User login
Monthly injection therapy for HIV found noninferior to daily oral dosing
Two international phase 3 randomized trials of according to reports published in the New England Journal of Medicine.
The Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection (FLAIR) study and the Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression (ATLAS) study looked at a separate facet of the use of a monthly therapeutic injection as a replacement for daily oral HIV therapy.
The FLAIR trial (ClinicalTrials.gov number, NCT02938520) was a phase 3, randomized, open-label study in which adults with HIV-1 infection who had not previously received antiretroviral therapy were given 20 weeks of daily oral induction therapy with dolutegravir–abacavir–lamivudine. Those patients with an HIV-1 RNA level of less than 50 copies per milliliter after 16 weeks were then randomly assigned (1:1) to continue their current oral therapy or switch to oral cabotegravir plus rilpivirine for 1 month followed by monthly intramuscular injections of long-acting cabotegravir, an HIV-1 integrase strand-transfer inhibitor, and rilpivirine, a nonnucleoside reverse-transcriptase inhibitor.
At week 48, an HIV-1 RNA level of 50 copies per milliliter or higher was found in 6 of 283 patients (2.1%) who received the long-acting therapy and in 7 of 283 (2.5%) who received oral therapy, which met the criterion for noninferiority for the primary endpoint. An HIV-1 RNA level of less than 50 copies per milliliter at week 48 was found in 93.6% of patients who received long-acting therapy and in 93.3% who received oral therapy, which also met the criterion for noninferiority, according to the study published in the New England Journal of Medicine.
Injection site reactions were reported in 86% of the long-acting therapy patients, 4 of whom withdrew from the trial for injection-related reasons. Grade 3 or higher adverse events and events that met liver-related stopping criteria occurred in 11% and 2%, respectively, of those who received long-acting therapy and in 4% and 1% of those who received oral therapy.
An assessment of treatment satisfaction at 48 weeks showed that 91% of the patients who switched to long-acting therapy preferred it to their daily oral therapy.
The ATLAS trial (ClinicalTrials.gov number, NCT02951052) was a phase 3, open-label, multicenter, noninferiority trial involving patients who had plasma HIV-1 RNA levels of less than 50 copies per milliliter for at least 6 months while taking standard oral antiretroviral therapy. These patients were randomized (308 in each group) to the long-acting cabotegravir plus rilpivirine injection therapy or daily oral therapy.
At 48 weeks, HIV-1 RNA levels of 50 copies per milliliter or higher were found in five participants (1.6%) receiving long-acting therapy and in three (1.0%) receiving oral therapy, which met the criterion for noninferiority for the primary endpoint, according to a study reported in the New England Journal of Medicine.
HIV-1 RNA levels of less than 50 copies per milliliter at week 48 occurred in 92.5% of patients on long-acting therapy and in 95.5% of those receiving oral therapy, which also met the criterion for noninferiority for this endpoint. Three patients in the long-acting therapy group had virologic failure, compared with four participants who received oral therapy.
Adverse events were more common in the long-acting–therapy group and included injection-site pain, which occurred in 231 recipients (75%) of long-acting therapy. This was mild or moderate in most cases, according to the authors. However, 1% of the participants in this group withdrew because of it. Overall, serious adverse events were reported in no more than 5% of participants in each group.
Together, the ATLAS and the FLAIR trials show that long-acting intramuscular injection therapy is noninferior to oral therapy as both an early regimen for HIV treatment, as well as for later, maintenance dosing. The use of long-acting therapy may improve patient adherence to treatment, according to both sets of study authors.
The ATLAS and FLAIR trials were funded by ViiV Healthcare and Janssen. The authors of both studies reported ties to pharmaceutical associations, and some authors are employees of the two funding sources.
SOURCE: Orkin C et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512 and Swindells S et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
Two international phase 3 randomized trials of according to reports published in the New England Journal of Medicine.
The Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection (FLAIR) study and the Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression (ATLAS) study looked at a separate facet of the use of a monthly therapeutic injection as a replacement for daily oral HIV therapy.
The FLAIR trial (ClinicalTrials.gov number, NCT02938520) was a phase 3, randomized, open-label study in which adults with HIV-1 infection who had not previously received antiretroviral therapy were given 20 weeks of daily oral induction therapy with dolutegravir–abacavir–lamivudine. Those patients with an HIV-1 RNA level of less than 50 copies per milliliter after 16 weeks were then randomly assigned (1:1) to continue their current oral therapy or switch to oral cabotegravir plus rilpivirine for 1 month followed by monthly intramuscular injections of long-acting cabotegravir, an HIV-1 integrase strand-transfer inhibitor, and rilpivirine, a nonnucleoside reverse-transcriptase inhibitor.
At week 48, an HIV-1 RNA level of 50 copies per milliliter or higher was found in 6 of 283 patients (2.1%) who received the long-acting therapy and in 7 of 283 (2.5%) who received oral therapy, which met the criterion for noninferiority for the primary endpoint. An HIV-1 RNA level of less than 50 copies per milliliter at week 48 was found in 93.6% of patients who received long-acting therapy and in 93.3% who received oral therapy, which also met the criterion for noninferiority, according to the study published in the New England Journal of Medicine.
Injection site reactions were reported in 86% of the long-acting therapy patients, 4 of whom withdrew from the trial for injection-related reasons. Grade 3 or higher adverse events and events that met liver-related stopping criteria occurred in 11% and 2%, respectively, of those who received long-acting therapy and in 4% and 1% of those who received oral therapy.
An assessment of treatment satisfaction at 48 weeks showed that 91% of the patients who switched to long-acting therapy preferred it to their daily oral therapy.
The ATLAS trial (ClinicalTrials.gov number, NCT02951052) was a phase 3, open-label, multicenter, noninferiority trial involving patients who had plasma HIV-1 RNA levels of less than 50 copies per milliliter for at least 6 months while taking standard oral antiretroviral therapy. These patients were randomized (308 in each group) to the long-acting cabotegravir plus rilpivirine injection therapy or daily oral therapy.
At 48 weeks, HIV-1 RNA levels of 50 copies per milliliter or higher were found in five participants (1.6%) receiving long-acting therapy and in three (1.0%) receiving oral therapy, which met the criterion for noninferiority for the primary endpoint, according to a study reported in the New England Journal of Medicine.
HIV-1 RNA levels of less than 50 copies per milliliter at week 48 occurred in 92.5% of patients on long-acting therapy and in 95.5% of those receiving oral therapy, which also met the criterion for noninferiority for this endpoint. Three patients in the long-acting therapy group had virologic failure, compared with four participants who received oral therapy.
Adverse events were more common in the long-acting–therapy group and included injection-site pain, which occurred in 231 recipients (75%) of long-acting therapy. This was mild or moderate in most cases, according to the authors. However, 1% of the participants in this group withdrew because of it. Overall, serious adverse events were reported in no more than 5% of participants in each group.
Together, the ATLAS and the FLAIR trials show that long-acting intramuscular injection therapy is noninferior to oral therapy as both an early regimen for HIV treatment, as well as for later, maintenance dosing. The use of long-acting therapy may improve patient adherence to treatment, according to both sets of study authors.
The ATLAS and FLAIR trials were funded by ViiV Healthcare and Janssen. The authors of both studies reported ties to pharmaceutical associations, and some authors are employees of the two funding sources.
SOURCE: Orkin C et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512 and Swindells S et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
Two international phase 3 randomized trials of according to reports published in the New England Journal of Medicine.
The Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection (FLAIR) study and the Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression (ATLAS) study looked at a separate facet of the use of a monthly therapeutic injection as a replacement for daily oral HIV therapy.
The FLAIR trial (ClinicalTrials.gov number, NCT02938520) was a phase 3, randomized, open-label study in which adults with HIV-1 infection who had not previously received antiretroviral therapy were given 20 weeks of daily oral induction therapy with dolutegravir–abacavir–lamivudine. Those patients with an HIV-1 RNA level of less than 50 copies per milliliter after 16 weeks were then randomly assigned (1:1) to continue their current oral therapy or switch to oral cabotegravir plus rilpivirine for 1 month followed by monthly intramuscular injections of long-acting cabotegravir, an HIV-1 integrase strand-transfer inhibitor, and rilpivirine, a nonnucleoside reverse-transcriptase inhibitor.
At week 48, an HIV-1 RNA level of 50 copies per milliliter or higher was found in 6 of 283 patients (2.1%) who received the long-acting therapy and in 7 of 283 (2.5%) who received oral therapy, which met the criterion for noninferiority for the primary endpoint. An HIV-1 RNA level of less than 50 copies per milliliter at week 48 was found in 93.6% of patients who received long-acting therapy and in 93.3% who received oral therapy, which also met the criterion for noninferiority, according to the study published in the New England Journal of Medicine.
Injection site reactions were reported in 86% of the long-acting therapy patients, 4 of whom withdrew from the trial for injection-related reasons. Grade 3 or higher adverse events and events that met liver-related stopping criteria occurred in 11% and 2%, respectively, of those who received long-acting therapy and in 4% and 1% of those who received oral therapy.
An assessment of treatment satisfaction at 48 weeks showed that 91% of the patients who switched to long-acting therapy preferred it to their daily oral therapy.
The ATLAS trial (ClinicalTrials.gov number, NCT02951052) was a phase 3, open-label, multicenter, noninferiority trial involving patients who had plasma HIV-1 RNA levels of less than 50 copies per milliliter for at least 6 months while taking standard oral antiretroviral therapy. These patients were randomized (308 in each group) to the long-acting cabotegravir plus rilpivirine injection therapy or daily oral therapy.
At 48 weeks, HIV-1 RNA levels of 50 copies per milliliter or higher were found in five participants (1.6%) receiving long-acting therapy and in three (1.0%) receiving oral therapy, which met the criterion for noninferiority for the primary endpoint, according to a study reported in the New England Journal of Medicine.
HIV-1 RNA levels of less than 50 copies per milliliter at week 48 occurred in 92.5% of patients on long-acting therapy and in 95.5% of those receiving oral therapy, which also met the criterion for noninferiority for this endpoint. Three patients in the long-acting therapy group had virologic failure, compared with four participants who received oral therapy.
Adverse events were more common in the long-acting–therapy group and included injection-site pain, which occurred in 231 recipients (75%) of long-acting therapy. This was mild or moderate in most cases, according to the authors. However, 1% of the participants in this group withdrew because of it. Overall, serious adverse events were reported in no more than 5% of participants in each group.
Together, the ATLAS and the FLAIR trials show that long-acting intramuscular injection therapy is noninferior to oral therapy as both an early regimen for HIV treatment, as well as for later, maintenance dosing. The use of long-acting therapy may improve patient adherence to treatment, according to both sets of study authors.
The ATLAS and FLAIR trials were funded by ViiV Healthcare and Janssen. The authors of both studies reported ties to pharmaceutical associations, and some authors are employees of the two funding sources.
SOURCE: Orkin C et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512 and Swindells S et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
RT plus checkpoint blockade active in head and neck cancer
The combination of a phase 2 trial suggests.
“There are convincing arguments that radiation sensitizes patients to immunotherapy and can enhance its effects,” Jared Weiss, MD, associate professor of medicine, UNC Lineberger Comprehensive Cancer, Chapel Hill, North Carolina, said in a statement.
“And the opposite direction also seems to be true – radiation therapy needs a functional immune system to work. Our hope was that pembrolizumab might be a radiation sensitizer for these patients,” he said.
The study was presented at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Both modalities have had some outstanding results in the past, observed Weiss. “If you look back to the historic studies, radiation alone often cures patients with this disease, while some of the first patients treated with pembrolizumab for recurrent/metastatic cancer are still alive many years out, with no evidence of disease,” he said.
“Our concept was that, in addition to whatever synergy the immunotherapy might provide with radiation, we also conceived of it as a ‘second shot on goal’ towards a cure, because there is durable control with drug alone,” he added.
Single-arm trial
The single-arm trial included 29 patients with locally advanced HNSCC.
Only about 10% of patients were current smokers, but more than half of the study group had a history of smoking. Of those, more than 55% had a history of 10 pack-years or more.
In slightly more than one third of patients, the primary site of the cancer was the base of the tongue. The tonsils were the primary site in slightly more than one third.
Platinum ineligibility was defined by provider and standard measures.
More than two thirds of patients were ineligible to receive cisplatin because of preexisting otopathy, including hearing impairment and tinnitus.
The combination of cisplatin and definitive-dose radiotherapy is standard treatment for locally advanced head and neck cancer, but contraindications to cisplatin are common in everyday clinical practice. Weiss noted that contraindications are present in about one third of his patients.
“We replaced standard, every-3-week cisplatin with pembrolizumab every 3 weeks,” Weiss explained, “and we hypothesized that with the ongoing effects of radiation therapy after completion, that additional adjuvant cycles could further sensitize patients [to the effects of radiation] without impairing recovery, so we added three adjuvant cycles as well,” he added.
With six cycles of an every-3-week drug, patients received 18 weeks of pembrolizumab in total.
Echoing results from the previously reported KEYNOTE-48 trial, pembrolizumab given with radiotherapy instead of chemotherapy led to an overall progression-free survival (PFS) rate of 76% at 1 year and an estimated PFS of 71% at 2 years.
At 1 year, 86% of patients were still alive, and at 2 years, an estimated 75% of patients were still alive, Weiss added.
For patients with human papillomavirus 16–positive cancer, rates of PFS and overall survival were slightly better, at 88% and 94%, respectively.
