart failure (2017). www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.V8cFhfkrLRY. Accessed March 7, 2018.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994.
8. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
9. Bashore T, Granger C, Hranitzy P, Patel M. Heart diseases. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment. 55th ed. New York, NY: McGraw-Hill Education; 2016:402.
10. Colucci S. Patient education: heart failure (beyond the basics). Waltham, MA: UpToDate; 2017. www.uptodate.com/contents/heart-failure-beyond-the-basics?view=print. Accessed March 7, 2018.
11. Butman S, Ewy GA, Standen J, et al. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol. 1993;22(4):968-974.
12. Vinayak A, Levitt J, Gehlbach B, et al. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166(19):2132-2137.
13. Bickley LA. Bates’ Guide to Physical Examination and History Taking. 11th ed. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2012:364-365.
14. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961-1970.
15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-204.
16. Cowie M, Struthers A, Wood D, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350(9088):1349-1353.
17. Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
18. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47(1):85-90.
19. Fonseca C, Mota T, Morais H; EPICA Investigators. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2006;6(6):807-812.
20. Rihal CS, Davis KB, Kennedy JW, Gersch BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223.
21. Joint Commission on Accreditation of Healthcare Organizations. A Comprehensive Review of Development and Testing for National Implementation of Hospital Core Measures. 2002. www.jointcommission.org/assets/1/18/A_Comprehensive_Review_of_Development_for_Core_Measures.pdf. Accessed March 6, 2018.
22. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371.
23. Gibbs CR, Jackson G, Lip GY. ABC of heart failure: Nondrug management. BMJ. 2000;320(7231):365-369.
24. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631.
25. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716-1722.
26. Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193-198.
27. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
28. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
29. Bissessor N, White H. Valsartan in the treatment of heart failure or left ventricular dysfunction after myocardial infarction. Vasc Health Risk Manag. 2007;3(4):425-430.
30. McMurray JJ, Ostegren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
31. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073.
32. McMurray J, Packer M, Desai A, et al; PARADIGM-HF Committees and Investigators. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
33. Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657-1658.
34. Packer M, McMurray JJ, Desai AS, et al; PARADIGM-HF Committees and Investigators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61.
35. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199.
36. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
37. Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
CE/CME No: CR-1805
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Develop an understanding of the classification of heart failure and its associated signs and symptoms.
• Describe clinical findings of heart failure from the physical exam.
• Understand the utility of laboratory and imaging studies for diagnosis of heart failure and its underlying causes.
• Demonstrate knowledge of nonpharmacotherapeutic and pharmacotherapeutic options for managing heart failure.
FACULTY
Michael Roscoe is Chair/Program Director and Associate Professor, Andrew Lampkins is Associate Professor, Sean Harper is Assistant Professor, and Gina Niemeier is Associate Program Director and Assistant Professor, in the PA Department at the University of Evansville in Indiana.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through April 30, 2019.
Article begins on next page >>
Heart failure is a complex syndrome with a spectrum of signs and symptoms that range from asymptomatic to terminal. This variability of presentation, paired with the irreversibility of the process, make it both difficult and critical to identify this syndrome early to prevent progression. Here is an overview of the classification and common presentations of heart failure, as well as a guide to diagnostic modalities and treatment options.
Heart failure (HF) is a complex syndrome, not a specific disease; it is always associated with an underlying cause. Hypertension and coronary artery disease are the two most common causes in all age groups, but a number of other conditions—valvular disease, unrecognized obstructive sleep apnea, obesity, chronic kidney disease, anemia, hyperlipidemia, diabetes, and atrial fibrillation—have been identified as secondary causes.1-3
Often, however, clinicians do not identify HF until the syndrome reaches an advanced stage—at which point, the damage is irreversible and pharmacotherapeutic management is limited to control of signs and symptoms. The ramifications are concerning, since HF has achieved near-epidemic scope in the United States. An estimated 5.7 million Americans ages 20 and older have HF; prevalence is projected to increase by 46% between 2012 and 2030—resulting in more than 8 million affected individuals (ages 18 and older).4,5 More than 1 million patients are discharged from the hospital annually with a primary diagnosis of HF.4 And in 2013, one in nine death certificates in the US mentioned HF.4
Most cases of HF are managed in primary care. Established, evidence-based therapies should be implemented in the outpatient setting when possible, as early in the course as possible. Referral to a cardiologist is needed when the underlying cause of HF remains undetermined, or when specialized treatment is required.
CLASSIFICATION OF HF
The two most widely recognized classification systems for HF are those of the American College of Cardiology and the American Heart Association (ACC/AHA) and of the New York Heart Association (NYHA). Both focus on one-year mortality. The stages of the ACC/AHA system (A to D) are based on worsening of both structural heart disease and clinical symptoms of HF. The NYHA designations (Class I to IV) are based on the functional capability associated with physical activity. Both systems are outlined in Table 1.3,6,7

While these systems are used to “stage” HF, there are several ways the syndrome is classified in the medical literature. For example, HF can be described by
- Anatomy (left- or right-sided)
- Physiology (dilated and hypertrophic)
- Course (chronic or acute heart failure [cardiogenic shock])
- Output (high- or low-output failure)
- Ejection fraction (reduced or preserved)
- Pressure phase (systolic and diastolic).
All these classifications have merit; however, this article will attempt to simplify the approach to patients with HF and focus on systolic HF, defined as a reduced left ventricular ejection fraction (LVEF), and diastolic HF, defined as a preserved LVEF. Although the common perception of HF among clinicians—and thus the traditional diagnostic focus—is reduced LVEF (systolic HF), preserved LVEF (diastolic HF) in fact represents approximately 50% of cases.1 Diastolic HF is estimated to be increasing in prevalence and is expected to become the more common phenotype.8
Continue to: SIGNS AND SYMPTOMS
SIGNS AND SYMPTOMS
Heart failure is characterized by a constellation of signs and symptoms of pulmonary and/or systemic venous congestion caused by impaired ability of the heart to fill with or eject blood in proportion to the metabolic needs of the body.1 Manifestations include fatigue, dyspnea, fluid retention, and cachexia, and patients can present anywhere on the spectrum—from asymptomatic at rest to severely symptomatic.
Symptoms
In both reduced LVEF and preserved LVEF HF, common early symptoms include dyspnea and fatigue on exertion, with or without some degree of lower-extremity swelling.2 Lack of treatment and disease progression increase symptom severity—to the extent that dyspnea and fatigue start to occur at rest. Reviewing the anatomic classifications/findings of HF facilitates understanding of the clinical symptoms.
Right HF. Right ventricular dysfunction is rarely found in isolation; when symptoms are present, further evaluation of the left ventricle and the pulmonary system (to look for cor pulmonale) is warranted. Right HF is associated with an inability to manage venous return and move volume into the pulmonary circuit. This produces the predominant symptom of fluid retention. Peripheral edema is a cardinal symptom of right HF; edema can also present systemically, mostly as hepatic congestion or general gastrointestinal complaints resulting from impaired gastrointestinal perfusion.9
Left HF. Left ventricular dysfunction is the more common anatomic finding in HF and is often generalized to represent all cases. The dysfunction can be found in isolation and is actually the leading cause of right HF. In left HF (regardless of etiology), the heart does not produce enough “forward” pressure (ie, cannot pump or fill with enough blood) for the cardiovascular system to remain in balance. Thus, “back” pressure into the pulmonary circuit develops.
The combination of insufficient systemic perfusion capability with a dysfunctional left pump means that the most common symptom in all cases of left HF is shortness of breath—specifically, exertional dyspnea. Dyspnea can progress to orthopnea, paroxysmal nocturnal dyspnea, and, finally, dyspnea at rest. Patients often present with a cough that is worse while lying down and that can be nonproductive or productive, depending on volume status.9,10
Signs
Vital signs range from normal to indicative of shock. Increased sympathetic nervous system activity is common; this may manifest as coldness of the extremities and diaphoresis. Keep in mind that HF is a clinical diagnosis: The physical examination, focusing on peripheral signs, is key in all HF patients for both diagnosis and management.
PHYSICAL EXAMINATION
The physical exam includes the heart, neck, lungs, abdomen, and extremities. The cardiac exam includes evaluation for hypertrophy, by palpating for the point of maximum impulse to assess for lifts, heaves, valvular disease, and S3 and S4 sounds. Respiratory, abdominal, and extremity exams all focus on the evaluation of fluid status and edema.
A key, often underutilized measurement is jugular venous pressure (JVP). Elevated JVP has been identified as the most specific sign of fluid overload in HF and the most important physical finding in the initial and subsequent examinations of a patient with HF.11 (For good reason, clinicians who treat patients with HF must be able to recognize volume overload and hypovolemia; euvolemia allows patients to remain symptom-free and makes it possible to initiate life-prolonging therapy, which will be discussed in the Treatment section.2) JVP is an indirect measure of pressure within the right side of the heart (central venous pressure).
Most texts on performing the physical exam recommend measuring JVP using the right internal jugular vein. However, use of the internal jugular vein is limiting in HF patients, because it is covered by the sternocleidomastoid muscle for most of its course in the neck and visible only in a small triangle between the two heads in the root of the neck (see Figure). Conversely, the external jugular veins are subcutaneous along their entire course and pulsations are easily visible—but superficial and prone to external pressure and internal occlusion. A nonpulsatile, distended jugular vein should not be used to estimate venous pressure.2

What, then, is the best method? Vinayak et al evaluated the comparative effectiveness of the internal and external jugular veins for detection of central venous pressure. They found that the external jugular vein is easier to visualize and has excellent reliability for determining low and high venous pressures.12
The process for estimating JVP is the same regardless of which jugular vein (internal or external) is used. The technique is described by Bickley in Bates’ Guide to Physical Examination and History Taking:
The patient lies supine and at an angle between 30° and 45°. Turn the head slightly, and with tangential lighting, identify the external and internal jugular veins. Identify the highest point of pulsation in the right jugular vein and extend a long rectangular object or card horizontally from this point and a centimeter ruler vertically from the sternal angle, making an exact right angle. Measure the vertical distance (in centimeters) above the sternal angle where the horizontal object crosses the ruler and add this distance to 4 cm. Measurements of > 3-4 cm above the sternal angle or > 8 cm in total distance are considered elevated.13
Continue to: DIAGNOSTIC AND SCREENING TESTS
DIAGNOSTIC AND SCREENING TESTS
Several diagnostic studies can identify HF or elucidate underlying causes. However, not all tests yield sufficient information for diagnosis—and commonly held “maxims” about findings that definitively rule in or out the diagnosis have been confounded by the available evidence.
Laboratory tests. The lab parameter most associated with HF is brain natriuretic peptide (BNP). Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension.14 In contrast to atrial natriuretic peptide (ANP), BNP is secreted not only from the atria but also from the ventricles, especially in patients with HF.15
Circulating concentrations of several cardiac natriuretic peptides—including ANP, BNP, and the two peptides’ N-terminal pro-hormones (N-terminal pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide, respectively) are elevated in both symptomatic and asymptomatic patients with left ventricular dysfunction.16 These levels are generally elevated for both systolic and diastolic HF. However, in one study, as many as 30% of diastolic HF patients had a low BNP level, despite signs and symptoms of HF and significantly elevated left ventricular filling pressures, as determined by invasive hemodynamic monitoring.17 Comorbid obesity is associated with low levels of natriuretic peptides.18
Additional lab tests can provide information about underlying causes of HF or reveal contraindications to certain treatment options (to be discussed in the Treatment section). A complete blood count (CBC) might reveal anemia, which can cause or aggravate HF, and which is an important consideration in management because of its association with decreased renal function, hemodilution, and proinflammatory cytokines. Leukocytosis can signal underlying infection. A troponin assay is helpful for ruling out acute MI as a cause of worsening HF in acute cases. Thyroid function tests and iron studies can be considered to rule out secondary causes of HF.
A serum electrolyte screen should be ordered; results are usually within normal ranges. Hyponatremia is an indicator of activation of the renin–angiotensin–aldosterone system (RAAS) and may be seen in the context of prolonged salt restriction and diuretic therapy. Hyperkalemia and hypokalemia are also prevalent in HF; both are limiting factors for some treatment options. A low sodium level is often the result of increased congestion and release of vasopressin; a level of ≤ 135 mEq/L predicts a poorer outcome.
Kidney function tests can determine whether HF is associated with impaired kidney function, secondary to poor renal perfusion. Poor renal function may limit treatment options. Patients with severe HF, particularly those receiving a high dosage of a diuretic for a long period, may have elevated levels of blood urea nitrogen and creatinine, indicating renal insufficiency.
Electrocardiography and chest radiography. ECGs and chest radiographs are noninvasive tests that have been used widely in the diagnosis of HF. They might indicate an underlying cause (eg, acute MI, ischemia, secondary arrhythmia) but are often nonspecific—and thus may be unhelpful in the diagnosis and treatment of HF.
The most common ECG findings in HF are nonspecific ST-T wave abnormalities.19 Other findings often consist of low-voltage left ventricular hypertrophy, conduction defects, and repolarization changes. With chest radiography, primary findings in HF include pulmonary edema—seen as perivascular edema, peribronchial cuffing, perihilar haze, interstitial edema (Kerley B, or septal, lines), and alveolar fluid—and pleural effusion.19,20
For both these modalities, however, there are commonly held conceptions that particular findings rule out HF—which has been disproven by Fonseca and colleagues.19 Because most patients with HF have an abnormal ECG, some studies have proposed that a normal ECG virtually rules out left ventricular systolic dysfunction.20 Evaluating the value of ECG in HF diagnosis, Fonseca et al found that about 85% of patients with an abnormal ECG had HF—but so did 30% of patients with a normal ECG. They concluded that, if used alone, ECG could have missed as many as 25% of patients with HF.19
Likewise, it has been suggested that a patient cannot have HF if heart size is normal on a chest radiograph.20 Fonseca et al found that cardiac enlargement, while the most informative radiologic measurement in HF, was present in only half of patients with HF.19 About 57% of patients who had an abnormal chest radiograph had HF, but so did about 40% of those who had a normal chest radiograph.19 Therefore, abnormal chest radiograph for identification of HF had an estimated sensitivity of 57%, a specificity of 78%, positive predictive value of 50%, and negative predictive value of 83%.19 The conclusion: Caution should be taken regarding the use of chest radiography in isolation to make a diagnosis of HF.
Echocardiography. The most useful test in evaluating HF is the echocardiogram, because it can distinguish between HF with and without preserved left ventricular systolic function. This is critical: The most clinically relevant classification of HF differentiates systolic and diastolic HF, based on LVEF.2 This determination has both prognostic and therapeutic implications.21
Echocardiography is widely available, safe, and noninvasive. The “echo” can identify the size of the atria and ventricles, valve function and dysfunction, and any associated shunting. Pericardial effusions and heart wall-motion abnormalities (for example, an old MI) are also easily identified.9
A normal ejection fraction does not rule out HF. Therefore, assessment of LVEF should not be considered until after a clinical diagnosis has been made, because more than half of HF patients have a normal LVEF—evidence that can confound the diagnostic process.2
Continue to: TREATMENT OF HEART FAILURE WITH REDUCED LVEF
TREATMENT OF HEART FAILURE WITH REDUCED LVEF
The body’s neurohormonal system, including the RAAS and the sympathetic nervous system, is activated to compensate for the insufficient cardiac performance found in HF. However, activation of these systems contributes to worsening HF, deterioration of quality of life, and poor outcomes.22 Therefore, therapies that suppress these responses can reduce the progression of HF.
Treatment of HF is generally divided into symptom-relieving treatment and disease-modifying/life-prolonging treatment.1 Symptom relief is similar in both systolic and diastolic HF. However, most evidence-based, disease-modifying treatment focuses on systolic HF; guidelines for disease-modifying treatment of diastolic HF are minimal.1
Treatment of reversible causes
Since HF is caused by something else, the primary focus of management is addressing underlying causes. The primary goal is to relieve symptoms while improving functional status—which should lead to a decrease in hospitalization and premature death.
The first step is to evaluate patients’ use of medications that can contribute to a worsening of HF.9 The most common offending medications are calcium-channel blockers with negative inotropy (non-dihydropyridine calcium-channel blockers, eg, verapamil and diltiazem); some antiarrhythmic drugs (eg, amiodarone); thiazolidinediones (glitazones); and NSAIDs.9 If identified as a possible contributor to HF symptoms, these agents should be stopped (if possible) or replaced.
Nonpharmacotherapy
Effective counseling and education of patients with HF may help with long-term adherence to treatment plans. Patients can be taught to monitor their weight at home and to adjust the dosage of diuretics as advised: A sudden increase in weight (> 2 kg in one to three d), for example, should alert a patient to alter treatment or seek advice.23
Diet modification is a multifactorial recommendation. Proper nutrition is critical because HF patients are at increased risk for malnutrition due to poor appetite, malabsorption, and increased nutritional requirements.23 Weight reduction in obese patients helps reduce cardiac workload. Patients should be placed on salt restriction (2 to 2.6 g/d of sodium).9,23
Exercise has been shown to relieve symptoms, provide a greater sense of well-being, and improve functional capacity. It does not, however, result in obvious improvement in cardiac function.22
Alcohol consumption should be restricted because of the myocardial depressant properties of alcohol and its direct toxic effect on the myocardium.22 Smoking should be discouraged because it has a direct effect on coronary artery disease.
Influenza and pneumococcal vaccination should be considered in all patients with HF.23 Heart failure predisposes patients to, and can be exacerbated by, pulmonary infection and exacerbation of chronic obstructive pulmonary disease.
Evaluation and management of obstructive sleep apnea should be performed. Sleep-disordered breathing, an umbrella term that covers obstructive and central sleep apneas, has been found to increase the risk for poor prognosis in HF.24 All patients with HF should be tested for obstructive sleep apnea because, often, only the patient’s bed partner is aware of disordered sleep. For unknown reasons, patients with HF do not report subjective sleepiness.25
Continue to: Pharmacotherapy
Pharmacotherapy
Diuretics have not been found to have benefit for reducing early mortality but are the most common agents used for symptomatic relief of sodium and water retention.26 In fact, few patients with signs and symptoms of fluid retention can be managed without a diuretic.9 Caution must be observed, however, because excessive diuresis can lead to electrolyte imbalance and neurohormonal activation.
Treating mild fluid retention with a thiazide diuretic (hydrochlorothiazide, metolazone, or chlorthalidone) is often sufficient (see Table 2 for dosing and other information on these and other drugs for treating HF).9 Thiazide diuretics are dependent on the glomerular filtration rate and are ineffective when it falls below 30-40 mL/min. Adverse reactions to diuretics include hypokalemia, dehydration (intravascular volume depletion) with prerenal azotemia, skin rash, neutropenia, thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.

In cases of moderate or severe HF, or failure of thiazide diuretics to relieve mild symptoms, an oral loop diuretic (furosemide, bumetanide, or torsemide; see Table 2 for dosing) can be used if kidney function is preserved.9 These agents are best administered in two or more divided doses. Major adverse reactions are similar to those of thiazide diuretics, plus ototoxicity. Special caution must be used when a loop diuretic is co-administered with digitalis because the combination can cause significant hypokalemia.9
A potassium-sparing diuretic (triamterene or amiloride; see Table 2) can be used in combination with thiazide and loop diuretics.9 The location of their action is at the distal tubule, but diuretic potency is mild. Potassium-sparing agents can minimize hypokalemia induced by other diuretics. Adverse effects include hyperkalemia, kidney dysfunction, and gastrointestinal symptoms.
The aldosterone-receptor antagonists spironolactone and eplerenone are specific inhibitors of aldosterone, an effect that has been shown to improve clinical outcomes.9 Aldosterone-receptor antagonists are indicated for patients with NYHA class II-IV HF who have LVEF ≤ 35% or those with a history of acute MI, LVEF < 40%, and symptoms of HF.27 In these patients, a reduction in mortality and relative risk has been demonstrated with the use of aldosterone-receptor antagonists, unrelated to their role in diuresis. The adverse effect profile of spironolactone includes gynecomastia.
Continue to: Inhibitors of the RAAS
Inhibitors of the RAAS. As noted, the RAAS plays a key role during the development and worsening of HF.22
Angiotensin-converting enzyme inhibitors (ACE inhibitors) constitute the cornerstone of modern HF pharmacotherapy and have compelling evidence of survival benefit.28 These agents (captopril, enalapril, lisinopril, and ramipril; see Table 2 for dosing) have been shown to be effective therapy for HF and post-MI left ventricular dysfunction.29 They reduce early mortality by approximately 20% in symptomatic HF and can prevent hospitalization, increase exercise tolerance, and reduce adverse events (symptoms).9
Because of these benefits, ACE inhibitors should be firstline therapy in all HF patients with left ventricular dysfunction. They are usually used in combination with a diuretic. In addition, ACE inhibitors should be used in patients with reduced LVEF, even if they are asymptomatic, because these agents prevent progression to clinical HF.
Since ACE inhibitors carry the risk for severe hypotension, caution must be used, especially during treatment initiation. Patients whose systolic blood pressure is < 100 mm Hg, or who are hypovolemic, should be started at a low dosage (captopril, 6.25 mg tid; enalapril, 2.5 mg/d; or other equivalent ACE inhibitor dose); for other patients, these dosages can be doubled at initiation.9 Within days of initiation, but no longer than two weeks later, patients should be screened for hypotension and have both kidney function and potassium levels monitored. The dosage of ACE inhibitors should be increased over one to three months to an effective dosage (eg, captopril, 25 mg tid; enalapril, 10 mg bid; ramipril, 10 mg/d; and lisinopril, 20 mg/d, or other equivalent ACE inhibitor dose).9
Asymptomatic hypotension is not a contraindication to continuation or uptitration of the dosage of ACE inhibitors. Patients may also experience an increase in the serum creatinine or potassium level; likewise, this should not prompt a change in dosage if the elevated level stabilizes. The most common adverse effects of ACE inhibitors are dizziness and cough.9
Angiotensin II-receptor blockers (ARBs) have been shown to be as effective as ACE inhibitors for the management of hypertension, congestive HF, and chronic renal failure.30 ARBs decrease the adverse effects of angiotensin II, but do not have the same effects that ACE inhibitors do on other pathways found in HF (specifically, on bradykinin, prostaglandins, and nitric oxide).29 Candesartan and valsartan have been shown to have benefits in HF and are an equivalent alternative to ACE inhibitors; they are often used when a patient cannot tolerate an ACE inhibitor. Because ARBs and ACE inhibitors affect the RAAS at different points in the pathway, there is a theoretical basis for ARB and ACE inhibitor combination therapy; in clinical studies to date, however, the combination has shown no beneficial effect and rather was associated with more adverse effects.29
Neprilysin inhibitor. Recently, the combination of the neprilysin inhibitor sacubitril and an ARB, valsartan, has surfaced as an alternative to ACE inhibitor or single-agent ARB therapy.31,32 Inhibition of the enzyme neprilysin results in an increase in levels of endogenous vasoactive peptides (eg, natriuretic peptides and bradykinin), which may benefit hemodynamics in patients with HF. However, use of an ARB in combination with sacubitril is necessary to counteract the increase in angiotensin II levels that also results from inhibition of neprilysin.33
Sacubitril–valsartan is typically reserved for patients with mild-to-moderate HF who have either an elevated BNP (≥ 50 pg/mL) or an HF-related hospitalization within the past year. Upon transitioning from an ACE inhibitor (or single-agent ARB), a 36-hour washout period must be observed before starting sacubitril–valsartan to minimize the risk for angioedema. Because therapy with sacubitril–valsartan leads directly to an elevation in the BNP level, using the level of N-terminal pro-brain natriuretic peptide—which is not degraded by neprilysin—to monitor disease progression is recommended.34
ß-
Initiation of ß-blockers in stable patients can cause general deterioration; initiation must therefore be done gradually:
- Carvedilol, initiated at 3.125 mg bid, can be increased to 6.25 mg, then 12.5 mg, and then 25 mg, all bid, at intervals of approximately two weeks
- Sustained-release metoprolol can be started at 12.5 mg/d or 25 mg/d and doubled at two weeks to a target of 200 mg/d
- Bisoprolol, initiated at 1.25 mg/d, can be increased incrementally to 2.5 mg/d, 3.75 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d at one-to-four-week intervals.9
Patients taking ß-blockers need to monitor their weight at home as an indicator of fluid retention. If HF becomes worse, an increase in the dosage of the accompanying diuretic, a delay in the increase of the ß-blocker, or downward adjustment of the ß-blocker is usually sufficient.
Continue to: Digitalis glycoside
Digitalis glycoside. Digoxin has been shown to relieve the symptoms of HF, decrease the risk for hospitalization, and increase exercise tolerance; however, it has not been shown to offer a mortality benefit.36 Digoxin should be considered for patients who remain symptomatic when taking a diuretic and an ACE inhibitor and for patients who are also in atrial fibrillation and need rate control.
Digoxin is cleared almost entirely by the kidneys and therefore must be used with care in patients with renal dysfunction. Patients usually can be started on the expected maintenance dosage (0.125-0.25 mg/d). An oral loading dose of 0.75-1.25 mg over 24 to 48 hours can be used if an early effect is needed.9
Digoxin can induce ventricular arrhythmias (especially in a setting of hypokalemia and ischemia) and is sensitive to other medications that can drastically increase its level—most notably, amiodarone, quinidine, propafenone, and verapamil.9 The digoxin blood level should be measured every seven to 14 days until a maintenance dosage is established, and again whenever there is a change in medication or kidney function. The optimum serum level is 0.7-1.2 ng/mL; toxicity is not usually seen at < 1.8 ng/mL.9
Hydralazine and nitrates. The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in African-American patients with HF, but efficacy is less well-established in this population than for ACE inhibitor and ARB therapy.9 This combination can be considered in patients who are unable to tolerate ACE inhibitor or ARB therapy. It can also be considered in those who have persistent symptoms despite treatment with a ß-blocker, ACE inhibitor, or aldosterone antagonist.9
Intravenous nitrates are used primarily for acute HF, especially when accompanied by hypertension or myocardial ischemia. The starting dosage for nitroglycerin is approximately 10 μcg/min, titrated upward by 10-20 μcg/min to a maximum of 200 μcg/min. Isosorbide dinitrate (20-40 mg tid) and nitroglycerine ointment 2% (1.4 in applied every six to eight hours [although generally reserved for inpatient therapy]) are equally effective.9 Adverse effects that may limit the use of these agents are headache and hypotension. Patients also develop tolerance for nitrates, which can be mitigated if a daily 8-to-12–hour nitrate-free period is instituted.9
TREATMENT OF HEART FAILURE WITH PRESERVED LVEF
Treatment options for patients with preserved LVEF (diastolic HF) are not as clear as those for patients with reduced LVEF (systolic HF). No traditional therapies (ACE inhibitors, ARBs, ß-blockers, digoxin) have been shown to improve survival in this population, although a recent study on the effects of spironolactone in diastolic HF did show some improvement of diastolic dysfunction without adverse effects.17,37 In the absense of clear evidence-based therapies, treatment focuses on managing comorbidities, addressing reversible causes, and alleviating fluid overload with a diuretic.9,17
Diuretic therapy is critical to control fluid overload; regimens are similar to those for HF with reduced LVEF. ACE inhibitors and ARBs have not been shown to improve outcomes in this population but can be used to manage comorbid hypertension. Spironolactone has also not been shown to improve outcomes in patients with diastolic HF.9
The principal conditions that can lead to HF with preserved LVEF are hypertension, pericardial disease, and atrial tachycardia. Tachycardia is associated with overall shorter diastolic filling time; controlling an accelerated heart rate, therefore, is theorized to be an important therapeutic goal.9 Other disease states, including diabetes mellitus, sleep-disordered breathing, obesity, and chronic kidney disease, can all lead to HF with preserved LVEF.
CONCLUSION
Managing HF today requires an “upstream” model of care, by which providers consider the diagnosis/syndrome of HF in the asymptomatic patient with risk factors (Stage A/Class I and Stage B/Class II). Perhaps a better way to state this is that providers must change their approach from ruling-in to ruling-out HF. Any person in whom a decrease in activity level, mild shortness of breath, or edema are observed, and who has known risk factors, should be considered to have HF until proven otherwise.
Because of the wide variability in the underlying causes of HF, providers must be dynamic in both their clinical approach and their treatment plans to optimize care for the individual patient. Finally, providers must also take note of the increasing prevalence of diastolic HF and, again, focus on early diagnosis.
CE/CME No: CR-1805
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Develop an understanding of the classification of heart failure and its associated signs and symptoms.
• Describe clinical findings of heart failure from the physical exam.
• Understand the utility of laboratory and imaging studies for diagnosis of heart failure and its underlying causes.
• Demonstrate knowledge of nonpharmacotherapeutic and pharmacotherapeutic options for managing heart failure.
FACULTY
Michael Roscoe is Chair/Program Director and Associate Professor, Andrew Lampkins is Associate Professor, Sean Harper is Assistant Professor, and Gina Niemeier is Associate Program Director and Assistant Professor, in the PA Department at the University of Evansville in Indiana.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through April 30, 2019.
Article begins on next page >>
Heart failure is a complex syndrome with a spectrum of signs and symptoms that range from asymptomatic to terminal. This variability of presentation, paired with the irreversibility of the process, make it both difficult and critical to identify this syndrome early to prevent progression. Here is an overview of the classification and common presentations of heart failure, as well as a guide to diagnostic modalities and treatment options.
Heart failure (HF) is a complex syndrome, not a specific disease; it is always associated with an underlying cause. Hypertension and coronary artery disease are the two most common causes in all age groups, but a number of other conditions—valvular disease, unrecognized obstructive sleep apnea, obesity, chronic kidney disease, anemia, hyperlipidemia, diabetes, and atrial fibrillation—have been identified as secondary causes.1-3
Often, however, clinicians do not identify HF until the syndrome reaches an advanced stage—at which point, the damage is irreversible and pharmacotherapeutic management is limited to control of signs and symptoms. The ramifications are concerning, since HF has achieved near-epidemic scope in the United States. An estimated 5.7 million Americans ages 20 and older have HF; prevalence is projected to increase by 46% between 2012 and 2030—resulting in more than 8 million affected individuals (ages 18 and older).4,5 More than 1 million patients are discharged from the hospital annually with a primary diagnosis of HF.4 And in 2013, one in nine death certificates in the US mentioned HF.4
Most cases of HF are managed in primary care. Established, evidence-based therapies should be implemented in the outpatient setting when possible, as early in the course as possible. Referral to a cardiologist is needed when the underlying cause of HF remains undetermined, or when specialized treatment is required.
CLASSIFICATION OF HF
The two most widely recognized classification systems for HF are those of the American College of Cardiology and the American Heart Association (ACC/AHA) and of the New York Heart Association (NYHA). Both focus on one-year mortality. The stages of the ACC/AHA system (A to D) are based on worsening of both structural heart disease and clinical symptoms of HF. The NYHA designations (Class I to IV) are based on the functional capability associated with physical activity. Both systems are outlined in Table 1.3,6,7

While these systems are used to “stage” HF, there are several ways the syndrome is classified in the medical literature. For example, HF can be described by
- Anatomy (left- or right-sided)
- Physiology (dilated and hypertrophic)
- Course (chronic or acute heart failure [cardiogenic shock])
- Output (high- or low-output failure)
- Ejection fraction (reduced or preserved)
- Pressure phase (systolic and diastolic).
All these classifications have merit; however, this article will attempt to simplify the approach to patients with HF and focus on systolic HF, defined as a reduced left ventricular ejection fraction (LVEF), and diastolic HF, defined as a preserved LVEF. Although the common perception of HF among clinicians—and thus the traditional diagnostic focus—is reduced LVEF (systolic HF), preserved LVEF (diastolic HF) in fact represents approximately 50% of cases.1 Diastolic HF is estimated to be increasing in prevalence and is expected to become the more common phenotype.8
Continue to: SIGNS AND SYMPTOMS
SIGNS AND SYMPTOMS
Heart failure is characterized by a constellation of signs and symptoms of pulmonary and/or systemic venous congestion caused by impaired ability of the heart to fill with or eject blood in proportion to the metabolic needs of the body.1 Manifestations include fatigue, dyspnea, fluid retention, and cachexia, and patients can present anywhere on the spectrum—from asymptomatic at rest to severely symptomatic.
Symptoms
In both reduced LVEF and preserved LVEF HF, common early symptoms include dyspnea and fatigue on exertion, with or without some degree of lower-extremity swelling.2 Lack of treatment and disease progression increase symptom severity—to the extent that dyspnea and fatigue start to occur at rest. Reviewing the anatomic classifications/findings of HF facilitates understanding of the clinical symptoms.
Right HF. Right ventricular dysfunction is rarely found in isolation; when symptoms are present, further evaluation of the left ventricle and the pulmonary system (to look for cor pulmonale) is warranted. Right HF is associated with an inability to manage venous return and move volume into the pulmonary circuit. This produces the predominant symptom of fluid retention. Peripheral edema is a cardinal symptom of right HF; edema can also present systemically, mostly as hepatic congestion or general gastrointestinal complaints resulting from impaired gastrointestinal perfusion.9
Left HF. Left ventricular dysfunction is the more common anatomic finding in HF and is often generalized to represent all cases. The dysfunction can be found in isolation and is actually the leading cause of right HF. In left HF (regardless of etiology), the heart does not produce enough “forward” pressure (ie, cannot pump or fill with enough blood) for the cardiovascular system to remain in balance. Thus, “back” pressure into the pulmonary circuit develops.
The combination of insufficient systemic perfusion capability with a dysfunctional left pump means that the most common symptom in all cases of left HF is shortness of breath—specifically, exertional dyspnea. Dyspnea can progress to orthopnea, paroxysmal nocturnal dyspnea, and, finally, dyspnea at rest. Patients often present with a cough that is worse while lying down and that can be nonproductive or productive, depending on volume status.9,10
Signs
Vital signs range from normal to indicative of shock. Increased sympathetic nervous system activity is common; this may manifest as coldness of the extremities and diaphoresis. Keep in mind that HF is a clinical diagnosis: The physical examination, focusing on peripheral signs, is key in all HF patients for both diagnosis and management.
PHYSICAL EXAMINATION
The physical exam includes the heart, neck, lungs, abdomen, and extremities. The cardiac exam includes evaluation for hypertrophy, by palpating for the point of maximum impulse to assess for lifts, heaves, valvular disease, and S3 and S4 sounds. Respiratory, abdominal, and extremity exams all focus on the evaluation of fluid status and edema.
A key, often underutilized measurement is jugular venous pressure (JVP). Elevated JVP has been identified as the most specific sign of fluid overload in HF and the most important physical finding in the initial and subsequent examinations of a patient with HF.11 (For good reason, clinicians who treat patients with HF must be able to recognize volume overload and hypovolemia; euvolemia allows patients to remain symptom-free and makes it possible to initiate life-prolonging therapy, which will be discussed in the Treatment section.2) JVP is an indirect measure of pressure within the right side of the heart (central venous pressure).
Most texts on performing the physical exam recommend measuring JVP using the right internal jugular vein. However, use of the internal jugular vein is limiting in HF patients, because it is covered by the sternocleidomastoid muscle for most of its course in the neck and visible only in a small triangle between the two heads in the root of the neck (see Figure). Conversely, the external jugular veins are subcutaneous along their entire course and pulsations are easily visible—but superficial and prone to external pressure and internal occlusion. A nonpulsatile, distended jugular vein should not be used to estimate venous pressure.2

What, then, is the best method? Vinayak et al evaluated the comparative effectiveness of the internal and external jugular veins for detection of central venous pressure. They found that the external jugular vein is easier to visualize and has excellent reliability for determining low and high venous pressures.12
The process for estimating JVP is the same regardless of which jugular vein (internal or external) is used. The technique is described by Bickley in Bates’ Guide to Physical Examination and History Taking:
The patient lies supine and at an angle between 30° and 45°. Turn the head slightly, and with tangential lighting, identify the external and internal jugular veins. Identify the highest point of pulsation in the right jugular vein and extend a long rectangular object or card horizontally from this point and a centimeter ruler vertically from the sternal angle, making an exact right angle. Measure the vertical distance (in centimeters) above the sternal angle where the horizontal object crosses the ruler and add this distance to 4 cm. Measurements of > 3-4 cm above the sternal angle or > 8 cm in total distance are considered elevated.13
Continue to: DIAGNOSTIC AND SCREENING TESTS
DIAGNOSTIC AND SCREENING TESTS
Several diagnostic studies can identify HF or elucidate underlying causes. However, not all tests yield sufficient information for diagnosis—and commonly held “maxims” about findings that definitively rule in or out the diagnosis have been confounded by the available evidence.
Laboratory tests. The lab parameter most associated with HF is brain natriuretic peptide (BNP). Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension.14 In contrast to atrial natriuretic peptide (ANP), BNP is secreted not only from the atria but also from the ventricles, especially in patients with HF.15
Circulating concentrations of several cardiac natriuretic peptides—including ANP, BNP, and the two peptides’ N-terminal pro-hormones (N-terminal pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide, respectively) are elevated in both symptomatic and asymptomatic patients with left ventricular dysfunction.16 These levels are generally elevated for both systolic and diastolic HF. However, in one study, as many as 30% of diastolic HF patients had a low BNP level, despite signs and symptoms of HF and significantly elevated left ventricular filling pressures, as determined by invasive hemodynamic monitoring.17 Comorbid obesity is associated with low levels of natriuretic peptides.18
Additional lab tests can provide information about underlying causes of HF or reveal contraindications to certain treatment options (to be discussed in the Treatment section). A complete blood count (CBC) might reveal anemia, which can cause or aggravate HF, and which is an important consideration in management because of its association with decreased renal function, hemodilution, and proinflammatory cytokines. Leukocytosis can signal underlying infection. A troponin assay is helpful for ruling out acute MI as a cause of worsening HF in acute cases. Thyroid function tests and iron studies can be considered to rule out secondary causes of HF.
A serum electrolyte screen should be ordered; results are usually within normal ranges. Hyponatremia is an indicator of activation of the renin–angiotensin–aldosterone system (RAAS) and may be seen in the context of prolonged salt restriction and diuretic therapy. Hyperkalemia and hypokalemia are also prevalent in HF; both are limiting factors for some treatment options. A low sodium level is often the result of increased congestion and release of vasopressin; a level of ≤ 135 mEq/L predicts a poorer outcome.
Kidney function tests can determine whether HF is associated with impaired kidney function, secondary to poor renal perfusion. Poor renal function may limit treatment options. Patients with severe HF, particularly those receiving a high dosage of a diuretic for a long period, may have elevated levels of blood urea nitrogen and creatinine, indicating renal insufficiency.
Electrocardiography and chest radiography. ECGs and chest radiographs are noninvasive tests that have been used widely in the diagnosis of HF. They might indicate an underlying cause (eg, acute MI, ischemia, secondary arrhythmia) but are often nonspecific—and thus may be unhelpful in the diagnosis and treatment of HF.
The most common ECG findings in HF are nonspecific ST-T wave abnormalities.19 Other findings often consist of low-voltage left ventricular hypertrophy, conduction defects, and repolarization changes. With chest radiography, primary findings in HF include pulmonary edema—seen as perivascular edema, peribronchial cuffing, perihilar haze, interstitial edema (Kerley B, or septal, lines), and alveolar fluid—and pleural effusion.19,20
For both these modalities, however, there are commonly held conceptions that particular findings rule out HF—which has been disproven by Fonseca and colleagues.19 Because most patients with HF have an abnormal ECG, some studies have proposed that a normal ECG virtually rules out left ventricular systolic dysfunction.20 Evaluating the value of ECG in HF diagnosis, Fonseca et al found that about 85% of patients with an abnormal ECG had HF—but so did 30% of patients with a normal ECG. They concluded that, if used alone, ECG could have missed as many as 25% of patients with HF.19
Likewise, it has been suggested that a patient cannot have HF if heart size is normal on a chest radiograph.20 Fonseca et al found that cardiac enlargement, while the most informative radiologic measurement in HF, was present in only half of patients with HF.19 About 57% of patients who had an abnormal chest radiograph had HF, but so did about 40% of those who had a normal chest radiograph.19 Therefore, abnormal chest radiograph for identification of HF had an estimated sensitivity of 57%, a specificity of 78%, positive predictive value of 50%, and negative predictive value of 83%.19 The conclusion: Caution should be taken regarding the use of chest radiography in isolation to make a diagnosis of HF.
Echocardiography. The most useful test in evaluating HF is the echocardiogram, because it can distinguish between HF with and without preserved left ventricular systolic function. This is critical: The most clinically relevant classification of HF differentiates systolic and diastolic HF, based on LVEF.2 This determination has both prognostic and therapeutic implications.21
Echocardiography is widely available, safe, and noninvasive. The “echo” can identify the size of the atria and ventricles, valve function and dysfunction, and any associated shunting. Pericardial effusions and heart wall-motion abnormalities (for example, an old MI) are also easily identified.9
A normal ejection fraction does not rule out HF. Therefore, assessment of LVEF should not be considered until after a clinical diagnosis has been made, because more than half of HF patients have a normal LVEF—evidence that can confound the diagnostic process.2
Continue to: TREATMENT OF HEART FAILURE WITH REDUCED LVEF
TREATMENT OF HEART FAILURE WITH REDUCED LVEF
The body’s neurohormonal system, including the RAAS and the sympathetic nervous system, is activated to compensate for the insufficient cardiac performance found in HF. However, activation of these systems contributes to worsening HF, deterioration of quality of life, and poor outcomes.22 Therefore, therapies that suppress these responses can reduce the progression of HF.
Treatment of HF is generally divided into symptom-relieving treatment and disease-modifying/life-prolonging treatment.1 Symptom relief is similar in both systolic and diastolic HF. However, most evidence-based, disease-modifying treatment focuses on systolic HF; guidelines for disease-modifying treatment of diastolic HF are minimal.1
Treatment of reversible causes
Since HF is caused by something else, the primary focus of management is addressing underlying causes. The primary goal is to relieve symptoms while improving functional status—which should lead to a decrease in hospitalization and premature death.
The first step is to evaluate patients’ use of medications that can contribute to a worsening of HF.9 The most common offending medications are calcium-channel blockers with negative inotropy (non-dihydropyridine calcium-channel blockers, eg, verapamil and diltiazem); some antiarrhythmic drugs (eg, amiodarone); thiazolidinediones (glitazones); and NSAIDs.9 If identified as a possible contributor to HF symptoms, these agents should be stopped (if possible) or replaced.
Nonpharmacotherapy
Effective counseling and education of patients with HF may help with long-term adherence to treatment plans. Patients can be taught to monitor their weight at home and to adjust the dosage of diuretics as advised: A sudden increase in weight (> 2 kg in one to three d), for example, should alert a patient to alter treatment or seek advice.23
Diet modification is a multifactorial recommendation. Proper nutrition is critical because HF patients are at increased risk for malnutrition due to poor appetite, malabsorption, and increased nutritional requirements.23 Weight reduction in obese patients helps reduce cardiac workload. Patients should be placed on salt restriction (2 to 2.6 g/d of sodium).9,23
Exercise has been shown to relieve symptoms, provide a greater sense of well-being, and improve functional capacity. It does not, however, result in obvious improvement in cardiac function.22
Alcohol consumption should be restricted because of the myocardial depressant properties of alcohol and its direct toxic effect on the myocardium.22 Smoking should be discouraged because it has a direct effect on coronary artery disease.
Influenza and pneumococcal vaccination should be considered in all patients with HF.23 Heart failure predisposes patients to, and can be exacerbated by, pulmonary infection and exacerbation of chronic obstructive pulmonary disease.
Evaluation and management of obstructive sleep apnea should be performed. Sleep-disordered breathing, an umbrella term that covers obstructive and central sleep apneas, has been found to increase the risk for poor prognosis in HF.24 All patients with HF should be tested for obstructive sleep apnea because, often, only the patient’s bed partner is aware of disordered sleep. For unknown reasons, patients with HF do not report subjective sleepiness.25
Continue to: Pharmacotherapy
Pharmacotherapy
Diuretics have not been found to have benefit for reducing early mortality but are the most common agents used for symptomatic relief of sodium and water retention.26 In fact, few patients with signs and symptoms of fluid retention can be managed without a diuretic.9 Caution must be observed, however, because excessive diuresis can lead to electrolyte imbalance and neurohormonal activation.
Treating mild fluid retention with a thiazide diuretic (hydrochlorothiazide, metolazone, or chlorthalidone) is often sufficient (see Table 2 for dosing and other information on these and other drugs for treating HF).9 Thiazide diuretics are dependent on the glomerular filtration rate and are ineffective when it falls below 30-40 mL/min. Adverse reactions to diuretics include hypokalemia, dehydration (intravascular volume depletion) with prerenal azotemia, skin rash, neutropenia, thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.

