User login
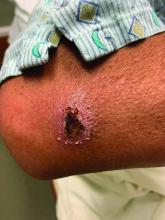
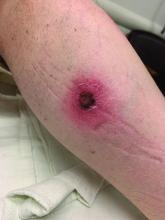
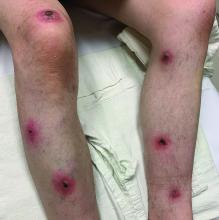
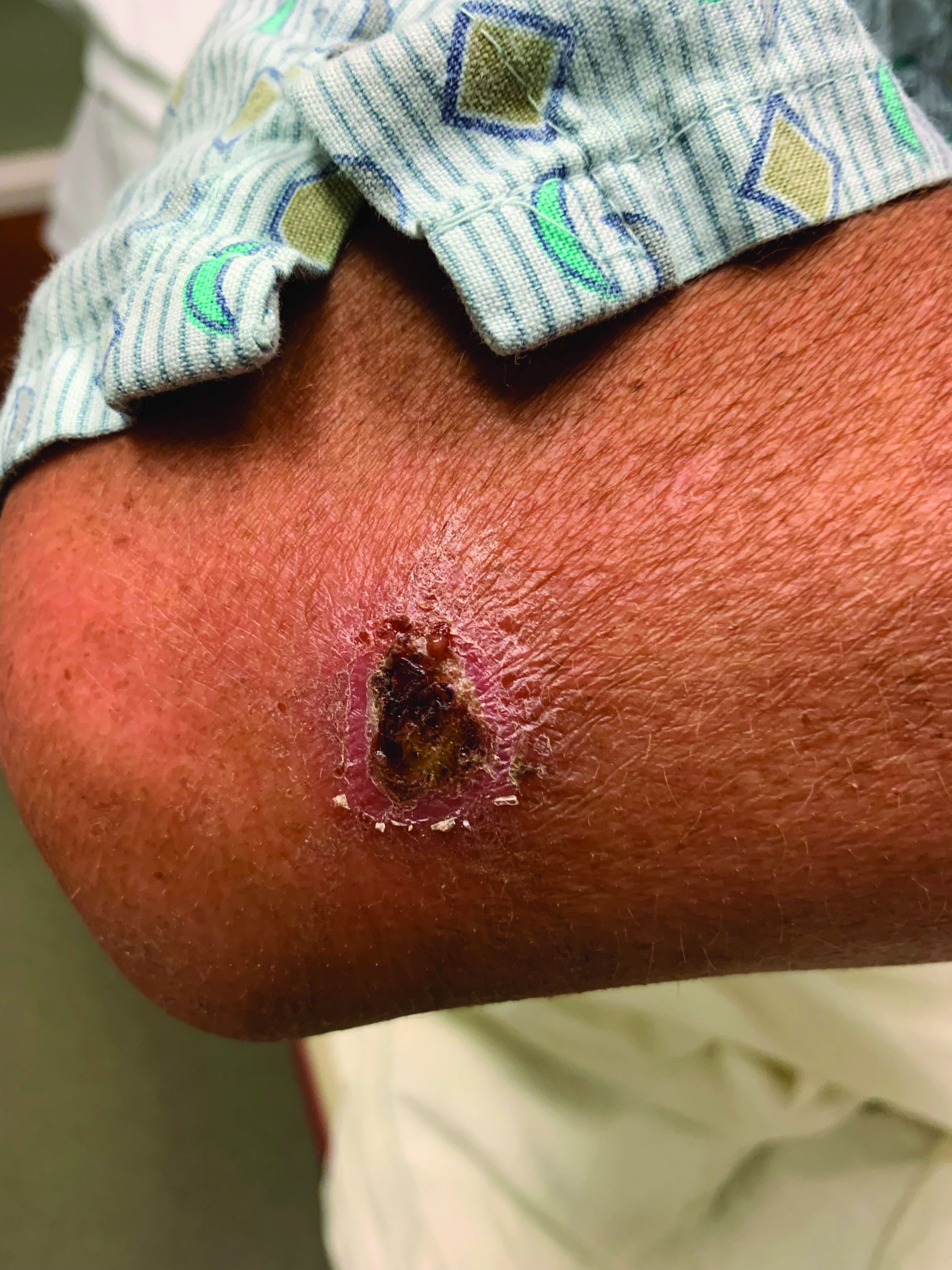
A White male presented with a 1-month history of recurrent, widespread, painful sores
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Natural fertility: When less can be more
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
EHR: A progress report
I wrote my first column on electronic health records in the mid-1990s. At the time, it seemed like an idea whose time had come. After all, in an era when just about every essential process in medicine had already been computerized, we physicians continued to process clinical data – our key asset – with pen and paper. Most of us were reluctant to make the switch, and for good reason:
Then, the government stepped in. Shortly after his inauguration in 2000, President George W. Bush outlined a plan to ensure that most Americans had electronic health records within 10 years. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” The goal was to eliminate missing charts, duplication of lab testing, ineffective documentation, and inordinate amounts of time spent on paperwork, not to mention illegible handwriting, poor coordination of care between physicians, and many other problems. Studies were quoted, suggesting that EHR shortened inpatient stays, decreased risk of adverse drug interactions, improved the consistency and content of records, and improved continuity of care and follow-up.
The EHR Incentive Program (later renamed the Promoting Interoperability Program) was introduced to encourage physicians and hospitals “to adopt, implement, upgrade, and demonstrate meaningful use of certified electronic health record technology.”
Nearly a quarter-century later, implementation is well behind schedule. According to a 2019 federal study, while nearly all hospitals (96%) have adopted a certified EHR, only 72% of office-based physicians have done so.
There are multiple reasons for this. For one thing, EHR is still by and large slower than pen and paper, because direct data entry is still primarily done by keyboard. Voice recognition, hand-held and wireless devices have been developed, but most work only on specialized tasks. Even the best systems take more clinician time per encounter than the manual processes they replace.
Physicians have been slow to warm to a system that slows them down and forces them to change the way they think and work. In addition, paper systems never crash; the prospect of a server malfunction or Internet failure bringing an entire clinic to a grinding halt is not particularly inviting.
The special needs of dermatology – high patient volumes, multiple diagnoses and prescriptions per patient, the wide variety of procedures we perform, and digital image storage – present further hurdles.
Nevertheless, the march toward electronic record keeping continues, and I continue to receive many questions about choosing a good EHR system. As always, I cannot recommend any specific products since every office has unique needs and requirements.
The key phrase to keep in mind is caveat emptor. Several regulatory bodies exist to test vendor claims and certify system behaviors, but different agencies use different criteria that may or may not be relevant to your requirements. Vaporware is still as common as real software; beware the “feature in the next release” that might never appear, particularly if you need it right now.
Avoid the temptation to buy a flashy new system and then try to adapt it to your office; figure out your needs first, then find a system that meets them.
Unfortunately, there is no easy way around doing the work of comparing one system with another. The most important information a vendor can give you is the names and addresses of two or more offices where you can go watch their system in action. Site visits are time-consuming, but they are only way to pick the best EHR the first time around.
Don’t be the first office using a new system. Let the vendor work out the bugs somewhere else.
Above all, if you have disorganized paper records, don’t count on EHR to automatically solve your problems. Well-designed paper systems usually lend themselves to effective automation, but automating a poorly designed system just increases the chaos. If your paper system is in disarray, solve that problem before considering EHR.
With all of its problems and hurdles, EHRs will inevitably be a part of most of our lives. And for those who take the time to do it right, it will ultimately be an improvement.
Think of information technologies as power tools: They can help you to do things better, but they can also amplify your errors. So choose carefully.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I wrote my first column on electronic health records in the mid-1990s. At the time, it seemed like an idea whose time had come. After all, in an era when just about every essential process in medicine had already been computerized, we physicians continued to process clinical data – our key asset – with pen and paper. Most of us were reluctant to make the switch, and for good reason:
Then, the government stepped in. Shortly after his inauguration in 2000, President George W. Bush outlined a plan to ensure that most Americans had electronic health records within 10 years. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” The goal was to eliminate missing charts, duplication of lab testing, ineffective documentation, and inordinate amounts of time spent on paperwork, not to mention illegible handwriting, poor coordination of care between physicians, and many other problems. Studies were quoted, suggesting that EHR shortened inpatient stays, decreased risk of adverse drug interactions, improved the consistency and content of records, and improved continuity of care and follow-up.
The EHR Incentive Program (later renamed the Promoting Interoperability Program) was introduced to encourage physicians and hospitals “to adopt, implement, upgrade, and demonstrate meaningful use of certified electronic health record technology.”
Nearly a quarter-century later, implementation is well behind schedule. According to a 2019 federal study, while nearly all hospitals (96%) have adopted a certified EHR, only 72% of office-based physicians have done so.
There are multiple reasons for this. For one thing, EHR is still by and large slower than pen and paper, because direct data entry is still primarily done by keyboard. Voice recognition, hand-held and wireless devices have been developed, but most work only on specialized tasks. Even the best systems take more clinician time per encounter than the manual processes they replace.
Physicians have been slow to warm to a system that slows them down and forces them to change the way they think and work. In addition, paper systems never crash; the prospect of a server malfunction or Internet failure bringing an entire clinic to a grinding halt is not particularly inviting.
The special needs of dermatology – high patient volumes, multiple diagnoses and prescriptions per patient, the wide variety of procedures we perform, and digital image storage – present further hurdles.
Nevertheless, the march toward electronic record keeping continues, and I continue to receive many questions about choosing a good EHR system. As always, I cannot recommend any specific products since every office has unique needs and requirements.
The key phrase to keep in mind is caveat emptor. Several regulatory bodies exist to test vendor claims and certify system behaviors, but different agencies use different criteria that may or may not be relevant to your requirements. Vaporware is still as common as real software; beware the “feature in the next release” that might never appear, particularly if you need it right now.
Avoid the temptation to buy a flashy new system and then try to adapt it to your office; figure out your needs first, then find a system that meets them.
Unfortunately, there is no easy way around doing the work of comparing one system with another. The most important information a vendor can give you is the names and addresses of two or more offices where you can go watch their system in action. Site visits are time-consuming, but they are only way to pick the best EHR the first time around.
Don’t be the first office using a new system. Let the vendor work out the bugs somewhere else.
Above all, if you have disorganized paper records, don’t count on EHR to automatically solve your problems. Well-designed paper systems usually lend themselves to effective automation, but automating a poorly designed system just increases the chaos. If your paper system is in disarray, solve that problem before considering EHR.
With all of its problems and hurdles, EHRs will inevitably be a part of most of our lives. And for those who take the time to do it right, it will ultimately be an improvement.
Think of information technologies as power tools: They can help you to do things better, but they can also amplify your errors. So choose carefully.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I wrote my first column on electronic health records in the mid-1990s. At the time, it seemed like an idea whose time had come. After all, in an era when just about every essential process in medicine had already been computerized, we physicians continued to process clinical data – our key asset – with pen and paper. Most of us were reluctant to make the switch, and for good reason:
Then, the government stepped in. Shortly after his inauguration in 2000, President George W. Bush outlined a plan to ensure that most Americans had electronic health records within 10 years. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” The goal was to eliminate missing charts, duplication of lab testing, ineffective documentation, and inordinate amounts of time spent on paperwork, not to mention illegible handwriting, poor coordination of care between physicians, and many other problems. Studies were quoted, suggesting that EHR shortened inpatient stays, decreased risk of adverse drug interactions, improved the consistency and content of records, and improved continuity of care and follow-up.
