User login
Nodding Off While Feeding an Infant
In a recent survey of 1259 mothers published in the journal Pediatrics, 28% reported they had fallen asleep while feeding their babies, and 83% of those mothers reported that the sleep was unplanned. Although the study sample was small, the investigators found that sociodemographic factors did not increase the odds that a mother would fall asleep while feeding.
These numbers are not surprising, but nonetheless they are concerning because co-sleeping is a known risk factor for sudden unexplained infant death (SUID). Every parent will tell you during the first 6 months of their adventure in parenting they didn’t get enough sleep. In fact some will tell you that sleep deprivation was their chronic state for the child’s first year.
Falling asleep easily at times and places not intended for sleep is the primary symptom of sleep deprivation. SUID is the most tragic event associated with parental sleep deprivation, but it is certainly not the only one. Overtired parents are more likely to be involved in accidents and are more likely to make poor decisions, particularly those regarding how to respond to a crying or misbehaving child.
The investigators found that 24% of mothers who reported that their usual nighttime feeding location was a chair or sofa (14%). Not surprisingly, mothers who fed in chairs were less likely to fall asleep while feeding. Many of these mothers reported that they chose the chair because they thought they would be less likely to fall asleep and/or disturb other family members. One wonders how we should interpret these numbers in light of other research that has found it is “relatively less hazardous to fall asleep with an infant in the adult bed than on a chair or sofa.” Had these chair feeding mothers made the better choice under the circumstances? It would take a much larger and more granular study to answer that question.
Mothers who exclusively breastfed were more likely to fall asleep feeding than were those who partially breastfed or used formula. The investigators postulated that the infants of mothers who exclusively breastfed may have required more feedings because breast milk is more easily and quickly digested. I know this is a common explanation, but in my experience I have found that exclusively breastfed infants often use nursing as pacification and a sleep trigger and spend more time at the breast regardless of how quickly they emptied their stomachs.
This study also examined the effect of repeated educational interventions and support and found that mothers who received an intervention based on safe sleep practices were less likely to fall asleep while feeding than were the mothers who had received the intervention focused on exclusive breastfeeding value and barriers to its adoption.
Education is one avenue, particularly when it includes the mother’s partner who can play an important role as standby lifeguard to make sure the mother doesn’t fall asleep. Obviously, this is easier said than done because when there is a new baby in the house sleep deprivation is usually a shared experience.
Although I believe that my family is on the verge of gifting me a smartwatch to protect me from my own misadventures, I don’t have any personal experience with these wonders of modern technology. However, I suspect with very little tweaking a wearable sensor could be easily programmed to detect when a mother is beginning to fall asleep while she is feeding her infant. A smartwatch would be an expensive intervention and is unlikely to filter down to economically challenged families. On the other hand, this paper has reinforced our suspicions that sleep-deprived infant feeding is a significant problem. A subsidized loaner program for those families that can’t afford a smartwatch is an option that should be considered.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a recent survey of 1259 mothers published in the journal Pediatrics, 28% reported they had fallen asleep while feeding their babies, and 83% of those mothers reported that the sleep was unplanned. Although the study sample was small, the investigators found that sociodemographic factors did not increase the odds that a mother would fall asleep while feeding.
These numbers are not surprising, but nonetheless they are concerning because co-sleeping is a known risk factor for sudden unexplained infant death (SUID). Every parent will tell you during the first 6 months of their adventure in parenting they didn’t get enough sleep. In fact some will tell you that sleep deprivation was their chronic state for the child’s first year.
Falling asleep easily at times and places not intended for sleep is the primary symptom of sleep deprivation. SUID is the most tragic event associated with parental sleep deprivation, but it is certainly not the only one. Overtired parents are more likely to be involved in accidents and are more likely to make poor decisions, particularly those regarding how to respond to a crying or misbehaving child.
The investigators found that 24% of mothers who reported that their usual nighttime feeding location was a chair or sofa (14%). Not surprisingly, mothers who fed in chairs were less likely to fall asleep while feeding. Many of these mothers reported that they chose the chair because they thought they would be less likely to fall asleep and/or disturb other family members. One wonders how we should interpret these numbers in light of other research that has found it is “relatively less hazardous to fall asleep with an infant in the adult bed than on a chair or sofa.” Had these chair feeding mothers made the better choice under the circumstances? It would take a much larger and more granular study to answer that question.
Mothers who exclusively breastfed were more likely to fall asleep feeding than were those who partially breastfed or used formula. The investigators postulated that the infants of mothers who exclusively breastfed may have required more feedings because breast milk is more easily and quickly digested. I know this is a common explanation, but in my experience I have found that exclusively breastfed infants often use nursing as pacification and a sleep trigger and spend more time at the breast regardless of how quickly they emptied their stomachs.
This study also examined the effect of repeated educational interventions and support and found that mothers who received an intervention based on safe sleep practices were less likely to fall asleep while feeding than were the mothers who had received the intervention focused on exclusive breastfeeding value and barriers to its adoption.
Education is one avenue, particularly when it includes the mother’s partner who can play an important role as standby lifeguard to make sure the mother doesn’t fall asleep. Obviously, this is easier said than done because when there is a new baby in the house sleep deprivation is usually a shared experience.
Although I believe that my family is on the verge of gifting me a smartwatch to protect me from my own misadventures, I don’t have any personal experience with these wonders of modern technology. However, I suspect with very little tweaking a wearable sensor could be easily programmed to detect when a mother is beginning to fall asleep while she is feeding her infant. A smartwatch would be an expensive intervention and is unlikely to filter down to economically challenged families. On the other hand, this paper has reinforced our suspicions that sleep-deprived infant feeding is a significant problem. A subsidized loaner program for those families that can’t afford a smartwatch is an option that should be considered.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a recent survey of 1259 mothers published in the journal Pediatrics, 28% reported they had fallen asleep while feeding their babies, and 83% of those mothers reported that the sleep was unplanned. Although the study sample was small, the investigators found that sociodemographic factors did not increase the odds that a mother would fall asleep while feeding.
These numbers are not surprising, but nonetheless they are concerning because co-sleeping is a known risk factor for sudden unexplained infant death (SUID). Every parent will tell you during the first 6 months of their adventure in parenting they didn’t get enough sleep. In fact some will tell you that sleep deprivation was their chronic state for the child’s first year.
