User login
How to Discuss Lifestyle Modifications in MASLD
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).
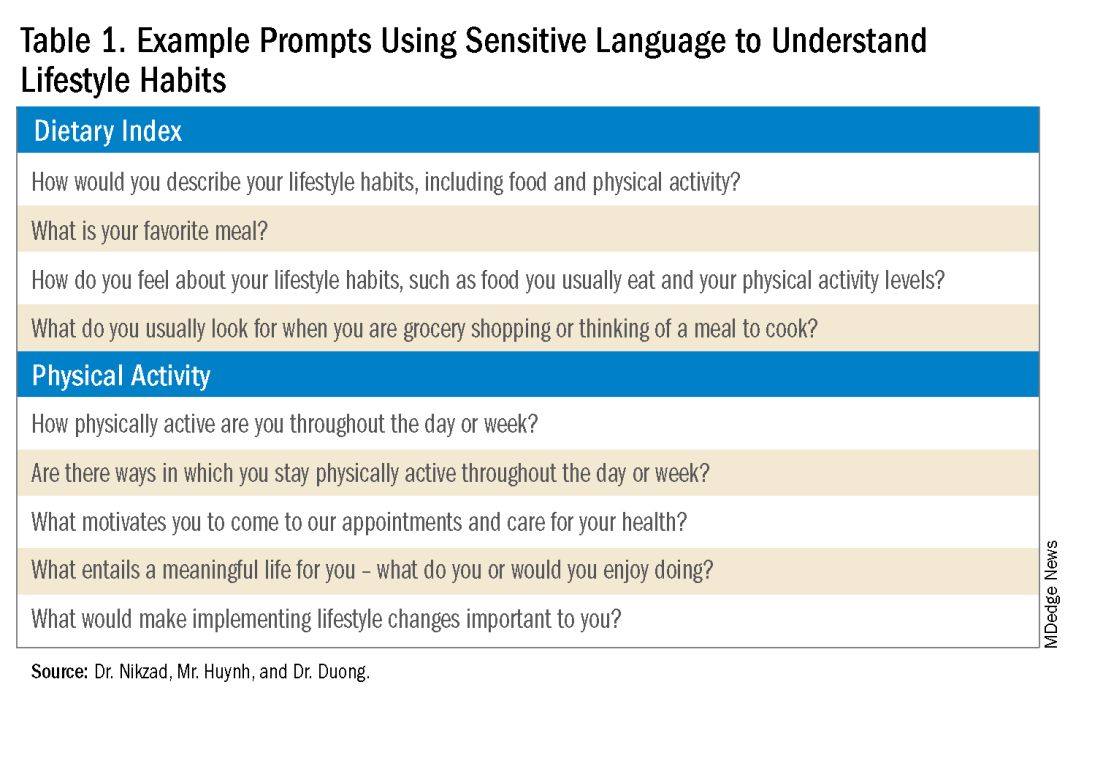
Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
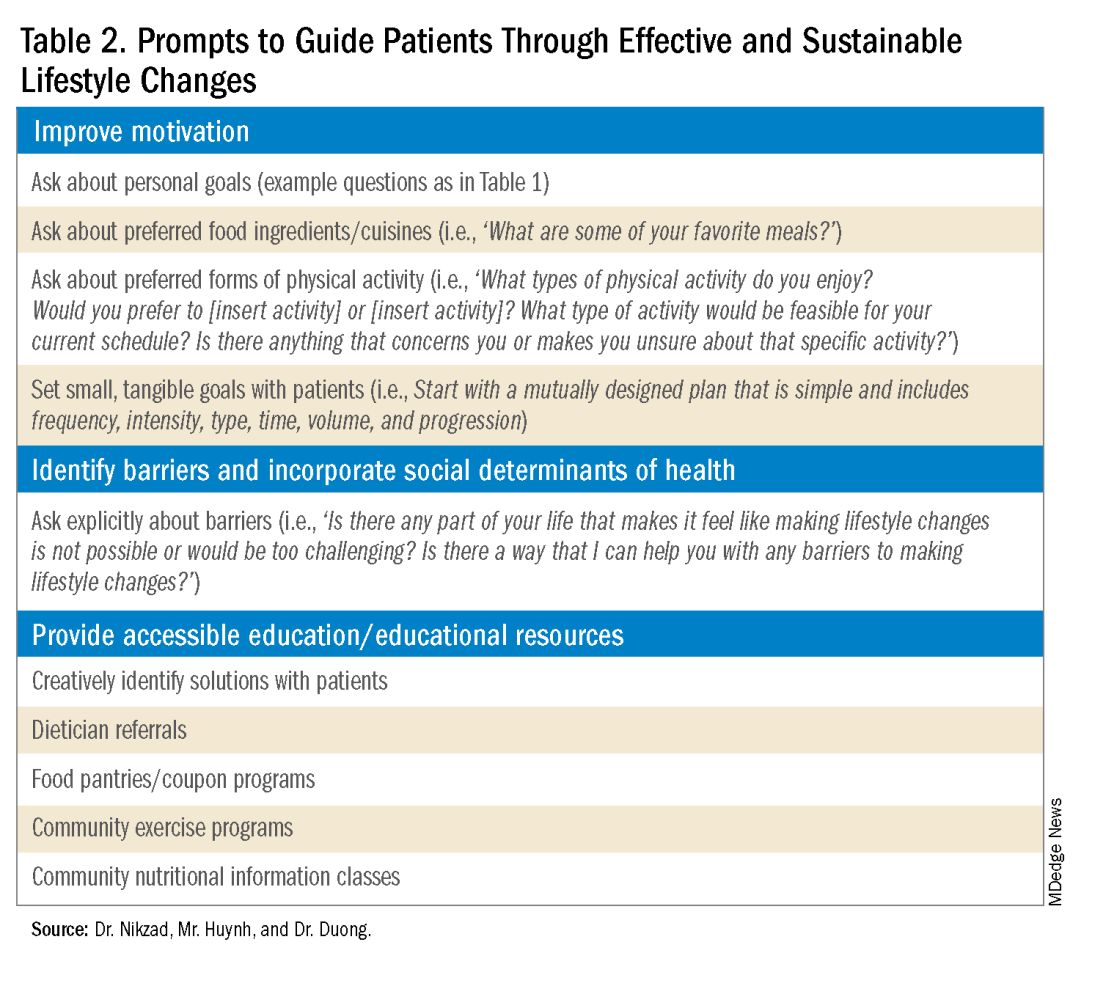
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.
Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Aliens, Ian McShane, and Heart Disease Risk
This transcript has been edited for clarity.
I was really struggling to think of a good analogy to explain the glaring problem of polygenic risk scores (PRS) this week. But I think I have it now. Go with me on this.
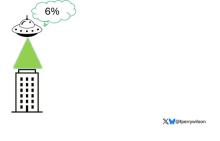
An alien spaceship parks itself, Independence Day style, above a local office building.
But unlike the aliens that gave such a hard time to Will Smith and Brent Spiner, these are benevolent, technologically superior guys. They shine a mysterious green light down on the building and then announce, maybe via telepathy, that 6% of the people in that building will have a heart attack in the next year.
They move on to the next building. “Five percent will have a heart attack in the next year.” And the next, 7%. And the next, 2%.
Let’s assume the aliens are entirely accurate. What do you do with this information?
Most of us would suggest that you find out who was in the buildings with the higher percentages. You check their cholesterol levels, get them to exercise more, do some stress tests, and so on.
But that said, you’d still be spending a lot of money on a bunch of people who were not going to have heart attacks. So, a crack team of spies — in my mind, this is definitely led by a grizzled Ian McShane — infiltrate the alien ship, steal this predictive ray gun, and start pointing it, not at buildings but at people.
In this scenario, one person could have a 10% chance of having a heart attack in the next year. Another person has a 50% chance. The aliens, seeing this, leave us one final message before flying into the great beyond: “No, you guys are doing it wrong.”
This week: The people and companies using an advanced predictive technology, PRS , wrong — and a study that shows just how problematic this is.
We all know that genes play a significant role in our health outcomes. Some diseases (Huntington disease, cystic fibrosis, sickle cell disease, hemochromatosis, and Duchenne muscular dystrophy, for example) are entirely driven by genetic mutations.
The vast majority of chronic diseases we face are not driven by genetics, but they may be enhanced by genetics. Coronary heart disease (CHD) is a prime example. There are clearly environmental risk factors, like smoking, that dramatically increase risk. But there are also genetic underpinnings; about half the risk for CHD comes from genetic variation, according to one study.
But in the case of those common diseases, it’s not one gene that leads to increased risk; it’s the aggregate effect of multiple risk genes, each contributing a small amount of risk to the final total.
The promise of PRS was based on this fact. Take the genome of an individual, identify all the risk genes, and integrate them into some final number that represents your genetic risk of developing CHD.
The way you derive a PRS is take a big group of people and sequence their genomes. Then, you see who develops the disease of interest — in this case, CHD. If the people who develop CHD are more likely to have a particular mutation, that mutation goes in the risk score. Risk scores can integrate tens, hundreds, even thousands of individual mutations to create that final score.
There are literally dozens of PRS for CHD. And there are companies that will calculate yours right now for a reasonable fee.
The accuracy of these scores is assessed at the population level. It’s the alien ray gun thing. Researchers apply the PRS to a big group of people and say 20% of them should develop CHD. If indeed 20% develop CHD, they say the score is accurate. And that’s true.
But what happens next is the problem. Companies and even doctors have been marketing PRS to individuals. And honestly, it sounds amazing. “We’ll use sophisticated techniques to analyze your genetic code and integrate the information to give you your personal risk for CHD.” Or dementia. Or other diseases. A lot of people would want to know this information.
It turns out, though, that this is where the system breaks down. And it is nicely illustrated by this study, appearing November 16 in JAMA.
The authors wanted to see how PRS, which are developed to predict disease in a group of people, work when applied to an individual.
They identified 48 previously published PRS for CHD. They applied those scores to more than 170,000 individuals across multiple genetic databases. And, by and large, the scores worked as advertised, at least across the entire group. The weighted accuracy of all 48 scores was around 78%. They aren’t perfect, of course. We wouldn’t expect them to be, since CHD is not entirely driven by genetics. But 78% accurate isn’t too bad.
But that accuracy is at the population level. At the level of the office building. At the individual level, it was a vastly different story.
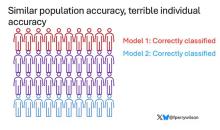
This is best illustrated by this plot, which shows the score from 48 different PRS for CHD within the same person. A note here: It is arranged by the publication date of the risk score, but these were all assessed on a single blood sample at a single point in time in this study participant.
The individual scores are all over the map. Using one risk score gives an individual a risk that is near the 99th percentile — a ticking time bomb of CHD. Another score indicates a level of risk at the very bottom of the spectrum — highly reassuring. A bunch of scores fall somewhere in between. In other words, as a doctor, the risk I will discuss with this patient is more strongly determined by which PRS I happen to choose than by his actual genetic risk, whatever that is.
This may seem counterintuitive. All these risk scores were similarly accurate within a population; how can they all give different results to an individual? The answer is simpler than you may think. As long as a given score makes one extra good prediction for each extra bad prediction, its accuracy is not changed.
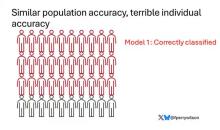
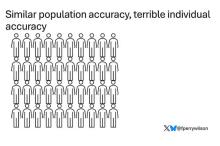
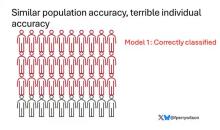
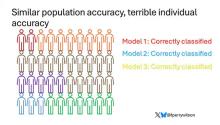
Let’s imagine we have a population of 40 people.
Risk score model 1 correctly classified 30 of them for 75% accuracy. Great.
Risk score model 2 also correctly classified 30 of our 40 individuals, for 75% accuracy. It’s just a different 30.
Risk score model 3 also correctly classified 30 of 40, but another different 30.
I’ve colored this to show you all the different overlaps. What you can see is that although each score has similar accuracy, the individual people have a bunch of different colors, indicating that some scores worked for them and some didn’t. That’s a real problem.
This has not stopped companies from advertising PRS for all sorts of diseases. Companies are even using PRS to decide which fetuses to implant during IVF therapy, which is a particularly egregiously wrong use of this technology that I have written about before.
How do you fix this? Our aliens tried to warn us. This is not how you are supposed to use this ray gun. You are supposed to use it to identify groups of people at higher risk to direct more resources to that group. That’s really all you can do.
It’s also possible that we need to match the risk score to the individual in a better way. This is likely driven by the fact that risk scores tend to work best in the populations in which they were developed, and many of them were developed in people of largely European ancestry.
It is worth noting that if a PRS had perfect accuracy at the population level, it would also necessarily have perfect accuracy at the individual level. But there aren’t any scores like that. It’s possible that combining various scores may increase the individual accuracy, but that hasn’t been demonstrated yet either.
Look, genetics is and will continue to play a major role in healthcare. At the same time, sequencing entire genomes is a technology that is ripe for hype and thus misuse. Or even abuse. Fundamentally, this JAMA study reminds us that accuracy in a population and accuracy in an individual are not the same. But more deeply, it reminds us that just because a technology is new or cool or expensive doesn’t mean it will work in the clinic.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I was really struggling to think of a good analogy to explain the glaring problem of polygenic risk scores (PRS) this week. But I think I have it now. Go with me on this.
An alien spaceship parks itself, Independence Day style, above a local office building.
But unlike the aliens that gave such a hard time to Will Smith and Brent Spiner, these are benevolent, technologically superior guys. They shine a mysterious green light down on the building and then announce, maybe via telepathy, that 6% of the people in that building will have a heart attack in the next year.
They move on to the next building. “Five percent will have a heart attack in the next year.” And the next, 7%. And the next, 2%.
Let’s assume the aliens are entirely accurate. What do you do with this information?
Most of us would suggest that you find out who was in the buildings with the higher percentages. You check their cholesterol levels, get them to exercise more, do some stress tests, and so on.
But that said, you’d still be spending a lot of money on a bunch of people who were not going to have heart attacks. So, a crack team of spies — in my mind, this is definitely led by a grizzled Ian McShane — infiltrate the alien ship, steal this predictive ray gun, and start pointing it, not at buildings but at people.
In this scenario, one person could have a 10% chance of having a heart attack in the next year. Another person has a 50% chance. The aliens, seeing this, leave us one final message before flying into the great beyond: “No, you guys are doing it wrong.”
This week: The people and companies using an advanced predictive technology, PRS , wrong — and a study that shows just how problematic this is.
We all know that genes play a significant role in our health outcomes. Some diseases (Huntington disease, cystic fibrosis, sickle cell disease, hemochromatosis, and Duchenne muscular dystrophy, for example) are entirely driven by genetic mutations.
The vast majority of chronic diseases we face are not driven by genetics, but they may be enhanced by genetics. Coronary heart disease (CHD) is a prime example. There are clearly environmental risk factors, like smoking, that dramatically increase risk. But there are also genetic underpinnings; about half the risk for CHD comes from genetic variation, according to one study.
But in the case of those common diseases, it’s not one gene that leads to increased risk; it’s the aggregate effect of multiple risk genes, each contributing a small amount of risk to the final total.
The promise of PRS was based on this fact. Take the genome of an individual, identify all the risk genes, and integrate them into some final number that represents your genetic risk of developing CHD.
The way you derive a PRS is take a big group of people and sequence their genomes. Then, you see who develops the disease of interest — in this case, CHD. If the people who develop CHD are more likely to have a particular mutation, that mutation goes in the risk score. Risk scores can integrate tens, hundreds, even thousands of individual mutations to create that final score.
There are literally dozens of PRS for CHD. And there are companies that will calculate yours right now for a reasonable fee.
The accuracy of these scores is assessed at the population level. It’s the alien ray gun thing. Researchers apply the PRS to a big group of people and say 20% of them should develop CHD. If indeed 20% develop CHD, they say the score is accurate. And that’s true.
But what happens next is the problem. Companies and even doctors have been marketing PRS to individuals. And honestly, it sounds amazing. “We’ll use sophisticated techniques to analyze your genetic code and integrate the information to give you your personal risk for CHD.” Or dementia. Or other diseases. A lot of people would want to know this information.
It turns out, though, that this is where the system breaks down. And it is nicely illustrated by this study, appearing November 16 in JAMA.
The authors wanted to see how PRS, which are developed to predict disease in a group of people, work when applied to an individual.
They identified 48 previously published PRS for CHD. They applied those scores to more than 170,000 individuals across multiple genetic databases. And, by and large, the scores worked as advertised, at least across the entire group. The weighted accuracy of all 48 scores was around 78%. They aren’t perfect, of course. We wouldn’t expect them to be, since CHD is not entirely driven by genetics. But 78% accurate isn’t too bad.
But that accuracy is at the population level. At the level of the office building. At the individual level, it was a vastly different story.
This is best illustrated by this plot, which shows the score from 48 different PRS for CHD within the same person. A note here: It is arranged by the publication date of the risk score, but these were all assessed on a single blood sample at a single point in time in this study participant.
The individual scores are all over the map. Using one risk score gives an individual a risk that is near the 99th percentile — a ticking time bomb of CHD. Another score indicates a level of risk at the very bottom of the spectrum — highly reassuring. A bunch of scores fall somewhere in between. In other words, as a doctor, the risk I will discuss with this patient is more strongly determined by which PRS I happen to choose than by his actual genetic risk, whatever that is.
This may seem counterintuitive. All these risk scores were similarly accurate within a population; how can they all give different results to an individual? The answer is simpler than you may think. As long as a given score makes one extra good prediction for each extra bad prediction, its accuracy is not changed.
Let’s imagine we have a population of 40 people.
Risk score model 1 correctly classified 30 of them for 75% accuracy. Great.
Risk score model 2 also correctly classified 30 of our 40 individuals, for 75% accuracy. It’s just a different 30.
Risk score model 3 also correctly classified 30 of 40, but another different 30.
I’ve colored this to show you all the different overlaps. What you can see is that although each score has similar accuracy, the individual people have a bunch of different colors, indicating that some scores worked for them and some didn’t. That’s a real problem.
This has not stopped companies from advertising PRS for all sorts of diseases. Companies are even using PRS to decide which fetuses to implant during IVF therapy, which is a particularly egregiously wrong use of this technology that I have written about before.
How do you fix this? Our aliens tried to warn us. This is not how you are supposed to use this ray gun. You are supposed to use it to identify groups of people at higher risk to direct more resources to that group. That’s really all you can do.
It’s also possible that we need to match the risk score to the individual in a better way. This is likely driven by the fact that risk scores tend to work best in the populations in which they were developed, and many of them were developed in people of largely European ancestry.
It is worth noting that if a PRS had perfect accuracy at the population level, it would also necessarily have perfect accuracy at the individual level. But there aren’t any scores like that. It’s possible that combining various scores may increase the individual accuracy, but that hasn’t been demonstrated yet either.
Look, genetics is and will continue to play a major role in healthcare. At the same time, sequencing entire genomes is a technology that is ripe for hype and thus misuse. Or even abuse. Fundamentally, this JAMA study reminds us that accuracy in a population and accuracy in an individual are not the same. But more deeply, it reminds us that just because a technology is new or cool or expensive doesn’t mean it will work in the clinic.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I was really struggling to think of a good analogy to explain the glaring problem of polygenic risk scores (PRS) this week. But I think I have it now. Go with me on this.
An alien spaceship parks itself, Independence Day style, above a local office building.
But unlike the aliens that gave such a hard time to Will Smith and Brent Spiner, these are benevolent, technologically superior guys. They shine a mysterious green light down on the building and then announce, maybe via telepathy, that 6% of the people in that building will have a heart attack in the next year.
They move on to the next building. “Five percent will have a heart attack in the next year.” And the next, 7%. And the next, 2%.
Let’s assume the aliens are entirely accurate. What do you do with this information?
Most of us would suggest that you find out who was in the buildings with the higher percentages. You check their cholesterol levels, get them to exercise more, do some stress tests, and so on.
But that said, you’d still be spending a lot of money on a bunch of people who were not going to have heart attacks. So, a crack team of spies — in my mind, this is definitely led by a grizzled Ian McShane — infiltrate the alien ship, steal this predictive ray gun, and start pointing it, not at buildings but at people.
In this scenario, one person could have a 10% chance of having a heart attack in the next year. Another person has a 50% chance. The aliens, seeing this, leave us one final message before flying into the great beyond: “No, you guys are doing it wrong.”