The EHR Incentive Program (later renamed the Promoting Interoperability Program) was introduced to encourage physicians and hospitals “to adopt, implement, upgrade, and demonstrate meaningful use of certified electronic health record technology.”
Nearly a quarter-century later, implementation is well behind schedule. According to a 2019 federal study, while nearly all hospitals (96%) have adopted a certified EHR, only 72% of office-based physicians have done so.
There are multiple reasons for this. For one thing, EHR is still by and large slower than pen and paper, because direct data entry is still primarily done by keyboard. Voice recognition, hand-held and wireless devices have been developed, but most work only on specialized tasks. Even the best systems take more clinician time per encounter than the manual processes they replace.
Physicians have been slow to warm to a system that slows them down and forces them to change the way they think and work. In addition, paper systems never crash; the prospect of a server malfunction or Internet failure bringing an entire clinic to a grinding halt is not particularly inviting.
The special needs of dermatology – high patient volumes, multiple diagnoses and prescriptions per patient, the wide variety of procedures we perform, and digital image storage – present further hurdles.
Nevertheless, the march toward electronic record keeping continues, and I continue to receive many questions about choosing a good EHR system. As always, I cannot recommend any specific products since every office has unique needs and requirements.
The key phrase to keep in mind is caveat emptor. Several regulatory bodies exist to test vendor claims and certify system behaviors, but different agencies use different criteria that may or may not be relevant to your requirements. Vaporware is still as common as real software; beware the “feature in the next release” that might never appear, particularly if you need it right now.
Avoid the temptation to buy a flashy new system and then try to adapt it to your office; figure out your needs first, then find a system that meets them.
Unfortunately, there is no easy way around doing the work of comparing one system with another. The most important information a vendor can give you is the names and addresses of two or more offices where you can go watch their system in action. Site visits are time-consuming, but they are only way to pick the best EHR the first time around.
Don’t be the first office using a new system. Let the vendor work out the bugs somewhere else.
Above all, if you have disorganized paper records, don’t count on EHR to automatically solve your problems. Well-designed paper systems usually lend themselves to effective automation, but automating a poorly designed system just increases the chaos. If your paper system is in disarray, solve that problem before considering EHR.
With all of its problems and hurdles, EHRs will inevitably be a part of most of our lives. And for those who take the time to do it right, it will ultimately be an improvement.
Think of information technologies as power tools: They can help you to do things better, but they can also amplify your errors. So choose carefully.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Quiet quitting: Are physicians dying inside bit by bit? Or setting healthy boundaries?
In the past few months, “quiet quitting” has garnered increasing traction across social media platforms. My morning review of social media revealed thousands of posts ranging from “Why doing less at work could be good for you – and your employer” to “After ‘quiet quitting’ here comes ‘quiet firing.’ ”
But quiet quitting is neither quiet nor quitting.
Quiet quitting is a misnomer. In addition, quiet quitters are firmer with their boundaries, do not take on work above and beyond clearly stated expectations, do not respond after hours, and do not feel like they are “not doing their job” when they are not immediately available.
Individuals who “quiet quit” continue to meet the demands of their job but reject the hustle-culture mentality that you must always be available for more work and, most importantly, that your value as person and self-worth are defined and determined by your work. Quiet quitters believe that it is possible to have good boundaries and yet remain productive, engaged, and active within the workplace.
Earlier this month, NPR’s posted tutorial on how to set better boundaries at work garnered 491,000 views, reflecting employees’ difficulties in communicating their needs, thoughts, and availability to their employers. Quiet quitting refers to not only rejecting the idea of going above and beyond in the workplace but also feeling confident that there will not be negative ramifications for not consistently working beyond the expected requirements.
A focus on balance, life, loves, and family is rarely addressed or emphasized by traditional employers; employees have little skill in addressing boundaries and clarifying their value and availability. For decades, “needing” flexibility of any kind or valuing activities as much as your job were viewed as negative attributes, making those individuals less-desired employees.
Data support the quiet quitting trend. Gallup data reveal that employee engagement has fallen for 2 consecutive years in the U.S. workforce. Across the first quarter of 2022, Generation Z and younger Millennials report the lowest engagement across populations at 31%. More than half of this cohort, 54%, classified as “not engaged” in their workplace.
Why is quiet quitting gaining prominence now? COVID may play a role.
Many suggest that self-evaluation and establishing firmer boundaries is a logical response to emotional sequelae caused by COVID. Quiet quitting appears to have been fueled by the pandemic. Employees were forced into crisis mode by COVID; the lines between work, life, and home evaporated, allowing or forcing workers to evaluate their efficacy and satisfaction. With the structural impact of COVID reducing and a return to more standard work practices, it is expected that the job “rules” once held as truths come under evaluation and scrutiny.
Perhaps COVID has forced, and provided, another opportunity for us to closely examine our routines and habits and take stock of what really matters. Generations expectedly differ in their values and definitions of success. COVID has set prior established rules on fire, by forcing patterns and expectations that were neither expected nor wanted, within the context of a global health crisis. Within this backdrop, should we really believe our worth is determined by our job?
The truth is, we are still grieving what we lost during COVID and we have expectedly not assimilated to “the new normal.” Psychology has long recognized that losing structures and supports, routines and habits, causes symptoms of significant discomfort.
The idea that we would return to prior workplace expectations is naive. The idea we would “return to life as it was” is naive. It seems expected, then, that both employers and employees should evaluate their goals and communicate more openly about how each can be met.
It is incumbent upon the employers to set up clear guidelines regarding expectations, including rewards for performance and expectations for time, both within and outside of the work schedule. Employers must recognize symptoms of detachment in their employees and engage in the process of continuing clarifying roles and expectations while providing necessities for employees to succeed at their highest level. Employees, in turn, must self-examine their goals, communicate their needs, meet their responsibilities fully, and take on the challenge of determining their own definition of balance.
Maybe instead of quiet quitting, we should call it this new movement “self-awareness, growth, and evolution.” Hmmm, there’s an intriguing thought.
Dr. Calvery is professor of pediatrics at the University of Louisville (Ky.) She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the past few months, “quiet quitting” has garnered increasing traction across social media platforms. My morning review of social media revealed thousands of posts ranging from “Why doing less at work could be good for you – and your employer” to “After ‘quiet quitting’ here comes ‘quiet firing.’ ”
But quiet quitting is neither quiet nor quitting.
Quiet quitting is a misnomer. In addition, quiet quitters are firmer with their boundaries, do not take on work above and beyond clearly stated expectations, do not respond after hours, and do not feel like they are “not doing their job” when they are not immediately available.
Individuals who “quiet quit” continue to meet the demands of their job but reject the hustle-culture mentality that you must always be available for more work and, most importantly, that your value as person and self-worth are defined and determined by your work. Quiet quitters believe that it is possible to have good boundaries and yet remain productive, engaged, and active within the workplace.
Earlier this month, NPR’s posted tutorial on how to set better boundaries at work garnered 491,000 views, reflecting employees’ difficulties in communicating their needs, thoughts, and availability to their employers. Quiet quitting refers to not only rejecting the idea of going above and beyond in the workplace but also feeling confident that there will not be negative ramifications for not consistently working beyond the expected requirements.
A focus on balance, life, loves, and family is rarely addressed or emphasized by traditional employers; employees have little skill in addressing boundaries and clarifying their value and availability. For decades, “needing” flexibility of any kind or valuing activities as much as your job were viewed as negative attributes, making those individuals less-desired employees.
Data support the quiet quitting trend. Gallup data reveal that employee engagement has fallen for 2 consecutive years in the U.S. workforce. Across the first quarter of 2022, Generation Z and younger Millennials report the lowest engagement across populations at 31%. More than half of this cohort, 54%, classified as “not engaged” in their workplace.
Why is quiet quitting gaining prominence now? COVID may play a role.
Many suggest that self-evaluation and establishing firmer boundaries is a logical response to emotional sequelae caused by COVID. Quiet quitting appears to have been fueled by the pandemic. Employees were forced into crisis mode by COVID; the lines between work, life, and home evaporated, allowing or forcing workers to evaluate their efficacy and satisfaction. With the structural impact of COVID reducing and a return to more standard work practices, it is expected that the job “rules” once held as truths come under evaluation and scrutiny.
Perhaps COVID has forced, and provided, another opportunity for us to closely examine our routines and habits and take stock of what really matters. Generations expectedly differ in their values and definitions of success. COVID has set prior established rules on fire, by forcing patterns and expectations that were neither expected nor wanted, within the context of a global health crisis. Within this backdrop, should we really believe our worth is determined by our job?
The truth is, we are still grieving what we lost during COVID and we have expectedly not assimilated to “the new normal.” Psychology has long recognized that losing structures and supports, routines and habits, causes symptoms of significant discomfort.
The idea that we would return to prior workplace expectations is naive. The idea we would “return to life as it was” is naive. It seems expected, then, that both employers and employees should evaluate their goals and communicate more openly about how each can be met.
It is incumbent upon the employers to set up clear guidelines regarding expectations, including rewards for performance and expectations for time, both within and outside of the work schedule. Employers must recognize symptoms of detachment in their employees and engage in the process of continuing clarifying roles and expectations while providing necessities for employees to succeed at their highest level. Employees, in turn, must self-examine their goals, communicate their needs, meet their responsibilities fully, and take on the challenge of determining their own definition of balance.
Maybe instead of quiet quitting, we should call it this new movement “self-awareness, growth, and evolution.” Hmmm, there’s an intriguing thought.
Dr. Calvery is professor of pediatrics at the University of Louisville (Ky.) She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the past few months, “quiet quitting” has garnered increasing traction across social media platforms. My morning review of social media revealed thousands of posts ranging from “Why doing less at work could be good for you – and your employer” to “After ‘quiet quitting’ here comes ‘quiet firing.’ ”
But quiet quitting is neither quiet nor quitting.
Quiet quitting is a misnomer. In addition, quiet quitters are firmer with their boundaries, do not take on work above and beyond clearly stated expectations, do not respond after hours, and do not feel like they are “not doing their job” when they are not immediately available.
Individuals who “quiet quit” continue to meet the demands of their job but reject the hustle-culture mentality that you must always be available for more work and, most importantly, that your value as person and self-worth are defined and determined by your work. Quiet quitters believe that it is possible to have good boundaries and yet remain productive, engaged, and active within the workplace.
Earlier this month, NPR’s posted tutorial on how to set better boundaries at work garnered 491,000 views, reflecting employees’ difficulties in communicating their needs, thoughts, and availability to their employers. Quiet quitting refers to not only rejecting the idea of going above and beyond in the workplace but also feeling confident that there will not be negative ramifications for not consistently working beyond the expected requirements.
A focus on balance, life, loves, and family is rarely addressed or emphasized by traditional employers; employees have little skill in addressing boundaries and clarifying their value and availability. For decades, “needing” flexibility of any kind or valuing activities as much as your job were viewed as negative attributes, making those individuals less-desired employees.
Data support the quiet quitting trend. Gallup data reveal that employee engagement has fallen for 2 consecutive years in the U.S. workforce. Across the first quarter of 2022, Generation Z and younger Millennials report the lowest engagement across populations at 31%. More than half of this cohort, 54%, classified as “not engaged” in their workplace.
Why is quiet quitting gaining prominence now? COVID may play a role.
Many suggest that self-evaluation and establishing firmer boundaries is a logical response to emotional sequelae caused by COVID. Quiet quitting appears to have been fueled by the pandemic. Employees were forced into crisis mode by COVID; the lines between work, life, and home evaporated, allowing or forcing workers to evaluate their efficacy and satisfaction. With the structural impact of COVID reducing and a return to more standard work practices, it is expected that the job “rules” once held as truths come under evaluation and scrutiny.
Perhaps COVID has forced, and provided, another opportunity for us to closely examine our routines and habits and take stock of what really matters. Generations expectedly differ in their values and definitions of success. COVID has set prior established rules on fire, by forcing patterns and expectations that were neither expected nor wanted, within the context of a global health crisis. Within this backdrop, should we really believe our worth is determined by our job?
The truth is, we are still grieving what we lost during COVID and we have expectedly not assimilated to “the new normal.” Psychology has long recognized that losing structures and supports, routines and habits, causes symptoms of significant discomfort.
The idea that we would return to prior workplace expectations is naive. The idea we would “return to life as it was” is naive. It seems expected, then, that both employers and employees should evaluate their goals and communicate more openly about how each can be met.
It is incumbent upon the employers to set up clear guidelines regarding expectations, including rewards for performance and expectations for time, both within and outside of the work schedule. Employers must recognize symptoms of detachment in their employees and engage in the process of continuing clarifying roles and expectations while providing necessities for employees to succeed at their highest level. Employees, in turn, must self-examine their goals, communicate their needs, meet their responsibilities fully, and take on the challenge of determining their own definition of balance.
Maybe instead of quiet quitting, we should call it this new movement “self-awareness, growth, and evolution.” Hmmm, there’s an intriguing thought.
Dr. Calvery is professor of pediatrics at the University of Louisville (Ky.) She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Polio in 2022: Some concerns but vaccine still works
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Artemisia capillaris extract
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Talking to teens
After 15 years as a high school teacher at urban schools, I realized adults widely misunderstand that teenagers do not want to talk to them. In fact, most crave finding an adult they can trust and have serious conversations about issues like sex, drugs, and death. G was a sophomore who was going blind from a rare degenerative disease and one day sought my guidance about a sexual orgy he accidentally got in involved in. Was it wrong? Would God send him to hell? Why was he now so anxious after?
Because I was an openly gay teacher, students every semester would come out to me, asking what the “gay scene” was like, or how to deal with a homophobic family. Sometimes, students would seek counsel about an unplanned pregnancy, about abortion. In one instance, a student sought counsel about her violent thoughts, and eventually checked herself into a psychiatric ward. Five separate times, students in my class were murdered and I accompanied my classes through mourning.
Unlike many pediatricians, a teacher has a lot of time with these young adults: daily, sometimes over years. Students often admit they spend more time with their teachers than their parents. I can’t give you that time, but here are some general tips.
Attitude promoting trust
My guiding attitude toward teens was that they were my equals. I would “do unto them as I would have them do unto me.” Not less, but also not more – because sometimes “more” can cloak condescension. When I was a student, I trusted teachers who shared their fears and mistakes, not performing under a confessional spotlight, but to establish commonality, to flatten hierarchy. I also trusted those who could set boundaries and wield authority compassionately. Because sometimes I needed a firm hand. And so, as an adult I tried to give this to my students as well.
Although my students and I were equals, our situations are different. That is true with gender, race, and class, and it is also true with adults versus teens. The first step toward treating someone authentically as an equal when in a position of authority is to understand the unique stressors of their life. That means asking questions and listening to what they need.
Stressors in a teen’s life
A typical high school junior or senior goes to work 8-10 hours a day. Unpaid. They sit for hours at a small desk in a small room with sometimes 34 others. Most of the time they cannot eat or use their phone. If they need to pee, they need to ask permission. They have to ask permission to speak. And then when they go home, they sit at a small desk again for homework. They often do not even have their own room. They also have to ask for permission to buy something for themselves, for money, for a ride anywhere. Their values are often compromised so they won’t get kicked out of a house or a class. The life of a teen is not at all “carefree” but largely prescribed and with little control.
When I think about my youth and how little freedom, privacy, and control I had compared with now, it softens my attitude to even the rudest student. (Isn’t rudeness often a sign of resistance against an oppressive system?) But, some may say, these teens do not have to worry about bills. But if I think back honestly to my teen years, would I trade the responsibilities I have now for those supposed carefree years? Carefree is not how most teens describe their lives but a nostalgic rosy retrospection adults assign. Almost all teens I taught would rather work to gain some control over their lives. Which is why so many work 4-5 hours after school on top of homework, giving up their weekends, and binding themselves to a “carefree” 60- to 80-hour work week.
Talking about drugs, sex, and mental health
Drugs
It’s a good idea to first disarm teens of their fear of judgment or punishment, saying things like: “It’s normal to experiment with drugs, even hard ones.” The most successful, respected adults you see now have, so it’s not a reflection of who you are. Tell me what you’re worried about and it’ll be just between us.
After rapport is established, follow-up questions that elicit and affirm their feelings and thoughts can encourage more revelations: Do you think you have a problem? Why? How do you get your drugs and I’m curious only because finding that out can help us understand risks and solutions. What made you start? And keep on using?
Sex
Again, first disarm their fears: You can talk to me freely and confidently about sex: What you do, who you do it with, how you do it, and how often – I know that people are very different in their sexual interests and activities.
It is also good to set up clear boundaries. I had instances where students had romantic interest in me and would use these conversations as overtures. If you feel like your patient may be interested in you, then be explicit about boundaries: I’m a doctor who can point you to resources or offer treatments related to any sexual practice and its consequences, but that is all I am. Anything else is illegal and would end our patient-doctor relationship. (I would also immediately document the interaction and tell it to a witness.)
I never escalated incidences like this because I understood that most teens are naturally curious and often not taught about sexual boundaries, so I tried to make these encounters “teachable moments,” not punitive ones. Many teens are more aware of health consequences, like STDs or pregnancy, than psychological ones. So, it’s useful to ask: When you have sex outside your relationship, how does that make you feel? Does sex with multiple partners make you anxious or guilty afterwards? I like to use straightforward language and normalize taboo sexual practices with an even tone to allow teens to speak truthfully.
Suicide/depression
First, disarm and normalize: It is very common for people to have depression or thoughts of suicide. Most of the adults around you probably have and so have I (if that is true). Have you experienced this? Older teens often crave an intelligent open discussion about depression and suicide. If they look particularly distressed, I also tell them that I, and countless others, found strategies to deal with these thoughts. For most older teens, talking about causes of mental health issues and treatments is a breath of fresh air. This is especially true for teens from urban communities who have dealt precociously with death and violence, minority communities where mental health is often stigmatized, and young males whose machismo code can prevent them from acknowledging their feelings.
Some follow-up questions: Where do you think these thoughts come from? And if they don’t know: It’s perfectly normal for there to be no reason. The important thing is that they don’t last too long and that you know that. And if they do, then I can provide you resources and potential treatments.
Summary
Treating teens as equals by understanding their situation allows understanding and compassion for their stressors. This motivates an inquisitive and collaborative patient-centric approach that allows a sharing of sensitive topics like drugs, sex, and mental health.
Dr. Nguyen is a resident in psychiatry at the University of California, San Francisco.
*This story was updated on Nov. 3, 2022.
After 15 years as a high school teacher at urban schools, I realized adults widely misunderstand that teenagers do not want to talk to them. In fact, most crave finding an adult they can trust and have serious conversations about issues like sex, drugs, and death. G was a sophomore who was going blind from a rare degenerative disease and one day sought my guidance about a sexual orgy he accidentally got in involved in. Was it wrong? Would God send him to hell? Why was he now so anxious after?
Because I was an openly gay teacher, students every semester would come out to me, asking what the “gay scene” was like, or how to deal with a homophobic family. Sometimes, students would seek counsel about an unplanned pregnancy, about abortion. In one instance, a student sought counsel about her violent thoughts, and eventually checked herself into a psychiatric ward. Five separate times, students in my class were murdered and I accompanied my classes through mourning.
Unlike many pediatricians, a teacher has a lot of time with these young adults: daily, sometimes over years. Students often admit they spend more time with their teachers than their parents. I can’t give you that time, but here are some general tips.
Attitude promoting trust
My guiding attitude toward teens was that they were my equals. I would “do unto them as I would have them do unto me.” Not less, but also not more – because sometimes “more” can cloak condescension. When I was a student, I trusted teachers who shared their fears and mistakes, not performing under a confessional spotlight, but to establish commonality, to flatten hierarchy. I also trusted those who could set boundaries and wield authority compassionately. Because sometimes I needed a firm hand. And so, as an adult I tried to give this to my students as well.
Although my students and I were equals, our situations are different. That is true with gender, race, and class, and it is also true with adults versus teens. The first step toward treating someone authentically as an equal when in a position of authority is to understand the unique stressors of their life. That means asking questions and listening to what they need.
Stressors in a teen’s life
A typical high school junior or senior goes to work 8-10 hours a day. Unpaid. They sit for hours at a small desk in a small room with sometimes 34 others. Most of the time they cannot eat or use their phone. If they need to pee, they need to ask permission. They have to ask permission to speak. And then when they go home, they sit at a small desk again for homework. They often do not even have their own room. They also have to ask for permission to buy something for themselves, for money, for a ride anywhere. Their values are often compromised so they won’t get kicked out of a house or a class. The life of a teen is not at all “carefree” but largely prescribed and with little control.
When I think about my youth and how little freedom, privacy, and control I had compared with now, it softens my attitude to even the rudest student. (Isn’t rudeness often a sign of resistance against an oppressive system?) But, some may say, these teens do not have to worry about bills. But if I think back honestly to my teen years, would I trade the responsibilities I have now for those supposed carefree years? Carefree is not how most teens describe their lives but a nostalgic rosy retrospection adults assign. Almost all teens I taught would rather work to gain some control over their lives. Which is why so many work 4-5 hours after school on top of homework, giving up their weekends, and binding themselves to a “carefree” 60- to 80-hour work week.
Talking about drugs, sex, and mental health
Drugs
It’s a good idea to first disarm teens of their fear of judgment or punishment, saying things like: “It’s normal to experiment with drugs, even hard ones.” The most successful, respected adults you see now have, so it’s not a reflection of who you are. Tell me what you’re worried about and it’ll be just between us.
After rapport is established, follow-up questions that elicit and affirm their feelings and thoughts can encourage more revelations: Do you think you have a problem? Why? How do you get your drugs and I’m curious only because finding that out can help us understand risks and solutions. What made you start? And keep on using?
Sex
Again, first disarm their fears: You can talk to me freely and confidently about sex: What you do, who you do it with, how you do it, and how often – I know that people are very different in their sexual interests and activities.
It is also good to set up clear boundaries. I had instances where students had romantic interest in me and would use these conversations as overtures. If you feel like your patient may be interested in you, then be explicit about boundaries: I’m a doctor who can point you to resources or offer treatments related to any sexual practice and its consequences, but that is all I am. Anything else is illegal and would end our patient-doctor relationship. (I would also immediately document the interaction and tell it to a witness.)
I never escalated incidences like this because I understood that most teens are naturally curious and often not taught about sexual boundaries, so I tried to make these encounters “teachable moments,” not punitive ones. Many teens are more aware of health consequences, like STDs or pregnancy, than psychological ones. So, it’s useful to ask: When you have sex outside your relationship, how does that make you feel? Does sex with multiple partners make you anxious or guilty afterwards? I like to use straightforward language and normalize taboo sexual practices with an even tone to allow teens to speak truthfully.
Suicide/depression
First, disarm and normalize: It is very common for people to have depression or thoughts of suicide. Most of the adults around you probably have and so have I (if that is true). Have you experienced this? Older teens often crave an intelligent open discussion about depression and suicide. If they look particularly distressed, I also tell them that I, and countless others, found strategies to deal with these thoughts. For most older teens, talking about causes of mental health issues and treatments is a breath of fresh air. This is especially true for teens from urban communities who have dealt precociously with death and violence, minority communities where mental health is often stigmatized, and young males whose machismo code can prevent them from acknowledging their feelings.
Some follow-up questions: Where do you think these thoughts come from? And if they don’t know: It’s perfectly normal for there to be no reason. The important thing is that they don’t last too long and that you know that. And if they do, then I can provide you resources and potential treatments.
Summary
Treating teens as equals by understanding their situation allows understanding and compassion for their stressors. This motivates an inquisitive and collaborative patient-centric approach that allows a sharing of sensitive topics like drugs, sex, and mental health.
Dr. Nguyen is a resident in psychiatry at the University of California, San Francisco.
*This story was updated on Nov. 3, 2022.
After 15 years as a high school teacher at urban schools, I realized adults widely misunderstand that teenagers do not want to talk to them. In fact, most crave finding an adult they can trust and have serious conversations about issues like sex, drugs, and death. G was a sophomore who was going blind from a rare degenerative disease and one day sought my guidance about a sexual orgy he accidentally got in involved in. Was it wrong? Would God send him to hell? Why was he now so anxious after?
Because I was an openly gay teacher, students every semester would come out to me, asking what the “gay scene” was like, or how to deal with a homophobic family. Sometimes, students would seek counsel about an unplanned pregnancy, about abortion. In one instance, a student sought counsel about her violent thoughts, and eventually checked herself into a psychiatric ward. Five separate times, students in my class were murdered and I accompanied my classes through mourning.
Unlike many pediatricians, a teacher has a lot of time with these young adults: daily, sometimes over years. Students often admit they spend more time with their teachers than their parents. I can’t give you that time, but here are some general tips.
Attitude promoting trust
My guiding attitude toward teens was that they were my equals. I would “do unto them as I would have them do unto me.” Not less, but also not more – because sometimes “more” can cloak condescension. When I was a student, I trusted teachers who shared their fears and mistakes, not performing under a confessional spotlight, but to establish commonality, to flatten hierarchy. I also trusted those who could set boundaries and wield authority compassionately. Because sometimes I needed a firm hand. And so, as an adult I tried to give this to my students as well.
Although my students and I were equals, our situations are different. That is true with gender, race, and class, and it is also true with adults versus teens. The first step toward treating someone authentically as an equal when in a position of authority is to understand the unique stressors of their life. That means asking questions and listening to what they need.
Stressors in a teen’s life
A typical high school junior or senior goes to work 8-10 hours a day. Unpaid. They sit for hours at a small desk in a small room with sometimes 34 others. Most of the time they cannot eat or use their phone. If they need to pee, they need to ask permission. They have to ask permission to speak. And then when they go home, they sit at a small desk again for homework. They often do not even have their own room. They also have to ask for permission to buy something for themselves, for money, for a ride anywhere. Their values are often compromised so they won’t get kicked out of a house or a class. The life of a teen is not at all “carefree” but largely prescribed and with little control.
When I think about my youth and how little freedom, privacy, and control I had compared with now, it softens my attitude to even the rudest student. (Isn’t rudeness often a sign of resistance against an oppressive system?) But, some may say, these teens do not have to worry about bills. But if I think back honestly to my teen years, would I trade the responsibilities I have now for those supposed carefree years? Carefree is not how most teens describe their lives but a nostalgic rosy retrospection adults assign. Almost all teens I taught would rather work to gain some control over their lives. Which is why so many work 4-5 hours after school on top of homework, giving up their weekends, and binding themselves to a “carefree” 60- to 80-hour work week.
Talking about drugs, sex, and mental health
Drugs
It’s a good idea to first disarm teens of their fear of judgment or punishment, saying things like: “It’s normal to experiment with drugs, even hard ones.” The most successful, respected adults you see now have, so it’s not a reflection of who you are. Tell me what you’re worried about and it’ll be just between us.
After rapport is established, follow-up questions that elicit and affirm their feelings and thoughts can encourage more revelations: Do you think you have a problem? Why? How do you get your drugs and I’m curious only because finding that out can help us understand risks and solutions. What made you start? And keep on using?
Sex
Again, first disarm their fears: You can talk to me freely and confidently about sex: What you do, who you do it with, how you do it, and how often – I know that people are very different in their sexual interests and activities.
It is also good to set up clear boundaries. I had instances where students had romantic interest in me and would use these conversations as overtures. If you feel like your patient may be interested in you, then be explicit about boundaries: I’m a doctor who can point you to resources or offer treatments related to any sexual practice and its consequences, but that is all I am. Anything else is illegal and would end our patient-doctor relationship. (I would also immediately document the interaction and tell it to a witness.)
I never escalated incidences like this because I understood that most teens are naturally curious and often not taught about sexual boundaries, so I tried to make these encounters “teachable moments,” not punitive ones. Many teens are more aware of health consequences, like STDs or pregnancy, than psychological ones. So, it’s useful to ask: When you have sex outside your relationship, how does that make you feel? Does sex with multiple partners make you anxious or guilty afterwards? I like to use straightforward language and normalize taboo sexual practices with an even tone to allow teens to speak truthfully.
Suicide/depression
First, disarm and normalize: It is very common for people to have depression or thoughts of suicide. Most of the adults around you probably have and so have I (if that is true). Have you experienced this? Older teens often crave an intelligent open discussion about depression and suicide. If they look particularly distressed, I also tell them that I, and countless others, found strategies to deal with these thoughts. For most older teens, talking about causes of mental health issues and treatments is a breath of fresh air. This is especially true for teens from urban communities who have dealt precociously with death and violence, minority communities where mental health is often stigmatized, and young males whose machismo code can prevent them from acknowledging their feelings.
Some follow-up questions: Where do you think these thoughts come from? And if they don’t know: It’s perfectly normal for there to be no reason. The important thing is that they don’t last too long and that you know that. And if they do, then I can provide you resources and potential treatments.
Summary
Treating teens as equals by understanding their situation allows understanding and compassion for their stressors. This motivates an inquisitive and collaborative patient-centric approach that allows a sharing of sensitive topics like drugs, sex, and mental health.
Dr. Nguyen is a resident in psychiatry at the University of California, San Francisco.
*This story was updated on Nov. 3, 2022.
Depression as a terminal illness
Is there a place for palliative care?
In 2020, there were 5,224 suicide deaths registered in England and Wales.1 The Mental Health Foundation, a London-based charitable organization, reports that approximately 70% of such deaths are in patients with depression.2 The number of attempted suicides is much higher – the South West London and St. George’s Mental Health Trust estimates that at least 140,000 people attempt suicide in England and Wales every year.3
In suicidal depression, the psychological pain is often unbearable and feels overwhelmingly incompatible with life. One is no longer living but merely surviving, and eventually the exhaustion will lead to decompensation. This is marked by suicide. The goal is to end the suffering permanently and this is achieved through death.
Depression, like all other physical and mental illnesses, runs a course. This is highly variable between individuals and can be the case even between separate relapse episodes in the same patient. Like many diagnoses, depression is known to lead to death in a significant number of people. Many suicidally depressed patients feel that death will be an inevitable result of the illness.
Suicide is often viewed as a symptom of severe depression, but what if we considered death as part of the disease process itself? Consequently, would it be justifiable to consider depression in these patients as a form of terminal illness, since without treatment, the condition would lead to death? Accordingly, could there be a place for palliative care in a small minority of suicidally depressed patients? Taking such a perspective would mean that instead of placing the focus on the prevention of deaths and prolonging of lifespan, the focus would be on making the patients comfortable as the disease progresses, maintaining their dignity, and promoting autonomy.
Suicidal depression and rights
The rationale for this is that psychiatric patients do not have the capacity to make such decisions in the acute setting, because of the direct effects of the unwell mind on their decision-making processes and cognitive faculties. While this may be true in some cases, there is limited evidence that this applies to all suicidally depressed patients in all cases.
Another argument against allowing suicidally depressed patients to decline treatment is the notion that the episode of depression can be successfully treated, and the patients can return to their normal level of functioning. However, in individuals with a previous history of severe depression, it is possible that they will relapse again at some point. In the same way, a cancer can be treated, and patients could return to their baseline level of functioning, only for the cancer to then return later in life. In both cases, these relapses are emotionally and physically exhausting and painful to get through. The difference is that a cancer patient can decline further treatment and opt for no treatment or for palliative treatment, knowing that the disease will shorten life expectancy. For suicidal depression, this is not an option. Such patients may be sectioned, admitted, and treated against their will. Suicide, which could be considered a natural endpoint of the depressive illness, is unacceptable.
Is it fair to confiscate one’s right to decline treatment, solely because that person suffers from a mental illness, as opposed to a physical one? Numerous studies have demonstrated clear structural, neurological, and neurochemical changes in suicidal depression. This is evidence that such a condition encompasses a clear physical property. Other conditions, such as dementia and chronic pain, have previously been accepted for euthanasia in certain countries. Pain is a subjective experience of nociceptive and neurochemical signaling. In the same way, depression is a subjective experience involving aberrant neurochemical signaling. The difference is that physical pain can often be localized. However, patients with suicidal depression often experience very severe and tangible pain that can be difficult to articulate and for others to understand if they have never experienced it themselves.
Like distinct forms of physical pain, suicidal depression creates a different form of pain, but it is pain, nonetheless. Is it therefore fair for suicidally depressed patients to be given lesser rights than those suffering from physical illnesses in determining their fate?
Suicidal depression and capacity
A patient is assumed to have capacity unless proven otherwise. This is often the reverse when managing psychiatric patients. However, if patients are able to fulfill all criteria required for demonstrating capacity (understanding the information, retaining, weighing up, and communicating the decision), surely they have demonstrated capacity to make their decisions, whether that is to receive or to refuse treatment.
For physical illnesses, adults with capacity are permitted to make decisions that their treating teams may not agree with, but this disagreement alone is generally insufficient to override the decisions. These patients, unlike in suicidal depression, have the right to refuse lifesaving or life-prolonging treatment.
An argument for this is that in terminal physical illnesses, death is a passive process and neither the patient nor the physician are actively causing it. However, in many palliative settings, patients can be given medications and treatment for symptomatic relief, even if these may hasten their death. The principle that makes this permissible is that the primary aim is to improve the symptoms and ensure comfort. The unintended effect includes side effects and hastened death. Similarly, in suicidal depression, one could argue that the patient should be permitted medications that may hasten or lead to death, so long as the primary aim is to improve the symptoms of the unbearable mental pain and suffering.
Let us consider an alternative scenario. What if previously suicidal patients are currently in remission from depression and make advanced directives? In their current healthy state, they assert that if, in the future, they were to relapse, they would not want any form of treatment. Instead, they wish for the disease to run its course, which may end in death through suicide.
In this case, the circumstances in which the statement was made would be entirely valid – the patients at that moment have capacity, are not under coercion, are able to articulate logical thought processes, and their reasoning would not be affected by a concurrent psychiatric pathology. Furthermore, they can demonstrate that suicide is not an impulsive decision and have considered the consequences of suicide on themselves and others. If the patients can demonstrate all the above, what would the ethical grounds be for refusing this advanced directive?
Medical ethics
Below, I consider this debate in the context of four pillars of medical ethics.
Non-maleficence
To determine whether an action is in line with non-maleficence, one must ask whether the proposed treatment will improve or resolve one’s condition. In the case of severe suicidal depression, the treatment may help patients in the short term, but what happens if or when they relapse? The treatment will likely prolong life, but also inadvertently prolong suffering. What if the patients do not wish to go through this again? The treatment regime can be profoundly taxing for the patients, the loved ones, and sometimes even for the treating team. Are we doing more harm by forcing these patients to stay alive against their will?
Beneficence
Beneficence is the moral duty to promote the action that is in the patient’s best interest. But who should determine what the patient’s best interests are if the patient and the doctor disagree? Usually, this decision is made by the treating doctor, who considers the patient’s past and present wishes, beliefs and values, and capacity assessment. Supposing that the law was not a restriction, could one’s psychiatrist ever agree on psychiatric grounds alone that it is indeed in the patient’s best interests to die?
Doctors play a central role in the duty of care. But care does not always mean active treatment. Caring encompasses physical, psychological, and spiritual welfare and includes considering an individual patient’s dignity, personal circumstances, and wishes. In certain circumstances, keeping patients with capacity alive against their wishes could be more harmful than caring.
Autonomy
Autonomy gives the patients ultimate decision-making responsibility for their own lives. It allows patients with capacity to decline treatment that is recommended by their physicians and to make decisions regarding their own death. However, in suicidally depressed patients, this autonomy is confiscated. Severely unwell patients, at high risk of committing suicide, are not permitted the autonomy to make the decision regarding their treatment, suicide, and death.
Justice
A justice-orientated and utilitarian view questions whether spending resources on these patients wastes time, resources, and expertise, and whether resources should instead be spent on patients who do want treatment.
For example, the British National Health Service holds an outstanding debt of £13.4 billion.4 The financial cost of treating mental illness in 2020/2021 was £14.31 billion.5 The NHS estimates that wider costs to national economy, including welfare benefits, housing support, social workers, community support, lost productivity at work, etc., amounts to approximately £77 billion annually.6 Many severely depressed patients are so unwell that their ability to contribute to society, financially, socially, and otherwise, is minimal. If patients with capacity genuinely want to die and society would benefit from a reduction in the pressures on health and social care services, would it not be in both their best interests to allow them to die? This way, resources could be redirected to service users who would appreciate and benefit from them the most.
A consequentialist view focuses on whether the action will benefit the patient overall; the action itself is not so relevant. According to this view, keeping suicidally depressed patients alive against their wishes would be ethical if the patients lack capacity. Keeping them safe and treating them until they are better would overall be in the patients’ best interests. However, if the patients do have capacity and wish to die, forcing them to stay alive and undergo treatment against their wishes would merely prolong their suffering and thus could be considered unethical.
When enough is enough
In suicidal treatment-resistant depression, where the patient has tried multiple treatments over time and carefully considered alternatives, when is it time to stop trying? For physical illness, patients can refuse treatment provided they can demonstrate capacity. In depression, they can refuse treatment only if they can demonstrate that they are not at serious risk to themselves or others. Most societies consider suicide as a serious risk to self and therefore unacceptable. However, if we considered suicide as a natural endpoint of the disease process, should the patient have the right to refuse treatment and allow the disease to progress to death?
The treatment regime can be a lengthy process and the repeated failures to improve can be physically and mentally exhausting and further compound the hopelessness. Treatments often have side effects, which further erode the patient’s physical and mental wellbeing. Is there a time when giving up and withdrawing active treatment is in the patient’s best interests, especially if that is what the patient wants?
Terminal diseases are incurable and likely to hasten one’s death. Severe suicidal treatment-resistant depression conforms to both conditions – it is unresponsive to treatment and has a high likelihood of precipitating premature death through suicide. Most terminal illnesses can be managed with palliative treatment. In the context of severe suicidal depression, euthanasia and assisted suicide could be considered as means of palliative care.
Palliative care involves managing the patient’s symptomatology, dignity, and comfort. Euthanasia and assisted suicide help to address all of these. Like palliative care, euthanasia and assisted suicide aim to improve symptoms of depression by alleviating pain and suffering, even if they may hasten death.
Euthanasia and assisted suicide in severe depression
Euthanasia and assisted suicide are legal in seven countries. Two countries (Belgium and the Netherlands) permit euthanasia for psychiatric illnesses. Passive euthanasia is practiced in most countries, e.g., withholding artificial life support. In suicidal depression, it could be considered that this withholding of treatment may directly lead to death by suicide.
In active euthanasia and assisted suicide, the patient is given a chemical that will directly lead to death. Euthanasia and assisted suicide allow individuals to die with dignity in a controlled and organized manner. It ends the patients’ suffering and allows them to finally find peace. The difficulties that led them to seek euthanasia/assisted suicide indicate a loss of control of the pain and suffering in life, and euthanasia allows them to regain this control and autonomy through death. It allows these individuals to properly say goodbye to their loved ones, and a chance to share their thoughts and feelings.
In contrast, suicide is often covert, clandestine, and planned in secret, and it frequently requires individuals to be dishonest with their closest loved ones. The suicide often comes as a shock to the loved ones and profound grief, questions, anger, pain, sorrow, and guilt follow. These are due to questions that have been left unanswered, thoughts that were never shared, regret that they had not done more to help, and anguish knowing that their loved one died alone, in unbearable mental agony, unable to speak to anyone about this final hurdle.
Euthanasia and assisted suicide provide a path to overcome all these issues. They encourage open conversations between the patients, their loved ones, and the treating team. They promote transparency, mutual support, and help prepare the loved ones for the death. In this way, euthanasia and assisted suicide can benefit both the patient and the loved ones.
A significant proportion of severely suicidally depressed patients will eventually go on to commit or attempt suicide. Thus, giving them the autonomy to choose euthanasia or assisted suicide could be considered a kind, fair, and compassionate course of action, as it respects their wishes, and allows them to escape their suffering and to die with dignity.
Conclusion
Depression has historically never been considered a terminal illness, but there is undeniable evidence that a significant number of deaths every year are directly caused by depression. Should we therefore shift the focus from lifesaving and life-prolonging treatment to ensuring comfort and maintaining dignity by exploring palliative options for extremely suicidally depressed patients with capacity, who are adamant on ending their lives?
Euthanasia and assisted suicide for depression pose a profound paradox when viewed through a deontological lens. According to this, the correct course of action directly corresponds to what the most “moral” action would be. The moral stance would be to help those who are suffering. But what exactly constitutes “help”? Are euthanasia and assisted suicide helping or harming? Likewise, is keeping patients with capacity alive against their wishes helping or harming? Many believe that euthanasia, assisted suicide, and suicide itself are intrinsically and morally wrong. But this poses another clear impasse. Who should be the ones to decide whether an action is moral or not? Should it be the individual? The treating physician? Or society?
Dr. Chang graduated from Imperial College London with an MBBS (medicine and surgery) and a BSc (gastroenterology and hepatology) degree.
References
1. Office for National Statistics. Suicides in England and Wales – Office for National Statistics, 2021.
2. Faulkner, A. Suicide and Deliberate Self Harm: The Fundamental Facts. Mental Health Foundation; 1997.
3. NHS. Suicide Factsheet. Southwest London and St. George’s Mental Health NHS Trust [ebook], 2022.
4. The King’s Fund. Financial debts and loans in the NHS. 2020.
5. NHS England. Mental Health Five Year Forward View Dashboard. 2018.
6. National Mental Health, Policy into Practice. The costs of mental ill health.
Is there a place for palliative care?
Is there a place for palliative care?
In 2020, there were 5,224 suicide deaths registered in England and Wales.1 The Mental Health Foundation, a London-based charitable organization, reports that approximately 70% of such deaths are in patients with depression.2 The number of attempted suicides is much higher – the South West London and St. George’s Mental Health Trust estimates that at least 140,000 people attempt suicide in England and Wales every year.3
In suicidal depression, the psychological pain is often unbearable and feels overwhelmingly incompatible with life. One is no longer living but merely surviving, and eventually the exhaustion will lead to decompensation. This is marked by suicide. The goal is to end the suffering permanently and this is achieved through death.
Depression, like all other physical and mental illnesses, runs a course. This is highly variable between individuals and can be the case even between separate relapse episodes in the same patient. Like many diagnoses, depression is known to lead to death in a significant number of people. Many suicidally depressed patients feel that death will be an inevitable result of the illness.
Suicide is often viewed as a symptom of severe depression, but what if we considered death as part of the disease process itself? Consequently, would it be justifiable to consider depression in these patients as a form of terminal illness, since without treatment, the condition would lead to death? Accordingly, could there be a place for palliative care in a small minority of suicidally depressed patients? Taking such a perspective would mean that instead of placing the focus on the prevention of deaths and prolonging of lifespan, the focus would be on making the patients comfortable as the disease progresses, maintaining their dignity, and promoting autonomy.
Suicidal depression and rights
The rationale for this is that psychiatric patients do not have the capacity to make such decisions in the acute setting, because of the direct effects of the unwell mind on their decision-making processes and cognitive faculties. While this may be true in some cases, there is limited evidence that this applies to all suicidally depressed patients in all cases.
Another argument against allowing suicidally depressed patients to decline treatment is the notion that the episode of depression can be successfully treated, and the patients can return to their normal level of functioning. However, in individuals with a previous history of severe depression, it is possible that they will relapse again at some point. In the same way, a cancer can be treated, and patients could return to their baseline level of functioning, only for the cancer to then return later in life. In both cases, these relapses are emotionally and physically exhausting and painful to get through. The difference is that a cancer patient can decline further treatment and opt for no treatment or for palliative treatment, knowing that the disease will shorten life expectancy. For suicidal depression, this is not an option. Such patients may be sectioned, admitted, and treated against their will. Suicide, which could be considered a natural endpoint of the depressive illness, is unacceptable.
