User login
Hepatitis C Virus Infections Underreported
Formal surveillance reporting of acute hepatitis C virus (HCV) infection clinical diagnoses were grossly underascertained, according to a review of cases reported to the Massachusetts Department of Public Health (MDPH) and Centers for Disease Control and Prevention.
Investigators assessed rates of electronic case reporting of acute HCV infection to MDPH and rate of subsequent confirmation according to the national case definition and found:
• Of 183 clinical cases, 149 were reported to MDPH.
• Of 149 reports, MDPH investigated 43 reports based on surveillance requirements.
• Only 1 case met the national criteria and was reported to the CDC.
The authors noted hurdles to accurate case ascertainment included incomplete clinician reporting, problematic case definitions, limitations of diagnostic testing, and imperfect data captures.
Citation: Onofrey S, Aneja J, Haney GA, et al. Underascertainment of acute hepatitis C virus infections in the U.S. Surveillance System: a case series and chart review. Ann Intern Med. 2015. doi:10.7326/M14-2939. [Epub ahead of print]
Commentary: Hepatitis C, for which there is now effective and well-tolerated treatment, is an important public health problem affecting over 180 million people worldwide. The USPSTF now recommends screening for Hep C in persons at high risk for infection and also a 1-time screening for HCV infection to adults born between 1945 and 1965.1 Unlike Hep A and Hep B, where the definition of an acute infection is clear with detection of IgM antibody, the definition of acute Hep C is based on a combination of symptoms, laboratory assessments including antibodies to HCV and nucleic acid testing, as well as elevated aminotransferase levels. This study shows that the current estimates of the incidence of new Hepatitis C infections are very inexact. —Neil Skolnik, MD
Citation: Hepatitis C: Screening. US Preventive Services Task Force website.www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/hepatitis-c-screening. Updated June 2013. Accessed July 13, 2015.
Formal surveillance reporting of acute hepatitis C virus (HCV) infection clinical diagnoses were grossly underascertained, according to a review of cases reported to the Massachusetts Department of Public Health (MDPH) and Centers for Disease Control and Prevention.
Investigators assessed rates of electronic case reporting of acute HCV infection to MDPH and rate of subsequent confirmation according to the national case definition and found:
• Of 183 clinical cases, 149 were reported to MDPH.
• Of 149 reports, MDPH investigated 43 reports based on surveillance requirements.
• Only 1 case met the national criteria and was reported to the CDC.
The authors noted hurdles to accurate case ascertainment included incomplete clinician reporting, problematic case definitions, limitations of diagnostic testing, and imperfect data captures.
Citation: Onofrey S, Aneja J, Haney GA, et al. Underascertainment of acute hepatitis C virus infections in the U.S. Surveillance System: a case series and chart review. Ann Intern Med. 2015. doi:10.7326/M14-2939. [Epub ahead of print]
Commentary: Hepatitis C, for which there is now effective and well-tolerated treatment, is an important public health problem affecting over 180 million people worldwide. The USPSTF now recommends screening for Hep C in persons at high risk for infection and also a 1-time screening for HCV infection to adults born between 1945 and 1965.1 Unlike Hep A and Hep B, where the definition of an acute infection is clear with detection of IgM antibody, the definition of acute Hep C is based on a combination of symptoms, laboratory assessments including antibodies to HCV and nucleic acid testing, as well as elevated aminotransferase levels. This study shows that the current estimates of the incidence of new Hepatitis C infections are very inexact. —Neil Skolnik, MD
Citation: Hepatitis C: Screening. US Preventive Services Task Force website.www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/hepatitis-c-screening. Updated June 2013. Accessed July 13, 2015.
Formal surveillance reporting of acute hepatitis C virus (HCV) infection clinical diagnoses were grossly underascertained, according to a review of cases reported to the Massachusetts Department of Public Health (MDPH) and Centers for Disease Control and Prevention.
Investigators assessed rates of electronic case reporting of acute HCV infection to MDPH and rate of subsequent confirmation according to the national case definition and found:
• Of 183 clinical cases, 149 were reported to MDPH.
• Of 149 reports, MDPH investigated 43 reports based on surveillance requirements.
• Only 1 case met the national criteria and was reported to the CDC.
The authors noted hurdles to accurate case ascertainment included incomplete clinician reporting, problematic case definitions, limitations of diagnostic testing, and imperfect data captures.
Citation: Onofrey S, Aneja J, Haney GA, et al. Underascertainment of acute hepatitis C virus infections in the U.S. Surveillance System: a case series and chart review. Ann Intern Med. 2015. doi:10.7326/M14-2939. [Epub ahead of print]
Commentary: Hepatitis C, for which there is now effective and well-tolerated treatment, is an important public health problem affecting over 180 million people worldwide. The USPSTF now recommends screening for Hep C in persons at high risk for infection and also a 1-time screening for HCV infection to adults born between 1945 and 1965.1 Unlike Hep A and Hep B, where the definition of an acute infection is clear with detection of IgM antibody, the definition of acute Hep C is based on a combination of symptoms, laboratory assessments including antibodies to HCV and nucleic acid testing, as well as elevated aminotransferase levels. This study shows that the current estimates of the incidence of new Hepatitis C infections are very inexact. —Neil Skolnik, MD
Citation: Hepatitis C: Screening. US Preventive Services Task Force website.www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/hepatitis-c-screening. Updated June 2013. Accessed July 13, 2015.
Pharmacist Pain E-Consults That Result in a Therapy Change
The enormity of chronic pain among the veteran population makes pain management within the VA a critical issue. Of the veterans returning from Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF), chronic pain is the most common report.1 Of equal concern is the lack of available pain specialists in the U.S. There are < 4,000 pain specialists in the U.S., and even fewer pain specialists are available within the VA system, making it difficult for veterans to access pain care and timely treatment.2
Furthermore, one of the biggest challenges surrounding pain management is the lack of proper training received by generalists and primary care providers (PCPs). Whereas opioid therapy was previously prescribed mainly by specialists and mainly to cancer patients, that is no longer the case. Today, nonspecialists frequently prescribe opioids, and 95% of long-acting opioids are for chronic, noncancer pain.1 In a majority of reviewed pain electronic consultations (e-consults) completed by the pain specialty pharmacist at the Bay Pines VA Healthcare System (BPVAHCS), the patient was not currently receiving opioid therapy, suggesting PCPs’ lack of comfort and training in chronic pain management. Effective and appropriate pain management from the patient perspective and confidence and reassurance from the prescriber standpoint cannot be successfully achieved without drastic improvements in education and training.
Related: Urologist Workforce Variation Across the VHA
The inception of the E-Consult Pain Service arose from a grant from VA National Innovations in Consult Management to 3 VA facilities in Florida: BPVAHCS; Orlando VAMC, and North Florida/South Georgia Veterans Health Systems.3 At the Orlando VAMC, PCPs needed advice on pain management for patients while they waited to see a pain clinic specialist, so a Pain Help Line was implemented to provide immediate consults, but miscommunication between recommendations given and their implementation limited its utility. That eventually led to an E-Consult Pain Service, which provided formal full chart reviews for pain management cases.
The E-Consult Pain Service program included 2 full-time pharmacists, a part-time pain psychologist, and a pain physician. Its goal was to assist PCPs with patient-specific pain management recommendations. The consult service did not replace specialty pain clinics, nor was it meant to provide continual pain management. Additional, separate pain e-consults could be scheduled as a follow-up to a previous consult or for new pain management issues. Although the recommendations in the consults were available for the provider’s use, it was at the provider’s discretion as to whether the recommendations were accepted and implemented.
The E-Consult Pain Service
The E-Consult Pain Service at the BPVAHCS started July 2011. Staffed by a full-time physician and pain specialty pharmacist, the program provides electronic chart review and recommendations to PCPs regarding complex pain management issues. About three-fourths of their time was spent directly on the consults, which took between 1 and 5 business days to complete. Their remaining time was spent on educational initiatives and administrative duties.
The BPVAHCS is a complexity level 1a facility providing comprehensive health care. The facility comprises a 192-acute care bed hospital (includes intensive care, medical, surgical, and psychiatric units); a 112-bed community living center; a 65-bed domiciliary; and a 34-bed residential treatment program.
Related: A Medical Specialty e-Consult Program in a VA Health Care System
Initially, the program was developed to provide pain management support to PCPs in the community-based outpatient clinics (CBOCs) but expanded to all BPVAHCS providers. With the expansion, the program helped reduce a 3-month delay for patients waiting to be seen in the pain clinic. Goals of the program included improving patient outcomes and safety while minimizing opioid therapy risks. These goals are met through an individual case consultation as well as formal educational programs for providers.
The purpose of this study was to obtain data evaluating the characteristics of recommendations made by a pharmacist through pain e-consults at the BPVAHCS and the percentage of consults that resulted in a change in therapy. Future research is warranted to provide clarity on why specific recommendations are or are not being accepted, patient outcomes, and PCPs’ perception on the program’s utility.
Methods
An institutional review board-exempt, retrospective chart review was conducted at the BPVAHCS to determine the percentage of patients whose pain regimen changed as a result of a pain e-consult completed by a pain specialty pharmacist. Although the BPVAHCS E-Consult Pain Service comprised a physician and a pain specialty pharmacist, this study was focused solely on the role and recommendations by the pharmacist. The characteristics of those recommendations and their acceptance/rejection rate were then recorded. Of note, the physician completed separate e-consults, and the frequency of input by the physician on pharmacist recommendations was not collected.
Patients who had a pain e-consult regarding chronic, noncancer pain between January 1, 2012, and March 31, 2012, were identified for inclusion in the study. Charts were selected based on consults submitted by providers. No consults for headaches were requested in the selected time frame.
Chronic pain is defined as persistent pain with or without an identifiable organic cause, lasting longer than 3 to 6 months.1 Use of the term chronic pain throughout this article refers to chronic, noncancer pain. Criteria for exclusion included patients who received a pain e-consult but died before September 30, 2012.
Data Collection
Patients identified for inclusion had their charts reviewed 6 months after completion of the consult in order to allow sufficient time for potential implementation of recommendations. Patient demographics, name and dose of pain medications, requesting practitioner, type of chronic pain, recommendation of pain e-consult, and consult outcome(s) were recorded for all participants.
The primary outcome of the study was the percentage of recommendations, both pharmacologic and nonpharmacologic, made by the pain specialty pharmacist and accepted and implemented by the consulting provider.
Results
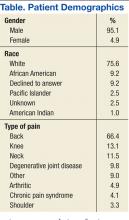
A total of 127 patient charts were identified for inclusion. Five patients died prior to September 30, 2012, and were excluded from the study, leaving 122 charts for review. All 122 charts reviewed by the pain specialty pharmacist included recommendations. Most patients were male (95.1%) and white (75.6%). The most common source of chronic pain was back pain (66.4%) (Table).
Primary Outcome
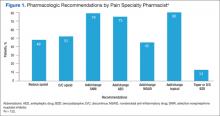
The pain specialty pharmacist pharmacologic treatment option recommendations varied significantly: add and/or change topical (80% of the 122 patients); add and/or change selective norepinephrine reuptake inhibitor (SNRI) (79%); add and/or change antiepileptic drug (AED) therapy (75%); discontinue opioid (52%); reduce opioid (48%); add and/or change nonsteroidal anti- inflammatory drug (NSAID) (45%); and taper/discontinue benzodiazepine (BZD) (13%) (Figure 1).
Primary care providers could choose to accept or ignore the recommendation, and of all the pharmacologic recommendations made, about 50% were implemented. The rate of PCP implementation of the pharmacist’s recommendations varied: add and/or change AED therapy (54%); add and/or change topical (44%); reduce opioid (42%); discontinue opioid (41%); taper/discontinue BZD (38%); add and/or change SNRI (36%); and add and/or change NSAID (33%).
Despite the most frequent recommendations made by pharmacists, the 3 most accepted and implemented by providers were addition and/or change in AED therapy, addition and/or change in topical therapy, and a decrease in opioid dose. Changes in therapy were identified as either a dose decrease or increase in the existing agent, whereas a new agent was considered an addition to existing pain therapy.
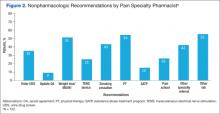
The rates of nonpharmacologic recommendations made by the pharmacist were as follows: ordering additional labs, primarily vitamin D and testosterone levels (55%); referral to physical therapy (PT) (54%); weight loss (51%); smoking cessation (43%); specialty referral (42%); order new urine drug screen (UDS) (35%); referral to pain school education program (26%); transcutaneous electrical nerve stimulation (TENS) (25%); referral to the substance abuse treatment program (SATP) (15%); and update opioid agreement (OA) (9%) (Figure 2).
Nonpharmacologic treatment acceptance rates were as follows: order new UDS (67%); update OA (45%); referral to SATP (33%); referral to PT (33%); order additional labs, primarily vitamin D and testosterone levels (27%); specialty referral (22%); TENS (19%); weight loss (18%); referral to pain school education program (16%); smoking cessation (6%); and music therapy (0%). The top 3 accepted recommendations were obtainment of a new UDS; updating the patient’s OA; and tied for third, referral to PT or the SATP.
Discussion
Including a pharmacist as part of a pain e-consult team may provide support to PCPs for managing chronic pain as well as for measuring improved adherence to VA/DoD guidelines for chronic pain. Pharmacists can offer recommendations for nontraditional pain therapies that PCPs may be unaware of or are unfamiliar with, such as the use of nonnarcotic agents and various nonpharmacologic options. For example, recommend testing for vitamin D levels. Vitamin D deficiency is common among the general population, and a project completed by Roesel and Engel specifically addressed vitamin D deficiency and pain in OEF/OIF veterans.4 Other studies have shown a correlation between vitamin D supplementation and a reduction in musculoskeletal pain or the association between low vitamin D levels and hypersensitivity in patients with chronic pain.5-7 Studies have also demonstrated a link between vitamin D deficiency and depression, which is well known to augment or increase patient awareness of somatic reports, like pain.8
Of all the pharmacologic recommendations made, about 50% were implemented. It is noteworthy to mention that although a change/ addition in SNRI therapy was recommended by the pharmacist 79% of the time, it was accepted and implemented by the PCP only one-third of the time. Many veterans have co-occurring mental health conditions, which are often managed by a psychiatrist, who is typically not the consulting provider (the PCP is). The PCP may be hesitant to change antidepressant therapy for fear of destabilizing the patient or because the PCP was not the antidepressant therapy prescriber. However, the incidence of co-occurring seizure disorders among veterans is much less than that of mental health disorders, making PCPs much more likely to accept and/or change AEDs. Interestingly, the majority of pain specialty pharmacist e-consults involved chronic pain management, further demonstrating the lack of comfort, time, and/or proper training for PCPs in general pain management.
Related: The Rapid Rise of e-Consults Across Specialty Care
Although the e-consult program at the BPVAHCS consisted solely of a physician and a pain specialty pharmacist, the purpose of this project was to evaluate the characteristics of recommendations made by a pharmacist and the percentage of consults that resulted in a therapy change. The physician was responsible for separate consults, and their recommendations were not collected. However, it is important to recognize that the pain specialty pharmacist and physician performed identical roles on the team, each recommending both pharmacologic and nonpharmacologic treatment options in every consult.
Related Programs
The VA Boston Healthcare System (VABHS) is composed of 3 main facilities and 5 CBOCs across eastern Massachusetts. Although veterans in the eastern part of the state are able to receive primary care at a CBOC, specialty care is provided primarily at 2 of the main locations in the Boston area. Therefore, the VABHS began an e-consult program in order to facilitate patient access to specialty providers for patients unable to participate in a face-to-face visit.
The purpose of the VABHS study was to examine the implementation and provider perception of an e-consult program within a large VA system, to provide timely patient access to specialty care. The pilot program was initiated in 2 specialty clinics in 2011 but expanded to 12 specialty clinics within 9 months. The specialty clinics included allergy, cardiology, endocrinology, gastroenterology, hematology, infectious disease, nephrology, oncology, palliative care, pulmonary disease, rheumatology, and sleep medicine. Outcomes of the VABHS e-consult program revealed that a majority of PCPs were satisfied with the use of e-consults, whereas specialists were less satisfied. The PCP-perceived benefits to patients included avoidance of unnecessary travel, faster clinical input, and avoidance of unnecessary copays.9
Like the VABHS, the use of pain e-consults at BPVAHCS helps reduce the burden of face-to-face clinic visits and eliminate accessibility barriers for veterans. This study differs from the VABHS in that PCPs requested onetime consults focused solely on pain management. The pain specialty pharmacist at BPVAHCS did not provide longitudinal care; measure patient outcomes, such as satisfaction, reduction in pain, or improved functionally; or examine provider satisfaction. Additionally, unlike the BPVAHCS program, there was no indication whether a pharmacist played a role in the program.9
Other studies have explored the role of a pain pharmacist in the inpatient setting offering consults on patient-controlled analgesia and in patients with a history of substance abuse.10,11 Another recent study similarly looked at the effectiveness of a pharmacist-led medication review in chronic pain management. The aim was to assess patient outcomes: decrease in pain intensity and improvement in physical functioning.12 Another study involving a nurse and pharmacist-led chronic pain clinic in a primary care setting conducted in England showed improvement in patient-reported pain and reduction in secondary referrals.13
Limitations
Limitations of the study included short study duration (6 months); use of a newly implemented E-Consult Pain Service; lack of pharmacist follow-up on acceptance/rejection of their recommendation; inability to determine patient outcome(s), as consults were for a single point in time, regarding a therapy recommendation; lack of access to non-VA medications; and patient refusal to change current pain regimen.
Patient refusal inhibited providers from implementing therapy changes recommended by the pharmacist and therefore could have negatively impacted study outcomes. Raw data were used for this study, and there were no statistical analyses conducted. Furthermore, lack of other formal e-consult programs within BPVAHCS to compare the acceptance/rejection of pharmacist recommendations for other conditions and lack of a third-party review of pharmacist recommendations to ensure standard of care may have limited this study.
Future Research
As the E-Consult Pain Service continues, research regarding the value of the pain specialty pharmacist may be warranted. Additional research is needed to identify the reasons that recommendations were accepted or ignored, whether the recommendations were beneficial to the patients, and the PCPs’ perception on the usefulness of a pain e-consult program. When the program started, 14% of patients at BPVAHCS were taking opioids. The pain e-consult program handled a small percentage of these patients.
The authors considered proactively reviewing all patients on > 100 mg morphine equivalents of opioids daily but did not have adequate staff to support the review. It may be helpful to identify those patients taking opioids but do not have a consult. The role of the pain e-consult pharmacist may be expanded to assist PCPs, including leading patient education classes to explain the concept and purpose of the opioid treatment agreement; reinforcing expected behaviors and outcomes of patients prescribed opioids; assisting providers with interpreting UDSs and notifying providers of aberrant behaviors; and educating providers on opioid risk mitigation through seminars or academic detailing.
Furthermore, future research may be warranted to determine the prospective role of a pain specialty pharmacist on longitudinal measures, such as pain outcomes, patient satisfaction, improvement in quality of life and/or function, as well as to determine provider perspective and satisfaction of the program. Finally, it would be interesting to compare and contrast the recommendations and outcomes between the pharmacist and physician team at the BPVAHCS.
Conclusion
The addition of a pain specialty pharmacist as part of the E-Consult Pain Service seems to provide support to prescribing PCPs in general chronic pain management, as well as measuring improved adherence to VA/DoD guidelines for chronic pain.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. Management of opioid therapy for chronic pain. U.S. Department of Veterans Affairs Website. http://www.healthquality.va.gov/guidelines/Pain/cot. Published May 2010. Accessed May 25, 2015.
2. Boyle AM. VA ahead of schedule in improving chronic pain care. U.S. Medicine. 2012. http://www.usmedicine.com/agencies/department-of-veterans-affairs/va-ahead-of-schedule-in-improving -chronic-pain-care. Accessed June 16, 2015.
3. Sproul RD. Bridging the gap: e-consult pain service for the primary care physician. Am Soc Pain Educ. 2012;8(1).
4. Roesel T, Engel C. Vitamin D levels and their correlation to pain, fatigue, anxiety, and other co-morbidities in specialized care program service members seen at the Deployment Health Clinical Center. In: Deployment Health Clinical Center Annual Report 2011: pp28-29. http://www.pdhealth.mil/downloads/DHCC_AR_2011.pdf. Accessed May 25, 2015.
5. Le Goaziou MF, Kellou N, Flori M, et al. Vitamin D supplementation for diffuse musculoskeletal pain: results of a before-and-after study. Eur J Gen Pract. 2014;20(1):3-9.
6. Abbasi M, Hashemipour S, Hajmanuchehri F, Kazemifar AM. Is vitamin D deficiency associated with non specific musculoskeletal pain? Glob J Health Sci. 2012;11;5(1):107-111.
7. Von Känel R, Müller-Hartmannsgruber V, Kokinogenis G, Egloff N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med. 2014;15(9):1609-1618.
8. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
9. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA healthcare system. Fed Pract. 2014;31(5):26-31.
10. Fan T, Elgourt T. Pain management pharmacy service in a community hospital. Am J Health Syst Pharm. 2008;65(16):1560-1565.
11. Andrews LB, Bridgeman MB, Dalal KS, et al. Implementation of pharmacist-driven pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342-1349.
12. Hadi M, Alldred D, Briggs M, Munyombwe T, Closs SJ. Effectiveness of pharmacist-led medication review in chronic pain management: systematic review and meta-analysis. Clin J Pain. 2014;30(11):1006-1014.
13. Briggs M, Closs SJ, Marczewski K, Barratt J. A feasibility study of a combined nurse/pharmacist-led chronic pain clinic in primary care. Qual Prim Care. 2008;16(2):91-94.
The enormity of chronic pain among the veteran population makes pain management within the VA a critical issue. Of the veterans returning from Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF), chronic pain is the most common report.1 Of equal concern is the lack of available pain specialists in the U.S. There are < 4,000 pain specialists in the U.S., and even fewer pain specialists are available within the VA system, making it difficult for veterans to access pain care and timely treatment.2
Furthermore, one of the biggest challenges surrounding pain management is the lack of proper training received by generalists and primary care providers (PCPs). Whereas opioid therapy was previously prescribed mainly by specialists and mainly to cancer patients, that is no longer the case. Today, nonspecialists frequently prescribe opioids, and 95% of long-acting opioids are for chronic, noncancer pain.1 In a majority of reviewed pain electronic consultations (e-consults) completed by the pain specialty pharmacist at the Bay Pines VA Healthcare System (BPVAHCS), the patient was not currently receiving opioid therapy, suggesting PCPs’ lack of comfort and training in chronic pain management. Effective and appropriate pain management from the patient perspective and confidence and reassurance from the prescriber standpoint cannot be successfully achieved without drastic improvements in education and training.
Related: Urologist Workforce Variation Across the VHA
The inception of the E-Consult Pain Service arose from a grant from VA National Innovations in Consult Management to 3 VA facilities in Florida: BPVAHCS; Orlando VAMC, and North Florida/South Georgia Veterans Health Systems.3 At the Orlando VAMC, PCPs needed advice on pain management for patients while they waited to see a pain clinic specialist, so a Pain Help Line was implemented to provide immediate consults, but miscommunication between recommendations given and their implementation limited its utility. That eventually led to an E-Consult Pain Service, which provided formal full chart reviews for pain management cases.
The E-Consult Pain Service program included 2 full-time pharmacists, a part-time pain psychologist, and a pain physician. Its goal was to assist PCPs with patient-specific pain management recommendations. The consult service did not replace specialty pain clinics, nor was it meant to provide continual pain management. Additional, separate pain e-consults could be scheduled as a follow-up to a previous consult or for new pain management issues. Although the recommendations in the consults were available for the provider’s use, it was at the provider’s discretion as to whether the recommendations were accepted and implemented.
The E-Consult Pain Service
The E-Consult Pain Service at the BPVAHCS started July 2011. Staffed by a full-time physician and pain specialty pharmacist, the program provides electronic chart review and recommendations to PCPs regarding complex pain management issues. About three-fourths of their time was spent directly on the consults, which took between 1 and 5 business days to complete. Their remaining time was spent on educational initiatives and administrative duties.
The BPVAHCS is a complexity level 1a facility providing comprehensive health care. The facility comprises a 192-acute care bed hospital (includes intensive care, medical, surgical, and psychiatric units); a 112-bed community living center; a 65-bed domiciliary; and a 34-bed residential treatment program.
Related: A Medical Specialty e-Consult Program in a VA Health Care System
Initially, the program was developed to provide pain management support to PCPs in the community-based outpatient clinics (CBOCs) but expanded to all BPVAHCS providers. With the expansion, the program helped reduce a 3-month delay for patients waiting to be seen in the pain clinic. Goals of the program included improving patient outcomes and safety while minimizing opioid therapy risks. These goals are met through an individual case consultation as well as formal educational programs for providers.
The purpose of this study was to obtain data evaluating the characteristics of recommendations made by a pharmacist through pain e-consults at the BPVAHCS and the percentage of consults that resulted in a change in therapy. Future research is warranted to provide clarity on why specific recommendations are or are not being accepted, patient outcomes, and PCPs’ perception on the program’s utility.
Methods
An institutional review board-exempt, retrospective chart review was conducted at the BPVAHCS to determine the percentage of patients whose pain regimen changed as a result of a pain e-consult completed by a pain specialty pharmacist. Although the BPVAHCS E-Consult Pain Service comprised a physician and a pain specialty pharmacist, this study was focused solely on the role and recommendations by the pharmacist. The characteristics of those recommendations and their acceptance/rejection rate were then recorded. Of note, the physician completed separate e-consults, and the frequency of input by the physician on pharmacist recommendations was not collected.
Patients who had a pain e-consult regarding chronic, noncancer pain between January 1, 2012, and March 31, 2012, were identified for inclusion in the study. Charts were selected based on consults submitted by providers. No consults for headaches were requested in the selected time frame.
Chronic pain is defined as persistent pain with or without an identifiable organic cause, lasting longer than 3 to 6 months.1 Use of the term chronic pain throughout this article refers to chronic, noncancer pain. Criteria for exclusion included patients who received a pain e-consult but died before September 30, 2012.
Data Collection
Patients identified for inclusion had their charts reviewed 6 months after completion of the consult in order to allow sufficient time for potential implementation of recommendations. Patient demographics, name and dose of pain medications, requesting practitioner, type of chronic pain, recommendation of pain e-consult, and consult outcome(s) were recorded for all participants.
The primary outcome of the study was the percentage of recommendations, both pharmacologic and nonpharmacologic, made by the pain specialty pharmacist and accepted and implemented by the consulting provider.
Results
A total of 127 patient charts were identified for inclusion. Five patients died prior to September 30, 2012, and were excluded from the study, leaving 122 charts for review. All 122 charts reviewed by the pain specialty pharmacist included recommendations. Most patients were male (95.1%) and white (75.6%). The most common source of chronic pain was back pain (66.4%) (Table).
Primary Outcome
The pain specialty pharmacist pharmacologic treatment option recommendations varied significantly: add and/or change topical (80% of the 122 patients); add and/or change selective norepinephrine reuptake inhibitor (SNRI) (79%); add and/or change antiepileptic drug (AED) therapy (75%); discontinue opioid (52%); reduce opioid (48%); add and/or change nonsteroidal anti- inflammatory drug (NSAID) (45%); and taper/discontinue benzodiazepine (BZD) (13%) (Figure 1).
Primary care providers could choose to accept or ignore the recommendation, and of all the pharmacologic recommendations made, about 50% were implemented. The rate of PCP implementation of the pharmacist’s recommendations varied: add and/or change AED therapy (54%); add and/or change topical (44%); reduce opioid (42%); discontinue opioid (41%); taper/discontinue BZD (38%); add and/or change SNRI (36%); and add and/or change NSAID (33%).
Despite the most frequent recommendations made by pharmacists, the 3 most accepted and implemented by providers were addition and/or change in AED therapy, addition and/or change in topical therapy, and a decrease in opioid dose. Changes in therapy were identified as either a dose decrease or increase in the existing agent, whereas a new agent was considered an addition to existing pain therapy.
The rates of nonpharmacologic recommendations made by the pharmacist were as follows: ordering additional labs, primarily vitamin D and testosterone levels (55%); referral to physical therapy (PT) (54%); weight loss (51%); smoking cessation (43%); specialty referral (42%); order new urine drug screen (UDS) (35%); referral to pain school education program (26%); transcutaneous electrical nerve stimulation (TENS) (25%); referral to the substance abuse treatment program (SATP) (15%); and update opioid agreement (OA) (9%) (Figure 2).
Nonpharmacologic treatment acceptance rates were as follows: order new UDS (67%); update OA (45%); referral to SATP (33%); referral to PT (33%); order additional labs, primarily vitamin D and testosterone levels (27%); specialty referral (22%); TENS (19%); weight loss (18%); referral to pain school education program (16%); smoking cessation (6%); and music therapy (0%). The top 3 accepted recommendations were obtainment of a new UDS; updating the patient’s OA; and tied for third, referral to PT or the SATP.
Discussion
Including a pharmacist as part of a pain e-consult team may provide support to PCPs for managing chronic pain as well as for measuring improved adherence to VA/DoD guidelines for chronic pain. Pharmacists can offer recommendations for nontraditional pain therapies that PCPs may be unaware of or are unfamiliar with, such as the use of nonnarcotic agents and various nonpharmacologic options. For example, recommend testing for vitamin D levels. Vitamin D deficiency is common among the general population, and a project completed by Roesel and Engel specifically addressed vitamin D deficiency and pain in OEF/OIF veterans.4 Other studies have shown a correlation between vitamin D supplementation and a reduction in musculoskeletal pain or the association between low vitamin D levels and hypersensitivity in patients with chronic pain.5-7 Studies have also demonstrated a link between vitamin D deficiency and depression, which is well known to augment or increase patient awareness of somatic reports, like pain.8
Of all the pharmacologic recommendations made, about 50% were implemented. It is noteworthy to mention that although a change/ addition in SNRI therapy was recommended by the pharmacist 79% of the time, it was accepted and implemented by the PCP only one-third of the time. Many veterans have co-occurring mental health conditions, which are often managed by a psychiatrist, who is typically not the consulting provider (the PCP is). The PCP may be hesitant to change antidepressant therapy for fear of destabilizing the patient or because the PCP was not the antidepressant therapy prescriber. However, the incidence of co-occurring seizure disorders among veterans is much less than that of mental health disorders, making PCPs much more likely to accept and/or change AEDs. Interestingly, the majority of pain specialty pharmacist e-consults involved chronic pain management, further demonstrating the lack of comfort, time, and/or proper training for PCPs in general pain management.
Related: The Rapid Rise of e-Consults Across Specialty Care
Although the e-consult program at the BPVAHCS consisted solely of a physician and a pain specialty pharmacist, the purpose of this project was to evaluate the characteristics of recommendations made by a pharmacist and the percentage of consults that resulted in a therapy change. The physician was responsible for separate consults, and their recommendations were not collected. However, it is important to recognize that the pain specialty pharmacist and physician performed identical roles on the team, each recommending both pharmacologic and nonpharmacologic treatment options in every consult.
Related Programs
The VA Boston Healthcare System (VABHS) is composed of 3 main facilities and 5 CBOCs across eastern Massachusetts. Although veterans in the eastern part of the state are able to receive primary care at a CBOC, specialty care is provided primarily at 2 of the main locations in the Boston area. Therefore, the VABHS began an e-consult program in order to facilitate patient access to specialty providers for patients unable to participate in a face-to-face visit.
The purpose of the VABHS study was to examine the implementation and provider perception of an e-consult program within a large VA system, to provide timely patient access to specialty care. The pilot program was initiated in 2 specialty clinics in 2011 but expanded to 12 specialty clinics within 9 months. The specialty clinics included allergy, cardiology, endocrinology, gastroenterology, hematology, infectious disease, nephrology, oncology, palliative care, pulmonary disease, rheumatology, and sleep medicine. Outcomes of the VABHS e-consult program revealed that a majority of PCPs were satisfied with the use of e-consults, whereas specialists were less satisfied. The PCP-perceived benefits to patients included avoidance of unnecessary travel, faster clinical input, and avoidance of unnecessary copays.9
Like the VABHS, the use of pain e-consults at BPVAHCS helps reduce the burden of face-to-face clinic visits and eliminate accessibility barriers for veterans. This study differs from the VABHS in that PCPs requested onetime consults focused solely on pain management. The pain specialty pharmacist at BPVAHCS did not provide longitudinal care; measure patient outcomes, such as satisfaction, reduction in pain, or improved functionally; or examine provider satisfaction. Additionally, unlike the BPVAHCS program, there was no indication whether a pharmacist played a role in the program.9
Other studies have explored the role of a pain pharmacist in the inpatient setting offering consults on patient-controlled analgesia and in patients with a history of substance abuse.10,11 Another recent study similarly looked at the effectiveness of a pharmacist-led medication review in chronic pain management. The aim was to assess patient outcomes: decrease in pain intensity and improvement in physical functioning.12 Another study involving a nurse and pharmacist-led chronic pain clinic in a primary care setting conducted in England showed improvement in patient-reported pain and reduction in secondary referrals.13
Limitations
Limitations of the study included short study duration (6 months); use of a newly implemented E-Consult Pain Service; lack of pharmacist follow-up on acceptance/rejection of their recommendation; inability to determine patient outcome(s), as consults were for a single point in time, regarding a therapy recommendation; lack of access to non-VA medications; and patient refusal to change current pain regimen.
Patient refusal inhibited providers from implementing therapy changes recommended by the pharmacist and therefore could have negatively impacted study outcomes. Raw data were used for this study, and there were no statistical analyses conducted. Furthermore, lack of other formal e-consult programs within BPVAHCS to compare the acceptance/rejection of pharmacist recommendations for other conditions and lack of a third-party review of pharmacist recommendations to ensure standard of care may have limited this study.
Future Research
As the E-Consult Pain Service continues, research regarding the value of the pain specialty pharmacist may be warranted. Additional research is needed to identify the reasons that recommendations were accepted or ignored, whether the recommendations were beneficial to the patients, and the PCPs’ perception on the usefulness of a pain e-consult program. When the program started, 14% of patients at BPVAHCS were taking opioids. The pain e-consult program handled a small percentage of these patients.
The authors considered proactively reviewing all patients on > 100 mg morphine equivalents of opioids daily but did not have adequate staff to support the review. It may be helpful to identify those patients taking opioids but do not have a consult. The role of the pain e-consult pharmacist may be expanded to assist PCPs, including leading patient education classes to explain the concept and purpose of the opioid treatment agreement; reinforcing expected behaviors and outcomes of patients prescribed opioids; assisting providers with interpreting UDSs and notifying providers of aberrant behaviors; and educating providers on opioid risk mitigation through seminars or academic detailing.
Furthermore, future research may be warranted to determine the prospective role of a pain specialty pharmacist on longitudinal measures, such as pain outcomes, patient satisfaction, improvement in quality of life and/or function, as well as to determine provider perspective and satisfaction of the program. Finally, it would be interesting to compare and contrast the recommendations and outcomes between the pharmacist and physician team at the BPVAHCS.
Conclusion
The addition of a pain specialty pharmacist as part of the E-Consult Pain Service seems to provide support to prescribing PCPs in general chronic pain management, as well as measuring improved adherence to VA/DoD guidelines for chronic pain.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The enormity of chronic pain among the veteran population makes pain management within the VA a critical issue. Of the veterans returning from Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF), chronic pain is the most common report.1 Of equal concern is the lack of available pain specialists in the U.S. There are < 4,000 pain specialists in the U.S., and even fewer pain specialists are available within the VA system, making it difficult for veterans to access pain care and timely treatment.2
Furthermore, one of the biggest challenges surrounding pain management is the lack of proper training received by generalists and primary care providers (PCPs). Whereas opioid therapy was previously prescribed mainly by specialists and mainly to cancer patients, that is no longer the case. Today, nonspecialists frequently prescribe opioids, and 95% of long-acting opioids are for chronic, noncancer pain.1 In a majority of reviewed pain electronic consultations (e-consults) completed by the pain specialty pharmacist at the Bay Pines VA Healthcare System (BPVAHCS), the patient was not currently receiving opioid therapy, suggesting PCPs’ lack of comfort and training in chronic pain management. Effective and appropriate pain management from the patient perspective and confidence and reassurance from the prescriber standpoint cannot be successfully achieved without drastic improvements in education and training.
Related: Urologist Workforce Variation Across the VHA
The inception of the E-Consult Pain Service arose from a grant from VA National Innovations in Consult Management to 3 VA facilities in Florida: BPVAHCS; Orlando VAMC, and North Florida/South Georgia Veterans Health Systems.3 At the Orlando VAMC, PCPs needed advice on pain management for patients while they waited to see a pain clinic specialist, so a Pain Help Line was implemented to provide immediate consults, but miscommunication between recommendations given and their implementation limited its utility. That eventually led to an E-Consult Pain Service, which provided formal full chart reviews for pain management cases.
The E-Consult Pain Service program included 2 full-time pharmacists, a part-time pain psychologist, and a pain physician. Its goal was to assist PCPs with patient-specific pain management recommendations. The consult service did not replace specialty pain clinics, nor was it meant to provide continual pain management. Additional, separate pain e-consults could be scheduled as a follow-up to a previous consult or for new pain management issues. Although the recommendations in the consults were available for the provider’s use, it was at the provider’s discretion as to whether the recommendations were accepted and implemented.
The E-Consult Pain Service
The E-Consult Pain Service at the BPVAHCS started July 2011. Staffed by a full-time physician and pain specialty pharmacist, the program provides electronic chart review and recommendations to PCPs regarding complex pain management issues. About three-fourths of their time was spent directly on the consults, which took between 1 and 5 business days to complete. Their remaining time was spent on educational initiatives and administrative duties.
The BPVAHCS is a complexity level 1a facility providing comprehensive health care. The facility comprises a 192-acute care bed hospital (includes intensive care, medical, surgical, and psychiatric units); a 112-bed community living center; a 65-bed domiciliary; and a 34-bed residential treatment program.
Related: A Medical Specialty e-Consult Program in a VA Health Care System
Initially, the program was developed to provide pain management support to PCPs in the community-based outpatient clinics (CBOCs) but expanded to all BPVAHCS providers. With the expansion, the program helped reduce a 3-month delay for patients waiting to be seen in the pain clinic. Goals of the program included improving patient outcomes and safety while minimizing opioid therapy risks. These goals are met through an individual case consultation as well as formal educational programs for providers.
The purpose of this study was to obtain data evaluating the characteristics of recommendations made by a pharmacist through pain e-consults at the BPVAHCS and the percentage of consults that resulted in a change in therapy. Future research is warranted to provide clarity on why specific recommendations are or are not being accepted, patient outcomes, and PCPs’ perception on the program’s utility.
Methods
An institutional review board-exempt, retrospective chart review was conducted at the BPVAHCS to determine the percentage of patients whose pain regimen changed as a result of a pain e-consult completed by a pain specialty pharmacist. Although the BPVAHCS E-Consult Pain Service comprised a physician and a pain specialty pharmacist, this study was focused solely on the role and recommendations by the pharmacist. The characteristics of those recommendations and their acceptance/rejection rate were then recorded. Of note, the physician completed separate e-consults, and the frequency of input by the physician on pharmacist recommendations was not collected.
Patients who had a pain e-consult regarding chronic, noncancer pain between January 1, 2012, and March 31, 2012, were identified for inclusion in the study. Charts were selected based on consults submitted by providers. No consults for headaches were requested in the selected time frame.
Chronic pain is defined as persistent pain with or without an identifiable organic cause, lasting longer than 3 to 6 months.1 Use of the term chronic pain throughout this article refers to chronic, noncancer pain. Criteria for exclusion included patients who received a pain e-consult but died before September 30, 2012.
Data Collection
Patients identified for inclusion had their charts reviewed 6 months after completion of the consult in order to allow sufficient time for potential implementation of recommendations. Patient demographics, name and dose of pain medications, requesting practitioner, type of chronic pain, recommendation of pain e-consult, and consult outcome(s) were recorded for all participants.
The primary outcome of the study was the percentage of recommendations, both pharmacologic and nonpharmacologic, made by the pain specialty pharmacist and accepted and implemented by the consulting provider.
Results
A total of 127 patient charts were identified for inclusion. Five patients died prior to September 30, 2012, and were excluded from the study, leaving 122 charts for review. All 122 charts reviewed by the pain specialty pharmacist included recommendations. Most patients were male (95.1%) and white (75.6%). The most common source of chronic pain was back pain (66.4%) (Table).
Primary Outcome
The pain specialty pharmacist pharmacologic treatment option recommendations varied significantly: add and/or change topical (80% of the 122 patients); add and/or change selective norepinephrine reuptake inhibitor (SNRI) (79%); add and/or change antiepileptic drug (AED) therapy (75%); discontinue opioid (52%); reduce opioid (48%); add and/or change nonsteroidal anti- inflammatory drug (NSAID) (45%); and taper/discontinue benzodiazepine (BZD) (13%) (Figure 1).
