User login
Prostate Cancer in Seniors, Part 1: Epidemiology, Pathology, and Screening
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
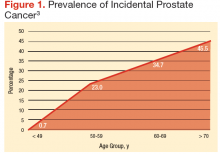
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
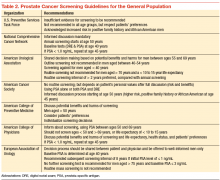
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
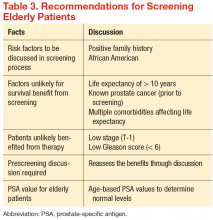
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
Reflectance Confocal Microscopy: An Overview of Technology and Advances in Telepathology
Reflectance confocal microscopy (RCM) creates an image by detecting backscattered light from illuminated tissue and displaying it on a monitor at high resolution and contrast. The grayscale images, oriented in a horizontal (en face) plane, reveal cellular and morphologic architecture in progressive depths from the epidermis to the papillary dermis.1,2 Analyses of confocal features have shown good correlation with histologic and dermoscopic findings, allowing key features of normal skin topography as well as cutaneous lesions to be delineated.1-15 Most research has focused on differentiating benign and malignant lesions, but RCM also has proven utility in presurgical mapping and in monitoring therapeutic efficacy of topical treatments of malignancies.16-19 Most recently, this US Food and Drug Administration 510(k)-cleared tool for imaging in vivo unstained epithelium (including blood, collagen, and pigment) has an added adjunct: a telepathology network dedicated to the transfer of confocal images from a private practice to a remote confocal diagnostic reader for image interpretation. As a noninvasive technique, RCM is a promising tool, not only in the field of dermatology but also in primary care.
Comparison of Diagnostic Modalities
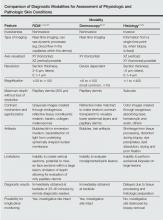
Historically, diagnostic modalities have included visual and histopathologic examination; however, basing a diagnosis solely on clinical grounds may not be reliable, and obtaining a tissue specimen may not be feasible or practical. Thus, noninvasive modalities as adjuncts for evaluation were developed, including high-frequency ultrasound, high-definition optical coherence tomography, dermoscopy, and in vivo RCM. Sonography was not reliable in clinical practice due to poor echogenicity and insufficient resolution, and although high-definition optical coherence tomography is emerging as an important tool for the evaluation of lesions with high clinical suspicion for nonmelanoma skin cancers (eg, basal cell carcinoma [BCC]), resolution is still insufficient for definitive diagnosis; therefore, these devices cannot be reliably used on pigmented lesions suspicious for melanoma.20-23
Reflectance confocal microscopy has many properties similar to both dermoscopy and histology (Table).1-3,24-28 Dermoscopy and RCM are used by physicians to noninvasively analyze lesions en face in real time; both modalities operate through optical magnification and liquid immersion without exogenous contrast agents and can be used to monitor lesion progression over time.25,29 However, when comparing these modalities for melanoma identification among equivocal melanocytic lesions, they revealed statistically similar sensitivities (dermoscopy, 88%; RCM, 91%) but notably different specificities with RCM achieving more than double the specificity (dermoscopy, 32%; RCM, 68%).30
Similar to histology images, RCM images provide high axial and lateral resolution, delineating cellular and morphologic architecture in both vertical and horizontal planes.29,31 Unlike histology, RCM does not require tissue removal and processing, thus the images are immediately available for analysis. Although RCM is noninvasive similar to dermoscopy and has resolutions comparable to histology, it uniquely demonstrates the dynamic processes of living skin in real time.1-3
Technical Properties of RCM
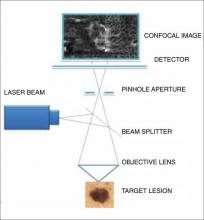
There are 7 components to the microscope: a laser light source, scanning elements, a relay telescope, a beam splitter, a pinhole aperture, an objective lens, and a detector (Figure 1).1,2,32 A low-power laser beam illuminates a point on or within the skin. The scattered light reflected back into the optical system is imaged on a detector. A pinhole aperture in front of this detector filters out the scattered light and allows only the light from the image plane (a thin, in-focus plane in the tissue) to pass through, creating a high-resolution image (3–5 μm horizontal optical sections) of the target lesion. The optical parameters include an 830-nm laser with an operating power of 22 mW and an immersion objective lens with a 0.9 numerical aperture. Each image has a 500-μm field of view with approximately ×30 magnification. A larger 2-dimensional image is created when the laser rapidly scans across the plane of the skin lesion, sequentially capturing multiple images. These individual images are stitched together to create a mosaic with a field of up to 8×8 mm.1,2,32
The maximum imaging depth extends into the papillary dermis (up to 350 mm, depending on tissue thickness).1,2,27,32 Depth of light penetration is limited by wavelength and intensity to maximize resolution of discernible structures and to avoid tissue damage. Contrast is dependent on light scattering, which is generated in 2 ways: (1) by differences between the refractive index of water and tissue constituents, and (2) by diffraction of light by structures similar in size to the illuminating light wavelength. Thus, highly reflective or diffractive structures such as melanin, keratin, hydrated collagen, and melanosomes produce backscattered light that appears bright (white) compared to their surroundings.1,2,27,32
RCM Image Acquisition and Interpretation
After patient and lesion history are obtained, visual and dermoscopic evaluation of the lesion is performed. Index fluid (mineral oil) is applied to the lesion and a metal ring with an optically clear, nonbirefringent polymer window is attached to the skin.32 A 5-megapixel dermoscopic-quality image is captured through this ring and window. A water-based immersion medium (ultrasound gel) for the objective lens is then placed on the window and the confocal microscope is magnetically attached to the ring. The index fluid has a refractive index similar to the stratum corneum and the window, allowing for optimal imaging down to the papillary dermis. The adhesive and magnetic attachments stabilize the skin lesion as the confocal microscope captures sequential 2-dimensional images. A mosaic is then created at the specified anatomic level. Levels of imaging are determined by the depth (in micrometers) from the stratum corneum and correspond to each anatomic layer. En face images also can be vertically stacked, generally in 3- to 5-μm increments.32
The number of mosaics and stacks obtained are based on a preset standardized protocol. Once captured, images are saved and then sent over the telemedicine network to a remote confocal diagnostic reader for image interpretation, which can be done immediately or the images can be stored and sent (known as store and forward) at a later time.
Lesion evaluation begins with a review of patient and lesion history and dermoscopic images, followed by review of confocal images for additional information through visualization of cellular structures and architecture. Confocal interpretation commonly begins with examination of the mosaic at the level of the dermoepidermal junction, as it often provides the most diagnostic information. The papillary epidermal layers can then be used to confirm the working diagnosis, to further enhance the description, or to refine the diagnosis. Areas of interest may then be further examined in a vertical plane, which is achieved by observing a specific spot at different depths. Image interpretation can be approached in a variety of ways; however, the most critical aspect to any method is the recognition of skin morphology.
Confocal Images
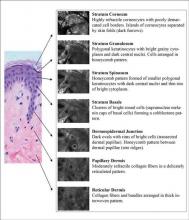
Epidermal and dermal structures identified with in vivo confocal images are comparable to histology. The first consensus terminology glossary with illustrative images was published in 2007 with descriptions and definitions of image quality, normal skin morphology, lesional architecture, and cellular details of melanocytic lesions.33 Figure 2 shows the normal structures that comprise the different layers of skin as seen on RCM.
Stratum Corneum
At a thickness of 0 to 15 μm, the stratum corneum is the first bright image seen on RCM.1,2 The individual anucleate corneocytes often cannot be delineated; thus, sheets of cells appear as islands separated by dark furrows (wrinkles).1,2
Stratum Granulosum
The first layer of viable cells is located 15 to 20 μm below the skin’s surface.1,2 The large 25- to 35-μm polygonal structures (granular keratinocytes) contain bright border zones (cytoplasm), a central dark oval (nucleus), and a grainy appearance (keratohyalin granules, organelles, and melanosomes).1-3 A honeycomb pattern is seen within the normal epidermis, whereas in darkly pigmented lesions where keratinocytes may contain some pigment, it has been described as cobblestone pattern.3
Stratum Spinosum
At a depth of 20 to 50 μm, the honeycomb pattern consists of smaller 15- to 25-μm polygonal structures (spinous keratinocytes) with thinner bright borders (cytoplasm) and a darker oval nucleus.1-3
Stratum Basale
Below the spinous cells is a single layer of brighter round structures (basal keratinocytes), each 7 to 12 mm in diameter.1,2 Due to the supranuclear melanin caps, the basal keratinocytes have increased reflectivity, appearing brighter than granular and spinous cells. The more abundant the melanin within the basal keratinocytes, the brighter the appearance.1,2
Dermoepidermal Junction
Below the stratum corneum (50–150 μm) is the dermoepidermal junction.1 The “peaks” of dermal papillae emerge as clusters of bright cells (basal keratinocytes). With deeper sectioning, the dark round-oval spaces rimmed by bright basal cells (dermal papillae) progressively enlarge. They continue to enlarge until neighboring papillae touch each other tangentially, corresponding to the valleys of rete ridges.1
Papillary Dermis
At a depth of 60 to 80 μm, blood vessels and collagen fibers are seen.1-3 Collagen and elastin fibers present as thin, delicately intertwined, highly reflective fibrillar structures (1–5 μm). Blood vessels appear as weakly reflective, round or canalicular structures within dermal papillae. Within the lumina, serum appears dark, but blood cells can be seen in real time as continually moving, weakly reflective or bright round structures corresponding to leukocytes, erythrocytes, and platelets. With real-time imaging, cells also can be identified based on their movement; leukocytes can fill or distend the lumen and roll slowly along vessel walls, whereas erythrocytes move rapidly within vessel lumina.1-3
Reticular Dermis
Further below the stratum corneum (100–350 μm), similar highly reflective collagen fibers and bundles are present, with diameters of 1 to 5 μm and 5 to 25 μm, respectively.2,3
Adnexal Structures
The limitation of imaging depth by wavelength and intensity restricts visualization to upper portions of sebaceous glands, sweat ducts, and hair shafts within hair follicles.2,32
Clinical Applications of RCM
Diagnosis of Lesions
Since the inception of RCM, confocal-based diagnostic criteria have been established for allergic and irritant contact dermatitis,4,5 malignant melanoma,6 BCC,7 actinic keratosis,8 and squamous cell carcinoma.8 Much of the research has focused on skin cancers, including the differentiation of benign and malignant skin lesions,34-38 to help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.10,11,28,35,38 In 2008 Guitera et al39 used RCM and dermoscopy to detect melanoma with a sensitivity of 98% and in 2012 determined that biopsies of benign nevi and lesions clinically suspicious for BCC could be reduced by as much as 68% in a series of 710 equivocal lesions.35 In 2014, in a prospective study including more than 1000 patients, Pellacani et al38 demonstrated that biopsies of equivocal benign lesions were reduced by more that 50%, and all of the melanomas and BCCs excised in the study were correctly detected by RCM interpretation. Additionally, in both studies, the sensitivity of the RCM interpretation for detecting BCC was 100%. Amelanotic melanoma can be diagnostically challenging because clinical and dermoscopic features often are nondescript. In 2001, Busam et al17 successfully used RCM for amelanotic melanoma detection and margin assessment. A subsequent study by Braga et al24 positively demonstrated that RCM may aid in the detection and diagnosis of various solitary pink lesions.
Adjunct to Mohs Micrographic Surgery
When excisional biopsies are impractical, incisional biopsies may be performed, which may lead to sampling errors. Atypical lesions with poorly defined clinical borders dictates standard of care with surgical excision and microscopic evaluation of margins. For malignancies requiring treatment with Mohs micrographic surgery, further staging often is required. These limitations may be overcome with RCM. Early detection of amelanotic malignant melanoma with margin assessment has been successfully demonstrated.17 Curiel-Lewandroski et al16 reported 3 successful cases wherein RCM was used for diagnosis and monitoring of topical treatment, delineation of surgical margins, and guidance in tissue-sparing surgical excision with amelanotic melanoma, locally recurrent melanoma, and lentigo maligna melanoma, respectively. In 2013, Guitera et al40 demonstrated that mapping lentigo maligna margins prior to Mohs surgery changed the surgical management of 73% of patients in a study that included 37 patients with clinically or dermoscopically visible lesions.
Monitoring Topical Treatment
Unlike conventional histology, RCM does not involve tissue destruction, allowing for longitudinal surveillance when treating a malignancy with topical therapy. In a 2003 case study, RCM was used to confirm a previously diagnosed BCC, map tumor periphery, visualize the inflammatory response to imiquimod cream 5%, and confirm posttreatment clearance. Reflectance confocal microscopy features were confirmed with biopsy before and after treatment, and clinical findings during treatment precisely correlated with RCM findings.18 A similar study the following year demonstrated the efficacy of imiquimod cream 5% as an adjunct to BCC treatment by reducing or eliminating the lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.19 To date, several studies have been performed by physicians throughout the world that have used RCM to monitor therapeutic outcomes of topically applied treatments such as imiquimod and hyaluronic acid as well as photodynamic therapy.41-43
A Clinical Tool
In vivo reflectance confocal microscopy, previously used only in the research setting, is now being used as a clinical tool for the evaluation of lesions suspicious for skin cancer by several academic centers and private practices throughout the United States. With clearance from the US Food and Drug Administration, physicians can use the device clinically for in vivo microscopic examination of skin lesions. The telepathology network allows for images to be acquired by a trained technician in a clinician’s office and then to be evaluated remotely by a diagnostic reader. The clinician can receive a diagnosis in as little as 30 minutes. The potential to noninvasively monitor tumor response to topical therapies, to delineate tumor margins prior to surgery, and to monitor lesions over time is an attractive option to patients.
The technology and telepathology network of RCM continues to be developed as diagnostic criteria are established and diagnostic readers are trained; however, diagnostic confocal features of various lesions have yet to be described, refined, or validated. Consequently, an extensive library of reference images has not yet been constructed.
Practical Application
A dermatology practice collaborated with a dermatopathology office to examine the feasibility of incorporating RCM and the telepathology network into the workflow of a private practice while creating a comprehensive library of cutaneous pathologies. A physician who did not have prior knowledge of RCM was selected for training with the goal to become proficient at operating the confocal microscope and interpreting the images. A dermatopathologist (also a confocal diagnostic reader) performed the histopathologic diagnoses of the lesions and correlated findings to confocal images.
Once images were captured using a standardized protocol, the lesion was biopsied according to standard of care. The images were sent over the telepathology network for interpretation and correlation to the histologic specimen by the dermatopathologist. These images were then stored on a secure server for use as a reference and educational tool for other diagnostic readers. We successfully achieved our goal of assisting with the development and integration of RCM and the telepathology network into the workflow of a busy private practice while building an extensive image library, thus showing potential use for other private practitioners.
Limitations of RCM
Although RCM may provide diagnostic information for many epidermal and papillary dermal lesions, it is not practical for predominantly dermal lesions or for providing prognostic information of invasive malignancies. Maximal imaging depth is 350 mm, but structures can truly be delineated at only approximately 250 μm (papillary dermis).2 Evaluation is further challenged with hypertrophic or hyperkeratotic lesions as well as those located on glabrous skin. Compared to histology, RCM resolution is slightly lower and nuclear features are not easily seen due to their weak backscattering effect.2 There are no adverse effects related to operator use; however, use may be limited if the patient has an allergy to the mediums used or to adhesive tape.
Challenges faced in integrating the technology into our practice include the machine size, time constraints, and reimbursement issues. Although not available in our office, smaller clinical devices exist (including a handheld RCM device that launched in 2007) and continue to be developed for future implementation. In our practice, capturing an image of 1 lesion took up to 20 minutes, but other protocols may necessitate only 10 minutes. Reimbursement for the imaging and image-reading procedures currently is being pursued.
Conclusion
In vivo RCM was developed as a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin. Cellular and subcellular structures as well as dynamic processes are observed without destruction of tissue. The morphologic features seen in RCM are comparable to those demonstrated with histology and dermoscopy. Despite current challenges, RCM has been shown to be an advantageous diagnostic tool, a guide to evaluating benign and malignant lesions, an adjunct to Mohs micrographic surgery via presurgical mapping of tumor margins, and a monitoring tool to establish treatment responses and efficacy. Reflectance confocal microscopy has steadily gained acceptance in clinical dermatology over the last decade, and the number of users continues to grow. With the continued efforts in advancing research, including usage of the telepathology network, we believe these tools will prove to be valuable in the private practice setting, both in the fields of dermatology and primary care.
Acknowledgment
The authors thank Caliber Imaging & Diagnostics, Inc (Rochester, New York), for providing the RCM imaging system with associated disposable supplies and the reader workstation for this review.
1. Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
2. Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison to histology. J Invest Dermatol. 1999;113:293-303.
3. Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846-852.
4. Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
5. Astner S, González E, Cheung AC, et al. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351-359.
6. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-985.
7. González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869-874.
8. Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766-772.
9. Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862-868.
10. Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365-376.
11. Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469-474.
12. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532-537.
13. Scope A, Benvenuto-Andrade C, Agero AL, et al. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176-185.
14. Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008:144:1597-1608.
15. Scope A, Gill M, Benveuto-Andrade C, et al. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727-734.
16. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140:1127-1132.
17. Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
18. Goldgeier M, Fox CA, Zavislan JM, et al. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol Surg. 2003;29:205-210.
19. Torres A, Niemeyer A, Berkes B, et al. 5% Imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
20. Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457-461.
21. Ruocco E, Argenziano G, Pellacani G, et al. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30(2, pt 2):301-310.
22. Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1-9.
23. Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions [published online ahead of print September 18, 2014]. J Biophotonics. doi:10.1002/jbio.201400085.
24. Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230-241.
25. Argenyl ZB. Dermoscopy (epiluminescence microscopy) of pigmented skin lesions. current status and evolving trends. Dermatologic Clin. 1997;15:79-95.
26. Grin CM, Kopf AW, Welkovich B, et al. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763-766.
27. Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
28. Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88-97.
29. Scope A, Halpern AC. Diagnostic procedures and devices. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:40-42.
30. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2008;129:131-138.
31. Junqueira LC, Carneiro J. Histology and its methods of study. In: Junqueira LC, Carneiro J, eds. Basic Histology: Text and Atlas. 11 ed. New York, NY: McGraw-Hill; 2005:1-21.
32. González S, Gill M, Halpern A, eds. Reflectance Confocal Microscopy of Cutaneous Tumors: An Atlas With Clinical, Dermoscopic and Histological Correlations. London, UK: Informa Healthcare; 2008.
33. Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
34. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365-372.
35. Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases [published online ahead of print June 21, 2012]. J Invest Dermatol. 2012;132:2386-2394.
36. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face [published online ahead of print April 15, 2010]. J Invest Dermatol. 2010;130:2080-2091.
37. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions [published online ahead of print July 26, 2007]. J Invest Dermatol. 2007;127:2759-2765.
38. Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study [published online ahead of print October 19, 2014]. Br J Dermatol. 2014;171:1044-1051.
39. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions [published online ahead of print July 17, 2008]. J Invest Dermatol. 2009;129:131-138
40. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
41. Malvehy J, Roldán-Marín R, Iglesias-García P, et al. Monitoring treatment of field cancerisation with 3% diclofenac sodium 2.5% hyaluronic acid by reflectance confocal microscopy: a histologic correlation. Acta Derm Venereol. 2015;95:45-50.
42. Zalaudek I, Piana S, Moscarella E, et al. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87.
43. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma tophotodynamic therapy [published online ahead of print December 13, 2012]. Dermatology. 2012;225:264-270.
Reflectance confocal microscopy (RCM) creates an image by detecting backscattered light from illuminated tissue and displaying it on a monitor at high resolution and contrast. The grayscale images, oriented in a horizontal (en face) plane, reveal cellular and morphologic architecture in progressive depths from the epidermis to the papillary dermis.1,2 Analyses of confocal features have shown good correlation with histologic and dermoscopic findings, allowing key features of normal skin topography as well as cutaneous lesions to be delineated.1-15 Most research has focused on differentiating benign and malignant lesions, but RCM also has proven utility in presurgical mapping and in monitoring therapeutic efficacy of topical treatments of malignancies.16-19 Most recently, this US Food and Drug Administration 510(k)-cleared tool for imaging in vivo unstained epithelium (including blood, collagen, and pigment) has an added adjunct: a telepathology network dedicated to the transfer of confocal images from a private practice to a remote confocal diagnostic reader for image interpretation. As a noninvasive technique, RCM is a promising tool, not only in the field of dermatology but also in primary care.
Comparison of Diagnostic Modalities
Historically, diagnostic modalities have included visual and histopathologic examination; however, basing a diagnosis solely on clinical grounds may not be reliable, and obtaining a tissue specimen may not be feasible or practical. Thus, noninvasive modalities as adjuncts for evaluation were developed, including high-frequency ultrasound, high-definition optical coherence tomography, dermoscopy, and in vivo RCM. Sonography was not reliable in clinical practice due to poor echogenicity and insufficient resolution, and although high-definition optical coherence tomography is emerging as an important tool for the evaluation of lesions with high clinical suspicion for nonmelanoma skin cancers (eg, basal cell carcinoma [BCC]), resolution is still insufficient for definitive diagnosis; therefore, these devices cannot be reliably used on pigmented lesions suspicious for melanoma.20-23
Reflectance confocal microscopy has many properties similar to both dermoscopy and histology (Table).1-3,24-28 Dermoscopy and RCM are used by physicians to noninvasively analyze lesions en face in real time; both modalities operate through optical magnification and liquid immersion without exogenous contrast agents and can be used to monitor lesion progression over time.25,29 However, when comparing these modalities for melanoma identification among equivocal melanocytic lesions, they revealed statistically similar sensitivities (dermoscopy, 88%; RCM, 91%) but notably different specificities with RCM achieving more than double the specificity (dermoscopy, 32%; RCM, 68%).30
Similar to histology images, RCM images provide high axial and lateral resolution, delineating cellular and morphologic architecture in both vertical and horizontal planes.29,31 Unlike histology, RCM does not require tissue removal and processing, thus the images are immediately available for analysis. Although RCM is noninvasive similar to dermoscopy and has resolutions comparable to histology, it uniquely demonstrates the dynamic processes of living skin in real time.1-3
Technical Properties of RCM
There are 7 components to the microscope: a laser light source, scanning elements, a relay telescope, a beam splitter, a pinhole aperture, an objective lens, and a detector (Figure 1).1,2,32 A low-power laser beam illuminates a point on or within the skin. The scattered light reflected back into the optical system is imaged on a detector. A pinhole aperture in front of this detector filters out the scattered light and allows only the light from the image plane (a thin, in-focus plane in the tissue) to pass through, creating a high-resolution image (3–5 μm horizontal optical sections) of the target lesion. The optical parameters include an 830-nm laser with an operating power of 22 mW and an immersion objective lens with a 0.9 numerical aperture. Each image has a 500-μm field of view with approximately ×30 magnification. A larger 2-dimensional image is created when the laser rapidly scans across the plane of the skin lesion, sequentially capturing multiple images. These individual images are stitched together to create a mosaic with a field of up to 8×8 mm.1,2,32
The maximum imaging depth extends into the papillary dermis (up to 350 mm, depending on tissue thickness).1,2,27,32 Depth of light penetration is limited by wavelength and intensity to maximize resolution of discernible structures and to avoid tissue damage. Contrast is dependent on light scattering, which is generated in 2 ways: (1) by differences between the refractive index of water and tissue constituents, and (2) by diffraction of light by structures similar in size to the illuminating light wavelength. Thus, highly reflective or diffractive structures such as melanin, keratin, hydrated collagen, and melanosomes produce backscattered light that appears bright (white) compared to their surroundings.1,2,27,32
RCM Image Acquisition and Interpretation
After patient and lesion history are obtained, visual and dermoscopic evaluation of the lesion is performed. Index fluid (mineral oil) is applied to the lesion and a metal ring with an optically clear, nonbirefringent polymer window is attached to the skin.32 A 5-megapixel dermoscopic-quality image is captured through this ring and window. A water-based immersion medium (ultrasound gel) for the objective lens is then placed on the window and the confocal microscope is magnetically attached to the ring. The index fluid has a refractive index similar to the stratum corneum and the window, allowing for optimal imaging down to the papillary dermis. The adhesive and magnetic attachments stabilize the skin lesion as the confocal microscope captures sequential 2-dimensional images. A mosaic is then created at the specified anatomic level. Levels of imaging are determined by the depth (in micrometers) from the stratum corneum and correspond to each anatomic layer. En face images also can be vertically stacked, generally in 3- to 5-μm increments.32
The number of mosaics and stacks obtained are based on a preset standardized protocol. Once captured, images are saved and then sent over the telemedicine network to a remote confocal diagnostic reader for image interpretation, which can be done immediately or the images can be stored and sent (known as store and forward) at a later time.
Lesion evaluation begins with a review of patient and lesion history and dermoscopic images, followed by review of confocal images for additional information through visualization of cellular structures and architecture. Confocal interpretation commonly begins with examination of the mosaic at the level of the dermoepidermal junction, as it often provides the most diagnostic information. The papillary epidermal layers can then be used to confirm the working diagnosis, to further enhance the description, or to refine the diagnosis. Areas of interest may then be further examined in a vertical plane, which is achieved by observing a specific spot at different depths. Image interpretation can be approached in a variety of ways; however, the most critical aspect to any method is the recognition of skin morphology.
Confocal Images
Epidermal and dermal structures identified with in vivo confocal images are comparable to histology. The first consensus terminology glossary with illustrative images was published in 2007 with descriptions and definitions of image quality, normal skin morphology, lesional architecture, and cellular details of melanocytic lesions.33 Figure 2 shows the normal structures that comprise the different layers of skin as seen on RCM.
Stratum Corneum
At a thickness of 0 to 15 μm, the stratum corneum is the first bright image seen on RCM.1,2 The individual anucleate corneocytes often cannot be delineated; thus, sheets of cells appear as islands separated by dark furrows (wrinkles).1,2
Stratum Granulosum
The first layer of viable cells is located 15 to 20 μm below the skin’s surface.1,2 The large 25- to 35-μm polygonal structures (granular keratinocytes) contain bright border zones (cytoplasm), a central dark oval (nucleus), and a grainy appearance (keratohyalin granules, organelles, and melanosomes).1-3 A honeycomb pattern is seen within the normal epidermis, whereas in darkly pigmented lesions where keratinocytes may contain some pigment, it has been described as cobblestone pattern.3
Stratum Spinosum
At a depth of 20 to 50 μm, the honeycomb pattern consists of smaller 15- to 25-μm polygonal structures (spinous keratinocytes) with thinner bright borders (cytoplasm) and a darker oval nucleus.1-3
Stratum Basale
Below the spinous cells is a single layer of brighter round structures (basal keratinocytes), each 7 to 12 mm in diameter.1,2 Due to the supranuclear melanin caps, the basal keratinocytes have increased reflectivity, appearing brighter than granular and spinous cells. The more abundant the melanin within the basal keratinocytes, the brighter the appearance.1,2
Dermoepidermal Junction
Below the stratum corneum (50–150 μm) is the dermoepidermal junction.1 The “peaks” of dermal papillae emerge as clusters of bright cells (basal keratinocytes). With deeper sectioning, the dark round-oval spaces rimmed by bright basal cells (dermal papillae) progressively enlarge. They continue to enlarge until neighboring papillae touch each other tangentially, corresponding to the valleys of rete ridges.1
Papillary Dermis
At a depth of 60 to 80 μm, blood vessels and collagen fibers are seen.1-3 Collagen and elastin fibers present as thin, delicately intertwined, highly reflective fibrillar structures (1–5 μm). Blood vessels appear as weakly reflective, round or canalicular structures within dermal papillae. Within the lumina, serum appears dark, but blood cells can be seen in real time as continually moving, weakly reflective or bright round structures corresponding to leukocytes, erythrocytes, and platelets. With real-time imaging, cells also can be identified based on their movement; leukocytes can fill or distend the lumen and roll slowly along vessel walls, whereas erythrocytes move rapidly within vessel lumina.1-3
Reticular Dermis
Further below the stratum corneum (100–350 μm), similar highly reflective collagen fibers and bundles are present, with diameters of 1 to 5 μm and 5 to 25 μm, respectively.2,3
Adnexal Structures
The limitation of imaging depth by wavelength and intensity restricts visualization to upper portions of sebaceous glands, sweat ducts, and hair shafts within hair follicles.2,32
Clinical Applications of RCM
Diagnosis of Lesions
Since the inception of RCM, confocal-based diagnostic criteria have been established for allergic and irritant contact dermatitis,4,5 malignant melanoma,6 BCC,7 actinic keratosis,8 and squamous cell carcinoma.8 Much of the research has focused on skin cancers, including the differentiation of benign and malignant skin lesions,34-38 to help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.10,11,28,35,38 In 2008 Guitera et al39 used RCM and dermoscopy to detect melanoma with a sensitivity of 98% and in 2012 determined that biopsies of benign nevi and lesions clinically suspicious for BCC could be reduced by as much as 68% in a series of 710 equivocal lesions.35 In 2014, in a prospective study including more than 1000 patients, Pellacani et al38 demonstrated that biopsies of equivocal benign lesions were reduced by more that 50%, and all of the melanomas and BCCs excised in the study were correctly detected by RCM interpretation. Additionally, in both studies, the sensitivity of the RCM interpretation for detecting BCC was 100%. Amelanotic melanoma can be diagnostically challenging because clinical and dermoscopic features often are nondescript. In 2001, Busam et al17 successfully used RCM for amelanotic melanoma detection and margin assessment. A subsequent study by Braga et al24 positively demonstrated that RCM may aid in the detection and diagnosis of various solitary pink lesions.
Adjunct to Mohs Micrographic Surgery
When excisional biopsies are impractical, incisional biopsies may be performed, which may lead to sampling errors. Atypical lesions with poorly defined clinical borders dictates standard of care with surgical excision and microscopic evaluation of margins. For malignancies requiring treatment with Mohs micrographic surgery, further staging often is required. These limitations may be overcome with RCM. Early detection of amelanotic malignant melanoma with margin assessment has been successfully demonstrated.17 Curiel-Lewandroski et al16 reported 3 successful cases wherein RCM was used for diagnosis and monitoring of topical treatment, delineation of surgical margins, and guidance in tissue-sparing surgical excision with amelanotic melanoma, locally recurrent melanoma, and lentigo maligna melanoma, respectively. In 2013, Guitera et al40 demonstrated that mapping lentigo maligna margins prior to Mohs surgery changed the surgical management of 73% of patients in a study that included 37 patients with clinically or dermoscopically visible lesions.
Monitoring Topical Treatment
Unlike conventional histology, RCM does not involve tissue destruction, allowing for longitudinal surveillance when treating a malignancy with topical therapy. In a 2003 case study, RCM was used to confirm a previously diagnosed BCC, map tumor periphery, visualize the inflammatory response to imiquimod cream 5%, and confirm posttreatment clearance. Reflectance confocal microscopy features were confirmed with biopsy before and after treatment, and clinical findings during treatment precisely correlated with RCM findings.18 A similar study the following year demonstrated the efficacy of imiquimod cream 5% as an adjunct to BCC treatment by reducing or eliminating the lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.19 To date, several studies have been performed by physicians throughout the world that have used RCM to monitor therapeutic outcomes of topically applied treatments such as imiquimod and hyaluronic acid as well as photodynamic therapy.41-43
A Clinical Tool
In vivo reflectance confocal microscopy, previously used only in the research setting, is now being used as a clinical tool for the evaluation of lesions suspicious for skin cancer by several academic centers and private practices throughout the United States. With clearance from the US Food and Drug Administration, physicians can use the device clinically for in vivo microscopic examination of skin lesions. The telepathology network allows for images to be acquired by a trained technician in a clinician’s office and then to be evaluated remotely by a diagnostic reader. The clinician can receive a diagnosis in as little as 30 minutes. The potential to noninvasively monitor tumor response to topical therapies, to delineate tumor margins prior to surgery, and to monitor lesions over time is an attractive option to patients.
The technology and telepathology network of RCM continues to be developed as diagnostic criteria are established and diagnostic readers are trained; however, diagnostic confocal features of various lesions have yet to be described, refined, or validated. Consequently, an extensive library of reference images has not yet been constructed.
Practical Application
A dermatology practice collaborated with a dermatopathology office to examine the feasibility of incorporating RCM and the telepathology network into the workflow of a private practice while creating a comprehensive library of cutaneous pathologies. A physician who did not have prior knowledge of RCM was selected for training with the goal to become proficient at operating the confocal microscope and interpreting the images. A dermatopathologist (also a confocal diagnostic reader) performed the histopathologic diagnoses of the lesions and correlated findings to confocal images.
Once images were captured using a standardized protocol, the lesion was biopsied according to standard of care. The images were sent over the telepathology network for interpretation and correlation to the histologic specimen by the dermatopathologist. These images were then stored on a secure server for use as a reference and educational tool for other diagnostic readers. We successfully achieved our goal of assisting with the development and integration of RCM and the telepathology network into the workflow of a busy private practice while building an extensive image library, thus showing potential use for other private practitioners.
Limitations of RCM
Although RCM may provide diagnostic information for many epidermal and papillary dermal lesions, it is not practical for predominantly dermal lesions or for providing prognostic information of invasive malignancies. Maximal imaging depth is 350 mm, but structures can truly be delineated at only approximately 250 μm (papillary dermis).2 Evaluation is further challenged with hypertrophic or hyperkeratotic lesions as well as those located on glabrous skin. Compared to histology, RCM resolution is slightly lower and nuclear features are not easily seen due to their weak backscattering effect.2 There are no adverse effects related to operator use; however, use may be limited if the patient has an allergy to the mediums used or to adhesive tape.
Challenges faced in integrating the technology into our practice include the machine size, time constraints, and reimbursement issues. Although not available in our office, smaller clinical devices exist (including a handheld RCM device that launched in 2007) and continue to be developed for future implementation. In our practice, capturing an image of 1 lesion took up to 20 minutes, but other protocols may necessitate only 10 minutes. Reimbursement for the imaging and image-reading procedures currently is being pursued.
Conclusion
In vivo RCM was developed as a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin. Cellular and subcellular structures as well as dynamic processes are observed without destruction of tissue. The morphologic features seen in RCM are comparable to those demonstrated with histology and dermoscopy. Despite current challenges, RCM has been shown to be an advantageous diagnostic tool, a guide to evaluating benign and malignant lesions, an adjunct to Mohs micrographic surgery via presurgical mapping of tumor margins, and a monitoring tool to establish treatment responses and efficacy. Reflectance confocal microscopy has steadily gained acceptance in clinical dermatology over the last decade, and the number of users continues to grow. With the continued efforts in advancing research, including usage of the telepathology network, we believe these tools will prove to be valuable in the private practice setting, both in the fields of dermatology and primary care.
Acknowledgment
The authors thank Caliber Imaging & Diagnostics, Inc (Rochester, New York), for providing the RCM imaging system with associated disposable supplies and the reader workstation for this review.
Reflectance confocal microscopy (RCM) creates an image by detecting backscattered light from illuminated tissue and displaying it on a monitor at high resolution and contrast. The grayscale images, oriented in a horizontal (en face) plane, reveal cellular and morphologic architecture in progressive depths from the epidermis to the papillary dermis.1,2 Analyses of confocal features have shown good correlation with histologic and dermoscopic findings, allowing key features of normal skin topography as well as cutaneous lesions to be delineated.1-15 Most research has focused on differentiating benign and malignant lesions, but RCM also has proven utility in presurgical mapping and in monitoring therapeutic efficacy of topical treatments of malignancies.16-19 Most recently, this US Food and Drug Administration 510(k)-cleared tool for imaging in vivo unstained epithelium (including blood, collagen, and pigment) has an added adjunct: a telepathology network dedicated to the transfer of confocal images from a private practice to a remote confocal diagnostic reader for image interpretation. As a noninvasive technique, RCM is a promising tool, not only in the field of dermatology but also in primary care.
Comparison of Diagnostic Modalities
Historically, diagnostic modalities have included visual and histopathologic examination; however, basing a diagnosis solely on clinical grounds may not be reliable, and obtaining a tissue specimen may not be feasible or practical. Thus, noninvasive modalities as adjuncts for evaluation were developed, including high-frequency ultrasound, high-definition optical coherence tomography, dermoscopy, and in vivo RCM. Sonography was not reliable in clinical practice due to poor echogenicity and insufficient resolution, and although high-definition optical coherence tomography is emerging as an important tool for the evaluation of lesions with high clinical suspicion for nonmelanoma skin cancers (eg, basal cell carcinoma [BCC]), resolution is still insufficient for definitive diagnosis; therefore, these devices cannot be reliably used on pigmented lesions suspicious for melanoma.20-23
Reflectance confocal microscopy has many properties similar to both dermoscopy and histology (Table).1-3,24-28 Dermoscopy and RCM are used by physicians to noninvasively analyze lesions en face in real time; both modalities operate through optical magnification and liquid immersion without exogenous contrast agents and can be used to monitor lesion progression over time.25,29 However, when comparing these modalities for melanoma identification among equivocal melanocytic lesions, they revealed statistically similar sensitivities (dermoscopy, 88%; RCM, 91%) but notably different specificities with RCM achieving more than double the specificity (dermoscopy, 32%; RCM, 68%).30
Similar to histology images, RCM images provide high axial and lateral resolution, delineating cellular and morphologic architecture in both vertical and horizontal planes.29,31 Unlike histology, RCM does not require tissue removal and processing, thus the images are immediately available for analysis. Although RCM is noninvasive similar to dermoscopy and has resolutions comparable to histology, it uniquely demonstrates the dynamic processes of living skin in real time.1-3
Technical Properties of RCM
There are 7 components to the microscope: a laser light source, scanning elements, a relay telescope, a beam splitter, a pinhole aperture, an objective lens, and a detector (Figure 1).1,2,32 A low-power laser beam illuminates a point on or within the skin. The scattered light reflected back into the optical system is imaged on a detector. A pinhole aperture in front of this detector filters out the scattered light and allows only the light from the image plane (a thin, in-focus plane in the tissue) to pass through, creating a high-resolution image (3–5 μm horizontal optical sections) of the target lesion. The optical parameters include an 830-nm laser with an operating power of 22 mW and an immersion objective lens with a 0.9 numerical aperture. Each image has a 500-μm field of view with approximately ×30 magnification. A larger 2-dimensional image is created when the laser rapidly scans across the plane of the skin lesion, sequentially capturing multiple images. These individual images are stitched together to create a mosaic with a field of up to 8×8 mm.1,2,32
The maximum imaging depth extends into the papillary dermis (up to 350 mm, depending on tissue thickness).1,2,27,32 Depth of light penetration is limited by wavelength and intensity to maximize resolution of discernible structures and to avoid tissue damage. Contrast is dependent on light scattering, which is generated in 2 ways: (1) by differences between the refractive index of water and tissue constituents, and (2) by diffraction of light by structures similar in size to the illuminating light wavelength. Thus, highly reflective or diffractive structures such as melanin, keratin, hydrated collagen, and melanosomes produce backscattered light that appears bright (white) compared to their surroundings.1,2,27,32
RCM Image Acquisition and Interpretation
After patient and lesion history are obtained, visual and dermoscopic evaluation of the lesion is performed. Index fluid (mineral oil) is applied to the lesion and a metal ring with an optically clear, nonbirefringent polymer window is attached to the skin.32 A 5-megapixel dermoscopic-quality image is captured through this ring and window. A water-based immersion medium (ultrasound gel) for the objective lens is then placed on the window and the confocal microscope is magnetically attached to the ring. The index fluid has a refractive index similar to the stratum corneum and the window, allowing for optimal imaging down to the papillary dermis. The adhesive and magnetic attachments stabilize the skin lesion as the confocal microscope captures sequential 2-dimensional images. A mosaic is then created at the specified anatomic level. Levels of imaging are determined by the depth (in micrometers) from the stratum corneum and correspond to each anatomic layer. En face images also can be vertically stacked, generally in 3- to 5-μm increments.32
The number of mosaics and stacks obtained are based on a preset standardized protocol. Once captured, images are saved and then sent over the telemedicine network to a remote confocal diagnostic reader for image interpretation, which can be done immediately or the images can be stored and sent (known as store and forward) at a later time.
Lesion evaluation begins with a review of patient and lesion history and dermoscopic images, followed by review of confocal images for additional information through visualization of cellular structures and architecture. Confocal interpretation commonly begins with examination of the mosaic at the level of the dermoepidermal junction, as it often provides the most diagnostic information. The papillary epidermal layers can then be used to confirm the working diagnosis, to further enhance the description, or to refine the diagnosis. Areas of interest may then be further examined in a vertical plane, which is achieved by observing a specific spot at different depths. Image interpretation can be approached in a variety of ways; however, the most critical aspect to any method is the recognition of skin morphology.
Confocal Images
Epidermal and dermal structures identified with in vivo confocal images are comparable to histology. The first consensus terminology glossary with illustrative images was published in 2007 with descriptions and definitions of image quality, normal skin morphology, lesional architecture, and cellular details of melanocytic lesions.33 Figure 2 shows the normal structures that comprise the different layers of skin as seen on RCM.
Stratum Corneum
At a thickness of 0 to 15 μm, the stratum corneum is the first bright image seen on RCM.1,2 The individual anucleate corneocytes often cannot be delineated; thus, sheets of cells appear as islands separated by dark furrows (wrinkles).1,2
Stratum Granulosum
The first layer of viable cells is located 15 to 20 μm below the skin’s surface.1,2 The large 25- to 35-μm polygonal structures (granular keratinocytes) contain bright border zones (cytoplasm), a central dark oval (nucleus), and a grainy appearance (keratohyalin granules, organelles, and melanosomes).1-3 A honeycomb pattern is seen within the normal epidermis, whereas in darkly pigmented lesions where keratinocytes may contain some pigment, it has been described as cobblestone pattern.3
Stratum Spinosum
At a depth of 20 to 50 μm, the honeycomb pattern consists of smaller 15- to 25-μm polygonal structures (spinous keratinocytes) with thinner bright borders (cytoplasm) and a darker oval nucleus.1-3
Stratum Basale
Below the spinous cells is a single layer of brighter round structures (basal keratinocytes), each 7 to 12 mm in diameter.1,2 Due to the supranuclear melanin caps, the basal keratinocytes have increased reflectivity, appearing brighter than granular and spinous cells. The more abundant the melanin within the basal keratinocytes, the brighter the appearance.1,2
Dermoepidermal Junction
Below the stratum corneum (50–150 μm) is the dermoepidermal junction.1 The “peaks” of dermal papillae emerge as clusters of bright cells (basal keratinocytes). With deeper sectioning, the dark round-oval spaces rimmed by bright basal cells (dermal papillae) progressively enlarge. They continue to enlarge until neighboring papillae touch each other tangentially, corresponding to the valleys of rete ridges.1
Papillary Dermis
At a depth of 60 to 80 μm, blood vessels and collagen fibers are seen.1-3 Collagen and elastin fibers present as thin, delicately intertwined, highly reflective fibrillar structures (1–5 μm). Blood vessels appear as weakly reflective, round or canalicular structures within dermal papillae. Within the lumina, serum appears dark, but blood cells can be seen in real time as continually moving, weakly reflective or bright round structures corresponding to leukocytes, erythrocytes, and platelets. With real-time imaging, cells also can be identified based on their movement; leukocytes can fill or distend the lumen and roll slowly along vessel walls, whereas erythrocytes move rapidly within vessel lumina.1-3
Reticular Dermis
Further below the stratum corneum (100–350 μm), similar highly reflective collagen fibers and bundles are present, with diameters of 1 to 5 μm and 5 to 25 μm, respectively.2,3
Adnexal Structures
The limitation of imaging depth by wavelength and intensity restricts visualization to upper portions of sebaceous glands, sweat ducts, and hair shafts within hair follicles.2,32
Clinical Applications of RCM
Diagnosis of Lesions
Since the inception of RCM, confocal-based diagnostic criteria have been established for allergic and irritant contact dermatitis,4,5 malignant melanoma,6 BCC,7 actinic keratosis,8 and squamous cell carcinoma.8 Much of the research has focused on skin cancers, including the differentiation of benign and malignant skin lesions,34-38 to help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.10,11,28,35,38 In 2008 Guitera et al39 used RCM and dermoscopy to detect melanoma with a sensitivity of 98% and in 2012 determined that biopsies of benign nevi and lesions clinically suspicious for BCC could be reduced by as much as 68% in a series of 710 equivocal lesions.35 In 2014, in a prospective study including more than 1000 patients, Pellacani et al38 demonstrated that biopsies of equivocal benign lesions were reduced by more that 50%, and all of the melanomas and BCCs excised in the study were correctly detected by RCM interpretation. Additionally, in both studies, the sensitivity of the RCM interpretation for detecting BCC was 100%. Amelanotic melanoma can be diagnostically challenging because clinical and dermoscopic features often are nondescript. In 2001, Busam et al17 successfully used RCM for amelanotic melanoma detection and margin assessment. A subsequent study by Braga et al24 positively demonstrated that RCM may aid in the detection and diagnosis of various solitary pink lesions.