Is it fair to confiscate one’s right to decline treatment, solely because that person suffers from a mental illness, as opposed to a physical one? Numerous studies have demonstrated clear structural, neurological, and neurochemical changes in suicidal depression. This is evidence that such a condition encompasses a clear physical property. Other conditions, such as dementia and chronic pain, have previously been accepted for euthanasia in certain countries. Pain is a subjective experience of nociceptive and neurochemical signaling. In the same way, depression is a subjective experience involving aberrant neurochemical signaling. The difference is that physical pain can often be localized. However, patients with suicidal depression often experience very severe and tangible pain that can be difficult to articulate and for others to understand if they have never experienced it themselves.
Like distinct forms of physical pain, suicidal depression creates a different form of pain, but it is pain, nonetheless. Is it therefore fair for suicidally depressed patients to be given lesser rights than those suffering from physical illnesses in determining their fate?
Suicidal depression and capacity
A patient is assumed to have capacity unless proven otherwise. This is often the reverse when managing psychiatric patients. However, if patients are able to fulfill all criteria required for demonstrating capacity (understanding the information, retaining, weighing up, and communicating the decision), surely they have demonstrated capacity to make their decisions, whether that is to receive or to refuse treatment.
For physical illnesses, adults with capacity are permitted to make decisions that their treating teams may not agree with, but this disagreement alone is generally insufficient to override the decisions. These patients, unlike in suicidal depression, have the right to refuse lifesaving or life-prolonging treatment.
An argument for this is that in terminal physical illnesses, death is a passive process and neither the patient nor the physician are actively causing it. However, in many palliative settings, patients can be given medications and treatment for symptomatic relief, even if these may hasten their death. The principle that makes this permissible is that the primary aim is to improve the symptoms and ensure comfort. The unintended effect includes side effects and hastened death. Similarly, in suicidal depression, one could argue that the patient should be permitted medications that may hasten or lead to death, so long as the primary aim is to improve the symptoms of the unbearable mental pain and suffering.
Let us consider an alternative scenario. What if previously suicidal patients are currently in remission from depression and make advanced directives? In their current healthy state, they assert that if, in the future, they were to relapse, they would not want any form of treatment. Instead, they wish for the disease to run its course, which may end in death through suicide.
In this case, the circumstances in which the statement was made would be entirely valid – the patients at that moment have capacity, are not under coercion, are able to articulate logical thought processes, and their reasoning would not be affected by a concurrent psychiatric pathology. Furthermore, they can demonstrate that suicide is not an impulsive decision and have considered the consequences of suicide on themselves and others. If the patients can demonstrate all the above, what would the ethical grounds be for refusing this advanced directive?
Medical ethics
Below, I consider this debate in the context of four pillars of medical ethics.
Non-maleficence
To determine whether an action is in line with non-maleficence, one must ask whether the proposed treatment will improve or resolve one’s condition. In the case of severe suicidal depression, the treatment may help patients in the short term, but what happens if or when they relapse? The treatment will likely prolong life, but also inadvertently prolong suffering. What if the patients do not wish to go through this again? The treatment regime can be profoundly taxing for the patients, the loved ones, and sometimes even for the treating team. Are we doing more harm by forcing these patients to stay alive against their will?
Beneficence
Beneficence is the moral duty to promote the action that is in the patient’s best interest. But who should determine what the patient’s best interests are if the patient and the doctor disagree? Usually, this decision is made by the treating doctor, who considers the patient’s past and present wishes, beliefs and values, and capacity assessment. Supposing that the law was not a restriction, could one’s psychiatrist ever agree on psychiatric grounds alone that it is indeed in the patient’s best interests to die?
Doctors play a central role in the duty of care. But care does not always mean active treatment. Caring encompasses physical, psychological, and spiritual welfare and includes considering an individual patient’s dignity, personal circumstances, and wishes. In certain circumstances, keeping patients with capacity alive against their wishes could be more harmful than caring.
Autonomy
Autonomy gives the patients ultimate decision-making responsibility for their own lives. It allows patients with capacity to decline treatment that is recommended by their physicians and to make decisions regarding their own death. However, in suicidally depressed patients, this autonomy is confiscated. Severely unwell patients, at high risk of committing suicide, are not permitted the autonomy to make the decision regarding their treatment, suicide, and death.
Justice
A justice-orientated and utilitarian view questions whether spending resources on these patients wastes time, resources, and expertise, and whether resources should instead be spent on patients who do want treatment.
For example, the British National Health Service holds an outstanding debt of £13.4 billion.4 The financial cost of treating mental illness in 2020/2021 was £14.31 billion.5 The NHS estimates that wider costs to national economy, including welfare benefits, housing support, social workers, community support, lost productivity at work, etc., amounts to approximately £77 billion annually.6 Many severely depressed patients are so unwell that their ability to contribute to society, financially, socially, and otherwise, is minimal. If patients with capacity genuinely want to die and society would benefit from a reduction in the pressures on health and social care services, would it not be in both their best interests to allow them to die? This way, resources could be redirected to service users who would appreciate and benefit from them the most.
A consequentialist view focuses on whether the action will benefit the patient overall; the action itself is not so relevant. According to this view, keeping suicidally depressed patients alive against their wishes would be ethical if the patients lack capacity. Keeping them safe and treating them until they are better would overall be in the patients’ best interests. However, if the patients do have capacity and wish to die, forcing them to stay alive and undergo treatment against their wishes would merely prolong their suffering and thus could be considered unethical.
When enough is enough
In suicidal treatment-resistant depression, where the patient has tried multiple treatments over time and carefully considered alternatives, when is it time to stop trying? For physical illness, patients can refuse treatment provided they can demonstrate capacity. In depression, they can refuse treatment only if they can demonstrate that they are not at serious risk to themselves or others. Most societies consider suicide as a serious risk to self and therefore unacceptable. However, if we considered suicide as a natural endpoint of the disease process, should the patient have the right to refuse treatment and allow the disease to progress to death?
The treatment regime can be a lengthy process and the repeated failures to improve can be physically and mentally exhausting and further compound the hopelessness. Treatments often have side effects, which further erode the patient’s physical and mental wellbeing. Is there a time when giving up and withdrawing active treatment is in the patient’s best interests, especially if that is what the patient wants?
Terminal diseases are incurable and likely to hasten one’s death. Severe suicidal treatment-resistant depression conforms to both conditions – it is unresponsive to treatment and has a high likelihood of precipitating premature death through suicide. Most terminal illnesses can be managed with palliative treatment. In the context of severe suicidal depression, euthanasia and assisted suicide could be considered as means of palliative care.
Palliative care involves managing the patient’s symptomatology, dignity, and comfort. Euthanasia and assisted suicide help to address all of these. Like palliative care, euthanasia and assisted suicide aim to improve symptoms of depression by alleviating pain and suffering, even if they may hasten death.
Euthanasia and assisted suicide in severe depression
Euthanasia and assisted suicide are legal in seven countries. Two countries (Belgium and the Netherlands) permit euthanasia for psychiatric illnesses. Passive euthanasia is practiced in most countries, e.g., withholding artificial life support. In suicidal depression, it could be considered that this withholding of treatment may directly lead to death by suicide.
In active euthanasia and assisted suicide, the patient is given a chemical that will directly lead to death. Euthanasia and assisted suicide allow individuals to die with dignity in a controlled and organized manner. It ends the patients’ suffering and allows them to finally find peace. The difficulties that led them to seek euthanasia/assisted suicide indicate a loss of control of the pain and suffering in life, and euthanasia allows them to regain this control and autonomy through death. It allows these individuals to properly say goodbye to their loved ones, and a chance to share their thoughts and feelings.
In contrast, suicide is often covert, clandestine, and planned in secret, and it frequently requires individuals to be dishonest with their closest loved ones. The suicide often comes as a shock to the loved ones and profound grief, questions, anger, pain, sorrow, and guilt follow. These are due to questions that have been left unanswered, thoughts that were never shared, regret that they had not done more to help, and anguish knowing that their loved one died alone, in unbearable mental agony, unable to speak to anyone about this final hurdle.
Euthanasia and assisted suicide provide a path to overcome all these issues. They encourage open conversations between the patients, their loved ones, and the treating team. They promote transparency, mutual support, and help prepare the loved ones for the death. In this way, euthanasia and assisted suicide can benefit both the patient and the loved ones.
A significant proportion of severely suicidally depressed patients will eventually go on to commit or attempt suicide. Thus, giving them the autonomy to choose euthanasia or assisted suicide could be considered a kind, fair, and compassionate course of action, as it respects their wishes, and allows them to escape their suffering and to die with dignity.
Conclusion
Depression has historically never been considered a terminal illness, but there is undeniable evidence that a significant number of deaths every year are directly caused by depression. Should we therefore shift the focus from lifesaving and life-prolonging treatment to ensuring comfort and maintaining dignity by exploring palliative options for extremely suicidally depressed patients with capacity, who are adamant on ending their lives?
Euthanasia and assisted suicide for depression pose a profound paradox when viewed through a deontological lens. According to this, the correct course of action directly corresponds to what the most “moral” action would be. The moral stance would be to help those who are suffering. But what exactly constitutes “help”? Are euthanasia and assisted suicide helping or harming? Likewise, is keeping patients with capacity alive against their wishes helping or harming? Many believe that euthanasia, assisted suicide, and suicide itself are intrinsically and morally wrong. But this poses another clear impasse. Who should be the ones to decide whether an action is moral or not? Should it be the individual? The treating physician? Or society?
Dr. Chang graduated from Imperial College London with an MBBS (medicine and surgery) and a BSc (gastroenterology and hepatology) degree.
References
1. Office for National Statistics. Suicides in England and Wales – Office for National Statistics, 2021.
2. Faulkner, A. Suicide and Deliberate Self Harm: The Fundamental Facts. Mental Health Foundation; 1997.
3. NHS. Suicide Factsheet. Southwest London and St. George’s Mental Health NHS Trust [ebook], 2022.
4. The King’s Fund. Financial debts and loans in the NHS. 2020.
5. NHS England. Mental Health Five Year Forward View Dashboard. 2018.
6. National Mental Health, Policy into Practice. The costs of mental ill health.
In 2020, there were 5,224 suicide deaths registered in England and Wales.1 The Mental Health Foundation, a London-based charitable organization, reports that approximately 70% of such deaths are in patients with depression.2 The number of attempted suicides is much higher – the South West London and St. George’s Mental Health Trust estimates that at least 140,000 people attempt suicide in England and Wales every year.3
In suicidal depression, the psychological pain is often unbearable and feels overwhelmingly incompatible with life. One is no longer living but merely surviving, and eventually the exhaustion will lead to decompensation. This is marked by suicide. The goal is to end the suffering permanently and this is achieved through death.
Depression, like all other physical and mental illnesses, runs a course. This is highly variable between individuals and can be the case even between separate relapse episodes in the same patient. Like many diagnoses, depression is known to lead to death in a significant number of people. Many suicidally depressed patients feel that death will be an inevitable result of the illness.
