User login
ERRATUM
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
Guidelines are not mandates
Just like the 2018 hypertension treatment guidelines, the 2018 Guidelines on the Management of Blood Cholesterol developed by the American College of Cardiology and the American Heart Association (ACC/AHA) have made treatment decisions much more complicated. In this issue of JFP, Wójcik and Shapiro summarize the 70-page document to help family physicians and other primary health care professionals use these complex guidelines in everyday practice.
The good news is that not much has changed from the 2013 ACC/AHA cholesterol guidelines regarding the treatment of patients with established cardiovascular disease and diabetes mellitus, and those with familial hyperlipidemia—the groups at highest risk for major cardiovascular events. Most of these patients should be treated aggressively, and a target low-density lipoprotein of 70 mg/dL is recommended.
The new guidelines recommend using ezetimibe or a PCSK9 inhibitor if the goal of 70 mg/dL cannot be achieved with a statin alone. There is randomized trial evidence to support the benefit of this aggressive approach. Generic ezetimibe costs about $20 per month,1 but the PCSK9 inhibitors are about $500 per month,2,3 so cost may be a treatment barrier for the 2 monoclonal antibodies approved for cardiovascular prevention: evolocumab and alirocumab.
For primary prevention, the new guidelines are much more complicated. They divide cardiovascular risk into 4 tiers depending on the 10-year risk for atherosclerotic cardiovascular disease calculated using the “pooled cohort equation.” Treatment recommendations are more aggressive for those at higher risk. Although it intuitively makes sense to treat those at higher risk more aggressively, there is no clinical trial evidence to support this approach’s superiority over the simpler approach recommended in the 2013 guidelines.
I find the recommendations for screening and primary prevention in adults ages 75 and older and for children and teens to be problematic. A meta-analysis of 28 studies found no statin treatment benefit for primary prevention in those older than 70.4 And there are no randomized trials showing benefit of screening and treating children and teens for hyperlipidemia.
On a positive note, most patients do not need to fast prior to having their lipids measured.
Read the 2018 cholesterol treatment guideline summary in this issue of JFP. But as you do so, remember that guidelines are guidelines; they are not mandates for treatment. You may need to customize these guidelines for your practice and your patients. In my opinion, the simpler 2013 cholesterol guidelines remain good guidelines.
1. Ezetimibe prices. GoodRx. www.goodrx.com/ezetimibe. Accessed April 24, 2019.
2. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed May 1, 2019.
3. Kuchler H. Sanofi and Regeneron cut price of Praluent by 60%. Financial Times. February 11, 2019. https://www.ft.com/content/d1b34cca-2e18-11e9-8744-e7016697f225. Accessed May 1, 2019.
4. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
Just like the 2018 hypertension treatment guidelines, the 2018 Guidelines on the Management of Blood Cholesterol developed by the American College of Cardiology and the American Heart Association (ACC/AHA) have made treatment decisions much more complicated. In this issue of JFP, Wójcik and Shapiro summarize the 70-page document to help family physicians and other primary health care professionals use these complex guidelines in everyday practice.
The good news is that not much has changed from the 2013 ACC/AHA cholesterol guidelines regarding the treatment of patients with established cardiovascular disease and diabetes mellitus, and those with familial hyperlipidemia—the groups at highest risk for major cardiovascular events. Most of these patients should be treated aggressively, and a target low-density lipoprotein of 70 mg/dL is recommended.
The new guidelines recommend using ezetimibe or a PCSK9 inhibitor if the goal of 70 mg/dL cannot be achieved with a statin alone. There is randomized trial evidence to support the benefit of this aggressive approach. Generic ezetimibe costs about $20 per month,1 but the PCSK9 inhibitors are about $500 per month,2,3 so cost may be a treatment barrier for the 2 monoclonal antibodies approved for cardiovascular prevention: evolocumab and alirocumab.
For primary prevention, the new guidelines are much more complicated. They divide cardiovascular risk into 4 tiers depending on the 10-year risk for atherosclerotic cardiovascular disease calculated using the “pooled cohort equation.” Treatment recommendations are more aggressive for those at higher risk. Although it intuitively makes sense to treat those at higher risk more aggressively, there is no clinical trial evidence to support this approach’s superiority over the simpler approach recommended in the 2013 guidelines.
I find the recommendations for screening and primary prevention in adults ages 75 and older and for children and teens to be problematic. A meta-analysis of 28 studies found no statin treatment benefit for primary prevention in those older than 70.4 And there are no randomized trials showing benefit of screening and treating children and teens for hyperlipidemia.
On a positive note, most patients do not need to fast prior to having their lipids measured.
Read the 2018 cholesterol treatment guideline summary in this issue of JFP. But as you do so, remember that guidelines are guidelines; they are not mandates for treatment. You may need to customize these guidelines for your practice and your patients. In my opinion, the simpler 2013 cholesterol guidelines remain good guidelines.
Just like the 2018 hypertension treatment guidelines, the 2018 Guidelines on the Management of Blood Cholesterol developed by the American College of Cardiology and the American Heart Association (ACC/AHA) have made treatment decisions much more complicated. In this issue of JFP, Wójcik and Shapiro summarize the 70-page document to help family physicians and other primary health care professionals use these complex guidelines in everyday practice.
The good news is that not much has changed from the 2013 ACC/AHA cholesterol guidelines regarding the treatment of patients with established cardiovascular disease and diabetes mellitus, and those with familial hyperlipidemia—the groups at highest risk for major cardiovascular events. Most of these patients should be treated aggressively, and a target low-density lipoprotein of 70 mg/dL is recommended.
The new guidelines recommend using ezetimibe or a PCSK9 inhibitor if the goal of 70 mg/dL cannot be achieved with a statin alone. There is randomized trial evidence to support the benefit of this aggressive approach. Generic ezetimibe costs about $20 per month,1 but the PCSK9 inhibitors are about $500 per month,2,3 so cost may be a treatment barrier for the 2 monoclonal antibodies approved for cardiovascular prevention: evolocumab and alirocumab.
For primary prevention, the new guidelines are much more complicated. They divide cardiovascular risk into 4 tiers depending on the 10-year risk for atherosclerotic cardiovascular disease calculated using the “pooled cohort equation.” Treatment recommendations are more aggressive for those at higher risk. Although it intuitively makes sense to treat those at higher risk more aggressively, there is no clinical trial evidence to support this approach’s superiority over the simpler approach recommended in the 2013 guidelines.
I find the recommendations for screening and primary prevention in adults ages 75 and older and for children and teens to be problematic. A meta-analysis of 28 studies found no statin treatment benefit for primary prevention in those older than 70.4 And there are no randomized trials showing benefit of screening and treating children and teens for hyperlipidemia.
On a positive note, most patients do not need to fast prior to having their lipids measured.
Read the 2018 cholesterol treatment guideline summary in this issue of JFP. But as you do so, remember that guidelines are guidelines; they are not mandates for treatment. You may need to customize these guidelines for your practice and your patients. In my opinion, the simpler 2013 cholesterol guidelines remain good guidelines.
1. Ezetimibe prices. GoodRx. www.goodrx.com/ezetimibe. Accessed April 24, 2019.
2. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed May 1, 2019.
3. Kuchler H. Sanofi and Regeneron cut price of Praluent by 60%. Financial Times. February 11, 2019. https://www.ft.com/content/d1b34cca-2e18-11e9-8744-e7016697f225. Accessed May 1, 2019.
4. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
1. Ezetimibe prices. GoodRx. www.goodrx.com/ezetimibe. Accessed April 24, 2019.
2. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed May 1, 2019.
3. Kuchler H. Sanofi and Regeneron cut price of Praluent by 60%. Financial Times. February 11, 2019. https://www.ft.com/content/d1b34cca-2e18-11e9-8744-e7016697f225. Accessed May 1, 2019.
4. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
Can Medicine Bring Good Out of War?
The title of this essay is more often posed as “Is War Good for Medicine?”2 The career VA physician in me, and the daughter and granddaughter of combat veterans, finds this question historically accurate, but ethically problematic. So I have rewritten the question to one that enables us to examine the historic relationship of medical advances and war from a more ethically justifiable posture. I am by no means ascribing to authors of other publications with this title anything but the highest motives of education and edification.
Yet the more I read and thought about the question(s), I realized that the moral assumptions underlying and supporting each concept are significantly different. What led me to that realization was a story my father told me when I was young which in my youthful ignorance I either dismissed or ignored. I now see that the narrative captured a profound truth about how war is not good especially for those who must wage it, but good may come from it for those who now live in peace.
My father was one of the founders of military pediatrics. Surprisingly, pediatricians were valuable members of the military medical forces because of their knowledge of infectious diseases.3 My father had gone in to the then new specialty of pediatrics because in the 1930s, infectious diseases were the primary cause of death in children. Before antibiotics, children would often die of common infections. Service as a combat medical officer in World War II stationed in the European Theater, my father had experience with and access to penicillin. After returning from the war to work in an Army hospital, he and his staff went into the acute pediatric ward and gave the drug to several very sick children, many of whom were likely to die. The next morning on rounds, they noted that many of the children were feeling much better, some even bouncing on their beds.
Perhaps either his telling or my remembering of these events is partly apocryphal, but the reality is that those lethal microbes had no idea what had hit them. Before human physicians overused the new drugs and nature struck back with antibiotic resistance, penicillin seemed miraculous.
Most likely, in 1945 those children would never have been prescribed penicillin, much less survived, if not for the unprecedented and war-driven consortium of industry and government that mass-produced penicillin to treat the troops with infections. Without a doubt then, from the sacrifice and devastation of World War II came the benefits and boons of the antibiotic era—one of the greatest discoveries in medical science.4
Penicillin is but one of legions of scientific discoveries that emerged during wartime. Many of these dramatic improvements, especially those in surgical techniques and emergency medicine, quickly entered the civilian sector. The French surgeon Amboise Paré, for example, revived an old Roman Army practice of using ligatures or tourniquets to stop excessive blood loss, now a staple of emergency responders in disasters. The ambulance services that transported wounded troops to the hospital began on the battlefields of the Civil War.5
These impressive contributions are the direct result of military medicine intended to preserve fighting strength. There are also indirect, although just as revolutionary, efforts of DoD and VA scientists and health care professionals to minimize disability and prevent progression especially of service-connected injuries and illnesses. Among the most groundbreaking is the VA’s 3D-printed artificial lung. I have to admit at first I thought that it was futuristic, but quickly I learned that it was a realistic possibility for the coming decades.6 VA researchers hope the lung will offer a treatment option for patients with chronic obstructive pulmonary disease (COPD), a lung condition more prevalent in veterans than in the civilian population.7 One contributing factor to the increased risk of COPD among former military is the higher rate of smoking among both active duty and veterans than that in the civilian population.8 And the last chain in the link of causation is that smoking is more common in those service members who have posttraumatic stress disorder.9
However, there also is a very dark side to the link between wartime research and medicine—most infamously the Nazi hypothermia experiments conducted at concentration camps. The proposed publication aroused a decades long ethical controversy regarding whether the data should be published, much less used, in research and practice even if it could save the lives of present or future warriors. In 1990, Marcia Angel, MD, then editor-in-chief of the prestigious New England Journal of Medicine, published the information with an accompanying ethical justification. “Finally, refusal to publish the unethical work serves notice to society at large that even scientists do not consider science the primary measure of a civilization. Knowledge, although important, may be less important to a decent society than the way it is obtained.”10 Ethicist Stephen Post writing on behalf of Holocaust victims strenuously disagreed with the decision to publish the research, “Because the Nazi experiments on human beings were so appallingly unethical, it follows, prima facie, that the use of the records is unethical.”11
This debate is key to the distinction between the 2 questions posed at the beginning of this column. Few who have been on a battlefield or who have cared for those who were can suggest or defend that wars should be fought as a catalyst for scientific research or an impetus to medical advancement. Such an instrumentalist view justifies the end of healing with the means of death, which is an intrinsic contradiction that would eventually corrode the integrity of the medical and scientific professions. Conversely, the second question challenges all of us in federal practice to assume a mantle of obligation to take the interventions that enabled combat medicine to save soldiers and apply them to improve the health and save the lives of veterans and civilians alike. It summons scientists laboring in the hundreds of DoD and VA laboratories to use the unparalleled funding and infrastructure of the institutions to develop promising therapeutics to treat the psychological toll and physical cost of war. And finally it charges the citizens whose family and friends have and will serve in uniform to enlist in a political process that enables military medicine and science to achieve the greatest good-health in peace.
1. Remarque EM. All Quiet on the Western Front. New York, NY: Fawcett Books; 1929:228.
2. Connell C. Is war good for medicine: war’s medical legacy? http://sm.stanford.edu/archive/stanmed/2007summer/main.html. Published 2007. Accessed April 18, 2019.
3. Burnett MW, Callahan CW. American pediatricians at war; a legacy of service. Pediatrics. 2012;129(suppl 1):S33-S49.
4. Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52-57.
5. Samuel L. 6 medical innovations that moved from the battlefield to mainstream medicine. https://www.scientificamercan.com/article/6-medical-innovations-that-moved-from-the-battlefield-to-mainstream-medicine. Published November 11, 2017. Accessed April 18, 2019.
6. Richman M. Breathing easier. https://www.research.va.gov/currents/0818-Researchers-strive-to-make-3D-printed-artificial-lung-to-help-Vets-with-respiratory-disease.cfm. Published August 1, 2018. Accessed April 18, 2019.
7. Murphy DE, Chaudry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban Midwest. Mill Med. 2011;176(5):552-560.
8. Thompson WH, St-Hilaire C. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555-560.
9. Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189-1195.
10. Angell M. The Nazi hypothermia experiments and unethical research today. N Eng J Med 1990;322(20):1462-1464.
11. Post SG. The echo of Nuremberg: Nazi data and ethics. J Med Ethics. 1991;17(1):42-44.
The title of this essay is more often posed as “Is War Good for Medicine?”2 The career VA physician in me, and the daughter and granddaughter of combat veterans, finds this question historically accurate, but ethically problematic. So I have rewritten the question to one that enables us to examine the historic relationship of medical advances and war from a more ethically justifiable posture. I am by no means ascribing to authors of other publications with this title anything but the highest motives of education and edification.
Yet the more I read and thought about the question(s), I realized that the moral assumptions underlying and supporting each concept are significantly different. What led me to that realization was a story my father told me when I was young which in my youthful ignorance I either dismissed or ignored. I now see that the narrative captured a profound truth about how war is not good especially for those who must wage it, but good may come from it for those who now live in peace.
