User login
Retail neurosis
When I stopped by last August to pick up new eyeglass lenses, Harold the optician sat alone in his shop.
“Business slow in the summer?” I asked.
Harold looked morose. “I knew it would be like this when I bought the business,” he said. “We’re open Saturdays, but summers I close at 2. Everybody’s at the Cape.”
Working in retail makes people more neurotic than necessary. I should know. I’ve been in retail for 40 years.
My patient Myrtle once explained to me how retail induces neurosis by deforming incentives. Myrtle used to work in management at a big department store. (Older readers may recall going to stores in buildings to buy things. The same readers may recall newspapers.)
“The month between Thanksgiving and Christmas makes or breaks the whole year,” Myrtle said. “If you do worse than last year, you feel bad. But if you do better than last year you also feel bad, because you worry you won’t be able to top it next year.”
She paused. “I guess that’s not a very healthy way to live, is it?”
I was too polite to agree.
Early in my career I had few patients on my schedule, maybe five on a good day. Then three of them would cancel. That was the start of my retail neurosis. Of course, I was a solo practitioner who started my own practice. The likes of me will someday be found in a museum, stuffed and mounted, along with other extinct species, under the label Medicus Cutaneous Solipsisticus (North America c. 20th century).
Over time, I got busier and dropped each of my eleven part-time jobs. By now I’ve been busy for decades, even though I’ve never had much of a waiting list. Don’t know why that is, but it no longer matters.
Except it does, psychologically. You won’t find this code in the DSM, but my working definition for the malady I describe is as follows:
Retail Neurosis (billable ICD-10 code F48.8. Other unspecified nonpsychotic mental disorders, along with writer’s block and psychasthenia):
Definition: The unquenchable fear that even the tiniest break in an endless churn of patients means that all patients will disappear later this afternoon, reverting the practice to the empty, formless void from whence it came. Other than retirement, there is no treatment for this disorder. And maybe not then either.
You might think to classify Retail Neurosis under Financial Insecurity, but that disorder has a different code. (F40.248, Fear of Failing, Life-Circumstance Problem). After all, a single well-remunerated patient (53 actinic keratoses!) can outreimburse half a dozen others with only E/M codes and big deductibles. Treat one of the former, take the rest of the hour off, and you’re financially just as well off, or even better. Yes?
No. Taking the rest of the hour off leaves you with too much time to ponder what every retailer knows: Each idle minute is another lost chance to make another sale and generate revenue. That minute (and revenue) can never be retrieved. Never!
As Myrtle would say, “Not a very healthy way to live, is it?”
Maybe not, but here as elsewhere, knowing something and fixing it are different things. Besides, brisk retail business brings a buzz, along with a sense of mastery and accomplishment, which is pleasantly addictive. Until it isn’t.
New generations of physicians and other medical providers will work in different settings than mine; they will be wage-earners in large organizations. These conglomerations bring their own neurosis-inducing incentives. Their managers measure providers’ productivity in various deforming and crazy-making ways. (See RVU-penia, ICD-10 M26.56: “Nonworking side interference.” This is actually a dental code that refers to jaw position, but billing demands creativity.) Practitioner anxieties will center on being docked for not generating enough relative value units or for failure to bundle enough comorbidities for maximizing capitation payments (e.g., Plaque Psoriasis plus Morbid Obesity plus Writer’s Block). But the youngsters will learn to get along. They’ll have to.
“Taking any time off this summer?” I asked my optician Harold.
“My wife and daughter are going out to Michigan in mid-August,” he said.
“Aren’t you going with them?”
“I can’t swing it that week,” he said. “By then, people are coming back to town, getting their kids ready for school. If I go away, I would miss some customers.”
Harold, you are my kind of guy!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
When I stopped by last August to pick up new eyeglass lenses, Harold the optician sat alone in his shop.
“Business slow in the summer?” I asked.
Harold looked morose. “I knew it would be like this when I bought the business,” he said. “We’re open Saturdays, but summers I close at 2. Everybody’s at the Cape.”
Working in retail makes people more neurotic than necessary. I should know. I’ve been in retail for 40 years.
My patient Myrtle once explained to me how retail induces neurosis by deforming incentives. Myrtle used to work in management at a big department store. (Older readers may recall going to stores in buildings to buy things. The same readers may recall newspapers.)
“The month between Thanksgiving and Christmas makes or breaks the whole year,” Myrtle said. “If you do worse than last year, you feel bad. But if you do better than last year you also feel bad, because you worry you won’t be able to top it next year.”
She paused. “I guess that’s not a very healthy way to live, is it?”
I was too polite to agree.
Early in my career I had few patients on my schedule, maybe five on a good day. Then three of them would cancel. That was the start of my retail neurosis. Of course, I was a solo practitioner who started my own practice. The likes of me will someday be found in a museum, stuffed and mounted, along with other extinct species, under the label Medicus Cutaneous Solipsisticus (North America c. 20th century).
Over time, I got busier and dropped each of my eleven part-time jobs. By now I’ve been busy for decades, even though I’ve never had much of a waiting list. Don’t know why that is, but it no longer matters.
Except it does, psychologically. You won’t find this code in the DSM, but my working definition for the malady I describe is as follows:
Retail Neurosis (billable ICD-10 code F48.8. Other unspecified nonpsychotic mental disorders, along with writer’s block and psychasthenia):
Definition: The unquenchable fear that even the tiniest break in an endless churn of patients means that all patients will disappear later this afternoon, reverting the practice to the empty, formless void from whence it came. Other than retirement, there is no treatment for this disorder. And maybe not then either.
You might think to classify Retail Neurosis under Financial Insecurity, but that disorder has a different code. (F40.248, Fear of Failing, Life-Circumstance Problem). After all, a single well-remunerated patient (53 actinic keratoses!) can outreimburse half a dozen others with only E/M codes and big deductibles. Treat one of the former, take the rest of the hour off, and you’re financially just as well off, or even better. Yes?
No. Taking the rest of the hour off leaves you with too much time to ponder what every retailer knows: Each idle minute is another lost chance to make another sale and generate revenue. That minute (and revenue) can never be retrieved. Never!
As Myrtle would say, “Not a very healthy way to live, is it?”
Maybe not, but here as elsewhere, knowing something and fixing it are different things. Besides, brisk retail business brings a buzz, along with a sense of mastery and accomplishment, which is pleasantly addictive. Until it isn’t.
New generations of physicians and other medical providers will work in different settings than mine; they will be wage-earners in large organizations. These conglomerations bring their own neurosis-inducing incentives. Their managers measure providers’ productivity in various deforming and crazy-making ways. (See RVU-penia, ICD-10 M26.56: “Nonworking side interference.” This is actually a dental code that refers to jaw position, but billing demands creativity.) Practitioner anxieties will center on being docked for not generating enough relative value units or for failure to bundle enough comorbidities for maximizing capitation payments (e.g., Plaque Psoriasis plus Morbid Obesity plus Writer’s Block). But the youngsters will learn to get along. They’ll have to.
“Taking any time off this summer?” I asked my optician Harold.
“My wife and daughter are going out to Michigan in mid-August,” he said.
“Aren’t you going with them?”
“I can’t swing it that week,” he said. “By then, people are coming back to town, getting their kids ready for school. If I go away, I would miss some customers.”
Harold, you are my kind of guy!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
When I stopped by last August to pick up new eyeglass lenses, Harold the optician sat alone in his shop.
“Business slow in the summer?” I asked.
Harold looked morose. “I knew it would be like this when I bought the business,” he said. “We’re open Saturdays, but summers I close at 2. Everybody’s at the Cape.”
Working in retail makes people more neurotic than necessary. I should know. I’ve been in retail for 40 years.
My patient Myrtle once explained to me how retail induces neurosis by deforming incentives. Myrtle used to work in management at a big department store. (Older readers may recall going to stores in buildings to buy things. The same readers may recall newspapers.)
“The month between Thanksgiving and Christmas makes or breaks the whole year,” Myrtle said. “If you do worse than last year, you feel bad. But if you do better than last year you also feel bad, because you worry you won’t be able to top it next year.”
She paused. “I guess that’s not a very healthy way to live, is it?”
I was too polite to agree.
Early in my career I had few patients on my schedule, maybe five on a good day. Then three of them would cancel. That was the start of my retail neurosis. Of course, I was a solo practitioner who started my own practice. The likes of me will someday be found in a museum, stuffed and mounted, along with other extinct species, under the label Medicus Cutaneous Solipsisticus (North America c. 20th century).
Over time, I got busier and dropped each of my eleven part-time jobs. By now I’ve been busy for decades, even though I’ve never had much of a waiting list. Don’t know why that is, but it no longer matters.
Except it does, psychologically. You won’t find this code in the DSM, but my working definition for the malady I describe is as follows:
Retail Neurosis (billable ICD-10 code F48.8. Other unspecified nonpsychotic mental disorders, along with writer’s block and psychasthenia):
Definition: The unquenchable fear that even the tiniest break in an endless churn of patients means that all patients will disappear later this afternoon, reverting the practice to the empty, formless void from whence it came. Other than retirement, there is no treatment for this disorder. And maybe not then either.
You might think to classify Retail Neurosis under Financial Insecurity, but that disorder has a different code. (F40.248, Fear of Failing, Life-Circumstance Problem). After all, a single well-remunerated patient (53 actinic keratoses!) can outreimburse half a dozen others with only E/M codes and big deductibles. Treat one of the former, take the rest of the hour off, and you’re financially just as well off, or even better. Yes?
No. Taking the rest of the hour off leaves you with too much time to ponder what every retailer knows: Each idle minute is another lost chance to make another sale and generate revenue. That minute (and revenue) can never be retrieved. Never!
As Myrtle would say, “Not a very healthy way to live, is it?”
Maybe not, but here as elsewhere, knowing something and fixing it are different things. Besides, brisk retail business brings a buzz, along with a sense of mastery and accomplishment, which is pleasantly addictive. Until it isn’t.
New generations of physicians and other medical providers will work in different settings than mine; they will be wage-earners in large organizations. These conglomerations bring their own neurosis-inducing incentives. Their managers measure providers’ productivity in various deforming and crazy-making ways. (See RVU-penia, ICD-10 M26.56: “Nonworking side interference.” This is actually a dental code that refers to jaw position, but billing demands creativity.) Practitioner anxieties will center on being docked for not generating enough relative value units or for failure to bundle enough comorbidities for maximizing capitation payments (e.g., Plaque Psoriasis plus Morbid Obesity plus Writer’s Block). But the youngsters will learn to get along. They’ll have to.
“Taking any time off this summer?” I asked my optician Harold.
“My wife and daughter are going out to Michigan in mid-August,” he said.
“Aren’t you going with them?”
“I can’t swing it that week,” he said. “By then, people are coming back to town, getting their kids ready for school. If I go away, I would miss some customers.”
Harold, you are my kind of guy!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Navigating the Oncology Care Model
Care of the cancer patient is complex and expensive. During 2001-2011, medical spending to treat cancer increased from $56.8 billion to $88.3 billion in the United States. During this time, ambulatory expenditures for care and treatment increased while inpatient hospital expenditures decreased.1,2 Treatments for cancer have advanced, but costs do not correlate with outcomes. Advanced payment models aimed at ensuring high quality while lowering costs may be the vehicle to help mitigate the financial burden of cancer treatment on patients and society at large.
Oncology Care Model
The Center for Medicare and Medicaid Innovation designed the Oncology Care Model (OCM), which allows practices and payers in the United States to partner with the Centers for Medicare & Medicaid Services. The goal of the OCM is to provide high quality, highly coordinated cancer care at the same or lower cost. Practice partnerships with the CMS involve payment arrangements that include financial and performance accountability for episodes of cancer care surrounding chemotherapy delivery to patients.3
Practices that have been selected by the CMS have attested to providing a number of enhanced services from 24/7 patient access to an appropriate clinician who can access medical records to having a documented care plan for every patient.4
Payment methodology
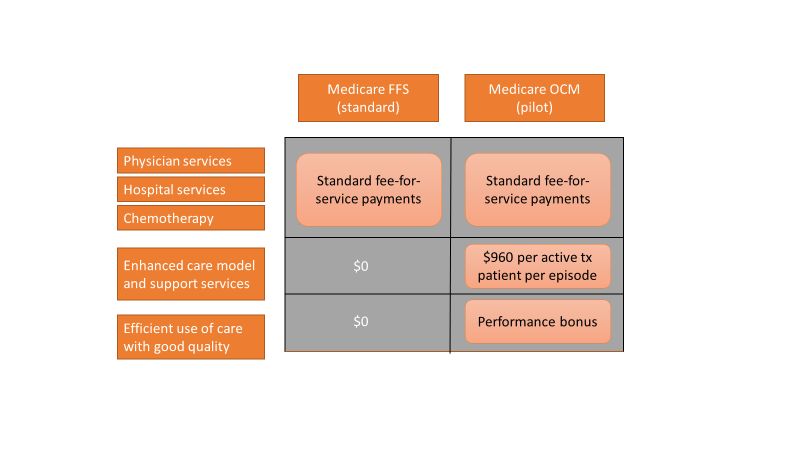
An episode of care is defined as a 6-month period that starts at the time of chemotherapy administration. In addition to the standard fee-for-service payment, practices have the ability to earn two other types of payments during an oncology episode.
The per-beneficiary Monthly Enhanced Oncology Services payment is $960 for the entire episode but is paid to practices at $160 per month.
Practices have the potential to earn additional performance-based payments (PBP) based on the difference in cost between the projected and actual cost of the episode. The PBP also incorporates performance on quality metrics, based on Medicare claims and other information submitted by the practice. For example, claims-based measures include hospital, emergency department (ED), and hospice utilization.
To participate in the OCM, practices must choose either a one-sided or two-sided risk model. In the one-sided risk model, practices take on no downside risk but need to achieve a greater reduction in expenditures (4% below the benchmark price). In the two-side risk model, practices need only to reduce expenditures by 2.75% below the benchmark price. But if they fail to meet their savings goals, they must pay the difference to the CMS. The recoupment is capped at 20% of the benchmark amount.
Feedback reports
The CMS sends quarterly feedback reports that contain information on practice demographics, outcomes, expenditures, chemotherapy use, and patient satisfaction. The outcomes include the mortality rate for Medicare beneficiaries treated at the practice, compared with other practices nationally. In addition, the reports include end-of-life metrics and patient satisfaction, as well as details of expenditures on drugs, hospital use, imaging and laboratory services, and a description of chemotherapy usage.
These reports can be a helpful tool for measuring your own use of services, as well as benchmarking it against national figures.
Practice modifications
According to CMS feedback reports, the cost of care per beneficiary per month has increased across all practices since the inception of the OCM. However, there are practices that have been successful in reducing cost of care without negatively affecting mortality.
Drugs, hospital, and ED visits, along with imaging and laboratory evaluation, account for 75% of the cost. Some strategies to reduce expenditure involve targeting those areas.
Consider prescribing drugs conservatively without affecting outcomes. For instance, bisphosphonates for bone metastasis can be given every 12 weeks instead of 4 weeks.5 Similarly, adjuvant chemotherapy can be given for 3 months, instead of 6 months in appropriate stage 3 colon cancer patients.6
Another potential opportunity for savings is the judicious use of pertuzumab in early-stage breast cancer patients.7 These are all evidence-based recommendations with potential for cost savings. Clinical pathways can aid in this process, but physician buy-in is imperative.
In terms of imaging, avoid PET scans when they will not affect your clinical decision making, avoid staging scans in early-stage breast and prostate cancer patients, and avoid surveillance scans among early-stage breast cancer and lymphoma patients. The Choosing Wisely campaign can help guide some of these decisions.8
Another area where good care meets cost effective care is in the early engagement of palliative care. Several studies have shown that early involvement of palliative care improves survival and quality of life.9,10 Palliative care involvement also decreases the emotional burden for patients and oncologists. Appropriate symptom control, particularly of pain, decreases hospitalizations during treatment.
Investing in a robust supportive care team – financial advocates, social work, nutrition, behavioral health, as well as various community services – can help reduce the financial, physical, and emotional distress levels for patients. All of these services ultimately lead to reduced hospitalizations.11 The Monthly Enhanced Oncology Services payment can be put toward these expenses.
Care teams working at the highest level of competence and license can also save time and money. Consider using registered nurses to implement triage pathways to assess side effects and symptom management, or using nurse practitioners, registered nurses, and physician assistants for same-day appointments and to assess symptoms rather than referring patients to the emergency department.
Avoid the ED and hospitalizations by using the infusion center to provide hydration and blood transfusions in a timely fashion.
Telemedicine can be used for symptom management as well as leveraging supportive care services.
Cost for cancer care is very difficult to sustain. The OCM provides early insights into expenditures, challenges, and opportunities. Practices should use this information to build infrastructure and provide high quality, cost-effective care. Value-based cancer care should be the overarching goal for oncology practices and health care organizations.
Dr. Mahesh is the director of hematology-oncology and program director of the Oncology Care Model at Summa Health in Akron, Ohio.
References
1. Siegel RL et al. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Medical Expenditure Panel Survey, Statistical Brief #443. 2014 Jun.
3. CMS: Oncology Care Model.
4. CMS: OCM Frequently Asked Questions.
5. Himelstein AL et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases. JAMA. 2017 Jan 3;317(1):48-58.
6. Grothey A et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-88.
7. Von Minckwitz G et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-31.
8. American Society of Clinical Oncology: Ten Things Physician and Patients Should Question.
9. Temel JS et al. Early palliative care for patients with metastatic non–small cell lung cancer. N Engl J Med. 2010;363(8):733-42.
