User login
The power and promise of person-generated health data – part 1
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
Storytelling tool can assist elderly in the ICU
SAN FRANCISCO – A “Best Case/Worst Case” (BCWC) framework tool has been adapted for use with geriatric trauma patients in the ICU, where it can help track a patient’s progress and enable better communication with patients and loved ones. The tool relies on a combination of graphics and text that surgeons update daily during rounds, and creates a longitudinal view of a patient’s trajectory during their stay in the ICU.
– for example, after a complication has arisen.
“Each day during rounds, the ICU team records important events on the graphic aid that change the patient’s course. The team draws a star to represent the best case, and a line to represent prognostic uncertainty. The attending trauma surgeon then uses the geriatric trauma outcome score, their knowledge of the health state of the patient, and their own clinical experience to tell a story about treatments, recovery, and outcomes if everything goes as well as we might hope. This story is written down in the best-case scenario box,” Christopher Zimmerman, MD, a general surgery resident at the University of Wisconsin–Madison, said during a presentation about the BCWC tool at the annual clinical congress of the American College of Surgeons
“We often like to talk to patients and their families [about best- and worst-case scenarios] anyway, but [the research team] have tried to formalize it,” said Tam Pham, MD, professor of surgery at the University of Washington, in an interview. Dr. Pham comoderated the session where the research was presented.
“When we’re able to communicate where the uncertainty is and where the boundaries are around the course of care and possible outcomes, we can build an alliance with patients and families that will be helpful when there is a big decision to make, say about a laparotomy for a perforated viscus,” said Dr. Zimmerman.
Dr. Zimmerman gave an example of a patient who came into the ICU after suffering multiple fractures from falling down a set of stairs. The team created an initial BCWC with a hoped-for best-case scenario. Later, the patient developed hypoxemic respiratory failure and had to be intubated overnight. “This event is recorded on the graphic, and her star representing the best case has changed position, the line representing uncertainty has shortened, and the contents of her best-case scenario has changed. Each day in rounds, this process is repeated,” said Dr. Zimmerman.
Palliative care physicians, education experts, and surgeons at the University of Wisconsin–Madison developed the tool in an effort to reduce unwanted care at the end of life, in the context of high-risk surgeries. The researchers adapted the tool to the trauma setting by gathering six focus groups of trauma practitioners at the University of Wisconsin; University of Texas, Dallas; and Oregon Health & Science University, Portland. They modified the tool after incorporating comments, and then iteratively modified it through tasks carried out in the ICU as part of a qualitative improvement initiative at the University of Wisconsin–Madison. They generated a change to the tool, implemented it in the ICU during subsequent rounds, then collected observations and field notes, then revised and repeated the process, streamlining it to fit into the ICU environment, according to Dr. Zimmerman.
The back side of the tool is available for family members to write important details about their loved ones, leading insight into the patient’s personality and desires, such as favorite music or affection for a family pet.
The work was supported by the National Institutes of Health. Dr. Zimmerman and Dr. Pham have no relevant financial disclosures.
SOURCE: Zimmerman C et al. Clinical Congress 2019, Abstract.
SAN FRANCISCO – A “Best Case/Worst Case” (BCWC) framework tool has been adapted for use with geriatric trauma patients in the ICU, where it can help track a patient’s progress and enable better communication with patients and loved ones. The tool relies on a combination of graphics and text that surgeons update daily during rounds, and creates a longitudinal view of a patient’s trajectory during their stay in the ICU.
– for example, after a complication has arisen.
“Each day during rounds, the ICU team records important events on the graphic aid that change the patient’s course. The team draws a star to represent the best case, and a line to represent prognostic uncertainty. The attending trauma surgeon then uses the geriatric trauma outcome score, their knowledge of the health state of the patient, and their own clinical experience to tell a story about treatments, recovery, and outcomes if everything goes as well as we might hope. This story is written down in the best-case scenario box,” Christopher Zimmerman, MD, a general surgery resident at the University of Wisconsin–Madison, said during a presentation about the BCWC tool at the annual clinical congress of the American College of Surgeons
“We often like to talk to patients and their families [about best- and worst-case scenarios] anyway, but [the research team] have tried to formalize it,” said Tam Pham, MD, professor of surgery at the University of Washington, in an interview. Dr. Pham comoderated the session where the research was presented.
