User login
When Patients Make Unexpected Medical Choices
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
Age competency exams for physicians – yes or no?
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Insights From the 2020-2021 Dermatology Residency Match
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.
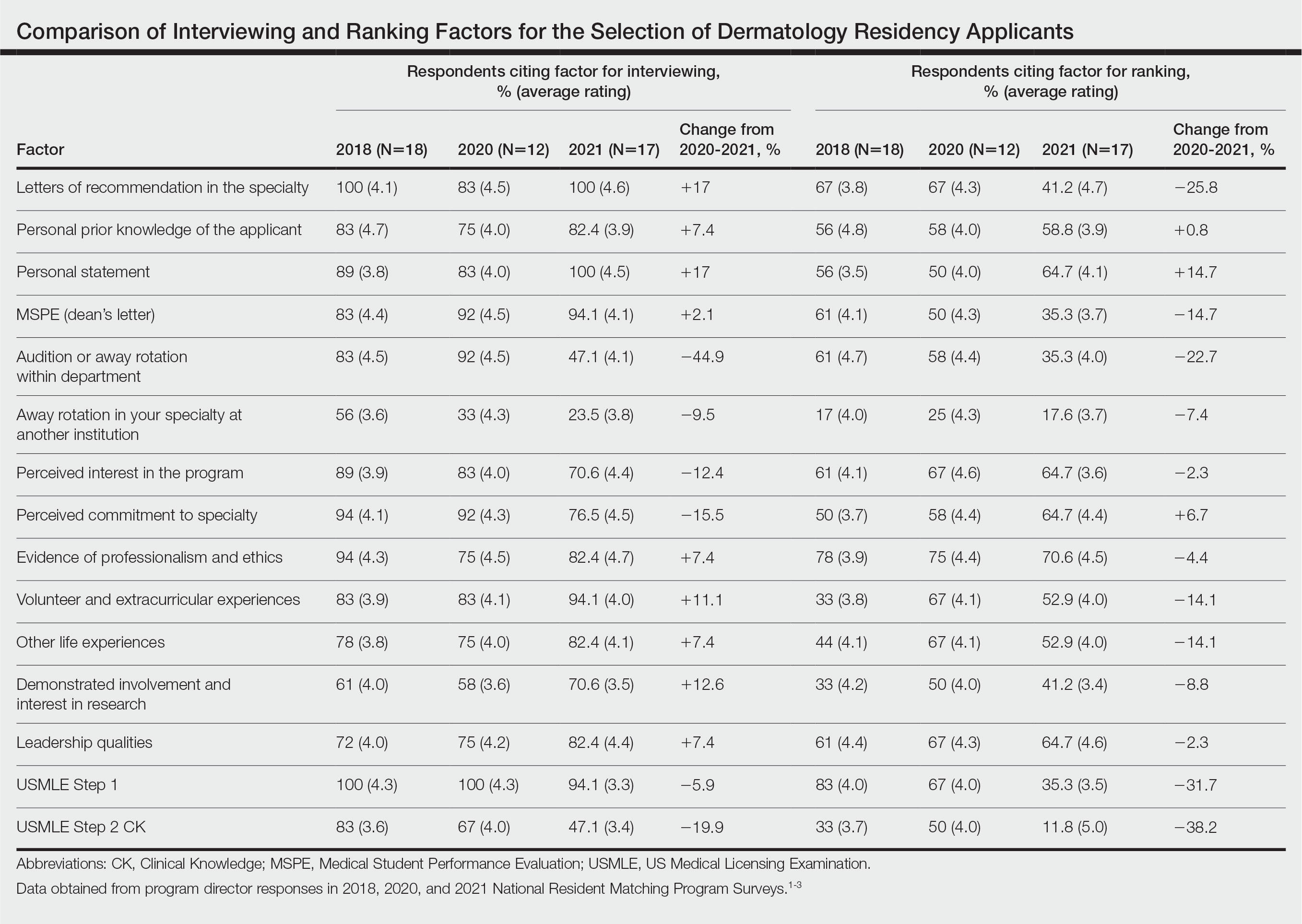
We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
PRACTICE POINTS
- Although there have been numerous changes to the dermatology interview process due to the COVID-19 pandemic, the overall fill rate for postgraduate year 2 positions remained unchanged from 2018 (prepandemic) to 2021 (postpandemic).
- Strategies to accommodate new safety recommendations for interviews may reduce the financial burden (approximately $10,000 for each senior applicant) and time constraints on applicants. These strategies should be considered for implementation in future cycles.