With regard to toxicities, “For the most part, this [treatment regimen] looks like radiation alone with one very notable exception, which was lymphopenia,” Weiss observed. Grade 3-4 lymphopenia affected 59% of patients.
Lymphocyte count hit bottom at week 4, he added, with only partial recovery at week 20 and no further recovery at 40 weeks. Lymphocyte count alone or any change in it was not predictive of early progression.
However, in comparing patients who experienced early disease progression to patients who did not experience progression, levels of baseline naive B cells in peripheral blood were higher and levels of circulating marginal zone B cells were lower in patients with progressive disease, Weiss reported.
Patient-reported outcomes indicated that common symptoms of treatment peaked at week 10, and there was relative recovery by week 20.
As reflected by Functional Assessment of Cancer Therapy (FACT) scores, which include social, emotional, and functional well-being, as well as the head and neck cancer scale, “we again see a nadir at 10 weeks with relative recovery at 20 weeks,” Weiss noted.
“We found that concurrent pembrolizumab with radiotherapy is a safe and feasible option for locally advanced head and neck cancer patients with cisplatin ineligibility,” Weiss concluded.
More research is being conducted in this area, and multiple ongoing studies will further elucidate the value of PD-1 or PD-L1 checkpoint blockade with definitive radiation therapy, he added.
The study was funded by Merck & Co. Weiss’ institution has received research funding from Celgene, Pfizer, Merck, AZ/Medimmmune, Amgen, Carefusion, G1 Therapeutics, Immunicum, Loxo/Lilly, and the Jimmy V Foundation. Weiss has received honoraria for consulting from AstraZeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Loxo/Lilly, Pfizer, and Blueprint had has stock in Nektar and Vesselon.
This article first appeared on Medscape.com.
The combination of a phase 2 trial suggests.
“There are convincing arguments that radiation sensitizes patients to immunotherapy and can enhance its effects,” Jared Weiss, MD, associate professor of medicine, UNC Lineberger Comprehensive Cancer, Chapel Hill, North Carolina, said in a statement.
“And the opposite direction also seems to be true – radiation therapy needs a functional immune system to work. Our hope was that pembrolizumab might be a radiation sensitizer for these patients,” he said.
The study was presented at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Both modalities have had some outstanding results in the past, observed Weiss. “If you look back to the historic studies, radiation alone often cures patients with this disease, while some of the first patients treated with pembrolizumab for recurrent/metastatic cancer are still alive many years out, with no evidence of disease,” he said.
“Our concept was that, in addition to whatever synergy the immunotherapy might provide with radiation, we also conceived of it as a ‘second shot on goal’ towards a cure, because there is durable control with drug alone,” he added.
Single-arm trial
The single-arm trial included 29 patients with locally advanced HNSCC.
Only about 10% of patients were current smokers, but more than half of the study group had a history of smoking. Of those, more than 55% had a history of 10 pack-years or more.
In slightly more than one third of patients, the primary site of the cancer was the base of the tongue. The tonsils were the primary site in slightly more than one third.
Platinum ineligibility was defined by provider and standard measures.
More than two thirds of patients were ineligible to receive cisplatin because of preexisting otopathy, including hearing impairment and tinnitus.
The combination of cisplatin and definitive-dose radiotherapy is standard treatment for locally advanced head and neck cancer, but contraindications to cisplatin are common in everyday clinical practice. Weiss noted that contraindications are present in about one third of his patients.
“We replaced standard, every-3-week cisplatin with pembrolizumab every 3 weeks,” Weiss explained, “and we hypothesized that with the ongoing effects of radiation therapy after completion, that additional adjuvant cycles could further sensitize patients [to the effects of radiation] without impairing recovery, so we added three adjuvant cycles as well,” he added.
With six cycles of an every-3-week drug, patients received 18 weeks of pembrolizumab in total.
Echoing results from the previously reported KEYNOTE-48 trial, pembrolizumab given with radiotherapy instead of chemotherapy led to an overall progression-free survival (PFS) rate of 76% at 1 year and an estimated PFS of 71% at 2 years.
At 1 year, 86% of patients were still alive, and at 2 years, an estimated 75% of patients were still alive, Weiss added.
For patients with human papillomavirus 16–positive cancer, rates of PFS and overall survival were slightly better, at 88% and 94%, respectively.
With regard to toxicities, “For the most part, this [treatment regimen] looks like radiation alone with one very notable exception, which was lymphopenia,” Weiss observed. Grade 3-4 lymphopenia affected 59% of patients.
Lymphocyte count hit bottom at week 4, he added, with only partial recovery at week 20 and no further recovery at 40 weeks. Lymphocyte count alone or any change in it was not predictive of early progression.
However, in comparing patients who experienced early disease progression to patients who did not experience progression, levels of baseline naive B cells in peripheral blood were higher and levels of circulating marginal zone B cells were lower in patients with progressive disease, Weiss reported.
Patient-reported outcomes indicated that common symptoms of treatment peaked at week 10, and there was relative recovery by week 20.
As reflected by Functional Assessment of Cancer Therapy (FACT) scores, which include social, emotional, and functional well-being, as well as the head and neck cancer scale, “we again see a nadir at 10 weeks with relative recovery at 20 weeks,” Weiss noted.
“We found that concurrent pembrolizumab with radiotherapy is a safe and feasible option for locally advanced head and neck cancer patients with cisplatin ineligibility,” Weiss concluded.
More research is being conducted in this area, and multiple ongoing studies will further elucidate the value of PD-1 or PD-L1 checkpoint blockade with definitive radiation therapy, he added.
The study was funded by Merck & Co. Weiss’ institution has received research funding from Celgene, Pfizer, Merck, AZ/Medimmmune, Amgen, Carefusion, G1 Therapeutics, Immunicum, Loxo/Lilly, and the Jimmy V Foundation. Weiss has received honoraria for consulting from AstraZeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Loxo/Lilly, Pfizer, and Blueprint had has stock in Nektar and Vesselon.
This article first appeared on Medscape.com.
The combination of a phase 2 trial suggests.
“There are convincing arguments that radiation sensitizes patients to immunotherapy and can enhance its effects,” Jared Weiss, MD, associate professor of medicine, UNC Lineberger Comprehensive Cancer, Chapel Hill, North Carolina, said in a statement.
“And the opposite direction also seems to be true – radiation therapy needs a functional immune system to work. Our hope was that pembrolizumab might be a radiation sensitizer for these patients,” he said.
The study was presented at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Both modalities have had some outstanding results in the past, observed Weiss. “If you look back to the historic studies, radiation alone often cures patients with this disease, while some of the first patients treated with pembrolizumab for recurrent/metastatic cancer are still alive many years out, with no evidence of disease,” he said.
“Our concept was that, in addition to whatever synergy the immunotherapy might provide with radiation, we also conceived of it as a ‘second shot on goal’ towards a cure, because there is durable control with drug alone,” he added.
Single-arm trial
The single-arm trial included 29 patients with locally advanced HNSCC.
Only about 10% of patients were current smokers, but more than half of the study group had a history of smoking. Of those, more than 55% had a history of 10 pack-years or more.
In slightly more than one third of patients, the primary site of the cancer was the base of the tongue. The tonsils were the primary site in slightly more than one third.
Platinum ineligibility was defined by provider and standard measures.
More than two thirds of patients were ineligible to receive cisplatin because of preexisting otopathy, including hearing impairment and tinnitus.
The combination of cisplatin and definitive-dose radiotherapy is standard treatment for locally advanced head and neck cancer, but contraindications to cisplatin are common in everyday clinical practice. Weiss noted that contraindications are present in about one third of his patients.
“We replaced standard, every-3-week cisplatin with pembrolizumab every 3 weeks,” Weiss explained, “and we hypothesized that with the ongoing effects of radiation therapy after completion, that additional adjuvant cycles could further sensitize patients [to the effects of radiation] without impairing recovery, so we added three adjuvant cycles as well,” he added.
With six cycles of an every-3-week drug, patients received 18 weeks of pembrolizumab in total.
Echoing results from the previously reported KEYNOTE-48 trial, pembrolizumab given with radiotherapy instead of chemotherapy led to an overall progression-free survival (PFS) rate of 76% at 1 year and an estimated PFS of 71% at 2 years.
At 1 year, 86% of patients were still alive, and at 2 years, an estimated 75% of patients were still alive, Weiss added.
For patients with human papillomavirus 16–positive cancer, rates of PFS and overall survival were slightly better, at 88% and 94%, respectively.
With regard to toxicities, “For the most part, this [treatment regimen] looks like radiation alone with one very notable exception, which was lymphopenia,” Weiss observed. Grade 3-4 lymphopenia affected 59% of patients.
Lymphocyte count hit bottom at week 4, he added, with only partial recovery at week 20 and no further recovery at 40 weeks. Lymphocyte count alone or any change in it was not predictive of early progression.
However, in comparing patients who experienced early disease progression to patients who did not experience progression, levels of baseline naive B cells in peripheral blood were higher and levels of circulating marginal zone B cells were lower in patients with progressive disease, Weiss reported.
Patient-reported outcomes indicated that common symptoms of treatment peaked at week 10, and there was relative recovery by week 20.
As reflected by Functional Assessment of Cancer Therapy (FACT) scores, which include social, emotional, and functional well-being, as well as the head and neck cancer scale, “we again see a nadir at 10 weeks with relative recovery at 20 weeks,” Weiss noted.
“We found that concurrent pembrolizumab with radiotherapy is a safe and feasible option for locally advanced head and neck cancer patients with cisplatin ineligibility,” Weiss concluded.
More research is being conducted in this area, and multiple ongoing studies will further elucidate the value of PD-1 or PD-L1 checkpoint blockade with definitive radiation therapy, he added.
The study was funded by Merck & Co. Weiss’ institution has received research funding from Celgene, Pfizer, Merck, AZ/Medimmmune, Amgen, Carefusion, G1 Therapeutics, Immunicum, Loxo/Lilly, and the Jimmy V Foundation. Weiss has received honoraria for consulting from AstraZeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Loxo/Lilly, Pfizer, and Blueprint had has stock in Nektar and Vesselon.
This article first appeared on Medscape.com.
REPORTING FROM HEAD AND NECK CANCERS SYMPOSIUM 2020
MACE benefits with dapagliflozin improve with disease duration
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
FROM DIABETES, OBESITY AND METABOLISM
Pembro ups survival in NSCLC: ‘Really extraordinary’ results
More than a third (35%) of patients with relapsed non–small cell lung cancer (NSCLC) treated with pembrolizumab (Keytruda, Merck) were still alive at 3 years, according to long-term results from a pivotal clinical trial.
The results also showed that, among the 10% of patients who completed all 35 cycles of pembrolizumab, the 3-year overall survival was approximately 99%, with progression-free survival (PFS) at around 70%.
“It is too soon to say that pembrolizumab is a potential cure...and we know that it doesn’t work for all patients, but the agent remains very, very promising,” said lead investigator Roy Herbst, MD, PhD, Department of Medical Oncology, Yale Comprehensive Cancer Center, New Haven, Connecticut.
These new results come from the KEYNOTE-010 trial, conducted in more than 1000 patients with NSCLC who had progressed on chemotherapy, randomized to receive immunotherapy with pembrolizumab or chemotherapy with docetaxel.
The results were published online on February 20 in the Journal of Clinical Oncology and were previously presented at the 2018 annual meeting of the European Society of Medical Oncology.
Overall survival at 3 years was 35% in patients with PD-L1 expression ≥ 50% in the tumor, and 23% in those with PD-L1 ≥ 1%.
This compares with 3-year overall survival of 11-13% with docetaxel.
These results are “really extraordinary,” Herbst commented to Medscape Medical News.
The 3-year overall survival rate of 35% in patients with PD-L1 ≥ 50% “is huge,” he said. “It really shows the durability of the response.”
Herbst commented that the “almost 100%” survival at 3 years among patients who completed 35 cycles of pembrolizumab shows that this treatment period (of about 2 years) is “probably about the right time to treat.”
“Currently, the agent is being used in all potential settings, before any other treatment, after other treatment, and with other treatments,” he said.
“Our hope is to find the very best way to use pembrolizumab to treat individual lung cancer patients, assessing how much PD-L1 a tumor expresses, what stage the patient is in, as well as other variables and biomarkers we are working on. This is the story of tailored therapy,” Herbst said.
Approached for comment, Solange Peters, MD, PhD, Oncology Department, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said that the results are “very good” and “confirm the paradigms we have been seeing in melanoma,” with good long-term control, which is “very reassuring.”
However, she told Medscape Medical News that the trial raises an important question: «How long do you need to expose your patient with lung cancer to immunotherapy in order to get this long-term control?»
She said the “good news” is that, for the 10% of patients who completed 2 years of treatment per protocol, almost all of them are still alive at 3 years, “which is not observed with chemotherapy.”
The question for Peters is “more about the definition of long-term control,” as it was seen that almost one in three patients nevertheless had some form of progression.
This suggests that you have a group of people “who are nicely controlled, you stop the drug, and 1 year later a third of them have progressed.”
Peters said: “So how long do you need to treat these patients? I would say I still don’t know.”
“If I were one of these patients probably I would still want to continue [on the drug]. Of course, some might have progressed even while remaining on the drug, but the proportion who would have progressed is probably smaller than this one.”
Responses on Re-introduction of Therapy
The study also allowed patients who had completed 35 cycles of pembrolizumab to be restarted on the drug if they experienced progression.