“When we’re able to communicate where the uncertainty is and where the boundaries are around the course of care and possible outcomes, we can build an alliance with patients and families that will be helpful when there is a big decision to make, say about a laparotomy for a perforated viscus,” said Dr. Zimmerman.
Dr. Zimmerman gave an example of a patient who came into the ICU after suffering multiple fractures from falling down a set of stairs. The team created an initial BCWC with a hoped-for best-case scenario. Later, the patient developed hypoxemic respiratory failure and had to be intubated overnight. “This event is recorded on the graphic, and her star representing the best case has changed position, the line representing uncertainty has shortened, and the contents of her best-case scenario has changed. Each day in rounds, this process is repeated,” said Dr. Zimmerman.
Palliative care physicians, education experts, and surgeons at the University of Wisconsin–Madison developed the tool in an effort to reduce unwanted care at the end of life, in the context of high-risk surgeries. The researchers adapted the tool to the trauma setting by gathering six focus groups of trauma practitioners at the University of Wisconsin; University of Texas, Dallas; and Oregon Health & Science University, Portland. They modified the tool after incorporating comments, and then iteratively modified it through tasks carried out in the ICU as part of a qualitative improvement initiative at the University of Wisconsin–Madison. They generated a change to the tool, implemented it in the ICU during subsequent rounds, then collected observations and field notes, then revised and repeated the process, streamlining it to fit into the ICU environment, according to Dr. Zimmerman.
The back side of the tool is available for family members to write important details about their loved ones, leading insight into the patient’s personality and desires, such as favorite music or affection for a family pet.
The work was supported by the National Institutes of Health. Dr. Zimmerman and Dr. Pham have no relevant financial disclosures.
SOURCE: Zimmerman C et al. Clinical Congress 2019, Abstract.
SAN FRANCISCO – A “Best Case/Worst Case” (BCWC) framework tool has been adapted for use with geriatric trauma patients in the ICU, where it can help track a patient’s progress and enable better communication with patients and loved ones. The tool relies on a combination of graphics and text that surgeons update daily during rounds, and creates a longitudinal view of a patient’s trajectory during their stay in the ICU.
– for example, after a complication has arisen.
“Each day during rounds, the ICU team records important events on the graphic aid that change the patient’s course. The team draws a star to represent the best case, and a line to represent prognostic uncertainty. The attending trauma surgeon then uses the geriatric trauma outcome score, their knowledge of the health state of the patient, and their own clinical experience to tell a story about treatments, recovery, and outcomes if everything goes as well as we might hope. This story is written down in the best-case scenario box,” Christopher Zimmerman, MD, a general surgery resident at the University of Wisconsin–Madison, said during a presentation about the BCWC tool at the annual clinical congress of the American College of Surgeons
“We often like to talk to patients and their families [about best- and worst-case scenarios] anyway, but [the research team] have tried to formalize it,” said Tam Pham, MD, professor of surgery at the University of Washington, in an interview. Dr. Pham comoderated the session where the research was presented.
“When we’re able to communicate where the uncertainty is and where the boundaries are around the course of care and possible outcomes, we can build an alliance with patients and families that will be helpful when there is a big decision to make, say about a laparotomy for a perforated viscus,” said Dr. Zimmerman.
Dr. Zimmerman gave an example of a patient who came into the ICU after suffering multiple fractures from falling down a set of stairs. The team created an initial BCWC with a hoped-for best-case scenario. Later, the patient developed hypoxemic respiratory failure and had to be intubated overnight. “This event is recorded on the graphic, and her star representing the best case has changed position, the line representing uncertainty has shortened, and the contents of her best-case scenario has changed. Each day in rounds, this process is repeated,” said Dr. Zimmerman.
