User login
New Stroke Prevention: Clopidogrel-Aspirin Within 72 Hours
TOPLINE:
Dual antiplatelet therapy (DAPT) with clopidogrel-aspirin given within 72 hours of a mild ischemic stroke or a high-risk transient ischemic attack (TIA) shows a greater risk reduction for new stroke than aspirin alone, although with a higher bleeding risk.
METHODOLOGY:
- The INSPIRES, a double-blind, placebo-controlled trial, involved patients with mild ischemic stroke or high-risk TIA of presumed atherosclerotic cause who had not undergone thrombolysis or thrombectomy.
- A total of 6100 patients were randomly assigned to receive clopidogrel plus aspirin or matching clopidogrel placebo plus aspirin within 72 hours after symptom onset.
- The occurrence of any new stroke (ischemic or hemorrhagic) within 90 days was the primary efficacy outcome.
- The primary safety outcome was moderate to severe bleeding, also assessed within 90 days.
TAKEAWAY:
- Within 24 hours of symptom onset, 12.8% of patients were assigned to each treatment group, and the remaining 87.2% were assigned within the time window of 24-72 hours.
- (7.3% vs 9.2%; marginal estimated hazard ratio [HR], 0.79; P =.008).
- The risk of a composite cardiovascular event and ischemic stroke were also 20%-25% lower with aspirin-clopidogrel combo vs aspirin alone.
- Moderate to severe bleeding was low in both groups (<1%), but the risk was double in patients who received DAPT vs aspirin alone (HR, 2.08; P =.03).
IN PRACTICE:
In an accompanying editorial, Anthony S. Kim, MD from the UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, commented, “The current trial provides evidence to support expanding the time window for dual antiplatelet therapy to 72 hours.” He also warned against administering DAPT to “patients with heightened bleeding risks, such as those with a history of cerebral or systemic hemorrhage.”
SOURCE:
Yilong Wang, MD, PhD, who held positions in the Department of Neurology, Beijing Tiantan Hospital, and several other institutions, was the corresponding author of this study. This study was published online December 28 in the New England Journal of Medicine.
LIMITATIONS:
- Patients with stroke of presumed cardioembolic origin, those with moderate or severe stroke, and those who had undergone thrombolysis or thrombectomy were excluded from this study.
- Of the enrolled participants, 98.5% belonged to the Han Chinese ethnic group.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the National Key R&D Program of China, and other sources. Some authors declared receiving grants or contracts or serving as consultants in various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Dual antiplatelet therapy (DAPT) with clopidogrel-aspirin given within 72 hours of a mild ischemic stroke or a high-risk transient ischemic attack (TIA) shows a greater risk reduction for new stroke than aspirin alone, although with a higher bleeding risk.
METHODOLOGY:
- The INSPIRES, a double-blind, placebo-controlled trial, involved patients with mild ischemic stroke or high-risk TIA of presumed atherosclerotic cause who had not undergone thrombolysis or thrombectomy.
- A total of 6100 patients were randomly assigned to receive clopidogrel plus aspirin or matching clopidogrel placebo plus aspirin within 72 hours after symptom onset.
- The occurrence of any new stroke (ischemic or hemorrhagic) within 90 days was the primary efficacy outcome.
- The primary safety outcome was moderate to severe bleeding, also assessed within 90 days.
TAKEAWAY:
- Within 24 hours of symptom onset, 12.8% of patients were assigned to each treatment group, and the remaining 87.2% were assigned within the time window of 24-72 hours.
- (7.3% vs 9.2%; marginal estimated hazard ratio [HR], 0.79; P =.008).
- The risk of a composite cardiovascular event and ischemic stroke were also 20%-25% lower with aspirin-clopidogrel combo vs aspirin alone.
- Moderate to severe bleeding was low in both groups (<1%), but the risk was double in patients who received DAPT vs aspirin alone (HR, 2.08; P =.03).
IN PRACTICE:
In an accompanying editorial, Anthony S. Kim, MD from the UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, commented, “The current trial provides evidence to support expanding the time window for dual antiplatelet therapy to 72 hours.” He also warned against administering DAPT to “patients with heightened bleeding risks, such as those with a history of cerebral or systemic hemorrhage.”
SOURCE:
Yilong Wang, MD, PhD, who held positions in the Department of Neurology, Beijing Tiantan Hospital, and several other institutions, was the corresponding author of this study. This study was published online December 28 in the New England Journal of Medicine.
LIMITATIONS:
- Patients with stroke of presumed cardioembolic origin, those with moderate or severe stroke, and those who had undergone thrombolysis or thrombectomy were excluded from this study.