The team found that, among 14 patients, 43% had a partial response and 36% had stable disease.
Herbst highlighted this finding and told Medscape Medical News that this «could be very important to physicians because they might want to think about using the drug again» in patients who have progressed on it.
He believes that the progression was not because of any resistance per se but rather a slowing down of the adaptive immune response.
“It’s just that it needs a boost,” he said, while noting that tissue specimens will nevertheless be required to demonstrate the theory.
Peters agreed that these results are “very promising,” but questioned their overall significance, as it is “a very small number of patients” from a subset whose disease was controlled while on treatment and then progressed after stopping.
She also pointed out that, in another study in patients with lung cancer (CheckMate-153), some patients were rechallenged with immunotherapy after having stopped treatment at 1 year “with very poor results.”
Peters said studies in melanoma have shown “rechallenge can be useful in a significant proportion of patients, but still you have not demonstrated that stopping and rechallenging is the same as not stopping.”
Study Details
KEYNOTE-010 involved patients with NSCLC from 202 centers in 24 countries with stage IIIB/IV disease expressing PD-L1 who had experienced disease progression after at least two cycles of platinum-based chemotherapy.
They were randomized 1:1:1 to open-label pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks.
Pembrolizumab was continued for 35 treatment cycles over 2 years and docetaxel was continued for the maximum duration allowed by local regulators.
Patients who stopped pembrolizumab after a complete response or completing all 35 cycles, and who subsequently experienced disease progression, could receive up to 17 additional cycles over 1 year if they had not received another anticancer therapy in the meantime.
Among the 1,034 patients originally recruited between August 2013 and February 2015, 691 were assigned to pembrolizumab at 3 mg/kg or 10 mg/kg and 343 to docetaxel.
For the intention-to-treat analysis in 1033 patients, the mean duration of follow-up was 42.6 months, with a median treatment duration of 3.5 months in the pembrolizumab group and 2.0 months in the docetaxel group.
Compared with docetaxel, pembrolizumab was associated with a significant reduction in the risk of death, at a hazard ratio of 0.53 in patients with PD-L1 ≥ 50% and 0.69 in those with PD-L1 ≥ 1% (both P < .0001).
In patients with PD-L1 ≥ 50%, median overall survival was 16.9 months in those given pembrolizumab and 8.2 months with docetaxel. Among those with PD-L1 ≥ 1%, median overall survival was 11.8 months with pembrolizumab versus 8.4 months with docetaxel.
Overall survival on Kaplan-Meier analysis was 34.5% with pembrolizumab and 12.7% with docetaxel in the PD-L1 ≥ 50% group, and 22.9% versus 11.0% in the PD-L1 ≥ 1% group.
PFS significantly improved with pembrolizumab versus docetaxel, at a hazard ratio of 0.57 (P < .00001) among patients with PD-L1 ≥ 50% and 0.83 (P < .005) in those with PD-L1 ≥ 1%.
In terms of safety, 17.7% of patients who completed 2 years of pembrolizumab had grade 3-5 treatment-related adverse events, compared with 16.6% among all pembrolizumab-treated patients and 36.6% of those given docetaxel.
The team reports that 79 patients completed 35 cycles of pembrolizumab, with a median follow-up of 43.4 months.
Compared with the overall patient group, these patients were less likely to be aged ≥ 65 years and to have received two or more prior treatment lines, although they were more likely to be current or former smokers and to have squamous tumor histology.
Patients who completed 35 cycles had an objective response rate of 94.9%, and 91.0% were still alive at the data cutoff. Overall survival rates were 98.7% at 12 months and 86.3% at 24 months.
Of 71 patients eligible for analysis, 23 experienced progression after completing pembrolizumab, at PFS rates at 12 and 24 months of 72.5% and 57.7%, respectively.
A total of 14 patients were given a second course of pembrolizumab, of whom six had a partial response and five had stable disease. At the data cutoff, five patients had completed 17 additional cycles and 11 were alive.
Pembro Approved at Fixed Dose
One notable aspect of the study is that patients in the pembrolizumab arm were given two different doses of the drug based on body weight, whereas the drug is approved in the United States at a fixed dose of 200 mg.
Herbst told Medscape Medical News he considers the 200-mg dose to be appropriate.
“I didn’t think that the 3-mg versus 10-mg dose per kg that we used in our study made much difference in an average-sized person,” he said, adding that the 200-mg dose “is something a little bit more than 3 mg/kg.”
“So I think that this is clearly the right dos, and I don’t think more would make any difference,” he said.
The study was funded by Merck, the manufacturer of pembrolizumab. Herbst has reported having a consulting or advisory role for many pharmaceutical companies. Other coauthors have also reported relationships with industry, and some of the authors are Merck employees. Peters has reported receiving education grants, providing consultation, attending advisory boards, and/or providing lectures for many pharmaceutical companies.
This article first appeared on Medscape.com.
More than a third (35%) of patients with relapsed non–small cell lung cancer (NSCLC) treated with pembrolizumab (Keytruda, Merck) were still alive at 3 years, according to long-term results from a pivotal clinical trial.
The results also showed that, among the 10% of patients who completed all 35 cycles of pembrolizumab, the 3-year overall survival was approximately 99%, with progression-free survival (PFS) at around 70%.
“It is too soon to say that pembrolizumab is a potential cure...and we know that it doesn’t work for all patients, but the agent remains very, very promising,” said lead investigator Roy Herbst, MD, PhD, Department of Medical Oncology, Yale Comprehensive Cancer Center, New Haven, Connecticut.
These new results come from the KEYNOTE-010 trial, conducted in more than 1000 patients with NSCLC who had progressed on chemotherapy, randomized to receive immunotherapy with pembrolizumab or chemotherapy with docetaxel.
The results were published online on February 20 in the Journal of Clinical Oncology and were previously presented at the 2018 annual meeting of the European Society of Medical Oncology.
Overall survival at 3 years was 35% in patients with PD-L1 expression ≥ 50% in the tumor, and 23% in those with PD-L1 ≥ 1%.
This compares with 3-year overall survival of 11-13% with docetaxel.
These results are “really extraordinary,” Herbst commented to Medscape Medical News.
The 3-year overall survival rate of 35% in patients with PD-L1 ≥ 50% “is huge,” he said. “It really shows the durability of the response.”
Herbst commented that the “almost 100%” survival at 3 years among patients who completed 35 cycles of pembrolizumab shows that this treatment period (of about 2 years) is “probably about the right time to treat.”
“Currently, the agent is being used in all potential settings, before any other treatment, after other treatment, and with other treatments,” he said.
“Our hope is to find the very best way to use pembrolizumab to treat individual lung cancer patients, assessing how much PD-L1 a tumor expresses, what stage the patient is in, as well as other variables and biomarkers we are working on. This is the story of tailored therapy,” Herbst said.
Approached for comment, Solange Peters, MD, PhD, Oncology Department, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said that the results are “very good” and “confirm the paradigms we have been seeing in melanoma,” with good long-term control, which is “very reassuring.”
However, she told Medscape Medical News that the trial raises an important question: «How long do you need to expose your patient with lung cancer to immunotherapy in order to get this long-term control?»
She said the “good news” is that, for the 10% of patients who completed 2 years of treatment per protocol, almost all of them are still alive at 3 years, “which is not observed with chemotherapy.”
The question for Peters is “more about the definition of long-term control,” as it was seen that almost one in three patients nevertheless had some form of progression.
This suggests that you have a group of people “who are nicely controlled, you stop the drug, and 1 year later a third of them have progressed.”
Peters said: “So how long do you need to treat these patients? I would say I still don’t know.”
“If I were one of these patients probably I would still want to continue [on the drug]. Of course, some might have progressed even while remaining on the drug, but the proportion who would have progressed is probably smaller than this one.”
Responses on Re-introduction of Therapy
The study also allowed patients who had completed 35 cycles of pembrolizumab to be restarted on the drug if they experienced progression.
The team found that, among 14 patients, 43% had a partial response and 36% had stable disease.
Herbst highlighted this finding and told Medscape Medical News that this «could be very important to physicians because they might want to think about using the drug again» in patients who have progressed on it.
He believes that the progression was not because of any resistance per se but rather a slowing down of the adaptive immune response.
“It’s just that it needs a boost,” he said, while noting that tissue specimens will nevertheless be required to demonstrate the theory.
Peters agreed that these results are “very promising,” but questioned their overall significance, as it is “a very small number of patients” from a subset whose disease was controlled while on treatment and then progressed after stopping.
She also pointed out that, in another study in patients with lung cancer (CheckMate-153), some patients were rechallenged with immunotherapy after having stopped treatment at 1 year “with very poor results.”
Peters said studies in melanoma have shown “rechallenge can be useful in a significant proportion of patients, but still you have not demonstrated that stopping and rechallenging is the same as not stopping.”
Study Details
KEYNOTE-010 involved patients with NSCLC from 202 centers in 24 countries with stage IIIB/IV disease expressing PD-L1 who had experienced disease progression after at least two cycles of platinum-based chemotherapy.
They were randomized 1:1:1 to open-label pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks.
Pembrolizumab was continued for 35 treatment cycles over 2 years and docetaxel was continued for the maximum duration allowed by local regulators.
Patients who stopped pembrolizumab after a complete response or completing all 35 cycles, and who subsequently experienced disease progression, could receive up to 17 additional cycles over 1 year if they had not received another anticancer therapy in the meantime.
Among the 1,034 patients originally recruited between August 2013 and February 2015, 691 were assigned to pembrolizumab at 3 mg/kg or 10 mg/kg and 343 to docetaxel.
For the intention-to-treat analysis in 1033 patients, the mean duration of follow-up was 42.6 months, with a median treatment duration of 3.5 months in the pembrolizumab group and 2.0 months in the docetaxel group.
Compared with docetaxel, pembrolizumab was associated with a significant reduction in the risk of death, at a hazard ratio of 0.53 in patients with PD-L1 ≥ 50% and 0.69 in those with PD-L1 ≥ 1% (both P < .0001).
In patients with PD-L1 ≥ 50%, median overall survival was 16.9 months in those given pembrolizumab and 8.2 months with docetaxel. Among those with PD-L1 ≥ 1%, median overall survival was 11.8 months with pembrolizumab versus 8.4 months with docetaxel.
Overall survival on Kaplan-Meier analysis was 34.5% with pembrolizumab and 12.7% with docetaxel in the PD-L1 ≥ 50% group, and 22.9% versus 11.0% in the PD-L1 ≥ 1% group.
PFS significantly improved with pembrolizumab versus docetaxel, at a hazard ratio of 0.57 (P < .00001) among patients with PD-L1 ≥ 50% and 0.83 (P < .005) in those with PD-L1 ≥ 1%.
In terms of safety, 17.7% of patients who completed 2 years of pembrolizumab had grade 3-5 treatment-related adverse events, compared with 16.6% among all pembrolizumab-treated patients and 36.6% of those given docetaxel.
The team reports that 79 patients completed 35 cycles of pembrolizumab, with a median follow-up of 43.4 months.
Compared with the overall patient group, these patients were less likely to be aged ≥ 65 years and to have received two or more prior treatment lines, although they were more likely to be current or former smokers and to have squamous tumor histology.
Patients who completed 35 cycles had an objective response rate of 94.9%, and 91.0% were still alive at the data cutoff. Overall survival rates were 98.7% at 12 months and 86.3% at 24 months.
Of 71 patients eligible for analysis, 23 experienced progression after completing pembrolizumab, at PFS rates at 12 and 24 months of 72.5% and 57.7%, respectively.
A total of 14 patients were given a second course of pembrolizumab, of whom six had a partial response and five had stable disease. At the data cutoff, five patients had completed 17 additional cycles and 11 were alive.
Pembro Approved at Fixed Dose
One notable aspect of the study is that patients in the pembrolizumab arm were given two different doses of the drug based on body weight, whereas the drug is approved in the United States at a fixed dose of 200 mg.
Herbst told Medscape Medical News he considers the 200-mg dose to be appropriate.
“I didn’t think that the 3-mg versus 10-mg dose per kg that we used in our study made much difference in an average-sized person,” he said, adding that the 200-mg dose “is something a little bit more than 3 mg/kg.”
“So I think that this is clearly the right dos, and I don’t think more would make any difference,” he said.
The study was funded by Merck, the manufacturer of pembrolizumab. Herbst has reported having a consulting or advisory role for many pharmaceutical companies. Other coauthors have also reported relationships with industry, and some of the authors are Merck employees. Peters has reported receiving education grants, providing consultation, attending advisory boards, and/or providing lectures for many pharmaceutical companies.
This article first appeared on Medscape.com.
More than a third (35%) of patients with relapsed non–small cell lung cancer (NSCLC) treated with pembrolizumab (Keytruda, Merck) were still alive at 3 years, according to long-term results from a pivotal clinical trial.
The results also showed that, among the 10% of patients who completed all 35 cycles of pembrolizumab, the 3-year overall survival was approximately 99%, with progression-free survival (PFS) at around 70%.
“It is too soon to say that pembrolizumab is a potential cure...and we know that it doesn’t work for all patients, but the agent remains very, very promising,” said lead investigator Roy Herbst, MD, PhD, Department of Medical Oncology, Yale Comprehensive Cancer Center, New Haven, Connecticut.
These new results come from the KEYNOTE-010 trial, conducted in more than 1000 patients with NSCLC who had progressed on chemotherapy, randomized to receive immunotherapy with pembrolizumab or chemotherapy with docetaxel.
