User login
Delivery of Care: The Ethical Imperative in Healthcare
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
Helping Patients Cut Down on Sodium: Useful Substitutes and Strategies
Humans have used salt for centuries, to preserve or cure food before refrigeration was readily available, and even as currency in some cultures. Though modern food preservation efforts have decreased our reliance on salt, we still heavily incorporate it as a flavor enhancer.
It’s only relatively recently that we’ve begun limiting salt in our diets, as research has linked high sodium intake with chronic, preventable conditions like hypertension, heart disease, and kidney disease.
How to Recommend Restriction in a Helpful Way
The US Department of Agriculture’s Dietary Guidelines for Americans recommends intake of no more than 2300 mg of sodium daily for adults and children aged 14 years or older. This echoes similar recommendations for people at risk for heart disease, kidney disease, and hypertension. However, the sodium intake of the average American still sits at a whopping 3400 mg daily.
High sodium intake is primarily the result of modern commercial food processing. Food prepared outside the home accounts for up to 70% of sodium intake in the United States, whereas only about 10% comes from salt that is added to food either during or after cooking. For this reason, I hesitate to recommend withholding salt as a primary focus when counseling on a low-sodium diet.
To many people, certain foods just taste better with salt. Many of my patients in the southern United States simply will not eat foods like eggs and tomatoes if they cannot salt them. We can spend every moment of patient interaction time explaining why excess sodium is unhealthy, but the fact remains that humans prefer food that tastes good. This is why I try to avoid counseling a “no-added-salt” diet; instead, I recommend a low-sodium diet with a focus on fresh, whole foods and limiting salt to only a few food items.
Patients should be counseled to slowly restrict their salt intake and be made aware that doing so may increase the time it takes for their sensitivity to the taste of less salty foods to return. But it is also important for them to know that it will return. The surest way to kill progress is for an unprepared patient to believe that their food will taste bland forever. A prepared patient understands that their food may taste different for a couple of weeks, but that the change will not last forever.
Types of Salt
I have often worked with patients who insist that their sodium intake is acceptable because they are using sea salt instead of table salt. This is the result of exceptional marketing and misinformation.
Specialty salts like sea salt and Himalayan pink salt contain about 560 mg and 590 mg of sodium, respectively, per quarter teaspoon. These products do have a slightly different mineral content, with sea salt typically having a negligible amount of calcium, magnesium, or potassium. The very small amount of these minerals offers no obvious health benefits compared with more affordable table salt.
The sodium content of iodized table salt is comparable to these products, with about 590 mg of sodium per quarter teaspoon. Though its high sodium content will put some practitioners off, it is also an excellent source of iodine, at about 75 mg per serving. It has been estimated that upward of 35% of the US population has iodine deficiency, most commonly due to pregnancy, avoidance of dairy products, increasing rates of vegetarianism, intake of highly processed foods, and avoidance of added salt. For this reason, and its relative affordability, I find table salt to be far more appropriate for the average American than specialty salts.
Salt Substitutes
Monosodium glutamate (MSG). MSG was previously at the center of public health concern owing to reports of “Chinese restaurant syndrome” that have since been debunked. I often recommend MSG to people trying to decrease sodium intake because the US Food and Drug Administration has designated it as GRAS (“generally recognized as safe”), and it has about one quarter of the sodium content of table salt at 125 mg per quarter teaspoon. Its crystalline structure makes it a lower-sodium salt substitute in savory applications like soups, stews, and gravies.
Hot sauce. These sauces are generally composed of peppers, vinegar, salt, and sugar. There may be some variation and occasionally added ingredients depending upon the brand. However, I find most hot sauces to be a low-sodium seasoning option that works especially well on proteins like eggs, chicken, and pork.
Potassium-based substitutes. Salt alternatives such as Nu-Salt and Morton Salt Substitute are sodium-free options with a significant amount of potassium, at 525 mg per quarter-teaspoon serving. These alternatives may not be ideal for patients with kidney problems, but they can be very helpful for those with potassium deficiency.
Herb-based seasonings. Garlic and onion powder are both sodium-free seasonings that many of my patients have found help to increase palatability while decreasing salt use. Black pepper; lemon and lime juice; salt-free herb mixes like Mrs. Dash; and spices like cumin, paprika, dill, chili powder, and ginger are also sodium-free or low-sodium alternatives that can help to alleviate blandness for someone new to a low-sodium diet. I recommend them often and use them in my own cooking at home.
Plant-based diet. If the goal of care is to improve cardiovascular or kidney health, then I find that working with patients to increase intake of plant foods to be a helpful option. This way of eating encourages replacing highly processed foods that may be high in sodium and sugar with plants that tend to be higher in potassium and calcium. The Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and other plant-based diets have been shown to increase cardiovascular and metabolic health by significantly decreasing serum lipids, blood pressure, and hemoglobin A1c and promoting weight loss. They have also been shown to increase the gut microbiome and promote increased cognitive function.
I rarely encourage the use of added salt. However, research shows that putting down the salt shaker is probably not the most effective option to restrict sodium intake. For those who can cut back, these options can help keep food flavorful and patients compliant.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Humans have used salt for centuries, to preserve or cure food before refrigeration was readily available, and even as currency in some cultures. Though modern food preservation efforts have decreased our reliance on salt, we still heavily incorporate it as a flavor enhancer.
It’s only relatively recently that we’ve begun limiting salt in our diets, as research has linked high sodium intake with chronic, preventable conditions like hypertension, heart disease, and kidney disease.
How to Recommend Restriction in a Helpful Way
The US Department of Agriculture’s Dietary Guidelines for Americans recommends intake of no more than 2300 mg of sodium daily for adults and children aged 14 years or older. This echoes similar recommendations for people at risk for heart disease, kidney disease, and hypertension. However, the sodium intake of the average American still sits at a whopping 3400 mg daily.
High sodium intake is primarily the result of modern commercial food processing. Food prepared outside the home accounts for up to 70% of sodium intake in the United States, whereas only about 10% comes from salt that is added to food either during or after cooking. For this reason, I hesitate to recommend withholding salt as a primary focus when counseling on a low-sodium diet.
To many people, certain foods just taste better with salt. Many of my patients in the southern United States simply will not eat foods like eggs and tomatoes if they cannot salt them. We can spend every moment of patient interaction time explaining why excess sodium is unhealthy, but the fact remains that humans prefer food that tastes good. This is why I try to avoid counseling a “no-added-salt” diet; instead, I recommend a low-sodium diet with a focus on fresh, whole foods and limiting salt to only a few food items.
Patients should be counseled to slowly restrict their salt intake and be made aware that doing so may increase the time it takes for their sensitivity to the taste of less salty foods to return. But it is also important for them to know that it will return. The surest way to kill progress is for an unprepared patient to believe that their food will taste bland forever. A prepared patient understands that their food may taste different for a couple of weeks, but that the change will not last forever.
Types of Salt
I have often worked with patients who insist that their sodium intake is acceptable because they are using sea salt instead of table salt. This is the result of exceptional marketing and misinformation.
Specialty salts like sea salt and Himalayan pink salt contain about 560 mg and 590 mg of sodium, respectively, per quarter teaspoon. These products do have a slightly different mineral content, with sea salt typically having a negligible amount of calcium, magnesium, or potassium. The very small amount of these minerals offers no obvious health benefits compared with more affordable table salt.
The sodium content of iodized table salt is comparable to these products, with about 590 mg of sodium per quarter teaspoon. Though its high sodium content will put some practitioners off, it is also an excellent source of iodine, at about 75 mg per serving. It has been estimated that upward of 35% of the US population has iodine deficiency, most commonly due to pregnancy, avoidance of dairy products, increasing rates of vegetarianism, intake of highly processed foods, and avoidance of added salt. For this reason, and its relative affordability, I find table salt to be far more appropriate for the average American than specialty salts.
Salt Substitutes
Monosodium glutamate (MSG). MSG was previously at the center of public health concern owing to reports of “Chinese restaurant syndrome” that have since been debunked. I often recommend MSG to people trying to decrease sodium intake because the US Food and Drug Administration has designated it as GRAS (“generally recognized as safe”), and it has about one quarter of the sodium content of table salt at 125 mg per quarter teaspoon. Its crystalline structure makes it a lower-sodium salt substitute in savory applications like soups, stews, and gravies.
Hot sauce. These sauces are generally composed of peppers, vinegar, salt, and sugar. There may be some variation and occasionally added ingredients depending upon the brand. However, I find most hot sauces to be a low-sodium seasoning option that works especially well on proteins like eggs, chicken, and pork.
Potassium-based substitutes. Salt alternatives such as Nu-Salt and Morton Salt Substitute are sodium-free options with a significant amount of potassium, at 525 mg per quarter-teaspoon serving. These alternatives may not be ideal for patients with kidney problems, but they can be very helpful for those with potassium deficiency.
Herb-based seasonings. Garlic and onion powder are both sodium-free seasonings that many of my patients have found help to increase palatability while decreasing salt use. Black pepper; lemon and lime juice; salt-free herb mixes like Mrs. Dash; and spices like cumin, paprika, dill, chili powder, and ginger are also sodium-free or low-sodium alternatives that can help to alleviate blandness for someone new to a low-sodium diet. I recommend them often and use them in my own cooking at home.
Plant-based diet. If the goal of care is to improve cardiovascular or kidney health, then I find that working with patients to increase intake of plant foods to be a helpful option. This way of eating encourages replacing highly processed foods that may be high in sodium and sugar with plants that tend to be higher in potassium and calcium. The Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and other plant-based diets have been shown to increase cardiovascular and metabolic health by significantly decreasing serum lipids, blood pressure, and hemoglobin A1c and promoting weight loss. They have also been shown to increase the gut microbiome and promote increased cognitive function.
I rarely encourage the use of added salt. However, research shows that putting down the salt shaker is probably not the most effective option to restrict sodium intake. For those who can cut back, these options can help keep food flavorful and patients compliant.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Humans have used salt for centuries, to preserve or cure food before refrigeration was readily available, and even as currency in some cultures. Though modern food preservation efforts have decreased our reliance on salt, we still heavily incorporate it as a flavor enhancer.
It’s only relatively recently that we’ve begun limiting salt in our diets, as research has linked high sodium intake with chronic, preventable conditions like hypertension, heart disease, and kidney disease.
How to Recommend Restriction in a Helpful Way
The US Department of Agriculture’s Dietary Guidelines for Americans recommends intake of no more than 2300 mg of sodium daily for adults and children aged 14 years or older. This echoes similar recommendations for people at risk for heart disease, kidney disease, and hypertension. However, the sodium intake of the average American still sits at a whopping 3400 mg daily.
High sodium intake is primarily the result of modern commercial food processing. Food prepared outside the home accounts for up to 70% of sodium intake in the United States, whereas only about 10% comes from salt that is added to food either during or after cooking. For this reason, I hesitate to recommend withholding salt as a primary focus when counseling on a low-sodium diet.
To many people, certain foods just taste better with salt. Many of my patients in the southern United States simply will not eat foods like eggs and tomatoes if they cannot salt them. We can spend every moment of patient interaction time explaining why excess sodium is unhealthy, but the fact remains that humans prefer food that tastes good. This is why I try to avoid counseling a “no-added-salt” diet; instead, I recommend a low-sodium diet with a focus on fresh, whole foods and limiting salt to only a few food items.
Patients should be counseled to slowly restrict their salt intake and be made aware that doing so may increase the time it takes for their sensitivity to the taste of less salty foods to return. But it is also important for them to know that it will return. The surest way to kill progress is for an unprepared patient to believe that their food will taste bland forever. A prepared patient understands that their food may taste different for a couple of weeks, but that the change will not last forever.
Types of Salt
I have often worked with patients who insist that their sodium intake is acceptable because they are using sea salt instead of table salt. This is the result of exceptional marketing and misinformation.
Specialty salts like sea salt and Himalayan pink salt contain about 560 mg and 590 mg of sodium, respectively, per quarter teaspoon. These products do have a slightly different mineral content, with sea salt typically having a negligible amount of calcium, magnesium, or potassium. The very small amount of these minerals offers no obvious health benefits compared with more affordable table salt.
The sodium content of iodized table salt is comparable to these products, with about 590 mg of sodium per quarter teaspoon. Though its high sodium content will put some practitioners off, it is also an excellent source of iodine, at about 75 mg per serving. It has been estimated that upward of 35% of the US population has iodine deficiency, most commonly due to pregnancy, avoidance of dairy products, increasing rates of vegetarianism, intake of highly processed foods, and avoidance of added salt. For this reason, and its relative affordability, I find table salt to be far more appropriate for the average American than specialty salts.
Salt Substitutes
Monosodium glutamate (MSG). MSG was previously at the center of public health concern owing to reports of “Chinese restaurant syndrome” that have since been debunked. I often recommend MSG to people trying to decrease sodium intake because the US Food and Drug Administration has designated it as GRAS (“generally recognized as safe”), and it has about one quarter of the sodium content of table salt at 125 mg per quarter teaspoon. Its crystalline structure makes it a lower-sodium salt substitute in savory applications like soups, stews, and gravies.
Hot sauce. These sauces are generally composed of peppers, vinegar, salt, and sugar. There may be some variation and occasionally added ingredients depending upon the brand. However, I find most hot sauces to be a low-sodium seasoning option that works especially well on proteins like eggs, chicken, and pork.