This week: The people and companies using an advanced predictive technology, PRS , wrong — and a study that shows just how problematic this is.
We all know that genes play a significant role in our health outcomes. Some diseases (Huntington disease, cystic fibrosis, sickle cell disease, hemochromatosis, and Duchenne muscular dystrophy, for example) are entirely driven by genetic mutations.
The vast majority of chronic diseases we face are not driven by genetics, but they may be enhanced by genetics. Coronary heart disease (CHD) is a prime example. There are clearly environmental risk factors, like smoking, that dramatically increase risk. But there are also genetic underpinnings; about half the risk for CHD comes from genetic variation, according to one study.
But in the case of those common diseases, it’s not one gene that leads to increased risk; it’s the aggregate effect of multiple risk genes, each contributing a small amount of risk to the final total.
The promise of PRS was based on this fact. Take the genome of an individual, identify all the risk genes, and integrate them into some final number that represents your genetic risk of developing CHD.
The way you derive a PRS is take a big group of people and sequence their genomes. Then, you see who develops the disease of interest — in this case, CHD. If the people who develop CHD are more likely to have a particular mutation, that mutation goes in the risk score. Risk scores can integrate tens, hundreds, even thousands of individual mutations to create that final score.
There are literally dozens of PRS for CHD. And there are companies that will calculate yours right now for a reasonable fee.
The accuracy of these scores is assessed at the population level. It’s the alien ray gun thing. Researchers apply the PRS to a big group of people and say 20% of them should develop CHD. If indeed 20% develop CHD, they say the score is accurate. And that’s true.
But what happens next is the problem. Companies and even doctors have been marketing PRS to individuals. And honestly, it sounds amazing. “We’ll use sophisticated techniques to analyze your genetic code and integrate the information to give you your personal risk for CHD.” Or dementia. Or other diseases. A lot of people would want to know this information.
It turns out, though, that this is where the system breaks down. And it is nicely illustrated by this study, appearing November 16 in JAMA.
The authors wanted to see how PRS, which are developed to predict disease in a group of people, work when applied to an individual.
They identified 48 previously published PRS for CHD. They applied those scores to more than 170,000 individuals across multiple genetic databases. And, by and large, the scores worked as advertised, at least across the entire group. The weighted accuracy of all 48 scores was around 78%. They aren’t perfect, of course. We wouldn’t expect them to be, since CHD is not entirely driven by genetics. But 78% accurate isn’t too bad.
But that accuracy is at the population level. At the level of the office building. At the individual level, it was a vastly different story.
This is best illustrated by this plot, which shows the score from 48 different PRS for CHD within the same person. A note here: It is arranged by the publication date of the risk score, but these were all assessed on a single blood sample at a single point in time in this study participant.
The individual scores are all over the map. Using one risk score gives an individual a risk that is near the 99th percentile — a ticking time bomb of CHD. Another score indicates a level of risk at the very bottom of the spectrum — highly reassuring. A bunch of scores fall somewhere in between. In other words, as a doctor, the risk I will discuss with this patient is more strongly determined by which PRS I happen to choose than by his actual genetic risk, whatever that is.
This may seem counterintuitive. All these risk scores were similarly accurate within a population; how can they all give different results to an individual? The answer is simpler than you may think. As long as a given score makes one extra good prediction for each extra bad prediction, its accuracy is not changed.
Let’s imagine we have a population of 40 people.
Risk score model 1 correctly classified 30 of them for 75% accuracy. Great.
Risk score model 2 also correctly classified 30 of our 40 individuals, for 75% accuracy. It’s just a different 30.
Risk score model 3 also correctly classified 30 of 40, but another different 30.
I’ve colored this to show you all the different overlaps. What you can see is that although each score has similar accuracy, the individual people have a bunch of different colors, indicating that some scores worked for them and some didn’t. That’s a real problem.
This has not stopped companies from advertising PRS for all sorts of diseases. Companies are even using PRS to decide which fetuses to implant during IVF therapy, which is a particularly egregiously wrong use of this technology that I have written about before.
How do you fix this? Our aliens tried to warn us. This is not how you are supposed to use this ray gun. You are supposed to use it to identify groups of people at higher risk to direct more resources to that group. That’s really all you can do.
It’s also possible that we need to match the risk score to the individual in a better way. This is likely driven by the fact that risk scores tend to work best in the populations in which they were developed, and many of them were developed in people of largely European ancestry.
It is worth noting that if a PRS had perfect accuracy at the population level, it would also necessarily have perfect accuracy at the individual level. But there aren’t any scores like that. It’s possible that combining various scores may increase the individual accuracy, but that hasn’t been demonstrated yet either.
Look, genetics is and will continue to play a major role in healthcare. At the same time, sequencing entire genomes is a technology that is ripe for hype and thus misuse. Or even abuse. Fundamentally, this JAMA study reminds us that accuracy in a population and accuracy in an individual are not the same. But more deeply, it reminds us that just because a technology is new or cool or expensive doesn’t mean it will work in the clinic.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Portrait of the Patient
Most of my writing starts on paper. I’ve stacks of Docket Gold legal pads, yellow and college ruled, filled with Sharpie S-Gel black ink. There are many scratch-outs and arrows, but no doodles. I’m genetically not a doodler. The draft of this essay however was interrupted by a graphic. It is a round figure with stick arms and legs. Somewhat centered are two intense scribbles, which represent eyes. A few loopy curls rest on top. It looks like a Mr. Potato Head, with owl eyes.
“Ah, art!” I say when I flip up the page and discover this spontaneous self-portrait of my 4-year-old. Using the media she had on hand, she let free her stored creative energy, an energy we all seem to have. “Tell me about what you’ve drawn here,” I say. She’s eager to share. Art is a natural way to connect.
My patients have shown me many similar self-portraits. Last week, the artist was a 71-year-old woman. She came with her friend, a 73-year-old woman, who is also my patient. They accompany each other on all their visits. She chose a small realtor pad with a color photo of a blonde with her arms folded and back against a graphic of a house. My patient managed to fit her sketch on the small, lined space, noting with tiny scribbles the lesions she wanted me to check. Although unnecessary, she added eyes, nose, and mouth.
Another drawing was from a middle-aged white man. He has a look that suggests he rises early. His was on white printer paper, which he withdrew from a folder. He drew both a front and back view indicating with precision where I might find the spots he had mapped on his portrait. A retired teacher brought hers with a notably proportional anatomy and uniform tick marks on her face, arms, and legs. It reminded me of a self-portrait by the artist Frida Kahlo’s “The Broken Column.”
Kahlo was born with polio and suffered a severe bus accident as a young woman. She is one of many artists who shared their suffering through their art. “The Broken Column” depicts her with nails running from her face down her right short, weak leg. They look like the ticks my patient had added to her own self-portrait.
I remember in my neurology rotation asking patients to draw a clock. Stroke patients leave a whole half missing. Patients with dementia often crunch all the numbers into a little corner of the circle or forget to add the hands. Some of my dermatology patient self-portraits looked like that. I sometimes wonder if they also need a neurologist.
These pieces of patient art are utilitarian, drawn to narrate the story of what brought them to see me. Yet patients often add superfluous detail, demonstrating that utility and aesthetics are inseparable. I hold their drawings in the best light and notice the features and attributes. It helps me see their concerns from their point of view and primes me to notice other details during the physical exam. Viewing patients’ drawings can help build something called narrative competence the “ability to acknowledge, absorb, interpret, and act on the stories and plights of others.” Like Kahlo, patients are trying to share something with us, universal and recognizable. Art is how we connect to each other.
A few months ago, I walked in a room to see a consult. A white man in his 30s, he had prematurely graying hair and 80s-hip frames for glasses. He explained he was there for a skin screening and stood without warning, taking a step toward me. Like Michelangelo on wet plaster, he had grabbed a purple surgical marker to draw a self-portrait on the exam paper, the table set to just the right height and pitch to be an easel. It was the ginger-bread-man-type portrait with thick arms and legs and frosting-like dots marking the spots of concern. He marked L and R on the sheet, which were opposite what they would be if he was sitting facing me. But this was a self-portrait and he was drawing as it was with him facing the canvas, of course. “Ah, art!” I thought, and said, “Delightful! Tell me about what you’ve drawn here.” And so he did. A faint shadow of his portrait remains on that exam table to this day for every patient to see.
Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Most of my writing starts on paper. I’ve stacks of Docket Gold legal pads, yellow and college ruled, filled with Sharpie S-Gel black ink. There are many scratch-outs and arrows, but no doodles. I’m genetically not a doodler. The draft of this essay however was interrupted by a graphic. It is a round figure with stick arms and legs. Somewhat centered are two intense scribbles, which represent eyes. A few loopy curls rest on top. It looks like a Mr. Potato Head, with owl eyes.
“Ah, art!” I say when I flip up the page and discover this spontaneous self-portrait of my 4-year-old. Using the media she had on hand, she let free her stored creative energy, an energy we all seem to have. “Tell me about what you’ve drawn here,” I say. She’s eager to share. Art is a natural way to connect.
My patients have shown me many similar self-portraits. Last week, the artist was a 71-year-old woman. She came with her friend, a 73-year-old woman, who is also my patient. They accompany each other on all their visits. She chose a small realtor pad with a color photo of a blonde with her arms folded and back against a graphic of a house. My patient managed to fit her sketch on the small, lined space, noting with tiny scribbles the lesions she wanted me to check. Although unnecessary, she added eyes, nose, and mouth.
Another drawing was from a middle-aged white man. He has a look that suggests he rises early. His was on white printer paper, which he withdrew from a folder. He drew both a front and back view indicating with precision where I might find the spots he had mapped on his portrait. A retired teacher brought hers with a notably proportional anatomy and uniform tick marks on her face, arms, and legs. It reminded me of a self-portrait by the artist Frida Kahlo’s “The Broken Column.”
Kahlo was born with polio and suffered a severe bus accident as a young woman. She is one of many artists who shared their suffering through their art. “The Broken Column” depicts her with nails running from her face down her right short, weak leg. They look like the ticks my patient had added to her own self-portrait.
I remember in my neurology rotation asking patients to draw a clock. Stroke patients leave a whole half missing. Patients with dementia often crunch all the numbers into a little corner of the circle or forget to add the hands. Some of my dermatology patient self-portraits looked like that. I sometimes wonder if they also need a neurologist.
These pieces of patient art are utilitarian, drawn to narrate the story of what brought them to see me. Yet patients often add superfluous detail, demonstrating that utility and aesthetics are inseparable. I hold their drawings in the best light and notice the features and attributes. It helps me see their concerns from their point of view and primes me to notice other details during the physical exam. Viewing patients’ drawings can help build something called narrative competence the “ability to acknowledge, absorb, interpret, and act on the stories and plights of others.” Like Kahlo, patients are trying to share something with us, universal and recognizable. Art is how we connect to each other.
A few months ago, I walked in a room to see a consult. A white man in his 30s, he had prematurely graying hair and 80s-hip frames for glasses. He explained he was there for a skin screening and stood without warning, taking a step toward me. Like Michelangelo on wet plaster, he had grabbed a purple surgical marker to draw a self-portrait on the exam paper, the table set to just the right height and pitch to be an easel. It was the ginger-bread-man-type portrait with thick arms and legs and frosting-like dots marking the spots of concern. He marked L and R on the sheet, which were opposite what they would be if he was sitting facing me. But this was a self-portrait and he was drawing as it was with him facing the canvas, of course. “Ah, art!” I thought, and said, “Delightful! Tell me about what you’ve drawn here.” And so he did. A faint shadow of his portrait remains on that exam table to this day for every patient to see.
Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Most of my writing starts on paper. I’ve stacks of Docket Gold legal pads, yellow and college ruled, filled with Sharpie S-Gel black ink. There are many scratch-outs and arrows, but no doodles. I’m genetically not a doodler. The draft of this essay however was interrupted by a graphic. It is a round figure with stick arms and legs. Somewhat centered are two intense scribbles, which represent eyes. A few loopy curls rest on top. It looks like a Mr. Potato Head, with owl eyes.
“Ah, art!” I say when I flip up the page and discover this spontaneous self-portrait of my 4-year-old. Using the media she had on hand, she let free her stored creative energy, an energy we all seem to have. “Tell me about what you’ve drawn here,” I say. She’s eager to share. Art is a natural way to connect.
My patients have shown me many similar self-portraits. Last week, the artist was a 71-year-old woman. She came with her friend, a 73-year-old woman, who is also my patient. They accompany each other on all their visits. She chose a small realtor pad with a color photo of a blonde with her arms folded and back against a graphic of a house. My patient managed to fit her sketch on the small, lined space, noting with tiny scribbles the lesions she wanted me to check. Although unnecessary, she added eyes, nose, and mouth.
Another drawing was from a middle-aged white man. He has a look that suggests he rises early. His was on white printer paper, which he withdrew from a folder. He drew both a front and back view indicating with precision where I might find the spots he had mapped on his portrait. A retired teacher brought hers with a notably proportional anatomy and uniform tick marks on her face, arms, and legs. It reminded me of a self-portrait by the artist Frida Kahlo’s “The Broken Column.”
Kahlo was born with polio and suffered a severe bus accident as a young woman. She is one of many artists who shared their suffering through their art. “The Broken Column” depicts her with nails running from her face down her right short, weak leg. They look like the ticks my patient had added to her own self-portrait.
I remember in my neurology rotation asking patients to draw a clock. Stroke patients leave a whole half missing. Patients with dementia often crunch all the numbers into a little corner of the circle or forget to add the hands. Some of my dermatology patient self-portraits looked like that. I sometimes wonder if they also need a neurologist.
These pieces of patient art are utilitarian, drawn to narrate the story of what brought them to see me. Yet patients often add superfluous detail, demonstrating that utility and aesthetics are inseparable. I hold their drawings in the best light and notice the features and attributes. It helps me see their concerns from their point of view and primes me to notice other details during the physical exam. Viewing patients’ drawings can help build something called narrative competence the “ability to acknowledge, absorb, interpret, and act on the stories and plights of others.” Like Kahlo, patients are trying to share something with us, universal and recognizable. Art is how we connect to each other.
A few months ago, I walked in a room to see a consult. A white man in his 30s, he had prematurely graying hair and 80s-hip frames for glasses. He explained he was there for a skin screening and stood without warning, taking a step toward me. Like Michelangelo on wet plaster, he had grabbed a purple surgical marker to draw a self-portrait on the exam paper, the table set to just the right height and pitch to be an easel. It was the ginger-bread-man-type portrait with thick arms and legs and frosting-like dots marking the spots of concern. He marked L and R on the sheet, which were opposite what they would be if he was sitting facing me. But this was a self-portrait and he was drawing as it was with him facing the canvas, of course. “Ah, art!” I thought, and said, “Delightful! Tell me about what you’ve drawn here.” And so he did. A faint shadow of his portrait remains on that exam table to this day for every patient to see.
Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
The Veteran’s Canon Under Fire
The Veteran’s Canon Under Fire
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
The Veteran’s Canon Under Fire
The Veteran’s Canon Under Fire
Gentle Parenting
In one my recent Letters, I concluded with the concern that infant-led weaning, which makes some sense, can be confused with child-led family meals, which make none. I referred to an increasingly popular style of parenting overemphasizing child autonomy that seems to be a major contributor to the mealtime chaos that occurs when pleasing every palate at the table becomes the goal.
In the intervening weeks, I have learned that this parenting style is called “gentle parenting.” Despite its growing popularity, possibly fueled by the pandemic, it has not been well-defined nor its effectiveness investigated. In a recent paper published in PLOS ONE, two professors of developmental psychology have attempted correct this deficit in our understanding of this parenting style, which doesn’t appear to make sense to many of us with experience in child behavior and development.
Gentle Parents
By surveying a group of 100 parents of young children, the investigators were able to sort out a group of parents (n = 49) who self-identified as employing gentle parenting. Their responses emphasized a high level of parental affection and emotional regulation by both their children and themselves.
Investigators found that 40% of the self-defined gentle parents “had negative difference scores indicating misbehavior response descriptions that included more child directed responses. I interpret this to mean that almost half of the time the parents failed to evenly include themselves in a solution to a conflict, which indicates incomplete or unsuccessful emotional regulation on their part. The investigators also observed that, like many other parenting styles, gentle parenting includes an emphasis on boundaries “yet, enactment of those boundaries is not uniform.”
More telling was the authors’ observation that “statements of parenting uncertainty and burnout were present in over one third of the gentle of the gentle parenting sample.” While some parents were pleased with their experience, the downside seems unacceptable to me. When asked to explain this finding, Annie Pezalla, PhD, one of the coauthors, has said “gentle parenting practices work best when a parent is emotionally regulated and unconstrained for time — commodities that parents struggle with the most.”
Abundance Advice on Parenting Styles
I find this to be a very sad story. Parenting can be difficult. Creating and then gently and effectively policing those boundaries is often the hardest part. It is not surprising to me that of the four books I have written for parents, the one titled How to Say No to Your Toddler is the only one popular enough to be published in four languages.
Of course I am troubled, as I suspect you may be, with the label “gentle parenting.” It implies that the rest of us are doing something terrible, “harsh” maybe, “cruel” maybe. We can dispense with the “affectionate” descriptor immediately because gentle parenting can’t claim sole ownership to it. Every, behavior management scheme I am aware of touts being caring and loving at its core.
I completely agree that emotional regulation for both parent and child are worthy goals, but I’m not hearing much on how that is to be achieved other than by trying to avoid the inevitable conflict by failing to even say “No” when poorly crafted boundaries are breached.
There are scores of parenting styles out there. And there should be, because we are all different. Parents have strengths and weaknesses and they have begotten children with different personalities and vulnerabilities. And, families come from different cultures and socioeconomic backgrounds.
Across all of these differences there are two primary roles for every parent. The first is to lead by example. If a parent wants his/her child to be kind and caring and polite, then the parent has no choice but to behave that way. If the parent can’t always be present, the environment where the child spends most of his/her day should model the desired behavior. I’m not talking about teaching because you can’t preach good behavior. It must be modeled.
The second role for the parent is to keep his/her child safe from dangers that exist in every environment. This can mean accepting vaccines and seeking available medical care. But, it also means creating some limits — the current buzzword is “guardrails” — to keep the child from veering into the ditch.
Setting Limits
Limits will, of necessity, vary with the environment. The risks of a child growing on a farm differ from those of child living in the city. And they must be tailored to the personality and developmental stage of the child. A parent may need advice from someone experienced in child behavior to create individualized limits. You may be able to allow your 3-year-old to roam freely in an environment in which I would have to monitor my risk-taking 3-year-old every second. A parent must learn and accept his/her child’s personality and the environment they can provide.
Limits should be inanimate objects whenever possible. Fences, gates, doors with latches, and locked cabinets to keep temptations out of view, etc. Creative environmental manipulations should be employed to keep the annoying verbal warnings, unenforceable threats, and direct child-to-parent confrontations to a minimum.
Consequences
Challenges to even the most carefully crafted limits are inevitable, and this is where we get to the third-rail topic of consequences. Yes, when prevention has failed for whatever reason, I believe that an intelligently and affectionately applied time-out is the most efficient and most effective consequence. This is not the place for me to explore or defend the details, but before you write me off as an octogenarian hard-ass (or hard-liner if you prefer) I urge you to read a few chapters in How to Say No to Your Toddler.
Far more important than which consequence a parent chooses are the steps the family has taken to keep both parent and child in a state of balanced emotional regulation. Is everyone well rested and getting enough sleep? Sleep deprivation is one of the most potent triggers of a tantrum; it also leaves parents vulnerable to saying things and making threats they will regret later. Does the child’s schedule leave him or her enough time to decompress? Does the parent’s schedule sync with a developmentally appropriate schedule for the child? Is he/she getting the right kind of attention when it makes the most sense to him/her?
Intelligent Parenting
If a family has created an environment in which limits are appropriate for the child’s personality and developmental stage, used physical barriers whenever possible, and kept everyone as well rested as possible, both challenges to the limits and consequences can be kept to a minimum.
But achieving this state requires time as free of constraints as possible. For the few families that have the luxury of meeting these conditions, gentle parenting might be the answer. For the rest of us, intelligent parenting that acknowledges the realities and limits of our own abilities and our children’s vulnerabilities is the better answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In one my recent Letters, I concluded with the concern that infant-led weaning, which makes some sense, can be confused with child-led family meals, which make none. I referred to an increasingly popular style of parenting overemphasizing child autonomy that seems to be a major contributor to the mealtime chaos that occurs when pleasing every palate at the table becomes the goal.