Primary care providers could choose to accept or ignore the recommendation, and of all the pharmacologic recommendations made, about 50% were implemented. The rate of PCP implementation of the pharmacist’s recommendations varied: add and/or change AED therapy (54%); add and/or change topical (44%); reduce opioid (42%); discontinue opioid (41%); taper/discontinue BZD (38%); add and/or change SNRI (36%); and add and/or change NSAID (33%).
Despite the most frequent recommendations made by pharmacists, the 3 most accepted and implemented by providers were addition and/or change in AED therapy, addition and/or change in topical therapy, and a decrease in opioid dose. Changes in therapy were identified as either a dose decrease or increase in the existing agent, whereas a new agent was considered an addition to existing pain therapy.
The rates of nonpharmacologic recommendations made by the pharmacist were as follows: ordering additional labs, primarily vitamin D and testosterone levels (55%); referral to physical therapy (PT) (54%); weight loss (51%); smoking cessation (43%); specialty referral (42%); order new urine drug screen (UDS) (35%); referral to pain school education program (26%); transcutaneous electrical nerve stimulation (TENS) (25%); referral to the substance abuse treatment program (SATP) (15%); and update opioid agreement (OA) (9%) (Figure 2).
Nonpharmacologic treatment acceptance rates were as follows: order new UDS (67%); update OA (45%); referral to SATP (33%); referral to PT (33%); order additional labs, primarily vitamin D and testosterone levels (27%); specialty referral (22%); TENS (19%); weight loss (18%); referral to pain school education program (16%); smoking cessation (6%); and music therapy (0%). The top 3 accepted recommendations were obtainment of a new UDS; updating the patient’s OA; and tied for third, referral to PT or the SATP.
Discussion
Including a pharmacist as part of a pain e-consult team may provide support to PCPs for managing chronic pain as well as for measuring improved adherence to VA/DoD guidelines for chronic pain. Pharmacists can offer recommendations for nontraditional pain therapies that PCPs may be unaware of or are unfamiliar with, such as the use of nonnarcotic agents and various nonpharmacologic options. For example, recommend testing for vitamin D levels. Vitamin D deficiency is common among the general population, and a project completed by Roesel and Engel specifically addressed vitamin D deficiency and pain in OEF/OIF veterans.4 Other studies have shown a correlation between vitamin D supplementation and a reduction in musculoskeletal pain or the association between low vitamin D levels and hypersensitivity in patients with chronic pain.5-7 Studies have also demonstrated a link between vitamin D deficiency and depression, which is well known to augment or increase patient awareness of somatic reports, like pain.8
Of all the pharmacologic recommendations made, about 50% were implemented. It is noteworthy to mention that although a change/ addition in SNRI therapy was recommended by the pharmacist 79% of the time, it was accepted and implemented by the PCP only one-third of the time. Many veterans have co-occurring mental health conditions, which are often managed by a psychiatrist, who is typically not the consulting provider (the PCP is). The PCP may be hesitant to change antidepressant therapy for fear of destabilizing the patient or because the PCP was not the antidepressant therapy prescriber. However, the incidence of co-occurring seizure disorders among veterans is much less than that of mental health disorders, making PCPs much more likely to accept and/or change AEDs. Interestingly, the majority of pain specialty pharmacist e-consults involved chronic pain management, further demonstrating the lack of comfort, time, and/or proper training for PCPs in general pain management.
Related: The Rapid Rise of e-Consults Across Specialty Care
Although the e-consult program at the BPVAHCS consisted solely of a physician and a pain specialty pharmacist, the purpose of this project was to evaluate the characteristics of recommendations made by a pharmacist and the percentage of consults that resulted in a therapy change. The physician was responsible for separate consults, and their recommendations were not collected. However, it is important to recognize that the pain specialty pharmacist and physician performed identical roles on the team, each recommending both pharmacologic and nonpharmacologic treatment options in every consult.
Related Programs
The VA Boston Healthcare System (VABHS) is composed of 3 main facilities and 5 CBOCs across eastern Massachusetts. Although veterans in the eastern part of the state are able to receive primary care at a CBOC, specialty care is provided primarily at 2 of the main locations in the Boston area. Therefore, the VABHS began an e-consult program in order to facilitate patient access to specialty providers for patients unable to participate in a face-to-face visit.
The purpose of the VABHS study was to examine the implementation and provider perception of an e-consult program within a large VA system, to provide timely patient access to specialty care. The pilot program was initiated in 2 specialty clinics in 2011 but expanded to 12 specialty clinics within 9 months. The specialty clinics included allergy, cardiology, endocrinology, gastroenterology, hematology, infectious disease, nephrology, oncology, palliative care, pulmonary disease, rheumatology, and sleep medicine. Outcomes of the VABHS e-consult program revealed that a majority of PCPs were satisfied with the use of e-consults, whereas specialists were less satisfied. The PCP-perceived benefits to patients included avoidance of unnecessary travel, faster clinical input, and avoidance of unnecessary copays.9
Like the VABHS, the use of pain e-consults at BPVAHCS helps reduce the burden of face-to-face clinic visits and eliminate accessibility barriers for veterans. This study differs from the VABHS in that PCPs requested onetime consults focused solely on pain management. The pain specialty pharmacist at BPVAHCS did not provide longitudinal care; measure patient outcomes, such as satisfaction, reduction in pain, or improved functionally; or examine provider satisfaction. Additionally, unlike the BPVAHCS program, there was no indication whether a pharmacist played a role in the program.9
Other studies have explored the role of a pain pharmacist in the inpatient setting offering consults on patient-controlled analgesia and in patients with a history of substance abuse.10,11 Another recent study similarly looked at the effectiveness of a pharmacist-led medication review in chronic pain management. The aim was to assess patient outcomes: decrease in pain intensity and improvement in physical functioning.12 Another study involving a nurse and pharmacist-led chronic pain clinic in a primary care setting conducted in England showed improvement in patient-reported pain and reduction in secondary referrals.13
Limitations
Limitations of the study included short study duration (6 months); use of a newly implemented E-Consult Pain Service; lack of pharmacist follow-up on acceptance/rejection of their recommendation; inability to determine patient outcome(s), as consults were for a single point in time, regarding a therapy recommendation; lack of access to non-VA medications; and patient refusal to change current pain regimen.
Patient refusal inhibited providers from implementing therapy changes recommended by the pharmacist and therefore could have negatively impacted study outcomes. Raw data were used for this study, and there were no statistical analyses conducted. Furthermore, lack of other formal e-consult programs within BPVAHCS to compare the acceptance/rejection of pharmacist recommendations for other conditions and lack of a third-party review of pharmacist recommendations to ensure standard of care may have limited this study.
Future Research
As the E-Consult Pain Service continues, research regarding the value of the pain specialty pharmacist may be warranted. Additional research is needed to identify the reasons that recommendations were accepted or ignored, whether the recommendations were beneficial to the patients, and the PCPs’ perception on the usefulness of a pain e-consult program. When the program started, 14% of patients at BPVAHCS were taking opioids. The pain e-consult program handled a small percentage of these patients.
The authors considered proactively reviewing all patients on > 100 mg morphine equivalents of opioids daily but did not have adequate staff to support the review. It may be helpful to identify those patients taking opioids but do not have a consult. The role of the pain e-consult pharmacist may be expanded to assist PCPs, including leading patient education classes to explain the concept and purpose of the opioid treatment agreement; reinforcing expected behaviors and outcomes of patients prescribed opioids; assisting providers with interpreting UDSs and notifying providers of aberrant behaviors; and educating providers on opioid risk mitigation through seminars or academic detailing.
Furthermore, future research may be warranted to determine the prospective role of a pain specialty pharmacist on longitudinal measures, such as pain outcomes, patient satisfaction, improvement in quality of life and/or function, as well as to determine provider perspective and satisfaction of the program. Finally, it would be interesting to compare and contrast the recommendations and outcomes between the pharmacist and physician team at the BPVAHCS.
Conclusion
The addition of a pain specialty pharmacist as part of the E-Consult Pain Service seems to provide support to prescribing PCPs in general chronic pain management, as well as measuring improved adherence to VA/DoD guidelines for chronic pain.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. Management of opioid therapy for chronic pain. U.S. Department of Veterans Affairs Website. http://www.healthquality.va.gov/guidelines/Pain/cot. Published May 2010. Accessed May 25, 2015.
2. Boyle AM. VA ahead of schedule in improving chronic pain care. U.S. Medicine. 2012. http://www.usmedicine.com/agencies/department-of-veterans-affairs/va-ahead-of-schedule-in-improving -chronic-pain-care. Accessed June 16, 2015.
3. Sproul RD. Bridging the gap: e-consult pain service for the primary care physician. Am Soc Pain Educ. 2012;8(1).
4. Roesel T, Engel C. Vitamin D levels and their correlation to pain, fatigue, anxiety, and other co-morbidities in specialized care program service members seen at the Deployment Health Clinical Center. In: Deployment Health Clinical Center Annual Report 2011: pp28-29. http://www.pdhealth.mil/downloads/DHCC_AR_2011.pdf. Accessed May 25, 2015.
5. Le Goaziou MF, Kellou N, Flori M, et al. Vitamin D supplementation for diffuse musculoskeletal pain: results of a before-and-after study. Eur J Gen Pract. 2014;20(1):3-9.
6. Abbasi M, Hashemipour S, Hajmanuchehri F, Kazemifar AM. Is vitamin D deficiency associated with non specific musculoskeletal pain? Glob J Health Sci. 2012;11;5(1):107-111.
7. Von Känel R, Müller-Hartmannsgruber V, Kokinogenis G, Egloff N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med. 2014;15(9):1609-1618.
8. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
9. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA healthcare system. Fed Pract. 2014;31(5):26-31.
10. Fan T, Elgourt T. Pain management pharmacy service in a community hospital. Am J Health Syst Pharm. 2008;65(16):1560-1565.
11. Andrews LB, Bridgeman MB, Dalal KS, et al. Implementation of pharmacist-driven pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342-1349.
12. Hadi M, Alldred D, Briggs M, Munyombwe T, Closs SJ. Effectiveness of pharmacist-led medication review in chronic pain management: systematic review and meta-analysis. Clin J Pain. 2014;30(11):1006-1014.
13. Briggs M, Closs SJ, Marczewski K, Barratt J. A feasibility study of a combined nurse/pharmacist-led chronic pain clinic in primary care. Qual Prim Care. 2008;16(2):91-94.
1. U.S. Department of Veterans Affairs. Management of opioid therapy for chronic pain. U.S. Department of Veterans Affairs Website. http://www.healthquality.va.gov/guidelines/Pain/cot. Published May 2010. Accessed May 25, 2015.
2. Boyle AM. VA ahead of schedule in improving chronic pain care. U.S. Medicine. 2012. http://www.usmedicine.com/agencies/department-of-veterans-affairs/va-ahead-of-schedule-in-improving -chronic-pain-care. Accessed June 16, 2015.
3. Sproul RD. Bridging the gap: e-consult pain service for the primary care physician. Am Soc Pain Educ. 2012;8(1).
4. Roesel T, Engel C. Vitamin D levels and their correlation to pain, fatigue, anxiety, and other co-morbidities in specialized care program service members seen at the Deployment Health Clinical Center. In: Deployment Health Clinical Center Annual Report 2011: pp28-29. http://www.pdhealth.mil/downloads/DHCC_AR_2011.pdf. Accessed May 25, 2015.
5. Le Goaziou MF, Kellou N, Flori M, et al. Vitamin D supplementation for diffuse musculoskeletal pain: results of a before-and-after study. Eur J Gen Pract. 2014;20(1):3-9.
6. Abbasi M, Hashemipour S, Hajmanuchehri F, Kazemifar AM. Is vitamin D deficiency associated with non specific musculoskeletal pain? Glob J Health Sci. 2012;11;5(1):107-111.
7. Von Känel R, Müller-Hartmannsgruber V, Kokinogenis G, Egloff N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med. 2014;15(9):1609-1618.
8. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
9. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA healthcare system. Fed Pract. 2014;31(5):26-31.
10. Fan T, Elgourt T. Pain management pharmacy service in a community hospital. Am J Health Syst Pharm. 2008;65(16):1560-1565.
11. Andrews LB, Bridgeman MB, Dalal KS, et al. Implementation of pharmacist-driven pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342-1349.
12. Hadi M, Alldred D, Briggs M, Munyombwe T, Closs SJ. Effectiveness of pharmacist-led medication review in chronic pain management: systematic review and meta-analysis. Clin J Pain. 2014;30(11):1006-1014.
13. Briggs M, Closs SJ, Marczewski K, Barratt J. A feasibility study of a combined nurse/pharmacist-led chronic pain clinic in primary care. Qual Prim Care. 2008;16(2):91-94.
Development of a Virtual Pharmacy Resident Conference
The VHA is the nation’s largest provider of pharmacy residency programs offering > 150 programs.1 The American Society of Health-System Pharmacists (ASHP) is the accreditation body for these pharmacy residency programs. One of the several ASHP residency standards is the presentation of a resident project at an annual conference.2,3 To meet the requirement, U.S. residency programs send pharmacy residents to regional conferences to present their projects.
Often only pharmacy residents and their project preceptors attend the regional conferences. Most pharmacists who work at each institution are not able to attend and do not have the opportunity to benefit from resident research directly related to the pharmacy profession and the facilities where the research is conducted.
Related: Treatment of Ampicillin-Resistant Enterococcus faecium Urinary Tract Infections
Reasons for not being able to attend these regional resident conferences include financial limitations as well as staffing and work requirements. The expenses associated with attending regional resident conferences include conference registration, transportation, lodging, meals, and other incidental expenses. These expenses could easily surpass several hundred dollars per attendee.
The requirements to obtain travel reimbursement for VHA employees to attend conferences for professional development have become increasingly more complex. This has presented the VHA with a unique challenge to provide its employees with professional development opportunities that do not require travel.
One option is to develop virtual learning environments, which eliminate the need for travel and conference-related expenses. Virtual learning has been a successful and convenient platform for professional development and has recently emerged within the pharmacy profession.4 In 2012, the American College of Clinical Pharmacy hosted its first Virtual Poster Symposium, which allowed participants to visit posters and interact with presenters online.5 At the VHA, pharmacists also have the opportunity to deliver and attend virtual presentations through the VA Learning University system.
To provide increased exposure and understanding to pharmacy resident research within the limitations of the VHA employee travel reimbursement system, a virtual pharmacy resident conference was developed. This article describes the steps taken to develop a conference and its impact on the pharmacists and pharmacy residents of VISN 11.
Methods
Planning for the VISN 11 Virtual Pharmacy Resident Conference started in June 2013 during the annual call for education programming from the VHA Employee Education System (EES). A proposal for the virtual conference, explaining its purpose and structure, was submitted to EES at that time, and approval was granted in August 2013.
Planning Process
Once approved, an EES representative provided guidance through the planning process and serve as a liaison between the planning committee and the desired educational accreditation body, the Accreditation Council for Pharmacy Education (ACPE). For any educational program receiving continuing education (CE) credit from ACPE, an ACPE Planning Committee must be formed that includes a licensed pharmacist.6
The ACPE credit approval process then requires a needs assessment. The needs assessment identified a current gap within the profession and highlighted how the proposed education programming filled this gap. For the VISN 11 Virtual Pharmacy Resident Conference, the needs assessment included the challenges surrounding professional travel reimbursement and the missed learning opportunity for VA pharmacists who were not able to learn from resident research projects. Developing a virtual conference was proposed to fill this gap; pharmacists within the VISN could attend presentations from their workstations in order to stay abreast of pharmacy resident projects while gaining required CE hours for license renewal.
After the needs assessment was approved, a brochure and a content alignment worksheet was developed. The brochure identified the date and time of the conference, the target audience, and included a statement of purpose. The content alignment worksheet listed the program (presentation) title, the faculty delivering the presentation, and objectives. The completed brochure and content alignment worksheet was submitted to ACPE for credit hours approval. In the VHA, it is a VHA employee who coordinates ACPE activities for the entire health system.
Gaining Support
Another important step was to gain the support of VHA pharmacy leadership. In September 2013, an informational meeting was held to discuss the proposal and request feedback from the pharmacy chiefs, supervisors, and residency program directors at each facility within VISN 11. Following this meeting, each facility was given 1 month to determine whether the pharmacy residents at each respective facility would participate in the virtual conference. Once the planning committee had a final list of participating residents, an official announcement of the virtual conference was made to the pharmacy residents, chiefs of pharmacy, supervisors, and residency pharmacy directors.
Participating pharmacy residents submitted presentation titles to the ACPE planning committee and identified which of the 3 content tracks the research fell into: ambulatory care, acute care, or pharmacy administration. A presentation schedule was then developed.
VISN 11 pharmacists were invited to register for each of the presentations. Registration took place through the VHA Talent Management System. Presentations were delivered through Microsoft Lync (Redmond, WA), a web-based communication and conferencing platform. The VA eHealth University could have been used to achieve the same outcome.
Statistical Analysis
Presentation content breakdown and attendance rates from the VISN 11 Virtual Pharmacy Resident Conference were analyzed using descriptive statistics. The comparison of attendance rates at the virtual conference with those expected for the regional face-to-face conference was analyzed using a single sample t test.
Results
The VISN 11 Virtual Pharmacy Resident Conference took place May 5-7, 2014. Twenty-six of the 29 pharmacy residents in VISN 11 delivered 23 presentations. Three presentations had 2 presenters each, as these had completed their research as a team. Each presentation was approved by ACPE for 0.5 CE hours for a total of 11.5 CE hours available to participants.
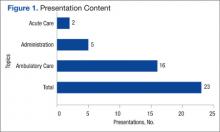
Of the 23 presentations, 16 (69.6%) focused on ambulatory care, 5 (21.7%) on pharmacy administration, and 2 (8.7%) on acute care (Figure 1). The ambulatory care presentations were divided into subgroups of diabetes (n = 6), mental health (n = 4), anticoagulation (n = 4), and cardiology (n = 2). Diabetes and cardiology presentations were delivered on day 1 of the conference, mental health and anticoagulation on day 2, and acute care and pharmacy administration on day 3.
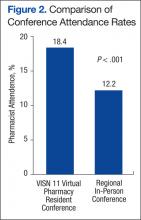
A total of 386 VISN 11 pharmacists were invited to attend the virtual conference and 71 pharmacists (18.4%) registered for at least 1 presentation. VISN 11 pharmacy participation at the virtual conference was increased by 50% compared with the attendance at the 29th Annual Great Lakes Pharmacy Resident Conference, hosted by Purdue University, where only 47 VISN 11 pharmacists (12.2%) were expected to attend, based on results of a VISN-wide survey (95% confidence interval, 0.15-0.23; P < .001) (Figure 2).
On average, each participant attended 7 presentations and earned 3.5 hours of ACPE credit. Of the pharmacists who registered, 14 (19.7%) were pharmacy residents. Of note, registration was not required to deliver a presentation, which explains why the number of pharmacy residents registered to attend (14) was less than the number of pharmacy residents that delivered presentations (26).
More pharmacists registered for ambulatory care presentations (76.2%), followed by pharmacy administration (16.4%) and acute care (7.4%). These differences may be explained by the variability in the number of presentations within each content track. The registration for ambulatory care presentations, when stratified by content subgroup, was 45.7% for diabetes, 22.6% for mental health, 21.2% for anticoagulation, and 10.5% for cardiology.
The first day of the conference had the largest number of participants with 42.8% of all registrants, followed by 33.4% of registrants attending presentations on day 2 and 23.8% on day 3. The presentation with the largest number of registrants was in the diabetes subgroup, which was presented on the first day of the conference. The pharmacy administration presentation was held on the third and final day of the conference and had the lowest number of registrants. An average of 21.2 pharmacists registered for each presentation.
Discussion
The VISN 11 Virtual Pharmacy Resident Conference was structured in a way that offered benefits to multiple groups. First, the virtual conference served as a medium for pharmacy residents to present their yearlong research projects and meet an ASHP residency requirement. Second, the virtual conference greatly expanded the audience size and potential impact of the presentations. Traditionally, resident research projects have been available to the few pharmacists who are able to attend an in-person conference. Almost 20% of all VISN 11 pharmacists were able to attend at least 1 presentation over the course of the 3-day conference. Attendance may increase as the virtual conference becomes more familiar to the VISN 11 pharmacy staff.
Access to a larger audience may help more pharmacists understand veteran-specific research. The information discovered through these research projects may be valuable to advance the clinical and administrative role of pharmacy within each facility as well as the entire VISN. Previously, staff pharmacists could not easily learn about resident research projects taking place at their local and neighboring VA facilities. In addition to the increased impact having a larger audience size also increases staff buy-in and feedback toward the projects.
Related:Non–Daily-Dosed Rosuvastatin in Statin-Intolerant Veterans
Individual VHA facilities frequently try to find ways to increase collaboration between VISN sites. The virtual conference format can help this collaboration. Sharing information between sites through a virtual conference may decrease duplication of projects across facilities, and each facility can learn from the mistakes of the others as well as the successes.
The VHA has a standing contract with ACPE, and therefore, registration fees were not required for this conference. For health systems that may not have such a contract, an ACPE registration fee may be required; however, this fee would still be considerably lower than the travel costs of an in-person conference.
Experience preparing and delivering a virtual presentation is useful for pharmacy residents. Delivering a virtual presentation offers its own set of challenges, such as learning how to engage an audience. Exposure to this type of public speaking may benefit residents as they progress on their career paths.
To prepare for this conference, a tutorial was created to help develop presentations. Residents were encouraged to learn how to not only deliver the presentation using the web conference technology, but also incorporate active learning exercises throughout the presentation to maximize involvement and engagement of the audience. For most resident presenters, this was the first experience delivering a virtual presentation.
Finally, a virtual conference format allows pharmacists to obtain ACPE credit hours required for license renewal.
In addition to the many benefits offered through virtual conferences, there are also some limitations. Many learners enjoy the personal element that comes with an in-person presentation. Although the use of webcams is available for virtual conferences, some of this human element may still be lost. Additionally, in-person conferences provide professional networking opportunities, which are not as readily available through virtual conferences.
The majority of presentations for this conference were related to ambulatory care, which is to be expected in a VHA setting, given the multitude of outpatient clinics in the VHA health system. Of ambulatory care presentations, most participants attended presentations that focused on diabetes or cardiology (day 1 of the conference).
However, some technology difficulties occurred on the first day of the conference, which might explain the decreased participation on subsequent days. Afternoon hours were selected as the time to host the virtual conference, because it was believed this would increase the opportunity for participation, as several pharmacists were expected to be unavailable in the morning hours due to increased workload and/or clinical responsibilities.
A follow-up questionnaire was available to participants after the conference. The majority of responses received indicated positive feedback in regards to the ease of conference participation, applicability of information gained to specific facilities, as well as availability of ACPE CE hours. In the future, the intent is to expand the VISN 11 Virtual Pharmacy Resident Conference to also include CE credit for pharmacy technicians, which requires some additional steps in the ACPE credit approval process. Also, presentations will be recorded and available either live or on-demand for CE credit.
Conclusion
The VISN 11 Virtual Pharmacy Resident Conference was an innovative, educational program that allowed pharmacy residents to meet the ASHP requirement to present residency research at an annual conference, while also providing the opportunity for pharmacists to have a more encompassing understanding of research taking place within the VISN and meet their CE requirements.
The virtual conference format may be applied to any multisite health system where members from pharmacy services would benefit from the presentations. Last, pharmacy residents will gain new techniques and experience in developing and delivering a virtual presentation, which will prove be a useful skill set for the future.
Author Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. Discover more options with a VA pharmacy residency. U.S. Department of Veterans Affairs Website. http://www.vacareers.va.gov/careers/pharmacists /residency.asp. Updated January 2, 2014. Accessed June 8, 2015.
2. American Society of Health-System Pharmacists. ASHP Accreditation Standard For Postgraduate Year One (PGY1) Pharmacy Residency Programs. American Society of Health-System Pharmacists Website. http://www.ashp.org/DocLibrary/Accreditation/ASD-PGY1-Standard.aspx. Updated April 13, 2012. Accessed June 8, 2015.
3. American Society of Health-System Pharmacists. ASHP Accreditation Standard For Postgraduate Year Two (PGY2) Pharmacy Residency Programs. American Society of Health-System Pharmacists Website. http://www.ashp.org/DocLibrary/Accreditation/ASD-PGY2-Standard.aspx. Updated April 13, 2012. Accessed June 8, 2015.
4. Sloan R. Poster presentations in the virtual world. J Contin Educ Nurs. 2012;43(11):485-486.
5. American College of Clinical Pharmacy. Virtual poster symposium. American College of Clinical Pharmacy Website. http://www.accp.com/meetings/virtual.aspx. Accessed June 8, 2015.
6. Accreditation Council for Pharmacy Education. Accreditation Standards for Continuing Pharmacy Education, Version 2. Chicago, IL: Accreditation Council for Pharmacy Education; 2007. https://www.acpe-accredit.org/pdf/CPE_Standards_Final.pdf. Updated March 2014. Accessed June 8, 2015.
The VHA is the nation’s largest provider of pharmacy residency programs offering > 150 programs.1 The American Society of Health-System Pharmacists (ASHP) is the accreditation body for these pharmacy residency programs. One of the several ASHP residency standards is the presentation of a resident project at an annual conference.2,3 To meet the requirement, U.S. residency programs send pharmacy residents to regional conferences to present their projects.
Often only pharmacy residents and their project preceptors attend the regional conferences. Most pharmacists who work at each institution are not able to attend and do not have the opportunity to benefit from resident research directly related to the pharmacy profession and the facilities where the research is conducted.
Related: Treatment of Ampicillin-Resistant Enterococcus faecium Urinary Tract Infections
Reasons for not being able to attend these regional resident conferences include financial limitations as well as staffing and work requirements. The expenses associated with attending regional resident conferences include conference registration, transportation, lodging, meals, and other incidental expenses. These expenses could easily surpass several hundred dollars per attendee.
The requirements to obtain travel reimbursement for VHA employees to attend conferences for professional development have become increasingly more complex. This has presented the VHA with a unique challenge to provide its employees with professional development opportunities that do not require travel.
One option is to develop virtual learning environments, which eliminate the need for travel and conference-related expenses. Virtual learning has been a successful and convenient platform for professional development and has recently emerged within the pharmacy profession.4 In 2012, the American College of Clinical Pharmacy hosted its first Virtual Poster Symposium, which allowed participants to visit posters and interact with presenters online.5 At the VHA, pharmacists also have the opportunity to deliver and attend virtual presentations through the VA Learning University system.
To provide increased exposure and understanding to pharmacy resident research within the limitations of the VHA employee travel reimbursement system, a virtual pharmacy resident conference was developed. This article describes the steps taken to develop a conference and its impact on the pharmacists and pharmacy residents of VISN 11.
Methods
Planning for the VISN 11 Virtual Pharmacy Resident Conference started in June 2013 during the annual call for education programming from the VHA Employee Education System (EES). A proposal for the virtual conference, explaining its purpose and structure, was submitted to EES at that time, and approval was granted in August 2013.
Planning Process
Once approved, an EES representative provided guidance through the planning process and serve as a liaison between the planning committee and the desired educational accreditation body, the Accreditation Council for Pharmacy Education (ACPE). For any educational program receiving continuing education (CE) credit from ACPE, an ACPE Planning Committee must be formed that includes a licensed pharmacist.6
The ACPE credit approval process then requires a needs assessment. The needs assessment identified a current gap within the profession and highlighted how the proposed education programming filled this gap. For the VISN 11 Virtual Pharmacy Resident Conference, the needs assessment included the challenges surrounding professional travel reimbursement and the missed learning opportunity for VA pharmacists who were not able to learn from resident research projects. Developing a virtual conference was proposed to fill this gap; pharmacists within the VISN could attend presentations from their workstations in order to stay abreast of pharmacy resident projects while gaining required CE hours for license renewal.
After the needs assessment was approved, a brochure and a content alignment worksheet was developed. The brochure identified the date and time of the conference, the target audience, and included a statement of purpose. The content alignment worksheet listed the program (presentation) title, the faculty delivering the presentation, and objectives. The completed brochure and content alignment worksheet was submitted to ACPE for credit hours approval. In the VHA, it is a VHA employee who coordinates ACPE activities for the entire health system.
Gaining Support
Another important step was to gain the support of VHA pharmacy leadership. In September 2013, an informational meeting was held to discuss the proposal and request feedback from the pharmacy chiefs, supervisors, and residency program directors at each facility within VISN 11. Following this meeting, each facility was given 1 month to determine whether the pharmacy residents at each respective facility would participate in the virtual conference. Once the planning committee had a final list of participating residents, an official announcement of the virtual conference was made to the pharmacy residents, chiefs of pharmacy, supervisors, and residency pharmacy directors.
Participating pharmacy residents submitted presentation titles to the ACPE planning committee and identified which of the 3 content tracks the research fell into: ambulatory care, acute care, or pharmacy administration. A presentation schedule was then developed.
VISN 11 pharmacists were invited to register for each of the presentations. Registration took place through the VHA Talent Management System. Presentations were delivered through Microsoft Lync (Redmond, WA), a web-based communication and conferencing platform. The VA eHealth University could have been used to achieve the same outcome.
Statistical Analysis
Presentation content breakdown and attendance rates from the VISN 11 Virtual Pharmacy Resident Conference were analyzed using descriptive statistics. The comparison of attendance rates at the virtual conference with those expected for the regional face-to-face conference was analyzed using a single sample t test.
Results
The VISN 11 Virtual Pharmacy Resident Conference took place May 5-7, 2014. Twenty-six of the 29 pharmacy residents in VISN 11 delivered 23 presentations. Three presentations had 2 presenters each, as these had completed their research as a team. Each presentation was approved by ACPE for 0.5 CE hours for a total of 11.5 CE hours available to participants.
Of the 23 presentations, 16 (69.6%) focused on ambulatory care, 5 (21.7%) on pharmacy administration, and 2 (8.7%) on acute care (Figure 1). The ambulatory care presentations were divided into subgroups of diabetes (n = 6), mental health (n = 4), anticoagulation (n = 4), and cardiology (n = 2). Diabetes and cardiology presentations were delivered on day 1 of the conference, mental health and anticoagulation on day 2, and acute care and pharmacy administration on day 3.
A total of 386 VISN 11 pharmacists were invited to attend the virtual conference and 71 pharmacists (18.4%) registered for at least 1 presentation. VISN 11 pharmacy participation at the virtual conference was increased by 50% compared with the attendance at the 29th Annual Great Lakes Pharmacy Resident Conference, hosted by Purdue University, where only 47 VISN 11 pharmacists (12.2%) were expected to attend, based on results of a VISN-wide survey (95% confidence interval, 0.15-0.23; P < .001) (Figure 2).
On average, each participant attended 7 presentations and earned 3.5 hours of ACPE credit. Of the pharmacists who registered, 14 (19.7%) were pharmacy residents. Of note, registration was not required to deliver a presentation, which explains why the number of pharmacy residents registered to attend (14) was less than the number of pharmacy residents that delivered presentations (26).
More pharmacists registered for ambulatory care presentations (76.2%), followed by pharmacy administration (16.4%) and acute care (7.4%). These differences may be explained by the variability in the number of presentations within each content track. The registration for ambulatory care presentations, when stratified by content subgroup, was 45.7% for diabetes, 22.6% for mental health, 21.2% for anticoagulation, and 10.5% for cardiology.
The first day of the conference had the largest number of participants with 42.8% of all registrants, followed by 33.4% of registrants attending presentations on day 2 and 23.8% on day 3. The presentation with the largest number of registrants was in the diabetes subgroup, which was presented on the first day of the conference. The pharmacy administration presentation was held on the third and final day of the conference and had the lowest number of registrants. An average of 21.2 pharmacists registered for each presentation.
Discussion
The VISN 11 Virtual Pharmacy Resident Conference was structured in a way that offered benefits to multiple groups. First, the virtual conference served as a medium for pharmacy residents to present their yearlong research projects and meet an ASHP residency requirement. Second, the virtual conference greatly expanded the audience size and potential impact of the presentations. Traditionally, resident research projects have been available to the few pharmacists who are able to attend an in-person conference. Almost 20% of all VISN 11 pharmacists were able to attend at least 1 presentation over the course of the 3-day conference. Attendance may increase as the virtual conference becomes more familiar to the VISN 11 pharmacy staff.
Access to a larger audience may help more pharmacists understand veteran-specific research. The information discovered through these research projects may be valuable to advance the clinical and administrative role of pharmacy within each facility as well as the entire VISN. Previously, staff pharmacists could not easily learn about resident research projects taking place at their local and neighboring VA facilities. In addition to the increased impact having a larger audience size also increases staff buy-in and feedback toward the projects.
Related:Non–Daily-Dosed Rosuvastatin in Statin-Intolerant Veterans
Individual VHA facilities frequently try to find ways to increase collaboration between VISN sites. The virtual conference format can help this collaboration. Sharing information between sites through a virtual conference may decrease duplication of projects across facilities, and each facility can learn from the mistakes of the others as well as the successes.
The VHA has a standing contract with ACPE, and therefore, registration fees were not required for this conference. For health systems that may not have such a contract, an ACPE registration fee may be required; however, this fee would still be considerably lower than the travel costs of an in-person conference.
Experience preparing and delivering a virtual presentation is useful for pharmacy residents. Delivering a virtual presentation offers its own set of challenges, such as learning how to engage an audience. Exposure to this type of public speaking may benefit residents as they progress on their career paths.
To prepare for this conference, a tutorial was created to help develop presentations. Residents were encouraged to learn how to not only deliver the presentation using the web conference technology, but also incorporate active learning exercises throughout the presentation to maximize involvement and engagement of the audience. For most resident presenters, this was the first experience delivering a virtual presentation.
Finally, a virtual conference format allows pharmacists to obtain ACPE credit hours required for license renewal.
In addition to the many benefits offered through virtual conferences, there are also some limitations. Many learners enjoy the personal element that comes with an in-person presentation. Although the use of webcams is available for virtual conferences, some of this human element may still be lost. Additionally, in-person conferences provide professional networking opportunities, which are not as readily available through virtual conferences.
The majority of presentations for this conference were related to ambulatory care, which is to be expected in a VHA setting, given the multitude of outpatient clinics in the VHA health system. Of ambulatory care presentations, most participants attended presentations that focused on diabetes or cardiology (day 1 of the conference).
However, some technology difficulties occurred on the first day of the conference, which might explain the decreased participation on subsequent days. Afternoon hours were selected as the time to host the virtual conference, because it was believed this would increase the opportunity for participation, as several pharmacists were expected to be unavailable in the morning hours due to increased workload and/or clinical responsibilities.
A follow-up questionnaire was available to participants after the conference. The majority of responses received indicated positive feedback in regards to the ease of conference participation, applicability of information gained to specific facilities, as well as availability of ACPE CE hours. In the future, the intent is to expand the VISN 11 Virtual Pharmacy Resident Conference to also include CE credit for pharmacy technicians, which requires some additional steps in the ACPE credit approval process. Also, presentations will be recorded and available either live or on-demand for CE credit.
Conclusion
The VISN 11 Virtual Pharmacy Resident Conference was an innovative, educational program that allowed pharmacy residents to meet the ASHP requirement to present residency research at an annual conference, while also providing the opportunity for pharmacists to have a more encompassing understanding of research taking place within the VISN and meet their CE requirements.
The virtual conference format may be applied to any multisite health system where members from pharmacy services would benefit from the presentations. Last, pharmacy residents will gain new techniques and experience in developing and delivering a virtual presentation, which will prove be a useful skill set for the future.
Author Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The VHA is the nation’s largest provider of pharmacy residency programs offering > 150 programs.1 The American Society of Health-System Pharmacists (ASHP) is the accreditation body for these pharmacy residency programs. One of the several ASHP residency standards is the presentation of a resident project at an annual conference.2,3 To meet the requirement, U.S. residency programs send pharmacy residents to regional conferences to present their projects.
Often only pharmacy residents and their project preceptors attend the regional conferences. Most pharmacists who work at each institution are not able to attend and do not have the opportunity to benefit from resident research directly related to the pharmacy profession and the facilities where the research is conducted.
Related: Treatment of Ampicillin-Resistant Enterococcus faecium Urinary Tract Infections
Reasons for not being able to attend these regional resident conferences include financial limitations as well as staffing and work requirements. The expenses associated with attending regional resident conferences include conference registration, transportation, lodging, meals, and other incidental expenses. These expenses could easily surpass several hundred dollars per attendee.
The requirements to obtain travel reimbursement for VHA employees to attend conferences for professional development have become increasingly more complex. This has presented the VHA with a unique challenge to provide its employees with professional development opportunities that do not require travel.
One option is to develop virtual learning environments, which eliminate the need for travel and conference-related expenses. Virtual learning has been a successful and convenient platform for professional development and has recently emerged within the pharmacy profession.4 In 2012, the American College of Clinical Pharmacy hosted its first Virtual Poster Symposium, which allowed participants to visit posters and interact with presenters online.5 At the VHA, pharmacists also have the opportunity to deliver and attend virtual presentations through the VA Learning University system.
To provide increased exposure and understanding to pharmacy resident research within the limitations of the VHA employee travel reimbursement system, a virtual pharmacy resident conference was developed. This article describes the steps taken to develop a conference and its impact on the pharmacists and pharmacy residents of VISN 11.
Methods
Planning for the VISN 11 Virtual Pharmacy Resident Conference started in June 2013 during the annual call for education programming from the VHA Employee Education System (EES). A proposal for the virtual conference, explaining its purpose and structure, was submitted to EES at that time, and approval was granted in August 2013.
Planning Process
Once approved, an EES representative provided guidance through the planning process and serve as a liaison between the planning committee and the desired educational accreditation body, the Accreditation Council for Pharmacy Education (ACPE). For any educational program receiving continuing education (CE) credit from ACPE, an ACPE Planning Committee must be formed that includes a licensed pharmacist.6
The ACPE credit approval process then requires a needs assessment. The needs assessment identified a current gap within the profession and highlighted how the proposed education programming filled this gap. For the VISN 11 Virtual Pharmacy Resident Conference, the needs assessment included the challenges surrounding professional travel reimbursement and the missed learning opportunity for VA pharmacists who were not able to learn from resident research projects. Developing a virtual conference was proposed to fill this gap; pharmacists within the VISN could attend presentations from their workstations in order to stay abreast of pharmacy resident projects while gaining required CE hours for license renewal.
After the needs assessment was approved, a brochure and a content alignment worksheet was developed. The brochure identified the date and time of the conference, the target audience, and included a statement of purpose. The content alignment worksheet listed the program (presentation) title, the faculty delivering the presentation, and objectives. The completed brochure and content alignment worksheet was submitted to ACPE for credit hours approval. In the VHA, it is a VHA employee who coordinates ACPE activities for the entire health system.
Gaining Support
Another important step was to gain the support of VHA pharmacy leadership. In September 2013, an informational meeting was held to discuss the proposal and request feedback from the pharmacy chiefs, supervisors, and residency program directors at each facility within VISN 11. Following this meeting, each facility was given 1 month to determine whether the pharmacy residents at each respective facility would participate in the virtual conference. Once the planning committee had a final list of participating residents, an official announcement of the virtual conference was made to the pharmacy residents, chiefs of pharmacy, supervisors, and residency pharmacy directors.
Participating pharmacy residents submitted presentation titles to the ACPE planning committee and identified which of the 3 content tracks the research fell into: ambulatory care, acute care, or pharmacy administration. A presentation schedule was then developed.
VISN 11 pharmacists were invited to register for each of the presentations. Registration took place through the VHA Talent Management System. Presentations were delivered through Microsoft Lync (Redmond, WA), a web-based communication and conferencing platform. The VA eHealth University could have been used to achieve the same outcome.
Statistical Analysis
Presentation content breakdown and attendance rates from the VISN 11 Virtual Pharmacy Resident Conference were analyzed using descriptive statistics. The comparison of attendance rates at the virtual conference with those expected for the regional face-to-face conference was analyzed using a single sample t test.
Results
The VISN 11 Virtual Pharmacy Resident Conference took place May 5-7, 2014. Twenty-six of the 29 pharmacy residents in VISN 11 delivered 23 presentations. Three presentations had 2 presenters each, as these had completed their research as a team. Each presentation was approved by ACPE for 0.5 CE hours for a total of 11.5 CE hours available to participants.
Of the 23 presentations, 16 (69.6%) focused on ambulatory care, 5 (21.7%) on pharmacy administration, and 2 (8.7%) on acute care (Figure 1). The ambulatory care presentations were divided into subgroups of diabetes (n = 6), mental health (n = 4), anticoagulation (n = 4), and cardiology (n = 2). Diabetes and cardiology presentations were delivered on day 1 of the conference, mental health and anticoagulation on day 2, and acute care and pharmacy administration on day 3.