Potassium-based substitutes. Salt alternatives such as Nu-Salt and Morton Salt Substitute are sodium-free options with a significant amount of potassium, at 525 mg per quarter-teaspoon serving. These alternatives may not be ideal for patients with kidney problems, but they can be very helpful for those with potassium deficiency.
Herb-based seasonings. Garlic and onion powder are both sodium-free seasonings that many of my patients have found help to increase palatability while decreasing salt use. Black pepper; lemon and lime juice; salt-free herb mixes like Mrs. Dash; and spices like cumin, paprika, dill, chili powder, and ginger are also sodium-free or low-sodium alternatives that can help to alleviate blandness for someone new to a low-sodium diet. I recommend them often and use them in my own cooking at home.
Plant-based diet. If the goal of care is to improve cardiovascular or kidney health, then I find that working with patients to increase intake of plant foods to be a helpful option. This way of eating encourages replacing highly processed foods that may be high in sodium and sugar with plants that tend to be higher in potassium and calcium. The Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and other plant-based diets have been shown to increase cardiovascular and metabolic health by significantly decreasing serum lipids, blood pressure, and hemoglobin A1c and promoting weight loss. They have also been shown to increase the gut microbiome and promote increased cognitive function.
I rarely encourage the use of added salt. However, research shows that putting down the salt shaker is probably not the most effective option to restrict sodium intake. For those who can cut back, these options can help keep food flavorful and patients compliant.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Latest Breakthroughs in Molluscum Contagiosum Therapy
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Losing Weight, Decreasing Alcohol, and Improving Sex Life?
Richard* was a master-of-the-universe type. He went to Wharton, ran a large hedge fund, and lived in Greenwich, Connecticut. His three children attended Ivy League schools. He played golf on the weekends and ate three healthy meals per day. There was just one issue: He had gained 90 pounds since the 1990s from consuming six to seven alcoholic beverages per day. He already had one DUI under his belt, and his marriage was on shaky ground. He had tried to address his alcohol abuse disorder on multiple occasions: He went to a yearlong class on alcoholism, saw a psychologist for cognitive-behavioral therapy, and joined Alcoholics Anonymous, all to no avail.
When I met him in December 2023, he had hit rock bottom and was willing to try anything.
At our first visit, I prescribed him weekly tirzepatide (Zepbound) off label, along with a small dose of naltrexone.
Richard shared some feedback after his first 2 weeks:
The naltrexone works great and is strong ... small dose for me effective ... I haven’t wanted to drink and when I do I can’t finish a glass over 2 hours … went from 25 drinks a week to about 4 … don’t notice other side effects … sleeping better too.
And after 6 weeks:
Some more feedback … on week 6-7 and all going well ... drinking very little alcohol and still on half tab of naltrexone ... that works well and have no side effects ... the Zepbound works well too. I do get hungry a few days after the shot but still don’t crave sugar or bad snacks … weight down 21 pounds since started … 292 to 271.
And finally, after 8 weeks:
Looking at my last text to you I see the progress … been incredible ... now down 35 pounds and at 257 … continue to feel excellent with plenty of energy … want to exercise more ... and no temptation to eat or drink unhealthy stuff ... I’m very happy this has surpassed my expectations on how fast it’s worked and I don’t feel any side effects. Marriage has never been better … all thanks to you.
Tirzepatide contains two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are naturally produced by our bodies after meals. Scientists recently learned that the GLP-1 system contributes to the feedback loop of addictive behaviors. Increasing synthetic GLP-1, through medications like tirzepatide, appears to minimize addictive behaviors by limiting their ability to upregulate the brain’s production of dopamine.
Dopamine is a neurotransmitter produced in the brain’s reward center, which regulates how people experience pleasure and control impulses. Dopamine reinforces the pleasure experienced by certain behaviors like drinking, smoking, and eating sweets. These new medications reduce the amount of dopamine released after these activities and thereby lower the motivation to repeat these behaviors.
Contrary to some reports in the news, the vast majority of my male patients using these medications for alcohol abuse disorder experience concurrent increases in testosterone, for two reasons: (1) testosterone increases as body mass index decreases and (2) chronic alcohol use can damage the cells in the testicles that produce testosterone and also decrease the brain’s ability to stimulate the testicles to produce testosterone.
At his most recent checkup last month, Richard’s testosterone had risen from borderline to robust levels, his libido and sleep had improved, and he reported never having felt so healthy or confident. Fingers crossed that the US Food and Drug Administration won’t wait too long before approving this class of medications for more than just diabetes, heart disease, and obesity.
*Patient’s name has been changed.
Dr. Messer is clinical assistant professor, Icahn School of Medicine at Mount Sinai, New York, and associate professor, Zucker School of Medicine at Hofstra University, Hempstead, New York. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Richard* was a master-of-the-universe type. He went to Wharton, ran a large hedge fund, and lived in Greenwich, Connecticut. His three children attended Ivy League schools. He played golf on the weekends and ate three healthy meals per day. There was just one issue: He had gained 90 pounds since the 1990s from consuming six to seven alcoholic beverages per day. He already had one DUI under his belt, and his marriage was on shaky ground. He had tried to address his alcohol abuse disorder on multiple occasions: He went to a yearlong class on alcoholism, saw a psychologist for cognitive-behavioral therapy, and joined Alcoholics Anonymous, all to no avail.
When I met him in December 2023, he had hit rock bottom and was willing to try anything.
At our first visit, I prescribed him weekly tirzepatide (Zepbound) off label, along with a small dose of naltrexone.
Richard shared some feedback after his first 2 weeks:
The naltrexone works great and is strong ... small dose for me effective ... I haven’t wanted to drink and when I do I can’t finish a glass over 2 hours … went from 25 drinks a week to about 4 … don’t notice other side effects … sleeping better too.
And after 6 weeks:
Some more feedback … on week 6-7 and all going well ... drinking very little alcohol and still on half tab of naltrexone ... that works well and have no side effects ... the Zepbound works well too. I do get hungry a few days after the shot but still don’t crave sugar or bad snacks … weight down 21 pounds since started … 292 to 271.
And finally, after 8 weeks:
Looking at my last text to you I see the progress … been incredible ... now down 35 pounds and at 257 … continue to feel excellent with plenty of energy … want to exercise more ... and no temptation to eat or drink unhealthy stuff ... I’m very happy this has surpassed my expectations on how fast it’s worked and I don’t feel any side effects. Marriage has never been better … all thanks to you.
Tirzepatide contains two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are naturally produced by our bodies after meals. Scientists recently learned that the GLP-1 system contributes to the feedback loop of addictive behaviors. Increasing synthetic GLP-1, through medications like tirzepatide, appears to minimize addictive behaviors by limiting their ability to upregulate the brain’s production of dopamine.
Dopamine is a neurotransmitter produced in the brain’s reward center, which regulates how people experience pleasure and control impulses. Dopamine reinforces the pleasure experienced by certain behaviors like drinking, smoking, and eating sweets. These new medications reduce the amount of dopamine released after these activities and thereby lower the motivation to repeat these behaviors.
Contrary to some reports in the news, the vast majority of my male patients using these medications for alcohol abuse disorder experience concurrent increases in testosterone, for two reasons: (1) testosterone increases as body mass index decreases and (2) chronic alcohol use can damage the cells in the testicles that produce testosterone and also decrease the brain’s ability to stimulate the testicles to produce testosterone.
At his most recent checkup last month, Richard’s testosterone had risen from borderline to robust levels, his libido and sleep had improved, and he reported never having felt so healthy or confident. Fingers crossed that the US Food and Drug Administration won’t wait too long before approving this class of medications for more than just diabetes, heart disease, and obesity.
*Patient’s name has been changed.
Dr. Messer is clinical assistant professor, Icahn School of Medicine at Mount Sinai, New York, and associate professor, Zucker School of Medicine at Hofstra University, Hempstead, New York. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Richard* was a master-of-the-universe type. He went to Wharton, ran a large hedge fund, and lived in Greenwich, Connecticut. His three children attended Ivy League schools. He played golf on the weekends and ate three healthy meals per day. There was just one issue: He had gained 90 pounds since the 1990s from consuming six to seven alcoholic beverages per day. He already had one DUI under his belt, and his marriage was on shaky ground. He had tried to address his alcohol abuse disorder on multiple occasions: He went to a yearlong class on alcoholism, saw a psychologist for cognitive-behavioral therapy, and joined Alcoholics Anonymous, all to no avail.
When I met him in December 2023, he had hit rock bottom and was willing to try anything.
At our first visit, I prescribed him weekly tirzepatide (Zepbound) off label, along with a small dose of naltrexone.
Richard shared some feedback after his first 2 weeks:
The naltrexone works great and is strong ... small dose for me effective ... I haven’t wanted to drink and when I do I can’t finish a glass over 2 hours … went from 25 drinks a week to about 4 … don’t notice other side effects … sleeping better too.
And after 6 weeks:
Some more feedback … on week 6-7 and all going well ... drinking very little alcohol and still on half tab of naltrexone ... that works well and have no side effects ... the Zepbound works well too. I do get hungry a few days after the shot but still don’t crave sugar or bad snacks … weight down 21 pounds since started … 292 to 271.
And finally, after 8 weeks:
Looking at my last text to you I see the progress … been incredible ... now down 35 pounds and at 257 … continue to feel excellent with plenty of energy … want to exercise more ... and no temptation to eat or drink unhealthy stuff ... I’m very happy this has surpassed my expectations on how fast it’s worked and I don’t feel any side effects. Marriage has never been better … all thanks to you.
Tirzepatide contains two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are naturally produced by our bodies after meals. Scientists recently learned that the GLP-1 system contributes to the feedback loop of addictive behaviors. Increasing synthetic GLP-1, through medications like tirzepatide, appears to minimize addictive behaviors by limiting their ability to upregulate the brain’s production of dopamine.
Dopamine is a neurotransmitter produced in the brain’s reward center, which regulates how people experience pleasure and control impulses. Dopamine reinforces the pleasure experienced by certain behaviors like drinking, smoking, and eating sweets. These new medications reduce the amount of dopamine released after these activities and thereby lower the motivation to repeat these behaviors.
Contrary to some reports in the news, the vast majority of my male patients using these medications for alcohol abuse disorder experience concurrent increases in testosterone, for two reasons: (1) testosterone increases as body mass index decreases and (2) chronic alcohol use can damage the cells in the testicles that produce testosterone and also decrease the brain’s ability to stimulate the testicles to produce testosterone.
At his most recent checkup last month, Richard’s testosterone had risen from borderline to robust levels, his libido and sleep had improved, and he reported never having felt so healthy or confident. Fingers crossed that the US Food and Drug Administration won’t wait too long before approving this class of medications for more than just diabetes, heart disease, and obesity.
*Patient’s name has been changed.
Dr. Messer is clinical assistant professor, Icahn School of Medicine at Mount Sinai, New York, and associate professor, Zucker School of Medicine at Hofstra University, Hempstead, New York. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Would Making Tuition Free Address the Primary Care Shortage?
This transcript has been edited for clarity.
Would free medical school encourage more students to pursue primary care? Overpriced medical training in this country definitely contributes to burnout and the physician shortage, and all that debt may influence what type of specialty somebody goes into.
Assumptions Behind Free Tuition Initiatives
Now, this question is based on an assumption that there already is a large group of medical students who want to go into primary care, and the reason they’re not or the reason they’re reluctant to is because of tuition. If you take that stress away, you fix the problem.
This was at least part of the assumption made when NYU announced free tuition for all medical students back in 2018. One goal was to encourage more students to go into primary care. The other was to broaden the application pool to include more diverse students from different socioeconomic and racial backgrounds. We›ll get back to that.
Quick numbers. In NYU’s 2022 match, the first tuition-free class, about 25% of students matched into a primary care specialty— internal medicine or pediatrics — and there were zero matches into a family medicine residency. I can’t find the data, but anecdotally that 25% is a slight increase from prior years.
Primary Care Match Rates Post–Tuition Waiver
There’s some fine print to consider. Some of the residents who matched into internal medicine or pediatric programs may subspecialize and not work in outpatient medicine at all. Also, a majority of students matched in major urban areas such as New York, Boston, or Los Angeles. We know that historically, people tend to work where they train, so that’s not looking too great for recruitment to rural communities or underserved areas.
There is some hope. In 2024, slightly more students from NYU matched into primary care specialties, including to family medicine. This is amidst a new record in family med residency matches nationwide. Rightfully, in the beginning, NYU was applauded for this tuition-free decision, but there was some criticism about who should be prioritized for financial assistance. Consider a future surgeon from a wealthy family vs a first-generation student committed to rural primary care.
NYU said, “No, equality for all.” Even acclaimed physician and bioethicist, Dr Ezekiel Emanuel weighed in and suggested “forgiving medical school debt for students who commit to a career in primary care in an underserved area. Two years of service for each year of free tuition.” At least this would allow resources to be focused only on building a primary care pool.
Also, after the tuition-free announcement, applications to NYU increased by almost 50%, and from underrepresented groups, 100%. Not that surprising. The average MCAT score and GPA also increased, but the acceptance rate stayed at around 2%.
How much difference will this tuition-free program really make in the future? Time will tell, but I do think that NYU set an important precedent here. Medical schools should be critically looking at where tuition money goes and what financial incentives could be used to attract a more diverse student body, with more hopefully going into primary care.
Let’s take a look at NYU’s Grossman Long Island campus. They have an accelerated 3-year program, tuition-free, primary care focused, and 67% of their graduates went into primary care.