In cases of moderate or severe HF, or failure of thiazide diuretics to relieve mild symptoms, an oral loop diuretic (furosemide, bumetanide, or torsemide; see Table 2 for dosing) can be used if kidney function is preserved.9 These agents are best administered in two or more divided doses. Major adverse reactions are similar to those of thiazide diuretics, plus ototoxicity. Special caution must be used when a loop diuretic is co-administered with digitalis because the combination can cause significant hypokalemia.9
A potassium-sparing diuretic (triamterene or amiloride; see Table 2) can be used in combination with thiazide and loop diuretics.9 The location of their action is at the distal tubule, but diuretic potency is mild. Potassium-sparing agents can minimize hypokalemia induced by other diuretics. Adverse effects include hyperkalemia, kidney dysfunction, and gastrointestinal symptoms.
The aldosterone-receptor antagonists spironolactone and eplerenone are specific inhibitors of aldosterone, an effect that has been shown to improve clinical outcomes.9 Aldosterone-receptor antagonists are indicated for patients with NYHA class II-IV HF who have LVEF ≤ 35% or those with a history of acute MI, LVEF < 40%, and symptoms of HF.27 In these patients, a reduction in mortality and relative risk has been demonstrated with the use of aldosterone-receptor antagonists, unrelated to their role in diuresis. The adverse effect profile of spironolactone includes gynecomastia.
Continue to: Inhibitors of the RAAS
Inhibitors of the RAAS. As noted, the RAAS plays a key role during the development and worsening of HF.22
Angiotensin-converting enzyme inhibitors (ACE inhibitors) constitute the cornerstone of modern HF pharmacotherapy and have compelling evidence of survival benefit.28 These agents (captopril, enalapril, lisinopril, and ramipril; see Table 2 for dosing) have been shown to be effective therapy for HF and post-MI left ventricular dysfunction.29 They reduce early mortality by approximately 20% in symptomatic HF and can prevent hospitalization, increase exercise tolerance, and reduce adverse events (symptoms).9
Because of these benefits, ACE inhibitors should be firstline therapy in all HF patients with left ventricular dysfunction. They are usually used in combination with a diuretic. In addition, ACE inhibitors should be used in patients with reduced LVEF, even if they are asymptomatic, because these agents prevent progression to clinical HF.
Since ACE inhibitors carry the risk for severe hypotension, caution must be used, especially during treatment initiation. Patients whose systolic blood pressure is < 100 mm Hg, or who are hypovolemic, should be started at a low dosage (captopril, 6.25 mg tid; enalapril, 2.5 mg/d; or other equivalent ACE inhibitor dose); for other patients, these dosages can be doubled at initiation.9 Within days of initiation, but no longer than two weeks later, patients should be screened for hypotension and have both kidney function and potassium levels monitored. The dosage of ACE inhibitors should be increased over one to three months to an effective dosage (eg, captopril, 25 mg tid; enalapril, 10 mg bid; ramipril, 10 mg/d; and lisinopril, 20 mg/d, or other equivalent ACE inhibitor dose).9
Asymptomatic hypotension is not a contraindication to continuation or uptitration of the dosage of ACE inhibitors. Patients may also experience an increase in the serum creatinine or potassium level; likewise, this should not prompt a change in dosage if the elevated level stabilizes. The most common adverse effects of ACE inhibitors are dizziness and cough.9
Angiotensin II-receptor blockers (ARBs) have been shown to be as effective as ACE inhibitors for the management of hypertension, congestive HF, and chronic renal failure.30 ARBs decrease the adverse effects of angiotensin II, but do not have the same effects that ACE inhibitors do on other pathways found in HF (specifically, on bradykinin, prostaglandins, and nitric oxide).29 Candesartan and valsartan have been shown to have benefits in HF and are an equivalent alternative to ACE inhibitors; they are often used when a patient cannot tolerate an ACE inhibitor. Because ARBs and ACE inhibitors affect the RAAS at different points in the pathway, there is a theoretical basis for ARB and ACE inhibitor combination therapy; in clinical studies to date, however, the combination has shown no beneficial effect and rather was associated with more adverse effects.29
Neprilysin inhibitor. Recently, the combination of the neprilysin inhibitor sacubitril and an ARB, valsartan, has surfaced as an alternative to ACE inhibitor or single-agent ARB therapy.31,32 Inhibition of the enzyme neprilysin results in an increase in levels of endogenous vasoactive peptides (eg, natriuretic peptides and bradykinin), which may benefit hemodynamics in patients with HF. However, use of an ARB in combination with sacubitril is necessary to counteract the increase in angiotensin II levels that also results from inhibition of neprilysin.33
Sacubitril–valsartan is typically reserved for patients with mild-to-moderate HF who have either an elevated BNP (≥ 50 pg/mL) or an HF-related hospitalization within the past year. Upon transitioning from an ACE inhibitor (or single-agent ARB), a 36-hour washout period must be observed before starting sacubitril–valsartan to minimize the risk for angioedema. Because therapy with sacubitril–valsartan leads directly to an elevation in the BNP level, using the level of N-terminal pro-brain natriuretic peptide—which is not degraded by neprilysin—to monitor disease progression is recommended.34
ß-
Initiation of ß-blockers in stable patients can cause general deterioration; initiation must therefore be done gradually:
- Carvedilol, initiated at 3.125 mg bid, can be increased to 6.25 mg, then 12.5 mg, and then 25 mg, all bid, at intervals of approximately two weeks
- Sustained-release metoprolol can be started at 12.5 mg/d or 25 mg/d and doubled at two weeks to a target of 200 mg/d
- Bisoprolol, initiated at 1.25 mg/d, can be increased incrementally to 2.5 mg/d, 3.75 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d at one-to-four-week intervals.9
Patients taking ß-blockers need to monitor their weight at home as an indicator of fluid retention. If HF becomes worse, an increase in the dosage of the accompanying diuretic, a delay in the increase of the ß-blocker, or downward adjustment of the ß-blocker is usually sufficient.
Continue to: Digitalis glycoside
Digitalis glycoside. Digoxin has been shown to relieve the symptoms of HF, decrease the risk for hospitalization, and increase exercise tolerance; however, it has not been shown to offer a mortality benefit.36 Digoxin should be considered for patients who remain symptomatic when taking a diuretic and an ACE inhibitor and for patients who are also in atrial fibrillation and need rate control.
Digoxin is cleared almost entirely by the kidneys and therefore must be used with care in patients with renal dysfunction. Patients usually can be started on the expected maintenance dosage (0.125-0.25 mg/d). An oral loading dose of 0.75-1.25 mg over 24 to 48 hours can be used if an early effect is needed.9
Digoxin can induce ventricular arrhythmias (especially in a setting of hypokalemia and ischemia) and is sensitive to other medications that can drastically increase its level—most notably, amiodarone, quinidine, propafenone, and verapamil.9 The digoxin blood level should be measured every seven to 14 days until a maintenance dosage is established, and again whenever there is a change in medication or kidney function. The optimum serum level is 0.7-1.2 ng/mL; toxicity is not usually seen at < 1.8 ng/mL.9
Hydralazine and nitrates. The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in African-American patients with HF, but efficacy is less well-established in this population than for ACE inhibitor and ARB therapy.9 This combination can be considered in patients who are unable to tolerate ACE inhibitor or ARB therapy. It can also be considered in those who have persistent symptoms despite treatment with a ß-blocker, ACE inhibitor, or aldosterone antagonist.9
Intravenous nitrates are used primarily for acute HF, especially when accompanied by hypertension or myocardial ischemia. The starting dosage for nitroglycerin is approximately 10 μcg/min, titrated upward by 10-20 μcg/min to a maximum of 200 μcg/min. Isosorbide dinitrate (20-40 mg tid) and nitroglycerine ointment 2% (1.4 in applied every six to eight hours [although generally reserved for inpatient therapy]) are equally effective.9 Adverse effects that may limit the use of these agents are headache and hypotension. Patients also develop tolerance for nitrates, which can be mitigated if a daily 8-to-12–hour nitrate-free period is instituted.9
TREATMENT OF HEART FAILURE WITH PRESERVED LVEF
Treatment options for patients with preserved LVEF (diastolic HF) are not as clear as those for patients with reduced LVEF (systolic HF). No traditional therapies (ACE inhibitors, ARBs, ß-blockers, digoxin) have been shown to improve survival in this population, although a recent study on the effects of spironolactone in diastolic HF did show some improvement of diastolic dysfunction without adverse effects.17,37 In the absense of clear evidence-based therapies, treatment focuses on managing comorbidities, addressing reversible causes, and alleviating fluid overload with a diuretic.9,17
Diuretic therapy is critical to control fluid overload; regimens are similar to those for HF with reduced LVEF. ACE inhibitors and ARBs have not been shown to improve outcomes in this population but can be used to manage comorbid hypertension. Spironolactone has also not been shown to improve outcomes in patients with diastolic HF.9
The principal conditions that can lead to HF with preserved LVEF are hypertension, pericardial disease, and atrial tachycardia. Tachycardia is associated with overall shorter diastolic filling time; controlling an accelerated heart rate, therefore, is theorized to be an important therapeutic goal.9 Other disease states, including diabetes mellitus, sleep-disordered breathing, obesity, and chronic kidney disease, can all lead to HF with preserved LVEF.
CONCLUSION
Managing HF today requires an “upstream” model of care, by which providers consider the diagnosis/syndrome of HF in the asymptomatic patient with risk factors (Stage A/Class I and Stage B/Class II). Perhaps a better way to state this is that providers must change their approach from ruling-in to ruling-out HF. Any person in whom a decrease in activity level, mild shortness of breath, or edema are observed, and who has known risk factors, should be considered to have HF until proven otherwise.
Because of the wide variability in the underlying causes of HF, providers must be dynamic in both their clinical approach and their treatment plans to optimize care for the individual patient. Finally, providers must also take note of the increasing prevalence of diastolic HF and, again, focus on early diagnosis.
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28.
2. Ahmed A. DEFEAT - Heart failure: a guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100(1):39-50.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001; 38(7):2101-2113.
4. Mozaffarian D, Benjamin EJ, Go AS, et al; Writing Group Members, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;26: 133(4):e38-e360.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
6. American Heart Association. Classes of heart failure (2017). www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.V8cFhfkrLRY. Accessed March 7, 2018.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994.
8. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
9. Bashore T, Granger C, Hranitzy P, Patel M. Heart diseases. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment. 55th ed. New York, NY: McGraw-Hill Education; 2016:402.
10. Colucci S. Patient education: heart failure (beyond the basics). Waltham, MA: UpToDate; 2017. www.uptodate.com/contents/heart-failure-beyond-the-basics?view=print. Accessed March 7, 2018.
11. Butman S, Ewy GA, Standen J, et al. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol. 1993;22(4):968-974.
12. Vinayak A, Levitt J, Gehlbach B, et al. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166(19):2132-2137.
13. Bickley LA. Bates’ Guide to Physical Examination and History Taking. 11th ed. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2012:364-365.
14. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961-1970.
15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-204.
16. Cowie M, Struthers A, Wood D, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350(9088):1349-1353.
17. Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
18. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47(1):85-90.
19. Fonseca C, Mota T, Morais H; EPICA Investigators. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2006;6(6):807-812.
20. Rihal CS, Davis KB, Kennedy JW, Gersch BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223.
21. Joint Commission on Accreditation of Healthcare Organizations. A Comprehensive Review of Development and Testing for National Implementation of Hospital Core Measures. 2002. www.jointcommission.org/assets/1/18/A_Comprehensive_Review_of_Development_for_Core_Measures.pdf. Accessed March 6, 2018.
22. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371.
23. Gibbs CR, Jackson G, Lip GY. ABC of heart failure: Nondrug management. BMJ. 2000;320(7231):365-369.
24. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631.
25. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716-1722.
26. Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193-198.
27. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
28. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
29. Bissessor N, White H. Valsartan in the treatment of heart failure or left ventricular dysfunction after myocardial infarction. Vasc Health Risk Manag. 2007;3(4):425-430.
30. McMurray JJ, Ostegren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
31. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073.
32. McMurray J, Packer M, Desai A, et al; PARADIGM-HF Committees and Investigators. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
33. Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657-1658.
34. Packer M, McMurray JJ, Desai AS, et al; PARADIGM-HF Committees and Investigators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61.
35. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199.
36. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
37. Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28.
2. Ahmed A. DEFEAT - Heart failure: a guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100(1):39-50.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001; 38(7):2101-2113.
4. Mozaffarian D, Benjamin EJ, Go AS, et al; Writing Group Members, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;26: 133(4):e38-e360.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
6. American Heart Association. Classes of heart failure (2017). www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.V8cFhfkrLRY. Accessed March 7, 2018.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994.
8. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
9. Bashore T, Granger C, Hranitzy P, Patel M. Heart diseases. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment. 55th ed. New York, NY: McGraw-Hill Education; 2016:402.
10. Colucci S. Patient education: heart failure (beyond the basics). Waltham, MA: UpToDate; 2017. www.uptodate.com/contents/heart-failure-beyond-the-basics?view=print. Accessed March 7, 2018.
11. Butman S, Ewy GA, Standen J, et al. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol. 1993;22(4):968-974.
12. Vinayak A, Levitt J, Gehlbach B, et al. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166(19):2132-2137.
13. Bickley LA. Bates’ Guide to Physical Examination and History Taking. 11th ed. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2012:364-365.
14. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961-1970.
15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-204.
16. Cowie M, Struthers A, Wood D, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350(9088):1349-1353.
17. Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
18. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47(1):85-90.
19. Fonseca C, Mota T, Morais H; EPICA Investigators. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2006;6(6):807-812.
20. Rihal CS, Davis KB, Kennedy JW, Gersch BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223.
21. Joint Commission on Accreditation of Healthcare Organizations. A Comprehensive Review of Development and Testing for National Implementation of Hospital Core Measures. 2002. www.jointcommission.org/assets/1/18/A_Comprehensive_Review_of_Development_for_Core_Measures.pdf. Accessed March 6, 2018.
22. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371.
23. Gibbs CR, Jackson G, Lip GY. ABC of heart failure: Nondrug management. BMJ. 2000;320(7231):365-369.
24. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631.
25. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716-1722.
26. Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193-198.
27. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
28. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
29. Bissessor N, White H. Valsartan in the treatment of heart failure or left ventricular dysfunction after myocardial infarction. Vasc Health Risk Manag. 2007;3(4):425-430.
30. McMurray JJ, Ostegren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
31. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073.
32. McMurray J, Packer M, Desai A, et al; PARADIGM-HF Committees and Investigators. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
33. Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657-1658.
34. Packer M, McMurray JJ, Desai AS, et al; PARADIGM-HF Committees and Investigators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61.
35. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199.
36. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
37. Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Pain Management in an Opioid Epidemic What’s Appropriate, What’s Safe
CE/CME No: CR-1804
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the basic pharmacology of opioid medications and how they affect pain.
• Apply a stepwise approach to pain management, based on the World Health Organization's "pain ladder."
• Communicate to patients the key educational points on the risks of opioid use.
• Identify strategies to deter or detect opioid misuse or abubse.
FACULTY
Deborah Salani is an Associate Professor of Clinical and Director of the Accelerated BSN Program, Nichole A. Crenshaw is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Acute Care Nurse Practitioner Program, Brenda Owusu is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Primary Care Nurse Practitioner Program, and Juan M. Gonzalez is an Assistant Professor of Clinical and Program Director for the Family Nurse Practitioner Program, at the University of Miami School of Nursing and Health Studies in Coral Gables, Florida.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through March 31, 2019.
Article begins on next page >>
Abuse of prescribed controlled substances—particularly opioid analgesics—and associated morbidity and mortality are a serious public health problem. The response to this crisis must include prevention, early identification, and appropriate treatment of addiction. Prescribing NPs and PAs must understand how to manage acute and chronic pain while also being attentive to signs of drug seeking and opioid misuse and abuse. The information and tools outlined in this article can equip providers to combat the opioid epidemic.
Controlled prescription drug abuse and its associated morbidity and mortality are a serious public health problem globally. In 2015, more than 29 million people worldwide misused and abused drugs, according to the United Nations Office on Drugs and Crime.1 Opioid use disorders account for approximately 70% of that estimate.
In the US, the mortality associated with this abuse has been devastating. Between 1999 and 2014, drug overdose deaths nearly tripled; in 2014 alone, there were 47,055 such fatalities, 61% of which involved opioids.2,3 Since 2000, unintentional overdose deaths from opioids have increased by 200%.3 Overdose deaths associated with natural and semisynthetic opioids (the most commonly prescribed pain relievers) increased 9% from 2013 to 2014, while those associated with synthetic opioids (fentanyl and tramadol) nearly doubled in the same period.3
Further contributing to the problem, a person addicted to prescription opioid drugs is 40 times more likely to be addicted to heroin, compared to someone who is not addicted to opioids.4 Deaths related to heroin overdose continue to dramatically increase.3
A call to action
In August 2016, former US Surgeon General Vivek Murthy, MD, sent a personal letter to more than 2.3 million health care providers, seeking their assistance in addressing the prescription opioid crisis.5 Murthy acknowledged the challenges providers face when attempting to strike a balance between treating a patient’s pain and reducing the risk for opioid addiction. He explained that clinicians are uniquely situated to end this crisis, and he asked providers to pledge to “turn the tide” by taking three actions
- Become more educated about treating pain safely and effectively.
- Screen patients for opioid use disorder and make the appropriate evidence-based treatment referrals.
- Discuss and treat addiction as a chronic disorder.5
To help stem the epidemic of controlled prescription drug abuse, NPs and PAs must be knowledgeable about patient safety issues, including how to identify patients at risk for opioid misuse and recognize signs of misuse or abuse. This article aims to educate providers who have prescriptive authority about the pharmacology of opioids; safe and effective prescribing of these drugs; and how to identify and manage misuse and abuse.
Continue to: OVERVIEW OF PAIN
OVERVIEW OF PAIN
Pain, considered the fifth vital sign, is one of the more common reasons that people seek treatment from a health care provider. Pain is a personal, individual, subjective experience: It is whatever the patient says it is and exists whenever the patient says it does. Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
Pain is classified as acute or chronic. Acute pain is a sudden but temporary, self-limiting response to some type of bodily injury; it generally lasts less than six months. Chronic pain is often associated with prolonged diseases such as cancer, fibromyalgia, and osteoarthritis; it persists for six months or longer.
Pain can be separated into two categories: nociceptive and neuropathic. Nociceptive pain originates from peripheral or visceral nociceptors as a result of injury and comprises somatic and visceral pain. Somatic pain is caused by injury to soft tissue, connective tissue, and bone; the classic description is a sharp, well-localized discomfort. Visceral pain originates from an organ or deeper structure; it is commonly described as dull, poorly localized, and sensitive to stretch, ischemia, and inflammation.
Neuropathic pain is an abnormal processing of pain stimuli by the peripheral nervous system or central nervous system (CNS) and can result from injury or inflammation to a nerve. Neuropathic pain is usually described by patients as electric, burning, and/or shooting. Examples include pain associated with cancer, diabetic neuropathy, and phantom-limb sensation (following amputation).
The physiologic experience of pain follows a defined set of phases. First is transduction, which occurs at the moment of injury or trauma; sensory nerve endings convert the noxious stimulus into a nerve impulse. Second is transmission of the pain impulse to the spinal column by means of chemical messengers known as neurotransmitters.
After the pain impulse reaches the spinal tract, it continues to the brain, at which point there is perception, the third step in the process. This leads to modulation (also known as anti-nociception). In this fourth step, neurons that originate in the brainstem are activated, releasing neurotransmitters that inhibit transmission of pain. Modulation occurs in several areas of the CNS and involves the neurotransmitters serotonin, norepinephrine, and endogenous opioids (eg, ß-endorphin).6 During modulation, the limbic nervous system provokes a response to the painful stimulus, triggering endogenous opioids to bind to opioid receptors.7
Continue to: ROLE OF OPIOIDS IN PAIN MANAGEMENT
ROLE OF OPIOIDS IN PAIN MANAGEMENT
Opioids have been used to control pain for centuries. They are extracted from the opium poppy plant, Papaver somniferum. From the substance extracted, roughly 9% to 14% is morphine and 0.8% to 2.5% is codeine.7 Opioids are used to treat many symptoms and ailments, including diarrhea, moderate to severe pain, and persistent cough.
Opioids work through receptors in the CNS, including mu, kappa, and delta opioid receptors and the opioid-like receptor nociceptin.7 The principal receptors associated with pain physiology and inhibition are mu and kappa. (Morphine, the gold standard for treating severe pain, is an opioid agonist that binds to mu and kappa receptors.)
Most opioids that are used clinically bind to mu receptors; these drugs provide analgesia but also present the risk for adverse effects, such as decreased respiratory drive, miosis, and decreased motor function of the gastrointestinal (GI) tract, which can lead to constipation.8 Because mu receptors are located mainly in the brain and spinal cord (as well as the GI tract), opioids also produce a feeling of euphoria that can lead to dependence.
Kappa receptors, in contrast, are located mainly in the limbic system, diencephalic area, and spinal cord. When these receptors are activated, they can produce spinal analgesia, dyspnea, dependence, and dysphoria.
Delta receptors also play a role in pain management and are associated with emotional and affective components of the experience of pain.7 They are largely located in the brain; when activated, they can lead to spinal and supraspinal anesthesia, as well as decreased gastric motility.8 Delta receptors have not been studied as much as mu and kappa receptors, but it has been suggested that they play a role in psychologic dependency.
Depending on the effect that a drug has on these receptors, it can be considered a full (or pure) opioid agonist, a partial agonist, or a mixed agonist–antagonist. By binding to opioid receptors, opioid agonists provide pain relief. Health care providers often prescribe a full agonist, such as morphine, hydrocodone, codeine, or oxycodone, to treat pain. Partial agonists, such as buprenorphine and butorphanol, often decrease activity at mu receptor sites. Mixed agonist–antagonists either block or bind opioids at receptor sites.9 Medications that block mu and kappa receptors are considered opioid antagonists, which are used not to treat pain but rather to reverse the effect of opioids (eg, naloxone).6
Table 1 lists commonly used opioids, their analgesic duration, and the standard approved dosages.10
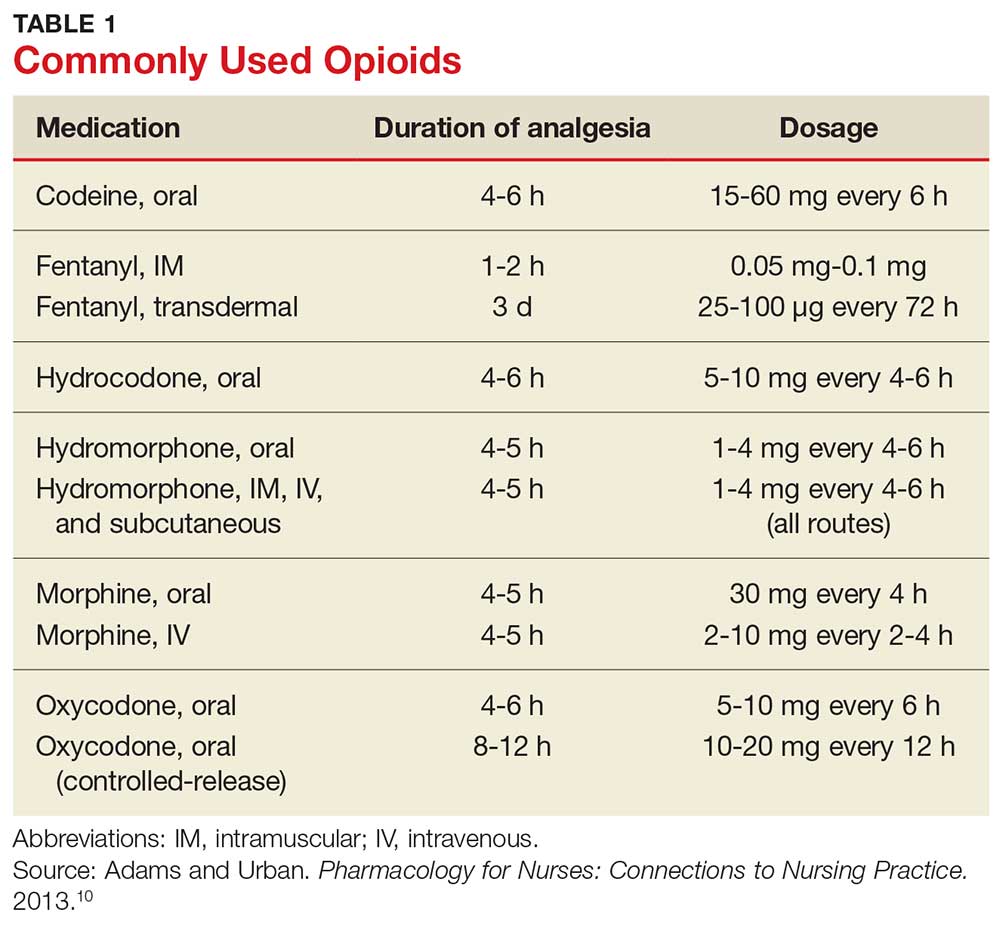
Continue to: A STEPWISE APPROACH TO PAIN MANAGEMENT
A STEPWISE APPROACH TO PAIN MANAGEMENT
On January 1, 2018, The Joint Commission (JNC) implemented new and revised standards to ensure that all patients receive appropriate assessment and management of their pain. While these standards apply to accredited hospitals, they provide a solid framework for assessing and treating pain in any patient. JNC now requires that patients be included in the development of treatment plans, which should encompass realistic expectations and reasonable goals, and that providers promote safe opioid use by identifying and monitoring high-risk patients.11
One valuable tool that can help clinicians fulfill the obligation to provide safe and effective pain management is the World Health Organization’s “pain ladder” (see Figure 1).12 Originally released in 1986 to address cancer pain in the pediatric population, this tool has proven validity. It has since been expanded to guide treatment of pain in other patient populations. In addition, the steps of the “pain ladder” provide useful information on the clinical examination and documentation of pain, principles of pharmacotherapeutic management, and considerations when using different analgesics.
Pain is assessed on a scale of 1 to 10, with 1 representing the least pain. Medication recommendations are as follows
- For mild pain (ie, a score of 1-3): acetaminophen, NSAIDs, or other nonopioids.
- For moderate pain (pain score, 4-6): an opioid (eg, hydrocodone), with or without an adjunct medication.
- For severe pain (pain score, 7-10) or pain that has not responded to previous therapies: a stronger opioid (eg, morphine, hydromorphone, fentanyl), with or without an adjuvant drug.12
In all cases, patients should be informed about both pharmacotherapeutic and nonpharmacotherapeutic options. The latter include hypnosis, relaxation techniques, acupuncture, physical therapy, application of heat and cold, and electro-analgesia.
Pharmacologic options at any “step” of the ladder carry the risk for adverse effects. Thus, NPs and PAs who prescribe these medications need to apprise patients of the potential harms associated with their treatment.
Acetaminophen. Patients should be instructed on the safe use of acetaminophen, particularly with regard to dosing, since liver damage can occur. Patients should not take more than 4,000 mg in a 24-hour period, and each dose should not exceed 1,000 mg.
NSAIDs. These drugs are often used for short-term management of mild and moderate pain. Patients should be instructed to take these agents with food to decrease GI upset. Other common adverse effects include GI bleed or perforation and renal insufficiency or failure.
Opioids. Depending on which class of receptors an opioid medication targets, patients may develop any of the following: constipation, decreased GI motility, nausea, hypotension, urinary retention, euphoria, pruritus, miosis, dependence, respiratory depression, and sedation. It is important for NPs and PAs who prescribe these medications to remain vigilant for adverse effects and complications from opioid use and to educate the patient and his/her family about possible complications.6
Patients must be instructed not to drink alcohol or take other CNS depressants while taking an opioid. They should be advised about the dangers of operating heavy equipment or engaging in other activities that require mental and physical alertness, since opioids can cause drowsiness. Among the GI effects of some opioids (nausea, vomiting) is constipation—so patients should also be educated on the need to increase fluid intake and include high-fiber foods in their diet.9
But most important of all, patients taking an opioid should be informed that there is the potential for physical dependency and abuse with these agents, and these agents should be used only for acute, severe pain.
Continue to: DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
Every patient has a right to adequate and safe pain control—but NPs and PAs must be aware of the potential for some patients to misuse opioids by taking them in a different way than intended, in a different quantity than prescribed, or without a prescription.13 Having prescriptive authority confers an obligation for NPs and PAs to recognize the prevalence of drug misuse and its impact on patients, families, and society.
Regrettably, there is lack of clarity in the literature about specific characteristics and demographic data that can help determine who is at risk for opioid misuse.14 For example, risk factors that have been associated with drug misuse include a personal or family history of substance abuse; younger age; and an ongoing psychiatric condition.
In contrast, Kennedy and colleagues determined that patients seeking prescription opioids for misuse or abuse tend to be older; be of Caucasian background; have a history of overdose; be receiving methadone maintenance therapy; and have been incarcerated.15 In addition, several characteristics—having moderate or extreme pain, disability, or a history of being refused pain medication—were also associated with a history of seeking prescription opioids to abuse.15
This diverse set of variables underscores the importance of obtaining and documenting a complete history from patients who are experiencing (and seeking relief of) pain; performing a thorough physical exam; and asking specific questions about the patient’s level of pain and the potential for misuse of pain medication.
Gathering this information may help identify patients at risk for opioid misuse or abuse. Furthermore, it ensures that a patient’s chronic pain is not being undertreated and that he/she is not being undeservedly labeled or judged as a drug seeker or abuser.
Continue to: Tools and strategies for appropriate use of opioids
Tools and strategies for appropriate use of opioids
There are tools and strategies available to ensure proper use of opioids for managing chronic noncancer pain. Urine drug testing, screening tools for opioid abuse, prescription drug monitoring programs, and opioid treatment agreements should be considered for patients who require prescription opioids to treat pain.15
Urine drug testing. The CDC recommends that prescribing clinicians perform urine drug testing before initiating opioid therapy and at least annually afterward. It can be used to assess for prescription medications generally, controlled prescription drugs specifically, and substances of abuse.16 Urine drug testing can mitigate the risk for misuse or overdose of opioids, as well as identify patients who were prescribed an opioid but are not taking it. The prescribing provider is responsible for explaining to the patient why urine testing is being done, performing confirmatory testing, and discussing results with the patient.
Risk-assessment tools. A number of web-based tools help the prescribing provider assess a patient’s risk for misuse or abuse of opioids and other substances. They fall into three general categories of use: assessing patients being considered for long-term opioid therapy; assessing for misuse once opioid treatment is initiated; and addressing the potential for substance abuse generally.17-24 Table 2 lists examples. Although screening tools are not 100% accurate at identifying who is a substance abuser, they do alert the provider that a potential problem exists and needs to be explored. As such, they should be considered one component of comprehensive risk assessment, monitoring, and mitigation.25
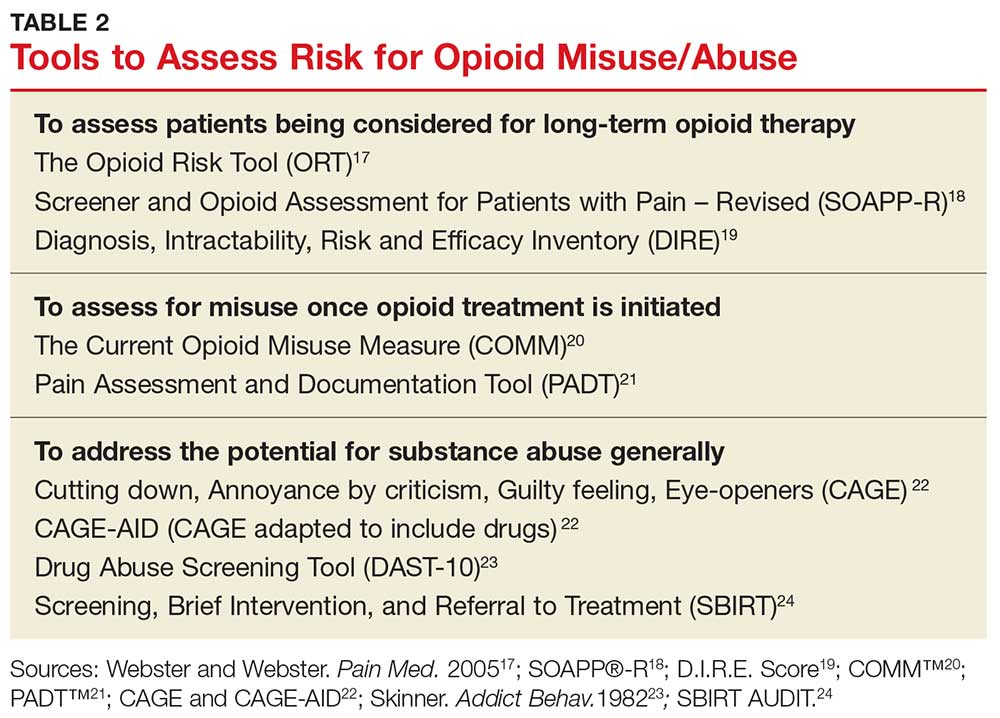
Prescription drug monitoring programs (PDMPs). NPs and PAs must also be aware of “doctor shopping,” in which a person seeks prescriptions from multiple providers (often under false pretenses) and has them filled at multiple pharmacies. PDMPs are designed to monitor for suspected abuse, diversion, or inappropriate prescribing. These state-run electronic databases track the amount of controlled substances prescribed, dispensed, and refilled for a given patient.26 This information can assist providers in identifying high-risk patients who may benefit from an early intervention program.27 Once a patient is identified as having an opioid use disorder, NPs and PAs must provide appropriate referral to an evidence-based practice for treatment of abuse. It is essential to recognize that an opioid use disorder is a chronic illness and that relapses occur.
Opioid treatment agreements. These have been presented as a strategy to prevent prescription drug abuse; however, there is little evidence to support their effectiveness in preventing medication misuse, abuse, or diversion of opioids. In fact, research has shown that such agreements can put the patient–provider therapeutic relationship at risk for disruption, since patients may feel mistrusted or stigmatized by the suggestion that they might behave inappropriately.28 The position of the American Pain Society and the American Academy of Pain Management is that patients and clinicians should have ongoing discussions about chronic opioid therapy that include goals, expectations, risks, and alternatives to opioids.29 If a written agreement is used, it needs to address the patient’s and the clinician’s responsibilities and expectations in managing chronic pain.28
Continue to: Additional resources for providers
Additional resources for providers
Many other resources are available for prescribers of controlled substances. For example, the CDC has published guidelines for prescribing opioids to patients with chronic pain, with a goal of increasing patient–provider communication.16 Additional goals include improving the safety of opioid use, maintaining the effectiveness of treatment, and reducing the necessity and practice of long-term therapy.
The FDA has also published a blueprint on how opioid analgesics can be formulated to deter abuse and, thus, be safer.30 Although directed at the pharmaceutical industry—the FDA encourages manufacturers to develop abuse-deterrent mechanisms, such as physical and chemical barriers, aversion technology, and new delivery systems—the guidance may enlighten providers on how abusers can alter or manipulate oral opioids to achieve the desired effects.30

CONCLUSION
Because NPs and PAs are authorized to prescribe Schedule II-V drugs in their scope of practice, they must have knowledge of drug-seeking behaviors and drug misuse before they prescribe opioids for pain relief. They must be attentive to patients’ pain-control needs and consider how to avoid or reduce the potential for misuse and abuse. Understanding the experience of pain and how opioids modulate it, as well as using available risk-assessment strategies, will help providers offer safe, effective treatment to their patients.
1. United Nations Office on Drugs and Crime. World drug report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed March 21, 2018.
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051): 1445-1452.
3. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(5051):1378-1382.
4. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725.
5. US Department of Health and Human Services. United States Surgeon General. Letter from the Surgeon General. 2016. https://turnthetiderx.org/#. Accessed March 21, 2018.
6. Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA. Pharmacotherapeutics for Advanced Practice. 4th ed. Philadelphia, PA: Wolters Kluwer; 2017:1-23.
7. Adams MP, Holland N, Urban CQ. Pharmacology for Nurses: A Pathophysiologic Approach. 5th ed. Upper Saddle River, NJ: Pearson Education; 2016:239-252.
8. Grossman S, Porth CM. Somatosensory function, pain, and headache. In: Porth’s Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014:441.
9. Woo TM, Robinson MV. Pain management: Acute and chronic pain. In: Pharmacotherapeutics for Advanced Practice Nurse Prescribers. 4th ed. Philadelphia, PA: FA Davis; 2015:1361.
10. Adams MP, Urban CQ. Pharmacology for Nurses: Connections to Nursing Practice. 2nd ed. Upper Saddle River, NJ: Pearson Education; 2013:437.
11. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):1-4. www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Accessed March 21, 2018.
12. World Health Organization. WHO’s cancer pain ladder for adults. 2017. www.who.int/cancer/palliative/painladder/en/. Accessed March 21, 2018.
13. National Institutes of Health. National Institute on Drug Abuse. Opioids: brief description. www.drugabuse.gov/drugs-abuse/opioids. Accessed March 21, 2018.
14. Hudspeth RS. Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. J Nurse Pract. 2016;12(3):141-148.
15. Kennedy MC, Kerr T, DeBeck K, et al. Seeking prescription opioids from physicians for nonmedical use among people who inject drugs in a Canadian setting. Am J Addict. 2016;25(4):275-282.
16. US Department of Health and Human Services. CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed March 21, 2018.
17. Webster LR, Webster R. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432. [Tool available at www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf.] Accessed March 21, 2018.
18. Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP®-R). http://nationalpaincentre.mcmaster.ca/documents/soapp_r_sample_watermark.pdf. Accessed March 21, 2018.
19. D.I.R.E. Score: Patient Selection for Chronic Opioid Analgesia. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/D.I.R.E.%20Score.pdf. Accessed March 21, 2018.
20. Current Opioid Misuse Measure (COMM)™. www.opioidprescribing.com/documents/09-comm-inflexxion.pdf. Accessed March 21, 2018.
21. Pain Assessment and Documentation Tool (PADT™). www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/Pain%20Assess ment%20Documentation%20Tool%20%28PADT%29.pdf. Accessed March 21, 2018.
22. The CAGE and CAGE-AID Questionnaires. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/CAGE-AID.pdf. Accessed March 21, 2018.
23. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. [DAST-10 available at https://cde.drugabuse.gov/sites/nida_cde/files/DrugAbuseScreeningTest_2014Mar24.pdf.] Accessed March 21, 2018.
24. SBIRT AUDIT forms (English and Spanish). www.communitycarenc.org/media/tool-resource-files/sbirt-audit-forms.pdf. Accessed March 21, 2018.
25. Cheattle MD. Risk assessment: safe opioid prescribing tools. 2017. https://www.practicalpainmanagement.com/resource-centers/opioid-prescribing-monitoring/risk-assessment-safe-opioid-prescribing-tools. Accessed March 21, 2018.
26. Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65-77.
27. US Department of Health and Human Services. CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed March 21, 2018.
28. McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 2015;16(1):25-29.
29. Chou R, Fanciullo GJ, Fine PG, et al; for the American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
30. US Department of Health and Human Services. FDA Center for Drug Evaluation and Research. Abuse-deterrent opioids—evaluation and labeling guidance for industry. 2015. www.fda.gov/downloads/Drugs/Guid ances/UCM334743.pdf. Accessed March 21, 2018.
CE/CME No: CR-1804
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the basic pharmacology of opioid medications and how they affect pain.
• Apply a stepwise approach to pain management, based on the World Health Organization's "pain ladder."
• Communicate to patients the key educational points on the risks of opioid use.
• Identify strategies to deter or detect opioid misuse or abubse.
FACULTY
Deborah Salani is an Associate Professor of Clinical and Director of the Accelerated BSN Program, Nichole A. Crenshaw is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Acute Care Nurse Practitioner Program, Brenda Owusu is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Primary Care Nurse Practitioner Program, and Juan M. Gonzalez is an Assistant Professor of Clinical and Program Director for the Family Nurse Practitioner Program, at the University of Miami School of Nursing and Health Studies in Coral Gables, Florida.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through March 31, 2019.
Article begins on next page >>
Abuse of prescribed controlled substances—particularly opioid analgesics—and associated morbidity and mortality are a serious public health problem. The response to this crisis must include prevention, early identification, and appropriate treatment of addiction. Prescribing NPs and PAs must understand how to manage acute and chronic pain while also being attentive to signs of drug seeking and opioid misuse and abuse. The information and tools outlined in this article can equip providers to combat the opioid epidemic.
Controlled prescription drug abuse and its associated morbidity and mortality are a serious public health problem globally. In 2015, more than 29 million people worldwide misused and abused drugs, according to the United Nations Office on Drugs and Crime.1 Opioid use disorders account for approximately 70% of that estimate.
In the US, the mortality associated with this abuse has been devastating. Between 1999 and 2014, drug overdose deaths nearly tripled; in 2014 alone, there were 47,055 such fatalities, 61% of which involved opioids.2,3 Since 2000, unintentional overdose deaths from opioids have increased by 200%.3 Overdose deaths associated with natural and semisynthetic opioids (the most commonly prescribed pain relievers) increased 9% from 2013 to 2014, while those associated with synthetic opioids (fentanyl and tramadol) nearly doubled in the same period.3
Further contributing to the problem, a person addicted to prescription opioid drugs is 40 times more likely to be addicted to heroin, compared to someone who is not addicted to opioids.4 Deaths related to heroin overdose continue to dramatically increase.3
A call to action
In August 2016, former US Surgeon General Vivek Murthy, MD, sent a personal letter to more than 2.3 million health care providers, seeking their assistance in addressing the prescription opioid crisis.5 Murthy acknowledged the challenges providers face when attempting to strike a balance between treating a patient’s pain and reducing the risk for opioid addiction. He explained that clinicians are uniquely situated to end this crisis, and he asked providers to pledge to “turn the tide” by taking three actions
- Become more educated about treating pain safely and effectively.
- Screen patients for opioid use disorder and make the appropriate evidence-based treatment referrals.
- Discuss and treat addiction as a chronic disorder.5
To help stem the epidemic of controlled prescription drug abuse, NPs and PAs must be knowledgeable about patient safety issues, including how to identify patients at risk for opioid misuse and recognize signs of misuse or abuse. This article aims to educate providers who have prescriptive authority about the pharmacology of opioids; safe and effective prescribing of these drugs; and how to identify and manage misuse and abuse.
Continue to: OVERVIEW OF PAIN
OVERVIEW OF PAIN
Pain, considered the fifth vital sign, is one of the more common reasons that people seek treatment from a health care provider. Pain is a personal, individual, subjective experience: It is whatever the patient says it is and exists whenever the patient says it does. Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
Pain is classified as acute or chronic. Acute pain is a sudden but temporary, self-limiting response to some type of bodily injury; it generally lasts less than six months. Chronic pain is often associated with prolonged diseases such as cancer, fibromyalgia, and osteoarthritis; it persists for six months or longer.
Pain can be separated into two categories: nociceptive and neuropathic. Nociceptive pain originates from peripheral or visceral nociceptors as a result of injury and comprises somatic and visceral pain. Somatic pain is caused by injury to soft tissue, connective tissue, and bone; the classic description is a sharp, well-localized discomfort. Visceral pain originates from an organ or deeper structure; it is commonly described as dull, poorly localized, and sensitive to stretch, ischemia, and inflammation.
Neuropathic pain is an abnormal processing of pain stimuli by the peripheral nervous system or central nervous system (CNS) and can result from injury or inflammation to a nerve. Neuropathic pain is usually described by patients as electric, burning, and/or shooting. Examples include pain associated with cancer, diabetic neuropathy, and phantom-limb sensation (following amputation).
The physiologic experience of pain follows a defined set of phases. First is transduction, which occurs at the moment of injury or trauma; sensory nerve endings convert the noxious stimulus into a nerve impulse. Second is transmission of the pain impulse to the spinal column by means of chemical messengers known as neurotransmitters.
After the pain impulse reaches the spinal tract, it continues to the brain, at which point there is perception, the third step in the process. This leads to modulation (also known as anti-nociception). In this fourth step, neurons that originate in the brainstem are activated, releasing neurotransmitters that inhibit transmission of pain. Modulation occurs in several areas of the CNS and involves the neurotransmitters serotonin, norepinephrine, and endogenous opioids (eg, ß-endorphin).6 During modulation, the limbic nervous system provokes a response to the painful stimulus, triggering endogenous opioids to bind to opioid receptors.7
Continue to: ROLE OF OPIOIDS IN PAIN MANAGEMENT
ROLE OF OPIOIDS IN PAIN MANAGEMENT
Opioids have been used to control pain for centuries. They are extracted from the opium poppy plant, Papaver somniferum. From the substance extracted, roughly 9% to 14% is morphine and 0.8% to 2.5% is codeine.7 Opioids are used to treat many symptoms and ailments, including diarrhea, moderate to severe pain, and persistent cough.
Opioids work through receptors in the CNS, including mu, kappa, and delta opioid receptors and the opioid-like receptor nociceptin.7 The principal receptors associated with pain physiology and inhibition are mu and kappa. (Morphine, the gold standard for treating severe pain, is an opioid agonist that binds to mu and kappa receptors.)
Most opioids that are used clinically bind to mu receptors; these drugs provide analgesia but also present the risk for adverse effects, such as decreased respiratory drive, miosis, and decreased motor function of the gastrointestinal (GI) tract, which can lead to constipation.8 Because mu receptors are located mainly in the brain and spinal cord (as well as the GI tract), opioids also produce a feeling of euphoria that can lead to dependence.
Kappa receptors, in contrast, are located mainly in the limbic system, diencephalic area, and spinal cord. When these receptors are activated, they can produce spinal analgesia, dyspnea, dependence, and dysphoria.
Delta receptors also play a role in pain management and are associated with emotional and affective components of the experience of pain.7 They are largely located in the brain; when activated, they can lead to spinal and supraspinal anesthesia, as well as decreased gastric motility.8 Delta receptors have not been studied as much as mu and kappa receptors, but it has been suggested that they play a role in psychologic dependency.
Depending on the effect that a drug has on these receptors, it can be considered a full (or pure) opioid agonist, a partial agonist, or a mixed agonist–antagonist. By binding to opioid receptors, opioid agonists provide pain relief. Health care providers often prescribe a full agonist, such as morphine, hydrocodone, codeine, or oxycodone, to treat pain. Partial agonists, such as buprenorphine and butorphanol, often decrease activity at mu receptor sites. Mixed agonist–antagonists either block or bind opioids at receptor sites.9 Medications that block mu and kappa receptors are considered opioid antagonists, which are used not to treat pain but rather to reverse the effect of opioids (eg, naloxone).6
Table 1 lists commonly used opioids, their analgesic duration, and the standard approved dosages.10

Continue to: A STEPWISE APPROACH TO PAIN MANAGEMENT
A STEPWISE APPROACH TO PAIN MANAGEMENT
On January 1, 2018, The Joint Commission (JNC) implemented new and revised standards to ensure that all patients receive appropriate assessment and management of their pain. While these standards apply to accredited hospitals, they provide a solid framework for assessing and treating pain in any patient. JNC now requires that patients be included in the development of treatment plans, which should encompass realistic expectations and reasonable goals, and that providers promote safe opioid use by identifying and monitoring high-risk patients.11
One valuable tool that can help clinicians fulfill the obligation to provide safe and effective pain management is the World Health Organization’s “pain ladder” (see Figure 1).12 Originally released in 1986 to address cancer pain in the pediatric population, this tool has proven validity. It has since been expanded to guide treatment of pain in other patient populations. In addition, the steps of the “pain ladder” provide useful information on the clinical examination and documentation of pain, principles of pharmacotherapeutic management, and considerations when using different analgesics.

Pain is assessed on a scale of 1 to 10, with 1 representing the least pain. Medication recommendations are as follows
- For mild pain (ie, a score of 1-3): acetaminophen, NSAIDs, or other nonopioids.
- For moderate pain (pain score, 4-6): an opioid (eg, hydrocodone), with or without an adjunct medication.
- For severe pain (pain score, 7-10) or pain that has not responded to previous therapies: a stronger opioid (eg, morphine, hydromorphone, fentanyl), with or without an adjuvant drug.12
In all cases, patients should be informed about both pharmacotherapeutic and nonpharmacotherapeutic options. The latter include hypnosis, relaxation techniques, acupuncture, physical therapy, application of heat and cold, and electro-analgesia.
Pharmacologic options at any “step” of the ladder carry the risk for adverse effects. Thus, NPs and PAs who prescribe these medications need to apprise patients of the potential harms associated with their treatment.
Acetaminophen. Patients should be instructed on the safe use of acetaminophen, particularly with regard to dosing, since liver damage can occur. Patients should not take more than 4,000 mg in a 24-hour period, and each dose should not exceed 1,000 mg.
NSAIDs. These drugs are often used for short-term management of mild and moderate pain. Patients should be instructed to take these agents with food to decrease GI upset. Other common adverse effects include GI bleed or perforation and renal insufficiency or failure.
Opioids. Depending on which class of receptors an opioid medication targets, patients may develop any of the following: constipation, decreased GI motility, nausea, hypotension, urinary retention, euphoria, pruritus, miosis, dependence, respiratory depression, and sedation. It is important for NPs and PAs who prescribe these medications to remain vigilant for adverse effects and complications from opioid use and to educate the patient and his/her family about possible complications.6
Patients must be instructed not to drink alcohol or take other CNS depressants while taking an opioid. They should be advised about the dangers of operating heavy equipment or engaging in other activities that require mental and physical alertness, since opioids can cause drowsiness. Among the GI effects of some opioids (nausea, vomiting) is constipation—so patients should also be educated on the need to increase fluid intake and include high-fiber foods in their diet.9
But most important of all, patients taking an opioid should be informed that there is the potential for physical dependency and abuse with these agents, and these agents should be used only for acute, severe pain.
Continue to: DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
Every patient has a right to adequate and safe pain control—but NPs and PAs must be aware of the potential for some patients to misuse opioids by taking them in a different way than intended, in a different quantity than prescribed, or without a prescription.13 Having prescriptive authority confers an obligation for NPs and PAs to recognize the prevalence of drug misuse and its impact on patients, families, and society.
Regrettably, there is lack of clarity in the literature about specific characteristics and demographic data that can help determine who is at risk for opioid misuse.14 For example, risk factors that have been associated with drug misuse include a personal or family history of substance abuse; younger age; and an ongoing psychiatric condition.
In contrast, Kennedy and colleagues determined that patients seeking prescription opioids for misuse or abuse tend to be older; be of Caucasian background; have a history of overdose; be receiving methadone maintenance therapy; and have been incarcerated.15 In addition, several characteristics—having moderate or extreme pain, disability, or a history of being refused pain medication—were also associated with a history of seeking prescription opioids to abuse.15
This diverse set of variables underscores the importance of obtaining and documenting a complete history from patients who are experiencing (and seeking relief of) pain; performing a thorough physical exam; and asking specific questions about the patient’s level of pain and the potential for misuse of pain medication.
Gathering this information may help identify patients at risk for opioid misuse or abuse. Furthermore, it ensures that a patient’s chronic pain is not being undertreated and that he/she is not being undeservedly labeled or judged as a drug seeker or abuser.
Continue to: Tools and strategies for appropriate use of opioids
Tools and strategies for appropriate use of opioids
There are tools and strategies available to ensure proper use of opioids for managing chronic noncancer pain. Urine drug testing, screening tools for opioid abuse, prescription drug monitoring programs, and opioid treatment agreements should be considered for patients who require prescription opioids to treat pain.15
Urine drug testing. The CDC recommends that prescribing clinicians perform urine drug testing before initiating opioid therapy and at least annually afterward. It can be used to assess for prescription medications generally, controlled prescription drugs specifically, and substances of abuse.16 Urine drug testing can mitigate the risk for misuse or overdose of opioids, as well as identify patients who were prescribed an opioid but are not taking it. The prescribing provider is responsible for explaining to the patient why urine testing is being done, performing confirmatory testing, and discussing results with the patient.
Risk-assessment tools. A number of web-based tools help the prescribing provider assess a patient’s risk for misuse or abuse of opioids and other substances. They fall into three general categories of use: assessing patients being considered for long-term opioid therapy; assessing for misuse once opioid treatment is initiated; and addressing the potential for substance abuse generally.17-24 Table 2 lists examples. Although screening tools are not 100% accurate at identifying who is a substance abuser, they do alert the provider that a potential problem exists and needs to be explored. As such, they should be considered one component of comprehensive risk assessment, monitoring, and mitigation.25

Prescription drug monitoring programs (PDMPs). NPs and PAs must also be aware of “doctor shopping,” in which a person seeks prescriptions from multiple providers (often under false pretenses) and has them filled at multiple pharmacies. PDMPs are designed to monitor for suspected abuse, diversion, or inappropriate prescribing. These state-run electronic databases track the amount of controlled substances prescribed, dispensed, and refilled for a given patient.26 This information can assist providers in identifying high-risk patients who may benefit from an early intervention program.27 Once a patient is identified as having an opioid use disorder, NPs and PAs must provide appropriate referral to an evidence-based practice for treatment of abuse. It is essential to recognize that an opioid use disorder is a chronic illness and that relapses occur.
Opioid treatment agreements. These have been presented as a strategy to prevent prescription drug abuse; however, there is little evidence to support their effectiveness in preventing medication misuse, abuse, or diversion of opioids. In fact, research has shown that such agreements can put the patient–provider therapeutic relationship at risk for disruption, since patients may feel mistrusted or stigmatized by the suggestion that they might behave inappropriately.28 The position of the American Pain Society and the American Academy of Pain Management is that patients and clinicians should have ongoing discussions about chronic opioid therapy that include goals, expectations, risks, and alternatives to opioids.29 If a written agreement is used, it needs to address the patient’s and the clinician’s responsibilities and expectations in managing chronic pain.28
Continue to: Additional resources for providers
Additional resources for providers
Many other resources are available for prescribers of controlled substances. For example, the CDC has published guidelines for prescribing opioids to patients with chronic pain, with a goal of increasing patient–provider communication.16 Additional goals include improving the safety of opioid use, maintaining the effectiveness of treatment, and reducing the necessity and practice of long-term therapy.
The FDA has also published a blueprint on how opioid analgesics can be formulated to deter abuse and, thus, be safer.30 Although directed at the pharmaceutical industry—the FDA encourages manufacturers to develop abuse-deterrent mechanisms, such as physical and chemical barriers, aversion technology, and new delivery systems—the guidance may enlighten providers on how abusers can alter or manipulate oral opioids to achieve the desired effects.30

CONCLUSION
Because NPs and PAs are authorized to prescribe Schedule II-V drugs in their scope of practice, they must have knowledge of drug-seeking behaviors and drug misuse before they prescribe opioids for pain relief. They must be attentive to patients’ pain-control needs and consider how to avoid or reduce the potential for misuse and abuse. Understanding the experience of pain and how opioids modulate it, as well as using available risk-assessment strategies, will help providers offer safe, effective treatment to their patients.
CE/CME No: CR-1804
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the basic pharmacology of opioid medications and how they affect pain.
• Apply a stepwise approach to pain management, based on the World Health Organization's "pain ladder."
• Communicate to patients the key educational points on the risks of opioid use.
• Identify strategies to deter or detect opioid misuse or abubse.
FACULTY
Deborah Salani is an Associate Professor of Clinical and Director of the Accelerated BSN Program, Nichole A. Crenshaw is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Acute Care Nurse Practitioner Program, Brenda Owusu is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Primary Care Nurse Practitioner Program, and Juan M. Gonzalez is an Assistant Professor of Clinical and Program Director for the Family Nurse Practitioner Program, at the University of Miami School of Nursing and Health Studies in Coral Gables, Florida.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through March 31, 2019.
Article begins on next page >>
Abuse of prescribed controlled substances—particularly opioid analgesics—and associated morbidity and mortality are a serious public health problem. The response to this crisis must include prevention, early identification, and appropriate treatment of addiction. Prescribing NPs and PAs must understand how to manage acute and chronic pain while also being attentive to signs of drug seeking and opioid misuse and abuse. The information and tools outlined in this article can equip providers to combat the opioid epidemic.
Controlled prescription drug abuse and its associated morbidity and mortality are a serious public health problem globally. In 2015, more than 29 million people worldwide misused and abused drugs, according to the United Nations Office on Drugs and Crime.1 Opioid use disorders account for approximately 70% of that estimate.
In the US, the mortality associated with this abuse has been devastating. Between 1999 and 2014, drug overdose deaths nearly tripled; in 2014 alone, there were 47,055 such fatalities, 61% of which involved opioids.2,3 Since 2000, unintentional overdose deaths from opioids have increased by 200%.3 Overdose deaths associated with natural and semisynthetic opioids (the most commonly prescribed pain relievers) increased 9% from 2013 to 2014, while those associated with synthetic opioids (fentanyl and tramadol) nearly doubled in the same period.3
Further contributing to the problem, a person addicted to prescription opioid drugs is 40 times more likely to be addicted to heroin, compared to someone who is not addicted to opioids.4 Deaths related to heroin overdose continue to dramatically increase.3
A call to action
In August 2016, former US Surgeon General Vivek Murthy, MD, sent a personal letter to more than 2.3 million health care providers, seeking their assistance in addressing the prescription opioid crisis.5 Murthy acknowledged the challenges providers face when attempting to strike a balance between treating a patient’s pain and reducing the risk for opioid addiction. He explained that clinicians are uniquely situated to end this crisis, and he asked providers to pledge to “turn the tide” by taking three actions
- Become more educated about treating pain safely and effectively.
- Screen patients for opioid use disorder and make the appropriate evidence-based treatment referrals.
- Discuss and treat addiction as a chronic disorder.5
To help stem the epidemic of controlled prescription drug abuse, NPs and PAs must be knowledgeable about patient safety issues, including how to identify patients at risk for opioid misuse and recognize signs of misuse or abuse. This article aims to educate providers who have prescriptive authority about the pharmacology of opioids; safe and effective prescribing of these drugs; and how to identify and manage misuse and abuse.
Continue to: OVERVIEW OF PAIN
OVERVIEW OF PAIN
Pain, considered the fifth vital sign, is one of the more common reasons that people seek treatment from a health care provider. Pain is a personal, individual, subjective experience: It is whatever the patient says it is and exists whenever the patient says it does. Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
Pain is classified as acute or chronic. Acute pain is a sudden but temporary, self-limiting response to some type of bodily injury; it generally lasts less than six months. Chronic pain is often associated with prolonged diseases such as cancer, fibromyalgia, and osteoarthritis; it persists for six months or longer.
Pain can be separated into two categories: nociceptive and neuropathic. Nociceptive pain originates from peripheral or visceral nociceptors as a result of injury and comprises somatic and visceral pain. Somatic pain is caused by injury to soft tissue, connective tissue, and bone; the classic description is a sharp, well-localized discomfort. Visceral pain originates from an organ or deeper structure; it is commonly described as dull, poorly localized, and sensitive to stretch, ischemia, and inflammation.
Neuropathic pain is an abnormal processing of pain stimuli by the peripheral nervous system or central nervous system (CNS) and can result from injury or inflammation to a nerve. Neuropathic pain is usually described by patients as electric, burning, and/or shooting. Examples include pain associated with cancer, diabetic neuropathy, and phantom-limb sensation (following amputation).
The physiologic experience of pain follows a defined set of phases. First is transduction, which occurs at the moment of injury or trauma; sensory nerve endings convert the noxious stimulus into a nerve impulse. Second is transmission of the pain impulse to the spinal column by means of chemical messengers known as neurotransmitters.
After the pain impulse reaches the spinal tract, it continues to the brain, at which point there is perception, the third step in the process. This leads to modulation (also known as anti-nociception). In this fourth step, neurons that originate in the brainstem are activated, releasing neurotransmitters that inhibit transmission of pain. Modulation occurs in several areas of the CNS and involves the neurotransmitters serotonin, norepinephrine, and endogenous opioids (eg, ß-endorphin).6 During modulation, the limbic nervous system provokes a response to the painful stimulus, triggering endogenous opioids to bind to opioid receptors.7
Continue to: ROLE OF OPIOIDS IN PAIN MANAGEMENT
ROLE OF OPIOIDS IN PAIN MANAGEMENT
Opioids have been used to control pain for centuries. They are extracted from the opium poppy plant, Papaver somniferum. From the substance extracted, roughly 9% to 14% is morphine and 0.8% to 2.5% is codeine.7 Opioids are used to treat many symptoms and ailments, including diarrhea, moderate to severe pain, and persistent cough.
Opioids work through receptors in the CNS, including mu, kappa, and delta opioid receptors and the opioid-like receptor nociceptin.7 The principal receptors associated with pain physiology and inhibition are mu and kappa. (Morphine, the gold standard for treating severe pain, is an opioid agonist that binds to mu and kappa receptors.)
Most opioids that are used clinically bind to mu receptors; these drugs provide analgesia but also present the risk for adverse effects, such as decreased respiratory drive, miosis, and decreased motor function of the gastrointestinal (GI) tract, which can lead to constipation.8 Because mu receptors are located mainly in the brain and spinal cord (as well as the GI tract), opioids also produce a feeling of euphoria that can lead to dependence.
Kappa receptors, in contrast, are located mainly in the limbic system, diencephalic area, and spinal cord. When these receptors are activated, they can produce spinal analgesia, dyspnea, dependence, and dysphoria.
Delta receptors also play a role in pain management and are associated with emotional and affective components of the experience of pain.7 They are largely located in the brain; when activated, they can lead to spinal and supraspinal anesthesia, as well as decreased gastric motility.8 Delta receptors have not been studied as much as mu and kappa receptors, but it has been suggested that they play a role in psychologic dependency.
Depending on the effect that a drug has on these receptors, it can be considered a full (or pure) opioid agonist, a partial agonist, or a mixed agonist–antagonist. By binding to opioid receptors, opioid agonists provide pain relief. Health care providers often prescribe a full agonist, such as morphine, hydrocodone, codeine, or oxycodone, to treat pain. Partial agonists, such as buprenorphine and butorphanol, often decrease activity at mu receptor sites. Mixed agonist–antagonists either block or bind opioids at receptor sites.9 Medications that block mu and kappa receptors are considered opioid antagonists, which are used not to treat pain but rather to reverse the effect of opioids (eg, naloxone).6
Table 1 lists commonly used opioids, their analgesic duration, and the standard approved dosages.10

Continue to: A STEPWISE APPROACH TO PAIN MANAGEMENT
A STEPWISE APPROACH TO PAIN MANAGEMENT
On January 1, 2018, The Joint Commission (JNC) implemented new and revised standards to ensure that all patients receive appropriate assessment and management of their pain. While these standards apply to accredited hospitals, they provide a solid framework for assessing and treating pain in any patient. JNC now requires that patients be included in the development of treatment plans, which should encompass realistic expectations and reasonable goals, and that providers promote safe opioid use by identifying and monitoring high-risk patients.11
One valuable tool that can help clinicians fulfill the obligation to provide safe and effective pain management is the World Health Organization’s “pain ladder” (see Figure 1).12 Originally released in 1986 to address cancer pain in the pediatric population, this tool has proven validity. It has since been expanded to guide treatment of pain in other patient populations. In addition, the steps of the “pain ladder” provide useful information on the clinical examination and documentation of pain, principles of pharmacotherapeutic management, and considerations when using different analgesics.