My father was one of the founders of military pediatrics. Surprisingly, pediatricians were valuable members of the military medical forces because of their knowledge of infectious diseases.3 My father had gone in to the then new specialty of pediatrics because in the 1930s, infectious diseases were the primary cause of death in children. Before antibiotics, children would often die of common infections. Service as a combat medical officer in World War II stationed in the European Theater, my father had experience with and access to penicillin. After returning from the war to work in an Army hospital, he and his staff went into the acute pediatric ward and gave the drug to several very sick children, many of whom were likely to die. The next morning on rounds, they noted that many of the children were feeling much better, some even bouncing on their beds.
Perhaps either his telling or my remembering of these events is partly apocryphal, but the reality is that those lethal microbes had no idea what had hit them. Before human physicians overused the new drugs and nature struck back with antibiotic resistance, penicillin seemed miraculous.
Most likely, in 1945 those children would never have been prescribed penicillin, much less survived, if not for the unprecedented and war-driven consortium of industry and government that mass-produced penicillin to treat the troops with infections. Without a doubt then, from the sacrifice and devastation of World War II came the benefits and boons of the antibiotic era—one of the greatest discoveries in medical science.4
Penicillin is but one of legions of scientific discoveries that emerged during wartime. Many of these dramatic improvements, especially those in surgical techniques and emergency medicine, quickly entered the civilian sector. The French surgeon Amboise Paré, for example, revived an old Roman Army practice of using ligatures or tourniquets to stop excessive blood loss, now a staple of emergency responders in disasters. The ambulance services that transported wounded troops to the hospital began on the battlefields of the Civil War.5
These impressive contributions are the direct result of military medicine intended to preserve fighting strength. There are also indirect, although just as revolutionary, efforts of DoD and VA scientists and health care professionals to minimize disability and prevent progression especially of service-connected injuries and illnesses. Among the most groundbreaking is the VA’s 3D-printed artificial lung. I have to admit at first I thought that it was futuristic, but quickly I learned that it was a realistic possibility for the coming decades.6 VA researchers hope the lung will offer a treatment option for patients with chronic obstructive pulmonary disease (COPD), a lung condition more prevalent in veterans than in the civilian population.7 One contributing factor to the increased risk of COPD among former military is the higher rate of smoking among both active duty and veterans than that in the civilian population.8 And the last chain in the link of causation is that smoking is more common in those service members who have posttraumatic stress disorder.9
However, there also is a very dark side to the link between wartime research and medicine—most infamously the Nazi hypothermia experiments conducted at concentration camps. The proposed publication aroused a decades long ethical controversy regarding whether the data should be published, much less used, in research and practice even if it could save the lives of present or future warriors. In 1990, Marcia Angel, MD, then editor-in-chief of the prestigious New England Journal of Medicine, published the information with an accompanying ethical justification. “Finally, refusal to publish the unethical work serves notice to society at large that even scientists do not consider science the primary measure of a civilization. Knowledge, although important, may be less important to a decent society than the way it is obtained.”10 Ethicist Stephen Post writing on behalf of Holocaust victims strenuously disagreed with the decision to publish the research, “Because the Nazi experiments on human beings were so appallingly unethical, it follows, prima facie, that the use of the records is unethical.”11
This debate is key to the distinction between the 2 questions posed at the beginning of this column. Few who have been on a battlefield or who have cared for those who were can suggest or defend that wars should be fought as a catalyst for scientific research or an impetus to medical advancement. Such an instrumentalist view justifies the end of healing with the means of death, which is an intrinsic contradiction that would eventually corrode the integrity of the medical and scientific professions. Conversely, the second question challenges all of us in federal practice to assume a mantle of obligation to take the interventions that enabled combat medicine to save soldiers and apply them to improve the health and save the lives of veterans and civilians alike. It summons scientists laboring in the hundreds of DoD and VA laboratories to use the unparalleled funding and infrastructure of the institutions to develop promising therapeutics to treat the psychological toll and physical cost of war. And finally it charges the citizens whose family and friends have and will serve in uniform to enlist in a political process that enables military medicine and science to achieve the greatest good-health in peace.
The title of this essay is more often posed as “Is War Good for Medicine?”2 The career VA physician in me, and the daughter and granddaughter of combat veterans, finds this question historically accurate, but ethically problematic. So I have rewritten the question to one that enables us to examine the historic relationship of medical advances and war from a more ethically justifiable posture. I am by no means ascribing to authors of other publications with this title anything but the highest motives of education and edification.
Yet the more I read and thought about the question(s), I realized that the moral assumptions underlying and supporting each concept are significantly different. What led me to that realization was a story my father told me when I was young which in my youthful ignorance I either dismissed or ignored. I now see that the narrative captured a profound truth about how war is not good especially for those who must wage it, but good may come from it for those who now live in peace.
My father was one of the founders of military pediatrics. Surprisingly, pediatricians were valuable members of the military medical forces because of their knowledge of infectious diseases.3 My father had gone in to the then new specialty of pediatrics because in the 1930s, infectious diseases were the primary cause of death in children. Before antibiotics, children would often die of common infections. Service as a combat medical officer in World War II stationed in the European Theater, my father had experience with and access to penicillin. After returning from the war to work in an Army hospital, he and his staff went into the acute pediatric ward and gave the drug to several very sick children, many of whom were likely to die. The next morning on rounds, they noted that many of the children were feeling much better, some even bouncing on their beds.
Perhaps either his telling or my remembering of these events is partly apocryphal, but the reality is that those lethal microbes had no idea what had hit them. Before human physicians overused the new drugs and nature struck back with antibiotic resistance, penicillin seemed miraculous.
Most likely, in 1945 those children would never have been prescribed penicillin, much less survived, if not for the unprecedented and war-driven consortium of industry and government that mass-produced penicillin to treat the troops with infections. Without a doubt then, from the sacrifice and devastation of World War II came the benefits and boons of the antibiotic era—one of the greatest discoveries in medical science.4
Penicillin is but one of legions of scientific discoveries that emerged during wartime. Many of these dramatic improvements, especially those in surgical techniques and emergency medicine, quickly entered the civilian sector. The French surgeon Amboise Paré, for example, revived an old Roman Army practice of using ligatures or tourniquets to stop excessive blood loss, now a staple of emergency responders in disasters. The ambulance services that transported wounded troops to the hospital began on the battlefields of the Civil War.5
These impressive contributions are the direct result of military medicine intended to preserve fighting strength. There are also indirect, although just as revolutionary, efforts of DoD and VA scientists and health care professionals to minimize disability and prevent progression especially of service-connected injuries and illnesses. Among the most groundbreaking is the VA’s 3D-printed artificial lung. I have to admit at first I thought that it was futuristic, but quickly I learned that it was a realistic possibility for the coming decades.6 VA researchers hope the lung will offer a treatment option for patients with chronic obstructive pulmonary disease (COPD), a lung condition more prevalent in veterans than in the civilian population.7 One contributing factor to the increased risk of COPD among former military is the higher rate of smoking among both active duty and veterans than that in the civilian population.8 And the last chain in the link of causation is that smoking is more common in those service members who have posttraumatic stress disorder.9
However, there also is a very dark side to the link between wartime research and medicine—most infamously the Nazi hypothermia experiments conducted at concentration camps. The proposed publication aroused a decades long ethical controversy regarding whether the data should be published, much less used, in research and practice even if it could save the lives of present or future warriors. In 1990, Marcia Angel, MD, then editor-in-chief of the prestigious New England Journal of Medicine, published the information with an accompanying ethical justification. “Finally, refusal to publish the unethical work serves notice to society at large that even scientists do not consider science the primary measure of a civilization. Knowledge, although important, may be less important to a decent society than the way it is obtained.”10 Ethicist Stephen Post writing on behalf of Holocaust victims strenuously disagreed with the decision to publish the research, “Because the Nazi experiments on human beings were so appallingly unethical, it follows, prima facie, that the use of the records is unethical.”11
This debate is key to the distinction between the 2 questions posed at the beginning of this column. Few who have been on a battlefield or who have cared for those who were can suggest or defend that wars should be fought as a catalyst for scientific research or an impetus to medical advancement. Such an instrumentalist view justifies the end of healing with the means of death, which is an intrinsic contradiction that would eventually corrode the integrity of the medical and scientific professions. Conversely, the second question challenges all of us in federal practice to assume a mantle of obligation to take the interventions that enabled combat medicine to save soldiers and apply them to improve the health and save the lives of veterans and civilians alike. It summons scientists laboring in the hundreds of DoD and VA laboratories to use the unparalleled funding and infrastructure of the institutions to develop promising therapeutics to treat the psychological toll and physical cost of war. And finally it charges the citizens whose family and friends have and will serve in uniform to enlist in a political process that enables military medicine and science to achieve the greatest good-health in peace.