In the intervening weeks, I have learned that this parenting style is called “gentle parenting.” Despite its growing popularity, possibly fueled by the pandemic, it has not been well-defined nor its effectiveness investigated. In a recent paper published in PLOS ONE, two professors of developmental psychology have attempted correct this deficit in our understanding of this parenting style, which doesn’t appear to make sense to many of us with experience in child behavior and development.
Gentle Parents
By surveying a group of 100 parents of young children, the investigators were able to sort out a group of parents (n = 49) who self-identified as employing gentle parenting. Their responses emphasized a high level of parental affection and emotional regulation by both their children and themselves.
Investigators found that 40% of the self-defined gentle parents “had negative difference scores indicating misbehavior response descriptions that included more child directed responses. I interpret this to mean that almost half of the time the parents failed to evenly include themselves in a solution to a conflict, which indicates incomplete or unsuccessful emotional regulation on their part. The investigators also observed that, like many other parenting styles, gentle parenting includes an emphasis on boundaries “yet, enactment of those boundaries is not uniform.”
More telling was the authors’ observation that “statements of parenting uncertainty and burnout were present in over one third of the gentle of the gentle parenting sample.” While some parents were pleased with their experience, the downside seems unacceptable to me. When asked to explain this finding, Annie Pezalla, PhD, one of the coauthors, has said “gentle parenting practices work best when a parent is emotionally regulated and unconstrained for time — commodities that parents struggle with the most.”
Abundance Advice on Parenting Styles
I find this to be a very sad story. Parenting can be difficult. Creating and then gently and effectively policing those boundaries is often the hardest part. It is not surprising to me that of the four books I have written for parents, the one titled How to Say No to Your Toddler is the only one popular enough to be published in four languages.
Of course I am troubled, as I suspect you may be, with the label “gentle parenting.” It implies that the rest of us are doing something terrible, “harsh” maybe, “cruel” maybe. We can dispense with the “affectionate” descriptor immediately because gentle parenting can’t claim sole ownership to it. Every, behavior management scheme I am aware of touts being caring and loving at its core.
I completely agree that emotional regulation for both parent and child are worthy goals, but I’m not hearing much on how that is to be achieved other than by trying to avoid the inevitable conflict by failing to even say “No” when poorly crafted boundaries are breached.
There are scores of parenting styles out there. And there should be, because we are all different. Parents have strengths and weaknesses and they have begotten children with different personalities and vulnerabilities. And, families come from different cultures and socioeconomic backgrounds.
Across all of these differences there are two primary roles for every parent. The first is to lead by example. If a parent wants his/her child to be kind and caring and polite, then the parent has no choice but to behave that way. If the parent can’t always be present, the environment where the child spends most of his/her day should model the desired behavior. I’m not talking about teaching because you can’t preach good behavior. It must be modeled.
The second role for the parent is to keep his/her child safe from dangers that exist in every environment. This can mean accepting vaccines and seeking available medical care. But, it also means creating some limits — the current buzzword is “guardrails” — to keep the child from veering into the ditch.
Setting Limits
Limits will, of necessity, vary with the environment. The risks of a child growing on a farm differ from those of child living in the city. And they must be tailored to the personality and developmental stage of the child. A parent may need advice from someone experienced in child behavior to create individualized limits. You may be able to allow your 3-year-old to roam freely in an environment in which I would have to monitor my risk-taking 3-year-old every second. A parent must learn and accept his/her child’s personality and the environment they can provide.
Limits should be inanimate objects whenever possible. Fences, gates, doors with latches, and locked cabinets to keep temptations out of view, etc. Creative environmental manipulations should be employed to keep the annoying verbal warnings, unenforceable threats, and direct child-to-parent confrontations to a minimum.
Consequences
Challenges to even the most carefully crafted limits are inevitable, and this is where we get to the third-rail topic of consequences. Yes, when prevention has failed for whatever reason, I believe that an intelligently and affectionately applied time-out is the most efficient and most effective consequence. This is not the place for me to explore or defend the details, but before you write me off as an octogenarian hard-ass (or hard-liner if you prefer) I urge you to read a few chapters in How to Say No to Your Toddler.
Far more important than which consequence a parent chooses are the steps the family has taken to keep both parent and child in a state of balanced emotional regulation. Is everyone well rested and getting enough sleep? Sleep deprivation is one of the most potent triggers of a tantrum; it also leaves parents vulnerable to saying things and making threats they will regret later. Does the child’s schedule leave him or her enough time to decompress? Does the parent’s schedule sync with a developmentally appropriate schedule for the child? Is he/she getting the right kind of attention when it makes the most sense to him/her?
Intelligent Parenting
If a family has created an environment in which limits are appropriate for the child’s personality and developmental stage, used physical barriers whenever possible, and kept everyone as well rested as possible, both challenges to the limits and consequences can be kept to a minimum.
But achieving this state requires time as free of constraints as possible. For the few families that have the luxury of meeting these conditions, gentle parenting might be the answer. For the rest of us, intelligent parenting that acknowledges the realities and limits of our own abilities and our children’s vulnerabilities is the better answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In one my recent Letters, I concluded with the concern that infant-led weaning, which makes some sense, can be confused with child-led family meals, which make none. I referred to an increasingly popular style of parenting overemphasizing child autonomy that seems to be a major contributor to the mealtime chaos that occurs when pleasing every palate at the table becomes the goal.
In the intervening weeks, I have learned that this parenting style is called “gentle parenting.” Despite its growing popularity, possibly fueled by the pandemic, it has not been well-defined nor its effectiveness investigated. In a recent paper published in PLOS ONE, two professors of developmental psychology have attempted correct this deficit in our understanding of this parenting style, which doesn’t appear to make sense to many of us with experience in child behavior and development.
Gentle Parents
By surveying a group of 100 parents of young children, the investigators were able to sort out a group of parents (n = 49) who self-identified as employing gentle parenting. Their responses emphasized a high level of parental affection and emotional regulation by both their children and themselves.
Investigators found that 40% of the self-defined gentle parents “had negative difference scores indicating misbehavior response descriptions that included more child directed responses. I interpret this to mean that almost half of the time the parents failed to evenly include themselves in a solution to a conflict, which indicates incomplete or unsuccessful emotional regulation on their part. The investigators also observed that, like many other parenting styles, gentle parenting includes an emphasis on boundaries “yet, enactment of those boundaries is not uniform.”
More telling was the authors’ observation that “statements of parenting uncertainty and burnout were present in over one third of the gentle of the gentle parenting sample.” While some parents were pleased with their experience, the downside seems unacceptable to me. When asked to explain this finding, Annie Pezalla, PhD, one of the coauthors, has said “gentle parenting practices work best when a parent is emotionally regulated and unconstrained for time — commodities that parents struggle with the most.”
Abundance Advice on Parenting Styles
I find this to be a very sad story. Parenting can be difficult. Creating and then gently and effectively policing those boundaries is often the hardest part. It is not surprising to me that of the four books I have written for parents, the one titled How to Say No to Your Toddler is the only one popular enough to be published in four languages.
Of course I am troubled, as I suspect you may be, with the label “gentle parenting.” It implies that the rest of us are doing something terrible, “harsh” maybe, “cruel” maybe. We can dispense with the “affectionate” descriptor immediately because gentle parenting can’t claim sole ownership to it. Every, behavior management scheme I am aware of touts being caring and loving at its core.
I completely agree that emotional regulation for both parent and child are worthy goals, but I’m not hearing much on how that is to be achieved other than by trying to avoid the inevitable conflict by failing to even say “No” when poorly crafted boundaries are breached.
There are scores of parenting styles out there. And there should be, because we are all different. Parents have strengths and weaknesses and they have begotten children with different personalities and vulnerabilities. And, families come from different cultures and socioeconomic backgrounds.
Across all of these differences there are two primary roles for every parent. The first is to lead by example. If a parent wants his/her child to be kind and caring and polite, then the parent has no choice but to behave that way. If the parent can’t always be present, the environment where the child spends most of his/her day should model the desired behavior. I’m not talking about teaching because you can’t preach good behavior. It must be modeled.
The second role for the parent is to keep his/her child safe from dangers that exist in every environment. This can mean accepting vaccines and seeking available medical care. But, it also means creating some limits — the current buzzword is “guardrails” — to keep the child from veering into the ditch.
Setting Limits
Limits will, of necessity, vary with the environment. The risks of a child growing on a farm differ from those of child living in the city. And they must be tailored to the personality and developmental stage of the child. A parent may need advice from someone experienced in child behavior to create individualized limits. You may be able to allow your 3-year-old to roam freely in an environment in which I would have to monitor my risk-taking 3-year-old every second. A parent must learn and accept his/her child’s personality and the environment they can provide.
Limits should be inanimate objects whenever possible. Fences, gates, doors with latches, and locked cabinets to keep temptations out of view, etc. Creative environmental manipulations should be employed to keep the annoying verbal warnings, unenforceable threats, and direct child-to-parent confrontations to a minimum.
Consequences
Challenges to even the most carefully crafted limits are inevitable, and this is where we get to the third-rail topic of consequences. Yes, when prevention has failed for whatever reason, I believe that an intelligently and affectionately applied time-out is the most efficient and most effective consequence. This is not the place for me to explore or defend the details, but before you write me off as an octogenarian hard-ass (or hard-liner if you prefer) I urge you to read a few chapters in How to Say No to Your Toddler.
Far more important than which consequence a parent chooses are the steps the family has taken to keep both parent and child in a state of balanced emotional regulation. Is everyone well rested and getting enough sleep? Sleep deprivation is one of the most potent triggers of a tantrum; it also leaves parents vulnerable to saying things and making threats they will regret later. Does the child’s schedule leave him or her enough time to decompress? Does the parent’s schedule sync with a developmentally appropriate schedule for the child? Is he/she getting the right kind of attention when it makes the most sense to him/her?
Intelligent Parenting
If a family has created an environment in which limits are appropriate for the child’s personality and developmental stage, used physical barriers whenever possible, and kept everyone as well rested as possible, both challenges to the limits and consequences can be kept to a minimum.
But achieving this state requires time as free of constraints as possible. For the few families that have the luxury of meeting these conditions, gentle parenting might be the answer. For the rest of us, intelligent parenting that acknowledges the realities and limits of our own abilities and our children’s vulnerabilities is the better answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Navigating the Physician Mortgage Loan
Navigating the path to homeownership can be particularly challenging for physicians, who often face a unique set of financial circumstances. With substantial student loan debt, limited savings, and a delayed peak earning potential, traditional mortgage options may seem out of reach.
Enter physician mortgage loans—specialized financing designed specifically for medical professionals. These loans offer tailored solutions that address the common barriers faced by doctors, making it easier for them to achieve their homeownership goals. In this article, we’ll
What Is a Physician Mortgage Loan?
A physician mortgage loan, also known as a ‘doctor loan,’ is a specialized mortgage product designed for a specific group of qualifying medical professionals. These loans are particularly attractive to new doctors who may have substantial student loan debt, limited savings, and an income that is expected to increase significantly over time. As unique portfolio loans, physician mortgage products can vary considerably between lending institutions. However, a common feature is that they typically require little to no down payment and do not require private mortgage insurance (PMI).
Beyond the common features, loan options and qualifying parameters can vary significantly from one institution to another. Therefore, it’s important to start gathering information as early as possible, giving you ample time to evaluate which institution and loan option best meet your needs.
How Do I Know if I Am Eligible for a Physician Mortgage Loan?
Physician loans are typically offered to MDs, DOs, DDSs, DMDs, and ODs, though some institutions expand this list to include DPMs, PAs, CRNAs, NPs, PharmDs, and DVMs. Additionally, most of these loan products are available to residents, fellows, and attending or practicing physicians.
How Do I Know What Physician Mortgage Loan Is Best for Me?
When selecting the optimal physician loan option for your home purchase, consider several important metrics:
- Duration of Stay: Consider how long you expect to live in the home. If you’re in a lengthy residency or fellowship program, or if you plan to move for a new job soon after, a 30-year fixed-rate loan might not be ideal. Instead, evaluate loan options that match your anticipated duration of stay. For example, a 5-year or 7-year ARM (adjustable rate mortgage) could offer a lower interest rate and reduced monthly payments for the initial fixed period, which aligns with your shorter-term stay. This can result in substantial savings if you do not plan to stay in the home for the full term of a traditional mortgage.
- Underwriting Guidelines: Each lender has different underwriting standards and qualifying criteria, so it’s essential to understand these differences. For instance, some lenders may have higher minimum credit score requirements or stricter debt-to-income (DTI) ratio limits. Others might require a larger down payment or have different rules regarding student loan payments and closing costs. Flexibility in these guidelines can impact your ability to qualify for a loan and the terms you receive. For example, some lenders may allow you to include student loan payments at a lower percentage of your income, which could improve your DTI ratio and help you secure a better loan offer.
- Closing Timing: The timing of your home closing relative to your job start date can be crucial, especially if you’re relocating. Some lenders permit closing up to 60-90 days before your job begins, while others offer up to 120 days. If you need to relocate your family before starting your new position, having the ability to close earlier can provide you with more flexibility in finding and moving into a home. This additional time can ease the transition and allow you to settle in before your new job starts.
Given the wide range of options and standards, it’s important to strategically identify which factors are most meaningful to you. Beyond interest rates, consider the overall cost of the loan, the flexibility of terms, and how well the loan aligns with your financial goals and career plans. For example, if you value lower monthly payments over a longer period or need to accommodate significant student loan debt, ensure that the loan program you choose aligns with these priorities.
What Attributes Should I Look for in My Loan Officer?
When interviewing multiple loan officers for your upcoming loan needs, it’s essential to use the right metrics—beyond just the interest rate—to determine the best fit for your situation. Some critical factors to consider include the loan officer’s experience working with physicians, that person’s availability and responsiveness, and the potential for building a long-term relationship.
As in most professions, experience is paramount—it’s something that cannot be taught or simply read in a training manual. Physicians, especially those in training or just stepping into an attending role, often have unique financial situations. This makes it crucial to work with a loan officer who has extensive experience serving physician clients. An experienced loan officer will better understand how to customize a loan solution that aligns with your specific needs, resulting in a much more tailored and meaningful mortgage. There is no one-size-fits-all mortgage. You are unique, and your loan officer should be crafting a mortgage solution that reflects your individuality and financial circumstances.
In my opinion, availability and responsiveness are among the most critical attributes your chosen loan officer should possess. Interestingly, this factor doesn’t directly influence the ‘cost’ of your loan but can significantly impact your experience. As a physician with a demanding schedule, it’s unrealistic to expect that all communication will take place strictly during business hours—this is true for any consumer. Pay close attention to how promptly loan officers respond during your initial interactions, and evaluate how thoroughly they explain loan terms, out-of-pocket costs, and the overall loan process. Your loan officer should be your trusted guide as you navigate through the complexities of the loan process, so setting yourself up for success starts with choosing someone who meets your expectations in this regard.
It’s crucial to build a good rapport with the loan officer you choose, as this likely won’t be the last mortgage or financial need you encounter in your lifetime. Establishing a personal connection with your loan officer fosters a level of trust that is invaluable. Whether you’re considering refinancing your current mortgage or exploring additional loan products for other financial needs, having a trusted advisor you can rely on as a financial resource is immensely beneficial as you progress in your career. A strong, long-standing relationship with a loan officer ensures you receive reliable and sound financial advice tailored to your unique needs.
Additional Things to Consider if You Are a First-Time Home Buyer
Interview multiple lenders and make those conversations about more than just interest rates. This approach will help you gauge their knowledge of physician mortgage loans while allowing you to assess who might be the best fit for you in terms of compatibility. Relying solely on an email blast to inquire about rates could easily lead you to a subpar lender and result in an unfavorable experience.
Don’t be afraid to ask a lot of questions! As a first-time home buyer, it’s natural to feel a bit overwhelmed by the process—it can seem daunting if you’ve never been through it before. That’s why it’s crucial to ask any questions that come to mind and to work with a lender who is willing to take the time to answer them while educating you throughout the home-buying journey. With a trusted guide and the right education, the process will feel far less overwhelming, leading to a smoother and more positive experience from start to finish.
In conclusion, choosing the right lender for a physician mortgage loan is a crucial step in securing your financial future and achieving homeownership. By thoroughly evaluating interest rates, down payment requirements, loan terms, and other key metrics, you can find a lender that offers competitive rates and favorable terms tailored to your unique needs. Consider factors such as customer service, closing costs, and the lender’s experience with physician loans to ensure a smooth and supportive mortgage process. By taking the time to compare options and select the best fit for your financial situation, you can confidently move forward in your home-buying journey and set the stage for a successful and fulfilling homeownership experience.
Mr. Kelley is vice president of mortgage lending and a physician mortgage specialist at Arvest Bank in Overland Park, Kansas.
Navigating the path to homeownership can be particularly challenging for physicians, who often face a unique set of financial circumstances. With substantial student loan debt, limited savings, and a delayed peak earning potential, traditional mortgage options may seem out of reach.
Enter physician mortgage loans—specialized financing designed specifically for medical professionals. These loans offer tailored solutions that address the common barriers faced by doctors, making it easier for them to achieve their homeownership goals. In this article, we’ll
What Is a Physician Mortgage Loan?
A physician mortgage loan, also known as a ‘doctor loan,’ is a specialized mortgage product designed for a specific group of qualifying medical professionals. These loans are particularly attractive to new doctors who may have substantial student loan debt, limited savings, and an income that is expected to increase significantly over time. As unique portfolio loans, physician mortgage products can vary considerably between lending institutions. However, a common feature is that they typically require little to no down payment and do not require private mortgage insurance (PMI).
Beyond the common features, loan options and qualifying parameters can vary significantly from one institution to another. Therefore, it’s important to start gathering information as early as possible, giving you ample time to evaluate which institution and loan option best meet your needs.
How Do I Know if I Am Eligible for a Physician Mortgage Loan?
Physician loans are typically offered to MDs, DOs, DDSs, DMDs, and ODs, though some institutions expand this list to include DPMs, PAs, CRNAs, NPs, PharmDs, and DVMs. Additionally, most of these loan products are available to residents, fellows, and attending or practicing physicians.
How Do I Know What Physician Mortgage Loan Is Best for Me?
When selecting the optimal physician loan option for your home purchase, consider several important metrics:
- Duration of Stay: Consider how long you expect to live in the home. If you’re in a lengthy residency or fellowship program, or if you plan to move for a new job soon after, a 30-year fixed-rate loan might not be ideal. Instead, evaluate loan options that match your anticipated duration of stay. For example, a 5-year or 7-year ARM (adjustable rate mortgage) could offer a lower interest rate and reduced monthly payments for the initial fixed period, which aligns with your shorter-term stay. This can result in substantial savings if you do not plan to stay in the home for the full term of a traditional mortgage.
- Underwriting Guidelines: Each lender has different underwriting standards and qualifying criteria, so it’s essential to understand these differences. For instance, some lenders may have higher minimum credit score requirements or stricter debt-to-income (DTI) ratio limits. Others might require a larger down payment or have different rules regarding student loan payments and closing costs. Flexibility in these guidelines can impact your ability to qualify for a loan and the terms you receive. For example, some lenders may allow you to include student loan payments at a lower percentage of your income, which could improve your DTI ratio and help you secure a better loan offer.
- Closing Timing: The timing of your home closing relative to your job start date can be crucial, especially if you’re relocating. Some lenders permit closing up to 60-90 days before your job begins, while others offer up to 120 days. If you need to relocate your family before starting your new position, having the ability to close earlier can provide you with more flexibility in finding and moving into a home. This additional time can ease the transition and allow you to settle in before your new job starts.
Given the wide range of options and standards, it’s important to strategically identify which factors are most meaningful to you. Beyond interest rates, consider the overall cost of the loan, the flexibility of terms, and how well the loan aligns with your financial goals and career plans. For example, if you value lower monthly payments over a longer period or need to accommodate significant student loan debt, ensure that the loan program you choose aligns with these priorities.
What Attributes Should I Look for in My Loan Officer?
When interviewing multiple loan officers for your upcoming loan needs, it’s essential to use the right metrics—beyond just the interest rate—to determine the best fit for your situation. Some critical factors to consider include the loan officer’s experience working with physicians, that person’s availability and responsiveness, and the potential for building a long-term relationship.