10. Blayney DW et al. Critical lessons from high-value oncology practices. JAMA Oncol. 2018 Feb 1;4(2):164-71.
11. Sherman DE. Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract. 2017 Aug;13(8):519-22.
Care of the cancer patient is complex and expensive. During 2001-2011, medical spending to treat cancer increased from $56.8 billion to $88.3 billion in the United States. During this time, ambulatory expenditures for care and treatment increased while inpatient hospital expenditures decreased.1,2 Treatments for cancer have advanced, but costs do not correlate with outcomes. Advanced payment models aimed at ensuring high quality while lowering costs may be the vehicle to help mitigate the financial burden of cancer treatment on patients and society at large.

Oncology Care Model
The Center for Medicare and Medicaid Innovation designed the Oncology Care Model (OCM), which allows practices and payers in the United States to partner with the Centers for Medicare & Medicaid Services. The goal of the OCM is to provide high quality, highly coordinated cancer care at the same or lower cost. Practice partnerships with the CMS involve payment arrangements that include financial and performance accountability for episodes of cancer care surrounding chemotherapy delivery to patients.3
Practices that have been selected by the CMS have attested to providing a number of enhanced services from 24/7 patient access to an appropriate clinician who can access medical records to having a documented care plan for every patient.4
Payment methodology
An episode of care is defined as a 6-month period that starts at the time of chemotherapy administration. In addition to the standard fee-for-service payment, practices have the ability to earn two other types of payments during an oncology episode.
The per-beneficiary Monthly Enhanced Oncology Services payment is $960 for the entire episode but is paid to practices at $160 per month.
Practices have the potential to earn additional performance-based payments (PBP) based on the difference in cost between the projected and actual cost of the episode. The PBP also incorporates performance on quality metrics, based on Medicare claims and other information submitted by the practice. For example, claims-based measures include hospital, emergency department (ED), and hospice utilization.
To participate in the OCM, practices must choose either a one-sided or two-sided risk model. In the one-sided risk model, practices take on no downside risk but need to achieve a greater reduction in expenditures (4% below the benchmark price). In the two-side risk model, practices need only to reduce expenditures by 2.75% below the benchmark price. But if they fail to meet their savings goals, they must pay the difference to the CMS. The recoupment is capped at 20% of the benchmark amount.
Feedback reports
The CMS sends quarterly feedback reports that contain information on practice demographics, outcomes, expenditures, chemotherapy use, and patient satisfaction. The outcomes include the mortality rate for Medicare beneficiaries treated at the practice, compared with other practices nationally. In addition, the reports include end-of-life metrics and patient satisfaction, as well as details of expenditures on drugs, hospital use, imaging and laboratory services, and a description of chemotherapy usage.
These reports can be a helpful tool for measuring your own use of services, as well as benchmarking it against national figures.
Practice modifications
According to CMS feedback reports, the cost of care per beneficiary per month has increased across all practices since the inception of the OCM. However, there are practices that have been successful in reducing cost of care without negatively affecting mortality.
Drugs, hospital, and ED visits, along with imaging and laboratory evaluation, account for 75% of the cost. Some strategies to reduce expenditure involve targeting those areas.
Consider prescribing drugs conservatively without affecting outcomes. For instance, bisphosphonates for bone metastasis can be given every 12 weeks instead of 4 weeks.5 Similarly, adjuvant chemotherapy can be given for 3 months, instead of 6 months in appropriate stage 3 colon cancer patients.6
Another potential opportunity for savings is the judicious use of pertuzumab in early-stage breast cancer patients.7 These are all evidence-based recommendations with potential for cost savings. Clinical pathways can aid in this process, but physician buy-in is imperative.
In terms of imaging, avoid PET scans when they will not affect your clinical decision making, avoid staging scans in early-stage breast and prostate cancer patients, and avoid surveillance scans among early-stage breast cancer and lymphoma patients. The Choosing Wisely campaign can help guide some of these decisions.8
Another area where good care meets cost effective care is in the early engagement of palliative care. Several studies have shown that early involvement of palliative care improves survival and quality of life.9,10 Palliative care involvement also decreases the emotional burden for patients and oncologists. Appropriate symptom control, particularly of pain, decreases hospitalizations during treatment.
Investing in a robust supportive care team – financial advocates, social work, nutrition, behavioral health, as well as various community services – can help reduce the financial, physical, and emotional distress levels for patients. All of these services ultimately lead to reduced hospitalizations.11 The Monthly Enhanced Oncology Services payment can be put toward these expenses.
Care teams working at the highest level of competence and license can also save time and money. Consider using registered nurses to implement triage pathways to assess side effects and symptom management, or using nurse practitioners, registered nurses, and physician assistants for same-day appointments and to assess symptoms rather than referring patients to the emergency department.
Avoid the ED and hospitalizations by using the infusion center to provide hydration and blood transfusions in a timely fashion.
Telemedicine can be used for symptom management as well as leveraging supportive care services.
Cost for cancer care is very difficult to sustain. The OCM provides early insights into expenditures, challenges, and opportunities. Practices should use this information to build infrastructure and provide high quality, cost-effective care. Value-based cancer care should be the overarching goal for oncology practices and health care organizations.
Dr. Mahesh is the director of hematology-oncology and program director of the Oncology Care Model at Summa Health in Akron, Ohio.
References
1. Siegel RL et al. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Medical Expenditure Panel Survey, Statistical Brief #443. 2014 Jun.
3. CMS: Oncology Care Model.
4. CMS: OCM Frequently Asked Questions.
5. Himelstein AL et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases. JAMA. 2017 Jan 3;317(1):48-58.
6. Grothey A et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-88.
7. Von Minckwitz G et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-31.
8. American Society of Clinical Oncology: Ten Things Physician and Patients Should Question.
9. Temel JS et al. Early palliative care for patients with metastatic non–small cell lung cancer. N Engl J Med. 2010;363(8):733-42.
10. Blayney DW et al. Critical lessons from high-value oncology practices. JAMA Oncol. 2018 Feb 1;4(2):164-71.
11. Sherman DE. Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract. 2017 Aug;13(8):519-22.
Care of the cancer patient is complex and expensive. During 2001-2011, medical spending to treat cancer increased from $56.8 billion to $88.3 billion in the United States. During this time, ambulatory expenditures for care and treatment increased while inpatient hospital expenditures decreased.1,2 Treatments for cancer have advanced, but costs do not correlate with outcomes. Advanced payment models aimed at ensuring high quality while lowering costs may be the vehicle to help mitigate the financial burden of cancer treatment on patients and society at large.

Oncology Care Model
The Center for Medicare and Medicaid Innovation designed the Oncology Care Model (OCM), which allows practices and payers in the United States to partner with the Centers for Medicare & Medicaid Services. The goal of the OCM is to provide high quality, highly coordinated cancer care at the same or lower cost. Practice partnerships with the CMS involve payment arrangements that include financial and performance accountability for episodes of cancer care surrounding chemotherapy delivery to patients.3
Practices that have been selected by the CMS have attested to providing a number of enhanced services from 24/7 patient access to an appropriate clinician who can access medical records to having a documented care plan for every patient.4
Payment methodology
An episode of care is defined as a 6-month period that starts at the time of chemotherapy administration. In addition to the standard fee-for-service payment, practices have the ability to earn two other types of payments during an oncology episode.
The per-beneficiary Monthly Enhanced Oncology Services payment is $960 for the entire episode but is paid to practices at $160 per month.
Practices have the potential to earn additional performance-based payments (PBP) based on the difference in cost between the projected and actual cost of the episode. The PBP also incorporates performance on quality metrics, based on Medicare claims and other information submitted by the practice. For example, claims-based measures include hospital, emergency department (ED), and hospice utilization.
To participate in the OCM, practices must choose either a one-sided or two-sided risk model. In the one-sided risk model, practices take on no downside risk but need to achieve a greater reduction in expenditures (4% below the benchmark price). In the two-side risk model, practices need only to reduce expenditures by 2.75% below the benchmark price. But if they fail to meet their savings goals, they must pay the difference to the CMS. The recoupment is capped at 20% of the benchmark amount.
Feedback reports
The CMS sends quarterly feedback reports that contain information on practice demographics, outcomes, expenditures, chemotherapy use, and patient satisfaction. The outcomes include the mortality rate for Medicare beneficiaries treated at the practice, compared with other practices nationally. In addition, the reports include end-of-life metrics and patient satisfaction, as well as details of expenditures on drugs, hospital use, imaging and laboratory services, and a description of chemotherapy usage.
These reports can be a helpful tool for measuring your own use of services, as well as benchmarking it against national figures.
Practice modifications
According to CMS feedback reports, the cost of care per beneficiary per month has increased across all practices since the inception of the OCM. However, there are practices that have been successful in reducing cost of care without negatively affecting mortality.
Drugs, hospital, and ED visits, along with imaging and laboratory evaluation, account for 75% of the cost. Some strategies to reduce expenditure involve targeting those areas.
Consider prescribing drugs conservatively without affecting outcomes. For instance, bisphosphonates for bone metastasis can be given every 12 weeks instead of 4 weeks.5 Similarly, adjuvant chemotherapy can be given for 3 months, instead of 6 months in appropriate stage 3 colon cancer patients.6
Another potential opportunity for savings is the judicious use of pertuzumab in early-stage breast cancer patients.7 These are all evidence-based recommendations with potential for cost savings. Clinical pathways can aid in this process, but physician buy-in is imperative.
In terms of imaging, avoid PET scans when they will not affect your clinical decision making, avoid staging scans in early-stage breast and prostate cancer patients, and avoid surveillance scans among early-stage breast cancer and lymphoma patients. The Choosing Wisely campaign can help guide some of these decisions.8
Another area where good care meets cost effective care is in the early engagement of palliative care. Several studies have shown that early involvement of palliative care improves survival and quality of life.9,10 Palliative care involvement also decreases the emotional burden for patients and oncologists. Appropriate symptom control, particularly of pain, decreases hospitalizations during treatment.
Investing in a robust supportive care team – financial advocates, social work, nutrition, behavioral health, as well as various community services – can help reduce the financial, physical, and emotional distress levels for patients. All of these services ultimately lead to reduced hospitalizations.11 The Monthly Enhanced Oncology Services payment can be put toward these expenses.
Care teams working at the highest level of competence and license can also save time and money. Consider using registered nurses to implement triage pathways to assess side effects and symptom management, or using nurse practitioners, registered nurses, and physician assistants for same-day appointments and to assess symptoms rather than referring patients to the emergency department.
Avoid the ED and hospitalizations by using the infusion center to provide hydration and blood transfusions in a timely fashion.
Telemedicine can be used for symptom management as well as leveraging supportive care services.
Cost for cancer care is very difficult to sustain. The OCM provides early insights into expenditures, challenges, and opportunities. Practices should use this information to build infrastructure and provide high quality, cost-effective care. Value-based cancer care should be the overarching goal for oncology practices and health care organizations.
Dr. Mahesh is the director of hematology-oncology and program director of the Oncology Care Model at Summa Health in Akron, Ohio.
References
1. Siegel RL et al. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Medical Expenditure Panel Survey, Statistical Brief #443. 2014 Jun.
3. CMS: Oncology Care Model.
4. CMS: OCM Frequently Asked Questions.
5. Himelstein AL et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases. JAMA. 2017 Jan 3;317(1):48-58.
6. Grothey A et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-88.
7. Von Minckwitz G et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-31.
8. American Society of Clinical Oncology: Ten Things Physician and Patients Should Question.
9. Temel JS et al. Early palliative care for patients with metastatic non–small cell lung cancer. N Engl J Med. 2010;363(8):733-42.
10. Blayney DW et al. Critical lessons from high-value oncology practices. JAMA Oncol. 2018 Feb 1;4(2):164-71.
11. Sherman DE. Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract. 2017 Aug;13(8):519-22.
Part 4: Misguided Research or Missed Opportunities?
I have been ruminating about the Bai et al article on independent billing in the emergency department (ED) for weeks.1 I keep wondering why the data analysis seems so off base. Don’t get me wrong: The data gathered from Medicare is what it is—but a key piece of information is not present in the pure numbers input to the Medicare database.
So, I continued to probe this study with my colleagues. To a person, their comments supported that the intent of the study is unclear. The authors posit their objective to be an examination of the “involvement of NPs and PAs” in emergency services, using billing data. But to use billing data as a measure of “involvement” does not tell the whole story.
Independence in billing does not mean that the care NPs and PAs are providing is “beyond their scope of practice.” Moreover, the billing does not capture whether, or to what extent, physician consultation or assistance was involved. If the NP or PA dictated the chart, then they are by default the “only” (independent) provider. However, billing independently does not mean a physician (or other provider) was not consulted about the plan of care.
Case in point: Years ago, I had a young woman present to the ED with a sore throat. Her presenting complaint was a symptom of a peritonsillar abscess. So I phoned an ENT colleague (a physician) and asked him about the best treatment and follow-up in this case. Did he make a note in or sign the chart? No. Was I the only provider of record? Yes. Was that care “independent,” if you only look at the billing (done by a coder, for the record)? Yes.
Admittedly, Bai and colleagues do add in their conclusion that “independence in billing … does not necessarily indicate [NPs’/PAs’] independence in care delivery.”1 And they do note that the true challenge in the ED is determining how best to “blend” the expertise of the three professions (MD, NP, and PA) to provide efficient and cost-effective care.
However, throughout the article, there is an underpinning of inference that NPs and PAs are potentially practicing beyond their scope. Their comment that the increase in billing for NP and PA services results in a “reduction of the proportion of emergency physicians” speaks volumes.1 Perhaps there is more concern here about ED physician job security than about independent billing!
Regardless of the intention by Bai et al—and acknowledging that the analysis they presented is somewhat interesting—I see two missed opportunities to “actionalize” the data.2 One is to use the information to identify whether a problem with billing exists (ie, is there upcharging as a result of more details contained within the electronic health record?). The second is to use the data to investigate innovative ways to improve access to care across the continuum. Essentially, how do we use the results of any data analysis in a way that can be useful? That is the real challenge.
Continue to: The biggest conclusion I've drawn...
The biggest conclusion I’ve drawn from my exploration of these study findings? The opportunity to investigate the competencies of all ED providers, with the goal of improving access and controlling costs, is there. And as the NPs and PAs providing the care, we should undertake the next research study or data analysis and not leave the research on us to other professions!
I’d love to hear your thoughts on the Bai et al study or any aspect of this 4-part discussion! Drop me a line at [email protected].
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. The Wharton School at the University of Pennsylvania. Big data’s biggest challenge: how to avoid getting lost in the weeds. Knowledge@Wharton podcast. March 14, 2019. http://knowledge.wharton.upenn.edu/article/data-analytics-challenges. Accessed April 1, 2019.
I have been ruminating about the Bai et al article on independent billing in the emergency department (ED) for weeks.1 I keep wondering why the data analysis seems so off base. Don’t get me wrong: The data gathered from Medicare is what it is—but a key piece of information is not present in the pure numbers input to the Medicare database.
So, I continued to probe this study with my colleagues. To a person, their comments supported that the intent of the study is unclear. The authors posit their objective to be an examination of the “involvement of NPs and PAs” in emergency services, using billing data. But to use billing data as a measure of “involvement” does not tell the whole story.
Independence in billing does not mean that the care NPs and PAs are providing is “beyond their scope of practice.” Moreover, the billing does not capture whether, or to what extent, physician consultation or assistance was involved. If the NP or PA dictated the chart, then they are by default the “only” (independent) provider. However, billing independently does not mean a physician (or other provider) was not consulted about the plan of care.
Case in point: Years ago, I had a young woman present to the ED with a sore throat. Her presenting complaint was a symptom of a peritonsillar abscess. So I phoned an ENT colleague (a physician) and asked him about the best treatment and follow-up in this case. Did he make a note in or sign the chart? No. Was I the only provider of record? Yes. Was that care “independent,” if you only look at the billing (done by a coder, for the record)? Yes.
Admittedly, Bai and colleagues do add in their conclusion that “independence in billing … does not necessarily indicate [NPs’/PAs’] independence in care delivery.”1 And they do note that the true challenge in the ED is determining how best to “blend” the expertise of the three professions (MD, NP, and PA) to provide efficient and cost-effective care.
However, throughout the article, there is an underpinning of inference that NPs and PAs are potentially practicing beyond their scope. Their comment that the increase in billing for NP and PA services results in a “reduction of the proportion of emergency physicians” speaks volumes.1 Perhaps there is more concern here about ED physician job security than about independent billing!
Regardless of the intention by Bai et al—and acknowledging that the analysis they presented is somewhat interesting—I see two missed opportunities to “actionalize” the data.2 One is to use the information to identify whether a problem with billing exists (ie, is there upcharging as a result of more details contained within the electronic health record?). The second is to use the data to investigate innovative ways to improve access to care across the continuum. Essentially, how do we use the results of any data analysis in a way that can be useful? That is the real challenge.
Continue to: The biggest conclusion I've drawn...
The biggest conclusion I’ve drawn from my exploration of these study findings? The opportunity to investigate the competencies of all ED providers, with the goal of improving access and controlling costs, is there. And as the NPs and PAs providing the care, we should undertake the next research study or data analysis and not leave the research on us to other professions!
I’d love to hear your thoughts on the Bai et al study or any aspect of this 4-part discussion! Drop me a line at [email protected].
I have been ruminating about the Bai et al article on independent billing in the emergency department (ED) for weeks.1 I keep wondering why the data analysis seems so off base. Don’t get me wrong: The data gathered from Medicare is what it is—but a key piece of information is not present in the pure numbers input to the Medicare database.
