User login
Polydactyly of the Hand
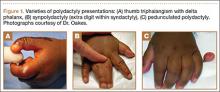
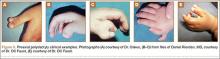
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
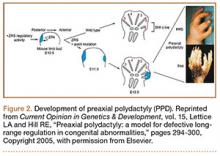
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
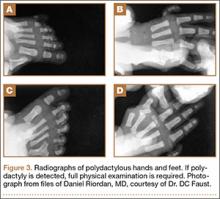
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
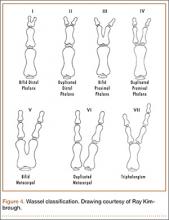
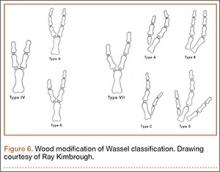
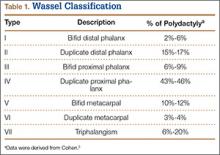
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
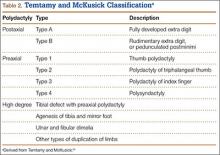
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
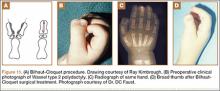
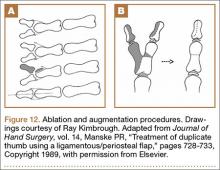
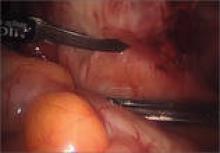
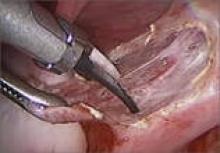
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
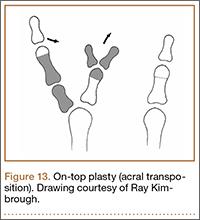
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
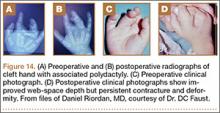
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
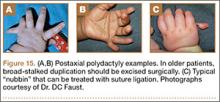
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
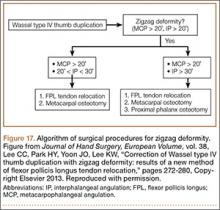
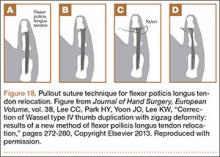
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
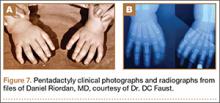
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
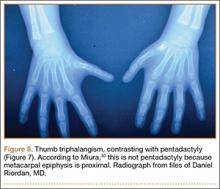
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
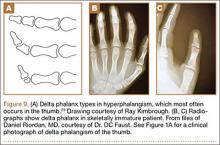
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
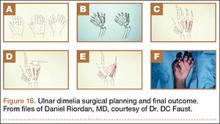
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Decision Making in Venous Thromboembolism
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
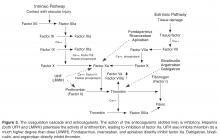
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
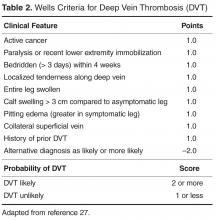
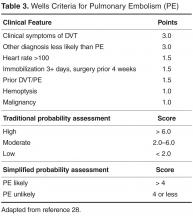
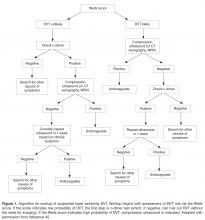
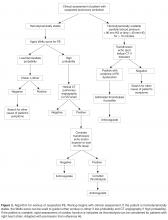
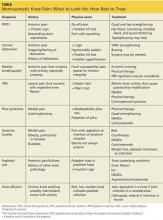
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
Healing the Broken Places
May is Mental Health Month; in recognition of this—and our 25th anniversary—we decided to reprint this 2008 feature article on the state of mental health care in the United States. It’s the story I am personally most proud of telling, but rereading it today leads me to wonder: How much progress have we actually made? Please share your thoughts and experiences with me at [email protected]. —AMH
Broken. Grossly underfunded. In crisis. That’s how psychiatric and behavioral health specialists describe the current state of mental health care in the United States. The problems that plague the health care system in general—workforce shortages, barriers to access, and inadequate reimbursement—are only exacerbated in mental health.
“Mental illness isn’t glamorous,” says Don St. John, MA, PA-C, who practices in adult outpatient psychiatry at the University of Iowa Behavioral Health in Iowa City. Taking an example from the academic medical center setting, he adds, “It’s nice to have the cardiac surgery wing dedicated to or named after your family—but nobody wants a mental health wing named after them.”
Stigma is perhaps the greatest challenge with this patient population. “Until this country really embraces the notion that mental health is inherent in every aspect of a person’s general health,” says Gail W. Stuart, PhD, APRN, FAAN, Professor and Dean of the College of Nursing at Medical University of South Carolina, Charleston, “I think the stigma issue is going to continue to make it difficult to overcome these problems.”
FROM HOSPITALS TO JAILS
The current state of mental health care in the US is perhaps a direct result of the deinstitutionalization that occurred in the 1980s. By that time, most mental health hospitals were overcrowded and, in the worst cases, patients were subject to neglect and even abuse. (Recall, if you can, Geraldo Rivera skulking through the dark at Willowbrook State School in Staten Island, NY; his 1972 exposé brought the issue to the forefront.)
Following the public outcry over the treatment of these patients who—mentally ill or not—were people, there was a movement to reduce the number of long-term hospitalizations for mental illness. Along the way, the number of hospital beds available for mentally ill patients also declined, as freestanding hospitals and private facilities closed. What have these patients been left with?
“The thought was that people would be maintained in the community—there would be community support services, halfway houses, boarding homes, community-based programs,” says Catherine R. Judd, MS, PA-C, President of the Association of Psychiatric PAs, who works in the Department of Psychiatry at the University of Texas Southwestern Medical Center, Dallas. “The idea is good, but unfortunately, most of those programs have really not materialized to the extent or with the capacity to take care of the people who are out there.”
Without these services—and with an overtaxed health care system in general—many patients with mental illness find themselves adrift. And, eventually, incarcerated. An estimate from the US Department of Justice indicates that 24% of state and 21% of local prisoners have a recent history of mental illness. The largest psychiatric hospital in the country is the Los Angeles County Jail.
The problem is so widespread and so serious that both Judd and Jeanne Clement, EdD, APRN, BC, FAAN, President of the American Psychiatric Nurses Association (APNA), describe it in identical terms: “The jails and prisons have become the de facto mental health system.”
“More and more mentally ill people are in the streets, not receiving services, not taking medication as prescribed, with less-than-optimal case management in the community,” says Judd, who also works with the chronic mentally ill at the Dallas County Jail. “So, they are picked up on substance abuse–related charges or criminal trespassing or burglary. Consequently, they’re brought to jail.”
In Dallas, a divert court has been established, with the aim of getting chronic, persistent mentally ill patients “back to clinics and back on medication as quickly as possible without incarcerating them,” Judd notes. For such a program to succeed, of course, you need clinics—and providers—to divert these patients to.
Next: Problems of access >>
PROBLEMS OF ACCESS
Talk with clinicians who work in psychiatry, mental health, or behavioral health settings, and you’ll hear a familiar litany of problems. For one thing, there is the shortage of providers. “Here in Iowa, we’ve got areas where we have one psychiatrist covering five counties,” St. John says. “It’s almost impossible to get in with someone, and then when you do, it’s a five- or 10-minute appointment, because they’re just so busy.”
The number of clinicians choosing psychiatry—particularly psychiatric nursing—has declined significantly, perhaps due to insufficient funding for educational programs. “The highest number we had going into psychiatric nursing was when the National Institute of Mental Health, which was then separate from the NIH, had training grants,” explains Clement, who is the Director of the Graduate Specialty Program in Psychiatric–Mental Health Nursing at Ohio State University, Columbus. “And many of us who had those training grants are getting way past retirement age!”
The allure of other specialties also keeps people from mental health fields. “There are a lot of jobs and openings for PAs in psychiatry,” St. John says, “but there are a lot of jobs in orthopedics or surgery, too—and that’s what tends to draw them.”
The shortage of mental health care providers and subsequent lack of access to services means a larger role for primary care providers. High-profile expert panels have highlighted the need for integration of mental health into primary care settings—which St. John says is already largely the case.
“Most mental illness is treated in primary care, not in mental health settings,” he points out. “Mental health settings should really be reserved for the more challenging patients, the more difficult diagnoses and problems, and co-occurring illnesses.”
“Most primary care clinicians have some education in relationship to diagnosing and treating mild to moderate mental health issues, and then they refer on when needed,” Clement says. “The problem is, referring on is more and more difficult if there aren’t any people to refer to, or if waiting lists are as long as they currently are.”
Time is just as much of a problem in primary care as it is in specialty care, and when it comes to psychiatric and behavioral disorders, you can’t just order a lab test or an x-ray. “In psychiatry, you have to talk with the person and try to figure out what’s going on in their head and how that’s affecting their function,” St. John says. “It takes more time, and in primary care, that’s the problem they have. They’ve got appointments that may only have 10 minutes uled, and that’s not adequate to obtain a decent psychiatric history.”
The importance of both primary care providers and mental health specialists cannot be downplayed, because mental illnesses are among the most disabling and deadly. “If you look at disabling conditions, depression is right up there at the top,” St. John says. “Actually, it’s predicted that in the next three or four years, worldwide, depression will be the number one disabling illness.”
Anorexia is associated with a 15% death rate, and the completed suicide rate for persons with severe depression is also 15%. “If you were to look at one issue alone that we’re missing the boat on, it’s suicide,” Stuart says. “There are more suicides globally than there are deaths from war and violence combined—and the incidence of suicide is rising. So if, for example, a primary care provider sees someone who’s depressed, they have to go the next step and also ask about potential suicidal thought.”
Clement says it is equally important to integrate primary care services into mental health settings, since many patients with mental illnesses “are not going to show up in a private office in a primary care setting. And people with mental illness die 25 years earlier than the general population, from treatable medical illnesses.”
This is why, for example, the APNA is partnering with the Smoking Cessation Leadership Center. “Persons with mental illness are purchasing approximately half of the cigarettes that are being bought in the US,” Clement says. “And many of the treatable medical illnesses that people are dying from are related to smoking. It’s a whole person you’re working with, not just a brain or a body.”
REIMBURSEMENT ISSUE
Reimbursement is one of the major deterrents to the pursuit of a career in mental health care. “The whole reimbursement issue makes it difficult to attract people to work in mental health, particularly in community-based clinics, state hospitals, prisons, and jails,” which Judd says results in a lack of services for the seriously mentally ill and decreased access for people of low income.
The biggest problem is parity—or rather, the lack of it. What services are covered and at what rate tends to vary by state, and mental health is often not covered at the same rate as physical health. “There are a number of states that now have parity in mental health,” Clement observes. “If insurance is offered for physical health and [includes] mental health coverage, it has to be at exactly the same level as physical health, in terms of copays and lifetime limits.” But even so, there is not always parity in parity.
Furthermore, many people who need mental health services fall under the Medicaid program, which is state-based and just as variable. “Definitions of ‘medical necessity’ differ, and providers don’t get paid unless they can document according to medical necessity,” Clement says. “Even though what people—particularly those in the Medicaid and public mental health systems—need, along with their treatment, is a community-based program that helps people find jobs and housing. But that’s not ‘medical necessity.’”
Another problem is the sheer expense of some of the medications for mental disorders. “A lot of the drugs that we use to treat serious mental illnesses are horrendously expensive,” St. John notes. “They’ll almost bankrupt some states.…We just don’t have those budgets.”
Achieving parity and improving reimbursement is a slow process. Clement has been involved at the federal level with a parity bill, but as she notes, “that has not been resolved in terms of the differences between the House and the Senate.” Since so many of the programs are administered at a state level anyway, some suggest that might be a good place to begin working on reform.
In October 2007, the Annapolis Coalition, of which Stuart is President of the Board of Directors, released an action plan for reforming the mental health system—particularly for addressing workforce needs. The report (available at www.annapoliscoalition.org) includes the most specific recommendations possible in an overarching “framework” document, and Stuart says the coalition is currently working with some states—including North Carolina, Connecticut, New Mexico, and California—to identify and prioritize their needs and determine how best to tailor the plan to them.
“We’re really approaching it not at a federal level but seeing that the true change would come about at a state level,” Stuart says. “The need is derived differently by each state. If I can use the analogy, it’s a little bit like having a general way of approaching hypertension, but then you tailor it to the individual.”
Whether at the state or federal level, St. John thinks major changes to reimbursement for mental health care will require a cultural shift. “We reimburse for activity, we reimburse for procedures; we don’t reimburse for time spent or for decision making or thinking,” he points out. When a clinician is being reimbursed 50% (compared to 90% for other medical care), or $12 to $15 per visit for providing medication management, “You have to see large volumes of people in order to get reimbursed enough to pay for yourself and your staff.”
In the current economic climate, finding the money is going to take some shuffling. “It would be unrealistic to say that there are new dollars out there, because clearly there are not,” Stuart says. “So I think the issue is to reallocate the current resources that are out there and evaluate, Are we getting the best return on our investment of these dollars?”
The irony is that the people with the greatest needs for treatment, monitoring, and support services are the ones who face the biggest barriers to accessing care. “Services are more readily available to people who have jobs, have insurance—which would tell you in and of itself they’re probably higher functioning to start with,” Judd says. “I mean, if you’re having stress holding down a job, you’re probably higher functioning at your baseline than the homeless person who is living in the streets and under bridges and doesn’t go to shelters because they’re too paranoid to be around other people.”
Continue for taking the shame out of mental illness >>
TAKING THE SHAME OUT OF MENTAL ILLNESS
No discussion of mental health care can be complete without addressing the stigma associated with mental illness. Americans may have responded with outrage when they saw the deplorable conditions at mental hospitals, but many are still leery of being associated with a mental illness, whether in themselves or in a family member. And the cases that garner the most media attention are not necessarily the ones that reduce stigma.
What Americans see on the nightly news is the schizophrenic man who stops taking his medication and then stabs another man to death while he’s waiting for a train. Or the mother with chronic depression who can’t get out of bed until someone notices her kids look dirty and underfed, and Social Services steps in to remove them from the home. Do we, as a society, recognize the double tragedy of those situations? Or do we shake our heads in disgust, slap on a “crazy guy” or “bad mom” label, and change the channel?
Public service campaigns are trying to reduce the stigma associated with mental illness, to point out that it can affect anyone. The faces of the mentally ill are diverse: There’s the grandfather with Alzheimer’s disease who mistakes his granddaughter for his daughter. The 2-year-old autistic girl who has difficulty connecting with family and friends. The soldiers returning from the war zones in Iraq and Afghanistan, struggling with posttraumatic stress disorder (PTSD).
“The message that is being sent that needs to be broadcast more and heard with a different ear is that there is no health without mental health,” Clement says. St. John adds that it will take “a lot of time and education” to get that message out to the public, to let people know that it’s OK—in fact, it’s better—to acknowledge mental illness and seek help for it.
Stuart thinks the troops’ return from overseas, which is generating more stories about traumatic brain injury, PTSD, depression, and suicide, may start to turn the tide. “Perhaps because these are our veterans and our heroes, they’ve served the country, it’s opening up a public discussion in a way that’s different from seeing the aberrant, violent patient who does something very disruptive,” she says. “So, in a sense—and this sounds odd—we’re normalizing mental health problems, saying that all kinds of people from all walks of life can develop mental health problems, just as they can develop physical health problems.”