As in most professions, experience is paramount—it’s something that cannot be taught or simply read in a training manual. Physicians, especially those in training or just stepping into an attending role, often have unique financial situations. This makes it crucial to work with a loan officer who has extensive experience serving physician clients. An experienced loan officer will better understand how to customize a loan solution that aligns with your specific needs, resulting in a much more tailored and meaningful mortgage. There is no one-size-fits-all mortgage. You are unique, and your loan officer should be crafting a mortgage solution that reflects your individuality and financial circumstances.
In my opinion, availability and responsiveness are among the most critical attributes your chosen loan officer should possess. Interestingly, this factor doesn’t directly influence the ‘cost’ of your loan but can significantly impact your experience. As a physician with a demanding schedule, it’s unrealistic to expect that all communication will take place strictly during business hours—this is true for any consumer. Pay close attention to how promptly loan officers respond during your initial interactions, and evaluate how thoroughly they explain loan terms, out-of-pocket costs, and the overall loan process. Your loan officer should be your trusted guide as you navigate through the complexities of the loan process, so setting yourself up for success starts with choosing someone who meets your expectations in this regard.
It’s crucial to build a good rapport with the loan officer you choose, as this likely won’t be the last mortgage or financial need you encounter in your lifetime. Establishing a personal connection with your loan officer fosters a level of trust that is invaluable. Whether you’re considering refinancing your current mortgage or exploring additional loan products for other financial needs, having a trusted advisor you can rely on as a financial resource is immensely beneficial as you progress in your career. A strong, long-standing relationship with a loan officer ensures you receive reliable and sound financial advice tailored to your unique needs.
Additional Things to Consider if You Are a First-Time Home Buyer
Interview multiple lenders and make those conversations about more than just interest rates. This approach will help you gauge their knowledge of physician mortgage loans while allowing you to assess who might be the best fit for you in terms of compatibility. Relying solely on an email blast to inquire about rates could easily lead you to a subpar lender and result in an unfavorable experience.
Don’t be afraid to ask a lot of questions! As a first-time home buyer, it’s natural to feel a bit overwhelmed by the process—it can seem daunting if you’ve never been through it before. That’s why it’s crucial to ask any questions that come to mind and to work with a lender who is willing to take the time to answer them while educating you throughout the home-buying journey. With a trusted guide and the right education, the process will feel far less overwhelming, leading to a smoother and more positive experience from start to finish.
In conclusion, choosing the right lender for a physician mortgage loan is a crucial step in securing your financial future and achieving homeownership. By thoroughly evaluating interest rates, down payment requirements, loan terms, and other key metrics, you can find a lender that offers competitive rates and favorable terms tailored to your unique needs. Consider factors such as customer service, closing costs, and the lender’s experience with physician loans to ensure a smooth and supportive mortgage process. By taking the time to compare options and select the best fit for your financial situation, you can confidently move forward in your home-buying journey and set the stage for a successful and fulfilling homeownership experience.
Mr. Kelley is vice president of mortgage lending and a physician mortgage specialist at Arvest Bank in Overland Park, Kansas.
Navigating the path to homeownership can be particularly challenging for physicians, who often face a unique set of financial circumstances. With substantial student loan debt, limited savings, and a delayed peak earning potential, traditional mortgage options may seem out of reach.
Enter physician mortgage loans—specialized financing designed specifically for medical professionals. These loans offer tailored solutions that address the common barriers faced by doctors, making it easier for them to achieve their homeownership goals. In this article, we’ll
What Is a Physician Mortgage Loan?
A physician mortgage loan, also known as a ‘doctor loan,’ is a specialized mortgage product designed for a specific group of qualifying medical professionals. These loans are particularly attractive to new doctors who may have substantial student loan debt, limited savings, and an income that is expected to increase significantly over time. As unique portfolio loans, physician mortgage products can vary considerably between lending institutions. However, a common feature is that they typically require little to no down payment and do not require private mortgage insurance (PMI).
Beyond the common features, loan options and qualifying parameters can vary significantly from one institution to another. Therefore, it’s important to start gathering information as early as possible, giving you ample time to evaluate which institution and loan option best meet your needs.
How Do I Know if I Am Eligible for a Physician Mortgage Loan?
Physician loans are typically offered to MDs, DOs, DDSs, DMDs, and ODs, though some institutions expand this list to include DPMs, PAs, CRNAs, NPs, PharmDs, and DVMs. Additionally, most of these loan products are available to residents, fellows, and attending or practicing physicians.
How Do I Know What Physician Mortgage Loan Is Best for Me?
When selecting the optimal physician loan option for your home purchase, consider several important metrics:
- Duration of Stay: Consider how long you expect to live in the home. If you’re in a lengthy residency or fellowship program, or if you plan to move for a new job soon after, a 30-year fixed-rate loan might not be ideal. Instead, evaluate loan options that match your anticipated duration of stay. For example, a 5-year or 7-year ARM (adjustable rate mortgage) could offer a lower interest rate and reduced monthly payments for the initial fixed period, which aligns with your shorter-term stay. This can result in substantial savings if you do not plan to stay in the home for the full term of a traditional mortgage.
- Underwriting Guidelines: Each lender has different underwriting standards and qualifying criteria, so it’s essential to understand these differences. For instance, some lenders may have higher minimum credit score requirements or stricter debt-to-income (DTI) ratio limits. Others might require a larger down payment or have different rules regarding student loan payments and closing costs. Flexibility in these guidelines can impact your ability to qualify for a loan and the terms you receive. For example, some lenders may allow you to include student loan payments at a lower percentage of your income, which could improve your DTI ratio and help you secure a better loan offer.
- Closing Timing: The timing of your home closing relative to your job start date can be crucial, especially if you’re relocating. Some lenders permit closing up to 60-90 days before your job begins, while others offer up to 120 days. If you need to relocate your family before starting your new position, having the ability to close earlier can provide you with more flexibility in finding and moving into a home. This additional time can ease the transition and allow you to settle in before your new job starts.
Given the wide range of options and standards, it’s important to strategically identify which factors are most meaningful to you. Beyond interest rates, consider the overall cost of the loan, the flexibility of terms, and how well the loan aligns with your financial goals and career plans. For example, if you value lower monthly payments over a longer period or need to accommodate significant student loan debt, ensure that the loan program you choose aligns with these priorities.
What Attributes Should I Look for in My Loan Officer?
When interviewing multiple loan officers for your upcoming loan needs, it’s essential to use the right metrics—beyond just the interest rate—to determine the best fit for your situation. Some critical factors to consider include the loan officer’s experience working with physicians, that person’s availability and responsiveness, and the potential for building a long-term relationship.
As in most professions, experience is paramount—it’s something that cannot be taught or simply read in a training manual. Physicians, especially those in training or just stepping into an attending role, often have unique financial situations. This makes it crucial to work with a loan officer who has extensive experience serving physician clients. An experienced loan officer will better understand how to customize a loan solution that aligns with your specific needs, resulting in a much more tailored and meaningful mortgage. There is no one-size-fits-all mortgage. You are unique, and your loan officer should be crafting a mortgage solution that reflects your individuality and financial circumstances.
In my opinion, availability and responsiveness are among the most critical attributes your chosen loan officer should possess. Interestingly, this factor doesn’t directly influence the ‘cost’ of your loan but can significantly impact your experience. As a physician with a demanding schedule, it’s unrealistic to expect that all communication will take place strictly during business hours—this is true for any consumer. Pay close attention to how promptly loan officers respond during your initial interactions, and evaluate how thoroughly they explain loan terms, out-of-pocket costs, and the overall loan process. Your loan officer should be your trusted guide as you navigate through the complexities of the loan process, so setting yourself up for success starts with choosing someone who meets your expectations in this regard.
It’s crucial to build a good rapport with the loan officer you choose, as this likely won’t be the last mortgage or financial need you encounter in your lifetime. Establishing a personal connection with your loan officer fosters a level of trust that is invaluable. Whether you’re considering refinancing your current mortgage or exploring additional loan products for other financial needs, having a trusted advisor you can rely on as a financial resource is immensely beneficial as you progress in your career. A strong, long-standing relationship with a loan officer ensures you receive reliable and sound financial advice tailored to your unique needs.
Additional Things to Consider if You Are a First-Time Home Buyer
Interview multiple lenders and make those conversations about more than just interest rates. This approach will help you gauge their knowledge of physician mortgage loans while allowing you to assess who might be the best fit for you in terms of compatibility. Relying solely on an email blast to inquire about rates could easily lead you to a subpar lender and result in an unfavorable experience.
Don’t be afraid to ask a lot of questions! As a first-time home buyer, it’s natural to feel a bit overwhelmed by the process—it can seem daunting if you’ve never been through it before. That’s why it’s crucial to ask any questions that come to mind and to work with a lender who is willing to take the time to answer them while educating you throughout the home-buying journey. With a trusted guide and the right education, the process will feel far less overwhelming, leading to a smoother and more positive experience from start to finish.
In conclusion, choosing the right lender for a physician mortgage loan is a crucial step in securing your financial future and achieving homeownership. By thoroughly evaluating interest rates, down payment requirements, loan terms, and other key metrics, you can find a lender that offers competitive rates and favorable terms tailored to your unique needs. Consider factors such as customer service, closing costs, and the lender’s experience with physician loans to ensure a smooth and supportive mortgage process. By taking the time to compare options and select the best fit for your financial situation, you can confidently move forward in your home-buying journey and set the stage for a successful and fulfilling homeownership experience.
Mr. Kelley is vice president of mortgage lending and a physician mortgage specialist at Arvest Bank in Overland Park, Kansas.
Screen Use and Toddler Bedtimes
For decades I have suspected that there is a strong association between sleep deprivation and pediatric attention disorders. More recently I have wondered whether screen time, particularly at bedtime might be a significant contributor to sleep quantity and quality in both children and adults. There is a growing body of research that combines my two observations and suggests that bedtime screen time through its effect on sleep may be linked to pediatric attention problems. However, most of this work is preliminary and needs to be confirmed.
Stumbling across a paper from England titled “Toddler Screen Use Before Bed and Its Effect on Sleep and Attention” renewed my hope that we finally have evidence to close that knowledge gap. My bubble burst quickly however when I jumped ahead and read the conclusion portion of the abstract and learned that authors observed “no clear difference in parent reported attention” in the group of children in which screen time before bedtime had been eliminated. The authors wonder if their small study sample may be to blame.
Disappointed, I persisted and read the paper in its entirety and found that despite their failure to link bedtime screen time with attention disorders, the investigators have made a significant contribution to our understanding of how we can better encourage good pediatric sleep hygiene.
The Study
One hundred and five families with a toddler who was being exposed to a video screen in the hour before bedtime were divided into three groups. One group received guidance and advice from a pediatric team about the potential benefit of eliminating bedtime screen time. They were also given a box of activities that contained “activity cards and age appropriate toys” to replace the screen use. The family also received periodic support and follow-up contacts. A second group received only the “bedtime box.” And the third received no intervention.
It is important to note that the investigators modeled their intervention on one developed in a previous study using older children that was “co-created with caregivers and early years practitioners”(my italics).
The intervention resulted in reductions in parent-reported screen time, sleep efficiency, night awakenings, and daytime sleep. The decrease in nap time was a surprise to the investigators.
These reductions were small. However, the investigators were most impressed (and I share in their sentiment) with the finding that 99% (104/105) of the families stayed with the study until completion, demonstrating that future studies using this format were highly feasible. The authors of the study were pleased also and possibly surprised that 94% (33/35) of the families who received the intervention adhered to the recommendations.
One Suggestion: ‘Just Shut the TV Off’
If you are a cynic, you might be tempted to explain the investigators’ (and my) excitement over the feasibility and adherence numbers as an attempt to pump up the importance of a set of otherwise lackluster numbers regarding sleep and the failure to find any association between the intervention and attention. However, having spent a large part of my career trying to encourage parents to improve their child’s sleep hygiene, often with little success, I am encouraged by this study’s success in getting families to accept and then adhere to the intervention.
I must admit that when presented with a child who appeared to be having some attention difficulties and was watching television as part of his or her bedtime ritual, there’s a good chance I would have simply told the parents, “Just shut the TV off.” This certainly worked with some families, particularly those who had already bought into my preaching about the importance of sleep. However, my acceptance and adherence rates were no where near the 99% and 94% these investigators where achieving.
I did try to make follow-up phone calls, as these investigators did, but generally only to the most seriously effected families or in situations in which felt I was going to have the greatest chance of success. I am sorry to say that I didn’t involve the parents in crafting my overly simplistic intervention. Had I been more open to parental input, I suspect my results would have improved.
An Alternative
I think another reason for these investigators’ success was the clever ploy of offering a replacement (in this case the bedtime box of alternative activities) when they asked the parents to remove the screen time. Getting anyone to break an unhealthy habit, be they parents or patients, it often helps to offer them an alternative. The activity may not be as appealing as their current behavior but it can fill the gap until a new even healthier behavior develops.
Building an efficient and effective bedtime ritual begins in the first months of life. The initial challenge could be separating nursing or a bottle from the settling in process. Later on it could mean helping a parent who is out of the home all day understand that they may have to suppress their natural urge to engage in vigorous play with his/her child at a time that is best devoted to winding down into a healthy bedtime ritual. Although screen time may not be physically stimulating, there is increasing evidence that it shouldn’t be part of a pre-bedtime ritual. The question of if and how it contributes to attention problems will have to wait until another day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
For decades I have suspected that there is a strong association between sleep deprivation and pediatric attention disorders. More recently I have wondered whether screen time, particularly at bedtime might be a significant contributor to sleep quantity and quality in both children and adults. There is a growing body of research that combines my two observations and suggests that bedtime screen time through its effect on sleep may be linked to pediatric attention problems. However, most of this work is preliminary and needs to be confirmed.
Stumbling across a paper from England titled “Toddler Screen Use Before Bed and Its Effect on Sleep and Attention” renewed my hope that we finally have evidence to close that knowledge gap. My bubble burst quickly however when I jumped ahead and read the conclusion portion of the abstract and learned that authors observed “no clear difference in parent reported attention” in the group of children in which screen time before bedtime had been eliminated. The authors wonder if their small study sample may be to blame.
Disappointed, I persisted and read the paper in its entirety and found that despite their failure to link bedtime screen time with attention disorders, the investigators have made a significant contribution to our understanding of how we can better encourage good pediatric sleep hygiene.
The Study
One hundred and five families with a toddler who was being exposed to a video screen in the hour before bedtime were divided into three groups. One group received guidance and advice from a pediatric team about the potential benefit of eliminating bedtime screen time. They were also given a box of activities that contained “activity cards and age appropriate toys” to replace the screen use. The family also received periodic support and follow-up contacts. A second group received only the “bedtime box.” And the third received no intervention.
It is important to note that the investigators modeled their intervention on one developed in a previous study using older children that was “co-created with caregivers and early years practitioners”(my italics).
The intervention resulted in reductions in parent-reported screen time, sleep efficiency, night awakenings, and daytime sleep. The decrease in nap time was a surprise to the investigators.
These reductions were small. However, the investigators were most impressed (and I share in their sentiment) with the finding that 99% (104/105) of the families stayed with the study until completion, demonstrating that future studies using this format were highly feasible. The authors of the study were pleased also and possibly surprised that 94% (33/35) of the families who received the intervention adhered to the recommendations.
One Suggestion: ‘Just Shut the TV Off’
If you are a cynic, you might be tempted to explain the investigators’ (and my) excitement over the feasibility and adherence numbers as an attempt to pump up the importance of a set of otherwise lackluster numbers regarding sleep and the failure to find any association between the intervention and attention. However, having spent a large part of my career trying to encourage parents to improve their child’s sleep hygiene, often with little success, I am encouraged by this study’s success in getting families to accept and then adhere to the intervention.
I must admit that when presented with a child who appeared to be having some attention difficulties and was watching television as part of his or her bedtime ritual, there’s a good chance I would have simply told the parents, “Just shut the TV off.” This certainly worked with some families, particularly those who had already bought into my preaching about the importance of sleep. However, my acceptance and adherence rates were no where near the 99% and 94% these investigators where achieving.
I did try to make follow-up phone calls, as these investigators did, but generally only to the most seriously effected families or in situations in which felt I was going to have the greatest chance of success. I am sorry to say that I didn’t involve the parents in crafting my overly simplistic intervention. Had I been more open to parental input, I suspect my results would have improved.
An Alternative
I think another reason for these investigators’ success was the clever ploy of offering a replacement (in this case the bedtime box of alternative activities) when they asked the parents to remove the screen time. Getting anyone to break an unhealthy habit, be they parents or patients, it often helps to offer them an alternative. The activity may not be as appealing as their current behavior but it can fill the gap until a new even healthier behavior develops.
Building an efficient and effective bedtime ritual begins in the first months of life. The initial challenge could be separating nursing or a bottle from the settling in process. Later on it could mean helping a parent who is out of the home all day understand that they may have to suppress their natural urge to engage in vigorous play with his/her child at a time that is best devoted to winding down into a healthy bedtime ritual. Although screen time may not be physically stimulating, there is increasing evidence that it shouldn’t be part of a pre-bedtime ritual. The question of if and how it contributes to attention problems will have to wait until another day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
For decades I have suspected that there is a strong association between sleep deprivation and pediatric attention disorders. More recently I have wondered whether screen time, particularly at bedtime might be a significant contributor to sleep quantity and quality in both children and adults. There is a growing body of research that combines my two observations and suggests that bedtime screen time through its effect on sleep may be linked to pediatric attention problems. However, most of this work is preliminary and needs to be confirmed.
Stumbling across a paper from England titled “Toddler Screen Use Before Bed and Its Effect on Sleep and Attention” renewed my hope that we finally have evidence to close that knowledge gap. My bubble burst quickly however when I jumped ahead and read the conclusion portion of the abstract and learned that authors observed “no clear difference in parent reported attention” in the group of children in which screen time before bedtime had been eliminated. The authors wonder if their small study sample may be to blame.
Disappointed, I persisted and read the paper in its entirety and found that despite their failure to link bedtime screen time with attention disorders, the investigators have made a significant contribution to our understanding of how we can better encourage good pediatric sleep hygiene.
The Study
One hundred and five families with a toddler who was being exposed to a video screen in the hour before bedtime were divided into three groups. One group received guidance and advice from a pediatric team about the potential benefit of eliminating bedtime screen time. They were also given a box of activities that contained “activity cards and age appropriate toys” to replace the screen use. The family also received periodic support and follow-up contacts. A second group received only the “bedtime box.” And the third received no intervention.
It is important to note that the investigators modeled their intervention on one developed in a previous study using older children that was “co-created with caregivers and early years practitioners”(my italics).
The intervention resulted in reductions in parent-reported screen time, sleep efficiency, night awakenings, and daytime sleep. The decrease in nap time was a surprise to the investigators.
These reductions were small. However, the investigators were most impressed (and I share in their sentiment) with the finding that 99% (104/105) of the families stayed with the study until completion, demonstrating that future studies using this format were highly feasible. The authors of the study were pleased also and possibly surprised that 94% (33/35) of the families who received the intervention adhered to the recommendations.
One Suggestion: ‘Just Shut the TV Off’
If you are a cynic, you might be tempted to explain the investigators’ (and my) excitement over the feasibility and adherence numbers as an attempt to pump up the importance of a set of otherwise lackluster numbers regarding sleep and the failure to find any association between the intervention and attention. However, having spent a large part of my career trying to encourage parents to improve their child’s sleep hygiene, often with little success, I am encouraged by this study’s success in getting families to accept and then adhere to the intervention.
I must admit that when presented with a child who appeared to be having some attention difficulties and was watching television as part of his or her bedtime ritual, there’s a good chance I would have simply told the parents, “Just shut the TV off.” This certainly worked with some families, particularly those who had already bought into my preaching about the importance of sleep. However, my acceptance and adherence rates were no where near the 99% and 94% these investigators where achieving.
I did try to make follow-up phone calls, as these investigators did, but generally only to the most seriously effected families or in situations in which felt I was going to have the greatest chance of success. I am sorry to say that I didn’t involve the parents in crafting my overly simplistic intervention. Had I been more open to parental input, I suspect my results would have improved.
An Alternative
I think another reason for these investigators’ success was the clever ploy of offering a replacement (in this case the bedtime box of alternative activities) when they asked the parents to remove the screen time. Getting anyone to break an unhealthy habit, be they parents or patients, it often helps to offer them an alternative. The activity may not be as appealing as their current behavior but it can fill the gap until a new even healthier behavior develops.
