User login
Promoting a child’s resilience
As an inpatient child psychiatrist, I see children with some of the most difficult emotional and behavioral issues. And among them, children with adverse childhood experiences (ACE) make up a significant portion. But early childhood adversity is common not just among children who present to the hospital. In the landmark ACE study, which was an ongoing collaboration between Kaiser Permanente and the Centers for Disease Control and Prevention to assess impact of ACEs on various health outcomes, 40% percent of the participants reported experiencing two or more ACEs.1 Subsequent studies have shown even higher numbers. The study by Copeland et al. on traumatic events based on the Great Smokey Mountains Study showed that more than two-thirds of children reported at least one traumatic event by the age of 16 years.2
The significance of this finding cannot be overstated. It is clear that the cumulative incidences of ACEs are associated with poorer health outcomes in a graded dose-response relationship. Those exposed are at great risk of developing PTSD, ADHD, mood disorders, anxiety disorders, and substance use disorder.3 Furthermore, they also are at risk for developing asthma, obesity, ischemic heart disease, diabetes, chronic obstructive pulmonary disease, autoimmune disease, and sexually transmitted disease.4 They have lower quality of life, use more health care services, and die nearly 20 years younger.5
Currently, the biology of adverse childhood experiences is being elucidated. The deleterious effects of chronically elevated cortisol leading to smaller hippocampal volume through atrophy, neurotoxicity, and disruption of neurogenesis has been demonstrated in adults, but children and adolescents have been found to have reduced medial and posterior corpus callosum.6-10 Other alterations include changes in EEG activity, and dysregulation of the sympathetic nervous system.11-13 New systems or neuropeptides that could potentially be beneficial in the treatment of trauma include tempering down of the locus coeruleus–norepinephrine system, the anxiolytic effect of neuropeptide Y, and brain-derived neurotrophic factor.14,15
Despite all the progress, the treatment for trauma remains imperfect. Depending on the presenting symptoms, stimulants, alpha-agonists (guanfacine or clonidine), alpha-1 blocker, and/or SSRIs all could be a good first step. Medication could reduce the burden of some of the symptoms, but its effects are limited. During the 1970s, researchers started noticing that some children were able to thrive despite substantial risk factors for mental illness. This led to research identifying individual and environmental factors that could be protective against ACEs.
So, what is resilience? It is the development of positive adaptations in the context of significant adversity.16 But this ability is not purely incumbent on the child. The individual characteristics that lead to resilience such as internal locus of control, optimism, and determination also are dependent on their environment. As such, it is a complex dynamic interplay of genetics, temperament, experience, and environmental supports. As much as the environment can affect resilience, this gives us opportunities to help the child be more resilient, perhaps before an adverse event happens.
One, emphasize the family! A strong family relationship is among the most robust predictor of resilient adaptation. Early experiences and attachments will shape the lens through which people view their subsequent relationships and place them on probabilistic trajectories of “relatively good or bad adaptation.”17 And just what constitutes “good parenting”? The authoritative parenting style that balances appropriate controls and warmth with consistency and responsiveness generally lead to better outcomes.18 Other important features include reasonable limit-setting, monitoring, and containment.19, 20 Clinicians with expertise in one of the parent-coaching manuals (i.e., “Helping the Noncompliant Child,” by McMahon and Forehand; and “Your Defiant Child: Eight Steps to Better Behavior,” by Barkley and Benton and others) can be helpful in answering parenting questions whether individually or in the group setting.
In addition, parental mental health issues also can adversely affect family relationships. Based on previous studies, mothers who suffer from depression have more difficulty being responsive and warm to their child.21 They are often times more punitive and less consistent. Children of mothers with depression are at risk for internalizing, externalizing, and general psychopathology.22 Mothers with history of ACEs are less able to modulate stress and model coping skills. As such, it can be just as important to screen the parents for mental health issues and refer to the appropriate clinician.
Two, community supports also can facilitate development of resilience. Studies have shown participants in the Big Brothers and Big Sisters programs of America exhibit more positive behavior such as better academic behaviors, better relationships with family and friends, and decreased substance use.23 Furthermore, studies on minority students (African American and Hispanic) suggest improved relationship with teachers led to less behavioral problems and improved social competence. Religious affiliations and other social supports can serve similar purposes as well.24
Three, keep in mind the malleability of the child. Many child attributes are dependent on environmental influences and resilience should focus more on what adults can do to bolster the child’s own efforts.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Arch Gen Psychiatry. 2007 May;64(5):577-84.
3. Eur Arch Psychiatry Clin Neurosci. 2006 Apr;256(3):174-86.
4. Am J Prev Med. 1998 May;14(4):245-58.
5. Pediatr Res. 2016 Jan;79(1-2):227-33.
6. Prog Neuropsychopharmacol Biol Psychiatry. 2010 Oct 1;34(7):1181-8.
7. Brain Res. 1992 Aug 21;588(2):341-5.
8. J Neurosci. 1985 May;5(5):1222-7.
9. J Comp Neurol. 1996 May 20;369(1):56-63.
10. Biol Psychiatry. 2002 Dec 1;52(11):1066-78.
11. J Neuropsychiatry Clin Neurosci. 1998 Summer;10(3):298-307.
12. Biol Psychiatry. 2002 Apr 1;51(7):575-82.
13. Brain Res. 2009 Oct 13;1293:13-23.
14. Neuropeptides. 2016 Apr;56:19-24.
15. Cell. 2007 Oct 19;131(2):391-404.
16. Child Dev. 2000; 71(3): 543-62.
17. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook, 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2007)
18. Early Hum Dev. 2010 Nov;86(11):689-93.
19. Clin Child Fam Psychol Rev. 1998 Mar;1(1):61-75.
20. Dev Psychopathol. 2003 Winter;15(1):95-117.
21. Clin Psychol Rev. 2000 Aug;20(5):561-92.
22. Clin Child Fam Psychol Rev. 2011 Mar;14(1):1-27.
23. “Making a Difference: An Impact Study of Big Brothers Big Sisters,” (Philadelphia: Public/Private Ventures, 1995).
24. Child Dev. 2003 Nov-Dec;74(6):1682-96.
As an inpatient child psychiatrist, I see children with some of the most difficult emotional and behavioral issues. And among them, children with adverse childhood experiences (ACE) make up a significant portion. But early childhood adversity is common not just among children who present to the hospital. In the landmark ACE study, which was an ongoing collaboration between Kaiser Permanente and the Centers for Disease Control and Prevention to assess impact of ACEs on various health outcomes, 40% percent of the participants reported experiencing two or more ACEs.1 Subsequent studies have shown even higher numbers. The study by Copeland et al. on traumatic events based on the Great Smokey Mountains Study showed that more than two-thirds of children reported at least one traumatic event by the age of 16 years.2
The significance of this finding cannot be overstated. It is clear that the cumulative incidences of ACEs are associated with poorer health outcomes in a graded dose-response relationship. Those exposed are at great risk of developing PTSD, ADHD, mood disorders, anxiety disorders, and substance use disorder.3 Furthermore, they also are at risk for developing asthma, obesity, ischemic heart disease, diabetes, chronic obstructive pulmonary disease, autoimmune disease, and sexually transmitted disease.4 They have lower quality of life, use more health care services, and die nearly 20 years younger.5
Currently, the biology of adverse childhood experiences is being elucidated. The deleterious effects of chronically elevated cortisol leading to smaller hippocampal volume through atrophy, neurotoxicity, and disruption of neurogenesis has been demonstrated in adults, but children and adolescents have been found to have reduced medial and posterior corpus callosum.6-10 Other alterations include changes in EEG activity, and dysregulation of the sympathetic nervous system.11-13 New systems or neuropeptides that could potentially be beneficial in the treatment of trauma include tempering down of the locus coeruleus–norepinephrine system, the anxiolytic effect of neuropeptide Y, and brain-derived neurotrophic factor.14,15
Despite all the progress, the treatment for trauma remains imperfect. Depending on the presenting symptoms, stimulants, alpha-agonists (guanfacine or clonidine), alpha-1 blocker, and/or SSRIs all could be a good first step. Medication could reduce the burden of some of the symptoms, but its effects are limited. During the 1970s, researchers started noticing that some children were able to thrive despite substantial risk factors for mental illness. This led to research identifying individual and environmental factors that could be protective against ACEs.
So, what is resilience? It is the development of positive adaptations in the context of significant adversity.16 But this ability is not purely incumbent on the child. The individual characteristics that lead to resilience such as internal locus of control, optimism, and determination also are dependent on their environment. As such, it is a complex dynamic interplay of genetics, temperament, experience, and environmental supports. As much as the environment can affect resilience, this gives us opportunities to help the child be more resilient, perhaps before an adverse event happens.
One, emphasize the family! A strong family relationship is among the most robust predictor of resilient adaptation. Early experiences and attachments will shape the lens through which people view their subsequent relationships and place them on probabilistic trajectories of “relatively good or bad adaptation.”17 And just what constitutes “good parenting”? The authoritative parenting style that balances appropriate controls and warmth with consistency and responsiveness generally lead to better outcomes.18 Other important features include reasonable limit-setting, monitoring, and containment.19, 20 Clinicians with expertise in one of the parent-coaching manuals (i.e., “Helping the Noncompliant Child,” by McMahon and Forehand; and “Your Defiant Child: Eight Steps to Better Behavior,” by Barkley and Benton and others) can be helpful in answering parenting questions whether individually or in the group setting.
In addition, parental mental health issues also can adversely affect family relationships. Based on previous studies, mothers who suffer from depression have more difficulty being responsive and warm to their child.21 They are often times more punitive and less consistent. Children of mothers with depression are at risk for internalizing, externalizing, and general psychopathology.22 Mothers with history of ACEs are less able to modulate stress and model coping skills. As such, it can be just as important to screen the parents for mental health issues and refer to the appropriate clinician.
Two, community supports also can facilitate development of resilience. Studies have shown participants in the Big Brothers and Big Sisters programs of America exhibit more positive behavior such as better academic behaviors, better relationships with family and friends, and decreased substance use.23 Furthermore, studies on minority students (African American and Hispanic) suggest improved relationship with teachers led to less behavioral problems and improved social competence. Religious affiliations and other social supports can serve similar purposes as well.24
Three, keep in mind the malleability of the child. Many child attributes are dependent on environmental influences and resilience should focus more on what adults can do to bolster the child’s own efforts.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Arch Gen Psychiatry. 2007 May;64(5):577-84.
3. Eur Arch Psychiatry Clin Neurosci. 2006 Apr;256(3):174-86.
4. Am J Prev Med. 1998 May;14(4):245-58.
5. Pediatr Res. 2016 Jan;79(1-2):227-33.
6. Prog Neuropsychopharmacol Biol Psychiatry. 2010 Oct 1;34(7):1181-8.
7. Brain Res. 1992 Aug 21;588(2):341-5.
8. J Neurosci. 1985 May;5(5):1222-7.
9. J Comp Neurol. 1996 May 20;369(1):56-63.
10. Biol Psychiatry. 2002 Dec 1;52(11):1066-78.
11. J Neuropsychiatry Clin Neurosci. 1998 Summer;10(3):298-307.
12. Biol Psychiatry. 2002 Apr 1;51(7):575-82.
13. Brain Res. 2009 Oct 13;1293:13-23.
14. Neuropeptides. 2016 Apr;56:19-24.
15. Cell. 2007 Oct 19;131(2):391-404.
16. Child Dev. 2000; 71(3): 543-62.
17. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook, 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2007)
18. Early Hum Dev. 2010 Nov;86(11):689-93.
19. Clin Child Fam Psychol Rev. 1998 Mar;1(1):61-75.
20. Dev Psychopathol. 2003 Winter;15(1):95-117.
21. Clin Psychol Rev. 2000 Aug;20(5):561-92.
22. Clin Child Fam Psychol Rev. 2011 Mar;14(1):1-27.
23. “Making a Difference: An Impact Study of Big Brothers Big Sisters,” (Philadelphia: Public/Private Ventures, 1995).
24. Child Dev. 2003 Nov-Dec;74(6):1682-96.
As an inpatient child psychiatrist, I see children with some of the most difficult emotional and behavioral issues. And among them, children with adverse childhood experiences (ACE) make up a significant portion. But early childhood adversity is common not just among children who present to the hospital. In the landmark ACE study, which was an ongoing collaboration between Kaiser Permanente and the Centers for Disease Control and Prevention to assess impact of ACEs on various health outcomes, 40% percent of the participants reported experiencing two or more ACEs.1 Subsequent studies have shown even higher numbers. The study by Copeland et al. on traumatic events based on the Great Smokey Mountains Study showed that more than two-thirds of children reported at least one traumatic event by the age of 16 years.2
The significance of this finding cannot be overstated. It is clear that the cumulative incidences of ACEs are associated with poorer health outcomes in a graded dose-response relationship. Those exposed are at great risk of developing PTSD, ADHD, mood disorders, anxiety disorders, and substance use disorder.3 Furthermore, they also are at risk for developing asthma, obesity, ischemic heart disease, diabetes, chronic obstructive pulmonary disease, autoimmune disease, and sexually transmitted disease.4 They have lower quality of life, use more health care services, and die nearly 20 years younger.5
Currently, the biology of adverse childhood experiences is being elucidated. The deleterious effects of chronically elevated cortisol leading to smaller hippocampal volume through atrophy, neurotoxicity, and disruption of neurogenesis has been demonstrated in adults, but children and adolescents have been found to have reduced medial and posterior corpus callosum.6-10 Other alterations include changes in EEG activity, and dysregulation of the sympathetic nervous system.11-13 New systems or neuropeptides that could potentially be beneficial in the treatment of trauma include tempering down of the locus coeruleus–norepinephrine system, the anxiolytic effect of neuropeptide Y, and brain-derived neurotrophic factor.14,15
Despite all the progress, the treatment for trauma remains imperfect. Depending on the presenting symptoms, stimulants, alpha-agonists (guanfacine or clonidine), alpha-1 blocker, and/or SSRIs all could be a good first step. Medication could reduce the burden of some of the symptoms, but its effects are limited. During the 1970s, researchers started noticing that some children were able to thrive despite substantial risk factors for mental illness. This led to research identifying individual and environmental factors that could be protective against ACEs.
So, what is resilience? It is the development of positive adaptations in the context of significant adversity.16 But this ability is not purely incumbent on the child. The individual characteristics that lead to resilience such as internal locus of control, optimism, and determination also are dependent on their environment. As such, it is a complex dynamic interplay of genetics, temperament, experience, and environmental supports. As much as the environment can affect resilience, this gives us opportunities to help the child be more resilient, perhaps before an adverse event happens.
One, emphasize the family! A strong family relationship is among the most robust predictor of resilient adaptation. Early experiences and attachments will shape the lens through which people view their subsequent relationships and place them on probabilistic trajectories of “relatively good or bad adaptation.”17 And just what constitutes “good parenting”? The authoritative parenting style that balances appropriate controls and warmth with consistency and responsiveness generally lead to better outcomes.18 Other important features include reasonable limit-setting, monitoring, and containment.19, 20 Clinicians with expertise in one of the parent-coaching manuals (i.e., “Helping the Noncompliant Child,” by McMahon and Forehand; and “Your Defiant Child: Eight Steps to Better Behavior,” by Barkley and Benton and others) can be helpful in answering parenting questions whether individually or in the group setting.
In addition, parental mental health issues also can adversely affect family relationships. Based on previous studies, mothers who suffer from depression have more difficulty being responsive and warm to their child.21 They are often times more punitive and less consistent. Children of mothers with depression are at risk for internalizing, externalizing, and general psychopathology.22 Mothers with history of ACEs are less able to modulate stress and model coping skills. As such, it can be just as important to screen the parents for mental health issues and refer to the appropriate clinician.
Two, community supports also can facilitate development of resilience. Studies have shown participants in the Big Brothers and Big Sisters programs of America exhibit more positive behavior such as better academic behaviors, better relationships with family and friends, and decreased substance use.23 Furthermore, studies on minority students (African American and Hispanic) suggest improved relationship with teachers led to less behavioral problems and improved social competence. Religious affiliations and other social supports can serve similar purposes as well.24
Three, keep in mind the malleability of the child. Many child attributes are dependent on environmental influences and resilience should focus more on what adults can do to bolster the child’s own efforts.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Arch Gen Psychiatry. 2007 May;64(5):577-84.
3. Eur Arch Psychiatry Clin Neurosci. 2006 Apr;256(3):174-86.
4. Am J Prev Med. 1998 May;14(4):245-58.
5. Pediatr Res. 2016 Jan;79(1-2):227-33.
6. Prog Neuropsychopharmacol Biol Psychiatry. 2010 Oct 1;34(7):1181-8.
7. Brain Res. 1992 Aug 21;588(2):341-5.
8. J Neurosci. 1985 May;5(5):1222-7.
9. J Comp Neurol. 1996 May 20;369(1):56-63.
10. Biol Psychiatry. 2002 Dec 1;52(11):1066-78.
11. J Neuropsychiatry Clin Neurosci. 1998 Summer;10(3):298-307.
12. Biol Psychiatry. 2002 Apr 1;51(7):575-82.
13. Brain Res. 2009 Oct 13;1293:13-23.
14. Neuropeptides. 2016 Apr;56:19-24.
15. Cell. 2007 Oct 19;131(2):391-404.
16. Child Dev. 2000; 71(3): 543-62.
17. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook, 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2007)
18. Early Hum Dev. 2010 Nov;86(11):689-93.
19. Clin Child Fam Psychol Rev. 1998 Mar;1(1):61-75.
20. Dev Psychopathol. 2003 Winter;15(1):95-117.
21. Clin Psychol Rev. 2000 Aug;20(5):561-92.
22. Clin Child Fam Psychol Rev. 2011 Mar;14(1):1-27.
23. “Making a Difference: An Impact Study of Big Brothers Big Sisters,” (Philadelphia: Public/Private Ventures, 1995).
24. Child Dev. 2003 Nov-Dec;74(6):1682-96.
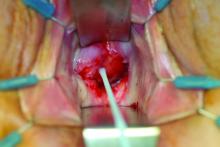
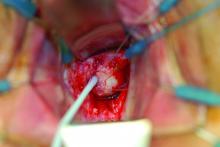
What is your diagnosis?
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
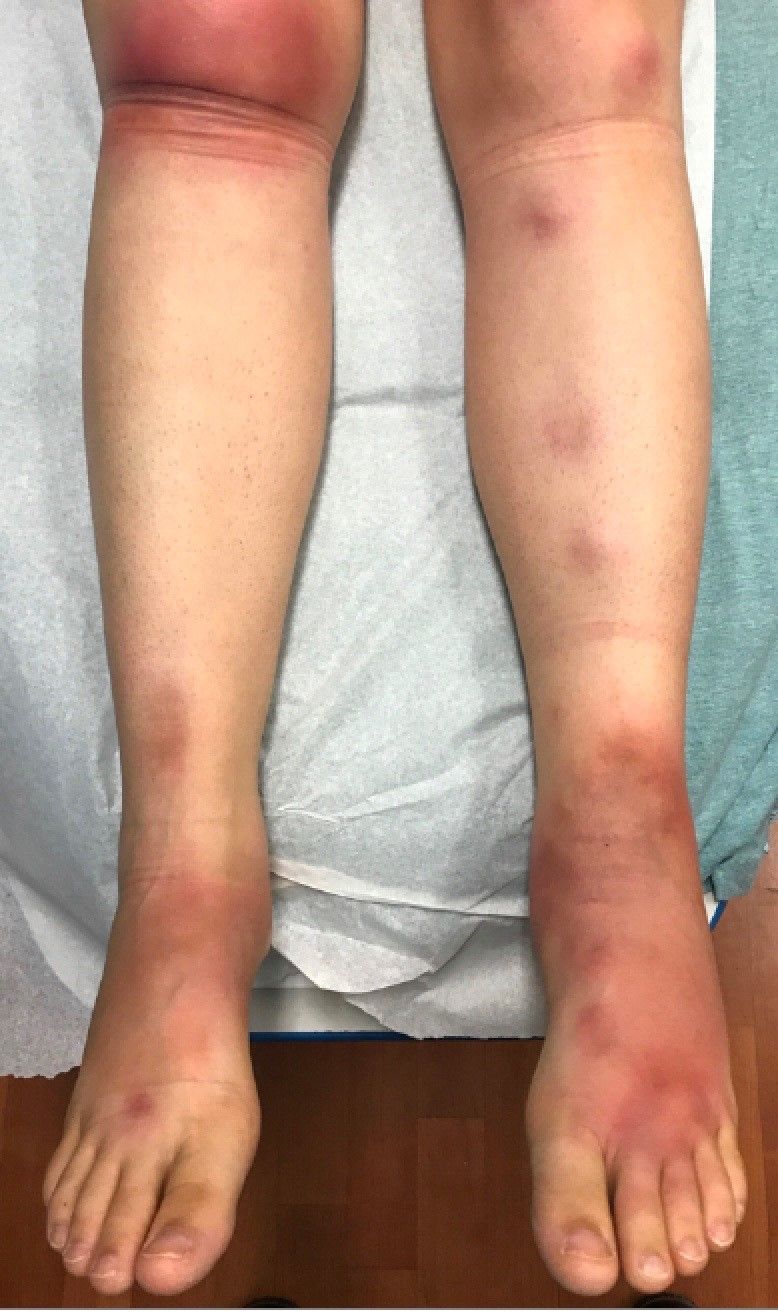
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
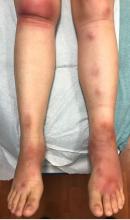
A 16-year-old female came to the dermatology clinic for acne follow-up. She reported some improvement on her acne since she started taking OCs. She also had been using benzoyl peroxide and tretinoin on her face. In addition to the acne, she also wanted us to check some tender bumps she had been getting on her shins after she came back from a camping trip. Initially she thought they were bug bites, but the lesions were getting larger, more tender, and not improving with diphenhydramine.
The physical exam did not reveal acute distress. She was afebrile. On skin examination, she had comedones, papules and scars on her face, chest, and back. On her shins there were several erythematous tender nodules and plaques. There was no edema on her legs and pulses were present.
Focus on symptoms over stories is detrimental to separated families
The horrors faced by migrant families forced to separate under the new U.S. “zero tolerance” policy continue to unfold. Tragic emblems of this policy include tapes of crying children and the reported suicide of a father who had been separated from his children.
A federal judge had issued an injunction requiring the reunification of thousands of families by July 26. Despite that deadline, hundreds of adults are no longer in the United States, and hundreds of children are scattered in shelters across the country.
In response to those events, mental health and medical organizations have released powerful statements. The American Psychological Association stated: “The administration’s policy ... is not only needless and cruel, it threatens the mental and physical health of both the children and their caregivers.” The American Medical Association issued a call asserting that separating children from their parents “will do great harm” and “create negative health impacts that will last an individual’s entire lifespan.” Meanwhile, the American Psychiatric Association’s president, Altha J. Stewart, MD, released a statement affirming that “any forced separation is highly stressful for children and can cause lifelong trauma, as well as an increased risk of other mental illnesses, such as depression, anxiety, and posttraumatic stress disorder.”
As forensic experts who testify about the mental well-being of immigration detainees, we applaud those powerful and unambiguous messages from the leaders in our fields. Yet, their statements also underscore the limitations of our diagnostic models: Our field is caught in the difficult position of either applying ill-fitting diagnostic labels or overpathologizing a normal reaction to horrific circumstances. While not applying diagnoses potentially minimizes the enormous psychological burden of separation, diagnosing depression or PTSD as catchalls for suffering incorrectly defines the experience of many survivors of ongoing trauma.
Currently, most providers, in trying to communicate the effects of ongoing trauma, rely on the diagnoses of depression or PTSD. Both of these diagnoses, however, are problematic. The diagnosis of major depressive disorder, for example, is useful in communicating a loss of hope, and the inability to enjoy pleasurable things. However, depression is an episodic illness, often part of a larger chronic disorder.1 Depression often has a genetic-hereditary component. On the other hand, children suffering from childhood traumas often present lifelong and wide-ranging problems, which may be triggered by reminders but are not episodic. For example, children experiencing parental separation have difficulty forming attachments, which, in turn, leads to subsequent difficulty forming meaningful interpersonal relationships.
The diagnosis of PTSD is useful in communicating a myriad of possible symptoms, which may accompany the trauma. However, PTSD implies a traumatic event as described in criteria A of the DSM-5: “exposure to actual or threatened death, serious injury, or sexual violence.” As such, PTSD poorly encompasses the wide array of smaller yet repetitive traumas experienced by victims of ongoing trauma, such as those youth separated from their parents at the U.S. border. Furthermore, PTSD is a disorder with specific symptoms that, based on a vast body of research,2,3 inadequately describes the multitude of interpersonal, psychological, and physical consequences associated with the type of trauma caused by family separations.
Our understanding of the long-term sequelae of childhood trauma has been greatly influenced by the adverse childhood experiences (ACE) study. The ACE study, one of the largest investigations ever conducted to assess associations between childhood maltreatment and later-life health and well-being, collected the life histories of more than 17,000 patients in a collaborative effort between the Centers for Disease Control and Prevention and Kaiser Permanente’s Health Appraisal Clinic.
The ACE study identified 10 forms of childhood trauma, including: abuse, neglect, abandonment, household dysfunction, and exposure to violence, that were strongly associated with negative psychological outcomes such as depression, suicide attempts, and engagement in high-risk behaviors, as well as significant medical consequences, including higher incidence of heart disease, diabetes, and stroke. Ultimately, having four or more ACEs was associated with early death.
In response to the emerging body of research on childhood trauma, various terms, including complex trauma, type-II trauma, and complex PTSD, have entered our professional lexicon as a means of communicating the wide-ranging consequences of developmental trauma. On the one hand, the less defined and rigid nature of these terms permits mental health providers to develop a rich narrative of a patient’s background, encompassing the patient’s behavior, character, and symptoms. However, the absence of formal terminology also has its drawbacks: Courts and juries have grown accustomed to diagnoses, labels, and syndromes. Most forensic mental health providers who testify about developmental trauma in court can predict the question: “So doctor, you are saying that the individual’s presentation is not severe enough to be considered PTSD, am I correct?” Disorders justify treatment, can explain disability, and warrant empathy; concomitantly, “complex trauma” runs the risk of being considered an academic explanation for trauma victims’ lifelong problems, rather than a societal failure that merits care.
Recognizing the limitations of our current diagnoses, the forthcoming update to the International Classification of Diseases (ICD-11) will add a new category: complex PTSD. The ICD-11 will attempt to widen the concept of trauma to include “conditions of prolonged adversity, in the form of sustained, repeated, or multiple forms of traumatic exposure.” Trauma exposure examples include genocide campaigns, childhood sexual abuse, child soldiering, severe domestic violence, torture, or slavery. The ICD-11 also expands our understanding of the consequences of trauma to include “affective dysregulation,” “negative self-concept,” and “disturbances in relationships” as part of a concept called “disturbances in self-organization.” Those are important steps in acknowledging the consequences of different forms of trauma as well as noticing a richer array of damages from those incidents.4
While the World Health Organization’s latest iteration of the ICD takes an important step in widening the scope of our diagnostic tools, we are cognizant that our field’s obsessional search for diagnoses, labels, and nomenclature reinforces a detrimental focus on symptoms over stories. However, as forensic mental health providers, we also are keenly aware that a failure to adopt common definitions impedes forensic evaluations, patient advocacy, public policy, and most importantly, patient care.
In the end, we have trained society to understand pathology through narrow lenses, and therefore, . So, despite the limitations of labels, let’s be encouraged by the World Health Organization’s efforts and continue in that direction.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Lehman is a licensed clinical and forensic psychologist in San Diego. Her practice consists of conducting forensic psychological evaluations for the courts with children, adolescents, and adults. Dr. Lehman has been qualified as an expert witness in California as well as in the federal courts. She previously was a supervisor at Sharper Future, a forensic rehabilitation program, and previously served as an adjunct faculty member at Alliant International University, San Diego. Dr. Lehman can be reached at [email protected].
References
1. Am J Psychiatry. 2000 Sep;157(9):1501-4.
2. Psychiatr Ann. 2005;35(5):390-8.
3. Psychiatr Ann. 2005;35(5):401-8.
4. Eur J Psychotraumatol. 2018 Jan 15. doi: 10.1080/20008198.2017.1418103.
The horrors faced by migrant families forced to separate under the new U.S. “zero tolerance” policy continue to unfold. Tragic emblems of this policy include tapes of crying children and the reported suicide of a father who had been separated from his children.
A federal judge had issued an injunction requiring the reunification of thousands of families by July 26. Despite that deadline, hundreds of adults are no longer in the United States, and hundreds of children are scattered in shelters across the country.
In response to those events, mental health and medical organizations have released powerful statements. The American Psychological Association stated: “The administration’s policy ... is not only needless and cruel, it threatens the mental and physical health of both the children and their caregivers.” The American Medical Association issued a call asserting that separating children from their parents “will do great harm” and “create negative health impacts that will last an individual’s entire lifespan.” Meanwhile, the American Psychiatric Association’s president, Altha J. Stewart, MD, released a statement affirming that “any forced separation is highly stressful for children and can cause lifelong trauma, as well as an increased risk of other mental illnesses, such as depression, anxiety, and posttraumatic stress disorder.”
As forensic experts who testify about the mental well-being of immigration detainees, we applaud those powerful and unambiguous messages from the leaders in our fields. Yet, their statements also underscore the limitations of our diagnostic models: Our field is caught in the difficult position of either applying ill-fitting diagnostic labels or overpathologizing a normal reaction to horrific circumstances. While not applying diagnoses potentially minimizes the enormous psychological burden of separation, diagnosing depression or PTSD as catchalls for suffering incorrectly defines the experience of many survivors of ongoing trauma.
Currently, most providers, in trying to communicate the effects of ongoing trauma, rely on the diagnoses of depression or PTSD. Both of these diagnoses, however, are problematic. The diagnosis of major depressive disorder, for example, is useful in communicating a loss of hope, and the inability to enjoy pleasurable things. However, depression is an episodic illness, often part of a larger chronic disorder.1 Depression often has a genetic-hereditary component. On the other hand, children suffering from childhood traumas often present lifelong and wide-ranging problems, which may be triggered by reminders but are not episodic. For example, children experiencing parental separation have difficulty forming attachments, which, in turn, leads to subsequent difficulty forming meaningful interpersonal relationships.
The diagnosis of PTSD is useful in communicating a myriad of possible symptoms, which may accompany the trauma. However, PTSD implies a traumatic event as described in criteria A of the DSM-5: “exposure to actual or threatened death, serious injury, or sexual violence.” As such, PTSD poorly encompasses the wide array of smaller yet repetitive traumas experienced by victims of ongoing trauma, such as those youth separated from their parents at the U.S. border. Furthermore, PTSD is a disorder with specific symptoms that, based on a vast body of research,2,3 inadequately describes the multitude of interpersonal, psychological, and physical consequences associated with the type of trauma caused by family separations.
Our understanding of the long-term sequelae of childhood trauma has been greatly influenced by the adverse childhood experiences (ACE) study. The ACE study, one of the largest investigations ever conducted to assess associations between childhood maltreatment and later-life health and well-being, collected the life histories of more than 17,000 patients in a collaborative effort between the Centers for Disease Control and Prevention and Kaiser Permanente’s Health Appraisal Clinic.
The ACE study identified 10 forms of childhood trauma, including: abuse, neglect, abandonment, household dysfunction, and exposure to violence, that were strongly associated with negative psychological outcomes such as depression, suicide attempts, and engagement in high-risk behaviors, as well as significant medical consequences, including higher incidence of heart disease, diabetes, and stroke. Ultimately, having four or more ACEs was associated with early death.
In response to the emerging body of research on childhood trauma, various terms, including complex trauma, type-II trauma, and complex PTSD, have entered our professional lexicon as a means of communicating the wide-ranging consequences of developmental trauma. On the one hand, the less defined and rigid nature of these terms permits mental health providers to develop a rich narrative of a patient’s background, encompassing the patient’s behavior, character, and symptoms. However, the absence of formal terminology also has its drawbacks: Courts and juries have grown accustomed to diagnoses, labels, and syndromes. Most forensic mental health providers who testify about developmental trauma in court can predict the question: “So doctor, you are saying that the individual’s presentation is not severe enough to be considered PTSD, am I correct?” Disorders justify treatment, can explain disability, and warrant empathy; concomitantly, “complex trauma” runs the risk of being considered an academic explanation for trauma victims’ lifelong problems, rather than a societal failure that merits care.
Recognizing the limitations of our current diagnoses, the forthcoming update to the International Classification of Diseases (ICD-11) will add a new category: complex PTSD. The ICD-11 will attempt to widen the concept of trauma to include “conditions of prolonged adversity, in the form of sustained, repeated, or multiple forms of traumatic exposure.” Trauma exposure examples include genocide campaigns, childhood sexual abuse, child soldiering, severe domestic violence, torture, or slavery. The ICD-11 also expands our understanding of the consequences of trauma to include “affective dysregulation,” “negative self-concept,” and “disturbances in relationships” as part of a concept called “disturbances in self-organization.” Those are important steps in acknowledging the consequences of different forms of trauma as well as noticing a richer array of damages from those incidents.4
While the World Health Organization’s latest iteration of the ICD takes an important step in widening the scope of our diagnostic tools, we are cognizant that our field’s obsessional search for diagnoses, labels, and nomenclature reinforces a detrimental focus on symptoms over stories. However, as forensic mental health providers, we also are keenly aware that a failure to adopt common definitions impedes forensic evaluations, patient advocacy, public policy, and most importantly, patient care.
In the end, we have trained society to understand pathology through narrow lenses, and therefore, . So, despite the limitations of labels, let’s be encouraged by the World Health Organization’s efforts and continue in that direction.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Lehman is a licensed clinical and forensic psychologist in San Diego. Her practice consists of conducting forensic psychological evaluations for the courts with children, adolescents, and adults. Dr. Lehman has been qualified as an expert witness in California as well as in the federal courts. She previously was a supervisor at Sharper Future, a forensic rehabilitation program, and previously served as an adjunct faculty member at Alliant International University, San Diego. Dr. Lehman can be reached at [email protected].
References
1. Am J Psychiatry. 2000 Sep;157(9):1501-4.
2. Psychiatr Ann. 2005;35(5):390-8.
3. Psychiatr Ann. 2005;35(5):401-8.
4. Eur J Psychotraumatol. 2018 Jan 15. doi: 10.1080/20008198.2017.1418103.
The horrors faced by migrant families forced to separate under the new U.S. “zero tolerance” policy continue to unfold. Tragic emblems of this policy include tapes of crying children and the reported suicide of a father who had been separated from his children.
A federal judge had issued an injunction requiring the reunification of thousands of families by July 26. Despite that deadline, hundreds of adults are no longer in the United States, and hundreds of children are scattered in shelters across the country.
In response to those events, mental health and medical organizations have released powerful statements. The American Psychological Association stated: “The administration’s policy ... is not only needless and cruel, it threatens the mental and physical health of both the children and their caregivers.” The American Medical Association issued a call asserting that separating children from their parents “will do great harm” and “create negative health impacts that will last an individual’s entire lifespan.” Meanwhile, the American Psychiatric Association’s president, Altha J. Stewart, MD, released a statement affirming that “any forced separation is highly stressful for children and can cause lifelong trauma, as well as an increased risk of other mental illnesses, such as depression, anxiety, and posttraumatic stress disorder.”
As forensic experts who testify about the mental well-being of immigration detainees, we applaud those powerful and unambiguous messages from the leaders in our fields. Yet, their statements also underscore the limitations of our diagnostic models: Our field is caught in the difficult position of either applying ill-fitting diagnostic labels or overpathologizing a normal reaction to horrific circumstances. While not applying diagnoses potentially minimizes the enormous psychological burden of separation, diagnosing depression or PTSD as catchalls for suffering incorrectly defines the experience of many survivors of ongoing trauma.
Currently, most providers, in trying to communicate the effects of ongoing trauma, rely on the diagnoses of depression or PTSD. Both of these diagnoses, however, are problematic. The diagnosis of major depressive disorder, for example, is useful in communicating a loss of hope, and the inability to enjoy pleasurable things. However, depression is an episodic illness, often part of a larger chronic disorder.1 Depression often has a genetic-hereditary component. On the other hand, children suffering from childhood traumas often present lifelong and wide-ranging problems, which may be triggered by reminders but are not episodic. For example, children experiencing parental separation have difficulty forming attachments, which, in turn, leads to subsequent difficulty forming meaningful interpersonal relationships.
The diagnosis of PTSD is useful in communicating a myriad of possible symptoms, which may accompany the trauma. However, PTSD implies a traumatic event as described in criteria A of the DSM-5: “exposure to actual or threatened death, serious injury, or sexual violence.” As such, PTSD poorly encompasses the wide array of smaller yet repetitive traumas experienced by victims of ongoing trauma, such as those youth separated from their parents at the U.S. border. Furthermore, PTSD is a disorder with specific symptoms that, based on a vast body of research,2,3 inadequately describes the multitude of interpersonal, psychological, and physical consequences associated with the type of trauma caused by family separations.
Our understanding of the long-term sequelae of childhood trauma has been greatly influenced by the adverse childhood experiences (ACE) study. The ACE study, one of the largest investigations ever conducted to assess associations between childhood maltreatment and later-life health and well-being, collected the life histories of more than 17,000 patients in a collaborative effort between the Centers for Disease Control and Prevention and Kaiser Permanente’s Health Appraisal Clinic.
The ACE study identified 10 forms of childhood trauma, including: abuse, neglect, abandonment, household dysfunction, and exposure to violence, that were strongly associated with negative psychological outcomes such as depression, suicide attempts, and engagement in high-risk behaviors, as well as significant medical consequences, including higher incidence of heart disease, diabetes, and stroke. Ultimately, having four or more ACEs was associated with early death.
In response to the emerging body of research on childhood trauma, various terms, including complex trauma, type-II trauma, and complex PTSD, have entered our professional lexicon as a means of communicating the wide-ranging consequences of developmental trauma. On the one hand, the less defined and rigid nature of these terms permits mental health providers to develop a rich narrative of a patient’s background, encompassing the patient’s behavior, character, and symptoms. However, the absence of formal terminology also has its drawbacks: Courts and juries have grown accustomed to diagnoses, labels, and syndromes. Most forensic mental health providers who testify about developmental trauma in court can predict the question: “So doctor, you are saying that the individual’s presentation is not severe enough to be considered PTSD, am I correct?” Disorders justify treatment, can explain disability, and warrant empathy; concomitantly, “complex trauma” runs the risk of being considered an academic explanation for trauma victims’ lifelong problems, rather than a societal failure that merits care.
Recognizing the limitations of our current diagnoses, the forthcoming update to the International Classification of Diseases (ICD-11) will add a new category: complex PTSD. The ICD-11 will attempt to widen the concept of trauma to include “conditions of prolonged adversity, in the form of sustained, repeated, or multiple forms of traumatic exposure.” Trauma exposure examples include genocide campaigns, childhood sexual abuse, child soldiering, severe domestic violence, torture, or slavery. The ICD-11 also expands our understanding of the consequences of trauma to include “affective dysregulation,” “negative self-concept,” and “disturbances in relationships” as part of a concept called “disturbances in self-organization.” Those are important steps in acknowledging the consequences of different forms of trauma as well as noticing a richer array of damages from those incidents.4
While the World Health Organization’s latest iteration of the ICD takes an important step in widening the scope of our diagnostic tools, we are cognizant that our field’s obsessional search for diagnoses, labels, and nomenclature reinforces a detrimental focus on symptoms over stories. However, as forensic mental health providers, we also are keenly aware that a failure to adopt common definitions impedes forensic evaluations, patient advocacy, public policy, and most importantly, patient care.
In the end, we have trained society to understand pathology through narrow lenses, and therefore, . So, despite the limitations of labels, let’s be encouraged by the World Health Organization’s efforts and continue in that direction.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Lehman is a licensed clinical and forensic psychologist in San Diego. Her practice consists of conducting forensic psychological evaluations for the courts with children, adolescents, and adults. Dr. Lehman has been qualified as an expert witness in California as well as in the federal courts. She previously was a supervisor at Sharper Future, a forensic rehabilitation program, and previously served as an adjunct faculty member at Alliant International University, San Diego. Dr. Lehman can be reached at [email protected].
References
1. Am J Psychiatry. 2000 Sep;157(9):1501-4.
2. Psychiatr Ann. 2005;35(5):390-8.
3. Psychiatr Ann. 2005;35(5):401-8.
4. Eur J Psychotraumatol. 2018 Jan 15. doi: 10.1080/20008198.2017.1418103.
Preventative Care in Orthopedics: Treating Injuries Before They Happen
By 2025, it is estimated that the annual cost of treating osteoporosis-related fractures in the United States will be 25 billion dollars, which is 10 billion dollars more than was spent in 2010.1 As healthcare costs in the United States continue to skyrocket, it is imperative that orthopedic surgeons take an active role in avoiding preventable injury and disease. For orthopedic surgeons, preventative medicine will include promoting bone health and educating patients on injury prevention. By incorporating these principles into residency and fellowship education, and by leveraging the electronic medical record to support preventive care through systematic reminders, orthopedic surgeons have a critical opportunity to take a leading role in promoting prevention to our patients.
In 2009, the American Orthopaedic Association (AOA) launched a “Own the Bone” campaign, a national quality improvement program designed to optimize the treatment of osteoporosis.2 This program came about following the Surgeon General’s call, in 2004, for orthopedic surgeons to take a more active role in treating osteoporosis. The program primarily aims to improve treatment of osteoporosis after a fragility fracture in an inpatient setting. Early results from a 2010 follow-up study showed that the new emphasis on prevention inspired by this program is effective. As compared with patients who had osteoporosis work-up and treatment initiated during their hospital admission, the group of patients who were referred for osteoporosis treatment after discharge were found to have a significantly lower rate of diagnosis and treatment.3 The loss of aftercare for patients who do not obtain immediate diagnosis and treatment for osteoporosis can and should be avoided. Many hospitals now have hip fracture services with multidisciplinary input. The successful outcomes of these programs include shorter times to the operating room, shorter hospital stays, decreased readmission, and decreased 30-day mortality.4-6 These services provide an excellent opportunity to ensure that each patient has initiated management of osteoporosis before discharge. Ideally, patients would be scheduled for bone mineral density testing prior to leaving the hospital, when applicable, and would begin calcium and vitamin D supplementation or bisphosphonate treatment in the hospital, when appropriate. As part of these hip fracture services, a goal of clearly initiating or managing treatment for osteoporosis should be routinely addressed.
While patients presenting with hip fractures are an easily identifiable high-risk population, other patients present in an outpatient setting following fragility fractures, such as distal radius or vertebral compression fractures. These patients should be considered for osteoporosis work-up and counseled accordingly. A recent study compared the efficacy of the orthopedic surgeon initiating bone mineral density testing after a distal radius fracture, compared with referring the patient back to their primary care physician for testing. The study found a significantly higher rate of patients going on to bone mineral density testing when the surgeon initiated this process.7 In the era of improved digital communication, the outpatient setting offers an opportunity for clinicians to communicate with patients’ primary care physicians and initiate a multidisciplinary approach to bone health and prevention. In the outpatient setting, the orthopedist can address nutritional issues and screening on a repeated basis. Studies have demonstrated that physician counseling can be very effective in changing behavior and helping patients to stop using tobacco.8 In this vein, efforts by the physician to encourage calcium and vitamin D intake and weight-bearing exercise have the potential to be very effective.
Programs such as “Own the Bone” are crucial to orthopedists’ treatment of osteoporosis, but prevention of bone disease and fragility fracture must extend even further. Individual practitioners must be cognizant that many patients may benefit from outpatient diagnosis of osteoporosis and initiation of appropriate treatment, before fragility fractures occur. Moreover, although patients at high risk include post-menopausal women, orthopedists need to be consistently aware of osteoporosis as a disease of both genders. An estimated 2.8 million men in the United States have osteoporosis.9 A 2012 study published out of Washington, DC found a significant disparity in the rate of osteoporosis screening between men and women. Among the elderly men and women in their patient population, 60% of women underwent screening compared with only 18.4% of men.10 This gender disparity potentially represents significant physician bias regarding the risk of osteoporosis and offers an important opportunity for orthopedic surgeons to improve preventative care for this population.
Preventative care in terms of advocating for bone health should not be limited to patients presenting with fragility fractures. Education regarding smoking cessation, resistance exercise, and calcium intake are relevant to many orthopedic patients. With the advent of the electronic medical record system, a simple intervention could easily ensure that patients report on their calcium intake. A trial published in 2006 found that a simple reminder from the electronic medical record improved osteoporosis management following a fragility fracture.11 This type of intervention could certainly be expanded to include counseling on calcium and vitamin D for any orthopedic patient.
Another area in which orthopedic surgeons have an opportunity to practice good preventative care is injury prevention. Several studies examining fall prevention among the elderly have shown that physical therapy or exercise may decrease the rate of falls.12 Promotion of activity and therapy among high-risk patients by orthopedic surgeons may help to reduce fracture incidence. Injury prevention is also relevant to young, healthy patients. It is well established that neuromuscular training helps to prevent anterior cruciate ligament injuries.13 Orthopedic surgeons have an opportunity during sports physicals or as team physicians to help promote injury prevention strategies. Discussion of training regimens may prevent overuse injuries among athletes. Moreover, faced with many patients who present with significant musculoskeletal trauma, orthopedic surgeons have the opportunity to offer education regarding motorcycle helmets, seatbelt use, and avoidance of drunk driving.
New orthopedic residency educational goals were recently published to include core competencies in resident education. Among these goals is to educate residents on care of a patient with hip fracture, including counseling and management of osteoporosis.14 These milestones could be expanded to include a thorough understanding of bone health. Residents should be able to make nutritional recommendations for any patient seen as an inpatient or outpatient, identify when a referral to an endocrinologist is needed, and educate patients regarding injury and fall prevention.
As healthcare expenditures rise, so does the impetus for physicians to work to improve efficiency in the healthcare system. Furthermore, the best possible care for our patients is to prevent injury and disability before it arises, rather than to depend on our ability to intervene after the fact. Residencies and training programs should work to incorporate preventative strategies into trainee education. Hospitals and outpatient settings should include a basic bone health questionnaire in the electronic medical record. The identification and management of risk factors for injury has the potential to help our patients and to help our healthcare system, but such intervention needs to start with the clinician.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi:10.1359/jbmr.061113.
- Bunta AD. It is time for everyone to own the bone. Osteoporos Int. 2011;22 Suppl 3:477-482. doi:10.1007/s00198-011-1704-0.
- Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.” J Bone Joint Surg Am. 2011;93(15):e87. doi:10.2106/JBJS.I.00540.
- Sivakumar BS, McDermott LM, Bell JJ, Pulle CR, Jayamaha S, Ottley MC. Dedicated hip fracture service: implementing a novel model of care. ANZ J Surg. 2013;83(7-8):559-563. doi:10.1111/j.1445-2197.2012.06201.x.
- Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31.
- Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. doi:10.1111/j.1532-5415.2005.53466.x.
- Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953-961. doi:10.2106/JBJS.G.01121.
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2012-2022.
- Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900-1905. doi:10.1007/s11999-011-1780-7.
- Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311-313. doi:10.1007/s11657-012-0113-0.
- Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi:10.1111/j.1532-5415.2005.00618.x.
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community-dwelling elderly Japanese women. J Bone Miner Metab. 2004;22(6):602-611. doi:10.1007/s00774-004-0530-2.
- Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498. doi:10.1177/0363546505282619.
- Stern PJ, Albanese S, Bostrom M, et al. Orthopaedic surgery milestones. J Grad Med Educ. 2013;5(1 Suppl 1):36-58. doi:10.4300/JGME-05-01s1-05.
By 2025, it is estimated that the annual cost of treating osteoporosis-related fractures in the United States will be 25 billion dollars, which is 10 billion dollars more than was spent in 2010.1 As healthcare costs in the United States continue to skyrocket, it is imperative that orthopedic surgeons take an active role in avoiding preventable injury and disease. For orthopedic surgeons, preventative medicine will include promoting bone health and educating patients on injury prevention. By incorporating these principles into residency and fellowship education, and by leveraging the electronic medical record to support preventive care through systematic reminders, orthopedic surgeons have a critical opportunity to take a leading role in promoting prevention to our patients.
In 2009, the American Orthopaedic Association (AOA) launched a “Own the Bone” campaign, a national quality improvement program designed to optimize the treatment of osteoporosis.2 This program came about following the Surgeon General’s call, in 2004, for orthopedic surgeons to take a more active role in treating osteoporosis. The program primarily aims to improve treatment of osteoporosis after a fragility fracture in an inpatient setting. Early results from a 2010 follow-up study showed that the new emphasis on prevention inspired by this program is effective. As compared with patients who had osteoporosis work-up and treatment initiated during their hospital admission, the group of patients who were referred for osteoporosis treatment after discharge were found to have a significantly lower rate of diagnosis and treatment.3 The loss of aftercare for patients who do not obtain immediate diagnosis and treatment for osteoporosis can and should be avoided. Many hospitals now have hip fracture services with multidisciplinary input. The successful outcomes of these programs include shorter times to the operating room, shorter hospital stays, decreased readmission, and decreased 30-day mortality.4-6 These services provide an excellent opportunity to ensure that each patient has initiated management of osteoporosis before discharge. Ideally, patients would be scheduled for bone mineral density testing prior to leaving the hospital, when applicable, and would begin calcium and vitamin D supplementation or bisphosphonate treatment in the hospital, when appropriate. As part of these hip fracture services, a goal of clearly initiating or managing treatment for osteoporosis should be routinely addressed.
While patients presenting with hip fractures are an easily identifiable high-risk population, other patients present in an outpatient setting following fragility fractures, such as distal radius or vertebral compression fractures. These patients should be considered for osteoporosis work-up and counseled accordingly. A recent study compared the efficacy of the orthopedic surgeon initiating bone mineral density testing after a distal radius fracture, compared with referring the patient back to their primary care physician for testing. The study found a significantly higher rate of patients going on to bone mineral density testing when the surgeon initiated this process.7 In the era of improved digital communication, the outpatient setting offers an opportunity for clinicians to communicate with patients’ primary care physicians and initiate a multidisciplinary approach to bone health and prevention. In the outpatient setting, the orthopedist can address nutritional issues and screening on a repeated basis. Studies have demonstrated that physician counseling can be very effective in changing behavior and helping patients to stop using tobacco.8 In this vein, efforts by the physician to encourage calcium and vitamin D intake and weight-bearing exercise have the potential to be very effective.
Programs such as “Own the Bone” are crucial to orthopedists’ treatment of osteoporosis, but prevention of bone disease and fragility fracture must extend even further. Individual practitioners must be cognizant that many patients may benefit from outpatient diagnosis of osteoporosis and initiation of appropriate treatment, before fragility fractures occur. Moreover, although patients at high risk include post-menopausal women, orthopedists need to be consistently aware of osteoporosis as a disease of both genders. An estimated 2.8 million men in the United States have osteoporosis.9 A 2012 study published out of Washington, DC found a significant disparity in the rate of osteoporosis screening between men and women. Among the elderly men and women in their patient population, 60% of women underwent screening compared with only 18.4% of men.10 This gender disparity potentially represents significant physician bias regarding the risk of osteoporosis and offers an important opportunity for orthopedic surgeons to improve preventative care for this population.
Preventative care in terms of advocating for bone health should not be limited to patients presenting with fragility fractures. Education regarding smoking cessation, resistance exercise, and calcium intake are relevant to many orthopedic patients. With the advent of the electronic medical record system, a simple intervention could easily ensure that patients report on their calcium intake. A trial published in 2006 found that a simple reminder from the electronic medical record improved osteoporosis management following a fragility fracture.11 This type of intervention could certainly be expanded to include counseling on calcium and vitamin D for any orthopedic patient.
Another area in which orthopedic surgeons have an opportunity to practice good preventative care is injury prevention. Several studies examining fall prevention among the elderly have shown that physical therapy or exercise may decrease the rate of falls.12 Promotion of activity and therapy among high-risk patients by orthopedic surgeons may help to reduce fracture incidence. Injury prevention is also relevant to young, healthy patients. It is well established that neuromuscular training helps to prevent anterior cruciate ligament injuries.13 Orthopedic surgeons have an opportunity during sports physicals or as team physicians to help promote injury prevention strategies. Discussion of training regimens may prevent overuse injuries among athletes. Moreover, faced with many patients who present with significant musculoskeletal trauma, orthopedic surgeons have the opportunity to offer education regarding motorcycle helmets, seatbelt use, and avoidance of drunk driving.
New orthopedic residency educational goals were recently published to include core competencies in resident education. Among these goals is to educate residents on care of a patient with hip fracture, including counseling and management of osteoporosis.14 These milestones could be expanded to include a thorough understanding of bone health. Residents should be able to make nutritional recommendations for any patient seen as an inpatient or outpatient, identify when a referral to an endocrinologist is needed, and educate patients regarding injury and fall prevention.
As healthcare expenditures rise, so does the impetus for physicians to work to improve efficiency in the healthcare system. Furthermore, the best possible care for our patients is to prevent injury and disability before it arises, rather than to depend on our ability to intervene after the fact. Residencies and training programs should work to incorporate preventative strategies into trainee education. Hospitals and outpatient settings should include a basic bone health questionnaire in the electronic medical record. The identification and management of risk factors for injury has the potential to help our patients and to help our healthcare system, but such intervention needs to start with the clinician.
By 2025, it is estimated that the annual cost of treating osteoporosis-related fractures in the United States will be 25 billion dollars, which is 10 billion dollars more than was spent in 2010.1 As healthcare costs in the United States continue to skyrocket, it is imperative that orthopedic surgeons take an active role in avoiding preventable injury and disease. For orthopedic surgeons, preventative medicine will include promoting bone health and educating patients on injury prevention. By incorporating these principles into residency and fellowship education, and by leveraging the electronic medical record to support preventive care through systematic reminders, orthopedic surgeons have a critical opportunity to take a leading role in promoting prevention to our patients.
In 2009, the American Orthopaedic Association (AOA) launched a “Own the Bone” campaign, a national quality improvement program designed to optimize the treatment of osteoporosis.2 This program came about following the Surgeon General’s call, in 2004, for orthopedic surgeons to take a more active role in treating osteoporosis. The program primarily aims to improve treatment of osteoporosis after a fragility fracture in an inpatient setting. Early results from a 2010 follow-up study showed that the new emphasis on prevention inspired by this program is effective. As compared with patients who had osteoporosis work-up and treatment initiated during their hospital admission, the group of patients who were referred for osteoporosis treatment after discharge were found to have a significantly lower rate of diagnosis and treatment.3 The loss of aftercare for patients who do not obtain immediate diagnosis and treatment for osteoporosis can and should be avoided. Many hospitals now have hip fracture services with multidisciplinary input. The successful outcomes of these programs include shorter times to the operating room, shorter hospital stays, decreased readmission, and decreased 30-day mortality.4-6 These services provide an excellent opportunity to ensure that each patient has initiated management of osteoporosis before discharge. Ideally, patients would be scheduled for bone mineral density testing prior to leaving the hospital, when applicable, and would begin calcium and vitamin D supplementation or bisphosphonate treatment in the hospital, when appropriate. As part of these hip fracture services, a goal of clearly initiating or managing treatment for osteoporosis should be routinely addressed.
While patients presenting with hip fractures are an easily identifiable high-risk population, other patients present in an outpatient setting following fragility fractures, such as distal radius or vertebral compression fractures. These patients should be considered for osteoporosis work-up and counseled accordingly. A recent study compared the efficacy of the orthopedic surgeon initiating bone mineral density testing after a distal radius fracture, compared with referring the patient back to their primary care physician for testing. The study found a significantly higher rate of patients going on to bone mineral density testing when the surgeon initiated this process.7 In the era of improved digital communication, the outpatient setting offers an opportunity for clinicians to communicate with patients’ primary care physicians and initiate a multidisciplinary approach to bone health and prevention. In the outpatient setting, the orthopedist can address nutritional issues and screening on a repeated basis. Studies have demonstrated that physician counseling can be very effective in changing behavior and helping patients to stop using tobacco.8 In this vein, efforts by the physician to encourage calcium and vitamin D intake and weight-bearing exercise have the potential to be very effective.
Programs such as “Own the Bone” are crucial to orthopedists’ treatment of osteoporosis, but prevention of bone disease and fragility fracture must extend even further. Individual practitioners must be cognizant that many patients may benefit from outpatient diagnosis of osteoporosis and initiation of appropriate treatment, before fragility fractures occur. Moreover, although patients at high risk include post-menopausal women, orthopedists need to be consistently aware of osteoporosis as a disease of both genders. An estimated 2.8 million men in the United States have osteoporosis.9 A 2012 study published out of Washington, DC found a significant disparity in the rate of osteoporosis screening between men and women. Among the elderly men and women in their patient population, 60% of women underwent screening compared with only 18.4% of men.10 This gender disparity potentially represents significant physician bias regarding the risk of osteoporosis and offers an important opportunity for orthopedic surgeons to improve preventative care for this population.
Preventative care in terms of advocating for bone health should not be limited to patients presenting with fragility fractures. Education regarding smoking cessation, resistance exercise, and calcium intake are relevant to many orthopedic patients. With the advent of the electronic medical record system, a simple intervention could easily ensure that patients report on their calcium intake. A trial published in 2006 found that a simple reminder from the electronic medical record improved osteoporosis management following a fragility fracture.11 This type of intervention could certainly be expanded to include counseling on calcium and vitamin D for any orthopedic patient.
Another area in which orthopedic surgeons have an opportunity to practice good preventative care is injury prevention. Several studies examining fall prevention among the elderly have shown that physical therapy or exercise may decrease the rate of falls.12 Promotion of activity and therapy among high-risk patients by orthopedic surgeons may help to reduce fracture incidence. Injury prevention is also relevant to young, healthy patients. It is well established that neuromuscular training helps to prevent anterior cruciate ligament injuries.13 Orthopedic surgeons have an opportunity during sports physicals or as team physicians to help promote injury prevention strategies. Discussion of training regimens may prevent overuse injuries among athletes. Moreover, faced with many patients who present with significant musculoskeletal trauma, orthopedic surgeons have the opportunity to offer education regarding motorcycle helmets, seatbelt use, and avoidance of drunk driving.
New orthopedic residency educational goals were recently published to include core competencies in resident education. Among these goals is to educate residents on care of a patient with hip fracture, including counseling and management of osteoporosis.14 These milestones could be expanded to include a thorough understanding of bone health. Residents should be able to make nutritional recommendations for any patient seen as an inpatient or outpatient, identify when a referral to an endocrinologist is needed, and educate patients regarding injury and fall prevention.
As healthcare expenditures rise, so does the impetus for physicians to work to improve efficiency in the healthcare system. Furthermore, the best possible care for our patients is to prevent injury and disability before it arises, rather than to depend on our ability to intervene after the fact. Residencies and training programs should work to incorporate preventative strategies into trainee education. Hospitals and outpatient settings should include a basic bone health questionnaire in the electronic medical record. The identification and management of risk factors for injury has the potential to help our patients and to help our healthcare system, but such intervention needs to start with the clinician.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi:10.1359/jbmr.061113.
- Bunta AD. It is time for everyone to own the bone. Osteoporos Int. 2011;22 Suppl 3:477-482. doi:10.1007/s00198-011-1704-0.
- Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.” J Bone Joint Surg Am. 2011;93(15):e87. doi:10.2106/JBJS.I.00540.
- Sivakumar BS, McDermott LM, Bell JJ, Pulle CR, Jayamaha S, Ottley MC. Dedicated hip fracture service: implementing a novel model of care. ANZ J Surg. 2013;83(7-8):559-563. doi:10.1111/j.1445-2197.2012.06201.x.
- Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31.
- Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. doi:10.1111/j.1532-5415.2005.53466.x.
- Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953-961. doi:10.2106/JBJS.G.01121.
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2012-2022.
- Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900-1905. doi:10.1007/s11999-011-1780-7.
- Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311-313. doi:10.1007/s11657-012-0113-0.
- Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi:10.1111/j.1532-5415.2005.00618.x.
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community-dwelling elderly Japanese women. J Bone Miner Metab. 2004;22(6):602-611. doi:10.1007/s00774-004-0530-2.
- Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498. doi:10.1177/0363546505282619.
- Stern PJ, Albanese S, Bostrom M, et al. Orthopaedic surgery milestones. J Grad Med Educ. 2013;5(1 Suppl 1):36-58. doi:10.4300/JGME-05-01s1-05.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi:10.1359/jbmr.061113.
- Bunta AD. It is time for everyone to own the bone. Osteoporos Int. 2011;22 Suppl 3:477-482. doi:10.1007/s00198-011-1704-0.
- Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.” J Bone Joint Surg Am. 2011;93(15):e87. doi:10.2106/JBJS.I.00540.
- Sivakumar BS, McDermott LM, Bell JJ, Pulle CR, Jayamaha S, Ottley MC. Dedicated hip fracture service: implementing a novel model of care. ANZ J Surg. 2013;83(7-8):559-563. doi:10.1111/j.1445-2197.2012.06201.x.
- Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31.
- Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. doi:10.1111/j.1532-5415.2005.53466.x.
- Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953-961. doi:10.2106/JBJS.G.01121.
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2012-2022.
- Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900-1905. doi:10.1007/s11999-011-1780-7.
- Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311-313. doi:10.1007/s11657-012-0113-0.
- Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi:10.1111/j.1532-5415.2005.00618.x.
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community-dwelling elderly Japanese women. J Bone Miner Metab. 2004;22(6):602-611. doi:10.1007/s00774-004-0530-2.
- Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498. doi:10.1177/0363546505282619.
- Stern PJ, Albanese S, Bostrom M, et al. Orthopaedic surgery milestones. J Grad Med Educ. 2013;5(1 Suppl 1):36-58. doi:10.4300/JGME-05-01s1-05.
Benzodiazepines for anxious depression
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Pseudotumor cerebri pediatric rates are rising
Pseudotumor cerebri, benign intracranial hypertension, and idiopathic intracranial hypertension are all terms to describe a syndrome of increased intracranial pressure, headaches, vision loss, or changes without an associated mass lesion.1 The condition was considered relatively rare, presenting most commonly in obese women in childbearing years. Surprisingly, 2
Obesity is the fastest growing morbidity among adolescents. The Centers for Disease Control and Prevention reported 32% of children 2-19 years were obese.1 This reality is impacting many areas of an adolescent’s health, but it also is changing the landscape of diseases that present in this age group. Although pediatric and adult pseudotumor cerebri always have had slightly varied features, many features were similar such as the papilledema, vision loss, headaches, and sixth nerve palsy. Obesity and female predominance tended to present more in the adult population, as many pediatric patients were not obese,2 and had fewer associated symptoms at the time of diagnosis, and the cause was thought to idiopathic.
Now, with the increase in obesity, more adolescents and more male patients are presenting with pseudotumor cerebri as a cause for their headache, and 57%-100% are obese, making it a compounding factor.3
Pediatric populations also are at risk of secondary pseudotumor cerebri, which is an increase in intracranial pressure from the use of medication, or other disease states such as anemia, kidney disease, or Down syndrome. Minocycline use is the most common medication cause and usually presents 1-2 months after normal use.4 Discontinuing the drug does lead to resolution. Retinoids, vitamin A products, growth hormone, and steroids also have been implicated. Given that acne is a common complaint amongst teens, knowledge of these side effects is important.4
In 2013, the criteria for diagnosis of pseudotumor cerebri was revised. Currently, the presence of papilledema, normal neurologic exam except for abnormal sixth cranial nerve, normal cerebral spinal fluid, elevated lumbar opening pressure, and normal imaging are needed for a definitive diagnosis. A probable diagnosis can be made if papilledema is not present but there abducens nerve palsy.2
In a routine physical exam, when I questioned a patient on any medication that was used daily, she replied she took ibuprofen daily for headaches and that she had been doing this for several months. Headaches were not in her chief complaints as she had learned to live with and ignore this symptom. Upon further evaluation, she was slightly overweight and has a questionable fundoscopic exam. After further evaluation by an ophthalmologist and a neurologist, pseudotumor cerebri was diagnosed.
Index of suspicion is key in correctly diagnosing patients, and understanding the changing landscape of medicine will lead to more thoughtful questioning during routine health exams and better outcomes for your patients.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Am J Ophthalmol. 2015 Feb;159(2):344-52.e1.
2. Horm Res Paediatr. 2014;81(4):217-25.
3. Clin Imaging. 2018 May 24. doi: 10.1016/j.clinimag.2018.05.020.
4. Am J Ophthalmol. 1998 Jul;126(1):116-21.
5. Glob Pediatr Health. 2018. doi:10.1177/2333794X18785550.
Pseudotumor cerebri, benign intracranial hypertension, and idiopathic intracranial hypertension are all terms to describe a syndrome of increased intracranial pressure, headaches, vision loss, or changes without an associated mass lesion.1 The condition was considered relatively rare, presenting most commonly in obese women in childbearing years. Surprisingly, 2
Obesity is the fastest growing morbidity among adolescents. The Centers for Disease Control and Prevention reported 32% of children 2-19 years were obese.1 This reality is impacting many areas of an adolescent’s health, but it also is changing the landscape of diseases that present in this age group. Although pediatric and adult pseudotumor cerebri always have had slightly varied features, many features were similar such as the papilledema, vision loss, headaches, and sixth nerve palsy. Obesity and female predominance tended to present more in the adult population, as many pediatric patients were not obese,2 and had fewer associated symptoms at the time of diagnosis, and the cause was thought to idiopathic.
Now, with the increase in obesity, more adolescents and more male patients are presenting with pseudotumor cerebri as a cause for their headache, and 57%-100% are obese, making it a compounding factor.3
Pediatric populations also are at risk of secondary pseudotumor cerebri, which is an increase in intracranial pressure from the use of medication, or other disease states such as anemia, kidney disease, or Down syndrome. Minocycline use is the most common medication cause and usually presents 1-2 months after normal use.4 Discontinuing the drug does lead to resolution. Retinoids, vitamin A products, growth hormone, and steroids also have been implicated. Given that acne is a common complaint amongst teens, knowledge of these side effects is important.4
In 2013, the criteria for diagnosis of pseudotumor cerebri was revised. Currently, the presence of papilledema, normal neurologic exam except for abnormal sixth cranial nerve, normal cerebral spinal fluid, elevated lumbar opening pressure, and normal imaging are needed for a definitive diagnosis. A probable diagnosis can be made if papilledema is not present but there abducens nerve palsy.2
In a routine physical exam, when I questioned a patient on any medication that was used daily, she replied she took ibuprofen daily for headaches and that she had been doing this for several months. Headaches were not in her chief complaints as she had learned to live with and ignore this symptom. Upon further evaluation, she was slightly overweight and has a questionable fundoscopic exam. After further evaluation by an ophthalmologist and a neurologist, pseudotumor cerebri was diagnosed.
Index of suspicion is key in correctly diagnosing patients, and understanding the changing landscape of medicine will lead to more thoughtful questioning during routine health exams and better outcomes for your patients.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Am J Ophthalmol. 2015 Feb;159(2):344-52.e1.
2. Horm Res Paediatr. 2014;81(4):217-25.
3. Clin Imaging. 2018 May 24. doi: 10.1016/j.clinimag.2018.05.020.
4. Am J Ophthalmol. 1998 Jul;126(1):116-21.
5. Glob Pediatr Health. 2018. doi:10.1177/2333794X18785550.
Pseudotumor cerebri, benign intracranial hypertension, and idiopathic intracranial hypertension are all terms to describe a syndrome of increased intracranial pressure, headaches, vision loss, or changes without an associated mass lesion.1 The condition was considered relatively rare, presenting most commonly in obese women in childbearing years. Surprisingly, 2
Obesity is the fastest growing morbidity among adolescents. The Centers for Disease Control and Prevention reported 32% of children 2-19 years were obese.1 This reality is impacting many areas of an adolescent’s health, but it also is changing the landscape of diseases that present in this age group. Although pediatric and adult pseudotumor cerebri always have had slightly varied features, many features were similar such as the papilledema, vision loss, headaches, and sixth nerve palsy. Obesity and female predominance tended to present more in the adult population, as many pediatric patients were not obese,2 and had fewer associated symptoms at the time of diagnosis, and the cause was thought to idiopathic.
Now, with the increase in obesity, more adolescents and more male patients are presenting with pseudotumor cerebri as a cause for their headache, and 57%-100% are obese, making it a compounding factor.3
Pediatric populations also are at risk of secondary pseudotumor cerebri, which is an increase in intracranial pressure from the use of medication, or other disease states such as anemia, kidney disease, or Down syndrome. Minocycline use is the most common medication cause and usually presents 1-2 months after normal use.4 Discontinuing the drug does lead to resolution. Retinoids, vitamin A products, growth hormone, and steroids also have been implicated. Given that acne is a common complaint amongst teens, knowledge of these side effects is important.4
In 2013, the criteria for diagnosis of pseudotumor cerebri was revised. Currently, the presence of papilledema, normal neurologic exam except for abnormal sixth cranial nerve, normal cerebral spinal fluid, elevated lumbar opening pressure, and normal imaging are needed for a definitive diagnosis. A probable diagnosis can be made if papilledema is not present but there abducens nerve palsy.2
In a routine physical exam, when I questioned a patient on any medication that was used daily, she replied she took ibuprofen daily for headaches and that she had been doing this for several months. Headaches were not in her chief complaints as she had learned to live with and ignore this symptom. Upon further evaluation, she was slightly overweight and has a questionable fundoscopic exam. After further evaluation by an ophthalmologist and a neurologist, pseudotumor cerebri was diagnosed.
Index of suspicion is key in correctly diagnosing patients, and understanding the changing landscape of medicine will lead to more thoughtful questioning during routine health exams and better outcomes for your patients.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Am J Ophthalmol. 2015 Feb;159(2):344-52.e1.
2. Horm Res Paediatr. 2014;81(4):217-25.
3. Clin Imaging. 2018 May 24. doi: 10.1016/j.clinimag.2018.05.020.
4. Am J Ophthalmol. 1998 Jul;126(1):116-21.
5. Glob Pediatr Health. 2018. doi:10.1177/2333794X18785550.
How to bring behavioral care into your office
There is consensus within both the medical and public health communities that an integrated model of health care, in which behavioral health is integrated into primary care settings, is the optimal way to improve the health of a population (not just treat disease) while managing costs and improving the patient’s experience of care. Such a model is especially compelling for pediatric care.
There are 74 million children under 18 years in the United States and the prevalence of psychiatric disorders in youth is 20%, or 15 million; after vaccinations and following development, managing psychiatric symptoms is the most common issue in pediatric primary care.
While some psychiatric illnesses can be well managed by primary care clinicians alone, some illnesses require specialized therapy or more complex pharmacologic treatment. Untreated or inadequately treated childhood mental illness can lead to a longer and worse course of illness, academic difficulties, emergence of associated illnesses (such as substance use disorders), and legal problems. For those children with chronic medical conditions, emotional disorders cause distress, and affect adherence and family functioning. We will discuss some practical strategies to begin to bring behavioral health care into the pediatric primary care setting.
Start by implementing behavioral health screening into annual and sick visits. Broad instruments, such as the Pediatric Symptom Checklist (PSC, 35 items) or the Child Behavior Check List (CBCL, 113 items) can be filled out by caregivers in the waiting room or online before a visit, and can suggest specific disorders or simply the need for a full psychiatric assessment. Electronic medical records may have publicly available questionnaires such as PSC built into their software, facilitating use of a tablet or home computer, and may ease scoring and downloading of results. Depending on the structure of your practice, you could have one clinician in charge of managing screening. You may become comfortable diagnosing certain disorders, such as ADHD, a major depressive episode, or an anxiety disorder, and you may begin medication treatment when appropriate. You can use instruments developed for specific disease entities (such as ADHD, obsessive compulsive disorder [OCD], anxiety, or depression) to monitor your patient’s treatment response, and they may be done virtually to minimize unnecessary visits.
Treatment algorithms for most psychiatric illnesses are available through the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry, and can guide you through the early stages of treatment. Psychotherapy is the first-line treatment for mild to moderate anxiety and mood disorders, and it is critical to the treatment of more severe disorders. Difficulty in finding a therapist who is skilled in a specific treatment, is a good fit, and accepts insurance can be a significant barrier to care. Establishing a coordinated relationship with a team of therapists can facilitate referrals. Some states have programs in which primary care physicians can have telephone consultations with mental health clinicians or to access referral services for therapy, such as the Massachusetts Child Psychiatry Access Project.
If you have a busy enough practice, consider bringing a social worker or psychologist to work with you. Such a clinician could perform diagnostic assessments, ongoing therapy, parent guidance, family work, or care coordination. Consider how to make it cost-effective for this clinician and your group, whether by inviting that person to sublet one of your offices, or having that person directly employed by you and benefiting from your office staff and patient flow. Many states now reimburse for screening and these funds could contribute to the expense of a social worker. This approach would bring you from coordination to true colocation, which greatly improves the likelihood of compliance with therapy, enhances coordination of a patient’s care, creates opportunities for ongoing education between disciplines, and diminishes stigma of acknowledging a mental illness. Anxiety disorders are the most common illnesses of youth, with mood disorders emerging in adolescence, and substance use disorders in later adolescence. Consider this in seeking a clinician with a specific interest or skill set (such as cognitive behavioral therapy for anxiety or mood problems, dialectical behavior therapy for chronic suicidality, or motivational interviewing for substance abuse).
Beyond diagnosing and treating psychiatric illness in your patients, a primary care pediatric setting with integrated behavioral health would improve the health of our young patients by investing in prevention and parental support. Universal prevention efforts are a hallmark of good pediatric care, from vaccines to educating parents and children about injury prevention (bike helmets, smoke detectors, and car seats) and risky behaviors (smoking). Educate your patients and their parents about best practices to promote good mental health, from good sleep hygiene to regular exercise and healthy stress management techniques. You could use posters and pamphlets, videos and smartphone apps, or screening instruments and discussion.
If you invest in a colocated mental health clinician, you can expand your prevention efforts beyond the universal. Screen for a family history of anxiety, mood, and substance use disorders, and screen for adverse childhood experiences scores. Chronic stress and a family history of specific psychiatric illnesses significantly increase the risk of specific illnesses in your patients. There are evidence-based interventions that can be used to prevent the emergence of many disorders in young people at specific risk. For example, parents who have struggled with anxiety can learn specific strategies for managing their children’s anxiety, significantly lowering the risk of anxiety disorders in their children. These skills can be taught individually or in groups, depending on the prevalence in your practice. Those insurers who reimburse for therapy have a reimbursement schedule for work with parents as well.
There may be funds available to support your investment in integrated care. Under the Affordable Care Act, Medicaid enhanced funding for Health Homes for enrolled children. There have been federal grants for primary care offices to engage in different levels of integration and measure outcomes (Project LAUNCH – Linking Actions for Unmet Needs in Children’s Health). There may be funding at the state level or from private foundations dedicated to public health research and initiatives. Even if you do not invest in procuring outside funding, you should consider how to measure patient outcomes once you are making any efforts at integrating behavioral health care into your practice. Outcome measures include questionnaire scores, treatment adherence, number of school absences, number of office or ED visits, or global measurements, such as the Child Global Assessment Scale (CGAS). Such data can inform you about how to adjust your approach, and could contribute to the larger effort to understand what strategies are most effective and feasible. Addressing the behavioral health needs of your patients could meaningfully contribute to the efforts to make the vision of integrated care – that which truly promotes health in our young people – a reality.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
There is consensus within both the medical and public health communities that an integrated model of health care, in which behavioral health is integrated into primary care settings, is the optimal way to improve the health of a population (not just treat disease) while managing costs and improving the patient’s experience of care. Such a model is especially compelling for pediatric care.
There are 74 million children under 18 years in the United States and the prevalence of psychiatric disorders in youth is 20%, or 15 million; after vaccinations and following development, managing psychiatric symptoms is the most common issue in pediatric primary care.
While some psychiatric illnesses can be well managed by primary care clinicians alone, some illnesses require specialized therapy or more complex pharmacologic treatment. Untreated or inadequately treated childhood mental illness can lead to a longer and worse course of illness, academic difficulties, emergence of associated illnesses (such as substance use disorders), and legal problems. For those children with chronic medical conditions, emotional disorders cause distress, and affect adherence and family functioning. We will discuss some practical strategies to begin to bring behavioral health care into the pediatric primary care setting.
Start by implementing behavioral health screening into annual and sick visits. Broad instruments, such as the Pediatric Symptom Checklist (PSC, 35 items) or the Child Behavior Check List (CBCL, 113 items) can be filled out by caregivers in the waiting room or online before a visit, and can suggest specific disorders or simply the need for a full psychiatric assessment. Electronic medical records may have publicly available questionnaires such as PSC built into their software, facilitating use of a tablet or home computer, and may ease scoring and downloading of results. Depending on the structure of your practice, you could have one clinician in charge of managing screening. You may become comfortable diagnosing certain disorders, such as ADHD, a major depressive episode, or an anxiety disorder, and you may begin medication treatment when appropriate. You can use instruments developed for specific disease entities (such as ADHD, obsessive compulsive disorder [OCD], anxiety, or depression) to monitor your patient’s treatment response, and they may be done virtually to minimize unnecessary visits.
Treatment algorithms for most psychiatric illnesses are available through the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry, and can guide you through the early stages of treatment. Psychotherapy is the first-line treatment for mild to moderate anxiety and mood disorders, and it is critical to the treatment of more severe disorders. Difficulty in finding a therapist who is skilled in a specific treatment, is a good fit, and accepts insurance can be a significant barrier to care. Establishing a coordinated relationship with a team of therapists can facilitate referrals. Some states have programs in which primary care physicians can have telephone consultations with mental health clinicians or to access referral services for therapy, such as the Massachusetts Child Psychiatry Access Project.
If you have a busy enough practice, consider bringing a social worker or psychologist to work with you. Such a clinician could perform diagnostic assessments, ongoing therapy, parent guidance, family work, or care coordination. Consider how to make it cost-effective for this clinician and your group, whether by inviting that person to sublet one of your offices, or having that person directly employed by you and benefiting from your office staff and patient flow. Many states now reimburse for screening and these funds could contribute to the expense of a social worker. This approach would bring you from coordination to true colocation, which greatly improves the likelihood of compliance with therapy, enhances coordination of a patient’s care, creates opportunities for ongoing education between disciplines, and diminishes stigma of acknowledging a mental illness. Anxiety disorders are the most common illnesses of youth, with mood disorders emerging in adolescence, and substance use disorders in later adolescence. Consider this in seeking a clinician with a specific interest or skill set (such as cognitive behavioral therapy for anxiety or mood problems, dialectical behavior therapy for chronic suicidality, or motivational interviewing for substance abuse).
Beyond diagnosing and treating psychiatric illness in your patients, a primary care pediatric setting with integrated behavioral health would improve the health of our young patients by investing in prevention and parental support. Universal prevention efforts are a hallmark of good pediatric care, from vaccines to educating parents and children about injury prevention (bike helmets, smoke detectors, and car seats) and risky behaviors (smoking). Educate your patients and their parents about best practices to promote good mental health, from good sleep hygiene to regular exercise and healthy stress management techniques. You could use posters and pamphlets, videos and smartphone apps, or screening instruments and discussion.
If you invest in a colocated mental health clinician, you can expand your prevention efforts beyond the universal. Screen for a family history of anxiety, mood, and substance use disorders, and screen for adverse childhood experiences scores. Chronic stress and a family history of specific psychiatric illnesses significantly increase the risk of specific illnesses in your patients. There are evidence-based interventions that can be used to prevent the emergence of many disorders in young people at specific risk. For example, parents who have struggled with anxiety can learn specific strategies for managing their children’s anxiety, significantly lowering the risk of anxiety disorders in their children. These skills can be taught individually or in groups, depending on the prevalence in your practice. Those insurers who reimburse for therapy have a reimbursement schedule for work with parents as well.
There may be funds available to support your investment in integrated care. Under the Affordable Care Act, Medicaid enhanced funding for Health Homes for enrolled children. There have been federal grants for primary care offices to engage in different levels of integration and measure outcomes (Project LAUNCH – Linking Actions for Unmet Needs in Children’s Health). There may be funding at the state level or from private foundations dedicated to public health research and initiatives. Even if you do not invest in procuring outside funding, you should consider how to measure patient outcomes once you are making any efforts at integrating behavioral health care into your practice. Outcome measures include questionnaire scores, treatment adherence, number of school absences, number of office or ED visits, or global measurements, such as the Child Global Assessment Scale (CGAS). Such data can inform you about how to adjust your approach, and could contribute to the larger effort to understand what strategies are most effective and feasible. Addressing the behavioral health needs of your patients could meaningfully contribute to the efforts to make the vision of integrated care – that which truly promotes health in our young people – a reality.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
There is consensus within both the medical and public health communities that an integrated model of health care, in which behavioral health is integrated into primary care settings, is the optimal way to improve the health of a population (not just treat disease) while managing costs and improving the patient’s experience of care. Such a model is especially compelling for pediatric care.
There are 74 million children under 18 years in the United States and the prevalence of psychiatric disorders in youth is 20%, or 15 million; after vaccinations and following development, managing psychiatric symptoms is the most common issue in pediatric primary care.
While some psychiatric illnesses can be well managed by primary care clinicians alone, some illnesses require specialized therapy or more complex pharmacologic treatment. Untreated or inadequately treated childhood mental illness can lead to a longer and worse course of illness, academic difficulties, emergence of associated illnesses (such as substance use disorders), and legal problems. For those children with chronic medical conditions, emotional disorders cause distress, and affect adherence and family functioning. We will discuss some practical strategies to begin to bring behavioral health care into the pediatric primary care setting.
Start by implementing behavioral health screening into annual and sick visits. Broad instruments, such as the Pediatric Symptom Checklist (PSC, 35 items) or the Child Behavior Check List (CBCL, 113 items) can be filled out by caregivers in the waiting room or online before a visit, and can suggest specific disorders or simply the need for a full psychiatric assessment. Electronic medical records may have publicly available questionnaires such as PSC built into their software, facilitating use of a tablet or home computer, and may ease scoring and downloading of results. Depending on the structure of your practice, you could have one clinician in charge of managing screening. You may become comfortable diagnosing certain disorders, such as ADHD, a major depressive episode, or an anxiety disorder, and you may begin medication treatment when appropriate. You can use instruments developed for specific disease entities (such as ADHD, obsessive compulsive disorder [OCD], anxiety, or depression) to monitor your patient’s treatment response, and they may be done virtually to minimize unnecessary visits.
Treatment algorithms for most psychiatric illnesses are available through the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry, and can guide you through the early stages of treatment. Psychotherapy is the first-line treatment for mild to moderate anxiety and mood disorders, and it is critical to the treatment of more severe disorders. Difficulty in finding a therapist who is skilled in a specific treatment, is a good fit, and accepts insurance can be a significant barrier to care. Establishing a coordinated relationship with a team of therapists can facilitate referrals. Some states have programs in which primary care physicians can have telephone consultations with mental health clinicians or to access referral services for therapy, such as the Massachusetts Child Psychiatry Access Project.
If you have a busy enough practice, consider bringing a social worker or psychologist to work with you. Such a clinician could perform diagnostic assessments, ongoing therapy, parent guidance, family work, or care coordination. Consider how to make it cost-effective for this clinician and your group, whether by inviting that person to sublet one of your offices, or having that person directly employed by you and benefiting from your office staff and patient flow. Many states now reimburse for screening and these funds could contribute to the expense of a social worker. This approach would bring you from coordination to true colocation, which greatly improves the likelihood of compliance with therapy, enhances coordination of a patient’s care, creates opportunities for ongoing education between disciplines, and diminishes stigma of acknowledging a mental illness. Anxiety disorders are the most common illnesses of youth, with mood disorders emerging in adolescence, and substance use disorders in later adolescence. Consider this in seeking a clinician with a specific interest or skill set (such as cognitive behavioral therapy for anxiety or mood problems, dialectical behavior therapy for chronic suicidality, or motivational interviewing for substance abuse).
Beyond diagnosing and treating psychiatric illness in your patients, a primary care pediatric setting with integrated behavioral health would improve the health of our young patients by investing in prevention and parental support. Universal prevention efforts are a hallmark of good pediatric care, from vaccines to educating parents and children about injury prevention (bike helmets, smoke detectors, and car seats) and risky behaviors (smoking). Educate your patients and their parents about best practices to promote good mental health, from good sleep hygiene to regular exercise and healthy stress management techniques. You could use posters and pamphlets, videos and smartphone apps, or screening instruments and discussion.
If you invest in a colocated mental health clinician, you can expand your prevention efforts beyond the universal. Screen for a family history of anxiety, mood, and substance use disorders, and screen for adverse childhood experiences scores. Chronic stress and a family history of specific psychiatric illnesses significantly increase the risk of specific illnesses in your patients. There are evidence-based interventions that can be used to prevent the emergence of many disorders in young people at specific risk. For example, parents who have struggled with anxiety can learn specific strategies for managing their children’s anxiety, significantly lowering the risk of anxiety disorders in their children. These skills can be taught individually or in groups, depending on the prevalence in your practice. Those insurers who reimburse for therapy have a reimbursement schedule for work with parents as well.
There may be funds available to support your investment in integrated care. Under the Affordable Care Act, Medicaid enhanced funding for Health Homes for enrolled children. There have been federal grants for primary care offices to engage in different levels of integration and measure outcomes (Project LAUNCH – Linking Actions for Unmet Needs in Children’s Health). There may be funding at the state level or from private foundations dedicated to public health research and initiatives. Even if you do not invest in procuring outside funding, you should consider how to measure patient outcomes once you are making any efforts at integrating behavioral health care into your practice. Outcome measures include questionnaire scores, treatment adherence, number of school absences, number of office or ED visits, or global measurements, such as the Child Global Assessment Scale (CGAS). Such data can inform you about how to adjust your approach, and could contribute to the larger effort to understand what strategies are most effective and feasible. Addressing the behavioral health needs of your patients could meaningfully contribute to the efforts to make the vision of integrated care – that which truly promotes health in our young people – a reality.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
The diagnosis and surgical repair of vesicovaginal fistula
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.