Adjunct to Mohs Micrographic Surgery
When excisional biopsies are impractical, incisional biopsies may be performed, which may lead to sampling errors. Atypical lesions with poorly defined clinical borders dictates standard of care with surgical excision and microscopic evaluation of margins. For malignancies requiring treatment with Mohs micrographic surgery, further staging often is required. These limitations may be overcome with RCM. Early detection of amelanotic malignant melanoma with margin assessment has been successfully demonstrated.17 Curiel-Lewandroski et al16 reported 3 successful cases wherein RCM was used for diagnosis and monitoring of topical treatment, delineation of surgical margins, and guidance in tissue-sparing surgical excision with amelanotic melanoma, locally recurrent melanoma, and lentigo maligna melanoma, respectively. In 2013, Guitera et al40 demonstrated that mapping lentigo maligna margins prior to Mohs surgery changed the surgical management of 73% of patients in a study that included 37 patients with clinically or dermoscopically visible lesions.
Monitoring Topical Treatment
Unlike conventional histology, RCM does not involve tissue destruction, allowing for longitudinal surveillance when treating a malignancy with topical therapy. In a 2003 case study, RCM was used to confirm a previously diagnosed BCC, map tumor periphery, visualize the inflammatory response to imiquimod cream 5%, and confirm posttreatment clearance. Reflectance confocal microscopy features were confirmed with biopsy before and after treatment, and clinical findings during treatment precisely correlated with RCM findings.18 A similar study the following year demonstrated the efficacy of imiquimod cream 5% as an adjunct to BCC treatment by reducing or eliminating the lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.19 To date, several studies have been performed by physicians throughout the world that have used RCM to monitor therapeutic outcomes of topically applied treatments such as imiquimod and hyaluronic acid as well as photodynamic therapy.41-43
A Clinical Tool
In vivo reflectance confocal microscopy, previously used only in the research setting, is now being used as a clinical tool for the evaluation of lesions suspicious for skin cancer by several academic centers and private practices throughout the United States. With clearance from the US Food and Drug Administration, physicians can use the device clinically for in vivo microscopic examination of skin lesions. The telepathology network allows for images to be acquired by a trained technician in a clinician’s office and then to be evaluated remotely by a diagnostic reader. The clinician can receive a diagnosis in as little as 30 minutes. The potential to noninvasively monitor tumor response to topical therapies, to delineate tumor margins prior to surgery, and to monitor lesions over time is an attractive option to patients.
The technology and telepathology network of RCM continues to be developed as diagnostic criteria are established and diagnostic readers are trained; however, diagnostic confocal features of various lesions have yet to be described, refined, or validated. Consequently, an extensive library of reference images has not yet been constructed.
Practical Application
A dermatology practice collaborated with a dermatopathology office to examine the feasibility of incorporating RCM and the telepathology network into the workflow of a private practice while creating a comprehensive library of cutaneous pathologies. A physician who did not have prior knowledge of RCM was selected for training with the goal to become proficient at operating the confocal microscope and interpreting the images. A dermatopathologist (also a confocal diagnostic reader) performed the histopathologic diagnoses of the lesions and correlated findings to confocal images.
Once images were captured using a standardized protocol, the lesion was biopsied according to standard of care. The images were sent over the telepathology network for interpretation and correlation to the histologic specimen by the dermatopathologist. These images were then stored on a secure server for use as a reference and educational tool for other diagnostic readers. We successfully achieved our goal of assisting with the development and integration of RCM and the telepathology network into the workflow of a busy private practice while building an extensive image library, thus showing potential use for other private practitioners.
Limitations of RCM
Although RCM may provide diagnostic information for many epidermal and papillary dermal lesions, it is not practical for predominantly dermal lesions or for providing prognostic information of invasive malignancies. Maximal imaging depth is 350 mm, but structures can truly be delineated at only approximately 250 μm (papillary dermis).2 Evaluation is further challenged with hypertrophic or hyperkeratotic lesions as well as those located on glabrous skin. Compared to histology, RCM resolution is slightly lower and nuclear features are not easily seen due to their weak backscattering effect.2 There are no adverse effects related to operator use; however, use may be limited if the patient has an allergy to the mediums used or to adhesive tape.
Challenges faced in integrating the technology into our practice include the machine size, time constraints, and reimbursement issues. Although not available in our office, smaller clinical devices exist (including a handheld RCM device that launched in 2007) and continue to be developed for future implementation. In our practice, capturing an image of 1 lesion took up to 20 minutes, but other protocols may necessitate only 10 minutes. Reimbursement for the imaging and image-reading procedures currently is being pursued.
Conclusion
In vivo RCM was developed as a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin. Cellular and subcellular structures as well as dynamic processes are observed without destruction of tissue. The morphologic features seen in RCM are comparable to those demonstrated with histology and dermoscopy. Despite current challenges, RCM has been shown to be an advantageous diagnostic tool, a guide to evaluating benign and malignant lesions, an adjunct to Mohs micrographic surgery via presurgical mapping of tumor margins, and a monitoring tool to establish treatment responses and efficacy. Reflectance confocal microscopy has steadily gained acceptance in clinical dermatology over the last decade, and the number of users continues to grow. With the continued efforts in advancing research, including usage of the telepathology network, we believe these tools will prove to be valuable in the private practice setting, both in the fields of dermatology and primary care.
Acknowledgment
The authors thank Caliber Imaging & Diagnostics, Inc (Rochester, New York), for providing the RCM imaging system with associated disposable supplies and the reader workstation for this review.
1. Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
2. Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison to histology. J Invest Dermatol. 1999;113:293-303.
3. Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846-852.
4. Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
5. Astner S, González E, Cheung AC, et al. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351-359.
6. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-985.
7. González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869-874.
8. Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766-772.
9. Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862-868.
10. Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365-376.
11. Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469-474.
12. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532-537.
13. Scope A, Benvenuto-Andrade C, Agero AL, et al. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176-185.
14. Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008:144:1597-1608.
15. Scope A, Gill M, Benveuto-Andrade C, et al. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727-734.
16. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140:1127-1132.
17. Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
18. Goldgeier M, Fox CA, Zavislan JM, et al. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol Surg. 2003;29:205-210.
19. Torres A, Niemeyer A, Berkes B, et al. 5% Imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
20. Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457-461.
21. Ruocco E, Argenziano G, Pellacani G, et al. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30(2, pt 2):301-310.
22. Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1-9.
23. Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions [published online ahead of print September 18, 2014]. J Biophotonics. doi:10.1002/jbio.201400085.
24. Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230-241.
25. Argenyl ZB. Dermoscopy (epiluminescence microscopy) of pigmented skin lesions. current status and evolving trends. Dermatologic Clin. 1997;15:79-95.
26. Grin CM, Kopf AW, Welkovich B, et al. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763-766.
27. Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
28. Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88-97.
29. Scope A, Halpern AC. Diagnostic procedures and devices. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:40-42.
30. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2008;129:131-138.
31. Junqueira LC, Carneiro J. Histology and its methods of study. In: Junqueira LC, Carneiro J, eds. Basic Histology: Text and Atlas. 11 ed. New York, NY: McGraw-Hill; 2005:1-21.
32. González S, Gill M, Halpern A, eds. Reflectance Confocal Microscopy of Cutaneous Tumors: An Atlas With Clinical, Dermoscopic and Histological Correlations. London, UK: Informa Healthcare; 2008.
33. Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
34. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365-372.
35. Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases [published online ahead of print June 21, 2012]. J Invest Dermatol. 2012;132:2386-2394.
36. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face [published online ahead of print April 15, 2010]. J Invest Dermatol. 2010;130:2080-2091.
37. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions [published online ahead of print July 26, 2007]. J Invest Dermatol. 2007;127:2759-2765.
38. Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study [published online ahead of print October 19, 2014]. Br J Dermatol. 2014;171:1044-1051.
39. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions [published online ahead of print July 17, 2008]. J Invest Dermatol. 2009;129:131-138
40. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
41. Malvehy J, Roldán-Marín R, Iglesias-García P, et al. Monitoring treatment of field cancerisation with 3% diclofenac sodium 2.5% hyaluronic acid by reflectance confocal microscopy: a histologic correlation. Acta Derm Venereol. 2015;95:45-50.
42. Zalaudek I, Piana S, Moscarella E, et al. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87.
43. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma tophotodynamic therapy [published online ahead of print December 13, 2012]. Dermatology. 2012;225:264-270.
1. Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
2. Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison to histology. J Invest Dermatol. 1999;113:293-303.
3. Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846-852.
4. Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
5. Astner S, González E, Cheung AC, et al. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351-359.
6. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-985.
7. González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869-874.
8. Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766-772.
9. Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862-868.
10. Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365-376.
11. Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469-474.
12. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532-537.
13. Scope A, Benvenuto-Andrade C, Agero AL, et al. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176-185.
14. Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008:144:1597-1608.
15. Scope A, Gill M, Benveuto-Andrade C, et al. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727-734.
16. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140:1127-1132.
17. Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
18. Goldgeier M, Fox CA, Zavislan JM, et al. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol Surg. 2003;29:205-210.
19. Torres A, Niemeyer A, Berkes B, et al. 5% Imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
20. Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457-461.
21. Ruocco E, Argenziano G, Pellacani G, et al. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30(2, pt 2):301-310.
22. Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1-9.
23. Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions [published online ahead of print September 18, 2014]. J Biophotonics. doi:10.1002/jbio.201400085.
24. Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230-241.
25. Argenyl ZB. Dermoscopy (epiluminescence microscopy) of pigmented skin lesions. current status and evolving trends. Dermatologic Clin. 1997;15:79-95.
26. Grin CM, Kopf AW, Welkovich B, et al. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763-766.
27. Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
28. Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88-97.
29. Scope A, Halpern AC. Diagnostic procedures and devices. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:40-42.
30. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2008;129:131-138.
31. Junqueira LC, Carneiro J. Histology and its methods of study. In: Junqueira LC, Carneiro J, eds. Basic Histology: Text and Atlas. 11 ed. New York, NY: McGraw-Hill; 2005:1-21.
32. González S, Gill M, Halpern A, eds. Reflectance Confocal Microscopy of Cutaneous Tumors: An Atlas With Clinical, Dermoscopic and Histological Correlations. London, UK: Informa Healthcare; 2008.
33. Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
34. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365-372.
35. Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases [published online ahead of print June 21, 2012]. J Invest Dermatol. 2012;132:2386-2394.
36. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face [published online ahead of print April 15, 2010]. J Invest Dermatol. 2010;130:2080-2091.
37. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions [published online ahead of print July 26, 2007]. J Invest Dermatol. 2007;127:2759-2765.
38. Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study [published online ahead of print October 19, 2014]. Br J Dermatol. 2014;171:1044-1051.
39. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions [published online ahead of print July 17, 2008]. J Invest Dermatol. 2009;129:131-138
40. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
41. Malvehy J, Roldán-Marín R, Iglesias-García P, et al. Monitoring treatment of field cancerisation with 3% diclofenac sodium 2.5% hyaluronic acid by reflectance confocal microscopy: a histologic correlation. Acta Derm Venereol. 2015;95:45-50.
42. Zalaudek I, Piana S, Moscarella E, et al. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87.
43. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma tophotodynamic therapy [published online ahead of print December 13, 2012]. Dermatology. 2012;225:264-270.
Practice Points
- In vivo reflectance confocal microscopy (RCM) is a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin.
- Similar to dermoscopy, RCM allows lesions to be analyzed noninvasively, and similar to histology, RCM images provide high resolution in both vertical and horizontal planes.
- Utilizing RCM as an adjunctive tool can help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.
- Incorporating RCM and a telepathology network into the workflow of a private practice may be valuable for dermatologists and primary care physicians.
Novel Psoriasis Therapies and Patient Outcomes, Part 2: Biologic Treatments
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
Practice Points
- Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and psoriatic arthritis (PsA).
- Although biologics currently in use for treatment of psoriasis and PsA are in the form of tumor necrosis factor α inhibitors, other drugs in phase 2 through phase 4 clinical trials aim to target alternative pathways underlying the pathogenesis of these disorders, including IL-12/IL-23 inhibition, IL-17 inhibition, inhibition of T-cell activation in antigen-presenting cells, regulatory T-cell activation, toll-like receptor inhibition, and granulocyte-macrophage colony-stimulating factor inhibition.
- New approaches to the management of psoriasis and PsA offer patients hope for more targeted treatment regimens.
Building evidence-based medicine skills in gynecology
Although evidence-based medicine, or EBM, is not a new concept, the phrase is tossed about frequently in today’s culture of quality improvement initiatives and metrics. What does EBM really mean, however, and how do we ensure we are practicing it?
At its heart, EBM integrates 3 components:
- the individual clinician’s expertise
- the patient’s values and preferences
- the best external evidence to guide treatment decisions.
Because each clinician’s skillset and each patient’s issues and preferences may be quite varied, in this article we target the third piece—determining the best external evidence.
Our focus on EBM is not meant to negate the importance of the clinician’s expertise, which has been gained through years of practice. Indeed, without expertise, “practice risks becoming tyrannized by evidence.”1 However, without current best evidence, “practice risks becoming rapidly out of date, to the detriment of patients.”1 With the integration of evidence, expertise, and patient choice, EBM is not “cookbook” medicine, and it is not conducted only from armchairs and ivory towers. Rather, EBM is, or should be, at the frontline of clinical care.
EBM begins with a specific clinical question, such as “What is the best treatment option for my patient?” The answer can be honed with the “PICO” approach, which considers Population, Intervention, Comparators, and Outcomes of interest. Specifically, in a particular patient population (similar to your own patient), how does an intervention impact key outcomes?
For directly comparing intervention options, such as surgery A versus surgery B, a randomized controlled trial (RCT) is one of the best methods to address clinical questions (FIGURE).2 Systematic reviews are more generalizable than single studies since they compare a range of relevant interventions across populations and settings. Evaluations of diagnostic test accuracy3,4or analyses of risk factors or natural history are best addressed by other study designs, which also can provide important evidence, but will not be discussed in depth here.
In this article, we focus on the benefits of RCTs and systematic reviews, as well as when to exhibit caution, for instance when RCTs report “surrogate outcomes” or make analyses drawn from subgroups of the original population. In addition, we discuss the inability to adequately assess treatment harms (versus benefits) from available evidence as well as the practicalities of how to apply EBM to patients.
RCTs: The good, the bad, and the ugly
RCTs are prospective experiments with a predefined protocol in which patients are randomly allocated to groups where the only difference is the intervention (vs comparators). This design helps to minimize the effects of known and unknown confounders and selection bias.
Ideally, the group into which a study participant is allocated is concealed from the patient and from the caregiver, minimizing the risk that the randomization is broken and the treatment allocation is biased. (Frequently this is not possible, however, particularly for surgical interventions.) Similarly, ideally, the outcome assessors are blinded to the treatment whenever possible. This minimizes the risk of a patient’s outcome being consciously or unconsciously altered due to the outcome assessor’s beliefs about the effectiveness of the intervention.
The reported clinical or surrogate outcomes (which will be discussed in more depth on the next page) for an RCT may be objective or subjective. Preferably, outcomes are patient-centered—important from the patient’s perspective of benefits and harms. Examples of these types of outcomes include survival, function, symptoms, and health-related quality of life, as well as impact on work and family, convenience, and cost. Patients likely are less interested in estimated blood loss, surgical time, biochemistry results, and other clinical or surrogate outcomes.
There are disadvantages to RCTs. For instance, each study provides only a snapshot of the evidence on a given topic. One study rarely, if ever, provides a definitive conclusion. The study’s findings are subject to random error and to biases introduced by study design or analytic methods, and they will not be generalizable to all patients and settings. In addition, the study likely has evaluated only 1 or 2 specific interventions among a plethora of available options, and is unlikely to have analyzed all outcomes of interest.
It becomes your burden to assess whether a trial’s findings are applicable to an actual patient (known as “external validity”). Because an RCT must artificially constrain the underlying clinical questions into a testable research question, translation to the specific patient is often flawed. Perhaps the patient does not precisely fit the inclusion criteria of the trial, for instance, or the exact intervention tested is not fully reproducible. From a practicality perspective, an RCT is often immensely costly to execute, which may be reflected in relatively small numbers of patients and short-term duration of follow-up. These disadvantages limit the ability of RCTs to assess harms, rare events, and long-term outcomes.
Surrogate outcomes
Outcomes measured in a trial should be relevant, easy to interpret and diagnose, sensitive to treatment differences, and measurable within a reasonable period of time. However, these characteristics are not always achievable for important clinical outcomes in an RCT. Therefore, a surrogate outcome may take the place of the true clinical efficacy measurement.
For example, in studies of interventions for infertility in patients with polycystic ovary syndrome (PCOS), common surrogates to the “true” desired outcome of a healthy live birth may include ovulation, implantation, or pregnancy rates. These surrogate outcomes may correlate with live birth but clearly ignore other factors extrinsic and intrinsic to PCOS that affect the chance for a healthy term delivery; the possible increased risk for miscarriage in PCOS; and increased risks of other pregnancy complications, such as preeclampsia and gestational diabetes.
Similarly, many trials of oral contraceptives that aim to study the clinical endpoint of pulmonary embolism or venous thromboembolism, which are rare events, instead use the surrogates of results of coagulation tests or levels of sex hormone-binding globulin. Clearly, caution must be exercised when interpreting studies that use surrogate outcomes. As the clinician, you must recognize that a change in a biologic or physical measurement may not be clinically relevant. Some judgment is required about causal pathways: The less that is known about the causal pathway of a disease, the less confident one should be in any surrogate outcome.
Finally, clinicians also must recognize that a valid surrogate for one treatment may not be valid for another treatment or another population.5 For example, ovulation inhibition would be an appropriate surrogate endpoint for contraceptive efficacy for a method that reliably prevents ovulation; however, this would not be a good surrogate outcome to evaluate the progestin-only pill, which fails to inhibit ovulation completely and yet is highly effective in contraceptive trials.
Avoiding pitfalls with subgroup analyses
It is common, particularly in large RCTs, to evaluate treatment effects for a specific endpoint in a subgroup of patients included in the trial. The goal is to determine whether the findings of the larger study apply more or less to a specific patient (who may differ from the total population by some important characteristic, such as age, weight, parity, or menopausal or smoking status). The variability in study results when stratified by these patient factors is known as heterogeneity of treatment effect, which may be quantitative or qualitative.6
In the former, one treatment is always better than the other, although by varying degrees depending on the subgroup. (For example, a stronger effect could be seen in those aged 65 and younger than in those older than 65.) In the latter, the treatment fares better than the comparator in one subgroup but worse or no different for another subgroup. In either case, the appropriate statistical tool to identify heterogeneity of treatment effect is a test for interaction between the characteristic and the treatment effect, rather than claiming heterogeneity on the basis of separate tests of treatment effects within the different subpopulations.
One problem with dividing the original population into smaller subpopulations is that the number of participants decreases—thus there is less power, or less statistical strength, to identify a treatment effect. More accurately, there is a greater likelihood of a type II error (a false negative) when these small subpopulations have too few patients to demonstrate a clinical treatment effect that actually may exist.
False positives. Paradoxically, another problem with subgroup analyses is a greater chance for false positives due to the multiple statistical testing that is performed. The original study is rarely powered appropriately to do this (see “Error rates in subgroup analyses”). According to Wang and colleagues, “It is common practice to conduct a subgroup analysis for each of several (and often many) baseline characteristics, for each of several endpoints, or for both.”7 The more subgroup analyses performed, the more likely that differences found are due to chance only. Unfortunately, in unplanned post hoc analyses, the number of tests performed is often unreported; therefore, the error rates are unknown. There are statistical methods to try and correct for this “multiplicity” problem but, ideally, only a few key subgroup analyses are performed, and they are planned a priori in the original study design. In these cases, the study’s size can be adjusted accordingly. In most instances, findings from subgroup analyses, whether positive or negative, should be considered as “hypothesis generating” and interpreted with caution.
Error rates in subgroup analyses
With “k” independent subgroups and no difference in treatments, the probability of at least one “significant” subgroup (such as a false positive) is 1 – (1-α)k.
If α = 0.05 and there are k = 10 subgroups, then 1 – (0.95)10 = 0.40. That is, if 10 subgroup analyses are performed, there is a 40% likelihood that 1 will demonstrate a “significant” difference in treatment effect, even though no difference exists.
Systematic reviews: What, why, and how?
Systematic reviews aim to overcome the deficiencies of single studies in a comprehensive and unbiased manner. They critically evaluate, summarize, and, when possible, combine all available studies addressing a given topic. By comparing a range of relevant interventions across populations and settings, systematic reviews may be more generalizable than single studies. Meta-analysis, or quantitatively combining study results, increases sample size and usually provides more precise estimates of effect sizes than the single studies. Critical appraisal of the combined studies can highlight methodologic and other concerns about the body of evidence to assess the overall confidence in the included studies.
A systematic review, like a well-conducted RCT, has a protocol that lays out the scope of the review and defines a priori criteria and analytic plans—all with the goal of minimizing bias. It starts with a well-formulated research question, explicitly defining the PICO elements—population, interventions, comparators, outcomes—in addition to the setting and study designs of interest.8 Based on these eligibility criteria, several sources of evidence (such as electronic databases and reference lists) are searched to find all potentially eligible studies.
Typically, several thousand citations are found that must be matched against the eligibility criteria. Potentially eligible studies are then rescreened in full text to further scrutinize their eligibility. The goal is to be highly sensitive to avoid missing relevant studies—even at the time cost of screening many articles. The individual study designs (including the study eligibility criteria, interventions, outcomes, and analytic methods) and the results for all outcomes of interest are extracted from each study.
For most systematic reviews, researchers also will assess the quality, or risk of bias, of each study for each outcome.9,10 Study data are summarized across all included studies, with study results meta-analyzed and reasons for heterogeneity across studies explored. Several consensus statements detail the proper methodology to conduct and report a systematic review.11,12 Ultimately, the review’s conclusions are based on analyses of all available evidence. By contrast, narrative reviews typically start with a conclusion and then select evidence to support that conclusion, and are therefore more likely to be biased.13
As noted, systematic reviews often include meta-analysis, which may allow an exploration of some reasons for study heterogeneity. The meta-analysis is usually presented graphically in a forest plot, which displays point estimates for each study with their associated 95% confidence intervals and a description of each study.14 In a forest plot, one can see the estimate and precision of each study, assess the heterogeneity of results across studies, and compare individual studies to each other and to the overall summary estimate.
Systematic reviews should be read as critically as primary studies. Some important questions you should consider are:
- Did the review address the populations, interventions, comparators, outcomes, and settings relevant to your practice?
- Have studies been included in a nonbiased manner, and is the described body of evidence likely to be complete?
- Did the study authors evaluate and summarize the underlying risks of bias of the studies?
- Did the researchers avoid combining studies that are too different from each other to allow a coherent interpretation of the summary results?
- Did the researchers attempt to explain how and why studies differed from one another?
Of note, systematic reviews and meta-analyses are subject to the same biases as all retrospective studies. Also, the systematic reviewers’ own biases—due to factors such as funding source, researchers’ agendas, or specialties—may subtly affect systematic reviews just as biases may affect an individual study. Furthermore, the confidence you have in a systematic review’s conclusions may be limited by the quality and generalizability of the underlying studies.
Assessing harms
You make the ultimate management decisions for your patient (though, of course, with her participation). The likely benefit of a specific treatment—determined in an experimental trial and refined further in a systematic review and meta-analysis—must be balanced with the risk of harms. RCTs usually do not provide the highest quality evidence of harms due to their limited sample sizes and short follow-up duration. Rather, large observational studies, case series, and case reports commonly provide these important details. Increasingly, patient registries are being created to prospectively follow patients and gather uniform safety data. By providing a true denominator, more accurate estimates of adverse event incidence are possible. However, the disadvantages of all of these modalities are 1) there usually are no comparators (that is, “How does the adverse event incidence for surgery A compare to that for surgery B?”) and 2) data usually are gleaned from medical records and not directly from patients.
As a result, these studies typically lack information on subjective harms, such as impaired sexual function. The reporting of treatment harms suffers from inconsistent and imprecise terminology, making it hard to reliably gather all reports of similar adverse events. Adverse event reporting in clinical trials is often driven by regulatory definitions and requirements instead of patient-centered definitions. In fact, there has been little work to date that assesses which adverse events or complications may be most relevant or important from the patient perspective.
Taken together, it is clear that the medical literature tends to emphasize treatment benefits (with robust methodologies and data to detect these benefits) but does not reliably or adequately assess harms. For rare events, risk estimates always will be imprecise. Nonetheless, better systematic reviews and today’s larger comparative effectiveness reviews strive to gather harms data from the multiple available sources described above.
Applying the evidence and your expertise to your patient
Now that you have identified the best valid and important evidence to support or refute a clinical decision (TABLE15), and have coupled this with your own expert knowledge and judgment in shared decision making with your patient, you must communicate to her the personalized information about outcomes, probabilities, and scientific uncertainties of her available treatment options.15 Patients, in turn, should be allowed to communicate their values and the relative importance they place on benefits and harms.16 This conversation, of course, is built on the foundation of a sound physician–patient relationship and is a part of every informed consent process.
Is this evidence applicable to my patient? A decision guide.15
- Is my patient so different from those in the study that the trial results cannot be applied?
- Is the treatment feasible in my setting?
- What are my patient’s likely benefits and harms from the therapy?
- How will my patient’s values influence the final treatment decision?
Decision tools
Increasingly, decision-aid tools are being developed to support this process. These aids must express the helpful and harmful effects of a treatment, including alternative options, in statements that are valid and concise. Furthermore, they must be intelligible to both the clinician and patient and modifiable to the patient’s values and wishes.17 Two examples of counseling aids are the Gail model of breast cancer risk prediction18 and the Framingham Coronary Heart Disease Prediction Score.19 Web-based decision aids that can be accessed in real-time in busy clinical settings also are being developed for gynecology.20
Never stop re-evaluating
The final piece of EBM is to “close the loop”—meaning to evaluate the effectiveness of applying the evidence in clinical practice. To do this, watch for clinical practice guidelines that are based on systematic reviews and the EBM approach and stay abreast of ACOG’s and other professional societies’ guideline statements. Ultimately, guidelines beget performance measures. Organizations such as the National Quality Forum are working to define these standards of performance measurement and seek feedback from individual clinicians to ensure measures are meaningful and accurate. By 2017, 9% of all Medicare payments are scheduled to be performance based.21
Conclusion
During the course of reading medical literature, stay attuned to comparative effectiveness research and recognize studies with active comparators that examine clinical questions that could impact your day-to-day practice and that can be applied to your patient population. While there is no such thing as a perfect research study, and it is rare that one trial can address any one clinician’s specific patients precisely, increasingly we are seeing better systematic reviews and meta-analyses. It is these studies that provide the high quality data for you to couple with your clinical expertise and your patients’ values and preferences to truly deliver evidence-based medicine.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72.
- Sackett DL, et al. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed. Edinburgh, UK: Churchill Livingstone; 2000.
- Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ. 2002;324(7335):477–480.
- Bossuyt PM, Reitsma JB, Bruns DE, et al; Standards for Reporting of Diagnostic Accuracy Group. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1-W12.
- Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women’s health research: science, protoscience, and pseudoscience. Fertil Steril. 2010;93(6):1731–1734.
- Lagakos SW. The challenge of subgroup analyses—reporting without distorting. N Engl J Med. 2006;354(16):1667–1669.
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194.
- Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–387.
- Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD)2008. AHRQ Methods for Effective Health Care. 2013 Nov 18.
- Liberati A1, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Institute of Medicine of the National Academies. Finding What Works in Health Care: Standards for Systematic Reviews. iom.edu/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews.aspx. Published March 23, 2011. Accessed March 20, 2015.
- Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987;106(3):485–488.
- Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322(7300):1479–1480.
- Glasziou P, Guyatt GH, Dans AL, Dans LF, Straus S, Sackett DL. Applying the results of trials and systematic reviews to individual patients. ACP J Club. 1998;129(3):A15–A16.
- What is shared decision making? Informed Medical Decisions Foundation Web site. http://www.informed medicaldecisions.org/what-is-shared-decision-making. Published 2015. Accessed January 23, 2015.
- Straus SE, Sackett DL. Applying evidence to the individual patient. Ann Oncol. 1999;10(1):29–32.
- Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358–366.
- D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187.
- Jelovsek JE, Chagin K, Brubaker L, et al; Pelvic Floor Disorders Network. A model for predicting the risk of de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(2 Pt 1):279–287.
- National Quality Forum. What we do. National Quality Forum Web sight. http://www.qualityforum.org/what_we_do.aspx. Published 2015. Accessed January 29, 2015.
Although evidence-based medicine, or EBM, is not a new concept, the phrase is tossed about frequently in today’s culture of quality improvement initiatives and metrics. What does EBM really mean, however, and how do we ensure we are practicing it?
At its heart, EBM integrates 3 components:
- the individual clinician’s expertise
- the patient’s values and preferences
- the best external evidence to guide treatment decisions.
Because each clinician’s skillset and each patient’s issues and preferences may be quite varied, in this article we target the third piece—determining the best external evidence.
Our focus on EBM is not meant to negate the importance of the clinician’s expertise, which has been gained through years of practice. Indeed, without expertise, “practice risks becoming tyrannized by evidence.”1 However, without current best evidence, “practice risks becoming rapidly out of date, to the detriment of patients.”1 With the integration of evidence, expertise, and patient choice, EBM is not “cookbook” medicine, and it is not conducted only from armchairs and ivory towers. Rather, EBM is, or should be, at the frontline of clinical care.
EBM begins with a specific clinical question, such as “What is the best treatment option for my patient?” The answer can be honed with the “PICO” approach, which considers Population, Intervention, Comparators, and Outcomes of interest. Specifically, in a particular patient population (similar to your own patient), how does an intervention impact key outcomes?
For directly comparing intervention options, such as surgery A versus surgery B, a randomized controlled trial (RCT) is one of the best methods to address clinical questions (FIGURE).2 Systematic reviews are more generalizable than single studies since they compare a range of relevant interventions across populations and settings. Evaluations of diagnostic test accuracy3,4or analyses of risk factors or natural history are best addressed by other study designs, which also can provide important evidence, but will not be discussed in depth here.
In this article, we focus on the benefits of RCTs and systematic reviews, as well as when to exhibit caution, for instance when RCTs report “surrogate outcomes” or make analyses drawn from subgroups of the original population. In addition, we discuss the inability to adequately assess treatment harms (versus benefits) from available evidence as well as the practicalities of how to apply EBM to patients.
RCTs: The good, the bad, and the ugly
RCTs are prospective experiments with a predefined protocol in which patients are randomly allocated to groups where the only difference is the intervention (vs comparators). This design helps to minimize the effects of known and unknown confounders and selection bias.
Ideally, the group into which a study participant is allocated is concealed from the patient and from the caregiver, minimizing the risk that the randomization is broken and the treatment allocation is biased. (Frequently this is not possible, however, particularly for surgical interventions.) Similarly, ideally, the outcome assessors are blinded to the treatment whenever possible. This minimizes the risk of a patient’s outcome being consciously or unconsciously altered due to the outcome assessor’s beliefs about the effectiveness of the intervention.
The reported clinical or surrogate outcomes (which will be discussed in more depth on the next page) for an RCT may be objective or subjective. Preferably, outcomes are patient-centered—important from the patient’s perspective of benefits and harms. Examples of these types of outcomes include survival, function, symptoms, and health-related quality of life, as well as impact on work and family, convenience, and cost. Patients likely are less interested in estimated blood loss, surgical time, biochemistry results, and other clinical or surrogate outcomes.
There are disadvantages to RCTs. For instance, each study provides only a snapshot of the evidence on a given topic. One study rarely, if ever, provides a definitive conclusion. The study’s findings are subject to random error and to biases introduced by study design or analytic methods, and they will not be generalizable to all patients and settings. In addition, the study likely has evaluated only 1 or 2 specific interventions among a plethora of available options, and is unlikely to have analyzed all outcomes of interest.
It becomes your burden to assess whether a trial’s findings are applicable to an actual patient (known as “external validity”). Because an RCT must artificially constrain the underlying clinical questions into a testable research question, translation to the specific patient is often flawed. Perhaps the patient does not precisely fit the inclusion criteria of the trial, for instance, or the exact intervention tested is not fully reproducible. From a practicality perspective, an RCT is often immensely costly to execute, which may be reflected in relatively small numbers of patients and short-term duration of follow-up. These disadvantages limit the ability of RCTs to assess harms, rare events, and long-term outcomes.
Surrogate outcomes
Outcomes measured in a trial should be relevant, easy to interpret and diagnose, sensitive to treatment differences, and measurable within a reasonable period of time. However, these characteristics are not always achievable for important clinical outcomes in an RCT. Therefore, a surrogate outcome may take the place of the true clinical efficacy measurement.
For example, in studies of interventions for infertility in patients with polycystic ovary syndrome (PCOS), common surrogates to the “true” desired outcome of a healthy live birth may include ovulation, implantation, or pregnancy rates. These surrogate outcomes may correlate with live birth but clearly ignore other factors extrinsic and intrinsic to PCOS that affect the chance for a healthy term delivery; the possible increased risk for miscarriage in PCOS; and increased risks of other pregnancy complications, such as preeclampsia and gestational diabetes.
Similarly, many trials of oral contraceptives that aim to study the clinical endpoint of pulmonary embolism or venous thromboembolism, which are rare events, instead use the surrogates of results of coagulation tests or levels of sex hormone-binding globulin. Clearly, caution must be exercised when interpreting studies that use surrogate outcomes. As the clinician, you must recognize that a change in a biologic or physical measurement may not be clinically relevant. Some judgment is required about causal pathways: The less that is known about the causal pathway of a disease, the less confident one should be in any surrogate outcome.
Finally, clinicians also must recognize that a valid surrogate for one treatment may not be valid for another treatment or another population.5 For example, ovulation inhibition would be an appropriate surrogate endpoint for contraceptive efficacy for a method that reliably prevents ovulation; however, this would not be a good surrogate outcome to evaluate the progestin-only pill, which fails to inhibit ovulation completely and yet is highly effective in contraceptive trials.
Avoiding pitfalls with subgroup analyses
It is common, particularly in large RCTs, to evaluate treatment effects for a specific endpoint in a subgroup of patients included in the trial. The goal is to determine whether the findings of the larger study apply more or less to a specific patient (who may differ from the total population by some important characteristic, such as age, weight, parity, or menopausal or smoking status). The variability in study results when stratified by these patient factors is known as heterogeneity of treatment effect, which may be quantitative or qualitative.6
In the former, one treatment is always better than the other, although by varying degrees depending on the subgroup. (For example, a stronger effect could be seen in those aged 65 and younger than in those older than 65.) In the latter, the treatment fares better than the comparator in one subgroup but worse or no different for another subgroup. In either case, the appropriate statistical tool to identify heterogeneity of treatment effect is a test for interaction between the characteristic and the treatment effect, rather than claiming heterogeneity on the basis of separate tests of treatment effects within the different subpopulations.
One problem with dividing the original population into smaller subpopulations is that the number of participants decreases—thus there is less power, or less statistical strength, to identify a treatment effect. More accurately, there is a greater likelihood of a type II error (a false negative) when these small subpopulations have too few patients to demonstrate a clinical treatment effect that actually may exist.
False positives. Paradoxically, another problem with subgroup analyses is a greater chance for false positives due to the multiple statistical testing that is performed. The original study is rarely powered appropriately to do this (see “Error rates in subgroup analyses”). According to Wang and colleagues, “It is common practice to conduct a subgroup analysis for each of several (and often many) baseline characteristics, for each of several endpoints, or for both.”7 The more subgroup analyses performed, the more likely that differences found are due to chance only. Unfortunately, in unplanned post hoc analyses, the number of tests performed is often unreported; therefore, the error rates are unknown. There are statistical methods to try and correct for this “multiplicity” problem but, ideally, only a few key subgroup analyses are performed, and they are planned a priori in the original study design. In these cases, the study’s size can be adjusted accordingly. In most instances, findings from subgroup analyses, whether positive or negative, should be considered as “hypothesis generating” and interpreted with caution.
Error rates in subgroup analyses
With “k” independent subgroups and no difference in treatments, the probability of at least one “significant” subgroup (such as a false positive) is 1 – (1-α)k.
If α = 0.05 and there are k = 10 subgroups, then 1 – (0.95)10 = 0.40. That is, if 10 subgroup analyses are performed, there is a 40% likelihood that 1 will demonstrate a “significant” difference in treatment effect, even though no difference exists.
Systematic reviews: What, why, and how?
Systematic reviews aim to overcome the deficiencies of single studies in a comprehensive and unbiased manner. They critically evaluate, summarize, and, when possible, combine all available studies addressing a given topic. By comparing a range of relevant interventions across populations and settings, systematic reviews may be more generalizable than single studies. Meta-analysis, or quantitatively combining study results, increases sample size and usually provides more precise estimates of effect sizes than the single studies. Critical appraisal of the combined studies can highlight methodologic and other concerns about the body of evidence to assess the overall confidence in the included studies.
A systematic review, like a well-conducted RCT, has a protocol that lays out the scope of the review and defines a priori criteria and analytic plans—all with the goal of minimizing bias. It starts with a well-formulated research question, explicitly defining the PICO elements—population, interventions, comparators, outcomes—in addition to the setting and study designs of interest.8 Based on these eligibility criteria, several sources of evidence (such as electronic databases and reference lists) are searched to find all potentially eligible studies.
Typically, several thousand citations are found that must be matched against the eligibility criteria. Potentially eligible studies are then rescreened in full text to further scrutinize their eligibility. The goal is to be highly sensitive to avoid missing relevant studies—even at the time cost of screening many articles. The individual study designs (including the study eligibility criteria, interventions, outcomes, and analytic methods) and the results for all outcomes of interest are extracted from each study.
For most systematic reviews, researchers also will assess the quality, or risk of bias, of each study for each outcome.9,10 Study data are summarized across all included studies, with study results meta-analyzed and reasons for heterogeneity across studies explored. Several consensus statements detail the proper methodology to conduct and report a systematic review.11,12 Ultimately, the review’s conclusions are based on analyses of all available evidence. By contrast, narrative reviews typically start with a conclusion and then select evidence to support that conclusion, and are therefore more likely to be biased.13
As noted, systematic reviews often include meta-analysis, which may allow an exploration of some reasons for study heterogeneity. The meta-analysis is usually presented graphically in a forest plot, which displays point estimates for each study with their associated 95% confidence intervals and a description of each study.14 In a forest plot, one can see the estimate and precision of each study, assess the heterogeneity of results across studies, and compare individual studies to each other and to the overall summary estimate.
Systematic reviews should be read as critically as primary studies. Some important questions you should consider are:
- Did the review address the populations, interventions, comparators, outcomes, and settings relevant to your practice?
- Have studies been included in a nonbiased manner, and is the described body of evidence likely to be complete?
- Did the study authors evaluate and summarize the underlying risks of bias of the studies?
- Did the researchers avoid combining studies that are too different from each other to allow a coherent interpretation of the summary results?
- Did the researchers attempt to explain how and why studies differed from one another?
Of note, systematic reviews and meta-analyses are subject to the same biases as all retrospective studies. Also, the systematic reviewers’ own biases—due to factors such as funding source, researchers’ agendas, or specialties—may subtly affect systematic reviews just as biases may affect an individual study. Furthermore, the confidence you have in a systematic review’s conclusions may be limited by the quality and generalizability of the underlying studies.
Assessing harms
You make the ultimate management decisions for your patient (though, of course, with her participation). The likely benefit of a specific treatment—determined in an experimental trial and refined further in a systematic review and meta-analysis—must be balanced with the risk of harms. RCTs usually do not provide the highest quality evidence of harms due to their limited sample sizes and short follow-up duration. Rather, large observational studies, case series, and case reports commonly provide these important details. Increasingly, patient registries are being created to prospectively follow patients and gather uniform safety data. By providing a true denominator, more accurate estimates of adverse event incidence are possible. However, the disadvantages of all of these modalities are 1) there usually are no comparators (that is, “How does the adverse event incidence for surgery A compare to that for surgery B?”) and 2) data usually are gleaned from medical records and not directly from patients.
As a result, these studies typically lack information on subjective harms, such as impaired sexual function. The reporting of treatment harms suffers from inconsistent and imprecise terminology, making it hard to reliably gather all reports of similar adverse events. Adverse event reporting in clinical trials is often driven by regulatory definitions and requirements instead of patient-centered definitions. In fact, there has been little work to date that assesses which adverse events or complications may be most relevant or important from the patient perspective.
Taken together, it is clear that the medical literature tends to emphasize treatment benefits (with robust methodologies and data to detect these benefits) but does not reliably or adequately assess harms. For rare events, risk estimates always will be imprecise. Nonetheless, better systematic reviews and today’s larger comparative effectiveness reviews strive to gather harms data from the multiple available sources described above.
Applying the evidence and your expertise to your patient
Now that you have identified the best valid and important evidence to support or refute a clinical decision (TABLE15), and have coupled this with your own expert knowledge and judgment in shared decision making with your patient, you must communicate to her the personalized information about outcomes, probabilities, and scientific uncertainties of her available treatment options.15 Patients, in turn, should be allowed to communicate their values and the relative importance they place on benefits and harms.16 This conversation, of course, is built on the foundation of a sound physician–patient relationship and is a part of every informed consent process.
Is this evidence applicable to my patient? A decision guide.15
- Is my patient so different from those in the study that the trial results cannot be applied?
- Is the treatment feasible in my setting?
- What are my patient’s likely benefits and harms from the therapy?
- How will my patient’s values influence the final treatment decision?
Decision tools
Increasingly, decision-aid tools are being developed to support this process. These aids must express the helpful and harmful effects of a treatment, including alternative options, in statements that are valid and concise. Furthermore, they must be intelligible to both the clinician and patient and modifiable to the patient’s values and wishes.17 Two examples of counseling aids are the Gail model of breast cancer risk prediction18 and the Framingham Coronary Heart Disease Prediction Score.19 Web-based decision aids that can be accessed in real-time in busy clinical settings also are being developed for gynecology.20
Never stop re-evaluating
The final piece of EBM is to “close the loop”—meaning to evaluate the effectiveness of applying the evidence in clinical practice. To do this, watch for clinical practice guidelines that are based on systematic reviews and the EBM approach and stay abreast of ACOG’s and other professional societies’ guideline statements. Ultimately, guidelines beget performance measures. Organizations such as the National Quality Forum are working to define these standards of performance measurement and seek feedback from individual clinicians to ensure measures are meaningful and accurate. By 2017, 9% of all Medicare payments are scheduled to be performance based.21
Conclusion
During the course of reading medical literature, stay attuned to comparative effectiveness research and recognize studies with active comparators that examine clinical questions that could impact your day-to-day practice and that can be applied to your patient population. While there is no such thing as a perfect research study, and it is rare that one trial can address any one clinician’s specific patients precisely, increasingly we are seeing better systematic reviews and meta-analyses. It is these studies that provide the high quality data for you to couple with your clinical expertise and your patients’ values and preferences to truly deliver evidence-based medicine.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Although evidence-based medicine, or EBM, is not a new concept, the phrase is tossed about frequently in today’s culture of quality improvement initiatives and metrics. What does EBM really mean, however, and how do we ensure we are practicing it?