Spikes out: A COVID mystery
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Measles
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
ADHD beyond medications
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Atrial fibrillation: Sex differences and modifiable risk factors
This transcript has been edited for clarity.
Hello. This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital.
We looked at these questions in our vitamin D and omega-3 trial VITAL in an ancillary study called VITAL Rhythm, led by Dr. Christine Albert at Cedars-Sinai. And this particular project was led by Dr. Hasan Siddiqi at Vanderbilt.
As you know, AF is the most common arrhythmia in the world, and it’s burgeoning in numbers, primarily because of the aging of the population. It’s also a major cause of stroke, heart failure, and cardiovascular mortality. Although women are known to have lower rates of AF than men, they’re also known to have a higher risk for cardiovascular complications and sequelae, such as higher risk for stroke and CVD mortality. Therefore, we thought that understanding sex differences in risk and modifiable risk factors for AF that could reduce the burden of disease would be important.
It’s known that greater height is a risk factor for AF, but the extent to which it explains the differences in AF risk between men and women isn’t really known. So we looked at these questions in the VITAL cohort. VITAL has more than 25,000 participants. It’s a large, diverse, nationwide cohort. About 51% are women, and all are aged 50 years or older, with a mean age of 67. All were free of known clinical cardiovascular disease at the start of the study.
AF reports were confirmed by medical records and also supplemented by Medicare CMS linkage for fuller ascertainment of outcomes. We had 900 incident cases of AF in the study, and we did see that women were less likely to be diagnosed with AF. They had a 32% lower risk – strongly statistically significant compared with men, with a P < .001. Women were also more likely to be symptomatic: About 77% of women vs. 63% of men had symptoms prior to or at diagnosis.
It was very interesting that adjustment for height eliminated the lower risk for AF in women compared with men. After accounting for height, there was not only no reduction in risk for AF among the women, there was actually a reversal of the association so that there was a slightly higher risk for AF in the women. Other risk factors for AF in the cohort included older age, higher body mass index, hypertension, and higher consumption of alcohol. We did not see an association between diabetes and higher risk for AF. We also saw no clear association with physical activity, although very strenuous physical activity has been linked to AF in some other studies.
We looked at the interventions of vitamin D (2,000 IU/day) and omega-3 fatty acids (460 mg/day of EPA and 380 mg/day of DHA) and found no association with AF, although some other studies have seen increased risk for AF with higher doses of the marine omega-3s > 1 g/day and certainly at doses of 4 g/day. So overall, the findings highlight the fact that many of the risk factors for AF do seem to be modifiable, and it is really important to identify and try to reduce these risk factors in order to reduce the burden of AF. This may be particularly important in women because women are more likely to have stroke and cardiovascular mortality in these adverse cardiovascular outcomes.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital.
We looked at these questions in our vitamin D and omega-3 trial VITAL in an ancillary study called VITAL Rhythm, led by Dr. Christine Albert at Cedars-Sinai. And this particular project was led by Dr. Hasan Siddiqi at Vanderbilt.
As you know, AF is the most common arrhythmia in the world, and it’s burgeoning in numbers, primarily because of the aging of the population. It’s also a major cause of stroke, heart failure, and cardiovascular mortality. Although women are known to have lower rates of AF than men, they’re also known to have a higher risk for cardiovascular complications and sequelae, such as higher risk for stroke and CVD mortality. Therefore, we thought that understanding sex differences in risk and modifiable risk factors for AF that could reduce the burden of disease would be important.
It’s known that greater height is a risk factor for AF, but the extent to which it explains the differences in AF risk between men and women isn’t really known. So we looked at these questions in the VITAL cohort. VITAL has more than 25,000 participants. It’s a large, diverse, nationwide cohort. About 51% are women, and all are aged 50 years or older, with a mean age of 67. All were free of known clinical cardiovascular disease at the start of the study.
AF reports were confirmed by medical records and also supplemented by Medicare CMS linkage for fuller ascertainment of outcomes. We had 900 incident cases of AF in the study, and we did see that women were less likely to be diagnosed with AF. They had a 32% lower risk – strongly statistically significant compared with men, with a P < .001. Women were also more likely to be symptomatic: About 77% of women vs. 63% of men had symptoms prior to or at diagnosis.
