User login
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products

Focus on Nutrient Density Instead of Limiting Certain Foods
The word “malnutrition” probably brings to mind images of very thin patients with catabolic illness. But it really just means “poor nutrition,” which can — and often does — apply to patients with overweight or obesity.
That’s because malnutrition doesn’t occur simply because of a lack of calories, but rather because there is a gap in the nutrition the body requires and the nutrition it receives.
Each day, clinicians see patients with chronic conditions related to malnutrition. That list includes diabetes and hypertension, which can be promoted by excess intake of certain nutrients (carbohydrates and sodium) or inadequate intake of others (fiber, protein, potassium, magnesium, and calcium).
Diet Education Is Vital in Chronic Disease Management
Diet education is without a doubt a core pillar of chronic disease management. Nutrition therapy is recommended in treatment guidelines for the management of some of the most commonly seen chronic conditions such as hypertension, diabetes, and kidney disease. But in one study, only 58% of physicians, nurses and other health professionals surveyed had received formal nutrition education and only 40% were confident in their ability to provide nutrition education to patients.
As a registered dietitian, I welcome referrals for both prevention and management of chronic diseases with open arms. But medical nutrition therapy with a registered dietitian may not be realistic for all patients owing to financial, geographic, or other constraints. So, their best option may be the few minutes that a physician or physician extender has to spare at the end of their appointment.
But time constraints may result in clinicians turning to short, easy-to-remember messages such as “Don’t eat anything white” or “Only shop the edges of the grocery store.” Although catchy, this type of advice can inadvertently encourage patients to skip over foods that are actually very nutrient dense. For example, white foods such as onions, turnips, mushrooms, cauliflower, and even popcorn are low in calories and high in nutritional value. The center aisles of the grocery store may harbor high-carbohydrate breakfast cereals and potato chips, but they are also home to legumes, nuts, and canned and frozen fruits and vegetables.
What may be more effective is educating the patient on the importance of focusing on the nutrient density of foods, rather than simply limiting certain food groups or colors.
How to Work Nutrient Density into the Conversation
Nutrient density is a concept that refers to the proportion of nutrients to calories in a food item: essentially, a food’s qualitative nutritional value. It provides more depth than simply referring to foods as being high or low in calories, healthy or unhealthy, or good or bad.
Educating patients about nutrition density and encouraging a focus on foods that are low in calories and high in vitamins and minerals can help address micronutrient deficiencies, which may be more common than previously thought and linked to the chronic diseases that we see daily. It is worth noting that some foods that are not low in calories are still nutrient dense. Avocados, liver, and nuts come to mind as foods that are high in calories, but they have additional nutrients such as fiber, potassium, antioxidants, vitamin A, iron, and selenium that can still make them an excellent choice if they are part of a well-balanced diet.
I fear that we often underestimate our patients. We worry that not providing them with a list of acceptable foods will set them up for failure. But, in my experience, that list of “good” and “bad” foods may be useful for a week or so but will eventually become lost on the fridge under children’s artwork and save-the-dates.
Patients know that potato chips offer little more than fat, carbs, and salt and that they’re a poor choice for long-term health. What they might not know is that cocktail peanuts can also satisfy the craving for a salty snack, with more than four times the protein, twice the fiber, and just over half of the sodium found in the same serving size of regular salted potato chips. Peanuts have the added bonus of being high in heart-healthy monounsaturated fatty acids.
The best thing that clinicians can do with just a few minutes of time for diet education is to talk to patients about the nutrient density of whole foods and caution patients against highly processed foods, because processing can decrease nutritional content. Our most effective option is to explain why a varied diet with focus on fruits, vegetables, lean protein, nuts, legumes, and healthy fats is beneficial for cardiovascular and metabolic health. After that, all that is left is to trust the patient to make the right choices for their health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The word “malnutrition” probably brings to mind images of very thin patients with catabolic illness. But it really just means “poor nutrition,” which can — and often does — apply to patients with overweight or obesity.
That’s because malnutrition doesn’t occur simply because of a lack of calories, but rather because there is a gap in the nutrition the body requires and the nutrition it receives.
Each day, clinicians see patients with chronic conditions related to malnutrition. That list includes diabetes and hypertension, which can be promoted by excess intake of certain nutrients (carbohydrates and sodium) or inadequate intake of others (fiber, protein, potassium, magnesium, and calcium).
Diet Education Is Vital in Chronic Disease Management
Diet education is without a doubt a core pillar of chronic disease management. Nutrition therapy is recommended in treatment guidelines for the management of some of the most commonly seen chronic conditions such as hypertension, diabetes, and kidney disease. But in one study, only 58% of physicians, nurses and other health professionals surveyed had received formal nutrition education and only 40% were confident in their ability to provide nutrition education to patients.
As a registered dietitian, I welcome referrals for both prevention and management of chronic diseases with open arms. But medical nutrition therapy with a registered dietitian may not be realistic for all patients owing to financial, geographic, or other constraints. So, their best option may be the few minutes that a physician or physician extender has to spare at the end of their appointment.
But time constraints may result in clinicians turning to short, easy-to-remember messages such as “Don’t eat anything white” or “Only shop the edges of the grocery store.” Although catchy, this type of advice can inadvertently encourage patients to skip over foods that are actually very nutrient dense. For example, white foods such as onions, turnips, mushrooms, cauliflower, and even popcorn are low in calories and high in nutritional value. The center aisles of the grocery store may harbor high-carbohydrate breakfast cereals and potato chips, but they are also home to legumes, nuts, and canned and frozen fruits and vegetables.
What may be more effective is educating the patient on the importance of focusing on the nutrient density of foods, rather than simply limiting certain food groups or colors.
How to Work Nutrient Density into the Conversation
Nutrient density is a concept that refers to the proportion of nutrients to calories in a food item: essentially, a food’s qualitative nutritional value. It provides more depth than simply referring to foods as being high or low in calories, healthy or unhealthy, or good or bad.
Educating patients about nutrition density and encouraging a focus on foods that are low in calories and high in vitamins and minerals can help address micronutrient deficiencies, which may be more common than previously thought and linked to the chronic diseases that we see daily. It is worth noting that some foods that are not low in calories are still nutrient dense. Avocados, liver, and nuts come to mind as foods that are high in calories, but they have additional nutrients such as fiber, potassium, antioxidants, vitamin A, iron, and selenium that can still make them an excellent choice if they are part of a well-balanced diet.
I fear that we often underestimate our patients. We worry that not providing them with a list of acceptable foods will set them up for failure. But, in my experience, that list of “good” and “bad” foods may be useful for a week or so but will eventually become lost on the fridge under children’s artwork and save-the-dates.
Patients know that potato chips offer little more than fat, carbs, and salt and that they’re a poor choice for long-term health. What they might not know is that cocktail peanuts can also satisfy the craving for a salty snack, with more than four times the protein, twice the fiber, and just over half of the sodium found in the same serving size of regular salted potato chips. Peanuts have the added bonus of being high in heart-healthy monounsaturated fatty acids.
The best thing that clinicians can do with just a few minutes of time for diet education is to talk to patients about the nutrient density of whole foods and caution patients against highly processed foods, because processing can decrease nutritional content. Our most effective option is to explain why a varied diet with focus on fruits, vegetables, lean protein, nuts, legumes, and healthy fats is beneficial for cardiovascular and metabolic health. After that, all that is left is to trust the patient to make the right choices for their health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The word “malnutrition” probably brings to mind images of very thin patients with catabolic illness. But it really just means “poor nutrition,” which can — and often does — apply to patients with overweight or obesity.
That’s because malnutrition doesn’t occur simply because of a lack of calories, but rather because there is a gap in the nutrition the body requires and the nutrition it receives.
Each day, clinicians see patients with chronic conditions related to malnutrition. That list includes diabetes and hypertension, which can be promoted by excess intake of certain nutrients (carbohydrates and sodium) or inadequate intake of others (fiber, protein, potassium, magnesium, and calcium).
Diet Education Is Vital in Chronic Disease Management
Diet education is without a doubt a core pillar of chronic disease management. Nutrition therapy is recommended in treatment guidelines for the management of some of the most commonly seen chronic conditions such as hypertension, diabetes, and kidney disease. But in one study, only 58% of physicians, nurses and other health professionals surveyed had received formal nutrition education and only 40% were confident in their ability to provide nutrition education to patients.
As a registered dietitian, I welcome referrals for both prevention and management of chronic diseases with open arms. But medical nutrition therapy with a registered dietitian may not be realistic for all patients owing to financial, geographic, or other constraints. So, their best option may be the few minutes that a physician or physician extender has to spare at the end of their appointment.
But time constraints may result in clinicians turning to short, easy-to-remember messages such as “Don’t eat anything white” or “Only shop the edges of the grocery store.” Although catchy, this type of advice can inadvertently encourage patients to skip over foods that are actually very nutrient dense. For example, white foods such as onions, turnips, mushrooms, cauliflower, and even popcorn are low in calories and high in nutritional value. The center aisles of the grocery store may harbor high-carbohydrate breakfast cereals and potato chips, but they are also home to legumes, nuts, and canned and frozen fruits and vegetables.
What may be more effective is educating the patient on the importance of focusing on the nutrient density of foods, rather than simply limiting certain food groups or colors.
How to Work Nutrient Density into the Conversation
Nutrient density is a concept that refers to the proportion of nutrients to calories in a food item: essentially, a food’s qualitative nutritional value. It provides more depth than simply referring to foods as being high or low in calories, healthy or unhealthy, or good or bad.
Educating patients about nutrition density and encouraging a focus on foods that are low in calories and high in vitamins and minerals can help address micronutrient deficiencies, which may be more common than previously thought and linked to the chronic diseases that we see daily. It is worth noting that some foods that are not low in calories are still nutrient dense. Avocados, liver, and nuts come to mind as foods that are high in calories, but they have additional nutrients such as fiber, potassium, antioxidants, vitamin A, iron, and selenium that can still make them an excellent choice if they are part of a well-balanced diet.
I fear that we often underestimate our patients. We worry that not providing them with a list of acceptable foods will set them up for failure. But, in my experience, that list of “good” and “bad” foods may be useful for a week or so but will eventually become lost on the fridge under children’s artwork and save-the-dates.
Patients know that potato chips offer little more than fat, carbs, and salt and that they’re a poor choice for long-term health. What they might not know is that cocktail peanuts can also satisfy the craving for a salty snack, with more than four times the protein, twice the fiber, and just over half of the sodium found in the same serving size of regular salted potato chips. Peanuts have the added bonus of being high in heart-healthy monounsaturated fatty acids.
The best thing that clinicians can do with just a few minutes of time for diet education is to talk to patients about the nutrient density of whole foods and caution patients against highly processed foods, because processing can decrease nutritional content. Our most effective option is to explain why a varied diet with focus on fruits, vegetables, lean protein, nuts, legumes, and healthy fats is beneficial for cardiovascular and metabolic health. After that, all that is left is to trust the patient to make the right choices for their health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Maintaining Weight Loss With GLP-1s Needs Lifestyle Changes
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
New Approaches to Research Beyond Massive Clinical Trials
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
How Metals Affect the Brain
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.
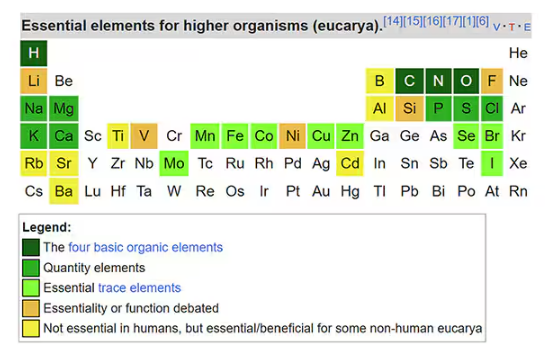
Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.
This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.
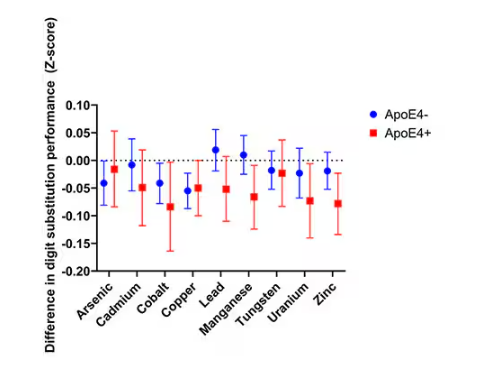
Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.
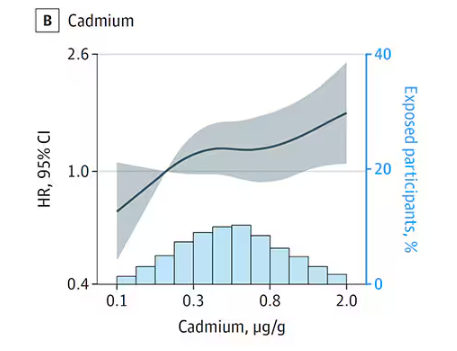
We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).
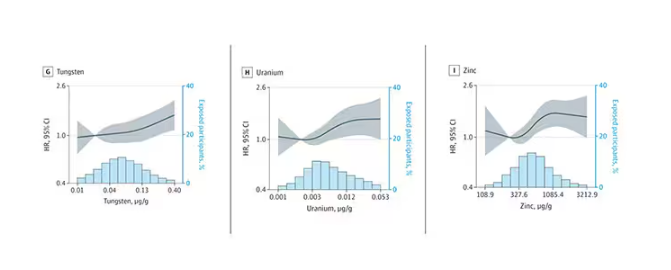
But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.
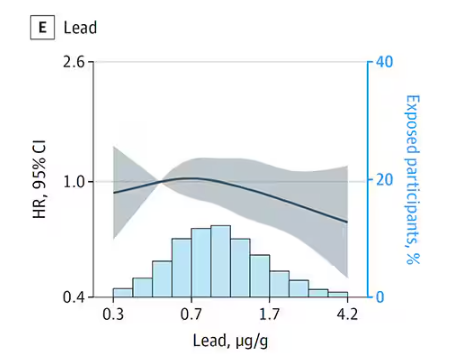
This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.
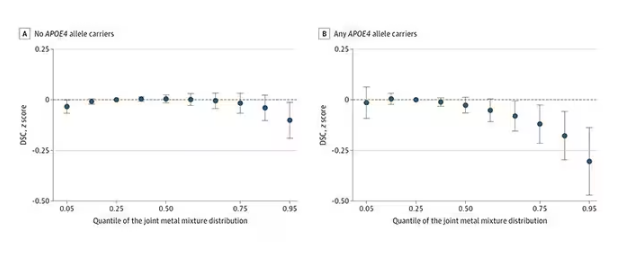
So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Exercise or Inactivity?
The answer one gets often depends on how one crafts the question. For example, Jeffrey D. Johnson PhD, a professor of communications at Portland State University in Oregon has found that if patients are asked “Is there something else you would like to address today?” 80% had their unmet questions addressed. However, if the question was worded “Is there anything else ...?” Very few had their unmet concerns addressed.
I recently encountered two studies that provide another striking example of how differently structured questions aimed at same topic can result in dramatically different results. In this case both studies used one database, the UK Biobank cohort study which contains “de-identified genetic, lifestyle, and health information” collected from a half million adults in the UK. A subgroup of nearly 90,000 who had undergone a week long activity measurement using a wrist accelerometer was the focus of both groups of investigators who asked the same broad question “What is the relationship between physical activity and disease?”
The first study I found has already received some publicity in the lay press and dealt with those individuals who, for a variety of reasons, pack all of their exercise into just a few days, usually the weekend, aka weekend warriors. The investigators found that when compared with generally inactive individuals those who were able to achieve activity volumes that met current guidelines were at lower risk for more than 200 diseases, particularly those that were cardiac based. I guess that shouldn’t surprise us. The finding that has received most of the publicity to date in the lay press was that “Associations were similar whether the activity followed a weekend warrior pattern or was spread out evenly through the week.”
The second study, using the same database, found that those individuals who spent more than 10.6 hours per day sitting had 60% an increased risk of heart failure and cardiovascular related death. And, here’s the real news, that risk remained even in people who were otherwise physically active.