So, I continued to probe this study with my colleagues. To a person, their comments supported that the intent of the study is unclear. The authors posit their objective to be an examination of the “involvement of NPs and PAs” in emergency services, using billing data. But to use billing data as a measure of “involvement” does not tell the whole story.
Independence in billing does not mean that the care NPs and PAs are providing is “beyond their scope of practice.” Moreover, the billing does not capture whether, or to what extent, physician consultation or assistance was involved. If the NP or PA dictated the chart, then they are by default the “only” (independent) provider. However, billing independently does not mean a physician (or other provider) was not consulted about the plan of care.
Case in point: Years ago, I had a young woman present to the ED with a sore throat. Her presenting complaint was a symptom of a peritonsillar abscess. So I phoned an ENT colleague (a physician) and asked him about the best treatment and follow-up in this case. Did he make a note in or sign the chart? No. Was I the only provider of record? Yes. Was that care “independent,” if you only look at the billing (done by a coder, for the record)? Yes.
Admittedly, Bai and colleagues do add in their conclusion that “independence in billing … does not necessarily indicate [NPs’/PAs’] independence in care delivery.”1 And they do note that the true challenge in the ED is determining how best to “blend” the expertise of the three professions (MD, NP, and PA) to provide efficient and cost-effective care.
However, throughout the article, there is an underpinning of inference that NPs and PAs are potentially practicing beyond their scope. Their comment that the increase in billing for NP and PA services results in a “reduction of the proportion of emergency physicians” speaks volumes.1 Perhaps there is more concern here about ED physician job security than about independent billing!
Regardless of the intention by Bai et al—and acknowledging that the analysis they presented is somewhat interesting—I see two missed opportunities to “actionalize” the data.2 One is to use the information to identify whether a problem with billing exists (ie, is there upcharging as a result of more details contained within the electronic health record?). The second is to use the data to investigate innovative ways to improve access to care across the continuum. Essentially, how do we use the results of any data analysis in a way that can be useful? That is the real challenge.
Continue to: The biggest conclusion I've drawn...
The biggest conclusion I’ve drawn from my exploration of these study findings? The opportunity to investigate the competencies of all ED providers, with the goal of improving access and controlling costs, is there. And as the NPs and PAs providing the care, we should undertake the next research study or data analysis and not leave the research on us to other professions!
I’d love to hear your thoughts on the Bai et al study or any aspect of this 4-part discussion! Drop me a line at [email protected].
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. The Wharton School at the University of Pennsylvania. Big data’s biggest challenge: how to avoid getting lost in the weeds. Knowledge@Wharton podcast. March 14, 2019. http://knowledge.wharton.upenn.edu/article/data-analytics-challenges. Accessed April 1, 2019.
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. The Wharton School at the University of Pennsylvania. Big data’s biggest challenge: how to avoid getting lost in the weeds. Knowledge@Wharton podcast. March 14, 2019. http://knowledge.wharton.upenn.edu/article/data-analytics-challenges. Accessed April 1, 2019.
Suicide barriers on the Golden Gate Bridge: Will they save lives?
Ultimately, we need to find better treatments for depression and anxiety
San Francisco entrances people. Photographers capture more images of the Golden Gate Bridge than any other bridge in the world.1 And only the Nanjing Yangtze River Bridge in China surpasses the Golden Gate as a destination for dying by suicide.2 At least 1,700 people reportedly have plunged from the bridge to their deaths since its opening in 1937.3
Despite concerted efforts by bridge security, the local mental health community, and a volunteer organization – Bridgewatch Angels – suicides continue at the pace of about 1 every 2 weeks. After more than 60 years of discussion, transportation officials allocated funding and have started building a suicide prevention barrier system on the Golden Gate.
Extrapolating from the success of barriers built on other bridges that were “suicide magnets,” we should be able to assure people that suicide deaths from the Golden Gate will dramatically decrease, and perhaps cease completely.4 Certainly, some in the mental health community think this barrier will save lives. They support this claim by citing research showing that removing highly accessible and lethal means of suicide reduces overall suicide rates, and that suicidal individuals, when thwarted, do not seek alternate modes of death.
I support building the Golden Gate suicide barrier, partly because symbolically, it should deliver a powerful message that we value all human life. But will the barrier save lives? I don’t think it will. As the American Psychiatric Association prepares to gather for its annual meeting in San Francisco, I would like to share my reasoning.
What the evidence shows
The most robust evidence that restricting availability of highly lethal and accessible means of suicide reduces overall suicide deaths comes from studies looking at self-poisoning in Asian countries and Great Britain. In many parts of Asia, ingestion of pesticides constitutes a significant proportion of suicide deaths, and several studies have found that, in localities where sales of highly lethal pesticides were restricted, overall suicide deaths decreased.5,6 Conversely, suicide rates increased when more lethal varieties of pesticides became more available. In Great Britain, overall suicide rates decreased when natural gas replaced coal gas for home heating and cooking.7 For decades preceding this change, more Britons had killed themselves by inhaling coal gas than by any other method.
Strong correlations exist between regional levels of gun ownership and suicide rates by shooting,8 but several potentially confounding sociopolitical factors explain some portion of this connection. Stronger evidence of gun availability affecting suicide rates has been demonstrated by decreases in suicide rates after restrictions in gun access in Switzerland,9 Israel,10 and other areas. These studies show correlations – not causality. However, the number of studies, links between increases and decreases in suicide rates with changes in access to guns, absence of changes in suicide rates during the same time periods among ostensibly similar control populations, and lack of other compelling explanations support the argument that restricting access to highly lethal and accessible means of suicide prevents suicide deaths overall.
The installation of suicide barriers on bridges that have been the sites of multiple suicides robustly reduces or even eliminates suicide deaths from those bridges,11 but the effect on overall suicide rates remains less clear. Various studies have found subsequent increases or no changes12-14 in suicide deaths from other bridges or tall buildings in the vicinity after the installation of suicide barriers on a “suicide magnet.” Many of the studies failed to find any impact on overall suicide rates in the regions investigated. Deaths from jumping off tall structures constitute a tiny proportion of total suicide deaths, making it difficult to detect any changes in overall suicide rates. In the United States, suicides by jumping/falling constituted 1%-2% of total suicides over the last several decades.15
If we know that restricting highly lethal and accessible methods of killing reduces suicide deaths, why would I question the value of the Golden Gate suicide barrier in preventing overall suicide deaths?
Unique aspects of the bridge
The World High Dive Federation recommends keeping dives to less than 20 meters (65.5 feet), with a few exceptions.16 The rail of the Golden Gate Bridge stands 67 meters (220 feet) above the water, and assuming minimal wind resistance, a falling person traverses that distance in about 3.7 seconds and lands with an impact of 130 km/hour (81 miles per hour).17 Only about 1%-2% of those jumping from the Golden Gate survive that fall.18
A 99% likelihood of death sounds pretty lethal; however, death by jumping from the Golden Gate inherently takes place in a public space, with the opportunity for interventions by other people. A more realistic calculation of the lethality would start the instant that someone initiates a sequence of behaviors leading to the intended death. By that criteria, measuring the lethality of the Golden Gate would begin when an individual enters a vehicle or sets off on foot with the plan of going over the railing.
Unless our surveillance-oriented society makes substantially greater advances (which I oppose), we will remain unable to assess suicide lethality by starting at the moment of inception. However, we do have data showing what happens once someone with suicidal intentions walks onto the bridge.
Between 2000 and 2018, observers noted 2,546 people on the Golden Gate who appeared to be considering a suicide attempt, the San Francisco Chronicle has reported. Five hundred sixty-four confirmed suicides occurred. In an additional 71 cases, suicide is presumed but bodies were not recovered. In the 1,911 remaining instances, mental health interventions were made, with individuals taken to local hospitals and psychiatric wards, and released when no longer overtly suicidal. Interventions successfully diverted 75% (1,911/2,546) of those intending to end their own lives, which suggests that the current lethality of the Golden Gate as a means of suicide is only 25%. Even in the bridge’s first half-century, without constant camera monitoring, and a cadre of volunteers and professionals scanning for those attempting suicide, the lethality rate approached about 50%.19
We face even more difficulties measuring accessibility than in determining lethality. The Golden Gate appears to be accessible to almost anyone – drivers have to pay a toll only when traveling from the north, and then only after they have traversed the span. Pedestrians retain unfettered admittance to the east sidewalk (facing San Francisco city and bay) throughout daylight hours. But any determination of accessibility must include how quickly and easily one can make use of an opportunity.
Both entrances to the Golden Gate are embedded in the Golden Gate National Recreational Area, part of the National Park system. The south entrance to the bridge arises from The Presidio, a former military installation that housed about 4,000 people.20 Even fewer people live in the parklands at the north end of the bridge. The Presidio extends far enough so that the closest San Francisco neighborhoods outside of the park are a full 2.2 km (1.36 miles) from the bridge railing. A brisk walk would still require a minimum of about 20 minutes to get to the bridge; it is difficult to arrive at the bridge without a trek.
Researchers define impulsivity, like accessibility, inconsistently – and often imprecisely. Impulsivity, which clearly exists on a spectrum, connotes overvaluing of immediate feelings and thoughts at the expense of longer term goals and aspirations. Some suicide research appears to define impulsivity as the antithesis of planned behavior;21,22 others define it pragmatically as behaviors executed within 5 minutes of a decision,23 and still others contend that “suicidal behavior is rarely if ever impulsive.”24 Furthermore, when we assess impulsivity, we must acknowledge a fundamental difference between “impulsive” shootings and poisonings that are accomplished at home and within seconds or minutes, from “impulsive” Golden Gate Bridge suicide attempts, which require substantial travel and time commitments, and inherently involve the potential for others to intervene.
Those arguing that the bridge suicide barrier will save lives often bring up two additional sets of numbers to back up their assertions. They provide evidence that most of those people who were stopped in their attempts at suicide at the Golden Gate do not go on to commit suicide elsewhere, and that many of those who survived their attempts express regret at having tried to kill themselves. Specifically, 94% of those who were prevented from jumping from the Golden Gate had not committed suicide after a median follow-up of 26 years, according to a follow-up study published a few years ago. On the other hand, those who have made a serious suicide attempt have a substantially increased risk, relative to the general population, of dying from a later attempt,25,26 and the strongest predictor for death by suicide is having made a previous, serious suicide attempt.27
While all of these studies provide important and interesting information regarding suicide, none directly address the question of whether individuals will substitute attempts by other methods if the Golden Gate Bridge were no longer available. Many discussions blur the distinction between how individuals behave after a thwarted Golden Gate suicide attempt and how other people might act if we secured the bridge from any potential future suicide attempts. I hope that the following analogy makes this distinction clearer without trivializing: Imagine that we know that everyone who was interrupted while eating their dinner in a particular restaurant never went back and ate out anywhere, ever again. We could not conclude from this that another individual, who learned that the intended restaurant was indefinitely closed, would never dine out again. Once effective suicide barriers exist on the Golden Gate, this will likely become widely known, thereby greatly reducing the likelihood that any individuals will consider the possibility of jumping from the bridge. But it seems very unlikely that this would vanquish all suicidal impulses from the northern California population.
Lessons from patients
Two former patients of mine ended their lives by suicide from the Golden Gate. P, a solitary and lonely man in his 50s, was referred to me by his neighbor, Q, one of my long-term patients. P had a history of repeated assessments for lifelong depression, with minimal follow-up. I made a treatment plan with P that we hoped would address both his depression and his reluctance to engage with mental health professionals. He did not return for his follow-up appointment and ignored all my attempts to contact him.
P continued to have intermittent contact with Q. A decade after I had evaluated him, P was finally hospitalized for depression. Since P had no local family or friends, he asked Q to pick him up from the hospital at the time of his discharge. P asked Q to drive him to the Golden Gate Bridge, ostensibly to relish his release by partaking of the panoramic view of San Francisco from the bridge. They parked in the lot at the north end of the bridge, where Q stayed with the car at the vista point. The last that anyone saw of P was when Q noticed him walking on the bridge; nobody saw him go over and his body was not recovered.
In contrast to my brief connection with P, I worked with S over the course of 8 years to deal with her very severe attention-deficit/hyperactivity disorder and associated depression, which destroyed jobs and friendships, and estranged her from her family. She moved to Hawaii in hopes of “starting over with less baggage,” but I received a few phone calls over the next few years detailing suicide attempts, including driving her car off a bridge. Floundering in life, she returned to San Francisco and was hospitalized with suicidal ideation. The inpatient team sedated her heavily, ignored her past treatments and diagnoses, and discharged her after several days. Within a day of discharge, S’s sister called to say that S’s body had been recovered from the water below the bridge.
I don’t think that suicide was inevitable for either P or S, but I also lack any indication that either would be alive today had we installed suicide barriers on the Golden Gate years ago. Unless we eliminate access to guns, cars, trains, poisons, ropes, tall buildings and cliffs, people contemplating suicide will have numerous options at their disposal. We are likely to save lives by continuing to find ways to restrict access to means of death that can be used within seconds and have a high degree of lethality, and we should persist with such efforts. Buying a $5 trigger lock for every gun in California, and spending tens of millions on a public service campaign would cost less and may well save more lives than the Golden Gate suicide barrier. Unfortunately, we still possess very limited knowledge regarding which suicide prevention measures have an “impact on actual deaths or behavior.”28
To increase our efficacy in reducing suicide, we need to find better treatments for depression and anxiety. We also need to identify better ways of targeting those most at risk for suicide,29 improve our delivery of such treatments, and mitigate the social factors that contribute to such misery and unhappiness.
As a psychiatrist who has lost not only patients but also family members to suicide, I appreciate the hole in the soul these deaths create. I understand the drive to find ways to prevent additional deaths and save future survivors from such grief. But we must design psychiatric interventions that do the maximum good. To be imprecise in the lessons we learn from those who have killed themselves doubles down on the disservice to those lives already lost.
Dr. Kruse is a psychiatrist who practices in San Francisco. Several key details about the patients were changed to protect confidentiality.
References
1. Frommer’s Comprehensive Travel Guide, California. New York: Prentice Hall Travel, 1993.
2. “Chen Si, the ‘Angel of Nanjing,’ has saved more than 330 people from suicide,” by Matt Young, News.com.au. May 14, 2017.
3. “Finding Kyle,” by Lizzie Johnson, San Francisco Chronicle. Feb 8, 2019.
4. Beautrais A. Suicide by jumping. A review of research and prevention strategies. Crisis. 2007 Jan;28 Suppl 1:58-63. Crisis: The J of Crisis Interven Suicide Preven. 2007 Jan. (28)[Suppl1]:58-63.
5. Gunnell D et al. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007 Dec. 21;7:357.
6. Vijayakumar L and Satheesh-Babu R. Does ‘no pesticide’ reduce suicides? Int J Soc Psychiatry. 2009 Jul 17;55:401-6.
7. Kreitman N. The coal gas story. United Kingdom suicide rates, 1960-71. Br J Prev Soc Med. 1976 Jun;30(2)86-93.
8. Ajdacic-Gross V et al. Changing times: A longitudinal analysis of international firearm suicide data. Am J Public Health. 2006 Oct;96(10):1752-5.
9. Reisch T et al. Change in suicide rates in Switzerland before and after firearm restriction resulting from the 2003 “Army XXI” reform. Am J Psychiatry. 2013 Sep170(9):977-84.
10. Lubin G et al. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: A naturalistic epidemiological study. Suicide Life Threat Behav. 2010 Oct;40(5):421-4.
11. Sinyor M and Levitt A. Effect of a barrier at Bloor Street Viaduct on suicide rates in Toronto: Natural experiment BMJ. 2010;341. doi: 1136/bmjc2884.
12. O’Carroll P and Silverman M. Community suicide prevention: The effectiveness of bridge barriers. Suicide Life Threat Behav. 1994 Spring;24(1):89-91; discussion 91-9.
13. Pelletier A. Preventing suicide by jumping: The effect of a bridge safety fence. Inj Prev. 2007 Feb;13(1):57-9.
14. Bennewith O et al. Effect of barriers on the Clifton suspension bridge, England, on local patterns of suicide: Implications for prevention. Br J Psychiatry. 2007 Mar;190:266-7.
15. Harvard T.H. Chan School of Public Health. 2004. “How do people most commonly complete suicide?”
16. “How cliff diving works,” by Heather Kolich, HowStuffWorks.com. Oct 5, 2009.
17. “Bridge design and construction statistics.” Goldengate.org
18. “How did teen survive fall from Golden Gate Bridge?” by Remy Molina, Live Science. Apr 19, 2011.
19. Seiden R. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978 Winter;8(4):203-16.
20. Presidio demographics. Point2homes.com.
21. Baca-García E et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001 Jul;62(7):560-4.
22. Lim M et al. Differences between impulsive and non-impulsive suicide attempts among individuals treated in emergency rooms of South Korea. Psychiatry Investig. 2016 Jul;13(4):389-96.
23. Simon O et al. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(1 Suppl):49-59.
24. Anestis M et al. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014 Nov;18(4):366-86.
25. Ostamo A et al. Excess mortality of suicide attempters. Psychiatry Psychiatr Epidemiol. 2001 Jan;36(1):29-35.
26. Leon A et al. Statistical issues in the identification of risk factors for suicidal behavior: The application of survival analysis. Psychiatry Res. 1990 Jan;31(1):99-108.
27. Bostwick J et al. Suicide attempt as a risk factor for completed suicide: Even more lethal than we knew. Am J Psychiatry. 2016 Nov 1;173(11):1094-100.