A total of 386 VISN 11 pharmacists were invited to attend the virtual conference and 71 pharmacists (18.4%) registered for at least 1 presentation. VISN 11 pharmacy participation at the virtual conference was increased by 50% compared with the attendance at the 29th Annual Great Lakes Pharmacy Resident Conference, hosted by Purdue University, where only 47 VISN 11 pharmacists (12.2%) were expected to attend, based on results of a VISN-wide survey (95% confidence interval, 0.15-0.23; P < .001) (Figure 2).
On average, each participant attended 7 presentations and earned 3.5 hours of ACPE credit. Of the pharmacists who registered, 14 (19.7%) were pharmacy residents. Of note, registration was not required to deliver a presentation, which explains why the number of pharmacy residents registered to attend (14) was less than the number of pharmacy residents that delivered presentations (26).
More pharmacists registered for ambulatory care presentations (76.2%), followed by pharmacy administration (16.4%) and acute care (7.4%). These differences may be explained by the variability in the number of presentations within each content track. The registration for ambulatory care presentations, when stratified by content subgroup, was 45.7% for diabetes, 22.6% for mental health, 21.2% for anticoagulation, and 10.5% for cardiology.
The first day of the conference had the largest number of participants with 42.8% of all registrants, followed by 33.4% of registrants attending presentations on day 2 and 23.8% on day 3. The presentation with the largest number of registrants was in the diabetes subgroup, which was presented on the first day of the conference. The pharmacy administration presentation was held on the third and final day of the conference and had the lowest number of registrants. An average of 21.2 pharmacists registered for each presentation.
Discussion
The VISN 11 Virtual Pharmacy Resident Conference was structured in a way that offered benefits to multiple groups. First, the virtual conference served as a medium for pharmacy residents to present their yearlong research projects and meet an ASHP residency requirement. Second, the virtual conference greatly expanded the audience size and potential impact of the presentations. Traditionally, resident research projects have been available to the few pharmacists who are able to attend an in-person conference. Almost 20% of all VISN 11 pharmacists were able to attend at least 1 presentation over the course of the 3-day conference. Attendance may increase as the virtual conference becomes more familiar to the VISN 11 pharmacy staff.
Access to a larger audience may help more pharmacists understand veteran-specific research. The information discovered through these research projects may be valuable to advance the clinical and administrative role of pharmacy within each facility as well as the entire VISN. Previously, staff pharmacists could not easily learn about resident research projects taking place at their local and neighboring VA facilities. In addition to the increased impact having a larger audience size also increases staff buy-in and feedback toward the projects.
Related:Non–Daily-Dosed Rosuvastatin in Statin-Intolerant Veterans
Individual VHA facilities frequently try to find ways to increase collaboration between VISN sites. The virtual conference format can help this collaboration. Sharing information between sites through a virtual conference may decrease duplication of projects across facilities, and each facility can learn from the mistakes of the others as well as the successes.
The VHA has a standing contract with ACPE, and therefore, registration fees were not required for this conference. For health systems that may not have such a contract, an ACPE registration fee may be required; however, this fee would still be considerably lower than the travel costs of an in-person conference.
Experience preparing and delivering a virtual presentation is useful for pharmacy residents. Delivering a virtual presentation offers its own set of challenges, such as learning how to engage an audience. Exposure to this type of public speaking may benefit residents as they progress on their career paths.
To prepare for this conference, a tutorial was created to help develop presentations. Residents were encouraged to learn how to not only deliver the presentation using the web conference technology, but also incorporate active learning exercises throughout the presentation to maximize involvement and engagement of the audience. For most resident presenters, this was the first experience delivering a virtual presentation.
Finally, a virtual conference format allows pharmacists to obtain ACPE credit hours required for license renewal.
In addition to the many benefits offered through virtual conferences, there are also some limitations. Many learners enjoy the personal element that comes with an in-person presentation. Although the use of webcams is available for virtual conferences, some of this human element may still be lost. Additionally, in-person conferences provide professional networking opportunities, which are not as readily available through virtual conferences.
The majority of presentations for this conference were related to ambulatory care, which is to be expected in a VHA setting, given the multitude of outpatient clinics in the VHA health system. Of ambulatory care presentations, most participants attended presentations that focused on diabetes or cardiology (day 1 of the conference).
However, some technology difficulties occurred on the first day of the conference, which might explain the decreased participation on subsequent days. Afternoon hours were selected as the time to host the virtual conference, because it was believed this would increase the opportunity for participation, as several pharmacists were expected to be unavailable in the morning hours due to increased workload and/or clinical responsibilities.
A follow-up questionnaire was available to participants after the conference. The majority of responses received indicated positive feedback in regards to the ease of conference participation, applicability of information gained to specific facilities, as well as availability of ACPE CE hours. In the future, the intent is to expand the VISN 11 Virtual Pharmacy Resident Conference to also include CE credit for pharmacy technicians, which requires some additional steps in the ACPE credit approval process. Also, presentations will be recorded and available either live or on-demand for CE credit.
Conclusion
The VISN 11 Virtual Pharmacy Resident Conference was an innovative, educational program that allowed pharmacy residents to meet the ASHP requirement to present residency research at an annual conference, while also providing the opportunity for pharmacists to have a more encompassing understanding of research taking place within the VISN and meet their CE requirements.
The virtual conference format may be applied to any multisite health system where members from pharmacy services would benefit from the presentations. Last, pharmacy residents will gain new techniques and experience in developing and delivering a virtual presentation, which will prove be a useful skill set for the future.
Author Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. Discover more options with a VA pharmacy residency. U.S. Department of Veterans Affairs Website. http://www.vacareers.va.gov/careers/pharmacists /residency.asp. Updated January 2, 2014. Accessed June 8, 2015.
2. American Society of Health-System Pharmacists. ASHP Accreditation Standard For Postgraduate Year One (PGY1) Pharmacy Residency Programs. American Society of Health-System Pharmacists Website. http://www.ashp.org/DocLibrary/Accreditation/ASD-PGY1-Standard.aspx. Updated April 13, 2012. Accessed June 8, 2015.
3. American Society of Health-System Pharmacists. ASHP Accreditation Standard For Postgraduate Year Two (PGY2) Pharmacy Residency Programs. American Society of Health-System Pharmacists Website. http://www.ashp.org/DocLibrary/Accreditation/ASD-PGY2-Standard.aspx. Updated April 13, 2012. Accessed June 8, 2015.
4. Sloan R. Poster presentations in the virtual world. J Contin Educ Nurs. 2012;43(11):485-486.
5. American College of Clinical Pharmacy. Virtual poster symposium. American College of Clinical Pharmacy Website. http://www.accp.com/meetings/virtual.aspx. Accessed June 8, 2015.
6. Accreditation Council for Pharmacy Education. Accreditation Standards for Continuing Pharmacy Education, Version 2. Chicago, IL: Accreditation Council for Pharmacy Education; 2007. https://www.acpe-accredit.org/pdf/CPE_Standards_Final.pdf. Updated March 2014. Accessed June 8, 2015.
1. U.S. Department of Veterans Affairs. Discover more options with a VA pharmacy residency. U.S. Department of Veterans Affairs Website. http://www.vacareers.va.gov/careers/pharmacists /residency.asp. Updated January 2, 2014. Accessed June 8, 2015.
2. American Society of Health-System Pharmacists. ASHP Accreditation Standard For Postgraduate Year One (PGY1) Pharmacy Residency Programs. American Society of Health-System Pharmacists Website. http://www.ashp.org/DocLibrary/Accreditation/ASD-PGY1-Standard.aspx. Updated April 13, 2012. Accessed June 8, 2015.
3. American Society of Health-System Pharmacists. ASHP Accreditation Standard For Postgraduate Year Two (PGY2) Pharmacy Residency Programs. American Society of Health-System Pharmacists Website. http://www.ashp.org/DocLibrary/Accreditation/ASD-PGY2-Standard.aspx. Updated April 13, 2012. Accessed June 8, 2015.
4. Sloan R. Poster presentations in the virtual world. J Contin Educ Nurs. 2012;43(11):485-486.
5. American College of Clinical Pharmacy. Virtual poster symposium. American College of Clinical Pharmacy Website. http://www.accp.com/meetings/virtual.aspx. Accessed June 8, 2015.
6. Accreditation Council for Pharmacy Education. Accreditation Standards for Continuing Pharmacy Education, Version 2. Chicago, IL: Accreditation Council for Pharmacy Education; 2007. https://www.acpe-accredit.org/pdf/CPE_Standards_Final.pdf. Updated March 2014. Accessed June 8, 2015.
Telejustice: Reaching Incarcerated Veterans via Telehealth
The mission of the veterans justice programs (VJPs), which began in 2007 with the initiation of Health Care for Re-entry Veterans (HCRV) and expanded in 2009 to include Veterans Justice Outreach (VJO), is to prevent homelessness and provide justice-involved veterans with timely access to mental health and substance abuse services or other VA benefits.1 About 50% of homeless veterans have a history with the criminal justice system, and about 10% of all individuals incarcerated in the U.S. are veterans.2
Related: Redesign of a Screening Process for VA Homeless Housing
As the VA’ s use of telehealth services increases at non-VA settings, new opportunities emerge to reach veterans. One such population is incarcerated veterans, who can receive VJO and HCRV services. This article focuses primarily on the implementation of jail/prison outreach via clinical video telehealth (CVT) by VJO, which has already expanded to court liaison work (also provided by VJO) and may further expand to jail/prison outreach by HCRV. The article also describes the development and implementation of the VA’ s first telejustice program (TJP) at the VA Portland Health Care System (HCS) and briefly presents a second TJP at the VA New Jersey HCS Lyons Campus. Currently, there are about 15 known telejustice programs across the VA (Table 1).
Background
Overall, there were about 57,000 veterans seen in VJPs in fiscal year (FY) 2014, an estimated 11% increase over the previous year and an estimated 45% increase from FY12.3 Until November 2012, incarcerated veterans were able to access VJO services only by a face-to-face visit with traveling VA providers. Clinical video telehealth, conducted between a patient and a provider through real-time two-way communication, is a viable option to help improve access to care. In FY14, there were about 248,000 unique veterans who used CVT technologies to access about 660,000 appointments.4
Telemental health (TMH) via clinic-based CVT was first implemented in the VA in 2003. To date, more than 500,000 TMH encounters have occurred. Clinical video telehealth into the home (CVT-IH), which is focused on nonclinic settings was implemented nationally by VHA telehealth services in February 2013. Utilization of CVT-IH has increased from about 1,300 veterans seen (about 6,900 visits) in FY12 to about 4,200 veterans seen (about 20,000 visits) in FY14.4 Nationally, from June 2011 to April 2014, about 150 veterans received some form of VJO services via telehealth through a total of about 500 visits.
Each VAMC has a VJO specialist who serves as a liaison between the VA and law enforcement, court (particularly Veterans Treatment Courts and other collaborative treatment courts), and jails. About 225 VJO specialists provide a variety of services, including outreach, treatment matching/linkage assessment, court liaison and court team participation, and education/training to law enforcement on veteran-centric issues such as posttraumatic stress disorder (PTSD) and traumatic brain injury. Specialists spend significant time traveling to provide these jail/prison
outreach services.
The VA Portland HCS specialist, a licensed clinical social worker, had been in contact with representatives at the Deschutes County Adult Jail in Oregon and had determined there were veterans who could benefit from VA services, but she was unable to make the 322-mile round-trip in 1 day to conduct her visits. She contacted Peter Shore, PsyD, in 2010, then a clinical psychologist at the same VA who was conducting home-based TMH visits, and inquired whether it would be possible to see veterans in the jail via a webcam and personal computer. That was the start of the first VA telejustice program.
Portland Pilot
The VJO specialist at the VA Portland HCS initiated this project in early 2012. The Portland TJP used the same technology, staff, and approach that had been implemented in December 2009 through the Home-Based TMH (HBTMH) pilot. The HBTMH pilot (2009-2012), which predated the national CVT-IH program, included about 40 mental health care providers. It was the first VA pilot to successfully connect providers with veterans in their homes via Internet, webcam, and personal computer. During this period, about 250 veterans were seen in an estimated 750 clinical encounters. About 80% of those enrolled indicated they would not have received any mental health treatment were it not for the availability of HBMTH.
In May 2012, Dr. Shore was awarded a VHA Innovation grant through the VA Office for Innovation to expand the HBTMH pilot to VISN 20 via VHA Innovation 669. The Portland TJP was able to expedite implementation through the grant. In addition to continuing the mission of the HBTMH pilot to deliver behavioral health services into the homes of veterans, the Innovation 669 program was established to focus on the advancement of clinical video visits into a variety of non-VA settings, using alternative technologies, including iPads, netbooks, and alternatives to Cisco Jabber (San Jose, CA), the VA-approved videoconferencing software.
The Portland VJO specialist saw the first veteran on November 27, 2012. Through May 28, 2015, she has conducted 28 assessments with incarcerated veterans via CVT. Among the 28 individuals were 15 army, 11 navy, and 2 marine
veterans aged 24 to 70 years (mean 49.6 y). All 28 veterans were identified as male and white (non-Hispanic). Fifty percent of the veterans seen had at least 1 service-connected disability, and all 28 veterans had at least 1 recorded mental health diagnosis (Tables 2 and 3) (Belinda Maddy, LCSW, written communication, June 9, 2015).
Many of the veterans enrolled in the Portland pilot were able to successfully access services at the VA for substance abuse treatment, PTSD treatment, other mental health services, and/or medical services (Belinda Maddy, LCSW, written communication, June 9, 2015). Like the HBTMH pilot, the Portland VJO pilot has also yielded numerous unexpected patient outcomes, including access to services otherwise not available, access to community resources, enrollment in VA services, and an increase in social connectedness.
“This saved my life,” said one veteran in a testimonial. “Now I have a chance to get treatment instead of prison.” Another veteran noted, “I need to not live in this area to be able to learn how to be sober. Going to a long-term treatment program will help me learn how to live sober so I can stay out of trouble.”
New Jersey Pilot
Similar to the Portland pilot, the VA New Jersey HCS VJP specialist spearheaded the pilot and in June 2013 visited with the warden at the Mercer County Correction Center. In November 2014, a year and a half later and with numerous steps in between, a memorandum of understanding (MOU) and telehealth service agreement (TSA) were signed (Mark Correale, LICSW, written communication, June 10, 2015).
For documentation, the New Jersey specialist established a VA MOU and a TSA; whereas the Portland pilot used documentation specific to the Innovation 669 program (http://vaww.visn20.portal.va.gov/sites/clinical/TH/TeleJustice/SitePages/Home.aspx). From the outset, the specialist explored the CVT-IH model of using an Internet connection, Jabber software, and webcam. According to Mr. Correale (June 10, 2015), VA telehealth-issued webcams and Jabber video used on a VA campus did not work. In testing, it failed to provide synced video and audio but was successful after switching to the Cisco EX90 (San Jose, CA).
Mr. Correale was able to get access to desktop technology in the jail, where a stand-alone monitor was connected to a network inside the facility. As of June 2015, the New Jersey pilot has successfully made 9 videoconferencing connections in Monmouth County and has an additional signed MOU for Hudson County.
Related: Using Life Stories to Connect Veterans and Providers
Mr. Correale (June 10, 2015) indicated, “Dialing into a web-based system from the VA would have been outside the traditional VA telehealth arrangement and was therefore not pursued further.” This indicated that the web-based system used by the Portland VJO specialist may not be accepted at all VA facilities.
In the New Jersey pilot, a video-telephone booth was used, which had an EX90 desktop monitor and connection to the jail’s network. The VA information technology (IT) personnel obtained contact numbers for videoconferencing locations within the New Jersey justice system through an arrangement with the New Jersey State Parole Board. The specialist coordinated with the correctional officers (COs) responsible for escorting veterans to the chosen locations regarding privacy and visit scheduling. The CO would escort the veteran to the video-telephone booth in the jail. For scheduling and completing the encounter, the specialist scheduled the appointment time in VISTA, as did the specialist in Portland.
Discussion
A key element of the Portland pilot was autonomy. The pilot was implemented in the context of a VHA Innovation grant, which reduced a number of required approvals. Utilizing a web-based solution also eliminated significant technical obstacles. A peer technical consultant was on-call during each scheduled appointment and provided all technical support. The peer technical consultant, who had logged 2,500 hours of volunteer services in the HBTMH pilot and who worked full-time as a contractor in the Innovation 669 program, was a critical component to the success of the Portland pilot. The Portland pilot demonstrated an effective, simple, and cost-responsible clinical pathway to connect VA providers with incarcerated veterans through telehealth technologies.
The New Jersey pilot also demonstrated a feasible pathway to connect with incarcerated veterans, achieved through a different approach. The New Jersey pilot accessed incarcerated veterans through the correction center’s internal videoconferencing system, whereas the Portland pilot used VA-approved software over the Internet.
In both cases, the driver for success was the VJO specialist. The New Jersey specialist, Mr. Correale (June 10, 2015) suggested, “It’s helpful to find out what works locally and try to adapt the telehealth model that’s already working.” It is also important for the VJO specialist to get to know local and VISN telehealth staff, as they potentially could provide access to a variety of resources, including assistance with the TSA, locating appropriate equipment, etc. In both pilots, the specialists were very flexible in amending their protocols, documentation, and/or clinic times.
Related: Funding for Innovative Federal Employees
Although there is no national mandate to establish TJPs, there is support from VJO leadership for specialists to investigate the need in their local communities. Information contained in this article and supporting documentation and resources available on the Tele-Justice SharePoint site can provide an adequate starting point for any VJO specialist to initiate their own pilot. Communication through the various VJO listservs is also another mode of acquiring information for those interested in pursuing similar telejustice services.
Compensation and Pension (C&P) examinations for mental health provide a rich opportunity for further exploration. Much of the same operational guidance in this article may be applied to a C&P for mental health clinics. The only significant difference would be the referral source and how the encounter is charted.
Program Launch
A successful telehealth program launch is achieved through distinct development, planning, and implementation stages. Embedded throughout the process are building good relationships, consistent and transparent communication, and coordination. Clinical services drive the need for telehealth; telehealth should not drive the need for clinical services.
A descriptive analysis was approved as a quality improvement project by the institutional review board of the VA Portland HCS. Using information from the 2 pilot projects, the intent is to furnish practical guidance for those developing a TJP. If there is anything the reader should take away from the following guide, it is that implementing a VA TJP is very possible.
Development
Identify the need. With every telehealth program comes a fundamental question: Is there a need to deliver clinical services from a distance? The need can be viewed in many ways, but at the core is access. Identifying a need can be any of the following: travel burden, a judge interested in addressing the increasing number of veterans on their docket, limited resources at the jail/prison for transporting veterans to court hearings, inability to identify a C&P examiner willing to see a veteran in a correctional facility, or a need for the VJP to increase the number of veterans served. In the New Jersey pilot, the local VJO specialist spent time with the New Jersey County Jail Wardens Association to describe how screening justice-involved veterans via telehealth may create more opportunities for veterans and positively impact recidivism.
Evaluate feasibility. Is there buy-in from local leadership and local telehealth personnel? Does the distant site (non-VA) administration agree to a telehealth program? Does the technology at the distant site permit a videoconferencing connection? Are there individuals at the court/jail/prison who can serve as points of contact to assist with a variety of tasks?
Planning
Coordinate with the local facility telehealth coordinator (FTC). All VAMCs have an FTC whose primary role is to implement telehealth programs at the facility. As with any relatively new initiative, the FTC may be unfamiliar with the feasibility of a TJP. It is important to work closely with the FTC to ensure all necessary steps are taken, consistent with national policy regarding telehealth in non-VA settings. For most, the national CVT-IH platform will be the logical approach to establishing a TJP. In some instances, involvement with either the VISN telehealth program manager and/or the VISN behavioral health director may be recommended in addition to the VJP specialist’s supervisor.
In general, the FTC will assist with all required documentation, establishment of a clinic, and ongoing technical support. The FTC may also provide needed guidance with logistics around technical specifications at the distant site.
Conduct a site visit. Meeting with administrative and technology decision makers at the site is an important part of the process and is an opportunity to alleviate any apprehension. They may want to hear more about how telehealth is used at the VA, telehealth research in general, and/or other active TJPs.
Identify a suitable space. There will be a variety of appointments that may be considered for the TJP. If the appointment is an encounter between the provider and veteran, it is recommended to find a space at the detention facility that is as private as possible. The law library, which has windows and a cage, was the designated space for the Portland pilot. The desktop computer and webcam were situated on the outside of the cage. The veteran entered the cage with the correctional officer, who established the Internet connection (Figure).
Identify a point of contact and staff at the distant site. As feasibility is evaluated, identifying a point of contact at the distant site is vital. During the site visit, it is important to meet with the point of contact to review any ongoing logistic issues. One or more staff members should be available to escort the veteran into the space where the telehealth appointment will occur. In some cases, a correctional officer will be on standby during the appointment to address any technical issues with the VA provider and/or in case of a medical or behavioral emergency. In most if not all cases, it is important for the staff member at the distant site to have telephone contact information for the VA provider and/or their respective technical support contact person.
Evaluate technology. Does the distant site facility have Internet access for the space under consideration? If it does, it is likely the FTC will follow the protocols outlined in the CVT-IH platform. Although Jabber is currently the only nationally accepted video teleconferencing software, VISN 20 has successfully used Vidyo (Hackensack, NJ) and VSee (Sunnyvale, CA) on iPads for VISN 20 mobile telehealth programs and is in the process to deploy alternative software solutions and iPads for all VISN 20 TJPs. If the jail/prison facility is open to discussing using their own videoconferencing technologies to bridge into the VA system, these efforts should be coordinated through the FTC. In some cases, the correctional facility will request a desktop computer or laptop with Internet access. The most common issue that prevents a program from being further developed is the lack of viable technology.
VA facility preparation. After the site visit is complete, the FTC should assist with the planning process. This will include identifying a telehealth clinical technician on the VA side and development of appropriate documentation and emergency management protocols. The planning package will also include a MOU between the local VA program leadership representatives and the institution/justice entity where the veteran is being served.
Emergency management protocols must include, at a minimum, a point of contact at the distant site and a contingency point of contact. Phone numbers for each should be acquired well in advance. At the beginning of each session, the provider should have access to those names and numbers in case an emergency arises during the session. At the same time, the VA provider should communicate the emergency protocols to the veteran receiving the services. In the event of an emergency, the provider should do whatever possible to remain connected via video with the veteran and call the distant site point of contact to assist with the emergency. Importantly, participants should follow emergency protocols as outlined by the correctional facility. Readers may contact the author at
[email protected] for a copy of the Portland pilot emergency protocol.
A telehealth clinic will need to be built to capture workload. In most cases, this will be a CVT-IH clinic at the facility. Typically, the FTC will initiate the process with the facility clinical applications coordinator to establish the appropriate clinic build. As the program begins to take shape, an operations manual or practice guidelines will need to be created and updated regularly.
Implementation
After the setup documentation has been completed and before the first appointment, the technology support person and the distant site point of contact should be contacted to confirm the appointment and assist in establishing the Internet connection. (An implementation checklist is available at http://wp.me/p6jTLD-5.)
As the TJP grows, it will be important to evaluate and test the technology, technical support, staffing, and modifications to local protocols to ensure the safety and welfare of the veteran and the provider. Also consider whether the program will collect data and if so, what type.
The Portland pilot collected a variety of data sets, including provider and veteran perspectives on their experiences with the technology. The VHA Innovation 669 program used a brief technology impact questionnaire, designed to monitor how technology has impacted quality of care.5 Data was collected iteratively and used in part to improve aspects of the program. This included both veteran and provider satisfaction, clinical outcomes, quality of life, and levels of occupational and social functioning.
Conclusion
Reaching incarcerated veterans sends an important message: The VA will go to great lengths to ensure that veterans have access to services to help ease the transition back into the community. Connecting with incarcerated veterans via telehealth takes the VA mission to a new level.
Given the wide array of technical solutions currently being used in correctional facilities, VA TJPs may benefit from exploring a consumer-based technology solution. However, one factor in expanding VA TJPs is that current telehealth systems rely on VA Office of Information and Technology resources that build systems within the VA network. There are privacy and security standards the VA adheres to in order to maintain a safe clinical video connection.
There are private sector companies that offer Internet-based access to secure video to connect physicians and mental health professionals. Given the significant variability in IT across correctional facilities, VA TJPs could expand their access into correctional facilities by deploying a web-based clinical video solution, similar to those currently available in the private sector. The technology already exists, and although taking this approach would need to be thoroughly vetted, it could significantly expand VA TJPs and eliminate several obstacles outlined in
this article.
Acknowledgments
Joel Rosenthal, PhD, national training director for Veterans Justice Outreach programs, has provided enduring support to VJPs. To Linda Maddy, the Portland VJO specialist—Being the first to do anything in the VA takes courage and tenacity. To Mark Correale, the NJ VJO specialist, for his tireless efforts. A special thanks to the VHA Innovation 669 team: Chuck Brown, Kit Teague, Trevor Davis, Sean O’Connor, Tracy Dekelboum, and William “Bear” Cannon for providing ongoing support to the Portland telejustice providers and distant jail staff. Mary Lu is providing editorial and research support on this and other telehealth-related projects. This article is dedicated to justice-involved men and women who have served our nation and to those VA employees who dare to be disruptive in order to reach them.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. McGuire J, Clark S. Veterans Justice Outreach Initiative (VJO). Washington, DC: Veterans Health Administration; 2009.
2. U.S. Department of Veterans Affairs. Veterans Health Administration Fact Sheet. VA Services for Veterans Involved in the Justice System: VA’s Veterans Justice Outreach Program. Washington, DC: Veterans Health Administration; 2011.
3. Veterans Health Administration Support Service Center. Homeless Services Briefing Book. Veterans Affairs Intranet Website. http://vaww.fcdm.med.va.gov/pas/en/src/Proclarity.asp. Accessed June 8, 2015.
4. Veterans Health Administration Support Service Center. Virtual care and telehealth report. https://securereports2.vssc.med.va.gov/Reports/Pages/Folder.aspx?ViewMode=Detail. Accessed June 8, 2015.
5. Shore P, Davis T. Technology Impact Questionnaire (TIQ). http://wp.me/p2toog-1f. Published 2014. Accessed June 8, 2015.
The mission of the veterans justice programs (VJPs), which began in 2007 with the initiation of Health Care for Re-entry Veterans (HCRV) and expanded in 2009 to include Veterans Justice Outreach (VJO), is to prevent homelessness and provide justice-involved veterans with timely access to mental health and substance abuse services or other VA benefits.1 About 50% of homeless veterans have a history with the criminal justice system, and about 10% of all individuals incarcerated in the U.S. are veterans.2
Related: Redesign of a Screening Process for VA Homeless Housing
As the VA’ s use of telehealth services increases at non-VA settings, new opportunities emerge to reach veterans. One such population is incarcerated veterans, who can receive VJO and HCRV services. This article focuses primarily on the implementation of jail/prison outreach via clinical video telehealth (CVT) by VJO, which has already expanded to court liaison work (also provided by VJO) and may further expand to jail/prison outreach by HCRV. The article also describes the development and implementation of the VA’ s first telejustice program (TJP) at the VA Portland Health Care System (HCS) and briefly presents a second TJP at the VA New Jersey HCS Lyons Campus. Currently, there are about 15 known telejustice programs across the VA (Table 1).
Background
Overall, there were about 57,000 veterans seen in VJPs in fiscal year (FY) 2014, an estimated 11% increase over the previous year and an estimated 45% increase from FY12.3 Until November 2012, incarcerated veterans were able to access VJO services only by a face-to-face visit with traveling VA providers. Clinical video telehealth, conducted between a patient and a provider through real-time two-way communication, is a viable option to help improve access to care. In FY14, there were about 248,000 unique veterans who used CVT technologies to access about 660,000 appointments.4
Telemental health (TMH) via clinic-based CVT was first implemented in the VA in 2003. To date, more than 500,000 TMH encounters have occurred. Clinical video telehealth into the home (CVT-IH), which is focused on nonclinic settings was implemented nationally by VHA telehealth services in February 2013. Utilization of CVT-IH has increased from about 1,300 veterans seen (about 6,900 visits) in FY12 to about 4,200 veterans seen (about 20,000 visits) in FY14.4 Nationally, from June 2011 to April 2014, about 150 veterans received some form of VJO services via telehealth through a total of about 500 visits.
Each VAMC has a VJO specialist who serves as a liaison between the VA and law enforcement, court (particularly Veterans Treatment Courts and other collaborative treatment courts), and jails. About 225 VJO specialists provide a variety of services, including outreach, treatment matching/linkage assessment, court liaison and court team participation, and education/training to law enforcement on veteran-centric issues such as posttraumatic stress disorder (PTSD) and traumatic brain injury. Specialists spend significant time traveling to provide these jail/prison
outreach services.
The VA Portland HCS specialist, a licensed clinical social worker, had been in contact with representatives at the Deschutes County Adult Jail in Oregon and had determined there were veterans who could benefit from VA services, but she was unable to make the 322-mile round-trip in 1 day to conduct her visits. She contacted Peter Shore, PsyD, in 2010, then a clinical psychologist at the same VA who was conducting home-based TMH visits, and inquired whether it would be possible to see veterans in the jail via a webcam and personal computer. That was the start of the first VA telejustice program.
Portland Pilot
The VJO specialist at the VA Portland HCS initiated this project in early 2012. The Portland TJP used the same technology, staff, and approach that had been implemented in December 2009 through the Home-Based TMH (HBTMH) pilot. The HBTMH pilot (2009-2012), which predated the national CVT-IH program, included about 40 mental health care providers. It was the first VA pilot to successfully connect providers with veterans in their homes via Internet, webcam, and personal computer. During this period, about 250 veterans were seen in an estimated 750 clinical encounters. About 80% of those enrolled indicated they would not have received any mental health treatment were it not for the availability of HBMTH.
In May 2012, Dr. Shore was awarded a VHA Innovation grant through the VA Office for Innovation to expand the HBTMH pilot to VISN 20 via VHA Innovation 669. The Portland TJP was able to expedite implementation through the grant. In addition to continuing the mission of the HBTMH pilot to deliver behavioral health services into the homes of veterans, the Innovation 669 program was established to focus on the advancement of clinical video visits into a variety of non-VA settings, using alternative technologies, including iPads, netbooks, and alternatives to Cisco Jabber (San Jose, CA), the VA-approved videoconferencing software.
The Portland VJO specialist saw the first veteran on November 27, 2012. Through May 28, 2015, she has conducted 28 assessments with incarcerated veterans via CVT. Among the 28 individuals were 15 army, 11 navy, and 2 marine
veterans aged 24 to 70 years (mean 49.6 y). All 28 veterans were identified as male and white (non-Hispanic). Fifty percent of the veterans seen had at least 1 service-connected disability, and all 28 veterans had at least 1 recorded mental health diagnosis (Tables 2 and 3) (Belinda Maddy, LCSW, written communication, June 9, 2015).
Many of the veterans enrolled in the Portland pilot were able to successfully access services at the VA for substance abuse treatment, PTSD treatment, other mental health services, and/or medical services (Belinda Maddy, LCSW, written communication, June 9, 2015). Like the HBTMH pilot, the Portland VJO pilot has also yielded numerous unexpected patient outcomes, including access to services otherwise not available, access to community resources, enrollment in VA services, and an increase in social connectedness.
“This saved my life,” said one veteran in a testimonial. “Now I have a chance to get treatment instead of prison.” Another veteran noted, “I need to not live in this area to be able to learn how to be sober. Going to a long-term treatment program will help me learn how to live sober so I can stay out of trouble.”
New Jersey Pilot
Similar to the Portland pilot, the VA New Jersey HCS VJP specialist spearheaded the pilot and in June 2013 visited with the warden at the Mercer County Correction Center. In November 2014, a year and a half later and with numerous steps in between, a memorandum of understanding (MOU) and telehealth service agreement (TSA) were signed (Mark Correale, LICSW, written communication, June 10, 2015).
For documentation, the New Jersey specialist established a VA MOU and a TSA; whereas the Portland pilot used documentation specific to the Innovation 669 program (http://vaww.visn20.portal.va.gov/sites/clinical/TH/TeleJustice/SitePages/Home.aspx). From the outset, the specialist explored the CVT-IH model of using an Internet connection, Jabber software, and webcam. According to Mr. Correale (June 10, 2015), VA telehealth-issued webcams and Jabber video used on a VA campus did not work. In testing, it failed to provide synced video and audio but was successful after switching to the Cisco EX90 (San Jose, CA).
Mr. Correale was able to get access to desktop technology in the jail, where a stand-alone monitor was connected to a network inside the facility. As of June 2015, the New Jersey pilot has successfully made 9 videoconferencing connections in Monmouth County and has an additional signed MOU for Hudson County.
Related: Using Life Stories to Connect Veterans and Providers
Mr. Correale (June 10, 2015) indicated, “Dialing into a web-based system from the VA would have been outside the traditional VA telehealth arrangement and was therefore not pursued further.” This indicated that the web-based system used by the Portland VJO specialist may not be accepted at all VA facilities.
In the New Jersey pilot, a video-telephone booth was used, which had an EX90 desktop monitor and connection to the jail’s network. The VA information technology (IT) personnel obtained contact numbers for videoconferencing locations within the New Jersey justice system through an arrangement with the New Jersey State Parole Board. The specialist coordinated with the correctional officers (COs) responsible for escorting veterans to the chosen locations regarding privacy and visit scheduling. The CO would escort the veteran to the video-telephone booth in the jail. For scheduling and completing the encounter, the specialist scheduled the appointment time in VISTA, as did the specialist in Portland.
Discussion
A key element of the Portland pilot was autonomy. The pilot was implemented in the context of a VHA Innovation grant, which reduced a number of required approvals. Utilizing a web-based solution also eliminated significant technical obstacles. A peer technical consultant was on-call during each scheduled appointment and provided all technical support. The peer technical consultant, who had logged 2,500 hours of volunteer services in the HBTMH pilot and who worked full-time as a contractor in the Innovation 669 program, was a critical component to the success of the Portland pilot. The Portland pilot demonstrated an effective, simple, and cost-responsible clinical pathway to connect VA providers with incarcerated veterans through telehealth technologies.
The New Jersey pilot also demonstrated a feasible pathway to connect with incarcerated veterans, achieved through a different approach. The New Jersey pilot accessed incarcerated veterans through the correction center’s internal videoconferencing system, whereas the Portland pilot used VA-approved software over the Internet.
In both cases, the driver for success was the VJO specialist. The New Jersey specialist, Mr. Correale (June 10, 2015) suggested, “It’s helpful to find out what works locally and try to adapt the telehealth model that’s already working.” It is also important for the VJO specialist to get to know local and VISN telehealth staff, as they potentially could provide access to a variety of resources, including assistance with the TSA, locating appropriate equipment, etc. In both pilots, the specialists were very flexible in amending their protocols, documentation, and/or clinic times.
Related: Funding for Innovative Federal Employees
Although there is no national mandate to establish TJPs, there is support from VJO leadership for specialists to investigate the need in their local communities. Information contained in this article and supporting documentation and resources available on the Tele-Justice SharePoint site can provide an adequate starting point for any VJO specialist to initiate their own pilot. Communication through the various VJO listservs is also another mode of acquiring information for those interested in pursuing similar telejustice services.
Compensation and Pension (C&P) examinations for mental health provide a rich opportunity for further exploration. Much of the same operational guidance in this article may be applied to a C&P for mental health clinics. The only significant difference would be the referral source and how the encounter is charted.
Program Launch
A successful telehealth program launch is achieved through distinct development, planning, and implementation stages. Embedded throughout the process are building good relationships, consistent and transparent communication, and coordination. Clinical services drive the need for telehealth; telehealth should not drive the need for clinical services.
A descriptive analysis was approved as a quality improvement project by the institutional review board of the VA Portland HCS. Using information from the 2 pilot projects, the intent is to furnish practical guidance for those developing a TJP. If there is anything the reader should take away from the following guide, it is that implementing a VA TJP is very possible.
Development
Identify the need. With every telehealth program comes a fundamental question: Is there a need to deliver clinical services from a distance? The need can be viewed in many ways, but at the core is access. Identifying a need can be any of the following: travel burden, a judge interested in addressing the increasing number of veterans on their docket, limited resources at the jail/prison for transporting veterans to court hearings, inability to identify a C&P examiner willing to see a veteran in a correctional facility, or a need for the VJP to increase the number of veterans served. In the New Jersey pilot, the local VJO specialist spent time with the New Jersey County Jail Wardens Association to describe how screening justice-involved veterans via telehealth may create more opportunities for veterans and positively impact recidivism.
Evaluate feasibility. Is there buy-in from local leadership and local telehealth personnel? Does the distant site (non-VA) administration agree to a telehealth program? Does the technology at the distant site permit a videoconferencing connection? Are there individuals at the court/jail/prison who can serve as points of contact to assist with a variety of tasks?
Planning
Coordinate with the local facility telehealth coordinator (FTC). All VAMCs have an FTC whose primary role is to implement telehealth programs at the facility. As with any relatively new initiative, the FTC may be unfamiliar with the feasibility of a TJP. It is important to work closely with the FTC to ensure all necessary steps are taken, consistent with national policy regarding telehealth in non-VA settings. For most, the national CVT-IH platform will be the logical approach to establishing a TJP. In some instances, involvement with either the VISN telehealth program manager and/or the VISN behavioral health director may be recommended in addition to the VJP specialist’s supervisor.
In general, the FTC will assist with all required documentation, establishment of a clinic, and ongoing technical support. The FTC may also provide needed guidance with logistics around technical specifications at the distant site.
Conduct a site visit. Meeting with administrative and technology decision makers at the site is an important part of the process and is an opportunity to alleviate any apprehension. They may want to hear more about how telehealth is used at the VA, telehealth research in general, and/or other active TJPs.
Identify a suitable space. There will be a variety of appointments that may be considered for the TJP. If the appointment is an encounter between the provider and veteran, it is recommended to find a space at the detention facility that is as private as possible. The law library, which has windows and a cage, was the designated space for the Portland pilot. The desktop computer and webcam were situated on the outside of the cage. The veteran entered the cage with the correctional officer, who established the Internet connection (Figure).
Identify a point of contact and staff at the distant site. As feasibility is evaluated, identifying a point of contact at the distant site is vital. During the site visit, it is important to meet with the point of contact to review any ongoing logistic issues. One or more staff members should be available to escort the veteran into the space where the telehealth appointment will occur. In some cases, a correctional officer will be on standby during the appointment to address any technical issues with the VA provider and/or in case of a medical or behavioral emergency. In most if not all cases, it is important for the staff member at the distant site to have telephone contact information for the VA provider and/or their respective technical support contact person.
Evaluate technology. Does the distant site facility have Internet access for the space under consideration? If it does, it is likely the FTC will follow the protocols outlined in the CVT-IH platform. Although Jabber is currently the only nationally accepted video teleconferencing software, VISN 20 has successfully used Vidyo (Hackensack, NJ) and VSee (Sunnyvale, CA) on iPads for VISN 20 mobile telehealth programs and is in the process to deploy alternative software solutions and iPads for all VISN 20 TJPs. If the jail/prison facility is open to discussing using their own videoconferencing technologies to bridge into the VA system, these efforts should be coordinated through the FTC. In some cases, the correctional facility will request a desktop computer or laptop with Internet access. The most common issue that prevents a program from being further developed is the lack of viable technology.
VA facility preparation. After the site visit is complete, the FTC should assist with the planning process. This will include identifying a telehealth clinical technician on the VA side and development of appropriate documentation and emergency management protocols. The planning package will also include a MOU between the local VA program leadership representatives and the institution/justice entity where the veteran is being served.
Emergency management protocols must include, at a minimum, a point of contact at the distant site and a contingency point of contact. Phone numbers for each should be acquired well in advance. At the beginning of each session, the provider should have access to those names and numbers in case an emergency arises during the session. At the same time, the VA provider should communicate the emergency protocols to the veteran receiving the services. In the event of an emergency, the provider should do whatever possible to remain connected via video with the veteran and call the distant site point of contact to assist with the emergency. Importantly, participants should follow emergency protocols as outlined by the correctional facility. Readers may contact the author at
[email protected] for a copy of the Portland pilot emergency protocol.
A telehealth clinic will need to be built to capture workload. In most cases, this will be a CVT-IH clinic at the facility. Typically, the FTC will initiate the process with the facility clinical applications coordinator to establish the appropriate clinic build. As the program begins to take shape, an operations manual or practice guidelines will need to be created and updated regularly.
Implementation
After the setup documentation has been completed and before the first appointment, the technology support person and the distant site point of contact should be contacted to confirm the appointment and assist in establishing the Internet connection. (An implementation checklist is available at http://wp.me/p6jTLD-5.)
As the TJP grows, it will be important to evaluate and test the technology, technical support, staffing, and modifications to local protocols to ensure the safety and welfare of the veteran and the provider. Also consider whether the program will collect data and if so, what type.