At its heart, EBM integrates 3 components:
- the individual clinician’s expertise
- the patient’s values and preferences
- the best external evidence to guide treatment decisions.
Because each clinician’s skillset and each patient’s issues and preferences may be quite varied, in this article we target the third piece—determining the best external evidence.
Our focus on EBM is not meant to negate the importance of the clinician’s expertise, which has been gained through years of practice. Indeed, without expertise, “practice risks becoming tyrannized by evidence.”1 However, without current best evidence, “practice risks becoming rapidly out of date, to the detriment of patients.”1 With the integration of evidence, expertise, and patient choice, EBM is not “cookbook” medicine, and it is not conducted only from armchairs and ivory towers. Rather, EBM is, or should be, at the frontline of clinical care.
EBM begins with a specific clinical question, such as “What is the best treatment option for my patient?” The answer can be honed with the “PICO” approach, which considers Population, Intervention, Comparators, and Outcomes of interest. Specifically, in a particular patient population (similar to your own patient), how does an intervention impact key outcomes?
For directly comparing intervention options, such as surgery A versus surgery B, a randomized controlled trial (RCT) is one of the best methods to address clinical questions (FIGURE).2 Systematic reviews are more generalizable than single studies since they compare a range of relevant interventions across populations and settings. Evaluations of diagnostic test accuracy3,4or analyses of risk factors or natural history are best addressed by other study designs, which also can provide important evidence, but will not be discussed in depth here.
In this article, we focus on the benefits of RCTs and systematic reviews, as well as when to exhibit caution, for instance when RCTs report “surrogate outcomes” or make analyses drawn from subgroups of the original population. In addition, we discuss the inability to adequately assess treatment harms (versus benefits) from available evidence as well as the practicalities of how to apply EBM to patients.
RCTs: The good, the bad, and the ugly
RCTs are prospective experiments with a predefined protocol in which patients are randomly allocated to groups where the only difference is the intervention (vs comparators). This design helps to minimize the effects of known and unknown confounders and selection bias.
Ideally, the group into which a study participant is allocated is concealed from the patient and from the caregiver, minimizing the risk that the randomization is broken and the treatment allocation is biased. (Frequently this is not possible, however, particularly for surgical interventions.) Similarly, ideally, the outcome assessors are blinded to the treatment whenever possible. This minimizes the risk of a patient’s outcome being consciously or unconsciously altered due to the outcome assessor’s beliefs about the effectiveness of the intervention.
The reported clinical or surrogate outcomes (which will be discussed in more depth on the next page) for an RCT may be objective or subjective. Preferably, outcomes are patient-centered—important from the patient’s perspective of benefits and harms. Examples of these types of outcomes include survival, function, symptoms, and health-related quality of life, as well as impact on work and family, convenience, and cost. Patients likely are less interested in estimated blood loss, surgical time, biochemistry results, and other clinical or surrogate outcomes.
There are disadvantages to RCTs. For instance, each study provides only a snapshot of the evidence on a given topic. One study rarely, if ever, provides a definitive conclusion. The study’s findings are subject to random error and to biases introduced by study design or analytic methods, and they will not be generalizable to all patients and settings. In addition, the study likely has evaluated only 1 or 2 specific interventions among a plethora of available options, and is unlikely to have analyzed all outcomes of interest.
It becomes your burden to assess whether a trial’s findings are applicable to an actual patient (known as “external validity”). Because an RCT must artificially constrain the underlying clinical questions into a testable research question, translation to the specific patient is often flawed. Perhaps the patient does not precisely fit the inclusion criteria of the trial, for instance, or the exact intervention tested is not fully reproducible. From a practicality perspective, an RCT is often immensely costly to execute, which may be reflected in relatively small numbers of patients and short-term duration of follow-up. These disadvantages limit the ability of RCTs to assess harms, rare events, and long-term outcomes.
Surrogate outcomes
Outcomes measured in a trial should be relevant, easy to interpret and diagnose, sensitive to treatment differences, and measurable within a reasonable period of time. However, these characteristics are not always achievable for important clinical outcomes in an RCT. Therefore, a surrogate outcome may take the place of the true clinical efficacy measurement.
For example, in studies of interventions for infertility in patients with polycystic ovary syndrome (PCOS), common surrogates to the “true” desired outcome of a healthy live birth may include ovulation, implantation, or pregnancy rates. These surrogate outcomes may correlate with live birth but clearly ignore other factors extrinsic and intrinsic to PCOS that affect the chance for a healthy term delivery; the possible increased risk for miscarriage in PCOS; and increased risks of other pregnancy complications, such as preeclampsia and gestational diabetes.
Similarly, many trials of oral contraceptives that aim to study the clinical endpoint of pulmonary embolism or venous thromboembolism, which are rare events, instead use the surrogates of results of coagulation tests or levels of sex hormone-binding globulin. Clearly, caution must be exercised when interpreting studies that use surrogate outcomes. As the clinician, you must recognize that a change in a biologic or physical measurement may not be clinically relevant. Some judgment is required about causal pathways: The less that is known about the causal pathway of a disease, the less confident one should be in any surrogate outcome.
Finally, clinicians also must recognize that a valid surrogate for one treatment may not be valid for another treatment or another population.5 For example, ovulation inhibition would be an appropriate surrogate endpoint for contraceptive efficacy for a method that reliably prevents ovulation; however, this would not be a good surrogate outcome to evaluate the progestin-only pill, which fails to inhibit ovulation completely and yet is highly effective in contraceptive trials.
Avoiding pitfalls with subgroup analyses
It is common, particularly in large RCTs, to evaluate treatment effects for a specific endpoint in a subgroup of patients included in the trial. The goal is to determine whether the findings of the larger study apply more or less to a specific patient (who may differ from the total population by some important characteristic, such as age, weight, parity, or menopausal or smoking status). The variability in study results when stratified by these patient factors is known as heterogeneity of treatment effect, which may be quantitative or qualitative.6
In the former, one treatment is always better than the other, although by varying degrees depending on the subgroup. (For example, a stronger effect could be seen in those aged 65 and younger than in those older than 65.) In the latter, the treatment fares better than the comparator in one subgroup but worse or no different for another subgroup. In either case, the appropriate statistical tool to identify heterogeneity of treatment effect is a test for interaction between the characteristic and the treatment effect, rather than claiming heterogeneity on the basis of separate tests of treatment effects within the different subpopulations.
One problem with dividing the original population into smaller subpopulations is that the number of participants decreases—thus there is less power, or less statistical strength, to identify a treatment effect. More accurately, there is a greater likelihood of a type II error (a false negative) when these small subpopulations have too few patients to demonstrate a clinical treatment effect that actually may exist.
False positives. Paradoxically, another problem with subgroup analyses is a greater chance for false positives due to the multiple statistical testing that is performed. The original study is rarely powered appropriately to do this (see “Error rates in subgroup analyses”). According to Wang and colleagues, “It is common practice to conduct a subgroup analysis for each of several (and often many) baseline characteristics, for each of several endpoints, or for both.”7 The more subgroup analyses performed, the more likely that differences found are due to chance only. Unfortunately, in unplanned post hoc analyses, the number of tests performed is often unreported; therefore, the error rates are unknown. There are statistical methods to try and correct for this “multiplicity” problem but, ideally, only a few key subgroup analyses are performed, and they are planned a priori in the original study design. In these cases, the study’s size can be adjusted accordingly. In most instances, findings from subgroup analyses, whether positive or negative, should be considered as “hypothesis generating” and interpreted with caution.
Error rates in subgroup analyses
With “k” independent subgroups and no difference in treatments, the probability of at least one “significant” subgroup (such as a false positive) is 1 – (1-α)k.
If α = 0.05 and there are k = 10 subgroups, then 1 – (0.95)10 = 0.40. That is, if 10 subgroup analyses are performed, there is a 40% likelihood that 1 will demonstrate a “significant” difference in treatment effect, even though no difference exists.
Systematic reviews: What, why, and how?
Systematic reviews aim to overcome the deficiencies of single studies in a comprehensive and unbiased manner. They critically evaluate, summarize, and, when possible, combine all available studies addressing a given topic. By comparing a range of relevant interventions across populations and settings, systematic reviews may be more generalizable than single studies. Meta-analysis, or quantitatively combining study results, increases sample size and usually provides more precise estimates of effect sizes than the single studies. Critical appraisal of the combined studies can highlight methodologic and other concerns about the body of evidence to assess the overall confidence in the included studies.
A systematic review, like a well-conducted RCT, has a protocol that lays out the scope of the review and defines a priori criteria and analytic plans—all with the goal of minimizing bias. It starts with a well-formulated research question, explicitly defining the PICO elements—population, interventions, comparators, outcomes—in addition to the setting and study designs of interest.8 Based on these eligibility criteria, several sources of evidence (such as electronic databases and reference lists) are searched to find all potentially eligible studies.
Typically, several thousand citations are found that must be matched against the eligibility criteria. Potentially eligible studies are then rescreened in full text to further scrutinize their eligibility. The goal is to be highly sensitive to avoid missing relevant studies—even at the time cost of screening many articles. The individual study designs (including the study eligibility criteria, interventions, outcomes, and analytic methods) and the results for all outcomes of interest are extracted from each study.
For most systematic reviews, researchers also will assess the quality, or risk of bias, of each study for each outcome.9,10 Study data are summarized across all included studies, with study results meta-analyzed and reasons for heterogeneity across studies explored. Several consensus statements detail the proper methodology to conduct and report a systematic review.11,12 Ultimately, the review’s conclusions are based on analyses of all available evidence. By contrast, narrative reviews typically start with a conclusion and then select evidence to support that conclusion, and are therefore more likely to be biased.13
As noted, systematic reviews often include meta-analysis, which may allow an exploration of some reasons for study heterogeneity. The meta-analysis is usually presented graphically in a forest plot, which displays point estimates for each study with their associated 95% confidence intervals and a description of each study.14 In a forest plot, one can see the estimate and precision of each study, assess the heterogeneity of results across studies, and compare individual studies to each other and to the overall summary estimate.
Systematic reviews should be read as critically as primary studies. Some important questions you should consider are:
- Did the review address the populations, interventions, comparators, outcomes, and settings relevant to your practice?
- Have studies been included in a nonbiased manner, and is the described body of evidence likely to be complete?
- Did the study authors evaluate and summarize the underlying risks of bias of the studies?
- Did the researchers avoid combining studies that are too different from each other to allow a coherent interpretation of the summary results?
- Did the researchers attempt to explain how and why studies differed from one another?
Of note, systematic reviews and meta-analyses are subject to the same biases as all retrospective studies. Also, the systematic reviewers’ own biases—due to factors such as funding source, researchers’ agendas, or specialties—may subtly affect systematic reviews just as biases may affect an individual study. Furthermore, the confidence you have in a systematic review’s conclusions may be limited by the quality and generalizability of the underlying studies.
Assessing harms
You make the ultimate management decisions for your patient (though, of course, with her participation). The likely benefit of a specific treatment—determined in an experimental trial and refined further in a systematic review and meta-analysis—must be balanced with the risk of harms. RCTs usually do not provide the highest quality evidence of harms due to their limited sample sizes and short follow-up duration. Rather, large observational studies, case series, and case reports commonly provide these important details. Increasingly, patient registries are being created to prospectively follow patients and gather uniform safety data. By providing a true denominator, more accurate estimates of adverse event incidence are possible. However, the disadvantages of all of these modalities are 1) there usually are no comparators (that is, “How does the adverse event incidence for surgery A compare to that for surgery B?”) and 2) data usually are gleaned from medical records and not directly from patients.
As a result, these studies typically lack information on subjective harms, such as impaired sexual function. The reporting of treatment harms suffers from inconsistent and imprecise terminology, making it hard to reliably gather all reports of similar adverse events. Adverse event reporting in clinical trials is often driven by regulatory definitions and requirements instead of patient-centered definitions. In fact, there has been little work to date that assesses which adverse events or complications may be most relevant or important from the patient perspective.
Taken together, it is clear that the medical literature tends to emphasize treatment benefits (with robust methodologies and data to detect these benefits) but does not reliably or adequately assess harms. For rare events, risk estimates always will be imprecise. Nonetheless, better systematic reviews and today’s larger comparative effectiveness reviews strive to gather harms data from the multiple available sources described above.
Applying the evidence and your expertise to your patient
Now that you have identified the best valid and important evidence to support or refute a clinical decision (TABLE15), and have coupled this with your own expert knowledge and judgment in shared decision making with your patient, you must communicate to her the personalized information about outcomes, probabilities, and scientific uncertainties of her available treatment options.15 Patients, in turn, should be allowed to communicate their values and the relative importance they place on benefits and harms.16 This conversation, of course, is built on the foundation of a sound physician–patient relationship and is a part of every informed consent process.
Is this evidence applicable to my patient? A decision guide.15
- Is my patient so different from those in the study that the trial results cannot be applied?
- Is the treatment feasible in my setting?
- What are my patient’s likely benefits and harms from the therapy?
- How will my patient’s values influence the final treatment decision?
Decision tools
Increasingly, decision-aid tools are being developed to support this process. These aids must express the helpful and harmful effects of a treatment, including alternative options, in statements that are valid and concise. Furthermore, they must be intelligible to both the clinician and patient and modifiable to the patient’s values and wishes.17 Two examples of counseling aids are the Gail model of breast cancer risk prediction18 and the Framingham Coronary Heart Disease Prediction Score.19 Web-based decision aids that can be accessed in real-time in busy clinical settings also are being developed for gynecology.20
Never stop re-evaluating
The final piece of EBM is to “close the loop”—meaning to evaluate the effectiveness of applying the evidence in clinical practice. To do this, watch for clinical practice guidelines that are based on systematic reviews and the EBM approach and stay abreast of ACOG’s and other professional societies’ guideline statements. Ultimately, guidelines beget performance measures. Organizations such as the National Quality Forum are working to define these standards of performance measurement and seek feedback from individual clinicians to ensure measures are meaningful and accurate. By 2017, 9% of all Medicare payments are scheduled to be performance based.21
Conclusion
During the course of reading medical literature, stay attuned to comparative effectiveness research and recognize studies with active comparators that examine clinical questions that could impact your day-to-day practice and that can be applied to your patient population. While there is no such thing as a perfect research study, and it is rare that one trial can address any one clinician’s specific patients precisely, increasingly we are seeing better systematic reviews and meta-analyses. It is these studies that provide the high quality data for you to couple with your clinical expertise and your patients’ values and preferences to truly deliver evidence-based medicine.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72.
- Sackett DL, et al. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed. Edinburgh, UK: Churchill Livingstone; 2000.
- Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ. 2002;324(7335):477–480.
- Bossuyt PM, Reitsma JB, Bruns DE, et al; Standards for Reporting of Diagnostic Accuracy Group. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1-W12.
- Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women’s health research: science, protoscience, and pseudoscience. Fertil Steril. 2010;93(6):1731–1734.
- Lagakos SW. The challenge of subgroup analyses—reporting without distorting. N Engl J Med. 2006;354(16):1667–1669.
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194.
- Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–387.
- Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD)2008. AHRQ Methods for Effective Health Care. 2013 Nov 18.
- Liberati A1, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Institute of Medicine of the National Academies. Finding What Works in Health Care: Standards for Systematic Reviews. iom.edu/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews.aspx. Published March 23, 2011. Accessed March 20, 2015.
- Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987;106(3):485–488.
- Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322(7300):1479–1480.
- Glasziou P, Guyatt GH, Dans AL, Dans LF, Straus S, Sackett DL. Applying the results of trials and systematic reviews to individual patients. ACP J Club. 1998;129(3):A15–A16.
- What is shared decision making? Informed Medical Decisions Foundation Web site. http://www.informed medicaldecisions.org/what-is-shared-decision-making. Published 2015. Accessed January 23, 2015.
- Straus SE, Sackett DL. Applying evidence to the individual patient. Ann Oncol. 1999;10(1):29–32.
- Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358–366.
- D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187.
- Jelovsek JE, Chagin K, Brubaker L, et al; Pelvic Floor Disorders Network. A model for predicting the risk of de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(2 Pt 1):279–287.
- National Quality Forum. What we do. National Quality Forum Web sight. http://www.qualityforum.org/what_we_do.aspx. Published 2015. Accessed January 29, 2015.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72.
- Sackett DL, et al. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed. Edinburgh, UK: Churchill Livingstone; 2000.
- Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ. 2002;324(7335):477–480.
- Bossuyt PM, Reitsma JB, Bruns DE, et al; Standards for Reporting of Diagnostic Accuracy Group. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1-W12.
- Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women’s health research: science, protoscience, and pseudoscience. Fertil Steril. 2010;93(6):1731–1734.
- Lagakos SW. The challenge of subgroup analyses—reporting without distorting. N Engl J Med. 2006;354(16):1667–1669.
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194.
- Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–387.
- Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD)2008. AHRQ Methods for Effective Health Care. 2013 Nov 18.
- Liberati A1, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Institute of Medicine of the National Academies. Finding What Works in Health Care: Standards for Systematic Reviews. iom.edu/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews.aspx. Published March 23, 2011. Accessed March 20, 2015.
- Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987;106(3):485–488.
- Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322(7300):1479–1480.
- Glasziou P, Guyatt GH, Dans AL, Dans LF, Straus S, Sackett DL. Applying the results of trials and systematic reviews to individual patients. ACP J Club. 1998;129(3):A15–A16.
- What is shared decision making? Informed Medical Decisions Foundation Web site. http://www.informed medicaldecisions.org/what-is-shared-decision-making. Published 2015. Accessed January 23, 2015.
- Straus SE, Sackett DL. Applying evidence to the individual patient. Ann Oncol. 1999;10(1):29–32.
- Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358–366.
- D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187.
- Jelovsek JE, Chagin K, Brubaker L, et al; Pelvic Floor Disorders Network. A model for predicting the risk of de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(2 Pt 1):279–287.
- National Quality Forum. What we do. National Quality Forum Web sight. http://www.qualityforum.org/what_we_do.aspx. Published 2015. Accessed January 29, 2015.
In thIS article
- RCTs: The good, the bad, and the ugly
- Systematic reviews: What, why, and how?
- Assessing harms
- Applying the evidence and your expertise to your patient
Early Recognition: The Rate-Limiting Step to Quality Care for Severe Sepsis Patients in the Emergency Department
From the Department of Medicine, University of Pennsylvania, and the Department of Emergency Medicine, Thomas Jefferson University Hospital, Philadelphia, PA.
Abstract
- Objective: To detail strategies to improve sepsis recognition and the quality of care provided to the septic patient.
- Methods: Review of the literature.
- Results: Severe sepsis affects nearly 3 million individuals each year in the United States, and cost estimates for these hospitalizations exceed $24 billion. Effective management is predicated on timely recognition. In this review, we detail strategies to improve early identification of potentially septic patients as well as the quality of care provided to the septic patient in the emergency department (ED). The strategies discussed are based upon an understanding of the signs and symptoms of sepsis and the clinical risk factors associated with sepsis, which can be used to design novel strategies to screen patients for sepsis and risk stratify patients at risk for clinical deterioration.
- Conclusion: ED structures and processes can be used to increase adherence with sepsis management guidelines to improve patient outcomes.
Severe sepsis affects nearly 3 million individuals each year in the United States and cost estimates for these hospitalizations exceed $24 billion [1–3]. Sepsis is a life-threatening condition characterized by a suspected or identified infection accompanied by a vigorous host inflammatory response. In severe sepsis, end-organ dysfunction manifests in myriad forms, including altered mental status, acute kidney injury, liver dysfunction, pulmonary dysfunction, and hemodynamic compromise [4,5]. This protean presentation of a deadly condition makes identification and risk stratification both challenging and essential to improving patient outcomes. The majority of patients with severe sepsis will receive their initial care within an emergency department (ED) [6,7]. It is essential that emergency medicine providers have the means to appropriately identify patients presenting with severe sepsis in a timely manner—thus facilitating life-saving measures such as early intravenous fluid resuscitation and administration of timely and appropriate antimicrobials.
In this review, we detail strategies to improve sepsis recognition and the quality of care provided to the septic patient in the ED. The strategies discussed are based upon an understanding of the signs and symptoms of sepsis and the clinical risk factors associated with sepsis, which can be used to design novel strategies to screen patients for sepsis and risk stratify patients for clinical deterioration. Then, we review suggested ED structures and processes to increase adherence with sepsis-based guidelines to improve patient outcomes. Successful implementation is predicated on hospital administrative support towards the efforts given the time and resources required and strong and committed leadership across the health care system.
Epidemiology of Severe Sepsis
Estimates of annual cases of severe sepsis vary, ranging from 1 million to 3 million cases in the United States [1–3]. In-hospital mortality for this condition ranges from 14% to 30% [5]. The incidence of severe sepsis in the United States has been increasing at a rate of 13% annually, with an estimated cost of greater than $24 billion per year [1,2]. In 2 large cohorts of hospitalized patients, it was found that sepsis contributed to 1 in every 2 to 3 deaths following inpatient admission [8]. Coincident with these increased estimates, advances in the early identification and treatment of sepsis have led to decreasing mortality rates over the past decade [1,9].
Of importance to the ED clinician, an episode of sepsis has long-term effects on cognitive and physical function, quality-of-life, and survival [10,11]. Post-discharge, approximately one-quarter of sepsis survivors will be readmitted within 30 days [12–14]. In as many as half of these instances, another life-threatening infection is the cause for readmission, making the past medical history, including a detailed accounting of recent episodes of sepsis, an important part of the initial ED evaluation [12]. Furthermore, severe sepsis survivors spend a large proportion of their time following discharge within a health care facility, and will frequently present to the ED with an acute condition from such an environment. Important factors for predicting readmission after a sepsis hospitalization include patient age, severity of illness, hospital length of stay, and the need for intensive care during the initial hospitalization [12–14].
Principles of Effective Sepsis Management
The principles of effective sepsis management begin with early identification in the pre-hospital setting, at triage, or when a patient begins to decompensate in the hospital. After the point of initial recognition, core principles include risk stratification, timely and appropriate antimicrobial administration, initial intravenous fluid boluses and ongoing resuscitation guided by physical examination and objective resuscitation end-points [4,5]. These practices have been operationalized in the care bundles of the Surviving Sepsis Campaign Guidelines [4]. Within 3 hours, the resuscitation bundle includes measuring serum lactate to risk stratify patients, obtaining blood cultures, administering broad-spectrum antibiotics, and administering 30 mL/kg crystalloid in patients with hypotension or hyperlactatemia [4]. The 6-hour bundle expands upon these initial measures and includes additional management recommendations based on resuscitation end-points.
As effective management is predicated on timely recognition, an understanding of the impact of delayed recognition is essential to provide optimal care for the severe sepsis patient in the ED. Decades of research has revealed that certain markers predict adverse outcomes, including transition to septic shock and death, as do delayed processes of care. Importantly, while early quantitative resuscitation was demonstrated to improve outcomes in a meta-analysis, there was no demonstrable benefit when resuscitation was initiated late (> 24 hours) in the course in the ICU (odds ratio of death, 1.16 [95% confidence interval, 0.60–2.22]) [15].
Strategies To Improve Recognition
Pre-Hospital Environment
From EMS to ED Triage
Borrowing the principle “time equals tissue” from a variety of time sensitive conditions (eg, myocardial infarction management [“time equals muscle”] and stroke care [“time equals brain”]), clinicians and researchers have realized that expedited recognition of severe sepsis patients begins at the time of initial contact with the health care system. For severe sepsis patients, clinicians need to think “time equals organ function.” Given the frequency with which sepsis patients arrive to the ED via EMS, effective communication between EMS and ED providers could be leveraged to prepare the ED team to provide timely care for the sepsis patient via a “sepsis alert.” While confirmation of its applicability to sepsis care is required in the absence of a regionalized network of sepsis centers, the rationale is based on the experience of the effectiveness of trauma and stroke alert systems [20–22]. For patients not recognized as potentially being infected by EMS providers during transport, repeat vital signs during ED triage can be screened to identify patients exhibiting signs of the systemic inflammatory response syndrome (SIRS) [4,23]. The same principles of effective communication apply for patients being sent from medical clinics to the ED for evaluation and treatment of potential severe sepsis. For patients arriving independent of EMS, focused triage and initial vital signs are the starting point for identifying severe sepsis at the most proximal phase of entry into the health care system.
Vital Signs and SIRS Criteria in the ED
The Afferent Arm: Multimodal Screening Strategies
While institutional practice improvement initiatives to facilitate sepsis recognition and care should incorporate educational strategies, led by champions with expertise in sepsis, the complex presentation of sepsis requires multimodal approaches [29]. These multimodal approaches, beginning at the time of ED triage, should be designed to harness information technology to screen patients to improve severe sepsis recognition (the afferent arm) and to utilize structures and processes of care efficiently and effectively (the efferent arm) to guide severe sepsis management according to sepsis-care bundles espoused by guidelines (Figure) [4].
Operational processes to screen for sepsis in the ED will need to account for ED organizational flow (eg, average time from registration to triage, average time from triage to being seen by a physician, average length of stay in the ED, number of hospital beds) and hand-off practices (eg, care transition from ED team to floor or ICU team, or within ED at shift change). For ED organizations with shorter ED lengths of stay (eg, < 2 hours), screening practices at ED triage will serve as the focal point to identify cases of sepsis. Boarding, defined as caring for a patient in the ED pending transfer, is common, increasing as a result of ED closures [30,31], and associated with prolonged hospital length of stay and increased in-hospital mortality when ICU transfer is delayed [32]. Sepsis patients in particular appear to be a vulnerable group of patients. While many explanations exist to account for the relationship between delayed transfer and adverse outcomes, timely recognition and management of the septic patient could be compromised with prolonged boarding. To combat this potential effect, continual assessment during the entire ED stay may unmask an initially unclear presentation of sepsis.
One strategy to identify sepsis in ED organizations with prolonged ED lengths of stay is through the use of a track-and-trigger system, or early warning system. Traditionally, track-and-trigger systems were implemented on the hospital wards, as means to identify physiological deterioration in a timely manner to prevent clinical deterioration [33]. More recently, early warning systems have been used to identify patients with sepsis on the hospital wards and within EDs, as these systems rely on physiological parameters such as SIRS that are cardinal features of sepsis [34]. However, given the potential for alert fatigue, designing a system that operates with high accuracy is imperative.
Efforts are underway to redefine sepsis, using a simplified approach and readily available physiological variables, with the main goal of targeting those most at-risk of an adverse outcome during the hospitalization. Simultaneously, an understanding of the overt and more occult manifestations are essential to incorporate into the clinical decision-making and pattern recognition required to identify sepsis in a timely and accurate manner. In Table 2, the signs and symptoms that may serve as flags for severe sepsis are presented.
Mature early warning systems, designed to leverage the electronic medical record (EMR) by capturing vital signs, laboratory measures, (eg, elevated serum creatinine compared to a recent hospitalization) and symptoms (eg, altered mental status), are well-positioned to herald clinical deterioration (eg, cardiac arrest) with improved accuracy [35] and to be applied to sepsis specifically [34]. While sophisticated analytical strategies, such as machine learning, are being used to improve the test characteristics of these early warning systems, iterative, prospective chart review is an essential and complementary performance improvement step to refine the process. Further, chart review affords the opportunity to ensure compliance with sepsis care bundles.
Knowledge of the risk factors associated with development of sepsis is critical for the front-line emergency physician and nurse. Additionally, as many of these risk factors are associated with adverse outcomes, including unplanned ICU transfer and in-hospital mortality, which occur in as many as one out of 8 patients admitted directly to the ward, they have utility for early risk-stratification and triaging purposes in the ED. Advanced age and pre-existing comorbid conditions, particularly an oncologic diagnosis and/or chronic organ dysfunction, are major risk factors for sepsis and worse outcomes result in those who develop sepsis [2]. Further, illness severity, including an elevated serum lactate level, is associated with adverse outcomes. These factors can be incorporated into triage decisions and/or close monitoring for patients admitted to the general ward [36]. Conversely, because patients admitted to the ICU setting and subsequently stepped down through their hospitalization may experience better outcomes compared to patients admitted to the general ward who then require step-up to an ICU setting (37,38), attention to triage practices is critical.
These complementary strategies, which serve as the afferent arm of the system, summon health care providers to the bedside of a vulnerable patient. However, clinical effectiveness in the management of severe sepsis requires a robust, sophisticated, and mature efferent arm capable of delivering expert care to the now recognized septic patient.
Principles of Effective Management Post-Recognition
Risk Stratification
An elevated serum lactate level was initially described in pathological states in the mid 19th century by Johann Joseph Scherer [39] and has long been associated with increased mortality in hospitalized patients [40]. Lactate is a useful biomarker for risk stratification in a variety of patients arriving to the ED, particularly those who have been identified at high risk for sepsis. Jansen and colleagues examined the measurement of pre-hospital serum lactate at the time of paramedic on-scene assessment in a group of acutely ill patients [41]. Patients with point-of-care lactate levels of 3.5 mmol/L or greater were found to have an in-hospital mortality of 41% versus 12% for those with lactate levels less than 3.5 mmol/L. Within the population with an elevated lactate, patients with a systolic blood pressure greater than 100 mgHg experienced a mortality of nearly 30%, while it was greater than 50% in hypotensive patients with an elevated lactate, highlighting the value of both hemodynamic and serum lactate measures. Upon arrival to the ED, lactate measurements have a strong correlation with mortality. In one retrospective cohort, lactate level was linearly associated with mortality in a broad array of patients older than age 65 years [42]. An initial serum lactate level in the ED in the intermediate (2.0 – 3.9 mmol/L) or high range (≥ 4 mmol/L) has been associated with increased odds of death 2 to 5 times higher independent of organ dysfunction in severe sepsis specifically [43].
As the association between serum lactate levels and death is independent of organ dysfunction, serum lactate is a simple and reliable tool to both enhance detection and risk-stratify patients presenting to the ED with severe sepsis. Given the frequency with which hyperlactatemia is present in patients with suspected infection [43], operationalizing serum lactate measures with the initial phlebotomy draw is an important step to risk-stratify patients. This step can be coupled later with intravenous fluid resuscitation for those with marked elevations (≥ 4 mmol/L), in accord with guideline recommendations [4]. Screening of initial lactate values can be further expedited by utilizing fingerstick point-of-care lactate devices [44]. Last, while serial lactate measures can be incorporated into triage decisions, there is no clear threshold that warrants ICU admission. Rather, persistent elevations in serum lactate can be used to identify patients who require close observation regardless of their admission location.
Several scoring systems have been developed to augment sepsis risk stratification within the ED. The most prominent of these are the Predisposition Insult Response and Organ failure (PIRO), Sequential Organ Failure Assessment (SOFA), and Mortality in the Emergency Department Sepsis (MEDS) scores, and the National early warning score (NEWS) [45-48]. The MEDS score incorporates host factors including age and co-morbid illness, as well as physiologic and laboratory tests which can be obtained rapidly in an ED setting. Multiple prospective and retrospective examinations of the MEDS scoring systems have demonstrated that it performs optimally in ED patients with sepsis but not those with severe sepsis, in terms of predicting 30-day mortality [46,47]. The PIRO score more extensively incorporates predisposing co-morbidities, physiologic and laboratory parameters, and has been modified to consider presumed source of infection, leading to a stronger predictive ability for mortality in more severely ill patients. In patients presenting to the ED with severe sepsis and septic shock, a prospective observational study found the PIRO to be the best predictor of mortality, compared to SOFA and MEDS scores [45]. In a recent study by Corfield et al, sepsis patients with a higher NEWS, according to initial ED vital signs (temperature, pulse, respiratory rate, systolic blood pressure, oxyhemoglobin saturation) and consciousness level, were significantly more likely to be admitted to an ICU within 48 hours or to experience in-hospital mortality [48].
Timely and Appropriate Antibiotics
In a landmark study published by Kumar and colleagues in 2006, the relationship between timing of antibiotics and mortality was established [49]. In 2731 adult septic shock patients, mortality increased 7.6% for every hour delay in effective antimicrobial administration. A striking finding, given that the study population was limited to patients cared for in the ICU, was the fact that only 50% of patients received appropriate antibiotics within 6 hours of onset of shock and nearly one-quarter of patients did not receive antibiotics until the 15th hour. As a direct result, in-hospital mortality was observed to be 58% in this study.
Over the ensuing decade, a series of studies have demonstrated a narrowing of the quality gap in this regard, and the result has coincided with a significant improvement in survival. In 2010, Gaieski and colleagues demonstrated a significant improvement in the prompt administration of antibiotic delivery in patients presenting to an ED with severe sepsis, with the median time from shock onset (sustained hypotension or lactate ≥ 4 mmol/L) to antibiotics down to 42 minutes [50]. Importantly, consistent with the Kumar study, time to appropriate antibiotics, rather than simply initial antibiotics, remained associated with in-hospital mortality independent of initiating early goal-directed therapy. In 2011, Puskarich and colleagues revealed that time to antibiotics continued to improve and, as a result, the investigators did not identify a relationship between time from triage to antibiotics and in-hospital mortality [51]. However, when antibiotics were delayed until after shock recognition, consistent with the study by Kumar and colleagues, survival decreased. Until recently, this important observation was challenging to operationalize clinically as little was known about how to facilitate risk-stratification of those at risk to develop shock. However, Capp and colleagues recently found that deterioration to septic shock 48 hours after ED presentation occurs in approximately one out of eight patients and identified gender (female), transient hypotension, and/or hyperlactatemia upon presentation as risk factors associated with such a deterioration [52].
As an essential element of sepsis care bundles, a focus on timely use of antibiotics in patients with suspected infection, has the potential to increase the use of antibiotics in the ED in patients determined subsequently to not be infected. To combat this acknowledged downstream effect, reconsideration of the utility of empiric antibiotics 48 to 72 hours after admission is required. This step can be accomplished through the use of a sepsis care pathway and/or a formal antibiotic stewardship program.
Quantitative Resuscitation
Rivers and colleagues, in a landmark 2001 trial, examined the effectiveness of a protocolized resuscitation strategy in the most proximal phase of severe sepsis and septic shock [53]. A distinguishing characteristic between the usual care arm and the intervention in this ED-based study, in addition to whether mixed central venous oxygen saturation was measured as a resuscitation end-point, was the inclusion of an ED provider at the bedside to attend to clinical management. The intervention, aimed at achieving physiologic targets, resulted in significantly more fluid resuscitation (3.5 L vs. 5.0 L within the first 6 hours) and a significant decrease in in-hospital mortality compared to the usual care arm (46.5 vs. 30.5%). The study revolutionized the culture and practice of sepsis care, in part by shining a light on the importance of timely resuscitation at the most proximal point of contact between the patient and the healthcare system. It also highlighted the importance of integrating serum lactate measurement into the early screening and risk stratification processes for sepsis care delivery.
The 2014 randomized trial of Protocol-Based Care for Early Septic Shock (ProCESS) revisited this concept, comparing the Rivers 2001 protocol to both a current guideline-based non-invasive algorithmic protocol and what had become usual ED care in the interim [54]. The ProCESS trial, which operationalized a team of bedside providers to direct care for each of the 3 distinct arms, found no significant difference between the arms in terms of 90-day and 1-year mortality, but mortality was approximately 10% less in all arms compared with the intervention arm of the Rivers trial. Further, subjects in each of the 3 arms received in excess of 2 L intravenous fluid resuscitation pre-randomization and 4.4–5.5 L when resuscitation spanned from pre-randomization to 6 hours post-randomization. The conclusion drawn is that the commonalities between the arms—early fluid resuscitation, early antibiotics, and the option to use physiologic measures as markers of the adequacy of treatment, all guided by bedside ED providers—are the most important factors for surviving sepsis. And the result is that practitioners have refined these tools over a decade, leading to steady improvements in survival.
Consistent with the ProCESS trial, a recent Australia and New Zealand trial confirmed no significant difference in 90-day mortality between protocolized EGDT and current usual care for septic shock within an ED [55]. Consistent with ProCESS and ProMISe [56], subjects enrolled in ARISE received in excess of 2.5 L in resuscitation pre-randomization, which when paired with fluid resuscitation in the 0-6 hour post-randomization period (1.96 L in the EGDT arm and 1.71 in the usual-care arm) resulted in resuscitation in the 4.5 to 5L range during the initial resuscitation. The ARISE trial was unique in that appropriate antibiotic administration was a requirement prior to randomization, ensuring that this important driver of mortality reduction was standardized between the two arms of the trial. In summary, while the ideal fluid resuscitation amount is unknown, requires a personalized approach, and further investigation is required to effectively incorporate non-invasive measures to guide fluid responsiveness, early and aggressive resuscitation paired with early antibiotic administration are essential aspects of effective sepsis management.
The Efferent Arm: Structure And Processes To Improve Outcomes
Personnel and Staffing
Quality care for the septic patient requires immediate availability of a multidisciplinary care team, including physicians and nurses with critical care experience who can be rapidly deployed to the bedside. The location of care provision may include on-going care in the initial ED room assignment or transfer to a dedicated area for the care of the critically ill patient within the ED.
To provide optimal care in the era of overcrowding and delayed transfer to an ICU, a movement towards ED intensive care units (ED-ICUs) has emerged [57]. The models of practice range from a model based upon ED intensivists, with expertise in critical care medicine, providing care within the traditional structure of an ED, to a model wherein a portion of the ED is assigned for the care of the critically ill for extended periods of time beyond the initial resuscitation. As these models mature from resuscitation bays capable of scaling up based on need to dedicated ED-ICUs, investments in shared Unit leadership (physician and nursing), staffing (physician, critical care nursing, respiratory therapy, critical care pharmacist) and processes of care (eg, multidisciplinary rounds) in line with established ICUs will be necessary.
While attractive conceptually, large-scale implementation of this movement is unlikely to occur outside of tertiary care academic medical centers. In the many EDs across the US without ED intensivists, and confronted with limited clinician resources, flexible physician and nursing staffing models will be necessary to ensure that care provisions are in accord with established guidelines. Potential solutions to provide the resources to meet the needs of these high-intensity patients include critical care consultation and a strategy traditionally applied to the ICU, telemedicine [58]. Last, given the relationship between hospital volume and mortality in severe sepsis [59,60], timely transfer to a high-volume center for specific cases may be appropriate, although the optimal timing, case selection, and impact of transfer on outcomes warrant further examination.
Clinical Decision Support Strategies
To complement the identification and risk-stratification available by screening and scoring systems, clinical decision support systems are novel tools to improve outcomes in the era of electronic medical records (EMR). Specific to sepsis care delivery, performance improvement initiatives including audit-and-feedback practice can increase severe sepsis guideline adherence, and even modest improvements in adherence appear to lead to sustained improvements that contributed to a 25% relative risk reduction in the observed mortality rate [61,62]. Clinical decision support tools can be used to link early recognition to optimal care processes, such as the Surviving Sepsis Campaign resuscitation and management bundles. The use of prompts as strategies to ensure that bundles of care are ordered and carried out is an important aspect to operationalize during the design phase [63].
Significant preparation is required to effectively carry out the clinical decision support design strategy. For example, to ensure timely antibiotic dispensing, a number of process steps will be required, including prompt notification to a central pharmacist or preferably, an ED pharmacist with access to a local pharmacy pre-stocked with commonly used antibiotics [64]. In addition, the use of an institution-specific antibiogram within the physician computer-order entry sepsis order set, that includes site-specific recommendations (eg, pulmonary, gastrointestinal source) and susceptibility patterns, is an essential aspect of optimal sepsis processes of care. Last, the antibiogram will need to be frequently updated to include season-specific (eg, oseltamivir administration for high-risk cases during influenza season) recommendations to ensure that providers are prompted with the most up-to-date clinical information.
Audit and Feedback and Continuous Performance Improvement
The multimodal approach required to translate knowledge (eg, guidelines) into sepsis care implemented at the bedside is an iterative process. An ED armed with a robust track-and-trigger system and an effective efferent arm, including sophisticated clinical decision support strategies, will require frequent auditing in the plan-do-study-act model of quality improvement to yield clinical effectiveness [61,62,65]. Auditing, paired with feedback to frontline providers, is essential to refine and improve the complex process required to provide expert care to the septic patient [29,65]. Sustained success in optimizing sepsis care delivery is the goal, yet significant work is required to determine the best strategies to achieve this endpoint.
Conclusion
Severe sepsis affects millions of individuals each year in the United States. Delays in recognition result in increased morbidity and mortality, at a tremendous cost to the patient and society. By designing strategies to identify sepsis in a timely, efficient, and effective manner, and by implementing ED structures and processes to increase adherence with sepsis-based guidelines, improved patient-centered outcomes can be realized.
Corresponding author: Mark E. Mikkelsen, MD, MSCE, Gates 05.042, 3400 Spruce St., Philadelphia, PA 19104, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JHM, MEM; analysis and interpretation of data, DFG; drafting of article, JHM, DFG, MEM; critical revision of the article, JHM, MEM.
1. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167–74.
2. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10.
3. Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754–61.
4. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228.
5. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51.
6. Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928–36.
7. Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–50.
8. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014;312:90–2.
9. Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308–16.
10. Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep 2007;9:382–6.
11. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–94.
12. Ortego A, Gaieski DF, Fuchs BD, et al. Hospital-based acute care use in survivors of septic shock. Crit Care Med 2015;43:729–37.
13. Prescott HC, Langa KM, Liu V, et al. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 2014;190:62–9.
14. Liu V, Lei X, Prescott HC, et al. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med 2014;9:502–7.
15. Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008;36:2734–9.
16. Seymour CW, Rea TD, Kahn JM, et al. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med 2012;186:1264–71.
17. Seymour CW, Cooke CR, Mikkelsen ME, et al. Out-of-hospital fluid in severe sepsis: effect on early resuscitation in the emergency department. Prehosp Emerg Care 2010;14:145–52.
18. Seymour CW, Cooke CR, Heckbert SR, et al. Prehospital intravenous access and fluid resuscitation in severe sepsis: an observational cohort study. Crit Care 2014;18:533
19. Studnek JR, Artho MR, Garner CL, Jones AE. The impact of emergency medical services on the ED care of severe sepsis. Am J Emerg Med 2012;30:51–6.
20. Guss DA, Meyer FT, Neuman TS, et al. The impact of a regionalized trauma system on trauma care in San Diego County. Ann Emerg Med 1989;18:1141–5.
21. Liberman M, Mulder DS, Jurkovich GJ, Sampalis JS. The association between trauma system and trauma center components and outcome in a mature regionalized trauma system. Surgery 2005;137:647–58.
22. Hachinski V, Donnan GA, Gorelick PB, et al. Stroke: working toward a prioritized world agenda. Stroke 2010;41:1084–99.
23. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6.
24. Sibbald W, Doig G, Inman K. Sepsis, SIRS, and infection. Intensive Care Med 1995;21:299–301.
25. Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; online March 17, 2015.
26. Shapiro NI, Howell MD, Bates D, et al. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med 2006;48:583–90.
27. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168–74.
28. Farley H, Zubrow MT, Gies J, et al. Emergency department tachypnea predicts transfer to a higher level of care in the first 24 hours after ED admission. Acad Emerg Med 2010;17:718–22.
29. Sinuff T, Muscadere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med 2013;41:2627–40.
30. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med 2008;52:126–36.
31. Hsia RY, Kellermann AL, Shen YC. Factors associated with closures of emergency departments in the United States. JAMA 2011;305:1978–85.
32. Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007;35:1477–83.
33. Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified early warning score in medical admissions. Q J Med 2001;94:521–6.
34. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 2015;10:26–31.
35. Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med 2014;190:649–55.
36. Whittaker SA, Fuchs BD, Gaieski DF, et al. Epidemiology and outcomes in patients with severe sepsis admitted to the hospital wards. J Crit Care 2015;30:78–84.
37. Delgado MK, Liu V, Pines JM, et al. Risk factors for unplanned transfer to intensive care within 24 hours of admission from the emergency department in an integrated healthcare system. J Hosp Med 2013;8:13–9.