Palliative care physicians, education experts, and surgeons at the University of Wisconsin–Madison developed the tool in an effort to reduce unwanted care at the end of life, in the context of high-risk surgeries. The researchers adapted the tool to the trauma setting by gathering six focus groups of trauma practitioners at the University of Wisconsin; University of Texas, Dallas; and Oregon Health & Science University, Portland. They modified the tool after incorporating comments, and then iteratively modified it through tasks carried out in the ICU as part of a qualitative improvement initiative at the University of Wisconsin–Madison. They generated a change to the tool, implemented it in the ICU during subsequent rounds, then collected observations and field notes, then revised and repeated the process, streamlining it to fit into the ICU environment, according to Dr. Zimmerman.
The back side of the tool is available for family members to write important details about their loved ones, leading insight into the patient’s personality and desires, such as favorite music or affection for a family pet.
The work was supported by the National Institutes of Health. Dr. Zimmerman and Dr. Pham have no relevant financial disclosures.
SOURCE: Zimmerman C et al. Clinical Congress 2019, Abstract.
REPORTING FROM CLINICAL CONGRESS 2019
Primary care for the declining cancer survivor
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
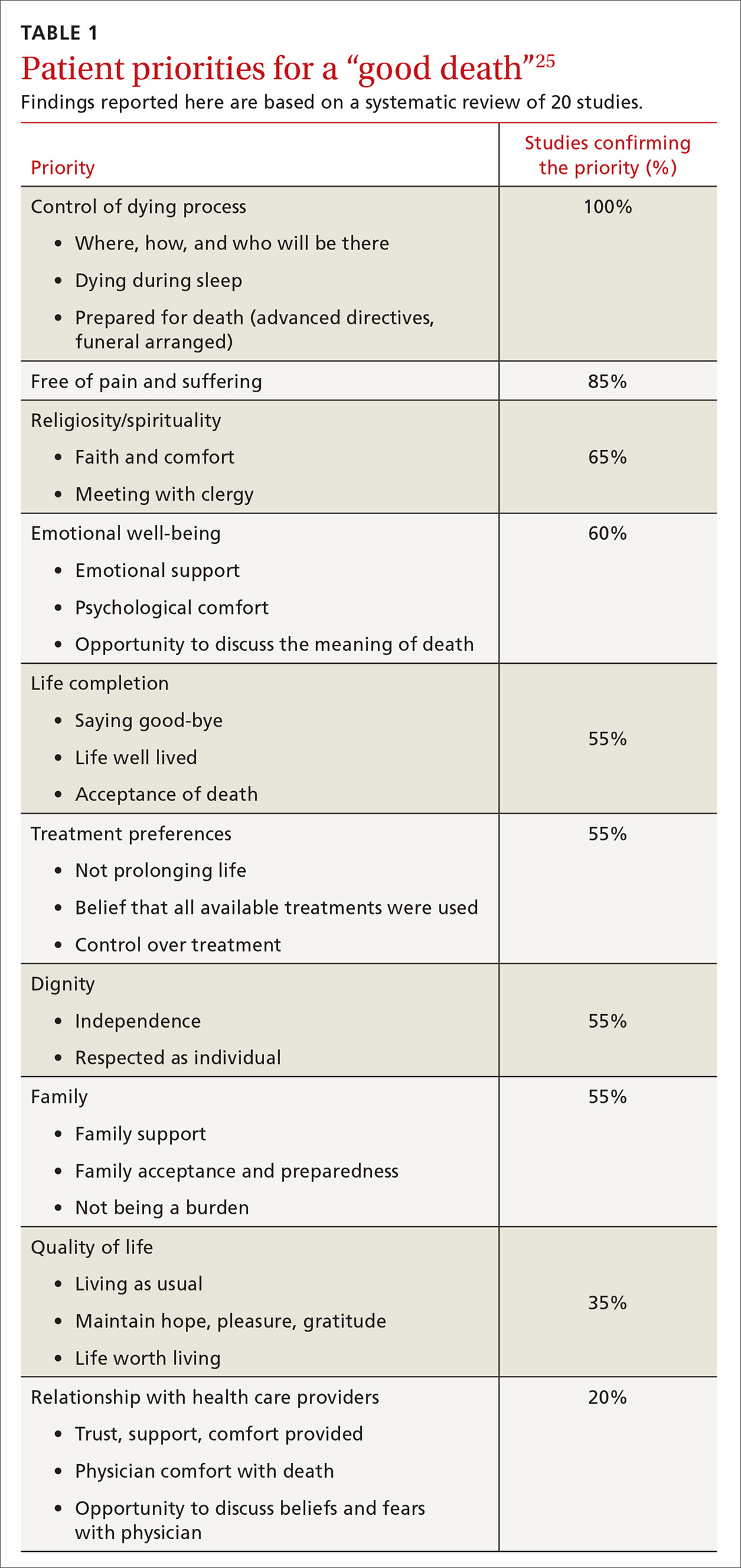
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25
Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28

Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