I suspect these two groups of investigators, both associated with Harvard-related institutions, knew of each other’s work and would agree that their findings are not incompatible. However, it is interesting that, when presented with the same database, one group chose to focus its attention on the exercise end of the spectrum while the other looked at the effect of inactivity.
I have always tried to include a “healthy” amount of exercise in my day. However, more recently my professional interest has been drawn to the increasing number of studies I read that deal with the risks of inactivity and sedentarism. For example, just in the last 2 years I have written about a study in children that showed that sedentary time is responsible for 70% of the total increase in cholesterol as children advance into young adulthood. Another study in adults found that every 2-hour increase in sedentary behavior was associated with a 12% decrease in the patient’s likelihood of achieving healthy aging.
If I were asked to place relative values on these two studies, I would say that the study highlighting the risk of prolonged sitting is potentially far more relevant to the population at large, which is for the most part sedentary. Of course, while I have no data to support my contention, I see the weekend warrior population as a niche group.
So what are the take-home messages from these two studies? One is for the weekend warrior. “You can take some comfort in the results that support your exercise schedule but don’t feel too comfortable about it if most of the week you are sitting at a desk.”
For the rest of us — It’s beginning to feel like we should be including accelerometers in our regular diagnostic and therapeutic weaponry. Sending home patients with a Holter cardiac monitor has become commonplace. We should be sending more folks home with accelerometers or asking the more affluent to share the data from their smart watches. “You’ve been bragging about your “steps. Show me your sitting time.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The answer one gets often depends on how one crafts the question. For example, Jeffrey D. Johnson PhD, a professor of communications at Portland State University in Oregon has found that if patients are asked “Is there something else you would like to address today?” 80% had their unmet questions addressed. However, if the question was worded “Is there anything else ...?” Very few had their unmet concerns addressed.
I recently encountered two studies that provide another striking example of how differently structured questions aimed at same topic can result in dramatically different results. In this case both studies used one database, the UK Biobank cohort study which contains “de-identified genetic, lifestyle, and health information” collected from a half million adults in the UK. A subgroup of nearly 90,000 who had undergone a week long activity measurement using a wrist accelerometer was the focus of both groups of investigators who asked the same broad question “What is the relationship between physical activity and disease?”
The first study I found has already received some publicity in the lay press and dealt with those individuals who, for a variety of reasons, pack all of their exercise into just a few days, usually the weekend, aka weekend warriors. The investigators found that when compared with generally inactive individuals those who were able to achieve activity volumes that met current guidelines were at lower risk for more than 200 diseases, particularly those that were cardiac based. I guess that shouldn’t surprise us. The finding that has received most of the publicity to date in the lay press was that “Associations were similar whether the activity followed a weekend warrior pattern or was spread out evenly through the week.”
The second study, using the same database, found that those individuals who spent more than 10.6 hours per day sitting had 60% an increased risk of heart failure and cardiovascular related death. And, here’s the real news, that risk remained even in people who were otherwise physically active.
I suspect these two groups of investigators, both associated with Harvard-related institutions, knew of each other’s work and would agree that their findings are not incompatible. However, it is interesting that, when presented with the same database, one group chose to focus its attention on the exercise end of the spectrum while the other looked at the effect of inactivity.
I have always tried to include a “healthy” amount of exercise in my day. However, more recently my professional interest has been drawn to the increasing number of studies I read that deal with the risks of inactivity and sedentarism. For example, just in the last 2 years I have written about a study in children that showed that sedentary time is responsible for 70% of the total increase in cholesterol as children advance into young adulthood. Another study in adults found that every 2-hour increase in sedentary behavior was associated with a 12% decrease in the patient’s likelihood of achieving healthy aging.
If I were asked to place relative values on these two studies, I would say that the study highlighting the risk of prolonged sitting is potentially far more relevant to the population at large, which is for the most part sedentary. Of course, while I have no data to support my contention, I see the weekend warrior population as a niche group.
So what are the take-home messages from these two studies? One is for the weekend warrior. “You can take some comfort in the results that support your exercise schedule but don’t feel too comfortable about it if most of the week you are sitting at a desk.”
For the rest of us — It’s beginning to feel like we should be including accelerometers in our regular diagnostic and therapeutic weaponry. Sending home patients with a Holter cardiac monitor has become commonplace. We should be sending more folks home with accelerometers or asking the more affluent to share the data from their smart watches. “You’ve been bragging about your “steps. Show me your sitting time.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The answer one gets often depends on how one crafts the question. For example, Jeffrey D. Johnson PhD, a professor of communications at Portland State University in Oregon has found that if patients are asked “Is there something else you would like to address today?” 80% had their unmet questions addressed. However, if the question was worded “Is there anything else ...?” Very few had their unmet concerns addressed.
I recently encountered two studies that provide another striking example of how differently structured questions aimed at same topic can result in dramatically different results. In this case both studies used one database, the UK Biobank cohort study which contains “de-identified genetic, lifestyle, and health information” collected from a half million adults in the UK. A subgroup of nearly 90,000 who had undergone a week long activity measurement using a wrist accelerometer was the focus of both groups of investigators who asked the same broad question “What is the relationship between physical activity and disease?”
The first study I found has already received some publicity in the lay press and dealt with those individuals who, for a variety of reasons, pack all of their exercise into just a few days, usually the weekend, aka weekend warriors. The investigators found that when compared with generally inactive individuals those who were able to achieve activity volumes that met current guidelines were at lower risk for more than 200 diseases, particularly those that were cardiac based. I guess that shouldn’t surprise us. The finding that has received most of the publicity to date in the lay press was that “Associations were similar whether the activity followed a weekend warrior pattern or was spread out evenly through the week.”
The second study, using the same database, found that those individuals who spent more than 10.6 hours per day sitting had 60% an increased risk of heart failure and cardiovascular related death. And, here’s the real news, that risk remained even in people who were otherwise physically active.
I suspect these two groups of investigators, both associated with Harvard-related institutions, knew of each other’s work and would agree that their findings are not incompatible. However, it is interesting that, when presented with the same database, one group chose to focus its attention on the exercise end of the spectrum while the other looked at the effect of inactivity.
I have always tried to include a “healthy” amount of exercise in my day. However, more recently my professional interest has been drawn to the increasing number of studies I read that deal with the risks of inactivity and sedentarism. For example, just in the last 2 years I have written about a study in children that showed that sedentary time is responsible for 70% of the total increase in cholesterol as children advance into young adulthood. Another study in adults found that every 2-hour increase in sedentary behavior was associated with a 12% decrease in the patient’s likelihood of achieving healthy aging.
If I were asked to place relative values on these two studies, I would say that the study highlighting the risk of prolonged sitting is potentially far more relevant to the population at large, which is for the most part sedentary. Of course, while I have no data to support my contention, I see the weekend warrior population as a niche group.
So what are the take-home messages from these two studies? One is for the weekend warrior. “You can take some comfort in the results that support your exercise schedule but don’t feel too comfortable about it if most of the week you are sitting at a desk.”
For the rest of us — It’s beginning to feel like we should be including accelerometers in our regular diagnostic and therapeutic weaponry. Sending home patients with a Holter cardiac monitor has become commonplace. We should be sending more folks home with accelerometers or asking the more affluent to share the data from their smart watches. “You’ve been bragging about your “steps. Show me your sitting time.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
CRC Screening: Right Patient, Right Test, Right Time
It has been three and a half years since the US Preventive Services Task Force (USPSTF) lowered the age to start colorectal cancer (CRC) screening from 50 to 45. As I mentioned in a previous commentary, two major medical groups — the American Academy of Family Physicians and the American College of Physicians — felt that the evidence was insufficient to support this change.
Comparing CRC screening rates in more than 10 million adults aged 45-49 during the 20 months preceding and 20 months following the USPSTF recommendation, researchers found significant increases during the latter time period, with the greatest increases among persons of high socioeconomic status or living in metropolitan areas.
Another study addressed concerns that younger adults may be less likely to follow up on positive screening results or more likely to have false positives on a fecal immunochemical test (FIT). Patients aged 45-49 years were slightly less likely to have a positive FIT result than 50-year-olds, but they had similar rates of colonoscopy completion and similar percentages of abnormal findings on colonoscopy.
Although the sensitivity and specificity of FIT varies quite a bit across different test brands, its overall effectiveness at reducing colorectal cancer deaths is well established. In 2024, the Food and Drug Administration approved three new screening options: a blood-based screening test (Shield), a next-generation multitarget stool DNA test (Cologuard Plus), and a multitarget stool RNA test (ColoSense) with similar performance characteristics as Cologuard Plus. The latter two tests will become available early next year.
This profusion of noninvasive options for CRC screening will challenge those tasked with developing the next iteration of the USPSTF recommendations. Not only must future guidelines establish what evidence threshold is sufficient to recommend a new screening strategy, but they also will need to consider the population-level consequences of relative utilization of different tests. For example, a cost-effectiveness analysis found that more CRC deaths would occur if people who would have otherwise accepted colonoscopy or fecal tests chose to be screened with Shield instead; however, this negative outcome could be offset if for every three of these test substitutions, two other people chose Shield who would otherwise have not been screened at all.
In the meantime, it is important for primary care clinicians to be familiar with evidence-based intervals for CRC screening tests and test eligibility criteria. A troubling study of patients who completed a multitarget stool DNA test in a Midwestern health system in 2021 found that more than one in five had the test ordered inappropriately, based on USPSTF guidelines. Reasons for inappropriate testing included having had a colonoscopy within the past 10 years, a family history of CRC, symptoms suggestive of possible CRC, age younger than 45, and a prior diagnosis of colonic adenomas.
Just as a medication works best when the patient takes it as prescribed, a CRC screening test is most likely to yield more benefit than harm when it’s provided to the right patient at the right time.
Dr. Lin is Associate Director, Family Medicine Residency Program, at Lancaster General Hospital in Pennsylvania. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
It has been three and a half years since the US Preventive Services Task Force (USPSTF) lowered the age to start colorectal cancer (CRC) screening from 50 to 45. As I mentioned in a previous commentary, two major medical groups — the American Academy of Family Physicians and the American College of Physicians — felt that the evidence was insufficient to support this change.
Comparing CRC screening rates in more than 10 million adults aged 45-49 during the 20 months preceding and 20 months following the USPSTF recommendation, researchers found significant increases during the latter time period, with the greatest increases among persons of high socioeconomic status or living in metropolitan areas.
Another study addressed concerns that younger adults may be less likely to follow up on positive screening results or more likely to have false positives on a fecal immunochemical test (FIT). Patients aged 45-49 years were slightly less likely to have a positive FIT result than 50-year-olds, but they had similar rates of colonoscopy completion and similar percentages of abnormal findings on colonoscopy.
Although the sensitivity and specificity of FIT varies quite a bit across different test brands, its overall effectiveness at reducing colorectal cancer deaths is well established. In 2024, the Food and Drug Administration approved three new screening options: a blood-based screening test (Shield), a next-generation multitarget stool DNA test (Cologuard Plus), and a multitarget stool RNA test (ColoSense) with similar performance characteristics as Cologuard Plus. The latter two tests will become available early next year.
This profusion of noninvasive options for CRC screening will challenge those tasked with developing the next iteration of the USPSTF recommendations. Not only must future guidelines establish what evidence threshold is sufficient to recommend a new screening strategy, but they also will need to consider the population-level consequences of relative utilization of different tests. For example, a cost-effectiveness analysis found that more CRC deaths would occur if people who would have otherwise accepted colonoscopy or fecal tests chose to be screened with Shield instead; however, this negative outcome could be offset if for every three of these test substitutions, two other people chose Shield who would otherwise have not been screened at all.
In the meantime, it is important for primary care clinicians to be familiar with evidence-based intervals for CRC screening tests and test eligibility criteria. A troubling study of patients who completed a multitarget stool DNA test in a Midwestern health system in 2021 found that more than one in five had the test ordered inappropriately, based on USPSTF guidelines. Reasons for inappropriate testing included having had a colonoscopy within the past 10 years, a family history of CRC, symptoms suggestive of possible CRC, age younger than 45, and a prior diagnosis of colonic adenomas.
Just as a medication works best when the patient takes it as prescribed, a CRC screening test is most likely to yield more benefit than harm when it’s provided to the right patient at the right time.
Dr. Lin is Associate Director, Family Medicine Residency Program, at Lancaster General Hospital in Pennsylvania. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
It has been three and a half years since the US Preventive Services Task Force (USPSTF) lowered the age to start colorectal cancer (CRC) screening from 50 to 45. As I mentioned in a previous commentary, two major medical groups — the American Academy of Family Physicians and the American College of Physicians — felt that the evidence was insufficient to support this change.
Comparing CRC screening rates in more than 10 million adults aged 45-49 during the 20 months preceding and 20 months following the USPSTF recommendation, researchers found significant increases during the latter time period, with the greatest increases among persons of high socioeconomic status or living in metropolitan areas.