It was very interesting that adjustment for height eliminated the lower risk for AF in women compared with men. After accounting for height, there was not only no reduction in risk for AF among the women, there was actually a reversal of the association so that there was a slightly higher risk for AF in the women. Other risk factors for AF in the cohort included older age, higher body mass index, hypertension, and higher consumption of alcohol. We did not see an association between diabetes and higher risk for AF. We also saw no clear association with physical activity, although very strenuous physical activity has been linked to AF in some other studies.
We looked at the interventions of vitamin D (2,000 IU/day) and omega-3 fatty acids (460 mg/day of EPA and 380 mg/day of DHA) and found no association with AF, although some other studies have seen increased risk for AF with higher doses of the marine omega-3s > 1 g/day and certainly at doses of 4 g/day. So overall, the findings highlight the fact that many of the risk factors for AF do seem to be modifiable, and it is really important to identify and try to reduce these risk factors in order to reduce the burden of AF. This may be particularly important in women because women are more likely to have stroke and cardiovascular mortality in these adverse cardiovascular outcomes.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital.
We looked at these questions in our vitamin D and omega-3 trial VITAL in an ancillary study called VITAL Rhythm, led by Dr. Christine Albert at Cedars-Sinai. And this particular project was led by Dr. Hasan Siddiqi at Vanderbilt.
As you know, AF is the most common arrhythmia in the world, and it’s burgeoning in numbers, primarily because of the aging of the population. It’s also a major cause of stroke, heart failure, and cardiovascular mortality. Although women are known to have lower rates of AF than men, they’re also known to have a higher risk for cardiovascular complications and sequelae, such as higher risk for stroke and CVD mortality. Therefore, we thought that understanding sex differences in risk and modifiable risk factors for AF that could reduce the burden of disease would be important.
It’s known that greater height is a risk factor for AF, but the extent to which it explains the differences in AF risk between men and women isn’t really known. So we looked at these questions in the VITAL cohort. VITAL has more than 25,000 participants. It’s a large, diverse, nationwide cohort. About 51% are women, and all are aged 50 years or older, with a mean age of 67. All were free of known clinical cardiovascular disease at the start of the study.
AF reports were confirmed by medical records and also supplemented by Medicare CMS linkage for fuller ascertainment of outcomes. We had 900 incident cases of AF in the study, and we did see that women were less likely to be diagnosed with AF. They had a 32% lower risk – strongly statistically significant compared with men, with a P < .001. Women were also more likely to be symptomatic: About 77% of women vs. 63% of men had symptoms prior to or at diagnosis.
It was very interesting that adjustment for height eliminated the lower risk for AF in women compared with men. After accounting for height, there was not only no reduction in risk for AF among the women, there was actually a reversal of the association so that there was a slightly higher risk for AF in the women. Other risk factors for AF in the cohort included older age, higher body mass index, hypertension, and higher consumption of alcohol. We did not see an association between diabetes and higher risk for AF. We also saw no clear association with physical activity, although very strenuous physical activity has been linked to AF in some other studies.
We looked at the interventions of vitamin D (2,000 IU/day) and omega-3 fatty acids (460 mg/day of EPA and 380 mg/day of DHA) and found no association with AF, although some other studies have seen increased risk for AF with higher doses of the marine omega-3s > 1 g/day and certainly at doses of 4 g/day. So overall, the findings highlight the fact that many of the risk factors for AF do seem to be modifiable, and it is really important to identify and try to reduce these risk factors in order to reduce the burden of AF. This may be particularly important in women because women are more likely to have stroke and cardiovascular mortality in these adverse cardiovascular outcomes.
A version of this article first appeared on Medscape.com.
Medical student well-being during the COVID-19 pandemic
During the initial stage of the COVID-19 pandemic U.S. medical students were suspended from in-person clinical interaction. This decision was based on specific guidance from the Association of American Medical Colleges and subsequently implemented in medical schools across the United States.1 Our research project addressed students’ stress level before and after clinical in-person suspension and assessed medical students perceived COVID-19–related risk level. We were particularly curious to learn about students’ emotional struggles as they navigated the initial pedagogical uncertainty associated with the pandemic.
It is likely that heightened stress was greater than before and the rationale was likely multifactorial in nature.2
One key stressor U.S. medical students faced was the negative impacts of COVID-19 on medical education. U.S. Medical Licensing Examination exam-taking was severely impacted, and some students needed to reschedule their test dates because of increased restrictions at testing centers. Third-year medical students in particular were worried about how COVID-19 would influence their residency application; for example, in-person residency interviews and away rotations as fourth-year medical students. Another concern was not being able to be involved in clinical work during the direst stage of this public health emergency because of personal protective equipment shortages and attempts to reduce community spread of COVID-19.