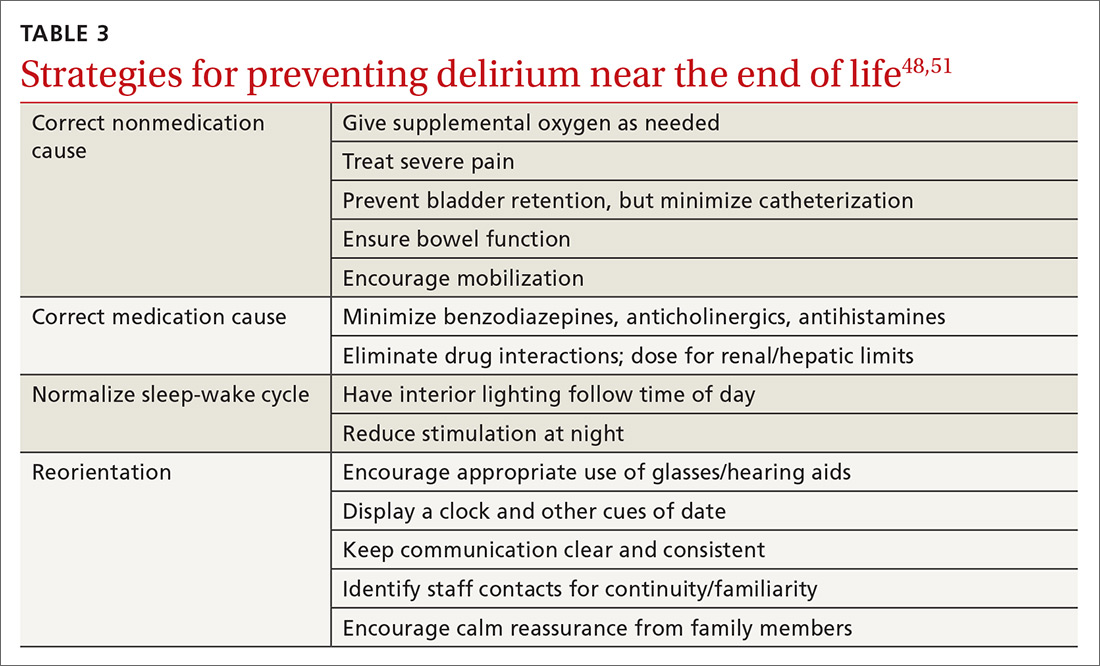
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51
Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25

Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28

Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51

Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25

Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28

Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51

Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
PRACTICE RECOMMENDATIONS
› Implement palliative/ supportive care shortly after the diagnosis of an incurable cancer. A
› Candidly communicate prognoses to patients and help them adjust their goals of care. B
› Recommend hospice care for patients who likely have less than 6 months to live, especially with treatmentrelated complications or significant caregiver stress. B
› Delay opioid therapy— if possible—to better control symptoms near the end of life. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Disclosure After Adverse Medical Outcomes: A Multidimensional Challenge
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
Assessing decisional capacity in patients with substance use disorders
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Patient and family education of asthma management is critical
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
“Every contact with them is a teachable moment,” Mary Lou Hayden, RN, MS, FNP-BC, AE-C, a board-certified nurse practitioner and asthma educator, said in her presentation. “You want to make sure you’re involving the important people in their lives to help them.”
