User login
Long COVID Can’t Be Solved Until We Decide What It Is
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.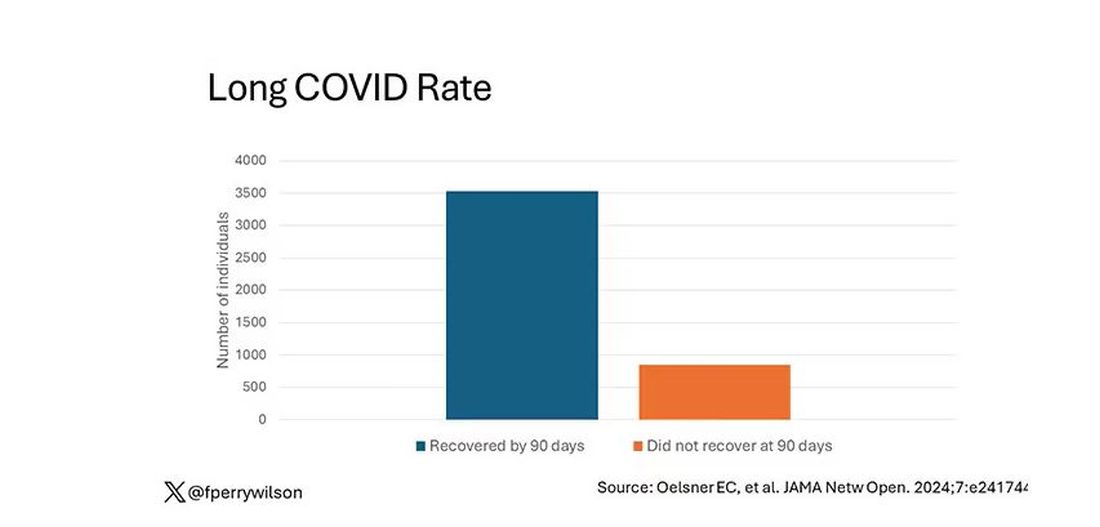
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.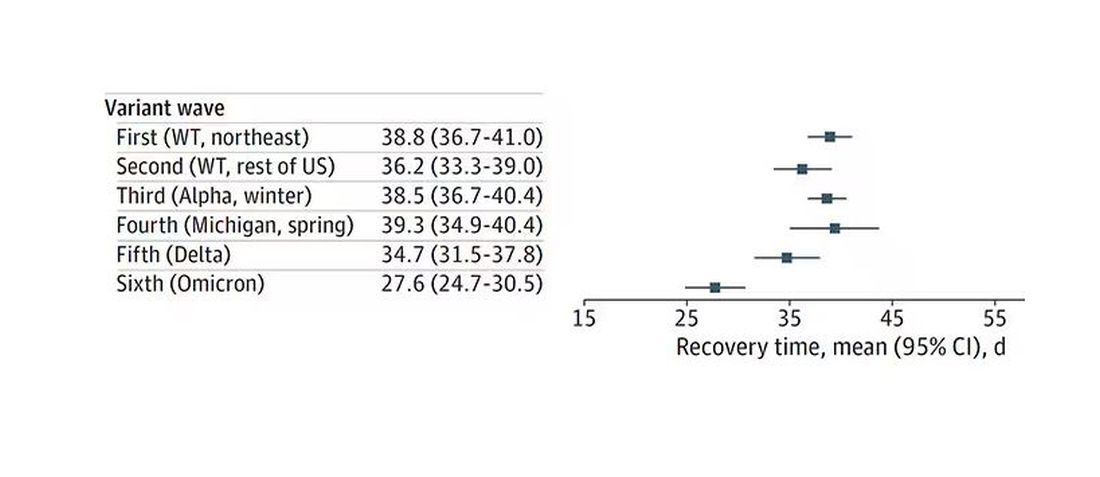
Recovery times were longer for smokers, those with diabetes, and those who were obese.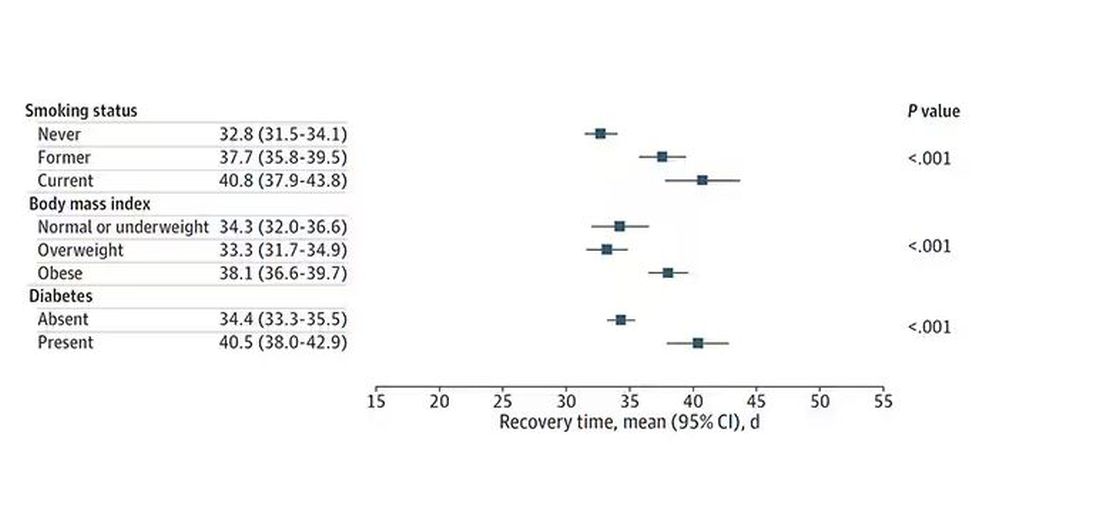
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.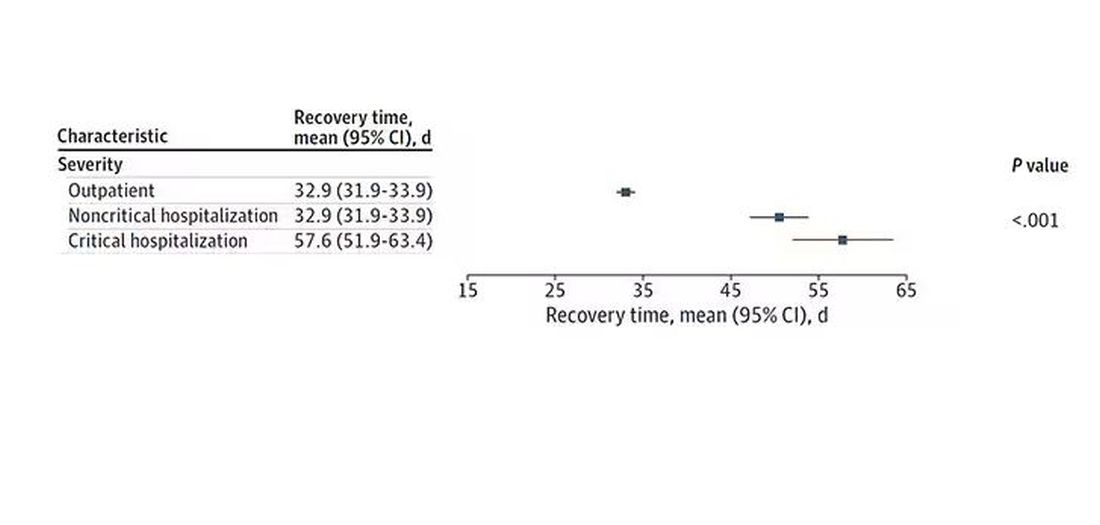
Vaccination was associated with shorter recovery times, as you can see here. 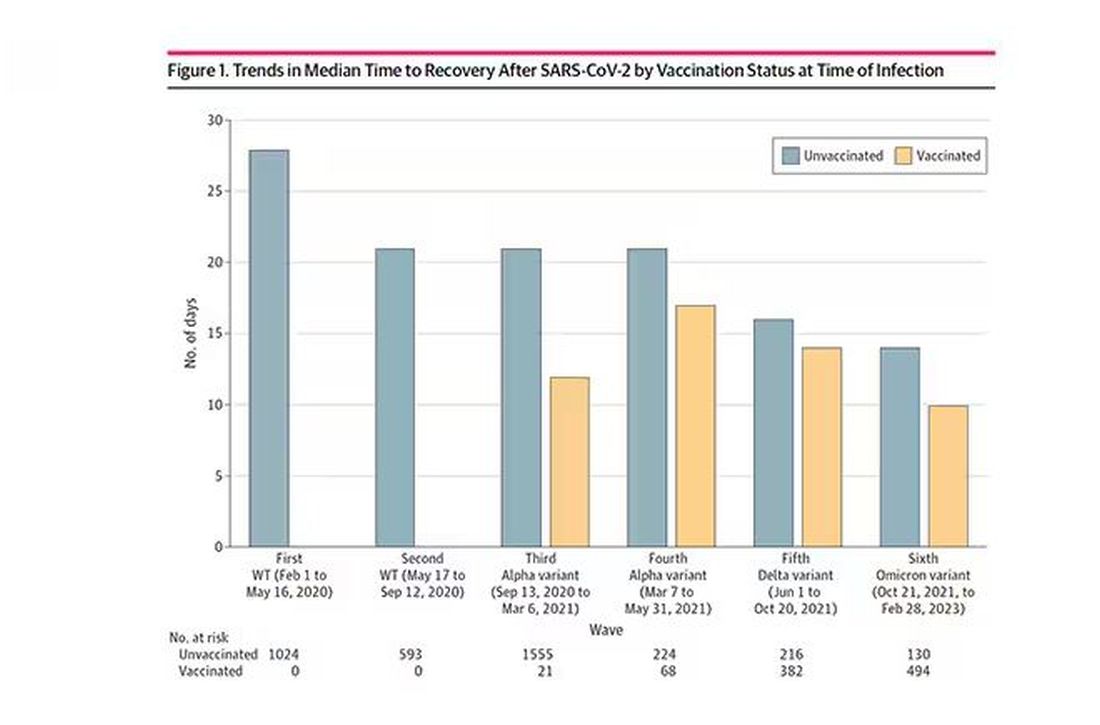
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.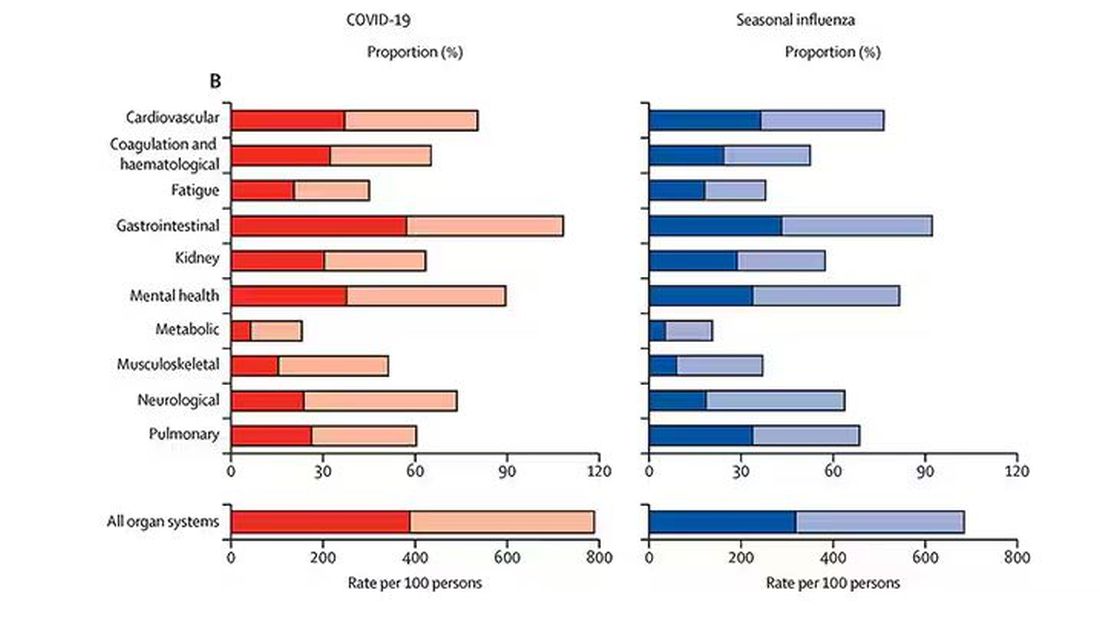
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here. 
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here. 
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Acute Sore Throat in Primary Care: When to Reach for the Antibiotics
This transcript has been edited for clarity.
There is a helpful consensus from experts on the best management of patients with acute sore throat. This is a common problem in primary care, and one for which there is a lot of evidence, opinion, and ultimately overprescribing of antibiotics. This consensus presents a pragmatic clinical approach aimed at decreasing overprescribing, yet detecting which patients are likely to benefit from treatment with antibiotics.
Let’s first go over the evidence that forms the basis for the recommendations, then the recommended approach. First, a sore throat can be caused by many different viruses, as well as group A streptococcus (GAS), the group C streptococcus S dysgalactiae, and fusobacterium. We sometimes think of throat cultures as telling us the definitive etiology of a sore throat. In fact, children commonly are colonized with GAS even when not infected — 35% of the time, when GAS is detected on throat swab in a child, GAS is not the cause of the sore throat. Very few adults are colonized with GAS.