28. Stone D and Crosby A. Suicide prevention. Am J Lifestyle Med. 2014;8(6):404-20.
29. Belsher B et al. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. doi: 10.1001/jamapsychiatry.2019.0174.
Ultimately, we need to find better treatments for depression and anxiety
Ultimately, we need to find better treatments for depression and anxiety
San Francisco entrances people. Photographers capture more images of the Golden Gate Bridge than any other bridge in the world.1 And only the Nanjing Yangtze River Bridge in China surpasses the Golden Gate as a destination for dying by suicide.2 At least 1,700 people reportedly have plunged from the bridge to their deaths since its opening in 1937.3
Despite concerted efforts by bridge security, the local mental health community, and a volunteer organization – Bridgewatch Angels – suicides continue at the pace of about 1 every 2 weeks. After more than 60 years of discussion, transportation officials allocated funding and have started building a suicide prevention barrier system on the Golden Gate.
Extrapolating from the success of barriers built on other bridges that were “suicide magnets,” we should be able to assure people that suicide deaths from the Golden Gate will dramatically decrease, and perhaps cease completely.4 Certainly, some in the mental health community think this barrier will save lives. They support this claim by citing research showing that removing highly accessible and lethal means of suicide reduces overall suicide rates, and that suicidal individuals, when thwarted, do not seek alternate modes of death.
I support building the Golden Gate suicide barrier, partly because symbolically, it should deliver a powerful message that we value all human life. But will the barrier save lives? I don’t think it will. As the American Psychiatric Association prepares to gather for its annual meeting in San Francisco, I would like to share my reasoning.
What the evidence shows
The most robust evidence that restricting availability of highly lethal and accessible means of suicide reduces overall suicide deaths comes from studies looking at self-poisoning in Asian countries and Great Britain. In many parts of Asia, ingestion of pesticides constitutes a significant proportion of suicide deaths, and several studies have found that, in localities where sales of highly lethal pesticides were restricted, overall suicide deaths decreased.5,6 Conversely, suicide rates increased when more lethal varieties of pesticides became more available. In Great Britain, overall suicide rates decreased when natural gas replaced coal gas for home heating and cooking.7 For decades preceding this change, more Britons had killed themselves by inhaling coal gas than by any other method.
Strong correlations exist between regional levels of gun ownership and suicide rates by shooting,8 but several potentially confounding sociopolitical factors explain some portion of this connection. Stronger evidence of gun availability affecting suicide rates has been demonstrated by decreases in suicide rates after restrictions in gun access in Switzerland,9 Israel,10 and other areas. These studies show correlations – not causality. However, the number of studies, links between increases and decreases in suicide rates with changes in access to guns, absence of changes in suicide rates during the same time periods among ostensibly similar control populations, and lack of other compelling explanations support the argument that restricting access to highly lethal and accessible means of suicide prevents suicide deaths overall.
The installation of suicide barriers on bridges that have been the sites of multiple suicides robustly reduces or even eliminates suicide deaths from those bridges,11 but the effect on overall suicide rates remains less clear. Various studies have found subsequent increases or no changes12-14 in suicide deaths from other bridges or tall buildings in the vicinity after the installation of suicide barriers on a “suicide magnet.” Many of the studies failed to find any impact on overall suicide rates in the regions investigated. Deaths from jumping off tall structures constitute a tiny proportion of total suicide deaths, making it difficult to detect any changes in overall suicide rates. In the United States, suicides by jumping/falling constituted 1%-2% of total suicides over the last several decades.15
If we know that restricting highly lethal and accessible methods of killing reduces suicide deaths, why would I question the value of the Golden Gate suicide barrier in preventing overall suicide deaths?
Unique aspects of the bridge
The World High Dive Federation recommends keeping dives to less than 20 meters (65.5 feet), with a few exceptions.16 The rail of the Golden Gate Bridge stands 67 meters (220 feet) above the water, and assuming minimal wind resistance, a falling person traverses that distance in about 3.7 seconds and lands with an impact of 130 km/hour (81 miles per hour).17 Only about 1%-2% of those jumping from the Golden Gate survive that fall.18
A 99% likelihood of death sounds pretty lethal; however, death by jumping from the Golden Gate inherently takes place in a public space, with the opportunity for interventions by other people. A more realistic calculation of the lethality would start the instant that someone initiates a sequence of behaviors leading to the intended death. By that criteria, measuring the lethality of the Golden Gate would begin when an individual enters a vehicle or sets off on foot with the plan of going over the railing.
Unless our surveillance-oriented society makes substantially greater advances (which I oppose), we will remain unable to assess suicide lethality by starting at the moment of inception. However, we do have data showing what happens once someone with suicidal intentions walks onto the bridge.
Between 2000 and 2018, observers noted 2,546 people on the Golden Gate who appeared to be considering a suicide attempt, the San Francisco Chronicle has reported. Five hundred sixty-four confirmed suicides occurred. In an additional 71 cases, suicide is presumed but bodies were not recovered. In the 1,911 remaining instances, mental health interventions were made, with individuals taken to local hospitals and psychiatric wards, and released when no longer overtly suicidal. Interventions successfully diverted 75% (1,911/2,546) of those intending to end their own lives, which suggests that the current lethality of the Golden Gate as a means of suicide is only 25%. Even in the bridge’s first half-century, without constant camera monitoring, and a cadre of volunteers and professionals scanning for those attempting suicide, the lethality rate approached about 50%.19
We face even more difficulties measuring accessibility than in determining lethality. The Golden Gate appears to be accessible to almost anyone – drivers have to pay a toll only when traveling from the north, and then only after they have traversed the span. Pedestrians retain unfettered admittance to the east sidewalk (facing San Francisco city and bay) throughout daylight hours. But any determination of accessibility must include how quickly and easily one can make use of an opportunity.
Both entrances to the Golden Gate are embedded in the Golden Gate National Recreational Area, part of the National Park system. The south entrance to the bridge arises from The Presidio, a former military installation that housed about 4,000 people.20 Even fewer people live in the parklands at the north end of the bridge. The Presidio extends far enough so that the closest San Francisco neighborhoods outside of the park are a full 2.2 km (1.36 miles) from the bridge railing. A brisk walk would still require a minimum of about 20 minutes to get to the bridge; it is difficult to arrive at the bridge without a trek.
Researchers define impulsivity, like accessibility, inconsistently – and often imprecisely. Impulsivity, which clearly exists on a spectrum, connotes overvaluing of immediate feelings and thoughts at the expense of longer term goals and aspirations. Some suicide research appears to define impulsivity as the antithesis of planned behavior;21,22 others define it pragmatically as behaviors executed within 5 minutes of a decision,23 and still others contend that “suicidal behavior is rarely if ever impulsive.”24 Furthermore, when we assess impulsivity, we must acknowledge a fundamental difference between “impulsive” shootings and poisonings that are accomplished at home and within seconds or minutes, from “impulsive” Golden Gate Bridge suicide attempts, which require substantial travel and time commitments, and inherently involve the potential for others to intervene.
Those arguing that the bridge suicide barrier will save lives often bring up two additional sets of numbers to back up their assertions. They provide evidence that most of those people who were stopped in their attempts at suicide at the Golden Gate do not go on to commit suicide elsewhere, and that many of those who survived their attempts express regret at having tried to kill themselves. Specifically, 94% of those who were prevented from jumping from the Golden Gate had not committed suicide after a median follow-up of 26 years, according to a follow-up study published a few years ago. On the other hand, those who have made a serious suicide attempt have a substantially increased risk, relative to the general population, of dying from a later attempt,25,26 and the strongest predictor for death by suicide is having made a previous, serious suicide attempt.27
While all of these studies provide important and interesting information regarding suicide, none directly address the question of whether individuals will substitute attempts by other methods if the Golden Gate Bridge were no longer available. Many discussions blur the distinction between how individuals behave after a thwarted Golden Gate suicide attempt and how other people might act if we secured the bridge from any potential future suicide attempts. I hope that the following analogy makes this distinction clearer without trivializing: Imagine that we know that everyone who was interrupted while eating their dinner in a particular restaurant never went back and ate out anywhere, ever again. We could not conclude from this that another individual, who learned that the intended restaurant was indefinitely closed, would never dine out again. Once effective suicide barriers exist on the Golden Gate, this will likely become widely known, thereby greatly reducing the likelihood that any individuals will consider the possibility of jumping from the bridge. But it seems very unlikely that this would vanquish all suicidal impulses from the northern California population.
Lessons from patients
Two former patients of mine ended their lives by suicide from the Golden Gate. P, a solitary and lonely man in his 50s, was referred to me by his neighbor, Q, one of my long-term patients. P had a history of repeated assessments for lifelong depression, with minimal follow-up. I made a treatment plan with P that we hoped would address both his depression and his reluctance to engage with mental health professionals. He did not return for his follow-up appointment and ignored all my attempts to contact him.
P continued to have intermittent contact with Q. A decade after I had evaluated him, P was finally hospitalized for depression. Since P had no local family or friends, he asked Q to pick him up from the hospital at the time of his discharge. P asked Q to drive him to the Golden Gate Bridge, ostensibly to relish his release by partaking of the panoramic view of San Francisco from the bridge. They parked in the lot at the north end of the bridge, where Q stayed with the car at the vista point. The last that anyone saw of P was when Q noticed him walking on the bridge; nobody saw him go over and his body was not recovered.
In contrast to my brief connection with P, I worked with S over the course of 8 years to deal with her very severe attention-deficit/hyperactivity disorder and associated depression, which destroyed jobs and friendships, and estranged her from her family. She moved to Hawaii in hopes of “starting over with less baggage,” but I received a few phone calls over the next few years detailing suicide attempts, including driving her car off a bridge. Floundering in life, she returned to San Francisco and was hospitalized with suicidal ideation. The inpatient team sedated her heavily, ignored her past treatments and diagnoses, and discharged her after several days. Within a day of discharge, S’s sister called to say that S’s body had been recovered from the water below the bridge.
I don’t think that suicide was inevitable for either P or S, but I also lack any indication that either would be alive today had we installed suicide barriers on the Golden Gate years ago. Unless we eliminate access to guns, cars, trains, poisons, ropes, tall buildings and cliffs, people contemplating suicide will have numerous options at their disposal. We are likely to save lives by continuing to find ways to restrict access to means of death that can be used within seconds and have a high degree of lethality, and we should persist with such efforts. Buying a $5 trigger lock for every gun in California, and spending tens of millions on a public service campaign would cost less and may well save more lives than the Golden Gate suicide barrier. Unfortunately, we still possess very limited knowledge regarding which suicide prevention measures have an “impact on actual deaths or behavior.”28
To increase our efficacy in reducing suicide, we need to find better treatments for depression and anxiety. We also need to identify better ways of targeting those most at risk for suicide,29 improve our delivery of such treatments, and mitigate the social factors that contribute to such misery and unhappiness.
As a psychiatrist who has lost not only patients but also family members to suicide, I appreciate the hole in the soul these deaths create. I understand the drive to find ways to prevent additional deaths and save future survivors from such grief. But we must design psychiatric interventions that do the maximum good. To be imprecise in the lessons we learn from those who have killed themselves doubles down on the disservice to those lives already lost.
Dr. Kruse is a psychiatrist who practices in San Francisco. Several key details about the patients were changed to protect confidentiality.
References
1. Frommer’s Comprehensive Travel Guide, California. New York: Prentice Hall Travel, 1993.
2. “Chen Si, the ‘Angel of Nanjing,’ has saved more than 330 people from suicide,” by Matt Young, News.com.au. May 14, 2017.
3. “Finding Kyle,” by Lizzie Johnson, San Francisco Chronicle. Feb 8, 2019.
4. Beautrais A. Suicide by jumping. A review of research and prevention strategies. Crisis. 2007 Jan;28 Suppl 1:58-63. Crisis: The J of Crisis Interven Suicide Preven. 2007 Jan. (28)[Suppl1]:58-63.
5. Gunnell D et al. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007 Dec. 21;7:357.
6. Vijayakumar L and Satheesh-Babu R. Does ‘no pesticide’ reduce suicides? Int J Soc Psychiatry. 2009 Jul 17;55:401-6.
7. Kreitman N. The coal gas story. United Kingdom suicide rates, 1960-71. Br J Prev Soc Med. 1976 Jun;30(2)86-93.
8. Ajdacic-Gross V et al. Changing times: A longitudinal analysis of international firearm suicide data. Am J Public Health. 2006 Oct;96(10):1752-5.
9. Reisch T et al. Change in suicide rates in Switzerland before and after firearm restriction resulting from the 2003 “Army XXI” reform. Am J Psychiatry. 2013 Sep170(9):977-84.
10. Lubin G et al. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: A naturalistic epidemiological study. Suicide Life Threat Behav. 2010 Oct;40(5):421-4.
11. Sinyor M and Levitt A. Effect of a barrier at Bloor Street Viaduct on suicide rates in Toronto: Natural experiment BMJ. 2010;341. doi: 1136/bmjc2884.
12. O’Carroll P and Silverman M. Community suicide prevention: The effectiveness of bridge barriers. Suicide Life Threat Behav. 1994 Spring;24(1):89-91; discussion 91-9.
13. Pelletier A. Preventing suicide by jumping: The effect of a bridge safety fence. Inj Prev. 2007 Feb;13(1):57-9.
14. Bennewith O et al. Effect of barriers on the Clifton suspension bridge, England, on local patterns of suicide: Implications for prevention. Br J Psychiatry. 2007 Mar;190:266-7.
15. Harvard T.H. Chan School of Public Health. 2004. “How do people most commonly complete suicide?”
16. “How cliff diving works,” by Heather Kolich, HowStuffWorks.com. Oct 5, 2009.
17. “Bridge design and construction statistics.” Goldengate.org
18. “How did teen survive fall from Golden Gate Bridge?” by Remy Molina, Live Science. Apr 19, 2011.
19. Seiden R. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978 Winter;8(4):203-16.
20. Presidio demographics. Point2homes.com.
21. Baca-García E et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001 Jul;62(7):560-4.
22. Lim M et al. Differences between impulsive and non-impulsive suicide attempts among individuals treated in emergency rooms of South Korea. Psychiatry Investig. 2016 Jul;13(4):389-96.
23. Simon O et al. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(1 Suppl):49-59.
24. Anestis M et al. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014 Nov;18(4):366-86.
25. Ostamo A et al. Excess mortality of suicide attempters. Psychiatry Psychiatr Epidemiol. 2001 Jan;36(1):29-35.
26. Leon A et al. Statistical issues in the identification of risk factors for suicidal behavior: The application of survival analysis. Psychiatry Res. 1990 Jan;31(1):99-108.
27. Bostwick J et al. Suicide attempt as a risk factor for completed suicide: Even more lethal than we knew. Am J Psychiatry. 2016 Nov 1;173(11):1094-100.
28. Stone D and Crosby A. Suicide prevention. Am J Lifestyle Med. 2014;8(6):404-20.
29. Belsher B et al. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. doi: 10.1001/jamapsychiatry.2019.0174.
San Francisco entrances people. Photographers capture more images of the Golden Gate Bridge than any other bridge in the world.1 And only the Nanjing Yangtze River Bridge in China surpasses the Golden Gate as a destination for dying by suicide.2 At least 1,700 people reportedly have plunged from the bridge to their deaths since its opening in 1937.3
Despite concerted efforts by bridge security, the local mental health community, and a volunteer organization – Bridgewatch Angels – suicides continue at the pace of about 1 every 2 weeks. After more than 60 years of discussion, transportation officials allocated funding and have started building a suicide prevention barrier system on the Golden Gate.
Extrapolating from the success of barriers built on other bridges that were “suicide magnets,” we should be able to assure people that suicide deaths from the Golden Gate will dramatically decrease, and perhaps cease completely.4 Certainly, some in the mental health community think this barrier will save lives. They support this claim by citing research showing that removing highly accessible and lethal means of suicide reduces overall suicide rates, and that suicidal individuals, when thwarted, do not seek alternate modes of death.
I support building the Golden Gate suicide barrier, partly because symbolically, it should deliver a powerful message that we value all human life. But will the barrier save lives? I don’t think it will. As the American Psychiatric Association prepares to gather for its annual meeting in San Francisco, I would like to share my reasoning.
What the evidence shows
The most robust evidence that restricting availability of highly lethal and accessible means of suicide reduces overall suicide deaths comes from studies looking at self-poisoning in Asian countries and Great Britain. In many parts of Asia, ingestion of pesticides constitutes a significant proportion of suicide deaths, and several studies have found that, in localities where sales of highly lethal pesticides were restricted, overall suicide deaths decreased.5,6 Conversely, suicide rates increased when more lethal varieties of pesticides became more available. In Great Britain, overall suicide rates decreased when natural gas replaced coal gas for home heating and cooking.7 For decades preceding this change, more Britons had killed themselves by inhaling coal gas than by any other method.
Strong correlations exist between regional levels of gun ownership and suicide rates by shooting,8 but several potentially confounding sociopolitical factors explain some portion of this connection. Stronger evidence of gun availability affecting suicide rates has been demonstrated by decreases in suicide rates after restrictions in gun access in Switzerland,9 Israel,10 and other areas. These studies show correlations – not causality. However, the number of studies, links between increases and decreases in suicide rates with changes in access to guns, absence of changes in suicide rates during the same time periods among ostensibly similar control populations, and lack of other compelling explanations support the argument that restricting access to highly lethal and accessible means of suicide prevents suicide deaths overall.
The installation of suicide barriers on bridges that have been the sites of multiple suicides robustly reduces or even eliminates suicide deaths from those bridges,11 but the effect on overall suicide rates remains less clear. Various studies have found subsequent increases or no changes12-14 in suicide deaths from other bridges or tall buildings in the vicinity after the installation of suicide barriers on a “suicide magnet.” Many of the studies failed to find any impact on overall suicide rates in the regions investigated. Deaths from jumping off tall structures constitute a tiny proportion of total suicide deaths, making it difficult to detect any changes in overall suicide rates. In the United States, suicides by jumping/falling constituted 1%-2% of total suicides over the last several decades.15
If we know that restricting highly lethal and accessible methods of killing reduces suicide deaths, why would I question the value of the Golden Gate suicide barrier in preventing overall suicide deaths?