Pain is assessed on a scale of 1 to 10, with 1 representing the least pain. Medication recommendations are as follows
- For mild pain (ie, a score of 1-3): acetaminophen, NSAIDs, or other nonopioids.
- For moderate pain (pain score, 4-6): an opioid (eg, hydrocodone), with or without an adjunct medication.
- For severe pain (pain score, 7-10) or pain that has not responded to previous therapies: a stronger opioid (eg, morphine, hydromorphone, fentanyl), with or without an adjuvant drug.12
In all cases, patients should be informed about both pharmacotherapeutic and nonpharmacotherapeutic options. The latter include hypnosis, relaxation techniques, acupuncture, physical therapy, application of heat and cold, and electro-analgesia.
Pharmacologic options at any “step” of the ladder carry the risk for adverse effects. Thus, NPs and PAs who prescribe these medications need to apprise patients of the potential harms associated with their treatment.
Acetaminophen. Patients should be instructed on the safe use of acetaminophen, particularly with regard to dosing, since liver damage can occur. Patients should not take more than 4,000 mg in a 24-hour period, and each dose should not exceed 1,000 mg.
NSAIDs. These drugs are often used for short-term management of mild and moderate pain. Patients should be instructed to take these agents with food to decrease GI upset. Other common adverse effects include GI bleed or perforation and renal insufficiency or failure.
Opioids. Depending on which class of receptors an opioid medication targets, patients may develop any of the following: constipation, decreased GI motility, nausea, hypotension, urinary retention, euphoria, pruritus, miosis, dependence, respiratory depression, and sedation. It is important for NPs and PAs who prescribe these medications to remain vigilant for adverse effects and complications from opioid use and to educate the patient and his/her family about possible complications.6
Patients must be instructed not to drink alcohol or take other CNS depressants while taking an opioid. They should be advised about the dangers of operating heavy equipment or engaging in other activities that require mental and physical alertness, since opioids can cause drowsiness. Among the GI effects of some opioids (nausea, vomiting) is constipation—so patients should also be educated on the need to increase fluid intake and include high-fiber foods in their diet.9
But most important of all, patients taking an opioid should be informed that there is the potential for physical dependency and abuse with these agents, and these agents should be used only for acute, severe pain.
Continue to: DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
Every patient has a right to adequate and safe pain control—but NPs and PAs must be aware of the potential for some patients to misuse opioids by taking them in a different way than intended, in a different quantity than prescribed, or without a prescription.13 Having prescriptive authority confers an obligation for NPs and PAs to recognize the prevalence of drug misuse and its impact on patients, families, and society.
Regrettably, there is lack of clarity in the literature about specific characteristics and demographic data that can help determine who is at risk for opioid misuse.14 For example, risk factors that have been associated with drug misuse include a personal or family history of substance abuse; younger age; and an ongoing psychiatric condition.
In contrast, Kennedy and colleagues determined that patients seeking prescription opioids for misuse or abuse tend to be older; be of Caucasian background; have a history of overdose; be receiving methadone maintenance therapy; and have been incarcerated.15 In addition, several characteristics—having moderate or extreme pain, disability, or a history of being refused pain medication—were also associated with a history of seeking prescription opioids to abuse.15
This diverse set of variables underscores the importance of obtaining and documenting a complete history from patients who are experiencing (and seeking relief of) pain; performing a thorough physical exam; and asking specific questions about the patient’s level of pain and the potential for misuse of pain medication.
Gathering this information may help identify patients at risk for opioid misuse or abuse. Furthermore, it ensures that a patient’s chronic pain is not being undertreated and that he/she is not being undeservedly labeled or judged as a drug seeker or abuser.
Continue to: Tools and strategies for appropriate use of opioids
Tools and strategies for appropriate use of opioids
There are tools and strategies available to ensure proper use of opioids for managing chronic noncancer pain. Urine drug testing, screening tools for opioid abuse, prescription drug monitoring programs, and opioid treatment agreements should be considered for patients who require prescription opioids to treat pain.15
Urine drug testing. The CDC recommends that prescribing clinicians perform urine drug testing before initiating opioid therapy and at least annually afterward. It can be used to assess for prescription medications generally, controlled prescription drugs specifically, and substances of abuse.16 Urine drug testing can mitigate the risk for misuse or overdose of opioids, as well as identify patients who were prescribed an opioid but are not taking it. The prescribing provider is responsible for explaining to the patient why urine testing is being done, performing confirmatory testing, and discussing results with the patient.
Risk-assessment tools. A number of web-based tools help the prescribing provider assess a patient’s risk for misuse or abuse of opioids and other substances. They fall into three general categories of use: assessing patients being considered for long-term opioid therapy; assessing for misuse once opioid treatment is initiated; and addressing the potential for substance abuse generally.17-24 Table 2 lists examples. Although screening tools are not 100% accurate at identifying who is a substance abuser, they do alert the provider that a potential problem exists and needs to be explored. As such, they should be considered one component of comprehensive risk assessment, monitoring, and mitigation.25

Prescription drug monitoring programs (PDMPs). NPs and PAs must also be aware of “doctor shopping,” in which a person seeks prescriptions from multiple providers (often under false pretenses) and has them filled at multiple pharmacies. PDMPs are designed to monitor for suspected abuse, diversion, or inappropriate prescribing. These state-run electronic databases track the amount of controlled substances prescribed, dispensed, and refilled for a given patient.26 This information can assist providers in identifying high-risk patients who may benefit from an early intervention program.27 Once a patient is identified as having an opioid use disorder, NPs and PAs must provide appropriate referral to an evidence-based practice for treatment of abuse. It is essential to recognize that an opioid use disorder is a chronic illness and that relapses occur.
Opioid treatment agreements. These have been presented as a strategy to prevent prescription drug abuse; however, there is little evidence to support their effectiveness in preventing medication misuse, abuse, or diversion of opioids. In fact, research has shown that such agreements can put the patient–provider therapeutic relationship at risk for disruption, since patients may feel mistrusted or stigmatized by the suggestion that they might behave inappropriately.28 The position of the American Pain Society and the American Academy of Pain Management is that patients and clinicians should have ongoing discussions about chronic opioid therapy that include goals, expectations, risks, and alternatives to opioids.29 If a written agreement is used, it needs to address the patient’s and the clinician’s responsibilities and expectations in managing chronic pain.28
Continue to: Additional resources for providers
Additional resources for providers
Many other resources are available for prescribers of controlled substances. For example, the CDC has published guidelines for prescribing opioids to patients with chronic pain, with a goal of increasing patient–provider communication.16 Additional goals include improving the safety of opioid use, maintaining the effectiveness of treatment, and reducing the necessity and practice of long-term therapy.
The FDA has also published a blueprint on how opioid analgesics can be formulated to deter abuse and, thus, be safer.30 Although directed at the pharmaceutical industry—the FDA encourages manufacturers to develop abuse-deterrent mechanisms, such as physical and chemical barriers, aversion technology, and new delivery systems—the guidance may enlighten providers on how abusers can alter or manipulate oral opioids to achieve the desired effects.30

CONCLUSION
Because NPs and PAs are authorized to prescribe Schedule II-V drugs in their scope of practice, they must have knowledge of drug-seeking behaviors and drug misuse before they prescribe opioids for pain relief. They must be attentive to patients’ pain-control needs and consider how to avoid or reduce the potential for misuse and abuse. Understanding the experience of pain and how opioids modulate it, as well as using available risk-assessment strategies, will help providers offer safe, effective treatment to their patients.
1. United Nations Office on Drugs and Crime. World drug report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed March 21, 2018.
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051): 1445-1452.
3. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(5051):1378-1382.
4. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725.
5. US Department of Health and Human Services. United States Surgeon General. Letter from the Surgeon General. 2016. https://turnthetiderx.org/#. Accessed March 21, 2018.
6. Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA. Pharmacotherapeutics for Advanced Practice. 4th ed. Philadelphia, PA: Wolters Kluwer; 2017:1-23.
7. Adams MP, Holland N, Urban CQ. Pharmacology for Nurses: A Pathophysiologic Approach. 5th ed. Upper Saddle River, NJ: Pearson Education; 2016:239-252.
8. Grossman S, Porth CM. Somatosensory function, pain, and headache. In: Porth’s Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014:441.
9. Woo TM, Robinson MV. Pain management: Acute and chronic pain. In: Pharmacotherapeutics for Advanced Practice Nurse Prescribers. 4th ed. Philadelphia, PA: FA Davis; 2015:1361.
10. Adams MP, Urban CQ. Pharmacology for Nurses: Connections to Nursing Practice. 2nd ed. Upper Saddle River, NJ: Pearson Education; 2013:437.
11. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):1-4. www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Accessed March 21, 2018.
12. World Health Organization. WHO’s cancer pain ladder for adults. 2017. www.who.int/cancer/palliative/painladder/en/. Accessed March 21, 2018.
13. National Institutes of Health. National Institute on Drug Abuse. Opioids: brief description. www.drugabuse.gov/drugs-abuse/opioids. Accessed March 21, 2018.
14. Hudspeth RS. Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. J Nurse Pract. 2016;12(3):141-148.
15. Kennedy MC, Kerr T, DeBeck K, et al. Seeking prescription opioids from physicians for nonmedical use among people who inject drugs in a Canadian setting. Am J Addict. 2016;25(4):275-282.
16. US Department of Health and Human Services. CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed March 21, 2018.
17. Webster LR, Webster R. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432. [Tool available at www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf.] Accessed March 21, 2018.
18. Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP®-R). http://nationalpaincentre.mcmaster.ca/documents/soapp_r_sample_watermark.pdf. Accessed March 21, 2018.
19. D.I.R.E. Score: Patient Selection for Chronic Opioid Analgesia. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/D.I.R.E.%20Score.pdf. Accessed March 21, 2018.
20. Current Opioid Misuse Measure (COMM)™. www.opioidprescribing.com/documents/09-comm-inflexxion.pdf. Accessed March 21, 2018.
21. Pain Assessment and Documentation Tool (PADT™). www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/Pain%20Assess ment%20Documentation%20Tool%20%28PADT%29.pdf. Accessed March 21, 2018.
22. The CAGE and CAGE-AID Questionnaires. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/CAGE-AID.pdf. Accessed March 21, 2018.
23. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. [DAST-10 available at https://cde.drugabuse.gov/sites/nida_cde/files/DrugAbuseScreeningTest_2014Mar24.pdf.] Accessed March 21, 2018.
24. SBIRT AUDIT forms (English and Spanish). www.communitycarenc.org/media/tool-resource-files/sbirt-audit-forms.pdf. Accessed March 21, 2018.
25. Cheattle MD. Risk assessment: safe opioid prescribing tools. 2017. https://www.practicalpainmanagement.com/resource-centers/opioid-prescribing-monitoring/risk-assessment-safe-opioid-prescribing-tools. Accessed March 21, 2018.
26. Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65-77.
27. US Department of Health and Human Services. CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed March 21, 2018.
28. McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 2015;16(1):25-29.
29. Chou R, Fanciullo GJ, Fine PG, et al; for the American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
30. US Department of Health and Human Services. FDA Center for Drug Evaluation and Research. Abuse-deterrent opioids—evaluation and labeling guidance for industry. 2015. www.fda.gov/downloads/Drugs/Guid ances/UCM334743.pdf. Accessed March 21, 2018.
1. United Nations Office on Drugs and Crime. World drug report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed March 21, 2018.
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051): 1445-1452.
3. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(5051):1378-1382.
4. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725.
5. US Department of Health and Human Services. United States Surgeon General. Letter from the Surgeon General. 2016. https://turnthetiderx.org/#. Accessed March 21, 2018.
6. Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA. Pharmacotherapeutics for Advanced Practice. 4th ed. Philadelphia, PA: Wolters Kluwer; 2017:1-23.
7. Adams MP, Holland N, Urban CQ. Pharmacology for Nurses: A Pathophysiologic Approach. 5th ed. Upper Saddle River, NJ: Pearson Education; 2016:239-252.
8. Grossman S, Porth CM. Somatosensory function, pain, and headache. In: Porth’s Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014:441.
9. Woo TM, Robinson MV. Pain management: Acute and chronic pain. In: Pharmacotherapeutics for Advanced Practice Nurse Prescribers. 4th ed. Philadelphia, PA: FA Davis; 2015:1361.
10. Adams MP, Urban CQ. Pharmacology for Nurses: Connections to Nursing Practice. 2nd ed. Upper Saddle River, NJ: Pearson Education; 2013:437.
11. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):1-4. www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Accessed March 21, 2018.
12. World Health Organization. WHO’s cancer pain ladder for adults. 2017. www.who.int/cancer/palliative/painladder/en/. Accessed March 21, 2018.
13. National Institutes of Health. National Institute on Drug Abuse. Opioids: brief description. www.drugabuse.gov/drugs-abuse/opioids. Accessed March 21, 2018.
14. Hudspeth RS. Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. J Nurse Pract. 2016;12(3):141-148.
15. Kennedy MC, Kerr T, DeBeck K, et al. Seeking prescription opioids from physicians for nonmedical use among people who inject drugs in a Canadian setting. Am J Addict. 2016;25(4):275-282.
16. US Department of Health and Human Services. CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed March 21, 2018.
17. Webster LR, Webster R. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432. [Tool available at www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf.] Accessed March 21, 2018.
18. Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP®-R). http://nationalpaincentre.mcmaster.ca/documents/soapp_r_sample_watermark.pdf. Accessed March 21, 2018.
19. D.I.R.E. Score: Patient Selection for Chronic Opioid Analgesia. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/D.I.R.E.%20Score.pdf. Accessed March 21, 2018.
20. Current Opioid Misuse Measure (COMM)™. www.opioidprescribing.com/documents/09-comm-inflexxion.pdf. Accessed March 21, 2018.
21. Pain Assessment and Documentation Tool (PADT™). www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/Pain%20Assess ment%20Documentation%20Tool%20%28PADT%29.pdf. Accessed March 21, 2018.
22. The CAGE and CAGE-AID Questionnaires. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/CAGE-AID.pdf. Accessed March 21, 2018.
23. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. [DAST-10 available at https://cde.drugabuse.gov/sites/nida_cde/files/DrugAbuseScreeningTest_2014Mar24.pdf.] Accessed March 21, 2018.
24. SBIRT AUDIT forms (English and Spanish). www.communitycarenc.org/media/tool-resource-files/sbirt-audit-forms.pdf. Accessed March 21, 2018.
25. Cheattle MD. Risk assessment: safe opioid prescribing tools. 2017. https://www.practicalpainmanagement.com/resource-centers/opioid-prescribing-monitoring/risk-assessment-safe-opioid-prescribing-tools. Accessed March 21, 2018.
26. Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65-77.
27. US Department of Health and Human Services. CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed March 21, 2018.
28. McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 2015;16(1):25-29.
29. Chou R, Fanciullo GJ, Fine PG, et al; for the American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
30. US Department of Health and Human Services. FDA Center for Drug Evaluation and Research. Abuse-deterrent opioids—evaluation and labeling guidance for industry. 2015. www.fda.gov/downloads/Drugs/Guid ances/UCM334743.pdf. Accessed March 21, 2018.
March 2018: Click for Credit
Here are 4 articles in the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Prenatal Maternal Anxiety Linked to Hyperactivity in Offspring as Teenagers
To take the posttest, go to: http://bit.ly/2BLXsRs
Expires November 15, 2018
2. The Better Mammogram: Experts Explore Sensitivity of New Modalities
To take the posttest, go to: http://bit.ly/2nQaJii
Expires November 14, 2018
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
To take the posttest, go to: http://bit.ly/2E3tcmJ
Expires November 14, 2018
4. Salivary Biomarker for Huntington Disease Identified
To take the posttest, go to: http://bit.ly/2BGQpJP
Expires November 13, 2018
Here are 4 articles in the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Prenatal Maternal Anxiety Linked to Hyperactivity in Offspring as Teenagers
To take the posttest, go to: http://bit.ly/2BLXsRs
Expires November 15, 2018
2. The Better Mammogram: Experts Explore Sensitivity of New Modalities
To take the posttest, go to: http://bit.ly/2nQaJii
Expires November 14, 2018
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
To take the posttest, go to: http://bit.ly/2E3tcmJ
Expires November 14, 2018
4. Salivary Biomarker for Huntington Disease Identified
To take the posttest, go to: http://bit.ly/2BGQpJP
Expires November 13, 2018
Here are 4 articles in the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Prenatal Maternal Anxiety Linked to Hyperactivity in Offspring as Teenagers
To take the posttest, go to: http://bit.ly/2BLXsRs
Expires November 15, 2018
2. The Better Mammogram: Experts Explore Sensitivity of New Modalities
To take the posttest, go to: http://bit.ly/2nQaJii
Expires November 14, 2018
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
To take the posttest, go to: http://bit.ly/2E3tcmJ
Expires November 14, 2018
4. Salivary Biomarker for Huntington Disease Identified
To take the posttest, go to: http://bit.ly/2BGQpJP
Expires November 13, 2018
Polypharmacy in the Elderly: How to Reduce Adverse Drug Events
CE/CME No: CR-1802
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients who are at the greatest risk for the effects of polypharmacy.
• Recognize which medications are most likely to cause adverse drug events (ADEs) in the elderly population.
• Understand the effects of aging on the pharmacokinetics and pharmacodynamics of medications.
• Learn strategies to reduce the risk for polypharmacy and ADEs, including use of the Beers Criteria and the STOPP/START Criteria.
FACULTY
Kelsey Barclay practices in orthopedic surgery at Stanford Medical Center in Palo Alto, California. Amy Frassetto practices in Ob-Gyn at NewYork-Presbyterian in New York City. Julie Robb practices in emergency medicine at South Nassau Communities Hospital in Oceanside, New York. Ellen D. Mandel is a Clinical Professor in the Department of PA Studies at Pace University-Lenox Hill Hospital in New York City.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through January 31, 2019.
Article begins on next page >>
Managing medications in the elderly can be complicated by the physiologic effects of aging and the prevalence of comorbidities. Consistent use of tools such as the Beers criteria and the STOPP/START criteria, as well as medication reconciliation, can reduce polypharmacy and its adverse drug effects, improving health outcomes in this population.
Older adults (those 65 and older) often have a number of comorbidities requiring pharmacologic intervention, making medication management a complicated but essential part of caring for the elderly. A recent analysis of trends in prescription drug use by community-dwelling adults found that 39% of older adults used five or more prescribed medications.1 Furthermore, about 72% of older adults also take a nonprescription medication (OTC or supplement); while OTC medication use has declined in this population in recent years, dietary supplement use has increased.2
These patients are also more susceptible to adverse drug events (ADEs)—including adverse drug reactions (ADRs)—resulting from the physiologic changes of aging. By one estimate, ADRs are about seven times more common in those older than 70 than in younger persons.3 One out of every 30 urgent hospital admissions in patients ages 65 and older is related to an ADR.4
Providers must therefore be cognizant of drug indications, dosing, and drug interactions when prescribing medications to elderly patients. Fortunately, tools and methods to avoid polypharmacy and the adverse effects of commonly prescribed medications—such as anticholinergics and psychotropic drugs—are available.
POLYPHARMACY AND PRESCRIPTION CASCADING
While there is no specific number of medications required to define polypharmacy, the term is generally used when a nonhospitalized individual is taking five or more medications.5 The more medications a patient is taking, the more at risk he or she will be for ADRs, drug interactions, and prescription cascading.
Prescription cascading begins when an ADR is thought to be a new symptom and a new drug is prescribed to control it. Ultimately, a cascade of prescriptions occurs to control avoidable ADRs, resulting in polypharmacy. As many as 57% of women older than 65 in the United States are currently prescribed five or more medications, with 12% prescribed nine or more drugs.6 Not only do these medications cause independent ADRs, but there is also increased risk for drug interactions—and potentially, additional avoidable ADRs.
The elderly population is at greater risk for ADEs because these patients are more likely to have multiple comorbidities and chronic diseases, requiring multiple therapies.7 Polypharmacy is also more dangerous in the elderly because the physiologic changes that occur during natural aging can affect both the pharmacokinetics and pharmacodynamics of medications. The absorption, distribution, metabolism, and excretion of drugs within the human body changes as a person ages, while certain drug classes can alter the way the body functions. For example, muscle mass naturally declines and the proportion of body fat to muscle increases; this change affects the distribution of drugs such as benzodiazepines or lithium.7 If the medication dosage is not corrected, the toxicity of the drug will be increased.7
Medication excretion is largely controlled by the kidneys. Renal perfusion and function decline with age, leading to a decrease in glomerular filtration rate—which requires closer monitoring of medication selection and dosing. The risk is heightened when the elderly patient becomes acutely ill. An acute decrease in kidney function results in decreased excretion of medications, leading to an increase in ADRs.7
Ultimately, the safety of many medications in the elderly patient is unknown.8 But there is a growing body of knowledge on the adverse effects of some classes of medication in this population.
COMMONLY PRESCRIBED MEDICATIONS—AND RISKS
ADEs result from medication errors, ADRs, allergic reactions, and overdoses. The incidence of ADEs—specifically ADRs and medication errors—is elevated in elderly patients who are prescribed certain classes of medications or multiple drugs simultaneously.8 Anticholinergic drugs and psychotropic drugs (specifically antipsychotics and benzodiazepines) are among the medications most commonly prescribed to elderly patients—and among the most likely to contribute to ADEs.9 Diabetes is a chronic condition whose treatment may also put elderly patients at risk for ADEs.10
Anticholinergic medications
Anticholinergic drugs—commonly prescribed for Parkinson disease, depression, urinary incontinence, pulmonary disorders, intestinal motility, and muscle spasms—competitively inhibit the binding of acetylcholine to muscarinic acetylcholine receptors.9 Because this mechanism tends to be nonselective, the adverse effects may be widespread. Central adverse effects include cognitive impairment, confusion, and delirium; peripheral adverse effects include constipation, urinary retention, dry mouth, blurred vision, peristaltic reduction, and tachycardia.9
Anticholinergic drugs are commonly prescribed to elderly patients for cardiovascular (CV) and neurologic disorders. (Medications for the former include ß-blockers, calcium channel blockers, diuretics, and ACE inhibitors; for the latter, amitriptyline, quetiapine, nortriptyline, prochlorperazine, haloperidol, and paroxetine.) An assessment of anticholinergic activity classified most neurologic medications as high activity and most CV medications as low—however, the latter are usually given in conjunction with other anticholinergic medications, increasing their ability to cause ADRs.11
In many cases, patients are prescribed anticholinergic medications to control symptoms of a disease, not to cure it—which means patients may be taking these medications for years. This cumulative exposure is called the anticholinergic burden. Many studies show that the anticholinergic burden is a predictor of cognitive and physical decline; a 2016 study of adults older than 65 who were exposed to 5 mg/d of oxybutynin for more than three years had a 23% increased risk for dementia, compared to low-risk or no exposure groups.9
In a retrospective, population-level study conducted in New Zealand, researchers assessed the anticholinergic effects of delirium, urinary retention, and constipation in 2,248 patients (65 and older) who were admitted to the hospital with at least one prescribed medication. Anticholinergic burden was found to be a significant independent predictor; patients taking five anticholinergic medications were more than three times as likely to develop an anticholinergic effect than those taking just one such medication (adjusted odds ratio, 3.21).11
Psychotropic drugs
Another often-prescribed medication group is psychotropic drugs, specifically antipsychotics and benzodiazepines, for agitation and behavioral disturbances in dementia. A year-long study of 851 patients in two long-term care nursing homes in Boston found that risk for ADRs—specifically, falls—was increased in those who had a change (initiation or dose increase) in psychotropic medication (ie, benzodiazepine, antipsychotic, or antidepressant).12
Second-generation antipsychotics, which are more commonly prescribed than first-generation agents, work on a postsynaptic blockade of brain dopamine D2 receptors and have an increased affinity for serotonin 5-HT2A receptors (see Table 1 for pharmacology of these medications).13,14 Adverse effects of these drugs include hypotension, sedation, and anticholinergic effects. Second-generation antipsychotics also carry a “black box warning” for increased risk for death in elderly patients with dementia-related psychosis.15
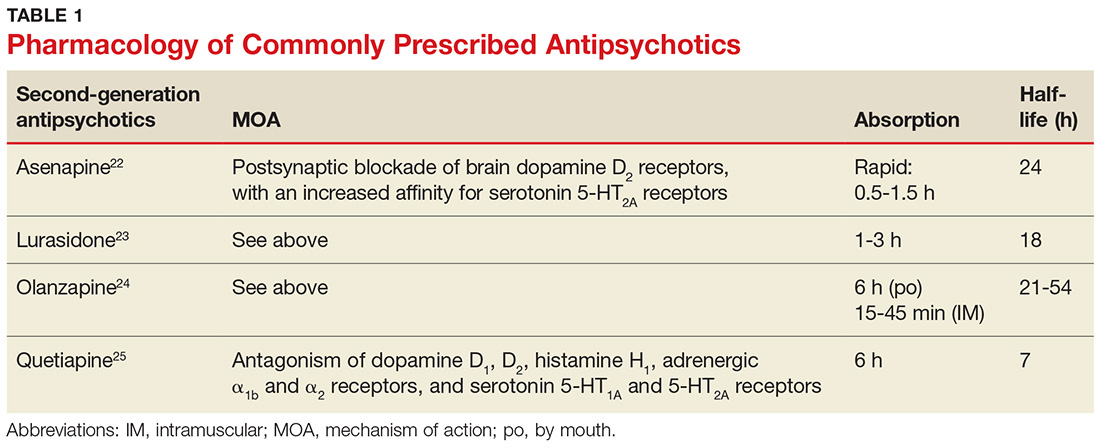
Benzodiazepines bind to receptors in the gamma-aminobutyric acid receptor complex, which enhances the binding of this inhibitory neurotransmitter (see Table 2 for pharmacology). Of this class of drugs, lorazepam has the highest potency, whereas midazolam and diazepam have lower potencies. Use of benzodiazepines increases risk for delirium and respiratory depression.16 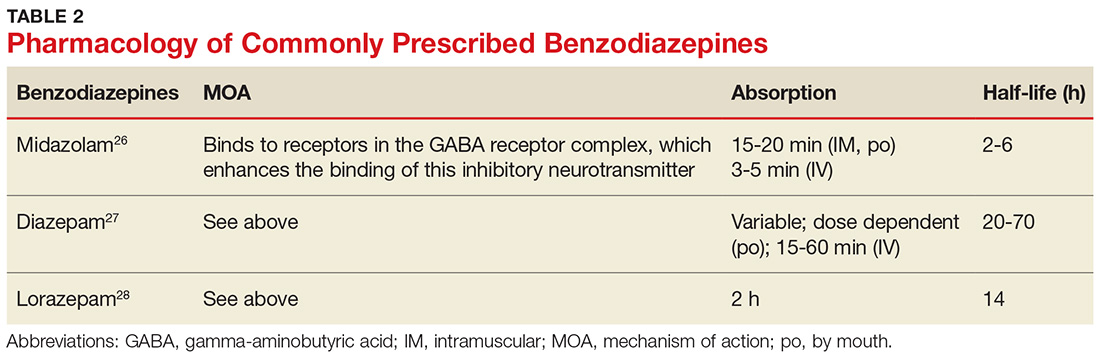
Diabetes treatment
People with diabetes have an increased risk for ADEs; this risk is elevated in older adults due to comorbidities such as peripheral neuropathy, retinopathy, coronary artery disease, and peripheral vascular disease.10 Hypoglycemic agents, such as insulin and insulin secretagogues, confer a higher risk for falls due to their hypoglycemic effect.10 Furthermore, metformin is known to increase risk for cognitive impairment in patients with diabetes.10
PREVENTING ADEs AND UNNECESSARY POLYPHARMACY
Predicting and preventing ADEs should be a health care provider’s priority when treating an elderly patient taking multiple medications—but it is often overlooked. Electronic medical records (EMRs) are helpful in preventing ADEs, specifically prescription errors, by flagging the patient’s chart when potentially problematic medications are ordered; however, this captures only a portion of ADEs occurring in this population.7
Other options to evaluate a patient for polypharmacy and possible ADRs include the Beers Criteria and the STOPP/START Criteria.17,18 Additionally, performing thorough and frequent medication reviews helps ensure that patients are prescribed essential medications to treat their comorbidities with the most opportunistic risk-benefit ratio. Patients’ medication lists across settings (eg, hospital, primary care, urgent care) can be accessed more easily, efficiently, and accurately with the integration of EMRs.
Beers Criteria
First published by Dr. Mark Beers in 1991 and endorsed by the American Geriatrics Society, the Beers Criteria identifies possible harmful effects of certain commonly prescribed medications to help guide and modify pharmacologic treatments, particularly in adults older than 65. The Beers Criteria classifies medications into three categories:
- Drugs that should be avoided or dose-adjusted
- Drugs that are potentially inappropriate in patients with certain conditions or syndromes
- Drugs that should be prescribed with caution in older adults.17
In the most recent update (2015), possible adverse effects of medications based on a patient’s hepatic or renal function, the effectiveness of the medication, and possible drug interactions were added. For example, nitrofurantoin and antiarrhythmics (eg, amiodarone and digoxin) should be avoided at a lower threshold of hepatic and renal impairment than previously recommended. The criteria suggest avoiding use of zolpidem, a nonbenzodiazepine receptor agonist, because of its elevated risk for adverse effects and minimal effectiveness in treating insomnia. More information about the 2015 criteria is available from the American Geriatrics Society (http://online library.wiley.com/doi/10.1111/jgs. 13702/full).19
The latest update also takes into account recently published evidence of increased ADEs resulting from drugs such as antipsychotics and proton pump inhibitors (PPIs).20 Antipsychotics are associated with an increased risk for morbidity and mortality, and PPIs are now recommended only for treatment duration of up to two months because of the possible increased risk for Clostridium difficile infection, as well as falls and fractures in patients older than 65.20 (PPIs indirectly reduce calcium absorption, which may lead to an increased fracture risk, particularly in postmenopausal women.20)
As with any guideline, the Beers Criteria was designed to supplement, not replace, clinical expertise and judgment. The risks and benefits of a medication should be weighed for the individual patient.
STOPP/START Criteria
Less widely used is the STOPP/START Criteria, an evidence-based set of guidelines consisting of 65 STOPP (Screening Tool of Older Person’s potentially inappropriate Prescriptions) and 22 START (Screening Tool to Alert doctors to the Right Treatment) criteria. Although they may be used individually, STOPP and START are best used together to determine the most appropriate medications for an elderly patient.
The STOPP guidelines help determine when the risks of a medication may outweigh the benefits in a given patient. STOPP includes recommendations for the appropriate length of time to use a medication; for example, PPIs should not be used for more than eight weeks (similar to the Beers recommendation) and benzodiazepines and neuroleptics for more than four weeks.18
START helps clinicians recognize potential prescribing omissions and to identify when a medication regimen should be implemented based on a patient’s history.18 Examples of START criteria include suggestions of when to initiate calcium and vitamin D supplementation for prevention of osteoporosis and when to begin statins in patients with diabetes, coronary artery disease, and cardiovascular disease.18
STOPP/START is organized by physiologic system, which allows for greater usability, and it addresses medications by class rather than specific medications. (The Beers Criteria was criticized for these reasons, as well as its limited transferability outside the United States.) When assessed in systematic reviews, the STOPP/START criteria were found to be fundamentally more sensitive than the Beers Criteria. Overall, it was concluded that the use of the STOPP/START criteria resulted in an absolute risk reduction of 21.2% to 35.7% and greatly improved the appropriateness of prescribing medication to the elderly. Its use also resulted in fewer follow-up appointments with a primary care physician (PCP).18
iPhone and Android applications such as iGeriatrics and Medstopper provide clinicians with easy access to Beers Criteria and STOPP/START Criteria, respectively.
Medication reconciliation
Medication reconciliation—in which health care providers review a patient’s medication list at hospital admission and discharge, and even at routine office visits—is an increasingly common practice, especially with the implementation of EMRs. The patient’s prescribed and OTC medications, as well as dose, route, frequency, and indication, are updated, with the goal of maintaining the most accurate list. Health care providers can utilize both the Beers Criteria and the STOPP/START criteria in their reconciliation process to help reduce polypharmacy in the elderly. It is an essential step in maintaining communication between providers and ultimately decreasing the incidence of ADEs.17
IMPROVE … continuity of care
Polypharmacy can decrease patient likelihood to adhere to the regimen, whether due to confusion or intolerance.8 Patients should be included, along with caregivers and all medical providers, in a holistic assessment of the patient’s best interests in terms of long-term care and pharmacologic treatment, since those who have a sense of control in their treatment goals and expectations often achieve a better understanding of their medical status.10
However, educating patients about their medications is time-consuming, and time is often at a premium during a typical office visit. A pilot study of 28 male veterans (ages 85 and older)—the Integrated Management and Polypharmacy Review of Vulnerable Elders (IMPROVE) project—devised a model to combat this problem.21 As an adjunct to a visit with the PCP, a clinical pharmacist trained in patient education and medication management performed face-to-face clinical consults with patients and their caregivers. The results indicated that medical management by both the PCP and the pharmacist resulted in better medication management. The pharmacist was able to spend time with the patient and caregiver, resulting in individualized instructions, education, and strategies for safe and effective medication use. The PCP remained involved by cosigning the note with the pharmacist and was available for consultation, if needed.
In IMPROVE, 79% of patients had at least one medication discontinued and 75% had one or more dosing or timing adjustments made. Potentially inappropriate medications were reduced by 14%.21 When the researchers compared the six-month period before the trial with the six-month period afterward, they found an average pharmacy cost savings of $64 per veteran per month. There was also a decreasing trend in phone calls and visits to the PCP. Cost savings were comparable to or greater than those reported for similar interventions.21 There has not been sufficient long-term follow-up to assess this method’s effects on ADEs, morbidity, and mortality, however.
CONCLUSION
Managing medications in the elderly population is difficult, and polypharmacy is common due to the prevalence of patients with comorbidities. It is important for providers to be aware of possible drug interactions, prescribing cascades, and ADEs. Medications such as anticholinergics and antipsychotics pose an increased risk for ADEs, but the regular implementation of criteria such as Beers or STOPP/START in clinical practice will minimize overprescribing and improve health outcomes. These criteria should be used to supplement the clinical judgment and expertise of providers as a mainstay of patient care in the elderly.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818-1830.
2. Qato DM, Wilder J, Schumm LP. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
3. Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992;2(4):356-367.
4. Pedros C, Formiga F, Corbella X, Arnau J. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016:72(2):219-226.
5. Maher RL Jr, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
6. Nguyen PV-Q, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016;149(3):122-124.
7. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866.
8. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137.
9. Koronkowski M, Eisenhower C, Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann Longterm Care. 2016; 24(3):37-40.
10. Peron EP, Ogbonna KC, Donohoe KL. Diabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1): 17-vii.
11. Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3): 530-541.
12. Echt MA, Samelson EJ, Hannan MT, et al. Psychotropic drug initiation or increased dosage and the acute risk of falls: a prospective cohort study of nursing home residents. BMC Geriatrics. 2013;13:19.
13. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of antipsychotics: an update. EXCLI J. 2014;13: 1163-1191.
14. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:29-40.
15. FDA. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances (2005). www. fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 28, 2017.
16. Griffin CE III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-223.
17. Flanagan N, Beizer J. Medication reconciliation and education for older adults: using the 2015 AGS Beers Criteria as a guide. Home Healthc Now. 2016;34(10): 542-549.
18. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360-372.
19. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227-2246.
20. Salbu RL, Feuer J. A closer look at the 2015 Beers criteria. J Pharm Pract. 2017;30(4):419-424.
21. Mirk A, Echt KV, Vandenberg AE, et al. Polypharmacy review of vulnerable elders: can we IMPROVE outcomes? Fed Pract. 2016;33(3):39-41.
22. Saphris [package insert]. Irvine, CA: Allergan, USA, Inc; 2017.
23. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc; 2017.
24. Zyprexa [package insert]. Indianapolis, IN: Lilly USA LLC; 2017.
25. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017.
26. Midazolam hydrochloride injection solution [package insert]. Lake Forest, IL: Hospira Inc; 2017.
27. Diazepam oral solution and Diazepam Intensol oral solution concentrate [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corp; 2016.
28. Ativan tablet [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2013.
CE/CME No: CR-1802
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients who are at the greatest risk for the effects of polypharmacy.
• Recognize which medications are most likely to cause adverse drug events (ADEs) in the elderly population.
• Understand the effects of aging on the pharmacokinetics and pharmacodynamics of medications.
• Learn strategies to reduce the risk for polypharmacy and ADEs, including use of the Beers Criteria and the STOPP/START Criteria.
FACULTY
Kelsey Barclay practices in orthopedic surgery at Stanford Medical Center in Palo Alto, California. Amy Frassetto practices in Ob-Gyn at NewYork-Presbyterian in New York City. Julie Robb practices in emergency medicine at South Nassau Communities Hospital in Oceanside, New York. Ellen D. Mandel is a Clinical Professor in the Department of PA Studies at Pace University-Lenox Hill Hospital in New York City.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through January 31, 2019.
Article begins on next page >>
Managing medications in the elderly can be complicated by the physiologic effects of aging and the prevalence of comorbidities. Consistent use of tools such as the Beers criteria and the STOPP/START criteria, as well as medication reconciliation, can reduce polypharmacy and its adverse drug effects, improving health outcomes in this population.
Older adults (those 65 and older) often have a number of comorbidities requiring pharmacologic intervention, making medication management a complicated but essential part of caring for the elderly. A recent analysis of trends in prescription drug use by community-dwelling adults found that 39% of older adults used five or more prescribed medications.1 Furthermore, about 72% of older adults also take a nonprescription medication (OTC or supplement); while OTC medication use has declined in this population in recent years, dietary supplement use has increased.2
These patients are also more susceptible to adverse drug events (ADEs)—including adverse drug reactions (ADRs)—resulting from the physiologic changes of aging. By one estimate, ADRs are about seven times more common in those older than 70 than in younger persons.3 One out of every 30 urgent hospital admissions in patients ages 65 and older is related to an ADR.4
Providers must therefore be cognizant of drug indications, dosing, and drug interactions when prescribing medications to elderly patients. Fortunately, tools and methods to avoid polypharmacy and the adverse effects of commonly prescribed medications—such as anticholinergics and psychotropic drugs—are available.
POLYPHARMACY AND PRESCRIPTION CASCADING
While there is no specific number of medications required to define polypharmacy, the term is generally used when a nonhospitalized individual is taking five or more medications.5 The more medications a patient is taking, the more at risk he or she will be for ADRs, drug interactions, and prescription cascading.
Prescription cascading begins when an ADR is thought to be a new symptom and a new drug is prescribed to control it. Ultimately, a cascade of prescriptions occurs to control avoidable ADRs, resulting in polypharmacy. As many as 57% of women older than 65 in the United States are currently prescribed five or more medications, with 12% prescribed nine or more drugs.6 Not only do these medications cause independent ADRs, but there is also increased risk for drug interactions—and potentially, additional avoidable ADRs.
The elderly population is at greater risk for ADEs because these patients are more likely to have multiple comorbidities and chronic diseases, requiring multiple therapies.7 Polypharmacy is also more dangerous in the elderly because the physiologic changes that occur during natural aging can affect both the pharmacokinetics and pharmacodynamics of medications. The absorption, distribution, metabolism, and excretion of drugs within the human body changes as a person ages, while certain drug classes can alter the way the body functions. For example, muscle mass naturally declines and the proportion of body fat to muscle increases; this change affects the distribution of drugs such as benzodiazepines or lithium.7 If the medication dosage is not corrected, the toxicity of the drug will be increased.7
Medication excretion is largely controlled by the kidneys. Renal perfusion and function decline with age, leading to a decrease in glomerular filtration rate—which requires closer monitoring of medication selection and dosing. The risk is heightened when the elderly patient becomes acutely ill. An acute decrease in kidney function results in decreased excretion of medications, leading to an increase in ADRs.7
Ultimately, the safety of many medications in the elderly patient is unknown.8 But there is a growing body of knowledge on the adverse effects of some classes of medication in this population.
COMMONLY PRESCRIBED MEDICATIONS—AND RISKS
ADEs result from medication errors, ADRs, allergic reactions, and overdoses. The incidence of ADEs—specifically ADRs and medication errors—is elevated in elderly patients who are prescribed certain classes of medications or multiple drugs simultaneously.8 Anticholinergic drugs and psychotropic drugs (specifically antipsychotics and benzodiazepines) are among the medications most commonly prescribed to elderly patients—and among the most likely to contribute to ADEs.9 Diabetes is a chronic condition whose treatment may also put elderly patients at risk for ADEs.10
Anticholinergic medications
Anticholinergic drugs—commonly prescribed for Parkinson disease, depression, urinary incontinence, pulmonary disorders, intestinal motility, and muscle spasms—competitively inhibit the binding of acetylcholine to muscarinic acetylcholine receptors.9 Because this mechanism tends to be nonselective, the adverse effects may be widespread. Central adverse effects include cognitive impairment, confusion, and delirium; peripheral adverse effects include constipation, urinary retention, dry mouth, blurred vision, peristaltic reduction, and tachycardia.9
Anticholinergic drugs are commonly prescribed to elderly patients for cardiovascular (CV) and neurologic disorders. (Medications for the former include ß-blockers, calcium channel blockers, diuretics, and ACE inhibitors; for the latter, amitriptyline, quetiapine, nortriptyline, prochlorperazine, haloperidol, and paroxetine.) An assessment of anticholinergic activity classified most neurologic medications as high activity and most CV medications as low—however, the latter are usually given in conjunction with other anticholinergic medications, increasing their ability to cause ADRs.11
In many cases, patients are prescribed anticholinergic medications to control symptoms of a disease, not to cure it—which means patients may be taking these medications for years. This cumulative exposure is called the anticholinergic burden. Many studies show that the anticholinergic burden is a predictor of cognitive and physical decline; a 2016 study of adults older than 65 who were exposed to 5 mg/d of oxybutynin for more than three years had a 23% increased risk for dementia, compared to low-risk or no exposure groups.9
In a retrospective, population-level study conducted in New Zealand, researchers assessed the anticholinergic effects of delirium, urinary retention, and constipation in 2,248 patients (65 and older) who were admitted to the hospital with at least one prescribed medication. Anticholinergic burden was found to be a significant independent predictor; patients taking five anticholinergic medications were more than three times as likely to develop an anticholinergic effect than those taking just one such medication (adjusted odds ratio, 3.21).11
Psychotropic drugs
Another often-prescribed medication group is psychotropic drugs, specifically antipsychotics and benzodiazepines, for agitation and behavioral disturbances in dementia. A year-long study of 851 patients in two long-term care nursing homes in Boston found that risk for ADRs—specifically, falls—was increased in those who had a change (initiation or dose increase) in psychotropic medication (ie, benzodiazepine, antipsychotic, or antidepressant).12
Second-generation antipsychotics, which are more commonly prescribed than first-generation agents, work on a postsynaptic blockade of brain dopamine D2 receptors and have an increased affinity for serotonin 5-HT2A receptors (see Table 1 for pharmacology of these medications).13,14 Adverse effects of these drugs include hypotension, sedation, and anticholinergic effects. Second-generation antipsychotics also carry a “black box warning” for increased risk for death in elderly patients with dementia-related psychosis.15

Benzodiazepines bind to receptors in the gamma-aminobutyric acid receptor complex, which enhances the binding of this inhibitory neurotransmitter (see Table 2 for pharmacology). Of this class of drugs, lorazepam has the highest potency, whereas midazolam and diazepam have lower potencies. Use of benzodiazepines increases risk for delirium and respiratory depression.16 
Diabetes treatment
People with diabetes have an increased risk for ADEs; this risk is elevated in older adults due to comorbidities such as peripheral neuropathy, retinopathy, coronary artery disease, and peripheral vascular disease.10 Hypoglycemic agents, such as insulin and insulin secretagogues, confer a higher risk for falls due to their hypoglycemic effect.10 Furthermore, metformin is known to increase risk for cognitive impairment in patients with diabetes.10
PREVENTING ADEs AND UNNECESSARY POLYPHARMACY
Predicting and preventing ADEs should be a health care provider’s priority when treating an elderly patient taking multiple medications—but it is often overlooked. Electronic medical records (EMRs) are helpful in preventing ADEs, specifically prescription errors, by flagging the patient’s chart when potentially problematic medications are ordered; however, this captures only a portion of ADEs occurring in this population.7
Other options to evaluate a patient for polypharmacy and possible ADRs include the Beers Criteria and the STOPP/START Criteria.17,18 Additionally, performing thorough and frequent medication reviews helps ensure that patients are prescribed essential medications to treat their comorbidities with the most opportunistic risk-benefit ratio. Patients’ medication lists across settings (eg, hospital, primary care, urgent care) can be accessed more easily, efficiently, and accurately with the integration of EMRs.
Beers Criteria
First published by Dr. Mark Beers in 1991 and endorsed by the American Geriatrics Society, the Beers Criteria identifies possible harmful effects of certain commonly prescribed medications to help guide and modify pharmacologic treatments, particularly in adults older than 65. The Beers Criteria classifies medications into three categories:
- Drugs that should be avoided or dose-adjusted
- Drugs that are potentially inappropriate in patients with certain conditions or syndromes
- Drugs that should be prescribed with caution in older adults.17
In the most recent update (2015), possible adverse effects of medications based on a patient’s hepatic or renal function, the effectiveness of the medication, and possible drug interactions were added. For example, nitrofurantoin and antiarrhythmics (eg, amiodarone and digoxin) should be avoided at a lower threshold of hepatic and renal impairment than previously recommended. The criteria suggest avoiding use of zolpidem, a nonbenzodiazepine receptor agonist, because of its elevated risk for adverse effects and minimal effectiveness in treating insomnia. More information about the 2015 criteria is available from the American Geriatrics Society (http://online library.wiley.com/doi/10.1111/jgs. 13702/full).19
The latest update also takes into account recently published evidence of increased ADEs resulting from drugs such as antipsychotics and proton pump inhibitors (PPIs).20 Antipsychotics are associated with an increased risk for morbidity and mortality, and PPIs are now recommended only for treatment duration of up to two months because of the possible increased risk for Clostridium difficile infection, as well as falls and fractures in patients older than 65.20 (PPIs indirectly reduce calcium absorption, which may lead to an increased fracture risk, particularly in postmenopausal women.20)
As with any guideline, the Beers Criteria was designed to supplement, not replace, clinical expertise and judgment. The risks and benefits of a medication should be weighed for the individual patient.
STOPP/START Criteria
Less widely used is the STOPP/START Criteria, an evidence-based set of guidelines consisting of 65 STOPP (Screening Tool of Older Person’s potentially inappropriate Prescriptions) and 22 START (Screening Tool to Alert doctors to the Right Treatment) criteria. Although they may be used individually, STOPP and START are best used together to determine the most appropriate medications for an elderly patient.
The STOPP guidelines help determine when the risks of a medication may outweigh the benefits in a given patient. STOPP includes recommendations for the appropriate length of time to use a medication; for example, PPIs should not be used for more than eight weeks (similar to the Beers recommendation) and benzodiazepines and neuroleptics for more than four weeks.18
START helps clinicians recognize potential prescribing omissions and to identify when a medication regimen should be implemented based on a patient’s history.18 Examples of START criteria include suggestions of when to initiate calcium and vitamin D supplementation for prevention of osteoporosis and when to begin statins in patients with diabetes, coronary artery disease, and cardiovascular disease.18
STOPP/START is organized by physiologic system, which allows for greater usability, and it addresses medications by class rather than specific medications. (The Beers Criteria was criticized for these reasons, as well as its limited transferability outside the United States.) When assessed in systematic reviews, the STOPP/START criteria were found to be fundamentally more sensitive than the Beers Criteria. Overall, it was concluded that the use of the STOPP/START criteria resulted in an absolute risk reduction of 21.2% to 35.7% and greatly improved the appropriateness of prescribing medication to the elderly. Its use also resulted in fewer follow-up appointments with a primary care physician (PCP).18
iPhone and Android applications such as iGeriatrics and Medstopper provide clinicians with easy access to Beers Criteria and STOPP/START Criteria, respectively.
Medication reconciliation
Medication reconciliation—in which health care providers review a patient’s medication list at hospital admission and discharge, and even at routine office visits—is an increasingly common practice, especially with the implementation of EMRs. The patient’s prescribed and OTC medications, as well as dose, route, frequency, and indication, are updated, with the goal of maintaining the most accurate list. Health care providers can utilize both the Beers Criteria and the STOPP/START criteria in their reconciliation process to help reduce polypharmacy in the elderly. It is an essential step in maintaining communication between providers and ultimately decreasing the incidence of ADEs.17
IMPROVE … continuity of care
Polypharmacy can decrease patient likelihood to adhere to the regimen, whether due to confusion or intolerance.8 Patients should be included, along with caregivers and all medical providers, in a holistic assessment of the patient’s best interests in terms of long-term care and pharmacologic treatment, since those who have a sense of control in their treatment goals and expectations often achieve a better understanding of their medical status.10
However, educating patients about their medications is time-consuming, and time is often at a premium during a typical office visit. A pilot study of 28 male veterans (ages 85 and older)—the Integrated Management and Polypharmacy Review of Vulnerable Elders (IMPROVE) project—devised a model to combat this problem.21 As an adjunct to a visit with the PCP, a clinical pharmacist trained in patient education and medication management performed face-to-face clinical consults with patients and their caregivers. The results indicated that medical management by both the PCP and the pharmacist resulted in better medication management. The pharmacist was able to spend time with the patient and caregiver, resulting in individualized instructions, education, and strategies for safe and effective medication use. The PCP remained involved by cosigning the note with the pharmacist and was available for consultation, if needed.
In IMPROVE, 79% of patients had at least one medication discontinued and 75% had one or more dosing or timing adjustments made. Potentially inappropriate medications were reduced by 14%.21 When the researchers compared the six-month period before the trial with the six-month period afterward, they found an average pharmacy cost savings of $64 per veteran per month. There was also a decreasing trend in phone calls and visits to the PCP. Cost savings were comparable to or greater than those reported for similar interventions.21 There has not been sufficient long-term follow-up to assess this method’s effects on ADEs, morbidity, and mortality, however.
CONCLUSION
Managing medications in the elderly population is difficult, and polypharmacy is common due to the prevalence of patients with comorbidities. It is important for providers to be aware of possible drug interactions, prescribing cascades, and ADEs. Medications such as anticholinergics and antipsychotics pose an increased risk for ADEs, but the regular implementation of criteria such as Beers or STOPP/START in clinical practice will minimize overprescribing and improve health outcomes. These criteria should be used to supplement the clinical judgment and expertise of providers as a mainstay of patient care in the elderly.
CE/CME No: CR-1802
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients who are at the greatest risk for the effects of polypharmacy.
• Recognize which medications are most likely to cause adverse drug events (ADEs) in the elderly population.
• Understand the effects of aging on the pharmacokinetics and pharmacodynamics of medications.
• Learn strategies to reduce the risk for polypharmacy and ADEs, including use of the Beers Criteria and the STOPP/START Criteria.
FACULTY
Kelsey Barclay practices in orthopedic surgery at Stanford Medical Center in Palo Alto, California. Amy Frassetto practices in Ob-Gyn at NewYork-Presbyterian in New York City. Julie Robb practices in emergency medicine at South Nassau Communities Hospital in Oceanside, New York. Ellen D. Mandel is a Clinical Professor in the Department of PA Studies at Pace University-Lenox Hill Hospital in New York City.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through January 31, 2019.
Article begins on next page >>
Managing medications in the elderly can be complicated by the physiologic effects of aging and the prevalence of comorbidities. Consistent use of tools such as the Beers criteria and the STOPP/START criteria, as well as medication reconciliation, can reduce polypharmacy and its adverse drug effects, improving health outcomes in this population.
Older adults (those 65 and older) often have a number of comorbidities requiring pharmacologic intervention, making medication management a complicated but essential part of caring for the elderly. A recent analysis of trends in prescription drug use by community-dwelling adults found that 39% of older adults used five or more prescribed medications.1 Furthermore, about 72% of older adults also take a nonprescription medication (OTC or supplement); while OTC medication use has declined in this population in recent years, dietary supplement use has increased.2
These patients are also more susceptible to adverse drug events (ADEs)—including adverse drug reactions (ADRs)—resulting from the physiologic changes of aging. By one estimate, ADRs are about seven times more common in those older than 70 than in younger persons.3 One out of every 30 urgent hospital admissions in patients ages 65 and older is related to an ADR.4
Providers must therefore be cognizant of drug indications, dosing, and drug interactions when prescribing medications to elderly patients. Fortunately, tools and methods to avoid polypharmacy and the adverse effects of commonly prescribed medications—such as anticholinergics and psychotropic drugs—are available.
POLYPHARMACY AND PRESCRIPTION CASCADING
While there is no specific number of medications required to define polypharmacy, the term is generally used when a nonhospitalized individual is taking five or more medications.5 The more medications a patient is taking, the more at risk he or she will be for ADRs, drug interactions, and prescription cascading.
Prescription cascading begins when an ADR is thought to be a new symptom and a new drug is prescribed to control it. Ultimately, a cascade of prescriptions occurs to control avoidable ADRs, resulting in polypharmacy. As many as 57% of women older than 65 in the United States are currently prescribed five or more medications, with 12% prescribed nine or more drugs.6 Not only do these medications cause independent ADRs, but there is also increased risk for drug interactions—and potentially, additional avoidable ADRs.
The elderly population is at greater risk for ADEs because these patients are more likely to have multiple comorbidities and chronic diseases, requiring multiple therapies.7 Polypharmacy is also more dangerous in the elderly because the physiologic changes that occur during natural aging can affect both the pharmacokinetics and pharmacodynamics of medications. The absorption, distribution, metabolism, and excretion of drugs within the human body changes as a person ages, while certain drug classes can alter the way the body functions. For example, muscle mass naturally declines and the proportion of body fat to muscle increases; this change affects the distribution of drugs such as benzodiazepines or lithium.7 If the medication dosage is not corrected, the toxicity of the drug will be increased.7
Medication excretion is largely controlled by the kidneys. Renal perfusion and function decline with age, leading to a decrease in glomerular filtration rate—which requires closer monitoring of medication selection and dosing. The risk is heightened when the elderly patient becomes acutely ill. An acute decrease in kidney function results in decreased excretion of medications, leading to an increase in ADRs.7
Ultimately, the safety of many medications in the elderly patient is unknown.8 But there is a growing body of knowledge on the adverse effects of some classes of medication in this population.
COMMONLY PRESCRIBED MEDICATIONS—AND RISKS
ADEs result from medication errors, ADRs, allergic reactions, and overdoses. The incidence of ADEs—specifically ADRs and medication errors—is elevated in elderly patients who are prescribed certain classes of medications or multiple drugs simultaneously.8 Anticholinergic drugs and psychotropic drugs (specifically antipsychotics and benzodiazepines) are among the medications most commonly prescribed to elderly patients—and among the most likely to contribute to ADEs.9 Diabetes is a chronic condition whose treatment may also put elderly patients at risk for ADEs.10
Anticholinergic medications
Anticholinergic drugs—commonly prescribed for Parkinson disease, depression, urinary incontinence, pulmonary disorders, intestinal motility, and muscle spasms—competitively inhibit the binding of acetylcholine to muscarinic acetylcholine receptors.9 Because this mechanism tends to be nonselective, the adverse effects may be widespread. Central adverse effects include cognitive impairment, confusion, and delirium; peripheral adverse effects include constipation, urinary retention, dry mouth, blurred vision, peristaltic reduction, and tachycardia.9
Anticholinergic drugs are commonly prescribed to elderly patients for cardiovascular (CV) and neurologic disorders. (Medications for the former include ß-blockers, calcium channel blockers, diuretics, and ACE inhibitors; for the latter, amitriptyline, quetiapine, nortriptyline, prochlorperazine, haloperidol, and paroxetine.) An assessment of anticholinergic activity classified most neurologic medications as high activity and most CV medications as low—however, the latter are usually given in conjunction with other anticholinergic medications, increasing their ability to cause ADRs.11
In many cases, patients are prescribed anticholinergic medications to control symptoms of a disease, not to cure it—which means patients may be taking these medications for years. This cumulative exposure is called the anticholinergic burden. Many studies show that the anticholinergic burden is a predictor of cognitive and physical decline; a 2016 study of adults older than 65 who were exposed to 5 mg/d of oxybutynin for more than three years had a 23% increased risk for dementia, compared to low-risk or no exposure groups.9
In a retrospective, population-level study conducted in New Zealand, researchers assessed the anticholinergic effects of delirium, urinary retention, and constipation in 2,248 patients (65 and older) who were admitted to the hospital with at least one prescribed medication. Anticholinergic burden was found to be a significant independent predictor; patients taking five anticholinergic medications were more than three times as likely to develop an anticholinergic effect than those taking just one such medication (adjusted odds ratio, 3.21).11
Psychotropic drugs
Another often-prescribed medication group is psychotropic drugs, specifically antipsychotics and benzodiazepines, for agitation and behavioral disturbances in dementia. A year-long study of 851 patients in two long-term care nursing homes in Boston found that risk for ADRs—specifically, falls—was increased in those who had a change (initiation or dose increase) in psychotropic medication (ie, benzodiazepine, antipsychotic, or antidepressant).12
Second-generation antipsychotics, which are more commonly prescribed than first-generation agents, work on a postsynaptic blockade of brain dopamine D2 receptors and have an increased affinity for serotonin 5-HT2A receptors (see Table 1 for pharmacology of these medications).13,14 Adverse effects of these drugs include hypotension, sedation, and anticholinergic effects. Second-generation antipsychotics also carry a “black box warning” for increased risk for death in elderly patients with dementia-related psychosis.15

