User login
Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
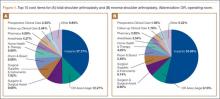
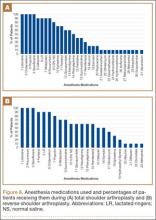
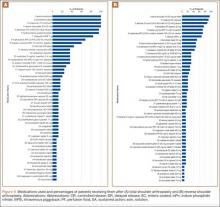
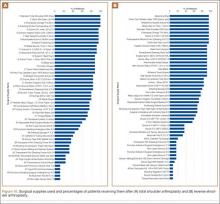
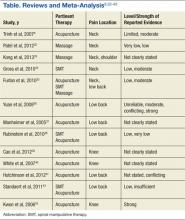
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
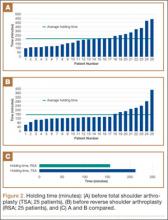
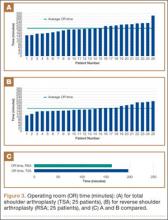
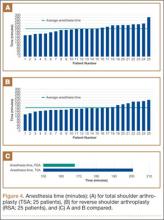
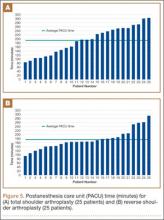
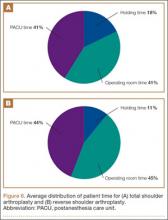
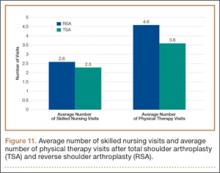
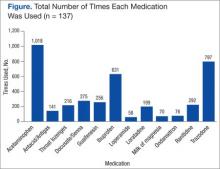
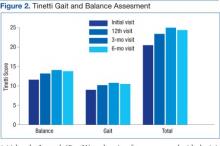
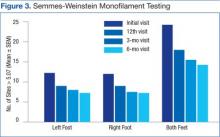
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
The Pathobiology of Diabetes Mellitus in Bone Metabolism, Fracture Healing, and Complications
Diabetes mellitus (DM) affects a significant portion of the world’s people, and the problem is increasing in magnitude as the population ages and becomes more obese.1 An estimated 347 million people have diabetes.1 In the United States, 26 million (roughly 8% of the population) are affected, making DM a major health issue.2 Given the prevalence of diabetes in the general population, it is not surprising that increasing numbers of fracture patients have DM. Unfortunately, for these patients, many relatively simple fractures can have disastrous outcomes. Infections and wound complications occur in disproportionate numbers, healing time is delayed, and risk for nonunion or malunion is substantially higher.3
It is imperative to understand the pathophysiology of DM to appreciate potential interventions and strategies aimed at decreasing complications and improving outcomes of fractures in patients with the disease. In type 1 DM (T1DM), autoimmune destruction of the insulin-secreting β cells in the pancreas results in a complete absence of insulin. Patients with T1DM are dependent on exogenous insulin, and, despite hyperglycemia, most cells in the body are starved for energy. This leads to a catabolic condition, high lipid and protein metabolism, and, in many cases, ketoacidosis. When insulin resistance develops, the β cells are forced to secrete large amounts of insulin; when they fail to keep up, type 2 DM (T2DM) develops. T2DM is often associated with obesity, as excess adipose tissue leads to insulin resistance. Although exogenous insulin may be necessary to treat advanced T2DM, other medications are commonly used to effectively lower blood glucose: Secretagogues (eg, sulfonylureas) facilitate insulin release from β cells, and sensitizers (eg, metformin) increase insulin sensitivity.4,5
The potential morbidity of fractures in patients with DM can be appreciated with the example of ankle fractures. These typically uncomplicated fractures can have very poor outcomes in the setting of DM. In a prospective study of approximately 1500 patients with ankle fractures treated with open reduction and internal fixation, Wukich and colleagues6 found that 9.5% of patients with DM (vs 2.4% of patients without DM) developed surgical site infections. As defined by Jones and colleagues,7 major complications of treating ankle fractures in patients with DM include infection, malunion, nonunion, Charcot arthropathy, and amputation. The authors reported major complications in 31% and 17% of patients with and without DM, respectively. Highlighting the importance of glycemic control, Wukich and colleagues6 found relative risks of 3.8 for infection, 3.4 for noninfectious complications, and 5.0 for revision in complicated (vs uncomplicated) fractures in patients with DM.
Given the magnitude of problems in the treatment of fractures in patients with DM, we focus our review on the pathobiology of diabetes in terms of bone metabolism and fracture healing, wound healing and vasculopathy, infection, and potential new treatment modalities.
Bone Metabolism and Fracture Healing in Diabetes
Insulin appears to play a role in bone metabolism and fracture healing. Therefore, absence of insulin in T1DM and elevated insulin levels associated with T2DM likely influence these metabolic and fracture-healing processes. Insulin has been hypothesized to have an anabolic effect on bone, and in both human and animal models bone mineral density (BMD) is significantly lower in T1DM. Furthermore, BMD in T2DM has been shown to be normal or even elevated.8 Other metabolic effects of insulin on bone metabolism and growth include slower growth rates and lower BMD in pediatric patients with T1DM versus patients without diabetes, and some animal models show bone microarchitecture altered in the absence of insulin (and reversible with insulin supplementation).9 These factors seem to contradict the markedly elevated risk for osteoporotic fracture in patients with T2DM, but the mechanisms responsible for this have not been elucidated.8
In terms of fracture healing, resorption of cartilage during transition to hard callus appears to be influenced by diabetes. It has been hypothesized that the smaller callus observed in diabetic mice may be secondary to upregulation of osteoclasts. Initial callus size appears not to differ between mice with streptozotocin-induced diabetes, which exhibit a complete absence of insulin, and control mice, but levels of osteoclast and osteoclastogenesis mediators were significantly higher in the diabetic mice.10 Some investigators think that the reduction in cartilage callus size in diabetic mice is caused by altered mRNA expression and collagen production.11 Diabetic mice, in addition to showing increased resorption by osteoclasts, demonstrate increased chondrocyte apoptosis, which is thought to activate cartilage resorption events. Exogenous insulin effectively reverses this cartilage loss to baseline levels.12
Osteoblasts are a crucial component of the fracture-healing cascade, and acute and chronic hyperglycemia, the hallmark of diabetes, has a variety of effects on osteoblasts.13 Genes for cell-signal proteins such as osteocalcin, MMP-13, and vascular endothelial growth factor are downregulated in the presence of chronic hyperglycemia, whereas genes for alkaline phosphate are upregulated. Acute hyperglycemia by way of hyperosmolarity is associated with MMP-13 downregulation. Thus, osteoblasts appear to respond to hyperglycemia through 2 different processes: Hyperosmolarity, through osteoblast cell shrinkage, influences the acute response, and hyperglycemia itself, through pathways such as nonenzymatic glycosylation, protein kinase C (PKC) signaling, and the polyol pathway, is the force behind the chronic response.14 The lineage of osteoblasts from mesenchymal stem cells also can be affected by hyperglycemia, with lower growth rates for mesenchymal stem cells and preferential development toward the adipocyte lineage, while the osteoblast and chondrocyte lineages are downregulated.15
Increased osteoblast apoptosis has been associated with diabetes through advanced glycation end-products (AGEs), which modify the structure and function of bioactive compounds through AGE receptors that cross-link and bond to amino groups on bioactive molecules.16 It has been reported that AGEs interfere with osteoblast development and collagen and osteocalcin production.17 A common AGE, carboxymethyl lysine-modified collagen, has been associated with a significant increase in apoptosis through the mitogen-activated protein kinase (MAPK) pathway. Although most of the literature suggests that osteoblast apoptosis is activated by hypoxia, nitric oxide, or integrins, these factors all have the MAPK pathway in common.18
Osteoclasts are also influenced by diabetes. Recent work in T1DM demonstrated that osteoclasts are hyperactive and more sensitive to receptor activator of nuclear factor kB ligand (RANKL) compared with osteoclasts from the population without diabetes. It is also known that osteoclasts are under the control of immunologic mediators like lipopolysaccharide (LPS), a surface component of gram-negative bacteria, and various other proinflammatory cytokines. In patients with diabetes, osteoclasts react differently to LPS and other proinflammatory cytokines, at times with opposing effects, including secretion of RANKL to stimulate resorption by the osteoclast, and precursors preventing progression into osteoclasts. In healthy people, high LPS levels not only prevent precursors from producing more osteoclasts, but promote them to mature into immune-like cells that actually phagocytose bacteria. So, in a state of infection, precursors shift from bone-resorbing osteoclasts to protective immune cells. This phenomenon does not occur in patients with diabetes, in whom the osteoclasts instead resorb more bone and stimulate inflammation by releasing cytokines.19
Interestingly, osteoblasts and osteoclasts are also affected by medications commonly used to treat diabetes. Thiazolidinediones are a class of sensitizers often used to treat patients with T2DM. Thiazolidinediones, particularly rosiglitazone, have been associated with increased bone loss primarily caused by increased bone resorption by osteoclasts.20 In addition, some investigators think that thiazolidinediones induce osteocyte apoptosis, contributing to impaired bone growth.8 Metformin, an insulin sensitizer, appears to have a positive effect on bone growth and fracture risk by enhancing osteoblastogenesis and inhibiting osteoclastogenesis, leading to a protective effect on bone.8
Peripheral neuropathy, which is often associated with diabetes, appears to play a major role in fracture-healing complications, even more so than hyperglycemia does. A recent clinical paper found that patients with diabetic neuropathy had a 44% risk of foot and ankle fracture-healing complications.21 Regardless of the risk, the pathogenesis of diabetic neuropathy can be caused by several mechanisms. Neural tissue does not require insulin for glucose uptake; therefore, in a state of hyperglycemia, aldose reductase shunts glucose to sorbitol while using protective glutathione and generating reactive oxygen species. This oxidative stress results in nerve damage or neuropathy. Microangiopathy, which we discuss in more detail later, also contributes to the development of neuropathy, through compromised flow of blood to neural tissue.22 Another mechanism contributing to diabetic neuropathy involves PKC, which is activated by 1,2-diacylglycerol in the presence of glucose, leading to vascular changes that restrict the flow of blood to peripheral nerves.23 Finally, AGEs may also participate by altering nerve function after binding to neural tissue.
Charcot neuroarthropathy is a complication associated with diabetes, particularly after injury in which chronic inflammation results in damage to the joint through fracture, dislocation, and osteolytic bony destruction. The pathophysiology is attributed to repeated microtrauma caused by loss of protective sensibility and hyperemia caused by dysregulation.24 Sympathetic and sensory nerve fibers are associated with bone, but a few serve as mechanoreceptors and nociceptors, which can activate substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide—neuropeptides all thought to be involved in the inflammatory process, and in the activation of osteoblasts and osteoclasts. In diabetic neuropathy, many of these neuropeptides show a reduced regulation response, which can lead to impaired fracture healing. In particular, osteoclast activity is upregulated, and consequently bone resorption is increased. In addition to the neuropeptides mentioned, RANKL is one mechanism by which this upregulation occurs.25
It is clear that bone metabolism and fracture healing are complex processes. In the patient with diabetes, many factors are affected, including BMD, bone microarchitecture and bone growth, cartilage resorption during callus formation, osteoblast and osteoclast activation through both altered responses to cell signals and pharmacologic interactions, and, finally, peripheral neuropathy. Given the complex interactions described, it is likely that these factors in combination, as well as those yet undiscovered, negatively affect fracture healing.
Wound Healing and Vasculopathy in Diabetes
Bone healing and soft-tissue healing depend on many of the same factors. Therefore, interactions between neuropathy and vasculopathy can have a tremendous influence on wound healing in patients with diabetes. The vascular pathology that occurs in diabetes depends in part on the fact that endothelial cells do not require insulin for glucose uptake and therefore are more susceptible to damage by hyperglycemia. As already discussed, shunting of glucose through the polyol pathway with the resultant oxidative stress is partly responsible for angiopathy in diabetes.
Also as already discussed, AGEs affect intracellular processes by protein binding and gene regulation and by disrupting the communication between cells and the surrounding matrix. From an extracellular standpoint, AGEs bind to circulating proteins, promoting inflammation and upregulation/downregulation of growth factors, including endothelial nitric oxide synthase, a critical vasodilator. Endothelin 1, on the other hand, is a potent vasoconstrictor. It is upregulated while transforming growth factor b and plasminogen activator inhibitor 1 are upregulated, resulting in further vascular damage.26 The common mechanism for this vasculopathy appears to be superoxide production in the mitochondria, caused by excess glucose oxidation forcing coenzyme Q to donate electrons to oxygen, producing the superoxides. Superoxides in turn inhibit glyceraldehyde 3-phosphate dehydrogenase, which activates the polyol pathway, AGE formation, PKC, and the hexosamine pathway.26 In addition to coenzyme Q, several other enzymes generate reactive oxygen species, including nicotinamide adenine dinucleotide phosphate oxidase, aldehyde oxidase, xanthine oxidase, and glucose oxidase.27 These reactive oxygen species exacerbate oxidative stress, leading to further endothelial cell damage, and cause vascular smooth muscle injury.28
Further influencing the wound-healing environment are the effects of diabetes on blood vessel maintenance and repair as well as angiogenesis in response to local-tissue hypoxia. Vessel-repair mechanisms require endothelial progenitor cells (EPCs), which are released in response to cytokines and neural impulses.29 Bone marrow–derived EPCs have inadequate proliferative and migratory ability in patients with diabetes.28,30 In a diabetic mouse model, EPCs appear in the bone marrow at normal levels, but levels in circulation are lower than anticipated, because of poor proliferation and mobilization, it is thought. In terms of local-tissue hypoxia, hypoxia-inducible factor 1 (HIF-1) is an important transcription factor that promotes the expression of genes that in turn induce angiogenesis. The mechanism of this response is complex, and hyperglycemia has the potential to interfere in various steps of the cycle. In response to local-tissue hypoxia, the HIF-1a subunit must localize to the target site, where it combines with HIF-1b to create the active dimer, HIF-1.31 This active dimer is regulated through degradation of the a subunit in the presence of normal oxygen levels. However, in a state of hypoxia, the molecule is stabilized, promoting angiogenesis and fibroblast migration.32 Recent evidence suggests that hyperglycemia interferes with the dimerization process and that there is a failure of HIF-1a to locate into the nucleus, which is crucial for gene upregulation.31-33
Infection in Diabetes
Throughout the literature, the risk for infection after fracture is consistently higher in patients with diabetes than without diabetes. There likely are many contributing factors, including diminished blood flow and vasculopathy as well as a dampened immune response as a result of defective granulocytic, phagocytic, and chemotactic functions and defective macrophagic activity. Typically, polymorphonuclear leukocytes (PMNs) migrate to bacteria and initiate bacteriocidal activity, and then macrophages phagocytize PMNs and other damaged cells. PMNs demonstrate impaired function in patients with diabetes—reduced phagocytic response and respiratory burst as well as chemotaxis impairment. The diminished phagocytic potential is substantial, with experiments showing an almost 50% reduction in ingestion of Staphylococcus aureus in a patient with diabetes than in one without diabetes.34 Expression of surface integrins, which mediate PMN adhesion to the basement membrane of the tissue, appears to be negatively altered in both T1DM and T2DM, furthering diminishing the chemotactic response of PMNs.35 Impaired leukocyte function may also be a downstream effect of vasculopathy and associated hypoxia/hypoxemia as PMNs use superoxide radicals and other oxidizing agents to create a bacteriocidal environment that is negatively impacted in a low oxygen state.3 In addition, macrophages are disabled in patients with diabetes. (In rats with streptozotocin-induced diabetes, there is inadequate activation of macrophages in the early stages of healing.36) Furthermore, AGEs similar to those mentioned earlier have a significant negative impact on macrophagic function.37 Thus, both the activation and the activity of macrophages appear to be impeded in the setting of diabetes.
Potential New Treatment Modalities
There is tremendous potential for clinical intervention to prevent pathologic outcomes in patients with diabetes, given the complex tissue, cellular, and molecular interactions, particularly those caused by hyperglycemia. At the bone tissue level, increased osteoclastic activity in patients with diabetes has been associated with many complications, including Charcot arthropathy. RANKL modulates differentiation and activation of osteoclasts; thus, RANKL inhibition is a possible therapeutic target.38 Elevated AGE levels have also been observed in patients with Charcot arthropathy, and RAGE, the receptor for AGE, has been seen at lower than expected levels in patients with diabetes. RAGE appears to provide a protective effect against excessive bone resorption; therefore, treatment that increases RAGE levels—such as angiotensin-converting-enzyme inhibitors, statins, and glitazones—may be capable of mitigating the osteoclastic effects in Charcot arthropathy.39
AGE formation appears to be central to many pathologic processes in diabetes, so it is a logical therapeutic target, particularly for pathologic processes at the vascular tissue level. Aminoguanidine is an anti-AGE agent that was initially used to prevent diabetic retinopathy, but it has also been shown to prevent general vascular complications in diabetic animal models. The terminal amino residue in the compound specifically binds glucose-derived reactive intermediates and prevents cross-linking, which renders them inactive. Disrupting those cross-links is another treatment strategy. N-phenacylthiazolium bromide and 3-phenacyl-4,5-dimethylthiazolium chloride (ALT-711 or alagebrium) are compounds that have been shown to break cross-links in a diabetic rat model.16
Another tactic for reducing vascular pathology involves mitigating superoxide radicals, as these radicals are generated from the glycolytic intermediates in hyperglycemic states. It has been reasoned that, if the concentration of these intermediates can be decreased, there would be less substrate available for the pathways that lead to radical formation. One approach is to use transketolase, an enzyme that shunts intermediates to pathways that do not produce superoxide radicals. In the treatment of patients with diabetic retinopathy, early data appear promising with benfotiamine, a thiamine derivative, which upregulates transketolase 250%. An additional tactic involves catalytic antioxidants—namely, superoxide dismutase/catalase mimetic, which has been shown to reduce hyperglycemia-induced superoxides. These interventions are appealing because of their nonstoichiometric reactions, which render them potentially more potent antioxidants.26
Potential neurologic interventions include recombinant human nerve growth factor, neurotrophic factors, and gene therapy, all directed toward preventing or regenerating neuropathic tissues in patients with diabetes. Most of these interventions, however, remain theoretical. Few trials have demonstrated clinically significant improvement. In patients with T1DM, however, the absence of circulating C-peptide is thought to contribute to diabetic neuropathy. Results of trials with subcutaneous C-peptide treatment suggest improvement in both sural sensory and vibration perception after only 12 weeks.40 These novel treatments further emphasize the potential for intervention at the tissue, cellular, and molecular levels.
Conclusion
Whereas most fractures are uncomplicated in healthy patients, they can have devastating consequences in patients with diabetes. In this review, we have highlighted many of the pathologic processes that can influence outcomes of fractures in patients with diabetes. These problems will become more common as the population ages, age-related risks for osteoporosis and fragility fracture increase, and diabetes becomes nearly epidemic in our increasingly obese, sedentary society. Although some progress has been made, a more thorough intervention strategy is needed to improve both bone and soft-tissue outcomes of fractures in patients with diabetes.
1. Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31-40.
2. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2011.
3. Wukich DK, Joseph A, Ryan M, Ramirez C, Irrgang JJ. Outcomes of ankle fractures in patients with uncomplicated versus complicated diabetes. Foot Ankle Int. 2011;32(2):120-130.
4. Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2010.
5. Diabetes basics. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/basics/index.html. Updated October 25, 2014. Accessed August 24, 2015.
6. Wukich DK, McMillen RL, Lowery NJ, Frykberg RG. Surgical site infections after foot and ankle surgery. Diabetes Care. 2001;34(10):2211-2213.
7. Jones KB, Maiers-Yelden KA, Marsh JL, et al. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87(4):489-495.
8. Yan W, Li X. Impact of diabetes and its treatments on skeletal diseases. Front Med. 2013;7(1):81-90.
9. Thrailkill K, Lumpkin C Jr, Bunn R, Kemp S, Fowlkes J. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735-E745.
10. Kayal RA, Tsatsas D, Bauer MA, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560-568.
11. Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41(2):81-91.
12. Kayal RA, Alblowi J, McKenzie E, et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009;44(2):357-363.
13. Motyl K, Botolin S, Irwin R, et al. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218(3):575-583.
14. Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99(2):411-424.
15. Keats E, Khanz ZA. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS One. 2012;7(6):e38752.
16. Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87-101.
17. Fong Y, Edelstein D, Wang E, Brownlee M. Inhibition of matrix-induced bone differentiation by advanced glycation end-products in rats. Diabetologia. 1993;36(9):802-807.
18. Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation endproducts stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345-353.
19. Catalfamo DL, Calderon NL, Harden SW, Sorenson HL, Neiva KG, Wallet SM. Augmented LPS responsiveness in type 1 diabetes-derived osteoclasts. J Cell Physiol. 2013;228(2):349-361.
20. Kahn SE, Lachin JM, Zinman B, et al; ADOPT Study Group. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552-1560.
21. Shibuya N, Humphers JM, Fluhman BL, Jupiter DC. Factors associated with nonunion, delayed union, and malunion in foot and ankle surgery in diabetic patients. J Foot Ankle Surg. 2013;52(2):207-211.
22. Shami SK, Chittenden SJ. Microangiopathy in diabetes mellitus: II. Features, complications and investigation. Diabetes Res. 1991;17(4):157-168.
23. Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404(6779):787-790.
24. Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep. 2005;5(6):430-435.
25. Lerner UH, Persson E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Musculoskelet Neuronal Interact. 2008;8(2):154-165.
26. Brownlee M. The pathobiology of diabetic complications—a unifying mechanism. Diabetes. 2005;54(6):1615-1625.
27. Tsuji S, Taniuchi S, Hasui M, Yamamoto A, Kobayashi Y. Increased nitric oxide production by neutrophils from patients with chronic granulomatous disease on trimethoprim-sulfamethoxazole. Nitric Oxide. 2002;7(4):283-288.
28. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
29. Westerweel PE. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One. 2013;8(3):e60357.
30. Fadini GP, Avogaro A. It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications. Exp Diabetes Res. 2012;2012:742976.
31. Gadad PC, Matthews KH, Knott RM. Role of HIF1α and PKCβ in mediating the effect of oxygen and glucose in a novel wound assay. Microvasc Res. 2013;88:61-69.
32. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426-19431.
33. Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53(12):3226-3232.
34. Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15(2):256-260.
35. Calmi G, Montana M, Citarella R, Porretto F, Catania A, Lo Presti R. Polymorphonuclear leukocyte integrin profile in diabetes mellitus. Clin Hemorheol Microcirc. 2002;27(2):83-89.
36. Miao M, Niu Y, Xie T, Yuan B, Qing C, Lu S. Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012;20(2):203-213.
37. Liu BF, Miyata S, Kojima H, et al. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes. 1999;48(10):2074-2082.
38. Mabilleau G, Petrova NL, Edmonds ME, Sabokbar A. Increased osteoclastic activity in acute Charcot’s osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia. 2008;51(6):1035-1040.
39. Witzke KA, Vinik AI, Grant LM, et al. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. 2011;34(7):1617-1621.
40. Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4(4):271-285.
Diabetes mellitus (DM) affects a significant portion of the world’s people, and the problem is increasing in magnitude as the population ages and becomes more obese.1 An estimated 347 million people have diabetes.1 In the United States, 26 million (roughly 8% of the population) are affected, making DM a major health issue.2 Given the prevalence of diabetes in the general population, it is not surprising that increasing numbers of fracture patients have DM. Unfortunately, for these patients, many relatively simple fractures can have disastrous outcomes. Infections and wound complications occur in disproportionate numbers, healing time is delayed, and risk for nonunion or malunion is substantially higher.3
It is imperative to understand the pathophysiology of DM to appreciate potential interventions and strategies aimed at decreasing complications and improving outcomes of fractures in patients with the disease. In type 1 DM (T1DM), autoimmune destruction of the insulin-secreting β cells in the pancreas results in a complete absence of insulin. Patients with T1DM are dependent on exogenous insulin, and, despite hyperglycemia, most cells in the body are starved for energy. This leads to a catabolic condition, high lipid and protein metabolism, and, in many cases, ketoacidosis. When insulin resistance develops, the β cells are forced to secrete large amounts of insulin; when they fail to keep up, type 2 DM (T2DM) develops. T2DM is often associated with obesity, as excess adipose tissue leads to insulin resistance. Although exogenous insulin may be necessary to treat advanced T2DM, other medications are commonly used to effectively lower blood glucose: Secretagogues (eg, sulfonylureas) facilitate insulin release from β cells, and sensitizers (eg, metformin) increase insulin sensitivity.4,5
The potential morbidity of fractures in patients with DM can be appreciated with the example of ankle fractures. These typically uncomplicated fractures can have very poor outcomes in the setting of DM. In a prospective study of approximately 1500 patients with ankle fractures treated with open reduction and internal fixation, Wukich and colleagues6 found that 9.5% of patients with DM (vs 2.4% of patients without DM) developed surgical site infections. As defined by Jones and colleagues,7 major complications of treating ankle fractures in patients with DM include infection, malunion, nonunion, Charcot arthropathy, and amputation. The authors reported major complications in 31% and 17% of patients with and without DM, respectively. Highlighting the importance of glycemic control, Wukich and colleagues6 found relative risks of 3.8 for infection, 3.4 for noninfectious complications, and 5.0 for revision in complicated (vs uncomplicated) fractures in patients with DM.
Given the magnitude of problems in the treatment of fractures in patients with DM, we focus our review on the pathobiology of diabetes in terms of bone metabolism and fracture healing, wound healing and vasculopathy, infection, and potential new treatment modalities.
Bone Metabolism and Fracture Healing in Diabetes
Insulin appears to play a role in bone metabolism and fracture healing. Therefore, absence of insulin in T1DM and elevated insulin levels associated with T2DM likely influence these metabolic and fracture-healing processes. Insulin has been hypothesized to have an anabolic effect on bone, and in both human and animal models bone mineral density (BMD) is significantly lower in T1DM. Furthermore, BMD in T2DM has been shown to be normal or even elevated.8 Other metabolic effects of insulin on bone metabolism and growth include slower growth rates and lower BMD in pediatric patients with T1DM versus patients without diabetes, and some animal models show bone microarchitecture altered in the absence of insulin (and reversible with insulin supplementation).9 These factors seem to contradict the markedly elevated risk for osteoporotic fracture in patients with T2DM, but the mechanisms responsible for this have not been elucidated.8
In terms of fracture healing, resorption of cartilage during transition to hard callus appears to be influenced by diabetes. It has been hypothesized that the smaller callus observed in diabetic mice may be secondary to upregulation of osteoclasts. Initial callus size appears not to differ between mice with streptozotocin-induced diabetes, which exhibit a complete absence of insulin, and control mice, but levels of osteoclast and osteoclastogenesis mediators were significantly higher in the diabetic mice.10 Some investigators think that the reduction in cartilage callus size in diabetic mice is caused by altered mRNA expression and collagen production.11 Diabetic mice, in addition to showing increased resorption by osteoclasts, demonstrate increased chondrocyte apoptosis, which is thought to activate cartilage resorption events. Exogenous insulin effectively reverses this cartilage loss to baseline levels.12
Osteoblasts are a crucial component of the fracture-healing cascade, and acute and chronic hyperglycemia, the hallmark of diabetes, has a variety of effects on osteoblasts.13 Genes for cell-signal proteins such as osteocalcin, MMP-13, and vascular endothelial growth factor are downregulated in the presence of chronic hyperglycemia, whereas genes for alkaline phosphate are upregulated. Acute hyperglycemia by way of hyperosmolarity is associated with MMP-13 downregulation. Thus, osteoblasts appear to respond to hyperglycemia through 2 different processes: Hyperosmolarity, through osteoblast cell shrinkage, influences the acute response, and hyperglycemia itself, through pathways such as nonenzymatic glycosylation, protein kinase C (PKC) signaling, and the polyol pathway, is the force behind the chronic response.14 The lineage of osteoblasts from mesenchymal stem cells also can be affected by hyperglycemia, with lower growth rates for mesenchymal stem cells and preferential development toward the adipocyte lineage, while the osteoblast and chondrocyte lineages are downregulated.15
Increased osteoblast apoptosis has been associated with diabetes through advanced glycation end-products (AGEs), which modify the structure and function of bioactive compounds through AGE receptors that cross-link and bond to amino groups on bioactive molecules.16 It has been reported that AGEs interfere with osteoblast development and collagen and osteocalcin production.17 A common AGE, carboxymethyl lysine-modified collagen, has been associated with a significant increase in apoptosis through the mitogen-activated protein kinase (MAPK) pathway. Although most of the literature suggests that osteoblast apoptosis is activated by hypoxia, nitric oxide, or integrins, these factors all have the MAPK pathway in common.18
Osteoclasts are also influenced by diabetes. Recent work in T1DM demonstrated that osteoclasts are hyperactive and more sensitive to receptor activator of nuclear factor kB ligand (RANKL) compared with osteoclasts from the population without diabetes. It is also known that osteoclasts are under the control of immunologic mediators like lipopolysaccharide (LPS), a surface component of gram-negative bacteria, and various other proinflammatory cytokines. In patients with diabetes, osteoclasts react differently to LPS and other proinflammatory cytokines, at times with opposing effects, including secretion of RANKL to stimulate resorption by the osteoclast, and precursors preventing progression into osteoclasts. In healthy people, high LPS levels not only prevent precursors from producing more osteoclasts, but promote them to mature into immune-like cells that actually phagocytose bacteria. So, in a state of infection, precursors shift from bone-resorbing osteoclasts to protective immune cells. This phenomenon does not occur in patients with diabetes, in whom the osteoclasts instead resorb more bone and stimulate inflammation by releasing cytokines.19
Interestingly, osteoblasts and osteoclasts are also affected by medications commonly used to treat diabetes. Thiazolidinediones are a class of sensitizers often used to treat patients with T2DM. Thiazolidinediones, particularly rosiglitazone, have been associated with increased bone loss primarily caused by increased bone resorption by osteoclasts.20 In addition, some investigators think that thiazolidinediones induce osteocyte apoptosis, contributing to impaired bone growth.8 Metformin, an insulin sensitizer, appears to have a positive effect on bone growth and fracture risk by enhancing osteoblastogenesis and inhibiting osteoclastogenesis, leading to a protective effect on bone.8
Peripheral neuropathy, which is often associated with diabetes, appears to play a major role in fracture-healing complications, even more so than hyperglycemia does. A recent clinical paper found that patients with diabetic neuropathy had a 44% risk of foot and ankle fracture-healing complications.21 Regardless of the risk, the pathogenesis of diabetic neuropathy can be caused by several mechanisms. Neural tissue does not require insulin for glucose uptake; therefore, in a state of hyperglycemia, aldose reductase shunts glucose to sorbitol while using protective glutathione and generating reactive oxygen species. This oxidative stress results in nerve damage or neuropathy. Microangiopathy, which we discuss in more detail later, also contributes to the development of neuropathy, through compromised flow of blood to neural tissue.22 Another mechanism contributing to diabetic neuropathy involves PKC, which is activated by 1,2-diacylglycerol in the presence of glucose, leading to vascular changes that restrict the flow of blood to peripheral nerves.23 Finally, AGEs may also participate by altering nerve function after binding to neural tissue.
Charcot neuroarthropathy is a complication associated with diabetes, particularly after injury in which chronic inflammation results in damage to the joint through fracture, dislocation, and osteolytic bony destruction. The pathophysiology is attributed to repeated microtrauma caused by loss of protective sensibility and hyperemia caused by dysregulation.24 Sympathetic and sensory nerve fibers are associated with bone, but a few serve as mechanoreceptors and nociceptors, which can activate substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide—neuropeptides all thought to be involved in the inflammatory process, and in the activation of osteoblasts and osteoclasts. In diabetic neuropathy, many of these neuropeptides show a reduced regulation response, which can lead to impaired fracture healing. In particular, osteoclast activity is upregulated, and consequently bone resorption is increased. In addition to the neuropeptides mentioned, RANKL is one mechanism by which this upregulation occurs.25
It is clear that bone metabolism and fracture healing are complex processes. In the patient with diabetes, many factors are affected, including BMD, bone microarchitecture and bone growth, cartilage resorption during callus formation, osteoblast and osteoclast activation through both altered responses to cell signals and pharmacologic interactions, and, finally, peripheral neuropathy. Given the complex interactions described, it is likely that these factors in combination, as well as those yet undiscovered, negatively affect fracture healing.
Wound Healing and Vasculopathy in Diabetes
Bone healing and soft-tissue healing depend on many of the same factors. Therefore, interactions between neuropathy and vasculopathy can have a tremendous influence on wound healing in patients with diabetes. The vascular pathology that occurs in diabetes depends in part on the fact that endothelial cells do not require insulin for glucose uptake and therefore are more susceptible to damage by hyperglycemia. As already discussed, shunting of glucose through the polyol pathway with the resultant oxidative stress is partly responsible for angiopathy in diabetes.
Also as already discussed, AGEs affect intracellular processes by protein binding and gene regulation and by disrupting the communication between cells and the surrounding matrix. From an extracellular standpoint, AGEs bind to circulating proteins, promoting inflammation and upregulation/downregulation of growth factors, including endothelial nitric oxide synthase, a critical vasodilator. Endothelin 1, on the other hand, is a potent vasoconstrictor. It is upregulated while transforming growth factor b and plasminogen activator inhibitor 1 are upregulated, resulting in further vascular damage.26 The common mechanism for this vasculopathy appears to be superoxide production in the mitochondria, caused by excess glucose oxidation forcing coenzyme Q to donate electrons to oxygen, producing the superoxides. Superoxides in turn inhibit glyceraldehyde 3-phosphate dehydrogenase, which activates the polyol pathway, AGE formation, PKC, and the hexosamine pathway.26 In addition to coenzyme Q, several other enzymes generate reactive oxygen species, including nicotinamide adenine dinucleotide phosphate oxidase, aldehyde oxidase, xanthine oxidase, and glucose oxidase.27 These reactive oxygen species exacerbate oxidative stress, leading to further endothelial cell damage, and cause vascular smooth muscle injury.28
Further influencing the wound-healing environment are the effects of diabetes on blood vessel maintenance and repair as well as angiogenesis in response to local-tissue hypoxia. Vessel-repair mechanisms require endothelial progenitor cells (EPCs), which are released in response to cytokines and neural impulses.29 Bone marrow–derived EPCs have inadequate proliferative and migratory ability in patients with diabetes.28,30 In a diabetic mouse model, EPCs appear in the bone marrow at normal levels, but levels in circulation are lower than anticipated, because of poor proliferation and mobilization, it is thought. In terms of local-tissue hypoxia, hypoxia-inducible factor 1 (HIF-1) is an important transcription factor that promotes the expression of genes that in turn induce angiogenesis. The mechanism of this response is complex, and hyperglycemia has the potential to interfere in various steps of the cycle. In response to local-tissue hypoxia, the HIF-1a subunit must localize to the target site, where it combines with HIF-1b to create the active dimer, HIF-1.31 This active dimer is regulated through degradation of the a subunit in the presence of normal oxygen levels. However, in a state of hypoxia, the molecule is stabilized, promoting angiogenesis and fibroblast migration.32 Recent evidence suggests that hyperglycemia interferes with the dimerization process and that there is a failure of HIF-1a to locate into the nucleus, which is crucial for gene upregulation.31-33
Infection in Diabetes
Throughout the literature, the risk for infection after fracture is consistently higher in patients with diabetes than without diabetes. There likely are many contributing factors, including diminished blood flow and vasculopathy as well as a dampened immune response as a result of defective granulocytic, phagocytic, and chemotactic functions and defective macrophagic activity. Typically, polymorphonuclear leukocytes (PMNs) migrate to bacteria and initiate bacteriocidal activity, and then macrophages phagocytize PMNs and other damaged cells. PMNs demonstrate impaired function in patients with diabetes—reduced phagocytic response and respiratory burst as well as chemotaxis impairment. The diminished phagocytic potential is substantial, with experiments showing an almost 50% reduction in ingestion of Staphylococcus aureus in a patient with diabetes than in one without diabetes.34 Expression of surface integrins, which mediate PMN adhesion to the basement membrane of the tissue, appears to be negatively altered in both T1DM and T2DM, furthering diminishing the chemotactic response of PMNs.35 Impaired leukocyte function may also be a downstream effect of vasculopathy and associated hypoxia/hypoxemia as PMNs use superoxide radicals and other oxidizing agents to create a bacteriocidal environment that is negatively impacted in a low oxygen state.3 In addition, macrophages are disabled in patients with diabetes. (In rats with streptozotocin-induced diabetes, there is inadequate activation of macrophages in the early stages of healing.36) Furthermore, AGEs similar to those mentioned earlier have a significant negative impact on macrophagic function.37 Thus, both the activation and the activity of macrophages appear to be impeded in the setting of diabetes.
Potential New Treatment Modalities
There is tremendous potential for clinical intervention to prevent pathologic outcomes in patients with diabetes, given the complex tissue, cellular, and molecular interactions, particularly those caused by hyperglycemia. At the bone tissue level, increased osteoclastic activity in patients with diabetes has been associated with many complications, including Charcot arthropathy. RANKL modulates differentiation and activation of osteoclasts; thus, RANKL inhibition is a possible therapeutic target.38 Elevated AGE levels have also been observed in patients with Charcot arthropathy, and RAGE, the receptor for AGE, has been seen at lower than expected levels in patients with diabetes. RAGE appears to provide a protective effect against excessive bone resorption; therefore, treatment that increases RAGE levels—such as angiotensin-converting-enzyme inhibitors, statins, and glitazones—may be capable of mitigating the osteoclastic effects in Charcot arthropathy.39
AGE formation appears to be central to many pathologic processes in diabetes, so it is a logical therapeutic target, particularly for pathologic processes at the vascular tissue level. Aminoguanidine is an anti-AGE agent that was initially used to prevent diabetic retinopathy, but it has also been shown to prevent general vascular complications in diabetic animal models. The terminal amino residue in the compound specifically binds glucose-derived reactive intermediates and prevents cross-linking, which renders them inactive. Disrupting those cross-links is another treatment strategy. N-phenacylthiazolium bromide and 3-phenacyl-4,5-dimethylthiazolium chloride (ALT-711 or alagebrium) are compounds that have been shown to break cross-links in a diabetic rat model.16
Another tactic for reducing vascular pathology involves mitigating superoxide radicals, as these radicals are generated from the glycolytic intermediates in hyperglycemic states. It has been reasoned that, if the concentration of these intermediates can be decreased, there would be less substrate available for the pathways that lead to radical formation. One approach is to use transketolase, an enzyme that shunts intermediates to pathways that do not produce superoxide radicals. In the treatment of patients with diabetic retinopathy, early data appear promising with benfotiamine, a thiamine derivative, which upregulates transketolase 250%. An additional tactic involves catalytic antioxidants—namely, superoxide dismutase/catalase mimetic, which has been shown to reduce hyperglycemia-induced superoxides. These interventions are appealing because of their nonstoichiometric reactions, which render them potentially more potent antioxidants.26
Potential neurologic interventions include recombinant human nerve growth factor, neurotrophic factors, and gene therapy, all directed toward preventing or regenerating neuropathic tissues in patients with diabetes. Most of these interventions, however, remain theoretical. Few trials have demonstrated clinically significant improvement. In patients with T1DM, however, the absence of circulating C-peptide is thought to contribute to diabetic neuropathy. Results of trials with subcutaneous C-peptide treatment suggest improvement in both sural sensory and vibration perception after only 12 weeks.40 These novel treatments further emphasize the potential for intervention at the tissue, cellular, and molecular levels.
Conclusion
Whereas most fractures are uncomplicated in healthy patients, they can have devastating consequences in patients with diabetes. In this review, we have highlighted many of the pathologic processes that can influence outcomes of fractures in patients with diabetes. These problems will become more common as the population ages, age-related risks for osteoporosis and fragility fracture increase, and diabetes becomes nearly epidemic in our increasingly obese, sedentary society. Although some progress has been made, a more thorough intervention strategy is needed to improve both bone and soft-tissue outcomes of fractures in patients with diabetes.
Diabetes mellitus (DM) affects a significant portion of the world’s people, and the problem is increasing in magnitude as the population ages and becomes more obese.1 An estimated 347 million people have diabetes.1 In the United States, 26 million (roughly 8% of the population) are affected, making DM a major health issue.2 Given the prevalence of diabetes in the general population, it is not surprising that increasing numbers of fracture patients have DM. Unfortunately, for these patients, many relatively simple fractures can have disastrous outcomes. Infections and wound complications occur in disproportionate numbers, healing time is delayed, and risk for nonunion or malunion is substantially higher.3
It is imperative to understand the pathophysiology of DM to appreciate potential interventions and strategies aimed at decreasing complications and improving outcomes of fractures in patients with the disease. In type 1 DM (T1DM), autoimmune destruction of the insulin-secreting β cells in the pancreas results in a complete absence of insulin. Patients with T1DM are dependent on exogenous insulin, and, despite hyperglycemia, most cells in the body are starved for energy. This leads to a catabolic condition, high lipid and protein metabolism, and, in many cases, ketoacidosis. When insulin resistance develops, the β cells are forced to secrete large amounts of insulin; when they fail to keep up, type 2 DM (T2DM) develops. T2DM is often associated with obesity, as excess adipose tissue leads to insulin resistance. Although exogenous insulin may be necessary to treat advanced T2DM, other medications are commonly used to effectively lower blood glucose: Secretagogues (eg, sulfonylureas) facilitate insulin release from β cells, and sensitizers (eg, metformin) increase insulin sensitivity.4,5
The potential morbidity of fractures in patients with DM can be appreciated with the example of ankle fractures. These typically uncomplicated fractures can have very poor outcomes in the setting of DM. In a prospective study of approximately 1500 patients with ankle fractures treated with open reduction and internal fixation, Wukich and colleagues6 found that 9.5% of patients with DM (vs 2.4% of patients without DM) developed surgical site infections. As defined by Jones and colleagues,7 major complications of treating ankle fractures in patients with DM include infection, malunion, nonunion, Charcot arthropathy, and amputation. The authors reported major complications in 31% and 17% of patients with and without DM, respectively. Highlighting the importance of glycemic control, Wukich and colleagues6 found relative risks of 3.8 for infection, 3.4 for noninfectious complications, and 5.0 for revision in complicated (vs uncomplicated) fractures in patients with DM.
Given the magnitude of problems in the treatment of fractures in patients with DM, we focus our review on the pathobiology of diabetes in terms of bone metabolism and fracture healing, wound healing and vasculopathy, infection, and potential new treatment modalities.
Bone Metabolism and Fracture Healing in Diabetes
Insulin appears to play a role in bone metabolism and fracture healing. Therefore, absence of insulin in T1DM and elevated insulin levels associated with T2DM likely influence these metabolic and fracture-healing processes. Insulin has been hypothesized to have an anabolic effect on bone, and in both human and animal models bone mineral density (BMD) is significantly lower in T1DM. Furthermore, BMD in T2DM has been shown to be normal or even elevated.8 Other metabolic effects of insulin on bone metabolism and growth include slower growth rates and lower BMD in pediatric patients with T1DM versus patients without diabetes, and some animal models show bone microarchitecture altered in the absence of insulin (and reversible with insulin supplementation).9 These factors seem to contradict the markedly elevated risk for osteoporotic fracture in patients with T2DM, but the mechanisms responsible for this have not been elucidated.8
In terms of fracture healing, resorption of cartilage during transition to hard callus appears to be influenced by diabetes. It has been hypothesized that the smaller callus observed in diabetic mice may be secondary to upregulation of osteoclasts. Initial callus size appears not to differ between mice with streptozotocin-induced diabetes, which exhibit a complete absence of insulin, and control mice, but levels of osteoclast and osteoclastogenesis mediators were significantly higher in the diabetic mice.10 Some investigators think that the reduction in cartilage callus size in diabetic mice is caused by altered mRNA expression and collagen production.11 Diabetic mice, in addition to showing increased resorption by osteoclasts, demonstrate increased chondrocyte apoptosis, which is thought to activate cartilage resorption events. Exogenous insulin effectively reverses this cartilage loss to baseline levels.12
Osteoblasts are a crucial component of the fracture-healing cascade, and acute and chronic hyperglycemia, the hallmark of diabetes, has a variety of effects on osteoblasts.13 Genes for cell-signal proteins such as osteocalcin, MMP-13, and vascular endothelial growth factor are downregulated in the presence of chronic hyperglycemia, whereas genes for alkaline phosphate are upregulated. Acute hyperglycemia by way of hyperosmolarity is associated with MMP-13 downregulation. Thus, osteoblasts appear to respond to hyperglycemia through 2 different processes: Hyperosmolarity, through osteoblast cell shrinkage, influences the acute response, and hyperglycemia itself, through pathways such as nonenzymatic glycosylation, protein kinase C (PKC) signaling, and the polyol pathway, is the force behind the chronic response.14 The lineage of osteoblasts from mesenchymal stem cells also can be affected by hyperglycemia, with lower growth rates for mesenchymal stem cells and preferential development toward the adipocyte lineage, while the osteoblast and chondrocyte lineages are downregulated.15
Increased osteoblast apoptosis has been associated with diabetes through advanced glycation end-products (AGEs), which modify the structure and function of bioactive compounds through AGE receptors that cross-link and bond to amino groups on bioactive molecules.16 It has been reported that AGEs interfere with osteoblast development and collagen and osteocalcin production.17 A common AGE, carboxymethyl lysine-modified collagen, has been associated with a significant increase in apoptosis through the mitogen-activated protein kinase (MAPK) pathway. Although most of the literature suggests that osteoblast apoptosis is activated by hypoxia, nitric oxide, or integrins, these factors all have the MAPK pathway in common.18
Osteoclasts are also influenced by diabetes. Recent work in T1DM demonstrated that osteoclasts are hyperactive and more sensitive to receptor activator of nuclear factor kB ligand (RANKL) compared with osteoclasts from the population without diabetes. It is also known that osteoclasts are under the control of immunologic mediators like lipopolysaccharide (LPS), a surface component of gram-negative bacteria, and various other proinflammatory cytokines. In patients with diabetes, osteoclasts react differently to LPS and other proinflammatory cytokines, at times with opposing effects, including secretion of RANKL to stimulate resorption by the osteoclast, and precursors preventing progression into osteoclasts. In healthy people, high LPS levels not only prevent precursors from producing more osteoclasts, but promote them to mature into immune-like cells that actually phagocytose bacteria. So, in a state of infection, precursors shift from bone-resorbing osteoclasts to protective immune cells. This phenomenon does not occur in patients with diabetes, in whom the osteoclasts instead resorb more bone and stimulate inflammation by releasing cytokines.19
Interestingly, osteoblasts and osteoclasts are also affected by medications commonly used to treat diabetes. Thiazolidinediones are a class of sensitizers often used to treat patients with T2DM. Thiazolidinediones, particularly rosiglitazone, have been associated with increased bone loss primarily caused by increased bone resorption by osteoclasts.20 In addition, some investigators think that thiazolidinediones induce osteocyte apoptosis, contributing to impaired bone growth.8 Metformin, an insulin sensitizer, appears to have a positive effect on bone growth and fracture risk by enhancing osteoblastogenesis and inhibiting osteoclastogenesis, leading to a protective effect on bone.8
Peripheral neuropathy, which is often associated with diabetes, appears to play a major role in fracture-healing complications, even more so than hyperglycemia does. A recent clinical paper found that patients with diabetic neuropathy had a 44% risk of foot and ankle fracture-healing complications.21 Regardless of the risk, the pathogenesis of diabetic neuropathy can be caused by several mechanisms. Neural tissue does not require insulin for glucose uptake; therefore, in a state of hyperglycemia, aldose reductase shunts glucose to sorbitol while using protective glutathione and generating reactive oxygen species. This oxidative stress results in nerve damage or neuropathy. Microangiopathy, which we discuss in more detail later, also contributes to the development of neuropathy, through compromised flow of blood to neural tissue.22 Another mechanism contributing to diabetic neuropathy involves PKC, which is activated by 1,2-diacylglycerol in the presence of glucose, leading to vascular changes that restrict the flow of blood to peripheral nerves.23 Finally, AGEs may also participate by altering nerve function after binding to neural tissue.
Charcot neuroarthropathy is a complication associated with diabetes, particularly after injury in which chronic inflammation results in damage to the joint through fracture, dislocation, and osteolytic bony destruction. The pathophysiology is attributed to repeated microtrauma caused by loss of protective sensibility and hyperemia caused by dysregulation.24 Sympathetic and sensory nerve fibers are associated with bone, but a few serve as mechanoreceptors and nociceptors, which can activate substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide—neuropeptides all thought to be involved in the inflammatory process, and in the activation of osteoblasts and osteoclasts. In diabetic neuropathy, many of these neuropeptides show a reduced regulation response, which can lead to impaired fracture healing. In particular, osteoclast activity is upregulated, and consequently bone resorption is increased. In addition to the neuropeptides mentioned, RANKL is one mechanism by which this upregulation occurs.25
It is clear that bone metabolism and fracture healing are complex processes. In the patient with diabetes, many factors are affected, including BMD, bone microarchitecture and bone growth, cartilage resorption during callus formation, osteoblast and osteoclast activation through both altered responses to cell signals and pharmacologic interactions, and, finally, peripheral neuropathy. Given the complex interactions described, it is likely that these factors in combination, as well as those yet undiscovered, negatively affect fracture healing.
Wound Healing and Vasculopathy in Diabetes
Bone healing and soft-tissue healing depend on many of the same factors. Therefore, interactions between neuropathy and vasculopathy can have a tremendous influence on wound healing in patients with diabetes. The vascular pathology that occurs in diabetes depends in part on the fact that endothelial cells do not require insulin for glucose uptake and therefore are more susceptible to damage by hyperglycemia. As already discussed, shunting of glucose through the polyol pathway with the resultant oxidative stress is partly responsible for angiopathy in diabetes.
Also as already discussed, AGEs affect intracellular processes by protein binding and gene regulation and by disrupting the communication between cells and the surrounding matrix. From an extracellular standpoint, AGEs bind to circulating proteins, promoting inflammation and upregulation/downregulation of growth factors, including endothelial nitric oxide synthase, a critical vasodilator. Endothelin 1, on the other hand, is a potent vasoconstrictor. It is upregulated while transforming growth factor b and plasminogen activator inhibitor 1 are upregulated, resulting in further vascular damage.26 The common mechanism for this vasculopathy appears to be superoxide production in the mitochondria, caused by excess glucose oxidation forcing coenzyme Q to donate electrons to oxygen, producing the superoxides. Superoxides in turn inhibit glyceraldehyde 3-phosphate dehydrogenase, which activates the polyol pathway, AGE formation, PKC, and the hexosamine pathway.26 In addition to coenzyme Q, several other enzymes generate reactive oxygen species, including nicotinamide adenine dinucleotide phosphate oxidase, aldehyde oxidase, xanthine oxidase, and glucose oxidase.27 These reactive oxygen species exacerbate oxidative stress, leading to further endothelial cell damage, and cause vascular smooth muscle injury.28
Further influencing the wound-healing environment are the effects of diabetes on blood vessel maintenance and repair as well as angiogenesis in response to local-tissue hypoxia. Vessel-repair mechanisms require endothelial progenitor cells (EPCs), which are released in response to cytokines and neural impulses.29 Bone marrow–derived EPCs have inadequate proliferative and migratory ability in patients with diabetes.28,30 In a diabetic mouse model, EPCs appear in the bone marrow at normal levels, but levels in circulation are lower than anticipated, because of poor proliferation and mobilization, it is thought. In terms of local-tissue hypoxia, hypoxia-inducible factor 1 (HIF-1) is an important transcription factor that promotes the expression of genes that in turn induce angiogenesis. The mechanism of this response is complex, and hyperglycemia has the potential to interfere in various steps of the cycle. In response to local-tissue hypoxia, the HIF-1a subunit must localize to the target site, where it combines with HIF-1b to create the active dimer, HIF-1.31 This active dimer is regulated through degradation of the a subunit in the presence of normal oxygen levels. However, in a state of hypoxia, the molecule is stabilized, promoting angiogenesis and fibroblast migration.32 Recent evidence suggests that hyperglycemia interferes with the dimerization process and that there is a failure of HIF-1a to locate into the nucleus, which is crucial for gene upregulation.31-33
Infection in Diabetes
Throughout the literature, the risk for infection after fracture is consistently higher in patients with diabetes than without diabetes. There likely are many contributing factors, including diminished blood flow and vasculopathy as well as a dampened immune response as a result of defective granulocytic, phagocytic, and chemotactic functions and defective macrophagic activity. Typically, polymorphonuclear leukocytes (PMNs) migrate to bacteria and initiate bacteriocidal activity, and then macrophages phagocytize PMNs and other damaged cells. PMNs demonstrate impaired function in patients with diabetes—reduced phagocytic response and respiratory burst as well as chemotaxis impairment. The diminished phagocytic potential is substantial, with experiments showing an almost 50% reduction in ingestion of Staphylococcus aureus in a patient with diabetes than in one without diabetes.34 Expression of surface integrins, which mediate PMN adhesion to the basement membrane of the tissue, appears to be negatively altered in both T1DM and T2DM, furthering diminishing the chemotactic response of PMNs.35 Impaired leukocyte function may also be a downstream effect of vasculopathy and associated hypoxia/hypoxemia as PMNs use superoxide radicals and other oxidizing agents to create a bacteriocidal environment that is negatively impacted in a low oxygen state.3 In addition, macrophages are disabled in patients with diabetes. (In rats with streptozotocin-induced diabetes, there is inadequate activation of macrophages in the early stages of healing.36) Furthermore, AGEs similar to those mentioned earlier have a significant negative impact on macrophagic function.37 Thus, both the activation and the activity of macrophages appear to be impeded in the setting of diabetes.
Potential New Treatment Modalities
There is tremendous potential for clinical intervention to prevent pathologic outcomes in patients with diabetes, given the complex tissue, cellular, and molecular interactions, particularly those caused by hyperglycemia. At the bone tissue level, increased osteoclastic activity in patients with diabetes has been associated with many complications, including Charcot arthropathy. RANKL modulates differentiation and activation of osteoclasts; thus, RANKL inhibition is a possible therapeutic target.38 Elevated AGE levels have also been observed in patients with Charcot arthropathy, and RAGE, the receptor for AGE, has been seen at lower than expected levels in patients with diabetes. RAGE appears to provide a protective effect against excessive bone resorption; therefore, treatment that increases RAGE levels—such as angiotensin-converting-enzyme inhibitors, statins, and glitazones—may be capable of mitigating the osteoclastic effects in Charcot arthropathy.39
AGE formation appears to be central to many pathologic processes in diabetes, so it is a logical therapeutic target, particularly for pathologic processes at the vascular tissue level. Aminoguanidine is an anti-AGE agent that was initially used to prevent diabetic retinopathy, but it has also been shown to prevent general vascular complications in diabetic animal models. The terminal amino residue in the compound specifically binds glucose-derived reactive intermediates and prevents cross-linking, which renders them inactive. Disrupting those cross-links is another treatment strategy. N-phenacylthiazolium bromide and 3-phenacyl-4,5-dimethylthiazolium chloride (ALT-711 or alagebrium) are compounds that have been shown to break cross-links in a diabetic rat model.16
Another tactic for reducing vascular pathology involves mitigating superoxide radicals, as these radicals are generated from the glycolytic intermediates in hyperglycemic states. It has been reasoned that, if the concentration of these intermediates can be decreased, there would be less substrate available for the pathways that lead to radical formation. One approach is to use transketolase, an enzyme that shunts intermediates to pathways that do not produce superoxide radicals. In the treatment of patients with diabetic retinopathy, early data appear promising with benfotiamine, a thiamine derivative, which upregulates transketolase 250%. An additional tactic involves catalytic antioxidants—namely, superoxide dismutase/catalase mimetic, which has been shown to reduce hyperglycemia-induced superoxides. These interventions are appealing because of their nonstoichiometric reactions, which render them potentially more potent antioxidants.26
Potential neurologic interventions include recombinant human nerve growth factor, neurotrophic factors, and gene therapy, all directed toward preventing or regenerating neuropathic tissues in patients with diabetes. Most of these interventions, however, remain theoretical. Few trials have demonstrated clinically significant improvement. In patients with T1DM, however, the absence of circulating C-peptide is thought to contribute to diabetic neuropathy. Results of trials with subcutaneous C-peptide treatment suggest improvement in both sural sensory and vibration perception after only 12 weeks.40 These novel treatments further emphasize the potential for intervention at the tissue, cellular, and molecular levels.
Conclusion
Whereas most fractures are uncomplicated in healthy patients, they can have devastating consequences in patients with diabetes. In this review, we have highlighted many of the pathologic processes that can influence outcomes of fractures in patients with diabetes. These problems will become more common as the population ages, age-related risks for osteoporosis and fragility fracture increase, and diabetes becomes nearly epidemic in our increasingly obese, sedentary society. Although some progress has been made, a more thorough intervention strategy is needed to improve both bone and soft-tissue outcomes of fractures in patients with diabetes.
1. Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31-40.
2. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2011.
