User login
Noninvasive prenatal testing: Where we are and where we’re going
The introduction of amniocentesis in the 1960s brought to prenatal diagnosticians the ability to detect fetal chromosome abnormalities and certain structural defects (including neural tube defects). Since that time, a goal for these practitioners has been the development of effective screening algorithms to better identify women at high risk for detectable fetal abnormalities in concert with the advent of safer and more accessible diagnostic tests, with the eventual aim being the development of a noninvasive prenatal diagnostic test.
Postamniocentesis advancements have included the identification of maternal serum analytes as well as the incorporation of first-trimester ultrasonographic measurements of the fetal nuchal translucency (NT) and nasal bone, all associated with an improved ability to identify women at increased risk for fetal trisomies 21 and 18 as well as some other fetal abnormalities. In addition, targeted ultrasound has greatly improved the ability to detect fetal structural and growth abnormalities in women of all risk levels, although it remains a highly subjective process with considerable inter/intraoperator and equipment variability.
Related article: NIPT is expanding rapidly--but don't throw out that CVS kit just yet! (Update on Obstetrics; Jaimey M. Pauli, MD, and John T. Repke, MD; January 2014)
Noninvasive prenatal screening has the advantages of being noninvasive and carrying no increased risk for fetal loss compared with chorionic villus sampling (CVS) and amniocentesis, which are associated with a small increased risk for pregnancy loss (1/500 to 1/1,500 over baseline risk for loss). However, noninvasive screening is limited compared with diagnostic procedures because it provides only a risk adjustment rather than a definitive diagnostic outcome and is mostly limited to assessment for fetal trisomies 18 and 21.
Targeted ultrasound can identify structural abnormalities associated with other chromosomal, genetic, and genomic abnormalities, but again depends on operator experience, equipment used, maternal habitus, and fetal position. Accordingly, considerable interest has remained in developing a more effective approach for detecting fetal aneuploidy and other fetal abnormalities, including assays that eventually could serve to provide noninvasive prenatal diagnosis.
RECENT ADVANCES BRING US CLOSER TO OUR ULTIMATE GOAL
The recent introduction of circulating cell-free nucleic acids (ccfna) technologies for prenatal screening for common fetal aneuploidies, better known as noninvasive prenatal testing, or NIPT, has presented a far more effective prenatal screening protocol for certain groups of women compared with the aforementioned screening algorithms that rely on measurements of the fetal NT in the late first trimester and maternal serum measurements of analytes in the first and second trimesters.
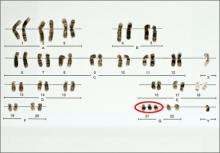
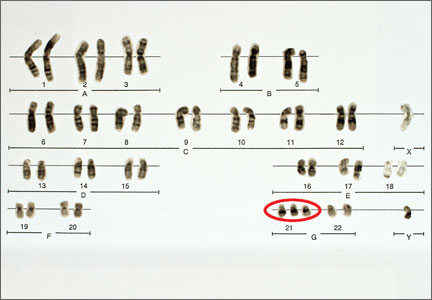
Currently, four NIPT screening products are available commercially in the United States: MaterniT21 Plus (Sequenom, San Diego, California); Verifi (Illumina, San Diego, California); Harmony Prenatal Test (Ariosa Diagnostics, San Jose, California); and Panorama Prenatal Test (Natera, San Carlos, California). While the technologies and algorithms used by each of the companies differ, they all rely on the premise that 5% to 10% of ccfna in maternal blood are fetal in nature.1 Calculating the ratios of the expected amount of each chromosome-specific nucleic acid to that actually measured in the sample, a prediction of a normal or abnormal complement for that specific chromosome is then made. None of the commercially available tests specifically identify fetal DNA or differentiate fetal from maternal DNA.
Current validation studies have thus far limited the offering of NIPT to women at increased risk for fetal aneuploidy, including those:2–6
- of advanced maternal age
- with a positive conventional screening test
- with abnormal ultrasound results suggestive of aneuploidy, or
- who have had a prior pregnancy with a chromosome aneuploidy found in the NIPT panel.
Studies of all available technologies tested on women at increased risk for fetal aneuploidy have thus far shown considerably higher sensitivities and specificities and detection rates for fetal trisomies 21, 18, and 13 than conventional screening algorithms, although detection rates for trisomy 13 are slightly lower than those observed for trisomies 21 and 18.
WE STILL HAVE MANY HURDLES TO LEAP
However, the groups of women at high risk for fetal aneuploidy just outlined represent only a small segment of the community of pregnant women. A multicenter study involving 1,914 women published February 2014 in the New England Journal of Medicine7 showed considerably and significantly lower false-positive rates and higher positive predictive values for the detection of trisomies 21 and 18 by NIPT compared with conventional fetal aneuploidy screening. This study incorporated women at low risk for fetal aneuploidies in the study cohort, although women at high risk (based on the stated range of maternal age) also were included in the cohort. Unfortunately, no information was provided in the report about the percentage of low-risk women among the study participants.
Related articles:
Noninvasive prenatal DNA testing: Who is using it, and how? Audiocast, June 2013
Noninvasive prenatal DNA tests are unproven and costly David A. Carpenter, MD (Comment & Controversy; September 2013)
Another concern about the published accuracy of NIPT clinical assays was recently sounded by Menutti and colleagues.8 The authors cited recent cases of positive NIPT outcomes for fetal trisomies 18 and 13 that were not confirmed by diagnostic testing of the pregnancies in question. The authors pondered whether such cases may reflect a limitation of the positive predictive values attributed to NIPT assays and that such limitations may carry profound inaccuracies in determining the accuracy of such protocols for rare aneuploidies.
While the improved detection rates for NIPT compared with conventional screening are not surprising, guidelines published by the American College of Obstetricians and Gynecologists still do not recommend the use of NIPT for the screening of low-risk women because of insufficient evaluation of ccfna technologies in the screening of such pregnancies.3 This also applies to twin pregnancies, despite preliminary studies showing comparable detection of trisomies 18 and 21 in such pregnancies compared with singleton pregnancies.3,9
There are no direct comparative studies of the four commercially available screening products, thus precluding a robust comparison and determination of the best existing method to use.
SO, WHERE ARE WE WITH NIPT EXACTLY?
The recent introduction of NIPT into routine obstetric care has left many clinicians with a wide range of questions, many of which cannot be answered because of little or no information, robust or otherwise, to formulate an accurate and cogent response. So let’s state what we know based on the available evidence, recognizing that this will likely change, perhaps considerably, in the weeks and months ahead.
NIPT is a far superior approach, compared with conventional screening approaches, to screening for fetal trisomies 21, 18, and 13 in women carrying singleton pregnancies who are at an increased risk for fetal chromosome abnormalities.
In our current understanding of prenatal screening and diagnosis, NIPT does not provide either the comprehensive approach or the diagnostic accuracy associated with CVS and amniocentesis. As such, NIPT is not a suitable replacement for prenatal diagnostic procedures.
However, its application to screening a low-risk population for the common fetal aneuploidies, as well as in twin pregnancies, has been supported by initial studies, and the inclusion of other clinical outcomes—including other chromosome abnormalities, such as X and Y aneuploidies, trisomy 16, and triploidy10,11 and certain genomic abnormalities (eg, 22q deletions)—in the screening algorithm will expand the future clinical applications of NIPT screening.
DOES NIPT CHANGE OUR CONCEPTS OF SCREENING AND DIAGNOSIS?
This question is simple but profound and is perhaps the most important to be asked and addressed. Is a screening algorithm that has a similar sensitivity and specificity to that of CVS and amniocentesis for the most common fetal trisomies in the first and second trimesters sufficient to replace invasive testing for most women? Does the ability to detect fetal genomic abnormalities with microarray analyses of fetal cells obtained by CVS or amniocentesis provide a far greater benefit than that possible with any screening algorithm?
With renewed interest in the cost of health-care screening and diagnosis, we need to consider how comprehensive and accurate our prenatal screening and diagnostic tests should be and whether such improvements are desired or even possible from a clinical or economic viewpoint. In addition, the development of new technologies, such as the capture and analysis of fetal cells in maternal blood, presents the potential for a direct diagnostic fetal assay without the risks of an invasive procedure.
BIAS-FREE COUNSELING CANNOT BE OVERLOOKED
That being said, the current role of NIPT and other screening protocols in obstetric care needs to be clearly communicated to women who are considering their fetal assessment options, with emphasis placed on the capabilities and limitations of prenatal screening (even the newer ccfna-based options), the actual risks associated with invasive testing, and the ability of invasive testing to provide expanded fetal information with the use of microarray analyses.
As it has been from the beginning of prenatal testing in the 1960s, counseling continues to be the most important part of the prenatal screening and diagnostic process and it is needed to facilitate clinical decisions made by women and couples. Counseling must include an accurate communication of the risks, benefits, and limitations of the aforementioned options and issues, and should be provided in a manner that strives to be free of bias, direction, and the personal opinions of the counselor.
In order to provide such counseling, we must remain informed of the ongoing work in the field of prenatal testing, a task that has become more challenging with the rapid release of a considerable amount of new information on prenatal screening technologies over the past 2 years. This will likely continue, and perhaps become even more frenetic, with the expected release of additional information on the clinical applications of ccfna technologies in the near future as well as the development of new technologies applicable for the screening and diagnosis of fetal abnormalities.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487.
- Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell–free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120(6):1532–1534.
- Bianchi DW, Platt LD, Goldberg JD, et al; MatErnal Blood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA-sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13(11):913–920.
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet Med. 2012;14(3):296–305.
- Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Menutti MT, Cherry AM, Morrissette JJ, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy. Am J Obstet Gynecol. 2013;209(5):415−419.
- Canick JA, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn. 2012;32(8):730–734.
- Nicolaides KH, Syngelaki A, Gil MM, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood [published online ahead of print October 10, 2013]. Fetal Diagn Ther.
- Semango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–649.
The introduction of amniocentesis in the 1960s brought to prenatal diagnosticians the ability to detect fetal chromosome abnormalities and certain structural defects (including neural tube defects). Since that time, a goal for these practitioners has been the development of effective screening algorithms to better identify women at high risk for detectable fetal abnormalities in concert with the advent of safer and more accessible diagnostic tests, with the eventual aim being the development of a noninvasive prenatal diagnostic test.
Postamniocentesis advancements have included the identification of maternal serum analytes as well as the incorporation of first-trimester ultrasonographic measurements of the fetal nuchal translucency (NT) and nasal bone, all associated with an improved ability to identify women at increased risk for fetal trisomies 21 and 18 as well as some other fetal abnormalities. In addition, targeted ultrasound has greatly improved the ability to detect fetal structural and growth abnormalities in women of all risk levels, although it remains a highly subjective process with considerable inter/intraoperator and equipment variability.
Related article: NIPT is expanding rapidly--but don't throw out that CVS kit just yet! (Update on Obstetrics; Jaimey M. Pauli, MD, and John T. Repke, MD; January 2014)
Noninvasive prenatal screening has the advantages of being noninvasive and carrying no increased risk for fetal loss compared with chorionic villus sampling (CVS) and amniocentesis, which are associated with a small increased risk for pregnancy loss (1/500 to 1/1,500 over baseline risk for loss). However, noninvasive screening is limited compared with diagnostic procedures because it provides only a risk adjustment rather than a definitive diagnostic outcome and is mostly limited to assessment for fetal trisomies 18 and 21.
Targeted ultrasound can identify structural abnormalities associated with other chromosomal, genetic, and genomic abnormalities, but again depends on operator experience, equipment used, maternal habitus, and fetal position. Accordingly, considerable interest has remained in developing a more effective approach for detecting fetal aneuploidy and other fetal abnormalities, including assays that eventually could serve to provide noninvasive prenatal diagnosis.
RECENT ADVANCES BRING US CLOSER TO OUR ULTIMATE GOAL
The recent introduction of circulating cell-free nucleic acids (ccfna) technologies for prenatal screening for common fetal aneuploidies, better known as noninvasive prenatal testing, or NIPT, has presented a far more effective prenatal screening protocol for certain groups of women compared with the aforementioned screening algorithms that rely on measurements of the fetal NT in the late first trimester and maternal serum measurements of analytes in the first and second trimesters.
Currently, four NIPT screening products are available commercially in the United States: MaterniT21 Plus (Sequenom, San Diego, California); Verifi (Illumina, San Diego, California); Harmony Prenatal Test (Ariosa Diagnostics, San Jose, California); and Panorama Prenatal Test (Natera, San Carlos, California). While the technologies and algorithms used by each of the companies differ, they all rely on the premise that 5% to 10% of ccfna in maternal blood are fetal in nature.1 Calculating the ratios of the expected amount of each chromosome-specific nucleic acid to that actually measured in the sample, a prediction of a normal or abnormal complement for that specific chromosome is then made. None of the commercially available tests specifically identify fetal DNA or differentiate fetal from maternal DNA.
Current validation studies have thus far limited the offering of NIPT to women at increased risk for fetal aneuploidy, including those:2–6
- of advanced maternal age
- with a positive conventional screening test
- with abnormal ultrasound results suggestive of aneuploidy, or
- who have had a prior pregnancy with a chromosome aneuploidy found in the NIPT panel.
Studies of all available technologies tested on women at increased risk for fetal aneuploidy have thus far shown considerably higher sensitivities and specificities and detection rates for fetal trisomies 21, 18, and 13 than conventional screening algorithms, although detection rates for trisomy 13 are slightly lower than those observed for trisomies 21 and 18.
WE STILL HAVE MANY HURDLES TO LEAP
However, the groups of women at high risk for fetal aneuploidy just outlined represent only a small segment of the community of pregnant women. A multicenter study involving 1,914 women published February 2014 in the New England Journal of Medicine7 showed considerably and significantly lower false-positive rates and higher positive predictive values for the detection of trisomies 21 and 18 by NIPT compared with conventional fetal aneuploidy screening. This study incorporated women at low risk for fetal aneuploidies in the study cohort, although women at high risk (based on the stated range of maternal age) also were included in the cohort. Unfortunately, no information was provided in the report about the percentage of low-risk women among the study participants.
Related articles:
Noninvasive prenatal DNA testing: Who is using it, and how? Audiocast, June 2013
Noninvasive prenatal DNA tests are unproven and costly David A. Carpenter, MD (Comment & Controversy; September 2013)
Another concern about the published accuracy of NIPT clinical assays was recently sounded by Menutti and colleagues.8 The authors cited recent cases of positive NIPT outcomes for fetal trisomies 18 and 13 that were not confirmed by diagnostic testing of the pregnancies in question. The authors pondered whether such cases may reflect a limitation of the positive predictive values attributed to NIPT assays and that such limitations may carry profound inaccuracies in determining the accuracy of such protocols for rare aneuploidies.
While the improved detection rates for NIPT compared with conventional screening are not surprising, guidelines published by the American College of Obstetricians and Gynecologists still do not recommend the use of NIPT for the screening of low-risk women because of insufficient evaluation of ccfna technologies in the screening of such pregnancies.3 This also applies to twin pregnancies, despite preliminary studies showing comparable detection of trisomies 18 and 21 in such pregnancies compared with singleton pregnancies.3,9
There are no direct comparative studies of the four commercially available screening products, thus precluding a robust comparison and determination of the best existing method to use.
SO, WHERE ARE WE WITH NIPT EXACTLY?
The recent introduction of NIPT into routine obstetric care has left many clinicians with a wide range of questions, many of which cannot be answered because of little or no information, robust or otherwise, to formulate an accurate and cogent response. So let’s state what we know based on the available evidence, recognizing that this will likely change, perhaps considerably, in the weeks and months ahead.
NIPT is a far superior approach, compared with conventional screening approaches, to screening for fetal trisomies 21, 18, and 13 in women carrying singleton pregnancies who are at an increased risk for fetal chromosome abnormalities.
In our current understanding of prenatal screening and diagnosis, NIPT does not provide either the comprehensive approach or the diagnostic accuracy associated with CVS and amniocentesis. As such, NIPT is not a suitable replacement for prenatal diagnostic procedures.
However, its application to screening a low-risk population for the common fetal aneuploidies, as well as in twin pregnancies, has been supported by initial studies, and the inclusion of other clinical outcomes—including other chromosome abnormalities, such as X and Y aneuploidies, trisomy 16, and triploidy10,11 and certain genomic abnormalities (eg, 22q deletions)—in the screening algorithm will expand the future clinical applications of NIPT screening.
DOES NIPT CHANGE OUR CONCEPTS OF SCREENING AND DIAGNOSIS?
This question is simple but profound and is perhaps the most important to be asked and addressed. Is a screening algorithm that has a similar sensitivity and specificity to that of CVS and amniocentesis for the most common fetal trisomies in the first and second trimesters sufficient to replace invasive testing for most women? Does the ability to detect fetal genomic abnormalities with microarray analyses of fetal cells obtained by CVS or amniocentesis provide a far greater benefit than that possible with any screening algorithm?
With renewed interest in the cost of health-care screening and diagnosis, we need to consider how comprehensive and accurate our prenatal screening and diagnostic tests should be and whether such improvements are desired or even possible from a clinical or economic viewpoint. In addition, the development of new technologies, such as the capture and analysis of fetal cells in maternal blood, presents the potential for a direct diagnostic fetal assay without the risks of an invasive procedure.
BIAS-FREE COUNSELING CANNOT BE OVERLOOKED
That being said, the current role of NIPT and other screening protocols in obstetric care needs to be clearly communicated to women who are considering their fetal assessment options, with emphasis placed on the capabilities and limitations of prenatal screening (even the newer ccfna-based options), the actual risks associated with invasive testing, and the ability of invasive testing to provide expanded fetal information with the use of microarray analyses.
As it has been from the beginning of prenatal testing in the 1960s, counseling continues to be the most important part of the prenatal screening and diagnostic process and it is needed to facilitate clinical decisions made by women and couples. Counseling must include an accurate communication of the risks, benefits, and limitations of the aforementioned options and issues, and should be provided in a manner that strives to be free of bias, direction, and the personal opinions of the counselor.
In order to provide such counseling, we must remain informed of the ongoing work in the field of prenatal testing, a task that has become more challenging with the rapid release of a considerable amount of new information on prenatal screening technologies over the past 2 years. This will likely continue, and perhaps become even more frenetic, with the expected release of additional information on the clinical applications of ccfna technologies in the near future as well as the development of new technologies applicable for the screening and diagnosis of fetal abnormalities.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
The introduction of amniocentesis in the 1960s brought to prenatal diagnosticians the ability to detect fetal chromosome abnormalities and certain structural defects (including neural tube defects). Since that time, a goal for these practitioners has been the development of effective screening algorithms to better identify women at high risk for detectable fetal abnormalities in concert with the advent of safer and more accessible diagnostic tests, with the eventual aim being the development of a noninvasive prenatal diagnostic test.
Postamniocentesis advancements have included the identification of maternal serum analytes as well as the incorporation of first-trimester ultrasonographic measurements of the fetal nuchal translucency (NT) and nasal bone, all associated with an improved ability to identify women at increased risk for fetal trisomies 21 and 18 as well as some other fetal abnormalities. In addition, targeted ultrasound has greatly improved the ability to detect fetal structural and growth abnormalities in women of all risk levels, although it remains a highly subjective process with considerable inter/intraoperator and equipment variability.