Our study also showed that students had a relatively lower perceived risk level when it comes to COVID-19 than health care workers in the 2003 SARS epidemic, which we suspect is mostly attributable to the suspension of clinical in-person interaction. We also found that female gender and self-reported mental illness diagnosis were two risk factors for perceived stress level, consistent with our current literature.
The reality of clinical in-person inaction caused by PPE shortage and limited telehealth options, together with social isolation and uncertainty regarding future education opportunities, appear to have had a detrimental effect on medical students’ psychological wellbeing. This did not have to be the case. Some medical students found innovative ways to stay involved.
For example, in 2020 some of Dr. Zhang’s classmates helped proctor virtual group therapy sessions held by the local National Alliance on Mental Illness chapter. Medical students at the Icahn School of Medicine at Mount Sinai, New York were not only able to engage in telehealth but also join other task forces, such as PPE supply, distribution, and coordination, morale promotion, and administrative services.3 Finally, many medical students in New York volunteered in providing child care for frontline doctors to help relieve their burden.4 These actions, if implemented more widely, may have had a protective effect on the stress and well-being of medical students at that time.
While our study focused on the academic side of things, the personal impacts from COVID-19 need to be acknowledged – sickness from COVID-19 and its sequelae, family loss fromCOVID-19, financial struggle, and racial targeting of Asians to name a few. COVID-19 has influenced many families’ livelihood and changed our understanding of ourselves, others, and the world in unprecedented ways.
Fast forward to today – medical students are used to learning and living in a world with an ongoing pandemic, and medical education and residency application process have adapted to this new normal. The once-crippling uncertainty surrounding COVID-19 and disastrous PPE shortages have passed. Yet, COVID-19 continues to be a stressor. In fact, burnout related to “COVID-19 fatigue” has been on the rise and one recent national survey shows one in five physicians intends to leave practice within 2 years.5
Meanwhile, uncertainty continued to persist, as in August 2022 monkeypox was declared a public health emergency in the United States.6 What Dr. Zhang learned as a medical student during the initial months of COVID-19 continues to be relevant: connect with loved ones, understand the changing reality, process the emotions, recognize what is under one’s control, have a solution-oriented mindset, and be forgiving and patient with oneself and others.
Dr. Zhang is a second-year psychiatry resident physician at Saint Elizabeth’s Hospital/DC DBH, Washington. Dr. Himelhoch serves as professor and chair of the department of psychiatry at the University of Kentucky, Lexington. His research focuses on developing and studying the efficacy of innovative strategies aimed at improving the health and welfare among people with co-occurring psychiatric and substance use disorders.
References
1. Association of American Medical Colleges. Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID-19) Outbreak. 2020 Mar 17.
2. Zhang Y et al. Psychiatry Res. 2022;313:114595. doi: 10.1016/j.psychres.2022.114595.
3. Bahethi RR et al. Acad Med. 2021 Jun 1;96(6):859-63. doi: 10.1097/ACM.0000000000003863.
4. Krieger P and Goodnough A. Medical Students, Sidelined for Now, Find New Ways to Fight Coronavirus. The New York Times. 2020 Mar 23.
5. Abbasi J. JAMA. 2022 Apr 19;327(15):1435-7. doi: 10.1001/jama.2022.5074.
6. Department of Health & Human Services. Biden-Harris Administration Bolsters Monkeypox Response; HHS Secretary Becerra Declares Public Health Emergency. 2022 Aug 4.
During the initial stage of the COVID-19 pandemic U.S. medical students were suspended from in-person clinical interaction. This decision was based on specific guidance from the Association of American Medical Colleges and subsequently implemented in medical schools across the United States.1 Our research project addressed students’ stress level before and after clinical in-person suspension and assessed medical students perceived COVID-19–related risk level. We were particularly curious to learn about students’ emotional struggles as they navigated the initial pedagogical uncertainty associated with the pandemic.
It is likely that heightened stress was greater than before and the rationale was likely multifactorial in nature.2
One key stressor U.S. medical students faced was the negative impacts of COVID-19 on medical education. U.S. Medical Licensing Examination exam-taking was severely impacted, and some students needed to reschedule their test dates because of increased restrictions at testing centers. Third-year medical students in particular were worried about how COVID-19 would influence their residency application; for example, in-person residency interviews and away rotations as fourth-year medical students. Another concern was not being able to be involved in clinical work during the direst stage of this public health emergency because of personal protective equipment shortages and attempts to reduce community spread of COVID-19.