Education for asthma includes teaching patients and their families the difference between long-term control and reliever medications; the proper timing and technique with the medications, as well as the importance of adherence; how to recognize and avoid triggers for asthma; how to self-monitor their asthma and control the disease; and when to seek medication care, she said.
“We review their inhaler technique every time they come in,” she added.
According to the American Lung Association, patients learn in visual, auditory, and kinesthetic styles. Teaching patients in a kinesthetic style by actually showing the patient how to take the medication through example will help the patient learn through feeling, or muscle memory. This also method works even if patients do not have the medication with them at the time, said Ms. Hayden.
“Let’s say, you don’t have [the medication], but you prescribe it,” she said. “When they come back, tell them to bring their bag of medications and make sure you go back through because if they can kinesthetically use it correctly, they’ve already mastered the visual and the auditory piece.
Written action plans are also important to successful asthma management. The plan should be tailored to the patient’s disease severity, loss of control, and include information like the peak expiratory flow and medication types, dosages, and frequencies. The action plan should also be available at home, daycare, and school. “You want them to know how to recognize their symptoms, what to do about their symptoms, and when to contact you or go to urgent care or [the emergency room],” said Ms. Hayden.
To simplify the plan, Ms. Hayden recommended zoning actions based on color, like the asthma action plan provided by the American Academy of Allergy, Asthma & Immunology. The AAAAI plan uses traffic colors to signify how well controlled a patient’s asthma is, with green indicating well-controlled disease, yellow denoting worsening asthma, and red indicating that the asthma needs to be treated right away.
Action plans should also address a patient’s health literacy level and culture. “Think about who’s going to be using it,” said Ms. Hayden.
The goal of asthma therapy is to prevent chronic or problematic symptoms, lower use of short-acting beta-agonists, maintain good pulmonary function, normalize activity levels at school and work, prevent exacerbations and hospitalizations, and meet the patient’s expectations, as well as those of their family. “If you’re thinking only severe patients have exacerbations that are near fatal or fatal, that’s not true,” she said. It’s “very common for somebody with a very mild and intermittent asthma to go to severe in a very short period of time.”
When properly implemented, patient education is performed at the time of diagnosis, is done according to a plan, is integrated into care, reinforces important information, improves adherence, is individualized to the patient and addresses their needs, and builds a partnership between provider and patient.
“We really are thinking of the team concept: us, the patient and the important people the patient’s lives, and other clinicians that might be involved with other diseases to care for the patient,” said Ms. Hayden.
Ms. Hayden reports no relevant conflicts of interest. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
“Every contact with them is a teachable moment,” Mary Lou Hayden, RN, MS, FNP-BC, AE-C, a board-certified nurse practitioner and asthma educator, said in her presentation. “You want to make sure you’re involving the important people in their lives to help them.”
Education for asthma includes teaching patients and their families the difference between long-term control and reliever medications; the proper timing and technique with the medications, as well as the importance of adherence; how to recognize and avoid triggers for asthma; how to self-monitor their asthma and control the disease; and when to seek medication care, she said.
“We review their inhaler technique every time they come in,” she added.