The results were published online on February 20 in the Journal of Clinical Oncology and were previously presented at the 2018 annual meeting of the European Society of Medical Oncology.
Overall survival at 3 years was 35% in patients with PD-L1 expression ≥ 50% in the tumor, and 23% in those with PD-L1 ≥ 1%.
This compares with 3-year overall survival of 11-13% with docetaxel.
These results are “really extraordinary,” Herbst commented to Medscape Medical News.
The 3-year overall survival rate of 35% in patients with PD-L1 ≥ 50% “is huge,” he said. “It really shows the durability of the response.”
Herbst commented that the “almost 100%” survival at 3 years among patients who completed 35 cycles of pembrolizumab shows that this treatment period (of about 2 years) is “probably about the right time to treat.”
“Currently, the agent is being used in all potential settings, before any other treatment, after other treatment, and with other treatments,” he said.
“Our hope is to find the very best way to use pembrolizumab to treat individual lung cancer patients, assessing how much PD-L1 a tumor expresses, what stage the patient is in, as well as other variables and biomarkers we are working on. This is the story of tailored therapy,” Herbst said.
Approached for comment, Solange Peters, MD, PhD, Oncology Department, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said that the results are “very good” and “confirm the paradigms we have been seeing in melanoma,” with good long-term control, which is “very reassuring.”
However, she told Medscape Medical News that the trial raises an important question: «How long do you need to expose your patient with lung cancer to immunotherapy in order to get this long-term control?»
She said the “good news” is that, for the 10% of patients who completed 2 years of treatment per protocol, almost all of them are still alive at 3 years, “which is not observed with chemotherapy.”
The question for Peters is “more about the definition of long-term control,” as it was seen that almost one in three patients nevertheless had some form of progression.
This suggests that you have a group of people “who are nicely controlled, you stop the drug, and 1 year later a third of them have progressed.”
Peters said: “So how long do you need to treat these patients? I would say I still don’t know.”
“If I were one of these patients probably I would still want to continue [on the drug]. Of course, some might have progressed even while remaining on the drug, but the proportion who would have progressed is probably smaller than this one.”
Responses on Re-introduction of Therapy
The study also allowed patients who had completed 35 cycles of pembrolizumab to be restarted on the drug if they experienced progression.
The team found that, among 14 patients, 43% had a partial response and 36% had stable disease.
Herbst highlighted this finding and told Medscape Medical News that this «could be very important to physicians because they might want to think about using the drug again» in patients who have progressed on it.
He believes that the progression was not because of any resistance per se but rather a slowing down of the adaptive immune response.
“It’s just that it needs a boost,” he said, while noting that tissue specimens will nevertheless be required to demonstrate the theory.
Peters agreed that these results are “very promising,” but questioned their overall significance, as it is “a very small number of patients” from a subset whose disease was controlled while on treatment and then progressed after stopping.
She also pointed out that, in another study in patients with lung cancer (CheckMate-153), some patients were rechallenged with immunotherapy after having stopped treatment at 1 year “with very poor results.”
Peters said studies in melanoma have shown “rechallenge can be useful in a significant proportion of patients, but still you have not demonstrated that stopping and rechallenging is the same as not stopping.”
Study Details
KEYNOTE-010 involved patients with NSCLC from 202 centers in 24 countries with stage IIIB/IV disease expressing PD-L1 who had experienced disease progression after at least two cycles of platinum-based chemotherapy.
They were randomized 1:1:1 to open-label pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks.
Pembrolizumab was continued for 35 treatment cycles over 2 years and docetaxel was continued for the maximum duration allowed by local regulators.
Patients who stopped pembrolizumab after a complete response or completing all 35 cycles, and who subsequently experienced disease progression, could receive up to 17 additional cycles over 1 year if they had not received another anticancer therapy in the meantime.
Among the 1,034 patients originally recruited between August 2013 and February 2015, 691 were assigned to pembrolizumab at 3 mg/kg or 10 mg/kg and 343 to docetaxel.
For the intention-to-treat analysis in 1033 patients, the mean duration of follow-up was 42.6 months, with a median treatment duration of 3.5 months in the pembrolizumab group and 2.0 months in the docetaxel group.
Compared with docetaxel, pembrolizumab was associated with a significant reduction in the risk of death, at a hazard ratio of 0.53 in patients with PD-L1 ≥ 50% and 0.69 in those with PD-L1 ≥ 1% (both P < .0001).
In patients with PD-L1 ≥ 50%, median overall survival was 16.9 months in those given pembrolizumab and 8.2 months with docetaxel. Among those with PD-L1 ≥ 1%, median overall survival was 11.8 months with pembrolizumab versus 8.4 months with docetaxel.
Overall survival on Kaplan-Meier analysis was 34.5% with pembrolizumab and 12.7% with docetaxel in the PD-L1 ≥ 50% group, and 22.9% versus 11.0% in the PD-L1 ≥ 1% group.
PFS significantly improved with pembrolizumab versus docetaxel, at a hazard ratio of 0.57 (P < .00001) among patients with PD-L1 ≥ 50% and 0.83 (P < .005) in those with PD-L1 ≥ 1%.
In terms of safety, 17.7% of patients who completed 2 years of pembrolizumab had grade 3-5 treatment-related adverse events, compared with 16.6% among all pembrolizumab-treated patients and 36.6% of those given docetaxel.
The team reports that 79 patients completed 35 cycles of pembrolizumab, with a median follow-up of 43.4 months.
Compared with the overall patient group, these patients were less likely to be aged ≥ 65 years and to have received two or more prior treatment lines, although they were more likely to be current or former smokers and to have squamous tumor histology.
Patients who completed 35 cycles had an objective response rate of 94.9%, and 91.0% were still alive at the data cutoff. Overall survival rates were 98.7% at 12 months and 86.3% at 24 months.
Of 71 patients eligible for analysis, 23 experienced progression after completing pembrolizumab, at PFS rates at 12 and 24 months of 72.5% and 57.7%, respectively.
A total of 14 patients were given a second course of pembrolizumab, of whom six had a partial response and five had stable disease. At the data cutoff, five patients had completed 17 additional cycles and 11 were alive.
Pembro Approved at Fixed Dose
One notable aspect of the study is that patients in the pembrolizumab arm were given two different doses of the drug based on body weight, whereas the drug is approved in the United States at a fixed dose of 200 mg.
Herbst told Medscape Medical News he considers the 200-mg dose to be appropriate.
“I didn’t think that the 3-mg versus 10-mg dose per kg that we used in our study made much difference in an average-sized person,” he said, adding that the 200-mg dose “is something a little bit more than 3 mg/kg.”
“So I think that this is clearly the right dos, and I don’t think more would make any difference,” he said.
The study was funded by Merck, the manufacturer of pembrolizumab. Herbst has reported having a consulting or advisory role for many pharmaceutical companies. Other coauthors have also reported relationships with industry, and some of the authors are Merck employees. Peters has reported receiving education grants, providing consultation, attending advisory boards, and/or providing lectures for many pharmaceutical companies.
This article first appeared on Medscape.com.
Prescription cascade more likely after CCBs than other hypertension meds
Elderly adults with hypertension who are newly prescribed a calcium-channel blocker (CCB), compared to other antihypertensive agents, are at least twice as likely to be given a loop diuretic over the following months, a large cohort study suggests.
The likelihood remained elevated for as long as a year after the start of a CCB and was more pronounced when comparing CCBs to any other kind of medication.
“Our findings suggest that many older adults who begin taking a CCB may subsequently experience a prescribing cascade” when loop diuretics are prescribed for peripheral edema, a known CCB adverse effect, that is misinterpreted as a new medical condition, Rachel D. Savage, PhD, Women’s College Hospital, Toronto, Canada, told theheart.org/Medscape Cardiology.
Edema caused by CCBs is caused by fluid redistribution, not overload, and “treating euvolemic individuals with a diuretic places them at increased risk of overdiuresis, leading to falls, urinary incontinence, acute kidney injury, electrolyte imbalances, and a cascade of other downstream consequences to which older adults are especially vulnerable,” explain Savage and coauthors of the analysis published online February 24 in JAMA Internal Medicine.
However, 1.4% of the cohort had been prescribed a loop diuretic, and 4.5% had been given any diuretic within 90 days after the start of CCBs. The corresponding rates were 0.7% and 3.4%, respectively, for patients who had started on ACE inhibitors or angiotensin receptor blocker (ARB) rather than a CCB.
Also, Savage observed, “the likelihood of being prescribed a loop diuretic following initiation of a CCB changed over time and was greatest 61 to 90 days postinitiation.” At that point, it was increased 2.4 times compared with initiation of an ACE inhibitor or an ARB in an adjusted analysis and increased almost 4 times compared with starting on any non-CCB agent.
Importantly, the actual prevalence of peripheral edema among those started on CCBs, ACE inhibitors, ARBs, or any non-CCB medication was not available in the data sets.
However, “the main message for clinicians is to consider medication side effects as a potential cause for new symptoms when patients present. We also encourage patients to ask prescribers about whether new symptoms could be caused by a medication,” senior author Lisa M. McCarthy, PharmD, told theheart.org/Medscape Cardiology.
“If a patient experiences peripheral edema while taking a CCB, we would encourage clinicians to consider whether the calcium-channel blocker is still necessary, whether it could be discontinued or the dose reduced, or whether the patient can be switched to another therapy,” she said.
Based on the current analysis, if the rate of CCB-induced peripheral edema is assumed to be 10%, which is consistent with the literature, then “potentially 7% to 14% of people who develop edema while taking a calcium channel blocker may then receive a loop diuretic,” an accompanying editorial notes.
“Patients with polypharmacy are at heightened risk of being exposed to [a] series of prescribing cascades if their current use of medications is not carefully discussed before the decision to add a new antihypertensive,” observe Timothy S. Anderson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, and Michael A. Steinman, MD, San Francisco Veterans Affairs Medical Center and University of California, San Francisco.
“The initial prescribing cascade can set off many other negative consequences, including adverse drug events, potentially avoidable diagnostic testing, and hospitalizations,” the editorialists caution.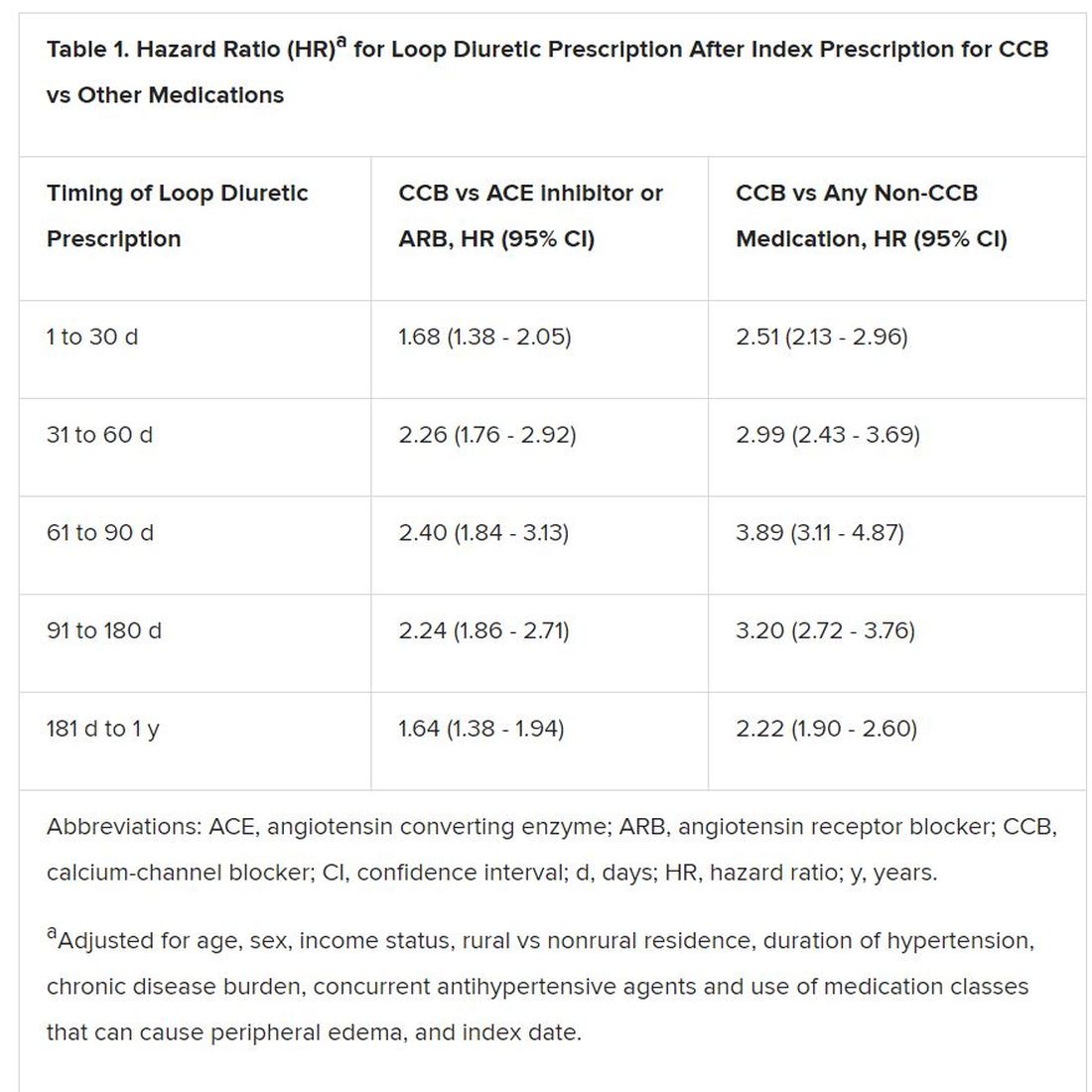
“Identifying prescribing cascades and their consequences is an important step to stem the tide of polypharmacy and inform deprescribing efforts.”