The key will be ensuring that the pendulum doesn’t swing too far the other way and cause the “stigma reduction” movement to generate its own problems. “On the one hand, we’re trying to destigmatize mental illness, but on the other hand, it [sometimes] seems like we’re calling any aberrant behavior or problems in life, stress or problems of adjustment, a mental illness,” Judd observes.
There are certain niches in which mental illness seems almost “trendy,” and industry advertising may encourage that. “Pharmaceutical companies are putting advertisements out there that would imply, ‘Gee, you’re getting divorced because you had conflict in your marriage—maybe you have bipolar disorder’ or ‘Your child isn’t doing well in school, so surely he has ADHD and needs to be on medication,’” Judd says. “There’s this promoting of drugs for anything and everything. And so that’s kind of the other extreme, where any problems in life in functioning must be because of a mental illness, and therefore you need a drug.”
RESTORED TO LIFE
With such a grim picture of mental health care in the US, it hardly seems surprising that clinicians don’t flock to the specialty. Yet, Clement, Judd, St. John, and Stuart did. Why?
For Judd, “the science of it is extremely interesting.” She thinks that as psychiatry becomes more biological and clinicians delve more deeply into what is affecting a patient’s function (Is it trauma, prenatal influences, infection, genetics?) and how that impacts treatment choices, more practitioners might choose mental health care. But the biggest reward, she says, is seeing people “return to a higher level of functioning.”
“I have never, ever sat down with a client that I have not felt privileged to be allowed into their lives,” says Clement, who has been a nurse for 49 years and a psychiatric nurse for 47 of them. “People allow clinicians into their lives in a very different way than they do anybody else.”
That can be especially true in mental health, when clinicians must interact on a very intimate level with their patients. It can be challenging, frustrating, even devastating (such as when a patient takes his or her own life). But it can also be infinitely rewarding. That is why St. John moved from family practice and emergency settings to psychiatry, where he has spent the past 15 years.
“When you see people who kind of get back into life and start working more toward their life goals, and you start seeing them get back into their family and their work and their social function, perking up and engaging in the world,” he says, his voice conveying a deep sense of fulfillment, “there’s just nothing more rewarding than that.”
Reprinted from Clinician Reviews. 2008;18(10):cover, 6-8.
May is Mental Health Month; in recognition of this—and our 25th anniversary—we decided to reprint this 2008 feature article on the state of mental health care in the United States. It’s the story I am personally most proud of telling, but rereading it today leads me to wonder: How much progress have we actually made? Please share your thoughts and experiences with me at [email protected]. —AMH
Broken. Grossly underfunded. In crisis. That’s how psychiatric and behavioral health specialists describe the current state of mental health care in the United States. The problems that plague the health care system in general—workforce shortages, barriers to access, and inadequate reimbursement—are only exacerbated in mental health.
“Mental illness isn’t glamorous,” says Don St. John, MA, PA-C, who practices in adult outpatient psychiatry at the University of Iowa Behavioral Health in Iowa City. Taking an example from the academic medical center setting, he adds, “It’s nice to have the cardiac surgery wing dedicated to or named after your family—but nobody wants a mental health wing named after them.”
Stigma is perhaps the greatest challenge with this patient population. “Until this country really embraces the notion that mental health is inherent in every aspect of a person’s general health,” says Gail W. Stuart, PhD, APRN, FAAN, Professor and Dean of the College of Nursing at Medical University of South Carolina, Charleston, “I think the stigma issue is going to continue to make it difficult to overcome these problems.”
FROM HOSPITALS TO JAILS
The current state of mental health care in the US is perhaps a direct result of the deinstitutionalization that occurred in the 1980s. By that time, most mental health hospitals were overcrowded and, in the worst cases, patients were subject to neglect and even abuse. (Recall, if you can, Geraldo Rivera skulking through the dark at Willowbrook State School in Staten Island, NY; his 1972 exposé brought the issue to the forefront.)
Following the public outcry over the treatment of these patients who—mentally ill or not—were people, there was a movement to reduce the number of long-term hospitalizations for mental illness. Along the way, the number of hospital beds available for mentally ill patients also declined, as freestanding hospitals and private facilities closed. What have these patients been left with?
“The thought was that people would be maintained in the community—there would be community support services, halfway houses, boarding homes, community-based programs,” says Catherine R. Judd, MS, PA-C, President of the Association of Psychiatric PAs, who works in the Department of Psychiatry at the University of Texas Southwestern Medical Center, Dallas. “The idea is good, but unfortunately, most of those programs have really not materialized to the extent or with the capacity to take care of the people who are out there.”
Without these services—and with an overtaxed health care system in general—many patients with mental illness find themselves adrift. And, eventually, incarcerated. An estimate from the US Department of Justice indicates that 24% of state and 21% of local prisoners have a recent history of mental illness. The largest psychiatric hospital in the country is the Los Angeles County Jail.
The problem is so widespread and so serious that both Judd and Jeanne Clement, EdD, APRN, BC, FAAN, President of the American Psychiatric Nurses Association (APNA), describe it in identical terms: “The jails and prisons have become the de facto mental health system.”
“More and more mentally ill people are in the streets, not receiving services, not taking medication as prescribed, with less-than-optimal case management in the community,” says Judd, who also works with the chronic mentally ill at the Dallas County Jail. “So, they are picked up on substance abuse–related charges or criminal trespassing or burglary. Consequently, they’re brought to jail.”
In Dallas, a divert court has been established, with the aim of getting chronic, persistent mentally ill patients “back to clinics and back on medication as quickly as possible without incarcerating them,” Judd notes. For such a program to succeed, of course, you need clinics—and providers—to divert these patients to.
Next: Problems of access >>
PROBLEMS OF ACCESS
Talk with clinicians who work in psychiatry, mental health, or behavioral health settings, and you’ll hear a familiar litany of problems. For one thing, there is the shortage of providers. “Here in Iowa, we’ve got areas where we have one psychiatrist covering five counties,” St. John says. “It’s almost impossible to get in with someone, and then when you do, it’s a five- or 10-minute appointment, because they’re just so busy.”
The number of clinicians choosing psychiatry—particularly psychiatric nursing—has declined significantly, perhaps due to insufficient funding for educational programs. “The highest number we had going into psychiatric nursing was when the National Institute of Mental Health, which was then separate from the NIH, had training grants,” explains Clement, who is the Director of the Graduate Specialty Program in Psychiatric–Mental Health Nursing at Ohio State University, Columbus. “And many of us who had those training grants are getting way past retirement age!”
The allure of other specialties also keeps people from mental health fields. “There are a lot of jobs and openings for PAs in psychiatry,” St. John says, “but there are a lot of jobs in orthopedics or surgery, too—and that’s what tends to draw them.”
The shortage of mental health care providers and subsequent lack of access to services means a larger role for primary care providers. High-profile expert panels have highlighted the need for integration of mental health into primary care settings—which St. John says is already largely the case.
“Most mental illness is treated in primary care, not in mental health settings,” he points out. “Mental health settings should really be reserved for the more challenging patients, the more difficult diagnoses and problems, and co-occurring illnesses.”
“Most primary care clinicians have some education in relationship to diagnosing and treating mild to moderate mental health issues, and then they refer on when needed,” Clement says. “The problem is, referring on is more and more difficult if there aren’t any people to refer to, or if waiting lists are as long as they currently are.”
Time is just as much of a problem in primary care as it is in specialty care, and when it comes to psychiatric and behavioral disorders, you can’t just order a lab test or an x-ray. “In psychiatry, you have to talk with the person and try to figure out what’s going on in their head and how that’s affecting their function,” St. John says. “It takes more time, and in primary care, that’s the problem they have. They’ve got appointments that may only have 10 minutes uled, and that’s not adequate to obtain a decent psychiatric history.”
The importance of both primary care providers and mental health specialists cannot be downplayed, because mental illnesses are among the most disabling and deadly. “If you look at disabling conditions, depression is right up there at the top,” St. John says. “Actually, it’s predicted that in the next three or four years, worldwide, depression will be the number one disabling illness.”
Anorexia is associated with a 15% death rate, and the completed suicide rate for persons with severe depression is also 15%. “If you were to look at one issue alone that we’re missing the boat on, it’s suicide,” Stuart says. “There are more suicides globally than there are deaths from war and violence combined—and the incidence of suicide is rising. So if, for example, a primary care provider sees someone who’s depressed, they have to go the next step and also ask about potential suicidal thought.”
Clement says it is equally important to integrate primary care services into mental health settings, since many patients with mental illnesses “are not going to show up in a private office in a primary care setting. And people with mental illness die 25 years earlier than the general population, from treatable medical illnesses.”
This is why, for example, the APNA is partnering with the Smoking Cessation Leadership Center. “Persons with mental illness are purchasing approximately half of the cigarettes that are being bought in the US,” Clement says. “And many of the treatable medical illnesses that people are dying from are related to smoking. It’s a whole person you’re working with, not just a brain or a body.”
REIMBURSEMENT ISSUE
Reimbursement is one of the major deterrents to the pursuit of a career in mental health care. “The whole reimbursement issue makes it difficult to attract people to work in mental health, particularly in community-based clinics, state hospitals, prisons, and jails,” which Judd says results in a lack of services for the seriously mentally ill and decreased access for people of low income.
The biggest problem is parity—or rather, the lack of it. What services are covered and at what rate tends to vary by state, and mental health is often not covered at the same rate as physical health. “There are a number of states that now have parity in mental health,” Clement observes. “If insurance is offered for physical health and [includes] mental health coverage, it has to be at exactly the same level as physical health, in terms of copays and lifetime limits.” But even so, there is not always parity in parity.
Furthermore, many people who need mental health services fall under the Medicaid program, which is state-based and just as variable. “Definitions of ‘medical necessity’ differ, and providers don’t get paid unless they can document according to medical necessity,” Clement says. “Even though what people—particularly those in the Medicaid and public mental health systems—need, along with their treatment, is a community-based program that helps people find jobs and housing. But that’s not ‘medical necessity.’”
Another problem is the sheer expense of some of the medications for mental disorders. “A lot of the drugs that we use to treat serious mental illnesses are horrendously expensive,” St. John notes. “They’ll almost bankrupt some states.…We just don’t have those budgets.”
Achieving parity and improving reimbursement is a slow process. Clement has been involved at the federal level with a parity bill, but as she notes, “that has not been resolved in terms of the differences between the House and the Senate.” Since so many of the programs are administered at a state level anyway, some suggest that might be a good place to begin working on reform.
In October 2007, the Annapolis Coalition, of which Stuart is President of the Board of Directors, released an action plan for reforming the mental health system—particularly for addressing workforce needs. The report (available at www.annapoliscoalition.org) includes the most specific recommendations possible in an overarching “framework” document, and Stuart says the coalition is currently working with some states—including North Carolina, Connecticut, New Mexico, and California—to identify and prioritize their needs and determine how best to tailor the plan to them.
“We’re really approaching it not at a federal level but seeing that the true change would come about at a state level,” Stuart says. “The need is derived differently by each state. If I can use the analogy, it’s a little bit like having a general way of approaching hypertension, but then you tailor it to the individual.”
Whether at the state or federal level, St. John thinks major changes to reimbursement for mental health care will require a cultural shift. “We reimburse for activity, we reimburse for procedures; we don’t reimburse for time spent or for decision making or thinking,” he points out. When a clinician is being reimbursed 50% (compared to 90% for other medical care), or $12 to $15 per visit for providing medication management, “You have to see large volumes of people in order to get reimbursed enough to pay for yourself and your staff.”
In the current economic climate, finding the money is going to take some shuffling. “It would be unrealistic to say that there are new dollars out there, because clearly there are not,” Stuart says. “So I think the issue is to reallocate the current resources that are out there and evaluate, Are we getting the best return on our investment of these dollars?”
The irony is that the people with the greatest needs for treatment, monitoring, and support services are the ones who face the biggest barriers to accessing care. “Services are more readily available to people who have jobs, have insurance—which would tell you in and of itself they’re probably higher functioning to start with,” Judd says. “I mean, if you’re having stress holding down a job, you’re probably higher functioning at your baseline than the homeless person who is living in the streets and under bridges and doesn’t go to shelters because they’re too paranoid to be around other people.”
Continue for taking the shame out of mental illness >>
TAKING THE SHAME OUT OF MENTAL ILLNESS
No discussion of mental health care can be complete without addressing the stigma associated with mental illness. Americans may have responded with outrage when they saw the deplorable conditions at mental hospitals, but many are still leery of being associated with a mental illness, whether in themselves or in a family member. And the cases that garner the most media attention are not necessarily the ones that reduce stigma.
What Americans see on the nightly news is the schizophrenic man who stops taking his medication and then stabs another man to death while he’s waiting for a train. Or the mother with chronic depression who can’t get out of bed until someone notices her kids look dirty and underfed, and Social Services steps in to remove them from the home. Do we, as a society, recognize the double tragedy of those situations? Or do we shake our heads in disgust, slap on a “crazy guy” or “bad mom” label, and change the channel?
Public service campaigns are trying to reduce the stigma associated with mental illness, to point out that it can affect anyone. The faces of the mentally ill are diverse: There’s the grandfather with Alzheimer’s disease who mistakes his granddaughter for his daughter. The 2-year-old autistic girl who has difficulty connecting with family and friends. The soldiers returning from the war zones in Iraq and Afghanistan, struggling with posttraumatic stress disorder (PTSD).
“The message that is being sent that needs to be broadcast more and heard with a different ear is that there is no health without mental health,” Clement says. St. John adds that it will take “a lot of time and education” to get that message out to the public, to let people know that it’s OK—in fact, it’s better—to acknowledge mental illness and seek help for it.
Stuart thinks the troops’ return from overseas, which is generating more stories about traumatic brain injury, PTSD, depression, and suicide, may start to turn the tide. “Perhaps because these are our veterans and our heroes, they’ve served the country, it’s opening up a public discussion in a way that’s different from seeing the aberrant, violent patient who does something very disruptive,” she says. “So, in a sense—and this sounds odd—we’re normalizing mental health problems, saying that all kinds of people from all walks of life can develop mental health problems, just as they can develop physical health problems.”
The key will be ensuring that the pendulum doesn’t swing too far the other way and cause the “stigma reduction” movement to generate its own problems. “On the one hand, we’re trying to destigmatize mental illness, but on the other hand, it [sometimes] seems like we’re calling any aberrant behavior or problems in life, stress or problems of adjustment, a mental illness,” Judd observes.
There are certain niches in which mental illness seems almost “trendy,” and industry advertising may encourage that. “Pharmaceutical companies are putting advertisements out there that would imply, ‘Gee, you’re getting divorced because you had conflict in your marriage—maybe you have bipolar disorder’ or ‘Your child isn’t doing well in school, so surely he has ADHD and needs to be on medication,’” Judd says. “There’s this promoting of drugs for anything and everything. And so that’s kind of the other extreme, where any problems in life in functioning must be because of a mental illness, and therefore you need a drug.”
RESTORED TO LIFE
With such a grim picture of mental health care in the US, it hardly seems surprising that clinicians don’t flock to the specialty. Yet, Clement, Judd, St. John, and Stuart did. Why?