Sore throats are usually self-limited, whether they are treated with antibiotics or not, but occasionally complications can occur. Suppurative complications include peritonsillar abscess, sinusitis and sepsis. Nonsuppurative complications are primarily glomerulonephritis and rheumatic fever, which can lead to rheumatic heart disease.
Antibiotics. Antibiotics have three potential benefits in acute sore throat: to reduce the risk of developing rheumatic heart disease, reduce the duration and severity of symptoms, and treat suppurative complications. The risk for rheumatic heart disease has almost vanished in high-income countries, but not in low-income countries. Thus, antibiotic treatment of acute sore throat due to GAS may benefit those in living in, and those who recently emigrated from, low-income countries.
Patients with suppurative complications should be identified because antibiotics are important for this group. Although antibiotics are prescribed primarily to prevent rheumatic fever in this population, they may be mildly helpful in reducing a patient’s symptoms.
Testing. The sensitivity and specificity of high-quality point-of-care tests (POCTs) are on par with those of cultures, with the advantage that the results are available within minutes. Negative tests reduce unneeded antibiotic prescriptions.
Given this evidence, the authors recommend an approach that puts a lot of emphasis on two major things: the risk for rheumatic fever, and clinical assessment. On the basis of these factors, a decision is made about the utility of POCTs and treatment with antibiotics for GAS. The risk for rheumatic fever is based on epidemiology: If the patient is in a low-income country or has recently immigrated from one, then the risk is high, and if not, the risk is low.
Complicated vs uncomplicated? This is determined by clinical assessment of the severity of the patient’s illness, including general appearance. Uncomplicated sore throat means that the patient:
- Is not getting worse after 3 days of illness
- Has a duration of illness ≤ 5 days or is getting better after day 5
- Has mild to moderate symptom severity (bilateral throat pain, the ability to open the mouth fully, and absence of a sandpaper or scarlatiniform rash or strawberry tongue)
For patients with uncomplicated sore throat and low risk for rheumatic fever, the main goals are to reduce antibiotic use and provide symptomatic relief. For these patients, an assessment such as the Centor score can be done. Those with a low Centor score (0-2) can be treated with analgesics and there is no need for a POCT.
In patients with a higher Centor score, the consensus gives two choices: They can either be tested (and treated if the testing is positive), or it is reasonable to forgo testing and use a wait-and-see strategy, with reevaluation if they are getting worse after day 3 or not improving after day 5 days of their illness. Illnesses that last longer than 5 days with sore throat and fatigue should prompt consideration of alternative diagnoses, such as infectious mononucleosis.
For patients with potentially complicated sore throat — including indicators such as worsening symptoms after 3 days or worsening after initiation of antibiotics, inability to open the mouth fully, unilateral neck pain or swelling, or rigors — should undergo a careful evaluation. The need for further testing in these patients, including labs and imaging, should be decided on a case-by-case basis. If the patient appears seriously ill, don’t rely solely on POCT for GAS, but think about other diagnoses.
Rheumatic fever. The approach is very different in patients at high risk for rheumatic fever. POCT for GAS is recommended irrespective of their clinical score, and antibiotics should be prescribed if it’s positive for GAS. If a POCT is unavailable, then the consensus recommends prescribing antibiotics for all high-risk patients who have acute sore throat.
This approach is sensible and puts a lot of emphasis on clinical evaluation, though it should be noted that this approach is considerably different from that in the 2012 Infectious Diseases Society of America guidelines.
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
There is a helpful consensus from experts on the best management of patients with acute sore throat. This is a common problem in primary care, and one for which there is a lot of evidence, opinion, and ultimately overprescribing of antibiotics. This consensus presents a pragmatic clinical approach aimed at decreasing overprescribing, yet detecting which patients are likely to benefit from treatment with antibiotics.
Let’s first go over the evidence that forms the basis for the recommendations, then the recommended approach. First, a sore throat can be caused by many different viruses, as well as group A streptococcus (GAS), the group C streptococcus S dysgalactiae, and fusobacterium. We sometimes think of throat cultures as telling us the definitive etiology of a sore throat. In fact, children commonly are colonized with GAS even when not infected — 35% of the time, when GAS is detected on throat swab in a child, GAS is not the cause of the sore throat. Very few adults are colonized with GAS.
Sore throats are usually self-limited, whether they are treated with antibiotics or not, but occasionally complications can occur. Suppurative complications include peritonsillar abscess, sinusitis and sepsis. Nonsuppurative complications are primarily glomerulonephritis and rheumatic fever, which can lead to rheumatic heart disease.
Antibiotics. Antibiotics have three potential benefits in acute sore throat: to reduce the risk of developing rheumatic heart disease, reduce the duration and severity of symptoms, and treat suppurative complications. The risk for rheumatic heart disease has almost vanished in high-income countries, but not in low-income countries. Thus, antibiotic treatment of acute sore throat due to GAS may benefit those in living in, and those who recently emigrated from, low-income countries.
Patients with suppurative complications should be identified because antibiotics are important for this group. Although antibiotics are prescribed primarily to prevent rheumatic fever in this population, they may be mildly helpful in reducing a patient’s symptoms.
Testing. The sensitivity and specificity of high-quality point-of-care tests (POCTs) are on par with those of cultures, with the advantage that the results are available within minutes. Negative tests reduce unneeded antibiotic prescriptions.
Given this evidence, the authors recommend an approach that puts a lot of emphasis on two major things: the risk for rheumatic fever, and clinical assessment. On the basis of these factors, a decision is made about the utility of POCTs and treatment with antibiotics for GAS. The risk for rheumatic fever is based on epidemiology: If the patient is in a low-income country or has recently immigrated from one, then the risk is high, and if not, the risk is low.
Complicated vs uncomplicated? This is determined by clinical assessment of the severity of the patient’s illness, including general appearance. Uncomplicated sore throat means that the patient:
- Is not getting worse after 3 days of illness
- Has a duration of illness ≤ 5 days or is getting better after day 5
- Has mild to moderate symptom severity (bilateral throat pain, the ability to open the mouth fully, and absence of a sandpaper or scarlatiniform rash or strawberry tongue)
For patients with uncomplicated sore throat and low risk for rheumatic fever, the main goals are to reduce antibiotic use and provide symptomatic relief. For these patients, an assessment such as the Centor score can be done. Those with a low Centor score (0-2) can be treated with analgesics and there is no need for a POCT.
In patients with a higher Centor score, the consensus gives two choices: They can either be tested (and treated if the testing is positive), or it is reasonable to forgo testing and use a wait-and-see strategy, with reevaluation if they are getting worse after day 3 or not improving after day 5 days of their illness. Illnesses that last longer than 5 days with sore throat and fatigue should prompt consideration of alternative diagnoses, such as infectious mononucleosis.
For patients with potentially complicated sore throat — including indicators such as worsening symptoms after 3 days or worsening after initiation of antibiotics, inability to open the mouth fully, unilateral neck pain or swelling, or rigors — should undergo a careful evaluation. The need for further testing in these patients, including labs and imaging, should be decided on a case-by-case basis. If the patient appears seriously ill, don’t rely solely on POCT for GAS, but think about other diagnoses.
Rheumatic fever. The approach is very different in patients at high risk for rheumatic fever. POCT for GAS is recommended irrespective of their clinical score, and antibiotics should be prescribed if it’s positive for GAS. If a POCT is unavailable, then the consensus recommends prescribing antibiotics for all high-risk patients who have acute sore throat.
This approach is sensible and puts a lot of emphasis on clinical evaluation, though it should be noted that this approach is considerably different from that in the 2012 Infectious Diseases Society of America guidelines.
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
There is a helpful consensus from experts on the best management of patients with acute sore throat. This is a common problem in primary care, and one for which there is a lot of evidence, opinion, and ultimately overprescribing of antibiotics. This consensus presents a pragmatic clinical approach aimed at decreasing overprescribing, yet detecting which patients are likely to benefit from treatment with antibiotics.
Let’s first go over the evidence that forms the basis for the recommendations, then the recommended approach. First, a sore throat can be caused by many different viruses, as well as group A streptococcus (GAS), the group C streptococcus S dysgalactiae, and fusobacterium. We sometimes think of throat cultures as telling us the definitive etiology of a sore throat. In fact, children commonly are colonized with GAS even when not infected — 35% of the time, when GAS is detected on throat swab in a child, GAS is not the cause of the sore throat. Very few adults are colonized with GAS.
Sore throats are usually self-limited, whether they are treated with antibiotics or not, but occasionally complications can occur. Suppurative complications include peritonsillar abscess, sinusitis and sepsis. Nonsuppurative complications are primarily glomerulonephritis and rheumatic fever, which can lead to rheumatic heart disease.
Antibiotics. Antibiotics have three potential benefits in acute sore throat: to reduce the risk of developing rheumatic heart disease, reduce the duration and severity of symptoms, and treat suppurative complications. The risk for rheumatic heart disease has almost vanished in high-income countries, but not in low-income countries. Thus, antibiotic treatment of acute sore throat due to GAS may benefit those in living in, and those who recently emigrated from, low-income countries.
Patients with suppurative complications should be identified because antibiotics are important for this group. Although antibiotics are prescribed primarily to prevent rheumatic fever in this population, they may be mildly helpful in reducing a patient’s symptoms.
Testing. The sensitivity and specificity of high-quality point-of-care tests (POCTs) are on par with those of cultures, with the advantage that the results are available within minutes. Negative tests reduce unneeded antibiotic prescriptions.
Given this evidence, the authors recommend an approach that puts a lot of emphasis on two major things: the risk for rheumatic fever, and clinical assessment. On the basis of these factors, a decision is made about the utility of POCTs and treatment with antibiotics for GAS. The risk for rheumatic fever is based on epidemiology: If the patient is in a low-income country or has recently immigrated from one, then the risk is high, and if not, the risk is low.
Complicated vs uncomplicated? This is determined by clinical assessment of the severity of the patient’s illness, including general appearance. Uncomplicated sore throat means that the patient:
- Is not getting worse after 3 days of illness
- Has a duration of illness ≤ 5 days or is getting better after day 5
- Has mild to moderate symptom severity (bilateral throat pain, the ability to open the mouth fully, and absence of a sandpaper or scarlatiniform rash or strawberry tongue)
For patients with uncomplicated sore throat and low risk for rheumatic fever, the main goals are to reduce antibiotic use and provide symptomatic relief. For these patients, an assessment such as the Centor score can be done. Those with a low Centor score (0-2) can be treated with analgesics and there is no need for a POCT.
In patients with a higher Centor score, the consensus gives two choices: They can either be tested (and treated if the testing is positive), or it is reasonable to forgo testing and use a wait-and-see strategy, with reevaluation if they are getting worse after day 3 or not improving after day 5 days of their illness. Illnesses that last longer than 5 days with sore throat and fatigue should prompt consideration of alternative diagnoses, such as infectious mononucleosis.
For patients with potentially complicated sore throat — including indicators such as worsening symptoms after 3 days or worsening after initiation of antibiotics, inability to open the mouth fully, unilateral neck pain or swelling, or rigors — should undergo a careful evaluation. The need for further testing in these patients, including labs and imaging, should be decided on a case-by-case basis. If the patient appears seriously ill, don’t rely solely on POCT for GAS, but think about other diagnoses.
Rheumatic fever. The approach is very different in patients at high risk for rheumatic fever. POCT for GAS is recommended irrespective of their clinical score, and antibiotics should be prescribed if it’s positive for GAS. If a POCT is unavailable, then the consensus recommends prescribing antibiotics for all high-risk patients who have acute sore throat.
This approach is sensible and puts a lot of emphasis on clinical evaluation, though it should be noted that this approach is considerably different from that in the 2012 Infectious Diseases Society of America guidelines.
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
Gynecologic Oncology Consult: Update on Endometrial Cancer Treatment
While rates of most other cancers have declined or plateaued, the incidence and mortality rate of endometrial cancer continue to rise.1 The landscape of endometrial cancer treatment has evolved quickly over the past 2-3 years. While surgery and radiation therapy remain the mainstay of treatment for early-stage disease, the development of multiple targeted therapeutics has led to additional treatment options in advanced-stage disease and more aggressive tumor types, which are both associated with a poorer prognosis.
In this update, we highlight the recent advances in these targeted therapies in endometrial cancer. We review the landmark NRG-GY018 and RUBY trials, which demonstrated that checkpoint inhibitors improve outcomes in women with advanced endometrial cancer. We discuss an immunotherapy and antivascular endothelial growth factor (VEGF) combination useful in certain tumors lacking biomarker expression. We also highlight progress against endometrial cancers that overexpress human epidermal growth factor receptor 2 (HER2), demonstrated in the DESTINY PanTumor-02 trial.
PD-1 inhibitors
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells that binds to programmed cell death ligand 1 (PD-L1). PD-L1 is expressed on many immune cells but can also be expressed on tumor cells. The interaction of PD-L1 expressed on the surface of endometrial cancer cells with the PD-1 receptor on T cells results in diminished T-cell function, eliminating the immune system’s ability to attack the tumor cells. Treatment with a PD-1 inhibitor prevents this ligand-receptor interaction and restores cancer-fighting function to T cells.
Pembrolizumab, an antibody against the PD-1 receptor, has been used as single-agent treatment for recurrent endometrial cancer since the KEYNOTE-158 study demonstrated clinical benefit in patients with mismatch repair deficient (dMMR) tumors.2
Additionally, in 2022, Makker et al. published results from a phase 3 trial3 evaluating immunotherapy in the treatment of recurrent endometrial cancer, specifically in patients with mismatch repair proficient (pMMR) tumors. They compared the combination of pembrolizumab and lenvatinib, an oral inhibitor of VEGF, to physician’s choice next-line chemotherapy in over 800 patients with advanced or recurrent endometrial cancer. They found that progression-free survival (PFS) was significantly improved from a median of 3.8 months with chemotherapy to a median of 6.6 months with pembrolizumab and lenvatinib in the pMMR population. Overall survival was also improved from 12 months to 17.4 months in the pMMR population.