Another study addressed concerns that younger adults may be less likely to follow up on positive screening results or more likely to have false positives on a fecal immunochemical test (FIT). Patients aged 45-49 years were slightly less likely to have a positive FIT result than 50-year-olds, but they had similar rates of colonoscopy completion and similar percentages of abnormal findings on colonoscopy.
Although the sensitivity and specificity of FIT varies quite a bit across different test brands, its overall effectiveness at reducing colorectal cancer deaths is well established. In 2024, the Food and Drug Administration approved three new screening options: a blood-based screening test (Shield), a next-generation multitarget stool DNA test (Cologuard Plus), and a multitarget stool RNA test (ColoSense) with similar performance characteristics as Cologuard Plus. The latter two tests will become available early next year.
This profusion of noninvasive options for CRC screening will challenge those tasked with developing the next iteration of the USPSTF recommendations. Not only must future guidelines establish what evidence threshold is sufficient to recommend a new screening strategy, but they also will need to consider the population-level consequences of relative utilization of different tests. For example, a cost-effectiveness analysis found that more CRC deaths would occur if people who would have otherwise accepted colonoscopy or fecal tests chose to be screened with Shield instead; however, this negative outcome could be offset if for every three of these test substitutions, two other people chose Shield who would otherwise have not been screened at all.
In the meantime, it is important for primary care clinicians to be familiar with evidence-based intervals for CRC screening tests and test eligibility criteria. A troubling study of patients who completed a multitarget stool DNA test in a Midwestern health system in 2021 found that more than one in five had the test ordered inappropriately, based on USPSTF guidelines. Reasons for inappropriate testing included having had a colonoscopy within the past 10 years, a family history of CRC, symptoms suggestive of possible CRC, age younger than 45, and a prior diagnosis of colonic adenomas.
Just as a medication works best when the patient takes it as prescribed, a CRC screening test is most likely to yield more benefit than harm when it’s provided to the right patient at the right time.
Dr. Lin is Associate Director, Family Medicine Residency Program, at Lancaster General Hospital in Pennsylvania. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New Gel Stops Severe Bleeding in Seconds
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me today to discuss a novel, plant-based approach to stopping moderate to severe bleeding is Joe Landolina, CEO and cofounder of Cresilon. Welcome, Joe.
Joe Landolina, MS: Thank you so much for taking the time. It’s great to be here.
Educational Background and Inception of Cresilon
Glatter: It’s a pleasure to have you join me, and I want to congratulate you on your recent 510(k) FDA clearance for your novel product to save lives and stop bleeding. To begin with, can you explain how the idea for launching your company came about?
Landolina: The way that Cresilon came about was a little bit unorthodox, because I was 17 years old when I invented the technology behind the product that eventually became Traumagel®.
My grandfather was an ex-pharmaceutical executive, who later in life started a vineyard. I grew up on a vineyard with a winery chemistry lab across the street from my house and a grandfather who learned lab safety in the 60s. So, that meant that the day I learned how to walk, I was tossed into a lab and I fell head over heels in love with lab research.
That started experimentation and my academic pursuits. That led to discovering a blend of two plant-based polymers derived from algae that stop bleeding on contact, effectively creating a mechanical barrier and allowing anything from a gunshot wound to anything quite a bit more minor to stop in a matter of seconds.
Glatter: Your background is in biomedical engineering. How is it that you started tinkering and doing all this type of work?
Landolina: That’s correct. I did my undergrad in chemical engineering, and my graduate studies were in biomedical engineering. For me, that was supposed to be a pathway into medical school. I always wanted to be a surgeon myself, and I love the field of medicine.
As a freshman in college at NYU Engineering, I had this idea. I entered it into NYU’s business plan competition, and we won at the engineering school. That gave us just enough capital to start developing and researching Traumagel more, and Cresilon was born out of that research.
Techniques for Stopping Hemorrhage
Glatter: In terms of stopping hemorrhage, which takes so many lives in the United States and globally — certainly, uncontrolled hemorrhage — what are the techniques that you see, prior to the arrival of your product, as being effective? Can you elucidate some of these techniques?
Landolina: In emergency medicine, the primary mode of controlling hemorrhage is passive. It’s what, in Brooklyn, we like to call “pressure and a prayer”, where you have a material that’s either gauze or an impregnated gauze in most cases, where the mode of action is absorbing blood, with the adjunct of pressure by the first responder or by the clinician who’s providing aid.
These types of technologies are widespread. There are many versions of this technology carried by EMS agencies, trauma bays, US military soldiers, and soldiers across NATO countries. But these types of technologies tend to be relatively inefficient, meaning that they’re very difficult to get into wounds because of the gauze or the powder form of the devices, and it’s very hard to get them in contact with the form of bleeding.
On top of that, if the patient is clotting compromised or immunocompromised in some way, the ability to create a durable clot that will not be ripped off when you remove the product at the next level of care is also of concern. And so, this type of technology or the type of treatment of massive hemorrhage hasn’t changed in decades.
Current Applications and Potential Use
Glatter: I envision this product will be carried by paramedics, used on the battlefield at some point after your FDA clearance, and recently it went through.
Do you see any possibility that this could be an AED equivalent to Stop the Bleed? In other words, could the average lay person be trained to use your product if kits are available?
Landolina: To be very clear, Traumagel today is only approved or cleared under a “prescription-only” indication, which means that it will not initially be available OTC. However, that is our goal. Our goal is to make this product available and usable by someone with no medical training whatsoever.
The form factor of being a gel in a syringe lends itself well to that, meaning that we try to make it as easy as point and shoot to control hemorrhage, where there’s not as much technique to be learned in the application of a product like Traumagel as there is in current hemorrhage control techniques.
Mechanism of Action and Physiology
Glatter: Once you apply Traumagel, can you explain what happens to the product after it’s applied and the bleeding has stopped? Does it get reabsorbed by the body? What’s the process here?
Landolina: Under Traumagel’s indication, because it’s used in traumatic injury, it must be removed within 24 hours.
One of the big benefits of Traumagel is that when the patient produces a blood clot underneath Traumagel, it doesn’t become incorporated within the gel itself. To contrast that with the use of gauze, gauze is porous. The clot ends up wrapped around the fibers of the gauze, so if you peel the gauze away, it’s very likely that clot is coming off with it. The surgeon or the clinician at the next level of care is going to have to deal with the re-bleed.
You can remove Traumagel cleanly and entirely without disturbing the underlying clot. That’s a major benefit, not only to the patient but also to the next level of care, to the next clinician or physician that is required to remove the product.
Glatter: How is it possible to remove the substance without disturbing the clot? Can you explain in more detail?
Landolina: That’s one of the hallmarks of these plant-based polymers and the way that we design Traumagel itself. Traumagel is completely nonporous, and it has no fibrous nature to it. What that means is when the patient produces a blood clot or fibrin next to or on top of Traumagel, that fibrin ends up not incorporated within the polymers of Traumagel itself.
Over time, because Traumagel is a hydrogel, meaning that by weight it’s mostly water, you end up having less adhesion to the clot over time. When it’s time to remove Traumagel from the injury, it has lost almost all of its adhesive capabilities, meaning that when you peel it away, that clot is going to stick better to tissue than it will to the gel itself.
Glatter: Can you explain a little bit about the matrix that’s formed, the physiology, and how the polymers work to form this matrix?
Landolina: Sure. Traumagel is made of two polysaccharides that are plant derived. One polysaccharide is polyanionic, and the other is polycationic, meaning one has negative charges and the other has positive charges, which together create almost a Lego block effect, where when the material comes in contact with tissue, it adheres strongly and allows for itself to effectively create a mechanical barrier against bleeding.
Courtesy of Cresilon
Landolina: Even in the face of major arterial blood flow, Traumagel will stay where it needs to stay, and it’s not going to get washed away. This means that it is much more easily appliable to these types of surfaces and will allow the patient to produce their own endogenous fibrin clot at that location.
Like I mentioned before, when that fibrin clot is formed, because the gel itself has no pores or fibers, it doesn’t become incorporated within the fibrin clot. You can take the gel away, leaving that clot behind without the chance of a rebleed.
Testing With Major Bleeds
Glatter: In terms of bleeding itself, have you tested your product with major aortic bleeds or carotid bleeds in preclinical work?
Landolina: We have used the US military’s model for lethal hemorrhage, and the idea there is to create a model that is just that — lethal. These are the worst types of bleeds that you can possibly imagine, where the patients are clotting compromised, and where you have, in most cases, a very strong arterial component, so something like a femoral artery bleed.
We’ve also tested in carotid artery, aortic applications, as well as combinations of venous and arterial bleeds. The idea here is to show the use of the product in the absolute worst-case scenario so that when this translates into the clinic, the models that we’ve used for evaluation, hopefully, are worse than what actually rolls into the trauma bay.
Glatter: Excellent. What’s the mean time to stop an arterial vs a venous bleed? Are we talking a matter of seconds?
Landolina: In the case of a healthy patient, meaning a patient without clotting compromise, you’re in a matter of seconds. It’s less than 10 seconds.
In the case where you have clotting compromise, a deep, complicated wound geometry, we recommend holding a pressure bandage on for 3 minutes just because it increases the chance of Traumagel coming into contact with the bleed, especially when you can’t visualize the bleed in the bleed source. Because of that pressure time, that becomes the mean. But again, it’s highly dependent on the type of bleed and the style of application.
Failure Rates and Effectiveness
Glatter: As a segue to that, what is the failure rate based on your studies and internal research using Traumagel? Have there been cases where bleeding has not been able to be stopped?
Landolina: It depends on the study, but the failure rates are incredibly low with Traumagel, assuming that it’s correctly used. That’s one of the benefits to this product, where with proper technique, with overwrap with gauze, you nearly always get control of hemorrhage with a product like this.
Glatter: Is manual pressure required in that sense? From what you described earlier, manual pressure would not be required.
Landolina: It depends on the injury. What we recommend is that, if you have a very deep wound where you cannot visualize the source of bleed, you use pressure to seat Traumagel into the source of bleeding, meaning that you’re following Committee on Tactical Combat Casualty Care (Co-TCCC) regulations or requirements, where you’re over wrapping with gauze, and you’re providing a pressure wrapping to ensure that the Traumagel is in contact with the bleed while it’s doing what it’s doing.
In most cases, it doesn’t hurt to apply pressure on top of Traumagel as well. In more surface level bleeds, you don’t need pressure at all.
Applications Beyond Trauma
Glatter: Interesting. In terms of further applications (eg, nose bleeds or GYN bleeding, which are life-threatening), do you see this coming as an application for the future?
Landolina: That’s where we’re working. Traumagel is the successor to an animal health product called Vetigel. The formulations of the gel behind Vetigel and Traumagel are identical. Vetigel has a full surgical indication, and that’s everything from epistaxis to neuro and spine procedures, into cardiovascular and soft tissue surgeries, orthopedic medicine, and so on.
Cresilon’s goal is to eventually expand the indication of our technology to include surgical indications and other indications where we can help any patient that’s bleeding.
Glatter: That’s important, because we use prehospital whole blood, low titer, specifically, when patients have life-threatening hemorrhage. With your product, that would reduce the amount of blood products that would need to be administered. This could be a real game changer.
Landolina: Definitely, that’s the goal we’re working on.
Infection Risks and Biocompatibility
Glatter: In terms of any risk for infection, has that been studied as well? Does Traumagel in any way lead to increased rates of infection?
Landolina: Traumagel is biocompatible. It’s a sterile product. We’ve done the full suite of biocompatibility testing as required by FDA. On top of that, remember that Vetigel, which is the same formulation, is an implantable product. As a result, that has even extended biocompatibility testing beyond what would be necessary for an external product.
In Vetigel’s use case, which has been used now in over 60,000 patients, primarily companion animals, dogs and cats, we haven’t seen instances of infection. There’s no reason to believe that we would see that clinically with Traumagel.
Research Collaborations and Future Applications
Glatter: In terms of other research that your company’s embarked on preclinically, I understand there were some studies done at Walter Reed Army Institute of Research. I was wondering if you could expand on these, specifically, in terms of traumatic brain injury (TBI) and hemorrhage related to that. For example, with shrapnel or even a gunshot wound.
Landolina: The Walter Reed collaboration with Cresilon is something that I’m particularly excited about, because it marks Cresilon’s first project that’s outside the scope of just hemostasis. Walter Reed came to us with this proposal where there’s a big challenge in a subset of TBI called penetrating ballistic-like brain injury, where the brain has been penetrated by a bullet, shrapnel, or some other projectile, and there’s an injury that exposes the brain to the outside.
Today, there is no standard of care to treat patients with those types of injuries. In many cases, mortality is caused through swelling of the brain, or collapse of the brain. What they came to us with was the potential of using our technology, not primarily as a hemostatic agent, but to be able to stabilize that patient enough to get to the next level of care to be treated by a neurosurgeon.
That study Walter Reed did was just a pilot that was done in small animals. In that pilot, they showed that over the period of treatment, there was no negative change in vital signs, no increase in edema or in swelling, or in any of the biomarkers that were being monitored at that time.
At the very least, this is not full indication that this indication will work for Cresilon, but it shows that there’s promise. It’s something that we’re working on and hopefully we’ll be able to bring to market soon.
Glatter: Certainly, maintaining intracranial pressure and cerebral perfusion pressures are very critical. In the future, do you think this product would be able to be deployed endovascularly? Imagine this in terms of stopping bleeding from some source, whether it’s from a stroke or another intracranial source.
Landolina: That’s been an area of interest for us. We have no evidence to prove that indication works at this point, but there’s also nothing to say that it wouldn’t be possible for our technology. At this point, we’ve only looked at a cursory level at those indications.
Glatter: Does the use of Traumagel obviate the need for a more definitive repair (eg, with sutures) or something that’s more permanent?
Landolina: I always say that Traumagel — and Vetigel, for that matter — is not a replacement for good surgical technique. The surgeon always needs to make his or her best judgment when reviewing the patient. That doesn’t mean that there won’t need to be sutures or vascular repair in most of these cases, especially in major trauma.