Benzodiazepines bind to receptors in the gamma-aminobutyric acid receptor complex, which enhances the binding of this inhibitory neurotransmitter (see Table 2 for pharmacology). Of this class of drugs, lorazepam has the highest potency, whereas midazolam and diazepam have lower potencies. Use of benzodiazepines increases risk for delirium and respiratory depression.16 
Diabetes treatment
People with diabetes have an increased risk for ADEs; this risk is elevated in older adults due to comorbidities such as peripheral neuropathy, retinopathy, coronary artery disease, and peripheral vascular disease.10 Hypoglycemic agents, such as insulin and insulin secretagogues, confer a higher risk for falls due to their hypoglycemic effect.10 Furthermore, metformin is known to increase risk for cognitive impairment in patients with diabetes.10
PREVENTING ADEs AND UNNECESSARY POLYPHARMACY
Predicting and preventing ADEs should be a health care provider’s priority when treating an elderly patient taking multiple medications—but it is often overlooked. Electronic medical records (EMRs) are helpful in preventing ADEs, specifically prescription errors, by flagging the patient’s chart when potentially problematic medications are ordered; however, this captures only a portion of ADEs occurring in this population.7
Other options to evaluate a patient for polypharmacy and possible ADRs include the Beers Criteria and the STOPP/START Criteria.17,18 Additionally, performing thorough and frequent medication reviews helps ensure that patients are prescribed essential medications to treat their comorbidities with the most opportunistic risk-benefit ratio. Patients’ medication lists across settings (eg, hospital, primary care, urgent care) can be accessed more easily, efficiently, and accurately with the integration of EMRs.
Beers Criteria
First published by Dr. Mark Beers in 1991 and endorsed by the American Geriatrics Society, the Beers Criteria identifies possible harmful effects of certain commonly prescribed medications to help guide and modify pharmacologic treatments, particularly in adults older than 65. The Beers Criteria classifies medications into three categories:
- Drugs that should be avoided or dose-adjusted
- Drugs that are potentially inappropriate in patients with certain conditions or syndromes
- Drugs that should be prescribed with caution in older adults.17
In the most recent update (2015), possible adverse effects of medications based on a patient’s hepatic or renal function, the effectiveness of the medication, and possible drug interactions were added. For example, nitrofurantoin and antiarrhythmics (eg, amiodarone and digoxin) should be avoided at a lower threshold of hepatic and renal impairment than previously recommended. The criteria suggest avoiding use of zolpidem, a nonbenzodiazepine receptor agonist, because of its elevated risk for adverse effects and minimal effectiveness in treating insomnia. More information about the 2015 criteria is available from the American Geriatrics Society (http://online library.wiley.com/doi/10.1111/jgs. 13702/full).19
The latest update also takes into account recently published evidence of increased ADEs resulting from drugs such as antipsychotics and proton pump inhibitors (PPIs).20 Antipsychotics are associated with an increased risk for morbidity and mortality, and PPIs are now recommended only for treatment duration of up to two months because of the possible increased risk for Clostridium difficile infection, as well as falls and fractures in patients older than 65.20 (PPIs indirectly reduce calcium absorption, which may lead to an increased fracture risk, particularly in postmenopausal women.20)
As with any guideline, the Beers Criteria was designed to supplement, not replace, clinical expertise and judgment. The risks and benefits of a medication should be weighed for the individual patient.
STOPP/START Criteria
Less widely used is the STOPP/START Criteria, an evidence-based set of guidelines consisting of 65 STOPP (Screening Tool of Older Person’s potentially inappropriate Prescriptions) and 22 START (Screening Tool to Alert doctors to the Right Treatment) criteria. Although they may be used individually, STOPP and START are best used together to determine the most appropriate medications for an elderly patient.
The STOPP guidelines help determine when the risks of a medication may outweigh the benefits in a given patient. STOPP includes recommendations for the appropriate length of time to use a medication; for example, PPIs should not be used for more than eight weeks (similar to the Beers recommendation) and benzodiazepines and neuroleptics for more than four weeks.18
START helps clinicians recognize potential prescribing omissions and to identify when a medication regimen should be implemented based on a patient’s history.18 Examples of START criteria include suggestions of when to initiate calcium and vitamin D supplementation for prevention of osteoporosis and when to begin statins in patients with diabetes, coronary artery disease, and cardiovascular disease.18
STOPP/START is organized by physiologic system, which allows for greater usability, and it addresses medications by class rather than specific medications. (The Beers Criteria was criticized for these reasons, as well as its limited transferability outside the United States.) When assessed in systematic reviews, the STOPP/START criteria were found to be fundamentally more sensitive than the Beers Criteria. Overall, it was concluded that the use of the STOPP/START criteria resulted in an absolute risk reduction of 21.2% to 35.7% and greatly improved the appropriateness of prescribing medication to the elderly. Its use also resulted in fewer follow-up appointments with a primary care physician (PCP).18
iPhone and Android applications such as iGeriatrics and Medstopper provide clinicians with easy access to Beers Criteria and STOPP/START Criteria, respectively.
Medication reconciliation
Medication reconciliation—in which health care providers review a patient’s medication list at hospital admission and discharge, and even at routine office visits—is an increasingly common practice, especially with the implementation of EMRs. The patient’s prescribed and OTC medications, as well as dose, route, frequency, and indication, are updated, with the goal of maintaining the most accurate list. Health care providers can utilize both the Beers Criteria and the STOPP/START criteria in their reconciliation process to help reduce polypharmacy in the elderly. It is an essential step in maintaining communication between providers and ultimately decreasing the incidence of ADEs.17
IMPROVE … continuity of care
Polypharmacy can decrease patient likelihood to adhere to the regimen, whether due to confusion or intolerance.8 Patients should be included, along with caregivers and all medical providers, in a holistic assessment of the patient’s best interests in terms of long-term care and pharmacologic treatment, since those who have a sense of control in their treatment goals and expectations often achieve a better understanding of their medical status.10
However, educating patients about their medications is time-consuming, and time is often at a premium during a typical office visit. A pilot study of 28 male veterans (ages 85 and older)—the Integrated Management and Polypharmacy Review of Vulnerable Elders (IMPROVE) project—devised a model to combat this problem.21 As an adjunct to a visit with the PCP, a clinical pharmacist trained in patient education and medication management performed face-to-face clinical consults with patients and their caregivers. The results indicated that medical management by both the PCP and the pharmacist resulted in better medication management. The pharmacist was able to spend time with the patient and caregiver, resulting in individualized instructions, education, and strategies for safe and effective medication use. The PCP remained involved by cosigning the note with the pharmacist and was available for consultation, if needed.
In IMPROVE, 79% of patients had at least one medication discontinued and 75% had one or more dosing or timing adjustments made. Potentially inappropriate medications were reduced by 14%.21 When the researchers compared the six-month period before the trial with the six-month period afterward, they found an average pharmacy cost savings of $64 per veteran per month. There was also a decreasing trend in phone calls and visits to the PCP. Cost savings were comparable to or greater than those reported for similar interventions.21 There has not been sufficient long-term follow-up to assess this method’s effects on ADEs, morbidity, and mortality, however.
CONCLUSION
Managing medications in the elderly population is difficult, and polypharmacy is common due to the prevalence of patients with comorbidities. It is important for providers to be aware of possible drug interactions, prescribing cascades, and ADEs. Medications such as anticholinergics and antipsychotics pose an increased risk for ADEs, but the regular implementation of criteria such as Beers or STOPP/START in clinical practice will minimize overprescribing and improve health outcomes. These criteria should be used to supplement the clinical judgment and expertise of providers as a mainstay of patient care in the elderly.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818-1830.
2. Qato DM, Wilder J, Schumm LP. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
3. Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992;2(4):356-367.
4. Pedros C, Formiga F, Corbella X, Arnau J. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016:72(2):219-226.
5. Maher RL Jr, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
6. Nguyen PV-Q, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016;149(3):122-124.
7. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866.
8. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137.
9. Koronkowski M, Eisenhower C, Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann Longterm Care. 2016; 24(3):37-40.
10. Peron EP, Ogbonna KC, Donohoe KL. Diabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1): 17-vii.
11. Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3): 530-541.
12. Echt MA, Samelson EJ, Hannan MT, et al. Psychotropic drug initiation or increased dosage and the acute risk of falls: a prospective cohort study of nursing home residents. BMC Geriatrics. 2013;13:19.
13. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of antipsychotics: an update. EXCLI J. 2014;13: 1163-1191.
14. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:29-40.
15. FDA. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances (2005). www. fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 28, 2017.
16. Griffin CE III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-223.
17. Flanagan N, Beizer J. Medication reconciliation and education for older adults: using the 2015 AGS Beers Criteria as a guide. Home Healthc Now. 2016;34(10): 542-549.
18. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360-372.
19. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227-2246.
20. Salbu RL, Feuer J. A closer look at the 2015 Beers criteria. J Pharm Pract. 2017;30(4):419-424.
21. Mirk A, Echt KV, Vandenberg AE, et al. Polypharmacy review of vulnerable elders: can we IMPROVE outcomes? Fed Pract. 2016;33(3):39-41.
22. Saphris [package insert]. Irvine, CA: Allergan, USA, Inc; 2017.
23. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc; 2017.
24. Zyprexa [package insert]. Indianapolis, IN: Lilly USA LLC; 2017.
25. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017.
26. Midazolam hydrochloride injection solution [package insert]. Lake Forest, IL: Hospira Inc; 2017.
27. Diazepam oral solution and Diazepam Intensol oral solution concentrate [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corp; 2016.
28. Ativan tablet [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2013.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818-1830.
2. Qato DM, Wilder J, Schumm LP. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
3. Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992;2(4):356-367.
4. Pedros C, Formiga F, Corbella X, Arnau J. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016:72(2):219-226.
5. Maher RL Jr, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
6. Nguyen PV-Q, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016;149(3):122-124.
7. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866.
8. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137.
9. Koronkowski M, Eisenhower C, Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann Longterm Care. 2016; 24(3):37-40.
10. Peron EP, Ogbonna KC, Donohoe KL. Diabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1): 17-vii.
11. Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3): 530-541.
12. Echt MA, Samelson EJ, Hannan MT, et al. Psychotropic drug initiation or increased dosage and the acute risk of falls: a prospective cohort study of nursing home residents. BMC Geriatrics. 2013;13:19.
13. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of antipsychotics: an update. EXCLI J. 2014;13: 1163-1191.
14. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:29-40.
15. FDA. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances (2005). www. fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 28, 2017.
16. Griffin CE III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-223.
17. Flanagan N, Beizer J. Medication reconciliation and education for older adults: using the 2015 AGS Beers Criteria as a guide. Home Healthc Now. 2016;34(10): 542-549.
18. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360-372.
19. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227-2246.
20. Salbu RL, Feuer J. A closer look at the 2015 Beers criteria. J Pharm Pract. 2017;30(4):419-424.
21. Mirk A, Echt KV, Vandenberg AE, et al. Polypharmacy review of vulnerable elders: can we IMPROVE outcomes? Fed Pract. 2016;33(3):39-41.
22. Saphris [package insert]. Irvine, CA: Allergan, USA, Inc; 2017.
23. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc; 2017.
24. Zyprexa [package insert]. Indianapolis, IN: Lilly USA LLC; 2017.
25. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017.
26. Midazolam hydrochloride injection solution [package insert]. Lake Forest, IL: Hospira Inc; 2017.
27. Diazepam oral solution and Diazepam Intensol oral solution concentrate [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corp; 2016.
28. Ativan tablet [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2013.
Chronic Urticaria: It’s More Than Just Antihistamines!
CE/CME No: CR-1801
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between acute and chronic urticaria.
• List common history questions required for the diagnosis of chronic urticaria.
• Explain a stepwise plan for treatment of chronic urticaria.
• Describe serologic testing that should be ordered for chronic urticaria.
• Demonstrate knowledge of when to refer patients to a specialist for alternative treatment options.
FACULTY
Randy D. Danielsen is Professor and Dean of the Arizona School of Health Sciences, and Director of the Center for the Future of the Health Professions at A.T. Still University in Mesa, Arizona. Gabriel Ortiz practices at Breathe America El Paso in Texas and is a former AAPA liaison to the American Academy of Allergy, Asthma & Immunology (AAAAI) and National Institutes of Health/National Asthma Education and Prevention Program—Coordinating Committee. Susan Symington has practiced in allergy, asthma, and immunology for more than 10 years. She is the current AAPA liaison to theAAAAI and is President-Elect of the AAPA-Allergy, Asthma, and Immunology subspecialty organization.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through December 31, 2018.
Article begins on next page >>
The discomfort caused by an urticarial rash, along with its unpredictable course, can interfere with a patient’s sleep and work/school. Adding to the frustration of patients and providers alike, an underlying cause is seldom identified. But a stepwise treatment approach can bring relief to all.
Urticaria, often referred to as hives, is a common cutaneous disorder with a lifetime incidence between 15% and 25%.1 Urticaria is characterized by recurring pruritic wheals that arise due to allergic and nonallergic reactions to internal and external agents. The name urticaria comes from the Latin word for “nettle,” urtica, derived from the Latin word uro, meaning “to burn.”2
Urticaria can be debilitating for patients, who may complain of a burning sensation. It can last for years in some and reduces quality of life for many. Recently, more successful treatments for urticaria have emerged that can provide tremendous relief.
It is important to understand some of the ways to diagnose and treat patients in a primary care setting and also to know when referral is appropriate. This article will discuss the diagnosis, treatment, and referral process for patients with chronic urticaria.
PATHOPHYSIOLOGY
Hives most commonly arise from an immunologic reaction in the superficial skin layers that results in the release of histamine, which causes swelling, itching, and erythema. The mast cell is the major effector cell in the pathophysiology of urticaria.3 In immunologic urticaria, the antigen binds to immunoglobulin (Ig) E on the mast cell surface, causing degranulation and release of histamine, which accounts for the wheals and itching associated with the condition. Histamine binds to H1 and H2 receptors in the skin to cause arteriolar dilation, venous constriction, and increased capillary permeability, accounting for the accompanying swelling.3 Not all urticaria is mediated by IgE; it can result from systemic disease processes in the body that are immune related but not related to IgE. An example would be autoimmune urticaria.
Urticaria commonly occurs with angioedema, which is marked by a greater degree of swelling and results from mast cell activation in the deeper dermis and subcutaneous tissue. Either condition can occur independently, however. Angioedema typically affects the lips, tongue, face, pharynx, and bilateral extremities; rarely, it affects the gastrointestinal tract. Angioedema may be hereditary, but its nonhereditary causes can be similar to those of urticaria.3 For example, a patient could be severely allergic to cat dander and, when exposed to this allergic trigger, develop swelling of the lips, facial edema, and flushing.
FORMS OF URTICARIA
Urticaria can be broadly divided based on the duration of illness: less than six weeks is termed acute urticaria, and continuous or intermittent presence for six weeks or more, chronic urticaria.4
Acute urticaria may occur in any age group but is most often seen in children.1 Acute urticaria and angioedema frequently resolve within a few days, without an identified cause. An inciting cause can be identified in only about 15% to 20% of cases; the most common cause is viral infection, followed by foods, drugs, insect stings, transfusion reactions, and, rarely, contactants and inhalants (see Table 1).1,5 Acute urticaria that is not associated with angioedema or respiratory distress is usually self-limited. The condition typically resolves before extensive evaluation, including testing for possible allergic triggers, can be done. The associated skin lesions are often self-limited or can be controlled symptomatically with antihistamines and avoidance of known possible triggers.1
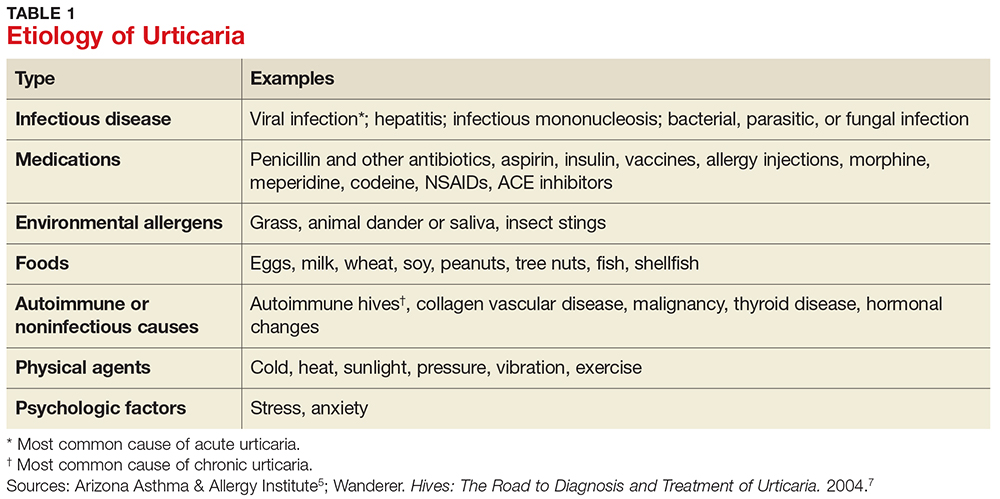
Chronic urticaria, sometimes called chronic idiopathic urticaria, is more common in adults, occurs on most days of the week, and, as noted, persists for more than six weeks with no identifiable triggers.6 It affects about 0.5% to 1% of the population (lifetime prevalence).3 Approximately 45% of patients with chronic urticaria have accompanying episodes of angioedema, and 30% to 50% have an autoimmune process involving autoantibodies against the thyroid, IgE, or the high-affinity IgE receptor (FcR1).3 The diagnosis is based primarily on clinical history and presentation; this will guide the determination of what types of diagnostic testing are necessary.
Chronic urticaria requires an extensive, but not indiscriminate, evaluation with history, physical examination, allergy testing, and laboratory testing for immune system, liver, kidney, thyroid, and collagen vascular diseases.3 Unfortunately, an identifiable cause of chronic urticaria is found in only 10% to 20% of patients; most cases are idiopathic.3,7
Several forms of chronic urticaria can be precipitated by physical stimuli, such as exercise, generalized heat, or sweating (cholinergic urticaria); localized heat (localized heat urticaria); low temperatures (cold urticaria); sun exposure (solar urticaria); water (aquagenic urticaria); and vibration.1 In another form (pressure urticaria), pressure on the skin increases histamine release, leading to the development of wheals and itching; this form is also called dermatographism, which means “write on skin” (see Figure 1). These types of urticaria should be evaluated and treated by a board-certified allergist, as there are special evaluations that can confirm the diagnosis.
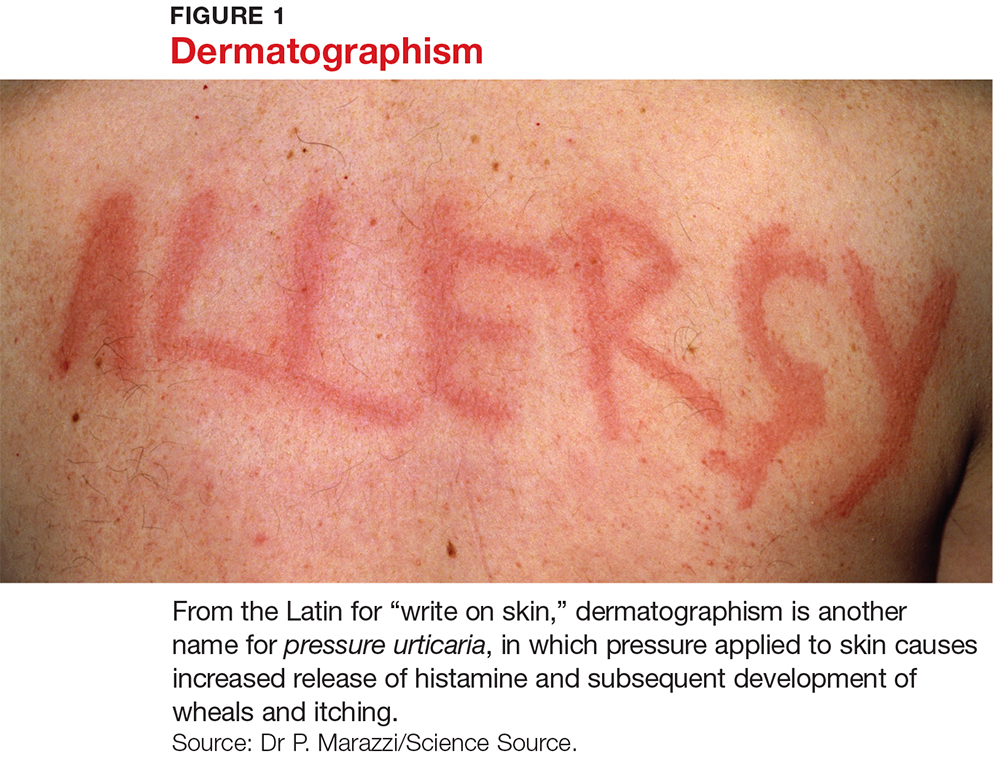
CLINICAL FEATURES
The main feature of urticaria is raised skin lesions that appear pale to pink to erythematous and most commonly are intensely pruritic (see Figure 2). These lesions range from a few millimeters to several centimeters in size and may coalesce.
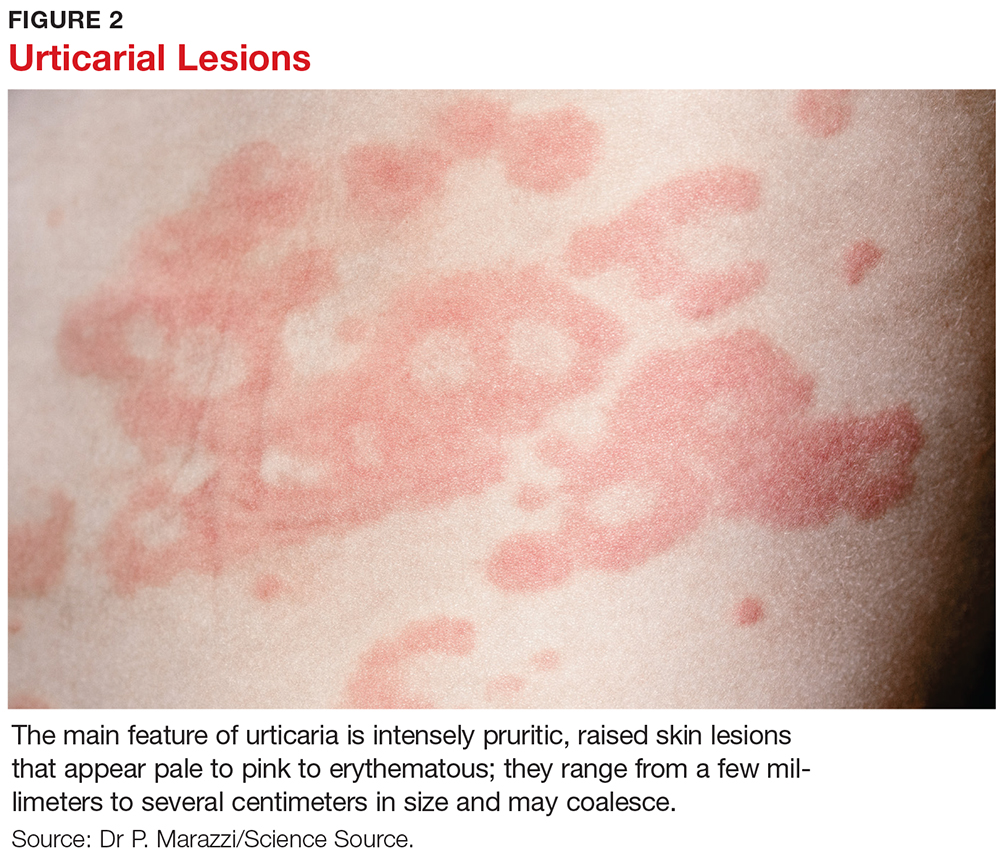
Characteristically, evanescent old lesions resolve, and new ones develop over 24 hours, usually without scarring. Scratching generally worsens dermatographism, with new urticaria produced over the scratched area. Any area of the body may be involved.
The lesions of early urticaria may vary in size and blanch when pressure is applied. An individual hive may last minutes or up to 24 hours and may reoccur intermittently on various sites on the body for an unspecified period of time.1,6
DIFFERENTIAL DIAGNOSIS
Other dermatologic conditions may be mistaken for chronic urticaria. Common rashes that may mimic it include anaphylaxis, atopic dermatitis, medication allergy or fixed drug eruption, ACE inhibitor–related angioedema, mastocytosis, contact dermatitis, autoimmune thyroid disease, bullous pemphigoid, and dermatitis herpetiformis.
Patients should be encouraged to bring pictures of the rash to the office visit, since the rash may have waned at the time of the visit and diagnosis based on the patient’s description alone can be challenging. Most rashes in the differential can be identified or eliminated through a careful history and complete physical exam. When necessary, serologic testing and skin punch biopsies can elucidate and confirm the diagnosis.
EVALUATION
History and physical examination
The medical history is the most important part of the evaluation of a patient with urticaria. The information that should be elicited and documented during the history is shown in Table 2.
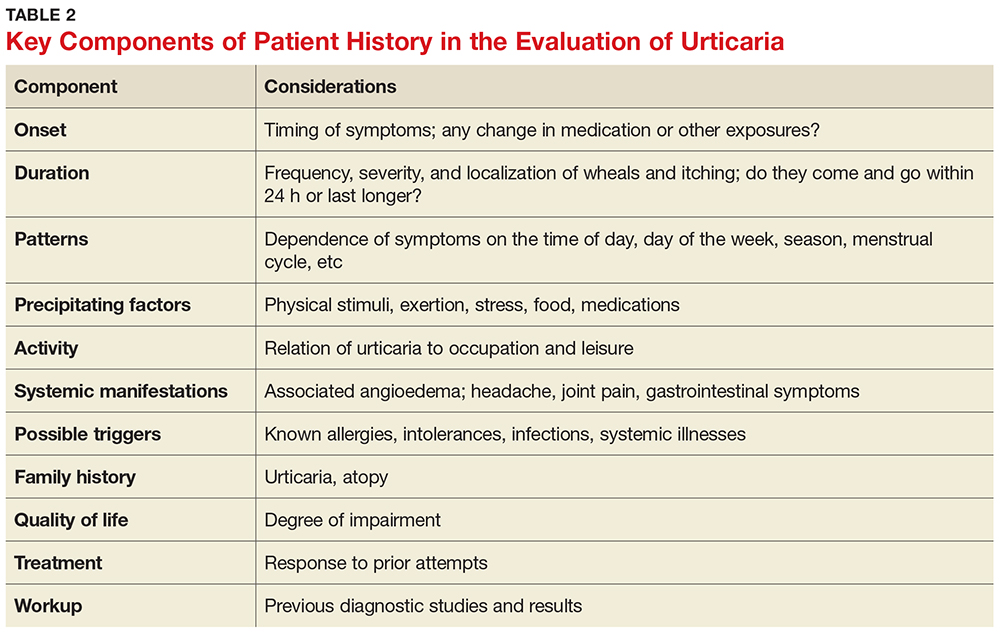
A general comprehensive physical exam should be undertaken and the findings carefully documented. As noted, it can be helpful for patients to bring in pictures of the rash if the lesions wax and wane. It is also important to assess whether the urticarial lesions blanch when palpated, since this is a characteristic feature of acute and chronic urticarial lesions (but not of those with an autoimmune, cholinergic, or vasculitic cause). Thus, blanching of the wheal is a key finding on physical exam to discriminate between possible causes.8 Lesions pigmented with purpuric areas that scar or last longer than 24 hours suggest urticarial vasculitis; other features that distinguish urticarial vasculitis from chronic urticaria are listed in Table 3.2
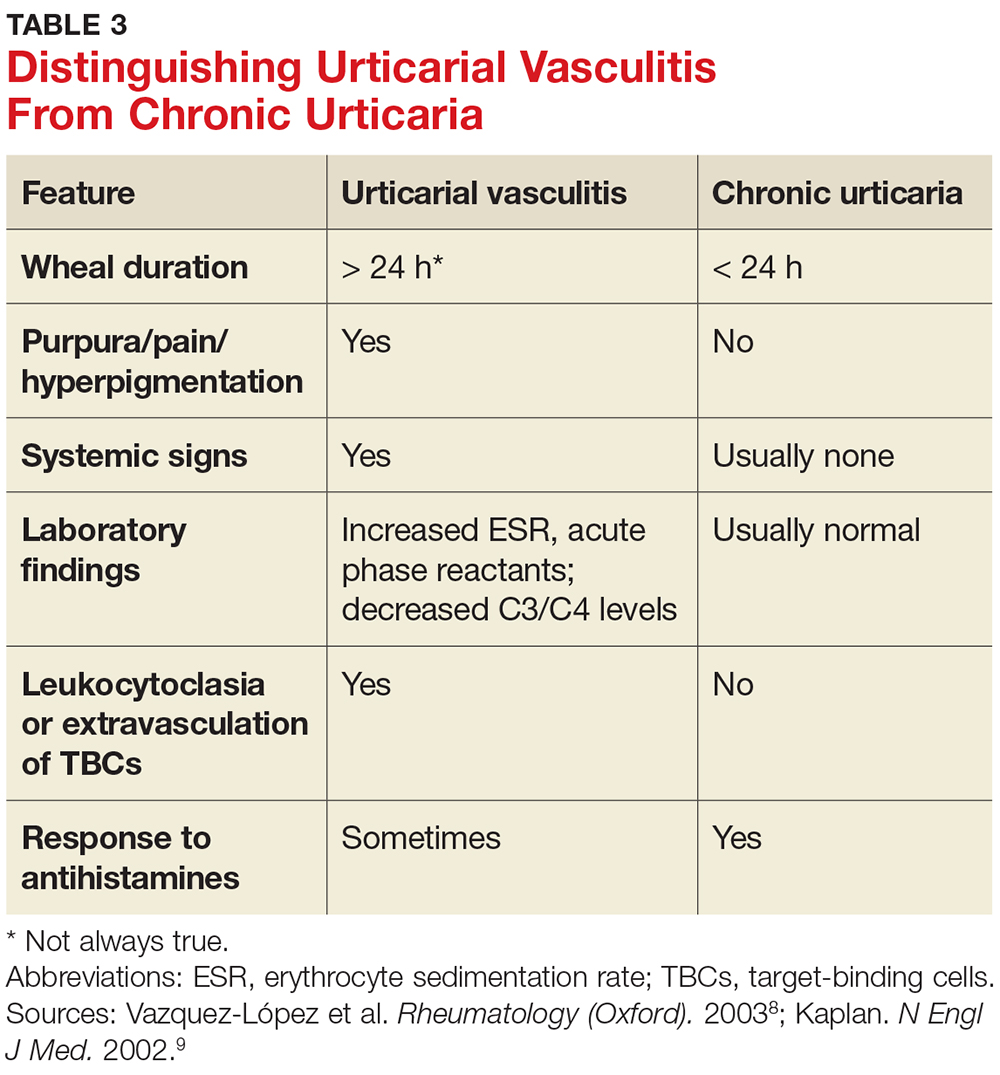
Laboratory evaluation
Although there is no consensus regarding appropriate laboratory testing, the following tests should be considered for patients with chronic urticaria after completion of a thorough history and physical exam: complete blood count (CBC) with differential; erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP); chemistry panel and hepatic panel; and thyroid-stimulating hormone, antimicrosomal antibodies, and antithyroglobulin antibodies measurements.7
While the CBC is usually within normal limits, if eosinophilia is present, a workup for an atopic disorder or parasitic infection should be considered. If the ESR/CRP results are positive, consider ordering a larger antinuclear antibody (ANA) panel. Note: The utility of performing these tests routinely for chronic urticaria patients is unclear, as studies have demonstrated that results are usually normal. But it is important to order the appropriate tests to help you rule in or out a likely diagnosis.
Additional testing may be indicated by non-IgE or possible autoimmune findings on the history and/or physical exam. This can include a functional autoantibody assay (for autoantibodies to the high-affinity IgE receptor [FcR1]); complement analysis (eg, C3, C4, CH50), especially when concerned about hereditary angioedema; stool analysis for ova and parasites; Helicobacter pylori workup (there is limited experimental evidence to recommend this, however); hepatitis B and C workup; chest radiograph and/or other imaging studies; ANA panel; rheumatoid factor; cryoglobulin levels; skin biopsy; and urinalysis.7
Local urticaria can occur following contact with allergens via an IgE-mediated mechanism. If an allergen is suspected as a possible trigger, serologic testing to assess allergen-specific IgE levels that may be contributing to the urticaria can be performed in a primary care setting. The specific IgE levels most commonly assessed are for the endemic outdoor aeroallergens (eg, pets [cat, dog], dust mites); measurement of food-specific IgE levels can be ordered if a specific allergy is a concern. Allergy skin prick testing for immediate hypersensitivity and a physical challenge test are usually performed in an allergy office by board-certified allergists.
Skin biopsy should be done on all lesions concerning for urticarial vasculitis (see Table 4).2 Biopsy is also important if the hives are painful rather than pruritic, as this may suggest a different cause. The clinician should consider more detailed lab testing and skin biopsy if urticaria does not respond to therapy as anticipated. Also, specific lab testing may be required screening for certain planned medical therapies (eg, glucose-6-phosphate dehydrogenase enzyme deficiency screening before dapsone or hydroxychloroquine therapy).3
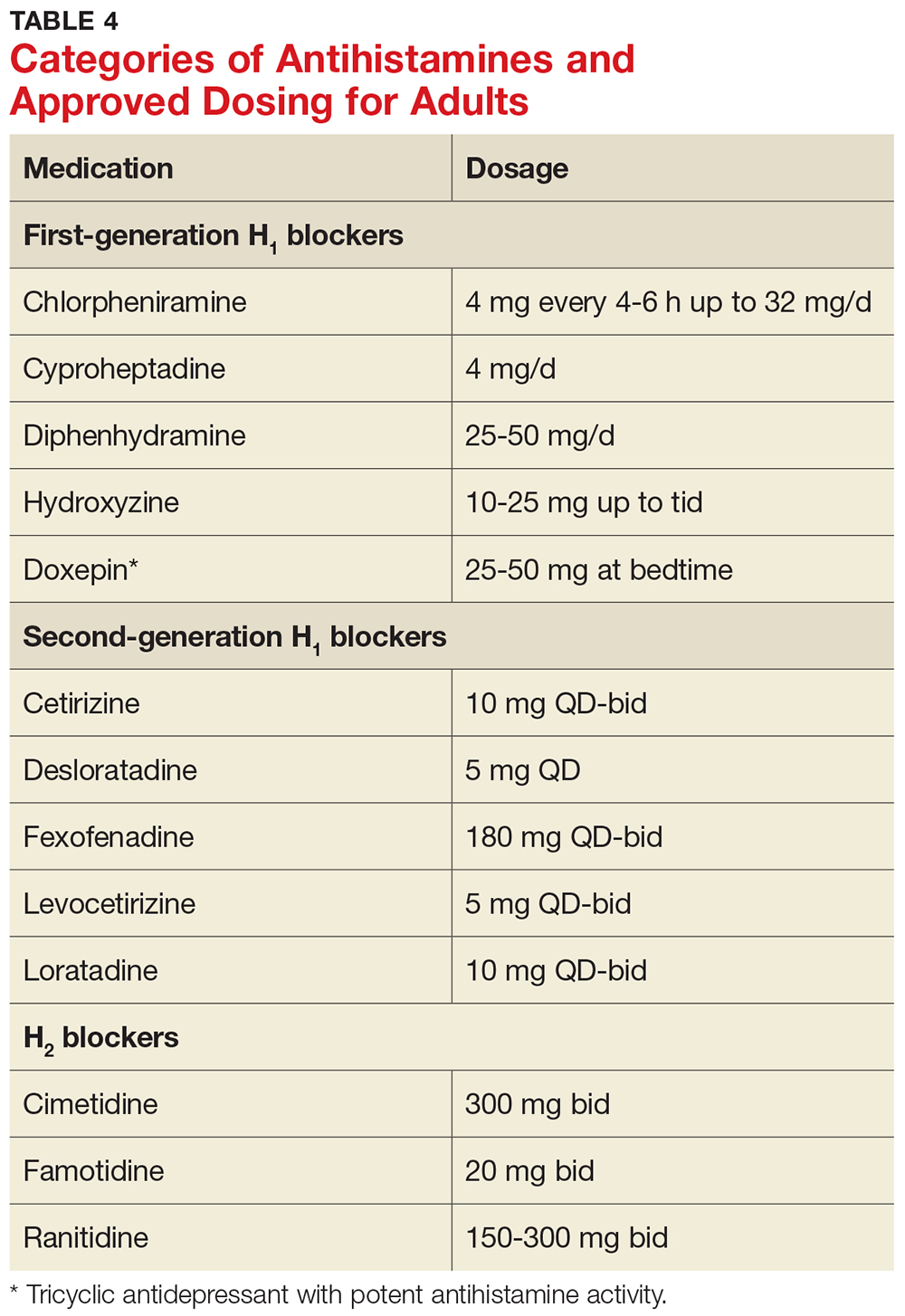
MANAGEMENT
Nonpharmacologic therapy
Treatment of the underlying cause, if identified, may be helpful and should be considered. For example, if a thyroid disorder is found on serologic testing, correcting the disorder may resolve the urticaria.9 Similarly, if a complement deficiency consistent with hereditary angioedema is detected, there are medications to correct it, which can be life-saving.3 Medications for treating hereditary angioedema are best prescribed in an allergy practice.
If triggers are discovered, the patient must be made aware of them and advised to avoid them as much as possible; however, total avoidance can be very difficult. Other common potentiating factors—such as alcohol overuse, excessive tiredness, emotional stress, hyperthermia, and use of aspirin and NSAIDs—should be avoided.10 These factors can worsen what is already triggering the urticaria and make it more difficult to treat; an example would be a patient who develops urticaria from a new household dog and is taking anti-inflammatory drugs for arthritis symptoms.
Topical agents rarely result in any improvement, and their use is therefore discouraged. In fact, high-potency corticosteroids may cause dermal atrophy.11 Also, dietary changes are not indicated for most patients with chronic urticaria, because undiscovered allergy to food or food additives is not likely to be responsible.4
Antihistamines
Antihistamines are the most commonly used pharmacologic treatment for chronic urticaria (see Table 4). H2-receptor blockers, taken in combination with first- and second-generation H1-receptor blockers, have been reported to be more efficacious than H1 antihistamines alone for the treatment of chronic urticaria.6 This added efficacy may be related to pharmacologic interactions and increased blood levels achieved with first-generation antihistamines. Increased doses of second-generation antihistamines—as high as four times the standard dose—are advocated by the 2014 Joint Task Force on Practice Parameters (JTFPP) for the diagnosis and management of acute and chronic urticaria.4
A stepwise approach to treatment is imperative. The JTFPP guidelines (available at www.allergyparameters.org) are summarized below.
Step 1: Administer a second-generation antihistamine at the standard therapeutic dose (see Table 4) and avoid triggers, NSAIDs, and other exacerbating factors.
If symptom control is not achieved in one to two weeks, move on to
Step 2: Increase therapy by one or more of the following methods: increase the dose of the second-generation antihistamine used in Step 1 (up to 4x the standard dose); add another second-generation antihistamine to the regimen; add an H2 blocker (ranitidine, famotidine, cimetidine); and/or add a leukotriene-receptor antagonist (montelukast 10 mg/d).
If these measures do not result in adequate symptom control, it’s time for
Step 3: Gradually increase the dose of H1 antihistamine(s) and discontinue any medications added in Step 2 that did not appear beneficial. Add a first-generation antihistamine (hydroxyzine, doxepin, cyproheptadine), which should be taken at bedtime due to risk for sedation.12
If symptoms are not controlled by Step 3 measures, or if the patient is unable to tolerate an increased dose of first-generation antihistamines, the urticaria is considered refractory. At this point, the clinician should consider referral to an allergy specialist for
Step 4: Add an alternative medication, such as cyclosporine (an anti-inflammatory, immunosuppressive agent) or omalizumab (a monoclonal antibody that selectively binds to IgE).
It should be noted that while the recent FDA approval of omalizumab for treatment of chronic urticaria has been life-changing for many patients, the product label does carry a black box warning about anaphylaxis. Because special monitoring is needed (and prior authorization will likely be required by the patient’s insurer), omalizumab is best prescribed in an allergy office.
It is not uncommon for patients with chronic urticaria to require multiple medications to control their symptoms. Once controlled, they will require maintenance and reevaluation on a regular basis.13
When to refer
Clinicians must know when to refer a patient with chronic urticaria to an allergist/immunologist. Referral is indicated when an underlying disorder is suspected, when symptoms are not controlled with Steps 1 to 3 of the management guidelines, or when the patient requires repeated or prolonged treatment with glucocorticoids.
Unfortunately, out of frustration on both the provider and the patient side, glucocorticoids may be started, after determining that that is “all that works” for the patient. There appears to be a limited role for glucocorticoids, so they should be avoided unless absolutely necessary (ie, if there is no response to antihistamines).
If signs and symptoms suggest urticarial vasculitis, it is prudent to consider referral to a specialist in rheumatology. Urticarial vasculitis requires a special skin punch biopsy to confirm the diagnosis.8 The biopsy procedure may be performed by a primary care provider; if the clinician is not comfortable doing so, referral to an appropriate dermatology provider is indicated.
PATIENT EDUCATION/REASSURANCE
Effective patient education is critical, because patients often experience considerable distress as the symptoms of chronic urticaria wax and wane unpredictably. It is not uncommon for patients with this condition to complain of symptoms that interfere with work, school, and sleep. Reassurance can help to alleviate frustration and anxiety. Patients should understand that the symptoms of chronic urticaria can be successfully managed in the majority of patients, and that chronic idiopathic urticaria is rarely permanent, with about 50% of patients experiencing remission within one year.6
CONCLUSION
The diagnosis of chronic urticaria is based primarily on the presentation, clinical history, and laboratory workup. Management of this chronic and uncomfortable condition requires the identification and exclusion of possible triggers, followed by effective patient education/counseling and a personalized management plan. By knowing when to suspect chronic urticaria, being familiar with the approach to evaluation and initial treatment, and knowing when referral to a specialist is indicated, primary care providers can help their patients find a path to relief.
1. Riedl MA, Ortiz G, Casillas AM. A primary care guide to managing chronic urticaria. JAAPA. 2003;16:WEB.
2. Grieve M. Nettles. http://botanical.com/botanical/mgmh/n/nettle03.html. Accessed December 19, 2017.
3. Powell RJ, Du Toit GL, Siddique N, et al; British Society for Allergy and Clinical Immunology. BSACI guidelines for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2007;37(5):631-650.
4. Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270-1277.
5. Arizona Asthma & Allergy Institute. Possible causes of hives. www.azsneeze.com/hives. Accessed December 19, 2017.
6. Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45(3):387-391.
7. Wanderer AA. Hives: The Road to Diagnosis and Treatment of Urticaria. Bozeman, MT: Anson Publishing; 2004.
8. Vazquez-López F, Maldonado-Seral C, Soler-Sánchez T, et al. Surface microscopy for discriminating between common urticaria and urticarial vasculitis. Rheumatology (Oxford). 2003;42(9):1079-1082.
9. Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346(3):175-179.
10. Yadav S, Bajaj AK. Management of difficult urticaria. Indian J Dermatol. 2009;54(3):275-279.
11. Ellingsen AR, Thestrup-Pedersen K. Treatment of chronic idiopathic urticaria with topical steroids. An open trial. Acta Derm Venereol. 1996;76(1):43-44.
12. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 pt 1):867-873.
13. Ferrer M, Bartra J, Gimenez-Arnau A, et al. Management of urticaria: not too complicated, not too simple. Clin Exp Allergy. 2015;45(4):731-743.
CE/CME No: CR-1801
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between acute and chronic urticaria.
• List common history questions required for the diagnosis of chronic urticaria.
• Explain a stepwise plan for treatment of chronic urticaria.
• Describe serologic testing that should be ordered for chronic urticaria.
• Demonstrate knowledge of when to refer patients to a specialist for alternative treatment options.
FACULTY
Randy D. Danielsen is Professor and Dean of the Arizona School of Health Sciences, and Director of the Center for the Future of the Health Professions at A.T. Still University in Mesa, Arizona. Gabriel Ortiz practices at Breathe America El Paso in Texas and is a former AAPA liaison to the American Academy of Allergy, Asthma & Immunology (AAAAI) and National Institutes of Health/National Asthma Education and Prevention Program—Coordinating Committee. Susan Symington has practiced in allergy, asthma, and immunology for more than 10 years. She is the current AAPA liaison to theAAAAI and is President-Elect of the AAPA-Allergy, Asthma, and Immunology subspecialty organization.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through December 31, 2018.
Article begins on next page >>
The discomfort caused by an urticarial rash, along with its unpredictable course, can interfere with a patient’s sleep and work/school. Adding to the frustration of patients and providers alike, an underlying cause is seldom identified. But a stepwise treatment approach can bring relief to all.
Urticaria, often referred to as hives, is a common cutaneous disorder with a lifetime incidence between 15% and 25%.1 Urticaria is characterized by recurring pruritic wheals that arise due to allergic and nonallergic reactions to internal and external agents. The name urticaria comes from the Latin word for “nettle,” urtica, derived from the Latin word uro, meaning “to burn.”2
Urticaria can be debilitating for patients, who may complain of a burning sensation. It can last for years in some and reduces quality of life for many. Recently, more successful treatments for urticaria have emerged that can provide tremendous relief.
It is important to understand some of the ways to diagnose and treat patients in a primary care setting and also to know when referral is appropriate. This article will discuss the diagnosis, treatment, and referral process for patients with chronic urticaria.
PATHOPHYSIOLOGY
Hives most commonly arise from an immunologic reaction in the superficial skin layers that results in the release of histamine, which causes swelling, itching, and erythema. The mast cell is the major effector cell in the pathophysiology of urticaria.3 In immunologic urticaria, the antigen binds to immunoglobulin (Ig) E on the mast cell surface, causing degranulation and release of histamine, which accounts for the wheals and itching associated with the condition. Histamine binds to H1 and H2 receptors in the skin to cause arteriolar dilation, venous constriction, and increased capillary permeability, accounting for the accompanying swelling.3 Not all urticaria is mediated by IgE; it can result from systemic disease processes in the body that are immune related but not related to IgE. An example would be autoimmune urticaria.
Urticaria commonly occurs with angioedema, which is marked by a greater degree of swelling and results from mast cell activation in the deeper dermis and subcutaneous tissue. Either condition can occur independently, however. Angioedema typically affects the lips, tongue, face, pharynx, and bilateral extremities; rarely, it affects the gastrointestinal tract. Angioedema may be hereditary, but its nonhereditary causes can be similar to those of urticaria.3 For example, a patient could be severely allergic to cat dander and, when exposed to this allergic trigger, develop swelling of the lips, facial edema, and flushing.
FORMS OF URTICARIA
Urticaria can be broadly divided based on the duration of illness: less than six weeks is termed acute urticaria, and continuous or intermittent presence for six weeks or more, chronic urticaria.4
Acute urticaria may occur in any age group but is most often seen in children.1 Acute urticaria and angioedema frequently resolve within a few days, without an identified cause. An inciting cause can be identified in only about 15% to 20% of cases; the most common cause is viral infection, followed by foods, drugs, insect stings, transfusion reactions, and, rarely, contactants and inhalants (see Table 1).1,5 Acute urticaria that is not associated with angioedema or respiratory distress is usually self-limited. The condition typically resolves before extensive evaluation, including testing for possible allergic triggers, can be done. The associated skin lesions are often self-limited or can be controlled symptomatically with antihistamines and avoidance of known possible triggers.1

Chronic urticaria, sometimes called chronic idiopathic urticaria, is more common in adults, occurs on most days of the week, and, as noted, persists for more than six weeks with no identifiable triggers.6 It affects about 0.5% to 1% of the population (lifetime prevalence).3 Approximately 45% of patients with chronic urticaria have accompanying episodes of angioedema, and 30% to 50% have an autoimmune process involving autoantibodies against the thyroid, IgE, or the high-affinity IgE receptor (FcR1).3 The diagnosis is based primarily on clinical history and presentation; this will guide the determination of what types of diagnostic testing are necessary.
Chronic urticaria requires an extensive, but not indiscriminate, evaluation with history, physical examination, allergy testing, and laboratory testing for immune system, liver, kidney, thyroid, and collagen vascular diseases.3 Unfortunately, an identifiable cause of chronic urticaria is found in only 10% to 20% of patients; most cases are idiopathic.3,7
Several forms of chronic urticaria can be precipitated by physical stimuli, such as exercise, generalized heat, or sweating (cholinergic urticaria); localized heat (localized heat urticaria); low temperatures (cold urticaria); sun exposure (solar urticaria); water (aquagenic urticaria); and vibration.1 In another form (pressure urticaria), pressure on the skin increases histamine release, leading to the development of wheals and itching; this form is also called dermatographism, which means “write on skin” (see Figure 1). These types of urticaria should be evaluated and treated by a board-certified allergist, as there are special evaluations that can confirm the diagnosis.

CLINICAL FEATURES
The main feature of urticaria is raised skin lesions that appear pale to pink to erythematous and most commonly are intensely pruritic (see Figure 2). These lesions range from a few millimeters to several centimeters in size and may coalesce.

Characteristically, evanescent old lesions resolve, and new ones develop over 24 hours, usually without scarring. Scratching generally worsens dermatographism, with new urticaria produced over the scratched area. Any area of the body may be involved.
The lesions of early urticaria may vary in size and blanch when pressure is applied. An individual hive may last minutes or up to 24 hours and may reoccur intermittently on various sites on the body for an unspecified period of time.1,6
DIFFERENTIAL DIAGNOSIS
Other dermatologic conditions may be mistaken for chronic urticaria. Common rashes that may mimic it include anaphylaxis, atopic dermatitis, medication allergy or fixed drug eruption, ACE inhibitor–related angioedema, mastocytosis, contact dermatitis, autoimmune thyroid disease, bullous pemphigoid, and dermatitis herpetiformis.
Patients should be encouraged to bring pictures of the rash to the office visit, since the rash may have waned at the time of the visit and diagnosis based on the patient’s description alone can be challenging. Most rashes in the differential can be identified or eliminated through a careful history and complete physical exam. When necessary, serologic testing and skin punch biopsies can elucidate and confirm the diagnosis.
EVALUATION
History and physical examination
The medical history is the most important part of the evaluation of a patient with urticaria. The information that should be elicited and documented during the history is shown in Table 2.

A general comprehensive physical exam should be undertaken and the findings carefully documented. As noted, it can be helpful for patients to bring in pictures of the rash if the lesions wax and wane. It is also important to assess whether the urticarial lesions blanch when palpated, since this is a characteristic feature of acute and chronic urticarial lesions (but not of those with an autoimmune, cholinergic, or vasculitic cause). Thus, blanching of the wheal is a key finding on physical exam to discriminate between possible causes.8 Lesions pigmented with purpuric areas that scar or last longer than 24 hours suggest urticarial vasculitis; other features that distinguish urticarial vasculitis from chronic urticaria are listed in Table 3.2

Laboratory evaluation
Although there is no consensus regarding appropriate laboratory testing, the following tests should be considered for patients with chronic urticaria after completion of a thorough history and physical exam: complete blood count (CBC) with differential; erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP); chemistry panel and hepatic panel; and thyroid-stimulating hormone, antimicrosomal antibodies, and antithyroglobulin antibodies measurements.7
While the CBC is usually within normal limits, if eosinophilia is present, a workup for an atopic disorder or parasitic infection should be considered. If the ESR/CRP results are positive, consider ordering a larger antinuclear antibody (ANA) panel. Note: The utility of performing these tests routinely for chronic urticaria patients is unclear, as studies have demonstrated that results are usually normal. But it is important to order the appropriate tests to help you rule in or out a likely diagnosis.
Additional testing may be indicated by non-IgE or possible autoimmune findings on the history and/or physical exam. This can include a functional autoantibody assay (for autoantibodies to the high-affinity IgE receptor [FcR1]); complement analysis (eg, C3, C4, CH50), especially when concerned about hereditary angioedema; stool analysis for ova and parasites; Helicobacter pylori workup (there is limited experimental evidence to recommend this, however); hepatitis B and C workup; chest radiograph and/or other imaging studies; ANA panel; rheumatoid factor; cryoglobulin levels; skin biopsy; and urinalysis.7
Local urticaria can occur following contact with allergens via an IgE-mediated mechanism. If an allergen is suspected as a possible trigger, serologic testing to assess allergen-specific IgE levels that may be contributing to the urticaria can be performed in a primary care setting. The specific IgE levels most commonly assessed are for the endemic outdoor aeroallergens (eg, pets [cat, dog], dust mites); measurement of food-specific IgE levels can be ordered if a specific allergy is a concern. Allergy skin prick testing for immediate hypersensitivity and a physical challenge test are usually performed in an allergy office by board-certified allergists.
Skin biopsy should be done on all lesions concerning for urticarial vasculitis (see Table 4).2 Biopsy is also important if the hives are painful rather than pruritic, as this may suggest a different cause. The clinician should consider more detailed lab testing and skin biopsy if urticaria does not respond to therapy as anticipated. Also, specific lab testing may be required screening for certain planned medical therapies (eg, glucose-6-phosphate dehydrogenase enzyme deficiency screening before dapsone or hydroxychloroquine therapy).3

MANAGEMENT
Nonpharmacologic therapy
Treatment of the underlying cause, if identified, may be helpful and should be considered. For example, if a thyroid disorder is found on serologic testing, correcting the disorder may resolve the urticaria.9 Similarly, if a complement deficiency consistent with hereditary angioedema is detected, there are medications to correct it, which can be life-saving.3 Medications for treating hereditary angioedema are best prescribed in an allergy practice.
If triggers are discovered, the patient must be made aware of them and advised to avoid them as much as possible; however, total avoidance can be very difficult. Other common potentiating factors—such as alcohol overuse, excessive tiredness, emotional stress, hyperthermia, and use of aspirin and NSAIDs—should be avoided.10 These factors can worsen what is already triggering the urticaria and make it more difficult to treat; an example would be a patient who develops urticaria from a new household dog and is taking anti-inflammatory drugs for arthritis symptoms.
Topical agents rarely result in any improvement, and their use is therefore discouraged. In fact, high-potency corticosteroids may cause dermal atrophy.11 Also, dietary changes are not indicated for most patients with chronic urticaria, because undiscovered allergy to food or food additives is not likely to be responsible.4
Antihistamines
Antihistamines are the most commonly used pharmacologic treatment for chronic urticaria (see Table 4). H2-receptor blockers, taken in combination with first- and second-generation H1-receptor blockers, have been reported to be more efficacious than H1 antihistamines alone for the treatment of chronic urticaria.6 This added efficacy may be related to pharmacologic interactions and increased blood levels achieved with first-generation antihistamines. Increased doses of second-generation antihistamines—as high as four times the standard dose—are advocated by the 2014 Joint Task Force on Practice Parameters (JTFPP) for the diagnosis and management of acute and chronic urticaria.4
A stepwise approach to treatment is imperative. The JTFPP guidelines (available at www.allergyparameters.org) are summarized below.
Step 1: Administer a second-generation antihistamine at the standard therapeutic dose (see Table 4) and avoid triggers, NSAIDs, and other exacerbating factors.
If symptom control is not achieved in one to two weeks, move on to
Step 2: Increase therapy by one or more of the following methods: increase the dose of the second-generation antihistamine used in Step 1 (up to 4x the standard dose); add another second-generation antihistamine to the regimen; add an H2 blocker (ranitidine, famotidine, cimetidine); and/or add a leukotriene-receptor antagonist (montelukast 10 mg/d).
If these measures do not result in adequate symptom control, it’s time for
Step 3: Gradually increase the dose of H1 antihistamine(s) and discontinue any medications added in Step 2 that did not appear beneficial. Add a first-generation antihistamine (hydroxyzine, doxepin, cyproheptadine), which should be taken at bedtime due to risk for sedation.12
If symptoms are not controlled by Step 3 measures, or if the patient is unable to tolerate an increased dose of first-generation antihistamines, the urticaria is considered refractory. At this point, the clinician should consider referral to an allergy specialist for
Step 4: Add an alternative medication, such as cyclosporine (an anti-inflammatory, immunosuppressive agent) or omalizumab (a monoclonal antibody that selectively binds to IgE).
It should be noted that while the recent FDA approval of omalizumab for treatment of chronic urticaria has been life-changing for many patients, the product label does carry a black box warning about anaphylaxis. Because special monitoring is needed (and prior authorization will likely be required by the patient’s insurer), omalizumab is best prescribed in an allergy office.
It is not uncommon for patients with chronic urticaria to require multiple medications to control their symptoms. Once controlled, they will require maintenance and reevaluation on a regular basis.13
When to refer
Clinicians must know when to refer a patient with chronic urticaria to an allergist/immunologist. Referral is indicated when an underlying disorder is suspected, when symptoms are not controlled with Steps 1 to 3 of the management guidelines, or when the patient requires repeated or prolonged treatment with glucocorticoids.
Unfortunately, out of frustration on both the provider and the patient side, glucocorticoids may be started, after determining that that is “all that works” for the patient. There appears to be a limited role for glucocorticoids, so they should be avoided unless absolutely necessary (ie, if there is no response to antihistamines).
If signs and symptoms suggest urticarial vasculitis, it is prudent to consider referral to a specialist in rheumatology. Urticarial vasculitis requires a special skin punch biopsy to confirm the diagnosis.8 The biopsy procedure may be performed by a primary care provider; if the clinician is not comfortable doing so, referral to an appropriate dermatology provider is indicated.
PATIENT EDUCATION/REASSURANCE
Effective patient education is critical, because patients often experience considerable distress as the symptoms of chronic urticaria wax and wane unpredictably. It is not uncommon for patients with this condition to complain of symptoms that interfere with work, school, and sleep. Reassurance can help to alleviate frustration and anxiety. Patients should understand that the symptoms of chronic urticaria can be successfully managed in the majority of patients, and that chronic idiopathic urticaria is rarely permanent, with about 50% of patients experiencing remission within one year.6
CONCLUSION
The diagnosis of chronic urticaria is based primarily on the presentation, clinical history, and laboratory workup. Management of this chronic and uncomfortable condition requires the identification and exclusion of possible triggers, followed by effective patient education/counseling and a personalized management plan. By knowing when to suspect chronic urticaria, being familiar with the approach to evaluation and initial treatment, and knowing when referral to a specialist is indicated, primary care providers can help their patients find a path to relief.
CE/CME No: CR-1801
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between acute and chronic urticaria.
• List common history questions required for the diagnosis of chronic urticaria.
• Explain a stepwise plan for treatment of chronic urticaria.
• Describe serologic testing that should be ordered for chronic urticaria.
• Demonstrate knowledge of when to refer patients to a specialist for alternative treatment options.
FACULTY
Randy D. Danielsen is Professor and Dean of the Arizona School of Health Sciences, and Director of the Center for the Future of the Health Professions at A.T. Still University in Mesa, Arizona. Gabriel Ortiz practices at Breathe America El Paso in Texas and is a former AAPA liaison to the American Academy of Allergy, Asthma & Immunology (AAAAI) and National Institutes of Health/National Asthma Education and Prevention Program—Coordinating Committee. Susan Symington has practiced in allergy, asthma, and immunology for more than 10 years. She is the current AAPA liaison to theAAAAI and is President-Elect of the AAPA-Allergy, Asthma, and Immunology subspecialty organization.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through December 31, 2018.
Article begins on next page >>
The discomfort caused by an urticarial rash, along with its unpredictable course, can interfere with a patient’s sleep and work/school. Adding to the frustration of patients and providers alike, an underlying cause is seldom identified. But a stepwise treatment approach can bring relief to all.
Urticaria, often referred to as hives, is a common cutaneous disorder with a lifetime incidence between 15% and 25%.1 Urticaria is characterized by recurring pruritic wheals that arise due to allergic and nonallergic reactions to internal and external agents. The name urticaria comes from the Latin word for “nettle,” urtica, derived from the Latin word uro, meaning “to burn.”2
Urticaria can be debilitating for patients, who may complain of a burning sensation. It can last for years in some and reduces quality of life for many. Recently, more successful treatments for urticaria have emerged that can provide tremendous relief.
It is important to understand some of the ways to diagnose and treat patients in a primary care setting and also to know when referral is appropriate. This article will discuss the diagnosis, treatment, and referral process for patients with chronic urticaria.
PATHOPHYSIOLOGY
Hives most commonly arise from an immunologic reaction in the superficial skin layers that results in the release of histamine, which causes swelling, itching, and erythema. The mast cell is the major effector cell in the pathophysiology of urticaria.3 In immunologic urticaria, the antigen binds to immunoglobulin (Ig) E on the mast cell surface, causing degranulation and release of histamine, which accounts for the wheals and itching associated with the condition. Histamine binds to H1 and H2 receptors in the skin to cause arteriolar dilation, venous constriction, and increased capillary permeability, accounting for the accompanying swelling.3 Not all urticaria is mediated by IgE; it can result from systemic disease processes in the body that are immune related but not related to IgE. An example would be autoimmune urticaria.
Urticaria commonly occurs with angioedema, which is marked by a greater degree of swelling and results from mast cell activation in the deeper dermis and subcutaneous tissue. Either condition can occur independently, however. Angioedema typically affects the lips, tongue, face, pharynx, and bilateral extremities; rarely, it affects the gastrointestinal tract. Angioedema may be hereditary, but its nonhereditary causes can be similar to those of urticaria.3 For example, a patient could be severely allergic to cat dander and, when exposed to this allergic trigger, develop swelling of the lips, facial edema, and flushing.
FORMS OF URTICARIA
Urticaria can be broadly divided based on the duration of illness: less than six weeks is termed acute urticaria, and continuous or intermittent presence for six weeks or more, chronic urticaria.4
Acute urticaria may occur in any age group but is most often seen in children.1 Acute urticaria and angioedema frequently resolve within a few days, without an identified cause. An inciting cause can be identified in only about 15% to 20% of cases; the most common cause is viral infection, followed by foods, drugs, insect stings, transfusion reactions, and, rarely, contactants and inhalants (see Table 1).1,5 Acute urticaria that is not associated with angioedema or respiratory distress is usually self-limited. The condition typically resolves before extensive evaluation, including testing for possible allergic triggers, can be done. The associated skin lesions are often self-limited or can be controlled symptomatically with antihistamines and avoidance of known possible triggers.1

Chronic urticaria, sometimes called chronic idiopathic urticaria, is more common in adults, occurs on most days of the week, and, as noted, persists for more than six weeks with no identifiable triggers.6 It affects about 0.5% to 1% of the population (lifetime prevalence).3 Approximately 45% of patients with chronic urticaria have accompanying episodes of angioedema, and 30% to 50% have an autoimmune process involving autoantibodies against the thyroid, IgE, or the high-affinity IgE receptor (FcR1).3 The diagnosis is based primarily on clinical history and presentation; this will guide the determination of what types of diagnostic testing are necessary.
Chronic urticaria requires an extensive, but not indiscriminate, evaluation with history, physical examination, allergy testing, and laboratory testing for immune system, liver, kidney, thyroid, and collagen vascular diseases.3 Unfortunately, an identifiable cause of chronic urticaria is found in only 10% to 20% of patients; most cases are idiopathic.3,7
Several forms of chronic urticaria can be precipitated by physical stimuli, such as exercise, generalized heat, or sweating (cholinergic urticaria); localized heat (localized heat urticaria); low temperatures (cold urticaria); sun exposure (solar urticaria); water (aquagenic urticaria); and vibration.1 In another form (pressure urticaria), pressure on the skin increases histamine release, leading to the development of wheals and itching; this form is also called dermatographism, which means “write on skin” (see Figure 1). These types of urticaria should be evaluated and treated by a board-certified allergist, as there are special evaluations that can confirm the diagnosis.

CLINICAL FEATURES
The main feature of urticaria is raised skin lesions that appear pale to pink to erythematous and most commonly are intensely pruritic (see Figure 2). These lesions range from a few millimeters to several centimeters in size and may coalesce.

Characteristically, evanescent old lesions resolve, and new ones develop over 24 hours, usually without scarring. Scratching generally worsens dermatographism, with new urticaria produced over the scratched area. Any area of the body may be involved.
The lesions of early urticaria may vary in size and blanch when pressure is applied. An individual hive may last minutes or up to 24 hours and may reoccur intermittently on various sites on the body for an unspecified period of time.1,6
DIFFERENTIAL DIAGNOSIS
Other dermatologic conditions may be mistaken for chronic urticaria. Common rashes that may mimic it include anaphylaxis, atopic dermatitis, medication allergy or fixed drug eruption, ACE inhibitor–related angioedema, mastocytosis, contact dermatitis, autoimmune thyroid disease, bullous pemphigoid, and dermatitis herpetiformis.
Patients should be encouraged to bring pictures of the rash to the office visit, since the rash may have waned at the time of the visit and diagnosis based on the patient’s description alone can be challenging. Most rashes in the differential can be identified or eliminated through a careful history and complete physical exam. When necessary, serologic testing and skin punch biopsies can elucidate and confirm the diagnosis.
EVALUATION
History and physical examination
The medical history is the most important part of the evaluation of a patient with urticaria. The information that should be elicited and documented during the history is shown in Table 2.