With the clear benefit of immunotherapy in the recurrent setting established, Eskander and colleagues were the first to evaluate treatment with pembrolizumab as upfront treatment in the NRG-GY018 trial,4 comparing standard first-line chemotherapy (carboplatin and paclitaxel) with or without the addition of pembrolizumab. This randomized, international, phase 3 trial included over 800 patients with advanced or recurrent endometrial cancer of any histology except carcinosarcoma. Patients received carboplatin and paclitaxel with either pembrolizumab or placebo, followed by maintenance pembrolizumab or placebo. The results showed an improvement in PFS with the addition of immunotherapy, with a risk of disease progression or death with pembrolizumab 70% lower than with placebo in patients with dMMR tumors and 46% lower than with placebo in patients with pMMR tumors.
In the similar randomized, international, phase 3 RUBY trial,5 Mirza and colleagues compared standard chemotherapy with or without the addition of another PD-1 inhibitor, dostarlimab, in almost 500 patients with advanced or recurrent endometrial cancer of any histology. They found that the addition of dostarlimab to standard chemotherapy significantly improved PFS. Unpublished data presented at the Society of Gynecologic Oncology annual meeting in March also demonstrated an improvement in overall survival.6 As in NRG-GY018, they found a substantial benefit again in the dMMR population.
The results of these three landmark trials demonstrate the increasing role for immunotherapy in endometrial cancer, especially at the time of initial treatment, and how biomarkers can help direct treatment options.
Takeaway
Use of PD-1 inhibitors improves clinical outcomes in both the upfront and recurrent treatment settings. The magnitude of benefit of treatment with PD-1 inhibitors is greater in patients with dMMR tumors.
Anti-HER2 therapies
HER2 is a cell surface protein that can become overexpressed and promote tumorigenesis. It is used as a prognostic biomarker and a therapeutic target in breast, stomach, and colon cancer, but it has also been identified at high rates (20%-30%) in the most aggressive histologic subtypes in endometrial cancer (serous, clear cell, and carcinosarcoma). Trastuzumab is a monoclonal antibody directed against HER2, most commonly used in HER2-positive breast cancer. In 2018, a phase 2 trial demonstrated that trastuzumab combined with standard chemotherapy improved PFS in serous endometrial cancers that overexpress HER2.7 These results were important and promising given both the poor prognosis associated with the aggressive serous histology and the relative lack of effective therapies at the time of recurrence.
Recently, antibody-drug conjugates (ADCs) have come to the forefront of cancer-directed therapies. ADCs deliver chemotherapy agents directly to cancer cells via a monoclonal antibody that binds to a specific target on the cancer cells. Trastuzumab-deruxtecan (T-DXd) is an ADC consisting of an anti-HER2 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker. T-DXd was evaluated in the tumor-agnostic phase 2 DESTINY-PanTumor02 trial,8 which included endometrial, ovarian, and cervical cancer cohorts, in addition to four other nongynecologic malignancies. In this study, 40 patients with advanced or recurrent malignancies overexpressing HER2 in each cohort were treated with T-DXd.
The results within the endometrial cancer cohort were particularly promising. The overall response rate in endometrial cancer was an astounding 57.5% with a median PFS of over 11 months. Even higher response rates were seen in endometrial cancer patients whose tumors demonstrated higher rates of HER2 overexpression. These results are unprecedented in a cohort in which most patients had seen at least 2 prior lines of therapy. Ocular and pulmonary toxicities are of particular interest with use of ADCs, but they were mostly low grade and manageable in this study.
Takeaway
Anti-HER2 therapies, including antibody-drug conjugates, are effective in treating patients with some of the highest-risk endometrial cancers when they overexpress this protein.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Siegel R et al. CA Cancer J. 2024;74(1):12-49.
2. Marabelle A et al. J Clin Oncol. 2020;38(1):1-10.
3. Makker V et al. N Engl J Med. 2022;386(5):437-48.
4. Eskander RN et al. N Engl J Med. 2023;388(23):2159-70.
5. Mirza MR et al. N Engl J Med. 2023;388(23):2145-58.
6. Powell MA et al, editors. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, 2024; San Diego, CA.
7. Fader AN et al. J Clin Oncol. 2018;36(20):2044-51.
8. Meric-Bernstam F et al. J Clin Oncol. 2024;42(1):47-58.
While rates of most other cancers have declined or plateaued, the incidence and mortality rate of endometrial cancer continue to rise.1 The landscape of endometrial cancer treatment has evolved quickly over the past 2-3 years. While surgery and radiation therapy remain the mainstay of treatment for early-stage disease, the development of multiple targeted therapeutics has led to additional treatment options in advanced-stage disease and more aggressive tumor types, which are both associated with a poorer prognosis.
In this update, we highlight the recent advances in these targeted therapies in endometrial cancer. We review the landmark NRG-GY018 and RUBY trials, which demonstrated that checkpoint inhibitors improve outcomes in women with advanced endometrial cancer. We discuss an immunotherapy and antivascular endothelial growth factor (VEGF) combination useful in certain tumors lacking biomarker expression. We also highlight progress against endometrial cancers that overexpress human epidermal growth factor receptor 2 (HER2), demonstrated in the DESTINY PanTumor-02 trial.
PD-1 inhibitors
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells that binds to programmed cell death ligand 1 (PD-L1). PD-L1 is expressed on many immune cells but can also be expressed on tumor cells. The interaction of PD-L1 expressed on the surface of endometrial cancer cells with the PD-1 receptor on T cells results in diminished T-cell function, eliminating the immune system’s ability to attack the tumor cells. Treatment with a PD-1 inhibitor prevents this ligand-receptor interaction and restores cancer-fighting function to T cells.
Pembrolizumab, an antibody against the PD-1 receptor, has been used as single-agent treatment for recurrent endometrial cancer since the KEYNOTE-158 study demonstrated clinical benefit in patients with mismatch repair deficient (dMMR) tumors.2
Additionally, in 2022, Makker et al. published results from a phase 3 trial3 evaluating immunotherapy in the treatment of recurrent endometrial cancer, specifically in patients with mismatch repair proficient (pMMR) tumors. They compared the combination of pembrolizumab and lenvatinib, an oral inhibitor of VEGF, to physician’s choice next-line chemotherapy in over 800 patients with advanced or recurrent endometrial cancer. They found that progression-free survival (PFS) was significantly improved from a median of 3.8 months with chemotherapy to a median of 6.6 months with pembrolizumab and lenvatinib in the pMMR population. Overall survival was also improved from 12 months to 17.4 months in the pMMR population.
With the clear benefit of immunotherapy in the recurrent setting established, Eskander and colleagues were the first to evaluate treatment with pembrolizumab as upfront treatment in the NRG-GY018 trial,4 comparing standard first-line chemotherapy (carboplatin and paclitaxel) with or without the addition of pembrolizumab. This randomized, international, phase 3 trial included over 800 patients with advanced or recurrent endometrial cancer of any histology except carcinosarcoma. Patients received carboplatin and paclitaxel with either pembrolizumab or placebo, followed by maintenance pembrolizumab or placebo. The results showed an improvement in PFS with the addition of immunotherapy, with a risk of disease progression or death with pembrolizumab 70% lower than with placebo in patients with dMMR tumors and 46% lower than with placebo in patients with pMMR tumors.
In the similar randomized, international, phase 3 RUBY trial,5 Mirza and colleagues compared standard chemotherapy with or without the addition of another PD-1 inhibitor, dostarlimab, in almost 500 patients with advanced or recurrent endometrial cancer of any histology. They found that the addition of dostarlimab to standard chemotherapy significantly improved PFS. Unpublished data presented at the Society of Gynecologic Oncology annual meeting in March also demonstrated an improvement in overall survival.6 As in NRG-GY018, they found a substantial benefit again in the dMMR population.
The results of these three landmark trials demonstrate the increasing role for immunotherapy in endometrial cancer, especially at the time of initial treatment, and how biomarkers can help direct treatment options.
Takeaway
Use of PD-1 inhibitors improves clinical outcomes in both the upfront and recurrent treatment settings. The magnitude of benefit of treatment with PD-1 inhibitors is greater in patients with dMMR tumors.
Anti-HER2 therapies
HER2 is a cell surface protein that can become overexpressed and promote tumorigenesis. It is used as a prognostic biomarker and a therapeutic target in breast, stomach, and colon cancer, but it has also been identified at high rates (20%-30%) in the most aggressive histologic subtypes in endometrial cancer (serous, clear cell, and carcinosarcoma). Trastuzumab is a monoclonal antibody directed against HER2, most commonly used in HER2-positive breast cancer. In 2018, a phase 2 trial demonstrated that trastuzumab combined with standard chemotherapy improved PFS in serous endometrial cancers that overexpress HER2.7 These results were important and promising given both the poor prognosis associated with the aggressive serous histology and the relative lack of effective therapies at the time of recurrence.
Recently, antibody-drug conjugates (ADCs) have come to the forefront of cancer-directed therapies. ADCs deliver chemotherapy agents directly to cancer cells via a monoclonal antibody that binds to a specific target on the cancer cells. Trastuzumab-deruxtecan (T-DXd) is an ADC consisting of an anti-HER2 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker. T-DXd was evaluated in the tumor-agnostic phase 2 DESTINY-PanTumor02 trial,8 which included endometrial, ovarian, and cervical cancer cohorts, in addition to four other nongynecologic malignancies. In this study, 40 patients with advanced or recurrent malignancies overexpressing HER2 in each cohort were treated with T-DXd.
The results within the endometrial cancer cohort were particularly promising. The overall response rate in endometrial cancer was an astounding 57.5% with a median PFS of over 11 months. Even higher response rates were seen in endometrial cancer patients whose tumors demonstrated higher rates of HER2 overexpression. These results are unprecedented in a cohort in which most patients had seen at least 2 prior lines of therapy. Ocular and pulmonary toxicities are of particular interest with use of ADCs, but they were mostly low grade and manageable in this study.
Takeaway
Anti-HER2 therapies, including antibody-drug conjugates, are effective in treating patients with some of the highest-risk endometrial cancers when they overexpress this protein.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Siegel R et al. CA Cancer J. 2024;74(1):12-49.
2. Marabelle A et al. J Clin Oncol. 2020;38(1):1-10.
3. Makker V et al. N Engl J Med. 2022;386(5):437-48.
4. Eskander RN et al. N Engl J Med. 2023;388(23):2159-70.
5. Mirza MR et al. N Engl J Med. 2023;388(23):2145-58.
6. Powell MA et al, editors. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, 2024; San Diego, CA.
7. Fader AN et al. J Clin Oncol. 2018;36(20):2044-51.
8. Meric-Bernstam F et al. J Clin Oncol. 2024;42(1):47-58.
While rates of most other cancers have declined or plateaued, the incidence and mortality rate of endometrial cancer continue to rise.1 The landscape of endometrial cancer treatment has evolved quickly over the past 2-3 years. While surgery and radiation therapy remain the mainstay of treatment for early-stage disease, the development of multiple targeted therapeutics has led to additional treatment options in advanced-stage disease and more aggressive tumor types, which are both associated with a poorer prognosis.
In this update, we highlight the recent advances in these targeted therapies in endometrial cancer. We review the landmark NRG-GY018 and RUBY trials, which demonstrated that checkpoint inhibitors improve outcomes in women with advanced endometrial cancer. We discuss an immunotherapy and antivascular endothelial growth factor (VEGF) combination useful in certain tumors lacking biomarker expression. We also highlight progress against endometrial cancers that overexpress human epidermal growth factor receptor 2 (HER2), demonstrated in the DESTINY PanTumor-02 trial.
PD-1 inhibitors
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells that binds to programmed cell death ligand 1 (PD-L1). PD-L1 is expressed on many immune cells but can also be expressed on tumor cells. The interaction of PD-L1 expressed on the surface of endometrial cancer cells with the PD-1 receptor on T cells results in diminished T-cell function, eliminating the immune system’s ability to attack the tumor cells. Treatment with a PD-1 inhibitor prevents this ligand-receptor interaction and restores cancer-fighting function to T cells.
Pembrolizumab, an antibody against the PD-1 receptor, has been used as single-agent treatment for recurrent endometrial cancer since the KEYNOTE-158 study demonstrated clinical benefit in patients with mismatch repair deficient (dMMR) tumors.2
Additionally, in 2022, Makker et al. published results from a phase 3 trial3 evaluating immunotherapy in the treatment of recurrent endometrial cancer, specifically in patients with mismatch repair proficient (pMMR) tumors. They compared the combination of pembrolizumab and lenvatinib, an oral inhibitor of VEGF, to physician’s choice next-line chemotherapy in over 800 patients with advanced or recurrent endometrial cancer. They found that progression-free survival (PFS) was significantly improved from a median of 3.8 months with chemotherapy to a median of 6.6 months with pembrolizumab and lenvatinib in the pMMR population. Overall survival was also improved from 12 months to 17.4 months in the pMMR population.