Final Takeaways
Glatter: Do you have some bullet points or pearls you could give our audience as a takeaway?
Landolina: When Cresilon looks at Traumagel — and for us, Traumagel is the next generation of hemostatic agent, especially in trauma care and in emergency medicine — it allows for a far-simplified application of the product and much faster control of hemorrhage with better patient outcomes.
As we roll this out through EMS agencies, trauma hospitals, military agencies, and eventually to the general public through a future indication, it’s something we’re very excited about. Personally, I started this business 14 years ago, and so it’s great to see our mission of saving lives transitioning to saving human lives.
Glatter: I look forward to seeing this product in the emergency department, but also in other settings, such as in the operating room where we can really help patients who are dying from hemorrhage, certainly on the battlefield, and the lay public. If someone were to come upon a patient who’s bleeding out, this could be certainly a game changer and a lifesaver.
I want to thank you for your time. This is a really important product that’s transformed the lives of so many animals, but also people in the future.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He reported no relevant conflicts of interest. Mr. Landolina is the CEO and co-founder of Cresilon, a biotechnology company specializing in plant-based solutions for emergency bleeding control.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me today to discuss a novel, plant-based approach to stopping moderate to severe bleeding is Joe Landolina, CEO and cofounder of Cresilon. Welcome, Joe.
Joe Landolina, MS: Thank you so much for taking the time. It’s great to be here.
Educational Background and Inception of Cresilon
Glatter: It’s a pleasure to have you join me, and I want to congratulate you on your recent 510(k) FDA clearance for your novel product to save lives and stop bleeding. To begin with, can you explain how the idea for launching your company came about?
Landolina: The way that Cresilon came about was a little bit unorthodox, because I was 17 years old when I invented the technology behind the product that eventually became Traumagel®.
My grandfather was an ex-pharmaceutical executive, who later in life started a vineyard. I grew up on a vineyard with a winery chemistry lab across the street from my house and a grandfather who learned lab safety in the 60s. So, that meant that the day I learned how to walk, I was tossed into a lab and I fell head over heels in love with lab research.
That started experimentation and my academic pursuits. That led to discovering a blend of two plant-based polymers derived from algae that stop bleeding on contact, effectively creating a mechanical barrier and allowing anything from a gunshot wound to anything quite a bit more minor to stop in a matter of seconds.
Glatter: Your background is in biomedical engineering. How is it that you started tinkering and doing all this type of work?
Landolina: That’s correct. I did my undergrad in chemical engineering, and my graduate studies were in biomedical engineering. For me, that was supposed to be a pathway into medical school. I always wanted to be a surgeon myself, and I love the field of medicine.
As a freshman in college at NYU Engineering, I had this idea. I entered it into NYU’s business plan competition, and we won at the engineering school. That gave us just enough capital to start developing and researching Traumagel more, and Cresilon was born out of that research.
Techniques for Stopping Hemorrhage
Glatter: In terms of stopping hemorrhage, which takes so many lives in the United States and globally — certainly, uncontrolled hemorrhage — what are the techniques that you see, prior to the arrival of your product, as being effective? Can you elucidate some of these techniques?
Landolina: In emergency medicine, the primary mode of controlling hemorrhage is passive. It’s what, in Brooklyn, we like to call “pressure and a prayer”, where you have a material that’s either gauze or an impregnated gauze in most cases, where the mode of action is absorbing blood, with the adjunct of pressure by the first responder or by the clinician who’s providing aid.
These types of technologies are widespread. There are many versions of this technology carried by EMS agencies, trauma bays, US military soldiers, and soldiers across NATO countries. But these types of technologies tend to be relatively inefficient, meaning that they’re very difficult to get into wounds because of the gauze or the powder form of the devices, and it’s very hard to get them in contact with the form of bleeding.
On top of that, if the patient is clotting compromised or immunocompromised in some way, the ability to create a durable clot that will not be ripped off when you remove the product at the next level of care is also of concern. And so, this type of technology or the type of treatment of massive hemorrhage hasn’t changed in decades.
Current Applications and Potential Use
Glatter: I envision this product will be carried by paramedics, used on the battlefield at some point after your FDA clearance, and recently it went through.
Do you see any possibility that this could be an AED equivalent to Stop the Bleed? In other words, could the average lay person be trained to use your product if kits are available?
Landolina: To be very clear, Traumagel today is only approved or cleared under a “prescription-only” indication, which means that it will not initially be available OTC. However, that is our goal. Our goal is to make this product available and usable by someone with no medical training whatsoever.
The form factor of being a gel in a syringe lends itself well to that, meaning that we try to make it as easy as point and shoot to control hemorrhage, where there’s not as much technique to be learned in the application of a product like Traumagel as there is in current hemorrhage control techniques.
Mechanism of Action and Physiology
Glatter: Once you apply Traumagel, can you explain what happens to the product after it’s applied and the bleeding has stopped? Does it get reabsorbed by the body? What’s the process here?
Landolina: Under Traumagel’s indication, because it’s used in traumatic injury, it must be removed within 24 hours.
One of the big benefits of Traumagel is that when the patient produces a blood clot underneath Traumagel, it doesn’t become incorporated within the gel itself. To contrast that with the use of gauze, gauze is porous. The clot ends up wrapped around the fibers of the gauze, so if you peel the gauze away, it’s very likely that clot is coming off with it. The surgeon or the clinician at the next level of care is going to have to deal with the re-bleed.
You can remove Traumagel cleanly and entirely without disturbing the underlying clot. That’s a major benefit, not only to the patient but also to the next level of care, to the next clinician or physician that is required to remove the product.
Glatter: How is it possible to remove the substance without disturbing the clot? Can you explain in more detail?
Landolina: That’s one of the hallmarks of these plant-based polymers and the way that we design Traumagel itself. Traumagel is completely nonporous, and it has no fibrous nature to it. What that means is when the patient produces a blood clot or fibrin next to or on top of Traumagel, that fibrin ends up not incorporated within the polymers of Traumagel itself.
Over time, because Traumagel is a hydrogel, meaning that by weight it’s mostly water, you end up having less adhesion to the clot over time. When it’s time to remove Traumagel from the injury, it has lost almost all of its adhesive capabilities, meaning that when you peel it away, that clot is going to stick better to tissue than it will to the gel itself.
Glatter: Can you explain a little bit about the matrix that’s formed, the physiology, and how the polymers work to form this matrix?
Landolina: Sure. Traumagel is made of two polysaccharides that are plant derived. One polysaccharide is polyanionic, and the other is polycationic, meaning one has negative charges and the other has positive charges, which together create almost a Lego block effect, where when the material comes in contact with tissue, it adheres strongly and allows for itself to effectively create a mechanical barrier against bleeding.
Courtesy of Cresilon
Landolina: Even in the face of major arterial blood flow, Traumagel will stay where it needs to stay, and it’s not going to get washed away. This means that it is much more easily appliable to these types of surfaces and will allow the patient to produce their own endogenous fibrin clot at that location.
Like I mentioned before, when that fibrin clot is formed, because the gel itself has no pores or fibers, it doesn’t become incorporated within the fibrin clot. You can take the gel away, leaving that clot behind without the chance of a rebleed.
Testing With Major Bleeds
Glatter: In terms of bleeding itself, have you tested your product with major aortic bleeds or carotid bleeds in preclinical work?
Landolina: We have used the US military’s model for lethal hemorrhage, and the idea there is to create a model that is just that — lethal. These are the worst types of bleeds that you can possibly imagine, where the patients are clotting compromised, and where you have, in most cases, a very strong arterial component, so something like a femoral artery bleed.
We’ve also tested in carotid artery, aortic applications, as well as combinations of venous and arterial bleeds. The idea here is to show the use of the product in the absolute worst-case scenario so that when this translates into the clinic, the models that we’ve used for evaluation, hopefully, are worse than what actually rolls into the trauma bay.
Glatter: Excellent. What’s the mean time to stop an arterial vs a venous bleed? Are we talking a matter of seconds?
Landolina: In the case of a healthy patient, meaning a patient without clotting compromise, you’re in a matter of seconds. It’s less than 10 seconds.
In the case where you have clotting compromise, a deep, complicated wound geometry, we recommend holding a pressure bandage on for 3 minutes just because it increases the chance of Traumagel coming into contact with the bleed, especially when you can’t visualize the bleed in the bleed source. Because of that pressure time, that becomes the mean. But again, it’s highly dependent on the type of bleed and the style of application.
Failure Rates and Effectiveness
Glatter: As a segue to that, what is the failure rate based on your studies and internal research using Traumagel? Have there been cases where bleeding has not been able to be stopped?
Landolina: It depends on the study, but the failure rates are incredibly low with Traumagel, assuming that it’s correctly used. That’s one of the benefits to this product, where with proper technique, with overwrap with gauze, you nearly always get control of hemorrhage with a product like this.
Glatter: Is manual pressure required in that sense? From what you described earlier, manual pressure would not be required.
Landolina: It depends on the injury. What we recommend is that, if you have a very deep wound where you cannot visualize the source of bleed, you use pressure to seat Traumagel into the source of bleeding, meaning that you’re following Committee on Tactical Combat Casualty Care (Co-TCCC) regulations or requirements, where you’re over wrapping with gauze, and you’re providing a pressure wrapping to ensure that the Traumagel is in contact with the bleed while it’s doing what it’s doing.
In most cases, it doesn’t hurt to apply pressure on top of Traumagel as well. In more surface level bleeds, you don’t need pressure at all.
Applications Beyond Trauma
Glatter: Interesting. In terms of further applications (eg, nose bleeds or GYN bleeding, which are life-threatening), do you see this coming as an application for the future?
Landolina: That’s where we’re working. Traumagel is the successor to an animal health product called Vetigel. The formulations of the gel behind Vetigel and Traumagel are identical. Vetigel has a full surgical indication, and that’s everything from epistaxis to neuro and spine procedures, into cardiovascular and soft tissue surgeries, orthopedic medicine, and so on.
Cresilon’s goal is to eventually expand the indication of our technology to include surgical indications and other indications where we can help any patient that’s bleeding.
Glatter: That’s important, because we use prehospital whole blood, low titer, specifically, when patients have life-threatening hemorrhage. With your product, that would reduce the amount of blood products that would need to be administered. This could be a real game changer.
Landolina: Definitely, that’s the goal we’re working on.
Infection Risks and Biocompatibility
Glatter: In terms of any risk for infection, has that been studied as well? Does Traumagel in any way lead to increased rates of infection?
Landolina: Traumagel is biocompatible. It’s a sterile product. We’ve done the full suite of biocompatibility testing as required by FDA. On top of that, remember that Vetigel, which is the same formulation, is an implantable product. As a result, that has even extended biocompatibility testing beyond what would be necessary for an external product.
In Vetigel’s use case, which has been used now in over 60,000 patients, primarily companion animals, dogs and cats, we haven’t seen instances of infection. There’s no reason to believe that we would see that clinically with Traumagel.
Research Collaborations and Future Applications
Glatter: In terms of other research that your company’s embarked on preclinically, I understand there were some studies done at Walter Reed Army Institute of Research. I was wondering if you could expand on these, specifically, in terms of traumatic brain injury (TBI) and hemorrhage related to that. For example, with shrapnel or even a gunshot wound.
Landolina: The Walter Reed collaboration with Cresilon is something that I’m particularly excited about, because it marks Cresilon’s first project that’s outside the scope of just hemostasis. Walter Reed came to us with this proposal where there’s a big challenge in a subset of TBI called penetrating ballistic-like brain injury, where the brain has been penetrated by a bullet, shrapnel, or some other projectile, and there’s an injury that exposes the brain to the outside.
Today, there is no standard of care to treat patients with those types of injuries. In many cases, mortality is caused through swelling of the brain, or collapse of the brain. What they came to us with was the potential of using our technology, not primarily as a hemostatic agent, but to be able to stabilize that patient enough to get to the next level of care to be treated by a neurosurgeon.
That study Walter Reed did was just a pilot that was done in small animals. In that pilot, they showed that over the period of treatment, there was no negative change in vital signs, no increase in edema or in swelling, or in any of the biomarkers that were being monitored at that time.
At the very least, this is not full indication that this indication will work for Cresilon, but it shows that there’s promise. It’s something that we’re working on and hopefully we’ll be able to bring to market soon.
Glatter: Certainly, maintaining intracranial pressure and cerebral perfusion pressures are very critical. In the future, do you think this product would be able to be deployed endovascularly? Imagine this in terms of stopping bleeding from some source, whether it’s from a stroke or another intracranial source.
Landolina: That’s been an area of interest for us. We have no evidence to prove that indication works at this point, but there’s also nothing to say that it wouldn’t be possible for our technology. At this point, we’ve only looked at a cursory level at those indications.
Glatter: Does the use of Traumagel obviate the need for a more definitive repair (eg, with sutures) or something that’s more permanent?
Landolina: I always say that Traumagel — and Vetigel, for that matter — is not a replacement for good surgical technique. The surgeon always needs to make his or her best judgment when reviewing the patient. That doesn’t mean that there won’t need to be sutures or vascular repair in most of these cases, especially in major trauma.
Final Takeaways
Glatter: Do you have some bullet points or pearls you could give our audience as a takeaway?
Landolina: When Cresilon looks at Traumagel — and for us, Traumagel is the next generation of hemostatic agent, especially in trauma care and in emergency medicine — it allows for a far-simplified application of the product and much faster control of hemorrhage with better patient outcomes.
As we roll this out through EMS agencies, trauma hospitals, military agencies, and eventually to the general public through a future indication, it’s something we’re very excited about. Personally, I started this business 14 years ago, and so it’s great to see our mission of saving lives transitioning to saving human lives.
Glatter: I look forward to seeing this product in the emergency department, but also in other settings, such as in the operating room where we can really help patients who are dying from hemorrhage, certainly on the battlefield, and the lay public. If someone were to come upon a patient who’s bleeding out, this could be certainly a game changer and a lifesaver.