A general comprehensive physical exam should be undertaken and the findings carefully documented. As noted, it can be helpful for patients to bring in pictures of the rash if the lesions wax and wane. It is also important to assess whether the urticarial lesions blanch when palpated, since this is a characteristic feature of acute and chronic urticarial lesions (but not of those with an autoimmune, cholinergic, or vasculitic cause). Thus, blanching of the wheal is a key finding on physical exam to discriminate between possible causes.8 Lesions pigmented with purpuric areas that scar or last longer than 24 hours suggest urticarial vasculitis; other features that distinguish urticarial vasculitis from chronic urticaria are listed in Table 3.2

Laboratory evaluation
Although there is no consensus regarding appropriate laboratory testing, the following tests should be considered for patients with chronic urticaria after completion of a thorough history and physical exam: complete blood count (CBC) with differential; erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP); chemistry panel and hepatic panel; and thyroid-stimulating hormone, antimicrosomal antibodies, and antithyroglobulin antibodies measurements.7
While the CBC is usually within normal limits, if eosinophilia is present, a workup for an atopic disorder or parasitic infection should be considered. If the ESR/CRP results are positive, consider ordering a larger antinuclear antibody (ANA) panel. Note: The utility of performing these tests routinely for chronic urticaria patients is unclear, as studies have demonstrated that results are usually normal. But it is important to order the appropriate tests to help you rule in or out a likely diagnosis.
Additional testing may be indicated by non-IgE or possible autoimmune findings on the history and/or physical exam. This can include a functional autoantibody assay (for autoantibodies to the high-affinity IgE receptor [FcR1]); complement analysis (eg, C3, C4, CH50), especially when concerned about hereditary angioedema; stool analysis for ova and parasites; Helicobacter pylori workup (there is limited experimental evidence to recommend this, however); hepatitis B and C workup; chest radiograph and/or other imaging studies; ANA panel; rheumatoid factor; cryoglobulin levels; skin biopsy; and urinalysis.7
Local urticaria can occur following contact with allergens via an IgE-mediated mechanism. If an allergen is suspected as a possible trigger, serologic testing to assess allergen-specific IgE levels that may be contributing to the urticaria can be performed in a primary care setting. The specific IgE levels most commonly assessed are for the endemic outdoor aeroallergens (eg, pets [cat, dog], dust mites); measurement of food-specific IgE levels can be ordered if a specific allergy is a concern. Allergy skin prick testing for immediate hypersensitivity and a physical challenge test are usually performed in an allergy office by board-certified allergists.
Skin biopsy should be done on all lesions concerning for urticarial vasculitis (see Table 4).2 Biopsy is also important if the hives are painful rather than pruritic, as this may suggest a different cause. The clinician should consider more detailed lab testing and skin biopsy if urticaria does not respond to therapy as anticipated. Also, specific lab testing may be required screening for certain planned medical therapies (eg, glucose-6-phosphate dehydrogenase enzyme deficiency screening before dapsone or hydroxychloroquine therapy).3

MANAGEMENT
Nonpharmacologic therapy
Treatment of the underlying cause, if identified, may be helpful and should be considered. For example, if a thyroid disorder is found on serologic testing, correcting the disorder may resolve the urticaria.9 Similarly, if a complement deficiency consistent with hereditary angioedema is detected, there are medications to correct it, which can be life-saving.3 Medications for treating hereditary angioedema are best prescribed in an allergy practice.
If triggers are discovered, the patient must be made aware of them and advised to avoid them as much as possible; however, total avoidance can be very difficult. Other common potentiating factors—such as alcohol overuse, excessive tiredness, emotional stress, hyperthermia, and use of aspirin and NSAIDs—should be avoided.10 These factors can worsen what is already triggering the urticaria and make it more difficult to treat; an example would be a patient who develops urticaria from a new household dog and is taking anti-inflammatory drugs for arthritis symptoms.
Topical agents rarely result in any improvement, and their use is therefore discouraged. In fact, high-potency corticosteroids may cause dermal atrophy.11 Also, dietary changes are not indicated for most patients with chronic urticaria, because undiscovered allergy to food or food additives is not likely to be responsible.4
Antihistamines
Antihistamines are the most commonly used pharmacologic treatment for chronic urticaria (see Table 4). H2-receptor blockers, taken in combination with first- and second-generation H1-receptor blockers, have been reported to be more efficacious than H1 antihistamines alone for the treatment of chronic urticaria.6 This added efficacy may be related to pharmacologic interactions and increased blood levels achieved with first-generation antihistamines. Increased doses of second-generation antihistamines—as high as four times the standard dose—are advocated by the 2014 Joint Task Force on Practice Parameters (JTFPP) for the diagnosis and management of acute and chronic urticaria.4
A stepwise approach to treatment is imperative. The JTFPP guidelines (available at www.allergyparameters.org) are summarized below.
Step 1: Administer a second-generation antihistamine at the standard therapeutic dose (see Table 4) and avoid triggers, NSAIDs, and other exacerbating factors.
If symptom control is not achieved in one to two weeks, move on to
Step 2: Increase therapy by one or more of the following methods: increase the dose of the second-generation antihistamine used in Step 1 (up to 4x the standard dose); add another second-generation antihistamine to the regimen; add an H2 blocker (ranitidine, famotidine, cimetidine); and/or add a leukotriene-receptor antagonist (montelukast 10 mg/d).
If these measures do not result in adequate symptom control, it’s time for
Step 3: Gradually increase the dose of H1 antihistamine(s) and discontinue any medications added in Step 2 that did not appear beneficial. Add a first-generation antihistamine (hydroxyzine, doxepin, cyproheptadine), which should be taken at bedtime due to risk for sedation.12
If symptoms are not controlled by Step 3 measures, or if the patient is unable to tolerate an increased dose of first-generation antihistamines, the urticaria is considered refractory. At this point, the clinician should consider referral to an allergy specialist for
Step 4: Add an alternative medication, such as cyclosporine (an anti-inflammatory, immunosuppressive agent) or omalizumab (a monoclonal antibody that selectively binds to IgE).
It should be noted that while the recent FDA approval of omalizumab for treatment of chronic urticaria has been life-changing for many patients, the product label does carry a black box warning about anaphylaxis. Because special monitoring is needed (and prior authorization will likely be required by the patient’s insurer), omalizumab is best prescribed in an allergy office.
It is not uncommon for patients with chronic urticaria to require multiple medications to control their symptoms. Once controlled, they will require maintenance and reevaluation on a regular basis.13
When to refer
Clinicians must know when to refer a patient with chronic urticaria to an allergist/immunologist. Referral is indicated when an underlying disorder is suspected, when symptoms are not controlled with Steps 1 to 3 of the management guidelines, or when the patient requires repeated or prolonged treatment with glucocorticoids.
Unfortunately, out of frustration on both the provider and the patient side, glucocorticoids may be started, after determining that that is “all that works” for the patient. There appears to be a limited role for glucocorticoids, so they should be avoided unless absolutely necessary (ie, if there is no response to antihistamines).
If signs and symptoms suggest urticarial vasculitis, it is prudent to consider referral to a specialist in rheumatology. Urticarial vasculitis requires a special skin punch biopsy to confirm the diagnosis.8 The biopsy procedure may be performed by a primary care provider; if the clinician is not comfortable doing so, referral to an appropriate dermatology provider is indicated.
PATIENT EDUCATION/REASSURANCE
Effective patient education is critical, because patients often experience considerable distress as the symptoms of chronic urticaria wax and wane unpredictably. It is not uncommon for patients with this condition to complain of symptoms that interfere with work, school, and sleep. Reassurance can help to alleviate frustration and anxiety. Patients should understand that the symptoms of chronic urticaria can be successfully managed in the majority of patients, and that chronic idiopathic urticaria is rarely permanent, with about 50% of patients experiencing remission within one year.6
CONCLUSION
The diagnosis of chronic urticaria is based primarily on the presentation, clinical history, and laboratory workup. Management of this chronic and uncomfortable condition requires the identification and exclusion of possible triggers, followed by effective patient education/counseling and a personalized management plan. By knowing when to suspect chronic urticaria, being familiar with the approach to evaluation and initial treatment, and knowing when referral to a specialist is indicated, primary care providers can help their patients find a path to relief.
1. Riedl MA, Ortiz G, Casillas AM. A primary care guide to managing chronic urticaria. JAAPA. 2003;16:WEB.
2. Grieve M. Nettles. http://botanical.com/botanical/mgmh/n/nettle03.html. Accessed December 19, 2017.
3. Powell RJ, Du Toit GL, Siddique N, et al; British Society for Allergy and Clinical Immunology. BSACI guidelines for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2007;37(5):631-650.
4. Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270-1277.
5. Arizona Asthma & Allergy Institute. Possible causes of hives. www.azsneeze.com/hives. Accessed December 19, 2017.
6. Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45(3):387-391.
7. Wanderer AA. Hives: The Road to Diagnosis and Treatment of Urticaria. Bozeman, MT: Anson Publishing; 2004.
8. Vazquez-López F, Maldonado-Seral C, Soler-Sánchez T, et al. Surface microscopy for discriminating between common urticaria and urticarial vasculitis. Rheumatology (Oxford). 2003;42(9):1079-1082.
9. Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346(3):175-179.
10. Yadav S, Bajaj AK. Management of difficult urticaria. Indian J Dermatol. 2009;54(3):275-279.
11. Ellingsen AR, Thestrup-Pedersen K. Treatment of chronic idiopathic urticaria with topical steroids. An open trial. Acta Derm Venereol. 1996;76(1):43-44.
12. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 pt 1):867-873.
13. Ferrer M, Bartra J, Gimenez-Arnau A, et al. Management of urticaria: not too complicated, not too simple. Clin Exp Allergy. 2015;45(4):731-743.
1. Riedl MA, Ortiz G, Casillas AM. A primary care guide to managing chronic urticaria. JAAPA. 2003;16:WEB.
2. Grieve M. Nettles. http://botanical.com/botanical/mgmh/n/nettle03.html. Accessed December 19, 2017.
3. Powell RJ, Du Toit GL, Siddique N, et al; British Society for Allergy and Clinical Immunology. BSACI guidelines for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2007;37(5):631-650.
4. Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270-1277.
5. Arizona Asthma & Allergy Institute. Possible causes of hives. www.azsneeze.com/hives. Accessed December 19, 2017.
6. Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45(3):387-391.
7. Wanderer AA. Hives: The Road to Diagnosis and Treatment of Urticaria. Bozeman, MT: Anson Publishing; 2004.
8. Vazquez-López F, Maldonado-Seral C, Soler-Sánchez T, et al. Surface microscopy for discriminating between common urticaria and urticarial vasculitis. Rheumatology (Oxford). 2003;42(9):1079-1082.
9. Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346(3):175-179.
10. Yadav S, Bajaj AK. Management of difficult urticaria. Indian J Dermatol. 2009;54(3):275-279.
11. Ellingsen AR, Thestrup-Pedersen K. Treatment of chronic idiopathic urticaria with topical steroids. An open trial. Acta Derm Venereol. 1996;76(1):43-44.
12. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 pt 1):867-873.
13. Ferrer M, Bartra J, Gimenez-Arnau A, et al. Management of urticaria: not too complicated, not too simple. Clin Exp Allergy. 2015;45(4):731-743.
December 2017: Click for Credit
Here are 5 articles in the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. When Is It Really Recurrent Strep Throat?
To take the posttest, go to: http://bit.ly/2lHFh8i
Expires September 21, 2018
2. Revised Bethesda System Resets Thyroid Malignancy Risks
To take the posttest, go to: http://bit.ly/2iSLOvM
Expires August 10, 2018
3. Tips for Avoiding Potentially Dangerous Patients
To take the posttest, go to: http://bit.ly/2lH1Fi7
Expires August 10, 2018
4. Study Findings Support Uncapping MELD Score
To take the posttest, go to: http://bit.ly/2xOA7sI
Expires September 12, 2018
5. 'Motivational Pharmacotherapy' Engages Latino Patients With Depression
To take the posttest, go to: http://bit.ly/2zs2ly4
Expires August 14, 2018
Here are 5 articles in the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. When Is It Really Recurrent Strep Throat?
To take the posttest, go to: http://bit.ly/2lHFh8i
Expires September 21, 2018
2. Revised Bethesda System Resets Thyroid Malignancy Risks
To take the posttest, go to: http://bit.ly/2iSLOvM
Expires August 10, 2018
3. Tips for Avoiding Potentially Dangerous Patients
To take the posttest, go to: http://bit.ly/2lH1Fi7
Expires August 10, 2018
4. Study Findings Support Uncapping MELD Score
To take the posttest, go to: http://bit.ly/2xOA7sI
Expires September 12, 2018
5. 'Motivational Pharmacotherapy' Engages Latino Patients With Depression
To take the posttest, go to: http://bit.ly/2zs2ly4
Expires August 14, 2018
Here are 5 articles in the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. When Is It Really Recurrent Strep Throat?
To take the posttest, go to: http://bit.ly/2lHFh8i
Expires September 21, 2018
2. Revised Bethesda System Resets Thyroid Malignancy Risks
To take the posttest, go to: http://bit.ly/2iSLOvM
Expires August 10, 2018
3. Tips for Avoiding Potentially Dangerous Patients
To take the posttest, go to: http://bit.ly/2lH1Fi7
Expires August 10, 2018
4. Study Findings Support Uncapping MELD Score
To take the posttest, go to: http://bit.ly/2xOA7sI
Expires September 12, 2018
5. 'Motivational Pharmacotherapy' Engages Latino Patients With Depression
To take the posttest, go to: http://bit.ly/2zs2ly4
Expires August 14, 2018
Anorectal Evaluations: Diagnosing & Treating Benign Conditions
CE/CME No: CR-1711
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand basic anorectal anatomy and how to perform a thorough anorectal exam.
• Describe the physical exam findings of common benign anorectal conditions.
• Discuss the different treatment options for benign anorectal conditions.
• Differentiate between common benign anorectal symptoms and red flags that should prompt referral to a colorectal specialist.
FACULTY
Priscilla Marsicovetere is an Assistant Professor of Medical Education and of Surgery at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire; Program Director for the Franklin Pierce University PA Program in Lebanon, New Hampshire; and practices with Emergency Services of New England at Springfield Hospital in Vermont. Srinivas Joga Ivatury is an Assistant Professor of Surgery at the Geisel School of Medicine at Dartmouth and practices in the Department of Surgery at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through October 31, 2018.
Article begins on next page >>
Anorectal pain and discomfort can stem from several possible etiologies, most of which are benign. As such, many patients with anorectal complaints who present in the primary care setting can be adequately evaluated, diagnosed, and treated without referral to a colorectal specialist. However, the clinician must be able to differentiate between common benign anorectal symptoms and red flags that should prompt referral.
Anorectal disorders are common in the United States and result in numerous primary care visits each year. Presentations range from pain and itching to bleeding and lesions. Common anorectal conditions include hemorrhoids, perianal skin tags, fissures, pruritus ani, perianal abscess, and condyloma. Most are benign and can be managed in the primary care setting.
Before a provider can competently diagnose and treat anorectal conditions, however, a comprehensive history and physical examination must be conducted. Grucela and colleagues documented that physicians’ diagnostic accuracy with anorectal conditions is about 50%—highlighting the need for providers to become more familiar with the history and clinical elements associated with anorectal complaints.1
This article reviews the assessment of the anorectum, diagnosis of common disorders and their recommended treatments, and red flags for referral to a colorectal specialist.
ANORECTAL ANATOMY
The beginning of the anal canal is demarcated by its moist, hairless appearance. Just inside the anal opening are two palpable circular muscles, the internal and external anal sphincters, separated by an intersphincteric groove. The sphincters are firmly closed in the resting state, which helps maintain continence.
The anal canal is generally 3 to 4 cm long and ends at the dentate line, a series of crypts in the anal mucosa.2 The crypts are openings into the anal glands, which are mucus-secreting structures in the anus. The dentate line is easily identified on anoscopy as a discrete change in the appearance of the mucosa. The dentate line is an important landmark because it delineates the boundary between somatic and visceral nerve supplies.3 Tissue proximal to the dentate line is innervated by visceral nerves and is insensate, and thus usually not a cause of pain; tissue distal to the dentate line, however, is highly innervated by somatic nerves and can be intensely painful.2
The anorectal canal is lined by three fibrovascular cushions, located in the left lateral, right posterior, and right anterior positions.4 Inside each cushion is a venous structure, called a hemorrhoid, which allows the cushion to enlarge and help maintain continence.5
Proximal to the anus is the rectum, the 12- to 15-cm long terminus of the colon. Anorectal examination in the primary care setting will typically not progress beyond the last 2 to 3 cm of the rectum.
TAKING THE HISTORY
A thorough history will provide clues about potential underlying anorectal pathology. Patients may not be forthcoming about symptoms due to embarrassment, fear of a cancer diagnosis, or cultural customs or habits. A thorough history should elicit information about all of the patient’s symptoms (see Table 1), including bleeding, change in bowel habits, and unintended weight loss.

PHYSICAL EXAM
Positioning the patient
Undergoing an anorectal examination can be embarrassing, whether it be from exposure of sensitive body parts or the less-than-desirable prone jackknife positioning. Patients often have preconceived notions that the exam will be humiliating and/or painful. Care should be taken to minimize any embarrassment and discomfort.
Positioning of the patient is a matter of provider preference. Options include the left lateral decubitus, prone jackknife, or lithotomy positions.
Positioning should always be done with draping. Regardless of position, ensure the draping exposes only the perineum. This can be achieved by encircling the patient’s bare bottom with a plain white sheet that exposes only the anus and surrounding skin, keeping the lower back, lateral buttocks, and thighs covered.
Interestingly, data on patient preference for positioning during anorectal exams are limited. In a 2009 study of 178 patients undergoing anorectal exam, more than half of patients (up to 71.4%) expecting to or having already had a proctologic exam reported that no specific type of positioning (eg, Sims, lithotomy with lifted legs, knee-chest, knee-chest with patient’s body bent forward) was most embarrassing to them.6 The report revealed that while most patients would favor the Sims position if they had a choice, they deferred to their examiner to choose the position that seemed most suitable to get a reliable diagnosis.6
Inspection of the perineum
Once the patient is properly positioned and draped, inspection of the perineum can occur. Begin by gently spreading the buttocks. Describe any abnormality seen (eg, ulcer, lesion, dermatitis, prolapsing tissue, or blood), including size, color, and location.
A common pitfall is to describe the location of abnormalities using a clock face, such as “at 4 o’clock.” This is misleading and should be avoided, because depending on patient position, the clock face can point to different locations (eg, if the patient is in the lithotomy versus prone jackknife position).
A better approach is to divide the perianal area into four anatomic quadrants: right anterior, right posterior, left anterior, left posterior. Using this schematic, the patient's position is irrelevant, and accurate documentation of lesion location is assured.
Digital rectal exam
After visual inspection of the perianal skin, a digital rectal exam (DRE) should be performed. Slowly insert a gloved, lubricated index finger into the anus and lower rectum. Note the tone of the anus at rest (eg, excessively tight vs lax). Palpate the circumference of the anus, sweeping side to side while assessing for any tenderness, mass, or induration—if present, note the anatomic quadrant. If a mass is felt, note whether it is firm or soft, fixed or mobile, and broad-based or pedunculated. When the lubricated finger is removed from the anus, note whether blood is present.
Anoscopy
After DRE, visually inspect the anorectum. The instrument used varies from anoscope to rigid proctoscope to flexible sigmoidoscope. In a primary care setting, the most likely available instrument is an anoscope. The average anoscope is about 7 cm long and 2 cm in diameter, with a beveled tip and an obturator (see Figure 1), and allows a 360° view of the anal canal.7
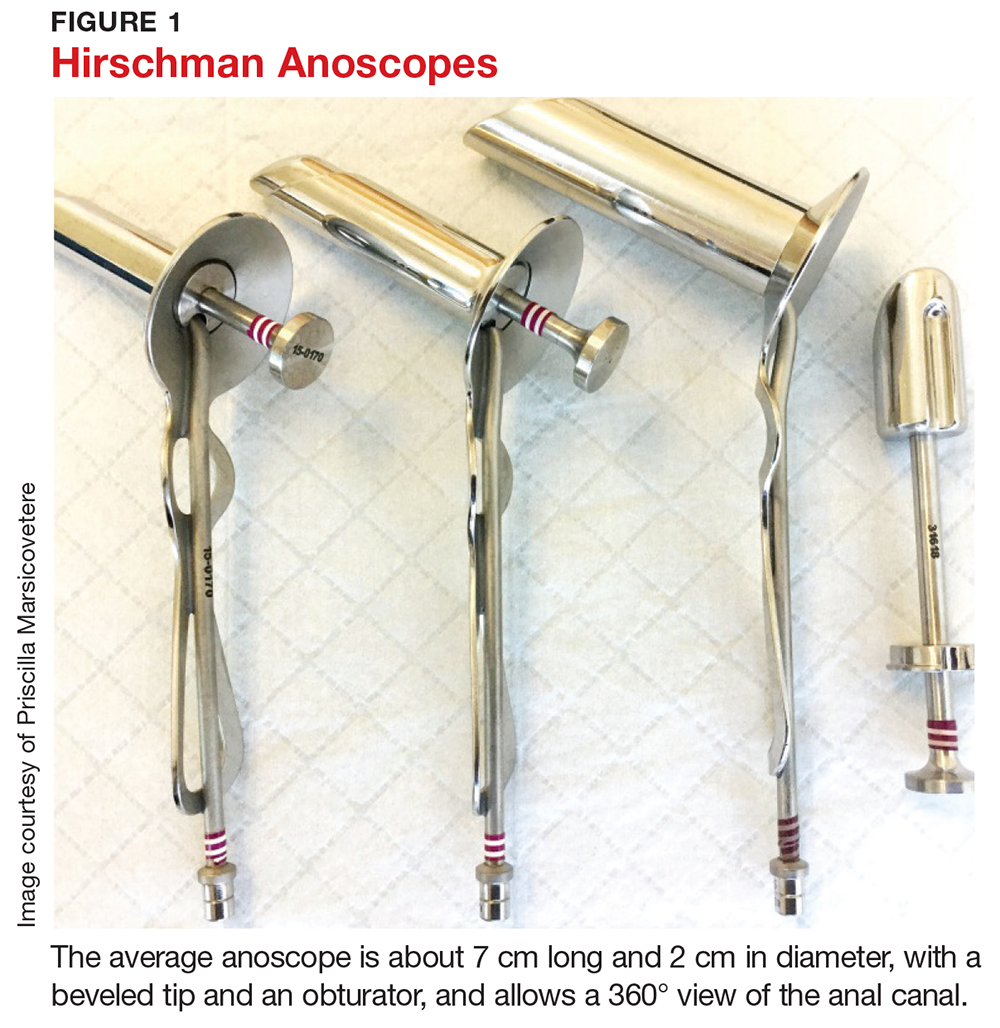
Examination of the anal canal is accomplished by dividing the canal into the four anatomic quadrants described earlier and inserting the lubricated anoscope for inspection of each of the four quadrants. Observe the rectal mucosa and the anus as the scope is slowly withdrawn. If abnormalities are seen, note the location, size, shape, and any other descriptive features.
It is not necessary to perform a Hemoccult test after examination of the anorectum, as the presence of minor blood may be the direct result of the exam itself and thus provides no useful information to the examiner.
COMMON PATHOLOGIES
Once the history and physical exam are complete, a differential diagnosis can be formulated. Most anorectal disorders are benign conditions that pose no immediate health threat and can be managed in the primary care setting. Others, however, can be more serious and should prompt referral to a colorectal specialist for further evaluation. Knowing the difference can spare a patient unnecessary anxiety and referral; it can also lead to prompt, lifesaving interventions if red flags are recognized.
Hemorrhoids
Hemorrhoids are a common anorectal complaint.8,9 It is estimated that up to 75% of the population will experience symptoms of hemorrhoids during their lifetime.5,8 Whether internal or external, in their normal, nonpathologic, quiescent state, hemorrhoids are asymptomatic. Hemorrhoids become symptomatic when the supporting structures of hemorrhoidal tissue (ie, the anal cushions) deteriorate, resulting in venous dilation, inflammation, and thrombosis, which in turn lead to swelling, bright red bleeding, and/or prolapse.2,10 The most common causes of hemorrhoidal disease are chronic constipation and prolonged straining with bowel movements, though chronic diarrhea and pregnancy have also been identified as risk factors.2,8,11
External hemorrhoids, which are located distal to the dentate line, are typically only visible when they become thrombosed or swollen. In this state, they may manifest as acute-onset, exquisitely painful, large, purple-to-blue bulges at the anal outlet (see Figure 2). The number and size of the lesions can vary. The patient may report pain when sitting or wiping, as well as bleeding from the lesion.12,13 The pain is typically severe in the first couple of days, then slowly starts to subside.2,12
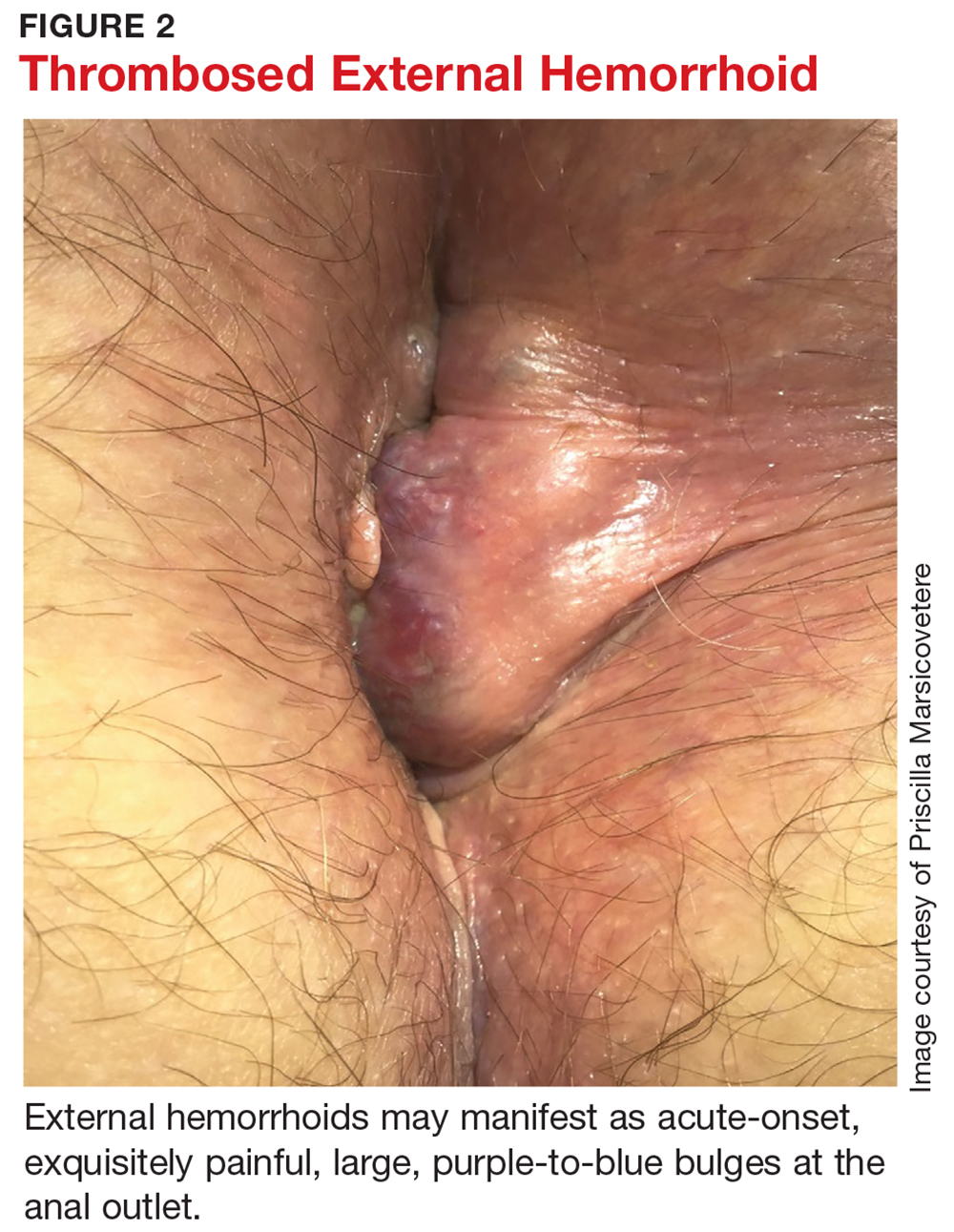
For internal hemorrhoids, which are located proximal to the dentate line, the main symptom is usually painless bright red blood per rectum.8,11,12 Patients may also report a sensation of rectal fullness or experience prolapse of the hemorrhoid through the anus. Prolapse typically occurs with defecation; in more severe cases, it can also occur between bowel movements, usually with any activity that increases intra-abdominal pressure (eg, coughing, heavy lifting, pregnancy, portal hypertension). The prolapse may reduce spontaneously, or may have to be manually reduced. If it cannot be reduced, there is a risk for incarceration or strangulation, potentially leading to gangrene.
The presence of bleeding and/or prolapse determines the classification of internal hemorrhoids (see Table 2). Dietary and lifestyle modification are used in the management of all grades of hemorrhoids. In addition, for grade 1 and 2 lesions, topical medication (eg, anti-inflammatory cream) can be used, whereas grade 3 (and selected grade 2) lesions respond well to rubber band ligation. Given the severity of grade 4 lesions, surgical intervention (eg, hemorrhoidectomy) is usually indicated.10
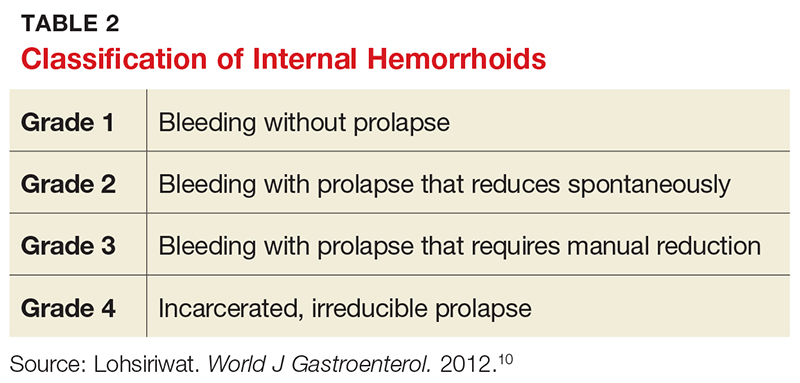
About a third of patients with symptomatic hemorrhoids seek clinical treatment.14 Most are hemodynamically stable and require no imaging and usually no labs (unless anemia is suspected).2 Management depends on the location and degree of symptoms (eg, internal vs external, prolapse, or thrombosis). In the event of an acutely thrombosed external hemorrhoid, clot excision for pain relief is appropriate if symptoms have been present for less than 48 to 72 hours; after that amount of time, the pain from the procedure will likely exceed the degree of relief provided, and conservative management should instead be recommended.2,8,11
Firstline treatment consists of lifestyle modification with a high-fiber diet and daily fiber supplement to ensure stool is soft and easy to pass.2,8 A meta-analysis of seven clinical trials with a total of 378 patients with hemorrhoids showed that fiber supplementation resulted in a 50% decrease in bleeding risk from internal hemorrhoids.15
Adequate hydration, preferably with noncaffeinated liquids, is also recommended. This will prevent constipation and the need to strain or spend excessive time on the toilet. Sitz baths can help alleviate pain and discomfort.
Several OTC topical medications are marketed for hemorrhoid relief. Many of these preparations contain steroids for their anti-inflammatory effects or astringents to address skin irritation that can result from anal leakage if prolapsing hemorrhoids prevent the anal outlet from closing. Steroid use should be limited to five to seven days, due to atrophic effects on the skin. While OTC preparations may temporarily alleviate discomfort, they will not address the underlying cause of symptoms.
Indications for referral to a colorectal specialist for symptomatic hemorrhoids include failure to improve with conservative management, persistent patient discomfort, and prolapse, as these indicate potential need for more invasive treatment.
Perianal skin tags
Perianal skin tags, while a nuisance, are not pathologic in most instances and pose no threat to health. They are an outgrowth of normal skin, appearing as loose, flesh-colored perianal tissues (see Figure 3). Tags range in size from a few millimeters to a centimeter long and can occur alone or in multiples.
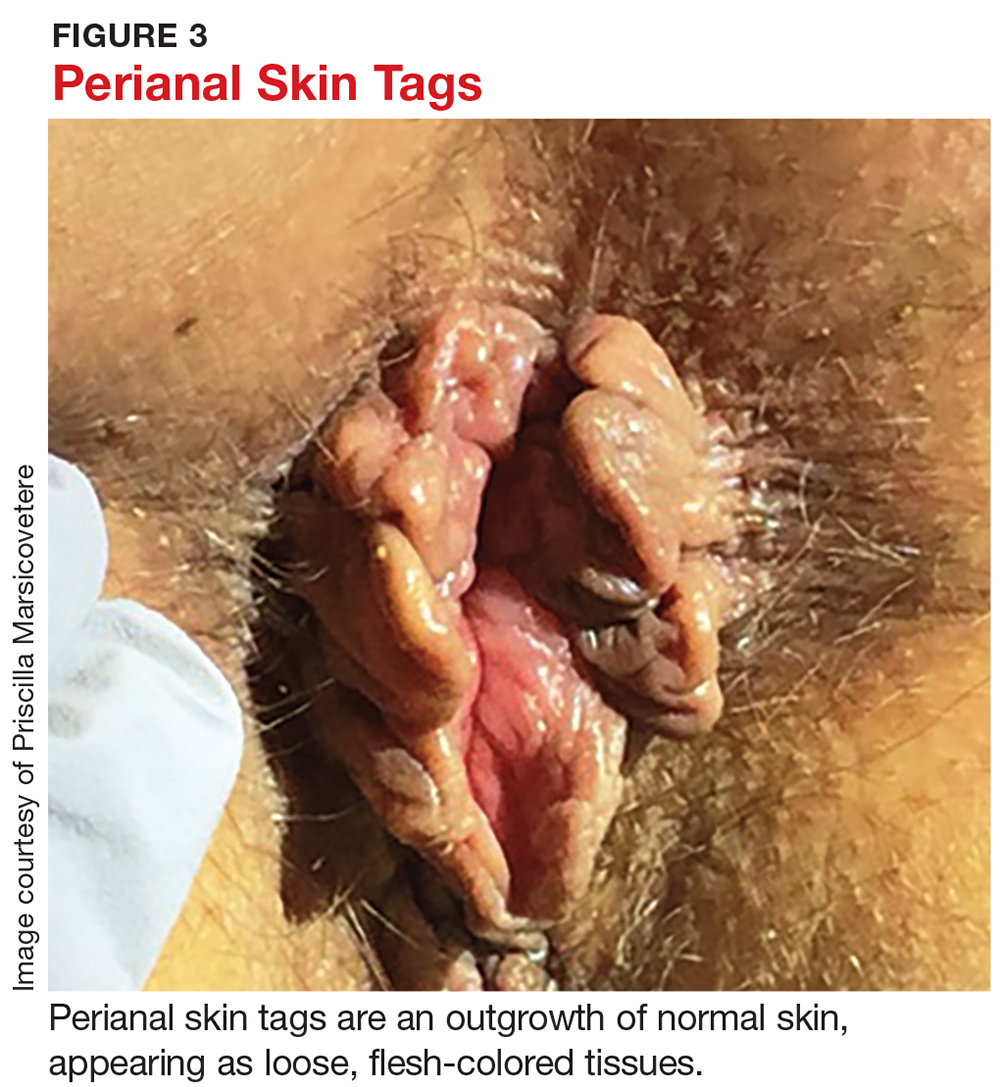
Perianal skin tags are diagnosed clinically and require no labwork or imaging. Visual inspection is typically sufficient to distinguish tags from pathologic lesions such as condyloma or abscess. If there is uncertainty, however, biopsy or referral to a specialist is warranted.
Certain medical conditions can predispose a patient to development of perianal skin tags. They can be sequelae of thrombosed external hemorrhoids.8,11 They are also common in patients with Crohn disease.11 Perianal skin tags are not, however, the result of anal intercourse or sexually transmitted infections.
Treatment is usually not indicated for perianal skin tags. If the tags interfere with hygiene or cause perianal discomfort or significantly decreased quality of life, however, patients may seek removal. These patients should be referred to a colorectal specialist for evaluation for excision.
Anal fissures (fissure in ano)
The most common cause of severe anorectal pain is fissure.4 A fissure is an elliptical tear, or split, in the lining of the anal canal that causes spasm of the anal sphincters. The tear is distal to the dentate line and thus intensely painful.2,5 Common cited causes of fissures are trauma from passage of large, hard stools, straining, or diarrhea.16
Fissures can usually be visualized by spreading the posterior anus apart (see Figure 4). They are most commonly located in the posterior or anterior midline, though they can occur anywhere around the anus.2,4 Often, a sentinel tag—appearing as a taut, flesh-colored skin tag—is present at the external pole of the fissure.5,11 DRE and anoscopy should be avoided, as they will trigger intense pain and spasm of the sphincters.
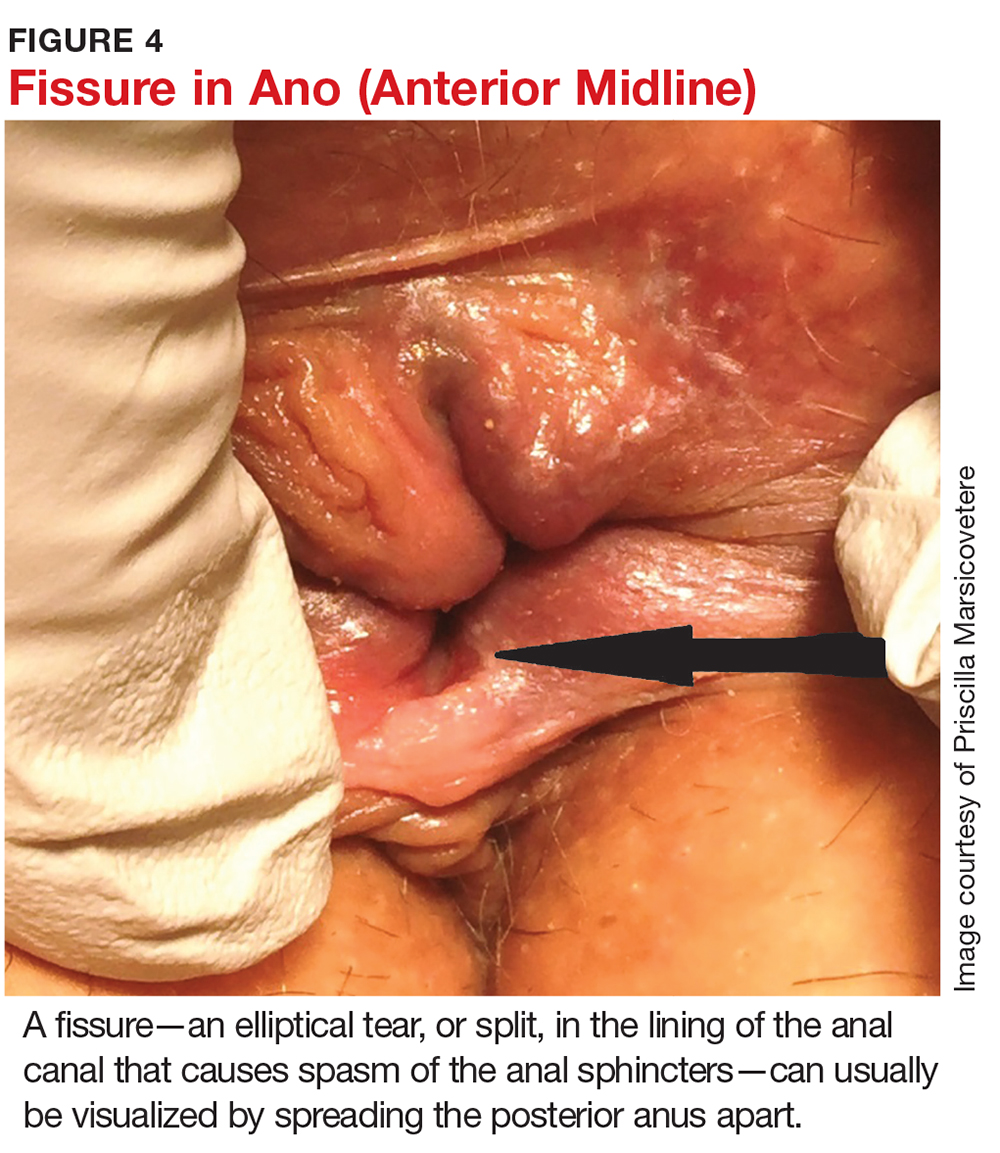
Fissures are characterized as acute (present ≤ 3 months) or chronic (> 3 months).9,11 Visually, acute fissures typically have clean edges, with the appearance of a paper cut to the mucosa, while chronic fissures have indurated, heaped-up edges, often with exposure of the underlying sphincter muscle.17
They tend to be exquisitely painful, as the mucosa distal to the dentate line is highly innervated. Patients report pain akin to “passing shards of broken glass” with bowel movements, which is often accompanied by a fear of defecation and bright red blood on the toilet paper or dripping into the water.11 The pain, caused by spasm of the sphincters, typically starts during a bowel movement and lasts minutes to hours afterward.
Initial treatment is aimed at relaxing the sphincters, as well as softening stool to prevent further trauma and allow the fissure to heal. Patients should be educated about the importance of adequate fiber intake to prevent constipation and straining. A daily bulk fiber supplement, in addition to a high-fiber diet (20-25 g/d), has been shown to result in healing of 87% of acute fissures.16 Sitz baths in plain warm water, three to four times a day, can encourage relaxation of the sphincters and increase local blood flow, both of which help with fissure healing.16 Topical medications can also be prescribed. These include compounded nitroglycerin 0.2% or nifedipine 2.0%, which act to reduce the spasm by relaxing smooth muscle, as well as increase blood flow to the lesion.12,14
Most acute fissures will heal with the regimen of high fiber intake, sitz baths, and topical medication. For refractory or chronic fissures, referral to a colorectal specialist for more invasive treatment is appropriate. Additionally, fissures that are not located in the typical posterior or anterior midline might indicate an atypical etiology, such as Crohn disease, tuberculosis, leukemia, or HIV, and thus patients who present with fissures in these locations should also be referred to a colorectal specialist.2,4,11,18
Pruritus ani
Pruritus ani, known as perianal dermatitis, is a benign condition that presents with intense perianal itching and burning. It is the second most common anorectal condition after hemorrhoids and affects up to 5% of the US population.2,9,11,19
Pruritus ani often develops secondary to local irritation of the skin (eg, from prolonged exposure to moisture), leading to an inflammatory response within the superficial skin layers. The irritation causes patients to scratch the skin, resulting in trauma, excoriation, and ulcer formation and leading to a cycle of further inflammation, exacerbation of symptoms, and persistent scratching.
Physical exam may reveal circumferential erythematous and irritated perianal skin (see Figure 5). Linear or deep, punched-out excoriations may be present. Chronically, patients may develop lichenification with thick, whitened patches of skin.11 In the absence of red flags such as unintentional weight loss, anemia, rectal bleeding, or a family history of colon cancer, no additional evaluation is required during the initial visit, though anoscopy can be used to rule out associated anorectal pathology.
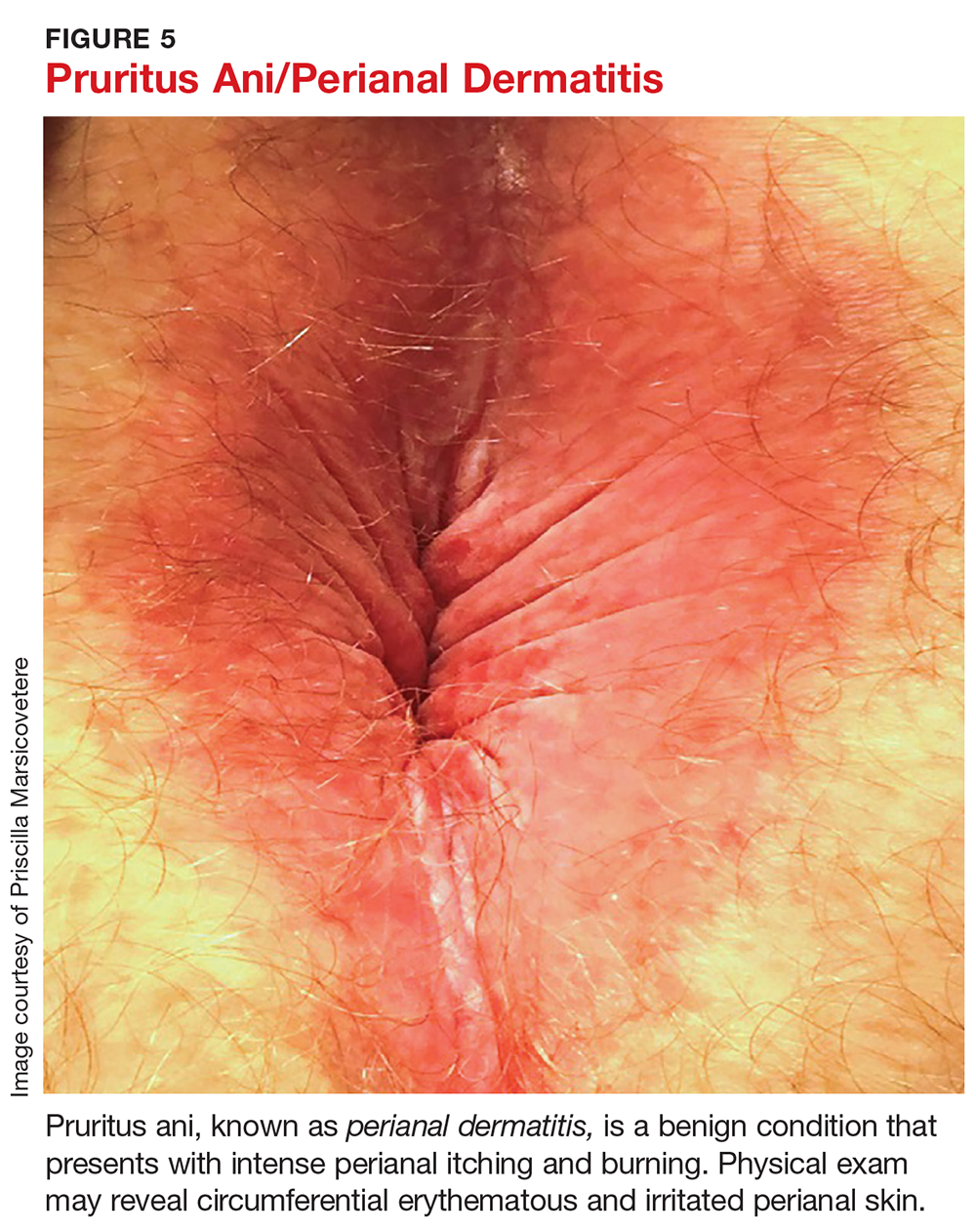
Many different causes of pruritus ani have been reported (see Table 3). In the case of an identifiable cause, symptoms tend to resolve once the offending agent is eliminated. Up to a quarter of cases, however, are idiopathic, with no identifiable trigger.20
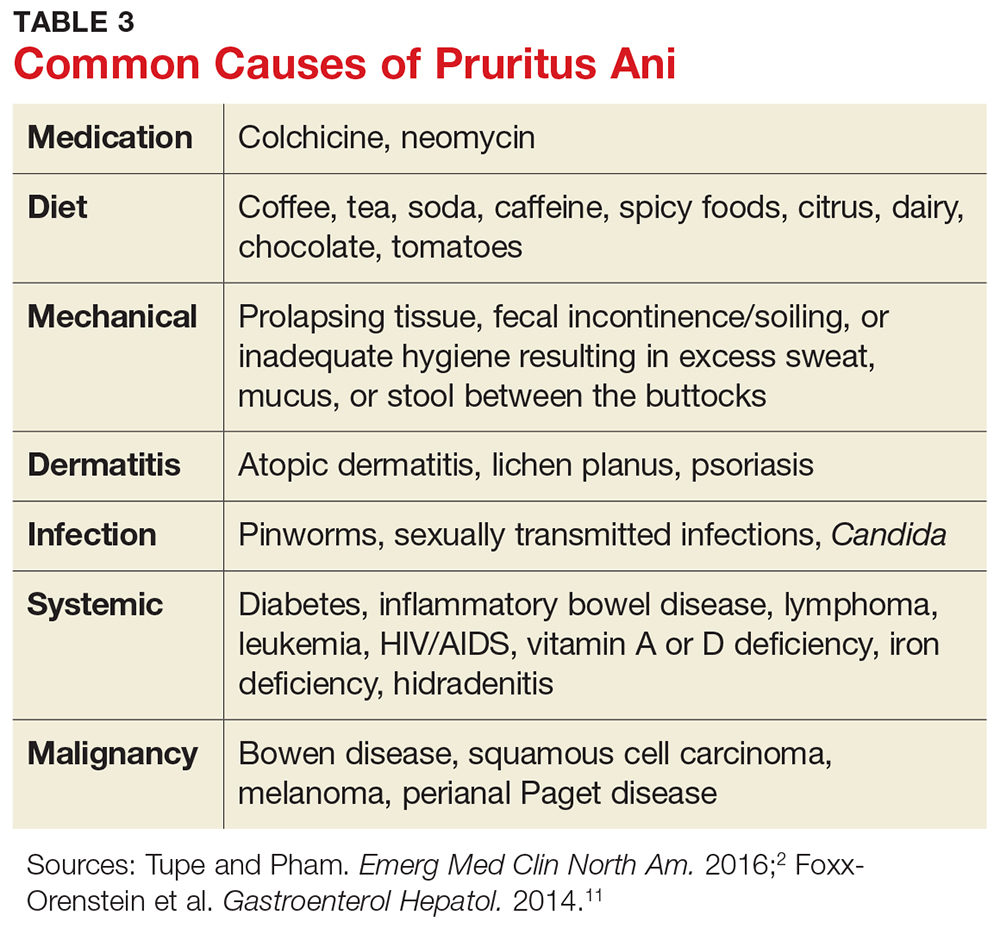
Symptom management is thus key. Patients should be educated about lifestyle modification and informed that scratching will irritate the skin further and aggravate symptoms.5,11 If incontinence or diarrhea is thought to cause symptoms, dietary modifications, a fiber supplement, dysmotility agents, and Kegel exercises to strengthen the sphincters and decrease anal leakage can be recommended.
The affected skin should be kept clean and dry at all times. Aggressive wiping and overzealous hygiene should be avoided. Sitz baths can help with hygiene. A topical astringent such as witch hazel can help remove excess moisture from the skin. A layer of protective skin barrier cream with zinc oxide, when applied over dry skin, can help protect the skin from leakage throughout the day.
A sedating antihistamine can reduce scratching during sleep. Topical hydrocortisone 1% cream is effective for itch relief; this should be limited to five to seven days of consecutive use, however, as it can lead to pathologic thinning of the perianal skin. Topical capsaicin 0.006% cream has been shown to help alleviate intractable pruritus.2,21
The goal of these measures is to break the cycle of irritation and inflammation and give the skin an opportunity to heal. If the patient fails to improve, referral to a colorectal specialist or, alternatively, a dermatologist, is warranted for perianal skin biopsy and more invasive treatment options.
Perianal abscess
A perianal abscess is an infected cavity filled with pus under pressure, located near the anus or rectum. It most often results from an acute infection of the anorectal glands located at the dentate line that tracks outward to the perianal skin.12 Abscesses can also result from another disease process, such as Crohn disease, diabetes, or rectal trauma.2,11
Localized pain, swelling, and drainage are common presenting symptoms of an abscess.2,11 Systemic symptoms such as fever and chills may present later in the course but are rare.2
Treatment of the acute process is incision and drainage (I&D).11,12 This can be accomplished in the office with injection of local anesthesia, followed by excision of an ellipse of skin overlying the cavity large enough to allow full drainage of the abscess. Use of drains and packing of the wound are usually not necessary. There is also no role for antibiotics unless the patient is diabetic or immunosuppressed or cellulitis is present.11 In patients with systemic signs of illness, imaging such as CT or MRI of the pelvis can be used to assess for a deeper infection.2,12
For most patients, I&D resolves the process. However, up to half of perianal abscesses progress to form a fistula, a tunnel connecting the infected anal gland to the external skin.2,11,22 Indeed, abscesses and fistulae are part of the same infectious process, with the abscess representing the acute phase of infection and the fistula representing the chronic phase. On physical exam, in addition to the abscess site on the skin, a fistula may manifest as a palpable cord beneath the skin between the anus and the abscess opening.9 Additionally, the patient may report the abscess has been recurrent in nature, cyclically increasing in size and pain, then spontaneously draining.
A fistula requires surgical intervention for definitive treatment and should therefore prompt referral to a colorectal specialist. In the absence of fistula, however, a simple perianal abscess can be treated with I&D in the primary care setting.
Condyloma acuminatum
Condyloma acuminatum, also known as genital warts, is the most common sexually transmitted infection in the United States and a frequent anorectal complaint.23-25 More than 6 million new infections occur annually.25
Condyloma is caused by the human papillomavirus (HPV).24 More than 100 HPV subtypes have been identified.25 Types 6 and 11 are associated with typical condyloma acuminatum, while types 16 and 18 are found more commonly with dysplasia and malignant transformation.3,26
The lesions are spread by direct skin-to-skin or mucosa-to-mucosa contact, including anal intercourse. They appear as tiny finger-like projections on the perianal or genital skin, often with a cauliflower-like appearance, in clusters or as single entities, and ranging in size from a few millimeters to several centimeters (see Figure 6).
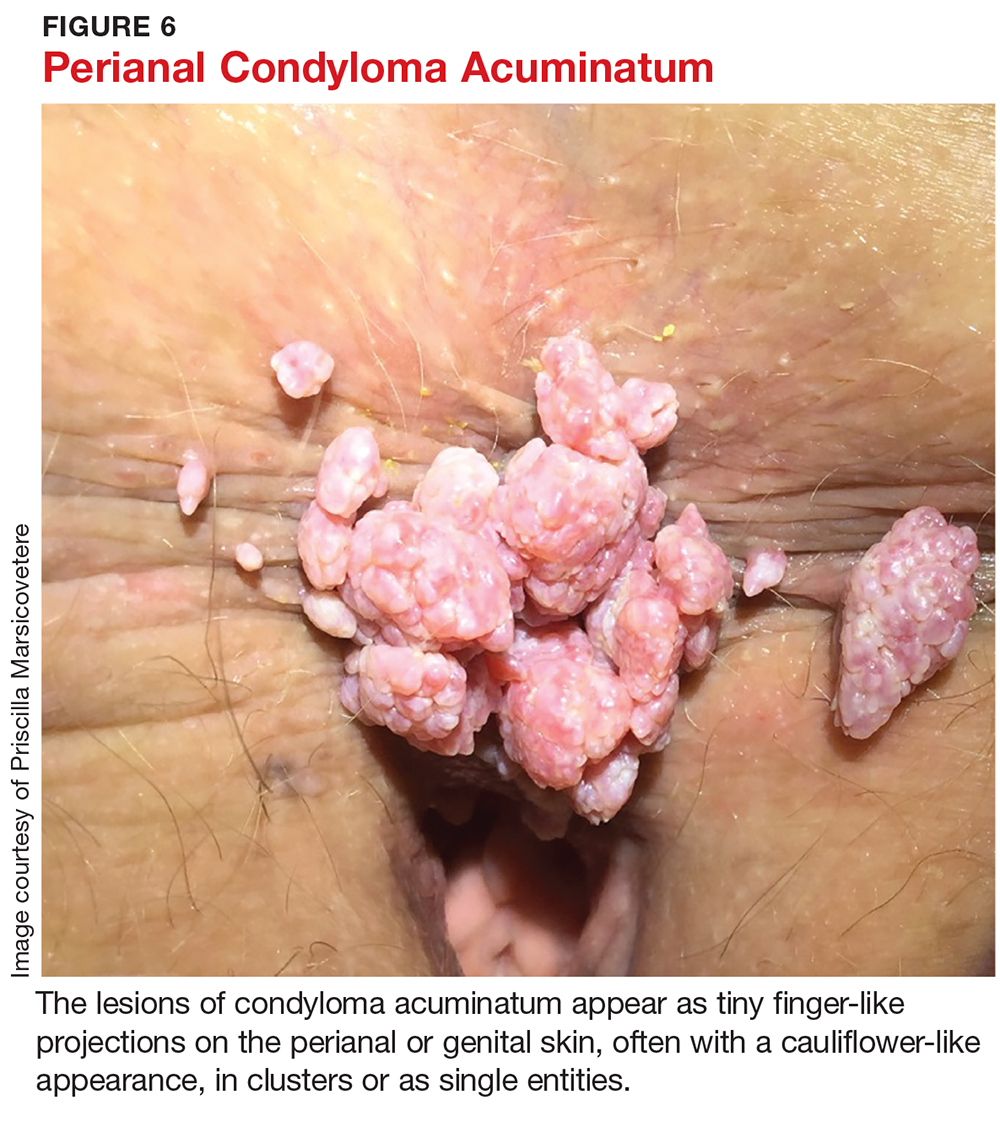
The patient may be asymptomatic or may complain of bleeding with defecation or leakage from the rectum between bowel movements. In up to 90% of perianal condyloma, concomitant anorectal lesions are present.3 Anoscopy is therefore indicated whenever perianal lesions are seen.
Treatment of genital warts varies depending on the number and location of lesions. The goal is lesion removal, but none of the available treatments are curative.24 If only a few small (diameter < 2 mm) warts are seen in the pe
Trichloroacetic acid (TCA), which is considered a form of chemical cautery, has been deemed more effective for tiny warts (diameter 1-2 mm) and better tolerated than podophyllin.24 Unlike podophyllin, however, TCA must be applied in clinic by a specialist, not at home by the patient. It works by chemically burning, cauterizing, and eroding skin and mucosa, thereby destroying old infected cells and allowing for growth of new, healthy cells.24 Using a solution with a higher concentration of TCA (80%-90%) has been shown to be more effective than lower concentration solutions (30%).24 The recurrence rate is high, generally about 36%.27 Due to its low risk for systemic absorption, TCA is safe for use during pregnancy.24
Local application of an immunomodulator, such as imiquimod 2%, 3.75%, or 5% cream, can also be used. Imiquimod works by upregulating tumor necrosis factor, leading to decreased viral replication and subsequent regression of warts.24 The most common side effects of imiquimod are erythema, erosions, burning, and pruritus.24 The higher concentration is associated with increased clearance rates as well as increased adverse effects.27 Therefore, the 3.75% cream is the preferred concentration of most providers.27 Recurrence rates range from 13% to 19%.28
In 2006, the FDA approved polyphenon E, a botanical ointment, to treat anogenital warts. The formulation is composed of eight different sinecatechins extracted from green tea leaves.29 Green tea sinecatechins have been shown to have antioxidant, antiproliferative, and antiviral properties, though the specific mechanisms of action in inhibiting anogenital wart growth have not been well studied to date. In a multicenter, randomized, double-blind, vehicle-controlled, three-arm parallel group phase 3 trial of 495 patients with anogenital warts, sinecatechins ointment 10% or 15% applied three times daily for 16 weeks resulted in complete clearance of warts in 57.2% and 56.3% of patients, respectively, compared to 33.7% clearance in the vehicle patients.30 Partial clearance (≥ 50%) of warts was observed in 78.4% of patients who used the 15% ointment, 74.0% of those who used the 10% ointment, and 51.5% of the vehicle patients. Rates of recurrence were 6.5% and 8.3% with use of the 10% and 15% sinecatechins ointment, respectively, which are much lower than recurrence rates observed with other topical applications (eg, imiquimod and podophyllotoxin).30
For larger warts or when there are anal lesions present, surgical excision and fulguration is required and referral to a colorectal specialist is warranted.
Warts recur from 4.6% to more than 70% of the time, most commonly due to activation of latent virus.23 Therefore, whether initially treated in an office or operating room, condyloma patients require follow-up on a regular basis, generally every six to 12 months, to assess for recurrent anal lesions so that treatment can be promptly initiated.
In the absence of a cure for anogenital warts, prevention through an HPV vaccine is important. The quadrivalent HPV vaccine (Gardasil) was approved by the FDA in 2006 for prophylactic vaccination of females ages 9 to 26. The vaccine triggers host formation of antibodies against four common subtypes of HPV: 6, 11, 16, and 18.31 In 2009, the FDA expanded the use of Gardasil to include males ages 9 to 26; subsequently, Gardasil 9 (which protects against nine types of HPV) was approved for males ages 9 to 15.32 In a group of 4,065 healthy males between the ages of 16 and 26, Gardasil reduced the incidence of external anogenital warts by 90.4%.33
HPV vaccine is now recommended for females through age 26 and males through age 21. The CDC also recommends HPV vaccine for the following individuals through age 26 if they did not get vaccinated when they were younger:
- Young men who have sex with men
- Young men who identify as gay or bisexual or who intend to have sex with men
- Young adults who are transgender
- Young adults with immunocompromising conditions (including HIV).34
CONCLUSION
Anorectal conditions are a common presentation in the primary care setting. A thorough history and physical exam will usually determine the etiology, and most benign pathologies can be successfully treated in the primary care clinic. Knowing how to perform an anorectal exam and having a thorough understanding of the most common anorectal pathologies can help alleviate examiner discomfort, while ensuring that patients receive prompt and adequate care. At the same time, recognizing the red flags can expedite referral for colorectal specialty evaluation when appropriate. Red flags that should prompt immediate referral to a colorectal specialist include older age, unintentional weight loss, iron deficiency anemia, family history of inflammatory bowel disease or colorectal cancer, and persistent anorectal bleeding or persistent symptoms despite adequate treatment of a suspected benign condition.
1. Grucela A, Salinas H, Khaitov S, et al. Prospective analysis of clinical accuracy in the diagnosis of benign anal pathology, comparison across specialties and years of experience. Dis Colon Rectum. 2010;53(1):47-52.
2. Tupe CL, Pham TV. Anorectal complaints in the emergency department. Emerg Med Clin North Am. 2016;34(2):251-270.
3. Billingham RP, Isler JT, Kimmins MH, et al. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(7):586-645.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15(26):3201-3209.
5. Henderson PK, Cash BD. Common anorectal conditions: evaluation and treatment. Curr Gastroenterol Rep. 2014; 16(10):408.
6. Gebbensleben O, Hilger Y, Rohde H. Patients’ view of medical positioning for proctologic examination. Clin Exp Gastroenterol. 2009;2:133-138.
7. Gopal DV. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4): 34-48.
8. Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol. 2015;21(31):9245-9252.
9. Fargo MV, Latimer K. Evaluation and management of common anorectal conditions. Am Fam Physician. 2012;85(6):624-630.
10. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17): 2009-2017.
11. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294-301.
12. Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22(26):5867-5878.
13. Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative treatment or surgical management. Dis Colon Rectum. 2004(9);47:1493-1498.
14. Summers A. Assessment and treatment of three common anorectal conditions. Emerg Nurse. 2013;21(2):28-33.
15. Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101(1):181-188.
16. Medhi B, Rao RS, Prakash A, et al. Recent advances in the pharmacotherapy of chronic anal fissure: an update. Asian J Surg. 2008;31(3):154-163.
17. Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014;2014. pii: 0407.
18. deRosa M, Cestaro G, Vitiello C, et al. Conservative versus surgical treatment for chronic idiopathic anal fissure: a prospective randomized trial. Updates Surg. 2013;65(3):197-200.
19. Lacy B, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413-419.
20. Siddiqi S, Vijay V, Ward M, et al. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457-463.
21. Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomized, placebo controlled, crossover study. Gut. 2003;52(9):1323-1326.
22. Burnstein M. Managing anorectal emergencies. Can Fam Physician. 1993;39:1782-1785.
23. Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636-2641.
24. Kollipara R, Ekhlassi E, Downing C, et al. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J Clin Med. 2015;4(5):832-846.
25. CDC. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 5: Human papillomavirus (HPV). www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed October 18, 2017.
26. Leszczyszyn J, Lebski I, Lysenko L, et al. Anal warts (condyloma acuminatum)—current issues and treatment modalities. Adv Clin Exp Med. 2014;23(2):307-311.
27. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105.
28. Yan J, Chen SL, Wang HN, et al. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminate. Dermatology. 2006;213(3):218-223.
29. Hoy SM. Polyphenon E 10% ointment: in immunocompetent adults with external genital and perianal warts. Am J Clin Dermatol. 2012;13(4):275-281.
30. Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet Gynecol. 2008;111(6):1371-1379.
31. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed October 18, 2017.
32. National Institutes of Health, National Cancer Institute. Human papillomavirus (HPV) vaccine. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet#q5. Accessed October 18, 2017.
33. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
34. CDC. HPV vaccines: vaccinating your preteen or teen. www.cdc.gov/hpv/parents/vaccine.html. Accessed October 18, 2017.
CE/CME No: CR-1711
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand basic anorectal anatomy and how to perform a thorough anorectal exam.
• Describe the physical exam findings of common benign anorectal conditions.
• Discuss the different treatment options for benign anorectal conditions.
• Differentiate between common benign anorectal symptoms and red flags that should prompt referral to a colorectal specialist.
FACULTY
Priscilla Marsicovetere is an Assistant Professor of Medical Education and of Surgery at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire; Program Director for the Franklin Pierce University PA Program in Lebanon, New Hampshire; and practices with Emergency Services of New England at Springfield Hospital in Vermont. Srinivas Joga Ivatury is an Assistant Professor of Surgery at the Geisel School of Medicine at Dartmouth and practices in the Department of Surgery at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through October 31, 2018.
Article begins on next page >>
Anorectal pain and discomfort can stem from several possible etiologies, most of which are benign. As such, many patients with anorectal complaints who present in the primary care setting can be adequately evaluated, diagnosed, and treated without referral to a colorectal specialist. However, the clinician must be able to differentiate between common benign anorectal symptoms and red flags that should prompt referral.
Anorectal disorders are common in the United States and result in numerous primary care visits each year. Presentations range from pain and itching to bleeding and lesions. Common anorectal conditions include hemorrhoids, perianal skin tags, fissures, pruritus ani, perianal abscess, and condyloma. Most are benign and can be managed in the primary care setting.
Before a provider can competently diagnose and treat anorectal conditions, however, a comprehensive history and physical examination must be conducted. Grucela and colleagues documented that physicians’ diagnostic accuracy with anorectal conditions is about 50%—highlighting the need for providers to become more familiar with the history and clinical elements associated with anorectal complaints.1
This article reviews the assessment of the anorectum, diagnosis of common disorders and their recommended treatments, and red flags for referral to a colorectal specialist.
ANORECTAL ANATOMY
The beginning of the anal canal is demarcated by its moist, hairless appearance. Just inside the anal opening are two palpable circular muscles, the internal and external anal sphincters, separated by an intersphincteric groove. The sphincters are firmly closed in the resting state, which helps maintain continence.
The anal canal is generally 3 to 4 cm long and ends at the dentate line, a series of crypts in the anal mucosa.2 The crypts are openings into the anal glands, which are mucus-secreting structures in the anus. The dentate line is easily identified on anoscopy as a discrete change in the appearance of the mucosa. The dentate line is an important landmark because it delineates the boundary between somatic and visceral nerve supplies.3 Tissue proximal to the dentate line is innervated by visceral nerves and is insensate, and thus usually not a cause of pain; tissue distal to the dentate line, however, is highly innervated by somatic nerves and can be intensely painful.2
The anorectal canal is lined by three fibrovascular cushions, located in the left lateral, right posterior, and right anterior positions.4 Inside each cushion is a venous structure, called a hemorrhoid, which allows the cushion to enlarge and help maintain continence.5
Proximal to the anus is the rectum, the 12- to 15-cm long terminus of the colon. Anorectal examination in the primary care setting will typically not progress beyond the last 2 to 3 cm of the rectum.
TAKING THE HISTORY
A thorough history will provide clues about potential underlying anorectal pathology. Patients may not be forthcoming about symptoms due to embarrassment, fear of a cancer diagnosis, or cultural customs or habits. A thorough history should elicit information about all of the patient’s symptoms (see Table 1), including bleeding, change in bowel habits, and unintended weight loss.