- Of the enrolled participants, 98.5% belonged to the Han Chinese ethnic group.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the National Key R&D Program of China, and other sources. Some authors declared receiving grants or contracts or serving as consultants in various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Dual antiplatelet therapy (DAPT) with clopidogrel-aspirin given within 72 hours of a mild ischemic stroke or a high-risk transient ischemic attack (TIA) shows a greater risk reduction for new stroke than aspirin alone, although with a higher bleeding risk.
METHODOLOGY:
- The INSPIRES, a double-blind, placebo-controlled trial, involved patients with mild ischemic stroke or high-risk TIA of presumed atherosclerotic cause who had not undergone thrombolysis or thrombectomy.
- A total of 6100 patients were randomly assigned to receive clopidogrel plus aspirin or matching clopidogrel placebo plus aspirin within 72 hours after symptom onset.
- The occurrence of any new stroke (ischemic or hemorrhagic) within 90 days was the primary efficacy outcome.
- The primary safety outcome was moderate to severe bleeding, also assessed within 90 days.
TAKEAWAY:
- Within 24 hours of symptom onset, 12.8% of patients were assigned to each treatment group, and the remaining 87.2% were assigned within the time window of 24-72 hours.
- (7.3% vs 9.2%; marginal estimated hazard ratio [HR], 0.79; P =.008).
- The risk of a composite cardiovascular event and ischemic stroke were also 20%-25% lower with aspirin-clopidogrel combo vs aspirin alone.
- Moderate to severe bleeding was low in both groups (<1%), but the risk was double in patients who received DAPT vs aspirin alone (HR, 2.08; P =.03).
IN PRACTICE:
In an accompanying editorial, Anthony S. Kim, MD from the UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, commented, “The current trial provides evidence to support expanding the time window for dual antiplatelet therapy to 72 hours.” He also warned against administering DAPT to “patients with heightened bleeding risks, such as those with a history of cerebral or systemic hemorrhage.”
SOURCE:
Yilong Wang, MD, PhD, who held positions in the Department of Neurology, Beijing Tiantan Hospital, and several other institutions, was the corresponding author of this study. This study was published online December 28 in the New England Journal of Medicine.
LIMITATIONS:
- Patients with stroke of presumed cardioembolic origin, those with moderate or severe stroke, and those who had undergone thrombolysis or thrombectomy were excluded from this study.
- Of the enrolled participants, 98.5% belonged to the Han Chinese ethnic group.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the National Key R&D Program of China, and other sources. Some authors declared receiving grants or contracts or serving as consultants in various sources.
A version of this article appeared on Medscape.com.
FDA mandates five changes to iPLEDGE program for isotretinoin
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
Thiazide Diuretic Utilization Within the VA
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
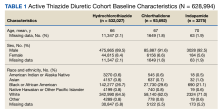
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
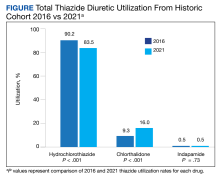
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
FDA warns of potentially lethal reaction to seizure meds
that can be life threatening if not detected and treated promptly, the Food and Drug Administration warns in an alert.
Known as drug reaction with eosinophilia and systemic symptoms (DRESS), it may start as a rash but can quickly progress and cause injury to internal organs, the need for hospitalization, and death, the FDA notes.
A search of the FDA Adverse Event Reporting System (FAERS) and the medical literature through March 2023 identified 32 serious cases of DRESS worldwide that were associated with levetiracetam.
Three cases occurred in the United States, and 29 occurred abroad. In all 32 cases, the patients were hospitalized and received medical treatment; in 2 cases, the patients died.
The median time to onset of DRESS in the levetiracetam cases was 24 days; times ranged from 7 to 170 days. The reported signs and symptoms included skin rash (n = 22), fever (n = 20), eosinophilia (n = 17), lymph node swelling (n = 9), and atypical lymphocytes (n = 4).
Twenty-two levetiracetam-associated cases of DRESS involved injury to one or more organs, including the liver, lungs, kidneys, and gallbladder.
In 25 of the 29 cases for which information on treatment discontinuation was available, DRESS symptoms resolved when levetiracetam was discontinued.
As for clobazam, a search of FAERS and the medical literature through July 2023 identified 10 serious cases of DRESS worldwide – 1 in the United States and 9 abroad. All 10 patients were hospitalized and received medical treatment. No deaths were reported.
The median time to onset of clobazam-associated DRESS was 21.5 days (range, 7-103 days). The reported signs and symptoms included skin rash (n = 10), fever (n = 8), eosinophilia (n = 7), facial swelling (n = 7), leukocytosis (n = 4), lymph node swelling (n = 4), and leukopenia/thrombocytopenia (n = 1).
In nine cases, there was injury to one or more organs, including the liver, kidneys, and gastrointestinal tract.