I want to thank you for your time. This is a really important product that’s transformed the lives of so many animals, but also people in the future.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He reported no relevant conflicts of interest. Mr. Landolina is the CEO and co-founder of Cresilon, a biotechnology company specializing in plant-based solutions for emergency bleeding control.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me today to discuss a novel, plant-based approach to stopping moderate to severe bleeding is Joe Landolina, CEO and cofounder of Cresilon. Welcome, Joe.
Joe Landolina, MS: Thank you so much for taking the time. It’s great to be here.
Educational Background and Inception of Cresilon
Glatter: It’s a pleasure to have you join me, and I want to congratulate you on your recent 510(k) FDA clearance for your novel product to save lives and stop bleeding. To begin with, can you explain how the idea for launching your company came about?
Landolina: The way that Cresilon came about was a little bit unorthodox, because I was 17 years old when I invented the technology behind the product that eventually became Traumagel®.
My grandfather was an ex-pharmaceutical executive, who later in life started a vineyard. I grew up on a vineyard with a winery chemistry lab across the street from my house and a grandfather who learned lab safety in the 60s. So, that meant that the day I learned how to walk, I was tossed into a lab and I fell head over heels in love with lab research.
That started experimentation and my academic pursuits. That led to discovering a blend of two plant-based polymers derived from algae that stop bleeding on contact, effectively creating a mechanical barrier and allowing anything from a gunshot wound to anything quite a bit more minor to stop in a matter of seconds.
Glatter: Your background is in biomedical engineering. How is it that you started tinkering and doing all this type of work?
Landolina: That’s correct. I did my undergrad in chemical engineering, and my graduate studies were in biomedical engineering. For me, that was supposed to be a pathway into medical school. I always wanted to be a surgeon myself, and I love the field of medicine.
As a freshman in college at NYU Engineering, I had this idea. I entered it into NYU’s business plan competition, and we won at the engineering school. That gave us just enough capital to start developing and researching Traumagel more, and Cresilon was born out of that research.
Techniques for Stopping Hemorrhage
Glatter: In terms of stopping hemorrhage, which takes so many lives in the United States and globally — certainly, uncontrolled hemorrhage — what are the techniques that you see, prior to the arrival of your product, as being effective? Can you elucidate some of these techniques?
Landolina: In emergency medicine, the primary mode of controlling hemorrhage is passive. It’s what, in Brooklyn, we like to call “pressure and a prayer”, where you have a material that’s either gauze or an impregnated gauze in most cases, where the mode of action is absorbing blood, with the adjunct of pressure by the first responder or by the clinician who’s providing aid.
These types of technologies are widespread. There are many versions of this technology carried by EMS agencies, trauma bays, US military soldiers, and soldiers across NATO countries. But these types of technologies tend to be relatively inefficient, meaning that they’re very difficult to get into wounds because of the gauze or the powder form of the devices, and it’s very hard to get them in contact with the form of bleeding.
On top of that, if the patient is clotting compromised or immunocompromised in some way, the ability to create a durable clot that will not be ripped off when you remove the product at the next level of care is also of concern. And so, this type of technology or the type of treatment of massive hemorrhage hasn’t changed in decades.
Current Applications and Potential Use
Glatter: I envision this product will be carried by paramedics, used on the battlefield at some point after your FDA clearance, and recently it went through.
Do you see any possibility that this could be an AED equivalent to Stop the Bleed? In other words, could the average lay person be trained to use your product if kits are available?
Landolina: To be very clear, Traumagel today is only approved or cleared under a “prescription-only” indication, which means that it will not initially be available OTC. However, that is our goal. Our goal is to make this product available and usable by someone with no medical training whatsoever.
The form factor of being a gel in a syringe lends itself well to that, meaning that we try to make it as easy as point and shoot to control hemorrhage, where there’s not as much technique to be learned in the application of a product like Traumagel as there is in current hemorrhage control techniques.
Mechanism of Action and Physiology
Glatter: Once you apply Traumagel, can you explain what happens to the product after it’s applied and the bleeding has stopped? Does it get reabsorbed by the body? What’s the process here?
Landolina: Under Traumagel’s indication, because it’s used in traumatic injury, it must be removed within 24 hours.
One of the big benefits of Traumagel is that when the patient produces a blood clot underneath Traumagel, it doesn’t become incorporated within the gel itself. To contrast that with the use of gauze, gauze is porous. The clot ends up wrapped around the fibers of the gauze, so if you peel the gauze away, it’s very likely that clot is coming off with it. The surgeon or the clinician at the next level of care is going to have to deal with the re-bleed.
You can remove Traumagel cleanly and entirely without disturbing the underlying clot. That’s a major benefit, not only to the patient but also to the next level of care, to the next clinician or physician that is required to remove the product.
Glatter: How is it possible to remove the substance without disturbing the clot? Can you explain in more detail?
Landolina: That’s one of the hallmarks of these plant-based polymers and the way that we design Traumagel itself. Traumagel is completely nonporous, and it has no fibrous nature to it. What that means is when the patient produces a blood clot or fibrin next to or on top of Traumagel, that fibrin ends up not incorporated within the polymers of Traumagel itself.
Over time, because Traumagel is a hydrogel, meaning that by weight it’s mostly water, you end up having less adhesion to the clot over time. When it’s time to remove Traumagel from the injury, it has lost almost all of its adhesive capabilities, meaning that when you peel it away, that clot is going to stick better to tissue than it will to the gel itself.
Glatter: Can you explain a little bit about the matrix that’s formed, the physiology, and how the polymers work to form this matrix?
Landolina: Sure. Traumagel is made of two polysaccharides that are plant derived. One polysaccharide is polyanionic, and the other is polycationic, meaning one has negative charges and the other has positive charges, which together create almost a Lego block effect, where when the material comes in contact with tissue, it adheres strongly and allows for itself to effectively create a mechanical barrier against bleeding.
Courtesy of Cresilon
Landolina: Even in the face of major arterial blood flow, Traumagel will stay where it needs to stay, and it’s not going to get washed away. This means that it is much more easily appliable to these types of surfaces and will allow the patient to produce their own endogenous fibrin clot at that location.
Like I mentioned before, when that fibrin clot is formed, because the gel itself has no pores or fibers, it doesn’t become incorporated within the fibrin clot. You can take the gel away, leaving that clot behind without the chance of a rebleed.
Testing With Major Bleeds
Glatter: In terms of bleeding itself, have you tested your product with major aortic bleeds or carotid bleeds in preclinical work?
Landolina: We have used the US military’s model for lethal hemorrhage, and the idea there is to create a model that is just that — lethal. These are the worst types of bleeds that you can possibly imagine, where the patients are clotting compromised, and where you have, in most cases, a very strong arterial component, so something like a femoral artery bleed.
We’ve also tested in carotid artery, aortic applications, as well as combinations of venous and arterial bleeds. The idea here is to show the use of the product in the absolute worst-case scenario so that when this translates into the clinic, the models that we’ve used for evaluation, hopefully, are worse than what actually rolls into the trauma bay.
Glatter: Excellent. What’s the mean time to stop an arterial vs a venous bleed? Are we talking a matter of seconds?
Landolina: In the case of a healthy patient, meaning a patient without clotting compromise, you’re in a matter of seconds. It’s less than 10 seconds.
In the case where you have clotting compromise, a deep, complicated wound geometry, we recommend holding a pressure bandage on for 3 minutes just because it increases the chance of Traumagel coming into contact with the bleed, especially when you can’t visualize the bleed in the bleed source. Because of that pressure time, that becomes the mean. But again, it’s highly dependent on the type of bleed and the style of application.
Failure Rates and Effectiveness
Glatter: As a segue to that, what is the failure rate based on your studies and internal research using Traumagel? Have there been cases where bleeding has not been able to be stopped?
Landolina: It depends on the study, but the failure rates are incredibly low with Traumagel, assuming that it’s correctly used. That’s one of the benefits to this product, where with proper technique, with overwrap with gauze, you nearly always get control of hemorrhage with a product like this.
Glatter: Is manual pressure required in that sense? From what you described earlier, manual pressure would not be required.
Landolina: It depends on the injury. What we recommend is that, if you have a very deep wound where you cannot visualize the source of bleed, you use pressure to seat Traumagel into the source of bleeding, meaning that you’re following Committee on Tactical Combat Casualty Care (Co-TCCC) regulations or requirements, where you’re over wrapping with gauze, and you’re providing a pressure wrapping to ensure that the Traumagel is in contact with the bleed while it’s doing what it’s doing.
In most cases, it doesn’t hurt to apply pressure on top of Traumagel as well. In more surface level bleeds, you don’t need pressure at all.
Applications Beyond Trauma
Glatter: Interesting. In terms of further applications (eg, nose bleeds or GYN bleeding, which are life-threatening), do you see this coming as an application for the future?
Landolina: That’s where we’re working. Traumagel is the successor to an animal health product called Vetigel. The formulations of the gel behind Vetigel and Traumagel are identical. Vetigel has a full surgical indication, and that’s everything from epistaxis to neuro and spine procedures, into cardiovascular and soft tissue surgeries, orthopedic medicine, and so on.
Cresilon’s goal is to eventually expand the indication of our technology to include surgical indications and other indications where we can help any patient that’s bleeding.
Glatter: That’s important, because we use prehospital whole blood, low titer, specifically, when patients have life-threatening hemorrhage. With your product, that would reduce the amount of blood products that would need to be administered. This could be a real game changer.
Landolina: Definitely, that’s the goal we’re working on.
Infection Risks and Biocompatibility
Glatter: In terms of any risk for infection, has that been studied as well? Does Traumagel in any way lead to increased rates of infection?
Landolina: Traumagel is biocompatible. It’s a sterile product. We’ve done the full suite of biocompatibility testing as required by FDA. On top of that, remember that Vetigel, which is the same formulation, is an implantable product. As a result, that has even extended biocompatibility testing beyond what would be necessary for an external product.
In Vetigel’s use case, which has been used now in over 60,000 patients, primarily companion animals, dogs and cats, we haven’t seen instances of infection. There’s no reason to believe that we would see that clinically with Traumagel.
Research Collaborations and Future Applications
Glatter: In terms of other research that your company’s embarked on preclinically, I understand there were some studies done at Walter Reed Army Institute of Research. I was wondering if you could expand on these, specifically, in terms of traumatic brain injury (TBI) and hemorrhage related to that. For example, with shrapnel or even a gunshot wound.
Landolina: The Walter Reed collaboration with Cresilon is something that I’m particularly excited about, because it marks Cresilon’s first project that’s outside the scope of just hemostasis. Walter Reed came to us with this proposal where there’s a big challenge in a subset of TBI called penetrating ballistic-like brain injury, where the brain has been penetrated by a bullet, shrapnel, or some other projectile, and there’s an injury that exposes the brain to the outside.
Today, there is no standard of care to treat patients with those types of injuries. In many cases, mortality is caused through swelling of the brain, or collapse of the brain. What they came to us with was the potential of using our technology, not primarily as a hemostatic agent, but to be able to stabilize that patient enough to get to the next level of care to be treated by a neurosurgeon.
That study Walter Reed did was just a pilot that was done in small animals. In that pilot, they showed that over the period of treatment, there was no negative change in vital signs, no increase in edema or in swelling, or in any of the biomarkers that were being monitored at that time.
At the very least, this is not full indication that this indication will work for Cresilon, but it shows that there’s promise. It’s something that we’re working on and hopefully we’ll be able to bring to market soon.
Glatter: Certainly, maintaining intracranial pressure and cerebral perfusion pressures are very critical. In the future, do you think this product would be able to be deployed endovascularly? Imagine this in terms of stopping bleeding from some source, whether it’s from a stroke or another intracranial source.
Landolina: That’s been an area of interest for us. We have no evidence to prove that indication works at this point, but there’s also nothing to say that it wouldn’t be possible for our technology. At this point, we’ve only looked at a cursory level at those indications.
Glatter: Does the use of Traumagel obviate the need for a more definitive repair (eg, with sutures) or something that’s more permanent?
Landolina: I always say that Traumagel — and Vetigel, for that matter — is not a replacement for good surgical technique. The surgeon always needs to make his or her best judgment when reviewing the patient. That doesn’t mean that there won’t need to be sutures or vascular repair in most of these cases, especially in major trauma.
Final Takeaways
Glatter: Do you have some bullet points or pearls you could give our audience as a takeaway?
Landolina: When Cresilon looks at Traumagel — and for us, Traumagel is the next generation of hemostatic agent, especially in trauma care and in emergency medicine — it allows for a far-simplified application of the product and much faster control of hemorrhage with better patient outcomes.
As we roll this out through EMS agencies, trauma hospitals, military agencies, and eventually to the general public through a future indication, it’s something we’re very excited about. Personally, I started this business 14 years ago, and so it’s great to see our mission of saving lives transitioning to saving human lives.
Glatter: I look forward to seeing this product in the emergency department, but also in other settings, such as in the operating room where we can really help patients who are dying from hemorrhage, certainly on the battlefield, and the lay public. If someone were to come upon a patient who’s bleeding out, this could be certainly a game changer and a lifesaver.
I want to thank you for your time. This is a really important product that’s transformed the lives of so many animals, but also people in the future.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He reported no relevant conflicts of interest. Mr. Landolina is the CEO and co-founder of Cresilon, a biotechnology company specializing in plant-based solutions for emergency bleeding control.
A version of this article first appeared on Medscape.com.
How Much Water Should We Drink in a Day?
This transcript has been edited for clarity.
It’s just about the easiest, safest medical advice you can give: “Drink more water.” You have a headache? Drink more water. Tired? Drink more water. Cold coming on? Drink more water. Tom Brady famously attributed his QB longevity to water drinking, among some other less ordinary practices.
I’m a nephrologist — a kidney doctor. I think about water all the time. I can tell you how your brain senses how much water is in your body and exactly how it communicates that information to your kidneys to control how dilute your urine is. I can explain the miraculous ability of the kidney to concentrate urine across a range from 50 mOsm/L to 1200 mOsm/L and the physiology that makes it all work.