Building an efficient and effective bedtime ritual begins in the first months of life. The initial challenge could be separating nursing or a bottle from the settling in process. Later on it could mean helping a parent who is out of the home all day understand that they may have to suppress their natural urge to engage in vigorous play with his/her child at a time that is best devoted to winding down into a healthy bedtime ritual. Although screen time may not be physically stimulating, there is increasing evidence that it shouldn’t be part of a pre-bedtime ritual. The question of if and how it contributes to attention problems will have to wait until another day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
An Epidemiologist’s Guide to Debunking Nutritional Research
You’re invited to a dinner party but you struggle to make small talk. Do not worry; that will invariably crop up over cocktails. Because all journalism has been reduced to listicles, here are four ways to seem clever at dinner parties.
1. The Predinner Cocktails: A Lesson in Reverse Causation
Wine connoisseurs sniff, swirl, and gently swish the wine in their mouths before spitting out and cleansing their palates to better appreciate the subtlety of each vintage. If you’re not an oenophile, no matter. Whenever somebody claims that moderate amounts of alcohol are good for your heart, this is your moment to pounce. Interject yourself in the conversation and tell everybody about reverse causation.
Reverse causation, also known as protopathic bias, involves misinterpreting the directionality of an association. You assume that X leads to Y, when in fact Y leads to X. Temporal paradoxes are useful plot devices in science fiction movies, but they have no place in medical research. In our bland world, cause must precede effect. As such, smoking leads to lung cancer; lung cancer doesn’t make you smoke more.
But with alcohol, directionality is less obvious. Many studies of alcohol and cardiovascular disease have demonstrated a U-shaped association, with risk being lowest among those who drink moderate amounts of alcohol (usually one to two drinks per day) and higher in those who drink more and also those who drink very little.
But one must ask why some people drink little or no alcohol. There is an important difference between former drinkers and never drinkers. Former drinkers cut back for a reason. More likely than not, the reason for this newfound sobriety was medical. A new cancer diagnosis, the emergence of atrial fibrillation, the development of diabetes, or rising blood pressure are all good reasons to reduce or eliminate alcohol. A cross-sectional study will fail to capture that alcohol consumption changes over time — people who now don’t drink may have imbibed alcohol in years past. It was not abstinence that led to an increased risk for heart disease; it was the increased risk for heart disease that led to abstinence.
You see the same phenomenon with the so-called obesity paradox. The idea that being a little overweight is good for you may appeal when you no longer fit into last year’s pants. But people who are underweight are so for a reason. Malnutrition, cachexia from cancer, or some other cause is almost certainly driving up the risk at the left-hand side of the U-shaped curve that makes the middle part seem better than it actually is.
Food consumption changes over time. A cross-sectional survey at one point in time cannot accurately capture past habits and distant exposures, especially for diseases such as heart disease and cancer that develop slowly over time. Studies on alcohol that try to overcome these shortcomings by eliminating former drinkers, or by using Mendelian randomization to better account for past exposure, do not show a cardiovascular benefit for moderate red wine drinking.
2. The Hors D’oeuvres — The Importance of RCTs
Now that you have made yourself the center of attention, it is time to cement your newfound reputation as a font of scientific knowledge. Most self-respecting hosts will serve smoked salmon as an amuse-bouche before the main meal. When someone mentions the health benefits of fish oils, you should take the opportunity to teach them about confounding.
Fish, especially cold-water fish from northern climates, have relatively high amounts of omega-3 fatty acids. Despite the plethora of observational studies suggesting a cardiovascular benefit, it’s now relatively clear that fish oil or omega-3 supplements have no medical benefit.
This will probably come as a shock to the worried well, but many studies, including VITAL and ASCEND, have demonstrated no cardiovascular or cancer benefit to supplementation with omega-3s. The reason is straightforward and explains why hormone replacement therapy, vitamin D, and myriad purported game-changers never panned out. Confounding is hard to overcome in observational research.
Prior to the publication of the Women’s Health Initiative (WHI) Study, hormone replacement therapy was routinely prescribed to postmenopausal women because numerous observational studies suggested a cardiovascular benefit. But with the publication of the WHI study, it became clear that much of that “benefit” was due to confounding. The women choosing to take hormones were more health conscious at baseline and healthier overall.
A similar phenomenon occurred during COVID. Patients with low serum vitamin D levels had worse outcomes, prompting many to suggest vitamin D supplementation as a possible treatment. Trials did not support the intervention because we’d overlooked the obvious. People with vitamin D deficiency have underlying health problems that contribute to the vitamin D deficiency. They are probably older, frailer, possibly with a poorer diet. No amount of statistical adjustment can account for all those differences, and some degree of residual confounding will always persist.
The only way to overcome confounding is with randomization. When patients are randomly assigned to one group or another, their baseline differences largely balance out if the randomization was performed properly and the groups were large enough. There is a role for observational research, such as in situations where ethics, cost, and practicality do not allow for a randomized controlled trial. But randomized controlled trials have largely put to rest the purported health benefits of over-the-counter fish oils, omega-3s, and vitamin D.
3. The Main Course — Absolute vs Relative Risk
When you get to the main course, all eyes will now be on you. You will almost certainly be called upon to pronounce on the harms or benefits of red meat consumption. Begin by regaling your guests with a little trivia. Ask them if they know the definition of red meat and white meat. When someone says pork is white meat, you can reveal that “pork, the other white meat,” was a marketing slogan with no scientific underpinning. Now that everyone is lulled into a stupefied silence, tell them that red meat comes from mammals and white meat comes from birds. As they process this revelation, you can now launch into the deeply mathematical concept of absolute vs relative risk.
Many etiquette books will caution against bringing up math at a dinner party. These books are wrong. Everyone finds math interesting if they are primed properly. For example, you can point to a study claiming that berries reduce cardiovascular risk in women. Even if true — and there is reason to be cautious, given the observational nature of the research — we need to understand what the authors meant by a 32% risk reduction. (Side note: It was a reduction in hazard, with a hazard ratio of 0.68 (95% CI, 0.49-0.96), but we won’t dwell on the difference between hazard ratios and risk ratios right now.)
This relative risk reduction has to be interpreted carefully. The authors divided the population into quintiles based on their consumption of anthocyanins (the antioxidant in blueberries and strawberries) and compared the bottom fifth (average consumption, 2.5 mg/d) with the top fifth (average consumption, 25 mg/d). The bottom quintile had 126 myocardial infarctions (MIs) over 324,793 patient-years compared with 59 MIs over 332,143 patient-years. Some quick math shows an approximate reduction from 39 to 18 MIs per 100,000 patient-years. Or to put it another way, you must get 4762 women to increase their berry consumption 10-fold for 1 year to prevent one heart attack. Feel free to show people how you calculated this number. They will be impressed by your head for numbers. It is nothing more than 39 minus 18, divided by 100,000, to get the absolute risk reduction. Take the reciprocal of this (ie, 1 divided by this number) to get the number needed to treat.
Describing risks in absolute terms or using number needed to treat (or harm) can help conceptualize statistics that are sometimes hard to wrap your head around.
4. Dessert — Funding
By the time the coffee is served, everyone will be hanging on to your every word. This is as it should be, and you should not be afraid of your newfound power and influence.
Dessert will probably involve some form of chocolate, possibly in cake format. (Anyone who serves fruit as dessert is not someone you should associate with.) Take the opportunity to tell your follow diners that chocolate is not actually good for you and will not boost brain performance.
The health benefits of chocolate are often repeated but rarely scrutinized. In fact, much of the scientific research purporting to show that chocolate is good for you did not actually study chocolate. It usually involved a cocoa bean extract because the chocolate manufacturing process destroys the supposedly health-promoting antioxidants in the cocoa bean. It is true that dark chocolate has more antioxidants than milk chocolate, and that the addition of milk to chocolate further inactivates the potentially healthy antioxidants. But the amount of sugar and fat that has to be added to chocolate to make it palatable precludes any serious consideration about health benefits. Dark chocolate may have less fat and sugar than milk chocolate, but it still has a lot.
But even the cocoa bean extract doesn’t seem to do much for your heart or your brain. The long-awaited COSMOS study was published with surprisingly little fanfare. The largest randomized controlled trial of chocolate (or rather cocoa bean extract) was supposed to settle the issue definitively.
COSMOS showed no cardiovascular or neurocognitive benefit to the cocoa bean extract. But the health halo of chocolate continues to be bolstered by many studies funded by chocolate manufacturers.
We are appropriately critical of the pharmaceutical industry’s involvement in drug research. However, we should not forget that any private entity is prone to the same self-interest regardless of its product’s tastiness. How many of you knew that there was an avocado lobby funding research? No matter how many industry-funded observational studies using surrogate endpoints are out there telling you that chocolate is healthy, a randomized trial with hard clinical endpoints such as COSMOS should generally win the day.
The Final Goodbyes — Summarizing Your Case
As the party slowly winds down and everyone is saddened that you will soon take your leave, synthesize everything you have taught them over the evening. Like movies, not all studies are good. Some are just bad. They can be prone to reverse causation or confounding, and they may report relative risks when absolute risks would be more telling. Reading research studies critically is essential for separating the wheat from the chaff. With the knowledge you have now imparted to your friends, they will be much better consumers of medical news, especially when it comes to food.
And they will no doubt thank you for it by never inviting you to another dinner party!
Labos, a cardiologist at Hôpital, Notre-Dame, Montreal, Quebec, Canada, has disclosed no relevant financial relationships. He has a degree in epidemiology.
A version of this article appeared on Medscape.com.
You’re invited to a dinner party but you struggle to make small talk. Do not worry; that will invariably crop up over cocktails. Because all journalism has been reduced to listicles, here are four ways to seem clever at dinner parties.
1. The Predinner Cocktails: A Lesson in Reverse Causation
Wine connoisseurs sniff, swirl, and gently swish the wine in their mouths before spitting out and cleansing their palates to better appreciate the subtlety of each vintage. If you’re not an oenophile, no matter. Whenever somebody claims that moderate amounts of alcohol are good for your heart, this is your moment to pounce. Interject yourself in the conversation and tell everybody about reverse causation.
Reverse causation, also known as protopathic bias, involves misinterpreting the directionality of an association. You assume that X leads to Y, when in fact Y leads to X. Temporal paradoxes are useful plot devices in science fiction movies, but they have no place in medical research. In our bland world, cause must precede effect. As such, smoking leads to lung cancer; lung cancer doesn’t make you smoke more.
But with alcohol, directionality is less obvious. Many studies of alcohol and cardiovascular disease have demonstrated a U-shaped association, with risk being lowest among those who drink moderate amounts of alcohol (usually one to two drinks per day) and higher in those who drink more and also those who drink very little.
But one must ask why some people drink little or no alcohol. There is an important difference between former drinkers and never drinkers. Former drinkers cut back for a reason. More likely than not, the reason for this newfound sobriety was medical. A new cancer diagnosis, the emergence of atrial fibrillation, the development of diabetes, or rising blood pressure are all good reasons to reduce or eliminate alcohol. A cross-sectional study will fail to capture that alcohol consumption changes over time — people who now don’t drink may have imbibed alcohol in years past. It was not abstinence that led to an increased risk for heart disease; it was the increased risk for heart disease that led to abstinence.
You see the same phenomenon with the so-called obesity paradox. The idea that being a little overweight is good for you may appeal when you no longer fit into last year’s pants. But people who are underweight are so for a reason. Malnutrition, cachexia from cancer, or some other cause is almost certainly driving up the risk at the left-hand side of the U-shaped curve that makes the middle part seem better than it actually is.
Food consumption changes over time. A cross-sectional survey at one point in time cannot accurately capture past habits and distant exposures, especially for diseases such as heart disease and cancer that develop slowly over time. Studies on alcohol that try to overcome these shortcomings by eliminating former drinkers, or by using Mendelian randomization to better account for past exposure, do not show a cardiovascular benefit for moderate red wine drinking.
2. The Hors D’oeuvres — The Importance of RCTs
Now that you have made yourself the center of attention, it is time to cement your newfound reputation as a font of scientific knowledge. Most self-respecting hosts will serve smoked salmon as an amuse-bouche before the main meal. When someone mentions the health benefits of fish oils, you should take the opportunity to teach them about confounding.
Fish, especially cold-water fish from northern climates, have relatively high amounts of omega-3 fatty acids. Despite the plethora of observational studies suggesting a cardiovascular benefit, it’s now relatively clear that fish oil or omega-3 supplements have no medical benefit.
This will probably come as a shock to the worried well, but many studies, including VITAL and ASCEND, have demonstrated no cardiovascular or cancer benefit to supplementation with omega-3s. The reason is straightforward and explains why hormone replacement therapy, vitamin D, and myriad purported game-changers never panned out. Confounding is hard to overcome in observational research.
Prior to the publication of the Women’s Health Initiative (WHI) Study, hormone replacement therapy was routinely prescribed to postmenopausal women because numerous observational studies suggested a cardiovascular benefit. But with the publication of the WHI study, it became clear that much of that “benefit” was due to confounding. The women choosing to take hormones were more health conscious at baseline and healthier overall.
A similar phenomenon occurred during COVID. Patients with low serum vitamin D levels had worse outcomes, prompting many to suggest vitamin D supplementation as a possible treatment. Trials did not support the intervention because we’d overlooked the obvious. People with vitamin D deficiency have underlying health problems that contribute to the vitamin D deficiency. They are probably older, frailer, possibly with a poorer diet. No amount of statistical adjustment can account for all those differences, and some degree of residual confounding will always persist.
The only way to overcome confounding is with randomization. When patients are randomly assigned to one group or another, their baseline differences largely balance out if the randomization was performed properly and the groups were large enough. There is a role for observational research, such as in situations where ethics, cost, and practicality do not allow for a randomized controlled trial. But randomized controlled trials have largely put to rest the purported health benefits of over-the-counter fish oils, omega-3s, and vitamin D.
3. The Main Course — Absolute vs Relative Risk
When you get to the main course, all eyes will now be on you. You will almost certainly be called upon to pronounce on the harms or benefits of red meat consumption. Begin by regaling your guests with a little trivia. Ask them if they know the definition of red meat and white meat. When someone says pork is white meat, you can reveal that “pork, the other white meat,” was a marketing slogan with no scientific underpinning. Now that everyone is lulled into a stupefied silence, tell them that red meat comes from mammals and white meat comes from birds. As they process this revelation, you can now launch into the deeply mathematical concept of absolute vs relative risk.
Many etiquette books will caution against bringing up math at a dinner party. These books are wrong. Everyone finds math interesting if they are primed properly. For example, you can point to a study claiming that berries reduce cardiovascular risk in women. Even if true — and there is reason to be cautious, given the observational nature of the research — we need to understand what the authors meant by a 32% risk reduction. (Side note: It was a reduction in hazard, with a hazard ratio of 0.68 (95% CI, 0.49-0.96), but we won’t dwell on the difference between hazard ratios and risk ratios right now.)
This relative risk reduction has to be interpreted carefully. The authors divided the population into quintiles based on their consumption of anthocyanins (the antioxidant in blueberries and strawberries) and compared the bottom fifth (average consumption, 2.5 mg/d) with the top fifth (average consumption, 25 mg/d). The bottom quintile had 126 myocardial infarctions (MIs) over 324,793 patient-years compared with 59 MIs over 332,143 patient-years. Some quick math shows an approximate reduction from 39 to 18 MIs per 100,000 patient-years. Or to put it another way, you must get 4762 women to increase their berry consumption 10-fold for 1 year to prevent one heart attack. Feel free to show people how you calculated this number. They will be impressed by your head for numbers. It is nothing more than 39 minus 18, divided by 100,000, to get the absolute risk reduction. Take the reciprocal of this (ie, 1 divided by this number) to get the number needed to treat.
Describing risks in absolute terms or using number needed to treat (or harm) can help conceptualize statistics that are sometimes hard to wrap your head around.
4. Dessert — Funding
By the time the coffee is served, everyone will be hanging on to your every word. This is as it should be, and you should not be afraid of your newfound power and influence.
Dessert will probably involve some form of chocolate, possibly in cake format. (Anyone who serves fruit as dessert is not someone you should associate with.) Take the opportunity to tell your follow diners that chocolate is not actually good for you and will not boost brain performance.
The health benefits of chocolate are often repeated but rarely scrutinized. In fact, much of the scientific research purporting to show that chocolate is good for you did not actually study chocolate. It usually involved a cocoa bean extract because the chocolate manufacturing process destroys the supposedly health-promoting antioxidants in the cocoa bean. It is true that dark chocolate has more antioxidants than milk chocolate, and that the addition of milk to chocolate further inactivates the potentially healthy antioxidants. But the amount of sugar and fat that has to be added to chocolate to make it palatable precludes any serious consideration about health benefits. Dark chocolate may have less fat and sugar than milk chocolate, but it still has a lot.
But even the cocoa bean extract doesn’t seem to do much for your heart or your brain. The long-awaited COSMOS study was published with surprisingly little fanfare. The largest randomized controlled trial of chocolate (or rather cocoa bean extract) was supposed to settle the issue definitively.
COSMOS showed no cardiovascular or neurocognitive benefit to the cocoa bean extract. But the health halo of chocolate continues to be bolstered by many studies funded by chocolate manufacturers.
We are appropriately critical of the pharmaceutical industry’s involvement in drug research. However, we should not forget that any private entity is prone to the same self-interest regardless of its product’s tastiness. How many of you knew that there was an avocado lobby funding research? No matter how many industry-funded observational studies using surrogate endpoints are out there telling you that chocolate is healthy, a randomized trial with hard clinical endpoints such as COSMOS should generally win the day.
The Final Goodbyes — Summarizing Your Case
As the party slowly winds down and everyone is saddened that you will soon take your leave, synthesize everything you have taught them over the evening. Like movies, not all studies are good. Some are just bad. They can be prone to reverse causation or confounding, and they may report relative risks when absolute risks would be more telling. Reading research studies critically is essential for separating the wheat from the chaff. With the knowledge you have now imparted to your friends, they will be much better consumers of medical news, especially when it comes to food.
And they will no doubt thank you for it by never inviting you to another dinner party!
Labos, a cardiologist at Hôpital, Notre-Dame, Montreal, Quebec, Canada, has disclosed no relevant financial relationships. He has a degree in epidemiology.
A version of this article appeared on Medscape.com.
You’re invited to a dinner party but you struggle to make small talk. Do not worry; that will invariably crop up over cocktails. Because all journalism has been reduced to listicles, here are four ways to seem clever at dinner parties.
1. The Predinner Cocktails: A Lesson in Reverse Causation
Wine connoisseurs sniff, swirl, and gently swish the wine in their mouths before spitting out and cleansing their palates to better appreciate the subtlety of each vintage. If you’re not an oenophile, no matter. Whenever somebody claims that moderate amounts of alcohol are good for your heart, this is your moment to pounce. Interject yourself in the conversation and tell everybody about reverse causation.
Reverse causation, also known as protopathic bias, involves misinterpreting the directionality of an association. You assume that X leads to Y, when in fact Y leads to X. Temporal paradoxes are useful plot devices in science fiction movies, but they have no place in medical research. In our bland world, cause must precede effect. As such, smoking leads to lung cancer; lung cancer doesn’t make you smoke more.
But with alcohol, directionality is less obvious. Many studies of alcohol and cardiovascular disease have demonstrated a U-shaped association, with risk being lowest among those who drink moderate amounts of alcohol (usually one to two drinks per day) and higher in those who drink more and also those who drink very little.
But one must ask why some people drink little or no alcohol. There is an important difference between former drinkers and never drinkers. Former drinkers cut back for a reason. More likely than not, the reason for this newfound sobriety was medical. A new cancer diagnosis, the emergence of atrial fibrillation, the development of diabetes, or rising blood pressure are all good reasons to reduce or eliminate alcohol. A cross-sectional study will fail to capture that alcohol consumption changes over time — people who now don’t drink may have imbibed alcohol in years past. It was not abstinence that led to an increased risk for heart disease; it was the increased risk for heart disease that led to abstinence.
You see the same phenomenon with the so-called obesity paradox. The idea that being a little overweight is good for you may appeal when you no longer fit into last year’s pants. But people who are underweight are so for a reason. Malnutrition, cachexia from cancer, or some other cause is almost certainly driving up the risk at the left-hand side of the U-shaped curve that makes the middle part seem better than it actually is.