The Portland pilot collected a variety of data sets, including provider and veteran perspectives on their experiences with the technology. The VHA Innovation 669 program used a brief technology impact questionnaire, designed to monitor how technology has impacted quality of care.5 Data was collected iteratively and used in part to improve aspects of the program. This included both veteran and provider satisfaction, clinical outcomes, quality of life, and levels of occupational and social functioning.
Conclusion
Reaching incarcerated veterans sends an important message: The VA will go to great lengths to ensure that veterans have access to services to help ease the transition back into the community. Connecting with incarcerated veterans via telehealth takes the VA mission to a new level.
Given the wide array of technical solutions currently being used in correctional facilities, VA TJPs may benefit from exploring a consumer-based technology solution. However, one factor in expanding VA TJPs is that current telehealth systems rely on VA Office of Information and Technology resources that build systems within the VA network. There are privacy and security standards the VA adheres to in order to maintain a safe clinical video connection.
There are private sector companies that offer Internet-based access to secure video to connect physicians and mental health professionals. Given the significant variability in IT across correctional facilities, VA TJPs could expand their access into correctional facilities by deploying a web-based clinical video solution, similar to those currently available in the private sector. The technology already exists, and although taking this approach would need to be thoroughly vetted, it could significantly expand VA TJPs and eliminate several obstacles outlined in
this article.
Acknowledgments
Joel Rosenthal, PhD, national training director for Veterans Justice Outreach programs, has provided enduring support to VJPs. To Linda Maddy, the Portland VJO specialist—Being the first to do anything in the VA takes courage and tenacity. To Mark Correale, the NJ VJO specialist, for his tireless efforts. A special thanks to the VHA Innovation 669 team: Chuck Brown, Kit Teague, Trevor Davis, Sean O’Connor, Tracy Dekelboum, and William “Bear” Cannon for providing ongoing support to the Portland telejustice providers and distant jail staff. Mary Lu is providing editorial and research support on this and other telehealth-related projects. This article is dedicated to justice-involved men and women who have served our nation and to those VA employees who dare to be disruptive in order to reach them.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The mission of the veterans justice programs (VJPs), which began in 2007 with the initiation of Health Care for Re-entry Veterans (HCRV) and expanded in 2009 to include Veterans Justice Outreach (VJO), is to prevent homelessness and provide justice-involved veterans with timely access to mental health and substance abuse services or other VA benefits.1 About 50% of homeless veterans have a history with the criminal justice system, and about 10% of all individuals incarcerated in the U.S. are veterans.2
Related: Redesign of a Screening Process for VA Homeless Housing
As the VA’ s use of telehealth services increases at non-VA settings, new opportunities emerge to reach veterans. One such population is incarcerated veterans, who can receive VJO and HCRV services. This article focuses primarily on the implementation of jail/prison outreach via clinical video telehealth (CVT) by VJO, which has already expanded to court liaison work (also provided by VJO) and may further expand to jail/prison outreach by HCRV. The article also describes the development and implementation of the VA’ s first telejustice program (TJP) at the VA Portland Health Care System (HCS) and briefly presents a second TJP at the VA New Jersey HCS Lyons Campus. Currently, there are about 15 known telejustice programs across the VA (Table 1).
Background
Overall, there were about 57,000 veterans seen in VJPs in fiscal year (FY) 2014, an estimated 11% increase over the previous year and an estimated 45% increase from FY12.3 Until November 2012, incarcerated veterans were able to access VJO services only by a face-to-face visit with traveling VA providers. Clinical video telehealth, conducted between a patient and a provider through real-time two-way communication, is a viable option to help improve access to care. In FY14, there were about 248,000 unique veterans who used CVT technologies to access about 660,000 appointments.4
Telemental health (TMH) via clinic-based CVT was first implemented in the VA in 2003. To date, more than 500,000 TMH encounters have occurred. Clinical video telehealth into the home (CVT-IH), which is focused on nonclinic settings was implemented nationally by VHA telehealth services in February 2013. Utilization of CVT-IH has increased from about 1,300 veterans seen (about 6,900 visits) in FY12 to about 4,200 veterans seen (about 20,000 visits) in FY14.4 Nationally, from June 2011 to April 2014, about 150 veterans received some form of VJO services via telehealth through a total of about 500 visits.
Each VAMC has a VJO specialist who serves as a liaison between the VA and law enforcement, court (particularly Veterans Treatment Courts and other collaborative treatment courts), and jails. About 225 VJO specialists provide a variety of services, including outreach, treatment matching/linkage assessment, court liaison and court team participation, and education/training to law enforcement on veteran-centric issues such as posttraumatic stress disorder (PTSD) and traumatic brain injury. Specialists spend significant time traveling to provide these jail/prison
outreach services.
The VA Portland HCS specialist, a licensed clinical social worker, had been in contact with representatives at the Deschutes County Adult Jail in Oregon and had determined there were veterans who could benefit from VA services, but she was unable to make the 322-mile round-trip in 1 day to conduct her visits. She contacted Peter Shore, PsyD, in 2010, then a clinical psychologist at the same VA who was conducting home-based TMH visits, and inquired whether it would be possible to see veterans in the jail via a webcam and personal computer. That was the start of the first VA telejustice program.
Portland Pilot
The VJO specialist at the VA Portland HCS initiated this project in early 2012. The Portland TJP used the same technology, staff, and approach that had been implemented in December 2009 through the Home-Based TMH (HBTMH) pilot. The HBTMH pilot (2009-2012), which predated the national CVT-IH program, included about 40 mental health care providers. It was the first VA pilot to successfully connect providers with veterans in their homes via Internet, webcam, and personal computer. During this period, about 250 veterans were seen in an estimated 750 clinical encounters. About 80% of those enrolled indicated they would not have received any mental health treatment were it not for the availability of HBMTH.
In May 2012, Dr. Shore was awarded a VHA Innovation grant through the VA Office for Innovation to expand the HBTMH pilot to VISN 20 via VHA Innovation 669. The Portland TJP was able to expedite implementation through the grant. In addition to continuing the mission of the HBTMH pilot to deliver behavioral health services into the homes of veterans, the Innovation 669 program was established to focus on the advancement of clinical video visits into a variety of non-VA settings, using alternative technologies, including iPads, netbooks, and alternatives to Cisco Jabber (San Jose, CA), the VA-approved videoconferencing software.
The Portland VJO specialist saw the first veteran on November 27, 2012. Through May 28, 2015, she has conducted 28 assessments with incarcerated veterans via CVT. Among the 28 individuals were 15 army, 11 navy, and 2 marine
veterans aged 24 to 70 years (mean 49.6 y). All 28 veterans were identified as male and white (non-Hispanic). Fifty percent of the veterans seen had at least 1 service-connected disability, and all 28 veterans had at least 1 recorded mental health diagnosis (Tables 2 and 3) (Belinda Maddy, LCSW, written communication, June 9, 2015).
Many of the veterans enrolled in the Portland pilot were able to successfully access services at the VA for substance abuse treatment, PTSD treatment, other mental health services, and/or medical services (Belinda Maddy, LCSW, written communication, June 9, 2015). Like the HBTMH pilot, the Portland VJO pilot has also yielded numerous unexpected patient outcomes, including access to services otherwise not available, access to community resources, enrollment in VA services, and an increase in social connectedness.
“This saved my life,” said one veteran in a testimonial. “Now I have a chance to get treatment instead of prison.” Another veteran noted, “I need to not live in this area to be able to learn how to be sober. Going to a long-term treatment program will help me learn how to live sober so I can stay out of trouble.”
New Jersey Pilot
Similar to the Portland pilot, the VA New Jersey HCS VJP specialist spearheaded the pilot and in June 2013 visited with the warden at the Mercer County Correction Center. In November 2014, a year and a half later and with numerous steps in between, a memorandum of understanding (MOU) and telehealth service agreement (TSA) were signed (Mark Correale, LICSW, written communication, June 10, 2015).
For documentation, the New Jersey specialist established a VA MOU and a TSA; whereas the Portland pilot used documentation specific to the Innovation 669 program (http://vaww.visn20.portal.va.gov/sites/clinical/TH/TeleJustice/SitePages/Home.aspx). From the outset, the specialist explored the CVT-IH model of using an Internet connection, Jabber software, and webcam. According to Mr. Correale (June 10, 2015), VA telehealth-issued webcams and Jabber video used on a VA campus did not work. In testing, it failed to provide synced video and audio but was successful after switching to the Cisco EX90 (San Jose, CA).
Mr. Correale was able to get access to desktop technology in the jail, where a stand-alone monitor was connected to a network inside the facility. As of June 2015, the New Jersey pilot has successfully made 9 videoconferencing connections in Monmouth County and has an additional signed MOU for Hudson County.
Related: Using Life Stories to Connect Veterans and Providers
Mr. Correale (June 10, 2015) indicated, “Dialing into a web-based system from the VA would have been outside the traditional VA telehealth arrangement and was therefore not pursued further.” This indicated that the web-based system used by the Portland VJO specialist may not be accepted at all VA facilities.
In the New Jersey pilot, a video-telephone booth was used, which had an EX90 desktop monitor and connection to the jail’s network. The VA information technology (IT) personnel obtained contact numbers for videoconferencing locations within the New Jersey justice system through an arrangement with the New Jersey State Parole Board. The specialist coordinated with the correctional officers (COs) responsible for escorting veterans to the chosen locations regarding privacy and visit scheduling. The CO would escort the veteran to the video-telephone booth in the jail. For scheduling and completing the encounter, the specialist scheduled the appointment time in VISTA, as did the specialist in Portland.
Discussion
A key element of the Portland pilot was autonomy. The pilot was implemented in the context of a VHA Innovation grant, which reduced a number of required approvals. Utilizing a web-based solution also eliminated significant technical obstacles. A peer technical consultant was on-call during each scheduled appointment and provided all technical support. The peer technical consultant, who had logged 2,500 hours of volunteer services in the HBTMH pilot and who worked full-time as a contractor in the Innovation 669 program, was a critical component to the success of the Portland pilot. The Portland pilot demonstrated an effective, simple, and cost-responsible clinical pathway to connect VA providers with incarcerated veterans through telehealth technologies.
The New Jersey pilot also demonstrated a feasible pathway to connect with incarcerated veterans, achieved through a different approach. The New Jersey pilot accessed incarcerated veterans through the correction center’s internal videoconferencing system, whereas the Portland pilot used VA-approved software over the Internet.
In both cases, the driver for success was the VJO specialist. The New Jersey specialist, Mr. Correale (June 10, 2015) suggested, “It’s helpful to find out what works locally and try to adapt the telehealth model that’s already working.” It is also important for the VJO specialist to get to know local and VISN telehealth staff, as they potentially could provide access to a variety of resources, including assistance with the TSA, locating appropriate equipment, etc. In both pilots, the specialists were very flexible in amending their protocols, documentation, and/or clinic times.
Related: Funding for Innovative Federal Employees
Although there is no national mandate to establish TJPs, there is support from VJO leadership for specialists to investigate the need in their local communities. Information contained in this article and supporting documentation and resources available on the Tele-Justice SharePoint site can provide an adequate starting point for any VJO specialist to initiate their own pilot. Communication through the various VJO listservs is also another mode of acquiring information for those interested in pursuing similar telejustice services.
Compensation and Pension (C&P) examinations for mental health provide a rich opportunity for further exploration. Much of the same operational guidance in this article may be applied to a C&P for mental health clinics. The only significant difference would be the referral source and how the encounter is charted.
Program Launch
A successful telehealth program launch is achieved through distinct development, planning, and implementation stages. Embedded throughout the process are building good relationships, consistent and transparent communication, and coordination. Clinical services drive the need for telehealth; telehealth should not drive the need for clinical services.
A descriptive analysis was approved as a quality improvement project by the institutional review board of the VA Portland HCS. Using information from the 2 pilot projects, the intent is to furnish practical guidance for those developing a TJP. If there is anything the reader should take away from the following guide, it is that implementing a VA TJP is very possible.
Development
Identify the need. With every telehealth program comes a fundamental question: Is there a need to deliver clinical services from a distance? The need can be viewed in many ways, but at the core is access. Identifying a need can be any of the following: travel burden, a judge interested in addressing the increasing number of veterans on their docket, limited resources at the jail/prison for transporting veterans to court hearings, inability to identify a C&P examiner willing to see a veteran in a correctional facility, or a need for the VJP to increase the number of veterans served. In the New Jersey pilot, the local VJO specialist spent time with the New Jersey County Jail Wardens Association to describe how screening justice-involved veterans via telehealth may create more opportunities for veterans and positively impact recidivism.
Evaluate feasibility. Is there buy-in from local leadership and local telehealth personnel? Does the distant site (non-VA) administration agree to a telehealth program? Does the technology at the distant site permit a videoconferencing connection? Are there individuals at the court/jail/prison who can serve as points of contact to assist with a variety of tasks?
Planning
Coordinate with the local facility telehealth coordinator (FTC). All VAMCs have an FTC whose primary role is to implement telehealth programs at the facility. As with any relatively new initiative, the FTC may be unfamiliar with the feasibility of a TJP. It is important to work closely with the FTC to ensure all necessary steps are taken, consistent with national policy regarding telehealth in non-VA settings. For most, the national CVT-IH platform will be the logical approach to establishing a TJP. In some instances, involvement with either the VISN telehealth program manager and/or the VISN behavioral health director may be recommended in addition to the VJP specialist’s supervisor.
In general, the FTC will assist with all required documentation, establishment of a clinic, and ongoing technical support. The FTC may also provide needed guidance with logistics around technical specifications at the distant site.
Conduct a site visit. Meeting with administrative and technology decision makers at the site is an important part of the process and is an opportunity to alleviate any apprehension. They may want to hear more about how telehealth is used at the VA, telehealth research in general, and/or other active TJPs.
Identify a suitable space. There will be a variety of appointments that may be considered for the TJP. If the appointment is an encounter between the provider and veteran, it is recommended to find a space at the detention facility that is as private as possible. The law library, which has windows and a cage, was the designated space for the Portland pilot. The desktop computer and webcam were situated on the outside of the cage. The veteran entered the cage with the correctional officer, who established the Internet connection (Figure).
Identify a point of contact and staff at the distant site. As feasibility is evaluated, identifying a point of contact at the distant site is vital. During the site visit, it is important to meet with the point of contact to review any ongoing logistic issues. One or more staff members should be available to escort the veteran into the space where the telehealth appointment will occur. In some cases, a correctional officer will be on standby during the appointment to address any technical issues with the VA provider and/or in case of a medical or behavioral emergency. In most if not all cases, it is important for the staff member at the distant site to have telephone contact information for the VA provider and/or their respective technical support contact person.
Evaluate technology. Does the distant site facility have Internet access for the space under consideration? If it does, it is likely the FTC will follow the protocols outlined in the CVT-IH platform. Although Jabber is currently the only nationally accepted video teleconferencing software, VISN 20 has successfully used Vidyo (Hackensack, NJ) and VSee (Sunnyvale, CA) on iPads for VISN 20 mobile telehealth programs and is in the process to deploy alternative software solutions and iPads for all VISN 20 TJPs. If the jail/prison facility is open to discussing using their own videoconferencing technologies to bridge into the VA system, these efforts should be coordinated through the FTC. In some cases, the correctional facility will request a desktop computer or laptop with Internet access. The most common issue that prevents a program from being further developed is the lack of viable technology.
VA facility preparation. After the site visit is complete, the FTC should assist with the planning process. This will include identifying a telehealth clinical technician on the VA side and development of appropriate documentation and emergency management protocols. The planning package will also include a MOU between the local VA program leadership representatives and the institution/justice entity where the veteran is being served.
Emergency management protocols must include, at a minimum, a point of contact at the distant site and a contingency point of contact. Phone numbers for each should be acquired well in advance. At the beginning of each session, the provider should have access to those names and numbers in case an emergency arises during the session. At the same time, the VA provider should communicate the emergency protocols to the veteran receiving the services. In the event of an emergency, the provider should do whatever possible to remain connected via video with the veteran and call the distant site point of contact to assist with the emergency. Importantly, participants should follow emergency protocols as outlined by the correctional facility. Readers may contact the author at
[email protected] for a copy of the Portland pilot emergency protocol.
A telehealth clinic will need to be built to capture workload. In most cases, this will be a CVT-IH clinic at the facility. Typically, the FTC will initiate the process with the facility clinical applications coordinator to establish the appropriate clinic build. As the program begins to take shape, an operations manual or practice guidelines will need to be created and updated regularly.
Implementation
After the setup documentation has been completed and before the first appointment, the technology support person and the distant site point of contact should be contacted to confirm the appointment and assist in establishing the Internet connection. (An implementation checklist is available at http://wp.me/p6jTLD-5.)
As the TJP grows, it will be important to evaluate and test the technology, technical support, staffing, and modifications to local protocols to ensure the safety and welfare of the veteran and the provider. Also consider whether the program will collect data and if so, what type.
The Portland pilot collected a variety of data sets, including provider and veteran perspectives on their experiences with the technology. The VHA Innovation 669 program used a brief technology impact questionnaire, designed to monitor how technology has impacted quality of care.5 Data was collected iteratively and used in part to improve aspects of the program. This included both veteran and provider satisfaction, clinical outcomes, quality of life, and levels of occupational and social functioning.
Conclusion
Reaching incarcerated veterans sends an important message: The VA will go to great lengths to ensure that veterans have access to services to help ease the transition back into the community. Connecting with incarcerated veterans via telehealth takes the VA mission to a new level.
Given the wide array of technical solutions currently being used in correctional facilities, VA TJPs may benefit from exploring a consumer-based technology solution. However, one factor in expanding VA TJPs is that current telehealth systems rely on VA Office of Information and Technology resources that build systems within the VA network. There are privacy and security standards the VA adheres to in order to maintain a safe clinical video connection.
There are private sector companies that offer Internet-based access to secure video to connect physicians and mental health professionals. Given the significant variability in IT across correctional facilities, VA TJPs could expand their access into correctional facilities by deploying a web-based clinical video solution, similar to those currently available in the private sector. The technology already exists, and although taking this approach would need to be thoroughly vetted, it could significantly expand VA TJPs and eliminate several obstacles outlined in
this article.
Acknowledgments
Joel Rosenthal, PhD, national training director for Veterans Justice Outreach programs, has provided enduring support to VJPs. To Linda Maddy, the Portland VJO specialist—Being the first to do anything in the VA takes courage and tenacity. To Mark Correale, the NJ VJO specialist, for his tireless efforts. A special thanks to the VHA Innovation 669 team: Chuck Brown, Kit Teague, Trevor Davis, Sean O’Connor, Tracy Dekelboum, and William “Bear” Cannon for providing ongoing support to the Portland telejustice providers and distant jail staff. Mary Lu is providing editorial and research support on this and other telehealth-related projects. This article is dedicated to justice-involved men and women who have served our nation and to those VA employees who dare to be disruptive in order to reach them.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. McGuire J, Clark S. Veterans Justice Outreach Initiative (VJO). Washington, DC: Veterans Health Administration; 2009.
2. U.S. Department of Veterans Affairs. Veterans Health Administration Fact Sheet. VA Services for Veterans Involved in the Justice System: VA’s Veterans Justice Outreach Program. Washington, DC: Veterans Health Administration; 2011.
3. Veterans Health Administration Support Service Center. Homeless Services Briefing Book. Veterans Affairs Intranet Website. http://vaww.fcdm.med.va.gov/pas/en/src/Proclarity.asp. Accessed June 8, 2015.
4. Veterans Health Administration Support Service Center. Virtual care and telehealth report. https://securereports2.vssc.med.va.gov/Reports/Pages/Folder.aspx?ViewMode=Detail. Accessed June 8, 2015.
5. Shore P, Davis T. Technology Impact Questionnaire (TIQ). http://wp.me/p2toog-1f. Published 2014. Accessed June 8, 2015.
1. McGuire J, Clark S. Veterans Justice Outreach Initiative (VJO). Washington, DC: Veterans Health Administration; 2009.
2. U.S. Department of Veterans Affairs. Veterans Health Administration Fact Sheet. VA Services for Veterans Involved in the Justice System: VA’s Veterans Justice Outreach Program. Washington, DC: Veterans Health Administration; 2011.
3. Veterans Health Administration Support Service Center. Homeless Services Briefing Book. Veterans Affairs Intranet Website. http://vaww.fcdm.med.va.gov/pas/en/src/Proclarity.asp. Accessed June 8, 2015.
4. Veterans Health Administration Support Service Center. Virtual care and telehealth report. https://securereports2.vssc.med.va.gov/Reports/Pages/Folder.aspx?ViewMode=Detail. Accessed June 8, 2015.
5. Shore P, Davis T. Technology Impact Questionnaire (TIQ). http://wp.me/p2toog-1f. Published 2014. Accessed June 8, 2015.
Evaluation of E-Consults in the VHA: Provider Perspectives
Electronic consultations (e-consults), also called e-referrals, are an alternative method of obtaining general patient information through the electronic health record (EHR) shared by primary care providers (PCPs) and specialists in the VHA. In the e-consult system, test results, medication lists, and other pertinent data are available.1 Many PCPs are willing to use new technologies to maximize practice efficiency and patient convenience.2 In the VHA’s hub-and-spoke model of care, e-consults have the potential to make delivery of specialty care more efficient by prearranging or completing necessary diagnostic testing and redirecting inappropriate referrals to the correct specialists.1
Some early studies of e-consults report better communication, improved referral appropriateness, and greater access to specialty care as well as better continuity of care and information transfer between patients and PCPs.3-5 Researchers at the VA Boston Healthcare System in Massachusetts found that 61% of specialists surveyed agreed that e-consults improve quality of care and found the approach beneficial to help initiate diagnostic testing prior to a face-to-face visit.6 However, researchers at the Michael E. DeBakey VAMC in Houston, Texas, found no improvement in care coordination.7 To date, there have been no large-scale evaluations of e-consult programs or assessments of implementation of e-consult programs.
Related: HHS Grants Fund Health IT in Communities
In early 2011, the VHA Office of Specialty Care Services (OSCS), Office of Specialty Care Transformation launched a national e-consult pilot as part of a broader effort to improve the delivery of patient-centered specialty care. This initiative was based on core concepts advanced by the American College of Physicians, which highlighted the importance of specialty care within a patient- centered medical home and provided a framework for collaboration.8,9 The goals of the e-consult program were to improve access to specialty care for veterans and their PCPs, to enhance the collaborative relationship between PCPs and specialists, and to augment PCP education.
The OSCS created an Electronic Consultation Implementation Guide to help sites develop and implement each of their e-consult programs.10 The Implementation Guide established operating rules, strategies for engaging key stakeholders, and recommendations for provider education and training.
As with face-to-face referrals, e-consults are organized in a hub-and-spoke model, where community-based outpatient clinics (CBOCs) are linked to a central VAMC. An e-consult can be accessed by any CBOC, VAMC, medical center-based primary care clinic or specialist, and between medical centers that share the same EHR. There were 217,014 completed e-consults between May 2011 and December 2013 across VHA.11
Some programs created an e-consult template to aid in the transition to electronic referrals (Figure). Although not mandatory, the template helped organize needed information to expedite the e-consult.
The objective of this evaluation is to describe the implementation of e-consults from the perspectives of PCPs, specialists, and other key staff involved in the pilot. Key findings were related to: (1) how the e-consult pilot was implemented; (2) how implementation of the e-consult pilot affected providers; and (3) to what extent the e-consult pilot achieved programmatic objectives from the provider’s perspective.
Methods
The authors conducted a key informant analysis with 2 waves of interviews at 8 e-consult pilot sites across the U.S., selected for variation on early progress in implementation. The sites cannot be identified based on an agreement with the VA Office of Labor-Management Relations.
Setting
The e-consult pilot involved 15 VAMCs in 2 cohorts: alpha sites, which began using e-consults in May 2011, and beta sites, which began using e-consults in July 2011. The alpha sites included 10 VAMCs in 12 medical specialties, with a total of 21 facility-specialty combinations. For the evaluation, sites were defined based on specialty, regardless of location within the same medical center (eg, cardiology and diabetes at the same VAMC would be 2 sites). Beta sites included 5 VAMCs with 6 medical specialties for a total of 6 sites. For 1 year, alpha sites received $175,000 and beta sites received $150,000 to support start-up activities.
Initial specialties included diabetes, hepatitis C, geriatrics, cardiology, liver transplant, dementia, gastrointestinal disease, pulmonary medicine, rheumatology, pain management, neurosurgery, infectious diseases, hematology/oncology, and vascular surgery. Facilities could add additional e-consult specialties but did not receive further funding.
Sample
Study participants were selected from 8 of the 15 pilot sites (geographic site/specialty combinations). Site selection was based on 2 measures of baseline e-consult implementation: (1) overall e-consult implementation rates, measured as the ratio of e-consults to all consults for the specialties of interest; and (2) CBOC participation, measured as the ratio of e-consults for patients from CBOCs vs e-consults for patients from primary care clinics located within the 152 VAMCs. Participation with CBOCs was important for ensuring that implementation factors that influenced uptake of e-consults within tertiary medical centers and between VAMCs and CBOCs could be identified. Two e-consult sites were randomly selected from each of the 4 resulting categories (VAMC high volume, VAMC low volume, CBOC high volume, and CBOC low volume). Volume data of e-consults were obtained from the VA Corporate Data Warehouse and assessed from the beginning of the pilot period to initial site selection, May 2011 to February 2012.
Respondents were identified using a modified snowball sampling process. Snowball sampling is a qualitative sampling technique that identifies study participants, who then identify other potential participants to participate in the study. The researchers started with the local e-consult initiative lead and then contacted the directors of primary care and specialty care services for help identifying PCPs, specialists, and support staff (nurse practitioners, pharmacists, program managers, informatics staff, and medical support personnel) engaged in the initiative. The goal for follow-up interviews was to interview at least 2 of the following respondents at each site: e-consult project manager, PCP, and/or specialist. Due to turnover and changes in clinic roles, some follow-up interviews were conducted with different individuals from the baseline interviews.
Data Collection
Interviews followed semistructured interview guidelines and included open-ended questions designed to elicit rich responses to a variety of aspects related to e-consult implementation, including patient needs, communication, leadership, resources, priorities, knowledge about the program, and unintended consequences. Follow-up interviews addressed how e-consults impacted the quality of specialty care; the impact of e-consults on Patient Aligned Care Teams (PACTs), the VHA patient-centered medical home initiative for primary care; and how e-consults have been used, eg, whether patients were involved in the decision to seek an e-consult.
Two interviewers who had participated in a 1-day, in-person training covering both data collection and analyzing key informant data conducted the 40 to 60 minute telephone interviews. One team member conducted the interview while the other took field notes. Interviews were also recorded. Follow-up probes were used to elicit specific examples and ensure sufficiently rich data. Following each interview, the notetaker reviewed the audio recording and filled in details in the field notes. The interview team debriefed and reviewed the augmented field notes and audio recordings, which became the primary data sources for the study.
Analysis
This was a qualitative descriptive analysis.12 Interview data were analyzed using an iterative, inductive content analysis method using an open coding approach (ie, a priori codes were not defined for this portion of the analysis).13 Two members of the research team used audio recordings and summary transcripts simultaneously to code data. Summary transcripts were compared with the recorded interviews to assure fidelity.
The researchers used Atlas.ti (Berlin, Germany) qualitative data analysis software to organize the coding process. Emergent codes were iteratively added throughout the analysis to reflect quotations that did not adequately fit previously developed codes. Codes were combined weekly to biweekly. After the combinations were completed, the analytics team met to review meanings of codes to ensure consistency of coding and interpretations.
To create categories, broad themes were identified from interview responses and grouped under high- order headings that described distinct aspects of participant experience. The analysis was intentionally kept close to the original data to reflect and describe the participant’s experience as accurately as possible. In support of analytical rigor, members of the multidisciplinary research team, composed of clinicians, implementation scientists, and mixed methodologists, reviewed findings to assess their thoroughness, comprehensiveness, and representativeness across roles and participating sites.14
Results
The e-consult evaluation period was from November 1, 2011, to July 31, 2013. Key conclusions were drawn from both alpha and beta sites (Table). Baseline interviews were conducted with 37 participants at 8 sites from April 10, 2012, to August 6, 2012. Follow-up interviews were conducted with 21 of the 37 participants at the 8 sites. Follow-up interviews with either a PCP or specialist could not be scheduled at 1 site. Follow-up interviews were conducted from April 16, 2013, to June 18, 2013. Open coding continued until saturation (the point at which subsequent data failed to produce new findings).15 This occurred after analysis of 22 baseline interviews (12 PCPs, 6 specialists, 1 pharmacist, and 4 other staff members) and 17 follow-up interviews (10 PCPs, 4 specialists, 1 pharmacist, and 2 other staff members).
Implementation
The e-consults provided a programmatic structure to the more informal practice of obtaining diagnostic or therapeutic advice from a specialist. Several of the specialists interviewed described having previously used existing informal consult processes that were “like e-consult.” These specialists reported that their practice patterns did not change significantly since implementing e-consults, because they have been “using the Computerized Patient Record System (CPRS) in an e-consult way for many years.” In these cases, the primary change resulting from the initiative was that national VHA workload policy was revised so that e-consults were assigned a CPT (Current Procedural Terminology) code and specialists began receiving workload credit for completing e-consults.
At sites where an informal e-consult practice was already in place, the initiative was consistently described as flexible. Many specialists reported that this degree of flexibility allowed them to make a relatively easy transition to e-consults by adopting new mechanisms to support existing processes. The e-consult initiative also allowed specialists to formally document this work and to increase the efficiency of specialty care.
Specialists drove the implementation process across sites. The e-consults were envisioned as a collaborative process; however, during initial interviews, few specialists mentioned PCPs when describing the development and implementation of the e-consult program. Primary care providers also reported having little awareness of or input into how the initiative was implemented, although this had little consequence on the use of e-consults.
In a rare case, a PCP reported that poorly designed, lengthy e-consult templates were a major barrier to using e-consults for specific specialties. The PCP said, “E-consults have created an elaborate but extraordinarily cumbersome tool that is difficult for PCPs to actually accomplish, because you have a consult menu that requires a lot of data to be entered—a lot of history from the chart, a lot of exam findings, a lot of previous cognitive testing scores; neurologic findings—lab and imaging tests.”
Still, many other PCPs described receiving detailed information and guidance from e-consults. “E-consults help me to be more accurate. Many providers don’t have a comfort with pain management. To get guidance and education and to really hold our hand, this is how to do this…this has been a big change. If they give you a great response, then [for] the next patient [with that condition], you go back to that note and then follow what was said there,” said one PCP.
In follow-up interviews, providers and other key staff stated there were more data available on the patient as a result of the e-consult and, consequently, even when specialists determined that a patient needed an in-person visit, the data obtained in the e-consult improved the quality of the in-person consultation.
Enhanced Communication and Collaboration
Neither the PCPs nor the specialists were aware of the collaborative intent of the initiative. They focused, instead, on other key aims, such as increasing accessibility and minimizing unnecessary patient travel. Most participants were generally positive about e-consults during baseline interviews, and this perception increased over time.
Both the PCPs and the specialists reported improved communication following the launch of e-consults. In follow-up interviews, some PCPs reported that before e-consults, they had trouble getting timely responses from specialists unless they knew them personally. “You had to know the person in the old days,” one respondent said. “After e-consults, responses improved…e-consult is available to have the resources to tap that knowledge base, and the team is answering the question. I think it opens up access and information and knowledge to everybody.”
Many PCPs spoke positively about this new communication tool as an opportunity to learn from specialists and said they valued the input they received. They felt the increased interaction between the 2 groups positively benefited patient care. One example cited that collaborative communication improved care coordination for veterans: “We are able to step in with e-consults to coordinate services, and this has been huge in improving care.”
Furthermore, follow-up interviews found that all participating PCPs and specialists were communicating more frequently and effectively. “Services that have embraced e-consult give a lot of great information flowing back; it’s closer to a real-time conversation,” said one respondent.
Related: Home-Based Video Telehealth for Veterans With Dementia
In baseline interviews, some specialists described how e-consults went against their belief that patient care is synonymous with face-to-face medical treatment and voiced dissatisfaction with e-consults as “sitting in front of a computer” rather than “seeing patients.” Others were concerned that medical center administration would not recognize the time it takes to conduct an e-consult and therefore not add necessary specialists staff. “E-consults take work and time, just like seeing a patient. I worry that won’t be seen,” one specialist said.
In order to successfully implement the e-consult initiative, providers and staff needed to incorporate new processes into their daily workflow.
Most sites did not develop a mechanism in which specialists received feedback regarding the outcome of their consultations. This lack of response created anxiety for some specialists in the absence of the face-to-face encounter, leaving some wondering whether they or the PCP had missed anything. According to one specialist, “That’s always in the back of your head: ‘Have I [the specialist] missed something?’”
In follow-up interviews, none of these concerns were raised. Primary care providers tended to speak of the care provided by specialists through e-consults in very positive terms, except in those instances where PCPs felt the e-consult template was difficult to use and required too much time to complete. “I was worried in the beginning about patients thinking less of me, but we ask for help all the time. We’re asking for help and not inconveniencing the patient; they seem to like it very much,” one PCP said.
The e-consults also complement PACTs. Initially, a few participants described soliciting patient input regarding the choice to have an e-consult or a face-to-face visit. During follow-up interviews, participants highlighted how well e-consults fit in to the PACT philosophy. One participant said, “The PACT team seeks to improve quality of care. E-consult fits very well with this, because answers to questions can come quickly, and the veteran may not need to come back to the clinic to be seen, even though things are still getting accomplished. E-consult works very well. E-consults were credited with improving access to specialty care as a tool for PACT.”
Achieving Program Objectives
Based on interviews, support for the e-consult program has increased over time as providers have gained experience with the program and have seen its benefits. Respondents at all sites consistently supported the concept of e-consults and expressed their belief in the importance and value of e-consults in improving patient-c entered care, primarily by reducing the need for patients to travel to see specialists, reducing the time to obtain feedback from specialists, and maintaining the provision of high quality care.
“Last year we only had 2 clinics categorized as e-consults. As of now we have 14 e-consults available for our providers. I think the numbers are growing. They are realizing the value of e-consults as far as the provider’s needs being met,” said one respondent.
The e-consults were credited with improving access to specialty care for veterans. Several participants stated that e-consults improved access to specialty care services and decreased travel for veterans. “It’s another way of getting care to the patient when the patient needs it without having to wait,” said one respondent.
Many PCPs described how difficult it was for patients to get to specialty appointments—particularly for their elderly, disabled, and rural patients—before the implementation of e-consults. “I like the fact that patients who live very far don’t have to come back. A lot of our patients are older…diabetic, see me Monday and back on Thursday. Now, they are able to stay home and follow the recommendations I write,” said one PCP.
Most providers were of the opinion that patients liked the program. “I think e-consults are helping patients...It’s been very successful regarding decreasing travel…Quicker response time for specialty care,” said a PCP. Several providers also stated in follow-up interviews that there was a greater degree of patient participation in the e-consult process and that “patients are definitely informed.”
Discussion
Most PCPs reported that the e-consults were an effective means of consultation and contained the information they needed to provide high-quality coordinated care. Most also found e-consult templates easy to complete. A majority of PCPs felt sufficient control over the choice of whether to use e-consults or an in-person visit, and a minority of patients were involved in the decision to receive an e-consult. Although the OSCS outlined guiding principles and operational rules in the Implementation Guide to help sites implement the e-consult program, its contribution was limited. Few examples were found that engaged PCPs in development of the e-consult program locally; involving patients in the decision to obtain a specialty consult electronically or in person; and PCPs feeding back results to specialists.
Implementing e-consults posed a number of challenges, including lack of resources to respond to referral requests, lack of referral policies and standardized procedures, and confusion related to roles and responsibilities. This is consistent with findings from another VHA research project of e-consults in 2 VHA health systems that was conducted prior to this national level e-consult pilot.7
Related: Using Facilitative Coaching to Support Patient Aligned Care Teams
Communication by OSCS of key aspects of the e-consult initiative will be critical as more sites implement e-consults. Since initiation of this pilot, workload specifications and credit have changed from 1 code to 3 codes, to more accurately reflect the amount of time a specialist consultant spends reviewing the EHR and responding to the consult. Without seeing the patient directly, specialists are more reliant on the PCP to describe the problem and provide adequate information in the e-consult request in order to provide recommendations back to the PCP.
Primary care physicians need to know that e-consults are available and determine when they are appropriate. A template or other guidance may be helpful to ensure adequate information is provided in the e-consult request; and the information provided by the specialist in response to the e-consult has to be sufficient for the PCP to provide care. VHA continues to expand the use of e-consults throughout the system, as this pilot found that the electronic option was often more timely than were face-to-face consultations. The result of this evaluation has informed national implementation of this effort.
Limitations
There are 3 main limitations to this study. First, because there was no practical way to preidentify participants who participated in implementing e-consults, a modified snowball sampling was used. However, this limited the degree to which the group was representative of the pilot participants. Second, the authors reported findings from a real-world initiative, not an experimental study. As such, not all participants in the first wave of key informant interviews were available for follow-up interview, which may have introduced bias. Third, the VHA is unlike most of the rest of the U.S. health care system in that it is a fully integrated system with salaried PCPs and specialists and an EHR.
Generalizability of the study may be limited, as a modified snowball sampling approach is not entirely random and has potential for community bias, because initial participants influence subsequent sampling. Additionally, though the sample size (n = 37) was sufficient for qualitative, in-depth analysis, it may be too small for confident generalization of findings. However, as health care moves toward an accountable care organization system, the authors’ analysis may provide insights.
Issues include revision of reimbursement policy for e-consults and developing or coordinating informational technology infrastructures to permit e-consults. It is also important to note that this evaluation reports solely on the extent of implementation of e-consults and the effects of e-consult implementation from the perspectives of staff, including specialists and PCPs.
Evaluating the effectiveness of the program in improving access, care coordination, and patient satisfaction was beyond the scope of the study. Further research is needed, because findings on those outcomes are critical for drawing inferences about this study’s implementation results.
Conclusion
The assessment of the e-consult system by providers and staff was based on a perception that e-consults are a valuable tool in providing greater access to quality care. Currently, e-consults have been expanded across VHA in medical and surgical specialties. VHA policymakers have drafted field guidance and a communication plan to support these efforts.
Acknowledgement
This material is based on work supported by the VA Office of Specialty Care Transformation, the office overseeing the e-consult initiative, and the Office of Research and Development Quality Enhancement Research Initiative.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453.
2. Hanna L, May C, Fairhurst K. The place of information and communication technology-mediated consultations in primary care: GPs’ perspectives. Fam Pract. 2012;29(3):361-366.
3. Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123-1128.
4. Straus SG, Chen AH, Yee HF Jr, Kushel MB, Bell DS. Implementation of an electronic referral system for outpatient specialty care. AMIA Annu Symp Proc. 2011;2011:1337-1346.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014;31(5):26-31.
7. Hysong SJ, Esquivel A, Sittig DF, et al. Towards successful coordination of electronic health record based-referrals: a qualitative analysis. Implement Sci. 2011;6:84.
8. American College of Physicians. The Patient- Centered Medical Home Neighbor: The Interface of the Patient-Centered Medical Home with Specialty/Subspecialty Practices. Philadelphia, PA: American College of Physicians; 2010. Policy paper.
9. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12): 1202-1205.
10. Department of Veterans Affairs. Electronic Consultation (E-Consult) Implementation Guide, Version 1.2. Washington, DC: Department of Veterans Affairs, Office of Specialty Care Services, Specialty Care Transformation. 2013.
11. Kirsh S, Cary E, Aron DC et al. Results of a national pilot project for specialty care e-consultation in primary care medical homes: the impact of specialty e-consultation on access. Am J Manag Care. In press.
12. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
13. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
14. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000;284(3):357-362.
15. Sandelowski M. The problem of rigor in qualitative research. ANS Adv Nurs Sci. 1986;8(3):27-37.
Electronic consultations (e-consults), also called e-referrals, are an alternative method of obtaining general patient information through the electronic health record (EHR) shared by primary care providers (PCPs) and specialists in the VHA. In the e-consult system, test results, medication lists, and other pertinent data are available.1 Many PCPs are willing to use new technologies to maximize practice efficiency and patient convenience.2 In the VHA’s hub-and-spoke model of care, e-consults have the potential to make delivery of specialty care more efficient by prearranging or completing necessary diagnostic testing and redirecting inappropriate referrals to the correct specialists.1
Some early studies of e-consults report better communication, improved referral appropriateness, and greater access to specialty care as well as better continuity of care and information transfer between patients and PCPs.3-5 Researchers at the VA Boston Healthcare System in Massachusetts found that 61% of specialists surveyed agreed that e-consults improve quality of care and found the approach beneficial to help initiate diagnostic testing prior to a face-to-face visit.6 However, researchers at the Michael E. DeBakey VAMC in Houston, Texas, found no improvement in care coordination.7 To date, there have been no large-scale evaluations of e-consult programs or assessments of implementation of e-consult programs.