But I can’t really tell you how much water you’re supposed to drink. And believe me, I get asked all the time.
I’m sure of a couple of things when it comes to water: You need to drink some. Though some animals, such as kangaroo rats, can get virtually all the water they need from the food they eat, we are not such animals. Without water, we die. I’m also sure that you can die from drinking too much water. Drinking excessive amounts of water dilutes the sodium in your blood, which messes with the electrical system in your brain and heart. I actually had a patient who went on a “water cleanse” and gave herself a seizure.
But, to be fair, assuming your kidneys are working reasonably well and you’re otherwise healthy, you’d need to drink around 20 liters of water a day to get into mortal trouble. The dose is the poison, as they say.
So, somewhere between zero and 20 liters of water is the amount you should be drinking in a day. That much I’m sure of.
But the evidence on where in that range you should target is actually pretty skimpy. You wouldn’t think so if you look at the online wellness influencers, with their Stanleys and their strict water intake regimens. You’d think the evidence for the benefits of drinking extra water is overwhelming.
The venerated National Academy of Medicine suggests that men drink thirteen 8 oz cups a day (that’s about 3 liters) and women drink nine 8 oz cups a day (a bit more than 2 liters). From what I can tell, this recommendation — like the old “8 cups of water per day” recommendation — is pulled out of thin air.
I’m not arguing that we shouldn’t drink water. Of course, water is important. I’m just wondering what data there are to really prove that drinking more water is better.
Fortunately, a team from UCSF has finally done the legwork for us. They break down the actual evidence in this paper, appearing in JAMA Network Open.
The team scoured the medical literature for randomized controlled trials of water intake. This is critical; we don’t want anecdotes about how clear someone’s skin became after they increased their water intake. We want icy cold, clear data. Randomized trials take a group of people and, at random, assign some to the intervention — in this case, drinking more water — and others to keep doing what they would normally do.