With the clear benefit of immunotherapy in the recurrent setting established, Eskander and colleagues were the first to evaluate treatment with pembrolizumab as upfront treatment in the NRG-GY018 trial,4 comparing standard first-line chemotherapy (carboplatin and paclitaxel) with or without the addition of pembrolizumab. This randomized, international, phase 3 trial included over 800 patients with advanced or recurrent endometrial cancer of any histology except carcinosarcoma. Patients received carboplatin and paclitaxel with either pembrolizumab or placebo, followed by maintenance pembrolizumab or placebo. The results showed an improvement in PFS with the addition of immunotherapy, with a risk of disease progression or death with pembrolizumab 70% lower than with placebo in patients with dMMR tumors and 46% lower than with placebo in patients with pMMR tumors.
In the similar randomized, international, phase 3 RUBY trial,5 Mirza and colleagues compared standard chemotherapy with or without the addition of another PD-1 inhibitor, dostarlimab, in almost 500 patients with advanced or recurrent endometrial cancer of any histology. They found that the addition of dostarlimab to standard chemotherapy significantly improved PFS. Unpublished data presented at the Society of Gynecologic Oncology annual meeting in March also demonstrated an improvement in overall survival.6 As in NRG-GY018, they found a substantial benefit again in the dMMR population.
The results of these three landmark trials demonstrate the increasing role for immunotherapy in endometrial cancer, especially at the time of initial treatment, and how biomarkers can help direct treatment options.
Takeaway
Use of PD-1 inhibitors improves clinical outcomes in both the upfront and recurrent treatment settings. The magnitude of benefit of treatment with PD-1 inhibitors is greater in patients with dMMR tumors.
Anti-HER2 therapies
HER2 is a cell surface protein that can become overexpressed and promote tumorigenesis. It is used as a prognostic biomarker and a therapeutic target in breast, stomach, and colon cancer, but it has also been identified at high rates (20%-30%) in the most aggressive histologic subtypes in endometrial cancer (serous, clear cell, and carcinosarcoma). Trastuzumab is a monoclonal antibody directed against HER2, most commonly used in HER2-positive breast cancer. In 2018, a phase 2 trial demonstrated that trastuzumab combined with standard chemotherapy improved PFS in serous endometrial cancers that overexpress HER2.7 These results were important and promising given both the poor prognosis associated with the aggressive serous histology and the relative lack of effective therapies at the time of recurrence.
Recently, antibody-drug conjugates (ADCs) have come to the forefront of cancer-directed therapies. ADCs deliver chemotherapy agents directly to cancer cells via a monoclonal antibody that binds to a specific target on the cancer cells. Trastuzumab-deruxtecan (T-DXd) is an ADC consisting of an anti-HER2 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker. T-DXd was evaluated in the tumor-agnostic phase 2 DESTINY-PanTumor02 trial,8 which included endometrial, ovarian, and cervical cancer cohorts, in addition to four other nongynecologic malignancies. In this study, 40 patients with advanced or recurrent malignancies overexpressing HER2 in each cohort were treated with T-DXd.
The results within the endometrial cancer cohort were particularly promising. The overall response rate in endometrial cancer was an astounding 57.5% with a median PFS of over 11 months. Even higher response rates were seen in endometrial cancer patients whose tumors demonstrated higher rates of HER2 overexpression. These results are unprecedented in a cohort in which most patients had seen at least 2 prior lines of therapy. Ocular and pulmonary toxicities are of particular interest with use of ADCs, but they were mostly low grade and manageable in this study.
Takeaway
Anti-HER2 therapies, including antibody-drug conjugates, are effective in treating patients with some of the highest-risk endometrial cancers when they overexpress this protein.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Siegel R et al. CA Cancer J. 2024;74(1):12-49.
2. Marabelle A et al. J Clin Oncol. 2020;38(1):1-10.
3. Makker V et al. N Engl J Med. 2022;386(5):437-48.
4. Eskander RN et al. N Engl J Med. 2023;388(23):2159-70.
5. Mirza MR et al. N Engl J Med. 2023;388(23):2145-58.
6. Powell MA et al, editors. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, 2024; San Diego, CA.
7. Fader AN et al. J Clin Oncol. 2018;36(20):2044-51.
8. Meric-Bernstam F et al. J Clin Oncol. 2024;42(1):47-58.
The Management of Anxiety in Primary Care
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about anxiety?
Paul N. Williams, MD: Always. It’s one of my favorite topics.
Dr. Watto: We had a great guest for this podcast on anxiety — Dr. Jessi Gold, who gave us a lot of practical tips. The way she talks to her patients about anxiety is really useful. When patients say “my anxiety” or “I feel anxious,” she considers that a symptom. Anxiety can be a diagnosis or a symptom. You need to clarify what they mean when they refer to their anxiety and dig into how it affects their life.
We asked her about the Generalized Anxiety Disorder (GAD)-7 score. Like most of the experts we’ve talked to, she’s internalized that, so she doesn’t need to rely on a questionnaire. But I still rely on a questionnaire when I’m taking a history for anxiety.
We also asked her how she explains anxiety to patients. I don’t know about you, Paul, but I’ve never really thought about explaining to patients why they have anxiety.
Dr. Williams: I’ve done my best to try to normalize it, but I haven’t actually talked to patients about the evolutionary advantage of anxiety.
Dr. Watto: She frames it to patients this way: As we were evolving, it was somewhat of an advantage to be hypervigilant, to have some anxiety and a healthy amount of fear so that you weren’t killed or eaten. But now, in the modern world, anxiety isn’t playing to our advantage. Anxiety is not making them safer; it’s making their lives worse. She explains to patients that she’s trying to help them overcome that.
In terms of pharmacotherapy for anxiety, I always think about SSRIs as one of the first steps. Why not use an SNRI as first-line treatment?
Dr. Williams: I was glad we had this conversation because I feel, for whatever reason, a bit more comfortable treating depression than anxiety. In any case, Dr. Gold reaches for the SSRI first, in part because getting off an SNRI (for example, to switch to something else) can be absolutely miserable. The discontinuation effects can be severe enough to have to bridge some patients with a benzodiazepine to get them fully off the SNRI. So, an SNRI is not the first drug you should necessarily reach for.
She thinks about using an SNRI if she has tried a couple of SSRIs that have been ineffective, or if the patient has a comorbid condition that might also benefit from the SNRI in the same way that you might use a tricyclic antidepressant in the patient with both migraines and anxiety. An SNRI might be a good medication to consider in the patient with neuropathic pain and anxiety but rarely as a first-line treatment, because if it doesn’t work out, getting the patient off that medication can be a challenge.
Dr. Watto: She mentioned venlafaxine as being especially difficult to get people off of. I’ve heard that bupropion should never be used in anxiety, and if you give it, you are a terrible doctor. What did we learn about that?
Dr. Williams: It’s a drug I’ve hesitated to prescribe to patients with anxiety or even comorbid anxiety. I’m a little bit nervous for someone who has depression and anxiety to prescribe bupropion because it can be activating and make things worse. But Dr. Gold says that she has seen bupropion work for some patients so she will consider it, especially for patients who don’t want to gain weight, or for whom sexual side effects would be bothersome. So, it’s not always the wrong answer. In her expert opinion, you can try it and see how the patient responds, using shared decision-making and letting the patient know that they may not tolerate it as well as other medications.
Dr. Watto: She sees a lot of younger people — students, working professionals — who do not want to gain weight, and that’s understandable. She will tell patients, “We can try bupropion, but if you get more anxious, we might not be able to continue it. We might have to use one of the first-line agents instead.”
Dr. Williams: We talked about mirtazapine as well. She tells patients they are going to gain weight with it. You have to have that conversation with the patient to see whether that is something they are willing to tolerate. If so, mirtazapine might be worth a try, but you have to be upfront about the potential side effects and know what the medications you’re prescribing will do to patients.
Dr. Watto: We asked her about benzodiazepines. For as-needed medication for people who are experiencing panic or anxiety attacks, she prescribes propranolol 10-20 mg twice a day as needed, which is a low dose. In primary care, we use higher doses for migraine prophylaxis.
She uses propranolol because for some patients, it’s the physical symptoms of anxiety that are bothering them. She can calm down the physical symptoms with that and get by without needing to use a benzodiazepine.
But what about thoughts that make people anxious? Can we change people’s thoughts with medication?
Dr. Williams: Dr. Gold made the point that we can medicate away insomnia, for the most part. We can medicate away the physical symptoms of anxiety, which can be really bothersome. But we can’t medicate away thoughts and thought patterns. You can make patients feel better with medications, but you may not be able to get rid of the persistent bothersome thoughts. That’s where cognitive-behavioral therapy can be especially helpful. Most of these patients would benefit from therapy.
Dr. Watto: I completely agree with that. We talked about so many great things with Dr. Gold, but we can’t recap all of it here. Please click on this link to hear the full podcast episode.
Dr. Watto is Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania. He has disclosed no relevant financial relationships. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania. He disclosed receiving income from The Curbsiders. The Curbsiders is an internal medicine podcast, in which three board-certified internists interview experts on clinically important topics. In a collaboration with Medscape, the Curbsiders share clinical pearls and practice-changing knowledge from selected podcasts.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about anxiety?
Paul N. Williams, MD: Always. It’s one of my favorite topics.
Dr. Watto: We had a great guest for this podcast on anxiety — Dr. Jessi Gold, who gave us a lot of practical tips. The way she talks to her patients about anxiety is really useful. When patients say “my anxiety” or “I feel anxious,” she considers that a symptom. Anxiety can be a diagnosis or a symptom. You need to clarify what they mean when they refer to their anxiety and dig into how it affects their life.
We asked her about the Generalized Anxiety Disorder (GAD)-7 score. Like most of the experts we’ve talked to, she’s internalized that, so she doesn’t need to rely on a questionnaire. But I still rely on a questionnaire when I’m taking a history for anxiety.
We also asked her how she explains anxiety to patients. I don’t know about you, Paul, but I’ve never really thought about explaining to patients why they have anxiety.
Dr. Williams: I’ve done my best to try to normalize it, but I haven’t actually talked to patients about the evolutionary advantage of anxiety.
Dr. Watto: She frames it to patients this way: As we were evolving, it was somewhat of an advantage to be hypervigilant, to have some anxiety and a healthy amount of fear so that you weren’t killed or eaten. But now, in the modern world, anxiety isn’t playing to our advantage. Anxiety is not making them safer; it’s making their lives worse. She explains to patients that she’s trying to help them overcome that.
In terms of pharmacotherapy for anxiety, I always think about SSRIs as one of the first steps. Why not use an SNRI as first-line treatment?
Dr. Williams: I was glad we had this conversation because I feel, for whatever reason, a bit more comfortable treating depression than anxiety. In any case, Dr. Gold reaches for the SSRI first, in part because getting off an SNRI (for example, to switch to something else) can be absolutely miserable. The discontinuation effects can be severe enough to have to bridge some patients with a benzodiazepine to get them fully off the SNRI. So, an SNRI is not the first drug you should necessarily reach for.
She thinks about using an SNRI if she has tried a couple of SSRIs that have been ineffective, or if the patient has a comorbid condition that might also benefit from the SNRI in the same way that you might use a tricyclic antidepressant in the patient with both migraines and anxiety. An SNRI might be a good medication to consider in the patient with neuropathic pain and anxiety but rarely as a first-line treatment, because if it doesn’t work out, getting the patient off that medication can be a challenge.
Dr. Watto: She mentioned venlafaxine as being especially difficult to get people off of. I’ve heard that bupropion should never be used in anxiety, and if you give it, you are a terrible doctor. What did we learn about that?
Dr. Williams: It’s a drug I’ve hesitated to prescribe to patients with anxiety or even comorbid anxiety. I’m a little bit nervous for someone who has depression and anxiety to prescribe bupropion because it can be activating and make things worse. But Dr. Gold says that she has seen bupropion work for some patients so she will consider it, especially for patients who don’t want to gain weight, or for whom sexual side effects would be bothersome. So, it’s not always the wrong answer. In her expert opinion, you can try it and see how the patient responds, using shared decision-making and letting the patient know that they may not tolerate it as well as other medications.
Dr. Watto: She sees a lot of younger people — students, working professionals — who do not want to gain weight, and that’s understandable. She will tell patients, “We can try bupropion, but if you get more anxious, we might not be able to continue it. We might have to use one of the first-line agents instead.”
Dr. Williams: We talked about mirtazapine as well. She tells patients they are going to gain weight with it. You have to have that conversation with the patient to see whether that is something they are willing to tolerate. If so, mirtazapine might be worth a try, but you have to be upfront about the potential side effects and know what the medications you’re prescribing will do to patients.
Dr. Watto: We asked her about benzodiazepines. For as-needed medication for people who are experiencing panic or anxiety attacks, she prescribes propranolol 10-20 mg twice a day as needed, which is a low dose. In primary care, we use higher doses for migraine prophylaxis.
She uses propranolol because for some patients, it’s the physical symptoms of anxiety that are bothering them. She can calm down the physical symptoms with that and get by without needing to use a benzodiazepine.
But what about thoughts that make people anxious? Can we change people’s thoughts with medication?
Dr. Williams: Dr. Gold made the point that we can medicate away insomnia, for the most part. We can medicate away the physical symptoms of anxiety, which can be really bothersome. But we can’t medicate away thoughts and thought patterns. You can make patients feel better with medications, but you may not be able to get rid of the persistent bothersome thoughts. That’s where cognitive-behavioral therapy can be especially helpful. Most of these patients would benefit from therapy.
Dr. Watto: I completely agree with that. We talked about so many great things with Dr. Gold, but we can’t recap all of it here. Please click on this link to hear the full podcast episode.