PHYSICAL EXAM
Positioning the patient
Undergoing an anorectal examination can be embarrassing, whether it be from exposure of sensitive body parts or the less-than-desirable prone jackknife positioning. Patients often have preconceived notions that the exam will be humiliating and/or painful. Care should be taken to minimize any embarrassment and discomfort.
Positioning of the patient is a matter of provider preference. Options include the left lateral decubitus, prone jackknife, or lithotomy positions.
Positioning should always be done with draping. Regardless of position, ensure the draping exposes only the perineum. This can be achieved by encircling the patient’s bare bottom with a plain white sheet that exposes only the anus and surrounding skin, keeping the lower back, lateral buttocks, and thighs covered.
Interestingly, data on patient preference for positioning during anorectal exams are limited. In a 2009 study of 178 patients undergoing anorectal exam, more than half of patients (up to 71.4%) expecting to or having already had a proctologic exam reported that no specific type of positioning (eg, Sims, lithotomy with lifted legs, knee-chest, knee-chest with patient’s body bent forward) was most embarrassing to them.6 The report revealed that while most patients would favor the Sims position if they had a choice, they deferred to their examiner to choose the position that seemed most suitable to get a reliable diagnosis.6
Inspection of the perineum
Once the patient is properly positioned and draped, inspection of the perineum can occur. Begin by gently spreading the buttocks. Describe any abnormality seen (eg, ulcer, lesion, dermatitis, prolapsing tissue, or blood), including size, color, and location.
A common pitfall is to describe the location of abnormalities using a clock face, such as “at 4 o’clock.” This is misleading and should be avoided, because depending on patient position, the clock face can point to different locations (eg, if the patient is in the lithotomy versus prone jackknife position).
A better approach is to divide the perianal area into four anatomic quadrants: right anterior, right posterior, left anterior, left posterior. Using this schematic, the patient's position is irrelevant, and accurate documentation of lesion location is assured.
Digital rectal exam
After visual inspection of the perianal skin, a digital rectal exam (DRE) should be performed. Slowly insert a gloved, lubricated index finger into the anus and lower rectum. Note the tone of the anus at rest (eg, excessively tight vs lax). Palpate the circumference of the anus, sweeping side to side while assessing for any tenderness, mass, or induration—if present, note the anatomic quadrant. If a mass is felt, note whether it is firm or soft, fixed or mobile, and broad-based or pedunculated. When the lubricated finger is removed from the anus, note whether blood is present.
Anoscopy
After DRE, visually inspect the anorectum. The instrument used varies from anoscope to rigid proctoscope to flexible sigmoidoscope. In a primary care setting, the most likely available instrument is an anoscope. The average anoscope is about 7 cm long and 2 cm in diameter, with a beveled tip and an obturator (see Figure 1), and allows a 360° view of the anal canal.7

Examination of the anal canal is accomplished by dividing the canal into the four anatomic quadrants described earlier and inserting the lubricated anoscope for inspection of each of the four quadrants. Observe the rectal mucosa and the anus as the scope is slowly withdrawn. If abnormalities are seen, note the location, size, shape, and any other descriptive features.
It is not necessary to perform a Hemoccult test after examination of the anorectum, as the presence of minor blood may be the direct result of the exam itself and thus provides no useful information to the examiner.
COMMON PATHOLOGIES
Once the history and physical exam are complete, a differential diagnosis can be formulated. Most anorectal disorders are benign conditions that pose no immediate health threat and can be managed in the primary care setting. Others, however, can be more serious and should prompt referral to a colorectal specialist for further evaluation. Knowing the difference can spare a patient unnecessary anxiety and referral; it can also lead to prompt, lifesaving interventions if red flags are recognized.
Hemorrhoids
Hemorrhoids are a common anorectal complaint.8,9 It is estimated that up to 75% of the population will experience symptoms of hemorrhoids during their lifetime.5,8 Whether internal or external, in their normal, nonpathologic, quiescent state, hemorrhoids are asymptomatic. Hemorrhoids become symptomatic when the supporting structures of hemorrhoidal tissue (ie, the anal cushions) deteriorate, resulting in venous dilation, inflammation, and thrombosis, which in turn lead to swelling, bright red bleeding, and/or prolapse.2,10 The most common causes of hemorrhoidal disease are chronic constipation and prolonged straining with bowel movements, though chronic diarrhea and pregnancy have also been identified as risk factors.2,8,11
External hemorrhoids, which are located distal to the dentate line, are typically only visible when they become thrombosed or swollen. In this state, they may manifest as acute-onset, exquisitely painful, large, purple-to-blue bulges at the anal outlet (see Figure 2). The number and size of the lesions can vary. The patient may report pain when sitting or wiping, as well as bleeding from the lesion.12,13 The pain is typically severe in the first couple of days, then slowly starts to subside.2,12

For internal hemorrhoids, which are located proximal to the dentate line, the main symptom is usually painless bright red blood per rectum.8,11,12 Patients may also report a sensation of rectal fullness or experience prolapse of the hemorrhoid through the anus. Prolapse typically occurs with defecation; in more severe cases, it can also occur between bowel movements, usually with any activity that increases intra-abdominal pressure (eg, coughing, heavy lifting, pregnancy, portal hypertension). The prolapse may reduce spontaneously, or may have to be manually reduced. If it cannot be reduced, there is a risk for incarceration or strangulation, potentially leading to gangrene.
The presence of bleeding and/or prolapse determines the classification of internal hemorrhoids (see Table 2). Dietary and lifestyle modification are used in the management of all grades of hemorrhoids. In addition, for grade 1 and 2 lesions, topical medication (eg, anti-inflammatory cream) can be used, whereas grade 3 (and selected grade 2) lesions respond well to rubber band ligation. Given the severity of grade 4 lesions, surgical intervention (eg, hemorrhoidectomy) is usually indicated.10

About a third of patients with symptomatic hemorrhoids seek clinical treatment.14 Most are hemodynamically stable and require no imaging and usually no labs (unless anemia is suspected).2 Management depends on the location and degree of symptoms (eg, internal vs external, prolapse, or thrombosis). In the event of an acutely thrombosed external hemorrhoid, clot excision for pain relief is appropriate if symptoms have been present for less than 48 to 72 hours; after that amount of time, the pain from the procedure will likely exceed the degree of relief provided, and conservative management should instead be recommended.2,8,11
Firstline treatment consists of lifestyle modification with a high-fiber diet and daily fiber supplement to ensure stool is soft and easy to pass.2,8 A meta-analysis of seven clinical trials with a total of 378 patients with hemorrhoids showed that fiber supplementation resulted in a 50% decrease in bleeding risk from internal hemorrhoids.15
Adequate hydration, preferably with noncaffeinated liquids, is also recommended. This will prevent constipation and the need to strain or spend excessive time on the toilet. Sitz baths can help alleviate pain and discomfort.
Several OTC topical medications are marketed for hemorrhoid relief. Many of these preparations contain steroids for their anti-inflammatory effects or astringents to address skin irritation that can result from anal leakage if prolapsing hemorrhoids prevent the anal outlet from closing. Steroid use should be limited to five to seven days, due to atrophic effects on the skin. While OTC preparations may temporarily alleviate discomfort, they will not address the underlying cause of symptoms.
Indications for referral to a colorectal specialist for symptomatic hemorrhoids include failure to improve with conservative management, persistent patient discomfort, and prolapse, as these indicate potential need for more invasive treatment.
Perianal skin tags
Perianal skin tags, while a nuisance, are not pathologic in most instances and pose no threat to health. They are an outgrowth of normal skin, appearing as loose, flesh-colored perianal tissues (see Figure 3). Tags range in size from a few millimeters to a centimeter long and can occur alone or in multiples.

Perianal skin tags are diagnosed clinically and require no labwork or imaging. Visual inspection is typically sufficient to distinguish tags from pathologic lesions such as condyloma or abscess. If there is uncertainty, however, biopsy or referral to a specialist is warranted.
Certain medical conditions can predispose a patient to development of perianal skin tags. They can be sequelae of thrombosed external hemorrhoids.8,11 They are also common in patients with Crohn disease.11 Perianal skin tags are not, however, the result of anal intercourse or sexually transmitted infections.
Treatment is usually not indicated for perianal skin tags. If the tags interfere with hygiene or cause perianal discomfort or significantly decreased quality of life, however, patients may seek removal. These patients should be referred to a colorectal specialist for evaluation for excision.
Anal fissures (fissure in ano)
The most common cause of severe anorectal pain is fissure.4 A fissure is an elliptical tear, or split, in the lining of the anal canal that causes spasm of the anal sphincters. The tear is distal to the dentate line and thus intensely painful.2,5 Common cited causes of fissures are trauma from passage of large, hard stools, straining, or diarrhea.16
Fissures can usually be visualized by spreading the posterior anus apart (see Figure 4). They are most commonly located in the posterior or anterior midline, though they can occur anywhere around the anus.2,4 Often, a sentinel tag—appearing as a taut, flesh-colored skin tag—is present at the external pole of the fissure.5,11 DRE and anoscopy should be avoided, as they will trigger intense pain and spasm of the sphincters.

Fissures are characterized as acute (present ≤ 3 months) or chronic (> 3 months).9,11 Visually, acute fissures typically have clean edges, with the appearance of a paper cut to the mucosa, while chronic fissures have indurated, heaped-up edges, often with exposure of the underlying sphincter muscle.17
They tend to be exquisitely painful, as the mucosa distal to the dentate line is highly innervated. Patients report pain akin to “passing shards of broken glass” with bowel movements, which is often accompanied by a fear of defecation and bright red blood on the toilet paper or dripping into the water.11 The pain, caused by spasm of the sphincters, typically starts during a bowel movement and lasts minutes to hours afterward.
Initial treatment is aimed at relaxing the sphincters, as well as softening stool to prevent further trauma and allow the fissure to heal. Patients should be educated about the importance of adequate fiber intake to prevent constipation and straining. A daily bulk fiber supplement, in addition to a high-fiber diet (20-25 g/d), has been shown to result in healing of 87% of acute fissures.16 Sitz baths in plain warm water, three to four times a day, can encourage relaxation of the sphincters and increase local blood flow, both of which help with fissure healing.16 Topical medications can also be prescribed. These include compounded nitroglycerin 0.2% or nifedipine 2.0%, which act to reduce the spasm by relaxing smooth muscle, as well as increase blood flow to the lesion.12,14
Most acute fissures will heal with the regimen of high fiber intake, sitz baths, and topical medication. For refractory or chronic fissures, referral to a colorectal specialist for more invasive treatment is appropriate. Additionally, fissures that are not located in the typical posterior or anterior midline might indicate an atypical etiology, such as Crohn disease, tuberculosis, leukemia, or HIV, and thus patients who present with fissures in these locations should also be referred to a colorectal specialist.2,4,11,18
Pruritus ani
Pruritus ani, known as perianal dermatitis, is a benign condition that presents with intense perianal itching and burning. It is the second most common anorectal condition after hemorrhoids and affects up to 5% of the US population.2,9,11,19
Pruritus ani often develops secondary to local irritation of the skin (eg, from prolonged exposure to moisture), leading to an inflammatory response within the superficial skin layers. The irritation causes patients to scratch the skin, resulting in trauma, excoriation, and ulcer formation and leading to a cycle of further inflammation, exacerbation of symptoms, and persistent scratching.
Physical exam may reveal circumferential erythematous and irritated perianal skin (see Figure 5). Linear or deep, punched-out excoriations may be present. Chronically, patients may develop lichenification with thick, whitened patches of skin.11 In the absence of red flags such as unintentional weight loss, anemia, rectal bleeding, or a family history of colon cancer, no additional evaluation is required during the initial visit, though anoscopy can be used to rule out associated anorectal pathology.

Many different causes of pruritus ani have been reported (see Table 3). In the case of an identifiable cause, symptoms tend to resolve once the offending agent is eliminated. Up to a quarter of cases, however, are idiopathic, with no identifiable trigger.20

Symptom management is thus key. Patients should be educated about lifestyle modification and informed that scratching will irritate the skin further and aggravate symptoms.5,11 If incontinence or diarrhea is thought to cause symptoms, dietary modifications, a fiber supplement, dysmotility agents, and Kegel exercises to strengthen the sphincters and decrease anal leakage can be recommended.
The affected skin should be kept clean and dry at all times. Aggressive wiping and overzealous hygiene should be avoided. Sitz baths can help with hygiene. A topical astringent such as witch hazel can help remove excess moisture from the skin. A layer of protective skin barrier cream with zinc oxide, when applied over dry skin, can help protect the skin from leakage throughout the day.
A sedating antihistamine can reduce scratching during sleep. Topical hydrocortisone 1% cream is effective for itch relief; this should be limited to five to seven days of consecutive use, however, as it can lead to pathologic thinning of the perianal skin. Topical capsaicin 0.006% cream has been shown to help alleviate intractable pruritus.2,21
The goal of these measures is to break the cycle of irritation and inflammation and give the skin an opportunity to heal. If the patient fails to improve, referral to a colorectal specialist or, alternatively, a dermatologist, is warranted for perianal skin biopsy and more invasive treatment options.
Perianal abscess
A perianal abscess is an infected cavity filled with pus under pressure, located near the anus or rectum. It most often results from an acute infection of the anorectal glands located at the dentate line that tracks outward to the perianal skin.12 Abscesses can also result from another disease process, such as Crohn disease, diabetes, or rectal trauma.2,11
Localized pain, swelling, and drainage are common presenting symptoms of an abscess.2,11 Systemic symptoms such as fever and chills may present later in the course but are rare.2
Treatment of the acute process is incision and drainage (I&D).11,12 This can be accomplished in the office with injection of local anesthesia, followed by excision of an ellipse of skin overlying the cavity large enough to allow full drainage of the abscess. Use of drains and packing of the wound are usually not necessary. There is also no role for antibiotics unless the patient is diabetic or immunosuppressed or cellulitis is present.11 In patients with systemic signs of illness, imaging such as CT or MRI of the pelvis can be used to assess for a deeper infection.2,12
For most patients, I&D resolves the process. However, up to half of perianal abscesses progress to form a fistula, a tunnel connecting the infected anal gland to the external skin.2,11,22 Indeed, abscesses and fistulae are part of the same infectious process, with the abscess representing the acute phase of infection and the fistula representing the chronic phase. On physical exam, in addition to the abscess site on the skin, a fistula may manifest as a palpable cord beneath the skin between the anus and the abscess opening.9 Additionally, the patient may report the abscess has been recurrent in nature, cyclically increasing in size and pain, then spontaneously draining.
A fistula requires surgical intervention for definitive treatment and should therefore prompt referral to a colorectal specialist. In the absence of fistula, however, a simple perianal abscess can be treated with I&D in the primary care setting.
Condyloma acuminatum
Condyloma acuminatum, also known as genital warts, is the most common sexually transmitted infection in the United States and a frequent anorectal complaint.23-25 More than 6 million new infections occur annually.25
Condyloma is caused by the human papillomavirus (HPV).24 More than 100 HPV subtypes have been identified.25 Types 6 and 11 are associated with typical condyloma acuminatum, while types 16 and 18 are found more commonly with dysplasia and malignant transformation.3,26
The lesions are spread by direct skin-to-skin or mucosa-to-mucosa contact, including anal intercourse. They appear as tiny finger-like projections on the perianal or genital skin, often with a cauliflower-like appearance, in clusters or as single entities, and ranging in size from a few millimeters to several centimeters (see Figure 6).

The patient may be asymptomatic or may complain of bleeding with defecation or leakage from the rectum between bowel movements. In up to 90% of perianal condyloma, concomitant anorectal lesions are present.3 Anoscopy is therefore indicated whenever perianal lesions are seen.
Treatment of genital warts varies depending on the number and location of lesions. The goal is lesion removal, but none of the available treatments are curative.24 If only a few small (diameter < 2 mm) warts are seen in the pe
Trichloroacetic acid (TCA), which is considered a form of chemical cautery, has been deemed more effective for tiny warts (diameter 1-2 mm) and better tolerated than podophyllin.24 Unlike podophyllin, however, TCA must be applied in clinic by a specialist, not at home by the patient. It works by chemically burning, cauterizing, and eroding skin and mucosa, thereby destroying old infected cells and allowing for growth of new, healthy cells.24 Using a solution with a higher concentration of TCA (80%-90%) has been shown to be more effective than lower concentration solutions (30%).24 The recurrence rate is high, generally about 36%.27 Due to its low risk for systemic absorption, TCA is safe for use during pregnancy.24
Local application of an immunomodulator, such as imiquimod 2%, 3.75%, or 5% cream, can also be used. Imiquimod works by upregulating tumor necrosis factor, leading to decreased viral replication and subsequent regression of warts.24 The most common side effects of imiquimod are erythema, erosions, burning, and pruritus.24 The higher concentration is associated with increased clearance rates as well as increased adverse effects.27 Therefore, the 3.75% cream is the preferred concentration of most providers.27 Recurrence rates range from 13% to 19%.28
In 2006, the FDA approved polyphenon E, a botanical ointment, to treat anogenital warts. The formulation is composed of eight different sinecatechins extracted from green tea leaves.29 Green tea sinecatechins have been shown to have antioxidant, antiproliferative, and antiviral properties, though the specific mechanisms of action in inhibiting anogenital wart growth have not been well studied to date. In a multicenter, randomized, double-blind, vehicle-controlled, three-arm parallel group phase 3 trial of 495 patients with anogenital warts, sinecatechins ointment 10% or 15% applied three times daily for 16 weeks resulted in complete clearance of warts in 57.2% and 56.3% of patients, respectively, compared to 33.7% clearance in the vehicle patients.30 Partial clearance (≥ 50%) of warts was observed in 78.4% of patients who used the 15% ointment, 74.0% of those who used the 10% ointment, and 51.5% of the vehicle patients. Rates of recurrence were 6.5% and 8.3% with use of the 10% and 15% sinecatechins ointment, respectively, which are much lower than recurrence rates observed with other topical applications (eg, imiquimod and podophyllotoxin).30
For larger warts or when there are anal lesions present, surgical excision and fulguration is required and referral to a colorectal specialist is warranted.
Warts recur from 4.6% to more than 70% of the time, most commonly due to activation of latent virus.23 Therefore, whether initially treated in an office or operating room, condyloma patients require follow-up on a regular basis, generally every six to 12 months, to assess for recurrent anal lesions so that treatment can be promptly initiated.
In the absence of a cure for anogenital warts, prevention through an HPV vaccine is important. The quadrivalent HPV vaccine (Gardasil) was approved by the FDA in 2006 for prophylactic vaccination of females ages 9 to 26. The vaccine triggers host formation of antibodies against four common subtypes of HPV: 6, 11, 16, and 18.31 In 2009, the FDA expanded the use of Gardasil to include males ages 9 to 26; subsequently, Gardasil 9 (which protects against nine types of HPV) was approved for males ages 9 to 15.32 In a group of 4,065 healthy males between the ages of 16 and 26, Gardasil reduced the incidence of external anogenital warts by 90.4%.33
HPV vaccine is now recommended for females through age 26 and males through age 21. The CDC also recommends HPV vaccine for the following individuals through age 26 if they did not get vaccinated when they were younger:
- Young men who have sex with men
- Young men who identify as gay or bisexual or who intend to have sex with men
- Young adults who are transgender
- Young adults with immunocompromising conditions (including HIV).34
CONCLUSION
Anorectal conditions are a common presentation in the primary care setting. A thorough history and physical exam will usually determine the etiology, and most benign pathologies can be successfully treated in the primary care clinic. Knowing how to perform an anorectal exam and having a thorough understanding of the most common anorectal pathologies can help alleviate examiner discomfort, while ensuring that patients receive prompt and adequate care. At the same time, recognizing the red flags can expedite referral for colorectal specialty evaluation when appropriate. Red flags that should prompt immediate referral to a colorectal specialist include older age, unintentional weight loss, iron deficiency anemia, family history of inflammatory bowel disease or colorectal cancer, and persistent anorectal bleeding or persistent symptoms despite adequate treatment of a suspected benign condition.
CE/CME No: CR-1711
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand basic anorectal anatomy and how to perform a thorough anorectal exam.
• Describe the physical exam findings of common benign anorectal conditions.
• Discuss the different treatment options for benign anorectal conditions.
• Differentiate between common benign anorectal symptoms and red flags that should prompt referral to a colorectal specialist.
FACULTY
Priscilla Marsicovetere is an Assistant Professor of Medical Education and of Surgery at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire; Program Director for the Franklin Pierce University PA Program in Lebanon, New Hampshire; and practices with Emergency Services of New England at Springfield Hospital in Vermont. Srinivas Joga Ivatury is an Assistant Professor of Surgery at the Geisel School of Medicine at Dartmouth and practices in the Department of Surgery at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through October 31, 2018.
Article begins on next page >>
Anorectal pain and discomfort can stem from several possible etiologies, most of which are benign. As such, many patients with anorectal complaints who present in the primary care setting can be adequately evaluated, diagnosed, and treated without referral to a colorectal specialist. However, the clinician must be able to differentiate between common benign anorectal symptoms and red flags that should prompt referral.
Anorectal disorders are common in the United States and result in numerous primary care visits each year. Presentations range from pain and itching to bleeding and lesions. Common anorectal conditions include hemorrhoids, perianal skin tags, fissures, pruritus ani, perianal abscess, and condyloma. Most are benign and can be managed in the primary care setting.
Before a provider can competently diagnose and treat anorectal conditions, however, a comprehensive history and physical examination must be conducted. Grucela and colleagues documented that physicians’ diagnostic accuracy with anorectal conditions is about 50%—highlighting the need for providers to become more familiar with the history and clinical elements associated with anorectal complaints.1
This article reviews the assessment of the anorectum, diagnosis of common disorders and their recommended treatments, and red flags for referral to a colorectal specialist.
ANORECTAL ANATOMY
The beginning of the anal canal is demarcated by its moist, hairless appearance. Just inside the anal opening are two palpable circular muscles, the internal and external anal sphincters, separated by an intersphincteric groove. The sphincters are firmly closed in the resting state, which helps maintain continence.
The anal canal is generally 3 to 4 cm long and ends at the dentate line, a series of crypts in the anal mucosa.2 The crypts are openings into the anal glands, which are mucus-secreting structures in the anus. The dentate line is easily identified on anoscopy as a discrete change in the appearance of the mucosa. The dentate line is an important landmark because it delineates the boundary between somatic and visceral nerve supplies.3 Tissue proximal to the dentate line is innervated by visceral nerves and is insensate, and thus usually not a cause of pain; tissue distal to the dentate line, however, is highly innervated by somatic nerves and can be intensely painful.2
The anorectal canal is lined by three fibrovascular cushions, located in the left lateral, right posterior, and right anterior positions.4 Inside each cushion is a venous structure, called a hemorrhoid, which allows the cushion to enlarge and help maintain continence.5
Proximal to the anus is the rectum, the 12- to 15-cm long terminus of the colon. Anorectal examination in the primary care setting will typically not progress beyond the last 2 to 3 cm of the rectum.
TAKING THE HISTORY
A thorough history will provide clues about potential underlying anorectal pathology. Patients may not be forthcoming about symptoms due to embarrassment, fear of a cancer diagnosis, or cultural customs or habits. A thorough history should elicit information about all of the patient’s symptoms (see Table 1), including bleeding, change in bowel habits, and unintended weight loss.

PHYSICAL EXAM
Positioning the patient
Undergoing an anorectal examination can be embarrassing, whether it be from exposure of sensitive body parts or the less-than-desirable prone jackknife positioning. Patients often have preconceived notions that the exam will be humiliating and/or painful. Care should be taken to minimize any embarrassment and discomfort.
Positioning of the patient is a matter of provider preference. Options include the left lateral decubitus, prone jackknife, or lithotomy positions.
Positioning should always be done with draping. Regardless of position, ensure the draping exposes only the perineum. This can be achieved by encircling the patient’s bare bottom with a plain white sheet that exposes only the anus and surrounding skin, keeping the lower back, lateral buttocks, and thighs covered.
Interestingly, data on patient preference for positioning during anorectal exams are limited. In a 2009 study of 178 patients undergoing anorectal exam, more than half of patients (up to 71.4%) expecting to or having already had a proctologic exam reported that no specific type of positioning (eg, Sims, lithotomy with lifted legs, knee-chest, knee-chest with patient’s body bent forward) was most embarrassing to them.6 The report revealed that while most patients would favor the Sims position if they had a choice, they deferred to their examiner to choose the position that seemed most suitable to get a reliable diagnosis.6
Inspection of the perineum
Once the patient is properly positioned and draped, inspection of the perineum can occur. Begin by gently spreading the buttocks. Describe any abnormality seen (eg, ulcer, lesion, dermatitis, prolapsing tissue, or blood), including size, color, and location.
A common pitfall is to describe the location of abnormalities using a clock face, such as “at 4 o’clock.” This is misleading and should be avoided, because depending on patient position, the clock face can point to different locations (eg, if the patient is in the lithotomy versus prone jackknife position).
A better approach is to divide the perianal area into four anatomic quadrants: right anterior, right posterior, left anterior, left posterior. Using this schematic, the patient's position is irrelevant, and accurate documentation of lesion location is assured.
Digital rectal exam
After visual inspection of the perianal skin, a digital rectal exam (DRE) should be performed. Slowly insert a gloved, lubricated index finger into the anus and lower rectum. Note the tone of the anus at rest (eg, excessively tight vs lax). Palpate the circumference of the anus, sweeping side to side while assessing for any tenderness, mass, or induration—if present, note the anatomic quadrant. If a mass is felt, note whether it is firm or soft, fixed or mobile, and broad-based or pedunculated. When the lubricated finger is removed from the anus, note whether blood is present.
Anoscopy
After DRE, visually inspect the anorectum. The instrument used varies from anoscope to rigid proctoscope to flexible sigmoidoscope. In a primary care setting, the most likely available instrument is an anoscope. The average anoscope is about 7 cm long and 2 cm in diameter, with a beveled tip and an obturator (see Figure 1), and allows a 360° view of the anal canal.7

Examination of the anal canal is accomplished by dividing the canal into the four anatomic quadrants described earlier and inserting the lubricated anoscope for inspection of each of the four quadrants. Observe the rectal mucosa and the anus as the scope is slowly withdrawn. If abnormalities are seen, note the location, size, shape, and any other descriptive features.
It is not necessary to perform a Hemoccult test after examination of the anorectum, as the presence of minor blood may be the direct result of the exam itself and thus provides no useful information to the examiner.
COMMON PATHOLOGIES
Once the history and physical exam are complete, a differential diagnosis can be formulated. Most anorectal disorders are benign conditions that pose no immediate health threat and can be managed in the primary care setting. Others, however, can be more serious and should prompt referral to a colorectal specialist for further evaluation. Knowing the difference can spare a patient unnecessary anxiety and referral; it can also lead to prompt, lifesaving interventions if red flags are recognized.
Hemorrhoids
Hemorrhoids are a common anorectal complaint.8,9 It is estimated that up to 75% of the population will experience symptoms of hemorrhoids during their lifetime.5,8 Whether internal or external, in their normal, nonpathologic, quiescent state, hemorrhoids are asymptomatic. Hemorrhoids become symptomatic when the supporting structures of hemorrhoidal tissue (ie, the anal cushions) deteriorate, resulting in venous dilation, inflammation, and thrombosis, which in turn lead to swelling, bright red bleeding, and/or prolapse.2,10 The most common causes of hemorrhoidal disease are chronic constipation and prolonged straining with bowel movements, though chronic diarrhea and pregnancy have also been identified as risk factors.2,8,11
External hemorrhoids, which are located distal to the dentate line, are typically only visible when they become thrombosed or swollen. In this state, they may manifest as acute-onset, exquisitely painful, large, purple-to-blue bulges at the anal outlet (see Figure 2). The number and size of the lesions can vary. The patient may report pain when sitting or wiping, as well as bleeding from the lesion.12,13 The pain is typically severe in the first couple of days, then slowly starts to subside.2,12

For internal hemorrhoids, which are located proximal to the dentate line, the main symptom is usually painless bright red blood per rectum.8,11,12 Patients may also report a sensation of rectal fullness or experience prolapse of the hemorrhoid through the anus. Prolapse typically occurs with defecation; in more severe cases, it can also occur between bowel movements, usually with any activity that increases intra-abdominal pressure (eg, coughing, heavy lifting, pregnancy, portal hypertension). The prolapse may reduce spontaneously, or may have to be manually reduced. If it cannot be reduced, there is a risk for incarceration or strangulation, potentially leading to gangrene.
The presence of bleeding and/or prolapse determines the classification of internal hemorrhoids (see Table 2). Dietary and lifestyle modification are used in the management of all grades of hemorrhoids. In addition, for grade 1 and 2 lesions, topical medication (eg, anti-inflammatory cream) can be used, whereas grade 3 (and selected grade 2) lesions respond well to rubber band ligation. Given the severity of grade 4 lesions, surgical intervention (eg, hemorrhoidectomy) is usually indicated.10

About a third of patients with symptomatic hemorrhoids seek clinical treatment.14 Most are hemodynamically stable and require no imaging and usually no labs (unless anemia is suspected).2 Management depends on the location and degree of symptoms (eg, internal vs external, prolapse, or thrombosis). In the event of an acutely thrombosed external hemorrhoid, clot excision for pain relief is appropriate if symptoms have been present for less than 48 to 72 hours; after that amount of time, the pain from the procedure will likely exceed the degree of relief provided, and conservative management should instead be recommended.2,8,11
Firstline treatment consists of lifestyle modification with a high-fiber diet and daily fiber supplement to ensure stool is soft and easy to pass.2,8 A meta-analysis of seven clinical trials with a total of 378 patients with hemorrhoids showed that fiber supplementation resulted in a 50% decrease in bleeding risk from internal hemorrhoids.15
Adequate hydration, preferably with noncaffeinated liquids, is also recommended. This will prevent constipation and the need to strain or spend excessive time on the toilet. Sitz baths can help alleviate pain and discomfort.
Several OTC topical medications are marketed for hemorrhoid relief. Many of these preparations contain steroids for their anti-inflammatory effects or astringents to address skin irritation that can result from anal leakage if prolapsing hemorrhoids prevent the anal outlet from closing. Steroid use should be limited to five to seven days, due to atrophic effects on the skin. While OTC preparations may temporarily alleviate discomfort, they will not address the underlying cause of symptoms.
Indications for referral to a colorectal specialist for symptomatic hemorrhoids include failure to improve with conservative management, persistent patient discomfort, and prolapse, as these indicate potential need for more invasive treatment.
Perianal skin tags
Perianal skin tags, while a nuisance, are not pathologic in most instances and pose no threat to health. They are an outgrowth of normal skin, appearing as loose, flesh-colored perianal tissues (see Figure 3). Tags range in size from a few millimeters to a centimeter long and can occur alone or in multiples.

Perianal skin tags are diagnosed clinically and require no labwork or imaging. Visual inspection is typically sufficient to distinguish tags from pathologic lesions such as condyloma or abscess. If there is uncertainty, however, biopsy or referral to a specialist is warranted.
Certain medical conditions can predispose a patient to development of perianal skin tags. They can be sequelae of thrombosed external hemorrhoids.8,11 They are also common in patients with Crohn disease.11 Perianal skin tags are not, however, the result of anal intercourse or sexually transmitted infections.
Treatment is usually not indicated for perianal skin tags. If the tags interfere with hygiene or cause perianal discomfort or significantly decreased quality of life, however, patients may seek removal. These patients should be referred to a colorectal specialist for evaluation for excision.
Anal fissures (fissure in ano)
The most common cause of severe anorectal pain is fissure.4 A fissure is an elliptical tear, or split, in the lining of the anal canal that causes spasm of the anal sphincters. The tear is distal to the dentate line and thus intensely painful.2,5 Common cited causes of fissures are trauma from passage of large, hard stools, straining, or diarrhea.16
Fissures can usually be visualized by spreading the posterior anus apart (see Figure 4). They are most commonly located in the posterior or anterior midline, though they can occur anywhere around the anus.2,4 Often, a sentinel tag—appearing as a taut, flesh-colored skin tag—is present at the external pole of the fissure.5,11 DRE and anoscopy should be avoided, as they will trigger intense pain and spasm of the sphincters.

Fissures are characterized as acute (present ≤ 3 months) or chronic (> 3 months).9,11 Visually, acute fissures typically have clean edges, with the appearance of a paper cut to the mucosa, while chronic fissures have indurated, heaped-up edges, often with exposure of the underlying sphincter muscle.17
They tend to be exquisitely painful, as the mucosa distal to the dentate line is highly innervated. Patients report pain akin to “passing shards of broken glass” with bowel movements, which is often accompanied by a fear of defecation and bright red blood on the toilet paper or dripping into the water.11 The pain, caused by spasm of the sphincters, typically starts during a bowel movement and lasts minutes to hours afterward.
Initial treatment is aimed at relaxing the sphincters, as well as softening stool to prevent further trauma and allow the fissure to heal. Patients should be educated about the importance of adequate fiber intake to prevent constipation and straining. A daily bulk fiber supplement, in addition to a high-fiber diet (20-25 g/d), has been shown to result in healing of 87% of acute fissures.16 Sitz baths in plain warm water, three to four times a day, can encourage relaxation of the sphincters and increase local blood flow, both of which help with fissure healing.16 Topical medications can also be prescribed. These include compounded nitroglycerin 0.2% or nifedipine 2.0%, which act to reduce the spasm by relaxing smooth muscle, as well as increase blood flow to the lesion.12,14
Most acute fissures will heal with the regimen of high fiber intake, sitz baths, and topical medication. For refractory or chronic fissures, referral to a colorectal specialist for more invasive treatment is appropriate. Additionally, fissures that are not located in the typical posterior or anterior midline might indicate an atypical etiology, such as Crohn disease, tuberculosis, leukemia, or HIV, and thus patients who present with fissures in these locations should also be referred to a colorectal specialist.2,4,11,18
Pruritus ani
Pruritus ani, known as perianal dermatitis, is a benign condition that presents with intense perianal itching and burning. It is the second most common anorectal condition after hemorrhoids and affects up to 5% of the US population.2,9,11,19
Pruritus ani often develops secondary to local irritation of the skin (eg, from prolonged exposure to moisture), leading to an inflammatory response within the superficial skin layers. The irritation causes patients to scratch the skin, resulting in trauma, excoriation, and ulcer formation and leading to a cycle of further inflammation, exacerbation of symptoms, and persistent scratching.
Physical exam may reveal circumferential erythematous and irritated perianal skin (see Figure 5). Linear or deep, punched-out excoriations may be present. Chronically, patients may develop lichenification with thick, whitened patches of skin.11 In the absence of red flags such as unintentional weight loss, anemia, rectal bleeding, or a family history of colon cancer, no additional evaluation is required during the initial visit, though anoscopy can be used to rule out associated anorectal pathology.

Many different causes of pruritus ani have been reported (see Table 3). In the case of an identifiable cause, symptoms tend to resolve once the offending agent is eliminated. Up to a quarter of cases, however, are idiopathic, with no identifiable trigger.20

Symptom management is thus key. Patients should be educated about lifestyle modification and informed that scratching will irritate the skin further and aggravate symptoms.5,11 If incontinence or diarrhea is thought to cause symptoms, dietary modifications, a fiber supplement, dysmotility agents, and Kegel exercises to strengthen the sphincters and decrease anal leakage can be recommended.
The affected skin should be kept clean and dry at all times. Aggressive wiping and overzealous hygiene should be avoided. Sitz baths can help with hygiene. A topical astringent such as witch hazel can help remove excess moisture from the skin. A layer of protective skin barrier cream with zinc oxide, when applied over dry skin, can help protect the skin from leakage throughout the day.
A sedating antihistamine can reduce scratching during sleep. Topical hydrocortisone 1% cream is effective for itch relief; this should be limited to five to seven days of consecutive use, however, as it can lead to pathologic thinning of the perianal skin. Topical capsaicin 0.006% cream has been shown to help alleviate intractable pruritus.2,21
The goal of these measures is to break the cycle of irritation and inflammation and give the skin an opportunity to heal. If the patient fails to improve, referral to a colorectal specialist or, alternatively, a dermatologist, is warranted for perianal skin biopsy and more invasive treatment options.
Perianal abscess
A perianal abscess is an infected cavity filled with pus under pressure, located near the anus or rectum. It most often results from an acute infection of the anorectal glands located at the dentate line that tracks outward to the perianal skin.12 Abscesses can also result from another disease process, such as Crohn disease, diabetes, or rectal trauma.2,11
Localized pain, swelling, and drainage are common presenting symptoms of an abscess.2,11 Systemic symptoms such as fever and chills may present later in the course but are rare.2
Treatment of the acute process is incision and drainage (I&D).11,12 This can be accomplished in the office with injection of local anesthesia, followed by excision of an ellipse of skin overlying the cavity large enough to allow full drainage of the abscess. Use of drains and packing of the wound are usually not necessary. There is also no role for antibiotics unless the patient is diabetic or immunosuppressed or cellulitis is present.11 In patients with systemic signs of illness, imaging such as CT or MRI of the pelvis can be used to assess for a deeper infection.2,12
For most patients, I&D resolves the process. However, up to half of perianal abscesses progress to form a fistula, a tunnel connecting the infected anal gland to the external skin.2,11,22 Indeed, abscesses and fistulae are part of the same infectious process, with the abscess representing the acute phase of infection and the fistula representing the chronic phase. On physical exam, in addition to the abscess site on the skin, a fistula may manifest as a palpable cord beneath the skin between the anus and the abscess opening.9 Additionally, the patient may report the abscess has been recurrent in nature, cyclically increasing in size and pain, then spontaneously draining.
A fistula requires surgical intervention for definitive treatment and should therefore prompt referral to a colorectal specialist. In the absence of fistula, however, a simple perianal abscess can be treated with I&D in the primary care setting.
Condyloma acuminatum
Condyloma acuminatum, also known as genital warts, is the most common sexually transmitted infection in the United States and a frequent anorectal complaint.23-25 More than 6 million new infections occur annually.25
Condyloma is caused by the human papillomavirus (HPV).24 More than 100 HPV subtypes have been identified.25 Types 6 and 11 are associated with typical condyloma acuminatum, while types 16 and 18 are found more commonly with dysplasia and malignant transformation.3,26
The lesions are spread by direct skin-to-skin or mucosa-to-mucosa contact, including anal intercourse. They appear as tiny finger-like projections on the perianal or genital skin, often with a cauliflower-like appearance, in clusters or as single entities, and ranging in size from a few millimeters to several centimeters (see Figure 6).