In California, Kaiser Permanente School of Medicine, which is also tuition-free and focused, had 38% of their graduates go to primary care.
What are these programs doing differently? Well, they’re tuition-free, they have focused tracks, and they have enough accredited sites so that students can get a realistic and broad look of what it’s actually like to practice primary care.
Attracting Med Students to Primary Care
This leads to a broader question: How do we create an environment beyond tuition that encourages more students to go into internal medicine, pediatrics, or family medicine?
Right now, across those three specialties, the average salary is $250,000, which is lower than in other subspecialties. There’s a high amount of administrative workload, loss of autonomy, and plenty of burnout. You want to get more students to go into primary care? We need to fix primary care.
That involves many factors. Get ready for this. I actually had to make a list based on what I’ve read in articles and heard from my colleagues.
If we want to attract more students to primary care, we need to talk about:
- Improving reimbursement;
- Better mental health support;
- Highlighting the importance primary care plays in public health;
- Expanding care teams;
- Creating more medical students and training sites in rural and underserved areas;
- Expanding the use of telehealth services;
- Creating early exposure programs for high school and college students; and
- Paying attention to how local policies and statistics, such as crime, housing, and abortion bans, may push people away from practicing in certain areas or states.
Clearly, this is a large number of considerations that goes far beyond the altruistic tuition-free gifts.
Look, it’s no surprise we have a physician shortage that affects multiple specialties, but it is alarming that by 2034, there’s going to be an estimated shortage of 50,000 primary care doctors.
Stay Tuned
What do you all think? Is free tuition enough to actually move the needle in the long term, or should NYU have made a more focused gift? Comment below.
I know what you’re all wondering why I didn’t talk about tuition waivers in terms of diversifying the student body. That’s because that deserves its own video. Stay tuned for part 2.
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He hosts The Hospitalist Retort video blog on Medscape.
Dr. Patel has disclosed the following relevant financial relationships: serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Medumo Inc.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Would free medical school encourage more students to pursue primary care? Overpriced medical training in this country definitely contributes to burnout and the physician shortage, and all that debt may influence what type of specialty somebody goes into.
Assumptions Behind Free Tuition Initiatives
Now, this question is based on an assumption that there already is a large group of medical students who want to go into primary care, and the reason they’re not or the reason they’re reluctant to is because of tuition. If you take that stress away, you fix the problem.
This was at least part of the assumption made when NYU announced free tuition for all medical students back in 2018. One goal was to encourage more students to go into primary care. The other was to broaden the application pool to include more diverse students from different socioeconomic and racial backgrounds. We›ll get back to that.
Quick numbers. In NYU’s 2022 match, the first tuition-free class, about 25% of students matched into a primary care specialty— internal medicine or pediatrics — and there were zero matches into a family medicine residency. I can’t find the data, but anecdotally that 25% is a slight increase from prior years.
Primary Care Match Rates Post–Tuition Waiver
There’s some fine print to consider. Some of the residents who matched into internal medicine or pediatric programs may subspecialize and not work in outpatient medicine at all. Also, a majority of students matched in major urban areas such as New York, Boston, or Los Angeles. We know that historically, people tend to work where they train, so that’s not looking too great for recruitment to rural communities or underserved areas.
There is some hope. In 2024, slightly more students from NYU matched into primary care specialties, including to family medicine. This is amidst a new record in family med residency matches nationwide. Rightfully, in the beginning, NYU was applauded for this tuition-free decision, but there was some criticism about who should be prioritized for financial assistance. Consider a future surgeon from a wealthy family vs a first-generation student committed to rural primary care.
NYU said, “No, equality for all.” Even acclaimed physician and bioethicist, Dr Ezekiel Emanuel weighed in and suggested “forgiving medical school debt for students who commit to a career in primary care in an underserved area. Two years of service for each year of free tuition.” At least this would allow resources to be focused only on building a primary care pool.
Also, after the tuition-free announcement, applications to NYU increased by almost 50%, and from underrepresented groups, 100%. Not that surprising. The average MCAT score and GPA also increased, but the acceptance rate stayed at around 2%.
How much difference will this tuition-free program really make in the future? Time will tell, but I do think that NYU set an important precedent here. Medical schools should be critically looking at where tuition money goes and what financial incentives could be used to attract a more diverse student body, with more hopefully going into primary care.
Let’s take a look at NYU’s Grossman Long Island campus. They have an accelerated 3-year program, tuition-free, primary care focused, and 67% of their graduates went into primary care.
In California, Kaiser Permanente School of Medicine, which is also tuition-free and focused, had 38% of their graduates go to primary care.
What are these programs doing differently? Well, they’re tuition-free, they have focused tracks, and they have enough accredited sites so that students can get a realistic and broad look of what it’s actually like to practice primary care.
Attracting Med Students to Primary Care
This leads to a broader question: How do we create an environment beyond tuition that encourages more students to go into internal medicine, pediatrics, or family medicine?
Right now, across those three specialties, the average salary is $250,000, which is lower than in other subspecialties. There’s a high amount of administrative workload, loss of autonomy, and plenty of burnout. You want to get more students to go into primary care? We need to fix primary care.
That involves many factors. Get ready for this. I actually had to make a list based on what I’ve read in articles and heard from my colleagues.
If we want to attract more students to primary care, we need to talk about:
- Improving reimbursement;
- Better mental health support;
- Highlighting the importance primary care plays in public health;
- Expanding care teams;
- Creating more medical students and training sites in rural and underserved areas;
- Expanding the use of telehealth services;
- Creating early exposure programs for high school and college students; and
- Paying attention to how local policies and statistics, such as crime, housing, and abortion bans, may push people away from practicing in certain areas or states.
Clearly, this is a large number of considerations that goes far beyond the altruistic tuition-free gifts.
Look, it’s no surprise we have a physician shortage that affects multiple specialties, but it is alarming that by 2034, there’s going to be an estimated shortage of 50,000 primary care doctors.
Stay Tuned
What do you all think? Is free tuition enough to actually move the needle in the long term, or should NYU have made a more focused gift? Comment below.
I know what you’re all wondering why I didn’t talk about tuition waivers in terms of diversifying the student body. That’s because that deserves its own video. Stay tuned for part 2.
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He hosts The Hospitalist Retort video blog on Medscape.
Dr. Patel has disclosed the following relevant financial relationships: serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Medumo Inc.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Would free medical school encourage more students to pursue primary care? Overpriced medical training in this country definitely contributes to burnout and the physician shortage, and all that debt may influence what type of specialty somebody goes into.
Assumptions Behind Free Tuition Initiatives
Now, this question is based on an assumption that there already is a large group of medical students who want to go into primary care, and the reason they’re not or the reason they’re reluctant to is because of tuition. If you take that stress away, you fix the problem.
This was at least part of the assumption made when NYU announced free tuition for all medical students back in 2018. One goal was to encourage more students to go into primary care. The other was to broaden the application pool to include more diverse students from different socioeconomic and racial backgrounds. We›ll get back to that.
Quick numbers. In NYU’s 2022 match, the first tuition-free class, about 25% of students matched into a primary care specialty— internal medicine or pediatrics — and there were zero matches into a family medicine residency. I can’t find the data, but anecdotally that 25% is a slight increase from prior years.
Primary Care Match Rates Post–Tuition Waiver
There’s some fine print to consider. Some of the residents who matched into internal medicine or pediatric programs may subspecialize and not work in outpatient medicine at all. Also, a majority of students matched in major urban areas such as New York, Boston, or Los Angeles. We know that historically, people tend to work where they train, so that’s not looking too great for recruitment to rural communities or underserved areas.
There is some hope. In 2024, slightly more students from NYU matched into primary care specialties, including to family medicine. This is amidst a new record in family med residency matches nationwide. Rightfully, in the beginning, NYU was applauded for this tuition-free decision, but there was some criticism about who should be prioritized for financial assistance. Consider a future surgeon from a wealthy family vs a first-generation student committed to rural primary care.
NYU said, “No, equality for all.” Even acclaimed physician and bioethicist, Dr Ezekiel Emanuel weighed in and suggested “forgiving medical school debt for students who commit to a career in primary care in an underserved area. Two years of service for each year of free tuition.” At least this would allow resources to be focused only on building a primary care pool.
Also, after the tuition-free announcement, applications to NYU increased by almost 50%, and from underrepresented groups, 100%. Not that surprising. The average MCAT score and GPA also increased, but the acceptance rate stayed at around 2%.
How much difference will this tuition-free program really make in the future? Time will tell, but I do think that NYU set an important precedent here. Medical schools should be critically looking at where tuition money goes and what financial incentives could be used to attract a more diverse student body, with more hopefully going into primary care.
Let’s take a look at NYU’s Grossman Long Island campus. They have an accelerated 3-year program, tuition-free, primary care focused, and 67% of their graduates went into primary care.
In California, Kaiser Permanente School of Medicine, which is also tuition-free and focused, had 38% of their graduates go to primary care.
What are these programs doing differently? Well, they’re tuition-free, they have focused tracks, and they have enough accredited sites so that students can get a realistic and broad look of what it’s actually like to practice primary care.
Attracting Med Students to Primary Care
This leads to a broader question: How do we create an environment beyond tuition that encourages more students to go into internal medicine, pediatrics, or family medicine?
Right now, across those three specialties, the average salary is $250,000, which is lower than in other subspecialties. There’s a high amount of administrative workload, loss of autonomy, and plenty of burnout. You want to get more students to go into primary care? We need to fix primary care.
That involves many factors. Get ready for this. I actually had to make a list based on what I’ve read in articles and heard from my colleagues.
If we want to attract more students to primary care, we need to talk about:
- Improving reimbursement;
- Better mental health support;
- Highlighting the importance primary care plays in public health;
- Expanding care teams;
- Creating more medical students and training sites in rural and underserved areas;
- Expanding the use of telehealth services;
- Creating early exposure programs for high school and college students; and
- Paying attention to how local policies and statistics, such as crime, housing, and abortion bans, may push people away from practicing in certain areas or states.
Clearly, this is a large number of considerations that goes far beyond the altruistic tuition-free gifts.
Look, it’s no surprise we have a physician shortage that affects multiple specialties, but it is alarming that by 2034, there’s going to be an estimated shortage of 50,000 primary care doctors.
Stay Tuned
What do you all think? Is free tuition enough to actually move the needle in the long term, or should NYU have made a more focused gift? Comment below.
I know what you’re all wondering why I didn’t talk about tuition waivers in terms of diversifying the student body. That’s because that deserves its own video. Stay tuned for part 2.
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He hosts The Hospitalist Retort video blog on Medscape.
Dr. Patel has disclosed the following relevant financial relationships: serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Medumo Inc.
A version of this article appeared on Medscape.com.
Who Benefits From Omega-3/Fish Oil Supplements?
I’d like to talk with you about a recent report in the British Medical Journal on the regular use of omega-3 fish oil supplements and the course of cardiovascular disease (CVD).
This is an observational study from the large-scale UK Biobank. The authors divided the participants into those with and those without CVD. In participants without CVD at baseline, those using fish oil supplements regularly had an increased incidence of both atrial fibrillation (AF) and stroke, whereas those with prevalent CVD had a reduction in the progression to major adverse cardiovascular events, which offset any increase in the risk for AF.
Observational studies of omega-3 supplements have potential limitations and confounding, and correlation in these studies does not prove causation. What do the randomized clinical trials of omega-3 supplements show? At least seven randomized trials have looked at AF. A meta-analysis published in Circulation in 2021 showed a dose-response relationship. In trials testing > 1 g/d of marine omega-3 fatty acids, there was close to a 50% overall increase in risk for AF. In studies testing lower doses, there was a very modest 12% increase and a significant dose-response gradient.
For the relationship between omega-3 supplements and major cardiovascular events, at least 15 individual randomized trials have been conducted. There actually have been more meta-analyses of these randomized trials than individual trials. The meta-analyses tend to show a significant reduction of coronary events with omega-3 supplementation, but no reduction in stroke. This is true in both primary and secondary prevention trials.
The one exception to this finding is the REDUCE-IT trial testing high-dose eicosapentaenoic acid (EPA) (4 g/day of icosapent ethyl), and there was a 25%-30% reduction in both cardiovascular events and stroke. But there has been some criticism of the mineral oil placebo used in the REDUCE-IT trial that it may have had adverse effects on biomarkers and might have interfered with the absorption of statins in the placebo group. So, it will be important to have a replication trial of the high-dose EPA, findings in a trial using an inert placebo such as corn oil.
What should be done in the meantime? It’s important to think about prescription omega-3s vs over-the-counter fish oil. The US Food and Drug Administration (FDA) has approved prescription omega-3 medications for several indications, including severely elevated triglyceride levels (> 500 mg/dL). In the REDUCE-IT trial, those who had moderate elevations of triglycerides (≥ 150 mg/dL) or prevalent CVD or diabetes, plus two additional risk factors, were also considered to have indications based on the FDA labeling for icosapent ethyl.
What about patients who don’t meet these criteria for prescription omega-3s? In the VITAL trial (the large-scale primary prevention trial), there was a similar reduction in coronary events but no effect on stroke. Those who seemed to benefit the most in terms of at least 40% reduction in coronary events were participants who had low fish consumption at baseline, had two or more risk factors for cardiovascular disease, or were African American.
There is a national recommendation for one to two servings of fish per week. For those planning to take fish oil, it’s important to use reputable sources of the supplement, and check the bottle for a quality control seal. It’s also really important to avoid megadoses of fish oil, because high doses have been linked to an increased risk for AF and bleeding.
Dr. Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston, disclosed ties with Mars Symbioscience for the COSMOS trial.
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report in the British Medical Journal on the regular use of omega-3 fish oil supplements and the course of cardiovascular disease (CVD).
This is an observational study from the large-scale UK Biobank. The authors divided the participants into those with and those without CVD. In participants without CVD at baseline, those using fish oil supplements regularly had an increased incidence of both atrial fibrillation (AF) and stroke, whereas those with prevalent CVD had a reduction in the progression to major adverse cardiovascular events, which offset any increase in the risk for AF.
Observational studies of omega-3 supplements have potential limitations and confounding, and correlation in these studies does not prove causation. What do the randomized clinical trials of omega-3 supplements show? At least seven randomized trials have looked at AF. A meta-analysis published in Circulation in 2021 showed a dose-response relationship. In trials testing > 1 g/d of marine omega-3 fatty acids, there was close to a 50% overall increase in risk for AF. In studies testing lower doses, there was a very modest 12% increase and a significant dose-response gradient.
For the relationship between omega-3 supplements and major cardiovascular events, at least 15 individual randomized trials have been conducted. There actually have been more meta-analyses of these randomized trials than individual trials. The meta-analyses tend to show a significant reduction of coronary events with omega-3 supplementation, but no reduction in stroke. This is true in both primary and secondary prevention trials.
The one exception to this finding is the REDUCE-IT trial testing high-dose eicosapentaenoic acid (EPA) (4 g/day of icosapent ethyl), and there was a 25%-30% reduction in both cardiovascular events and stroke. But there has been some criticism of the mineral oil placebo used in the REDUCE-IT trial that it may have had adverse effects on biomarkers and might have interfered with the absorption of statins in the placebo group. So, it will be important to have a replication trial of the high-dose EPA, findings in a trial using an inert placebo such as corn oil.
What should be done in the meantime? It’s important to think about prescription omega-3s vs over-the-counter fish oil. The US Food and Drug Administration (FDA) has approved prescription omega-3 medications for several indications, including severely elevated triglyceride levels (> 500 mg/dL). In the REDUCE-IT trial, those who had moderate elevations of triglycerides (≥ 150 mg/dL) or prevalent CVD or diabetes, plus two additional risk factors, were also considered to have indications based on the FDA labeling for icosapent ethyl.
What about patients who don’t meet these criteria for prescription omega-3s? In the VITAL trial (the large-scale primary prevention trial), there was a similar reduction in coronary events but no effect on stroke. Those who seemed to benefit the most in terms of at least 40% reduction in coronary events were participants who had low fish consumption at baseline, had two or more risk factors for cardiovascular disease, or were African American.
There is a national recommendation for one to two servings of fish per week. For those planning to take fish oil, it’s important to use reputable sources of the supplement, and check the bottle for a quality control seal. It’s also really important to avoid megadoses of fish oil, because high doses have been linked to an increased risk for AF and bleeding.
Dr. Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston, disclosed ties with Mars Symbioscience for the COSMOS trial.
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report in the British Medical Journal on the regular use of omega-3 fish oil supplements and the course of cardiovascular disease (CVD).
This is an observational study from the large-scale UK Biobank. The authors divided the participants into those with and those without CVD. In participants without CVD at baseline, those using fish oil supplements regularly had an increased incidence of both atrial fibrillation (AF) and stroke, whereas those with prevalent CVD had a reduction in the progression to major adverse cardiovascular events, which offset any increase in the risk for AF.
Observational studies of omega-3 supplements have potential limitations and confounding, and correlation in these studies does not prove causation. What do the randomized clinical trials of omega-3 supplements show? At least seven randomized trials have looked at AF. A meta-analysis published in Circulation in 2021 showed a dose-response relationship. In trials testing > 1 g/d of marine omega-3 fatty acids, there was close to a 50% overall increase in risk for AF. In studies testing lower doses, there was a very modest 12% increase and a significant dose-response gradient.
For the relationship between omega-3 supplements and major cardiovascular events, at least 15 individual randomized trials have been conducted. There actually have been more meta-analyses of these randomized trials than individual trials. The meta-analyses tend to show a significant reduction of coronary events with omega-3 supplementation, but no reduction in stroke. This is true in both primary and secondary prevention trials.
The one exception to this finding is the REDUCE-IT trial testing high-dose eicosapentaenoic acid (EPA) (4 g/day of icosapent ethyl), and there was a 25%-30% reduction in both cardiovascular events and stroke. But there has been some criticism of the mineral oil placebo used in the REDUCE-IT trial that it may have had adverse effects on biomarkers and might have interfered with the absorption of statins in the placebo group. So, it will be important to have a replication trial of the high-dose EPA, findings in a trial using an inert placebo such as corn oil.
What should be done in the meantime? It’s important to think about prescription omega-3s vs over-the-counter fish oil. The US Food and Drug Administration (FDA) has approved prescription omega-3 medications for several indications, including severely elevated triglyceride levels (> 500 mg/dL). In the REDUCE-IT trial, those who had moderate elevations of triglycerides (≥ 150 mg/dL) or prevalent CVD or diabetes, plus two additional risk factors, were also considered to have indications based on the FDA labeling for icosapent ethyl.
What about patients who don’t meet these criteria for prescription omega-3s? In the VITAL trial (the large-scale primary prevention trial), there was a similar reduction in coronary events but no effect on stroke. Those who seemed to benefit the most in terms of at least 40% reduction in coronary events were participants who had low fish consumption at baseline, had two or more risk factors for cardiovascular disease, or were African American.
There is a national recommendation for one to two servings of fish per week. For those planning to take fish oil, it’s important to use reputable sources of the supplement, and check the bottle for a quality control seal. It’s also really important to avoid megadoses of fish oil, because high doses have been linked to an increased risk for AF and bleeding.
Dr. Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston, disclosed ties with Mars Symbioscience for the COSMOS trial.
A version of this article appeared on Medscape.com.
Is Semaglutide the ‘New Statin’? Not So Fast
There has been much hyperbole since the presentation of results from the SELECT cardiovascular outcomes trial (CVOT) at this year’s European Congress on Obesity, which led many to herald semaglutide as the “new statin.”
In the SELECT CVOT, participants with overweight or obesity (body mass index [BMI] ≥ 27), established cardiovascular disease (CVD), and no history of type 2 diabetes were administered the injectable glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) at a 2.4-mg dose weekly. Treatment resulted in a significant 20% relative risk reduction in major adverse CV events (a composite endpoint comprising CV death, nonfatal myocardial infarction, or nonfatal stroke). Importantly, SELECT was a trial on secondary prevention of CVD.
The CV benefits of semaglutide were notably independent of baseline weight or amount of weight lost. This suggests that the underlying driver of improved CV outcomes with semaglutide extends beyond simple reduction in obesity and perhaps indicates a direct effect on vasculature and reduction in atherosclerosis, although this remains unproven.
Not All Risk Reduction Is Equal
Much of the sensationalist coverage in the lay press focused on the 20% relative risk reduction figure. This endpoint is often more impressive and headline-grabbing than the absolute risk reduction, which provides a clearer view of a treatment’s real-world impact.
In SELECT, the absolute risk reduction was 1.5 percentage points, which translated into a number needed to treat (NNT) of 67 over 34 months to prevent one primary outcome of a major adverse CV event.
Lower NNTs suggest more effective treatments because fewer people need to be treated to prevent one clinical event, such as the major adverse CV events used in SELECT.
Semaglutide vs Statins
How does the clinical effectiveness observed in the SELECT trial compare with that observed in statin trials when it comes to the secondary prevention of CVD?
The seminal 4S study published in 1994 explored the impact of simvastatin on all-cause mortality among people with previous myocardial infarction or angina and hyperlipidemia (mean baseline BMI, 26). After 5.4 years of follow-up, the trial was stopped early owing to a 3.3-percentage point absolute risk reduction in all-cause mortality (NNT, 30; relative risk reduction, 28%). The NNT to prevent one death from CV causes was 31, and the NNT to prevent one major coronary event was lower, at 15.
Other statin secondary prevention trials, such as the LIPID and MIRACL studies, demonstrated similarly low NNTs.
So, you can see that the NNTs for statins in secondary prevention are much lower than with semaglutide in SELECT. Furthermore, the benefits of semaglutide in preventing CVD in people living with overweight/obesity have yet to be elucidated.
In contrast, we already have published evidence showing the benefits of statins in the primary prevention of CVD, albeit with higher and more variable NNTs than in the statin secondary prevention studies.
The benefits of statins are also postulated to extend beyond their impact on lowering low-density lipoprotein cholesterol. Statins have been suggested to have anti-inflammatory and plaque-stabilizing effects, among other pleiotropic benefits.
We also currently lack evidence for the cost-effectiveness of semaglutide for CV risk reduction. Assessing economic viability and use in health care systems, such as the UK’s National Health Service, involves comparing the cost of semaglutide against the health care savings from prevented CV events. Health economic studies are vital to determine whether the benefits justify the expense. In contrast, the cost-effectiveness of statins is well established, particularly for high-risk individuals.
Advantages of GLP-1s Should Not Be Overlooked
Of course, statins don’t provide the significant weight loss benefits of semaglutide.
Additional data from SELECT presented at the 2024 European Congress on Obesity demonstrated that participants lost a mean of 10.2% body weight and 7.7 cm from their waist circumference after 4 years. Moreover, after 2 years, 12% of individuals randomized to semaglutide had returned to a normal BMI, and nearly half were no longer living with obesity.
Although the CV benefits of semaglutide were independent of weight reduction, this level of weight loss is clinically meaningful and will reduce the risk of many other cardiometabolic conditions including type 2 diabetes, metabolic dysfunction–associated steatotic liver disease, and obstructive sleep apnea/hypopnea syndrome, as well as improve low mood, depression, and overall quality of life. Additionally, obesity is now a risk factor for 13 different types of cancer, including bowel, breast, and pancreatic cancer, so facilitating a return to a healthier body weight will also mitigate future risk for cancer.
Sticking With Our Cornerstone Therapy, For Now
In conclusion, I do not believe that semaglutide is the “new statin.” Statins are the cornerstone of primary and secondary prevention of CVD in a wide range of comorbidities, as evidenced in multiple large and high-quality trials dating back over 30 years.
However, there is no doubt that the GLP-1 receptor agonist class is the most significant therapeutic advance for the management of obesity and comorbidities to date.
The SELECT CVOT data uniquely position semaglutide as a secondary CVD prevention agent on top of guideline-driven management for people living with overweight/obesity and established CVD. Additionally, the clinically meaningful weight loss achieved with semaglutide will impact the risk of developing many other cardiometabolic conditions, as well as improve mental health and overall quality of life.
Dr. Fernando, GP Partner, North Berwick Health Centre, North Berwick, Scotland, creates concise clinical aide-mémoire for primary and secondary care to make life easier for health care professionals and ultimately to improve the lives of patients. He is very active on social media (X handle @drkevinfernando), where he posts hot topics in type 2 diabetes and CVRM. He recently has forayed into YouTube (@DrKevinFernando) and TikTok (@drkevinfernando) with patient-facing video content. Dr. Fernando has been elected to Fellowship of the Royal College of General Practitioners, the Royal College of Physicians of Edinburgh, and the Academy of Medical Educators for his work in diabetes and medical education. He has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo; received income in an amount equal to or greater than $250 from AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There has been much hyperbole since the presentation of results from the SELECT cardiovascular outcomes trial (CVOT) at this year’s European Congress on Obesity, which led many to herald semaglutide as the “new statin.”
In the SELECT CVOT, participants with overweight or obesity (body mass index [BMI] ≥ 27), established cardiovascular disease (CVD), and no history of type 2 diabetes were administered the injectable glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) at a 2.4-mg dose weekly. Treatment resulted in a significant 20% relative risk reduction in major adverse CV events (a composite endpoint comprising CV death, nonfatal myocardial infarction, or nonfatal stroke). Importantly, SELECT was a trial on secondary prevention of CVD.
The CV benefits of semaglutide were notably independent of baseline weight or amount of weight lost. This suggests that the underlying driver of improved CV outcomes with semaglutide extends beyond simple reduction in obesity and perhaps indicates a direct effect on vasculature and reduction in atherosclerosis, although this remains unproven.
Not All Risk Reduction Is Equal
Much of the sensationalist coverage in the lay press focused on the 20% relative risk reduction figure. This endpoint is often more impressive and headline-grabbing than the absolute risk reduction, which provides a clearer view of a treatment’s real-world impact.
In SELECT, the absolute risk reduction was 1.5 percentage points, which translated into a number needed to treat (NNT) of 67 over 34 months to prevent one primary outcome of a major adverse CV event.
Lower NNTs suggest more effective treatments because fewer people need to be treated to prevent one clinical event, such as the major adverse CV events used in SELECT.
Semaglutide vs Statins
How does the clinical effectiveness observed in the SELECT trial compare with that observed in statin trials when it comes to the secondary prevention of CVD?
The seminal 4S study published in 1994 explored the impact of simvastatin on all-cause mortality among people with previous myocardial infarction or angina and hyperlipidemia (mean baseline BMI, 26). After 5.4 years of follow-up, the trial was stopped early owing to a 3.3-percentage point absolute risk reduction in all-cause mortality (NNT, 30; relative risk reduction, 28%). The NNT to prevent one death from CV causes was 31, and the NNT to prevent one major coronary event was lower, at 15.
Other statin secondary prevention trials, such as the LIPID and MIRACL studies, demonstrated similarly low NNTs.
So, you can see that the NNTs for statins in secondary prevention are much lower than with semaglutide in SELECT. Furthermore, the benefits of semaglutide in preventing CVD in people living with overweight/obesity have yet to be elucidated.
In contrast, we already have published evidence showing the benefits of statins in the primary prevention of CVD, albeit with higher and more variable NNTs than in the statin secondary prevention studies.
The benefits of statins are also postulated to extend beyond their impact on lowering low-density lipoprotein cholesterol. Statins have been suggested to have anti-inflammatory and plaque-stabilizing effects, among other pleiotropic benefits.
We also currently lack evidence for the cost-effectiveness of semaglutide for CV risk reduction. Assessing economic viability and use in health care systems, such as the UK’s National Health Service, involves comparing the cost of semaglutide against the health care savings from prevented CV events. Health economic studies are vital to determine whether the benefits justify the expense. In contrast, the cost-effectiveness of statins is well established, particularly for high-risk individuals.
Advantages of GLP-1s Should Not Be Overlooked
Of course, statins don’t provide the significant weight loss benefits of semaglutide.
Additional data from SELECT presented at the 2024 European Congress on Obesity demonstrated that participants lost a mean of 10.2% body weight and 7.7 cm from their waist circumference after 4 years. Moreover, after 2 years, 12% of individuals randomized to semaglutide had returned to a normal BMI, and nearly half were no longer living with obesity.
Although the CV benefits of semaglutide were independent of weight reduction, this level of weight loss is clinically meaningful and will reduce the risk of many other cardiometabolic conditions including type 2 diabetes, metabolic dysfunction–associated steatotic liver disease, and obstructive sleep apnea/hypopnea syndrome, as well as improve low mood, depression, and overall quality of life. Additionally, obesity is now a risk factor for 13 different types of cancer, including bowel, breast, and pancreatic cancer, so facilitating a return to a healthier body weight will also mitigate future risk for cancer.
Sticking With Our Cornerstone Therapy, For Now
In conclusion, I do not believe that semaglutide is the “new statin.” Statins are the cornerstone of primary and secondary prevention of CVD in a wide range of comorbidities, as evidenced in multiple large and high-quality trials dating back over 30 years.
However, there is no doubt that the GLP-1 receptor agonist class is the most significant therapeutic advance for the management of obesity and comorbidities to date.
The SELECT CVOT data uniquely position semaglutide as a secondary CVD prevention agent on top of guideline-driven management for people living with overweight/obesity and established CVD. Additionally, the clinically meaningful weight loss achieved with semaglutide will impact the risk of developing many other cardiometabolic conditions, as well as improve mental health and overall quality of life.
Dr. Fernando, GP Partner, North Berwick Health Centre, North Berwick, Scotland, creates concise clinical aide-mémoire for primary and secondary care to make life easier for health care professionals and ultimately to improve the lives of patients. He is very active on social media (X handle @drkevinfernando), where he posts hot topics in type 2 diabetes and CVRM. He recently has forayed into YouTube (@DrKevinFernando) and TikTok (@drkevinfernando) with patient-facing video content. Dr. Fernando has been elected to Fellowship of the Royal College of General Practitioners, the Royal College of Physicians of Edinburgh, and the Academy of Medical Educators for his work in diabetes and medical education. He has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo; received income in an amount equal to or greater than $250 from AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There has been much hyperbole since the presentation of results from the SELECT cardiovascular outcomes trial (CVOT) at this year’s European Congress on Obesity, which led many to herald semaglutide as the “new statin.”
In the SELECT CVOT, participants with overweight or obesity (body mass index [BMI] ≥ 27), established cardiovascular disease (CVD), and no history of type 2 diabetes were administered the injectable glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) at a 2.4-mg dose weekly. Treatment resulted in a significant 20% relative risk reduction in major adverse CV events (a composite endpoint comprising CV death, nonfatal myocardial infarction, or nonfatal stroke). Importantly, SELECT was a trial on secondary prevention of CVD.
The CV benefits of semaglutide were notably independent of baseline weight or amount of weight lost. This suggests that the underlying driver of improved CV outcomes with semaglutide extends beyond simple reduction in obesity and perhaps indicates a direct effect on vasculature and reduction in atherosclerosis, although this remains unproven.
Not All Risk Reduction Is Equal
Much of the sensationalist coverage in the lay press focused on the 20% relative risk reduction figure. This endpoint is often more impressive and headline-grabbing than the absolute risk reduction, which provides a clearer view of a treatment’s real-world impact.
In SELECT, the absolute risk reduction was 1.5 percentage points, which translated into a number needed to treat (NNT) of 67 over 34 months to prevent one primary outcome of a major adverse CV event.
Lower NNTs suggest more effective treatments because fewer people need to be treated to prevent one clinical event, such as the major adverse CV events used in SELECT.
Semaglutide vs Statins
How does the clinical effectiveness observed in the SELECT trial compare with that observed in statin trials when it comes to the secondary prevention of CVD?
The seminal 4S study published in 1994 explored the impact of simvastatin on all-cause mortality among people with previous myocardial infarction or angina and hyperlipidemia (mean baseline BMI, 26). After 5.4 years of follow-up, the trial was stopped early owing to a 3.3-percentage point absolute risk reduction in all-cause mortality (NNT, 30; relative risk reduction, 28%). The NNT to prevent one death from CV causes was 31, and the NNT to prevent one major coronary event was lower, at 15.
Other statin secondary prevention trials, such as the LIPID and MIRACL studies, demonstrated similarly low NNTs.
So, you can see that the NNTs for statins in secondary prevention are much lower than with semaglutide in SELECT. Furthermore, the benefits of semaglutide in preventing CVD in people living with overweight/obesity have yet to be elucidated.
In contrast, we already have published evidence showing the benefits of statins in the primary prevention of CVD, albeit with higher and more variable NNTs than in the statin secondary prevention studies.
The benefits of statins are also postulated to extend beyond their impact on lowering low-density lipoprotein cholesterol. Statins have been suggested to have anti-inflammatory and plaque-stabilizing effects, among other pleiotropic benefits.
We also currently lack evidence for the cost-effectiveness of semaglutide for CV risk reduction. Assessing economic viability and use in health care systems, such as the UK’s National Health Service, involves comparing the cost of semaglutide against the health care savings from prevented CV events. Health economic studies are vital to determine whether the benefits justify the expense. In contrast, the cost-effectiveness of statins is well established, particularly for high-risk individuals.
Advantages of GLP-1s Should Not Be Overlooked
Of course, statins don’t provide the significant weight loss benefits of semaglutide.
Additional data from SELECT presented at the 2024 European Congress on Obesity demonstrated that participants lost a mean of 10.2% body weight and 7.7 cm from their waist circumference after 4 years. Moreover, after 2 years, 12% of individuals randomized to semaglutide had returned to a normal BMI, and nearly half were no longer living with obesity.
Although the CV benefits of semaglutide were independent of weight reduction, this level of weight loss is clinically meaningful and will reduce the risk of many other cardiometabolic conditions including type 2 diabetes, metabolic dysfunction–associated steatotic liver disease, and obstructive sleep apnea/hypopnea syndrome, as well as improve low mood, depression, and overall quality of life. Additionally, obesity is now a risk factor for 13 different types of cancer, including bowel, breast, and pancreatic cancer, so facilitating a return to a healthier body weight will also mitigate future risk for cancer.
Sticking With Our Cornerstone Therapy, For Now
In conclusion, I do not believe that semaglutide is the “new statin.” Statins are the cornerstone of primary and secondary prevention of CVD in a wide range of comorbidities, as evidenced in multiple large and high-quality trials dating back over 30 years.
However, there is no doubt that the GLP-1 receptor agonist class is the most significant therapeutic advance for the management of obesity and comorbidities to date.
The SELECT CVOT data uniquely position semaglutide as a secondary CVD prevention agent on top of guideline-driven management for people living with overweight/obesity and established CVD. Additionally, the clinically meaningful weight loss achieved with semaglutide will impact the risk of developing many other cardiometabolic conditions, as well as improve mental health and overall quality of life.
Dr. Fernando, GP Partner, North Berwick Health Centre, North Berwick, Scotland, creates concise clinical aide-mémoire for primary and secondary care to make life easier for health care professionals and ultimately to improve the lives of patients. He is very active on social media (X handle @drkevinfernando), where he posts hot topics in type 2 diabetes and CVRM. He recently has forayed into YouTube (@DrKevinFernando) and TikTok (@drkevinfernando) with patient-facing video content. Dr. Fernando has been elected to Fellowship of the Royal College of General Practitioners, the Royal College of Physicians of Edinburgh, and the Academy of Medical Educators for his work in diabetes and medical education. He has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo; received income in an amount equal to or greater than $250 from AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
Calcium and CV Risk: Are Supplements and Vitamin D to Blame?
This transcript has been edited for clarity.
Tricia Ward: Hi. I’m Tricia Ward, from theheart.org/Medscape Cardiology. I’m joined today by Dr Matthew Budoff. He is professor of medicine at UCLA and the endowed chair of preventive cardiology at the Lundquist Institute. Welcome, Dr Budoff.
Matthew J. Budoff, MD: Thank you.
Dietary Calcium vs Coronary Calcium
Ms. Ward: The reason I wanted to talk to you today is because there have been some recent studies linking calcium supplements to an increased risk for cardiovascular disease. I’m old enough to remember when we used to tell people that dietary calcium and coronary calcium weren’t connected and weren’t the same. Were we wrong?
Dr. Budoff: I think there’s a large amount of mixed data out there still. The US Preventive Services Task Force looked into this a number of years ago and said there’s no association between calcium supplementation and increased risk for cardiovascular disease.
As you mentioned, there are a couple of newer studies that point us toward a relationship. I think that we still have a little bit of a mixed bag, but we need to dive a little deeper into that to figure out what’s going on.
Ms. Ward: Does it appear to be connected to calcium in the form of supplements vs calcium from foods?
Dr. Budoff: We looked very carefully at dietary calcium in the MESA study, the multiethnic study of atherosclerosis. There is no relationship between dietary calcium intake and coronary calcium or cardiovascular events. We’re talking mostly about supplements now when we talk about this increased risk that we’re seeing.
Does Vitamin D Exacerbate Risk?
Ms. Ward: Because it’s seen with supplements, is that likely because that’s a much higher concentration of calcium coming in or do you think it’s something inherent in its being in the form of a supplement?
Dr. Budoff: I think there are two things. One, it’s definitely a higher concentration all at once. You get many more milligrams at a time when you take a supplement than if you had a high-calcium food or drink.
Also, most supplements have vitamin D as well. I think vitamin D and calcium work synergistically. When you give them both together simultaneously, I think that may have more of a potentiating effect that might exacerbate any potential risk.
Ms. Ward: Is there any reason to think there might be a difference in type of calcium supplement? I always think of the chalky tablet form vs calcium chews.
Dr. Budoff: I’m not aware of a difference in the supplement type. I think the vitamin D issue is a big problem because we all have patients who take thousands of units of vitamin D — just crazy numbers. People advocate really high numbers and that stays in the system.
Personally, I think part of the explanation is that with very high levels of vitamin D on top of calcium supplementation, you now absorb it better. You now get it into the bone, but maybe also into the coronary arteries. If you’re very high in vitamin D and then are taking a large calcium supplement, it might be the calcium/vitamin D combination that’s giving us some trouble. I think people on vitamin D supplements really need to watch their levels and not get supratherapeutic.
Ms. Ward: With the vitamin D?
Dr. Budoff: With the vitamin D.
Diabetes and Renal Function
Ms. Ward: In some of the studies, there seems to be a higher risk in patients with diabetes. Is there any reason why that would be?
Dr. Budoff: I can’t think of a reason exactly why with diabetes per se, except for renal disease. Patients with diabetes have more intrinsic renal disease, proteinuria, and even a reduced eGFR. We’ve seen that the kidney is very strongly tied to this. We have a very strong relationship, in work I’ve done a decade ago now, showing that calcium supplementation (in the form of phosphate binders) in patients on dialysis or with advanced renal disease is linked to much higher coronary calcium progression.
We did prospective, randomized trials showing that calcium intake as binders to reduce phosphorus led to more coronary calcium. We always thought that was just relegated to the renal population, and there might be an overlap here with the diabetes and more renal disease. I have a feeling that it has to do with more of that. It might be regulation of parathyroid hormone as well, which might be more abnormal in patients with diabetes.
Avoid Supratherapeutic Vitamin D Levels
Ms. Ward:: What are you telling your patients?
Dr. Budoff: I tell patients with normal kidney function that the bone will modulate 99.9% of the calcium uptake. If they have osteopenia or osteoporosis, regardless of their calcium score, I’m very comfortable putting them on supplements.
I’m a little more cautious with the vitamin D levels, and I keep an eye on that and regulate how much vitamin D they get based on their levels. I get them into the normal range, but I don’t want them supratherapeutic. You can even follow their calcium score. Again, we’ve shown that if you’re taking too much calcium, your calcium score will go up. I can just check it again in a couple of years to make sure that it’s safe.
Ms. Ward:: In terms of vitamin D levels, when you’re saying “supratherapeutic,” what levels do you consider a safe amount to take?
Dr. Budoff: I’d like them under 100 ng/mL as far as their upper level. Normal is around 70 ng/mL at most labs. I try to keep them in the normal range. I don’t even want them to be high-normal if I’m going to be concomitantly giving them calcium supplements. Of course, if they have renal insufficiency, then I’m much more cautious. We’ve even seen calcium supplements raise the serum calcium, which you never see with dietary calcium. That’s another potential proof that it might be too much too fast.
For renal patients, even in mild renal insufficiency, maybe even in diabetes where we’ve seen a signal, maybe aim lower in the amount of calcium supplementation if diet is insufficient, and aim a little lower in vitamin D targets, and I think you’ll be in a safer place.
Ms. Ward: Is there anything else you want to add?
Dr. Budoff: The evidence is still evolving. I’d say that it’s interesting and maybe a little frustrating that we don’t have a final answer on all of this. I would stay tuned for more data because we’re looking at many of the epidemiologic studies to try to see what happens in the real world, with both dietary intake of calcium and calcium supplementation.
Ms. Ward: Thank you very much for joining me today.
Dr. Budoff: It’s a pleasure. Thanks for having me.
Dr. Budoff disclosed being a speaker for Amarin Pharma.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Tricia Ward: Hi. I’m Tricia Ward, from theheart.org/Medscape Cardiology. I’m joined today by Dr Matthew Budoff. He is professor of medicine at UCLA and the endowed chair of preventive cardiology at the Lundquist Institute. Welcome, Dr Budoff.
Matthew J. Budoff, MD: Thank you.
Dietary Calcium vs Coronary Calcium
Ms. Ward: The reason I wanted to talk to you today is because there have been some recent studies linking calcium supplements to an increased risk for cardiovascular disease. I’m old enough to remember when we used to tell people that dietary calcium and coronary calcium weren’t connected and weren’t the same. Were we wrong?
Dr. Budoff: I think there’s a large amount of mixed data out there still. The US Preventive Services Task Force looked into this a number of years ago and said there’s no association between calcium supplementation and increased risk for cardiovascular disease.
As you mentioned, there are a couple of newer studies that point us toward a relationship. I think that we still have a little bit of a mixed bag, but we need to dive a little deeper into that to figure out what’s going on.
Ms. Ward: Does it appear to be connected to calcium in the form of supplements vs calcium from foods?
Dr. Budoff: We looked very carefully at dietary calcium in the MESA study, the multiethnic study of atherosclerosis. There is no relationship between dietary calcium intake and coronary calcium or cardiovascular events. We’re talking mostly about supplements now when we talk about this increased risk that we’re seeing.
Does Vitamin D Exacerbate Risk?
Ms. Ward: Because it’s seen with supplements, is that likely because that’s a much higher concentration of calcium coming in or do you think it’s something inherent in its being in the form of a supplement?
Dr. Budoff: I think there are two things. One, it’s definitely a higher concentration all at once. You get many more milligrams at a time when you take a supplement than if you had a high-calcium food or drink.
Also, most supplements have vitamin D as well. I think vitamin D and calcium work synergistically. When you give them both together simultaneously, I think that may have more of a potentiating effect that might exacerbate any potential risk.
Ms. Ward: Is there any reason to think there might be a difference in type of calcium supplement? I always think of the chalky tablet form vs calcium chews.
Dr. Budoff: I’m not aware of a difference in the supplement type. I think the vitamin D issue is a big problem because we all have patients who take thousands of units of vitamin D — just crazy numbers. People advocate really high numbers and that stays in the system.
Personally, I think part of the explanation is that with very high levels of vitamin D on top of calcium supplementation, you now absorb it better. You now get it into the bone, but maybe also into the coronary arteries. If you’re very high in vitamin D and then are taking a large calcium supplement, it might be the calcium/vitamin D combination that’s giving us some trouble. I think people on vitamin D supplements really need to watch their levels and not get supratherapeutic.
Ms. Ward: With the vitamin D?
Dr. Budoff: With the vitamin D.
Diabetes and Renal Function
Ms. Ward: In some of the studies, there seems to be a higher risk in patients with diabetes. Is there any reason why that would be?
Dr. Budoff: I can’t think of a reason exactly why with diabetes per se, except for renal disease. Patients with diabetes have more intrinsic renal disease, proteinuria, and even a reduced eGFR. We’ve seen that the kidney is very strongly tied to this. We have a very strong relationship, in work I’ve done a decade ago now, showing that calcium supplementation (in the form of phosphate binders) in patients on dialysis or with advanced renal disease is linked to much higher coronary calcium progression.
We did prospective, randomized trials showing that calcium intake as binders to reduce phosphorus led to more coronary calcium. We always thought that was just relegated to the renal population, and there might be an overlap here with the diabetes and more renal disease. I have a feeling that it has to do with more of that. It might be regulation of parathyroid hormone as well, which might be more abnormal in patients with diabetes.
Avoid Supratherapeutic Vitamin D Levels
Ms. Ward:: What are you telling your patients?
Dr. Budoff: I tell patients with normal kidney function that the bone will modulate 99.9% of the calcium uptake. If they have osteopenia or osteoporosis, regardless of their calcium score, I’m very comfortable putting them on supplements.
I’m a little more cautious with the vitamin D levels, and I keep an eye on that and regulate how much vitamin D they get based on their levels. I get them into the normal range, but I don’t want them supratherapeutic. You can even follow their calcium score. Again, we’ve shown that if you’re taking too much calcium, your calcium score will go up. I can just check it again in a couple of years to make sure that it’s safe.
Ms. Ward:: In terms of vitamin D levels, when you’re saying “supratherapeutic,” what levels do you consider a safe amount to take?
Dr. Budoff: I’d like them under 100 ng/mL as far as their upper level. Normal is around 70 ng/mL at most labs. I try to keep them in the normal range. I don’t even want them to be high-normal if I’m going to be concomitantly giving them calcium supplements. Of course, if they have renal insufficiency, then I’m much more cautious. We’ve even seen calcium supplements raise the serum calcium, which you never see with dietary calcium. That’s another potential proof that it might be too much too fast.
For renal patients, even in mild renal insufficiency, maybe even in diabetes where we’ve seen a signal, maybe aim lower in the amount of calcium supplementation if diet is insufficient, and aim a little lower in vitamin D targets, and I think you’ll be in a safer place.
Ms. Ward: Is there anything else you want to add?
Dr. Budoff: The evidence is still evolving. I’d say that it’s interesting and maybe a little frustrating that we don’t have a final answer on all of this. I would stay tuned for more data because we’re looking at many of the epidemiologic studies to try to see what happens in the real world, with both dietary intake of calcium and calcium supplementation.
Ms. Ward: Thank you very much for joining me today.
Dr. Budoff: It’s a pleasure. Thanks for having me.
Dr. Budoff disclosed being a speaker for Amarin Pharma.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Tricia Ward: Hi. I’m Tricia Ward, from theheart.org/Medscape Cardiology. I’m joined today by Dr Matthew Budoff. He is professor of medicine at UCLA and the endowed chair of preventive cardiology at the Lundquist Institute. Welcome, Dr Budoff.
Matthew J. Budoff, MD: Thank you.
Dietary Calcium vs Coronary Calcium
Ms. Ward: The reason I wanted to talk to you today is because there have been some recent studies linking calcium supplements to an increased risk for cardiovascular disease. I’m old enough to remember when we used to tell people that dietary calcium and coronary calcium weren’t connected and weren’t the same. Were we wrong?
Dr. Budoff: I think there’s a large amount of mixed data out there still. The US Preventive Services Task Force looked into this a number of years ago and said there’s no association between calcium supplementation and increased risk for cardiovascular disease.
As you mentioned, there are a couple of newer studies that point us toward a relationship. I think that we still have a little bit of a mixed bag, but we need to dive a little deeper into that to figure out what’s going on.
Ms. Ward: Does it appear to be connected to calcium in the form of supplements vs calcium from foods?
Dr. Budoff: We looked very carefully at dietary calcium in the MESA study, the multiethnic study of atherosclerosis. There is no relationship between dietary calcium intake and coronary calcium or cardiovascular events. We’re talking mostly about supplements now when we talk about this increased risk that we’re seeing.
Does Vitamin D Exacerbate Risk?
Ms. Ward: Because it’s seen with supplements, is that likely because that’s a much higher concentration of calcium coming in or do you think it’s something inherent in its being in the form of a supplement?
Dr. Budoff: I think there are two things. One, it’s definitely a higher concentration all at once. You get many more milligrams at a time when you take a supplement than if you had a high-calcium food or drink.
Also, most supplements have vitamin D as well. I think vitamin D and calcium work synergistically. When you give them both together simultaneously, I think that may have more of a potentiating effect that might exacerbate any potential risk.
Ms. Ward: Is there any reason to think there might be a difference in type of calcium supplement? I always think of the chalky tablet form vs calcium chews.
Dr. Budoff: I’m not aware of a difference in the supplement type. I think the vitamin D issue is a big problem because we all have patients who take thousands of units of vitamin D — just crazy numbers. People advocate really high numbers and that stays in the system.
Personally, I think part of the explanation is that with very high levels of vitamin D on top of calcium supplementation, you now absorb it better. You now get it into the bone, but maybe also into the coronary arteries. If you’re very high in vitamin D and then are taking a large calcium supplement, it might be the calcium/vitamin D combination that’s giving us some trouble. I think people on vitamin D supplements really need to watch their levels and not get supratherapeutic.
Ms. Ward: With the vitamin D?
Dr. Budoff: With the vitamin D.
Diabetes and Renal Function
Ms. Ward: In some of the studies, there seems to be a higher risk in patients with diabetes. Is there any reason why that would be?
Dr. Budoff: I can’t think of a reason exactly why with diabetes per se, except for renal disease. Patients with diabetes have more intrinsic renal disease, proteinuria, and even a reduced eGFR. We’ve seen that the kidney is very strongly tied to this. We have a very strong relationship, in work I’ve done a decade ago now, showing that calcium supplementation (in the form of phosphate binders) in patients on dialysis or with advanced renal disease is linked to much higher coronary calcium progression.
We did prospective, randomized trials showing that calcium intake as binders to reduce phosphorus led to more coronary calcium. We always thought that was just relegated to the renal population, and there might be an overlap here with the diabetes and more renal disease. I have a feeling that it has to do with more of that. It might be regulation of parathyroid hormone as well, which might be more abnormal in patients with diabetes.
Avoid Supratherapeutic Vitamin D Levels
Ms. Ward:: What are you telling your patients?
Dr. Budoff: I tell patients with normal kidney function that the bone will modulate 99.9% of the calcium uptake. If they have osteopenia or osteoporosis, regardless of their calcium score, I’m very comfortable putting them on supplements.
I’m a little more cautious with the vitamin D levels, and I keep an eye on that and regulate how much vitamin D they get based on their levels. I get them into the normal range, but I don’t want them supratherapeutic. You can even follow their calcium score. Again, we’ve shown that if you’re taking too much calcium, your calcium score will go up. I can just check it again in a couple of years to make sure that it’s safe.
Ms. Ward:: In terms of vitamin D levels, when you’re saying “supratherapeutic,” what levels do you consider a safe amount to take?
Dr. Budoff: I’d like them under 100 ng/mL as far as their upper level. Normal is around 70 ng/mL at most labs. I try to keep them in the normal range. I don’t even want them to be high-normal if I’m going to be concomitantly giving them calcium supplements. Of course, if they have renal insufficiency, then I’m much more cautious. We’ve even seen calcium supplements raise the serum calcium, which you never see with dietary calcium. That’s another potential proof that it might be too much too fast.
For renal patients, even in mild renal insufficiency, maybe even in diabetes where we’ve seen a signal, maybe aim lower in the amount of calcium supplementation if diet is insufficient, and aim a little lower in vitamin D targets, and I think you’ll be in a safer place.
Ms. Ward: Is there anything else you want to add?
Dr. Budoff: The evidence is still evolving. I’d say that it’s interesting and maybe a little frustrating that we don’t have a final answer on all of this. I would stay tuned for more data because we’re looking at many of the epidemiologic studies to try to see what happens in the real world, with both dietary intake of calcium and calcium supplementation.
Ms. Ward: Thank you very much for joining me today.
Dr. Budoff: It’s a pleasure. Thanks for having me.
Dr. Budoff disclosed being a speaker for Amarin Pharma.
A version of this article appeared on Medscape.com.
In the Future, a Robot Intensivist May Save Your Life
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.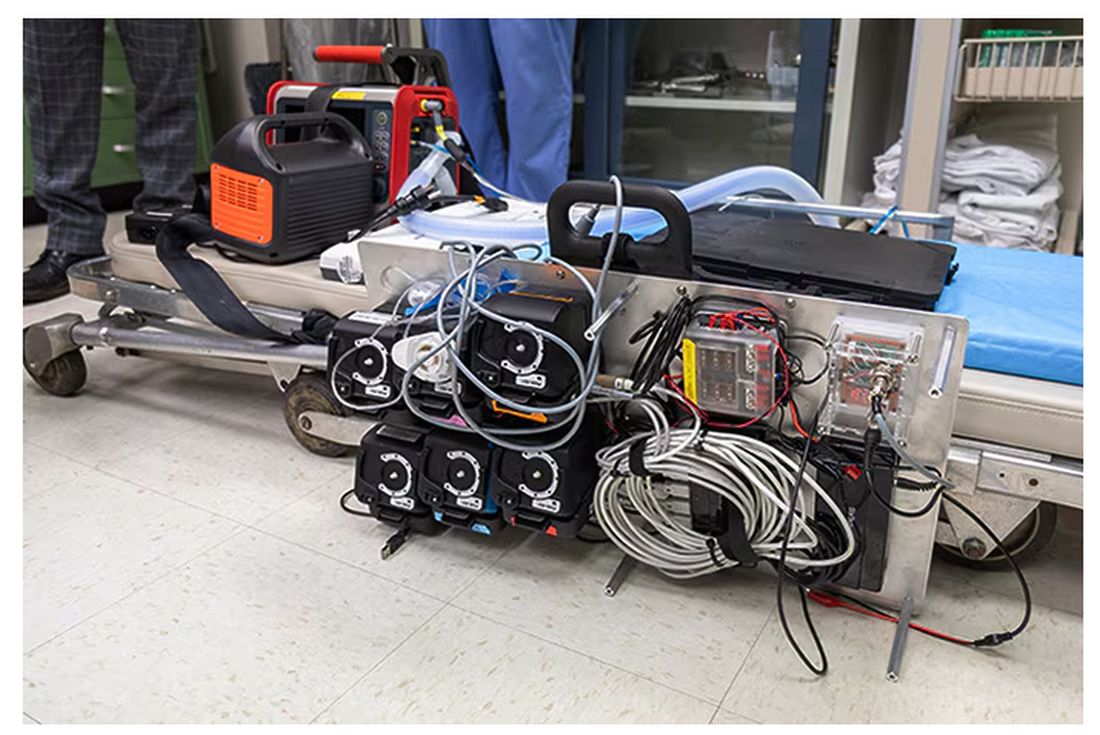
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.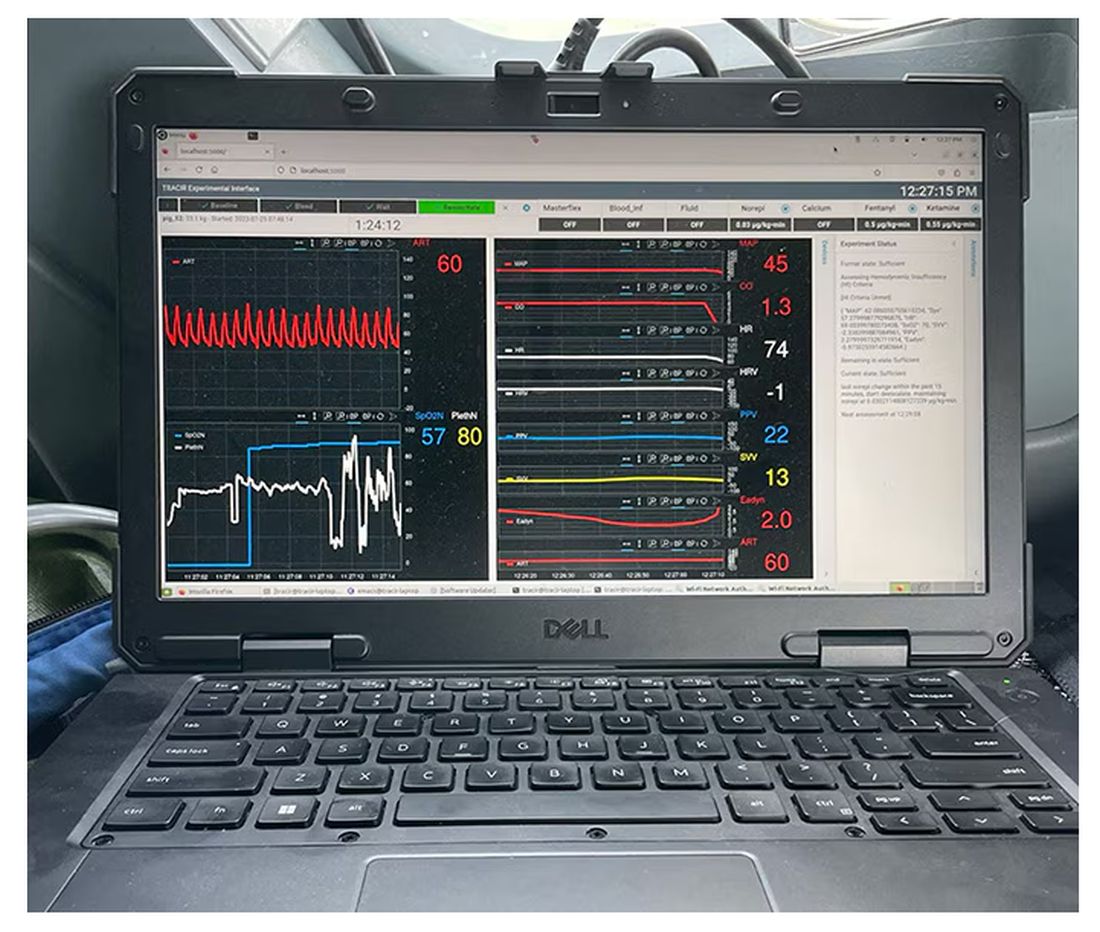
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.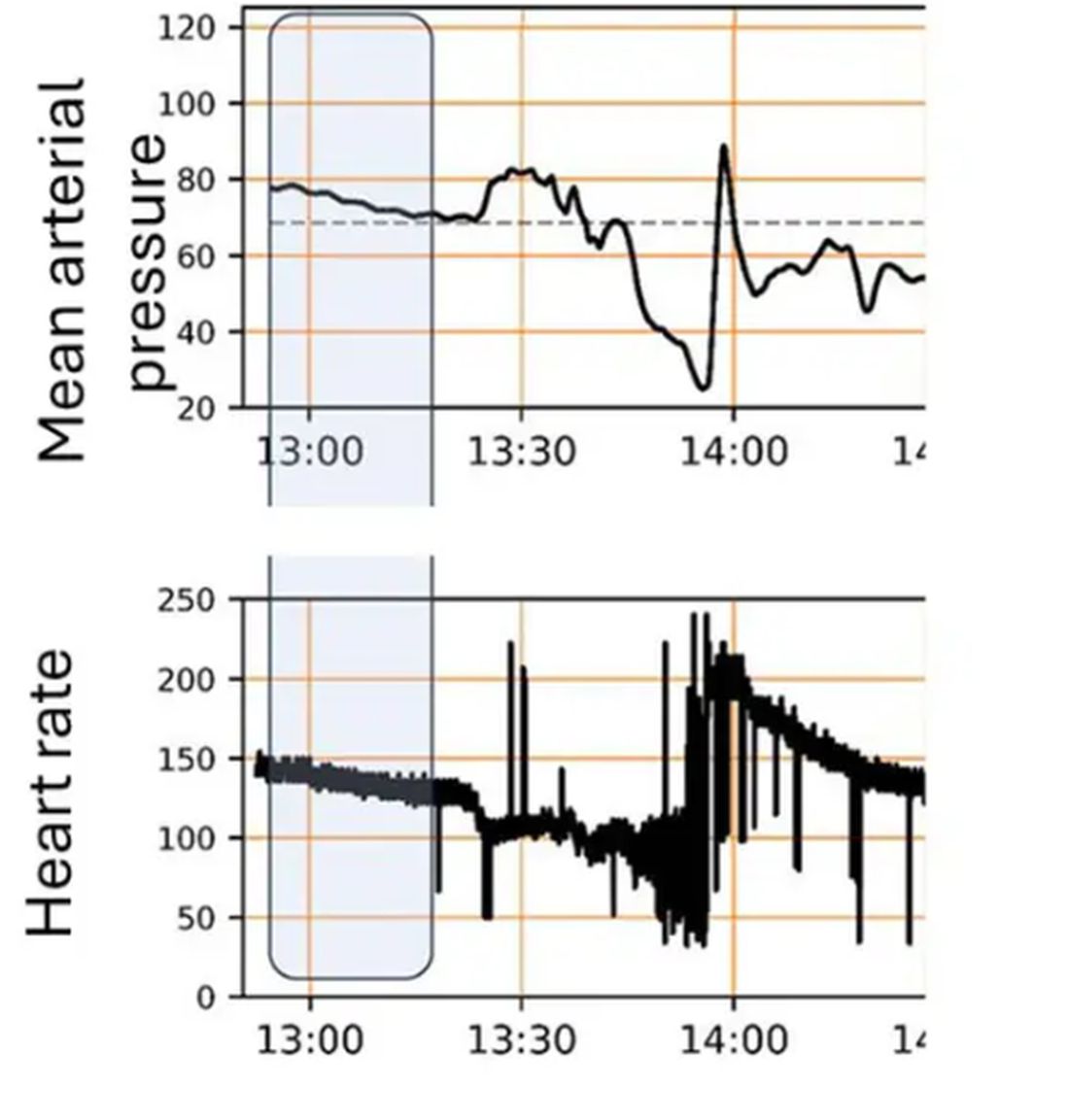
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 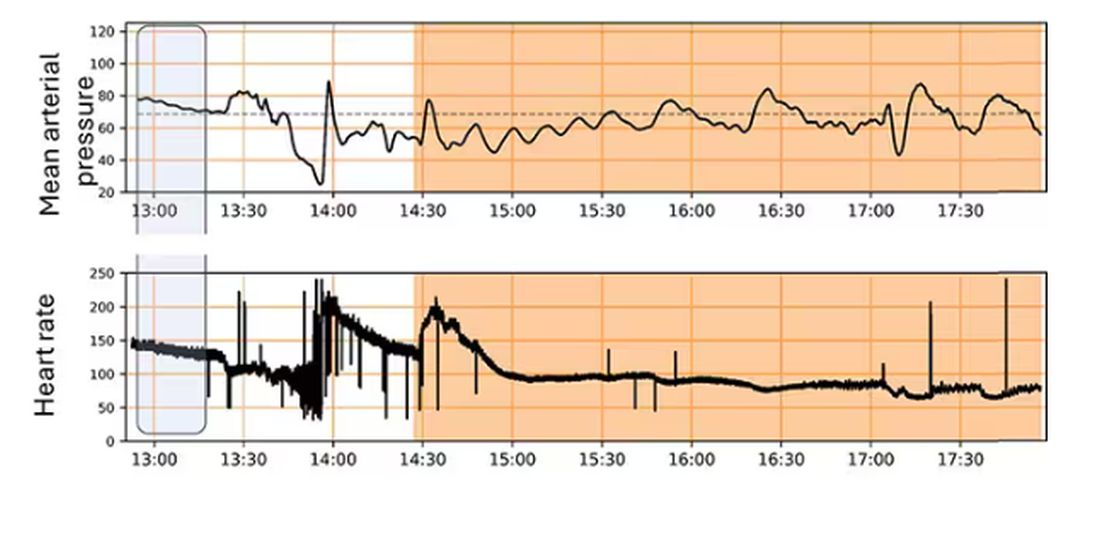
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When It Comes to Medicine, ‘Women Are Not Small Men’
Welcome everyone. I’m Dr. John White. I’m the chief medical officer at WebMD. Does your biologic sex impact your health? Does it have any play in how you’re diagnosed, how you’re treated in terms of what symptoms you have? Of course it does. We all know that. But that’s not something that many people believed 5, 10 years ago, certainly not 20 years ago. And it was only because of leaders like my guest today, Phyllis Greenberger, who really championed the need for research on women’s health. She has a new book out, which I love. It’s called Sex Cells: the Fight to Overcome Bias and Discrimination in Women’s Healthcare. Please welcome my very good friend, Phyllis Greenberger.
Thank you.
Phyllis, It’s great to see you today.
It’s great to see you as well.
Now, you and I have been talking about this for easily 2 decades.
At least.
And some people think, oh, of course it makes sense. Although I saw you disagreeing that not everyone still believes that. But what has been that journey? Why has it been so hard to make people understand, as you point out early on in your book, women are not smaller men?
I think the basic reason was that it was just believed that men and women were the same except for their reproductive organs. So minus the reproductive organs, whether it was a device, a diagnostic, or therapeutic, if it was used and successful on a male, that it would be successful on a female. We’re really very far from understanding the differences, and there’s still a lot of distrust and disbelief and ignorance about it. And so there’s still a long way to go.
But you talk about that in the book, that there’s still a long way to go. Why is that? What’s the biggest obstacle? Is it just misinformation, lack of information? People don’t understand the science? There’s still resistance in some areas. Why is that?
I think it’s misinformation, and I gave a presentation, I don’t know how many years ago, at least 20 years ago, about the curriculum. And at the time, there was no women’s health in the curriculum. It was health. So if it was on cardiovascular issues or on osteoporosis, it was sort of the basic. And at the time, there would maybe be one woman whose job was women’s health, and she’d have an office, and otherwise there was nothing. And maybe they talked about breast cancer, who knows. But I spoke to someone just the other day, in view of all the attention that the book is getting now, whether that’s changed, whether it’s necessary and required. And she said it’s not. So, it’s not necessarily on the curriculum of all research and medical institutions, and even if women’s health, quote unquote, is on the curriculum, it doesn’t mean that they’re really looking at sex differences. And the difference is obvious. I mean, gender is really, it’s a social construct, but biological sex is how disease occurs and develops. And so if you’re not looking, and because there’s so little research now on sex differences that I don’t even know, I mean, how much you could actually teach.
So what needs to change? This book is a manifesto in many ways in how we need to include women; we need to make research more inclusive of everyone. But we’re not there yet. So what needs to change, Phyllis?
During this whole saga of trying to get people to listen to me and to the society, we really started out just looking at clinical trials and that, as you mentioned, I mean, there are issues in rural communities. There’s travel issues for women and child care. There’s a lot of disbelief or fear of clinical trials in some ethnicities. I do think, going to the future, that technology can help that. I mean, if people have broadband, which of course is also an issue in rural areas.
What could women do today? What should women listeners hear and then be doing? Should they be saying something to their doctor? Should they be asking specific questions? When they interact with the health care system, how can they make sure they’re getting the best care that’s appropriate for them when we know that sex cells matter?
Well, that’s a good question. It depends on, frankly, if your doctor is aware of this, if he or she has learned anything about this in school, which, I had already said, we’re not sure about that because research is still ongoing and there’s so much we don’t know. So I mean, you used to think, or I used to think, that you go to, you want a physician who’s older and more experienced. But now I think you should be going to a physician who’s younger and hopefully has learned about this, because the physicians that were educated years ago and have been practicing for 20, 30 years, I don’t know how much they know about this, whether they’re even aware of it.
Phyllis, you are a woman of action. You’ve lived in the DC area. You have championed legislative reforms, executive agendas. What do you want done now? What needs to be changed today? The curriculum is going to take time, but what else needs to change?
That’s a good question. I mean, if curriculum is going to take a while and you can ask your doctor if he prescribes the medication, whether it’s been tested on women, but then if it hasn’t been tested on women, but it’s the only thing that there is for your condition, I mean, so it’s very difficult. The Biden administration, as you know, just allocated a hundred million dollars for women’s health research.
What do you hope to accomplish with this book?
Well, what I’m hoping is that I spoke to someone at AMWA and I’m hoping — and AMWA is an association for women medical students. And I’m hoping that’s the audience. The audience needs to be. I mean, obviously everybody that I know that’s not a doctor that’s read it, found it fascinating and didn’t know a lot of the stuff that was in it. So I think it’s an interesting book anyway, and I think women should be aware of it. But really I think it needs to be for medical students.
And to your credit, you built the Society for Women’s Health Research into a powerful force in Washington under your tenure in really promoting the need for Office of Women’s Health and Research in general. The book is entitled Sex Cells, the Fight to Overcome Bias and Discrimination in Women’s Healthcare. Phyllis Greenberger, thank you so much for all that you’ve done for women’s health, for women’s research. We wouldn’t be where we are today if it wasn’t for you. So thanks.
Thank you very much, John. Thank you. I appreciate the opportunity.
Dr. Whyte, is chief medical officer, WebMD, New York, NY. He has disclosed no relevant financial relationships. Ms. Greenberger is a women’s health advocate and author of “Sex Cells: The Fight to Overcome Bias and Discrimination in Women’s Healthcare”
This interview originally appeared on WebMD on May 23, 2024. A version of this article appeared on Medscape.com .
Welcome everyone. I’m Dr. John White. I’m the chief medical officer at WebMD. Does your biologic sex impact your health? Does it have any play in how you’re diagnosed, how you’re treated in terms of what symptoms you have? Of course it does. We all know that. But that’s not something that many people believed 5, 10 years ago, certainly not 20 years ago. And it was only because of leaders like my guest today, Phyllis Greenberger, who really championed the need for research on women’s health. She has a new book out, which I love. It’s called Sex Cells: the Fight to Overcome Bias and Discrimination in Women’s Healthcare. Please welcome my very good friend, Phyllis Greenberger.
Thank you.
Phyllis, It’s great to see you today.
It’s great to see you as well.
Now, you and I have been talking about this for easily 2 decades.
At least.
And some people think, oh, of course it makes sense. Although I saw you disagreeing that not everyone still believes that. But what has been that journey? Why has it been so hard to make people understand, as you point out early on in your book, women are not smaller men?
I think the basic reason was that it was just believed that men and women were the same except for their reproductive organs. So minus the reproductive organs, whether it was a device, a diagnostic, or therapeutic, if it was used and successful on a male, that it would be successful on a female. We’re really very far from understanding the differences, and there’s still a lot of distrust and disbelief and ignorance about it. And so there’s still a long way to go.
But you talk about that in the book, that there’s still a long way to go. Why is that? What’s the biggest obstacle? Is it just misinformation, lack of information? People don’t understand the science? There’s still resistance in some areas. Why is that?
I think it’s misinformation, and I gave a presentation, I don’t know how many years ago, at least 20 years ago, about the curriculum. And at the time, there was no women’s health in the curriculum. It was health. So if it was on cardiovascular issues or on osteoporosis, it was sort of the basic. And at the time, there would maybe be one woman whose job was women’s health, and she’d have an office, and otherwise there was nothing. And maybe they talked about breast cancer, who knows. But I spoke to someone just the other day, in view of all the attention that the book is getting now, whether that’s changed, whether it’s necessary and required. And she said it’s not. So, it’s not necessarily on the curriculum of all research and medical institutions, and even if women’s health, quote unquote, is on the curriculum, it doesn’t mean that they’re really looking at sex differences. And the difference is obvious. I mean, gender is really, it’s a social construct, but biological sex is how disease occurs and develops. And so if you’re not looking, and because there’s so little research now on sex differences that I don’t even know, I mean, how much you could actually teach.
So what needs to change? This book is a manifesto in many ways in how we need to include women; we need to make research more inclusive of everyone. But we’re not there yet. So what needs to change, Phyllis?
During this whole saga of trying to get people to listen to me and to the society, we really started out just looking at clinical trials and that, as you mentioned, I mean, there are issues in rural communities. There’s travel issues for women and child care. There’s a lot of disbelief or fear of clinical trials in some ethnicities. I do think, going to the future, that technology can help that. I mean, if people have broadband, which of course is also an issue in rural areas.
What could women do today? What should women listeners hear and then be doing? Should they be saying something to their doctor? Should they be asking specific questions? When they interact with the health care system, how can they make sure they’re getting the best care that’s appropriate for them when we know that sex cells matter?
Well, that’s a good question. It depends on, frankly, if your doctor is aware of this, if he or she has learned anything about this in school, which, I had already said, we’re not sure about that because research is still ongoing and there’s so much we don’t know. So I mean, you used to think, or I used to think, that you go to, you want a physician who’s older and more experienced. But now I think you should be going to a physician who’s younger and hopefully has learned about this, because the physicians that were educated years ago and have been practicing for 20, 30 years, I don’t know how much they know about this, whether they’re even aware of it.
Phyllis, you are a woman of action. You’ve lived in the DC area. You have championed legislative reforms, executive agendas. What do you want done now? What needs to be changed today? The curriculum is going to take time, but what else needs to change?
That’s a good question. I mean, if curriculum is going to take a while and you can ask your doctor if he prescribes the medication, whether it’s been tested on women, but then if it hasn’t been tested on women, but it’s the only thing that there is for your condition, I mean, so it’s very difficult. The Biden administration, as you know, just allocated a hundred million dollars for women’s health research.
What do you hope to accomplish with this book?
Well, what I’m hoping is that I spoke to someone at AMWA and I’m hoping — and AMWA is an association for women medical students. And I’m hoping that’s the audience. The audience needs to be. I mean, obviously everybody that I know that’s not a doctor that’s read it, found it fascinating and didn’t know a lot of the stuff that was in it. So I think it’s an interesting book anyway, and I think women should be aware of it. But really I think it needs to be for medical students.
And to your credit, you built the Society for Women’s Health Research into a powerful force in Washington under your tenure in really promoting the need for Office of Women’s Health and Research in general. The book is entitled Sex Cells, the Fight to Overcome Bias and Discrimination in Women’s Healthcare. Phyllis Greenberger, thank you so much for all that you’ve done for women’s health, for women’s research. We wouldn’t be where we are today if it wasn’t for you. So thanks.
Thank you very much, John. Thank you. I appreciate the opportunity.
Dr. Whyte, is chief medical officer, WebMD, New York, NY. He has disclosed no relevant financial relationships. Ms. Greenberger is a women’s health advocate and author of “Sex Cells: The Fight to Overcome Bias and Discrimination in Women’s Healthcare”
This interview originally appeared on WebMD on May 23, 2024. A version of this article appeared on Medscape.com .
Welcome everyone. I’m Dr. John White. I’m the chief medical officer at WebMD. Does your biologic sex impact your health? Does it have any play in how you’re diagnosed, how you’re treated in terms of what symptoms you have? Of course it does. We all know that. But that’s not something that many people believed 5, 10 years ago, certainly not 20 years ago. And it was only because of leaders like my guest today, Phyllis Greenberger, who really championed the need for research on women’s health. She has a new book out, which I love. It’s called Sex Cells: the Fight to Overcome Bias and Discrimination in Women’s Healthcare. Please welcome my very good friend, Phyllis Greenberger.
Thank you.
Phyllis, It’s great to see you today.
It’s great to see you as well.
Now, you and I have been talking about this for easily 2 decades.
At least.
And some people think, oh, of course it makes sense. Although I saw you disagreeing that not everyone still believes that. But what has been that journey? Why has it been so hard to make people understand, as you point out early on in your book, women are not smaller men?
I think the basic reason was that it was just believed that men and women were the same except for their reproductive organs. So minus the reproductive organs, whether it was a device, a diagnostic, or therapeutic, if it was used and successful on a male, that it would be successful on a female. We’re really very far from understanding the differences, and there’s still a lot of distrust and disbelief and ignorance about it. And so there’s still a long way to go.
But you talk about that in the book, that there’s still a long way to go. Why is that? What’s the biggest obstacle? Is it just misinformation, lack of information? People don’t understand the science? There’s still resistance in some areas. Why is that?
I think it’s misinformation, and I gave a presentation, I don’t know how many years ago, at least 20 years ago, about the curriculum. And at the time, there was no women’s health in the curriculum. It was health. So if it was on cardiovascular issues or on osteoporosis, it was sort of the basic. And at the time, there would maybe be one woman whose job was women’s health, and she’d have an office, and otherwise there was nothing. And maybe they talked about breast cancer, who knows. But I spoke to someone just the other day, in view of all the attention that the book is getting now, whether that’s changed, whether it’s necessary and required. And she said it’s not. So, it’s not necessarily on the curriculum of all research and medical institutions, and even if women’s health, quote unquote, is on the curriculum, it doesn’t mean that they’re really looking at sex differences. And the difference is obvious. I mean, gender is really, it’s a social construct, but biological sex is how disease occurs and develops. And so if you’re not looking, and because there’s so little research now on sex differences that I don’t even know, I mean, how much you could actually teach.
So what needs to change? This book is a manifesto in many ways in how we need to include women; we need to make research more inclusive of everyone. But we’re not there yet. So what needs to change, Phyllis?
During this whole saga of trying to get people to listen to me and to the society, we really started out just looking at clinical trials and that, as you mentioned, I mean, there are issues in rural communities. There’s travel issues for women and child care. There’s a lot of disbelief or fear of clinical trials in some ethnicities. I do think, going to the future, that technology can help that. I mean, if people have broadband, which of course is also an issue in rural areas.
What could women do today? What should women listeners hear and then be doing? Should they be saying something to their doctor? Should they be asking specific questions? When they interact with the health care system, how can they make sure they’re getting the best care that’s appropriate for them when we know that sex cells matter?
Well, that’s a good question. It depends on, frankly, if your doctor is aware of this, if he or she has learned anything about this in school, which, I had already said, we’re not sure about that because research is still ongoing and there’s so much we don’t know. So I mean, you used to think, or I used to think, that you go to, you want a physician who’s older and more experienced. But now I think you should be going to a physician who’s younger and hopefully has learned about this, because the physicians that were educated years ago and have been practicing for 20, 30 years, I don’t know how much they know about this, whether they’re even aware of it.
Phyllis, you are a woman of action. You’ve lived in the DC area. You have championed legislative reforms, executive agendas. What do you want done now? What needs to be changed today? The curriculum is going to take time, but what else needs to change?
That’s a good question. I mean, if curriculum is going to take a while and you can ask your doctor if he prescribes the medication, whether it’s been tested on women, but then if it hasn’t been tested on women, but it’s the only thing that there is for your condition, I mean, so it’s very difficult. The Biden administration, as you know, just allocated a hundred million dollars for women’s health research.
What do you hope to accomplish with this book?
Well, what I’m hoping is that I spoke to someone at AMWA and I’m hoping — and AMWA is an association for women medical students. And I’m hoping that’s the audience. The audience needs to be. I mean, obviously everybody that I know that’s not a doctor that’s read it, found it fascinating and didn’t know a lot of the stuff that was in it. So I think it’s an interesting book anyway, and I think women should be aware of it. But really I think it needs to be for medical students.
And to your credit, you built the Society for Women’s Health Research into a powerful force in Washington under your tenure in really promoting the need for Office of Women’s Health and Research in general. The book is entitled Sex Cells, the Fight to Overcome Bias and Discrimination in Women’s Healthcare. Phyllis Greenberger, thank you so much for all that you’ve done for women’s health, for women’s research. We wouldn’t be where we are today if it wasn’t for you. So thanks.
Thank you very much, John. Thank you. I appreciate the opportunity.
Dr. Whyte, is chief medical officer, WebMD, New York, NY. He has disclosed no relevant financial relationships. Ms. Greenberger is a women’s health advocate and author of “Sex Cells: The Fight to Overcome Bias and Discrimination in Women’s Healthcare”
This interview originally appeared on WebMD on May 23, 2024. A version of this article appeared on Medscape.com .