Our study also showed that students had a relatively lower perceived risk level when it comes to COVID-19 than health care workers in the 2003 SARS epidemic, which we suspect is mostly attributable to the suspension of clinical in-person interaction. We also found that female gender and self-reported mental illness diagnosis were two risk factors for perceived stress level, consistent with our current literature.
The reality of clinical in-person inaction caused by PPE shortage and limited telehealth options, together with social isolation and uncertainty regarding future education opportunities, appear to have had a detrimental effect on medical students’ psychological wellbeing. This did not have to be the case. Some medical students found innovative ways to stay involved.
For example, in 2020 some of Dr. Zhang’s classmates helped proctor virtual group therapy sessions held by the local National Alliance on Mental Illness chapter. Medical students at the Icahn School of Medicine at Mount Sinai, New York were not only able to engage in telehealth but also join other task forces, such as PPE supply, distribution, and coordination, morale promotion, and administrative services.3 Finally, many medical students in New York volunteered in providing child care for frontline doctors to help relieve their burden.4 These actions, if implemented more widely, may have had a protective effect on the stress and well-being of medical students at that time.
While our study focused on the academic side of things, the personal impacts from COVID-19 need to be acknowledged – sickness from COVID-19 and its sequelae, family loss fromCOVID-19, financial struggle, and racial targeting of Asians to name a few. COVID-19 has influenced many families’ livelihood and changed our understanding of ourselves, others, and the world in unprecedented ways.
Fast forward to today – medical students are used to learning and living in a world with an ongoing pandemic, and medical education and residency application process have adapted to this new normal. The once-crippling uncertainty surrounding COVID-19 and disastrous PPE shortages have passed. Yet, COVID-19 continues to be a stressor. In fact, burnout related to “COVID-19 fatigue” has been on the rise and one recent national survey shows one in five physicians intends to leave practice within 2 years.5
Meanwhile, uncertainty continued to persist, as in August 2022 monkeypox was declared a public health emergency in the United States.6 What Dr. Zhang learned as a medical student during the initial months of COVID-19 continues to be relevant: connect with loved ones, understand the changing reality, process the emotions, recognize what is under one’s control, have a solution-oriented mindset, and be forgiving and patient with oneself and others.
Dr. Zhang is a second-year psychiatry resident physician at Saint Elizabeth’s Hospital/DC DBH, Washington. Dr. Himelhoch serves as professor and chair of the department of psychiatry at the University of Kentucky, Lexington. His research focuses on developing and studying the efficacy of innovative strategies aimed at improving the health and welfare among people with co-occurring psychiatric and substance use disorders.
References
1. Association of American Medical Colleges. Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID-19) Outbreak. 2020 Mar 17.
2. Zhang Y et al. Psychiatry Res. 2022;313:114595. doi: 10.1016/j.psychres.2022.114595.
3. Bahethi RR et al. Acad Med. 2021 Jun 1;96(6):859-63. doi: 10.1097/ACM.0000000000003863.
4. Krieger P and Goodnough A. Medical Students, Sidelined for Now, Find New Ways to Fight Coronavirus. The New York Times. 2020 Mar 23.
5. Abbasi J. JAMA. 2022 Apr 19;327(15):1435-7. doi: 10.1001/jama.2022.5074.
6. Department of Health & Human Services. Biden-Harris Administration Bolsters Monkeypox Response; HHS Secretary Becerra Declares Public Health Emergency. 2022 Aug 4.
During the initial stage of the COVID-19 pandemic U.S. medical students were suspended from in-person clinical interaction. This decision was based on specific guidance from the Association of American Medical Colleges and subsequently implemented in medical schools across the United States.1 Our research project addressed students’ stress level before and after clinical in-person suspension and assessed medical students perceived COVID-19–related risk level. We were particularly curious to learn about students’ emotional struggles as they navigated the initial pedagogical uncertainty associated with the pandemic.
It is likely that heightened stress was greater than before and the rationale was likely multifactorial in nature.2
One key stressor U.S. medical students faced was the negative impacts of COVID-19 on medical education. U.S. Medical Licensing Examination exam-taking was severely impacted, and some students needed to reschedule their test dates because of increased restrictions at testing centers. Third-year medical students in particular were worried about how COVID-19 would influence their residency application; for example, in-person residency interviews and away rotations as fourth-year medical students. Another concern was not being able to be involved in clinical work during the direst stage of this public health emergency because of personal protective equipment shortages and attempts to reduce community spread of COVID-19.