The team reviewed nearly 1500 papers but only 18 (!) met the rigorous criteria to be included in the analysis, as you can see from this flow chart.

This is the first important finding; not many high-quality studies have investigated how much water we should drink. Of course, water isn’t a prescription product, so funding is likely hard to come by. Can we do a trial of Dasani?
In any case, these 18 trials all looked at different outcomes of interest. Four studies looked at the impact of drinking more water on weight loss, two on fasting blood glucose, two on headache, two on urinary tract infection, two on kidney stones, and six studies on various other outcomes. None of the studies looked at energy, skin tone, or overall wellness, though one did measure a quality-of-life score.
And if I could sum up all these studies in a word, that word would be “meh.”

One of four weight loss studies showed that increasing water intake had no effect on weight loss. Two studies showed an effect, but drinking extra water was combined with a low-calorie diet, so that feels a bit like cheating to me. One study randomized participants to drink half a liter of water before meals, and that group did lose more weight than the control group — about a kilogram more over 12 weeks. That’s not exactly Ozempic.
For fasting blood glucose, although one trial suggested that higher premeal water intake lowered glucose levels, the other study (which looked just at increasing water overall) didn’t.
For headache — and, cards on the table here, I’m a big believer in water for headaches — one study showed nothing. The other showed that increasing water intake by 1.5 liters per day improved migraine-related quality of life but didn’t change the number of headache days per month.
For urinary tract infections, one positive trial and one negative one.
The best evidence comes from the kidney stone trials. Increasing water intake to achieve more than two liters of urine a day was associated with a significant reduction in kidney stone recurrence. I consider this a positive finding, more or less. You would be hard-pressed to find a kidney doctor who doesn’t think that people with a history of kidney stones should drink more water.
What about that quality-of-life study? They randomized participants to either drink 1.5 liters of extra water per day (intervention group) or not (control group). Six months later, the scores on the quality-of-life survey were no different between those two groups.
Thirsty yet?
So, what’s going on here? There are a few possibilities.
First, I need to point out that clinical trials are really hard. All the studies in this review were relatively small, with most enrolling fewer than 100 people. The effect of extra water would need to be pretty potent to detect it with those small samples.
I can’t help but point out that our bodies are actually exquisitely tuned to manage how much water we carry. As we lose water throughout the day from sweat and exhalation, our blood becomes a tiny bit more concentrated — the sodium level goes up. Our brains detect that and create a sensation we call thirst. Thirst is one of the most powerful drives we have. Animals, including humans, when thirsty, will choose water over food, over drugs, and over sex. It is incredibly hard to resist, and assuming that we have ready access to water, there is no need to resist it. We drink when we are thirsty. And that may be enough.
Of course, pushing beyond thirst is possible. We are sapient beings who can drink more than we want to. But what we can’t do, assuming our kidneys work, is hold onto that water. It passes right through us. In the case of preventing kidney stones, this is a good thing. Putting more water into your body leads to more water coming out — more dilute urine — which means it’s harder for stones to form.
But for all that other stuff? The wellness, the skin tone, and so on? It just doesn’t make much sense. If you drink an extra liter of water, you pee an extra liter of water. Net net? Zero.
Some folks will argue that the extra pee gets rid of extra toxins or something like that, but — sorry, kidney doctor Perry here again — that’s not how pee works. The clearance of toxins from the blood happens way upstream of where your urine is diluted or concentrated.

If you drink more, the same toxins come out, just with more water around them. In fact, one of the largest studies in this JAMA Network Open review assessed whether increasing water consumption in people with chronic kidney disease would improve kidney function. It didn’t.
I am left, then, with only a bit more confidence than when I began. Beyond that, it seems reasonable to trust the millions of years of evolution that have made water homeostasis central to life itself. Give yourself access to water. Drink when you’re thirsty. Drink a bit more if you’d like. But no need to push it. Your kidneys won’t let you anyway.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
It’s just about the easiest, safest medical advice you can give: “Drink more water.” You have a headache? Drink more water. Tired? Drink more water. Cold coming on? Drink more water. Tom Brady famously attributed his QB longevity to water drinking, among some other less ordinary practices.
I’m a nephrologist — a kidney doctor. I think about water all the time. I can tell you how your brain senses how much water is in your body and exactly how it communicates that information to your kidneys to control how dilute your urine is. I can explain the miraculous ability of the kidney to concentrate urine across a range from 50 mOsm/L to 1200 mOsm/L and the physiology that makes it all work.