3. Wukich DK, Joseph A, Ryan M, Ramirez C, Irrgang JJ. Outcomes of ankle fractures in patients with uncomplicated versus complicated diabetes. Foot Ankle Int. 2011;32(2):120-130.
4. Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2010.
5. Diabetes basics. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/basics/index.html. Updated October 25, 2014. Accessed August 24, 2015.
6. Wukich DK, McMillen RL, Lowery NJ, Frykberg RG. Surgical site infections after foot and ankle surgery. Diabetes Care. 2001;34(10):2211-2213.
7. Jones KB, Maiers-Yelden KA, Marsh JL, et al. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87(4):489-495.
8. Yan W, Li X. Impact of diabetes and its treatments on skeletal diseases. Front Med. 2013;7(1):81-90.
9. Thrailkill K, Lumpkin C Jr, Bunn R, Kemp S, Fowlkes J. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735-E745.
10. Kayal RA, Tsatsas D, Bauer MA, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560-568.
11. Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41(2):81-91.
12. Kayal RA, Alblowi J, McKenzie E, et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009;44(2):357-363.
13. Motyl K, Botolin S, Irwin R, et al. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218(3):575-583.
14. Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99(2):411-424.
15. Keats E, Khanz ZA. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS One. 2012;7(6):e38752.
16. Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87-101.
17. Fong Y, Edelstein D, Wang E, Brownlee M. Inhibition of matrix-induced bone differentiation by advanced glycation end-products in rats. Diabetologia. 1993;36(9):802-807.
18. Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation endproducts stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345-353.
19. Catalfamo DL, Calderon NL, Harden SW, Sorenson HL, Neiva KG, Wallet SM. Augmented LPS responsiveness in type 1 diabetes-derived osteoclasts. J Cell Physiol. 2013;228(2):349-361.
20. Kahn SE, Lachin JM, Zinman B, et al; ADOPT Study Group. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552-1560.
21. Shibuya N, Humphers JM, Fluhman BL, Jupiter DC. Factors associated with nonunion, delayed union, and malunion in foot and ankle surgery in diabetic patients. J Foot Ankle Surg. 2013;52(2):207-211.
22. Shami SK, Chittenden SJ. Microangiopathy in diabetes mellitus: II. Features, complications and investigation. Diabetes Res. 1991;17(4):157-168.
23. Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404(6779):787-790.
24. Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep. 2005;5(6):430-435.
25. Lerner UH, Persson E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Musculoskelet Neuronal Interact. 2008;8(2):154-165.
26. Brownlee M. The pathobiology of diabetic complications—a unifying mechanism. Diabetes. 2005;54(6):1615-1625.
27. Tsuji S, Taniuchi S, Hasui M, Yamamoto A, Kobayashi Y. Increased nitric oxide production by neutrophils from patients with chronic granulomatous disease on trimethoprim-sulfamethoxazole. Nitric Oxide. 2002;7(4):283-288.
28. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
29. Westerweel PE. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One. 2013;8(3):e60357.
30. Fadini GP, Avogaro A. It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications. Exp Diabetes Res. 2012;2012:742976.
31. Gadad PC, Matthews KH, Knott RM. Role of HIF1α and PKCβ in mediating the effect of oxygen and glucose in a novel wound assay. Microvasc Res. 2013;88:61-69.
32. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426-19431.
33. Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53(12):3226-3232.
34. Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15(2):256-260.
35. Calmi G, Montana M, Citarella R, Porretto F, Catania A, Lo Presti R. Polymorphonuclear leukocyte integrin profile in diabetes mellitus. Clin Hemorheol Microcirc. 2002;27(2):83-89.
36. Miao M, Niu Y, Xie T, Yuan B, Qing C, Lu S. Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012;20(2):203-213.
37. Liu BF, Miyata S, Kojima H, et al. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes. 1999;48(10):2074-2082.
38. Mabilleau G, Petrova NL, Edmonds ME, Sabokbar A. Increased osteoclastic activity in acute Charcot’s osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia. 2008;51(6):1035-1040.
39. Witzke KA, Vinik AI, Grant LM, et al. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. 2011;34(7):1617-1621.
40. Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4(4):271-285.
1. Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31-40.
2. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2011.
3. Wukich DK, Joseph A, Ryan M, Ramirez C, Irrgang JJ. Outcomes of ankle fractures in patients with uncomplicated versus complicated diabetes. Foot Ankle Int. 2011;32(2):120-130.
4. Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2010.
5. Diabetes basics. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/basics/index.html. Updated October 25, 2014. Accessed August 24, 2015.
6. Wukich DK, McMillen RL, Lowery NJ, Frykberg RG. Surgical site infections after foot and ankle surgery. Diabetes Care. 2001;34(10):2211-2213.
7. Jones KB, Maiers-Yelden KA, Marsh JL, et al. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87(4):489-495.
8. Yan W, Li X. Impact of diabetes and its treatments on skeletal diseases. Front Med. 2013;7(1):81-90.
9. Thrailkill K, Lumpkin C Jr, Bunn R, Kemp S, Fowlkes J. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735-E745.
10. Kayal RA, Tsatsas D, Bauer MA, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560-568.
11. Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41(2):81-91.
12. Kayal RA, Alblowi J, McKenzie E, et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009;44(2):357-363.
13. Motyl K, Botolin S, Irwin R, et al. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218(3):575-583.
14. Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99(2):411-424.
15. Keats E, Khanz ZA. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS One. 2012;7(6):e38752.
16. Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87-101.
17. Fong Y, Edelstein D, Wang E, Brownlee M. Inhibition of matrix-induced bone differentiation by advanced glycation end-products in rats. Diabetologia. 1993;36(9):802-807.
18. Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation endproducts stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345-353.
19. Catalfamo DL, Calderon NL, Harden SW, Sorenson HL, Neiva KG, Wallet SM. Augmented LPS responsiveness in type 1 diabetes-derived osteoclasts. J Cell Physiol. 2013;228(2):349-361.
20. Kahn SE, Lachin JM, Zinman B, et al; ADOPT Study Group. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552-1560.
21. Shibuya N, Humphers JM, Fluhman BL, Jupiter DC. Factors associated with nonunion, delayed union, and malunion in foot and ankle surgery in diabetic patients. J Foot Ankle Surg. 2013;52(2):207-211.
22. Shami SK, Chittenden SJ. Microangiopathy in diabetes mellitus: II. Features, complications and investigation. Diabetes Res. 1991;17(4):157-168.
23. Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404(6779):787-790.
24. Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep. 2005;5(6):430-435.
25. Lerner UH, Persson E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Musculoskelet Neuronal Interact. 2008;8(2):154-165.
26. Brownlee M. The pathobiology of diabetic complications—a unifying mechanism. Diabetes. 2005;54(6):1615-1625.
27. Tsuji S, Taniuchi S, Hasui M, Yamamoto A, Kobayashi Y. Increased nitric oxide production by neutrophils from patients with chronic granulomatous disease on trimethoprim-sulfamethoxazole. Nitric Oxide. 2002;7(4):283-288.
28. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
29. Westerweel PE. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One. 2013;8(3):e60357.
30. Fadini GP, Avogaro A. It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications. Exp Diabetes Res. 2012;2012:742976.
31. Gadad PC, Matthews KH, Knott RM. Role of HIF1α and PKCβ in mediating the effect of oxygen and glucose in a novel wound assay. Microvasc Res. 2013;88:61-69.
32. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426-19431.
33. Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53(12):3226-3232.
34. Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15(2):256-260.
35. Calmi G, Montana M, Citarella R, Porretto F, Catania A, Lo Presti R. Polymorphonuclear leukocyte integrin profile in diabetes mellitus. Clin Hemorheol Microcirc. 2002;27(2):83-89.
36. Miao M, Niu Y, Xie T, Yuan B, Qing C, Lu S. Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012;20(2):203-213.
37. Liu BF, Miyata S, Kojima H, et al. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes. 1999;48(10):2074-2082.
38. Mabilleau G, Petrova NL, Edmonds ME, Sabokbar A. Increased osteoclastic activity in acute Charcot’s osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia. 2008;51(6):1035-1040.
39. Witzke KA, Vinik AI, Grant LM, et al. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. 2011;34(7):1617-1621.
40. Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4(4):271-285.
An Analysis of the Clinical Trial Landscape for Cutaneous Melanoma
The incidence of cutaneous melanoma, the deadliest form of skin cancer, has been steadily increasing over the last several decades.1 Currently, there are 73,870 new diagnoses of melanoma anticipated in the United States in 2015 alone.2 Many cases of melanoma are caught at early, actionable, and curable stages thanks in part to patient education and screening by dermatologists.3 However, until recently, few options existed for the treatment of locally advanced and metastatic melanomas, with a median survival rate of less than 1 year.4
Clinical trials represent the most reliable method for advancing treatment and improving outcomes for patients with disease; however, patient accrual and access to clinical trials remain formidable barriers. Studies have suggested that patients in rural areas perceive both an increased distance to clinical trial sites and a lack of awareness of available trials compared to their urban counterparts. Additionally, studies have shown that provider awareness of actively enrolling clinical trials in their respective fields is a key determinate in patient enrollment.5 Finally, insufficient funding and lack of collaboration has resulted in many small phase 1 or phase 2 single-center trials, which are less likely to quickly impact clinical care.6 Increased awareness of the ClinicalTrials.gov registry, a publicly available and easily accessible database, can facilitate referral, enrollment, and collaboration among physicians, patients, and researchers alike.
Using the ClinicalTrials.gov database, we sought to analyze the clinical trial landscape for cutaneous melanoma to understand the current state of melanoma research, future direction, and potential barriers that may impede success.
Methods
The primary objective was to provide a snapshot of the melanoma clinical research landscape from 2005 to 2013, including the number of registered trials, phase distribution, recruitment status, location of trials, type of intervention, and disease state being studied. Secondary objectives included describing patterns of clinical trial distribution within the United States in the context of melanoma mortality and examining changing trends in interventions studied in trials over time.
ClinicalTrials.gov is a comprehensive online registry of clinical trials conducted in the United States and abroad that is maintained by the National Library of Medicine.7 Although the initiative was launched in 2000, the registry became effectively comprehensive in September 2005 when the International Committee of Medical Journal Editors declared prospective registration of clinical trials as a prerequisite for publication. The US Food and Drug Administration followed suit in September 2007, expanding the requirements for registration and declaring penalties for parties who did not comply.8 Each registered trial can be found through searchable keywords, and each study page contains details of study design, principal investigators, and inclusion and exclusion criteria, as well as contact information for enrollment.
Study Selection
Clinical trials registered between September 15, 2005, and December 31, 2013, were evaluated; a total of 138,312 trials were found to be registered on ClinicalTrials.gov during that time period. We limited our study selection to interventional studies, which were filtered by topic to yield only those pertaining to melanoma patients. To minimize reporting bias, trials registered prior to the implementation of the International Committee of Medical Journal Editors’ reporting requirements were excluded. To focus specifically on the landscape of trials in cutaneous melanoma, trials investigating multiple advanced malignancies, uveal or ocular melanoma, and mucosal melanoma were manually excluded.
Study Variables
Information on each clinical trial was extracted from ClinicalTrials.gov. Each trial was manually reviewed by an investigator to determine the disease state and type of intervention being studied. Studies investigating multiple modalities concurrently were classified as “other.”
Data Analysis
Study variables were first analyzed among the entire cohort as a whole. Using each trial location and a python script based on open-source code, the number of actively recruiting melanoma trials in each US county was identified and mapped. County-level, melanoma-specific mortality data from 2001 to 2010 was extracted from the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research) mortality database (wonder.cdc.gov). Finally, to analyze changing trends in cutaneous melanoma investigation, trials were grouped into 3 categories based on the date they were received on ClinicalTrials.gov: (1) 2005-2007, (2) 2008-2010, and (3) 2011-2013. Disease state and type of intervention were analyzed and compared among each group using the χ2 statistic.
Results
Of the 138,312 trials registered on ClinicalTrials.gov between September 15, 2005, and December 31, 2013, only 931 were identified as interventional studies pertaining to melanoma patients. Of these, 154 were excluded because of a focus on uveal, ocular, or mucosal melanoma or because of the inclusion of participants with multiple types of advanced malignancies. The final analysis included 777 trials specifically focusing on cutaneous melanoma.
Characteristics of these 777 trials were varied. Many interventions were in the early stages of development, with 339 (44%) trials classified as phase 0, phase 1, or phase 1/phase 2; 306 (39%) as phase 2; and 71 (9%) as nonpharmacologic (nonphase) trials. Only 58 trials (8%) were classified as phase 3 or phase 4. The majority of the trials were actively recruiting (225 [29%]), active but not yet recruiting (172 [22%]), or completed (255 [33%]); however, 98 trials (13%) had been suspended, terminated, or withdrawn. Additionally, 22 trials (3%) were not yet recruiting and 5 (<1%) were classified as “other” because they did not have a recruitment status listed.
The distribution of actively enrolling clinical trials corresponds to major metropolitan areas within the Northeast, Upper Midwest, and Coastal California (Figure 1A). Figure 1B demonstrates the melanoma-specific mortality across the United States. Areas in the Southwest and Florida shared some of the greatest disease burden.
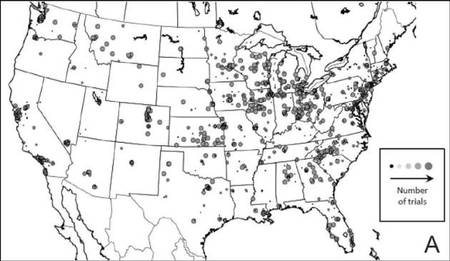 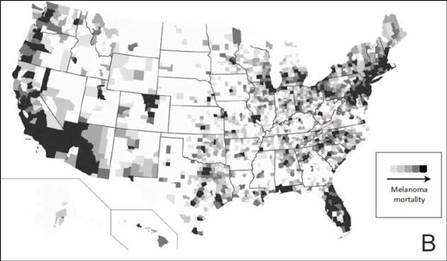 |
| Figure 1. Geographical representation of US clinical trial enrollment with the number of actively recruiting trials for each unique US zip code presented. The circle size corresponds to the number of trials. The largest circles indicate more than 5 trials within a given zip code (A). County-level melanoma-specific mortality data are presented for 2001 to 2010 (B). Darkest areas represent the highest numbers of melanoma deaths.
|
The disease state and type of intervention for all the included trials are summarized in Figure 2. The vast majority of trials (633/777 [82%]) enrolled participants with metastatic melanoma. Unlike many other tumor types, only 64 (8%) trials enrolled patients specifically in the adjuvant setting. Most trials focused on targeted (175 [23%]), immune (180 [23%]), and vaccine (117 [15%]) therapy.
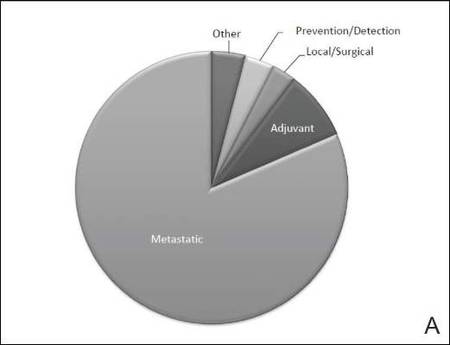 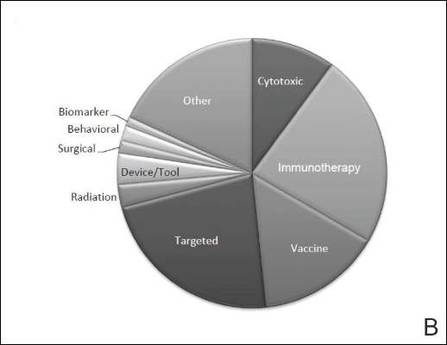 |
| Figure 2. Trial distribution stratified by disease state (A) and type of intervention (B). Trial distribution is shown for 777 interventional clinical trials including melanoma patients. The majority of clinical trials involved patients with metastatic melanoma. The majority of trials investigated targeted therapy, immunotherapy, and vaccine therapy. |
We subsequently analyzed changes in trial characteristics over time. We noted a decrease in the number of trials investigating cytotoxic and vaccine-based therapies, and increasing numbers of trials investigating immunotherapy (P=.041). Between 2005 and 2007, 14% (27/201) of all trials investigated cytotoxic therapies compared to just 7% (20/294) of trials between 2011 and 2013. With the approval of ipilimumab, 29% (85/294) of all clinical trials between 2011 and 2013 investigated immunotherapies, which comprised only 18% (37/201) of clinical trials between 2005 and 2007. The majority of trials continued to enroll patients in the metastatic setting where outcomes remain poor. Importantly, only 6% (49/777) of all clinical trials have focused on prevention, early detection, and local management of melanoma, which has remained constant over time.
Comment
Cutaneous melanoma remains an area of active investigation, interdisciplinary collaboration, and great promise. The ClinicalTrials.gov registry serves not only to increase transparency among interested parties but also as a rich resource to study the clinical research landscape as demonstrated in this study.
Greater understanding of the underlying genetic and immunogenic properties of melanoma tissues has led to the US Food and Drug Administration approval of several novel agents to treat metastatic disease. BRAF inhibitors such as vemurafenib and dabrafenib target more than 50% of all melanoma tumors harboring mutations in the BRAF gene and have shown unparalleled efficacy in clinical trials; however, durability of response and adverse effects still remain a concern.4,9-11 Ipilimumab, a CTLA-4 inhibitor, enhances antitumor immunity and demonstrated improved survival in clinical trials.12,13 Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody, also demonstrated improved overall and progression-free survival.14 Finally, trametinib, a MEK inhibitor, used in combination with BRAF inhibitors has demonstrated improved response over BRAF inhibitors alone.15
Although traditional cytotoxic chemotherapy was one of the few available treatment options before 2011, response was infrequent.16 Our data indicate that the melanoma research landscape has shifted to follow advances in targeted therapy and immunotherapy. We noted a decrease in the study of cytotoxic chemotherapy in metastatic melanoma, with a compensatory increase in immunotherapy trials and a continued commitment to targeted therapy. Further, with the approval of BRAF inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors for metastatic disease, some have pushed to move these agents into the adjuvant setting to prevent micrometastases from evolving into clinically significant disease.17 Early results from EORTC (European Organisation for Research and Treatment of Cancer) 18071 comparing adjuvant ipilimumab to placebo demonstrated a 26.1-month versus 17.1-month improvement in relapse-free survival, respectively.18 However, this finding has important implications for clinical dermatologists. Patients treated with BRAF inhibitors are at increased risk for keratoacanthomas, invasive squamous cell carcinomas, and secondary primary melanomas.19,20 Caring for these patients requires increased vigilance and collaboration between dermatologists and oncologists.
Our study also highlights the dynamic nature of the field. For example, novel vaccine therapies have demonstrated promise in the metastatic/ unresectable tumor setting, with some herpes simplex virus–based vaccines generating durable antitumor immune responses in patients with melanoma.21 Combination therapy with CTLA-4 and PD-1 inhibitors has demonstrated improved objective response rates and progression-free survival over monotherapy.22 As the status of actively recruiting trials changes on a regular basis, we encourage physicians to access ClinicalTrials.gov to find details and contact information for actively recruiting trials and results on completed trials.
Early detection and management, however, still remain our primary option for cure, and the role of community dermatologists cannot be overstated.23 Patients with stage I and stage II disease have excellent long-term survival rates, yet only 6% of all clinical trials in cutaneous melanoma have focused on patient education, disease prevention, early detection, and local management. With an increasing incidence of melanoma among an aging population, the disease burden remains of substantial concern.24 Optimizing disease prevention, appropriate screening, and early detection are critical roles for dermatologists.
Finally, our data offer some insight into accrual barriers often faced by clinical trials. Actively enrolling clinical trials cluster within major metropolitan areas, presumably with large academic medical centers; however, areas in the southwestern United States and Florida, for example, have some of the highest burden of disease, likely secondary to sun exposure and aging populations.25 Integration of community dermatologists and oncologists may decrease both actual and patient-perceived barriers to care, which requires further exploration.6
Conclusion
Melanoma incidence and disease burden is increasing, and the field of melanoma research is incredibly dynamic. Going forward, we believe dermatologists will continue to play a critical role both in primary disease prevention and detection as well as in detection of secondary treatment-related skin toxicities. ClinicalTrials.gov is an invaluable resource to keep interested parties informed, foster collaboration, identify potential barriers to success, and suggest future directions.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271.
2. American Cancer Society. Cancer Facts and Figures 2015. American Cancer Society Web site. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed August 26, 2015.
3. Cheng MY, Moreau JF, McGuire ST, et al. Melanoma depth in patients with an established dermatologist. J Am Acad Dermatol. 2014;70:841-846.
4. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60-e69.
5. Kim SH, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. 2014;39:562-571.
6. Gregg JR, Horn L, Davidson MA, et al. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ. 2014;29:74-79.
7. Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the
Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994-1998.
8. Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364:852-860.
9. Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717-725.
10. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
11. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775-787.
12. Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
15. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
16. Espinosa E, Berrocal A, López Martin JA, et al. Advances in cutaneous melanoma. Clin Transl Oncol. 2012;14:325-332.
17. Chapman PB. Treating metastatic melanoma in 2014: what just happened and what is next? Am Soc Clin Oncol Educ Book. 2014:16-19.
18. Eggermont A, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
19. Curry JL, Tetzlaff MT, Nicholson K, et al. Histological features associated with vemurafenib-induced skin toxicities: examination of 141 cutaneous lesions biopsied during therapy. Am J Dermatopathol. 2014; 36:557-561.
20. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-1358.
21. Ross MI, Andtbacka RI, Puzanov I, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): results from a phase III trial in patients with stage IIIb-IV melanoma. Paper presented at: ASCO Annual Meeting; June 2, 2014; Boston, MA.
22. Postow MA, Chesney J, Pavlick AC, et al. Novolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
23. Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415-435.
24. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw. 2013; 11(5 suppl):645-648.
25. Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5, suppl 1):S6-S16.
The incidence of cutaneous melanoma, the deadliest form of skin cancer, has been steadily increasing over the last several decades.1 Currently, there are 73,870 new diagnoses of melanoma anticipated in the United States in 2015 alone.2 Many cases of melanoma are caught at early, actionable, and curable stages thanks in part to patient education and screening by dermatologists.3 However, until recently, few options existed for the treatment of locally advanced and metastatic melanomas, with a median survival rate of less than 1 year.4
Clinical trials represent the most reliable method for advancing treatment and improving outcomes for patients with disease; however, patient accrual and access to clinical trials remain formidable barriers. Studies have suggested that patients in rural areas perceive both an increased distance to clinical trial sites and a lack of awareness of available trials compared to their urban counterparts. Additionally, studies have shown that provider awareness of actively enrolling clinical trials in their respective fields is a key determinate in patient enrollment.5 Finally, insufficient funding and lack of collaboration has resulted in many small phase 1 or phase 2 single-center trials, which are less likely to quickly impact clinical care.6 Increased awareness of the ClinicalTrials.gov registry, a publicly available and easily accessible database, can facilitate referral, enrollment, and collaboration among physicians, patients, and researchers alike.
Using the ClinicalTrials.gov database, we sought to analyze the clinical trial landscape for cutaneous melanoma to understand the current state of melanoma research, future direction, and potential barriers that may impede success.
Methods
The primary objective was to provide a snapshot of the melanoma clinical research landscape from 2005 to 2013, including the number of registered trials, phase distribution, recruitment status, location of trials, type of intervention, and disease state being studied. Secondary objectives included describing patterns of clinical trial distribution within the United States in the context of melanoma mortality and examining changing trends in interventions studied in trials over time.
ClinicalTrials.gov is a comprehensive online registry of clinical trials conducted in the United States and abroad that is maintained by the National Library of Medicine.7 Although the initiative was launched in 2000, the registry became effectively comprehensive in September 2005 when the International Committee of Medical Journal Editors declared prospective registration of clinical trials as a prerequisite for publication. The US Food and Drug Administration followed suit in September 2007, expanding the requirements for registration and declaring penalties for parties who did not comply.8 Each registered trial can be found through searchable keywords, and each study page contains details of study design, principal investigators, and inclusion and exclusion criteria, as well as contact information for enrollment.
Study Selection
Clinical trials registered between September 15, 2005, and December 31, 2013, were evaluated; a total of 138,312 trials were found to be registered on ClinicalTrials.gov during that time period. We limited our study selection to interventional studies, which were filtered by topic to yield only those pertaining to melanoma patients. To minimize reporting bias, trials registered prior to the implementation of the International Committee of Medical Journal Editors’ reporting requirements were excluded. To focus specifically on the landscape of trials in cutaneous melanoma, trials investigating multiple advanced malignancies, uveal or ocular melanoma, and mucosal melanoma were manually excluded.
Study Variables
Information on each clinical trial was extracted from ClinicalTrials.gov. Each trial was manually reviewed by an investigator to determine the disease state and type of intervention being studied. Studies investigating multiple modalities concurrently were classified as “other.”
Data Analysis
Study variables were first analyzed among the entire cohort as a whole. Using each trial location and a python script based on open-source code, the number of actively recruiting melanoma trials in each US county was identified and mapped. County-level, melanoma-specific mortality data from 2001 to 2010 was extracted from the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research) mortality database (wonder.cdc.gov). Finally, to analyze changing trends in cutaneous melanoma investigation, trials were grouped into 3 categories based on the date they were received on ClinicalTrials.gov: (1) 2005-2007, (2) 2008-2010, and (3) 2011-2013. Disease state and type of intervention were analyzed and compared among each group using the χ2 statistic.
Results
Of the 138,312 trials registered on ClinicalTrials.gov between September 15, 2005, and December 31, 2013, only 931 were identified as interventional studies pertaining to melanoma patients. Of these, 154 were excluded because of a focus on uveal, ocular, or mucosal melanoma or because of the inclusion of participants with multiple types of advanced malignancies. The final analysis included 777 trials specifically focusing on cutaneous melanoma.
Characteristics of these 777 trials were varied. Many interventions were in the early stages of development, with 339 (44%) trials classified as phase 0, phase 1, or phase 1/phase 2; 306 (39%) as phase 2; and 71 (9%) as nonpharmacologic (nonphase) trials. Only 58 trials (8%) were classified as phase 3 or phase 4. The majority of the trials were actively recruiting (225 [29%]), active but not yet recruiting (172 [22%]), or completed (255 [33%]); however, 98 trials (13%) had been suspended, terminated, or withdrawn. Additionally, 22 trials (3%) were not yet recruiting and 5 (<1%) were classified as “other” because they did not have a recruitment status listed.
The distribution of actively enrolling clinical trials corresponds to major metropolitan areas within the Northeast, Upper Midwest, and Coastal California (Figure 1A). Figure 1B demonstrates the melanoma-specific mortality across the United States. Areas in the Southwest and Florida shared some of the greatest disease burden.
  |
| Figure 1. Geographical representation of US clinical trial enrollment with the number of actively recruiting trials for each unique US zip code presented. The circle size corresponds to the number of trials. The largest circles indicate more than 5 trials within a given zip code (A). County-level melanoma-specific mortality data are presented for 2001 to 2010 (B). Darkest areas represent the highest numbers of melanoma deaths.
|
The disease state and type of intervention for all the included trials are summarized in Figure 2. The vast majority of trials (633/777 [82%]) enrolled participants with metastatic melanoma. Unlike many other tumor types, only 64 (8%) trials enrolled patients specifically in the adjuvant setting. Most trials focused on targeted (175 [23%]), immune (180 [23%]), and vaccine (117 [15%]) therapy.
  |
| Figure 2. Trial distribution stratified by disease state (A) and type of intervention (B). Trial distribution is shown for 777 interventional clinical trials including melanoma patients. The majority of clinical trials involved patients with metastatic melanoma. The majority of trials investigated targeted therapy, immunotherapy, and vaccine therapy. |
We subsequently analyzed changes in trial characteristics over time. We noted a decrease in the number of trials investigating cytotoxic and vaccine-based therapies, and increasing numbers of trials investigating immunotherapy (P=.041). Between 2005 and 2007, 14% (27/201) of all trials investigated cytotoxic therapies compared to just 7% (20/294) of trials between 2011 and 2013. With the approval of ipilimumab, 29% (85/294) of all clinical trials between 2011 and 2013 investigated immunotherapies, which comprised only 18% (37/201) of clinical trials between 2005 and 2007. The majority of trials continued to enroll patients in the metastatic setting where outcomes remain poor. Importantly, only 6% (49/777) of all clinical trials have focused on prevention, early detection, and local management of melanoma, which has remained constant over time.
Comment
Cutaneous melanoma remains an area of active investigation, interdisciplinary collaboration, and great promise. The ClinicalTrials.gov registry serves not only to increase transparency among interested parties but also as a rich resource to study the clinical research landscape as demonstrated in this study.
Greater understanding of the underlying genetic and immunogenic properties of melanoma tissues has led to the US Food and Drug Administration approval of several novel agents to treat metastatic disease. BRAF inhibitors such as vemurafenib and dabrafenib target more than 50% of all melanoma tumors harboring mutations in the BRAF gene and have shown unparalleled efficacy in clinical trials; however, durability of response and adverse effects still remain a concern.4,9-11 Ipilimumab, a CTLA-4 inhibitor, enhances antitumor immunity and demonstrated improved survival in clinical trials.12,13 Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody, also demonstrated improved overall and progression-free survival.14 Finally, trametinib, a MEK inhibitor, used in combination with BRAF inhibitors has demonstrated improved response over BRAF inhibitors alone.15
Although traditional cytotoxic chemotherapy was one of the few available treatment options before 2011, response was infrequent.16 Our data indicate that the melanoma research landscape has shifted to follow advances in targeted therapy and immunotherapy. We noted a decrease in the study of cytotoxic chemotherapy in metastatic melanoma, with a compensatory increase in immunotherapy trials and a continued commitment to targeted therapy. Further, with the approval of BRAF inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors for metastatic disease, some have pushed to move these agents into the adjuvant setting to prevent micrometastases from evolving into clinically significant disease.17 Early results from EORTC (European Organisation for Research and Treatment of Cancer) 18071 comparing adjuvant ipilimumab to placebo demonstrated a 26.1-month versus 17.1-month improvement in relapse-free survival, respectively.18 However, this finding has important implications for clinical dermatologists. Patients treated with BRAF inhibitors are at increased risk for keratoacanthomas, invasive squamous cell carcinomas, and secondary primary melanomas.19,20 Caring for these patients requires increased vigilance and collaboration between dermatologists and oncologists.
Our study also highlights the dynamic nature of the field. For example, novel vaccine therapies have demonstrated promise in the metastatic/ unresectable tumor setting, with some herpes simplex virus–based vaccines generating durable antitumor immune responses in patients with melanoma.21 Combination therapy with CTLA-4 and PD-1 inhibitors has demonstrated improved objective response rates and progression-free survival over monotherapy.22 As the status of actively recruiting trials changes on a regular basis, we encourage physicians to access ClinicalTrials.gov to find details and contact information for actively recruiting trials and results on completed trials.
Early detection and management, however, still remain our primary option for cure, and the role of community dermatologists cannot be overstated.23 Patients with stage I and stage II disease have excellent long-term survival rates, yet only 6% of all clinical trials in cutaneous melanoma have focused on patient education, disease prevention, early detection, and local management. With an increasing incidence of melanoma among an aging population, the disease burden remains of substantial concern.24 Optimizing disease prevention, appropriate screening, and early detection are critical roles for dermatologists.
Finally, our data offer some insight into accrual barriers often faced by clinical trials. Actively enrolling clinical trials cluster within major metropolitan areas, presumably with large academic medical centers; however, areas in the southwestern United States and Florida, for example, have some of the highest burden of disease, likely secondary to sun exposure and aging populations.25 Integration of community dermatologists and oncologists may decrease both actual and patient-perceived barriers to care, which requires further exploration.6
Conclusion
Melanoma incidence and disease burden is increasing, and the field of melanoma research is incredibly dynamic. Going forward, we believe dermatologists will continue to play a critical role both in primary disease prevention and detection as well as in detection of secondary treatment-related skin toxicities. ClinicalTrials.gov is an invaluable resource to keep interested parties informed, foster collaboration, identify potential barriers to success, and suggest future directions.
The incidence of cutaneous melanoma, the deadliest form of skin cancer, has been steadily increasing over the last several decades.1 Currently, there are 73,870 new diagnoses of melanoma anticipated in the United States in 2015 alone.2 Many cases of melanoma are caught at early, actionable, and curable stages thanks in part to patient education and screening by dermatologists.3 However, until recently, few options existed for the treatment of locally advanced and metastatic melanomas, with a median survival rate of less than 1 year.4
Clinical trials represent the most reliable method for advancing treatment and improving outcomes for patients with disease; however, patient accrual and access to clinical trials remain formidable barriers. Studies have suggested that patients in rural areas perceive both an increased distance to clinical trial sites and a lack of awareness of available trials compared to their urban counterparts. Additionally, studies have shown that provider awareness of actively enrolling clinical trials in their respective fields is a key determinate in patient enrollment.5 Finally, insufficient funding and lack of collaboration has resulted in many small phase 1 or phase 2 single-center trials, which are less likely to quickly impact clinical care.6 Increased awareness of the ClinicalTrials.gov registry, a publicly available and easily accessible database, can facilitate referral, enrollment, and collaboration among physicians, patients, and researchers alike.
Using the ClinicalTrials.gov database, we sought to analyze the clinical trial landscape for cutaneous melanoma to understand the current state of melanoma research, future direction, and potential barriers that may impede success.
Methods
The primary objective was to provide a snapshot of the melanoma clinical research landscape from 2005 to 2013, including the number of registered trials, phase distribution, recruitment status, location of trials, type of intervention, and disease state being studied. Secondary objectives included describing patterns of clinical trial distribution within the United States in the context of melanoma mortality and examining changing trends in interventions studied in trials over time.
ClinicalTrials.gov is a comprehensive online registry of clinical trials conducted in the United States and abroad that is maintained by the National Library of Medicine.7 Although the initiative was launched in 2000, the registry became effectively comprehensive in September 2005 when the International Committee of Medical Journal Editors declared prospective registration of clinical trials as a prerequisite for publication. The US Food and Drug Administration followed suit in September 2007, expanding the requirements for registration and declaring penalties for parties who did not comply.8 Each registered trial can be found through searchable keywords, and each study page contains details of study design, principal investigators, and inclusion and exclusion criteria, as well as contact information for enrollment.
Study Selection
Clinical trials registered between September 15, 2005, and December 31, 2013, were evaluated; a total of 138,312 trials were found to be registered on ClinicalTrials.gov during that time period. We limited our study selection to interventional studies, which were filtered by topic to yield only those pertaining to melanoma patients. To minimize reporting bias, trials registered prior to the implementation of the International Committee of Medical Journal Editors’ reporting requirements were excluded. To focus specifically on the landscape of trials in cutaneous melanoma, trials investigating multiple advanced malignancies, uveal or ocular melanoma, and mucosal melanoma were manually excluded.
Study Variables
Information on each clinical trial was extracted from ClinicalTrials.gov. Each trial was manually reviewed by an investigator to determine the disease state and type of intervention being studied. Studies investigating multiple modalities concurrently were classified as “other.”
Data Analysis
Study variables were first analyzed among the entire cohort as a whole. Using each trial location and a python script based on open-source code, the number of actively recruiting melanoma trials in each US county was identified and mapped. County-level, melanoma-specific mortality data from 2001 to 2010 was extracted from the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research) mortality database (wonder.cdc.gov). Finally, to analyze changing trends in cutaneous melanoma investigation, trials were grouped into 3 categories based on the date they were received on ClinicalTrials.gov: (1) 2005-2007, (2) 2008-2010, and (3) 2011-2013. Disease state and type of intervention were analyzed and compared among each group using the χ2 statistic.
Results
Of the 138,312 trials registered on ClinicalTrials.gov between September 15, 2005, and December 31, 2013, only 931 were identified as interventional studies pertaining to melanoma patients. Of these, 154 were excluded because of a focus on uveal, ocular, or mucosal melanoma or because of the inclusion of participants with multiple types of advanced malignancies. The final analysis included 777 trials specifically focusing on cutaneous melanoma.
Characteristics of these 777 trials were varied. Many interventions were in the early stages of development, with 339 (44%) trials classified as phase 0, phase 1, or phase 1/phase 2; 306 (39%) as phase 2; and 71 (9%) as nonpharmacologic (nonphase) trials. Only 58 trials (8%) were classified as phase 3 or phase 4. The majority of the trials were actively recruiting (225 [29%]), active but not yet recruiting (172 [22%]), or completed (255 [33%]); however, 98 trials (13%) had been suspended, terminated, or withdrawn. Additionally, 22 trials (3%) were not yet recruiting and 5 (<1%) were classified as “other” because they did not have a recruitment status listed.
The distribution of actively enrolling clinical trials corresponds to major metropolitan areas within the Northeast, Upper Midwest, and Coastal California (Figure 1A). Figure 1B demonstrates the melanoma-specific mortality across the United States. Areas in the Southwest and Florida shared some of the greatest disease burden.
  |
| Figure 1. Geographical representation of US clinical trial enrollment with the number of actively recruiting trials for each unique US zip code presented. The circle size corresponds to the number of trials. The largest circles indicate more than 5 trials within a given zip code (A). County-level melanoma-specific mortality data are presented for 2001 to 2010 (B). Darkest areas represent the highest numbers of melanoma deaths.
|
The disease state and type of intervention for all the included trials are summarized in Figure 2. The vast majority of trials (633/777 [82%]) enrolled participants with metastatic melanoma. Unlike many other tumor types, only 64 (8%) trials enrolled patients specifically in the adjuvant setting. Most trials focused on targeted (175 [23%]), immune (180 [23%]), and vaccine (117 [15%]) therapy.
  |
| Figure 2. Trial distribution stratified by disease state (A) and type of intervention (B). Trial distribution is shown for 777 interventional clinical trials including melanoma patients. The majority of clinical trials involved patients with metastatic melanoma. The majority of trials investigated targeted therapy, immunotherapy, and vaccine therapy. |
We subsequently analyzed changes in trial characteristics over time. We noted a decrease in the number of trials investigating cytotoxic and vaccine-based therapies, and increasing numbers of trials investigating immunotherapy (P=.041). Between 2005 and 2007, 14% (27/201) of all trials investigated cytotoxic therapies compared to just 7% (20/294) of trials between 2011 and 2013. With the approval of ipilimumab, 29% (85/294) of all clinical trials between 2011 and 2013 investigated immunotherapies, which comprised only 18% (37/201) of clinical trials between 2005 and 2007. The majority of trials continued to enroll patients in the metastatic setting where outcomes remain poor. Importantly, only 6% (49/777) of all clinical trials have focused on prevention, early detection, and local management of melanoma, which has remained constant over time.
Comment
Cutaneous melanoma remains an area of active investigation, interdisciplinary collaboration, and great promise. The ClinicalTrials.gov registry serves not only to increase transparency among interested parties but also as a rich resource to study the clinical research landscape as demonstrated in this study.
Greater understanding of the underlying genetic and immunogenic properties of melanoma tissues has led to the US Food and Drug Administration approval of several novel agents to treat metastatic disease. BRAF inhibitors such as vemurafenib and dabrafenib target more than 50% of all melanoma tumors harboring mutations in the BRAF gene and have shown unparalleled efficacy in clinical trials; however, durability of response and adverse effects still remain a concern.4,9-11 Ipilimumab, a CTLA-4 inhibitor, enhances antitumor immunity and demonstrated improved survival in clinical trials.12,13 Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody, also demonstrated improved overall and progression-free survival.14 Finally, trametinib, a MEK inhibitor, used in combination with BRAF inhibitors has demonstrated improved response over BRAF inhibitors alone.15
Although traditional cytotoxic chemotherapy was one of the few available treatment options before 2011, response was infrequent.16 Our data indicate that the melanoma research landscape has shifted to follow advances in targeted therapy and immunotherapy. We noted a decrease in the study of cytotoxic chemotherapy in metastatic melanoma, with a compensatory increase in immunotherapy trials and a continued commitment to targeted therapy. Further, with the approval of BRAF inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors for metastatic disease, some have pushed to move these agents into the adjuvant setting to prevent micrometastases from evolving into clinically significant disease.17 Early results from EORTC (European Organisation for Research and Treatment of Cancer) 18071 comparing adjuvant ipilimumab to placebo demonstrated a 26.1-month versus 17.1-month improvement in relapse-free survival, respectively.18 However, this finding has important implications for clinical dermatologists. Patients treated with BRAF inhibitors are at increased risk for keratoacanthomas, invasive squamous cell carcinomas, and secondary primary melanomas.19,20 Caring for these patients requires increased vigilance and collaboration between dermatologists and oncologists.
Our study also highlights the dynamic nature of the field. For example, novel vaccine therapies have demonstrated promise in the metastatic/ unresectable tumor setting, with some herpes simplex virus–based vaccines generating durable antitumor immune responses in patients with melanoma.21 Combination therapy with CTLA-4 and PD-1 inhibitors has demonstrated improved objective response rates and progression-free survival over monotherapy.22 As the status of actively recruiting trials changes on a regular basis, we encourage physicians to access ClinicalTrials.gov to find details and contact information for actively recruiting trials and results on completed trials.
Early detection and management, however, still remain our primary option for cure, and the role of community dermatologists cannot be overstated.23 Patients with stage I and stage II disease have excellent long-term survival rates, yet only 6% of all clinical trials in cutaneous melanoma have focused on patient education, disease prevention, early detection, and local management. With an increasing incidence of melanoma among an aging population, the disease burden remains of substantial concern.24 Optimizing disease prevention, appropriate screening, and early detection are critical roles for dermatologists.
Finally, our data offer some insight into accrual barriers often faced by clinical trials. Actively enrolling clinical trials cluster within major metropolitan areas, presumably with large academic medical centers; however, areas in the southwestern United States and Florida, for example, have some of the highest burden of disease, likely secondary to sun exposure and aging populations.25 Integration of community dermatologists and oncologists may decrease both actual and patient-perceived barriers to care, which requires further exploration.6
Conclusion
Melanoma incidence and disease burden is increasing, and the field of melanoma research is incredibly dynamic. Going forward, we believe dermatologists will continue to play a critical role both in primary disease prevention and detection as well as in detection of secondary treatment-related skin toxicities. ClinicalTrials.gov is an invaluable resource to keep interested parties informed, foster collaboration, identify potential barriers to success, and suggest future directions.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271.
2. American Cancer Society. Cancer Facts and Figures 2015. American Cancer Society Web site. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed August 26, 2015.
3. Cheng MY, Moreau JF, McGuire ST, et al. Melanoma depth in patients with an established dermatologist. J Am Acad Dermatol. 2014;70:841-846.
4. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60-e69.
5. Kim SH, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. 2014;39:562-571.
6. Gregg JR, Horn L, Davidson MA, et al. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ. 2014;29:74-79.
7. Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the
Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994-1998.
8. Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364:852-860.
9. Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717-725.
10. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
11. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775-787.
12. Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
15. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
16. Espinosa E, Berrocal A, López Martin JA, et al. Advances in cutaneous melanoma. Clin Transl Oncol. 2012;14:325-332.
17. Chapman PB. Treating metastatic melanoma in 2014: what just happened and what is next? Am Soc Clin Oncol Educ Book. 2014:16-19.
18. Eggermont A, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
19. Curry JL, Tetzlaff MT, Nicholson K, et al. Histological features associated with vemurafenib-induced skin toxicities: examination of 141 cutaneous lesions biopsied during therapy. Am J Dermatopathol. 2014; 36:557-561.
20. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-1358.
21. Ross MI, Andtbacka RI, Puzanov I, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): results from a phase III trial in patients with stage IIIb-IV melanoma. Paper presented at: ASCO Annual Meeting; June 2, 2014; Boston, MA.
22. Postow MA, Chesney J, Pavlick AC, et al. Novolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
23. Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415-435.
24. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw. 2013; 11(5 suppl):645-648.
25. Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5, suppl 1):S6-S16.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271.
2. American Cancer Society. Cancer Facts and Figures 2015. American Cancer Society Web site. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed August 26, 2015.
3. Cheng MY, Moreau JF, McGuire ST, et al. Melanoma depth in patients with an established dermatologist. J Am Acad Dermatol. 2014;70:841-846.
4. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60-e69.
5. Kim SH, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. 2014;39:562-571.
6. Gregg JR, Horn L, Davidson MA, et al. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ. 2014;29:74-79.
7. Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the
Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994-1998.
8. Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364:852-860.
9. Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717-725.
10. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
11. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775-787.
12. Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
15. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
16. Espinosa E, Berrocal A, López Martin JA, et al. Advances in cutaneous melanoma. Clin Transl Oncol. 2012;14:325-332.
17. Chapman PB. Treating metastatic melanoma in 2014: what just happened and what is next? Am Soc Clin Oncol Educ Book. 2014:16-19.
18. Eggermont A, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
19. Curry JL, Tetzlaff MT, Nicholson K, et al. Histological features associated with vemurafenib-induced skin toxicities: examination of 141 cutaneous lesions biopsied during therapy. Am J Dermatopathol. 2014; 36:557-561.
20. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-1358.
21. Ross MI, Andtbacka RI, Puzanov I, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): results from a phase III trial in patients with stage IIIb-IV melanoma. Paper presented at: ASCO Annual Meeting; June 2, 2014; Boston, MA.
22. Postow MA, Chesney J, Pavlick AC, et al. Novolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
23. Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415-435.
24. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw. 2013; 11(5 suppl):645-648.
25. Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5, suppl 1):S6-S16.
Practice Points
- The landscape of melanoma clinical trial research has shifted to follow advances in targeted therapy and immunotherapy.
- With these new treatments there is an increased risk for nonmelanoma skin toxicities requiring increased vigilance and collaboration between dermatologists and oncologists.
- Physicians are encouraged to use ClinicalTrials.gov to find details and contact information for actively recruiting clinical trials and results on completed trials.
The Importance of an Antimicrobial Stewardship Program
An antimicrobial stewardship program (ASP) is designed to provide guidance for the safe andcost-effective use of antimicrobial agents. This evidence-based approach addresses the correct selection of antimicrobial agents, dosages, routes of administration, and duration of therapy. In other words, the ASP necessitates the right drug, the right time, the right amount, and the right duration.1 The ASP reduces the development of multidrug-resistant organisms (MDROs), adverse drug events (such as antibiotic-associated diarrhea and renal toxicity), hospital length of stay, collateral damage (development of Clostridium difficile colitis), and health care costs. Review of the literature has shown the ASP reduces hospital stays among patients with acute bacterial-skin and skin-structure infections along with other costly infections.2
The ASP is not a new concept, but it is a hot topic. A successful ASP cannot be achieved without the support of the hospital leadership to determine and provide the needed resources. Its success stems from being a joint collaborative effort between pharmacy, medicine, infection control (IC), microbiology, and information technology. The purpose of the ASP is to ensure proper use of antimicrobials within the health care system through the development of a formal, interdisciplinary team. The primary goal of the ASP is to optimize clinical outcomes while minimizing unintended consequences related to antimicrobial usage, such as toxicities or the emergence of resistance.
In today’s world, health care clinicians are dealing with a global challenge of MDROs such as Enterococcus faecium, Staphylococcus aureus (S aureus), Klebsiella pneumonia, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species (ESKAPE), better known as “bugs without borders.”3 According to the CDC, antibiotic-resistant infections affect at least 2 million people in the U.S. annually and result in > 23,000 deaths.2 According to Thomas Frieden, director of the CDC, the pipeline of new antibiotics is nearly empty for the short term, and new drugs could be a decade away from discovery and approval by the FDA.2
Literature Review
Pasquale and colleagues conducted a retrospective, observational chart review on 62 patients who were admitted for bacterial-skin and skin-structure infections (S aureus, MRSA, MSSA, and Pseudomonas aeruginosa).4 The data examined patient demographic characteristics, comorbidities, specific type of skin infection (the most common being cellulitis, major or deep abscesses, and surgical site infections), microbiology, surgical interventions, and recommendations obtained from the ASP committee.
The ASP recommendations were divided into 5 categories, including dosage changes, de-escalation, antibiotic regimen changes, infectious disease (ID) consults, and other (not described). The ASP offered 85 recommendations, and acceptance of the ASP recommendations by physicians was 95%. The intervention group had a significantly lower length of stay (4.4 days vs 6.2 days, P < .001); and the 30-day all-cause readmission rate was also significantly lower (6.5% vs 16.71%, P = .05). However, the skin and skin-related structures readmission rate did not differ significantly (3.33% vs 6.27%). It was impossible for the investigators to determine exact differences in the amount of antimicrobials used in the intervention group vs the historical controls, because the historical data were based on ICD-9 codes, which may explain the nonsignificant finding.4
D’Agata reviewed the antimicrobial usage and ASP programs in dialysis centers.5 Chronic hemodialysis patients with central lines were noted to have the greatest rate of infections and antibiotic usage (6.4 per 100 patient months). The next highest group was dialysis patients with grafts (2.4 per 100 patient months), followed by patients with fistulas (1.8 per 100 patient months). Vancomycin was most commonly chosen for all antibiotic starts (73%). Interestingly, vancomycin was followed by cefazolin and third- and/or fourth-generation cephalosporin, which are risk factors for the emergence of multidrug-resistant, Gram-negative bacteria that are highly linked to increased morbidity and mortality rates. The U.S. Renal Data System stated in its 2009 report that the use of antibiotic therapy has increased from 31% in 1994 to 41% in 2007.5
In reviewing inappropriate choices of antimicrobial prescribing, D’Agata compared prescriptions given to the Healthcare Infection Control Practices Advisory Committee to determine whether the correct antibiotic was chosen. In 164 vancomycin prescriptions, 20% were categorized as inappropriate.5 In another study done by Zvonar and colleagues, 163 prescriptions of vancomycin were reviewed, and 12% were considered inappropriate.6
Snyder and colleagues examined 278 patients on hemodialysis, and over a 1-year period, 32% of these patients received ≥ 1 antimicrobial with 29.8% of the doses classified as inappropriate.7 The most common category for inappropriate prescribing of antimicrobials was not meeting the criteria for diagnosing infections (52.9% of cases). The second leading cause of inappropriate prescription for infections was not meeting criteria for diagnosing specific skin and skin-structure infections (51.6% of cases). Another common category was failure to choose a narrower spectrum antimicrobial prescription (26.8%).7 Attention to the indications and duration of antimicrobial treatment accounted for 20.3% of all inappropriate doses. Correction of these problems with use of an ASP could reduce the patient’s exposure to unneeded or inappropriate antibiotics by 22% to 36% and decrease hospital costs between $200,000 to $900,000.5
Rosa and colleagues discussed adherence to an ASP and the effects on mortality in hospitalized cancer patients with febrile neutropenia (FN).8 A prospective cohort study was performed in a single facility over a 2-year period. Patients admitted with cancer and FN were followed for 28 days. The mortality rates of those treated with ASP protocol antibiotics were compared with those treated with other antibiotic regimens. One hundred sixty-nine patients with 307 episodes of FN were included. The rate of adherence to ASP recommendations was 53% with the mortality of this cohort 9.4% (29 patients).8
Older patients were more likely to be treated according to ASP recommendations, whereas patients with comorbidities were not treated with ASP guidelines, Rosa and colleagues noted.8 No explanation was given, but statistical testing did uphold these findings, ensuring that the results were correctly interpreted. The 28-day mortality during FN was related to several factors, including nonadherence with ASP recommendations (P = .001) relapsing diseases stages (P = .001), and time to antibiotic start therapy > 1 hour (P = .001). Adherence to the ASP was independently associated with a higher survival rate (P = .03), whereas mortality was attributable to infection in all 29 patients who died.
Nowak and colleagues reviewed the clinical and economic benefits of an ASP using a pre- and postanalysis of potential patients who might benefit from recommendations of the ASP.9 Subjects included adult inpatients with pneumonia or abdominal sepsis. Recommendations from ASP that were followed decreased expenditures by 9.75% during the first year and remained stable in the following years. The cumulative cost savings was about $1.7 million. Rates of nosocomial infections decreased, and pre- and postcomparison of survival and lengths of stay for patients with pneumonia (n = 2,186) or abdominal sepsis (n = 225) revealed no significant differences. Investigators argued that this finding may have been due to the hospital’s initiation of other concurrent IC programs.
Doron and colleagues conducted a survey identifying characteristics of ASP practices and factors associated with the presence of an ASP.10 Surveys were received from 48 states (North and South Dakota were not included) and Puerto Rico. Surveys were received from 406 providers, and 96.4% identified some form of ASP. Barriers to implementation included staffing constraints (69.4%) and insufficient funding (0.6%).10
About 38% of the responses stated ASP was being used for adults and pediatric patients, whereas 58.8% were used for adults only.10 The ASP teams were composed of a variety of providers, including infectious disease (ID) physicians (70.7%), IC professionals (51.1%), and clinical microbiologists (38.6%). Additional barriers to implementing an ASP were found as insufficient medical staff buy-in (32.8%), not high on the priority list (22.2%), and too many other things to consider or deal with at the time (42.8%). Interestingly, 41.1% of the subjects in facilities without an ASP responded that providers agree with limiting the use of antimicrobials compared with 66.9% of subjects in hospitals with an ASP. Factors linked to having an ASP included having an ID consultation service, an ID fellowship program, an ID pharmacist, larger hospitals, annual admissions > 10,000, having a published antibiogram, and being a teaching hospital.
Establishment of an ASP
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) issued guidelines in 2007 for developing an institutional ASP to enhance antimicrobial stewardship and help prevent antimicrobial resistance in hospitals.11 The ASP may vary among facilities based on available resources.
When developing an ASP, 2 core strategies are necessary. The core measures are proactive and are usually conducted by an ID clinical pharmacist assigned to the ASP in collaboration with the ID physician. These strategies are not mutually exclusive and include a prospective audit with interventions provided to the clinicians, resulting in decreased inappropriate use of antimicrobials or a formulary restriction and preauthorization to help reduce antimicrobial usage and related cost.
Supplemental elements may be considered and prioritized as to the core antimicrobial stewardship strategies based on local practice pattern and resources.11 Factors to consider include education, which is considered to be an essential element of the ASP. Although education is important, it alone is only somewhat effective in changing clinicians’ prescribing practices. Guidelines and clinical pathways are elements set forth in institutional management protocols for common and potentially serious infections such as intravascular catheter-related infections, hospital- and community-acquired pneumonia, bloodstream infections, and complicated urinary tract infections among other types.
Another consideration is antimicrobial cycling. This process refers to the specific schedule of alternating specific antimicrobials or antimicrobial classes to prevent or reverse the development of antimicrobial resistance. Insufficient data on antimicrobial cycling currently exist to affect major changes in practice. This element, however, could be implemented in certain institutions if needed based on the reported bacterial resistance pattern.
Antimicrobial order forms can be used to help monitor the implementation of formulated institutional clinical practice pathways. However, the authors feel that documenting this indication in the clinician notes may be adequate and save time for everyone involved; additionally, reviewing combination therapy, which if avoided, may prevent the emergence of resistance. Although combination therapy is needed in certain clinical diagnostic situations, careful consideration of its use is essential.
Streamlining or de-escalation of therapy by using a narrower spectrum agent, based on culture and sensitivity results, prevents duplicative therapy with a patient when double coverage is not indicated or intended. Another goal is the discontinuation of therapy based on negative culture results and lack of supporting clinical signs and symptoms of infection. Dose optimization and adjustment should also be reviewed. Using the appropriate antimicrobial dose based on the specific pathogen, patient characteristic, source of infection, along with the pharmacokinetic and pharmacodynamics should be reviewed to prevent antimicrobial overuse and subsequent potentially avoidable adverse effects.
Parenteral to oral conversion from IV to oral administration (IV to oral) antimicrobials must be considered when the patient is clinically and hemodynamically stable, thus limiting the length of hospital stays and health care costs. However, it is important to keep in mind pharmacokinetic studies examining the bioavailability of antibiotics are usually conducted with healthy volunteers. Therefore, when treating patients who are elderly, on multiple medications, or severely ill, proper usage of these antibiotics is required. Also, having antibiotics with excellent bioavailability does not necessarily mean switching from IV to oral routes when treating serious infections such as bacteremia. Special consideration should be given when changing the route of administration. In addition, approval—or at least notification by the treating physician or ID specialist—should be included in the absence of an institutional policy, allowing for automatic IV to oral conversion.
The ASP Team
The participation of specific clinicians has been suggested as key to having a successful ASP team.12 Members should include an ID physician (director) who serves as the lead physician and supervises the overall function of the ASP, makes recommendations to the ASP team, and contributes to the educational activities. A clinical ID pharmacist (codirector) provides suggestions to clinicians on preferred first-line antimicrobials and reviews medication orders for antimicrobials and resistance patterns, microbiological data, patient data, and clinical information. The codirector also tracks any ASP-related data and submits monitoring reports on a regular basis.
If accessible, an IC professional should participate, implementing and monitoring prevention strategies that decrease health care-associated infections. These infections play a significant role in reducing MDROs and decreasing the use of antibiotics. Additionally, the IC professional can assist in the early identification of patients with MDROs, aid patient placement on transmission-based precautions, and flag a patient in the medical record for hightened awareness. Also, IC professionals promote hand hygiene, standard precautions, and contribute to infection prevention strategies, such as hospital bundle practices, to prevent catheter-associated bloodstream infections and ventilator-associated pneumonias, among others.