For Judd, “the science of it is extremely interesting.” She thinks that as psychiatry becomes more biological and clinicians delve more deeply into what is affecting a patient’s function (Is it trauma, prenatal influences, infection, genetics?) and how that impacts treatment choices, more practitioners might choose mental health care. But the biggest reward, she says, is seeing people “return to a higher level of functioning.”
“I have never, ever sat down with a client that I have not felt privileged to be allowed into their lives,” says Clement, who has been a nurse for 49 years and a psychiatric nurse for 47 of them. “People allow clinicians into their lives in a very different way than they do anybody else.”
That can be especially true in mental health, when clinicians must interact on a very intimate level with their patients. It can be challenging, frustrating, even devastating (such as when a patient takes his or her own life). But it can also be infinitely rewarding. That is why St. John moved from family practice and emergency settings to psychiatry, where he has spent the past 15 years.
“When you see people who kind of get back into life and start working more toward their life goals, and you start seeing them get back into their family and their work and their social function, perking up and engaging in the world,” he says, his voice conveying a deep sense of fulfillment, “there’s just nothing more rewarding than that.”
Reprinted from Clinician Reviews. 2008;18(10):cover, 6-8.
May is Mental Health Month; in recognition of this—and our 25th anniversary—we decided to reprint this 2008 feature article on the state of mental health care in the United States. It’s the story I am personally most proud of telling, but rereading it today leads me to wonder: How much progress have we actually made? Please share your thoughts and experiences with me at [email protected]. —AMH
Broken. Grossly underfunded. In crisis. That’s how psychiatric and behavioral health specialists describe the current state of mental health care in the United States. The problems that plague the health care system in general—workforce shortages, barriers to access, and inadequate reimbursement—are only exacerbated in mental health.
“Mental illness isn’t glamorous,” says Don St. John, MA, PA-C, who practices in adult outpatient psychiatry at the University of Iowa Behavioral Health in Iowa City. Taking an example from the academic medical center setting, he adds, “It’s nice to have the cardiac surgery wing dedicated to or named after your family—but nobody wants a mental health wing named after them.”
Stigma is perhaps the greatest challenge with this patient population. “Until this country really embraces the notion that mental health is inherent in every aspect of a person’s general health,” says Gail W. Stuart, PhD, APRN, FAAN, Professor and Dean of the College of Nursing at Medical University of South Carolina, Charleston, “I think the stigma issue is going to continue to make it difficult to overcome these problems.”
FROM HOSPITALS TO JAILS
The current state of mental health care in the US is perhaps a direct result of the deinstitutionalization that occurred in the 1980s. By that time, most mental health hospitals were overcrowded and, in the worst cases, patients were subject to neglect and even abuse. (Recall, if you can, Geraldo Rivera skulking through the dark at Willowbrook State School in Staten Island, NY; his 1972 exposé brought the issue to the forefront.)
Following the public outcry over the treatment of these patients who—mentally ill or not—were people, there was a movement to reduce the number of long-term hospitalizations for mental illness. Along the way, the number of hospital beds available for mentally ill patients also declined, as freestanding hospitals and private facilities closed. What have these patients been left with?
“The thought was that people would be maintained in the community—there would be community support services, halfway houses, boarding homes, community-based programs,” says Catherine R. Judd, MS, PA-C, President of the Association of Psychiatric PAs, who works in the Department of Psychiatry at the University of Texas Southwestern Medical Center, Dallas. “The idea is good, but unfortunately, most of those programs have really not materialized to the extent or with the capacity to take care of the people who are out there.”
Without these services—and with an overtaxed health care system in general—many patients with mental illness find themselves adrift. And, eventually, incarcerated. An estimate from the US Department of Justice indicates that 24% of state and 21% of local prisoners have a recent history of mental illness. The largest psychiatric hospital in the country is the Los Angeles County Jail.
The problem is so widespread and so serious that both Judd and Jeanne Clement, EdD, APRN, BC, FAAN, President of the American Psychiatric Nurses Association (APNA), describe it in identical terms: “The jails and prisons have become the de facto mental health system.”
“More and more mentally ill people are in the streets, not receiving services, not taking medication as prescribed, with less-than-optimal case management in the community,” says Judd, who also works with the chronic mentally ill at the Dallas County Jail. “So, they are picked up on substance abuse–related charges or criminal trespassing or burglary. Consequently, they’re brought to jail.”
In Dallas, a divert court has been established, with the aim of getting chronic, persistent mentally ill patients “back to clinics and back on medication as quickly as possible without incarcerating them,” Judd notes. For such a program to succeed, of course, you need clinics—and providers—to divert these patients to.
Next: Problems of access >>
PROBLEMS OF ACCESS
Talk with clinicians who work in psychiatry, mental health, or behavioral health settings, and you’ll hear a familiar litany of problems. For one thing, there is the shortage of providers. “Here in Iowa, we’ve got areas where we have one psychiatrist covering five counties,” St. John says. “It’s almost impossible to get in with someone, and then when you do, it’s a five- or 10-minute appointment, because they’re just so busy.”
The number of clinicians choosing psychiatry—particularly psychiatric nursing—has declined significantly, perhaps due to insufficient funding for educational programs. “The highest number we had going into psychiatric nursing was when the National Institute of Mental Health, which was then separate from the NIH, had training grants,” explains Clement, who is the Director of the Graduate Specialty Program in Psychiatric–Mental Health Nursing at Ohio State University, Columbus. “And many of us who had those training grants are getting way past retirement age!”
The allure of other specialties also keeps people from mental health fields. “There are a lot of jobs and openings for PAs in psychiatry,” St. John says, “but there are a lot of jobs in orthopedics or surgery, too—and that’s what tends to draw them.”
The shortage of mental health care providers and subsequent lack of access to services means a larger role for primary care providers. High-profile expert panels have highlighted the need for integration of mental health into primary care settings—which St. John says is already largely the case.
“Most mental illness is treated in primary care, not in mental health settings,” he points out. “Mental health settings should really be reserved for the more challenging patients, the more difficult diagnoses and problems, and co-occurring illnesses.”
“Most primary care clinicians have some education in relationship to diagnosing and treating mild to moderate mental health issues, and then they refer on when needed,” Clement says. “The problem is, referring on is more and more difficult if there aren’t any people to refer to, or if waiting lists are as long as they currently are.”
Time is just as much of a problem in primary care as it is in specialty care, and when it comes to psychiatric and behavioral disorders, you can’t just order a lab test or an x-ray. “In psychiatry, you have to talk with the person and try to figure out what’s going on in their head and how that’s affecting their function,” St. John says. “It takes more time, and in primary care, that’s the problem they have. They’ve got appointments that may only have 10 minutes uled, and that’s not adequate to obtain a decent psychiatric history.”
The importance of both primary care providers and mental health specialists cannot be downplayed, because mental illnesses are among the most disabling and deadly. “If you look at disabling conditions, depression is right up there at the top,” St. John says. “Actually, it’s predicted that in the next three or four years, worldwide, depression will be the number one disabling illness.”
Anorexia is associated with a 15% death rate, and the completed suicide rate for persons with severe depression is also 15%. “If you were to look at one issue alone that we’re missing the boat on, it’s suicide,” Stuart says. “There are more suicides globally than there are deaths from war and violence combined—and the incidence of suicide is rising. So if, for example, a primary care provider sees someone who’s depressed, they have to go the next step and also ask about potential suicidal thought.”
Clement says it is equally important to integrate primary care services into mental health settings, since many patients with mental illnesses “are not going to show up in a private office in a primary care setting. And people with mental illness die 25 years earlier than the general population, from treatable medical illnesses.”
This is why, for example, the APNA is partnering with the Smoking Cessation Leadership Center. “Persons with mental illness are purchasing approximately half of the cigarettes that are being bought in the US,” Clement says. “And many of the treatable medical illnesses that people are dying from are related to smoking. It’s a whole person you’re working with, not just a brain or a body.”
REIMBURSEMENT ISSUE
Reimbursement is one of the major deterrents to the pursuit of a career in mental health care. “The whole reimbursement issue makes it difficult to attract people to work in mental health, particularly in community-based clinics, state hospitals, prisons, and jails,” which Judd says results in a lack of services for the seriously mentally ill and decreased access for people of low income.
The biggest problem is parity—or rather, the lack of it. What services are covered and at what rate tends to vary by state, and mental health is often not covered at the same rate as physical health. “There are a number of states that now have parity in mental health,” Clement observes. “If insurance is offered for physical health and [includes] mental health coverage, it has to be at exactly the same level as physical health, in terms of copays and lifetime limits.” But even so, there is not always parity in parity.
Furthermore, many people who need mental health services fall under the Medicaid program, which is state-based and just as variable. “Definitions of ‘medical necessity’ differ, and providers don’t get paid unless they can document according to medical necessity,” Clement says. “Even though what people—particularly those in the Medicaid and public mental health systems—need, along with their treatment, is a community-based program that helps people find jobs and housing. But that’s not ‘medical necessity.’”
Another problem is the sheer expense of some of the medications for mental disorders. “A lot of the drugs that we use to treat serious mental illnesses are horrendously expensive,” St. John notes. “They’ll almost bankrupt some states.…We just don’t have those budgets.”
Achieving parity and improving reimbursement is a slow process. Clement has been involved at the federal level with a parity bill, but as she notes, “that has not been resolved in terms of the differences between the House and the Senate.” Since so many of the programs are administered at a state level anyway, some suggest that might be a good place to begin working on reform.
In October 2007, the Annapolis Coalition, of which Stuart is President of the Board of Directors, released an action plan for reforming the mental health system—particularly for addressing workforce needs. The report (available at www.annapoliscoalition.org) includes the most specific recommendations possible in an overarching “framework” document, and Stuart says the coalition is currently working with some states—including North Carolina, Connecticut, New Mexico, and California—to identify and prioritize their needs and determine how best to tailor the plan to them.
“We’re really approaching it not at a federal level but seeing that the true change would come about at a state level,” Stuart says. “The need is derived differently by each state. If I can use the analogy, it’s a little bit like having a general way of approaching hypertension, but then you tailor it to the individual.”
Whether at the state or federal level, St. John thinks major changes to reimbursement for mental health care will require a cultural shift. “We reimburse for activity, we reimburse for procedures; we don’t reimburse for time spent or for decision making or thinking,” he points out. When a clinician is being reimbursed 50% (compared to 90% for other medical care), or $12 to $15 per visit for providing medication management, “You have to see large volumes of people in order to get reimbursed enough to pay for yourself and your staff.”
In the current economic climate, finding the money is going to take some shuffling. “It would be unrealistic to say that there are new dollars out there, because clearly there are not,” Stuart says. “So I think the issue is to reallocate the current resources that are out there and evaluate, Are we getting the best return on our investment of these dollars?”
The irony is that the people with the greatest needs for treatment, monitoring, and support services are the ones who face the biggest barriers to accessing care. “Services are more readily available to people who have jobs, have insurance—which would tell you in and of itself they’re probably higher functioning to start with,” Judd says. “I mean, if you’re having stress holding down a job, you’re probably higher functioning at your baseline than the homeless person who is living in the streets and under bridges and doesn’t go to shelters because they’re too paranoid to be around other people.”
Continue for taking the shame out of mental illness >>
TAKING THE SHAME OUT OF MENTAL ILLNESS
No discussion of mental health care can be complete without addressing the stigma associated with mental illness. Americans may have responded with outrage when they saw the deplorable conditions at mental hospitals, but many are still leery of being associated with a mental illness, whether in themselves or in a family member. And the cases that garner the most media attention are not necessarily the ones that reduce stigma.
What Americans see on the nightly news is the schizophrenic man who stops taking his medication and then stabs another man to death while he’s waiting for a train. Or the mother with chronic depression who can’t get out of bed until someone notices her kids look dirty and underfed, and Social Services steps in to remove them from the home. Do we, as a society, recognize the double tragedy of those situations? Or do we shake our heads in disgust, slap on a “crazy guy” or “bad mom” label, and change the channel?
Public service campaigns are trying to reduce the stigma associated with mental illness, to point out that it can affect anyone. The faces of the mentally ill are diverse: There’s the grandfather with Alzheimer’s disease who mistakes his granddaughter for his daughter. The 2-year-old autistic girl who has difficulty connecting with family and friends. The soldiers returning from the war zones in Iraq and Afghanistan, struggling with posttraumatic stress disorder (PTSD).
“The message that is being sent that needs to be broadcast more and heard with a different ear is that there is no health without mental health,” Clement says. St. John adds that it will take “a lot of time and education” to get that message out to the public, to let people know that it’s OK—in fact, it’s better—to acknowledge mental illness and seek help for it.
Stuart thinks the troops’ return from overseas, which is generating more stories about traumatic brain injury, PTSD, depression, and suicide, may start to turn the tide. “Perhaps because these are our veterans and our heroes, they’ve served the country, it’s opening up a public discussion in a way that’s different from seeing the aberrant, violent patient who does something very disruptive,” she says. “So, in a sense—and this sounds odd—we’re normalizing mental health problems, saying that all kinds of people from all walks of life can develop mental health problems, just as they can develop physical health problems.”
The key will be ensuring that the pendulum doesn’t swing too far the other way and cause the “stigma reduction” movement to generate its own problems. “On the one hand, we’re trying to destigmatize mental illness, but on the other hand, it [sometimes] seems like we’re calling any aberrant behavior or problems in life, stress or problems of adjustment, a mental illness,” Judd observes.
There are certain niches in which mental illness seems almost “trendy,” and industry advertising may encourage that. “Pharmaceutical companies are putting advertisements out there that would imply, ‘Gee, you’re getting divorced because you had conflict in your marriage—maybe you have bipolar disorder’ or ‘Your child isn’t doing well in school, so surely he has ADHD and needs to be on medication,’” Judd says. “There’s this promoting of drugs for anything and everything. And so that’s kind of the other extreme, where any problems in life in functioning must be because of a mental illness, and therefore you need a drug.”
RESTORED TO LIFE
With such a grim picture of mental health care in the US, it hardly seems surprising that clinicians don’t flock to the specialty. Yet, Clement, Judd, St. John, and Stuart did. Why?
For Judd, “the science of it is extremely interesting.” She thinks that as psychiatry becomes more biological and clinicians delve more deeply into what is affecting a patient’s function (Is it trauma, prenatal influences, infection, genetics?) and how that impacts treatment choices, more practitioners might choose mental health care. But the biggest reward, she says, is seeing people “return to a higher level of functioning.”
“I have never, ever sat down with a client that I have not felt privileged to be allowed into their lives,” says Clement, who has been a nurse for 49 years and a psychiatric nurse for 47 of them. “People allow clinicians into their lives in a very different way than they do anybody else.”
That can be especially true in mental health, when clinicians must interact on a very intimate level with their patients. It can be challenging, frustrating, even devastating (such as when a patient takes his or her own life). But it can also be infinitely rewarding. That is why St. John moved from family practice and emergency settings to psychiatry, where he has spent the past 15 years.
“When you see people who kind of get back into life and start working more toward their life goals, and you start seeing them get back into their family and their work and their social function, perking up and engaging in the world,” he says, his voice conveying a deep sense of fulfillment, “there’s just nothing more rewarding than that.”
Reprinted from Clinician Reviews. 2008;18(10):cover, 6-8.