But I can’t really tell you how much water you’re supposed to drink. And believe me, I get asked all the time.
I’m sure of a couple of things when it comes to water: You need to drink some. Though some animals, such as kangaroo rats, can get virtually all the water they need from the food they eat, we are not such animals. Without water, we die. I’m also sure that you can die from drinking too much water. Drinking excessive amounts of water dilutes the sodium in your blood, which messes with the electrical system in your brain and heart. I actually had a patient who went on a “water cleanse” and gave herself a seizure.
But, to be fair, assuming your kidneys are working reasonably well and you’re otherwise healthy, you’d need to drink around 20 liters of water a day to get into mortal trouble. The dose is the poison, as they say.
So, somewhere between zero and 20 liters of water is the amount you should be drinking in a day. That much I’m sure of.
But the evidence on where in that range you should target is actually pretty skimpy. You wouldn’t think so if you look at the online wellness influencers, with their Stanleys and their strict water intake regimens. You’d think the evidence for the benefits of drinking extra water is overwhelming.
The venerated National Academy of Medicine suggests that men drink thirteen 8 oz cups a day (that’s about 3 liters) and women drink nine 8 oz cups a day (a bit more than 2 liters). From what I can tell, this recommendation — like the old “8 cups of water per day” recommendation — is pulled out of thin air.
I’m not arguing that we shouldn’t drink water. Of course, water is important. I’m just wondering what data there are to really prove that drinking more water is better.
Fortunately, a team from UCSF has finally done the legwork for us. They break down the actual evidence in this paper, appearing in JAMA Network Open.
The team scoured the medical literature for randomized controlled trials of water intake. This is critical; we don’t want anecdotes about how clear someone’s skin became after they increased their water intake. We want icy cold, clear data. Randomized trials take a group of people and, at random, assign some to the intervention — in this case, drinking more water — and others to keep doing what they would normally do.

The team reviewed nearly 1500 papers but only 18 (!) met the rigorous criteria to be included in the analysis, as you can see from this flow chart.

This is the first important finding; not many high-quality studies have investigated how much water we should drink. Of course, water isn’t a prescription product, so funding is likely hard to come by. Can we do a trial of Dasani?
In any case, these 18 trials all looked at different outcomes of interest. Four studies looked at the impact of drinking more water on weight loss, two on fasting blood glucose, two on headache, two on urinary tract infection, two on kidney stones, and six studies on various other outcomes. None of the studies looked at energy, skin tone, or overall wellness, though one did measure a quality-of-life score.
And if I could sum up all these studies in a word, that word would be “meh.”

One of four weight loss studies showed that increasing water intake had no effect on weight loss. Two studies showed an effect, but drinking extra water was combined with a low-calorie diet, so that feels a bit like cheating to me. One study randomized participants to drink half a liter of water before meals, and that group did lose more weight than the control group — about a kilogram more over 12 weeks. That’s not exactly Ozempic.
For fasting blood glucose, although one trial suggested that higher premeal water intake lowered glucose levels, the other study (which looked just at increasing water overall) didn’t.
For headache — and, cards on the table here, I’m a big believer in water for headaches — one study showed nothing. The other showed that increasing water intake by 1.5 liters per day improved migraine-related quality of life but didn’t change the number of headache days per month.
For urinary tract infections, one positive trial and one negative one.
The best evidence comes from the kidney stone trials. Increasing water intake to achieve more than two liters of urine a day was associated with a significant reduction in kidney stone recurrence. I consider this a positive finding, more or less. You would be hard-pressed to find a kidney doctor who doesn’t think that people with a history of kidney stones should drink more water.
What about that quality-of-life study? They randomized participants to either drink 1.5 liters of extra water per day (intervention group) or not (control group). Six months later, the scores on the quality-of-life survey were no different between those two groups.
Thirsty yet?
So, what’s going on here? There are a few possibilities.
First, I need to point out that clinical trials are really hard. All the studies in this review were relatively small, with most enrolling fewer than 100 people. The effect of extra water would need to be pretty potent to detect it with those small samples.
I can’t help but point out that our bodies are actually exquisitely tuned to manage how much water we carry. As we lose water throughout the day from sweat and exhalation, our blood becomes a tiny bit more concentrated — the sodium level goes up. Our brains detect that and create a sensation we call thirst. Thirst is one of the most powerful drives we have. Animals, including humans, when thirsty, will choose water over food, over drugs, and over sex. It is incredibly hard to resist, and assuming that we have ready access to water, there is no need to resist it. We drink when we are thirsty. And that may be enough.
Of course, pushing beyond thirst is possible. We are sapient beings who can drink more than we want to. But what we can’t do, assuming our kidneys work, is hold onto that water. It passes right through us. In the case of preventing kidney stones, this is a good thing. Putting more water into your body leads to more water coming out — more dilute urine — which means it’s harder for stones to form.
But for all that other stuff? The wellness, the skin tone, and so on? It just doesn’t make much sense. If you drink an extra liter of water, you pee an extra liter of water. Net net? Zero.
Some folks will argue that the extra pee gets rid of extra toxins or something like that, but — sorry, kidney doctor Perry here again — that’s not how pee works. The clearance of toxins from the blood happens way upstream of where your urine is diluted or concentrated.

If you drink more, the same toxins come out, just with more water around them. In fact, one of the largest studies in this JAMA Network Open review assessed whether increasing water consumption in people with chronic kidney disease would improve kidney function. It didn’t.
I am left, then, with only a bit more confidence than when I began. Beyond that, it seems reasonable to trust the millions of years of evolution that have made water homeostasis central to life itself. Give yourself access to water. Drink when you’re thirsty. Drink a bit more if you’d like. But no need to push it. Your kidneys won’t let you anyway.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
It’s just about the easiest, safest medical advice you can give: “Drink more water.” You have a headache? Drink more water. Tired? Drink more water. Cold coming on? Drink more water. Tom Brady famously attributed his QB longevity to water drinking, among some other less ordinary practices.
I’m a nephrologist — a kidney doctor. I think about water all the time. I can tell you how your brain senses how much water is in your body and exactly how it communicates that information to your kidneys to control how dilute your urine is. I can explain the miraculous ability of the kidney to concentrate urine across a range from 50 mOsm/L to 1200 mOsm/L and the physiology that makes it all work.

But I can’t really tell you how much water you’re supposed to drink. And believe me, I get asked all the time.
I’m sure of a couple of things when it comes to water: You need to drink some. Though some animals, such as kangaroo rats, can get virtually all the water they need from the food they eat, we are not such animals. Without water, we die. I’m also sure that you can die from drinking too much water. Drinking excessive amounts of water dilutes the sodium in your blood, which messes with the electrical system in your brain and heart. I actually had a patient who went on a “water cleanse” and gave herself a seizure.
But, to be fair, assuming your kidneys are working reasonably well and you’re otherwise healthy, you’d need to drink around 20 liters of water a day to get into mortal trouble. The dose is the poison, as they say.
So, somewhere between zero and 20 liters of water is the amount you should be drinking in a day. That much I’m sure of.
But the evidence on where in that range you should target is actually pretty skimpy. You wouldn’t think so if you look at the online wellness influencers, with their Stanleys and their strict water intake regimens. You’d think the evidence for the benefits of drinking extra water is overwhelming.
The venerated National Academy of Medicine suggests that men drink thirteen 8 oz cups a day (that’s about 3 liters) and women drink nine 8 oz cups a day (a bit more than 2 liters). From what I can tell, this recommendation — like the old “8 cups of water per day” recommendation — is pulled out of thin air.
I’m not arguing that we shouldn’t drink water. Of course, water is important. I’m just wondering what data there are to really prove that drinking more water is better.
Fortunately, a team from UCSF has finally done the legwork for us. They break down the actual evidence in this paper, appearing in JAMA Network Open.
The team scoured the medical literature for randomized controlled trials of water intake. This is critical; we don’t want anecdotes about how clear someone’s skin became after they increased their water intake. We want icy cold, clear data. Randomized trials take a group of people and, at random, assign some to the intervention — in this case, drinking more water — and others to keep doing what they would normally do.

The team reviewed nearly 1500 papers but only 18 (!) met the rigorous criteria to be included in the analysis, as you can see from this flow chart.

This is the first important finding; not many high-quality studies have investigated how much water we should drink. Of course, water isn’t a prescription product, so funding is likely hard to come by. Can we do a trial of Dasani?
In any case, these 18 trials all looked at different outcomes of interest. Four studies looked at the impact of drinking more water on weight loss, two on fasting blood glucose, two on headache, two on urinary tract infection, two on kidney stones, and six studies on various other outcomes. None of the studies looked at energy, skin tone, or overall wellness, though one did measure a quality-of-life score.
And if I could sum up all these studies in a word, that word would be “meh.”

One of four weight loss studies showed that increasing water intake had no effect on weight loss. Two studies showed an effect, but drinking extra water was combined with a low-calorie diet, so that feels a bit like cheating to me. One study randomized participants to drink half a liter of water before meals, and that group did lose more weight than the control group — about a kilogram more over 12 weeks. That’s not exactly Ozempic.
For fasting blood glucose, although one trial suggested that higher premeal water intake lowered glucose levels, the other study (which looked just at increasing water overall) didn’t.
For headache — and, cards on the table here, I’m a big believer in water for headaches — one study showed nothing. The other showed that increasing water intake by 1.5 liters per day improved migraine-related quality of life but didn’t change the number of headache days per month.
For urinary tract infections, one positive trial and one negative one.
The best evidence comes from the kidney stone trials. Increasing water intake to achieve more than two liters of urine a day was associated with a significant reduction in kidney stone recurrence. I consider this a positive finding, more or less. You would be hard-pressed to find a kidney doctor who doesn’t think that people with a history of kidney stones should drink more water.
What about that quality-of-life study? They randomized participants to either drink 1.5 liters of extra water per day (intervention group) or not (control group). Six months later, the scores on the quality-of-life survey were no different between those two groups.
Thirsty yet?
So, what’s going on here? There are a few possibilities.
First, I need to point out that clinical trials are really hard. All the studies in this review were relatively small, with most enrolling fewer than 100 people. The effect of extra water would need to be pretty potent to detect it with those small samples.
I can’t help but point out that our bodies are actually exquisitely tuned to manage how much water we carry. As we lose water throughout the day from sweat and exhalation, our blood becomes a tiny bit more concentrated — the sodium level goes up. Our brains detect that and create a sensation we call thirst. Thirst is one of the most powerful drives we have. Animals, including humans, when thirsty, will choose water over food, over drugs, and over sex. It is incredibly hard to resist, and assuming that we have ready access to water, there is no need to resist it. We drink when we are thirsty. And that may be enough.
Of course, pushing beyond thirst is possible. We are sapient beings who can drink more than we want to. But what we can’t do, assuming our kidneys work, is hold onto that water. It passes right through us. In the case of preventing kidney stones, this is a good thing. Putting more water into your body leads to more water coming out — more dilute urine — which means it’s harder for stones to form.
But for all that other stuff? The wellness, the skin tone, and so on? It just doesn’t make much sense. If you drink an extra liter of water, you pee an extra liter of water. Net net? Zero.
Some folks will argue that the extra pee gets rid of extra toxins or something like that, but — sorry, kidney doctor Perry here again — that’s not how pee works. The clearance of toxins from the blood happens way upstream of where your urine is diluted or concentrated.

If you drink more, the same toxins come out, just with more water around them. In fact, one of the largest studies in this JAMA Network Open review assessed whether increasing water consumption in people with chronic kidney disease would improve kidney function. It didn’t.
I am left, then, with only a bit more confidence than when I began. Beyond that, it seems reasonable to trust the millions of years of evolution that have made water homeostasis central to life itself. Give yourself access to water. Drink when you’re thirsty. Drink a bit more if you’d like. But no need to push it. Your kidneys won’t let you anyway.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Six Updates on Stroke Management
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