Unique aspects of the bridge
The World High Dive Federation recommends keeping dives to less than 20 meters (65.5 feet), with a few exceptions.16 The rail of the Golden Gate Bridge stands 67 meters (220 feet) above the water, and assuming minimal wind resistance, a falling person traverses that distance in about 3.7 seconds and lands with an impact of 130 km/hour (81 miles per hour).17 Only about 1%-2% of those jumping from the Golden Gate survive that fall.18
A 99% likelihood of death sounds pretty lethal; however, death by jumping from the Golden Gate inherently takes place in a public space, with the opportunity for interventions by other people. A more realistic calculation of the lethality would start the instant that someone initiates a sequence of behaviors leading to the intended death. By that criteria, measuring the lethality of the Golden Gate would begin when an individual enters a vehicle or sets off on foot with the plan of going over the railing.
Unless our surveillance-oriented society makes substantially greater advances (which I oppose), we will remain unable to assess suicide lethality by starting at the moment of inception. However, we do have data showing what happens once someone with suicidal intentions walks onto the bridge.
Between 2000 and 2018, observers noted 2,546 people on the Golden Gate who appeared to be considering a suicide attempt, the San Francisco Chronicle has reported. Five hundred sixty-four confirmed suicides occurred. In an additional 71 cases, suicide is presumed but bodies were not recovered. In the 1,911 remaining instances, mental health interventions were made, with individuals taken to local hospitals and psychiatric wards, and released when no longer overtly suicidal. Interventions successfully diverted 75% (1,911/2,546) of those intending to end their own lives, which suggests that the current lethality of the Golden Gate as a means of suicide is only 25%. Even in the bridge’s first half-century, without constant camera monitoring, and a cadre of volunteers and professionals scanning for those attempting suicide, the lethality rate approached about 50%.19
We face even more difficulties measuring accessibility than in determining lethality. The Golden Gate appears to be accessible to almost anyone – drivers have to pay a toll only when traveling from the north, and then only after they have traversed the span. Pedestrians retain unfettered admittance to the east sidewalk (facing San Francisco city and bay) throughout daylight hours. But any determination of accessibility must include how quickly and easily one can make use of an opportunity.
Both entrances to the Golden Gate are embedded in the Golden Gate National Recreational Area, part of the National Park system. The south entrance to the bridge arises from The Presidio, a former military installation that housed about 4,000 people.20 Even fewer people live in the parklands at the north end of the bridge. The Presidio extends far enough so that the closest San Francisco neighborhoods outside of the park are a full 2.2 km (1.36 miles) from the bridge railing. A brisk walk would still require a minimum of about 20 minutes to get to the bridge; it is difficult to arrive at the bridge without a trek.
Researchers define impulsivity, like accessibility, inconsistently – and often imprecisely. Impulsivity, which clearly exists on a spectrum, connotes overvaluing of immediate feelings and thoughts at the expense of longer term goals and aspirations. Some suicide research appears to define impulsivity as the antithesis of planned behavior;21,22 others define it pragmatically as behaviors executed within 5 minutes of a decision,23 and still others contend that “suicidal behavior is rarely if ever impulsive.”24 Furthermore, when we assess impulsivity, we must acknowledge a fundamental difference between “impulsive” shootings and poisonings that are accomplished at home and within seconds or minutes, from “impulsive” Golden Gate Bridge suicide attempts, which require substantial travel and time commitments, and inherently involve the potential for others to intervene.
Those arguing that the bridge suicide barrier will save lives often bring up two additional sets of numbers to back up their assertions. They provide evidence that most of those people who were stopped in their attempts at suicide at the Golden Gate do not go on to commit suicide elsewhere, and that many of those who survived their attempts express regret at having tried to kill themselves. Specifically, 94% of those who were prevented from jumping from the Golden Gate had not committed suicide after a median follow-up of 26 years, according to a follow-up study published a few years ago. On the other hand, those who have made a serious suicide attempt have a substantially increased risk, relative to the general population, of dying from a later attempt,25,26 and the strongest predictor for death by suicide is having made a previous, serious suicide attempt.27
While all of these studies provide important and interesting information regarding suicide, none directly address the question of whether individuals will substitute attempts by other methods if the Golden Gate Bridge were no longer available. Many discussions blur the distinction between how individuals behave after a thwarted Golden Gate suicide attempt and how other people might act if we secured the bridge from any potential future suicide attempts. I hope that the following analogy makes this distinction clearer without trivializing: Imagine that we know that everyone who was interrupted while eating their dinner in a particular restaurant never went back and ate out anywhere, ever again. We could not conclude from this that another individual, who learned that the intended restaurant was indefinitely closed, would never dine out again. Once effective suicide barriers exist on the Golden Gate, this will likely become widely known, thereby greatly reducing the likelihood that any individuals will consider the possibility of jumping from the bridge. But it seems very unlikely that this would vanquish all suicidal impulses from the northern California population.
Lessons from patients
Two former patients of mine ended their lives by suicide from the Golden Gate. P, a solitary and lonely man in his 50s, was referred to me by his neighbor, Q, one of my long-term patients. P had a history of repeated assessments for lifelong depression, with minimal follow-up. I made a treatment plan with P that we hoped would address both his depression and his reluctance to engage with mental health professionals. He did not return for his follow-up appointment and ignored all my attempts to contact him.
P continued to have intermittent contact with Q. A decade after I had evaluated him, P was finally hospitalized for depression. Since P had no local family or friends, he asked Q to pick him up from the hospital at the time of his discharge. P asked Q to drive him to the Golden Gate Bridge, ostensibly to relish his release by partaking of the panoramic view of San Francisco from the bridge. They parked in the lot at the north end of the bridge, where Q stayed with the car at the vista point. The last that anyone saw of P was when Q noticed him walking on the bridge; nobody saw him go over and his body was not recovered.
In contrast to my brief connection with P, I worked with S over the course of 8 years to deal with her very severe attention-deficit/hyperactivity disorder and associated depression, which destroyed jobs and friendships, and estranged her from her family. She moved to Hawaii in hopes of “starting over with less baggage,” but I received a few phone calls over the next few years detailing suicide attempts, including driving her car off a bridge. Floundering in life, she returned to San Francisco and was hospitalized with suicidal ideation. The inpatient team sedated her heavily, ignored her past treatments and diagnoses, and discharged her after several days. Within a day of discharge, S’s sister called to say that S’s body had been recovered from the water below the bridge.
I don’t think that suicide was inevitable for either P or S, but I also lack any indication that either would be alive today had we installed suicide barriers on the Golden Gate years ago. Unless we eliminate access to guns, cars, trains, poisons, ropes, tall buildings and cliffs, people contemplating suicide will have numerous options at their disposal. We are likely to save lives by continuing to find ways to restrict access to means of death that can be used within seconds and have a high degree of lethality, and we should persist with such efforts. Buying a $5 trigger lock for every gun in California, and spending tens of millions on a public service campaign would cost less and may well save more lives than the Golden Gate suicide barrier. Unfortunately, we still possess very limited knowledge regarding which suicide prevention measures have an “impact on actual deaths or behavior.”28
To increase our efficacy in reducing suicide, we need to find better treatments for depression and anxiety. We also need to identify better ways of targeting those most at risk for suicide,29 improve our delivery of such treatments, and mitigate the social factors that contribute to such misery and unhappiness.
As a psychiatrist who has lost not only patients but also family members to suicide, I appreciate the hole in the soul these deaths create. I understand the drive to find ways to prevent additional deaths and save future survivors from such grief. But we must design psychiatric interventions that do the maximum good. To be imprecise in the lessons we learn from those who have killed themselves doubles down on the disservice to those lives already lost.
Dr. Kruse is a psychiatrist who practices in San Francisco. Several key details about the patients were changed to protect confidentiality.
References
1. Frommer’s Comprehensive Travel Guide, California. New York: Prentice Hall Travel, 1993.
2. “Chen Si, the ‘Angel of Nanjing,’ has saved more than 330 people from suicide,” by Matt Young, News.com.au. May 14, 2017.
3. “Finding Kyle,” by Lizzie Johnson, San Francisco Chronicle. Feb 8, 2019.
4. Beautrais A. Suicide by jumping. A review of research and prevention strategies. Crisis. 2007 Jan;28 Suppl 1:58-63. Crisis: The J of Crisis Interven Suicide Preven. 2007 Jan. (28)[Suppl1]:58-63.
5. Gunnell D et al. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007 Dec. 21;7:357.
6. Vijayakumar L and Satheesh-Babu R. Does ‘no pesticide’ reduce suicides? Int J Soc Psychiatry. 2009 Jul 17;55:401-6.
7. Kreitman N. The coal gas story. United Kingdom suicide rates, 1960-71. Br J Prev Soc Med. 1976 Jun;30(2)86-93.
8. Ajdacic-Gross V et al. Changing times: A longitudinal analysis of international firearm suicide data. Am J Public Health. 2006 Oct;96(10):1752-5.
9. Reisch T et al. Change in suicide rates in Switzerland before and after firearm restriction resulting from the 2003 “Army XXI” reform. Am J Psychiatry. 2013 Sep170(9):977-84.
10. Lubin G et al. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: A naturalistic epidemiological study. Suicide Life Threat Behav. 2010 Oct;40(5):421-4.
11. Sinyor M and Levitt A. Effect of a barrier at Bloor Street Viaduct on suicide rates in Toronto: Natural experiment BMJ. 2010;341. doi: 1136/bmjc2884.
12. O’Carroll P and Silverman M. Community suicide prevention: The effectiveness of bridge barriers. Suicide Life Threat Behav. 1994 Spring;24(1):89-91; discussion 91-9.
13. Pelletier A. Preventing suicide by jumping: The effect of a bridge safety fence. Inj Prev. 2007 Feb;13(1):57-9.
14. Bennewith O et al. Effect of barriers on the Clifton suspension bridge, England, on local patterns of suicide: Implications for prevention. Br J Psychiatry. 2007 Mar;190:266-7.
15. Harvard T.H. Chan School of Public Health. 2004. “How do people most commonly complete suicide?”
16. “How cliff diving works,” by Heather Kolich, HowStuffWorks.com. Oct 5, 2009.
17. “Bridge design and construction statistics.” Goldengate.org
18. “How did teen survive fall from Golden Gate Bridge?” by Remy Molina, Live Science. Apr 19, 2011.
19. Seiden R. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978 Winter;8(4):203-16.
20. Presidio demographics. Point2homes.com.
21. Baca-García E et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001 Jul;62(7):560-4.
22. Lim M et al. Differences between impulsive and non-impulsive suicide attempts among individuals treated in emergency rooms of South Korea. Psychiatry Investig. 2016 Jul;13(4):389-96.
23. Simon O et al. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(1 Suppl):49-59.
24. Anestis M et al. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014 Nov;18(4):366-86.
25. Ostamo A et al. Excess mortality of suicide attempters. Psychiatry Psychiatr Epidemiol. 2001 Jan;36(1):29-35.
26. Leon A et al. Statistical issues in the identification of risk factors for suicidal behavior: The application of survival analysis. Psychiatry Res. 1990 Jan;31(1):99-108.
27. Bostwick J et al. Suicide attempt as a risk factor for completed suicide: Even more lethal than we knew. Am J Psychiatry. 2016 Nov 1;173(11):1094-100.
28. Stone D and Crosby A. Suicide prevention. Am J Lifestyle Med. 2014;8(6):404-20.
29. Belsher B et al. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. doi: 10.1001/jamapsychiatry.2019.0174.
Make your evaluations and progress notes sing
I was talking to a physical therapy (PT) colleague and she was lamenting how much she hated doing documentation on patients she was treating. I suggested to her that she make her evaluations and progress notes sing. This is a concept I would sometimes use with patients who might be depressed, for example, I would ask them if anything made their heart sing to get an idea how “depressed” they might be. If they were unhappy or sad, I would advise that they engage in “heart singing” activities and behaviors, as I believe it is the “simple pleasures” in life that keep us resilient and persistent.
It is funny, when I was a resident and working in Jackson Park Hospital’s first psychiatric ward in 1972, one day in a note I wrote “I am going to give this acutely psychotic patient the big T – Thorazine to help them get some sleep at night,” I did not really think much about it until one of the nurses brought it to my attention because she thought it was unique – and a funny way of reporting plans in my progress notes.
My PT colleague told me that she remembered the first time she read one of my notes on a patient we were treating together (she needed to know the patient’s psychiatric status before she engaged them in physical therapy), and it struck her that I reported the patient was “befuddled,” and she wondered who would use befuddled in a note (lately, I have started using “flummoxed”). Another time, I was charting on a patient, and I used the word “flapdoodle” to describe the nonsense the patient was spewing (I recall this particular patient told me they graduated from grammar school at 5 years old). Another favorite word of mine that I use to describe nonsense is “claptrap.”
So, I have been making my evaluations and progress notes sing for a very long time, as doing so improves my writing skills, stimulates my thinking, turns the drudgery of charting into some fun, and creates an adventure in writing.
I have also been a big user of mental status templates to cut down my time. The essential elements of a mental status are in the narrative template, and all I need to do is to edit the verbiage in the template to fit the patient’s presentation so that the mental status sings. Early on, I understood that, to be a good psychiatrist, you needed a good vocabulary so you can speak with as much precision as possible when describing a patient’s mental status.
I was seeing many Alzheimer’s patients at one point. So I developed a special mental status template for them (female and male), so all I had to do to it was cut and paste, and then edit the template to fit the patient like a glove. Template example: This is a xx-year-old female who was appropriately groomed and who was cooperative with the interview, but she could not give much information. She was not hyperactive or lethargic. Her mood was bland, and her affect was flat and bland. Her speech did not contain any relevant information. Thought processes were not evident, although she was awake. I could not get a history of delusions or current auditory or visual hallucinations. Her thought content was nondescript. She was attentive, and her recent and remote memory were poor. Clinical estimate of her intelligence could not be determined. Her judgment and insight were poor. I could not determine whether there was any suicidal or homicidal ideation.
Formulation: This is a xx-year-old female who has a major neurocognitive disorder (formerly known as dementia). She is not overtly psychotic, suicidal, homicidal, or gravely disabled, but her level of functioning leaves a lot to be desired, which is why she needs a sheltered living circumstance.
Dx: Major neurocognitive disorder (formerly known as dementia).
Here’s another example: This is a xx-year-old male who was appropriately groomed and who was cooperative with the interview. He was not hyperactive or lethargic. His mood was euthymic and he had a wide range of affect as he was able to smile, get serious, and be sad (about xxx). His speech was relevant, linear, and goal directed. Thought processes did not show any signs of loose associations, tangentiality, or circumstantiality. He denies any delusions or current auditory or visual hallucinations. His thought content was surrounding xxx. He was attentive, and his recent and remote memory were intact. Clinical estimate of his intelligence was xxx average. His judgment and insight were poor as xxx. No report of suicidal or homicidal ideation.
Formulation: xxx. He is not overtly psychotic, suicidal, homicidal, or gravely disabled so I will clear him for psychiatric discharge.
Dx: xxx.
Just me trying to make work a little easier for myself and everyone else.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
I was talking to a physical therapy (PT) colleague and she was lamenting how much she hated doing documentation on patients she was treating. I suggested to her that she make her evaluations and progress notes sing. This is a concept I would sometimes use with patients who might be depressed, for example, I would ask them if anything made their heart sing to get an idea how “depressed” they might be. If they were unhappy or sad, I would advise that they engage in “heart singing” activities and behaviors, as I believe it is the “simple pleasures” in life that keep us resilient and persistent.
It is funny, when I was a resident and working in Jackson Park Hospital’s first psychiatric ward in 1972, one day in a note I wrote “I am going to give this acutely psychotic patient the big T – Thorazine to help them get some sleep at night,” I did not really think much about it until one of the nurses brought it to my attention because she thought it was unique – and a funny way of reporting plans in my progress notes.
My PT colleague told me that she remembered the first time she read one of my notes on a patient we were treating together (she needed to know the patient’s psychiatric status before she engaged them in physical therapy), and it struck her that I reported the patient was “befuddled,” and she wondered who would use befuddled in a note (lately, I have started using “flummoxed”). Another time, I was charting on a patient, and I used the word “flapdoodle” to describe the nonsense the patient was spewing (I recall this particular patient told me they graduated from grammar school at 5 years old). Another favorite word of mine that I use to describe nonsense is “claptrap.”
So, I have been making my evaluations and progress notes sing for a very long time, as doing so improves my writing skills, stimulates my thinking, turns the drudgery of charting into some fun, and creates an adventure in writing.
I have also been a big user of mental status templates to cut down my time. The essential elements of a mental status are in the narrative template, and all I need to do is to edit the verbiage in the template to fit the patient’s presentation so that the mental status sings. Early on, I understood that, to be a good psychiatrist, you needed a good vocabulary so you can speak with as much precision as possible when describing a patient’s mental status.
I was seeing many Alzheimer’s patients at one point. So I developed a special mental status template for them (female and male), so all I had to do to it was cut and paste, and then edit the template to fit the patient like a glove. Template example: This is a xx-year-old female who was appropriately groomed and who was cooperative with the interview, but she could not give much information. She was not hyperactive or lethargic. Her mood was bland, and her affect was flat and bland. Her speech did not contain any relevant information. Thought processes were not evident, although she was awake. I could not get a history of delusions or current auditory or visual hallucinations. Her thought content was nondescript. She was attentive, and her recent and remote memory were poor. Clinical estimate of her intelligence could not be determined. Her judgment and insight were poor. I could not determine whether there was any suicidal or homicidal ideation.