Our study also showed that students had a relatively lower perceived risk level when it comes to COVID-19 than health care workers in the 2003 SARS epidemic, which we suspect is mostly attributable to the suspension of clinical in-person interaction. We also found that female gender and self-reported mental illness diagnosis were two risk factors for perceived stress level, consistent with our current literature.
The reality of clinical in-person inaction caused by PPE shortage and limited telehealth options, together with social isolation and uncertainty regarding future education opportunities, appear to have had a detrimental effect on medical students’ psychological wellbeing. This did not have to be the case. Some medical students found innovative ways to stay involved.
For example, in 2020 some of Dr. Zhang’s classmates helped proctor virtual group therapy sessions held by the local National Alliance on Mental Illness chapter. Medical students at the Icahn School of Medicine at Mount Sinai, New York were not only able to engage in telehealth but also join other task forces, such as PPE supply, distribution, and coordination, morale promotion, and administrative services.3 Finally, many medical students in New York volunteered in providing child care for frontline doctors to help relieve their burden.4 These actions, if implemented more widely, may have had a protective effect on the stress and well-being of medical students at that time.
While our study focused on the academic side of things, the personal impacts from COVID-19 need to be acknowledged – sickness from COVID-19 and its sequelae, family loss fromCOVID-19, financial struggle, and racial targeting of Asians to name a few. COVID-19 has influenced many families’ livelihood and changed our understanding of ourselves, others, and the world in unprecedented ways.
Fast forward to today – medical students are used to learning and living in a world with an ongoing pandemic, and medical education and residency application process have adapted to this new normal. The once-crippling uncertainty surrounding COVID-19 and disastrous PPE shortages have passed. Yet, COVID-19 continues to be a stressor. In fact, burnout related to “COVID-19 fatigue” has been on the rise and one recent national survey shows one in five physicians intends to leave practice within 2 years.5
Meanwhile, uncertainty continued to persist, as in August 2022 monkeypox was declared a public health emergency in the United States.6 What Dr. Zhang learned as a medical student during the initial months of COVID-19 continues to be relevant: connect with loved ones, understand the changing reality, process the emotions, recognize what is under one’s control, have a solution-oriented mindset, and be forgiving and patient with oneself and others.
Dr. Zhang is a second-year psychiatry resident physician at Saint Elizabeth’s Hospital/DC DBH, Washington. Dr. Himelhoch serves as professor and chair of the department of psychiatry at the University of Kentucky, Lexington. His research focuses on developing and studying the efficacy of innovative strategies aimed at improving the health and welfare among people with co-occurring psychiatric and substance use disorders.
References
1. Association of American Medical Colleges. Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID-19) Outbreak. 2020 Mar 17.
2. Zhang Y et al. Psychiatry Res. 2022;313:114595. doi: 10.1016/j.psychres.2022.114595.
3. Bahethi RR et al. Acad Med. 2021 Jun 1;96(6):859-63. doi: 10.1097/ACM.0000000000003863.
4. Krieger P and Goodnough A. Medical Students, Sidelined for Now, Find New Ways to Fight Coronavirus. The New York Times. 2020 Mar 23.
5. Abbasi J. JAMA. 2022 Apr 19;327(15):1435-7. doi: 10.1001/jama.2022.5074.
6. Department of Health & Human Services. Biden-Harris Administration Bolsters Monkeypox Response; HHS Secretary Becerra Declares Public Health Emergency. 2022 Aug 4.
Pediatric vaccination rates have failed to recover
I guess we shouldn’t be surprised that vaccination rates in this country fell during the frenzy created by the COVID pandemic. We had a lot on our plates. Schools closed and many of us retreated into what seemed to be the safety of our homes. Parents were reluctant to take their children anywhere, let alone a pediatrician’s office. State health agencies wisely focused on collecting case figures and then shepherding the efforts to immunize against SARS-CoV-2 once vaccines were available. Tracking and promoting the existing children’s vaccinations fell off the priority list, even in places with exemplary vaccination rates.
Whether or not the pandemic is over continues to be a topic for debate, but there is clearly a general shift toward a new normalcy. However, vaccination rates of our children have not rebounded to prepandemic levels. In fact, in some areas they are continuing to fall.
In a recent guest essay in the New York Times, Ezekiel J. Emmanuel, MD, PhD, a physician and professor of medical ethics and health policy at the University of Pennsylvania, and Matthew Guido, his research assistant, explore the reasons for this lack of a significant rebound. The authors cite recent outbreaks of measles in Ohio and polio in New York City as examples of the peril we are facing if we fail to reverse the trend. In some areas measles vaccine rates alarmingly have dipped below the threshold for herd immunity.