Related: HHS Grants Fund Health IT in Communities
In early 2011, the VHA Office of Specialty Care Services (OSCS), Office of Specialty Care Transformation launched a national e-consult pilot as part of a broader effort to improve the delivery of patient-centered specialty care. This initiative was based on core concepts advanced by the American College of Physicians, which highlighted the importance of specialty care within a patient- centered medical home and provided a framework for collaboration.8,9 The goals of the e-consult program were to improve access to specialty care for veterans and their PCPs, to enhance the collaborative relationship between PCPs and specialists, and to augment PCP education.
The OSCS created an Electronic Consultation Implementation Guide to help sites develop and implement each of their e-consult programs.10 The Implementation Guide established operating rules, strategies for engaging key stakeholders, and recommendations for provider education and training.
As with face-to-face referrals, e-consults are organized in a hub-and-spoke model, where community-based outpatient clinics (CBOCs) are linked to a central VAMC. An e-consult can be accessed by any CBOC, VAMC, medical center-based primary care clinic or specialist, and between medical centers that share the same EHR. There were 217,014 completed e-consults between May 2011 and December 2013 across VHA.11
Some programs created an e-consult template to aid in the transition to electronic referrals (Figure). Although not mandatory, the template helped organize needed information to expedite the e-consult.
The objective of this evaluation is to describe the implementation of e-consults from the perspectives of PCPs, specialists, and other key staff involved in the pilot. Key findings were related to: (1) how the e-consult pilot was implemented; (2) how implementation of the e-consult pilot affected providers; and (3) to what extent the e-consult pilot achieved programmatic objectives from the provider’s perspective.
Methods
The authors conducted a key informant analysis with 2 waves of interviews at 8 e-consult pilot sites across the U.S., selected for variation on early progress in implementation. The sites cannot be identified based on an agreement with the VA Office of Labor-Management Relations.
Setting
The e-consult pilot involved 15 VAMCs in 2 cohorts: alpha sites, which began using e-consults in May 2011, and beta sites, which began using e-consults in July 2011. The alpha sites included 10 VAMCs in 12 medical specialties, with a total of 21 facility-specialty combinations. For the evaluation, sites were defined based on specialty, regardless of location within the same medical center (eg, cardiology and diabetes at the same VAMC would be 2 sites). Beta sites included 5 VAMCs with 6 medical specialties for a total of 6 sites. For 1 year, alpha sites received $175,000 and beta sites received $150,000 to support start-up activities.
Initial specialties included diabetes, hepatitis C, geriatrics, cardiology, liver transplant, dementia, gastrointestinal disease, pulmonary medicine, rheumatology, pain management, neurosurgery, infectious diseases, hematology/oncology, and vascular surgery. Facilities could add additional e-consult specialties but did not receive further funding.
Sample
Study participants were selected from 8 of the 15 pilot sites (geographic site/specialty combinations). Site selection was based on 2 measures of baseline e-consult implementation: (1) overall e-consult implementation rates, measured as the ratio of e-consults to all consults for the specialties of interest; and (2) CBOC participation, measured as the ratio of e-consults for patients from CBOCs vs e-consults for patients from primary care clinics located within the 152 VAMCs. Participation with CBOCs was important for ensuring that implementation factors that influenced uptake of e-consults within tertiary medical centers and between VAMCs and CBOCs could be identified. Two e-consult sites were randomly selected from each of the 4 resulting categories (VAMC high volume, VAMC low volume, CBOC high volume, and CBOC low volume). Volume data of e-consults were obtained from the VA Corporate Data Warehouse and assessed from the beginning of the pilot period to initial site selection, May 2011 to February 2012.
Respondents were identified using a modified snowball sampling process. Snowball sampling is a qualitative sampling technique that identifies study participants, who then identify other potential participants to participate in the study. The researchers started with the local e-consult initiative lead and then contacted the directors of primary care and specialty care services for help identifying PCPs, specialists, and support staff (nurse practitioners, pharmacists, program managers, informatics staff, and medical support personnel) engaged in the initiative. The goal for follow-up interviews was to interview at least 2 of the following respondents at each site: e-consult project manager, PCP, and/or specialist. Due to turnover and changes in clinic roles, some follow-up interviews were conducted with different individuals from the baseline interviews.
Data Collection
Interviews followed semistructured interview guidelines and included open-ended questions designed to elicit rich responses to a variety of aspects related to e-consult implementation, including patient needs, communication, leadership, resources, priorities, knowledge about the program, and unintended consequences. Follow-up interviews addressed how e-consults impacted the quality of specialty care; the impact of e-consults on Patient Aligned Care Teams (PACTs), the VHA patient-centered medical home initiative for primary care; and how e-consults have been used, eg, whether patients were involved in the decision to seek an e-consult.
Two interviewers who had participated in a 1-day, in-person training covering both data collection and analyzing key informant data conducted the 40 to 60 minute telephone interviews. One team member conducted the interview while the other took field notes. Interviews were also recorded. Follow-up probes were used to elicit specific examples and ensure sufficiently rich data. Following each interview, the notetaker reviewed the audio recording and filled in details in the field notes. The interview team debriefed and reviewed the augmented field notes and audio recordings, which became the primary data sources for the study.
Analysis
This was a qualitative descriptive analysis.12 Interview data were analyzed using an iterative, inductive content analysis method using an open coding approach (ie, a priori codes were not defined for this portion of the analysis).13 Two members of the research team used audio recordings and summary transcripts simultaneously to code data. Summary transcripts were compared with the recorded interviews to assure fidelity.
The researchers used Atlas.ti (Berlin, Germany) qualitative data analysis software to organize the coding process. Emergent codes were iteratively added throughout the analysis to reflect quotations that did not adequately fit previously developed codes. Codes were combined weekly to biweekly. After the combinations were completed, the analytics team met to review meanings of codes to ensure consistency of coding and interpretations.
To create categories, broad themes were identified from interview responses and grouped under high- order headings that described distinct aspects of participant experience. The analysis was intentionally kept close to the original data to reflect and describe the participant’s experience as accurately as possible. In support of analytical rigor, members of the multidisciplinary research team, composed of clinicians, implementation scientists, and mixed methodologists, reviewed findings to assess their thoroughness, comprehensiveness, and representativeness across roles and participating sites.14
Results
The e-consult evaluation period was from November 1, 2011, to July 31, 2013. Key conclusions were drawn from both alpha and beta sites (Table). Baseline interviews were conducted with 37 participants at 8 sites from April 10, 2012, to August 6, 2012. Follow-up interviews were conducted with 21 of the 37 participants at the 8 sites. Follow-up interviews with either a PCP or specialist could not be scheduled at 1 site. Follow-up interviews were conducted from April 16, 2013, to June 18, 2013. Open coding continued until saturation (the point at which subsequent data failed to produce new findings).15 This occurred after analysis of 22 baseline interviews (12 PCPs, 6 specialists, 1 pharmacist, and 4 other staff members) and 17 follow-up interviews (10 PCPs, 4 specialists, 1 pharmacist, and 2 other staff members).
Implementation
The e-consults provided a programmatic structure to the more informal practice of obtaining diagnostic or therapeutic advice from a specialist. Several of the specialists interviewed described having previously used existing informal consult processes that were “like e-consult.” These specialists reported that their practice patterns did not change significantly since implementing e-consults, because they have been “using the Computerized Patient Record System (CPRS) in an e-consult way for many years.” In these cases, the primary change resulting from the initiative was that national VHA workload policy was revised so that e-consults were assigned a CPT (Current Procedural Terminology) code and specialists began receiving workload credit for completing e-consults.
At sites where an informal e-consult practice was already in place, the initiative was consistently described as flexible. Many specialists reported that this degree of flexibility allowed them to make a relatively easy transition to e-consults by adopting new mechanisms to support existing processes. The e-consult initiative also allowed specialists to formally document this work and to increase the efficiency of specialty care.
Specialists drove the implementation process across sites. The e-consults were envisioned as a collaborative process; however, during initial interviews, few specialists mentioned PCPs when describing the development and implementation of the e-consult program. Primary care providers also reported having little awareness of or input into how the initiative was implemented, although this had little consequence on the use of e-consults.
In a rare case, a PCP reported that poorly designed, lengthy e-consult templates were a major barrier to using e-consults for specific specialties. The PCP said, “E-consults have created an elaborate but extraordinarily cumbersome tool that is difficult for PCPs to actually accomplish, because you have a consult menu that requires a lot of data to be entered—a lot of history from the chart, a lot of exam findings, a lot of previous cognitive testing scores; neurologic findings—lab and imaging tests.”
Still, many other PCPs described receiving detailed information and guidance from e-consults. “E-consults help me to be more accurate. Many providers don’t have a comfort with pain management. To get guidance and education and to really hold our hand, this is how to do this…this has been a big change. If they give you a great response, then [for] the next patient [with that condition], you go back to that note and then follow what was said there,” said one PCP.
In follow-up interviews, providers and other key staff stated there were more data available on the patient as a result of the e-consult and, consequently, even when specialists determined that a patient needed an in-person visit, the data obtained in the e-consult improved the quality of the in-person consultation.
Enhanced Communication and Collaboration
Neither the PCPs nor the specialists were aware of the collaborative intent of the initiative. They focused, instead, on other key aims, such as increasing accessibility and minimizing unnecessary patient travel. Most participants were generally positive about e-consults during baseline interviews, and this perception increased over time.
Both the PCPs and the specialists reported improved communication following the launch of e-consults. In follow-up interviews, some PCPs reported that before e-consults, they had trouble getting timely responses from specialists unless they knew them personally. “You had to know the person in the old days,” one respondent said. “After e-consults, responses improved…e-consult is available to have the resources to tap that knowledge base, and the team is answering the question. I think it opens up access and information and knowledge to everybody.”
Many PCPs spoke positively about this new communication tool as an opportunity to learn from specialists and said they valued the input they received. They felt the increased interaction between the 2 groups positively benefited patient care. One example cited that collaborative communication improved care coordination for veterans: “We are able to step in with e-consults to coordinate services, and this has been huge in improving care.”
Furthermore, follow-up interviews found that all participating PCPs and specialists were communicating more frequently and effectively. “Services that have embraced e-consult give a lot of great information flowing back; it’s closer to a real-time conversation,” said one respondent.
Related: Home-Based Video Telehealth for Veterans With Dementia
In baseline interviews, some specialists described how e-consults went against their belief that patient care is synonymous with face-to-face medical treatment and voiced dissatisfaction with e-consults as “sitting in front of a computer” rather than “seeing patients.” Others were concerned that medical center administration would not recognize the time it takes to conduct an e-consult and therefore not add necessary specialists staff. “E-consults take work and time, just like seeing a patient. I worry that won’t be seen,” one specialist said.
In order to successfully implement the e-consult initiative, providers and staff needed to incorporate new processes into their daily workflow.
Most sites did not develop a mechanism in which specialists received feedback regarding the outcome of their consultations. This lack of response created anxiety for some specialists in the absence of the face-to-face encounter, leaving some wondering whether they or the PCP had missed anything. According to one specialist, “That’s always in the back of your head: ‘Have I [the specialist] missed something?’”
In follow-up interviews, none of these concerns were raised. Primary care providers tended to speak of the care provided by specialists through e-consults in very positive terms, except in those instances where PCPs felt the e-consult template was difficult to use and required too much time to complete. “I was worried in the beginning about patients thinking less of me, but we ask for help all the time. We’re asking for help and not inconveniencing the patient; they seem to like it very much,” one PCP said.
The e-consults also complement PACTs. Initially, a few participants described soliciting patient input regarding the choice to have an e-consult or a face-to-face visit. During follow-up interviews, participants highlighted how well e-consults fit in to the PACT philosophy. One participant said, “The PACT team seeks to improve quality of care. E-consult fits very well with this, because answers to questions can come quickly, and the veteran may not need to come back to the clinic to be seen, even though things are still getting accomplished. E-consult works very well. E-consults were credited with improving access to specialty care as a tool for PACT.”
Achieving Program Objectives
Based on interviews, support for the e-consult program has increased over time as providers have gained experience with the program and have seen its benefits. Respondents at all sites consistently supported the concept of e-consults and expressed their belief in the importance and value of e-consults in improving patient-c entered care, primarily by reducing the need for patients to travel to see specialists, reducing the time to obtain feedback from specialists, and maintaining the provision of high quality care.
“Last year we only had 2 clinics categorized as e-consults. As of now we have 14 e-consults available for our providers. I think the numbers are growing. They are realizing the value of e-consults as far as the provider’s needs being met,” said one respondent.
The e-consults were credited with improving access to specialty care for veterans. Several participants stated that e-consults improved access to specialty care services and decreased travel for veterans. “It’s another way of getting care to the patient when the patient needs it without having to wait,” said one respondent.
Many PCPs described how difficult it was for patients to get to specialty appointments—particularly for their elderly, disabled, and rural patients—before the implementation of e-consults. “I like the fact that patients who live very far don’t have to come back. A lot of our patients are older…diabetic, see me Monday and back on Thursday. Now, they are able to stay home and follow the recommendations I write,” said one PCP.
Most providers were of the opinion that patients liked the program. “I think e-consults are helping patients...It’s been very successful regarding decreasing travel…Quicker response time for specialty care,” said a PCP. Several providers also stated in follow-up interviews that there was a greater degree of patient participation in the e-consult process and that “patients are definitely informed.”
Discussion
Most PCPs reported that the e-consults were an effective means of consultation and contained the information they needed to provide high-quality coordinated care. Most also found e-consult templates easy to complete. A majority of PCPs felt sufficient control over the choice of whether to use e-consults or an in-person visit, and a minority of patients were involved in the decision to receive an e-consult. Although the OSCS outlined guiding principles and operational rules in the Implementation Guide to help sites implement the e-consult program, its contribution was limited. Few examples were found that engaged PCPs in development of the e-consult program locally; involving patients in the decision to obtain a specialty consult electronically or in person; and PCPs feeding back results to specialists.
Implementing e-consults posed a number of challenges, including lack of resources to respond to referral requests, lack of referral policies and standardized procedures, and confusion related to roles and responsibilities. This is consistent with findings from another VHA research project of e-consults in 2 VHA health systems that was conducted prior to this national level e-consult pilot.7
Related: Using Facilitative Coaching to Support Patient Aligned Care Teams
Communication by OSCS of key aspects of the e-consult initiative will be critical as more sites implement e-consults. Since initiation of this pilot, workload specifications and credit have changed from 1 code to 3 codes, to more accurately reflect the amount of time a specialist consultant spends reviewing the EHR and responding to the consult. Without seeing the patient directly, specialists are more reliant on the PCP to describe the problem and provide adequate information in the e-consult request in order to provide recommendations back to the PCP.
Primary care physicians need to know that e-consults are available and determine when they are appropriate. A template or other guidance may be helpful to ensure adequate information is provided in the e-consult request; and the information provided by the specialist in response to the e-consult has to be sufficient for the PCP to provide care. VHA continues to expand the use of e-consults throughout the system, as this pilot found that the electronic option was often more timely than were face-to-face consultations. The result of this evaluation has informed national implementation of this effort.
Limitations
There are 3 main limitations to this study. First, because there was no practical way to preidentify participants who participated in implementing e-consults, a modified snowball sampling was used. However, this limited the degree to which the group was representative of the pilot participants. Second, the authors reported findings from a real-world initiative, not an experimental study. As such, not all participants in the first wave of key informant interviews were available for follow-up interview, which may have introduced bias. Third, the VHA is unlike most of the rest of the U.S. health care system in that it is a fully integrated system with salaried PCPs and specialists and an EHR.
Generalizability of the study may be limited, as a modified snowball sampling approach is not entirely random and has potential for community bias, because initial participants influence subsequent sampling. Additionally, though the sample size (n = 37) was sufficient for qualitative, in-depth analysis, it may be too small for confident generalization of findings. However, as health care moves toward an accountable care organization system, the authors’ analysis may provide insights.
Issues include revision of reimbursement policy for e-consults and developing or coordinating informational technology infrastructures to permit e-consults. It is also important to note that this evaluation reports solely on the extent of implementation of e-consults and the effects of e-consult implementation from the perspectives of staff, including specialists and PCPs.
Evaluating the effectiveness of the program in improving access, care coordination, and patient satisfaction was beyond the scope of the study. Further research is needed, because findings on those outcomes are critical for drawing inferences about this study’s implementation results.
Conclusion
The assessment of the e-consult system by providers and staff was based on a perception that e-consults are a valuable tool in providing greater access to quality care. Currently, e-consults have been expanded across VHA in medical and surgical specialties. VHA policymakers have drafted field guidance and a communication plan to support these efforts.
Acknowledgement
This material is based on work supported by the VA Office of Specialty Care Transformation, the office overseeing the e-consult initiative, and the Office of Research and Development Quality Enhancement Research Initiative.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Electronic consultations (e-consults), also called e-referrals, are an alternative method of obtaining general patient information through the electronic health record (EHR) shared by primary care providers (PCPs) and specialists in the VHA. In the e-consult system, test results, medication lists, and other pertinent data are available.1 Many PCPs are willing to use new technologies to maximize practice efficiency and patient convenience.2 In the VHA’s hub-and-spoke model of care, e-consults have the potential to make delivery of specialty care more efficient by prearranging or completing necessary diagnostic testing and redirecting inappropriate referrals to the correct specialists.1
Some early studies of e-consults report better communication, improved referral appropriateness, and greater access to specialty care as well as better continuity of care and information transfer between patients and PCPs.3-5 Researchers at the VA Boston Healthcare System in Massachusetts found that 61% of specialists surveyed agreed that e-consults improve quality of care and found the approach beneficial to help initiate diagnostic testing prior to a face-to-face visit.6 However, researchers at the Michael E. DeBakey VAMC in Houston, Texas, found no improvement in care coordination.7 To date, there have been no large-scale evaluations of e-consult programs or assessments of implementation of e-consult programs.
Related: HHS Grants Fund Health IT in Communities
In early 2011, the VHA Office of Specialty Care Services (OSCS), Office of Specialty Care Transformation launched a national e-consult pilot as part of a broader effort to improve the delivery of patient-centered specialty care. This initiative was based on core concepts advanced by the American College of Physicians, which highlighted the importance of specialty care within a patient- centered medical home and provided a framework for collaboration.8,9 The goals of the e-consult program were to improve access to specialty care for veterans and their PCPs, to enhance the collaborative relationship between PCPs and specialists, and to augment PCP education.
The OSCS created an Electronic Consultation Implementation Guide to help sites develop and implement each of their e-consult programs.10 The Implementation Guide established operating rules, strategies for engaging key stakeholders, and recommendations for provider education and training.
As with face-to-face referrals, e-consults are organized in a hub-and-spoke model, where community-based outpatient clinics (CBOCs) are linked to a central VAMC. An e-consult can be accessed by any CBOC, VAMC, medical center-based primary care clinic or specialist, and between medical centers that share the same EHR. There were 217,014 completed e-consults between May 2011 and December 2013 across VHA.11
Some programs created an e-consult template to aid in the transition to electronic referrals (Figure). Although not mandatory, the template helped organize needed information to expedite the e-consult.
The objective of this evaluation is to describe the implementation of e-consults from the perspectives of PCPs, specialists, and other key staff involved in the pilot. Key findings were related to: (1) how the e-consult pilot was implemented; (2) how implementation of the e-consult pilot affected providers; and (3) to what extent the e-consult pilot achieved programmatic objectives from the provider’s perspective.
Methods
The authors conducted a key informant analysis with 2 waves of interviews at 8 e-consult pilot sites across the U.S., selected for variation on early progress in implementation. The sites cannot be identified based on an agreement with the VA Office of Labor-Management Relations.
Setting
The e-consult pilot involved 15 VAMCs in 2 cohorts: alpha sites, which began using e-consults in May 2011, and beta sites, which began using e-consults in July 2011. The alpha sites included 10 VAMCs in 12 medical specialties, with a total of 21 facility-specialty combinations. For the evaluation, sites were defined based on specialty, regardless of location within the same medical center (eg, cardiology and diabetes at the same VAMC would be 2 sites). Beta sites included 5 VAMCs with 6 medical specialties for a total of 6 sites. For 1 year, alpha sites received $175,000 and beta sites received $150,000 to support start-up activities.
Initial specialties included diabetes, hepatitis C, geriatrics, cardiology, liver transplant, dementia, gastrointestinal disease, pulmonary medicine, rheumatology, pain management, neurosurgery, infectious diseases, hematology/oncology, and vascular surgery. Facilities could add additional e-consult specialties but did not receive further funding.
Sample
Study participants were selected from 8 of the 15 pilot sites (geographic site/specialty combinations). Site selection was based on 2 measures of baseline e-consult implementation: (1) overall e-consult implementation rates, measured as the ratio of e-consults to all consults for the specialties of interest; and (2) CBOC participation, measured as the ratio of e-consults for patients from CBOCs vs e-consults for patients from primary care clinics located within the 152 VAMCs. Participation with CBOCs was important for ensuring that implementation factors that influenced uptake of e-consults within tertiary medical centers and between VAMCs and CBOCs could be identified. Two e-consult sites were randomly selected from each of the 4 resulting categories (VAMC high volume, VAMC low volume, CBOC high volume, and CBOC low volume). Volume data of e-consults were obtained from the VA Corporate Data Warehouse and assessed from the beginning of the pilot period to initial site selection, May 2011 to February 2012.
Respondents were identified using a modified snowball sampling process. Snowball sampling is a qualitative sampling technique that identifies study participants, who then identify other potential participants to participate in the study. The researchers started with the local e-consult initiative lead and then contacted the directors of primary care and specialty care services for help identifying PCPs, specialists, and support staff (nurse practitioners, pharmacists, program managers, informatics staff, and medical support personnel) engaged in the initiative. The goal for follow-up interviews was to interview at least 2 of the following respondents at each site: e-consult project manager, PCP, and/or specialist. Due to turnover and changes in clinic roles, some follow-up interviews were conducted with different individuals from the baseline interviews.
Data Collection
Interviews followed semistructured interview guidelines and included open-ended questions designed to elicit rich responses to a variety of aspects related to e-consult implementation, including patient needs, communication, leadership, resources, priorities, knowledge about the program, and unintended consequences. Follow-up interviews addressed how e-consults impacted the quality of specialty care; the impact of e-consults on Patient Aligned Care Teams (PACTs), the VHA patient-centered medical home initiative for primary care; and how e-consults have been used, eg, whether patients were involved in the decision to seek an e-consult.
Two interviewers who had participated in a 1-day, in-person training covering both data collection and analyzing key informant data conducted the 40 to 60 minute telephone interviews. One team member conducted the interview while the other took field notes. Interviews were also recorded. Follow-up probes were used to elicit specific examples and ensure sufficiently rich data. Following each interview, the notetaker reviewed the audio recording and filled in details in the field notes. The interview team debriefed and reviewed the augmented field notes and audio recordings, which became the primary data sources for the study.
Analysis
This was a qualitative descriptive analysis.12 Interview data were analyzed using an iterative, inductive content analysis method using an open coding approach (ie, a priori codes were not defined for this portion of the analysis).13 Two members of the research team used audio recordings and summary transcripts simultaneously to code data. Summary transcripts were compared with the recorded interviews to assure fidelity.
The researchers used Atlas.ti (Berlin, Germany) qualitative data analysis software to organize the coding process. Emergent codes were iteratively added throughout the analysis to reflect quotations that did not adequately fit previously developed codes. Codes were combined weekly to biweekly. After the combinations were completed, the analytics team met to review meanings of codes to ensure consistency of coding and interpretations.
To create categories, broad themes were identified from interview responses and grouped under high- order headings that described distinct aspects of participant experience. The analysis was intentionally kept close to the original data to reflect and describe the participant’s experience as accurately as possible. In support of analytical rigor, members of the multidisciplinary research team, composed of clinicians, implementation scientists, and mixed methodologists, reviewed findings to assess their thoroughness, comprehensiveness, and representativeness across roles and participating sites.14
Results
The e-consult evaluation period was from November 1, 2011, to July 31, 2013. Key conclusions were drawn from both alpha and beta sites (Table). Baseline interviews were conducted with 37 participants at 8 sites from April 10, 2012, to August 6, 2012. Follow-up interviews were conducted with 21 of the 37 participants at the 8 sites. Follow-up interviews with either a PCP or specialist could not be scheduled at 1 site. Follow-up interviews were conducted from April 16, 2013, to June 18, 2013. Open coding continued until saturation (the point at which subsequent data failed to produce new findings).15 This occurred after analysis of 22 baseline interviews (12 PCPs, 6 specialists, 1 pharmacist, and 4 other staff members) and 17 follow-up interviews (10 PCPs, 4 specialists, 1 pharmacist, and 2 other staff members).
Implementation
The e-consults provided a programmatic structure to the more informal practice of obtaining diagnostic or therapeutic advice from a specialist. Several of the specialists interviewed described having previously used existing informal consult processes that were “like e-consult.” These specialists reported that their practice patterns did not change significantly since implementing e-consults, because they have been “using the Computerized Patient Record System (CPRS) in an e-consult way for many years.” In these cases, the primary change resulting from the initiative was that national VHA workload policy was revised so that e-consults were assigned a CPT (Current Procedural Terminology) code and specialists began receiving workload credit for completing e-consults.
At sites where an informal e-consult practice was already in place, the initiative was consistently described as flexible. Many specialists reported that this degree of flexibility allowed them to make a relatively easy transition to e-consults by adopting new mechanisms to support existing processes. The e-consult initiative also allowed specialists to formally document this work and to increase the efficiency of specialty care.
Specialists drove the implementation process across sites. The e-consults were envisioned as a collaborative process; however, during initial interviews, few specialists mentioned PCPs when describing the development and implementation of the e-consult program. Primary care providers also reported having little awareness of or input into how the initiative was implemented, although this had little consequence on the use of e-consults.
In a rare case, a PCP reported that poorly designed, lengthy e-consult templates were a major barrier to using e-consults for specific specialties. The PCP said, “E-consults have created an elaborate but extraordinarily cumbersome tool that is difficult for PCPs to actually accomplish, because you have a consult menu that requires a lot of data to be entered—a lot of history from the chart, a lot of exam findings, a lot of previous cognitive testing scores; neurologic findings—lab and imaging tests.”
Still, many other PCPs described receiving detailed information and guidance from e-consults. “E-consults help me to be more accurate. Many providers don’t have a comfort with pain management. To get guidance and education and to really hold our hand, this is how to do this…this has been a big change. If they give you a great response, then [for] the next patient [with that condition], you go back to that note and then follow what was said there,” said one PCP.
In follow-up interviews, providers and other key staff stated there were more data available on the patient as a result of the e-consult and, consequently, even when specialists determined that a patient needed an in-person visit, the data obtained in the e-consult improved the quality of the in-person consultation.
Enhanced Communication and Collaboration
Neither the PCPs nor the specialists were aware of the collaborative intent of the initiative. They focused, instead, on other key aims, such as increasing accessibility and minimizing unnecessary patient travel. Most participants were generally positive about e-consults during baseline interviews, and this perception increased over time.
Both the PCPs and the specialists reported improved communication following the launch of e-consults. In follow-up interviews, some PCPs reported that before e-consults, they had trouble getting timely responses from specialists unless they knew them personally. “You had to know the person in the old days,” one respondent said. “After e-consults, responses improved…e-consult is available to have the resources to tap that knowledge base, and the team is answering the question. I think it opens up access and information and knowledge to everybody.”
Many PCPs spoke positively about this new communication tool as an opportunity to learn from specialists and said they valued the input they received. They felt the increased interaction between the 2 groups positively benefited patient care. One example cited that collaborative communication improved care coordination for veterans: “We are able to step in with e-consults to coordinate services, and this has been huge in improving care.”
Furthermore, follow-up interviews found that all participating PCPs and specialists were communicating more frequently and effectively. “Services that have embraced e-consult give a lot of great information flowing back; it’s closer to a real-time conversation,” said one respondent.
Related: Home-Based Video Telehealth for Veterans With Dementia
In baseline interviews, some specialists described how e-consults went against their belief that patient care is synonymous with face-to-face medical treatment and voiced dissatisfaction with e-consults as “sitting in front of a computer” rather than “seeing patients.” Others were concerned that medical center administration would not recognize the time it takes to conduct an e-consult and therefore not add necessary specialists staff. “E-consults take work and time, just like seeing a patient. I worry that won’t be seen,” one specialist said.
In order to successfully implement the e-consult initiative, providers and staff needed to incorporate new processes into their daily workflow.
Most sites did not develop a mechanism in which specialists received feedback regarding the outcome of their consultations. This lack of response created anxiety for some specialists in the absence of the face-to-face encounter, leaving some wondering whether they or the PCP had missed anything. According to one specialist, “That’s always in the back of your head: ‘Have I [the specialist] missed something?’”
In follow-up interviews, none of these concerns were raised. Primary care providers tended to speak of the care provided by specialists through e-consults in very positive terms, except in those instances where PCPs felt the e-consult template was difficult to use and required too much time to complete. “I was worried in the beginning about patients thinking less of me, but we ask for help all the time. We’re asking for help and not inconveniencing the patient; they seem to like it very much,” one PCP said.
The e-consults also complement PACTs. Initially, a few participants described soliciting patient input regarding the choice to have an e-consult or a face-to-face visit. During follow-up interviews, participants highlighted how well e-consults fit in to the PACT philosophy. One participant said, “The PACT team seeks to improve quality of care. E-consult fits very well with this, because answers to questions can come quickly, and the veteran may not need to come back to the clinic to be seen, even though things are still getting accomplished. E-consult works very well. E-consults were credited with improving access to specialty care as a tool for PACT.”
Achieving Program Objectives
Based on interviews, support for the e-consult program has increased over time as providers have gained experience with the program and have seen its benefits. Respondents at all sites consistently supported the concept of e-consults and expressed their belief in the importance and value of e-consults in improving patient-c entered care, primarily by reducing the need for patients to travel to see specialists, reducing the time to obtain feedback from specialists, and maintaining the provision of high quality care.
“Last year we only had 2 clinics categorized as e-consults. As of now we have 14 e-consults available for our providers. I think the numbers are growing. They are realizing the value of e-consults as far as the provider’s needs being met,” said one respondent.
The e-consults were credited with improving access to specialty care for veterans. Several participants stated that e-consults improved access to specialty care services and decreased travel for veterans. “It’s another way of getting care to the patient when the patient needs it without having to wait,” said one respondent.
Many PCPs described how difficult it was for patients to get to specialty appointments—particularly for their elderly, disabled, and rural patients—before the implementation of e-consults. “I like the fact that patients who live very far don’t have to come back. A lot of our patients are older…diabetic, see me Monday and back on Thursday. Now, they are able to stay home and follow the recommendations I write,” said one PCP.
Most providers were of the opinion that patients liked the program. “I think e-consults are helping patients...It’s been very successful regarding decreasing travel…Quicker response time for specialty care,” said a PCP. Several providers also stated in follow-up interviews that there was a greater degree of patient participation in the e-consult process and that “patients are definitely informed.”
Discussion
Most PCPs reported that the e-consults were an effective means of consultation and contained the information they needed to provide high-quality coordinated care. Most also found e-consult templates easy to complete. A majority of PCPs felt sufficient control over the choice of whether to use e-consults or an in-person visit, and a minority of patients were involved in the decision to receive an e-consult. Although the OSCS outlined guiding principles and operational rules in the Implementation Guide to help sites implement the e-consult program, its contribution was limited. Few examples were found that engaged PCPs in development of the e-consult program locally; involving patients in the decision to obtain a specialty consult electronically or in person; and PCPs feeding back results to specialists.
Implementing e-consults posed a number of challenges, including lack of resources to respond to referral requests, lack of referral policies and standardized procedures, and confusion related to roles and responsibilities. This is consistent with findings from another VHA research project of e-consults in 2 VHA health systems that was conducted prior to this national level e-consult pilot.7
Related: Using Facilitative Coaching to Support Patient Aligned Care Teams
Communication by OSCS of key aspects of the e-consult initiative will be critical as more sites implement e-consults. Since initiation of this pilot, workload specifications and credit have changed from 1 code to 3 codes, to more accurately reflect the amount of time a specialist consultant spends reviewing the EHR and responding to the consult. Without seeing the patient directly, specialists are more reliant on the PCP to describe the problem and provide adequate information in the e-consult request in order to provide recommendations back to the PCP.
Primary care physicians need to know that e-consults are available and determine when they are appropriate. A template or other guidance may be helpful to ensure adequate information is provided in the e-consult request; and the information provided by the specialist in response to the e-consult has to be sufficient for the PCP to provide care. VHA continues to expand the use of e-consults throughout the system, as this pilot found that the electronic option was often more timely than were face-to-face consultations. The result of this evaluation has informed national implementation of this effort.
Limitations
There are 3 main limitations to this study. First, because there was no practical way to preidentify participants who participated in implementing e-consults, a modified snowball sampling was used. However, this limited the degree to which the group was representative of the pilot participants. Second, the authors reported findings from a real-world initiative, not an experimental study. As such, not all participants in the first wave of key informant interviews were available for follow-up interview, which may have introduced bias. Third, the VHA is unlike most of the rest of the U.S. health care system in that it is a fully integrated system with salaried PCPs and specialists and an EHR.
Generalizability of the study may be limited, as a modified snowball sampling approach is not entirely random and has potential for community bias, because initial participants influence subsequent sampling. Additionally, though the sample size (n = 37) was sufficient for qualitative, in-depth analysis, it may be too small for confident generalization of findings. However, as health care moves toward an accountable care organization system, the authors’ analysis may provide insights.
Issues include revision of reimbursement policy for e-consults and developing or coordinating informational technology infrastructures to permit e-consults. It is also important to note that this evaluation reports solely on the extent of implementation of e-consults and the effects of e-consult implementation from the perspectives of staff, including specialists and PCPs.
Evaluating the effectiveness of the program in improving access, care coordination, and patient satisfaction was beyond the scope of the study. Further research is needed, because findings on those outcomes are critical for drawing inferences about this study’s implementation results.
Conclusion
The assessment of the e-consult system by providers and staff was based on a perception that e-consults are a valuable tool in providing greater access to quality care. Currently, e-consults have been expanded across VHA in medical and surgical specialties. VHA policymakers have drafted field guidance and a communication plan to support these efforts.
Acknowledgement
This material is based on work supported by the VA Office of Specialty Care Transformation, the office overseeing the e-consult initiative, and the Office of Research and Development Quality Enhancement Research Initiative.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453.
2. Hanna L, May C, Fairhurst K. The place of information and communication technology-mediated consultations in primary care: GPs’ perspectives. Fam Pract. 2012;29(3):361-366.
3. Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123-1128.
4. Straus SG, Chen AH, Yee HF Jr, Kushel MB, Bell DS. Implementation of an electronic referral system for outpatient specialty care. AMIA Annu Symp Proc. 2011;2011:1337-1346.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014;31(5):26-31.
7. Hysong SJ, Esquivel A, Sittig DF, et al. Towards successful coordination of electronic health record based-referrals: a qualitative analysis. Implement Sci. 2011;6:84.
8. American College of Physicians. The Patient- Centered Medical Home Neighbor: The Interface of the Patient-Centered Medical Home with Specialty/Subspecialty Practices. Philadelphia, PA: American College of Physicians; 2010. Policy paper.
9. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12): 1202-1205.
10. Department of Veterans Affairs. Electronic Consultation (E-Consult) Implementation Guide, Version 1.2. Washington, DC: Department of Veterans Affairs, Office of Specialty Care Services, Specialty Care Transformation. 2013.
11. Kirsh S, Cary E, Aron DC et al. Results of a national pilot project for specialty care e-consultation in primary care medical homes: the impact of specialty e-consultation on access. Am J Manag Care. In press.
12. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
13. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
14. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000;284(3):357-362.
15. Sandelowski M. The problem of rigor in qualitative research. ANS Adv Nurs Sci. 1986;8(3):27-37.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453.
2. Hanna L, May C, Fairhurst K. The place of information and communication technology-mediated consultations in primary care: GPs’ perspectives. Fam Pract. 2012;29(3):361-366.
3. Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123-1128.
4. Straus SG, Chen AH, Yee HF Jr, Kushel MB, Bell DS. Implementation of an electronic referral system for outpatient specialty care. AMIA Annu Symp Proc. 2011;2011:1337-1346.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014;31(5):26-31.
7. Hysong SJ, Esquivel A, Sittig DF, et al. Towards successful coordination of electronic health record based-referrals: a qualitative analysis. Implement Sci. 2011;6:84.
8. American College of Physicians. The Patient- Centered Medical Home Neighbor: The Interface of the Patient-Centered Medical Home with Specialty/Subspecialty Practices. Philadelphia, PA: American College of Physicians; 2010. Policy paper.
9. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12): 1202-1205.
10. Department of Veterans Affairs. Electronic Consultation (E-Consult) Implementation Guide, Version 1.2. Washington, DC: Department of Veterans Affairs, Office of Specialty Care Services, Specialty Care Transformation. 2013.
11. Kirsh S, Cary E, Aron DC et al. Results of a national pilot project for specialty care e-consultation in primary care medical homes: the impact of specialty e-consultation on access. Am J Manag Care. In press.
12. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
13. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
14. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000;284(3):357-362.
15. Sandelowski M. The problem of rigor in qualitative research. ANS Adv Nurs Sci. 1986;8(3):27-37.
Novel Psoriasis Therapies and Patient Outcomes, Part 3: Systemic Medications
Evolving knowledge of the underlying pathogenesis of psoriasis has afforded the development of a broad spectrum of nonbiologic systemic medications with therapeutic potential in moderate to severe psoriasis and psoriatic arthritis (PsA). The targets for these medications are antagonists of proinflammatory mediators such as tyrosine kinase, protein kinase, Janus kinase (JAK), p38α mitogen-activated protein (MAP) kinase, phosphodiesterase 4 (PDE-4), calcineurin, Rho-associated kinase (ROCK) 2, cytochrome P450 26 (CYP26), and purine nucleoside phosphorylase (PNP); agonists of anti-inflammatory mediators such as sphingosine-1-phosphate receptor 1 (S1P1) and adeno-sine A3 receptor; and myriad other mechanisms. A brief introduction to some of these therapies presently in phase 2 and phase 3 clinical trials are presented (Table).1
Masitinib (Tyrosine Kinase Inhibitor)
Masitinib (formerly known as AB1010)(AB Sciences) is a tyrosine kinase inhibitor that is purported to decrease inflammation by inhibiting stem cell factor receptor (c-kit) and consequently limiting mast cell degranulation.2 A randomized, placebo-controlled, double-blind phase 3 study evaluating its efficacy as an oral formulation was completed, but the results were not available at the time of publication (registered at www.clinicaltrials.gov with the identifier NCT01045577).
Ponesimod (S1P1 Receptor Agonist)
Ponesimod (formerly known as ACT-128800)(Actelion Pharmaceuticals US, Inc) is an orally formulated S1P1 receptor agonist. Sphingosine-1-phosphate receptor 1 is necessary for lymphoid chemotaxis.3 A randomized, placebo-controlled, double-blind phase 2 trial of 326 patients demonstrated 75% improvement in psoriasis area and severity index (PASI) score at week 16 in 13.4%, 46.0%, and 48.1% of participants receiving placebo, ponesimod 20 mg, and ponesimod 40 mg, respectively.4 At week 28, PASI 75 scores for participants transitioned from ponesimod 40 mg to placebo or ponesimod 20 mg to placebo and those maintained on ponesimod 20 mg and 40 mg were 40.4%, 42.2%, 77.4%, and 71.4%, respectively. This study demonstrated benefit of treatment with ponesimod versus placebo with increased efficacy using maintenance therapy.4
Sotrastaurin (Protein Kinase C Inhibitor)
Sotrastaurin (formerly known as AEB071)(Novartis AG) is an oral medication that inhibitsprotein kinase C, thereby limiting CD28-induced activation of T cells. Furthermore, it increases forkhead box P3 expression, which is important, as proinflammatory IL-17 production is stimulated in regulatory T cells with lost forkhead box P3 expression.5 In a study of 32 patients who received placebo or sotrastaurin 25, 100, 200, or 300 mg twice daily for 2 weeks, the mean PASI score was reduced by 69% for the 300-mg group versus 5.3% for placebo.6 A randomized, placebo-controlled, double-blind phase 2 study was completed for patients with moderate to severe psoriasis, but the results were not available at the time of publication (NCT00885196).
Alitretinoin (Retinoid)
Alitretinoin (9-cis-retinoic acid; Stiefel, a GSK company) is an oral retinoid with purported promise for patients with palmoplantar pustular psoriasis recalcitrant to conventional therapies. In one study, 7 participants with palmoplantar psoriasis received alitretinoin 30 mg daily for 12 weeks with 60% to 90% clinical improvement noted using the visual analog scale and palmoplantar pustular PASI to assess response.7 A randomized, placebo-controlled, double-blind phase 2 trial of alitretinoin assessing the success of alitretinoin in patients with palmoplantar psoriasis recalcitrant to topical treatments was completed, but the results were not available at the time of publication (NCT01245140).
