User login
Q&A: Cancer screening in older patients – who to screen and when to stop
More than 1 in 10 Americans over age 60 years will be diagnosed with cancer, according to the National Cancer Institute, making screening for the disease in older patients imperative. Much of the burden of cancer screening falls on primary care physicians. This news organization spoke recently with William L. Dahut, MD, chief scientific officer of the American Cancer Society, about the particular challenges of screening in older patients.
Question: How much does cancer screening change with age? What are the considerations for clinicians – what risks and comorbidities are important to consider in older populations?
Answer: We at the American Cancer Society are giving a lot of thought to how to help primary care practices keep up with screening, particularly with respect to guidelines, but also best practices where judgment is required, such as cancer screening in their older patients.
We’ve had a lot of conversations recently about cancer risk in the young, largely because data show rates are going up for colorectal and breast cancer in this population. But it’s not one size fits all. Screening for young women who have a BRCA gene, if they have dense breasts, or if they have a strong family history of breast cancer should be different from those who are at average risk of the disease.
But statistically, there are about 15 per 100,000 breast cancer diagnoses in women under the age of 40 while over the age of 65 it’s 443 per 100,000. So, the risk significantly increases with age but we should not have an arbitrary cut-off. The life expectancy of a woman at age 75 is about 13.5 years. If you’re over the age of 70 or 75, then it’s going to be comorbidities that you look at, as well as individual patient decisions. Patients may say, “I don’t want to ever go through a mammogram again, because I don’t want to have a biopsy again, and I’m not going to get treated.” Or they may say, “My mom died of metastatic breast cancer when she was 82 and I want to know.”
Q: How should primary care physicians interpret conflicting guidance from the major medical groups? For example, the American College of Gastroenterology and your own organization recommend colorectal cancer screening start at age 45 now. But the American College of Physicians recently came out and said 50. What is a well-meaning primary care physician supposed to do?
A: We make more of guideline differences than we should. Sometimes guideline differences aren’t a reflection of different judgments, but rather what data were available when the most recent update took place. For colorectal cancer screening, the ACS dropped the age to begin screening to 45 in 2018 based on a very careful consideration of disease burden data and within several years most other guideline developers reached the same conclusion.
However, I think it’s good for family practice and internal medicine doctors to know that significant GI symptoms in a young patient could be colorectal cancer. It’s not as if nobody sees a 34-year-old or 27-year-old with colorectal cancer. They should be aware that if something goes away in a day or two, that’s fine, but persistent GI symptoms need a cancer workup – colonoscopy or referral to a gastroenterologist. So that’s why I think age 45 is the time when folks should begin screening.
Q: What are the medical-legal issues for a physician who is trying to follow guideline-based care when there are different guidelines?
A: Any physician can say, “We follow the guidelines of this particular organization.” I don’t think anyone can say that an organization’s guidelines are malpractice. For individual physicians, following a set of office-based guidelines will hopefully keep them out of legal difficulty.
Q: What are the risks of overscreening, especially in breast cancer where false positives may result in invasive testing?
A: What people think of as overscreening takes a number of different forms. What one guideline would imply is overscreening is recommended screening by another guideline. I think we would all agree that in an average-risk population, beginning screening before it is recommended would be overscreening, and continuing screening when a patient has life-limiting comorbidities would constitute overscreening. Screening too frequently can constitute overscreening.
For example, many women report that their doctors still are advising a baseline mammogram at age 35. Most guideline-developing organizations would regard this as overscreening in an average-risk population.
I think we are also getting better, certainly in prostate cancer, about knowing who needs to be treated and not treated. There are a lot of cancers that would have been treated 20-30 years ago but now are being safely followed with PSA and MRI. We may be able to get to that point with breast cancer over time, too.
Q: Are you saying that there may be breast cancers for which active surveillance is appropriate? Is that already the case?
A: We’re not there yet. I think some of the DCIS breast cancers are part of the discussion on whether hormonal treatment or surgeries are done. I think people do have those discussions in the context of morbidity and life expectancy. Over time, we’re likely to have more cancers for which we won’t need surgical treatments.
Q: Why did the American Cancer Society change the upper limit for lung cancer screening from 75 to 80 years of age?
A: For an individual older than 65, screening will now continue until the patient is 80, assuming the patient is in good health. According to the previous guideline, if a patient was 65 and more than 15 years beyond smoking cessation, then screening would end. This is exactly the time when we see lung cancers increase in the population and so a curable lung cancer would not previously have been detected by a screening CT scan. *
Q: What role do the multicancer blood and DNA tests play in screening now?
A: As you know, the Exact Sciences Cologuard test is already included in major guidelines for colorectal cancer screening and covered by insurance. Our philosophy on multicancer early detection tests is that we’re supportive of Medicare reimbursement when two things occur: 1. When we know there’s clinical benefit, and 2. When the test has been approved by the FDA.
The multicancer early detection tests in development and undergoing prospective research would not now replace screening for the cancers with established screening programs, but if they are shown to have clinical utility for the cancers in their panel, we would be able to reduce deaths from cancers that mostly are diagnosed at late stages and have poor prognoses.
There’s going to be a need for expertise in primary care practices to help interpret the tests. These are new questions, which are well beyond what even the typical oncologist is trained in, much less primary care physicians. We and other organizations are working on providing those answers.
Q: While we’re on the subject of the future, how do you envision AI helping or hindering cancer screening specifically in primary care?
A: I think AI is going to help things for a couple of reasons. The ability of AI is to get through data quickly and get you information that’s personalized and useful. If AI tools could let a patient know their individual risk of a cancer in the near and long term, that would help the primary care doctor screen in an individualized way. I think AI is going to be able to improve both diagnostic radiology and pathology, and could make a very big difference in settings outside of large cancer centers that operate at high volume every day. The data look very promising for AI to contribute to risk estimation by operating like a second reader in imaging and pathology.
Q: Anything else you’d like to say on this subject that clinicians should know?
A: The questions about whether or not patients should be screened is being pushed on family practice doctors and internists and these questions require a relationship with the patient. A hard stopping point at age 70 when lots of people will live 20 years or more doesn’t make sense.
There’s very little data from randomized clinical trials of screening people over the age of 70. We know that cancer risk does obviously increase with age, particularly prostate and breast cancer. And these are the cancers that are going to be the most common in your practices. If someone has a known mutation, I think you’re going to look differently at screening them. And first-degree family members, particularly for the more aggressive cancers, should be considered for screening.
My philosophy on cancer screening in the elderly is that I think the guidelines are guidelines. If patients have very limited life expectancy, then they shouldn’t be screened. There are calculators that estimate life expectancy in the context of current age and current health status, and these can be useful for decision making and counseling. Patients never think their life expectancy is shorter than 10 years. If their life expectancy is longer than 10 years, then I think, all things being equal, they should continue screening, but the question of ongoing screening needs to be periodically revisited.
*This story was updated on Nov. 1, 2023.
More than 1 in 10 Americans over age 60 years will be diagnosed with cancer, according to the National Cancer Institute, making screening for the disease in older patients imperative. Much of the burden of cancer screening falls on primary care physicians. This news organization spoke recently with William L. Dahut, MD, chief scientific officer of the American Cancer Society, about the particular challenges of screening in older patients.
Question: How much does cancer screening change with age? What are the considerations for clinicians – what risks and comorbidities are important to consider in older populations?
Answer: We at the American Cancer Society are giving a lot of thought to how to help primary care practices keep up with screening, particularly with respect to guidelines, but also best practices where judgment is required, such as cancer screening in their older patients.