The analysis was based on administrative data from almost 340,000 adults in the community aged 66 years or older with hypertension and new drug prescriptions over 5 years ending in September 2016, the report notes. Their mean age was 74.5 years and 56.5% were women.
The data set included 41,086 patients who were newly prescribed a CCB; 66,494 who were newly prescribed an ACE inhibitor or ARB; and 231,439 newly prescribed any medication other than a CCB. The prescribed CCB was amlodipine in 79.6% of patients.
Although loop diuretics could possibly have been prescribed sometimes as a second-tier antihypertensive in the absence of peripheral edema, “we made efforts, through the design of our study, to limit this where possible,” Savage said in an interview.
For example, the focus was on loop diuretics, which aren’t generally recommended for blood-pressure lowering. Also, patients with heart failure and those with a recent history of diuretic or other antihypertensive medication use had been excluded, she said.
“As such, our cohort comprised individuals with new-onset or milder hypertension for whom diuretics would unlikely to be prescribed as part of guideline-based hypertension management.”
Although amlodipine was the most commonly prescribed CCB, the potential for a prescribing cascade seemed to be a class effect and to apply at a range of dosages.
That was unexpected, McCarthy observed, because “peripheral edema occurs more commonly in people taking dihydropyridine CCBs, like amlodipine, compared to non–dihydropyridine CCBs, such as verapamil and diltiazem.”
Savage, McCarthy, their coauthors, and the editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Elderly adults with hypertension who are newly prescribed a calcium-channel blocker (CCB), compared to other antihypertensive agents, are at least twice as likely to be given a loop diuretic over the following months, a large cohort study suggests.
The likelihood remained elevated for as long as a year after the start of a CCB and was more pronounced when comparing CCBs to any other kind of medication.
“Our findings suggest that many older adults who begin taking a CCB may subsequently experience a prescribing cascade” when loop diuretics are prescribed for peripheral edema, a known CCB adverse effect, that is misinterpreted as a new medical condition, Rachel D. Savage, PhD, Women’s College Hospital, Toronto, Canada, told theheart.org/Medscape Cardiology.
Edema caused by CCBs is caused by fluid redistribution, not overload, and “treating euvolemic individuals with a diuretic places them at increased risk of overdiuresis, leading to falls, urinary incontinence, acute kidney injury, electrolyte imbalances, and a cascade of other downstream consequences to which older adults are especially vulnerable,” explain Savage and coauthors of the analysis published online February 24 in JAMA Internal Medicine.
However, 1.4% of the cohort had been prescribed a loop diuretic, and 4.5% had been given any diuretic within 90 days after the start of CCBs. The corresponding rates were 0.7% and 3.4%, respectively, for patients who had started on ACE inhibitors or angiotensin receptor blocker (ARB) rather than a CCB.
Also, Savage observed, “the likelihood of being prescribed a loop diuretic following initiation of a CCB changed over time and was greatest 61 to 90 days postinitiation.” At that point, it was increased 2.4 times compared with initiation of an ACE inhibitor or an ARB in an adjusted analysis and increased almost 4 times compared with starting on any non-CCB agent.
Importantly, the actual prevalence of peripheral edema among those started on CCBs, ACE inhibitors, ARBs, or any non-CCB medication was not available in the data sets.
However, “the main message for clinicians is to consider medication side effects as a potential cause for new symptoms when patients present. We also encourage patients to ask prescribers about whether new symptoms could be caused by a medication,” senior author Lisa M. McCarthy, PharmD, told theheart.org/Medscape Cardiology.
“If a patient experiences peripheral edema while taking a CCB, we would encourage clinicians to consider whether the calcium-channel blocker is still necessary, whether it could be discontinued or the dose reduced, or whether the patient can be switched to another therapy,” she said.
Based on the current analysis, if the rate of CCB-induced peripheral edema is assumed to be 10%, which is consistent with the literature, then “potentially 7% to 14% of people who develop edema while taking a calcium channel blocker may then receive a loop diuretic,” an accompanying editorial notes.
“Patients with polypharmacy are at heightened risk of being exposed to [a] series of prescribing cascades if their current use of medications is not carefully discussed before the decision to add a new antihypertensive,” observe Timothy S. Anderson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, and Michael A. Steinman, MD, San Francisco Veterans Affairs Medical Center and University of California, San Francisco.
“The initial prescribing cascade can set off many other negative consequences, including adverse drug events, potentially avoidable diagnostic testing, and hospitalizations,” the editorialists caution.
“Identifying prescribing cascades and their consequences is an important step to stem the tide of polypharmacy and inform deprescribing efforts.”
The analysis was based on administrative data from almost 340,000 adults in the community aged 66 years or older with hypertension and new drug prescriptions over 5 years ending in September 2016, the report notes. Their mean age was 74.5 years and 56.5% were women.
The data set included 41,086 patients who were newly prescribed a CCB; 66,494 who were newly prescribed an ACE inhibitor or ARB; and 231,439 newly prescribed any medication other than a CCB. The prescribed CCB was amlodipine in 79.6% of patients.
Although loop diuretics could possibly have been prescribed sometimes as a second-tier antihypertensive in the absence of peripheral edema, “we made efforts, through the design of our study, to limit this where possible,” Savage said in an interview.
For example, the focus was on loop diuretics, which aren’t generally recommended for blood-pressure lowering. Also, patients with heart failure and those with a recent history of diuretic or other antihypertensive medication use had been excluded, she said.
“As such, our cohort comprised individuals with new-onset or milder hypertension for whom diuretics would unlikely to be prescribed as part of guideline-based hypertension management.”
Although amlodipine was the most commonly prescribed CCB, the potential for a prescribing cascade seemed to be a class effect and to apply at a range of dosages.
That was unexpected, McCarthy observed, because “peripheral edema occurs more commonly in people taking dihydropyridine CCBs, like amlodipine, compared to non–dihydropyridine CCBs, such as verapamil and diltiazem.”
Savage, McCarthy, their coauthors, and the editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Elderly adults with hypertension who are newly prescribed a calcium-channel blocker (CCB), compared to other antihypertensive agents, are at least twice as likely to be given a loop diuretic over the following months, a large cohort study suggests.
The likelihood remained elevated for as long as a year after the start of a CCB and was more pronounced when comparing CCBs to any other kind of medication.
“Our findings suggest that many older adults who begin taking a CCB may subsequently experience a prescribing cascade” when loop diuretics are prescribed for peripheral edema, a known CCB adverse effect, that is misinterpreted as a new medical condition, Rachel D. Savage, PhD, Women’s College Hospital, Toronto, Canada, told theheart.org/Medscape Cardiology.
Edema caused by CCBs is caused by fluid redistribution, not overload, and “treating euvolemic individuals with a diuretic places them at increased risk of overdiuresis, leading to falls, urinary incontinence, acute kidney injury, electrolyte imbalances, and a cascade of other downstream consequences to which older adults are especially vulnerable,” explain Savage and coauthors of the analysis published online February 24 in JAMA Internal Medicine.
However, 1.4% of the cohort had been prescribed a loop diuretic, and 4.5% had been given any diuretic within 90 days after the start of CCBs. The corresponding rates were 0.7% and 3.4%, respectively, for patients who had started on ACE inhibitors or angiotensin receptor blocker (ARB) rather than a CCB.
Also, Savage observed, “the likelihood of being prescribed a loop diuretic following initiation of a CCB changed over time and was greatest 61 to 90 days postinitiation.” At that point, it was increased 2.4 times compared with initiation of an ACE inhibitor or an ARB in an adjusted analysis and increased almost 4 times compared with starting on any non-CCB agent.
Importantly, the actual prevalence of peripheral edema among those started on CCBs, ACE inhibitors, ARBs, or any non-CCB medication was not available in the data sets.
However, “the main message for clinicians is to consider medication side effects as a potential cause for new symptoms when patients present. We also encourage patients to ask prescribers about whether new symptoms could be caused by a medication,” senior author Lisa M. McCarthy, PharmD, told theheart.org/Medscape Cardiology.
“If a patient experiences peripheral edema while taking a CCB, we would encourage clinicians to consider whether the calcium-channel blocker is still necessary, whether it could be discontinued or the dose reduced, or whether the patient can be switched to another therapy,” she said.
Based on the current analysis, if the rate of CCB-induced peripheral edema is assumed to be 10%, which is consistent with the literature, then “potentially 7% to 14% of people who develop edema while taking a calcium channel blocker may then receive a loop diuretic,” an accompanying editorial notes.
“Patients with polypharmacy are at heightened risk of being exposed to [a] series of prescribing cascades if their current use of medications is not carefully discussed before the decision to add a new antihypertensive,” observe Timothy S. Anderson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, and Michael A. Steinman, MD, San Francisco Veterans Affairs Medical Center and University of California, San Francisco.
“The initial prescribing cascade can set off many other negative consequences, including adverse drug events, potentially avoidable diagnostic testing, and hospitalizations,” the editorialists caution.
“Identifying prescribing cascades and their consequences is an important step to stem the tide of polypharmacy and inform deprescribing efforts.”
The analysis was based on administrative data from almost 340,000 adults in the community aged 66 years or older with hypertension and new drug prescriptions over 5 years ending in September 2016, the report notes. Their mean age was 74.5 years and 56.5% were women.
The data set included 41,086 patients who were newly prescribed a CCB; 66,494 who were newly prescribed an ACE inhibitor or ARB; and 231,439 newly prescribed any medication other than a CCB. The prescribed CCB was amlodipine in 79.6% of patients.
Although loop diuretics could possibly have been prescribed sometimes as a second-tier antihypertensive in the absence of peripheral edema, “we made efforts, through the design of our study, to limit this where possible,” Savage said in an interview.
For example, the focus was on loop diuretics, which aren’t generally recommended for blood-pressure lowering. Also, patients with heart failure and those with a recent history of diuretic or other antihypertensive medication use had been excluded, she said.
“As such, our cohort comprised individuals with new-onset or milder hypertension for whom diuretics would unlikely to be prescribed as part of guideline-based hypertension management.”
Although amlodipine was the most commonly prescribed CCB, the potential for a prescribing cascade seemed to be a class effect and to apply at a range of dosages.
That was unexpected, McCarthy observed, because “peripheral edema occurs more commonly in people taking dihydropyridine CCBs, like amlodipine, compared to non–dihydropyridine CCBs, such as verapamil and diltiazem.”
Savage, McCarthy, their coauthors, and the editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Gene therapy appears effective in bladder cancer patients with few options
SAN FRANCISCO – (BCG), new research suggests.
The therapy, nadofaragene firadenovec, is a recombinant adenovirus that is instilled into the bladder and delivers the human interferon alpha-2b gene, leading to expression of the immune cytokine.
At 3 months, nadofaragene firadenovec had produced a complete response in 53.4% of patients with carcinoma in situ (CIS), and the rate of high-grade recurrence-free survival was 59.6% in all patients. Although most patients (70.1%) experienced adverse events in this trial, few had serious events (1.9%).
Stephen A. Boorjian, MD, of the Mayo Clinic in Rochester, Minn., presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“The optimal management for patients with BCG-unresponsive non–muscle invasive bladder cancer remains to be established,” Dr. Boorjian said. “National and organizational guidelines recommend radical cystectomy in this setting, but we have to acknowledge that many of our patients will be either unwilling or unfit to undergo what is often a highly morbid operation.”
Dr. Boorjian and coinvestigators tested nadofaragene firadenovec in 107 patients with CIS, with or without high-grade Ta or T1 papillary disease, as well as 50 patients with high-grade Ta or T1 papillary disease only.
All patients received nadofaragene firadenovec once every 3 months for up four doses, with additional dosing at the investigator’s discretion. Although the trial was designated as phase 3, it did not have the typical randomized comparative design.
“The challenge in the BCG-unresponsive disease state is that, historically, there has been no validated good comparator to use here; there’s no standard of care for these patients,” Dr. Boorjian explained, noting that the design was chosen after discussion with the Food and Drug Administration.
Efficacy
The complete response rate among CIS patients at any time after instillation, the trial’s primary objective, was 53.4%. All of these responses occurred after a single dose of nadofaragene firadenovec. Responses were considered durable, as 45.5% of CIS patients who had a complete response at 3 months still had a complete response at 12 months.
The rate of high-grade recurrence-free survival at 3 months was 59.6% in the overall population, 53.4% in patients with CIS, and 72.9% in patients with papillary disease. At 12 months, the rate of high-grade recurrence-free survival was 30.5%, 24.3%, and 43.8%, respectively.
The rate of high-grade recurrence without muscle invasive bladder cancer was 71.8% in the CIS group and 52.1% in the papillary group. The rate of progression to muscle invasive bladder cancer or more advanced disease was 4.9% and 6.3%, respectively. The overall 2-year rate of cystectomy-free survival was 64%, and there were no bladder cancer deaths.
Safety
Nadofaragene firadenovec was considered safe and well tolerated. The most common study drug–related adverse events were irritative voiding symptoms, such as discharge, bladder spasm, micturition urgency, and hematuria.