38. Valentini I, Pacilli AM, Carbonara P, et al. Influence of the admission pattern on the outcome of patients admitted to a respiratory intensive care unit: does a step-down admission differ from a step-up one? Respir Care 2013;58:2053–60.
39. Kompanje EJO, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med 2007;33:1967–71.
40. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014;371:2309–19.
41. Jansen TC, van Bommel J, Mulder PG, et al. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care 2008;12:R160.
42. del Portal DA, Shofer F, Mikkelsen ME, et al. Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 2010;17:260–8.
43. Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37:1670–7.
44. Gaieski DF, Drumheller BC, Goyal M, et al. Accuracy of handheld point-of-care fingertip lactate measurement in the emergency department. West J Emerg Med 2013;14:58–62.
45. Macdonald SP, Arendts G, Fatovich DM, Brown SG. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med 2014;21:1257–63.
46. Carpenter CR, Keim SM, Upadhye S, Nguyen HB, Group BEiEMI. Risk stratification of the potentially septic patient in the emergency department: the Mortality in the Emergency Department Sepsis (MEDS) score. J Emerg Med 2009;37:319–27.
47. Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med 2008;36:421–6.
48. Corfield AR, Lees F, Zealley I, et al. Utility of a single early warning score in patients with sepsis in the emergency department. Emerg Med J 2014;31:482–7.
49. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589–96.
50. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010;38:1045–53.
51. Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011;39:2066–71.
52. Capp R, Horton CL, Takhar SS, et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med 2015 Jan 30.
53. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
54. The ProCESS Investigators. A ranodmized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683–93.
55. The ARISE Investigators and the ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496–506.
56. Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; online March 17, 2015.
57. Weingart SD, Sherwin RL, Emlet LL, et al. ED intensivists and ED intensive care units. Amer J Emerg Med 2013;31:617–20.
58. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA 2011;305:2175–85.
59. Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med 2014;189:548–55.
60. Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med 2014;190:665–74.
61. Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222–31.
62. Levy MM, Rhodes A, Phillips GS, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3–12.
63. Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med 2011;184:680–6.
64. Weant KA, Baker SN. Emergency medicine pharmacists and sepsis management. J Pharm Pract 2013;26:401–5.
65. Marwick CA, Guthrie B, Pringle JE, et al. A multifaceted intervention to improve sepsis management in general hospital wards with evaluation using segmented regression of interrupted time series. BMJ Qual Saf 2014;23:e2.
From the Department of Medicine, University of Pennsylvania, and the Department of Emergency Medicine, Thomas Jefferson University Hospital, Philadelphia, PA.
Abstract
- Objective: To detail strategies to improve sepsis recognition and the quality of care provided to the septic patient.
- Methods: Review of the literature.
- Results: Severe sepsis affects nearly 3 million individuals each year in the United States, and cost estimates for these hospitalizations exceed $24 billion. Effective management is predicated on timely recognition. In this review, we detail strategies to improve early identification of potentially septic patients as well as the quality of care provided to the septic patient in the emergency department (ED). The strategies discussed are based upon an understanding of the signs and symptoms of sepsis and the clinical risk factors associated with sepsis, which can be used to design novel strategies to screen patients for sepsis and risk stratify patients at risk for clinical deterioration.
- Conclusion: ED structures and processes can be used to increase adherence with sepsis management guidelines to improve patient outcomes.
Severe sepsis affects nearly 3 million individuals each year in the United States and cost estimates for these hospitalizations exceed $24 billion [1–3]. Sepsis is a life-threatening condition characterized by a suspected or identified infection accompanied by a vigorous host inflammatory response. In severe sepsis, end-organ dysfunction manifests in myriad forms, including altered mental status, acute kidney injury, liver dysfunction, pulmonary dysfunction, and hemodynamic compromise [4,5]. This protean presentation of a deadly condition makes identification and risk stratification both challenging and essential to improving patient outcomes. The majority of patients with severe sepsis will receive their initial care within an emergency department (ED) [6,7]. It is essential that emergency medicine providers have the means to appropriately identify patients presenting with severe sepsis in a timely manner—thus facilitating life-saving measures such as early intravenous fluid resuscitation and administration of timely and appropriate antimicrobials.
In this review, we detail strategies to improve sepsis recognition and the quality of care provided to the septic patient in the ED. The strategies discussed are based upon an understanding of the signs and symptoms of sepsis and the clinical risk factors associated with sepsis, which can be used to design novel strategies to screen patients for sepsis and risk stratify patients for clinical deterioration. Then, we review suggested ED structures and processes to increase adherence with sepsis-based guidelines to improve patient outcomes. Successful implementation is predicated on hospital administrative support towards the efforts given the time and resources required and strong and committed leadership across the health care system.
Epidemiology of Severe Sepsis
Estimates of annual cases of severe sepsis vary, ranging from 1 million to 3 million cases in the United States [1–3]. In-hospital mortality for this condition ranges from 14% to 30% [5]. The incidence of severe sepsis in the United States has been increasing at a rate of 13% annually, with an estimated cost of greater than $24 billion per year [1,2]. In 2 large cohorts of hospitalized patients, it was found that sepsis contributed to 1 in every 2 to 3 deaths following inpatient admission [8]. Coincident with these increased estimates, advances in the early identification and treatment of sepsis have led to decreasing mortality rates over the past decade [1,9].
Of importance to the ED clinician, an episode of sepsis has long-term effects on cognitive and physical function, quality-of-life, and survival [10,11]. Post-discharge, approximately one-quarter of sepsis survivors will be readmitted within 30 days [12–14]. In as many as half of these instances, another life-threatening infection is the cause for readmission, making the past medical history, including a detailed accounting of recent episodes of sepsis, an important part of the initial ED evaluation [12]. Furthermore, severe sepsis survivors spend a large proportion of their time following discharge within a health care facility, and will frequently present to the ED with an acute condition from such an environment. Important factors for predicting readmission after a sepsis hospitalization include patient age, severity of illness, hospital length of stay, and the need for intensive care during the initial hospitalization [12–14].
Principles of Effective Sepsis Management
The principles of effective sepsis management begin with early identification in the pre-hospital setting, at triage, or when a patient begins to decompensate in the hospital. After the point of initial recognition, core principles include risk stratification, timely and appropriate antimicrobial administration, initial intravenous fluid boluses and ongoing resuscitation guided by physical examination and objective resuscitation end-points [4,5]. These practices have been operationalized in the care bundles of the Surviving Sepsis Campaign Guidelines [4]. Within 3 hours, the resuscitation bundle includes measuring serum lactate to risk stratify patients, obtaining blood cultures, administering broad-spectrum antibiotics, and administering 30 mL/kg crystalloid in patients with hypotension or hyperlactatemia [4]. The 6-hour bundle expands upon these initial measures and includes additional management recommendations based on resuscitation end-points.
As effective management is predicated on timely recognition, an understanding of the impact of delayed recognition is essential to provide optimal care for the severe sepsis patient in the ED. Decades of research has revealed that certain markers predict adverse outcomes, including transition to septic shock and death, as do delayed processes of care. Importantly, while early quantitative resuscitation was demonstrated to improve outcomes in a meta-analysis, there was no demonstrable benefit when resuscitation was initiated late (> 24 hours) in the course in the ICU (odds ratio of death, 1.16 [95% confidence interval, 0.60–2.22]) [15].
Strategies To Improve Recognition
Pre-Hospital Environment
From EMS to ED Triage
Borrowing the principle “time equals tissue” from a variety of time sensitive conditions (eg, myocardial infarction management [“time equals muscle”] and stroke care [“time equals brain”]), clinicians and researchers have realized that expedited recognition of severe sepsis patients begins at the time of initial contact with the health care system. For severe sepsis patients, clinicians need to think “time equals organ function.” Given the frequency with which sepsis patients arrive to the ED via EMS, effective communication between EMS and ED providers could be leveraged to prepare the ED team to provide timely care for the sepsis patient via a “sepsis alert.” While confirmation of its applicability to sepsis care is required in the absence of a regionalized network of sepsis centers, the rationale is based on the experience of the effectiveness of trauma and stroke alert systems [20–22]. For patients not recognized as potentially being infected by EMS providers during transport, repeat vital signs during ED triage can be screened to identify patients exhibiting signs of the systemic inflammatory response syndrome (SIRS) [4,23]. The same principles of effective communication apply for patients being sent from medical clinics to the ED for evaluation and treatment of potential severe sepsis. For patients arriving independent of EMS, focused triage and initial vital signs are the starting point for identifying severe sepsis at the most proximal phase of entry into the health care system.
Vital Signs and SIRS Criteria in the ED
The Afferent Arm: Multimodal Screening Strategies
While institutional practice improvement initiatives to facilitate sepsis recognition and care should incorporate educational strategies, led by champions with expertise in sepsis, the complex presentation of sepsis requires multimodal approaches [29]. These multimodal approaches, beginning at the time of ED triage, should be designed to harness information technology to screen patients to improve severe sepsis recognition (the afferent arm) and to utilize structures and processes of care efficiently and effectively (the efferent arm) to guide severe sepsis management according to sepsis-care bundles espoused by guidelines (Figure) [4].
Operational processes to screen for sepsis in the ED will need to account for ED organizational flow (eg, average time from registration to triage, average time from triage to being seen by a physician, average length of stay in the ED, number of hospital beds) and hand-off practices (eg, care transition from ED team to floor or ICU team, or within ED at shift change). For ED organizations with shorter ED lengths of stay (eg, < 2 hours), screening practices at ED triage will serve as the focal point to identify cases of sepsis. Boarding, defined as caring for a patient in the ED pending transfer, is common, increasing as a result of ED closures [30,31], and associated with prolonged hospital length of stay and increased in-hospital mortality when ICU transfer is delayed [32]. Sepsis patients in particular appear to be a vulnerable group of patients. While many explanations exist to account for the relationship between delayed transfer and adverse outcomes, timely recognition and management of the septic patient could be compromised with prolonged boarding. To combat this potential effect, continual assessment during the entire ED stay may unmask an initially unclear presentation of sepsis.
One strategy to identify sepsis in ED organizations with prolonged ED lengths of stay is through the use of a track-and-trigger system, or early warning system. Traditionally, track-and-trigger systems were implemented on the hospital wards, as means to identify physiological deterioration in a timely manner to prevent clinical deterioration [33]. More recently, early warning systems have been used to identify patients with sepsis on the hospital wards and within EDs, as these systems rely on physiological parameters such as SIRS that are cardinal features of sepsis [34]. However, given the potential for alert fatigue, designing a system that operates with high accuracy is imperative.
Efforts are underway to redefine sepsis, using a simplified approach and readily available physiological variables, with the main goal of targeting those most at-risk of an adverse outcome during the hospitalization. Simultaneously, an understanding of the overt and more occult manifestations are essential to incorporate into the clinical decision-making and pattern recognition required to identify sepsis in a timely and accurate manner. In Table 2, the signs and symptoms that may serve as flags for severe sepsis are presented.
Mature early warning systems, designed to leverage the electronic medical record (EMR) by capturing vital signs, laboratory measures, (eg, elevated serum creatinine compared to a recent hospitalization) and symptoms (eg, altered mental status), are well-positioned to herald clinical deterioration (eg, cardiac arrest) with improved accuracy [35] and to be applied to sepsis specifically [34]. While sophisticated analytical strategies, such as machine learning, are being used to improve the test characteristics of these early warning systems, iterative, prospective chart review is an essential and complementary performance improvement step to refine the process. Further, chart review affords the opportunity to ensure compliance with sepsis care bundles.
Knowledge of the risk factors associated with development of sepsis is critical for the front-line emergency physician and nurse. Additionally, as many of these risk factors are associated with adverse outcomes, including unplanned ICU transfer and in-hospital mortality, which occur in as many as one out of 8 patients admitted directly to the ward, they have utility for early risk-stratification and triaging purposes in the ED. Advanced age and pre-existing comorbid conditions, particularly an oncologic diagnosis and/or chronic organ dysfunction, are major risk factors for sepsis and worse outcomes result in those who develop sepsis [2]. Further, illness severity, including an elevated serum lactate level, is associated with adverse outcomes. These factors can be incorporated into triage decisions and/or close monitoring for patients admitted to the general ward [36]. Conversely, because patients admitted to the ICU setting and subsequently stepped down through their hospitalization may experience better outcomes compared to patients admitted to the general ward who then require step-up to an ICU setting (37,38), attention to triage practices is critical.
These complementary strategies, which serve as the afferent arm of the system, summon health care providers to the bedside of a vulnerable patient. However, clinical effectiveness in the management of severe sepsis requires a robust, sophisticated, and mature efferent arm capable of delivering expert care to the now recognized septic patient.
Principles of Effective Management Post-Recognition
Risk Stratification
An elevated serum lactate level was initially described in pathological states in the mid 19th century by Johann Joseph Scherer [39] and has long been associated with increased mortality in hospitalized patients [40]. Lactate is a useful biomarker for risk stratification in a variety of patients arriving to the ED, particularly those who have been identified at high risk for sepsis. Jansen and colleagues examined the measurement of pre-hospital serum lactate at the time of paramedic on-scene assessment in a group of acutely ill patients [41]. Patients with point-of-care lactate levels of 3.5 mmol/L or greater were found to have an in-hospital mortality of 41% versus 12% for those with lactate levels less than 3.5 mmol/L. Within the population with an elevated lactate, patients with a systolic blood pressure greater than 100 mgHg experienced a mortality of nearly 30%, while it was greater than 50% in hypotensive patients with an elevated lactate, highlighting the value of both hemodynamic and serum lactate measures. Upon arrival to the ED, lactate measurements have a strong correlation with mortality. In one retrospective cohort, lactate level was linearly associated with mortality in a broad array of patients older than age 65 years [42]. An initial serum lactate level in the ED in the intermediate (2.0 – 3.9 mmol/L) or high range (≥ 4 mmol/L) has been associated with increased odds of death 2 to 5 times higher independent of organ dysfunction in severe sepsis specifically [43].
As the association between serum lactate levels and death is independent of organ dysfunction, serum lactate is a simple and reliable tool to both enhance detection and risk-stratify patients presenting to the ED with severe sepsis. Given the frequency with which hyperlactatemia is present in patients with suspected infection [43], operationalizing serum lactate measures with the initial phlebotomy draw is an important step to risk-stratify patients. This step can be coupled later with intravenous fluid resuscitation for those with marked elevations (≥ 4 mmol/L), in accord with guideline recommendations [4]. Screening of initial lactate values can be further expedited by utilizing fingerstick point-of-care lactate devices [44]. Last, while serial lactate measures can be incorporated into triage decisions, there is no clear threshold that warrants ICU admission. Rather, persistent elevations in serum lactate can be used to identify patients who require close observation regardless of their admission location.
Several scoring systems have been developed to augment sepsis risk stratification within the ED. The most prominent of these are the Predisposition Insult Response and Organ failure (PIRO), Sequential Organ Failure Assessment (SOFA), and Mortality in the Emergency Department Sepsis (MEDS) scores, and the National early warning score (NEWS) [45-48]. The MEDS score incorporates host factors including age and co-morbid illness, as well as physiologic and laboratory tests which can be obtained rapidly in an ED setting. Multiple prospective and retrospective examinations of the MEDS scoring systems have demonstrated that it performs optimally in ED patients with sepsis but not those with severe sepsis, in terms of predicting 30-day mortality [46,47]. The PIRO score more extensively incorporates predisposing co-morbidities, physiologic and laboratory parameters, and has been modified to consider presumed source of infection, leading to a stronger predictive ability for mortality in more severely ill patients. In patients presenting to the ED with severe sepsis and septic shock, a prospective observational study found the PIRO to be the best predictor of mortality, compared to SOFA and MEDS scores [45]. In a recent study by Corfield et al, sepsis patients with a higher NEWS, according to initial ED vital signs (temperature, pulse, respiratory rate, systolic blood pressure, oxyhemoglobin saturation) and consciousness level, were significantly more likely to be admitted to an ICU within 48 hours or to experience in-hospital mortality [48].
Timely and Appropriate Antibiotics
In a landmark study published by Kumar and colleagues in 2006, the relationship between timing of antibiotics and mortality was established [49]. In 2731 adult septic shock patients, mortality increased 7.6% for every hour delay in effective antimicrobial administration. A striking finding, given that the study population was limited to patients cared for in the ICU, was the fact that only 50% of patients received appropriate antibiotics within 6 hours of onset of shock and nearly one-quarter of patients did not receive antibiotics until the 15th hour. As a direct result, in-hospital mortality was observed to be 58% in this study.
Over the ensuing decade, a series of studies have demonstrated a narrowing of the quality gap in this regard, and the result has coincided with a significant improvement in survival. In 2010, Gaieski and colleagues demonstrated a significant improvement in the prompt administration of antibiotic delivery in patients presenting to an ED with severe sepsis, with the median time from shock onset (sustained hypotension or lactate ≥ 4 mmol/L) to antibiotics down to 42 minutes [50]. Importantly, consistent with the Kumar study, time to appropriate antibiotics, rather than simply initial antibiotics, remained associated with in-hospital mortality independent of initiating early goal-directed therapy. In 2011, Puskarich and colleagues revealed that time to antibiotics continued to improve and, as a result, the investigators did not identify a relationship between time from triage to antibiotics and in-hospital mortality [51]. However, when antibiotics were delayed until after shock recognition, consistent with the study by Kumar and colleagues, survival decreased. Until recently, this important observation was challenging to operationalize clinically as little was known about how to facilitate risk-stratification of those at risk to develop shock. However, Capp and colleagues recently found that deterioration to septic shock 48 hours after ED presentation occurs in approximately one out of eight patients and identified gender (female), transient hypotension, and/or hyperlactatemia upon presentation as risk factors associated with such a deterioration [52].
As an essential element of sepsis care bundles, a focus on timely use of antibiotics in patients with suspected infection, has the potential to increase the use of antibiotics in the ED in patients determined subsequently to not be infected. To combat this acknowledged downstream effect, reconsideration of the utility of empiric antibiotics 48 to 72 hours after admission is required. This step can be accomplished through the use of a sepsis care pathway and/or a formal antibiotic stewardship program.
Quantitative Resuscitation
Rivers and colleagues, in a landmark 2001 trial, examined the effectiveness of a protocolized resuscitation strategy in the most proximal phase of severe sepsis and septic shock [53]. A distinguishing characteristic between the usual care arm and the intervention in this ED-based study, in addition to whether mixed central venous oxygen saturation was measured as a resuscitation end-point, was the inclusion of an ED provider at the bedside to attend to clinical management. The intervention, aimed at achieving physiologic targets, resulted in significantly more fluid resuscitation (3.5 L vs. 5.0 L within the first 6 hours) and a significant decrease in in-hospital mortality compared to the usual care arm (46.5 vs. 30.5%). The study revolutionized the culture and practice of sepsis care, in part by shining a light on the importance of timely resuscitation at the most proximal point of contact between the patient and the healthcare system. It also highlighted the importance of integrating serum lactate measurement into the early screening and risk stratification processes for sepsis care delivery.
The 2014 randomized trial of Protocol-Based Care for Early Septic Shock (ProCESS) revisited this concept, comparing the Rivers 2001 protocol to both a current guideline-based non-invasive algorithmic protocol and what had become usual ED care in the interim [54]. The ProCESS trial, which operationalized a team of bedside providers to direct care for each of the 3 distinct arms, found no significant difference between the arms in terms of 90-day and 1-year mortality, but mortality was approximately 10% less in all arms compared with the intervention arm of the Rivers trial. Further, subjects in each of the 3 arms received in excess of 2 L intravenous fluid resuscitation pre-randomization and 4.4–5.5 L when resuscitation spanned from pre-randomization to 6 hours post-randomization. The conclusion drawn is that the commonalities between the arms—early fluid resuscitation, early antibiotics, and the option to use physiologic measures as markers of the adequacy of treatment, all guided by bedside ED providers—are the most important factors for surviving sepsis. And the result is that practitioners have refined these tools over a decade, leading to steady improvements in survival.
Consistent with the ProCESS trial, a recent Australia and New Zealand trial confirmed no significant difference in 90-day mortality between protocolized EGDT and current usual care for septic shock within an ED [55]. Consistent with ProCESS and ProMISe [56], subjects enrolled in ARISE received in excess of 2.5 L in resuscitation pre-randomization, which when paired with fluid resuscitation in the 0-6 hour post-randomization period (1.96 L in the EGDT arm and 1.71 in the usual-care arm) resulted in resuscitation in the 4.5 to 5L range during the initial resuscitation. The ARISE trial was unique in that appropriate antibiotic administration was a requirement prior to randomization, ensuring that this important driver of mortality reduction was standardized between the two arms of the trial. In summary, while the ideal fluid resuscitation amount is unknown, requires a personalized approach, and further investigation is required to effectively incorporate non-invasive measures to guide fluid responsiveness, early and aggressive resuscitation paired with early antibiotic administration are essential aspects of effective sepsis management.
The Efferent Arm: Structure And Processes To Improve Outcomes
Personnel and Staffing
Quality care for the septic patient requires immediate availability of a multidisciplinary care team, including physicians and nurses with critical care experience who can be rapidly deployed to the bedside. The location of care provision may include on-going care in the initial ED room assignment or transfer to a dedicated area for the care of the critically ill patient within the ED.
To provide optimal care in the era of overcrowding and delayed transfer to an ICU, a movement towards ED intensive care units (ED-ICUs) has emerged [57]. The models of practice range from a model based upon ED intensivists, with expertise in critical care medicine, providing care within the traditional structure of an ED, to a model wherein a portion of the ED is assigned for the care of the critically ill for extended periods of time beyond the initial resuscitation. As these models mature from resuscitation bays capable of scaling up based on need to dedicated ED-ICUs, investments in shared Unit leadership (physician and nursing), staffing (physician, critical care nursing, respiratory therapy, critical care pharmacist) and processes of care (eg, multidisciplinary rounds) in line with established ICUs will be necessary.
While attractive conceptually, large-scale implementation of this movement is unlikely to occur outside of tertiary care academic medical centers. In the many EDs across the US without ED intensivists, and confronted with limited clinician resources, flexible physician and nursing staffing models will be necessary to ensure that care provisions are in accord with established guidelines. Potential solutions to provide the resources to meet the needs of these high-intensity patients include critical care consultation and a strategy traditionally applied to the ICU, telemedicine [58]. Last, given the relationship between hospital volume and mortality in severe sepsis [59,60], timely transfer to a high-volume center for specific cases may be appropriate, although the optimal timing, case selection, and impact of transfer on outcomes warrant further examination.
Clinical Decision Support Strategies
To complement the identification and risk-stratification available by screening and scoring systems, clinical decision support systems are novel tools to improve outcomes in the era of electronic medical records (EMR). Specific to sepsis care delivery, performance improvement initiatives including audit-and-feedback practice can increase severe sepsis guideline adherence, and even modest improvements in adherence appear to lead to sustained improvements that contributed to a 25% relative risk reduction in the observed mortality rate [61,62]. Clinical decision support tools can be used to link early recognition to optimal care processes, such as the Surviving Sepsis Campaign resuscitation and management bundles. The use of prompts as strategies to ensure that bundles of care are ordered and carried out is an important aspect to operationalize during the design phase [63].
Significant preparation is required to effectively carry out the clinical decision support design strategy. For example, to ensure timely antibiotic dispensing, a number of process steps will be required, including prompt notification to a central pharmacist or preferably, an ED pharmacist with access to a local pharmacy pre-stocked with commonly used antibiotics [64]. In addition, the use of an institution-specific antibiogram within the physician computer-order entry sepsis order set, that includes site-specific recommendations (eg, pulmonary, gastrointestinal source) and susceptibility patterns, is an essential aspect of optimal sepsis processes of care. Last, the antibiogram will need to be frequently updated to include season-specific (eg, oseltamivir administration for high-risk cases during influenza season) recommendations to ensure that providers are prompted with the most up-to-date clinical information.
Audit and Feedback and Continuous Performance Improvement
The multimodal approach required to translate knowledge (eg, guidelines) into sepsis care implemented at the bedside is an iterative process. An ED armed with a robust track-and-trigger system and an effective efferent arm, including sophisticated clinical decision support strategies, will require frequent auditing in the plan-do-study-act model of quality improvement to yield clinical effectiveness [61,62,65]. Auditing, paired with feedback to frontline providers, is essential to refine and improve the complex process required to provide expert care to the septic patient [29,65]. Sustained success in optimizing sepsis care delivery is the goal, yet significant work is required to determine the best strategies to achieve this endpoint.
Conclusion
Severe sepsis affects millions of individuals each year in the United States. Delays in recognition result in increased morbidity and mortality, at a tremendous cost to the patient and society. By designing strategies to identify sepsis in a timely, efficient, and effective manner, and by implementing ED structures and processes to increase adherence with sepsis-based guidelines, improved patient-centered outcomes can be realized.
Corresponding author: Mark E. Mikkelsen, MD, MSCE, Gates 05.042, 3400 Spruce St., Philadelphia, PA 19104, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JHM, MEM; analysis and interpretation of data, DFG; drafting of article, JHM, DFG, MEM; critical revision of the article, JHM, MEM.
From the Department of Medicine, University of Pennsylvania, and the Department of Emergency Medicine, Thomas Jefferson University Hospital, Philadelphia, PA.
Abstract
- Objective: To detail strategies to improve sepsis recognition and the quality of care provided to the septic patient.
- Methods: Review of the literature.
- Results: Severe sepsis affects nearly 3 million individuals each year in the United States, and cost estimates for these hospitalizations exceed $24 billion. Effective management is predicated on timely recognition. In this review, we detail strategies to improve early identification of potentially septic patients as well as the quality of care provided to the septic patient in the emergency department (ED). The strategies discussed are based upon an understanding of the signs and symptoms of sepsis and the clinical risk factors associated with sepsis, which can be used to design novel strategies to screen patients for sepsis and risk stratify patients at risk for clinical deterioration.
- Conclusion: ED structures and processes can be used to increase adherence with sepsis management guidelines to improve patient outcomes.
Severe sepsis affects nearly 3 million individuals each year in the United States and cost estimates for these hospitalizations exceed $24 billion [1–3]. Sepsis is a life-threatening condition characterized by a suspected or identified infection accompanied by a vigorous host inflammatory response. In severe sepsis, end-organ dysfunction manifests in myriad forms, including altered mental status, acute kidney injury, liver dysfunction, pulmonary dysfunction, and hemodynamic compromise [4,5]. This protean presentation of a deadly condition makes identification and risk stratification both challenging and essential to improving patient outcomes. The majority of patients with severe sepsis will receive their initial care within an emergency department (ED) [6,7]. It is essential that emergency medicine providers have the means to appropriately identify patients presenting with severe sepsis in a timely manner—thus facilitating life-saving measures such as early intravenous fluid resuscitation and administration of timely and appropriate antimicrobials.
In this review, we detail strategies to improve sepsis recognition and the quality of care provided to the septic patient in the ED. The strategies discussed are based upon an understanding of the signs and symptoms of sepsis and the clinical risk factors associated with sepsis, which can be used to design novel strategies to screen patients for sepsis and risk stratify patients for clinical deterioration. Then, we review suggested ED structures and processes to increase adherence with sepsis-based guidelines to improve patient outcomes. Successful implementation is predicated on hospital administrative support towards the efforts given the time and resources required and strong and committed leadership across the health care system.
Epidemiology of Severe Sepsis
Estimates of annual cases of severe sepsis vary, ranging from 1 million to 3 million cases in the United States [1–3]. In-hospital mortality for this condition ranges from 14% to 30% [5]. The incidence of severe sepsis in the United States has been increasing at a rate of 13% annually, with an estimated cost of greater than $24 billion per year [1,2]. In 2 large cohorts of hospitalized patients, it was found that sepsis contributed to 1 in every 2 to 3 deaths following inpatient admission [8]. Coincident with these increased estimates, advances in the early identification and treatment of sepsis have led to decreasing mortality rates over the past decade [1,9].
Of importance to the ED clinician, an episode of sepsis has long-term effects on cognitive and physical function, quality-of-life, and survival [10,11]. Post-discharge, approximately one-quarter of sepsis survivors will be readmitted within 30 days [12–14]. In as many as half of these instances, another life-threatening infection is the cause for readmission, making the past medical history, including a detailed accounting of recent episodes of sepsis, an important part of the initial ED evaluation [12]. Furthermore, severe sepsis survivors spend a large proportion of their time following discharge within a health care facility, and will frequently present to the ED with an acute condition from such an environment. Important factors for predicting readmission after a sepsis hospitalization include patient age, severity of illness, hospital length of stay, and the need for intensive care during the initial hospitalization [12–14].
Principles of Effective Sepsis Management
The principles of effective sepsis management begin with early identification in the pre-hospital setting, at triage, or when a patient begins to decompensate in the hospital. After the point of initial recognition, core principles include risk stratification, timely and appropriate antimicrobial administration, initial intravenous fluid boluses and ongoing resuscitation guided by physical examination and objective resuscitation end-points [4,5]. These practices have been operationalized in the care bundles of the Surviving Sepsis Campaign Guidelines [4]. Within 3 hours, the resuscitation bundle includes measuring serum lactate to risk stratify patients, obtaining blood cultures, administering broad-spectrum antibiotics, and administering 30 mL/kg crystalloid in patients with hypotension or hyperlactatemia [4]. The 6-hour bundle expands upon these initial measures and includes additional management recommendations based on resuscitation end-points.
As effective management is predicated on timely recognition, an understanding of the impact of delayed recognition is essential to provide optimal care for the severe sepsis patient in the ED. Decades of research has revealed that certain markers predict adverse outcomes, including transition to septic shock and death, as do delayed processes of care. Importantly, while early quantitative resuscitation was demonstrated to improve outcomes in a meta-analysis, there was no demonstrable benefit when resuscitation was initiated late (> 24 hours) in the course in the ICU (odds ratio of death, 1.16 [95% confidence interval, 0.60–2.22]) [15].
Strategies To Improve Recognition
Pre-Hospital Environment
From EMS to ED Triage
Borrowing the principle “time equals tissue” from a variety of time sensitive conditions (eg, myocardial infarction management [“time equals muscle”] and stroke care [“time equals brain”]), clinicians and researchers have realized that expedited recognition of severe sepsis patients begins at the time of initial contact with the health care system. For severe sepsis patients, clinicians need to think “time equals organ function.” Given the frequency with which sepsis patients arrive to the ED via EMS, effective communication between EMS and ED providers could be leveraged to prepare the ED team to provide timely care for the sepsis patient via a “sepsis alert.” While confirmation of its applicability to sepsis care is required in the absence of a regionalized network of sepsis centers, the rationale is based on the experience of the effectiveness of trauma and stroke alert systems [20–22]. For patients not recognized as potentially being infected by EMS providers during transport, repeat vital signs during ED triage can be screened to identify patients exhibiting signs of the systemic inflammatory response syndrome (SIRS) [4,23]. The same principles of effective communication apply for patients being sent from medical clinics to the ED for evaluation and treatment of potential severe sepsis. For patients arriving independent of EMS, focused triage and initial vital signs are the starting point for identifying severe sepsis at the most proximal phase of entry into the health care system.
Vital Signs and SIRS Criteria in the ED
The Afferent Arm: Multimodal Screening Strategies
While institutional practice improvement initiatives to facilitate sepsis recognition and care should incorporate educational strategies, led by champions with expertise in sepsis, the complex presentation of sepsis requires multimodal approaches [29]. These multimodal approaches, beginning at the time of ED triage, should be designed to harness information technology to screen patients to improve severe sepsis recognition (the afferent arm) and to utilize structures and processes of care efficiently and effectively (the efferent arm) to guide severe sepsis management according to sepsis-care bundles espoused by guidelines (Figure) [4].
Operational processes to screen for sepsis in the ED will need to account for ED organizational flow (eg, average time from registration to triage, average time from triage to being seen by a physician, average length of stay in the ED, number of hospital beds) and hand-off practices (eg, care transition from ED team to floor or ICU team, or within ED at shift change). For ED organizations with shorter ED lengths of stay (eg, < 2 hours), screening practices at ED triage will serve as the focal point to identify cases of sepsis. Boarding, defined as caring for a patient in the ED pending transfer, is common, increasing as a result of ED closures [30,31], and associated with prolonged hospital length of stay and increased in-hospital mortality when ICU transfer is delayed [32]. Sepsis patients in particular appear to be a vulnerable group of patients. While many explanations exist to account for the relationship between delayed transfer and adverse outcomes, timely recognition and management of the septic patient could be compromised with prolonged boarding. To combat this potential effect, continual assessment during the entire ED stay may unmask an initially unclear presentation of sepsis.
One strategy to identify sepsis in ED organizations with prolonged ED lengths of stay is through the use of a track-and-trigger system, or early warning system. Traditionally, track-and-trigger systems were implemented on the hospital wards, as means to identify physiological deterioration in a timely manner to prevent clinical deterioration [33]. More recently, early warning systems have been used to identify patients with sepsis on the hospital wards and within EDs, as these systems rely on physiological parameters such as SIRS that are cardinal features of sepsis [34]. However, given the potential for alert fatigue, designing a system that operates with high accuracy is imperative.
Efforts are underway to redefine sepsis, using a simplified approach and readily available physiological variables, with the main goal of targeting those most at-risk of an adverse outcome during the hospitalization. Simultaneously, an understanding of the overt and more occult manifestations are essential to incorporate into the clinical decision-making and pattern recognition required to identify sepsis in a timely and accurate manner. In Table 2, the signs and symptoms that may serve as flags for severe sepsis are presented.
Mature early warning systems, designed to leverage the electronic medical record (EMR) by capturing vital signs, laboratory measures, (eg, elevated serum creatinine compared to a recent hospitalization) and symptoms (eg, altered mental status), are well-positioned to herald clinical deterioration (eg, cardiac arrest) with improved accuracy [35] and to be applied to sepsis specifically [34]. While sophisticated analytical strategies, such as machine learning, are being used to improve the test characteristics of these early warning systems, iterative, prospective chart review is an essential and complementary performance improvement step to refine the process. Further, chart review affords the opportunity to ensure compliance with sepsis care bundles.
Knowledge of the risk factors associated with development of sepsis is critical for the front-line emergency physician and nurse. Additionally, as many of these risk factors are associated with adverse outcomes, including unplanned ICU transfer and in-hospital mortality, which occur in as many as one out of 8 patients admitted directly to the ward, they have utility for early risk-stratification and triaging purposes in the ED. Advanced age and pre-existing comorbid conditions, particularly an oncologic diagnosis and/or chronic organ dysfunction, are major risk factors for sepsis and worse outcomes result in those who develop sepsis [2]. Further, illness severity, including an elevated serum lactate level, is associated with adverse outcomes. These factors can be incorporated into triage decisions and/or close monitoring for patients admitted to the general ward [36]. Conversely, because patients admitted to the ICU setting and subsequently stepped down through their hospitalization may experience better outcomes compared to patients admitted to the general ward who then require step-up to an ICU setting (37,38), attention to triage practices is critical.
These complementary strategies, which serve as the afferent arm of the system, summon health care providers to the bedside of a vulnerable patient. However, clinical effectiveness in the management of severe sepsis requires a robust, sophisticated, and mature efferent arm capable of delivering expert care to the now recognized septic patient.
Principles of Effective Management Post-Recognition
Risk Stratification
An elevated serum lactate level was initially described in pathological states in the mid 19th century by Johann Joseph Scherer [39] and has long been associated with increased mortality in hospitalized patients [40]. Lactate is a useful biomarker for risk stratification in a variety of patients arriving to the ED, particularly those who have been identified at high risk for sepsis. Jansen and colleagues examined the measurement of pre-hospital serum lactate at the time of paramedic on-scene assessment in a group of acutely ill patients [41]. Patients with point-of-care lactate levels of 3.5 mmol/L or greater were found to have an in-hospital mortality of 41% versus 12% for those with lactate levels less than 3.5 mmol/L. Within the population with an elevated lactate, patients with a systolic blood pressure greater than 100 mgHg experienced a mortality of nearly 30%, while it was greater than 50% in hypotensive patients with an elevated lactate, highlighting the value of both hemodynamic and serum lactate measures. Upon arrival to the ED, lactate measurements have a strong correlation with mortality. In one retrospective cohort, lactate level was linearly associated with mortality in a broad array of patients older than age 65 years [42]. An initial serum lactate level in the ED in the intermediate (2.0 – 3.9 mmol/L) or high range (≥ 4 mmol/L) has been associated with increased odds of death 2 to 5 times higher independent of organ dysfunction in severe sepsis specifically [43].
As the association between serum lactate levels and death is independent of organ dysfunction, serum lactate is a simple and reliable tool to both enhance detection and risk-stratify patients presenting to the ED with severe sepsis. Given the frequency with which hyperlactatemia is present in patients with suspected infection [43], operationalizing serum lactate measures with the initial phlebotomy draw is an important step to risk-stratify patients. This step can be coupled later with intravenous fluid resuscitation for those with marked elevations (≥ 4 mmol/L), in accord with guideline recommendations [4]. Screening of initial lactate values can be further expedited by utilizing fingerstick point-of-care lactate devices [44]. Last, while serial lactate measures can be incorporated into triage decisions, there is no clear threshold that warrants ICU admission. Rather, persistent elevations in serum lactate can be used to identify patients who require close observation regardless of their admission location.
Several scoring systems have been developed to augment sepsis risk stratification within the ED. The most prominent of these are the Predisposition Insult Response and Organ failure (PIRO), Sequential Organ Failure Assessment (SOFA), and Mortality in the Emergency Department Sepsis (MEDS) scores, and the National early warning score (NEWS) [45-48]. The MEDS score incorporates host factors including age and co-morbid illness, as well as physiologic and laboratory tests which can be obtained rapidly in an ED setting. Multiple prospective and retrospective examinations of the MEDS scoring systems have demonstrated that it performs optimally in ED patients with sepsis but not those with severe sepsis, in terms of predicting 30-day mortality [46,47]. The PIRO score more extensively incorporates predisposing co-morbidities, physiologic and laboratory parameters, and has been modified to consider presumed source of infection, leading to a stronger predictive ability for mortality in more severely ill patients. In patients presenting to the ED with severe sepsis and septic shock, a prospective observational study found the PIRO to be the best predictor of mortality, compared to SOFA and MEDS scores [45]. In a recent study by Corfield et al, sepsis patients with a higher NEWS, according to initial ED vital signs (temperature, pulse, respiratory rate, systolic blood pressure, oxyhemoglobin saturation) and consciousness level, were significantly more likely to be admitted to an ICU within 48 hours or to experience in-hospital mortality [48].
Timely and Appropriate Antibiotics
In a landmark study published by Kumar and colleagues in 2006, the relationship between timing of antibiotics and mortality was established [49]. In 2731 adult septic shock patients, mortality increased 7.6% for every hour delay in effective antimicrobial administration. A striking finding, given that the study population was limited to patients cared for in the ICU, was the fact that only 50% of patients received appropriate antibiotics within 6 hours of onset of shock and nearly one-quarter of patients did not receive antibiotics until the 15th hour. As a direct result, in-hospital mortality was observed to be 58% in this study.
Over the ensuing decade, a series of studies have demonstrated a narrowing of the quality gap in this regard, and the result has coincided with a significant improvement in survival. In 2010, Gaieski and colleagues demonstrated a significant improvement in the prompt administration of antibiotic delivery in patients presenting to an ED with severe sepsis, with the median time from shock onset (sustained hypotension or lactate ≥ 4 mmol/L) to antibiotics down to 42 minutes [50]. Importantly, consistent with the Kumar study, time to appropriate antibiotics, rather than simply initial antibiotics, remained associated with in-hospital mortality independent of initiating early goal-directed therapy. In 2011, Puskarich and colleagues revealed that time to antibiotics continued to improve and, as a result, the investigators did not identify a relationship between time from triage to antibiotics and in-hospital mortality [51]. However, when antibiotics were delayed until after shock recognition, consistent with the study by Kumar and colleagues, survival decreased. Until recently, this important observation was challenging to operationalize clinically as little was known about how to facilitate risk-stratification of those at risk to develop shock. However, Capp and colleagues recently found that deterioration to septic shock 48 hours after ED presentation occurs in approximately one out of eight patients and identified gender (female), transient hypotension, and/or hyperlactatemia upon presentation as risk factors associated with such a deterioration [52].
As an essential element of sepsis care bundles, a focus on timely use of antibiotics in patients with suspected infection, has the potential to increase the use of antibiotics in the ED in patients determined subsequently to not be infected. To combat this acknowledged downstream effect, reconsideration of the utility of empiric antibiotics 48 to 72 hours after admission is required. This step can be accomplished through the use of a sepsis care pathway and/or a formal antibiotic stewardship program.
Quantitative Resuscitation
Rivers and colleagues, in a landmark 2001 trial, examined the effectiveness of a protocolized resuscitation strategy in the most proximal phase of severe sepsis and septic shock [53]. A distinguishing characteristic between the usual care arm and the intervention in this ED-based study, in addition to whether mixed central venous oxygen saturation was measured as a resuscitation end-point, was the inclusion of an ED provider at the bedside to attend to clinical management. The intervention, aimed at achieving physiologic targets, resulted in significantly more fluid resuscitation (3.5 L vs. 5.0 L within the first 6 hours) and a significant decrease in in-hospital mortality compared to the usual care arm (46.5 vs. 30.5%). The study revolutionized the culture and practice of sepsis care, in part by shining a light on the importance of timely resuscitation at the most proximal point of contact between the patient and the healthcare system. It also highlighted the importance of integrating serum lactate measurement into the early screening and risk stratification processes for sepsis care delivery.
The 2014 randomized trial of Protocol-Based Care for Early Septic Shock (ProCESS) revisited this concept, comparing the Rivers 2001 protocol to both a current guideline-based non-invasive algorithmic protocol and what had become usual ED care in the interim [54]. The ProCESS trial, which operationalized a team of bedside providers to direct care for each of the 3 distinct arms, found no significant difference between the arms in terms of 90-day and 1-year mortality, but mortality was approximately 10% less in all arms compared with the intervention arm of the Rivers trial. Further, subjects in each of the 3 arms received in excess of 2 L intravenous fluid resuscitation pre-randomization and 4.4–5.5 L when resuscitation spanned from pre-randomization to 6 hours post-randomization. The conclusion drawn is that the commonalities between the arms—early fluid resuscitation, early antibiotics, and the option to use physiologic measures as markers of the adequacy of treatment, all guided by bedside ED providers—are the most important factors for surviving sepsis. And the result is that practitioners have refined these tools over a decade, leading to steady improvements in survival.
Consistent with the ProCESS trial, a recent Australia and New Zealand trial confirmed no significant difference in 90-day mortality between protocolized EGDT and current usual care for septic shock within an ED [55]. Consistent with ProCESS and ProMISe [56], subjects enrolled in ARISE received in excess of 2.5 L in resuscitation pre-randomization, which when paired with fluid resuscitation in the 0-6 hour post-randomization period (1.96 L in the EGDT arm and 1.71 in the usual-care arm) resulted in resuscitation in the 4.5 to 5L range during the initial resuscitation. The ARISE trial was unique in that appropriate antibiotic administration was a requirement prior to randomization, ensuring that this important driver of mortality reduction was standardized between the two arms of the trial. In summary, while the ideal fluid resuscitation amount is unknown, requires a personalized approach, and further investigation is required to effectively incorporate non-invasive measures to guide fluid responsiveness, early and aggressive resuscitation paired with early antibiotic administration are essential aspects of effective sepsis management.
The Efferent Arm: Structure And Processes To Improve Outcomes
Personnel and Staffing
Quality care for the septic patient requires immediate availability of a multidisciplinary care team, including physicians and nurses with critical care experience who can be rapidly deployed to the bedside. The location of care provision may include on-going care in the initial ED room assignment or transfer to a dedicated area for the care of the critically ill patient within the ED.