Dr. Watto is Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania. He has disclosed no relevant financial relationships. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania. He disclosed receiving income from The Curbsiders. The Curbsiders is an internal medicine podcast, in which three board-certified internists interview experts on clinically important topics. In a collaboration with Medscape, the Curbsiders share clinical pearls and practice-changing knowledge from selected podcasts.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about anxiety?
Paul N. Williams, MD: Always. It’s one of my favorite topics.
Dr. Watto: We had a great guest for this podcast on anxiety — Dr. Jessi Gold, who gave us a lot of practical tips. The way she talks to her patients about anxiety is really useful. When patients say “my anxiety” or “I feel anxious,” she considers that a symptom. Anxiety can be a diagnosis or a symptom. You need to clarify what they mean when they refer to their anxiety and dig into how it affects their life.
We asked her about the Generalized Anxiety Disorder (GAD)-7 score. Like most of the experts we’ve talked to, she’s internalized that, so she doesn’t need to rely on a questionnaire. But I still rely on a questionnaire when I’m taking a history for anxiety.
We also asked her how she explains anxiety to patients. I don’t know about you, Paul, but I’ve never really thought about explaining to patients why they have anxiety.
Dr. Williams: I’ve done my best to try to normalize it, but I haven’t actually talked to patients about the evolutionary advantage of anxiety.
Dr. Watto: She frames it to patients this way: As we were evolving, it was somewhat of an advantage to be hypervigilant, to have some anxiety and a healthy amount of fear so that you weren’t killed or eaten. But now, in the modern world, anxiety isn’t playing to our advantage. Anxiety is not making them safer; it’s making their lives worse. She explains to patients that she’s trying to help them overcome that.
In terms of pharmacotherapy for anxiety, I always think about SSRIs as one of the first steps. Why not use an SNRI as first-line treatment?
Dr. Williams: I was glad we had this conversation because I feel, for whatever reason, a bit more comfortable treating depression than anxiety. In any case, Dr. Gold reaches for the SSRI first, in part because getting off an SNRI (for example, to switch to something else) can be absolutely miserable. The discontinuation effects can be severe enough to have to bridge some patients with a benzodiazepine to get them fully off the SNRI. So, an SNRI is not the first drug you should necessarily reach for.
She thinks about using an SNRI if she has tried a couple of SSRIs that have been ineffective, or if the patient has a comorbid condition that might also benefit from the SNRI in the same way that you might use a tricyclic antidepressant in the patient with both migraines and anxiety. An SNRI might be a good medication to consider in the patient with neuropathic pain and anxiety but rarely as a first-line treatment, because if it doesn’t work out, getting the patient off that medication can be a challenge.
Dr. Watto: She mentioned venlafaxine as being especially difficult to get people off of. I’ve heard that bupropion should never be used in anxiety, and if you give it, you are a terrible doctor. What did we learn about that?
Dr. Williams: It’s a drug I’ve hesitated to prescribe to patients with anxiety or even comorbid anxiety. I’m a little bit nervous for someone who has depression and anxiety to prescribe bupropion because it can be activating and make things worse. But Dr. Gold says that she has seen bupropion work for some patients so she will consider it, especially for patients who don’t want to gain weight, or for whom sexual side effects would be bothersome. So, it’s not always the wrong answer. In her expert opinion, you can try it and see how the patient responds, using shared decision-making and letting the patient know that they may not tolerate it as well as other medications.
Dr. Watto: She sees a lot of younger people — students, working professionals — who do not want to gain weight, and that’s understandable. She will tell patients, “We can try bupropion, but if you get more anxious, we might not be able to continue it. We might have to use one of the first-line agents instead.”
Dr. Williams: We talked about mirtazapine as well. She tells patients they are going to gain weight with it. You have to have that conversation with the patient to see whether that is something they are willing to tolerate. If so, mirtazapine might be worth a try, but you have to be upfront about the potential side effects and know what the medications you’re prescribing will do to patients.
Dr. Watto: We asked her about benzodiazepines. For as-needed medication for people who are experiencing panic or anxiety attacks, she prescribes propranolol 10-20 mg twice a day as needed, which is a low dose. In primary care, we use higher doses for migraine prophylaxis.
She uses propranolol because for some patients, it’s the physical symptoms of anxiety that are bothering them. She can calm down the physical symptoms with that and get by without needing to use a benzodiazepine.
But what about thoughts that make people anxious? Can we change people’s thoughts with medication?
Dr. Williams: Dr. Gold made the point that we can medicate away insomnia, for the most part. We can medicate away the physical symptoms of anxiety, which can be really bothersome. But we can’t medicate away thoughts and thought patterns. You can make patients feel better with medications, but you may not be able to get rid of the persistent bothersome thoughts. That’s where cognitive-behavioral therapy can be especially helpful. Most of these patients would benefit from therapy.
Dr. Watto: I completely agree with that. We talked about so many great things with Dr. Gold, but we can’t recap all of it here. Please click on this link to hear the full podcast episode.
Dr. Watto is Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania. He has disclosed no relevant financial relationships. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania. He disclosed receiving income from The Curbsiders. The Curbsiders is an internal medicine podcast, in which three board-certified internists interview experts on clinically important topics. In a collaboration with Medscape, the Curbsiders share clinical pearls and practice-changing knowledge from selected podcasts.
A version of this article appeared on Medscape.com.
How to Better Diagnose and Manage Rumination Syndrome
Rumination syndrome is a well-recognized functional disorder characterized by the regurgitation of food or liquid in the absence of retching or nausea.
Evidence suggests that the prevalence of rumination syndrome is increasing. In a 2022 health survey study conducted across 26 countries — the largest epidemiologic study to date on rumination syndrome — investigators reported that it had a global prevalence of 3.1% in adults. This was higher than reported in most prior country-specific studies. More recently, a systematic review and meta-analysis from 2024 reported the pooled prevalence of rumination syndrome as 3.7% in adults and 0.4% in children. Both reports noted that female gender, anxiety, and depression were independent risk factors associated with rumination syndrome.
Recognition of this disorder is crucial in order for clinicians to better diagnose and manage it in their patients.
Making the Diagnosis
The diagnosis of rumination syndrome is currently based on the Rome IV consensus criteria, which were last updated in 2016. These include three diagnostic criteria essential to remember as discriminant for rumination syndrome:
- Regurgitation is the effortless return of gastric contents (recognizable food) retrograde back into the esophagus and/or mouth.
- This is not preceded by retching and not associated with nausea.
- These symptoms must have started at least 6 months before evaluation, been evident over the past 3 months, and occurred at least two to three times per month.
Although this diagnosis will be highly suspected after taking an astute clinical history, you will still need to rule out the presence of underlying organic disease.
Nearly one quarter of patients with eating disorders — which commonly accompany gastrointestinal disorders — will not have been diagnosed by the time they visit with a gastroenterologist. Therefore, gastroenterologists should be vigilant in screening for eating disorders. Notably, severe weight loss, malnutrition, electrolyte abnormalities, and dental erosions (due to acid etching) are uncommon in rumination syndrome. If such symptoms are present, it increases the possibility of an underlying eating disorder rather than primary regurgitation.
Previously, there were no published, validated questionnaires to assess the diagnosis or symptomatic response to therapies for rumination syndrome. This has recently changed with the development of a novel eight-point questionnaire that assesses frequency, severity, type of regurgitant, timing of regurgitation in relation to the meal, weight loss, and use of and response to proton pump inhibitors.
This questionnaire was recently implemented in five patients diagnosed with rumination syndrome. Albeit an extremely small trial, it nonetheless showed clinical improvement in scores associated with therapeutic intervention. Further evaluation of this tool is needed.
The diagnosis of rumination syndrome can be confirmed using impedance manometry in persons with evidence of reflux extending to the proximal esophagus, which is associated with an intragastric pressure > 30 mmHg in adults or > 25 mmHg in children.
Gastric emptying studies are typically not required to make a diagnosis unless the clinical symptoms are atypical and an alternative motility disorder is suspected. Endoscopy is performed to rule out a mechanical disorder.
Histopathologic Evidence
New data indicate that there may be specific histologic changes associated with rumination syndrome. A 2023 meta-analysis reported that patients with rumination syndrome had duodenal histologic evidence of increased lymphocytes and eosinophils, which have been associated with epithelial barrier dysfunction, microbial changes, and systemic immune activation in eosinophilic duodenitis.
If these histologic changes are validated, they may suggest future novel diagnostic and treatment approaches, at least for a subset of people with rumination syndrome.
Best Available Treatments
The first-line therapeutic treatment for rumination syndrome is diaphragmatic breathing.
I recommended using diaphragmatic breathing for this indication in a previous commentary, in which I noted that it can essentially serve as yoga for the diaphragm and abdominal muscles and advised patients to focus on breathing “through” their belly button.
Patients are instructed to breath in through their nose for 4-6 seconds, hold their breath for 2-3 seconds, and then breath out slowly against pursed lips. They can be supine or upright but should sense their abdominal muscles expand with inhaling, not move their chest wall, and completely relax their abdominal muscles upon exhaling.
Although there is no standard frequency or duration for diaphragmatic breathing, I routinely recommend patients try it after each meal for 10-15 minutes and, if possible, more during the day and in times of stress or anxiety.
Cognitive-behavioral therapies have been shown to be effective alternatives to diaphragmatic breathing.
There is some evidence that hypnosis and biofeedback-guided control of abdominothoracic muscle activity can also be effective options in treating rumination syndrome.
Robust data on pharmacologic treatments for rumination syndrome are lacking, with the exception of a randomized crossover study of baclofen. In this study, baclofen (10 mg three times daily) was significantly more effective than placebo (P = .04) in reducing regurgitation events. Investigators theorized that baclofen counteracts transient lower esophageal sphincter (LES) relaxations by increasing basal LES pressure, thereby potentially reducing regurgitation episodes. The most notable treatment side effects were somnolence, confusion, and dizziness, which may limit its extended use.
A Potentially Reversible Habit
Rumination syndrome is considered an acquired habit and, therefore, should be reversible.
Although there is no recent evidence in the literature that rumination syndrome contributes to a reduced survival rate, older data suggested adult mortality rates of 12%-20% (mostly in patients who were institutionalized). Additionally, rumination syndrome has been shown to diminish quality of life.
The best approach to improving the clinical outcomes of patients with rumination syndrome is to enlist a collaborative interprofessional team that includes physicians, behavioral therapists, and nurses to coordinate and optimize existing treatment strategies.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed the following relevant financial relationships: advisor to ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Rumination syndrome is a well-recognized functional disorder characterized by the regurgitation of food or liquid in the absence of retching or nausea.
Evidence suggests that the prevalence of rumination syndrome is increasing. In a 2022 health survey study conducted across 26 countries — the largest epidemiologic study to date on rumination syndrome — investigators reported that it had a global prevalence of 3.1% in adults. This was higher than reported in most prior country-specific studies. More recently, a systematic review and meta-analysis from 2024 reported the pooled prevalence of rumination syndrome as 3.7% in adults and 0.4% in children. Both reports noted that female gender, anxiety, and depression were independent risk factors associated with rumination syndrome.
Recognition of this disorder is crucial in order for clinicians to better diagnose and manage it in their patients.
Making the Diagnosis
The diagnosis of rumination syndrome is currently based on the Rome IV consensus criteria, which were last updated in 2016. These include three diagnostic criteria essential to remember as discriminant for rumination syndrome:
- Regurgitation is the effortless return of gastric contents (recognizable food) retrograde back into the esophagus and/or mouth.
- This is not preceded by retching and not associated with nausea.
- These symptoms must have started at least 6 months before evaluation, been evident over the past 3 months, and occurred at least two to three times per month.
Although this diagnosis will be highly suspected after taking an astute clinical history, you will still need to rule out the presence of underlying organic disease.
Nearly one quarter of patients with eating disorders — which commonly accompany gastrointestinal disorders — will not have been diagnosed by the time they visit with a gastroenterologist. Therefore, gastroenterologists should be vigilant in screening for eating disorders. Notably, severe weight loss, malnutrition, electrolyte abnormalities, and dental erosions (due to acid etching) are uncommon in rumination syndrome. If such symptoms are present, it increases the possibility of an underlying eating disorder rather than primary regurgitation.
Previously, there were no published, validated questionnaires to assess the diagnosis or symptomatic response to therapies for rumination syndrome. This has recently changed with the development of a novel eight-point questionnaire that assesses frequency, severity, type of regurgitant, timing of regurgitation in relation to the meal, weight loss, and use of and response to proton pump inhibitors.
This questionnaire was recently implemented in five patients diagnosed with rumination syndrome. Albeit an extremely small trial, it nonetheless showed clinical improvement in scores associated with therapeutic intervention. Further evaluation of this tool is needed.
The diagnosis of rumination syndrome can be confirmed using impedance manometry in persons with evidence of reflux extending to the proximal esophagus, which is associated with an intragastric pressure > 30 mmHg in adults or > 25 mmHg in children.
Gastric emptying studies are typically not required to make a diagnosis unless the clinical symptoms are atypical and an alternative motility disorder is suspected. Endoscopy is performed to rule out a mechanical disorder.
Histopathologic Evidence
New data indicate that there may be specific histologic changes associated with rumination syndrome. A 2023 meta-analysis reported that patients with rumination syndrome had duodenal histologic evidence of increased lymphocytes and eosinophils, which have been associated with epithelial barrier dysfunction, microbial changes, and systemic immune activation in eosinophilic duodenitis.
If these histologic changes are validated, they may suggest future novel diagnostic and treatment approaches, at least for a subset of people with rumination syndrome.