Although 70.1% of patients experienced a local or systemic adverse event related to the study drug or procedure, few experienced serious adverse events (1.9%), grade 3 adverse events (3.8%), or treatment-emergent adverse events leading to discontinuation (1.9%). The serious adverse events were sepsis, syncope, and hematuria.
Next steps
“Nadofaragene firadenovec represents a promising option for patients with BCG-unresponsive [non–muscle invasive bladder cancer],” Dr. Boorjian said. “What we want to see going forward is durability of response, cystectomy-free survival, and metastasis-free and cancer-specific survival. Those are the harder endpoints that, as these data mature, we will want to see so that we are able to counsel our patients accordingly.”
The FDA recently approved pembrolizumab for this patient population, noted session cochair Guru Sonpavde, MD, of the Dana-Farber Cancer Institute in Boston.
“But nadofaragene firadenovec is much less toxic, it is given intravesically, and it’s only given once every 3 months,” he said. “The outcomes at the 1-year point look similar in terms of the durable complete response rate. So we hope that this therapy will be able to get to the clinic, but we have to wait for the FDA to decide.”
It remains to be seen how nadofaragene firadenovec compares with other emerging therapies for high-risk BCG-unresponsive non–muscle invasive bladder cancer, including pembrolizumab and potentially intravesical oportuzumab monatox (a targeted immunotoxin) and intravesical combination BCG and superagonist interleukin-15 (ALT-803), according to Dr. Boorjian.
“A unique feature of the trial I presented was that the results were validated by a mandatory 12-month study biopsy, which has not been the case in other trials,” he pointed out. “So this separates it a little bit when you are looking at pathologic response rates and high-grade recurrence–free rates. They are based on pathologic confirmation.”
Evaluators will ultimately compare these therapies on safety, efficacy, ease of administration, delivery schedule, and cost, Dr. Boorjian said. “At this stage, it’s very difficult to start to say which agent is going to be placed where in our management paradigm. We have to go step by step: Do the trial that we did, show our data, put it out for review, and then allow the community to engage in a discussion about which agent, and why, for which patient.”
This trial was funded by FKD Therapies Oy. Dr. Boorjian disclosed relationships with Ferring and Sanofi. Dr. Sonpavde disclosed relationships with many companies.
SOURCE: Boorjian SA et al. GUCS 2020, Abstract 442.
SAN FRANCISCO – (BCG), new research suggests.
The therapy, nadofaragene firadenovec, is a recombinant adenovirus that is instilled into the bladder and delivers the human interferon alpha-2b gene, leading to expression of the immune cytokine.
At 3 months, nadofaragene firadenovec had produced a complete response in 53.4% of patients with carcinoma in situ (CIS), and the rate of high-grade recurrence-free survival was 59.6% in all patients. Although most patients (70.1%) experienced adverse events in this trial, few had serious events (1.9%).
Stephen A. Boorjian, MD, of the Mayo Clinic in Rochester, Minn., presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“The optimal management for patients with BCG-unresponsive non–muscle invasive bladder cancer remains to be established,” Dr. Boorjian said. “National and organizational guidelines recommend radical cystectomy in this setting, but we have to acknowledge that many of our patients will be either unwilling or unfit to undergo what is often a highly morbid operation.”
Dr. Boorjian and coinvestigators tested nadofaragene firadenovec in 107 patients with CIS, with or without high-grade Ta or T1 papillary disease, as well as 50 patients with high-grade Ta or T1 papillary disease only.
All patients received nadofaragene firadenovec once every 3 months for up four doses, with additional dosing at the investigator’s discretion. Although the trial was designated as phase 3, it did not have the typical randomized comparative design.
“The challenge in the BCG-unresponsive disease state is that, historically, there has been no validated good comparator to use here; there’s no standard of care for these patients,” Dr. Boorjian explained, noting that the design was chosen after discussion with the Food and Drug Administration.
Efficacy
The complete response rate among CIS patients at any time after instillation, the trial’s primary objective, was 53.4%. All of these responses occurred after a single dose of nadofaragene firadenovec. Responses were considered durable, as 45.5% of CIS patients who had a complete response at 3 months still had a complete response at 12 months.
The rate of high-grade recurrence-free survival at 3 months was 59.6% in the overall population, 53.4% in patients with CIS, and 72.9% in patients with papillary disease. At 12 months, the rate of high-grade recurrence-free survival was 30.5%, 24.3%, and 43.8%, respectively.
The rate of high-grade recurrence without muscle invasive bladder cancer was 71.8% in the CIS group and 52.1% in the papillary group. The rate of progression to muscle invasive bladder cancer or more advanced disease was 4.9% and 6.3%, respectively. The overall 2-year rate of cystectomy-free survival was 64%, and there were no bladder cancer deaths.
Safety
Nadofaragene firadenovec was considered safe and well tolerated. The most common study drug–related adverse events were irritative voiding symptoms, such as discharge, bladder spasm, micturition urgency, and hematuria.
Although 70.1% of patients experienced a local or systemic adverse event related to the study drug or procedure, few experienced serious adverse events (1.9%), grade 3 adverse events (3.8%), or treatment-emergent adverse events leading to discontinuation (1.9%). The serious adverse events were sepsis, syncope, and hematuria.
Next steps
“Nadofaragene firadenovec represents a promising option for patients with BCG-unresponsive [non–muscle invasive bladder cancer],” Dr. Boorjian said. “What we want to see going forward is durability of response, cystectomy-free survival, and metastasis-free and cancer-specific survival. Those are the harder endpoints that, as these data mature, we will want to see so that we are able to counsel our patients accordingly.”
The FDA recently approved pembrolizumab for this patient population, noted session cochair Guru Sonpavde, MD, of the Dana-Farber Cancer Institute in Boston.
“But nadofaragene firadenovec is much less toxic, it is given intravesically, and it’s only given once every 3 months,” he said. “The outcomes at the 1-year point look similar in terms of the durable complete response rate. So we hope that this therapy will be able to get to the clinic, but we have to wait for the FDA to decide.”
It remains to be seen how nadofaragene firadenovec compares with other emerging therapies for high-risk BCG-unresponsive non–muscle invasive bladder cancer, including pembrolizumab and potentially intravesical oportuzumab monatox (a targeted immunotoxin) and intravesical combination BCG and superagonist interleukin-15 (ALT-803), according to Dr. Boorjian.
“A unique feature of the trial I presented was that the results were validated by a mandatory 12-month study biopsy, which has not been the case in other trials,” he pointed out. “So this separates it a little bit when you are looking at pathologic response rates and high-grade recurrence–free rates. They are based on pathologic confirmation.”
Evaluators will ultimately compare these therapies on safety, efficacy, ease of administration, delivery schedule, and cost, Dr. Boorjian said. “At this stage, it’s very difficult to start to say which agent is going to be placed where in our management paradigm. We have to go step by step: Do the trial that we did, show our data, put it out for review, and then allow the community to engage in a discussion about which agent, and why, for which patient.”
This trial was funded by FKD Therapies Oy. Dr. Boorjian disclosed relationships with Ferring and Sanofi. Dr. Sonpavde disclosed relationships with many companies.
SOURCE: Boorjian SA et al. GUCS 2020, Abstract 442.
SAN FRANCISCO – (BCG), new research suggests.
The therapy, nadofaragene firadenovec, is a recombinant adenovirus that is instilled into the bladder and delivers the human interferon alpha-2b gene, leading to expression of the immune cytokine.
At 3 months, nadofaragene firadenovec had produced a complete response in 53.4% of patients with carcinoma in situ (CIS), and the rate of high-grade recurrence-free survival was 59.6% in all patients. Although most patients (70.1%) experienced adverse events in this trial, few had serious events (1.9%).
Stephen A. Boorjian, MD, of the Mayo Clinic in Rochester, Minn., presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“The optimal management for patients with BCG-unresponsive non–muscle invasive bladder cancer remains to be established,” Dr. Boorjian said. “National and organizational guidelines recommend radical cystectomy in this setting, but we have to acknowledge that many of our patients will be either unwilling or unfit to undergo what is often a highly morbid operation.”
Dr. Boorjian and coinvestigators tested nadofaragene firadenovec in 107 patients with CIS, with or without high-grade Ta or T1 papillary disease, as well as 50 patients with high-grade Ta or T1 papillary disease only.
All patients received nadofaragene firadenovec once every 3 months for up four doses, with additional dosing at the investigator’s discretion. Although the trial was designated as phase 3, it did not have the typical randomized comparative design.
“The challenge in the BCG-unresponsive disease state is that, historically, there has been no validated good comparator to use here; there’s no standard of care for these patients,” Dr. Boorjian explained, noting that the design was chosen after discussion with the Food and Drug Administration.
Efficacy
The complete response rate among CIS patients at any time after instillation, the trial’s primary objective, was 53.4%. All of these responses occurred after a single dose of nadofaragene firadenovec. Responses were considered durable, as 45.5% of CIS patients who had a complete response at 3 months still had a complete response at 12 months.
The rate of high-grade recurrence-free survival at 3 months was 59.6% in the overall population, 53.4% in patients with CIS, and 72.9% in patients with papillary disease. At 12 months, the rate of high-grade recurrence-free survival was 30.5%, 24.3%, and 43.8%, respectively.
The rate of high-grade recurrence without muscle invasive bladder cancer was 71.8% in the CIS group and 52.1% in the papillary group. The rate of progression to muscle invasive bladder cancer or more advanced disease was 4.9% and 6.3%, respectively. The overall 2-year rate of cystectomy-free survival was 64%, and there were no bladder cancer deaths.
Safety
Nadofaragene firadenovec was considered safe and well tolerated. The most common study drug–related adverse events were irritative voiding symptoms, such as discharge, bladder spasm, micturition urgency, and hematuria.
Although 70.1% of patients experienced a local or systemic adverse event related to the study drug or procedure, few experienced serious adverse events (1.9%), grade 3 adverse events (3.8%), or treatment-emergent adverse events leading to discontinuation (1.9%). The serious adverse events were sepsis, syncope, and hematuria.
Next steps
“Nadofaragene firadenovec represents a promising option for patients with BCG-unresponsive [non–muscle invasive bladder cancer],” Dr. Boorjian said. “What we want to see going forward is durability of response, cystectomy-free survival, and metastasis-free and cancer-specific survival. Those are the harder endpoints that, as these data mature, we will want to see so that we are able to counsel our patients accordingly.”
The FDA recently approved pembrolizumab for this patient population, noted session cochair Guru Sonpavde, MD, of the Dana-Farber Cancer Institute in Boston.
“But nadofaragene firadenovec is much less toxic, it is given intravesically, and it’s only given once every 3 months,” he said. “The outcomes at the 1-year point look similar in terms of the durable complete response rate. So we hope that this therapy will be able to get to the clinic, but we have to wait for the FDA to decide.”
It remains to be seen how nadofaragene firadenovec compares with other emerging therapies for high-risk BCG-unresponsive non–muscle invasive bladder cancer, including pembrolizumab and potentially intravesical oportuzumab monatox (a targeted immunotoxin) and intravesical combination BCG and superagonist interleukin-15 (ALT-803), according to Dr. Boorjian.
“A unique feature of the trial I presented was that the results were validated by a mandatory 12-month study biopsy, which has not been the case in other trials,” he pointed out. “So this separates it a little bit when you are looking at pathologic response rates and high-grade recurrence–free rates. They are based on pathologic confirmation.”
Evaluators will ultimately compare these therapies on safety, efficacy, ease of administration, delivery schedule, and cost, Dr. Boorjian said. “At this stage, it’s very difficult to start to say which agent is going to be placed where in our management paradigm. We have to go step by step: Do the trial that we did, show our data, put it out for review, and then allow the community to engage in a discussion about which agent, and why, for which patient.”
This trial was funded by FKD Therapies Oy. Dr. Boorjian disclosed relationships with Ferring and Sanofi. Dr. Sonpavde disclosed relationships with many companies.
SOURCE: Boorjian SA et al. GUCS 2020, Abstract 442.
REPORTING FROM GUCS 2020
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
Prescription osteoarthritis relief gets OTC approval
The Food and Drug Administration has approved formerly prescription-only Voltaren Arthritis Pain (diclofenac sodium topical gel, 1%) for nonprescription use via a process known as a prescription to over-the-counter (Rx-to-OTC) switch, according to a news release from the agency.
“As a result of the Rx-to-OTC switch process, many products sold over the counter today use ingredients or dosage strengths that were available only by prescription 30 years ago,” Karen Mahoney, MD, acting deputy director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the release.
This switch to nonprescription status is usually initiated by the manufacturer, who must provide data that demonstrates the drug in question is both safe and effective as self-medication in accordance with the proposed labeling and that consumers can use it safely and effectively without the supervision of a health care professional.
This particular therapy is a topical NSAID gel and was first approved by the FDA in 2007 with the indication for relief of osteoarthritis pain. It can take 7 days to have an effect, but if patients find it takes longer than that or they need to use it for more than 21 days, they should seek medical attention. The gel can cause severe allergic reactions, especially in people allergic to aspirin; patients who experience such reactions are advised to stop use and seek immediate medical care. Other concerns include potential for liver damage with extended use; the possibility of severe stomach bleeds; and risk of heart attack, heart failure, and stroke.
The gel will no longer be available in prescription form.
Full prescribing information can be found on the FDA website, as can the full news release regarding this approval.