Apo805K1 (Unknown Mechanism of Action)
Apo805K1 (ApoPharma Inc) is an oral agent whose mechanism of action has not been disclosed. A randomized, placebo-controlled, double-blind phase 2 trial in patients with moderate to severe psoriasis with a treatment duration of 14 days has been completed. For 12 weeks there was a daily dosing regimen of Apo805K1 10, 30, 60, or 100 mg or placebo, with 12 patients in each treatment group. The proportion of patients achieving PASI 75 was 16.7%, 0%, 0%, 8.3%, and 16.7%, respectively (P=.1975). The number of participants with adverse events reported ranged between 4 to 7, with the greatest number of adverse events reported in the 30-mg subset.8 It may be of interest to repeat the study with a larger sample size.
ASP015K (JAK 1 and JAK 3 Inhibitor)
ASP015K (Astellas Pharma US, Inc) is the first of the JAK inhibitors that will be discussed in this section. Janus kinase inhibitors represent a group of tyrosine kinases that regulate cytokine-mediated signaling pathways through the activation of signal transducer and activator of transcription proteins via phosphorylation in the cytoplasm, which in turn control the transcription of genes that generate inflammation. ASP015K and tofacitinib (formerly known as CP-690550)(Pfizer, Inc) inhibit JAK 1 and JAK 3, whereas GSK2586184 (GlaxoSmithKline) inhibits JAK 1 and baricitinib (formerly known as LY3009104 or INCB28050)(Eli Lilly and Company) inhibits JAK 1 and JAK 2.9 In a 6-week, dose-escalation phase 2 trial in patients with moderate to severe psoriasis, ASP015K showed a dose-dependent decline in PASI, psoriasis static global assessment, and body surface area.10
BMS-582949 (p38α MAP Kinase Inhibitor)
BMS-582949 (Bristol-Myers Squibb Company) is an oral p38α MAP kinase inhibitor.11 Along with c-Jun N-terminal kinase and extracellular signal-regulated protein kinases 1 and 2, MAP kinase plays a role in the pathogenesis of psoriasis.12 A randomized, placebo-controlled, double-blind, 12-week phase 2a study of placebo versus BMS-582949 dosed at 10, 30, and 100 mg has been completed, but the results were not available at the time of publication (NCT00399906).
Apremilast (PDE-4 Inhibitor)
Apremilast (formerly known as CC-10004)(Celgene Corporation) is an oral PDE-4 inhibitor that acts to inhibit the degradation of cyclic adenosine monophosphate. It is approved by the US Food and Drug Administration for moderate to severe plaque psoriasis13 and is a particularly good treatment for patients with recalcitrant disease.14 Several studies on apremilast have been published, including a phase 2 trial of 204 participants receiving placebo or apremilast 20 mg or 40 mg twice daily with American College of Rheumatology (ACR) 20% improvement response of 11.8%, 35.8%, and 43.5%, respectively.15 The phase 3 PALACE 1, 2, 3, and 4 trials also demonstrated therapeutic efficacy.16 In PALACE 1, 504 patients receiving placebo or apremilast 20 mg and 30 mg twice daily had an ACR20 of 19%, 31%, and 40%, respectively. In the PALACE 4 study, ACR20 was achieved by 58% of participants at week 52, but the ACR50 and ACR70 rates were less impressive.16
Lestaurtinib (Multikinase Inhibitor)
Lestaurtinib (formerly known as CEP-701)(Teva Pharmaceutical Industries) is an oral multikinase inhibitor for which a 12-week, nonrandomized, dose-escalation phase 2 study was completed, but the results were not available at the time of publication (NCT00236119).
CF101 (Adenosine A3 Receptor Agonist)
CF101 (IB-MECA; Can-Fite BioPharma Ltd) is an oral adenosine A3 receptor agonist. Adenosine A3 is a G protein–coupled receptor that has an anti-inflammatory role, which lends itself to the treatment of inflammatory conditions such as rheumatoid arthritis.17 In a randomized, placebo-controlled, double-blind phase 2 trial of 75 patients who received placebo or CF101 1, 2, or 4 mg twice daily for 12 weeks, PASI 50 or greater was reported in 35.3% of participants in the 2-mg group, which was statistically significant at weeks 8 (P=.047) and 12 (P=.031).18 A randomized, placebo-controlled, double-blind, 16-week phase 2/phase 3 trial in patients with moderate to severe psoriasis treated with CF101 2 mg twice daily versus placebo is ongoing but not recruiting participants (NCT01265667).
Tofacitinib (JAK 1 and JAK 3 Inhibitor)
Tofacitinib is a JAK 1 and JAK 3 inhibitor that is available in both topical and oral formulations. Many studies have been published on tofacitinib, including a dose-ranging phase 2b trial of 197 patients randomized to placebo or tofacitinib 2, 5, or 15 mg daily in which 2.0%, 25.0% (P<.001), 40.8% (P<.0001), and 66.7% (P<.0001) of participants in each treatment group, respectively, achieved PASI 75 at week 12.19 Another 12-week phase 2b trial in patients with moderate to severe psoriasis explored the effectiveness of oral formulations of tofacitinib based on body location, namely the head and neck, arms, trunk, and legs.20 Designed with twice-daily placebo and tofacitinib 2-, 5-, and 15-mg treatment arms, the target plaque severity score demonstrated a statistically significant dose-responsive improvement in each of the 4 anatomic regions (P<.01).
Two randomized, double-blinded, placebo-controlled phase 3 trials of tofacitinib have been completed. One trial compared the safety and effectiveness of tofacitinib and etanercept in patients with moderate to severe psoriasis.21 The 329 participants were randomized to treatment with oral tofacitinib 5 mg or 10 mg twice daily with placebo subcutaneous (SQ) injections twice weekly; oral placebo twice daily with etanercept 50-mg SQ injections twice weekly; or oral placebo twice daily with placebo SQ injection twice weekly. At week 12, PASI 75 was achieved in 39.51%, 63.64%, 58.81%, and 5.61% of participants in each treatment group, respectively. Thus, tofacitinib 10 mg and etanercept 50 mg were the first and second best performers in this study design.21
In the other phase 3 trial, the efficacy and safety of treatment, treatment withdrawal, and subsequent resumption of treatment with tofacitinib was examined in 290 patients who received either tofacitinib 5 mg or 10 mg or placebo twice daily for 24 weeks.22 In the withdrawal period, the percentage of patients who maintained a PASI 75 response in the tofacitinib 5-mg group was 56.2% versus 23.3% in the placebo group at week 16 (P<.0008). In the 10-mg group, 62.3% of participants maintained PASI 75 at week 16 versus 26.1% in the placebo group (P<.0001). During the re-treatment period for those who showed a greater than 50% reduction in week 24 PASI during treatment withdrawal (N=102), 50.0% of the tofacitinib 5-mg group showed PASI 75 versus 31.58% of the placebo group at week 16. In the tofacitinib 10-mg group, PASI 75 was seen in 0% versus 50.85% in the placebo group. Of note, only 5 participants who demonstrated a greater than 50% reduction in week 24 PASI response following withdrawal of therapy were in the tofacitinib 5- or 10-mg groups, as the rest were in the placebo group.22
FP187 (Fumaric Acid Ester)
FP187 (Forward Pharma A/S) is an oral fumaric acid ester whose underlying mechanism is thought to be elevation of glutathione levels that decreases the amount of inflammatory cytokines by blocking the translocation of nuclear factor κB. Other fumaric acid esters such as monoethyl fumarate and dimethyl fumarate have been used in Europe for the management of psoriasis.23 A phase 2 study of the safety and effectiveness of FP187 for moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01230138). A phase 3 trial examining the efficacy of FP187 in managing moderate to severe psoriasis was not yet open for participant recruitment at the time of publication (NCT01815723).
Doxercalciferol (Vitamin D2 Analogue)
Doxercalciferol (1α-hydroxyvitamin D2; Genzyme Corporation, a Sanofi company) is an oral prodrug of vitamin D.24 Topical preparations of vitamin D analogues approved by the US Food and Drug Administration such as calcipotriene are mainstays in psoriasis management. Doxercalciferol has been used for other therapeutic applications such as decreasing elevated parathyroid hormone levels.25 Research has demonstrated that CYP24A1 can extrahepatically regulate the activation of vitamin D prodrugs in cutaneous tissues.26 In a phase 2 study examining the efficacy and safety of doxercalciferol in patients with moderate to severe psoriasis, 111 patients were randomized to placebo or doxercalciferol 2.5, 5, or 7.5 mg daily for 24 weeks (NCT00601107). At week 12, PASI 50 was similar regardless of the treatment administered, reported at 20.0%, 20.0%, 17.9%, and 20.0%, respectively (P=1.000).27
GSK2586184 (JAK 1 Inhibitor)
GSK2586184 is an oral JAK 1 inhibitor. A placebo-controlled, double-blind, dose-dependent phase 2 study of GSK2586184 in patients with chronic plaque psoriasis who were randomized to placebo or GSK2586184 100, 200, and 400 mg twice daily for 84 days has been completed, but the results were not available at the time of publication (NCT01782664). Of note, despite clinical promise, due to potential adverse effects of the medication that included a possible interaction with statin medication, the manufacturer decided against pursuing GSK2586184 as a treatment in the management of psoriasis.28
Voclosporin (Calcineurin Inhibitor)
Voclosporin (formerly known as ISA247)(Aurinia Pharmaceuticals Inc) is an oral calcineurin inhibitor that is purported to be as effective as cyclospor-ine A with less associated toxicity. A 12-week randomized, placebo-controlled, double-blind phase 2 trial demonstrated PASI 75 in 0%, 18.2%, and 66.7% of 201 participants receiving placebo, voclospo-rin 0.5 mg/kg, and voclosporin 1.5 mg/kg twice daily, respectively (P<.0001). Elevated serum creatinine levels within the high range of normal were noted in the 1.5-mg/kg group.29 A 12-week phase 3 study of 451 patients randomized to placebo or voclospo-rin 0.2-, 0.3-, or 0.4-mg/kg treatment groups similarly demonstrated a dose-responsive PASI 75 of 4%, 16%, 25%, and 47%, respectively, that was maintained at week 24. Mild to moderate decreases in glomerular filtration rates were noted in 8 patients in the 0.3- and 0.4-mg/kg subsets.30 Another phase 2 study of voclosporin in patients with moderate to severe psoriasis showed improvements according to the psoriasis disability index and dermatology life quality index.31
Three randomized, placebo-controlled, double-blind phase 3 trials have been completed for voclosporin, but the results were not available at the time of publication. The phase 3 ESSENCE trial compared voclosporin to placebo and ciclosporin controls (NCT00408187). Two phase 3 trials known as the SPIRIT trials—one a 12-week study (NCT00244842) and the other a 36-week extension trial (NCT00258713)—compared the efficacy of placebo to voclosporin administered at 0.2-, 0.3-, and 0.4-mg/kg doses.
KD025 (ROCK 2 Inhibitor)
KD025 (Kadmon Corporation, LLC) is an oral ROCK 2 inhibitor. The inhibition of ROCK in the ras homolog gene family, member A/ROCK pathway has been targeted therapeutically for pulmonary arterial hypertension,32 glaucoma,33 and many other uses. A phase 2a study assessing the tolerability and safety profile of KD025 in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT02106195).
LAS41008 (Fumaric Acid Ester)
LAS41008 (Almirall, S.A.) is purported to be an oral dimethyl fumarate that inhibits endothelial cell proliferation, migration, and differentiation.34 A phase 3 trial comparing the safety and effectiveness of LAS41008, LASW1835 (an active comparator), and placebo in patients with moderate to severe psoriasis is ongoing but not recruiting participants (NCT01726933).
LEO 22811 (Anti-inflammatory)
LEO 22811 (LEO Pharma) is an oral anti-inflammatory agent for the treatment of psoriasis. Two clinical trials have been completed, the results of which have not yet been published. A phase 1 trial assessing the tolerability and safety profile of LEO 22811 (NCT00833872) and a phase 2 proof-of-concept study assessing dose response of LEO 22811 versus placebo (NCT01116895) were completed, but the results were not available at the time of publication.
Baricitinib (JAK 1 and JAK 2 Inhibitor)
As noted above, baricitinib (LY3009104 or INCB28050) is a JAK 1 and JAK 2 inhibitor. A phase 2b trial examining dose response for LY3009104 or baricitinib administration in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01490632).
Talarozole (CYP26 Inhibitor)
Talarozole (formerly known as R115866)(GlaxoSmithKline) is a selective oral CYP26 inhibitor that could serve to regulate the degradation of all-trans retinoic acid in the management of conditions such as acne and psoriasis. One phase 2 nonrandomized, open-label study (NCT00725348) and another phase 2 randomized, placebo-controlled, double-blind, dose-dependent trial examining the effectiveness and safety profile of 12-week talarozole treatment in patients with severe plaque psoriasis have been completed, but the results were not available at the time of publication (NCT00716144).
R3421 or BCX-4208 (PNP Inhibitor)
R3421 or BCX-4208 (BioCryst Pharmaceuticals, Inc/Hoffman–La Roche) is an oral PNP inhibitor that may help regulate apoptosis of T cells and B cells.35 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT00504270).
RWJ-445380 (Cathepsin S Inhibitor)
RWJ-445380 (Alza Corporation, DE) is an oral cathepsin S inhibitor. Cathepsin S is produced by antigen-presenting cells and activates CD4+ T cells via presentation of antigen by class II major histocompatibility complex.36 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial evaluating the tolerability, safety, pharmacodynamics, and pharmacokinetics of RWJ-445380 in patients with plaque psoriasis has been completed, but the results were not available at the time of publication (NCT00396422).
SRT2104 (Sirtuin 1 Activator)
SRT2104 (GlaxoSmithKline) is a selective oral NAD+-dependent deacetylase sirtuin 1 activator. Sirtuins help regulate apoptosis, inflammation, and other important cellular mechanisms.37 A randomized, open-label phase 1 trial examining drug bioavailability (NCT01702493) and a randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial studying the efficacy, tolerability, and safety of SRT2104 in patients with moderate to severe psoriasis have been completed (NCT01154101), but the results were not available at the time of publication.
VB-201 (Oxidized Phospholipid)
VB-201 (VBL Therapeutics) is an orally administered oxidized phospholipid analogue with anti-inflammatory effects.38 Oxidized phospholipids are another element in the intricate spectrum of inflammatory mediators. A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 20 mg or 80 mg daily in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT01001468). Another randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 80 mg and 160 mg twice daily in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01837420).
Conclusion
We are living in an exciting time in the treatment of psoriasis and PsA, with promising novel therapies afforded by the discovery of new therapeutic targets. In this 3-part series, we have reviewed topical, systemic, and biologic therapies that currently are in phase 2 through phase 4 clinical trials or have recently been approved by the US Food and Drug Administration for the management of psoriasis and PsA. These treatments offer patients and their caregivers the prospect of more targeted therapeutic regimens that offer enhanced clinical outcomes with more favorable side-effect profiles. As clinicians and researchers build upon this knowledge in the years to come, we can offer psoriasis patients an increasingly diverse and powerful therapeutic armamentarium.
1. Lu PD, Mazza JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:227-242.
2. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194-1201.
3. Krause A, Brossard P, D’Ambrosio D, et al. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. J Pharmacokinet Pharmacodyn. 2014;41:261-278.
4. Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036-2045.
5. He X, Koenen HJ, Smeets RL, et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J Invest Dermatol. 2014;134:975-983.
6. Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118:3151-3159.
7. Irla N, Navarini AA, Yawalkar N. Alitretinoin abrogates innate inflammation in palmoplantar pustular psoriasis. Br J Dermatol. 2012;167:1170-1174.
8. Study to evaluate Apo805K1 in subjects with moderate to severe chronic plaque psoriasis (NCT01483924). https://clinicaltrials.gov/ct2/results?term=NCT01483924&Search=Search. Updated February 9, 2015. Accessed June 23, 2015.
9. Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133-139.
10. Gooderham M. Small molecules: an overview of emerging therapeutic options in the treatment of psoriasis. Skin Therapy Lett. 2013;18:1-4.
11. Liu C, Lin J, Wrobleski ST, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629-6639.
12. Mavropoulos A, Rigopoulou EI, Liaskos C, et al. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751.
13. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celegene Corporation; September 23, 2014. http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed June 23, 2015.
14. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888-897.
15. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
16. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
17. Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359-366.
18. David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
19. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
20. Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252-256.
21. A phase 3, multi site, randomized, double blind, placebo controlled study of the efficacy and safety comparing CP-690,550 and etanercept in subjects with moderate to severe chronic plaque psoriasis (NCT01241591). https://clinicaltrials.gov/ct2/show/NCT01241591?term=01241591&rank=1. Updated May 8, 2014. Accessed June 16, 2015.
22. A study to evaluate the effects and safety of treatment, treatment withdrawal, followed by re-treatment with CP-690,550 in subjects with moderate to severe chronic plaque psoriasis (NCT01186744). https://clinicaltrials.gov/ct2/show/NCT01186744?term=01186744&rank=1. Updated May 14, 2014. Accessed June 16, 2015.
23. Rostami Yazdi M, Mrowietz U. Fumaric acid esters. Clin Dermatol. 2008;26:522-526.
24. Kubodera N. A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol. Molecules. 2009;14:3869-3880.
25. Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 suppl 5):S3-S19.
26. Masuda S, Strugnell SA, Knutson JC, et al. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221-234.
27. A study to evaluate the safety and effectiveness of doxercalciferol capsules in participants with moderate to severe psoriasis (NCT00601107). https://clinicaltrials.gov/ct2/show/NCT00601107?term=00601107&rank=1. Updated April 2, 2014. Accessed June 16, 2015.
28. GSK discloses good efficacy in psoriasis with GSK2856184 [news release]. Mechelen, Belgium: Galapagos NV; November 6, 2014. http://www.globenewswire.com/news-release/2014/11/06/680358/10106744/en/GSK-discloses-good-efficacy-in-psoriasis-with-GSK2856184.html. Accessed June 24, 2015.
29. Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472-478.
30. Papp K, Bissonnette R, Rosoph L, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337-1342.
31. Gupta AK, Langley RG, Lynde C, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. J Cutan Med Surg. 2008;12:268-275.
32. Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363.
33. Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81-95.
34. National Institute for Health Research Horizon Scanning Centre. Dimethyl fumarate for plaque psoriasis. http://www.hsc.nihr.ac.uk/files/downloads/2228/2526.163fbff8.Dimethylfumarate_Nov13.pdf. Published November 2013. Accessed June 15, 2015.
35. Bantia S, Parker C, Upshaw R, et al. Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol. 2010;10:784-790.
36. García-Pérez ME, Stevanovic T, Poubelle PE. New therapies under development for psoriasis treatment. Curr Opin Pediatr. 2013;25:480-487.
37. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359.
38. Feige E, Mendel I, George J, et al. Modified phospholipids as anti-inflammatory compounds. Curr Opin Lipidol. 2010;21:525-529.
Evolving knowledge of the underlying pathogenesis of psoriasis has afforded the development of a broad spectrum of nonbiologic systemic medications with therapeutic potential in moderate to severe psoriasis and psoriatic arthritis (PsA). The targets for these medications are antagonists of proinflammatory mediators such as tyrosine kinase, protein kinase, Janus kinase (JAK), p38α mitogen-activated protein (MAP) kinase, phosphodiesterase 4 (PDE-4), calcineurin, Rho-associated kinase (ROCK) 2, cytochrome P450 26 (CYP26), and purine nucleoside phosphorylase (PNP); agonists of anti-inflammatory mediators such as sphingosine-1-phosphate receptor 1 (S1P1) and adeno-sine A3 receptor; and myriad other mechanisms. A brief introduction to some of these therapies presently in phase 2 and phase 3 clinical trials are presented (Table).1
Masitinib (Tyrosine Kinase Inhibitor)
Masitinib (formerly known as AB1010)(AB Sciences) is a tyrosine kinase inhibitor that is purported to decrease inflammation by inhibiting stem cell factor receptor (c-kit) and consequently limiting mast cell degranulation.2 A randomized, placebo-controlled, double-blind phase 3 study evaluating its efficacy as an oral formulation was completed, but the results were not available at the time of publication (registered at www.clinicaltrials.gov with the identifier NCT01045577).
Ponesimod (S1P1 Receptor Agonist)
Ponesimod (formerly known as ACT-128800)(Actelion Pharmaceuticals US, Inc) is an orally formulated S1P1 receptor agonist. Sphingosine-1-phosphate receptor 1 is necessary for lymphoid chemotaxis.3 A randomized, placebo-controlled, double-blind phase 2 trial of 326 patients demonstrated 75% improvement in psoriasis area and severity index (PASI) score at week 16 in 13.4%, 46.0%, and 48.1% of participants receiving placebo, ponesimod 20 mg, and ponesimod 40 mg, respectively.4 At week 28, PASI 75 scores for participants transitioned from ponesimod 40 mg to placebo or ponesimod 20 mg to placebo and those maintained on ponesimod 20 mg and 40 mg were 40.4%, 42.2%, 77.4%, and 71.4%, respectively. This study demonstrated benefit of treatment with ponesimod versus placebo with increased efficacy using maintenance therapy.4
Sotrastaurin (Protein Kinase C Inhibitor)
Sotrastaurin (formerly known as AEB071)(Novartis AG) is an oral medication that inhibitsprotein kinase C, thereby limiting CD28-induced activation of T cells. Furthermore, it increases forkhead box P3 expression, which is important, as proinflammatory IL-17 production is stimulated in regulatory T cells with lost forkhead box P3 expression.5 In a study of 32 patients who received placebo or sotrastaurin 25, 100, 200, or 300 mg twice daily for 2 weeks, the mean PASI score was reduced by 69% for the 300-mg group versus 5.3% for placebo.6 A randomized, placebo-controlled, double-blind phase 2 study was completed for patients with moderate to severe psoriasis, but the results were not available at the time of publication (NCT00885196).
Alitretinoin (Retinoid)
Alitretinoin (9-cis-retinoic acid; Stiefel, a GSK company) is an oral retinoid with purported promise for patients with palmoplantar pustular psoriasis recalcitrant to conventional therapies. In one study, 7 participants with palmoplantar psoriasis received alitretinoin 30 mg daily for 12 weeks with 60% to 90% clinical improvement noted using the visual analog scale and palmoplantar pustular PASI to assess response.7 A randomized, placebo-controlled, double-blind phase 2 trial of alitretinoin assessing the success of alitretinoin in patients with palmoplantar psoriasis recalcitrant to topical treatments was completed, but the results were not available at the time of publication (NCT01245140).
Apo805K1 (Unknown Mechanism of Action)
Apo805K1 (ApoPharma Inc) is an oral agent whose mechanism of action has not been disclosed. A randomized, placebo-controlled, double-blind phase 2 trial in patients with moderate to severe psoriasis with a treatment duration of 14 days has been completed. For 12 weeks there was a daily dosing regimen of Apo805K1 10, 30, 60, or 100 mg or placebo, with 12 patients in each treatment group. The proportion of patients achieving PASI 75 was 16.7%, 0%, 0%, 8.3%, and 16.7%, respectively (P=.1975). The number of participants with adverse events reported ranged between 4 to 7, with the greatest number of adverse events reported in the 30-mg subset.8 It may be of interest to repeat the study with a larger sample size.
ASP015K (JAK 1 and JAK 3 Inhibitor)
ASP015K (Astellas Pharma US, Inc) is the first of the JAK inhibitors that will be discussed in this section. Janus kinase inhibitors represent a group of tyrosine kinases that regulate cytokine-mediated signaling pathways through the activation of signal transducer and activator of transcription proteins via phosphorylation in the cytoplasm, which in turn control the transcription of genes that generate inflammation. ASP015K and tofacitinib (formerly known as CP-690550)(Pfizer, Inc) inhibit JAK 1 and JAK 3, whereas GSK2586184 (GlaxoSmithKline) inhibits JAK 1 and baricitinib (formerly known as LY3009104 or INCB28050)(Eli Lilly and Company) inhibits JAK 1 and JAK 2.9 In a 6-week, dose-escalation phase 2 trial in patients with moderate to severe psoriasis, ASP015K showed a dose-dependent decline in PASI, psoriasis static global assessment, and body surface area.10
BMS-582949 (p38α MAP Kinase Inhibitor)
BMS-582949 (Bristol-Myers Squibb Company) is an oral p38α MAP kinase inhibitor.11 Along with c-Jun N-terminal kinase and extracellular signal-regulated protein kinases 1 and 2, MAP kinase plays a role in the pathogenesis of psoriasis.12 A randomized, placebo-controlled, double-blind, 12-week phase 2a study of placebo versus BMS-582949 dosed at 10, 30, and 100 mg has been completed, but the results were not available at the time of publication (NCT00399906).
Apremilast (PDE-4 Inhibitor)
Apremilast (formerly known as CC-10004)(Celgene Corporation) is an oral PDE-4 inhibitor that acts to inhibit the degradation of cyclic adenosine monophosphate. It is approved by the US Food and Drug Administration for moderate to severe plaque psoriasis13 and is a particularly good treatment for patients with recalcitrant disease.14 Several studies on apremilast have been published, including a phase 2 trial of 204 participants receiving placebo or apremilast 20 mg or 40 mg twice daily with American College of Rheumatology (ACR) 20% improvement response of 11.8%, 35.8%, and 43.5%, respectively.15 The phase 3 PALACE 1, 2, 3, and 4 trials also demonstrated therapeutic efficacy.16 In PALACE 1, 504 patients receiving placebo or apremilast 20 mg and 30 mg twice daily had an ACR20 of 19%, 31%, and 40%, respectively. In the PALACE 4 study, ACR20 was achieved by 58% of participants at week 52, but the ACR50 and ACR70 rates were less impressive.16
Lestaurtinib (Multikinase Inhibitor)
Lestaurtinib (formerly known as CEP-701)(Teva Pharmaceutical Industries) is an oral multikinase inhibitor for which a 12-week, nonrandomized, dose-escalation phase 2 study was completed, but the results were not available at the time of publication (NCT00236119).
CF101 (Adenosine A3 Receptor Agonist)
CF101 (IB-MECA; Can-Fite BioPharma Ltd) is an oral adenosine A3 receptor agonist. Adenosine A3 is a G protein–coupled receptor that has an anti-inflammatory role, which lends itself to the treatment of inflammatory conditions such as rheumatoid arthritis.17 In a randomized, placebo-controlled, double-blind phase 2 trial of 75 patients who received placebo or CF101 1, 2, or 4 mg twice daily for 12 weeks, PASI 50 or greater was reported in 35.3% of participants in the 2-mg group, which was statistically significant at weeks 8 (P=.047) and 12 (P=.031).18 A randomized, placebo-controlled, double-blind, 16-week phase 2/phase 3 trial in patients with moderate to severe psoriasis treated with CF101 2 mg twice daily versus placebo is ongoing but not recruiting participants (NCT01265667).
Tofacitinib (JAK 1 and JAK 3 Inhibitor)
Tofacitinib is a JAK 1 and JAK 3 inhibitor that is available in both topical and oral formulations. Many studies have been published on tofacitinib, including a dose-ranging phase 2b trial of 197 patients randomized to placebo or tofacitinib 2, 5, or 15 mg daily in which 2.0%, 25.0% (P<.001), 40.8% (P<.0001), and 66.7% (P<.0001) of participants in each treatment group, respectively, achieved PASI 75 at week 12.19 Another 12-week phase 2b trial in patients with moderate to severe psoriasis explored the effectiveness of oral formulations of tofacitinib based on body location, namely the head and neck, arms, trunk, and legs.20 Designed with twice-daily placebo and tofacitinib 2-, 5-, and 15-mg treatment arms, the target plaque severity score demonstrated a statistically significant dose-responsive improvement in each of the 4 anatomic regions (P<.01).
Two randomized, double-blinded, placebo-controlled phase 3 trials of tofacitinib have been completed. One trial compared the safety and effectiveness of tofacitinib and etanercept in patients with moderate to severe psoriasis.21 The 329 participants were randomized to treatment with oral tofacitinib 5 mg or 10 mg twice daily with placebo subcutaneous (SQ) injections twice weekly; oral placebo twice daily with etanercept 50-mg SQ injections twice weekly; or oral placebo twice daily with placebo SQ injection twice weekly. At week 12, PASI 75 was achieved in 39.51%, 63.64%, 58.81%, and 5.61% of participants in each treatment group, respectively. Thus, tofacitinib 10 mg and etanercept 50 mg were the first and second best performers in this study design.21
In the other phase 3 trial, the efficacy and safety of treatment, treatment withdrawal, and subsequent resumption of treatment with tofacitinib was examined in 290 patients who received either tofacitinib 5 mg or 10 mg or placebo twice daily for 24 weeks.22 In the withdrawal period, the percentage of patients who maintained a PASI 75 response in the tofacitinib 5-mg group was 56.2% versus 23.3% in the placebo group at week 16 (P<.0008). In the 10-mg group, 62.3% of participants maintained PASI 75 at week 16 versus 26.1% in the placebo group (P<.0001). During the re-treatment period for those who showed a greater than 50% reduction in week 24 PASI during treatment withdrawal (N=102), 50.0% of the tofacitinib 5-mg group showed PASI 75 versus 31.58% of the placebo group at week 16. In the tofacitinib 10-mg group, PASI 75 was seen in 0% versus 50.85% in the placebo group. Of note, only 5 participants who demonstrated a greater than 50% reduction in week 24 PASI response following withdrawal of therapy were in the tofacitinib 5- or 10-mg groups, as the rest were in the placebo group.22
FP187 (Fumaric Acid Ester)
FP187 (Forward Pharma A/S) is an oral fumaric acid ester whose underlying mechanism is thought to be elevation of glutathione levels that decreases the amount of inflammatory cytokines by blocking the translocation of nuclear factor κB. Other fumaric acid esters such as monoethyl fumarate and dimethyl fumarate have been used in Europe for the management of psoriasis.23 A phase 2 study of the safety and effectiveness of FP187 for moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01230138). A phase 3 trial examining the efficacy of FP187 in managing moderate to severe psoriasis was not yet open for participant recruitment at the time of publication (NCT01815723).
Doxercalciferol (Vitamin D2 Analogue)
Doxercalciferol (1α-hydroxyvitamin D2; Genzyme Corporation, a Sanofi company) is an oral prodrug of vitamin D.24 Topical preparations of vitamin D analogues approved by the US Food and Drug Administration such as calcipotriene are mainstays in psoriasis management. Doxercalciferol has been used for other therapeutic applications such as decreasing elevated parathyroid hormone levels.25 Research has demonstrated that CYP24A1 can extrahepatically regulate the activation of vitamin D prodrugs in cutaneous tissues.26 In a phase 2 study examining the efficacy and safety of doxercalciferol in patients with moderate to severe psoriasis, 111 patients were randomized to placebo or doxercalciferol 2.5, 5, or 7.5 mg daily for 24 weeks (NCT00601107). At week 12, PASI 50 was similar regardless of the treatment administered, reported at 20.0%, 20.0%, 17.9%, and 20.0%, respectively (P=1.000).27
GSK2586184 (JAK 1 Inhibitor)
GSK2586184 is an oral JAK 1 inhibitor. A placebo-controlled, double-blind, dose-dependent phase 2 study of GSK2586184 in patients with chronic plaque psoriasis who were randomized to placebo or GSK2586184 100, 200, and 400 mg twice daily for 84 days has been completed, but the results were not available at the time of publication (NCT01782664). Of note, despite clinical promise, due to potential adverse effects of the medication that included a possible interaction with statin medication, the manufacturer decided against pursuing GSK2586184 as a treatment in the management of psoriasis.28
Voclosporin (Calcineurin Inhibitor)
Voclosporin (formerly known as ISA247)(Aurinia Pharmaceuticals Inc) is an oral calcineurin inhibitor that is purported to be as effective as cyclospor-ine A with less associated toxicity. A 12-week randomized, placebo-controlled, double-blind phase 2 trial demonstrated PASI 75 in 0%, 18.2%, and 66.7% of 201 participants receiving placebo, voclospo-rin 0.5 mg/kg, and voclosporin 1.5 mg/kg twice daily, respectively (P<.0001). Elevated serum creatinine levels within the high range of normal were noted in the 1.5-mg/kg group.29 A 12-week phase 3 study of 451 patients randomized to placebo or voclospo-rin 0.2-, 0.3-, or 0.4-mg/kg treatment groups similarly demonstrated a dose-responsive PASI 75 of 4%, 16%, 25%, and 47%, respectively, that was maintained at week 24. Mild to moderate decreases in glomerular filtration rates were noted in 8 patients in the 0.3- and 0.4-mg/kg subsets.30 Another phase 2 study of voclosporin in patients with moderate to severe psoriasis showed improvements according to the psoriasis disability index and dermatology life quality index.31
Three randomized, placebo-controlled, double-blind phase 3 trials have been completed for voclosporin, but the results were not available at the time of publication. The phase 3 ESSENCE trial compared voclosporin to placebo and ciclosporin controls (NCT00408187). Two phase 3 trials known as the SPIRIT trials—one a 12-week study (NCT00244842) and the other a 36-week extension trial (NCT00258713)—compared the efficacy of placebo to voclosporin administered at 0.2-, 0.3-, and 0.4-mg/kg doses.
KD025 (ROCK 2 Inhibitor)
KD025 (Kadmon Corporation, LLC) is an oral ROCK 2 inhibitor. The inhibition of ROCK in the ras homolog gene family, member A/ROCK pathway has been targeted therapeutically for pulmonary arterial hypertension,32 glaucoma,33 and many other uses. A phase 2a study assessing the tolerability and safety profile of KD025 in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT02106195).
LAS41008 (Fumaric Acid Ester)
LAS41008 (Almirall, S.A.) is purported to be an oral dimethyl fumarate that inhibits endothelial cell proliferation, migration, and differentiation.34 A phase 3 trial comparing the safety and effectiveness of LAS41008, LASW1835 (an active comparator), and placebo in patients with moderate to severe psoriasis is ongoing but not recruiting participants (NCT01726933).
LEO 22811 (Anti-inflammatory)
LEO 22811 (LEO Pharma) is an oral anti-inflammatory agent for the treatment of psoriasis. Two clinical trials have been completed, the results of which have not yet been published. A phase 1 trial assessing the tolerability and safety profile of LEO 22811 (NCT00833872) and a phase 2 proof-of-concept study assessing dose response of LEO 22811 versus placebo (NCT01116895) were completed, but the results were not available at the time of publication.
Baricitinib (JAK 1 and JAK 2 Inhibitor)
As noted above, baricitinib (LY3009104 or INCB28050) is a JAK 1 and JAK 2 inhibitor. A phase 2b trial examining dose response for LY3009104 or baricitinib administration in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01490632).
Talarozole (CYP26 Inhibitor)
Talarozole (formerly known as R115866)(GlaxoSmithKline) is a selective oral CYP26 inhibitor that could serve to regulate the degradation of all-trans retinoic acid in the management of conditions such as acne and psoriasis. One phase 2 nonrandomized, open-label study (NCT00725348) and another phase 2 randomized, placebo-controlled, double-blind, dose-dependent trial examining the effectiveness and safety profile of 12-week talarozole treatment in patients with severe plaque psoriasis have been completed, but the results were not available at the time of publication (NCT00716144).
R3421 or BCX-4208 (PNP Inhibitor)
R3421 or BCX-4208 (BioCryst Pharmaceuticals, Inc/Hoffman–La Roche) is an oral PNP inhibitor that may help regulate apoptosis of T cells and B cells.35 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT00504270).
RWJ-445380 (Cathepsin S Inhibitor)
RWJ-445380 (Alza Corporation, DE) is an oral cathepsin S inhibitor. Cathepsin S is produced by antigen-presenting cells and activates CD4+ T cells via presentation of antigen by class II major histocompatibility complex.36 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial evaluating the tolerability, safety, pharmacodynamics, and pharmacokinetics of RWJ-445380 in patients with plaque psoriasis has been completed, but the results were not available at the time of publication (NCT00396422).
SRT2104 (Sirtuin 1 Activator)
SRT2104 (GlaxoSmithKline) is a selective oral NAD+-dependent deacetylase sirtuin 1 activator. Sirtuins help regulate apoptosis, inflammation, and other important cellular mechanisms.37 A randomized, open-label phase 1 trial examining drug bioavailability (NCT01702493) and a randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial studying the efficacy, tolerability, and safety of SRT2104 in patients with moderate to severe psoriasis have been completed (NCT01154101), but the results were not available at the time of publication.
VB-201 (Oxidized Phospholipid)
VB-201 (VBL Therapeutics) is an orally administered oxidized phospholipid analogue with anti-inflammatory effects.38 Oxidized phospholipids are another element in the intricate spectrum of inflammatory mediators. A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 20 mg or 80 mg daily in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT01001468). Another randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 80 mg and 160 mg twice daily in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01837420).
Conclusion
We are living in an exciting time in the treatment of psoriasis and PsA, with promising novel therapies afforded by the discovery of new therapeutic targets. In this 3-part series, we have reviewed topical, systemic, and biologic therapies that currently are in phase 2 through phase 4 clinical trials or have recently been approved by the US Food and Drug Administration for the management of psoriasis and PsA. These treatments offer patients and their caregivers the prospect of more targeted therapeutic regimens that offer enhanced clinical outcomes with more favorable side-effect profiles. As clinicians and researchers build upon this knowledge in the years to come, we can offer psoriasis patients an increasingly diverse and powerful therapeutic armamentarium.
Evolving knowledge of the underlying pathogenesis of psoriasis has afforded the development of a broad spectrum of nonbiologic systemic medications with therapeutic potential in moderate to severe psoriasis and psoriatic arthritis (PsA). The targets for these medications are antagonists of proinflammatory mediators such as tyrosine kinase, protein kinase, Janus kinase (JAK), p38α mitogen-activated protein (MAP) kinase, phosphodiesterase 4 (PDE-4), calcineurin, Rho-associated kinase (ROCK) 2, cytochrome P450 26 (CYP26), and purine nucleoside phosphorylase (PNP); agonists of anti-inflammatory mediators such as sphingosine-1-phosphate receptor 1 (S1P1) and adeno-sine A3 receptor; and myriad other mechanisms. A brief introduction to some of these therapies presently in phase 2 and phase 3 clinical trials are presented (Table).1
Masitinib (Tyrosine Kinase Inhibitor)
Masitinib (formerly known as AB1010)(AB Sciences) is a tyrosine kinase inhibitor that is purported to decrease inflammation by inhibiting stem cell factor receptor (c-kit) and consequently limiting mast cell degranulation.2 A randomized, placebo-controlled, double-blind phase 3 study evaluating its efficacy as an oral formulation was completed, but the results were not available at the time of publication (registered at www.clinicaltrials.gov with the identifier NCT01045577).
Ponesimod (S1P1 Receptor Agonist)
Ponesimod (formerly known as ACT-128800)(Actelion Pharmaceuticals US, Inc) is an orally formulated S1P1 receptor agonist. Sphingosine-1-phosphate receptor 1 is necessary for lymphoid chemotaxis.3 A randomized, placebo-controlled, double-blind phase 2 trial of 326 patients demonstrated 75% improvement in psoriasis area and severity index (PASI) score at week 16 in 13.4%, 46.0%, and 48.1% of participants receiving placebo, ponesimod 20 mg, and ponesimod 40 mg, respectively.4 At week 28, PASI 75 scores for participants transitioned from ponesimod 40 mg to placebo or ponesimod 20 mg to placebo and those maintained on ponesimod 20 mg and 40 mg were 40.4%, 42.2%, 77.4%, and 71.4%, respectively. This study demonstrated benefit of treatment with ponesimod versus placebo with increased efficacy using maintenance therapy.4
Sotrastaurin (Protein Kinase C Inhibitor)
Sotrastaurin (formerly known as AEB071)(Novartis AG) is an oral medication that inhibitsprotein kinase C, thereby limiting CD28-induced activation of T cells. Furthermore, it increases forkhead box P3 expression, which is important, as proinflammatory IL-17 production is stimulated in regulatory T cells with lost forkhead box P3 expression.5 In a study of 32 patients who received placebo or sotrastaurin 25, 100, 200, or 300 mg twice daily for 2 weeks, the mean PASI score was reduced by 69% for the 300-mg group versus 5.3% for placebo.6 A randomized, placebo-controlled, double-blind phase 2 study was completed for patients with moderate to severe psoriasis, but the results were not available at the time of publication (NCT00885196).
Alitretinoin (Retinoid)
Alitretinoin (9-cis-retinoic acid; Stiefel, a GSK company) is an oral retinoid with purported promise for patients with palmoplantar pustular psoriasis recalcitrant to conventional therapies. In one study, 7 participants with palmoplantar psoriasis received alitretinoin 30 mg daily for 12 weeks with 60% to 90% clinical improvement noted using the visual analog scale and palmoplantar pustular PASI to assess response.7 A randomized, placebo-controlled, double-blind phase 2 trial of alitretinoin assessing the success of alitretinoin in patients with palmoplantar psoriasis recalcitrant to topical treatments was completed, but the results were not available at the time of publication (NCT01245140).