The patient may be asymptomatic or may complain of bleeding with defecation or leakage from the rectum between bowel movements. In up to 90% of perianal condyloma, concomitant anorectal lesions are present.3 Anoscopy is therefore indicated whenever perianal lesions are seen.
Treatment of genital warts varies depending on the number and location of lesions. The goal is lesion removal, but none of the available treatments are curative.24 If only a few small (diameter < 2 mm) warts are seen in the pe
Trichloroacetic acid (TCA), which is considered a form of chemical cautery, has been deemed more effective for tiny warts (diameter 1-2 mm) and better tolerated than podophyllin.24 Unlike podophyllin, however, TCA must be applied in clinic by a specialist, not at home by the patient. It works by chemically burning, cauterizing, and eroding skin and mucosa, thereby destroying old infected cells and allowing for growth of new, healthy cells.24 Using a solution with a higher concentration of TCA (80%-90%) has been shown to be more effective than lower concentration solutions (30%).24 The recurrence rate is high, generally about 36%.27 Due to its low risk for systemic absorption, TCA is safe for use during pregnancy.24
Local application of an immunomodulator, such as imiquimod 2%, 3.75%, or 5% cream, can also be used. Imiquimod works by upregulating tumor necrosis factor, leading to decreased viral replication and subsequent regression of warts.24 The most common side effects of imiquimod are erythema, erosions, burning, and pruritus.24 The higher concentration is associated with increased clearance rates as well as increased adverse effects.27 Therefore, the 3.75% cream is the preferred concentration of most providers.27 Recurrence rates range from 13% to 19%.28
In 2006, the FDA approved polyphenon E, a botanical ointment, to treat anogenital warts. The formulation is composed of eight different sinecatechins extracted from green tea leaves.29 Green tea sinecatechins have been shown to have antioxidant, antiproliferative, and antiviral properties, though the specific mechanisms of action in inhibiting anogenital wart growth have not been well studied to date. In a multicenter, randomized, double-blind, vehicle-controlled, three-arm parallel group phase 3 trial of 495 patients with anogenital warts, sinecatechins ointment 10% or 15% applied three times daily for 16 weeks resulted in complete clearance of warts in 57.2% and 56.3% of patients, respectively, compared to 33.7% clearance in the vehicle patients.30 Partial clearance (≥ 50%) of warts was observed in 78.4% of patients who used the 15% ointment, 74.0% of those who used the 10% ointment, and 51.5% of the vehicle patients. Rates of recurrence were 6.5% and 8.3% with use of the 10% and 15% sinecatechins ointment, respectively, which are much lower than recurrence rates observed with other topical applications (eg, imiquimod and podophyllotoxin).30
For larger warts or when there are anal lesions present, surgical excision and fulguration is required and referral to a colorectal specialist is warranted.
Warts recur from 4.6% to more than 70% of the time, most commonly due to activation of latent virus.23 Therefore, whether initially treated in an office or operating room, condyloma patients require follow-up on a regular basis, generally every six to 12 months, to assess for recurrent anal lesions so that treatment can be promptly initiated.
In the absence of a cure for anogenital warts, prevention through an HPV vaccine is important. The quadrivalent HPV vaccine (Gardasil) was approved by the FDA in 2006 for prophylactic vaccination of females ages 9 to 26. The vaccine triggers host formation of antibodies against four common subtypes of HPV: 6, 11, 16, and 18.31 In 2009, the FDA expanded the use of Gardasil to include males ages 9 to 26; subsequently, Gardasil 9 (which protects against nine types of HPV) was approved for males ages 9 to 15.32 In a group of 4,065 healthy males between the ages of 16 and 26, Gardasil reduced the incidence of external anogenital warts by 90.4%.33
HPV vaccine is now recommended for females through age 26 and males through age 21. The CDC also recommends HPV vaccine for the following individuals through age 26 if they did not get vaccinated when they were younger:
- Young men who have sex with men
- Young men who identify as gay or bisexual or who intend to have sex with men
- Young adults who are transgender
- Young adults with immunocompromising conditions (including HIV).34
CONCLUSION
Anorectal conditions are a common presentation in the primary care setting. A thorough history and physical exam will usually determine the etiology, and most benign pathologies can be successfully treated in the primary care clinic. Knowing how to perform an anorectal exam and having a thorough understanding of the most common anorectal pathologies can help alleviate examiner discomfort, while ensuring that patients receive prompt and adequate care. At the same time, recognizing the red flags can expedite referral for colorectal specialty evaluation when appropriate. Red flags that should prompt immediate referral to a colorectal specialist include older age, unintentional weight loss, iron deficiency anemia, family history of inflammatory bowel disease or colorectal cancer, and persistent anorectal bleeding or persistent symptoms despite adequate treatment of a suspected benign condition.
1. Grucela A, Salinas H, Khaitov S, et al. Prospective analysis of clinical accuracy in the diagnosis of benign anal pathology, comparison across specialties and years of experience. Dis Colon Rectum. 2010;53(1):47-52.
2. Tupe CL, Pham TV. Anorectal complaints in the emergency department. Emerg Med Clin North Am. 2016;34(2):251-270.
3. Billingham RP, Isler JT, Kimmins MH, et al. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(7):586-645.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15(26):3201-3209.
5. Henderson PK, Cash BD. Common anorectal conditions: evaluation and treatment. Curr Gastroenterol Rep. 2014; 16(10):408.
6. Gebbensleben O, Hilger Y, Rohde H. Patients’ view of medical positioning for proctologic examination. Clin Exp Gastroenterol. 2009;2:133-138.
7. Gopal DV. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4): 34-48.
8. Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol. 2015;21(31):9245-9252.
9. Fargo MV, Latimer K. Evaluation and management of common anorectal conditions. Am Fam Physician. 2012;85(6):624-630.
10. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17): 2009-2017.
11. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294-301.
12. Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22(26):5867-5878.
13. Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative treatment or surgical management. Dis Colon Rectum. 2004(9);47:1493-1498.
14. Summers A. Assessment and treatment of three common anorectal conditions. Emerg Nurse. 2013;21(2):28-33.
15. Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101(1):181-188.
16. Medhi B, Rao RS, Prakash A, et al. Recent advances in the pharmacotherapy of chronic anal fissure: an update. Asian J Surg. 2008;31(3):154-163.
17. Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014;2014. pii: 0407.
18. deRosa M, Cestaro G, Vitiello C, et al. Conservative versus surgical treatment for chronic idiopathic anal fissure: a prospective randomized trial. Updates Surg. 2013;65(3):197-200.
19. Lacy B, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413-419.
20. Siddiqi S, Vijay V, Ward M, et al. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457-463.
21. Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomized, placebo controlled, crossover study. Gut. 2003;52(9):1323-1326.
22. Burnstein M. Managing anorectal emergencies. Can Fam Physician. 1993;39:1782-1785.
23. Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636-2641.
24. Kollipara R, Ekhlassi E, Downing C, et al. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J Clin Med. 2015;4(5):832-846.
25. CDC. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 5: Human papillomavirus (HPV). www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed October 18, 2017.
26. Leszczyszyn J, Lebski I, Lysenko L, et al. Anal warts (condyloma acuminatum)—current issues and treatment modalities. Adv Clin Exp Med. 2014;23(2):307-311.
27. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105.
28. Yan J, Chen SL, Wang HN, et al. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminate. Dermatology. 2006;213(3):218-223.
29. Hoy SM. Polyphenon E 10% ointment: in immunocompetent adults with external genital and perianal warts. Am J Clin Dermatol. 2012;13(4):275-281.
30. Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet Gynecol. 2008;111(6):1371-1379.
31. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed October 18, 2017.
32. National Institutes of Health, National Cancer Institute. Human papillomavirus (HPV) vaccine. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet#q5. Accessed October 18, 2017.
33. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
34. CDC. HPV vaccines: vaccinating your preteen or teen. www.cdc.gov/hpv/parents/vaccine.html. Accessed October 18, 2017.
1. Grucela A, Salinas H, Khaitov S, et al. Prospective analysis of clinical accuracy in the diagnosis of benign anal pathology, comparison across specialties and years of experience. Dis Colon Rectum. 2010;53(1):47-52.
2. Tupe CL, Pham TV. Anorectal complaints in the emergency department. Emerg Med Clin North Am. 2016;34(2):251-270.
3. Billingham RP, Isler JT, Kimmins MH, et al. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(7):586-645.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15(26):3201-3209.
5. Henderson PK, Cash BD. Common anorectal conditions: evaluation and treatment. Curr Gastroenterol Rep. 2014; 16(10):408.
6. Gebbensleben O, Hilger Y, Rohde H. Patients’ view of medical positioning for proctologic examination. Clin Exp Gastroenterol. 2009;2:133-138.
7. Gopal DV. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4): 34-48.
8. Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol. 2015;21(31):9245-9252.
9. Fargo MV, Latimer K. Evaluation and management of common anorectal conditions. Am Fam Physician. 2012;85(6):624-630.
10. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17): 2009-2017.
11. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294-301.
12. Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22(26):5867-5878.
13. Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative treatment or surgical management. Dis Colon Rectum. 2004(9);47:1493-1498.
14. Summers A. Assessment and treatment of three common anorectal conditions. Emerg Nurse. 2013;21(2):28-33.
15. Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101(1):181-188.
16. Medhi B, Rao RS, Prakash A, et al. Recent advances in the pharmacotherapy of chronic anal fissure: an update. Asian J Surg. 2008;31(3):154-163.
17. Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014;2014. pii: 0407.
18. deRosa M, Cestaro G, Vitiello C, et al. Conservative versus surgical treatment for chronic idiopathic anal fissure: a prospective randomized trial. Updates Surg. 2013;65(3):197-200.
19. Lacy B, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413-419.
20. Siddiqi S, Vijay V, Ward M, et al. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457-463.
21. Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomized, placebo controlled, crossover study. Gut. 2003;52(9):1323-1326.
22. Burnstein M. Managing anorectal emergencies. Can Fam Physician. 1993;39:1782-1785.
23. Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636-2641.
24. Kollipara R, Ekhlassi E, Downing C, et al. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J Clin Med. 2015;4(5):832-846.
25. CDC. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 5: Human papillomavirus (HPV). www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed October 18, 2017.
26. Leszczyszyn J, Lebski I, Lysenko L, et al. Anal warts (condyloma acuminatum)—current issues and treatment modalities. Adv Clin Exp Med. 2014;23(2):307-311.
27. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105.
28. Yan J, Chen SL, Wang HN, et al. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminate. Dermatology. 2006;213(3):218-223.
29. Hoy SM. Polyphenon E 10% ointment: in immunocompetent adults with external genital and perianal warts. Am J Clin Dermatol. 2012;13(4):275-281.
30. Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet Gynecol. 2008;111(6):1371-1379.
31. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed October 18, 2017.
32. National Institutes of Health, National Cancer Institute. Human papillomavirus (HPV) vaccine. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet#q5. Accessed October 18, 2017.
33. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
34. CDC. HPV vaccines: vaccinating your preteen or teen. www.cdc.gov/hpv/parents/vaccine.html. Accessed October 18, 2017.
Pharmacologic Therapy for Acne: A Primer for Primary Care
CE/CME No: CR-1710
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the main factors involved in the pathogenesis of acne.
• Assess acne severity and classify acne as mild, moderate, or severe.
• Describe available acne therapies, including their mechanisms of action, indications, and potential adverse effects.
• Identify strategies patients can employ to mitigate the adverse effects of acne treatments.
FACULTY
Janet Purath is an Associate Professor at Washington State University in Spokane, Washington. Theresa Coyner practices at Randall Dermatology, West Lafayette, Indiana.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2018.
Article begins on next page >>
Many of the 50 million persons affected by acne in the United States present to primary care. Acne severity guides treatment choices, which include topical antibiotics and retinoids, hormonal agents, and systemic antibiotics and retinoids. Formulating a treatment plan requires a thorough understanding of the dosing, mechanism of action, and potential adverse effects of available medications.
Acne vulgaris (acne) is a common skin condition that is frequently encountered in primary care. Acne affects up to 50 million people in the United States, and about 85% of teenagers experience it at some point.1 Costs for treatment exceed $3 billion per year.2 Although commonly considered a condition of adolescence and young adults (85% prevalence), acne may persist in both men and women well into their 30s and 40s (43% prevalence). In fact, 5% of women ages 40 and older may experience acne.3
Acne is associated with considerable, long-lasting psychological sequelae, even in those with mild conditions, as many affected patients experience self-esteem issues and may avoid social interactions.4 Recognition of patients’ concerns about acne will help to promote a trusting patient-provider relationship. This article describes the pathophysiology and classifications of acne and reviews therapeutic options, enabling the practitioner to initiate treatment.
PRESENTATION AND ASSESSMENT
Acne lesions may occur on the face, neck, trunk, and extremities. The severity of acne is assessed based on lesion type, number, and size, and this grading is used to inform decisions about treatment options. Mild acne is characterized by plugging of the sebaceous gland (comedones), with small numbers of inflammatory papules and pustules. Moderate acne involves a larger number of inflammatory papules/pustules as well as the presence of small cystic nodules. Severe acne is marked by the presence of large numbers of noninflammatory and inflammatory lesions and cystic nodules or widespread involvement of these lesions.5 Examples of mild, moderate, and severe acne are shown in Figure 1. Assessment should include questions about the patient’s experiences with prior therapies.
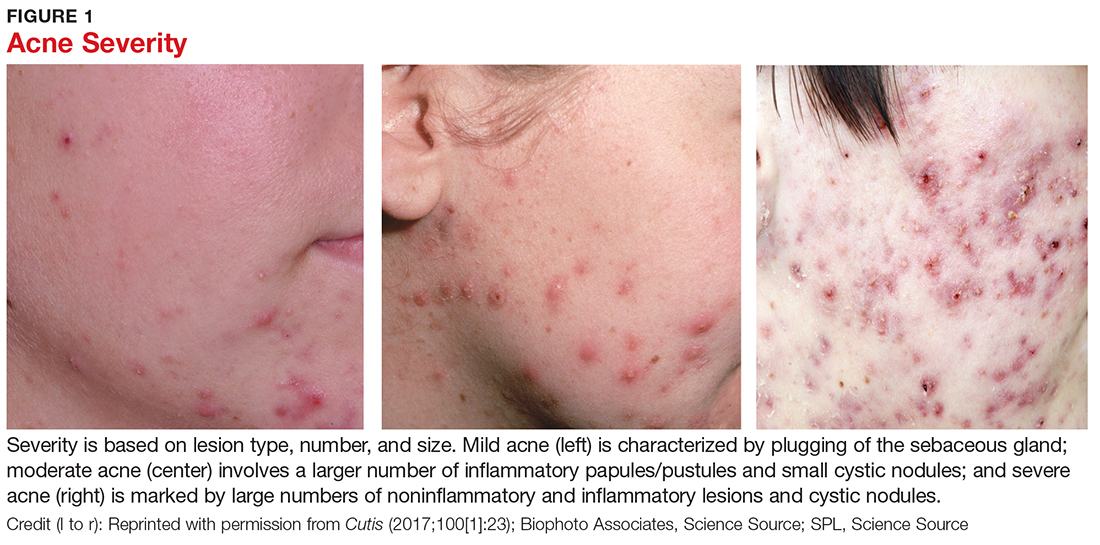
PATHOGENESIS
The pathogenesis of acne is a complex process involving multiple factors (see Figure 2). Knowledge about acne pathogenesis continues to evolve, but the current view is that a combination of simultaneous noninflammatory and inflammatory events involving pilosebaceous units (which consist of sebaceous glands and hair follicles) contribute to its development.6 Activation of the sebaceous glands is influenced by androgens, which increase sebum production and shedding of the keratinocytes lining the gland. Plugging of the pilosebaceous canal ensues, leading to the development of a microcomedone. Increased proliferation of Propionibacterium acnes occurs within the obstructed gland. The inflammatory response to this process includes a cascade of numerous cytokines, most notably toll-like receptor 2 (TLR-2).7 The plug at the opening of the sebaceous gland creates either an open comedone (blackhead) or a closed comedone (whitehead). Eventually, the follicular wall ruptures, leading to the formation of erythematous papules and pustules on the skin surface or deep-seated cystic structures under the skin surface. Current pharmacologic agents target one or more of these identified factors underlying acne pathogenesis.
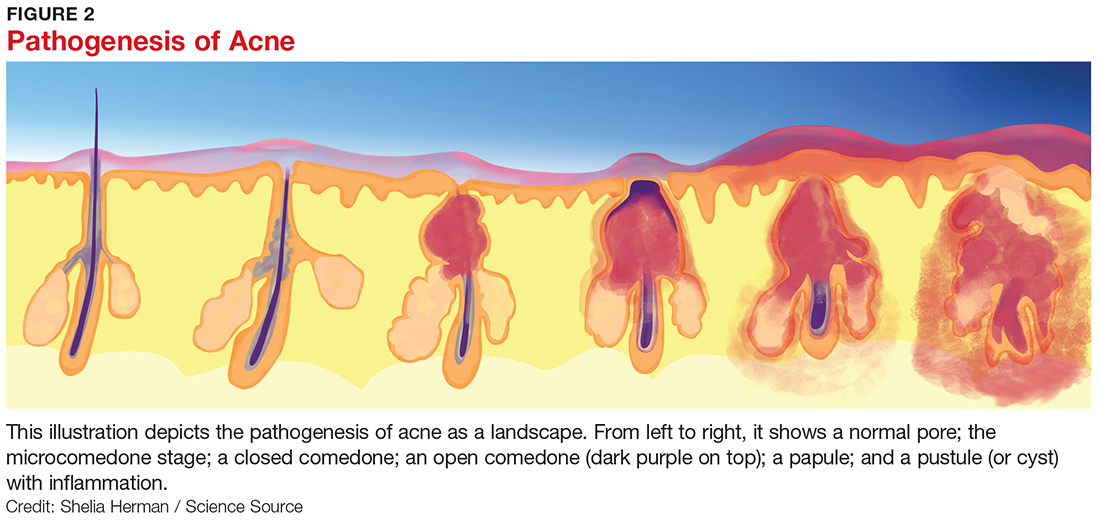
THERAPEUTIC OPTIONS
Pharmacologic treatment options for acne include topical, systemic, and hormonal agents. Topical and systemic therapies reduce inflammation and follicular plugging. Topical treatments include antibiotics, anti-inflammatories, and retinoids. Oral treatments include antibiotics, hormones, and retinoids. The clinician must have a thorough understanding of the actions, potential adverse reactions, and drug interactions of each proposed therapy prior to formulating a treatment plan.
Topical retinoids
Topical retinoids are the most effective comedolytic agents available.1 Since comedones are thought to be the precursor of all other acne lesions, retinoids are appropriate for cases in which comedones are seen.1 Retinoids belong to a class of compounds structurally related to vitamin A. Topical retinoids act by promoting normal follicular keratinocyte desquamation, which prevents obstruction of the pilosebaceous canal and thereby inhibits the formation of microcomedones.8
They also exhibit anti-inflammatory action via inhibition of TLR-2.9 The comedolytic and anti-inflammatory actions of topical retinoids make them a mainstay of acne treatment, although some patients are unable to tolerate their adverse effects, which include erythema and dryness related to increases in transepidermal water loss. Application of noncomedogenic emollients can improve these common effects.10 The newer micronized and time-release retinoid formulations may have less potential for irritation.8 Vehicle formulation and concentration also play a role in skin irritation, with gels and liquids and formulations with higher concentrations of retinoids generally causing more drying than creams and lower potency formulations.8 Table 1 summarizes the mechanisms of action, available formulations, and potential adverse effects of the topical retinoids and other topical agents.1,6,9-16
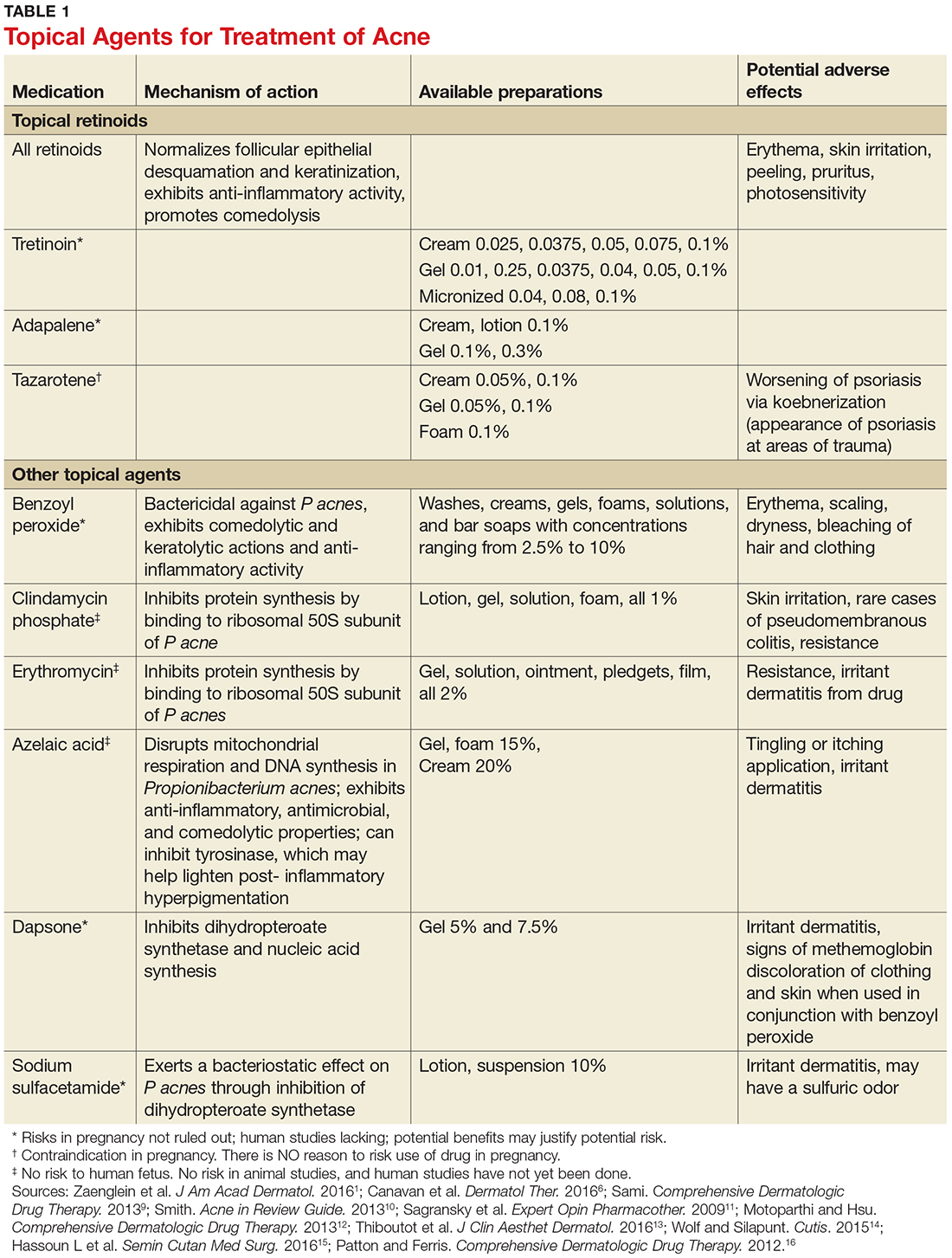
It is important to note that retinoids can adversely affect the developing fetus when absorbed in large quantities. Notably, tazarotene is assigned to pregnancy category X because when it is used to treat psoriasis, one of its approved indications, large surface areas may be treated, increasing absorption. Absorption amounts are extremely low when tazarotene is used to treat acne. Nevertheless, verification of a negative pregnancy test is recommended prior to initiating tazarotene therapy. Effective birth control measures should be utilized throughout therapy. Even though other commonly used retinoids (tretinoin and adapalene) are assigned to pregnancy category C, all topical retinoids should be avoided during pregnancy.9
As noted, patient education is key for increasing patient adherence to therapy. Patients should be instructed to use a small (pea-sized) amount of medication for the entire face. Providers should also inform patients that transient erythema and dryness can be expected, and that application of a noncomedolytic moisturizer may reduce irritation. Tretinoin is best used at night,1 and it is useful to advise that erythema and irritation associated with retinoid use can be reduced by initially using the medication every other night to every third night, gradually building up to nightly use.1
Topical antibiotic and anti-inflammatory agents
Topical agents used to treat inflammatory lesions include benzoyl peroxide, erythromycin, clindamycin, dapsone, azelaic acid, and sulfacetamide (Table 1).1,6,9-16 These topical agents are generally well tolerated, with most adverse reactions limited to facial irritation and erythema. They come in an array of vehicle formulations, including washes, creams, gels, solutions, foams, and lotions. Vehicle selection should be based upon patient preference and skin type. Gels and solutions have a drying effect, making them more appropriate for individuals with oily skin, whereas creams are moisturizing and appropriate for individuals with dry skin. Lotions are appropriate for all skin types.11
Benzoyl peroxide (BPO) has both keratolytic and comedolytic activity and is available in concentrations ranging from 2.5% to 10%. It is available OTC, as well as by prescription, and is thus readily accessed by the patient. Because BPO is bactericidal for P acnes, resistance to BPO among P acnes has not occurred.1 All concentrations are equally effective, but the higher concentrations are more likely to cause skin dryness and other adverse effects.12 Combination therapy with topical antibiotics, tretinoin, and BPO is more clinically effective than monotherapy.17 Combination products reduce the complexity of acne treatment and likely increase therapy adherence.11 Currently available combination products in various percentages are erythromycin with BPO, clindamycin with BPO, adapalene with BPO, and clindamycin with tretinoin.1
Oral antibiotics
Oral antibiotics should be reserved for use in situations where topical therapy is ineffective. All antibiotics are effective in treating acne due to their antimicrobial activity against P acnes.1 These agents play a key role in managing moderate to severe acne that is likely to scar, as well as in cases of widespread acne involving the face, arms, and trunk. Note that the use of oral antibiotics in acne treatment is controversial, as chronic use contributes to rising rates of bacterial resistance.18 For this reason, antibiotic therapy for acne should be limited to a duration of three months or less, and these agents should not be used as monotherapy.6 In particular, recent recommendations restrict the use of erythromycin for acne treatment due to an increase of P acnes resistance.1 Cephalosporins, macrolides, and penicillin class antibiotics are not routinely recommended due to lack of data regarding their clinical effectiveness in treating acne.1
Tetracycline class antibiotics are the most commonly used oral antibiotics for acne therapy, particularly doxycycline and minocycline.5 Common adverse effects include gastrointestinal upset, photosensitivity, and some pigmentation issues.19 Trimethoprim-sulfamethoxazole (TMP-SMX) is a folate synthesis inhibitor class antibiotic also used to treat acne. Its use should be reserved for individuals who are allergic to tetracyclines or in cases of acne resistant to other antimicrobials.1 Potential adverse reactions include photosensitivity and severe hypersensitivity conditions ranging from a mild rash to toxic epidermal necrolysis.19 Table 2 summarizes the dosage ranges, pregnancy category risk, and potential adverse effects of oral antibiotics used to treat acne.1,19,20
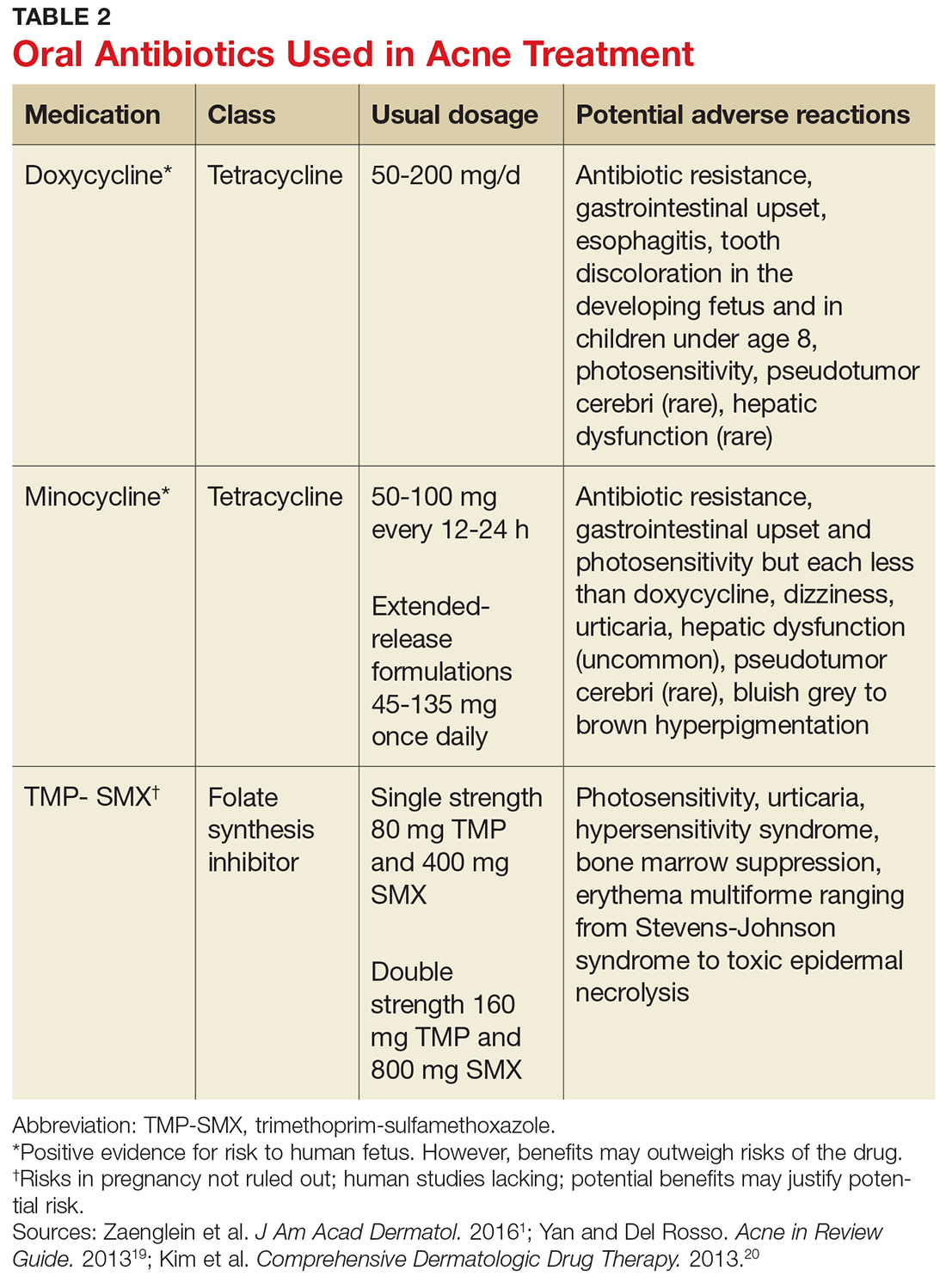
The firstline choice for treating moderate acne with papules and pustules is oral antibiotics with topical retinoids and BPO.5 Patients should be educated about potential adverse effects of these agents, including the development of antibiotic resistance.
Hormonal agents
Hormonal therapies should be reserved for females with acne lesions influenced by fluctuations in hormone levels.21 Pubertal changes initiate the production of adrenal dehydroepiandrosterone, which leads to increased testosterone production. Testosterone is converted to dihydrotestosterone (DHT), which binds to androgen receptors in the sebaceous glands, stimulating the glands and potentially increasing production of sebum. Hormonal agents act by reducing androgen activity in the sebaceous gland. Combined oral hormones, those containing both estrogen and progesterone, reduce the amount of free testosterone and ovarian androgens by suppressing ovulation.1 Hormonal therapy can be quite effective for females of childbearing age. Females who report acne flares with their menstrual cycles may be good candidates for hormonal therapy.1
The estrogen agent most frequently used in oral contraceptives is ethinyl estradiol. Numerous progesterone agents can also be used, but those with low
Spironolactone, a potassium-sparing diuretic, may also be appropriate for treating acne in women due to its antiandrogenic properties. The drug binds androgen receptors in the skin, which then blocks testosterone and DHT. Spironolactone can be an effective firstline agent in treating hormonal-pattern acne, which presents as inflammatory lesions located on the lower face and neck. In particular, it can be an appropriate choice for women with adult-onset acne.15 Spironolactone is not approved by the FDA for acne treatment, but it has been used successfully for many years.5 Spironolactone was found in rodent studies to cause feminization of the male rat fetus, so patients taking this drug should use reliable birth control methods. It can be used concomitantly with oral contraceptives.5 Common side effects include breast tenderness, diuretic effects, headaches, and menstrual irregularities. Although the risk for hypokalemia is low in healthy young women, it may be prudent to periodically assess potassium, sodium, and renal function in patients.1 Spironolactone should be avoided in patients with renal disease and those on other diuretics.15
Isotretinoin
Isotretinoin is an oral systemic retinoid that modulates nuclear receptors and regulates gene transcription in the epidermis.16 Isotretinoin’s mechanisms of action target the main pathogenic factors underlying acne, including reduction of follicular hyperkeratosis, comedogenesis, sebum production, and inflammation and suppression of P acnes.22 These combined actions make isotretinoin a highly effective treatment option for acne.
The drug is approved by the FDA for treatment of nodular acne refractory to traditional acne therapies.23 Isotretinoin is available in 10, 20, 25, 30, and 40 mg capsules, and the recommended dosing is 0.5 to 2.0 mg/kg/d. The usual course of therapy is 15 to 20 weeks or until an accumulative dosage of 120 to 150 mg/kg is attained.23 Patients should be instructed to take isotretinoin with meals, as oral availability is increased with high-fat foods.23
Isotretinoin has major adverse effects. It is a teratogenic medication that can cause congenital anomalies in exposed fetuses, including craniofacial, cardiac, and neurologic issues.16 Due to the seriousness of the congenital anomalies, all prescribers must be registered in the iPledge program, a computer-based risk management program instituted in 2006 by the FDA and the companies that manufacture isotretinoin to eliminate congenital risks associated with isotretinoin. All patients, both male and female, must sign an informed consent form when they register in the program.
Although the iPledge program does not mandate consistent condom use for male patients, they should be informed that minute amounts of isotretinoin can be found in semen. The risk for fathering a fetus with congenital anomalies when taking isotretinoin appears to be extremely low.16 Women of childbearing potential must commit to the use of two highly reliable forms of birth control when taking the medication, including one month before starting therapy and one month after completing therapy.16 Monthly pregnancy testing is mandatory throughout the course of treatment.24 Further information regarding the risk management program can be found at iPledgeprogram.com.
Isotretinoin is metabolized by the liver and may cause lipid abnormalities and hepatic enzyme elevations. Baseline and monthly laboratory monitoring of liver enzymes and cholesterol and triglyceride levels are recommended.24 The process of initiating and monitoring isotretinoin therapy is quite complex, and unless the practitioner plans to routinely prescribe this medication, patients needing isotretinoin therapy should be referred to a dermatology practice.
PATIENT EDUCATION
Patients are more likely to adhere to treatment when simplified regimens are used and when they have realistic expectations for therapy outcomes. Providers need to educate patients that all treatments may require at least two to three months of use before visible results occur. Initial and subsequent visits should include discussions about clear expectations and strategies to reduce potential adverse effects.
PUTTING IT ALL TOGETHER
Acne therapy starts with the use of a topical retinoid in mild acne cases, unless the patient is unable to tolerate the associated skin irritability. Addition of a topical antibiotic or anti-inflammatory agent, preferably BPO, either alone or with a combination product, is also recommended for mild to moderate acne. Patients with moderate to severe acne may benefit from a short course (three months or less) of antibiotics.
Oral hormones may be an excellent therapy choice when acne treatment is needed for women of childbearing age. Isotretinoin is indicated in select cases of severe acne resistant to other treatments.
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
2. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
3. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dertmatol. 2008;58(1):56-59.
4. Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
5. Baldwin HE, Zanglein AL, Leyden JJ, Webster GF. Pharmacologic treatment options in mild, moderate, and severe acne vulgaris. Semin Cutan Med Surg. 2015;34(supp5):S82-S85.
6. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther. 2016;6(4):555-578.
7. Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582-585.
8. Russell JJ. Topical therapy for acne. Am Fam Phys. 2000; 61(2):357-365.
9. Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St. Louis: Elsevier; 2013:505-517.
10. Smith RI. Treatments: Retinoids. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:59-63.
11. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555-2562.
12. Motoparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:452-459.
13. Thiboutot DM, Kircik L, McMichale A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10):18-27.
14. Wolf K, Silapunt S. The use of sodium sulfacetamide in dermatology. Cutis. 2015;96(2):128-130.
15. Hassoun LA, Chahal DS, Sivamani RK, Larsen LN. The use of hormonal agents in the treatment of acne. Semin Cutan Med Surg. 2016;35(2):68-73.
16. Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2012:252-268.
17. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-S50.
18. Walsh TR, Efthimious J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e22-32.
19. Yan AC, Del Rosso JQ. Prescription oral treatments: Antibiotics. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:77-87.
20. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:62-67, 70-74, 77-85.
21. Hecht CT, Sidbury R, Del Rosso JQ. Prescription oral treatments: Hormonal therapies. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:89-95.
22. Webster GF, Leyden JJ, Baldwin HE, Zaenglein AL. Isotretinoin: mechanism of action and patient selection. Semin Cutan Med Surg. 2015;34(supp 5):S86-S88.
23. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris. J Clin and Aesthet Dermatol. 2014;7(2 Suppl):S3-S21.
24. Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016-1022.
CE/CME No: CR-1710
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the main factors involved in the pathogenesis of acne.
• Assess acne severity and classify acne as mild, moderate, or severe.
• Describe available acne therapies, including their mechanisms of action, indications, and potential adverse effects.
• Identify strategies patients can employ to mitigate the adverse effects of acne treatments.
FACULTY
Janet Purath is an Associate Professor at Washington State University in Spokane, Washington. Theresa Coyner practices at Randall Dermatology, West Lafayette, Indiana.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2018.
Article begins on next page >>
Many of the 50 million persons affected by acne in the United States present to primary care. Acne severity guides treatment choices, which include topical antibiotics and retinoids, hormonal agents, and systemic antibiotics and retinoids. Formulating a treatment plan requires a thorough understanding of the dosing, mechanism of action, and potential adverse effects of available medications.
Acne vulgaris (acne) is a common skin condition that is frequently encountered in primary care. Acne affects up to 50 million people in the United States, and about 85% of teenagers experience it at some point.1 Costs for treatment exceed $3 billion per year.2 Although commonly considered a condition of adolescence and young adults (85% prevalence), acne may persist in both men and women well into their 30s and 40s (43% prevalence). In fact, 5% of women ages 40 and older may experience acne.3
Acne is associated with considerable, long-lasting psychological sequelae, even in those with mild conditions, as many affected patients experience self-esteem issues and may avoid social interactions.4 Recognition of patients’ concerns about acne will help to promote a trusting patient-provider relationship. This article describes the pathophysiology and classifications of acne and reviews therapeutic options, enabling the practitioner to initiate treatment.
PRESENTATION AND ASSESSMENT
Acne lesions may occur on the face, neck, trunk, and extremities. The severity of acne is assessed based on lesion type, number, and size, and this grading is used to inform decisions about treatment options. Mild acne is characterized by plugging of the sebaceous gland (comedones), with small numbers of inflammatory papules and pustules. Moderate acne involves a larger number of inflammatory papules/pustules as well as the presence of small cystic nodules. Severe acne is marked by the presence of large numbers of noninflammatory and inflammatory lesions and cystic nodules or widespread involvement of these lesions.5 Examples of mild, moderate, and severe acne are shown in Figure 1. Assessment should include questions about the patient’s experiences with prior therapies.

PATHOGENESIS
The pathogenesis of acne is a complex process involving multiple factors (see Figure 2). Knowledge about acne pathogenesis continues to evolve, but the current view is that a combination of simultaneous noninflammatory and inflammatory events involving pilosebaceous units (which consist of sebaceous glands and hair follicles) contribute to its development.6 Activation of the sebaceous glands is influenced by androgens, which increase sebum production and shedding of the keratinocytes lining the gland. Plugging of the pilosebaceous canal ensues, leading to the development of a microcomedone. Increased proliferation of Propionibacterium acnes occurs within the obstructed gland. The inflammatory response to this process includes a cascade of numerous cytokines, most notably toll-like receptor 2 (TLR-2).7 The plug at the opening of the sebaceous gland creates either an open comedone (blackhead) or a closed comedone (whitehead). Eventually, the follicular wall ruptures, leading to the formation of erythematous papules and pustules on the skin surface or deep-seated cystic structures under the skin surface. Current pharmacologic agents target one or more of these identified factors underlying acne pathogenesis.

THERAPEUTIC OPTIONS
Pharmacologic treatment options for acne include topical, systemic, and hormonal agents. Topical and systemic therapies reduce inflammation and follicular plugging. Topical treatments include antibiotics, anti-inflammatories, and retinoids. Oral treatments include antibiotics, hormones, and retinoids. The clinician must have a thorough understanding of the actions, potential adverse reactions, and drug interactions of each proposed therapy prior to formulating a treatment plan.
Topical retinoids
Topical retinoids are the most effective comedolytic agents available.1 Since comedones are thought to be the precursor of all other acne lesions, retinoids are appropriate for cases in which comedones are seen.1 Retinoids belong to a class of compounds structurally related to vitamin A. Topical retinoids act by promoting normal follicular keratinocyte desquamation, which prevents obstruction of the pilosebaceous canal and thereby inhibits the formation of microcomedones.8
They also exhibit anti-inflammatory action via inhibition of TLR-2.9 The comedolytic and anti-inflammatory actions of topical retinoids make them a mainstay of acne treatment, although some patients are unable to tolerate their adverse effects, which include erythema and dryness related to increases in transepidermal water loss. Application of noncomedogenic emollients can improve these common effects.10 The newer micronized and time-release retinoid formulations may have less potential for irritation.8 Vehicle formulation and concentration also play a role in skin irritation, with gels and liquids and formulations with higher concentrations of retinoids generally causing more drying than creams and lower potency formulations.8 Table 1 summarizes the mechanisms of action, available formulations, and potential adverse effects of the topical retinoids and other topical agents.1,6,9-16

It is important to note that retinoids can adversely affect the developing fetus when absorbed in large quantities. Notably, tazarotene is assigned to pregnancy category X because when it is used to treat psoriasis, one of its approved indications, large surface areas may be treated, increasing absorption. Absorption amounts are extremely low when tazarotene is used to treat acne. Nevertheless, verification of a negative pregnancy test is recommended prior to initiating tazarotene therapy. Effective birth control measures should be utilized throughout therapy. Even though other commonly used retinoids (tretinoin and adapalene) are assigned to pregnancy category C, all topical retinoids should be avoided during pregnancy.9
As noted, patient education is key for increasing patient adherence to therapy. Patients should be instructed to use a small (pea-sized) amount of medication for the entire face. Providers should also inform patients that transient erythema and dryness can be expected, and that application of a noncomedolytic moisturizer may reduce irritation. Tretinoin is best used at night,1 and it is useful to advise that erythema and irritation associated with retinoid use can be reduced by initially using the medication every other night to every third night, gradually building up to nightly use.1
Topical antibiotic and anti-inflammatory agents
Topical agents used to treat inflammatory lesions include benzoyl peroxide, erythromycin, clindamycin, dapsone, azelaic acid, and sulfacetamide (Table 1).1,6,9-16 These topical agents are generally well tolerated, with most adverse reactions limited to facial irritation and erythema. They come in an array of vehicle formulations, including washes, creams, gels, solutions, foams, and lotions. Vehicle selection should be based upon patient preference and skin type. Gels and solutions have a drying effect, making them more appropriate for individuals with oily skin, whereas creams are moisturizing and appropriate for individuals with dry skin. Lotions are appropriate for all skin types.11
Benzoyl peroxide (BPO) has both keratolytic and comedolytic activity and is available in concentrations ranging from 2.5% to 10%. It is available OTC, as well as by prescription, and is thus readily accessed by the patient. Because BPO is bactericidal for P acnes, resistance to BPO among P acnes has not occurred.1 All concentrations are equally effective, but the higher concentrations are more likely to cause skin dryness and other adverse effects.12 Combination therapy with topical antibiotics, tretinoin, and BPO is more clinically effective than monotherapy.17 Combination products reduce the complexity of acne treatment and likely increase therapy adherence.11 Currently available combination products in various percentages are erythromycin with BPO, clindamycin with BPO, adapalene with BPO, and clindamycin with tretinoin.1
Oral antibiotics
Oral antibiotics should be reserved for use in situations where topical therapy is ineffective. All antibiotics are effective in treating acne due to their antimicrobial activity against P acnes.1 These agents play a key role in managing moderate to severe acne that is likely to scar, as well as in cases of widespread acne involving the face, arms, and trunk. Note that the use of oral antibiotics in acne treatment is controversial, as chronic use contributes to rising rates of bacterial resistance.18 For this reason, antibiotic therapy for acne should be limited to a duration of three months or less, and these agents should not be used as monotherapy.6 In particular, recent recommendations restrict the use of erythromycin for acne treatment due to an increase of P acnes resistance.1 Cephalosporins, macrolides, and penicillin class antibiotics are not routinely recommended due to lack of data regarding their clinical effectiveness in treating acne.1
Tetracycline class antibiotics are the most commonly used oral antibiotics for acne therapy, particularly doxycycline and minocycline.5 Common adverse effects include gastrointestinal upset, photosensitivity, and some pigmentation issues.19 Trimethoprim-sulfamethoxazole (TMP-SMX) is a folate synthesis inhibitor class antibiotic also used to treat acne. Its use should be reserved for individuals who are allergic to tetracyclines or in cases of acne resistant to other antimicrobials.1 Potential adverse reactions include photosensitivity and severe hypersensitivity conditions ranging from a mild rash to toxic epidermal necrolysis.19 Table 2 summarizes the dosage ranges, pregnancy category risk, and potential adverse effects of oral antibiotics used to treat acne.1,19,20

The firstline choice for treating moderate acne with papules and pustules is oral antibiotics with topical retinoids and BPO.5 Patients should be educated about potential adverse effects of these agents, including the development of antibiotic resistance.
Hormonal agents
Hormonal therapies should be reserved for females with acne lesions influenced by fluctuations in hormone levels.21 Pubertal changes initiate the production of adrenal dehydroepiandrosterone, which leads to increased testosterone production. Testosterone is converted to dihydrotestosterone (DHT), which binds to androgen receptors in the sebaceous glands, stimulating the glands and potentially increasing production of sebum. Hormonal agents act by reducing androgen activity in the sebaceous gland. Combined oral hormones, those containing both estrogen and progesterone, reduce the amount of free testosterone and ovarian androgens by suppressing ovulation.1 Hormonal therapy can be quite effective for females of childbearing age. Females who report acne flares with their menstrual cycles may be good candidates for hormonal therapy.1
The estrogen agent most frequently used in oral contraceptives is ethinyl estradiol. Numerous progesterone agents can also be used, but those with low
Spironolactone, a potassium-sparing diuretic, may also be appropriate for treating acne in women due to its antiandrogenic properties. The drug binds androgen receptors in the skin, which then blocks testosterone and DHT. Spironolactone can be an effective firstline agent in treating hormonal-pattern acne, which presents as inflammatory lesions located on the lower face and neck. In particular, it can be an appropriate choice for women with adult-onset acne.15 Spironolactone is not approved by the FDA for acne treatment, but it has been used successfully for many years.5 Spironolactone was found in rodent studies to cause feminization of the male rat fetus, so patients taking this drug should use reliable birth control methods. It can be used concomitantly with oral contraceptives.5 Common side effects include breast tenderness, diuretic effects, headaches, and menstrual irregularities. Although the risk for hypokalemia is low in healthy young women, it may be prudent to periodically assess potassium, sodium, and renal function in patients.1 Spironolactone should be avoided in patients with renal disease and those on other diuretics.15
Isotretinoin
Isotretinoin is an oral systemic retinoid that modulates nuclear receptors and regulates gene transcription in the epidermis.16 Isotretinoin’s mechanisms of action target the main pathogenic factors underlying acne, including reduction of follicular hyperkeratosis, comedogenesis, sebum production, and inflammation and suppression of P acnes.22 These combined actions make isotretinoin a highly effective treatment option for acne.
The drug is approved by the FDA for treatment of nodular acne refractory to traditional acne therapies.23 Isotretinoin is available in 10, 20, 25, 30, and 40 mg capsules, and the recommended dosing is 0.5 to 2.0 mg/kg/d. The usual course of therapy is 15 to 20 weeks or until an accumulative dosage of 120 to 150 mg/kg is attained.23 Patients should be instructed to take isotretinoin with meals, as oral availability is increased with high-fat foods.23
Isotretinoin has major adverse effects. It is a teratogenic medication that can cause congenital anomalies in exposed fetuses, including craniofacial, cardiac, and neurologic issues.16 Due to the seriousness of the congenital anomalies, all prescribers must be registered in the iPledge program, a computer-based risk management program instituted in 2006 by the FDA and the companies that manufacture isotretinoin to eliminate congenital risks associated with isotretinoin. All patients, both male and female, must sign an informed consent form when they register in the program.
Although the iPledge program does not mandate consistent condom use for male patients, they should be informed that minute amounts of isotretinoin can be found in semen. The risk for fathering a fetus with congenital anomalies when taking isotretinoin appears to be extremely low.16 Women of childbearing potential must commit to the use of two highly reliable forms of birth control when taking the medication, including one month before starting therapy and one month after completing therapy.16 Monthly pregnancy testing is mandatory throughout the course of treatment.24 Further information regarding the risk management program can be found at iPledgeprogram.com.
Isotretinoin is metabolized by the liver and may cause lipid abnormalities and hepatic enzyme elevations. Baseline and monthly laboratory monitoring of liver enzymes and cholesterol and triglyceride levels are recommended.24 The process of initiating and monitoring isotretinoin therapy is quite complex, and unless the practitioner plans to routinely prescribe this medication, patients needing isotretinoin therapy should be referred to a dermatology practice.
PATIENT EDUCATION
Patients are more likely to adhere to treatment when simplified regimens are used and when they have realistic expectations for therapy outcomes. Providers need to educate patients that all treatments may require at least two to three months of use before visible results occur. Initial and subsequent visits should include discussions about clear expectations and strategies to reduce potential adverse effects.
PUTTING IT ALL TOGETHER
Acne therapy starts with the use of a topical retinoid in mild acne cases, unless the patient is unable to tolerate the associated skin irritability. Addition of a topical antibiotic or anti-inflammatory agent, preferably BPO, either alone or with a combination product, is also recommended for mild to moderate acne. Patients with moderate to severe acne may benefit from a short course (three months or less) of antibiotics.
Oral hormones may be an excellent therapy choice when acne treatment is needed for women of childbearing age. Isotretinoin is indicated in select cases of severe acne resistant to other treatments.
CE/CME No: CR-1710
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the main factors involved in the pathogenesis of acne.
• Assess acne severity and classify acne as mild, moderate, or severe.
• Describe available acne therapies, including their mechanisms of action, indications, and potential adverse effects.
• Identify strategies patients can employ to mitigate the adverse effects of acne treatments.
FACULTY
Janet Purath is an Associate Professor at Washington State University in Spokane, Washington. Theresa Coyner practices at Randall Dermatology, West Lafayette, Indiana.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2018.
Article begins on next page >>
Many of the 50 million persons affected by acne in the United States present to primary care. Acne severity guides treatment choices, which include topical antibiotics and retinoids, hormonal agents, and systemic antibiotics and retinoids. Formulating a treatment plan requires a thorough understanding of the dosing, mechanism of action, and potential adverse effects of available medications.
Acne vulgaris (acne) is a common skin condition that is frequently encountered in primary care. Acne affects up to 50 million people in the United States, and about 85% of teenagers experience it at some point.1 Costs for treatment exceed $3 billion per year.2 Although commonly considered a condition of adolescence and young adults (85% prevalence), acne may persist in both men and women well into their 30s and 40s (43% prevalence). In fact, 5% of women ages 40 and older may experience acne.3
Acne is associated with considerable, long-lasting psychological sequelae, even in those with mild conditions, as many affected patients experience self-esteem issues and may avoid social interactions.4 Recognition of patients’ concerns about acne will help to promote a trusting patient-provider relationship. This article describes the pathophysiology and classifications of acne and reviews therapeutic options, enabling the practitioner to initiate treatment.
PRESENTATION AND ASSESSMENT
Acne lesions may occur on the face, neck, trunk, and extremities. The severity of acne is assessed based on lesion type, number, and size, and this grading is used to inform decisions about treatment options. Mild acne is characterized by plugging of the sebaceous gland (comedones), with small numbers of inflammatory papules and pustules. Moderate acne involves a larger number of inflammatory papules/pustules as well as the presence of small cystic nodules. Severe acne is marked by the presence of large numbers of noninflammatory and inflammatory lesions and cystic nodules or widespread involvement of these lesions.5 Examples of mild, moderate, and severe acne are shown in Figure 1. Assessment should include questions about the patient’s experiences with prior therapies.

PATHOGENESIS
The pathogenesis of acne is a complex process involving multiple factors (see Figure 2). Knowledge about acne pathogenesis continues to evolve, but the current view is that a combination of simultaneous noninflammatory and inflammatory events involving pilosebaceous units (which consist of sebaceous glands and hair follicles) contribute to its development.6 Activation of the sebaceous glands is influenced by androgens, which increase sebum production and shedding of the keratinocytes lining the gland. Plugging of the pilosebaceous canal ensues, leading to the development of a microcomedone. Increased proliferation of Propionibacterium acnes occurs within the obstructed gland. The inflammatory response to this process includes a cascade of numerous cytokines, most notably toll-like receptor 2 (TLR-2).7 The plug at the opening of the sebaceous gland creates either an open comedone (blackhead) or a closed comedone (whitehead). Eventually, the follicular wall ruptures, leading to the formation of erythematous papules and pustules on the skin surface or deep-seated cystic structures under the skin surface. Current pharmacologic agents target one or more of these identified factors underlying acne pathogenesis.

THERAPEUTIC OPTIONS
Pharmacologic treatment options for acne include topical, systemic, and hormonal agents. Topical and systemic therapies reduce inflammation and follicular plugging. Topical treatments include antibiotics, anti-inflammatories, and retinoids. Oral treatments include antibiotics, hormones, and retinoids. The clinician must have a thorough understanding of the actions, potential adverse reactions, and drug interactions of each proposed therapy prior to formulating a treatment plan.
Topical retinoids
Topical retinoids are the most effective comedolytic agents available.1 Since comedones are thought to be the precursor of all other acne lesions, retinoids are appropriate for cases in which comedones are seen.1 Retinoids belong to a class of compounds structurally related to vitamin A. Topical retinoids act by promoting normal follicular keratinocyte desquamation, which prevents obstruction of the pilosebaceous canal and thereby inhibits the formation of microcomedones.8
They also exhibit anti-inflammatory action via inhibition of TLR-2.9 The comedolytic and anti-inflammatory actions of topical retinoids make them a mainstay of acne treatment, although some patients are unable to tolerate their adverse effects, which include erythema and dryness related to increases in transepidermal water loss. Application of noncomedogenic emollients can improve these common effects.10 The newer micronized and time-release retinoid formulations may have less potential for irritation.8 Vehicle formulation and concentration also play a role in skin irritation, with gels and liquids and formulations with higher concentrations of retinoids generally causing more drying than creams and lower potency formulations.8 Table 1 summarizes the mechanisms of action, available formulations, and potential adverse effects of the topical retinoids and other topical agents.1,6,9-16

It is important to note that retinoids can adversely affect the developing fetus when absorbed in large quantities. Notably, tazarotene is assigned to pregnancy category X because when it is used to treat psoriasis, one of its approved indications, large surface areas may be treated, increasing absorption. Absorption amounts are extremely low when tazarotene is used to treat acne. Nevertheless, verification of a negative pregnancy test is recommended prior to initiating tazarotene therapy. Effective birth control measures should be utilized throughout therapy. Even though other commonly used retinoids (tretinoin and adapalene) are assigned to pregnancy category C, all topical retinoids should be avoided during pregnancy.9
As noted, patient education is key for increasing patient adherence to therapy. Patients should be instructed to use a small (pea-sized) amount of medication for the entire face. Providers should also inform patients that transient erythema and dryness can be expected, and that application of a noncomedolytic moisturizer may reduce irritation. Tretinoin is best used at night,1 and it is useful to advise that erythema and irritation associated with retinoid use can be reduced by initially using the medication every other night to every third night, gradually building up to nightly use.1
Topical antibiotic and anti-inflammatory agents
Topical agents used to treat inflammatory lesions include benzoyl peroxide, erythromycin, clindamycin, dapsone, azelaic acid, and sulfacetamide (Table 1).1,6,9-16 These topical agents are generally well tolerated, with most adverse reactions limited to facial irritation and erythema. They come in an array of vehicle formulations, including washes, creams, gels, solutions, foams, and lotions. Vehicle selection should be based upon patient preference and skin type. Gels and solutions have a drying effect, making them more appropriate for individuals with oily skin, whereas creams are moisturizing and appropriate for individuals with dry skin. Lotions are appropriate for all skin types.11
Benzoyl peroxide (BPO) has both keratolytic and comedolytic activity and is available in concentrations ranging from 2.5% to 10%. It is available OTC, as well as by prescription, and is thus readily accessed by the patient. Because BPO is bactericidal for P acnes, resistance to BPO among P acnes has not occurred.1 All concentrations are equally effective, but the higher concentrations are more likely to cause skin dryness and other adverse effects.12 Combination therapy with topical antibiotics, tretinoin, and BPO is more clinically effective than monotherapy.17 Combination products reduce the complexity of acne treatment and likely increase therapy adherence.11 Currently available combination products in various percentages are erythromycin with BPO, clindamycin with BPO, adapalene with BPO, and clindamycin with tretinoin.1
Oral antibiotics
Oral antibiotics should be reserved for use in situations where topical therapy is ineffective. All antibiotics are effective in treating acne due to their antimicrobial activity against P acnes.1 These agents play a key role in managing moderate to severe acne that is likely to scar, as well as in cases of widespread acne involving the face, arms, and trunk. Note that the use of oral antibiotics in acne treatment is controversial, as chronic use contributes to rising rates of bacterial resistance.18 For this reason, antibiotic therapy for acne should be limited to a duration of three months or less, and these agents should not be used as monotherapy.6 In particular, recent recommendations restrict the use of erythromycin for acne treatment due to an increase of P acnes resistance.1 Cephalosporins, macrolides, and penicillin class antibiotics are not routinely recommended due to lack of data regarding their clinical effectiveness in treating acne.1
Tetracycline class antibiotics are the most commonly used oral antibiotics for acne therapy, particularly doxycycline and minocycline.5 Common adverse effects include gastrointestinal upset, photosensitivity, and some pigmentation issues.19 Trimethoprim-sulfamethoxazole (TMP-SMX) is a folate synthesis inhibitor class antibiotic also used to treat acne. Its use should be reserved for individuals who are allergic to tetracyclines or in cases of acne resistant to other antimicrobials.1 Potential adverse reactions include photosensitivity and severe hypersensitivity conditions ranging from a mild rash to toxic epidermal necrolysis.19 Table 2 summarizes the dosage ranges, pregnancy category risk, and potential adverse effects of oral antibiotics used to treat acne.1,19,20