The Food and Drug Administration has approved formerly prescription-only Voltaren Arthritis Pain (diclofenac sodium topical gel, 1%) for nonprescription use via a process known as a prescription to over-the-counter (Rx-to-OTC) switch, according to a news release from the agency.
“As a result of the Rx-to-OTC switch process, many products sold over the counter today use ingredients or dosage strengths that were available only by prescription 30 years ago,” Karen Mahoney, MD, acting deputy director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the release.
This switch to nonprescription status is usually initiated by the manufacturer, who must provide data that demonstrates the drug in question is both safe and effective as self-medication in accordance with the proposed labeling and that consumers can use it safely and effectively without the supervision of a health care professional.
This particular therapy is a topical NSAID gel and was first approved by the FDA in 2007 with the indication for relief of osteoarthritis pain. It can take 7 days to have an effect, but if patients find it takes longer than that or they need to use it for more than 21 days, they should seek medical attention. The gel can cause severe allergic reactions, especially in people allergic to aspirin; patients who experience such reactions are advised to stop use and seek immediate medical care. Other concerns include potential for liver damage with extended use; the possibility of severe stomach bleeds; and risk of heart attack, heart failure, and stroke.
The gel will no longer be available in prescription form.
Full prescribing information can be found on the FDA website, as can the full news release regarding this approval.
The Food and Drug Administration has approved formerly prescription-only Voltaren Arthritis Pain (diclofenac sodium topical gel, 1%) for nonprescription use via a process known as a prescription to over-the-counter (Rx-to-OTC) switch, according to a news release from the agency.
“As a result of the Rx-to-OTC switch process, many products sold over the counter today use ingredients or dosage strengths that were available only by prescription 30 years ago,” Karen Mahoney, MD, acting deputy director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the release.
This switch to nonprescription status is usually initiated by the manufacturer, who must provide data that demonstrates the drug in question is both safe and effective as self-medication in accordance with the proposed labeling and that consumers can use it safely and effectively without the supervision of a health care professional.
This particular therapy is a topical NSAID gel and was first approved by the FDA in 2007 with the indication for relief of osteoarthritis pain. It can take 7 days to have an effect, but if patients find it takes longer than that or they need to use it for more than 21 days, they should seek medical attention. The gel can cause severe allergic reactions, especially in people allergic to aspirin; patients who experience such reactions are advised to stop use and seek immediate medical care. Other concerns include potential for liver damage with extended use; the possibility of severe stomach bleeds; and risk of heart attack, heart failure, and stroke.
The gel will no longer be available in prescription form.
Full prescribing information can be found on the FDA website, as can the full news release regarding this approval.
Low-dose methotrexate trial pins down adverse event rates
A new study has found an elevated risk of some adverse events in patients treated with low-dose methotrexate, compared with patients treated with placebo.
“The data presented here provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX [low-dose methotrexate],” wrote Daniel H. Solomon, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, and coauthors. The study was published in Annals of Internal Medicine.
To determine the rates of adverse events (AEs) among LD-MTX users, along with assessing the risks of certain predefined AEs, the researchers enrolled 6,158 patients in the Cardiovascular Inflammation Reduction Trial (CIRT) and randomized 4,786 of those patients to two groups: those receiving LD-MTX (n = 2,391) and those receiving placebo (n = 2,395). The median dose was 15 mg per week, and median follow-up was 23 months. All participants in CIRT had a history of cardiovascular disease, along with diabetes or metabolic syndrome. Just over 81% of the participants were male, and nearly 85% were white. Their median age was nearly 66 years.
Of the participants in the LD-MTX group, 2,156 (90.2%) had an AE and 2,080 (87.0%) had an AE of interest, which included infectious, hematologic, pulmonary, hepatic, cancerous, and gastrointestinal AEs. Of the participants in the placebo group, 2,076 (86.7%) had an AE and 1,951 (81.5%) had an AE of interest. As such, the relative rate of an AE of interest was 17% higher in the LD-MTX group (hazard ratio, 1.17; 95% confidence interval, 1.10-1.25).
In regard to specific types of AEs, the rates of gastrointestinal (HR, 1.23; 95% CI, 1.03-1.47), pulmonary (HR, 1.42; 95% CI, 1.14-1.77), infectious (HR, 1.15; 95% CI, 1.01-1.30) and hematologic (HR, 1.22; 95% CI, 1.11-1.34) were higher for participants in the LD-MTX group. Five cases of cirrhosis were found in the LD-MTX group, compared with none in the placebo group; none of the patients with cirrhosis had severe liver test abnormalities before their diagnosis. While the risk of cancer overall was not elevated in the LD-MTX group, 53 participants in that group developed skin cancer, compared with 26 in the placebo group (HR, 2.04; 95% CI, 1.28-3.26). Renal AEs were among the few that decreased in LD-MTX users (HR, 0.85; 95% CI, 0.78-0.93).
“Methotrexate has become the standard of care for RA patients,” Dr. Solomon said in an interview, “and because it worked so well, we accepted it without large placebo-controlled trials and without a precise understanding of the risk factors for AEs. Until this study, our evidence basis for the side-effect profile was relatively weak.
“We had a limited data set but decades of experience,” he added. “Now we have better evidence, for example, that methotrexate is associated with elevations in liver function tests. We even found five cases of cirrhosis. And the people who developed cirrhosis didn’t have severe test abnormalities; just minor ones over many months. So now we have a better understanding of the potential impact of minor, yet chronic abnormalities.”
Dr. Solomon and coauthors acknowledged their study’s limitations, including CIRT not including patients with systemic rheumatic disease and the possibility that participants did not report AEs that occurred in between routine study visits. In addition, although the median follow-up of nearly 2 years was longer than in other LD-MTX trials, they noted that “it may still be too short to observe some AEs that require long-term exposure.”
Dr. Solomon and colleagues should be commended for undertaking a long-awaited randomized, placebo-controlled trial that adds much-needed insight into how and when to monitor patients being treated with MTX, Vivian P. Bykerk, MD, of the Hospital for Special Surgery and Weill Cornell Medical College in New York, wrote in an editorial (Ann Intern Med. 2020 Feb 17. doi: 10.7326/M20-0435).
Dr. Bykerk noted that although the results may not be applicable to patients with RA and other inflammatory arthritides who are treated with MTX – RA patients in particular are younger, more often female, have lower rates of diabetes, and usually receive higher doses than those used in CIRT — the risk estimates from the CIRT study are “largely congruent with those expected in MTX-treated patients with rheumatic diseases.”
Regardless, she emphasized that this is a step in a much-needed direction, reminding physicians that “MTX use has inherent risks” and that its AEs, although infrequent, are clinically serious.
The National Institutes of Health funded the study. Various authors reported receiving grants from the National Heart, Lung, and Blood Institute, along with grants, research support, and personal fees from numerous pharmaceutical companies before and during the study. Dr. Bykerk reported receiving personal fees, grants, and nonfinancial support from pharmaceutical companies, foundations, and the NIH.
SOURCE: Solomon DH et al. Ann Intern Med. 2020 Feb 17. doi: 10.7326/M19-3369.
A new study has found an elevated risk of some adverse events in patients treated with low-dose methotrexate, compared with patients treated with placebo.
“The data presented here provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX [low-dose methotrexate],” wrote Daniel H. Solomon, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, and coauthors. The study was published in Annals of Internal Medicine.
To determine the rates of adverse events (AEs) among LD-MTX users, along with assessing the risks of certain predefined AEs, the researchers enrolled 6,158 patients in the Cardiovascular Inflammation Reduction Trial (CIRT) and randomized 4,786 of those patients to two groups: those receiving LD-MTX (n = 2,391) and those receiving placebo (n = 2,395). The median dose was 15 mg per week, and median follow-up was 23 months. All participants in CIRT had a history of cardiovascular disease, along with diabetes or metabolic syndrome. Just over 81% of the participants were male, and nearly 85% were white. Their median age was nearly 66 years.
Of the participants in the LD-MTX group, 2,156 (90.2%) had an AE and 2,080 (87.0%) had an AE of interest, which included infectious, hematologic, pulmonary, hepatic, cancerous, and gastrointestinal AEs. Of the participants in the placebo group, 2,076 (86.7%) had an AE and 1,951 (81.5%) had an AE of interest. As such, the relative rate of an AE of interest was 17% higher in the LD-MTX group (hazard ratio, 1.17; 95% confidence interval, 1.10-1.25).
In regard to specific types of AEs, the rates of gastrointestinal (HR, 1.23; 95% CI, 1.03-1.47), pulmonary (HR, 1.42; 95% CI, 1.14-1.77), infectious (HR, 1.15; 95% CI, 1.01-1.30) and hematologic (HR, 1.22; 95% CI, 1.11-1.34) were higher for participants in the LD-MTX group. Five cases of cirrhosis were found in the LD-MTX group, compared with none in the placebo group; none of the patients with cirrhosis had severe liver test abnormalities before their diagnosis. While the risk of cancer overall was not elevated in the LD-MTX group, 53 participants in that group developed skin cancer, compared with 26 in the placebo group (HR, 2.04; 95% CI, 1.28-3.26). Renal AEs were among the few that decreased in LD-MTX users (HR, 0.85; 95% CI, 0.78-0.93).
“Methotrexate has become the standard of care for RA patients,” Dr. Solomon said in an interview, “and because it worked so well, we accepted it without large placebo-controlled trials and without a precise understanding of the risk factors for AEs. Until this study, our evidence basis for the side-effect profile was relatively weak.
“We had a limited data set but decades of experience,” he added. “Now we have better evidence, for example, that methotrexate is associated with elevations in liver function tests. We even found five cases of cirrhosis. And the people who developed cirrhosis didn’t have severe test abnormalities; just minor ones over many months. So now we have a better understanding of the potential impact of minor, yet chronic abnormalities.”
Dr. Solomon and coauthors acknowledged their study’s limitations, including CIRT not including patients with systemic rheumatic disease and the possibility that participants did not report AEs that occurred in between routine study visits. In addition, although the median follow-up of nearly 2 years was longer than in other LD-MTX trials, they noted that “it may still be too short to observe some AEs that require long-term exposure.”
Dr. Solomon and colleagues should be commended for undertaking a long-awaited randomized, placebo-controlled trial that adds much-needed insight into how and when to monitor patients being treated with MTX, Vivian P. Bykerk, MD, of the Hospital for Special Surgery and Weill Cornell Medical College in New York, wrote in an editorial (Ann Intern Med. 2020 Feb 17. doi: 10.7326/M20-0435).
Dr. Bykerk noted that although the results may not be applicable to patients with RA and other inflammatory arthritides who are treated with MTX – RA patients in particular are younger, more often female, have lower rates of diabetes, and usually receive higher doses than those used in CIRT — the risk estimates from the CIRT study are “largely congruent with those expected in MTX-treated patients with rheumatic diseases.”
Regardless, she emphasized that this is a step in a much-needed direction, reminding physicians that “MTX use has inherent risks” and that its AEs, although infrequent, are clinically serious.
The National Institutes of Health funded the study. Various authors reported receiving grants from the National Heart, Lung, and Blood Institute, along with grants, research support, and personal fees from numerous pharmaceutical companies before and during the study. Dr. Bykerk reported receiving personal fees, grants, and nonfinancial support from pharmaceutical companies, foundations, and the NIH.
SOURCE: Solomon DH et al. Ann Intern Med. 2020 Feb 17. doi: 10.7326/M19-3369.
A new study has found an elevated risk of some adverse events in patients treated with low-dose methotrexate, compared with patients treated with placebo.
“The data presented here provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX [low-dose methotrexate],” wrote Daniel H. Solomon, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, and coauthors. The study was published in Annals of Internal Medicine.
To determine the rates of adverse events (AEs) among LD-MTX users, along with assessing the risks of certain predefined AEs, the researchers enrolled 6,158 patients in the Cardiovascular Inflammation Reduction Trial (CIRT) and randomized 4,786 of those patients to two groups: those receiving LD-MTX (n = 2,391) and those receiving placebo (n = 2,395). The median dose was 15 mg per week, and median follow-up was 23 months. All participants in CIRT had a history of cardiovascular disease, along with diabetes or metabolic syndrome. Just over 81% of the participants were male, and nearly 85% were white. Their median age was nearly 66 years.
Of the participants in the LD-MTX group, 2,156 (90.2%) had an AE and 2,080 (87.0%) had an AE of interest, which included infectious, hematologic, pulmonary, hepatic, cancerous, and gastrointestinal AEs. Of the participants in the placebo group, 2,076 (86.7%) had an AE and 1,951 (81.5%) had an AE of interest. As such, the relative rate of an AE of interest was 17% higher in the LD-MTX group (hazard ratio, 1.17; 95% confidence interval, 1.10-1.25).
In regard to specific types of AEs, the rates of gastrointestinal (HR, 1.23; 95% CI, 1.03-1.47), pulmonary (HR, 1.42; 95% CI, 1.14-1.77), infectious (HR, 1.15; 95% CI, 1.01-1.30) and hematologic (HR, 1.22; 95% CI, 1.11-1.34) were higher for participants in the LD-MTX group. Five cases of cirrhosis were found in the LD-MTX group, compared with none in the placebo group; none of the patients with cirrhosis had severe liver test abnormalities before their diagnosis. While the risk of cancer overall was not elevated in the LD-MTX group, 53 participants in that group developed skin cancer, compared with 26 in the placebo group (HR, 2.04; 95% CI, 1.28-3.26). Renal AEs were among the few that decreased in LD-MTX users (HR, 0.85; 95% CI, 0.78-0.93).