DRESS symptoms resolved in all 10 cases when treatment with clobazam was stopped. DRESS and other serious skin reactions reported with clobazam, a benzodiazepine, have not generally been associated with other benzodiazepines, the FDA notes.
Label updates
As a result of these cases, warnings about the risk of DRESS will be added to the prescribing information and patient medication guides for these medicines, the FDA announced.
“Health care professionals should be aware that prompt recognition and early treatment is important for improving DRESS outcomes and decreasing mortality,” the FDA said.
They noted that diagnosis is often difficult because early signs and symptoms, such as fever and swollen lymph nodes, may be present without evidence of a rash.
DRESS may develop 2-8 weeks after starting levetiracetam or clobazam. Symptoms and intensity can vary widely.
DRESS can also be confused with other serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.
The FDA says patients should be advised of the signs and symptoms of DRESS and be told to stop taking the medicine and seek immediate medical attention if DRESS is suspected during treatment with levetiracetam or clobazam.
Adverse reactions with these medications should be reported to the FDA’s MedWatch program.
A version of this article appeared on Medscape.com.
that can be life threatening if not detected and treated promptly, the Food and Drug Administration warns in an alert.
Known as drug reaction with eosinophilia and systemic symptoms (DRESS), it may start as a rash but can quickly progress and cause injury to internal organs, the need for hospitalization, and death, the FDA notes.
A search of the FDA Adverse Event Reporting System (FAERS) and the medical literature through March 2023 identified 32 serious cases of DRESS worldwide that were associated with levetiracetam.
Three cases occurred in the United States, and 29 occurred abroad. In all 32 cases, the patients were hospitalized and received medical treatment; in 2 cases, the patients died.
The median time to onset of DRESS in the levetiracetam cases was 24 days; times ranged from 7 to 170 days. The reported signs and symptoms included skin rash (n = 22), fever (n = 20), eosinophilia (n = 17), lymph node swelling (n = 9), and atypical lymphocytes (n = 4).
Twenty-two levetiracetam-associated cases of DRESS involved injury to one or more organs, including the liver, lungs, kidneys, and gallbladder.
In 25 of the 29 cases for which information on treatment discontinuation was available, DRESS symptoms resolved when levetiracetam was discontinued.
As for clobazam, a search of FAERS and the medical literature through July 2023 identified 10 serious cases of DRESS worldwide – 1 in the United States and 9 abroad. All 10 patients were hospitalized and received medical treatment. No deaths were reported.
The median time to onset of clobazam-associated DRESS was 21.5 days (range, 7-103 days). The reported signs and symptoms included skin rash (n = 10), fever (n = 8), eosinophilia (n = 7), facial swelling (n = 7), leukocytosis (n = 4), lymph node swelling (n = 4), and leukopenia/thrombocytopenia (n = 1).
In nine cases, there was injury to one or more organs, including the liver, kidneys, and gastrointestinal tract.
DRESS symptoms resolved in all 10 cases when treatment with clobazam was stopped. DRESS and other serious skin reactions reported with clobazam, a benzodiazepine, have not generally been associated with other benzodiazepines, the FDA notes.
Label updates
As a result of these cases, warnings about the risk of DRESS will be added to the prescribing information and patient medication guides for these medicines, the FDA announced.
“Health care professionals should be aware that prompt recognition and early treatment is important for improving DRESS outcomes and decreasing mortality,” the FDA said.
They noted that diagnosis is often difficult because early signs and symptoms, such as fever and swollen lymph nodes, may be present without evidence of a rash.
DRESS may develop 2-8 weeks after starting levetiracetam or clobazam. Symptoms and intensity can vary widely.
DRESS can also be confused with other serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.
The FDA says patients should be advised of the signs and symptoms of DRESS and be told to stop taking the medicine and seek immediate medical attention if DRESS is suspected during treatment with levetiracetam or clobazam.
Adverse reactions with these medications should be reported to the FDA’s MedWatch program.
A version of this article appeared on Medscape.com.
that can be life threatening if not detected and treated promptly, the Food and Drug Administration warns in an alert.
Known as drug reaction with eosinophilia and systemic symptoms (DRESS), it may start as a rash but can quickly progress and cause injury to internal organs, the need for hospitalization, and death, the FDA notes.
A search of the FDA Adverse Event Reporting System (FAERS) and the medical literature through March 2023 identified 32 serious cases of DRESS worldwide that were associated with levetiracetam.
Three cases occurred in the United States, and 29 occurred abroad. In all 32 cases, the patients were hospitalized and received medical treatment; in 2 cases, the patients died.