Nontraumatic Knee Pain: A Diagnostic & Treatment Guide
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Epithelial Ovarian Cancer: Management of Advanced Disease
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Mind the Gap: Case Study in Toxicology
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Malpractice Counsel
Sepsis Following Vaginal Hysterectomy
| A 45-year-old woman presented to the ED complaining of lower abdominal pain, which she described as gradual, aching, and intermittent. The patient stated that she had undergone a vaginal hysterectomy a few days prior and that the pain started less than 24 hours after discharge from the hospital. She denied fever or chills, nausea, or vomiting, and said that she had a bowel movement earlier that day. She also denied any urinary symptoms. Her medical history was significant only for hypothyroidism, for which she was taking levothyroxine. The patient denied cigarette smoking or alcohol consumption. She said she had been taking acetaminophen-hydrocodone for postoperative pain, but that it did not provide any relief. |
The patient’s vital signs were: temperature, 98.6˚F; blood pressure, 112/65 mm Hg; heart rate, 98 beats/minute; and respiratory rate, 20 breaths/minute. The head, eyes, ears, nose, and throat examination was normal, as were the heart and lung examinations. The patient’s abdomen was soft, with mild diffuse lower abdominal tenderness. There was no guarding, rebound, or mass present. A gross nonspeculum examination of the vaginal area did not reveal any discharge or erythema; a rectal examination was not performed.
The EP ordered a complete blood count (CBC), lipase evaluation, and urinalysis. All test results were normal. The emergency physician (EP) then contacted the obstetrician-gynecologist (OB/GYN) who had performed the hysterectomy. The OB/GYN recommended the EP change the analgesic agent to acetaminophen-oxycodone and to encourage the patient to keep her follow-up postoperative appointment in 1 week. The EP followed these instructions and discharged the patient home with a prescription for the new analgesic.
Three days later, however, the patient presented back to the same ED complaining of increased and now generalized abdominal pain, nausea, and vomiting. She was noted to be febrile, tachycardic, and hypotensive. On physical examination, her abdomen was diffusely tender with guarding and rebound. She was given a 2-L bolus of intravenous (IV) normal saline and started on broad spectrum IV antibiotics. After another consultation with the patient’s OB/GYN surgeon, the patient was taken immediately to the operating room. On exploration, she was found to have a segment of perforated bowel and peritonitis. A portion of the bowel was resected, but her postoperative course was complicated by sepsis. After a 1-month stay in the hospital, she was discharged home.
The patient sued the EP—but not her OB/GYN—for failure to obtain a CT scan of the abdomen/pelvis on her initial ED visit, or at least to admit her to the hospital for observation. The EP argued that even if a computed tomography (CT) scan had been performed on the initial visit, it probably would have been normal, since the bowel had not yet perforated. After trial, a defense verdict was returned.
Discussion
This case illustrates two important points. First, not every patient with abdominal pain requires a CT scan of the abdomen/pelvis. So many malpractice cases against EPs involve the failure to perform advanced imaging. Unfortunately, that is usually only through the benefit of hindsight. For a patient with mild abdominal pain, only minimal tenderness on examination, and a negative laboratory workup, it can be perfectly appropriate to treat him or her symptomatically with close follow-up and specific instructions to return to the ED if his or her condition worsens (as was the case with this patient).
The second important point is to not over-rely on a consultant(s), especially if she or he has not independently examined the patient. When calling a consultant, it is best to have a specific question (ie, “Can you see the patient in the morning?”) or action (ie, “I would like to admit the patient to your service”). In general, the EP should not rely on the consultant to give “permission” to discharge the patient. As the physician seeing the patient, the EP is the most well-equipped to work up the patient and determine the needed disposition. Rare is the consultant that can arrive at a better disposition than the EP who performed the history and physical examination on the patient.
Regarding the patient’s GYN surgery, vaginal hysterectomy (VH) is preferred over abdominal hysterectomy (AH) for benign disease as it is associated with reduced infective morbidity and earlier return to normal activities.1 With respect to postoperative events, clinicians typically employ the Clavien-Dindo grading system for the classification of surgical complications.2 The system consists of five grades, ranging from Grade I (any deviation from normal postoperative course, without the need for pharmacological intervention) to Grade V (death).
Following hysterectomy, postoperative urinary or pelvic infections are not uncommon, with an incidence of 15% to 20%.1 In the Clavien-Dindo system, these complications would typically be considered Grade II (pharmacological treatment other than what is considered an acceptable therapeutic regimen), requiring antibiotics and no surgical intervention. Grade III complications, however, usually involve postoperative issues that require surgical, endoscopic, or radiological intervention, which in VH would include ureteral, bladder, or bowel injury.1 In a study by Gendy et al,1 the incidence of such complications posthysterectomy, ranged from 1.7% to 5.7%. So while not extremely common, serious complications can occur postoperatively.
The last point is a minor one, but a truth every EP needs to remember: While it may be difficult for a patient to sue her or his own physician, especially one with whom she or he has a longstanding patient-physician relationship, it is much easier for her or him to place blame upon and sue another physician—for example, the EP.
Missed Testicular Torsion?
| A 14-year-old boy presented to the ED with a several day history of abdominal pain with radiation to the right testicle. The patient denied any nausea, vomiting, or changes in bowel habits. He also denied any genitourinary symptoms, including dysuria or urinary frequency. The boy was otherwise in good health, on no medications, and up to date on his immunizations. |
The patient was a well appearing teenager in no acute distress. All vital signs were normal, as were the heart and lung examinations. The abdominal examination revealed mild, generalized tenderness without guarding or rebound. The genitalia examination was normal.
The EP ordered a CBC, urinalysis, and a testicular ultrasound, the results of which were all normal. The patient was discharged home with instructions to follow up with his pediatrician in 2 days and to return to the ED if his symptoms worsened.
The patient was seen by his pediatrician approximately 1 month later for his scheduled annual physical examination. The pediatrician, who was aware of the boy’s prior ED visit, found the patient in good health, and performed no additional testing.
Approximately 9 months after the initial ED visit, the patient was accidently kicked in the groin while jumping on a trampoline. He experienced immediate onset of severe, excruciating right testicular pain and presented to the ED approximately 24 hours later with continued pain and swelling. A testicular ultrasound was immediately ordered and demonstrated an enlarged right testicle due to torsion.
The patient underwent surgery to remove the right testicle. His family sued the EP and hospital from the initial visit (9 months earlier) for missed intermittent testicular torsion. They argued that the patient should have been referred to a urologist for further evaluation. In addition, the plaintiff claimed he could no longer participate in sports and suffered disfigurement as a result of the surgery. The EP asserted that the patient’s pain during that initial visit was primarily abdominal in nature and that an ultrasound of the testicles was normal, and did not reveal any evidence of testicular torsion. The EP further argued that the testicular torsion was due to the trauma incurred on the trampoline. According to published accounts, a defense verdict was returned.
Discussion
Testicular torsion occurs in a bimodal age distribution—during the first year of life (perinatal) and between ages 13 and 16 years (as was the case with this patient).1 In approximately 4% to 8% of patients, there is a history of an athletic event, strenuous physical activity, or trauma just prior to the onset of scrotal pain.2
Patients typically present with sudden onset of testicular pain that is frequently associated with nausea and vomiting. However, this condition can present with only lower abdominal pain—in part be due to the fact that adolescents and children may be reluctant to complain of testicular or scrotal pain out of fear or embarrassment.1 In all cases, a genital examination should be performed on every adolescent male with a chief complaint of lower abdominal pain.3
On physical examination, the patient will usually have a swollen tender testicle. In comparison to the opposite side, the affected testicle is frequently raised and rests on a horizontal axis. The cremasteric reflex (ie, scratching the proximal inner thigh causes the ipsilateral testicle to rise) is frequently absent.4
Because of the time sensitive nature of the disease process, in classic presentations, a urologist should be immediately consulted. Ischemic changes to the testicle can begin within hours, and complete testicular atrophy occurs after 24 hours in most cases.4 Detorsion within 6 hours of onset of symptoms has a salvage rate of 90% to 100%, which drops to 25% to 50% after 12 hours and to less than 10% after 24 hours.4
For less obvious cases, color duplex testicular ultrasonography can be very helpful. Demonstration of decreased or absent blood flow is diagnostic and requires operative intervention. If untwisting the testis restores blood flow, then the condition is resolved; if this procedure fails, the testis is removed. Regardless of the outcome, the contralateral testis is fixed to prevent future torsion.
Intermittent testicular torsion is a difficult diagnosis to make. A history of recurrent unilateral scrotal pain is highly suspicious and warrants referral to a urologist. This patient had only one previous episode, which was primarily abdominal pain—not scrotal or testicular pain.
In this case, it appears the jury came to the correct decision. Given the patient had only one previous episode of abdominal pain, and an inciting event (trauma to the testicle) on the second presentation, this does not appear to be a case of missed intermittent testicular torsion. Rather, this was a correctly diagnosed testicular torsion with a delayed presentation, resulting in an unsalvageable testicle.
Reference - Sepsis Following Vaginal Hysterectomy
- Gendy R, Walsh CA, Walsh SR, Karantanis E. Vaginal hysterectomy versus total laparoscopic hysterectomy for benign disease: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):388.e1-8.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009:250(2):187-196.
Reference - Missed Testicular Torsion?
- Pogorelić Z, Mrklić I, Jurić I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J Pediatr Urol. 2013;9(6 Pt B):1161-1165.
- Nicks BA, Manthey DE. Male genital problems. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cine DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill Medical; 2011:649.
- Lopez RN, Beasley SW. Testicular torsion: potential pitfalls in its diagnosis and management. J Paediatr Child Health. 2012;48(2):E30-E32.
- Somani BK, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213.
Sepsis Following Vaginal Hysterectomy
| A 45-year-old woman presented to the ED complaining of lower abdominal pain, which she described as gradual, aching, and intermittent. The patient stated that she had undergone a vaginal hysterectomy a few days prior and that the pain started less than 24 hours after discharge from the hospital. She denied fever or chills, nausea, or vomiting, and said that she had a bowel movement earlier that day. She also denied any urinary symptoms. Her medical history was significant only for hypothyroidism, for which she was taking levothyroxine. The patient denied cigarette smoking or alcohol consumption. She said she had been taking acetaminophen-hydrocodone for postoperative pain, but that it did not provide any relief. |
The patient’s vital signs were: temperature, 98.6˚F; blood pressure, 112/65 mm Hg; heart rate, 98 beats/minute; and respiratory rate, 20 breaths/minute. The head, eyes, ears, nose, and throat examination was normal, as were the heart and lung examinations. The patient’s abdomen was soft, with mild diffuse lower abdominal tenderness. There was no guarding, rebound, or mass present. A gross nonspeculum examination of the vaginal area did not reveal any discharge or erythema; a rectal examination was not performed.
The EP ordered a complete blood count (CBC), lipase evaluation, and urinalysis. All test results were normal. The emergency physician (EP) then contacted the obstetrician-gynecologist (OB/GYN) who had performed the hysterectomy. The OB/GYN recommended the EP change the analgesic agent to acetaminophen-oxycodone and to encourage the patient to keep her follow-up postoperative appointment in 1 week. The EP followed these instructions and discharged the patient home with a prescription for the new analgesic.
Three days later, however, the patient presented back to the same ED complaining of increased and now generalized abdominal pain, nausea, and vomiting. She was noted to be febrile, tachycardic, and hypotensive. On physical examination, her abdomen was diffusely tender with guarding and rebound. She was given a 2-L bolus of intravenous (IV) normal saline and started on broad spectrum IV antibiotics. After another consultation with the patient’s OB/GYN surgeon, the patient was taken immediately to the operating room. On exploration, she was found to have a segment of perforated bowel and peritonitis. A portion of the bowel was resected, but her postoperative course was complicated by sepsis. After a 1-month stay in the hospital, she was discharged home.
The patient sued the EP—but not her OB/GYN—for failure to obtain a CT scan of the abdomen/pelvis on her initial ED visit, or at least to admit her to the hospital for observation. The EP argued that even if a computed tomography (CT) scan had been performed on the initial visit, it probably would have been normal, since the bowel had not yet perforated. After trial, a defense verdict was returned.
Discussion
This case illustrates two important points. First, not every patient with abdominal pain requires a CT scan of the abdomen/pelvis. So many malpractice cases against EPs involve the failure to perform advanced imaging. Unfortunately, that is usually only through the benefit of hindsight. For a patient with mild abdominal pain, only minimal tenderness on examination, and a negative laboratory workup, it can be perfectly appropriate to treat him or her symptomatically with close follow-up and specific instructions to return to the ED if his or her condition worsens (as was the case with this patient).
The second important point is to not over-rely on a consultant(s), especially if she or he has not independently examined the patient. When calling a consultant, it is best to have a specific question (ie, “Can you see the patient in the morning?”) or action (ie, “I would like to admit the patient to your service”). In general, the EP should not rely on the consultant to give “permission” to discharge the patient. As the physician seeing the patient, the EP is the most well-equipped to work up the patient and determine the needed disposition. Rare is the consultant that can arrive at a better disposition than the EP who performed the history and physical examination on the patient.
Regarding the patient’s GYN surgery, vaginal hysterectomy (VH) is preferred over abdominal hysterectomy (AH) for benign disease as it is associated with reduced infective morbidity and earlier return to normal activities.1 With respect to postoperative events, clinicians typically employ the Clavien-Dindo grading system for the classification of surgical complications.2 The system consists of five grades, ranging from Grade I (any deviation from normal postoperative course, without the need for pharmacological intervention) to Grade V (death).
Following hysterectomy, postoperative urinary or pelvic infections are not uncommon, with an incidence of 15% to 20%.1 In the Clavien-Dindo system, these complications would typically be considered Grade II (pharmacological treatment other than what is considered an acceptable therapeutic regimen), requiring antibiotics and no surgical intervention. Grade III complications, however, usually involve postoperative issues that require surgical, endoscopic, or radiological intervention, which in VH would include ureteral, bladder, or bowel injury.1 In a study by Gendy et al,1 the incidence of such complications posthysterectomy, ranged from 1.7% to 5.7%. So while not extremely common, serious complications can occur postoperatively.
The last point is a minor one, but a truth every EP needs to remember: While it may be difficult for a patient to sue her or his own physician, especially one with whom she or he has a longstanding patient-physician relationship, it is much easier for her or him to place blame upon and sue another physician—for example, the EP.
Missed Testicular Torsion?
| A 14-year-old boy presented to the ED with a several day history of abdominal pain with radiation to the right testicle. The patient denied any nausea, vomiting, or changes in bowel habits. He also denied any genitourinary symptoms, including dysuria or urinary frequency. The boy was otherwise in good health, on no medications, and up to date on his immunizations. |
The patient was a well appearing teenager in no acute distress. All vital signs were normal, as were the heart and lung examinations. The abdominal examination revealed mild, generalized tenderness without guarding or rebound. The genitalia examination was normal.
The EP ordered a CBC, urinalysis, and a testicular ultrasound, the results of which were all normal. The patient was discharged home with instructions to follow up with his pediatrician in 2 days and to return to the ED if his symptoms worsened.
The patient was seen by his pediatrician approximately 1 month later for his scheduled annual physical examination. The pediatrician, who was aware of the boy’s prior ED visit, found the patient in good health, and performed no additional testing.
Approximately 9 months after the initial ED visit, the patient was accidently kicked in the groin while jumping on a trampoline. He experienced immediate onset of severe, excruciating right testicular pain and presented to the ED approximately 24 hours later with continued pain and swelling. A testicular ultrasound was immediately ordered and demonstrated an enlarged right testicle due to torsion.