According to the American Lung Association, patients learn in visual, auditory, and kinesthetic styles. Teaching patients in a kinesthetic style by actually showing the patient how to take the medication through example will help the patient learn through feeling, or muscle memory. This also method works even if patients do not have the medication with them at the time, said Ms. Hayden.
“Let’s say, you don’t have [the medication], but you prescribe it,” she said. “When they come back, tell them to bring their bag of medications and make sure you go back through because if they can kinesthetically use it correctly, they’ve already mastered the visual and the auditory piece.
Written action plans are also important to successful asthma management. The plan should be tailored to the patient’s disease severity, loss of control, and include information like the peak expiratory flow and medication types, dosages, and frequencies. The action plan should also be available at home, daycare, and school. “You want them to know how to recognize their symptoms, what to do about their symptoms, and when to contact you or go to urgent care or [the emergency room],” said Ms. Hayden.
To simplify the plan, Ms. Hayden recommended zoning actions based on color, like the asthma action plan provided by the American Academy of Allergy, Asthma & Immunology. The AAAAI plan uses traffic colors to signify how well controlled a patient’s asthma is, with green indicating well-controlled disease, yellow denoting worsening asthma, and red indicating that the asthma needs to be treated right away.
Action plans should also address a patient’s health literacy level and culture. “Think about who’s going to be using it,” said Ms. Hayden.
The goal of asthma therapy is to prevent chronic or problematic symptoms, lower use of short-acting beta-agonists, maintain good pulmonary function, normalize activity levels at school and work, prevent exacerbations and hospitalizations, and meet the patient’s expectations, as well as those of their family. “If you’re thinking only severe patients have exacerbations that are near fatal or fatal, that’s not true,” she said. It’s “very common for somebody with a very mild and intermittent asthma to go to severe in a very short period of time.”
When properly implemented, patient education is performed at the time of diagnosis, is done according to a plan, is integrated into care, reinforces important information, improves adherence, is individualized to the patient and addresses their needs, and builds a partnership between provider and patient.
“We really are thinking of the team concept: us, the patient and the important people the patient’s lives, and other clinicians that might be involved with other diseases to care for the patient,” said Ms. Hayden.
Ms. Hayden reports no relevant conflicts of interest. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
“Every contact with them is a teachable moment,” Mary Lou Hayden, RN, MS, FNP-BC, AE-C, a board-certified nurse practitioner and asthma educator, said in her presentation. “You want to make sure you’re involving the important people in their lives to help them.”
Education for asthma includes teaching patients and their families the difference between long-term control and reliever medications; the proper timing and technique with the medications, as well as the importance of adherence; how to recognize and avoid triggers for asthma; how to self-monitor their asthma and control the disease; and when to seek medication care, she said.
“We review their inhaler technique every time they come in,” she added.
According to the American Lung Association, patients learn in visual, auditory, and kinesthetic styles. Teaching patients in a kinesthetic style by actually showing the patient how to take the medication through example will help the patient learn through feeling, or muscle memory. This also method works even if patients do not have the medication with them at the time, said Ms. Hayden.
“Let’s say, you don’t have [the medication], but you prescribe it,” she said. “When they come back, tell them to bring their bag of medications and make sure you go back through because if they can kinesthetically use it correctly, they’ve already mastered the visual and the auditory piece.
Written action plans are also important to successful asthma management. The plan should be tailored to the patient’s disease severity, loss of control, and include information like the peak expiratory flow and medication types, dosages, and frequencies. The action plan should also be available at home, daycare, and school. “You want them to know how to recognize their symptoms, what to do about their symptoms, and when to contact you or go to urgent care or [the emergency room],” said Ms. Hayden.
To simplify the plan, Ms. Hayden recommended zoning actions based on color, like the asthma action plan provided by the American Academy of Allergy, Asthma & Immunology. The AAAAI plan uses traffic colors to signify how well controlled a patient’s asthma is, with green indicating well-controlled disease, yellow denoting worsening asthma, and red indicating that the asthma needs to be treated right away.
Action plans should also address a patient’s health literacy level and culture. “Think about who’s going to be using it,” said Ms. Hayden.
The goal of asthma therapy is to prevent chronic or problematic symptoms, lower use of short-acting beta-agonists, maintain good pulmonary function, normalize activity levels at school and work, prevent exacerbations and hospitalizations, and meet the patient’s expectations, as well as those of their family. “If you’re thinking only severe patients have exacerbations that are near fatal or fatal, that’s not true,” she said. It’s “very common for somebody with a very mild and intermittent asthma to go to severe in a very short period of time.”
When properly implemented, patient education is performed at the time of diagnosis, is done according to a plan, is integrated into care, reinforces important information, improves adherence, is individualized to the patient and addresses their needs, and builds a partnership between provider and patient.
“We really are thinking of the team concept: us, the patient and the important people the patient’s lives, and other clinicians that might be involved with other diseases to care for the patient,” said Ms. Hayden.
Ms. Hayden reports no relevant conflicts of interest. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Gaps in patient-provider survivorship communication persist
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
To help patients stay on diabetes regimens: Communicate, educate, and use technology
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
REPORTING FROM ADA 2019
How to have ‘the talk’ with vaccine skeptics
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
EXPERT ANALYSIS FROM ESPID 2019
Cultural competence behaviors linked to higher patient satisfaction scores
results from a single-center study showed.
“Cultural competence is valued by patients, and there is a potential for training providers to be more culturally competent, focusing on their behaviors,” lead study author Miquell O. Miller, MD, said at the annual Digestive Disease Week.
While the Society for Surgery of the Alimentary Tract (J Gastrointest Surg 2016;20[5]:879-84) and other medical organizations have recognized the importance of workforce diversity and cultural competence of providers, little is known of the relationship between cultural competence and patient-reported outcomes in surgery, said Dr. Miller, who is a general surgery resident at Stanford (Calif.) University. To investigate this relationship, she and her colleagues recruited surgeons, oncologists, gastroenterologists, and advanced practice providers to complete a validated online survey that measured two domains of cultural competency: awareness and behaviors. They matched these scores with the 10-item Press Ganey provider care scores from 2017 to 2018. Next, the researchers conducted a linear regression analysis with mixed effects to account for clustering of patients within providers. They also adjusted for provider bias by measuring social desirability, “which is the tendency for respondents to put a more socially appropriate answer as opposed to the true answer on the cultural competence survey,” Dr. Miller explained.
A total of 1,322 Press Ganey satisfaction surveys were available for 29 providers. Their mean age was 48 years, 59% were white, 72% were physicians, and 28% were advanced practice providers. They practiced in GI oncology (41%), gastroenterology (31%) and colorectal surgery (28%). Dr. Miller reported that providers who participated in the survey had a mean cultural awareness score of 6.2 out of a possible 7 points, while the mean cultural behavior score was a 4.1 out of a possible 7 points. She and her colleagues observed that providers who had high levels of cultural competence on the behavioral assessment were positively associated with Press Ganey patient satisfaction (P = .039).
“I think we do a poor job of training our providers to be culturally competent, but there are multiple ways to improve behaviors, by teaching people and by having real training for our providers,” Dr. Miller said.
She acknowledged certain limitations of the study, including its single-center design and the fact that not all providers had Press Ganey scores available. The study was funded by the Society for Surgery of the Alimentary Tract and the Black Academic Surgeons Resident Research Award. Dr. Miller reported having no financial disclosures.
results from a single-center study showed.
“Cultural competence is valued by patients, and there is a potential for training providers to be more culturally competent, focusing on their behaviors,” lead study author Miquell O. Miller, MD, said at the annual Digestive Disease Week.