Falling asleep easily at times and places not intended for sleep is the primary symptom of sleep deprivation. SUID is the most tragic event associated with parental sleep deprivation, but it is certainly not the only one. Overtired parents are more likely to be involved in accidents and are more likely to make poor decisions, particularly those regarding how to respond to a crying or misbehaving child.
The investigators found that 24% of mothers who reported that their usual nighttime feeding location was a chair or sofa (14%). Not surprisingly, mothers who fed in chairs were less likely to fall asleep while feeding. Many of these mothers reported that they chose the chair because they thought they would be less likely to fall asleep and/or disturb other family members. One wonders how we should interpret these numbers in light of other research that has found it is “relatively less hazardous to fall asleep with an infant in the adult bed than on a chair or sofa.” Had these chair feeding mothers made the better choice under the circumstances? It would take a much larger and more granular study to answer that question.
Mothers who exclusively breastfed were more likely to fall asleep feeding than were those who partially breastfed or used formula. The investigators postulated that the infants of mothers who exclusively breastfed may have required more feedings because breast milk is more easily and quickly digested. I know this is a common explanation, but in my experience I have found that exclusively breastfed infants often use nursing as pacification and a sleep trigger and spend more time at the breast regardless of how quickly they emptied their stomachs.
This study also examined the effect of repeated educational interventions and support and found that mothers who received an intervention based on safe sleep practices were less likely to fall asleep while feeding than were the mothers who had received the intervention focused on exclusive breastfeeding value and barriers to its adoption.
Education is one avenue, particularly when it includes the mother’s partner who can play an important role as standby lifeguard to make sure the mother doesn’t fall asleep. Obviously, this is easier said than done because when there is a new baby in the house sleep deprivation is usually a shared experience.
Although I believe that my family is on the verge of gifting me a smartwatch to protect me from my own misadventures, I don’t have any personal experience with these wonders of modern technology. However, I suspect with very little tweaking a wearable sensor could be easily programmed to detect when a mother is beginning to fall asleep while she is feeding her infant. A smartwatch would be an expensive intervention and is unlikely to filter down to economically challenged families. On the other hand, this paper has reinforced our suspicions that sleep-deprived infant feeding is a significant problem. A subsidized loaner program for those families that can’t afford a smartwatch is an option that should be considered.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
What To Do With Lipoprotein(a)?
Case: 45-year-old woman comes to clinic and requests lipoprotein(a) [Lp(a)] testing. She has a family history of early coronary disease (mother age 50, sister age 48) and has hypertension with home blood pressure readings of 130-140/70-75. She had a lipid panel checked last year which showed a total cholesterol of 210 mg/dL, LDL 145 mg/dL, HDL 45 mg/dL, and triglycerides of 100 mg/dL. She does not smoke and is currently taking irbesartan, chlorthalidone, sertraline, a multivitamin, and vitamin D.
What do you recommend?
There has been a great deal of media attention on testing for Lp(a). Many of my patients are requesting testing although many of them do not need it. This patient is an exception. I think Lp(a) testing would help inform her medical care. She has a family history of early coronary disease in her mother and sister, but her own lipid profile is not worrisome.
Her 10-year cardiovascular disease risk is 2%. The cardiac risk calculator does not incorporate family history; I think this is a situation where testing for Lp(a)(as well as apolipoprotein B) can be helpful. If her Lp(a) is elevated, it helps reassess her risk and that information would be helpful in targeting aggressive interventions for other CV risk factors, including optimal blood pressure control. In her case, pushing for a goal systolic blood pressure below 120 mm Hg and making sure she is doing regular exercise and eating a heart-healthy diet. The current consensus statement on Lp(a) recommends that patients with elevated levels have aggressive lifestyle and cardiovascular risk management.1
Currently, there are no medical treatments available for high Lp(a) for primary prevention. Apheresis has been approved by the US Food and Drug Administration (FDA) for patients with familial hyperlipidemia who have LDL ≥ 100 mg/dL, Lp(a) ≥ 60 mg/dL, and coronary or other artery disease.
PCSK9 inhibitors have shown a reduction in major cardiovascular events in patients who have established coronary artery disease and high Lp(a) levels, albeit with limited data. Unlike statins, which increase Lp(a) levels, PCSK9 inhibitors reduce Lp(a) levels.2 There are promising early results in a phase 2 trial of the oral drug muvalaplin lowering Lp(a) levels by up to 85% for the highest dose, but there are no peer-reviewed articles confirming these results and no outcome trials at this time.
In patients who are already recognized as high risk, especially those with established coronary artery disease, measuring Lp(a) levels offer little benefit. These patients should already be receiving aggressive medical therapy to reach blood pressure targets if hypertensive, maximal lifestyle modifications, and statin therapy.
If these patients need more therapy because of continued coronary events, despite maximal conventional medical therapy, then adding a PCSK9 inhibitor would be appropriate whether or not a patient has a high Lp(a) level. Once Lp(a) targeted therapies are available and show clinical benefit, then the role of Lp(a) measurement and treatment in this population will be clearer.
Pearl: Most patients do not need Lp(a) testing. There are no FDA-approved treatments for high Lp(a) levels.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Kronenberg F et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-46.
2. Ruscica M et al. Lipoprotein(a) and PCSK9 inhibition: Clinical evidence Eur Heart J Suppl 2020;Nov 18(Suppl L):L53–L56.
Case: 45-year-old woman comes to clinic and requests lipoprotein(a) [Lp(a)] testing. She has a family history of early coronary disease (mother age 50, sister age 48) and has hypertension with home blood pressure readings of 130-140/70-75. She had a lipid panel checked last year which showed a total cholesterol of 210 mg/dL, LDL 145 mg/dL, HDL 45 mg/dL, and triglycerides of 100 mg/dL. She does not smoke and is currently taking irbesartan, chlorthalidone, sertraline, a multivitamin, and vitamin D.
What do you recommend?
There has been a great deal of media attention on testing for Lp(a). Many of my patients are requesting testing although many of them do not need it. This patient is an exception. I think Lp(a) testing would help inform her medical care. She has a family history of early coronary disease in her mother and sister, but her own lipid profile is not worrisome.
Her 10-year cardiovascular disease risk is 2%. The cardiac risk calculator does not incorporate family history; I think this is a situation where testing for Lp(a)(as well as apolipoprotein B) can be helpful. If her Lp(a) is elevated, it helps reassess her risk and that information would be helpful in targeting aggressive interventions for other CV risk factors, including optimal blood pressure control. In her case, pushing for a goal systolic blood pressure below 120 mm Hg and making sure she is doing regular exercise and eating a heart-healthy diet. The current consensus statement on Lp(a) recommends that patients with elevated levels have aggressive lifestyle and cardiovascular risk management.1
Currently, there are no medical treatments available for high Lp(a) for primary prevention. Apheresis has been approved by the US Food and Drug Administration (FDA) for patients with familial hyperlipidemia who have LDL ≥ 100 mg/dL, Lp(a) ≥ 60 mg/dL, and coronary or other artery disease.
PCSK9 inhibitors have shown a reduction in major cardiovascular events in patients who have established coronary artery disease and high Lp(a) levels, albeit with limited data. Unlike statins, which increase Lp(a) levels, PCSK9 inhibitors reduce Lp(a) levels.2 There are promising early results in a phase 2 trial of the oral drug muvalaplin lowering Lp(a) levels by up to 85% for the highest dose, but there are no peer-reviewed articles confirming these results and no outcome trials at this time.
In patients who are already recognized as high risk, especially those with established coronary artery disease, measuring Lp(a) levels offer little benefit. These patients should already be receiving aggressive medical therapy to reach blood pressure targets if hypertensive, maximal lifestyle modifications, and statin therapy.
If these patients need more therapy because of continued coronary events, despite maximal conventional medical therapy, then adding a PCSK9 inhibitor would be appropriate whether or not a patient has a high Lp(a) level. Once Lp(a) targeted therapies are available and show clinical benefit, then the role of Lp(a) measurement and treatment in this population will be clearer.
Pearl: Most patients do not need Lp(a) testing. There are no FDA-approved treatments for high Lp(a) levels.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Kronenberg F et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-46.
2. Ruscica M et al. Lipoprotein(a) and PCSK9 inhibition: Clinical evidence Eur Heart J Suppl 2020;Nov 18(Suppl L):L53–L56.
Case: 45-year-old woman comes to clinic and requests lipoprotein(a) [Lp(a)] testing. She has a family history of early coronary disease (mother age 50, sister age 48) and has hypertension with home blood pressure readings of 130-140/70-75. She had a lipid panel checked last year which showed a total cholesterol of 210 mg/dL, LDL 145 mg/dL, HDL 45 mg/dL, and triglycerides of 100 mg/dL. She does not smoke and is currently taking irbesartan, chlorthalidone, sertraline, a multivitamin, and vitamin D.
What do you recommend?
There has been a great deal of media attention on testing for Lp(a). Many of my patients are requesting testing although many of them do not need it. This patient is an exception. I think Lp(a) testing would help inform her medical care. She has a family history of early coronary disease in her mother and sister, but her own lipid profile is not worrisome.
Her 10-year cardiovascular disease risk is 2%. The cardiac risk calculator does not incorporate family history; I think this is a situation where testing for Lp(a)(as well as apolipoprotein B) can be helpful. If her Lp(a) is elevated, it helps reassess her risk and that information would be helpful in targeting aggressive interventions for other CV risk factors, including optimal blood pressure control. In her case, pushing for a goal systolic blood pressure below 120 mm Hg and making sure she is doing regular exercise and eating a heart-healthy diet. The current consensus statement on Lp(a) recommends that patients with elevated levels have aggressive lifestyle and cardiovascular risk management.1
Currently, there are no medical treatments available for high Lp(a) for primary prevention. Apheresis has been approved by the US Food and Drug Administration (FDA) for patients with familial hyperlipidemia who have LDL ≥ 100 mg/dL, Lp(a) ≥ 60 mg/dL, and coronary or other artery disease.
PCSK9 inhibitors have shown a reduction in major cardiovascular events in patients who have established coronary artery disease and high Lp(a) levels, albeit with limited data. Unlike statins, which increase Lp(a) levels, PCSK9 inhibitors reduce Lp(a) levels.2 There are promising early results in a phase 2 trial of the oral drug muvalaplin lowering Lp(a) levels by up to 85% for the highest dose, but there are no peer-reviewed articles confirming these results and no outcome trials at this time.
In patients who are already recognized as high risk, especially those with established coronary artery disease, measuring Lp(a) levels offer little benefit. These patients should already be receiving aggressive medical therapy to reach blood pressure targets if hypertensive, maximal lifestyle modifications, and statin therapy.
If these patients need more therapy because of continued coronary events, despite maximal conventional medical therapy, then adding a PCSK9 inhibitor would be appropriate whether or not a patient has a high Lp(a) level. Once Lp(a) targeted therapies are available and show clinical benefit, then the role of Lp(a) measurement and treatment in this population will be clearer.
Pearl: Most patients do not need Lp(a) testing. There are no FDA-approved treatments for high Lp(a) levels.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Kronenberg F et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-46.
2. Ruscica M et al. Lipoprotein(a) and PCSK9 inhibition: Clinical evidence Eur Heart J Suppl 2020;Nov 18(Suppl L):L53–L56.
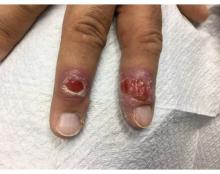
A 58-year-old White male presented with lesions on his index and middle finger for 3 months
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
A 58-year-old White male with no significant past medical history presented with lesions on his right index and middle fingers, which had been present for 3 months. The lesions were painless. The patient has a history of hand dermatitis. Upon questioning, the patient said he had not fished or cleaned fish tanks. He did garden occasionally (no roses). He has been using Neosporin on the lesions. He denied any fever or systemic symptoms and had no lymphadenopathy.
What's your diagnosis?
ASH 2024 Myeloma Studies: My Top 10 Picks
First, let me place my selected studies in context by acknowledging my biases. As a clinician, I’m prone to choosing clinical rather than basic science or translational work — even if translational work might well end up exerting a pivotal impact on practice in the future. And now — in no particular order — here are my picks:
IFM 2017-03 Phase 3 Study
Frail patients are underrepresented in most myeloma studies, yet in this randomized trial for newly diagnosed myeloma, exclusively frail patients were enrolled. The trial compared daratumumab/lenalidomide to lenalidomide/dexamethasone, and the most recent follow/up shows a progression-free survival (PFS) (48.5 vs 21 months) and overall survival (OS) (not reached vs 36 months) benefit to daratumumab/lenalidomide. What I see in practice is that anti-CD38 monoclonal antibodies are the best-tolerated drugs in this space and should be the backbone of any regimen for frail patients. Steroids should be omitted as early as possible. Future trials may optimize what to give in addition to the anti-CD38 therapy, and how to adapt/escalate therapy to frailty and clinical status, as lenalidomide remains difficult for such patients to tolerate.
AQUILA Study
This is a randomized, phase 3 comparison of daratumumab to observation for patients with smoldering myeloma. The endpoint was PFS. For context, similar studies done in asymptomatic CLL have shown improved PFS, but not OS, and the authors of such studies have concluded that improvement in PFS alone should not justify a change in approach.
This study shows that daratumumab can improve laboratory markers and reduce progression (60-month PFS rates of 63.1% for daratumumab vs 40.8% with observation). However, several important caveats remain. The protocol only mandated spine/pelvis MRI imaging, not whole-body MRI imaging, and such imaging was only performed once a year, which may not be frequent enough to catch lesions at an earlier stage. These details have important implications, as previous research shows that up to half of lesions can be missed by doing only a spine MRI, as opposed to a whole-body MRI.
All of this means that had more comprehensive imaging been done, many more patients may have been diagnosed with myeloma. Such patients may have been undertreated, and single agent daratumumab, with a response rate of just 63%, may not have been enough. Conversely, some patients may also have been overtreated using this approach, as the protocol allowed patients who had been diagnosed with smoldering myeloma up to 5 years earlier to be enrolled. Many of these people could have had indolent disease for years prior to enrollment and may not have ever progressed.
Further information is needed to help us understand this study better. What was the nature of the progression events: asymptomatic lab changes or morbid end organ damage? Was daratumumab given when patients in the control arm progressed to myeloma? My concern is that if patients in the control arm do not universally receive modern daratumumab-containing therapies when they develop myeloma, then an overall survival advantage may be shown simply because patients in the intervention arm are getting a good drug earlier in the disease, while those in the control arm are not getting a good drug at all. Nevertheless, despite these limitations, it is likely this trial will lead to regulatory approval of daratumumab in this space, and lots of discussions from patients and clinicians alike.
Extended Follow-Up of Anito-Cel in its First In-Human study
Two chimeric antigen receptor therapy (CAR-T) products are currently approved for myeloma. Cilta-cel is clearly effective but is associated with problematic late-onset neurological toxicities. Ide-cel appears much less effective. There is clearly a need for a product that is both effective and safe.
Anito-cel has two relevant abstracts this meeting that show much promise. Extended follow-up of anito-cel from its first in human study shows a promising 27-month PFS of 52%, and with no cases of delayed neurotoxicity. I also eagerly await further information from the registrational single-arm study of anti-cel being presented at ASH 2024, which should (hopefully) lead to its accelerated approval.
Screening for Myeloma for all People With Vertebral Fractures Likely Unnecessary
This elegant study of over 9,000 people with vertebral fractures shows that absolute risks for myeloma were 0.43% and 0.63% in women and men with grade 2-3 fractures, respectively, indicating that there is likely little benefit in evaluating asymptomatic individuals with incidentally discovered vertebral fractures for myeloma, unless other features are present. Spread the word.
Further Information on Cevostamab, Another Bispecific Option
We do need effective treatments for targets beyond just BCMA and GPRC5D. Cevostamab, a bispecific targeting FCRH5, represents another option, with updated data on 167 patients. With an overall response rate of 43% (duration of response, 10 months), and a response rate of about 30% in those with prior bispecific exposure, this data helps us contextualize expected benefits as we look forward to the eventual approval of this drug. The efficacy is relatively modest in those who have already progressed on bispecifics, but cevostamab would still be a welcome addition to our arsenal.
Is GPRC5D a Better Target for Car T Rather Than Bispecifics?
Our currently available GPRC5D bispecific (talquetamab) leads to high rates of skin, oral, and nail toxicity. This drug can also bring significant weight loss. These side effects make me consider that continuous targeting of GPRC5D through a bispecific may not be ideal, and that GPRC5D may be better as a one-time CAR T target. At ASH 2024, we will have 15-month follow-up data from BMS-986393, a GPRC5D CAR T. Response rates for this heavily pretreated population (76% of whom had triple refractory disease) were at 87%, with a median PFS of 14.5 months. Only 6% of patients experienced treatment-related weight loss, and nail (19%), skin (30%), and oral (31%) toxicities were relatively low. I look forward to updated data, as well as data on the resolution of the toxicities seen.
Daratumumab, a Game-Changer for AL Amyloidosis
A truly effective drug given early can change the natural history of disease, even if patients in the control arm only receive the drug later. A case in point is daratumumab. The 5-year survival rate was 76.1% for the daratumumab/cyclophosphamide/bortezomib/dexamethasone arm and 64.7% for cyclophosphamide/bortezomib/dexamethasone arm. This happened despite the fact that 67% of the control arm patients (among those who received therapy) went on to receive daratumumab later in the disease course.
Understanding how SMM Progresses to MM
We often hear that we should treat SMM and not just watch carefully because fractures may suddenly happen, or a patient may end up on dialysis. What this retrospective study tells us that amongst 427 patients with SMM, 42 had progression to myeloma, and amongst those 42, only 1 developed renal dysfunction (unclear if this resolved), and only 1 had lytic lesions that were symptomatic. The remainder were all asymptomatic lab and imaging changes. This is a retrospective study, and one should assume that follow-up was thus highly variable. It does not appear that diffusion weighted whole-body MRI imaging (our most sensitive imaging test) was employed universally or very frequently. Nevertheless, these powerful findings reassure us that, with close observation, morbidity is unlikely. Our group has designed a prospective study incorporating frequent diffusion weighted whole body MRI imaging to formally test this hypothesis (SPOTLIGHT, NCT06212323).
The Underperformance of Daratumumab/Lenalidomide/Dexamethasone in the Real World
At every major meeting I am reminded of the disconnect between real-world efficacy and clinical trial efficacy. Case in point: In this Austrian experience, daratumumab/lenalidomide/dexamethasone led to a PFS of 22.7 months vs 61.9 months in the MAIA trial of daratumumab/lenalidomide/dexamethasone. Such a sobering difference! And if you think this is an isolated experience, even in a US real-world cohort, consider that in a recently published comparative study dara/len/dex underperformed, although the time to next treatment or death was longer (37.8 months).
Delayed Neurotoxicity may not be Just a Consequence of high tumor burden
We currently think that some of the scariest side effects of cilta-cel, such as delayed neurotoxicity, may be a consequence of a high number of cancer cells and may be prevented by better disease control at the time of infusion. This study, a sobering analysis of 52 patients with delayed neurotoxicity occurring after CAR T, included 8 patients (15%) who were not heavily pretreated, and all had less than 5% plasma cells at the time of infusion. None of these patients had extramedullary disease. This result worries me, especially because cilta-cel is being studied and is poised for future approval in earlier line settings. It suggests that this toxicity may not always be a product of disease burden, contrary to our current belief.
I will be paying close attention to these 10 myeloma studies at ASH 2024, where I look forward to meeting you and learning more.Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
First, let me place my selected studies in context by acknowledging my biases. As a clinician, I’m prone to choosing clinical rather than basic science or translational work — even if translational work might well end up exerting a pivotal impact on practice in the future. And now — in no particular order — here are my picks:
IFM 2017-03 Phase 3 Study
Frail patients are underrepresented in most myeloma studies, yet in this randomized trial for newly diagnosed myeloma, exclusively frail patients were enrolled. The trial compared daratumumab/lenalidomide to lenalidomide/dexamethasone, and the most recent follow/up shows a progression-free survival (PFS) (48.5 vs 21 months) and overall survival (OS) (not reached vs 36 months) benefit to daratumumab/lenalidomide. What I see in practice is that anti-CD38 monoclonal antibodies are the best-tolerated drugs in this space and should be the backbone of any regimen for frail patients. Steroids should be omitted as early as possible. Future trials may optimize what to give in addition to the anti-CD38 therapy, and how to adapt/escalate therapy to frailty and clinical status, as lenalidomide remains difficult for such patients to tolerate.
AQUILA Study
This is a randomized, phase 3 comparison of daratumumab to observation for patients with smoldering myeloma. The endpoint was PFS. For context, similar studies done in asymptomatic CLL have shown improved PFS, but not OS, and the authors of such studies have concluded that improvement in PFS alone should not justify a change in approach.
This study shows that daratumumab can improve laboratory markers and reduce progression (60-month PFS rates of 63.1% for daratumumab vs 40.8% with observation). However, several important caveats remain. The protocol only mandated spine/pelvis MRI imaging, not whole-body MRI imaging, and such imaging was only performed once a year, which may not be frequent enough to catch lesions at an earlier stage. These details have important implications, as previous research shows that up to half of lesions can be missed by doing only a spine MRI, as opposed to a whole-body MRI.
All of this means that had more comprehensive imaging been done, many more patients may have been diagnosed with myeloma. Such patients may have been undertreated, and single agent daratumumab, with a response rate of just 63%, may not have been enough. Conversely, some patients may also have been overtreated using this approach, as the protocol allowed patients who had been diagnosed with smoldering myeloma up to 5 years earlier to be enrolled. Many of these people could have had indolent disease for years prior to enrollment and may not have ever progressed.
Further information is needed to help us understand this study better. What was the nature of the progression events: asymptomatic lab changes or morbid end organ damage? Was daratumumab given when patients in the control arm progressed to myeloma? My concern is that if patients in the control arm do not universally receive modern daratumumab-containing therapies when they develop myeloma, then an overall survival advantage may be shown simply because patients in the intervention arm are getting a good drug earlier in the disease, while those in the control arm are not getting a good drug at all. Nevertheless, despite these limitations, it is likely this trial will lead to regulatory approval of daratumumab in this space, and lots of discussions from patients and clinicians alike.
Extended Follow-Up of Anito-Cel in its First In-Human study
Two chimeric antigen receptor therapy (CAR-T) products are currently approved for myeloma. Cilta-cel is clearly effective but is associated with problematic late-onset neurological toxicities. Ide-cel appears much less effective. There is clearly a need for a product that is both effective and safe.
Anito-cel has two relevant abstracts this meeting that show much promise. Extended follow-up of anito-cel from its first in human study shows a promising 27-month PFS of 52%, and with no cases of delayed neurotoxicity. I also eagerly await further information from the registrational single-arm study of anti-cel being presented at ASH 2024, which should (hopefully) lead to its accelerated approval.
Screening for Myeloma for all People With Vertebral Fractures Likely Unnecessary
This elegant study of over 9,000 people with vertebral fractures shows that absolute risks for myeloma were 0.43% and 0.63% in women and men with grade 2-3 fractures, respectively, indicating that there is likely little benefit in evaluating asymptomatic individuals with incidentally discovered vertebral fractures for myeloma, unless other features are present. Spread the word.
Further Information on Cevostamab, Another Bispecific Option
We do need effective treatments for targets beyond just BCMA and GPRC5D. Cevostamab, a bispecific targeting FCRH5, represents another option, with updated data on 167 patients. With an overall response rate of 43% (duration of response, 10 months), and a response rate of about 30% in those with prior bispecific exposure, this data helps us contextualize expected benefits as we look forward to the eventual approval of this drug. The efficacy is relatively modest in those who have already progressed on bispecifics, but cevostamab would still be a welcome addition to our arsenal.
Is GPRC5D a Better Target for Car T Rather Than Bispecifics?
Our currently available GPRC5D bispecific (talquetamab) leads to high rates of skin, oral, and nail toxicity. This drug can also bring significant weight loss. These side effects make me consider that continuous targeting of GPRC5D through a bispecific may not be ideal, and that GPRC5D may be better as a one-time CAR T target. At ASH 2024, we will have 15-month follow-up data from BMS-986393, a GPRC5D CAR T. Response rates for this heavily pretreated population (76% of whom had triple refractory disease) were at 87%, with a median PFS of 14.5 months. Only 6% of patients experienced treatment-related weight loss, and nail (19%), skin (30%), and oral (31%) toxicities were relatively low. I look forward to updated data, as well as data on the resolution of the toxicities seen.
Daratumumab, a Game-Changer for AL Amyloidosis
A truly effective drug given early can change the natural history of disease, even if patients in the control arm only receive the drug later. A case in point is daratumumab. The 5-year survival rate was 76.1% for the daratumumab/cyclophosphamide/bortezomib/dexamethasone arm and 64.7% for cyclophosphamide/bortezomib/dexamethasone arm. This happened despite the fact that 67% of the control arm patients (among those who received therapy) went on to receive daratumumab later in the disease course.
Understanding how SMM Progresses to MM
We often hear that we should treat SMM and not just watch carefully because fractures may suddenly happen, or a patient may end up on dialysis. What this retrospective study tells us that amongst 427 patients with SMM, 42 had progression to myeloma, and amongst those 42, only 1 developed renal dysfunction (unclear if this resolved), and only 1 had lytic lesions that were symptomatic. The remainder were all asymptomatic lab and imaging changes. This is a retrospective study, and one should assume that follow-up was thus highly variable. It does not appear that diffusion weighted whole-body MRI imaging (our most sensitive imaging test) was employed universally or very frequently. Nevertheless, these powerful findings reassure us that, with close observation, morbidity is unlikely. Our group has designed a prospective study incorporating frequent diffusion weighted whole body MRI imaging to formally test this hypothesis (SPOTLIGHT, NCT06212323).
The Underperformance of Daratumumab/Lenalidomide/Dexamethasone in the Real World
At every major meeting I am reminded of the disconnect between real-world efficacy and clinical trial efficacy. Case in point: In this Austrian experience, daratumumab/lenalidomide/dexamethasone led to a PFS of 22.7 months vs 61.9 months in the MAIA trial of daratumumab/lenalidomide/dexamethasone. Such a sobering difference! And if you think this is an isolated experience, even in a US real-world cohort, consider that in a recently published comparative study dara/len/dex underperformed, although the time to next treatment or death was longer (37.8 months).
Delayed Neurotoxicity may not be Just a Consequence of high tumor burden
We currently think that some of the scariest side effects of cilta-cel, such as delayed neurotoxicity, may be a consequence of a high number of cancer cells and may be prevented by better disease control at the time of infusion. This study, a sobering analysis of 52 patients with delayed neurotoxicity occurring after CAR T, included 8 patients (15%) who were not heavily pretreated, and all had less than 5% plasma cells at the time of infusion. None of these patients had extramedullary disease. This result worries me, especially because cilta-cel is being studied and is poised for future approval in earlier line settings. It suggests that this toxicity may not always be a product of disease burden, contrary to our current belief.
I will be paying close attention to these 10 myeloma studies at ASH 2024, where I look forward to meeting you and learning more.Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
First, let me place my selected studies in context by acknowledging my biases. As a clinician, I’m prone to choosing clinical rather than basic science or translational work — even if translational work might well end up exerting a pivotal impact on practice in the future. And now — in no particular order — here are my picks:
IFM 2017-03 Phase 3 Study
Frail patients are underrepresented in most myeloma studies, yet in this randomized trial for newly diagnosed myeloma, exclusively frail patients were enrolled. The trial compared daratumumab/lenalidomide to lenalidomide/dexamethasone, and the most recent follow/up shows a progression-free survival (PFS) (48.5 vs 21 months) and overall survival (OS) (not reached vs 36 months) benefit to daratumumab/lenalidomide. What I see in practice is that anti-CD38 monoclonal antibodies are the best-tolerated drugs in this space and should be the backbone of any regimen for frail patients. Steroids should be omitted as early as possible. Future trials may optimize what to give in addition to the anti-CD38 therapy, and how to adapt/escalate therapy to frailty and clinical status, as lenalidomide remains difficult for such patients to tolerate.
AQUILA Study
This is a randomized, phase 3 comparison of daratumumab to observation for patients with smoldering myeloma. The endpoint was PFS. For context, similar studies done in asymptomatic CLL have shown improved PFS, but not OS, and the authors of such studies have concluded that improvement in PFS alone should not justify a change in approach.
This study shows that daratumumab can improve laboratory markers and reduce progression (60-month PFS rates of 63.1% for daratumumab vs 40.8% with observation). However, several important caveats remain. The protocol only mandated spine/pelvis MRI imaging, not whole-body MRI imaging, and such imaging was only performed once a year, which may not be frequent enough to catch lesions at an earlier stage. These details have important implications, as previous research shows that up to half of lesions can be missed by doing only a spine MRI, as opposed to a whole-body MRI.
All of this means that had more comprehensive imaging been done, many more patients may have been diagnosed with myeloma. Such patients may have been undertreated, and single agent daratumumab, with a response rate of just 63%, may not have been enough. Conversely, some patients may also have been overtreated using this approach, as the protocol allowed patients who had been diagnosed with smoldering myeloma up to 5 years earlier to be enrolled. Many of these people could have had indolent disease for years prior to enrollment and may not have ever progressed.
Further information is needed to help us understand this study better. What was the nature of the progression events: asymptomatic lab changes or morbid end organ damage? Was daratumumab given when patients in the control arm progressed to myeloma? My concern is that if patients in the control arm do not universally receive modern daratumumab-containing therapies when they develop myeloma, then an overall survival advantage may be shown simply because patients in the intervention arm are getting a good drug earlier in the disease, while those in the control arm are not getting a good drug at all. Nevertheless, despite these limitations, it is likely this trial will lead to regulatory approval of daratumumab in this space, and lots of discussions from patients and clinicians alike.
Extended Follow-Up of Anito-Cel in its First In-Human study
Two chimeric antigen receptor therapy (CAR-T) products are currently approved for myeloma. Cilta-cel is clearly effective but is associated with problematic late-onset neurological toxicities. Ide-cel appears much less effective. There is clearly a need for a product that is both effective and safe.
Anito-cel has two relevant abstracts this meeting that show much promise. Extended follow-up of anito-cel from its first in human study shows a promising 27-month PFS of 52%, and with no cases of delayed neurotoxicity. I also eagerly await further information from the registrational single-arm study of anti-cel being presented at ASH 2024, which should (hopefully) lead to its accelerated approval.
Screening for Myeloma for all People With Vertebral Fractures Likely Unnecessary
This elegant study of over 9,000 people with vertebral fractures shows that absolute risks for myeloma were 0.43% and 0.63% in women and men with grade 2-3 fractures, respectively, indicating that there is likely little benefit in evaluating asymptomatic individuals with incidentally discovered vertebral fractures for myeloma, unless other features are present. Spread the word.
Further Information on Cevostamab, Another Bispecific Option
We do need effective treatments for targets beyond just BCMA and GPRC5D. Cevostamab, a bispecific targeting FCRH5, represents another option, with updated data on 167 patients. With an overall response rate of 43% (duration of response, 10 months), and a response rate of about 30% in those with prior bispecific exposure, this data helps us contextualize expected benefits as we look forward to the eventual approval of this drug. The efficacy is relatively modest in those who have already progressed on bispecifics, but cevostamab would still be a welcome addition to our arsenal.
Is GPRC5D a Better Target for Car T Rather Than Bispecifics?
Our currently available GPRC5D bispecific (talquetamab) leads to high rates of skin, oral, and nail toxicity. This drug can also bring significant weight loss. These side effects make me consider that continuous targeting of GPRC5D through a bispecific may not be ideal, and that GPRC5D may be better as a one-time CAR T target. At ASH 2024, we will have 15-month follow-up data from BMS-986393, a GPRC5D CAR T. Response rates for this heavily pretreated population (76% of whom had triple refractory disease) were at 87%, with a median PFS of 14.5 months. Only 6% of patients experienced treatment-related weight loss, and nail (19%), skin (30%), and oral (31%) toxicities were relatively low. I look forward to updated data, as well as data on the resolution of the toxicities seen.
Daratumumab, a Game-Changer for AL Amyloidosis
A truly effective drug given early can change the natural history of disease, even if patients in the control arm only receive the drug later. A case in point is daratumumab. The 5-year survival rate was 76.1% for the daratumumab/cyclophosphamide/bortezomib/dexamethasone arm and 64.7% for cyclophosphamide/bortezomib/dexamethasone arm. This happened despite the fact that 67% of the control arm patients (among those who received therapy) went on to receive daratumumab later in the disease course.
Understanding how SMM Progresses to MM
We often hear that we should treat SMM and not just watch carefully because fractures may suddenly happen, or a patient may end up on dialysis. What this retrospective study tells us that amongst 427 patients with SMM, 42 had progression to myeloma, and amongst those 42, only 1 developed renal dysfunction (unclear if this resolved), and only 1 had lytic lesions that were symptomatic. The remainder were all asymptomatic lab and imaging changes. This is a retrospective study, and one should assume that follow-up was thus highly variable. It does not appear that diffusion weighted whole-body MRI imaging (our most sensitive imaging test) was employed universally or very frequently. Nevertheless, these powerful findings reassure us that, with close observation, morbidity is unlikely. Our group has designed a prospective study incorporating frequent diffusion weighted whole body MRI imaging to formally test this hypothesis (SPOTLIGHT, NCT06212323).
The Underperformance of Daratumumab/Lenalidomide/Dexamethasone in the Real World
At every major meeting I am reminded of the disconnect between real-world efficacy and clinical trial efficacy. Case in point: In this Austrian experience, daratumumab/lenalidomide/dexamethasone led to a PFS of 22.7 months vs 61.9 months in the MAIA trial of daratumumab/lenalidomide/dexamethasone. Such a sobering difference! And if you think this is an isolated experience, even in a US real-world cohort, consider that in a recently published comparative study dara/len/dex underperformed, although the time to next treatment or death was longer (37.8 months).
Delayed Neurotoxicity may not be Just a Consequence of high tumor burden
We currently think that some of the scariest side effects of cilta-cel, such as delayed neurotoxicity, may be a consequence of a high number of cancer cells and may be prevented by better disease control at the time of infusion. This study, a sobering analysis of 52 patients with delayed neurotoxicity occurring after CAR T, included 8 patients (15%) who were not heavily pretreated, and all had less than 5% plasma cells at the time of infusion. None of these patients had extramedullary disease. This result worries me, especially because cilta-cel is being studied and is poised for future approval in earlier line settings. It suggests that this toxicity may not always be a product of disease burden, contrary to our current belief.
I will be paying close attention to these 10 myeloma studies at ASH 2024, where I look forward to meeting you and learning more.Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
Recognizing Burnout: Why Physicians Often Miss the Signs in Themselves
Summary and Key Highlights
Summary: This section explores why physicians often struggle to recognize burnout within themselves, partly due to stigma and a tendency to focus on productivity over well-being. Dr. Tyra Fainstad shares personal experiences of burnout symptoms, emphasizing the importance of awareness and self-reflection. Recognizing and addressing burnout early can help physicians find healthier coping strategies, avoid productivity traps, and seek support.
Key Takeaways:
- Many physicians struggle to identify burnout due to stigma and self-blame.
- Awareness of burnout symptoms is essential for early intervention and healthy coping.
- Seeking support can prevent burnout from worsening and improve quality of life.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: This section explores why physicians often struggle to recognize burnout within themselves, partly due to stigma and a tendency to focus on productivity over well-being. Dr. Tyra Fainstad shares personal experiences of burnout symptoms, emphasizing the importance of awareness and self-reflection. Recognizing and addressing burnout early can help physicians find healthier coping strategies, avoid productivity traps, and seek support.
Key Takeaways:
- Many physicians struggle to identify burnout due to stigma and self-blame.
- Awareness of burnout symptoms is essential for early intervention and healthy coping.
- Seeking support can prevent burnout from worsening and improve quality of life.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: This section explores why physicians often struggle to recognize burnout within themselves, partly due to stigma and a tendency to focus on productivity over well-being. Dr. Tyra Fainstad shares personal experiences of burnout symptoms, emphasizing the importance of awareness and self-reflection. Recognizing and addressing burnout early can help physicians find healthier coping strategies, avoid productivity traps, and seek support.
Key Takeaways:
- Many physicians struggle to identify burnout due to stigma and self-blame.
- Awareness of burnout symptoms is essential for early intervention and healthy coping.
- Seeking support can prevent burnout from worsening and improve quality of life.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Breaking the Cycle: Why Self-Compassion Is Essential for Today’s Physicians
Summary and Key Highlights
Summary: Dr Tyra Fainstad explores the ingrained culture in medicine that encourages self-criticism, with many physicians feeling that they must be hard on themselves to succeed. Dr Fainstad challenges this belief, advocating for self-compassion as a healthier alternative. The evolving medical field now includes physicians who prioritize well-being without sacrificing quality of care, underscoring the importance of self-kindness for sustainable practice.
Key Takeaways:
- Many physicians believe that self-criticism is necessary for success, a mindset rooted in medical culture.
- Practicing self-compassion can improve long-term resilience and prevent burnout.
- The changing landscape of healthcare supports a more balanced approach to physician well-being.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad explores the ingrained culture in medicine that encourages self-criticism, with many physicians feeling that they must be hard on themselves to succeed. Dr Fainstad challenges this belief, advocating for self-compassion as a healthier alternative. The evolving medical field now includes physicians who prioritize well-being without sacrificing quality of care, underscoring the importance of self-kindness for sustainable practice.
Key Takeaways:
- Many physicians believe that self-criticism is necessary for success, a mindset rooted in medical culture.
- Practicing self-compassion can improve long-term resilience and prevent burnout.
- The changing landscape of healthcare supports a more balanced approach to physician well-being.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad explores the ingrained culture in medicine that encourages self-criticism, with many physicians feeling that they must be hard on themselves to succeed. Dr Fainstad challenges this belief, advocating for self-compassion as a healthier alternative. The evolving medical field now includes physicians who prioritize well-being without sacrificing quality of care, underscoring the importance of self-kindness for sustainable practice.
Key Takeaways:
- Many physicians believe that self-criticism is necessary for success, a mindset rooted in medical culture.
- Practicing self-compassion can improve long-term resilience and prevent burnout.
- The changing landscape of healthcare supports a more balanced approach to physician well-being.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Finding Fulfillment Beyond Metrics: A Physician’s Path to Lasting Well-Being
Summary and Key Highlights
Summary: Dr Tyra Fainstad shares her personal experience with burnout and the journey to recovery through coaching and self-compassion. She describes the pressures of seeking validation through external achievements, which ultimately led to a crisis in self-worth after medical training. Through coaching, she learned to cultivate a sense of internal fulfillment, reconnecting with her passion for medicine and achieving a healthier balance.
Key Takeaways:
- Relying solely on external validation can deepen burnout and affect well-being.
- Coaching empowers physicians to develop self-compassion and sustainable coping strategies.
- Shifting from external to internal validation strengthens long-term fulfillment and job satisfaction.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad shares her personal experience with burnout and the journey to recovery through coaching and self-compassion. She describes the pressures of seeking validation through external achievements, which ultimately led to a crisis in self-worth after medical training. Through coaching, she learned to cultivate a sense of internal fulfillment, reconnecting with her passion for medicine and achieving a healthier balance.
Key Takeaways:
- Relying solely on external validation can deepen burnout and affect well-being.
- Coaching empowers physicians to develop self-compassion and sustainable coping strategies.
- Shifting from external to internal validation strengthens long-term fulfillment and job satisfaction.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad shares her personal experience with burnout and the journey to recovery through coaching and self-compassion. She describes the pressures of seeking validation through external achievements, which ultimately led to a crisis in self-worth after medical training. Through coaching, she learned to cultivate a sense of internal fulfillment, reconnecting with her passion for medicine and achieving a healthier balance.
Key Takeaways:
- Relying solely on external validation can deepen burnout and affect well-being.
- Coaching empowers physicians to develop self-compassion and sustainable coping strategies.
- Shifting from external to internal validation strengthens long-term fulfillment and job satisfaction.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
A version of this article first appeared on Medscape.com.
To Hold or Not to Hold GLP-1s Before Surgery
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
Negotiating for a Successful Career in Private Practice Gastroenterology
In this video, Aja McCutchen, MD, of Atlanta Gastroenterology Associates in Georgia, discusses why she chose to enter private practice gastroenterology, and identifies some key considerations on the road to a successful career.
Dr. McCutchen is vice chair of the AGA Research Foundation. She has no financial conflicts relative to the topics in this video.

In this video, Aja McCutchen, MD, of Atlanta Gastroenterology Associates in Georgia, discusses why she chose to enter private practice gastroenterology, and identifies some key considerations on the road to a successful career.
Dr. McCutchen is vice chair of the AGA Research Foundation. She has no financial conflicts relative to the topics in this video.

In this video, Aja McCutchen, MD, of Atlanta Gastroenterology Associates in Georgia, discusses why she chose to enter private practice gastroenterology, and identifies some key considerations on the road to a successful career.
Dr. McCutchen is vice chair of the AGA Research Foundation. She has no financial conflicts relative to the topics in this video.

We Haven’t Kicked Our Pandemic Drinking Habit
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.
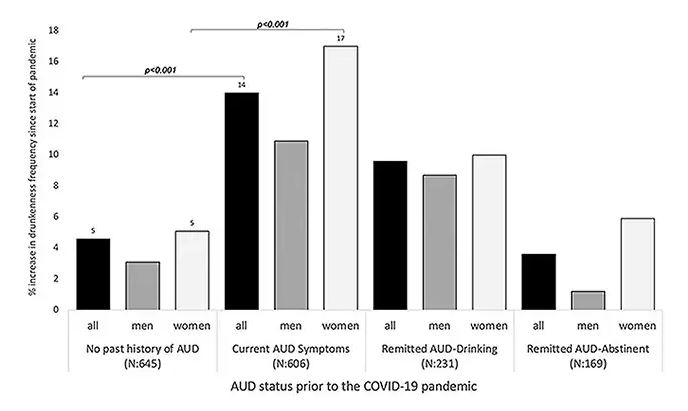
Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.
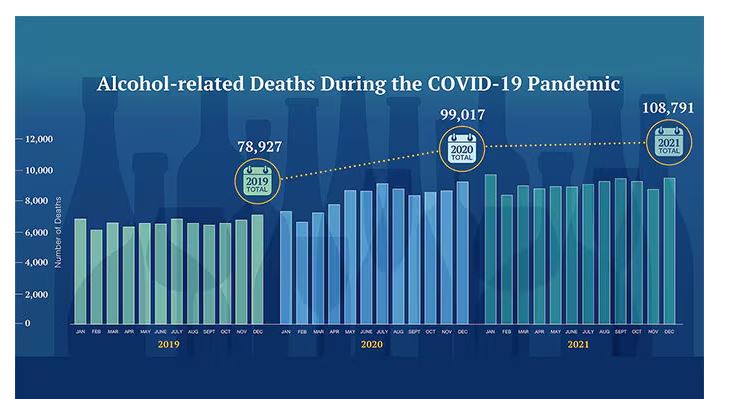
But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.
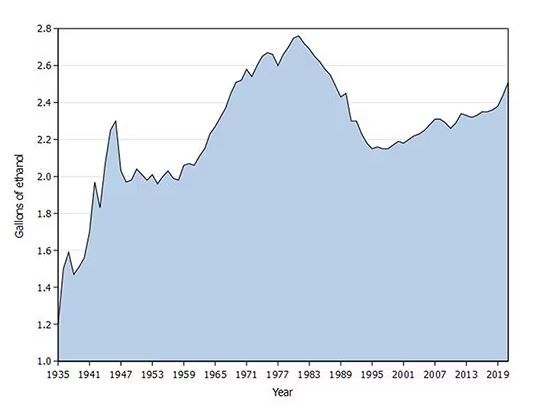
What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.
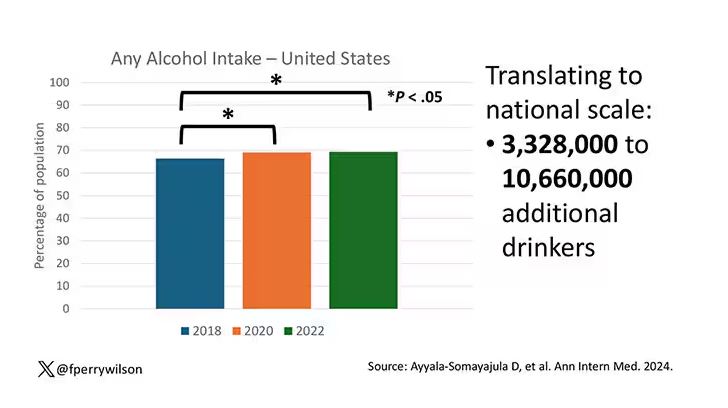
This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.
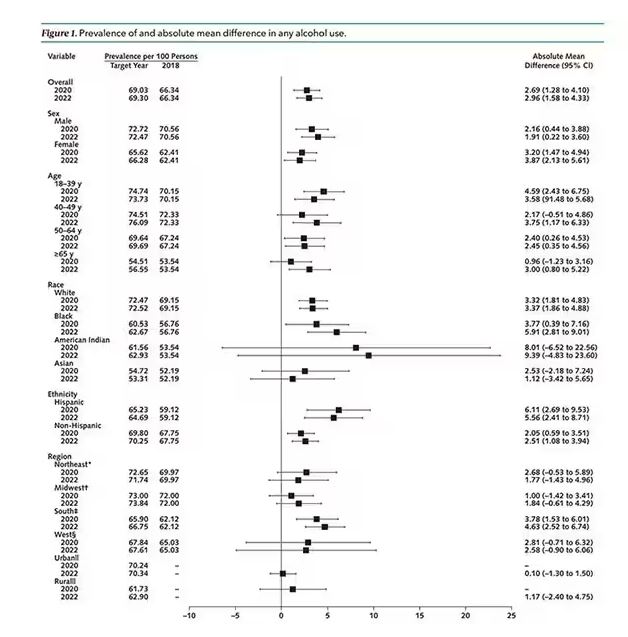
But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.
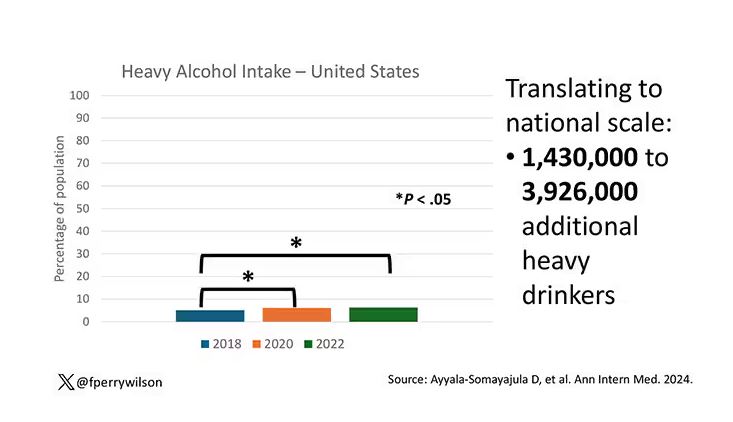
Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.
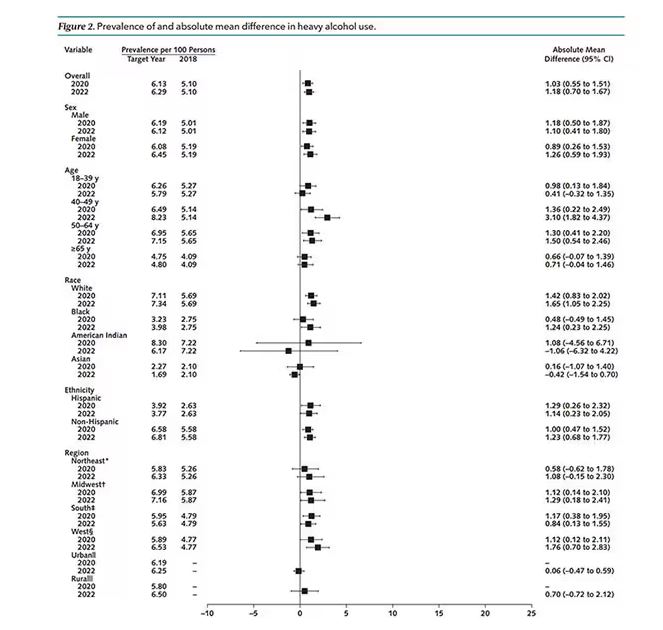
The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.

Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.

But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.

What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.

This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.

But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.

Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.

The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.

Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.

But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.

What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.

This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.

But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.

Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.

The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.