Related article: NIPT is expanding rapidly--but don't throw out that CVS kit just yet! (Update on Obstetrics; Jaimey M. Pauli, MD, and John T. Repke, MD; January 2014)
Noninvasive prenatal screening has the advantages of being noninvasive and carrying no increased risk for fetal loss compared with chorionic villus sampling (CVS) and amniocentesis, which are associated with a small increased risk for pregnancy loss (1/500 to 1/1,500 over baseline risk for loss). However, noninvasive screening is limited compared with diagnostic procedures because it provides only a risk adjustment rather than a definitive diagnostic outcome and is mostly limited to assessment for fetal trisomies 18 and 21.
Targeted ultrasound can identify structural abnormalities associated with other chromosomal, genetic, and genomic abnormalities, but again depends on operator experience, equipment used, maternal habitus, and fetal position. Accordingly, considerable interest has remained in developing a more effective approach for detecting fetal aneuploidy and other fetal abnormalities, including assays that eventually could serve to provide noninvasive prenatal diagnosis.
RECENT ADVANCES BRING US CLOSER TO OUR ULTIMATE GOAL
The recent introduction of circulating cell-free nucleic acids (ccfna) technologies for prenatal screening for common fetal aneuploidies, better known as noninvasive prenatal testing, or NIPT, has presented a far more effective prenatal screening protocol for certain groups of women compared with the aforementioned screening algorithms that rely on measurements of the fetal NT in the late first trimester and maternal serum measurements of analytes in the first and second trimesters.
Currently, four NIPT screening products are available commercially in the United States: MaterniT21 Plus (Sequenom, San Diego, California); Verifi (Illumina, San Diego, California); Harmony Prenatal Test (Ariosa Diagnostics, San Jose, California); and Panorama Prenatal Test (Natera, San Carlos, California). While the technologies and algorithms used by each of the companies differ, they all rely on the premise that 5% to 10% of ccfna in maternal blood are fetal in nature.1 Calculating the ratios of the expected amount of each chromosome-specific nucleic acid to that actually measured in the sample, a prediction of a normal or abnormal complement for that specific chromosome is then made. None of the commercially available tests specifically identify fetal DNA or differentiate fetal from maternal DNA.
Current validation studies have thus far limited the offering of NIPT to women at increased risk for fetal aneuploidy, including those:2–6
- of advanced maternal age
- with a positive conventional screening test
- with abnormal ultrasound results suggestive of aneuploidy, or
- who have had a prior pregnancy with a chromosome aneuploidy found in the NIPT panel.
Studies of all available technologies tested on women at increased risk for fetal aneuploidy have thus far shown considerably higher sensitivities and specificities and detection rates for fetal trisomies 21, 18, and 13 than conventional screening algorithms, although detection rates for trisomy 13 are slightly lower than those observed for trisomies 21 and 18.
WE STILL HAVE MANY HURDLES TO LEAP
However, the groups of women at high risk for fetal aneuploidy just outlined represent only a small segment of the community of pregnant women. A multicenter study involving 1,914 women published February 2014 in the New England Journal of Medicine7 showed considerably and significantly lower false-positive rates and higher positive predictive values for the detection of trisomies 21 and 18 by NIPT compared with conventional fetal aneuploidy screening. This study incorporated women at low risk for fetal aneuploidies in the study cohort, although women at high risk (based on the stated range of maternal age) also were included in the cohort. Unfortunately, no information was provided in the report about the percentage of low-risk women among the study participants.
Related articles:
Noninvasive prenatal DNA testing: Who is using it, and how? Audiocast, June 2013
Noninvasive prenatal DNA tests are unproven and costly David A. Carpenter, MD (Comment & Controversy; September 2013)
Another concern about the published accuracy of NIPT clinical assays was recently sounded by Menutti and colleagues.8 The authors cited recent cases of positive NIPT outcomes for fetal trisomies 18 and 13 that were not confirmed by diagnostic testing of the pregnancies in question. The authors pondered whether such cases may reflect a limitation of the positive predictive values attributed to NIPT assays and that such limitations may carry profound inaccuracies in determining the accuracy of such protocols for rare aneuploidies.
While the improved detection rates for NIPT compared with conventional screening are not surprising, guidelines published by the American College of Obstetricians and Gynecologists still do not recommend the use of NIPT for the screening of low-risk women because of insufficient evaluation of ccfna technologies in the screening of such pregnancies.3 This also applies to twin pregnancies, despite preliminary studies showing comparable detection of trisomies 18 and 21 in such pregnancies compared with singleton pregnancies.3,9
There are no direct comparative studies of the four commercially available screening products, thus precluding a robust comparison and determination of the best existing method to use.
SO, WHERE ARE WE WITH NIPT EXACTLY?
The recent introduction of NIPT into routine obstetric care has left many clinicians with a wide range of questions, many of which cannot be answered because of little or no information, robust or otherwise, to formulate an accurate and cogent response. So let’s state what we know based on the available evidence, recognizing that this will likely change, perhaps considerably, in the weeks and months ahead.
NIPT is a far superior approach, compared with conventional screening approaches, to screening for fetal trisomies 21, 18, and 13 in women carrying singleton pregnancies who are at an increased risk for fetal chromosome abnormalities.
In our current understanding of prenatal screening and diagnosis, NIPT does not provide either the comprehensive approach or the diagnostic accuracy associated with CVS and amniocentesis. As such, NIPT is not a suitable replacement for prenatal diagnostic procedures.
However, its application to screening a low-risk population for the common fetal aneuploidies, as well as in twin pregnancies, has been supported by initial studies, and the inclusion of other clinical outcomes—including other chromosome abnormalities, such as X and Y aneuploidies, trisomy 16, and triploidy10,11 and certain genomic abnormalities (eg, 22q deletions)—in the screening algorithm will expand the future clinical applications of NIPT screening.
DOES NIPT CHANGE OUR CONCEPTS OF SCREENING AND DIAGNOSIS?
This question is simple but profound and is perhaps the most important to be asked and addressed. Is a screening algorithm that has a similar sensitivity and specificity to that of CVS and amniocentesis for the most common fetal trisomies in the first and second trimesters sufficient to replace invasive testing for most women? Does the ability to detect fetal genomic abnormalities with microarray analyses of fetal cells obtained by CVS or amniocentesis provide a far greater benefit than that possible with any screening algorithm?
With renewed interest in the cost of health-care screening and diagnosis, we need to consider how comprehensive and accurate our prenatal screening and diagnostic tests should be and whether such improvements are desired or even possible from a clinical or economic viewpoint. In addition, the development of new technologies, such as the capture and analysis of fetal cells in maternal blood, presents the potential for a direct diagnostic fetal assay without the risks of an invasive procedure.
BIAS-FREE COUNSELING CANNOT BE OVERLOOKED
That being said, the current role of NIPT and other screening protocols in obstetric care needs to be clearly communicated to women who are considering their fetal assessment options, with emphasis placed on the capabilities and limitations of prenatal screening (even the newer ccfna-based options), the actual risks associated with invasive testing, and the ability of invasive testing to provide expanded fetal information with the use of microarray analyses.
As it has been from the beginning of prenatal testing in the 1960s, counseling continues to be the most important part of the prenatal screening and diagnostic process and it is needed to facilitate clinical decisions made by women and couples. Counseling must include an accurate communication of the risks, benefits, and limitations of the aforementioned options and issues, and should be provided in a manner that strives to be free of bias, direction, and the personal opinions of the counselor.
In order to provide such counseling, we must remain informed of the ongoing work in the field of prenatal testing, a task that has become more challenging with the rapid release of a considerable amount of new information on prenatal screening technologies over the past 2 years. This will likely continue, and perhaps become even more frenetic, with the expected release of additional information on the clinical applications of ccfna technologies in the near future as well as the development of new technologies applicable for the screening and diagnosis of fetal abnormalities.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487.
- Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell–free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120(6):1532–1534.
- Bianchi DW, Platt LD, Goldberg JD, et al; MatErnal Blood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA-sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13(11):913–920.
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet Med. 2012;14(3):296–305.
- Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Menutti MT, Cherry AM, Morrissette JJ, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy. Am J Obstet Gynecol. 2013;209(5):415−419.
- Canick JA, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn. 2012;32(8):730–734.
- Nicolaides KH, Syngelaki A, Gil MM, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood [published online ahead of print October 10, 2013]. Fetal Diagn Ther.
- Semango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–649.
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487.
- Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell–free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120(6):1532–1534.
- Bianchi DW, Platt LD, Goldberg JD, et al; MatErnal Blood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA-sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13(11):913–920.
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet Med. 2012;14(3):296–305.
- Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Menutti MT, Cherry AM, Morrissette JJ, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy. Am J Obstet Gynecol. 2013;209(5):415−419.
- Canick JA, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn. 2012;32(8):730–734.
- Nicolaides KH, Syngelaki A, Gil MM, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood [published online ahead of print October 10, 2013]. Fetal Diagn Ther.
- Semango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–649.
Does your obstetric unit have a protocol for treating amniotic fluid embolism?
Amniotic fluid embolism (AFE) occurs in about 1 in 20,000 to 1 in 40,000 deliveries.1,2 Although the condition is rare, the case fatality rate is high, and AFE is a common cause of maternal death in developed countries. AFE cannot be predicted or prevented. Moreover, the condition is difficult to precisely define and is often a diagnosis of exclusion.
AFE should be considered in the differential diagnosis of a pregnant woman with sudden onset of shortness of breath, hypotension, or cardiac arrhythmia or arrest, followed by coagulopathy and hemorrhage. Premonitory symptoms, including restlessness, confusion, disorientation, agitation, chills, nausea, numbness, and tingling, are commonly reported just before the cardiorespiratory collapse. AFE is less likely if the initial obstetric event is hemorrhage in the absence of cardiorespiratory compromise or a preceding coagulopathy.3
Typically, the onset is just before birth, during birth, or within the first few hours after delivery. In the United Kingdom, which has a robust centralized registry for reporting AFE, about 56% of cases occur before birth and 44% after birth.4
Related article: Is the incidence of amniotic fluid embolism rising? John T. Repke, MD (Examining the Evidence, August 2010)
The resources available to obstetric units vary greatly. Each unit needs to assess its resources and develop an AFE treatment protocol that builds on the unique strengths of the unit. Treatment of AFE requires the coordinated actions of anesthesiologists, obstetricians, nurses, the blood bank, pharmacy, and cardiovascular specialists. Coordinated activity among the members of such a large multidisciplinary team requires a written protocol that is practiced on a regular basis.
Six important components of a multidisciplinary response to AFE treatment protocol are:
- high-quality cardiopulmonary resuscitation (CPR)
- a protocol for massive transfusion
- treatment of diffuse bleeding and coagulopathy
- treatment of uterine and pelvic bleeding
- extracorporeal lung and heart support
- post-AFE intensive care.
1. Initiate high-quality CPR
Hypotension and hypoxemia due to cardiac and pulmonary dysfunction are prominent features of AFE. Dysrythmias such as pulseless electrical activity, bradycardia, ventricular fibrillation, and asystole are common. Rapid institution of high-quality CPR is critical to the survival of women with AFE.
Interventions often used in CPR of patients with AFE include initiation of high-quality chest compressions, early defibrillation if indicated, immediate administration of 100% oxygen by mask ventilation followed by early intubation, and rapid establishment of peripheral, arterial, and central venous access. Volume assessment, fluid replacement, and administration of vasopressors and inotropes are also important.
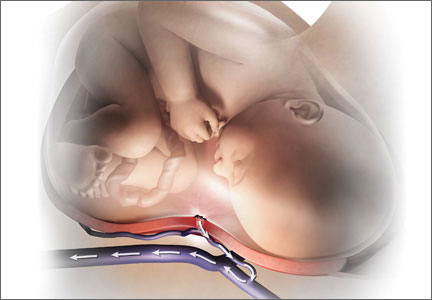
CPR of pregnant women requires special interventions, including maximal left lateral displacement of the uterus to reduce compression of the descending aorta and vena cava. Lateral displacement of the uterus can be accomplished by left lateral tilt or by manual uterine displacement. To optimize the effectiveness of chest compressions, many experts recommend placing the woman in a supine position and using manual uterine displacement rather than a left lateral tilt.5 For chest compressions, the hands should be placed just above the center of the sternum to adjust for the elevation of the diaphragm caused by the gravid uterus.
The gravid uterus can compromise the effectiveness of CPR. Fetal viability and neurologic outcome are best if delivery occurs within 5 minutes of the onset of cardiopulmonary arrest. If the gestational age of the fetus is consistent with extrauterine viability and initial CPR has not restored cardiac function, it is best to initiate fetal delivery within 4 minutes of the onset of cardiopulmonary arrest with the intent to deliver the fetus within 5 minutes.6,7 If the fetus is beyond 20 weeks’ gestational age, delivery early in the course of CPR improves the effectiveness of maternal resuscitation and may increase the probability of maternal survival.
In one study of the response of anesthesiologists, obstetricians, and nurses to a simulated cardiac arrest caused by an AFE, the participants did not routinely use defibrillation when indicated, did not place a firm support under the back for chest compressions, and did not switch the provider of chest compressions every 2 minutes.8 This study indicates that additional training and routinely scheduled multidisciplinary simulation of the response to cardiopulmonary arrest could improve the quality of our CPR.
2. Use a massive transfusion protocol
Severe coagulopathy and diffuse bleeding are commonly encountered in AFE. Target goals for the replacement of blood products include:
- hemoglobin concentration ≥8 g/dL
- fibrinogen ≥150 to 200 mg/dL
- platelets ≥50,000/μL
- prothrombin time international normalized ratio (INR) ≤1.5.
Most massive transfusion protocols provide for the rapid delivery of 4 to 8 units of red blood cells and a similar number of units of fresh frozen plasma to the patient’s bedside. In the management of AFE, 20 to 30 units of red blood cells and a similar quantity of fresh frozen plasma may need to be transfused. Cryoprecipitate takes 20 to 30 minutes to thaw, so preparations to transfuse cryoprecipitate should be initiated as soon as the massive transfusion protocol is triggered. A case of AFE can completely empty the blood bank of all available blood products and necessitate the use of alternative agents.
Lyophilized fibrinogen concentrate (RiaSTAP) is approved by the US Food and Drug Administration for the treatment of congenital hypofibrinogenemia and also may be useful to replace fibrinogen in cases of AFE. In many hospitals, large quantities of fresh frozen plasma are not immediately available; lyophilized fibrinogen concentrate may be especially useful in these settings. Another advantage of fibrinogen concentrate is that large amounts of fibrinogen can be administered in a small volume of intravenous fluid. Fibrinogen concentrate typically is used at a dose of 70 mg/kg of body weight.9,10
Intraoperative red cell salvage occasionally is used in cases of obstetric hemorrhage. In one case report of the use of red cell salvage with leukocyte depletion filtration during treatment of an AFE, acute hypotension developed in the patient after the transfusion of salvaged red cells.11 This case report raises safety concerns about the use of salvaged cells in women with severe AFE.
Related article: 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage Baha M. Sibai, MD (June 2011)
3. Treat diffuse bleeding and coagulopathy
In addition to the initiation of the massive transfusion protocol, additional treatments that may be helpful in managing the coagulopathy of AFE include tranexamic acid, recombinant factor VIIa (rFVIIa), and exchange transfusion.
AFE is often associated with hyperfibrinolysis, which can cause excessive bleeding.12 Tranexamic acid blocks the lysine binding sites on plasminogen and thereby reduces the lysis of fibrin clots. Clinical trials in patients who have undergone trauma have demonstrated that the administration of tranexamic acid reduces blood loss.13 The dose of tranexamic acid is approximately 10 to 20 mg/kg of body weight, or approximately 1 g.
Controversy exists about the use of rFVIIa to treat the coagulopathy and bleeding caused by AFE. Some authorities believe that rFVIIa is associated with an increased AFE case fatality rate.14 Other authorities believe rFVIIa may be useful in the treatment of AFE coagulopathy, especially when bleeding persists despite aggressive blood and component replacement.”15 The dose of rFVIIa is approximately 90 µg/kg of body weight. rFVIIa is extremely expensive.
Exchange transfusion has been used successfully to treat AFE.16 In women with AFE, exchange transfusion removes circulating cells, cell fragments, and substances that trigger systemic anaphylaxis and coagulopathy, thereby enhancing rapid recovery.
Related article: Act fast when confronted by a coagulopathy postpartum Robert L. Barbieri, MD (Editorial; March 2012)
4. Treat uterine and pelvic bleeding
Obstetrician-gynecologists are experts in the control of uterine and pelvic bleeding. Interventions that commonly are used to control uterine and pelvic bleeding in cases of postpartum hemorrhage, uterine rupture, or placenta accreta also can be applied in cases of AFE with uncontrolled uterine and pelvic bleeding. These techniques include:
- use of uterine compression sutures
- the Bakri balloon
- a uterine tourniquet
- vascular clamps on the ovarian vessels.17,18
In many cases of AFE, total or supracervical hysterectomy is necessary to control uterine bleeding. Uterine artery embolization, if available, has been reported to be helpful in select cases. However, many women with AFE are too unstable to survive transfer to an interventional radiology suite. Additional interventions to control bleeding include hypogastric artery ligation, infrarenal aortic compression, and pelvic packing.
Cross-clamping the aorta below the renal vessels can reduce blood flow to the pelvis and provide time for cardiopulmonary and volume resuscitation. Alternatively, placing pressure on the infrarenal aorta with a sponge or directly by hand can help reduce blood flow to the pelvis.19
In many cases of AFE, pelvic hemorrhage is difficult to control. Even if surgical pedicles are ligated securely, the coagulopathy of AFE may cause persistent oozing from areas of minor tissue trauma. Uncontrolled blood loss can be a proximate cause of death in women with AFE. All written protocols for responding to an AFE should include a plan to use pelvic packing for patients in whom standard operative procedures do not produce adequate control of bleeding. A “mushroom,” “parachute,” or “umbrella” pack has been reported to help stabilize the severely ill patient with pelvic bleeding and permit effective resuscitation and blood product replacement.20
Related articles:
A stitch in time: The B-Lynch, Hayman and Pereira uterine compression sutures Robert L. Barbieri, MD (Editorial, December 2012)
Have you made the best use of the Bakri balloon in PPH? Robert L. Barbieri, MD (Editorial, July 2011)
5. Consider extracorporeal lung and heart support
In many cases of AFE, both lung and cardiac function are severely compromised. Both veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and full cardiopulmonary bypass provide support for the failing lung and heart. Based on a small number of case reports, extracorporeal lung and heart support appear to be useful in the treatment of AFE.21–26 Using the Seldinger technique,27 it is technically feasible to rapidly access a major vein and artery to provide the input and output ports for VA-ECMO. Unlike the cardiopulmonary bypass pump, the VA-ECMO pump does not have a reservoir that needs to be primed with blood and is smaller and more portable. To provide a patient with VA-ECMO or cardiopulmonary bypass, a cardiac interventionist and a perfusionist must be available. Extracorporeal lung and heart support require heparinization of the patient’s blood, which may result in increased bleeding. Both VA-ECMO and cardiopulmonary bypass, along with the diseases for which they are used, may cause renal dysfunction, neurologic injury, and infection.28
Alternative approaches that provide support of the heart—but not lung—are the Impella pump, TandemHeart, and the intra-aortic balloon pump. An alternative that provides lung support—but not cardiac support—is veno-venous ECMO.