The patient underwent surgery to remove the right testicle. His family sued the EP and hospital from the initial visit (9 months earlier) for missed intermittent testicular torsion. They argued that the patient should have been referred to a urologist for further evaluation. In addition, the plaintiff claimed he could no longer participate in sports and suffered disfigurement as a result of the surgery. The EP asserted that the patient’s pain during that initial visit was primarily abdominal in nature and that an ultrasound of the testicles was normal, and did not reveal any evidence of testicular torsion. The EP further argued that the testicular torsion was due to the trauma incurred on the trampoline. According to published accounts, a defense verdict was returned.
Discussion
Testicular torsion occurs in a bimodal age distribution—during the first year of life (perinatal) and between ages 13 and 16 years (as was the case with this patient).1 In approximately 4% to 8% of patients, there is a history of an athletic event, strenuous physical activity, or trauma just prior to the onset of scrotal pain.2
Patients typically present with sudden onset of testicular pain that is frequently associated with nausea and vomiting. However, this condition can present with only lower abdominal pain—in part be due to the fact that adolescents and children may be reluctant to complain of testicular or scrotal pain out of fear or embarrassment.1 In all cases, a genital examination should be performed on every adolescent male with a chief complaint of lower abdominal pain.3
On physical examination, the patient will usually have a swollen tender testicle. In comparison to the opposite side, the affected testicle is frequently raised and rests on a horizontal axis. The cremasteric reflex (ie, scratching the proximal inner thigh causes the ipsilateral testicle to rise) is frequently absent.4
Because of the time sensitive nature of the disease process, in classic presentations, a urologist should be immediately consulted. Ischemic changes to the testicle can begin within hours, and complete testicular atrophy occurs after 24 hours in most cases.4 Detorsion within 6 hours of onset of symptoms has a salvage rate of 90% to 100%, which drops to 25% to 50% after 12 hours and to less than 10% after 24 hours.4
For less obvious cases, color duplex testicular ultrasonography can be very helpful. Demonstration of decreased or absent blood flow is diagnostic and requires operative intervention. If untwisting the testis restores blood flow, then the condition is resolved; if this procedure fails, the testis is removed. Regardless of the outcome, the contralateral testis is fixed to prevent future torsion.
Intermittent testicular torsion is a difficult diagnosis to make. A history of recurrent unilateral scrotal pain is highly suspicious and warrants referral to a urologist. This patient had only one previous episode, which was primarily abdominal pain—not scrotal or testicular pain.
In this case, it appears the jury came to the correct decision. Given the patient had only one previous episode of abdominal pain, and an inciting event (trauma to the testicle) on the second presentation, this does not appear to be a case of missed intermittent testicular torsion. Rather, this was a correctly diagnosed testicular torsion with a delayed presentation, resulting in an unsalvageable testicle.
Sepsis Following Vaginal Hysterectomy
| A 45-year-old woman presented to the ED complaining of lower abdominal pain, which she described as gradual, aching, and intermittent. The patient stated that she had undergone a vaginal hysterectomy a few days prior and that the pain started less than 24 hours after discharge from the hospital. She denied fever or chills, nausea, or vomiting, and said that she had a bowel movement earlier that day. She also denied any urinary symptoms. Her medical history was significant only for hypothyroidism, for which she was taking levothyroxine. The patient denied cigarette smoking or alcohol consumption. She said she had been taking acetaminophen-hydrocodone for postoperative pain, but that it did not provide any relief. |
The patient’s vital signs were: temperature, 98.6˚F; blood pressure, 112/65 mm Hg; heart rate, 98 beats/minute; and respiratory rate, 20 breaths/minute. The head, eyes, ears, nose, and throat examination was normal, as were the heart and lung examinations. The patient’s abdomen was soft, with mild diffuse lower abdominal tenderness. There was no guarding, rebound, or mass present. A gross nonspeculum examination of the vaginal area did not reveal any discharge or erythema; a rectal examination was not performed.
The EP ordered a complete blood count (CBC), lipase evaluation, and urinalysis. All test results were normal. The emergency physician (EP) then contacted the obstetrician-gynecologist (OB/GYN) who had performed the hysterectomy. The OB/GYN recommended the EP change the analgesic agent to acetaminophen-oxycodone and to encourage the patient to keep her follow-up postoperative appointment in 1 week. The EP followed these instructions and discharged the patient home with a prescription for the new analgesic.
Three days later, however, the patient presented back to the same ED complaining of increased and now generalized abdominal pain, nausea, and vomiting. She was noted to be febrile, tachycardic, and hypotensive. On physical examination, her abdomen was diffusely tender with guarding and rebound. She was given a 2-L bolus of intravenous (IV) normal saline and started on broad spectrum IV antibiotics. After another consultation with the patient’s OB/GYN surgeon, the patient was taken immediately to the operating room. On exploration, she was found to have a segment of perforated bowel and peritonitis. A portion of the bowel was resected, but her postoperative course was complicated by sepsis. After a 1-month stay in the hospital, she was discharged home.
The patient sued the EP—but not her OB/GYN—for failure to obtain a CT scan of the abdomen/pelvis on her initial ED visit, or at least to admit her to the hospital for observation. The EP argued that even if a computed tomography (CT) scan had been performed on the initial visit, it probably would have been normal, since the bowel had not yet perforated. After trial, a defense verdict was returned.
Discussion
This case illustrates two important points. First, not every patient with abdominal pain requires a CT scan of the abdomen/pelvis. So many malpractice cases against EPs involve the failure to perform advanced imaging. Unfortunately, that is usually only through the benefit of hindsight. For a patient with mild abdominal pain, only minimal tenderness on examination, and a negative laboratory workup, it can be perfectly appropriate to treat him or her symptomatically with close follow-up and specific instructions to return to the ED if his or her condition worsens (as was the case with this patient).
The second important point is to not over-rely on a consultant(s), especially if she or he has not independently examined the patient. When calling a consultant, it is best to have a specific question (ie, “Can you see the patient in the morning?”) or action (ie, “I would like to admit the patient to your service”). In general, the EP should not rely on the consultant to give “permission” to discharge the patient. As the physician seeing the patient, the EP is the most well-equipped to work up the patient and determine the needed disposition. Rare is the consultant that can arrive at a better disposition than the EP who performed the history and physical examination on the patient.
Regarding the patient’s GYN surgery, vaginal hysterectomy (VH) is preferred over abdominal hysterectomy (AH) for benign disease as it is associated with reduced infective morbidity and earlier return to normal activities.1 With respect to postoperative events, clinicians typically employ the Clavien-Dindo grading system for the classification of surgical complications.2 The system consists of five grades, ranging from Grade I (any deviation from normal postoperative course, without the need for pharmacological intervention) to Grade V (death).
Following hysterectomy, postoperative urinary or pelvic infections are not uncommon, with an incidence of 15% to 20%.1 In the Clavien-Dindo system, these complications would typically be considered Grade II (pharmacological treatment other than what is considered an acceptable therapeutic regimen), requiring antibiotics and no surgical intervention. Grade III complications, however, usually involve postoperative issues that require surgical, endoscopic, or radiological intervention, which in VH would include ureteral, bladder, or bowel injury.1 In a study by Gendy et al,1 the incidence of such complications posthysterectomy, ranged from 1.7% to 5.7%. So while not extremely common, serious complications can occur postoperatively.
The last point is a minor one, but a truth every EP needs to remember: While it may be difficult for a patient to sue her or his own physician, especially one with whom she or he has a longstanding patient-physician relationship, it is much easier for her or him to place blame upon and sue another physician—for example, the EP.
Missed Testicular Torsion?
| A 14-year-old boy presented to the ED with a several day history of abdominal pain with radiation to the right testicle. The patient denied any nausea, vomiting, or changes in bowel habits. He also denied any genitourinary symptoms, including dysuria or urinary frequency. The boy was otherwise in good health, on no medications, and up to date on his immunizations. |
The patient was a well appearing teenager in no acute distress. All vital signs were normal, as were the heart and lung examinations. The abdominal examination revealed mild, generalized tenderness without guarding or rebound. The genitalia examination was normal.
The EP ordered a CBC, urinalysis, and a testicular ultrasound, the results of which were all normal. The patient was discharged home with instructions to follow up with his pediatrician in 2 days and to return to the ED if his symptoms worsened.
The patient was seen by his pediatrician approximately 1 month later for his scheduled annual physical examination. The pediatrician, who was aware of the boy’s prior ED visit, found the patient in good health, and performed no additional testing.
Approximately 9 months after the initial ED visit, the patient was accidently kicked in the groin while jumping on a trampoline. He experienced immediate onset of severe, excruciating right testicular pain and presented to the ED approximately 24 hours later with continued pain and swelling. A testicular ultrasound was immediately ordered and demonstrated an enlarged right testicle due to torsion.
The patient underwent surgery to remove the right testicle. His family sued the EP and hospital from the initial visit (9 months earlier) for missed intermittent testicular torsion. They argued that the patient should have been referred to a urologist for further evaluation. In addition, the plaintiff claimed he could no longer participate in sports and suffered disfigurement as a result of the surgery. The EP asserted that the patient’s pain during that initial visit was primarily abdominal in nature and that an ultrasound of the testicles was normal, and did not reveal any evidence of testicular torsion. The EP further argued that the testicular torsion was due to the trauma incurred on the trampoline. According to published accounts, a defense verdict was returned.
Discussion
Testicular torsion occurs in a bimodal age distribution—during the first year of life (perinatal) and between ages 13 and 16 years (as was the case with this patient).1 In approximately 4% to 8% of patients, there is a history of an athletic event, strenuous physical activity, or trauma just prior to the onset of scrotal pain.2
Patients typically present with sudden onset of testicular pain that is frequently associated with nausea and vomiting. However, this condition can present with only lower abdominal pain—in part be due to the fact that adolescents and children may be reluctant to complain of testicular or scrotal pain out of fear or embarrassment.1 In all cases, a genital examination should be performed on every adolescent male with a chief complaint of lower abdominal pain.3
On physical examination, the patient will usually have a swollen tender testicle. In comparison to the opposite side, the affected testicle is frequently raised and rests on a horizontal axis. The cremasteric reflex (ie, scratching the proximal inner thigh causes the ipsilateral testicle to rise) is frequently absent.4
Because of the time sensitive nature of the disease process, in classic presentations, a urologist should be immediately consulted. Ischemic changes to the testicle can begin within hours, and complete testicular atrophy occurs after 24 hours in most cases.4 Detorsion within 6 hours of onset of symptoms has a salvage rate of 90% to 100%, which drops to 25% to 50% after 12 hours and to less than 10% after 24 hours.4
For less obvious cases, color duplex testicular ultrasonography can be very helpful. Demonstration of decreased or absent blood flow is diagnostic and requires operative intervention. If untwisting the testis restores blood flow, then the condition is resolved; if this procedure fails, the testis is removed. Regardless of the outcome, the contralateral testis is fixed to prevent future torsion.
Intermittent testicular torsion is a difficult diagnosis to make. A history of recurrent unilateral scrotal pain is highly suspicious and warrants referral to a urologist. This patient had only one previous episode, which was primarily abdominal pain—not scrotal or testicular pain.
In this case, it appears the jury came to the correct decision. Given the patient had only one previous episode of abdominal pain, and an inciting event (trauma to the testicle) on the second presentation, this does not appear to be a case of missed intermittent testicular torsion. Rather, this was a correctly diagnosed testicular torsion with a delayed presentation, resulting in an unsalvageable testicle.
Reference - Sepsis Following Vaginal Hysterectomy
- Gendy R, Walsh CA, Walsh SR, Karantanis E. Vaginal hysterectomy versus total laparoscopic hysterectomy for benign disease: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):388.e1-8.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009:250(2):187-196.
Reference - Missed Testicular Torsion?
- Pogorelić Z, Mrklić I, Jurić I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J Pediatr Urol. 2013;9(6 Pt B):1161-1165.
- Nicks BA, Manthey DE. Male genital problems. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cine DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill Medical; 2011:649.
- Lopez RN, Beasley SW. Testicular torsion: potential pitfalls in its diagnosis and management. J Paediatr Child Health. 2012;48(2):E30-E32.
- Somani BK, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213.
Reference - Sepsis Following Vaginal Hysterectomy
- Gendy R, Walsh CA, Walsh SR, Karantanis E. Vaginal hysterectomy versus total laparoscopic hysterectomy for benign disease: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):388.e1-8.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009:250(2):187-196.
Reference - Missed Testicular Torsion?
- Pogorelić Z, Mrklić I, Jurić I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J Pediatr Urol. 2013;9(6 Pt B):1161-1165.
- Nicks BA, Manthey DE. Male genital problems. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cine DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill Medical; 2011:649.
- Lopez RN, Beasley SW. Testicular torsion: potential pitfalls in its diagnosis and management. J Paediatr Child Health. 2012;48(2):E30-E32.
- Somani BK, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213.
Baby Don’t Cry: Evaluation of Prolonged, Unexplained Crying in Infants
Case
A previously healthy 4-month-old infant was brought to the ED late on a Sunday afternoon by his father, who reported that his son had been crying hysterically since the previous evening. The father stated that the infant was well until he awoke crying in his crib around midnight. Since then, the baby had not slept for more than 20 minutes uninterrupted and had not breastfed as usual due to the persistent crying. According to the father, the patient had been a “good baby” since birth, eating well and even sleeping through the night for the past month; specifically, his son’s continual crying was not typical of his usual behavior.
There was no known trauma, fever, congestion, cough, vomiting, or diarrhea at home, and the father confirmed the patient had been making wet diapers throughout the day. The baby had received his 4-month vaccines and, according to his pediatrician, had been following a normal growth pattern.
As the patient’s father related his son’s history, he appeared exhausted and extremely anxious, pacing the room as he spoke and trying unsuccessfully to quiet the baby. He stated that the patient’s mother, who was at home in bed with mastitis, was afraid their son had become ill because she had breastfed him while she had a fever. The father confessed that he was concerned that their older son, a rambunctious 2-year-old who shares the same room as the patient, may have accidentally done something to harm the baby unbeknownst to him or his wife.
In relating the history, the infant’s father was almost tearful as he admitted that he and his wife felt completely overwhelmed and helpless that they were not able to soothe or comfort the patient.
Overview
While often the healthiest of patients seen in the ED, infants with unexplained, prolonged crying are challenging to evaluate and discharge—especially in the care of distraught parents. The following review is intended to provide a basis for understanding the scope of normal crying in infants, how and if to diagnose infantile colic in the ED, and how to avoid missing common pathology requiring acute management in the infant with new, unexplained crying.
Normal Crying: How Much Is Too Much?
During the first 3 to 4 months of life, infants cry more than at any other time. Developmental pediatrician Harvey Karp, MD, dubs the first 3 months of an infant’s life as the “fourth trimester,” during which period the baby yearns for the calming acoustic and tactile sensations of the womb. He notes that crying in this fourth trimester is merely a response to the abruptly distinct stimulation of the outside world.1 Just as each baby tolerates these new sensations differently after birth, and is soothed in her or his own measure, each parent too has a different perception and tolerance of an infant’s crying—making for a clinical parameter that is difficult to clearly assess.