1. Remarque EM. All Quiet on the Western Front. New York, NY: Fawcett Books; 1929:228.
2. Connell C. Is war good for medicine: war’s medical legacy? http://sm.stanford.edu/archive/stanmed/2007summer/main.html. Published 2007. Accessed April 18, 2019.
3. Burnett MW, Callahan CW. American pediatricians at war; a legacy of service. Pediatrics. 2012;129(suppl 1):S33-S49.
4. Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52-57.
5. Samuel L. 6 medical innovations that moved from the battlefield to mainstream medicine. https://www.scientificamercan.com/article/6-medical-innovations-that-moved-from-the-battlefield-to-mainstream-medicine. Published November 11, 2017. Accessed April 18, 2019.
6. Richman M. Breathing easier. https://www.research.va.gov/currents/0818-Researchers-strive-to-make-3D-printed-artificial-lung-to-help-Vets-with-respiratory-disease.cfm. Published August 1, 2018. Accessed April 18, 2019.
7. Murphy DE, Chaudry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban Midwest. Mill Med. 2011;176(5):552-560.
8. Thompson WH, St-Hilaire C. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555-560.
9. Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189-1195.
10. Angell M. The Nazi hypothermia experiments and unethical research today. N Eng J Med 1990;322(20):1462-1464.
11. Post SG. The echo of Nuremberg: Nazi data and ethics. J Med Ethics. 1991;17(1):42-44.
1. Remarque EM. All Quiet on the Western Front. New York, NY: Fawcett Books; 1929:228.
2. Connell C. Is war good for medicine: war’s medical legacy? http://sm.stanford.edu/archive/stanmed/2007summer/main.html. Published 2007. Accessed April 18, 2019.
3. Burnett MW, Callahan CW. American pediatricians at war; a legacy of service. Pediatrics. 2012;129(suppl 1):S33-S49.
4. Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52-57.
5. Samuel L. 6 medical innovations that moved from the battlefield to mainstream medicine. https://www.scientificamercan.com/article/6-medical-innovations-that-moved-from-the-battlefield-to-mainstream-medicine. Published November 11, 2017. Accessed April 18, 2019.
6. Richman M. Breathing easier. https://www.research.va.gov/currents/0818-Researchers-strive-to-make-3D-printed-artificial-lung-to-help-Vets-with-respiratory-disease.cfm. Published August 1, 2018. Accessed April 18, 2019.
7. Murphy DE, Chaudry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban Midwest. Mill Med. 2011;176(5):552-560.
8. Thompson WH, St-Hilaire C. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555-560.
9. Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189-1195.
10. Angell M. The Nazi hypothermia experiments and unethical research today. N Eng J Med 1990;322(20):1462-1464.
11. Post SG. The echo of Nuremberg: Nazi data and ethics. J Med Ethics. 1991;17(1):42-44.
Topical Chemotherapy for Numerous Superficial Basal Cell Carcinomas Years After Isolated Limb Perfusion for Melanoma
Isolated limb perfusion (ILP) for the adjuvant treatment of melanoma involves isolating the blood flow of a limb from the rest of the body to allow for high concentrations of chemotherapeutic agents locally. Chemotherapy with nitrogen mustard is the preferred chemotherapeutic agent in ILP for the adjuvant treatment of locally advanced melanoma.1 Systemic exposure to nitrogen mustard has shown to be carcinogenic, and its topical application has been associated with the development of actinic keratosis, basal cell carcinoma (BCC), and squamous cell carcinoma.2,3 However, the long-term effects of ILP with nitrogen mustard are not well defined. In 1998, one of the authors (R.L.M.) described a patient with melanoma of the left leg that was treated with ILP with nitrogen mustard who subsequently developed numerous BCCs on the same leg.4 This same patient has since been successfully managed with only topical chemotherapeutic agents for the last 21 years.
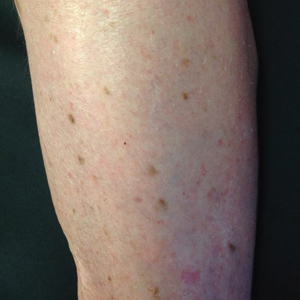
An 86-year-old man with a history of melanoma underwent wide resection, lymph node dissection, and adjuvant ILP with nitrogen mustard for the treatment of melanoma of the medial left thigh approximately 50 years ago. He denied any prior radiation treatment. He subsequently presented years later to our dermatology clinic with many biopsy-proven superficial and nodular BCCs of the left leg over the course of the last 30 years. On physical examination, the patient had several pink papules and macules on the left lower leg (Figure). The patient had previously undergone multiple invasive excisions with grafting for the treatment of BCCs by a plastic surgeon prior to presentation to our clinic but has since had many years of control under our care with only topical chemotherapeutic agents. His current medication regimen consists of 5-fluorouracil twice daily, which he tolerates without serious side effects. He also has used imiquimod in the past.
Isolated limb perfusion was first described by Creech et al5 in 1958. Chemotherapy in ILP is designed to maximize limb perfusion while minimizing systemic absorption.1 Meta
Topical use of nitrogen mustard has been linked to the development of nonmelanoma skin cancer (NMSC)2,3; however, a 30-year population-based study found no significant increase in secondary malignancies, including NMSC or melanoma, following use of topical nitrogen mustard.6 There also have been reported cases of secondary cancers following ILP reported in the literature, including pleomorphic sarcoma and Merkel cell carcinoma.7 We hypothesize that our patient’s exposure to nitrogen mustard during ILP led to the development of numerous BCCs, but further research is necessary to confirm this relationship.
Treatment modalities for NMSC include surgical excision with defined margins, Mohs micrographic surgery, radiotherapy, electrodesiccation and curettage, cryotherapy, photodynamic therapy, and topical therapy. Our patient experienced such a high volume of superficial BCCs that the decision was made to avoid frequent surgical procedures and to treat with topical chemotherapeutic agents. He had an excellent response to topical 5-fluorouracil, and the treatment has been well tolerated. This case is valuable for clinicians, as it demonstrates that topical chemotherapy can be a well-tolerated option for patients who present with frequent superficial BCCs to prevent numerous invasive surgical treatments.
- Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157-163.
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapy for mycosis fungoides. J Am Acad Dermatol. 1986;14:1029-1038.
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. 1982;7:590-598.
- Lamb PM, Menaker GM, Moy RL. Multiple basal cell carcinomas of the limb after adjuvant treatment of melanoma with isolated limb perfusion. J Am Acad Dermatol. 1998;38:767-768.
- Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporal circuit. Ann Surg. 1958;148:616-632.
- Lindahl L, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170:699-704.
- Lenormand C, Pelletier C, Goeldel AL, et al. Second malignant neoplasm occurring years after hyperthermic isolated limb perfusion for melanoma. Arch Dermatol. 2010;146:319-321.
Isolated limb perfusion (ILP) for the adjuvant treatment of melanoma involves isolating the blood flow of a limb from the rest of the body to allow for high concentrations of chemotherapeutic agents locally. Chemotherapy with nitrogen mustard is the preferred chemotherapeutic agent in ILP for the adjuvant treatment of locally advanced melanoma.1 Systemic exposure to nitrogen mustard has shown to be carcinogenic, and its topical application has been associated with the development of actinic keratosis, basal cell carcinoma (BCC), and squamous cell carcinoma.2,3 However, the long-term effects of ILP with nitrogen mustard are not well defined. In 1998, one of the authors (R.L.M.) described a patient with melanoma of the left leg that was treated with ILP with nitrogen mustard who subsequently developed numerous BCCs on the same leg.4 This same patient has since been successfully managed with only topical chemotherapeutic agents for the last 21 years.
An 86-year-old man with a history of melanoma underwent wide resection, lymph node dissection, and adjuvant ILP with nitrogen mustard for the treatment of melanoma of the medial left thigh approximately 50 years ago. He denied any prior radiation treatment. He subsequently presented years later to our dermatology clinic with many biopsy-proven superficial and nodular BCCs of the left leg over the course of the last 30 years. On physical examination, the patient had several pink papules and macules on the left lower leg (Figure). The patient had previously undergone multiple invasive excisions with grafting for the treatment of BCCs by a plastic surgeon prior to presentation to our clinic but has since had many years of control under our care with only topical chemotherapeutic agents. His current medication regimen consists of 5-fluorouracil twice daily, which he tolerates without serious side effects. He also has used imiquimod in the past.

Isolated limb perfusion was first described by Creech et al5 in 1958. Chemotherapy in ILP is designed to maximize limb perfusion while minimizing systemic absorption.1 Meta
Topical use of nitrogen mustard has been linked to the development of nonmelanoma skin cancer (NMSC)2,3; however, a 30-year population-based study found no significant increase in secondary malignancies, including NMSC or melanoma, following use of topical nitrogen mustard.6 There also have been reported cases of secondary cancers following ILP reported in the literature, including pleomorphic sarcoma and Merkel cell carcinoma.7 We hypothesize that our patient’s exposure to nitrogen mustard during ILP led to the development of numerous BCCs, but further research is necessary to confirm this relationship.
Treatment modalities for NMSC include surgical excision with defined margins, Mohs micrographic surgery, radiotherapy, electrodesiccation and curettage, cryotherapy, photodynamic therapy, and topical therapy. Our patient experienced such a high volume of superficial BCCs that the decision was made to avoid frequent surgical procedures and to treat with topical chemotherapeutic agents. He had an excellent response to topical 5-fluorouracil, and the treatment has been well tolerated. This case is valuable for clinicians, as it demonstrates that topical chemotherapy can be a well-tolerated option for patients who present with frequent superficial BCCs to prevent numerous invasive surgical treatments.
Isolated limb perfusion (ILP) for the adjuvant treatment of melanoma involves isolating the blood flow of a limb from the rest of the body to allow for high concentrations of chemotherapeutic agents locally. Chemotherapy with nitrogen mustard is the preferred chemotherapeutic agent in ILP for the adjuvant treatment of locally advanced melanoma.1 Systemic exposure to nitrogen mustard has shown to be carcinogenic, and its topical application has been associated with the development of actinic keratosis, basal cell carcinoma (BCC), and squamous cell carcinoma.2,3 However, the long-term effects of ILP with nitrogen mustard are not well defined. In 1998, one of the authors (R.L.M.) described a patient with melanoma of the left leg that was treated with ILP with nitrogen mustard who subsequently developed numerous BCCs on the same leg.4 This same patient has since been successfully managed with only topical chemotherapeutic agents for the last 21 years.
An 86-year-old man with a history of melanoma underwent wide resection, lymph node dissection, and adjuvant ILP with nitrogen mustard for the treatment of melanoma of the medial left thigh approximately 50 years ago. He denied any prior radiation treatment. He subsequently presented years later to our dermatology clinic with many biopsy-proven superficial and nodular BCCs of the left leg over the course of the last 30 years. On physical examination, the patient had several pink papules and macules on the left lower leg (Figure). The patient had previously undergone multiple invasive excisions with grafting for the treatment of BCCs by a plastic surgeon prior to presentation to our clinic but has since had many years of control under our care with only topical chemotherapeutic agents. His current medication regimen consists of 5-fluorouracil twice daily, which he tolerates without serious side effects. He also has used imiquimod in the past.

Isolated limb perfusion was first described by Creech et al5 in 1958. Chemotherapy in ILP is designed to maximize limb perfusion while minimizing systemic absorption.1 Meta
Topical use of nitrogen mustard has been linked to the development of nonmelanoma skin cancer (NMSC)2,3; however, a 30-year population-based study found no significant increase in secondary malignancies, including NMSC or melanoma, following use of topical nitrogen mustard.6 There also have been reported cases of secondary cancers following ILP reported in the literature, including pleomorphic sarcoma and Merkel cell carcinoma.7 We hypothesize that our patient’s exposure to nitrogen mustard during ILP led to the development of numerous BCCs, but further research is necessary to confirm this relationship.
Treatment modalities for NMSC include surgical excision with defined margins, Mohs micrographic surgery, radiotherapy, electrodesiccation and curettage, cryotherapy, photodynamic therapy, and topical therapy. Our patient experienced such a high volume of superficial BCCs that the decision was made to avoid frequent surgical procedures and to treat with topical chemotherapeutic agents. He had an excellent response to topical 5-fluorouracil, and the treatment has been well tolerated. This case is valuable for clinicians, as it demonstrates that topical chemotherapy can be a well-tolerated option for patients who present with frequent superficial BCCs to prevent numerous invasive surgical treatments.
- Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157-163.
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapy for mycosis fungoides. J Am Acad Dermatol. 1986;14:1029-1038.
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. 1982;7:590-598.
- Lamb PM, Menaker GM, Moy RL. Multiple basal cell carcinomas of the limb after adjuvant treatment of melanoma with isolated limb perfusion. J Am Acad Dermatol. 1998;38:767-768.
- Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporal circuit. Ann Surg. 1958;148:616-632.
- Lindahl L, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170:699-704.
- Lenormand C, Pelletier C, Goeldel AL, et al. Second malignant neoplasm occurring years after hyperthermic isolated limb perfusion for melanoma. Arch Dermatol. 2010;146:319-321.
- Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157-163.
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapy for mycosis fungoides. J Am Acad Dermatol. 1986;14:1029-1038.
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. 1982;7:590-598.
- Lamb PM, Menaker GM, Moy RL. Multiple basal cell carcinomas of the limb after adjuvant treatment of melanoma with isolated limb perfusion. J Am Acad Dermatol. 1998;38:767-768.
- Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporal circuit. Ann Surg. 1958;148:616-632.
- Lindahl L, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170:699-704.
- Lenormand C, Pelletier C, Goeldel AL, et al. Second malignant neoplasm occurring years after hyperthermic isolated limb perfusion for melanoma. Arch Dermatol. 2010;146:319-321.
The Dayanara Effect: Increasing Skin Cancer Awareness in the Hispanic Community
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
A telemedicine compromise
It’s late on a Thursday afternoon. Even through the six walls that separate you from the waiting room you can feel the impatient throng of families as you struggle to see the tympanic membrane of a feverish and uncooperative 3-year-old. You already have scraped his auditory canal once with your curette. Your gut tells you that he must have an otitis but deeper in your soul there are other voices reminding you that to make the diagnosis you must visualize his ear drum. Your skill and the technology on hand has failed you.
It’s a Sunday morning, weekend hours, and you are seeing a 12-year-old with a sore throat and fever. Her physical exam suggests that she has strep pharyngitis but the team member in charge of restocking supplies has forgotten to reorder rapid strep kits and you used the last one yesterday afternoon.
Do you ignore your training and treat these sick children with antibiotics?
If you are someone who perceives the world in black and white, your response to these scenarios is simple because you NEVER prescribe antibiotics without seeing a tympanic membrane or confirming your suspicion with a rapid strep test. There are unrealistic solutions that could include requesting an immediate ear/nose/throat consult or sending the patient on an hour-long odyssey to the hospital lab. But for the rest of us who see in shades of gray, we may have to compromise our principles and temporarily become poor antibiotic stewards. The question is, how often do you compromise? Once a week, once a month, twice a year, or twice a day?
A study published in Pediatrics looks at the issue of antibiotic stewardship as it relates to telemedicine (“Antibiotic Prescribing During Pediatrics Direct-to-Consumer Telemedicine Visits,” Pediatrics. 2019 May. doi: 10.1542/peds.2018-2491).
The investigators found that children with acute respiratory infections were more likely to receive antibiotics and less likely to receive guideline concordant management at direct-to-consumer (DTC) telemedicine visits than when they were seen by their primary care physician or at an urgent care center.
In their discussion, the researchers note several possible explanations for the discrepancies they observed. DTC telemedicine visits are limited by the devices used by the families and physicians and generally lack availability of otoscopy and strep testing. The authors also wonder whether “there may be differential expectations for antibiotics among children and parents who use DTC telemedicine versus in person care.” Does this mean that families who utilize DTC telemedicine undervalue in-person care and/or are willing to compromise by accepting what they may suspect is substandard care for the convenience of DTC telemedicine?
Which brings me to my point. A physician who accepts the challenge of seeing pediatric patients with acute respiratory illnesses knowing that he or she will not be able to visualize tympanic membranes or perform strep testing also has accepted the fact that he or she will be compromising the principles of antibiotic stewardship he or she must have – or maybe should have – learned in medical school or residency.
We all occasionally compromise our principles when technology fails us or when the situations are extraordinary. But I am troubled that there some physicians who are willing to practice in an environment in which they are aware that they will be compromising their antibiotic stewardship on a daily or even hourly basis.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s late on a Thursday afternoon. Even through the six walls that separate you from the waiting room you can feel the impatient throng of families as you struggle to see the tympanic membrane of a feverish and uncooperative 3-year-old. You already have scraped his auditory canal once with your curette. Your gut tells you that he must have an otitis but deeper in your soul there are other voices reminding you that to make the diagnosis you must visualize his ear drum. Your skill and the technology on hand has failed you.
It’s a Sunday morning, weekend hours, and you are seeing a 12-year-old with a sore throat and fever. Her physical exam suggests that she has strep pharyngitis but the team member in charge of restocking supplies has forgotten to reorder rapid strep kits and you used the last one yesterday afternoon.
Do you ignore your training and treat these sick children with antibiotics?
If you are someone who perceives the world in black and white, your response to these scenarios is simple because you NEVER prescribe antibiotics without seeing a tympanic membrane or confirming your suspicion with a rapid strep test. There are unrealistic solutions that could include requesting an immediate ear/nose/throat consult or sending the patient on an hour-long odyssey to the hospital lab. But for the rest of us who see in shades of gray, we may have to compromise our principles and temporarily become poor antibiotic stewards. The question is, how often do you compromise? Once a week, once a month, twice a year, or twice a day?
A study published in Pediatrics looks at the issue of antibiotic stewardship as it relates to telemedicine (“Antibiotic Prescribing During Pediatrics Direct-to-Consumer Telemedicine Visits,” Pediatrics. 2019 May. doi: 10.1542/peds.2018-2491).
The investigators found that children with acute respiratory infections were more likely to receive antibiotics and less likely to receive guideline concordant management at direct-to-consumer (DTC) telemedicine visits than when they were seen by their primary care physician or at an urgent care center.
In their discussion, the researchers note several possible explanations for the discrepancies they observed. DTC telemedicine visits are limited by the devices used by the families and physicians and generally lack availability of otoscopy and strep testing. The authors also wonder whether “there may be differential expectations for antibiotics among children and parents who use DTC telemedicine versus in person care.” Does this mean that families who utilize DTC telemedicine undervalue in-person care and/or are willing to compromise by accepting what they may suspect is substandard care for the convenience of DTC telemedicine?
Which brings me to my point. A physician who accepts the challenge of seeing pediatric patients with acute respiratory illnesses knowing that he or she will not be able to visualize tympanic membranes or perform strep testing also has accepted the fact that he or she will be compromising the principles of antibiotic stewardship he or she must have – or maybe should have – learned in medical school or residency.
We all occasionally compromise our principles when technology fails us or when the situations are extraordinary. But I am troubled that there some physicians who are willing to practice in an environment in which they are aware that they will be compromising their antibiotic stewardship on a daily or even hourly basis.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s late on a Thursday afternoon. Even through the six walls that separate you from the waiting room you can feel the impatient throng of families as you struggle to see the tympanic membrane of a feverish and uncooperative 3-year-old. You already have scraped his auditory canal once with your curette. Your gut tells you that he must have an otitis but deeper in your soul there are other voices reminding you that to make the diagnosis you must visualize his ear drum. Your skill and the technology on hand has failed you.
It’s a Sunday morning, weekend hours, and you are seeing a 12-year-old with a sore throat and fever. Her physical exam suggests that she has strep pharyngitis but the team member in charge of restocking supplies has forgotten to reorder rapid strep kits and you used the last one yesterday afternoon.
Do you ignore your training and treat these sick children with antibiotics?
If you are someone who perceives the world in black and white, your response to these scenarios is simple because you NEVER prescribe antibiotics without seeing a tympanic membrane or confirming your suspicion with a rapid strep test. There are unrealistic solutions that could include requesting an immediate ear/nose/throat consult or sending the patient on an hour-long odyssey to the hospital lab. But for the rest of us who see in shades of gray, we may have to compromise our principles and temporarily become poor antibiotic stewards. The question is, how often do you compromise? Once a week, once a month, twice a year, or twice a day?
A study published in Pediatrics looks at the issue of antibiotic stewardship as it relates to telemedicine (“Antibiotic Prescribing During Pediatrics Direct-to-Consumer Telemedicine Visits,” Pediatrics. 2019 May. doi: 10.1542/peds.2018-2491).
The investigators found that children with acute respiratory infections were more likely to receive antibiotics and less likely to receive guideline concordant management at direct-to-consumer (DTC) telemedicine visits than when they were seen by their primary care physician or at an urgent care center.
In their discussion, the researchers note several possible explanations for the discrepancies they observed. DTC telemedicine visits are limited by the devices used by the families and physicians and generally lack availability of otoscopy and strep testing. The authors also wonder whether “there may be differential expectations for antibiotics among children and parents who use DTC telemedicine versus in person care.” Does this mean that families who utilize DTC telemedicine undervalue in-person care and/or are willing to compromise by accepting what they may suspect is substandard care for the convenience of DTC telemedicine?
Which brings me to my point. A physician who accepts the challenge of seeing pediatric patients with acute respiratory illnesses knowing that he or she will not be able to visualize tympanic membranes or perform strep testing also has accepted the fact that he or she will be compromising the principles of antibiotic stewardship he or she must have – or maybe should have – learned in medical school or residency.
We all occasionally compromise our principles when technology fails us or when the situations are extraordinary. But I am troubled that there some physicians who are willing to practice in an environment in which they are aware that they will be compromising their antibiotic stewardship on a daily or even hourly basis.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Are guidelines relevant?
The recent publication of the changes and current levels of evidence for the guidelines from the American College of Cardiology, American Heart Association, and the European Society of Cardiology once again indicates that the level of high-quality scientific data supporting the decision making process is limited.
Of the thousands of recommendations among the guidelines provided, level A data – which is supported by at least two randomized, controlled trials (RCT), arguably the gold standard for the proof of benefit of a treatment program – was available in only 8.5% of ACC/AHA recommendations and in 14.2% of ESC recommendations. The recent review covering guidelines published from 2008 to 2018 has changed very little since the previous review carried out on data collected between 1999 and 2014 (JAMA. 2019 Mar 19;321[11]:1069-80). Admittedly, by the nature of their design, RCTs represent a very special subset of any disease process but they are the best we can do. The remainder of the guidelines is supported by nonrandomized data and consensus opinion of “experts.” The absence of randomized data in the rest of the guidelines creates an area of uncertainty between clinical practice and outcomes.
Guidelines have been with us for almost 30 years and have provided a near “cookbook recipe” for the treatment of almost the totality of cardiovascular care, both in the United States and Europe. There is little evidence that guideline dissemination results in a beneficial effect on cardiovascular care, instead reflecting the contemporary published research and informed opinion of our generation.
Despite the limitation of hard data based on RCTs, cardiovascular mortality has leveled off in the last few years. Although there are a number of areas that could be investigated with more focused and practical RCTs, there has been little enthusiasm by either the National Heart, Lung, and Blood Institute or the pharmaceutical or device industries to investigate existing cardiovascular drugs and/or devices to better elucidate nuanced therapy for subsets of heart disease that remain in the uncertain data areas. In fact, the current report shows that there has been little change in guidelines in the last 10 years. Most effort has been focused on new drug development and application.
Much of the advance in cardiovascular care and decrease in mortality is a result of the development of new drugs for the treatment of hypertension, management of hypercholesterolemia, and MI, together with coronary angiography and its interventional permutations of the previous 50 years. Those advances are now part of our knowledge base, our therapeutic DNA, and in the absence of some new scientific breakthrough, there is little to expect from future guideline development. Medical students and house officers are being taught as fact what we found as exciting new therapy just a few short years ago. Guidelines are now part of textbook knowledge and until new clinical outcome are reported, guidelines will probably serve a useful reference material but will not lead to much change in clinical care.
Even so, treating the patient in the clinic and in the hospital still requires the doctor to practice the “art” of medicine and to synthesize therapy based on the paucity of scientific data and quasi knowledge acquired over a generation.
The appreciation that much is unknown gives the doctor the humility required to be sensitive to patient needs and their symptoms and the aspiration for new, science-based data. This is still part of the excitement of being a doctor.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The recent publication of the changes and current levels of evidence for the guidelines from the American College of Cardiology, American Heart Association, and the European Society of Cardiology once again indicates that the level of high-quality scientific data supporting the decision making process is limited.
Of the thousands of recommendations among the guidelines provided, level A data – which is supported by at least two randomized, controlled trials (RCT), arguably the gold standard for the proof of benefit of a treatment program – was available in only 8.5% of ACC/AHA recommendations and in 14.2% of ESC recommendations. The recent review covering guidelines published from 2008 to 2018 has changed very little since the previous review carried out on data collected between 1999 and 2014 (JAMA. 2019 Mar 19;321[11]:1069-80). Admittedly, by the nature of their design, RCTs represent a very special subset of any disease process but they are the best we can do. The remainder of the guidelines is supported by nonrandomized data and consensus opinion of “experts.” The absence of randomized data in the rest of the guidelines creates an area of uncertainty between clinical practice and outcomes.
Guidelines have been with us for almost 30 years and have provided a near “cookbook recipe” for the treatment of almost the totality of cardiovascular care, both in the United States and Europe. There is little evidence that guideline dissemination results in a beneficial effect on cardiovascular care, instead reflecting the contemporary published research and informed opinion of our generation.
Despite the limitation of hard data based on RCTs, cardiovascular mortality has leveled off in the last few years. Although there are a number of areas that could be investigated with more focused and practical RCTs, there has been little enthusiasm by either the National Heart, Lung, and Blood Institute or the pharmaceutical or device industries to investigate existing cardiovascular drugs and/or devices to better elucidate nuanced therapy for subsets of heart disease that remain in the uncertain data areas. In fact, the current report shows that there has been little change in guidelines in the last 10 years. Most effort has been focused on new drug development and application.
Much of the advance in cardiovascular care and decrease in mortality is a result of the development of new drugs for the treatment of hypertension, management of hypercholesterolemia, and MI, together with coronary angiography and its interventional permutations of the previous 50 years. Those advances are now part of our knowledge base, our therapeutic DNA, and in the absence of some new scientific breakthrough, there is little to expect from future guideline development. Medical students and house officers are being taught as fact what we found as exciting new therapy just a few short years ago. Guidelines are now part of textbook knowledge and until new clinical outcome are reported, guidelines will probably serve a useful reference material but will not lead to much change in clinical care.
Even so, treating the patient in the clinic and in the hospital still requires the doctor to practice the “art” of medicine and to synthesize therapy based on the paucity of scientific data and quasi knowledge acquired over a generation.
The appreciation that much is unknown gives the doctor the humility required to be sensitive to patient needs and their symptoms and the aspiration for new, science-based data. This is still part of the excitement of being a doctor.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The recent publication of the changes and current levels of evidence for the guidelines from the American College of Cardiology, American Heart Association, and the European Society of Cardiology once again indicates that the level of high-quality scientific data supporting the decision making process is limited.
Of the thousands of recommendations among the guidelines provided, level A data – which is supported by at least two randomized, controlled trials (RCT), arguably the gold standard for the proof of benefit of a treatment program – was available in only 8.5% of ACC/AHA recommendations and in 14.2% of ESC recommendations. The recent review covering guidelines published from 2008 to 2018 has changed very little since the previous review carried out on data collected between 1999 and 2014 (JAMA. 2019 Mar 19;321[11]:1069-80). Admittedly, by the nature of their design, RCTs represent a very special subset of any disease process but they are the best we can do. The remainder of the guidelines is supported by nonrandomized data and consensus opinion of “experts.” The absence of randomized data in the rest of the guidelines creates an area of uncertainty between clinical practice and outcomes.