Suicide is often viewed as a symptom of severe depression, but what if we considered death as part of the disease process itself? Consequently, would it be justifiable to consider depression in these patients as a form of terminal illness, since without treatment, the condition would lead to death? Accordingly, could there be a place for palliative care in a small minority of suicidally depressed patients? Taking such a perspective would mean that instead of placing the focus on the prevention of deaths and prolonging of lifespan, the focus would be on making the patients comfortable as the disease progresses, maintaining their dignity, and promoting autonomy.
Suicidal depression and rights
The rationale for this is that psychiatric patients do not have the capacity to make such decisions in the acute setting, because of the direct effects of the unwell mind on their decision-making processes and cognitive faculties. While this may be true in some cases, there is limited evidence that this applies to all suicidally depressed patients in all cases.
Another argument against allowing suicidally depressed patients to decline treatment is the notion that the episode of depression can be successfully treated, and the patients can return to their normal level of functioning. However, in individuals with a previous history of severe depression, it is possible that they will relapse again at some point. In the same way, a cancer can be treated, and patients could return to their baseline level of functioning, only for the cancer to then return later in life. In both cases, these relapses are emotionally and physically exhausting and painful to get through. The difference is that a cancer patient can decline further treatment and opt for no treatment or for palliative treatment, knowing that the disease will shorten life expectancy. For suicidal depression, this is not an option. Such patients may be sectioned, admitted, and treated against their will. Suicide, which could be considered a natural endpoint of the depressive illness, is unacceptable.
Is it fair to confiscate one’s right to decline treatment, solely because that person suffers from a mental illness, as opposed to a physical one? Numerous studies have demonstrated clear structural, neurological, and neurochemical changes in suicidal depression. This is evidence that such a condition encompasses a clear physical property. Other conditions, such as dementia and chronic pain, have previously been accepted for euthanasia in certain countries. Pain is a subjective experience of nociceptive and neurochemical signaling. In the same way, depression is a subjective experience involving aberrant neurochemical signaling. The difference is that physical pain can often be localized. However, patients with suicidal depression often experience very severe and tangible pain that can be difficult to articulate and for others to understand if they have never experienced it themselves.
Like distinct forms of physical pain, suicidal depression creates a different form of pain, but it is pain, nonetheless. Is it therefore fair for suicidally depressed patients to be given lesser rights than those suffering from physical illnesses in determining their fate?
Suicidal depression and capacity
A patient is assumed to have capacity unless proven otherwise. This is often the reverse when managing psychiatric patients. However, if patients are able to fulfill all criteria required for demonstrating capacity (understanding the information, retaining, weighing up, and communicating the decision), surely they have demonstrated capacity to make their decisions, whether that is to receive or to refuse treatment.
For physical illnesses, adults with capacity are permitted to make decisions that their treating teams may not agree with, but this disagreement alone is generally insufficient to override the decisions. These patients, unlike in suicidal depression, have the right to refuse lifesaving or life-prolonging treatment.
An argument for this is that in terminal physical illnesses, death is a passive process and neither the patient nor the physician are actively causing it. However, in many palliative settings, patients can be given medications and treatment for symptomatic relief, even if these may hasten their death. The principle that makes this permissible is that the primary aim is to improve the symptoms and ensure comfort. The unintended effect includes side effects and hastened death. Similarly, in suicidal depression, one could argue that the patient should be permitted medications that may hasten or lead to death, so long as the primary aim is to improve the symptoms of the unbearable mental pain and suffering.
Let us consider an alternative scenario. What if previously suicidal patients are currently in remission from depression and make advanced directives? In their current healthy state, they assert that if, in the future, they were to relapse, they would not want any form of treatment. Instead, they wish for the disease to run its course, which may end in death through suicide.
In this case, the circumstances in which the statement was made would be entirely valid – the patients at that moment have capacity, are not under coercion, are able to articulate logical thought processes, and their reasoning would not be affected by a concurrent psychiatric pathology. Furthermore, they can demonstrate that suicide is not an impulsive decision and have considered the consequences of suicide on themselves and others. If the patients can demonstrate all the above, what would the ethical grounds be for refusing this advanced directive?
Medical ethics
Below, I consider this debate in the context of four pillars of medical ethics.
Non-maleficence
To determine whether an action is in line with non-maleficence, one must ask whether the proposed treatment will improve or resolve one’s condition. In the case of severe suicidal depression, the treatment may help patients in the short term, but what happens if or when they relapse? The treatment will likely prolong life, but also inadvertently prolong suffering. What if the patients do not wish to go through this again? The treatment regime can be profoundly taxing for the patients, the loved ones, and sometimes even for the treating team. Are we doing more harm by forcing these patients to stay alive against their will?
Beneficence
Beneficence is the moral duty to promote the action that is in the patient’s best interest. But who should determine what the patient’s best interests are if the patient and the doctor disagree? Usually, this decision is made by the treating doctor, who considers the patient’s past and present wishes, beliefs and values, and capacity assessment. Supposing that the law was not a restriction, could one’s psychiatrist ever agree on psychiatric grounds alone that it is indeed in the patient’s best interests to die?
Doctors play a central role in the duty of care. But care does not always mean active treatment. Caring encompasses physical, psychological, and spiritual welfare and includes considering an individual patient’s dignity, personal circumstances, and wishes. In certain circumstances, keeping patients with capacity alive against their wishes could be more harmful than caring.
Autonomy
Autonomy gives the patients ultimate decision-making responsibility for their own lives. It allows patients with capacity to decline treatment that is recommended by their physicians and to make decisions regarding their own death. However, in suicidally depressed patients, this autonomy is confiscated. Severely unwell patients, at high risk of committing suicide, are not permitted the autonomy to make the decision regarding their treatment, suicide, and death.
Justice
A justice-orientated and utilitarian view questions whether spending resources on these patients wastes time, resources, and expertise, and whether resources should instead be spent on patients who do want treatment.
For example, the British National Health Service holds an outstanding debt of £13.4 billion.4 The financial cost of treating mental illness in 2020/2021 was £14.31 billion.5 The NHS estimates that wider costs to national economy, including welfare benefits, housing support, social workers, community support, lost productivity at work, etc., amounts to approximately £77 billion annually.6 Many severely depressed patients are so unwell that their ability to contribute to society, financially, socially, and otherwise, is minimal. If patients with capacity genuinely want to die and society would benefit from a reduction in the pressures on health and social care services, would it not be in both their best interests to allow them to die? This way, resources could be redirected to service users who would appreciate and benefit from them the most.
A consequentialist view focuses on whether the action will benefit the patient overall; the action itself is not so relevant. According to this view, keeping suicidally depressed patients alive against their wishes would be ethical if the patients lack capacity. Keeping them safe and treating them until they are better would overall be in the patients’ best interests. However, if the patients do have capacity and wish to die, forcing them to stay alive and undergo treatment against their wishes would merely prolong their suffering and thus could be considered unethical.
When enough is enough
In suicidal treatment-resistant depression, where the patient has tried multiple treatments over time and carefully considered alternatives, when is it time to stop trying? For physical illness, patients can refuse treatment provided they can demonstrate capacity. In depression, they can refuse treatment only if they can demonstrate that they are not at serious risk to themselves or others. Most societies consider suicide as a serious risk to self and therefore unacceptable. However, if we considered suicide as a natural endpoint of the disease process, should the patient have the right to refuse treatment and allow the disease to progress to death?
The treatment regime can be a lengthy process and the repeated failures to improve can be physically and mentally exhausting and further compound the hopelessness. Treatments often have side effects, which further erode the patient’s physical and mental wellbeing. Is there a time when giving up and withdrawing active treatment is in the patient’s best interests, especially if that is what the patient wants?
Terminal diseases are incurable and likely to hasten one’s death. Severe suicidal treatment-resistant depression conforms to both conditions – it is unresponsive to treatment and has a high likelihood of precipitating premature death through suicide. Most terminal illnesses can be managed with palliative treatment. In the context of severe suicidal depression, euthanasia and assisted suicide could be considered as means of palliative care.
Palliative care involves managing the patient’s symptomatology, dignity, and comfort. Euthanasia and assisted suicide help to address all of these. Like palliative care, euthanasia and assisted suicide aim to improve symptoms of depression by alleviating pain and suffering, even if they may hasten death.
Euthanasia and assisted suicide in severe depression
Euthanasia and assisted suicide are legal in seven countries. Two countries (Belgium and the Netherlands) permit euthanasia for psychiatric illnesses. Passive euthanasia is practiced in most countries, e.g., withholding artificial life support. In suicidal depression, it could be considered that this withholding of treatment may directly lead to death by suicide.
In active euthanasia and assisted suicide, the patient is given a chemical that will directly lead to death. Euthanasia and assisted suicide allow individuals to die with dignity in a controlled and organized manner. It ends the patients’ suffering and allows them to finally find peace. The difficulties that led them to seek euthanasia/assisted suicide indicate a loss of control of the pain and suffering in life, and euthanasia allows them to regain this control and autonomy through death. It allows these individuals to properly say goodbye to their loved ones, and a chance to share their thoughts and feelings.
In contrast, suicide is often covert, clandestine, and planned in secret, and it frequently requires individuals to be dishonest with their closest loved ones. The suicide often comes as a shock to the loved ones and profound grief, questions, anger, pain, sorrow, and guilt follow. These are due to questions that have been left unanswered, thoughts that were never shared, regret that they had not done more to help, and anguish knowing that their loved one died alone, in unbearable mental agony, unable to speak to anyone about this final hurdle.
Euthanasia and assisted suicide provide a path to overcome all these issues. They encourage open conversations between the patients, their loved ones, and the treating team. They promote transparency, mutual support, and help prepare the loved ones for the death. In this way, euthanasia and assisted suicide can benefit both the patient and the loved ones.
A significant proportion of severely suicidally depressed patients will eventually go on to commit or attempt suicide. Thus, giving them the autonomy to choose euthanasia or assisted suicide could be considered a kind, fair, and compassionate course of action, as it respects their wishes, and allows them to escape their suffering and to die with dignity.
Conclusion
Depression has historically never been considered a terminal illness, but there is undeniable evidence that a significant number of deaths every year are directly caused by depression. Should we therefore shift the focus from lifesaving and life-prolonging treatment to ensuring comfort and maintaining dignity by exploring palliative options for extremely suicidally depressed patients with capacity, who are adamant on ending their lives?
Euthanasia and assisted suicide for depression pose a profound paradox when viewed through a deontological lens. According to this, the correct course of action directly corresponds to what the most “moral” action would be. The moral stance would be to help those who are suffering. But what exactly constitutes “help”? Are euthanasia and assisted suicide helping or harming? Likewise, is keeping patients with capacity alive against their wishes helping or harming? Many believe that euthanasia, assisted suicide, and suicide itself are intrinsically and morally wrong. But this poses another clear impasse. Who should be the ones to decide whether an action is moral or not? Should it be the individual? The treating physician? Or society?
Dr. Chang graduated from Imperial College London with an MBBS (medicine and surgery) and a BSc (gastroenterology and hepatology) degree.