What, then has been established as the upper limit of normal for crying in infancy? In the 1960s, Brazelton’s studies defined normal crying as 1 hour and 45 minutes per day at age 2 weeks; 2 hours and 45 minutes at age 6 weeks; and less than 1 hour per day by age 12 weeks.2 A more recent meta-analysis reinforces Brazelton’s criteria, indicating that the mean duration of crying is approximately 2 hours per day during the first 6 weeks of life and decreasing to a daily mean of 72 minutes by age 10 to 12 weeks.3
Infantile Colic
As the caregiver’s report of the duration of crying is often subjective, more emphasis has been placed on evaluating patterns of newborn crying by defining what is excessive. Infantile colic, or excessive crying in an otherwise healthy baby, is classically defined as fussing or crying lasting more than 3 hours per day and occurring on more than 3 days per week in a baby who is gaining weight and is otherwise well.4 Severe colic is further described as the persistence of the crying pattern for more than 3 weeks. When using this “rule of 3s,” excessive crying is estimated to be present in 1.5% to 11.9% of the infant population. There are, however, many other definitions of what constitutes excessive crying, including the more inclusive and subjective definition of colic as “intermittent, unexplained crying during the first 3 months of life that reaches a point where the parents complain about it.”5
Depending upon the definition utilized, as many as 43% of infants experience excessive crying.6 What is more uniformly accepted in the extensive literature on infantile colic is the observation that crying because of colic is concentrated during the hours of 3:00 pm to 11:00 pm and is associated with infant behaviors such as clenched fists, back-arching, passing gas, grimacing, and flexing legs, as well as with maternal anxiety.1,2,7,8
Diagnostic Certainty and Setting
Infantile colic is a diagnosis that is made retrospectively in the setting of an otherwise healthy infant who is growing and developing appropriately, and whose excessive crying ultimately resolves without medical intervention. Since colic is difficult to diagnose from any single medical encounter in the ED, it must be a diagnosis reached after excluding other possible causes.
Soothing the Colicky Infant
Extensive parental reassurance is required prior to discharging a colicky infant home—with the understanding that by the time of presentation in the ED, most parents have already exhausted their parental soothing abilities and personal coping mechanisms. Moreover, the typical physician’s promise that “this too, shall pass” is just not a sufficient addition to the parental armamentarium to manage their baby’s colic. Parents instead must be given effective techniques to calm colicky infants. Karp enumerates the following alliterative “5 Ss” guideline for soothing and calming fussy infants:
(1) Swaddling;
(2) Side/Stomach position (not while sleeping);
(3)“Shhhhhing” to provide a soothing sound that recalls the womb;
(4) Swinging the baby rhythmically in parents’ arms; and
(5) Sucking, either a pacifier or the mother’s breast.1
Equally important guidance prior to discharge from the ED is to inform parents that if they become overwhelmed by the baby’s fussing, it is always better to place an infant in the crib and let him or her cry alone for some time rather than allowing frustration to build with the baby in one's arms and increasing the potential of unintentionally harming the infant.
The “Don’t Miss” Differential Diagnoses
Whereas, as much as 43% of the infant population may experience excessive crying, only approximately 5% of infants with colic have underlying organic disease.8 The emergency physician (EP) is responsible for identifying this 5% when these infants present to the ED. A useful way to focus the initial evaluation of excessive crying is to determine the chronicity of the infant’s symptoms. To begin with, it is important to identify those babies who are in the ED merely because they have finally overwhelmed their parents with recurrent, intermittent bouts of prolonged crying despite being otherwise healthy and maintaining eating and sleeping patterns largely unaffected by crying. These are the infants who, after a thorough physical examination revealing none of the causes described below, should be swaddled, “shhhhhh’ed,” swung, suckled, and discharged with parental reassurance.
The EP, however, must be able to differentiate the classically colicky infant described above from the baby who has acutely developed unexplained crying and is at higher risk of serious disease or condition. In a study of afebrile infants experiencing an acute episode of excessive, prolonged crying, approximately 60% had an underlying disease process requiring management.9 Fortunately, many of these diagnoses can be made by an astute physical examination. In addition to evaluating infants for the most typical causes of new, prolonged crying, such as otitis media and anal fissures, the following common diagnoses should be clinically excluded in all infants presenting to the ED with acute, unexplained crying.
Corneal Abrasion
Performing a comprehensive eye examination on an inconsolable infant is not an easy task. However, corneal abrasions and foreign bodies in the eye are notorious causes of acute, excessive crying in infants—ones that are not always accompanied by conspicuous signs such as lacrimation or conjunctival injection.10
Fluorescein staining of both corneas should be performed to evaluate for a corneal abrasion. The infant’s eyelids should also be everted to look for retained foreign bodies, especially when vertical corneal abrasions have been visualized with fluorescein staining. Administering a topical ophthalmic anesthetic prior to fluorescein staining is advisable; this can be both therapeutic and diagnostic since resolution of crying after numbing the infant’s affected eye supports the diagnosis of corneal abrasion or foreign body.
Infants with corneal abrasions can be managed with a topical antibiotic ointment and 24-hour follow-up. Of note, recent studies indicate that asymptomatic corneal abrasions are extremely common in the infant period, suggesting that physicians should be careful to consider and exclude other potential causes of acute, excessive crying before attributing the symptoms to a corneal abrasion identified on examination.11
Hair Tourniquet Syndrome
Also referred to as hair thread tourniquet syndrome, the circumferential constriction of an infant’s appendage with hair, thread, or another fine material may present with the chief complaint of crying. Hair tourniquets most often involve the toes, followed by the fingers and external genitalia, and, if unrecognized or untreated, will lead to ischemia and necrosis of the distal tissue. Sleepwear that encloses the feet is a strong
risk factor for toe tourniquets, and the use of mittens is similarly linked to finger tourniquets.7,12
The crying or irritable infant merits a thorough examination of all digits and external genitalia in search of constricting bands and resultant tissue swelling. If the tourniquet has gone unnoticed over time, severe swelling, embedding of the thread, or re-epithelialization of the skin over lacerated tissue may obscure the band and make simple removal impossible. In cases when the constricting band cannot be directly unwound, treatment options for a hair tourniquet include application of a depilatory agent (eg, Nair), which has been shown to effectively dissolve the hair within 8 minutes.13 If a severe laceration is present and the thread cannot be removed by unwinding, the depilatory agent should not be applied to open tissue; instead, the clinician should perform a dorsal incision of the toe or finger to remove the constricting material in its entirety.
Testicular Torsion
Testicular torsion is a common urologic emergency in the male pediatric population, with up to 12% of all cases occurring within the first year of life. It should be considered in any infant who develops acute, prolonged, unexplained crying. Eighty-five percent of infants with torsion between 1 and 12 months old will present with irritability, and 92% will present with a tender scrotal or inguinal mass14 that may go unnoticed by the parents. This possibility alone is a compelling reason to remove the diaper of an infant with prolonged crying and to perform a thorough physical examination.
While testicular deformity or a high-riding testicle can be difficult to assess on a crying baby, any scrotal enlargement, tenderness, or color change merits an emergent urology consult—and likely ultrasound imaging with Doppler sonography. Efficient and rapid management of infants with testicular torsion is essential as the testicular salvage rate is historically very poor in this population.15
Inguinal Hernia
The reported incidence of pediatric inguinal hernia is between 0.8% and 4.4%, with most patients presenting with a chief complaint of inguinal swelling. Inguinal hernias alone are symptomatic in up to 25% of infants and may present as prolonged crying.16 In these cases, inguinal hernia surgery is usually scheduled as an elective outpatient procedure. Serious issues arise when the bowel becomes edematous and incarcerated in the hernial sac, which can lead to ischemic necrosis and intestinal perforation, intestinal obstruction, and gonadal necrosis.
More than half of all cases of pediatric incarcerated hernias occur within the first 6 months of life. Infants younger than age 12 months—especially neonates—have a more severe and a higher incidence of complications related to incarcerated inguinal hernias than the rest of the pediatric population. In a recent study, one third of newborn patients were diagnosed with incarceration as their initial presentation of an inguinal hernia.17 This is another reason to remove the patient’s diaper and perform a complete physical examination on any infant presenting with acute, unexplained crying. An incarcerated inguinal hernia will likely be identified as a tender, firm, inguinal or scrotal mass with overlying swelling or discoloration and is a surgical emergency if it cannot be reduced manually.
Nonaccidental Trauma
Emergency physicians must maintain a high index of suspicion for nonaccidental trauma in pediatric patients. Studies demonstrate that 95% of serious intracranial injuries and 64% of all head injuries in infants younger than age 12 months are the result of physical abuse. Equally discouraging is the finding that as many as 75% of child abuse cases presenting to EDs are unrecognized.18,19 Infants presenting with excessive crying have elevated risk for nonaccidental trauma both because their crying may be a result of injuries sustained from physical abuse and also because their prolonged crying itself may have been a precipitating factor in nonaccidental trauma.
Stress predisposes a caregiver to child abuse and is particularly important to identify when evaluating an infant with prolonged crying and also when providing guidance to the parents of a child with symptoms of infantile colic. Additional signs of possible nonaccidental trauma include an inconsistent history, a delay in seeking medical care, inappropriate affect of the caregiver, and an injury that is not well explained by the caregiver’s history or is incompatible with the developmental stage of the child.
Other Causes
While there are no specific laboratory tests required for the diagnosis of the abovementioned common neonatal causes of acute, prolonged crying, the EP must always consider life-threatening conditions in the inconsolable or irritable infant, including dehydration, early shock, meningitis, and a surgical abdomen. The pursuit of an appropriate workup for these diseases, accompanied by an awareness that infants may present atypically (eg, hypothermic instead of febrile) will often lead to comprehensive laboratory studies.
The EP additionally cannot undervalue the importance of trending vital signs and should never assume that tachycardia and tachypnea are due to crying alone. The onus is on the EP to consider and exclude serious illness, and nonemergent causes of prolonged crying should not be diagnosed in the setting of lethargy or poor feeding without a thorough workup. Any infant who is crying too much and eating too little per parental history is an infant who should be screened for possible underlying serious disease.
Case Conclusion
The patient in this case was found to be afebrile, tachycardic, tachypneic, and with a normal oxygen saturation while crying inconsolably on initial examination. Upon removal of the infant’s closed-footed pajamas, the distal second toe of his right foot was noted to be markedly swollen and erythematous. The EP recognized the presence of a hair tourniquet but was unable to unwind the constricting hair due to the small size of the infant’s toe and extent of tissue swelling. Topical anesthetic cream was first applied to the toe and, as there was no visible laceration of the skin, depilatory cream was subsequently applied to the tourniquet. After 10 minutes, the toe was rubbed and rinsed with water and the hair tourniquet dissolved. There was significant improvement in tissue swelling and color within 1 hour of removal of the constricting band. The affected toe demonstrated adequate capillary refill and no sign of persistent vascular compromise. The patient’s crying subsided and his vital signs and remainder of his physical examination were normal.
Since there was evidence of superficial skin breakdown at the site of the hair tourniquet, the patient’s father was instructed to apply topical antibiotic ointment to the site and to follow-up with the patient’s pediatrician in 24 hours. Of note, the father stated that the infant’s mother had been experiencing significant postpartum hair loss recently and that they would now be careful to remove loose hair from the baby’s clothing and bedding—especially when dressing him in closed-footed pajamas or mittens.
Dr Leader is a fellow, department of pediatric emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Karp H. The “fourth trimester”: a framework and strategy for understanding and resolving colic. Contemp Pediatrics. 2004;21:94.
- Brazelton TB. Crying in infancy. Pediatrics. 1962;29:579-588.
- Wolke D, Samara M, Alvarez Wolke M. Meta-analysis of fuss/cry durations and colic prevalence across countries. In: Proceedings of the 11th International Infant Cry Research Workshop. 8-10 June 2011. Zeist, The Netherlands. 2011.
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14(5):421-435.
- Schmitt BD. Colic: excessive crying in newborns. Clin Perinatol. 1985;12(2):441-451.
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics. 2001;108(4):893-897.
- Hicks M. An evidence-based, systematic approach to acute, unexplained, excessive crying in infants. Pediatr Emerg Med Pract. 2005;2(2-3):1-50.
- Barr RG. Colic and crying syndromes in infants. Pediatrics. 1998;102(5 Suppl E):1282-1286.
- Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88(3):450-455.
- Harkness MJ. Corneal abrasion in infancy as a cause of inconsolable crying. Pediatr Emerg Care. 1989;5(4):242-244.
- Shope TR, Rieg TS, Kathiria NN. Corneal abrasions in young infants. Pediatrics. 2012;125(3):e585-e589.
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics. 1988;82(6):925-928.
- Plesa JA, Shoup K, Manole MD, Hickey RW. Effect of depilatory agent on cotton, polyester, and rayon versus human hair in a laboratory setting. Ann Emerg Med. 2015;65(3):256-259.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular torsion in the first year of life—characteristics and treatment outcome. Urology. 2013;82(5):1132-1137.
- Saxena AK, Castellani C, Ruttenstock EM, Höllwarth ME. Testicular torsion: a 15-year single-centre clinical and histological analysis. Acta Paediatr. 2012;101(7):e282-e286.
- Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one pediatric inguinal hernias: a 35-year review. J Ped Surg. 2006;41(5):980-986.
- Erdoğan D, Karaman I, Aslan MK, Karaman A, Cavuşoğlu YH. Analysis of 3,776 pediatric inguinal hernia and hydrocele cases in a tertiary center. J Pediatr Surg. 2013;48(8):1767-1772.
- Sirotnak AP, Grigsby T, Krugman RD. Physical abuse of children. Pediatr Rev. 2004;25(8):264-277
- Kunen S, Hume P, Perret JN, Mandry CV, Patterson TR. Underdiagnosis of child abuse in emergency departments. Acad Emerg Med. 2003;10(5):546-a.
Case
A previously healthy 4-month-old infant was brought to the ED late on a Sunday afternoon by his father, who reported that his son had been crying hysterically since the previous evening. The father stated that the infant was well until he awoke crying in his crib around midnight. Since then, the baby had not slept for more than 20 minutes uninterrupted and had not breastfed as usual due to the persistent crying. According to the father, the patient had been a “good baby” since birth, eating well and even sleeping through the night for the past month; specifically, his son’s continual crying was not typical of his usual behavior.
There was no known trauma, fever, congestion, cough, vomiting, or diarrhea at home, and the father confirmed the patient had been making wet diapers throughout the day. The baby had received his 4-month vaccines and, according to his pediatrician, had been following a normal growth pattern.
As the patient’s father related his son’s history, he appeared exhausted and extremely anxious, pacing the room as he spoke and trying unsuccessfully to quiet the baby. He stated that the patient’s mother, who was at home in bed with mastitis, was afraid their son had become ill because she had breastfed him while she had a fever. The father confessed that he was concerned that their older son, a rambunctious 2-year-old who shares the same room as the patient, may have accidentally done something to harm the baby unbeknownst to him or his wife.
In relating the history, the infant’s father was almost tearful as he admitted that he and his wife felt completely overwhelmed and helpless that they were not able to soothe or comfort the patient.
Overview
While often the healthiest of patients seen in the ED, infants with unexplained, prolonged crying are challenging to evaluate and discharge—especially in the care of distraught parents. The following review is intended to provide a basis for understanding the scope of normal crying in infants, how and if to diagnose infantile colic in the ED, and how to avoid missing common pathology requiring acute management in the infant with new, unexplained crying.
Normal Crying: How Much Is Too Much?