While the Society for Surgery of the Alimentary Tract (J Gastrointest Surg 2016;20[5]:879-84) and other medical organizations have recognized the importance of workforce diversity and cultural competence of providers, little is known of the relationship between cultural competence and patient-reported outcomes in surgery, said Dr. Miller, who is a general surgery resident at Stanford (Calif.) University. To investigate this relationship, she and her colleagues recruited surgeons, oncologists, gastroenterologists, and advanced practice providers to complete a validated online survey that measured two domains of cultural competency: awareness and behaviors. They matched these scores with the 10-item Press Ganey provider care scores from 2017 to 2018. Next, the researchers conducted a linear regression analysis with mixed effects to account for clustering of patients within providers. They also adjusted for provider bias by measuring social desirability, “which is the tendency for respondents to put a more socially appropriate answer as opposed to the true answer on the cultural competence survey,” Dr. Miller explained.
A total of 1,322 Press Ganey satisfaction surveys were available for 29 providers. Their mean age was 48 years, 59% were white, 72% were physicians, and 28% were advanced practice providers. They practiced in GI oncology (41%), gastroenterology (31%) and colorectal surgery (28%). Dr. Miller reported that providers who participated in the survey had a mean cultural awareness score of 6.2 out of a possible 7 points, while the mean cultural behavior score was a 4.1 out of a possible 7 points. She and her colleagues observed that providers who had high levels of cultural competence on the behavioral assessment were positively associated with Press Ganey patient satisfaction (P = .039).
“I think we do a poor job of training our providers to be culturally competent, but there are multiple ways to improve behaviors, by teaching people and by having real training for our providers,” Dr. Miller said.
She acknowledged certain limitations of the study, including its single-center design and the fact that not all providers had Press Ganey scores available. The study was funded by the Society for Surgery of the Alimentary Tract and the Black Academic Surgeons Resident Research Award. Dr. Miller reported having no financial disclosures.
results from a single-center study showed.
“Cultural competence is valued by patients, and there is a potential for training providers to be more culturally competent, focusing on their behaviors,” lead study author Miquell O. Miller, MD, said at the annual Digestive Disease Week.
While the Society for Surgery of the Alimentary Tract (J Gastrointest Surg 2016;20[5]:879-84) and other medical organizations have recognized the importance of workforce diversity and cultural competence of providers, little is known of the relationship between cultural competence and patient-reported outcomes in surgery, said Dr. Miller, who is a general surgery resident at Stanford (Calif.) University. To investigate this relationship, she and her colleagues recruited surgeons, oncologists, gastroenterologists, and advanced practice providers to complete a validated online survey that measured two domains of cultural competency: awareness and behaviors. They matched these scores with the 10-item Press Ganey provider care scores from 2017 to 2018. Next, the researchers conducted a linear regression analysis with mixed effects to account for clustering of patients within providers. They also adjusted for provider bias by measuring social desirability, “which is the tendency for respondents to put a more socially appropriate answer as opposed to the true answer on the cultural competence survey,” Dr. Miller explained.
A total of 1,322 Press Ganey satisfaction surveys were available for 29 providers. Their mean age was 48 years, 59% were white, 72% were physicians, and 28% were advanced practice providers. They practiced in GI oncology (41%), gastroenterology (31%) and colorectal surgery (28%). Dr. Miller reported that providers who participated in the survey had a mean cultural awareness score of 6.2 out of a possible 7 points, while the mean cultural behavior score was a 4.1 out of a possible 7 points. She and her colleagues observed that providers who had high levels of cultural competence on the behavioral assessment were positively associated with Press Ganey patient satisfaction (P = .039).
“I think we do a poor job of training our providers to be culturally competent, but there are multiple ways to improve behaviors, by teaching people and by having real training for our providers,” Dr. Miller said.
She acknowledged certain limitations of the study, including its single-center design and the fact that not all providers had Press Ganey scores available. The study was funded by the Society for Surgery of the Alimentary Tract and the Black Academic Surgeons Resident Research Award. Dr. Miller reported having no financial disclosures.
REPORTING FROM DDW 2019
















