User login
Why Don’t Physicians Call In Sick?
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Cutting Across the Bias
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
‘Stop Teaching’ Children It’s Their Fault They’re Fat
The US Preventive Services Task Force (USPSTF) has published draft recommendations that 6-year-olds with obesity be lectured to about diet and exercise.
Never mind that there are no reproducible or scalable studies demonstrating durable and clinically meaningful benefits of this for adults let alone children. Never mind that children are not household decision-makers on matters of grocery shopping, cooking, or exercise. Never mind the corollary that many children so lectured who fail to see an impact on their weight will perceive that as their own personal failures. And of course, never mind that we’re privileged to be in an era with safe, effective, pharmacotherapeutic options for obesity. No. We must teach children it’s their fault if they’re fat. Because ultimately that’s what many of them will learn.
That’s not to say there’s no room for counseling. But with children as young as 6, that counseling should be delivered exclusively to their parents and caregivers. That counseling should focus as much if not more so on the impact of weight bias and the biological basis of obesity rather than diet and exercise, while explicitly teaching parents the means to discuss nutrition without risking their children feeling worse about themselves, increasing the risk for conflict over changes, or heightening their children’s chance of developing eating disorders or maladaptive relationships with food.
But back to the USPSTF’s actual recommendation for those 6 years old and up. They’re recommending “at least” 26 hours of lectures over a year-long interprofessional intervention. Putting aside the reality that this isn’t scalable time-wise or cost-wise to reach even a fraction of the roughly 15 million US children with obesity, there is also the issue of service provision. Because when it comes to obesity, if the intervention is purely educational, even if you want to believe there is a syllabus out there that would have a dramatic impact, its impact will vary wildly depending on the skill and approach of the service providers. This inconvenient truth is also the one that makes it impossible to meaningfully compare program outcomes even when they share the same content.
The USPSTF’s draft recommendations also explicitly avoid what the American Academy of Pediatrics has rightly embraced: the use where appropriate of medications or surgery. While opponents of the use of pharmacotherapy for childhood obesity tend to point to a lack of long-term data as rationale for its denial, something that the USPSTF has done, again, we have long-term data demonstrating a lack of scalable, clinically meaningful efficacy for service only based programs.
Childhood obesity is a flood and its ongoing current is relentless. Given its tremendous impact, especially at its extremes, on both physical and mental health, this is yet another example of systemic weight bias in action — it’s as if the USPSTF is recommending a swimming lesson–only approach while actively fearmongering, despite an absence of plausible mechanistic risk, about the long-term use of life jackets.
Dr. Freedhoff is associate professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. Dr. Freedhoff has disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
The US Preventive Services Task Force (USPSTF) has published draft recommendations that 6-year-olds with obesity be lectured to about diet and exercise.
Never mind that there are no reproducible or scalable studies demonstrating durable and clinically meaningful benefits of this for adults let alone children. Never mind that children are not household decision-makers on matters of grocery shopping, cooking, or exercise. Never mind the corollary that many children so lectured who fail to see an impact on their weight will perceive that as their own personal failures. And of course, never mind that we’re privileged to be in an era with safe, effective, pharmacotherapeutic options for obesity. No. We must teach children it’s their fault if they’re fat. Because ultimately that’s what many of them will learn.
That’s not to say there’s no room for counseling. But with children as young as 6, that counseling should be delivered exclusively to their parents and caregivers. That counseling should focus as much if not more so on the impact of weight bias and the biological basis of obesity rather than diet and exercise, while explicitly teaching parents the means to discuss nutrition without risking their children feeling worse about themselves, increasing the risk for conflict over changes, or heightening their children’s chance of developing eating disorders or maladaptive relationships with food.
But back to the USPSTF’s actual recommendation for those 6 years old and up. They’re recommending “at least” 26 hours of lectures over a year-long interprofessional intervention. Putting aside the reality that this isn’t scalable time-wise or cost-wise to reach even a fraction of the roughly 15 million US children with obesity, there is also the issue of service provision. Because when it comes to obesity, if the intervention is purely educational, even if you want to believe there is a syllabus out there that would have a dramatic impact, its impact will vary wildly depending on the skill and approach of the service providers. This inconvenient truth is also the one that makes it impossible to meaningfully compare program outcomes even when they share the same content.
The USPSTF’s draft recommendations also explicitly avoid what the American Academy of Pediatrics has rightly embraced: the use where appropriate of medications or surgery. While opponents of the use of pharmacotherapy for childhood obesity tend to point to a lack of long-term data as rationale for its denial, something that the USPSTF has done, again, we have long-term data demonstrating a lack of scalable, clinically meaningful efficacy for service only based programs.
Childhood obesity is a flood and its ongoing current is relentless. Given its tremendous impact, especially at its extremes, on both physical and mental health, this is yet another example of systemic weight bias in action — it’s as if the USPSTF is recommending a swimming lesson–only approach while actively fearmongering, despite an absence of plausible mechanistic risk, about the long-term use of life jackets.
Dr. Freedhoff is associate professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. Dr. Freedhoff has disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
The US Preventive Services Task Force (USPSTF) has published draft recommendations that 6-year-olds with obesity be lectured to about diet and exercise.
Never mind that there are no reproducible or scalable studies demonstrating durable and clinically meaningful benefits of this for adults let alone children. Never mind that children are not household decision-makers on matters of grocery shopping, cooking, or exercise. Never mind the corollary that many children so lectured who fail to see an impact on their weight will perceive that as their own personal failures. And of course, never mind that we’re privileged to be in an era with safe, effective, pharmacotherapeutic options for obesity. No. We must teach children it’s their fault if they’re fat. Because ultimately that’s what many of them will learn.
That’s not to say there’s no room for counseling. But with children as young as 6, that counseling should be delivered exclusively to their parents and caregivers. That counseling should focus as much if not more so on the impact of weight bias and the biological basis of obesity rather than diet and exercise, while explicitly teaching parents the means to discuss nutrition without risking their children feeling worse about themselves, increasing the risk for conflict over changes, or heightening their children’s chance of developing eating disorders or maladaptive relationships with food.
But back to the USPSTF’s actual recommendation for those 6 years old and up. They’re recommending “at least” 26 hours of lectures over a year-long interprofessional intervention. Putting aside the reality that this isn’t scalable time-wise or cost-wise to reach even a fraction of the roughly 15 million US children with obesity, there is also the issue of service provision. Because when it comes to obesity, if the intervention is purely educational, even if you want to believe there is a syllabus out there that would have a dramatic impact, its impact will vary wildly depending on the skill and approach of the service providers. This inconvenient truth is also the one that makes it impossible to meaningfully compare program outcomes even when they share the same content.
The USPSTF’s draft recommendations also explicitly avoid what the American Academy of Pediatrics has rightly embraced: the use where appropriate of medications or surgery. While opponents of the use of pharmacotherapy for childhood obesity tend to point to a lack of long-term data as rationale for its denial, something that the USPSTF has done, again, we have long-term data demonstrating a lack of scalable, clinically meaningful efficacy for service only based programs.
Childhood obesity is a flood and its ongoing current is relentless. Given its tremendous impact, especially at its extremes, on both physical and mental health, this is yet another example of systemic weight bias in action — it’s as if the USPSTF is recommending a swimming lesson–only approach while actively fearmongering, despite an absence of plausible mechanistic risk, about the long-term use of life jackets.
Dr. Freedhoff is associate professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. Dr. Freedhoff has disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
Tips and Techniques to Boost Colonoscopy Quality
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Biosimilar Business Deals Keep Up ‘Musical Chairs’ Game of Formulary Construction
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Coffee, COVID, and the Universal Antimicrobial
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).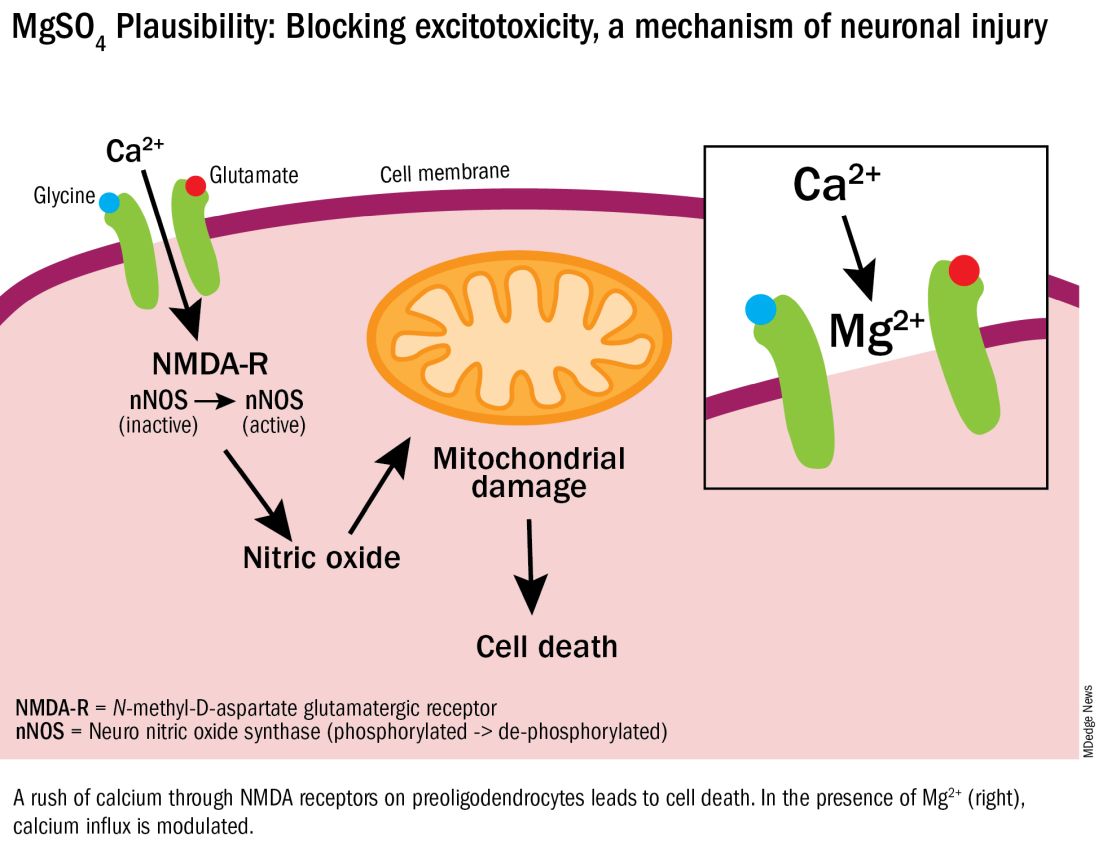
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.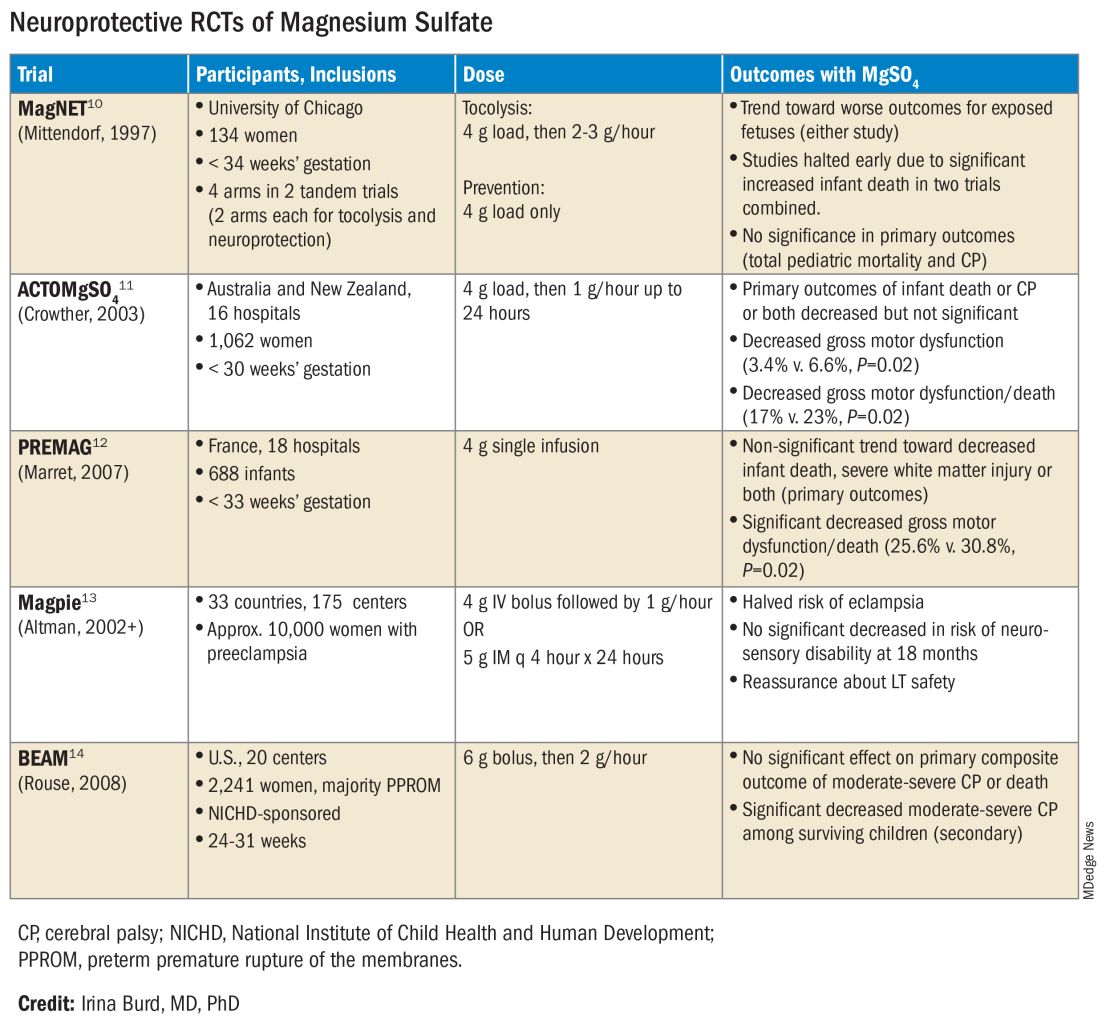
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Zoom: Convenient and Imperfect
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Yes, Patients Are Getting More Complicated
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Healthcare Violence: Doctors and Nurses Are Bearing the Brunt of Business Pressures
This transcript has been edited for clarity.
This month, I want to tackle the difficult subject of violence toward healthcare workers. There’s a reason this is top of mind for me in my practice, but I want to start by acknowledging that this has been a much larger issue for our profession and one that has been growing for a number of years now.
They also estimate that that rate doubled between 2011 and 2018. I think that range is important because it proves this was a problem, and a crescendoing problem, even before COVID.
Another thing I think is relevant is to look at where in the healthcare system are these attacks most likely. In the emergency room, ER staff have seen hostility toward them rise by at least 25% over the past several years. Some of the seeds of mistrust that were sown between the general public and the scientific and medical communities around the pandemic. I think there’s some explanation there for why that might be a particular crucible.
Perhaps most disturbingly of all, 60% of the victims of healthcare workplace violence are bedside nurses. There is something about the intensity of the inpatient setting that makes nerves particularly frayed and unfortunately makes patients and family members more likely to lash out. I think it’s actually the heightened sense of mortality.
I’m not excusing any of these behaviors, but maybe it’s akin to road rage. On the road, behind the wheel, tiny gestures can actually be, on some level, perceived as threats to our survival. Another driver swerving into your lane activates a fight-or-flight response, you feel threatened, and you might respond in the moment very rashly. I wonder if we’re not seeing that, quite unfairly, play out against bedside staff in our hospitals.
Here’s the thing. Those of us who practice in the outpatient setting — 95% of my work, for instance, happens in clinic — are not immune to this either. There are some very harrowing recent examples of physicians being killed, typically at gunpoint, often by patients, sometimes by aggrieved family members, in their offices. An orthopedist in Tennessee, a back surgeon in Tulsa, along with three of their colleagues. In the latter case, the assailant specifically blamed the surgeon for their pain.
This is where I think things get even more scary. We have to be the bearers of bad news in our profession. This has long been the task of the oncologist, in particular, to convey things that people don’t want to hear.
I think what brought this to my mind in terms of my reading was an incredible article in The ASCO Post and also in the Journal of Clinical Oncology by Dr. Noelle LoConte, who’s a medical oncologist in Wisconsin. The article is called, “I Want to Kill You,” and it recounts her telling a previously stage III colon cancer patient, with whom she thought she had good rapport, that the disease had recurred. The patient’s immediate reaction in the heat of that moment was to say, Dr LoConte, I want to kill you. I want to blow your face off.
Already, there’s clearly tension when we are telling people what they don’t want to hear. I think the final piece of the puzzle goes back to the intrusion of the business of healthcare on the practice of medicine. This is what I witnessed very recently. One of the things that’s interesting to think about is how what we do is now framed as customer service. I know there’s deriding of this model, but if perception is reality, we have a system where patients are set up to view themselves as consumers.
Let’s say, for instance, you’re in the unfortunate circumstance of being diagnosed with cancer and your insurer gives you the option to go to multiple oncologists. If you’re online browsing for oncologists, how do you differentiate me from some of my colleagues? The answer on these rating websites often has to do with domains that are about the overall experience — not just the patient-doctor interaction but also things like wait time, friendliness of staff, and promptness of care delivery.
That, I think, is the final piece of the puzzle, because what I really risk when I sit down with a patient and lay out a treatment plan is overpromising and underdelivering. I am long used to citing median overall survival for expectation of outcome. Of course, every patient wants to be an exceptional responder. Most patients want to be on the latter half of median survival. No one wants to be on the disappointingly shorter half.
My point is that I’ve long been able to mitigate that uncertainty for patients. What is getting harder and harder to explain away is the delay incurred between someone’s diagnosis, my meeting them and laying out a treatment plan, and their actual initiation of that therapy.
This finally brings me to my recent personal encounter. I have long taken care of a patient, much like Dr LoConte’s, with an extremely calm demeanor. I thought we had a great therapeutic alliance. I had to tell the patient that the disease had recurred, and then I laid out a treatment plan. It took weeks and then months for the insurer to approve this plan despite my providing my note in a timely fashion with a mountain of evidence behind the regimen that I’d selected.
This is where I think insurers — when they deny, deflect, and delay — are not taking adequate responsibility for the impact that has on the therapeutic alliance between a patient and their doctor. These people are trusting us with their lives. As an oncologist, I’ve already told them something they didn’t want to hear, and now I’m compounding that with the uncertainty of when we can actually begin treatment.
This gentleman — who, again, is normally extremely kind and affable — showed up at my office and was incredibly hostile toward me and my staff because of the delay that he was encountering. We literally couldn’t tell him when his insurer was going to approve his treatment, which would have been financially disastrous if he had tried to pay for it himself out of pocket. He needed his insurer’s approval before we could start, but we didn’t know when he could start. That uncertainty and not knowing was gnawing away at him until he was at the end of his rope.
What I’m here to say is that this has been a difficult couple of years in healthcare. I’m well aware that our ER staff are on the front lines, as are our bedside and inpatient teams. Even in the outpatient setting, I think we’re seeing this crucible and we’re seeing the pressure just grow, and grow, and grow. It’s like fracking. The more you increase the pressure, the more eventually you’re going to find out where the cracks are.
These patients are the ultimate stakeholders. It’s their lives on the line, and we should be concerned, but perhaps ultimately not surprised, that they’re lashing out to be heard. Given no other resort, they are taking out their frustration and their aggression on us. It›s not fair, but I am newly aware of it because, in a patient with whom I thought we had a superb rapport, I saw that vanish. As soon as he thought that his life was at risk, his fight-or-flight response kicked in. I was not dealing with the same man I knew. I was dealing with someone who was desperate and who just wanted to know when he could get the treatment.
I think this has taken the likelihood of workplace hostility to a whole other level for those of us in healthcare.
For any patients listening, I beg of you, please don’t shoot the messenger. We are here to serve you the best we can, but there are many external factors at play. We are doing our best to mitigate those for you so we can deliver the care that we promised in as timely a fashion as we can.
I hope everyone out there can stay safe. Thank you.
Dr. Lewis is director of gastrointestinal oncology at Intermountain Healthcare in Salt Lake City, Utah. He has an interest in neuroendocrine tumors, hereditary cancer syndromes, and patient-physician communication. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This month, I want to tackle the difficult subject of violence toward healthcare workers. There’s a reason this is top of mind for me in my practice, but I want to start by acknowledging that this has been a much larger issue for our profession and one that has been growing for a number of years now.
They also estimate that that rate doubled between 2011 and 2018. I think that range is important because it proves this was a problem, and a crescendoing problem, even before COVID.
Another thing I think is relevant is to look at where in the healthcare system are these attacks most likely. In the emergency room, ER staff have seen hostility toward them rise by at least 25% over the past several years. Some of the seeds of mistrust that were sown between the general public and the scientific and medical communities around the pandemic. I think there’s some explanation there for why that might be a particular crucible.
Perhaps most disturbingly of all, 60% of the victims of healthcare workplace violence are bedside nurses. There is something about the intensity of the inpatient setting that makes nerves particularly frayed and unfortunately makes patients and family members more likely to lash out. I think it’s actually the heightened sense of mortality.
I’m not excusing any of these behaviors, but maybe it’s akin to road rage. On the road, behind the wheel, tiny gestures can actually be, on some level, perceived as threats to our survival. Another driver swerving into your lane activates a fight-or-flight response, you feel threatened, and you might respond in the moment very rashly. I wonder if we’re not seeing that, quite unfairly, play out against bedside staff in our hospitals.
Here’s the thing. Those of us who practice in the outpatient setting — 95% of my work, for instance, happens in clinic — are not immune to this either. There are some very harrowing recent examples of physicians being killed, typically at gunpoint, often by patients, sometimes by aggrieved family members, in their offices. An orthopedist in Tennessee, a back surgeon in Tulsa, along with three of their colleagues. In the latter case, the assailant specifically blamed the surgeon for their pain.
This is where I think things get even more scary. We have to be the bearers of bad news in our profession. This has long been the task of the oncologist, in particular, to convey things that people don’t want to hear.
I think what brought this to my mind in terms of my reading was an incredible article in The ASCO Post and also in the Journal of Clinical Oncology by Dr. Noelle LoConte, who’s a medical oncologist in Wisconsin. The article is called, “I Want to Kill You,” and it recounts her telling a previously stage III colon cancer patient, with whom she thought she had good rapport, that the disease had recurred. The patient’s immediate reaction in the heat of that moment was to say, Dr LoConte, I want to kill you. I want to blow your face off.
Already, there’s clearly tension when we are telling people what they don’t want to hear. I think the final piece of the puzzle goes back to the intrusion of the business of healthcare on the practice of medicine. This is what I witnessed very recently. One of the things that’s interesting to think about is how what we do is now framed as customer service. I know there’s deriding of this model, but if perception is reality, we have a system where patients are set up to view themselves as consumers.
Let’s say, for instance, you’re in the unfortunate circumstance of being diagnosed with cancer and your insurer gives you the option to go to multiple oncologists. If you’re online browsing for oncologists, how do you differentiate me from some of my colleagues? The answer on these rating websites often has to do with domains that are about the overall experience — not just the patient-doctor interaction but also things like wait time, friendliness of staff, and promptness of care delivery.
That, I think, is the final piece of the puzzle, because what I really risk when I sit down with a patient and lay out a treatment plan is overpromising and underdelivering. I am long used to citing median overall survival for expectation of outcome. Of course, every patient wants to be an exceptional responder. Most patients want to be on the latter half of median survival. No one wants to be on the disappointingly shorter half.
My point is that I’ve long been able to mitigate that uncertainty for patients. What is getting harder and harder to explain away is the delay incurred between someone’s diagnosis, my meeting them and laying out a treatment plan, and their actual initiation of that therapy.
This finally brings me to my recent personal encounter. I have long taken care of a patient, much like Dr LoConte’s, with an extremely calm demeanor. I thought we had a great therapeutic alliance. I had to tell the patient that the disease had recurred, and then I laid out a treatment plan. It took weeks and then months for the insurer to approve this plan despite my providing my note in a timely fashion with a mountain of evidence behind the regimen that I’d selected.
This is where I think insurers — when they deny, deflect, and delay — are not taking adequate responsibility for the impact that has on the therapeutic alliance between a patient and their doctor. These people are trusting us with their lives. As an oncologist, I’ve already told them something they didn’t want to hear, and now I’m compounding that with the uncertainty of when we can actually begin treatment.
This gentleman — who, again, is normally extremely kind and affable — showed up at my office and was incredibly hostile toward me and my staff because of the delay that he was encountering. We literally couldn’t tell him when his insurer was going to approve his treatment, which would have been financially disastrous if he had tried to pay for it himself out of pocket. He needed his insurer’s approval before we could start, but we didn’t know when he could start. That uncertainty and not knowing was gnawing away at him until he was at the end of his rope.
What I’m here to say is that this has been a difficult couple of years in healthcare. I’m well aware that our ER staff are on the front lines, as are our bedside and inpatient teams. Even in the outpatient setting, I think we’re seeing this crucible and we’re seeing the pressure just grow, and grow, and grow. It’s like fracking. The more you increase the pressure, the more eventually you’re going to find out where the cracks are.
These patients are the ultimate stakeholders. It’s their lives on the line, and we should be concerned, but perhaps ultimately not surprised, that they’re lashing out to be heard. Given no other resort, they are taking out their frustration and their aggression on us. It›s not fair, but I am newly aware of it because, in a patient with whom I thought we had a superb rapport, I saw that vanish. As soon as he thought that his life was at risk, his fight-or-flight response kicked in. I was not dealing with the same man I knew. I was dealing with someone who was desperate and who just wanted to know when he could get the treatment.
I think this has taken the likelihood of workplace hostility to a whole other level for those of us in healthcare.
For any patients listening, I beg of you, please don’t shoot the messenger. We are here to serve you the best we can, but there are many external factors at play. We are doing our best to mitigate those for you so we can deliver the care that we promised in as timely a fashion as we can.
I hope everyone out there can stay safe. Thank you.
Dr. Lewis is director of gastrointestinal oncology at Intermountain Healthcare in Salt Lake City, Utah. He has an interest in neuroendocrine tumors, hereditary cancer syndromes, and patient-physician communication. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This month, I want to tackle the difficult subject of violence toward healthcare workers. There’s a reason this is top of mind for me in my practice, but I want to start by acknowledging that this has been a much larger issue for our profession and one that has been growing for a number of years now.
They also estimate that that rate doubled between 2011 and 2018. I think that range is important because it proves this was a problem, and a crescendoing problem, even before COVID.
Another thing I think is relevant is to look at where in the healthcare system are these attacks most likely. In the emergency room, ER staff have seen hostility toward them rise by at least 25% over the past several years. Some of the seeds of mistrust that were sown between the general public and the scientific and medical communities around the pandemic. I think there’s some explanation there for why that might be a particular crucible.
Perhaps most disturbingly of all, 60% of the victims of healthcare workplace violence are bedside nurses. There is something about the intensity of the inpatient setting that makes nerves particularly frayed and unfortunately makes patients and family members more likely to lash out. I think it’s actually the heightened sense of mortality.
I’m not excusing any of these behaviors, but maybe it’s akin to road rage. On the road, behind the wheel, tiny gestures can actually be, on some level, perceived as threats to our survival. Another driver swerving into your lane activates a fight-or-flight response, you feel threatened, and you might respond in the moment very rashly. I wonder if we’re not seeing that, quite unfairly, play out against bedside staff in our hospitals.
Here’s the thing. Those of us who practice in the outpatient setting — 95% of my work, for instance, happens in clinic — are not immune to this either. There are some very harrowing recent examples of physicians being killed, typically at gunpoint, often by patients, sometimes by aggrieved family members, in their offices. An orthopedist in Tennessee, a back surgeon in Tulsa, along with three of their colleagues. In the latter case, the assailant specifically blamed the surgeon for their pain.
This is where I think things get even more scary. We have to be the bearers of bad news in our profession. This has long been the task of the oncologist, in particular, to convey things that people don’t want to hear.
I think what brought this to my mind in terms of my reading was an incredible article in The ASCO Post and also in the Journal of Clinical Oncology by Dr. Noelle LoConte, who’s a medical oncologist in Wisconsin. The article is called, “I Want to Kill You,” and it recounts her telling a previously stage III colon cancer patient, with whom she thought she had good rapport, that the disease had recurred. The patient’s immediate reaction in the heat of that moment was to say, Dr LoConte, I want to kill you. I want to blow your face off.
Already, there’s clearly tension when we are telling people what they don’t want to hear. I think the final piece of the puzzle goes back to the intrusion of the business of healthcare on the practice of medicine. This is what I witnessed very recently. One of the things that’s interesting to think about is how what we do is now framed as customer service. I know there’s deriding of this model, but if perception is reality, we have a system where patients are set up to view themselves as consumers.
Let’s say, for instance, you’re in the unfortunate circumstance of being diagnosed with cancer and your insurer gives you the option to go to multiple oncologists. If you’re online browsing for oncologists, how do you differentiate me from some of my colleagues? The answer on these rating websites often has to do with domains that are about the overall experience — not just the patient-doctor interaction but also things like wait time, friendliness of staff, and promptness of care delivery.
That, I think, is the final piece of the puzzle, because what I really risk when I sit down with a patient and lay out a treatment plan is overpromising and underdelivering. I am long used to citing median overall survival for expectation of outcome. Of course, every patient wants to be an exceptional responder. Most patients want to be on the latter half of median survival. No one wants to be on the disappointingly shorter half.
My point is that I’ve long been able to mitigate that uncertainty for patients. What is getting harder and harder to explain away is the delay incurred between someone’s diagnosis, my meeting them and laying out a treatment plan, and their actual initiation of that therapy.
This finally brings me to my recent personal encounter. I have long taken care of a patient, much like Dr LoConte’s, with an extremely calm demeanor. I thought we had a great therapeutic alliance. I had to tell the patient that the disease had recurred, and then I laid out a treatment plan. It took weeks and then months for the insurer to approve this plan despite my providing my note in a timely fashion with a mountain of evidence behind the regimen that I’d selected.
This is where I think insurers — when they deny, deflect, and delay — are not taking adequate responsibility for the impact that has on the therapeutic alliance between a patient and their doctor. These people are trusting us with their lives. As an oncologist, I’ve already told them something they didn’t want to hear, and now I’m compounding that with the uncertainty of when we can actually begin treatment.
This gentleman — who, again, is normally extremely kind and affable — showed up at my office and was incredibly hostile toward me and my staff because of the delay that he was encountering. We literally couldn’t tell him when his insurer was going to approve his treatment, which would have been financially disastrous if he had tried to pay for it himself out of pocket. He needed his insurer’s approval before we could start, but we didn’t know when he could start. That uncertainty and not knowing was gnawing away at him until he was at the end of his rope.
What I’m here to say is that this has been a difficult couple of years in healthcare. I’m well aware that our ER staff are on the front lines, as are our bedside and inpatient teams. Even in the outpatient setting, I think we’re seeing this crucible and we’re seeing the pressure just grow, and grow, and grow. It’s like fracking. The more you increase the pressure, the more eventually you’re going to find out where the cracks are.
These patients are the ultimate stakeholders. It’s their lives on the line, and we should be concerned, but perhaps ultimately not surprised, that they’re lashing out to be heard. Given no other resort, they are taking out their frustration and their aggression on us. It›s not fair, but I am newly aware of it because, in a patient with whom I thought we had a superb rapport, I saw that vanish. As soon as he thought that his life was at risk, his fight-or-flight response kicked in. I was not dealing with the same man I knew. I was dealing with someone who was desperate and who just wanted to know when he could get the treatment.
I think this has taken the likelihood of workplace hostility to a whole other level for those of us in healthcare.
For any patients listening, I beg of you, please don’t shoot the messenger. We are here to serve you the best we can, but there are many external factors at play. We are doing our best to mitigate those for you so we can deliver the care that we promised in as timely a fashion as we can.
I hope everyone out there can stay safe. Thank you.
Dr. Lewis is director of gastrointestinal oncology at Intermountain Healthcare in Salt Lake City, Utah. He has an interest in neuroendocrine tumors, hereditary cancer syndromes, and patient-physician communication. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.