Best Available Treatments
The first-line therapeutic treatment for rumination syndrome is diaphragmatic breathing.
I recommended using diaphragmatic breathing for this indication in a previous commentary, in which I noted that it can essentially serve as yoga for the diaphragm and abdominal muscles and advised patients to focus on breathing “through” their belly button.
Patients are instructed to breath in through their nose for 4-6 seconds, hold their breath for 2-3 seconds, and then breath out slowly against pursed lips. They can be supine or upright but should sense their abdominal muscles expand with inhaling, not move their chest wall, and completely relax their abdominal muscles upon exhaling.
Although there is no standard frequency or duration for diaphragmatic breathing, I routinely recommend patients try it after each meal for 10-15 minutes and, if possible, more during the day and in times of stress or anxiety.
Cognitive-behavioral therapies have been shown to be effective alternatives to diaphragmatic breathing.
There is some evidence that hypnosis and biofeedback-guided control of abdominothoracic muscle activity can also be effective options in treating rumination syndrome.
Robust data on pharmacologic treatments for rumination syndrome are lacking, with the exception of a randomized crossover study of baclofen. In this study, baclofen (10 mg three times daily) was significantly more effective than placebo (P = .04) in reducing regurgitation events. Investigators theorized that baclofen counteracts transient lower esophageal sphincter (LES) relaxations by increasing basal LES pressure, thereby potentially reducing regurgitation episodes. The most notable treatment side effects were somnolence, confusion, and dizziness, which may limit its extended use.
A Potentially Reversible Habit
Rumination syndrome is considered an acquired habit and, therefore, should be reversible.
Although there is no recent evidence in the literature that rumination syndrome contributes to a reduced survival rate, older data suggested adult mortality rates of 12%-20% (mostly in patients who were institutionalized). Additionally, rumination syndrome has been shown to diminish quality of life.
The best approach to improving the clinical outcomes of patients with rumination syndrome is to enlist a collaborative interprofessional team that includes physicians, behavioral therapists, and nurses to coordinate and optimize existing treatment strategies.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed the following relevant financial relationships: advisor to ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Rumination syndrome is a well-recognized functional disorder characterized by the regurgitation of food or liquid in the absence of retching or nausea.
Evidence suggests that the prevalence of rumination syndrome is increasing. In a 2022 health survey study conducted across 26 countries — the largest epidemiologic study to date on rumination syndrome — investigators reported that it had a global prevalence of 3.1% in adults. This was higher than reported in most prior country-specific studies. More recently, a systematic review and meta-analysis from 2024 reported the pooled prevalence of rumination syndrome as 3.7% in adults and 0.4% in children. Both reports noted that female gender, anxiety, and depression were independent risk factors associated with rumination syndrome.
Recognition of this disorder is crucial in order for clinicians to better diagnose and manage it in their patients.
Making the Diagnosis
The diagnosis of rumination syndrome is currently based on the Rome IV consensus criteria, which were last updated in 2016. These include three diagnostic criteria essential to remember as discriminant for rumination syndrome:
- Regurgitation is the effortless return of gastric contents (recognizable food) retrograde back into the esophagus and/or mouth.
- This is not preceded by retching and not associated with nausea.
- These symptoms must have started at least 6 months before evaluation, been evident over the past 3 months, and occurred at least two to three times per month.
Although this diagnosis will be highly suspected after taking an astute clinical history, you will still need to rule out the presence of underlying organic disease.
Nearly one quarter of patients with eating disorders — which commonly accompany gastrointestinal disorders — will not have been diagnosed by the time they visit with a gastroenterologist. Therefore, gastroenterologists should be vigilant in screening for eating disorders. Notably, severe weight loss, malnutrition, electrolyte abnormalities, and dental erosions (due to acid etching) are uncommon in rumination syndrome. If such symptoms are present, it increases the possibility of an underlying eating disorder rather than primary regurgitation.
Previously, there were no published, validated questionnaires to assess the diagnosis or symptomatic response to therapies for rumination syndrome. This has recently changed with the development of a novel eight-point questionnaire that assesses frequency, severity, type of regurgitant, timing of regurgitation in relation to the meal, weight loss, and use of and response to proton pump inhibitors.
This questionnaire was recently implemented in five patients diagnosed with rumination syndrome. Albeit an extremely small trial, it nonetheless showed clinical improvement in scores associated with therapeutic intervention. Further evaluation of this tool is needed.
The diagnosis of rumination syndrome can be confirmed using impedance manometry in persons with evidence of reflux extending to the proximal esophagus, which is associated with an intragastric pressure > 30 mmHg in adults or > 25 mmHg in children.
Gastric emptying studies are typically not required to make a diagnosis unless the clinical symptoms are atypical and an alternative motility disorder is suspected. Endoscopy is performed to rule out a mechanical disorder.
Histopathologic Evidence
New data indicate that there may be specific histologic changes associated with rumination syndrome. A 2023 meta-analysis reported that patients with rumination syndrome had duodenal histologic evidence of increased lymphocytes and eosinophils, which have been associated with epithelial barrier dysfunction, microbial changes, and systemic immune activation in eosinophilic duodenitis.
If these histologic changes are validated, they may suggest future novel diagnostic and treatment approaches, at least for a subset of people with rumination syndrome.
Best Available Treatments
The first-line therapeutic treatment for rumination syndrome is diaphragmatic breathing.
I recommended using diaphragmatic breathing for this indication in a previous commentary, in which I noted that it can essentially serve as yoga for the diaphragm and abdominal muscles and advised patients to focus on breathing “through” their belly button.
Patients are instructed to breath in through their nose for 4-6 seconds, hold their breath for 2-3 seconds, and then breath out slowly against pursed lips. They can be supine or upright but should sense their abdominal muscles expand with inhaling, not move their chest wall, and completely relax their abdominal muscles upon exhaling.
Although there is no standard frequency or duration for diaphragmatic breathing, I routinely recommend patients try it after each meal for 10-15 minutes and, if possible, more during the day and in times of stress or anxiety.
Cognitive-behavioral therapies have been shown to be effective alternatives to diaphragmatic breathing.
There is some evidence that hypnosis and biofeedback-guided control of abdominothoracic muscle activity can also be effective options in treating rumination syndrome.
Robust data on pharmacologic treatments for rumination syndrome are lacking, with the exception of a randomized crossover study of baclofen. In this study, baclofen (10 mg three times daily) was significantly more effective than placebo (P = .04) in reducing regurgitation events. Investigators theorized that baclofen counteracts transient lower esophageal sphincter (LES) relaxations by increasing basal LES pressure, thereby potentially reducing regurgitation episodes. The most notable treatment side effects were somnolence, confusion, and dizziness, which may limit its extended use.
A Potentially Reversible Habit
Rumination syndrome is considered an acquired habit and, therefore, should be reversible.
Although there is no recent evidence in the literature that rumination syndrome contributes to a reduced survival rate, older data suggested adult mortality rates of 12%-20% (mostly in patients who were institutionalized). Additionally, rumination syndrome has been shown to diminish quality of life.
The best approach to improving the clinical outcomes of patients with rumination syndrome is to enlist a collaborative interprofessional team that includes physicians, behavioral therapists, and nurses to coordinate and optimize existing treatment strategies.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed the following relevant financial relationships: advisor to ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Continuous Glucose Monitors Should Not Be Normalized
Should we now recommend continuous glucose monitoring to all our patients, even those without diabetes? Most of us would instinctively say “no” to this question, but we are seeing opinions from doctors recommending it, and in recent years, scientific literature has focused on the subject.
Today, anyone can get an arm patch that continuously measures interstitial glucose, which is closely related to blood sugar. The information can be read on a dedicated reader or on a mobile phone by scanning the patch or, with some models, without even doing anything.
There is a consensus for prescribing continuous glucose monitoring for patients with type 1 or type 2 diabetes who are treated with at least three insulin injections. Not only is the use of continuous glucose monitoring much more comfortable than self-monitoring with finger sticks, but continuous monitoring also helps reduce glycosylated hemoglobin while decreasing the risk for hypoglycemia. Recently, another indication has begun to be reimbursed in France: Type 2 diabetes under mono-insulin injection when the diabetes is not well controlled.
But alongside these situations, there are two questions that are worth considering.
Untreated Type 2 Diabetes
First, is continuous glucose monitoring desirable for all patients with diabetes, even those not treated with insulin and even when blood sugar levels are well managed? Intuitively, one might think that it can’t hurt and that continuous monitoring of blood sugar can only improve things. We have some evidence supporting this idea, but the level of proof is quite weak. It is not clear that continuous monitoring can improve patients’ awareness of the impact of dietary choices or physical activity on blood sugar. Obviously, one can imagine that continuously monitoring glucose will encourage a shift toward more beneficial behaviors. But honestly, today, we do not have proof that wearing a continuous glucose monitor can improve behaviors in patients with type 2 diabetes who are treated with noninsulin antidiabetic medications.
Furthermore, a significant study has shown that while the effectiveness is more evident in patients treated with insulin, strong evidence suggests that continuous glucose monitoring could also reduce glycosylated hemoglobin in patients with type 2 diabetes who are not treated with insulin. A close examination of the results suggests that the benefits generally are less than those observed in insulin-treated patients with diabetes.
When we look at the scientific literature, two factors seem particularly important to consider if choosing to prescribe a continuous glucose monitoring sensor. The first is the method used, because the results can vary depending on the method. It appears that only self-monitoring that allows the patient to follow glucose in real time is effective, unlike blind monitoring that allows only a retrospective analysis of blood sugar levels. In the latter case, the patient wears the sensor, and after a week, 10 days, or 15 days, the results are analyzed, possibly with a health care provider. It seems that this is not very effective in improving glycosylated hemoglobin and dietary and physical activity behavior.
The second essential factor to consider is the need for an education program for the use of these sensors to be helpful. If sensors are used but nothing else is done, it does not seem logical. Seeing blood sugar levels without being able to understand them and act accordingly seems of little use. Scientific literature seems to confirm this idea.
Patients Without Diabetes
Now there is another question. We have discussed patients with type 2 diabetes without insulin. It’s trendy to talk about the potential benefits of continuous glucose monitors in patients without diabetes. The idea is emerging that these monitors could be used to refine the diagnosis of diabetes or to better predict the onset of diabetes in the subsequent years.
Others claim that continuous glucose monitors are an effective way to induce a change in dietary and physical activity behaviors in patients with prediabetes. One can, for example, tell a patient, “You are at risk of developing diabetes, so by monitoring your glucose, you will change your behavior.” Honestly, the scientific data we have today do not support these ideas, and I sincerely believe that it is not advisable today to recommend, as some would like, the mass use of monitors, whether in patients with overweight or obesity, or in patients with prediabetes. This goes for suggestions for using the monitor for 7-10 days per year, in the form of a session to try to reduce the risk for diabetes by motivating patients to change their behavior. We have no evidence at all that this can work. And in my opinion, with this kind of discourse, we ultimately risk, as usual, encouraging patients who are already “fans” of self-checks and self-monitoring to get health data, even if they do not know how to interpret it. Maybe even the doctor they ask for interpretation will not be trained to interpret the results of these monitors.
Spreading the idea that monitors are useful for preventing diabetes has a side effect: It hinders progress on the essential issue. Today, one of the problems in diabetes and prediabetes is that screening is not done often enough, and 20% of patients with diabetes are still unaware of their diagnosis. The management of early diabetes or prediabetes, in my opinion, is not optimal in routine care today. So, I think that adding the idea that using monitors could be beneficial dilutes the main information.
Having said that, I sometimes offer continuous glucose monitoring to some of my patients on a case-by-case basis. I believe that with proper support and an educational program, it can be beneficial for certain patients.
In Practice
In summary, I am totally opposed to the normalization of the use of monitors. I think it is our role as health care professionals to warn the public that even if it is accessible — anyone can buy a reader, a sensor — it is not necessarily beneficial, and it may even distract us from what is essential. But as a specialist, I think that using a monitor within a genuine care plan seems reasonable. Ultimately, it’s just personalized medicine.
Dr. Hansel is an endocrinologist-diabetologist and nutritionist, Department of Diabetology-Endocrinology-Nutrition, Hôpital Bichat, and a university lecturer and hospital practitioner, Université Paris-Diderot, France. He discloses ties with Iriade, Sanofi-Aventis, and Amgen.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should we now recommend continuous glucose monitoring to all our patients, even those without diabetes? Most of us would instinctively say “no” to this question, but we are seeing opinions from doctors recommending it, and in recent years, scientific literature has focused on the subject.
Today, anyone can get an arm patch that continuously measures interstitial glucose, which is closely related to blood sugar. The information can be read on a dedicated reader or on a mobile phone by scanning the patch or, with some models, without even doing anything.
There is a consensus for prescribing continuous glucose monitoring for patients with type 1 or type 2 diabetes who are treated with at least three insulin injections. Not only is the use of continuous glucose monitoring much more comfortable than self-monitoring with finger sticks, but continuous monitoring also helps reduce glycosylated hemoglobin while decreasing the risk for hypoglycemia. Recently, another indication has begun to be reimbursed in France: Type 2 diabetes under mono-insulin injection when the diabetes is not well controlled.
But alongside these situations, there are two questions that are worth considering.