If possible, a microbiologist who can prepare culture and susceptibility data to optimize antimicrobial management and conduct timely documentation of microbial pathogens should be a member of the team. Microbiologists can report organism susceptibility, assist in the surveillance of specific organisms, and provide early identification of patients with MDROs that require transmission-based precautions. The microbiologist can perform a semiannual update of a local antibiogram while reporting antimicrobial susceptibility profiles. Based on the information gathered, microbiologists can provide new drug panels to the members of the ASP, who will decide which antibiotic panel will be used. Another possible member of the ASP team is a program analyst who provides data retrieval, performs data analysis, and delivers necessary reports to the team.
It is the responsibility of medical staff to review and implement suggestions made by the ASP when appropriate. However, these suggestions are not considered a substitute for clinical decisions, and discretion is required when treating individual patients. The VHA, in response to the IDSA/SHEA published guidelines, chartered an antimicrobial stewardship task force in May 2011 with the sole purpose “To optimize the care of Veterans by developing, deploying and monitoring a national-level strategic plan for improvements in antimicrobial therapy management.”1 In 2011, the Office of Inspector General in a combined assessment program summary report for management of MDROs in VHA facilities recommended that “the Under Secretary for Health, in conjunction with VISN and facility senior managers, ensures that facilities develop policies and programs that control and reduce antimicrobial agent use.”13
In 2012, the VHA conducted a survey to obtain baseline data regarding ASP activities, presence of dedicated personnel, current related practice policies, available resources, and outcomes. There were 140 voluntary participating VA facilities, of which 130 had inpatient services. The survey found that 26 facilities (20%) did not have an attending ID physician, 49 facilities (38%) reported having an ASP, 19 facilities (15%) had developed policy in place addressing de-escalation of antimicrobials, 87 facilities (67%) had not developed a business plan for an ASP, and 61 facilities (47%) had completed a medication usage evaluation.14 Feedback following the analysis of the survey data recommended integrating more ID personnel as needed, along with the development of ASP teams for all facilities with inpatient services, who would have the authority to change the antimicrobial therapy selection and have policies in place related to ASP principles.
Conclusions
Increased MDROs and decreased anti-infective development requires stricter management of antibiotics. An ASP is essential in any hospital or health care facility to decrease the incidence of resistance and improve patient care. The ASP is a collaborative effort that involves multiple specialties and departments. A successful ASP is one that changes based on local prescribing trends and resistance patterns while focusing on a patient as an individual.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs, Veterans Health Administration. Antimicrobial Stewardship Programs (ASP). VHA Directive 1031. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Updated January 22, 2014. Accessed August 4, 2015.
2. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Published April 23, 2013. Accessed August 4, 2015.
3. Pyrek K. Bugs without borders: the global challenge of MDROs. Infect Control Today. 2013;17(2):1-8.
4. Pasquale T, Trienski TL, Olexia DE, et al. Impact of an antimicrobial stewardship program on patients with acute bacterial skin and skin structure infections. Am J Health Syst Pharm. 2014;71(13):1136-1139.
5. D’Agata EM. Antimicrobial use and stewardship programs among dialysis centers. Semin Dial. 2013;26(4):457-464.
6. Zvonar R, Natarajan S, Edwards C, Roth V. Assessment of vancomycin use in chronic hemodialysis patients: room for improvement. Nephrol Dial Transplant. 2008;23(11):3690-3695.
7. Snyder, GM, Patel PR, Kallen AJ, Strom JA, Tucker JK, D’Agata EM. Antimicrobial use in outpatient hemodialysis units. Infect Control Hosp Epidemiol. 2013;34(4):349-357.
8. Rosa RG, Goldani LZ, dos Santos RP. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febril neutropaenia: a prospective cohort study. BMC Infect Dis. 2014;14:286.
9. Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm. 2012;69(17):1500-1508.
10. Doron S, Nadkarni L, Lyn Price L, et al. A nationwide survey of antimicrobial stewardship practices. Clin Ther. 2013;35(6):758-765.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10(1):63-73.
13. U.S. Department of Veterans Affairs Office of Inspector General. Combined Assessment Program Summary Report: Management of Multidrug-Resistant Organisms in Veterans Health Administration Facilities. Report No. 11-02870-04. U.S. Department of Veterans Affairs Website. http://www.va.gov/oig/pubs/VAOIG-11-02870-04.pdf. Updated October 14, 2011. Accessed August 4, 2015.
14. Roselle GA, Neuhauser M, Kelly A, Vandenberg P. 2012 Survey of antimicrobial stewardship in VA. Washington, DC: Department of Veterans Affairs; 2013.
An antimicrobial stewardship program (ASP) is designed to provide guidance for the safe andcost-effective use of antimicrobial agents. This evidence-based approach addresses the correct selection of antimicrobial agents, dosages, routes of administration, and duration of therapy. In other words, the ASP necessitates the right drug, the right time, the right amount, and the right duration.1 The ASP reduces the development of multidrug-resistant organisms (MDROs), adverse drug events (such as antibiotic-associated diarrhea and renal toxicity), hospital length of stay, collateral damage (development of Clostridium difficile colitis), and health care costs. Review of the literature has shown the ASP reduces hospital stays among patients with acute bacterial-skin and skin-structure infections along with other costly infections.2
The ASP is not a new concept, but it is a hot topic. A successful ASP cannot be achieved without the support of the hospital leadership to determine and provide the needed resources. Its success stems from being a joint collaborative effort between pharmacy, medicine, infection control (IC), microbiology, and information technology. The purpose of the ASP is to ensure proper use of antimicrobials within the health care system through the development of a formal, interdisciplinary team. The primary goal of the ASP is to optimize clinical outcomes while minimizing unintended consequences related to antimicrobial usage, such as toxicities or the emergence of resistance.
In today’s world, health care clinicians are dealing with a global challenge of MDROs such as Enterococcus faecium, Staphylococcus aureus (S aureus), Klebsiella pneumonia, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species (ESKAPE), better known as “bugs without borders.”3 According to the CDC, antibiotic-resistant infections affect at least 2 million people in the U.S. annually and result in > 23,000 deaths.2 According to Thomas Frieden, director of the CDC, the pipeline of new antibiotics is nearly empty for the short term, and new drugs could be a decade away from discovery and approval by the FDA.2
Literature Review
Pasquale and colleagues conducted a retrospective, observational chart review on 62 patients who were admitted for bacterial-skin and skin-structure infections (S aureus, MRSA, MSSA, and Pseudomonas aeruginosa).4 The data examined patient demographic characteristics, comorbidities, specific type of skin infection (the most common being cellulitis, major or deep abscesses, and surgical site infections), microbiology, surgical interventions, and recommendations obtained from the ASP committee.
The ASP recommendations were divided into 5 categories, including dosage changes, de-escalation, antibiotic regimen changes, infectious disease (ID) consults, and other (not described). The ASP offered 85 recommendations, and acceptance of the ASP recommendations by physicians was 95%. The intervention group had a significantly lower length of stay (4.4 days vs 6.2 days, P < .001); and the 30-day all-cause readmission rate was also significantly lower (6.5% vs 16.71%, P = .05). However, the skin and skin-related structures readmission rate did not differ significantly (3.33% vs 6.27%). It was impossible for the investigators to determine exact differences in the amount of antimicrobials used in the intervention group vs the historical controls, because the historical data were based on ICD-9 codes, which may explain the nonsignificant finding.4
D’Agata reviewed the antimicrobial usage and ASP programs in dialysis centers.5 Chronic hemodialysis patients with central lines were noted to have the greatest rate of infections and antibiotic usage (6.4 per 100 patient months). The next highest group was dialysis patients with grafts (2.4 per 100 patient months), followed by patients with fistulas (1.8 per 100 patient months). Vancomycin was most commonly chosen for all antibiotic starts (73%). Interestingly, vancomycin was followed by cefazolin and third- and/or fourth-generation cephalosporin, which are risk factors for the emergence of multidrug-resistant, Gram-negative bacteria that are highly linked to increased morbidity and mortality rates. The U.S. Renal Data System stated in its 2009 report that the use of antibiotic therapy has increased from 31% in 1994 to 41% in 2007.5
In reviewing inappropriate choices of antimicrobial prescribing, D’Agata compared prescriptions given to the Healthcare Infection Control Practices Advisory Committee to determine whether the correct antibiotic was chosen. In 164 vancomycin prescriptions, 20% were categorized as inappropriate.5 In another study done by Zvonar and colleagues, 163 prescriptions of vancomycin were reviewed, and 12% were considered inappropriate.6
Snyder and colleagues examined 278 patients on hemodialysis, and over a 1-year period, 32% of these patients received ≥ 1 antimicrobial with 29.8% of the doses classified as inappropriate.7 The most common category for inappropriate prescribing of antimicrobials was not meeting the criteria for diagnosing infections (52.9% of cases). The second leading cause of inappropriate prescription for infections was not meeting criteria for diagnosing specific skin and skin-structure infections (51.6% of cases). Another common category was failure to choose a narrower spectrum antimicrobial prescription (26.8%).7 Attention to the indications and duration of antimicrobial treatment accounted for 20.3% of all inappropriate doses. Correction of these problems with use of an ASP could reduce the patient’s exposure to unneeded or inappropriate antibiotics by 22% to 36% and decrease hospital costs between $200,000 to $900,000.5
Rosa and colleagues discussed adherence to an ASP and the effects on mortality in hospitalized cancer patients with febrile neutropenia (FN).8 A prospective cohort study was performed in a single facility over a 2-year period. Patients admitted with cancer and FN were followed for 28 days. The mortality rates of those treated with ASP protocol antibiotics were compared with those treated with other antibiotic regimens. One hundred sixty-nine patients with 307 episodes of FN were included. The rate of adherence to ASP recommendations was 53% with the mortality of this cohort 9.4% (29 patients).8
Older patients were more likely to be treated according to ASP recommendations, whereas patients with comorbidities were not treated with ASP guidelines, Rosa and colleagues noted.8 No explanation was given, but statistical testing did uphold these findings, ensuring that the results were correctly interpreted. The 28-day mortality during FN was related to several factors, including nonadherence with ASP recommendations (P = .001) relapsing diseases stages (P = .001), and time to antibiotic start therapy > 1 hour (P = .001). Adherence to the ASP was independently associated with a higher survival rate (P = .03), whereas mortality was attributable to infection in all 29 patients who died.
Nowak and colleagues reviewed the clinical and economic benefits of an ASP using a pre- and postanalysis of potential patients who might benefit from recommendations of the ASP.9 Subjects included adult inpatients with pneumonia or abdominal sepsis. Recommendations from ASP that were followed decreased expenditures by 9.75% during the first year and remained stable in the following years. The cumulative cost savings was about $1.7 million. Rates of nosocomial infections decreased, and pre- and postcomparison of survival and lengths of stay for patients with pneumonia (n = 2,186) or abdominal sepsis (n = 225) revealed no significant differences. Investigators argued that this finding may have been due to the hospital’s initiation of other concurrent IC programs.
Doron and colleagues conducted a survey identifying characteristics of ASP practices and factors associated with the presence of an ASP.10 Surveys were received from 48 states (North and South Dakota were not included) and Puerto Rico. Surveys were received from 406 providers, and 96.4% identified some form of ASP. Barriers to implementation included staffing constraints (69.4%) and insufficient funding (0.6%).10
About 38% of the responses stated ASP was being used for adults and pediatric patients, whereas 58.8% were used for adults only.10 The ASP teams were composed of a variety of providers, including infectious disease (ID) physicians (70.7%), IC professionals (51.1%), and clinical microbiologists (38.6%). Additional barriers to implementing an ASP were found as insufficient medical staff buy-in (32.8%), not high on the priority list (22.2%), and too many other things to consider or deal with at the time (42.8%). Interestingly, 41.1% of the subjects in facilities without an ASP responded that providers agree with limiting the use of antimicrobials compared with 66.9% of subjects in hospitals with an ASP. Factors linked to having an ASP included having an ID consultation service, an ID fellowship program, an ID pharmacist, larger hospitals, annual admissions > 10,000, having a published antibiogram, and being a teaching hospital.
Establishment of an ASP
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) issued guidelines in 2007 for developing an institutional ASP to enhance antimicrobial stewardship and help prevent antimicrobial resistance in hospitals.11 The ASP may vary among facilities based on available resources.
When developing an ASP, 2 core strategies are necessary. The core measures are proactive and are usually conducted by an ID clinical pharmacist assigned to the ASP in collaboration with the ID physician. These strategies are not mutually exclusive and include a prospective audit with interventions provided to the clinicians, resulting in decreased inappropriate use of antimicrobials or a formulary restriction and preauthorization to help reduce antimicrobial usage and related cost.
Supplemental elements may be considered and prioritized as to the core antimicrobial stewardship strategies based on local practice pattern and resources.11 Factors to consider include education, which is considered to be an essential element of the ASP. Although education is important, it alone is only somewhat effective in changing clinicians’ prescribing practices. Guidelines and clinical pathways are elements set forth in institutional management protocols for common and potentially serious infections such as intravascular catheter-related infections, hospital- and community-acquired pneumonia, bloodstream infections, and complicated urinary tract infections among other types.
Another consideration is antimicrobial cycling. This process refers to the specific schedule of alternating specific antimicrobials or antimicrobial classes to prevent or reverse the development of antimicrobial resistance. Insufficient data on antimicrobial cycling currently exist to affect major changes in practice. This element, however, could be implemented in certain institutions if needed based on the reported bacterial resistance pattern.
Antimicrobial order forms can be used to help monitor the implementation of formulated institutional clinical practice pathways. However, the authors feel that documenting this indication in the clinician notes may be adequate and save time for everyone involved; additionally, reviewing combination therapy, which if avoided, may prevent the emergence of resistance. Although combination therapy is needed in certain clinical diagnostic situations, careful consideration of its use is essential.
Streamlining or de-escalation of therapy by using a narrower spectrum agent, based on culture and sensitivity results, prevents duplicative therapy with a patient when double coverage is not indicated or intended. Another goal is the discontinuation of therapy based on negative culture results and lack of supporting clinical signs and symptoms of infection. Dose optimization and adjustment should also be reviewed. Using the appropriate antimicrobial dose based on the specific pathogen, patient characteristic, source of infection, along with the pharmacokinetic and pharmacodynamics should be reviewed to prevent antimicrobial overuse and subsequent potentially avoidable adverse effects.
Parenteral to oral conversion from IV to oral administration (IV to oral) antimicrobials must be considered when the patient is clinically and hemodynamically stable, thus limiting the length of hospital stays and health care costs. However, it is important to keep in mind pharmacokinetic studies examining the bioavailability of antibiotics are usually conducted with healthy volunteers. Therefore, when treating patients who are elderly, on multiple medications, or severely ill, proper usage of these antibiotics is required. Also, having antibiotics with excellent bioavailability does not necessarily mean switching from IV to oral routes when treating serious infections such as bacteremia. Special consideration should be given when changing the route of administration. In addition, approval—or at least notification by the treating physician or ID specialist—should be included in the absence of an institutional policy, allowing for automatic IV to oral conversion.
The ASP Team
The participation of specific clinicians has been suggested as key to having a successful ASP team.12 Members should include an ID physician (director) who serves as the lead physician and supervises the overall function of the ASP, makes recommendations to the ASP team, and contributes to the educational activities. A clinical ID pharmacist (codirector) provides suggestions to clinicians on preferred first-line antimicrobials and reviews medication orders for antimicrobials and resistance patterns, microbiological data, patient data, and clinical information. The codirector also tracks any ASP-related data and submits monitoring reports on a regular basis.
If accessible, an IC professional should participate, implementing and monitoring prevention strategies that decrease health care-associated infections. These infections play a significant role in reducing MDROs and decreasing the use of antibiotics. Additionally, the IC professional can assist in the early identification of patients with MDROs, aid patient placement on transmission-based precautions, and flag a patient in the medical record for hightened awareness. Also, IC professionals promote hand hygiene, standard precautions, and contribute to infection prevention strategies, such as hospital bundle practices, to prevent catheter-associated bloodstream infections and ventilator-associated pneumonias, among others.
If possible, a microbiologist who can prepare culture and susceptibility data to optimize antimicrobial management and conduct timely documentation of microbial pathogens should be a member of the team. Microbiologists can report organism susceptibility, assist in the surveillance of specific organisms, and provide early identification of patients with MDROs that require transmission-based precautions. The microbiologist can perform a semiannual update of a local antibiogram while reporting antimicrobial susceptibility profiles. Based on the information gathered, microbiologists can provide new drug panels to the members of the ASP, who will decide which antibiotic panel will be used. Another possible member of the ASP team is a program analyst who provides data retrieval, performs data analysis, and delivers necessary reports to the team.
It is the responsibility of medical staff to review and implement suggestions made by the ASP when appropriate. However, these suggestions are not considered a substitute for clinical decisions, and discretion is required when treating individual patients. The VHA, in response to the IDSA/SHEA published guidelines, chartered an antimicrobial stewardship task force in May 2011 with the sole purpose “To optimize the care of Veterans by developing, deploying and monitoring a national-level strategic plan for improvements in antimicrobial therapy management.”1 In 2011, the Office of Inspector General in a combined assessment program summary report for management of MDROs in VHA facilities recommended that “the Under Secretary for Health, in conjunction with VISN and facility senior managers, ensures that facilities develop policies and programs that control and reduce antimicrobial agent use.”13
In 2012, the VHA conducted a survey to obtain baseline data regarding ASP activities, presence of dedicated personnel, current related practice policies, available resources, and outcomes. There were 140 voluntary participating VA facilities, of which 130 had inpatient services. The survey found that 26 facilities (20%) did not have an attending ID physician, 49 facilities (38%) reported having an ASP, 19 facilities (15%) had developed policy in place addressing de-escalation of antimicrobials, 87 facilities (67%) had not developed a business plan for an ASP, and 61 facilities (47%) had completed a medication usage evaluation.14 Feedback following the analysis of the survey data recommended integrating more ID personnel as needed, along with the development of ASP teams for all facilities with inpatient services, who would have the authority to change the antimicrobial therapy selection and have policies in place related to ASP principles.
Conclusions
Increased MDROs and decreased anti-infective development requires stricter management of antibiotics. An ASP is essential in any hospital or health care facility to decrease the incidence of resistance and improve patient care. The ASP is a collaborative effort that involves multiple specialties and departments. A successful ASP is one that changes based on local prescribing trends and resistance patterns while focusing on a patient as an individual.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
An antimicrobial stewardship program (ASP) is designed to provide guidance for the safe andcost-effective use of antimicrobial agents. This evidence-based approach addresses the correct selection of antimicrobial agents, dosages, routes of administration, and duration of therapy. In other words, the ASP necessitates the right drug, the right time, the right amount, and the right duration.1 The ASP reduces the development of multidrug-resistant organisms (MDROs), adverse drug events (such as antibiotic-associated diarrhea and renal toxicity), hospital length of stay, collateral damage (development of Clostridium difficile colitis), and health care costs. Review of the literature has shown the ASP reduces hospital stays among patients with acute bacterial-skin and skin-structure infections along with other costly infections.2
The ASP is not a new concept, but it is a hot topic. A successful ASP cannot be achieved without the support of the hospital leadership to determine and provide the needed resources. Its success stems from being a joint collaborative effort between pharmacy, medicine, infection control (IC), microbiology, and information technology. The purpose of the ASP is to ensure proper use of antimicrobials within the health care system through the development of a formal, interdisciplinary team. The primary goal of the ASP is to optimize clinical outcomes while minimizing unintended consequences related to antimicrobial usage, such as toxicities or the emergence of resistance.
In today’s world, health care clinicians are dealing with a global challenge of MDROs such as Enterococcus faecium, Staphylococcus aureus (S aureus), Klebsiella pneumonia, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species (ESKAPE), better known as “bugs without borders.”3 According to the CDC, antibiotic-resistant infections affect at least 2 million people in the U.S. annually and result in > 23,000 deaths.2 According to Thomas Frieden, director of the CDC, the pipeline of new antibiotics is nearly empty for the short term, and new drugs could be a decade away from discovery and approval by the FDA.2
Literature Review
Pasquale and colleagues conducted a retrospective, observational chart review on 62 patients who were admitted for bacterial-skin and skin-structure infections (S aureus, MRSA, MSSA, and Pseudomonas aeruginosa).4 The data examined patient demographic characteristics, comorbidities, specific type of skin infection (the most common being cellulitis, major or deep abscesses, and surgical site infections), microbiology, surgical interventions, and recommendations obtained from the ASP committee.
The ASP recommendations were divided into 5 categories, including dosage changes, de-escalation, antibiotic regimen changes, infectious disease (ID) consults, and other (not described). The ASP offered 85 recommendations, and acceptance of the ASP recommendations by physicians was 95%. The intervention group had a significantly lower length of stay (4.4 days vs 6.2 days, P < .001); and the 30-day all-cause readmission rate was also significantly lower (6.5% vs 16.71%, P = .05). However, the skin and skin-related structures readmission rate did not differ significantly (3.33% vs 6.27%). It was impossible for the investigators to determine exact differences in the amount of antimicrobials used in the intervention group vs the historical controls, because the historical data were based on ICD-9 codes, which may explain the nonsignificant finding.4
D’Agata reviewed the antimicrobial usage and ASP programs in dialysis centers.5 Chronic hemodialysis patients with central lines were noted to have the greatest rate of infections and antibiotic usage (6.4 per 100 patient months). The next highest group was dialysis patients with grafts (2.4 per 100 patient months), followed by patients with fistulas (1.8 per 100 patient months). Vancomycin was most commonly chosen for all antibiotic starts (73%). Interestingly, vancomycin was followed by cefazolin and third- and/or fourth-generation cephalosporin, which are risk factors for the emergence of multidrug-resistant, Gram-negative bacteria that are highly linked to increased morbidity and mortality rates. The U.S. Renal Data System stated in its 2009 report that the use of antibiotic therapy has increased from 31% in 1994 to 41% in 2007.5
In reviewing inappropriate choices of antimicrobial prescribing, D’Agata compared prescriptions given to the Healthcare Infection Control Practices Advisory Committee to determine whether the correct antibiotic was chosen. In 164 vancomycin prescriptions, 20% were categorized as inappropriate.5 In another study done by Zvonar and colleagues, 163 prescriptions of vancomycin were reviewed, and 12% were considered inappropriate.6
Snyder and colleagues examined 278 patients on hemodialysis, and over a 1-year period, 32% of these patients received ≥ 1 antimicrobial with 29.8% of the doses classified as inappropriate.7 The most common category for inappropriate prescribing of antimicrobials was not meeting the criteria for diagnosing infections (52.9% of cases). The second leading cause of inappropriate prescription for infections was not meeting criteria for diagnosing specific skin and skin-structure infections (51.6% of cases). Another common category was failure to choose a narrower spectrum antimicrobial prescription (26.8%).7 Attention to the indications and duration of antimicrobial treatment accounted for 20.3% of all inappropriate doses. Correction of these problems with use of an ASP could reduce the patient’s exposure to unneeded or inappropriate antibiotics by 22% to 36% and decrease hospital costs between $200,000 to $900,000.5
Rosa and colleagues discussed adherence to an ASP and the effects on mortality in hospitalized cancer patients with febrile neutropenia (FN).8 A prospective cohort study was performed in a single facility over a 2-year period. Patients admitted with cancer and FN were followed for 28 days. The mortality rates of those treated with ASP protocol antibiotics were compared with those treated with other antibiotic regimens. One hundred sixty-nine patients with 307 episodes of FN were included. The rate of adherence to ASP recommendations was 53% with the mortality of this cohort 9.4% (29 patients).8
Older patients were more likely to be treated according to ASP recommendations, whereas patients with comorbidities were not treated with ASP guidelines, Rosa and colleagues noted.8 No explanation was given, but statistical testing did uphold these findings, ensuring that the results were correctly interpreted. The 28-day mortality during FN was related to several factors, including nonadherence with ASP recommendations (P = .001) relapsing diseases stages (P = .001), and time to antibiotic start therapy > 1 hour (P = .001). Adherence to the ASP was independently associated with a higher survival rate (P = .03), whereas mortality was attributable to infection in all 29 patients who died.
Nowak and colleagues reviewed the clinical and economic benefits of an ASP using a pre- and postanalysis of potential patients who might benefit from recommendations of the ASP.9 Subjects included adult inpatients with pneumonia or abdominal sepsis. Recommendations from ASP that were followed decreased expenditures by 9.75% during the first year and remained stable in the following years. The cumulative cost savings was about $1.7 million. Rates of nosocomial infections decreased, and pre- and postcomparison of survival and lengths of stay for patients with pneumonia (n = 2,186) or abdominal sepsis (n = 225) revealed no significant differences. Investigators argued that this finding may have been due to the hospital’s initiation of other concurrent IC programs.
Doron and colleagues conducted a survey identifying characteristics of ASP practices and factors associated with the presence of an ASP.10 Surveys were received from 48 states (North and South Dakota were not included) and Puerto Rico. Surveys were received from 406 providers, and 96.4% identified some form of ASP. Barriers to implementation included staffing constraints (69.4%) and insufficient funding (0.6%).10
About 38% of the responses stated ASP was being used for adults and pediatric patients, whereas 58.8% were used for adults only.10 The ASP teams were composed of a variety of providers, including infectious disease (ID) physicians (70.7%), IC professionals (51.1%), and clinical microbiologists (38.6%). Additional barriers to implementing an ASP were found as insufficient medical staff buy-in (32.8%), not high on the priority list (22.2%), and too many other things to consider or deal with at the time (42.8%). Interestingly, 41.1% of the subjects in facilities without an ASP responded that providers agree with limiting the use of antimicrobials compared with 66.9% of subjects in hospitals with an ASP. Factors linked to having an ASP included having an ID consultation service, an ID fellowship program, an ID pharmacist, larger hospitals, annual admissions > 10,000, having a published antibiogram, and being a teaching hospital.
Establishment of an ASP
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) issued guidelines in 2007 for developing an institutional ASP to enhance antimicrobial stewardship and help prevent antimicrobial resistance in hospitals.11 The ASP may vary among facilities based on available resources.
When developing an ASP, 2 core strategies are necessary. The core measures are proactive and are usually conducted by an ID clinical pharmacist assigned to the ASP in collaboration with the ID physician. These strategies are not mutually exclusive and include a prospective audit with interventions provided to the clinicians, resulting in decreased inappropriate use of antimicrobials or a formulary restriction and preauthorization to help reduce antimicrobial usage and related cost.
Supplemental elements may be considered and prioritized as to the core antimicrobial stewardship strategies based on local practice pattern and resources.11 Factors to consider include education, which is considered to be an essential element of the ASP. Although education is important, it alone is only somewhat effective in changing clinicians’ prescribing practices. Guidelines and clinical pathways are elements set forth in institutional management protocols for common and potentially serious infections such as intravascular catheter-related infections, hospital- and community-acquired pneumonia, bloodstream infections, and complicated urinary tract infections among other types.
Another consideration is antimicrobial cycling. This process refers to the specific schedule of alternating specific antimicrobials or antimicrobial classes to prevent or reverse the development of antimicrobial resistance. Insufficient data on antimicrobial cycling currently exist to affect major changes in practice. This element, however, could be implemented in certain institutions if needed based on the reported bacterial resistance pattern.
Antimicrobial order forms can be used to help monitor the implementation of formulated institutional clinical practice pathways. However, the authors feel that documenting this indication in the clinician notes may be adequate and save time for everyone involved; additionally, reviewing combination therapy, which if avoided, may prevent the emergence of resistance. Although combination therapy is needed in certain clinical diagnostic situations, careful consideration of its use is essential.
Streamlining or de-escalation of therapy by using a narrower spectrum agent, based on culture and sensitivity results, prevents duplicative therapy with a patient when double coverage is not indicated or intended. Another goal is the discontinuation of therapy based on negative culture results and lack of supporting clinical signs and symptoms of infection. Dose optimization and adjustment should also be reviewed. Using the appropriate antimicrobial dose based on the specific pathogen, patient characteristic, source of infection, along with the pharmacokinetic and pharmacodynamics should be reviewed to prevent antimicrobial overuse and subsequent potentially avoidable adverse effects.
Parenteral to oral conversion from IV to oral administration (IV to oral) antimicrobials must be considered when the patient is clinically and hemodynamically stable, thus limiting the length of hospital stays and health care costs. However, it is important to keep in mind pharmacokinetic studies examining the bioavailability of antibiotics are usually conducted with healthy volunteers. Therefore, when treating patients who are elderly, on multiple medications, or severely ill, proper usage of these antibiotics is required. Also, having antibiotics with excellent bioavailability does not necessarily mean switching from IV to oral routes when treating serious infections such as bacteremia. Special consideration should be given when changing the route of administration. In addition, approval—or at least notification by the treating physician or ID specialist—should be included in the absence of an institutional policy, allowing for automatic IV to oral conversion.
The ASP Team
The participation of specific clinicians has been suggested as key to having a successful ASP team.12 Members should include an ID physician (director) who serves as the lead physician and supervises the overall function of the ASP, makes recommendations to the ASP team, and contributes to the educational activities. A clinical ID pharmacist (codirector) provides suggestions to clinicians on preferred first-line antimicrobials and reviews medication orders for antimicrobials and resistance patterns, microbiological data, patient data, and clinical information. The codirector also tracks any ASP-related data and submits monitoring reports on a regular basis.
If accessible, an IC professional should participate, implementing and monitoring prevention strategies that decrease health care-associated infections. These infections play a significant role in reducing MDROs and decreasing the use of antibiotics. Additionally, the IC professional can assist in the early identification of patients with MDROs, aid patient placement on transmission-based precautions, and flag a patient in the medical record for hightened awareness. Also, IC professionals promote hand hygiene, standard precautions, and contribute to infection prevention strategies, such as hospital bundle practices, to prevent catheter-associated bloodstream infections and ventilator-associated pneumonias, among others.
If possible, a microbiologist who can prepare culture and susceptibility data to optimize antimicrobial management and conduct timely documentation of microbial pathogens should be a member of the team. Microbiologists can report organism susceptibility, assist in the surveillance of specific organisms, and provide early identification of patients with MDROs that require transmission-based precautions. The microbiologist can perform a semiannual update of a local antibiogram while reporting antimicrobial susceptibility profiles. Based on the information gathered, microbiologists can provide new drug panels to the members of the ASP, who will decide which antibiotic panel will be used. Another possible member of the ASP team is a program analyst who provides data retrieval, performs data analysis, and delivers necessary reports to the team.
It is the responsibility of medical staff to review and implement suggestions made by the ASP when appropriate. However, these suggestions are not considered a substitute for clinical decisions, and discretion is required when treating individual patients. The VHA, in response to the IDSA/SHEA published guidelines, chartered an antimicrobial stewardship task force in May 2011 with the sole purpose “To optimize the care of Veterans by developing, deploying and monitoring a national-level strategic plan for improvements in antimicrobial therapy management.”1 In 2011, the Office of Inspector General in a combined assessment program summary report for management of MDROs in VHA facilities recommended that “the Under Secretary for Health, in conjunction with VISN and facility senior managers, ensures that facilities develop policies and programs that control and reduce antimicrobial agent use.”13
In 2012, the VHA conducted a survey to obtain baseline data regarding ASP activities, presence of dedicated personnel, current related practice policies, available resources, and outcomes. There were 140 voluntary participating VA facilities, of which 130 had inpatient services. The survey found that 26 facilities (20%) did not have an attending ID physician, 49 facilities (38%) reported having an ASP, 19 facilities (15%) had developed policy in place addressing de-escalation of antimicrobials, 87 facilities (67%) had not developed a business plan for an ASP, and 61 facilities (47%) had completed a medication usage evaluation.14 Feedback following the analysis of the survey data recommended integrating more ID personnel as needed, along with the development of ASP teams for all facilities with inpatient services, who would have the authority to change the antimicrobial therapy selection and have policies in place related to ASP principles.
Conclusions
Increased MDROs and decreased anti-infective development requires stricter management of antibiotics. An ASP is essential in any hospital or health care facility to decrease the incidence of resistance and improve patient care. The ASP is a collaborative effort that involves multiple specialties and departments. A successful ASP is one that changes based on local prescribing trends and resistance patterns while focusing on a patient as an individual.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs, Veterans Health Administration. Antimicrobial Stewardship Programs (ASP). VHA Directive 1031. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Updated January 22, 2014. Accessed August 4, 2015.
2. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Published April 23, 2013. Accessed August 4, 2015.
3. Pyrek K. Bugs without borders: the global challenge of MDROs. Infect Control Today. 2013;17(2):1-8.
4. Pasquale T, Trienski TL, Olexia DE, et al. Impact of an antimicrobial stewardship program on patients with acute bacterial skin and skin structure infections. Am J Health Syst Pharm. 2014;71(13):1136-1139.
5. D’Agata EM. Antimicrobial use and stewardship programs among dialysis centers. Semin Dial. 2013;26(4):457-464.
6. Zvonar R, Natarajan S, Edwards C, Roth V. Assessment of vancomycin use in chronic hemodialysis patients: room for improvement. Nephrol Dial Transplant. 2008;23(11):3690-3695.
7. Snyder, GM, Patel PR, Kallen AJ, Strom JA, Tucker JK, D’Agata EM. Antimicrobial use in outpatient hemodialysis units. Infect Control Hosp Epidemiol. 2013;34(4):349-357.
8. Rosa RG, Goldani LZ, dos Santos RP. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febril neutropaenia: a prospective cohort study. BMC Infect Dis. 2014;14:286.
9. Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm. 2012;69(17):1500-1508.
10. Doron S, Nadkarni L, Lyn Price L, et al. A nationwide survey of antimicrobial stewardship practices. Clin Ther. 2013;35(6):758-765.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10(1):63-73.
13. U.S. Department of Veterans Affairs Office of Inspector General. Combined Assessment Program Summary Report: Management of Multidrug-Resistant Organisms in Veterans Health Administration Facilities. Report No. 11-02870-04. U.S. Department of Veterans Affairs Website. http://www.va.gov/oig/pubs/VAOIG-11-02870-04.pdf. Updated October 14, 2011. Accessed August 4, 2015.
14. Roselle GA, Neuhauser M, Kelly A, Vandenberg P. 2012 Survey of antimicrobial stewardship in VA. Washington, DC: Department of Veterans Affairs; 2013.
1. U.S. Department of Veterans Affairs, Veterans Health Administration. Antimicrobial Stewardship Programs (ASP). VHA Directive 1031. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Updated January 22, 2014. Accessed August 4, 2015.
2. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Published April 23, 2013. Accessed August 4, 2015.
3. Pyrek K. Bugs without borders: the global challenge of MDROs. Infect Control Today. 2013;17(2):1-8.
4. Pasquale T, Trienski TL, Olexia DE, et al. Impact of an antimicrobial stewardship program on patients with acute bacterial skin and skin structure infections. Am J Health Syst Pharm. 2014;71(13):1136-1139.
5. D’Agata EM. Antimicrobial use and stewardship programs among dialysis centers. Semin Dial. 2013;26(4):457-464.
6. Zvonar R, Natarajan S, Edwards C, Roth V. Assessment of vancomycin use in chronic hemodialysis patients: room for improvement. Nephrol Dial Transplant. 2008;23(11):3690-3695.
7. Snyder, GM, Patel PR, Kallen AJ, Strom JA, Tucker JK, D’Agata EM. Antimicrobial use in outpatient hemodialysis units. Infect Control Hosp Epidemiol. 2013;34(4):349-357.
8. Rosa RG, Goldani LZ, dos Santos RP. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febril neutropaenia: a prospective cohort study. BMC Infect Dis. 2014;14:286.
9. Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm. 2012;69(17):1500-1508.
10. Doron S, Nadkarni L, Lyn Price L, et al. A nationwide survey of antimicrobial stewardship practices. Clin Ther. 2013;35(6):758-765.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10(1):63-73.
13. U.S. Department of Veterans Affairs Office of Inspector General. Combined Assessment Program Summary Report: Management of Multidrug-Resistant Organisms in Veterans Health Administration Facilities. Report No. 11-02870-04. U.S. Department of Veterans Affairs Website. http://www.va.gov/oig/pubs/VAOIG-11-02870-04.pdf. Updated October 14, 2011. Accessed August 4, 2015.
14. Roselle GA, Neuhauser M, Kelly A, Vandenberg P. 2012 Survey of antimicrobial stewardship in VA. Washington, DC: Department of Veterans Affairs; 2013.
Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Musculoskeletal pain affects one-quarter of the adult population and is the most common reason for self-medication and for seeking health care.1-3 It is also cited as the most common reason for the use of complementary and alternative medicine (CAM), and the lower back, head, neck, and knee are the most commonly reported areas of pain.4-8 In 2007, the estimated annual cost of managing chronic pain, adjusted for inflation, ranged from $560 to $635 billion; whereas the direct out-of-pocket cost for patients with back pain was $34 billion.9 Chronic pain persists beyond the usual course of disease or healing; generally about 3 months or longer.10-12 The most common forms of pain include those associated with musculoskeletal disorders, such as degenerative arthritis, rheumatoid arthritis, osteoarthritis, myofascial pain, chronic headache, low back pain, and bone pain.11,13-15
A large number of returning Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) veterans have reported chronic pain symptoms, with back and head being the most common pain locations.7 They experienced pain related to wearing heavy gear every day, being transported in armored vehicles over crater-ridden roads, and enduring less than optimal sleeping conditions.16 Studies have found a significant number of subjects in this population who reported using CAM therapy. The OIF/OEF veterans were likely to have outpatient visits for musculoskeletal system disorders and to use CAM as an additional tool in pain management—not as a reaction to perceived inadequacies of conventional care.17,18
Complementary and alternative medicine is often used to describe various substances, procedures, and approaches outside of mainstream, Western, or conventional medicine for health promotion, treating injuries, symptoms, and illnesses.18,19 Although complementary and alternative are often used interchangeably, the 2 terms refer to different concepts. Complementary refers to the use of a nonmainstream approach with conventional medicine, whereas alternative refers to the use of a nonmainstream approach instead of conventional medicine.19 About 40% of Americans use CAM for various reasons.19
The services and self-care forms of CAM account for a large portion of out-of-pocket costs; patients are willing to pay for it themselves. In 2007, the U.S. spent $33.9 billion on out-of-pocket expenses for CAM classes, products, materials, and visits to CAM providers.20 The costs are comparable with those of conventional health care services and prescription drug use.20 One national study concluded that many patients use CAM in accordance with their beliefs, values, and philosophy concerning health and life.21 Other studies found that many patients use CAM not only because of functional status, pain severity, or self-efficacy, but also because they perceive significant benefits in pain relief.6,17,22-25 Some authors reported that CAM is used to augment and not replace conventional medicine and that it has now become part of the accepted armamentarium for managing chronic musculoskeletal pain.6,17,25
The National Center for Complementary and Alternative Medicine at the National Institutes of Health (NIH) classifies CAM in 2 ways: (1) Mind and body practices, such as acupuncture, massage therapy, meditation, movement therapies, relaxation techniques, spinal manipulation, tai chi and qi gong, yoga, healing touch, and hypnotherapy; and (2) natural products, including probiotics, herbs, and vitamins and minerals usually sold as dietary supplements.19 These products are regulated by the FDA but not as drugs. They have a different set of regulations under the Dietary Supplement Health and Education Act of 1994.26
Mind and body practices or provider-based CAM therapies such as chiropractic care, acupuncture, and massage increased significantly between 2002 and 2007, and many more patients may be willing to try these therapies for chronic low back pain if they do not have to pay out of pocket.27,28 Multiple studies have also found that these treatments in addition to herbal medicine are the most commonly reported CAM treatments used for pain relief in adults.3,17,22,23
Other commonly reported CAM therapies are garlic preparations, exercise, and yoga and meditation.22,23 A large number of veterans have reported previous use or willingness to try chiropractic care, massage therapy, herbal medicines, and acupuncture for chronic noncancer pain.17 In addition to acute care with conventional treatment, the VHA has now expanded services to allow for CAM as available treatment options for chronic musculoskeletal pain.29 The majority of VHA facilities also provide and refer patients to CAM service providers.30
This review article explores the evidence supporting the use of the most commonly reported CAM therapies; specifically acupuncture, massage therapy, and spinal manipulation for musculoskeletal pain relief. Because of the plethora of herbs and dietary supplements in the literature, these were not included in this review, although they are also reported among the most commonly used CAM therapies.1,23,31 The investigators sought to examine the effectiveness of acupuncture, spinal manipulation, or massage compared with no treatment, sham therapy, or current noninvasive first-line treatment for chronic musculoskeletal pain.
Study Selection
To find research addressing this question, the authors searched the PubMed, MEDLINE, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases and the Cochrane Library for all relevant studies published between October 31, 2003, and October 31, 2013. The combined search from all sources for randomized controlled trials (RCTs) resulted in 1,157 studies with acupuncture and chronic pain, 343 studies with spinal manipulation and chronic pain, and 416 studies with massage and chronic pain. Acupuncture and chronic musculoskeletal pain yielded 94 studies, spinal manipulation and chronic musculoskeletal pain yielded 29 studies, and massage and chronic musculoskeletal pain yielded 55 RCTs.
Targeted searches were then conducted within the results for systematic reviews and meta-analysis of relevant studies of RCTs, focus on adults with any type of musculoskeletal pain, written in English, and had pain level or level of pain-related improvement as its primary outcome. The results were assessed for relevance to the review based on the information provided in the title, abstract, and the National Library of Medicine Medical Subject Headings. References of the search results were also searched manually for additional studies relevant to the review. Duplicated studies and those that looked at only acute or cancer pain were excluded. Thirteen systematic reviews and meta-analyses met the inclusion criteria (Table). The investigators reviewed the full reports and agreed to use the data that were abstracted from the studies.
Study Parameters
Four different categories of outcome measuring points for posttreatment follow-up are reported in the CAM studies: immediate, short-term, intermediate, and long-term. There are inconsistencies across studies for the timing of these 4 categories. Immediate posttreatment is defined as up to 1 day.8,32-34 The duration for the short-term follow-up period is defined as between 1 day and 3 months8,32,33; ≤ 3 months35,36; closest to 3 weeks37; closest to 4 weeks34; 1 month38; closest to 8 weeks, but < 3 months after randomization39; or up to 25 weeks, but nearest to 12 weeks.40Intermediate follow-up is between 3 months and 1 year8,33,35; between 3 and 6 months38; ≥ 3 months, but < 1 year36; or closest to 6 months.34Long term is defined as >12 months8,35; closest to 6 months37; 12 months38; 1 year or more36; closest to 6 months, but >3 months after randomization34,39; or between 26 weeks and 56 weeks.40
Pain intensity and pain relief was the treatment efficacy outcome for all the studies. A variety of measuring tools were reported across studies. Eight of the 13 studies reported measurement of pain intensity using the visual analog scale (VAS).8,33,35-37,41-43 In addition to the VAS, 2 studies also used the numerical rating scale (NRS).8,36 One study used the NRS alone.38 Other studies used the McGill Pain Questionnaire35; the SF-36 bodily pain dimension, Von Korff chronic pain grading scale, or low back pain rating scale36; or the Western Ontario and McMaster Universities Osteoarthritis Index subscale for pain.39,40,43
Authors from 8 of the systematic reviews and meta-analysis reported levels of evidence, or GRADE (Grades of Recommendation, Assessment, Development, and Evaluation), used to evaluate the overall quality of the evidence and the strength of the recommendations.8,32,34-36,38,42,43 Levels of evidence were based on RCTs. The various levels were (1) “strong evidence,” consistent findings in multiple high-quality RCTs; (2) “moderate evidence,” consistent findings among multiple high-quality RTCs and/or 1 high-quality RCT; (3) “limited evidence,” low-quality RCT; (4) “conflicting evidence,” inconsistent findings among multiple RCTs; and (5) “no evidence,” no RCTs or no studies.8,36
Most studies expressed the overall strength of the body of literature in 6 different categories: (1) “high quality,” confidence that the evidence reflected the true effect and that further research is very unlikely to change confidence in the effect of size; (2) “moderate quality,” further research is likely to have an impact on confidence in the estimate of effect and may change the estimate; (3) “low quality,” further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change it; (4) “very low quality,” great uncertainty about the estimate; (5) “insufficient evidence,” either the evidence is unavailable or does not allow for a conclusion; and (6)“no evidence,” no evidence from RTCs.32,34,35,38,42,43 Kwon and colleagues reported using a modified jaded score where a total of 5 points was awarded if a study was described as randomized, used an appropriate method, if subjects were blinded to the intervention, if the evaluator was blinded to the intervention, and if there was a description of withdrawals and dropouts.43
Acupuncture
About 3 million American adults receive acupuncture each year.44 The most commonly reported reason for its use is chronic pain.44,45 Trials that examined the characteristics of those seeking and using acupuncture as adjunct to conventional treatment have found that patients who experienced positive outcomes, such as improvement in pain subscale, included females, previous failure of other therapies, and prior positive acupuncture encounters.46
Six of the studies in this review examined the evidence of acupuncture for chronic low back pain.35-38,41,42 Two of those studies found moderate evidence that acupuncture was more effective than no treatment for short-term pain relief.35,36 Manheimer and colleagues found it to be significantly more effective than no additional treatment or sham treatment for short-term pain relief.37 They however, reported a lack of evidence to suggest that it was more effective than were other active therapies.37 Hutchinson and colleagues did not differentiate among data points for intermediate, short-term, or long-term follow-up in their study.41 However, they concluded that there was some evidence to support acupuncture as more effective than no treatment and conflicting evidence of its effectiveness over other treatment modalities. Different levels of evidence were reported for intermediate pain relief with 2 of the other studies. One study found that the evidence was limited.35 The other study reported conflicting evidence that it was more effective than no treatment for immediate pain relief for those with chronic low back pain.36
Rubinstein and colleagues reported low- to very-low-quality evidence that acupuncture provided a short-term clinically relevant effect compared with waiting list control or when it was added to another intervention for chronic low back pain.38 Standaert and colleagues concluded that there was insufficient evidence to determine the relative effectiveness of acupuncture compared with either exercise or spinal manipulative therapy (SMT) in relieving chronic low back pain.42 Yuan and colleagues reported strong evidence that acupuncture combined with conventional therapy was more effective than conventional therapy alone.36
Furlan and colleagues found moderate evidence for significant improvement in pain intensity compared with subjects in physical therapy or usual care groups at short-term or immediate follow-up for chronic back pain.35 Studies that evaluated the efficacy of acupuncture for knee osteoarthritis compared acupuncture with sham acupuncture controls or no additional treatment and found that acupuncture was significantly better at relieving knee pain.39,40,43 Cao and colleagues found it to be effective both in the short term and long term.39 White and colleagues and Kwon and colleagues were unable to draw a conclusion concerning long-term effects due to the data point included in the study or the heterogeneity in the results.40,43
Trinh and colleagues reported moderate evidence that acupuncture is more effective for relief of chronic neck pain compared with inactive, sham treatments at immediate posttreatment.8 They also found moderate evidence that acupuncture was more effective than some other types of sham controls immediately posttreatment and limited evidence that it was more effective than massage at short-term follow-up.8 Furlan and colleagues found trials that applied sham acupuncture tended to produce nonstatistically significant results.35 Their meta-analysis of 2 studies indicated no significant difference between acupuncture and sham acupuncture for immediate posttreatment pain intensity. They also reported inconsistent results for the effects of acupuncture compared with medication or with spinal manipulation for chronic neck pain.35
Massage
Massage promotes health and well-being through the use of mechanical manipulation of body tissues with rhythmic pressure and stroking.47 Treatment techniques include Hoffa massage, friction massage, connective tissue massage, transverse friction massage, and trigger point massage.48 Massage is one of the most popular CAM therapies for neck and back pain.49 In their survey, White and colleagues reported that active-duty military personnel listed massage as the most frequently used CAM therapy in the previous 12 months.18
Patel and colleagues reported that the overall methodology of the trials assessed in their study was either low- or very-low-GRADE level.32 They found very-low to low-quality evidence that there is no difference in effectiveness of 3 approaches of massage therapies (ischemic compression to upper fibre of trapezius trigger point, transverse friction massage to upper fibre of trapezius, and ischemic compression to upper fibre of trapezius) for neck muscle pain. They also reported no difference between conventional Western massage and acupuncture for generalized neck muscle pain at short-term follow-up, and no difference in pain intensity compared with other therapies such as acupuncture, manual therapy, exercise, education, and multimodal interventions. The investigators concluded that the effectiveness of massage therapy for improving neck pain remains unclear, as results could not be combined due to the wide range of techniques and comparative treatments. They were unable to make any firm statement to guide clinical practice.32
Two other studies compared massage to no treatment and found it significantly improved chronic neck pain immediately after the end of treatment.33,35 Kong and colleagues also found similar effects for shoulder pain at immediate and short-term
follow-up but not for neck or shoulder pain when massage was compared with active therapies.33 Furlan and colleagues’ meta-analysis found that massage compared with relaxation or physical therapy was significantly better at reducing chronic nonspecific low back pain immediately after treatment.35
Spinal Manipultaion
Spinal manipulation is high-velocity and low-amplitude localized force directed at specific spinal segments.34 It is performed by using the hands or a device to apply a controlled force to a joint of the spine and is practiced by osteopathic physicians, naturopathic physicians, chiropractors, physical therapists, and some medical doctors.19
In a study to assess its effectiveness, Rubinstein and colleagues found low-quality evidence to very-low-quality evidence to suggest that SMT does not provide a more clinically beneficial effect compared with sham, passive modalities, or other interventions for the relief of chronic low back pain.38 Comparative interventions included usual medical care, physical therapy, exercise, physiotherapy, and multimodal treatments. Standaert and colleagues also found no difference between motor control exercise and SMT in pain relief.42 They concluded that although the evidence is low, there is an indication that structured exercise and SMT seem to offer equivalent benefits in terms of pain for those with chronic lower back pain with clinical benefits evident within 8 weeks of care.42
Gross and colleagues found that when cervical manipulation was compared with control for chronic mechanical neck pain, there was moderate-quality evidence for similar effects at short-term and intermediate follow-up.34 They also reported low-quality evidence in support of thoracic manipulation alone or in combination with electrothermal or individualized physiotherapy and suggested cervical manipulation may provide short-term but not long-term pain relief.34 Furlan and colleagues reported moderate-quality evidence that spinal manipulation provided significantly better posttreatment neck pain relief compared with placebo.35 They also found low evidence that it was significantly better than placebo, acupuncture, and pain medication at immediate follow-up.35
Conclusion
Considerable effort was made to retrieve all studies; however, the authors cannot be certain that the review was exhaustive. They also relied on other analyses of primary studies for the conclusion.
The 3 types of musculoskeletal pain in the review were low back, neck, and knee pain related to osteoarthritis. The authors found that the most common CAM modality studied for chronic musculoskeletal pain was acupuncture. Studies on massage therapy and SMT that were relevant to the review were limited.
Two studies reported strong level of evidence for acupuncture.36,40 One study reported that acupuncture was superior to no treatment or to sham acupuncture for relief of chronic knee pain.40 The other study reported that acupuncture was more effective than conventional therapy alone when it was combined with conventional therapy for chronic low back pain, but there was no difference when compared with sham acupuncture for short-term pain relief.36 The strength of the evidence for acupuncture combined with conventional treatment for low back pain was conflicting. One other review found low evidence for its benefit. Similar to Hopton and MacPherson, this review found that acupuncture treatment seemed to provide effective short-term relief of chronic low back pain.14 Evidence would also seem to support acupuncture for the short-term relief of chronic neck pain and knee pain associated with osteoarthritis.
This review also found immediate and short-term benefits, although mostly with weak evidence, for the use of SMT in the treatment of chronic neck and low back pain. There was conflicting evidence for the support of massage therapy. Furlan and colleagues, however, found that acupuncture, SMT, and massage treatments were significantly more efficacious than no treatment, placebo, physical therapy, or usual care in reducing pain immediately or at short-term after treatment.35 Inconsistencies may be related to the methodologic and clinical diversity of RCTs, which limit the extent of quantitative synthesis and complicates result interpretation.35 Also, better conclusions could be drawn if future studies use head-to-head comparisons of CAM treatments and trials comparing CAM to widely used active treatments that report on all clinically relevant outcomes.35
Although the relationship between conventional treatment and the world of CAM remains equivocal, review of the evidence suggests acupuncture and SMT may be effective treatment for various chronic painful musculoskeletal conditions.35,44,50,51 These CAM modalities are reasonable referral options to supplement conventional therapy for the treatment of chronic musculoskeletal pain when conventional therapy has not yielded satisfactory results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Callahan LF, Wiley-Exley EK, Mielenz TJ, et al. Use of complementary and alternative medicine among patients with arthritis. Prev Chronic Dis. 2009;6(2):A44.
2. Walsh NE, Brooks P, Hazes JM, et al; Bone and Joint Decade Task Force for Standards of Care for Acute and Chronic Musculoskeletal Pain. Standards of care for acute and chronic musculoskeletal pain: the Bone and Joint Decade (2000-2010). Arch Phys Med Rehabil. 2008;89(9):1830-1845.
3. Williamson AT, Fletcher PC, Dawson KA. Complementary and alternative medicine. Use in an older population. J Gerontol Nurs. 2003;29(5):20-28.
4. Artus M, Croft P, Lewis M. The use of CAM and conventional treatments among primary care consulters with chronic musculoskeletal pain. BMC Fam Pract. 2007;8:26.
5. Cherkin DC, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized controlled trial. Ann Intern Med. 2011;155(1):1-9.
6. Fleming S, Rabago DP, Mundt MP, Fleming MF. CAM therapies among primary care patients using opioid therapy for chronic pain. BMC Complement Altern Med. 2007;7:15.
7. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent post concussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
8. Trinh K, Graham N, Gross A, et al. Acupuncture for neck disorders. Spine (Phila PA 1976). 2007;32(2):236-243.
9. Gaskin DJ, Richard P. Appendix C: The economic costs of pain in the United States. In: Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. National Center for Biotechnology Information Website. http://www. ncbi.nlm.nih.gov/books/NBK92521. Accessed July 31, 2015.
10. American Academy of Pain Medicine. AAPM facts and figures on pain. American Academy of Pain Medicine Website. http://www.painmed.org/files/facts-and-figures-on-pain.pdf. Accessed July 31, 2015.
11. Rosenquist RW, Vrooman MD. Chronic pain management. In: Butterworth JF, Mackey DC, Wasnick JD, eds. Morgan & Mikhail’s Clinical Anesthesiology. 5th ed. New York, NY: McGraw-Hill; 2013:chap 47.
12. Rasu RS, Sohraby R, Cunningham L, Knell ME. Assessing chronic pain treatment practices and evaluating adherence to chronic pain clinical guidelines in outpatient practices in the United States. J Pain. 2013;14(6):568-578.
13. Gureje O, Von Korff M, Simon, GE, Grater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280(2):147-151.
14. Hopton A, MacPherson H. Acupuncture for chronic pain: is acupuncture more than an effective placebo? A systematic review of pooled data from meta-analyses. Pain Pract. 2010;10(2):94-102.
15. Kumar N. WHO Normative guidelines on pain management. World Health Organization Website. http://www.who.int/medicines/areas/quality_safety/delphi_study_pain_guidelines.pdf. Published June 2007. Accessed August 3, 2015.
16. Koffman RL. Downrange acupuncture. Med Acupunct. 2011;23(4):215-218.
17. Denneson LM, Corson K, Dobscha SK. Complementary and alternative medicine use among veterans with chronic noncancer pain. J Rehabil Res Dev. 2011;(48)9:1119-1128.
18. White MR, Jacobson IG, Smith B, et al; Millennium Cohort Study Team. Health care utilization among complementary and alternative medicine users in a large military cohort. BMC Complement Alternat Med. 2011;11:27.
19. U.S. Department of Health and Human Services, National Institutes of Health, National Center for Complementary and Alternative Medicine. Complementary, alternative, or integrative health: What’s in a name? National Center for Complementary and Alternative Medicine Website. http://nccam.nih.gov/health/whatiscam. Updated March 2015. Modified July 8, 2015. Accessed August 4, 2015.
20. Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;30(18):1-14.
21. Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279(19):1548-1553.
22. Rosenberg EI, Genao I, Chen I, et al. Complementary and alternative medicine use in primary care patients with chronic pain. Pain Med. 2008;9(8):1065-1072.
23. Alaaeddine N, Okais J, Ballane L, Baddoura RM. Use of complementary and alternative therapy among patients with rheumatoid arthritis and osteoarthritis. J Clin Nurs. 2012;21(21-22):3198-3204.
24. Tan MG, Win MT, Khan SA. The use of complementary and alternative medicine in chronic pain patients in Singapore: a single-centre study. Ann Acad Med Singapore. 2013;42(3):133-137.
25. White P. A background to acupuncture and its use in chronic painful musculoskeletal conditions. J R Soc Promot Health. 2006;126(5):219-227.
26. U.S. Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Health and Education Act of 1994. National Institutes of Health Website. http://ods.od.nih.gov/About/DSHEA_Wording.aspx. Published October 25, 1994. Accessed August 4, 2015.