Food consumption changes over time. A cross-sectional survey at one point in time cannot accurately capture past habits and distant exposures, especially for diseases such as heart disease and cancer that develop slowly over time. Studies on alcohol that try to overcome these shortcomings by eliminating former drinkers, or by using Mendelian randomization to better account for past exposure, do not show a cardiovascular benefit for moderate red wine drinking.
2. The Hors D’oeuvres — The Importance of RCTs
Now that you have made yourself the center of attention, it is time to cement your newfound reputation as a font of scientific knowledge. Most self-respecting hosts will serve smoked salmon as an amuse-bouche before the main meal. When someone mentions the health benefits of fish oils, you should take the opportunity to teach them about confounding.
Fish, especially cold-water fish from northern climates, have relatively high amounts of omega-3 fatty acids. Despite the plethora of observational studies suggesting a cardiovascular benefit, it’s now relatively clear that fish oil or omega-3 supplements have no medical benefit.
This will probably come as a shock to the worried well, but many studies, including VITAL and ASCEND, have demonstrated no cardiovascular or cancer benefit to supplementation with omega-3s. The reason is straightforward and explains why hormone replacement therapy, vitamin D, and myriad purported game-changers never panned out. Confounding is hard to overcome in observational research.
Prior to the publication of the Women’s Health Initiative (WHI) Study, hormone replacement therapy was routinely prescribed to postmenopausal women because numerous observational studies suggested a cardiovascular benefit. But with the publication of the WHI study, it became clear that much of that “benefit” was due to confounding. The women choosing to take hormones were more health conscious at baseline and healthier overall.
A similar phenomenon occurred during COVID. Patients with low serum vitamin D levels had worse outcomes, prompting many to suggest vitamin D supplementation as a possible treatment. Trials did not support the intervention because we’d overlooked the obvious. People with vitamin D deficiency have underlying health problems that contribute to the vitamin D deficiency. They are probably older, frailer, possibly with a poorer diet. No amount of statistical adjustment can account for all those differences, and some degree of residual confounding will always persist.
The only way to overcome confounding is with randomization. When patients are randomly assigned to one group or another, their baseline differences largely balance out if the randomization was performed properly and the groups were large enough. There is a role for observational research, such as in situations where ethics, cost, and practicality do not allow for a randomized controlled trial. But randomized controlled trials have largely put to rest the purported health benefits of over-the-counter fish oils, omega-3s, and vitamin D.
3. The Main Course — Absolute vs Relative Risk
When you get to the main course, all eyes will now be on you. You will almost certainly be called upon to pronounce on the harms or benefits of red meat consumption. Begin by regaling your guests with a little trivia. Ask them if they know the definition of red meat and white meat. When someone says pork is white meat, you can reveal that “pork, the other white meat,” was a marketing slogan with no scientific underpinning. Now that everyone is lulled into a stupefied silence, tell them that red meat comes from mammals and white meat comes from birds. As they process this revelation, you can now launch into the deeply mathematical concept of absolute vs relative risk.
Many etiquette books will caution against bringing up math at a dinner party. These books are wrong. Everyone finds math interesting if they are primed properly. For example, you can point to a study claiming that berries reduce cardiovascular risk in women. Even if true — and there is reason to be cautious, given the observational nature of the research — we need to understand what the authors meant by a 32% risk reduction. (Side note: It was a reduction in hazard, with a hazard ratio of 0.68 (95% CI, 0.49-0.96), but we won’t dwell on the difference between hazard ratios and risk ratios right now.)
This relative risk reduction has to be interpreted carefully. The authors divided the population into quintiles based on their consumption of anthocyanins (the antioxidant in blueberries and strawberries) and compared the bottom fifth (average consumption, 2.5 mg/d) with the top fifth (average consumption, 25 mg/d). The bottom quintile had 126 myocardial infarctions (MIs) over 324,793 patient-years compared with 59 MIs over 332,143 patient-years. Some quick math shows an approximate reduction from 39 to 18 MIs per 100,000 patient-years. Or to put it another way, you must get 4762 women to increase their berry consumption 10-fold for 1 year to prevent one heart attack. Feel free to show people how you calculated this number. They will be impressed by your head for numbers. It is nothing more than 39 minus 18, divided by 100,000, to get the absolute risk reduction. Take the reciprocal of this (ie, 1 divided by this number) to get the number needed to treat.
Describing risks in absolute terms or using number needed to treat (or harm) can help conceptualize statistics that are sometimes hard to wrap your head around.
4. Dessert — Funding
By the time the coffee is served, everyone will be hanging on to your every word. This is as it should be, and you should not be afraid of your newfound power and influence.
Dessert will probably involve some form of chocolate, possibly in cake format. (Anyone who serves fruit as dessert is not someone you should associate with.) Take the opportunity to tell your follow diners that chocolate is not actually good for you and will not boost brain performance.
The health benefits of chocolate are often repeated but rarely scrutinized. In fact, much of the scientific research purporting to show that chocolate is good for you did not actually study chocolate. It usually involved a cocoa bean extract because the chocolate manufacturing process destroys the supposedly health-promoting antioxidants in the cocoa bean. It is true that dark chocolate has more antioxidants than milk chocolate, and that the addition of milk to chocolate further inactivates the potentially healthy antioxidants. But the amount of sugar and fat that has to be added to chocolate to make it palatable precludes any serious consideration about health benefits. Dark chocolate may have less fat and sugar than milk chocolate, but it still has a lot.
But even the cocoa bean extract doesn’t seem to do much for your heart or your brain. The long-awaited COSMOS study was published with surprisingly little fanfare. The largest randomized controlled trial of chocolate (or rather cocoa bean extract) was supposed to settle the issue definitively.
COSMOS showed no cardiovascular or neurocognitive benefit to the cocoa bean extract. But the health halo of chocolate continues to be bolstered by many studies funded by chocolate manufacturers.
We are appropriately critical of the pharmaceutical industry’s involvement in drug research. However, we should not forget that any private entity is prone to the same self-interest regardless of its product’s tastiness. How many of you knew that there was an avocado lobby funding research? No matter how many industry-funded observational studies using surrogate endpoints are out there telling you that chocolate is healthy, a randomized trial with hard clinical endpoints such as COSMOS should generally win the day.
The Final Goodbyes — Summarizing Your Case
As the party slowly winds down and everyone is saddened that you will soon take your leave, synthesize everything you have taught them over the evening. Like movies, not all studies are good. Some are just bad. They can be prone to reverse causation or confounding, and they may report relative risks when absolute risks would be more telling. Reading research studies critically is essential for separating the wheat from the chaff. With the knowledge you have now imparted to your friends, they will be much better consumers of medical news, especially when it comes to food.
And they will no doubt thank you for it by never inviting you to another dinner party!
Labos, a cardiologist at Hôpital, Notre-Dame, Montreal, Quebec, Canada, has disclosed no relevant financial relationships. He has a degree in epidemiology.
A version of this article appeared on Medscape.com.
8 New GI Studies With Practice-Shifting Implications
I’m just back from the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting in Philadelphia, Pennsylvania.
In part 2 of this series, I’m offering my highlights from this year’s meeting. (Part 1 is available here.) They are not presented in any particular order, but instead I am sharing what I found to be the most exciting among the thousands of abstracts and presentations.
Performing Capsule Endoscopy in Patients Taking GLP-1s
We’ve heard a lot about glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the possibility that they might contribute to an increased risk for retained gastric contents and aspiration during endoscopy.
In the first study I’d like to highlight, researchers from the Mayo Clinic in Jacksonville, Florida, investigated video capsule endoscopy in patients with diabetes who were taking GLP-1 RAs vs a control group with diabetes not taking GLP-1 Ras. Patients in this retrospective matched cohort study were well balanced for demographics and diabetes-related characteristics.
Researchers found that in 7% of the 68 patients in the GLP-1 RA cohort, the video capsule endoscopy actually failed to pass through the stomach, whereas it passed successfully in all 68 patients in the control group (P =.06). The GLP-1 RA cohort had a longer transit time by a factor of almost four times (P <.001).
Multivariate analysis also showed that gastric transit time was approximately 80 minutes longer in the GLP-1 RA cohort (P <.001). Interestingly, 23.5% (16 of 68 patients) in the GLP-1 RA group experienced incomplete passage of the video capsule endoscopy through the small intestine, which was significantly higher than the proportion observed in the control group (4.4%; P <.01).
We need to look at potential strategies to mitigate these effects. Be aware of these results as you perform capsule endoscopy in patients taking GLP-1 RAs.
Barrett Esophagus On the Rise in the Young
The second study that caught my eye revealed the increasing incidence of young-onset Barrett esophagus (BE).
This population-based study used data obtained from TriNetX, a multi-institutional national database that offers a composite of health records from 88 healthcare organizations. Eligible patients had to have a negative upper esophagogastroduodenoscopy for BE prior to subsequently developing BE. Researchers stratified patients as to whether they were younger (< 50 years) or older (≥ 50 years), with further age ranges analyzed within those groups.
Young-onset BE accounted for 20% of all incident cases. The majority (94%) had nondysplastic BE. The incidence rate was not significantly different depending on whether patients were in the 45- to 49-year or 50- to 54-year age group.
Regression analysis revealed that there was a significantly increased trend for young-onset BE with hiatal hernia (odds ratio [OR], 2.6), smoking (OR, 2.3), White race (OR, 2.3), obstructive sleep apnea (OR, 2.2), male gender (OR, 2.0), and — at relatively lower risk levels — gastroesophageal reflux disease symptoms (OR, 1.2) and body mass index (OR, 1.1). The researchers did not analyze patients based on the presence of obesity, which is one of the risk factors for BE mentioned in national guidelines.
Results also showed that 6% of those with young-onset BE had BE-related neoplasia.
ACG guidelines recommend screening for BE beginning at age 50 in those with some of the risk factors noted in this study, including the presence of chronic gastroesophageal reflux disease symptoms. However, doing so may not capture the growing number of patients with young-onset BE.
We’ve seen a similar rise in rates of young-onset colorectal cancer, which has caused us to reevaluate our screening methods. Maybe we should do this for BE as well, specifically for patients presenting with these risk factors.
There’s a caveat to be aware of, which comes from my personal experience. I was biopsied for short-segment BE, and because it came up on my health record, it increased my life insurance premiums. This was because I was identified as having the risk profile of, essentially, an otherwise healthy smoker.
Dr Nicholas J. Shaheen and colleagues published a study several years ago showing that many insurance companies would not certify young, otherwise healthy people once diagnosed with BE. This is something to be aware of when you start to screen for BE, especially among younger patients.
A Novel Biologic for Eosinophilic Esophagitis
The next study presented results from a randomized, placebo-controlled, phase 3 study of cendakimab, a biologic agent in development for the treatment of eosinophilic esophagitis (EoE).
Dupilumab, which is an anti–interleukin (IL)-4 antibody, is the first treatment approved by the US Food and Drug Administration (FDA) for the treatment of EoE. Cendakimab, in turn, is a monoclonal antibody that neutralizes IL-13, a cytokine that plays a key role in EoE.
The study was led by Dr Evan Dellon from the University of North Carolina at Chapel Hill. Dellon and colleagues analyzed two different dosing regimens of cendakimab — 360 mg once weekly for 48 weeks, or 360 mg once weekly for 24 weeks followed by 360 mg every other week for 24 weeks — vs placebo for 48 weeks.
There was a significant effect for both cendakimab regimens in terms of symptom improvement and histologic response. There wasn’t much difference between participants that maintained once-weekly dosing and those who switched over to receive cendakimab every other week at 24 weeks. Only a minimal number of serious adverse events leading to discontinuation were noted in the study, with no notable difference between the treatment groups.
I think we’ll probably see this drug become available to us soon after it goes through the FDA review process, at which point it will add to our ability to use formative biologics in patients with EoE.
No Clear Benefit to Adding Bezlotoxumab to Fecal Microbiota Transplantation
Next was a very interesting study, and I think a clinically relevant one, about using fecal microbiota transplantation (FMT) alone or in combination with bezlotoxumab in patients with inflammatory bowel disease (IBD) with recurrent Clostridioides difficile infection.
Bezlotoxumab is a fully human monoclonal antibody that binds to C difficile toxin B. This drug has been studied and is approved for use, but it’s also extremely expensive, at a cost of approximately $4000 per dose.
Patients with IBD were eligible for inclusion if they had had two or more episodes of C difficile infection. They were then randomized in a 1:1 ration to receive either a single infusion of bezlotoxumab or placebo prior to FMT. The primary endpoint was C difficile infection recurrence within 8 weeks, which was defined as diarrhea plus a positive enzyme immunoassay toxin test. The secondary outcome was C difficile decolonization following treatment.
Researchers observed no statistically significant difference between the two cohorts. Steroid use prior to FMT significantly increased the risk for ongoing C difficile colonization (P =.03).
In summary, this is a case where it doesn’t seem that more is better. Bezlotoxumab didn’t add much, which calls into question the justification for its combined use with FMT.
Additional Positive Data for Seladelpar in Primary Biliary Cholangitis and Cirrhosis
Reassuring findings were presented on seladelpar, which was granted accelerated approval by the FDA in August.
Seladelpar is a selective peroxisome proliferator–activated receptor delta agonist that works in biliary cholangitis by regulating the genes involved in blocking biliary bile acid synthesis and controlling inflammation and fibrosis.
Results from the phase 3 RESPONSE trial were published in The New England Journal of Medicine in February reporting on the use of seladelpar in primary biliary cholangitis.
Whereas the RESPONSE trial removed decompensated patients, the ongoing phase 3 ASSURE trial results presented at this year’s meeting included patients with compensated cirrhosis. Approximately 94% of the patients in this study had Child-Pugh class A and 6% had class B cirrhosis. Eligibility required that patients had an inadequate response or were intolerant to ursodeoxycholic acid. Patients were administered open-label seladelpar 10 mg orally daily and followed for up to 1 year.
The good news is that there were no safety signals, which is reassuring news for our patients with compensated cirrhosis.
Advantages to Respiratory Syncytial Virus Vaccination in IBD
Another study that offered results certainly worthy of attention dealt with vaccination recommendations in patients with IBD.
Vaccination for respiratory syncytial virus (RSV) is now available in the United States. Its use was recommended for patients with IBD as early as 2021 per the Canadian Association of Gastroenterology’s clinical practice guideline, which discusses both live and nonlive vaccines. We should be aggressive in recommending this vaccine to our patients with IBD, but we haven’t really had one until recently.
Researchers behind this retrospective cohort study used the TriNetX database, which includes over 100 million unique patient charts. They identified patients with IBD, who were then divided into two groups according to whether they received the RSV vaccine or not.
Although this analysis was conducted in patients > 60 years of age, the US Centers for Disease Control and Prevention recommends RSV vaccination for all those over the age of 75 years, as well as for those 60-74 years old based on severity of risk.
For the primary endpoint of risk for RSV pneumonia, the OR was dramatically better in those who were vaccinated, with an approximately 80% risk reduction. Additionally, vaccinated patients experienced risk reductions of approximately 60% for acute respiratory failure, 50% for hospital inpatient admission, and 70% for requiring intensive care unit services.
This is a strong study showing not only that RSV vaccine did not exacerbate IBD but also that it improved outcomes in these patients. There’s a live-attenuated RSV vaccination that’s administered intranasally, which wouldn’t be used in your biologic or immunosuppressed patients with IBD, but the intramuscularly administered RSV vaccine doesn’t have any risk.
I think we can immediately begin recommending the RSV vaccine for our patients with IBD, particularly in those 60 years of age or older.
The Impact of Palliative Care Consultations in Decompensated Cirrhosis
The next study I’d like to highlight offers important data on the impact of palliative care consultation on 30- and 90-day readmission in patients with decompensated cirrhosis, which is a major cause of morbidity and mortality.
Researchers queried the National Readmissions Database over a 10-year period (2010-2019) to determine whether patients received a palliative care consult during index admission. They drew on a population of over 1.6 million patients admitted with decompensated cirrhosis.
Of this group, only 7.4% received a palliative care consultation at the index admission. But if they had this consultation, it was associated with a dramatic effect on readmission at 30 and 90 days. There was statistically significant risk reduction of approximately 70% for both 30- and 90-day readmission compared with those who didn’t receive the palliative consult (P <.001).
The take-home message here is to get a palliative care consult with these patients when they come in. Your hospital will unquestionably experience value in this reduction in readmission, especially considering that readmission within 30 days may not even be covered. Look at these results and start to take advantage of this valuable consultation.
Auxora: A Novel Treatment for Acute Pancreatitis
The last study for discussion offered very interesting data related to a drug called Auxora, a calcium release–activated calcium-channel inhibitor.
There is growing data that overactive calcium release–activated calcium channels aggravate acute pancreatitis and accelerate systemic inflammatory response syndrome (SIRS).
Acute pancreatitis with necrosis encompasses both local and systemic inflammation and is associated with significant mortality and morbidity. It is estimated that among patients with acute pancreatitis, 20%-30% have pancreatic necrosis, 30% develop infection, and 25% develop organ failure.
The presence of SIRS seems to herald the activation of these complex inflammatory pathways, which then leads to organ failure and necrosis, which can potentially be stemmed through this calcium channel inhibitor. Phase 2 studies of Auxora found that its use was associated with significant reduction in the risk for progression.
This subsequent phase 3 study looked at patients with acute pancreatitis and accompanying grade ≥ 2 SIRS criteria. They were randomized to receive placebo or Auxora at doses of 2 mg/kg, 1 mg/kg, or 0.5 mg/kg, which was administered intravenously over 4 hours for 3 consecutive days.
The primary endpoint was time to solid food tolerance, which was defined as eating ≥ 50% of a ≥ 500-calorie low-fat solid meal without increased abdominal pain or emesis, which is an important target because we always aim for enteral nutrition in patients with acute pancreatitis. The key secondary endpoint was severe respiratory failure, which was defined as invasive mechanical ventilation or ≥ 48 hours of either high-flow nasal cannula or noninvasive mechanical ventilation.
The primary endpoint was dramatically improved among those receiving Auxora, who achieved early onset of refeed. It appears that the high-dose 2 mg/kg may be the most beneficial in achieving improvement.
There were no patients with suspected or unexpected adverse events in the study population. Additionally, no patients receiving Auxora at any dose level went on to develop respiratory failure.
The present results show that Auxora decreases the time for solid food tolerance, as well as the rates of respiratory failure and necrotizing pancreatitis in patients presenting with two or more SIRS criteria. We’ll certainly look forward to more data, but it provides hope for a new treatment for acute pancreatitis.
Some of the take-home messages I presented are actionable now, whereas for others, we’ll have to wait and see what the final data show as well as the results of ongoing FDA approval before applying them.
Dr Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article first appeared on Medscape.com.
I’m just back from the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting in Philadelphia, Pennsylvania.
In part 2 of this series, I’m offering my highlights from this year’s meeting. (Part 1 is available here.) They are not presented in any particular order, but instead I am sharing what I found to be the most exciting among the thousands of abstracts and presentations.
Performing Capsule Endoscopy in Patients Taking GLP-1s
We’ve heard a lot about glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the possibility that they might contribute to an increased risk for retained gastric contents and aspiration during endoscopy.
In the first study I’d like to highlight, researchers from the Mayo Clinic in Jacksonville, Florida, investigated video capsule endoscopy in patients with diabetes who were taking GLP-1 RAs vs a control group with diabetes not taking GLP-1 Ras. Patients in this retrospective matched cohort study were well balanced for demographics and diabetes-related characteristics.
Researchers found that in 7% of the 68 patients in the GLP-1 RA cohort, the video capsule endoscopy actually failed to pass through the stomach, whereas it passed successfully in all 68 patients in the control group (P =.06). The GLP-1 RA cohort had a longer transit time by a factor of almost four times (P <.001).
Multivariate analysis also showed that gastric transit time was approximately 80 minutes longer in the GLP-1 RA cohort (P <.001). Interestingly, 23.5% (16 of 68 patients) in the GLP-1 RA group experienced incomplete passage of the video capsule endoscopy through the small intestine, which was significantly higher than the proportion observed in the control group (4.4%; P <.01).
We need to look at potential strategies to mitigate these effects. Be aware of these results as you perform capsule endoscopy in patients taking GLP-1 RAs.
Barrett Esophagus On the Rise in the Young
The second study that caught my eye revealed the increasing incidence of young-onset Barrett esophagus (BE).