Apo805K1 (Unknown Mechanism of Action)
Apo805K1 (ApoPharma Inc) is an oral agent whose mechanism of action has not been disclosed. A randomized, placebo-controlled, double-blind phase 2 trial in patients with moderate to severe psoriasis with a treatment duration of 14 days has been completed. For 12 weeks there was a daily dosing regimen of Apo805K1 10, 30, 60, or 100 mg or placebo, with 12 patients in each treatment group. The proportion of patients achieving PASI 75 was 16.7%, 0%, 0%, 8.3%, and 16.7%, respectively (P=.1975). The number of participants with adverse events reported ranged between 4 to 7, with the greatest number of adverse events reported in the 30-mg subset.8 It may be of interest to repeat the study with a larger sample size.
ASP015K (JAK 1 and JAK 3 Inhibitor)
ASP015K (Astellas Pharma US, Inc) is the first of the JAK inhibitors that will be discussed in this section. Janus kinase inhibitors represent a group of tyrosine kinases that regulate cytokine-mediated signaling pathways through the activation of signal transducer and activator of transcription proteins via phosphorylation in the cytoplasm, which in turn control the transcription of genes that generate inflammation. ASP015K and tofacitinib (formerly known as CP-690550)(Pfizer, Inc) inhibit JAK 1 and JAK 3, whereas GSK2586184 (GlaxoSmithKline) inhibits JAK 1 and baricitinib (formerly known as LY3009104 or INCB28050)(Eli Lilly and Company) inhibits JAK 1 and JAK 2.9 In a 6-week, dose-escalation phase 2 trial in patients with moderate to severe psoriasis, ASP015K showed a dose-dependent decline in PASI, psoriasis static global assessment, and body surface area.10
BMS-582949 (p38α MAP Kinase Inhibitor)
BMS-582949 (Bristol-Myers Squibb Company) is an oral p38α MAP kinase inhibitor.11 Along with c-Jun N-terminal kinase and extracellular signal-regulated protein kinases 1 and 2, MAP kinase plays a role in the pathogenesis of psoriasis.12 A randomized, placebo-controlled, double-blind, 12-week phase 2a study of placebo versus BMS-582949 dosed at 10, 30, and 100 mg has been completed, but the results were not available at the time of publication (NCT00399906).
Apremilast (PDE-4 Inhibitor)
Apremilast (formerly known as CC-10004)(Celgene Corporation) is an oral PDE-4 inhibitor that acts to inhibit the degradation of cyclic adenosine monophosphate. It is approved by the US Food and Drug Administration for moderate to severe plaque psoriasis13 and is a particularly good treatment for patients with recalcitrant disease.14 Several studies on apremilast have been published, including a phase 2 trial of 204 participants receiving placebo or apremilast 20 mg or 40 mg twice daily with American College of Rheumatology (ACR) 20% improvement response of 11.8%, 35.8%, and 43.5%, respectively.15 The phase 3 PALACE 1, 2, 3, and 4 trials also demonstrated therapeutic efficacy.16 In PALACE 1, 504 patients receiving placebo or apremilast 20 mg and 30 mg twice daily had an ACR20 of 19%, 31%, and 40%, respectively. In the PALACE 4 study, ACR20 was achieved by 58% of participants at week 52, but the ACR50 and ACR70 rates were less impressive.16
Lestaurtinib (Multikinase Inhibitor)
Lestaurtinib (formerly known as CEP-701)(Teva Pharmaceutical Industries) is an oral multikinase inhibitor for which a 12-week, nonrandomized, dose-escalation phase 2 study was completed, but the results were not available at the time of publication (NCT00236119).
CF101 (Adenosine A3 Receptor Agonist)
CF101 (IB-MECA; Can-Fite BioPharma Ltd) is an oral adenosine A3 receptor agonist. Adenosine A3 is a G protein–coupled receptor that has an anti-inflammatory role, which lends itself to the treatment of inflammatory conditions such as rheumatoid arthritis.17 In a randomized, placebo-controlled, double-blind phase 2 trial of 75 patients who received placebo or CF101 1, 2, or 4 mg twice daily for 12 weeks, PASI 50 or greater was reported in 35.3% of participants in the 2-mg group, which was statistically significant at weeks 8 (P=.047) and 12 (P=.031).18 A randomized, placebo-controlled, double-blind, 16-week phase 2/phase 3 trial in patients with moderate to severe psoriasis treated with CF101 2 mg twice daily versus placebo is ongoing but not recruiting participants (NCT01265667).
Tofacitinib (JAK 1 and JAK 3 Inhibitor)
Tofacitinib is a JAK 1 and JAK 3 inhibitor that is available in both topical and oral formulations. Many studies have been published on tofacitinib, including a dose-ranging phase 2b trial of 197 patients randomized to placebo or tofacitinib 2, 5, or 15 mg daily in which 2.0%, 25.0% (P<.001), 40.8% (P<.0001), and 66.7% (P<.0001) of participants in each treatment group, respectively, achieved PASI 75 at week 12.19 Another 12-week phase 2b trial in patients with moderate to severe psoriasis explored the effectiveness of oral formulations of tofacitinib based on body location, namely the head and neck, arms, trunk, and legs.20 Designed with twice-daily placebo and tofacitinib 2-, 5-, and 15-mg treatment arms, the target plaque severity score demonstrated a statistically significant dose-responsive improvement in each of the 4 anatomic regions (P<.01).
Two randomized, double-blinded, placebo-controlled phase 3 trials of tofacitinib have been completed. One trial compared the safety and effectiveness of tofacitinib and etanercept in patients with moderate to severe psoriasis.21 The 329 participants were randomized to treatment with oral tofacitinib 5 mg or 10 mg twice daily with placebo subcutaneous (SQ) injections twice weekly; oral placebo twice daily with etanercept 50-mg SQ injections twice weekly; or oral placebo twice daily with placebo SQ injection twice weekly. At week 12, PASI 75 was achieved in 39.51%, 63.64%, 58.81%, and 5.61% of participants in each treatment group, respectively. Thus, tofacitinib 10 mg and etanercept 50 mg were the first and second best performers in this study design.21
In the other phase 3 trial, the efficacy and safety of treatment, treatment withdrawal, and subsequent resumption of treatment with tofacitinib was examined in 290 patients who received either tofacitinib 5 mg or 10 mg or placebo twice daily for 24 weeks.22 In the withdrawal period, the percentage of patients who maintained a PASI 75 response in the tofacitinib 5-mg group was 56.2% versus 23.3% in the placebo group at week 16 (P<.0008). In the 10-mg group, 62.3% of participants maintained PASI 75 at week 16 versus 26.1% in the placebo group (P<.0001). During the re-treatment period for those who showed a greater than 50% reduction in week 24 PASI during treatment withdrawal (N=102), 50.0% of the tofacitinib 5-mg group showed PASI 75 versus 31.58% of the placebo group at week 16. In the tofacitinib 10-mg group, PASI 75 was seen in 0% versus 50.85% in the placebo group. Of note, only 5 participants who demonstrated a greater than 50% reduction in week 24 PASI response following withdrawal of therapy were in the tofacitinib 5- or 10-mg groups, as the rest were in the placebo group.22
FP187 (Fumaric Acid Ester)
FP187 (Forward Pharma A/S) is an oral fumaric acid ester whose underlying mechanism is thought to be elevation of glutathione levels that decreases the amount of inflammatory cytokines by blocking the translocation of nuclear factor κB. Other fumaric acid esters such as monoethyl fumarate and dimethyl fumarate have been used in Europe for the management of psoriasis.23 A phase 2 study of the safety and effectiveness of FP187 for moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01230138). A phase 3 trial examining the efficacy of FP187 in managing moderate to severe psoriasis was not yet open for participant recruitment at the time of publication (NCT01815723).
Doxercalciferol (Vitamin D2 Analogue)
Doxercalciferol (1α-hydroxyvitamin D2; Genzyme Corporation, a Sanofi company) is an oral prodrug of vitamin D.24 Topical preparations of vitamin D analogues approved by the US Food and Drug Administration such as calcipotriene are mainstays in psoriasis management. Doxercalciferol has been used for other therapeutic applications such as decreasing elevated parathyroid hormone levels.25 Research has demonstrated that CYP24A1 can extrahepatically regulate the activation of vitamin D prodrugs in cutaneous tissues.26 In a phase 2 study examining the efficacy and safety of doxercalciferol in patients with moderate to severe psoriasis, 111 patients were randomized to placebo or doxercalciferol 2.5, 5, or 7.5 mg daily for 24 weeks (NCT00601107). At week 12, PASI 50 was similar regardless of the treatment administered, reported at 20.0%, 20.0%, 17.9%, and 20.0%, respectively (P=1.000).27
GSK2586184 (JAK 1 Inhibitor)
GSK2586184 is an oral JAK 1 inhibitor. A placebo-controlled, double-blind, dose-dependent phase 2 study of GSK2586184 in patients with chronic plaque psoriasis who were randomized to placebo or GSK2586184 100, 200, and 400 mg twice daily for 84 days has been completed, but the results were not available at the time of publication (NCT01782664). Of note, despite clinical promise, due to potential adverse effects of the medication that included a possible interaction with statin medication, the manufacturer decided against pursuing GSK2586184 as a treatment in the management of psoriasis.28
Voclosporin (Calcineurin Inhibitor)
Voclosporin (formerly known as ISA247)(Aurinia Pharmaceuticals Inc) is an oral calcineurin inhibitor that is purported to be as effective as cyclospor-ine A with less associated toxicity. A 12-week randomized, placebo-controlled, double-blind phase 2 trial demonstrated PASI 75 in 0%, 18.2%, and 66.7% of 201 participants receiving placebo, voclospo-rin 0.5 mg/kg, and voclosporin 1.5 mg/kg twice daily, respectively (P<.0001). Elevated serum creatinine levels within the high range of normal were noted in the 1.5-mg/kg group.29 A 12-week phase 3 study of 451 patients randomized to placebo or voclospo-rin 0.2-, 0.3-, or 0.4-mg/kg treatment groups similarly demonstrated a dose-responsive PASI 75 of 4%, 16%, 25%, and 47%, respectively, that was maintained at week 24. Mild to moderate decreases in glomerular filtration rates were noted in 8 patients in the 0.3- and 0.4-mg/kg subsets.30 Another phase 2 study of voclosporin in patients with moderate to severe psoriasis showed improvements according to the psoriasis disability index and dermatology life quality index.31
Three randomized, placebo-controlled, double-blind phase 3 trials have been completed for voclosporin, but the results were not available at the time of publication. The phase 3 ESSENCE trial compared voclosporin to placebo and ciclosporin controls (NCT00408187). Two phase 3 trials known as the SPIRIT trials—one a 12-week study (NCT00244842) and the other a 36-week extension trial (NCT00258713)—compared the efficacy of placebo to voclosporin administered at 0.2-, 0.3-, and 0.4-mg/kg doses.
KD025 (ROCK 2 Inhibitor)
KD025 (Kadmon Corporation, LLC) is an oral ROCK 2 inhibitor. The inhibition of ROCK in the ras homolog gene family, member A/ROCK pathway has been targeted therapeutically for pulmonary arterial hypertension,32 glaucoma,33 and many other uses. A phase 2a study assessing the tolerability and safety profile of KD025 in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT02106195).
LAS41008 (Fumaric Acid Ester)
LAS41008 (Almirall, S.A.) is purported to be an oral dimethyl fumarate that inhibits endothelial cell proliferation, migration, and differentiation.34 A phase 3 trial comparing the safety and effectiveness of LAS41008, LASW1835 (an active comparator), and placebo in patients with moderate to severe psoriasis is ongoing but not recruiting participants (NCT01726933).
LEO 22811 (Anti-inflammatory)
LEO 22811 (LEO Pharma) is an oral anti-inflammatory agent for the treatment of psoriasis. Two clinical trials have been completed, the results of which have not yet been published. A phase 1 trial assessing the tolerability and safety profile of LEO 22811 (NCT00833872) and a phase 2 proof-of-concept study assessing dose response of LEO 22811 versus placebo (NCT01116895) were completed, but the results were not available at the time of publication.
Baricitinib (JAK 1 and JAK 2 Inhibitor)
As noted above, baricitinib (LY3009104 or INCB28050) is a JAK 1 and JAK 2 inhibitor. A phase 2b trial examining dose response for LY3009104 or baricitinib administration in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01490632).
Talarozole (CYP26 Inhibitor)
Talarozole (formerly known as R115866)(GlaxoSmithKline) is a selective oral CYP26 inhibitor that could serve to regulate the degradation of all-trans retinoic acid in the management of conditions such as acne and psoriasis. One phase 2 nonrandomized, open-label study (NCT00725348) and another phase 2 randomized, placebo-controlled, double-blind, dose-dependent trial examining the effectiveness and safety profile of 12-week talarozole treatment in patients with severe plaque psoriasis have been completed, but the results were not available at the time of publication (NCT00716144).
R3421 or BCX-4208 (PNP Inhibitor)
R3421 or BCX-4208 (BioCryst Pharmaceuticals, Inc/Hoffman–La Roche) is an oral PNP inhibitor that may help regulate apoptosis of T cells and B cells.35 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT00504270).
RWJ-445380 (Cathepsin S Inhibitor)
RWJ-445380 (Alza Corporation, DE) is an oral cathepsin S inhibitor. Cathepsin S is produced by antigen-presenting cells and activates CD4+ T cells via presentation of antigen by class II major histocompatibility complex.36 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial evaluating the tolerability, safety, pharmacodynamics, and pharmacokinetics of RWJ-445380 in patients with plaque psoriasis has been completed, but the results were not available at the time of publication (NCT00396422).
SRT2104 (Sirtuin 1 Activator)
SRT2104 (GlaxoSmithKline) is a selective oral NAD+-dependent deacetylase sirtuin 1 activator. Sirtuins help regulate apoptosis, inflammation, and other important cellular mechanisms.37 A randomized, open-label phase 1 trial examining drug bioavailability (NCT01702493) and a randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial studying the efficacy, tolerability, and safety of SRT2104 in patients with moderate to severe psoriasis have been completed (NCT01154101), but the results were not available at the time of publication.
VB-201 (Oxidized Phospholipid)
VB-201 (VBL Therapeutics) is an orally administered oxidized phospholipid analogue with anti-inflammatory effects.38 Oxidized phospholipids are another element in the intricate spectrum of inflammatory mediators. A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 20 mg or 80 mg daily in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT01001468). Another randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 80 mg and 160 mg twice daily in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01837420).
Conclusion
We are living in an exciting time in the treatment of psoriasis and PsA, with promising novel therapies afforded by the discovery of new therapeutic targets. In this 3-part series, we have reviewed topical, systemic, and biologic therapies that currently are in phase 2 through phase 4 clinical trials or have recently been approved by the US Food and Drug Administration for the management of psoriasis and PsA. These treatments offer patients and their caregivers the prospect of more targeted therapeutic regimens that offer enhanced clinical outcomes with more favorable side-effect profiles. As clinicians and researchers build upon this knowledge in the years to come, we can offer psoriasis patients an increasingly diverse and powerful therapeutic armamentarium.
1. Lu PD, Mazza JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:227-242.
2. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194-1201.
3. Krause A, Brossard P, D’Ambrosio D, et al. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. J Pharmacokinet Pharmacodyn. 2014;41:261-278.
4. Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036-2045.
5. He X, Koenen HJ, Smeets RL, et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J Invest Dermatol. 2014;134:975-983.
6. Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118:3151-3159.
7. Irla N, Navarini AA, Yawalkar N. Alitretinoin abrogates innate inflammation in palmoplantar pustular psoriasis. Br J Dermatol. 2012;167:1170-1174.
8. Study to evaluate Apo805K1 in subjects with moderate to severe chronic plaque psoriasis (NCT01483924). https://clinicaltrials.gov/ct2/results?term=NCT01483924&Search=Search. Updated February 9, 2015. Accessed June 23, 2015.
9. Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133-139.
10. Gooderham M. Small molecules: an overview of emerging therapeutic options in the treatment of psoriasis. Skin Therapy Lett. 2013;18:1-4.
11. Liu C, Lin J, Wrobleski ST, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629-6639.
12. Mavropoulos A, Rigopoulou EI, Liaskos C, et al. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751.
13. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celegene Corporation; September 23, 2014. http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed June 23, 2015.
14. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888-897.
15. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
16. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
17. Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359-366.
18. David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
19. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
20. Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252-256.
21. A phase 3, multi site, randomized, double blind, placebo controlled study of the efficacy and safety comparing CP-690,550 and etanercept in subjects with moderate to severe chronic plaque psoriasis (NCT01241591). https://clinicaltrials.gov/ct2/show/NCT01241591?term=01241591&rank=1. Updated May 8, 2014. Accessed June 16, 2015.
22. A study to evaluate the effects and safety of treatment, treatment withdrawal, followed by re-treatment with CP-690,550 in subjects with moderate to severe chronic plaque psoriasis (NCT01186744). https://clinicaltrials.gov/ct2/show/NCT01186744?term=01186744&rank=1. Updated May 14, 2014. Accessed June 16, 2015.
23. Rostami Yazdi M, Mrowietz U. Fumaric acid esters. Clin Dermatol. 2008;26:522-526.
24. Kubodera N. A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol. Molecules. 2009;14:3869-3880.
25. Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 suppl 5):S3-S19.
26. Masuda S, Strugnell SA, Knutson JC, et al. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221-234.
27. A study to evaluate the safety and effectiveness of doxercalciferol capsules in participants with moderate to severe psoriasis (NCT00601107). https://clinicaltrials.gov/ct2/show/NCT00601107?term=00601107&rank=1. Updated April 2, 2014. Accessed June 16, 2015.
28. GSK discloses good efficacy in psoriasis with GSK2856184 [news release]. Mechelen, Belgium: Galapagos NV; November 6, 2014. http://www.globenewswire.com/news-release/2014/11/06/680358/10106744/en/GSK-discloses-good-efficacy-in-psoriasis-with-GSK2856184.html. Accessed June 24, 2015.
29. Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472-478.
30. Papp K, Bissonnette R, Rosoph L, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337-1342.
31. Gupta AK, Langley RG, Lynde C, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. J Cutan Med Surg. 2008;12:268-275.
32. Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363.
33. Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81-95.
34. National Institute for Health Research Horizon Scanning Centre. Dimethyl fumarate for plaque psoriasis. http://www.hsc.nihr.ac.uk/files/downloads/2228/2526.163fbff8.Dimethylfumarate_Nov13.pdf. Published November 2013. Accessed June 15, 2015.
35. Bantia S, Parker C, Upshaw R, et al. Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol. 2010;10:784-790.
36. García-Pérez ME, Stevanovic T, Poubelle PE. New therapies under development for psoriasis treatment. Curr Opin Pediatr. 2013;25:480-487.
37. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359.
38. Feige E, Mendel I, George J, et al. Modified phospholipids as anti-inflammatory compounds. Curr Opin Lipidol. 2010;21:525-529.
1. Lu PD, Mazza JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:227-242.
2. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194-1201.
3. Krause A, Brossard P, D’Ambrosio D, et al. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. J Pharmacokinet Pharmacodyn. 2014;41:261-278.
4. Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036-2045.
5. He X, Koenen HJ, Smeets RL, et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J Invest Dermatol. 2014;134:975-983.
6. Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118:3151-3159.
7. Irla N, Navarini AA, Yawalkar N. Alitretinoin abrogates innate inflammation in palmoplantar pustular psoriasis. Br J Dermatol. 2012;167:1170-1174.
8. Study to evaluate Apo805K1 in subjects with moderate to severe chronic plaque psoriasis (NCT01483924). https://clinicaltrials.gov/ct2/results?term=NCT01483924&Search=Search. Updated February 9, 2015. Accessed June 23, 2015.
9. Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133-139.
10. Gooderham M. Small molecules: an overview of emerging therapeutic options in the treatment of psoriasis. Skin Therapy Lett. 2013;18:1-4.
11. Liu C, Lin J, Wrobleski ST, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629-6639.
12. Mavropoulos A, Rigopoulou EI, Liaskos C, et al. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751.
13. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celegene Corporation; September 23, 2014. http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed June 23, 2015.
14. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888-897.
15. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
16. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
17. Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359-366.
18. David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
19. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
20. Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252-256.
21. A phase 3, multi site, randomized, double blind, placebo controlled study of the efficacy and safety comparing CP-690,550 and etanercept in subjects with moderate to severe chronic plaque psoriasis (NCT01241591). https://clinicaltrials.gov/ct2/show/NCT01241591?term=01241591&rank=1. Updated May 8, 2014. Accessed June 16, 2015.
22. A study to evaluate the effects and safety of treatment, treatment withdrawal, followed by re-treatment with CP-690,550 in subjects with moderate to severe chronic plaque psoriasis (NCT01186744). https://clinicaltrials.gov/ct2/show/NCT01186744?term=01186744&rank=1. Updated May 14, 2014. Accessed June 16, 2015.
23. Rostami Yazdi M, Mrowietz U. Fumaric acid esters. Clin Dermatol. 2008;26:522-526.
24. Kubodera N. A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol. Molecules. 2009;14:3869-3880.
25. Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 suppl 5):S3-S19.
26. Masuda S, Strugnell SA, Knutson JC, et al. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221-234.
27. A study to evaluate the safety and effectiveness of doxercalciferol capsules in participants with moderate to severe psoriasis (NCT00601107). https://clinicaltrials.gov/ct2/show/NCT00601107?term=00601107&rank=1. Updated April 2, 2014. Accessed June 16, 2015.
28. GSK discloses good efficacy in psoriasis with GSK2856184 [news release]. Mechelen, Belgium: Galapagos NV; November 6, 2014. http://www.globenewswire.com/news-release/2014/11/06/680358/10106744/en/GSK-discloses-good-efficacy-in-psoriasis-with-GSK2856184.html. Accessed June 24, 2015.
29. Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472-478.
30. Papp K, Bissonnette R, Rosoph L, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337-1342.
31. Gupta AK, Langley RG, Lynde C, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. J Cutan Med Surg. 2008;12:268-275.
32. Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363.
33. Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81-95.
34. National Institute for Health Research Horizon Scanning Centre. Dimethyl fumarate for plaque psoriasis. http://www.hsc.nihr.ac.uk/files/downloads/2228/2526.163fbff8.Dimethylfumarate_Nov13.pdf. Published November 2013. Accessed June 15, 2015.
35. Bantia S, Parker C, Upshaw R, et al. Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol. 2010;10:784-790.
36. García-Pérez ME, Stevanovic T, Poubelle PE. New therapies under development for psoriasis treatment. Curr Opin Pediatr. 2013;25:480-487.
37. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359.
38. Feige E, Mendel I, George J, et al. Modified phospholipids as anti-inflammatory compounds. Curr Opin Lipidol. 2010;21:525-529.
Practice Points
- A wide spectrum of promising nonbiologic systemic medications presently are in development or were recently approved for the management of moderate to severe psoriasis and psoriatic arthritis (PsA).
- Systemic medications broaden the therapeutic armamentarium for patients with psoriasis and PsA, offering targeted therapeutic regimens that afford enhanced clinical outcomes with favorable side-effect profiles.
What to Do After Basal Insulin: 3 Treatment Strategies for Type 2 Diabetes
Diabetes mellitus is a complex, progressive disease that affects every primary care provider’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every two to three months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many clinicians have become comfortable with using once-daily basal insulin, such as glargine or detemir, what to do after basal insulin is much more complex. This review explains three strategies to consider when basal insulin alone isn’t enough.
3 MAIN STRATEGIES FOR INTENSIFYING TREATMENT
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated A1C despite using two or more oral agents or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial A1C levels > 7% or > 8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk for hypoglycemia, effects on cardiovascular risk factors, and other nonglycemic effects should be considered in the shared decision-making process. There are three main strategies for intensifying treatment:
Basal plus incretin therapy. Add a newer injectable agent, such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
Basal-bolus combination. Add insulin prior to all meals.
Table 1 provides details of several studies that have documented the efficacy of these three strategies.5-8
Monitoring blood glucose to guide the way
Blood glucose monitoring using a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for three meals a day and an additional bedtime reading.9 This is typically performed for three to seven days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to two to three weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
BASAL PLUS INCRETIN THERAPY
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as add-on therapy for patients whose A1C exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL or those with a basal insulin dose > 0.5 U/kg/d. The five currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in Table 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance < 30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Continue for basal plus one strategy >>
BASAL PLUS ONE STRATEGY
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The delta value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤ 50 mg/dL.17 If the delta for a meal is > 75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of < 130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the postmeal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 U/kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one (or 10%-20% of TDD per meal for basal-bolus).16
Consider the timing of administration. Rapid-acting insulin analogues exhibit peak pharmacodynamic activity 60 minutes after injection (see Table 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analogue should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 U/d to a goal of either a 90-minute to 2-hour postprandial glucose of < 140 to 180 mg/dL or the next preprandial glucose of < 130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of < 50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of < 50 mg/dL.
BASAL-BOLUS COMBINATION
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and clinician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient to ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk for hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of < 70 mg/dL, and severe hypoglycemia as < 50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such a patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for two to three weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk for hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, injection site reactions and, rarely, allergy to insulin or its vehicle.16
REFERENCES
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014;63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes
management—exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin—options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. www.empr.com/insulins/article/123739/. Accessed June 19, 2015.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Fam Phys. 2014;1:19-25.
Diabetes mellitus is a complex, progressive disease that affects every primary care provider’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every two to three months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many clinicians have become comfortable with using once-daily basal insulin, such as glargine or detemir, what to do after basal insulin is much more complex. This review explains three strategies to consider when basal insulin alone isn’t enough.
3 MAIN STRATEGIES FOR INTENSIFYING TREATMENT
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated A1C despite using two or more oral agents or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial A1C levels > 7% or > 8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk for hypoglycemia, effects on cardiovascular risk factors, and other nonglycemic effects should be considered in the shared decision-making process. There are three main strategies for intensifying treatment:
Basal plus incretin therapy. Add a newer injectable agent, such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
Basal-bolus combination. Add insulin prior to all meals.
Table 1 provides details of several studies that have documented the efficacy of these three strategies.5-8
Monitoring blood glucose to guide the way
Blood glucose monitoring using a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for three meals a day and an additional bedtime reading.9 This is typically performed for three to seven days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to two to three weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
BASAL PLUS INCRETIN THERAPY
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as add-on therapy for patients whose A1C exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL or those with a basal insulin dose > 0.5 U/kg/d. The five currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in Table 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance < 30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Continue for basal plus one strategy >>
BASAL PLUS ONE STRATEGY
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The delta value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤ 50 mg/dL.17 If the delta for a meal is > 75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of < 130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the postmeal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 U/kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one (or 10%-20% of TDD per meal for basal-bolus).16
Consider the timing of administration. Rapid-acting insulin analogues exhibit peak pharmacodynamic activity 60 minutes after injection (see Table 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analogue should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 U/d to a goal of either a 90-minute to 2-hour postprandial glucose of < 140 to 180 mg/dL or the next preprandial glucose of < 130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of < 50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of < 50 mg/dL.
BASAL-BOLUS COMBINATION
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and clinician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient to ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk for hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of < 70 mg/dL, and severe hypoglycemia as < 50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such a patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for two to three weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk for hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, injection site reactions and, rarely, allergy to insulin or its vehicle.16
REFERENCES
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014;63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes
management—exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin—options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. www.empr.com/insulins/article/123739/. Accessed June 19, 2015.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Fam Phys. 2014;1:19-25.
Diabetes mellitus is a complex, progressive disease that affects every primary care provider’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every two to three months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many clinicians have become comfortable with using once-daily basal insulin, such as glargine or detemir, what to do after basal insulin is much more complex. This review explains three strategies to consider when basal insulin alone isn’t enough.
3 MAIN STRATEGIES FOR INTENSIFYING TREATMENT
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated A1C despite using two or more oral agents or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial A1C levels > 7% or > 8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk for hypoglycemia, effects on cardiovascular risk factors, and other nonglycemic effects should be considered in the shared decision-making process. There are three main strategies for intensifying treatment:
Basal plus incretin therapy. Add a newer injectable agent, such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
Basal-bolus combination. Add insulin prior to all meals.
Table 1 provides details of several studies that have documented the efficacy of these three strategies.5-8
Monitoring blood glucose to guide the way
Blood glucose monitoring using a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for three meals a day and an additional bedtime reading.9 This is typically performed for three to seven days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to two to three weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
BASAL PLUS INCRETIN THERAPY
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as add-on therapy for patients whose A1C exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL or those with a basal insulin dose > 0.5 U/kg/d. The five currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in Table 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance < 30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Continue for basal plus one strategy >>
BASAL PLUS ONE STRATEGY
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The delta value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤ 50 mg/dL.17 If the delta for a meal is > 75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of < 130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the postmeal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 U/kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one (or 10%-20% of TDD per meal for basal-bolus).16
Consider the timing of administration. Rapid-acting insulin analogues exhibit peak pharmacodynamic activity 60 minutes after injection (see Table 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analogue should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 U/d to a goal of either a 90-minute to 2-hour postprandial glucose of < 140 to 180 mg/dL or the next preprandial glucose of < 130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of < 50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of < 50 mg/dL.
BASAL-BOLUS COMBINATION
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and clinician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient to ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk for hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of < 70 mg/dL, and severe hypoglycemia as < 50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such a patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for two to three weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk for hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, injection site reactions and, rarely, allergy to insulin or its vehicle.16
REFERENCES
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014;63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes
management—exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin—options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. www.empr.com/insulins/article/123739/. Accessed June 19, 2015.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Fam Phys. 2014;1:19-25.
Pain: Updates on Diagnostic and Treatment Modalities
IS ACETAMINOPHEN EFFECTIVE IN EASING BACK PAIN AND KNEE PAIN?
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225.
Acetaminophen is ineffective in treating lower back pain and provides minimal short-term benefit for people with osteoarthritis, a systematic review of 13 randomized, placebo-controlled trials reports.
Two independent reviewers extracted data on pain, disability, and quality of life, as well as adverse effects, patient adherence, and use of rescue medication, and found high-quality evidence that:
• Acetaminophen is ineffective for reducing pain intensity and disability, or improving quality of life in patients with low back pain.
• Acetaminophen provides significant, but not clinically important, benefit for pain and disability in patients with hip or knee osteoarthritis.
• Patients taking acetaminophen are nearly four times more likely to have abnormal results on liver function tests.
COMMENTARY
This study adds to the literature a less potent effect of acetaminophen than we have previously assumed,1,2 suggesting a significant but not clinically important effect on pain. This result is at odds with the experience of many clinicians, who use acetaminophen regularly as a first-line agent for pain. When there is a dissonance between clinical experience and emerging evidence, one has to ask why. The explanation here may be that acetaminophen, NSAIDs, and opioid analgesics all have their problems and all seem to work better for some patients than others. In clinical practice, we often start with acetaminophen, which works for some patients, and go on to other agents for those in whom acetaminophen does not provide sufficient pain control. Studies that report a small mean effect may not detect the significant effect that can occur for many patients but gets hidden in the mean (which includes patients in whom there is no effect). I am reminded of the statistician who drowned in a river with a mean depth of 3 feet. A common clinical approach, often starting with well-tolerated acetaminophen and then progressing to other agents when needed, still seems sound. —NS
REFERENCES
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
2. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586-1596. doi: 10.1016/S0140-6736(14)60805-9.
Continue for Back pain: Does early imaging improve outcomes? >>
BACK PAIN: DOES EARLY IMAGING IMPROVE OUTCOMES?
Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313(11):1143-1153.
Early imaging for back pain is not associated with better one-year outcomes among patients ages 65 and older, according to a prospective cohort study of 5,239 older patients with a new primary care visit for back pain.
Investigators compared function and pain at the 12-month follow-up visit among 1,174 patients who had early radiographs, 349 patients who had early MRI/CT, and 3,719 controls.
The primary outcome was back or leg pain-related disability, as measured by a back and leg pain disability score. The mean score showed no significant differences between groups.
COMMENTARY
Many guidelines suggest consideration of imaging early on in the diagnosis of low back pain in older adults due to the high prevalence of important underlying causes such as cancer.1 This study looked at an older population and showed, as has been demonstrated in younger populations, that there is no advantage to early imaging with either x-ray or MRI, though costs were about 25% higher. —NS
REFERENCE
1. Davis PC, Wippold FJ II, Brunberg JA, et al. ACR Appropriateness Criteria on low back pain. J Am Coll Radiol. 2009;6(6):401-407.
Continue for anti-inflammatory drugs and antithrombotic therapy >>
ANTI-INFLAMMATORY DRUGS AND ANTITHROMBOTIC THERAPY
Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Combining prescription NSAIDs with antithrombotic therapy following myocardial infarction (MI) increases the risk for bleeding and excess thrombotic events, according to a study of 61,971 MI patients with ongoing antithrombotic therapy.
During an average of 3.5 years’ follow-up, patients who had taken NSAIDs had increased rates of bleeding and cardiovascular events, with incidence rates per 100-person years as follows:
The study authors note that clinicians should use caution when prescribing NSAIDs to patients who have recently experienced MI.
COMMENTARY
The use of NSAIDs in patients who have had coronary disease has been an area of concern for almost a decade. In 2007, the American Heart Association issued a scientific advisory update discouraging use of COX-2 inhibitors in patients with coronary disease and concluding that more data are needed on the cardiovascular safety of conventional NSAIDs.1 Non-COX-2 selective NSAIDs, such as naproxen, appear to have a better cardiovascular safety profile than those with more COX-2 inhibition. They do carry an increased risk for bleeding. The study reviewed above suggests that patients who have been given NSAIDs, even for a short amount of time, have an increased risk for both bleeding and cardiovascular events, reminding us to carefully weigh the risk and benefit of using these commonly prescribed medications. —NS
REFERENCE
1. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
Continue for chronic fatigue syndrome gets a new name >>
CHRONIC FATIGUE SYNDROME GETS A NEW NAME
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. www.iom.edu/~/media/Files/Report%20Files/2015/MECFS/MECFS_ReportBrief.pdf. Accessed June 4, 2015.
Myalgic encephalomyelitis/chronic fatigue syndrome has a new name and more clearcut diagnostic criteria following a report by the Institute of Medicine. The new name, systemic exertion intolerance disease (SEID), better reflects how exertion exacerbates symptoms.
According to the report, the proposed diagnostic criteria for SEID is all three of the following:
• A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than six months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest
• Post-exertional malaise
• Unrefreshing sleep
Plus, at least one of the following:
• Cognitive impairment
• Orthostatic intolerance
The group also recommended that a new code be assigned in the ICD-10 that is not linked to chronic fatigue or neurasthenia.
COMMENTARY
Systemic exertion intolerance disease (SEID) will likely take some time to be integrated into practice as the new name for this condition. This change will also refocus attention on this difficult illness, which has always been challenging because the symptoms of SEID are nonspecific and overlap with many other illnesses, from hypothyroidism to depression. The guidelines are a welcome addition to the literature, giving us better direction in diagnosing a difficult disease. —NS
Continue for a review of most effective treatments for knee OA >>
REVIEW: MOST EFFECTIVE TREATMENTS FOR KNEE OA
Bannuru RR, Schmid CH, Kent DM, Vet al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
Intra-articular hyaluronic acid offers the best relief for pain in patients with knee osteoarthritis (OA), a meta-analysis of 137 studies with 33,243 subjects reports.
Researchers reviewed randomized trials of adults with knee OA that compared two or more treatments, including acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, intra-articular (IA) corticosteroids, IA hyaluronic acid, oral placebo, and IA placebo. They found for pain, stiffness, and function all treatments fared better than oral placebo.
• For pain, IA hyaluronic acid was most effective (0.63); acetaminophen was least effective (0.18).
• For function, all of the treatments were superior to oral placebo except IA corticosteroids.
• For stiffness, there was no significant difference among the different treatments.
COMMENTARY
The decision about which medicine to use to treat a patient with osteoarthritis is made on an individual basis, based on effectiveness for pain, as well as safety and cost considerations. Acetaminophen, which is the least effective pain agent studied, probably deserves its place as the most commonly used analgesic for OA based on safety and cost. IA treatments were in general more effective than oral treatments, though it is important to recognize that these studies looked at months, not years, of treatment of OA, and most of our patients are treated over a course of years. —NS
IS ACETAMINOPHEN EFFECTIVE IN EASING BACK PAIN AND KNEE PAIN?
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225.
Acetaminophen is ineffective in treating lower back pain and provides minimal short-term benefit for people with osteoarthritis, a systematic review of 13 randomized, placebo-controlled trials reports.
Two independent reviewers extracted data on pain, disability, and quality of life, as well as adverse effects, patient adherence, and use of rescue medication, and found high-quality evidence that:
• Acetaminophen is ineffective for reducing pain intensity and disability, or improving quality of life in patients with low back pain.
• Acetaminophen provides significant, but not clinically important, benefit for pain and disability in patients with hip or knee osteoarthritis.
• Patients taking acetaminophen are nearly four times more likely to have abnormal results on liver function tests.
COMMENTARY
This study adds to the literature a less potent effect of acetaminophen than we have previously assumed,1,2 suggesting a significant but not clinically important effect on pain. This result is at odds with the experience of many clinicians, who use acetaminophen regularly as a first-line agent for pain. When there is a dissonance between clinical experience and emerging evidence, one has to ask why. The explanation here may be that acetaminophen, NSAIDs, and opioid analgesics all have their problems and all seem to work better for some patients than others. In clinical practice, we often start with acetaminophen, which works for some patients, and go on to other agents for those in whom acetaminophen does not provide sufficient pain control. Studies that report a small mean effect may not detect the significant effect that can occur for many patients but gets hidden in the mean (which includes patients in whom there is no effect). I am reminded of the statistician who drowned in a river with a mean depth of 3 feet. A common clinical approach, often starting with well-tolerated acetaminophen and then progressing to other agents when needed, still seems sound. —NS
REFERENCES
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
2. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586-1596. doi: 10.1016/S0140-6736(14)60805-9.
Continue for Back pain: Does early imaging improve outcomes? >>
BACK PAIN: DOES EARLY IMAGING IMPROVE OUTCOMES?
Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313(11):1143-1153.
Early imaging for back pain is not associated with better one-year outcomes among patients ages 65 and older, according to a prospective cohort study of 5,239 older patients with a new primary care visit for back pain.
Investigators compared function and pain at the 12-month follow-up visit among 1,174 patients who had early radiographs, 349 patients who had early MRI/CT, and 3,719 controls.
The primary outcome was back or leg pain-related disability, as measured by a back and leg pain disability score. The mean score showed no significant differences between groups.
COMMENTARY
Many guidelines suggest consideration of imaging early on in the diagnosis of low back pain in older adults due to the high prevalence of important underlying causes such as cancer.1 This study looked at an older population and showed, as has been demonstrated in younger populations, that there is no advantage to early imaging with either x-ray or MRI, though costs were about 25% higher. —NS
REFERENCE
1. Davis PC, Wippold FJ II, Brunberg JA, et al. ACR Appropriateness Criteria on low back pain. J Am Coll Radiol. 2009;6(6):401-407.
Continue for anti-inflammatory drugs and antithrombotic therapy >>
ANTI-INFLAMMATORY DRUGS AND ANTITHROMBOTIC THERAPY
Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Combining prescription NSAIDs with antithrombotic therapy following myocardial infarction (MI) increases the risk for bleeding and excess thrombotic events, according to a study of 61,971 MI patients with ongoing antithrombotic therapy.
During an average of 3.5 years’ follow-up, patients who had taken NSAIDs had increased rates of bleeding and cardiovascular events, with incidence rates per 100-person years as follows:
The study authors note that clinicians should use caution when prescribing NSAIDs to patients who have recently experienced MI.
COMMENTARY
The use of NSAIDs in patients who have had coronary disease has been an area of concern for almost a decade. In 2007, the American Heart Association issued a scientific advisory update discouraging use of COX-2 inhibitors in patients with coronary disease and concluding that more data are needed on the cardiovascular safety of conventional NSAIDs.1 Non-COX-2 selective NSAIDs, such as naproxen, appear to have a better cardiovascular safety profile than those with more COX-2 inhibition. They do carry an increased risk for bleeding. The study reviewed above suggests that patients who have been given NSAIDs, even for a short amount of time, have an increased risk for both bleeding and cardiovascular events, reminding us to carefully weigh the risk and benefit of using these commonly prescribed medications. —NS
REFERENCE
1. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
Continue for chronic fatigue syndrome gets a new name >>
CHRONIC FATIGUE SYNDROME GETS A NEW NAME
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. www.iom.edu/~/media/Files/Report%20Files/2015/MECFS/MECFS_ReportBrief.pdf. Accessed June 4, 2015.
Myalgic encephalomyelitis/chronic fatigue syndrome has a new name and more clearcut diagnostic criteria following a report by the Institute of Medicine. The new name, systemic exertion intolerance disease (SEID), better reflects how exertion exacerbates symptoms.
According to the report, the proposed diagnostic criteria for SEID is all three of the following:
• A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than six months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest
• Post-exertional malaise
• Unrefreshing sleep
Plus, at least one of the following:
• Cognitive impairment
• Orthostatic intolerance
The group also recommended that a new code be assigned in the ICD-10 that is not linked to chronic fatigue or neurasthenia.
COMMENTARY
Systemic exertion intolerance disease (SEID) will likely take some time to be integrated into practice as the new name for this condition. This change will also refocus attention on this difficult illness, which has always been challenging because the symptoms of SEID are nonspecific and overlap with many other illnesses, from hypothyroidism to depression. The guidelines are a welcome addition to the literature, giving us better direction in diagnosing a difficult disease. —NS
Continue for a review of most effective treatments for knee OA >>
REVIEW: MOST EFFECTIVE TREATMENTS FOR KNEE OA
Bannuru RR, Schmid CH, Kent DM, Vet al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
Intra-articular hyaluronic acid offers the best relief for pain in patients with knee osteoarthritis (OA), a meta-analysis of 137 studies with 33,243 subjects reports.
Researchers reviewed randomized trials of adults with knee OA that compared two or more treatments, including acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, intra-articular (IA) corticosteroids, IA hyaluronic acid, oral placebo, and IA placebo. They found for pain, stiffness, and function all treatments fared better than oral placebo.
• For pain, IA hyaluronic acid was most effective (0.63); acetaminophen was least effective (0.18).
• For function, all of the treatments were superior to oral placebo except IA corticosteroids.
• For stiffness, there was no significant difference among the different treatments.
COMMENTARY
The decision about which medicine to use to treat a patient with osteoarthritis is made on an individual basis, based on effectiveness for pain, as well as safety and cost considerations. Acetaminophen, which is the least effective pain agent studied, probably deserves its place as the most commonly used analgesic for OA based on safety and cost. IA treatments were in general more effective than oral treatments, though it is important to recognize that these studies looked at months, not years, of treatment of OA, and most of our patients are treated over a course of years. —NS
IS ACETAMINOPHEN EFFECTIVE IN EASING BACK PAIN AND KNEE PAIN?
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225.
Acetaminophen is ineffective in treating lower back pain and provides minimal short-term benefit for people with osteoarthritis, a systematic review of 13 randomized, placebo-controlled trials reports.
Two independent reviewers extracted data on pain, disability, and quality of life, as well as adverse effects, patient adherence, and use of rescue medication, and found high-quality evidence that:
• Acetaminophen is ineffective for reducing pain intensity and disability, or improving quality of life in patients with low back pain.
• Acetaminophen provides significant, but not clinically important, benefit for pain and disability in patients with hip or knee osteoarthritis.
• Patients taking acetaminophen are nearly four times more likely to have abnormal results on liver function tests.
COMMENTARY
This study adds to the literature a less potent effect of acetaminophen than we have previously assumed,1,2 suggesting a significant but not clinically important effect on pain. This result is at odds with the experience of many clinicians, who use acetaminophen regularly as a first-line agent for pain. When there is a dissonance between clinical experience and emerging evidence, one has to ask why. The explanation here may be that acetaminophen, NSAIDs, and opioid analgesics all have their problems and all seem to work better for some patients than others. In clinical practice, we often start with acetaminophen, which works for some patients, and go on to other agents for those in whom acetaminophen does not provide sufficient pain control. Studies that report a small mean effect may not detect the significant effect that can occur for many patients but gets hidden in the mean (which includes patients in whom there is no effect). I am reminded of the statistician who drowned in a river with a mean depth of 3 feet. A common clinical approach, often starting with well-tolerated acetaminophen and then progressing to other agents when needed, still seems sound. —NS
REFERENCES
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
2. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586-1596. doi: 10.1016/S0140-6736(14)60805-9.
Continue for Back pain: Does early imaging improve outcomes? >>
BACK PAIN: DOES EARLY IMAGING IMPROVE OUTCOMES?
Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313(11):1143-1153.
Early imaging for back pain is not associated with better one-year outcomes among patients ages 65 and older, according to a prospective cohort study of 5,239 older patients with a new primary care visit for back pain.
Investigators compared function and pain at the 12-month follow-up visit among 1,174 patients who had early radiographs, 349 patients who had early MRI/CT, and 3,719 controls.
The primary outcome was back or leg pain-related disability, as measured by a back and leg pain disability score. The mean score showed no significant differences between groups.
COMMENTARY
Many guidelines suggest consideration of imaging early on in the diagnosis of low back pain in older adults due to the high prevalence of important underlying causes such as cancer.1 This study looked at an older population and showed, as has been demonstrated in younger populations, that there is no advantage to early imaging with either x-ray or MRI, though costs were about 25% higher. —NS
REFERENCE
1. Davis PC, Wippold FJ II, Brunberg JA, et al. ACR Appropriateness Criteria on low back pain. J Am Coll Radiol. 2009;6(6):401-407.
Continue for anti-inflammatory drugs and antithrombotic therapy >>
ANTI-INFLAMMATORY DRUGS AND ANTITHROMBOTIC THERAPY
Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Combining prescription NSAIDs with antithrombotic therapy following myocardial infarction (MI) increases the risk for bleeding and excess thrombotic events, according to a study of 61,971 MI patients with ongoing antithrombotic therapy.
During an average of 3.5 years’ follow-up, patients who had taken NSAIDs had increased rates of bleeding and cardiovascular events, with incidence rates per 100-person years as follows:
The study authors note that clinicians should use caution when prescribing NSAIDs to patients who have recently experienced MI.
COMMENTARY
The use of NSAIDs in patients who have had coronary disease has been an area of concern for almost a decade. In 2007, the American Heart Association issued a scientific advisory update discouraging use of COX-2 inhibitors in patients with coronary disease and concluding that more data are needed on the cardiovascular safety of conventional NSAIDs.1 Non-COX-2 selective NSAIDs, such as naproxen, appear to have a better cardiovascular safety profile than those with more COX-2 inhibition. They do carry an increased risk for bleeding. The study reviewed above suggests that patients who have been given NSAIDs, even for a short amount of time, have an increased risk for both bleeding and cardiovascular events, reminding us to carefully weigh the risk and benefit of using these commonly prescribed medications. —NS
REFERENCE
1. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
Continue for chronic fatigue syndrome gets a new name >>
CHRONIC FATIGUE SYNDROME GETS A NEW NAME
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. www.iom.edu/~/media/Files/Report%20Files/2015/MECFS/MECFS_ReportBrief.pdf. Accessed June 4, 2015.
Myalgic encephalomyelitis/chronic fatigue syndrome has a new name and more clearcut diagnostic criteria following a report by the Institute of Medicine. The new name, systemic exertion intolerance disease (SEID), better reflects how exertion exacerbates symptoms.
According to the report, the proposed diagnostic criteria for SEID is all three of the following:
• A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than six months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest
• Post-exertional malaise
• Unrefreshing sleep
Plus, at least one of the following:
• Cognitive impairment
• Orthostatic intolerance
The group also recommended that a new code be assigned in the ICD-10 that is not linked to chronic fatigue or neurasthenia.
COMMENTARY
Systemic exertion intolerance disease (SEID) will likely take some time to be integrated into practice as the new name for this condition. This change will also refocus attention on this difficult illness, which has always been challenging because the symptoms of SEID are nonspecific and overlap with many other illnesses, from hypothyroidism to depression. The guidelines are a welcome addition to the literature, giving us better direction in diagnosing a difficult disease. —NS
Continue for a review of most effective treatments for knee OA >>
REVIEW: MOST EFFECTIVE TREATMENTS FOR KNEE OA
Bannuru RR, Schmid CH, Kent DM, Vet al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
Intra-articular hyaluronic acid offers the best relief for pain in patients with knee osteoarthritis (OA), a meta-analysis of 137 studies with 33,243 subjects reports.
Researchers reviewed randomized trials of adults with knee OA that compared two or more treatments, including acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, intra-articular (IA) corticosteroids, IA hyaluronic acid, oral placebo, and IA placebo. They found for pain, stiffness, and function all treatments fared better than oral placebo.
• For pain, IA hyaluronic acid was most effective (0.63); acetaminophen was least effective (0.18).
• For function, all of the treatments were superior to oral placebo except IA corticosteroids.
• For stiffness, there was no significant difference among the different treatments.
COMMENTARY
The decision about which medicine to use to treat a patient with osteoarthritis is made on an individual basis, based on effectiveness for pain, as well as safety and cost considerations. Acetaminophen, which is the least effective pain agent studied, probably deserves its place as the most commonly used analgesic for OA based on safety and cost. IA treatments were in general more effective than oral treatments, though it is important to recognize that these studies looked at months, not years, of treatment of OA, and most of our patients are treated over a course of years. —NS
Voices for Change: AANP Celebrates 25 Years
When I started editing Marie-Eileen Onieal’s editorial for this issue, her reference to the grassroots origins of the American Academy of Nurse Practitioners (now “Association”) reminded me of this article, which appeared in June 2010 to mark the organization’s 25th anniversary. Although five years have passed, I vividly recall the passion, enthusiasm, and wisdom of the founders, whose message about taking action to create positive change still resonates. They were inspiring to speak to, and never have I enjoyed working on an article more. —AMH
Picture it: Kansas City, 1984. At a meeting sponsored by the American Nurses Association (ANA), conversation among the NP attendees from across the United States focuses on the widely perceived need for “common representation” of their distinct interests. Who among the existing nursing organizations has the time, money, and/or inclination to serve as the voice of all NPs, providing a conduit for communication and leadership in legislative efforts to remove barriers to practice?
As it will turn out, the answer is no one—at least, not in the way these NPs envision. So they decide to do something about that … and since the result of their collaborative vision is the American Academy of Nurse Practitioners (AANP), you have a pretty good idea how it worked out.
AANP is celebrating its 25th anniversary this year—a milestone its founders probably never doubted would be reached, although others initially questioned the viability of such an organization.
“The feeling was that if it was needed, it would grow, and if it wasn’t needed, it wouldn’t grow,” recalls Jan Towers, PhD, NP-C, CRNP, FAANP, a founding member and past president who continues to serve AANP as Director of Health Policy. “And indeed it grew—so I think we know what our answer was!”
A SEPARATE BUT EQUAL NEED
Of course, creating a new professional organization was not as simple as a group of individuals putting their heads together. And yet, in a certain sense, it was that easy.
Shortly after those initial discussions in Kansas City, there was another conference in Washington, DC. During a panel discussion with executives from the insurance and advertising industries, it became evident that the biggest issue for NPs was “no one knew who we were,” says Clinician Reviews NP Editor-in-Chief Marie-Eileen Onieal, PhD, CPNP, FAANP, also a founder and past president of AANP. Clearly, that situation needed to be remedied—but how?
At that time, many NPs were members of ANA (and, it should be noted, many still are). However, since ANA represents all of nursing, the bulk of its resources could not be devoted to NPs. The organization had created a Council of Primary Care NPs in 1974, but the “renegade” NPs of 1984 felt that structure didn’t provide the latitude they wanted in their representation.
“We needed a way to really work together and have our undivided attention focused on NP issues,” Towers says. Onieal adds, “We weren’t abandoning our ‘nursing-ness,’ but clearly we had separate and distinctly different issues at hand than did the general populace of the nursing profession.”
The fact that NPs from across the country—remember, this is pre-Internet—shared this viewpoint added strength to the argument. If dozens of people who didn’t really know one another could see the same need, it must be real—and therefore, it had to be addressed.
“The [NP] role had been around since 1965, but nursing faculty were just beginning to define what NP education ought to look like,” says Carole Kain, DNS, ARNP, PNP-BC, a founding member and the first president of AANP. (She was Carole Kerwin then.) “A lot of things were changing in the profession, and we all wanted to be part of defining what that change meant. It was everybody coming together with a skill set that set us on this course.”
The time was certainly ripe. In her files, Kain still has a copy of a document discussing a referendum the American Medical Association passed in 1984, in which “they said they were actually going to try to inhibit the practice of NPs, PAs, nurse-midwives, and pharmacists, as to prescribing and taking care of patients.”
In addition to that restrictive attitude—a cause to rally around for NPs nationwide—the NP profession had reached a tipping point in terms of growth. “Enough of us had been prepared, but we were still small enough to be spread out [across the country],” Towers says. “We needed some way to connect.”
By the time they left Washington, DC, a steering committee had been formed to explore the need for an organization representing the nation’s approximately 24,000 NPs. They were expected to report back to the ANA one year later, at a meeting in Chicago. And so, the hard work began.
SO, YOU WANT TO START AN ORGANIZATION?
For a group of virtual strangers who had never started an organization before, they went about their business in a methodical and logical way. Different task forces were created, including one to look into how to do articles of incorporation and other “legalese” pieces, and another to develop a list of reasons why a separate organization was needed.
Onieal recalls a good deal of traveling that she and some other NPs did “to every NP meeting, every NP gathering, across the US for a year. We had a petition, and I’d say, ‘All I want to know is whether you think we should start an NP organization.’” The list grew over the course of the year.
In the meantime, contact was made with the handful of existing NP organizations, to see if any of them could meet the needs of the profession at large: the National Association of Pediatric NPs (1973), the National Association of NPs in Reproductive Health (which became the National Association of NPs in Women’s Health; 1980), and the National Conference of Gerontological NPs (1981). But obviously, since these groups had been tasked by their members with representing the interests of NPs in specific fields, their responses boiled down to “We can’t help you—but we wish you well.”
By the time the Chicago meeting rolled around in May 1985, about the only thing left to do was announce the new organization’s formation. Bylaws had already been drafted, and Onieal’s (now former) address in Lowell, Massachusetts, had been chosen as the original headquarters of the organization. (Trivia: AANP was originally incorporated in Lowell, before relocating to Austin, Texas.)
During a panel session in Chicago, Kain and Onieal told the assembled crowd, “This is what we need: We need to be heard as independent providers. We need to have some say in how our practice is regulated. We need … an organization that will allow us to learn from each other across the country,” as Kain recalls.
The official press release announcing AANP’s creation reads: “The overall purpose of the AANP is to promote high standards of health care as delivered by nurse practitioners and to act as a forum to enhance the identity and continuity of nurse practitioners. It is nationwide in scope and welcomes all nurse practitioners, regardless of specialty. The first year’s focus will be on networking and communications.”
AANP’s first elected officers were Kain (President), Towers (President-Elect), Onieal (Treasurer), Madeline D. Wiley, MSN, ARNP (Recording Secretary), and Robert T. Smithing, MSN, ARNP (Communications Secretary). Annual membership dues were $60.
25 YEARS OF PROGRESS
The leaders and members of the newly formed AANP wasted little time in setting out to accomplish their organizational goals. As Onieal says, “When we started, we had a five-year plan and a 10-year plan. And in five years, we got through all of that.”
With the initial focus on networking and communications, 10 geographical regions were established, based on the National Health Service Corps regions, and regional directors were found. So were individual reps from every state, each of whom then “started talking to people within their state,” Kain says.
For Towers, the focus was on legislative and regulatory issues, just as it is today. “One of the first things we started doing was finding ways to influence policy, both in Washington and in our states,” she says. “Early on, we started working on reimbursement.”
An early victory was getting NPs reimbursement through the Federal Employees Health Benefits Program (1986-1987). On the Medicare front, AANP had a significant impact in securing reimbursement for NPs working in rural areas and in long-term care facilities (1989-1990). Additional milestones include mandated Medicare payment for family and pediatric NPs regardless of supervision status (the early ’90s) and direct Medicare reimbursement for NPs regardless of setting (1997).
Seeing all that has been accomplished in 25 years is still a little amazing, even to the people who set the wheels in motion. “We’re funding research, and we have a foundation that gives scholarships,” Kain says. “Those are things we talked about as ‘someday’ dreams, but now they’re actualizations.”
She’s greeted every day by signs of how much has changed since 1985. “I can remember the initial discussions about having a certification program, talking to the psychometricians about how we’d start up the exam and which criteria we’d use,” she reflects. “And now, the NPs that I’m teaching are using that certification exam to get their licensure!”
As much as Onieal appreciates all the services that AANP has grown to offer its members, she also recognizes the core value of the organization. “One of our primary purposes was to let people know what NPs are all about—to clarify that we’re not LPNs, we’re not students, we’re not people who couldn’t go to medical school and decided to do this instead; this was our chosen profession,” she says. “I think we’ve been successful in doing that.”
THE ULTIMATE COLLABORATION
The founders of AANP never doubted that forming the organization was the right thing to do, which probably confirms that they were the best people to do the job. Instead of expending energy on doubts or infighting, they figured out what needed to be done and moved forward.
Kain still has a “vivid mental picture” of the early days in Kansas City and Washington, DC, when there was no organization and therefore no budget, so meetings were held in someone’s hotel room. “There would be people sitting on the bed, on the floor, standing up; we’re all crammed into the room,” she remembers with a laugh. “And people are talking, and we’re polite to one another, we’re respectful of one another. We’re passionate. We’re talking about the difficulties and why we need this. And if you could put that into a bottle, you could start a new organization right now!”
That spirit of collaboration is still a viable part of the organization, one that Onieal is proud of. “If anybody ever had an interest in doing something or being something within the organization, the doors were open,” she says. “Early on, a couple of students said, ‘But we have needs, too,’ so we said, ‘Fine, we’ll start a student special interest group. No problem.’” The point, after all, had always been to learn from one another.
If there is one lesson the AANP founders hope to pass along to future generations, it’s that great change can be brought about by a small group of (seemingly) ordinary people with extraordinary determination. They don’t want statues erected in their honor, but they do want others to be inspired to take an active role in the continuous evolution of the NP profession—especially since, as Towers observes, “There is still work to be done.”
“We need to get all the state practice acts on a level playing field,” she notes, “which we’re well on the way to doing now with our consensus document and the model rules and regulations. We need to have recognition within all the payment systems, and remove barriers within existing laws that prevent NPs from practicing to their fullest capability.”
The significant changes that have been brought about in the past 25 years can be difficult to fathom for those who did not witness them. In 2010, the NP profession is well established. (The same is true for PAs.) Students deciding their future choose these professions in part because of all they will be able to do and accomplish within their roles.
What they don’t always recognize is that it took hard work and continued vigilance on the part of those who have preceded them to achieve the privileges that today’s clinicians—and perhaps tomorrow’s—run the risk of taking for granted.
“We’re not done, and I don’t want anyone to think they can rest on the laurels of what has gone before or think that without their involvement it will continue,” says Kain. “It won’t. We need them. We need new blood, new thoughts, new ideas, to face the new challenges.”
When she reflects on how a group of near-strangers got together and made a mark on the history of their profession, she concludes, simply, “Others can do the same thing. They just have to want to, and see the need.”
Reprinted from Clinician Reviews. 2010;20(6):cover, 5-8.
When I started editing Marie-Eileen Onieal’s editorial for this issue, her reference to the grassroots origins of the American Academy of Nurse Practitioners (now “Association”) reminded me of this article, which appeared in June 2010 to mark the organization’s 25th anniversary. Although five years have passed, I vividly recall the passion, enthusiasm, and wisdom of the founders, whose message about taking action to create positive change still resonates. They were inspiring to speak to, and never have I enjoyed working on an article more. —AMH
Picture it: Kansas City, 1984. At a meeting sponsored by the American Nurses Association (ANA), conversation among the NP attendees from across the United States focuses on the widely perceived need for “common representation” of their distinct interests. Who among the existing nursing organizations has the time, money, and/or inclination to serve as the voice of all NPs, providing a conduit for communication and leadership in legislative efforts to remove barriers to practice?
As it will turn out, the answer is no one—at least, not in the way these NPs envision. So they decide to do something about that … and since the result of their collaborative vision is the American Academy of Nurse Practitioners (AANP), you have a pretty good idea how it worked out.
AANP is celebrating its 25th anniversary this year—a milestone its founders probably never doubted would be reached, although others initially questioned the viability of such an organization.
“The feeling was that if it was needed, it would grow, and if it wasn’t needed, it wouldn’t grow,” recalls Jan Towers, PhD, NP-C, CRNP, FAANP, a founding member and past president who continues to serve AANP as Director of Health Policy. “And indeed it grew—so I think we know what our answer was!”
A SEPARATE BUT EQUAL NEED
Of course, creating a new professional organization was not as simple as a group of individuals putting their heads together. And yet, in a certain sense, it was that easy.
Shortly after those initial discussions in Kansas City, there was another conference in Washington, DC. During a panel discussion with executives from the insurance and advertising industries, it became evident that the biggest issue for NPs was “no one knew who we were,” says Clinician Reviews NP Editor-in-Chief Marie-Eileen Onieal, PhD, CPNP, FAANP, also a founder and past president of AANP. Clearly, that situation needed to be remedied—but how?
At that time, many NPs were members of ANA (and, it should be noted, many still are). However, since ANA represents all of nursing, the bulk of its resources could not be devoted to NPs. The organization had created a Council of Primary Care NPs in 1974, but the “renegade” NPs of 1984 felt that structure didn’t provide the latitude they wanted in their representation.
“We needed a way to really work together and have our undivided attention focused on NP issues,” Towers says. Onieal adds, “We weren’t abandoning our ‘nursing-ness,’ but clearly we had separate and distinctly different issues at hand than did the general populace of the nursing profession.”
The fact that NPs from across the country—remember, this is pre-Internet—shared this viewpoint added strength to the argument. If dozens of people who didn’t really know one another could see the same need, it must be real—and therefore, it had to be addressed.
“The [NP] role had been around since 1965, but nursing faculty were just beginning to define what NP education ought to look like,” says Carole Kain, DNS, ARNP, PNP-BC, a founding member and the first president of AANP. (She was Carole Kerwin then.) “A lot of things were changing in the profession, and we all wanted to be part of defining what that change meant. It was everybody coming together with a skill set that set us on this course.”
The time was certainly ripe. In her files, Kain still has a copy of a document discussing a referendum the American Medical Association passed in 1984, in which “they said they were actually going to try to inhibit the practice of NPs, PAs, nurse-midwives, and pharmacists, as to prescribing and taking care of patients.”
In addition to that restrictive attitude—a cause to rally around for NPs nationwide—the NP profession had reached a tipping point in terms of growth. “Enough of us had been prepared, but we were still small enough to be spread out [across the country],” Towers says. “We needed some way to connect.”
By the time they left Washington, DC, a steering committee had been formed to explore the need for an organization representing the nation’s approximately 24,000 NPs. They were expected to report back to the ANA one year later, at a meeting in Chicago. And so, the hard work began.
SO, YOU WANT TO START AN ORGANIZATION?
For a group of virtual strangers who had never started an organization before, they went about their business in a methodical and logical way. Different task forces were created, including one to look into how to do articles of incorporation and other “legalese” pieces, and another to develop a list of reasons why a separate organization was needed.
Onieal recalls a good deal of traveling that she and some other NPs did “to every NP meeting, every NP gathering, across the US for a year. We had a petition, and I’d say, ‘All I want to know is whether you think we should start an NP organization.’” The list grew over the course of the year.
In the meantime, contact was made with the handful of existing NP organizations, to see if any of them could meet the needs of the profession at large: the National Association of Pediatric NPs (1973), the National Association of NPs in Reproductive Health (which became the National Association of NPs in Women’s Health; 1980), and the National Conference of Gerontological NPs (1981). But obviously, since these groups had been tasked by their members with representing the interests of NPs in specific fields, their responses boiled down to “We can’t help you—but we wish you well.”
By the time the Chicago meeting rolled around in May 1985, about the only thing left to do was announce the new organization’s formation. Bylaws had already been drafted, and Onieal’s (now former) address in Lowell, Massachusetts, had been chosen as the original headquarters of the organization. (Trivia: AANP was originally incorporated in Lowell, before relocating to Austin, Texas.)
During a panel session in Chicago, Kain and Onieal told the assembled crowd, “This is what we need: We need to be heard as independent providers. We need to have some say in how our practice is regulated. We need … an organization that will allow us to learn from each other across the country,” as Kain recalls.
The official press release announcing AANP’s creation reads: “The overall purpose of the AANP is to promote high standards of health care as delivered by nurse practitioners and to act as a forum to enhance the identity and continuity of nurse practitioners. It is nationwide in scope and welcomes all nurse practitioners, regardless of specialty. The first year’s focus will be on networking and communications.”
AANP’s first elected officers were Kain (President), Towers (President-Elect), Onieal (Treasurer), Madeline D. Wiley, MSN, ARNP (Recording Secretary), and Robert T. Smithing, MSN, ARNP (Communications Secretary). Annual membership dues were $60.
25 YEARS OF PROGRESS
The leaders and members of the newly formed AANP wasted little time in setting out to accomplish their organizational goals. As Onieal says, “When we started, we had a five-year plan and a 10-year plan. And in five years, we got through all of that.”
With the initial focus on networking and communications, 10 geographical regions were established, based on the National Health Service Corps regions, and regional directors were found. So were individual reps from every state, each of whom then “started talking to people within their state,” Kain says.
For Towers, the focus was on legislative and regulatory issues, just as it is today. “One of the first things we started doing was finding ways to influence policy, both in Washington and in our states,” she says. “Early on, we started working on reimbursement.”
An early victory was getting NPs reimbursement through the Federal Employees Health Benefits Program (1986-1987). On the Medicare front, AANP had a significant impact in securing reimbursement for NPs working in rural areas and in long-term care facilities (1989-1990). Additional milestones include mandated Medicare payment for family and pediatric NPs regardless of supervision status (the early ’90s) and direct Medicare reimbursement for NPs regardless of setting (1997).
Seeing all that has been accomplished in 25 years is still a little amazing, even to the people who set the wheels in motion. “We’re funding research, and we have a foundation that gives scholarships,” Kain says. “Those are things we talked about as ‘someday’ dreams, but now they’re actualizations.”
She’s greeted every day by signs of how much has changed since 1985. “I can remember the initial discussions about having a certification program, talking to the psychometricians about how we’d start up the exam and which criteria we’d use,” she reflects. “And now, the NPs that I’m teaching are using that certification exam to get their licensure!”
As much as Onieal appreciates all the services that AANP has grown to offer its members, she also recognizes the core value of the organization. “One of our primary purposes was to let people know what NPs are all about—to clarify that we’re not LPNs, we’re not students, we’re not people who couldn’t go to medical school and decided to do this instead; this was our chosen profession,” she says. “I think we’ve been successful in doing that.”
THE ULTIMATE COLLABORATION
The founders of AANP never doubted that forming the organization was the right thing to do, which probably confirms that they were the best people to do the job. Instead of expending energy on doubts or infighting, they figured out what needed to be done and moved forward.
Kain still has a “vivid mental picture” of the early days in Kansas City and Washington, DC, when there was no organization and therefore no budget, so meetings were held in someone’s hotel room. “There would be people sitting on the bed, on the floor, standing up; we’re all crammed into the room,” she remembers with a laugh. “And people are talking, and we’re polite to one another, we’re respectful of one another. We’re passionate. We’re talking about the difficulties and why we need this. And if you could put that into a bottle, you could start a new organization right now!”
That spirit of collaboration is still a viable part of the organization, one that Onieal is proud of. “If anybody ever had an interest in doing something or being something within the organization, the doors were open,” she says. “Early on, a couple of students said, ‘But we have needs, too,’ so we said, ‘Fine, we’ll start a student special interest group. No problem.’” The point, after all, had always been to learn from one another.
If there is one lesson the AANP founders hope to pass along to future generations, it’s that great change can be brought about by a small group of (seemingly) ordinary people with extraordinary determination. They don’t want statues erected in their honor, but they do want others to be inspired to take an active role in the continuous evolution of the NP profession—especially since, as Towers observes, “There is still work to be done.”
“We need to get all the state practice acts on a level playing field,” she notes, “which we’re well on the way to doing now with our consensus document and the model rules and regulations. We need to have recognition within all the payment systems, and remove barriers within existing laws that prevent NPs from practicing to their fullest capability.”
The significant changes that have been brought about in the past 25 years can be difficult to fathom for those who did not witness them. In 2010, the NP profession is well established. (The same is true for PAs.) Students deciding their future choose these professions in part because of all they will be able to do and accomplish within their roles.
What they don’t always recognize is that it took hard work and continued vigilance on the part of those who have preceded them to achieve the privileges that today’s clinicians—and perhaps tomorrow’s—run the risk of taking for granted.
“We’re not done, and I don’t want anyone to think they can rest on the laurels of what has gone before or think that without their involvement it will continue,” says Kain. “It won’t. We need them. We need new blood, new thoughts, new ideas, to face the new challenges.”
When she reflects on how a group of near-strangers got together and made a mark on the history of their profession, she concludes, simply, “Others can do the same thing. They just have to want to, and see the need.”
Reprinted from Clinician Reviews. 2010;20(6):cover, 5-8.
When I started editing Marie-Eileen Onieal’s editorial for this issue, her reference to the grassroots origins of the American Academy of Nurse Practitioners (now “Association”) reminded me of this article, which appeared in June 2010 to mark the organization’s 25th anniversary. Although five years have passed, I vividly recall the passion, enthusiasm, and wisdom of the founders, whose message about taking action to create positive change still resonates. They were inspiring to speak to, and never have I enjoyed working on an article more. —AMH
Picture it: Kansas City, 1984. At a meeting sponsored by the American Nurses Association (ANA), conversation among the NP attendees from across the United States focuses on the widely perceived need for “common representation” of their distinct interests. Who among the existing nursing organizations has the time, money, and/or inclination to serve as the voice of all NPs, providing a conduit for communication and leadership in legislative efforts to remove barriers to practice?
As it will turn out, the answer is no one—at least, not in the way these NPs envision. So they decide to do something about that … and since the result of their collaborative vision is the American Academy of Nurse Practitioners (AANP), you have a pretty good idea how it worked out.
AANP is celebrating its 25th anniversary this year—a milestone its founders probably never doubted would be reached, although others initially questioned the viability of such an organization.
“The feeling was that if it was needed, it would grow, and if it wasn’t needed, it wouldn’t grow,” recalls Jan Towers, PhD, NP-C, CRNP, FAANP, a founding member and past president who continues to serve AANP as Director of Health Policy. “And indeed it grew—so I think we know what our answer was!”
A SEPARATE BUT EQUAL NEED
Of course, creating a new professional organization was not as simple as a group of individuals putting their heads together. And yet, in a certain sense, it was that easy.
Shortly after those initial discussions in Kansas City, there was another conference in Washington, DC. During a panel discussion with executives from the insurance and advertising industries, it became evident that the biggest issue for NPs was “no one knew who we were,” says Clinician Reviews NP Editor-in-Chief Marie-Eileen Onieal, PhD, CPNP, FAANP, also a founder and past president of AANP. Clearly, that situation needed to be remedied—but how?
At that time, many NPs were members of ANA (and, it should be noted, many still are). However, since ANA represents all of nursing, the bulk of its resources could not be devoted to NPs. The organization had created a Council of Primary Care NPs in 1974, but the “renegade” NPs of 1984 felt that structure didn’t provide the latitude they wanted in their representation.
“We needed a way to really work together and have our undivided attention focused on NP issues,” Towers says. Onieal adds, “We weren’t abandoning our ‘nursing-ness,’ but clearly we had separate and distinctly different issues at hand than did the general populace of the nursing profession.”
The fact that NPs from across the country—remember, this is pre-Internet—shared this viewpoint added strength to the argument. If dozens of people who didn’t really know one another could see the same need, it must be real—and therefore, it had to be addressed.
“The [NP] role had been around since 1965, but nursing faculty were just beginning to define what NP education ought to look like,” says Carole Kain, DNS, ARNP, PNP-BC, a founding member and the first president of AANP. (She was Carole Kerwin then.) “A lot of things were changing in the profession, and we all wanted to be part of defining what that change meant. It was everybody coming together with a skill set that set us on this course.”
The time was certainly ripe. In her files, Kain still has a copy of a document discussing a referendum the American Medical Association passed in 1984, in which “they said they were actually going to try to inhibit the practice of NPs, PAs, nurse-midwives, and pharmacists, as to prescribing and taking care of patients.”
In addition to that restrictive attitude—a cause to rally around for NPs nationwide—the NP profession had reached a tipping point in terms of growth. “Enough of us had been prepared, but we were still small enough to be spread out [across the country],” Towers says. “We needed some way to connect.”
By the time they left Washington, DC, a steering committee had been formed to explore the need for an organization representing the nation’s approximately 24,000 NPs. They were expected to report back to the ANA one year later, at a meeting in Chicago. And so, the hard work began.
SO, YOU WANT TO START AN ORGANIZATION?
For a group of virtual strangers who had never started an organization before, they went about their business in a methodical and logical way. Different task forces were created, including one to look into how to do articles of incorporation and other “legalese” pieces, and another to develop a list of reasons why a separate organization was needed.
Onieal recalls a good deal of traveling that she and some other NPs did “to every NP meeting, every NP gathering, across the US for a year. We had a petition, and I’d say, ‘All I want to know is whether you think we should start an NP organization.’” The list grew over the course of the year.
In the meantime, contact was made with the handful of existing NP organizations, to see if any of them could meet the needs of the profession at large: the National Association of Pediatric NPs (1973), the National Association of NPs in Reproductive Health (which became the National Association of NPs in Women’s Health; 1980), and the National Conference of Gerontological NPs (1981). But obviously, since these groups had been tasked by their members with representing the interests of NPs in specific fields, their responses boiled down to “We can’t help you—but we wish you well.”
By the time the Chicago meeting rolled around in May 1985, about the only thing left to do was announce the new organization’s formation. Bylaws had already been drafted, and Onieal’s (now former) address in Lowell, Massachusetts, had been chosen as the original headquarters of the organization. (Trivia: AANP was originally incorporated in Lowell, before relocating to Austin, Texas.)
During a panel session in Chicago, Kain and Onieal told the assembled crowd, “This is what we need: We need to be heard as independent providers. We need to have some say in how our practice is regulated. We need … an organization that will allow us to learn from each other across the country,” as Kain recalls.
The official press release announcing AANP’s creation reads: “The overall purpose of the AANP is to promote high standards of health care as delivered by nurse practitioners and to act as a forum to enhance the identity and continuity of nurse practitioners. It is nationwide in scope and welcomes all nurse practitioners, regardless of specialty. The first year’s focus will be on networking and communications.”
AANP’s first elected officers were Kain (President), Towers (President-Elect), Onieal (Treasurer), Madeline D. Wiley, MSN, ARNP (Recording Secretary), and Robert T. Smithing, MSN, ARNP (Communications Secretary). Annual membership dues were $60.
25 YEARS OF PROGRESS
The leaders and members of the newly formed AANP wasted little time in setting out to accomplish their organizational goals. As Onieal says, “When we started, we had a five-year plan and a 10-year plan. And in five years, we got through all of that.”
With the initial focus on networking and communications, 10 geographical regions were established, based on the National Health Service Corps regions, and regional directors were found. So were individual reps from every state, each of whom then “started talking to people within their state,” Kain says.
For Towers, the focus was on legislative and regulatory issues, just as it is today. “One of the first things we started doing was finding ways to influence policy, both in Washington and in our states,” she says. “Early on, we started working on reimbursement.”
An early victory was getting NPs reimbursement through the Federal Employees Health Benefits Program (1986-1987). On the Medicare front, AANP had a significant impact in securing reimbursement for NPs working in rural areas and in long-term care facilities (1989-1990). Additional milestones include mandated Medicare payment for family and pediatric NPs regardless of supervision status (the early ’90s) and direct Medicare reimbursement for NPs regardless of setting (1997).
Seeing all that has been accomplished in 25 years is still a little amazing, even to the people who set the wheels in motion. “We’re funding research, and we have a foundation that gives scholarships,” Kain says. “Those are things we talked about as ‘someday’ dreams, but now they’re actualizations.”
She’s greeted every day by signs of how much has changed since 1985. “I can remember the initial discussions about having a certification program, talking to the psychometricians about how we’d start up the exam and which criteria we’d use,” she reflects. “And now, the NPs that I’m teaching are using that certification exam to get their licensure!”
As much as Onieal appreciates all the services that AANP has grown to offer its members, she also recognizes the core value of the organization. “One of our primary purposes was to let people know what NPs are all about—to clarify that we’re not LPNs, we’re not students, we’re not people who couldn’t go to medical school and decided to do this instead; this was our chosen profession,” she says. “I think we’ve been successful in doing that.”
THE ULTIMATE COLLABORATION
The founders of AANP never doubted that forming the organization was the right thing to do, which probably confirms that they were the best people to do the job. Instead of expending energy on doubts or infighting, they figured out what needed to be done and moved forward.
Kain still has a “vivid mental picture” of the early days in Kansas City and Washington, DC, when there was no organization and therefore no budget, so meetings were held in someone’s hotel room. “There would be people sitting on the bed, on the floor, standing up; we’re all crammed into the room,” she remembers with a laugh. “And people are talking, and we’re polite to one another, we’re respectful of one another. We’re passionate. We’re talking about the difficulties and why we need this. And if you could put that into a bottle, you could start a new organization right now!”
That spirit of collaboration is still a viable part of the organization, one that Onieal is proud of. “If anybody ever had an interest in doing something or being something within the organization, the doors were open,” she says. “Early on, a couple of students said, ‘But we have needs, too,’ so we said, ‘Fine, we’ll start a student special interest group. No problem.’” The point, after all, had always been to learn from one another.
If there is one lesson the AANP founders hope to pass along to future generations, it’s that great change can be brought about by a small group of (seemingly) ordinary people with extraordinary determination. They don’t want statues erected in their honor, but they do want others to be inspired to take an active role in the continuous evolution of the NP profession—especially since, as Towers observes, “There is still work to be done.”
“We need to get all the state practice acts on a level playing field,” she notes, “which we’re well on the way to doing now with our consensus document and the model rules and regulations. We need to have recognition within all the payment systems, and remove barriers within existing laws that prevent NPs from practicing to their fullest capability.”
The significant changes that have been brought about in the past 25 years can be difficult to fathom for those who did not witness them. In 2010, the NP profession is well established. (The same is true for PAs.) Students deciding their future choose these professions in part because of all they will be able to do and accomplish within their roles.
What they don’t always recognize is that it took hard work and continued vigilance on the part of those who have preceded them to achieve the privileges that today’s clinicians—and perhaps tomorrow’s—run the risk of taking for granted.
“We’re not done, and I don’t want anyone to think they can rest on the laurels of what has gone before or think that without their involvement it will continue,” says Kain. “It won’t. We need them. We need new blood, new thoughts, new ideas, to face the new challenges.”
When she reflects on how a group of near-strangers got together and made a mark on the history of their profession, she concludes, simply, “Others can do the same thing. They just have to want to, and see the need.”
Reprinted from Clinician Reviews. 2010;20(6):cover, 5-8.
Chronic Myeloid Leukemia
The first connection between cancer and a patient’s genome was documented by Peter Nowell and David Hungerford when they identified a unique chromosome in the metaphase spread of 7 patients diagnosed with chronic myeloid leukemia (CML). In 1973, renowned cytopathologist Janet Rowley determined that this chromosome is part of a chromosomal translocation between chromosome 9 and chromosome 22. Further delineation of this translocation showed that the gene ABL1, normally located on chromosome 9, is translocated to the Philadelphia (Ph+) chromosome in patients with CML. ABL1 was found to be located downstream of a specific genetic region in each patient, and this region became known as the BCR, or “breakpoint cluster region.” The BCR-ABL1 translocation found in patients with CML creates a constitutively active tyrosine kinase necessary for cellular transformation.
To read the full article in PDF:
The first connection between cancer and a patient’s genome was documented by Peter Nowell and David Hungerford when they identified a unique chromosome in the metaphase spread of 7 patients diagnosed with chronic myeloid leukemia (CML). In 1973, renowned cytopathologist Janet Rowley determined that this chromosome is part of a chromosomal translocation between chromosome 9 and chromosome 22. Further delineation of this translocation showed that the gene ABL1, normally located on chromosome 9, is translocated to the Philadelphia (Ph+) chromosome in patients with CML. ABL1 was found to be located downstream of a specific genetic region in each patient, and this region became known as the BCR, or “breakpoint cluster region.” The BCR-ABL1 translocation found in patients with CML creates a constitutively active tyrosine kinase necessary for cellular transformation.
To read the full article in PDF:
The first connection between cancer and a patient’s genome was documented by Peter Nowell and David Hungerford when they identified a unique chromosome in the metaphase spread of 7 patients diagnosed with chronic myeloid leukemia (CML). In 1973, renowned cytopathologist Janet Rowley determined that this chromosome is part of a chromosomal translocation between chromosome 9 and chromosome 22. Further delineation of this translocation showed that the gene ABL1, normally located on chromosome 9, is translocated to the Philadelphia (Ph+) chromosome in patients with CML. ABL1 was found to be located downstream of a specific genetic region in each patient, and this region became known as the BCR, or “breakpoint cluster region.” The BCR-ABL1 translocation found in patients with CML creates a constitutively active tyrosine kinase necessary for cellular transformation.
To read the full article in PDF: