User login
Guideline gives weak support to trying oral medical cannabis for chronic pain
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
FROM THE BMJ
Menopause society issues first osteoporosis advice in 10 years
In the first revision to its guidance on the management of osteoporosis in a decade, the North American Menopause Society has issued an updated position statement addressing evolving evidence on osteoporosis issues ranging from screening and risk assessment to appropriate use of preventive therapy in postmenopausal women.
“Since the 2010 statement, there have been important new developments in our field, including better delineation of risk factors for fracture, resulting in better strategies for assessing fracture risk,” Michael R. McClung, MD, who is a NAMS board member and colead of the editorial panel for the 2021 position statement, told this news organization. Dr. McClung is also director emeritus of the Oregon Osteoporosis Center in Portland.
“There is much more information about the long-term safety of therapies,” he added. Dr. McClung also noted “the availability of four new drugs for the prevention and treatment of osteoporosis and clinical experience informing us of the effects of using different treatments in various sequences.”
Osteoporosis is substantially underdiagnosed and undertreated
A basis for the update, recently published in Menopause: The Journal of the North American Menopause Society, is the need to tackle the troubling fact that approximately half of postmenopausal women will experience a fracture related to osteoporosis in their lifetime, yet the condition is “substantially underdiagnosed and undertreated,” NAMS underscores.
With that in mind, osteoporosis should be considered by practitioners treating menopausal and postmenopausal women at all levels of care.
“All physicians and advanced care providers caring for postmenopausal women should be comfortable assessing and managing their patients with, or at risk for, fractures,” Dr. McClung added.
Osteoporosis prevention in young menopausal women
The NAMS statement covers a broad range of issues, and while most recommendations generally follow those of other societies’ guidelines, a unique aspect is the emphasis on preventing osteoporosis in young menopausal women with estrogen or other drugs.
While underscoring that all menopausal women should be encouraged to adopt healthy lifestyles, with good diets and physical activity to reduce the risk of bone loss and fractures, pharmacologic interventions also have a role, NAMS says.
Though long an issue of debate, NAMS voices support for estrogen therapy as having an important role in osteoporosis prevention, as estrogen deficiency is the principal cause of bone loss in postmenopausal women.
“Hormone therapy is the most appropriate choice to prevent bone loss at the time of menopause for healthy women, particularly those who have menopause symptoms,” the group states. Drug interventions are specifically supported in women with premature menopause, at least until the average age of natural menopause, in addition to those with low bone mineral density (BMD) (T-score < –1.0) and those experiencing relatively rapid bone loss related to acute estrogen deficiency in the menopause transition or on discontinuing estrogen therapy.
“Although using drugs to prevent osteoporosis is not included in national osteoporosis guidelines, a strong clinical argument can be made for doing so, especially in women who come to menopause with low bone mass,” the report states.
And therapy is also recommended if patients have a low BMD and other risk factors for fracture, such as family history, but do not meet the criteria for osteoporosis treatment.
Ultimately, clinicians should work with patients when deciding the options, Dr. McClung said.
“After carefully weighing the small risks associated with hormone therapy or other therapies begun at the time of menopause, menopause practitioners and their patients can and should make informed decisions about the use of Food and Drug Administration–approved medications to prevent osteoporosis in women who are at risk for developing that condition,” he noted, adding that his view on the matter is his own and not necessarily that of NAMS.
New treatments endorsed for high-risk patients to avoid ‘bone attack’
While most patients are treated for osteoporosis with antiremodeling drugs such as bisphosphonates and denosumab, NAMS endorses “a new paradigm of beginning treatment with a bone-building agent followed by an antiremodeling agent” for women at very high risk of fracture.
“Consider osteoanabolic therapies for patients at very high risk of fracture, including older women with recent fractures, T-scores –3.0 and lower, or multiple other risk factors,” the statement suggests.
Among those at highest risk are women who have sustained a first fracture.
“A recent fracture in a postmenopausal woman is the strongest risk factor for another fracture,” Dr. McClung said.
In fact, “having a fracture should be thought of and assessed as a ‘bone attack,’ ” he asserted.
Therapy is recommended in such cases to rapidly increase bone density and reduce their subsequent fracture risk.
“For these patients, osteoanabolic or bone-building agents are more effective than bisphosphonates and are recommended as initial therapy,” Dr. McClung noted.
Treatment discontinuation?
On the issue of drug holidays and when or whether to stop therapy, as no therapies cure osteoporosis, medications should not be permanently stopped, even if bone density increases, NAMS recommends.
“By analogy, we do not stop diabetes therapy when A1c levels become normal,” Dr. McClung noted.
“Because the benefits of therapy on bone density and fracture protection wane, quickly for nonbisphosphonates and more slowly with bisphosphonates, short-term therapy, for instance 5 years, is not optimal treatment,” he said.
While the short-term interruption of bisphosphonate therapy may be considered in some patients, “the concept of ‘drug holidays’ does not pertain to nonbisphosphonate drugs,” Dr. McClung said.
NAMS adds that management of therapeutic choices should instead be ongoing.
“During therapy, reevaluate the treatment goals and the choice of medication on an ongoing basis through periodic medical examination and follow-up BMD testing,” NAMS recommends.
In terms of assessment, the measurement of bone mineral density while on treatment can gauge the current risk of fracture, and NAMS supports the use of the T-score at the hip as an appropriate clinical target in guiding choices of therapy.
Ultimately, “effective tools for diagnosing osteoporosis and assessing fracture risk are available, and well-studied strategies exist for managing bone health in women at both low and high risk of fracture,” NAMS concludes.
“By individualizing treatment approaches and monitoring and adjusting those approaches if the clinical picture changes, the consequences of osteoporosis on a menopausal woman’s activity and well-being can be minimized.”
Dr. McClung has reported receiving consulting fees from Amgen and Myovant, and honorarium for speaking from Amgen and Alexon. He serves on the boards of NAMS and the International Osteoporosis Foundation.
A version of this article first appeared on Medscape.com.
In the first revision to its guidance on the management of osteoporosis in a decade, the North American Menopause Society has issued an updated position statement addressing evolving evidence on osteoporosis issues ranging from screening and risk assessment to appropriate use of preventive therapy in postmenopausal women.
“Since the 2010 statement, there have been important new developments in our field, including better delineation of risk factors for fracture, resulting in better strategies for assessing fracture risk,” Michael R. McClung, MD, who is a NAMS board member and colead of the editorial panel for the 2021 position statement, told this news organization. Dr. McClung is also director emeritus of the Oregon Osteoporosis Center in Portland.
“There is much more information about the long-term safety of therapies,” he added. Dr. McClung also noted “the availability of four new drugs for the prevention and treatment of osteoporosis and clinical experience informing us of the effects of using different treatments in various sequences.”
Osteoporosis is substantially underdiagnosed and undertreated
A basis for the update, recently published in Menopause: The Journal of the North American Menopause Society, is the need to tackle the troubling fact that approximately half of postmenopausal women will experience a fracture related to osteoporosis in their lifetime, yet the condition is “substantially underdiagnosed and undertreated,” NAMS underscores.
With that in mind, osteoporosis should be considered by practitioners treating menopausal and postmenopausal women at all levels of care.
“All physicians and advanced care providers caring for postmenopausal women should be comfortable assessing and managing their patients with, or at risk for, fractures,” Dr. McClung added.
Osteoporosis prevention in young menopausal women
The NAMS statement covers a broad range of issues, and while most recommendations generally follow those of other societies’ guidelines, a unique aspect is the emphasis on preventing osteoporosis in young menopausal women with estrogen or other drugs.
While underscoring that all menopausal women should be encouraged to adopt healthy lifestyles, with good diets and physical activity to reduce the risk of bone loss and fractures, pharmacologic interventions also have a role, NAMS says.
Though long an issue of debate, NAMS voices support for estrogen therapy as having an important role in osteoporosis prevention, as estrogen deficiency is the principal cause of bone loss in postmenopausal women.
“Hormone therapy is the most appropriate choice to prevent bone loss at the time of menopause for healthy women, particularly those who have menopause symptoms,” the group states. Drug interventions are specifically supported in women with premature menopause, at least until the average age of natural menopause, in addition to those with low bone mineral density (BMD) (T-score < –1.0) and those experiencing relatively rapid bone loss related to acute estrogen deficiency in the menopause transition or on discontinuing estrogen therapy.
“Although using drugs to prevent osteoporosis is not included in national osteoporosis guidelines, a strong clinical argument can be made for doing so, especially in women who come to menopause with low bone mass,” the report states.
And therapy is also recommended if patients have a low BMD and other risk factors for fracture, such as family history, but do not meet the criteria for osteoporosis treatment.
Ultimately, clinicians should work with patients when deciding the options, Dr. McClung said.
“After carefully weighing the small risks associated with hormone therapy or other therapies begun at the time of menopause, menopause practitioners and their patients can and should make informed decisions about the use of Food and Drug Administration–approved medications to prevent osteoporosis in women who are at risk for developing that condition,” he noted, adding that his view on the matter is his own and not necessarily that of NAMS.
New treatments endorsed for high-risk patients to avoid ‘bone attack’
While most patients are treated for osteoporosis with antiremodeling drugs such as bisphosphonates and denosumab, NAMS endorses “a new paradigm of beginning treatment with a bone-building agent followed by an antiremodeling agent” for women at very high risk of fracture.
“Consider osteoanabolic therapies for patients at very high risk of fracture, including older women with recent fractures, T-scores –3.0 and lower, or multiple other risk factors,” the statement suggests.
Among those at highest risk are women who have sustained a first fracture.
“A recent fracture in a postmenopausal woman is the strongest risk factor for another fracture,” Dr. McClung said.
In fact, “having a fracture should be thought of and assessed as a ‘bone attack,’ ” he asserted.
Therapy is recommended in such cases to rapidly increase bone density and reduce their subsequent fracture risk.
“For these patients, osteoanabolic or bone-building agents are more effective than bisphosphonates and are recommended as initial therapy,” Dr. McClung noted.
Treatment discontinuation?
On the issue of drug holidays and when or whether to stop therapy, as no therapies cure osteoporosis, medications should not be permanently stopped, even if bone density increases, NAMS recommends.
“By analogy, we do not stop diabetes therapy when A1c levels become normal,” Dr. McClung noted.
“Because the benefits of therapy on bone density and fracture protection wane, quickly for nonbisphosphonates and more slowly with bisphosphonates, short-term therapy, for instance 5 years, is not optimal treatment,” he said.
While the short-term interruption of bisphosphonate therapy may be considered in some patients, “the concept of ‘drug holidays’ does not pertain to nonbisphosphonate drugs,” Dr. McClung said.
NAMS adds that management of therapeutic choices should instead be ongoing.
“During therapy, reevaluate the treatment goals and the choice of medication on an ongoing basis through periodic medical examination and follow-up BMD testing,” NAMS recommends.
In terms of assessment, the measurement of bone mineral density while on treatment can gauge the current risk of fracture, and NAMS supports the use of the T-score at the hip as an appropriate clinical target in guiding choices of therapy.
Ultimately, “effective tools for diagnosing osteoporosis and assessing fracture risk are available, and well-studied strategies exist for managing bone health in women at both low and high risk of fracture,” NAMS concludes.
“By individualizing treatment approaches and monitoring and adjusting those approaches if the clinical picture changes, the consequences of osteoporosis on a menopausal woman’s activity and well-being can be minimized.”
Dr. McClung has reported receiving consulting fees from Amgen and Myovant, and honorarium for speaking from Amgen and Alexon. He serves on the boards of NAMS and the International Osteoporosis Foundation.
A version of this article first appeared on Medscape.com.
In the first revision to its guidance on the management of osteoporosis in a decade, the North American Menopause Society has issued an updated position statement addressing evolving evidence on osteoporosis issues ranging from screening and risk assessment to appropriate use of preventive therapy in postmenopausal women.
“Since the 2010 statement, there have been important new developments in our field, including better delineation of risk factors for fracture, resulting in better strategies for assessing fracture risk,” Michael R. McClung, MD, who is a NAMS board member and colead of the editorial panel for the 2021 position statement, told this news organization. Dr. McClung is also director emeritus of the Oregon Osteoporosis Center in Portland.
“There is much more information about the long-term safety of therapies,” he added. Dr. McClung also noted “the availability of four new drugs for the prevention and treatment of osteoporosis and clinical experience informing us of the effects of using different treatments in various sequences.”
Osteoporosis is substantially underdiagnosed and undertreated
A basis for the update, recently published in Menopause: The Journal of the North American Menopause Society, is the need to tackle the troubling fact that approximately half of postmenopausal women will experience a fracture related to osteoporosis in their lifetime, yet the condition is “substantially underdiagnosed and undertreated,” NAMS underscores.
With that in mind, osteoporosis should be considered by practitioners treating menopausal and postmenopausal women at all levels of care.
“All physicians and advanced care providers caring for postmenopausal women should be comfortable assessing and managing their patients with, or at risk for, fractures,” Dr. McClung added.
Osteoporosis prevention in young menopausal women
The NAMS statement covers a broad range of issues, and while most recommendations generally follow those of other societies’ guidelines, a unique aspect is the emphasis on preventing osteoporosis in young menopausal women with estrogen or other drugs.
While underscoring that all menopausal women should be encouraged to adopt healthy lifestyles, with good diets and physical activity to reduce the risk of bone loss and fractures, pharmacologic interventions also have a role, NAMS says.
Though long an issue of debate, NAMS voices support for estrogen therapy as having an important role in osteoporosis prevention, as estrogen deficiency is the principal cause of bone loss in postmenopausal women.
“Hormone therapy is the most appropriate choice to prevent bone loss at the time of menopause for healthy women, particularly those who have menopause symptoms,” the group states. Drug interventions are specifically supported in women with premature menopause, at least until the average age of natural menopause, in addition to those with low bone mineral density (BMD) (T-score < –1.0) and those experiencing relatively rapid bone loss related to acute estrogen deficiency in the menopause transition or on discontinuing estrogen therapy.
“Although using drugs to prevent osteoporosis is not included in national osteoporosis guidelines, a strong clinical argument can be made for doing so, especially in women who come to menopause with low bone mass,” the report states.
And therapy is also recommended if patients have a low BMD and other risk factors for fracture, such as family history, but do not meet the criteria for osteoporosis treatment.
Ultimately, clinicians should work with patients when deciding the options, Dr. McClung said.
“After carefully weighing the small risks associated with hormone therapy or other therapies begun at the time of menopause, menopause practitioners and their patients can and should make informed decisions about the use of Food and Drug Administration–approved medications to prevent osteoporosis in women who are at risk for developing that condition,” he noted, adding that his view on the matter is his own and not necessarily that of NAMS.
New treatments endorsed for high-risk patients to avoid ‘bone attack’
While most patients are treated for osteoporosis with antiremodeling drugs such as bisphosphonates and denosumab, NAMS endorses “a new paradigm of beginning treatment with a bone-building agent followed by an antiremodeling agent” for women at very high risk of fracture.
“Consider osteoanabolic therapies for patients at very high risk of fracture, including older women with recent fractures, T-scores –3.0 and lower, or multiple other risk factors,” the statement suggests.
Among those at highest risk are women who have sustained a first fracture.
“A recent fracture in a postmenopausal woman is the strongest risk factor for another fracture,” Dr. McClung said.
In fact, “having a fracture should be thought of and assessed as a ‘bone attack,’ ” he asserted.
Therapy is recommended in such cases to rapidly increase bone density and reduce their subsequent fracture risk.
“For these patients, osteoanabolic or bone-building agents are more effective than bisphosphonates and are recommended as initial therapy,” Dr. McClung noted.
Treatment discontinuation?
On the issue of drug holidays and when or whether to stop therapy, as no therapies cure osteoporosis, medications should not be permanently stopped, even if bone density increases, NAMS recommends.
“By analogy, we do not stop diabetes therapy when A1c levels become normal,” Dr. McClung noted.
“Because the benefits of therapy on bone density and fracture protection wane, quickly for nonbisphosphonates and more slowly with bisphosphonates, short-term therapy, for instance 5 years, is not optimal treatment,” he said.
While the short-term interruption of bisphosphonate therapy may be considered in some patients, “the concept of ‘drug holidays’ does not pertain to nonbisphosphonate drugs,” Dr. McClung said.
NAMS adds that management of therapeutic choices should instead be ongoing.
“During therapy, reevaluate the treatment goals and the choice of medication on an ongoing basis through periodic medical examination and follow-up BMD testing,” NAMS recommends.
In terms of assessment, the measurement of bone mineral density while on treatment can gauge the current risk of fracture, and NAMS supports the use of the T-score at the hip as an appropriate clinical target in guiding choices of therapy.
Ultimately, “effective tools for diagnosing osteoporosis and assessing fracture risk are available, and well-studied strategies exist for managing bone health in women at both low and high risk of fracture,” NAMS concludes.
“By individualizing treatment approaches and monitoring and adjusting those approaches if the clinical picture changes, the consequences of osteoporosis on a menopausal woman’s activity and well-being can be minimized.”
Dr. McClung has reported receiving consulting fees from Amgen and Myovant, and honorarium for speaking from Amgen and Alexon. He serves on the boards of NAMS and the International Osteoporosis Foundation.
A version of this article first appeared on Medscape.com.
AGA Clinical Practice Update: Expert Review on IBD dysplasia surveillance, management
The American Gastroenterological Association recently published an expert review and clinical practice update addressing endoscopic surveillance and management of colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Because of practice-altering advances in therapy and surveillance over the past 2 decades, an updated approach is needed, according to authors led by Sanjay K. Murthy, MD, of Ottawa Hospital Research Institute and Fernando Velayos, MD, from Kaiser Permanente San Francisco Medical Center.
“Not long ago, notions of imperceptible CRC [colorectal cancer] development and urgent need for colectomy in the face of dysplasia dominated IBD practice,” the authors wrote in Gastroenterology. “However, improvements in disease management, as well as endoscopic technology and quality, have dramatically changed the way in which we conceptualize and manage IBD-related dysplasia over the past 20 years.”
Most notably, the authors called for a more conservative approach to sample collection and intervention.
“The practices of taking nontargeted biopsies and of referring patients for colectomy in the setting of low-grade or invisible dysplasia are being increasingly challenged in favor of ‘smart’ approaches that emphasize careful inspection and targeted sampling of visible and subtle lesions using newer technologies ... as well as endoscopic management of most lesions that appear endoscopically resectable,” the authors wrote. “Indeed, surgery is being increasingly reserved for lesions harboring strong risk factors for invasive cancer or when endoscopic clearance is not possible.”
The 14 best practice advice statements cover a variety of topics, including appropriate lesion terminology and characterization, endoscopy timing, and indications for biopsies, resection, and colectomy.
“The proposed conceptual model and best practice advice statements in this review are best used in conjunction with evolving literature and existing societal guidelines as part of a shared decision-making process,” the authors noted.
Lesion descriptions
First, the authors provided best practice advice for retirement of three older terms: “dysplasia-associated lesion or mass, adenoma-like mass, and flat dysplasia.” Instead, they advised sorting precancerous colorectal lesions into one of three categories: nonpolypoid (less than 2.5 mm tall), polypoid (at least 2.5 mm tall), or invisible (if detected by nontargeted biopsy).
According to the update, lesion descriptions should also include location, morphology, size, presence of ulceration, clarity of borders, presence within an area of past or current colitis, use of special visualization techniques, and perceived completeness of resection.
Surveillance timing
All patients with chronic IBD should undergo colonoscopy screening for dysplasia 8-10 years after diagnosis, the authors wrote. Subsequent colonoscopies should be performed every 1-5 years, depending on risk factors, such as family history of colorectal cancer and quality of prior surveillance exams.
Higher-risk patients may require colonoscopies earlier and more frequently, according to the update. Patients diagnosed with primary sclerosing cholangitis, for instance, should undergo immediate colonoscopy, while patients at high risk of dysplasia (such as those with prior CRC) should undergo annual pouch surveillance.
General principles and surveillance colonoscopy
“Conditions and practices for dysplasia detection should be optimized,” the authors wrote, “including control of inflammation, use of high-definition endoscopes, bowel preparation, careful washing and inspection of all colorectal mucosa, and targeted sampling of any suspicious mucosal irregularities.”
Endoscopists should consider use of dye spray chromoendoscopy, “particularly if a standard definition endoscope is used or if there is a history of dysplasia,” the authors wrote. Alternatively, virtual chromoendoscopy may be used in conjunction with high-definition endoscopy.
Biopsy, resection, and colectomy
According to the update, if chromoendoscopy is used, then biopsies should be targeted “where mucosal findings are suspicious for dysplasia or are inexplicably different from the surrounding mucosa.”
If chromoendoscopy isn’t used, then the authors advised clinicians to also perform nontargeted biopsies, ideally four per 10 cm of colon, in addition to targeted biopsies of suspicious areas.
When lesions are clearly demarcated and lack submucosal fibrosis or stigmata of invasive cancer, then endoscopic resection is preferred over biopsy. Following resection, mucosal biopsies are usually unnecessary, “unless there are concerns about resection completeness.”
“If the resectability of a lesion is in question, referral to a specialized endoscopist or inflammatory bowel disease center is suggested,” wrote the authors.
They noted that, if visible dysplasia is truly unresectable or if invisible multifocal/high-grade dysplasia is encountered, then colectomy should be performed.
IBD control
Finally, the authors emphasized the importance of adequately managing IBD activity to reduce dysplasia risk.
“Because CRC risk in IBD is primarily driven by inflammation, and available data do not demonstrate a clear independent chemopreventive effect of available agents, the focus of chemoprevention in IBD should be control of inflammation,” they wrote.
The expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed no conflicts of interest.
The American Gastroenterological Association recently published an expert review and clinical practice update addressing endoscopic surveillance and management of colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Because of practice-altering advances in therapy and surveillance over the past 2 decades, an updated approach is needed, according to authors led by Sanjay K. Murthy, MD, of Ottawa Hospital Research Institute and Fernando Velayos, MD, from Kaiser Permanente San Francisco Medical Center.
“Not long ago, notions of imperceptible CRC [colorectal cancer] development and urgent need for colectomy in the face of dysplasia dominated IBD practice,” the authors wrote in Gastroenterology. “However, improvements in disease management, as well as endoscopic technology and quality, have dramatically changed the way in which we conceptualize and manage IBD-related dysplasia over the past 20 years.”
Most notably, the authors called for a more conservative approach to sample collection and intervention.
“The practices of taking nontargeted biopsies and of referring patients for colectomy in the setting of low-grade or invisible dysplasia are being increasingly challenged in favor of ‘smart’ approaches that emphasize careful inspection and targeted sampling of visible and subtle lesions using newer technologies ... as well as endoscopic management of most lesions that appear endoscopically resectable,” the authors wrote. “Indeed, surgery is being increasingly reserved for lesions harboring strong risk factors for invasive cancer or when endoscopic clearance is not possible.”
The 14 best practice advice statements cover a variety of topics, including appropriate lesion terminology and characterization, endoscopy timing, and indications for biopsies, resection, and colectomy.
“The proposed conceptual model and best practice advice statements in this review are best used in conjunction with evolving literature and existing societal guidelines as part of a shared decision-making process,” the authors noted.
Lesion descriptions
First, the authors provided best practice advice for retirement of three older terms: “dysplasia-associated lesion or mass, adenoma-like mass, and flat dysplasia.” Instead, they advised sorting precancerous colorectal lesions into one of three categories: nonpolypoid (less than 2.5 mm tall), polypoid (at least 2.5 mm tall), or invisible (if detected by nontargeted biopsy).
According to the update, lesion descriptions should also include location, morphology, size, presence of ulceration, clarity of borders, presence within an area of past or current colitis, use of special visualization techniques, and perceived completeness of resection.
Surveillance timing
All patients with chronic IBD should undergo colonoscopy screening for dysplasia 8-10 years after diagnosis, the authors wrote. Subsequent colonoscopies should be performed every 1-5 years, depending on risk factors, such as family history of colorectal cancer and quality of prior surveillance exams.
Higher-risk patients may require colonoscopies earlier and more frequently, according to the update. Patients diagnosed with primary sclerosing cholangitis, for instance, should undergo immediate colonoscopy, while patients at high risk of dysplasia (such as those with prior CRC) should undergo annual pouch surveillance.
General principles and surveillance colonoscopy
“Conditions and practices for dysplasia detection should be optimized,” the authors wrote, “including control of inflammation, use of high-definition endoscopes, bowel preparation, careful washing and inspection of all colorectal mucosa, and targeted sampling of any suspicious mucosal irregularities.”
Endoscopists should consider use of dye spray chromoendoscopy, “particularly if a standard definition endoscope is used or if there is a history of dysplasia,” the authors wrote. Alternatively, virtual chromoendoscopy may be used in conjunction with high-definition endoscopy.
Biopsy, resection, and colectomy
According to the update, if chromoendoscopy is used, then biopsies should be targeted “where mucosal findings are suspicious for dysplasia or are inexplicably different from the surrounding mucosa.”
If chromoendoscopy isn’t used, then the authors advised clinicians to also perform nontargeted biopsies, ideally four per 10 cm of colon, in addition to targeted biopsies of suspicious areas.
When lesions are clearly demarcated and lack submucosal fibrosis or stigmata of invasive cancer, then endoscopic resection is preferred over biopsy. Following resection, mucosal biopsies are usually unnecessary, “unless there are concerns about resection completeness.”
“If the resectability of a lesion is in question, referral to a specialized endoscopist or inflammatory bowel disease center is suggested,” wrote the authors.
They noted that, if visible dysplasia is truly unresectable or if invisible multifocal/high-grade dysplasia is encountered, then colectomy should be performed.
IBD control
Finally, the authors emphasized the importance of adequately managing IBD activity to reduce dysplasia risk.
“Because CRC risk in IBD is primarily driven by inflammation, and available data do not demonstrate a clear independent chemopreventive effect of available agents, the focus of chemoprevention in IBD should be control of inflammation,” they wrote.
The expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed no conflicts of interest.
The American Gastroenterological Association recently published an expert review and clinical practice update addressing endoscopic surveillance and management of colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Because of practice-altering advances in therapy and surveillance over the past 2 decades, an updated approach is needed, according to authors led by Sanjay K. Murthy, MD, of Ottawa Hospital Research Institute and Fernando Velayos, MD, from Kaiser Permanente San Francisco Medical Center.
“Not long ago, notions of imperceptible CRC [colorectal cancer] development and urgent need for colectomy in the face of dysplasia dominated IBD practice,” the authors wrote in Gastroenterology. “However, improvements in disease management, as well as endoscopic technology and quality, have dramatically changed the way in which we conceptualize and manage IBD-related dysplasia over the past 20 years.”
Most notably, the authors called for a more conservative approach to sample collection and intervention.
“The practices of taking nontargeted biopsies and of referring patients for colectomy in the setting of low-grade or invisible dysplasia are being increasingly challenged in favor of ‘smart’ approaches that emphasize careful inspection and targeted sampling of visible and subtle lesions using newer technologies ... as well as endoscopic management of most lesions that appear endoscopically resectable,” the authors wrote. “Indeed, surgery is being increasingly reserved for lesions harboring strong risk factors for invasive cancer or when endoscopic clearance is not possible.”
The 14 best practice advice statements cover a variety of topics, including appropriate lesion terminology and characterization, endoscopy timing, and indications for biopsies, resection, and colectomy.
“The proposed conceptual model and best practice advice statements in this review are best used in conjunction with evolving literature and existing societal guidelines as part of a shared decision-making process,” the authors noted.
Lesion descriptions
First, the authors provided best practice advice for retirement of three older terms: “dysplasia-associated lesion or mass, adenoma-like mass, and flat dysplasia.” Instead, they advised sorting precancerous colorectal lesions into one of three categories: nonpolypoid (less than 2.5 mm tall), polypoid (at least 2.5 mm tall), or invisible (if detected by nontargeted biopsy).
According to the update, lesion descriptions should also include location, morphology, size, presence of ulceration, clarity of borders, presence within an area of past or current colitis, use of special visualization techniques, and perceived completeness of resection.
Surveillance timing
All patients with chronic IBD should undergo colonoscopy screening for dysplasia 8-10 years after diagnosis, the authors wrote. Subsequent colonoscopies should be performed every 1-5 years, depending on risk factors, such as family history of colorectal cancer and quality of prior surveillance exams.
Higher-risk patients may require colonoscopies earlier and more frequently, according to the update. Patients diagnosed with primary sclerosing cholangitis, for instance, should undergo immediate colonoscopy, while patients at high risk of dysplasia (such as those with prior CRC) should undergo annual pouch surveillance.
General principles and surveillance colonoscopy
“Conditions and practices for dysplasia detection should be optimized,” the authors wrote, “including control of inflammation, use of high-definition endoscopes, bowel preparation, careful washing and inspection of all colorectal mucosa, and targeted sampling of any suspicious mucosal irregularities.”
Endoscopists should consider use of dye spray chromoendoscopy, “particularly if a standard definition endoscope is used or if there is a history of dysplasia,” the authors wrote. Alternatively, virtual chromoendoscopy may be used in conjunction with high-definition endoscopy.
Biopsy, resection, and colectomy
According to the update, if chromoendoscopy is used, then biopsies should be targeted “where mucosal findings are suspicious for dysplasia or are inexplicably different from the surrounding mucosa.”
If chromoendoscopy isn’t used, then the authors advised clinicians to also perform nontargeted biopsies, ideally four per 10 cm of colon, in addition to targeted biopsies of suspicious areas.
When lesions are clearly demarcated and lack submucosal fibrosis or stigmata of invasive cancer, then endoscopic resection is preferred over biopsy. Following resection, mucosal biopsies are usually unnecessary, “unless there are concerns about resection completeness.”
“If the resectability of a lesion is in question, referral to a specialized endoscopist or inflammatory bowel disease center is suggested,” wrote the authors.
They noted that, if visible dysplasia is truly unresectable or if invisible multifocal/high-grade dysplasia is encountered, then colectomy should be performed.
IBD control
Finally, the authors emphasized the importance of adequately managing IBD activity to reduce dysplasia risk.
“Because CRC risk in IBD is primarily driven by inflammation, and available data do not demonstrate a clear independent chemopreventive effect of available agents, the focus of chemoprevention in IBD should be control of inflammation,” they wrote.
The expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”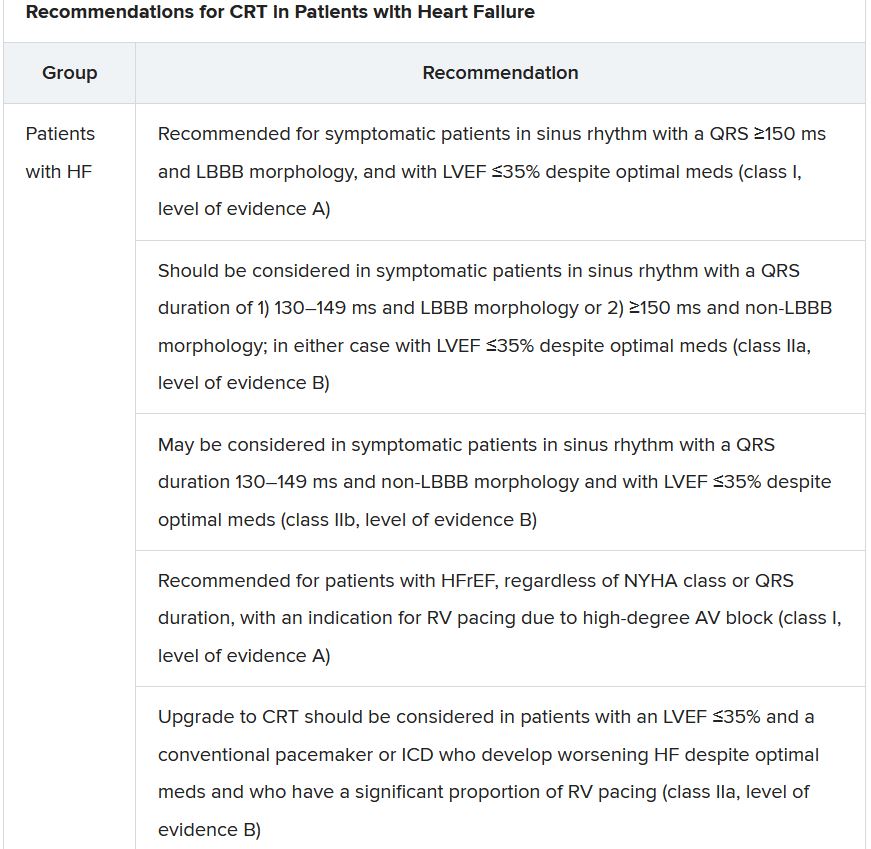
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.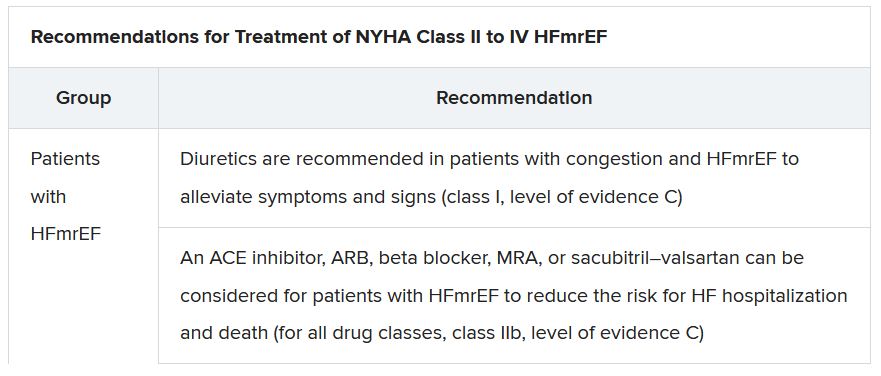
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
New European guidelines on CVD prevention
The new guidelines were published online Aug. 30 in the European Heart Journal to coincide with presentation at the European Stroke Congress (ESOC) 2021.
They were developed by an ESOC task force in collaboration with 12 medical societies and with special contribution of the European Association of Preventive Cardiology.
“A chief goal of the task force was to create a single CVD prevention guideline for everyone – for primary care, for hospital care, for guiding clinical practice – so one guideline for all,” said cochair of the guideline committee Frank Visseren, MD, PhD, University Medical Center Utrecht, Netherlands. “We also wanted to make a more personalized CVD prevention guideline, instead of a one-size-fits-all. In clinical practice, people are very, very different, and we really want to have a more individualized prevention guideline,” said Dr. Visseren, as well as provide “more room for shared decision-making.”
Prevention at the individual and population levels
The new guidelines also give more attention to CVD prevention in older persons. “Many of our patients are over 70 years old and we really want to have more detail, more guidance on older persons,” said Dr. Visseren.
The guideline is divided into two sections. One section covers CVD prevention at the individual level in apparently healthy people, in patients with established CVD, and in those with diabetes, familial hypercholesterolemia, or chronic kidney disease.
The other section covers CVD prevention at the population level, including public health policy, interventions, and the environment, including putting in place measures to reduce air pollution, use of fossil fuels, and limiting carbon dioxide emissions.
Targets for blood lipids, blood pressure, and glycemic control in diabetes remain in line with recent ESC guidelines on dyslipidemias, hypertension, or diabetes.
However, the guidelines introduce a new stepwise treatment-intensification approach to achieve these targets, with consideration of CVD risk, treatment benefit of risk factors, risk modifiers, comorbidities, and patient preferences.
The 2021 CVD prevention guidelines also embrace the recently published Systemic Coronary Risk Estimation 2 (SCORE2) and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) algorithms. “The algorithms we are using are a bit old and we want to have more updated risk prediction, because that’s the starting point of CVD prevention,” Dr. Visseren said.
The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people and provide estimation of lifetime CVD risk and treatment benefit. This will allow clinicians to have “an informed discussion with patients on lifetime risk and potential treatment benefits,” Dr. Visseren said.
For the first time, the guidelines recommend smoking cessation regardless of whether it leads to weight gain, as weight gain does not lessen the benefits of cessation.
Regarding exercise, adults of all ages should aim for at least 150-300 minutes a week of moderate, or 75-150 minutes a week of vigorous, aerobic physical activity. The guidelines recommend reducing sedentary time and engaging in at least light activity throughout the day.
Regarding nutrition, the guidelines advise adopting a Mediterranean or similar diet; restricting alcohol intake to a maximum of 100 g per week (a standard drink is 8-14 g); eating fish, preferably fatty fish, at least once a week; and restricting consumption of meat, particularly processed meat.
Also for the first time, the guidelines state that bariatric surgery should be considered for obese individuals at elevated risk of CVD when a healthy diet and exercise fail to lead to weight loss that is maintained.
They note that individuals with mental disorders need additional attention and support to improve adherence to lifestyle changes and drug treatment.
They advise consideration of referring patients with heart disease and significant stress and anxiety to psychotherapeutic stress management to reduce stress symptoms and improve CV outcomes.
Potential cost issues that could be considered when implementing the guidelines are also reviewed.
Dr. Visseren acknowledged and thanked the task force members for continuing their work on the guidelines over the 2 “challenging” years.
Setting the bar lower?
Discussant for the guideline presentation, Diederick Grobbee, MD, University Medical Center Utrecht, who was not involved in drafting the guidelines, said he does have one conflict of interest, which is a “passion for prevention.” From that perspective, he said the guideline panel “should be applauded; the once-every-5-year issuing of the prevention guidelines is a major event.”
Dr. Grobbee noted that the working group “really tried to follow their ambitions and goals, in a way making the guidelines simpler, or perhaps setting the bar not initially as high as we used to do, which may, in fact, sometimes scare off physicians and patients alike.”
“We’ve had prevention guidelines for quite some time now, yet looking at what is accomplished in practice is sobering,” said Dr. Grobbee. Introducing a stepwise approach is “really appealing,” he added.
A version of this article first appeared on Medscape.com.
The new guidelines were published online Aug. 30 in the European Heart Journal to coincide with presentation at the European Stroke Congress (ESOC) 2021.
They were developed by an ESOC task force in collaboration with 12 medical societies and with special contribution of the European Association of Preventive Cardiology.
“A chief goal of the task force was to create a single CVD prevention guideline for everyone – for primary care, for hospital care, for guiding clinical practice – so one guideline for all,” said cochair of the guideline committee Frank Visseren, MD, PhD, University Medical Center Utrecht, Netherlands. “We also wanted to make a more personalized CVD prevention guideline, instead of a one-size-fits-all. In clinical practice, people are very, very different, and we really want to have a more individualized prevention guideline,” said Dr. Visseren, as well as provide “more room for shared decision-making.”
Prevention at the individual and population levels
The new guidelines also give more attention to CVD prevention in older persons. “Many of our patients are over 70 years old and we really want to have more detail, more guidance on older persons,” said Dr. Visseren.
The guideline is divided into two sections. One section covers CVD prevention at the individual level in apparently healthy people, in patients with established CVD, and in those with diabetes, familial hypercholesterolemia, or chronic kidney disease.
The other section covers CVD prevention at the population level, including public health policy, interventions, and the environment, including putting in place measures to reduce air pollution, use of fossil fuels, and limiting carbon dioxide emissions.
Targets for blood lipids, blood pressure, and glycemic control in diabetes remain in line with recent ESC guidelines on dyslipidemias, hypertension, or diabetes.
However, the guidelines introduce a new stepwise treatment-intensification approach to achieve these targets, with consideration of CVD risk, treatment benefit of risk factors, risk modifiers, comorbidities, and patient preferences.
The 2021 CVD prevention guidelines also embrace the recently published Systemic Coronary Risk Estimation 2 (SCORE2) and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) algorithms. “The algorithms we are using are a bit old and we want to have more updated risk prediction, because that’s the starting point of CVD prevention,” Dr. Visseren said.
The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people and provide estimation of lifetime CVD risk and treatment benefit. This will allow clinicians to have “an informed discussion with patients on lifetime risk and potential treatment benefits,” Dr. Visseren said.
For the first time, the guidelines recommend smoking cessation regardless of whether it leads to weight gain, as weight gain does not lessen the benefits of cessation.
Regarding exercise, adults of all ages should aim for at least 150-300 minutes a week of moderate, or 75-150 minutes a week of vigorous, aerobic physical activity. The guidelines recommend reducing sedentary time and engaging in at least light activity throughout the day.
Regarding nutrition, the guidelines advise adopting a Mediterranean or similar diet; restricting alcohol intake to a maximum of 100 g per week (a standard drink is 8-14 g); eating fish, preferably fatty fish, at least once a week; and restricting consumption of meat, particularly processed meat.
Also for the first time, the guidelines state that bariatric surgery should be considered for obese individuals at elevated risk of CVD when a healthy diet and exercise fail to lead to weight loss that is maintained.
They note that individuals with mental disorders need additional attention and support to improve adherence to lifestyle changes and drug treatment.
They advise consideration of referring patients with heart disease and significant stress and anxiety to psychotherapeutic stress management to reduce stress symptoms and improve CV outcomes.
Potential cost issues that could be considered when implementing the guidelines are also reviewed.
Dr. Visseren acknowledged and thanked the task force members for continuing their work on the guidelines over the 2 “challenging” years.
Setting the bar lower?
Discussant for the guideline presentation, Diederick Grobbee, MD, University Medical Center Utrecht, who was not involved in drafting the guidelines, said he does have one conflict of interest, which is a “passion for prevention.” From that perspective, he said the guideline panel “should be applauded; the once-every-5-year issuing of the prevention guidelines is a major event.”
Dr. Grobbee noted that the working group “really tried to follow their ambitions and goals, in a way making the guidelines simpler, or perhaps setting the bar not initially as high as we used to do, which may, in fact, sometimes scare off physicians and patients alike.”
“We’ve had prevention guidelines for quite some time now, yet looking at what is accomplished in practice is sobering,” said Dr. Grobbee. Introducing a stepwise approach is “really appealing,” he added.
A version of this article first appeared on Medscape.com.
The new guidelines were published online Aug. 30 in the European Heart Journal to coincide with presentation at the European Stroke Congress (ESOC) 2021.
They were developed by an ESOC task force in collaboration with 12 medical societies and with special contribution of the European Association of Preventive Cardiology.
“A chief goal of the task force was to create a single CVD prevention guideline for everyone – for primary care, for hospital care, for guiding clinical practice – so one guideline for all,” said cochair of the guideline committee Frank Visseren, MD, PhD, University Medical Center Utrecht, Netherlands. “We also wanted to make a more personalized CVD prevention guideline, instead of a one-size-fits-all. In clinical practice, people are very, very different, and we really want to have a more individualized prevention guideline,” said Dr. Visseren, as well as provide “more room for shared decision-making.”
Prevention at the individual and population levels
The new guidelines also give more attention to CVD prevention in older persons. “Many of our patients are over 70 years old and we really want to have more detail, more guidance on older persons,” said Dr. Visseren.
The guideline is divided into two sections. One section covers CVD prevention at the individual level in apparently healthy people, in patients with established CVD, and in those with diabetes, familial hypercholesterolemia, or chronic kidney disease.
The other section covers CVD prevention at the population level, including public health policy, interventions, and the environment, including putting in place measures to reduce air pollution, use of fossil fuels, and limiting carbon dioxide emissions.
Targets for blood lipids, blood pressure, and glycemic control in diabetes remain in line with recent ESC guidelines on dyslipidemias, hypertension, or diabetes.
However, the guidelines introduce a new stepwise treatment-intensification approach to achieve these targets, with consideration of CVD risk, treatment benefit of risk factors, risk modifiers, comorbidities, and patient preferences.
The 2021 CVD prevention guidelines also embrace the recently published Systemic Coronary Risk Estimation 2 (SCORE2) and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) algorithms. “The algorithms we are using are a bit old and we want to have more updated risk prediction, because that’s the starting point of CVD prevention,” Dr. Visseren said.
The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people and provide estimation of lifetime CVD risk and treatment benefit. This will allow clinicians to have “an informed discussion with patients on lifetime risk and potential treatment benefits,” Dr. Visseren said.
For the first time, the guidelines recommend smoking cessation regardless of whether it leads to weight gain, as weight gain does not lessen the benefits of cessation.
Regarding exercise, adults of all ages should aim for at least 150-300 minutes a week of moderate, or 75-150 minutes a week of vigorous, aerobic physical activity. The guidelines recommend reducing sedentary time and engaging in at least light activity throughout the day.
Regarding nutrition, the guidelines advise adopting a Mediterranean or similar diet; restricting alcohol intake to a maximum of 100 g per week (a standard drink is 8-14 g); eating fish, preferably fatty fish, at least once a week; and restricting consumption of meat, particularly processed meat.
Also for the first time, the guidelines state that bariatric surgery should be considered for obese individuals at elevated risk of CVD when a healthy diet and exercise fail to lead to weight loss that is maintained.
They note that individuals with mental disorders need additional attention and support to improve adherence to lifestyle changes and drug treatment.
They advise consideration of referring patients with heart disease and significant stress and anxiety to psychotherapeutic stress management to reduce stress symptoms and improve CV outcomes.
Potential cost issues that could be considered when implementing the guidelines are also reviewed.
Dr. Visseren acknowledged and thanked the task force members for continuing their work on the guidelines over the 2 “challenging” years.
Setting the bar lower?
Discussant for the guideline presentation, Diederick Grobbee, MD, University Medical Center Utrecht, who was not involved in drafting the guidelines, said he does have one conflict of interest, which is a “passion for prevention.” From that perspective, he said the guideline panel “should be applauded; the once-every-5-year issuing of the prevention guidelines is a major event.”
Dr. Grobbee noted that the working group “really tried to follow their ambitions and goals, in a way making the guidelines simpler, or perhaps setting the bar not initially as high as we used to do, which may, in fact, sometimes scare off physicians and patients alike.”
“We’ve had prevention guidelines for quite some time now, yet looking at what is accomplished in practice is sobering,” said Dr. Grobbee. Introducing a stepwise approach is “really appealing,” he added.
A version of this article first appeared on Medscape.com.
FROM ESC 2021
2021 AGA Rapid Review and Guideline Update: Pre-endoscopy SARS-CoV-2 testing post vaccination
The American Gastroenterological Association recently updated their guideline for preendoscopy SARS-CoV-2 testing in light of population-wide vaccination programs, now recommending against routine viral screening regardless of patient vaccination status and local disease prevalence.
Centers electing to maintain a preprocedure testing strategy should use standard nucleic acid testing, preferably rapid reverse transcription polymerase chain reaction (RT-PCR) because this can be performed on the day of the procedure, thereby limiting patient testing burden, reported authors led by co–first authors Shahnaz Sultan, MD, of the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Healthcare System, and Shazia M. Siddique, MD, of the University of Pennsylvania, Philadelphia.
These new recommendations, both of which are conditional and based on very-low-certainty evidence, were drawn from a rapid evidence review of benefits and risks in the postvaccination period.
“Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety,” the guideline authors wrote in Gastroenterology. “Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a reevaluation of AGA’s prior recommendations was warranted.”
The 2020 AGA guideline, also led by Dr. Sultan, issued viral screening recommendations based on local prevalence rates of asymptomatic COVID-19, with pretesting reserved for moderately affected locations. Mildly affected areas were advised against pretesting, whereas centers in pandemic hot spots were cautioned against performing all but “emergency or time-sensitive procedures.”
Those recommendations have now been replaced by the present guideline, which no longer distinguishes between local prevalence rates. This decision was based on a variety of factors, the panelists noted, including endoscopy volumes, vaccine efficacy, HCW and patient anxiety, endoscopy-related risk of infection to both patients and HCWs, prevalence of asymptomatic COVID-19 among patients undergoing endoscopy, and the impact of delaying care on cancer burden.
“The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients,” Dr. Sultan and colleagues wrote.
The guideline includes a summary of evidence related to the two new recommendations, including several studies reporting prevalence of asymptomatic SARS-CoV-2 infection among patients tested prior to endoscopy procedures.
“Across 13 studies, asymptomatic prevalence ranged from 0% to 1.5%, but most studies reported a range from 0% to 0.5%,” the panelists wrote, “regardless of local surges of COVID-19 cases.”
Although Dr. Sultan and colleagues acknowledged that pretesting may be reassuring, they noted that, based on available evidence, “there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections.”
They went on to quantify the relationship between delays in care and cancer burden, reviewing data from 14 studies that demonstrated an overall reduction in endoscopic-detected colorectal cancers by 31%-71%, esophageal cancers by 27%-37%, and gastric cancer by 27%-52% since the start of the pandemic. A recent study by Ahmad Khan, MD, and colleagues, which focused on the United States from July to November 2020, demonstrated an 11.74% decrease in diagnoses of malignant colorectal cancer, and a 19.78% decline in diagnoses of esophageal and gastric cancer.
The second recommendation – calling for standard nucleic acid testing among centers electing to maintain a pretesting strategy – was also presented with a summary of supporting evidence, largely pertaining to test accuracy.
“Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour) are preferable as they pose less burden to patients,” the panelists wrote. “In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.”
For both new recommendations, it is assumed that “all centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators)” and that “all centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist, and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit.”
As COVID-19 cases rise in the United States because of the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. Stay tuned for updates and visit https://gastro.org/practice-guidance/practice-updates/covid-19/.
Guideline development was funded by the AGA. No panel members received any payments.
The American Gastroenterological Association recently updated their guideline for preendoscopy SARS-CoV-2 testing in light of population-wide vaccination programs, now recommending against routine viral screening regardless of patient vaccination status and local disease prevalence.
Centers electing to maintain a preprocedure testing strategy should use standard nucleic acid testing, preferably rapid reverse transcription polymerase chain reaction (RT-PCR) because this can be performed on the day of the procedure, thereby limiting patient testing burden, reported authors led by co–first authors Shahnaz Sultan, MD, of the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Healthcare System, and Shazia M. Siddique, MD, of the University of Pennsylvania, Philadelphia.
These new recommendations, both of which are conditional and based on very-low-certainty evidence, were drawn from a rapid evidence review of benefits and risks in the postvaccination period.
“Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety,” the guideline authors wrote in Gastroenterology. “Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a reevaluation of AGA’s prior recommendations was warranted.”
The 2020 AGA guideline, also led by Dr. Sultan, issued viral screening recommendations based on local prevalence rates of asymptomatic COVID-19, with pretesting reserved for moderately affected locations. Mildly affected areas were advised against pretesting, whereas centers in pandemic hot spots were cautioned against performing all but “emergency or time-sensitive procedures.”
Those recommendations have now been replaced by the present guideline, which no longer distinguishes between local prevalence rates. This decision was based on a variety of factors, the panelists noted, including endoscopy volumes, vaccine efficacy, HCW and patient anxiety, endoscopy-related risk of infection to both patients and HCWs, prevalence of asymptomatic COVID-19 among patients undergoing endoscopy, and the impact of delaying care on cancer burden.
“The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients,” Dr. Sultan and colleagues wrote.
The guideline includes a summary of evidence related to the two new recommendations, including several studies reporting prevalence of asymptomatic SARS-CoV-2 infection among patients tested prior to endoscopy procedures.
“Across 13 studies, asymptomatic prevalence ranged from 0% to 1.5%, but most studies reported a range from 0% to 0.5%,” the panelists wrote, “regardless of local surges of COVID-19 cases.”
Although Dr. Sultan and colleagues acknowledged that pretesting may be reassuring, they noted that, based on available evidence, “there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections.”
They went on to quantify the relationship between delays in care and cancer burden, reviewing data from 14 studies that demonstrated an overall reduction in endoscopic-detected colorectal cancers by 31%-71%, esophageal cancers by 27%-37%, and gastric cancer by 27%-52% since the start of the pandemic. A recent study by Ahmad Khan, MD, and colleagues, which focused on the United States from July to November 2020, demonstrated an 11.74% decrease in diagnoses of malignant colorectal cancer, and a 19.78% decline in diagnoses of esophageal and gastric cancer.
The second recommendation – calling for standard nucleic acid testing among centers electing to maintain a pretesting strategy – was also presented with a summary of supporting evidence, largely pertaining to test accuracy.
“Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour) are preferable as they pose less burden to patients,” the panelists wrote. “In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.”
For both new recommendations, it is assumed that “all centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators)” and that “all centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist, and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit.”
As COVID-19 cases rise in the United States because of the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. Stay tuned for updates and visit https://gastro.org/practice-guidance/practice-updates/covid-19/.
Guideline development was funded by the AGA. No panel members received any payments.
The American Gastroenterological Association recently updated their guideline for preendoscopy SARS-CoV-2 testing in light of population-wide vaccination programs, now recommending against routine viral screening regardless of patient vaccination status and local disease prevalence.
Centers electing to maintain a preprocedure testing strategy should use standard nucleic acid testing, preferably rapid reverse transcription polymerase chain reaction (RT-PCR) because this can be performed on the day of the procedure, thereby limiting patient testing burden, reported authors led by co–first authors Shahnaz Sultan, MD, of the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Healthcare System, and Shazia M. Siddique, MD, of the University of Pennsylvania, Philadelphia.
These new recommendations, both of which are conditional and based on very-low-certainty evidence, were drawn from a rapid evidence review of benefits and risks in the postvaccination period.
“Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety,” the guideline authors wrote in Gastroenterology. “Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a reevaluation of AGA’s prior recommendations was warranted.”
The 2020 AGA guideline, also led by Dr. Sultan, issued viral screening recommendations based on local prevalence rates of asymptomatic COVID-19, with pretesting reserved for moderately affected locations. Mildly affected areas were advised against pretesting, whereas centers in pandemic hot spots were cautioned against performing all but “emergency or time-sensitive procedures.”
Those recommendations have now been replaced by the present guideline, which no longer distinguishes between local prevalence rates. This decision was based on a variety of factors, the panelists noted, including endoscopy volumes, vaccine efficacy, HCW and patient anxiety, endoscopy-related risk of infection to both patients and HCWs, prevalence of asymptomatic COVID-19 among patients undergoing endoscopy, and the impact of delaying care on cancer burden.
“The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients,” Dr. Sultan and colleagues wrote.
The guideline includes a summary of evidence related to the two new recommendations, including several studies reporting prevalence of asymptomatic SARS-CoV-2 infection among patients tested prior to endoscopy procedures.
“Across 13 studies, asymptomatic prevalence ranged from 0% to 1.5%, but most studies reported a range from 0% to 0.5%,” the panelists wrote, “regardless of local surges of COVID-19 cases.”
Although Dr. Sultan and colleagues acknowledged that pretesting may be reassuring, they noted that, based on available evidence, “there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections.”
They went on to quantify the relationship between delays in care and cancer burden, reviewing data from 14 studies that demonstrated an overall reduction in endoscopic-detected colorectal cancers by 31%-71%, esophageal cancers by 27%-37%, and gastric cancer by 27%-52% since the start of the pandemic. A recent study by Ahmad Khan, MD, and colleagues, which focused on the United States from July to November 2020, demonstrated an 11.74% decrease in diagnoses of malignant colorectal cancer, and a 19.78% decline in diagnoses of esophageal and gastric cancer.
The second recommendation – calling for standard nucleic acid testing among centers electing to maintain a pretesting strategy – was also presented with a summary of supporting evidence, largely pertaining to test accuracy.
“Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour) are preferable as they pose less burden to patients,” the panelists wrote. “In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.”
For both new recommendations, it is assumed that “all centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators)” and that “all centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist, and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit.”
As COVID-19 cases rise in the United States because of the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. Stay tuned for updates and visit https://gastro.org/practice-guidance/practice-updates/covid-19/.
Guideline development was funded by the AGA. No panel members received any payments.
FROM GASTROENTEROLOGY
Type 2 diabetes ‘remission’ is a reality, say major organizations
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
ACR updates COVID vaccine guidance with booster schedule
Patients on immunosuppressive or immunomodulatory therapy should receive a third dose of either the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine at least 28 days after the second dose of either of these two mRNA vaccines, according to updated recommendations from the American College of Rheumatology.
The update follows the Centers for Disease Control and Prevention’s recommendation that certain immunocompromised patients receive a third dose of an mRNA vaccine to reduce their risk of contracting COVID-19.
Individuals receiving the Pfizer vaccine must be aged 12 years and older, while those receiving the Moderna vaccine must be 18 years and older, the ACR emphasized.
“These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment,” the authors wrote. “Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography, and time specific and, therefore, must be individualized as part of a shared decision-making process.”
The task force recommended using the same mRNA vaccine booster as the patient received for their initial two-dose series when possible, but notes that either mRNA vaccine is acceptable, and recommends the mRNA vaccine for patients who have yet to receive any vaccine because of the availability of the booster. The task force emphasized that they achieved no consensus on recommending a booster mRNA vaccine to patients who received a single dose of Johnson & Johnson vaccine because the safety data are uncertain.
The updated guidance also identifies the Food and Drug Administration’s emergency use authorization in August for the use of REGEN-COV monoclonal antibody treatment for emergency postexposure prophylaxis for COVID-19 in adults and adolescents aged 12 years and older who weigh at least 40 kg and are at increased risk for severe COVID-19, which includes patients receiving immunosuppressive or immunomodulatory therapies other than hydroxychloroquine. Patients who have been exposed to an individual with COVID-19 should discuss this treatment with their health care provider as an added precaution; however, the guidance emphasized that the prophylactic treatment is not a substitute for COVID-19 vaccination.
The recommendations advise clinicians to counsel their patients to refrain from taking certain immunomodulatory or immunosuppressive medications for 1-2 weeks after booster vaccination if disease activity allows, with the exception of glucocorticoids and anticytokines such as tumor necrosis factor inhibitors and others including interleukin-17, IL-12/23, IL-23, IL-1R, IL-6R antagonists, for which the task force did not achieve a consensus recommendation.
The guidance notes that patients on rituximab or other anti-CD20 medications “should discuss the optimal timing [of the booster] with their rheumatology provider” and that some practitioners measure CD19 B cells as a tool with which to time the booster and subsequent rituximab dosing. For those who elect to dose without such information, or for whom such measurement is not available or feasible, provide the booster 2-4 weeks before next anticipated rituximab dose (e.g., at month 5.0 or 5.5 for patients on an every-6-month rituximab dosing schedule).”
There was strong consensus from the task force that health care providers “should not routinely order any lab testing (e.g., antibody tests for IgM and/or IgG to spike or nucleocapsid proteins) to assess immunity to COVID-19 post vaccination, nor to assess the need for vaccination in a yet-unvaccinated person.”
“The updated information from the ACR addresses not only booster vaccination but also other important and practical issues facing rheumatology providers and their patients related to the pandemic,” said task force chair Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, in an ACR statement announcing the updates.
“Although the guidance is issued in light of the best evidence available, the science regarding COVID-19 vaccination as it affects the practice of rheumatology is undergoing rapid evolution,” he noted. “We need direct evidence such as that from randomized trials to inform the best practices of what we can do to protect our patients from SARS-CoV-2.”
The update retains the current recommendations that rheumatology patients follow all public health guidelines regarding physical distancing and other preventive measures following vaccination, but the task force did not recommend exceeding current public health guidance. “The appropriateness for continued preventive measures (e.g., masking, physical distancing) should be discussed with patients as their rheumatology providers deem appropriate,” they wrote.
The full updated version of the ACR’s COVID-19 Vaccine Clinical Guidance for Patients with Rheumatic and Musculoskeletal Diseases will be published in Arthritis & Rheumatology. The summary was developed by the ACR COVID-19 Vaccine Clinical Guidance Task Force, which included 9 rheumatologists, 2 infectious disease specialists, and 2 public health experts with current or past employment history with the CDC.
The ACR encourages clinicians with questions or concerns to email [email protected] for support.
Patients on immunosuppressive or immunomodulatory therapy should receive a third dose of either the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine at least 28 days after the second dose of either of these two mRNA vaccines, according to updated recommendations from the American College of Rheumatology.
The update follows the Centers for Disease Control and Prevention’s recommendation that certain immunocompromised patients receive a third dose of an mRNA vaccine to reduce their risk of contracting COVID-19.
Individuals receiving the Pfizer vaccine must be aged 12 years and older, while those receiving the Moderna vaccine must be 18 years and older, the ACR emphasized.
“These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment,” the authors wrote. “Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography, and time specific and, therefore, must be individualized as part of a shared decision-making process.”
The task force recommended using the same mRNA vaccine booster as the patient received for their initial two-dose series when possible, but notes that either mRNA vaccine is acceptable, and recommends the mRNA vaccine for patients who have yet to receive any vaccine because of the availability of the booster. The task force emphasized that they achieved no consensus on recommending a booster mRNA vaccine to patients who received a single dose of Johnson & Johnson vaccine because the safety data are uncertain.
The updated guidance also identifies the Food and Drug Administration’s emergency use authorization in August for the use of REGEN-COV monoclonal antibody treatment for emergency postexposure prophylaxis for COVID-19 in adults and adolescents aged 12 years and older who weigh at least 40 kg and are at increased risk for severe COVID-19, which includes patients receiving immunosuppressive or immunomodulatory therapies other than hydroxychloroquine. Patients who have been exposed to an individual with COVID-19 should discuss this treatment with their health care provider as an added precaution; however, the guidance emphasized that the prophylactic treatment is not a substitute for COVID-19 vaccination.
The recommendations advise clinicians to counsel their patients to refrain from taking certain immunomodulatory or immunosuppressive medications for 1-2 weeks after booster vaccination if disease activity allows, with the exception of glucocorticoids and anticytokines such as tumor necrosis factor inhibitors and others including interleukin-17, IL-12/23, IL-23, IL-1R, IL-6R antagonists, for which the task force did not achieve a consensus recommendation.
The guidance notes that patients on rituximab or other anti-CD20 medications “should discuss the optimal timing [of the booster] with their rheumatology provider” and that some practitioners measure CD19 B cells as a tool with which to time the booster and subsequent rituximab dosing. For those who elect to dose without such information, or for whom such measurement is not available or feasible, provide the booster 2-4 weeks before next anticipated rituximab dose (e.g., at month 5.0 or 5.5 for patients on an every-6-month rituximab dosing schedule).”
There was strong consensus from the task force that health care providers “should not routinely order any lab testing (e.g., antibody tests for IgM and/or IgG to spike or nucleocapsid proteins) to assess immunity to COVID-19 post vaccination, nor to assess the need for vaccination in a yet-unvaccinated person.”
“The updated information from the ACR addresses not only booster vaccination but also other important and practical issues facing rheumatology providers and their patients related to the pandemic,” said task force chair Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, in an ACR statement announcing the updates.
“Although the guidance is issued in light of the best evidence available, the science regarding COVID-19 vaccination as it affects the practice of rheumatology is undergoing rapid evolution,” he noted. “We need direct evidence such as that from randomized trials to inform the best practices of what we can do to protect our patients from SARS-CoV-2.”
The update retains the current recommendations that rheumatology patients follow all public health guidelines regarding physical distancing and other preventive measures following vaccination, but the task force did not recommend exceeding current public health guidance. “The appropriateness for continued preventive measures (e.g., masking, physical distancing) should be discussed with patients as their rheumatology providers deem appropriate,” they wrote.
The full updated version of the ACR’s COVID-19 Vaccine Clinical Guidance for Patients with Rheumatic and Musculoskeletal Diseases will be published in Arthritis & Rheumatology. The summary was developed by the ACR COVID-19 Vaccine Clinical Guidance Task Force, which included 9 rheumatologists, 2 infectious disease specialists, and 2 public health experts with current or past employment history with the CDC.
The ACR encourages clinicians with questions or concerns to email [email protected] for support.
Patients on immunosuppressive or immunomodulatory therapy should receive a third dose of either the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine at least 28 days after the second dose of either of these two mRNA vaccines, according to updated recommendations from the American College of Rheumatology.
The update follows the Centers for Disease Control and Prevention’s recommendation that certain immunocompromised patients receive a third dose of an mRNA vaccine to reduce their risk of contracting COVID-19.
Individuals receiving the Pfizer vaccine must be aged 12 years and older, while those receiving the Moderna vaccine must be 18 years and older, the ACR emphasized.
“These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment,” the authors wrote. “Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography, and time specific and, therefore, must be individualized as part of a shared decision-making process.”
The task force recommended using the same mRNA vaccine booster as the patient received for their initial two-dose series when possible, but notes that either mRNA vaccine is acceptable, and recommends the mRNA vaccine for patients who have yet to receive any vaccine because of the availability of the booster. The task force emphasized that they achieved no consensus on recommending a booster mRNA vaccine to patients who received a single dose of Johnson & Johnson vaccine because the safety data are uncertain.
The updated guidance also identifies the Food and Drug Administration’s emergency use authorization in August for the use of REGEN-COV monoclonal antibody treatment for emergency postexposure prophylaxis for COVID-19 in adults and adolescents aged 12 years and older who weigh at least 40 kg and are at increased risk for severe COVID-19, which includes patients receiving immunosuppressive or immunomodulatory therapies other than hydroxychloroquine. Patients who have been exposed to an individual with COVID-19 should discuss this treatment with their health care provider as an added precaution; however, the guidance emphasized that the prophylactic treatment is not a substitute for COVID-19 vaccination.
The recommendations advise clinicians to counsel their patients to refrain from taking certain immunomodulatory or immunosuppressive medications for 1-2 weeks after booster vaccination if disease activity allows, with the exception of glucocorticoids and anticytokines such as tumor necrosis factor inhibitors and others including interleukin-17, IL-12/23, IL-23, IL-1R, IL-6R antagonists, for which the task force did not achieve a consensus recommendation.
The guidance notes that patients on rituximab or other anti-CD20 medications “should discuss the optimal timing [of the booster] with their rheumatology provider” and that some practitioners measure CD19 B cells as a tool with which to time the booster and subsequent rituximab dosing. For those who elect to dose without such information, or for whom such measurement is not available or feasible, provide the booster 2-4 weeks before next anticipated rituximab dose (e.g., at month 5.0 or 5.5 for patients on an every-6-month rituximab dosing schedule).”
There was strong consensus from the task force that health care providers “should not routinely order any lab testing (e.g., antibody tests for IgM and/or IgG to spike or nucleocapsid proteins) to assess immunity to COVID-19 post vaccination, nor to assess the need for vaccination in a yet-unvaccinated person.”
“The updated information from the ACR addresses not only booster vaccination but also other important and practical issues facing rheumatology providers and their patients related to the pandemic,” said task force chair Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, in an ACR statement announcing the updates.
“Although the guidance is issued in light of the best evidence available, the science regarding COVID-19 vaccination as it affects the practice of rheumatology is undergoing rapid evolution,” he noted. “We need direct evidence such as that from randomized trials to inform the best practices of what we can do to protect our patients from SARS-CoV-2.”
The update retains the current recommendations that rheumatology patients follow all public health guidelines regarding physical distancing and other preventive measures following vaccination, but the task force did not recommend exceeding current public health guidance. “The appropriateness for continued preventive measures (e.g., masking, physical distancing) should be discussed with patients as their rheumatology providers deem appropriate,” they wrote.
The full updated version of the ACR’s COVID-19 Vaccine Clinical Guidance for Patients with Rheumatic and Musculoskeletal Diseases will be published in Arthritis & Rheumatology. The summary was developed by the ACR COVID-19 Vaccine Clinical Guidance Task Force, which included 9 rheumatologists, 2 infectious disease specialists, and 2 public health experts with current or past employment history with the CDC.
The ACR encourages clinicians with questions or concerns to email [email protected] for support.
New recommendations address ME/CFS diagnosis and management
New consensus recommendations address diagnosis and management of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), with advice that may also be helpful for patients with lingering symptoms following acute COVID-19 infection.
The document was published online Aug. 25, 2021, in the Mayo Clinic Proceedings by the 23-member U.S. ME/CFS Clinician Coalition, headed by Lucinda Bateman, MD, of the Bateman Horne Center of Excellence, Salt Lake City. The document is the culmination of work that began with a summit held at the center in March 2018.
The target audience is both generalist and specialist health care providers. While ME/CFS is estimated to affect up to 2.5 million Americans, more than 90% are either undiagnosed or misdiagnosed with other conditions such as depression. And those who are diagnosed often receive inappropriate, outdated treatments such as psychotherapy and exercise prescriptions.
“Despite myalgic encephalomyelitis/chronic fatigue syndrome affecting millions of people worldwide, many clinicians lack the knowledge to appropriately diagnose or manage ME/CFS. Unfortunately, clinical guidance has been scarce, obsolete, or potentially harmful,” Dr. Bateman and colleagues wrote.
The urgency of appropriate recognition and management of ME/CFS has increased as growing numbers of people are exhibiting signs and symptoms of ME/CFS following acute COVID-19 infection. This isn’t surprising because the illness has long been linked to other infections, including Epstein-Barr virus, the authors noted.
The document covers the epidemiology, impact, and prognosis of ME/CFS, as well as etiology and pathophysiology. “Scientific studies demonstrate multiple dysfunctional organ systems, including neuro, immune, and metabolic, in ME/CFS. These findings are not explained merely by deconditioning,” document coauthor Lily Chu, MD, an independent consultant in Burlingame, Calif., said in an interview.
The document reviews the 2015 U.S. Institute of Medicine (now Academy of Medicine) diagnostic criteria that are now also recommended by the Centers for Disease Control and Prevention. They are based on four main symptoms: substantial reduction or impairment in the ability to engage in preillness levels of occupational, educational, social or personal activities for longer than 6 months; postexertional malaise, a worsening of all current symptoms, that patients often describe as a “crash”; unrefreshing sleep; and cognitive impairment and/or orthostatic intolerance.
“The new diagnostic criteria focusing on the key symptom of postexertional malaise rather than chronic fatigue, which is common in many conditions, may make the diagnostic process quicker and more accurate. Diagnosis now is both an inclusionary and not just exclusionary process, so it’s not necessary to eliminate all causes of fatigue. Diagnose patients who fit the criteria and be alert for it in people with persistent symptoms post COVID,” Dr. Chu said.
The document provides advice for taking a clinical history to obtain the information necessary for making the diagnosis, including use of laboratory testing to rule out other conditions. Physical exams, while they may not reveal specific abnormalities, may help in identifying comorbidities and ruling out alternative diagnoses.
A long list of nonpharmacologic and pharmacologic treatment and management approaches is offered for each of the individual core and common ME/CFS symptoms, including postexertional malaise, orthostatic intolerance, sleep issues, cognitive dysfunction and fatigue, immune dysfunction, pain, and gastrointestinal issues.
The document recommends against using the “outdated standard of care” cognitive-behavioral therapy and graded exercise therapy as primary treatments for the illness. Instead, the authors recommend teaching patients “pacing,” an individualized approach to energy conservation aimed at minimizing the frequency, duration, and severity of postexertional malaise.
Clinicians are also advised to assess patients’ daily living needs and provide support, including acquiring handicap placards, work or school accommodations, and disability benefits.
“There are things clinicians can do now to help patients even without a disease-modifying treatment. These are actions they are already familiar with and carry out for people with other chronic diseases, which often have limited treatment options as well. Don’t underestimate the importance and value of supportive care for patients.” Dr. Chu said.
The recommendations are based primarily on clinical expertise because there are very few randomized trials, and much of the evidence from other types of trials has been flawed, document coauthor Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“The sad reality is there aren’t very many large randomized clinical trials with this illness and so what a group of very experienced clinicians did was to gather their collective experience and report it as that. It’s largely uncontrolled experience, but from people who have seen a lot of patients, for what it’s worth to the medical community.”
Dr. Komaroff also advised that clinicians watch out for ME/CFS in patients with long COVID. “If we find that those called long COVID meet ME/CFS criteria, the reason for knowing that is that there are already some treatments that according to experienced clinicians are helpful for ME/CFS, and it would be perfectly appropriate to try some of them in long COVID, particularly the ones that have minimal adverse reactions.”
The guidelines project was supported by the Open Medicine Foundation. Dr. Komaroff reported receiving personal fees from Serimmune outside the submitted work. Dr. Chu has no disclosures.
New consensus recommendations address diagnosis and management of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), with advice that may also be helpful for patients with lingering symptoms following acute COVID-19 infection.
The document was published online Aug. 25, 2021, in the Mayo Clinic Proceedings by the 23-member U.S. ME/CFS Clinician Coalition, headed by Lucinda Bateman, MD, of the Bateman Horne Center of Excellence, Salt Lake City. The document is the culmination of work that began with a summit held at the center in March 2018.
The target audience is both generalist and specialist health care providers. While ME/CFS is estimated to affect up to 2.5 million Americans, more than 90% are either undiagnosed or misdiagnosed with other conditions such as depression. And those who are diagnosed often receive inappropriate, outdated treatments such as psychotherapy and exercise prescriptions.
“Despite myalgic encephalomyelitis/chronic fatigue syndrome affecting millions of people worldwide, many clinicians lack the knowledge to appropriately diagnose or manage ME/CFS. Unfortunately, clinical guidance has been scarce, obsolete, or potentially harmful,” Dr. Bateman and colleagues wrote.
The urgency of appropriate recognition and management of ME/CFS has increased as growing numbers of people are exhibiting signs and symptoms of ME/CFS following acute COVID-19 infection. This isn’t surprising because the illness has long been linked to other infections, including Epstein-Barr virus, the authors noted.
The document covers the epidemiology, impact, and prognosis of ME/CFS, as well as etiology and pathophysiology. “Scientific studies demonstrate multiple dysfunctional organ systems, including neuro, immune, and metabolic, in ME/CFS. These findings are not explained merely by deconditioning,” document coauthor Lily Chu, MD, an independent consultant in Burlingame, Calif., said in an interview.
The document reviews the 2015 U.S. Institute of Medicine (now Academy of Medicine) diagnostic criteria that are now also recommended by the Centers for Disease Control and Prevention. They are based on four main symptoms: substantial reduction or impairment in the ability to engage in preillness levels of occupational, educational, social or personal activities for longer than 6 months; postexertional malaise, a worsening of all current symptoms, that patients often describe as a “crash”; unrefreshing sleep; and cognitive impairment and/or orthostatic intolerance.
“The new diagnostic criteria focusing on the key symptom of postexertional malaise rather than chronic fatigue, which is common in many conditions, may make the diagnostic process quicker and more accurate. Diagnosis now is both an inclusionary and not just exclusionary process, so it’s not necessary to eliminate all causes of fatigue. Diagnose patients who fit the criteria and be alert for it in people with persistent symptoms post COVID,” Dr. Chu said.
The document provides advice for taking a clinical history to obtain the information necessary for making the diagnosis, including use of laboratory testing to rule out other conditions. Physical exams, while they may not reveal specific abnormalities, may help in identifying comorbidities and ruling out alternative diagnoses.
A long list of nonpharmacologic and pharmacologic treatment and management approaches is offered for each of the individual core and common ME/CFS symptoms, including postexertional malaise, orthostatic intolerance, sleep issues, cognitive dysfunction and fatigue, immune dysfunction, pain, and gastrointestinal issues.
The document recommends against using the “outdated standard of care” cognitive-behavioral therapy and graded exercise therapy as primary treatments for the illness. Instead, the authors recommend teaching patients “pacing,” an individualized approach to energy conservation aimed at minimizing the frequency, duration, and severity of postexertional malaise.
Clinicians are also advised to assess patients’ daily living needs and provide support, including acquiring handicap placards, work or school accommodations, and disability benefits.
“There are things clinicians can do now to help patients even without a disease-modifying treatment. These are actions they are already familiar with and carry out for people with other chronic diseases, which often have limited treatment options as well. Don’t underestimate the importance and value of supportive care for patients.” Dr. Chu said.
The recommendations are based primarily on clinical expertise because there are very few randomized trials, and much of the evidence from other types of trials has been flawed, document coauthor Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“The sad reality is there aren’t very many large randomized clinical trials with this illness and so what a group of very experienced clinicians did was to gather their collective experience and report it as that. It’s largely uncontrolled experience, but from people who have seen a lot of patients, for what it’s worth to the medical community.”
Dr. Komaroff also advised that clinicians watch out for ME/CFS in patients with long COVID. “If we find that those called long COVID meet ME/CFS criteria, the reason for knowing that is that there are already some treatments that according to experienced clinicians are helpful for ME/CFS, and it would be perfectly appropriate to try some of them in long COVID, particularly the ones that have minimal adverse reactions.”
The guidelines project was supported by the Open Medicine Foundation. Dr. Komaroff reported receiving personal fees from Serimmune outside the submitted work. Dr. Chu has no disclosures.
New consensus recommendations address diagnosis and management of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), with advice that may also be helpful for patients with lingering symptoms following acute COVID-19 infection.
The document was published online Aug. 25, 2021, in the Mayo Clinic Proceedings by the 23-member U.S. ME/CFS Clinician Coalition, headed by Lucinda Bateman, MD, of the Bateman Horne Center of Excellence, Salt Lake City. The document is the culmination of work that began with a summit held at the center in March 2018.
The target audience is both generalist and specialist health care providers. While ME/CFS is estimated to affect up to 2.5 million Americans, more than 90% are either undiagnosed or misdiagnosed with other conditions such as depression. And those who are diagnosed often receive inappropriate, outdated treatments such as psychotherapy and exercise prescriptions.
“Despite myalgic encephalomyelitis/chronic fatigue syndrome affecting millions of people worldwide, many clinicians lack the knowledge to appropriately diagnose or manage ME/CFS. Unfortunately, clinical guidance has been scarce, obsolete, or potentially harmful,” Dr. Bateman and colleagues wrote.
The urgency of appropriate recognition and management of ME/CFS has increased as growing numbers of people are exhibiting signs and symptoms of ME/CFS following acute COVID-19 infection. This isn’t surprising because the illness has long been linked to other infections, including Epstein-Barr virus, the authors noted.
The document covers the epidemiology, impact, and prognosis of ME/CFS, as well as etiology and pathophysiology. “Scientific studies demonstrate multiple dysfunctional organ systems, including neuro, immune, and metabolic, in ME/CFS. These findings are not explained merely by deconditioning,” document coauthor Lily Chu, MD, an independent consultant in Burlingame, Calif., said in an interview.
The document reviews the 2015 U.S. Institute of Medicine (now Academy of Medicine) diagnostic criteria that are now also recommended by the Centers for Disease Control and Prevention. They are based on four main symptoms: substantial reduction or impairment in the ability to engage in preillness levels of occupational, educational, social or personal activities for longer than 6 months; postexertional malaise, a worsening of all current symptoms, that patients often describe as a “crash”; unrefreshing sleep; and cognitive impairment and/or orthostatic intolerance.
“The new diagnostic criteria focusing on the key symptom of postexertional malaise rather than chronic fatigue, which is common in many conditions, may make the diagnostic process quicker and more accurate. Diagnosis now is both an inclusionary and not just exclusionary process, so it’s not necessary to eliminate all causes of fatigue. Diagnose patients who fit the criteria and be alert for it in people with persistent symptoms post COVID,” Dr. Chu said.
The document provides advice for taking a clinical history to obtain the information necessary for making the diagnosis, including use of laboratory testing to rule out other conditions. Physical exams, while they may not reveal specific abnormalities, may help in identifying comorbidities and ruling out alternative diagnoses.
A long list of nonpharmacologic and pharmacologic treatment and management approaches is offered for each of the individual core and common ME/CFS symptoms, including postexertional malaise, orthostatic intolerance, sleep issues, cognitive dysfunction and fatigue, immune dysfunction, pain, and gastrointestinal issues.
The document recommends against using the “outdated standard of care” cognitive-behavioral therapy and graded exercise therapy as primary treatments for the illness. Instead, the authors recommend teaching patients “pacing,” an individualized approach to energy conservation aimed at minimizing the frequency, duration, and severity of postexertional malaise.
Clinicians are also advised to assess patients’ daily living needs and provide support, including acquiring handicap placards, work or school accommodations, and disability benefits.
“There are things clinicians can do now to help patients even without a disease-modifying treatment. These are actions they are already familiar with and carry out for people with other chronic diseases, which often have limited treatment options as well. Don’t underestimate the importance and value of supportive care for patients.” Dr. Chu said.
The recommendations are based primarily on clinical expertise because there are very few randomized trials, and much of the evidence from other types of trials has been flawed, document coauthor Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“The sad reality is there aren’t very many large randomized clinical trials with this illness and so what a group of very experienced clinicians did was to gather their collective experience and report it as that. It’s largely uncontrolled experience, but from people who have seen a lot of patients, for what it’s worth to the medical community.”
Dr. Komaroff also advised that clinicians watch out for ME/CFS in patients with long COVID. “If we find that those called long COVID meet ME/CFS criteria, the reason for knowing that is that there are already some treatments that according to experienced clinicians are helpful for ME/CFS, and it would be perfectly appropriate to try some of them in long COVID, particularly the ones that have minimal adverse reactions.”
The guidelines project was supported by the Open Medicine Foundation. Dr. Komaroff reported receiving personal fees from Serimmune outside the submitted work. Dr. Chu has no disclosures.
FROM THE MAYO CLINIC PROCEEDINGS
Eyes on ESC ‘21: Hope for EMPEROR-Preserved, guidelines remade
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.