The firstline choice for treating moderate acne with papules and pustules is oral antibiotics with topical retinoids and BPO.5 Patients should be educated about potential adverse effects of these agents, including the development of antibiotic resistance.
Hormonal agents
Hormonal therapies should be reserved for females with acne lesions influenced by fluctuations in hormone levels.21 Pubertal changes initiate the production of adrenal dehydroepiandrosterone, which leads to increased testosterone production. Testosterone is converted to dihydrotestosterone (DHT), which binds to androgen receptors in the sebaceous glands, stimulating the glands and potentially increasing production of sebum. Hormonal agents act by reducing androgen activity in the sebaceous gland. Combined oral hormones, those containing both estrogen and progesterone, reduce the amount of free testosterone and ovarian androgens by suppressing ovulation.1 Hormonal therapy can be quite effective for females of childbearing age. Females who report acne flares with their menstrual cycles may be good candidates for hormonal therapy.1
The estrogen agent most frequently used in oral contraceptives is ethinyl estradiol. Numerous progesterone agents can also be used, but those with low
Spironolactone, a potassium-sparing diuretic, may also be appropriate for treating acne in women due to its antiandrogenic properties. The drug binds androgen receptors in the skin, which then blocks testosterone and DHT. Spironolactone can be an effective firstline agent in treating hormonal-pattern acne, which presents as inflammatory lesions located on the lower face and neck. In particular, it can be an appropriate choice for women with adult-onset acne.15 Spironolactone is not approved by the FDA for acne treatment, but it has been used successfully for many years.5 Spironolactone was found in rodent studies to cause feminization of the male rat fetus, so patients taking this drug should use reliable birth control methods. It can be used concomitantly with oral contraceptives.5 Common side effects include breast tenderness, diuretic effects, headaches, and menstrual irregularities. Although the risk for hypokalemia is low in healthy young women, it may be prudent to periodically assess potassium, sodium, and renal function in patients.1 Spironolactone should be avoided in patients with renal disease and those on other diuretics.15
Isotretinoin
Isotretinoin is an oral systemic retinoid that modulates nuclear receptors and regulates gene transcription in the epidermis.16 Isotretinoin’s mechanisms of action target the main pathogenic factors underlying acne, including reduction of follicular hyperkeratosis, comedogenesis, sebum production, and inflammation and suppression of P acnes.22 These combined actions make isotretinoin a highly effective treatment option for acne.
The drug is approved by the FDA for treatment of nodular acne refractory to traditional acne therapies.23 Isotretinoin is available in 10, 20, 25, 30, and 40 mg capsules, and the recommended dosing is 0.5 to 2.0 mg/kg/d. The usual course of therapy is 15 to 20 weeks or until an accumulative dosage of 120 to 150 mg/kg is attained.23 Patients should be instructed to take isotretinoin with meals, as oral availability is increased with high-fat foods.23
Isotretinoin has major adverse effects. It is a teratogenic medication that can cause congenital anomalies in exposed fetuses, including craniofacial, cardiac, and neurologic issues.16 Due to the seriousness of the congenital anomalies, all prescribers must be registered in the iPledge program, a computer-based risk management program instituted in 2006 by the FDA and the companies that manufacture isotretinoin to eliminate congenital risks associated with isotretinoin. All patients, both male and female, must sign an informed consent form when they register in the program.
Although the iPledge program does not mandate consistent condom use for male patients, they should be informed that minute amounts of isotretinoin can be found in semen. The risk for fathering a fetus with congenital anomalies when taking isotretinoin appears to be extremely low.16 Women of childbearing potential must commit to the use of two highly reliable forms of birth control when taking the medication, including one month before starting therapy and one month after completing therapy.16 Monthly pregnancy testing is mandatory throughout the course of treatment.24 Further information regarding the risk management program can be found at iPledgeprogram.com.
Isotretinoin is metabolized by the liver and may cause lipid abnormalities and hepatic enzyme elevations. Baseline and monthly laboratory monitoring of liver enzymes and cholesterol and triglyceride levels are recommended.24 The process of initiating and monitoring isotretinoin therapy is quite complex, and unless the practitioner plans to routinely prescribe this medication, patients needing isotretinoin therapy should be referred to a dermatology practice.
PATIENT EDUCATION
Patients are more likely to adhere to treatment when simplified regimens are used and when they have realistic expectations for therapy outcomes. Providers need to educate patients that all treatments may require at least two to three months of use before visible results occur. Initial and subsequent visits should include discussions about clear expectations and strategies to reduce potential adverse effects.
PUTTING IT ALL TOGETHER
Acne therapy starts with the use of a topical retinoid in mild acne cases, unless the patient is unable to tolerate the associated skin irritability. Addition of a topical antibiotic or anti-inflammatory agent, preferably BPO, either alone or with a combination product, is also recommended for mild to moderate acne. Patients with moderate to severe acne may benefit from a short course (three months or less) of antibiotics.
Oral hormones may be an excellent therapy choice when acne treatment is needed for women of childbearing age. Isotretinoin is indicated in select cases of severe acne resistant to other treatments.
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
2. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
3. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dertmatol. 2008;58(1):56-59.
4. Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
5. Baldwin HE, Zanglein AL, Leyden JJ, Webster GF. Pharmacologic treatment options in mild, moderate, and severe acne vulgaris. Semin Cutan Med Surg. 2015;34(supp5):S82-S85.
6. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther. 2016;6(4):555-578.
7. Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582-585.
8. Russell JJ. Topical therapy for acne. Am Fam Phys. 2000; 61(2):357-365.
9. Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St. Louis: Elsevier; 2013:505-517.
10. Smith RI. Treatments: Retinoids. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:59-63.
11. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555-2562.
12. Motoparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:452-459.
13. Thiboutot DM, Kircik L, McMichale A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10):18-27.
14. Wolf K, Silapunt S. The use of sodium sulfacetamide in dermatology. Cutis. 2015;96(2):128-130.
15. Hassoun LA, Chahal DS, Sivamani RK, Larsen LN. The use of hormonal agents in the treatment of acne. Semin Cutan Med Surg. 2016;35(2):68-73.
16. Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2012:252-268.
17. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-S50.
18. Walsh TR, Efthimious J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e22-32.
19. Yan AC, Del Rosso JQ. Prescription oral treatments: Antibiotics. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:77-87.
20. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:62-67, 70-74, 77-85.
21. Hecht CT, Sidbury R, Del Rosso JQ. Prescription oral treatments: Hormonal therapies. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:89-95.
22. Webster GF, Leyden JJ, Baldwin HE, Zaenglein AL. Isotretinoin: mechanism of action and patient selection. Semin Cutan Med Surg. 2015;34(supp 5):S86-S88.
23. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris. J Clin and Aesthet Dermatol. 2014;7(2 Suppl):S3-S21.
24. Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016-1022.
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
2. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
3. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dertmatol. 2008;58(1):56-59.
4. Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
5. Baldwin HE, Zanglein AL, Leyden JJ, Webster GF. Pharmacologic treatment options in mild, moderate, and severe acne vulgaris. Semin Cutan Med Surg. 2015;34(supp5):S82-S85.
6. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther. 2016;6(4):555-578.
7. Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582-585.
8. Russell JJ. Topical therapy for acne. Am Fam Phys. 2000; 61(2):357-365.
9. Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St. Louis: Elsevier; 2013:505-517.
10. Smith RI. Treatments: Retinoids. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:59-63.
11. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555-2562.
12. Motoparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:452-459.
13. Thiboutot DM, Kircik L, McMichale A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10):18-27.
14. Wolf K, Silapunt S. The use of sodium sulfacetamide in dermatology. Cutis. 2015;96(2):128-130.
15. Hassoun LA, Chahal DS, Sivamani RK, Larsen LN. The use of hormonal agents in the treatment of acne. Semin Cutan Med Surg. 2016;35(2):68-73.
16. Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2012:252-268.
17. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-S50.
18. Walsh TR, Efthimious J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e22-32.
19. Yan AC, Del Rosso JQ. Prescription oral treatments: Antibiotics. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:77-87.
20. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:62-67, 70-74, 77-85.
21. Hecht CT, Sidbury R, Del Rosso JQ. Prescription oral treatments: Hormonal therapies. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:89-95.
22. Webster GF, Leyden JJ, Baldwin HE, Zaenglein AL. Isotretinoin: mechanism of action and patient selection. Semin Cutan Med Surg. 2015;34(supp 5):S86-S88.
23. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris. J Clin and Aesthet Dermatol. 2014;7(2 Suppl):S3-S21.
24. Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016-1022.
How to Increase HPV Vaccination Rates
CE/CME No: CR-1709
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand and identify the low- and high-risk human papillomavirus (HPV) types that can lead to benign and malignant manifestations.
• Know the recommended age range and dosing schedule for individuals who can and should receive the vaccination.
• Recognize important barriers to HPV vaccination in the health care setting.
• Understand how to promote HPV vaccination to parents/caregivers and patients.
• Find resources and educational material from national organizations that recommend and support HPV vaccination.
FACULTY
Tyler Cole practices at Coastal Community Health Services in Brunswick, Georgia, and is a clinical instructor in the DNP-APRN program at the Medical University of South Carolina (MUSC). Marie C. Thomas is a registered nurse on a surgical oncology unit at MUSC and will receive her DNP-FNP from MUSC in December 2017. Katlyn Straup practices at Roper St. Francis Healthcare and Southern Care Hospice in Charleston, South Carolina; she is also a clinical associate faculty member in the MUSC College of Nursing. Ashlyn Savage is an Associate Professor of Obstetrics and Gynecology at MUSC College of Nursing and is certified by the American Board of Obstetrics and Gynecology.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through August 31, 2018.
Article begins on next page >>
Although human papillomavirus (HPV) vaccine is a safe and effective means of preventing most HPV-related cancers, HPV vaccination rates lag well behind those of other vaccines recommended for children and adolescents. Understanding the barriers to HPV vaccine acceptance and effective strategies for overcoming them will improve vaccine uptake and completion in adolescents.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.1,2 HPV causes approximately 30,700 new cancer cases in the US annually.3 It is the primary cause of cervical cancer, which resulted in more than 4,000 deaths in the US in 2016.4 HPV is also associated with some vaginal, vulvar, penile, anal, and oropharyngeal cancers and causes anogenital warts.3
Although HPV vaccines are available to protect against infection with the HPV types that lead to these sequelae, HPV vaccination rates remain low compared with other routinely administered vaccines.5 Reasons for these lower rates include vaccine cost, lack of patient and provider education, providers’ failure to recommend, stigmas related to sexual behavior, and misconceptions about the vaccine, such as concerns about harm.5 This article discusses these barriers to better educate providers about the HPV vaccine and encourage them to assist in increasing vaccination rates.
EPIDEMIOLOGY
Approximately 79 million Americans are currently infected with HPV, and 14 million new cases are reported each year.2 In the US, the prevalence of HPV is highest among sexually active adolescents and young adults, especially those ages 20 to 24.2 Of the more than 150 types of HPV that have been identified, 40 infect the genital area. HPV genital infections are mainly spread through sexual intercourse but can also be spread through oral-to-genital contact.2
The genital HPV types are categorized as low-risk and high-risk based on their association with cervical cancer.2 High-risk types 16 and 18 are the most troublesome, accounting for 63% of all HPV-associated cancers, with HPV 16 posing the highest risk for cancer.3 High-risk types HPV 31, 33, 45, 52, and 58 account for another 10% of these cancers.3 Low-risk types, such as HPV 6 and 11, can cause low-grade cervical intraepithelial lesions, and HPV 6 and 11 account for more than 90% of genital warts.2
Most HPV infections, whether with high- or low-risk types, do not cause symptoms and resolve spontaneously in about two years.2 Persistent high-risk HPV infection is necessary for the development of cervical cancer precursor lesions—and therefore, once the infection has cleared, the risk for cancer declines.2
HPV VACCINES
Three HPV vaccines are licensed for use in the US: bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines (see Table 1).2,6,7 The bivalent, Cervarix, has recently been removed from the US market due to a decrease in product demand.6,8
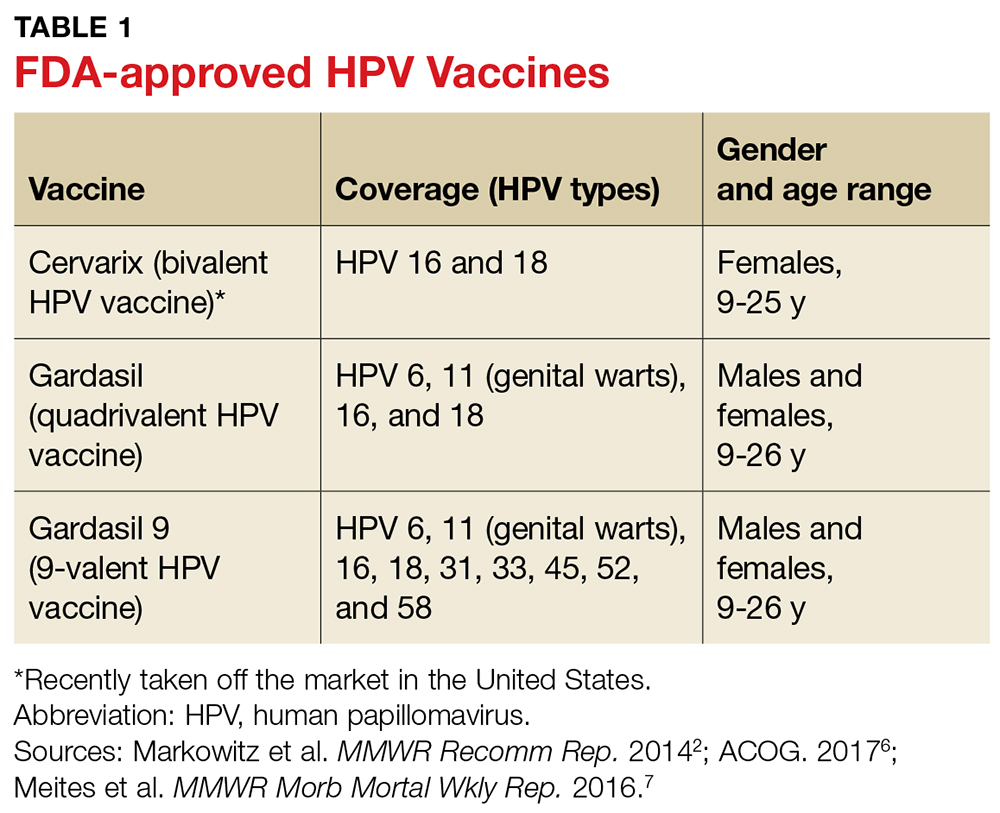
To ensure optimal protection, the vaccines must be administered in a series of scheduled doses over six to 12 months. The Advisory Committee on Immunization Practices (ACIP) recently updated their recommendations to include a two- or three-dose series based on age (see Table 2).7
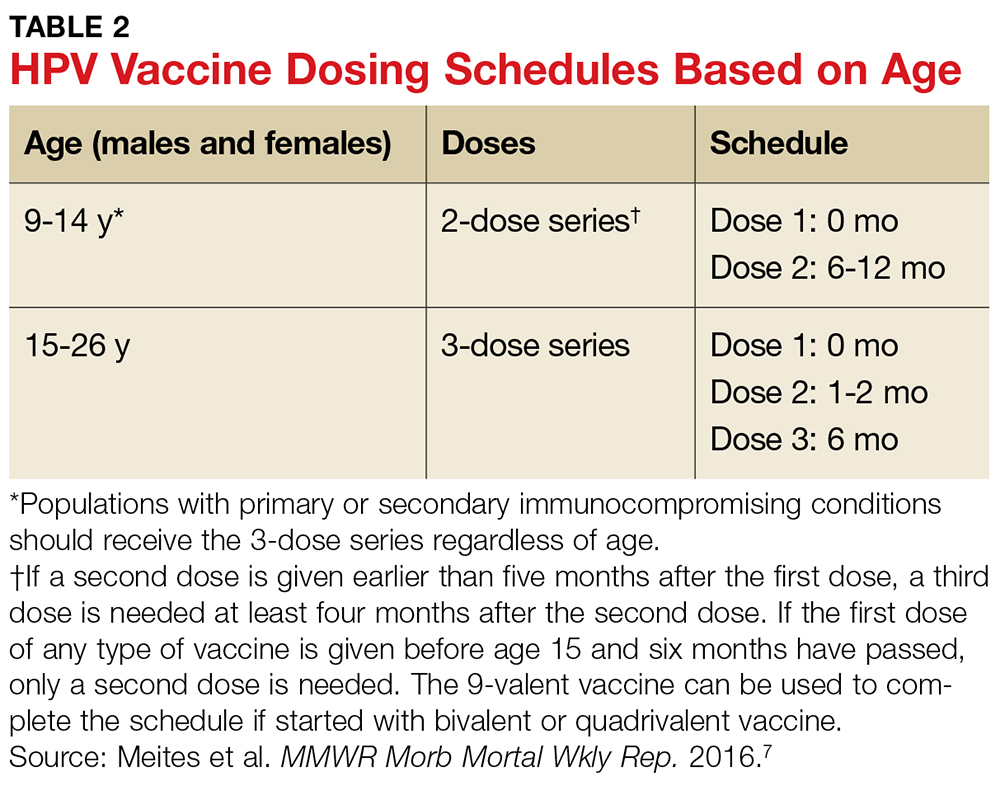
HPV vaccines are recommended for males and females between the ages of 9 and 26 years, but the ACIP and the American College of Obstetricians and Gynecologists (ACOG) strongly promote a targeted age range for vaccination between 11 and 12 years for both genders.6,7 Earlier vaccination is preferred because clinical data show a more rapid antibody response at a younger age, and because the vaccines are more effective if administered before an individual is exposed to or infected with HPV (ie, before the start of sexual activity).6,7
LOW VACCINATION RATES
HPV vaccination rates in the US are significantly lower than rates for other regularly administered vaccines; furthermore, they do not meet the Healthy People 2020 national goal of 80% for all vaccines.9 Immunization rates for most childhood vaccines range from 80% to 90%, but in 2015 only 28.1% of males and 41.9% of females ages 13 to 17 had completed the entire HPV vaccine series.9-11
The total HPV vaccination rates for male and female adolescents combined were 56.1% for one dose or more, 45.4% for two or more doses, and 34.9% for all three doses.9 In comparison, coverage rates for the meningococcal and Tdap (tetanus, diphtheria, and pertussis) immunizations, also recommended at the same age range as the HPV vaccine, were 81.3% and 86.4%, respectively.9
In addition to variation by gender and age, factors such as race, insurance coverage, and socioeconomic status influence vaccination rates.11 For the HPV vaccine specifically, Hispanic, non-Hispanic black, and American Indian/Alaska Native adolescents have higher rates of receiving each of the vaccine doses and higher rates of completing the vaccine series, compared to non-Hispanic white adolescents.9 Adolescents with Medicaid insurance and those living below the federal poverty level have better HPV vaccination coverage compared with adolescents with commercial insurance plans or those living at or above the poverty level.9,11
The HPV vaccine series completion rates in 2015 for males and females ages 13 to 17 living below the poverty level were 31.0% and 44.4%, respectively, compared to 27.4% and 41.3% for those living at or above the poverty level.9 One reason for increased rates among those living in lower-income households may be their eligibility for vaccinations at no cost through the Vaccines for Children (VFC) program, a federal program that provides vaccines to children who might otherwise forgo vaccination because of inability to pay.9
BARRIERS TO VACCINATION
Impediments that prevent adolescents and young adults from receiving the HPV vaccine exist throughout the vaccination process, with providers, parents, and the medical system itself contributing to low rates. Barriers to vaccination include fear and misconceptions, costs and socioeconomic status, lack of understanding and education, and logistic obstacles to completing the full series.5 Understanding these barriers, as well as discussing methods to overcome them, is key to increasing HPV vaccination rates and preventing the spread of this cancer-causing infection.
Health care provider barriers
Even though accredited national institutions and committees such as the CDC, ACIP, and ACOG strongly recommend vaccination based on current evidence, some health care providers still do not recommend the HPV vaccine to parents and patients.2,6,7,11 Lack of provider recommendation and the resulting lack of parental awareness of the vaccine account for many adolescents not receiving the vaccination.10,12
Providers do not recommend the vaccine for a number of reasons. Some have limited knowledge or conflicting ideas about the specific disease protection of the HPV vaccine, while others are hesitant to administer the vaccine before the onset of sexual activity, because they feel the suggested age for vaccination (11 to 12 years) is too young.10,11 Still other providers report difficulty approaching parents who they perceive as having concerns about the vaccine’s association with a sexually transmitted infection or believing that it might promote sexual activity.10
Some professionals simply claim that they forget to address the HPV vaccine at health visits, or that they propose it as optional and up to the parent’s discretion.5,10 Many providers do recommend and administer the initial dose of the vaccine, but have difficulties ensuring that patients complete the full multidose series.13 Evidence has shown that a strong provider recommendation is one of the most important incentives for parents and patients to accept vaccination.14
Parental and caregiver barriers
Lack of knowledge about the HPV vaccine and lack of recommendation from providers are two top reasons parents and caregivers cite for not vaccinating their children.5,10,14,15 In a national survey, almost all parents whose daughters completed the full vaccination series reported being counseled by their provider on the appropriate age for vaccination and the timeline of the series.14
Fears and apprehensions about side effects, especially with newer vaccines, can prevent some parents from having their children vaccinated.15 Although there is some stigma related to the vaccine’s association with the sexually transmitted HPV, this is a much less significant barrier than lack of provider recommendation or knowledge about the vaccine.5,11
Health care system barriers
Both providers and parents agree that system-level issues such as access, follow-up, and cost are barriers to initiating or completing the vaccination series.11,13 Many adolescents have few opportunities to receive the vaccine because they do not have a primary care provider.11 For those with access to primary care, visits are often problem-focused and frequently do not include a review of immunization history.13 Health care professionals also report challenges with scheduling follow-up visits for the second and third doses to complete the series within the recommended timeframe.13
Cost, insurance coverage, and reimbursement pose additional hurdles for both providers and patients, with some providers citing concerns about the cost of stocking the vaccine.16 Providers, both family practice providers and gynecologists, agree that reimbursement for administering the HPV vaccine in office poses a barrier when recommending the vaccine to patients.17 Lack of insurance coverage and type of insurance also pose barriers, with Medicaid patients more often completing the full series compared to those with private or no insurance, because Medicaid covers the cost of vaccination for men up to age 19.9,18 A national survey of males ages 9 to 17 found that the percentage of HPV vaccine initiation was double for those with public insurance compared to those with private insurance.19 Changes at the system-level, such as participation in the VFC program, in coordination with better provider recommendation should help increase HPV vaccination rates.9,11
STRATEGIES TO IMPROVE VACCINATION RATES
Many strategies for increasing HPV vaccination acceptance, decreasing barriers to access, and improving compliance with vaccine completion have been reported in the literature, with some strategies achieving more success than others. This section discusses interventions and strategies designed to help overcome provider-, parent-, and system-related barriers that have been shown to be effective (see Table 3).
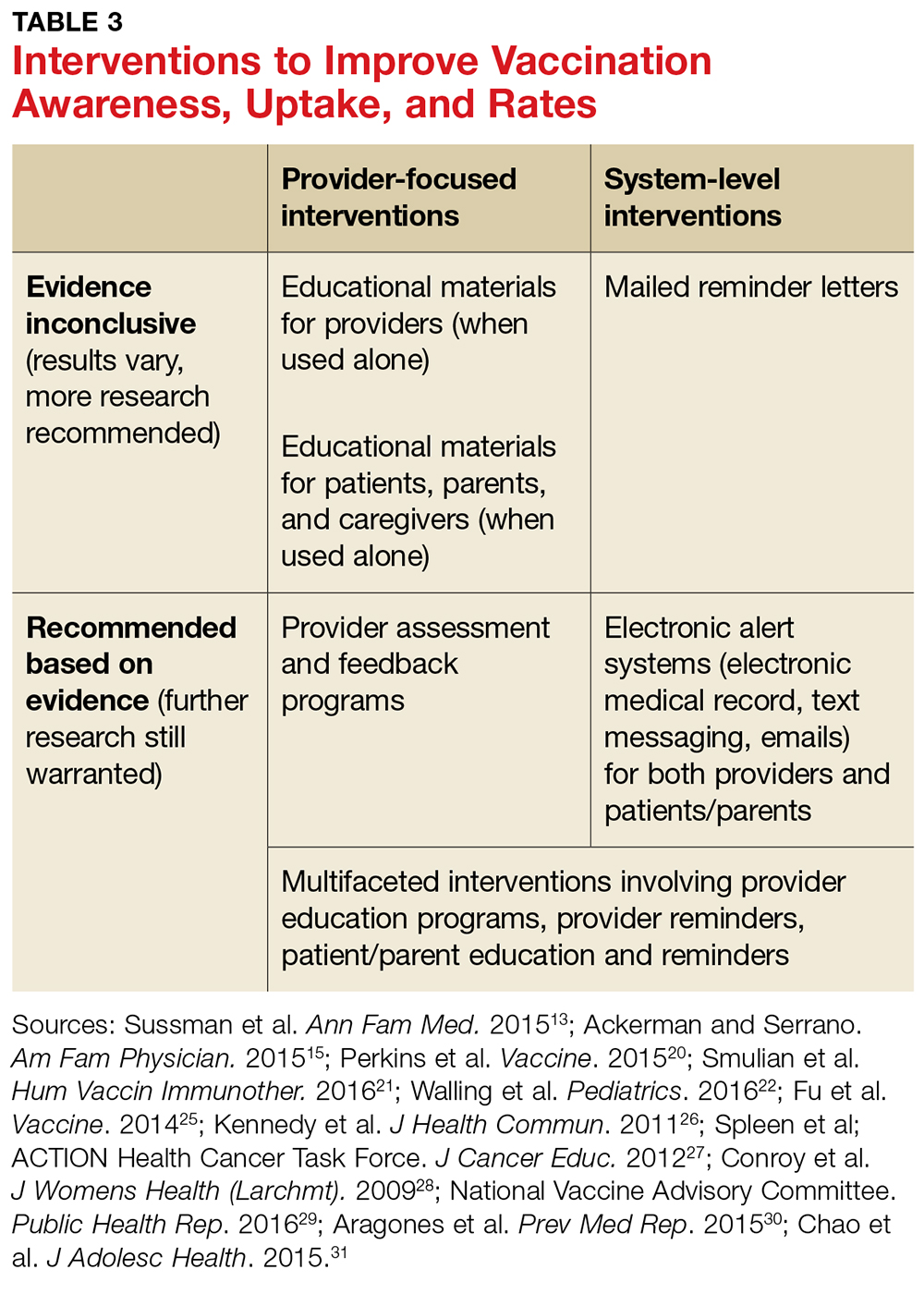
Health care provider interventions
Evidence supports a number of provider-level strategies to increase HPV vaccination rates (see Table 3). An improvement in vaccination acceptance was observed when providers promoted the vaccine as a safe, effective way to prevent cancer, rather than as a means to prevent a sexually transmitted infection.10,11,20
Some primary care providers found that encouraging the HPV vaccine at the same time as the meningococcal and Tdap vaccinations, which are also recommended at age 11 to 12 years, increased vaccination rates as well.13,20 Another successful strategy is reviewing vaccination history at every visit, whether the visit is for an acute event or an annual well exam.10,13,20 These tactics are most useful when providers practice them consistently, which may require them to change or adapt their way of practice.
Provider-based trainings that educate and prepare them to consistently recommend the vaccine have demonstrated success in increasing HPV vaccination uptake.21,22 The CDC’s Assessment/Feedback/Incentive/eXchange (AFIX) quality improvement program to increase vaccination rates, which includes Web-based or in-person consults, has been shown to increase HPV vaccination rates.20-23 The Assessment phase of the AFIX program determines a practice’s current immunization practices and rates, while the Feedback portion provides strategies for increasing vaccination rates.23 A study by Perkins and colleagues utilized AFIX strategies, specifically for the HPV vaccine, such as focusing provider education on HPV-related cancers and vaccine efficacy, as well as preparing providers to discuss and answer questions through basic motivational interviewing tactics.20
The CDC also offers PowerPoint presentations, flyers, posters, videos, and other informational resources to guide and educate providers, parents, and patients about the HPV vaccine.24 Educational resources, such as pamphlets, flyers, or fact sheets given to parents and patients, have been shown to improve intent to vaccinate as well as awareness of the vaccine.25-27
Although Fu and colleagues in a systematic review concluded that there was insufficient data to support a specific educational intervention for widespread use, the authors did recommend utilizing educational pieces and adapting them to specific populations.25 These simple interventions help increase awareness and can be implemented with other interventions in health care offices by providers and other staff.
System-level interventions
The use of systems that track patients for necessary vaccines and remind providers, parents, and patients about vaccine appointments have increased vaccination rates.13,28 Facility-based interventions, such as electronic medical records (EMR) that track patients for scheduled vaccines and remind providers when patients are due for vaccinations, will help increase provider recommendations and completion of the entire vaccination series.13
The National Vaccine Advisory Committee (NVAC) suggests that provider offices implement reminder-recall systems and provide educational material for parents and patients to increase vaccination rates.29 One specific study using both educational material and text-message reminders for parents found that these interventions significantly increased vaccination rates.30 Health care facilities could also incorporate reminder letters mailed to patients and parents to promote vaccine initiation and completion.31 The evidence supports the use of reminder alerts and EMR tracking systems to increase rates, but more research is warranted to determine the most cost-effective approach.
National programs, committees, and organizations have provided recommendations for overcoming system-related barriers to HPV vaccination, such as access and cost.29,32 The NVAC recommends incorporating alternative venues for vaccination delivery, such as pharmacies, schools, and health clinics, to increase availability to the adolescent population, especially to those who do not have primary care providers.29 One study that addressed parental opinions of vaccination administration in schools found that the majority of parents were in favor of this type of program.33 Although these recommendations seem promising and are accepted by parents, logistical barriers such as reimbursement to the pharmacies, schools, and clinics and accurate documentation of the doses received need to be addressed.29 The NVAC recommends continued evaluation and efforts to develop these programs in the future.29
In addition to school-based interventions, providing home visits for vaccination and implementing standing orders are other suggestions to overcome access and cost barriers for vaccinations, including HPV.32 Standing orders allow for individuals to receive a vaccine by a health care professional in an approved institution, where allowed by state law.32 This provides easier access to vaccinations, especially for those who do not see a primary care provider.
Although some of the system-level interventions mentioned in this article are outside the realm of what providers can do in the office, understanding and advocating for these advancements will promote vaccine uptake.
CONCLUSION
Lack of provider recommendation, coupled with poor or no parental knowledge about the HPV vaccine, are significant factors affecting vaccination uptake. Evidence supports the use of multifaceted interventions that promote and support provider recommendation and parent/patient education. Studies of interventions that incorporated educational resources and alert systems for both providers and patients or their caregivers have shown these strategies to be effective in increasing vaccination uptake and completion.
In addition to recommending the HPV vaccine, providers must educate parents/caregivers and patients about it, particularly by presenting the vaccine as a means of cancer prevention. Primary care facilities should implement reminder plans and provide educational literature to promote vaccine uptake. Although the interventions highlighted here have increased HPV vaccination rates, further research is warranted to evaluate more effective strategies for overcoming barriers and to determine which strategies are most cost-effective.
1. Juckett G, Hartsman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
4. American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016.
5. Holman DM, Benard V, Roland KB, et al. Barriers to the human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82.
6. American College of Obstetricians and Gynecologists. Human papillomavirus vaccination. Committee Opinion. Number 704. June 2017. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed August 17, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
8. Mulcahy N. GSK’s HPV vaccine, Cervarix, no longer available in the US. Medscape Medical News. October 26, 2016. www.medscape.com/viewarticle/870853. Accessed June 11, 2017.
9. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Muncie HL, Lebato AL. HPV vaccination: overcoming parental and physician impediments. Am Fam Physician. 2015;92(6):449-454.
11. Bratic JS, Seyferth ER, Bocchini JA Jr. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28(3):407-412.
12. Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr. 2015;54(4):371-375.
13. Sussman AL, Helitzer D, Bennett A, et al. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med. 2015;13(4):354-360.
14. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr. 2016;55(8):701-706.
15. Ackerman LK, Serrano JL. Update on routine childhood and adolescent immunizations. Am Fam Physician. 2015;92(6):460-468.
16. Tom A, Robinett H, Buenconsejo-Lum L, et al. Promoting and providing the HPV vaccination in Hawaii: barriers faced by health providers. J Community Health. 2016;41(5):1069-1077.
17. Young JL, Bernheim RG, Korte JE, et al. Human papillomavirus vaccination recommendation may be linked to reimbursement: A survey of Virginia family practitioners and gynecologists. J Pediat Adolesc Gynecol. 2011;24(6):380-385.
18. Thomas R, Higgins L, Ding L, et al. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Mens Health. 2016;1-9.
19. Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 9-17 year old males in the United States: the National Health Interview Survey, 2010. Hum Vaccin Immunother. 2013;9(4):874-878.
20. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33:1223-1229.
21. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12:1566-1588.
22. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1).
23. CDC. Overview of AFIX. www.cdc.gov/vaccines/programs/afix/index.html. Accessed August 17, 2017.
24. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed August 17, 2017.
25. Fu LY, Bonhomme LA, Cooper SC, et al. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901-1920.
26. Kennedy A, Sapsis KF, Stokley S, et al. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300-313.
27. Spleen AM, Kluhsman BC, Clark AD, et al; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, ages 9-17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312-319.
28. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18(10):1679-1686.
29. National Vaccine Advisory Committee. Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131(1):17-25.
30. Aragones A, Bruno DM, Ehrenberg M, et al. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554-558.
31. Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85-90.
32. Community Preventive Services Task Force. The community guide—guide to community preventive services: increasing appropriate vaccinations. Atlanta, GA: Community Preventive Services Task Force; 2016
33. Kelminson K, Saville A, Seewald L, et al. Parental views of school-located delivery of adolescent vaccines. J Adolesc Health. 2012;51(2):190-196.
CE/CME No: CR-1709
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand and identify the low- and high-risk human papillomavirus (HPV) types that can lead to benign and malignant manifestations.
• Know the recommended age range and dosing schedule for individuals who can and should receive the vaccination.
• Recognize important barriers to HPV vaccination in the health care setting.
• Understand how to promote HPV vaccination to parents/caregivers and patients.
• Find resources and educational material from national organizations that recommend and support HPV vaccination.
FACULTY
Tyler Cole practices at Coastal Community Health Services in Brunswick, Georgia, and is a clinical instructor in the DNP-APRN program at the Medical University of South Carolina (MUSC). Marie C. Thomas is a registered nurse on a surgical oncology unit at MUSC and will receive her DNP-FNP from MUSC in December 2017. Katlyn Straup practices at Roper St. Francis Healthcare and Southern Care Hospice in Charleston, South Carolina; she is also a clinical associate faculty member in the MUSC College of Nursing. Ashlyn Savage is an Associate Professor of Obstetrics and Gynecology at MUSC College of Nursing and is certified by the American Board of Obstetrics and Gynecology.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through August 31, 2018.
Article begins on next page >>
Although human papillomavirus (HPV) vaccine is a safe and effective means of preventing most HPV-related cancers, HPV vaccination rates lag well behind those of other vaccines recommended for children and adolescents. Understanding the barriers to HPV vaccine acceptance and effective strategies for overcoming them will improve vaccine uptake and completion in adolescents.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.1,2 HPV causes approximately 30,700 new cancer cases in the US annually.3 It is the primary cause of cervical cancer, which resulted in more than 4,000 deaths in the US in 2016.4 HPV is also associated with some vaginal, vulvar, penile, anal, and oropharyngeal cancers and causes anogenital warts.3
Although HPV vaccines are available to protect against infection with the HPV types that lead to these sequelae, HPV vaccination rates remain low compared with other routinely administered vaccines.5 Reasons for these lower rates include vaccine cost, lack of patient and provider education, providers’ failure to recommend, stigmas related to sexual behavior, and misconceptions about the vaccine, such as concerns about harm.5 This article discusses these barriers to better educate providers about the HPV vaccine and encourage them to assist in increasing vaccination rates.
EPIDEMIOLOGY
Approximately 79 million Americans are currently infected with HPV, and 14 million new cases are reported each year.2 In the US, the prevalence of HPV is highest among sexually active adolescents and young adults, especially those ages 20 to 24.2 Of the more than 150 types of HPV that have been identified, 40 infect the genital area. HPV genital infections are mainly spread through sexual intercourse but can also be spread through oral-to-genital contact.2
The genital HPV types are categorized as low-risk and high-risk based on their association with cervical cancer.2 High-risk types 16 and 18 are the most troublesome, accounting for 63% of all HPV-associated cancers, with HPV 16 posing the highest risk for cancer.3 High-risk types HPV 31, 33, 45, 52, and 58 account for another 10% of these cancers.3 Low-risk types, such as HPV 6 and 11, can cause low-grade cervical intraepithelial lesions, and HPV 6 and 11 account for more than 90% of genital warts.2
Most HPV infections, whether with high- or low-risk types, do not cause symptoms and resolve spontaneously in about two years.2 Persistent high-risk HPV infection is necessary for the development of cervical cancer precursor lesions—and therefore, once the infection has cleared, the risk for cancer declines.2
HPV VACCINES
Three HPV vaccines are licensed for use in the US: bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines (see Table 1).2,6,7 The bivalent, Cervarix, has recently been removed from the US market due to a decrease in product demand.6,8

To ensure optimal protection, the vaccines must be administered in a series of scheduled doses over six to 12 months. The Advisory Committee on Immunization Practices (ACIP) recently updated their recommendations to include a two- or three-dose series based on age (see Table 2).7

HPV vaccines are recommended for males and females between the ages of 9 and 26 years, but the ACIP and the American College of Obstetricians and Gynecologists (ACOG) strongly promote a targeted age range for vaccination between 11 and 12 years for both genders.6,7 Earlier vaccination is preferred because clinical data show a more rapid antibody response at a younger age, and because the vaccines are more effective if administered before an individual is exposed to or infected with HPV (ie, before the start of sexual activity).6,7
LOW VACCINATION RATES
HPV vaccination rates in the US are significantly lower than rates for other regularly administered vaccines; furthermore, they do not meet the Healthy People 2020 national goal of 80% for all vaccines.9 Immunization rates for most childhood vaccines range from 80% to 90%, but in 2015 only 28.1% of males and 41.9% of females ages 13 to 17 had completed the entire HPV vaccine series.9-11
The total HPV vaccination rates for male and female adolescents combined were 56.1% for one dose or more, 45.4% for two or more doses, and 34.9% for all three doses.9 In comparison, coverage rates for the meningococcal and Tdap (tetanus, diphtheria, and pertussis) immunizations, also recommended at the same age range as the HPV vaccine, were 81.3% and 86.4%, respectively.9
In addition to variation by gender and age, factors such as race, insurance coverage, and socioeconomic status influence vaccination rates.11 For the HPV vaccine specifically, Hispanic, non-Hispanic black, and American Indian/Alaska Native adolescents have higher rates of receiving each of the vaccine doses and higher rates of completing the vaccine series, compared to non-Hispanic white adolescents.9 Adolescents with Medicaid insurance and those living below the federal poverty level have better HPV vaccination coverage compared with adolescents with commercial insurance plans or those living at or above the poverty level.9,11
The HPV vaccine series completion rates in 2015 for males and females ages 13 to 17 living below the poverty level were 31.0% and 44.4%, respectively, compared to 27.4% and 41.3% for those living at or above the poverty level.9 One reason for increased rates among those living in lower-income households may be their eligibility for vaccinations at no cost through the Vaccines for Children (VFC) program, a federal program that provides vaccines to children who might otherwise forgo vaccination because of inability to pay.9
BARRIERS TO VACCINATION
Impediments that prevent adolescents and young adults from receiving the HPV vaccine exist throughout the vaccination process, with providers, parents, and the medical system itself contributing to low rates. Barriers to vaccination include fear and misconceptions, costs and socioeconomic status, lack of understanding and education, and logistic obstacles to completing the full series.5 Understanding these barriers, as well as discussing methods to overcome them, is key to increasing HPV vaccination rates and preventing the spread of this cancer-causing infection.
Health care provider barriers
Even though accredited national institutions and committees such as the CDC, ACIP, and ACOG strongly recommend vaccination based on current evidence, some health care providers still do not recommend the HPV vaccine to parents and patients.2,6,7,11 Lack of provider recommendation and the resulting lack of parental awareness of the vaccine account for many adolescents not receiving the vaccination.10,12
Providers do not recommend the vaccine for a number of reasons. Some have limited knowledge or conflicting ideas about the specific disease protection of the HPV vaccine, while others are hesitant to administer the vaccine before the onset of sexual activity, because they feel the suggested age for vaccination (11 to 12 years) is too young.10,11 Still other providers report difficulty approaching parents who they perceive as having concerns about the vaccine’s association with a sexually transmitted infection or believing that it might promote sexual activity.10
Some professionals simply claim that they forget to address the HPV vaccine at health visits, or that they propose it as optional and up to the parent’s discretion.5,10 Many providers do recommend and administer the initial dose of the vaccine, but have difficulties ensuring that patients complete the full multidose series.13 Evidence has shown that a strong provider recommendation is one of the most important incentives for parents and patients to accept vaccination.14
Parental and caregiver barriers
Lack of knowledge about the HPV vaccine and lack of recommendation from providers are two top reasons parents and caregivers cite for not vaccinating their children.5,10,14,15 In a national survey, almost all parents whose daughters completed the full vaccination series reported being counseled by their provider on the appropriate age for vaccination and the timeline of the series.14
Fears and apprehensions about side effects, especially with newer vaccines, can prevent some parents from having their children vaccinated.15 Although there is some stigma related to the vaccine’s association with the sexually transmitted HPV, this is a much less significant barrier than lack of provider recommendation or knowledge about the vaccine.5,11
Health care system barriers
Both providers and parents agree that system-level issues such as access, follow-up, and cost are barriers to initiating or completing the vaccination series.11,13 Many adolescents have few opportunities to receive the vaccine because they do not have a primary care provider.11 For those with access to primary care, visits are often problem-focused and frequently do not include a review of immunization history.13 Health care professionals also report challenges with scheduling follow-up visits for the second and third doses to complete the series within the recommended timeframe.13
Cost, insurance coverage, and reimbursement pose additional hurdles for both providers and patients, with some providers citing concerns about the cost of stocking the vaccine.16 Providers, both family practice providers and gynecologists, agree that reimbursement for administering the HPV vaccine in office poses a barrier when recommending the vaccine to patients.17 Lack of insurance coverage and type of insurance also pose barriers, with Medicaid patients more often completing the full series compared to those with private or no insurance, because Medicaid covers the cost of vaccination for men up to age 19.9,18 A national survey of males ages 9 to 17 found that the percentage of HPV vaccine initiation was double for those with public insurance compared to those with private insurance.19 Changes at the system-level, such as participation in the VFC program, in coordination with better provider recommendation should help increase HPV vaccination rates.9,11
STRATEGIES TO IMPROVE VACCINATION RATES
Many strategies for increasing HPV vaccination acceptance, decreasing barriers to access, and improving compliance with vaccine completion have been reported in the literature, with some strategies achieving more success than others. This section discusses interventions and strategies designed to help overcome provider-, parent-, and system-related barriers that have been shown to be effective (see Table 3).

Health care provider interventions
Evidence supports a number of provider-level strategies to increase HPV vaccination rates (see Table 3). An improvement in vaccination acceptance was observed when providers promoted the vaccine as a safe, effective way to prevent cancer, rather than as a means to prevent a sexually transmitted infection.10,11,20
Some primary care providers found that encouraging the HPV vaccine at the same time as the meningococcal and Tdap vaccinations, which are also recommended at age 11 to 12 years, increased vaccination rates as well.13,20 Another successful strategy is reviewing vaccination history at every visit, whether the visit is for an acute event or an annual well exam.10,13,20 These tactics are most useful when providers practice them consistently, which may require them to change or adapt their way of practice.
Provider-based trainings that educate and prepare them to consistently recommend the vaccine have demonstrated success in increasing HPV vaccination uptake.21,22 The CDC’s Assessment/Feedback/Incentive/eXchange (AFIX) quality improvement program to increase vaccination rates, which includes Web-based or in-person consults, has been shown to increase HPV vaccination rates.20-23 The Assessment phase of the AFIX program determines a practice’s current immunization practices and rates, while the Feedback portion provides strategies for increasing vaccination rates.23 A study by Perkins and colleagues utilized AFIX strategies, specifically for the HPV vaccine, such as focusing provider education on HPV-related cancers and vaccine efficacy, as well as preparing providers to discuss and answer questions through basic motivational interviewing tactics.20
The CDC also offers PowerPoint presentations, flyers, posters, videos, and other informational resources to guide and educate providers, parents, and patients about the HPV vaccine.24 Educational resources, such as pamphlets, flyers, or fact sheets given to parents and patients, have been shown to improve intent to vaccinate as well as awareness of the vaccine.25-27
Although Fu and colleagues in a systematic review concluded that there was insufficient data to support a specific educational intervention for widespread use, the authors did recommend utilizing educational pieces and adapting them to specific populations.25 These simple interventions help increase awareness and can be implemented with other interventions in health care offices by providers and other staff.

System-level interventions
The use of systems that track patients for necessary vaccines and remind providers, parents, and patients about vaccine appointments have increased vaccination rates.13,28 Facility-based interventions, such as electronic medical records (EMR) that track patients for scheduled vaccines and remind providers when patients are due for vaccinations, will help increase provider recommendations and completion of the entire vaccination series.13
The National Vaccine Advisory Committee (NVAC) suggests that provider offices implement reminder-recall systems and provide educational material for parents and patients to increase vaccination rates.29 One specific study using both educational material and text-message reminders for parents found that these interventions significantly increased vaccination rates.30 Health care facilities could also incorporate reminder letters mailed to patients and parents to promote vaccine initiation and completion.31 The evidence supports the use of reminder alerts and EMR tracking systems to increase rates, but more research is warranted to determine the most cost-effective approach.
National programs, committees, and organizations have provided recommendations for overcoming system-related barriers to HPV vaccination, such as access and cost.29,32 The NVAC recommends incorporating alternative venues for vaccination delivery, such as pharmacies, schools, and health clinics, to increase availability to the adolescent population, especially to those who do not have primary care providers.29 One study that addressed parental opinions of vaccination administration in schools found that the majority of parents were in favor of this type of program.33 Although these recommendations seem promising and are accepted by parents, logistical barriers such as reimbursement to the pharmacies, schools, and clinics and accurate documentation of the doses received need to be addressed.29 The NVAC recommends continued evaluation and efforts to develop these programs in the future.29
In addition to school-based interventions, providing home visits for vaccination and implementing standing orders are other suggestions to overcome access and cost barriers for vaccinations, including HPV.32 Standing orders allow for individuals to receive a vaccine by a health care professional in an approved institution, where allowed by state law.32 This provides easier access to vaccinations, especially for those who do not see a primary care provider.
Although some of the system-level interventions mentioned in this article are outside the realm of what providers can do in the office, understanding and advocating for these advancements will promote vaccine uptake.
CONCLUSION
Lack of provider recommendation, coupled with poor or no parental knowledge about the HPV vaccine, are significant factors affecting vaccination uptake. Evidence supports the use of multifaceted interventions that promote and support provider recommendation and parent/patient education. Studies of interventions that incorporated educational resources and alert systems for both providers and patients or their caregivers have shown these strategies to be effective in increasing vaccination uptake and completion.
In addition to recommending the HPV vaccine, providers must educate parents/caregivers and patients about it, particularly by presenting the vaccine as a means of cancer prevention. Primary care facilities should implement reminder plans and provide educational literature to promote vaccine uptake. Although the interventions highlighted here have increased HPV vaccination rates, further research is warranted to evaluate more effective strategies for overcoming barriers and to determine which strategies are most cost-effective.
CE/CME No: CR-1709
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand and identify the low- and high-risk human papillomavirus (HPV) types that can lead to benign and malignant manifestations.
• Know the recommended age range and dosing schedule for individuals who can and should receive the vaccination.
• Recognize important barriers to HPV vaccination in the health care setting.
• Understand how to promote HPV vaccination to parents/caregivers and patients.
• Find resources and educational material from national organizations that recommend and support HPV vaccination.
FACULTY
Tyler Cole practices at Coastal Community Health Services in Brunswick, Georgia, and is a clinical instructor in the DNP-APRN program at the Medical University of South Carolina (MUSC). Marie C. Thomas is a registered nurse on a surgical oncology unit at MUSC and will receive her DNP-FNP from MUSC in December 2017. Katlyn Straup practices at Roper St. Francis Healthcare and Southern Care Hospice in Charleston, South Carolina; she is also a clinical associate faculty member in the MUSC College of Nursing. Ashlyn Savage is an Associate Professor of Obstetrics and Gynecology at MUSC College of Nursing and is certified by the American Board of Obstetrics and Gynecology.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through August 31, 2018.
Article begins on next page >>
Although human papillomavirus (HPV) vaccine is a safe and effective means of preventing most HPV-related cancers, HPV vaccination rates lag well behind those of other vaccines recommended for children and adolescents. Understanding the barriers to HPV vaccine acceptance and effective strategies for overcoming them will improve vaccine uptake and completion in adolescents.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.1,2 HPV causes approximately 30,700 new cancer cases in the US annually.3 It is the primary cause of cervical cancer, which resulted in more than 4,000 deaths in the US in 2016.4 HPV is also associated with some vaginal, vulvar, penile, anal, and oropharyngeal cancers and causes anogenital warts.3
Although HPV vaccines are available to protect against infection with the HPV types that lead to these sequelae, HPV vaccination rates remain low compared with other routinely administered vaccines.5 Reasons for these lower rates include vaccine cost, lack of patient and provider education, providers’ failure to recommend, stigmas related to sexual behavior, and misconceptions about the vaccine, such as concerns about harm.5 This article discusses these barriers to better educate providers about the HPV vaccine and encourage them to assist in increasing vaccination rates.
EPIDEMIOLOGY
Approximately 79 million Americans are currently infected with HPV, and 14 million new cases are reported each year.2 In the US, the prevalence of HPV is highest among sexually active adolescents and young adults, especially those ages 20 to 24.2 Of the more than 150 types of HPV that have been identified, 40 infect the genital area. HPV genital infections are mainly spread through sexual intercourse but can also be spread through oral-to-genital contact.2
The genital HPV types are categorized as low-risk and high-risk based on their association with cervical cancer.2 High-risk types 16 and 18 are the most troublesome, accounting for 63% of all HPV-associated cancers, with HPV 16 posing the highest risk for cancer.3 High-risk types HPV 31, 33, 45, 52, and 58 account for another 10% of these cancers.3 Low-risk types, such as HPV 6 and 11, can cause low-grade cervical intraepithelial lesions, and HPV 6 and 11 account for more than 90% of genital warts.2
Most HPV infections, whether with high- or low-risk types, do not cause symptoms and resolve spontaneously in about two years.2 Persistent high-risk HPV infection is necessary for the development of cervical cancer precursor lesions—and therefore, once the infection has cleared, the risk for cancer declines.2
HPV VACCINES
Three HPV vaccines are licensed for use in the US: bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines (see Table 1).2,6,7 The bivalent, Cervarix, has recently been removed from the US market due to a decrease in product demand.6,8

To ensure optimal protection, the vaccines must be administered in a series of scheduled doses over six to 12 months. The Advisory Committee on Immunization Practices (ACIP) recently updated their recommendations to include a two- or three-dose series based on age (see Table 2).7

HPV vaccines are recommended for males and females between the ages of 9 and 26 years, but the ACIP and the American College of Obstetricians and Gynecologists (ACOG) strongly promote a targeted age range for vaccination between 11 and 12 years for both genders.6,7 Earlier vaccination is preferred because clinical data show a more rapid antibody response at a younger age, and because the vaccines are more effective if administered before an individual is exposed to or infected with HPV (ie, before the start of sexual activity).6,7
LOW VACCINATION RATES
HPV vaccination rates in the US are significantly lower than rates for other regularly administered vaccines; furthermore, they do not meet the Healthy People 2020 national goal of 80% for all vaccines.9 Immunization rates for most childhood vaccines range from 80% to 90%, but in 2015 only 28.1% of males and 41.9% of females ages 13 to 17 had completed the entire HPV vaccine series.9-11
The total HPV vaccination rates for male and female adolescents combined were 56.1% for one dose or more, 45.4% for two or more doses, and 34.9% for all three doses.9 In comparison, coverage rates for the meningococcal and Tdap (tetanus, diphtheria, and pertussis) immunizations, also recommended at the same age range as the HPV vaccine, were 81.3% and 86.4%, respectively.9
In addition to variation by gender and age, factors such as race, insurance coverage, and socioeconomic status influence vaccination rates.11 For the HPV vaccine specifically, Hispanic, non-Hispanic black, and American Indian/Alaska Native adolescents have higher rates of receiving each of the vaccine doses and higher rates of completing the vaccine series, compared to non-Hispanic white adolescents.9 Adolescents with Medicaid insurance and those living below the federal poverty level have better HPV vaccination coverage compared with adolescents with commercial insurance plans or those living at or above the poverty level.9,11
The HPV vaccine series completion rates in 2015 for males and females ages 13 to 17 living below the poverty level were 31.0% and 44.4%, respectively, compared to 27.4% and 41.3% for those living at or above the poverty level.9 One reason for increased rates among those living in lower-income households may be their eligibility for vaccinations at no cost through the Vaccines for Children (VFC) program, a federal program that provides vaccines to children who might otherwise forgo vaccination because of inability to pay.9
BARRIERS TO VACCINATION
Impediments that prevent adolescents and young adults from receiving the HPV vaccine exist throughout the vaccination process, with providers, parents, and the medical system itself contributing to low rates. Barriers to vaccination include fear and misconceptions, costs and socioeconomic status, lack of understanding and education, and logistic obstacles to completing the full series.5 Understanding these barriers, as well as discussing methods to overcome them, is key to increasing HPV vaccination rates and preventing the spread of this cancer-causing infection.
Health care provider barriers
Even though accredited national institutions and committees such as the CDC, ACIP, and ACOG strongly recommend vaccination based on current evidence, some health care providers still do not recommend the HPV vaccine to parents and patients.2,6,7,11 Lack of provider recommendation and the resulting lack of parental awareness of the vaccine account for many adolescents not receiving the vaccination.10,12
Providers do not recommend the vaccine for a number of reasons. Some have limited knowledge or conflicting ideas about the specific disease protection of the HPV vaccine, while others are hesitant to administer the vaccine before the onset of sexual activity, because they feel the suggested age for vaccination (11 to 12 years) is too young.10,11 Still other providers report difficulty approaching parents who they perceive as having concerns about the vaccine’s association with a sexually transmitted infection or believing that it might promote sexual activity.10
Some professionals simply claim that they forget to address the HPV vaccine at health visits, or that they propose it as optional and up to the parent’s discretion.5,10 Many providers do recommend and administer the initial dose of the vaccine, but have difficulties ensuring that patients complete the full multidose series.13 Evidence has shown that a strong provider recommendation is one of the most important incentives for parents and patients to accept vaccination.14
Parental and caregiver barriers
Lack of knowledge about the HPV vaccine and lack of recommendation from providers are two top reasons parents and caregivers cite for not vaccinating their children.5,10,14,15 In a national survey, almost all parents whose daughters completed the full vaccination series reported being counseled by their provider on the appropriate age for vaccination and the timeline of the series.14
Fears and apprehensions about side effects, especially with newer vaccines, can prevent some parents from having their children vaccinated.15 Although there is some stigma related to the vaccine’s association with the sexually transmitted HPV, this is a much less significant barrier than lack of provider recommendation or knowledge about the vaccine.5,11
Health care system barriers
Both providers and parents agree that system-level issues such as access, follow-up, and cost are barriers to initiating or completing the vaccination series.11,13 Many adolescents have few opportunities to receive the vaccine because they do not have a primary care provider.11 For those with access to primary care, visits are often problem-focused and frequently do not include a review of immunization history.13 Health care professionals also report challenges with scheduling follow-up visits for the second and third doses to complete the series within the recommended timeframe.13
Cost, insurance coverage, and reimbursement pose additional hurdles for both providers and patients, with some providers citing concerns about the cost of stocking the vaccine.16 Providers, both family practice providers and gynecologists, agree that reimbursement for administering the HPV vaccine in office poses a barrier when recommending the vaccine to patients.17 Lack of insurance coverage and type of insurance also pose barriers, with Medicaid patients more often completing the full series compared to those with private or no insurance, because Medicaid covers the cost of vaccination for men up to age 19.9,18 A national survey of males ages 9 to 17 found that the percentage of HPV vaccine initiation was double for those with public insurance compared to those with private insurance.19 Changes at the system-level, such as participation in the VFC program, in coordination with better provider recommendation should help increase HPV vaccination rates.9,11
STRATEGIES TO IMPROVE VACCINATION RATES
Many strategies for increasing HPV vaccination acceptance, decreasing barriers to access, and improving compliance with vaccine completion have been reported in the literature, with some strategies achieving more success than others. This section discusses interventions and strategies designed to help overcome provider-, parent-, and system-related barriers that have been shown to be effective (see Table 3).

Health care provider interventions
Evidence supports a number of provider-level strategies to increase HPV vaccination rates (see Table 3). An improvement in vaccination acceptance was observed when providers promoted the vaccine as a safe, effective way to prevent cancer, rather than as a means to prevent a sexually transmitted infection.10,11,20
Some primary care providers found that encouraging the HPV vaccine at the same time as the meningococcal and Tdap vaccinations, which are also recommended at age 11 to 12 years, increased vaccination rates as well.13,20 Another successful strategy is reviewing vaccination history at every visit, whether the visit is for an acute event or an annual well exam.10,13,20 These tactics are most useful when providers practice them consistently, which may require them to change or adapt their way of practice.
Provider-based trainings that educate and prepare them to consistently recommend the vaccine have demonstrated success in increasing HPV vaccination uptake.21,22 The CDC’s Assessment/Feedback/Incentive/eXchange (AFIX) quality improvement program to increase vaccination rates, which includes Web-based or in-person consults, has been shown to increase HPV vaccination rates.20-23 The Assessment phase of the AFIX program determines a practice’s current immunization practices and rates, while the Feedback portion provides strategies for increasing vaccination rates.23 A study by Perkins and colleagues utilized AFIX strategies, specifically for the HPV vaccine, such as focusing provider education on HPV-related cancers and vaccine efficacy, as well as preparing providers to discuss and answer questions through basic motivational interviewing tactics.20
The CDC also offers PowerPoint presentations, flyers, posters, videos, and other informational resources to guide and educate providers, parents, and patients about the HPV vaccine.24 Educational resources, such as pamphlets, flyers, or fact sheets given to parents and patients, have been shown to improve intent to vaccinate as well as awareness of the vaccine.25-27
Although Fu and colleagues in a systematic review concluded that there was insufficient data to support a specific educational intervention for widespread use, the authors did recommend utilizing educational pieces and adapting them to specific populations.25 These simple interventions help increase awareness and can be implemented with other interventions in health care offices by providers and other staff.

System-level interventions
The use of systems that track patients for necessary vaccines and remind providers, parents, and patients about vaccine appointments have increased vaccination rates.13,28 Facility-based interventions, such as electronic medical records (EMR) that track patients for scheduled vaccines and remind providers when patients are due for vaccinations, will help increase provider recommendations and completion of the entire vaccination series.13
The National Vaccine Advisory Committee (NVAC) suggests that provider offices implement reminder-recall systems and provide educational material for parents and patients to increase vaccination rates.29 One specific study using both educational material and text-message reminders for parents found that these interventions significantly increased vaccination rates.30 Health care facilities could also incorporate reminder letters mailed to patients and parents to promote vaccine initiation and completion.31 The evidence supports the use of reminder alerts and EMR tracking systems to increase rates, but more research is warranted to determine the most cost-effective approach.
National programs, committees, and organizations have provided recommendations for overcoming system-related barriers to HPV vaccination, such as access and cost.29,32 The NVAC recommends incorporating alternative venues for vaccination delivery, such as pharmacies, schools, and health clinics, to increase availability to the adolescent population, especially to those who do not have primary care providers.29 One study that addressed parental opinions of vaccination administration in schools found that the majority of parents were in favor of this type of program.33 Although these recommendations seem promising and are accepted by parents, logistical barriers such as reimbursement to the pharmacies, schools, and clinics and accurate documentation of the doses received need to be addressed.29 The NVAC recommends continued evaluation and efforts to develop these programs in the future.29
In addition to school-based interventions, providing home visits for vaccination and implementing standing orders are other suggestions to overcome access and cost barriers for vaccinations, including HPV.32 Standing orders allow for individuals to receive a vaccine by a health care professional in an approved institution, where allowed by state law.32 This provides easier access to vaccinations, especially for those who do not see a primary care provider.
Although some of the system-level interventions mentioned in this article are outside the realm of what providers can do in the office, understanding and advocating for these advancements will promote vaccine uptake.
CONCLUSION
Lack of provider recommendation, coupled with poor or no parental knowledge about the HPV vaccine, are significant factors affecting vaccination uptake. Evidence supports the use of multifaceted interventions that promote and support provider recommendation and parent/patient education. Studies of interventions that incorporated educational resources and alert systems for both providers and patients or their caregivers have shown these strategies to be effective in increasing vaccination uptake and completion.
In addition to recommending the HPV vaccine, providers must educate parents/caregivers and patients about it, particularly by presenting the vaccine as a means of cancer prevention. Primary care facilities should implement reminder plans and provide educational literature to promote vaccine uptake. Although the interventions highlighted here have increased HPV vaccination rates, further research is warranted to evaluate more effective strategies for overcoming barriers and to determine which strategies are most cost-effective.
1. Juckett G, Hartsman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
4. American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016.
5. Holman DM, Benard V, Roland KB, et al. Barriers to the human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82.
6. American College of Obstetricians and Gynecologists. Human papillomavirus vaccination. Committee Opinion. Number 704. June 2017. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed August 17, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
8. Mulcahy N. GSK’s HPV vaccine, Cervarix, no longer available in the US. Medscape Medical News. October 26, 2016. www.medscape.com/viewarticle/870853. Accessed June 11, 2017.
9. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Muncie HL, Lebato AL. HPV vaccination: overcoming parental and physician impediments. Am Fam Physician. 2015;92(6):449-454.
11. Bratic JS, Seyferth ER, Bocchini JA Jr. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28(3):407-412.
12. Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr. 2015;54(4):371-375.
13. Sussman AL, Helitzer D, Bennett A, et al. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med. 2015;13(4):354-360.
14. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr. 2016;55(8):701-706.
15. Ackerman LK, Serrano JL. Update on routine childhood and adolescent immunizations. Am Fam Physician. 2015;92(6):460-468.
16. Tom A, Robinett H, Buenconsejo-Lum L, et al. Promoting and providing the HPV vaccination in Hawaii: barriers faced by health providers. J Community Health. 2016;41(5):1069-1077.
17. Young JL, Bernheim RG, Korte JE, et al. Human papillomavirus vaccination recommendation may be linked to reimbursement: A survey of Virginia family practitioners and gynecologists. J Pediat Adolesc Gynecol. 2011;24(6):380-385.
18. Thomas R, Higgins L, Ding L, et al. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Mens Health. 2016;1-9.
19. Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 9-17 year old males in the United States: the National Health Interview Survey, 2010. Hum Vaccin Immunother. 2013;9(4):874-878.
20. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33:1223-1229.
21. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12:1566-1588.
22. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1).
23. CDC. Overview of AFIX. www.cdc.gov/vaccines/programs/afix/index.html. Accessed August 17, 2017.
24. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed August 17, 2017.
25. Fu LY, Bonhomme LA, Cooper SC, et al. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901-1920.
26. Kennedy A, Sapsis KF, Stokley S, et al. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300-313.
27. Spleen AM, Kluhsman BC, Clark AD, et al; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, ages 9-17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312-319.
28. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18(10):1679-1686.
29. National Vaccine Advisory Committee. Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131(1):17-25.
30. Aragones A, Bruno DM, Ehrenberg M, et al. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554-558.
31. Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85-90.
32. Community Preventive Services Task Force. The community guide—guide to community preventive services: increasing appropriate vaccinations. Atlanta, GA: Community Preventive Services Task Force; 2016
33. Kelminson K, Saville A, Seewald L, et al. Parental views of school-located delivery of adolescent vaccines. J Adolesc Health. 2012;51(2):190-196.
1. Juckett G, Hartsman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
4. American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016.
5. Holman DM, Benard V, Roland KB, et al. Barriers to the human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82.
6. American College of Obstetricians and Gynecologists. Human papillomavirus vaccination. Committee Opinion. Number 704. June 2017. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed August 17, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
8. Mulcahy N. GSK’s HPV vaccine, Cervarix, no longer available in the US. Medscape Medical News. October 26, 2016. www.medscape.com/viewarticle/870853. Accessed June 11, 2017.
9. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Muncie HL, Lebato AL. HPV vaccination: overcoming parental and physician impediments. Am Fam Physician. 2015;92(6):449-454.
11. Bratic JS, Seyferth ER, Bocchini JA Jr. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28(3):407-412.
12. Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr. 2015;54(4):371-375.
13. Sussman AL, Helitzer D, Bennett A, et al. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med. 2015;13(4):354-360.
14. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr. 2016;55(8):701-706.
15. Ackerman LK, Serrano JL. Update on routine childhood and adolescent immunizations. Am Fam Physician. 2015;92(6):460-468.
16. Tom A, Robinett H, Buenconsejo-Lum L, et al. Promoting and providing the HPV vaccination in Hawaii: barriers faced by health providers. J Community Health. 2016;41(5):1069-1077.
17. Young JL, Bernheim RG, Korte JE, et al. Human papillomavirus vaccination recommendation may be linked to reimbursement: A survey of Virginia family practitioners and gynecologists. J Pediat Adolesc Gynecol. 2011;24(6):380-385.
18. Thomas R, Higgins L, Ding L, et al. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Mens Health. 2016;1-9.
19. Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 9-17 year old males in the United States: the National Health Interview Survey, 2010. Hum Vaccin Immunother. 2013;9(4):874-878.
20. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33:1223-1229.
21. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12:1566-1588.
22. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1).
23. CDC. Overview of AFIX. www.cdc.gov/vaccines/programs/afix/index.html. Accessed August 17, 2017.
24. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed August 17, 2017.
25. Fu LY, Bonhomme LA, Cooper SC, et al. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901-1920.
26. Kennedy A, Sapsis KF, Stokley S, et al. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300-313.
27. Spleen AM, Kluhsman BC, Clark AD, et al; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, ages 9-17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312-319.
28. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18(10):1679-1686.
29. National Vaccine Advisory Committee. Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131(1):17-25.
30. Aragones A, Bruno DM, Ehrenberg M, et al. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554-558.
31. Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85-90.
32. Community Preventive Services Task Force. The community guide—guide to community preventive services: increasing appropriate vaccinations. Atlanta, GA: Community Preventive Services Task Force; 2016
33. Kelminson K, Saville A, Seewald L, et al. Parental views of school-located delivery of adolescent vaccines. J Adolesc Health. 2012;51(2):190-196.