This population-based study used data obtained from TriNetX, a multi-institutional national database that offers a composite of health records from 88 healthcare organizations. Eligible patients had to have a negative upper esophagogastroduodenoscopy for BE prior to subsequently developing BE. Researchers stratified patients as to whether they were younger (< 50 years) or older (≥ 50 years), with further age ranges analyzed within those groups.
Young-onset BE accounted for 20% of all incident cases. The majority (94%) had nondysplastic BE. The incidence rate was not significantly different depending on whether patients were in the 45- to 49-year or 50- to 54-year age group.
Regression analysis revealed that there was a significantly increased trend for young-onset BE with hiatal hernia (odds ratio [OR], 2.6), smoking (OR, 2.3), White race (OR, 2.3), obstructive sleep apnea (OR, 2.2), male gender (OR, 2.0), and — at relatively lower risk levels — gastroesophageal reflux disease symptoms (OR, 1.2) and body mass index (OR, 1.1). The researchers did not analyze patients based on the presence of obesity, which is one of the risk factors for BE mentioned in national guidelines.
Results also showed that 6% of those with young-onset BE had BE-related neoplasia.
ACG guidelines recommend screening for BE beginning at age 50 in those with some of the risk factors noted in this study, including the presence of chronic gastroesophageal reflux disease symptoms. However, doing so may not capture the growing number of patients with young-onset BE.
We’ve seen a similar rise in rates of young-onset colorectal cancer, which has caused us to reevaluate our screening methods. Maybe we should do this for BE as well, specifically for patients presenting with these risk factors.
There’s a caveat to be aware of, which comes from my personal experience. I was biopsied for short-segment BE, and because it came up on my health record, it increased my life insurance premiums. This was because I was identified as having the risk profile of, essentially, an otherwise healthy smoker.
Dr Nicholas J. Shaheen and colleagues published a study several years ago showing that many insurance companies would not certify young, otherwise healthy people once diagnosed with BE. This is something to be aware of when you start to screen for BE, especially among younger patients.
A Novel Biologic for Eosinophilic Esophagitis
The next study presented results from a randomized, placebo-controlled, phase 3 study of cendakimab, a biologic agent in development for the treatment of eosinophilic esophagitis (EoE).
Dupilumab, which is an anti–interleukin (IL)-4 antibody, is the first treatment approved by the US Food and Drug Administration (FDA) for the treatment of EoE. Cendakimab, in turn, is a monoclonal antibody that neutralizes IL-13, a cytokine that plays a key role in EoE.
The study was led by Dr Evan Dellon from the University of North Carolina at Chapel Hill. Dellon and colleagues analyzed two different dosing regimens of cendakimab — 360 mg once weekly for 48 weeks, or 360 mg once weekly for 24 weeks followed by 360 mg every other week for 24 weeks — vs placebo for 48 weeks.
There was a significant effect for both cendakimab regimens in terms of symptom improvement and histologic response. There wasn’t much difference between participants that maintained once-weekly dosing and those who switched over to receive cendakimab every other week at 24 weeks. Only a minimal number of serious adverse events leading to discontinuation were noted in the study, with no notable difference between the treatment groups.
I think we’ll probably see this drug become available to us soon after it goes through the FDA review process, at which point it will add to our ability to use formative biologics in patients with EoE.
No Clear Benefit to Adding Bezlotoxumab to Fecal Microbiota Transplantation
Next was a very interesting study, and I think a clinically relevant one, about using fecal microbiota transplantation (FMT) alone or in combination with bezlotoxumab in patients with inflammatory bowel disease (IBD) with recurrent Clostridioides difficile infection.
Bezlotoxumab is a fully human monoclonal antibody that binds to C difficile toxin B. This drug has been studied and is approved for use, but it’s also extremely expensive, at a cost of approximately $4000 per dose.
Patients with IBD were eligible for inclusion if they had had two or more episodes of C difficile infection. They were then randomized in a 1:1 ration to receive either a single infusion of bezlotoxumab or placebo prior to FMT. The primary endpoint was C difficile infection recurrence within 8 weeks, which was defined as diarrhea plus a positive enzyme immunoassay toxin test. The secondary outcome was C difficile decolonization following treatment.
Researchers observed no statistically significant difference between the two cohorts. Steroid use prior to FMT significantly increased the risk for ongoing C difficile colonization (P =.03).
In summary, this is a case where it doesn’t seem that more is better. Bezlotoxumab didn’t add much, which calls into question the justification for its combined use with FMT.
Additional Positive Data for Seladelpar in Primary Biliary Cholangitis and Cirrhosis
Reassuring findings were presented on seladelpar, which was granted accelerated approval by the FDA in August.
Seladelpar is a selective peroxisome proliferator–activated receptor delta agonist that works in biliary cholangitis by regulating the genes involved in blocking biliary bile acid synthesis and controlling inflammation and fibrosis.
Results from the phase 3 RESPONSE trial were published in The New England Journal of Medicine in February reporting on the use of seladelpar in primary biliary cholangitis.
Whereas the RESPONSE trial removed decompensated patients, the ongoing phase 3 ASSURE trial results presented at this year’s meeting included patients with compensated cirrhosis. Approximately 94% of the patients in this study had Child-Pugh class A and 6% had class B cirrhosis. Eligibility required that patients had an inadequate response or were intolerant to ursodeoxycholic acid. Patients were administered open-label seladelpar 10 mg orally daily and followed for up to 1 year.
The good news is that there were no safety signals, which is reassuring news for our patients with compensated cirrhosis.
Advantages to Respiratory Syncytial Virus Vaccination in IBD
Another study that offered results certainly worthy of attention dealt with vaccination recommendations in patients with IBD.
Vaccination for respiratory syncytial virus (RSV) is now available in the United States. Its use was recommended for patients with IBD as early as 2021 per the Canadian Association of Gastroenterology’s clinical practice guideline, which discusses both live and nonlive vaccines. We should be aggressive in recommending this vaccine to our patients with IBD, but we haven’t really had one until recently.
Researchers behind this retrospective cohort study used the TriNetX database, which includes over 100 million unique patient charts. They identified patients with IBD, who were then divided into two groups according to whether they received the RSV vaccine or not.
Although this analysis was conducted in patients > 60 years of age, the US Centers for Disease Control and Prevention recommends RSV vaccination for all those over the age of 75 years, as well as for those 60-74 years old based on severity of risk.
For the primary endpoint of risk for RSV pneumonia, the OR was dramatically better in those who were vaccinated, with an approximately 80% risk reduction. Additionally, vaccinated patients experienced risk reductions of approximately 60% for acute respiratory failure, 50% for hospital inpatient admission, and 70% for requiring intensive care unit services.
This is a strong study showing not only that RSV vaccine did not exacerbate IBD but also that it improved outcomes in these patients. There’s a live-attenuated RSV vaccination that’s administered intranasally, which wouldn’t be used in your biologic or immunosuppressed patients with IBD, but the intramuscularly administered RSV vaccine doesn’t have any risk.
I think we can immediately begin recommending the RSV vaccine for our patients with IBD, particularly in those 60 years of age or older.
The Impact of Palliative Care Consultations in Decompensated Cirrhosis
The next study I’d like to highlight offers important data on the impact of palliative care consultation on 30- and 90-day readmission in patients with decompensated cirrhosis, which is a major cause of morbidity and mortality.
Researchers queried the National Readmissions Database over a 10-year period (2010-2019) to determine whether patients received a palliative care consult during index admission. They drew on a population of over 1.6 million patients admitted with decompensated cirrhosis.
Of this group, only 7.4% received a palliative care consultation at the index admission. But if they had this consultation, it was associated with a dramatic effect on readmission at 30 and 90 days. There was statistically significant risk reduction of approximately 70% for both 30- and 90-day readmission compared with those who didn’t receive the palliative consult (P <.001).
The take-home message here is to get a palliative care consult with these patients when they come in. Your hospital will unquestionably experience value in this reduction in readmission, especially considering that readmission within 30 days may not even be covered. Look at these results and start to take advantage of this valuable consultation.
Auxora: A Novel Treatment for Acute Pancreatitis
The last study for discussion offered very interesting data related to a drug called Auxora, a calcium release–activated calcium-channel inhibitor.
There is growing data that overactive calcium release–activated calcium channels aggravate acute pancreatitis and accelerate systemic inflammatory response syndrome (SIRS).
Acute pancreatitis with necrosis encompasses both local and systemic inflammation and is associated with significant mortality and morbidity. It is estimated that among patients with acute pancreatitis, 20%-30% have pancreatic necrosis, 30% develop infection, and 25% develop organ failure.
The presence of SIRS seems to herald the activation of these complex inflammatory pathways, which then leads to organ failure and necrosis, which can potentially be stemmed through this calcium channel inhibitor. Phase 2 studies of Auxora found that its use was associated with significant reduction in the risk for progression.
This subsequent phase 3 study looked at patients with acute pancreatitis and accompanying grade ≥ 2 SIRS criteria. They were randomized to receive placebo or Auxora at doses of 2 mg/kg, 1 mg/kg, or 0.5 mg/kg, which was administered intravenously over 4 hours for 3 consecutive days.
The primary endpoint was time to solid food tolerance, which was defined as eating ≥ 50% of a ≥ 500-calorie low-fat solid meal without increased abdominal pain or emesis, which is an important target because we always aim for enteral nutrition in patients with acute pancreatitis. The key secondary endpoint was severe respiratory failure, which was defined as invasive mechanical ventilation or ≥ 48 hours of either high-flow nasal cannula or noninvasive mechanical ventilation.
The primary endpoint was dramatically improved among those receiving Auxora, who achieved early onset of refeed. It appears that the high-dose 2 mg/kg may be the most beneficial in achieving improvement.
There were no patients with suspected or unexpected adverse events in the study population. Additionally, no patients receiving Auxora at any dose level went on to develop respiratory failure.
The present results show that Auxora decreases the time for solid food tolerance, as well as the rates of respiratory failure and necrotizing pancreatitis in patients presenting with two or more SIRS criteria. We’ll certainly look forward to more data, but it provides hope for a new treatment for acute pancreatitis.
Some of the take-home messages I presented are actionable now, whereas for others, we’ll have to wait and see what the final data show as well as the results of ongoing FDA approval before applying them.
Dr Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article first appeared on Medscape.com.
I’m just back from the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting in Philadelphia, Pennsylvania.
In part 2 of this series, I’m offering my highlights from this year’s meeting. (Part 1 is available here.) They are not presented in any particular order, but instead I am sharing what I found to be the most exciting among the thousands of abstracts and presentations.
Performing Capsule Endoscopy in Patients Taking GLP-1s
We’ve heard a lot about glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the possibility that they might contribute to an increased risk for retained gastric contents and aspiration during endoscopy.
In the first study I’d like to highlight, researchers from the Mayo Clinic in Jacksonville, Florida, investigated video capsule endoscopy in patients with diabetes who were taking GLP-1 RAs vs a control group with diabetes not taking GLP-1 Ras. Patients in this retrospective matched cohort study were well balanced for demographics and diabetes-related characteristics.
Researchers found that in 7% of the 68 patients in the GLP-1 RA cohort, the video capsule endoscopy actually failed to pass through the stomach, whereas it passed successfully in all 68 patients in the control group (P =.06). The GLP-1 RA cohort had a longer transit time by a factor of almost four times (P <.001).
Multivariate analysis also showed that gastric transit time was approximately 80 minutes longer in the GLP-1 RA cohort (P <.001). Interestingly, 23.5% (16 of 68 patients) in the GLP-1 RA group experienced incomplete passage of the video capsule endoscopy through the small intestine, which was significantly higher than the proportion observed in the control group (4.4%; P <.01).
We need to look at potential strategies to mitigate these effects. Be aware of these results as you perform capsule endoscopy in patients taking GLP-1 RAs.
Barrett Esophagus On the Rise in the Young
The second study that caught my eye revealed the increasing incidence of young-onset Barrett esophagus (BE).
This population-based study used data obtained from TriNetX, a multi-institutional national database that offers a composite of health records from 88 healthcare organizations. Eligible patients had to have a negative upper esophagogastroduodenoscopy for BE prior to subsequently developing BE. Researchers stratified patients as to whether they were younger (< 50 years) or older (≥ 50 years), with further age ranges analyzed within those groups.
Young-onset BE accounted for 20% of all incident cases. The majority (94%) had nondysplastic BE. The incidence rate was not significantly different depending on whether patients were in the 45- to 49-year or 50- to 54-year age group.
Regression analysis revealed that there was a significantly increased trend for young-onset BE with hiatal hernia (odds ratio [OR], 2.6), smoking (OR, 2.3), White race (OR, 2.3), obstructive sleep apnea (OR, 2.2), male gender (OR, 2.0), and — at relatively lower risk levels — gastroesophageal reflux disease symptoms (OR, 1.2) and body mass index (OR, 1.1). The researchers did not analyze patients based on the presence of obesity, which is one of the risk factors for BE mentioned in national guidelines.
Results also showed that 6% of those with young-onset BE had BE-related neoplasia.
ACG guidelines recommend screening for BE beginning at age 50 in those with some of the risk factors noted in this study, including the presence of chronic gastroesophageal reflux disease symptoms. However, doing so may not capture the growing number of patients with young-onset BE.
We’ve seen a similar rise in rates of young-onset colorectal cancer, which has caused us to reevaluate our screening methods. Maybe we should do this for BE as well, specifically for patients presenting with these risk factors.
There’s a caveat to be aware of, which comes from my personal experience. I was biopsied for short-segment BE, and because it came up on my health record, it increased my life insurance premiums. This was because I was identified as having the risk profile of, essentially, an otherwise healthy smoker.
Dr Nicholas J. Shaheen and colleagues published a study several years ago showing that many insurance companies would not certify young, otherwise healthy people once diagnosed with BE. This is something to be aware of when you start to screen for BE, especially among younger patients.
A Novel Biologic for Eosinophilic Esophagitis
The next study presented results from a randomized, placebo-controlled, phase 3 study of cendakimab, a biologic agent in development for the treatment of eosinophilic esophagitis (EoE).
Dupilumab, which is an anti–interleukin (IL)-4 antibody, is the first treatment approved by the US Food and Drug Administration (FDA) for the treatment of EoE. Cendakimab, in turn, is a monoclonal antibody that neutralizes IL-13, a cytokine that plays a key role in EoE.
The study was led by Dr Evan Dellon from the University of North Carolina at Chapel Hill. Dellon and colleagues analyzed two different dosing regimens of cendakimab — 360 mg once weekly for 48 weeks, or 360 mg once weekly for 24 weeks followed by 360 mg every other week for 24 weeks — vs placebo for 48 weeks.
There was a significant effect for both cendakimab regimens in terms of symptom improvement and histologic response. There wasn’t much difference between participants that maintained once-weekly dosing and those who switched over to receive cendakimab every other week at 24 weeks. Only a minimal number of serious adverse events leading to discontinuation were noted in the study, with no notable difference between the treatment groups.
I think we’ll probably see this drug become available to us soon after it goes through the FDA review process, at which point it will add to our ability to use formative biologics in patients with EoE.
No Clear Benefit to Adding Bezlotoxumab to Fecal Microbiota Transplantation
Next was a very interesting study, and I think a clinically relevant one, about using fecal microbiota transplantation (FMT) alone or in combination with bezlotoxumab in patients with inflammatory bowel disease (IBD) with recurrent Clostridioides difficile infection.
Bezlotoxumab is a fully human monoclonal antibody that binds to C difficile toxin B. This drug has been studied and is approved for use, but it’s also extremely expensive, at a cost of approximately $4000 per dose.
Patients with IBD were eligible for inclusion if they had had two or more episodes of C difficile infection. They were then randomized in a 1:1 ration to receive either a single infusion of bezlotoxumab or placebo prior to FMT. The primary endpoint was C difficile infection recurrence within 8 weeks, which was defined as diarrhea plus a positive enzyme immunoassay toxin test. The secondary outcome was C difficile decolonization following treatment.
Researchers observed no statistically significant difference between the two cohorts. Steroid use prior to FMT significantly increased the risk for ongoing C difficile colonization (P =.03).
In summary, this is a case where it doesn’t seem that more is better. Bezlotoxumab didn’t add much, which calls into question the justification for its combined use with FMT.
Additional Positive Data for Seladelpar in Primary Biliary Cholangitis and Cirrhosis
Reassuring findings were presented on seladelpar, which was granted accelerated approval by the FDA in August.
Seladelpar is a selective peroxisome proliferator–activated receptor delta agonist that works in biliary cholangitis by regulating the genes involved in blocking biliary bile acid synthesis and controlling inflammation and fibrosis.
Results from the phase 3 RESPONSE trial were published in The New England Journal of Medicine in February reporting on the use of seladelpar in primary biliary cholangitis.
Whereas the RESPONSE trial removed decompensated patients, the ongoing phase 3 ASSURE trial results presented at this year’s meeting included patients with compensated cirrhosis. Approximately 94% of the patients in this study had Child-Pugh class A and 6% had class B cirrhosis. Eligibility required that patients had an inadequate response or were intolerant to ursodeoxycholic acid. Patients were administered open-label seladelpar 10 mg orally daily and followed for up to 1 year.
The good news is that there were no safety signals, which is reassuring news for our patients with compensated cirrhosis.
Advantages to Respiratory Syncytial Virus Vaccination in IBD
Another study that offered results certainly worthy of attention dealt with vaccination recommendations in patients with IBD.
Vaccination for respiratory syncytial virus (RSV) is now available in the United States. Its use was recommended for patients with IBD as early as 2021 per the Canadian Association of Gastroenterology’s clinical practice guideline, which discusses both live and nonlive vaccines. We should be aggressive in recommending this vaccine to our patients with IBD, but we haven’t really had one until recently.
Researchers behind this retrospective cohort study used the TriNetX database, which includes over 100 million unique patient charts. They identified patients with IBD, who were then divided into two groups according to whether they received the RSV vaccine or not.
Although this analysis was conducted in patients > 60 years of age, the US Centers for Disease Control and Prevention recommends RSV vaccination for all those over the age of 75 years, as well as for those 60-74 years old based on severity of risk.
For the primary endpoint of risk for RSV pneumonia, the OR was dramatically better in those who were vaccinated, with an approximately 80% risk reduction. Additionally, vaccinated patients experienced risk reductions of approximately 60% for acute respiratory failure, 50% for hospital inpatient admission, and 70% for requiring intensive care unit services.
This is a strong study showing not only that RSV vaccine did not exacerbate IBD but also that it improved outcomes in these patients. There’s a live-attenuated RSV vaccination that’s administered intranasally, which wouldn’t be used in your biologic or immunosuppressed patients with IBD, but the intramuscularly administered RSV vaccine doesn’t have any risk.
I think we can immediately begin recommending the RSV vaccine for our patients with IBD, particularly in those 60 years of age or older.
The Impact of Palliative Care Consultations in Decompensated Cirrhosis
The next study I’d like to highlight offers important data on the impact of palliative care consultation on 30- and 90-day readmission in patients with decompensated cirrhosis, which is a major cause of morbidity and mortality.
Researchers queried the National Readmissions Database over a 10-year period (2010-2019) to determine whether patients received a palliative care consult during index admission. They drew on a population of over 1.6 million patients admitted with decompensated cirrhosis.
Of this group, only 7.4% received a palliative care consultation at the index admission. But if they had this consultation, it was associated with a dramatic effect on readmission at 30 and 90 days. There was statistically significant risk reduction of approximately 70% for both 30- and 90-day readmission compared with those who didn’t receive the palliative consult (P <.001).
The take-home message here is to get a palliative care consult with these patients when they come in. Your hospital will unquestionably experience value in this reduction in readmission, especially considering that readmission within 30 days may not even be covered. Look at these results and start to take advantage of this valuable consultation.
Auxora: A Novel Treatment for Acute Pancreatitis
The last study for discussion offered very interesting data related to a drug called Auxora, a calcium release–activated calcium-channel inhibitor.
There is growing data that overactive calcium release–activated calcium channels aggravate acute pancreatitis and accelerate systemic inflammatory response syndrome (SIRS).
Acute pancreatitis with necrosis encompasses both local and systemic inflammation and is associated with significant mortality and morbidity. It is estimated that among patients with acute pancreatitis, 20%-30% have pancreatic necrosis, 30% develop infection, and 25% develop organ failure.
The presence of SIRS seems to herald the activation of these complex inflammatory pathways, which then leads to organ failure and necrosis, which can potentially be stemmed through this calcium channel inhibitor. Phase 2 studies of Auxora found that its use was associated with significant reduction in the risk for progression.