The median time to onset of DRESS in the levetiracetam cases was 24 days; times ranged from 7 to 170 days. The reported signs and symptoms included skin rash (n = 22), fever (n = 20), eosinophilia (n = 17), lymph node swelling (n = 9), and atypical lymphocytes (n = 4).
Twenty-two levetiracetam-associated cases of DRESS involved injury to one or more organs, including the liver, lungs, kidneys, and gallbladder.
In 25 of the 29 cases for which information on treatment discontinuation was available, DRESS symptoms resolved when levetiracetam was discontinued.
As for clobazam, a search of FAERS and the medical literature through July 2023 identified 10 serious cases of DRESS worldwide – 1 in the United States and 9 abroad. All 10 patients were hospitalized and received medical treatment. No deaths were reported.
The median time to onset of clobazam-associated DRESS was 21.5 days (range, 7-103 days). The reported signs and symptoms included skin rash (n = 10), fever (n = 8), eosinophilia (n = 7), facial swelling (n = 7), leukocytosis (n = 4), lymph node swelling (n = 4), and leukopenia/thrombocytopenia (n = 1).
In nine cases, there was injury to one or more organs, including the liver, kidneys, and gastrointestinal tract.
DRESS symptoms resolved in all 10 cases when treatment with clobazam was stopped. DRESS and other serious skin reactions reported with clobazam, a benzodiazepine, have not generally been associated with other benzodiazepines, the FDA notes.
Label updates
As a result of these cases, warnings about the risk of DRESS will be added to the prescribing information and patient medication guides for these medicines, the FDA announced.
“Health care professionals should be aware that prompt recognition and early treatment is important for improving DRESS outcomes and decreasing mortality,” the FDA said.
They noted that diagnosis is often difficult because early signs and symptoms, such as fever and swollen lymph nodes, may be present without evidence of a rash.
DRESS may develop 2-8 weeks after starting levetiracetam or clobazam. Symptoms and intensity can vary widely.
DRESS can also be confused with other serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.
The FDA says patients should be advised of the signs and symptoms of DRESS and be told to stop taking the medicine and seek immediate medical attention if DRESS is suspected during treatment with levetiracetam or clobazam.
Adverse reactions with these medications should be reported to the FDA’s MedWatch program.
A version of this article appeared on Medscape.com.
New consensus guide on rare drug hypersensitivity reaction
TOPLINE:
).
METHODOLOGY:
Data on the evaluation, assessment, and treatment of the rare but potentially life-threatening drug hypersensitivity reaction are lacking.
To support clinicians in diagnosing and managing DRESS, a steering committee conducted a literature review to examine current research, identify evidence, and develop consensus statements. They invited experts from 21 countries across four continents to participate in a Delphi consensus process.
An international panel of 54 experts (including 45 dermatologists) initially assessed 100 statements related to baseline workup, severity of the condition, and treatment. Two more statements were added in the second round.
After revisions and the second round, the group reached consensus for 93 statements overall.
TAKEAWAY:
The statements generating the most disagreement involved diagnosis. The group ultimately supported the value of measuring the viral load of Epstein-Barr virus, cytomegalovirus, and human herpesvirus 6 in all patients with suspected DRESS. The group also agreed on screening for hepatitis A, B, and C in cases of liver involvement and screening for hepatitis B and C before starting systemic therapy.
The group agreed with previous severity criteria that differentiate between mild, moderate, and severe DRESS based on the extent of liver, kidney, and blood involvement and the damage of other organs.
Consensus on treatment was reached for all 12 relevant statements in the first Delphi round. Recommendations included the use of corticosteroids and immediate discontinuation of the drugs causing the reaction.
IN PRACTICE:
“This Delphi exercise aimed to provide a common ground of consensus,” the authors noted. However, “each of the addressed categories needs more in-depth follow-up studies to improve the clinical management of patients.”
SOURCE:
The DRESS Delphi consensus group conducted its exercise under the leadership of Marie-Charlotte Brüggen, MD, of the University Hospital of Zürich. The consensus was published online in the JAMA Dermatology.
LIMITATIONS:
Published evidence was limited because of the low prevalence of DRESS. The consensus statements should therefore be considered with caution and in the context of a clinician’s expertise and available resources. Research gaps also persist in how DRESS may vary with region and ethnicity. The severity thresholds need validation in a revised multicenter statement.
DISCLOSURES:
The consensus review received no outside funding. Dr. Brüggen disclosed relationships with the Swiss National Science Foundation, Christine Kühne – Center for Allergy Research and Education, FreeNovation, LEO Foundation, Olga Mayenfisch Foundation, University of Zürich, LEO Pharma, Pierre Fabre Eczema Foundation, Eli Lilly, AbbVie, GSK, and AstraZeneca. Coauthors disclosed relationships with multiple pharmaceutical companies, foundations, and medical publishing companies.
A version of this article appeared on Medscape.com.
TOPLINE:
).
METHODOLOGY:
Data on the evaluation, assessment, and treatment of the rare but potentially life-threatening drug hypersensitivity reaction are lacking.
To support clinicians in diagnosing and managing DRESS, a steering committee conducted a literature review to examine current research, identify evidence, and develop consensus statements. They invited experts from 21 countries across four continents to participate in a Delphi consensus process.
An international panel of 54 experts (including 45 dermatologists) initially assessed 100 statements related to baseline workup, severity of the condition, and treatment. Two more statements were added in the second round.
After revisions and the second round, the group reached consensus for 93 statements overall.
TAKEAWAY:
The statements generating the most disagreement involved diagnosis. The group ultimately supported the value of measuring the viral load of Epstein-Barr virus, cytomegalovirus, and human herpesvirus 6 in all patients with suspected DRESS. The group also agreed on screening for hepatitis A, B, and C in cases of liver involvement and screening for hepatitis B and C before starting systemic therapy.
The group agreed with previous severity criteria that differentiate between mild, moderate, and severe DRESS based on the extent of liver, kidney, and blood involvement and the damage of other organs.
Consensus on treatment was reached for all 12 relevant statements in the first Delphi round. Recommendations included the use of corticosteroids and immediate discontinuation of the drugs causing the reaction.
IN PRACTICE:
“This Delphi exercise aimed to provide a common ground of consensus,” the authors noted. However, “each of the addressed categories needs more in-depth follow-up studies to improve the clinical management of patients.”
SOURCE:
The DRESS Delphi consensus group conducted its exercise under the leadership of Marie-Charlotte Brüggen, MD, of the University Hospital of Zürich. The consensus was published online in the JAMA Dermatology.
LIMITATIONS:
Published evidence was limited because of the low prevalence of DRESS. The consensus statements should therefore be considered with caution and in the context of a clinician’s expertise and available resources. Research gaps also persist in how DRESS may vary with region and ethnicity. The severity thresholds need validation in a revised multicenter statement.
DISCLOSURES:
The consensus review received no outside funding. Dr. Brüggen disclosed relationships with the Swiss National Science Foundation, Christine Kühne – Center for Allergy Research and Education, FreeNovation, LEO Foundation, Olga Mayenfisch Foundation, University of Zürich, LEO Pharma, Pierre Fabre Eczema Foundation, Eli Lilly, AbbVie, GSK, and AstraZeneca. Coauthors disclosed relationships with multiple pharmaceutical companies, foundations, and medical publishing companies.
A version of this article appeared on Medscape.com.
TOPLINE:
).
METHODOLOGY:
Data on the evaluation, assessment, and treatment of the rare but potentially life-threatening drug hypersensitivity reaction are lacking.
To support clinicians in diagnosing and managing DRESS, a steering committee conducted a literature review to examine current research, identify evidence, and develop consensus statements. They invited experts from 21 countries across four continents to participate in a Delphi consensus process.
An international panel of 54 experts (including 45 dermatologists) initially assessed 100 statements related to baseline workup, severity of the condition, and treatment. Two more statements were added in the second round.
After revisions and the second round, the group reached consensus for 93 statements overall.
TAKEAWAY:
The statements generating the most disagreement involved diagnosis. The group ultimately supported the value of measuring the viral load of Epstein-Barr virus, cytomegalovirus, and human herpesvirus 6 in all patients with suspected DRESS. The group also agreed on screening for hepatitis A, B, and C in cases of liver involvement and screening for hepatitis B and C before starting systemic therapy.
The group agreed with previous severity criteria that differentiate between mild, moderate, and severe DRESS based on the extent of liver, kidney, and blood involvement and the damage of other organs.
Consensus on treatment was reached for all 12 relevant statements in the first Delphi round. Recommendations included the use of corticosteroids and immediate discontinuation of the drugs causing the reaction.
IN PRACTICE:
“This Delphi exercise aimed to provide a common ground of consensus,” the authors noted. However, “each of the addressed categories needs more in-depth follow-up studies to improve the clinical management of patients.”
SOURCE:
The DRESS Delphi consensus group conducted its exercise under the leadership of Marie-Charlotte Brüggen, MD, of the University Hospital of Zürich. The consensus was published online in the JAMA Dermatology.
LIMITATIONS:
Published evidence was limited because of the low prevalence of DRESS. The consensus statements should therefore be considered with caution and in the context of a clinician’s expertise and available resources. Research gaps also persist in how DRESS may vary with region and ethnicity. The severity thresholds need validation in a revised multicenter statement.
DISCLOSURES:
The consensus review received no outside funding. Dr. Brüggen disclosed relationships with the Swiss National Science Foundation, Christine Kühne – Center for Allergy Research and Education, FreeNovation, LEO Foundation, Olga Mayenfisch Foundation, University of Zürich, LEO Pharma, Pierre Fabre Eczema Foundation, Eli Lilly, AbbVie, GSK, and AstraZeneca. Coauthors disclosed relationships with multiple pharmaceutical companies, foundations, and medical publishing companies.
A version of this article appeared on Medscape.com.
FDA OKs new agent to block chemotherapy-induced neutropenia
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Low-dose methotrexate carries higher risk for older patients with CKD
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Long-term use of ADHD meds and CVD risk: New data
results of a large Swedish nested case-control study suggest.
The increased risk was evident only for hypertension and arterial disease, was dose dependent, and was higher for stimulant than nonstimulant ADHD medications.
“Clinicians should be vigilant in monitoring signs and symptoms of cardiovascular diseases, particularly among those receiving higher doses,” Zheng Chang, PhD, principal researcher, department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, said in an interview.
“Treatment decisions, as always, should be based on careful weighing of potential benefits and risks at individual patient level, rather than simple one-size-fits-all recommendations,” Dr. Chang added.
The study was published online in JAMA Psychiatry
Filling in the research gaps
The use of medications to treat ADHD has increased markedly over the past decades in both children and adults. The potential risk for CVD associated with long-term ADHD medication use remains unclear. Most “longitudinal” studies that have looked at the association have an average follow-up time of no more than 2 years, the authors note.
In contrast, the Swedish study assessed the association between cumulative use of ADHD medication in children and adults followed for up to 14 years and also looked at whether associations differ across types of medication and dosages, types of CVD, gender, and age.
Among 278,027 individuals aged 6-64 years diagnosed with ADHD or dispensed ADHD medication, 10,388 with CVD were identified and matched to 51,672 controls without CVD.
Longer cumulative duration of ADHD medication use was associated with a statistically significant increased risk for CVD, compared with no use.
When the risk for specific CVDs was examined, long-term use of ADHD medication (compared with no use) was associated with an increased risk for hypertension and arterial disease but not arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease.
For hypertension, the adjusted odds ratio was 1.72 (95% confidence interval, 1.51-1.97) for 3 to ≤ 5 years and 1.80 (95% CI, 1.55-2.08) for > 5 years of medication use. For arterial disease, the AOR was 1.65 (95% CI, 1.11-2.45) for 3 to ≤ 5 years and 1.49 (95% CI, 0.96-2.32) for > 5 years of use.
Stimulants confer greatest risk
Across the 14-year follow-up period, each additional year of ADHD medication use was associated with an average 4% increased CVD risk, with a larger 8% increased risk in the first 3 years of cumulative use, followed by stable risk over the remaining follow-up.
Similar risks were observed in children and adults, as well as in females and males.
When focusing on specific ADHD medications, compared with no use, long-term use of the stimulant methylphenidate was associated with an increased risk for CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤ 5 years and 1.19 [95% CI, 1.08-1.31] for > 5 years).
The same was true for long-term use of the stimulant lisdexamfetamine (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤ 3 years and 1.17 [95% CI, 0.98-1.40] for > 3 years).
In contrast, use of the nonstimulant atomoxetine was associated with elevated CVD risk only for the first year of use (AOR, 1.07; 95% CI, 1.01-1.13).
The increased risk for CVD occurred only above certain average daily doses: 45 mg for methylphenidate and lisdexamfetamine, 22.5 mg for amphetamines, and 120 mg for atomoxetine.
The authors note that, although they accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, they could not prove causality.
‘Tricky trade-offs’
The coauthors of an editorial in JAMA Psychiatry (2023 Nov 22. doi: 10.1001/jamapsychiatry.2023.4126) note that the study “should remind us that clinical decision-making is often based on tricky trade-offs that should be considered at the individual patient level.”
Given that hypertension is the leading cause of CV morbidity and mortality worldwide, the increased likelihood of hypertension with long-term use of ADHD medications “cannot be disregarded,” write Samuele Cortese, MD, PhD, and Cristiano Fava, MD, PhD, with University of Southampton (England).
“These findings are especially relevant given the reported association between ADHD and physical conditions, such as obesity, which further contribute to increased cardiovascular risk,” they add.
Dr. Cortese and Dr. Fava say that the increased CV risk – averaging 4% per year and stabilizing after 3 years of treatment – “should be carefully weighed against the established benefits, on a case-by-case basis.”
“Importantly,” they write, “large real-world self-controlled studies have shown that individuals with ADHD experience significantly fewer unintentional physical injuries, motor vehicle crashes, substance use disorders, and criminal acts, as well as improved academic functioning, during periods when they are taking, compared with periods when they are not taking, methylphenidate.”
The risk-benefit ratio, however, may be lower in people with preexisting heart conditions. However, more evidence and precise recommendations are needed in relation to the treatment of individuals with ADHD and preexisting CV conditions, the editorial writers say.
This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare and the European Union’s Horizon 2020 research and innovation program. The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
results of a large Swedish nested case-control study suggest.
The increased risk was evident only for hypertension and arterial disease, was dose dependent, and was higher for stimulant than nonstimulant ADHD medications.
“Clinicians should be vigilant in monitoring signs and symptoms of cardiovascular diseases, particularly among those receiving higher doses,” Zheng Chang, PhD, principal researcher, department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, said in an interview.
“Treatment decisions, as always, should be based on careful weighing of potential benefits and risks at individual patient level, rather than simple one-size-fits-all recommendations,” Dr. Chang added.
The study was published online in JAMA Psychiatry
Filling in the research gaps
The use of medications to treat ADHD has increased markedly over the past decades in both children and adults. The potential risk for CVD associated with long-term ADHD medication use remains unclear. Most “longitudinal” studies that have looked at the association have an average follow-up time of no more than 2 years, the authors note.
In contrast, the Swedish study assessed the association between cumulative use of ADHD medication in children and adults followed for up to 14 years and also looked at whether associations differ across types of medication and dosages, types of CVD, gender, and age.
Among 278,027 individuals aged 6-64 years diagnosed with ADHD or dispensed ADHD medication, 10,388 with CVD were identified and matched to 51,672 controls without CVD.
Longer cumulative duration of ADHD medication use was associated with a statistically significant increased risk for CVD, compared with no use.
When the risk for specific CVDs was examined, long-term use of ADHD medication (compared with no use) was associated with an increased risk for hypertension and arterial disease but not arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease.
For hypertension, the adjusted odds ratio was 1.72 (95% confidence interval, 1.51-1.97) for 3 to ≤ 5 years and 1.80 (95% CI, 1.55-2.08) for > 5 years of medication use. For arterial disease, the AOR was 1.65 (95% CI, 1.11-2.45) for 3 to ≤ 5 years and 1.49 (95% CI, 0.96-2.32) for > 5 years of use.
Stimulants confer greatest risk
Across the 14-year follow-up period, each additional year of ADHD medication use was associated with an average 4% increased CVD risk, with a larger 8% increased risk in the first 3 years of cumulative use, followed by stable risk over the remaining follow-up.
Similar risks were observed in children and adults, as well as in females and males.
When focusing on specific ADHD medications, compared with no use, long-term use of the stimulant methylphenidate was associated with an increased risk for CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤ 5 years and 1.19 [95% CI, 1.08-1.31] for > 5 years).
The same was true for long-term use of the stimulant lisdexamfetamine (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤ 3 years and 1.17 [95% CI, 0.98-1.40] for > 3 years).
In contrast, use of the nonstimulant atomoxetine was associated with elevated CVD risk only for the first year of use (AOR, 1.07; 95% CI, 1.01-1.13).
The increased risk for CVD occurred only above certain average daily doses: 45 mg for methylphenidate and lisdexamfetamine, 22.5 mg for amphetamines, and 120 mg for atomoxetine.
The authors note that, although they accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, they could not prove causality.
‘Tricky trade-offs’
The coauthors of an editorial in JAMA Psychiatry (2023 Nov 22. doi: 10.1001/jamapsychiatry.2023.4126) note that the study “should remind us that clinical decision-making is often based on tricky trade-offs that should be considered at the individual patient level.”
Given that hypertension is the leading cause of CV morbidity and mortality worldwide, the increased likelihood of hypertension with long-term use of ADHD medications “cannot be disregarded,” write Samuele Cortese, MD, PhD, and Cristiano Fava, MD, PhD, with University of Southampton (England).
“These findings are especially relevant given the reported association between ADHD and physical conditions, such as obesity, which further contribute to increased cardiovascular risk,” they add.
Dr. Cortese and Dr. Fava say that the increased CV risk – averaging 4% per year and stabilizing after 3 years of treatment – “should be carefully weighed against the established benefits, on a case-by-case basis.”
“Importantly,” they write, “large real-world self-controlled studies have shown that individuals with ADHD experience significantly fewer unintentional physical injuries, motor vehicle crashes, substance use disorders, and criminal acts, as well as improved academic functioning, during periods when they are taking, compared with periods when they are not taking, methylphenidate.”
The risk-benefit ratio, however, may be lower in people with preexisting heart conditions. However, more evidence and precise recommendations are needed in relation to the treatment of individuals with ADHD and preexisting CV conditions, the editorial writers say.
This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare and the European Union’s Horizon 2020 research and innovation program. The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
results of a large Swedish nested case-control study suggest.
The increased risk was evident only for hypertension and arterial disease, was dose dependent, and was higher for stimulant than nonstimulant ADHD medications.
“Clinicians should be vigilant in monitoring signs and symptoms of cardiovascular diseases, particularly among those receiving higher doses,” Zheng Chang, PhD, principal researcher, department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, said in an interview.
“Treatment decisions, as always, should be based on careful weighing of potential benefits and risks at individual patient level, rather than simple one-size-fits-all recommendations,” Dr. Chang added.
The study was published online in JAMA Psychiatry
Filling in the research gaps
The use of medications to treat ADHD has increased markedly over the past decades in both children and adults. The potential risk for CVD associated with long-term ADHD medication use remains unclear. Most “longitudinal” studies that have looked at the association have an average follow-up time of no more than 2 years, the authors note.
In contrast, the Swedish study assessed the association between cumulative use of ADHD medication in children and adults followed for up to 14 years and also looked at whether associations differ across types of medication and dosages, types of CVD, gender, and age.
Among 278,027 individuals aged 6-64 years diagnosed with ADHD or dispensed ADHD medication, 10,388 with CVD were identified and matched to 51,672 controls without CVD.
Longer cumulative duration of ADHD medication use was associated with a statistically significant increased risk for CVD, compared with no use.
When the risk for specific CVDs was examined, long-term use of ADHD medication (compared with no use) was associated with an increased risk for hypertension and arterial disease but not arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease.
For hypertension, the adjusted odds ratio was 1.72 (95% confidence interval, 1.51-1.97) for 3 to ≤ 5 years and 1.80 (95% CI, 1.55-2.08) for > 5 years of medication use. For arterial disease, the AOR was 1.65 (95% CI, 1.11-2.45) for 3 to ≤ 5 years and 1.49 (95% CI, 0.96-2.32) for > 5 years of use.
Stimulants confer greatest risk
Across the 14-year follow-up period, each additional year of ADHD medication use was associated with an average 4% increased CVD risk, with a larger 8% increased risk in the first 3 years of cumulative use, followed by stable risk over the remaining follow-up.
Similar risks were observed in children and adults, as well as in females and males.
When focusing on specific ADHD medications, compared with no use, long-term use of the stimulant methylphenidate was associated with an increased risk for CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤ 5 years and 1.19 [95% CI, 1.08-1.31] for > 5 years).
The same was true for long-term use of the stimulant lisdexamfetamine (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤ 3 years and 1.17 [95% CI, 0.98-1.40] for > 3 years).
In contrast, use of the nonstimulant atomoxetine was associated with elevated CVD risk only for the first year of use (AOR, 1.07; 95% CI, 1.01-1.13).
The increased risk for CVD occurred only above certain average daily doses: 45 mg for methylphenidate and lisdexamfetamine, 22.5 mg for amphetamines, and 120 mg for atomoxetine.
The authors note that, although they accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, they could not prove causality.
‘Tricky trade-offs’
The coauthors of an editorial in JAMA Psychiatry (2023 Nov 22. doi: 10.1001/jamapsychiatry.2023.4126) note that the study “should remind us that clinical decision-making is often based on tricky trade-offs that should be considered at the individual patient level.”
Given that hypertension is the leading cause of CV morbidity and mortality worldwide, the increased likelihood of hypertension with long-term use of ADHD medications “cannot be disregarded,” write Samuele Cortese, MD, PhD, and Cristiano Fava, MD, PhD, with University of Southampton (England).
“These findings are especially relevant given the reported association between ADHD and physical conditions, such as obesity, which further contribute to increased cardiovascular risk,” they add.
Dr. Cortese and Dr. Fava say that the increased CV risk – averaging 4% per year and stabilizing after 3 years of treatment – “should be carefully weighed against the established benefits, on a case-by-case basis.”
“Importantly,” they write, “large real-world self-controlled studies have shown that individuals with ADHD experience significantly fewer unintentional physical injuries, motor vehicle crashes, substance use disorders, and criminal acts, as well as improved academic functioning, during periods when they are taking, compared with periods when they are not taking, methylphenidate.”
The risk-benefit ratio, however, may be lower in people with preexisting heart conditions. However, more evidence and precise recommendations are needed in relation to the treatment of individuals with ADHD and preexisting CV conditions, the editorial writers say.
This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare and the European Union’s Horizon 2020 research and innovation program. The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
At 52 weeks, complete hair regrowth rates still climbing on deuruxolitinib
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
At THE EADV CONGRESS
Tapinarof effective for AD in patients as young as 2 years
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
AT THE EADV CONGRESS