To provide optimal care in the era of overcrowding and delayed transfer to an ICU, a movement towards ED intensive care units (ED-ICUs) has emerged [57]. The models of practice range from a model based upon ED intensivists, with expertise in critical care medicine, providing care within the traditional structure of an ED, to a model wherein a portion of the ED is assigned for the care of the critically ill for extended periods of time beyond the initial resuscitation. As these models mature from resuscitation bays capable of scaling up based on need to dedicated ED-ICUs, investments in shared Unit leadership (physician and nursing), staffing (physician, critical care nursing, respiratory therapy, critical care pharmacist) and processes of care (eg, multidisciplinary rounds) in line with established ICUs will be necessary.
While attractive conceptually, large-scale implementation of this movement is unlikely to occur outside of tertiary care academic medical centers. In the many EDs across the US without ED intensivists, and confronted with limited clinician resources, flexible physician and nursing staffing models will be necessary to ensure that care provisions are in accord with established guidelines. Potential solutions to provide the resources to meet the needs of these high-intensity patients include critical care consultation and a strategy traditionally applied to the ICU, telemedicine [58]. Last, given the relationship between hospital volume and mortality in severe sepsis [59,60], timely transfer to a high-volume center for specific cases may be appropriate, although the optimal timing, case selection, and impact of transfer on outcomes warrant further examination.
Clinical Decision Support Strategies
To complement the identification and risk-stratification available by screening and scoring systems, clinical decision support systems are novel tools to improve outcomes in the era of electronic medical records (EMR). Specific to sepsis care delivery, performance improvement initiatives including audit-and-feedback practice can increase severe sepsis guideline adherence, and even modest improvements in adherence appear to lead to sustained improvements that contributed to a 25% relative risk reduction in the observed mortality rate [61,62]. Clinical decision support tools can be used to link early recognition to optimal care processes, such as the Surviving Sepsis Campaign resuscitation and management bundles. The use of prompts as strategies to ensure that bundles of care are ordered and carried out is an important aspect to operationalize during the design phase [63].
Significant preparation is required to effectively carry out the clinical decision support design strategy. For example, to ensure timely antibiotic dispensing, a number of process steps will be required, including prompt notification to a central pharmacist or preferably, an ED pharmacist with access to a local pharmacy pre-stocked with commonly used antibiotics [64]. In addition, the use of an institution-specific antibiogram within the physician computer-order entry sepsis order set, that includes site-specific recommendations (eg, pulmonary, gastrointestinal source) and susceptibility patterns, is an essential aspect of optimal sepsis processes of care. Last, the antibiogram will need to be frequently updated to include season-specific (eg, oseltamivir administration for high-risk cases during influenza season) recommendations to ensure that providers are prompted with the most up-to-date clinical information.
Audit and Feedback and Continuous Performance Improvement
The multimodal approach required to translate knowledge (eg, guidelines) into sepsis care implemented at the bedside is an iterative process. An ED armed with a robust track-and-trigger system and an effective efferent arm, including sophisticated clinical decision support strategies, will require frequent auditing in the plan-do-study-act model of quality improvement to yield clinical effectiveness [61,62,65]. Auditing, paired with feedback to frontline providers, is essential to refine and improve the complex process required to provide expert care to the septic patient [29,65]. Sustained success in optimizing sepsis care delivery is the goal, yet significant work is required to determine the best strategies to achieve this endpoint.
Conclusion
Severe sepsis affects millions of individuals each year in the United States. Delays in recognition result in increased morbidity and mortality, at a tremendous cost to the patient and society. By designing strategies to identify sepsis in a timely, efficient, and effective manner, and by implementing ED structures and processes to increase adherence with sepsis-based guidelines, improved patient-centered outcomes can be realized.
Corresponding author: Mark E. Mikkelsen, MD, MSCE, Gates 05.042, 3400 Spruce St., Philadelphia, PA 19104, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JHM, MEM; analysis and interpretation of data, DFG; drafting of article, JHM, DFG, MEM; critical revision of the article, JHM, MEM.
1. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167–74.
2. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10.
3. Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754–61.
4. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228.
5. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51.
6. Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928–36.
7. Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–50.
8. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014;312:90–2.
9. Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308–16.
10. Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep 2007;9:382–6.
11. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–94.
12. Ortego A, Gaieski DF, Fuchs BD, et al. Hospital-based acute care use in survivors of septic shock. Crit Care Med 2015;43:729–37.
13. Prescott HC, Langa KM, Liu V, et al. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 2014;190:62–9.
14. Liu V, Lei X, Prescott HC, et al. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med 2014;9:502–7.
15. Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008;36:2734–9.
16. Seymour CW, Rea TD, Kahn JM, et al. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med 2012;186:1264–71.
17. Seymour CW, Cooke CR, Mikkelsen ME, et al. Out-of-hospital fluid in severe sepsis: effect on early resuscitation in the emergency department. Prehosp Emerg Care 2010;14:145–52.
18. Seymour CW, Cooke CR, Heckbert SR, et al. Prehospital intravenous access and fluid resuscitation in severe sepsis: an observational cohort study. Crit Care 2014;18:533
19. Studnek JR, Artho MR, Garner CL, Jones AE. The impact of emergency medical services on the ED care of severe sepsis. Am J Emerg Med 2012;30:51–6.
20. Guss DA, Meyer FT, Neuman TS, et al. The impact of a regionalized trauma system on trauma care in San Diego County. Ann Emerg Med 1989;18:1141–5.
21. Liberman M, Mulder DS, Jurkovich GJ, Sampalis JS. The association between trauma system and trauma center components and outcome in a mature regionalized trauma system. Surgery 2005;137:647–58.
22. Hachinski V, Donnan GA, Gorelick PB, et al. Stroke: working toward a prioritized world agenda. Stroke 2010;41:1084–99.
23. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6.
24. Sibbald W, Doig G, Inman K. Sepsis, SIRS, and infection. Intensive Care Med 1995;21:299–301.
25. Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; online March 17, 2015.
26. Shapiro NI, Howell MD, Bates D, et al. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med 2006;48:583–90.
27. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168–74.
28. Farley H, Zubrow MT, Gies J, et al. Emergency department tachypnea predicts transfer to a higher level of care in the first 24 hours after ED admission. Acad Emerg Med 2010;17:718–22.
29. Sinuff T, Muscadere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med 2013;41:2627–40.
30. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med 2008;52:126–36.
31. Hsia RY, Kellermann AL, Shen YC. Factors associated with closures of emergency departments in the United States. JAMA 2011;305:1978–85.
32. Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007;35:1477–83.
33. Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified early warning score in medical admissions. Q J Med 2001;94:521–6.
34. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 2015;10:26–31.
35. Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med 2014;190:649–55.
36. Whittaker SA, Fuchs BD, Gaieski DF, et al. Epidemiology and outcomes in patients with severe sepsis admitted to the hospital wards. J Crit Care 2015;30:78–84.
37. Delgado MK, Liu V, Pines JM, et al. Risk factors for unplanned transfer to intensive care within 24 hours of admission from the emergency department in an integrated healthcare system. J Hosp Med 2013;8:13–9.
38. Valentini I, Pacilli AM, Carbonara P, et al. Influence of the admission pattern on the outcome of patients admitted to a respiratory intensive care unit: does a step-down admission differ from a step-up one? Respir Care 2013;58:2053–60.
39. Kompanje EJO, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med 2007;33:1967–71.
40. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014;371:2309–19.
41. Jansen TC, van Bommel J, Mulder PG, et al. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care 2008;12:R160.
42. del Portal DA, Shofer F, Mikkelsen ME, et al. Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 2010;17:260–8.
43. Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37:1670–7.
44. Gaieski DF, Drumheller BC, Goyal M, et al. Accuracy of handheld point-of-care fingertip lactate measurement in the emergency department. West J Emerg Med 2013;14:58–62.
45. Macdonald SP, Arendts G, Fatovich DM, Brown SG. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med 2014;21:1257–63.
46. Carpenter CR, Keim SM, Upadhye S, Nguyen HB, Group BEiEMI. Risk stratification of the potentially septic patient in the emergency department: the Mortality in the Emergency Department Sepsis (MEDS) score. J Emerg Med 2009;37:319–27.
47. Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med 2008;36:421–6.
48. Corfield AR, Lees F, Zealley I, et al. Utility of a single early warning score in patients with sepsis in the emergency department. Emerg Med J 2014;31:482–7.
49. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589–96.
50. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010;38:1045–53.
51. Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011;39:2066–71.
52. Capp R, Horton CL, Takhar SS, et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med 2015 Jan 30.
53. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
54. The ProCESS Investigators. A ranodmized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683–93.
55. The ARISE Investigators and the ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496–506.
56. Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; online March 17, 2015.
57. Weingart SD, Sherwin RL, Emlet LL, et al. ED intensivists and ED intensive care units. Amer J Emerg Med 2013;31:617–20.
58. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA 2011;305:2175–85.
59. Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med 2014;189:548–55.
60. Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med 2014;190:665–74.
61. Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222–31.
62. Levy MM, Rhodes A, Phillips GS, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3–12.
63. Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med 2011;184:680–6.
64. Weant KA, Baker SN. Emergency medicine pharmacists and sepsis management. J Pharm Pract 2013;26:401–5.
65. Marwick CA, Guthrie B, Pringle JE, et al. A multifaceted intervention to improve sepsis management in general hospital wards with evaluation using segmented regression of interrupted time series. BMJ Qual Saf 2014;23:e2.
1. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167–74.
2. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10.
3. Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754–61.
4. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228.
5. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51.
6. Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928–36.
7. Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–50.
8. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014;312:90–2.
9. Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308–16.
10. Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep 2007;9:382–6.
11. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–94.
12. Ortego A, Gaieski DF, Fuchs BD, et al. Hospital-based acute care use in survivors of septic shock. Crit Care Med 2015;43:729–37.
13. Prescott HC, Langa KM, Liu V, et al. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 2014;190:62–9.
14. Liu V, Lei X, Prescott HC, et al. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med 2014;9:502–7.
15. Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008;36:2734–9.
16. Seymour CW, Rea TD, Kahn JM, et al. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med 2012;186:1264–71.
17. Seymour CW, Cooke CR, Mikkelsen ME, et al. Out-of-hospital fluid in severe sepsis: effect on early resuscitation in the emergency department. Prehosp Emerg Care 2010;14:145–52.
18. Seymour CW, Cooke CR, Heckbert SR, et al. Prehospital intravenous access and fluid resuscitation in severe sepsis: an observational cohort study. Crit Care 2014;18:533
19. Studnek JR, Artho MR, Garner CL, Jones AE. The impact of emergency medical services on the ED care of severe sepsis. Am J Emerg Med 2012;30:51–6.
20. Guss DA, Meyer FT, Neuman TS, et al. The impact of a regionalized trauma system on trauma care in San Diego County. Ann Emerg Med 1989;18:1141–5.
21. Liberman M, Mulder DS, Jurkovich GJ, Sampalis JS. The association between trauma system and trauma center components and outcome in a mature regionalized trauma system. Surgery 2005;137:647–58.
22. Hachinski V, Donnan GA, Gorelick PB, et al. Stroke: working toward a prioritized world agenda. Stroke 2010;41:1084–99.
23. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6.
24. Sibbald W, Doig G, Inman K. Sepsis, SIRS, and infection. Intensive Care Med 1995;21:299–301.
25. Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; online March 17, 2015.
26. Shapiro NI, Howell MD, Bates D, et al. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med 2006;48:583–90.
27. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168–74.
28. Farley H, Zubrow MT, Gies J, et al. Emergency department tachypnea predicts transfer to a higher level of care in the first 24 hours after ED admission. Acad Emerg Med 2010;17:718–22.
29. Sinuff T, Muscadere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med 2013;41:2627–40.
30. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med 2008;52:126–36.
31. Hsia RY, Kellermann AL, Shen YC. Factors associated with closures of emergency departments in the United States. JAMA 2011;305:1978–85.
32. Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007;35:1477–83.
33. Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified early warning score in medical admissions. Q J Med 2001;94:521–6.
34. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 2015;10:26–31.
35. Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med 2014;190:649–55.
36. Whittaker SA, Fuchs BD, Gaieski DF, et al. Epidemiology and outcomes in patients with severe sepsis admitted to the hospital wards. J Crit Care 2015;30:78–84.
37. Delgado MK, Liu V, Pines JM, et al. Risk factors for unplanned transfer to intensive care within 24 hours of admission from the emergency department in an integrated healthcare system. J Hosp Med 2013;8:13–9.
38. Valentini I, Pacilli AM, Carbonara P, et al. Influence of the admission pattern on the outcome of patients admitted to a respiratory intensive care unit: does a step-down admission differ from a step-up one? Respir Care 2013;58:2053–60.
39. Kompanje EJO, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med 2007;33:1967–71.
40. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014;371:2309–19.
41. Jansen TC, van Bommel J, Mulder PG, et al. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care 2008;12:R160.
42. del Portal DA, Shofer F, Mikkelsen ME, et al. Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 2010;17:260–8.
43. Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37:1670–7.
44. Gaieski DF, Drumheller BC, Goyal M, et al. Accuracy of handheld point-of-care fingertip lactate measurement in the emergency department. West J Emerg Med 2013;14:58–62.
45. Macdonald SP, Arendts G, Fatovich DM, Brown SG. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med 2014;21:1257–63.
46. Carpenter CR, Keim SM, Upadhye S, Nguyen HB, Group BEiEMI. Risk stratification of the potentially septic patient in the emergency department: the Mortality in the Emergency Department Sepsis (MEDS) score. J Emerg Med 2009;37:319–27.
47. Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med 2008;36:421–6.
48. Corfield AR, Lees F, Zealley I, et al. Utility of a single early warning score in patients with sepsis in the emergency department. Emerg Med J 2014;31:482–7.
49. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589–96.
50. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010;38:1045–53.
51. Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011;39:2066–71.
52. Capp R, Horton CL, Takhar SS, et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med 2015 Jan 30.
53. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
54. The ProCESS Investigators. A ranodmized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683–93.
55. The ARISE Investigators and the ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496–506.
56. Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; online March 17, 2015.
57. Weingart SD, Sherwin RL, Emlet LL, et al. ED intensivists and ED intensive care units. Amer J Emerg Med 2013;31:617–20.
58. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA 2011;305:2175–85.
59. Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med 2014;189:548–55.
60. Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med 2014;190:665–74.
61. Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222–31.
62. Levy MM, Rhodes A, Phillips GS, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3–12.
63. Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med 2011;184:680–6.
64. Weant KA, Baker SN. Emergency medicine pharmacists and sepsis management. J Pharm Pract 2013;26:401–5.
65. Marwick CA, Guthrie B, Pringle JE, et al. A multifaceted intervention to improve sepsis management in general hospital wards with evaluation using segmented regression of interrupted time series. BMJ Qual Saf 2014;23:e2.
Management of Comorbid Sleep Disorders in Patients With PTSD
[This article originally published online ahead of print April 23, 2015.]
Sleep in the military has traditionally been thought of as a luxury and is sometimes considered at odds with optimal productivity. Every minute that a service member is asleep, he or she is not performing a primary duty, and getting a minimal amount of sleep is often seen as a badge of honor and strength. Research has recently been conducted, underscoring the importance of sleep management as an operational variable that must be accounted for in order to achieve optimal performance and promote resiliency. Both the quality and the duration of sleep must be considered, particularly given the increasingly complicated tasks that every service member must perform during both war and peace.
It has been well established that higher order mental tasks are the most vulnerable to sleep loss, as are those with little mental or physical stimulation, such as guard duty.1,2 Because service members are expected not only to perform in combat, but also to behave and operate ethically in spite of the challenges of war, the importance of adequate sleep must be considered. Many challenges are commonly encountered by service members when attempting to get adequate sleep (Table).3 This review highlights the recent diagnostic and treatment advances with respect to the overlap of sleep disorders and posttraumatic stress disorder (PTSD).
Culture of Sleep Loss
At the United States Military Academy in West Point, New York, a culture of poor sleep is instilled during initial military training; students typically get less than the recommended 7 to 8 hours of sleep per 24 hours.4,5 This sleep restriction continues for most of the time served on active duty: Military members get less sleep on average than does the rest of the U.S. population.6
Studies performed on pilots and during deployment have consistently shown a trend toward inadequate sleep, but only recently has inadequate sleep gained the attention of senior leadership.7,8 The Army Performance Triad, a public health campaign launched in 2013 by the Office of the U.S. Army Surgeon General, equally values sleep, nutrition, and activity. The goal of the Army Performance Triad is to influence behaviors by promoting healthy sleep, activity, and nutrition. Sleep is the apex of the Army Performance Triad.8
Those with chronic sleep restriction may not understand how impaired they are until objective testing is performed.9 In the civilian population, fatal sleep-related traffic accidents have been shown to exceed fatalities due to alcohol and illicit drug use combined.10 When poor sleep is combined with the trauma of war, symptoms exponentially worsen, and treatment becomes more complicated.11 Therefore, even before a formal sleep disorder or psychiatric condition develops, service members put themselves at risk by practicing poor sleep behaviors.11
Once insomnia develops, however, the potential negative health consequences are much more significant. Chronic insomnia, characterized by difficulty initiating or maintaining sleep or by waking too early, is the most common sleep disorder among adults. Thirty percent of adults experience occasional or transient insomnia, and between 9% and 12% of adults have severe chronic insomnia.12,13 This number is likely higher in the military and is much higher in those with PTSD.13
Related: How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
The etiology of chronic insomnia is multifactorial and is best conceptualized within a biopsychosocial framework. Physiologic abnormalities, such as increased activity in the central nervous system, hyperarousal of the hypothalamic-pituitary axis, and activation of proinflammatory cytokines, predispose individuals to developing insomnia. In addition, personality traits, such as anxious temperament or an internalizing stress-management style, make it more likely for individuals to respond negatively to stress, the most common precipitating cause of chronic insomnia.
Behavioral factors are also paramount. For example, individuals who experience acute sleep disturbance during deployment might develop maladaptive compensatory behaviors, such as spending excessive time in bed, “trying harder” to sleep, or overusing stimulants. These sleep behaviors can become a chronic condition.14
Comorbidities
Patients with insomnia are at increased risk for medical consequences, such as cardiovascular disease and mortality as well as psychiatric sequelae.15,16 Insomnia is also common among people who have attempted suicide.17 In the military, there was nearly a 20-fold increase in the rate of chronic insomnia among service members between 2000 and 2009, coincident with the dramatic uptick in operations tempo.18
Insomnia is one of the most common reports of returning Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans and is associated with the development of PTSD.19 Soldiers who reported symptoms of insomnia predeployment were more likely to develop anxiety, depression, and PTSD during deployment than were soldiers who did not report these symptoms.20
Empirically supported and evidence-based treatment options exist. Further, a robust evidence base supports the conclusion that treating insomnia improves not only sleep and quality of life (QOL), but also health-related outcomes in comorbid conditions, including depression, PTSD, chronic pain, and alcohol dependence.21-24 One historical barrier to effective treatment has been poor recognition of the scope of the problem. The army is looking to implement a more robust assessment of sleep in the primary care setting as part of the Army Performance Triad in order to intervene as early as possible. Other government organizations may also follow suit.
Although several FDA-approved medications for insomnia exist, the gold standard treatment for insomnia is cognitive behavioral therapy (CBT).25 Specific behavioral patient preferences that can be used to tailor treatment have been identified within a military population.26 Unfortunately, the most commonly used treatment for chronic insomnia in the military remains sedative- hypnotic medication. Multiple estimates suggest that 15% to 20% of all deployed service members have been prescribed a sedative-hypnotic to aid with sleep initiation, including many off-label antidepressants, antipsychotics, and antihistamines. Indeed, within VA, the use of quetiapine (an antipsychotic used off-label to treat insomnia) increased > 7-fold between 2001 and 2010, making it the second largest single drug expenditure in 2010. Many off-label medications have questionable risk-benefit ratios when used for sleep, and adverse effects can include infection,27 motor vehicle accidents,28 falls,29 and mortality.30 Further, some medications can limit deployability.
There are substantial challenges to incorporating behavioral approaches into the military armamentarium. There is a shortage of behavioral sleep specialists, although training initiatives seem promising.31 Most military facilities now have a medical home model of care with behavioral health providers as intrinsic team members. Their presence makes it easier to refer patients while reducing the stigma associated with behavioral health care. Leveraging technology will also facilitate the provision of quality, physician-directed insomnia treatment to an increasing number of military beneficiaries.
Nightmare Disorder
When patients with PTSD are able to get sleep, nightmares are a frequent occurrence and have been seen in up to 80% of individuals with this disorder.32 Nightmares usually occur during rapid eye movement (REM) sleep and are characterized by distressing dreams that threaten survival or security. They are often well remembered.33 After the nightmare, individuals typically wake up rapidly and report symptoms of distress, which can result in avoiding sleep (thereby perpetuating comorbid insomnia), daytime sleepiness, and fatigue.
Posttraumatic nightmares may have different dream mentation than do other disturbing dreams. The nightmare theme may involve actual events or reliving a prior traumatic experience. Most nightmares, however, have no associated movements or other complex behaviors, because during REM sleep, normal individuals are paralyzed, and thus do not move or act out their dreams.
Trauma-Associated Sleep Disorder
In some cases though, nightmares are accompanied by parasomnia activity.34 Parasomnias are abnormal and unintentional activities that occur during incomplete transitions between sleep stages and are seen more often in military personnel returning from deployment than in the general population. There is limited data regarding parasomnia activity in military personnel and veterans, although a study assessing sleep in 24 OIF/OEF veterans reported that 38% had either non-REM or REM parasomnia.34 Although in some instances these behaviors are simply a combination of genetics and insufficient sleep, in the majority of cases the clinical presentation is more complex.
In the authors’ clinical experience, patients described disruptive nocturnal behaviors (DNBs) which consisted of abnormal vocalizations (screaming, yelling), abnormal movements (tossing, turning, thrashing, sleep walking), or combative behaviors (striking the bed partner). These behaviors were strongly linked to symptoms of autonomic hyperarousal (night sweats, increased heart rate, or fast breathing). The DNBs often mimicked the content of the nightmares. The bed partner or spouse reported many of the cases after they had sustained unintended physical trauma from the combative behaviors.
Initially, REM behavior disorder (RBD) or nightmare disorder were considered potential diagnoses. However, RBD tends to occur in elderly males with neurodegenerative disorders (such as Parkinson disease). Dreams are relatively similar among patients with this disorder.35 Non-REM parasomnias are more common in young children and usually resolve prior to adolescence, although individuals who experienced parasomnias as children may see a reemergence during adulthood as a result of sleep fragmentation, medications, sleep-disordered breathing (SDB), recovery from sleep debt, or recreational drug or alcohol use.36,37
Since these posttraumatic nocturnal behaviors are not formally classified, a condition termed trauma-associated sleep disorder (TSD) was recently proposed.38 Trauma-associated sleep disorder is distinct from other parasomnias, because the onset must relate to a potentially traumatic event. On an overnight polysomnogram, increased muscle activity is seen during REM, and nightmares are almost invariably reported. Trauma-associated sleep disorder seems to involve not only DNB and traumatic dream enactment, but also insomnia and obstructive sleep apnea (OSA).
For patients who present with symptoms of TSD, a sleep study is recommended to evaluate for SDB as well as to characterize whether the patient has abnormal movements in REM sleep (lack of paralysis). There are currently no evidenced-based guidelines for treatment of this newly proposed sleep disorder. Behavioral and environmental modifications are the mainstay of treatment for individuals with any parasomnia. Obtaining an adequate quantity of sleep, avoiding triggers, and promoting a safe sleep environment are critical.
Substances that can lead to sleep fragmentation or impaired cognition, such as drugs and alcohol, should be avoided. Medical conditions that fragment sleep or cause nocturnal awakenings, such as sleep apnea, gastroesophageal reflux disease, and rhinitis should be treated to promote better sleep continuity.
When possible, medications with the potential to cause sleep fragmentation or disruption of normal sleep architecture should be reduced or discontinued. Weapons or objects that could be used as weapons should be removed from the bedroom, and padding should be placed on the sharp corners of furniture. Door and bed alarms, locks, and heavy curtains can minimize the risk of patients leaving the bedroom.
When these interventions are insufficient, medical therapy to suppress these events may be necessary. Some patients respond well to combined treatment with prazosin for nightmares and DNB, CBT for insomnia, and continuous positive airway pressure (CPAP) for OSA.39 Benzodiazepines, particularly clonazepam, may be effective for both slow-wave sleep parasomnias and RBD, but they should be used with caution in those with comorbid PTSD. Melatonin may also be effective, but there is a paucity of high-quality evidence supporting its use.
Obstructive Sleep Apnea
Another common sleep disorder that overlaps with PTSD is SDB. Obstructive sleep apnea is characterized by repetitive oxygen desaturations and arousals from sleep resulting from periodic upper airway collapse. Among middle-aged U.S. adults, about 9% of females and 24% of males have been estimated to have OSA, and rates increase with age and obesity.40 During the past decade, OSA in the military has risen dramatically, from 3,563 to 20,435 cases, with a 4-fold increase among those aged 20 to 24 years.17 Similar to the insomnia data, the increased rate of diagnosis during the recent wars in Southwest Asia coincides with an increase in the prevalence of traumatic brain injury (TBI) and PTSD. Additional reasons for the diagnostic increase may be heightened awareness of the diagnosis, increased availability of sleep disorders centers in the military, and even financial incentives for those undergoing a disability evaluation.
Obstructive sleep apnea is significantly more common in patients with PTSD compared with that in the general population, with rates of OSA ranging from 11.9% to 90%, depending on the study.41-43 Prevalence rates for OSA have been reported in several PTSD populations (violent crime, sexual assault, disasters, and combat). Military studies evaluating recent veterans have found OSA rates between 35% and 67%.44-46 In a recent study looking at SDB in those with PTSD, 53.8% had OSA (67.3% among those with polysomnograms).47 Although the other studies evaluated mixed populations of recent combat veterans, they were enriched for patients with PTSD.
Sleep disorders and PTSD have a “bidirectional” relationship.48 Sleep complaints preceding or temporally related to traumatic events increase the likelihood of subsequent mental health disorders, including PTSD.49-51 Sleep disorders are common in PTSD and are associated with symptoms of depression, relapse of depression, greater reductions in QOL, and suicide.52 Higher rates of OSA among patients who are not physically injured compared with the OSA rates of those with PTSD who also had physical injury (72.9% vs 38%) have also been seen, raising the possibility of different phenotypes of combat-related PTSD and a hypothetical role for premorbid OSA as a risk factor for PTSD.47
The pathophysiology linking SDB and PTSD is based on theories that poor sleep quality limits the ability to manage stress, promotes hyperarousal, confounds environmental stressors (trauma), and hinders the restorative qualities of sleep.49 Rapid eye movement sleep is believed to consolidate emotional memory, which may assist in recovery from traumatic events.53,54 Disrupted sleep architecture from OSA can diminish REM and hinder this process. Sleep fragmentation has been shown to cause upper airway instability and promote SDB.55 In addition, nighttime anxiety may induce hyperventilation with resultant hypocapnia, triggering apneic events.56 Taken together, disrupted sleep architecture, hyperarousal, respiratory instability, and nightmares may exacerbate one another and create a vicious cycle.57
Untreated OSA is associated with worse outcomes in PTSD. Continuous positive airway pressure has been shown to improve symptoms in this group.58-60 A study by Tamanna and colleagues evaluated clinical outcomes related to CPAP use, demonstrating improvements in nightmares, daytime sleepiness, and PTSD symptom severity with increasing adherence.61 Unfortunately, patients with PTSD generally have suboptimal medical adherence, and CPAP adherence decreases in psychiatric disease.62,63 Two recent studies have shown significantly lessened adherence in patients with both PTSD and OSA (compared with OSA alone), in both younger and older veteran populations.64,65 Limited insight and atypical clinical presentations of OSA also limit patient acceptance of treatment. Continuous positive airway pressure usage is decreased by comorbid insomnia, common in PTSD.66 Similarly, nightmares, mask discomfort, air hunger (the feeling of not being able to get a satisfying breath), and claustrophobia have all been associated with poor CPAP adherence in patients with PTSD.
Continuous positive airway pressure adherence is determined early (days to weeks), and initial use predicts long-term adherence.67-70 Patients are most likely to abandon therapy or fail to initiate therapy during this period. Given the potential adverse outcomes of comorbid mental illness and sleep disorders, including suicide, interventions should begin early.71 Continuous positive airway pressure devices with heated humidification, group education, peer success stories, and telephonic follow-up are all methods that improve adherence.72 There is conflicting evidence regarding the efficacy of nonbenzodiazepine sedative- hypnotics for improving diagnostic accuracy and CPAP adherence.73-76
Related: Using Light to Manage Sleep-Wake Issues in Patients With Dementia
Given this population’s high rate of comorbid insomnia, polypharmacy, and potentially pharmacotherapy refractory insomnia, the approach should be used cautiously in patients with PTSD OSA.77 Emerging efforts incorporate a biopsychosocial approach with an individualized focus on a patient’s unique barriers to adherence. Incorporating approaches such as motivational enhancement (for those ambivalent about change), educational approaches, and CBT may all be useful adjuncts.78-80
Ongoing VA trials have been designed to evaluate the impact of CPAP therapy on symptoms of PTSD and to compare CPAP and mandibular advancement devices with regards to efficacy in reducing the apneas and/or hypopneas per hour of sleep and improving symptoms.81,82
Discussion
Service members, like most adults, need about 8 hours of quality sleep per night to function at optimal levels and maximize operational readiness. The medical community is increasingly recognizing that sleep disturbances are inextricably linked to psychiatric disorders, particularly PTSD, depression, and anxiety.83,84 Balancing occupational performance and the demand of military missions with service member health remains a difficult leadership challenge.
Recent evidence suggests that disordered sleep may precede other PTSD symptom clusters.43,85 Sleep architecture in PTSD is disrupted, and abnormalities in both REM and non-REM sleep have been described.86,87 Insomnia not only is a component of depressive and anxiety disorders, but also impacts the course of disease severity.88 Sleep deprivation has been shown to be a risk factor for major depression in adolescents.89 In those with comorbid sleep problems, PTSD, and TBI, each disorder worsens QOL in an additive fashion.90
Severe mental illness impacts the military through a service member’s lost workdays, decreased productivity, impaired social relationships, and even suicide. Given that sleep quality is related to outcomes for patients with mental illnesses, access to medical professionals with specific training in sleep disorders becomes an integral part of a multidisciplinary approach to military health care. Encouragingly, treatment of insomnia and nightmares has been shown to improve PTSD symptom severity as well as headaches in veterans with mild TBI, even if neurologic deficits remain static.91 Similarly, treatment of insomnia is known to improve depressive symptoms in those with comorbid conditions.
Conclusion
The importance of sleep as a combat multiplier is increasingly recognized. The U.S. Army Surgeon General has acknowledged the interplay between inadequate sleep and impairments in other functional areas and placed specific emphasis on sleep as part of the Army Performance Triad. A core tenant of the Army Surgeon General’s message is that army medicine is on a mission to transform from a health care system to a system for health. The Army Wellness Centers, Army Medical Homes, Soldier-Centered Medical Homes, and embedded behavioral health are supporting the health of the force in these capacities. These functional areas treat behavioral health and sleep-related concerns across the continuum of disease from prevention, timely initial intervention once a condition has been identified, long-term treatment programs, and rehabilitative services.
Getting the proper quantity and quality of sleep, in addition to healthy activity and nutrition, increases readiness so that when called on to perform, soldiers are ready. A recent article by Wesensten and Balkin from the Walter Reed Army Institute of Research summarizes some guidelines for sleep from the Army Performance Triad Working Group to include sleep hygiene tips and judicious use of naps and caffeine.92 Efforts to improve soldier resiliency by improving sleep-related disorders have yet to be studied in a meaningful way, so additional research is needed to determine best practices and evidence-based guidelines.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
2. U.S. Department of the Army. Combat and Operational Stress Control Manual for Leaders and Soldiers. Washington, DC: Government Printing Office; 2009. FM 6-22.5.
3. Williams SG, Collen J, Wickwire E, Lettieri CJ, Mysliwiec V. The impact of sleep on soldier performance. Curr Psychiatry Rep. 2014;16(8):459.
4. Miller NL, Shattuck LG, Tvaryanas AP, Matsangas P. Effects of Sleep on Training Effectiveness in Soldiers at Fort Leonard Wood, Missouri. Monterey, CA: Naval Post Graduate School; 2010.
5. Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep. 2010;33(12):1623-1631.
6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. Fairfax, VA: Department of Defense; 2013.
7. Caldwell JL, Gilreath SR. Work and sleep hours of U.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166(2):159-166.
8. Mental Health Advisory Team 9. Mental Health Advisory Team 9 (MHAT 9), Operation Enduring Freedom (OEF) 2013 Afghanistan. Army Medicine Website. http://armymedicine.mil/Documents /MHAT_9_OEF_Report.pdf. Published October 10, 2013. Accessed March 3, 2015.
9. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126.
10. Czeisler CA. The Gordon Wilson lecture: work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159-188.
11. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11): 948-953.
12. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;229(suppl 2):S347-S353.
13. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484.
14. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
15. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4): 227-247.
16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213-218.
17. Sjöström N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep. 2007;30(1):91-95.
18. Armed Forces Health Surveillance Center. Insomnia, Active Component, U.S. Armed Forces, January 2000-December 2009 Medical Surveillance Monthly Report. 2010;17(5):12-15.
19. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759-762.
20. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
21. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495.
22. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327-341.
23. Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947-956.
24. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227-233.
25. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
26. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and p of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867-878.
27. Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-383.
28. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689-691.
29. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6.
30. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open. 2012;2:e000850.
31. Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917.
32. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
33. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
34. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12(9):850-859.
35. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. CCJM. 1990;57(suppl):S9-S23.
36. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(12):3494-3509.
37. Provini F, Piazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017-1031.
38. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-1148.
39. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
41. Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257-268.
42. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
43. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
44. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
45. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US Military Personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
46. Mysliwiec V, McGraw L, Pierce R, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
47. Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175-182.
48. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202.
49. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74.
50. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
51. Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240-1258.
52. Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Res. 2012;21(1):99-103.
53. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696-1701.
54. Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112-119.
55. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485.
56. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825-1832.
57. van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatology. 2012;3.
58. Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257-1270.
59. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298.
60. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379.
61. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
62. Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227-1232.
63. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260-273.
64. El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495-1500.
65. Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667-672.
66. Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772-776.
67. Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229-240.
68. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320-324.
69. Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138.
70. Pépin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124-1129.
71. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750.
72. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343-356.
73. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369-1376.
74. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263-1268.
75. Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310-1316.
76. Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696-702.
77. Mysliwiec V, Roth B. Pharmacotherapy refractory insomnia in soldiers with traumatic brain injury. Chest. 2013;143(2):582-583.
78. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep. 2013;36(11):1655-1662.
79. Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36(11):1647-1654.
80. Dickerson SS, Obeidat R, Dean G, et al. Development and usability testing of a self-management intervention to support individuals with obstructive sleep apnea in accommodating to CPAP treatment. Heart Lung. 2013;42(5):346-352.
81. Effects of CPAP therapy on PTSD symptoms. ClinicalTrials.gov Identifier NCT02019914. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT02019914. Updated September 3, 2014. Accessed March 4, 2015.
82. A randomized cross over trial of two treatments for obstructive sleep apnea in veterans with post-traumatic stress disorder. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT01535586. Updated February 16, 2012. Accessed March 4, 2015.
83. Babson KA, Boden MT, Woodward S, Alvarez J, Bonn-Miller M. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48-51.
84. Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126-132.
85. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474.
86. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
87. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382.
88. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9-15.
89. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239-244.
90. Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83-90.
91. Ruff RL, Riechers RG, Wang XF et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
92. Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013;109-118.
[This article originally published online ahead of print April 23, 2015.]
Sleep in the military has traditionally been thought of as a luxury and is sometimes considered at odds with optimal productivity. Every minute that a service member is asleep, he or she is not performing a primary duty, and getting a minimal amount of sleep is often seen as a badge of honor and strength. Research has recently been conducted, underscoring the importance of sleep management as an operational variable that must be accounted for in order to achieve optimal performance and promote resiliency. Both the quality and the duration of sleep must be considered, particularly given the increasingly complicated tasks that every service member must perform during both war and peace.
It has been well established that higher order mental tasks are the most vulnerable to sleep loss, as are those with little mental or physical stimulation, such as guard duty.1,2 Because service members are expected not only to perform in combat, but also to behave and operate ethically in spite of the challenges of war, the importance of adequate sleep must be considered. Many challenges are commonly encountered by service members when attempting to get adequate sleep (Table).3 This review highlights the recent diagnostic and treatment advances with respect to the overlap of sleep disorders and posttraumatic stress disorder (PTSD).
Culture of Sleep Loss
At the United States Military Academy in West Point, New York, a culture of poor sleep is instilled during initial military training; students typically get less than the recommended 7 to 8 hours of sleep per 24 hours.4,5 This sleep restriction continues for most of the time served on active duty: Military members get less sleep on average than does the rest of the U.S. population.6
Studies performed on pilots and during deployment have consistently shown a trend toward inadequate sleep, but only recently has inadequate sleep gained the attention of senior leadership.7,8 The Army Performance Triad, a public health campaign launched in 2013 by the Office of the U.S. Army Surgeon General, equally values sleep, nutrition, and activity. The goal of the Army Performance Triad is to influence behaviors by promoting healthy sleep, activity, and nutrition. Sleep is the apex of the Army Performance Triad.8
Those with chronic sleep restriction may not understand how impaired they are until objective testing is performed.9 In the civilian population, fatal sleep-related traffic accidents have been shown to exceed fatalities due to alcohol and illicit drug use combined.10 When poor sleep is combined with the trauma of war, symptoms exponentially worsen, and treatment becomes more complicated.11 Therefore, even before a formal sleep disorder or psychiatric condition develops, service members put themselves at risk by practicing poor sleep behaviors.11
Once insomnia develops, however, the potential negative health consequences are much more significant. Chronic insomnia, characterized by difficulty initiating or maintaining sleep or by waking too early, is the most common sleep disorder among adults. Thirty percent of adults experience occasional or transient insomnia, and between 9% and 12% of adults have severe chronic insomnia.12,13 This number is likely higher in the military and is much higher in those with PTSD.13
Related: How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
The etiology of chronic insomnia is multifactorial and is best conceptualized within a biopsychosocial framework. Physiologic abnormalities, such as increased activity in the central nervous system, hyperarousal of the hypothalamic-pituitary axis, and activation of proinflammatory cytokines, predispose individuals to developing insomnia. In addition, personality traits, such as anxious temperament or an internalizing stress-management style, make it more likely for individuals to respond negatively to stress, the most common precipitating cause of chronic insomnia.
Behavioral factors are also paramount. For example, individuals who experience acute sleep disturbance during deployment might develop maladaptive compensatory behaviors, such as spending excessive time in bed, “trying harder” to sleep, or overusing stimulants. These sleep behaviors can become a chronic condition.14
Comorbidities
Patients with insomnia are at increased risk for medical consequences, such as cardiovascular disease and mortality as well as psychiatric sequelae.15,16 Insomnia is also common among people who have attempted suicide.17 In the military, there was nearly a 20-fold increase in the rate of chronic insomnia among service members between 2000 and 2009, coincident with the dramatic uptick in operations tempo.18
Insomnia is one of the most common reports of returning Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans and is associated with the development of PTSD.19 Soldiers who reported symptoms of insomnia predeployment were more likely to develop anxiety, depression, and PTSD during deployment than were soldiers who did not report these symptoms.20
Empirically supported and evidence-based treatment options exist. Further, a robust evidence base supports the conclusion that treating insomnia improves not only sleep and quality of life (QOL), but also health-related outcomes in comorbid conditions, including depression, PTSD, chronic pain, and alcohol dependence.21-24 One historical barrier to effective treatment has been poor recognition of the scope of the problem. The army is looking to implement a more robust assessment of sleep in the primary care setting as part of the Army Performance Triad in order to intervene as early as possible. Other government organizations may also follow suit.
Although several FDA-approved medications for insomnia exist, the gold standard treatment for insomnia is cognitive behavioral therapy (CBT).25 Specific behavioral patient preferences that can be used to tailor treatment have been identified within a military population.26 Unfortunately, the most commonly used treatment for chronic insomnia in the military remains sedative- hypnotic medication. Multiple estimates suggest that 15% to 20% of all deployed service members have been prescribed a sedative-hypnotic to aid with sleep initiation, including many off-label antidepressants, antipsychotics, and antihistamines. Indeed, within VA, the use of quetiapine (an antipsychotic used off-label to treat insomnia) increased > 7-fold between 2001 and 2010, making it the second largest single drug expenditure in 2010. Many off-label medications have questionable risk-benefit ratios when used for sleep, and adverse effects can include infection,27 motor vehicle accidents,28 falls,29 and mortality.30 Further, some medications can limit deployability.
There are substantial challenges to incorporating behavioral approaches into the military armamentarium. There is a shortage of behavioral sleep specialists, although training initiatives seem promising.31 Most military facilities now have a medical home model of care with behavioral health providers as intrinsic team members. Their presence makes it easier to refer patients while reducing the stigma associated with behavioral health care. Leveraging technology will also facilitate the provision of quality, physician-directed insomnia treatment to an increasing number of military beneficiaries.
Nightmare Disorder
When patients with PTSD are able to get sleep, nightmares are a frequent occurrence and have been seen in up to 80% of individuals with this disorder.32 Nightmares usually occur during rapid eye movement (REM) sleep and are characterized by distressing dreams that threaten survival or security. They are often well remembered.33 After the nightmare, individuals typically wake up rapidly and report symptoms of distress, which can result in avoiding sleep (thereby perpetuating comorbid insomnia), daytime sleepiness, and fatigue.
Posttraumatic nightmares may have different dream mentation than do other disturbing dreams. The nightmare theme may involve actual events or reliving a prior traumatic experience. Most nightmares, however, have no associated movements or other complex behaviors, because during REM sleep, normal individuals are paralyzed, and thus do not move or act out their dreams.
Trauma-Associated Sleep Disorder
In some cases though, nightmares are accompanied by parasomnia activity.34 Parasomnias are abnormal and unintentional activities that occur during incomplete transitions between sleep stages and are seen more often in military personnel returning from deployment than in the general population. There is limited data regarding parasomnia activity in military personnel and veterans, although a study assessing sleep in 24 OIF/OEF veterans reported that 38% had either non-REM or REM parasomnia.34 Although in some instances these behaviors are simply a combination of genetics and insufficient sleep, in the majority of cases the clinical presentation is more complex.
In the authors’ clinical experience, patients described disruptive nocturnal behaviors (DNBs) which consisted of abnormal vocalizations (screaming, yelling), abnormal movements (tossing, turning, thrashing, sleep walking), or combative behaviors (striking the bed partner). These behaviors were strongly linked to symptoms of autonomic hyperarousal (night sweats, increased heart rate, or fast breathing). The DNBs often mimicked the content of the nightmares. The bed partner or spouse reported many of the cases after they had sustained unintended physical trauma from the combative behaviors.
Initially, REM behavior disorder (RBD) or nightmare disorder were considered potential diagnoses. However, RBD tends to occur in elderly males with neurodegenerative disorders (such as Parkinson disease). Dreams are relatively similar among patients with this disorder.35 Non-REM parasomnias are more common in young children and usually resolve prior to adolescence, although individuals who experienced parasomnias as children may see a reemergence during adulthood as a result of sleep fragmentation, medications, sleep-disordered breathing (SDB), recovery from sleep debt, or recreational drug or alcohol use.36,37
Since these posttraumatic nocturnal behaviors are not formally classified, a condition termed trauma-associated sleep disorder (TSD) was recently proposed.38 Trauma-associated sleep disorder is distinct from other parasomnias, because the onset must relate to a potentially traumatic event. On an overnight polysomnogram, increased muscle activity is seen during REM, and nightmares are almost invariably reported. Trauma-associated sleep disorder seems to involve not only DNB and traumatic dream enactment, but also insomnia and obstructive sleep apnea (OSA).