27. Su D, Li L. Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J Health Care Poor Underserved. 2011;22(1):296-310.
28. Sherman KJ, Cherkin DC, Connelly MT, et al. Complementary and alternative medical therapies for chronic low back pain: what treatments are patients willing to try? BMC Complement Alternat Med. 2004;4:9.
29. Smeeding SJ, Bradshaw DH, Kumpfer KL, Trevithick S, Stoddard GJ. Outcome evaluation of the Veterans Affairs Salt Lake City Integrative Health Clinic for Chronic Nonmalignant Pain. Clin J Pain. 2011;27(2):146-155.
30. U.S. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2011 complementary and alternative medicine. U.S. Department of Veterans Affairs Office of Research and Development Website. http://www.research.va.gov/research_topics/2011cam_finalreport.pdf. Published September 2011. Accessed August 4, 2015.
31. Unsal A, Gözüm S. Use of complementary and alternative medicine by patients with arthritis. J Clin Nurs. 2010;19(7-8):1129-1138.
32. Patel KC, Gross A, Graham N, et al. Massage for mechanical neck disorder. Cochrane Database Syst Rev. 2012;9:CD004871.
33. Kong LJ, Zhan HS, Cheng YW, Yuan WA, Chen B, Fang M. Massage therapy for neck and shoulder pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:613279.
34. Gross A, Miller J, D’Sylva J, et al; COG. Manipulation or mobilisation for neck pain: a Cochrane review. Man Ther. 2010;15(4):315-333.
35. Furlan A, Yazdi F, Tsertsvadze A, et al. Complementary and alternative therapies for back Pain II. Evidence report/technology assessment No. 194. (Prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-2007-10059-I (EPCIII). AHRQ Publication No. 10(11)E007. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
36. Yuan J, Purepong N, Kerr DP, Park J, Bradbury I, McDonough S. Effectiveness of acupuncture for low back pain. Spine (Phila PA 1976). 2008;33(23):E887-E900.
37. Manheimer E, White A, Berman B, Forys K, Ernst E. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142(8):651-663.
38. Rubinstein SM, van Middelkoop M, Kuijpers T, et al. A systematic review on the effectiveness of complementary and alternative medicine for chronic non-specific low-back pain. Eur Spine J. 2010;19(8):1213-1228.
39. Cao L, Zhang XL, Gao YS, Jiang Y. Needle acupuncture for osteoarthritis of the knee. A systematic review and updated meta-analysis. Saudi Med J. 2012;33(5):526-532.
40. White A, Foster NE, Cummings M, Barlas P. Acupuncture treatment for chronic knee pain: a systematic review. Rheumatology (Oxford). 2007;46(3):384-390.
41. Hutchinson AJ, Ball S, Andrews JC, Jones GG. The effectiveness of acupuncture in treating chronic non-specific low back pain: a systematic review of the literature. J Orthop Surg Res. 2012;7:36.
42. Standaert, CJ, Friedly J, Erwin MW, et al. Comparative effectiveness of exercise, acupuncture, and spinal manipulation for low back pain. Spine (Phila PA 1976). 2011;36(21 suppl):S120-S130.
43. Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2006;45(11):1331-1337.
44. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Individual patient data meta-analysis of acupuncture for chronic pain: protocol of the Acupuncture Trialists’ Collaboration. Trials. 2010;11:90.
45. Kelly RB. Acupuncture for pain. Am Fam Physician. 2009;80(5):481-484.
46. Witt CM, Schützler L, Lüdtke R, Wegscheider K, Willich SN. Patient characteristics and variation in treatment outcomes: which patients benefit most from acupuncture for chronic pain? Clin J Pain. 2011;27(6):550-555.
47. Cafarelli E, Flint F. The role of massage in preparation for and recovery from exercise: an overview. Sports Med. 1992;14(1):1-9.
48. Prentice WE. Therapeutic massage. In: Prentice WE. Therapeutic Modalities in Rehabilitation. 4th ed. New York, NY: McGraw-Hill; 2011:chap 16.
49. Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS. Patterns and perceptions of care for treatment of back and neck pain: results of a national survey. Spine (Phila PA 1976). 2003;28(3):292-297.
50. Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538.
51. Tsao JCI. Effectiveness of massage therapy for chronic, non-malignant pain: a review. Evid Based Complement Alternat Med. 2007;4(2):165-179.
Musculoskeletal pain affects one-quarter of the adult population and is the most common reason for self-medication and for seeking health care.1-3 It is also cited as the most common reason for the use of complementary and alternative medicine (CAM), and the lower back, head, neck, and knee are the most commonly reported areas of pain.4-8 In 2007, the estimated annual cost of managing chronic pain, adjusted for inflation, ranged from $560 to $635 billion; whereas the direct out-of-pocket cost for patients with back pain was $34 billion.9 Chronic pain persists beyond the usual course of disease or healing; generally about 3 months or longer.10-12 The most common forms of pain include those associated with musculoskeletal disorders, such as degenerative arthritis, rheumatoid arthritis, osteoarthritis, myofascial pain, chronic headache, low back pain, and bone pain.11,13-15
A large number of returning Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) veterans have reported chronic pain symptoms, with back and head being the most common pain locations.7 They experienced pain related to wearing heavy gear every day, being transported in armored vehicles over crater-ridden roads, and enduring less than optimal sleeping conditions.16 Studies have found a significant number of subjects in this population who reported using CAM therapy. The OIF/OEF veterans were likely to have outpatient visits for musculoskeletal system disorders and to use CAM as an additional tool in pain management—not as a reaction to perceived inadequacies of conventional care.17,18
Complementary and alternative medicine is often used to describe various substances, procedures, and approaches outside of mainstream, Western, or conventional medicine for health promotion, treating injuries, symptoms, and illnesses.18,19 Although complementary and alternative are often used interchangeably, the 2 terms refer to different concepts. Complementary refers to the use of a nonmainstream approach with conventional medicine, whereas alternative refers to the use of a nonmainstream approach instead of conventional medicine.19 About 40% of Americans use CAM for various reasons.19
The services and self-care forms of CAM account for a large portion of out-of-pocket costs; patients are willing to pay for it themselves. In 2007, the U.S. spent $33.9 billion on out-of-pocket expenses for CAM classes, products, materials, and visits to CAM providers.20 The costs are comparable with those of conventional health care services and prescription drug use.20 One national study concluded that many patients use CAM in accordance with their beliefs, values, and philosophy concerning health and life.21 Other studies found that many patients use CAM not only because of functional status, pain severity, or self-efficacy, but also because they perceive significant benefits in pain relief.6,17,22-25 Some authors reported that CAM is used to augment and not replace conventional medicine and that it has now become part of the accepted armamentarium for managing chronic musculoskeletal pain.6,17,25
The National Center for Complementary and Alternative Medicine at the National Institutes of Health (NIH) classifies CAM in 2 ways: (1) Mind and body practices, such as acupuncture, massage therapy, meditation, movement therapies, relaxation techniques, spinal manipulation, tai chi and qi gong, yoga, healing touch, and hypnotherapy; and (2) natural products, including probiotics, herbs, and vitamins and minerals usually sold as dietary supplements.19 These products are regulated by the FDA but not as drugs. They have a different set of regulations under the Dietary Supplement Health and Education Act of 1994.26
Mind and body practices or provider-based CAM therapies such as chiropractic care, acupuncture, and massage increased significantly between 2002 and 2007, and many more patients may be willing to try these therapies for chronic low back pain if they do not have to pay out of pocket.27,28 Multiple studies have also found that these treatments in addition to herbal medicine are the most commonly reported CAM treatments used for pain relief in adults.3,17,22,23
Other commonly reported CAM therapies are garlic preparations, exercise, and yoga and meditation.22,23 A large number of veterans have reported previous use or willingness to try chiropractic care, massage therapy, herbal medicines, and acupuncture for chronic noncancer pain.17 In addition to acute care with conventional treatment, the VHA has now expanded services to allow for CAM as available treatment options for chronic musculoskeletal pain.29 The majority of VHA facilities also provide and refer patients to CAM service providers.30
This review article explores the evidence supporting the use of the most commonly reported CAM therapies; specifically acupuncture, massage therapy, and spinal manipulation for musculoskeletal pain relief. Because of the plethora of herbs and dietary supplements in the literature, these were not included in this review, although they are also reported among the most commonly used CAM therapies.1,23,31 The investigators sought to examine the effectiveness of acupuncture, spinal manipulation, or massage compared with no treatment, sham therapy, or current noninvasive first-line treatment for chronic musculoskeletal pain.
Study Selection
To find research addressing this question, the authors searched the PubMed, MEDLINE, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases and the Cochrane Library for all relevant studies published between October 31, 2003, and October 31, 2013. The combined search from all sources for randomized controlled trials (RCTs) resulted in 1,157 studies with acupuncture and chronic pain, 343 studies with spinal manipulation and chronic pain, and 416 studies with massage and chronic pain. Acupuncture and chronic musculoskeletal pain yielded 94 studies, spinal manipulation and chronic musculoskeletal pain yielded 29 studies, and massage and chronic musculoskeletal pain yielded 55 RCTs.
Targeted searches were then conducted within the results for systematic reviews and meta-analysis of relevant studies of RCTs, focus on adults with any type of musculoskeletal pain, written in English, and had pain level or level of pain-related improvement as its primary outcome. The results were assessed for relevance to the review based on the information provided in the title, abstract, and the National Library of Medicine Medical Subject Headings. References of the search results were also searched manually for additional studies relevant to the review. Duplicated studies and those that looked at only acute or cancer pain were excluded. Thirteen systematic reviews and meta-analyses met the inclusion criteria (Table). The investigators reviewed the full reports and agreed to use the data that were abstracted from the studies.
Study Parameters
Four different categories of outcome measuring points for posttreatment follow-up are reported in the CAM studies: immediate, short-term, intermediate, and long-term. There are inconsistencies across studies for the timing of these 4 categories. Immediate posttreatment is defined as up to 1 day.8,32-34 The duration for the short-term follow-up period is defined as between 1 day and 3 months8,32,33; ≤ 3 months35,36; closest to 3 weeks37; closest to 4 weeks34; 1 month38; closest to 8 weeks, but < 3 months after randomization39; or up to 25 weeks, but nearest to 12 weeks.40Intermediate follow-up is between 3 months and 1 year8,33,35; between 3 and 6 months38; ≥ 3 months, but < 1 year36; or closest to 6 months.34Long term is defined as >12 months8,35; closest to 6 months37; 12 months38; 1 year or more36; closest to 6 months, but >3 months after randomization34,39; or between 26 weeks and 56 weeks.40
Pain intensity and pain relief was the treatment efficacy outcome for all the studies. A variety of measuring tools were reported across studies. Eight of the 13 studies reported measurement of pain intensity using the visual analog scale (VAS).8,33,35-37,41-43 In addition to the VAS, 2 studies also used the numerical rating scale (NRS).8,36 One study used the NRS alone.38 Other studies used the McGill Pain Questionnaire35; the SF-36 bodily pain dimension, Von Korff chronic pain grading scale, or low back pain rating scale36; or the Western Ontario and McMaster Universities Osteoarthritis Index subscale for pain.39,40,43
Authors from 8 of the systematic reviews and meta-analysis reported levels of evidence, or GRADE (Grades of Recommendation, Assessment, Development, and Evaluation), used to evaluate the overall quality of the evidence and the strength of the recommendations.8,32,34-36,38,42,43 Levels of evidence were based on RCTs. The various levels were (1) “strong evidence,” consistent findings in multiple high-quality RCTs; (2) “moderate evidence,” consistent findings among multiple high-quality RTCs and/or 1 high-quality RCT; (3) “limited evidence,” low-quality RCT; (4) “conflicting evidence,” inconsistent findings among multiple RCTs; and (5) “no evidence,” no RCTs or no studies.8,36
Most studies expressed the overall strength of the body of literature in 6 different categories: (1) “high quality,” confidence that the evidence reflected the true effect and that further research is very unlikely to change confidence in the effect of size; (2) “moderate quality,” further research is likely to have an impact on confidence in the estimate of effect and may change the estimate; (3) “low quality,” further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change it; (4) “very low quality,” great uncertainty about the estimate; (5) “insufficient evidence,” either the evidence is unavailable or does not allow for a conclusion; and (6)“no evidence,” no evidence from RTCs.32,34,35,38,42,43 Kwon and colleagues reported using a modified jaded score where a total of 5 points was awarded if a study was described as randomized, used an appropriate method, if subjects were blinded to the intervention, if the evaluator was blinded to the intervention, and if there was a description of withdrawals and dropouts.43
Acupuncture
About 3 million American adults receive acupuncture each year.44 The most commonly reported reason for its use is chronic pain.44,45 Trials that examined the characteristics of those seeking and using acupuncture as adjunct to conventional treatment have found that patients who experienced positive outcomes, such as improvement in pain subscale, included females, previous failure of other therapies, and prior positive acupuncture encounters.46
Six of the studies in this review examined the evidence of acupuncture for chronic low back pain.35-38,41,42 Two of those studies found moderate evidence that acupuncture was more effective than no treatment for short-term pain relief.35,36 Manheimer and colleagues found it to be significantly more effective than no additional treatment or sham treatment for short-term pain relief.37 They however, reported a lack of evidence to suggest that it was more effective than were other active therapies.37 Hutchinson and colleagues did not differentiate among data points for intermediate, short-term, or long-term follow-up in their study.41 However, they concluded that there was some evidence to support acupuncture as more effective than no treatment and conflicting evidence of its effectiveness over other treatment modalities. Different levels of evidence were reported for intermediate pain relief with 2 of the other studies. One study found that the evidence was limited.35 The other study reported conflicting evidence that it was more effective than no treatment for immediate pain relief for those with chronic low back pain.36
Rubinstein and colleagues reported low- to very-low-quality evidence that acupuncture provided a short-term clinically relevant effect compared with waiting list control or when it was added to another intervention for chronic low back pain.38 Standaert and colleagues concluded that there was insufficient evidence to determine the relative effectiveness of acupuncture compared with either exercise or spinal manipulative therapy (SMT) in relieving chronic low back pain.42 Yuan and colleagues reported strong evidence that acupuncture combined with conventional therapy was more effective than conventional therapy alone.36
Furlan and colleagues found moderate evidence for significant improvement in pain intensity compared with subjects in physical therapy or usual care groups at short-term or immediate follow-up for chronic back pain.35 Studies that evaluated the efficacy of acupuncture for knee osteoarthritis compared acupuncture with sham acupuncture controls or no additional treatment and found that acupuncture was significantly better at relieving knee pain.39,40,43 Cao and colleagues found it to be effective both in the short term and long term.39 White and colleagues and Kwon and colleagues were unable to draw a conclusion concerning long-term effects due to the data point included in the study or the heterogeneity in the results.40,43
Trinh and colleagues reported moderate evidence that acupuncture is more effective for relief of chronic neck pain compared with inactive, sham treatments at immediate posttreatment.8 They also found moderate evidence that acupuncture was more effective than some other types of sham controls immediately posttreatment and limited evidence that it was more effective than massage at short-term follow-up.8 Furlan and colleagues found trials that applied sham acupuncture tended to produce nonstatistically significant results.35 Their meta-analysis of 2 studies indicated no significant difference between acupuncture and sham acupuncture for immediate posttreatment pain intensity. They also reported inconsistent results for the effects of acupuncture compared with medication or with spinal manipulation for chronic neck pain.35
Massage
Massage promotes health and well-being through the use of mechanical manipulation of body tissues with rhythmic pressure and stroking.47 Treatment techniques include Hoffa massage, friction massage, connective tissue massage, transverse friction massage, and trigger point massage.48 Massage is one of the most popular CAM therapies for neck and back pain.49 In their survey, White and colleagues reported that active-duty military personnel listed massage as the most frequently used CAM therapy in the previous 12 months.18
Patel and colleagues reported that the overall methodology of the trials assessed in their study was either low- or very-low-GRADE level.32 They found very-low to low-quality evidence that there is no difference in effectiveness of 3 approaches of massage therapies (ischemic compression to upper fibre of trapezius trigger point, transverse friction massage to upper fibre of trapezius, and ischemic compression to upper fibre of trapezius) for neck muscle pain. They also reported no difference between conventional Western massage and acupuncture for generalized neck muscle pain at short-term follow-up, and no difference in pain intensity compared with other therapies such as acupuncture, manual therapy, exercise, education, and multimodal interventions. The investigators concluded that the effectiveness of massage therapy for improving neck pain remains unclear, as results could not be combined due to the wide range of techniques and comparative treatments. They were unable to make any firm statement to guide clinical practice.32
Two other studies compared massage to no treatment and found it significantly improved chronic neck pain immediately after the end of treatment.33,35 Kong and colleagues also found similar effects for shoulder pain at immediate and short-term
follow-up but not for neck or shoulder pain when massage was compared with active therapies.33 Furlan and colleagues’ meta-analysis found that massage compared with relaxation or physical therapy was significantly better at reducing chronic nonspecific low back pain immediately after treatment.35
Spinal Manipultaion
Spinal manipulation is high-velocity and low-amplitude localized force directed at specific spinal segments.34 It is performed by using the hands or a device to apply a controlled force to a joint of the spine and is practiced by osteopathic physicians, naturopathic physicians, chiropractors, physical therapists, and some medical doctors.19
In a study to assess its effectiveness, Rubinstein and colleagues found low-quality evidence to very-low-quality evidence to suggest that SMT does not provide a more clinically beneficial effect compared with sham, passive modalities, or other interventions for the relief of chronic low back pain.38 Comparative interventions included usual medical care, physical therapy, exercise, physiotherapy, and multimodal treatments. Standaert and colleagues also found no difference between motor control exercise and SMT in pain relief.42 They concluded that although the evidence is low, there is an indication that structured exercise and SMT seem to offer equivalent benefits in terms of pain for those with chronic lower back pain with clinical benefits evident within 8 weeks of care.42
Gross and colleagues found that when cervical manipulation was compared with control for chronic mechanical neck pain, there was moderate-quality evidence for similar effects at short-term and intermediate follow-up.34 They also reported low-quality evidence in support of thoracic manipulation alone or in combination with electrothermal or individualized physiotherapy and suggested cervical manipulation may provide short-term but not long-term pain relief.34 Furlan and colleagues reported moderate-quality evidence that spinal manipulation provided significantly better posttreatment neck pain relief compared with placebo.35 They also found low evidence that it was significantly better than placebo, acupuncture, and pain medication at immediate follow-up.35
Conclusion
Considerable effort was made to retrieve all studies; however, the authors cannot be certain that the review was exhaustive. They also relied on other analyses of primary studies for the conclusion.
The 3 types of musculoskeletal pain in the review were low back, neck, and knee pain related to osteoarthritis. The authors found that the most common CAM modality studied for chronic musculoskeletal pain was acupuncture. Studies on massage therapy and SMT that were relevant to the review were limited.
Two studies reported strong level of evidence for acupuncture.36,40 One study reported that acupuncture was superior to no treatment or to sham acupuncture for relief of chronic knee pain.40 The other study reported that acupuncture was more effective than conventional therapy alone when it was combined with conventional therapy for chronic low back pain, but there was no difference when compared with sham acupuncture for short-term pain relief.36 The strength of the evidence for acupuncture combined with conventional treatment for low back pain was conflicting. One other review found low evidence for its benefit. Similar to Hopton and MacPherson, this review found that acupuncture treatment seemed to provide effective short-term relief of chronic low back pain.14 Evidence would also seem to support acupuncture for the short-term relief of chronic neck pain and knee pain associated with osteoarthritis.
This review also found immediate and short-term benefits, although mostly with weak evidence, for the use of SMT in the treatment of chronic neck and low back pain. There was conflicting evidence for the support of massage therapy. Furlan and colleagues, however, found that acupuncture, SMT, and massage treatments were significantly more efficacious than no treatment, placebo, physical therapy, or usual care in reducing pain immediately or at short-term after treatment.35 Inconsistencies may be related to the methodologic and clinical diversity of RCTs, which limit the extent of quantitative synthesis and complicates result interpretation.35 Also, better conclusions could be drawn if future studies use head-to-head comparisons of CAM treatments and trials comparing CAM to widely used active treatments that report on all clinically relevant outcomes.35
Although the relationship between conventional treatment and the world of CAM remains equivocal, review of the evidence suggests acupuncture and SMT may be effective treatment for various chronic painful musculoskeletal conditions.35,44,50,51 These CAM modalities are reasonable referral options to supplement conventional therapy for the treatment of chronic musculoskeletal pain when conventional therapy has not yielded satisfactory results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Musculoskeletal pain affects one-quarter of the adult population and is the most common reason for self-medication and for seeking health care.1-3 It is also cited as the most common reason for the use of complementary and alternative medicine (CAM), and the lower back, head, neck, and knee are the most commonly reported areas of pain.4-8 In 2007, the estimated annual cost of managing chronic pain, adjusted for inflation, ranged from $560 to $635 billion; whereas the direct out-of-pocket cost for patients with back pain was $34 billion.9 Chronic pain persists beyond the usual course of disease or healing; generally about 3 months or longer.10-12 The most common forms of pain include those associated with musculoskeletal disorders, such as degenerative arthritis, rheumatoid arthritis, osteoarthritis, myofascial pain, chronic headache, low back pain, and bone pain.11,13-15
A large number of returning Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) veterans have reported chronic pain symptoms, with back and head being the most common pain locations.7 They experienced pain related to wearing heavy gear every day, being transported in armored vehicles over crater-ridden roads, and enduring less than optimal sleeping conditions.16 Studies have found a significant number of subjects in this population who reported using CAM therapy. The OIF/OEF veterans were likely to have outpatient visits for musculoskeletal system disorders and to use CAM as an additional tool in pain management—not as a reaction to perceived inadequacies of conventional care.17,18
Complementary and alternative medicine is often used to describe various substances, procedures, and approaches outside of mainstream, Western, or conventional medicine for health promotion, treating injuries, symptoms, and illnesses.18,19 Although complementary and alternative are often used interchangeably, the 2 terms refer to different concepts. Complementary refers to the use of a nonmainstream approach with conventional medicine, whereas alternative refers to the use of a nonmainstream approach instead of conventional medicine.19 About 40% of Americans use CAM for various reasons.19
The services and self-care forms of CAM account for a large portion of out-of-pocket costs; patients are willing to pay for it themselves. In 2007, the U.S. spent $33.9 billion on out-of-pocket expenses for CAM classes, products, materials, and visits to CAM providers.20 The costs are comparable with those of conventional health care services and prescription drug use.20 One national study concluded that many patients use CAM in accordance with their beliefs, values, and philosophy concerning health and life.21 Other studies found that many patients use CAM not only because of functional status, pain severity, or self-efficacy, but also because they perceive significant benefits in pain relief.6,17,22-25 Some authors reported that CAM is used to augment and not replace conventional medicine and that it has now become part of the accepted armamentarium for managing chronic musculoskeletal pain.6,17,25
The National Center for Complementary and Alternative Medicine at the National Institutes of Health (NIH) classifies CAM in 2 ways: (1) Mind and body practices, such as acupuncture, massage therapy, meditation, movement therapies, relaxation techniques, spinal manipulation, tai chi and qi gong, yoga, healing touch, and hypnotherapy; and (2) natural products, including probiotics, herbs, and vitamins and minerals usually sold as dietary supplements.19 These products are regulated by the FDA but not as drugs. They have a different set of regulations under the Dietary Supplement Health and Education Act of 1994.26
Mind and body practices or provider-based CAM therapies such as chiropractic care, acupuncture, and massage increased significantly between 2002 and 2007, and many more patients may be willing to try these therapies for chronic low back pain if they do not have to pay out of pocket.27,28 Multiple studies have also found that these treatments in addition to herbal medicine are the most commonly reported CAM treatments used for pain relief in adults.3,17,22,23
Other commonly reported CAM therapies are garlic preparations, exercise, and yoga and meditation.22,23 A large number of veterans have reported previous use or willingness to try chiropractic care, massage therapy, herbal medicines, and acupuncture for chronic noncancer pain.17 In addition to acute care with conventional treatment, the VHA has now expanded services to allow for CAM as available treatment options for chronic musculoskeletal pain.29 The majority of VHA facilities also provide and refer patients to CAM service providers.30
This review article explores the evidence supporting the use of the most commonly reported CAM therapies; specifically acupuncture, massage therapy, and spinal manipulation for musculoskeletal pain relief. Because of the plethora of herbs and dietary supplements in the literature, these were not included in this review, although they are also reported among the most commonly used CAM therapies.1,23,31 The investigators sought to examine the effectiveness of acupuncture, spinal manipulation, or massage compared with no treatment, sham therapy, or current noninvasive first-line treatment for chronic musculoskeletal pain.
Study Selection
To find research addressing this question, the authors searched the PubMed, MEDLINE, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases and the Cochrane Library for all relevant studies published between October 31, 2003, and October 31, 2013. The combined search from all sources for randomized controlled trials (RCTs) resulted in 1,157 studies with acupuncture and chronic pain, 343 studies with spinal manipulation and chronic pain, and 416 studies with massage and chronic pain. Acupuncture and chronic musculoskeletal pain yielded 94 studies, spinal manipulation and chronic musculoskeletal pain yielded 29 studies, and massage and chronic musculoskeletal pain yielded 55 RCTs.
Targeted searches were then conducted within the results for systematic reviews and meta-analysis of relevant studies of RCTs, focus on adults with any type of musculoskeletal pain, written in English, and had pain level or level of pain-related improvement as its primary outcome. The results were assessed for relevance to the review based on the information provided in the title, abstract, and the National Library of Medicine Medical Subject Headings. References of the search results were also searched manually for additional studies relevant to the review. Duplicated studies and those that looked at only acute or cancer pain were excluded. Thirteen systematic reviews and meta-analyses met the inclusion criteria (Table). The investigators reviewed the full reports and agreed to use the data that were abstracted from the studies.
Study Parameters
Four different categories of outcome measuring points for posttreatment follow-up are reported in the CAM studies: immediate, short-term, intermediate, and long-term. There are inconsistencies across studies for the timing of these 4 categories. Immediate posttreatment is defined as up to 1 day.8,32-34 The duration for the short-term follow-up period is defined as between 1 day and 3 months8,32,33; ≤ 3 months35,36; closest to 3 weeks37; closest to 4 weeks34; 1 month38; closest to 8 weeks, but < 3 months after randomization39; or up to 25 weeks, but nearest to 12 weeks.40Intermediate follow-up is between 3 months and 1 year8,33,35; between 3 and 6 months38; ≥ 3 months, but < 1 year36; or closest to 6 months.34Long term is defined as >12 months8,35; closest to 6 months37; 12 months38; 1 year or more36; closest to 6 months, but >3 months after randomization34,39; or between 26 weeks and 56 weeks.40
Pain intensity and pain relief was the treatment efficacy outcome for all the studies. A variety of measuring tools were reported across studies. Eight of the 13 studies reported measurement of pain intensity using the visual analog scale (VAS).8,33,35-37,41-43 In addition to the VAS, 2 studies also used the numerical rating scale (NRS).8,36 One study used the NRS alone.38 Other studies used the McGill Pain Questionnaire35; the SF-36 bodily pain dimension, Von Korff chronic pain grading scale, or low back pain rating scale36; or the Western Ontario and McMaster Universities Osteoarthritis Index subscale for pain.39,40,43
Authors from 8 of the systematic reviews and meta-analysis reported levels of evidence, or GRADE (Grades of Recommendation, Assessment, Development, and Evaluation), used to evaluate the overall quality of the evidence and the strength of the recommendations.8,32,34-36,38,42,43 Levels of evidence were based on RCTs. The various levels were (1) “strong evidence,” consistent findings in multiple high-quality RCTs; (2) “moderate evidence,” consistent findings among multiple high-quality RTCs and/or 1 high-quality RCT; (3) “limited evidence,” low-quality RCT; (4) “conflicting evidence,” inconsistent findings among multiple RCTs; and (5) “no evidence,” no RCTs or no studies.8,36
Most studies expressed the overall strength of the body of literature in 6 different categories: (1) “high quality,” confidence that the evidence reflected the true effect and that further research is very unlikely to change confidence in the effect of size; (2) “moderate quality,” further research is likely to have an impact on confidence in the estimate of effect and may change the estimate; (3) “low quality,” further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change it; (4) “very low quality,” great uncertainty about the estimate; (5) “insufficient evidence,” either the evidence is unavailable or does not allow for a conclusion; and (6)“no evidence,” no evidence from RTCs.32,34,35,38,42,43 Kwon and colleagues reported using a modified jaded score where a total of 5 points was awarded if a study was described as randomized, used an appropriate method, if subjects were blinded to the intervention, if the evaluator was blinded to the intervention, and if there was a description of withdrawals and dropouts.43
Acupuncture
About 3 million American adults receive acupuncture each year.44 The most commonly reported reason for its use is chronic pain.44,45 Trials that examined the characteristics of those seeking and using acupuncture as adjunct to conventional treatment have found that patients who experienced positive outcomes, such as improvement in pain subscale, included females, previous failure of other therapies, and prior positive acupuncture encounters.46
Six of the studies in this review examined the evidence of acupuncture for chronic low back pain.35-38,41,42 Two of those studies found moderate evidence that acupuncture was more effective than no treatment for short-term pain relief.35,36 Manheimer and colleagues found it to be significantly more effective than no additional treatment or sham treatment for short-term pain relief.37 They however, reported a lack of evidence to suggest that it was more effective than were other active therapies.37 Hutchinson and colleagues did not differentiate among data points for intermediate, short-term, or long-term follow-up in their study.41 However, they concluded that there was some evidence to support acupuncture as more effective than no treatment and conflicting evidence of its effectiveness over other treatment modalities. Different levels of evidence were reported for intermediate pain relief with 2 of the other studies. One study found that the evidence was limited.35 The other study reported conflicting evidence that it was more effective than no treatment for immediate pain relief for those with chronic low back pain.36
Rubinstein and colleagues reported low- to very-low-quality evidence that acupuncture provided a short-term clinically relevant effect compared with waiting list control or when it was added to another intervention for chronic low back pain.38 Standaert and colleagues concluded that there was insufficient evidence to determine the relative effectiveness of acupuncture compared with either exercise or spinal manipulative therapy (SMT) in relieving chronic low back pain.42 Yuan and colleagues reported strong evidence that acupuncture combined with conventional therapy was more effective than conventional therapy alone.36
Furlan and colleagues found moderate evidence for significant improvement in pain intensity compared with subjects in physical therapy or usual care groups at short-term or immediate follow-up for chronic back pain.35 Studies that evaluated the efficacy of acupuncture for knee osteoarthritis compared acupuncture with sham acupuncture controls or no additional treatment and found that acupuncture was significantly better at relieving knee pain.39,40,43 Cao and colleagues found it to be effective both in the short term and long term.39 White and colleagues and Kwon and colleagues were unable to draw a conclusion concerning long-term effects due to the data point included in the study or the heterogeneity in the results.40,43
Trinh and colleagues reported moderate evidence that acupuncture is more effective for relief of chronic neck pain compared with inactive, sham treatments at immediate posttreatment.8 They also found moderate evidence that acupuncture was more effective than some other types of sham controls immediately posttreatment and limited evidence that it was more effective than massage at short-term follow-up.8 Furlan and colleagues found trials that applied sham acupuncture tended to produce nonstatistically significant results.35 Their meta-analysis of 2 studies indicated no significant difference between acupuncture and sham acupuncture for immediate posttreatment pain intensity. They also reported inconsistent results for the effects of acupuncture compared with medication or with spinal manipulation for chronic neck pain.35
Massage
Massage promotes health and well-being through the use of mechanical manipulation of body tissues with rhythmic pressure and stroking.47 Treatment techniques include Hoffa massage, friction massage, connective tissue massage, transverse friction massage, and trigger point massage.48 Massage is one of the most popular CAM therapies for neck and back pain.49 In their survey, White and colleagues reported that active-duty military personnel listed massage as the most frequently used CAM therapy in the previous 12 months.18
Patel and colleagues reported that the overall methodology of the trials assessed in their study was either low- or very-low-GRADE level.32 They found very-low to low-quality evidence that there is no difference in effectiveness of 3 approaches of massage therapies (ischemic compression to upper fibre of trapezius trigger point, transverse friction massage to upper fibre of trapezius, and ischemic compression to upper fibre of trapezius) for neck muscle pain. They also reported no difference between conventional Western massage and acupuncture for generalized neck muscle pain at short-term follow-up, and no difference in pain intensity compared with other therapies such as acupuncture, manual therapy, exercise, education, and multimodal interventions. The investigators concluded that the effectiveness of massage therapy for improving neck pain remains unclear, as results could not be combined due to the wide range of techniques and comparative treatments. They were unable to make any firm statement to guide clinical practice.32
Two other studies compared massage to no treatment and found it significantly improved chronic neck pain immediately after the end of treatment.33,35 Kong and colleagues also found similar effects for shoulder pain at immediate and short-term
follow-up but not for neck or shoulder pain when massage was compared with active therapies.33 Furlan and colleagues’ meta-analysis found that massage compared with relaxation or physical therapy was significantly better at reducing chronic nonspecific low back pain immediately after treatment.35
Spinal Manipultaion
Spinal manipulation is high-velocity and low-amplitude localized force directed at specific spinal segments.34 It is performed by using the hands or a device to apply a controlled force to a joint of the spine and is practiced by osteopathic physicians, naturopathic physicians, chiropractors, physical therapists, and some medical doctors.19
In a study to assess its effectiveness, Rubinstein and colleagues found low-quality evidence to very-low-quality evidence to suggest that SMT does not provide a more clinically beneficial effect compared with sham, passive modalities, or other interventions for the relief of chronic low back pain.38 Comparative interventions included usual medical care, physical therapy, exercise, physiotherapy, and multimodal treatments. Standaert and colleagues also found no difference between motor control exercise and SMT in pain relief.42 They concluded that although the evidence is low, there is an indication that structured exercise and SMT seem to offer equivalent benefits in terms of pain for those with chronic lower back pain with clinical benefits evident within 8 weeks of care.42
Gross and colleagues found that when cervical manipulation was compared with control for chronic mechanical neck pain, there was moderate-quality evidence for similar effects at short-term and intermediate follow-up.34 They also reported low-quality evidence in support of thoracic manipulation alone or in combination with electrothermal or individualized physiotherapy and suggested cervical manipulation may provide short-term but not long-term pain relief.34 Furlan and colleagues reported moderate-quality evidence that spinal manipulation provided significantly better posttreatment neck pain relief compared with placebo.35 They also found low evidence that it was significantly better than placebo, acupuncture, and pain medication at immediate follow-up.35
Conclusion
Considerable effort was made to retrieve all studies; however, the authors cannot be certain that the review was exhaustive. They also relied on other analyses of primary studies for the conclusion.
The 3 types of musculoskeletal pain in the review were low back, neck, and knee pain related to osteoarthritis. The authors found that the most common CAM modality studied for chronic musculoskeletal pain was acupuncture. Studies on massage therapy and SMT that were relevant to the review were limited.
Two studies reported strong level of evidence for acupuncture.36,40 One study reported that acupuncture was superior to no treatment or to sham acupuncture for relief of chronic knee pain.40 The other study reported that acupuncture was more effective than conventional therapy alone when it was combined with conventional therapy for chronic low back pain, but there was no difference when compared with sham acupuncture for short-term pain relief.36 The strength of the evidence for acupuncture combined with conventional treatment for low back pain was conflicting. One other review found low evidence for its benefit. Similar to Hopton and MacPherson, this review found that acupuncture treatment seemed to provide effective short-term relief of chronic low back pain.14 Evidence would also seem to support acupuncture for the short-term relief of chronic neck pain and knee pain associated with osteoarthritis.
This review also found immediate and short-term benefits, although mostly with weak evidence, for the use of SMT in the treatment of chronic neck and low back pain. There was conflicting evidence for the support of massage therapy. Furlan and colleagues, however, found that acupuncture, SMT, and massage treatments were significantly more efficacious than no treatment, placebo, physical therapy, or usual care in reducing pain immediately or at short-term after treatment.35 Inconsistencies may be related to the methodologic and clinical diversity of RCTs, which limit the extent of quantitative synthesis and complicates result interpretation.35 Also, better conclusions could be drawn if future studies use head-to-head comparisons of CAM treatments and trials comparing CAM to widely used active treatments that report on all clinically relevant outcomes.35
Although the relationship between conventional treatment and the world of CAM remains equivocal, review of the evidence suggests acupuncture and SMT may be effective treatment for various chronic painful musculoskeletal conditions.35,44,50,51 These CAM modalities are reasonable referral options to supplement conventional therapy for the treatment of chronic musculoskeletal pain when conventional therapy has not yielded satisfactory results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Callahan LF, Wiley-Exley EK, Mielenz TJ, et al. Use of complementary and alternative medicine among patients with arthritis. Prev Chronic Dis. 2009;6(2):A44.
2. Walsh NE, Brooks P, Hazes JM, et al; Bone and Joint Decade Task Force for Standards of Care for Acute and Chronic Musculoskeletal Pain. Standards of care for acute and chronic musculoskeletal pain: the Bone and Joint Decade (2000-2010). Arch Phys Med Rehabil. 2008;89(9):1830-1845.
3. Williamson AT, Fletcher PC, Dawson KA. Complementary and alternative medicine. Use in an older population. J Gerontol Nurs. 2003;29(5):20-28.
4. Artus M, Croft P, Lewis M. The use of CAM and conventional treatments among primary care consulters with chronic musculoskeletal pain. BMC Fam Pract. 2007;8:26.
5. Cherkin DC, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized controlled trial. Ann Intern Med. 2011;155(1):1-9.
6. Fleming S, Rabago DP, Mundt MP, Fleming MF. CAM therapies among primary care patients using opioid therapy for chronic pain. BMC Complement Altern Med. 2007;7:15.
7. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent post concussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
8. Trinh K, Graham N, Gross A, et al. Acupuncture for neck disorders. Spine (Phila PA 1976). 2007;32(2):236-243.
9. Gaskin DJ, Richard P. Appendix C: The economic costs of pain in the United States. In: Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. National Center for Biotechnology Information Website. http://www. ncbi.nlm.nih.gov/books/NBK92521. Accessed July 31, 2015.
10. American Academy of Pain Medicine. AAPM facts and figures on pain. American Academy of Pain Medicine Website. http://www.painmed.org/files/facts-and-figures-on-pain.pdf. Accessed July 31, 2015.
11. Rosenquist RW, Vrooman MD. Chronic pain management. In: Butterworth JF, Mackey DC, Wasnick JD, eds. Morgan & Mikhail’s Clinical Anesthesiology. 5th ed. New York, NY: McGraw-Hill; 2013:chap 47.
12. Rasu RS, Sohraby R, Cunningham L, Knell ME. Assessing chronic pain treatment practices and evaluating adherence to chronic pain clinical guidelines in outpatient practices in the United States. J Pain. 2013;14(6):568-578.
13. Gureje O, Von Korff M, Simon, GE, Grater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280(2):147-151.
14. Hopton A, MacPherson H. Acupuncture for chronic pain: is acupuncture more than an effective placebo? A systematic review of pooled data from meta-analyses. Pain Pract. 2010;10(2):94-102.
15. Kumar N. WHO Normative guidelines on pain management. World Health Organization Website. http://www.who.int/medicines/areas/quality_safety/delphi_study_pain_guidelines.pdf. Published June 2007. Accessed August 3, 2015.
16. Koffman RL. Downrange acupuncture. Med Acupunct. 2011;23(4):215-218.
17. Denneson LM, Corson K, Dobscha SK. Complementary and alternative medicine use among veterans with chronic noncancer pain. J Rehabil Res Dev. 2011;(48)9:1119-1128.
18. White MR, Jacobson IG, Smith B, et al; Millennium Cohort Study Team. Health care utilization among complementary and alternative medicine users in a large military cohort. BMC Complement Alternat Med. 2011;11:27.
19. U.S. Department of Health and Human Services, National Institutes of Health, National Center for Complementary and Alternative Medicine. Complementary, alternative, or integrative health: What’s in a name? National Center for Complementary and Alternative Medicine Website. http://nccam.nih.gov/health/whatiscam. Updated March 2015. Modified July 8, 2015. Accessed August 4, 2015.
20. Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;30(18):1-14.
21. Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279(19):1548-1553.
22. Rosenberg EI, Genao I, Chen I, et al. Complementary and alternative medicine use in primary care patients with chronic pain. Pain Med. 2008;9(8):1065-1072.
23. Alaaeddine N, Okais J, Ballane L, Baddoura RM. Use of complementary and alternative therapy among patients with rheumatoid arthritis and osteoarthritis. J Clin Nurs. 2012;21(21-22):3198-3204.
24. Tan MG, Win MT, Khan SA. The use of complementary and alternative medicine in chronic pain patients in Singapore: a single-centre study. Ann Acad Med Singapore. 2013;42(3):133-137.
25. White P. A background to acupuncture and its use in chronic painful musculoskeletal conditions. J R Soc Promot Health. 2006;126(5):219-227.
26. U.S. Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Health and Education Act of 1994. National Institutes of Health Website. http://ods.od.nih.gov/About/DSHEA_Wording.aspx. Published October 25, 1994. Accessed August 4, 2015.
27. Su D, Li L. Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J Health Care Poor Underserved. 2011;22(1):296-310.
28. Sherman KJ, Cherkin DC, Connelly MT, et al. Complementary and alternative medical therapies for chronic low back pain: what treatments are patients willing to try? BMC Complement Alternat Med. 2004;4:9.
29. Smeeding SJ, Bradshaw DH, Kumpfer KL, Trevithick S, Stoddard GJ. Outcome evaluation of the Veterans Affairs Salt Lake City Integrative Health Clinic for Chronic Nonmalignant Pain. Clin J Pain. 2011;27(2):146-155.
30. U.S. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2011 complementary and alternative medicine. U.S. Department of Veterans Affairs Office of Research and Development Website. http://www.research.va.gov/research_topics/2011cam_finalreport.pdf. Published September 2011. Accessed August 4, 2015.
31. Unsal A, Gözüm S. Use of complementary and alternative medicine by patients with arthritis. J Clin Nurs. 2010;19(7-8):1129-1138.
32. Patel KC, Gross A, Graham N, et al. Massage for mechanical neck disorder. Cochrane Database Syst Rev. 2012;9:CD004871.
33. Kong LJ, Zhan HS, Cheng YW, Yuan WA, Chen B, Fang M. Massage therapy for neck and shoulder pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:613279.
34. Gross A, Miller J, D’Sylva J, et al; COG. Manipulation or mobilisation for neck pain: a Cochrane review. Man Ther. 2010;15(4):315-333.
35. Furlan A, Yazdi F, Tsertsvadze A, et al. Complementary and alternative therapies for back Pain II. Evidence report/technology assessment No. 194. (Prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-2007-10059-I (EPCIII). AHRQ Publication No. 10(11)E007. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
36. Yuan J, Purepong N, Kerr DP, Park J, Bradbury I, McDonough S. Effectiveness of acupuncture for low back pain. Spine (Phila PA 1976). 2008;33(23):E887-E900.
37. Manheimer E, White A, Berman B, Forys K, Ernst E. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142(8):651-663.
38. Rubinstein SM, van Middelkoop M, Kuijpers T, et al. A systematic review on the effectiveness of complementary and alternative medicine for chronic non-specific low-back pain. Eur Spine J. 2010;19(8):1213-1228.
39. Cao L, Zhang XL, Gao YS, Jiang Y. Needle acupuncture for osteoarthritis of the knee. A systematic review and updated meta-analysis. Saudi Med J. 2012;33(5):526-532.
40. White A, Foster NE, Cummings M, Barlas P. Acupuncture treatment for chronic knee pain: a systematic review. Rheumatology (Oxford). 2007;46(3):384-390.
41. Hutchinson AJ, Ball S, Andrews JC, Jones GG. The effectiveness of acupuncture in treating chronic non-specific low back pain: a systematic review of the literature. J Orthop Surg Res. 2012;7:36.
42. Standaert, CJ, Friedly J, Erwin MW, et al. Comparative effectiveness of exercise, acupuncture, and spinal manipulation for low back pain. Spine (Phila PA 1976). 2011;36(21 suppl):S120-S130.
43. Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2006;45(11):1331-1337.
44. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Individual patient data meta-analysis of acupuncture for chronic pain: protocol of the Acupuncture Trialists’ Collaboration. Trials. 2010;11:90.
45. Kelly RB. Acupuncture for pain. Am Fam Physician. 2009;80(5):481-484.
46. Witt CM, Schützler L, Lüdtke R, Wegscheider K, Willich SN. Patient characteristics and variation in treatment outcomes: which patients benefit most from acupuncture for chronic pain? Clin J Pain. 2011;27(6):550-555.
47. Cafarelli E, Flint F. The role of massage in preparation for and recovery from exercise: an overview. Sports Med. 1992;14(1):1-9.
48. Prentice WE. Therapeutic massage. In: Prentice WE. Therapeutic Modalities in Rehabilitation. 4th ed. New York, NY: McGraw-Hill; 2011:chap 16.
49. Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS. Patterns and perceptions of care for treatment of back and neck pain: results of a national survey. Spine (Phila PA 1976). 2003;28(3):292-297.
50. Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538.
51. Tsao JCI. Effectiveness of massage therapy for chronic, non-malignant pain: a review. Evid Based Complement Alternat Med. 2007;4(2):165-179.
1. Callahan LF, Wiley-Exley EK, Mielenz TJ, et al. Use of complementary and alternative medicine among patients with arthritis. Prev Chronic Dis. 2009;6(2):A44.
2. Walsh NE, Brooks P, Hazes JM, et al; Bone and Joint Decade Task Force for Standards of Care for Acute and Chronic Musculoskeletal Pain. Standards of care for acute and chronic musculoskeletal pain: the Bone and Joint Decade (2000-2010). Arch Phys Med Rehabil. 2008;89(9):1830-1845.
3. Williamson AT, Fletcher PC, Dawson KA. Complementary and alternative medicine. Use in an older population. J Gerontol Nurs. 2003;29(5):20-28.
4. Artus M, Croft P, Lewis M. The use of CAM and conventional treatments among primary care consulters with chronic musculoskeletal pain. BMC Fam Pract. 2007;8:26.
5. Cherkin DC, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized controlled trial. Ann Intern Med. 2011;155(1):1-9.
6. Fleming S, Rabago DP, Mundt MP, Fleming MF. CAM therapies among primary care patients using opioid therapy for chronic pain. BMC Complement Altern Med. 2007;7:15.
7. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent post concussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
8. Trinh K, Graham N, Gross A, et al. Acupuncture for neck disorders. Spine (Phila PA 1976). 2007;32(2):236-243.
9. Gaskin DJ, Richard P. Appendix C: The economic costs of pain in the United States. In: Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. National Center for Biotechnology Information Website. http://www. ncbi.nlm.nih.gov/books/NBK92521. Accessed July 31, 2015.
10. American Academy of Pain Medicine. AAPM facts and figures on pain. American Academy of Pain Medicine Website. http://www.painmed.org/files/facts-and-figures-on-pain.pdf. Accessed July 31, 2015.
11. Rosenquist RW, Vrooman MD. Chronic pain management. In: Butterworth JF, Mackey DC, Wasnick JD, eds. Morgan & Mikhail’s Clinical Anesthesiology. 5th ed. New York, NY: McGraw-Hill; 2013:chap 47.
12. Rasu RS, Sohraby R, Cunningham L, Knell ME. Assessing chronic pain treatment practices and evaluating adherence to chronic pain clinical guidelines in outpatient practices in the United States. J Pain. 2013;14(6):568-578.
13. Gureje O, Von Korff M, Simon, GE, Grater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280(2):147-151.
14. Hopton A, MacPherson H. Acupuncture for chronic pain: is acupuncture more than an effective placebo? A systematic review of pooled data from meta-analyses. Pain Pract. 2010;10(2):94-102.
15. Kumar N. WHO Normative guidelines on pain management. World Health Organization Website. http://www.who.int/medicines/areas/quality_safety/delphi_study_pain_guidelines.pdf. Published June 2007. Accessed August 3, 2015.
16. Koffman RL. Downrange acupuncture. Med Acupunct. 2011;23(4):215-218.
17. Denneson LM, Corson K, Dobscha SK. Complementary and alternative medicine use among veterans with chronic noncancer pain. J Rehabil Res Dev. 2011;(48)9:1119-1128.
18. White MR, Jacobson IG, Smith B, et al; Millennium Cohort Study Team. Health care utilization among complementary and alternative medicine users in a large military cohort. BMC Complement Alternat Med. 2011;11:27.
19. U.S. Department of Health and Human Services, National Institutes of Health, National Center for Complementary and Alternative Medicine. Complementary, alternative, or integrative health: What’s in a name? National Center for Complementary and Alternative Medicine Website. http://nccam.nih.gov/health/whatiscam. Updated March 2015. Modified July 8, 2015. Accessed August 4, 2015.
20. Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;30(18):1-14.
21. Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279(19):1548-1553.
22. Rosenberg EI, Genao I, Chen I, et al. Complementary and alternative medicine use in primary care patients with chronic pain. Pain Med. 2008;9(8):1065-1072.
23. Alaaeddine N, Okais J, Ballane L, Baddoura RM. Use of complementary and alternative therapy among patients with rheumatoid arthritis and osteoarthritis. J Clin Nurs. 2012;21(21-22):3198-3204.
24. Tan MG, Win MT, Khan SA. The use of complementary and alternative medicine in chronic pain patients in Singapore: a single-centre study. Ann Acad Med Singapore. 2013;42(3):133-137.
25. White P. A background to acupuncture and its use in chronic painful musculoskeletal conditions. J R Soc Promot Health. 2006;126(5):219-227.
26. U.S. Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Health and Education Act of 1994. National Institutes of Health Website. http://ods.od.nih.gov/About/DSHEA_Wording.aspx. Published October 25, 1994. Accessed August 4, 2015.
27. Su D, Li L. Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J Health Care Poor Underserved. 2011;22(1):296-310.
28. Sherman KJ, Cherkin DC, Connelly MT, et al. Complementary and alternative medical therapies for chronic low back pain: what treatments are patients willing to try? BMC Complement Alternat Med. 2004;4:9.
29. Smeeding SJ, Bradshaw DH, Kumpfer KL, Trevithick S, Stoddard GJ. Outcome evaluation of the Veterans Affairs Salt Lake City Integrative Health Clinic for Chronic Nonmalignant Pain. Clin J Pain. 2011;27(2):146-155.
30. U.S. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2011 complementary and alternative medicine. U.S. Department of Veterans Affairs Office of Research and Development Website. http://www.research.va.gov/research_topics/2011cam_finalreport.pdf. Published September 2011. Accessed August 4, 2015.
31. Unsal A, Gözüm S. Use of complementary and alternative medicine by patients with arthritis. J Clin Nurs. 2010;19(7-8):1129-1138.
32. Patel KC, Gross A, Graham N, et al. Massage for mechanical neck disorder. Cochrane Database Syst Rev. 2012;9:CD004871.
33. Kong LJ, Zhan HS, Cheng YW, Yuan WA, Chen B, Fang M. Massage therapy for neck and shoulder pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:613279.
34. Gross A, Miller J, D’Sylva J, et al; COG. Manipulation or mobilisation for neck pain: a Cochrane review. Man Ther. 2010;15(4):315-333.
35. Furlan A, Yazdi F, Tsertsvadze A, et al. Complementary and alternative therapies for back Pain II. Evidence report/technology assessment No. 194. (Prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-2007-10059-I (EPCIII). AHRQ Publication No. 10(11)E007. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
36. Yuan J, Purepong N, Kerr DP, Park J, Bradbury I, McDonough S. Effectiveness of acupuncture for low back pain. Spine (Phila PA 1976). 2008;33(23):E887-E900.
37. Manheimer E, White A, Berman B, Forys K, Ernst E. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142(8):651-663.
38. Rubinstein SM, van Middelkoop M, Kuijpers T, et al. A systematic review on the effectiveness of complementary and alternative medicine for chronic non-specific low-back pain. Eur Spine J. 2010;19(8):1213-1228.
39. Cao L, Zhang XL, Gao YS, Jiang Y. Needle acupuncture for osteoarthritis of the knee. A systematic review and updated meta-analysis. Saudi Med J. 2012;33(5):526-532.
40. White A, Foster NE, Cummings M, Barlas P. Acupuncture treatment for chronic knee pain: a systematic review. Rheumatology (Oxford). 2007;46(3):384-390.
41. Hutchinson AJ, Ball S, Andrews JC, Jones GG. The effectiveness of acupuncture in treating chronic non-specific low back pain: a systematic review of the literature. J Orthop Surg Res. 2012;7:36.
42. Standaert, CJ, Friedly J, Erwin MW, et al. Comparative effectiveness of exercise, acupuncture, and spinal manipulation for low back pain. Spine (Phila PA 1976). 2011;36(21 suppl):S120-S130.
43. Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2006;45(11):1331-1337.
44. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Individual patient data meta-analysis of acupuncture for chronic pain: protocol of the Acupuncture Trialists’ Collaboration. Trials. 2010;11:90.
45. Kelly RB. Acupuncture for pain. Am Fam Physician. 2009;80(5):481-484.
46. Witt CM, Schützler L, Lüdtke R, Wegscheider K, Willich SN. Patient characteristics and variation in treatment outcomes: which patients benefit most from acupuncture for chronic pain? Clin J Pain. 2011;27(6):550-555.
47. Cafarelli E, Flint F. The role of massage in preparation for and recovery from exercise: an overview. Sports Med. 1992;14(1):1-9.
48. Prentice WE. Therapeutic massage. In: Prentice WE. Therapeutic Modalities in Rehabilitation. 4th ed. New York, NY: McGraw-Hill; 2011:chap 16.
49. Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS. Patterns and perceptions of care for treatment of back and neck pain: results of a national survey. Spine (Phila PA 1976). 2003;28(3):292-297.
50. Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538.
51. Tsao JCI. Effectiveness of massage therapy for chronic, non-malignant pain: a review. Evid Based Complement Alternat Med. 2007;4(2):165-179.
Assessment of a Mental Health Residential Rehabilitation Treatment Program As Needed Medication List
The Mental Health Residential Rehabilitation Treatment Program (MHRRTP) is an essential part of the mental health services offered at the Clement J. Zablocki VAMC (ZVAMC) in Milwaukee, Wisconsin. Across the nation, there are about 250 MHRRTPs, which are designed to provide rehabilitation and treatment services to veterans ranging in age from 18 to 80 years, with medical conditions, mental illness, addiction, or psychosocial deficits.1 About 900 patients were admitted to the ZVAMC MHRRTP in 2013.
Background
Prior to 2010, pharmacy administrators recognized that many MHRRTP patients were inappropriately using emergency care services (ECS) to obtain treatments for simple ailments that often required only the use of over-the-counter medications. This was likely associated with the Safe Medication Management (SMM) Policy as defined in Professional Services Memorandum VII-29.2,3 This policy states that MHRRTP patients are not allowed to bring in any home medications—all medications are reconciled and readministered on admission in an effort to reduce diversion.
A lack of 24-hour-per-day provider availability forced patients to find treatment elsewhere. A 6-month review was completed in 2010, which identified all of the MHRRTP patients who used ECS, their chief medical condition, and the medication(s) that were administered to each patient. This review identified a total of 254 ECS visits made by MHRRTP patients during this period. Twenty percent of these visits resulted in prescriptions for over-the-counter medications. As a result, an as needed (PRN) medication list was created for patients to have medications readily available for simple ailments with nursing oversight (Box). The goal of the PRN medication list is to reduce the amount of unnecessary ECS visits, decrease unnecessary cost, and improve treatment efficiency and overall patient care.
Treatment Programs
The ZVAMC MHRRTP has 189 beds divided among 7 different 6-week treatment programs, including General Men’s Program (GEN), Substance Abuse Rehabilitation (SAR), Posttraumatic Stress Disorder (PTSD), Women’s Program (WOM), Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND), Domiciliary Care for Homeless Veterans (DCHV), and Individualized Addiction Consultation Team (I-ACT).4
The treatment programs within the MHRRTP at the ZVAMC address goals of rehabilitation, recovery, health maintenance, improved quality of life, and community integration in addition to specific treatment of medical conditions, mental illnesses, addictive disorders, and homelessness. Various levels of care are available through the program, based on the needs of each veteran. This care generally provides methods to enhance patients’ functional status and psychosocial rehabilitation.
A SMM program is used to ensure safe and effective medication use for all patients in the MHRRTP.2 As a result, the patients are admitted to the MHRRTP with inpatient status, and the medication delivery procedure varies based on the veteran’s ability to take medication independently. Veterans are assisted in developing self-care skills, which include comprehensive medication education. The goal of the SMM program is to give patients the assistance to eventually manage their medications independently.
MHRRTP Staffing
The MHRRTP must have adequate staffing in order to provide safe and effective patient care. Program staffing patterns are based on workload indicators and a bed-to-staff ratio.4 The MHRRTP is a multidisciplinary program; however, the only providers who can address medication issues are the 1.2 full-time employee equivalent MHRRTP psychiatrists. Unfortunately, the psychiatrists are not available for triage on nights, weekends, or holidays.
The role of the psychiatrist is to focus on the mental health needs of the MHRRTP patients, not the primary care medical concerns, which are the main reason for ECS visits. With the current model, providers are sometimes unavailable to meet the emergent needs of patients in the MHRRTP, and patients may be forced to choose between using ECS or leaving the concern unaddressed. Patients’ needs vary from mild to serious emergent needs but may not necessarily require full emergency assessments. For example, if a patient has a headache and a physician is not available to write an order for acetaminophen, the patient may need to visit the ECS to obtain a medication that otherwise would have been readily available at home. The restrictions are designed to promote medication safety, prevent medication diversion and misuse, and be in compliance with regulatory agencies (eg, The Joint Commission and the Commission on Accreditation of Rehabilitation Facilities).
ECS Use
During fiscal year 2010, pharmacy administrators discovered that many patients were using ECS to obtain medications for nonemergent conditions. Inappropriate and unnecessary use of ECS by MHRRTP patients delayed treatment, increased wait times for veterans in need of emergent care, and increased the cost of caring for simple ailments. To put this into perspective, the average cost of all conditions at the ZVAMC during the 2013 fiscal year was $657 per ECS visit, while the total cost of ECS was about $14 million.
In response to the inappropriate ECS use, the ZVAMC created a PRN medication list in 2010, which is offered to all MHRRTP patients, with the goal of reducing the number of patients inappropriately using ECS for minor ailments and providing more efficient and cost-effective patient care.2 The MHRRTP PRN medication list is initially evaluated by the admitting psychiatrist or nurse practitioner and mental health clinical pharmacy specialist completing the admission orders for appropriateness based on each patient’s comorbidities, medication regimen, and past medical history. For example, if a new patient with liver dysfunction is admitted to the MHRRTP, acetaminophen would not be made available due to an increased risk of hepatotoxicity. The other PRN medications would still be available for the patient if clinically appropriate.
Once the PRN medications are ordered, the MHRRTP nurse can assess a patient’s condition and administer the medication(s) to the patient as indicated. For instance, if a patient requests ibuprofen for pain, the nurse will document an initial pain score and administer the ibuprofendose. As a result, the patient obtains more efficient and convenient care and does not need to wait for a provider to become available or use ECS. Per ZVAMC policy, the nurse has 96 hours to reassess the PRN medication effectiveness; however, this is typically done within the same shift. Since the implementation of the PRN medication list, no formal assessment has been completed.
To the authors’ knowledge, the ZVAMC is the only MHRRTP in the VHA system that incorporates a PRN medication list in the admission orders to reduce unnecessary ECS visits. After completing a thorough literature review and contacting the national VA mental health pharmacist listserve, no studies discussing the use of PRN medication lists in this setting were identified, and no sites offered information as to a similar practice in place.
Methods
A randomized, retrospective case-controlled study involving a chart review was completed for patients admitted to the MHRRTP at the ZVAMC pre- and postimplementation of the MHRRTP PRN medication list between April 2010 and August 2010 and between April 2013 and August 2013, respectively. The ZVAMC is a teaching institution. This study was approved by the ZVAMC institutional review board.
Patients were eligible for the study if they were male, aged > 18 years, and admitted during the study period for treatment in the GEN or SAR programs at the ZVAMC for at least 4 weeks. Patients were excluded if they were female, admitted to the hospital after being seen by ECS, or if they were receiving treatment in the following programs: PTSD, WOM, OEF/OIF/OND, DCHV, and I-ACT. Patients studied in 2010 served as the control group, and patients studied in 2013 were the treatment group.
Objectives
The primary objective of this study was to evaluate the use of the current PRN medication list. Secondary objectives included the evaluation of the use of ECS by patients admitted to the MHRRTP pre- and postimplementation of the PRN medication list, the potential cost reduction due to avoided ECS use, and nurse and patient satisfaction with the PRN medication list.
Data
A list of all patients admitted to the MHRRTP at the ZVAMC between April and August of 2010 and 2013 was generated using the Veterans Health Information Systems and Technology Architecture (VISTA)system. The Computerized Patient Record System (CPRS) was used to evaluate the patient for inclusion and collect pertinent data. The PRN medication list was implemented on September 15, 2010. Data collection terminated as of September 14, 2010, regardless of discharge status. All data collected for this study were entered and stored in a database created by the authors. A table with set criteria to review was created for the 2010 and 2013 group to ensure standardization. The pharmacy resident reviewed all of the patient charts. The following data were collected for each patient in the 2010 group:
- Demographic data: Patient name, last 4 digits of their social security number, age
- Program information: Admitted to GEN or SAR program, admission and discharge date, duration of stay, reason for discharge
- ECS data: Date, type of visit, chief condition, medications administered during the visit, whether the visit resulted in a hospital admission, and whether the visit was avoidable
- Avoidable visit: visit in which the patient received or could have received medication(s) that are on the PRN medication list at the ECS visit to treat their illness
The same information was collected for each patient in the 2013 group in addition to the following: PRN medication data (medications administered from the PRN medication list and the number of times each medication was administered if applicable); and ECS data (along with the aforementioned data, it was noted if PRN medications were taken prior to the ECS visit).
In addition, nurse and patient satisfaction with the PRN medication list were assessed via a simple satisfaction survey. The survey was given to 120 patients admitted to the MHRRTP as well as to 32 nurses at the time of distribution. A cover letter on each survey explained the study and informed the patient that the survey was voluntary and anonymous. Satisfaction was based on 10-point scale, with 1 (lowest) and 10 (highest) in satisfaction. Additional questions were asked to identify areas of improvement (see eAppendixes A and B for patient and nurse surveys, respectively).
Statistical Analysis
Descriptive statistics were used to analyze collected data. The primary outcome was assessed for the group admitted postintervention by calculating the average number of times each medication on the PRN medication list was used per patient during their length of stay (LOS) as applicable. The administration totals for each medication on the PRN medication list during the postintervention study period were also recorded.
Secondary outcomes were assessed by comparing the recorded total number of ECS visits pre- and postimplementation. Additionally, the average number of ECS visits per admission and the number of avoidable ECS visits were recorded for each study group. The cost reduction from avoided ECS use was estimated by calculating the total cost of ECS used pre- and postimplementation. The difference between the number of avoidable ECS visits in the pre- and postintervention groups was assessed for statistical significance by using a chi-square test. The 2013 cost saving estimation was based on the average ECS visit cost in the 2013 fiscal year ($657). Of note, power for this study could not be calculated as this has not been studied prior; therefore, no precedence has been set.
Results
On completion of the data collection, 583 patients were assessed for inclusion into the study, 325 in the 2010 preimplementation group and 258 in the 2013 postimplementation group. A total of 200 patients were randomized in each group (n = 400); however, 69 (35%) and 63 (32%) were excluded from the 2010 group and 2013 group, respectively. Sample demographics are described in the Table.
PRN Medication and ECS Use
Between April 1, 2013, and September 14, 2013, 3,959 doses of PRN medications were administered to MHRRTP patients who were included in the study (Figure). Prior to accessing ECS for their problem, 22 (36%) of the 61 patients who used ECS had trialed the PRN medication(s).
When comparing the total number of ECS visits, the 2010 group had 145 visits and the 2013 group had 96 visits. The preimplementation group averaged 1.1 ECS visits per MHRRTP admission, whereas the postimplementation group averaged 0.7 ECS visits per admission. The difference in the number of avoidable ECS visits was statistically significant, with the 2010 group totaling 15 avoidable visits, while the 2013 group totaled 1 ECS visit (P = .0045).
It was estimated that 9 (9.3%) ECS visits were avoided due to the PRN medication list in 2013. Using 137 patients, who were included in the postimplementation group, it can be calculated that $5,867 was saved due to the PRN medication list, or $42.83 per patient in 2013. Using the 2013 MHRRTP census of 898 patients, the financial impact of the PRN medication list can be extrapolated to produce an estimated annual cost savings of $38,461.
Patient and Nurse Satisfaction
Of the 120 patients given the patient satisfaction questionnaire, 28 (23%) patients responded. Of the respondents, 25 (89%) stated they were aware of the PRN medication list. The median rank of satisfaction reported was 8 on a 10-point scale. Twenty-two (79%) patients felt that the PRN medication list had or may have reduced the need to go to ECS or urgent care. Twenty-three (82%) patients recommended not removing any drugs listed on the PRN medication list.
Of the 32 registered nurses and licensed practical nurses working in the MHRRTP, 7 (22%) responded to the nurse satisfaction questionnaire. Of the respondents, 6 (86%) stated they discuss the PRN medication list during admission assessments every time or most of the time. The median rank of satisfaction was 9 on a 10-point scale. Four (57%) nurses felt patients had a clear understanding of the PRN medication list, and 100% of nurses stated they had enough guidance on situations to administer the medications. Seven (100%) stated that the PRN medication list had not caused adverse events; however, 5 (71%) stated that the list had been used inappropriately.
Discussion
This retrospective case-controlled study of 400 patients revealed high use of the PRN medication list and a cost avoidance of nearly $40,000. Although this represents a small reduction of the annual ECS budget, the PRN medication list also improved patient care by providing more efficient and convenient access to medications. The most commonly used medications were acetaminophen, trazodone, and ibuprofen. In addition, the nursing and patient surveys demonstrated an overall satisfaction with the current PRN medication list. It is important to note that the number of avoidable ECS visits decreased significantly after the implementation of the PRN medication list in 2010.
Roughly 35% of patients in each group were excluded from the study. The main exclusion criteria included a < 4-week LOS, being admitted to the hospital, being female, and being admitted prior to the study period. Women veterans were treated through different programs prior to the implementation of the PRN medication list; therefore, they were excluded to decrease variability. Only patients in the GEN and SAR programs were included, because they were well established prior to and after the intervention. The other programs, which included PTSD, WOM, OEF/OIF/OND, DCHV, and I-ACT, accounted for about one-third of MHRRTP admissions. However, they were not all available or structured similarly in 2010. Including the other programs would have increased variability.
Survey Results
Although the response rates were low, the patient and nurse satisfaction surveys revealed useful information that may assist in identifying the strengths and weaknesses of the current program. More rigorous surveying needs to be conducted to make the results more generalizable. Fifty percent of patients reported using a PRN medication on a daily basis or 3 times per week. However, 28.6% stated they never used the PRN medication list, which was thought to be an overestimation due to an incomplete understanding of what medications are on the PRN medication list. This finding does not correlate with the high use demonstrated with the actual number of PRN medications used.
Two patients marked “other,” one reported using the list when they “need the medication,” and another did not mark an answer. Similarly, 57.1% of the nursing staff reported offering a PRN medication on a daily basis and discussing the list on admission every time. However, 28.6% of nursing staff stated they do not complete admission assessments or work in the medication room, most likely because they are licensed practical nurses and do not have those responsibilities. Interestingly, when asked about medications that should be removed from the PRN medication list, 1 nurse suggested removing trazodone, which was the second most used drug. Some of the medications patients suggested adding to the PRN medication list included creams for dry skin or fungal infections, calcium carbonate, and pain medications such as tramadol, aspirin, and naproxen. Nurses suggested adding aspirin, diphenhydramine, and nicotine gum. These responses will aid in enhancing the current PRN medication list by potentially increasing the types of medications offered.
Limitations
This study has several limitations that may affect its interpretation. The study was retrospective in nature and had a short study period. The data were collected from a single specialty program, which decreases the study’s generalizability, as not all VAMCs have a MHRRTP. Also, the average LOS in 2010 was longer than in 2013. This was related to the restructuring of the MHRRTP in the spring of 2013 to allow for more condensed programming. As a result, it may be reasonable to infer that there were more ECS visits prior to implementation of the PRN medication list due to the longer LOS in 2010. This confounding variable was minimized by normalizing the calculation for the number and percent of ECS visits avoided.
The patient population was limited to male veterans and the satisfaction questionnaires had low response rates. The low patient response rate may have been due to a lack of incentive, decreased health literacy, or possibly lack of time. The low nurse response rate may have been due to limited time and also lack of incentive. A larger response rate may have increased the PRN medication list use and satisfaction reported. This study looked at the change in the number of ECS visits; but, it did not investigate any changes in the number of primary care visits. Patients were able to go to their primary care appointments during their stay in the MHRRTP and may have received medications listed on the PRN medication list at these appointments, which could have been avoided. Last, the accuracy of the documentation in CPRS may be unclear and may have subjected the study to bias. Unfortunately, ECS does not use bar code medication administration, so the administration of medications has to be manually written into the ECS visit note. This method may be vulnerable to human error.
Future Directions
Future directions from this study include discussing the results with the MHRRTP staff and identifying areas of improvement to enhance the medication list. Some discussion points include the reasoning to remove trazodone and examples of inappropriate use. Furthermore, the questions asked by patients and general
suggestions made by the nursing staff identified that increased patient education of the PRN medication list should be implemented during the admission assessment process. This would improve patient understanding and awareness of the PRN medication list, because some patients did not know about the list or what medications it included. Moving forward, the results of this project may provide incentive for future implementation of PRN medication lists at other VA MHRRTPs.
Conclusion
This study confirms that the MHRRTP PRN medication list has been highly used since its implementation in 2010. The study also suggests that the nursing staff and patients are satisfied with the current process. Furthermore, these findings illustrate the PRN medication list’s success at decreasing unnecessary use of ECS and its association with avoiding cost. Further studies are needed to support the results seen in this analysis. Although these discoveries are preliminary, they may provide incentive for future implementation of PRN medication lists at other VA MHRRTPs.
Acknowledgements
Michelle Bury had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Department of Veterans Affairs. Mental Health Residential Rehabilitation Treatment Program. Washington, DC: Department of Veterans Affairs Website. https://vaww.portal.va.gov/sites/OMHS/mhrrtp/default.aspx. Accessed October 7, 2013.
2. Pharmacy Procedures for Safe Medication Management (SMM) in DOMs 123 and 43. Milwaukee, WI: Clement J. Zablocki VA Medical Center; September 2010.
3. Professional Services Memorandum VII-29. Milwaukee, WI: Clement J. Zablocki VA Medical Center; November 2010.
4. Petzel RA. Mental Health Residential Rehabilitation Treatment Program (MHRRTP): VHA Handbook 1162.02. Washington, DC: Veterans Health Administration; December 2010.
The Mental Health Residential Rehabilitation Treatment Program (MHRRTP) is an essential part of the mental health services offered at the Clement J. Zablocki VAMC (ZVAMC) in Milwaukee, Wisconsin. Across the nation, there are about 250 MHRRTPs, which are designed to provide rehabilitation and treatment services to veterans ranging in age from 18 to 80 years, with medical conditions, mental illness, addiction, or psychosocial deficits.1 About 900 patients were admitted to the ZVAMC MHRRTP in 2013.
Background
Prior to 2010, pharmacy administrators recognized that many MHRRTP patients were inappropriately using emergency care services (ECS) to obtain treatments for simple ailments that often required only the use of over-the-counter medications. This was likely associated with the Safe Medication Management (SMM) Policy as defined in Professional Services Memorandum VII-29.2,3 This policy states that MHRRTP patients are not allowed to bring in any home medications—all medications are reconciled and readministered on admission in an effort to reduce diversion.
A lack of 24-hour-per-day provider availability forced patients to find treatment elsewhere. A 6-month review was completed in 2010, which identified all of the MHRRTP patients who used ECS, their chief medical condition, and the medication(s) that were administered to each patient. This review identified a total of 254 ECS visits made by MHRRTP patients during this period. Twenty percent of these visits resulted in prescriptions for over-the-counter medications. As a result, an as needed (PRN) medication list was created for patients to have medications readily available for simple ailments with nursing oversight (Box). The goal of the PRN medication list is to reduce the amount of unnecessary ECS visits, decrease unnecessary cost, and improve treatment efficiency and overall patient care.
Treatment Programs
The ZVAMC MHRRTP has 189 beds divided among 7 different 6-week treatment programs, including General Men’s Program (GEN), Substance Abuse Rehabilitation (SAR), Posttraumatic Stress Disorder (PTSD), Women’s Program (WOM), Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND), Domiciliary Care for Homeless Veterans (DCHV), and Individualized Addiction Consultation Team (I-ACT).4
The treatment programs within the MHRRTP at the ZVAMC address goals of rehabilitation, recovery, health maintenance, improved quality of life, and community integration in addition to specific treatment of medical conditions, mental illnesses, addictive disorders, and homelessness. Various levels of care are available through the program, based on the needs of each veteran. This care generally provides methods to enhance patients’ functional status and psychosocial rehabilitation.
A SMM program is used to ensure safe and effective medication use for all patients in the MHRRTP.2 As a result, the patients are admitted to the MHRRTP with inpatient status, and the medication delivery procedure varies based on the veteran’s ability to take medication independently. Veterans are assisted in developing self-care skills, which include comprehensive medication education. The goal of the SMM program is to give patients the assistance to eventually manage their medications independently.
MHRRTP Staffing
The MHRRTP must have adequate staffing in order to provide safe and effective patient care. Program staffing patterns are based on workload indicators and a bed-to-staff ratio.4 The MHRRTP is a multidisciplinary program; however, the only providers who can address medication issues are the 1.2 full-time employee equivalent MHRRTP psychiatrists. Unfortunately, the psychiatrists are not available for triage on nights, weekends, or holidays.
The role of the psychiatrist is to focus on the mental health needs of the MHRRTP patients, not the primary care medical concerns, which are the main reason for ECS visits. With the current model, providers are sometimes unavailable to meet the emergent needs of patients in the MHRRTP, and patients may be forced to choose between using ECS or leaving the concern unaddressed. Patients’ needs vary from mild to serious emergent needs but may not necessarily require full emergency assessments. For example, if a patient has a headache and a physician is not available to write an order for acetaminophen, the patient may need to visit the ECS to obtain a medication that otherwise would have been readily available at home. The restrictions are designed to promote medication safety, prevent medication diversion and misuse, and be in compliance with regulatory agencies (eg, The Joint Commission and the Commission on Accreditation of Rehabilitation Facilities).
ECS Use
During fiscal year 2010, pharmacy administrators discovered that many patients were using ECS to obtain medications for nonemergent conditions. Inappropriate and unnecessary use of ECS by MHRRTP patients delayed treatment, increased wait times for veterans in need of emergent care, and increased the cost of caring for simple ailments. To put this into perspective, the average cost of all conditions at the ZVAMC during the 2013 fiscal year was $657 per ECS visit, while the total cost of ECS was about $14 million.
In response to the inappropriate ECS use, the ZVAMC created a PRN medication list in 2010, which is offered to all MHRRTP patients, with the goal of reducing the number of patients inappropriately using ECS for minor ailments and providing more efficient and cost-effective patient care.2 The MHRRTP PRN medication list is initially evaluated by the admitting psychiatrist or nurse practitioner and mental health clinical pharmacy specialist completing the admission orders for appropriateness based on each patient’s comorbidities, medication regimen, and past medical history. For example, if a new patient with liver dysfunction is admitted to the MHRRTP, acetaminophen would not be made available due to an increased risk of hepatotoxicity. The other PRN medications would still be available for the patient if clinically appropriate.
Once the PRN medications are ordered, the MHRRTP nurse can assess a patient’s condition and administer the medication(s) to the patient as indicated. For instance, if a patient requests ibuprofen for pain, the nurse will document an initial pain score and administer the ibuprofendose. As a result, the patient obtains more efficient and convenient care and does not need to wait for a provider to become available or use ECS. Per ZVAMC policy, the nurse has 96 hours to reassess the PRN medication effectiveness; however, this is typically done within the same shift. Since the implementation of the PRN medication list, no formal assessment has been completed.
To the authors’ knowledge, the ZVAMC is the only MHRRTP in the VHA system that incorporates a PRN medication list in the admission orders to reduce unnecessary ECS visits. After completing a thorough literature review and contacting the national VA mental health pharmacist listserve, no studies discussing the use of PRN medication lists in this setting were identified, and no sites offered information as to a similar practice in place.
Methods
A randomized, retrospective case-controlled study involving a chart review was completed for patients admitted to the MHRRTP at the ZVAMC pre- and postimplementation of the MHRRTP PRN medication list between April 2010 and August 2010 and between April 2013 and August 2013, respectively. The ZVAMC is a teaching institution. This study was approved by the ZVAMC institutional review board.
Patients were eligible for the study if they were male, aged > 18 years, and admitted during the study period for treatment in the GEN or SAR programs at the ZVAMC for at least 4 weeks. Patients were excluded if they were female, admitted to the hospital after being seen by ECS, or if they were receiving treatment in the following programs: PTSD, WOM, OEF/OIF/OND, DCHV, and I-ACT. Patients studied in 2010 served as the control group, and patients studied in 2013 were the treatment group.
Objectives
The primary objective of this study was to evaluate the use of the current PRN medication list. Secondary objectives included the evaluation of the use of ECS by patients admitted to the MHRRTP pre- and postimplementation of the PRN medication list, the potential cost reduction due to avoided ECS use, and nurse and patient satisfaction with the PRN medication list.
Data
A list of all patients admitted to the MHRRTP at the ZVAMC between April and August of 2010 and 2013 was generated using the Veterans Health Information Systems and Technology Architecture (VISTA)system. The Computerized Patient Record System (CPRS) was used to evaluate the patient for inclusion and collect pertinent data. The PRN medication list was implemented on September 15, 2010. Data collection terminated as of September 14, 2010, regardless of discharge status. All data collected for this study were entered and stored in a database created by the authors. A table with set criteria to review was created for the 2010 and 2013 group to ensure standardization. The pharmacy resident reviewed all of the patient charts. The following data were collected for each patient in the 2010 group:
- Demographic data: Patient name, last 4 digits of their social security number, age
- Program information: Admitted to GEN or SAR program, admission and discharge date, duration of stay, reason for discharge
- ECS data: Date, type of visit, chief condition, medications administered during the visit, whether the visit resulted in a hospital admission, and whether the visit was avoidable
- Avoidable visit: visit in which the patient received or could have received medication(s) that are on the PRN medication list at the ECS visit to treat their illness
The same information was collected for each patient in the 2013 group in addition to the following: PRN medication data (medications administered from the PRN medication list and the number of times each medication was administered if applicable); and ECS data (along with the aforementioned data, it was noted if PRN medications were taken prior to the ECS visit).
In addition, nurse and patient satisfaction with the PRN medication list were assessed via a simple satisfaction survey. The survey was given to 120 patients admitted to the MHRRTP as well as to 32 nurses at the time of distribution. A cover letter on each survey explained the study and informed the patient that the survey was voluntary and anonymous. Satisfaction was based on 10-point scale, with 1 (lowest) and 10 (highest) in satisfaction. Additional questions were asked to identify areas of improvement (see eAppendixes A and B for patient and nurse surveys, respectively).
Statistical Analysis
Descriptive statistics were used to analyze collected data. The primary outcome was assessed for the group admitted postintervention by calculating the average number of times each medication on the PRN medication list was used per patient during their length of stay (LOS) as applicable. The administration totals for each medication on the PRN medication list during the postintervention study period were also recorded.
Secondary outcomes were assessed by comparing the recorded total number of ECS visits pre- and postimplementation. Additionally, the average number of ECS visits per admission and the number of avoidable ECS visits were recorded for each study group. The cost reduction from avoided ECS use was estimated by calculating the total cost of ECS used pre- and postimplementation. The difference between the number of avoidable ECS visits in the pre- and postintervention groups was assessed for statistical significance by using a chi-square test. The 2013 cost saving estimation was based on the average ECS visit cost in the 2013 fiscal year ($657). Of note, power for this study could not be calculated as this has not been studied prior; therefore, no precedence has been set.
Results
On completion of the data collection, 583 patients were assessed for inclusion into the study, 325 in the 2010 preimplementation group and 258 in the 2013 postimplementation group. A total of 200 patients were randomized in each group (n = 400); however, 69 (35%) and 63 (32%) were excluded from the 2010 group and 2013 group, respectively. Sample demographics are described in the Table.
PRN Medication and ECS Use
Between April 1, 2013, and September 14, 2013, 3,959 doses of PRN medications were administered to MHRRTP patients who were included in the study (Figure). Prior to accessing ECS for their problem, 22 (36%) of the 61 patients who used ECS had trialed the PRN medication(s).
When comparing the total number of ECS visits, the 2010 group had 145 visits and the 2013 group had 96 visits. The preimplementation group averaged 1.1 ECS visits per MHRRTP admission, whereas the postimplementation group averaged 0.7 ECS visits per admission. The difference in the number of avoidable ECS visits was statistically significant, with the 2010 group totaling 15 avoidable visits, while the 2013 group totaled 1 ECS visit (P = .0045).
It was estimated that 9 (9.3%) ECS visits were avoided due to the PRN medication list in 2013. Using 137 patients, who were included in the postimplementation group, it can be calculated that $5,867 was saved due to the PRN medication list, or $42.83 per patient in 2013. Using the 2013 MHRRTP census of 898 patients, the financial impact of the PRN medication list can be extrapolated to produce an estimated annual cost savings of $38,461.
Patient and Nurse Satisfaction
Of the 120 patients given the patient satisfaction questionnaire, 28 (23%) patients responded. Of the respondents, 25 (89%) stated they were aware of the PRN medication list. The median rank of satisfaction reported was 8 on a 10-point scale. Twenty-two (79%) patients felt that the PRN medication list had or may have reduced the need to go to ECS or urgent care. Twenty-three (82%) patients recommended not removing any drugs listed on the PRN medication list.
Of the 32 registered nurses and licensed practical nurses working in the MHRRTP, 7 (22%) responded to the nurse satisfaction questionnaire. Of the respondents, 6 (86%) stated they discuss the PRN medication list during admission assessments every time or most of the time. The median rank of satisfaction was 9 on a 10-point scale. Four (57%) nurses felt patients had a clear understanding of the PRN medication list, and 100% of nurses stated they had enough guidance on situations to administer the medications. Seven (100%) stated that the PRN medication list had not caused adverse events; however, 5 (71%) stated that the list had been used inappropriately.
Discussion
This retrospective case-controlled study of 400 patients revealed high use of the PRN medication list and a cost avoidance of nearly $40,000. Although this represents a small reduction of the annual ECS budget, the PRN medication list also improved patient care by providing more efficient and convenient access to medications. The most commonly used medications were acetaminophen, trazodone, and ibuprofen. In addition, the nursing and patient surveys demonstrated an overall satisfaction with the current PRN medication list. It is important to note that the number of avoidable ECS visits decreased significantly after the implementation of the PRN medication list in 2010.
Roughly 35% of patients in each group were excluded from the study. The main exclusion criteria included a < 4-week LOS, being admitted to the hospital, being female, and being admitted prior to the study period. Women veterans were treated through different programs prior to the implementation of the PRN medication list; therefore, they were excluded to decrease variability. Only patients in the GEN and SAR programs were included, because they were well established prior to and after the intervention. The other programs, which included PTSD, WOM, OEF/OIF/OND, DCHV, and I-ACT, accounted for about one-third of MHRRTP admissions. However, they were not all available or structured similarly in 2010. Including the other programs would have increased variability.
Survey Results
Although the response rates were low, the patient and nurse satisfaction surveys revealed useful information that may assist in identifying the strengths and weaknesses of the current program. More rigorous surveying needs to be conducted to make the results more generalizable. Fifty percent of patients reported using a PRN medication on a daily basis or 3 times per week. However, 28.6% stated they never used the PRN medication list, which was thought to be an overestimation due to an incomplete understanding of what medications are on the PRN medication list. This finding does not correlate with the high use demonstrated with the actual number of PRN medications used.
Two patients marked “other,” one reported using the list when they “need the medication,” and another did not mark an answer. Similarly, 57.1% of the nursing staff reported offering a PRN medication on a daily basis and discussing the list on admission every time. However, 28.6% of nursing staff stated they do not complete admission assessments or work in the medication room, most likely because they are licensed practical nurses and do not have those responsibilities. Interestingly, when asked about medications that should be removed from the PRN medication list, 1 nurse suggested removing trazodone, which was the second most used drug. Some of the medications patients suggested adding to the PRN medication list included creams for dry skin or fungal infections, calcium carbonate, and pain medications such as tramadol, aspirin, and naproxen. Nurses suggested adding aspirin, diphenhydramine, and nicotine gum. These responses will aid in enhancing the current PRN medication list by potentially increasing the types of medications offered.
Limitations
This study has several limitations that may affect its interpretation. The study was retrospective in nature and had a short study period. The data were collected from a single specialty program, which decreases the study’s generalizability, as not all VAMCs have a MHRRTP. Also, the average LOS in 2010 was longer than in 2013. This was related to the restructuring of the MHRRTP in the spring of 2013 to allow for more condensed programming. As a result, it may be reasonable to infer that there were more ECS visits prior to implementation of the PRN medication list due to the longer LOS in 2010. This confounding variable was minimized by normalizing the calculation for the number and percent of ECS visits avoided.
The patient population was limited to male veterans and the satisfaction questionnaires had low response rates. The low patient response rate may have been due to a lack of incentive, decreased health literacy, or possibly lack of time. The low nurse response rate may have been due to limited time and also lack of incentive. A larger response rate may have increased the PRN medication list use and satisfaction reported. This study looked at the change in the number of ECS visits; but, it did not investigate any changes in the number of primary care visits. Patients were able to go to their primary care appointments during their stay in the MHRRTP and may have received medications listed on the PRN medication list at these appointments, which could have been avoided. Last, the accuracy of the documentation in CPRS may be unclear and may have subjected the study to bias. Unfortunately, ECS does not use bar code medication administration, so the administration of medications has to be manually written into the ECS visit note. This method may be vulnerable to human error.
Future Directions
Future directions from this study include discussing the results with the MHRRTP staff and identifying areas of improvement to enhance the medication list. Some discussion points include the reasoning to remove trazodone and examples of inappropriate use. Furthermore, the questions asked by patients and general
suggestions made by the nursing staff identified that increased patient education of the PRN medication list should be implemented during the admission assessment process. This would improve patient understanding and awareness of the PRN medication list, because some patients did not know about the list or what medications it included. Moving forward, the results of this project may provide incentive for future implementation of PRN medication lists at other VA MHRRTPs.
Conclusion
This study confirms that the MHRRTP PRN medication list has been highly used since its implementation in 2010. The study also suggests that the nursing staff and patients are satisfied with the current process. Furthermore, these findings illustrate the PRN medication list’s success at decreasing unnecessary use of ECS and its association with avoiding cost. Further studies are needed to support the results seen in this analysis. Although these discoveries are preliminary, they may provide incentive for future implementation of PRN medication lists at other VA MHRRTPs.
Acknowledgements
Michelle Bury had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The Mental Health Residential Rehabilitation Treatment Program (MHRRTP) is an essential part of the mental health services offered at the Clement J. Zablocki VAMC (ZVAMC) in Milwaukee, Wisconsin. Across the nation, there are about 250 MHRRTPs, which are designed to provide rehabilitation and treatment services to veterans ranging in age from 18 to 80 years, with medical conditions, mental illness, addiction, or psychosocial deficits.1 About 900 patients were admitted to the ZVAMC MHRRTP in 2013.
Background
Prior to 2010, pharmacy administrators recognized that many MHRRTP patients were inappropriately using emergency care services (ECS) to obtain treatments for simple ailments that often required only the use of over-the-counter medications. This was likely associated with the Safe Medication Management (SMM) Policy as defined in Professional Services Memorandum VII-29.2,3 This policy states that MHRRTP patients are not allowed to bring in any home medications—all medications are reconciled and readministered on admission in an effort to reduce diversion.
A lack of 24-hour-per-day provider availability forced patients to find treatment elsewhere. A 6-month review was completed in 2010, which identified all of the MHRRTP patients who used ECS, their chief medical condition, and the medication(s) that were administered to each patient. This review identified a total of 254 ECS visits made by MHRRTP patients during this period. Twenty percent of these visits resulted in prescriptions for over-the-counter medications. As a result, an as needed (PRN) medication list was created for patients to have medications readily available for simple ailments with nursing oversight (Box). The goal of the PRN medication list is to reduce the amount of unnecessary ECS visits, decrease unnecessary cost, and improve treatment efficiency and overall patient care.
Treatment Programs
The ZVAMC MHRRTP has 189 beds divided among 7 different 6-week treatment programs, including General Men’s Program (GEN), Substance Abuse Rehabilitation (SAR), Posttraumatic Stress Disorder (PTSD), Women’s Program (WOM), Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND), Domiciliary Care for Homeless Veterans (DCHV), and Individualized Addiction Consultation Team (I-ACT).4
The treatment programs within the MHRRTP at the ZVAMC address goals of rehabilitation, recovery, health maintenance, improved quality of life, and community integration in addition to specific treatment of medical conditions, mental illnesses, addictive disorders, and homelessness. Various levels of care are available through the program, based on the needs of each veteran. This care generally provides methods to enhance patients’ functional status and psychosocial rehabilitation.
A SMM program is used to ensure safe and effective medication use for all patients in the MHRRTP.2 As a result, the patients are admitted to the MHRRTP with inpatient status, and the medication delivery procedure varies based on the veteran’s ability to take medication independently. Veterans are assisted in developing self-care skills, which include comprehensive medication education. The goal of the SMM program is to give patients the assistance to eventually manage their medications independently.
MHRRTP Staffing
The MHRRTP must have adequate staffing in order to provide safe and effective patient care. Program staffing patterns are based on workload indicators and a bed-to-staff ratio.4 The MHRRTP is a multidisciplinary program; however, the only providers who can address medication issues are the 1.2 full-time employee equivalent MHRRTP psychiatrists. Unfortunately, the psychiatrists are not available for triage on nights, weekends, or holidays.
The role of the psychiatrist is to focus on the mental health needs of the MHRRTP patients, not the primary care medical concerns, which are the main reason for ECS visits. With the current model, providers are sometimes unavailable to meet the emergent needs of patients in the MHRRTP, and patients may be forced to choose between using ECS or leaving the concern unaddressed. Patients’ needs vary from mild to serious emergent needs but may not necessarily require full emergency assessments. For example, if a patient has a headache and a physician is not available to write an order for acetaminophen, the patient may need to visit the ECS to obtain a medication that otherwise would have been readily available at home. The restrictions are designed to promote medication safety, prevent medication diversion and misuse, and be in compliance with regulatory agencies (eg, The Joint Commission and the Commission on Accreditation of Rehabilitation Facilities).
ECS Use
During fiscal year 2010, pharmacy administrators discovered that many patients were using ECS to obtain medications for nonemergent conditions. Inappropriate and unnecessary use of ECS by MHRRTP patients delayed treatment, increased wait times for veterans in need of emergent care, and increased the cost of caring for simple ailments. To put this into perspective, the average cost of all conditions at the ZVAMC during the 2013 fiscal year was $657 per ECS visit, while the total cost of ECS was about $14 million.
In response to the inappropriate ECS use, the ZVAMC created a PRN medication list in 2010, which is offered to all MHRRTP patients, with the goal of reducing the number of patients inappropriately using ECS for minor ailments and providing more efficient and cost-effective patient care.2 The MHRRTP PRN medication list is initially evaluated by the admitting psychiatrist or nurse practitioner and mental health clinical pharmacy specialist completing the admission orders for appropriateness based on each patient’s comorbidities, medication regimen, and past medical history. For example, if a new patient with liver dysfunction is admitted to the MHRRTP, acetaminophen would not be made available due to an increased risk of hepatotoxicity. The other PRN medications would still be available for the patient if clinically appropriate.
Once the PRN medications are ordered, the MHRRTP nurse can assess a patient’s condition and administer the medication(s) to the patient as indicated. For instance, if a patient requests ibuprofen for pain, the nurse will document an initial pain score and administer the ibuprofendose. As a result, the patient obtains more efficient and convenient care and does not need to wait for a provider to become available or use ECS. Per ZVAMC policy, the nurse has 96 hours to reassess the PRN medication effectiveness; however, this is typically done within the same shift. Since the implementation of the PRN medication list, no formal assessment has been completed.
To the authors’ knowledge, the ZVAMC is the only MHRRTP in the VHA system that incorporates a PRN medication list in the admission orders to reduce unnecessary ECS visits. After completing a thorough literature review and contacting the national VA mental health pharmacist listserve, no studies discussing the use of PRN medication lists in this setting were identified, and no sites offered information as to a similar practice in place.
Methods
A randomized, retrospective case-controlled study involving a chart review was completed for patients admitted to the MHRRTP at the ZVAMC pre- and postimplementation of the MHRRTP PRN medication list between April 2010 and August 2010 and between April 2013 and August 2013, respectively. The ZVAMC is a teaching institution. This study was approved by the ZVAMC institutional review board.
Patients were eligible for the study if they were male, aged > 18 years, and admitted during the study period for treatment in the GEN or SAR programs at the ZVAMC for at least 4 weeks. Patients were excluded if they were female, admitted to the hospital after being seen by ECS, or if they were receiving treatment in the following programs: PTSD, WOM, OEF/OIF/OND, DCHV, and I-ACT. Patients studied in 2010 served as the control group, and patients studied in 2013 were the treatment group.
Objectives
The primary objective of this study was to evaluate the use of the current PRN medication list. Secondary objectives included the evaluation of the use of ECS by patients admitted to the MHRRTP pre- and postimplementation of the PRN medication list, the potential cost reduction due to avoided ECS use, and nurse and patient satisfaction with the PRN medication list.
Data
A list of all patients admitted to the MHRRTP at the ZVAMC between April and August of 2010 and 2013 was generated using the Veterans Health Information Systems and Technology Architecture (VISTA)system. The Computerized Patient Record System (CPRS) was used to evaluate the patient for inclusion and collect pertinent data. The PRN medication list was implemented on September 15, 2010. Data collection terminated as of September 14, 2010, regardless of discharge status. All data collected for this study were entered and stored in a database created by the authors. A table with set criteria to review was created for the 2010 and 2013 group to ensure standardization. The pharmacy resident reviewed all of the patient charts. The following data were collected for each patient in the 2010 group:
- Demographic data: Patient name, last 4 digits of their social security number, age
- Program information: Admitted to GEN or SAR program, admission and discharge date, duration of stay, reason for discharge
- ECS data: Date, type of visit, chief condition, medications administered during the visit, whether the visit resulted in a hospital admission, and whether the visit was avoidable
- Avoidable visit: visit in which the patient received or could have received medication(s) that are on the PRN medication list at the ECS visit to treat their illness
The same information was collected for each patient in the 2013 group in addition to the following: PRN medication data (medications administered from the PRN medication list and the number of times each medication was administered if applicable); and ECS data (along with the aforementioned data, it was noted if PRN medications were taken prior to the ECS visit).
In addition, nurse and patient satisfaction with the PRN medication list were assessed via a simple satisfaction survey. The survey was given to 120 patients admitted to the MHRRTP as well as to 32 nurses at the time of distribution. A cover letter on each survey explained the study and informed the patient that the survey was voluntary and anonymous. Satisfaction was based on 10-point scale, with 1 (lowest) and 10 (highest) in satisfaction. Additional questions were asked to identify areas of improvement (see eAppendixes A and B for patient and nurse surveys, respectively).
Statistical Analysis
Descriptive statistics were used to analyze collected data. The primary outcome was assessed for the group admitted postintervention by calculating the average number of times each medication on the PRN medication list was used per patient during their length of stay (LOS) as applicable. The administration totals for each medication on the PRN medication list during the postintervention study period were also recorded.
Secondary outcomes were assessed by comparing the recorded total number of ECS visits pre- and postimplementation. Additionally, the average number of ECS visits per admission and the number of avoidable ECS visits were recorded for each study group. The cost reduction from avoided ECS use was estimated by calculating the total cost of ECS used pre- and postimplementation. The difference between the number of avoidable ECS visits in the pre- and postintervention groups was assessed for statistical significance by using a chi-square test. The 2013 cost saving estimation was based on the average ECS visit cost in the 2013 fiscal year ($657). Of note, power for this study could not be calculated as this has not been studied prior; therefore, no precedence has been set.
Results
On completion of the data collection, 583 patients were assessed for inclusion into the study, 325 in the 2010 preimplementation group and 258 in the 2013 postimplementation group. A total of 200 patients were randomized in each group (n = 400); however, 69 (35%) and 63 (32%) were excluded from the 2010 group and 2013 group, respectively. Sample demographics are described in the Table.
PRN Medication and ECS Use
Between April 1, 2013, and September 14, 2013, 3,959 doses of PRN medications were administered to MHRRTP patients who were included in the study (Figure). Prior to accessing ECS for their problem, 22 (36%) of the 61 patients who used ECS had trialed the PRN medication(s).
When comparing the total number of ECS visits, the 2010 group had 145 visits and the 2013 group had 96 visits. The preimplementation group averaged 1.1 ECS visits per MHRRTP admission, whereas the postimplementation group averaged 0.7 ECS visits per admission. The difference in the number of avoidable ECS visits was statistically significant, with the 2010 group totaling 15 avoidable visits, while the 2013 group totaled 1 ECS visit (P = .0045).
It was estimated that 9 (9.3%) ECS visits were avoided due to the PRN medication list in 2013. Using 137 patients, who were included in the postimplementation group, it can be calculated that $5,867 was saved due to the PRN medication list, or $42.83 per patient in 2013. Using the 2013 MHRRTP census of 898 patients, the financial impact of the PRN medication list can be extrapolated to produce an estimated annual cost savings of $38,461.
Patient and Nurse Satisfaction
Of the 120 patients given the patient satisfaction questionnaire, 28 (23%) patients responded. Of the respondents, 25 (89%) stated they were aware of the PRN medication list. The median rank of satisfaction reported was 8 on a 10-point scale. Twenty-two (79%) patients felt that the PRN medication list had or may have reduced the need to go to ECS or urgent care. Twenty-three (82%) patients recommended not removing any drugs listed on the PRN medication list.
Of the 32 registered nurses and licensed practical nurses working in the MHRRTP, 7 (22%) responded to the nurse satisfaction questionnaire. Of the respondents, 6 (86%) stated they discuss the PRN medication list during admission assessments every time or most of the time. The median rank of satisfaction was 9 on a 10-point scale. Four (57%) nurses felt patients had a clear understanding of the PRN medication list, and 100% of nurses stated they had enough guidance on situations to administer the medications. Seven (100%) stated that the PRN medication list had not caused adverse events; however, 5 (71%) stated that the list had been used inappropriately.
Discussion
This retrospective case-controlled study of 400 patients revealed high use of the PRN medication list and a cost avoidance of nearly $40,000. Although this represents a small reduction of the annual ECS budget, the PRN medication list also improved patient care by providing more efficient and convenient access to medications. The most commonly used medications were acetaminophen, trazodone, and ibuprofen. In addition, the nursing and patient surveys demonstrated an overall satisfaction with the current PRN medication list. It is important to note that the number of avoidable ECS visits decreased significantly after the implementation of the PRN medication list in 2010.
Roughly 35% of patients in each group were excluded from the study. The main exclusion criteria included a < 4-week LOS, being admitted to the hospital, being female, and being admitted prior to the study period. Women veterans were treated through different programs prior to the implementation of the PRN medication list; therefore, they were excluded to decrease variability. Only patients in the GEN and SAR programs were included, because they were well established prior to and after the intervention. The other programs, which included PTSD, WOM, OEF/OIF/OND, DCHV, and I-ACT, accounted for about one-third of MHRRTP admissions. However, they were not all available or structured similarly in 2010. Including the other programs would have increased variability.
Survey Results
Although the response rates were low, the patient and nurse satisfaction surveys revealed useful information that may assist in identifying the strengths and weaknesses of the current program. More rigorous surveying needs to be conducted to make the results more generalizable. Fifty percent of patients reported using a PRN medication on a daily basis or 3 times per week. However, 28.6% stated they never used the PRN medication list, which was thought to be an overestimation due to an incomplete understanding of what medications are on the PRN medication list. This finding does not correlate with the high use demonstrated with the actual number of PRN medications used.
Two patients marked “other,” one reported using the list when they “need the medication,” and another did not mark an answer. Similarly, 57.1% of the nursing staff reported offering a PRN medication on a daily basis and discussing the list on admission every time. However, 28.6% of nursing staff stated they do not complete admission assessments or work in the medication room, most likely because they are licensed practical nurses and do not have those responsibilities. Interestingly, when asked about medications that should be removed from the PRN medication list, 1 nurse suggested removing trazodone, which was the second most used drug. Some of the medications patients suggested adding to the PRN medication list included creams for dry skin or fungal infections, calcium carbonate, and pain medications such as tramadol, aspirin, and naproxen. Nurses suggested adding aspirin, diphenhydramine, and nicotine gum. These responses will aid in enhancing the current PRN medication list by potentially increasing the types of medications offered.
Limitations
This study has several limitations that may affect its interpretation. The study was retrospective in nature and had a short study period. The data were collected from a single specialty program, which decreases the study’s generalizability, as not all VAMCs have a MHRRTP. Also, the average LOS in 2010 was longer than in 2013. This was related to the restructuring of the MHRRTP in the spring of 2013 to allow for more condensed programming. As a result, it may be reasonable to infer that there were more ECS visits prior to implementation of the PRN medication list due to the longer LOS in 2010. This confounding variable was minimized by normalizing the calculation for the number and percent of ECS visits avoided.
The patient population was limited to male veterans and the satisfaction questionnaires had low response rates. The low patient response rate may have been due to a lack of incentive, decreased health literacy, or possibly lack of time. The low nurse response rate may have been due to limited time and also lack of incentive. A larger response rate may have increased the PRN medication list use and satisfaction reported. This study looked at the change in the number of ECS visits; but, it did not investigate any changes in the number of primary care visits. Patients were able to go to their primary care appointments during their stay in the MHRRTP and may have received medications listed on the PRN medication list at these appointments, which could have been avoided. Last, the accuracy of the documentation in CPRS may be unclear and may have subjected the study to bias. Unfortunately, ECS does not use bar code medication administration, so the administration of medications has to be manually written into the ECS visit note. This method may be vulnerable to human error.
Future Directions
Future directions from this study include discussing the results with the MHRRTP staff and identifying areas of improvement to enhance the medication list. Some discussion points include the reasoning to remove trazodone and examples of inappropriate use. Furthermore, the questions asked by patients and general
suggestions made by the nursing staff identified that increased patient education of the PRN medication list should be implemented during the admission assessment process. This would improve patient understanding and awareness of the PRN medication list, because some patients did not know about the list or what medications it included. Moving forward, the results of this project may provide incentive for future implementation of PRN medication lists at other VA MHRRTPs.
Conclusion
This study confirms that the MHRRTP PRN medication list has been highly used since its implementation in 2010. The study also suggests that the nursing staff and patients are satisfied with the current process. Furthermore, these findings illustrate the PRN medication list’s success at decreasing unnecessary use of ECS and its association with avoiding cost. Further studies are needed to support the results seen in this analysis. Although these discoveries are preliminary, they may provide incentive for future implementation of PRN medication lists at other VA MHRRTPs.
Acknowledgements
Michelle Bury had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Department of Veterans Affairs. Mental Health Residential Rehabilitation Treatment Program. Washington, DC: Department of Veterans Affairs Website. https://vaww.portal.va.gov/sites/OMHS/mhrrtp/default.aspx. Accessed October 7, 2013.
2. Pharmacy Procedures for Safe Medication Management (SMM) in DOMs 123 and 43. Milwaukee, WI: Clement J. Zablocki VA Medical Center; September 2010.
3. Professional Services Memorandum VII-29. Milwaukee, WI: Clement J. Zablocki VA Medical Center; November 2010.
4. Petzel RA. Mental Health Residential Rehabilitation Treatment Program (MHRRTP): VHA Handbook 1162.02. Washington, DC: Veterans Health Administration; December 2010.
1. Department of Veterans Affairs. Mental Health Residential Rehabilitation Treatment Program. Washington, DC: Department of Veterans Affairs Website. https://vaww.portal.va.gov/sites/OMHS/mhrrtp/default.aspx. Accessed October 7, 2013.
2. Pharmacy Procedures for Safe Medication Management (SMM) in DOMs 123 and 43. Milwaukee, WI: Clement J. Zablocki VA Medical Center; September 2010.
3. Professional Services Memorandum VII-29. Milwaukee, WI: Clement J. Zablocki VA Medical Center; November 2010.
4. Petzel RA. Mental Health Residential Rehabilitation Treatment Program (MHRRTP): VHA Handbook 1162.02. Washington, DC: Veterans Health Administration; December 2010.
Assessing the Quality of VA Animal Care and Use Programs
Institutions conducting research involving animals have established operational frameworks, referred to as animal care and use programs (ACUPs), to ensure research animal welfare and high-quality research data and to meet ethical and regulatory requirements.1-4 The Institutional Animal Care and Use Committee (IACUC) is a critical component of the ACUP and is responsible for the oversight and evaluation of all aspects of the ACUP.5 However, investigators, IACUCs, institutions, the research sponsor, and the federal government share responsibilities for ensuring research animal welfare.
Effective policies, procedures, practices, and systems in the ACUP are critical to an institution’s ability to ensure that animal research is conducted humanely and complies with applicable regulations, policies, and guidelines. To this end, considerable effort and resources have been devoted to improve the effectiveness of ACUPs, including external accreditation of ACUPs by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and implementation of science-based performance standards, postapproval monitoring, and risk assessments and mitigation of identified vulnerability.6-9 However, the impact of these quality improvement measures remains unclear. There have been no valid, reliable, and quantifiable measures to assess the effectiveness and quality of ACUPs.
Compliance with federal regulations is not only required, but also essential in protecting laboratory animals. However, the goal is not to ensure compliance but to prevent unnecessary harm, injury, and suffering to those research animals. Overemphasis on compliance and documentation may negatively impact the system by diverting resources away from ensuring research animal welfare. The authors propose that although research animal welfare cannot be directly measured, it is possible to assess the quality of ACUPs. High-quality ACUPs are expected to minimize risk to research animals to the extent possible while maintaining the integrity of the research.
The authors previously developed a set of quality indicators (QIs) for human research protection programs (HRPPs) at the VA, emphasizing performance outcomes built on a foundation of compliance.10 Implementation of these QIs allowed the research team to collect data to assess the quality of VA HRPPs.11 It also allowed the team to answer important questions, such as whether there were significant differences in the quality of HRPPs among facilities using their own institutional review boards (IRBs) and those using affiliated university IRBs as their IRBs of record.12
Background
The VA health care system (VAHCS) is the largest integrated health care system in the U.S. Currently, there are 77 VA facilities conducting research involving laboratory animals. In addition to federal regulations governing research with animals, researchers in the VAHCS must comply with requirements established by VA.1-4 For example, in the VAHCS, the IACUC is a subcommittee of the Research and Development Committee (R&DC). Research involving animals may not be initiated until it has been approved by both the IACUC and the R&DC.13,14 All investigators, including animal research investigators, are required to have approved scopes of practice.14 Furthermore, all VA facilities that conduct animal research are required to have their ACUPs accredited by the AAALAC International.13
Based on the experience gained from the VA HRPP QIs, the authors developed a set of QIs that emphasize assessing the outcome of ACUPs rather than solely on IACUC review or compliance with animal research regulations and policies. This report describes the proposed QIs for assessing the quality of VA ACUPs and presents preliminary data using some of these QIs.
Methods
The VA ACUP QIs were developed through a process that included (1) identification of a set of potential indicators by the authors; (2) review and comments on the potential indicators by individuals within and outside VA who have expertise in protecting research animal welfare, including veterinarians with board certification in laboratory animal medicine, IACUC chairs, and individuals involved in the accreditation and oversight of ACUPs; and (3) review and revision by the authors of the proposed QIs in light of the suggestions and comments received. After 6 months of deliberation, a set of 13 QIs was finalized for consideration for implementation.
Data Collection
As part of the VA ACUP quality assurance program, each VA research facility is required to conduct regulatory audits of all animal research protocols once every 3 years by qualified research compliance officers (RCOs).15 Audit tools were developed for the triennial animal protocol regulatory audits (available at http://www.va.gov/oro/rcep.asp).11,12 Facility RCOs were then trained to use these tools to conduct audits throughout the year.
Results of the protocol regulatory audits, conducted between June 1, 2011, and May 31, 2012, were collected through a Web-based system from all 74 VA facilities conducting animal research during that period. Information collected included IACUC and R&DC initial approval of human research protocols; for-cause suspension or termination of animal research protocols; compliance with continuing review requirements; research personnel scopes of practice; and investigator animal research protection training requirements.
Because this study did not involve the use of laboratory animals, no ACUC review and approval was required.
Data Analysis
All data collected were entered into a database for analysis. When necessary, facilities were contacted to verify the accuracy and uniformity of data reported. Only descriptive statistics were obtained and presented.
Quality Indicators
As shown in the Box, a total of 13 QIs covering a broad range of areas that may have significant impact on research animal welfare were selected.
QI 1. ACUP accreditation status was chosen, because accreditation of an institutional ACUP by AAALAC International, the sole widely accepted ACUP accrediting organization, suggests that the institution establish acceptable operational frameworks to ensure research animal welfare. Because VA policy requires that all facilities conducting animal research be accredited, failure to achieve full accreditation may indicate that research animals are at an elevated risk due to a less than optimal system to protect research animals.13
QI 2. IACUC and R&DC initial approval of animal research protocols was chosen because of the importance of IACUC and R&DC review and approval in ensuring the scientific merit of the research and the adequacy of research animal protection. The number and the percentage of protocols conducted without or initiated prior to IACUC and/or R&DC approval, which may put animals at risk, is a good measure of the adequacy of the institution’s ACUP.
QI 3. For-cause suspension or termination of animal research protocols was chosen, because this is a serious event. Protocols can be suspended or prematurely terminated by IACUCs due to investigators’ serious or continuing noncompliance or due to serious adverse events/injuries to the animals or research personnel. The number and percentage of protocols suspended reflect the adequacy of the IACUC oversight of the institution’s animal research program.
QI 4. Investigator sanction was chosen, because investigators and research personnel play an important role in protecting research animals. The number and percentage of investigators or technicians whose research privileges were suspended due to noncompliance reflect the adequacy of the institution’s education and training program as well as oversight of the ACUP.
QI 5. Annual review requirement was chosen because of the importance of ongoing oversight of approved animal research by the IACUC. The number and percentage of protocols lapsed in annual reviews, particularly when research activities continued during the lapse reflects the adequacy of IACUC oversight.
QI 6. Unanticipated loss of animal lives was chosen, because loss of animal lives is the most serious harm to animals that the ACUP is intended to prevent. The number and percentage of animals whose lives are unnecessarily lost due to heating, ventilation, or air-conditioning failure reflect the adequacy of the institution’s animal care infrastructure and effectiveness of the emergency response plan.
QI 7. Serious or continuing noncompliance resulting in actual harm to animals was chosen, because actual harm to animals is an important outcome measure of the adequacy of ACUP. The number and percentage of animals harmed due to investigator noncompliance or inadequate care reflect the adequacy of the institution’s veterinarian and IACUC oversight.
QI 8. Semi-annual program review and facility inspection was chosen because of the importance of semi-annual program review and facility inspection in IACUC’s oversight of the institution’s ACUP. This QI emphasizes the timely correction and remediation of both major and minor deficiencies identified during semi-annual program reviews and facility inspections. Failure to promptly address identified deficiencies in a timely manner may place research animals at significant risk.
QI 9. Scope of practice was chosen because of the importance of the investigator’s qualification in ensuring not only high-quality research data, but also adequate protection of research animals. Certain animal procedures can be safely performed only by investigators with adequate training and experience. Allowing investigators who are unqualified to perform these procedures places animals at significant risk of being harmed.
QI 10. Work- or research-related injuries was chosen because of the importance of the safety of investigators and animal caretakers in the institution’s ACUP. The importance of the institution’s occupational health and safety program in protecting investigators and animal care workers cannot be overemphasized. The number and percentage of investigators and animal care workers covered by the occupational health and safety program and work- or research-related injuries reflect the adequacy of the ACUP.
QI 11. Investigator animal care and use education/training requirements was chosen because of the important role of investigators in protecting animal welfare. The number and percentage of investigators who fail to maintain required animal care and use education/training reflect the adequacy of the institution’s IACUC oversight.
QI 12. IACUC chair and members’ animal care and use education and training requirements was chosen because of the important role of the IACUC chair and members in the institution’s ACUP. To appropriately evaluate and approve/disapprove animal research protocols, the chair and members of IACUC must maintain sufficient knowledge of federal regulations and VA policies regarding animal protections.
QI 13. Veterinarian and veterinary medical unit staff qualification was chosen because of the important role of veterinarian and veterinary medical unit staff in the day-to-day care of research animals and the specialized knowledge and qualification they need to maintain the animal research facilities. The number of veterinarians and nonveterinary animal care staff with appropriate board certifications reflects the strength of an institution’s ACUP.
Results
Recognizing the importance of assessing the quality of VA ACUPs, the authors started to collect some QI data of VA ACUPs parallel to those of VA HRPPs before the aforementioned proposed QIs for VA ACUPs were fully developed. These preliminary data are included here to demonstrate the feasibility of implementing these proposed VA ACUP QIs.
IACUC and R&DC Approvals (QI 2)
VA policies require that all animal research protocols be reviewed and approved first by the IACUC and then by the R&DC.13,14 The IACUC is a subcommittee of the R&DC. No animal research activities in VA may be initiated before receiving both IACUC and R&DC approval.13,14
Between June 1, 2011, and May 31, 2012, regulatory audits were conducted on 1,286 animal research protocols. Among them, 1 (0.08%) protocol was conducted and completed without the required IACUC approval, 1 (0.08%) was conducted and completed without the required R&DC approval, 1 (0.08%) was initiated prior to IACUC approval, and 2 (0.16%) were initiated prior to R&DC approval.
For-Cause Suspension or Termination (QI 3)
Among the 1,286 animal research protocols audited, 14 (1.09%) protocols were suspended or terminated for cause; 10 (0.78%) protocols were suspended or terminated due to animal safety concerns; and 4 (0.31%) protocols were suspended or terminated due to investigator-related concerns.
Lapse in Continuing Reviews (QI 5)
Federal regulations and VA policies require that IACUC conduct continuing review of all animal research protocols annually.2,13 Of the 1,286 animal research protocols audited, 1,159 protocols required IACUC continuing reviews during the auditing period. Fifty-three protocols (4.57%) lapsed in IACUC annual reviews, and in 25 of these 53 protocols, investigators continued research activities during the lapse.
Scope of Practice (QI 9)
VA policies require all research personnel to have an approved research scope of practice or functional statement that defines the duties that the individual is qualified and allowed to perform for research purposes.14
A total of 4,604 research personnel records were reviewed from the 1,286 animal research protocols audited. Of these, 276 (5.99%) did not have an approved research scope of practice; 1 (0.02%) had an approved research scope of practice but was working outside the approved research scope of practice.
Training Requirements (QI 11)
VA policies require that all research personnel who participate in animal research complete initial and annual training to ensure that they can competently and humanely perform their duties related to animal research.14
Among the 4,604 animal research personnel records reviewed, 186 (4.04%) did not maintain their training requirements, including 26 (0.56%) without required initial training and 160 (3.48%) with lapses in required continuing training.
Discussion
Collectively, these proposed QIs should provide useful information about the overall quality of an ACUP. This allows semiquantitative assessment of the quality and performance of VA facilities’ ACUPs over time and comparison of the performance of ACUPs across research facilities in the VAHCS. The information obtained may also help administrators identify program vulnerabilities and make management decisions regarding where improvements are most needed. Specifically, QI data will be collected from all VA research facilities’ ACUPs annually. National averages for all QIs will be calculated. Each facility will then be provided with the results of its own ACUP QI data as well as the national averages, allowing the facility to compare its QI data with the national averages and determine how its ACUP performs compared with the overall VA ACUP performance.
These QIs were designed for use in assessing the quality of ACUPs at VA research facilities annually or at least once every other year. With the recent requirement that a full-time RCO at each VA research facility conduct regulatory audits of all animal research protocols once every 3 years, it is feasible that an assessment of the VA ACUPs using these QIs could be conducted annually as demonstrated by the preliminary data for QIs 2, 3, 5, 9, and 11 reported here.15,16 These preliminary data also showed high rates of lapses in IACUC continuing review (4.57%), lack of research personnel scopes of practice (5.99%), and noncompliance with training requirements (4.04%). These are areas that need improvements.
The size and complexity of animal research programs are different among different facilities, which can make it difficult to compare different facilities’ ACUPs using the same quality measures. In addition, VA facilities may use their own IACUCs or the affiliate university IACUCs as the IACUCs of record. However, based on the authors’ experience using HRPP QIs to assess the quality of VA HRPPs, the collected data using ACUP QIs will help determine whether such variables as the size and complexity of a program or the kind of IACUCs used (either VA, own IACUC, or affiliate IACUC) affect the quality of VA ACUPs.10-12
Limitations
There is no evidence proving that these QIs are the most optimal measures for evaluating the quality of a VA facility’s ACUP. It is also unknown whether these QIs correlate directly with the protection of research animals. Furthermore, a quantitative, numerical value cannot be put on each indicator to allow evaluators to rank facilities’ ACUPs.
Some QIs, such as QIs 3, 4, 7, and 8, may depend on how stringent an IACUC is. For example, it is possible that a conscientious IACUC may report more noncompliance or suspend more protocols, giving the appearance of a poor quality ACUP, whereas in fact it might be an excellent program. However, the authors want to emphasize that no single QI by itself is sufficient to assess the quality of a program. It is the combination of various QIs that provides information about the overall quality of a program. It is also through the data collected that the usefulness of any particular indicators may be determined.
Conclusion
These proposed QIs provide a useful first step toward developing a robust and valid assessment of VA ACUPs. As these QIs are used at VA facilities, they will likely be redefined and modified. The authors hope that other institutions will find these indicators useful as they develop instruments to assess their own ACUPs.
Acknowledgement
The authors thank Dr. Kathryn Bayne, Global Director, Association for Assessment and Accreditation of Laboratory Animal Care International, for her suggestions and comments during the development of these quality indicators and critical review of the manuscript, and Dr. J. Thomas Puglisi, Chief Officer, VA Office of Research Oversight, for his support and critical review of the manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Animal Welfare Act, 7 USC §2131-2156 (2008).
2. Animal Welfare Regulations, 9 CFR §1-4 (2008).
3. National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011.
4. Office of Laboratory Animal Welfare. Public Health Service Policy On Humane Care And Use Of Laboratory Animals. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; 2015. NIH publication 15-8013. http://grants.nih.gov/grants/olaw//PHSPolicyLabAnimals.pdf. Revised 2015. Accessed August 3, 2015.
5. Sandgren EP. Defining the animal care and use program. Lab Anim (NY). 2005;34(10):41-44.
6. Association for Assessment and Accreditation of Laboratory Animal Care International. The AAALAC International accreditation program. The Association for Assessment and Accreditation of Laboratory Animal Care International Website. http://www.aaalac.org/accreditation/index.cfm. Updated 2015. Accessed August 3, 2015.
7. Klein HJ, Bayne KA. Establishing a culture of care, conscience, and responsibility: addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007;48(1):3-11.
8. Banks RE, Norton JN. A sample postapproval monitoring program in academia. ILAR J. 2008;49(4):402-418.
9. Van Sluyters RC. A guide to risk assessment in animal care and use programs: the metaphor of the 3-legged stool. ILAR J. 2008;49(4):372-378.
10. Tsan MF, Smith K, Gao B. Assessing the quality of human research protection programs: the experience at the Department of Veterans Affairs. IRB. 2010;32(4):16-19.
11. Tsan MF, Nguyen Y, Brooks R. Using quality indicators to assess human research protection programs at the Department of Veterans Affairs. IRB. 2013;35(1):10-14.
12. Tsan MF, Nguyen Y, Brooks B. Assessing the quality of VA Human Research Protection Programs: VA vs. affiliated University Institutional Review Board. J Emp Res Hum Res Ethics. 2013;8(2):153-160.
13. VA Research and Development Service. Use of Animals in Research. VHA Handbook 1200.07. Washington, DC: Department of Veterans Affairs, Veterans Health Administration; 2011.
14. VA Research and Development Service. Research and Development (R&D) Committee. VHA Handbook 1200.01. Washington, DC: Veterans Health Administration; 2009.
15. Research Compliance Officers and the Auditing of VHA Human Subjects Research to Determine Compliance with Applicable Laws, Regulations, and Policies. VHA Directive 2008-064. Washington, DC: Veterans Health Administration; 2008.
16. VA Office of Research Oversight. Research Compliance Reporting Requirements. VHA Handbook 1058.01. Washington, DC: Veterans Health Administration; 2015.
Institutions conducting research involving animals have established operational frameworks, referred to as animal care and use programs (ACUPs), to ensure research animal welfare and high-quality research data and to meet ethical and regulatory requirements.1-4 The Institutional Animal Care and Use Committee (IACUC) is a critical component of the ACUP and is responsible for the oversight and evaluation of all aspects of the ACUP.5 However, investigators, IACUCs, institutions, the research sponsor, and the federal government share responsibilities for ensuring research animal welfare.
Effective policies, procedures, practices, and systems in the ACUP are critical to an institution’s ability to ensure that animal research is conducted humanely and complies with applicable regulations, policies, and guidelines. To this end, considerable effort and resources have been devoted to improve the effectiveness of ACUPs, including external accreditation of ACUPs by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and implementation of science-based performance standards, postapproval monitoring, and risk assessments and mitigation of identified vulnerability.6-9 However, the impact of these quality improvement measures remains unclear. There have been no valid, reliable, and quantifiable measures to assess the effectiveness and quality of ACUPs.
Compliance with federal regulations is not only required, but also essential in protecting laboratory animals. However, the goal is not to ensure compliance but to prevent unnecessary harm, injury, and suffering to those research animals. Overemphasis on compliance and documentation may negatively impact the system by diverting resources away from ensuring research animal welfare. The authors propose that although research animal welfare cannot be directly measured, it is possible to assess the quality of ACUPs. High-quality ACUPs are expected to minimize risk to research animals to the extent possible while maintaining the integrity of the research.
The authors previously developed a set of quality indicators (QIs) for human research protection programs (HRPPs) at the VA, emphasizing performance outcomes built on a foundation of compliance.10 Implementation of these QIs allowed the research team to collect data to assess the quality of VA HRPPs.11 It also allowed the team to answer important questions, such as whether there were significant differences in the quality of HRPPs among facilities using their own institutional review boards (IRBs) and those using affiliated university IRBs as their IRBs of record.12
Background
The VA health care system (VAHCS) is the largest integrated health care system in the U.S. Currently, there are 77 VA facilities conducting research involving laboratory animals. In addition to federal regulations governing research with animals, researchers in the VAHCS must comply with requirements established by VA.1-4 For example, in the VAHCS, the IACUC is a subcommittee of the Research and Development Committee (R&DC). Research involving animals may not be initiated until it has been approved by both the IACUC and the R&DC.13,14 All investigators, including animal research investigators, are required to have approved scopes of practice.14 Furthermore, all VA facilities that conduct animal research are required to have their ACUPs accredited by the AAALAC International.13
Based on the experience gained from the VA HRPP QIs, the authors developed a set of QIs that emphasize assessing the outcome of ACUPs rather than solely on IACUC review or compliance with animal research regulations and policies. This report describes the proposed QIs for assessing the quality of VA ACUPs and presents preliminary data using some of these QIs.
Methods
The VA ACUP QIs were developed through a process that included (1) identification of a set of potential indicators by the authors; (2) review and comments on the potential indicators by individuals within and outside VA who have expertise in protecting research animal welfare, including veterinarians with board certification in laboratory animal medicine, IACUC chairs, and individuals involved in the accreditation and oversight of ACUPs; and (3) review and revision by the authors of the proposed QIs in light of the suggestions and comments received. After 6 months of deliberation, a set of 13 QIs was finalized for consideration for implementation.
Data Collection
As part of the VA ACUP quality assurance program, each VA research facility is required to conduct regulatory audits of all animal research protocols once every 3 years by qualified research compliance officers (RCOs).15 Audit tools were developed for the triennial animal protocol regulatory audits (available at http://www.va.gov/oro/rcep.asp).11,12 Facility RCOs were then trained to use these tools to conduct audits throughout the year.
Results of the protocol regulatory audits, conducted between June 1, 2011, and May 31, 2012, were collected through a Web-based system from all 74 VA facilities conducting animal research during that period. Information collected included IACUC and R&DC initial approval of human research protocols; for-cause suspension or termination of animal research protocols; compliance with continuing review requirements; research personnel scopes of practice; and investigator animal research protection training requirements.
Because this study did not involve the use of laboratory animals, no ACUC review and approval was required.
Data Analysis
All data collected were entered into a database for analysis. When necessary, facilities were contacted to verify the accuracy and uniformity of data reported. Only descriptive statistics were obtained and presented.
Quality Indicators
As shown in the Box, a total of 13 QIs covering a broad range of areas that may have significant impact on research animal welfare were selected.
QI 1. ACUP accreditation status was chosen, because accreditation of an institutional ACUP by AAALAC International, the sole widely accepted ACUP accrediting organization, suggests that the institution establish acceptable operational frameworks to ensure research animal welfare. Because VA policy requires that all facilities conducting animal research be accredited, failure to achieve full accreditation may indicate that research animals are at an elevated risk due to a less than optimal system to protect research animals.13
QI 2. IACUC and R&DC initial approval of animal research protocols was chosen because of the importance of IACUC and R&DC review and approval in ensuring the scientific merit of the research and the adequacy of research animal protection. The number and the percentage of protocols conducted without or initiated prior to IACUC and/or R&DC approval, which may put animals at risk, is a good measure of the adequacy of the institution’s ACUP.
QI 3. For-cause suspension or termination of animal research protocols was chosen, because this is a serious event. Protocols can be suspended or prematurely terminated by IACUCs due to investigators’ serious or continuing noncompliance or due to serious adverse events/injuries to the animals or research personnel. The number and percentage of protocols suspended reflect the adequacy of the IACUC oversight of the institution’s animal research program.
QI 4. Investigator sanction was chosen, because investigators and research personnel play an important role in protecting research animals. The number and percentage of investigators or technicians whose research privileges were suspended due to noncompliance reflect the adequacy of the institution’s education and training program as well as oversight of the ACUP.
QI 5. Annual review requirement was chosen because of the importance of ongoing oversight of approved animal research by the IACUC. The number and percentage of protocols lapsed in annual reviews, particularly when research activities continued during the lapse reflects the adequacy of IACUC oversight.
QI 6. Unanticipated loss of animal lives was chosen, because loss of animal lives is the most serious harm to animals that the ACUP is intended to prevent. The number and percentage of animals whose lives are unnecessarily lost due to heating, ventilation, or air-conditioning failure reflect the adequacy of the institution’s animal care infrastructure and effectiveness of the emergency response plan.
QI 7. Serious or continuing noncompliance resulting in actual harm to animals was chosen, because actual harm to animals is an important outcome measure of the adequacy of ACUP. The number and percentage of animals harmed due to investigator noncompliance or inadequate care reflect the adequacy of the institution’s veterinarian and IACUC oversight.
QI 8. Semi-annual program review and facility inspection was chosen because of the importance of semi-annual program review and facility inspection in IACUC’s oversight of the institution’s ACUP. This QI emphasizes the timely correction and remediation of both major and minor deficiencies identified during semi-annual program reviews and facility inspections. Failure to promptly address identified deficiencies in a timely manner may place research animals at significant risk.
QI 9. Scope of practice was chosen because of the importance of the investigator’s qualification in ensuring not only high-quality research data, but also adequate protection of research animals. Certain animal procedures can be safely performed only by investigators with adequate training and experience. Allowing investigators who are unqualified to perform these procedures places animals at significant risk of being harmed.
QI 10. Work- or research-related injuries was chosen because of the importance of the safety of investigators and animal caretakers in the institution’s ACUP. The importance of the institution’s occupational health and safety program in protecting investigators and animal care workers cannot be overemphasized. The number and percentage of investigators and animal care workers covered by the occupational health and safety program and work- or research-related injuries reflect the adequacy of the ACUP.
QI 11. Investigator animal care and use education/training requirements was chosen because of the important role of investigators in protecting animal welfare. The number and percentage of investigators who fail to maintain required animal care and use education/training reflect the adequacy of the institution’s IACUC oversight.
QI 12. IACUC chair and members’ animal care and use education and training requirements was chosen because of the important role of the IACUC chair and members in the institution’s ACUP. To appropriately evaluate and approve/disapprove animal research protocols, the chair and members of IACUC must maintain sufficient knowledge of federal regulations and VA policies regarding animal protections.
QI 13. Veterinarian and veterinary medical unit staff qualification was chosen because of the important role of veterinarian and veterinary medical unit staff in the day-to-day care of research animals and the specialized knowledge and qualification they need to maintain the animal research facilities. The number of veterinarians and nonveterinary animal care staff with appropriate board certifications reflects the strength of an institution’s ACUP.
Results
Recognizing the importance of assessing the quality of VA ACUPs, the authors started to collect some QI data of VA ACUPs parallel to those of VA HRPPs before the aforementioned proposed QIs for VA ACUPs were fully developed. These preliminary data are included here to demonstrate the feasibility of implementing these proposed VA ACUP QIs.
IACUC and R&DC Approvals (QI 2)
VA policies require that all animal research protocols be reviewed and approved first by the IACUC and then by the R&DC.13,14 The IACUC is a subcommittee of the R&DC. No animal research activities in VA may be initiated before receiving both IACUC and R&DC approval.13,14
Between June 1, 2011, and May 31, 2012, regulatory audits were conducted on 1,286 animal research protocols. Among them, 1 (0.08%) protocol was conducted and completed without the required IACUC approval, 1 (0.08%) was conducted and completed without the required R&DC approval, 1 (0.08%) was initiated prior to IACUC approval, and 2 (0.16%) were initiated prior to R&DC approval.
For-Cause Suspension or Termination (QI 3)
Among the 1,286 animal research protocols audited, 14 (1.09%) protocols were suspended or terminated for cause; 10 (0.78%) protocols were suspended or terminated due to animal safety concerns; and 4 (0.31%) protocols were suspended or terminated due to investigator-related concerns.
Lapse in Continuing Reviews (QI 5)
Federal regulations and VA policies require that IACUC conduct continuing review of all animal research protocols annually.2,13 Of the 1,286 animal research protocols audited, 1,159 protocols required IACUC continuing reviews during the auditing period. Fifty-three protocols (4.57%) lapsed in IACUC annual reviews, and in 25 of these 53 protocols, investigators continued research activities during the lapse.
Scope of Practice (QI 9)
VA policies require all research personnel to have an approved research scope of practice or functional statement that defines the duties that the individual is qualified and allowed to perform for research purposes.14
A total of 4,604 research personnel records were reviewed from the 1,286 animal research protocols audited. Of these, 276 (5.99%) did not have an approved research scope of practice; 1 (0.02%) had an approved research scope of practice but was working outside the approved research scope of practice.
Training Requirements (QI 11)
VA policies require that all research personnel who participate in animal research complete initial and annual training to ensure that they can competently and humanely perform their duties related to animal research.14
Among the 4,604 animal research personnel records reviewed, 186 (4.04%) did not maintain their training requirements, including 26 (0.56%) without required initial training and 160 (3.48%) with lapses in required continuing training.
Discussion
Collectively, these proposed QIs should provide useful information about the overall quality of an ACUP. This allows semiquantitative assessment of the quality and performance of VA facilities’ ACUPs over time and comparison of the performance of ACUPs across research facilities in the VAHCS. The information obtained may also help administrators identify program vulnerabilities and make management decisions regarding where improvements are most needed. Specifically, QI data will be collected from all VA research facilities’ ACUPs annually. National averages for all QIs will be calculated. Each facility will then be provided with the results of its own ACUP QI data as well as the national averages, allowing the facility to compare its QI data with the national averages and determine how its ACUP performs compared with the overall VA ACUP performance.
These QIs were designed for use in assessing the quality of ACUPs at VA research facilities annually or at least once every other year. With the recent requirement that a full-time RCO at each VA research facility conduct regulatory audits of all animal research protocols once every 3 years, it is feasible that an assessment of the VA ACUPs using these QIs could be conducted annually as demonstrated by the preliminary data for QIs 2, 3, 5, 9, and 11 reported here.15,16 These preliminary data also showed high rates of lapses in IACUC continuing review (4.57%), lack of research personnel scopes of practice (5.99%), and noncompliance with training requirements (4.04%). These are areas that need improvements.
The size and complexity of animal research programs are different among different facilities, which can make it difficult to compare different facilities’ ACUPs using the same quality measures. In addition, VA facilities may use their own IACUCs or the affiliate university IACUCs as the IACUCs of record. However, based on the authors’ experience using HRPP QIs to assess the quality of VA HRPPs, the collected data using ACUP QIs will help determine whether such variables as the size and complexity of a program or the kind of IACUCs used (either VA, own IACUC, or affiliate IACUC) affect the quality of VA ACUPs.10-12
Limitations
There is no evidence proving that these QIs are the most optimal measures for evaluating the quality of a VA facility’s ACUP. It is also unknown whether these QIs correlate directly with the protection of research animals. Furthermore, a quantitative, numerical value cannot be put on each indicator to allow evaluators to rank facilities’ ACUPs.
Some QIs, such as QIs 3, 4, 7, and 8, may depend on how stringent an IACUC is. For example, it is possible that a conscientious IACUC may report more noncompliance or suspend more protocols, giving the appearance of a poor quality ACUP, whereas in fact it might be an excellent program. However, the authors want to emphasize that no single QI by itself is sufficient to assess the quality of a program. It is the combination of various QIs that provides information about the overall quality of a program. It is also through the data collected that the usefulness of any particular indicators may be determined.
Conclusion
These proposed QIs provide a useful first step toward developing a robust and valid assessment of VA ACUPs. As these QIs are used at VA facilities, they will likely be redefined and modified. The authors hope that other institutions will find these indicators useful as they develop instruments to assess their own ACUPs.
Acknowledgement
The authors thank Dr. Kathryn Bayne, Global Director, Association for Assessment and Accreditation of Laboratory Animal Care International, for her suggestions and comments during the development of these quality indicators and critical review of the manuscript, and Dr. J. Thomas Puglisi, Chief Officer, VA Office of Research Oversight, for his support and critical review of the manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Institutions conducting research involving animals have established operational frameworks, referred to as animal care and use programs (ACUPs), to ensure research animal welfare and high-quality research data and to meet ethical and regulatory requirements.1-4 The Institutional Animal Care and Use Committee (IACUC) is a critical component of the ACUP and is responsible for the oversight and evaluation of all aspects of the ACUP.5 However, investigators, IACUCs, institutions, the research sponsor, and the federal government share responsibilities for ensuring research animal welfare.
Effective policies, procedures, practices, and systems in the ACUP are critical to an institution’s ability to ensure that animal research is conducted humanely and complies with applicable regulations, policies, and guidelines. To this end, considerable effort and resources have been devoted to improve the effectiveness of ACUPs, including external accreditation of ACUPs by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and implementation of science-based performance standards, postapproval monitoring, and risk assessments and mitigation of identified vulnerability.6-9 However, the impact of these quality improvement measures remains unclear. There have been no valid, reliable, and quantifiable measures to assess the effectiveness and quality of ACUPs.
Compliance with federal regulations is not only required, but also essential in protecting laboratory animals. However, the goal is not to ensure compliance but to prevent unnecessary harm, injury, and suffering to those research animals. Overemphasis on compliance and documentation may negatively impact the system by diverting resources away from ensuring research animal welfare. The authors propose that although research animal welfare cannot be directly measured, it is possible to assess the quality of ACUPs. High-quality ACUPs are expected to minimize risk to research animals to the extent possible while maintaining the integrity of the research.
The authors previously developed a set of quality indicators (QIs) for human research protection programs (HRPPs) at the VA, emphasizing performance outcomes built on a foundation of compliance.10 Implementation of these QIs allowed the research team to collect data to assess the quality of VA HRPPs.11 It also allowed the team to answer important questions, such as whether there were significant differences in the quality of HRPPs among facilities using their own institutional review boards (IRBs) and those using affiliated university IRBs as their IRBs of record.12
Background
The VA health care system (VAHCS) is the largest integrated health care system in the U.S. Currently, there are 77 VA facilities conducting research involving laboratory animals. In addition to federal regulations governing research with animals, researchers in the VAHCS must comply with requirements established by VA.1-4 For example, in the VAHCS, the IACUC is a subcommittee of the Research and Development Committee (R&DC). Research involving animals may not be initiated until it has been approved by both the IACUC and the R&DC.13,14 All investigators, including animal research investigators, are required to have approved scopes of practice.14 Furthermore, all VA facilities that conduct animal research are required to have their ACUPs accredited by the AAALAC International.13
Based on the experience gained from the VA HRPP QIs, the authors developed a set of QIs that emphasize assessing the outcome of ACUPs rather than solely on IACUC review or compliance with animal research regulations and policies. This report describes the proposed QIs for assessing the quality of VA ACUPs and presents preliminary data using some of these QIs.
Methods
The VA ACUP QIs were developed through a process that included (1) identification of a set of potential indicators by the authors; (2) review and comments on the potential indicators by individuals within and outside VA who have expertise in protecting research animal welfare, including veterinarians with board certification in laboratory animal medicine, IACUC chairs, and individuals involved in the accreditation and oversight of ACUPs; and (3) review and revision by the authors of the proposed QIs in light of the suggestions and comments received. After 6 months of deliberation, a set of 13 QIs was finalized for consideration for implementation.
Data Collection
As part of the VA ACUP quality assurance program, each VA research facility is required to conduct regulatory audits of all animal research protocols once every 3 years by qualified research compliance officers (RCOs).15 Audit tools were developed for the triennial animal protocol regulatory audits (available at http://www.va.gov/oro/rcep.asp).11,12 Facility RCOs were then trained to use these tools to conduct audits throughout the year.
Results of the protocol regulatory audits, conducted between June 1, 2011, and May 31, 2012, were collected through a Web-based system from all 74 VA facilities conducting animal research during that period. Information collected included IACUC and R&DC initial approval of human research protocols; for-cause suspension or termination of animal research protocols; compliance with continuing review requirements; research personnel scopes of practice; and investigator animal research protection training requirements.
Because this study did not involve the use of laboratory animals, no ACUC review and approval was required.
Data Analysis
All data collected were entered into a database for analysis. When necessary, facilities were contacted to verify the accuracy and uniformity of data reported. Only descriptive statistics were obtained and presented.
Quality Indicators
As shown in the Box, a total of 13 QIs covering a broad range of areas that may have significant impact on research animal welfare were selected.
QI 1. ACUP accreditation status was chosen, because accreditation of an institutional ACUP by AAALAC International, the sole widely accepted ACUP accrediting organization, suggests that the institution establish acceptable operational frameworks to ensure research animal welfare. Because VA policy requires that all facilities conducting animal research be accredited, failure to achieve full accreditation may indicate that research animals are at an elevated risk due to a less than optimal system to protect research animals.13
QI 2. IACUC and R&DC initial approval of animal research protocols was chosen because of the importance of IACUC and R&DC review and approval in ensuring the scientific merit of the research and the adequacy of research animal protection. The number and the percentage of protocols conducted without or initiated prior to IACUC and/or R&DC approval, which may put animals at risk, is a good measure of the adequacy of the institution’s ACUP.
QI 3. For-cause suspension or termination of animal research protocols was chosen, because this is a serious event. Protocols can be suspended or prematurely terminated by IACUCs due to investigators’ serious or continuing noncompliance or due to serious adverse events/injuries to the animals or research personnel. The number and percentage of protocols suspended reflect the adequacy of the IACUC oversight of the institution’s animal research program.
QI 4. Investigator sanction was chosen, because investigators and research personnel play an important role in protecting research animals. The number and percentage of investigators or technicians whose research privileges were suspended due to noncompliance reflect the adequacy of the institution’s education and training program as well as oversight of the ACUP.
QI 5. Annual review requirement was chosen because of the importance of ongoing oversight of approved animal research by the IACUC. The number and percentage of protocols lapsed in annual reviews, particularly when research activities continued during the lapse reflects the adequacy of IACUC oversight.
QI 6. Unanticipated loss of animal lives was chosen, because loss of animal lives is the most serious harm to animals that the ACUP is intended to prevent. The number and percentage of animals whose lives are unnecessarily lost due to heating, ventilation, or air-conditioning failure reflect the adequacy of the institution’s animal care infrastructure and effectiveness of the emergency response plan.
QI 7. Serious or continuing noncompliance resulting in actual harm to animals was chosen, because actual harm to animals is an important outcome measure of the adequacy of ACUP. The number and percentage of animals harmed due to investigator noncompliance or inadequate care reflect the adequacy of the institution’s veterinarian and IACUC oversight.
QI 8. Semi-annual program review and facility inspection was chosen because of the importance of semi-annual program review and facility inspection in IACUC’s oversight of the institution’s ACUP. This QI emphasizes the timely correction and remediation of both major and minor deficiencies identified during semi-annual program reviews and facility inspections. Failure to promptly address identified deficiencies in a timely manner may place research animals at significant risk.
QI 9. Scope of practice was chosen because of the importance of the investigator’s qualification in ensuring not only high-quality research data, but also adequate protection of research animals. Certain animal procedures can be safely performed only by investigators with adequate training and experience. Allowing investigators who are unqualified to perform these procedures places animals at significant risk of being harmed.
QI 10. Work- or research-related injuries was chosen because of the importance of the safety of investigators and animal caretakers in the institution’s ACUP. The importance of the institution’s occupational health and safety program in protecting investigators and animal care workers cannot be overemphasized. The number and percentage of investigators and animal care workers covered by the occupational health and safety program and work- or research-related injuries reflect the adequacy of the ACUP.
QI 11. Investigator animal care and use education/training requirements was chosen because of the important role of investigators in protecting animal welfare. The number and percentage of investigators who fail to maintain required animal care and use education/training reflect the adequacy of the institution’s IACUC oversight.
QI 12. IACUC chair and members’ animal care and use education and training requirements was chosen because of the important role of the IACUC chair and members in the institution’s ACUP. To appropriately evaluate and approve/disapprove animal research protocols, the chair and members of IACUC must maintain sufficient knowledge of federal regulations and VA policies regarding animal protections.
QI 13. Veterinarian and veterinary medical unit staff qualification was chosen because of the important role of veterinarian and veterinary medical unit staff in the day-to-day care of research animals and the specialized knowledge and qualification they need to maintain the animal research facilities. The number of veterinarians and nonveterinary animal care staff with appropriate board certifications reflects the strength of an institution’s ACUP.
Results
Recognizing the importance of assessing the quality of VA ACUPs, the authors started to collect some QI data of VA ACUPs parallel to those of VA HRPPs before the aforementioned proposed QIs for VA ACUPs were fully developed. These preliminary data are included here to demonstrate the feasibility of implementing these proposed VA ACUP QIs.
IACUC and R&DC Approvals (QI 2)
VA policies require that all animal research protocols be reviewed and approved first by the IACUC and then by the R&DC.13,14 The IACUC is a subcommittee of the R&DC. No animal research activities in VA may be initiated before receiving both IACUC and R&DC approval.13,14
Between June 1, 2011, and May 31, 2012, regulatory audits were conducted on 1,286 animal research protocols. Among them, 1 (0.08%) protocol was conducted and completed without the required IACUC approval, 1 (0.08%) was conducted and completed without the required R&DC approval, 1 (0.08%) was initiated prior to IACUC approval, and 2 (0.16%) were initiated prior to R&DC approval.
For-Cause Suspension or Termination (QI 3)
Among the 1,286 animal research protocols audited, 14 (1.09%) protocols were suspended or terminated for cause; 10 (0.78%) protocols were suspended or terminated due to animal safety concerns; and 4 (0.31%) protocols were suspended or terminated due to investigator-related concerns.
Lapse in Continuing Reviews (QI 5)
Federal regulations and VA policies require that IACUC conduct continuing review of all animal research protocols annually.2,13 Of the 1,286 animal research protocols audited, 1,159 protocols required IACUC continuing reviews during the auditing period. Fifty-three protocols (4.57%) lapsed in IACUC annual reviews, and in 25 of these 53 protocols, investigators continued research activities during the lapse.
Scope of Practice (QI 9)
VA policies require all research personnel to have an approved research scope of practice or functional statement that defines the duties that the individual is qualified and allowed to perform for research purposes.14
A total of 4,604 research personnel records were reviewed from the 1,286 animal research protocols audited. Of these, 276 (5.99%) did not have an approved research scope of practice; 1 (0.02%) had an approved research scope of practice but was working outside the approved research scope of practice.
Training Requirements (QI 11)
VA policies require that all research personnel who participate in animal research complete initial and annual training to ensure that they can competently and humanely perform their duties related to animal research.14
Among the 4,604 animal research personnel records reviewed, 186 (4.04%) did not maintain their training requirements, including 26 (0.56%) without required initial training and 160 (3.48%) with lapses in required continuing training.
Discussion
Collectively, these proposed QIs should provide useful information about the overall quality of an ACUP. This allows semiquantitative assessment of the quality and performance of VA facilities’ ACUPs over time and comparison of the performance of ACUPs across research facilities in the VAHCS. The information obtained may also help administrators identify program vulnerabilities and make management decisions regarding where improvements are most needed. Specifically, QI data will be collected from all VA research facilities’ ACUPs annually. National averages for all QIs will be calculated. Each facility will then be provided with the results of its own ACUP QI data as well as the national averages, allowing the facility to compare its QI data with the national averages and determine how its ACUP performs compared with the overall VA ACUP performance.
These QIs were designed for use in assessing the quality of ACUPs at VA research facilities annually or at least once every other year. With the recent requirement that a full-time RCO at each VA research facility conduct regulatory audits of all animal research protocols once every 3 years, it is feasible that an assessment of the VA ACUPs using these QIs could be conducted annually as demonstrated by the preliminary data for QIs 2, 3, 5, 9, and 11 reported here.15,16 These preliminary data also showed high rates of lapses in IACUC continuing review (4.57%), lack of research personnel scopes of practice (5.99%), and noncompliance with training requirements (4.04%). These are areas that need improvements.
The size and complexity of animal research programs are different among different facilities, which can make it difficult to compare different facilities’ ACUPs using the same quality measures. In addition, VA facilities may use their own IACUCs or the affiliate university IACUCs as the IACUCs of record. However, based on the authors’ experience using HRPP QIs to assess the quality of VA HRPPs, the collected data using ACUP QIs will help determine whether such variables as the size and complexity of a program or the kind of IACUCs used (either VA, own IACUC, or affiliate IACUC) affect the quality of VA ACUPs.10-12
Limitations
There is no evidence proving that these QIs are the most optimal measures for evaluating the quality of a VA facility’s ACUP. It is also unknown whether these QIs correlate directly with the protection of research animals. Furthermore, a quantitative, numerical value cannot be put on each indicator to allow evaluators to rank facilities’ ACUPs.
Some QIs, such as QIs 3, 4, 7, and 8, may depend on how stringent an IACUC is. For example, it is possible that a conscientious IACUC may report more noncompliance or suspend more protocols, giving the appearance of a poor quality ACUP, whereas in fact it might be an excellent program. However, the authors want to emphasize that no single QI by itself is sufficient to assess the quality of a program. It is the combination of various QIs that provides information about the overall quality of a program. It is also through the data collected that the usefulness of any particular indicators may be determined.
Conclusion
These proposed QIs provide a useful first step toward developing a robust and valid assessment of VA ACUPs. As these QIs are used at VA facilities, they will likely be redefined and modified. The authors hope that other institutions will find these indicators useful as they develop instruments to assess their own ACUPs.
Acknowledgement
The authors thank Dr. Kathryn Bayne, Global Director, Association for Assessment and Accreditation of Laboratory Animal Care International, for her suggestions and comments during the development of these quality indicators and critical review of the manuscript, and Dr. J. Thomas Puglisi, Chief Officer, VA Office of Research Oversight, for his support and critical review of the manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Animal Welfare Act, 7 USC §2131-2156 (2008).
2. Animal Welfare Regulations, 9 CFR §1-4 (2008).
3. National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011.
4. Office of Laboratory Animal Welfare. Public Health Service Policy On Humane Care And Use Of Laboratory Animals. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; 2015. NIH publication 15-8013. http://grants.nih.gov/grants/olaw//PHSPolicyLabAnimals.pdf. Revised 2015. Accessed August 3, 2015.
5. Sandgren EP. Defining the animal care and use program. Lab Anim (NY). 2005;34(10):41-44.
6. Association for Assessment and Accreditation of Laboratory Animal Care International. The AAALAC International accreditation program. The Association for Assessment and Accreditation of Laboratory Animal Care International Website. http://www.aaalac.org/accreditation/index.cfm. Updated 2015. Accessed August 3, 2015.
7. Klein HJ, Bayne KA. Establishing a culture of care, conscience, and responsibility: addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007;48(1):3-11.
8. Banks RE, Norton JN. A sample postapproval monitoring program in academia. ILAR J. 2008;49(4):402-418.
9. Van Sluyters RC. A guide to risk assessment in animal care and use programs: the metaphor of the 3-legged stool. ILAR J. 2008;49(4):372-378.
10. Tsan MF, Smith K, Gao B. Assessing the quality of human research protection programs: the experience at the Department of Veterans Affairs. IRB. 2010;32(4):16-19.
11. Tsan MF, Nguyen Y, Brooks R. Using quality indicators to assess human research protection programs at the Department of Veterans Affairs. IRB. 2013;35(1):10-14.
12. Tsan MF, Nguyen Y, Brooks B. Assessing the quality of VA Human Research Protection Programs: VA vs. affiliated University Institutional Review Board. J Emp Res Hum Res Ethics. 2013;8(2):153-160.
13. VA Research and Development Service. Use of Animals in Research. VHA Handbook 1200.07. Washington, DC: Department of Veterans Affairs, Veterans Health Administration; 2011.
14. VA Research and Development Service. Research and Development (R&D) Committee. VHA Handbook 1200.01. Washington, DC: Veterans Health Administration; 2009.
15. Research Compliance Officers and the Auditing of VHA Human Subjects Research to Determine Compliance with Applicable Laws, Regulations, and Policies. VHA Directive 2008-064. Washington, DC: Veterans Health Administration; 2008.
16. VA Office of Research Oversight. Research Compliance Reporting Requirements. VHA Handbook 1058.01. Washington, DC: Veterans Health Administration; 2015.
1. Animal Welfare Act, 7 USC §2131-2156 (2008).
2. Animal Welfare Regulations, 9 CFR §1-4 (2008).
3. National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011.
4. Office of Laboratory Animal Welfare. Public Health Service Policy On Humane Care And Use Of Laboratory Animals. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; 2015. NIH publication 15-8013. http://grants.nih.gov/grants/olaw//PHSPolicyLabAnimals.pdf. Revised 2015. Accessed August 3, 2015.
5. Sandgren EP. Defining the animal care and use program. Lab Anim (NY). 2005;34(10):41-44.
6. Association for Assessment and Accreditation of Laboratory Animal Care International. The AAALAC International accreditation program. The Association for Assessment and Accreditation of Laboratory Animal Care International Website. http://www.aaalac.org/accreditation/index.cfm. Updated 2015. Accessed August 3, 2015.
7. Klein HJ, Bayne KA. Establishing a culture of care, conscience, and responsibility: addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007;48(1):3-11.
8. Banks RE, Norton JN. A sample postapproval monitoring program in academia. ILAR J. 2008;49(4):402-418.
9. Van Sluyters RC. A guide to risk assessment in animal care and use programs: the metaphor of the 3-legged stool. ILAR J. 2008;49(4):372-378.
10. Tsan MF, Smith K, Gao B. Assessing the quality of human research protection programs: the experience at the Department of Veterans Affairs. IRB. 2010;32(4):16-19.
11. Tsan MF, Nguyen Y, Brooks R. Using quality indicators to assess human research protection programs at the Department of Veterans Affairs. IRB. 2013;35(1):10-14.
12. Tsan MF, Nguyen Y, Brooks B. Assessing the quality of VA Human Research Protection Programs: VA vs. affiliated University Institutional Review Board. J Emp Res Hum Res Ethics. 2013;8(2):153-160.
13. VA Research and Development Service. Use of Animals in Research. VHA Handbook 1200.07. Washington, DC: Department of Veterans Affairs, Veterans Health Administration; 2011.
14. VA Research and Development Service. Research and Development (R&D) Committee. VHA Handbook 1200.01. Washington, DC: Veterans Health Administration; 2009.
15. Research Compliance Officers and the Auditing of VHA Human Subjects Research to Determine Compliance with Applicable Laws, Regulations, and Policies. VHA Directive 2008-064. Washington, DC: Veterans Health Administration; 2008.
16. VA Office of Research Oversight. Research Compliance Reporting Requirements. VHA Handbook 1058.01. Washington, DC: Veterans Health Administration; 2015.
A Treatment Protocol for Patients With Diabetic Peripheral Neuropathy
The progressive symptoms of diabetic peripheral neuropathy (DPN) are some of the most frequent presentations of patients seeking care at the VHA. Patients with DPN often experience unmanageable pain in the lower extremities, loss of sensation in the feet, loss of balance, and an inability to perform daily functional activities.1 In addition, these patients are at significant risk for lower extremity ulceration and amputation.2 The symptoms and consequences of DPN are strongly linked to chronic use of pain medications as well as increased fall risk and injury.3 The high health care usage of veterans with these complex issues makes DPN a significant burden for the patient, the VHA, and society as a whole.
At the William Jennings Bryan Dorn VA Medical Center (WJBDVAMC) in Columbia, South Carolina, 10,763 veterans were identified to be at risk for limb loss in 2014 due to loss of protective sensation and 5,667 veterans diagnosed with DPN were treated in 2014.4 Although WJBDVAMC offers multiple clinics and programs to address the complex issues of diabetes and DPN, veterans oftentimes continue to experience uncontrolled pain, loss of protective sensation, and a decline in function even after diagnosis.
One area of improvement the authors identified in the WJBDVAMC Physical Medicine and Rehabilitation Services Department was the need for an effective, nonpharmacologic treatment for patients who experience DPN. As a result, the authors designed a pilot research study to determine whether or not a combined physical therapy intervention of monochromatic near-infrared energy (MIRE) treatments and a standardized balance exercise program would help improve the protective sensation, reduce fall risk, and decrease the adverse impact of pain on daily function. The study was approved by the institutional review board (IRB) and had no outside source of funding.
Background
Current treatments for DPN are primarily pharmacologic and are viewed as only moderately effective, limited by significant adverse effects (AEs) and drug interactions.5 Patients in the VHA at risk for amputation in low-, moderate-, and high-risk groups total 541,475 and 363,468 have a history of neuropathy. They are considered at risk due to multiple, documented factors, including weakness, callus, foot deformity, loss of protective sensation, and/or history of amputation.4 Neuropathy can affect tissues throughout the body, including organs, sensory neurons, cardiovascular status, the autonomic system, and the gastrointestinal tract as it progresses.
Individuals who develop DPN often experience severe, uncontrolled pain in the lower extremities, insensate feet, and decreased proprioceptive skills. The functional status of individuals with DPN often declines insidiously while mortality rate increases.6 Increased levels of neuropathic pain often lead to decreased activity levels, which, in turn, contribute to decreased endurance, poorly managed glycemic indexes, decreased strength, and decreased independence.
Additional DPN complications, such as decreased sensation and muscle atrophy in the lower extremities, often lead to foot deformity and increased areas of pressure during weight bearing postures. These areas of increased pressure may develop unknowingly into ulceration. If a patient’s wound becomes chronic and nonhealing, it can also lead to amputation. In such cases, early mortality may result.6,7 The cascading effects of neuropathic pain and decreased sensation place a patient with diabetes at risk for falls. Injuries from falls are widely known to be a leading cause of hospitalization and mortality in the elderly.8
Physical therapy may be prescribed for DPN and its resulting sequelae. Several studies present conflicting results regarding the benefits of therapeutic exercise in the treatment of DPN. Akbari and colleagues showed that balance exercises can increase stability in patients with DPN; whereas, a study by Kruse and colleagues noted a training program consisting of lower-extremity exercises, balance training, and walking resulted in minimal improvement of participants’ balance and leg strength over a 12-month period.9,10 Recent studies have shown that weight bearing does not increase ulceration in patients with diabetes and DPN. This is contrary to previous assumptions that patients with diabetes and DPN need to avoid weight-bearing activities.11,12
Transcutaneous electrical nerve stimulation (TENS), a modality often used in physical therapy, has been studied in the treatment of DPN with conflicting results. Gossrau and colleagues found that pain reduction with micro-TENS applied peripherally is not superior to a placebo.13 However, a case study by Somers and Somers indicated that TENS applied to the lumbar area seemed to reduce pain and insomnia associated with diabetic neuropathy.14
Several recent research studies suggest that MIRE, another available modality, may be effective in treating symptoms of DPN. Monochromatic infrared energy therapy is a noninvasive, drug-free, FDA-approved medical device that emits monochromatic near-infrared light to improve local circulation and decrease pain. A large study of 2,239 patients with DPN reported an increase in foot sensation and decreased neuropathic pain levels when treated with MIRE.15
Leonard and colleagues found that the MIRE treatments resulted in a significant increase in sensation in individuals with baseline sensation of 6.65 Semmes-Weinstein Monofilament (SWM) after 6 and 12 active treatments as well as a decrease in neuropathic symptoms as measured by the Michigan Neuropathy Screening Instrument.16 Prendergast and colleagues noted improved electrophysical changes in both large and small myelinated nerve fibers of patients with DPN following 10 MIRE treatments.17 When studying 49 patients with DPN, Kochman and colleagues found 100% of participants had improved sensation after 12 MIRE treatments when tested with monofilaments.18
An additional benefit of MIRE treatment is that it can be safely performed at home once the patient is educated on proper use and application. Home DPN treatment has the potential to decrease the burden this population places on health care systems by reducing provider visits, medication, hospitalization secondary to pain, ulceration, fall injuries, and amputations.
Methods
This was a prospective, case series pilot study designed to measure changes in patient pain levels using the visual analog scale (VAS) and Pain Outcomes Questionnaire-VA (POQ-VA), degree of protective sensation loss as measured by SWM, and fall risk as denoted by Tinetti scores from entry to 6 months. Informed consent was obtained prior to treatment, and 33 patients referred by primary care providers and specialty clinics met the criteria and enrolled in the study. Twenty-one patients completed the entire 6-month study. The nonparametric Friedman test with a Dunn’s multiple comparison (DMC) post hoc test was used to analyze the data from the initial, 4-week, 3-month, and 6-month follow-up visits.
Setting and Participants
The study was performed in the Outpatient Physical Therapy Department at WJBDVAMC. Veterans with DPN who met the inclusion/exclusion criteria were enrolled. Inclusion criteria specified that the participant must be referred by a qualified health care provider for the treatment of DPN, be able to read and write in English, have consistent transportation to and from the study location, and be able to apply MIRE therapy as directed at home.
Exclusion criteria were subjects for whom MIRE or exercise were contraindicated. Subjects were excluded if they had medical conditions that suggested a possible decline in health status in the next 6 months. Such conditions included a current regimen of chemotherapy, radiation therapy, or dialysis; recent lower extremity amputation without prosthesis; documented active alcohol and/or drug misuse; advanced chronic obstructive pulmonary disease as defined as dyspnea at rest at least once per day; unstable angina; hemiplegia or other lower extremity paralysis; and a history of central nervous system or peripheral nervous system demyelinating disorders. Additional exclusion criteria included hospitalization in the past 60 days, use of any apparatus for continuous or patient-controlled analgesia; history of chronic low back pain with documented radiculopathy; and any change in pertinent medications in the past 60 days, including pain medications, insulin, metformin, and anti-inflammatories.
Interventions
Subjects participated in a combined physical therapy approach using MIRE and a standardized balance program. Patients received treatment in the outpatient clinic 3 times each week for 4 weeks. The treatment then continued at the same frequency at home until the scheduled 6-month follow-up visit. Clinic and home treatments included application of MIRE to bilateral lower extremities and feet for 30 minutes each as well as performance of a therapeutic exercise program for balance.
In the clinic, 2 pads from the MIRE device (Anodyne Therapy, LLC, Tampa, FL) were placed along the medial and lateral aspect of each lower leg, and an additional 2 pads were placed in a T formation on the plantar surface of each foot, per the manufacturer’s recommendations. The T formation consisted of the first pad placed horizontally across the metatarsal heads and the second placed vertically down the length of the foot. Each pad was protected by plastic wrap to ensure proper hygiene and secured. The intensity of clinic treatments was set at 7 bars, which minimized the risk of burns. Home treatments were similar to those in the clinic, except that each leg had to be treated individually instead of simultaneously and home MIRE units are preset and only function at an intensity that is equivalent to around 7 bars on the clinical unit.
The standardized balance program consisted of a progression of the following exercises: ankle alphabet/ankle range of motion, standing lateral weight shifts, bilateral heel raises, bilateral toe raises, unilateral heel raises, unilateral toe raises, partial wall squats, and single leg stance. Each participant performed these exercises 3 times per week in the clinic and then 3 times per week at home following the 12th visit.
Outcomes and Follow-up
The POQ-VA, a subjective quality of life (QOL) measure for veterans, as well as VAS, SWM testing, and the Tinetti Gait and Balance Assessment scores were used to measure outcomes. Data were collected for each of these measures during the initial and 12th clinic visits and at the 3-month and 6-month follow-up visits. The POQ-VA and VAS scores were self-reported and filled out by each participant at the initial, 12th, 3-month, and 6-month visits. The POQ-VA score has proven to be reliable and valid for the assessment of noncancer, chronic pain in veterans.19 The VAS scores were measured using a scale of 0 to 10 cm.
The SWM was standardized, and 7 sites were tested on each foot during the initial, 12th, 3-month, and 6-month visits: plantar surface of the distal great toe, the distal 3rd toe, the distal 5th toe, the 1st metatarsal head, the 3rd metatarsal head, the 5th metatarsal head, and the mid-plantar arch. At each site, the SWM was applied with just enough force to initiate a bending force and held for 1.5 seconds. Each site was tested 3 times. Participants had to detect the monofilament at least twice for the monofilament value to be recorded. Monofilament testing began with 6.65 SWM and decreased to 5.07, 4.56, 4.32, and lower until the patient was no longer able to detect sensation.
The Tinetti Gait and Balance Assessments was performed on each participant at the initial, 12th, 3-month, and 6-month visits. Tinetti balance, gait, and total scores were recorded at each interval.
Results
Thirty-three patients, referred by primary care providers and specialty clinics, met the inclusion criteria and enrolled in the study. Twenty-one patients (20 men and 1 woman) completed the entire 6-month study. Causes for withdrawal included travel difficulties (5), did not show up for follow-up visits (4), lumbar radiculopathy (1), perceived minimal/no benefit (1), and unrelated death (1). No AEs were reported.
The Friedman test with DMC post hoc test was performed on the POQ-VA total score and subscale scores. The POQ-VA subscale scores were divided into the following domains: pain, activities of daily living (ADL), fear, negative affect, mobility, and vitality. The POQ-VA domains were analyzed to compare data from the initial, 12th, 3-month, and 6-month visits. The POQ-VA total score significantly decreased from the initial to the 12th visit (P < .01), from the initial to the 3-month (P < .01), and from the initial to the 6-month visit (P < .05). However, there was no significant change from the 12th visit to the 3-month follow-up, 12th visit to the 6-month follow-up, or the 3-month to 6-month follow-up.
The POQ-VA pain score decreased significantly from the initial to the 12th visit (P < .05) and from the initial to the 6-month visit (P < .05). However, there was no significant interval change from the initial to the 3-month, the 12th to 3-month, 12th to 6-month, or 3-month to 6-month visit (Figure 1). The POQ-VA vitality scores and POQ-VA fear scores did not yield significant changes. The POQ-VA negative affect scores showed significant improvement only between the initial and the 3-month visit (P < .05) (Figure 2). The POQ-VA ADL scores showed significant improvement in the initial vs 3-month score (P < .05). The POQ-VA mobility scores were significantly improved for the initial vs 12th visit (P < .01), initial vs 3-month visit (P < .01), and the initial vs 6-month visit (P < .001) (Figure 1).
Analysis of VAS scores revealed a significant decrease at the 6-month time frame compared with the initial score for the left foot (P < .05). Further VAS analysis revealed no significant difference between the initial and 6-month right foot VAS score. When both feet were compared together, there was no significant difference in VAS ratings between any 2 points in time.
Analysis of Tinetti Total Score, Tinetti Balance Score, and Tinetti Gait Score revealed a significant difference between the initial vs 3-month visit for all 3 scores (P < .001, P < .001, and P < .05, respectively). In addition, Tinetti Total (P < .001) and Tinetti Balance (P < .01) scores were significantly improved from initial to the final 6-month visit. There were no significant findings between interim scores of the initial and 12th visits, the 12th and 3-month visits, or the 3-month and 6-month scores (Figure 2).
Analysis of SWM testing indicated a significant decrease in the total number of insensate sites (> 5.07) when both feet were grouped together between the initial and 3-month visits (P < .05) as well as the initial and 6-month (P < .01) visits. When the left and right feet were compared independently of each other, there was a significant decrease in the number of insensate sites between the initial and 6-month visits (P < .01 for both) (Figure 3).
Discussion
This study investigated whether or not a multimodal physical therapy approach would reduce several of the debilitating symptoms of DPN experienced by many veterans at WJBDVAMC. The results support the idea that a combined treatment protocol of MIRE and a standardized exercise program can lead to decreased POQ-VA pain levels, improved balance, and improved protective sensation in veterans with DPN. Alleviation of these DPN complications may ultimately decrease an individual’s risk of injury and improve overall QOL.
Because the POQ-VA is a reliable, valid self-reported measure for veterans, it was chosen to quantify the impact of pain. Overall, veterans who participated in this study perceived decreased pain interference in multiple areas of their lives. The most significant findings were in overall QOL, household and community mobility, and pain ratings. This suggests that the combined treatment protocol will help veterans maintain an active lifestyle despite poorly controlled diabetes and neuropathic pain.
Along with decreased pain interference with QOL, participants demonstrated a decrease in fall risk as quantified by the Tinetti Gait and Balance Assessment. The SWM testing showed improved protective sensation as early as 3 months and continued through the 6-month visit. As protective sensation improves and fall risk decreases, the risk of injury is lessened, fear of falling is decreased, and individuals are less likely to self-impose limitations on daily activity levels, which improves QOL. In addition, decreased fall risk and improved protective sensation can reduce the financial burden on both the patient and the health care system. Many individuals are hospitalized secondary to fall injury, nonhealing wounds, resulting infections, and/or secondary complications from prolonged immobility. This treatment protocol demonstrates how a standardized physical therapy protocol, including MIRE and balance exercises, can be used preventively to reduce both the personal and financial impact of DPN.
It is interesting to note that some POQ-VA and Tinetti subscores were significantly improved at 3 months but not at 6 months. The significance achieved at 3 months may be due to the time required (ie, > 12 visits) to make significant physiological changes. The lack of significance at 6 months may be due to the natural tendency of participants to less consistently perform the home exercise program and MIRE protocol when unsupervised in the home. Differences in the VAS and POQ-VA pain score ratings were noted in the data. The POQ-VA pain rating scale indicated significant improvement in pain levels over the course of the study. However, when asked about pain using the 10-cm VAS, patients reported no significant improvements. This may be because veterans are more familiar with the numerical pain rating scale and are rarely asked to use the 10-cm VAS. It may also be because the POQ-VA pain rating asks for an average pain level over the previous week, whereas the 10-cm VAS asks for pain level at a discrete point in time.
Historically, physical therapy has had little to offer individuals with DPN. As a result of this study, however, a standardized treatment program for DPN has been implemented at the WJBDVAMC Physical Therapy Clinic. Referred patients are seen in the clinic on a trial basis. If positive results are documented during the clinic treatments, a home MIRE unit and exercise program are provided. The patients are expected to continue performing home treatments of MIRE and exercise 3 times a week after discharge.
Strengths and Limitations
Strengths of the study include a stringent IRB review, control of medication changes during the study through alerts to all VA providers, and a standardized MIRE and exercise protocol. An additional strength is the long duration of the study, which included supervised and unsupervised interventions that simulate real-life scenarios.
Limitations of the study include a small sample size, case-controlled design rather than a randomized, double-blinded study, which can contribute to selection bias, inability to differentiate between the benefits of physical therapy alone vs physical therapy and MIRE treatments, and retention of participants due to travel difficulties across a wide catchment area.
This pilot study should be expanded to a multicenter, randomized, double-blinded study to clarify the most beneficial treatments for individuals with diabetic neuropathy. Examining the number of documented falls pre- and postintervention may also be helpful to determine actual effects on an individual’s fall risk.
Conclusion
The use of a multimodal physical therapy approach seems to be effective in reducing the impact of neuropathic pain, the risk of amputation, and the risk of falls in individuals who have pursued all standard medical options but still experience the long-term effects of DPN. By adhering to a standardized treatment protocol of MIRE and therapeutic exercise, it seems that the benefits of this intervention can be maintained over time. This offers new, nonconventional treatment options in the field of physical therapy for veterans whose QOL is negatively impacted by the devastating effects of diabetic neuropathy.
Acknowledgements
Clinical support was provided by David Metzelfeld, DPT, and Cam Lendrim, PTA of William Jennings Bryan Dorn VA Medical Center. Paul Bartels, PhD, of Warren Wilson College provided data analysis support. Anodyne Therapy, LLC, provided the MIRE unit used in the clinic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. National Institute of Neurological Disorders and Stroke Website. http://www.ninds.nih.gov/disorders/peripheralneuropath/detail_peripheralneuropathy.htm#183583208. Updated April 17, 2015. Accesssed August 8, 2015.
2. Armstrong DG, Lavery LA, and Wunderlich RP. Risk factors for diabetic foot ulceration: a logical approach to treatment. J Wound Ostomy Continence Nurs. 1998;25(3):123-128.
3. Pesa J, Meyer R, Quock T, Rattana SK, Mody SH. MBA Opioid utilization patterns among medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits. 2013;6(4):188-196.
4. VHA Support Service Center. The amputation risk by facility in the ProClarity amputation risk (PAVE) cube. Department of Veterans Affairs Nonpublic Intranet. http://vssc.med.va.gov.
5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892-900
6. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598-1604.
7. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
8. Centers for Disease Control and Prevention. Older adults falls: get the facts. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html. Updated July 1, 2015. Accessed August 8, 2015.
9. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333-338.
10. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568-1579.
11. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398.
12. Tuttle LG, Hastings MK, and Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92(1):133-141.
13. Gossrau G, Wähner M, Kuschke M, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011;12(6):953-960.
14. Somers DL, Somers MF. Treatment of neuropathic pain in a patient with diabetic neuropathy using transcutaneous electrical nerve stimulation applied to the skin of the lumbar region. Phys Ther. 1999;79(8):767-775.
15. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy—MIRE. J Diabetes Complications. 2006;20(2):81-87.
16. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168-172.
17. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract. 2004;10(1):24-30.
18. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92(3):125-130.
19. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395.
The progressive symptoms of diabetic peripheral neuropathy (DPN) are some of the most frequent presentations of patients seeking care at the VHA. Patients with DPN often experience unmanageable pain in the lower extremities, loss of sensation in the feet, loss of balance, and an inability to perform daily functional activities.1 In addition, these patients are at significant risk for lower extremity ulceration and amputation.2 The symptoms and consequences of DPN are strongly linked to chronic use of pain medications as well as increased fall risk and injury.3 The high health care usage of veterans with these complex issues makes DPN a significant burden for the patient, the VHA, and society as a whole.
At the William Jennings Bryan Dorn VA Medical Center (WJBDVAMC) in Columbia, South Carolina, 10,763 veterans were identified to be at risk for limb loss in 2014 due to loss of protective sensation and 5,667 veterans diagnosed with DPN were treated in 2014.4 Although WJBDVAMC offers multiple clinics and programs to address the complex issues of diabetes and DPN, veterans oftentimes continue to experience uncontrolled pain, loss of protective sensation, and a decline in function even after diagnosis.
One area of improvement the authors identified in the WJBDVAMC Physical Medicine and Rehabilitation Services Department was the need for an effective, nonpharmacologic treatment for patients who experience DPN. As a result, the authors designed a pilot research study to determine whether or not a combined physical therapy intervention of monochromatic near-infrared energy (MIRE) treatments and a standardized balance exercise program would help improve the protective sensation, reduce fall risk, and decrease the adverse impact of pain on daily function. The study was approved by the institutional review board (IRB) and had no outside source of funding.
Background
Current treatments for DPN are primarily pharmacologic and are viewed as only moderately effective, limited by significant adverse effects (AEs) and drug interactions.5 Patients in the VHA at risk for amputation in low-, moderate-, and high-risk groups total 541,475 and 363,468 have a history of neuropathy. They are considered at risk due to multiple, documented factors, including weakness, callus, foot deformity, loss of protective sensation, and/or history of amputation.4 Neuropathy can affect tissues throughout the body, including organs, sensory neurons, cardiovascular status, the autonomic system, and the gastrointestinal tract as it progresses.
Individuals who develop DPN often experience severe, uncontrolled pain in the lower extremities, insensate feet, and decreased proprioceptive skills. The functional status of individuals with DPN often declines insidiously while mortality rate increases.6 Increased levels of neuropathic pain often lead to decreased activity levels, which, in turn, contribute to decreased endurance, poorly managed glycemic indexes, decreased strength, and decreased independence.
Additional DPN complications, such as decreased sensation and muscle atrophy in the lower extremities, often lead to foot deformity and increased areas of pressure during weight bearing postures. These areas of increased pressure may develop unknowingly into ulceration. If a patient’s wound becomes chronic and nonhealing, it can also lead to amputation. In such cases, early mortality may result.6,7 The cascading effects of neuropathic pain and decreased sensation place a patient with diabetes at risk for falls. Injuries from falls are widely known to be a leading cause of hospitalization and mortality in the elderly.8
Physical therapy may be prescribed for DPN and its resulting sequelae. Several studies present conflicting results regarding the benefits of therapeutic exercise in the treatment of DPN. Akbari and colleagues showed that balance exercises can increase stability in patients with DPN; whereas, a study by Kruse and colleagues noted a training program consisting of lower-extremity exercises, balance training, and walking resulted in minimal improvement of participants’ balance and leg strength over a 12-month period.9,10 Recent studies have shown that weight bearing does not increase ulceration in patients with diabetes and DPN. This is contrary to previous assumptions that patients with diabetes and DPN need to avoid weight-bearing activities.11,12
Transcutaneous electrical nerve stimulation (TENS), a modality often used in physical therapy, has been studied in the treatment of DPN with conflicting results. Gossrau and colleagues found that pain reduction with micro-TENS applied peripherally is not superior to a placebo.13 However, a case study by Somers and Somers indicated that TENS applied to the lumbar area seemed to reduce pain and insomnia associated with diabetic neuropathy.14
Several recent research studies suggest that MIRE, another available modality, may be effective in treating symptoms of DPN. Monochromatic infrared energy therapy is a noninvasive, drug-free, FDA-approved medical device that emits monochromatic near-infrared light to improve local circulation and decrease pain. A large study of 2,239 patients with DPN reported an increase in foot sensation and decreased neuropathic pain levels when treated with MIRE.15
Leonard and colleagues found that the MIRE treatments resulted in a significant increase in sensation in individuals with baseline sensation of 6.65 Semmes-Weinstein Monofilament (SWM) after 6 and 12 active treatments as well as a decrease in neuropathic symptoms as measured by the Michigan Neuropathy Screening Instrument.16 Prendergast and colleagues noted improved electrophysical changes in both large and small myelinated nerve fibers of patients with DPN following 10 MIRE treatments.17 When studying 49 patients with DPN, Kochman and colleagues found 100% of participants had improved sensation after 12 MIRE treatments when tested with monofilaments.18
An additional benefit of MIRE treatment is that it can be safely performed at home once the patient is educated on proper use and application. Home DPN treatment has the potential to decrease the burden this population places on health care systems by reducing provider visits, medication, hospitalization secondary to pain, ulceration, fall injuries, and amputations.
Methods
This was a prospective, case series pilot study designed to measure changes in patient pain levels using the visual analog scale (VAS) and Pain Outcomes Questionnaire-VA (POQ-VA), degree of protective sensation loss as measured by SWM, and fall risk as denoted by Tinetti scores from entry to 6 months. Informed consent was obtained prior to treatment, and 33 patients referred by primary care providers and specialty clinics met the criteria and enrolled in the study. Twenty-one patients completed the entire 6-month study. The nonparametric Friedman test with a Dunn’s multiple comparison (DMC) post hoc test was used to analyze the data from the initial, 4-week, 3-month, and 6-month follow-up visits.
Setting and Participants
The study was performed in the Outpatient Physical Therapy Department at WJBDVAMC. Veterans with DPN who met the inclusion/exclusion criteria were enrolled. Inclusion criteria specified that the participant must be referred by a qualified health care provider for the treatment of DPN, be able to read and write in English, have consistent transportation to and from the study location, and be able to apply MIRE therapy as directed at home.
Exclusion criteria were subjects for whom MIRE or exercise were contraindicated. Subjects were excluded if they had medical conditions that suggested a possible decline in health status in the next 6 months. Such conditions included a current regimen of chemotherapy, radiation therapy, or dialysis; recent lower extremity amputation without prosthesis; documented active alcohol and/or drug misuse; advanced chronic obstructive pulmonary disease as defined as dyspnea at rest at least once per day; unstable angina; hemiplegia or other lower extremity paralysis; and a history of central nervous system or peripheral nervous system demyelinating disorders. Additional exclusion criteria included hospitalization in the past 60 days, use of any apparatus for continuous or patient-controlled analgesia; history of chronic low back pain with documented radiculopathy; and any change in pertinent medications in the past 60 days, including pain medications, insulin, metformin, and anti-inflammatories.
Interventions
Subjects participated in a combined physical therapy approach using MIRE and a standardized balance program. Patients received treatment in the outpatient clinic 3 times each week for 4 weeks. The treatment then continued at the same frequency at home until the scheduled 6-month follow-up visit. Clinic and home treatments included application of MIRE to bilateral lower extremities and feet for 30 minutes each as well as performance of a therapeutic exercise program for balance.
In the clinic, 2 pads from the MIRE device (Anodyne Therapy, LLC, Tampa, FL) were placed along the medial and lateral aspect of each lower leg, and an additional 2 pads were placed in a T formation on the plantar surface of each foot, per the manufacturer’s recommendations. The T formation consisted of the first pad placed horizontally across the metatarsal heads and the second placed vertically down the length of the foot. Each pad was protected by plastic wrap to ensure proper hygiene and secured. The intensity of clinic treatments was set at 7 bars, which minimized the risk of burns. Home treatments were similar to those in the clinic, except that each leg had to be treated individually instead of simultaneously and home MIRE units are preset and only function at an intensity that is equivalent to around 7 bars on the clinical unit.
The standardized balance program consisted of a progression of the following exercises: ankle alphabet/ankle range of motion, standing lateral weight shifts, bilateral heel raises, bilateral toe raises, unilateral heel raises, unilateral toe raises, partial wall squats, and single leg stance. Each participant performed these exercises 3 times per week in the clinic and then 3 times per week at home following the 12th visit.
Outcomes and Follow-up
The POQ-VA, a subjective quality of life (QOL) measure for veterans, as well as VAS, SWM testing, and the Tinetti Gait and Balance Assessment scores were used to measure outcomes. Data were collected for each of these measures during the initial and 12th clinic visits and at the 3-month and 6-month follow-up visits. The POQ-VA and VAS scores were self-reported and filled out by each participant at the initial, 12th, 3-month, and 6-month visits. The POQ-VA score has proven to be reliable and valid for the assessment of noncancer, chronic pain in veterans.19 The VAS scores were measured using a scale of 0 to 10 cm.
The SWM was standardized, and 7 sites were tested on each foot during the initial, 12th, 3-month, and 6-month visits: plantar surface of the distal great toe, the distal 3rd toe, the distal 5th toe, the 1st metatarsal head, the 3rd metatarsal head, the 5th metatarsal head, and the mid-plantar arch. At each site, the SWM was applied with just enough force to initiate a bending force and held for 1.5 seconds. Each site was tested 3 times. Participants had to detect the monofilament at least twice for the monofilament value to be recorded. Monofilament testing began with 6.65 SWM and decreased to 5.07, 4.56, 4.32, and lower until the patient was no longer able to detect sensation.
The Tinetti Gait and Balance Assessments was performed on each participant at the initial, 12th, 3-month, and 6-month visits. Tinetti balance, gait, and total scores were recorded at each interval.
Results
Thirty-three patients, referred by primary care providers and specialty clinics, met the inclusion criteria and enrolled in the study. Twenty-one patients (20 men and 1 woman) completed the entire 6-month study. Causes for withdrawal included travel difficulties (5), did not show up for follow-up visits (4), lumbar radiculopathy (1), perceived minimal/no benefit (1), and unrelated death (1). No AEs were reported.
The Friedman test with DMC post hoc test was performed on the POQ-VA total score and subscale scores. The POQ-VA subscale scores were divided into the following domains: pain, activities of daily living (ADL), fear, negative affect, mobility, and vitality. The POQ-VA domains were analyzed to compare data from the initial, 12th, 3-month, and 6-month visits. The POQ-VA total score significantly decreased from the initial to the 12th visit (P < .01), from the initial to the 3-month (P < .01), and from the initial to the 6-month visit (P < .05). However, there was no significant change from the 12th visit to the 3-month follow-up, 12th visit to the 6-month follow-up, or the 3-month to 6-month follow-up.
The POQ-VA pain score decreased significantly from the initial to the 12th visit (P < .05) and from the initial to the 6-month visit (P < .05). However, there was no significant interval change from the initial to the 3-month, the 12th to 3-month, 12th to 6-month, or 3-month to 6-month visit (Figure 1). The POQ-VA vitality scores and POQ-VA fear scores did not yield significant changes. The POQ-VA negative affect scores showed significant improvement only between the initial and the 3-month visit (P < .05) (Figure 2). The POQ-VA ADL scores showed significant improvement in the initial vs 3-month score (P < .05). The POQ-VA mobility scores were significantly improved for the initial vs 12th visit (P < .01), initial vs 3-month visit (P < .01), and the initial vs 6-month visit (P < .001) (Figure 1).
Analysis of VAS scores revealed a significant decrease at the 6-month time frame compared with the initial score for the left foot (P < .05). Further VAS analysis revealed no significant difference between the initial and 6-month right foot VAS score. When both feet were compared together, there was no significant difference in VAS ratings between any 2 points in time.
Analysis of Tinetti Total Score, Tinetti Balance Score, and Tinetti Gait Score revealed a significant difference between the initial vs 3-month visit for all 3 scores (P < .001, P < .001, and P < .05, respectively). In addition, Tinetti Total (P < .001) and Tinetti Balance (P < .01) scores were significantly improved from initial to the final 6-month visit. There were no significant findings between interim scores of the initial and 12th visits, the 12th and 3-month visits, or the 3-month and 6-month scores (Figure 2).
Analysis of SWM testing indicated a significant decrease in the total number of insensate sites (> 5.07) when both feet were grouped together between the initial and 3-month visits (P < .05) as well as the initial and 6-month (P < .01) visits. When the left and right feet were compared independently of each other, there was a significant decrease in the number of insensate sites between the initial and 6-month visits (P < .01 for both) (Figure 3).
Discussion
This study investigated whether or not a multimodal physical therapy approach would reduce several of the debilitating symptoms of DPN experienced by many veterans at WJBDVAMC. The results support the idea that a combined treatment protocol of MIRE and a standardized exercise program can lead to decreased POQ-VA pain levels, improved balance, and improved protective sensation in veterans with DPN. Alleviation of these DPN complications may ultimately decrease an individual’s risk of injury and improve overall QOL.
Because the POQ-VA is a reliable, valid self-reported measure for veterans, it was chosen to quantify the impact of pain. Overall, veterans who participated in this study perceived decreased pain interference in multiple areas of their lives. The most significant findings were in overall QOL, household and community mobility, and pain ratings. This suggests that the combined treatment protocol will help veterans maintain an active lifestyle despite poorly controlled diabetes and neuropathic pain.
Along with decreased pain interference with QOL, participants demonstrated a decrease in fall risk as quantified by the Tinetti Gait and Balance Assessment. The SWM testing showed improved protective sensation as early as 3 months and continued through the 6-month visit. As protective sensation improves and fall risk decreases, the risk of injury is lessened, fear of falling is decreased, and individuals are less likely to self-impose limitations on daily activity levels, which improves QOL. In addition, decreased fall risk and improved protective sensation can reduce the financial burden on both the patient and the health care system. Many individuals are hospitalized secondary to fall injury, nonhealing wounds, resulting infections, and/or secondary complications from prolonged immobility. This treatment protocol demonstrates how a standardized physical therapy protocol, including MIRE and balance exercises, can be used preventively to reduce both the personal and financial impact of DPN.
It is interesting to note that some POQ-VA and Tinetti subscores were significantly improved at 3 months but not at 6 months. The significance achieved at 3 months may be due to the time required (ie, > 12 visits) to make significant physiological changes. The lack of significance at 6 months may be due to the natural tendency of participants to less consistently perform the home exercise program and MIRE protocol when unsupervised in the home. Differences in the VAS and POQ-VA pain score ratings were noted in the data. The POQ-VA pain rating scale indicated significant improvement in pain levels over the course of the study. However, when asked about pain using the 10-cm VAS, patients reported no significant improvements. This may be because veterans are more familiar with the numerical pain rating scale and are rarely asked to use the 10-cm VAS. It may also be because the POQ-VA pain rating asks for an average pain level over the previous week, whereas the 10-cm VAS asks for pain level at a discrete point in time.
Historically, physical therapy has had little to offer individuals with DPN. As a result of this study, however, a standardized treatment program for DPN has been implemented at the WJBDVAMC Physical Therapy Clinic. Referred patients are seen in the clinic on a trial basis. If positive results are documented during the clinic treatments, a home MIRE unit and exercise program are provided. The patients are expected to continue performing home treatments of MIRE and exercise 3 times a week after discharge.
Strengths and Limitations
Strengths of the study include a stringent IRB review, control of medication changes during the study through alerts to all VA providers, and a standardized MIRE and exercise protocol. An additional strength is the long duration of the study, which included supervised and unsupervised interventions that simulate real-life scenarios.
Limitations of the study include a small sample size, case-controlled design rather than a randomized, double-blinded study, which can contribute to selection bias, inability to differentiate between the benefits of physical therapy alone vs physical therapy and MIRE treatments, and retention of participants due to travel difficulties across a wide catchment area.
This pilot study should be expanded to a multicenter, randomized, double-blinded study to clarify the most beneficial treatments for individuals with diabetic neuropathy. Examining the number of documented falls pre- and postintervention may also be helpful to determine actual effects on an individual’s fall risk.
Conclusion
The use of a multimodal physical therapy approach seems to be effective in reducing the impact of neuropathic pain, the risk of amputation, and the risk of falls in individuals who have pursued all standard medical options but still experience the long-term effects of DPN. By adhering to a standardized treatment protocol of MIRE and therapeutic exercise, it seems that the benefits of this intervention can be maintained over time. This offers new, nonconventional treatment options in the field of physical therapy for veterans whose QOL is negatively impacted by the devastating effects of diabetic neuropathy.
Acknowledgements
Clinical support was provided by David Metzelfeld, DPT, and Cam Lendrim, PTA of William Jennings Bryan Dorn VA Medical Center. Paul Bartels, PhD, of Warren Wilson College provided data analysis support. Anodyne Therapy, LLC, provided the MIRE unit used in the clinic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The progressive symptoms of diabetic peripheral neuropathy (DPN) are some of the most frequent presentations of patients seeking care at the VHA. Patients with DPN often experience unmanageable pain in the lower extremities, loss of sensation in the feet, loss of balance, and an inability to perform daily functional activities.1 In addition, these patients are at significant risk for lower extremity ulceration and amputation.2 The symptoms and consequences of DPN are strongly linked to chronic use of pain medications as well as increased fall risk and injury.3 The high health care usage of veterans with these complex issues makes DPN a significant burden for the patient, the VHA, and society as a whole.
At the William Jennings Bryan Dorn VA Medical Center (WJBDVAMC) in Columbia, South Carolina, 10,763 veterans were identified to be at risk for limb loss in 2014 due to loss of protective sensation and 5,667 veterans diagnosed with DPN were treated in 2014.4 Although WJBDVAMC offers multiple clinics and programs to address the complex issues of diabetes and DPN, veterans oftentimes continue to experience uncontrolled pain, loss of protective sensation, and a decline in function even after diagnosis.
One area of improvement the authors identified in the WJBDVAMC Physical Medicine and Rehabilitation Services Department was the need for an effective, nonpharmacologic treatment for patients who experience DPN. As a result, the authors designed a pilot research study to determine whether or not a combined physical therapy intervention of monochromatic near-infrared energy (MIRE) treatments and a standardized balance exercise program would help improve the protective sensation, reduce fall risk, and decrease the adverse impact of pain on daily function. The study was approved by the institutional review board (IRB) and had no outside source of funding.
Background
Current treatments for DPN are primarily pharmacologic and are viewed as only moderately effective, limited by significant adverse effects (AEs) and drug interactions.5 Patients in the VHA at risk for amputation in low-, moderate-, and high-risk groups total 541,475 and 363,468 have a history of neuropathy. They are considered at risk due to multiple, documented factors, including weakness, callus, foot deformity, loss of protective sensation, and/or history of amputation.4 Neuropathy can affect tissues throughout the body, including organs, sensory neurons, cardiovascular status, the autonomic system, and the gastrointestinal tract as it progresses.
Individuals who develop DPN often experience severe, uncontrolled pain in the lower extremities, insensate feet, and decreased proprioceptive skills. The functional status of individuals with DPN often declines insidiously while mortality rate increases.6 Increased levels of neuropathic pain often lead to decreased activity levels, which, in turn, contribute to decreased endurance, poorly managed glycemic indexes, decreased strength, and decreased independence.
Additional DPN complications, such as decreased sensation and muscle atrophy in the lower extremities, often lead to foot deformity and increased areas of pressure during weight bearing postures. These areas of increased pressure may develop unknowingly into ulceration. If a patient’s wound becomes chronic and nonhealing, it can also lead to amputation. In such cases, early mortality may result.6,7 The cascading effects of neuropathic pain and decreased sensation place a patient with diabetes at risk for falls. Injuries from falls are widely known to be a leading cause of hospitalization and mortality in the elderly.8
Physical therapy may be prescribed for DPN and its resulting sequelae. Several studies present conflicting results regarding the benefits of therapeutic exercise in the treatment of DPN. Akbari and colleagues showed that balance exercises can increase stability in patients with DPN; whereas, a study by Kruse and colleagues noted a training program consisting of lower-extremity exercises, balance training, and walking resulted in minimal improvement of participants’ balance and leg strength over a 12-month period.9,10 Recent studies have shown that weight bearing does not increase ulceration in patients with diabetes and DPN. This is contrary to previous assumptions that patients with diabetes and DPN need to avoid weight-bearing activities.11,12
Transcutaneous electrical nerve stimulation (TENS), a modality often used in physical therapy, has been studied in the treatment of DPN with conflicting results. Gossrau and colleagues found that pain reduction with micro-TENS applied peripherally is not superior to a placebo.13 However, a case study by Somers and Somers indicated that TENS applied to the lumbar area seemed to reduce pain and insomnia associated with diabetic neuropathy.14
Several recent research studies suggest that MIRE, another available modality, may be effective in treating symptoms of DPN. Monochromatic infrared energy therapy is a noninvasive, drug-free, FDA-approved medical device that emits monochromatic near-infrared light to improve local circulation and decrease pain. A large study of 2,239 patients with DPN reported an increase in foot sensation and decreased neuropathic pain levels when treated with MIRE.15
Leonard and colleagues found that the MIRE treatments resulted in a significant increase in sensation in individuals with baseline sensation of 6.65 Semmes-Weinstein Monofilament (SWM) after 6 and 12 active treatments as well as a decrease in neuropathic symptoms as measured by the Michigan Neuropathy Screening Instrument.16 Prendergast and colleagues noted improved electrophysical changes in both large and small myelinated nerve fibers of patients with DPN following 10 MIRE treatments.17 When studying 49 patients with DPN, Kochman and colleagues found 100% of participants had improved sensation after 12 MIRE treatments when tested with monofilaments.18
An additional benefit of MIRE treatment is that it can be safely performed at home once the patient is educated on proper use and application. Home DPN treatment has the potential to decrease the burden this population places on health care systems by reducing provider visits, medication, hospitalization secondary to pain, ulceration, fall injuries, and amputations.
Methods
This was a prospective, case series pilot study designed to measure changes in patient pain levels using the visual analog scale (VAS) and Pain Outcomes Questionnaire-VA (POQ-VA), degree of protective sensation loss as measured by SWM, and fall risk as denoted by Tinetti scores from entry to 6 months. Informed consent was obtained prior to treatment, and 33 patients referred by primary care providers and specialty clinics met the criteria and enrolled in the study. Twenty-one patients completed the entire 6-month study. The nonparametric Friedman test with a Dunn’s multiple comparison (DMC) post hoc test was used to analyze the data from the initial, 4-week, 3-month, and 6-month follow-up visits.
Setting and Participants
The study was performed in the Outpatient Physical Therapy Department at WJBDVAMC. Veterans with DPN who met the inclusion/exclusion criteria were enrolled. Inclusion criteria specified that the participant must be referred by a qualified health care provider for the treatment of DPN, be able to read and write in English, have consistent transportation to and from the study location, and be able to apply MIRE therapy as directed at home.
Exclusion criteria were subjects for whom MIRE or exercise were contraindicated. Subjects were excluded if they had medical conditions that suggested a possible decline in health status in the next 6 months. Such conditions included a current regimen of chemotherapy, radiation therapy, or dialysis; recent lower extremity amputation without prosthesis; documented active alcohol and/or drug misuse; advanced chronic obstructive pulmonary disease as defined as dyspnea at rest at least once per day; unstable angina; hemiplegia or other lower extremity paralysis; and a history of central nervous system or peripheral nervous system demyelinating disorders. Additional exclusion criteria included hospitalization in the past 60 days, use of any apparatus for continuous or patient-controlled analgesia; history of chronic low back pain with documented radiculopathy; and any change in pertinent medications in the past 60 days, including pain medications, insulin, metformin, and anti-inflammatories.
Interventions
Subjects participated in a combined physical therapy approach using MIRE and a standardized balance program. Patients received treatment in the outpatient clinic 3 times each week for 4 weeks. The treatment then continued at the same frequency at home until the scheduled 6-month follow-up visit. Clinic and home treatments included application of MIRE to bilateral lower extremities and feet for 30 minutes each as well as performance of a therapeutic exercise program for balance.
In the clinic, 2 pads from the MIRE device (Anodyne Therapy, LLC, Tampa, FL) were placed along the medial and lateral aspect of each lower leg, and an additional 2 pads were placed in a T formation on the plantar surface of each foot, per the manufacturer’s recommendations. The T formation consisted of the first pad placed horizontally across the metatarsal heads and the second placed vertically down the length of the foot. Each pad was protected by plastic wrap to ensure proper hygiene and secured. The intensity of clinic treatments was set at 7 bars, which minimized the risk of burns. Home treatments were similar to those in the clinic, except that each leg had to be treated individually instead of simultaneously and home MIRE units are preset and only function at an intensity that is equivalent to around 7 bars on the clinical unit.
The standardized balance program consisted of a progression of the following exercises: ankle alphabet/ankle range of motion, standing lateral weight shifts, bilateral heel raises, bilateral toe raises, unilateral heel raises, unilateral toe raises, partial wall squats, and single leg stance. Each participant performed these exercises 3 times per week in the clinic and then 3 times per week at home following the 12th visit.
Outcomes and Follow-up
The POQ-VA, a subjective quality of life (QOL) measure for veterans, as well as VAS, SWM testing, and the Tinetti Gait and Balance Assessment scores were used to measure outcomes. Data were collected for each of these measures during the initial and 12th clinic visits and at the 3-month and 6-month follow-up visits. The POQ-VA and VAS scores were self-reported and filled out by each participant at the initial, 12th, 3-month, and 6-month visits. The POQ-VA score has proven to be reliable and valid for the assessment of noncancer, chronic pain in veterans.19 The VAS scores were measured using a scale of 0 to 10 cm.
The SWM was standardized, and 7 sites were tested on each foot during the initial, 12th, 3-month, and 6-month visits: plantar surface of the distal great toe, the distal 3rd toe, the distal 5th toe, the 1st metatarsal head, the 3rd metatarsal head, the 5th metatarsal head, and the mid-plantar arch. At each site, the SWM was applied with just enough force to initiate a bending force and held for 1.5 seconds. Each site was tested 3 times. Participants had to detect the monofilament at least twice for the monofilament value to be recorded. Monofilament testing began with 6.65 SWM and decreased to 5.07, 4.56, 4.32, and lower until the patient was no longer able to detect sensation.
The Tinetti Gait and Balance Assessments was performed on each participant at the initial, 12th, 3-month, and 6-month visits. Tinetti balance, gait, and total scores were recorded at each interval.
Results
Thirty-three patients, referred by primary care providers and specialty clinics, met the inclusion criteria and enrolled in the study. Twenty-one patients (20 men and 1 woman) completed the entire 6-month study. Causes for withdrawal included travel difficulties (5), did not show up for follow-up visits (4), lumbar radiculopathy (1), perceived minimal/no benefit (1), and unrelated death (1). No AEs were reported.
The Friedman test with DMC post hoc test was performed on the POQ-VA total score and subscale scores. The POQ-VA subscale scores were divided into the following domains: pain, activities of daily living (ADL), fear, negative affect, mobility, and vitality. The POQ-VA domains were analyzed to compare data from the initial, 12th, 3-month, and 6-month visits. The POQ-VA total score significantly decreased from the initial to the 12th visit (P < .01), from the initial to the 3-month (P < .01), and from the initial to the 6-month visit (P < .05). However, there was no significant change from the 12th visit to the 3-month follow-up, 12th visit to the 6-month follow-up, or the 3-month to 6-month follow-up.
The POQ-VA pain score decreased significantly from the initial to the 12th visit (P < .05) and from the initial to the 6-month visit (P < .05). However, there was no significant interval change from the initial to the 3-month, the 12th to 3-month, 12th to 6-month, or 3-month to 6-month visit (Figure 1). The POQ-VA vitality scores and POQ-VA fear scores did not yield significant changes. The POQ-VA negative affect scores showed significant improvement only between the initial and the 3-month visit (P < .05) (Figure 2). The POQ-VA ADL scores showed significant improvement in the initial vs 3-month score (P < .05). The POQ-VA mobility scores were significantly improved for the initial vs 12th visit (P < .01), initial vs 3-month visit (P < .01), and the initial vs 6-month visit (P < .001) (Figure 1).
Analysis of VAS scores revealed a significant decrease at the 6-month time frame compared with the initial score for the left foot (P < .05). Further VAS analysis revealed no significant difference between the initial and 6-month right foot VAS score. When both feet were compared together, there was no significant difference in VAS ratings between any 2 points in time.
Analysis of Tinetti Total Score, Tinetti Balance Score, and Tinetti Gait Score revealed a significant difference between the initial vs 3-month visit for all 3 scores (P < .001, P < .001, and P < .05, respectively). In addition, Tinetti Total (P < .001) and Tinetti Balance (P < .01) scores were significantly improved from initial to the final 6-month visit. There were no significant findings between interim scores of the initial and 12th visits, the 12th and 3-month visits, or the 3-month and 6-month scores (Figure 2).
Analysis of SWM testing indicated a significant decrease in the total number of insensate sites (> 5.07) when both feet were grouped together between the initial and 3-month visits (P < .05) as well as the initial and 6-month (P < .01) visits. When the left and right feet were compared independently of each other, there was a significant decrease in the number of insensate sites between the initial and 6-month visits (P < .01 for both) (Figure 3).
Discussion
This study investigated whether or not a multimodal physical therapy approach would reduce several of the debilitating symptoms of DPN experienced by many veterans at WJBDVAMC. The results support the idea that a combined treatment protocol of MIRE and a standardized exercise program can lead to decreased POQ-VA pain levels, improved balance, and improved protective sensation in veterans with DPN. Alleviation of these DPN complications may ultimately decrease an individual’s risk of injury and improve overall QOL.
Because the POQ-VA is a reliable, valid self-reported measure for veterans, it was chosen to quantify the impact of pain. Overall, veterans who participated in this study perceived decreased pain interference in multiple areas of their lives. The most significant findings were in overall QOL, household and community mobility, and pain ratings. This suggests that the combined treatment protocol will help veterans maintain an active lifestyle despite poorly controlled diabetes and neuropathic pain.
Along with decreased pain interference with QOL, participants demonstrated a decrease in fall risk as quantified by the Tinetti Gait and Balance Assessment. The SWM testing showed improved protective sensation as early as 3 months and continued through the 6-month visit. As protective sensation improves and fall risk decreases, the risk of injury is lessened, fear of falling is decreased, and individuals are less likely to self-impose limitations on daily activity levels, which improves QOL. In addition, decreased fall risk and improved protective sensation can reduce the financial burden on both the patient and the health care system. Many individuals are hospitalized secondary to fall injury, nonhealing wounds, resulting infections, and/or secondary complications from prolonged immobility. This treatment protocol demonstrates how a standardized physical therapy protocol, including MIRE and balance exercises, can be used preventively to reduce both the personal and financial impact of DPN.
It is interesting to note that some POQ-VA and Tinetti subscores were significantly improved at 3 months but not at 6 months. The significance achieved at 3 months may be due to the time required (ie, > 12 visits) to make significant physiological changes. The lack of significance at 6 months may be due to the natural tendency of participants to less consistently perform the home exercise program and MIRE protocol when unsupervised in the home. Differences in the VAS and POQ-VA pain score ratings were noted in the data. The POQ-VA pain rating scale indicated significant improvement in pain levels over the course of the study. However, when asked about pain using the 10-cm VAS, patients reported no significant improvements. This may be because veterans are more familiar with the numerical pain rating scale and are rarely asked to use the 10-cm VAS. It may also be because the POQ-VA pain rating asks for an average pain level over the previous week, whereas the 10-cm VAS asks for pain level at a discrete point in time.
Historically, physical therapy has had little to offer individuals with DPN. As a result of this study, however, a standardized treatment program for DPN has been implemented at the WJBDVAMC Physical Therapy Clinic. Referred patients are seen in the clinic on a trial basis. If positive results are documented during the clinic treatments, a home MIRE unit and exercise program are provided. The patients are expected to continue performing home treatments of MIRE and exercise 3 times a week after discharge.
Strengths and Limitations
Strengths of the study include a stringent IRB review, control of medication changes during the study through alerts to all VA providers, and a standardized MIRE and exercise protocol. An additional strength is the long duration of the study, which included supervised and unsupervised interventions that simulate real-life scenarios.
Limitations of the study include a small sample size, case-controlled design rather than a randomized, double-blinded study, which can contribute to selection bias, inability to differentiate between the benefits of physical therapy alone vs physical therapy and MIRE treatments, and retention of participants due to travel difficulties across a wide catchment area.
This pilot study should be expanded to a multicenter, randomized, double-blinded study to clarify the most beneficial treatments for individuals with diabetic neuropathy. Examining the number of documented falls pre- and postintervention may also be helpful to determine actual effects on an individual’s fall risk.
Conclusion
The use of a multimodal physical therapy approach seems to be effective in reducing the impact of neuropathic pain, the risk of amputation, and the risk of falls in individuals who have pursued all standard medical options but still experience the long-term effects of DPN. By adhering to a standardized treatment protocol of MIRE and therapeutic exercise, it seems that the benefits of this intervention can be maintained over time. This offers new, nonconventional treatment options in the field of physical therapy for veterans whose QOL is negatively impacted by the devastating effects of diabetic neuropathy.
Acknowledgements
Clinical support was provided by David Metzelfeld, DPT, and Cam Lendrim, PTA of William Jennings Bryan Dorn VA Medical Center. Paul Bartels, PhD, of Warren Wilson College provided data analysis support. Anodyne Therapy, LLC, provided the MIRE unit used in the clinic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. National Institute of Neurological Disorders and Stroke Website. http://www.ninds.nih.gov/disorders/peripheralneuropath/detail_peripheralneuropathy.htm#183583208. Updated April 17, 2015. Accesssed August 8, 2015.
2. Armstrong DG, Lavery LA, and Wunderlich RP. Risk factors for diabetic foot ulceration: a logical approach to treatment. J Wound Ostomy Continence Nurs. 1998;25(3):123-128.
3. Pesa J, Meyer R, Quock T, Rattana SK, Mody SH. MBA Opioid utilization patterns among medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits. 2013;6(4):188-196.
4. VHA Support Service Center. The amputation risk by facility in the ProClarity amputation risk (PAVE) cube. Department of Veterans Affairs Nonpublic Intranet. http://vssc.med.va.gov.
5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892-900
6. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598-1604.
7. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
8. Centers for Disease Control and Prevention. Older adults falls: get the facts. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html. Updated July 1, 2015. Accessed August 8, 2015.
9. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333-338.
10. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568-1579.
11. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398.
12. Tuttle LG, Hastings MK, and Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92(1):133-141.
13. Gossrau G, Wähner M, Kuschke M, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011;12(6):953-960.
14. Somers DL, Somers MF. Treatment of neuropathic pain in a patient with diabetic neuropathy using transcutaneous electrical nerve stimulation applied to the skin of the lumbar region. Phys Ther. 1999;79(8):767-775.
15. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy—MIRE. J Diabetes Complications. 2006;20(2):81-87.
16. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168-172.
17. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract. 2004;10(1):24-30.
18. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92(3):125-130.
19. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395.
1. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. National Institute of Neurological Disorders and Stroke Website. http://www.ninds.nih.gov/disorders/peripheralneuropath/detail_peripheralneuropathy.htm#183583208. Updated April 17, 2015. Accesssed August 8, 2015.
2. Armstrong DG, Lavery LA, and Wunderlich RP. Risk factors for diabetic foot ulceration: a logical approach to treatment. J Wound Ostomy Continence Nurs. 1998;25(3):123-128.
3. Pesa J, Meyer R, Quock T, Rattana SK, Mody SH. MBA Opioid utilization patterns among medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits. 2013;6(4):188-196.
4. VHA Support Service Center. The amputation risk by facility in the ProClarity amputation risk (PAVE) cube. Department of Veterans Affairs Nonpublic Intranet. http://vssc.med.va.gov.
5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892-900
6. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598-1604.
7. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
8. Centers for Disease Control and Prevention. Older adults falls: get the facts. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html. Updated July 1, 2015. Accessed August 8, 2015.
9. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333-338.
10. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568-1579.
11. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398.
12. Tuttle LG, Hastings MK, and Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92(1):133-141.
13. Gossrau G, Wähner M, Kuschke M, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011;12(6):953-960.
14. Somers DL, Somers MF. Treatment of neuropathic pain in a patient with diabetic neuropathy using transcutaneous electrical nerve stimulation applied to the skin of the lumbar region. Phys Ther. 1999;79(8):767-775.
15. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy—MIRE. J Diabetes Complications. 2006;20(2):81-87.
16. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168-172.
17. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract. 2004;10(1):24-30.
18. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92(3):125-130.
19. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395.
2015 Update on vaginal hysterectomy
We’ve come a long way since Conrad Langebeck performed the first vaginal hysterectomy in 1813. For the inaugural surgery, Langebeck used no anesthesia, gloves, or other sterilization strategies, and he held the suture in his teeth at one point during the operation! (The patient survived.)1
Despite our dramatic progress since then, too many of us still perform benign hysterectomy by an approach other than vaginal. And too many of us still perform vaginal hysterectomy the way it was taught in the 1950s—frequently a backbreaking, frustrating undertaking.
That approach is unnecessary. In recent years, the technological world has developed many useful tools for minimally invasive gynecologic surgery, some of which greatly facilitate vaginal hysterectomy. In this Update, I focus on 3 of them:
- vessel-sealing devices
- a unique visualization system
- a lighted suction irrigator.
It is my hope that you will incorporate these tools into your vaginal hysterectomy cases and gain some of the significant benefits they have to offer. The revival of vaginal hysterectomy and vaginal surgery in general is all about using the best tools that we have available and using them well, cost- effectively, and thoughtfully to improve the experience of both surgeon and patient.
Why you should default to vaginal hysterectomy
Not only is vaginal hysterectomy more cosmetically pleasing but it also has a lower complication rate than laparoscopic, robot-assisted, or laparotomic hysterectomy, requiring no incisions through the abdominal wall. The original natural orifice translumenal endoscopic surgery (NOTES) procedure also is less expensive than laparoscopic or robot-assisted hysterectomy. Vaginal hysterectomy has so much to recommend it, in fact, that the biggest barrier to widespread use may simply be the lack of industry support.
According to the latest committee opinion from the American College of Obstetricians and Gynecologists (ACOG), “When choosing the route and method of hysterectomy, the physician should take into consideration how the procedure may be performed most safely and cost-effectively to fulfill the medical needs of the patient. Most literature supports the opinion that, when feasible, vaginal hysterectomy is the safest and most cost-effective route by which to remove the uterus.”2
A 2009 Cochrane review of surgical approaches to hysterectomy found that vaginal hysterectomy should be performed in preference to abdominal hysterectomy whenever possible.3 Yet data from 2008 indicate that almost 50% of all hysterectomies were still being performed using an open abdominal approach, and laparoscopic hysterectomy made up almost another 25%.4
To address the disparity between the evidence and practice, ACOG has joined forces with the AAGL and the Society for Gynecologic Surgeons (SGS) to present an online master course on vaginal hysterectomy, available at http://www.aagl.org/vaghystwebinar. This course features videos and live demonstrations on cadaveric models and is free to physicians, with continuing medical education (CME) credits available.
Vessel sealing offers real benefits over suturing
In any surgery, the need to achieve reliable hemostasis is critical. In vaginal hysterectomy, this goal traditionally has been attained by clamping and suturing of the vessels. In many respects, vaginal surgeons seem to have gotten trapped in the mindset that we need to suture during vaginal surgery—and train residents to suture, too. When it comes to laparoscopic surgery, however, the reverse is true. In that setting, vessel-sealing devices are used to seal blood vessels with “supraphysiologic burst pressure equal to that of previously used surgical clips or ligatures.”5
Why is vessel sealing necessarily better than suturing?
It’s safer, for one thing, eliminating the need to pass needles back and forth. It also frees the scrub technician to become the surgical assistant because there are no needles to load and unload. In order for suture to hold around a pedicle, it is necessary to have tissue adjacent to it. The surgeon ties and cuts but must have something beyond the suture or the suture won’t hold. That something is dead, devascularized tissue. Before healing can occur, all this tissue must be absorbed by the body. That is not the case with vessel sealing, which fuses the walls of the blood vessels, leaving less foreign material and dead tissue behind.
What the data show
The literature offers several randomized comparisons of bipolar vessel sealing and suturing during vaginal hysterectomy, and all of them find increased benefits for the vessel-sealing approach.
For example, in 2003, I published a randomized comparison looking specifically at blood loss and operative time.6 Sixty women in a single surgical practice were randomly allocated to vessel sealing or sutures for hemostasis during vaginal hysterectomy. In the vessel-sealing group, the mean operative time was 39.1 minutes (range, 22–93), compared with 53.6 minutes in the suturing group (range, 37–160; P = .003). Mean estimated blood loss also was significantly lower with vessel sealing, at 68.9 mL (range, 20–200) versus 126.7 mL for suturing (range, 25–600; P = .005). Complication rates and length of stay were similar between groups.6
In another randomized trial of vessel sealing versus suturing involving 68 women undergoing vaginal hysterectomy, pain was markedly reduced in the vessel-sealing group (median score, 4 vs 6; P<.0001). Operative time again was shorter with vessel sealing than with suturing (median of 32 vs 40 minutes; P = .003), but there were no differences in blood loss and hospitalization.7
Silva-Filho and colleagues randomly allocated 90 women to bipolar vessel sealing or suturing during vaginal hysterectomy.8 Vessel sealing provided reduced postoperative pain (pain score [SD] of 1.6 [0.4] vs 3.6 [0.4]; P<.001), shorter operative time (mean of 29.2 [2.1] vs 75.2 [5] minutes; P<.001), less blood loss (mean of 84 [5.9] vs 136.4 [89.1] mL; P = .001), and a shorter hospital stay (mean of 25.6 [0.9] vs 33.2 [1.7] hours; P<.001).8
A systematic review and meta-analysis by Kroft and Selk found that vessel sealing reduced: operative time by a mean of 17.2 minutes (95% confidence interval [CI], 7.5–27.0); blood loss by a mean of 47.7 mL (95% CI, 15.5–79.9); and hospital stay by a mean of 0.25 days (95% CI, 0.13–0.37) during vaginal hysterectomy.9
And in a randomized controlled trial from the Netherlands, women undergoing vaginal hysterectomy reported significantly less pain the evening after surgery in the vessel-sealing group, compared with the suturing group (pain score of 4.5 vs 5.7 on a scale of 1 to 10; P = .03).10 They also had a shorter operative time than women in the suturing group (60 vs 71 minutes; P = .05). Blood loss and hospital stays did not differ between groups, and there were no major differences in cost.
A reduction in pain is an especially important indicator of surgical success. In an interesting twist, Candiani and colleagues compared laparoscopic and vaginal hysterectomy for a number of variables, including pain, for benign pathology.11 They found less postoperative pain the day of surgery and a reduced number of days of analgesic request in the laparoscopic group, compared with vaginal hysterectomy. One reason: Hemostasis was achieved via vessel sealing in the laparoscopic group, compared with clamping and suturing in the vaginal group.11
Lighted suction irrigator facilitates visualization “around corners”
Many years ago, I conducted some informal studies for industry that showed—as one might guess intuitively—that the ability to see well during surgery cuts operative time. We all know that light is good. One useful lighting aid I’ve adopted of late is the Vital Vue (Covidien/Medtronic) suction irrigator. It has a disposable tip like all suction devices, but it includes 3 channels: one for a fiber optic cord, another for fluid, and the third for suction (FIGURE 1). It plugs into a regular suction machine, with a reusable box that provides the fiber optic light.
Because the suction tip is curved, the device makes it possible to illuminate the surgical field “around corners” if need be. Any bleeding can be irrigated to clear the field.
How to choose a vessel sealer
When selecting a vessel-sealing device for vaginal hysterectomy, keep in mind a number of factors:
- size of the vessels that will need to be controlled. Most devices on the market today control vessels 7 mm in size or smaller.
- amount of steam it releases, which can damage adjacent tissue
- overall size of the device
- size of the pedicles that will need to be controlled
- overall space required for use
- cost of the device.
In other words, to choose an appropriate device, you will need to think in advance about the specifics of the case you are planning, as not all hysterectomies are alike. The type of vessel sealer best for the surgery will vary with these details.
Both bipolar electrosurgical and ultrasonic devices now provide consistent hemostasis, increased functionality, and greater efficiency. What’s more, they cause minimal to no damage to surrounding tissue.
External scope offers visualization of vaginal procedures to entire OR
Designed for open surgeries, the VITOM system (Karl Storz) is an innovative tool for displaying procedures in which surgical access is limited. It’s an external telescope, or “exoscope,” with a 90° lens. It clips onto the table, providing visualization for the entire operative team (FIGURE 2).
As we all know, the advent of the camera made an enormous difference in laparoscopic procedures and in teaching because it enabled the assistant to see what the surgeon was doing and anticipate his or her needs. This device offers the same advantages for vaginal hysterectomy. In my opinion, it’s a game changer.
The VITOM system provides outstanding image quality and depth of view. It is placed at a distance of 25 cm to 75 cm from the surgical field and thus does not impinge on the surgeon’s workspace. Because it is compact, it facilitates the use of long instruments, if necessary. In addition, because it can be sterilized, the VITOM system can be manipulated directly by the surgeon or assistant.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www.brighamandwomens.org/Departments_and_Services/obgyn/ser vices/mininvgynsurg/mininvoptions/hysterectomy.aspx. Updated October 3, 2014. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- Nezhat C, Lewis M, King LP. Laparoscopic vessel sealing devices. Society of Laparoendoscopic Surgeons. http://laparoscopy.blogs.com/prevention_management_3/2010/10/laparoscopic-vessel-sealing-devices.html. Published 2010. Accessed August 6, 2015.
- Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147–151.
- Cronjé HS, de Coning EC. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. 2005;91(3):243–245.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Kroft J, Selk A. Energy-based vessel sealing in vaginal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
- Lakeman MM, The S, Schellart RP, et al. Electrosurgical bipolar vessel sealing versus conventional clamping and suturing for vaginal hysterectomy: a randomised controlled trial. BJOG. 2012;119(12):1473–1482.
- Candiani M, Izzo S, Bulfoni A, Riparini J, Ronzoni S, Marconi A. Laparoscopic vs vaginal hysterectomy for benign pathology. Am J Obstet Gynecol. 2009;200(4):368.e1–e7.
We’ve come a long way since Conrad Langebeck performed the first vaginal hysterectomy in 1813. For the inaugural surgery, Langebeck used no anesthesia, gloves, or other sterilization strategies, and he held the suture in his teeth at one point during the operation! (The patient survived.)1
Despite our dramatic progress since then, too many of us still perform benign hysterectomy by an approach other than vaginal. And too many of us still perform vaginal hysterectomy the way it was taught in the 1950s—frequently a backbreaking, frustrating undertaking.
That approach is unnecessary. In recent years, the technological world has developed many useful tools for minimally invasive gynecologic surgery, some of which greatly facilitate vaginal hysterectomy. In this Update, I focus on 3 of them:
- vessel-sealing devices
- a unique visualization system
- a lighted suction irrigator.
It is my hope that you will incorporate these tools into your vaginal hysterectomy cases and gain some of the significant benefits they have to offer. The revival of vaginal hysterectomy and vaginal surgery in general is all about using the best tools that we have available and using them well, cost- effectively, and thoughtfully to improve the experience of both surgeon and patient.
Why you should default to vaginal hysterectomy
Not only is vaginal hysterectomy more cosmetically pleasing but it also has a lower complication rate than laparoscopic, robot-assisted, or laparotomic hysterectomy, requiring no incisions through the abdominal wall. The original natural orifice translumenal endoscopic surgery (NOTES) procedure also is less expensive than laparoscopic or robot-assisted hysterectomy. Vaginal hysterectomy has so much to recommend it, in fact, that the biggest barrier to widespread use may simply be the lack of industry support.
According to the latest committee opinion from the American College of Obstetricians and Gynecologists (ACOG), “When choosing the route and method of hysterectomy, the physician should take into consideration how the procedure may be performed most safely and cost-effectively to fulfill the medical needs of the patient. Most literature supports the opinion that, when feasible, vaginal hysterectomy is the safest and most cost-effective route by which to remove the uterus.”2
A 2009 Cochrane review of surgical approaches to hysterectomy found that vaginal hysterectomy should be performed in preference to abdominal hysterectomy whenever possible.3 Yet data from 2008 indicate that almost 50% of all hysterectomies were still being performed using an open abdominal approach, and laparoscopic hysterectomy made up almost another 25%.4
To address the disparity between the evidence and practice, ACOG has joined forces with the AAGL and the Society for Gynecologic Surgeons (SGS) to present an online master course on vaginal hysterectomy, available at http://www.aagl.org/vaghystwebinar. This course features videos and live demonstrations on cadaveric models and is free to physicians, with continuing medical education (CME) credits available.
Vessel sealing offers real benefits over suturing
In any surgery, the need to achieve reliable hemostasis is critical. In vaginal hysterectomy, this goal traditionally has been attained by clamping and suturing of the vessels. In many respects, vaginal surgeons seem to have gotten trapped in the mindset that we need to suture during vaginal surgery—and train residents to suture, too. When it comes to laparoscopic surgery, however, the reverse is true. In that setting, vessel-sealing devices are used to seal blood vessels with “supraphysiologic burst pressure equal to that of previously used surgical clips or ligatures.”5
Why is vessel sealing necessarily better than suturing?
It’s safer, for one thing, eliminating the need to pass needles back and forth. It also frees the scrub technician to become the surgical assistant because there are no needles to load and unload. In order for suture to hold around a pedicle, it is necessary to have tissue adjacent to it. The surgeon ties and cuts but must have something beyond the suture or the suture won’t hold. That something is dead, devascularized tissue. Before healing can occur, all this tissue must be absorbed by the body. That is not the case with vessel sealing, which fuses the walls of the blood vessels, leaving less foreign material and dead tissue behind.
What the data show
The literature offers several randomized comparisons of bipolar vessel sealing and suturing during vaginal hysterectomy, and all of them find increased benefits for the vessel-sealing approach.
For example, in 2003, I published a randomized comparison looking specifically at blood loss and operative time.6 Sixty women in a single surgical practice were randomly allocated to vessel sealing or sutures for hemostasis during vaginal hysterectomy. In the vessel-sealing group, the mean operative time was 39.1 minutes (range, 22–93), compared with 53.6 minutes in the suturing group (range, 37–160; P = .003). Mean estimated blood loss also was significantly lower with vessel sealing, at 68.9 mL (range, 20–200) versus 126.7 mL for suturing (range, 25–600; P = .005). Complication rates and length of stay were similar between groups.6
In another randomized trial of vessel sealing versus suturing involving 68 women undergoing vaginal hysterectomy, pain was markedly reduced in the vessel-sealing group (median score, 4 vs 6; P<.0001). Operative time again was shorter with vessel sealing than with suturing (median of 32 vs 40 minutes; P = .003), but there were no differences in blood loss and hospitalization.7
Silva-Filho and colleagues randomly allocated 90 women to bipolar vessel sealing or suturing during vaginal hysterectomy.8 Vessel sealing provided reduced postoperative pain (pain score [SD] of 1.6 [0.4] vs 3.6 [0.4]; P<.001), shorter operative time (mean of 29.2 [2.1] vs 75.2 [5] minutes; P<.001), less blood loss (mean of 84 [5.9] vs 136.4 [89.1] mL; P = .001), and a shorter hospital stay (mean of 25.6 [0.9] vs 33.2 [1.7] hours; P<.001).8
A systematic review and meta-analysis by Kroft and Selk found that vessel sealing reduced: operative time by a mean of 17.2 minutes (95% confidence interval [CI], 7.5–27.0); blood loss by a mean of 47.7 mL (95% CI, 15.5–79.9); and hospital stay by a mean of 0.25 days (95% CI, 0.13–0.37) during vaginal hysterectomy.9
And in a randomized controlled trial from the Netherlands, women undergoing vaginal hysterectomy reported significantly less pain the evening after surgery in the vessel-sealing group, compared with the suturing group (pain score of 4.5 vs 5.7 on a scale of 1 to 10; P = .03).10 They also had a shorter operative time than women in the suturing group (60 vs 71 minutes; P = .05). Blood loss and hospital stays did not differ between groups, and there were no major differences in cost.
A reduction in pain is an especially important indicator of surgical success. In an interesting twist, Candiani and colleagues compared laparoscopic and vaginal hysterectomy for a number of variables, including pain, for benign pathology.11 They found less postoperative pain the day of surgery and a reduced number of days of analgesic request in the laparoscopic group, compared with vaginal hysterectomy. One reason: Hemostasis was achieved via vessel sealing in the laparoscopic group, compared with clamping and suturing in the vaginal group.11
Lighted suction irrigator facilitates visualization “around corners”
Many years ago, I conducted some informal studies for industry that showed—as one might guess intuitively—that the ability to see well during surgery cuts operative time. We all know that light is good. One useful lighting aid I’ve adopted of late is the Vital Vue (Covidien/Medtronic) suction irrigator. It has a disposable tip like all suction devices, but it includes 3 channels: one for a fiber optic cord, another for fluid, and the third for suction (FIGURE 1). It plugs into a regular suction machine, with a reusable box that provides the fiber optic light.
Because the suction tip is curved, the device makes it possible to illuminate the surgical field “around corners” if need be. Any bleeding can be irrigated to clear the field.
How to choose a vessel sealer
When selecting a vessel-sealing device for vaginal hysterectomy, keep in mind a number of factors:
- size of the vessels that will need to be controlled. Most devices on the market today control vessels 7 mm in size or smaller.
- amount of steam it releases, which can damage adjacent tissue
- overall size of the device
- size of the pedicles that will need to be controlled
- overall space required for use
- cost of the device.
In other words, to choose an appropriate device, you will need to think in advance about the specifics of the case you are planning, as not all hysterectomies are alike. The type of vessel sealer best for the surgery will vary with these details.
Both bipolar electrosurgical and ultrasonic devices now provide consistent hemostasis, increased functionality, and greater efficiency. What’s more, they cause minimal to no damage to surrounding tissue.
External scope offers visualization of vaginal procedures to entire OR
Designed for open surgeries, the VITOM system (Karl Storz) is an innovative tool for displaying procedures in which surgical access is limited. It’s an external telescope, or “exoscope,” with a 90° lens. It clips onto the table, providing visualization for the entire operative team (FIGURE 2).
As we all know, the advent of the camera made an enormous difference in laparoscopic procedures and in teaching because it enabled the assistant to see what the surgeon was doing and anticipate his or her needs. This device offers the same advantages for vaginal hysterectomy. In my opinion, it’s a game changer.
The VITOM system provides outstanding image quality and depth of view. It is placed at a distance of 25 cm to 75 cm from the surgical field and thus does not impinge on the surgeon’s workspace. Because it is compact, it facilitates the use of long instruments, if necessary. In addition, because it can be sterilized, the VITOM system can be manipulated directly by the surgeon or assistant.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
We’ve come a long way since Conrad Langebeck performed the first vaginal hysterectomy in 1813. For the inaugural surgery, Langebeck used no anesthesia, gloves, or other sterilization strategies, and he held the suture in his teeth at one point during the operation! (The patient survived.)1
Despite our dramatic progress since then, too many of us still perform benign hysterectomy by an approach other than vaginal. And too many of us still perform vaginal hysterectomy the way it was taught in the 1950s—frequently a backbreaking, frustrating undertaking.
That approach is unnecessary. In recent years, the technological world has developed many useful tools for minimally invasive gynecologic surgery, some of which greatly facilitate vaginal hysterectomy. In this Update, I focus on 3 of them:
- vessel-sealing devices
- a unique visualization system
- a lighted suction irrigator.
It is my hope that you will incorporate these tools into your vaginal hysterectomy cases and gain some of the significant benefits they have to offer. The revival of vaginal hysterectomy and vaginal surgery in general is all about using the best tools that we have available and using them well, cost- effectively, and thoughtfully to improve the experience of both surgeon and patient.
Why you should default to vaginal hysterectomy
Not only is vaginal hysterectomy more cosmetically pleasing but it also has a lower complication rate than laparoscopic, robot-assisted, or laparotomic hysterectomy, requiring no incisions through the abdominal wall. The original natural orifice translumenal endoscopic surgery (NOTES) procedure also is less expensive than laparoscopic or robot-assisted hysterectomy. Vaginal hysterectomy has so much to recommend it, in fact, that the biggest barrier to widespread use may simply be the lack of industry support.