In developing a written protocol for responding to an AFE, obstetricians should explore the potential availability of VA-ECMO, cardiopulmonary bypass, or other cardiopulmonary support devices as options for patients who have not responded to standard treatment of AFE and are at high risk of death.
6. Post-AFE intensive care
After stabilization, most women with AFE will require intensive care for 48 to 96 hours. Some experts have proposed that all survivors of cardiopulmonary arrest who are successfully resuscitated and stabilized be transferred to hospitals that specialize in post−cardiac arrest care to improve outcomes.
Assessment of organ injury is important after an AFE. In addition, encephalopathy is a common complication of AFE, and sequential neurologic examination is a priority. Therapeutic hypothermia (TH) may help to preserve neurologic function after AFE.29 However, TH may cause a mild coagulopathy by inhibiting platelet activation and enzyme activity of clotting factors. Because coagulopathy is a prominent feature of AFE, TH may be contraindicated if the patient has a clinically significant baseline coagulopathy.30
DEVELOP AN AFE PROTOCOL AND PRACTICE THE COMPONENTS
Practicing the components of obstetric protocols can improve unit performance and patient outcomes.31 The components of an AFE protocol, as described in this article, include high-quality CPR, a protocol for massive transfusion, treatment of diffuse bleeding and coagulopathy, treatment of uterine and pelvic bleeding, extracorporeal lung and heart support, and post-AFE intensive care. Practicing these components of an AFE protocol will enhance performance across many common obstetric complications including postpartum hemorrhage, uterine rupture, placenta accreta, and pulmonary embolism.
When Chesley “Sully” Sullenberger and his copilot landed Flight 1549 in the Hudson River in New York, he had never practiced that specific response to twin engine failure, but he had practiced many emergency responses involving related scenarios. The combination of exceptional flight experience and years of practicing the response to emergency scenarios in simulation exercises permitted him and his copilot to execute a uniquely clever plan to solve a life-threatening
emergency. In a related way, practicing the components of AFE treatment will help obstetricians, obstetric anesthesiologists, and their multidisciplinary team to improve the responses to all major obstetric emergencies.
INSTANT POLL
Does your obstetric unit have a written protocol for treating an amniotic fluid embolism (AFE)? Has your obstetric unit practiced any of the components of the AFE treatment protocol: 1) high-quality cardiopulmonary resuscitation, 2) a protocol for massive transfusion protocol, 3) treatment of diffuse bleeding and coagulopathy, 4) treatment of uterine and pelvic bleeding, 5) extracorporeal lung and heart support, and 6) post-AFE intensive care?
Tell us—at [email protected]. Please include your name and the city and state in which you practice.
- Kramer MS, Rouleau J, Baskett TF, Joseph KS; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Amniotic-fluid embolism and medical induction of labour: A retrospective, population-based cohort study. Lancet. 2006;368(9545):1444–1448.
- Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolism: A population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199(1):49.e1–49.e8.
- Tuffnell D, Knight M, Plaat F. Amniotic fluid embolism—An update. Anaesthesia. 2011;66(1):3–6.
- Knight M, Tuffnell D, Brocklehurst P, Spark P, Kurinczuk JJ; UK Obstetric Surveillance System. Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol. 2010;115(5):910–917.
- Kundra P, Khanna S, Habeebullahg S, Ravishankar M. Manual displacement of the uterus during Caesarean section. Anaesthesia. 2007;62(5):460–465.
- Katz VL, Dotters DJ, Droegemueller W. Perimortem cesarean delivery. Obstet Gynecol. 1986;68(4):571–576.
- Katz V, Balderston K, DeFreest M. Perimortem cesarean delivery: Were our assumptions correct? Am J Obstet Gynecol. 2005;192(6):1916–1920.
- Lipman SS, Daniels KI, Carvalho B, et al. Deficits in the provision of cardiopulmonary resuscitation during simulated obstetric crises. Am J Obstet Gynecol. 2010;203(2):179.e1–179.e5.
- Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anesth. 2010;19(2):218–223.
- Sorensen B, Tang M, Larsen OH, Laursen PN, Fenger-Eriksen C, Rea CJ. The role of fibrinogen: A new paradigm in the treatment of coagulopathic bleeding. Thromb Res. 2011;128(Suppl 1):S13–S16.
- Rogers WK, Wernimont SA, Kumar GC, Bennett E, Chestnut DH. Acute hypotension associated with intraoperative cell salvage using a leukocyte depletion filter during management of obstetric hemorrhage due to amniotic fluid embolism. Anesth Analg. 2013;117(2):449–452.
- Collins NF, Bloor M, McDonnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anesth. 2013;22(1):71–76.
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054.
- Leighton BL, Wall MH, Lockhart EM, Phillips LE, Zatta AJ. Use of recombinant factor VIIa in patients with amniotic fluid embolism: A systematic review of case reports. Anesthesiology. 2011;115(6):1201–1208.
- Huber AW, Raio L, Alberio L, Ghezzi F, Surbek DV. Recombinant human factor VIIa prevents hysterectomy in severe postpartum hemorrhage: single center study. J Perinat Med. 2011;40(1):43–49.
- Dodgson J, Martin J, Boswell J, Goodall HB, Smith R. Probable amniotic fluid embolism precipitated by amniocentesis and treated by exchange transfusion. Brit Med J (Clin Res Ed). 1987;294(6583):1322–1323.
- Barbieri RL. A stitch in time: The B-Lynch, Hayman and Pereira uterine compression sutures. OBG Manage. 2012;24(12):6, 8, 10, 11.
- Barbieri RL. Have you made the best use of the Bakri balloon in PPH? OBG Manage. 2011;23(7):6, 8, 9.
- Belfort MA, Zimmerman J, Schemmer G, Oldroyd R, Smilanich R, Pearce M. Aortic compression and cross clamping in a case of placenta percreta and amniotic fluid embolism: A case report. AJP Rep. 2011;1(1):33–36.
- Dildy GA, Scott JR, Saffer CS, Belfort MA. An
effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol. 2006;108(5):1222–1226. - Stanten RD, Iverson LI, Daugharty TM, Lovett SM, Terry C, Blumenstock E. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: Diagnosis by transesophageal echocardiogram and treatment by cardiopulmonary bypass. Obstet Gynecol. 2003;102(3):496–498.
- Ho CH, Chen KB, Liu SK, Liu YF, Cheng HC, Wu RS. Early application of extracorporeal membrane oxygenation in a patient with amniotic fluid embolism. Acta Anaesthesiol Taiwan. 2009;47(2):99–102.
- Shen HP, Chang WC, Yeh LS, Ho M. Amniotic fluid embolism treated with emergency extracorporeal membrane oxygenation: A case report.
J Reprod Med. 2009;54(11–12):706–708. - Lee PH, Shulman MS, Vellayappan U, Symes JF, Olenchock SA Jr. Surgical treatment of amniotic fluid embolism with cardiopulmonary collapse. Ann Thorac Surg. 2010;90(5):1694–1696.
- Firstenberg MS, Abel E, Blais D, et al. Temporary extracorporeal circulatory support and pulmonary embolectomy for catastrophic amniotic fluid embolism. Heart Surg Forum. 2011;14(3):E157–E159.
- Ecker JL, Solt K, Fitzsimons MG, MacGillivray TE. Case records of the Massachusetts General Hospital. Case 40-2012: A 43-year-old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med. 2012;367(26):2528–2536.
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953;39(5):368–376.
- Cheng R, Hachamovitch R, Kittelson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2013; epub Nov 8.
- Rittenberger JC, Kelly E, Jang D, Greer K, Heffner A. Successful outcome utilizing hypothermia after cardiac arrest in pregnancy: A case report. Crit Care Med. 2008;36(4):1354–1356.
- Michelson AD, MacGregor H, Barnard MR, Krestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71(5):633–640.
- Rivzi F, Mackey R, Barrett T, McKenna P, Geary M. Successful reduction of massive postpartum haemorrhage by use of guidelines and staff education. BJOG. 2004;111(5):495–498.
Amniotic fluid embolism (AFE) occurs in about 1 in 20,000 to 1 in 40,000 deliveries.1,2 Although the condition is rare, the case fatality rate is high, and AFE is a common cause of maternal death in developed countries. AFE cannot be predicted or prevented. Moreover, the condition is difficult to precisely define and is often a diagnosis of exclusion.
AFE should be considered in the differential diagnosis of a pregnant woman with sudden onset of shortness of breath, hypotension, or cardiac arrhythmia or arrest, followed by coagulopathy and hemorrhage. Premonitory symptoms, including restlessness, confusion, disorientation, agitation, chills, nausea, numbness, and tingling, are commonly reported just before the cardiorespiratory collapse. AFE is less likely if the initial obstetric event is hemorrhage in the absence of cardiorespiratory compromise or a preceding coagulopathy.3
Typically, the onset is just before birth, during birth, or within the first few hours after delivery. In the United Kingdom, which has a robust centralized registry for reporting AFE, about 56% of cases occur before birth and 44% after birth.4
Related article: Is the incidence of amniotic fluid embolism rising? John T. Repke, MD (Examining the Evidence, August 2010)
The resources available to obstetric units vary greatly. Each unit needs to assess its resources and develop an AFE treatment protocol that builds on the unique strengths of the unit. Treatment of AFE requires the coordinated actions of anesthesiologists, obstetricians, nurses, the blood bank, pharmacy, and cardiovascular specialists. Coordinated activity among the members of such a large multidisciplinary team requires a written protocol that is practiced on a regular basis.
Six important components of a multidisciplinary response to AFE treatment protocol are:
- high-quality cardiopulmonary resuscitation (CPR)
- a protocol for massive transfusion
- treatment of diffuse bleeding and coagulopathy
- treatment of uterine and pelvic bleeding
- extracorporeal lung and heart support
- post-AFE intensive care.
1. Initiate high-quality CPR
Hypotension and hypoxemia due to cardiac and pulmonary dysfunction are prominent features of AFE. Dysrythmias such as pulseless electrical activity, bradycardia, ventricular fibrillation, and asystole are common. Rapid institution of high-quality CPR is critical to the survival of women with AFE.
Interventions often used in CPR of patients with AFE include initiation of high-quality chest compressions, early defibrillation if indicated, immediate administration of 100% oxygen by mask ventilation followed by early intubation, and rapid establishment of peripheral, arterial, and central venous access. Volume assessment, fluid replacement, and administration of vasopressors and inotropes are also important.
CPR of pregnant women requires special interventions, including maximal left lateral displacement of the uterus to reduce compression of the descending aorta and vena cava. Lateral displacement of the uterus can be accomplished by left lateral tilt or by manual uterine displacement. To optimize the effectiveness of chest compressions, many experts recommend placing the woman in a supine position and using manual uterine displacement rather than a left lateral tilt.5 For chest compressions, the hands should be placed just above the center of the sternum to adjust for the elevation of the diaphragm caused by the gravid uterus.
The gravid uterus can compromise the effectiveness of CPR. Fetal viability and neurologic outcome are best if delivery occurs within 5 minutes of the onset of cardiopulmonary arrest. If the gestational age of the fetus is consistent with extrauterine viability and initial CPR has not restored cardiac function, it is best to initiate fetal delivery within 4 minutes of the onset of cardiopulmonary arrest with the intent to deliver the fetus within 5 minutes.6,7 If the fetus is beyond 20 weeks’ gestational age, delivery early in the course of CPR improves the effectiveness of maternal resuscitation and may increase the probability of maternal survival.
In one study of the response of anesthesiologists, obstetricians, and nurses to a simulated cardiac arrest caused by an AFE, the participants did not routinely use defibrillation when indicated, did not place a firm support under the back for chest compressions, and did not switch the provider of chest compressions every 2 minutes.8 This study indicates that additional training and routinely scheduled multidisciplinary simulation of the response to cardiopulmonary arrest could improve the quality of our CPR.
2. Use a massive transfusion protocol
Severe coagulopathy and diffuse bleeding are commonly encountered in AFE. Target goals for the replacement of blood products include:
- hemoglobin concentration ≥8 g/dL
- fibrinogen ≥150 to 200 mg/dL
- platelets ≥50,000/μL
- prothrombin time international normalized ratio (INR) ≤1.5.
Most massive transfusion protocols provide for the rapid delivery of 4 to 8 units of red blood cells and a similar number of units of fresh frozen plasma to the patient’s bedside. In the management of AFE, 20 to 30 units of red blood cells and a similar quantity of fresh frozen plasma may need to be transfused. Cryoprecipitate takes 20 to 30 minutes to thaw, so preparations to transfuse cryoprecipitate should be initiated as soon as the massive transfusion protocol is triggered. A case of AFE can completely empty the blood bank of all available blood products and necessitate the use of alternative agents.
Lyophilized fibrinogen concentrate (RiaSTAP) is approved by the US Food and Drug Administration for the treatment of congenital hypofibrinogenemia and also may be useful to replace fibrinogen in cases of AFE. In many hospitals, large quantities of fresh frozen plasma are not immediately available; lyophilized fibrinogen concentrate may be especially useful in these settings. Another advantage of fibrinogen concentrate is that large amounts of fibrinogen can be administered in a small volume of intravenous fluid. Fibrinogen concentrate typically is used at a dose of 70 mg/kg of body weight.9,10
Intraoperative red cell salvage occasionally is used in cases of obstetric hemorrhage. In one case report of the use of red cell salvage with leukocyte depletion filtration during treatment of an AFE, acute hypotension developed in the patient after the transfusion of salvaged red cells.11 This case report raises safety concerns about the use of salvaged cells in women with severe AFE.
Related article: 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage Baha M. Sibai, MD (June 2011)
3. Treat diffuse bleeding and coagulopathy
In addition to the initiation of the massive transfusion protocol, additional treatments that may be helpful in managing the coagulopathy of AFE include tranexamic acid, recombinant factor VIIa (rFVIIa), and exchange transfusion.
AFE is often associated with hyperfibrinolysis, which can cause excessive bleeding.12 Tranexamic acid blocks the lysine binding sites on plasminogen and thereby reduces the lysis of fibrin clots. Clinical trials in patients who have undergone trauma have demonstrated that the administration of tranexamic acid reduces blood loss.13 The dose of tranexamic acid is approximately 10 to 20 mg/kg of body weight, or approximately 1 g.
Controversy exists about the use of rFVIIa to treat the coagulopathy and bleeding caused by AFE. Some authorities believe that rFVIIa is associated with an increased AFE case fatality rate.14 Other authorities believe rFVIIa may be useful in the treatment of AFE coagulopathy, especially when bleeding persists despite aggressive blood and component replacement.”15 The dose of rFVIIa is approximately 90 µg/kg of body weight. rFVIIa is extremely expensive.
Exchange transfusion has been used successfully to treat AFE.16 In women with AFE, exchange transfusion removes circulating cells, cell fragments, and substances that trigger systemic anaphylaxis and coagulopathy, thereby enhancing rapid recovery.
Related article: Act fast when confronted by a coagulopathy postpartum Robert L. Barbieri, MD (Editorial; March 2012)
4. Treat uterine and pelvic bleeding
Obstetrician-gynecologists are experts in the control of uterine and pelvic bleeding. Interventions that commonly are used to control uterine and pelvic bleeding in cases of postpartum hemorrhage, uterine rupture, or placenta accreta also can be applied in cases of AFE with uncontrolled uterine and pelvic bleeding. These techniques include:
- use of uterine compression sutures
- the Bakri balloon
- a uterine tourniquet
- vascular clamps on the ovarian vessels.17,18
In many cases of AFE, total or supracervical hysterectomy is necessary to control uterine bleeding. Uterine artery embolization, if available, has been reported to be helpful in select cases. However, many women with AFE are too unstable to survive transfer to an interventional radiology suite. Additional interventions to control bleeding include hypogastric artery ligation, infrarenal aortic compression, and pelvic packing.
Cross-clamping the aorta below the renal vessels can reduce blood flow to the pelvis and provide time for cardiopulmonary and volume resuscitation. Alternatively, placing pressure on the infrarenal aorta with a sponge or directly by hand can help reduce blood flow to the pelvis.19
In many cases of AFE, pelvic hemorrhage is difficult to control. Even if surgical pedicles are ligated securely, the coagulopathy of AFE may cause persistent oozing from areas of minor tissue trauma. Uncontrolled blood loss can be a proximate cause of death in women with AFE. All written protocols for responding to an AFE should include a plan to use pelvic packing for patients in whom standard operative procedures do not produce adequate control of bleeding. A “mushroom,” “parachute,” or “umbrella” pack has been reported to help stabilize the severely ill patient with pelvic bleeding and permit effective resuscitation and blood product replacement.20
Related articles:
A stitch in time: The B-Lynch, Hayman and Pereira uterine compression sutures Robert L. Barbieri, MD (Editorial, December 2012)
Have you made the best use of the Bakri balloon in PPH? Robert L. Barbieri, MD (Editorial, July 2011)
5. Consider extracorporeal lung and heart support
In many cases of AFE, both lung and cardiac function are severely compromised. Both veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and full cardiopulmonary bypass provide support for the failing lung and heart. Based on a small number of case reports, extracorporeal lung and heart support appear to be useful in the treatment of AFE.21–26 Using the Seldinger technique,27 it is technically feasible to rapidly access a major vein and artery to provide the input and output ports for VA-ECMO. Unlike the cardiopulmonary bypass pump, the VA-ECMO pump does not have a reservoir that needs to be primed with blood and is smaller and more portable. To provide a patient with VA-ECMO or cardiopulmonary bypass, a cardiac interventionist and a perfusionist must be available. Extracorporeal lung and heart support require heparinization of the patient’s blood, which may result in increased bleeding. Both VA-ECMO and cardiopulmonary bypass, along with the diseases for which they are used, may cause renal dysfunction, neurologic injury, and infection.28
Alternative approaches that provide support of the heart—but not lung—are the Impella pump, TandemHeart, and the intra-aortic balloon pump. An alternative that provides lung support—but not cardiac support—is veno-venous ECMO.
In developing a written protocol for responding to an AFE, obstetricians should explore the potential availability of VA-ECMO, cardiopulmonary bypass, or other cardiopulmonary support devices as options for patients who have not responded to standard treatment of AFE and are at high risk of death.
6. Post-AFE intensive care
After stabilization, most women with AFE will require intensive care for 48 to 96 hours. Some experts have proposed that all survivors of cardiopulmonary arrest who are successfully resuscitated and stabilized be transferred to hospitals that specialize in post−cardiac arrest care to improve outcomes.
Assessment of organ injury is important after an AFE. In addition, encephalopathy is a common complication of AFE, and sequential neurologic examination is a priority. Therapeutic hypothermia (TH) may help to preserve neurologic function after AFE.29 However, TH may cause a mild coagulopathy by inhibiting platelet activation and enzyme activity of clotting factors. Because coagulopathy is a prominent feature of AFE, TH may be contraindicated if the patient has a clinically significant baseline coagulopathy.30
DEVELOP AN AFE PROTOCOL AND PRACTICE THE COMPONENTS
Practicing the components of obstetric protocols can improve unit performance and patient outcomes.31 The components of an AFE protocol, as described in this article, include high-quality CPR, a protocol for massive transfusion, treatment of diffuse bleeding and coagulopathy, treatment of uterine and pelvic bleeding, extracorporeal lung and heart support, and post-AFE intensive care. Practicing these components of an AFE protocol will enhance performance across many common obstetric complications including postpartum hemorrhage, uterine rupture, placenta accreta, and pulmonary embolism.
When Chesley “Sully” Sullenberger and his copilot landed Flight 1549 in the Hudson River in New York, he had never practiced that specific response to twin engine failure, but he had practiced many emergency responses involving related scenarios. The combination of exceptional flight experience and years of practicing the response to emergency scenarios in simulation exercises permitted him and his copilot to execute a uniquely clever plan to solve a life-threatening
emergency. In a related way, practicing the components of AFE treatment will help obstetricians, obstetric anesthesiologists, and their multidisciplinary team to improve the responses to all major obstetric emergencies.
INSTANT POLL
Does your obstetric unit have a written protocol for treating an amniotic fluid embolism (AFE)? Has your obstetric unit practiced any of the components of the AFE treatment protocol: 1) high-quality cardiopulmonary resuscitation, 2) a protocol for massive transfusion protocol, 3) treatment of diffuse bleeding and coagulopathy, 4) treatment of uterine and pelvic bleeding, 5) extracorporeal lung and heart support, and 6) post-AFE intensive care?
Tell us—at [email protected]. Please include your name and the city and state in which you practice.
Amniotic fluid embolism (AFE) occurs in about 1 in 20,000 to 1 in 40,000 deliveries.1,2 Although the condition is rare, the case fatality rate is high, and AFE is a common cause of maternal death in developed countries. AFE cannot be predicted or prevented. Moreover, the condition is difficult to precisely define and is often a diagnosis of exclusion.
AFE should be considered in the differential diagnosis of a pregnant woman with sudden onset of shortness of breath, hypotension, or cardiac arrhythmia or arrest, followed by coagulopathy and hemorrhage. Premonitory symptoms, including restlessness, confusion, disorientation, agitation, chills, nausea, numbness, and tingling, are commonly reported just before the cardiorespiratory collapse. AFE is less likely if the initial obstetric event is hemorrhage in the absence of cardiorespiratory compromise or a preceding coagulopathy.3
Typically, the onset is just before birth, during birth, or within the first few hours after delivery. In the United Kingdom, which has a robust centralized registry for reporting AFE, about 56% of cases occur before birth and 44% after birth.4
Related article: Is the incidence of amniotic fluid embolism rising? John T. Repke, MD (Examining the Evidence, August 2010)
The resources available to obstetric units vary greatly. Each unit needs to assess its resources and develop an AFE treatment protocol that builds on the unique strengths of the unit. Treatment of AFE requires the coordinated actions of anesthesiologists, obstetricians, nurses, the blood bank, pharmacy, and cardiovascular specialists. Coordinated activity among the members of such a large multidisciplinary team requires a written protocol that is practiced on a regular basis.
Six important components of a multidisciplinary response to AFE treatment protocol are:
- high-quality cardiopulmonary resuscitation (CPR)
- a protocol for massive transfusion
- treatment of diffuse bleeding and coagulopathy
- treatment of uterine and pelvic bleeding
- extracorporeal lung and heart support
- post-AFE intensive care.
1. Initiate high-quality CPR
Hypotension and hypoxemia due to cardiac and pulmonary dysfunction are prominent features of AFE. Dysrythmias such as pulseless electrical activity, bradycardia, ventricular fibrillation, and asystole are common. Rapid institution of high-quality CPR is critical to the survival of women with AFE.
Interventions often used in CPR of patients with AFE include initiation of high-quality chest compressions, early defibrillation if indicated, immediate administration of 100% oxygen by mask ventilation followed by early intubation, and rapid establishment of peripheral, arterial, and central venous access. Volume assessment, fluid replacement, and administration of vasopressors and inotropes are also important.
CPR of pregnant women requires special interventions, including maximal left lateral displacement of the uterus to reduce compression of the descending aorta and vena cava. Lateral displacement of the uterus can be accomplished by left lateral tilt or by manual uterine displacement. To optimize the effectiveness of chest compressions, many experts recommend placing the woman in a supine position and using manual uterine displacement rather than a left lateral tilt.5 For chest compressions, the hands should be placed just above the center of the sternum to adjust for the elevation of the diaphragm caused by the gravid uterus.
The gravid uterus can compromise the effectiveness of CPR. Fetal viability and neurologic outcome are best if delivery occurs within 5 minutes of the onset of cardiopulmonary arrest. If the gestational age of the fetus is consistent with extrauterine viability and initial CPR has not restored cardiac function, it is best to initiate fetal delivery within 4 minutes of the onset of cardiopulmonary arrest with the intent to deliver the fetus within 5 minutes.6,7 If the fetus is beyond 20 weeks’ gestational age, delivery early in the course of CPR improves the effectiveness of maternal resuscitation and may increase the probability of maternal survival.
In one study of the response of anesthesiologists, obstetricians, and nurses to a simulated cardiac arrest caused by an AFE, the participants did not routinely use defibrillation when indicated, did not place a firm support under the back for chest compressions, and did not switch the provider of chest compressions every 2 minutes.8 This study indicates that additional training and routinely scheduled multidisciplinary simulation of the response to cardiopulmonary arrest could improve the quality of our CPR.
2. Use a massive transfusion protocol
Severe coagulopathy and diffuse bleeding are commonly encountered in AFE. Target goals for the replacement of blood products include:
- hemoglobin concentration ≥8 g/dL
- fibrinogen ≥150 to 200 mg/dL
- platelets ≥50,000/μL
- prothrombin time international normalized ratio (INR) ≤1.5.
Most massive transfusion protocols provide for the rapid delivery of 4 to 8 units of red blood cells and a similar number of units of fresh frozen plasma to the patient’s bedside. In the management of AFE, 20 to 30 units of red blood cells and a similar quantity of fresh frozen plasma may need to be transfused. Cryoprecipitate takes 20 to 30 minutes to thaw, so preparations to transfuse cryoprecipitate should be initiated as soon as the massive transfusion protocol is triggered. A case of AFE can completely empty the blood bank of all available blood products and necessitate the use of alternative agents.
Lyophilized fibrinogen concentrate (RiaSTAP) is approved by the US Food and Drug Administration for the treatment of congenital hypofibrinogenemia and also may be useful to replace fibrinogen in cases of AFE. In many hospitals, large quantities of fresh frozen plasma are not immediately available; lyophilized fibrinogen concentrate may be especially useful in these settings. Another advantage of fibrinogen concentrate is that large amounts of fibrinogen can be administered in a small volume of intravenous fluid. Fibrinogen concentrate typically is used at a dose of 70 mg/kg of body weight.9,10
Intraoperative red cell salvage occasionally is used in cases of obstetric hemorrhage. In one case report of the use of red cell salvage with leukocyte depletion filtration during treatment of an AFE, acute hypotension developed in the patient after the transfusion of salvaged red cells.11 This case report raises safety concerns about the use of salvaged cells in women with severe AFE.
Related article: 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage Baha M. Sibai, MD (June 2011)
3. Treat diffuse bleeding and coagulopathy
In addition to the initiation of the massive transfusion protocol, additional treatments that may be helpful in managing the coagulopathy of AFE include tranexamic acid, recombinant factor VIIa (rFVIIa), and exchange transfusion.
AFE is often associated with hyperfibrinolysis, which can cause excessive bleeding.12 Tranexamic acid blocks the lysine binding sites on plasminogen and thereby reduces the lysis of fibrin clots. Clinical trials in patients who have undergone trauma have demonstrated that the administration of tranexamic acid reduces blood loss.13 The dose of tranexamic acid is approximately 10 to 20 mg/kg of body weight, or approximately 1 g.
Controversy exists about the use of rFVIIa to treat the coagulopathy and bleeding caused by AFE. Some authorities believe that rFVIIa is associated with an increased AFE case fatality rate.14 Other authorities believe rFVIIa may be useful in the treatment of AFE coagulopathy, especially when bleeding persists despite aggressive blood and component replacement.”15 The dose of rFVIIa is approximately 90 µg/kg of body weight. rFVIIa is extremely expensive.
Exchange transfusion has been used successfully to treat AFE.16 In women with AFE, exchange transfusion removes circulating cells, cell fragments, and substances that trigger systemic anaphylaxis and coagulopathy, thereby enhancing rapid recovery.
Related article: Act fast when confronted by a coagulopathy postpartum Robert L. Barbieri, MD (Editorial; March 2012)
4. Treat uterine and pelvic bleeding
Obstetrician-gynecologists are experts in the control of uterine and pelvic bleeding. Interventions that commonly are used to control uterine and pelvic bleeding in cases of postpartum hemorrhage, uterine rupture, or placenta accreta also can be applied in cases of AFE with uncontrolled uterine and pelvic bleeding. These techniques include:
- use of uterine compression sutures
- the Bakri balloon
- a uterine tourniquet
- vascular clamps on the ovarian vessels.17,18
In many cases of AFE, total or supracervical hysterectomy is necessary to control uterine bleeding. Uterine artery embolization, if available, has been reported to be helpful in select cases. However, many women with AFE are too unstable to survive transfer to an interventional radiology suite. Additional interventions to control bleeding include hypogastric artery ligation, infrarenal aortic compression, and pelvic packing.
Cross-clamping the aorta below the renal vessels can reduce blood flow to the pelvis and provide time for cardiopulmonary and volume resuscitation. Alternatively, placing pressure on the infrarenal aorta with a sponge or directly by hand can help reduce blood flow to the pelvis.19
In many cases of AFE, pelvic hemorrhage is difficult to control. Even if surgical pedicles are ligated securely, the coagulopathy of AFE may cause persistent oozing from areas of minor tissue trauma. Uncontrolled blood loss can be a proximate cause of death in women with AFE. All written protocols for responding to an AFE should include a plan to use pelvic packing for patients in whom standard operative procedures do not produce adequate control of bleeding. A “mushroom,” “parachute,” or “umbrella” pack has been reported to help stabilize the severely ill patient with pelvic bleeding and permit effective resuscitation and blood product replacement.20
Related articles:
A stitch in time: The B-Lynch, Hayman and Pereira uterine compression sutures Robert L. Barbieri, MD (Editorial, December 2012)
Have you made the best use of the Bakri balloon in PPH? Robert L. Barbieri, MD (Editorial, July 2011)
5. Consider extracorporeal lung and heart support
In many cases of AFE, both lung and cardiac function are severely compromised. Both veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and full cardiopulmonary bypass provide support for the failing lung and heart. Based on a small number of case reports, extracorporeal lung and heart support appear to be useful in the treatment of AFE.21–26 Using the Seldinger technique,27 it is technically feasible to rapidly access a major vein and artery to provide the input and output ports for VA-ECMO. Unlike the cardiopulmonary bypass pump, the VA-ECMO pump does not have a reservoir that needs to be primed with blood and is smaller and more portable. To provide a patient with VA-ECMO or cardiopulmonary bypass, a cardiac interventionist and a perfusionist must be available. Extracorporeal lung and heart support require heparinization of the patient’s blood, which may result in increased bleeding. Both VA-ECMO and cardiopulmonary bypass, along with the diseases for which they are used, may cause renal dysfunction, neurologic injury, and infection.28
Alternative approaches that provide support of the heart—but not lung—are the Impella pump, TandemHeart, and the intra-aortic balloon pump. An alternative that provides lung support—but not cardiac support—is veno-venous ECMO.
In developing a written protocol for responding to an AFE, obstetricians should explore the potential availability of VA-ECMO, cardiopulmonary bypass, or other cardiopulmonary support devices as options for patients who have not responded to standard treatment of AFE and are at high risk of death.
6. Post-AFE intensive care
After stabilization, most women with AFE will require intensive care for 48 to 96 hours. Some experts have proposed that all survivors of cardiopulmonary arrest who are successfully resuscitated and stabilized be transferred to hospitals that specialize in post−cardiac arrest care to improve outcomes.
Assessment of organ injury is important after an AFE. In addition, encephalopathy is a common complication of AFE, and sequential neurologic examination is a priority. Therapeutic hypothermia (TH) may help to preserve neurologic function after AFE.29 However, TH may cause a mild coagulopathy by inhibiting platelet activation and enzyme activity of clotting factors. Because coagulopathy is a prominent feature of AFE, TH may be contraindicated if the patient has a clinically significant baseline coagulopathy.30
DEVELOP AN AFE PROTOCOL AND PRACTICE THE COMPONENTS
Practicing the components of obstetric protocols can improve unit performance and patient outcomes.31 The components of an AFE protocol, as described in this article, include high-quality CPR, a protocol for massive transfusion, treatment of diffuse bleeding and coagulopathy, treatment of uterine and pelvic bleeding, extracorporeal lung and heart support, and post-AFE intensive care. Practicing these components of an AFE protocol will enhance performance across many common obstetric complications including postpartum hemorrhage, uterine rupture, placenta accreta, and pulmonary embolism.
When Chesley “Sully” Sullenberger and his copilot landed Flight 1549 in the Hudson River in New York, he had never practiced that specific response to twin engine failure, but he had practiced many emergency responses involving related scenarios. The combination of exceptional flight experience and years of practicing the response to emergency scenarios in simulation exercises permitted him and his copilot to execute a uniquely clever plan to solve a life-threatening
emergency. In a related way, practicing the components of AFE treatment will help obstetricians, obstetric anesthesiologists, and their multidisciplinary team to improve the responses to all major obstetric emergencies.
INSTANT POLL
Does your obstetric unit have a written protocol for treating an amniotic fluid embolism (AFE)? Has your obstetric unit practiced any of the components of the AFE treatment protocol: 1) high-quality cardiopulmonary resuscitation, 2) a protocol for massive transfusion protocol, 3) treatment of diffuse bleeding and coagulopathy, 4) treatment of uterine and pelvic bleeding, 5) extracorporeal lung and heart support, and 6) post-AFE intensive care?
Tell us—at [email protected]. Please include your name and the city and state in which you practice.
- Kramer MS, Rouleau J, Baskett TF, Joseph KS; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Amniotic-fluid embolism and medical induction of labour: A retrospective, population-based cohort study. Lancet. 2006;368(9545):1444–1448.
- Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolism: A population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199(1):49.e1–49.e8.
- Tuffnell D, Knight M, Plaat F. Amniotic fluid embolism—An update. Anaesthesia. 2011;66(1):3–6.
- Knight M, Tuffnell D, Brocklehurst P, Spark P, Kurinczuk JJ; UK Obstetric Surveillance System. Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol. 2010;115(5):910–917.
- Kundra P, Khanna S, Habeebullahg S, Ravishankar M. Manual displacement of the uterus during Caesarean section. Anaesthesia. 2007;62(5):460–465.
- Katz VL, Dotters DJ, Droegemueller W. Perimortem cesarean delivery. Obstet Gynecol. 1986;68(4):571–576.
- Katz V, Balderston K, DeFreest M. Perimortem cesarean delivery: Were our assumptions correct? Am J Obstet Gynecol. 2005;192(6):1916–1920.
- Lipman SS, Daniels KI, Carvalho B, et al. Deficits in the provision of cardiopulmonary resuscitation during simulated obstetric crises. Am J Obstet Gynecol. 2010;203(2):179.e1–179.e5.
- Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anesth. 2010;19(2):218–223.
- Sorensen B, Tang M, Larsen OH, Laursen PN, Fenger-Eriksen C, Rea CJ. The role of fibrinogen: A new paradigm in the treatment of coagulopathic bleeding. Thromb Res. 2011;128(Suppl 1):S13–S16.
- Rogers WK, Wernimont SA, Kumar GC, Bennett E, Chestnut DH. Acute hypotension associated with intraoperative cell salvage using a leukocyte depletion filter during management of obstetric hemorrhage due to amniotic fluid embolism. Anesth Analg. 2013;117(2):449–452.
- Collins NF, Bloor M, McDonnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anesth. 2013;22(1):71–76.
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054.
- Leighton BL, Wall MH, Lockhart EM, Phillips LE, Zatta AJ. Use of recombinant factor VIIa in patients with amniotic fluid embolism: A systematic review of case reports. Anesthesiology. 2011;115(6):1201–1208.
- Huber AW, Raio L, Alberio L, Ghezzi F, Surbek DV. Recombinant human factor VIIa prevents hysterectomy in severe postpartum hemorrhage: single center study. J Perinat Med. 2011;40(1):43–49.
- Dodgson J, Martin J, Boswell J, Goodall HB, Smith R. Probable amniotic fluid embolism precipitated by amniocentesis and treated by exchange transfusion. Brit Med J (Clin Res Ed). 1987;294(6583):1322–1323.
- Barbieri RL. A stitch in time: The B-Lynch, Hayman and Pereira uterine compression sutures. OBG Manage. 2012;24(12):6, 8, 10, 11.
- Barbieri RL. Have you made the best use of the Bakri balloon in PPH? OBG Manage. 2011;23(7):6, 8, 9.
- Belfort MA, Zimmerman J, Schemmer G, Oldroyd R, Smilanich R, Pearce M. Aortic compression and cross clamping in a case of placenta percreta and amniotic fluid embolism: A case report. AJP Rep. 2011;1(1):33–36.
- Dildy GA, Scott JR, Saffer CS, Belfort MA. An
effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol. 2006;108(5):1222–1226. - Stanten RD, Iverson LI, Daugharty TM, Lovett SM, Terry C, Blumenstock E. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: Diagnosis by transesophageal echocardiogram and treatment by cardiopulmonary bypass. Obstet Gynecol. 2003;102(3):496–498.
- Ho CH, Chen KB, Liu SK, Liu YF, Cheng HC, Wu RS. Early application of extracorporeal membrane oxygenation in a patient with amniotic fluid embolism. Acta Anaesthesiol Taiwan. 2009;47(2):99–102.
- Shen HP, Chang WC, Yeh LS, Ho M. Amniotic fluid embolism treated with emergency extracorporeal membrane oxygenation: A case report.
J Reprod Med. 2009;54(11–12):706–708. - Lee PH, Shulman MS, Vellayappan U, Symes JF, Olenchock SA Jr. Surgical treatment of amniotic fluid embolism with cardiopulmonary collapse. Ann Thorac Surg. 2010;90(5):1694–1696.
- Firstenberg MS, Abel E, Blais D, et al. Temporary extracorporeal circulatory support and pulmonary embolectomy for catastrophic amniotic fluid embolism. Heart Surg Forum. 2011;14(3):E157–E159.
- Ecker JL, Solt K, Fitzsimons MG, MacGillivray TE. Case records of the Massachusetts General Hospital. Case 40-2012: A 43-year-old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med. 2012;367(26):2528–2536.
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953;39(5):368–376.
- Cheng R, Hachamovitch R, Kittelson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2013; epub Nov 8.
- Rittenberger JC, Kelly E, Jang D, Greer K, Heffner A. Successful outcome utilizing hypothermia after cardiac arrest in pregnancy: A case report. Crit Care Med. 2008;36(4):1354–1356.
- Michelson AD, MacGregor H, Barnard MR, Krestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71(5):633–640.
- Rivzi F, Mackey R, Barrett T, McKenna P, Geary M. Successful reduction of massive postpartum haemorrhage by use of guidelines and staff education. BJOG. 2004;111(5):495–498.
- Kramer MS, Rouleau J, Baskett TF, Joseph KS; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Amniotic-fluid embolism and medical induction of labour: A retrospective, population-based cohort study. Lancet. 2006;368(9545):1444–1448.
- Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolism: A population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199(1):49.e1–49.e8.
- Tuffnell D, Knight M, Plaat F. Amniotic fluid embolism—An update. Anaesthesia. 2011;66(1):3–6.
- Knight M, Tuffnell D, Brocklehurst P, Spark P, Kurinczuk JJ; UK Obstetric Surveillance System. Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol. 2010;115(5):910–917.
- Kundra P, Khanna S, Habeebullahg S, Ravishankar M. Manual displacement of the uterus during Caesarean section. Anaesthesia. 2007;62(5):460–465.
- Katz VL, Dotters DJ, Droegemueller W. Perimortem cesarean delivery. Obstet Gynecol. 1986;68(4):571–576.
- Katz V, Balderston K, DeFreest M. Perimortem cesarean delivery: Were our assumptions correct? Am J Obstet Gynecol. 2005;192(6):1916–1920.
- Lipman SS, Daniels KI, Carvalho B, et al. Deficits in the provision of cardiopulmonary resuscitation during simulated obstetric crises. Am J Obstet Gynecol. 2010;203(2):179.e1–179.e5.
- Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anesth. 2010;19(2):218–223.
- Sorensen B, Tang M, Larsen OH, Laursen PN, Fenger-Eriksen C, Rea CJ. The role of fibrinogen: A new paradigm in the treatment of coagulopathic bleeding. Thromb Res. 2011;128(Suppl 1):S13–S16.
- Rogers WK, Wernimont SA, Kumar GC, Bennett E, Chestnut DH. Acute hypotension associated with intraoperative cell salvage using a leukocyte depletion filter during management of obstetric hemorrhage due to amniotic fluid embolism. Anesth Analg. 2013;117(2):449–452.
- Collins NF, Bloor M, McDonnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anesth. 2013;22(1):71–76.
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054.
- Leighton BL, Wall MH, Lockhart EM, Phillips LE, Zatta AJ. Use of recombinant factor VIIa in patients with amniotic fluid embolism: A systematic review of case reports. Anesthesiology. 2011;115(6):1201–1208.
- Huber AW, Raio L, Alberio L, Ghezzi F, Surbek DV. Recombinant human factor VIIa prevents hysterectomy in severe postpartum hemorrhage: single center study. J Perinat Med. 2011;40(1):43–49.
- Dodgson J, Martin J, Boswell J, Goodall HB, Smith R. Probable amniotic fluid embolism precipitated by amniocentesis and treated by exchange transfusion. Brit Med J (Clin Res Ed). 1987;294(6583):1322–1323.
- Barbieri RL. A stitch in time: The B-Lynch, Hayman and Pereira uterine compression sutures. OBG Manage. 2012;24(12):6, 8, 10, 11.
- Barbieri RL. Have you made the best use of the Bakri balloon in PPH? OBG Manage. 2011;23(7):6, 8, 9.
- Belfort MA, Zimmerman J, Schemmer G, Oldroyd R, Smilanich R, Pearce M. Aortic compression and cross clamping in a case of placenta percreta and amniotic fluid embolism: A case report. AJP Rep. 2011;1(1):33–36.
- Dildy GA, Scott JR, Saffer CS, Belfort MA. An
effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol. 2006;108(5):1222–1226. - Stanten RD, Iverson LI, Daugharty TM, Lovett SM, Terry C, Blumenstock E. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: Diagnosis by transesophageal echocardiogram and treatment by cardiopulmonary bypass. Obstet Gynecol. 2003;102(3):496–498.
- Ho CH, Chen KB, Liu SK, Liu YF, Cheng HC, Wu RS. Early application of extracorporeal membrane oxygenation in a patient with amniotic fluid embolism. Acta Anaesthesiol Taiwan. 2009;47(2):99–102.
- Shen HP, Chang WC, Yeh LS, Ho M. Amniotic fluid embolism treated with emergency extracorporeal membrane oxygenation: A case report.
J Reprod Med. 2009;54(11–12):706–708. - Lee PH, Shulman MS, Vellayappan U, Symes JF, Olenchock SA Jr. Surgical treatment of amniotic fluid embolism with cardiopulmonary collapse. Ann Thorac Surg. 2010;90(5):1694–1696.
- Firstenberg MS, Abel E, Blais D, et al. Temporary extracorporeal circulatory support and pulmonary embolectomy for catastrophic amniotic fluid embolism. Heart Surg Forum. 2011;14(3):E157–E159.
- Ecker JL, Solt K, Fitzsimons MG, MacGillivray TE. Case records of the Massachusetts General Hospital. Case 40-2012: A 43-year-old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med. 2012;367(26):2528–2536.
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953;39(5):368–376.
- Cheng R, Hachamovitch R, Kittelson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2013; epub Nov 8.
- Rittenberger JC, Kelly E, Jang D, Greer K, Heffner A. Successful outcome utilizing hypothermia after cardiac arrest in pregnancy: A case report. Crit Care Med. 2008;36(4):1354–1356.
- Michelson AD, MacGregor H, Barnard MR, Krestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71(5):633–640.
- Rivzi F, Mackey R, Barrett T, McKenna P, Geary M. Successful reduction of massive postpartum haemorrhage by use of guidelines and staff education. BJOG. 2004;111(5):495–498.
Editorial: “Pre” Prehospital Care
In recent months, state and federal legislation has been enacted to place lifesaving prescription medications and devices in the hands of nonhealthcare providers present at the scenes of medical emergencies. In 1973, the Emergency Medical Services Development Act ushered in the modern era of advanced prehospital care; current legislation may initiate a new era of advanced “pre-prehospital care.”
The 1970s
The origins of prehospital care can be traced to the years immediately following the Civil War when the horse-drawn wagons used to rapidly evacuate battlefield casualties were quickly adapted to civilian life—sometimes with a physician on board to administer care at the scene. But it wasn’t until the 1970s—more than 100 years later—that the most potent lifesaving medications and powerful electronic devices available to physicians would also be made available to paramedics operating at the scene with written protocols and online medical control.
The success in providing advanced treatment at the scene to those who might not survive transport to an ED led to the realization that in order to survive, patients with ventricular fibrillation (VF) and tachycardia, (VT), respiratory arrest from opioid overdoses, and anaphylaxis, required interventions even before paramedics could get to them.
The 1990s
A new era of “pre-prehospital care” began when the first automatic external defibrillators (AEDs) were placed in public spaces for use on any person who might be in cardiac arrest from VF or VT. The advances in computer programing of the 1990s made it possible to build compact defibrillators capable of delivering jolts of electricity only when indicated and at precisely the right time, without causing harm from inappropriate application or timing. Though initial deployment of AEDs was accompanied by an emphasis on training nonhealthcare workers in their use, many untrained people have since successfully defibrillated dying victims. In 2010, Weisfeldt et al (J Am Coll Cardiol. 55(16):1713-1720.) published the results of a study on survival after AED application prior to the arrival of EMS; of 13,769 out-of-hospital cardiac arrests, application of an AED in 259 cases was associated with a nearly doubling of survival, and the success rate of 40% by lay persons using the devices suggested that speed is more important than training.
Current Efforts
Though spring-loaded epinephrine syringes have been available for many years to individuals (and their families) at risk for anaphylaxis or severe asthma attacks, recent concerns have focused on the need to stock these devices in classrooms and other locations where they could be used on any child in need, with or without a prior history of severe allergic reactions.
At present, over 30 states permit or mandate stocking epi syringes in schools, and on November 13, 2013 President Obama signed into law the School Access to Emergency Epinephrine Act authorizing the Department of Health and Human Services to preferentially fund asthma treatment applications of states that both maintain emergency supplies of epi pens, and insure the availability of trained personnel to administer them throughout the school day.
Adding to the “pre-prehospital care” armamentarium, the FDA last month approved, by prescription, spring-loaded autoinjectors containing doses of naloxone for family members or care providers to rapidly administer to a person overdosed on a legal or illicit opioid. Also available, though not yet FDA approved, are intranasal doses of naloxone for use by nonhealthcare providers.
Emergency physicians should give all of these new developments our full support. Just as we frequently keep seriously traumatized patients alive until surgeons can arrive and operate, these newly available, lifesaving, “pre-prehospital care” measures will help patients survive until they can get to an ED. EPs can play an extremely important role in educating the public on their proper use, encouraging those who might be reluctant to apply them when needed, while decreasing any possible adverse effects from their use.
In recent months, state and federal legislation has been enacted to place lifesaving prescription medications and devices in the hands of nonhealthcare providers present at the scenes of medical emergencies. In 1973, the Emergency Medical Services Development Act ushered in the modern era of advanced prehospital care; current legislation may initiate a new era of advanced “pre-prehospital care.”
The 1970s
The origins of prehospital care can be traced to the years immediately following the Civil War when the horse-drawn wagons used to rapidly evacuate battlefield casualties were quickly adapted to civilian life—sometimes with a physician on board to administer care at the scene. But it wasn’t until the 1970s—more than 100 years later—that the most potent lifesaving medications and powerful electronic devices available to physicians would also be made available to paramedics operating at the scene with written protocols and online medical control.
The success in providing advanced treatment at the scene to those who might not survive transport to an ED led to the realization that in order to survive, patients with ventricular fibrillation (VF) and tachycardia, (VT), respiratory arrest from opioid overdoses, and anaphylaxis, required interventions even before paramedics could get to them.
The 1990s
A new era of “pre-prehospital care” began when the first automatic external defibrillators (AEDs) were placed in public spaces for use on any person who might be in cardiac arrest from VF or VT. The advances in computer programing of the 1990s made it possible to build compact defibrillators capable of delivering jolts of electricity only when indicated and at precisely the right time, without causing harm from inappropriate application or timing. Though initial deployment of AEDs was accompanied by an emphasis on training nonhealthcare workers in their use, many untrained people have since successfully defibrillated dying victims. In 2010, Weisfeldt et al (J Am Coll Cardiol. 55(16):1713-1720.) published the results of a study on survival after AED application prior to the arrival of EMS; of 13,769 out-of-hospital cardiac arrests, application of an AED in 259 cases was associated with a nearly doubling of survival, and the success rate of 40% by lay persons using the devices suggested that speed is more important than training.
Current Efforts
Though spring-loaded epinephrine syringes have been available for many years to individuals (and their families) at risk for anaphylaxis or severe asthma attacks, recent concerns have focused on the need to stock these devices in classrooms and other locations where they could be used on any child in need, with or without a prior history of severe allergic reactions.
At present, over 30 states permit or mandate stocking epi syringes in schools, and on November 13, 2013 President Obama signed into law the School Access to Emergency Epinephrine Act authorizing the Department of Health and Human Services to preferentially fund asthma treatment applications of states that both maintain emergency supplies of epi pens, and insure the availability of trained personnel to administer them throughout the school day.
Adding to the “pre-prehospital care” armamentarium, the FDA last month approved, by prescription, spring-loaded autoinjectors containing doses of naloxone for family members or care providers to rapidly administer to a person overdosed on a legal or illicit opioid. Also available, though not yet FDA approved, are intranasal doses of naloxone for use by nonhealthcare providers.
Emergency physicians should give all of these new developments our full support. Just as we frequently keep seriously traumatized patients alive until surgeons can arrive and operate, these newly available, lifesaving, “pre-prehospital care” measures will help patients survive until they can get to an ED. EPs can play an extremely important role in educating the public on their proper use, encouraging those who might be reluctant to apply them when needed, while decreasing any possible adverse effects from their use.
In recent months, state and federal legislation has been enacted to place lifesaving prescription medications and devices in the hands of nonhealthcare providers present at the scenes of medical emergencies. In 1973, the Emergency Medical Services Development Act ushered in the modern era of advanced prehospital care; current legislation may initiate a new era of advanced “pre-prehospital care.”
The 1970s
The origins of prehospital care can be traced to the years immediately following the Civil War when the horse-drawn wagons used to rapidly evacuate battlefield casualties were quickly adapted to civilian life—sometimes with a physician on board to administer care at the scene. But it wasn’t until the 1970s—more than 100 years later—that the most potent lifesaving medications and powerful electronic devices available to physicians would also be made available to paramedics operating at the scene with written protocols and online medical control.
The success in providing advanced treatment at the scene to those who might not survive transport to an ED led to the realization that in order to survive, patients with ventricular fibrillation (VF) and tachycardia, (VT), respiratory arrest from opioid overdoses, and anaphylaxis, required interventions even before paramedics could get to them.
The 1990s
A new era of “pre-prehospital care” began when the first automatic external defibrillators (AEDs) were placed in public spaces for use on any person who might be in cardiac arrest from VF or VT. The advances in computer programing of the 1990s made it possible to build compact defibrillators capable of delivering jolts of electricity only when indicated and at precisely the right time, without causing harm from inappropriate application or timing. Though initial deployment of AEDs was accompanied by an emphasis on training nonhealthcare workers in their use, many untrained people have since successfully defibrillated dying victims. In 2010, Weisfeldt et al (J Am Coll Cardiol. 55(16):1713-1720.) published the results of a study on survival after AED application prior to the arrival of EMS; of 13,769 out-of-hospital cardiac arrests, application of an AED in 259 cases was associated with a nearly doubling of survival, and the success rate of 40% by lay persons using the devices suggested that speed is more important than training.
Current Efforts
Though spring-loaded epinephrine syringes have been available for many years to individuals (and their families) at risk for anaphylaxis or severe asthma attacks, recent concerns have focused on the need to stock these devices in classrooms and other locations where they could be used on any child in need, with or without a prior history of severe allergic reactions.
At present, over 30 states permit or mandate stocking epi syringes in schools, and on November 13, 2013 President Obama signed into law the School Access to Emergency Epinephrine Act authorizing the Department of Health and Human Services to preferentially fund asthma treatment applications of states that both maintain emergency supplies of epi pens, and insure the availability of trained personnel to administer them throughout the school day.
Adding to the “pre-prehospital care” armamentarium, the FDA last month approved, by prescription, spring-loaded autoinjectors containing doses of naloxone for family members or care providers to rapidly administer to a person overdosed on a legal or illicit opioid. Also available, though not yet FDA approved, are intranasal doses of naloxone for use by nonhealthcare providers.
Emergency physicians should give all of these new developments our full support. Just as we frequently keep seriously traumatized patients alive until surgeons can arrive and operate, these newly available, lifesaving, “pre-prehospital care” measures will help patients survive until they can get to an ED. EPs can play an extremely important role in educating the public on their proper use, encouraging those who might be reluctant to apply them when needed, while decreasing any possible adverse effects from their use.
Who will be found at fault?
I am always interested in the Medical Verdicts section (it’s like watching a car accident—you can’t look away). I recently was involved in an argument in a physician-only online forum where a participant presented a case of a failed homebirth brought into a hospital with an obstetrics (OB) service. A call was allegedly placed to one of the ObGyns who refused to come to the hospital and attend the patient, claiming that since the hospital had negotiated no contract with the ObGyns for unassigned coverage, he was not obligated to do so. The baby died. A lawsuit was filed.
I argued that, per the Emergency Medical Treatment and Active Labor Act (EMTALA), the hospital is required to have an unassigned call roster for OB. Usually it is an obligation of privileges and is outlined in the medical staff bylaws; sometimes payment is offered by the hospital, sometimes not.
A spirited discussion ensued. A bunch of emergency department (ED) physicians said that they practice in hospitals where no one can be “forced to take a call for unassigned patients for free against their will” and that this scenario is quite common. They felt there is nothing in EMTALA that requires a hospital to have on-call physicians available. They said it is common to be unable to find specialists, surgeons, or ObGyns willing to come in and take these patients.
I still don’t know what our legal obligation is under EMTALA, but I had never heard of an OB service without a provision for unassigned patients.
If this story is true, and not just an apocryphal tale, who will be found at fault? (Probably everyone.)
Deborah Owen, MD
Spokane, Washington
Shirley Pruitt, RN, JD, responds
The obligation to maintain a call schedule is imposed on hospitals by a section of the Medicare statute that refers back to the EMTALA obligations.1 Generally, if a hospital provides specialized services to the public, it is required to provide these services through ED on-call coverage. Each hospital has the discretion to maintain the on-call list in a manner to best meet the needs of its patients. The medical staff bylaws or policies and procedures must define 1) the responsibility of on-call physicians to respond, examine, and treat patients with emergency medical conditions, and 2) the procedures to be followed when a particular specialty is not available or the on-call physician cannot respond because of situations beyond his or her control. The Centers for Medicare and Medicaid Services (CMS) may impose a penalty on a physician who fails to respond to an emergency situation when he or she is assigned as the on-call physician.
There is no Federal law through CMS/EMTALA requiring a specialist to participate in the on-call list. However, a specialist who refuses to participate in the on-call list may not take “selective call” and agree to see patients with whom the specialist has a prior existing relationship while refusing to see other patients with whom there is no such relationship.
Disclaimer
This information should not be construed as business, risk management, or legal advice or legal opinion.
Shirley M. Pruitt, RN, JD
Yates, McLamb & Weyher, LLP
Raleigh, North Carolina
OBG Management Contributing Editor
Do you have a DIAGNOSTIC IN-SIGHT?
Read What is causing her abdominal pain? by Chetan Narasanna, MD, Reginald Griffin, MD, Michael S. Nussbaum, MD, and Andrew M. Kaunitz, MD.
Submit a query for your image-based case to [email protected]
Reference
- The Public Health and Welfare, 42 USC §1395dd et seq (2011).
I am always interested in the Medical Verdicts section (it’s like watching a car accident—you can’t look away). I recently was involved in an argument in a physician-only online forum where a participant presented a case of a failed homebirth brought into a hospital with an obstetrics (OB) service. A call was allegedly placed to one of the ObGyns who refused to come to the hospital and attend the patient, claiming that since the hospital had negotiated no contract with the ObGyns for unassigned coverage, he was not obligated to do so. The baby died. A lawsuit was filed.
I argued that, per the Emergency Medical Treatment and Active Labor Act (EMTALA), the hospital is required to have an unassigned call roster for OB. Usually it is an obligation of privileges and is outlined in the medical staff bylaws; sometimes payment is offered by the hospital, sometimes not.
A spirited discussion ensued. A bunch of emergency department (ED) physicians said that they practice in hospitals where no one can be “forced to take a call for unassigned patients for free against their will” and that this scenario is quite common. They felt there is nothing in EMTALA that requires a hospital to have on-call physicians available. They said it is common to be unable to find specialists, surgeons, or ObGyns willing to come in and take these patients.
I still don’t know what our legal obligation is under EMTALA, but I had never heard of an OB service without a provision for unassigned patients.
If this story is true, and not just an apocryphal tale, who will be found at fault? (Probably everyone.)
Deborah Owen, MD
Spokane, Washington
Shirley Pruitt, RN, JD, responds
The obligation to maintain a call schedule is imposed on hospitals by a section of the Medicare statute that refers back to the EMTALA obligations.1 Generally, if a hospital provides specialized services to the public, it is required to provide these services through ED on-call coverage. Each hospital has the discretion to maintain the on-call list in a manner to best meet the needs of its patients. The medical staff bylaws or policies and procedures must define 1) the responsibility of on-call physicians to respond, examine, and treat patients with emergency medical conditions, and 2) the procedures to be followed when a particular specialty is not available or the on-call physician cannot respond because of situations beyond his or her control. The Centers for Medicare and Medicaid Services (CMS) may impose a penalty on a physician who fails to respond to an emergency situation when he or she is assigned as the on-call physician.
There is no Federal law through CMS/EMTALA requiring a specialist to participate in the on-call list. However, a specialist who refuses to participate in the on-call list may not take “selective call” and agree to see patients with whom the specialist has a prior existing relationship while refusing to see other patients with whom there is no such relationship.
Disclaimer
This information should not be construed as business, risk management, or legal advice or legal opinion.
Shirley M. Pruitt, RN, JD
Yates, McLamb & Weyher, LLP
Raleigh, North Carolina
OBG Management Contributing Editor
Do you have a DIAGNOSTIC IN-SIGHT?
Read What is causing her abdominal pain? by Chetan Narasanna, MD, Reginald Griffin, MD, Michael S. Nussbaum, MD, and Andrew M. Kaunitz, MD.
Submit a query for your image-based case to [email protected]
I am always interested in the Medical Verdicts section (it’s like watching a car accident—you can’t look away). I recently was involved in an argument in a physician-only online forum where a participant presented a case of a failed homebirth brought into a hospital with an obstetrics (OB) service. A call was allegedly placed to one of the ObGyns who refused to come to the hospital and attend the patient, claiming that since the hospital had negotiated no contract with the ObGyns for unassigned coverage, he was not obligated to do so. The baby died. A lawsuit was filed.
I argued that, per the Emergency Medical Treatment and Active Labor Act (EMTALA), the hospital is required to have an unassigned call roster for OB. Usually it is an obligation of privileges and is outlined in the medical staff bylaws; sometimes payment is offered by the hospital, sometimes not.
A spirited discussion ensued. A bunch of emergency department (ED) physicians said that they practice in hospitals where no one can be “forced to take a call for unassigned patients for free against their will” and that this scenario is quite common. They felt there is nothing in EMTALA that requires a hospital to have on-call physicians available. They said it is common to be unable to find specialists, surgeons, or ObGyns willing to come in and take these patients.
I still don’t know what our legal obligation is under EMTALA, but I had never heard of an OB service without a provision for unassigned patients.
If this story is true, and not just an apocryphal tale, who will be found at fault? (Probably everyone.)
Deborah Owen, MD
Spokane, Washington
Shirley Pruitt, RN, JD, responds
The obligation to maintain a call schedule is imposed on hospitals by a section of the Medicare statute that refers back to the EMTALA obligations.1 Generally, if a hospital provides specialized services to the public, it is required to provide these services through ED on-call coverage. Each hospital has the discretion to maintain the on-call list in a manner to best meet the needs of its patients. The medical staff bylaws or policies and procedures must define 1) the responsibility of on-call physicians to respond, examine, and treat patients with emergency medical conditions, and 2) the procedures to be followed when a particular specialty is not available or the on-call physician cannot respond because of situations beyond his or her control. The Centers for Medicare and Medicaid Services (CMS) may impose a penalty on a physician who fails to respond to an emergency situation when he or she is assigned as the on-call physician.
There is no Federal law through CMS/EMTALA requiring a specialist to participate in the on-call list. However, a specialist who refuses to participate in the on-call list may not take “selective call” and agree to see patients with whom the specialist has a prior existing relationship while refusing to see other patients with whom there is no such relationship.
Disclaimer
This information should not be construed as business, risk management, or legal advice or legal opinion.
Shirley M. Pruitt, RN, JD
Yates, McLamb & Weyher, LLP
Raleigh, North Carolina
OBG Management Contributing Editor
Do you have a DIAGNOSTIC IN-SIGHT?
Read What is causing her abdominal pain? by Chetan Narasanna, MD, Reginald Griffin, MD, Michael S. Nussbaum, MD, and Andrew M. Kaunitz, MD.
Submit a query for your image-based case to [email protected]
Reference
- The Public Health and Welfare, 42 USC §1395dd et seq (2011).
Reference
- The Public Health and Welfare, 42 USC §1395dd et seq (2011).
WE LIKE TO HEAR FROM YOU!
Readers are always encouraged to “Tell us what you think!” We want to know your feedback regarding current articles, topics you’d like to see covered in future issues, and what challenges you face in daily practice.
Here’s a letter from an avid reader about emergency department on-call obligations, and a response from our legal expert.
What’s your opinion? Email us at [email protected]. Please include your name and the city and state in which you practice.
Niacin's effect on cardiovascular risk: Have we finally learned our lesson?
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
Skin Deep
As the snow melts and the freezing temperatures begin to abate, I cannot help but look forward to temperate days and the ability to warm my chilblained body in the sun. Ah, I can see myself now: wearing only a bathing suit, sitting in a comfortable beach chair, with a cool beverage in one hand and a good book in the other, wriggling my toes in the warm sand while the bright yellow sun beams down on my bare skin. At least that’s what I would do in the “good old days,” when “getting a little color” wasn’t considered a bad thing.
Oh, how times have changed! We have since learned that the sun is the enemy of our skin and one of the leading causes of skin cancer (tanning beds are the other).1 These days, we get daily warnings about the ill effects of too much sun exposure and cautions about avoiding those harmful UV rays. Even exposure years ago can increase our risk for skin cancer now or in future.
Current estimates are that one in five Americans will develop skin cancer in his/her lifetime.2 The incidence of nonmelanoma (basal and squamous cell) skin cancer in the United States has been reported at 3.5 million new cases annually; for melanoma, nearly 77,000 new cases were expected to be diagnosed in 2013 alone.3 More than 9,000 people die of melanoma annually.3
While these statistics are disturbing, the cure rate offers some reassurance. If melanoma is detected and treated early, before it has the chance to spread to the lymph nodes, the cure rate can be as high as 100%. The five-year survival rate for localized melanoma is 98%.3 And while not all melanoma is preventable, we can take an active role in reducing our risk—and educating our patients on how to protect themselves.
Continued on next page >>
The American Academy of Dermatology (AAD) has designated May as National Melanoma Skin Cancer Prevention Month. As we progress from spring to summer, the position of the sun rises in the sky, the days become longer, and our vulnerability to skin cancer increases. Our colleagues at AAD have impeccable timing; we are a captive audience and eager to greet the summer and sunshine—responsibly. Here is what the AAD “strongly recommends” to accomplish this goal:
Seek shade when appropriate. This is especially important between 10 am and 2 pm, when the sun’s rays are strongest. The AAD’s tip: If your shadow appears to be shorter than you are, seek shade.
Wear protective clothing. It may not meet your fashion standards, but wearing a long-sleeved shirt and pants, as well as a wide-brimmed hat and sunglasses, offers better protection.
Generously apply a broad-spectrum, water-resistant sunscreen with an SPF of 30 or more to all exposed skin. (Broad-spectrum sunscreens provide protection from both UVA and UVB rays.) Sunscreen should be reapplied every two hours or so, even on cloudy days, and after you’ve gone swimming or gotten sweaty.
Use extra caution near water, snow, and sand. These reflect and intensify the sun’s damaging rays and can increase your risk for sunburn.
Avoid tanning beds. UV light from tanning beds (as well as the sun) can cause skin cancer and wrinkling. Resent being pale? Consider using a self-tanning product or spray—but continue to use sunscreen with it!4
Continued on next page >>
The American Cancer Society has a skin protection campaign with a catchy slogan (adapted from an Australian campaign that launched in the 1980s) that may appeal to the younger (and most vulnerable) population: Slip, Slop, Slap® and Wrap. It stands for: Slip on a shirt. Slop on sunscreen. Slap on a hat. Wrap on sunglasses (to protect the eyes and sensitive skin around them).5
If our own (or our patients’) history of sun exposure has increased our risk for melanoma, remember that early detection is vital. Learn the signs and symptoms of melanoma; teach them to your family, friends, and patients.
Know that any mole can be suspicious and should be evaluated. The following mnemonic (ABCDE) provides clues to potential malignancy:
Asymmetry: Is the mole asymmetrical?
Border: Does the border or edge of the mole look uneven?
Color: Is the mole one uniform color? Several colors or shades of color within a mole could be a warning sign.
Diameter: How big is the mole? Melanomas often have a diameter of 6 mm (0.25 in) or more.
Evolving: Has the mole changed in shape, size, or color? Are there any other changes (eg, bleeding, itching, or pus)?
Many—but not all—melanomas present with the signs and symptoms listed above. But as our resident derm guru Joe Monroe regularly points out, there are different types of melanoma. The key is to know your skin and your moles. The AAD suggests that your birthday is a great day to “check your birthday suit.”6 While this is good advice (and perhaps easier to remember), I suggest you check your skin more often—and if you notice something, however insignificant it may seem, get it checked out by a professional. (And don’t assume that skin color or type offers immunity from skin cancer.)
I never gave much thought to sun exposure when I was younger—despite the many painful sunburns I endured. Today, my skin bears the scars of my early ignorance. Now I wear a hat (ugh) and slather on sunscreen. And that bathing suit? Now it is covered as much and as often as feasible. As for the sun, well, it now beats down on the umbrella that provides shade for that comfortable beach chair. My dermatology NP would be so proud!
So I challenge you to observe Melanoma Month and save your (and your patients’) skin—and potentially, your lives.
References on next page >>
REFERENCES
1. American Academy of Dermatology. Skin cancer: who gets and causes. www.aad.org/dermatology-a-to-z/diseases-and-treatments/ q---t/skin-cancer/who-gets-causes. Accessed April 15, 2014.
2. American Academy of Dermatology. Skin cancer. www.aad.org/media-resources/stats-and-facts/conditions/skin-cancer. Accessed April 15, 2014.
3. American Cancer Society. Cancer Facts and Figures 2013. www.cancer.org/acs/groups/content/@epidemiologysurveilance/docu ments/document/acspc-036845.pdf. Accessed April 15, 2014.
4. American Academy of Dermatology. How do I prevent skin cancer? www.aad.org/spot-skin-cancer/understanding-skin-cancer/how-do-i-prevent-skin-cancer. Accessed April 15, 2014.
5. American Cancer Society. Skin cancer prevention activities. www.cancer.org/healthy/more waysacshelpsyoustaywell/acs-skin-cancer-prevention-activities. Accessed April 15, 2014.
6. American Academy of Dermatology. Skin cancer prevention tips. www.aad.org/spot-skin-cancer/understanding-skin-cancer/how-do-i-prevent-skin-cancer/skin-cancer-preven tion-tips. Accessed April 15, 2014.
As the snow melts and the freezing temperatures begin to abate, I cannot help but look forward to temperate days and the ability to warm my chilblained body in the sun. Ah, I can see myself now: wearing only a bathing suit, sitting in a comfortable beach chair, with a cool beverage in one hand and a good book in the other, wriggling my toes in the warm sand while the bright yellow sun beams down on my bare skin. At least that’s what I would do in the “good old days,” when “getting a little color” wasn’t considered a bad thing.
Oh, how times have changed! We have since learned that the sun is the enemy of our skin and one of the leading causes of skin cancer (tanning beds are the other).1 These days, we get daily warnings about the ill effects of too much sun exposure and cautions about avoiding those harmful UV rays. Even exposure years ago can increase our risk for skin cancer now or in future.
Current estimates are that one in five Americans will develop skin cancer in his/her lifetime.2 The incidence of nonmelanoma (basal and squamous cell) skin cancer in the United States has been reported at 3.5 million new cases annually; for melanoma, nearly 77,000 new cases were expected to be diagnosed in 2013 alone.3 More than 9,000 people die of melanoma annually.3
While these statistics are disturbing, the cure rate offers some reassurance. If melanoma is detected and treated early, before it has the chance to spread to the lymph nodes, the cure rate can be as high as 100%. The five-year survival rate for localized melanoma is 98%.3 And while not all melanoma is preventable, we can take an active role in reducing our risk—and educating our patients on how to protect themselves.
Continued on next page >>
The American Academy of Dermatology (AAD) has designated May as National Melanoma Skin Cancer Prevention Month. As we progress from spring to summer, the position of the sun rises in the sky, the days become longer, and our vulnerability to skin cancer increases. Our colleagues at AAD have impeccable timing; we are a captive audience and eager to greet the summer and sunshine—responsibly. Here is what the AAD “strongly recommends” to accomplish this goal:
Seek shade when appropriate. This is especially important between 10 am and 2 pm, when the sun’s rays are strongest. The AAD’s tip: If your shadow appears to be shorter than you are, seek shade.
Wear protective clothing. It may not meet your fashion standards, but wearing a long-sleeved shirt and pants, as well as a wide-brimmed hat and sunglasses, offers better protection.
Generously apply a broad-spectrum, water-resistant sunscreen with an SPF of 30 or more to all exposed skin. (Broad-spectrum sunscreens provide protection from both UVA and UVB rays.) Sunscreen should be reapplied every two hours or so, even on cloudy days, and after you’ve gone swimming or gotten sweaty.
Use extra caution near water, snow, and sand. These reflect and intensify the sun’s damaging rays and can increase your risk for sunburn.
Avoid tanning beds. UV light from tanning beds (as well as the sun) can cause skin cancer and wrinkling. Resent being pale? Consider using a self-tanning product or spray—but continue to use sunscreen with it!4
Continued on next page >>
The American Cancer Society has a skin protection campaign with a catchy slogan (adapted from an Australian campaign that launched in the 1980s) that may appeal to the younger (and most vulnerable) population: Slip, Slop, Slap® and Wrap. It stands for: Slip on a shirt. Slop on sunscreen. Slap on a hat. Wrap on sunglasses (to protect the eyes and sensitive skin around them).5
If our own (or our patients’) history of sun exposure has increased our risk for melanoma, remember that early detection is vital. Learn the signs and symptoms of melanoma; teach them to your family, friends, and patients.
Know that any mole can be suspicious and should be evaluated. The following mnemonic (ABCDE) provides clues to potential malignancy:
Asymmetry: Is the mole asymmetrical?
Border: Does the border or edge of the mole look uneven?
Color: Is the mole one uniform color? Several colors or shades of color within a mole could be a warning sign.
Diameter: How big is the mole? Melanomas often have a diameter of 6 mm (0.25 in) or more.
Evolving: Has the mole changed in shape, size, or color? Are there any other changes (eg, bleeding, itching, or pus)?
Many—but not all—melanomas present with the signs and symptoms listed above. But as our resident derm guru Joe Monroe regularly points out, there are different types of melanoma. The key is to know your skin and your moles. The AAD suggests that your birthday is a great day to “check your birthday suit.”6 While this is good advice (and perhaps easier to remember), I suggest you check your skin more often—and if you notice something, however insignificant it may seem, get it checked out by a professional. (And don’t assume that skin color or type offers immunity from skin cancer.)
I never gave much thought to sun exposure when I was younger—despite the many painful sunburns I endured. Today, my skin bears the scars of my early ignorance. Now I wear a hat (ugh) and slather on sunscreen. And that bathing suit? Now it is covered as much and as often as feasible. As for the sun, well, it now beats down on the umbrella that provides shade for that comfortable beach chair. My dermatology NP would be so proud!
So I challenge you to observe Melanoma Month and save your (and your patients’) skin—and potentially, your lives.
References on next page >>
REFERENCES
1. American Academy of Dermatology. Skin cancer: who gets and causes. www.aad.org/dermatology-a-to-z/diseases-and-treatments/ q---t/skin-cancer/who-gets-causes. Accessed April 15, 2014.
2. American Academy of Dermatology. Skin cancer. www.aad.org/media-resources/stats-and-facts/conditions/skin-cancer. Accessed April 15, 2014.
3. American Cancer Society. Cancer Facts and Figures 2013. www.cancer.org/acs/groups/content/@epidemiologysurveilance/docu ments/document/acspc-036845.pdf. Accessed April 15, 2014.
4. American Academy of Dermatology. How do I prevent skin cancer? www.aad.org/spot-skin-cancer/understanding-skin-cancer/how-do-i-prevent-skin-cancer. Accessed April 15, 2014.
5. American Cancer Society. Skin cancer prevention activities. www.cancer.org/healthy/more waysacshelpsyoustaywell/acs-skin-cancer-prevention-activities. Accessed April 15, 2014.
6. American Academy of Dermatology. Skin cancer prevention tips. www.aad.org/spot-skin-cancer/understanding-skin-cancer/how-do-i-prevent-skin-cancer/skin-cancer-preven tion-tips. Accessed April 15, 2014.
As the snow melts and the freezing temperatures begin to abate, I cannot help but look forward to temperate days and the ability to warm my chilblained body in the sun. Ah, I can see myself now: wearing only a bathing suit, sitting in a comfortable beach chair, with a cool beverage in one hand and a good book in the other, wriggling my toes in the warm sand while the bright yellow sun beams down on my bare skin. At least that’s what I would do in the “good old days,” when “getting a little color” wasn’t considered a bad thing.
Oh, how times have changed! We have since learned that the sun is the enemy of our skin and one of the leading causes of skin cancer (tanning beds are the other).1 These days, we get daily warnings about the ill effects of too much sun exposure and cautions about avoiding those harmful UV rays. Even exposure years ago can increase our risk for skin cancer now or in future.
Current estimates are that one in five Americans will develop skin cancer in his/her lifetime.2 The incidence of nonmelanoma (basal and squamous cell) skin cancer in the United States has been reported at 3.5 million new cases annually; for melanoma, nearly 77,000 new cases were expected to be diagnosed in 2013 alone.3 More than 9,000 people die of melanoma annually.3
While these statistics are disturbing, the cure rate offers some reassurance. If melanoma is detected and treated early, before it has the chance to spread to the lymph nodes, the cure rate can be as high as 100%. The five-year survival rate for localized melanoma is 98%.3 And while not all melanoma is preventable, we can take an active role in reducing our risk—and educating our patients on how to protect themselves.
Continued on next page >>
The American Academy of Dermatology (AAD) has designated May as National Melanoma Skin Cancer Prevention Month. As we progress from spring to summer, the position of the sun rises in the sky, the days become longer, and our vulnerability to skin cancer increases. Our colleagues at AAD have impeccable timing; we are a captive audience and eager to greet the summer and sunshine—responsibly. Here is what the AAD “strongly recommends” to accomplish this goal:
Seek shade when appropriate. This is especially important between 10 am and 2 pm, when the sun’s rays are strongest. The AAD’s tip: If your shadow appears to be shorter than you are, seek shade.
Wear protective clothing. It may not meet your fashion standards, but wearing a long-sleeved shirt and pants, as well as a wide-brimmed hat and sunglasses, offers better protection.
Generously apply a broad-spectrum, water-resistant sunscreen with an SPF of 30 or more to all exposed skin. (Broad-spectrum sunscreens provide protection from both UVA and UVB rays.) Sunscreen should be reapplied every two hours or so, even on cloudy days, and after you’ve gone swimming or gotten sweaty.
Use extra caution near water, snow, and sand. These reflect and intensify the sun’s damaging rays and can increase your risk for sunburn.
Avoid tanning beds. UV light from tanning beds (as well as the sun) can cause skin cancer and wrinkling. Resent being pale? Consider using a self-tanning product or spray—but continue to use sunscreen with it!4
Continued on next page >>
The American Cancer Society has a skin protection campaign with a catchy slogan (adapted from an Australian campaign that launched in the 1980s) that may appeal to the younger (and most vulnerable) population: Slip, Slop, Slap® and Wrap. It stands for: Slip on a shirt. Slop on sunscreen. Slap on a hat. Wrap on sunglasses (to protect the eyes and sensitive skin around them).5
If our own (or our patients’) history of sun exposure has increased our risk for melanoma, remember that early detection is vital. Learn the signs and symptoms of melanoma; teach them to your family, friends, and patients.
Know that any mole can be suspicious and should be evaluated. The following mnemonic (ABCDE) provides clues to potential malignancy:
Asymmetry: Is the mole asymmetrical?
Border: Does the border or edge of the mole look uneven?
Color: Is the mole one uniform color? Several colors or shades of color within a mole could be a warning sign.
Diameter: How big is the mole? Melanomas often have a diameter of 6 mm (0.25 in) or more.
Evolving: Has the mole changed in shape, size, or color? Are there any other changes (eg, bleeding, itching, or pus)?
Many—but not all—melanomas present with the signs and symptoms listed above. But as our resident derm guru Joe Monroe regularly points out, there are different types of melanoma. The key is to know your skin and your moles. The AAD suggests that your birthday is a great day to “check your birthday suit.”6 While this is good advice (and perhaps easier to remember), I suggest you check your skin more often—and if you notice something, however insignificant it may seem, get it checked out by a professional. (And don’t assume that skin color or type offers immunity from skin cancer.)
I never gave much thought to sun exposure when I was younger—despite the many painful sunburns I endured. Today, my skin bears the scars of my early ignorance. Now I wear a hat (ugh) and slather on sunscreen. And that bathing suit? Now it is covered as much and as often as feasible. As for the sun, well, it now beats down on the umbrella that provides shade for that comfortable beach chair. My dermatology NP would be so proud!
So I challenge you to observe Melanoma Month and save your (and your patients’) skin—and potentially, your lives.
References on next page >>
REFERENCES
1. American Academy of Dermatology. Skin cancer: who gets and causes. www.aad.org/dermatology-a-to-z/diseases-and-treatments/ q---t/skin-cancer/who-gets-causes. Accessed April 15, 2014.
2. American Academy of Dermatology. Skin cancer. www.aad.org/media-resources/stats-and-facts/conditions/skin-cancer. Accessed April 15, 2014.
3. American Cancer Society. Cancer Facts and Figures 2013. www.cancer.org/acs/groups/content/@epidemiologysurveilance/docu ments/document/acspc-036845.pdf. Accessed April 15, 2014.
4. American Academy of Dermatology. How do I prevent skin cancer? www.aad.org/spot-skin-cancer/understanding-skin-cancer/how-do-i-prevent-skin-cancer. Accessed April 15, 2014.
5. American Cancer Society. Skin cancer prevention activities. www.cancer.org/healthy/more waysacshelpsyoustaywell/acs-skin-cancer-prevention-activities. Accessed April 15, 2014.
6. American Academy of Dermatology. Skin cancer prevention tips. www.aad.org/spot-skin-cancer/understanding-skin-cancer/how-do-i-prevent-skin-cancer/skin-cancer-preven tion-tips. Accessed April 15, 2014.
Philadelphia story
It’s scary how much my 12-year-old son resents his younger brother right now, so much that he can’t stop explaining what a horror it is that they have to share a room, a back seat, and 50% of their DNA. “You just don’t understand,” he’ll cry, “how hard it is (pulls hair dramatically) to live with him!” I get it: 9-year-old boys aren’t always particular about where they throw their clothes, their action figures, their boogers. Maybe this experience will at least keep them both from ever joining a fraternity. Think of it: a whole house full of nothing but brothers.
Blurred guidelines
Back in 2006, the American College of Chest Physicians and the American Academy of Pediatrics both published guidelines on the use of codeine-containing medications in children, essentially three words long: “Don’t do it.” The rationale was pretty simple: Codeine is inferior to ibuprofen for pain, and when used for cough relief, codeine is exactly like placebo, except for the part where codeine sometimes kills children. That’s the difference, the accidental death part.
Dr. Sunitha V. Kaiser of the Hospital for Sick Children in Toronto just published a study that asked a simple question: If two large organizations published guidelines in the same year that said a certain drug sometimes kills children and doesn’t help them, how long would it take emergency physicians to prescribe less of that drug? A month? A year? You win a free tablet of acetaminophen-with-codeine if you guessed “never.”
Actually, that’s not quite fair. Over the study period (2001-2010), there was a (very) small decline in codeine prescriptions for kids nationwide, especially for children aged 3-7 years. For cough and URI, however, there was no measurable decline. It was akin to how much codeine helps cough and cold symptoms: none.
These study results put a huge kink in my plan for world domination. I figured that if I could just wheedle my way into a position where I authored AAP guidelines, I’d soon hold a dictatorial sway over the practice of medicine, which I could then parlay into unlimited power. Fortunately, I have a plan B: First, I become an elementary school teacher...
Galvanize
Don’t you hate it when a trend disappears just as you find out about it? Just now, for example, I came up with the greatest idea for a Harlem Shake video. So why did it take a proposed FDA ban for me to learn that you could legally get kids to behave by shocking them with electricity? Did you know about this? Is that why your children have such good manners?
Actually, probably not, since these “electrical stimulation devices” are only used in one place, the Judge Rotenberg Educational Center in Massachusetts. They reportedly use the devices as the behavioral control method of last resort for patients with severe developmental disorders who might otherwise injure themselves. Of course, since FDA investigators were told of victims suffering burns, scars, muscle spasms, and seizures, perhaps they were just trying to save the kids the effort.
The Food and Drug Administration is still deciding whether to ban the devices, but it’s possible that soon no one will be able to use an FDA-approved electrical shock device to control kids’ behavior. I guess I’ll just keep doing what I’ve been doing: threatening my kids with posting my Harlem Shake video on Instagram...and tagging them.
The kindest cut
You know what I love the most about science? It’s so weird! Who would think to test whether children might behave better during a meal if their food were cut up instead of whole? A behaviorist at Cornell named Brian Wansink, that’s who! And why would he wonder that? I know, you’re thinking what I’m thinking: because he’s on drugs.
But he’s not. He knows that facial expressions and emotions are a two-way street: Not only do our emotions show on our faces, but when we make our faces express emotions, our brains respond in kind. Try it now: Make a really silly face. Now take a selfie and post it on Instagram. Do you feel silly? Wait until you read the comments!
According to Wansink’s study, children who bare their teeth to eat food like corn on the cob, drumsticks, and apples succumbed to the aggression implied by their expressions, behaving like, well, like my boys anytime they’re together. Now I’m inspired: If the brothers don’t lay off each other, they’re never again going to eat anything larger than a pea. If that doesn’t work, there’s still Instagram, and if that fails, well, college is coming sooner than they think.
David L. Hill, M.D., FAAP is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
It’s scary how much my 12-year-old son resents his younger brother right now, so much that he can’t stop explaining what a horror it is that they have to share a room, a back seat, and 50% of their DNA. “You just don’t understand,” he’ll cry, “how hard it is (pulls hair dramatically) to live with him!” I get it: 9-year-old boys aren’t always particular about where they throw their clothes, their action figures, their boogers. Maybe this experience will at least keep them both from ever joining a fraternity. Think of it: a whole house full of nothing but brothers.
Blurred guidelines
Back in 2006, the American College of Chest Physicians and the American Academy of Pediatrics both published guidelines on the use of codeine-containing medications in children, essentially three words long: “Don’t do it.” The rationale was pretty simple: Codeine is inferior to ibuprofen for pain, and when used for cough relief, codeine is exactly like placebo, except for the part where codeine sometimes kills children. That’s the difference, the accidental death part.
Dr. Sunitha V. Kaiser of the Hospital for Sick Children in Toronto just published a study that asked a simple question: If two large organizations published guidelines in the same year that said a certain drug sometimes kills children and doesn’t help them, how long would it take emergency physicians to prescribe less of that drug? A month? A year? You win a free tablet of acetaminophen-with-codeine if you guessed “never.”
Actually, that’s not quite fair. Over the study period (2001-2010), there was a (very) small decline in codeine prescriptions for kids nationwide, especially for children aged 3-7 years. For cough and URI, however, there was no measurable decline. It was akin to how much codeine helps cough and cold symptoms: none.
These study results put a huge kink in my plan for world domination. I figured that if I could just wheedle my way into a position where I authored AAP guidelines, I’d soon hold a dictatorial sway over the practice of medicine, which I could then parlay into unlimited power. Fortunately, I have a plan B: First, I become an elementary school teacher...
Galvanize
Don’t you hate it when a trend disappears just as you find out about it? Just now, for example, I came up with the greatest idea for a Harlem Shake video. So why did it take a proposed FDA ban for me to learn that you could legally get kids to behave by shocking them with electricity? Did you know about this? Is that why your children have such good manners?
Actually, probably not, since these “electrical stimulation devices” are only used in one place, the Judge Rotenberg Educational Center in Massachusetts. They reportedly use the devices as the behavioral control method of last resort for patients with severe developmental disorders who might otherwise injure themselves. Of course, since FDA investigators were told of victims suffering burns, scars, muscle spasms, and seizures, perhaps they were just trying to save the kids the effort.
The Food and Drug Administration is still deciding whether to ban the devices, but it’s possible that soon no one will be able to use an FDA-approved electrical shock device to control kids’ behavior. I guess I’ll just keep doing what I’ve been doing: threatening my kids with posting my Harlem Shake video on Instagram...and tagging them.
The kindest cut
You know what I love the most about science? It’s so weird! Who would think to test whether children might behave better during a meal if their food were cut up instead of whole? A behaviorist at Cornell named Brian Wansink, that’s who! And why would he wonder that? I know, you’re thinking what I’m thinking: because he’s on drugs.
But he’s not. He knows that facial expressions and emotions are a two-way street: Not only do our emotions show on our faces, but when we make our faces express emotions, our brains respond in kind. Try it now: Make a really silly face. Now take a selfie and post it on Instagram. Do you feel silly? Wait until you read the comments!
According to Wansink’s study, children who bare their teeth to eat food like corn on the cob, drumsticks, and apples succumbed to the aggression implied by their expressions, behaving like, well, like my boys anytime they’re together. Now I’m inspired: If the brothers don’t lay off each other, they’re never again going to eat anything larger than a pea. If that doesn’t work, there’s still Instagram, and if that fails, well, college is coming sooner than they think.
David L. Hill, M.D., FAAP is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
It’s scary how much my 12-year-old son resents his younger brother right now, so much that he can’t stop explaining what a horror it is that they have to share a room, a back seat, and 50% of their DNA. “You just don’t understand,” he’ll cry, “how hard it is (pulls hair dramatically) to live with him!” I get it: 9-year-old boys aren’t always particular about where they throw their clothes, their action figures, their boogers. Maybe this experience will at least keep them both from ever joining a fraternity. Think of it: a whole house full of nothing but brothers.
Blurred guidelines
Back in 2006, the American College of Chest Physicians and the American Academy of Pediatrics both published guidelines on the use of codeine-containing medications in children, essentially three words long: “Don’t do it.” The rationale was pretty simple: Codeine is inferior to ibuprofen for pain, and when used for cough relief, codeine is exactly like placebo, except for the part where codeine sometimes kills children. That’s the difference, the accidental death part.
Dr. Sunitha V. Kaiser of the Hospital for Sick Children in Toronto just published a study that asked a simple question: If two large organizations published guidelines in the same year that said a certain drug sometimes kills children and doesn’t help them, how long would it take emergency physicians to prescribe less of that drug? A month? A year? You win a free tablet of acetaminophen-with-codeine if you guessed “never.”
Actually, that’s not quite fair. Over the study period (2001-2010), there was a (very) small decline in codeine prescriptions for kids nationwide, especially for children aged 3-7 years. For cough and URI, however, there was no measurable decline. It was akin to how much codeine helps cough and cold symptoms: none.
These study results put a huge kink in my plan for world domination. I figured that if I could just wheedle my way into a position where I authored AAP guidelines, I’d soon hold a dictatorial sway over the practice of medicine, which I could then parlay into unlimited power. Fortunately, I have a plan B: First, I become an elementary school teacher...
Galvanize
Don’t you hate it when a trend disappears just as you find out about it? Just now, for example, I came up with the greatest idea for a Harlem Shake video. So why did it take a proposed FDA ban for me to learn that you could legally get kids to behave by shocking them with electricity? Did you know about this? Is that why your children have such good manners?
Actually, probably not, since these “electrical stimulation devices” are only used in one place, the Judge Rotenberg Educational Center in Massachusetts. They reportedly use the devices as the behavioral control method of last resort for patients with severe developmental disorders who might otherwise injure themselves. Of course, since FDA investigators were told of victims suffering burns, scars, muscle spasms, and seizures, perhaps they were just trying to save the kids the effort.
The Food and Drug Administration is still deciding whether to ban the devices, but it’s possible that soon no one will be able to use an FDA-approved electrical shock device to control kids’ behavior. I guess I’ll just keep doing what I’ve been doing: threatening my kids with posting my Harlem Shake video on Instagram...and tagging them.
The kindest cut
You know what I love the most about science? It’s so weird! Who would think to test whether children might behave better during a meal if their food were cut up instead of whole? A behaviorist at Cornell named Brian Wansink, that’s who! And why would he wonder that? I know, you’re thinking what I’m thinking: because he’s on drugs.
But he’s not. He knows that facial expressions and emotions are a two-way street: Not only do our emotions show on our faces, but when we make our faces express emotions, our brains respond in kind. Try it now: Make a really silly face. Now take a selfie and post it on Instagram. Do you feel silly? Wait until you read the comments!
According to Wansink’s study, children who bare their teeth to eat food like corn on the cob, drumsticks, and apples succumbed to the aggression implied by their expressions, behaving like, well, like my boys anytime they’re together. Now I’m inspired: If the brothers don’t lay off each other, they’re never again going to eat anything larger than a pea. If that doesn’t work, there’s still Instagram, and if that fails, well, college is coming sooner than they think.
David L. Hill, M.D., FAAP is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
How’s that transparency thing working out for you?
The recent release of billing information by the Centers for Medicare & Medicaid Services was accompanied by a spurious headline on the cms.gov website: "Historic release of data gives consumers unprecedented transparency on the medical services physicians provide and how much they are paid."
Have you downloaded the Excel spreadsheets online? There are megafiles of megabytes. The data lack all context and are confusing. What does it all mean? How are patients supposed to make decisions based on the data (if at all)? Do you want a doctor with a lot of Medicare billing or very little? As a cognitive clinic "E&M"-oriented cardiologist, I perform very few procedures. So my billings are in a middle range (I guess). If one of my patients were to carry out a search and discuss with me, I think that I would be within my professional rights to quote a recent secretary of state and ask, "What difference does it make?"
Perhaps that is where the government gets it wrong. Are there high-end outliers? Sure, and if the Centers for Medicare & Medicaid Services wants to carry out an audit, it can. But the publication of these data on a website does nothing to improve transparency. They may create front page headline material for newspapers, but on a day-to-day basis, patients just want to have a physician with whom they can talk, a physician who advocates for them, a physician who is skilled and thoughtful and careful. Medicare billing? Not on anyone’s Top Ten list.
Next stop by the government? The sunshine law release of information. We might as well repeat this column when that happens.
Dr. Hauptman is professor of internal medicine and assistant dean of clinical-translational research at Saint Louis University and director of heart failure at Saint Louis University Hospital. He currently serves as an associate editor for Circulation: Heart Failure and blogs while staring out his office window at the Arch.
The recent release of billing information by the Centers for Medicare & Medicaid Services was accompanied by a spurious headline on the cms.gov website: "Historic release of data gives consumers unprecedented transparency on the medical services physicians provide and how much they are paid."
Have you downloaded the Excel spreadsheets online? There are megafiles of megabytes. The data lack all context and are confusing. What does it all mean? How are patients supposed to make decisions based on the data (if at all)? Do you want a doctor with a lot of Medicare billing or very little? As a cognitive clinic "E&M"-oriented cardiologist, I perform very few procedures. So my billings are in a middle range (I guess). If one of my patients were to carry out a search and discuss with me, I think that I would be within my professional rights to quote a recent secretary of state and ask, "What difference does it make?"
Perhaps that is where the government gets it wrong. Are there high-end outliers? Sure, and if the Centers for Medicare & Medicaid Services wants to carry out an audit, it can. But the publication of these data on a website does nothing to improve transparency. They may create front page headline material for newspapers, but on a day-to-day basis, patients just want to have a physician with whom they can talk, a physician who advocates for them, a physician who is skilled and thoughtful and careful. Medicare billing? Not on anyone’s Top Ten list.
Next stop by the government? The sunshine law release of information. We might as well repeat this column when that happens.
Dr. Hauptman is professor of internal medicine and assistant dean of clinical-translational research at Saint Louis University and director of heart failure at Saint Louis University Hospital. He currently serves as an associate editor for Circulation: Heart Failure and blogs while staring out his office window at the Arch.
The recent release of billing information by the Centers for Medicare & Medicaid Services was accompanied by a spurious headline on the cms.gov website: "Historic release of data gives consumers unprecedented transparency on the medical services physicians provide and how much they are paid."
Have you downloaded the Excel spreadsheets online? There are megafiles of megabytes. The data lack all context and are confusing. What does it all mean? How are patients supposed to make decisions based on the data (if at all)? Do you want a doctor with a lot of Medicare billing or very little? As a cognitive clinic "E&M"-oriented cardiologist, I perform very few procedures. So my billings are in a middle range (I guess). If one of my patients were to carry out a search and discuss with me, I think that I would be within my professional rights to quote a recent secretary of state and ask, "What difference does it make?"
Perhaps that is where the government gets it wrong. Are there high-end outliers? Sure, and if the Centers for Medicare & Medicaid Services wants to carry out an audit, it can. But the publication of these data on a website does nothing to improve transparency. They may create front page headline material for newspapers, but on a day-to-day basis, patients just want to have a physician with whom they can talk, a physician who advocates for them, a physician who is skilled and thoughtful and careful. Medicare billing? Not on anyone’s Top Ten list.
Next stop by the government? The sunshine law release of information. We might as well repeat this column when that happens.
Dr. Hauptman is professor of internal medicine and assistant dean of clinical-translational research at Saint Louis University and director of heart failure at Saint Louis University Hospital. He currently serves as an associate editor for Circulation: Heart Failure and blogs while staring out his office window at the Arch.
Management of pediatric gastroesophageal reflux disease
Gastroesophageal reflux, defined as passage of gastric contents into the esophagus, is normal and occurs in 66% of healthy infants. Gastroesophageal reflux disease (GERD), defined as reflux associated with worrisome symptoms or complications, is far less common and must be differentiated from simple gastroesophageal reflux (GER) when making decisions about further testing and treatment. A primary emphasis of the American Academy of Pediatrics guidelines is for clinicians to decrease unnecessary diagnostic testing and pharmacologic treatment by distinguishing between GER, which requires relatively mild or no treatment at all, and GERD, which may require more careful intervention.
Clinical presentation
Symptoms associated with GER vary by age group. In infants (younger than 1 year of age), common symptoms include spitting up and vomiting. In school-age children, symptoms may include regurgitation and vomiting. In older children and adults, symptoms include the feeling of "heartburn" and foul-tasting belches. GERD on the other hand has additional symptoms and consequences. GERD symptoms are classified as esophageal or extraesophageal. Esophageal symptoms include poor weight gain, persistent vomiting, dysphagia, severe pain, and esophagitis. Extraesophageal manifestations include respiratory symptoms including cough, laryngitis, pneumonia, wheezing, and dental erosions. In infants less than age 1, the most common presentations of GERD include feeding refusal, poor weight gain, persistent irritability, and sleep disturbances, arching of the back, choking, and respiratory symptoms. In children aged 1-5 years, common symptoms include feeding refusal, vomiting, regurgitation, and abdominal pain. In older children and adolescents, the most common symptoms of GERD include abdominal pain (heartburn), recurrent vomiting, dysphagia, asthma, dental erosions, recurrent pneumonia, and chronic cough.
Diagnostic testing
Diagnostic testing usually is not necessary to make a diagnosis of either GER or GERD. A careful history and physical exam suffice. The diagnostic choices for evaluation of pediatric GERD include upper GI contrast radiography, esophageal pH and/or impedance monitoring, and upper endoscopy. None of these tests are sufficiently sensitive or specific to serve as a reliable test for GERD. Upper GI series are too short in duration to adequately rule out reflux, and, since reflux can occur normally, the observation of reflux on an upper GI test can lead to false-positive interpretations of the test. Esophageal pH monitoring is also flawed because of similar issues in that there is not a clear cut-off point in changes in esophageal pH that distinguish GER from GERD. Upper endoscopy allows visualization of injury to the esophageal mucosa, but recent data suggest that 25% of infants younger than 1 year have histologic evidence of esophageal inflammation, so the test again suffers from both false-positive and false-negative results. The decision for further diagnostic testing and/or evaluation by specialists is generally determined by failure to respond to pharmacologic treatment or the need to determine with more certainty the diagnosis because of severe consequences of GERD including poor weight gain, unexplained anemia, positive fecal occult blood, recurrent pneumonia, or hematemesis.
Management
Management of GER and GERD should always begin conservatively with lifestyle modifications. Lifestyle modifications in older children and adults include weight loss, as well as avoidance of food triggers such as caffeine, chocolate, alcohol, and spicy foods. Lifestyle modifications vary based on the age of the child. In infants who have uncomplicated GER or GERD, the following treatments can be considered:
• Reducing the volume of feeds and increasing the frequency of feeding.
• Maternal dietary restriction of egg and milk in breastfed children and changing of formula to a non–milk-based formula in bottle-fed infants, because mild protein allergy may mimic GERD. The guidelines reference one study where simply changing to protein hydrolysate formula thickened with 1 tablespoon of rice per 1 ounce of formula, avoiding overfeeding, and emphasizing correct feeding position led to a 24% rate of resolution of symptoms over 2 weeks.
• Formula thickening with 1 tablespoon of rice cereal per 1 ounce of formula. This technique should be recommended to full term infants only, because of an association between thickened feedings and necrotizing enterocolitis in preterm infants. In addition, it is important to realize that thickening a 20 kcal/oz infant formula with 1 tablespoon of rice cereal per ounce increases the caloric density to 34 kcal/oz. There are commercially available thickened formulas that do not add excess calories per ounce.
• Positioning recommendations include keeping infant upright or placing them prone while supervised and awake. Recently, studies have shown that the semisupine position (such as in a car seat) exacerbates GER and should be avoided in infants especially after feeding.
Pharmacotherapeutic agents are the next line of treatment. The guidelines express concern about overprescription of medications for pediatric GERD and emphasize that medications should be reserved to treat GERD in infants and children who did not respond to lifestyle modifications or who have significant complications of GERD. It is important to understand that medications should not be recommended to healthy children with GER. When medications are chosen the following points should be considered:
• Histamine2 receptor antagonists are effective at achieving acid suppression within 30 minutes of administration. There is little clinical difference between different formulations. Tachyphylaxis can develop within 6 weeks of medication use, limiting long-term efficacy.
• Proton pump inhibitors (PPIs) are effective at achieving acid suppression and do not cause tachyphylaxis. They work best when dosed 30 minutes prior to meals. The FDA has approved omeprazole, lansoprazole, and esomeprazole for use in children above 1 year old. It is important to note that randomized trials have shown no improvement with PPIs over placebo for reduction in irritability. PPIs can cause headaches, diarrhea, constipation, and nausea in up to 14% of children. Again, a word of caution is in order because recent evidence suggests that long-term acid suppression may increase the risk of community-acquired pneumonia, gastroenteritis, candidemia, and in preterm infants, necrotizing enterocolitis.
• Antacids and prokinetic agents have insufficient evidence to support their use, as well as significant potential side effects.
The bottom line
Uncomplicated GER is a common entity in family medicine, especially in infants and children. The most important part of the guidelines is to distinguish between GER and GERD. GER requires education and sometimes lifestyle modification. Treatment of GERD starts with lifestyle modification, moving on to medications and referral when needed.
Reference
J.R. Lightdale and G.A. Gremse. Gastroesophageal Reflux: Management Guidance for the Pediatrician. (Pediatrics 2013;131:e1684-e95).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Carcia is a second-year resident in the family medicine residency program at Abington Memorial Hospital.
Gastroesophageal reflux, defined as passage of gastric contents into the esophagus, is normal and occurs in 66% of healthy infants. Gastroesophageal reflux disease (GERD), defined as reflux associated with worrisome symptoms or complications, is far less common and must be differentiated from simple gastroesophageal reflux (GER) when making decisions about further testing and treatment. A primary emphasis of the American Academy of Pediatrics guidelines is for clinicians to decrease unnecessary diagnostic testing and pharmacologic treatment by distinguishing between GER, which requires relatively mild or no treatment at all, and GERD, which may require more careful intervention.
Clinical presentation
Symptoms associated with GER vary by age group. In infants (younger than 1 year of age), common symptoms include spitting up and vomiting. In school-age children, symptoms may include regurgitation and vomiting. In older children and adults, symptoms include the feeling of "heartburn" and foul-tasting belches. GERD on the other hand has additional symptoms and consequences. GERD symptoms are classified as esophageal or extraesophageal. Esophageal symptoms include poor weight gain, persistent vomiting, dysphagia, severe pain, and esophagitis. Extraesophageal manifestations include respiratory symptoms including cough, laryngitis, pneumonia, wheezing, and dental erosions. In infants less than age 1, the most common presentations of GERD include feeding refusal, poor weight gain, persistent irritability, and sleep disturbances, arching of the back, choking, and respiratory symptoms. In children aged 1-5 years, common symptoms include feeding refusal, vomiting, regurgitation, and abdominal pain. In older children and adolescents, the most common symptoms of GERD include abdominal pain (heartburn), recurrent vomiting, dysphagia, asthma, dental erosions, recurrent pneumonia, and chronic cough.
Diagnostic testing
Diagnostic testing usually is not necessary to make a diagnosis of either GER or GERD. A careful history and physical exam suffice. The diagnostic choices for evaluation of pediatric GERD include upper GI contrast radiography, esophageal pH and/or impedance monitoring, and upper endoscopy. None of these tests are sufficiently sensitive or specific to serve as a reliable test for GERD. Upper GI series are too short in duration to adequately rule out reflux, and, since reflux can occur normally, the observation of reflux on an upper GI test can lead to false-positive interpretations of the test. Esophageal pH monitoring is also flawed because of similar issues in that there is not a clear cut-off point in changes in esophageal pH that distinguish GER from GERD. Upper endoscopy allows visualization of injury to the esophageal mucosa, but recent data suggest that 25% of infants younger than 1 year have histologic evidence of esophageal inflammation, so the test again suffers from both false-positive and false-negative results. The decision for further diagnostic testing and/or evaluation by specialists is generally determined by failure to respond to pharmacologic treatment or the need to determine with more certainty the diagnosis because of severe consequences of GERD including poor weight gain, unexplained anemia, positive fecal occult blood, recurrent pneumonia, or hematemesis.
Management
Management of GER and GERD should always begin conservatively with lifestyle modifications. Lifestyle modifications in older children and adults include weight loss, as well as avoidance of food triggers such as caffeine, chocolate, alcohol, and spicy foods. Lifestyle modifications vary based on the age of the child. In infants who have uncomplicated GER or GERD, the following treatments can be considered:
• Reducing the volume of feeds and increasing the frequency of feeding.
• Maternal dietary restriction of egg and milk in breastfed children and changing of formula to a non–milk-based formula in bottle-fed infants, because mild protein allergy may mimic GERD. The guidelines reference one study where simply changing to protein hydrolysate formula thickened with 1 tablespoon of rice per 1 ounce of formula, avoiding overfeeding, and emphasizing correct feeding position led to a 24% rate of resolution of symptoms over 2 weeks.
• Formula thickening with 1 tablespoon of rice cereal per 1 ounce of formula. This technique should be recommended to full term infants only, because of an association between thickened feedings and necrotizing enterocolitis in preterm infants. In addition, it is important to realize that thickening a 20 kcal/oz infant formula with 1 tablespoon of rice cereal per ounce increases the caloric density to 34 kcal/oz. There are commercially available thickened formulas that do not add excess calories per ounce.
• Positioning recommendations include keeping infant upright or placing them prone while supervised and awake. Recently, studies have shown that the semisupine position (such as in a car seat) exacerbates GER and should be avoided in infants especially after feeding.
Pharmacotherapeutic agents are the next line of treatment. The guidelines express concern about overprescription of medications for pediatric GERD and emphasize that medications should be reserved to treat GERD in infants and children who did not respond to lifestyle modifications or who have significant complications of GERD. It is important to understand that medications should not be recommended to healthy children with GER. When medications are chosen the following points should be considered:
• Histamine2 receptor antagonists are effective at achieving acid suppression within 30 minutes of administration. There is little clinical difference between different formulations. Tachyphylaxis can develop within 6 weeks of medication use, limiting long-term efficacy.
• Proton pump inhibitors (PPIs) are effective at achieving acid suppression and do not cause tachyphylaxis. They work best when dosed 30 minutes prior to meals. The FDA has approved omeprazole, lansoprazole, and esomeprazole for use in children above 1 year old. It is important to note that randomized trials have shown no improvement with PPIs over placebo for reduction in irritability. PPIs can cause headaches, diarrhea, constipation, and nausea in up to 14% of children. Again, a word of caution is in order because recent evidence suggests that long-term acid suppression may increase the risk of community-acquired pneumonia, gastroenteritis, candidemia, and in preterm infants, necrotizing enterocolitis.
• Antacids and prokinetic agents have insufficient evidence to support their use, as well as significant potential side effects.
The bottom line
Uncomplicated GER is a common entity in family medicine, especially in infants and children. The most important part of the guidelines is to distinguish between GER and GERD. GER requires education and sometimes lifestyle modification. Treatment of GERD starts with lifestyle modification, moving on to medications and referral when needed.
Reference
J.R. Lightdale and G.A. Gremse. Gastroesophageal Reflux: Management Guidance for the Pediatrician. (Pediatrics 2013;131:e1684-e95).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Carcia is a second-year resident in the family medicine residency program at Abington Memorial Hospital.
Gastroesophageal reflux, defined as passage of gastric contents into the esophagus, is normal and occurs in 66% of healthy infants. Gastroesophageal reflux disease (GERD), defined as reflux associated with worrisome symptoms or complications, is far less common and must be differentiated from simple gastroesophageal reflux (GER) when making decisions about further testing and treatment. A primary emphasis of the American Academy of Pediatrics guidelines is for clinicians to decrease unnecessary diagnostic testing and pharmacologic treatment by distinguishing between GER, which requires relatively mild or no treatment at all, and GERD, which may require more careful intervention.
Clinical presentation
Symptoms associated with GER vary by age group. In infants (younger than 1 year of age), common symptoms include spitting up and vomiting. In school-age children, symptoms may include regurgitation and vomiting. In older children and adults, symptoms include the feeling of "heartburn" and foul-tasting belches. GERD on the other hand has additional symptoms and consequences. GERD symptoms are classified as esophageal or extraesophageal. Esophageal symptoms include poor weight gain, persistent vomiting, dysphagia, severe pain, and esophagitis. Extraesophageal manifestations include respiratory symptoms including cough, laryngitis, pneumonia, wheezing, and dental erosions. In infants less than age 1, the most common presentations of GERD include feeding refusal, poor weight gain, persistent irritability, and sleep disturbances, arching of the back, choking, and respiratory symptoms. In children aged 1-5 years, common symptoms include feeding refusal, vomiting, regurgitation, and abdominal pain. In older children and adolescents, the most common symptoms of GERD include abdominal pain (heartburn), recurrent vomiting, dysphagia, asthma, dental erosions, recurrent pneumonia, and chronic cough.
Diagnostic testing
Diagnostic testing usually is not necessary to make a diagnosis of either GER or GERD. A careful history and physical exam suffice. The diagnostic choices for evaluation of pediatric GERD include upper GI contrast radiography, esophageal pH and/or impedance monitoring, and upper endoscopy. None of these tests are sufficiently sensitive or specific to serve as a reliable test for GERD. Upper GI series are too short in duration to adequately rule out reflux, and, since reflux can occur normally, the observation of reflux on an upper GI test can lead to false-positive interpretations of the test. Esophageal pH monitoring is also flawed because of similar issues in that there is not a clear cut-off point in changes in esophageal pH that distinguish GER from GERD. Upper endoscopy allows visualization of injury to the esophageal mucosa, but recent data suggest that 25% of infants younger than 1 year have histologic evidence of esophageal inflammation, so the test again suffers from both false-positive and false-negative results. The decision for further diagnostic testing and/or evaluation by specialists is generally determined by failure to respond to pharmacologic treatment or the need to determine with more certainty the diagnosis because of severe consequences of GERD including poor weight gain, unexplained anemia, positive fecal occult blood, recurrent pneumonia, or hematemesis.
Management
Management of GER and GERD should always begin conservatively with lifestyle modifications. Lifestyle modifications in older children and adults include weight loss, as well as avoidance of food triggers such as caffeine, chocolate, alcohol, and spicy foods. Lifestyle modifications vary based on the age of the child. In infants who have uncomplicated GER or GERD, the following treatments can be considered:
• Reducing the volume of feeds and increasing the frequency of feeding.
• Maternal dietary restriction of egg and milk in breastfed children and changing of formula to a non–milk-based formula in bottle-fed infants, because mild protein allergy may mimic GERD. The guidelines reference one study where simply changing to protein hydrolysate formula thickened with 1 tablespoon of rice per 1 ounce of formula, avoiding overfeeding, and emphasizing correct feeding position led to a 24% rate of resolution of symptoms over 2 weeks.
• Formula thickening with 1 tablespoon of rice cereal per 1 ounce of formula. This technique should be recommended to full term infants only, because of an association between thickened feedings and necrotizing enterocolitis in preterm infants. In addition, it is important to realize that thickening a 20 kcal/oz infant formula with 1 tablespoon of rice cereal per ounce increases the caloric density to 34 kcal/oz. There are commercially available thickened formulas that do not add excess calories per ounce.
• Positioning recommendations include keeping infant upright or placing them prone while supervised and awake. Recently, studies have shown that the semisupine position (such as in a car seat) exacerbates GER and should be avoided in infants especially after feeding.
Pharmacotherapeutic agents are the next line of treatment. The guidelines express concern about overprescription of medications for pediatric GERD and emphasize that medications should be reserved to treat GERD in infants and children who did not respond to lifestyle modifications or who have significant complications of GERD. It is important to understand that medications should not be recommended to healthy children with GER. When medications are chosen the following points should be considered:
• Histamine2 receptor antagonists are effective at achieving acid suppression within 30 minutes of administration. There is little clinical difference between different formulations. Tachyphylaxis can develop within 6 weeks of medication use, limiting long-term efficacy.
• Proton pump inhibitors (PPIs) are effective at achieving acid suppression and do not cause tachyphylaxis. They work best when dosed 30 minutes prior to meals. The FDA has approved omeprazole, lansoprazole, and esomeprazole for use in children above 1 year old. It is important to note that randomized trials have shown no improvement with PPIs over placebo for reduction in irritability. PPIs can cause headaches, diarrhea, constipation, and nausea in up to 14% of children. Again, a word of caution is in order because recent evidence suggests that long-term acid suppression may increase the risk of community-acquired pneumonia, gastroenteritis, candidemia, and in preterm infants, necrotizing enterocolitis.
• Antacids and prokinetic agents have insufficient evidence to support their use, as well as significant potential side effects.
The bottom line
Uncomplicated GER is a common entity in family medicine, especially in infants and children. The most important part of the guidelines is to distinguish between GER and GERD. GER requires education and sometimes lifestyle modification. Treatment of GERD starts with lifestyle modification, moving on to medications and referral when needed.
Reference
J.R. Lightdale and G.A. Gremse. Gastroesophageal Reflux: Management Guidance for the Pediatrician. (Pediatrics 2013;131:e1684-e95).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Carcia is a second-year resident in the family medicine residency program at Abington Memorial Hospital.
How often does otitis media cause fever?
I read with interest “Otitis media? Not likely” in your “What’s the Verdict” column (J Fam Pract. 2014;63:47). I agree with Dr. Hickner’s assessment that a 3-month-old child who has a fever of 103°F should raise concern for other serious illnesses, yet I disagree that otitis media rarely causes fever.
Fever is relatively common in children with acute otitis media. Schwartz et al,1 presented a case series of 671 children with otitis media. They could identify no cause of fever other than the ear infection in 23% of patients.
Thomas Bielanski, MD, FAAFP
Oak Park, Ill
1. Schwartz RH, Rodriguez WJ, Brook I, et al. The febrile response in acute otitis media. JAMA. 1981;245:2057-2058.
I read with interest “Otitis media? Not likely” in your “What’s the Verdict” column (J Fam Pract. 2014;63:47). I agree with Dr. Hickner’s assessment that a 3-month-old child who has a fever of 103°F should raise concern for other serious illnesses, yet I disagree that otitis media rarely causes fever.
Fever is relatively common in children with acute otitis media. Schwartz et al,1 presented a case series of 671 children with otitis media. They could identify no cause of fever other than the ear infection in 23% of patients.
Thomas Bielanski, MD, FAAFP
Oak Park, Ill
I read with interest “Otitis media? Not likely” in your “What’s the Verdict” column (J Fam Pract. 2014;63:47). I agree with Dr. Hickner’s assessment that a 3-month-old child who has a fever of 103°F should raise concern for other serious illnesses, yet I disagree that otitis media rarely causes fever.
Fever is relatively common in children with acute otitis media. Schwartz et al,1 presented a case series of 671 children with otitis media. They could identify no cause of fever other than the ear infection in 23% of patients.
Thomas Bielanski, MD, FAAFP
Oak Park, Ill
1. Schwartz RH, Rodriguez WJ, Brook I, et al. The febrile response in acute otitis media. JAMA. 1981;245:2057-2058.
1. Schwartz RH, Rodriguez WJ, Brook I, et al. The febrile response in acute otitis media. JAMA. 1981;245:2057-2058.