This subsequent phase 3 study looked at patients with acute pancreatitis and accompanying grade ≥ 2 SIRS criteria. They were randomized to receive placebo or Auxora at doses of 2 mg/kg, 1 mg/kg, or 0.5 mg/kg, which was administered intravenously over 4 hours for 3 consecutive days.
The primary endpoint was time to solid food tolerance, which was defined as eating ≥ 50% of a ≥ 500-calorie low-fat solid meal without increased abdominal pain or emesis, which is an important target because we always aim for enteral nutrition in patients with acute pancreatitis. The key secondary endpoint was severe respiratory failure, which was defined as invasive mechanical ventilation or ≥ 48 hours of either high-flow nasal cannula or noninvasive mechanical ventilation.
The primary endpoint was dramatically improved among those receiving Auxora, who achieved early onset of refeed. It appears that the high-dose 2 mg/kg may be the most beneficial in achieving improvement.
There were no patients with suspected or unexpected adverse events in the study population. Additionally, no patients receiving Auxora at any dose level went on to develop respiratory failure.
The present results show that Auxora decreases the time for solid food tolerance, as well as the rates of respiratory failure and necrotizing pancreatitis in patients presenting with two or more SIRS criteria. We’ll certainly look forward to more data, but it provides hope for a new treatment for acute pancreatitis.
Some of the take-home messages I presented are actionable now, whereas for others, we’ll have to wait and see what the final data show as well as the results of ongoing FDA approval before applying them.
Dr Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article first appeared on Medscape.com.
Thrombocytosis and Cancer Risk: Management in Primary Care
This transcript has been edited for clarity.
In this podcast, I’m going to talk about unexplained high platelet counts, or thrombocytosis, and the risk for cancer in primary care. Let’s start with a typical case we all might see in primary care.
Louisa is 47 years old and is the chief financial officer for a tech startup company. She presents to us in primary care feeling tired all the time — a very common presentation in primary care — with associated reduced appetite. Past medical history includes irritable bowel syndrome, and she’s an ex-smoker.
Systemic inquiry is unremarkable. Specifically, there is no history of weight loss. Louisa has not been prescribed any medication and uses over-the-counter remedies for her irritable bowel syndrome. Examination is also unremarkable. Blood tests were checked, which were all reassuring, except for a platelet count of 612 × 109 cells/L (usual normal range, about 150-450).
What do we do next? Do we refer for an urgent chest x-ray to exclude lung cancer? Do we check a quantitative immunohistochemical fecal occult blood test (qFIT) to identify any occult bleeding in her stool? Do we refer for a routine upper gastrointestinal endoscopy or pelvic ultrasound scan to exclude any upper gastrointestinal or endometrial malignancy?
Do we simply repeat the bloods? If so, do we repeat them routinely or urgently, and indeed, which ones should we recheck?
Louisa has an unexplained thrombocytosis. How do we manage this in primary care? Thrombocytosis is generally defined as a raised platelet count over 450. Importantly, thrombocytosis is a common incidental finding in around 2% of those over 40 years of age attending primary care. Reassuringly, 80%-90% of thrombocytosis is reactive, secondary to acute blood loss, infection, or inflammation, and the majority of cases resolve within 3 months.
Why the concern with Louisa then? Although most cases are reactive, clinical guidance (for example, NICE suspected cancer guidance in the UK and Scottish suspected cancer guidance in Scotland) reminds us that unexplained thrombocytosis is a risk marker for some solid-tumor malignancies.
Previous studies have demonstrated that unexplained thrombocytosis is associated with a 1-year cancer incidence of 11.6% in males and 6.2% in females, well exceeding the standard 3% threshold warranting investigation for underlying malignancy. However, thrombocytosis should not be used as a stand-alone diagnostic or screening test for cancer, or indeed to rule out cancer.
Instead, unexplained thrombocytosis should prompt us to think cancer. The Scottish suspected cancer referral guidelines include thrombocytosis in the investigation criteria for what they call the LEGO-C cancers — L for lung, E for endometrial, G for gastric, O for oesophageal, and C for colorectal, which is a useful reminder for us all.
What further history, examination, and investigations might we consider in primary care if we identify an unexplained high platelet count? As always, we should use our clinical judgment and trust our clinical acumen.
We should consider all the possible underlying causes, including infection, inflammation, and blood loss, including menstrual blood loss in women; myeloproliferative disorders such as polycythemia rubra vera, chronic myeloid leukemia, and essential thrombocythemia; and, of course, underlying malignancy. If a likely underlying reversible cause is present (for example, a recent lower respiratory tract infection), simply repeating the full blood count in 4-6 weeks is quite appropriate to see if the thrombocytosis has resolved.
Remember, 80%-90% of cases are reactive thrombocytosis, and most cases resolve within 3 months. If thrombocytosis is unexplained or not resolving, consider checking ferritin levels to exclude iron deficiency. Consider checking C-reactive protein (CRP) levels to exclude any inflammation, and also consider checking a blood film to exclude any hematologic disorders, in addition, of course, to more detailed history-taking and examination to elicit any red flags.
We can also consider a JAK2 gene mutation test, if it is available to you locally, or a hematology referral if we suspect a myeloproliferative disorder. JAK2 is a genetic mutation that may be present in people with essential thrombocythemia and can indicate a diagnosis of polycythemia rubra vera.
Subsequent to this, and again using our clinical judgment, we then need to exclude the LEGO-C cancers. Consider urgent chest x-ray to exclude lung cancer or pelvic ultrasound in women to exclude endometrial cancer. Also, we should consider an upper gastrointestinal endoscopy, particularly in those individuals who have associated upper gastrointestinal symptoms and/or weight loss.
Finally, consider a qFIT to identify any occult bleeding in the stool, again if it’s available to you, or certainly if not, urgent lower gastrointestinal investigations to exclude colorectal cancer.
Alongside these possible investigations, as always, we should safety-net appropriately within agreed timeframes and check for resolution of the thrombocytosis according to the condition being suspected. Remember, most cases resolve within 3 months.
Returning to Louisa, what did I do? After seeing a platelet count of 600, I subsequently telephoned her and reexplored her history, which yielded nil else of note. Specifically, there was no history of unexplained weight loss, no history of upper or lower gastrointestinal symptoms, and certainly nothing significantly different from her usual irritable bowel syndrome symptoms. There were also no respiratory or genitourinary symptoms of note.
I did arrange for Louisa to undergo a chest x-ray over the next few days, though, as she was an ex-smoker. This was subsequently reported as normal. I appreciate chest x-rays have poor sensitivity for detecting lung cancer, as highlighted in a number of recent papers, but it was mutually agreed with Louisa that we would simply repeat her blood test in around 6 weeks. As well as repeating the full blood count, I arranged to check her ferritin, CRP, and a blood film, and then I was planning to reassess her clinically in person.
These bloods and my subsequent clinical review were reassuring. In fact, her platelet count had normalized after that 6 weeks had elapsed. Her thrombocytosis had resolved.
I didn’t arrange any further follow-up for her, but I did give her the usual safety netting advice to re-present to me or one of my colleagues if she does develop any worrying symptoms or signs.
I appreciate these scenarios are not always this straightforward, but I wanted to outline what investigations and referrals we may need to consider in primary care if we encounter an unexplained high platelet count.
There are a couple of quality-improvement activities for us all to consider in primary care. Consider as a team how we would respond to an incidental finding of thrombocytosis on a full blood count. Also consider what are our safety-netting options for those found to have raised platelet counts but no other symptoms or risk factors for underlying malignancy.
Finally, I’ve produced a Medscape UK primary care hack or clinical aide-memoire on managing unexplained thrombocytosis and associated cancer risk in primary care for all healthcare professionals working in primary care. This can be found online. I hope you find this resource helpful.
Dr. Kevin Fernando, General practitioner partner with specialist interests in cardiovascular, renal, and metabolic medicine, North Berwick Group Practice in Scotland, has disclosed relevant financial relationships with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, Sanofi Menarini, and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In this podcast, I’m going to talk about unexplained high platelet counts, or thrombocytosis, and the risk for cancer in primary care. Let’s start with a typical case we all might see in primary care.
Louisa is 47 years old and is the chief financial officer for a tech startup company. She presents to us in primary care feeling tired all the time — a very common presentation in primary care — with associated reduced appetite. Past medical history includes irritable bowel syndrome, and she’s an ex-smoker.
Systemic inquiry is unremarkable. Specifically, there is no history of weight loss. Louisa has not been prescribed any medication and uses over-the-counter remedies for her irritable bowel syndrome. Examination is also unremarkable. Blood tests were checked, which were all reassuring, except for a platelet count of 612 × 109 cells/L (usual normal range, about 150-450).
What do we do next? Do we refer for an urgent chest x-ray to exclude lung cancer? Do we check a quantitative immunohistochemical fecal occult blood test (qFIT) to identify any occult bleeding in her stool? Do we refer for a routine upper gastrointestinal endoscopy or pelvic ultrasound scan to exclude any upper gastrointestinal or endometrial malignancy?
Do we simply repeat the bloods? If so, do we repeat them routinely or urgently, and indeed, which ones should we recheck?
Louisa has an unexplained thrombocytosis. How do we manage this in primary care? Thrombocytosis is generally defined as a raised platelet count over 450. Importantly, thrombocytosis is a common incidental finding in around 2% of those over 40 years of age attending primary care. Reassuringly, 80%-90% of thrombocytosis is reactive, secondary to acute blood loss, infection, or inflammation, and the majority of cases resolve within 3 months.
Why the concern with Louisa then? Although most cases are reactive, clinical guidance (for example, NICE suspected cancer guidance in the UK and Scottish suspected cancer guidance in Scotland) reminds us that unexplained thrombocytosis is a risk marker for some solid-tumor malignancies.
Previous studies have demonstrated that unexplained thrombocytosis is associated with a 1-year cancer incidence of 11.6% in males and 6.2% in females, well exceeding the standard 3% threshold warranting investigation for underlying malignancy. However, thrombocytosis should not be used as a stand-alone diagnostic or screening test for cancer, or indeed to rule out cancer.
Instead, unexplained thrombocytosis should prompt us to think cancer. The Scottish suspected cancer referral guidelines include thrombocytosis in the investigation criteria for what they call the LEGO-C cancers — L for lung, E for endometrial, G for gastric, O for oesophageal, and C for colorectal, which is a useful reminder for us all.
What further history, examination, and investigations might we consider in primary care if we identify an unexplained high platelet count? As always, we should use our clinical judgment and trust our clinical acumen.
We should consider all the possible underlying causes, including infection, inflammation, and blood loss, including menstrual blood loss in women; myeloproliferative disorders such as polycythemia rubra vera, chronic myeloid leukemia, and essential thrombocythemia; and, of course, underlying malignancy. If a likely underlying reversible cause is present (for example, a recent lower respiratory tract infection), simply repeating the full blood count in 4-6 weeks is quite appropriate to see if the thrombocytosis has resolved.
Remember, 80%-90% of cases are reactive thrombocytosis, and most cases resolve within 3 months. If thrombocytosis is unexplained or not resolving, consider checking ferritin levels to exclude iron deficiency. Consider checking C-reactive protein (CRP) levels to exclude any inflammation, and also consider checking a blood film to exclude any hematologic disorders, in addition, of course, to more detailed history-taking and examination to elicit any red flags.
We can also consider a JAK2 gene mutation test, if it is available to you locally, or a hematology referral if we suspect a myeloproliferative disorder. JAK2 is a genetic mutation that may be present in people with essential thrombocythemia and can indicate a diagnosis of polycythemia rubra vera.
Subsequent to this, and again using our clinical judgment, we then need to exclude the LEGO-C cancers. Consider urgent chest x-ray to exclude lung cancer or pelvic ultrasound in women to exclude endometrial cancer. Also, we should consider an upper gastrointestinal endoscopy, particularly in those individuals who have associated upper gastrointestinal symptoms and/or weight loss.
Finally, consider a qFIT to identify any occult bleeding in the stool, again if it’s available to you, or certainly if not, urgent lower gastrointestinal investigations to exclude colorectal cancer.
Alongside these possible investigations, as always, we should safety-net appropriately within agreed timeframes and check for resolution of the thrombocytosis according to the condition being suspected. Remember, most cases resolve within 3 months.
Returning to Louisa, what did I do? After seeing a platelet count of 600, I subsequently telephoned her and reexplored her history, which yielded nil else of note. Specifically, there was no history of unexplained weight loss, no history of upper or lower gastrointestinal symptoms, and certainly nothing significantly different from her usual irritable bowel syndrome symptoms. There were also no respiratory or genitourinary symptoms of note.
I did arrange for Louisa to undergo a chest x-ray over the next few days, though, as she was an ex-smoker. This was subsequently reported as normal. I appreciate chest x-rays have poor sensitivity for detecting lung cancer, as highlighted in a number of recent papers, but it was mutually agreed with Louisa that we would simply repeat her blood test in around 6 weeks. As well as repeating the full blood count, I arranged to check her ferritin, CRP, and a blood film, and then I was planning to reassess her clinically in person.
These bloods and my subsequent clinical review were reassuring. In fact, her platelet count had normalized after that 6 weeks had elapsed. Her thrombocytosis had resolved.
I didn’t arrange any further follow-up for her, but I did give her the usual safety netting advice to re-present to me or one of my colleagues if she does develop any worrying symptoms or signs.
I appreciate these scenarios are not always this straightforward, but I wanted to outline what investigations and referrals we may need to consider in primary care if we encounter an unexplained high platelet count.
There are a couple of quality-improvement activities for us all to consider in primary care. Consider as a team how we would respond to an incidental finding of thrombocytosis on a full blood count. Also consider what are our safety-netting options for those found to have raised platelet counts but no other symptoms or risk factors for underlying malignancy.
Finally, I’ve produced a Medscape UK primary care hack or clinical aide-memoire on managing unexplained thrombocytosis and associated cancer risk in primary care for all healthcare professionals working in primary care. This can be found online. I hope you find this resource helpful.
Dr. Kevin Fernando, General practitioner partner with specialist interests in cardiovascular, renal, and metabolic medicine, North Berwick Group Practice in Scotland, has disclosed relevant financial relationships with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, Sanofi Menarini, and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In this podcast, I’m going to talk about unexplained high platelet counts, or thrombocytosis, and the risk for cancer in primary care. Let’s start with a typical case we all might see in primary care.
Louisa is 47 years old and is the chief financial officer for a tech startup company. She presents to us in primary care feeling tired all the time — a very common presentation in primary care — with associated reduced appetite. Past medical history includes irritable bowel syndrome, and she’s an ex-smoker.
Systemic inquiry is unremarkable. Specifically, there is no history of weight loss. Louisa has not been prescribed any medication and uses over-the-counter remedies for her irritable bowel syndrome. Examination is also unremarkable. Blood tests were checked, which were all reassuring, except for a platelet count of 612 × 109 cells/L (usual normal range, about 150-450).
What do we do next? Do we refer for an urgent chest x-ray to exclude lung cancer? Do we check a quantitative immunohistochemical fecal occult blood test (qFIT) to identify any occult bleeding in her stool? Do we refer for a routine upper gastrointestinal endoscopy or pelvic ultrasound scan to exclude any upper gastrointestinal or endometrial malignancy?
Do we simply repeat the bloods? If so, do we repeat them routinely or urgently, and indeed, which ones should we recheck?
Louisa has an unexplained thrombocytosis. How do we manage this in primary care? Thrombocytosis is generally defined as a raised platelet count over 450. Importantly, thrombocytosis is a common incidental finding in around 2% of those over 40 years of age attending primary care. Reassuringly, 80%-90% of thrombocytosis is reactive, secondary to acute blood loss, infection, or inflammation, and the majority of cases resolve within 3 months.
Why the concern with Louisa then? Although most cases are reactive, clinical guidance (for example, NICE suspected cancer guidance in the UK and Scottish suspected cancer guidance in Scotland) reminds us that unexplained thrombocytosis is a risk marker for some solid-tumor malignancies.
Previous studies have demonstrated that unexplained thrombocytosis is associated with a 1-year cancer incidence of 11.6% in males and 6.2% in females, well exceeding the standard 3% threshold warranting investigation for underlying malignancy. However, thrombocytosis should not be used as a stand-alone diagnostic or screening test for cancer, or indeed to rule out cancer.
Instead, unexplained thrombocytosis should prompt us to think cancer. The Scottish suspected cancer referral guidelines include thrombocytosis in the investigation criteria for what they call the LEGO-C cancers — L for lung, E for endometrial, G for gastric, O for oesophageal, and C for colorectal, which is a useful reminder for us all.
What further history, examination, and investigations might we consider in primary care if we identify an unexplained high platelet count? As always, we should use our clinical judgment and trust our clinical acumen.
We should consider all the possible underlying causes, including infection, inflammation, and blood loss, including menstrual blood loss in women; myeloproliferative disorders such as polycythemia rubra vera, chronic myeloid leukemia, and essential thrombocythemia; and, of course, underlying malignancy. If a likely underlying reversible cause is present (for example, a recent lower respiratory tract infection), simply repeating the full blood count in 4-6 weeks is quite appropriate to see if the thrombocytosis has resolved.
Remember, 80%-90% of cases are reactive thrombocytosis, and most cases resolve within 3 months. If thrombocytosis is unexplained or not resolving, consider checking ferritin levels to exclude iron deficiency. Consider checking C-reactive protein (CRP) levels to exclude any inflammation, and also consider checking a blood film to exclude any hematologic disorders, in addition, of course, to more detailed history-taking and examination to elicit any red flags.
We can also consider a JAK2 gene mutation test, if it is available to you locally, or a hematology referral if we suspect a myeloproliferative disorder. JAK2 is a genetic mutation that may be present in people with essential thrombocythemia and can indicate a diagnosis of polycythemia rubra vera.
Subsequent to this, and again using our clinical judgment, we then need to exclude the LEGO-C cancers. Consider urgent chest x-ray to exclude lung cancer or pelvic ultrasound in women to exclude endometrial cancer. Also, we should consider an upper gastrointestinal endoscopy, particularly in those individuals who have associated upper gastrointestinal symptoms and/or weight loss.
Finally, consider a qFIT to identify any occult bleeding in the stool, again if it’s available to you, or certainly if not, urgent lower gastrointestinal investigations to exclude colorectal cancer.
Alongside these possible investigations, as always, we should safety-net appropriately within agreed timeframes and check for resolution of the thrombocytosis according to the condition being suspected. Remember, most cases resolve within 3 months.
Returning to Louisa, what did I do? After seeing a platelet count of 600, I subsequently telephoned her and reexplored her history, which yielded nil else of note. Specifically, there was no history of unexplained weight loss, no history of upper or lower gastrointestinal symptoms, and certainly nothing significantly different from her usual irritable bowel syndrome symptoms. There were also no respiratory or genitourinary symptoms of note.
I did arrange for Louisa to undergo a chest x-ray over the next few days, though, as she was an ex-smoker. This was subsequently reported as normal. I appreciate chest x-rays have poor sensitivity for detecting lung cancer, as highlighted in a number of recent papers, but it was mutually agreed with Louisa that we would simply repeat her blood test in around 6 weeks. As well as repeating the full blood count, I arranged to check her ferritin, CRP, and a blood film, and then I was planning to reassess her clinically in person.
These bloods and my subsequent clinical review were reassuring. In fact, her platelet count had normalized after that 6 weeks had elapsed. Her thrombocytosis had resolved.
I didn’t arrange any further follow-up for her, but I did give her the usual safety netting advice to re-present to me or one of my colleagues if she does develop any worrying symptoms or signs.
I appreciate these scenarios are not always this straightforward, but I wanted to outline what investigations and referrals we may need to consider in primary care if we encounter an unexplained high platelet count.
There are a couple of quality-improvement activities for us all to consider in primary care. Consider as a team how we would respond to an incidental finding of thrombocytosis on a full blood count. Also consider what are our safety-netting options for those found to have raised platelet counts but no other symptoms or risk factors for underlying malignancy.
Finally, I’ve produced a Medscape UK primary care hack or clinical aide-memoire on managing unexplained thrombocytosis and associated cancer risk in primary care for all healthcare professionals working in primary care. This can be found online. I hope you find this resource helpful.
Dr. Kevin Fernando, General practitioner partner with specialist interests in cardiovascular, renal, and metabolic medicine, North Berwick Group Practice in Scotland, has disclosed relevant financial relationships with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, Sanofi Menarini, and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.