According to the latest committee opinion from the American College of Obstetricians and Gynecologists (ACOG), “When choosing the route and method of hysterectomy, the physician should take into consideration how the procedure may be performed most safely and cost-effectively to fulfill the medical needs of the patient. Most literature supports the opinion that, when feasible, vaginal hysterectomy is the safest and most cost-effective route by which to remove the uterus.”2
A 2009 Cochrane review of surgical approaches to hysterectomy found that vaginal hysterectomy should be performed in preference to abdominal hysterectomy whenever possible.3 Yet data from 2008 indicate that almost 50% of all hysterectomies were still being performed using an open abdominal approach, and laparoscopic hysterectomy made up almost another 25%.4
To address the disparity between the evidence and practice, ACOG has joined forces with the AAGL and the Society for Gynecologic Surgeons (SGS) to present an online master course on vaginal hysterectomy, available at http://www.aagl.org/vaghystwebinar. This course features videos and live demonstrations on cadaveric models and is free to physicians, with continuing medical education (CME) credits available.
Vessel sealing offers real benefits over suturing
In any surgery, the need to achieve reliable hemostasis is critical. In vaginal hysterectomy, this goal traditionally has been attained by clamping and suturing of the vessels. In many respects, vaginal surgeons seem to have gotten trapped in the mindset that we need to suture during vaginal surgery—and train residents to suture, too. When it comes to laparoscopic surgery, however, the reverse is true. In that setting, vessel-sealing devices are used to seal blood vessels with “supraphysiologic burst pressure equal to that of previously used surgical clips or ligatures.”5
Why is vessel sealing necessarily better than suturing?
It’s safer, for one thing, eliminating the need to pass needles back and forth. It also frees the scrub technician to become the surgical assistant because there are no needles to load and unload. In order for suture to hold around a pedicle, it is necessary to have tissue adjacent to it. The surgeon ties and cuts but must have something beyond the suture or the suture won’t hold. That something is dead, devascularized tissue. Before healing can occur, all this tissue must be absorbed by the body. That is not the case with vessel sealing, which fuses the walls of the blood vessels, leaving less foreign material and dead tissue behind.
What the data show
The literature offers several randomized comparisons of bipolar vessel sealing and suturing during vaginal hysterectomy, and all of them find increased benefits for the vessel-sealing approach.
For example, in 2003, I published a randomized comparison looking specifically at blood loss and operative time.6 Sixty women in a single surgical practice were randomly allocated to vessel sealing or sutures for hemostasis during vaginal hysterectomy. In the vessel-sealing group, the mean operative time was 39.1 minutes (range, 22–93), compared with 53.6 minutes in the suturing group (range, 37–160; P = .003). Mean estimated blood loss also was significantly lower with vessel sealing, at 68.9 mL (range, 20–200) versus 126.7 mL for suturing (range, 25–600; P = .005). Complication rates and length of stay were similar between groups.6
In another randomized trial of vessel sealing versus suturing involving 68 women undergoing vaginal hysterectomy, pain was markedly reduced in the vessel-sealing group (median score, 4 vs 6; P<.0001). Operative time again was shorter with vessel sealing than with suturing (median of 32 vs 40 minutes; P = .003), but there were no differences in blood loss and hospitalization.7
Silva-Filho and colleagues randomly allocated 90 women to bipolar vessel sealing or suturing during vaginal hysterectomy.8 Vessel sealing provided reduced postoperative pain (pain score [SD] of 1.6 [0.4] vs 3.6 [0.4]; P<.001), shorter operative time (mean of 29.2 [2.1] vs 75.2 [5] minutes; P<.001), less blood loss (mean of 84 [5.9] vs 136.4 [89.1] mL; P = .001), and a shorter hospital stay (mean of 25.6 [0.9] vs 33.2 [1.7] hours; P<.001).8
A systematic review and meta-analysis by Kroft and Selk found that vessel sealing reduced: operative time by a mean of 17.2 minutes (95% confidence interval [CI], 7.5–27.0); blood loss by a mean of 47.7 mL (95% CI, 15.5–79.9); and hospital stay by a mean of 0.25 days (95% CI, 0.13–0.37) during vaginal hysterectomy.9
And in a randomized controlled trial from the Netherlands, women undergoing vaginal hysterectomy reported significantly less pain the evening after surgery in the vessel-sealing group, compared with the suturing group (pain score of 4.5 vs 5.7 on a scale of 1 to 10; P = .03).10 They also had a shorter operative time than women in the suturing group (60 vs 71 minutes; P = .05). Blood loss and hospital stays did not differ between groups, and there were no major differences in cost.
A reduction in pain is an especially important indicator of surgical success. In an interesting twist, Candiani and colleagues compared laparoscopic and vaginal hysterectomy for a number of variables, including pain, for benign pathology.11 They found less postoperative pain the day of surgery and a reduced number of days of analgesic request in the laparoscopic group, compared with vaginal hysterectomy. One reason: Hemostasis was achieved via vessel sealing in the laparoscopic group, compared with clamping and suturing in the vaginal group.11
Lighted suction irrigator facilitates visualization “around corners”
Many years ago, I conducted some informal studies for industry that showed—as one might guess intuitively—that the ability to see well during surgery cuts operative time. We all know that light is good. One useful lighting aid I’ve adopted of late is the Vital Vue (Covidien/Medtronic) suction irrigator. It has a disposable tip like all suction devices, but it includes 3 channels: one for a fiber optic cord, another for fluid, and the third for suction (FIGURE 1). It plugs into a regular suction machine, with a reusable box that provides the fiber optic light.
Because the suction tip is curved, the device makes it possible to illuminate the surgical field “around corners” if need be. Any bleeding can be irrigated to clear the field.
How to choose a vessel sealer
When selecting a vessel-sealing device for vaginal hysterectomy, keep in mind a number of factors:
- size of the vessels that will need to be controlled. Most devices on the market today control vessels 7 mm in size or smaller.
- amount of steam it releases, which can damage adjacent tissue
- overall size of the device
- size of the pedicles that will need to be controlled
- overall space required for use
- cost of the device.
In other words, to choose an appropriate device, you will need to think in advance about the specifics of the case you are planning, as not all hysterectomies are alike. The type of vessel sealer best for the surgery will vary with these details.
Both bipolar electrosurgical and ultrasonic devices now provide consistent hemostasis, increased functionality, and greater efficiency. What’s more, they cause minimal to no damage to surrounding tissue.
External scope offers visualization of vaginal procedures to entire OR
Designed for open surgeries, the VITOM system (Karl Storz) is an innovative tool for displaying procedures in which surgical access is limited. It’s an external telescope, or “exoscope,” with a 90° lens. It clips onto the table, providing visualization for the entire operative team (FIGURE 2).
As we all know, the advent of the camera made an enormous difference in laparoscopic procedures and in teaching because it enabled the assistant to see what the surgeon was doing and anticipate his or her needs. This device offers the same advantages for vaginal hysterectomy. In my opinion, it’s a game changer.
The VITOM system provides outstanding image quality and depth of view. It is placed at a distance of 25 cm to 75 cm from the surgical field and thus does not impinge on the surgeon’s workspace. Because it is compact, it facilitates the use of long instruments, if necessary. In addition, because it can be sterilized, the VITOM system can be manipulated directly by the surgeon or assistant.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www.brighamandwomens.org/Departments_and_Services/obgyn/ser vices/mininvgynsurg/mininvoptions/hysterectomy.aspx. Updated October 3, 2014. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- Nezhat C, Lewis M, King LP. Laparoscopic vessel sealing devices. Society of Laparoendoscopic Surgeons. http://laparoscopy.blogs.com/prevention_management_3/2010/10/laparoscopic-vessel-sealing-devices.html. Published 2010. Accessed August 6, 2015.
- Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147–151.
- Cronjé HS, de Coning EC. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. 2005;91(3):243–245.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Kroft J, Selk A. Energy-based vessel sealing in vaginal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
- Lakeman MM, The S, Schellart RP, et al. Electrosurgical bipolar vessel sealing versus conventional clamping and suturing for vaginal hysterectomy: a randomised controlled trial. BJOG. 2012;119(12):1473–1482.
- Candiani M, Izzo S, Bulfoni A, Riparini J, Ronzoni S, Marconi A. Laparoscopic vs vaginal hysterectomy for benign pathology. Am J Obstet Gynecol. 2009;200(4):368.e1–e7.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www.brighamandwomens.org/Departments_and_Services/obgyn/ser vices/mininvgynsurg/mininvoptions/hysterectomy.aspx. Updated October 3, 2014. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- Nezhat C, Lewis M, King LP. Laparoscopic vessel sealing devices. Society of Laparoendoscopic Surgeons. http://laparoscopy.blogs.com/prevention_management_3/2010/10/laparoscopic-vessel-sealing-devices.html. Published 2010. Accessed August 6, 2015.
- Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147–151.
- Cronjé HS, de Coning EC. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. 2005;91(3):243–245.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Kroft J, Selk A. Energy-based vessel sealing in vaginal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
- Lakeman MM, The S, Schellart RP, et al. Electrosurgical bipolar vessel sealing versus conventional clamping and suturing for vaginal hysterectomy: a randomised controlled trial. BJOG. 2012;119(12):1473–1482.
- Candiani M, Izzo S, Bulfoni A, Riparini J, Ronzoni S, Marconi A. Laparoscopic vs vaginal hysterectomy for benign pathology. Am J Obstet Gynecol. 2009;200(4):368.e1–e7.
In this Article
- Benefits of vessel sealing over suturing
- Lighted suction irrigator: visualization “around corners”
- External scope offers optimal visualization to the entire team
Your teenage patient and contraception: Think “long-acting” first
CASE: Teen patient asks to switch contraceptive methods
A 17-year-old nulliparous woman comes to your clinic for an annual examination. She has no significant health problems, and her examination is normal. She notes that she was started on oral contraceptives (OCs) the year before because of heavy menstrual flow and a desire for birth control but has trouble remembering to take them—though she does usually use condoms. She asks your advice about switching to a different method but indicates that she has lost her health insurance coverage.
What can you offer her as an effective, low-cost contraceptive?
Long-acting reversible contraception (LARC) methods are especially suited for adolescent and young adult women, for whom daily compliance with a shorter-acting contraceptive may be problematic. Five LARC methods are available in the United States, including a new levonorgestrel-releasing intrauterine system (LNG-IUS; Liletta), which received approval from the US Food and Drug Administration (FDA) this year. Like Mirena, Liletta contains 52 mg of levonorgestrel that is released over time. Liletta was introduced by the nonprofit organization Medicines360 and its commercial partner Actavis Pharma in response to evidence that poor women continue to lack access to LARC because of cost or problems with insurance coverage.1
For providers who practice in settings eligible for 340B pricing, Liletta costs $50, a fraction of the cost of alternative intrauterine devices (IUDs). The cost is slightly higher for non-340B providers but is still significantly lower than the cost of other IUDs. For health care practices, the reduced price of Liletta may make it feasible for them to offer LARC to more patients. The reduced pricing also makes Liletta an attractive option for women who choose to pay for the device directly rather than use insurance, such as the patient described above.
Patient experience with Liletta also is key. Not surprisingly, Liletta’s clinical trial found patient satisfaction to be similar to that of Mirena users.2 The failure rate is less than 1%, again comparable to Mirena. The rate of pelvic infection with Liletta use was 0.5%, also comparable to previously published data.3
One difference between Liletta and Mirena is that Liletta carries FDA approval for 3 years of contraceptive efficacy, compared with 5 years for Mirena. In order to make Liletta available to US patients now, Medicines360 decided to apply for 3-year contraceptive labeling while 5- and 7-year efficacy data are being collected. Like Mirena, Liletta is expected to provide excellent contraception for at least 5 years.
How to insert Liletta
- While still pinching the insertion tube, slide the tube through the cervical canal until the upper edge of the flange is approximately 1.5 to 2 cm from the cervix. Do not force the inserter. If necessary, dilate the cervical canal. Release your hold on the tenaculum.
- Hold the insertion tube with the fingers of one hand (Hand A) and the rod with the fingers of the other hand (Hand B).
- Holding the rod in place (Hand B), relax your pinch on the tube and pull the insertion tube back with Hand A to the edge of the second indent of the rod. This will allow the IUS arms to unfold in the lower uterine segment (FIGURE). Wait 10 to 15 seconds for the arms of the IUS to open fully.
- Apply gentle traction with the tenaculum before advancing the IUS. With Hand A still holding the proximal end of the tube, advance both the insertion tube and rod simultaneously up to the uterine fundus. You will feel slight resistance when the IUS is at the fundus. Make sure the flange is touching the cervix when the IUS reaches the uterine fundus. Fundal positioning is important to prevent expulsion.
- Hold the rod still (Hand B) while pulling the insertion tube back with Hand A to the ring of the rod. While holding the inserter tube with Hand A, withdraw the rod from the insertion tube all of the way out to prevent the rod from catching on the knot at the lower end of the IUS. Completely remove the insertion tube.
- Using blunt-tipped sharp scissors, cut the IUS threads perpendicular to the thread length, leaving about 3 cm outside of the cervix. Do not apply tension or pull on the threads when cutting to prevent displacing the IUS. Insertion is now complete.
Source: Liletta [package insert]. Actavis Pharma, Parsippany, NJ; 2015.
Skyla is another LARC option for womenseeking an LNG-IUS for contraception. It provides highly effective contraception for at least 3 years through the release of 13.5 mg of levonorgestrel over time. Skyla’s reduced levonorgestrel content, as compared with Mirena and Liletta, means that fewer users will experience amenorrhea (13% vs 25%).
Paragard is a nonhormonal IUD that uses copper for contraceptive efficacy. The device contains a total of 380 mm of copper. Possible mechanisms of action include interference with sperm migration in the uterus and damage to or destruction of ova. It is FDA-approved for at least 10 years of use. The lack of any hormone in Paragard IUDs may make them attractive to women who do not wish to experience amenorrhea.
Nexplanon is a subdermal implant containing 68 mg of etonogestrel; it is approved for at least 3 years of use. It is the only LARC method that does not require a pelvic examination. Providers are required to complete a training course offered by the manufacturer to ensure proper placement and removal technique.
LARC should be a first-line birth control option
The primary indication for LARC is preg-nancy prevention. Because LARC methods are the most effective reversible means to prevent pregnancy—apart from complete abstinence from sexual intercourse—they should be offered as first-line birth control options to patients who do not wish to conceive. The ability to discontinue LARC methods is an attractive option for women who may want to become pregnant in the future, such as the patient in the opening vignette.
Efficacy rates are high
Because LARC methods do not require users to take action daily or prior to intercourse, they carry a risk of pregnancy of less than 1% (TABLE 1)4-7—equal to or better than rates seen with tubal sterilization. In comparison, the OC pill has a typical use contraceptive failure rate of about 8%.
LARC still has a low utilization rate
It is unfortunate that barriers to LARC methods remain in the United States (see, for example, “National organization identifies barriers to LARC,” above). As recently as 2011 to 2013, only 7.2% of US women aged 15 to 44 years used a LARC method.8 Provider inexperience and patient fears surrounding LARC use remain major barriers. In the past, nulliparity and young age were thought to be contraindications to IUD use. Research and experience have demonstrated, however, that IUDs and contraceptive implants are safe for use in young women and those who have not had children.
Cost barriers also have significantly limited the use of LARC methods. Over time, however, these contraceptives have become less costly to patients, and most insurance providers routinely cover LARC devices and insertion fees. The contraceptive mandate of the Affordable Care Act ensures coverage of contraception, including LARC, for interested women. These trends suggest continued improvement in women’s access to LARC.
National organization identifies barriers to LARC
In 2014, the National Committee for Quality Assurance (NCQA), with support from Bayer Healthcare, organized a meeting of key opinion leaders to discuss ways to eliminate barriers to the most effective contraceptive methods, better known as long-acting reversible contraception (LARC). The resultant issue brief, Women’s Health: Approaches to improving unintended pregnancy rates in the United States, identified a number of key barriers:
- Financial and logistical obstacles. The consensus attendees agreed that LARC methods should be offered to all women not planning a pregnancy in the next 2 years, but acknowledged that operational or administrative process issues sometimes interfere with this goal. One of the most prominent of these issues was the lack of opportunity for same-day insertion of LARC. Other issues included the cost to stock LARC methods, a lack of understanding of billing and reimbursement for LARC, reimbursement policies that prohibit billing for the visit and placement on the same day, and an overabundance of paperwork.
- Timing of the contraceptive counseling session. Many women fail to return for the 6-week postpartum visit—the visit typically set aside for counseling about contraception.
- Lack of a quality measure that would “motivate change in clinical practice.”1 One option: Treat family planning as a “vital sign” that needs to be addressed during the annual visit. “This would lead to stronger evidence for effecting change,” the report notes.1
- Lack of adequate communication skills by the provider. According to the NCQA report, “There are strong positive relationships between a health care team member’s communication skills and a patient’s willingness to follow through with medical recommendations.”1 The establishment of a “current counseling approach” that emphasizes the efficiency and effectiveness of LARC methods as well as the tremendous impact an unintended pregnancy would have on a woman’s whole life course would help improve provider-patient communication and increase the likelihood of LARC methods being utilized.1
- Lack of receptivity among some patients. For some women, the person delivering the message is as important as the message itself, depending on social and cultural norms. Sensitivity of health care providers to these nuances of communication can help enhance patient receptivity to the key message. As the NCQA report notes, “Physicians and the health care system are not always the most trusted source of information, and understanding disparities in contraception care will be important in changing patient behavior.”1
- Basic issues such as cost and access to care.1 Not all women are covered by insurance, particularly in states that opted against expanding access to Medicaid. For these women, the cost of LARC methods and insertion may be prohibitive.
Reference
Noncontraceptive benefits include reduced bleeding
The 3 LNG-IUS methods and the subdermal implant offer several benefits beyond contraception. Because of their progestin content, these methods reduce or even eliminate menses. This benefit can be very helpful for women who experience heavy menstrual periods and the consequent risk of anemia. Because of reduced menstrual flow, users of hormonal LARC methods also commonly experience less cramping associated with menses.
Women with endometriosis often benefit from hormonal LARC methods, as the disease is suppressed by the progestin component. Users of IUDs also have a reduced risk of endometrial cancer.
Contraindications to LARC
There are few contraindications to LARC methods, making them an appropriate choice for most women. The US Medical Eligibility Criteria for Contraceptive Use, 2010, published by the Centers for Disease Control and Prevention (CDC), contain guidelines that are based on the best available evidence.9 Contraceptive methods that are labeled as Category 1 or 2 are not contraindicated for most women. Methods that fall into Category 3 (theoretical or proven risks outweigh the advantages) or Category 4 (unacceptable health risk) are contraindicated (TABLE 2).9
IUDs once were thought to expose women to an increased risk of pelvic inflammatory disease, but this fear has long been disproven. Screening for chlamydia can be performed at the time of placement, as recommended annually for women younger than 25 years. Unless there is concern for active cervical or uterine infection, there is no need to delay insertion of an IUD while awaiting test results. In most cases, women found to have positive cultures after insertion can be treated successfully without IUD removal.
Main adverse effect is altered bleeding patterns
Adverse effects vary depending on the method being used. All LARC methods may affect menstrual patterns. For example, clinical trials involving the copper IUD indicate that abnormal heavy bleeding may lead to discontinuation in up to 10% of users.5,10 Amenorrhea or oligomenorrhea is uncommon with this method and rarely leads to discontinuation. For example, in one trial involving more than 900 women using a copper IUD for up to 5 years, there were no discontinuations due to amenorrhea. Dysmenorrhea may arise, but data from clinical trials indicate that its frequency decreases over time. In one trial, the frequency of any menstrual pain decreased from about 9% of users to 5% after 8 months or more of use.
The LNG-IUS also can be associated with abnormal uterine bleeding. In contrast to the copper IUD, LNG devices tend to reduce menstrual bleeding and can be unpredictable. Clinical trials involving the 5-year 52-mg LNG-IUS indicate that bleeding decreases over time, with as many as 70% of users developing amenorrhea or oligomenorrhea.5,11 However, some women using an LNG-IUS experience heavy bleeding— although the frequency of such bleeding tends to be substantially less than that experienced by copper IUD users.7
A lack of comparative trials makes it unclear whether the newer 3-year LNG-IUS devices are associated with a significantly altered bleeding pattern. Noncomparative data from the package insert for Skyla suggest that women using it may have a higher frequency of heavy menstrual bleeding and less amenorrhea than users of the 5-year device.6
Data from a 3-year clinical trial of the newest 52-mg LNG-IUS (Liletta) indicate that bleeding and dysmenorrhea led to discontinuation 1% to 2% of the time.2
Although the concentration of progestincirculating systemically is low with the various LNG-IUS devices, some women may experience symptoms such as mood swings, headaches, acne, and breast tenderness.
Expulsions during the first year of use of the copper IUD and the 3 LNG-IUS devices range from 2% to 10%, with the higher rates associated with immediate postpartum insertion.5
Uterine perforation has been reported in about 1 of every 1,000 insertions. Other adverse events are uncommon.
Clinical trials indicate that about 11% of implant users will discontinue the method due to bleeding abnormalities.12 About 25% to 30% of users will experience heavy or prolonged bleeding, while up to 33% will experience infrequent bleeding or amenorrhea. About 50% of implant users will experience improved bleeding patterns over time.
Other reasons for discontinuation of implant use in a very small percentage of users include emotional lability, weight gain, acne, and headaches.4 Complications due to insertion and removal are rare and include pain, bleeding, and hematoma formation.
Public health impact of LARC methods
An important question in regard to LARC use is: How do we best provide safe and effective contraception for teens and young adult women? There is increasing evidence that, with appropriate counseling and the removal of cost barriers, LARC methods can have a significant public health impact in this population.
The Contraceptive CHOICE Project, a cohort study in a teenage population of women in the St. Louis, Missouri, area, achieved increased utilization of LARC methods and significantly lower rates of pregnancy, birth, and abortion.13 Investigators proactively counseled young women about the advantages of LARC methods and offered them free of charge. As a result, 72% of women in the study chose an IUD or implant as their method of contraception. Pregnancy, birth, and abortion rates among participants were 34.0, 19.4, and 9.7 per 1,000 teens, respectively. By comparison, national statistics during the same time frame for pregnancy, birth, and abortion were 158.5, 94.0, and 41.5 per 1,000 US teens, respectively.13
A similar project in Colorado received $23.6 million in 2009 from an outside donor to make LARC methods more affordable to patients in family planning clinics in the state.14 Between 2009 and 2014, 30,000 contraceptive implants or IUDs were made available at low or no cost to low-income women attending 68 family planning clinics statewide. The use of these methods at participating clinics quadrupled. Further, the teen birth rate declined by 40% between 2009 and 2013—from 37 to 22 births per 1,000 teens.14 Seventy-five percent of this decline was attributable to increased use of these methods. The teen abortion rate declined by 35% in the same time frame.
In 2014, the Colorado governor’s office indicated that the state had saved $42.5 million in health care expenditures associated with teen births. It was estimated that, for every dollar spent on contraceptives, the state saved $5.68 in Medicaid costs. However, a bipartisan bill to continue funding the project has failed so far in 2015 due to concerns among some legislators that these methods—particularly the IUDs—are abortifacients. The reduced cost of the 3-year LNG-IUS (Liletta) and recent guidance from the US Department of Health and Human Services mandating that at least 1 form of contraception in each of the FDA-approved categories must be covered by insurers may help to overcome this barrier.
CASE: Resolved
You counsel the patient about the value of each LARC method, letting her know that they are all highly effective in the prevention of pregnancy. You also let her know how each method would affect her menstrual cycle and acknowledge that she may have a preference for whether the contraceptive is placed in her uterus or under the skin of her arm. She chooses the contraceptive implant, which you insert during the same visit. At a follow-up visit 6 weeks later, she reports satisfaction with the method.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Dehlendorf C, Rodriguez MI, Levy K, Borrero S, Steinauer J.Disparities in family planning. Am J Obstet Gynecol. 2010;202(3):214–220.
2. Eisenberg DL, Schreiber CA, Turok DK, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
3. Sufrin C, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120(6):1314–1321.
4. Espey E, Ogburn T. Long-acting reversible contraceptives—intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117(3):705–719.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
6. Skyla [package insert]. Bayer Healthcare, Wayne, NJ; 2013.
7. Liletta [package insert]. Actavis Pharma, Parsippany, NJ; 2015.
8. Branum AM, Jones J. Centers for Disease Control and Prevention: NCHS Data Brief No. 188: Trends in Long-Acting Reversible Contraception Use Among US Women Aged 15–44. http://www.cdc.gov/nchs/data/databriefs/db188.htm. Published February 2015. Accessed August 14, 2015.
9. Centers for Disease Control and Prevention. US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR4):1–86.
10. Andersson K, Odlind VL, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use; a randomized comparative trial. Contraception. 1994;49(1):56–72.
11. Sivin I, Stern J, Diaz J, et al. Two years of intrauterine contraception with levonorgestrel and copper: a randomized comparison of the TCu 380Ag and levonorgestrel 20 mcg/day devices. Contraception. 1987;35(3):245–255.
12. Mansour F, Korver T, Marintcheva-Petrova M, Fraser IS. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13–28.
13. Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med. 2014;371:1316–1323.
14. Tavernise S. Colorado’s effort against teenage pregnancies is a startling success. New York Times. http://www.nytimes.com/2015/07/06/science/colorados-push-against-teenage-pregnancies-is-a-startling-success.html. Published July 6, 2015. Accessed August 17, 2015.
CASE: Teen patient asks to switch contraceptive methods
A 17-year-old nulliparous woman comes to your clinic for an annual examination. She has no significant health problems, and her examination is normal. She notes that she was started on oral contraceptives (OCs) the year before because of heavy menstrual flow and a desire for birth control but has trouble remembering to take them—though she does usually use condoms. She asks your advice about switching to a different method but indicates that she has lost her health insurance coverage.
What can you offer her as an effective, low-cost contraceptive?
Long-acting reversible contraception (LARC) methods are especially suited for adolescent and young adult women, for whom daily compliance with a shorter-acting contraceptive may be problematic. Five LARC methods are available in the United States, including a new levonorgestrel-releasing intrauterine system (LNG-IUS; Liletta), which received approval from the US Food and Drug Administration (FDA) this year. Like Mirena, Liletta contains 52 mg of levonorgestrel that is released over time. Liletta was introduced by the nonprofit organization Medicines360 and its commercial partner Actavis Pharma in response to evidence that poor women continue to lack access to LARC because of cost or problems with insurance coverage.1
For providers who practice in settings eligible for 340B pricing, Liletta costs $50, a fraction of the cost of alternative intrauterine devices (IUDs). The cost is slightly higher for non-340B providers but is still significantly lower than the cost of other IUDs. For health care practices, the reduced price of Liletta may make it feasible for them to offer LARC to more patients. The reduced pricing also makes Liletta an attractive option for women who choose to pay for the device directly rather than use insurance, such as the patient described above.
Patient experience with Liletta also is key. Not surprisingly, Liletta’s clinical trial found patient satisfaction to be similar to that of Mirena users.2 The failure rate is less than 1%, again comparable to Mirena. The rate of pelvic infection with Liletta use was 0.5%, also comparable to previously published data.3
One difference between Liletta and Mirena is that Liletta carries FDA approval for 3 years of contraceptive efficacy, compared with 5 years for Mirena. In order to make Liletta available to US patients now, Medicines360 decided to apply for 3-year contraceptive labeling while 5- and 7-year efficacy data are being collected. Like Mirena, Liletta is expected to provide excellent contraception for at least 5 years.
How to insert Liletta
- While still pinching the insertion tube, slide the tube through the cervical canal until the upper edge of the flange is approximately 1.5 to 2 cm from the cervix. Do not force the inserter. If necessary, dilate the cervical canal. Release your hold on the tenaculum.
- Hold the insertion tube with the fingers of one hand (Hand A) and the rod with the fingers of the other hand (Hand B).
- Holding the rod in place (Hand B), relax your pinch on the tube and pull the insertion tube back with Hand A to the edge of the second indent of the rod. This will allow the IUS arms to unfold in the lower uterine segment (FIGURE). Wait 10 to 15 seconds for the arms of the IUS to open fully.
- Apply gentle traction with the tenaculum before advancing the IUS. With Hand A still holding the proximal end of the tube, advance both the insertion tube and rod simultaneously up to the uterine fundus. You will feel slight resistance when the IUS is at the fundus. Make sure the flange is touching the cervix when the IUS reaches the uterine fundus. Fundal positioning is important to prevent expulsion.
- Hold the rod still (Hand B) while pulling the insertion tube back with Hand A to the ring of the rod. While holding the inserter tube with Hand A, withdraw the rod from the insertion tube all of the way out to prevent the rod from catching on the knot at the lower end of the IUS. Completely remove the insertion tube.
- Using blunt-tipped sharp scissors, cut the IUS threads perpendicular to the thread length, leaving about 3 cm outside of the cervix. Do not apply tension or pull on the threads when cutting to prevent displacing the IUS. Insertion is now complete.
Source: Liletta [package insert]. Actavis Pharma, Parsippany, NJ; 2015.
Skyla is another LARC option for womenseeking an LNG-IUS for contraception. It provides highly effective contraception for at least 3 years through the release of 13.5 mg of levonorgestrel over time. Skyla’s reduced levonorgestrel content, as compared with Mirena and Liletta, means that fewer users will experience amenorrhea (13% vs 25%).
Paragard is a nonhormonal IUD that uses copper for contraceptive efficacy. The device contains a total of 380 mm of copper. Possible mechanisms of action include interference with sperm migration in the uterus and damage to or destruction of ova. It is FDA-approved for at least 10 years of use. The lack of any hormone in Paragard IUDs may make them attractive to women who do not wish to experience amenorrhea.
Nexplanon is a subdermal implant containing 68 mg of etonogestrel; it is approved for at least 3 years of use. It is the only LARC method that does not require a pelvic examination. Providers are required to complete a training course offered by the manufacturer to ensure proper placement and removal technique.
LARC should be a first-line birth control option
The primary indication for LARC is preg-nancy prevention. Because LARC methods are the most effective reversible means to prevent pregnancy—apart from complete abstinence from sexual intercourse—they should be offered as first-line birth control options to patients who do not wish to conceive. The ability to discontinue LARC methods is an attractive option for women who may want to become pregnant in the future, such as the patient in the opening vignette.
Efficacy rates are high
Because LARC methods do not require users to take action daily or prior to intercourse, they carry a risk of pregnancy of less than 1% (TABLE 1)4-7—equal to or better than rates seen with tubal sterilization. In comparison, the OC pill has a typical use contraceptive failure rate of about 8%.
LARC still has a low utilization rate
It is unfortunate that barriers to LARC methods remain in the United States (see, for example, “National organization identifies barriers to LARC,” above). As recently as 2011 to 2013, only 7.2% of US women aged 15 to 44 years used a LARC method.8 Provider inexperience and patient fears surrounding LARC use remain major barriers. In the past, nulliparity and young age were thought to be contraindications to IUD use. Research and experience have demonstrated, however, that IUDs and contraceptive implants are safe for use in young women and those who have not had children.
Cost barriers also have significantly limited the use of LARC methods. Over time, however, these contraceptives have become less costly to patients, and most insurance providers routinely cover LARC devices and insertion fees. The contraceptive mandate of the Affordable Care Act ensures coverage of contraception, including LARC, for interested women. These trends suggest continued improvement in women’s access to LARC.
National organization identifies barriers to LARC
In 2014, the National Committee for Quality Assurance (NCQA), with support from Bayer Healthcare, organized a meeting of key opinion leaders to discuss ways to eliminate barriers to the most effective contraceptive methods, better known as long-acting reversible contraception (LARC). The resultant issue brief, Women’s Health: Approaches to improving unintended pregnancy rates in the United States, identified a number of key barriers:
- Financial and logistical obstacles. The consensus attendees agreed that LARC methods should be offered to all women not planning a pregnancy in the next 2 years, but acknowledged that operational or administrative process issues sometimes interfere with this goal. One of the most prominent of these issues was the lack of opportunity for same-day insertion of LARC. Other issues included the cost to stock LARC methods, a lack of understanding of billing and reimbursement for LARC, reimbursement policies that prohibit billing for the visit and placement on the same day, and an overabundance of paperwork.
- Timing of the contraceptive counseling session. Many women fail to return for the 6-week postpartum visit—the visit typically set aside for counseling about contraception.
- Lack of a quality measure that would “motivate change in clinical practice.”1 One option: Treat family planning as a “vital sign” that needs to be addressed during the annual visit. “This would lead to stronger evidence for effecting change,” the report notes.1
- Lack of adequate communication skills by the provider. According to the NCQA report, “There are strong positive relationships between a health care team member’s communication skills and a patient’s willingness to follow through with medical recommendations.”1 The establishment of a “current counseling approach” that emphasizes the efficiency and effectiveness of LARC methods as well as the tremendous impact an unintended pregnancy would have on a woman’s whole life course would help improve provider-patient communication and increase the likelihood of LARC methods being utilized.1
- Lack of receptivity among some patients. For some women, the person delivering the message is as important as the message itself, depending on social and cultural norms. Sensitivity of health care providers to these nuances of communication can help enhance patient receptivity to the key message. As the NCQA report notes, “Physicians and the health care system are not always the most trusted source of information, and understanding disparities in contraception care will be important in changing patient behavior.”1
- Basic issues such as cost and access to care.1 Not all women are covered by insurance, particularly in states that opted against expanding access to Medicaid. For these women, the cost of LARC methods and insertion may be prohibitive.
Reference
Noncontraceptive benefits include reduced bleeding
The 3 LNG-IUS methods and the subdermal implant offer several benefits beyond contraception. Because of their progestin content, these methods reduce or even eliminate menses. This benefit can be very helpful for women who experience heavy menstrual periods and the consequent risk of anemia. Because of reduced menstrual flow, users of hormonal LARC methods also commonly experience less cramping associated with menses.
Women with endometriosis often benefit from hormonal LARC methods, as the disease is suppressed by the progestin component. Users of IUDs also have a reduced risk of endometrial cancer.
Contraindications to LARC
There are few contraindications to LARC methods, making them an appropriate choice for most women. The US Medical Eligibility Criteria for Contraceptive Use, 2010, published by the Centers for Disease Control and Prevention (CDC), contain guidelines that are based on the best available evidence.9 Contraceptive methods that are labeled as Category 1 or 2 are not contraindicated for most women. Methods that fall into Category 3 (theoretical or proven risks outweigh the advantages) or Category 4 (unacceptable health risk) are contraindicated (TABLE 2).9
IUDs once were thought to expose women to an increased risk of pelvic inflammatory disease, but this fear has long been disproven. Screening for chlamydia can be performed at the time of placement, as recommended annually for women younger than 25 years. Unless there is concern for active cervical or uterine infection, there is no need to delay insertion of an IUD while awaiting test results. In most cases, women found to have positive cultures after insertion can be treated successfully without IUD removal.
Main adverse effect is altered bleeding patterns
Adverse effects vary depending on the method being used. All LARC methods may affect menstrual patterns. For example, clinical trials involving the copper IUD indicate that abnormal heavy bleeding may lead to discontinuation in up to 10% of users.5,10 Amenorrhea or oligomenorrhea is uncommon with this method and rarely leads to discontinuation. For example, in one trial involving more than 900 women using a copper IUD for up to 5 years, there were no discontinuations due to amenorrhea. Dysmenorrhea may arise, but data from clinical trials indicate that its frequency decreases over time. In one trial, the frequency of any menstrual pain decreased from about 9% of users to 5% after 8 months or more of use.
The LNG-IUS also can be associated with abnormal uterine bleeding. In contrast to the copper IUD, LNG devices tend to reduce menstrual bleeding and can be unpredictable. Clinical trials involving the 5-year 52-mg LNG-IUS indicate that bleeding decreases over time, with as many as 70% of users developing amenorrhea or oligomenorrhea.5,11 However, some women using an LNG-IUS experience heavy bleeding— although the frequency of such bleeding tends to be substantially less than that experienced by copper IUD users.7
A lack of comparative trials makes it unclear whether the newer 3-year LNG-IUS devices are associated with a significantly altered bleeding pattern. Noncomparative data from the package insert for Skyla suggest that women using it may have a higher frequency of heavy menstrual bleeding and less amenorrhea than users of the 5-year device.6
Data from a 3-year clinical trial of the newest 52-mg LNG-IUS (Liletta) indicate that bleeding and dysmenorrhea led to discontinuation 1% to 2% of the time.2
Although the concentration of progestincirculating systemically is low with the various LNG-IUS devices, some women may experience symptoms such as mood swings, headaches, acne, and breast tenderness.
Expulsions during the first year of use of the copper IUD and the 3 LNG-IUS devices range from 2% to 10%, with the higher rates associated with immediate postpartum insertion.5
Uterine perforation has been reported in about 1 of every 1,000 insertions. Other adverse events are uncommon.
Clinical trials indicate that about 11% of implant users will discontinue the method due to bleeding abnormalities.12 About 25% to 30% of users will experience heavy or prolonged bleeding, while up to 33% will experience infrequent bleeding or amenorrhea. About 50% of implant users will experience improved bleeding patterns over time.
Other reasons for discontinuation of implant use in a very small percentage of users include emotional lability, weight gain, acne, and headaches.4 Complications due to insertion and removal are rare and include pain, bleeding, and hematoma formation.
Public health impact of LARC methods
An important question in regard to LARC use is: How do we best provide safe and effective contraception for teens and young adult women? There is increasing evidence that, with appropriate counseling and the removal of cost barriers, LARC methods can have a significant public health impact in this population.
The Contraceptive CHOICE Project, a cohort study in a teenage population of women in the St. Louis, Missouri, area, achieved increased utilization of LARC methods and significantly lower rates of pregnancy, birth, and abortion.13 Investigators proactively counseled young women about the advantages of LARC methods and offered them free of charge. As a result, 72% of women in the study chose an IUD or implant as their method of contraception. Pregnancy, birth, and abortion rates among participants were 34.0, 19.4, and 9.7 per 1,000 teens, respectively. By comparison, national statistics during the same time frame for pregnancy, birth, and abortion were 158.5, 94.0, and 41.5 per 1,000 US teens, respectively.13
A similar project in Colorado received $23.6 million in 2009 from an outside donor to make LARC methods more affordable to patients in family planning clinics in the state.14 Between 2009 and 2014, 30,000 contraceptive implants or IUDs were made available at low or no cost to low-income women attending 68 family planning clinics statewide. The use of these methods at participating clinics quadrupled. Further, the teen birth rate declined by 40% between 2009 and 2013—from 37 to 22 births per 1,000 teens.14 Seventy-five percent of this decline was attributable to increased use of these methods. The teen abortion rate declined by 35% in the same time frame.
In 2014, the Colorado governor’s office indicated that the state had saved $42.5 million in health care expenditures associated with teen births. It was estimated that, for every dollar spent on contraceptives, the state saved $5.68 in Medicaid costs. However, a bipartisan bill to continue funding the project has failed so far in 2015 due to concerns among some legislators that these methods—particularly the IUDs—are abortifacients. The reduced cost of the 3-year LNG-IUS (Liletta) and recent guidance from the US Department of Health and Human Services mandating that at least 1 form of contraception in each of the FDA-approved categories must be covered by insurers may help to overcome this barrier.
CASE: Resolved
You counsel the patient about the value of each LARC method, letting her know that they are all highly effective in the prevention of pregnancy. You also let her know how each method would affect her menstrual cycle and acknowledge that she may have a preference for whether the contraceptive is placed in her uterus or under the skin of her arm. She chooses the contraceptive implant, which you insert during the same visit. At a follow-up visit 6 weeks later, she reports satisfaction with the method.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Teen patient asks to switch contraceptive methods
A 17-year-old nulliparous woman comes to your clinic for an annual examination. She has no significant health problems, and her examination is normal. She notes that she was started on oral contraceptives (OCs) the year before because of heavy menstrual flow and a desire for birth control but has trouble remembering to take them—though she does usually use condoms. She asks your advice about switching to a different method but indicates that she has lost her health insurance coverage.
What can you offer her as an effective, low-cost contraceptive?
Long-acting reversible contraception (LARC) methods are especially suited for adolescent and young adult women, for whom daily compliance with a shorter-acting contraceptive may be problematic. Five LARC methods are available in the United States, including a new levonorgestrel-releasing intrauterine system (LNG-IUS; Liletta), which received approval from the US Food and Drug Administration (FDA) this year. Like Mirena, Liletta contains 52 mg of levonorgestrel that is released over time. Liletta was introduced by the nonprofit organization Medicines360 and its commercial partner Actavis Pharma in response to evidence that poor women continue to lack access to LARC because of cost or problems with insurance coverage.1
For providers who practice in settings eligible for 340B pricing, Liletta costs $50, a fraction of the cost of alternative intrauterine devices (IUDs). The cost is slightly higher for non-340B providers but is still significantly lower than the cost of other IUDs. For health care practices, the reduced price of Liletta may make it feasible for them to offer LARC to more patients. The reduced pricing also makes Liletta an attractive option for women who choose to pay for the device directly rather than use insurance, such as the patient described above.
Patient experience with Liletta also is key. Not surprisingly, Liletta’s clinical trial found patient satisfaction to be similar to that of Mirena users.2 The failure rate is less than 1%, again comparable to Mirena. The rate of pelvic infection with Liletta use was 0.5%, also comparable to previously published data.3
One difference between Liletta and Mirena is that Liletta carries FDA approval for 3 years of contraceptive efficacy, compared with 5 years for Mirena. In order to make Liletta available to US patients now, Medicines360 decided to apply for 3-year contraceptive labeling while 5- and 7-year efficacy data are being collected. Like Mirena, Liletta is expected to provide excellent contraception for at least 5 years.
How to insert Liletta
- While still pinching the insertion tube, slide the tube through the cervical canal until the upper edge of the flange is approximately 1.5 to 2 cm from the cervix. Do not force the inserter. If necessary, dilate the cervical canal. Release your hold on the tenaculum.
- Hold the insertion tube with the fingers of one hand (Hand A) and the rod with the fingers of the other hand (Hand B).
- Holding the rod in place (Hand B), relax your pinch on the tube and pull the insertion tube back with Hand A to the edge of the second indent of the rod. This will allow the IUS arms to unfold in the lower uterine segment (FIGURE). Wait 10 to 15 seconds for the arms of the IUS to open fully.
- Apply gentle traction with the tenaculum before advancing the IUS. With Hand A still holding the proximal end of the tube, advance both the insertion tube and rod simultaneously up to the uterine fundus. You will feel slight resistance when the IUS is at the fundus. Make sure the flange is touching the cervix when the IUS reaches the uterine fundus. Fundal positioning is important to prevent expulsion.
- Hold the rod still (Hand B) while pulling the insertion tube back with Hand A to the ring of the rod. While holding the inserter tube with Hand A, withdraw the rod from the insertion tube all of the way out to prevent the rod from catching on the knot at the lower end of the IUS. Completely remove the insertion tube.
- Using blunt-tipped sharp scissors, cut the IUS threads perpendicular to the thread length, leaving about 3 cm outside of the cervix. Do not apply tension or pull on the threads when cutting to prevent displacing the IUS. Insertion is now complete.
Source: Liletta [package insert]. Actavis Pharma, Parsippany, NJ; 2015.
Skyla is another LARC option for womenseeking an LNG-IUS for contraception. It provides highly effective contraception for at least 3 years through the release of 13.5 mg of levonorgestrel over time. Skyla’s reduced levonorgestrel content, as compared with Mirena and Liletta, means that fewer users will experience amenorrhea (13% vs 25%).
Paragard is a nonhormonal IUD that uses copper for contraceptive efficacy. The device contains a total of 380 mm of copper. Possible mechanisms of action include interference with sperm migration in the uterus and damage to or destruction of ova. It is FDA-approved for at least 10 years of use. The lack of any hormone in Paragard IUDs may make them attractive to women who do not wish to experience amenorrhea.
Nexplanon is a subdermal implant containing 68 mg of etonogestrel; it is approved for at least 3 years of use. It is the only LARC method that does not require a pelvic examination. Providers are required to complete a training course offered by the manufacturer to ensure proper placement and removal technique.
LARC should be a first-line birth control option
The primary indication for LARC is preg-nancy prevention. Because LARC methods are the most effective reversible means to prevent pregnancy—apart from complete abstinence from sexual intercourse—they should be offered as first-line birth control options to patients who do not wish to conceive. The ability to discontinue LARC methods is an attractive option for women who may want to become pregnant in the future, such as the patient in the opening vignette.
Efficacy rates are high
Because LARC methods do not require users to take action daily or prior to intercourse, they carry a risk of pregnancy of less than 1% (TABLE 1)4-7—equal to or better than rates seen with tubal sterilization. In comparison, the OC pill has a typical use contraceptive failure rate of about 8%.
LARC still has a low utilization rate
It is unfortunate that barriers to LARC methods remain in the United States (see, for example, “National organization identifies barriers to LARC,” above). As recently as 2011 to 2013, only 7.2% of US women aged 15 to 44 years used a LARC method.8 Provider inexperience and patient fears surrounding LARC use remain major barriers. In the past, nulliparity and young age were thought to be contraindications to IUD use. Research and experience have demonstrated, however, that IUDs and contraceptive implants are safe for use in young women and those who have not had children.
Cost barriers also have significantly limited the use of LARC methods. Over time, however, these contraceptives have become less costly to patients, and most insurance providers routinely cover LARC devices and insertion fees. The contraceptive mandate of the Affordable Care Act ensures coverage of contraception, including LARC, for interested women. These trends suggest continued improvement in women’s access to LARC.
National organization identifies barriers to LARC
In 2014, the National Committee for Quality Assurance (NCQA), with support from Bayer Healthcare, organized a meeting of key opinion leaders to discuss ways to eliminate barriers to the most effective contraceptive methods, better known as long-acting reversible contraception (LARC). The resultant issue brief, Women’s Health: Approaches to improving unintended pregnancy rates in the United States, identified a number of key barriers:
- Financial and logistical obstacles. The consensus attendees agreed that LARC methods should be offered to all women not planning a pregnancy in the next 2 years, but acknowledged that operational or administrative process issues sometimes interfere with this goal. One of the most prominent of these issues was the lack of opportunity for same-day insertion of LARC. Other issues included the cost to stock LARC methods, a lack of understanding of billing and reimbursement for LARC, reimbursement policies that prohibit billing for the visit and placement on the same day, and an overabundance of paperwork.
- Timing of the contraceptive counseling session. Many women fail to return for the 6-week postpartum visit—the visit typically set aside for counseling about contraception.
- Lack of a quality measure that would “motivate change in clinical practice.”1 One option: Treat family planning as a “vital sign” that needs to be addressed during the annual visit. “This would lead to stronger evidence for effecting change,” the report notes.1
- Lack of adequate communication skills by the provider. According to the NCQA report, “There are strong positive relationships between a health care team member’s communication skills and a patient’s willingness to follow through with medical recommendations.”1 The establishment of a “current counseling approach” that emphasizes the efficiency and effectiveness of LARC methods as well as the tremendous impact an unintended pregnancy would have on a woman’s whole life course would help improve provider-patient communication and increase the likelihood of LARC methods being utilized.1
- Lack of receptivity among some patients. For some women, the person delivering the message is as important as the message itself, depending on social and cultural norms. Sensitivity of health care providers to these nuances of communication can help enhance patient receptivity to the key message. As the NCQA report notes, “Physicians and the health care system are not always the most trusted source of information, and understanding disparities in contraception care will be important in changing patient behavior.”1
- Basic issues such as cost and access to care.1 Not all women are covered by insurance, particularly in states that opted against expanding access to Medicaid. For these women, the cost of LARC methods and insertion may be prohibitive.
Reference
Noncontraceptive benefits include reduced bleeding
The 3 LNG-IUS methods and the subdermal implant offer several benefits beyond contraception. Because of their progestin content, these methods reduce or even eliminate menses. This benefit can be very helpful for women who experience heavy menstrual periods and the consequent risk of anemia. Because of reduced menstrual flow, users of hormonal LARC methods also commonly experience less cramping associated with menses.
Women with endometriosis often benefit from hormonal LARC methods, as the disease is suppressed by the progestin component. Users of IUDs also have a reduced risk of endometrial cancer.
Contraindications to LARC
There are few contraindications to LARC methods, making them an appropriate choice for most women. The US Medical Eligibility Criteria for Contraceptive Use, 2010, published by the Centers for Disease Control and Prevention (CDC), contain guidelines that are based on the best available evidence.9 Contraceptive methods that are labeled as Category 1 or 2 are not contraindicated for most women. Methods that fall into Category 3 (theoretical or proven risks outweigh the advantages) or Category 4 (unacceptable health risk) are contraindicated (TABLE 2).9
IUDs once were thought to expose women to an increased risk of pelvic inflammatory disease, but this fear has long been disproven. Screening for chlamydia can be performed at the time of placement, as recommended annually for women younger than 25 years. Unless there is concern for active cervical or uterine infection, there is no need to delay insertion of an IUD while awaiting test results. In most cases, women found to have positive cultures after insertion can be treated successfully without IUD removal.
Main adverse effect is altered bleeding patterns
Adverse effects vary depending on the method being used. All LARC methods may affect menstrual patterns. For example, clinical trials involving the copper IUD indicate that abnormal heavy bleeding may lead to discontinuation in up to 10% of users.5,10 Amenorrhea or oligomenorrhea is uncommon with this method and rarely leads to discontinuation. For example, in one trial involving more than 900 women using a copper IUD for up to 5 years, there were no discontinuations due to amenorrhea. Dysmenorrhea may arise, but data from clinical trials indicate that its frequency decreases over time. In one trial, the frequency of any menstrual pain decreased from about 9% of users to 5% after 8 months or more of use.
The LNG-IUS also can be associated with abnormal uterine bleeding. In contrast to the copper IUD, LNG devices tend to reduce menstrual bleeding and can be unpredictable. Clinical trials involving the 5-year 52-mg LNG-IUS indicate that bleeding decreases over time, with as many as 70% of users developing amenorrhea or oligomenorrhea.5,11 However, some women using an LNG-IUS experience heavy bleeding— although the frequency of such bleeding tends to be substantially less than that experienced by copper IUD users.7
A lack of comparative trials makes it unclear whether the newer 3-year LNG-IUS devices are associated with a significantly altered bleeding pattern. Noncomparative data from the package insert for Skyla suggest that women using it may have a higher frequency of heavy menstrual bleeding and less amenorrhea than users of the 5-year device.6
Data from a 3-year clinical trial of the newest 52-mg LNG-IUS (Liletta) indicate that bleeding and dysmenorrhea led to discontinuation 1% to 2% of the time.2
Although the concentration of progestincirculating systemically is low with the various LNG-IUS devices, some women may experience symptoms such as mood swings, headaches, acne, and breast tenderness.
Expulsions during the first year of use of the copper IUD and the 3 LNG-IUS devices range from 2% to 10%, with the higher rates associated with immediate postpartum insertion.5
Uterine perforation has been reported in about 1 of every 1,000 insertions. Other adverse events are uncommon.
Clinical trials indicate that about 11% of implant users will discontinue the method due to bleeding abnormalities.12 About 25% to 30% of users will experience heavy or prolonged bleeding, while up to 33% will experience infrequent bleeding or amenorrhea. About 50% of implant users will experience improved bleeding patterns over time.
Other reasons for discontinuation of implant use in a very small percentage of users include emotional lability, weight gain, acne, and headaches.4 Complications due to insertion and removal are rare and include pain, bleeding, and hematoma formation.
Public health impact of LARC methods
An important question in regard to LARC use is: How do we best provide safe and effective contraception for teens and young adult women? There is increasing evidence that, with appropriate counseling and the removal of cost barriers, LARC methods can have a significant public health impact in this population.
The Contraceptive CHOICE Project, a cohort study in a teenage population of women in the St. Louis, Missouri, area, achieved increased utilization of LARC methods and significantly lower rates of pregnancy, birth, and abortion.13 Investigators proactively counseled young women about the advantages of LARC methods and offered them free of charge. As a result, 72% of women in the study chose an IUD or implant as their method of contraception. Pregnancy, birth, and abortion rates among participants were 34.0, 19.4, and 9.7 per 1,000 teens, respectively. By comparison, national statistics during the same time frame for pregnancy, birth, and abortion were 158.5, 94.0, and 41.5 per 1,000 US teens, respectively.13
A similar project in Colorado received $23.6 million in 2009 from an outside donor to make LARC methods more affordable to patients in family planning clinics in the state.14 Between 2009 and 2014, 30,000 contraceptive implants or IUDs were made available at low or no cost to low-income women attending 68 family planning clinics statewide. The use of these methods at participating clinics quadrupled. Further, the teen birth rate declined by 40% between 2009 and 2013—from 37 to 22 births per 1,000 teens.14 Seventy-five percent of this decline was attributable to increased use of these methods. The teen abortion rate declined by 35% in the same time frame.
In 2014, the Colorado governor’s office indicated that the state had saved $42.5 million in health care expenditures associated with teen births. It was estimated that, for every dollar spent on contraceptives, the state saved $5.68 in Medicaid costs. However, a bipartisan bill to continue funding the project has failed so far in 2015 due to concerns among some legislators that these methods—particularly the IUDs—are abortifacients. The reduced cost of the 3-year LNG-IUS (Liletta) and recent guidance from the US Department of Health and Human Services mandating that at least 1 form of contraception in each of the FDA-approved categories must be covered by insurers may help to overcome this barrier.
CASE: Resolved
You counsel the patient about the value of each LARC method, letting her know that they are all highly effective in the prevention of pregnancy. You also let her know how each method would affect her menstrual cycle and acknowledge that she may have a preference for whether the contraceptive is placed in her uterus or under the skin of her arm. She chooses the contraceptive implant, which you insert during the same visit. At a follow-up visit 6 weeks later, she reports satisfaction with the method.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Dehlendorf C, Rodriguez MI, Levy K, Borrero S, Steinauer J.Disparities in family planning. Am J Obstet Gynecol. 2010;202(3):214–220.
2. Eisenberg DL, Schreiber CA, Turok DK, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
3. Sufrin C, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120(6):1314–1321.
4. Espey E, Ogburn T. Long-acting reversible contraceptives—intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117(3):705–719.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
6. Skyla [package insert]. Bayer Healthcare, Wayne, NJ; 2013.
7. Liletta [package insert]. Actavis Pharma, Parsippany, NJ; 2015.
8. Branum AM, Jones J. Centers for Disease Control and Prevention: NCHS Data Brief No. 188: Trends in Long-Acting Reversible Contraception Use Among US Women Aged 15–44. http://www.cdc.gov/nchs/data/databriefs/db188.htm. Published February 2015. Accessed August 14, 2015.
9. Centers for Disease Control and Prevention. US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR4):1–86.
10. Andersson K, Odlind VL, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use; a randomized comparative trial. Contraception. 1994;49(1):56–72.
11. Sivin I, Stern J, Diaz J, et al. Two years of intrauterine contraception with levonorgestrel and copper: a randomized comparison of the TCu 380Ag and levonorgestrel 20 mcg/day devices. Contraception. 1987;35(3):245–255.
12. Mansour F, Korver T, Marintcheva-Petrova M, Fraser IS. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13–28.
13. Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med. 2014;371:1316–1323.
14. Tavernise S. Colorado’s effort against teenage pregnancies is a startling success. New York Times. http://www.nytimes.com/2015/07/06/science/colorados-push-against-teenage-pregnancies-is-a-startling-success.html. Published July 6, 2015. Accessed August 17, 2015.
1. Dehlendorf C, Rodriguez MI, Levy K, Borrero S, Steinauer J.Disparities in family planning. Am J Obstet Gynecol. 2010;202(3):214–220.
2. Eisenberg DL, Schreiber CA, Turok DK, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
3. Sufrin C, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120(6):1314–1321.
4. Espey E, Ogburn T. Long-acting reversible contraceptives—intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117(3):705–719.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
6. Skyla [package insert]. Bayer Healthcare, Wayne, NJ; 2013.
7. Liletta [package insert]. Actavis Pharma, Parsippany, NJ; 2015.
8. Branum AM, Jones J. Centers for Disease Control and Prevention: NCHS Data Brief No. 188: Trends in Long-Acting Reversible Contraception Use Among US Women Aged 15–44. http://www.cdc.gov/nchs/data/databriefs/db188.htm. Published February 2015. Accessed August 14, 2015.
9. Centers for Disease Control and Prevention. US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR4):1–86.
10. Andersson K, Odlind VL, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use; a randomized comparative trial. Contraception. 1994;49(1):56–72.
11. Sivin I, Stern J, Diaz J, et al. Two years of intrauterine contraception with levonorgestrel and copper: a randomized comparison of the TCu 380Ag and levonorgestrel 20 mcg/day devices. Contraception. 1987;35(3):245–255.
12. Mansour F, Korver T, Marintcheva-Petrova M, Fraser IS. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13–28.
13. Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med. 2014;371:1316–1323.
14. Tavernise S. Colorado’s effort against teenage pregnancies is a startling success. New York Times. http://www.nytimes.com/2015/07/06/science/colorados-push-against-teenage-pregnancies-is-a-startling-success.html. Published July 6, 2015. Accessed August 17, 2015.
In this Article
- A comparative look at 5 LARC methods
- Common barriers to LARC
- How to insert Liletta