Untreated Type 2 Diabetes
First, is continuous glucose monitoring desirable for all patients with diabetes, even those not treated with insulin and even when blood sugar levels are well managed? Intuitively, one might think that it can’t hurt and that continuous monitoring of blood sugar can only improve things. We have some evidence supporting this idea, but the level of proof is quite weak. It is not clear that continuous monitoring can improve patients’ awareness of the impact of dietary choices or physical activity on blood sugar. Obviously, one can imagine that continuously monitoring glucose will encourage a shift toward more beneficial behaviors. But honestly, today, we do not have proof that wearing a continuous glucose monitor can improve behaviors in patients with type 2 diabetes who are treated with noninsulin antidiabetic medications.
Furthermore, a significant study has shown that while the effectiveness is more evident in patients treated with insulin, strong evidence suggests that continuous glucose monitoring could also reduce glycosylated hemoglobin in patients with type 2 diabetes who are not treated with insulin. A close examination of the results suggests that the benefits generally are less than those observed in insulin-treated patients with diabetes.
When we look at the scientific literature, two factors seem particularly important to consider if choosing to prescribe a continuous glucose monitoring sensor. The first is the method used, because the results can vary depending on the method. It appears that only self-monitoring that allows the patient to follow glucose in real time is effective, unlike blind monitoring that allows only a retrospective analysis of blood sugar levels. In the latter case, the patient wears the sensor, and after a week, 10 days, or 15 days, the results are analyzed, possibly with a health care provider. It seems that this is not very effective in improving glycosylated hemoglobin and dietary and physical activity behavior.
The second essential factor to consider is the need for an education program for the use of these sensors to be helpful. If sensors are used but nothing else is done, it does not seem logical. Seeing blood sugar levels without being able to understand them and act accordingly seems of little use. Scientific literature seems to confirm this idea.
Patients Without Diabetes
Now there is another question. We have discussed patients with type 2 diabetes without insulin. It’s trendy to talk about the potential benefits of continuous glucose monitors in patients without diabetes. The idea is emerging that these monitors could be used to refine the diagnosis of diabetes or to better predict the onset of diabetes in the subsequent years.
Others claim that continuous glucose monitors are an effective way to induce a change in dietary and physical activity behaviors in patients with prediabetes. One can, for example, tell a patient, “You are at risk of developing diabetes, so by monitoring your glucose, you will change your behavior.” Honestly, the scientific data we have today do not support these ideas, and I sincerely believe that it is not advisable today to recommend, as some would like, the mass use of monitors, whether in patients with overweight or obesity, or in patients with prediabetes. This goes for suggestions for using the monitor for 7-10 days per year, in the form of a session to try to reduce the risk for diabetes by motivating patients to change their behavior. We have no evidence at all that this can work. And in my opinion, with this kind of discourse, we ultimately risk, as usual, encouraging patients who are already “fans” of self-checks and self-monitoring to get health data, even if they do not know how to interpret it. Maybe even the doctor they ask for interpretation will not be trained to interpret the results of these monitors.
Spreading the idea that monitors are useful for preventing diabetes has a side effect: It hinders progress on the essential issue. Today, one of the problems in diabetes and prediabetes is that screening is not done often enough, and 20% of patients with diabetes are still unaware of their diagnosis. The management of early diabetes or prediabetes, in my opinion, is not optimal in routine care today. So, I think that adding the idea that using monitors could be beneficial dilutes the main information.
Having said that, I sometimes offer continuous glucose monitoring to some of my patients on a case-by-case basis. I believe that with proper support and an educational program, it can be beneficial for certain patients.
In Practice
In summary, I am totally opposed to the normalization of the use of monitors. I think it is our role as health care professionals to warn the public that even if it is accessible — anyone can buy a reader, a sensor — it is not necessarily beneficial, and it may even distract us from what is essential. But as a specialist, I think that using a monitor within a genuine care plan seems reasonable. Ultimately, it’s just personalized medicine.
Dr. Hansel is an endocrinologist-diabetologist and nutritionist, Department of Diabetology-Endocrinology-Nutrition, Hôpital Bichat, and a university lecturer and hospital practitioner, Université Paris-Diderot, France. He discloses ties with Iriade, Sanofi-Aventis, and Amgen.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should we now recommend continuous glucose monitoring to all our patients, even those without diabetes? Most of us would instinctively say “no” to this question, but we are seeing opinions from doctors recommending it, and in recent years, scientific literature has focused on the subject.
Today, anyone can get an arm patch that continuously measures interstitial glucose, which is closely related to blood sugar. The information can be read on a dedicated reader or on a mobile phone by scanning the patch or, with some models, without even doing anything.
There is a consensus for prescribing continuous glucose monitoring for patients with type 1 or type 2 diabetes who are treated with at least three insulin injections. Not only is the use of continuous glucose monitoring much more comfortable than self-monitoring with finger sticks, but continuous monitoring also helps reduce glycosylated hemoglobin while decreasing the risk for hypoglycemia. Recently, another indication has begun to be reimbursed in France: Type 2 diabetes under mono-insulin injection when the diabetes is not well controlled.
But alongside these situations, there are two questions that are worth considering.
Untreated Type 2 Diabetes
First, is continuous glucose monitoring desirable for all patients with diabetes, even those not treated with insulin and even when blood sugar levels are well managed? Intuitively, one might think that it can’t hurt and that continuous monitoring of blood sugar can only improve things. We have some evidence supporting this idea, but the level of proof is quite weak. It is not clear that continuous monitoring can improve patients’ awareness of the impact of dietary choices or physical activity on blood sugar. Obviously, one can imagine that continuously monitoring glucose will encourage a shift toward more beneficial behaviors. But honestly, today, we do not have proof that wearing a continuous glucose monitor can improve behaviors in patients with type 2 diabetes who are treated with noninsulin antidiabetic medications.
Furthermore, a significant study has shown that while the effectiveness is more evident in patients treated with insulin, strong evidence suggests that continuous glucose monitoring could also reduce glycosylated hemoglobin in patients with type 2 diabetes who are not treated with insulin. A close examination of the results suggests that the benefits generally are less than those observed in insulin-treated patients with diabetes.
When we look at the scientific literature, two factors seem particularly important to consider if choosing to prescribe a continuous glucose monitoring sensor. The first is the method used, because the results can vary depending on the method. It appears that only self-monitoring that allows the patient to follow glucose in real time is effective, unlike blind monitoring that allows only a retrospective analysis of blood sugar levels. In the latter case, the patient wears the sensor, and after a week, 10 days, or 15 days, the results are analyzed, possibly with a health care provider. It seems that this is not very effective in improving glycosylated hemoglobin and dietary and physical activity behavior.
The second essential factor to consider is the need for an education program for the use of these sensors to be helpful. If sensors are used but nothing else is done, it does not seem logical. Seeing blood sugar levels without being able to understand them and act accordingly seems of little use. Scientific literature seems to confirm this idea.
Patients Without Diabetes
Now there is another question. We have discussed patients with type 2 diabetes without insulin. It’s trendy to talk about the potential benefits of continuous glucose monitors in patients without diabetes. The idea is emerging that these monitors could be used to refine the diagnosis of diabetes or to better predict the onset of diabetes in the subsequent years.
Others claim that continuous glucose monitors are an effective way to induce a change in dietary and physical activity behaviors in patients with prediabetes. One can, for example, tell a patient, “You are at risk of developing diabetes, so by monitoring your glucose, you will change your behavior.” Honestly, the scientific data we have today do not support these ideas, and I sincerely believe that it is not advisable today to recommend, as some would like, the mass use of monitors, whether in patients with overweight or obesity, or in patients with prediabetes. This goes for suggestions for using the monitor for 7-10 days per year, in the form of a session to try to reduce the risk for diabetes by motivating patients to change their behavior. We have no evidence at all that this can work. And in my opinion, with this kind of discourse, we ultimately risk, as usual, encouraging patients who are already “fans” of self-checks and self-monitoring to get health data, even if they do not know how to interpret it. Maybe even the doctor they ask for interpretation will not be trained to interpret the results of these monitors.
Spreading the idea that monitors are useful for preventing diabetes has a side effect: It hinders progress on the essential issue. Today, one of the problems in diabetes and prediabetes is that screening is not done often enough, and 20% of patients with diabetes are still unaware of their diagnosis. The management of early diabetes or prediabetes, in my opinion, is not optimal in routine care today. So, I think that adding the idea that using monitors could be beneficial dilutes the main information.
Having said that, I sometimes offer continuous glucose monitoring to some of my patients on a case-by-case basis. I believe that with proper support and an educational program, it can be beneficial for certain patients.
In Practice
In summary, I am totally opposed to the normalization of the use of monitors. I think it is our role as health care professionals to warn the public that even if it is accessible — anyone can buy a reader, a sensor — it is not necessarily beneficial, and it may even distract us from what is essential. But as a specialist, I think that using a monitor within a genuine care plan seems reasonable. Ultimately, it’s just personalized medicine.
Dr. Hansel is an endocrinologist-diabetologist and nutritionist, Department of Diabetology-Endocrinology-Nutrition, Hôpital Bichat, and a university lecturer and hospital practitioner, Université Paris-Diderot, France. He discloses ties with Iriade, Sanofi-Aventis, and Amgen.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
DEA Training Mandate: 8 Hours of My Life I’d Like Back
It’s time to renew two of my three narcotic prescribing licenses. For the first time in my career, I’ve waffled on whether the financial outlay to the US Drug Enforcement Agency (DEA) is worth it.
At $888 each, I’ve considered letting two licenses lapse because I only work part-time in Montana. But several friends advised me to keep a “spare” in case I transfer to a new location.
I thought about just paying the fees until I could do a little more research, but there is no mechanism for a refund unless I die within the first year of the 3-year cycle, provide incorrect credit card digits, or accidentally duplicate payments.
The renewal fee is just part of the issue.
Mandatory 8-Hour Training
I also received an alert about the requirement for more “narcotics prescribing education” thanks to the Medication Access and Training Expansion Act (MATE).
The requirement seems counterintuitive because opioid prescribing has decreased for the 10th consecutive year, according to the AMA Overdose Epidemic Report. The continuing rise in overdose deaths is largely due to illegitimate manufacturing of synthetic opioids.
I’ve written zero outpatient narcotics prescriptions in the past 6 years, and I’ve written very few in my 33 years of practice. My use is limited to intravenous morphine for flash pulmonary edema or refractory angina, but unless you graduated from a training program within 5 years of the June 2023 mandate or are boarded in addiction medicine, there is no way to escape the 8-hour education requirement.
The problem is that these courses are never just 8 hours in duration. After signing up for one such CME course that cost $150, I was still dying of boredom and at risk for DVT 4 days later. That’s how long it took to sit through.
Instead of the 30 seconds it should have taken to review the simple instructions to deliver Narcan, there were scores of screens followed by juvenile quizlets and cartoons. All but about 2 hours out of the 4 days is now relegated to that category of “hours of my life that I can never get back.” Additionally, none of that mandatory “education” will change my prescribing habits one whit.
And beware the penalty. 
Of course, I would always be truthful when asked to check the box on the DEA renewal application attesting to my having completed the required education. On the outside chance that you plan to check the yes box without completing the relevant courses, those found guilty of such false claims could be fined up to $250,000 and subject to “not more than four years in prison,” or both. Yikes!
Larry Houck, a former DEA investigator, explained that “[t]here are lot of people who are coming up for renewal and log on but still don’t know this is a requirement.” Neither ignorance nor complacency is an acceptable defense.
Changes Needed
The only good thing that came of those 4 long days of opioid education was a motivation to drive change in our current licensing and educational experience. Why not use this opportunity to reform the DEA-physician/prescriber relationship?
The educational requirements should be curtailed for those of us who do not provide outpatient narcotic prescriptions even if we use inpatient opioids. Meds with low abuse potential should be rescheduled to minimize who gets caught in the broad net of the education requirement.
We should reduce overregulation of the legitimate prescribers by lowering, instead of increasing, licensing fees. We should change to a single license number that covers every state. In this digital age, there is no legitimate excuse to prevent this from happening.
After all, the settlements from opioid manufacturers and distributors will in time total $50 billion. It seems that at least some of the responsibilities of the DEA could shift to states, cities, and towns.
My friend Siamak Karimian, MD, who provides locum services in multiple states, pays for seven active DEA licenses every 3 years. He pointed out the hypocrisy in the current regulatory system: “It’s funny that you can have only one DEA or state license and work for the government in all other states or territories with no limits, including the VA, Indian healthcare systems, or prison systems.”
All other prescribers require a separate DEA number for every state. Ultimately, you’d think tracking prescriptions for a single DEA number should be far simpler than tracking someone with seven.
Competent physicians not guilty of criminal overprescribing seem to be the last to be considered in nearly every healthcare endeavor these days. It would be refreshing if they would reduce our fees and prevent this waste of our time.
And while we are at it, perhaps a more fitting punishment is due for Richard Sackler and all the Purdue Pharma–affiliated family members. The Sacklers will pay out $6 billion in exchange for immunity against civil litigation. That doesn’t seem like much when they are worth $11 billion.
Perhaps they should be made to take an 8-hour course on opioid prescribing, annually and in perpetuity. Let’s see them complete a few quizlets and sit through screens of instruction on how to administer Naloxone. Of course, that would be a mild punishment for those who manufactured a drug that killed hundreds of thousands. But it would be a start.
Dr. Walton-Shirley, a clinical cardiologist in Nashville, Tennessee, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
It’s time to renew two of my three narcotic prescribing licenses. For the first time in my career, I’ve waffled on whether the financial outlay to the US Drug Enforcement Agency (DEA) is worth it.
At $888 each, I’ve considered letting two licenses lapse because I only work part-time in Montana. But several friends advised me to keep a “spare” in case I transfer to a new location.