Guidelines have been with us for almost 30 years and have provided a near “cookbook recipe” for the treatment of almost the totality of cardiovascular care, both in the United States and Europe. There is little evidence that guideline dissemination results in a beneficial effect on cardiovascular care, instead reflecting the contemporary published research and informed opinion of our generation.
Despite the limitation of hard data based on RCTs, cardiovascular mortality has leveled off in the last few years. Although there are a number of areas that could be investigated with more focused and practical RCTs, there has been little enthusiasm by either the National Heart, Lung, and Blood Institute or the pharmaceutical or device industries to investigate existing cardiovascular drugs and/or devices to better elucidate nuanced therapy for subsets of heart disease that remain in the uncertain data areas. In fact, the current report shows that there has been little change in guidelines in the last 10 years. Most effort has been focused on new drug development and application.
Much of the advance in cardiovascular care and decrease in mortality is a result of the development of new drugs for the treatment of hypertension, management of hypercholesterolemia, and MI, together with coronary angiography and its interventional permutations of the previous 50 years. Those advances are now part of our knowledge base, our therapeutic DNA, and in the absence of some new scientific breakthrough, there is little to expect from future guideline development. Medical students and house officers are being taught as fact what we found as exciting new therapy just a few short years ago. Guidelines are now part of textbook knowledge and until new clinical outcome are reported, guidelines will probably serve a useful reference material but will not lead to much change in clinical care.
Even so, treating the patient in the clinic and in the hospital still requires the doctor to practice the “art” of medicine and to synthesize therapy based on the paucity of scientific data and quasi knowledge acquired over a generation.
The appreciation that much is unknown gives the doctor the humility required to be sensitive to patient needs and their symptoms and the aspiration for new, science-based data. This is still part of the excitement of being a doctor.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Bipolar disorder during pregnancy: Lessons learned
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Spring for GI
Spring has always been an exciting time for gastroenterologists, beginning with Colon Cancer Awareness month in March and finishing with our flagship scientific meeting in May. Gastroenterologists have led the fight against colon cancer; publishing seminal research (the National Polyp Study was published April 1, 26 years ago), building a distributed network of high-value ambulatory endoscopy centers, educating primary care physicians and the public about screening and early detection, and advocating continuously to make cancer prevention affordable for all people.
This year, AGA has sponsored two meetings where truly ground-breaking science was presented and we have highlighted them on our front page this month. On March 23-24, the AGA worked with the European Society of Neurogastroenterology and Motility to bring you the 8th annual Gut Microbiota for Health World Summit in Miami. World leaders in microbiome research presented a breath-taking array of clinically relevant research on topics that impact your patients. Dr. Stanley Hazen (Cleveland Clinic) presented his work linking dietary choices to a blood marker of atherosclerotic risk (TMAO) where the key associative link is the diet-influenced microbiome.
The AGA also brought you the 10th annual AGA Tech Summit from San Francisco, April 10-12. This meeting has become the best single source to learn about new technology emerging in our field. In this issue of GI & Hepatology News, we highlight two presentations about managing visceral pain with virtual reality technology and how predictive analysis is being used to personalize IBD therapy.
Spring wraps up with DDW® in San Diego (May 18-21). DDW begins with the AGA Postgraduate Course (May 18-19) that provides the best annual summary of both gastroenterology and hepatology combined in a single setting. The live meeting will feature key updates and new science about biosimilars, cancer prevention, celiac disease, endoscopy, the microbiome, hepatology, IBD, nutrition, and care delivery.
As usual, GIHN will feature key presentations from DDW including those from the Presidential Plenary session (Monday morning May 20).
John I. Allen, MD, MBA, AGAF
Editor in Chief
Spring has always been an exciting time for gastroenterologists, beginning with Colon Cancer Awareness month in March and finishing with our flagship scientific meeting in May. Gastroenterologists have led the fight against colon cancer; publishing seminal research (the National Polyp Study was published April 1, 26 years ago), building a distributed network of high-value ambulatory endoscopy centers, educating primary care physicians and the public about screening and early detection, and advocating continuously to make cancer prevention affordable for all people.
This year, AGA has sponsored two meetings where truly ground-breaking science was presented and we have highlighted them on our front page this month. On March 23-24, the AGA worked with the European Society of Neurogastroenterology and Motility to bring you the 8th annual Gut Microbiota for Health World Summit in Miami. World leaders in microbiome research presented a breath-taking array of clinically relevant research on topics that impact your patients. Dr. Stanley Hazen (Cleveland Clinic) presented his work linking dietary choices to a blood marker of atherosclerotic risk (TMAO) where the key associative link is the diet-influenced microbiome.
The AGA also brought you the 10th annual AGA Tech Summit from San Francisco, April 10-12. This meeting has become the best single source to learn about new technology emerging in our field. In this issue of GI & Hepatology News, we highlight two presentations about managing visceral pain with virtual reality technology and how predictive analysis is being used to personalize IBD therapy.
Spring wraps up with DDW® in San Diego (May 18-21). DDW begins with the AGA Postgraduate Course (May 18-19) that provides the best annual summary of both gastroenterology and hepatology combined in a single setting. The live meeting will feature key updates and new science about biosimilars, cancer prevention, celiac disease, endoscopy, the microbiome, hepatology, IBD, nutrition, and care delivery.
As usual, GIHN will feature key presentations from DDW including those from the Presidential Plenary session (Monday morning May 20).
John I. Allen, MD, MBA, AGAF
Editor in Chief
Spring has always been an exciting time for gastroenterologists, beginning with Colon Cancer Awareness month in March and finishing with our flagship scientific meeting in May. Gastroenterologists have led the fight against colon cancer; publishing seminal research (the National Polyp Study was published April 1, 26 years ago), building a distributed network of high-value ambulatory endoscopy centers, educating primary care physicians and the public about screening and early detection, and advocating continuously to make cancer prevention affordable for all people.
This year, AGA has sponsored two meetings where truly ground-breaking science was presented and we have highlighted them on our front page this month. On March 23-24, the AGA worked with the European Society of Neurogastroenterology and Motility to bring you the 8th annual Gut Microbiota for Health World Summit in Miami. World leaders in microbiome research presented a breath-taking array of clinically relevant research on topics that impact your patients. Dr. Stanley Hazen (Cleveland Clinic) presented his work linking dietary choices to a blood marker of atherosclerotic risk (TMAO) where the key associative link is the diet-influenced microbiome.
The AGA also brought you the 10th annual AGA Tech Summit from San Francisco, April 10-12. This meeting has become the best single source to learn about new technology emerging in our field. In this issue of GI & Hepatology News, we highlight two presentations about managing visceral pain with virtual reality technology and how predictive analysis is being used to personalize IBD therapy.
Spring wraps up with DDW® in San Diego (May 18-21). DDW begins with the AGA Postgraduate Course (May 18-19) that provides the best annual summary of both gastroenterology and hepatology combined in a single setting. The live meeting will feature key updates and new science about biosimilars, cancer prevention, celiac disease, endoscopy, the microbiome, hepatology, IBD, nutrition, and care delivery.
As usual, GIHN will feature key presentations from DDW including those from the Presidential Plenary session (Monday morning May 20).
John I. Allen, MD, MBA, AGAF
Editor in Chief
Discuss compounded bioidentical hormones and cancer risk
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.