Formulation: This is a xx-year-old female who has a major neurocognitive disorder (formerly known as dementia). She is not overtly psychotic, suicidal, homicidal, or gravely disabled, but her level of functioning leaves a lot to be desired, which is why she needs a sheltered living circumstance.
Dx: Major neurocognitive disorder (formerly known as dementia).
Here’s another example: This is a xx-year-old male who was appropriately groomed and who was cooperative with the interview. He was not hyperactive or lethargic. His mood was euthymic and he had a wide range of affect as he was able to smile, get serious, and be sad (about xxx). His speech was relevant, linear, and goal directed. Thought processes did not show any signs of loose associations, tangentiality, or circumstantiality. He denies any delusions or current auditory or visual hallucinations. His thought content was surrounding xxx. He was attentive, and his recent and remote memory were intact. Clinical estimate of his intelligence was xxx average. His judgment and insight were poor as xxx. No report of suicidal or homicidal ideation.
Formulation: xxx. He is not overtly psychotic, suicidal, homicidal, or gravely disabled so I will clear him for psychiatric discharge.
Dx: xxx.
Just me trying to make work a little easier for myself and everyone else.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
I was talking to a physical therapy (PT) colleague and she was lamenting how much she hated doing documentation on patients she was treating. I suggested to her that she make her evaluations and progress notes sing. This is a concept I would sometimes use with patients who might be depressed, for example, I would ask them if anything made their heart sing to get an idea how “depressed” they might be. If they were unhappy or sad, I would advise that they engage in “heart singing” activities and behaviors, as I believe it is the “simple pleasures” in life that keep us resilient and persistent.
It is funny, when I was a resident and working in Jackson Park Hospital’s first psychiatric ward in 1972, one day in a note I wrote “I am going to give this acutely psychotic patient the big T – Thorazine to help them get some sleep at night,” I did not really think much about it until one of the nurses brought it to my attention because she thought it was unique – and a funny way of reporting plans in my progress notes.
My PT colleague told me that she remembered the first time she read one of my notes on a patient we were treating together (she needed to know the patient’s psychiatric status before she engaged them in physical therapy), and it struck her that I reported the patient was “befuddled,” and she wondered who would use befuddled in a note (lately, I have started using “flummoxed”). Another time, I was charting on a patient, and I used the word “flapdoodle” to describe the nonsense the patient was spewing (I recall this particular patient told me they graduated from grammar school at 5 years old). Another favorite word of mine that I use to describe nonsense is “claptrap.”
So, I have been making my evaluations and progress notes sing for a very long time, as doing so improves my writing skills, stimulates my thinking, turns the drudgery of charting into some fun, and creates an adventure in writing.
I have also been a big user of mental status templates to cut down my time. The essential elements of a mental status are in the narrative template, and all I need to do is to edit the verbiage in the template to fit the patient’s presentation so that the mental status sings. Early on, I understood that, to be a good psychiatrist, you needed a good vocabulary so you can speak with as much precision as possible when describing a patient’s mental status.
I was seeing many Alzheimer’s patients at one point. So I developed a special mental status template for them (female and male), so all I had to do to it was cut and paste, and then edit the template to fit the patient like a glove. Template example: This is a xx-year-old female who was appropriately groomed and who was cooperative with the interview, but she could not give much information. She was not hyperactive or lethargic. Her mood was bland, and her affect was flat and bland. Her speech did not contain any relevant information. Thought processes were not evident, although she was awake. I could not get a history of delusions or current auditory or visual hallucinations. Her thought content was nondescript. She was attentive, and her recent and remote memory were poor. Clinical estimate of her intelligence could not be determined. Her judgment and insight were poor. I could not determine whether there was any suicidal or homicidal ideation.
Formulation: This is a xx-year-old female who has a major neurocognitive disorder (formerly known as dementia). She is not overtly psychotic, suicidal, homicidal, or gravely disabled, but her level of functioning leaves a lot to be desired, which is why she needs a sheltered living circumstance.
Dx: Major neurocognitive disorder (formerly known as dementia).
Here’s another example: This is a xx-year-old male who was appropriately groomed and who was cooperative with the interview. He was not hyperactive or lethargic. His mood was euthymic and he had a wide range of affect as he was able to smile, get serious, and be sad (about xxx). His speech was relevant, linear, and goal directed. Thought processes did not show any signs of loose associations, tangentiality, or circumstantiality. He denies any delusions or current auditory or visual hallucinations. His thought content was surrounding xxx. He was attentive, and his recent and remote memory were intact. Clinical estimate of his intelligence was xxx average. His judgment and insight were poor as xxx. No report of suicidal or homicidal ideation.
Formulation: xxx. He is not overtly psychotic, suicidal, homicidal, or gravely disabled so I will clear him for psychiatric discharge.
Dx: xxx.
Just me trying to make work a little easier for myself and everyone else.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Teen e-cigarette use: A public health crisis
After 2 decades of steady decline in adolescent and young adult use of tobacco products, e-cigarettes have dramatically altered the landscape of substance use in youth. E-cigarette use among teens has been on the rise for years but the recent exponential increase is unprecedented. From 2017 to 2018, adolescent e-cigarette use had the largest year-to-year increase (78%, from 12% to 21%) of any individual substance or class of substances at any time during the past 2 decades of nationwide monitoring.1 This has appropriately caught the nation’s attention. In 2016, Surgeon General Vivek H. Murthy, MD, commissioned an extensive report about electronic cigarettes, and in 2018 Surgeon General Jerome Adams, MD, MPH, issued an advisory declaring e-cigarettes a public health crisis for adolescents.2
E-cigarettes have received attention as a possible boon to adult cigarette smokers seeking a less hazardous product. We can consider the use of tobacco products along a continuum from smoked tobacco, dual use (both smoked tobacco and electronic nicotine delivery), electronic nicotine delivery only, and finally, nonuse. For some adults, transitioning from smoked tobacco products to electronic delivery systems has been a step toward less overall harm from substance use, with a small minority of that population going on to achieve abstinence from all nicotine products.3 For youth and teens, the story has been the opposite. With the rapid rise of e-cigarettes, adolescents overwhelmingly have been moving in the wrong direction at each potential step along this continuum.4 Less than 8% of teens who use e-cigarettes indicated that smoking cessation is a factor in their use.5 An estimated 1.3 million U.S. teens now are dependent or at high risk for dependence upon nicotine because of e-cigarette use. Furthermore, these teens are at a fourfold higher risk of progression to cigarette use, compared with their peers.6
One product in particular gives us information as to why this trend has accelerated so rapidly. Juul, now the sales leader among electronic nicotine delivery systems, rose from approximately 25% to a dominant 75% of market share in just over 1 fiscal year after a social media campaign targeted toward youth and young adults. The device is shaped like an elongated flash drive, is marketed as “sleek,” “looking cool,” and being “super easy” to use. This product touts its use of nicotine salts that can deliver higher concentrations of nicotine more rapidly to mimic the experience of smoking a cigarette as closely as possible. The fruity flavors in Juul “pods” and many other devices also appeal to teens. Many youth are left misinformed, thinking they are using a relatively harmless alternative to cigarettes.
E-cigarette use in youth carries many risks. Among the physical risks is exposure to harmful chemicals (even if less numerous than smoked tobacco products) such as diacetyl (a known cause of bronchiolitis obliterans, or “popcorn lung”), formaldehyde, acrolein, benzene, and metals such as nickel, tin and lead.7 “Safer than cigarettes” is a low bar indeed. Cognitive and emotional risks of early nicotine exposure include poor focus and attention, permanent lowering of impulse control, and a higher risk of mood and anxiety disorders.
Furthermore, nicotine is a gateway drug, with a clearly understood molecular basis for how it can potentiate the effects of later used substances, especially stimulants such as cocaine.8 The gateway and priming effect is compounded for youth because of ongoing brain development and plasticity during teen years. E-cigarette use also is associated with other risk behaviors including a manyfold higher likelihood of binge drinking, having multiple sexual partners in a short period of time, and using other substances such as cannabis, cocaine, methamphetamine, and heroin or nonprescribed opioids.9 An electronic system for vaporization also presents a risk for use of other substances. In just 1 year from 2017 to 2018, marijuana “vaping” increased by more than 50% among all ages surveyed.10
Pediatric health care providers are essential educators for both teens and parents regarding the risks of e-cigarette use. Many youth don’t know what they’re using; 66% of youth reported that the vapors they were inhaling contained only flavoring. Only 13% reported they were inhaling nicotine.10 In stark contrast to these self-reports, all Juul “pods” contain nicotine. As has been a pattern with nationwide surveys of substance use for decades, adolescent use is inversely correlated with perception of risk; 70% of 8th-12th graders do not foresee great harm in regular e-cigarette use. In addition, adolescents use substances less often when they know their parents disapprove. Parents also must be taught about the risks of e-cigarette use and can be provided with resources and taught effective strategies if they have difficulty communicating their disapproval to their children.
Age-appropriate screening in primary care settings must include specific language regarding the use of electronic cigarettes, with questions about “vaping” and “juuling.” Discussions with teens may be more effective with emphasis on issues that resonate with youth such as the financial cost, loss of freedom when dependence develops, and the fact that their generation is once again being targeted by the tobacco industry. Referral for further treatment, including individual and group therapy as well as family-focused interventions, should be considered for teens who use daily, use other substances regularly, or could benefit from treatment for co-occurring mental health disorders.
Electronic cigarette use should not be recommended as a smoking cessation strategy for teens.11 Pediatric health care providers must advocate for regulation of these products, including increasing the legal age of purchase and banning flavoring in e-cigarettes products, Internet sales, and advertisements targeted to youth.
The rapid rise in e-cigarette use among teens is of great concern. As with all classes of substances, early initiation of nicotine drastically increases the risk of developing a substance use disorder and portends a prolonged course and greater accumulation of adverse consequences. There is an urgent need for education, prevention, and early identification of e-cigarette use to protect the current and future well-being of children and adolescents.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email Dr. Jackson at [email protected].
References
1. MMWR Morb Mortal Wkly Rep. 2018;67:1276-7.
2. e-cigarettes.surgeongeneral.gov
3. N Engl J Med 2019;380:629-37.
4. Pediatrics. 2018 Dec; 142(6):e20180486.
5. MMWR Morb Mortal Wkly Rep 2018;67:196-200.
6. JAMA Pediatr. 2017 Aug 1;171(8):788-97.
7. “Public health consequences of e-cigarettes” (Washington, DC: National Academies Press, January 2018).
8. N Engl J Med 2014;371:932-43.
9. N Engl J Med 2019;380:689-90.
10. MMWR Morb Mortal Wkly Rep. 2016 Jan 8;64(52):1403-8.
11. Pediatrics. 2019 Feb;143(2). pii: e20183652.
After 2 decades of steady decline in adolescent and young adult use of tobacco products, e-cigarettes have dramatically altered the landscape of substance use in youth. E-cigarette use among teens has been on the rise for years but the recent exponential increase is unprecedented. From 2017 to 2018, adolescent e-cigarette use had the largest year-to-year increase (78%, from 12% to 21%) of any individual substance or class of substances at any time during the past 2 decades of nationwide monitoring.1 This has appropriately caught the nation’s attention. In 2016, Surgeon General Vivek H. Murthy, MD, commissioned an extensive report about electronic cigarettes, and in 2018 Surgeon General Jerome Adams, MD, MPH, issued an advisory declaring e-cigarettes a public health crisis for adolescents.2
E-cigarettes have received attention as a possible boon to adult cigarette smokers seeking a less hazardous product. We can consider the use of tobacco products along a continuum from smoked tobacco, dual use (both smoked tobacco and electronic nicotine delivery), electronic nicotine delivery only, and finally, nonuse. For some adults, transitioning from smoked tobacco products to electronic delivery systems has been a step toward less overall harm from substance use, with a small minority of that population going on to achieve abstinence from all nicotine products.3 For youth and teens, the story has been the opposite. With the rapid rise of e-cigarettes, adolescents overwhelmingly have been moving in the wrong direction at each potential step along this continuum.4 Less than 8% of teens who use e-cigarettes indicated that smoking cessation is a factor in their use.5 An estimated 1.3 million U.S. teens now are dependent or at high risk for dependence upon nicotine because of e-cigarette use. Furthermore, these teens are at a fourfold higher risk of progression to cigarette use, compared with their peers.6
One product in particular gives us information as to why this trend has accelerated so rapidly. Juul, now the sales leader among electronic nicotine delivery systems, rose from approximately 25% to a dominant 75% of market share in just over 1 fiscal year after a social media campaign targeted toward youth and young adults. The device is shaped like an elongated flash drive, is marketed as “sleek,” “looking cool,” and being “super easy” to use. This product touts its use of nicotine salts that can deliver higher concentrations of nicotine more rapidly to mimic the experience of smoking a cigarette as closely as possible. The fruity flavors in Juul “pods” and many other devices also appeal to teens. Many youth are left misinformed, thinking they are using a relatively harmless alternative to cigarettes.
E-cigarette use in youth carries many risks. Among the physical risks is exposure to harmful chemicals (even if less numerous than smoked tobacco products) such as diacetyl (a known cause of bronchiolitis obliterans, or “popcorn lung”), formaldehyde, acrolein, benzene, and metals such as nickel, tin and lead.7 “Safer than cigarettes” is a low bar indeed. Cognitive and emotional risks of early nicotine exposure include poor focus and attention, permanent lowering of impulse control, and a higher risk of mood and anxiety disorders.
Furthermore, nicotine is a gateway drug, with a clearly understood molecular basis for how it can potentiate the effects of later used substances, especially stimulants such as cocaine.8 The gateway and priming effect is compounded for youth because of ongoing brain development and plasticity during teen years. E-cigarette use also is associated with other risk behaviors including a manyfold higher likelihood of binge drinking, having multiple sexual partners in a short period of time, and using other substances such as cannabis, cocaine, methamphetamine, and heroin or nonprescribed opioids.9 An electronic system for vaporization also presents a risk for use of other substances. In just 1 year from 2017 to 2018, marijuana “vaping” increased by more than 50% among all ages surveyed.10
Pediatric health care providers are essential educators for both teens and parents regarding the risks of e-cigarette use. Many youth don’t know what they’re using; 66% of youth reported that the vapors they were inhaling contained only flavoring. Only 13% reported they were inhaling nicotine.10 In stark contrast to these self-reports, all Juul “pods” contain nicotine. As has been a pattern with nationwide surveys of substance use for decades, adolescent use is inversely correlated with perception of risk; 70% of 8th-12th graders do not foresee great harm in regular e-cigarette use. In addition, adolescents use substances less often when they know their parents disapprove. Parents also must be taught about the risks of e-cigarette use and can be provided with resources and taught effective strategies if they have difficulty communicating their disapproval to their children.
Age-appropriate screening in primary care settings must include specific language regarding the use of electronic cigarettes, with questions about “vaping” and “juuling.” Discussions with teens may be more effective with emphasis on issues that resonate with youth such as the financial cost, loss of freedom when dependence develops, and the fact that their generation is once again being targeted by the tobacco industry. Referral for further treatment, including individual and group therapy as well as family-focused interventions, should be considered for teens who use daily, use other substances regularly, or could benefit from treatment for co-occurring mental health disorders.
Electronic cigarette use should not be recommended as a smoking cessation strategy for teens.11 Pediatric health care providers must advocate for regulation of these products, including increasing the legal age of purchase and banning flavoring in e-cigarettes products, Internet sales, and advertisements targeted to youth.
The rapid rise in e-cigarette use among teens is of great concern. As with all classes of substances, early initiation of nicotine drastically increases the risk of developing a substance use disorder and portends a prolonged course and greater accumulation of adverse consequences. There is an urgent need for education, prevention, and early identification of e-cigarette use to protect the current and future well-being of children and adolescents.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email Dr. Jackson at [email protected].
References
1. MMWR Morb Mortal Wkly Rep. 2018;67:1276-7.
2. e-cigarettes.surgeongeneral.gov
3. N Engl J Med 2019;380:629-37.
4. Pediatrics. 2018 Dec; 142(6):e20180486.
5. MMWR Morb Mortal Wkly Rep 2018;67:196-200.
6. JAMA Pediatr. 2017 Aug 1;171(8):788-97.
7. “Public health consequences of e-cigarettes” (Washington, DC: National Academies Press, January 2018).