For patients who present with symptoms of TSD, a sleep study is recommended to evaluate for SDB as well as to characterize whether the patient has abnormal movements in REM sleep (lack of paralysis). There are currently no evidenced-based guidelines for treatment of this newly proposed sleep disorder. Behavioral and environmental modifications are the mainstay of treatment for individuals with any parasomnia. Obtaining an adequate quantity of sleep, avoiding triggers, and promoting a safe sleep environment are critical.
Substances that can lead to sleep fragmentation or impaired cognition, such as drugs and alcohol, should be avoided. Medical conditions that fragment sleep or cause nocturnal awakenings, such as sleep apnea, gastroesophageal reflux disease, and rhinitis should be treated to promote better sleep continuity.
When possible, medications with the potential to cause sleep fragmentation or disruption of normal sleep architecture should be reduced or discontinued. Weapons or objects that could be used as weapons should be removed from the bedroom, and padding should be placed on the sharp corners of furniture. Door and bed alarms, locks, and heavy curtains can minimize the risk of patients leaving the bedroom.
When these interventions are insufficient, medical therapy to suppress these events may be necessary. Some patients respond well to combined treatment with prazosin for nightmares and DNB, CBT for insomnia, and continuous positive airway pressure (CPAP) for OSA.39 Benzodiazepines, particularly clonazepam, may be effective for both slow-wave sleep parasomnias and RBD, but they should be used with caution in those with comorbid PTSD. Melatonin may also be effective, but there is a paucity of high-quality evidence supporting its use.
Obstructive Sleep Apnea
Another common sleep disorder that overlaps with PTSD is SDB. Obstructive sleep apnea is characterized by repetitive oxygen desaturations and arousals from sleep resulting from periodic upper airway collapse. Among middle-aged U.S. adults, about 9% of females and 24% of males have been estimated to have OSA, and rates increase with age and obesity.40 During the past decade, OSA in the military has risen dramatically, from 3,563 to 20,435 cases, with a 4-fold increase among those aged 20 to 24 years.17 Similar to the insomnia data, the increased rate of diagnosis during the recent wars in Southwest Asia coincides with an increase in the prevalence of traumatic brain injury (TBI) and PTSD. Additional reasons for the diagnostic increase may be heightened awareness of the diagnosis, increased availability of sleep disorders centers in the military, and even financial incentives for those undergoing a disability evaluation.
Obstructive sleep apnea is significantly more common in patients with PTSD compared with that in the general population, with rates of OSA ranging from 11.9% to 90%, depending on the study.41-43 Prevalence rates for OSA have been reported in several PTSD populations (violent crime, sexual assault, disasters, and combat). Military studies evaluating recent veterans have found OSA rates between 35% and 67%.44-46 In a recent study looking at SDB in those with PTSD, 53.8% had OSA (67.3% among those with polysomnograms).47 Although the other studies evaluated mixed populations of recent combat veterans, they were enriched for patients with PTSD.
Sleep disorders and PTSD have a “bidirectional” relationship.48 Sleep complaints preceding or temporally related to traumatic events increase the likelihood of subsequent mental health disorders, including PTSD.49-51 Sleep disorders are common in PTSD and are associated with symptoms of depression, relapse of depression, greater reductions in QOL, and suicide.52 Higher rates of OSA among patients who are not physically injured compared with the OSA rates of those with PTSD who also had physical injury (72.9% vs 38%) have also been seen, raising the possibility of different phenotypes of combat-related PTSD and a hypothetical role for premorbid OSA as a risk factor for PTSD.47
The pathophysiology linking SDB and PTSD is based on theories that poor sleep quality limits the ability to manage stress, promotes hyperarousal, confounds environmental stressors (trauma), and hinders the restorative qualities of sleep.49 Rapid eye movement sleep is believed to consolidate emotional memory, which may assist in recovery from traumatic events.53,54 Disrupted sleep architecture from OSA can diminish REM and hinder this process. Sleep fragmentation has been shown to cause upper airway instability and promote SDB.55 In addition, nighttime anxiety may induce hyperventilation with resultant hypocapnia, triggering apneic events.56 Taken together, disrupted sleep architecture, hyperarousal, respiratory instability, and nightmares may exacerbate one another and create a vicious cycle.57
Untreated OSA is associated with worse outcomes in PTSD. Continuous positive airway pressure has been shown to improve symptoms in this group.58-60 A study by Tamanna and colleagues evaluated clinical outcomes related to CPAP use, demonstrating improvements in nightmares, daytime sleepiness, and PTSD symptom severity with increasing adherence.61 Unfortunately, patients with PTSD generally have suboptimal medical adherence, and CPAP adherence decreases in psychiatric disease.62,63 Two recent studies have shown significantly lessened adherence in patients with both PTSD and OSA (compared with OSA alone), in both younger and older veteran populations.64,65 Limited insight and atypical clinical presentations of OSA also limit patient acceptance of treatment. Continuous positive airway pressure usage is decreased by comorbid insomnia, common in PTSD.66 Similarly, nightmares, mask discomfort, air hunger (the feeling of not being able to get a satisfying breath), and claustrophobia have all been associated with poor CPAP adherence in patients with PTSD.
Continuous positive airway pressure adherence is determined early (days to weeks), and initial use predicts long-term adherence.67-70 Patients are most likely to abandon therapy or fail to initiate therapy during this period. Given the potential adverse outcomes of comorbid mental illness and sleep disorders, including suicide, interventions should begin early.71 Continuous positive airway pressure devices with heated humidification, group education, peer success stories, and telephonic follow-up are all methods that improve adherence.72 There is conflicting evidence regarding the efficacy of nonbenzodiazepine sedative- hypnotics for improving diagnostic accuracy and CPAP adherence.73-76
Related: Using Light to Manage Sleep-Wake Issues in Patients With Dementia
Given this population’s high rate of comorbid insomnia, polypharmacy, and potentially pharmacotherapy refractory insomnia, the approach should be used cautiously in patients with PTSD OSA.77 Emerging efforts incorporate a biopsychosocial approach with an individualized focus on a patient’s unique barriers to adherence. Incorporating approaches such as motivational enhancement (for those ambivalent about change), educational approaches, and CBT may all be useful adjuncts.78-80
Ongoing VA trials have been designed to evaluate the impact of CPAP therapy on symptoms of PTSD and to compare CPAP and mandibular advancement devices with regards to efficacy in reducing the apneas and/or hypopneas per hour of sleep and improving symptoms.81,82
Discussion
Service members, like most adults, need about 8 hours of quality sleep per night to function at optimal levels and maximize operational readiness. The medical community is increasingly recognizing that sleep disturbances are inextricably linked to psychiatric disorders, particularly PTSD, depression, and anxiety.83,84 Balancing occupational performance and the demand of military missions with service member health remains a difficult leadership challenge.
Recent evidence suggests that disordered sleep may precede other PTSD symptom clusters.43,85 Sleep architecture in PTSD is disrupted, and abnormalities in both REM and non-REM sleep have been described.86,87 Insomnia not only is a component of depressive and anxiety disorders, but also impacts the course of disease severity.88 Sleep deprivation has been shown to be a risk factor for major depression in adolescents.89 In those with comorbid sleep problems, PTSD, and TBI, each disorder worsens QOL in an additive fashion.90
Severe mental illness impacts the military through a service member’s lost workdays, decreased productivity, impaired social relationships, and even suicide. Given that sleep quality is related to outcomes for patients with mental illnesses, access to medical professionals with specific training in sleep disorders becomes an integral part of a multidisciplinary approach to military health care. Encouragingly, treatment of insomnia and nightmares has been shown to improve PTSD symptom severity as well as headaches in veterans with mild TBI, even if neurologic deficits remain static.91 Similarly, treatment of insomnia is known to improve depressive symptoms in those with comorbid conditions.
Conclusion
The importance of sleep as a combat multiplier is increasingly recognized. The U.S. Army Surgeon General has acknowledged the interplay between inadequate sleep and impairments in other functional areas and placed specific emphasis on sleep as part of the Army Performance Triad. A core tenant of the Army Surgeon General’s message is that army medicine is on a mission to transform from a health care system to a system for health. The Army Wellness Centers, Army Medical Homes, Soldier-Centered Medical Homes, and embedded behavioral health are supporting the health of the force in these capacities. These functional areas treat behavioral health and sleep-related concerns across the continuum of disease from prevention, timely initial intervention once a condition has been identified, long-term treatment programs, and rehabilitative services.
Getting the proper quantity and quality of sleep, in addition to healthy activity and nutrition, increases readiness so that when called on to perform, soldiers are ready. A recent article by Wesensten and Balkin from the Walter Reed Army Institute of Research summarizes some guidelines for sleep from the Army Performance Triad Working Group to include sleep hygiene tips and judicious use of naps and caffeine.92 Efforts to improve soldier resiliency by improving sleep-related disorders have yet to be studied in a meaningful way, so additional research is needed to determine best practices and evidence-based guidelines.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[This article originally published online ahead of print April 23, 2015.]
Sleep in the military has traditionally been thought of as a luxury and is sometimes considered at odds with optimal productivity. Every minute that a service member is asleep, he or she is not performing a primary duty, and getting a minimal amount of sleep is often seen as a badge of honor and strength. Research has recently been conducted, underscoring the importance of sleep management as an operational variable that must be accounted for in order to achieve optimal performance and promote resiliency. Both the quality and the duration of sleep must be considered, particularly given the increasingly complicated tasks that every service member must perform during both war and peace.
It has been well established that higher order mental tasks are the most vulnerable to sleep loss, as are those with little mental or physical stimulation, such as guard duty.1,2 Because service members are expected not only to perform in combat, but also to behave and operate ethically in spite of the challenges of war, the importance of adequate sleep must be considered. Many challenges are commonly encountered by service members when attempting to get adequate sleep (Table).3 This review highlights the recent diagnostic and treatment advances with respect to the overlap of sleep disorders and posttraumatic stress disorder (PTSD).
Culture of Sleep Loss
At the United States Military Academy in West Point, New York, a culture of poor sleep is instilled during initial military training; students typically get less than the recommended 7 to 8 hours of sleep per 24 hours.4,5 This sleep restriction continues for most of the time served on active duty: Military members get less sleep on average than does the rest of the U.S. population.6
Studies performed on pilots and during deployment have consistently shown a trend toward inadequate sleep, but only recently has inadequate sleep gained the attention of senior leadership.7,8 The Army Performance Triad, a public health campaign launched in 2013 by the Office of the U.S. Army Surgeon General, equally values sleep, nutrition, and activity. The goal of the Army Performance Triad is to influence behaviors by promoting healthy sleep, activity, and nutrition. Sleep is the apex of the Army Performance Triad.8
Those with chronic sleep restriction may not understand how impaired they are until objective testing is performed.9 In the civilian population, fatal sleep-related traffic accidents have been shown to exceed fatalities due to alcohol and illicit drug use combined.10 When poor sleep is combined with the trauma of war, symptoms exponentially worsen, and treatment becomes more complicated.11 Therefore, even before a formal sleep disorder or psychiatric condition develops, service members put themselves at risk by practicing poor sleep behaviors.11
Once insomnia develops, however, the potential negative health consequences are much more significant. Chronic insomnia, characterized by difficulty initiating or maintaining sleep or by waking too early, is the most common sleep disorder among adults. Thirty percent of adults experience occasional or transient insomnia, and between 9% and 12% of adults have severe chronic insomnia.12,13 This number is likely higher in the military and is much higher in those with PTSD.13
Related: How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
The etiology of chronic insomnia is multifactorial and is best conceptualized within a biopsychosocial framework. Physiologic abnormalities, such as increased activity in the central nervous system, hyperarousal of the hypothalamic-pituitary axis, and activation of proinflammatory cytokines, predispose individuals to developing insomnia. In addition, personality traits, such as anxious temperament or an internalizing stress-management style, make it more likely for individuals to respond negatively to stress, the most common precipitating cause of chronic insomnia.
Behavioral factors are also paramount. For example, individuals who experience acute sleep disturbance during deployment might develop maladaptive compensatory behaviors, such as spending excessive time in bed, “trying harder” to sleep, or overusing stimulants. These sleep behaviors can become a chronic condition.14
Comorbidities
Patients with insomnia are at increased risk for medical consequences, such as cardiovascular disease and mortality as well as psychiatric sequelae.15,16 Insomnia is also common among people who have attempted suicide.17 In the military, there was nearly a 20-fold increase in the rate of chronic insomnia among service members between 2000 and 2009, coincident with the dramatic uptick in operations tempo.18
Insomnia is one of the most common reports of returning Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans and is associated with the development of PTSD.19 Soldiers who reported symptoms of insomnia predeployment were more likely to develop anxiety, depression, and PTSD during deployment than were soldiers who did not report these symptoms.20
Empirically supported and evidence-based treatment options exist. Further, a robust evidence base supports the conclusion that treating insomnia improves not only sleep and quality of life (QOL), but also health-related outcomes in comorbid conditions, including depression, PTSD, chronic pain, and alcohol dependence.21-24 One historical barrier to effective treatment has been poor recognition of the scope of the problem. The army is looking to implement a more robust assessment of sleep in the primary care setting as part of the Army Performance Triad in order to intervene as early as possible. Other government organizations may also follow suit.
Although several FDA-approved medications for insomnia exist, the gold standard treatment for insomnia is cognitive behavioral therapy (CBT).25 Specific behavioral patient preferences that can be used to tailor treatment have been identified within a military population.26 Unfortunately, the most commonly used treatment for chronic insomnia in the military remains sedative- hypnotic medication. Multiple estimates suggest that 15% to 20% of all deployed service members have been prescribed a sedative-hypnotic to aid with sleep initiation, including many off-label antidepressants, antipsychotics, and antihistamines. Indeed, within VA, the use of quetiapine (an antipsychotic used off-label to treat insomnia) increased > 7-fold between 2001 and 2010, making it the second largest single drug expenditure in 2010. Many off-label medications have questionable risk-benefit ratios when used for sleep, and adverse effects can include infection,27 motor vehicle accidents,28 falls,29 and mortality.30 Further, some medications can limit deployability.
There are substantial challenges to incorporating behavioral approaches into the military armamentarium. There is a shortage of behavioral sleep specialists, although training initiatives seem promising.31 Most military facilities now have a medical home model of care with behavioral health providers as intrinsic team members. Their presence makes it easier to refer patients while reducing the stigma associated with behavioral health care. Leveraging technology will also facilitate the provision of quality, physician-directed insomnia treatment to an increasing number of military beneficiaries.
Nightmare Disorder
When patients with PTSD are able to get sleep, nightmares are a frequent occurrence and have been seen in up to 80% of individuals with this disorder.32 Nightmares usually occur during rapid eye movement (REM) sleep and are characterized by distressing dreams that threaten survival or security. They are often well remembered.33 After the nightmare, individuals typically wake up rapidly and report symptoms of distress, which can result in avoiding sleep (thereby perpetuating comorbid insomnia), daytime sleepiness, and fatigue.
Posttraumatic nightmares may have different dream mentation than do other disturbing dreams. The nightmare theme may involve actual events or reliving a prior traumatic experience. Most nightmares, however, have no associated movements or other complex behaviors, because during REM sleep, normal individuals are paralyzed, and thus do not move or act out their dreams.
Trauma-Associated Sleep Disorder
In some cases though, nightmares are accompanied by parasomnia activity.34 Parasomnias are abnormal and unintentional activities that occur during incomplete transitions between sleep stages and are seen more often in military personnel returning from deployment than in the general population. There is limited data regarding parasomnia activity in military personnel and veterans, although a study assessing sleep in 24 OIF/OEF veterans reported that 38% had either non-REM or REM parasomnia.34 Although in some instances these behaviors are simply a combination of genetics and insufficient sleep, in the majority of cases the clinical presentation is more complex.
In the authors’ clinical experience, patients described disruptive nocturnal behaviors (DNBs) which consisted of abnormal vocalizations (screaming, yelling), abnormal movements (tossing, turning, thrashing, sleep walking), or combative behaviors (striking the bed partner). These behaviors were strongly linked to symptoms of autonomic hyperarousal (night sweats, increased heart rate, or fast breathing). The DNBs often mimicked the content of the nightmares. The bed partner or spouse reported many of the cases after they had sustained unintended physical trauma from the combative behaviors.
Initially, REM behavior disorder (RBD) or nightmare disorder were considered potential diagnoses. However, RBD tends to occur in elderly males with neurodegenerative disorders (such as Parkinson disease). Dreams are relatively similar among patients with this disorder.35 Non-REM parasomnias are more common in young children and usually resolve prior to adolescence, although individuals who experienced parasomnias as children may see a reemergence during adulthood as a result of sleep fragmentation, medications, sleep-disordered breathing (SDB), recovery from sleep debt, or recreational drug or alcohol use.36,37
Since these posttraumatic nocturnal behaviors are not formally classified, a condition termed trauma-associated sleep disorder (TSD) was recently proposed.38 Trauma-associated sleep disorder is distinct from other parasomnias, because the onset must relate to a potentially traumatic event. On an overnight polysomnogram, increased muscle activity is seen during REM, and nightmares are almost invariably reported. Trauma-associated sleep disorder seems to involve not only DNB and traumatic dream enactment, but also insomnia and obstructive sleep apnea (OSA).
For patients who present with symptoms of TSD, a sleep study is recommended to evaluate for SDB as well as to characterize whether the patient has abnormal movements in REM sleep (lack of paralysis). There are currently no evidenced-based guidelines for treatment of this newly proposed sleep disorder. Behavioral and environmental modifications are the mainstay of treatment for individuals with any parasomnia. Obtaining an adequate quantity of sleep, avoiding triggers, and promoting a safe sleep environment are critical.
Substances that can lead to sleep fragmentation or impaired cognition, such as drugs and alcohol, should be avoided. Medical conditions that fragment sleep or cause nocturnal awakenings, such as sleep apnea, gastroesophageal reflux disease, and rhinitis should be treated to promote better sleep continuity.
When possible, medications with the potential to cause sleep fragmentation or disruption of normal sleep architecture should be reduced or discontinued. Weapons or objects that could be used as weapons should be removed from the bedroom, and padding should be placed on the sharp corners of furniture. Door and bed alarms, locks, and heavy curtains can minimize the risk of patients leaving the bedroom.
When these interventions are insufficient, medical therapy to suppress these events may be necessary. Some patients respond well to combined treatment with prazosin for nightmares and DNB, CBT for insomnia, and continuous positive airway pressure (CPAP) for OSA.39 Benzodiazepines, particularly clonazepam, may be effective for both slow-wave sleep parasomnias and RBD, but they should be used with caution in those with comorbid PTSD. Melatonin may also be effective, but there is a paucity of high-quality evidence supporting its use.
Obstructive Sleep Apnea
Another common sleep disorder that overlaps with PTSD is SDB. Obstructive sleep apnea is characterized by repetitive oxygen desaturations and arousals from sleep resulting from periodic upper airway collapse. Among middle-aged U.S. adults, about 9% of females and 24% of males have been estimated to have OSA, and rates increase with age and obesity.40 During the past decade, OSA in the military has risen dramatically, from 3,563 to 20,435 cases, with a 4-fold increase among those aged 20 to 24 years.17 Similar to the insomnia data, the increased rate of diagnosis during the recent wars in Southwest Asia coincides with an increase in the prevalence of traumatic brain injury (TBI) and PTSD. Additional reasons for the diagnostic increase may be heightened awareness of the diagnosis, increased availability of sleep disorders centers in the military, and even financial incentives for those undergoing a disability evaluation.
Obstructive sleep apnea is significantly more common in patients with PTSD compared with that in the general population, with rates of OSA ranging from 11.9% to 90%, depending on the study.41-43 Prevalence rates for OSA have been reported in several PTSD populations (violent crime, sexual assault, disasters, and combat). Military studies evaluating recent veterans have found OSA rates between 35% and 67%.44-46 In a recent study looking at SDB in those with PTSD, 53.8% had OSA (67.3% among those with polysomnograms).47 Although the other studies evaluated mixed populations of recent combat veterans, they were enriched for patients with PTSD.
Sleep disorders and PTSD have a “bidirectional” relationship.48 Sleep complaints preceding or temporally related to traumatic events increase the likelihood of subsequent mental health disorders, including PTSD.49-51 Sleep disorders are common in PTSD and are associated with symptoms of depression, relapse of depression, greater reductions in QOL, and suicide.52 Higher rates of OSA among patients who are not physically injured compared with the OSA rates of those with PTSD who also had physical injury (72.9% vs 38%) have also been seen, raising the possibility of different phenotypes of combat-related PTSD and a hypothetical role for premorbid OSA as a risk factor for PTSD.47
The pathophysiology linking SDB and PTSD is based on theories that poor sleep quality limits the ability to manage stress, promotes hyperarousal, confounds environmental stressors (trauma), and hinders the restorative qualities of sleep.49 Rapid eye movement sleep is believed to consolidate emotional memory, which may assist in recovery from traumatic events.53,54 Disrupted sleep architecture from OSA can diminish REM and hinder this process. Sleep fragmentation has been shown to cause upper airway instability and promote SDB.55 In addition, nighttime anxiety may induce hyperventilation with resultant hypocapnia, triggering apneic events.56 Taken together, disrupted sleep architecture, hyperarousal, respiratory instability, and nightmares may exacerbate one another and create a vicious cycle.57
Untreated OSA is associated with worse outcomes in PTSD. Continuous positive airway pressure has been shown to improve symptoms in this group.58-60 A study by Tamanna and colleagues evaluated clinical outcomes related to CPAP use, demonstrating improvements in nightmares, daytime sleepiness, and PTSD symptom severity with increasing adherence.61 Unfortunately, patients with PTSD generally have suboptimal medical adherence, and CPAP adherence decreases in psychiatric disease.62,63 Two recent studies have shown significantly lessened adherence in patients with both PTSD and OSA (compared with OSA alone), in both younger and older veteran populations.64,65 Limited insight and atypical clinical presentations of OSA also limit patient acceptance of treatment. Continuous positive airway pressure usage is decreased by comorbid insomnia, common in PTSD.66 Similarly, nightmares, mask discomfort, air hunger (the feeling of not being able to get a satisfying breath), and claustrophobia have all been associated with poor CPAP adherence in patients with PTSD.
Continuous positive airway pressure adherence is determined early (days to weeks), and initial use predicts long-term adherence.67-70 Patients are most likely to abandon therapy or fail to initiate therapy during this period. Given the potential adverse outcomes of comorbid mental illness and sleep disorders, including suicide, interventions should begin early.71 Continuous positive airway pressure devices with heated humidification, group education, peer success stories, and telephonic follow-up are all methods that improve adherence.72 There is conflicting evidence regarding the efficacy of nonbenzodiazepine sedative- hypnotics for improving diagnostic accuracy and CPAP adherence.73-76
Related: Using Light to Manage Sleep-Wake Issues in Patients With Dementia
Given this population’s high rate of comorbid insomnia, polypharmacy, and potentially pharmacotherapy refractory insomnia, the approach should be used cautiously in patients with PTSD OSA.77 Emerging efforts incorporate a biopsychosocial approach with an individualized focus on a patient’s unique barriers to adherence. Incorporating approaches such as motivational enhancement (for those ambivalent about change), educational approaches, and CBT may all be useful adjuncts.78-80
Ongoing VA trials have been designed to evaluate the impact of CPAP therapy on symptoms of PTSD and to compare CPAP and mandibular advancement devices with regards to efficacy in reducing the apneas and/or hypopneas per hour of sleep and improving symptoms.81,82
Discussion
Service members, like most adults, need about 8 hours of quality sleep per night to function at optimal levels and maximize operational readiness. The medical community is increasingly recognizing that sleep disturbances are inextricably linked to psychiatric disorders, particularly PTSD, depression, and anxiety.83,84 Balancing occupational performance and the demand of military missions with service member health remains a difficult leadership challenge.
Recent evidence suggests that disordered sleep may precede other PTSD symptom clusters.43,85 Sleep architecture in PTSD is disrupted, and abnormalities in both REM and non-REM sleep have been described.86,87 Insomnia not only is a component of depressive and anxiety disorders, but also impacts the course of disease severity.88 Sleep deprivation has been shown to be a risk factor for major depression in adolescents.89 In those with comorbid sleep problems, PTSD, and TBI, each disorder worsens QOL in an additive fashion.90
Severe mental illness impacts the military through a service member’s lost workdays, decreased productivity, impaired social relationships, and even suicide. Given that sleep quality is related to outcomes for patients with mental illnesses, access to medical professionals with specific training in sleep disorders becomes an integral part of a multidisciplinary approach to military health care. Encouragingly, treatment of insomnia and nightmares has been shown to improve PTSD symptom severity as well as headaches in veterans with mild TBI, even if neurologic deficits remain static.91 Similarly, treatment of insomnia is known to improve depressive symptoms in those with comorbid conditions.
Conclusion
The importance of sleep as a combat multiplier is increasingly recognized. The U.S. Army Surgeon General has acknowledged the interplay between inadequate sleep and impairments in other functional areas and placed specific emphasis on sleep as part of the Army Performance Triad. A core tenant of the Army Surgeon General’s message is that army medicine is on a mission to transform from a health care system to a system for health. The Army Wellness Centers, Army Medical Homes, Soldier-Centered Medical Homes, and embedded behavioral health are supporting the health of the force in these capacities. These functional areas treat behavioral health and sleep-related concerns across the continuum of disease from prevention, timely initial intervention once a condition has been identified, long-term treatment programs, and rehabilitative services.
Getting the proper quantity and quality of sleep, in addition to healthy activity and nutrition, increases readiness so that when called on to perform, soldiers are ready. A recent article by Wesensten and Balkin from the Walter Reed Army Institute of Research summarizes some guidelines for sleep from the Army Performance Triad Working Group to include sleep hygiene tips and judicious use of naps and caffeine.92 Efforts to improve soldier resiliency by improving sleep-related disorders have yet to be studied in a meaningful way, so additional research is needed to determine best practices and evidence-based guidelines.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
2. U.S. Department of the Army. Combat and Operational Stress Control Manual for Leaders and Soldiers. Washington, DC: Government Printing Office; 2009. FM 6-22.5.
3. Williams SG, Collen J, Wickwire E, Lettieri CJ, Mysliwiec V. The impact of sleep on soldier performance. Curr Psychiatry Rep. 2014;16(8):459.
4. Miller NL, Shattuck LG, Tvaryanas AP, Matsangas P. Effects of Sleep on Training Effectiveness in Soldiers at Fort Leonard Wood, Missouri. Monterey, CA: Naval Post Graduate School; 2010.
5. Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep. 2010;33(12):1623-1631.
6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. Fairfax, VA: Department of Defense; 2013.
7. Caldwell JL, Gilreath SR. Work and sleep hours of U.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166(2):159-166.
8. Mental Health Advisory Team 9. Mental Health Advisory Team 9 (MHAT 9), Operation Enduring Freedom (OEF) 2013 Afghanistan. Army Medicine Website. http://armymedicine.mil/Documents /MHAT_9_OEF_Report.pdf. Published October 10, 2013. Accessed March 3, 2015.
9. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126.
10. Czeisler CA. The Gordon Wilson lecture: work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159-188.
11. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11): 948-953.
12. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;229(suppl 2):S347-S353.
13. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484.
14. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
15. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4): 227-247.
16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213-218.
17. Sjöström N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep. 2007;30(1):91-95.
18. Armed Forces Health Surveillance Center. Insomnia, Active Component, U.S. Armed Forces, January 2000-December 2009 Medical Surveillance Monthly Report. 2010;17(5):12-15.
19. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759-762.
20. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
21. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495.
22. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327-341.
23. Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947-956.
24. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227-233.
25. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
26. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and p of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867-878.
27. Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-383.
28. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689-691.
29. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6.
30. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open. 2012;2:e000850.
31. Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917.
32. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
33. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
34. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12(9):850-859.
35. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. CCJM. 1990;57(suppl):S9-S23.
36. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(12):3494-3509.
37. Provini F, Piazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017-1031.
38. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-1148.
39. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
41. Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257-268.
42. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
43. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
44. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
45. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US Military Personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
46. Mysliwiec V, McGraw L, Pierce R, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
47. Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175-182.
48. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202.
49. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74.
50. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
51. Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240-1258.
52. Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Res. 2012;21(1):99-103.
53. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696-1701.
54. Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112-119.
55. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485.
56. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825-1832.
57. van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatology. 2012;3.
58. Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257-1270.
59. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298.
60. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379.
61. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
62. Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227-1232.
63. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260-273.
64. El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495-1500.
65. Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667-672.
66. Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772-776.
67. Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229-240.
68. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320-324.
69. Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138.
70. Pépin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124-1129.
71. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750.
72. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343-356.
73. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369-1376.
74. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263-1268.
75. Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310-1316.
76. Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696-702.
77. Mysliwiec V, Roth B. Pharmacotherapy refractory insomnia in soldiers with traumatic brain injury. Chest. 2013;143(2):582-583.
78. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep. 2013;36(11):1655-1662.
79. Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36(11):1647-1654.
80. Dickerson SS, Obeidat R, Dean G, et al. Development and usability testing of a self-management intervention to support individuals with obstructive sleep apnea in accommodating to CPAP treatment. Heart Lung. 2013;42(5):346-352.
81. Effects of CPAP therapy on PTSD symptoms. ClinicalTrials.gov Identifier NCT02019914. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT02019914. Updated September 3, 2014. Accessed March 4, 2015.
82. A randomized cross over trial of two treatments for obstructive sleep apnea in veterans with post-traumatic stress disorder. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT01535586. Updated February 16, 2012. Accessed March 4, 2015.
83. Babson KA, Boden MT, Woodward S, Alvarez J, Bonn-Miller M. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48-51.
84. Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126-132.
85. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474.
86. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
87. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382.
88. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9-15.
89. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239-244.
90. Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83-90.
91. Ruff RL, Riechers RG, Wang XF et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
92. Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013;109-118.
1. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
2. U.S. Department of the Army. Combat and Operational Stress Control Manual for Leaders and Soldiers. Washington, DC: Government Printing Office; 2009. FM 6-22.5.
3. Williams SG, Collen J, Wickwire E, Lettieri CJ, Mysliwiec V. The impact of sleep on soldier performance. Curr Psychiatry Rep. 2014;16(8):459.
4. Miller NL, Shattuck LG, Tvaryanas AP, Matsangas P. Effects of Sleep on Training Effectiveness in Soldiers at Fort Leonard Wood, Missouri. Monterey, CA: Naval Post Graduate School; 2010.
5. Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep. 2010;33(12):1623-1631.
6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. Fairfax, VA: Department of Defense; 2013.
7. Caldwell JL, Gilreath SR. Work and sleep hours of U.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166(2):159-166.
8. Mental Health Advisory Team 9. Mental Health Advisory Team 9 (MHAT 9), Operation Enduring Freedom (OEF) 2013 Afghanistan. Army Medicine Website. http://armymedicine.mil/Documents /MHAT_9_OEF_Report.pdf. Published October 10, 2013. Accessed March 3, 2015.
9. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126.
10. Czeisler CA. The Gordon Wilson lecture: work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159-188.
11. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11): 948-953.
12. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;229(suppl 2):S347-S353.
13. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484.
14. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
15. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4): 227-247.
16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213-218.
17. Sjöström N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep. 2007;30(1):91-95.
18. Armed Forces Health Surveillance Center. Insomnia, Active Component, U.S. Armed Forces, January 2000-December 2009 Medical Surveillance Monthly Report. 2010;17(5):12-15.
19. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759-762.
20. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
21. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495.
22. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327-341.
23. Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947-956.
24. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227-233.
25. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
26. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and p of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867-878.
27. Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-383.
28. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689-691.
29. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6.
30. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open. 2012;2:e000850.
31. Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917.
32. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
33. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
34. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12(9):850-859.
35. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. CCJM. 1990;57(suppl):S9-S23.
36. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(12):3494-3509.
37. Provini F, Piazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017-1031.
38. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-1148.
39. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
41. Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257-268.
42. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
43. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
44. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
45. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US Military Personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
46. Mysliwiec V, McGraw L, Pierce R, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
47. Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175-182.
48. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202.
49. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74.
50. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
51. Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240-1258.
52. Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Res. 2012;21(1):99-103.
53. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696-1701.
54. Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112-119.
55. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485.
56. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825-1832.
57. van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatology. 2012;3.
58. Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257-1270.
59. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298.
60. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379.
61. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
62. Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227-1232.
63. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260-273.
64. El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495-1500.
65. Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667-672.
66. Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772-776.
67. Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229-240.
68. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320-324.
69. Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138.
70. Pépin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124-1129.
71. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750.
72. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343-356.
73. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369-1376.
74. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263-1268.
75. Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310-1316.
76. Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696-702.
77. Mysliwiec V, Roth B. Pharmacotherapy refractory insomnia in soldiers with traumatic brain injury. Chest. 2013;143(2):582-583.
78. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep. 2013;36(11):1655-1662.
79. Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36(11):1647-1654.
80. Dickerson SS, Obeidat R, Dean G, et al. Development and usability testing of a self-management intervention to support individuals with obstructive sleep apnea in accommodating to CPAP treatment. Heart Lung. 2013;42(5):346-352.
81. Effects of CPAP therapy on PTSD symptoms. ClinicalTrials.gov Identifier NCT02019914. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT02019914. Updated September 3, 2014. Accessed March 4, 2015.
82. A randomized cross over trial of two treatments for obstructive sleep apnea in veterans with post-traumatic stress disorder. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT01535586. Updated February 16, 2012. Accessed March 4, 2015.
83. Babson KA, Boden MT, Woodward S, Alvarez J, Bonn-Miller M. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48-51.
84. Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126-132.
85. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474.
86. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
87. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382.
88. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9-15.
89. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239-244.
90. Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83-90.
91. Ruff RL, Riechers RG, Wang XF et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
92. Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013;109-118.
Young Man With Headache, Confusion, and Hearing Loss
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
Insights and Implications of the VA Rheumatoid Arthritis Registry
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
Using Quality Indicators to Assess and Improve Human Research Protection Programs at the VA
Protection of human subjects participating in research is critically important during this era of rapid medical progress and the increasing emphasis on translating discoveries from basic science research into clinical practices. The Federal Policy for the Protection of Human Subjects, also known as the Common Rule, was established based on the Belmont Report’s ethical principles of respect for persons, beneficence, and justice.1,2 Under the Common Rule, institutional review boards (IRBs) are responsible for reviewing and approving human research protocols and providing oversight to ensure protection of human research subjects.1
In addition to IRBs, investigators, institutions, research volunteers, sponsors of research, and the federal government share responsibilities for protecting research subjects.3 Institutions conducting research involving human subjects have thus established operational frameworks, referred to as human research protection programs (HRPPs), to ensure the rights and welfare of research participants and to meet the ethical and regulatory requirements.3,4
Related: Empathic Disclosure of Adverse Events to Patients
In the late 1990s and early 2000s, a number of major academic institutions’ federally supported research programs were suspended due to persistent noncompliance with federal regulations, including some issues that resulted in the death of healthy volunteers.5,6 In response to increased public scrutiny of clinical research, considerable efforts have been made to improve the protection of research subjects.5,7-9 These efforts included stronger federal oversight of research, voluntary accreditation of institutional HRPPs, increased institutional support for HRPPs, improved training for investigators and IRB members, improved monitoring and reporting of adverse events (AEs), and greater involvement of research participants and the public.9
Despite considerable investment to improve research subject protections, scant data exist showing that these efforts have made human research safer than before. Although research subject protection cannot be directly measured, quality assessment of HRPPs is possible. High-quality HRPPs are expected to minimize risk to research participants to the extent possible while maintaining the integrity of the research.10
Related: Improving Veteran Access to Clinical Trials
The VA health care system is the largest integrated health care system in the country. Currently, there are 107 VA facilities conducting research involving human subjects. In addition to federal regulations governing research with human subjects, VA researchers must also comply with requirements established by the VA. For example, in the VA the IRB is a subcommittee of the research and development committee (R&DC). Research involving human subjects may not be initiated until approved by both the IRB and the R&DC.4,11 All VA investigators are required to have approved research scopes of practice and training in ethical principles and current good clinical practices.4
Recently, the VA Office of Research Oversight (VAORO) developed a set of indicators for assessing the quality of VA HRPPs.10 Since 2010, VAORO has been collecting quality indicator (QI) data from all VA research facilities for quality improvement purposes.12-14 In this study, VAORO analyzed these data to assess changes in VA HRPP QI data from 2010 to 2012 and identify areas for improvement.
Methods
As part of the VA HRPP quality assurance program, each VA research facility was required to conduct annual audits of all informed consent documents (ICDs) and regulatory audits of all human research protocols once every 3 years by qualified research compliance officers (RCOs).15 Protocol regulatory audits were limited to a 3-year look back of the protocols. Tools were developed for the annual ICD and triennial protocol regulatory audits (available at http://www.va.gov/ORO/Research_Compliance_Education.asp). Facility RCOs were then trained to use these tools to conduct audits.
Data Collection
Data were collected annually from all 107 VA research facilities. Information collected included compliance with ICD and Health Insurance Portability and Accountability Act authorization requirements; compliance with requirements for IRB and R&DC initial approval of human research protocols; compliance with selected informed consent requirements; for-cause suspension or termination of human research protocols; research-related serious AEs; compliance with continuing review requirements; subject enrollment according to inclusion and exclusion criteria; research personnel scopes of practice; investigator human research protection training; international research; and research involving vulnerable subjects. No individually identifiable personal information was collected. As this was a VA quality assurance project and no individually identifiable information was collected, no IRB review and approval of the project was required.16
All data collected were entered into a database for analysis. When necessary, facilities were contacted to verify the accuracy and uniformity of data reported.
Data Analysis
The Mantel-Haenszel chi-square test for trend was used to determine the trend of changes from 2010 through 2012.17 A P value of < .05 was considered to be statistically significant. For those QIs with statistically significant changes, VAORO calculated the percent changes and the actual numbers impacted, ie, the actual numbers of ICDs, human research protocols, case histories, or research personnel affected by these changes.18
Results
The HRPP QI data was collected from 2010 through 2012 from all 107 VA research facilities (Table 1). There were a total of 25 QIs; 18 had all 3-year data available and 7 lacked 2010 data. Only those 18 QI data available from all 3 years were included for this analysis. The 2010 data collected for QIs related to for-cause suspension or termination of protocols and research personnel scopes of practice and training requirements were derived from all human, animal, and safety research protocols audited, not just the human research protocols audited. However, these data were included for comparison with the 2011 and 2012 data, because nonhuman research protocols audited constituted < 30% of the total. Based on VAORO on-site routine reviews of facilities’ HRPPs, animal care and use programs, as well as research safety and security programs, the authors believe that the QI rates in these nonhuman research protocols were similar to those of human research protocols.
From a total of 18 QIs with all 3-year data available for analysis, 9 QIs did not show any statistically significant changes; whereas 9 QIs showed statistically significant changes from 2010 to 2012 (Table 1). These 9 QIs were: (1) incorrect ICDs used; (2) number of protocols suspended or terminated due to cause; (3) protocols suspended or terminated due to investigator concerns; (4) informed consent not obtained prior to initiation of the study; (5) research personnel without research scopes of practice; (6) research personnel working outside of scopes of practice; (7) required training not current for research personnel; (8) research personnel working without initial training; and (9) research personnel lapsed in continuing training.
Table 2 shows the percent changes and the actual numbers impacted by the changes in the 9 QIs that showed statistically significant changes. The percent changes describe the magnitude of changes, and the numbers impacted provide information on the actual numbers of events (ie, ICDs, human research protocols, case histories, or research personnel) affected by these changes in 2012 if the QI rates had stayed the same as those of 2010.
All 9 QIs with statistically significant changes showed improvement, ranging from 25% improvement in incorrect ICDs used to 92% improvement in research personnel without scopes of practice (Table 2). The actual numbers impacted (ie, the difference between numbers expected in 2012 based on 2010 QI rates and the actual numbers observed in 2012) ranged from 55 protocols suspended or terminated for cause to 1,177 research scopes of practice.
Of the 9 QIs with no statistically significant changes, all but 2 QIs had QI rates of < 1% in 2010, suggesting that these QI rates were already so low that further improvement was difficult to achieve. The 2 exceptions were lapses in IRB continuing reviews and international research conducted without VA Chief Research and Development Officer (CRADO) approval.
The rates of lapse in IRB continuing reviews remained high at 6% to 7% between 2010 and 2012 (Figure). In contrast, the rates of research personnel lacking scopes of practice and required training not current, which had comparable high rates in 2010, decreased sharply from 2010 to 2012.
Federal policies require that all individuals participating in research at international sites be provided with appropriate protections that are in accord with those given to research subjects within the U.S. as well as protections considered appropriate by local authorities and customary at the international site.1 VA policies require that permissions be obtained from the CRADO prior to initiating any VA-approved international research.4
Likewise, federal policies require additional protections when research involves vulnerable populations, such as children and prisoners.1 VA policies require that permission be obtained from the CRADO prior to initiating any research involving children or prisoners.4
Data on international research were available for all 3 years (Table 1). However, data on research involving children and prisoners were available only in 2011 and 2012. Although the numbers of these research protocols were small, ranging from 0 to 8 protocols, a high percentage of these protocols, ranging from 21% to 100%, did not receive CRADO approval prior to the initiation of the studies.
Discussion
The data presented in this report reveal that there has been considerable improvement in VA HRPPs since VAORO started to collect QI data in 2010. Of the QI data available from 2010 through 2012, 9 showed improvement, none showed deterioration. Of the 9 QIs that showed no statistically significant differences, 7 had very low QI rates in 2010 (most were < 1%). Consequently, further improvement may be difficult to achieve. On the other hand, VAORO identified 2 QIs to be in need of improvement.
The main purpose of collecting these data is to promote quality improvement. Each year VAORO provides feedback to VA research facilities by giving each facility its QI data along with the national and network averages so that each facility knows where it stands at the national and VISN level. It is hoped that with this information, facilities will be able to identify strengths and weaknesses and carry out quality improvement measures accordingly.
Several potential reasons exist for the observed improvements. Possibly, improvements could be due to reporting errors, for example, if facilities were underreporting noncompliance. However, underreporting is unlikely, because data were collected from independent RCO audits of ICDs and regulatory protocol audits. At VA, RCOs report directly to institutional officials and function independently of the Research Service.
Some facilities also may have been systematically “gaming the system” in order to make their programs look better. For example, some IRBs might become less likely to suspend a protocol when it should be suspended. While the above possibilities cannot be ruled out completely, the authors believe that they are unlikely. First, not all QIs were improved. Particularly, lapse in IRB continuing reviews remained high and unchanged from 2010 to 2012. In addition, routine on-site reviews of facility’s HRPPs have independently verified some of the improvements observed in these QI data.
Two areas in need of improvement have been identified: lapses in IRB continuing reviews and studies requiring CRADO. These 2 areas can be easily improved if facilities are willing to devote effort and resources to improve IRB procedures and practices. In a previous study based on 2011 QI data, the authors reported that VA facilities with a small human research program (active human research protocols of < 50) had a rate of lapse in IRB continuing reviews of 3.2%; facilities with a medium research program (50-200 active human research protocols) had a rate of 5.5%; and facilities with a large research program (> 200 active human research protocols) had a rate of 8.6%.14 Thus, facilities with a large research program particularly need to improve their IRB continuing review processes.
In addition to QI, these data provide opportunities to answer a number of important questions regarding HRPPs. For example, based on 2011 QI data, the authors had previously shown that HRPPs of facilities using their own VA IRBs and those using affiliated university IRBs as their IRBs of record performed equally well, providing scientific data for the first time to support the long-standing VA policy that it is acceptable for VA facilities to use their own IRB or the affiliated university IRB as the IRB of record.4,13 Likewise, there has been concern that facilities with small research programs may not have sufficient resources to support a vigorous HRPP.
In a previous study based on analysis of 2011 QI data, the authors showed that HRPPs of facilities with small research programs performed at least as well as facilities with medium and large research programs.14 Facilities with large research programs seemed to perform not as well as facilities with small and medium research programs, suggesting that facilities with large research programs may need to allocate additional resources to support HRPPs.