During the first 3 to 4 months of life, infants cry more than at any other time. Developmental pediatrician Harvey Karp, MD, dubs the first 3 months of an infant’s life as the “fourth trimester,” during which period the baby yearns for the calming acoustic and tactile sensations of the womb. He notes that crying in this fourth trimester is merely a response to the abruptly distinct stimulation of the outside world.1 Just as each baby tolerates these new sensations differently after birth, and is soothed in her or his own measure, each parent too has a different perception and tolerance of an infant’s crying—making for a clinical parameter that is difficult to clearly assess.
What, then has been established as the upper limit of normal for crying in infancy? In the 1960s, Brazelton’s studies defined normal crying as 1 hour and 45 minutes per day at age 2 weeks; 2 hours and 45 minutes at age 6 weeks; and less than 1 hour per day by age 12 weeks.2 A more recent meta-analysis reinforces Brazelton’s criteria, indicating that the mean duration of crying is approximately 2 hours per day during the first 6 weeks of life and decreasing to a daily mean of 72 minutes by age 10 to 12 weeks.3
Infantile Colic
As the caregiver’s report of the duration of crying is often subjective, more emphasis has been placed on evaluating patterns of newborn crying by defining what is excessive. Infantile colic, or excessive crying in an otherwise healthy baby, is classically defined as fussing or crying lasting more than 3 hours per day and occurring on more than 3 days per week in a baby who is gaining weight and is otherwise well.4 Severe colic is further described as the persistence of the crying pattern for more than 3 weeks. When using this “rule of 3s,” excessive crying is estimated to be present in 1.5% to 11.9% of the infant population. There are, however, many other definitions of what constitutes excessive crying, including the more inclusive and subjective definition of colic as “intermittent, unexplained crying during the first 3 months of life that reaches a point where the parents complain about it.”5
Depending upon the definition utilized, as many as 43% of infants experience excessive crying.6 What is more uniformly accepted in the extensive literature on infantile colic is the observation that crying because of colic is concentrated during the hours of 3:00 pm to 11:00 pm and is associated with infant behaviors such as clenched fists, back-arching, passing gas, grimacing, and flexing legs, as well as with maternal anxiety.1,2,7,8
Diagnostic Certainty and Setting
Infantile colic is a diagnosis that is made retrospectively in the setting of an otherwise healthy infant who is growing and developing appropriately, and whose excessive crying ultimately resolves without medical intervention. Since colic is difficult to diagnose from any single medical encounter in the ED, it must be a diagnosis reached after excluding other possible causes.
Soothing the Colicky Infant
Extensive parental reassurance is required prior to discharging a colicky infant home—with the understanding that by the time of presentation in the ED, most parents have already exhausted their parental soothing abilities and personal coping mechanisms. Moreover, the typical physician’s promise that “this too, shall pass” is just not a sufficient addition to the parental armamentarium to manage their baby’s colic. Parents instead must be given effective techniques to calm colicky infants. Karp enumerates the following alliterative “5 Ss” guideline for soothing and calming fussy infants:
(1) Swaddling;
(2) Side/Stomach position (not while sleeping);
(3)“Shhhhhing” to provide a soothing sound that recalls the womb;
(4) Swinging the baby rhythmically in parents’ arms; and
(5) Sucking, either a pacifier or the mother’s breast.1
Equally important guidance prior to discharge from the ED is to inform parents that if they become overwhelmed by the baby’s fussing, it is always better to place an infant in the crib and let him or her cry alone for some time rather than allowing frustration to build with the baby in one's arms and increasing the potential of unintentionally harming the infant.
The “Don’t Miss” Differential Diagnoses
Whereas, as much as 43% of the infant population may experience excessive crying, only approximately 5% of infants with colic have underlying organic disease.8 The emergency physician (EP) is responsible for identifying this 5% when these infants present to the ED. A useful way to focus the initial evaluation of excessive crying is to determine the chronicity of the infant’s symptoms. To begin with, it is important to identify those babies who are in the ED merely because they have finally overwhelmed their parents with recurrent, intermittent bouts of prolonged crying despite being otherwise healthy and maintaining eating and sleeping patterns largely unaffected by crying. These are the infants who, after a thorough physical examination revealing none of the causes described below, should be swaddled, “shhhhhh’ed,” swung, suckled, and discharged with parental reassurance.
The EP, however, must be able to differentiate the classically colicky infant described above from the baby who has acutely developed unexplained crying and is at higher risk of serious disease or condition. In a study of afebrile infants experiencing an acute episode of excessive, prolonged crying, approximately 60% had an underlying disease process requiring management.9 Fortunately, many of these diagnoses can be made by an astute physical examination. In addition to evaluating infants for the most typical causes of new, prolonged crying, such as otitis media and anal fissures, the following common diagnoses should be clinically excluded in all infants presenting to the ED with acute, unexplained crying.
Corneal Abrasion
Performing a comprehensive eye examination on an inconsolable infant is not an easy task. However, corneal abrasions and foreign bodies in the eye are notorious causes of acute, excessive crying in infants—ones that are not always accompanied by conspicuous signs such as lacrimation or conjunctival injection.10
Fluorescein staining of both corneas should be performed to evaluate for a corneal abrasion. The infant’s eyelids should also be everted to look for retained foreign bodies, especially when vertical corneal abrasions have been visualized with fluorescein staining. Administering a topical ophthalmic anesthetic prior to fluorescein staining is advisable; this can be both therapeutic and diagnostic since resolution of crying after numbing the infant’s affected eye supports the diagnosis of corneal abrasion or foreign body.
Infants with corneal abrasions can be managed with a topical antibiotic ointment and 24-hour follow-up. Of note, recent studies indicate that asymptomatic corneal abrasions are extremely common in the infant period, suggesting that physicians should be careful to consider and exclude other potential causes of acute, excessive crying before attributing the symptoms to a corneal abrasion identified on examination.11
Hair Tourniquet Syndrome
Also referred to as hair thread tourniquet syndrome, the circumferential constriction of an infant’s appendage with hair, thread, or another fine material may present with the chief complaint of crying. Hair tourniquets most often involve the toes, followed by the fingers and external genitalia, and, if unrecognized or untreated, will lead to ischemia and necrosis of the distal tissue. Sleepwear that encloses the feet is a strong
risk factor for toe tourniquets, and the use of mittens is similarly linked to finger tourniquets.7,12
The crying or irritable infant merits a thorough examination of all digits and external genitalia in search of constricting bands and resultant tissue swelling. If the tourniquet has gone unnoticed over time, severe swelling, embedding of the thread, or re-epithelialization of the skin over lacerated tissue may obscure the band and make simple removal impossible. In cases when the constricting band cannot be directly unwound, treatment options for a hair tourniquet include application of a depilatory agent (eg, Nair), which has been shown to effectively dissolve the hair within 8 minutes.13 If a severe laceration is present and the thread cannot be removed by unwinding, the depilatory agent should not be applied to open tissue; instead, the clinician should perform a dorsal incision of the toe or finger to remove the constricting material in its entirety.
Testicular Torsion
Testicular torsion is a common urologic emergency in the male pediatric population, with up to 12% of all cases occurring within the first year of life. It should be considered in any infant who develops acute, prolonged, unexplained crying. Eighty-five percent of infants with torsion between 1 and 12 months old will present with irritability, and 92% will present with a tender scrotal or inguinal mass14 that may go unnoticed by the parents. This possibility alone is a compelling reason to remove the diaper of an infant with prolonged crying and to perform a thorough physical examination.
While testicular deformity or a high-riding testicle can be difficult to assess on a crying baby, any scrotal enlargement, tenderness, or color change merits an emergent urology consult—and likely ultrasound imaging with Doppler sonography. Efficient and rapid management of infants with testicular torsion is essential as the testicular salvage rate is historically very poor in this population.15
Inguinal Hernia
The reported incidence of pediatric inguinal hernia is between 0.8% and 4.4%, with most patients presenting with a chief complaint of inguinal swelling. Inguinal hernias alone are symptomatic in up to 25% of infants and may present as prolonged crying.16 In these cases, inguinal hernia surgery is usually scheduled as an elective outpatient procedure. Serious issues arise when the bowel becomes edematous and incarcerated in the hernial sac, which can lead to ischemic necrosis and intestinal perforation, intestinal obstruction, and gonadal necrosis.
More than half of all cases of pediatric incarcerated hernias occur within the first 6 months of life. Infants younger than age 12 months—especially neonates—have a more severe and a higher incidence of complications related to incarcerated inguinal hernias than the rest of the pediatric population. In a recent study, one third of newborn patients were diagnosed with incarceration as their initial presentation of an inguinal hernia.17 This is another reason to remove the patient’s diaper and perform a complete physical examination on any infant presenting with acute, unexplained crying. An incarcerated inguinal hernia will likely be identified as a tender, firm, inguinal or scrotal mass with overlying swelling or discoloration and is a surgical emergency if it cannot be reduced manually.
Nonaccidental Trauma
Emergency physicians must maintain a high index of suspicion for nonaccidental trauma in pediatric patients. Studies demonstrate that 95% of serious intracranial injuries and 64% of all head injuries in infants younger than age 12 months are the result of physical abuse. Equally discouraging is the finding that as many as 75% of child abuse cases presenting to EDs are unrecognized.18,19 Infants presenting with excessive crying have elevated risk for nonaccidental trauma both because their crying may be a result of injuries sustained from physical abuse and also because their prolonged crying itself may have been a precipitating factor in nonaccidental trauma.
Stress predisposes a caregiver to child abuse and is particularly important to identify when evaluating an infant with prolonged crying and also when providing guidance to the parents of a child with symptoms of infantile colic. Additional signs of possible nonaccidental trauma include an inconsistent history, a delay in seeking medical care, inappropriate affect of the caregiver, and an injury that is not well explained by the caregiver’s history or is incompatible with the developmental stage of the child.
Other Causes
While there are no specific laboratory tests required for the diagnosis of the abovementioned common neonatal causes of acute, prolonged crying, the EP must always consider life-threatening conditions in the inconsolable or irritable infant, including dehydration, early shock, meningitis, and a surgical abdomen. The pursuit of an appropriate workup for these diseases, accompanied by an awareness that infants may present atypically (eg, hypothermic instead of febrile) will often lead to comprehensive laboratory studies.
The EP additionally cannot undervalue the importance of trending vital signs and should never assume that tachycardia and tachypnea are due to crying alone. The onus is on the EP to consider and exclude serious illness, and nonemergent causes of prolonged crying should not be diagnosed in the setting of lethargy or poor feeding without a thorough workup. Any infant who is crying too much and eating too little per parental history is an infant who should be screened for possible underlying serious disease.
Case Conclusion
The patient in this case was found to be afebrile, tachycardic, tachypneic, and with a normal oxygen saturation while crying inconsolably on initial examination. Upon removal of the infant’s closed-footed pajamas, the distal second toe of his right foot was noted to be markedly swollen and erythematous. The EP recognized the presence of a hair tourniquet but was unable to unwind the constricting hair due to the small size of the infant’s toe and extent of tissue swelling. Topical anesthetic cream was first applied to the toe and, as there was no visible laceration of the skin, depilatory cream was subsequently applied to the tourniquet. After 10 minutes, the toe was rubbed and rinsed with water and the hair tourniquet dissolved. There was significant improvement in tissue swelling and color within 1 hour of removal of the constricting band. The affected toe demonstrated adequate capillary refill and no sign of persistent vascular compromise. The patient’s crying subsided and his vital signs and remainder of his physical examination were normal.
Since there was evidence of superficial skin breakdown at the site of the hair tourniquet, the patient’s father was instructed to apply topical antibiotic ointment to the site and to follow-up with the patient’s pediatrician in 24 hours. Of note, the father stated that the infant’s mother had been experiencing significant postpartum hair loss recently and that they would now be careful to remove loose hair from the baby’s clothing and bedding—especially when dressing him in closed-footed pajamas or mittens.
Dr Leader is a fellow, department of pediatric emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Case
A previously healthy 4-month-old infant was brought to the ED late on a Sunday afternoon by his father, who reported that his son had been crying hysterically since the previous evening. The father stated that the infant was well until he awoke crying in his crib around midnight. Since then, the baby had not slept for more than 20 minutes uninterrupted and had not breastfed as usual due to the persistent crying. According to the father, the patient had been a “good baby” since birth, eating well and even sleeping through the night for the past month; specifically, his son’s continual crying was not typical of his usual behavior.
There was no known trauma, fever, congestion, cough, vomiting, or diarrhea at home, and the father confirmed the patient had been making wet diapers throughout the day. The baby had received his 4-month vaccines and, according to his pediatrician, had been following a normal growth pattern.
As the patient’s father related his son’s history, he appeared exhausted and extremely anxious, pacing the room as he spoke and trying unsuccessfully to quiet the baby. He stated that the patient’s mother, who was at home in bed with mastitis, was afraid their son had become ill because she had breastfed him while she had a fever. The father confessed that he was concerned that their older son, a rambunctious 2-year-old who shares the same room as the patient, may have accidentally done something to harm the baby unbeknownst to him or his wife.
In relating the history, the infant’s father was almost tearful as he admitted that he and his wife felt completely overwhelmed and helpless that they were not able to soothe or comfort the patient.
Overview
While often the healthiest of patients seen in the ED, infants with unexplained, prolonged crying are challenging to evaluate and discharge—especially in the care of distraught parents. The following review is intended to provide a basis for understanding the scope of normal crying in infants, how and if to diagnose infantile colic in the ED, and how to avoid missing common pathology requiring acute management in the infant with new, unexplained crying.
Normal Crying: How Much Is Too Much?
During the first 3 to 4 months of life, infants cry more than at any other time. Developmental pediatrician Harvey Karp, MD, dubs the first 3 months of an infant’s life as the “fourth trimester,” during which period the baby yearns for the calming acoustic and tactile sensations of the womb. He notes that crying in this fourth trimester is merely a response to the abruptly distinct stimulation of the outside world.1 Just as each baby tolerates these new sensations differently after birth, and is soothed in her or his own measure, each parent too has a different perception and tolerance of an infant’s crying—making for a clinical parameter that is difficult to clearly assess.
What, then has been established as the upper limit of normal for crying in infancy? In the 1960s, Brazelton’s studies defined normal crying as 1 hour and 45 minutes per day at age 2 weeks; 2 hours and 45 minutes at age 6 weeks; and less than 1 hour per day by age 12 weeks.2 A more recent meta-analysis reinforces Brazelton’s criteria, indicating that the mean duration of crying is approximately 2 hours per day during the first 6 weeks of life and decreasing to a daily mean of 72 minutes by age 10 to 12 weeks.3
Infantile Colic
As the caregiver’s report of the duration of crying is often subjective, more emphasis has been placed on evaluating patterns of newborn crying by defining what is excessive. Infantile colic, or excessive crying in an otherwise healthy baby, is classically defined as fussing or crying lasting more than 3 hours per day and occurring on more than 3 days per week in a baby who is gaining weight and is otherwise well.4 Severe colic is further described as the persistence of the crying pattern for more than 3 weeks. When using this “rule of 3s,” excessive crying is estimated to be present in 1.5% to 11.9% of the infant population. There are, however, many other definitions of what constitutes excessive crying, including the more inclusive and subjective definition of colic as “intermittent, unexplained crying during the first 3 months of life that reaches a point where the parents complain about it.”5
Depending upon the definition utilized, as many as 43% of infants experience excessive crying.6 What is more uniformly accepted in the extensive literature on infantile colic is the observation that crying because of colic is concentrated during the hours of 3:00 pm to 11:00 pm and is associated with infant behaviors such as clenched fists, back-arching, passing gas, grimacing, and flexing legs, as well as with maternal anxiety.1,2,7,8
Diagnostic Certainty and Setting
Infantile colic is a diagnosis that is made retrospectively in the setting of an otherwise healthy infant who is growing and developing appropriately, and whose excessive crying ultimately resolves without medical intervention. Since colic is difficult to diagnose from any single medical encounter in the ED, it must be a diagnosis reached after excluding other possible causes.