8. N Engl J Med 2014;371:932-43.
9. N Engl J Med 2019;380:689-90.
10. MMWR Morb Mortal Wkly Rep. 2016 Jan 8;64(52):1403-8.
11. Pediatrics. 2019 Feb;143(2). pii: e20183652.
After 2 decades of steady decline in adolescent and young adult use of tobacco products, e-cigarettes have dramatically altered the landscape of substance use in youth. E-cigarette use among teens has been on the rise for years but the recent exponential increase is unprecedented. From 2017 to 2018, adolescent e-cigarette use had the largest year-to-year increase (78%, from 12% to 21%) of any individual substance or class of substances at any time during the past 2 decades of nationwide monitoring.1 This has appropriately caught the nation’s attention. In 2016, Surgeon General Vivek H. Murthy, MD, commissioned an extensive report about electronic cigarettes, and in 2018 Surgeon General Jerome Adams, MD, MPH, issued an advisory declaring e-cigarettes a public health crisis for adolescents.2
E-cigarettes have received attention as a possible boon to adult cigarette smokers seeking a less hazardous product. We can consider the use of tobacco products along a continuum from smoked tobacco, dual use (both smoked tobacco and electronic nicotine delivery), electronic nicotine delivery only, and finally, nonuse. For some adults, transitioning from smoked tobacco products to electronic delivery systems has been a step toward less overall harm from substance use, with a small minority of that population going on to achieve abstinence from all nicotine products.3 For youth and teens, the story has been the opposite. With the rapid rise of e-cigarettes, adolescents overwhelmingly have been moving in the wrong direction at each potential step along this continuum.4 Less than 8% of teens who use e-cigarettes indicated that smoking cessation is a factor in their use.5 An estimated 1.3 million U.S. teens now are dependent or at high risk for dependence upon nicotine because of e-cigarette use. Furthermore, these teens are at a fourfold higher risk of progression to cigarette use, compared with their peers.6
One product in particular gives us information as to why this trend has accelerated so rapidly. Juul, now the sales leader among electronic nicotine delivery systems, rose from approximately 25% to a dominant 75% of market share in just over 1 fiscal year after a social media campaign targeted toward youth and young adults. The device is shaped like an elongated flash drive, is marketed as “sleek,” “looking cool,” and being “super easy” to use. This product touts its use of nicotine salts that can deliver higher concentrations of nicotine more rapidly to mimic the experience of smoking a cigarette as closely as possible. The fruity flavors in Juul “pods” and many other devices also appeal to teens. Many youth are left misinformed, thinking they are using a relatively harmless alternative to cigarettes.
E-cigarette use in youth carries many risks. Among the physical risks is exposure to harmful chemicals (even if less numerous than smoked tobacco products) such as diacetyl (a known cause of bronchiolitis obliterans, or “popcorn lung”), formaldehyde, acrolein, benzene, and metals such as nickel, tin and lead.7 “Safer than cigarettes” is a low bar indeed. Cognitive and emotional risks of early nicotine exposure include poor focus and attention, permanent lowering of impulse control, and a higher risk of mood and anxiety disorders.
Furthermore, nicotine is a gateway drug, with a clearly understood molecular basis for how it can potentiate the effects of later used substances, especially stimulants such as cocaine.8 The gateway and priming effect is compounded for youth because of ongoing brain development and plasticity during teen years. E-cigarette use also is associated with other risk behaviors including a manyfold higher likelihood of binge drinking, having multiple sexual partners in a short period of time, and using other substances such as cannabis, cocaine, methamphetamine, and heroin or nonprescribed opioids.9 An electronic system for vaporization also presents a risk for use of other substances. In just 1 year from 2017 to 2018, marijuana “vaping” increased by more than 50% among all ages surveyed.10
Pediatric health care providers are essential educators for both teens and parents regarding the risks of e-cigarette use. Many youth don’t know what they’re using; 66% of youth reported that the vapors they were inhaling contained only flavoring. Only 13% reported they were inhaling nicotine.10 In stark contrast to these self-reports, all Juul “pods” contain nicotine. As has been a pattern with nationwide surveys of substance use for decades, adolescent use is inversely correlated with perception of risk; 70% of 8th-12th graders do not foresee great harm in regular e-cigarette use. In addition, adolescents use substances less often when they know their parents disapprove. Parents also must be taught about the risks of e-cigarette use and can be provided with resources and taught effective strategies if they have difficulty communicating their disapproval to their children.
Age-appropriate screening in primary care settings must include specific language regarding the use of electronic cigarettes, with questions about “vaping” and “juuling.” Discussions with teens may be more effective with emphasis on issues that resonate with youth such as the financial cost, loss of freedom when dependence develops, and the fact that their generation is once again being targeted by the tobacco industry. Referral for further treatment, including individual and group therapy as well as family-focused interventions, should be considered for teens who use daily, use other substances regularly, or could benefit from treatment for co-occurring mental health disorders.
Electronic cigarette use should not be recommended as a smoking cessation strategy for teens.11 Pediatric health care providers must advocate for regulation of these products, including increasing the legal age of purchase and banning flavoring in e-cigarettes products, Internet sales, and advertisements targeted to youth.
The rapid rise in e-cigarette use among teens is of great concern. As with all classes of substances, early initiation of nicotine drastically increases the risk of developing a substance use disorder and portends a prolonged course and greater accumulation of adverse consequences. There is an urgent need for education, prevention, and early identification of e-cigarette use to protect the current and future well-being of children and adolescents.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email Dr. Jackson at [email protected].
References
1. MMWR Morb Mortal Wkly Rep. 2018;67:1276-7.
2. e-cigarettes.surgeongeneral.gov
3. N Engl J Med 2019;380:629-37.
4. Pediatrics. 2018 Dec; 142(6):e20180486.
5. MMWR Morb Mortal Wkly Rep 2018;67:196-200.
6. JAMA Pediatr. 2017 Aug 1;171(8):788-97.
7. “Public health consequences of e-cigarettes” (Washington, DC: National Academies Press, January 2018).
8. N Engl J Med 2014;371:932-43.
9. N Engl J Med 2019;380:689-90.
10. MMWR Morb Mortal Wkly Rep. 2016 Jan 8;64(52):1403-8.
11. Pediatrics. 2019 Feb;143(2). pii: e20183652.
The genesis of vaginal anomalies
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at [email protected].
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at [email protected].
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at [email protected].
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
Vaginal anomalies and their surgical correction
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Poster ads don’t belong in the clinic
In the last few months, I’ve received several posters. They’re always delivered by UPS, and come in a solid cardboard box to keep them from being crushed.
The boxes get opened, and once I know what they are, the whole thing gets tossed in the office recycling.
I know they’re presented as helpful patient information, with some bullet lists and glossy graphics showing brains, nerve transmitters, or patients. But the basic reality is that they’re just advertisements. Like infomercials on TV, they come across as professional and interesting, but at their heart and soul are just selling something.
No thanks.
Years ago, a company sent me a poster listing the warning signs of stroke. Although it was still an advertisement, I decided to hang it up in my exam room as a sort of public service announcement. Unfortunately, I soon discovered that any patient left staring at it for more than 1-2 minutes would start to complain of at least two of the symptoms listed. It got taken down after a few days.
I have nothing against advertising. It pays for websites, television shows, sporting events, newspapers, and magazines.
But my exam room isn’t the place for it. Patients are bombarded with direct-to-consumer advertising for many drugs in every media outlet. The doctor’s discussion room shouldn’t be one of the them.
The meeting between me and a patient should be frank, honest assessments about what should be done and what, specifically, is best for their individual case. I don’t need marketing for a drug that may or may not be appropriate, or easily covered by insurance, staring back at them.
It’s a thin line. Obviously, magazines out in my lobby are full of pharmaceutical ads, and that doesn’t bother me. But once a patient crosses the line into my consultation area it should just be between me and them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the last few months, I’ve received several posters. They’re always delivered by UPS, and come in a solid cardboard box to keep them from being crushed.
The boxes get opened, and once I know what they are, the whole thing gets tossed in the office recycling.
I know they’re presented as helpful patient information, with some bullet lists and glossy graphics showing brains, nerve transmitters, or patients. But the basic reality is that they’re just advertisements. Like infomercials on TV, they come across as professional and interesting, but at their heart and soul are just selling something.
No thanks.
Years ago, a company sent me a poster listing the warning signs of stroke. Although it was still an advertisement, I decided to hang it up in my exam room as a sort of public service announcement. Unfortunately, I soon discovered that any patient left staring at it for more than 1-2 minutes would start to complain of at least two of the symptoms listed. It got taken down after a few days.
I have nothing against advertising. It pays for websites, television shows, sporting events, newspapers, and magazines.
But my exam room isn’t the place for it. Patients are bombarded with direct-to-consumer advertising for many drugs in every media outlet. The doctor’s discussion room shouldn’t be one of the them.
The meeting between me and a patient should be frank, honest assessments about what should be done and what, specifically, is best for their individual case. I don’t need marketing for a drug that may or may not be appropriate, or easily covered by insurance, staring back at them.
It’s a thin line. Obviously, magazines out in my lobby are full of pharmaceutical ads, and that doesn’t bother me. But once a patient crosses the line into my consultation area it should just be between me and them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the last few months, I’ve received several posters. They’re always delivered by UPS, and come in a solid cardboard box to keep them from being crushed.
The boxes get opened, and once I know what they are, the whole thing gets tossed in the office recycling.
I know they’re presented as helpful patient information, with some bullet lists and glossy graphics showing brains, nerve transmitters, or patients. But the basic reality is that they’re just advertisements. Like infomercials on TV, they come across as professional and interesting, but at their heart and soul are just selling something.
No thanks.
Years ago, a company sent me a poster listing the warning signs of stroke. Although it was still an advertisement, I decided to hang it up in my exam room as a sort of public service announcement. Unfortunately, I soon discovered that any patient left staring at it for more than 1-2 minutes would start to complain of at least two of the symptoms listed. It got taken down after a few days.
I have nothing against advertising. It pays for websites, television shows, sporting events, newspapers, and magazines.
But my exam room isn’t the place for it. Patients are bombarded with direct-to-consumer advertising for many drugs in every media outlet. The doctor’s discussion room shouldn’t be one of the them.
The meeting between me and a patient should be frank, honest assessments about what should be done and what, specifically, is best for their individual case. I don’t need marketing for a drug that may or may not be appropriate, or easily covered by insurance, staring back at them.
It’s a thin line. Obviously, magazines out in my lobby are full of pharmaceutical ads, and that doesn’t bother me. But once a patient crosses the line into my consultation area it should just be between me and them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Sunscreen Regulations and Advice for Your Patients
If by now you have not had a patient ask, “Doctor, what sunscreen should I use NOW?” you will soon.
The US Food and Drug Administration (FDA) recently published a press release detailing a proposed rule on how manufacturers will be required to test and label sunscreens in the United States.1,2 Although the press release was complicated and contained much information, the media specifically latched onto the FDA’s consideration of only 2 active sunscreen ingredients—zinc oxide and titanium dioxide—as generally recognized as safe and effective (GRASE). In response, some patients may assume that most sunscreens on the market are dangerous.
How did this new proposed rule come about? To understand the process, it takes some explanation of the history of the FDA’s regulation of sunscreens.
How are sunscreens regulated by the FDA?
The regulatory process for sunscreens in the United States is complicated. The FDA regulates sunscreens as over-the-counter (OTC) drugs rather than as cosmetics, which is how they are regulated in most of the rest of the world.
The US sunscreen regulation process began in 1978 with an advance notice of proposed rulemaking from the FDA that included recommendations from an advisory review panel on the safe and effective use of OTC sunscreen products.3 At that time, 21 active sunscreen ingredients and their maximum use concentrations were listed and determined to be safe, or GRASE. It also gave manufacturers guidance on how to test for efficacy with the methodology for determining the sun protection factor (SPF) as well as various labeling requirements. Over the years, the FDA has issued a number of other sunscreen guidelines, such as removing padimate A and adding avobenzone and zinc oxide to the list of GRASE ingredients in the 1990s.4,5
In 1999, the FDA issued a final rule that listed 16 active sunscreen ingredients and concentrations as GRASE.6 There were some restrictions as to certain combinations of ingredients that could not be used in a finished product. Labeling requirements, including a maximum SPF of 30, also were put in place. This final rule established a final sunscreen monograph that was supposed to have been effective by 2002; however, in 2001 the agency delayed the effective date indefinitely because they had not yet established broad-spectrum (UVA) protection testing and labeling.7
The FDA published a proposed rule in 2007 as well as a final rule in 2011 that again listed the same 16 ingredients as GRASE and specified labeling and testing methods for establishing SPF, broad-spectrum protection, and water-resistance claims.8,9 The final rule limited product labels to a maximum SPF of 50+; provided directions for use with regard to other labeling elements (eg, warnings); and identified specific claims that would not be allowed on product labels, such as “waterproof” and “all-day protection.”9
Nevertheless, an effective final OTC monograph for sunscreen products has not yet been published.
What is the Sunscreen Innovation Act?
In 2014, the US Congress enacted the Sunscreen Innovation Act10 primarily to mandate that the FDA develop a more efficient way to determine the safety and efficacy of new active sunscreen ingredients that were commonly used in Europe and other parts of the world at the time. Many of these agents were thought to be more protective in the UVA and/or UVB spectrum, and if added to the list of GRASE ingredients available to US manufacturers, they would lead to the development of products that would improve the protection offered by sunscreens marketed to US consumers. The time and extent application (TEA) was established, a method that allowed manufacturers to apply for FDA approval of specific agents. The TEA also suggested allowing data generated in other countries where these agents were already in use for years to be considered in the FDA’s evaluation of the agents as GRASE. In addition, Congress mandated that a final monograph on OTC sunscreens be published by the end of 2019. A number of manufacturers have submitted TEAs for new active sunscreen ingredients, and so far, all have been rejected.
Why is the FDA interested in more safety data?
Since then, the FDA has become concerned not only with the safety and efficacy of newly proposed agents through the TEA but also with the original 16 active sunscreen ingredients listed as GRASE in the 2011 final rule. In the 1970s and 1980s, sunscreen use was limited to beach vacations or outdoor sporting events, but sun-protective behaviors have changed dramatically since that time, with health care providers now becoming cognizant of the growing threats of skin cancer and melanoma as well as the cosmetic concerns of photoaging, thereby recommending daily sunscreen use to their patients. In addition, the science behind sunscreens with higher concentrations of active ingredients intended to achieve higher and higher SPFs and their respective penetration of the skin has evolved, leading to new concerns about systemic toxicity. Early limited research frequently touted by the lay media has suggested that some of these agents might lead to hormonal changes, reproductive toxicity, and carcinogenicity.
In November 2016, the FDA issued a guidance for manufacturers that outlined the safety data that would be required to establish an OTC sunscreen active ingredient as GRASE.11 It also provided detailed information about both clinical and nonclinical safety testing, including human irritation and sensitization studies as well as human photosafety studies. In vitro dermal and systemic carcinogenicity studies and animal developmental and reproductive toxicity studies also were required as well studies regarding safety in children.
Many of these recommendations were already being utilized by manufacturers; however, one important change was the requirement for human absorption studies by a maximal usage trial, which more accurately addresses the absorption of sunscreen agents according to actual use. Such studies will be required at the highest allowable concentration of an agent in multiple vehicles and over large body surface areas for considerable exposure times.
This guidance to sunscreen manufacturers was announced to the public in a press release in May 2018.12
What are the new regulations?
All of this has culminated in the recent proposed rule, which includes several important proposals2:
- Of the 16 currently marketed active sunscreen ingredients, only 2—zinc oxide and titanium dioxide—are considered GRASE. Two ingredients—trolamine salicylate and para-aminobenzoic acid—are considered non-GRASE, but there is not enough information at this time to determine if the remaining 12 ingredients are GRASE. The FDA is working with manufacturers to obtain sufficient information to make this determination.
- Approved dosage formulations include sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Further information is needed regarding powders before they can be considered.
- The maximum SPF will be increased from 50+ to 60+.
- Sunscreens with an SPF of 15 or higher are required to provide broad-spectrum protection commensurate with the SPF, expanding on critical wavelength testing.
- There are new labeling changes, including a requirement that active ingredients be listed on the front of the packaging.
- Sunscreen products that contain insect repellents are considered non-GRASE.
What’s next?
The process for the proposed final rule has now entered a 90-day public comment period that will end on May 27, 2019; however, it is unlikely that a final monograph as mandated by Congress will be produced by the end of this year.
Sunscreen manufacturers currently are coordinating a response to the proposed rule through the Personal Care Products Council and the Consumer Healthcare Products Association Sunscreen Task Force. It is likely that the new required testing will be costly, with estimates exceeding tens or even hundreds of millions of dollars. In all likelihood, the number of active ingredients that the industry will agree to support with costly testing will be fewer than the 12 that are now on the list. It also is likely that this process will lead to fewer sunscreen products for consumers to choose from and almost certainly at a higher cost.
What do we tell patients in the meantime?
According to the FDA’s rules, it was necessary that this process was made public, but it will almost certainly concern our patients as to the safety of the sunscreen products they have been using. We should be concerned that some of our patients may limit their use of sunscreens because of safety concerns.
There is no question that, as physicians, we want to “first, do no harm,” so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data. The good news is that when this process is completed, a large number of agents will likely be found to be GRASE. When the FDA finally gives its imprimatur to sunscreens, it will hopefully help to silence those naysayers who report that sunscreens are dangerous for consumers; however, it has been suggested by some in industry that the new testing required may take at least 5 years.
What should dermatologists do when we are asked, “What sunscreen should I use NOW?” For most patients, I would explain the regulatory process and assure them that the risk-benefit ratio at this point suggests they should continue using the same sunscreens that they are currently using. For special situations such as pregnant women and children, it may be best to suggest products that contain only the 2 GRASE inorganic agents.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective [news release]. Silver Spring, MD: US Food and Drug Administration; February 21, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631736.htm. Accessed April 4, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 1978;43(166):38206-38269. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph. Fed Registr. 1996;60(180):48645-48655. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph; enforcement policy. Fed Registr. 1998;63(204):56584-56589. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; final monograph. Fed Registr. 1999;64(98):27666-27693. To be codified at 21 CFR §310, 352, 700, and 740.
- Sunscreen drug products for over-the-counter human use; final monograph; partial stay; final rule. Fed Registr. 2001;66:67485-67487. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Registr. 2007;72(165):49069-49122. To be codified at 21 CFR §347 and 352.
- Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Registr. 2011;76(117):35619-35665. To be codified at 21 CFR §201 and 310.
- Sunscreen Innovation Act, S 2141, 113th Cong, 2nd Sess (2014).
- Nonprescription sunscreen drug products-safety and effectiveness data; guidance for industry; availability. Fed Registr. 2016;81(226):84594-84595.
- Statement from Commissioner Scott Gottlieb, MD, on new FDA actions to keep consumers safe from the harmful effects of sun exposure, and ensure the long-term safety and benefits of sunscreens [news release]. Silver Spring, MD: US Food and Drug Administration; May 22, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm608499.htm. Accessed April 5, 2019.
If by now you have not had a patient ask, “Doctor, what sunscreen should I use NOW?” you will soon.
The US Food and Drug Administration (FDA) recently published a press release detailing a proposed rule on how manufacturers will be required to test and label sunscreens in the United States.1,2 Although the press release was complicated and contained much information, the media specifically latched onto the FDA’s consideration of only 2 active sunscreen ingredients—zinc oxide and titanium dioxide—as generally recognized as safe and effective (GRASE). In response, some patients may assume that most sunscreens on the market are dangerous.
How did this new proposed rule come about? To understand the process, it takes some explanation of the history of the FDA’s regulation of sunscreens.
How are sunscreens regulated by the FDA?
The regulatory process for sunscreens in the United States is complicated. The FDA regulates sunscreens as over-the-counter (OTC) drugs rather than as cosmetics, which is how they are regulated in most of the rest of the world.
The US sunscreen regulation process began in 1978 with an advance notice of proposed rulemaking from the FDA that included recommendations from an advisory review panel on the safe and effective use of OTC sunscreen products.3 At that time, 21 active sunscreen ingredients and their maximum use concentrations were listed and determined to be safe, or GRASE. It also gave manufacturers guidance on how to test for efficacy with the methodology for determining the sun protection factor (SPF) as well as various labeling requirements. Over the years, the FDA has issued a number of other sunscreen guidelines, such as removing padimate A and adding avobenzone and zinc oxide to the list of GRASE ingredients in the 1990s.4,5
In 1999, the FDA issued a final rule that listed 16 active sunscreen ingredients and concentrations as GRASE.6 There were some restrictions as to certain combinations of ingredients that could not be used in a finished product. Labeling requirements, including a maximum SPF of 30, also were put in place. This final rule established a final sunscreen monograph that was supposed to have been effective by 2002; however, in 2001 the agency delayed the effective date indefinitely because they had not yet established broad-spectrum (UVA) protection testing and labeling.7
The FDA published a proposed rule in 2007 as well as a final rule in 2011 that again listed the same 16 ingredients as GRASE and specified labeling and testing methods for establishing SPF, broad-spectrum protection, and water-resistance claims.8,9 The final rule limited product labels to a maximum SPF of 50+; provided directions for use with regard to other labeling elements (eg, warnings); and identified specific claims that would not be allowed on product labels, such as “waterproof” and “all-day protection.”9
Nevertheless, an effective final OTC monograph for sunscreen products has not yet been published.
What is the Sunscreen Innovation Act?
In 2014, the US Congress enacted the Sunscreen Innovation Act10 primarily to mandate that the FDA develop a more efficient way to determine the safety and efficacy of new active sunscreen ingredients that were commonly used in Europe and other parts of the world at the time. Many of these agents were thought to be more protective in the UVA and/or UVB spectrum, and if added to the list of GRASE ingredients available to US manufacturers, they would lead to the development of products that would improve the protection offered by sunscreens marketed to US consumers. The time and extent application (TEA) was established, a method that allowed manufacturers to apply for FDA approval of specific agents. The TEA also suggested allowing data generated in other countries where these agents were already in use for years to be considered in the FDA’s evaluation of the agents as GRASE. In addition, Congress mandated that a final monograph on OTC sunscreens be published by the end of 2019. A number of manufacturers have submitted TEAs for new active sunscreen ingredients, and so far, all have been rejected.
Why is the FDA interested in more safety data?
Since then, the FDA has become concerned not only with the safety and efficacy of newly proposed agents through the TEA but also with the original 16 active sunscreen ingredients listed as GRASE in the 2011 final rule. In the 1970s and 1980s, sunscreen use was limited to beach vacations or outdoor sporting events, but sun-protective behaviors have changed dramatically since that time, with health care providers now becoming cognizant of the growing threats of skin cancer and melanoma as well as the cosmetic concerns of photoaging, thereby recommending daily sunscreen use to their patients. In addition, the science behind sunscreens with higher concentrations of active ingredients intended to achieve higher and higher SPFs and their respective penetration of the skin has evolved, leading to new concerns about systemic toxicity. Early limited research frequently touted by the lay media has suggested that some of these agents might lead to hormonal changes, reproductive toxicity, and carcinogenicity.
In November 2016, the FDA issued a guidance for manufacturers that outlined the safety data that would be required to establish an OTC sunscreen active ingredient as GRASE.11 It also provided detailed information about both clinical and nonclinical safety testing, including human irritation and sensitization studies as well as human photosafety studies. In vitro dermal and systemic carcinogenicity studies and animal developmental and reproductive toxicity studies also were required as well studies regarding safety in children.
Many of these recommendations were already being utilized by manufacturers; however, one important change was the requirement for human absorption studies by a maximal usage trial, which more accurately addresses the absorption of sunscreen agents according to actual use. Such studies will be required at the highest allowable concentration of an agent in multiple vehicles and over large body surface areas for considerable exposure times.
This guidance to sunscreen manufacturers was announced to the public in a press release in May 2018.12
What are the new regulations?
All of this has culminated in the recent proposed rule, which includes several important proposals2:
- Of the 16 currently marketed active sunscreen ingredients, only 2—zinc oxide and titanium dioxide—are considered GRASE. Two ingredients—trolamine salicylate and para-aminobenzoic acid—are considered non-GRASE, but there is not enough information at this time to determine if the remaining 12 ingredients are GRASE. The FDA is working with manufacturers to obtain sufficient information to make this determination.
- Approved dosage formulations include sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Further information is needed regarding powders before they can be considered.
- The maximum SPF will be increased from 50+ to 60+.
- Sunscreens with an SPF of 15 or higher are required to provide broad-spectrum protection commensurate with the SPF, expanding on critical wavelength testing.
- There are new labeling changes, including a requirement that active ingredients be listed on the front of the packaging.
- Sunscreen products that contain insect repellents are considered non-GRASE.
What’s next?
The process for the proposed final rule has now entered a 90-day public comment period that will end on May 27, 2019; however, it is unlikely that a final monograph as mandated by Congress will be produced by the end of this year.
Sunscreen manufacturers currently are coordinating a response to the proposed rule through the Personal Care Products Council and the Consumer Healthcare Products Association Sunscreen Task Force. It is likely that the new required testing will be costly, with estimates exceeding tens or even hundreds of millions of dollars. In all likelihood, the number of active ingredients that the industry will agree to support with costly testing will be fewer than the 12 that are now on the list. It also is likely that this process will lead to fewer sunscreen products for consumers to choose from and almost certainly at a higher cost.
What do we tell patients in the meantime?
According to the FDA’s rules, it was necessary that this process was made public, but it will almost certainly concern our patients as to the safety of the sunscreen products they have been using. We should be concerned that some of our patients may limit their use of sunscreens because of safety concerns.
There is no question that, as physicians, we want to “first, do no harm,” so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data. The good news is that when this process is completed, a large number of agents will likely be found to be GRASE. When the FDA finally gives its imprimatur to sunscreens, it will hopefully help to silence those naysayers who report that sunscreens are dangerous for consumers; however, it has been suggested by some in industry that the new testing required may take at least 5 years.
What should dermatologists do when we are asked, “What sunscreen should I use NOW?” For most patients, I would explain the regulatory process and assure them that the risk-benefit ratio at this point suggests they should continue using the same sunscreens that they are currently using. For special situations such as pregnant women and children, it may be best to suggest products that contain only the 2 GRASE inorganic agents.
If by now you have not had a patient ask, “Doctor, what sunscreen should I use NOW?” you will soon.
The US Food and Drug Administration (FDA) recently published a press release detailing a proposed rule on how manufacturers will be required to test and label sunscreens in the United States.1,2 Although the press release was complicated and contained much information, the media specifically latched onto the FDA’s consideration of only 2 active sunscreen ingredients—zinc oxide and titanium dioxide—as generally recognized as safe and effective (GRASE). In response, some patients may assume that most sunscreens on the market are dangerous.
How did this new proposed rule come about? To understand the process, it takes some explanation of the history of the FDA’s regulation of sunscreens.
How are sunscreens regulated by the FDA?
The regulatory process for sunscreens in the United States is complicated. The FDA regulates sunscreens as over-the-counter (OTC) drugs rather than as cosmetics, which is how they are regulated in most of the rest of the world.
The US sunscreen regulation process began in 1978 with an advance notice of proposed rulemaking from the FDA that included recommendations from an advisory review panel on the safe and effective use of OTC sunscreen products.3 At that time, 21 active sunscreen ingredients and their maximum use concentrations were listed and determined to be safe, or GRASE. It also gave manufacturers guidance on how to test for efficacy with the methodology for determining the sun protection factor (SPF) as well as various labeling requirements. Over the years, the FDA has issued a number of other sunscreen guidelines, such as removing padimate A and adding avobenzone and zinc oxide to the list of GRASE ingredients in the 1990s.4,5
In 1999, the FDA issued a final rule that listed 16 active sunscreen ingredients and concentrations as GRASE.6 There were some restrictions as to certain combinations of ingredients that could not be used in a finished product. Labeling requirements, including a maximum SPF of 30, also were put in place. This final rule established a final sunscreen monograph that was supposed to have been effective by 2002; however, in 2001 the agency delayed the effective date indefinitely because they had not yet established broad-spectrum (UVA) protection testing and labeling.7
The FDA published a proposed rule in 2007 as well as a final rule in 2011 that again listed the same 16 ingredients as GRASE and specified labeling and testing methods for establishing SPF, broad-spectrum protection, and water-resistance claims.8,9 The final rule limited product labels to a maximum SPF of 50+; provided directions for use with regard to other labeling elements (eg, warnings); and identified specific claims that would not be allowed on product labels, such as “waterproof” and “all-day protection.”9
Nevertheless, an effective final OTC monograph for sunscreen products has not yet been published.
What is the Sunscreen Innovation Act?
In 2014, the US Congress enacted the Sunscreen Innovation Act10 primarily to mandate that the FDA develop a more efficient way to determine the safety and efficacy of new active sunscreen ingredients that were commonly used in Europe and other parts of the world at the time. Many of these agents were thought to be more protective in the UVA and/or UVB spectrum, and if added to the list of GRASE ingredients available to US manufacturers, they would lead to the development of products that would improve the protection offered by sunscreens marketed to US consumers. The time and extent application (TEA) was established, a method that allowed manufacturers to apply for FDA approval of specific agents. The TEA also suggested allowing data generated in other countries where these agents were already in use for years to be considered in the FDA’s evaluation of the agents as GRASE. In addition, Congress mandated that a final monograph on OTC sunscreens be published by the end of 2019. A number of manufacturers have submitted TEAs for new active sunscreen ingredients, and so far, all have been rejected.
Why is the FDA interested in more safety data?
Since then, the FDA has become concerned not only with the safety and efficacy of newly proposed agents through the TEA but also with the original 16 active sunscreen ingredients listed as GRASE in the 2011 final rule. In the 1970s and 1980s, sunscreen use was limited to beach vacations or outdoor sporting events, but sun-protective behaviors have changed dramatically since that time, with health care providers now becoming cognizant of the growing threats of skin cancer and melanoma as well as the cosmetic concerns of photoaging, thereby recommending daily sunscreen use to their patients. In addition, the science behind sunscreens with higher concentrations of active ingredients intended to achieve higher and higher SPFs and their respective penetration of the skin has evolved, leading to new concerns about systemic toxicity. Early limited research frequently touted by the lay media has suggested that some of these agents might lead to hormonal changes, reproductive toxicity, and carcinogenicity.
In November 2016, the FDA issued a guidance for manufacturers that outlined the safety data that would be required to establish an OTC sunscreen active ingredient as GRASE.11 It also provided detailed information about both clinical and nonclinical safety testing, including human irritation and sensitization studies as well as human photosafety studies. In vitro dermal and systemic carcinogenicity studies and animal developmental and reproductive toxicity studies also were required as well studies regarding safety in children.
Many of these recommendations were already being utilized by manufacturers; however, one important change was the requirement for human absorption studies by a maximal usage trial, which more accurately addresses the absorption of sunscreen agents according to actual use. Such studies will be required at the highest allowable concentration of an agent in multiple vehicles and over large body surface areas for considerable exposure times.
This guidance to sunscreen manufacturers was announced to the public in a press release in May 2018.12
What are the new regulations?
All of this has culminated in the recent proposed rule, which includes several important proposals2:
- Of the 16 currently marketed active sunscreen ingredients, only 2—zinc oxide and titanium dioxide—are considered GRASE. Two ingredients—trolamine salicylate and para-aminobenzoic acid—are considered non-GRASE, but there is not enough information at this time to determine if the remaining 12 ingredients are GRASE. The FDA is working with manufacturers to obtain sufficient information to make this determination.
- Approved dosage formulations include sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Further information is needed regarding powders before they can be considered.
- The maximum SPF will be increased from 50+ to 60+.
- Sunscreens with an SPF of 15 or higher are required to provide broad-spectrum protection commensurate with the SPF, expanding on critical wavelength testing.
- There are new labeling changes, including a requirement that active ingredients be listed on the front of the packaging.
- Sunscreen products that contain insect repellents are considered non-GRASE.
What’s next?
The process for the proposed final rule has now entered a 90-day public comment period that will end on May 27, 2019; however, it is unlikely that a final monograph as mandated by Congress will be produced by the end of this year.
Sunscreen manufacturers currently are coordinating a response to the proposed rule through the Personal Care Products Council and the Consumer Healthcare Products Association Sunscreen Task Force. It is likely that the new required testing will be costly, with estimates exceeding tens or even hundreds of millions of dollars. In all likelihood, the number of active ingredients that the industry will agree to support with costly testing will be fewer than the 12 that are now on the list. It also is likely that this process will lead to fewer sunscreen products for consumers to choose from and almost certainly at a higher cost.
What do we tell patients in the meantime?
According to the FDA’s rules, it was necessary that this process was made public, but it will almost certainly concern our patients as to the safety of the sunscreen products they have been using. We should be concerned that some of our patients may limit their use of sunscreens because of safety concerns.
There is no question that, as physicians, we want to “first, do no harm,” so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data. The good news is that when this process is completed, a large number of agents will likely be found to be GRASE. When the FDA finally gives its imprimatur to sunscreens, it will hopefully help to silence those naysayers who report that sunscreens are dangerous for consumers; however, it has been suggested by some in industry that the new testing required may take at least 5 years.
What should dermatologists do when we are asked, “What sunscreen should I use NOW?” For most patients, I would explain the regulatory process and assure them that the risk-benefit ratio at this point suggests they should continue using the same sunscreens that they are currently using. For special situations such as pregnant women and children, it may be best to suggest products that contain only the 2 GRASE inorganic agents.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective [news release]. Silver Spring, MD: US Food and Drug Administration; February 21, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631736.htm. Accessed April 4, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 1978;43(166):38206-38269. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph. Fed Registr. 1996;60(180):48645-48655. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph; enforcement policy. Fed Registr. 1998;63(204):56584-56589. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; final monograph. Fed Registr. 1999;64(98):27666-27693. To be codified at 21 CFR §310, 352, 700, and 740.
- Sunscreen drug products for over-the-counter human use; final monograph; partial stay; final rule. Fed Registr. 2001;66:67485-67487. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Registr. 2007;72(165):49069-49122. To be codified at 21 CFR §347 and 352.
- Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Registr. 2011;76(117):35619-35665. To be codified at 21 CFR §201 and 310.
- Sunscreen Innovation Act, S 2141, 113th Cong, 2nd Sess (2014).
- Nonprescription sunscreen drug products-safety and effectiveness data; guidance for industry; availability. Fed Registr. 2016;81(226):84594-84595.
- Statement from Commissioner Scott Gottlieb, MD, on new FDA actions to keep consumers safe from the harmful effects of sun exposure, and ensure the long-term safety and benefits of sunscreens [news release]. Silver Spring, MD: US Food and Drug Administration; May 22, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm608499.htm. Accessed April 5, 2019.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective [news release]. Silver Spring, MD: US Food and Drug Administration; February 21, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631736.htm. Accessed April 4, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 1978;43(166):38206-38269. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph. Fed Registr. 1996;60(180):48645-48655. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph; enforcement policy. Fed Registr. 1998;63(204):56584-56589. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; final monograph. Fed Registr. 1999;64(98):27666-27693. To be codified at 21 CFR §310, 352, 700, and 740.
- Sunscreen drug products for over-the-counter human use; final monograph; partial stay; final rule. Fed Registr. 2001;66:67485-67487. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Registr. 2007;72(165):49069-49122. To be codified at 21 CFR §347 and 352.
- Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Registr. 2011;76(117):35619-35665. To be codified at 21 CFR §201 and 310.
- Sunscreen Innovation Act, S 2141, 113th Cong, 2nd Sess (2014).
- Nonprescription sunscreen drug products-safety and effectiveness data; guidance for industry; availability. Fed Registr. 2016;81(226):84594-84595.
- Statement from Commissioner Scott Gottlieb, MD, on new FDA actions to keep consumers safe from the harmful effects of sun exposure, and ensure the long-term safety and benefits of sunscreens [news release]. Silver Spring, MD: US Food and Drug Administration; May 22, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm608499.htm. Accessed April 5, 2019.