Two fundamental questions remain unanswered. First, are these QIs the most optimal for evaluating HRPPs? Second, do high-quality HRPPs as measured using QIs actually provide better human research subject protections? Although no clear answers to these important questions exist at this time, there is a clear need to measure the quality of HRPPs. Undoubtedly, modification of current QIs or the addition of new ones is needed. However, the authors are sharing their experience with academic and other non-VA research institutions as they develop their own QIs for assessing the quality of their HRPPs.
Acknowledgement
The authors wish to thank J. Thomas Puglisi, PhD, chief officer, Office of Research Oversight, for his support and critical review of the manuscript and thank all VA research compliance officers for their contributions in conducting audits and collecting the data presented in this report.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Health and Human Services. 1991. Federal Policy for the Protection of Human Subjects. 45 Code of Federal Registration (CFR) 46.
2. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. U.S. Department of Health and Human Services Website. http://www.hhs.gov/ohrp /humansubjects/guidance/belmont.html. Published April 18, 1979. Accessed March 6, 2015.
3. Institute of Medicine (U.S.) Committee on Assessing the System for Protecting Human Research Subjects. Preserving Public Trust: Accreditation and Human Research Participant Protection Programs. Washington, DC: National Academies Press; 2001.
4. U.S. Department of Veterans Affairs, Veterans Health Administration. Requirements for the protection of human subjects in research. Handbook 1200.05. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications /ViewPublication.asp?pub_ID=3052. November 12, 2014. Accessed March 6, 2015.
5. Kizer KW. Statement on Oversight in the Veterans Health Administration before the Subcommittee on Veterans’ Affairs, U.S. House of Representatives. U.S. Department of Veterans Affairs Website. http://www .va.gov/OCA/testimony/hvac/sh/21AP9910.asp. April 21, 1999. Accessed March 19, 2015.
6. Steinbrook R. Protecting research subjects—The crisis at Johns Hopkins. N Engl J Med. 2002;346(9):716-720.
7. Kranish M. System for protecting humans in research faulted. Boston Globe. March 25, 2002:A1.
8. Shalala D. Protecting research subjects—What must be done. N Engl J Med. 2000;343(11):808-810.
9. Steinbrook R. Improving protection for research subjects. N Engl J Med. 2002;346(18):1425-1430.
10. Tsan MF, Smith K, Gao B. Assessing the quality of human research protection programs: the experience at the Department of Veterans Affairs. IRB. 2010;32(4):16-19.
11. U.S. Department of Veterans Affairs, Veterans Health Administration. Research and development committee. Handbook 1200.01 U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2038. Published June 16, 2009. Accessed March 6, 2015.
12. Tsan MF, Nguyen Y, Brooks R. Using quality indicators to assess human research protection programs at the Department of Veterans Affairs. IRB. 2013;35(1):10-14.
13. Tsan MF, Nguyen Y, Brooks R. Assessing the quality of VA human research protection programs: VA vs affiliated University Institutional Review Board. J Empir Res Hum Res Ethics. 2013;8(2):153-160.
14. Nguyen Y, Brooks R, Tsan MF. Human research protection programs at the Department of Veterans Affairs: quality indicators and program size. IRB. 2014;36(1):16-19.
15. U.S. Department of Veterans Affairs, Veterans Health Administration. Research compliance reporting requirements. Handbook 1058.01. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/viewpublication.asp?pub_id=2463. Published November 15, 2011. Accessed March 6, 2015.
16. Tsan MF, Puglisi JT. Health care operations activities that may constitute research: the Department of Veterans Affairs’s perspective. IRB. 2014;36(1):9-11.
17. Woodward M. Epidemiology. Study Design and Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2014.
18. Tsan L, Davis C, Langberg R, Pierce JR. Quality indicators in the Department of Veterans Affairs nursing home care units: a preliminary assessment. Am J Med Qual. 2007;22(5):344-350.
Protection of human subjects participating in research is critically important during this era of rapid medical progress and the increasing emphasis on translating discoveries from basic science research into clinical practices. The Federal Policy for the Protection of Human Subjects, also known as the Common Rule, was established based on the Belmont Report’s ethical principles of respect for persons, beneficence, and justice.1,2 Under the Common Rule, institutional review boards (IRBs) are responsible for reviewing and approving human research protocols and providing oversight to ensure protection of human research subjects.1
In addition to IRBs, investigators, institutions, research volunteers, sponsors of research, and the federal government share responsibilities for protecting research subjects.3 Institutions conducting research involving human subjects have thus established operational frameworks, referred to as human research protection programs (HRPPs), to ensure the rights and welfare of research participants and to meet the ethical and regulatory requirements.3,4
Related: Empathic Disclosure of Adverse Events to Patients
In the late 1990s and early 2000s, a number of major academic institutions’ federally supported research programs were suspended due to persistent noncompliance with federal regulations, including some issues that resulted in the death of healthy volunteers.5,6 In response to increased public scrutiny of clinical research, considerable efforts have been made to improve the protection of research subjects.5,7-9 These efforts included stronger federal oversight of research, voluntary accreditation of institutional HRPPs, increased institutional support for HRPPs, improved training for investigators and IRB members, improved monitoring and reporting of adverse events (AEs), and greater involvement of research participants and the public.9
Despite considerable investment to improve research subject protections, scant data exist showing that these efforts have made human research safer than before. Although research subject protection cannot be directly measured, quality assessment of HRPPs is possible. High-quality HRPPs are expected to minimize risk to research participants to the extent possible while maintaining the integrity of the research.10
Related: Improving Veteran Access to Clinical Trials
The VA health care system is the largest integrated health care system in the country. Currently, there are 107 VA facilities conducting research involving human subjects. In addition to federal regulations governing research with human subjects, VA researchers must also comply with requirements established by the VA. For example, in the VA the IRB is a subcommittee of the research and development committee (R&DC). Research involving human subjects may not be initiated until approved by both the IRB and the R&DC.4,11 All VA investigators are required to have approved research scopes of practice and training in ethical principles and current good clinical practices.4
Recently, the VA Office of Research Oversight (VAORO) developed a set of indicators for assessing the quality of VA HRPPs.10 Since 2010, VAORO has been collecting quality indicator (QI) data from all VA research facilities for quality improvement purposes.12-14 In this study, VAORO analyzed these data to assess changes in VA HRPP QI data from 2010 to 2012 and identify areas for improvement.
Methods
As part of the VA HRPP quality assurance program, each VA research facility was required to conduct annual audits of all informed consent documents (ICDs) and regulatory audits of all human research protocols once every 3 years by qualified research compliance officers (RCOs).15 Protocol regulatory audits were limited to a 3-year look back of the protocols. Tools were developed for the annual ICD and triennial protocol regulatory audits (available at http://www.va.gov/ORO/Research_Compliance_Education.asp). Facility RCOs were then trained to use these tools to conduct audits.
Data Collection
Data were collected annually from all 107 VA research facilities. Information collected included compliance with ICD and Health Insurance Portability and Accountability Act authorization requirements; compliance with requirements for IRB and R&DC initial approval of human research protocols; compliance with selected informed consent requirements; for-cause suspension or termination of human research protocols; research-related serious AEs; compliance with continuing review requirements; subject enrollment according to inclusion and exclusion criteria; research personnel scopes of practice; investigator human research protection training; international research; and research involving vulnerable subjects. No individually identifiable personal information was collected. As this was a VA quality assurance project and no individually identifiable information was collected, no IRB review and approval of the project was required.16
All data collected were entered into a database for analysis. When necessary, facilities were contacted to verify the accuracy and uniformity of data reported.
Data Analysis
The Mantel-Haenszel chi-square test for trend was used to determine the trend of changes from 2010 through 2012.17 A P value of < .05 was considered to be statistically significant. For those QIs with statistically significant changes, VAORO calculated the percent changes and the actual numbers impacted, ie, the actual numbers of ICDs, human research protocols, case histories, or research personnel affected by these changes.18
Results
The HRPP QI data was collected from 2010 through 2012 from all 107 VA research facilities (Table 1). There were a total of 25 QIs; 18 had all 3-year data available and 7 lacked 2010 data. Only those 18 QI data available from all 3 years were included for this analysis. The 2010 data collected for QIs related to for-cause suspension or termination of protocols and research personnel scopes of practice and training requirements were derived from all human, animal, and safety research protocols audited, not just the human research protocols audited. However, these data were included for comparison with the 2011 and 2012 data, because nonhuman research protocols audited constituted < 30% of the total. Based on VAORO on-site routine reviews of facilities’ HRPPs, animal care and use programs, as well as research safety and security programs, the authors believe that the QI rates in these nonhuman research protocols were similar to those of human research protocols.
From a total of 18 QIs with all 3-year data available for analysis, 9 QIs did not show any statistically significant changes; whereas 9 QIs showed statistically significant changes from 2010 to 2012 (Table 1). These 9 QIs were: (1) incorrect ICDs used; (2) number of protocols suspended or terminated due to cause; (3) protocols suspended or terminated due to investigator concerns; (4) informed consent not obtained prior to initiation of the study; (5) research personnel without research scopes of practice; (6) research personnel working outside of scopes of practice; (7) required training not current for research personnel; (8) research personnel working without initial training; and (9) research personnel lapsed in continuing training.
Table 2 shows the percent changes and the actual numbers impacted by the changes in the 9 QIs that showed statistically significant changes. The percent changes describe the magnitude of changes, and the numbers impacted provide information on the actual numbers of events (ie, ICDs, human research protocols, case histories, or research personnel) affected by these changes in 2012 if the QI rates had stayed the same as those of 2010.
All 9 QIs with statistically significant changes showed improvement, ranging from 25% improvement in incorrect ICDs used to 92% improvement in research personnel without scopes of practice (Table 2). The actual numbers impacted (ie, the difference between numbers expected in 2012 based on 2010 QI rates and the actual numbers observed in 2012) ranged from 55 protocols suspended or terminated for cause to 1,177 research scopes of practice.
Of the 9 QIs with no statistically significant changes, all but 2 QIs had QI rates of < 1% in 2010, suggesting that these QI rates were already so low that further improvement was difficult to achieve. The 2 exceptions were lapses in IRB continuing reviews and international research conducted without VA Chief Research and Development Officer (CRADO) approval.
The rates of lapse in IRB continuing reviews remained high at 6% to 7% between 2010 and 2012 (Figure). In contrast, the rates of research personnel lacking scopes of practice and required training not current, which had comparable high rates in 2010, decreased sharply from 2010 to 2012.
Federal policies require that all individuals participating in research at international sites be provided with appropriate protections that are in accord with those given to research subjects within the U.S. as well as protections considered appropriate by local authorities and customary at the international site.1 VA policies require that permissions be obtained from the CRADO prior to initiating any VA-approved international research.4
Likewise, federal policies require additional protections when research involves vulnerable populations, such as children and prisoners.1 VA policies require that permission be obtained from the CRADO prior to initiating any research involving children or prisoners.4
Data on international research were available for all 3 years (Table 1). However, data on research involving children and prisoners were available only in 2011 and 2012. Although the numbers of these research protocols were small, ranging from 0 to 8 protocols, a high percentage of these protocols, ranging from 21% to 100%, did not receive CRADO approval prior to the initiation of the studies.
Discussion
The data presented in this report reveal that there has been considerable improvement in VA HRPPs since VAORO started to collect QI data in 2010. Of the QI data available from 2010 through 2012, 9 showed improvement, none showed deterioration. Of the 9 QIs that showed no statistically significant differences, 7 had very low QI rates in 2010 (most were < 1%). Consequently, further improvement may be difficult to achieve. On the other hand, VAORO identified 2 QIs to be in need of improvement.
The main purpose of collecting these data is to promote quality improvement. Each year VAORO provides feedback to VA research facilities by giving each facility its QI data along with the national and network averages so that each facility knows where it stands at the national and VISN level. It is hoped that with this information, facilities will be able to identify strengths and weaknesses and carry out quality improvement measures accordingly.
Several potential reasons exist for the observed improvements. Possibly, improvements could be due to reporting errors, for example, if facilities were underreporting noncompliance. However, underreporting is unlikely, because data were collected from independent RCO audits of ICDs and regulatory protocol audits. At VA, RCOs report directly to institutional officials and function independently of the Research Service.
Some facilities also may have been systematically “gaming the system” in order to make their programs look better. For example, some IRBs might become less likely to suspend a protocol when it should be suspended. While the above possibilities cannot be ruled out completely, the authors believe that they are unlikely. First, not all QIs were improved. Particularly, lapse in IRB continuing reviews remained high and unchanged from 2010 to 2012. In addition, routine on-site reviews of facility’s HRPPs have independently verified some of the improvements observed in these QI data.
Two areas in need of improvement have been identified: lapses in IRB continuing reviews and studies requiring CRADO. These 2 areas can be easily improved if facilities are willing to devote effort and resources to improve IRB procedures and practices. In a previous study based on 2011 QI data, the authors reported that VA facilities with a small human research program (active human research protocols of < 50) had a rate of lapse in IRB continuing reviews of 3.2%; facilities with a medium research program (50-200 active human research protocols) had a rate of 5.5%; and facilities with a large research program (> 200 active human research protocols) had a rate of 8.6%.14 Thus, facilities with a large research program particularly need to improve their IRB continuing review processes.
In addition to QI, these data provide opportunities to answer a number of important questions regarding HRPPs. For example, based on 2011 QI data, the authors had previously shown that HRPPs of facilities using their own VA IRBs and those using affiliated university IRBs as their IRBs of record performed equally well, providing scientific data for the first time to support the long-standing VA policy that it is acceptable for VA facilities to use their own IRB or the affiliated university IRB as the IRB of record.4,13 Likewise, there has been concern that facilities with small research programs may not have sufficient resources to support a vigorous HRPP.
In a previous study based on analysis of 2011 QI data, the authors showed that HRPPs of facilities with small research programs performed at least as well as facilities with medium and large research programs.14 Facilities with large research programs seemed to perform not as well as facilities with small and medium research programs, suggesting that facilities with large research programs may need to allocate additional resources to support HRPPs.
Two fundamental questions remain unanswered. First, are these QIs the most optimal for evaluating HRPPs? Second, do high-quality HRPPs as measured using QIs actually provide better human research subject protections? Although no clear answers to these important questions exist at this time, there is a clear need to measure the quality of HRPPs. Undoubtedly, modification of current QIs or the addition of new ones is needed. However, the authors are sharing their experience with academic and other non-VA research institutions as they develop their own QIs for assessing the quality of their HRPPs.
Acknowledgement
The authors wish to thank J. Thomas Puglisi, PhD, chief officer, Office of Research Oversight, for his support and critical review of the manuscript and thank all VA research compliance officers for their contributions in conducting audits and collecting the data presented in this report.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Protection of human subjects participating in research is critically important during this era of rapid medical progress and the increasing emphasis on translating discoveries from basic science research into clinical practices. The Federal Policy for the Protection of Human Subjects, also known as the Common Rule, was established based on the Belmont Report’s ethical principles of respect for persons, beneficence, and justice.1,2 Under the Common Rule, institutional review boards (IRBs) are responsible for reviewing and approving human research protocols and providing oversight to ensure protection of human research subjects.1
In addition to IRBs, investigators, institutions, research volunteers, sponsors of research, and the federal government share responsibilities for protecting research subjects.3 Institutions conducting research involving human subjects have thus established operational frameworks, referred to as human research protection programs (HRPPs), to ensure the rights and welfare of research participants and to meet the ethical and regulatory requirements.3,4
Related: Empathic Disclosure of Adverse Events to Patients
In the late 1990s and early 2000s, a number of major academic institutions’ federally supported research programs were suspended due to persistent noncompliance with federal regulations, including some issues that resulted in the death of healthy volunteers.5,6 In response to increased public scrutiny of clinical research, considerable efforts have been made to improve the protection of research subjects.5,7-9 These efforts included stronger federal oversight of research, voluntary accreditation of institutional HRPPs, increased institutional support for HRPPs, improved training for investigators and IRB members, improved monitoring and reporting of adverse events (AEs), and greater involvement of research participants and the public.9
Despite considerable investment to improve research subject protections, scant data exist showing that these efforts have made human research safer than before. Although research subject protection cannot be directly measured, quality assessment of HRPPs is possible. High-quality HRPPs are expected to minimize risk to research participants to the extent possible while maintaining the integrity of the research.10
Related: Improving Veteran Access to Clinical Trials
The VA health care system is the largest integrated health care system in the country. Currently, there are 107 VA facilities conducting research involving human subjects. In addition to federal regulations governing research with human subjects, VA researchers must also comply with requirements established by the VA. For example, in the VA the IRB is a subcommittee of the research and development committee (R&DC). Research involving human subjects may not be initiated until approved by both the IRB and the R&DC.4,11 All VA investigators are required to have approved research scopes of practice and training in ethical principles and current good clinical practices.4
Recently, the VA Office of Research Oversight (VAORO) developed a set of indicators for assessing the quality of VA HRPPs.10 Since 2010, VAORO has been collecting quality indicator (QI) data from all VA research facilities for quality improvement purposes.12-14 In this study, VAORO analyzed these data to assess changes in VA HRPP QI data from 2010 to 2012 and identify areas for improvement.
Methods
As part of the VA HRPP quality assurance program, each VA research facility was required to conduct annual audits of all informed consent documents (ICDs) and regulatory audits of all human research protocols once every 3 years by qualified research compliance officers (RCOs).15 Protocol regulatory audits were limited to a 3-year look back of the protocols. Tools were developed for the annual ICD and triennial protocol regulatory audits (available at http://www.va.gov/ORO/Research_Compliance_Education.asp). Facility RCOs were then trained to use these tools to conduct audits.
Data Collection
Data were collected annually from all 107 VA research facilities. Information collected included compliance with ICD and Health Insurance Portability and Accountability Act authorization requirements; compliance with requirements for IRB and R&DC initial approval of human research protocols; compliance with selected informed consent requirements; for-cause suspension or termination of human research protocols; research-related serious AEs; compliance with continuing review requirements; subject enrollment according to inclusion and exclusion criteria; research personnel scopes of practice; investigator human research protection training; international research; and research involving vulnerable subjects. No individually identifiable personal information was collected. As this was a VA quality assurance project and no individually identifiable information was collected, no IRB review and approval of the project was required.16
All data collected were entered into a database for analysis. When necessary, facilities were contacted to verify the accuracy and uniformity of data reported.
Data Analysis
The Mantel-Haenszel chi-square test for trend was used to determine the trend of changes from 2010 through 2012.17 A P value of < .05 was considered to be statistically significant. For those QIs with statistically significant changes, VAORO calculated the percent changes and the actual numbers impacted, ie, the actual numbers of ICDs, human research protocols, case histories, or research personnel affected by these changes.18
Results
The HRPP QI data was collected from 2010 through 2012 from all 107 VA research facilities (Table 1). There were a total of 25 QIs; 18 had all 3-year data available and 7 lacked 2010 data. Only those 18 QI data available from all 3 years were included for this analysis. The 2010 data collected for QIs related to for-cause suspension or termination of protocols and research personnel scopes of practice and training requirements were derived from all human, animal, and safety research protocols audited, not just the human research protocols audited. However, these data were included for comparison with the 2011 and 2012 data, because nonhuman research protocols audited constituted < 30% of the total. Based on VAORO on-site routine reviews of facilities’ HRPPs, animal care and use programs, as well as research safety and security programs, the authors believe that the QI rates in these nonhuman research protocols were similar to those of human research protocols.
From a total of 18 QIs with all 3-year data available for analysis, 9 QIs did not show any statistically significant changes; whereas 9 QIs showed statistically significant changes from 2010 to 2012 (Table 1). These 9 QIs were: (1) incorrect ICDs used; (2) number of protocols suspended or terminated due to cause; (3) protocols suspended or terminated due to investigator concerns; (4) informed consent not obtained prior to initiation of the study; (5) research personnel without research scopes of practice; (6) research personnel working outside of scopes of practice; (7) required training not current for research personnel; (8) research personnel working without initial training; and (9) research personnel lapsed in continuing training.
Table 2 shows the percent changes and the actual numbers impacted by the changes in the 9 QIs that showed statistically significant changes. The percent changes describe the magnitude of changes, and the numbers impacted provide information on the actual numbers of events (ie, ICDs, human research protocols, case histories, or research personnel) affected by these changes in 2012 if the QI rates had stayed the same as those of 2010.
All 9 QIs with statistically significant changes showed improvement, ranging from 25% improvement in incorrect ICDs used to 92% improvement in research personnel without scopes of practice (Table 2). The actual numbers impacted (ie, the difference between numbers expected in 2012 based on 2010 QI rates and the actual numbers observed in 2012) ranged from 55 protocols suspended or terminated for cause to 1,177 research scopes of practice.
Of the 9 QIs with no statistically significant changes, all but 2 QIs had QI rates of < 1% in 2010, suggesting that these QI rates were already so low that further improvement was difficult to achieve. The 2 exceptions were lapses in IRB continuing reviews and international research conducted without VA Chief Research and Development Officer (CRADO) approval.
The rates of lapse in IRB continuing reviews remained high at 6% to 7% between 2010 and 2012 (Figure). In contrast, the rates of research personnel lacking scopes of practice and required training not current, which had comparable high rates in 2010, decreased sharply from 2010 to 2012.
Federal policies require that all individuals participating in research at international sites be provided with appropriate protections that are in accord with those given to research subjects within the U.S. as well as protections considered appropriate by local authorities and customary at the international site.1 VA policies require that permissions be obtained from the CRADO prior to initiating any VA-approved international research.4
Likewise, federal policies require additional protections when research involves vulnerable populations, such as children and prisoners.1 VA policies require that permission be obtained from the CRADO prior to initiating any research involving children or prisoners.4
Data on international research were available for all 3 years (Table 1). However, data on research involving children and prisoners were available only in 2011 and 2012. Although the numbers of these research protocols were small, ranging from 0 to 8 protocols, a high percentage of these protocols, ranging from 21% to 100%, did not receive CRADO approval prior to the initiation of the studies.
Discussion
The data presented in this report reveal that there has been considerable improvement in VA HRPPs since VAORO started to collect QI data in 2010. Of the QI data available from 2010 through 2012, 9 showed improvement, none showed deterioration. Of the 9 QIs that showed no statistically significant differences, 7 had very low QI rates in 2010 (most were < 1%). Consequently, further improvement may be difficult to achieve. On the other hand, VAORO identified 2 QIs to be in need of improvement.
The main purpose of collecting these data is to promote quality improvement. Each year VAORO provides feedback to VA research facilities by giving each facility its QI data along with the national and network averages so that each facility knows where it stands at the national and VISN level. It is hoped that with this information, facilities will be able to identify strengths and weaknesses and carry out quality improvement measures accordingly.
Several potential reasons exist for the observed improvements. Possibly, improvements could be due to reporting errors, for example, if facilities were underreporting noncompliance. However, underreporting is unlikely, because data were collected from independent RCO audits of ICDs and regulatory protocol audits. At VA, RCOs report directly to institutional officials and function independently of the Research Service.
Some facilities also may have been systematically “gaming the system” in order to make their programs look better. For example, some IRBs might become less likely to suspend a protocol when it should be suspended. While the above possibilities cannot be ruled out completely, the authors believe that they are unlikely. First, not all QIs were improved. Particularly, lapse in IRB continuing reviews remained high and unchanged from 2010 to 2012. In addition, routine on-site reviews of facility’s HRPPs have independently verified some of the improvements observed in these QI data.
Two areas in need of improvement have been identified: lapses in IRB continuing reviews and studies requiring CRADO. These 2 areas can be easily improved if facilities are willing to devote effort and resources to improve IRB procedures and practices. In a previous study based on 2011 QI data, the authors reported that VA facilities with a small human research program (active human research protocols of < 50) had a rate of lapse in IRB continuing reviews of 3.2%; facilities with a medium research program (50-200 active human research protocols) had a rate of 5.5%; and facilities with a large research program (> 200 active human research protocols) had a rate of 8.6%.14 Thus, facilities with a large research program particularly need to improve their IRB continuing review processes.
In addition to QI, these data provide opportunities to answer a number of important questions regarding HRPPs. For example, based on 2011 QI data, the authors had previously shown that HRPPs of facilities using their own VA IRBs and those using affiliated university IRBs as their IRBs of record performed equally well, providing scientific data for the first time to support the long-standing VA policy that it is acceptable for VA facilities to use their own IRB or the affiliated university IRB as the IRB of record.4,13 Likewise, there has been concern that facilities with small research programs may not have sufficient resources to support a vigorous HRPP.
In a previous study based on analysis of 2011 QI data, the authors showed that HRPPs of facilities with small research programs performed at least as well as facilities with medium and large research programs.14 Facilities with large research programs seemed to perform not as well as facilities with small and medium research programs, suggesting that facilities with large research programs may need to allocate additional resources to support HRPPs.
Two fundamental questions remain unanswered. First, are these QIs the most optimal for evaluating HRPPs? Second, do high-quality HRPPs as measured using QIs actually provide better human research subject protections? Although no clear answers to these important questions exist at this time, there is a clear need to measure the quality of HRPPs. Undoubtedly, modification of current QIs or the addition of new ones is needed. However, the authors are sharing their experience with academic and other non-VA research institutions as they develop their own QIs for assessing the quality of their HRPPs.
Acknowledgement
The authors wish to thank J. Thomas Puglisi, PhD, chief officer, Office of Research Oversight, for his support and critical review of the manuscript and thank all VA research compliance officers for their contributions in conducting audits and collecting the data presented in this report.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Health and Human Services. 1991. Federal Policy for the Protection of Human Subjects. 45 Code of Federal Registration (CFR) 46.
2. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. U.S. Department of Health and Human Services Website. http://www.hhs.gov/ohrp /humansubjects/guidance/belmont.html. Published April 18, 1979. Accessed March 6, 2015.
3. Institute of Medicine (U.S.) Committee on Assessing the System for Protecting Human Research Subjects. Preserving Public Trust: Accreditation and Human Research Participant Protection Programs. Washington, DC: National Academies Press; 2001.
4. U.S. Department of Veterans Affairs, Veterans Health Administration. Requirements for the protection of human subjects in research. Handbook 1200.05. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications /ViewPublication.asp?pub_ID=3052. November 12, 2014. Accessed March 6, 2015.
5. Kizer KW. Statement on Oversight in the Veterans Health Administration before the Subcommittee on Veterans’ Affairs, U.S. House of Representatives. U.S. Department of Veterans Affairs Website. http://www .va.gov/OCA/testimony/hvac/sh/21AP9910.asp. April 21, 1999. Accessed March 19, 2015.
6. Steinbrook R. Protecting research subjects—The crisis at Johns Hopkins. N Engl J Med. 2002;346(9):716-720.
7. Kranish M. System for protecting humans in research faulted. Boston Globe. March 25, 2002:A1.
8. Shalala D. Protecting research subjects—What must be done. N Engl J Med. 2000;343(11):808-810.
9. Steinbrook R. Improving protection for research subjects. N Engl J Med. 2002;346(18):1425-1430.
10. Tsan MF, Smith K, Gao B. Assessing the quality of human research protection programs: the experience at the Department of Veterans Affairs. IRB. 2010;32(4):16-19.
11. U.S. Department of Veterans Affairs, Veterans Health Administration. Research and development committee. Handbook 1200.01 U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2038. Published June 16, 2009. Accessed March 6, 2015.
12. Tsan MF, Nguyen Y, Brooks R. Using quality indicators to assess human research protection programs at the Department of Veterans Affairs. IRB. 2013;35(1):10-14.
13. Tsan MF, Nguyen Y, Brooks R. Assessing the quality of VA human research protection programs: VA vs affiliated University Institutional Review Board. J Empir Res Hum Res Ethics. 2013;8(2):153-160.
14. Nguyen Y, Brooks R, Tsan MF. Human research protection programs at the Department of Veterans Affairs: quality indicators and program size. IRB. 2014;36(1):16-19.
15. U.S. Department of Veterans Affairs, Veterans Health Administration. Research compliance reporting requirements. Handbook 1058.01. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/viewpublication.asp?pub_id=2463. Published November 15, 2011. Accessed March 6, 2015.
16. Tsan MF, Puglisi JT. Health care operations activities that may constitute research: the Department of Veterans Affairs’s perspective. IRB. 2014;36(1):9-11.
17. Woodward M. Epidemiology. Study Design and Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2014.
18. Tsan L, Davis C, Langberg R, Pierce JR. Quality indicators in the Department of Veterans Affairs nursing home care units: a preliminary assessment. Am J Med Qual. 2007;22(5):344-350.
1. U.S. Department of Health and Human Services. 1991. Federal Policy for the Protection of Human Subjects. 45 Code of Federal Registration (CFR) 46.
2. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. U.S. Department of Health and Human Services Website. http://www.hhs.gov/ohrp /humansubjects/guidance/belmont.html. Published April 18, 1979. Accessed March 6, 2015.
3. Institute of Medicine (U.S.) Committee on Assessing the System for Protecting Human Research Subjects. Preserving Public Trust: Accreditation and Human Research Participant Protection Programs. Washington, DC: National Academies Press; 2001.
4. U.S. Department of Veterans Affairs, Veterans Health Administration. Requirements for the protection of human subjects in research. Handbook 1200.05. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications /ViewPublication.asp?pub_ID=3052. November 12, 2014. Accessed March 6, 2015.
5. Kizer KW. Statement on Oversight in the Veterans Health Administration before the Subcommittee on Veterans’ Affairs, U.S. House of Representatives. U.S. Department of Veterans Affairs Website. http://www .va.gov/OCA/testimony/hvac/sh/21AP9910.asp. April 21, 1999. Accessed March 19, 2015.
6. Steinbrook R. Protecting research subjects—The crisis at Johns Hopkins. N Engl J Med. 2002;346(9):716-720.
7. Kranish M. System for protecting humans in research faulted. Boston Globe. March 25, 2002:A1.
8. Shalala D. Protecting research subjects—What must be done. N Engl J Med. 2000;343(11):808-810.
9. Steinbrook R. Improving protection for research subjects. N Engl J Med. 2002;346(18):1425-1430.
10. Tsan MF, Smith K, Gao B. Assessing the quality of human research protection programs: the experience at the Department of Veterans Affairs. IRB. 2010;32(4):16-19.
11. U.S. Department of Veterans Affairs, Veterans Health Administration. Research and development committee. Handbook 1200.01 U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2038. Published June 16, 2009. Accessed March 6, 2015.
12. Tsan MF, Nguyen Y, Brooks R. Using quality indicators to assess human research protection programs at the Department of Veterans Affairs. IRB. 2013;35(1):10-14.
13. Tsan MF, Nguyen Y, Brooks R. Assessing the quality of VA human research protection programs: VA vs affiliated University Institutional Review Board. J Empir Res Hum Res Ethics. 2013;8(2):153-160.
14. Nguyen Y, Brooks R, Tsan MF. Human research protection programs at the Department of Veterans Affairs: quality indicators and program size. IRB. 2014;36(1):16-19.
15. U.S. Department of Veterans Affairs, Veterans Health Administration. Research compliance reporting requirements. Handbook 1058.01. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/viewpublication.asp?pub_id=2463. Published November 15, 2011. Accessed March 6, 2015.
16. Tsan MF, Puglisi JT. Health care operations activities that may constitute research: the Department of Veterans Affairs’s perspective. IRB. 2014;36(1):9-11.
17. Woodward M. Epidemiology. Study Design and Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2014.
18. Tsan L, Davis C, Langberg R, Pierce JR. Quality indicators in the Department of Veterans Affairs nursing home care units: a preliminary assessment. Am J Med Qual. 2007;22(5):344-350.
Experiences of Veterans With Diabetes From Shared Medical Appointments
Treatment of diabetes can be difficult and challenging. Information to improve the self-management behavior of patients with diabetes is important, because the prevalence of diabetes is expected to increase as the population ages, along with rising medical costs, premature death, and morbidity due to complications. Veterans, as a group, present unique challenges in health care. A recent analysis at a VA setting found only 17.3% of veterans were meeting all 3 of their “ABC” goals—A1c, blood pressure, and cholesterol.1
Within the VA, diabetes is the third most common diagnosis, with a higher prevalence among veterans (25%) than among the general U.S. population (8.3%).2 However, little information exists about the barriers and motivations of the veterans who have completed a diabetes shared medical appointment (SMA) series.
The VA promotes SMAs as an effective alternative to one-on-one encounters for a cohort of patients with similar health conditions. In these SMAs, a multidisciplinary team meets with a group of patients for about 2 hours. These SMAs can be especially important for patients who need frequent encounters for care management, such as diabetes. Shared medical appointments focus on the American Association of Diabetes Educators 7 (AADE 7) self-care behaviors and provide a medium to foster improved self-management and healthy coping.3
Related: Education Pitfalls of Insulin Administration in Patients With Diabetes
Several systematic reviews of qualitative studies have identified and summarized factors that impact diabetes self-management.4,5 Behavioral science and social psychology provide rich examples of theories to influence and understand behaviors, including motivational interviewing and self-determinism.6,7 Other recent innovative approaches in primary care settings and diabetes self-management at the VA include companion (family or friend) participation in primary care visits, collaborative goal setting with patient and providers, age-matched patient pairing, and using a clinical pharmacist clinic as a midlevel provider to help meet VA national diabetes performance standards.8-11
In accordance with the Patient Aligned Care Team (PACT) focus on the delivery of patient care, the goal of this study was to understand the experiences of veterans and to learn about the tools and methods they perceive to be most useful in improving patient education and motivation for self-management of diabetes. A onetime diabetes focus group was held to inquire about these specific issues.
Methods
The focus group took place at the Vancouver, Washington, campus of VA Portland Health Care System (VAPHCS). All veteran participants and their family members who had completed at least 3 of a 4-session SMA series were invited. Out of 29 invited veterans, 18 participated in the discussion along with 3 family members (all wives), for a total of 21 participants. The SMAs focused on meeting primary care performance standards on A1c, blood pressure, and hyperlipidemia, in accordance with the new PACT model. The VA education division approved the use of the Conversation Map for SMAs, created by Merck (Whitehouse Station, NJ) in collaboration with the American Diabetes Association. Using the Conversation Map format in a VA setting has been shown to reduce mean A1c levels by -0.9 (± 1.9%; P < .001).12 The SMA team made lifestyle and medication changes weekly (under a scope of practice for the pharmacist).
Data Gathering and Analysis
Participants attended a 2-hour focus group facilitated by the same 4 clinic care providers (2 pharmacists, 1 clinical nurse, 1 dietician) who had led the SMAs. The decision to have the discussion led by these same providers was grounded in the belief that this format would be familiar to the participants, and the rapport already established between providers and participants would encourage greater participation than if the meeting were led by unfamiliar VA employees. Two trained VAPHCS qualitative researchers attended the focus group and took extensive verbatim notes.
During the first 45 minutes, participants used a dot-voting technique to provide general demographic and background information in response to questions posted on boards around the room. Participants then were asked to choose their top 3 answers in response to each of a series of questions about barriers, resources, and motivators in self-management. The group was divided into 2 smaller groups of 10 or 11 participants, each facilitated by 2 researchers and assisted by a note-taker trained to capture the verbatim discussion. Session audio was not recorded, because VA policy requires signed consent, and this requirement might have discouraged participation.
The following questions guided the discussion: (1) Thinking back to when you were diagnosed with diabetes, what could you have done then that would have made a difference? (2) Thinking about all your experiences with diabetes, what was most helpful in motivating you to take control? (3) What one thing helped education or information “stick” with you? (4) What additional resources that are not currently available at the VA would help you? and (5) Tell us about your diabetes management plan.
Related: Diabetes Patient-Centered Medical Home Approach
After the focus groups, the research team used a formal debriefing tool to identify both initial impressions of possible discussion themes and group dynamics potentially influencing the content of the discussion; no significant communication or participation issues were identified.13 All research team members read the discussion notes and met to iteratively develop a simple codebook of global themes, using an approach of general inductive thematic content analysis.14
Two team members coded the notes, paying attention to the need to capture divergent or minority positions voiced by participants. Both coders worked toward consensus on code definitions through repeated discussions with each other and with the full research team. Codes were then used to sort and analyze about 180 comments made by participants during the focus groups. The Institutional Review Board of the VAPHCS approved the protocol for this study.
Results
Most participants in the diabetes focus group had type 2 diabetes mellitus (T2DM) and were male—1 female veteran participated (eTable).
After the initial analysis, all participants were mailed a letter summarizing themes and suggestions from the meeting (see Box). Responses to questions posted on the board and responded to by voting are included in Table 1. Weight gain was most commonly chosen as a barrier to self-management. The A1c value was the highest rated motivator for self-management of diabetes, followed by face-to-face support from the care team and family support. Participants chose one-on-one appointments with the diabetes team and classes with instructors as the most helpful VA resources.
The final codebook resulted in 9 domains: diagnostic experience, what helps, perceived value of the SMA group, veteran identity, interaction with care providers, denial, fatalism, motivators, and barriers; each contained several related codes. Several themes emerged from the analysis of the focus group data for a desired experience of managing and coping with diabetes.
Identity as a Person With Diabetes
Participants were at various stages of their identity with a chronic illness. Over time, the veterans noted a transition from being a “diabetic person” to a “person living healthy with diabetes.” One veteran’s comment encapsulated the shift in diabetic identity over time: “[Initially] when looking up diabetes on the computer, there were scary things. It was very frightening, and I was thinking, oh, they’re going to cut off my legs. Years later you have more objectivity and control.”
Some participants responded with denial, rejecting that they had diabetes or that they needed to make lifestyle changes. When asked how he felt when first diagnosed, one veteran stated, “I resisted endlessly. I wouldn’t take my face out of the food.” Many veterans expressed that the diagnosis of diabetes was similar to an assault on their identity.
One veteran with long-standing diabetes shared the following: “I got a giant plastic box, and every needle [I use] goes into the box. Every day I look at this huge pile of needles. It’s my sign of weakness. If I kick it…I won’t keep adding them [to the pile].”
Similarly another veteran stated, “I felt like a failure, not a winner, when I started taking insulin.” The A1c test result was seen “like a hammer coming down,” an indication of the individual’s success or failure.
Identity as a Veteran
For some veterans in the focus group, the development of diabetes was considered to be service connected and related to chemical exposure to herbicides, including Agent Orange. One veteran emphasized, “This [diabetes] is ’cause of Agent Orange exposure.”
Another participant commented, “If you’ve served your country, you’re strong…[you think diabetes] can’t happen to you.” One participant explained, “Hearing it from [other veterans in focus group].... I don’t know if this was bred into me in the service. Probably. These guys have traversed the territory. I go to these guys for my answers. And hearing it from them, you could tell me everything I need to hear, and every one of these guys could tell me the exact same thing, and I would listen to them and not to [the clinical staff].”
Early Education
Another theme was a general desire for early information and education. Veterans suggested that information and self-management coaching when diagnosed with prediabetes would have been beneficial to reduce the risk of progression to diabetes. Many participants expressed regret that tools such as A1c monitoring were not available to them earlier: “I was also diagnosed borderline…that’s when I should have been hit by the 2 x 4...[they] should have done the A1c every 6 months.” Some participants described feelings that early education and, as one put it, “more emphasis on the seriousness of it,” would have helped them prevent their diabetes from worsening or develop healthier habits for self-management earlier.
Another veteran had what the group felt was the optimal experience: “The nurse told me I was diabetic...sat there for 45 minutes and just talked to me about it. It was the fact that she sat and talked with me and covered all the questions I had. That was the best thing bar none.”
Veterans expressed frustration with the time delay between the diagnosis and availability of clinical support and education: “When I first got the diagnosis, there was 4 or 5 weeks until the class. I’m thinking, what they should have done as soon as they sent that letter with the A1c, they should have sent me a packet saying, here’s what you can do NOW. Boom!”
Interventions
The chance to meet with other veterans with diabetes was something many participants said was helpful and provided a specific benefit that health care providers on their own could not give. One participant stated, “Classes make you feel more normal, when you sit with these people whose experiences you share.” Another stated, “When people have had a problem, get together and say how they’ve overcome it, I wanna hear about it.” The veterans agreed that someone who has specialty training in diabetes, not just in peer-to-peer support groups, should lead the education or support groups.
When the veterans were asked whether they thought that a veteran-led group would be beneficial, one veteran stated, “A support group must have a facilitator that has skills and resources.” Another stated, “You need at least one person to give you direction.”
One veteran explained that having weekly classes and hearing the same information several times helped information to “stick.” Another veteran, while expressing frustration about the lack of education he received on diet, stated, “What we eat directly affects us…classes like this are the greatest thing that ever happened. They give us more support than the doctors ever do.” One veteran described how having his weekly morning SMA class to look forward to was a strong motivation to pay attention to all the things that matter to his diabetes throughout the week. Another veteran emphasized the SMA as being important, because “being members of the military, you still have the civilians and they are them and we are us. ... With no family members, this [SMA] has made a big difference.”
Provider Relationship
Veterans expressed that having a positive provider relationship was an important element in diabetes self-management. The lack of time available for diabetes management in standard primary care encounters was cited as a barrier. One veteran stated, “[Providers] diagnose you with a blood test and [push you] out the door!” One veteran observed, “I think they need to turn you over to a nurse practitioner. You’re better off with someone like that who actually has time to talk to you instead of leaving you with someone who just gives you a prescription.”
The quality of the interaction mattered, and veterans felt that providers’ actions during the appointment could negatively affect the experience. One veteran summed up the groups’ feelings regarding their interactions with providers by saying, “[It is] key for our care providers to treat us like people. We should be able to ask them to get off of the computer and talk to us for a bit!” Other participants nodded in agreement, and one veteran remarked that he had a provider who had diabetes and “that was great.” The veterans also appreciated positive reinforcement from the primary care team. One participant remarked, “It’s nice to get the letter from my primary care provider with a little note saying you’re doing better.”
Resources
Participants had many suggestions regarding additional resources that they would like the VA to offer to help them self-manage diabetes. Many suggestions related to having greater access to resources for weight management through exercise or healthful eating. One participant stated, “An exercise facility…I think that’s key, and not just for diabetics.” Another participant noted, “In the VA, we have places to eat. Have you seen the food they give us to eat? Fatty, carbs, fried food.” However, many veterans were unclear about what resources the VA did offer, not knowing about certain resources such as diabetic shoes. When asked to prioritize what resources are most useful, given a scarcity, most participants insisted that a wide range of resources needed to be offered, because different people have different needs. One participant summed it up: “You can’t do away with primary care, you can’t do away with education, you can’t do away with pharmacy...[and] face-to-face makes all the difference in the world.”
Discussion
The purpose of this study was to analyze the veteran experience with diabetes self-management and identify motivating factors and barriers in a population that had attended a primary care SMA series. The focus group had several interesting findings. A person’s identity or self-worth can be disrupted by the experience of chronic illness. Chronic illness can be conceptualized as a threat to one’s sense of security and identity.15 This disruption of identity at various stages of diabetes duration, from new onset to living many years with this chronic illness, is illustrated in the study participants’ comments of negotiating, adapting, and integrating diabetes into their lives.
This outcome is similar to findings by Olshansky and colleagues in which individuals struggled with the transition of becoming “a person with diabetes” rather than a “diabetic person.”16 Olshansky and colleagues suggested emphasizing lifestyle changes as health-related benefits for all people, those with and without diabetes, as a strategy to deal with normalizing their new identity; this concept can be viewed as a form of empowerment.
Related: A Shared Diabetes Clinic at a Veterans Affairs Medical Center
Furthermore, veteran identity was found to be an important factor for driving behaviors in diabetes care. This layering or double identity of diabetes plus being a veteran can be particularly challenging. Several participants commented on Agent Orange exposure during their service time as the etiology of their diabetes. Some of the veterans placed more value on what other fellow veterans said vs what health care professionals said. A study of nonveteran insulin users found that narratives or sharing of life stories of diabetes to be beneficial to the described assault on personal identity.17
As only about one-third of participants had a support person for their diabetes in this focus group, veteran-only groups likely have additional benefits, especially for those without family support. An additional complication of diabetes self-management in the veteran population is a disproportionate prevalence of posttraumatic stress disorder, depression, and substance abuse comorbidities (including alcoholism).