I thought about just paying the fees until I could do a little more research, but there is no mechanism for a refund unless I die within the first year of the 3-year cycle, provide incorrect credit card digits, or accidentally duplicate payments.
The renewal fee is just part of the issue.
Mandatory 8-Hour Training
I also received an alert about the requirement for more “narcotics prescribing education” thanks to the Medication Access and Training Expansion Act (MATE).
The requirement seems counterintuitive because opioid prescribing has decreased for the 10th consecutive year, according to the AMA Overdose Epidemic Report. The continuing rise in overdose deaths is largely due to illegitimate manufacturing of synthetic opioids.
I’ve written zero outpatient narcotics prescriptions in the past 6 years, and I’ve written very few in my 33 years of practice. My use is limited to intravenous morphine for flash pulmonary edema or refractory angina, but unless you graduated from a training program within 5 years of the June 2023 mandate or are boarded in addiction medicine, there is no way to escape the 8-hour education requirement.
The problem is that these courses are never just 8 hours in duration. After signing up for one such CME course that cost $150, I was still dying of boredom and at risk for DVT 4 days later. That’s how long it took to sit through.
Instead of the 30 seconds it should have taken to review the simple instructions to deliver Narcan, there were scores of screens followed by juvenile quizlets and cartoons. All but about 2 hours out of the 4 days is now relegated to that category of “hours of my life that I can never get back.” Additionally, none of that mandatory “education” will change my prescribing habits one whit.
And beware the penalty. 
Of course, I would always be truthful when asked to check the box on the DEA renewal application attesting to my having completed the required education. On the outside chance that you plan to check the yes box without completing the relevant courses, those found guilty of such false claims could be fined up to $250,000 and subject to “not more than four years in prison,” or both. Yikes!
Larry Houck, a former DEA investigator, explained that “[t]here are lot of people who are coming up for renewal and log on but still don’t know this is a requirement.” Neither ignorance nor complacency is an acceptable defense.
Changes Needed
The only good thing that came of those 4 long days of opioid education was a motivation to drive change in our current licensing and educational experience. Why not use this opportunity to reform the DEA-physician/prescriber relationship?
The educational requirements should be curtailed for those of us who do not provide outpatient narcotic prescriptions even if we use inpatient opioids. Meds with low abuse potential should be rescheduled to minimize who gets caught in the broad net of the education requirement.
We should reduce overregulation of the legitimate prescribers by lowering, instead of increasing, licensing fees. We should change to a single license number that covers every state. In this digital age, there is no legitimate excuse to prevent this from happening.
After all, the settlements from opioid manufacturers and distributors will in time total $50 billion. It seems that at least some of the responsibilities of the DEA could shift to states, cities, and towns.
My friend Siamak Karimian, MD, who provides locum services in multiple states, pays for seven active DEA licenses every 3 years. He pointed out the hypocrisy in the current regulatory system: “It’s funny that you can have only one DEA or state license and work for the government in all other states or territories with no limits, including the VA, Indian healthcare systems, or prison systems.”
All other prescribers require a separate DEA number for every state. Ultimately, you’d think tracking prescriptions for a single DEA number should be far simpler than tracking someone with seven.
Competent physicians not guilty of criminal overprescribing seem to be the last to be considered in nearly every healthcare endeavor these days. It would be refreshing if they would reduce our fees and prevent this waste of our time.
And while we are at it, perhaps a more fitting punishment is due for Richard Sackler and all the Purdue Pharma–affiliated family members. The Sacklers will pay out $6 billion in exchange for immunity against civil litigation. That doesn’t seem like much when they are worth $11 billion.
Perhaps they should be made to take an 8-hour course on opioid prescribing, annually and in perpetuity. Let’s see them complete a few quizlets and sit through screens of instruction on how to administer Naloxone. Of course, that would be a mild punishment for those who manufactured a drug that killed hundreds of thousands. But it would be a start.
Dr. Walton-Shirley, a clinical cardiologist in Nashville, Tennessee, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
It’s time to renew two of my three narcotic prescribing licenses. For the first time in my career, I’ve waffled on whether the financial outlay to the US Drug Enforcement Agency (DEA) is worth it.
At $888 each, I’ve considered letting two licenses lapse because I only work part-time in Montana. But several friends advised me to keep a “spare” in case I transfer to a new location.
I thought about just paying the fees until I could do a little more research, but there is no mechanism for a refund unless I die within the first year of the 3-year cycle, provide incorrect credit card digits, or accidentally duplicate payments.
The renewal fee is just part of the issue.
Mandatory 8-Hour Training
I also received an alert about the requirement for more “narcotics prescribing education” thanks to the Medication Access and Training Expansion Act (MATE).
The requirement seems counterintuitive because opioid prescribing has decreased for the 10th consecutive year, according to the AMA Overdose Epidemic Report. The continuing rise in overdose deaths is largely due to illegitimate manufacturing of synthetic opioids.
I’ve written zero outpatient narcotics prescriptions in the past 6 years, and I’ve written very few in my 33 years of practice. My use is limited to intravenous morphine for flash pulmonary edema or refractory angina, but unless you graduated from a training program within 5 years of the June 2023 mandate or are boarded in addiction medicine, there is no way to escape the 8-hour education requirement.
The problem is that these courses are never just 8 hours in duration. After signing up for one such CME course that cost $150, I was still dying of boredom and at risk for DVT 4 days later. That’s how long it took to sit through.
Instead of the 30 seconds it should have taken to review the simple instructions to deliver Narcan, there were scores of screens followed by juvenile quizlets and cartoons. All but about 2 hours out of the 4 days is now relegated to that category of “hours of my life that I can never get back.” Additionally, none of that mandatory “education” will change my prescribing habits one whit.
And beware the penalty. 
Of course, I would always be truthful when asked to check the box on the DEA renewal application attesting to my having completed the required education. On the outside chance that you plan to check the yes box without completing the relevant courses, those found guilty of such false claims could be fined up to $250,000 and subject to “not more than four years in prison,” or both. Yikes!
Larry Houck, a former DEA investigator, explained that “[t]here are lot of people who are coming up for renewal and log on but still don’t know this is a requirement.” Neither ignorance nor complacency is an acceptable defense.
Changes Needed
The only good thing that came of those 4 long days of opioid education was a motivation to drive change in our current licensing and educational experience. Why not use this opportunity to reform the DEA-physician/prescriber relationship?
The educational requirements should be curtailed for those of us who do not provide outpatient narcotic prescriptions even if we use inpatient opioids. Meds with low abuse potential should be rescheduled to minimize who gets caught in the broad net of the education requirement.
We should reduce overregulation of the legitimate prescribers by lowering, instead of increasing, licensing fees. We should change to a single license number that covers every state. In this digital age, there is no legitimate excuse to prevent this from happening.
After all, the settlements from opioid manufacturers and distributors will in time total $50 billion. It seems that at least some of the responsibilities of the DEA could shift to states, cities, and towns.
My friend Siamak Karimian, MD, who provides locum services in multiple states, pays for seven active DEA licenses every 3 years. He pointed out the hypocrisy in the current regulatory system: “It’s funny that you can have only one DEA or state license and work for the government in all other states or territories with no limits, including the VA, Indian healthcare systems, or prison systems.”
All other prescribers require a separate DEA number for every state. Ultimately, you’d think tracking prescriptions for a single DEA number should be far simpler than tracking someone with seven.
Competent physicians not guilty of criminal overprescribing seem to be the last to be considered in nearly every healthcare endeavor these days. It would be refreshing if they would reduce our fees and prevent this waste of our time.
And while we are at it, perhaps a more fitting punishment is due for Richard Sackler and all the Purdue Pharma–affiliated family members. The Sacklers will pay out $6 billion in exchange for immunity against civil litigation. That doesn’t seem like much when they are worth $11 billion.
Perhaps they should be made to take an 8-hour course on opioid prescribing, annually and in perpetuity. Let’s see them complete a few quizlets and sit through screens of instruction on how to administer Naloxone. Of course, that would be a mild punishment for those who manufactured a drug that killed hundreds of thousands. But it would be a start.
Dr. Walton-Shirley, a clinical cardiologist in Nashville, Tennessee, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
How Media Coverage of Oral Minoxidil for Hair Loss Has Impacted Prescribing Habits
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
Practice Points
- Low-dose oral minoxidil (LDOM) prescriptions have increased due to rising attention to its efficacy and safety.
- Media outlets can have a powerful effect on prescribing habits of physicians.
- Physicians should be aware of media trends to help direct patient education.
Listen to earn your patients’ trust
Recently, I had an interesting conversation while getting my hair cut. It gave me a great deal of insight into some of the problems we have right now with how medical information is shared and some of the disconnect our patients may feel.
The young woman who was cutting my hair asked me what I did for an occupation. I said that I was a physician. She said, “Can I please ask you an important question?” She asked me what my thoughts were about the COVID vaccine. She prefaced it with “I am so confused on whether I should get the vaccine. I have seen a number of TikTok videos that talk about nano particles in the COVID vaccine that can be very dangerous.”
I discussed with her how the COVID vaccine actually works and shared with her the remarkable success of the vaccine. I asked her what side effects she was worried about from the vaccine and what her fears were. She said that she had heard that a lot of people had died from the vaccine. I told her that severe reactions from the vaccine were very uncommon.
She then made a very telling comment: “I wish I could talk to a doctor about my concerns. I have been going to the same health center for the last 5 years and every time I go I see a different person.” She added, “I rarely have more than 5-10 minutes with the person that I am seeing and I rarely get the opportunity to ask questions.”
She thanked me for the information and said that she would be getting the COVID vaccine in the future. She said it is so hard to know where to get information now and the very different things that she heard confused her. She told me that she thought her generation got most of its information from short sound bites or TikTok and Instagram videos.
Why did she trust me? I still think that the medical profession is respected. We are all pressured to do more with less time. Conversations where we can listen and then respond go a long way. We can always listen and learn what information people need and will appreciate. I was also struck by how alone this person felt in our health care system. She did not have a relationship with any one person whom she could trust and reach out to with questions. Relationships with our patients go a long way to establishing trust.
Pearl
It takes time to listen to and answer our patients’ questions. We need to do that to fight the waves of misinformation our patients face.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
Recently, I had an interesting conversation while getting my hair cut. It gave me a great deal of insight into some of the problems we have right now with how medical information is shared and some of the disconnect our patients may feel.
The young woman who was cutting my hair asked me what I did for an occupation. I said that I was a physician. She said, “Can I please ask you an important question?” She asked me what my thoughts were about the COVID vaccine. She prefaced it with “I am so confused on whether I should get the vaccine. I have seen a number of TikTok videos that talk about nano particles in the COVID vaccine that can be very dangerous.”
I discussed with her how the COVID vaccine actually works and shared with her the remarkable success of the vaccine. I asked her what side effects she was worried about from the vaccine and what her fears were. She said that she had heard that a lot of people had died from the vaccine. I told her that severe reactions from the vaccine were very uncommon.
She then made a very telling comment: “I wish I could talk to a doctor about my concerns. I have been going to the same health center for the last 5 years and every time I go I see a different person.” She added, “I rarely have more than 5-10 minutes with the person that I am seeing and I rarely get the opportunity to ask questions.”
She thanked me for the information and said that she would be getting the COVID vaccine in the future. She said it is so hard to know where to get information now and the very different things that she heard confused her. She told me that she thought her generation got most of its information from short sound bites or TikTok and Instagram videos.
Why did she trust me? I still think that the medical profession is respected. We are all pressured to do more with less time. Conversations where we can listen and then respond go a long way. We can always listen and learn what information people need and will appreciate. I was also struck by how alone this person felt in our health care system. She did not have a relationship with any one person whom she could trust and reach out to with questions. Relationships with our patients go a long way to establishing trust.
Pearl
It takes time to listen to and answer our patients’ questions. We need to do that to fight the waves of misinformation our patients face.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
Recently, I had an interesting conversation while getting my hair cut. It gave me a great deal of insight into some of the problems we have right now with how medical information is shared and some of the disconnect our patients may feel.
The young woman who was cutting my hair asked me what I did for an occupation. I said that I was a physician. She said, “Can I please ask you an important question?” She asked me what my thoughts were about the COVID vaccine. She prefaced it with “I am so confused on whether I should get the vaccine. I have seen a number of TikTok videos that talk about nano particles in the COVID vaccine that can be very dangerous.”
I discussed with her how the COVID vaccine actually works and shared with her the remarkable success of the vaccine. I asked her what side effects she was worried about from the vaccine and what her fears were. She said that she had heard that a lot of people had died from the vaccine. I told her that severe reactions from the vaccine were very uncommon.
She then made a very telling comment: “I wish I could talk to a doctor about my concerns. I have been going to the same health center for the last 5 years and every time I go I see a different person.” She added, “I rarely have more than 5-10 minutes with the person that I am seeing and I rarely get the opportunity to ask questions.”
She thanked me for the information and said that she would be getting the COVID vaccine in the future. She said it is so hard to know where to get information now and the very different things that she heard confused her. She told me that she thought her generation got most of its information from short sound bites or TikTok and Instagram videos.
Why did she trust me? I still think that the medical profession is respected. We are all pressured to do more with less time. Conversations where we can listen and then respond go a long way. We can always listen and learn what information people need and will appreciate. I was also struck by how alone this person felt in our health care system. She did not have a relationship with any one person whom she could trust and reach out to with questions. Relationships with our patients go a long way to establishing trust.
Pearl
It takes time to listen to and answer our patients’ questions. We need to do that to fight the waves of misinformation our patients face.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
The Tyranny of Beta-Blockers
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.