While Dr. Emmanuel and Mr. Guido acknowledge that the pandemic was a major driver of the falling vaccination rates they lay blame on the persistent decline on three factors that they view as correctable: nonmedical exemptions, our failure to vigorously enforce existing vaccine requirements, and inadequate public health campaigns.
The authors underestimate the lingering effect of the pandemic on parents’ vaccine hesitancy. As a septuagenarian who often hangs out with other septuagenarians I view the rapid development and effectiveness of the COVID vaccine as astounding and a boost for vaccines in general. However, were I much younger I might treat the vaccine’s success with a shrug. After some initial concern, the younger half of the population didn’t seem to see the illness as much of a threat to themselves or their peers. This attitude was reinforced by the fact that few of their peers, including those who were unvaccinated, were getting seriously ill. Despite all the hype, most parents and their children never ended up getting seriously ill.
You can understand why many parents might be quick to toss what you and I consider a successful COVID vaccine onto what they view as a growing pile of vaccines for diseases that in their experience have never sickened or killed anyone they have known.
Let’s be honest: Over the last half century we have produced several generations of parents who have little knowledge and certainly no personal experience with a childhood disease on the order or magnitude of polio. The vaccines that we have developed during their lifetimes have been targeted at diseases such as haemophilus influenzae meningitis that, while serious and anxiety provoking for pediatricians, occur so sporadically that most parents have no personal experience to motivate them to vaccinate their children.
Dr. Emmanuel and Mr. Guido are correct in advocating for the broader elimination of nonmedical exemptions and urging us to find the political will to vigorously enforce the vaccine requirements we have already enacted. I agree that our promotional campaigns need to be more robust. But, this will be a difficult challenge unless we can impress our audience with our straight talk and honesty. We must acknowledge and then explain why all vaccines are not created equal and that some are of more critical importance than others.
We are slowly learning that education isn’t the cure-all for vaccine hesitancy we once thought it was. And using scare tactics can backfire and create dysfunctional anxiety. We must choosing our words and target audience carefully. And ... having the political will to force parents into doing the right thing will be critical if we wish to restore our vaccination rates.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I guess we shouldn’t be surprised that vaccination rates in this country fell during the frenzy created by the COVID pandemic. We had a lot on our plates. Schools closed and many of us retreated into what seemed to be the safety of our homes. Parents were reluctant to take their children anywhere, let alone a pediatrician’s office. State health agencies wisely focused on collecting case figures and then shepherding the efforts to immunize against SARS-CoV-2 once vaccines were available. Tracking and promoting the existing children’s vaccinations fell off the priority list, even in places with exemplary vaccination rates.
Whether or not the pandemic is over continues to be a topic for debate, but there is clearly a general shift toward a new normalcy. However, vaccination rates of our children have not rebounded to prepandemic levels. In fact, in some areas they are continuing to fall.
In a recent guest essay in the New York Times, Ezekiel J. Emmanuel, MD, PhD, a physician and professor of medical ethics and health policy at the University of Pennsylvania, and Matthew Guido, his research assistant, explore the reasons for this lack of a significant rebound. The authors cite recent outbreaks of measles in Ohio and polio in New York City as examples of the peril we are facing if we fail to reverse the trend. In some areas measles vaccine rates alarmingly have dipped below the threshold for herd immunity.
While Dr. Emmanuel and Mr. Guido acknowledge that the pandemic was a major driver of the falling vaccination rates they lay blame on the persistent decline on three factors that they view as correctable: nonmedical exemptions, our failure to vigorously enforce existing vaccine requirements, and inadequate public health campaigns.
The authors underestimate the lingering effect of the pandemic on parents’ vaccine hesitancy. As a septuagenarian who often hangs out with other septuagenarians I view the rapid development and effectiveness of the COVID vaccine as astounding and a boost for vaccines in general. However, were I much younger I might treat the vaccine’s success with a shrug. After some initial concern, the younger half of the population didn’t seem to see the illness as much of a threat to themselves or their peers. This attitude was reinforced by the fact that few of their peers, including those who were unvaccinated, were getting seriously ill. Despite all the hype, most parents and their children never ended up getting seriously ill.
You can understand why many parents might be quick to toss what you and I consider a successful COVID vaccine onto what they view as a growing pile of vaccines for diseases that in their experience have never sickened or killed anyone they have known.
Let’s be honest: Over the last half century we have produced several generations of parents who have little knowledge and certainly no personal experience with a childhood disease on the order or magnitude of polio. The vaccines that we have developed during their lifetimes have been targeted at diseases such as haemophilus influenzae meningitis that, while serious and anxiety provoking for pediatricians, occur so sporadically that most parents have no personal experience to motivate them to vaccinate their children.
Dr. Emmanuel and Mr. Guido are correct in advocating for the broader elimination of nonmedical exemptions and urging us to find the political will to vigorously enforce the vaccine requirements we have already enacted. I agree that our promotional campaigns need to be more robust. But, this will be a difficult challenge unless we can impress our audience with our straight talk and honesty. We must acknowledge and then explain why all vaccines are not created equal and that some are of more critical importance than others.
We are slowly learning that education isn’t the cure-all for vaccine hesitancy we once thought it was. And using scare tactics can backfire and create dysfunctional anxiety. We must choosing our words and target audience carefully. And ... having the political will to force parents into doing the right thing will be critical if we wish to restore our vaccination rates.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I guess we shouldn’t be surprised that vaccination rates in this country fell during the frenzy created by the COVID pandemic. We had a lot on our plates. Schools closed and many of us retreated into what seemed to be the safety of our homes. Parents were reluctant to take their children anywhere, let alone a pediatrician’s office. State health agencies wisely focused on collecting case figures and then shepherding the efforts to immunize against SARS-CoV-2 once vaccines were available. Tracking and promoting the existing children’s vaccinations fell off the priority list, even in places with exemplary vaccination rates.
Whether or not the pandemic is over continues to be a topic for debate, but there is clearly a general shift toward a new normalcy. However, vaccination rates of our children have not rebounded to prepandemic levels. In fact, in some areas they are continuing to fall.
In a recent guest essay in the New York Times, Ezekiel J. Emmanuel, MD, PhD, a physician and professor of medical ethics and health policy at the University of Pennsylvania, and Matthew Guido, his research assistant, explore the reasons for this lack of a significant rebound. The authors cite recent outbreaks of measles in Ohio and polio in New York City as examples of the peril we are facing if we fail to reverse the trend. In some areas measles vaccine rates alarmingly have dipped below the threshold for herd immunity.
While Dr. Emmanuel and Mr. Guido acknowledge that the pandemic was a major driver of the falling vaccination rates they lay blame on the persistent decline on three factors that they view as correctable: nonmedical exemptions, our failure to vigorously enforce existing vaccine requirements, and inadequate public health campaigns.
The authors underestimate the lingering effect of the pandemic on parents’ vaccine hesitancy. As a septuagenarian who often hangs out with other septuagenarians I view the rapid development and effectiveness of the COVID vaccine as astounding and a boost for vaccines in general. However, were I much younger I might treat the vaccine’s success with a shrug. After some initial concern, the younger half of the population didn’t seem to see the illness as much of a threat to themselves or their peers. This attitude was reinforced by the fact that few of their peers, including those who were unvaccinated, were getting seriously ill. Despite all the hype, most parents and their children never ended up getting seriously ill.
You can understand why many parents might be quick to toss what you and I consider a successful COVID vaccine onto what they view as a growing pile of vaccines for diseases that in their experience have never sickened or killed anyone they have known.
Let’s be honest: Over the last half century we have produced several generations of parents who have little knowledge and certainly no personal experience with a childhood disease on the order or magnitude of polio. The vaccines that we have developed during their lifetimes have been targeted at diseases such as haemophilus influenzae meningitis that, while serious and anxiety provoking for pediatricians, occur so sporadically that most parents have no personal experience to motivate them to vaccinate their children.
Dr. Emmanuel and Mr. Guido are correct in advocating for the broader elimination of nonmedical exemptions and urging us to find the political will to vigorously enforce the vaccine requirements we have already enacted. I agree that our promotional campaigns need to be more robust. But, this will be a difficult challenge unless we can impress our audience with our straight talk and honesty. We must acknowledge and then explain why all vaccines are not created equal and that some are of more critical importance than others.
We are slowly learning that education isn’t the cure-all for vaccine hesitancy we once thought it was. And using scare tactics can backfire and create dysfunctional anxiety. We must choosing our words and target audience carefully. And ... having the political will to force parents into doing the right thing will be critical if we wish to restore our vaccination rates.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Five thoughts on the Damar Hamlin collapse
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.