Soothing the Colicky Infant
Extensive parental reassurance is required prior to discharging a colicky infant home—with the understanding that by the time of presentation in the ED, most parents have already exhausted their parental soothing abilities and personal coping mechanisms. Moreover, the typical physician’s promise that “this too, shall pass” is just not a sufficient addition to the parental armamentarium to manage their baby’s colic. Parents instead must be given effective techniques to calm colicky infants. Karp enumerates the following alliterative “5 Ss” guideline for soothing and calming fussy infants:
(1) Swaddling;
(2) Side/Stomach position (not while sleeping);
(3)“Shhhhhing” to provide a soothing sound that recalls the womb;
(4) Swinging the baby rhythmically in parents’ arms; and
(5) Sucking, either a pacifier or the mother’s breast.1
Equally important guidance prior to discharge from the ED is to inform parents that if they become overwhelmed by the baby’s fussing, it is always better to place an infant in the crib and let him or her cry alone for some time rather than allowing frustration to build with the baby in one's arms and increasing the potential of unintentionally harming the infant.
The “Don’t Miss” Differential Diagnoses
Whereas, as much as 43% of the infant population may experience excessive crying, only approximately 5% of infants with colic have underlying organic disease.8 The emergency physician (EP) is responsible for identifying this 5% when these infants present to the ED. A useful way to focus the initial evaluation of excessive crying is to determine the chronicity of the infant’s symptoms. To begin with, it is important to identify those babies who are in the ED merely because they have finally overwhelmed their parents with recurrent, intermittent bouts of prolonged crying despite being otherwise healthy and maintaining eating and sleeping patterns largely unaffected by crying. These are the infants who, after a thorough physical examination revealing none of the causes described below, should be swaddled, “shhhhhh’ed,” swung, suckled, and discharged with parental reassurance.
The EP, however, must be able to differentiate the classically colicky infant described above from the baby who has acutely developed unexplained crying and is at higher risk of serious disease or condition. In a study of afebrile infants experiencing an acute episode of excessive, prolonged crying, approximately 60% had an underlying disease process requiring management.9 Fortunately, many of these diagnoses can be made by an astute physical examination. In addition to evaluating infants for the most typical causes of new, prolonged crying, such as otitis media and anal fissures, the following common diagnoses should be clinically excluded in all infants presenting to the ED with acute, unexplained crying.
Corneal Abrasion
Performing a comprehensive eye examination on an inconsolable infant is not an easy task. However, corneal abrasions and foreign bodies in the eye are notorious causes of acute, excessive crying in infants—ones that are not always accompanied by conspicuous signs such as lacrimation or conjunctival injection.10
Fluorescein staining of both corneas should be performed to evaluate for a corneal abrasion. The infant’s eyelids should also be everted to look for retained foreign bodies, especially when vertical corneal abrasions have been visualized with fluorescein staining. Administering a topical ophthalmic anesthetic prior to fluorescein staining is advisable; this can be both therapeutic and diagnostic since resolution of crying after numbing the infant’s affected eye supports the diagnosis of corneal abrasion or foreign body.
Infants with corneal abrasions can be managed with a topical antibiotic ointment and 24-hour follow-up. Of note, recent studies indicate that asymptomatic corneal abrasions are extremely common in the infant period, suggesting that physicians should be careful to consider and exclude other potential causes of acute, excessive crying before attributing the symptoms to a corneal abrasion identified on examination.11
Hair Tourniquet Syndrome
Also referred to as hair thread tourniquet syndrome, the circumferential constriction of an infant’s appendage with hair, thread, or another fine material may present with the chief complaint of crying. Hair tourniquets most often involve the toes, followed by the fingers and external genitalia, and, if unrecognized or untreated, will lead to ischemia and necrosis of the distal tissue. Sleepwear that encloses the feet is a strong
risk factor for toe tourniquets, and the use of mittens is similarly linked to finger tourniquets.7,12
The crying or irritable infant merits a thorough examination of all digits and external genitalia in search of constricting bands and resultant tissue swelling. If the tourniquet has gone unnoticed over time, severe swelling, embedding of the thread, or re-epithelialization of the skin over lacerated tissue may obscure the band and make simple removal impossible. In cases when the constricting band cannot be directly unwound, treatment options for a hair tourniquet include application of a depilatory agent (eg, Nair), which has been shown to effectively dissolve the hair within 8 minutes.13 If a severe laceration is present and the thread cannot be removed by unwinding, the depilatory agent should not be applied to open tissue; instead, the clinician should perform a dorsal incision of the toe or finger to remove the constricting material in its entirety.
Testicular Torsion
Testicular torsion is a common urologic emergency in the male pediatric population, with up to 12% of all cases occurring within the first year of life. It should be considered in any infant who develops acute, prolonged, unexplained crying. Eighty-five percent of infants with torsion between 1 and 12 months old will present with irritability, and 92% will present with a tender scrotal or inguinal mass14 that may go unnoticed by the parents. This possibility alone is a compelling reason to remove the diaper of an infant with prolonged crying and to perform a thorough physical examination.
While testicular deformity or a high-riding testicle can be difficult to assess on a crying baby, any scrotal enlargement, tenderness, or color change merits an emergent urology consult—and likely ultrasound imaging with Doppler sonography. Efficient and rapid management of infants with testicular torsion is essential as the testicular salvage rate is historically very poor in this population.15
Inguinal Hernia
The reported incidence of pediatric inguinal hernia is between 0.8% and 4.4%, with most patients presenting with a chief complaint of inguinal swelling. Inguinal hernias alone are symptomatic in up to 25% of infants and may present as prolonged crying.16 In these cases, inguinal hernia surgery is usually scheduled as an elective outpatient procedure. Serious issues arise when the bowel becomes edematous and incarcerated in the hernial sac, which can lead to ischemic necrosis and intestinal perforation, intestinal obstruction, and gonadal necrosis.
More than half of all cases of pediatric incarcerated hernias occur within the first 6 months of life. Infants younger than age 12 months—especially neonates—have a more severe and a higher incidence of complications related to incarcerated inguinal hernias than the rest of the pediatric population. In a recent study, one third of newborn patients were diagnosed with incarceration as their initial presentation of an inguinal hernia.17 This is another reason to remove the patient’s diaper and perform a complete physical examination on any infant presenting with acute, unexplained crying. An incarcerated inguinal hernia will likely be identified as a tender, firm, inguinal or scrotal mass with overlying swelling or discoloration and is a surgical emergency if it cannot be reduced manually.
Nonaccidental Trauma
Emergency physicians must maintain a high index of suspicion for nonaccidental trauma in pediatric patients. Studies demonstrate that 95% of serious intracranial injuries and 64% of all head injuries in infants younger than age 12 months are the result of physical abuse. Equally discouraging is the finding that as many as 75% of child abuse cases presenting to EDs are unrecognized.18,19 Infants presenting with excessive crying have elevated risk for nonaccidental trauma both because their crying may be a result of injuries sustained from physical abuse and also because their prolonged crying itself may have been a precipitating factor in nonaccidental trauma.
Stress predisposes a caregiver to child abuse and is particularly important to identify when evaluating an infant with prolonged crying and also when providing guidance to the parents of a child with symptoms of infantile colic. Additional signs of possible nonaccidental trauma include an inconsistent history, a delay in seeking medical care, inappropriate affect of the caregiver, and an injury that is not well explained by the caregiver’s history or is incompatible with the developmental stage of the child.
Other Causes
While there are no specific laboratory tests required for the diagnosis of the abovementioned common neonatal causes of acute, prolonged crying, the EP must always consider life-threatening conditions in the inconsolable or irritable infant, including dehydration, early shock, meningitis, and a surgical abdomen. The pursuit of an appropriate workup for these diseases, accompanied by an awareness that infants may present atypically (eg, hypothermic instead of febrile) will often lead to comprehensive laboratory studies.
The EP additionally cannot undervalue the importance of trending vital signs and should never assume that tachycardia and tachypnea are due to crying alone. The onus is on the EP to consider and exclude serious illness, and nonemergent causes of prolonged crying should not be diagnosed in the setting of lethargy or poor feeding without a thorough workup. Any infant who is crying too much and eating too little per parental history is an infant who should be screened for possible underlying serious disease.
Case Conclusion
The patient in this case was found to be afebrile, tachycardic, tachypneic, and with a normal oxygen saturation while crying inconsolably on initial examination. Upon removal of the infant’s closed-footed pajamas, the distal second toe of his right foot was noted to be markedly swollen and erythematous. The EP recognized the presence of a hair tourniquet but was unable to unwind the constricting hair due to the small size of the infant’s toe and extent of tissue swelling. Topical anesthetic cream was first applied to the toe and, as there was no visible laceration of the skin, depilatory cream was subsequently applied to the tourniquet. After 10 minutes, the toe was rubbed and rinsed with water and the hair tourniquet dissolved. There was significant improvement in tissue swelling and color within 1 hour of removal of the constricting band. The affected toe demonstrated adequate capillary refill and no sign of persistent vascular compromise. The patient’s crying subsided and his vital signs and remainder of his physical examination were normal.
Since there was evidence of superficial skin breakdown at the site of the hair tourniquet, the patient’s father was instructed to apply topical antibiotic ointment to the site and to follow-up with the patient’s pediatrician in 24 hours. Of note, the father stated that the infant’s mother had been experiencing significant postpartum hair loss recently and that they would now be careful to remove loose hair from the baby’s clothing and bedding—especially when dressing him in closed-footed pajamas or mittens.
Dr Leader is a fellow, department of pediatric emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Karp H. The “fourth trimester”: a framework and strategy for understanding and resolving colic. Contemp Pediatrics. 2004;21:94.
- Brazelton TB. Crying in infancy. Pediatrics. 1962;29:579-588.
- Wolke D, Samara M, Alvarez Wolke M. Meta-analysis of fuss/cry durations and colic prevalence across countries. In: Proceedings of the 11th International Infant Cry Research Workshop. 8-10 June 2011. Zeist, The Netherlands. 2011.
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14(5):421-435.
- Schmitt BD. Colic: excessive crying in newborns. Clin Perinatol. 1985;12(2):441-451.
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics. 2001;108(4):893-897.
- Hicks M. An evidence-based, systematic approach to acute, unexplained, excessive crying in infants. Pediatr Emerg Med Pract. 2005;2(2-3):1-50.
- Barr RG. Colic and crying syndromes in infants. Pediatrics. 1998;102(5 Suppl E):1282-1286.
- Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88(3):450-455.
- Harkness MJ. Corneal abrasion in infancy as a cause of inconsolable crying. Pediatr Emerg Care. 1989;5(4):242-244.
- Shope TR, Rieg TS, Kathiria NN. Corneal abrasions in young infants. Pediatrics. 2012;125(3):e585-e589.
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics. 1988;82(6):925-928.
- Plesa JA, Shoup K, Manole MD, Hickey RW. Effect of depilatory agent on cotton, polyester, and rayon versus human hair in a laboratory setting. Ann Emerg Med. 2015;65(3):256-259.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular torsion in the first year of life—characteristics and treatment outcome. Urology. 2013;82(5):1132-1137.
- Saxena AK, Castellani C, Ruttenstock EM, Höllwarth ME. Testicular torsion: a 15-year single-centre clinical and histological analysis. Acta Paediatr. 2012;101(7):e282-e286.
- Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one pediatric inguinal hernias: a 35-year review. J Ped Surg. 2006;41(5):980-986.
- Erdoğan D, Karaman I, Aslan MK, Karaman A, Cavuşoğlu YH. Analysis of 3,776 pediatric inguinal hernia and hydrocele cases in a tertiary center. J Pediatr Surg. 2013;48(8):1767-1772.
- Sirotnak AP, Grigsby T, Krugman RD. Physical abuse of children. Pediatr Rev. 2004;25(8):264-277
- Kunen S, Hume P, Perret JN, Mandry CV, Patterson TR. Underdiagnosis of child abuse in emergency departments. Acad Emerg Med. 2003;10(5):546-a.
- Karp H. The “fourth trimester”: a framework and strategy for understanding and resolving colic. Contemp Pediatrics. 2004;21:94.
- Brazelton TB. Crying in infancy. Pediatrics. 1962;29:579-588.
- Wolke D, Samara M, Alvarez Wolke M. Meta-analysis of fuss/cry durations and colic prevalence across countries. In: Proceedings of the 11th International Infant Cry Research Workshop. 8-10 June 2011. Zeist, The Netherlands. 2011.
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14(5):421-435.
- Schmitt BD. Colic: excessive crying in newborns. Clin Perinatol. 1985;12(2):441-451.
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics. 2001;108(4):893-897.
- Hicks M. An evidence-based, systematic approach to acute, unexplained, excessive crying in infants. Pediatr Emerg Med Pract. 2005;2(2-3):1-50.
- Barr RG. Colic and crying syndromes in infants. Pediatrics. 1998;102(5 Suppl E):1282-1286.
- Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88(3):450-455.
- Harkness MJ. Corneal abrasion in infancy as a cause of inconsolable crying. Pediatr Emerg Care. 1989;5(4):242-244.
- Shope TR, Rieg TS, Kathiria NN. Corneal abrasions in young infants. Pediatrics. 2012;125(3):e585-e589.
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics. 1988;82(6):925-928.
- Plesa JA, Shoup K, Manole MD, Hickey RW. Effect of depilatory agent on cotton, polyester, and rayon versus human hair in a laboratory setting. Ann Emerg Med. 2015;65(3):256-259.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular torsion in the first year of life—characteristics and treatment outcome. Urology. 2013;82(5):1132-1137.
- Saxena AK, Castellani C, Ruttenstock EM, Höllwarth ME. Testicular torsion: a 15-year single-centre clinical and histological analysis. Acta Paediatr. 2012;101(7):e282-e286.
- Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one pediatric inguinal hernias: a 35-year review. J Ped Surg. 2006;41(5):980-986.
- Erdoğan D, Karaman I, Aslan MK, Karaman A, Cavuşoğlu YH. Analysis of 3,776 pediatric inguinal hernia and hydrocele cases in a tertiary center. J Pediatr Surg. 2013;48(8):1767-1772.
- Sirotnak AP, Grigsby T, Krugman RD. Physical abuse of children. Pediatr Rev. 2004;25(8):264-277
- Kunen S, Hume P, Perret JN, Mandry CV, Patterson TR. Underdiagnosis of child abuse in emergency departments. Acad Emerg Med. 2003;10(5):546-a.
2015 Update on cervical disease: New ammo for HPV prevention and screening
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
In this article
- Disease progression with 9-valent versus quadrivalent HPV vaccine
- How well do current screening strategies detect disease?
- Recommended primary HPV screening algorithm
Endometriosis and pain: Expert answers to 6 questions targeting your management options
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
 The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
 The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
 The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Endometriosis: 3 intraoperative videos
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu.