User login
Clock Watchers
The following scenario was discussed during a forum at a meeting recently:
Two employees managing the front desk are clock watchers, always the first to leave at 11:59 a.m. for lunch and at 4:59 p.m. for the end of the day no matter what is happening. This leaves the other employees stuck with their work.
I have seen clock watching often enough to know that it is widely practiced, and widely reviled by coworkers and managers alike. Generally, clock watchers — sometimes referred to in modern parlance as “quiet quitters” — radiate a palpable sense of “I don’t want to be here.”
; if that involves working past the usual “quitting time,” so be it. So your first task in dealing with this problem is to determine its cause. The clock watcher label may be unfair. There may be legitimate reasons for certain employees to leave work at precisely 4:59 every day. Perhaps they must pick up children, or they have a second job to get to. The label usually comes from a pattern of consistent, repeated behavior. And if more than one employee is exhibiting the same behavior in the same office, the likelihood of a valid explanation decreases proportionally.
A common cause of clock watching is a lack of employees’ commitment to their jobs. They don’t see the point in putting in extra effort, so they run out the door as soon as possible. There are many reasons why this might be the case. For example, the workload in your office may be too large to be accomplished in the time available by the number of people you employ. The solution might be to simply hire additional personnel.
Another common cause is a lack of communication between physicians, managers, and lower-level employees. If staffers are raising concerns or potential solutions, and management is not listening to their opinions or ideas, they will stop offering them. Alternatively, other staff members may not be pulling their weight. When there is a large imbalance in the contribution of team members, the higher performers will stop trying.
Over my 40 plus years in practice, I have had my share of clock watchers. I try the best I can not to let employees’ time commitment practices impact my valuation of their work. I always attempt to focus on quality and productivity. It isn’t easy, but I always try to address the issues behind clock watching behavior. As such, I can’t recall ever having to fire anyone for clock watching. Here are some of the strategies that have worked for me over the years:
1. Set clear expectations. Clearly communicate job responsibilities and expectations regarding time management and patient care. Ensure that all staff understand the importance of dedicating the necessary time to each patient, regardless of the time of day.
2. Foster a patient-centered culture. Cultivate a work environment that prioritizes patient care above all. This can help shift the focus from watching the clock to ensuring high-quality patient care.
3. Provide adequate breaks. Ensure that staff schedules include sufficient breaks. Overworked staff are more likely to watch the clock. Adequate rest periods can help alleviate this issue.
4. Offer flexibility where possible. If feasible, offer some degree of scheduling flexibility. This can help staff manage their personal time more effectively, potentially reducing the tendency to watch the clock.
5. Implement time management training. Offer training sessions focused on time management and efficiency. This can help staff manage their duties more effectively, reducing the need to constantly check the time.
6. Encourage open communication. Create an environment where staff feel comfortable discussing their concerns, including issues related to workload and time management. This can help identify and address specific factors contributing to clock watching.
7. Monitor and provide feedback. Regularly monitor staff performance and provide constructive feedback. If clock watching is observed, discuss it directly with the employee, focusing on the impact on patient care and the work environment.
8. Recognize and reward. Acknowledge and reward staff who consistently provide high-quality care and demonstrate effective time management. Recognition can motivate others to adjust their behavior.
9. Evaluate workloads. Regularly assess staff workloads to ensure they are manageable. Overburdened employees are more likely to engage in clock watching.
10. Lead by example. Management should model the behavior they wish to see in their staff. Demonstrating a commitment to patient care and effective time management can set a positive example.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The following scenario was discussed during a forum at a meeting recently:
Two employees managing the front desk are clock watchers, always the first to leave at 11:59 a.m. for lunch and at 4:59 p.m. for the end of the day no matter what is happening. This leaves the other employees stuck with their work.
I have seen clock watching often enough to know that it is widely practiced, and widely reviled by coworkers and managers alike. Generally, clock watchers — sometimes referred to in modern parlance as “quiet quitters” — radiate a palpable sense of “I don’t want to be here.”
; if that involves working past the usual “quitting time,” so be it. So your first task in dealing with this problem is to determine its cause. The clock watcher label may be unfair. There may be legitimate reasons for certain employees to leave work at precisely 4:59 every day. Perhaps they must pick up children, or they have a second job to get to. The label usually comes from a pattern of consistent, repeated behavior. And if more than one employee is exhibiting the same behavior in the same office, the likelihood of a valid explanation decreases proportionally.
A common cause of clock watching is a lack of employees’ commitment to their jobs. They don’t see the point in putting in extra effort, so they run out the door as soon as possible. There are many reasons why this might be the case. For example, the workload in your office may be too large to be accomplished in the time available by the number of people you employ. The solution might be to simply hire additional personnel.
Another common cause is a lack of communication between physicians, managers, and lower-level employees. If staffers are raising concerns or potential solutions, and management is not listening to their opinions or ideas, they will stop offering them. Alternatively, other staff members may not be pulling their weight. When there is a large imbalance in the contribution of team members, the higher performers will stop trying.
Over my 40 plus years in practice, I have had my share of clock watchers. I try the best I can not to let employees’ time commitment practices impact my valuation of their work. I always attempt to focus on quality and productivity. It isn’t easy, but I always try to address the issues behind clock watching behavior. As such, I can’t recall ever having to fire anyone for clock watching. Here are some of the strategies that have worked for me over the years:
1. Set clear expectations. Clearly communicate job responsibilities and expectations regarding time management and patient care. Ensure that all staff understand the importance of dedicating the necessary time to each patient, regardless of the time of day.
2. Foster a patient-centered culture. Cultivate a work environment that prioritizes patient care above all. This can help shift the focus from watching the clock to ensuring high-quality patient care.
3. Provide adequate breaks. Ensure that staff schedules include sufficient breaks. Overworked staff are more likely to watch the clock. Adequate rest periods can help alleviate this issue.
4. Offer flexibility where possible. If feasible, offer some degree of scheduling flexibility. This can help staff manage their personal time more effectively, potentially reducing the tendency to watch the clock.
5. Implement time management training. Offer training sessions focused on time management and efficiency. This can help staff manage their duties more effectively, reducing the need to constantly check the time.
6. Encourage open communication. Create an environment where staff feel comfortable discussing their concerns, including issues related to workload and time management. This can help identify and address specific factors contributing to clock watching.
7. Monitor and provide feedback. Regularly monitor staff performance and provide constructive feedback. If clock watching is observed, discuss it directly with the employee, focusing on the impact on patient care and the work environment.
8. Recognize and reward. Acknowledge and reward staff who consistently provide high-quality care and demonstrate effective time management. Recognition can motivate others to adjust their behavior.
9. Evaluate workloads. Regularly assess staff workloads to ensure they are manageable. Overburdened employees are more likely to engage in clock watching.
10. Lead by example. Management should model the behavior they wish to see in their staff. Demonstrating a commitment to patient care and effective time management can set a positive example.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The following scenario was discussed during a forum at a meeting recently:
Two employees managing the front desk are clock watchers, always the first to leave at 11:59 a.m. for lunch and at 4:59 p.m. for the end of the day no matter what is happening. This leaves the other employees stuck with their work.
I have seen clock watching often enough to know that it is widely practiced, and widely reviled by coworkers and managers alike. Generally, clock watchers — sometimes referred to in modern parlance as “quiet quitters” — radiate a palpable sense of “I don’t want to be here.”
; if that involves working past the usual “quitting time,” so be it. So your first task in dealing with this problem is to determine its cause. The clock watcher label may be unfair. There may be legitimate reasons for certain employees to leave work at precisely 4:59 every day. Perhaps they must pick up children, or they have a second job to get to. The label usually comes from a pattern of consistent, repeated behavior. And if more than one employee is exhibiting the same behavior in the same office, the likelihood of a valid explanation decreases proportionally.
A common cause of clock watching is a lack of employees’ commitment to their jobs. They don’t see the point in putting in extra effort, so they run out the door as soon as possible. There are many reasons why this might be the case. For example, the workload in your office may be too large to be accomplished in the time available by the number of people you employ. The solution might be to simply hire additional personnel.
Another common cause is a lack of communication between physicians, managers, and lower-level employees. If staffers are raising concerns or potential solutions, and management is not listening to their opinions or ideas, they will stop offering them. Alternatively, other staff members may not be pulling their weight. When there is a large imbalance in the contribution of team members, the higher performers will stop trying.
Over my 40 plus years in practice, I have had my share of clock watchers. I try the best I can not to let employees’ time commitment practices impact my valuation of their work. I always attempt to focus on quality and productivity. It isn’t easy, but I always try to address the issues behind clock watching behavior. As such, I can’t recall ever having to fire anyone for clock watching. Here are some of the strategies that have worked for me over the years:
1. Set clear expectations. Clearly communicate job responsibilities and expectations regarding time management and patient care. Ensure that all staff understand the importance of dedicating the necessary time to each patient, regardless of the time of day.
2. Foster a patient-centered culture. Cultivate a work environment that prioritizes patient care above all. This can help shift the focus from watching the clock to ensuring high-quality patient care.
3. Provide adequate breaks. Ensure that staff schedules include sufficient breaks. Overworked staff are more likely to watch the clock. Adequate rest periods can help alleviate this issue.
4. Offer flexibility where possible. If feasible, offer some degree of scheduling flexibility. This can help staff manage their personal time more effectively, potentially reducing the tendency to watch the clock.
5. Implement time management training. Offer training sessions focused on time management and efficiency. This can help staff manage their duties more effectively, reducing the need to constantly check the time.
6. Encourage open communication. Create an environment where staff feel comfortable discussing their concerns, including issues related to workload and time management. This can help identify and address specific factors contributing to clock watching.
7. Monitor and provide feedback. Regularly monitor staff performance and provide constructive feedback. If clock watching is observed, discuss it directly with the employee, focusing on the impact on patient care and the work environment.
8. Recognize and reward. Acknowledge and reward staff who consistently provide high-quality care and demonstrate effective time management. Recognition can motivate others to adjust their behavior.
9. Evaluate workloads. Regularly assess staff workloads to ensure they are manageable. Overburdened employees are more likely to engage in clock watching.
10. Lead by example. Management should model the behavior they wish to see in their staff. Demonstrating a commitment to patient care and effective time management can set a positive example.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Is It Possible to Reverse Osteoporosis?
Fractures, particularly hip and spine fractures, are a major cause of mortality and morbidity among older individuals. The term “osteoporosis” indicates increased porosity of bones resulting in low bone density; increased bone fragility; and an increased risk for fracture, often with minimal trauma.
During the adolescent years, bone accrues at a rapid rate, and optimal bone accrual during this time is essential to attain optimal peak bone mass, typically achieved in the third decade of life. Bone mass then stays stable until the 40s-50s, after which it starts to decline. One’s peak bone mass sets the stage for both immediate and future bone health. Individuals with lower peak bone mass tend to have less optimal bone health throughout their lives, and this becomes particularly problematic in older men and in the postmenopausal years for women.
One’s genes have a major impact on bone density and are currently not modifiable.
Modifiable factors include mechanical loading of bones through exercise activity, maintaining a normal body weight, and ensuring adequate intake of micronutrients (including calcium and vitamin D) and macronutrients. Medications such as glucocorticoids that have deleterious effects on bones should be limited as far as possible. Endocrine, gastrointestinal, renal, and rheumatologic conditions and others, such as cancer, which are known to be associated with reduced bone density and increased fracture risk, should be managed appropriately.
A deficiency of the gonadal hormones (estrogen and testosterone) and high blood concentrations of cortisol are particularly deleterious to bone. Hormone replacement therapy in those with gonadal hormone deficiency and strategies to reduce cortisol levels in those with hypercortisolemia are essential to prevent osteoporosis and also improve bone density over time. The same applies to management of conditions such as anorexia nervosa, relative energy deficiency in sports, inflammatory bowel disease, celiac disease, cystic fibrosis, chronic kidney disease, and chronic arthritis.
Once osteoporosis has developed, depending on the cause, these strategies may not be sufficient to completely reverse the condition, and pharmacologic therapy may be necessary to improve bone density and reduce fracture risk. This is particularly an issue with postmenopausal women and older men. In these individuals, medications that increase bone formation or reduce bone loss may be necessary.
Medications that reduce bone loss include bisphosphonates and denosumab; these are also called “antiresorptive medications” because they reduce bone resorption by cells called osteoclasts. Bisphosphonates include alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid, and these medications have direct effects on osteoclasts, reducing their activity. Some bisphosphonates, such as alendronate and risedronate, are taken orally (daily, weekly, or monthly, depending on the medication and its strength), whereas others, such as pamidronate and zoledronic acid, are administered intravenously: every 3-4 months for pamidronate and every 6-12 months for zoledronic acid. Ibandronate is available both orally and intravenously.
Denosumab is a medication that inhibits the action of receptor activator of nuclear factor-kappa ligand 1 (RANKL), which otherwise increases osteoclast activity. It is administered as a subcutaneous injection every 6 months to treat osteoporosis. One concern with denosumab is a rapid increase in bone loss after its discontinuation.
Medications that increase bone formation are called bone anabolics and include teriparatide, abaloparatide, and romosozumab. Teriparatide is a synthetic form of parathyroid hormone (recombinant PTH1-34) administered daily for up to 2 years. Abaloparatide is a synthetic analog of parathyroid hormone–related peptide (PTHrP), which is also administered daily as a subcutaneous injection. Romosozumab inhibits sclerostin (a substance that otherwise reduces bone formation and increases bone resorption) and is administered as a subcutaneous injection once a month. Effects of these medications tend to be lost after they are discontinued.
In 2019, the Endocrine Society published guidelines for managing postmenopausal osteoporosis. The guidelines recommend lifestyle modifications, including attention to diet, calcium and vitamin D supplements, and weight-bearing exercise for all postmenopausal women. They also recommend assessing fracture risk using country-specific existing models.
Guidelines vary depending on whether fracture risk is low, moderate, or high. Patients at low risk are followed and reassessed every 2-4 years for fracture risk. Those at moderate risk may be followed similarly or prescribed bisphosphonates. Those at high risk are prescribed an antiresorptive, such as a bisphosphonate or denosumab, or a bone anabolic, such as teriparatide or abaloparatide (for up to 2 years) or romosozumab (for a year), with calcium and vitamin D and are reassessed at defined intervals for fracture risk; subsequent management then depends on the assessed fracture risk.
People who are on a bone anabolic should typically follow this with an antiresorptive medication to maintain the gains achieved with the former after that medication is discontinued. Patients who discontinue denosumab should be switched to bisphosphonates to prevent the increase in bone loss that typically occurs.
In postmenopausal women who are intolerant to or inappropriate for use of these medications, guidelines vary depending on age (younger or older than 60 years) and presence or absence of vasomotor symptoms (such as hot flashes). Options could include the use of calcium and vitamin D supplements; hormone replacement therapy with estrogen with or without a progestin; or selective estrogen receptor modulators (such as raloxifene or bazedoxifene), tibolone, or calcitonin.
It’s important to recognize that all pharmacologic therapy carries the risk for adverse events, and it’s essential to take the necessary steps to prevent, monitor for, and manage any adverse effects that may develop.
Managing osteoporosis in older men could include the use of bone anabolics and/or antiresorptives. In younger individuals, use of pharmacologic therapy is less common but sometimes necessary, particularly when bone density is very low and associated with a problematic fracture history — for example, in those with genetic conditions such as osteogenesis imperfecta. Furthermore, the occurrence of vertebral compression fractures often requires bisphosphonate treatment regardless of bone density, particularly in patients on chronic glucocorticoid therapy.
Preventing osteoporosis is best managed by paying attention to lifestyle; optimizing nutrition and calcium and vitamin D intake; and managing conditions and limiting the use of medications that reduce bone density.
However, in certain patients, these measures are not enough, and pharmacologic therapy with bone anabolics or antiresorptives may be necessary to improve bone density and reduce fracture risk.
Dr. Misra, of the University of Virginia and UVA Health Children’s Hospital, Charlottesville, disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Fractures, particularly hip and spine fractures, are a major cause of mortality and morbidity among older individuals. The term “osteoporosis” indicates increased porosity of bones resulting in low bone density; increased bone fragility; and an increased risk for fracture, often with minimal trauma.
During the adolescent years, bone accrues at a rapid rate, and optimal bone accrual during this time is essential to attain optimal peak bone mass, typically achieved in the third decade of life. Bone mass then stays stable until the 40s-50s, after which it starts to decline. One’s peak bone mass sets the stage for both immediate and future bone health. Individuals with lower peak bone mass tend to have less optimal bone health throughout their lives, and this becomes particularly problematic in older men and in the postmenopausal years for women.
One’s genes have a major impact on bone density and are currently not modifiable.
Modifiable factors include mechanical loading of bones through exercise activity, maintaining a normal body weight, and ensuring adequate intake of micronutrients (including calcium and vitamin D) and macronutrients. Medications such as glucocorticoids that have deleterious effects on bones should be limited as far as possible. Endocrine, gastrointestinal, renal, and rheumatologic conditions and others, such as cancer, which are known to be associated with reduced bone density and increased fracture risk, should be managed appropriately.
A deficiency of the gonadal hormones (estrogen and testosterone) and high blood concentrations of cortisol are particularly deleterious to bone. Hormone replacement therapy in those with gonadal hormone deficiency and strategies to reduce cortisol levels in those with hypercortisolemia are essential to prevent osteoporosis and also improve bone density over time. The same applies to management of conditions such as anorexia nervosa, relative energy deficiency in sports, inflammatory bowel disease, celiac disease, cystic fibrosis, chronic kidney disease, and chronic arthritis.
Once osteoporosis has developed, depending on the cause, these strategies may not be sufficient to completely reverse the condition, and pharmacologic therapy may be necessary to improve bone density and reduce fracture risk. This is particularly an issue with postmenopausal women and older men. In these individuals, medications that increase bone formation or reduce bone loss may be necessary.
Medications that reduce bone loss include bisphosphonates and denosumab; these are also called “antiresorptive medications” because they reduce bone resorption by cells called osteoclasts. Bisphosphonates include alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid, and these medications have direct effects on osteoclasts, reducing their activity. Some bisphosphonates, such as alendronate and risedronate, are taken orally (daily, weekly, or monthly, depending on the medication and its strength), whereas others, such as pamidronate and zoledronic acid, are administered intravenously: every 3-4 months for pamidronate and every 6-12 months for zoledronic acid. Ibandronate is available both orally and intravenously.
Denosumab is a medication that inhibits the action of receptor activator of nuclear factor-kappa ligand 1 (RANKL), which otherwise increases osteoclast activity. It is administered as a subcutaneous injection every 6 months to treat osteoporosis. One concern with denosumab is a rapid increase in bone loss after its discontinuation.
Medications that increase bone formation are called bone anabolics and include teriparatide, abaloparatide, and romosozumab. Teriparatide is a synthetic form of parathyroid hormone (recombinant PTH1-34) administered daily for up to 2 years. Abaloparatide is a synthetic analog of parathyroid hormone–related peptide (PTHrP), which is also administered daily as a subcutaneous injection. Romosozumab inhibits sclerostin (a substance that otherwise reduces bone formation and increases bone resorption) and is administered as a subcutaneous injection once a month. Effects of these medications tend to be lost after they are discontinued.
In 2019, the Endocrine Society published guidelines for managing postmenopausal osteoporosis. The guidelines recommend lifestyle modifications, including attention to diet, calcium and vitamin D supplements, and weight-bearing exercise for all postmenopausal women. They also recommend assessing fracture risk using country-specific existing models.
Guidelines vary depending on whether fracture risk is low, moderate, or high. Patients at low risk are followed and reassessed every 2-4 years for fracture risk. Those at moderate risk may be followed similarly or prescribed bisphosphonates. Those at high risk are prescribed an antiresorptive, such as a bisphosphonate or denosumab, or a bone anabolic, such as teriparatide or abaloparatide (for up to 2 years) or romosozumab (for a year), with calcium and vitamin D and are reassessed at defined intervals for fracture risk; subsequent management then depends on the assessed fracture risk.
People who are on a bone anabolic should typically follow this with an antiresorptive medication to maintain the gains achieved with the former after that medication is discontinued. Patients who discontinue denosumab should be switched to bisphosphonates to prevent the increase in bone loss that typically occurs.
In postmenopausal women who are intolerant to or inappropriate for use of these medications, guidelines vary depending on age (younger or older than 60 years) and presence or absence of vasomotor symptoms (such as hot flashes). Options could include the use of calcium and vitamin D supplements; hormone replacement therapy with estrogen with or without a progestin; or selective estrogen receptor modulators (such as raloxifene or bazedoxifene), tibolone, or calcitonin.
It’s important to recognize that all pharmacologic therapy carries the risk for adverse events, and it’s essential to take the necessary steps to prevent, monitor for, and manage any adverse effects that may develop.
Managing osteoporosis in older men could include the use of bone anabolics and/or antiresorptives. In younger individuals, use of pharmacologic therapy is less common but sometimes necessary, particularly when bone density is very low and associated with a problematic fracture history — for example, in those with genetic conditions such as osteogenesis imperfecta. Furthermore, the occurrence of vertebral compression fractures often requires bisphosphonate treatment regardless of bone density, particularly in patients on chronic glucocorticoid therapy.
Preventing osteoporosis is best managed by paying attention to lifestyle; optimizing nutrition and calcium and vitamin D intake; and managing conditions and limiting the use of medications that reduce bone density.
However, in certain patients, these measures are not enough, and pharmacologic therapy with bone anabolics or antiresorptives may be necessary to improve bone density and reduce fracture risk.
Dr. Misra, of the University of Virginia and UVA Health Children’s Hospital, Charlottesville, disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Fractures, particularly hip and spine fractures, are a major cause of mortality and morbidity among older individuals. The term “osteoporosis” indicates increased porosity of bones resulting in low bone density; increased bone fragility; and an increased risk for fracture, often with minimal trauma.
During the adolescent years, bone accrues at a rapid rate, and optimal bone accrual during this time is essential to attain optimal peak bone mass, typically achieved in the third decade of life. Bone mass then stays stable until the 40s-50s, after which it starts to decline. One’s peak bone mass sets the stage for both immediate and future bone health. Individuals with lower peak bone mass tend to have less optimal bone health throughout their lives, and this becomes particularly problematic in older men and in the postmenopausal years for women.
One’s genes have a major impact on bone density and are currently not modifiable.
Modifiable factors include mechanical loading of bones through exercise activity, maintaining a normal body weight, and ensuring adequate intake of micronutrients (including calcium and vitamin D) and macronutrients. Medications such as glucocorticoids that have deleterious effects on bones should be limited as far as possible. Endocrine, gastrointestinal, renal, and rheumatologic conditions and others, such as cancer, which are known to be associated with reduced bone density and increased fracture risk, should be managed appropriately.
A deficiency of the gonadal hormones (estrogen and testosterone) and high blood concentrations of cortisol are particularly deleterious to bone. Hormone replacement therapy in those with gonadal hormone deficiency and strategies to reduce cortisol levels in those with hypercortisolemia are essential to prevent osteoporosis and also improve bone density over time. The same applies to management of conditions such as anorexia nervosa, relative energy deficiency in sports, inflammatory bowel disease, celiac disease, cystic fibrosis, chronic kidney disease, and chronic arthritis.
Once osteoporosis has developed, depending on the cause, these strategies may not be sufficient to completely reverse the condition, and pharmacologic therapy may be necessary to improve bone density and reduce fracture risk. This is particularly an issue with postmenopausal women and older men. In these individuals, medications that increase bone formation or reduce bone loss may be necessary.
Medications that reduce bone loss include bisphosphonates and denosumab; these are also called “antiresorptive medications” because they reduce bone resorption by cells called osteoclasts. Bisphosphonates include alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid, and these medications have direct effects on osteoclasts, reducing their activity. Some bisphosphonates, such as alendronate and risedronate, are taken orally (daily, weekly, or monthly, depending on the medication and its strength), whereas others, such as pamidronate and zoledronic acid, are administered intravenously: every 3-4 months for pamidronate and every 6-12 months for zoledronic acid. Ibandronate is available both orally and intravenously.
Denosumab is a medication that inhibits the action of receptor activator of nuclear factor-kappa ligand 1 (RANKL), which otherwise increases osteoclast activity. It is administered as a subcutaneous injection every 6 months to treat osteoporosis. One concern with denosumab is a rapid increase in bone loss after its discontinuation.
Medications that increase bone formation are called bone anabolics and include teriparatide, abaloparatide, and romosozumab. Teriparatide is a synthetic form of parathyroid hormone (recombinant PTH1-34) administered daily for up to 2 years. Abaloparatide is a synthetic analog of parathyroid hormone–related peptide (PTHrP), which is also administered daily as a subcutaneous injection. Romosozumab inhibits sclerostin (a substance that otherwise reduces bone formation and increases bone resorption) and is administered as a subcutaneous injection once a month. Effects of these medications tend to be lost after they are discontinued.
In 2019, the Endocrine Society published guidelines for managing postmenopausal osteoporosis. The guidelines recommend lifestyle modifications, including attention to diet, calcium and vitamin D supplements, and weight-bearing exercise for all postmenopausal women. They also recommend assessing fracture risk using country-specific existing models.
Guidelines vary depending on whether fracture risk is low, moderate, or high. Patients at low risk are followed and reassessed every 2-4 years for fracture risk. Those at moderate risk may be followed similarly or prescribed bisphosphonates. Those at high risk are prescribed an antiresorptive, such as a bisphosphonate or denosumab, or a bone anabolic, such as teriparatide or abaloparatide (for up to 2 years) or romosozumab (for a year), with calcium and vitamin D and are reassessed at defined intervals for fracture risk; subsequent management then depends on the assessed fracture risk.
People who are on a bone anabolic should typically follow this with an antiresorptive medication to maintain the gains achieved with the former after that medication is discontinued. Patients who discontinue denosumab should be switched to bisphosphonates to prevent the increase in bone loss that typically occurs.
In postmenopausal women who are intolerant to or inappropriate for use of these medications, guidelines vary depending on age (younger or older than 60 years) and presence or absence of vasomotor symptoms (such as hot flashes). Options could include the use of calcium and vitamin D supplements; hormone replacement therapy with estrogen with or without a progestin; or selective estrogen receptor modulators (such as raloxifene or bazedoxifene), tibolone, or calcitonin.
It’s important to recognize that all pharmacologic therapy carries the risk for adverse events, and it’s essential to take the necessary steps to prevent, monitor for, and manage any adverse effects that may develop.
Managing osteoporosis in older men could include the use of bone anabolics and/or antiresorptives. In younger individuals, use of pharmacologic therapy is less common but sometimes necessary, particularly when bone density is very low and associated with a problematic fracture history — for example, in those with genetic conditions such as osteogenesis imperfecta. Furthermore, the occurrence of vertebral compression fractures often requires bisphosphonate treatment regardless of bone density, particularly in patients on chronic glucocorticoid therapy.
Preventing osteoporosis is best managed by paying attention to lifestyle; optimizing nutrition and calcium and vitamin D intake; and managing conditions and limiting the use of medications that reduce bone density.
However, in certain patients, these measures are not enough, and pharmacologic therapy with bone anabolics or antiresorptives may be necessary to improve bone density and reduce fracture risk.
Dr. Misra, of the University of Virginia and UVA Health Children’s Hospital, Charlottesville, disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
When the Next Big Thing Falls Short
Recently, Acadia Pharmaceuticals announced it was stopping trials on Nuplazid for indications outside of Parkinson’s disease psychosis.
I was impressed with what I saw in my office. Although I know there’s some controversy over the drug, the majority of studies do show efficacy, and in my little practice I clearly noticed improvements in patients with Parkinson’s disease who’d previously failed the more standard agents (note - I have no financial affiliation with Acadia Pharmaceuticals).
So, as a lay-neurologist, I expected the drug to work for other kinds of psychosis, particularly Alzheimer’s disease. All of us in practice know how much we need new options for that.
But when the clinical trials came, the drug didn’t work. It didn’t work for schizophrenia, either, Finally, Acadia threw in the towel and gave up.
I have no idea what happened. I’m sure others are wondering the same thing. On paper, I’d have thought it would work for Alzheimer’s psychosis, but in the real world it didn’t.
Is psychosis between the two disorders that different, with different neurotransmitter causes? Are the benefits in my patients with Parkinson’s disease really just from my own selection bias? Or is there just a lot we still don’t know?
Look at the graveyard full of amyloid-targeting drugs. Yeah, I know Leqembi is out there, and donanemab is in the wings, but are they anywhere near as good as we thought they’d be? Not at all.
At the same time, we’ve been waiting for the BTK drugs (not to be confused with a Korean pop band) for multiple sclerosis. They sounded like they were the Next Big Thing.
They may be, but recent data on one of them, evobrutinib, was less than encouraging. Of course, that shouldn’t extrapolate to the group as a whole, but it does leave you wondering why.
Medicine is always improving, but it’s also still a trial-and-error process. Just because something should work doesn’t mean it will, and it may be years before we know why.
It’s just a reminder that, here in 2024, we still have a lot to learn.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, Acadia Pharmaceuticals announced it was stopping trials on Nuplazid for indications outside of Parkinson’s disease psychosis.
I was impressed with what I saw in my office. Although I know there’s some controversy over the drug, the majority of studies do show efficacy, and in my little practice I clearly noticed improvements in patients with Parkinson’s disease who’d previously failed the more standard agents (note - I have no financial affiliation with Acadia Pharmaceuticals).
So, as a lay-neurologist, I expected the drug to work for other kinds of psychosis, particularly Alzheimer’s disease. All of us in practice know how much we need new options for that.
But when the clinical trials came, the drug didn’t work. It didn’t work for schizophrenia, either, Finally, Acadia threw in the towel and gave up.
I have no idea what happened. I’m sure others are wondering the same thing. On paper, I’d have thought it would work for Alzheimer’s psychosis, but in the real world it didn’t.
Is psychosis between the two disorders that different, with different neurotransmitter causes? Are the benefits in my patients with Parkinson’s disease really just from my own selection bias? Or is there just a lot we still don’t know?
Look at the graveyard full of amyloid-targeting drugs. Yeah, I know Leqembi is out there, and donanemab is in the wings, but are they anywhere near as good as we thought they’d be? Not at all.
At the same time, we’ve been waiting for the BTK drugs (not to be confused with a Korean pop band) for multiple sclerosis. They sounded like they were the Next Big Thing.
They may be, but recent data on one of them, evobrutinib, was less than encouraging. Of course, that shouldn’t extrapolate to the group as a whole, but it does leave you wondering why.
Medicine is always improving, but it’s also still a trial-and-error process. Just because something should work doesn’t mean it will, and it may be years before we know why.
It’s just a reminder that, here in 2024, we still have a lot to learn.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, Acadia Pharmaceuticals announced it was stopping trials on Nuplazid for indications outside of Parkinson’s disease psychosis.
I was impressed with what I saw in my office. Although I know there’s some controversy over the drug, the majority of studies do show efficacy, and in my little practice I clearly noticed improvements in patients with Parkinson’s disease who’d previously failed the more standard agents (note - I have no financial affiliation with Acadia Pharmaceuticals).
So, as a lay-neurologist, I expected the drug to work for other kinds of psychosis, particularly Alzheimer’s disease. All of us in practice know how much we need new options for that.
But when the clinical trials came, the drug didn’t work. It didn’t work for schizophrenia, either, Finally, Acadia threw in the towel and gave up.
I have no idea what happened. I’m sure others are wondering the same thing. On paper, I’d have thought it would work for Alzheimer’s psychosis, but in the real world it didn’t.
Is psychosis between the two disorders that different, with different neurotransmitter causes? Are the benefits in my patients with Parkinson’s disease really just from my own selection bias? Or is there just a lot we still don’t know?
Look at the graveyard full of amyloid-targeting drugs. Yeah, I know Leqembi is out there, and donanemab is in the wings, but are they anywhere near as good as we thought they’d be? Not at all.
At the same time, we’ve been waiting for the BTK drugs (not to be confused with a Korean pop band) for multiple sclerosis. They sounded like they were the Next Big Thing.
They may be, but recent data on one of them, evobrutinib, was less than encouraging. Of course, that shouldn’t extrapolate to the group as a whole, but it does leave you wondering why.
Medicine is always improving, but it’s also still a trial-and-error process. Just because something should work doesn’t mean it will, and it may be years before we know why.
It’s just a reminder that, here in 2024, we still have a lot to learn.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Paid Parental Leave: Impact on Maternal Mental Health and Child Wellbeing
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
ERISA Health Plan Lawsuits: Why Should We Care?
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
A 35-year-old female presented with a 1-day history of eroded papules and vesicles distributed periorally
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
Rosemary, Part 2
Used as a spice in various, particularly Mediterranean, cuisines and in traditional medicine for hundreds of years, this aromatic shrub has been the focus of substantial research this century to clarify its roles in skin care. It is used broadly in cosmetic formulations, particularly to preserve the product, and acts as a skin conditioner and fragrance in safe concentrations.1 Rosemary essential oil is also a popular choice frequently used in aromatherapy.2,3 This column focuses on recent promising results supporting the antioxidant and anti-photoaging activities, especially, of rosemary.
UV Protection and Rosemary in Combination
A 2021 study in mice authored by Auh and Madhavan showed that a mixture of marigold and rosemary extracts yielded anti-photoaging effects, with the botanical formula suppressing UV-induced damage.4
Seven years earlier, Pérez-Sánchez et al. combined rosemary and citrus extracts and found that they exerted protective effects against UV damage in human HaCaT keratinocytes as well as human volunteers after oral consumption. Significant increases in minimal erythema dose (MED) were seen in participants, with daily intake of 250 mg of botanical combination, at 8 weeks (34%) and 12 weeks (56%). The investigators attributed the photoprotective effects of the formula to rosemary polyphenols and diterpenes as well as citrus flavonoids.5
Evaluation of a human skin cell model by Sánchez-Marzo et al. in 2020 revealed that rosemary diterpenes were instrumental in an herbal extract that combined citrus, olive, and rosemary in conferring genoprotection against UV-induced DNA damage. The authors note that human trials are needed to overcome the limitations of the cellular model in ascertaining whether the tested herbal formulations can yield oral and/or topical photoprotection.6
Anti-Photoaging and Anti-Pollution
In 2022, Ibrahim et al. assessed a hexane extract of rosemary leaves for anti-photoaging activity. Their evaluation showed an abundance of triterpenoids, monoterpenoids, and phenolic diterpenes in rosemary, with in vitro assays verifying the anti-aging, antioxidant, and wound healing functions of the extract. Further, topical rosemary hexane extract–loaded lipid nanocapsules protected rat skin from UV radiation, as epidermal and dermal histological parameters improved, antioxidant biochemical balance was restored, and inflammatory markers and wrinkling were diminished. The researchers concluded that the use of rosemary hexane extract represents a safe, efficient, and cost-effective way to deliver anti-aging, photoprotective functions to cosmeceutical formulations.7
In March 2021, Nobile et al. published a report on their randomized, double-blind, placebo-controlled parallel group study to investigate the efficacy of a marketed polyphenol-enriched dietary supplement (Zeropollution, which contains four standardized herbal extracts: Olea europaea leaf, Lippia citriodora, S. rosmarinus, and Sophora japonica) in diminishing pollution-induced oxidative stress and in improving skin aging in 100 White and Asian women who were outdoor workers living in a polluted environment (Milan, Italy). Statistically significant improvements in reducing wrinkle depth and hyperpigmentation, enhancing elasticity and firmness, as well as promoting skin moisturization and diminishing transepidermal water loss were noted as early as 2 weeks after product consumption began, with inter-group and intra-group analysis verifying that all skin parameters were ameliorated in Asian and White subjects.8
Previously, Nobile et al. conducted a randomized, parallel-group study on 90 subjects to evaluate the photoprotective effects of a combination of rosemary and grapefruit (Citrus paradisi) extracts (Nutroxsun). The investigators also performed a pilot, randomized crossover study on five participants. Both studies included only females with Fitzpatrick skin phototypes I-III who manifested mild to moderate chronological aging or photoaging. Within as little as 2 weeks, treated individuals exhibited reductions in UVA- and UVB-induced skin changes. Skin elasticity improved in this group, with wrinkles diminishing along with skin redness and lipoperoxides. The investigators concluded that the oral blend of rosemary and grapefruit consumed long term merits consideration as an adjuvant approach to preventing the deleterious effects of solar exposure.9
In 2021, Hoskin et al. used ex vivo human biopsies exposed to diesel engine exhaust to study the impact of spray-dried algae-rosemary particles against pollution-induced damage. The spirulina-rosemary gel that was developed lowered levels of 4-hydroxynonenal protein adducts (4HNE-PA) as well as matrix metalloproteinase-9 (MMP-9) and reduced the loss of filaggrin. The researchers concluded that their topically applied spirulina-rosemary gel was effective in mitigating or preventing skin aging and cutaneous damage caused by diesel air pollution.10
Antioxidant, Antibacterial, and Anti-Inflammatory Activity
Based on a 2023 literature search by Li Pomi et al. of in vitro as well as in vivo animal and human studies involving S. rosmarinus and the skin, researchers reported on substantial evidence buttressing the antioxidant role of the botanical agent. They cautioned that, while data support the harnessing of the bioactive constituents of rosemary to address inflammatory and infectious skin conditions, large controlled trials remain necessary to establish its potential functions in dermatologic clinical practice.11
Ten years earlier, Park et al. determined that a phenolic diterpene from rosemary (carnosic acid) prevented UV-induced expression of MMP-1, MMP-3, and MMP-9 in human skin fibroblasts and keratinocytes in a concentration-dependent manner by suppressing reactive oxygen species and blocking through the inhibition of ROS and the suppression of extracellular signal-regulated kinase (ERK)-mediated AP-1 activation.12
Around the same time, Sienkiewicz et al. showed that rosemary essential oil exhibits antibacterial activity against the standard strain Escherichia coli ATCC 25922 and 60 other clinical strains of the bacteria.13
Further, anti-inflammatory properties have been attributed to rosemary essential oil, which are thought to be due to its suppression of nuclear factor kappa B transcription and inhibition of the arachidonic acid cascade.14
Other Functions of Rosemary
In 2022, Sutkowska-Skolimowska et al. demonstrated that rosemary extract in concentrations of 50 and 100mcg/mL significantly diminished accumulated collagen in the fibroblasts of four patients with severe and fatal osteogenesis imperfecta, suggesting that the botanical agent may have a role targeting cellular stress and inducing autophagy in therapy for this condition.15
In 2015, Akbari et al. established that 0.5% and 1% concentrations of rosemary essential oil were effective in facilitating the percutaneous absorption of diclofenac sodium topical gel.16
Conclusion
In Western culture, rosemary is thought of more as a spice to add flavor to food. However, there appears to be an emerging body of evidence suggesting various possible functions for rosemary in the dermatologic armamentarium. Much more research is necessary, though, to ascertain the most appropriate and optimal roles for this popular herb in skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami, Florida. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
2. Sayorwan W et al. Sci Pharm. 2013 Apr-Jun;81(2):531-42.
3. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
4. Auh JH and Madhavan J Biomed Pharmacother. 2021 Mar;135:111178.
5. Pérez-Sánchez A et al. J Photochem Photobiol B. 2014 Jul 5;136:12-8.
6. Sánchez-Marzo N et al. Antioxidants (Basel). 2020 Mar 20;9(3):255.
7. Ibrahim N et al. Sci Rep. 2022 Jul 30;12(1):13102.
8. Nobile V et al. Food Nutr Res. 2021 Mar 29:65.
9. Nobile V et al. Food Nutr Res. 2016 Jul 1;60:31871.
10. Hoskin R et al. Molecules. 2021 Jun 22;26(13):3781.
11. Li Pomi F et al. Antioxidants (Basel). 2023 Mar 9;12(3):680.
12. Park M et al. Exp Dermatol. 2013 May;22(5):336-41.
13. Sienkiewicz M et al. Molecules. 2013 Aug 5;18(8):9334-51.
14. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
15. Sutkowska-Skolimowska. Int J Mol Sci. 2022 Sep 7;23(18):10341.
16. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
Used as a spice in various, particularly Mediterranean, cuisines and in traditional medicine for hundreds of years, this aromatic shrub has been the focus of substantial research this century to clarify its roles in skin care. It is used broadly in cosmetic formulations, particularly to preserve the product, and acts as a skin conditioner and fragrance in safe concentrations.1 Rosemary essential oil is also a popular choice frequently used in aromatherapy.2,3 This column focuses on recent promising results supporting the antioxidant and anti-photoaging activities, especially, of rosemary.
UV Protection and Rosemary in Combination
A 2021 study in mice authored by Auh and Madhavan showed that a mixture of marigold and rosemary extracts yielded anti-photoaging effects, with the botanical formula suppressing UV-induced damage.4
Seven years earlier, Pérez-Sánchez et al. combined rosemary and citrus extracts and found that they exerted protective effects against UV damage in human HaCaT keratinocytes as well as human volunteers after oral consumption. Significant increases in minimal erythema dose (MED) were seen in participants, with daily intake of 250 mg of botanical combination, at 8 weeks (34%) and 12 weeks (56%). The investigators attributed the photoprotective effects of the formula to rosemary polyphenols and diterpenes as well as citrus flavonoids.5
Evaluation of a human skin cell model by Sánchez-Marzo et al. in 2020 revealed that rosemary diterpenes were instrumental in an herbal extract that combined citrus, olive, and rosemary in conferring genoprotection against UV-induced DNA damage. The authors note that human trials are needed to overcome the limitations of the cellular model in ascertaining whether the tested herbal formulations can yield oral and/or topical photoprotection.6
Anti-Photoaging and Anti-Pollution
In 2022, Ibrahim et al. assessed a hexane extract of rosemary leaves for anti-photoaging activity. Their evaluation showed an abundance of triterpenoids, monoterpenoids, and phenolic diterpenes in rosemary, with in vitro assays verifying the anti-aging, antioxidant, and wound healing functions of the extract. Further, topical rosemary hexane extract–loaded lipid nanocapsules protected rat skin from UV radiation, as epidermal and dermal histological parameters improved, antioxidant biochemical balance was restored, and inflammatory markers and wrinkling were diminished. The researchers concluded that the use of rosemary hexane extract represents a safe, efficient, and cost-effective way to deliver anti-aging, photoprotective functions to cosmeceutical formulations.7
In March 2021, Nobile et al. published a report on their randomized, double-blind, placebo-controlled parallel group study to investigate the efficacy of a marketed polyphenol-enriched dietary supplement (Zeropollution, which contains four standardized herbal extracts: Olea europaea leaf, Lippia citriodora, S. rosmarinus, and Sophora japonica) in diminishing pollution-induced oxidative stress and in improving skin aging in 100 White and Asian women who were outdoor workers living in a polluted environment (Milan, Italy). Statistically significant improvements in reducing wrinkle depth and hyperpigmentation, enhancing elasticity and firmness, as well as promoting skin moisturization and diminishing transepidermal water loss were noted as early as 2 weeks after product consumption began, with inter-group and intra-group analysis verifying that all skin parameters were ameliorated in Asian and White subjects.8
Previously, Nobile et al. conducted a randomized, parallel-group study on 90 subjects to evaluate the photoprotective effects of a combination of rosemary and grapefruit (Citrus paradisi) extracts (Nutroxsun). The investigators also performed a pilot, randomized crossover study on five participants. Both studies included only females with Fitzpatrick skin phototypes I-III who manifested mild to moderate chronological aging or photoaging. Within as little as 2 weeks, treated individuals exhibited reductions in UVA- and UVB-induced skin changes. Skin elasticity improved in this group, with wrinkles diminishing along with skin redness and lipoperoxides. The investigators concluded that the oral blend of rosemary and grapefruit consumed long term merits consideration as an adjuvant approach to preventing the deleterious effects of solar exposure.9
In 2021, Hoskin et al. used ex vivo human biopsies exposed to diesel engine exhaust to study the impact of spray-dried algae-rosemary particles against pollution-induced damage. The spirulina-rosemary gel that was developed lowered levels of 4-hydroxynonenal protein adducts (4HNE-PA) as well as matrix metalloproteinase-9 (MMP-9) and reduced the loss of filaggrin. The researchers concluded that their topically applied spirulina-rosemary gel was effective in mitigating or preventing skin aging and cutaneous damage caused by diesel air pollution.10
Antioxidant, Antibacterial, and Anti-Inflammatory Activity
Based on a 2023 literature search by Li Pomi et al. of in vitro as well as in vivo animal and human studies involving S. rosmarinus and the skin, researchers reported on substantial evidence buttressing the antioxidant role of the botanical agent. They cautioned that, while data support the harnessing of the bioactive constituents of rosemary to address inflammatory and infectious skin conditions, large controlled trials remain necessary to establish its potential functions in dermatologic clinical practice.11
Ten years earlier, Park et al. determined that a phenolic diterpene from rosemary (carnosic acid) prevented UV-induced expression of MMP-1, MMP-3, and MMP-9 in human skin fibroblasts and keratinocytes in a concentration-dependent manner by suppressing reactive oxygen species and blocking through the inhibition of ROS and the suppression of extracellular signal-regulated kinase (ERK)-mediated AP-1 activation.12
Around the same time, Sienkiewicz et al. showed that rosemary essential oil exhibits antibacterial activity against the standard strain Escherichia coli ATCC 25922 and 60 other clinical strains of the bacteria.13
Further, anti-inflammatory properties have been attributed to rosemary essential oil, which are thought to be due to its suppression of nuclear factor kappa B transcription and inhibition of the arachidonic acid cascade.14
Other Functions of Rosemary
In 2022, Sutkowska-Skolimowska et al. demonstrated that rosemary extract in concentrations of 50 and 100mcg/mL significantly diminished accumulated collagen in the fibroblasts of four patients with severe and fatal osteogenesis imperfecta, suggesting that the botanical agent may have a role targeting cellular stress and inducing autophagy in therapy for this condition.15
In 2015, Akbari et al. established that 0.5% and 1% concentrations of rosemary essential oil were effective in facilitating the percutaneous absorption of diclofenac sodium topical gel.16
Conclusion
In Western culture, rosemary is thought of more as a spice to add flavor to food. However, there appears to be an emerging body of evidence suggesting various possible functions for rosemary in the dermatologic armamentarium. Much more research is necessary, though, to ascertain the most appropriate and optimal roles for this popular herb in skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami, Florida. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
2. Sayorwan W et al. Sci Pharm. 2013 Apr-Jun;81(2):531-42.
3. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
4. Auh JH and Madhavan J Biomed Pharmacother. 2021 Mar;135:111178.
5. Pérez-Sánchez A et al. J Photochem Photobiol B. 2014 Jul 5;136:12-8.
6. Sánchez-Marzo N et al. Antioxidants (Basel). 2020 Mar 20;9(3):255.
7. Ibrahim N et al. Sci Rep. 2022 Jul 30;12(1):13102.
8. Nobile V et al. Food Nutr Res. 2021 Mar 29:65.
9. Nobile V et al. Food Nutr Res. 2016 Jul 1;60:31871.
10. Hoskin R et al. Molecules. 2021 Jun 22;26(13):3781.
11. Li Pomi F et al. Antioxidants (Basel). 2023 Mar 9;12(3):680.
12. Park M et al. Exp Dermatol. 2013 May;22(5):336-41.
13. Sienkiewicz M et al. Molecules. 2013 Aug 5;18(8):9334-51.
14. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
15. Sutkowska-Skolimowska. Int J Mol Sci. 2022 Sep 7;23(18):10341.
16. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
Used as a spice in various, particularly Mediterranean, cuisines and in traditional medicine for hundreds of years, this aromatic shrub has been the focus of substantial research this century to clarify its roles in skin care. It is used broadly in cosmetic formulations, particularly to preserve the product, and acts as a skin conditioner and fragrance in safe concentrations.1 Rosemary essential oil is also a popular choice frequently used in aromatherapy.2,3 This column focuses on recent promising results supporting the antioxidant and anti-photoaging activities, especially, of rosemary.
UV Protection and Rosemary in Combination
A 2021 study in mice authored by Auh and Madhavan showed that a mixture of marigold and rosemary extracts yielded anti-photoaging effects, with the botanical formula suppressing UV-induced damage.4
Seven years earlier, Pérez-Sánchez et al. combined rosemary and citrus extracts and found that they exerted protective effects against UV damage in human HaCaT keratinocytes as well as human volunteers after oral consumption. Significant increases in minimal erythema dose (MED) were seen in participants, with daily intake of 250 mg of botanical combination, at 8 weeks (34%) and 12 weeks (56%). The investigators attributed the photoprotective effects of the formula to rosemary polyphenols and diterpenes as well as citrus flavonoids.5
Evaluation of a human skin cell model by Sánchez-Marzo et al. in 2020 revealed that rosemary diterpenes were instrumental in an herbal extract that combined citrus, olive, and rosemary in conferring genoprotection against UV-induced DNA damage. The authors note that human trials are needed to overcome the limitations of the cellular model in ascertaining whether the tested herbal formulations can yield oral and/or topical photoprotection.6
Anti-Photoaging and Anti-Pollution
In 2022, Ibrahim et al. assessed a hexane extract of rosemary leaves for anti-photoaging activity. Their evaluation showed an abundance of triterpenoids, monoterpenoids, and phenolic diterpenes in rosemary, with in vitro assays verifying the anti-aging, antioxidant, and wound healing functions of the extract. Further, topical rosemary hexane extract–loaded lipid nanocapsules protected rat skin from UV radiation, as epidermal and dermal histological parameters improved, antioxidant biochemical balance was restored, and inflammatory markers and wrinkling were diminished. The researchers concluded that the use of rosemary hexane extract represents a safe, efficient, and cost-effective way to deliver anti-aging, photoprotective functions to cosmeceutical formulations.7
In March 2021, Nobile et al. published a report on their randomized, double-blind, placebo-controlled parallel group study to investigate the efficacy of a marketed polyphenol-enriched dietary supplement (Zeropollution, which contains four standardized herbal extracts: Olea europaea leaf, Lippia citriodora, S. rosmarinus, and Sophora japonica) in diminishing pollution-induced oxidative stress and in improving skin aging in 100 White and Asian women who were outdoor workers living in a polluted environment (Milan, Italy). Statistically significant improvements in reducing wrinkle depth and hyperpigmentation, enhancing elasticity and firmness, as well as promoting skin moisturization and diminishing transepidermal water loss were noted as early as 2 weeks after product consumption began, with inter-group and intra-group analysis verifying that all skin parameters were ameliorated in Asian and White subjects.8
Previously, Nobile et al. conducted a randomized, parallel-group study on 90 subjects to evaluate the photoprotective effects of a combination of rosemary and grapefruit (Citrus paradisi) extracts (Nutroxsun). The investigators also performed a pilot, randomized crossover study on five participants. Both studies included only females with Fitzpatrick skin phototypes I-III who manifested mild to moderate chronological aging or photoaging. Within as little as 2 weeks, treated individuals exhibited reductions in UVA- and UVB-induced skin changes. Skin elasticity improved in this group, with wrinkles diminishing along with skin redness and lipoperoxides. The investigators concluded that the oral blend of rosemary and grapefruit consumed long term merits consideration as an adjuvant approach to preventing the deleterious effects of solar exposure.9
In 2021, Hoskin et al. used ex vivo human biopsies exposed to diesel engine exhaust to study the impact of spray-dried algae-rosemary particles against pollution-induced damage. The spirulina-rosemary gel that was developed lowered levels of 4-hydroxynonenal protein adducts (4HNE-PA) as well as matrix metalloproteinase-9 (MMP-9) and reduced the loss of filaggrin. The researchers concluded that their topically applied spirulina-rosemary gel was effective in mitigating or preventing skin aging and cutaneous damage caused by diesel air pollution.10
Antioxidant, Antibacterial, and Anti-Inflammatory Activity
Based on a 2023 literature search by Li Pomi et al. of in vitro as well as in vivo animal and human studies involving S. rosmarinus and the skin, researchers reported on substantial evidence buttressing the antioxidant role of the botanical agent. They cautioned that, while data support the harnessing of the bioactive constituents of rosemary to address inflammatory and infectious skin conditions, large controlled trials remain necessary to establish its potential functions in dermatologic clinical practice.11
Ten years earlier, Park et al. determined that a phenolic diterpene from rosemary (carnosic acid) prevented UV-induced expression of MMP-1, MMP-3, and MMP-9 in human skin fibroblasts and keratinocytes in a concentration-dependent manner by suppressing reactive oxygen species and blocking through the inhibition of ROS and the suppression of extracellular signal-regulated kinase (ERK)-mediated AP-1 activation.12
Around the same time, Sienkiewicz et al. showed that rosemary essential oil exhibits antibacterial activity against the standard strain Escherichia coli ATCC 25922 and 60 other clinical strains of the bacteria.13
Further, anti-inflammatory properties have been attributed to rosemary essential oil, which are thought to be due to its suppression of nuclear factor kappa B transcription and inhibition of the arachidonic acid cascade.14
Other Functions of Rosemary
In 2022, Sutkowska-Skolimowska et al. demonstrated that rosemary extract in concentrations of 50 and 100mcg/mL significantly diminished accumulated collagen in the fibroblasts of four patients with severe and fatal osteogenesis imperfecta, suggesting that the botanical agent may have a role targeting cellular stress and inducing autophagy in therapy for this condition.15
In 2015, Akbari et al. established that 0.5% and 1% concentrations of rosemary essential oil were effective in facilitating the percutaneous absorption of diclofenac sodium topical gel.16
Conclusion
In Western culture, rosemary is thought of more as a spice to add flavor to food. However, there appears to be an emerging body of evidence suggesting various possible functions for rosemary in the dermatologic armamentarium. Much more research is necessary, though, to ascertain the most appropriate and optimal roles for this popular herb in skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami, Florida. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
2. Sayorwan W et al. Sci Pharm. 2013 Apr-Jun;81(2):531-42.
3. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
4. Auh JH and Madhavan J Biomed Pharmacother. 2021 Mar;135:111178.
5. Pérez-Sánchez A et al. J Photochem Photobiol B. 2014 Jul 5;136:12-8.
6. Sánchez-Marzo N et al. Antioxidants (Basel). 2020 Mar 20;9(3):255.
7. Ibrahim N et al. Sci Rep. 2022 Jul 30;12(1):13102.
8. Nobile V et al. Food Nutr Res. 2021 Mar 29:65.
9. Nobile V et al. Food Nutr Res. 2016 Jul 1;60:31871.
10. Hoskin R et al. Molecules. 2021 Jun 22;26(13):3781.
11. Li Pomi F et al. Antioxidants (Basel). 2023 Mar 9;12(3):680.
12. Park M et al. Exp Dermatol. 2013 May;22(5):336-41.
13. Sienkiewicz M et al. Molecules. 2013 Aug 5;18(8):9334-51.
14. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
15. Sutkowska-Skolimowska. Int J Mol Sci. 2022 Sep 7;23(18):10341.
16. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.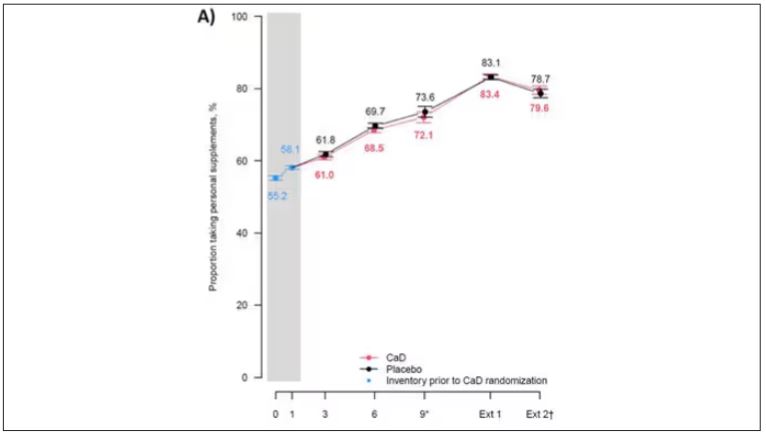
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.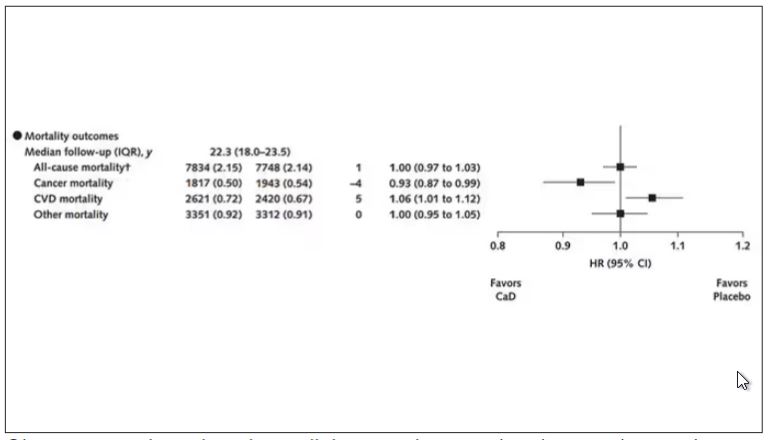
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.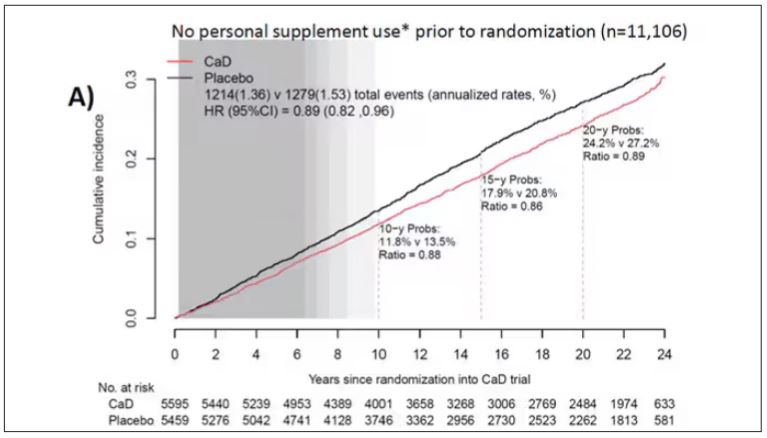
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.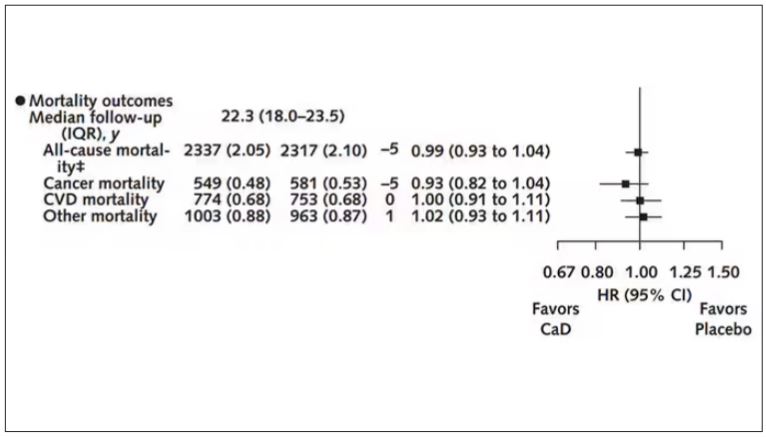
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
Survival Advantage of Adjuvant IO ‘Big News’ in Renal Cancer
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
The Urethra Is a Sex Organ; Why This Matters in Incontinence
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. We are coming to you live from the Mayo Clinic Urology Conference in Maui, Hawaii, with the world’s leading experts in men’s health, sexual health, and quality of life. I’m bringing in Dr. Allen Morey from the North Dallas area, one of the world’s leading experts in reconstructive urology. He deals with all things urinary incontinence, penile curvature, and sexual health.
Dr. Morey said something at this conference that really put my chin on the floor. He said, So, Dr. Morey, tell us more about why you made that comment and about the incontinence in men that you deal with all the time.
Allen F. Morey, MD: For many years, I’ve worked at cancer centers where, through the various treatments for prostate cancer, the men suffered from urinary incontinence and we put a lot of artificial urinary sphincters in those patients. I had one patient who asked, “Why do I keep having this erosion?” Of course, the erosion is where the cuff compresses the tissue surrounding the urethra, and the tissue gives way, leaving a hole in the urethra. I looked at him and noticed that he was pale. And I thought, Let me check his testosterone level. We started checking it on everybody who had this problem, and sure enough, the ones who had the cup erosion — who had the atrophic tissue around the urethra — most of them had low testosterone levels. Some of this was due to the cancer treatment, but in other men, it was just due to old age.
We started thinking that this is a causal relationship. And we tested it. I had a fellow who was a board-certified pathologist before becoming a urologist. He obtained some specimens from the urethra and did very sophisticated, elegant stains on that tissue. We found that it’s just erectile tissue surrounding the urethra. That’s why I call it a sex organ. I tell my patients, “When you are a teenager, this tissue is thin, like on your pinky finger. As you get older, it becomes thicker. And then as you get even older, and you may be having cancer treatment, that tissue is gone.” You can show them your little finger; it’s about that size. All the meat is off the bone. There’s nothing left protecting the urinary mucosa from the device.
That’s why it’s important to maintain the optimal health of those tissues and for them to remain dry. Because let’s face it: Urinary incontinence is a horrible quality of life. These are my happiest patients when we fix it. Before that, when they go on vacation, they have a separate suitcase filled with diapers. They can’t go anywhere. They can’t do anything. When they become incontinent after the prostatectomy, they gain weight. And when you put in the device to treat it, they lose weight and you can track it. The urinary incontinence patients really suffer. And we need to consider the medical optimization of those patients.
Dr. Rubin: It’s so important, and I love how analogous this is to our female patients. We know that incontinence is devastating to our female patients as well, and there’s a lot of hope. As we get older we start to pee every time we cough, laugh, or sneeze. Men are a little more bothered by it when it happens; they don’t expect it. Among women, it’s thought of as normal, but it’s not normal. There is so much we can do to help these patients, ranging from conservative treatment to surgical therapies.
The connection between hormones and urethral health is true on the female side as well. As you go through menopause, the urethral tissue thins out, gets dry, gets irritated, and can cause worsening incontinence, pain with sex, and genital urinary syndrome of menopause. It’s really important.
For our primary care doctors, how should we talk about stress incontinence in men? How do we diagnose it?
Dr. Morey: It’s easy to diagnose. Just do a quick history. Find out how many pads a day they are using. You have to ask the question, and then you have them stand up and look inside their underwear. You’ll see what kind of pad they are wearing. Is it just a shield or are they actually wearing full diapers? Then I have them do a standing cough test. I stand off to the side, holding a couple of towels, and have the patient cough four times. I can tell if it’s a full stream or just a couple of drops. Is it nothing but they are wearing a pad? You match up what you see with their experience, and in an instant it tells you how severe their problem is and it helps you direct them on to further treatment, because many patients have treatment fatigue. They’ve already been through the system. They have really suffered and they don’t know which way to go. They don’t know what’s available.
Dr. Rubin: On the female side, we have pelvic floor physical therapy. We have pads and devices that you can wear, and pessaries. We have surgical options, like bulking agents into the urethra as well as urethral slings, which can be quite helpful for women. So there’s a lot of hope out there for women, and from what I learned from you at this conference, there’s a lot of hope for men as well. So talk us through treatment, from conservative to surgical options.
Dr. Morey: There hasn’t been much innovation in male incontinence treatment over the past few decades, but we’re starting to see signs of new products appearing on the horizon, so I’m very optimistic that in the next 5 or 10 years, we’ll have more. But right now it comes down to slings and artificial sphincters, which are devices with little pumps and hydraulics, and they’re very good. But they’ve been around for 50 years, and they have this other potential risk factor of the erosion of the tissue.
We don’t have a pill that we can give the patient to tighten up those muscles. We can help them with overactive bladder. But maybe the hormonal influence is a way to optimize the health of the tissue so that these surgical treatments can really deliver the best outcomes. And as I always say, and having treated so many of these patients, it’s really a game of millimeters — how much coaptation you get. If you’re off by the slightest amount, that’s an unhappy patient. So it doesn’t take much to make it a lot better.
Dr. Rubin: There’s so much hope for our patients, and this can really have an effect on sexual health. You know the benefits you see in their quality of life and sexual health when you can stop leakage.
Dr. Morey: I always take care of the waterworks first. Many of the men have both urinary problems and erectile problems. Nobody feels sexy when they’re leaking urine all over their partner. So first we take care of that. And then, in the motivated younger patients, we bring them back and talk to them about potentially having a second operation.
Dr. Rubin: And so similarly, in women with urinary incontinence, it can have a major impact on sexual health — how they show up and how they talk to their partner. So, it is really important for our primary care docs to talk to patients about urinary incontinence and not just say, “Oh, well, you’re getting older. There’s nothing that you can do.” There’s actually no age at which there is nothing that we can do. And it’s really important to refer patients to those urologists who have extra training in incontinence and sexual health, because we do care about these quality-of-life measures and there is a lot we can do, ranging from conservative to more invasive treatments. But patients really should have options.
Dr. Morey: I heard during this meeting that urinary incontinence was the number-one source of treatment regret among patients who had their prostate treated for cancer. So, this is a really big deal for our patients. And it impacts wellness, quality of life, and overall well-being.
Dr. Rubin: When we are counseling patients for cancer surgeries or cancer treatments such as radiation therapy, it’s really hard for the patients who have never had urinary incontinence to imagine what that might be like. When you’re telling them they could have a stroke or a heart attack, or they could have erectile dysfunction or urinary incontinence, it all sounds similar to them. It could happen to someone else. It’s very hard to truly counsel patients on these quality-of-life issues that they’ve never encountered before.
Dr. Morey: We have found that it takes a long time for patients to get into our office for treatment, and it’s unbelievable — often 5 years in diapers before they find us.
Dr. Rubin: Hopefully videos like this will teach our docs and our patients that there is hope out there, that you don’t need to wait through years of suffering from incontinence. So, how does somebody find a reconstructive urologist or a sexual medicine urologist?
Dr. Morey: There are a couple of good websites out there, such as fixincontinence.com and edcure.org. The device manufacturers have pretty good information for patients.
Dr. Rubin: The Sexual Medicine Society of North America (SMSNA) has a great find-a-provider website, which provides a list of urologists who are trained in both sexual health and urinary incontinence, because they both matter. Our patients deeply care about these issues.
Rachel S. Rubin, MD, has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. We are coming to you live from the Mayo Clinic Urology Conference in Maui, Hawaii, with the world’s leading experts in men’s health, sexual health, and quality of life. I’m bringing in Dr. Allen Morey from the North Dallas area, one of the world’s leading experts in reconstructive urology. He deals with all things urinary incontinence, penile curvature, and sexual health.
Dr. Morey said something at this conference that really put my chin on the floor. He said, So, Dr. Morey, tell us more about why you made that comment and about the incontinence in men that you deal with all the time.
Allen F. Morey, MD: For many years, I’ve worked at cancer centers where, through the various treatments for prostate cancer, the men suffered from urinary incontinence and we put a lot of artificial urinary sphincters in those patients. I had one patient who asked, “Why do I keep having this erosion?” Of course, the erosion is where the cuff compresses the tissue surrounding the urethra, and the tissue gives way, leaving a hole in the urethra. I looked at him and noticed that he was pale. And I thought, Let me check his testosterone level. We started checking it on everybody who had this problem, and sure enough, the ones who had the cup erosion — who had the atrophic tissue around the urethra — most of them had low testosterone levels. Some of this was due to the cancer treatment, but in other men, it was just due to old age.
We started thinking that this is a causal relationship. And we tested it. I had a fellow who was a board-certified pathologist before becoming a urologist. He obtained some specimens from the urethra and did very sophisticated, elegant stains on that tissue. We found that it’s just erectile tissue surrounding the urethra. That’s why I call it a sex organ. I tell my patients, “When you are a teenager, this tissue is thin, like on your pinky finger. As you get older, it becomes thicker. And then as you get even older, and you may be having cancer treatment, that tissue is gone.” You can show them your little finger; it’s about that size. All the meat is off the bone. There’s nothing left protecting the urinary mucosa from the device.
That’s why it’s important to maintain the optimal health of those tissues and for them to remain dry. Because let’s face it: Urinary incontinence is a horrible quality of life. These are my happiest patients when we fix it. Before that, when they go on vacation, they have a separate suitcase filled with diapers. They can’t go anywhere. They can’t do anything. When they become incontinent after the prostatectomy, they gain weight. And when you put in the device to treat it, they lose weight and you can track it. The urinary incontinence patients really suffer. And we need to consider the medical optimization of those patients.
Dr. Rubin: It’s so important, and I love how analogous this is to our female patients. We know that incontinence is devastating to our female patients as well, and there’s a lot of hope. As we get older we start to pee every time we cough, laugh, or sneeze. Men are a little more bothered by it when it happens; they don’t expect it. Among women, it’s thought of as normal, but it’s not normal. There is so much we can do to help these patients, ranging from conservative treatment to surgical therapies.
The connection between hormones and urethral health is true on the female side as well. As you go through menopause, the urethral tissue thins out, gets dry, gets irritated, and can cause worsening incontinence, pain with sex, and genital urinary syndrome of menopause. It’s really important.
For our primary care doctors, how should we talk about stress incontinence in men? How do we diagnose it?
Dr. Morey: It’s easy to diagnose. Just do a quick history. Find out how many pads a day they are using. You have to ask the question, and then you have them stand up and look inside their underwear. You’ll see what kind of pad they are wearing. Is it just a shield or are they actually wearing full diapers? Then I have them do a standing cough test. I stand off to the side, holding a couple of towels, and have the patient cough four times. I can tell if it’s a full stream or just a couple of drops. Is it nothing but they are wearing a pad? You match up what you see with their experience, and in an instant it tells you how severe their problem is and it helps you direct them on to further treatment, because many patients have treatment fatigue. They’ve already been through the system. They have really suffered and they don’t know which way to go. They don’t know what’s available.
Dr. Rubin: On the female side, we have pelvic floor physical therapy. We have pads and devices that you can wear, and pessaries. We have surgical options, like bulking agents into the urethra as well as urethral slings, which can be quite helpful for women. So there’s a lot of hope out there for women, and from what I learned from you at this conference, there’s a lot of hope for men as well. So talk us through treatment, from conservative to surgical options.
Dr. Morey: There hasn’t been much innovation in male incontinence treatment over the past few decades, but we’re starting to see signs of new products appearing on the horizon, so I’m very optimistic that in the next 5 or 10 years, we’ll have more. But right now it comes down to slings and artificial sphincters, which are devices with little pumps and hydraulics, and they’re very good. But they’ve been around for 50 years, and they have this other potential risk factor of the erosion of the tissue.
We don’t have a pill that we can give the patient to tighten up those muscles. We can help them with overactive bladder. But maybe the hormonal influence is a way to optimize the health of the tissue so that these surgical treatments can really deliver the best outcomes. And as I always say, and having treated so many of these patients, it’s really a game of millimeters — how much coaptation you get. If you’re off by the slightest amount, that’s an unhappy patient. So it doesn’t take much to make it a lot better.
Dr. Rubin: There’s so much hope for our patients, and this can really have an effect on sexual health. You know the benefits you see in their quality of life and sexual health when you can stop leakage.
Dr. Morey: I always take care of the waterworks first. Many of the men have both urinary problems and erectile problems. Nobody feels sexy when they’re leaking urine all over their partner. So first we take care of that. And then, in the motivated younger patients, we bring them back and talk to them about potentially having a second operation.
Dr. Rubin: And so similarly, in women with urinary incontinence, it can have a major impact on sexual health — how they show up and how they talk to their partner. So, it is really important for our primary care docs to talk to patients about urinary incontinence and not just say, “Oh, well, you’re getting older. There’s nothing that you can do.” There’s actually no age at which there is nothing that we can do. And it’s really important to refer patients to those urologists who have extra training in incontinence and sexual health, because we do care about these quality-of-life measures and there is a lot we can do, ranging from conservative to more invasive treatments. But patients really should have options.
Dr. Morey: I heard during this meeting that urinary incontinence was the number-one source of treatment regret among patients who had their prostate treated for cancer. So, this is a really big deal for our patients. And it impacts wellness, quality of life, and overall well-being.
Dr. Rubin: When we are counseling patients for cancer surgeries or cancer treatments such as radiation therapy, it’s really hard for the patients who have never had urinary incontinence to imagine what that might be like. When you’re telling them they could have a stroke or a heart attack, or they could have erectile dysfunction or urinary incontinence, it all sounds similar to them. It could happen to someone else. It’s very hard to truly counsel patients on these quality-of-life issues that they’ve never encountered before.
Dr. Morey: We have found that it takes a long time for patients to get into our office for treatment, and it’s unbelievable — often 5 years in diapers before they find us.
Dr. Rubin: Hopefully videos like this will teach our docs and our patients that there is hope out there, that you don’t need to wait through years of suffering from incontinence. So, how does somebody find a reconstructive urologist or a sexual medicine urologist?
Dr. Morey: There are a couple of good websites out there, such as fixincontinence.com and edcure.org. The device manufacturers have pretty good information for patients.
Dr. Rubin: The Sexual Medicine Society of North America (SMSNA) has a great find-a-provider website, which provides a list of urologists who are trained in both sexual health and urinary incontinence, because they both matter. Our patients deeply care about these issues.
Rachel S. Rubin, MD, has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. We are coming to you live from the Mayo Clinic Urology Conference in Maui, Hawaii, with the world’s leading experts in men’s health, sexual health, and quality of life. I’m bringing in Dr. Allen Morey from the North Dallas area, one of the world’s leading experts in reconstructive urology. He deals with all things urinary incontinence, penile curvature, and sexual health.
Dr. Morey said something at this conference that really put my chin on the floor. He said, So, Dr. Morey, tell us more about why you made that comment and about the incontinence in men that you deal with all the time.
Allen F. Morey, MD: For many years, I’ve worked at cancer centers where, through the various treatments for prostate cancer, the men suffered from urinary incontinence and we put a lot of artificial urinary sphincters in those patients. I had one patient who asked, “Why do I keep having this erosion?” Of course, the erosion is where the cuff compresses the tissue surrounding the urethra, and the tissue gives way, leaving a hole in the urethra. I looked at him and noticed that he was pale. And I thought, Let me check his testosterone level. We started checking it on everybody who had this problem, and sure enough, the ones who had the cup erosion — who had the atrophic tissue around the urethra — most of them had low testosterone levels. Some of this was due to the cancer treatment, but in other men, it was just due to old age.
We started thinking that this is a causal relationship. And we tested it. I had a fellow who was a board-certified pathologist before becoming a urologist. He obtained some specimens from the urethra and did very sophisticated, elegant stains on that tissue. We found that it’s just erectile tissue surrounding the urethra. That’s why I call it a sex organ. I tell my patients, “When you are a teenager, this tissue is thin, like on your pinky finger. As you get older, it becomes thicker. And then as you get even older, and you may be having cancer treatment, that tissue is gone.” You can show them your little finger; it’s about that size. All the meat is off the bone. There’s nothing left protecting the urinary mucosa from the device.
That’s why it’s important to maintain the optimal health of those tissues and for them to remain dry. Because let’s face it: Urinary incontinence is a horrible quality of life. These are my happiest patients when we fix it. Before that, when they go on vacation, they have a separate suitcase filled with diapers. They can’t go anywhere. They can’t do anything. When they become incontinent after the prostatectomy, they gain weight. And when you put in the device to treat it, they lose weight and you can track it. The urinary incontinence patients really suffer. And we need to consider the medical optimization of those patients.
Dr. Rubin: It’s so important, and I love how analogous this is to our female patients. We know that incontinence is devastating to our female patients as well, and there’s a lot of hope. As we get older we start to pee every time we cough, laugh, or sneeze. Men are a little more bothered by it when it happens; they don’t expect it. Among women, it’s thought of as normal, but it’s not normal. There is so much we can do to help these patients, ranging from conservative treatment to surgical therapies.
The connection between hormones and urethral health is true on the female side as well. As you go through menopause, the urethral tissue thins out, gets dry, gets irritated, and can cause worsening incontinence, pain with sex, and genital urinary syndrome of menopause. It’s really important.
For our primary care doctors, how should we talk about stress incontinence in men? How do we diagnose it?
Dr. Morey: It’s easy to diagnose. Just do a quick history. Find out how many pads a day they are using. You have to ask the question, and then you have them stand up and look inside their underwear. You’ll see what kind of pad they are wearing. Is it just a shield or are they actually wearing full diapers? Then I have them do a standing cough test. I stand off to the side, holding a couple of towels, and have the patient cough four times. I can tell if it’s a full stream or just a couple of drops. Is it nothing but they are wearing a pad? You match up what you see with their experience, and in an instant it tells you how severe their problem is and it helps you direct them on to further treatment, because many patients have treatment fatigue. They’ve already been through the system. They have really suffered and they don’t know which way to go. They don’t know what’s available.
Dr. Rubin: On the female side, we have pelvic floor physical therapy. We have pads and devices that you can wear, and pessaries. We have surgical options, like bulking agents into the urethra as well as urethral slings, which can be quite helpful for women. So there’s a lot of hope out there for women, and from what I learned from you at this conference, there’s a lot of hope for men as well. So talk us through treatment, from conservative to surgical options.
Dr. Morey: There hasn’t been much innovation in male incontinence treatment over the past few decades, but we’re starting to see signs of new products appearing on the horizon, so I’m very optimistic that in the next 5 or 10 years, we’ll have more. But right now it comes down to slings and artificial sphincters, which are devices with little pumps and hydraulics, and they’re very good. But they’ve been around for 50 years, and they have this other potential risk factor of the erosion of the tissue.
We don’t have a pill that we can give the patient to tighten up those muscles. We can help them with overactive bladder. But maybe the hormonal influence is a way to optimize the health of the tissue so that these surgical treatments can really deliver the best outcomes. And as I always say, and having treated so many of these patients, it’s really a game of millimeters — how much coaptation you get. If you’re off by the slightest amount, that’s an unhappy patient. So it doesn’t take much to make it a lot better.
Dr. Rubin: There’s so much hope for our patients, and this can really have an effect on sexual health. You know the benefits you see in their quality of life and sexual health when you can stop leakage.
Dr. Morey: I always take care of the waterworks first. Many of the men have both urinary problems and erectile problems. Nobody feels sexy when they’re leaking urine all over their partner. So first we take care of that. And then, in the motivated younger patients, we bring them back and talk to them about potentially having a second operation.
Dr. Rubin: And so similarly, in women with urinary incontinence, it can have a major impact on sexual health — how they show up and how they talk to their partner. So, it is really important for our primary care docs to talk to patients about urinary incontinence and not just say, “Oh, well, you’re getting older. There’s nothing that you can do.” There’s actually no age at which there is nothing that we can do. And it’s really important to refer patients to those urologists who have extra training in incontinence and sexual health, because we do care about these quality-of-life measures and there is a lot we can do, ranging from conservative to more invasive treatments. But patients really should have options.
Dr. Morey: I heard during this meeting that urinary incontinence was the number-one source of treatment regret among patients who had their prostate treated for cancer. So, this is a really big deal for our patients. And it impacts wellness, quality of life, and overall well-being.
Dr. Rubin: When we are counseling patients for cancer surgeries or cancer treatments such as radiation therapy, it’s really hard for the patients who have never had urinary incontinence to imagine what that might be like. When you’re telling them they could have a stroke or a heart attack, or they could have erectile dysfunction or urinary incontinence, it all sounds similar to them. It could happen to someone else. It’s very hard to truly counsel patients on these quality-of-life issues that they’ve never encountered before.
Dr. Morey: We have found that it takes a long time for patients to get into our office for treatment, and it’s unbelievable — often 5 years in diapers before they find us.
Dr. Rubin: Hopefully videos like this will teach our docs and our patients that there is hope out there, that you don’t need to wait through years of suffering from incontinence. So, how does somebody find a reconstructive urologist or a sexual medicine urologist?
Dr. Morey: There are a couple of good websites out there, such as fixincontinence.com and edcure.org. The device manufacturers have pretty good information for patients.
Dr. Rubin: The Sexual Medicine Society of North America (SMSNA) has a great find-a-provider website, which provides a list of urologists who are trained in both sexual health and urinary incontinence, because they both matter. Our patients deeply care about these issues.
Rachel S. Rubin, MD, has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, Endo.
A version of this article appeared on Medscape.com.