We’ve had a lot of conversations recently about cancer risk in the young, largely because data show rates are going up for colorectal and breast cancer in this population. But it’s not one size fits all. Screening for young women who have a BRCA gene, if they have dense breasts, or if they have a strong family history of breast cancer should be different from those who are at average risk of the disease.
But statistically, there are about 15 per 100,000 breast cancer diagnoses in women under the age of 40 while over the age of 65 it’s 443 per 100,000. So, the risk significantly increases with age but we should not have an arbitrary cut-off. The life expectancy of a woman at age 75 is about 13.5 years. If you’re over the age of 70 or 75, then it’s going to be comorbidities that you look at, as well as individual patient decisions. Patients may say, “I don’t want to ever go through a mammogram again, because I don’t want to have a biopsy again, and I’m not going to get treated.” Or they may say, “My mom died of metastatic breast cancer when she was 82 and I want to know.”
Q: How should primary care physicians interpret conflicting guidance from the major medical groups? For example, the American College of Gastroenterology and your own organization recommend colorectal cancer screening start at age 45 now. But the American College of Physicians recently came out and said 50. What is a well-meaning primary care physician supposed to do?
A: We make more of guideline differences than we should. Sometimes guideline differences aren’t a reflection of different judgments, but rather what data were available when the most recent update took place. For colorectal cancer screening, the ACS dropped the age to begin screening to 45 in 2018 based on a very careful consideration of disease burden data and within several years most other guideline developers reached the same conclusion.
However, I think it’s good for family practice and internal medicine doctors to know that significant GI symptoms in a young patient could be colorectal cancer. It’s not as if nobody sees a 34-year-old or 27-year-old with colorectal cancer. They should be aware that if something goes away in a day or two, that’s fine, but persistent GI symptoms need a cancer workup – colonoscopy or referral to a gastroenterologist. So that’s why I think age 45 is the time when folks should begin screening.
Q: What are the medical-legal issues for a physician who is trying to follow guideline-based care when there are different guidelines?
A: Any physician can say, “We follow the guidelines of this particular organization.” I don’t think anyone can say that an organization’s guidelines are malpractice. For individual physicians, following a set of office-based guidelines will hopefully keep them out of legal difficulty.
Q: What are the risks of overscreening, especially in breast cancer where false positives may result in invasive testing?
A: What people think of as overscreening takes a number of different forms. What one guideline would imply is overscreening is recommended screening by another guideline. I think we would all agree that in an average-risk population, beginning screening before it is recommended would be overscreening, and continuing screening when a patient has life-limiting comorbidities would constitute overscreening. Screening too frequently can constitute overscreening.
For example, many women report that their doctors still are advising a baseline mammogram at age 35. Most guideline-developing organizations would regard this as overscreening in an average-risk population.
I think we are also getting better, certainly in prostate cancer, about knowing who needs to be treated and not treated. There are a lot of cancers that would have been treated 20-30 years ago but now are being safely followed with PSA and MRI. We may be able to get to that point with breast cancer over time, too.
Q: Are you saying that there may be breast cancers for which active surveillance is appropriate? Is that already the case?
A: We’re not there yet. I think some of the DCIS breast cancers are part of the discussion on whether hormonal treatment or surgeries are done. I think people do have those discussions in the context of morbidity and life expectancy. Over time, we’re likely to have more cancers for which we won’t need surgical treatments.
Q: Why did the American Cancer Society change the upper limit for lung cancer screening from 75 to 80 years of age?
A: For an individual older than 65, screening will now continue until the patient is 80, assuming the patient is in good health. According to the previous guideline, if a patient was 65 and more than 15 years beyond smoking cessation, then screening would end. This is exactly the time when we see lung cancers increase in the population and so a curable lung cancer would not previously have been detected by a screening CT scan. *
Q: What role do the multicancer blood and DNA tests play in screening now?
A: As you know, the Exact Sciences Cologuard test is already included in major guidelines for colorectal cancer screening and covered by insurance. Our philosophy on multicancer early detection tests is that we’re supportive of Medicare reimbursement when two things occur: 1. When we know there’s clinical benefit, and 2. When the test has been approved by the FDA.
The multicancer early detection tests in development and undergoing prospective research would not now replace screening for the cancers with established screening programs, but if they are shown to have clinical utility for the cancers in their panel, we would be able to reduce deaths from cancers that mostly are diagnosed at late stages and have poor prognoses.
There’s going to be a need for expertise in primary care practices to help interpret the tests. These are new questions, which are well beyond what even the typical oncologist is trained in, much less primary care physicians. We and other organizations are working on providing those answers.
Q: While we’re on the subject of the future, how do you envision AI helping or hindering cancer screening specifically in primary care?
A: I think AI is going to help things for a couple of reasons. The ability of AI is to get through data quickly and get you information that’s personalized and useful. If AI tools could let a patient know their individual risk of a cancer in the near and long term, that would help the primary care doctor screen in an individualized way. I think AI is going to be able to improve both diagnostic radiology and pathology, and could make a very big difference in settings outside of large cancer centers that operate at high volume every day. The data look very promising for AI to contribute to risk estimation by operating like a second reader in imaging and pathology.
Q: Anything else you’d like to say on this subject that clinicians should know?
A: The questions about whether or not patients should be screened is being pushed on family practice doctors and internists and these questions require a relationship with the patient. A hard stopping point at age 70 when lots of people will live 20 years or more doesn’t make sense.
There’s very little data from randomized clinical trials of screening people over the age of 70. We know that cancer risk does obviously increase with age, particularly prostate and breast cancer. And these are the cancers that are going to be the most common in your practices. If someone has a known mutation, I think you’re going to look differently at screening them. And first-degree family members, particularly for the more aggressive cancers, should be considered for screening.
My philosophy on cancer screening in the elderly is that I think the guidelines are guidelines. If patients have very limited life expectancy, then they shouldn’t be screened. There are calculators that estimate life expectancy in the context of current age and current health status, and these can be useful for decision making and counseling. Patients never think their life expectancy is shorter than 10 years. If their life expectancy is longer than 10 years, then I think, all things being equal, they should continue screening, but the question of ongoing screening needs to be periodically revisited.
*This story was updated on Nov. 1, 2023.
More than 1 in 10 Americans over age 60 years will be diagnosed with cancer, according to the National Cancer Institute, making screening for the disease in older patients imperative. Much of the burden of cancer screening falls on primary care physicians. This news organization spoke recently with William L. Dahut, MD, chief scientific officer of the American Cancer Society, about the particular challenges of screening in older patients.
Question: How much does cancer screening change with age? What are the considerations for clinicians – what risks and comorbidities are important to consider in older populations?
Answer: We at the American Cancer Society are giving a lot of thought to how to help primary care practices keep up with screening, particularly with respect to guidelines, but also best practices where judgment is required, such as cancer screening in their older patients.
We’ve had a lot of conversations recently about cancer risk in the young, largely because data show rates are going up for colorectal and breast cancer in this population. But it’s not one size fits all. Screening for young women who have a BRCA gene, if they have dense breasts, or if they have a strong family history of breast cancer should be different from those who are at average risk of the disease.
But statistically, there are about 15 per 100,000 breast cancer diagnoses in women under the age of 40 while over the age of 65 it’s 443 per 100,000. So, the risk significantly increases with age but we should not have an arbitrary cut-off. The life expectancy of a woman at age 75 is about 13.5 years. If you’re over the age of 70 or 75, then it’s going to be comorbidities that you look at, as well as individual patient decisions. Patients may say, “I don’t want to ever go through a mammogram again, because I don’t want to have a biopsy again, and I’m not going to get treated.” Or they may say, “My mom died of metastatic breast cancer when she was 82 and I want to know.”
Q: How should primary care physicians interpret conflicting guidance from the major medical groups? For example, the American College of Gastroenterology and your own organization recommend colorectal cancer screening start at age 45 now. But the American College of Physicians recently came out and said 50. What is a well-meaning primary care physician supposed to do?
A: We make more of guideline differences than we should. Sometimes guideline differences aren’t a reflection of different judgments, but rather what data were available when the most recent update took place. For colorectal cancer screening, the ACS dropped the age to begin screening to 45 in 2018 based on a very careful consideration of disease burden data and within several years most other guideline developers reached the same conclusion.
However, I think it’s good for family practice and internal medicine doctors to know that significant GI symptoms in a young patient could be colorectal cancer. It’s not as if nobody sees a 34-year-old or 27-year-old with colorectal cancer. They should be aware that if something goes away in a day or two, that’s fine, but persistent GI symptoms need a cancer workup – colonoscopy or referral to a gastroenterologist. So that’s why I think age 45 is the time when folks should begin screening.
Q: What are the medical-legal issues for a physician who is trying to follow guideline-based care when there are different guidelines?
A: Any physician can say, “We follow the guidelines of this particular organization.” I don’t think anyone can say that an organization’s guidelines are malpractice. For individual physicians, following a set of office-based guidelines will hopefully keep them out of legal difficulty.
Q: What are the risks of overscreening, especially in breast cancer where false positives may result in invasive testing?
A: What people think of as overscreening takes a number of different forms. What one guideline would imply is overscreening is recommended screening by another guideline. I think we would all agree that in an average-risk population, beginning screening before it is recommended would be overscreening, and continuing screening when a patient has life-limiting comorbidities would constitute overscreening. Screening too frequently can constitute overscreening.
For example, many women report that their doctors still are advising a baseline mammogram at age 35. Most guideline-developing organizations would regard this as overscreening in an average-risk population.
I think we are also getting better, certainly in prostate cancer, about knowing who needs to be treated and not treated. There are a lot of cancers that would have been treated 20-30 years ago but now are being safely followed with PSA and MRI. We may be able to get to that point with breast cancer over time, too.
Q: Are you saying that there may be breast cancers for which active surveillance is appropriate? Is that already the case?
A: We’re not there yet. I think some of the DCIS breast cancers are part of the discussion on whether hormonal treatment or surgeries are done. I think people do have those discussions in the context of morbidity and life expectancy. Over time, we’re likely to have more cancers for which we won’t need surgical treatments.
Q: Why did the American Cancer Society change the upper limit for lung cancer screening from 75 to 80 years of age?
A: For an individual older than 65, screening will now continue until the patient is 80, assuming the patient is in good health. According to the previous guideline, if a patient was 65 and more than 15 years beyond smoking cessation, then screening would end. This is exactly the time when we see lung cancers increase in the population and so a curable lung cancer would not previously have been detected by a screening CT scan. *
Q: What role do the multicancer blood and DNA tests play in screening now?
A: As you know, the Exact Sciences Cologuard test is already included in major guidelines for colorectal cancer screening and covered by insurance. Our philosophy on multicancer early detection tests is that we’re supportive of Medicare reimbursement when two things occur: 1. When we know there’s clinical benefit, and 2. When the test has been approved by the FDA.
The multicancer early detection tests in development and undergoing prospective research would not now replace screening for the cancers with established screening programs, but if they are shown to have clinical utility for the cancers in their panel, we would be able to reduce deaths from cancers that mostly are diagnosed at late stages and have poor prognoses.
There’s going to be a need for expertise in primary care practices to help interpret the tests. These are new questions, which are well beyond what even the typical oncologist is trained in, much less primary care physicians. We and other organizations are working on providing those answers.
Q: While we’re on the subject of the future, how do you envision AI helping or hindering cancer screening specifically in primary care?
A: I think AI is going to help things for a couple of reasons. The ability of AI is to get through data quickly and get you information that’s personalized and useful. If AI tools could let a patient know their individual risk of a cancer in the near and long term, that would help the primary care doctor screen in an individualized way. I think AI is going to be able to improve both diagnostic radiology and pathology, and could make a very big difference in settings outside of large cancer centers that operate at high volume every day. The data look very promising for AI to contribute to risk estimation by operating like a second reader in imaging and pathology.
Q: Anything else you’d like to say on this subject that clinicians should know?
A: The questions about whether or not patients should be screened is being pushed on family practice doctors and internists and these questions require a relationship with the patient. A hard stopping point at age 70 when lots of people will live 20 years or more doesn’t make sense.
There’s very little data from randomized clinical trials of screening people over the age of 70. We know that cancer risk does obviously increase with age, particularly prostate and breast cancer. And these are the cancers that are going to be the most common in your practices. If someone has a known mutation, I think you’re going to look differently at screening them. And first-degree family members, particularly for the more aggressive cancers, should be considered for screening.
My philosophy on cancer screening in the elderly is that I think the guidelines are guidelines. If patients have very limited life expectancy, then they shouldn’t be screened. There are calculators that estimate life expectancy in the context of current age and current health status, and these can be useful for decision making and counseling. Patients never think their life expectancy is shorter than 10 years. If their life expectancy is longer than 10 years, then I think, all things being equal, they should continue screening, but the question of ongoing screening needs to be periodically revisited.
*This story was updated on Nov. 1, 2023.
This drug works, but wait till you hear what’s in it
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.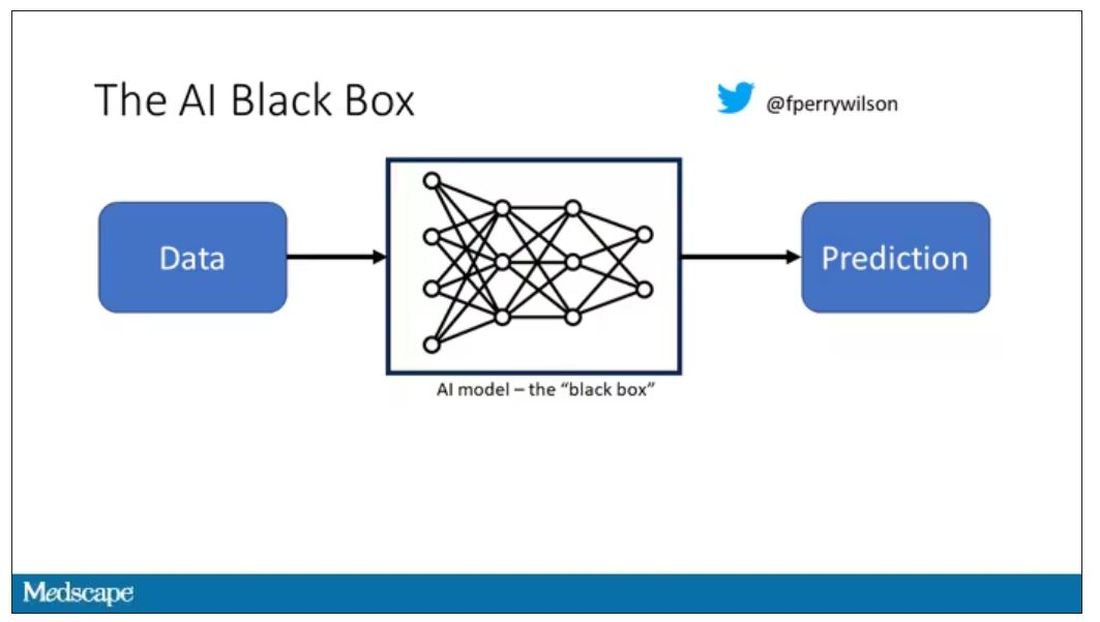
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.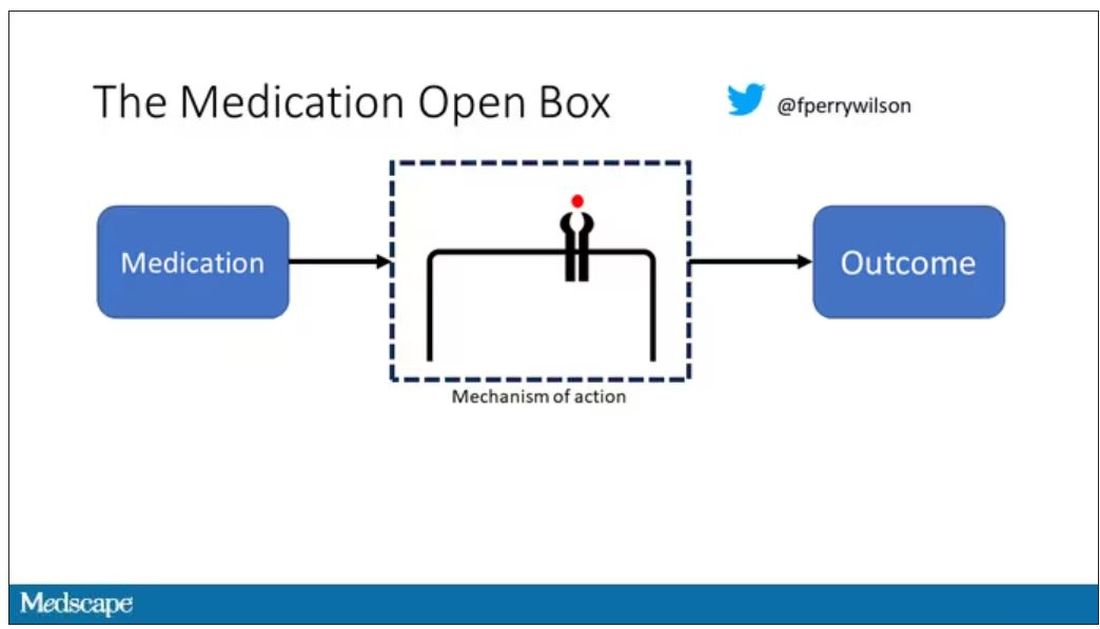
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.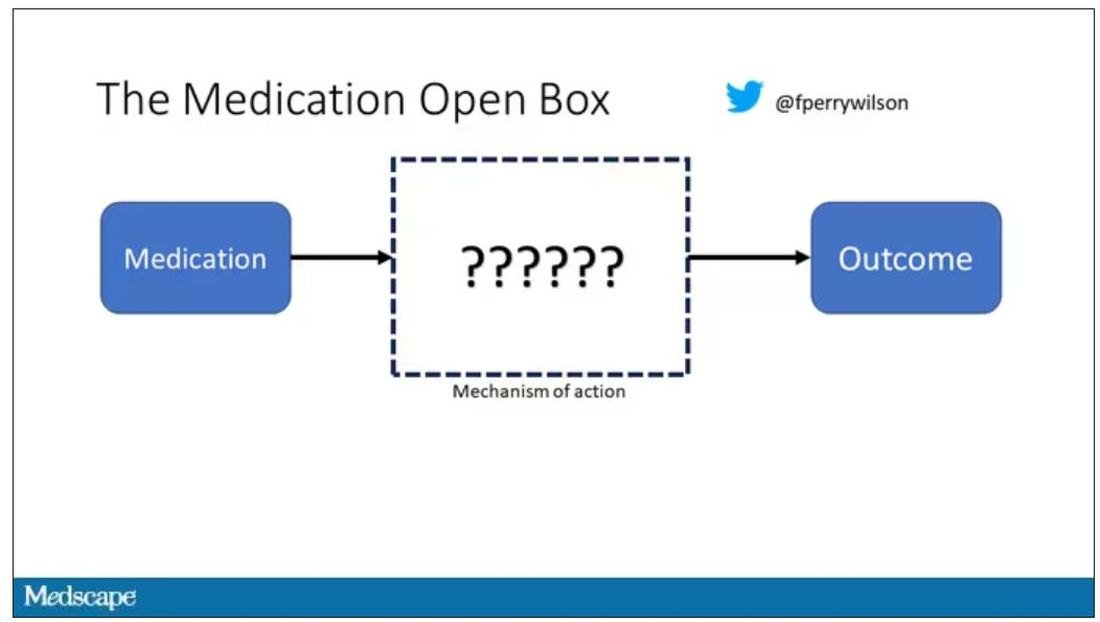
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.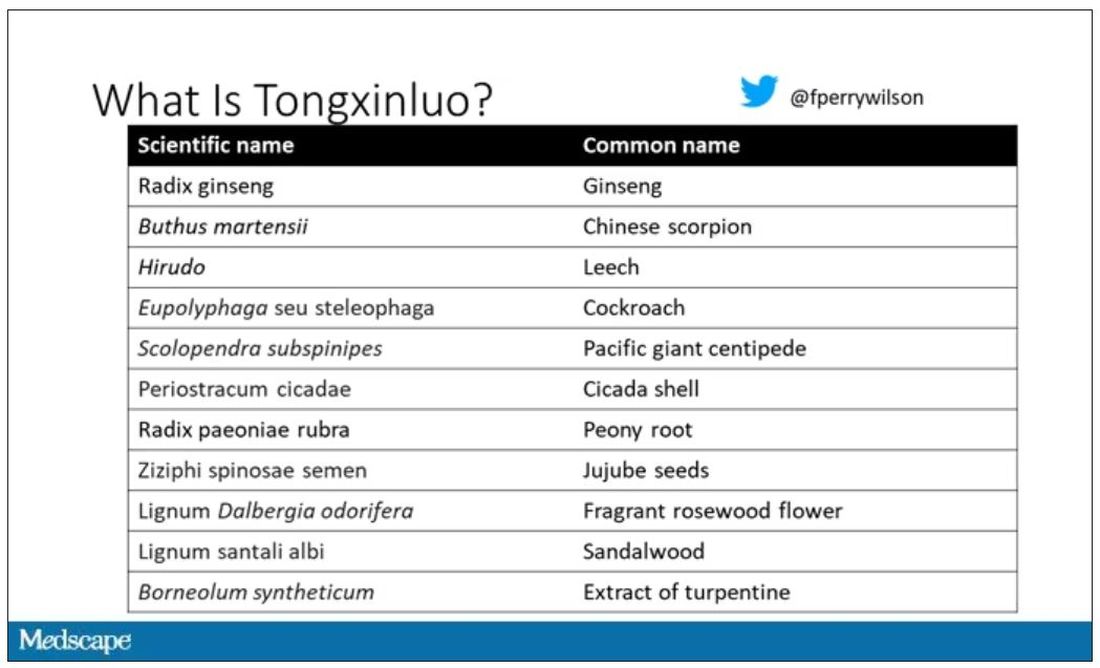
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.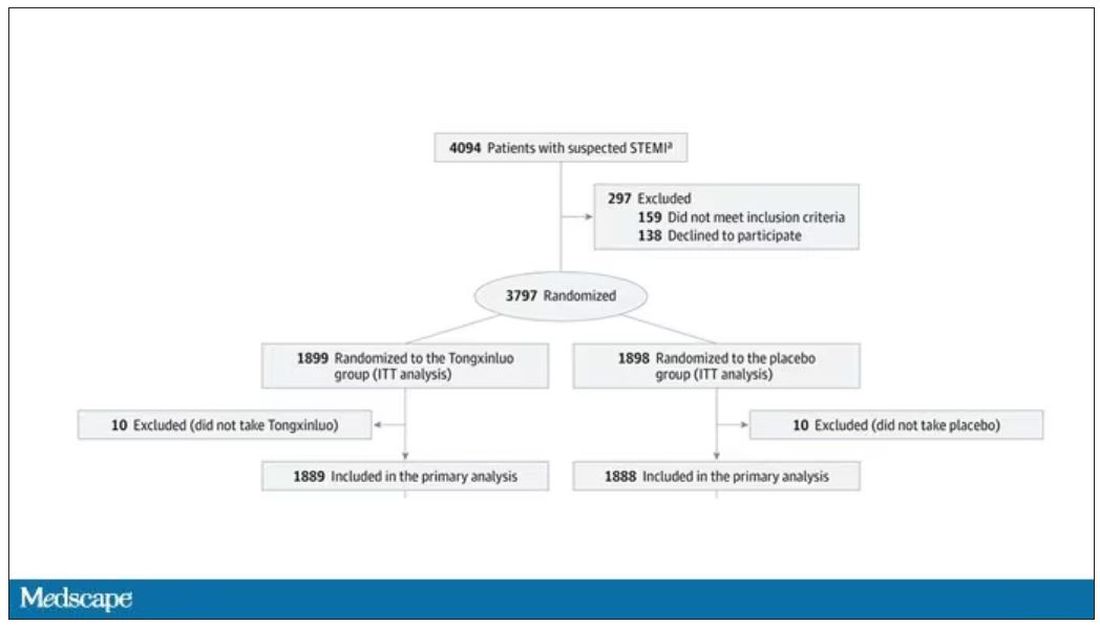
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.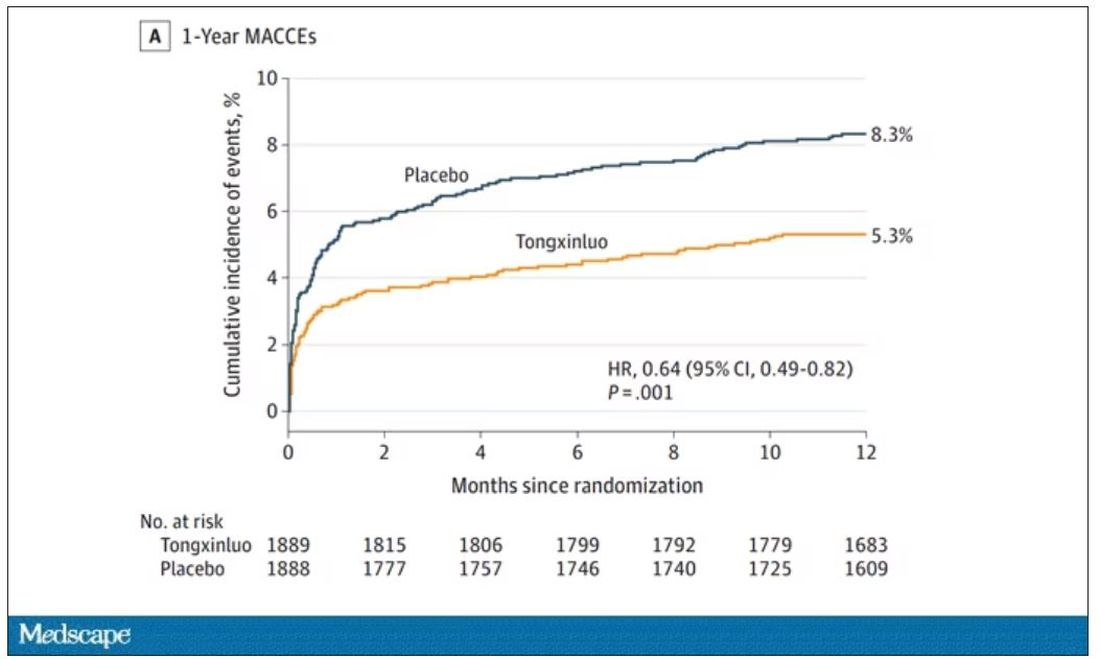
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.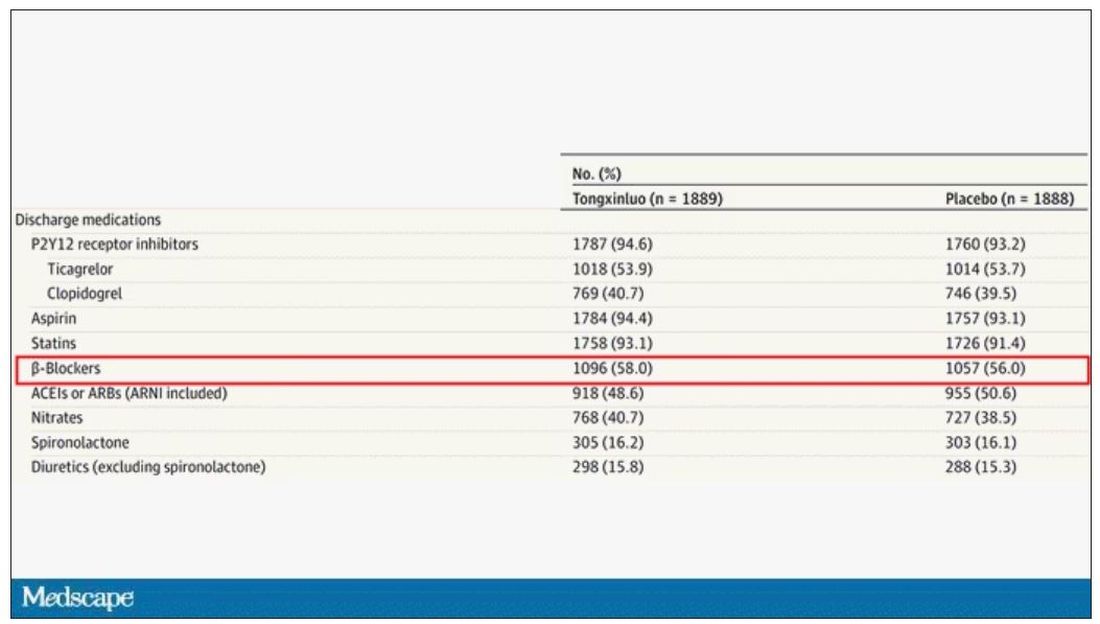
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
My pet peeves about the current state of primary care
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A focus on women with diabetes and their offspring
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 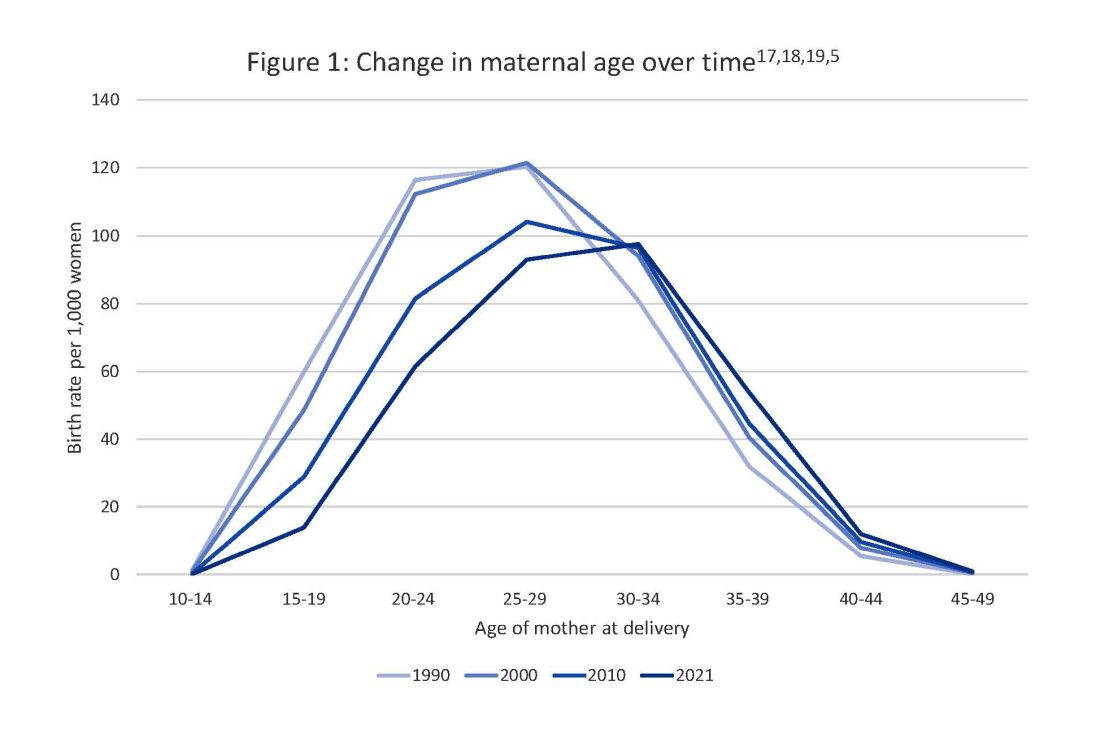
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 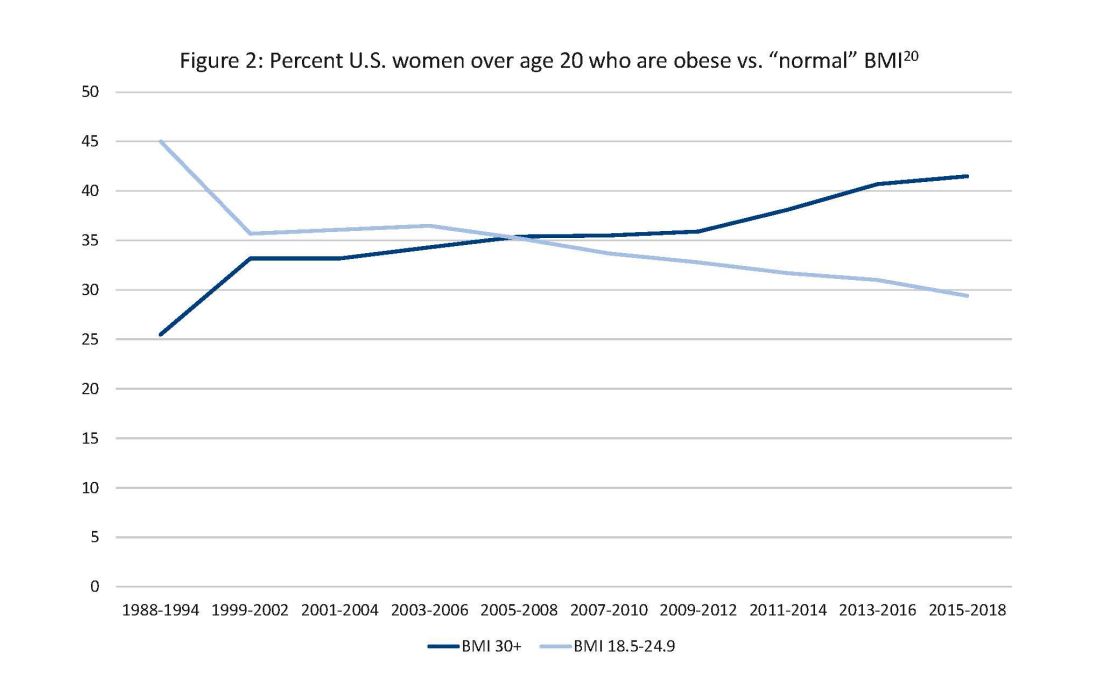
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
Employed physicians: A survival guide
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Why legal pot makes this physician sick
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Addressing obesity bias in health care
Obesity is a major factor affecting the health of many Americans. It is estimated by the Centers for Disease Control and Prevention that 41% of adults and 19.7% of children in our country now meet the criteria for being obese. Obesity costs the United States approximately $147 billion annually in health care costs. While these numbers are staggering, they continue to rise.
The recent craze over medications such as Ozempic, Wegovy, and Mounjaro shows how eager people are to lose weight. Yet, many of them face bias, not just in their daily lives, but from health care professionals who should do better. No one should feel stigmatized when they come for medical help. This just drives away patients who need us and who may then suffer more severe consequences of obesity-related illnesses.
Earlier this year, the American Association of Clinical Endocrinology issued a consensus statement on the role stigma and weight bias play in the management of obesity. They proposed a staging system to address the severity of obesity and suggested stigma and bias should be assessed in all patients.
While we are good at diagnosing obesity, many of us fail at addressing it empathetically with patients. I’ve seen many patients cry about past encounters they’ve had in the health care system. We need to address the emotional effect that obesity has as well as the physical complications.
Obesity is a major contributor to many diseases such as diabetes and heart disease, but we are finding it also plays a role in other diseases such as certain cancers. Treating obesity is imperative to prevent these diseases as well as to promote better treatment outcomes. We’ve all seen the diabetic patient lose weight and have their blood glucose levels come under control.
Many patients have tried hard to lose weight yet health care providers talk to them as if they haven’t made any efforts. This is very frustrating for patients. Simply telling a patient to diet and lose weight is a setup for failure. We need to address their past efforts and see what has worked and what hasn’t. Redoing the same thing over and over again is not a recipe for success.
Additionally, the focus on “diet and exercise” fails to account for emotional factors that may be contributing to a person’s obesity. Some people eat when they are stressed or depressed. It can become a habit or even an addiction. If this contributor to obesity isn’t fixed, nothing will work.
However, no medication will work well without the basic building blocks of diet and exercise. Routinely prescribing weight-loss medications without discussing diet and exercise will not result in much weight loss. Some patients simply don’t know how to eat healthfully or what they should do for exercise. A little education can go a long way. Ancillary staff, such as nutritionists or diabetic counselors, can help and free up the doctor’s time. In small practices, we can’t afford to provide those services in house but we should learn where patients can go for these services.
The AACE guidelines do a great job staging obesity. The guidelines make it easier to measure progress and decide on treatment plans. With this system, it is no longer necessary to use terms such as “excess weight” or “morbid obesity.” Patients already know they are overweight. What they need to know are clear steps so that they can reach goals. These guidelines greatly assist with providing those steps.
Most of us can do better when treating patients with obesity, We are probably not even aware of the times we have been guilty of stigmatization or weight bias. When we start treating obesity as a serious medical problem rather than something that’s the fault of the patient, it becomes much easier. When we remind ourselves what can happen to our patients when we fail to treat their obesity, we can become more serious about trying to help them reverse this critical medical problem. Bring an end to throwing out a “lose weight” or “eat healthier” suggestion to our already stressed patients. In order to address the obesity crisis that is here, we need to look inside ourselves and ask how we are going to contribute to the solution.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant of medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Obesity is a major factor affecting the health of many Americans. It is estimated by the Centers for Disease Control and Prevention that 41% of adults and 19.7% of children in our country now meet the criteria for being obese. Obesity costs the United States approximately $147 billion annually in health care costs. While these numbers are staggering, they continue to rise.
The recent craze over medications such as Ozempic, Wegovy, and Mounjaro shows how eager people are to lose weight. Yet, many of them face bias, not just in their daily lives, but from health care professionals who should do better. No one should feel stigmatized when they come for medical help. This just drives away patients who need us and who may then suffer more severe consequences of obesity-related illnesses.
Earlier this year, the American Association of Clinical Endocrinology issued a consensus statement on the role stigma and weight bias play in the management of obesity. They proposed a staging system to address the severity of obesity and suggested stigma and bias should be assessed in all patients.
While we are good at diagnosing obesity, many of us fail at addressing it empathetically with patients. I’ve seen many patients cry about past encounters they’ve had in the health care system. We need to address the emotional effect that obesity has as well as the physical complications.
Obesity is a major contributor to many diseases such as diabetes and heart disease, but we are finding it also plays a role in other diseases such as certain cancers. Treating obesity is imperative to prevent these diseases as well as to promote better treatment outcomes. We’ve all seen the diabetic patient lose weight and have their blood glucose levels come under control.
Many patients have tried hard to lose weight yet health care providers talk to them as if they haven’t made any efforts. This is very frustrating for patients. Simply telling a patient to diet and lose weight is a setup for failure. We need to address their past efforts and see what has worked and what hasn’t. Redoing the same thing over and over again is not a recipe for success.
Additionally, the focus on “diet and exercise” fails to account for emotional factors that may be contributing to a person’s obesity. Some people eat when they are stressed or depressed. It can become a habit or even an addiction. If this contributor to obesity isn’t fixed, nothing will work.
However, no medication will work well without the basic building blocks of diet and exercise. Routinely prescribing weight-loss medications without discussing diet and exercise will not result in much weight loss. Some patients simply don’t know how to eat healthfully or what they should do for exercise. A little education can go a long way. Ancillary staff, such as nutritionists or diabetic counselors, can help and free up the doctor’s time. In small practices, we can’t afford to provide those services in house but we should learn where patients can go for these services.
The AACE guidelines do a great job staging obesity. The guidelines make it easier to measure progress and decide on treatment plans. With this system, it is no longer necessary to use terms such as “excess weight” or “morbid obesity.” Patients already know they are overweight. What they need to know are clear steps so that they can reach goals. These guidelines greatly assist with providing those steps.
Most of us can do better when treating patients with obesity, We are probably not even aware of the times we have been guilty of stigmatization or weight bias. When we start treating obesity as a serious medical problem rather than something that’s the fault of the patient, it becomes much easier. When we remind ourselves what can happen to our patients when we fail to treat their obesity, we can become more serious about trying to help them reverse this critical medical problem. Bring an end to throwing out a “lose weight” or “eat healthier” suggestion to our already stressed patients. In order to address the obesity crisis that is here, we need to look inside ourselves and ask how we are going to contribute to the solution.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant of medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Obesity is a major factor affecting the health of many Americans. It is estimated by the Centers for Disease Control and Prevention that 41% of adults and 19.7% of children in our country now meet the criteria for being obese. Obesity costs the United States approximately $147 billion annually in health care costs. While these numbers are staggering, they continue to rise.
The recent craze over medications such as Ozempic, Wegovy, and Mounjaro shows how eager people are to lose weight. Yet, many of them face bias, not just in their daily lives, but from health care professionals who should do better. No one should feel stigmatized when they come for medical help. This just drives away patients who need us and who may then suffer more severe consequences of obesity-related illnesses.
Earlier this year, the American Association of Clinical Endocrinology issued a consensus statement on the role stigma and weight bias play in the management of obesity. They proposed a staging system to address the severity of obesity and suggested stigma and bias should be assessed in all patients.
While we are good at diagnosing obesity, many of us fail at addressing it empathetically with patients. I’ve seen many patients cry about past encounters they’ve had in the health care system. We need to address the emotional effect that obesity has as well as the physical complications.
Obesity is a major contributor to many diseases such as diabetes and heart disease, but we are finding it also plays a role in other diseases such as certain cancers. Treating obesity is imperative to prevent these diseases as well as to promote better treatment outcomes. We’ve all seen the diabetic patient lose weight and have their blood glucose levels come under control.
Many patients have tried hard to lose weight yet health care providers talk to them as if they haven’t made any efforts. This is very frustrating for patients. Simply telling a patient to diet and lose weight is a setup for failure. We need to address their past efforts and see what has worked and what hasn’t. Redoing the same thing over and over again is not a recipe for success.
Additionally, the focus on “diet and exercise” fails to account for emotional factors that may be contributing to a person’s obesity. Some people eat when they are stressed or depressed. It can become a habit or even an addiction. If this contributor to obesity isn’t fixed, nothing will work.
However, no medication will work well without the basic building blocks of diet and exercise. Routinely prescribing weight-loss medications without discussing diet and exercise will not result in much weight loss. Some patients simply don’t know how to eat healthfully or what they should do for exercise. A little education can go a long way. Ancillary staff, such as nutritionists or diabetic counselors, can help and free up the doctor’s time. In small practices, we can’t afford to provide those services in house but we should learn where patients can go for these services.
The AACE guidelines do a great job staging obesity. The guidelines make it easier to measure progress and decide on treatment plans. With this system, it is no longer necessary to use terms such as “excess weight” or “morbid obesity.” Patients already know they are overweight. What they need to know are clear steps so that they can reach goals. These guidelines greatly assist with providing those steps.
Most of us can do better when treating patients with obesity, We are probably not even aware of the times we have been guilty of stigmatization or weight bias. When we start treating obesity as a serious medical problem rather than something that’s the fault of the patient, it becomes much easier. When we remind ourselves what can happen to our patients when we fail to treat their obesity, we can become more serious about trying to help them reverse this critical medical problem. Bring an end to throwing out a “lose weight” or “eat healthier” suggestion to our already stressed patients. In order to address the obesity crisis that is here, we need to look inside ourselves and ask how we are going to contribute to the solution.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant of medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Artificial intelligence in the office: Part 2
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
Neoadjuvant advantages: Treating locally advanced lung cancer
Many of you saw the press release from Merck announcing that their randomized trial comparing chemo with chemo plus pembrolizumab in the neoadjuvant setting led to improved event-free survival and also improved pathologic complete response rate.
This comes in addition to the data from the AstraZeneca trial with durvalumab saying they’ve already achieved their endpoint of higher pathologic complete response rate vs. chemotherapy alone and also the data with nivolumab from Bristol-Myers Squibb saying that nivolumab plus chemotherapy leads to a better event-free survival and a better pathologic complete response rate. That information has led to Food and Drug Administration approval for their regimen.
We’re running the table with these very positive data, and I think it’s just a sign that the approach is safe and effective.
A huge question has come up. I just came from a meeting of lung cancer experts asking what to do if you have a patient with a small tumor, for example, a 3-cm tumor. Do you recommend immediate surgery followed by adjuvant therapy, chemotherapy, and then a checkpoint inhibitor if appropriate? Or do you proceed with neoadjuvant therapy if appropriate? The truth is that it’s a very difficult decision.
We have overwhelming data that the neoadjuvant approach works for that patient. Please remember that this is a clinically staged patient. This is not the patient after their surgery, where I think we have a very clear path. We have adjuvant data and adjuvant trials for those patients.
For the patient who’s in your office with a small tumor or a small tumor and only hilar lymphadenopathy, the decision there isn’t data driven, but rather it is experience driven. The data that are out there right now suggest that neoadjuvant therapy is a better way to go. Why is that?
Well, I think that the first reason is that it is probably a better regimen. I think many of you saw the recent clinical trial by Patel and colleagues in the New England Journal of Medicine with melanoma. It was an interesting trial. They gave a checkpoint inhibitor for 18 doses after surgery for melanoma versus three doses of checkpoint inhibitor, surgery, and then 15 doses of the checkpoint inhibitor.
It was 18 doses versus 18 doses, with the only difference being the three doses before surgery. Lo and behold, the three doses before surgery led to a better event-free survival.
There are preclinical data in lung cancer demonstrating that the same thing is true. Tina Cascone published on that years ago. We could talk about why, but it appears that neoadjuvant is just better.
There are other advantages to it as well. I think a big one is that all the information shows that it’s better tolerated, so you’re more likely to give all the drug. You can see if the drug isn’t working, and you can stop the drug. Also, if the drug is causing a side effect, you can see whether it’s working or not and use that decision to stop. It’s different than when you’re giving a drug in the adjuvant setting where you don’t really know whether it is working or not.
I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group. You need to weigh the pros and cons I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group coming in. It’s already an FDA-approved regimen with nivolumab and chemotherapy, and I think we’re moving to making that our standard of care now.
The way to handle it today, though, is to convene your multidisciplinary panel about every patient other than those with the tiniest of lung cancers and put your heads together to see what the best treatment is for that patient.
Dr. Kris is professor of medicine, Weill Cornell Medicine, and the William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, both in New York. He disclosed ties with Ariad Pharmaceuticals, AstraZeneca, Pfizer, PUMA, and Roche/Genentech.
A version of this article appeared on Medscape.com.
Many of you saw the press release from Merck announcing that their randomized trial comparing chemo with chemo plus pembrolizumab in the neoadjuvant setting led to improved event-free survival and also improved pathologic complete response rate.
This comes in addition to the data from the AstraZeneca trial with durvalumab saying they’ve already achieved their endpoint of higher pathologic complete response rate vs. chemotherapy alone and also the data with nivolumab from Bristol-Myers Squibb saying that nivolumab plus chemotherapy leads to a better event-free survival and a better pathologic complete response rate. That information has led to Food and Drug Administration approval for their regimen.
We’re running the table with these very positive data, and I think it’s just a sign that the approach is safe and effective.
A huge question has come up. I just came from a meeting of lung cancer experts asking what to do if you have a patient with a small tumor, for example, a 3-cm tumor. Do you recommend immediate surgery followed by adjuvant therapy, chemotherapy, and then a checkpoint inhibitor if appropriate? Or do you proceed with neoadjuvant therapy if appropriate? The truth is that it’s a very difficult decision.
We have overwhelming data that the neoadjuvant approach works for that patient. Please remember that this is a clinically staged patient. This is not the patient after their surgery, where I think we have a very clear path. We have adjuvant data and adjuvant trials for those patients.
For the patient who’s in your office with a small tumor or a small tumor and only hilar lymphadenopathy, the decision there isn’t data driven, but rather it is experience driven. The data that are out there right now suggest that neoadjuvant therapy is a better way to go. Why is that?
Well, I think that the first reason is that it is probably a better regimen. I think many of you saw the recent clinical trial by Patel and colleagues in the New England Journal of Medicine with melanoma. It was an interesting trial. They gave a checkpoint inhibitor for 18 doses after surgery for melanoma versus three doses of checkpoint inhibitor, surgery, and then 15 doses of the checkpoint inhibitor.
It was 18 doses versus 18 doses, with the only difference being the three doses before surgery. Lo and behold, the three doses before surgery led to a better event-free survival.
There are preclinical data in lung cancer demonstrating that the same thing is true. Tina Cascone published on that years ago. We could talk about why, but it appears that neoadjuvant is just better.
There are other advantages to it as well. I think a big one is that all the information shows that it’s better tolerated, so you’re more likely to give all the drug. You can see if the drug isn’t working, and you can stop the drug. Also, if the drug is causing a side effect, you can see whether it’s working or not and use that decision to stop. It’s different than when you’re giving a drug in the adjuvant setting where you don’t really know whether it is working or not.
I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group. You need to weigh the pros and cons I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group coming in. It’s already an FDA-approved regimen with nivolumab and chemotherapy, and I think we’re moving to making that our standard of care now.
The way to handle it today, though, is to convene your multidisciplinary panel about every patient other than those with the tiniest of lung cancers and put your heads together to see what the best treatment is for that patient.
Dr. Kris is professor of medicine, Weill Cornell Medicine, and the William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, both in New York. He disclosed ties with Ariad Pharmaceuticals, AstraZeneca, Pfizer, PUMA, and Roche/Genentech.
A version of this article appeared on Medscape.com.
Many of you saw the press release from Merck announcing that their randomized trial comparing chemo with chemo plus pembrolizumab in the neoadjuvant setting led to improved event-free survival and also improved pathologic complete response rate.
This comes in addition to the data from the AstraZeneca trial with durvalumab saying they’ve already achieved their endpoint of higher pathologic complete response rate vs. chemotherapy alone and also the data with nivolumab from Bristol-Myers Squibb saying that nivolumab plus chemotherapy leads to a better event-free survival and a better pathologic complete response rate. That information has led to Food and Drug Administration approval for their regimen.
We’re running the table with these very positive data, and I think it’s just a sign that the approach is safe and effective.
A huge question has come up. I just came from a meeting of lung cancer experts asking what to do if you have a patient with a small tumor, for example, a 3-cm tumor. Do you recommend immediate surgery followed by adjuvant therapy, chemotherapy, and then a checkpoint inhibitor if appropriate? Or do you proceed with neoadjuvant therapy if appropriate? The truth is that it’s a very difficult decision.
We have overwhelming data that the neoadjuvant approach works for that patient. Please remember that this is a clinically staged patient. This is not the patient after their surgery, where I think we have a very clear path. We have adjuvant data and adjuvant trials for those patients.
For the patient who’s in your office with a small tumor or a small tumor and only hilar lymphadenopathy, the decision there isn’t data driven, but rather it is experience driven. The data that are out there right now suggest that neoadjuvant therapy is a better way to go. Why is that?
Well, I think that the first reason is that it is probably a better regimen. I think many of you saw the recent clinical trial by Patel and colleagues in the New England Journal of Medicine with melanoma. It was an interesting trial. They gave a checkpoint inhibitor for 18 doses after surgery for melanoma versus three doses of checkpoint inhibitor, surgery, and then 15 doses of the checkpoint inhibitor.
It was 18 doses versus 18 doses, with the only difference being the three doses before surgery. Lo and behold, the three doses before surgery led to a better event-free survival.
There are preclinical data in lung cancer demonstrating that the same thing is true. Tina Cascone published on that years ago. We could talk about why, but it appears that neoadjuvant is just better.
There are other advantages to it as well. I think a big one is that all the information shows that it’s better tolerated, so you’re more likely to give all the drug. You can see if the drug isn’t working, and you can stop the drug. Also, if the drug is causing a side effect, you can see whether it’s working or not and use that decision to stop. It’s different than when you’re giving a drug in the adjuvant setting where you don’t really know whether it is working or not.
I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group. You need to weigh the pros and cons I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group coming in. It’s already an FDA-approved regimen with nivolumab and chemotherapy, and I think we’re moving to making that our standard of care now.
The way to handle it today, though, is to convene your multidisciplinary panel about every patient other than those with the tiniest of lung cancers and put your heads together to see what the best treatment is for that patient.
Dr. Kris is professor of medicine, Weill Cornell Medicine, and the William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, both in New York. He disclosed ties with Ariad Pharmaceuticals, AstraZeneca, Pfizer, PUMA, and Roche/Genentech.
A version of this article appeared on Medscape.com.