References
1. Office for National Statistics. Suicides in England and Wales – Office for National Statistics, 2021.
2. Faulkner, A. Suicide and Deliberate Self Harm: The Fundamental Facts. Mental Health Foundation; 1997.
3. NHS. Suicide Factsheet. Southwest London and St. George’s Mental Health NHS Trust [ebook], 2022.
4. The King’s Fund. Financial debts and loans in the NHS. 2020.
5. NHS England. Mental Health Five Year Forward View Dashboard. 2018.
6. National Mental Health, Policy into Practice. The costs of mental ill health.
Schizophrenia and postmodernism: A philosophical exercise in treatment
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Low-dose oral minoxidil for the treatment of alopecia
Other than oral finasteride, vitamins, and topicals, there has been little advancement in the treatment of AGA leaving many (including me) desperate for anything remotely new.
Oral minoxidil is a peripheral vasodilator approved by the Food and Drug Administration for use in patients with hypertensive disease taken at doses ranging between 10 mg to 40 mg daily. Animal studies have shown that minoxidil affects the hair growth cycle by shortening the telogen phase and prolonging the anagen phase.
Recent case studies have also shown growing evidence for the off-label use of low-dose oral minoxidil (LDOM) for treating different types of alopecia. Topical minoxidil is metabolized into its active metabolite minoxidil sulfate, by sulfotransferase enzymes located in the outer root sheath of hair follicles. The expression of sulfotransferase varies greatly in the scalp of different individuals, and this difference is directly correlated to the wide range of responses to minoxidil treatment. LDOM is, however, more widely effective because it requires decreased follicular enzymatic activity to form its active metabolite as compared with its topical form.
In a retrospective series by Beach and colleagues evaluating the efficacy and tolerability of LDOM for treating AGA, there was increased scalp hair growth in 33 of 51 patients (65%) and decreased hair shedding in 14 of the 51 patients (27%) with LDOM. Patients with nonscarring alopecia were most likely to show improvement. Side effects were dose dependent and infrequent. The most frequent adverse effects were hypertrichosis, lightheadedness, edema, and tachycardia. No life-threatening adverse effects were observed. Although there has been a recently reported case report of severe pericardial effusion, edema, and anasarca in a woman with frontal fibrosing alopecia treated with LDOM, life threatening side effects are rare.3
To compare the efficacy of topical versus oral minoxidil, Ramos and colleagues performed a 24-week prospective study of low-dose (1 mg/day) oral minoxidil, compared with topical 5% minoxidil, in the treatment of 52 women with female pattern hair loss. Blinded analysis of trichoscopic images were evaluated to compare the change in total hair density in a target area from baseline to week 24 by three dermatologists.
Results after 24 weeks of treatment showed an increase in total hair density (12%) among the women taking oral minoxidil, compared with 7.2% in women who applied topical minoxidil (P =.09).
In the armamentarium of hair-loss treatments, dermatologists have limited choices. LDOM can be used in patients with both scarring and nonscarring alopecia if monitored regularly. Treatment doses I recommend are 1.25-5 mg daily titrated up slowly in properly selected patients without contraindications and those who are not taking other vasodilators. Self-reported dizziness, edema, and headache are common and treatments for facial hypertrichosis in women are always discussed. Clinical efficacy can be evaluated after 10-12 months of therapy and concomitant spironolactone can be given to mitigate the side effect of hypertrichosis.Patient selection is crucial as patients with severe scarring alopecia and those with active inflammatory diseases of the scalp may not see similar results. Similar to other hair loss treatments, treatment courses of 10-12 months are often needed to see visible signs of hair growth.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Beach RA et al. J Am Acad Dermatol. 2021 Mar;84(3):761-3.
Dlova et al. JAAD Case Reports. 2022 Oct;28:94-6.
Jimenez-Cauhe J et al. J Am Acad Dermatol. 2021 Jan;84(1):222-3.
Ramos PM et al. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):e40-1.
Ramos PM et al. J Am Acad Dermatol. 2020 Jan;82(1):252-3.
Randolph M and Tosti A. J Am Acad Dermatol. 2021 Mar;84(3):737-46.
Vañó-Galván S et al. J Am Acad Dermatol. 2021 Jun;84(6):1644-51.
Other than oral finasteride, vitamins, and topicals, there has been little advancement in the treatment of AGA leaving many (including me) desperate for anything remotely new.
Oral minoxidil is a peripheral vasodilator approved by the Food and Drug Administration for use in patients with hypertensive disease taken at doses ranging between 10 mg to 40 mg daily. Animal studies have shown that minoxidil affects the hair growth cycle by shortening the telogen phase and prolonging the anagen phase.
Recent case studies have also shown growing evidence for the off-label use of low-dose oral minoxidil (LDOM) for treating different types of alopecia. Topical minoxidil is metabolized into its active metabolite minoxidil sulfate, by sulfotransferase enzymes located in the outer root sheath of hair follicles. The expression of sulfotransferase varies greatly in the scalp of different individuals, and this difference is directly correlated to the wide range of responses to minoxidil treatment. LDOM is, however, more widely effective because it requires decreased follicular enzymatic activity to form its active metabolite as compared with its topical form.
In a retrospective series by Beach and colleagues evaluating the efficacy and tolerability of LDOM for treating AGA, there was increased scalp hair growth in 33 of 51 patients (65%) and decreased hair shedding in 14 of the 51 patients (27%) with LDOM. Patients with nonscarring alopecia were most likely to show improvement. Side effects were dose dependent and infrequent. The most frequent adverse effects were hypertrichosis, lightheadedness, edema, and tachycardia. No life-threatening adverse effects were observed. Although there has been a recently reported case report of severe pericardial effusion, edema, and anasarca in a woman with frontal fibrosing alopecia treated with LDOM, life threatening side effects are rare.3
To compare the efficacy of topical versus oral minoxidil, Ramos and colleagues performed a 24-week prospective study of low-dose (1 mg/day) oral minoxidil, compared with topical 5% minoxidil, in the treatment of 52 women with female pattern hair loss. Blinded analysis of trichoscopic images were evaluated to compare the change in total hair density in a target area from baseline to week 24 by three dermatologists.
Results after 24 weeks of treatment showed an increase in total hair density (12%) among the women taking oral minoxidil, compared with 7.2% in women who applied topical minoxidil (P =.09).
In the armamentarium of hair-loss treatments, dermatologists have limited choices. LDOM can be used in patients with both scarring and nonscarring alopecia if monitored regularly. Treatment doses I recommend are 1.25-5 mg daily titrated up slowly in properly selected patients without contraindications and those who are not taking other vasodilators. Self-reported dizziness, edema, and headache are common and treatments for facial hypertrichosis in women are always discussed. Clinical efficacy can be evaluated after 10-12 months of therapy and concomitant spironolactone can be given to mitigate the side effect of hypertrichosis.Patient selection is crucial as patients with severe scarring alopecia and those with active inflammatory diseases of the scalp may not see similar results. Similar to other hair loss treatments, treatment courses of 10-12 months are often needed to see visible signs of hair growth.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Beach RA et al. J Am Acad Dermatol. 2021 Mar;84(3):761-3.
Dlova et al. JAAD Case Reports. 2022 Oct;28:94-6.
Jimenez-Cauhe J et al. J Am Acad Dermatol. 2021 Jan;84(1):222-3.
Ramos PM et al. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):e40-1.
Ramos PM et al. J Am Acad Dermatol. 2020 Jan;82(1):252-3.
Randolph M and Tosti A. J Am Acad Dermatol. 2021 Mar;84(3):737-46.
Vañó-Galván S et al. J Am Acad Dermatol. 2021 Jun;84(6):1644-51.
Other than oral finasteride, vitamins, and topicals, there has been little advancement in the treatment of AGA leaving many (including me) desperate for anything remotely new.
Oral minoxidil is a peripheral vasodilator approved by the Food and Drug Administration for use in patients with hypertensive disease taken at doses ranging between 10 mg to 40 mg daily. Animal studies have shown that minoxidil affects the hair growth cycle by shortening the telogen phase and prolonging the anagen phase.
Recent case studies have also shown growing evidence for the off-label use of low-dose oral minoxidil (LDOM) for treating different types of alopecia. Topical minoxidil is metabolized into its active metabolite minoxidil sulfate, by sulfotransferase enzymes located in the outer root sheath of hair follicles. The expression of sulfotransferase varies greatly in the scalp of different individuals, and this difference is directly correlated to the wide range of responses to minoxidil treatment. LDOM is, however, more widely effective because it requires decreased follicular enzymatic activity to form its active metabolite as compared with its topical form.
In a retrospective series by Beach and colleagues evaluating the efficacy and tolerability of LDOM for treating AGA, there was increased scalp hair growth in 33 of 51 patients (65%) and decreased hair shedding in 14 of the 51 patients (27%) with LDOM. Patients with nonscarring alopecia were most likely to show improvement. Side effects were dose dependent and infrequent. The most frequent adverse effects were hypertrichosis, lightheadedness, edema, and tachycardia. No life-threatening adverse effects were observed. Although there has been a recently reported case report of severe pericardial effusion, edema, and anasarca in a woman with frontal fibrosing alopecia treated with LDOM, life threatening side effects are rare.3
To compare the efficacy of topical versus oral minoxidil, Ramos and colleagues performed a 24-week prospective study of low-dose (1 mg/day) oral minoxidil, compared with topical 5% minoxidil, in the treatment of 52 women with female pattern hair loss. Blinded analysis of trichoscopic images were evaluated to compare the change in total hair density in a target area from baseline to week 24 by three dermatologists.
Results after 24 weeks of treatment showed an increase in total hair density (12%) among the women taking oral minoxidil, compared with 7.2% in women who applied topical minoxidil (P =.09).
In the armamentarium of hair-loss treatments, dermatologists have limited choices. LDOM can be used in patients with both scarring and nonscarring alopecia if monitored regularly. Treatment doses I recommend are 1.25-5 mg daily titrated up slowly in properly selected patients without contraindications and those who are not taking other vasodilators. Self-reported dizziness, edema, and headache are common and treatments for facial hypertrichosis in women are always discussed. Clinical efficacy can be evaluated after 10-12 months of therapy and concomitant spironolactone can be given to mitigate the side effect of hypertrichosis.Patient selection is crucial as patients with severe scarring alopecia and those with active inflammatory diseases of the scalp may not see similar results. Similar to other hair loss treatments, treatment courses of 10-12 months are often needed to see visible signs of hair growth.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Beach RA et al. J Am Acad Dermatol. 2021 Mar;84(3):761-3.
Dlova et al. JAAD Case Reports. 2022 Oct;28:94-6.
Jimenez-Cauhe J et al. J Am Acad Dermatol. 2021 Jan;84(1):222-3.
Ramos PM et al. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):e40-1.
Ramos PM et al. J Am Acad Dermatol. 2020 Jan;82(1):252-3.
Randolph M and Tosti A. J Am Acad Dermatol. 2021 Mar;84(3):737-46.
Vañó-Galván S et al. J Am Acad Dermatol. 2021 Jun;84(6):1644-51.