Of interest in this focus group was the low rating of peer-led groups as a motivator for successful diabetes self-management, perhaps because this was not offered at VAPHCS. Support or peer-led groups provide ongoing opportunities to address at least 2 of the AADE 7 self-care behaviors—problem solving and healthy coping. A recent 6-month study compared peer coaches, financial incentives, or usual care to promote behaviors for improved glucose control in African American veterans.18 Weekly telephone interventions by the peer mentors reduced A1c by 1.07% (95% confidence interval [CI], 1.84%-0.31%) compared with 0.45% (95% CI, 1.23%-0.32%) in the group with financial incentives. The authors suggest transitioning patients who achieve control from mentee to mentor roles to maintain the program’s sustainability.
Nearly all participants endorsed the SMAs as valuable for the expertise and education they offered as well as for the chance to meet regularly with a veteran diabetes cohort group for support. The SMAs could be viewed as an avenue for shared narratives that may assist individuals in understanding their experiences and adapting to their chronic illness.
Using social psychology interventions to change behaviors may be challenging in busy primary care settings and hampered when veterans perceive only pressure to do what their providers recommend in a controlled behavior fashion. Individuals in a SMA may be more apt to act in a self-determined manner when they feel they are in control and activities are done with volition and a choice consistent with their identity when supported by their fellow diabetic veterans. A previous survey of VA provider and student perceptions that used an SMA for diabetes education in a primary care setting also found benefits, but sustainability issues were identified, such as limited resources (space), organization issues with clinic structure redesign, and potential to alter long-standing patient-provider relationships.19,20
An emphasis on A1c goals may be appropriate, because this was the highest rated motivator in the focus group, although care should be taken to tailor care to the needs of the veteran. A veteran population may be even more driven by constant evaluation of their success in reaching target goals. Education may be useful about how A1c relates to diabetes, such as self-monitoring of blood sugar, complications, and medications.
A study by Heisler and colleagues found that knowing A1c values was useful to patients to assess their diabetes control but not sufficient to increase confidence or motivation.21 In this mail survey of patients with T2DM, where the VA was 1 of 5 sites, 66% did not know their last A1c value, and only 25% accurately reported that value. The authors stated that it was unknown why VA respondents had significantly lower odds than did patients at the other sites of knowing their last A1c value. This study’s focus group was anonymous, and participants were not asked whether they accurately knew their A1c value or goal.
Limitations
One of the strengths of this study is that to the authors’ knowledge, this is the first report of findings from a focus group on motivating factors and barriers for veterans with diabetes who had attended an SMA in a primary care setting. Although the study was small, the participation rate was high.
The study had a few limitations. The results might not be applicable to other populations, because all participants were veterans, predominantly male with T2DM. Selection bias is possible, because participants had already attended SMA classes. Participants may have been biased in their providing positive feedback of the SMA classes, since SMA facilitators held this focus group.
Conclusions
The study findings have several implications. Weight gain was ranked as the greatest barrier to self-managing diabetes in this focus group. Veterans stated they had limited resources, which could impact their AADE 7 self-care activities of being active and healthy eating. As resources allow, cooking classes, gym memberships, and VA-affiliated exercise facilities may be beneficial. Since there was heterogeneity in veteran experiences during diabetes diagnosis, consistent information should be provided upfront, including general concepts of diabetes and available resources.
This diabetes focus group highlighted the challenges of having a double identity, of being both a veteran and having diabetes. Shared medical appointments with veteran cohorts were identified as a promising intervention that allows for camaraderie and shared narratives to be enhanced by clinical guidance and education. By providing social support, SMAs may nudge fellow veterans to act on barriers that have them “stuck” in certain behaviors or situations. Many veterans view A1c as an important motivator, and this should be considered as a general educational tool.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Vouri SM, Shaw RF, Waterbury NV, Egge A, Alexander B. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
2. Kupersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: lessons from the Veterans Health Administration. Health Aff (Millwood). 2007;26(2):156-168.
3. Mulcahy K, Maryniuk M, Peeple M, et al. AADE Position Statement: standards for outcomes measurement of diabetes self-management education. Diabetes Educ. 2003;29(5):804-816.
4. Fitzpatrick SL, Schumann KP, Hill-Briggs F. Problem solving interventions for diabetes self-management and control: a systemic review of the literature. Diabetes Res Clin Pract. 2013;100(2):145-161.
5. Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: a systematic review. Prev Chronic Dis. 2013;10:120180.
6. Heisler M, Resnicow K. Helping patients make and sustain healthy changes: a brief introduction to motivational interviewing in clinical diabetes care. Clin Diabetes. 2008;26(4):161-165.
7. Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol. 1998;17(3):269-276.
8. Rosland AM, Piette JD, Choi HJ, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Med Care. 2011;49(1):37-45.
9. Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013;92(1):94-99.
10. Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507-515.
11. Collier IA, Baker DM. Implementation of a pharmacist-supervised outpatient diabetes treatment clinic. Am J Health Syst Pharm. 2014;71(1):27-36.
12. Kirsch S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16(5):349-353.
13. Moen J, Antonov K, Nilsson JLF. Interaction between participants in focus groups with older patients and general practitioners. Qual Health Res. 2010;20(5):607-616.
14. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
15. Aujoulat I, Marcolongo R, Bonadiman L, Deccache A. Reconsidering patient empowerment in chronic illness: a critique of models of self-efficacy and bodily control. Soc Sci Med. 2008;66(5):1228-1239.
16. Olshansky E, Sacco D, Fitzgerald K, et al. Living with diabetes: normalizing the process of managing diabetes. Diabetes Educ. 2008;34(6):1004-1012.
17. Goldman JB, Maclean HM. The significance of identity in the adjustment to diabetes among insulin users. Diabetes Educ. 1998;24(6):741-748.
18. Long JA, Jahnle EC, Richardson DM, Lowenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans. Ann Intern Med. 2012;156(6):416-424.
19. Kirsch SR, Schaub K, Aron DC. Shared medical appointments: a potential venue for education in interprofessional care. Qual Manag Health Care. 2009;18(3)217-224.
20. Kirsch SR, Lawrence RH, Aron DC. Tailoring an intervention to the context and system redesign related to the intervention: a case study of implementing shared medical appointments for diabetes. Implement Sci. 2008;3(suppl 1):34.
21. Heisler M, Piette JD, Spencer M, Kiefer E, Vijan S. The relationship between knowledge of recent HbA1c values and diabetes care understanding and self-management. Diabetes Care. 2005;28(4):816-822.
Treatment of diabetes can be difficult and challenging. Information to improve the self-management behavior of patients with diabetes is important, because the prevalence of diabetes is expected to increase as the population ages, along with rising medical costs, premature death, and morbidity due to complications. Veterans, as a group, present unique challenges in health care. A recent analysis at a VA setting found only 17.3% of veterans were meeting all 3 of their “ABC” goals—A1c, blood pressure, and cholesterol.1
Within the VA, diabetes is the third most common diagnosis, with a higher prevalence among veterans (25%) than among the general U.S. population (8.3%).2 However, little information exists about the barriers and motivations of the veterans who have completed a diabetes shared medical appointment (SMA) series.
The VA promotes SMAs as an effective alternative to one-on-one encounters for a cohort of patients with similar health conditions. In these SMAs, a multidisciplinary team meets with a group of patients for about 2 hours. These SMAs can be especially important for patients who need frequent encounters for care management, such as diabetes. Shared medical appointments focus on the American Association of Diabetes Educators 7 (AADE 7) self-care behaviors and provide a medium to foster improved self-management and healthy coping.3
Related: Education Pitfalls of Insulin Administration in Patients With Diabetes
Several systematic reviews of qualitative studies have identified and summarized factors that impact diabetes self-management.4,5 Behavioral science and social psychology provide rich examples of theories to influence and understand behaviors, including motivational interviewing and self-determinism.6,7 Other recent innovative approaches in primary care settings and diabetes self-management at the VA include companion (family or friend) participation in primary care visits, collaborative goal setting with patient and providers, age-matched patient pairing, and using a clinical pharmacist clinic as a midlevel provider to help meet VA national diabetes performance standards.8-11
In accordance with the Patient Aligned Care Team (PACT) focus on the delivery of patient care, the goal of this study was to understand the experiences of veterans and to learn about the tools and methods they perceive to be most useful in improving patient education and motivation for self-management of diabetes. A onetime diabetes focus group was held to inquire about these specific issues.
Methods
The focus group took place at the Vancouver, Washington, campus of VA Portland Health Care System (VAPHCS). All veteran participants and their family members who had completed at least 3 of a 4-session SMA series were invited. Out of 29 invited veterans, 18 participated in the discussion along with 3 family members (all wives), for a total of 21 participants. The SMAs focused on meeting primary care performance standards on A1c, blood pressure, and hyperlipidemia, in accordance with the new PACT model. The VA education division approved the use of the Conversation Map for SMAs, created by Merck (Whitehouse Station, NJ) in collaboration with the American Diabetes Association. Using the Conversation Map format in a VA setting has been shown to reduce mean A1c levels by -0.9 (± 1.9%; P < .001).12 The SMA team made lifestyle and medication changes weekly (under a scope of practice for the pharmacist).
Data Gathering and Analysis
Participants attended a 2-hour focus group facilitated by the same 4 clinic care providers (2 pharmacists, 1 clinical nurse, 1 dietician) who had led the SMAs. The decision to have the discussion led by these same providers was grounded in the belief that this format would be familiar to the participants, and the rapport already established between providers and participants would encourage greater participation than if the meeting were led by unfamiliar VA employees. Two trained VAPHCS qualitative researchers attended the focus group and took extensive verbatim notes.
During the first 45 minutes, participants used a dot-voting technique to provide general demographic and background information in response to questions posted on boards around the room. Participants then were asked to choose their top 3 answers in response to each of a series of questions about barriers, resources, and motivators in self-management. The group was divided into 2 smaller groups of 10 or 11 participants, each facilitated by 2 researchers and assisted by a note-taker trained to capture the verbatim discussion. Session audio was not recorded, because VA policy requires signed consent, and this requirement might have discouraged participation.
The following questions guided the discussion: (1) Thinking back to when you were diagnosed with diabetes, what could you have done then that would have made a difference? (2) Thinking about all your experiences with diabetes, what was most helpful in motivating you to take control? (3) What one thing helped education or information “stick” with you? (4) What additional resources that are not currently available at the VA would help you? and (5) Tell us about your diabetes management plan.
Related: Diabetes Patient-Centered Medical Home Approach
After the focus groups, the research team used a formal debriefing tool to identify both initial impressions of possible discussion themes and group dynamics potentially influencing the content of the discussion; no significant communication or participation issues were identified.13 All research team members read the discussion notes and met to iteratively develop a simple codebook of global themes, using an approach of general inductive thematic content analysis.14
Two team members coded the notes, paying attention to the need to capture divergent or minority positions voiced by participants. Both coders worked toward consensus on code definitions through repeated discussions with each other and with the full research team. Codes were then used to sort and analyze about 180 comments made by participants during the focus groups. The Institutional Review Board of the VAPHCS approved the protocol for this study.
Results
Most participants in the diabetes focus group had type 2 diabetes mellitus (T2DM) and were male—1 female veteran participated (eTable).
After the initial analysis, all participants were mailed a letter summarizing themes and suggestions from the meeting (see Box). Responses to questions posted on the board and responded to by voting are included in Table 1. Weight gain was most commonly chosen as a barrier to self-management. The A1c value was the highest rated motivator for self-management of diabetes, followed by face-to-face support from the care team and family support. Participants chose one-on-one appointments with the diabetes team and classes with instructors as the most helpful VA resources.
The final codebook resulted in 9 domains: diagnostic experience, what helps, perceived value of the SMA group, veteran identity, interaction with care providers, denial, fatalism, motivators, and barriers; each contained several related codes. Several themes emerged from the analysis of the focus group data for a desired experience of managing and coping with diabetes.
Identity as a Person With Diabetes
Participants were at various stages of their identity with a chronic illness. Over time, the veterans noted a transition from being a “diabetic person” to a “person living healthy with diabetes.” One veteran’s comment encapsulated the shift in diabetic identity over time: “[Initially] when looking up diabetes on the computer, there were scary things. It was very frightening, and I was thinking, oh, they’re going to cut off my legs. Years later you have more objectivity and control.”
Some participants responded with denial, rejecting that they had diabetes or that they needed to make lifestyle changes. When asked how he felt when first diagnosed, one veteran stated, “I resisted endlessly. I wouldn’t take my face out of the food.” Many veterans expressed that the diagnosis of diabetes was similar to an assault on their identity.
One veteran with long-standing diabetes shared the following: “I got a giant plastic box, and every needle [I use] goes into the box. Every day I look at this huge pile of needles. It’s my sign of weakness. If I kick it…I won’t keep adding them [to the pile].”
Similarly another veteran stated, “I felt like a failure, not a winner, when I started taking insulin.” The A1c test result was seen “like a hammer coming down,” an indication of the individual’s success or failure.
Identity as a Veteran
For some veterans in the focus group, the development of diabetes was considered to be service connected and related to chemical exposure to herbicides, including Agent Orange. One veteran emphasized, “This [diabetes] is ’cause of Agent Orange exposure.”
Another participant commented, “If you’ve served your country, you’re strong…[you think diabetes] can’t happen to you.” One participant explained, “Hearing it from [other veterans in focus group].... I don’t know if this was bred into me in the service. Probably. These guys have traversed the territory. I go to these guys for my answers. And hearing it from them, you could tell me everything I need to hear, and every one of these guys could tell me the exact same thing, and I would listen to them and not to [the clinical staff].”
Early Education
Another theme was a general desire for early information and education. Veterans suggested that information and self-management coaching when diagnosed with prediabetes would have been beneficial to reduce the risk of progression to diabetes. Many participants expressed regret that tools such as A1c monitoring were not available to them earlier: “I was also diagnosed borderline…that’s when I should have been hit by the 2 x 4...[they] should have done the A1c every 6 months.” Some participants described feelings that early education and, as one put it, “more emphasis on the seriousness of it,” would have helped them prevent their diabetes from worsening or develop healthier habits for self-management earlier.
Another veteran had what the group felt was the optimal experience: “The nurse told me I was diabetic...sat there for 45 minutes and just talked to me about it. It was the fact that she sat and talked with me and covered all the questions I had. That was the best thing bar none.”
Veterans expressed frustration with the time delay between the diagnosis and availability of clinical support and education: “When I first got the diagnosis, there was 4 or 5 weeks until the class. I’m thinking, what they should have done as soon as they sent that letter with the A1c, they should have sent me a packet saying, here’s what you can do NOW. Boom!”
Interventions
The chance to meet with other veterans with diabetes was something many participants said was helpful and provided a specific benefit that health care providers on their own could not give. One participant stated, “Classes make you feel more normal, when you sit with these people whose experiences you share.” Another stated, “When people have had a problem, get together and say how they’ve overcome it, I wanna hear about it.” The veterans agreed that someone who has specialty training in diabetes, not just in peer-to-peer support groups, should lead the education or support groups.
When the veterans were asked whether they thought that a veteran-led group would be beneficial, one veteran stated, “A support group must have a facilitator that has skills and resources.” Another stated, “You need at least one person to give you direction.”
One veteran explained that having weekly classes and hearing the same information several times helped information to “stick.” Another veteran, while expressing frustration about the lack of education he received on diet, stated, “What we eat directly affects us…classes like this are the greatest thing that ever happened. They give us more support than the doctors ever do.” One veteran described how having his weekly morning SMA class to look forward to was a strong motivation to pay attention to all the things that matter to his diabetes throughout the week. Another veteran emphasized the SMA as being important, because “being members of the military, you still have the civilians and they are them and we are us. ... With no family members, this [SMA] has made a big difference.”
Provider Relationship
Veterans expressed that having a positive provider relationship was an important element in diabetes self-management. The lack of time available for diabetes management in standard primary care encounters was cited as a barrier. One veteran stated, “[Providers] diagnose you with a blood test and [push you] out the door!” One veteran observed, “I think they need to turn you over to a nurse practitioner. You’re better off with someone like that who actually has time to talk to you instead of leaving you with someone who just gives you a prescription.”
The quality of the interaction mattered, and veterans felt that providers’ actions during the appointment could negatively affect the experience. One veteran summed up the groups’ feelings regarding their interactions with providers by saying, “[It is] key for our care providers to treat us like people. We should be able to ask them to get off of the computer and talk to us for a bit!” Other participants nodded in agreement, and one veteran remarked that he had a provider who had diabetes and “that was great.” The veterans also appreciated positive reinforcement from the primary care team. One participant remarked, “It’s nice to get the letter from my primary care provider with a little note saying you’re doing better.”
Resources
Participants had many suggestions regarding additional resources that they would like the VA to offer to help them self-manage diabetes. Many suggestions related to having greater access to resources for weight management through exercise or healthful eating. One participant stated, “An exercise facility…I think that’s key, and not just for diabetics.” Another participant noted, “In the VA, we have places to eat. Have you seen the food they give us to eat? Fatty, carbs, fried food.” However, many veterans were unclear about what resources the VA did offer, not knowing about certain resources such as diabetic shoes. When asked to prioritize what resources are most useful, given a scarcity, most participants insisted that a wide range of resources needed to be offered, because different people have different needs. One participant summed it up: “You can’t do away with primary care, you can’t do away with education, you can’t do away with pharmacy...[and] face-to-face makes all the difference in the world.”
Discussion
The purpose of this study was to analyze the veteran experience with diabetes self-management and identify motivating factors and barriers in a population that had attended a primary care SMA series. The focus group had several interesting findings. A person’s identity or self-worth can be disrupted by the experience of chronic illness. Chronic illness can be conceptualized as a threat to one’s sense of security and identity.15 This disruption of identity at various stages of diabetes duration, from new onset to living many years with this chronic illness, is illustrated in the study participants’ comments of negotiating, adapting, and integrating diabetes into their lives.
This outcome is similar to findings by Olshansky and colleagues in which individuals struggled with the transition of becoming “a person with diabetes” rather than a “diabetic person.”16 Olshansky and colleagues suggested emphasizing lifestyle changes as health-related benefits for all people, those with and without diabetes, as a strategy to deal with normalizing their new identity; this concept can be viewed as a form of empowerment.
Related: A Shared Diabetes Clinic at a Veterans Affairs Medical Center
Furthermore, veteran identity was found to be an important factor for driving behaviors in diabetes care. This layering or double identity of diabetes plus being a veteran can be particularly challenging. Several participants commented on Agent Orange exposure during their service time as the etiology of their diabetes. Some of the veterans placed more value on what other fellow veterans said vs what health care professionals said. A study of nonveteran insulin users found that narratives or sharing of life stories of diabetes to be beneficial to the described assault on personal identity.17
As only about one-third of participants had a support person for their diabetes in this focus group, veteran-only groups likely have additional benefits, especially for those without family support. An additional complication of diabetes self-management in the veteran population is a disproportionate prevalence of posttraumatic stress disorder, depression, and substance abuse comorbidities (including alcoholism).
Of interest in this focus group was the low rating of peer-led groups as a motivator for successful diabetes self-management, perhaps because this was not offered at VAPHCS. Support or peer-led groups provide ongoing opportunities to address at least 2 of the AADE 7 self-care behaviors—problem solving and healthy coping. A recent 6-month study compared peer coaches, financial incentives, or usual care to promote behaviors for improved glucose control in African American veterans.18 Weekly telephone interventions by the peer mentors reduced A1c by 1.07% (95% confidence interval [CI], 1.84%-0.31%) compared with 0.45% (95% CI, 1.23%-0.32%) in the group with financial incentives. The authors suggest transitioning patients who achieve control from mentee to mentor roles to maintain the program’s sustainability.
Nearly all participants endorsed the SMAs as valuable for the expertise and education they offered as well as for the chance to meet regularly with a veteran diabetes cohort group for support. The SMAs could be viewed as an avenue for shared narratives that may assist individuals in understanding their experiences and adapting to their chronic illness.
Using social psychology interventions to change behaviors may be challenging in busy primary care settings and hampered when veterans perceive only pressure to do what their providers recommend in a controlled behavior fashion. Individuals in a SMA may be more apt to act in a self-determined manner when they feel they are in control and activities are done with volition and a choice consistent with their identity when supported by their fellow diabetic veterans. A previous survey of VA provider and student perceptions that used an SMA for diabetes education in a primary care setting also found benefits, but sustainability issues were identified, such as limited resources (space), organization issues with clinic structure redesign, and potential to alter long-standing patient-provider relationships.19,20
An emphasis on A1c goals may be appropriate, because this was the highest rated motivator in the focus group, although care should be taken to tailor care to the needs of the veteran. A veteran population may be even more driven by constant evaluation of their success in reaching target goals. Education may be useful about how A1c relates to diabetes, such as self-monitoring of blood sugar, complications, and medications.
A study by Heisler and colleagues found that knowing A1c values was useful to patients to assess their diabetes control but not sufficient to increase confidence or motivation.21 In this mail survey of patients with T2DM, where the VA was 1 of 5 sites, 66% did not know their last A1c value, and only 25% accurately reported that value. The authors stated that it was unknown why VA respondents had significantly lower odds than did patients at the other sites of knowing their last A1c value. This study’s focus group was anonymous, and participants were not asked whether they accurately knew their A1c value or goal.
Limitations
One of the strengths of this study is that to the authors’ knowledge, this is the first report of findings from a focus group on motivating factors and barriers for veterans with diabetes who had attended an SMA in a primary care setting. Although the study was small, the participation rate was high.
The study had a few limitations. The results might not be applicable to other populations, because all participants were veterans, predominantly male with T2DM. Selection bias is possible, because participants had already attended SMA classes. Participants may have been biased in their providing positive feedback of the SMA classes, since SMA facilitators held this focus group.
Conclusions
The study findings have several implications. Weight gain was ranked as the greatest barrier to self-managing diabetes in this focus group. Veterans stated they had limited resources, which could impact their AADE 7 self-care activities of being active and healthy eating. As resources allow, cooking classes, gym memberships, and VA-affiliated exercise facilities may be beneficial. Since there was heterogeneity in veteran experiences during diabetes diagnosis, consistent information should be provided upfront, including general concepts of diabetes and available resources.
This diabetes focus group highlighted the challenges of having a double identity, of being both a veteran and having diabetes. Shared medical appointments with veteran cohorts were identified as a promising intervention that allows for camaraderie and shared narratives to be enhanced by clinical guidance and education. By providing social support, SMAs may nudge fellow veterans to act on barriers that have them “stuck” in certain behaviors or situations. Many veterans view A1c as an important motivator, and this should be considered as a general educational tool.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Treatment of diabetes can be difficult and challenging. Information to improve the self-management behavior of patients with diabetes is important, because the prevalence of diabetes is expected to increase as the population ages, along with rising medical costs, premature death, and morbidity due to complications. Veterans, as a group, present unique challenges in health care. A recent analysis at a VA setting found only 17.3% of veterans were meeting all 3 of their “ABC” goals—A1c, blood pressure, and cholesterol.1
Within the VA, diabetes is the third most common diagnosis, with a higher prevalence among veterans (25%) than among the general U.S. population (8.3%).2 However, little information exists about the barriers and motivations of the veterans who have completed a diabetes shared medical appointment (SMA) series.
The VA promotes SMAs as an effective alternative to one-on-one encounters for a cohort of patients with similar health conditions. In these SMAs, a multidisciplinary team meets with a group of patients for about 2 hours. These SMAs can be especially important for patients who need frequent encounters for care management, such as diabetes. Shared medical appointments focus on the American Association of Diabetes Educators 7 (AADE 7) self-care behaviors and provide a medium to foster improved self-management and healthy coping.3
Related: Education Pitfalls of Insulin Administration in Patients With Diabetes
Several systematic reviews of qualitative studies have identified and summarized factors that impact diabetes self-management.4,5 Behavioral science and social psychology provide rich examples of theories to influence and understand behaviors, including motivational interviewing and self-determinism.6,7 Other recent innovative approaches in primary care settings and diabetes self-management at the VA include companion (family or friend) participation in primary care visits, collaborative goal setting with patient and providers, age-matched patient pairing, and using a clinical pharmacist clinic as a midlevel provider to help meet VA national diabetes performance standards.8-11
In accordance with the Patient Aligned Care Team (PACT) focus on the delivery of patient care, the goal of this study was to understand the experiences of veterans and to learn about the tools and methods they perceive to be most useful in improving patient education and motivation for self-management of diabetes. A onetime diabetes focus group was held to inquire about these specific issues.
Methods
The focus group took place at the Vancouver, Washington, campus of VA Portland Health Care System (VAPHCS). All veteran participants and their family members who had completed at least 3 of a 4-session SMA series were invited. Out of 29 invited veterans, 18 participated in the discussion along with 3 family members (all wives), for a total of 21 participants. The SMAs focused on meeting primary care performance standards on A1c, blood pressure, and hyperlipidemia, in accordance with the new PACT model. The VA education division approved the use of the Conversation Map for SMAs, created by Merck (Whitehouse Station, NJ) in collaboration with the American Diabetes Association. Using the Conversation Map format in a VA setting has been shown to reduce mean A1c levels by -0.9 (± 1.9%; P < .001).12 The SMA team made lifestyle and medication changes weekly (under a scope of practice for the pharmacist).
Data Gathering and Analysis
Participants attended a 2-hour focus group facilitated by the same 4 clinic care providers (2 pharmacists, 1 clinical nurse, 1 dietician) who had led the SMAs. The decision to have the discussion led by these same providers was grounded in the belief that this format would be familiar to the participants, and the rapport already established between providers and participants would encourage greater participation than if the meeting were led by unfamiliar VA employees. Two trained VAPHCS qualitative researchers attended the focus group and took extensive verbatim notes.
During the first 45 minutes, participants used a dot-voting technique to provide general demographic and background information in response to questions posted on boards around the room. Participants then were asked to choose their top 3 answers in response to each of a series of questions about barriers, resources, and motivators in self-management. The group was divided into 2 smaller groups of 10 or 11 participants, each facilitated by 2 researchers and assisted by a note-taker trained to capture the verbatim discussion. Session audio was not recorded, because VA policy requires signed consent, and this requirement might have discouraged participation.
The following questions guided the discussion: (1) Thinking back to when you were diagnosed with diabetes, what could you have done then that would have made a difference? (2) Thinking about all your experiences with diabetes, what was most helpful in motivating you to take control? (3) What one thing helped education or information “stick” with you? (4) What additional resources that are not currently available at the VA would help you? and (5) Tell us about your diabetes management plan.
Related: Diabetes Patient-Centered Medical Home Approach
After the focus groups, the research team used a formal debriefing tool to identify both initial impressions of possible discussion themes and group dynamics potentially influencing the content of the discussion; no significant communication or participation issues were identified.13 All research team members read the discussion notes and met to iteratively develop a simple codebook of global themes, using an approach of general inductive thematic content analysis.14
Two team members coded the notes, paying attention to the need to capture divergent or minority positions voiced by participants. Both coders worked toward consensus on code definitions through repeated discussions with each other and with the full research team. Codes were then used to sort and analyze about 180 comments made by participants during the focus groups. The Institutional Review Board of the VAPHCS approved the protocol for this study.
Results
Most participants in the diabetes focus group had type 2 diabetes mellitus (T2DM) and were male—1 female veteran participated (eTable).
After the initial analysis, all participants were mailed a letter summarizing themes and suggestions from the meeting (see Box). Responses to questions posted on the board and responded to by voting are included in Table 1. Weight gain was most commonly chosen as a barrier to self-management. The A1c value was the highest rated motivator for self-management of diabetes, followed by face-to-face support from the care team and family support. Participants chose one-on-one appointments with the diabetes team and classes with instructors as the most helpful VA resources.
The final codebook resulted in 9 domains: diagnostic experience, what helps, perceived value of the SMA group, veteran identity, interaction with care providers, denial, fatalism, motivators, and barriers; each contained several related codes. Several themes emerged from the analysis of the focus group data for a desired experience of managing and coping with diabetes.
Identity as a Person With Diabetes
Participants were at various stages of their identity with a chronic illness. Over time, the veterans noted a transition from being a “diabetic person” to a “person living healthy with diabetes.” One veteran’s comment encapsulated the shift in diabetic identity over time: “[Initially] when looking up diabetes on the computer, there were scary things. It was very frightening, and I was thinking, oh, they’re going to cut off my legs. Years later you have more objectivity and control.”
Some participants responded with denial, rejecting that they had diabetes or that they needed to make lifestyle changes. When asked how he felt when first diagnosed, one veteran stated, “I resisted endlessly. I wouldn’t take my face out of the food.” Many veterans expressed that the diagnosis of diabetes was similar to an assault on their identity.
One veteran with long-standing diabetes shared the following: “I got a giant plastic box, and every needle [I use] goes into the box. Every day I look at this huge pile of needles. It’s my sign of weakness. If I kick it…I won’t keep adding them [to the pile].”
Similarly another veteran stated, “I felt like a failure, not a winner, when I started taking insulin.” The A1c test result was seen “like a hammer coming down,” an indication of the individual’s success or failure.
Identity as a Veteran
For some veterans in the focus group, the development of diabetes was considered to be service connected and related to chemical exposure to herbicides, including Agent Orange. One veteran emphasized, “This [diabetes] is ’cause of Agent Orange exposure.”
Another participant commented, “If you’ve served your country, you’re strong…[you think diabetes] can’t happen to you.” One participant explained, “Hearing it from [other veterans in focus group].... I don’t know if this was bred into me in the service. Probably. These guys have traversed the territory. I go to these guys for my answers. And hearing it from them, you could tell me everything I need to hear, and every one of these guys could tell me the exact same thing, and I would listen to them and not to [the clinical staff].”
Early Education
Another theme was a general desire for early information and education. Veterans suggested that information and self-management coaching when diagnosed with prediabetes would have been beneficial to reduce the risk of progression to diabetes. Many participants expressed regret that tools such as A1c monitoring were not available to them earlier: “I was also diagnosed borderline…that’s when I should have been hit by the 2 x 4...[they] should have done the A1c every 6 months.” Some participants described feelings that early education and, as one put it, “more emphasis on the seriousness of it,” would have helped them prevent their diabetes from worsening or develop healthier habits for self-management earlier.
Another veteran had what the group felt was the optimal experience: “The nurse told me I was diabetic...sat there for 45 minutes and just talked to me about it. It was the fact that she sat and talked with me and covered all the questions I had. That was the best thing bar none.”
Veterans expressed frustration with the time delay between the diagnosis and availability of clinical support and education: “When I first got the diagnosis, there was 4 or 5 weeks until the class. I’m thinking, what they should have done as soon as they sent that letter with the A1c, they should have sent me a packet saying, here’s what you can do NOW. Boom!”
Interventions
The chance to meet with other veterans with diabetes was something many participants said was helpful and provided a specific benefit that health care providers on their own could not give. One participant stated, “Classes make you feel more normal, when you sit with these people whose experiences you share.” Another stated, “When people have had a problem, get together and say how they’ve overcome it, I wanna hear about it.” The veterans agreed that someone who has specialty training in diabetes, not just in peer-to-peer support groups, should lead the education or support groups.
When the veterans were asked whether they thought that a veteran-led group would be beneficial, one veteran stated, “A support group must have a facilitator that has skills and resources.” Another stated, “You need at least one person to give you direction.”
One veteran explained that having weekly classes and hearing the same information several times helped information to “stick.” Another veteran, while expressing frustration about the lack of education he received on diet, stated, “What we eat directly affects us…classes like this are the greatest thing that ever happened. They give us more support than the doctors ever do.” One veteran described how having his weekly morning SMA class to look forward to was a strong motivation to pay attention to all the things that matter to his diabetes throughout the week. Another veteran emphasized the SMA as being important, because “being members of the military, you still have the civilians and they are them and we are us. ... With no family members, this [SMA] has made a big difference.”
Provider Relationship
Veterans expressed that having a positive provider relationship was an important element in diabetes self-management. The lack of time available for diabetes management in standard primary care encounters was cited as a barrier. One veteran stated, “[Providers] diagnose you with a blood test and [push you] out the door!” One veteran observed, “I think they need to turn you over to a nurse practitioner. You’re better off with someone like that who actually has time to talk to you instead of leaving you with someone who just gives you a prescription.”
The quality of the interaction mattered, and veterans felt that providers’ actions during the appointment could negatively affect the experience. One veteran summed up the groups’ feelings regarding their interactions with providers by saying, “[It is] key for our care providers to treat us like people. We should be able to ask them to get off of the computer and talk to us for a bit!” Other participants nodded in agreement, and one veteran remarked that he had a provider who had diabetes and “that was great.” The veterans also appreciated positive reinforcement from the primary care team. One participant remarked, “It’s nice to get the letter from my primary care provider with a little note saying you’re doing better.”
Resources
Participants had many suggestions regarding additional resources that they would like the VA to offer to help them self-manage diabetes. Many suggestions related to having greater access to resources for weight management through exercise or healthful eating. One participant stated, “An exercise facility…I think that’s key, and not just for diabetics.” Another participant noted, “In the VA, we have places to eat. Have you seen the food they give us to eat? Fatty, carbs, fried food.” However, many veterans were unclear about what resources the VA did offer, not knowing about certain resources such as diabetic shoes. When asked to prioritize what resources are most useful, given a scarcity, most participants insisted that a wide range of resources needed to be offered, because different people have different needs. One participant summed it up: “You can’t do away with primary care, you can’t do away with education, you can’t do away with pharmacy...[and] face-to-face makes all the difference in the world.”
Discussion
The purpose of this study was to analyze the veteran experience with diabetes self-management and identify motivating factors and barriers in a population that had attended a primary care SMA series. The focus group had several interesting findings. A person’s identity or self-worth can be disrupted by the experience of chronic illness. Chronic illness can be conceptualized as a threat to one’s sense of security and identity.15 This disruption of identity at various stages of diabetes duration, from new onset to living many years with this chronic illness, is illustrated in the study participants’ comments of negotiating, adapting, and integrating diabetes into their lives.
This outcome is similar to findings by Olshansky and colleagues in which individuals struggled with the transition of becoming “a person with diabetes” rather than a “diabetic person.”16 Olshansky and colleagues suggested emphasizing lifestyle changes as health-related benefits for all people, those with and without diabetes, as a strategy to deal with normalizing their new identity; this concept can be viewed as a form of empowerment.
Related: A Shared Diabetes Clinic at a Veterans Affairs Medical Center
Furthermore, veteran identity was found to be an important factor for driving behaviors in diabetes care. This layering or double identity of diabetes plus being a veteran can be particularly challenging. Several participants commented on Agent Orange exposure during their service time as the etiology of their diabetes. Some of the veterans placed more value on what other fellow veterans said vs what health care professionals said. A study of nonveteran insulin users found that narratives or sharing of life stories of diabetes to be beneficial to the described assault on personal identity.17
As only about one-third of participants had a support person for their diabetes in this focus group, veteran-only groups likely have additional benefits, especially for those without family support. An additional complication of diabetes self-management in the veteran population is a disproportionate prevalence of posttraumatic stress disorder, depression, and substance abuse comorbidities (including alcoholism).
Of interest in this focus group was the low rating of peer-led groups as a motivator for successful diabetes self-management, perhaps because this was not offered at VAPHCS. Support or peer-led groups provide ongoing opportunities to address at least 2 of the AADE 7 self-care behaviors—problem solving and healthy coping. A recent 6-month study compared peer coaches, financial incentives, or usual care to promote behaviors for improved glucose control in African American veterans.18 Weekly telephone interventions by the peer mentors reduced A1c by 1.07% (95% confidence interval [CI], 1.84%-0.31%) compared with 0.45% (95% CI, 1.23%-0.32%) in the group with financial incentives. The authors suggest transitioning patients who achieve control from mentee to mentor roles to maintain the program’s sustainability.
Nearly all participants endorsed the SMAs as valuable for the expertise and education they offered as well as for the chance to meet regularly with a veteran diabetes cohort group for support. The SMAs could be viewed as an avenue for shared narratives that may assist individuals in understanding their experiences and adapting to their chronic illness.
Using social psychology interventions to change behaviors may be challenging in busy primary care settings and hampered when veterans perceive only pressure to do what their providers recommend in a controlled behavior fashion. Individuals in a SMA may be more apt to act in a self-determined manner when they feel they are in control and activities are done with volition and a choice consistent with their identity when supported by their fellow diabetic veterans. A previous survey of VA provider and student perceptions that used an SMA for diabetes education in a primary care setting also found benefits, but sustainability issues were identified, such as limited resources (space), organization issues with clinic structure redesign, and potential to alter long-standing patient-provider relationships.19,20
An emphasis on A1c goals may be appropriate, because this was the highest rated motivator in the focus group, although care should be taken to tailor care to the needs of the veteran. A veteran population may be even more driven by constant evaluation of their success in reaching target goals. Education may be useful about how A1c relates to diabetes, such as self-monitoring of blood sugar, complications, and medications.
A study by Heisler and colleagues found that knowing A1c values was useful to patients to assess their diabetes control but not sufficient to increase confidence or motivation.21 In this mail survey of patients with T2DM, where the VA was 1 of 5 sites, 66% did not know their last A1c value, and only 25% accurately reported that value. The authors stated that it was unknown why VA respondents had significantly lower odds than did patients at the other sites of knowing their last A1c value. This study’s focus group was anonymous, and participants were not asked whether they accurately knew their A1c value or goal.
Limitations
One of the strengths of this study is that to the authors’ knowledge, this is the first report of findings from a focus group on motivating factors and barriers for veterans with diabetes who had attended an SMA in a primary care setting. Although the study was small, the participation rate was high.
The study had a few limitations. The results might not be applicable to other populations, because all participants were veterans, predominantly male with T2DM. Selection bias is possible, because participants had already attended SMA classes. Participants may have been biased in their providing positive feedback of the SMA classes, since SMA facilitators held this focus group.
Conclusions
The study findings have several implications. Weight gain was ranked as the greatest barrier to self-managing diabetes in this focus group. Veterans stated they had limited resources, which could impact their AADE 7 self-care activities of being active and healthy eating. As resources allow, cooking classes, gym memberships, and VA-affiliated exercise facilities may be beneficial. Since there was heterogeneity in veteran experiences during diabetes diagnosis, consistent information should be provided upfront, including general concepts of diabetes and available resources.
This diabetes focus group highlighted the challenges of having a double identity, of being both a veteran and having diabetes. Shared medical appointments with veteran cohorts were identified as a promising intervention that allows for camaraderie and shared narratives to be enhanced by clinical guidance and education. By providing social support, SMAs may nudge fellow veterans to act on barriers that have them “stuck” in certain behaviors or situations. Many veterans view A1c as an important motivator, and this should be considered as a general educational tool.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Vouri SM, Shaw RF, Waterbury NV, Egge A, Alexander B. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
2. Kupersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: lessons from the Veterans Health Administration. Health Aff (Millwood). 2007;26(2):156-168.
3. Mulcahy K, Maryniuk M, Peeple M, et al. AADE Position Statement: standards for outcomes measurement of diabetes self-management education. Diabetes Educ. 2003;29(5):804-816.
4. Fitzpatrick SL, Schumann KP, Hill-Briggs F. Problem solving interventions for diabetes self-management and control: a systemic review of the literature. Diabetes Res Clin Pract. 2013;100(2):145-161.
5. Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: a systematic review. Prev Chronic Dis. 2013;10:120180.
6. Heisler M, Resnicow K. Helping patients make and sustain healthy changes: a brief introduction to motivational interviewing in clinical diabetes care. Clin Diabetes. 2008;26(4):161-165.
7. Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol. 1998;17(3):269-276.
8. Rosland AM, Piette JD, Choi HJ, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Med Care. 2011;49(1):37-45.
9. Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013;92(1):94-99.
10. Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507-515.
11. Collier IA, Baker DM. Implementation of a pharmacist-supervised outpatient diabetes treatment clinic. Am J Health Syst Pharm. 2014;71(1):27-36.
12. Kirsch S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16(5):349-353.
13. Moen J, Antonov K, Nilsson JLF. Interaction between participants in focus groups with older patients and general practitioners. Qual Health Res. 2010;20(5):607-616.
14. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
15. Aujoulat I, Marcolongo R, Bonadiman L, Deccache A. Reconsidering patient empowerment in chronic illness: a critique of models of self-efficacy and bodily control. Soc Sci Med. 2008;66(5):1228-1239.
16. Olshansky E, Sacco D, Fitzgerald K, et al. Living with diabetes: normalizing the process of managing diabetes. Diabetes Educ. 2008;34(6):1004-1012.
17. Goldman JB, Maclean HM. The significance of identity in the adjustment to diabetes among insulin users. Diabetes Educ. 1998;24(6):741-748.
18. Long JA, Jahnle EC, Richardson DM, Lowenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans. Ann Intern Med. 2012;156(6):416-424.
19. Kirsch SR, Schaub K, Aron DC. Shared medical appointments: a potential venue for education in interprofessional care. Qual Manag Health Care. 2009;18(3)217-224.
20. Kirsch SR, Lawrence RH, Aron DC. Tailoring an intervention to the context and system redesign related to the intervention: a case study of implementing shared medical appointments for diabetes. Implement Sci. 2008;3(suppl 1):34.
21. Heisler M, Piette JD, Spencer M, Kiefer E, Vijan S. The relationship between knowledge of recent HbA1c values and diabetes care understanding and self-management. Diabetes Care. 2005;28(4):816-822.
1. Vouri SM, Shaw RF, Waterbury NV, Egge A, Alexander B. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
2. Kupersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: lessons from the Veterans Health Administration. Health Aff (Millwood). 2007;26(2):156-168.
3. Mulcahy K, Maryniuk M, Peeple M, et al. AADE Position Statement: standards for outcomes measurement of diabetes self-management education. Diabetes Educ. 2003;29(5):804-816.
4. Fitzpatrick SL, Schumann KP, Hill-Briggs F. Problem solving interventions for diabetes self-management and control: a systemic review of the literature. Diabetes Res Clin Pract. 2013;100(2):145-161.
5. Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: a systematic review. Prev Chronic Dis. 2013;10:120180.
6. Heisler M, Resnicow K. Helping patients make and sustain healthy changes: a brief introduction to motivational interviewing in clinical diabetes care. Clin Diabetes. 2008;26(4):161-165.
7. Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol. 1998;17(3):269-276.
8. Rosland AM, Piette JD, Choi HJ, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Med Care. 2011;49(1):37-45.
9. Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013;92(1):94-99.
10. Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507-515.
11. Collier IA, Baker DM. Implementation of a pharmacist-supervised outpatient diabetes treatment clinic. Am J Health Syst Pharm. 2014;71(1):27-36.
12. Kirsch S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16(5):349-353.
13. Moen J, Antonov K, Nilsson JLF. Interaction between participants in focus groups with older patients and general practitioners. Qual Health Res. 2010;20(5):607-616.
14. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
15. Aujoulat I, Marcolongo R, Bonadiman L, Deccache A. Reconsidering patient empowerment in chronic illness: a critique of models of self-efficacy and bodily control. Soc Sci Med. 2008;66(5):1228-1239.
16. Olshansky E, Sacco D, Fitzgerald K, et al. Living with diabetes: normalizing the process of managing diabetes. Diabetes Educ. 2008;34(6):1004-1012.
17. Goldman JB, Maclean HM. The significance of identity in the adjustment to diabetes among insulin users. Diabetes Educ. 1998;24(6):741-748.
18. Long JA, Jahnle EC, Richardson DM, Lowenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans. Ann Intern Med. 2012;156(6):416-424.
19. Kirsch SR, Schaub K, Aron DC. Shared medical appointments: a potential venue for education in interprofessional care. Qual Manag Health Care. 2009;18(3)217-224.
20. Kirsch SR, Lawrence RH, Aron DC. Tailoring an intervention to the context and system redesign related to the intervention: a case study of implementing shared medical appointments for diabetes. Implement Sci. 2008;3(suppl 1):34.
21. Heisler M, Piette JD, Spencer M, Kiefer E, Vijan S. The relationship between knowledge of recent HbA1c values and diabetes care understanding and self-management. Diabetes Care. 2005;28(4):816-822.