“Methotrexate has become the standard of care for RA patients,” Dr. Solomon said in an interview, “and because it worked so well, we accepted it without large placebo-controlled trials and without a precise understanding of the risk factors for AEs. Until this study, our evidence basis for the side-effect profile was relatively weak.
“We had a limited data set but decades of experience,” he added. “Now we have better evidence, for example, that methotrexate is associated with elevations in liver function tests. We even found five cases of cirrhosis. And the people who developed cirrhosis didn’t have severe test abnormalities; just minor ones over many months. So now we have a better understanding of the potential impact of minor, yet chronic abnormalities.”
Dr. Solomon and coauthors acknowledged their study’s limitations, including CIRT not including patients with systemic rheumatic disease and the possibility that participants did not report AEs that occurred in between routine study visits. In addition, although the median follow-up of nearly 2 years was longer than in other LD-MTX trials, they noted that “it may still be too short to observe some AEs that require long-term exposure.”
Dr. Solomon and colleagues should be commended for undertaking a long-awaited randomized, placebo-controlled trial that adds much-needed insight into how and when to monitor patients being treated with MTX, Vivian P. Bykerk, MD, of the Hospital for Special Surgery and Weill Cornell Medical College in New York, wrote in an editorial (Ann Intern Med. 2020 Feb 17. doi: 10.7326/M20-0435).
Dr. Bykerk noted that although the results may not be applicable to patients with RA and other inflammatory arthritides who are treated with MTX – RA patients in particular are younger, more often female, have lower rates of diabetes, and usually receive higher doses than those used in CIRT — the risk estimates from the CIRT study are “largely congruent with those expected in MTX-treated patients with rheumatic diseases.”
Regardless, she emphasized that this is a step in a much-needed direction, reminding physicians that “MTX use has inherent risks” and that its AEs, although infrequent, are clinically serious.
The National Institutes of Health funded the study. Various authors reported receiving grants from the National Heart, Lung, and Blood Institute, along with grants, research support, and personal fees from numerous pharmaceutical companies before and during the study. Dr. Bykerk reported receiving personal fees, grants, and nonfinancial support from pharmaceutical companies, foundations, and the NIH.
SOURCE: Solomon DH et al. Ann Intern Med. 2020 Feb 17. doi: 10.7326/M19-3369.
FROM ANNALS OF INTERNAL MEDICINE
Another round of research shows ketamine may help alcoholism
More research suggests that a single infusion of ketamine combined with counseling may help alcohol-dependent patients curb their drinking.
In a pilot study of 40 participants, those who were randomly assigned to receive intravenous ketamine plus outpatient motivational enhancement therapy (MET) showed greater abstinence rates, longer time to relapse, and fewer heavy drinking days than did those who received MET plus midazolam.
The findings support a U.K. study published late last year showing that a single dose of intravenous ketamine plus therapy that focused on reactivating drinking-related “maladaptive reward memories” reduced drinking urges and alcohol intake more than just ketamine or a placebo infusion alone (Nat Commun. 2019 Nov 26;10[1]:5187).
of the New York State Psychiatric Institute, Columbia University, New York, said in an interview.
“It’s an important area of research to understand in order to make behavioral treatments more effective, and ketamine appears to have the properties to address those vulnerabilities,” Dr. Dakwar said.
The study was published in the American Journal of Psychiatry (2019 Dec 2. doi: 10.1176/appi.ajp.2019.19070684).
Real-world approach
Pathologic alcohol use is responsible for an estimated 3.8% of all deaths globally, yet current interventions for alcohol use disorder have limited efficacy, the researchers noted.
New treatments with innovative mechanisms would be valuable, they added.
Ketamine is a high-affinity N-methyl-d-aspartate receptor (NMDAR) antagonist.
Previously, research offered “promising results” with the use of ketamine for cocaine use disorder, including increased motivation to quit and decreased craving, Dr. Dakwar noted.
“Those results led us to think about how ketamine might be helpful for other substance use disorders, especially given the overlap in clinical vulnerabilities and epidemiology,” he said.
The study from the U.K. researchers was conducted in 90 patients with harmful drinking behavior but who had not been diagnosed with alcohol use disorder.
Dr. Dakwar noted that this was “a nontreatment study. None of the people there had alcohol use disorder; they were heavy drinkers. Also, the effects there were fairly modest.
“My interest was how to integrate ketamine into a clinical, real-world framework that could be helpful for people,” he added.
The study included 40 participants (52.5% women; 70.3% white; mean age, 53 years) with alcohol dependence whose average consumption was five drinks per day.
All entered a 5-week outpatient program of MET, which involved engaging in strategies to promote motivation and self-directed change.
During the program’s second week, the participants were randomly assigned to received a 52-minute IV infusion of ketamine 0.71 mg/kg (n = 17) or the benzodiazepine midazolam 0.025 mg/kg (n = 23).
This ketamine dose was selected “because it was the highest dose tolerated by participants in preliminary studies,” the researchers reported.
“Midazolam was chosen as the active control because it alters consciousness without any known persistent ... effect on alcohol dependence,” they added.
The “timeline follow back method” was used to assess alcohol use after treatment. Abstinence was confirmed by measuring urine ethyl glucuronide levels with urine toxicology tests.
Other measures included use of a visual analogue scale, the Clinical Institute Withdrawal Assessment, and the modified Perceived Stress Scale.
Primary outcome met
Results showed that 47.1% of the ketamine group and 59.1% of the midazolam group used alcohol during the 21 days after treatment infusion; 17.6% and 40.9%, respectively, had a heavy drinking day.
For the primary outcome measure of alcohol abstinence, the “quadratic effect of time was significant” (P = .004), as was time-by-treatment interaction (P less than .001).
Although the model-estimated proportions of alcohol abstinence remained stable for the ketamine group for 21 days post infusion, the proportions decreased significantly for the control group.
The odds of having a heavy drinking day did not change significantly after treatment for the ketamine group (odds ratio, 0.98; P = .74) but increased significantly with each postinfusion day for the midazolam group (OR, 1.19; P less than .001).
For the ketamine group, time to relapse was also significantly longer (P = .04).
No significant differences were found between the groups in rates of withdrawal, craving, or stress sensitivity.
A new direction?
The most common adverse events after treatment were sedation, seen in 12 members of the midazolam group and in 8 members of the ketamine group, and headache, seen in four and six members, respectively.
Although two ketamine-group members experienced mild agitation for up to 1 hour post infusion, no incidents of persistent psychoactive effects were reported in either group.
No participants who received ketamine dropped out during the study period; among those who received midazolam, six dropped out.
“These preliminary data suggest new directions in integrated pharmacotherapy-behavioral treatments for alcohol use disorder,” the investigators wrote.
However, a larger patient population will be needed in future research in order to “replicate these promising results,” they added.
Dr. Dakwar noted that the time to first drink after treatment was comparable between the groups.
“But what was different in the ketamine group was that they didn’t continue drinking after that first drink. They didn’t initiate heavy drinking, they didn’t relapse, they were able to bounce back and stay with the program,” he said.
“It was surprising but still consistent with the central hypothesis that ketamine provides this opportunity for setting the foundation for the requisite commitment so that, once things become difficult, they’re still able to maintain recovery,” Dr. Dakwar said.
‘Provocative findings’
In an accompanying editorial, Sanjay J. Mathew, MD, of the department of psychiatry and behavioral sciences at Baylor College of Medicine in Houston, and Rebecca B. Price, PhD, of the department of psychiatry at the University of Pittsburgh, noted that ketamine’s effects on abstinence “were robust” in this trial.
“It is also noteworthy that, in spite of recruiting from a population of patients with active and significant substance use history (a group that has routinely been excluded from ketamine trials in depression), no participant showed evidence of new drug-seeking behaviors,” Dr. Mathew and Dr. Price wrote.
“Overall, these findings are provocative and hypothesis generating but certainly not definitive because of the small sample size,” they add.
Other limitations cited include the short follow-up period and the fact that only half of the participants were available for a 6-month follow-up telephone interview. In addition, generalizability was limited because the population did not have additional medical or psychiatric illnesses or additional substance use disorders, the editorialists wrote.
Because of the limitations, the investigators “are appropriately circumspect about the immediate clinical implications of this small pilot study.”
Still, the results “affirm the potential of rational combinatorial approaches for a vexing medical and public health problem,” Dr. Mathew and Dr. Price concluded.
The study was funded by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute. The study authors and Dr. Price reported no relevant financial relationships. Dr Mathew reported serving as a consultant to or having received research support from several companies, including Alkermes, Allergan, Clexio Biosciences, and Janssen. The original article includes a full list of his disclosures.
A version of this article first appeared on Medscape.com.
More research suggests that a single infusion of ketamine combined with counseling may help alcohol-dependent patients curb their drinking.
In a pilot study of 40 participants, those who were randomly assigned to receive intravenous ketamine plus outpatient motivational enhancement therapy (MET) showed greater abstinence rates, longer time to relapse, and fewer heavy drinking days than did those who received MET plus midazolam.
The findings support a U.K. study published late last year showing that a single dose of intravenous ketamine plus therapy that focused on reactivating drinking-related “maladaptive reward memories” reduced drinking urges and alcohol intake more than just ketamine or a placebo infusion alone (Nat Commun. 2019 Nov 26;10[1]:5187).
of the New York State Psychiatric Institute, Columbia University, New York, said in an interview.
“It’s an important area of research to understand in order to make behavioral treatments more effective, and ketamine appears to have the properties to address those vulnerabilities,” Dr. Dakwar said.
The study was published in the American Journal of Psychiatry (2019 Dec 2. doi: 10.1176/appi.ajp.2019.19070684).
Real-world approach
Pathologic alcohol use is responsible for an estimated 3.8% of all deaths globally, yet current interventions for alcohol use disorder have limited efficacy, the researchers noted.
New treatments with innovative mechanisms would be valuable, they added.
Ketamine is a high-affinity N-methyl-d-aspartate receptor (NMDAR) antagonist.
Previously, research offered “promising results” with the use of ketamine for cocaine use disorder, including increased motivation to quit and decreased craving, Dr. Dakwar noted.
“Those results led us to think about how ketamine might be helpful for other substance use disorders, especially given the overlap in clinical vulnerabilities and epidemiology,” he said.
The study from the U.K. researchers was conducted in 90 patients with harmful drinking behavior but who had not been diagnosed with alcohol use disorder.
Dr. Dakwar noted that this was “a nontreatment study. None of the people there had alcohol use disorder; they were heavy drinkers. Also, the effects there were fairly modest.
“My interest was how to integrate ketamine into a clinical, real-world framework that could be helpful for people,” he added.
The study included 40 participants (52.5% women; 70.3% white; mean age, 53 years) with alcohol dependence whose average consumption was five drinks per day.
All entered a 5-week outpatient program of MET, which involved engaging in strategies to promote motivation and self-directed change.
During the program’s second week, the participants were randomly assigned to received a 52-minute IV infusion of ketamine 0.71 mg/kg (n = 17) or the benzodiazepine midazolam 0.025 mg/kg (n = 23).
This ketamine dose was selected “because it was the highest dose tolerated by participants in preliminary studies,” the researchers reported.
“Midazolam was chosen as the active control because it alters consciousness without any known persistent ... effect on alcohol dependence,” they added.
The “timeline follow back method” was used to assess alcohol use after treatment. Abstinence was confirmed by measuring urine ethyl glucuronide levels with urine toxicology tests.
Other measures included use of a visual analogue scale, the Clinical Institute Withdrawal Assessment, and the modified Perceived Stress Scale.
Primary outcome met
Results showed that 47.1% of the ketamine group and 59.1% of the midazolam group used alcohol during the 21 days after treatment infusion; 17.6% and 40.9%, respectively, had a heavy drinking day.
For the primary outcome measure of alcohol abstinence, the “quadratic effect of time was significant” (P = .004), as was time-by-treatment interaction (P less than .001).
Although the model-estimated proportions of alcohol abstinence remained stable for the ketamine group for 21 days post infusion, the proportions decreased significantly for the control group.
The odds of having a heavy drinking day did not change significantly after treatment for the ketamine group (odds ratio, 0.98; P = .74) but increased significantly with each postinfusion day for the midazolam group (OR, 1.19; P less than .001).
For the ketamine group, time to relapse was also significantly longer (P = .04).
No significant differences were found between the groups in rates of withdrawal, craving, or stress sensitivity.
A new direction?
The most common adverse events after treatment were sedation, seen in 12 members of the midazolam group and in 8 members of the ketamine group, and headache, seen in four and six members, respectively.
Although two ketamine-group members experienced mild agitation for up to 1 hour post infusion, no incidents of persistent psychoactive effects were reported in either group.
No participants who received ketamine dropped out during the study period; among those who received midazolam, six dropped out.
“These preliminary data suggest new directions in integrated pharmacotherapy-behavioral treatments for alcohol use disorder,” the investigators wrote.
However, a larger patient population will be needed in future research in order to “replicate these promising results,” they added.
Dr. Dakwar noted that the time to first drink after treatment was comparable between the groups.
“But what was different in the ketamine group was that they didn’t continue drinking after that first drink. They didn’t initiate heavy drinking, they didn’t relapse, they were able to bounce back and stay with the program,” he said.
“It was surprising but still consistent with the central hypothesis that ketamine provides this opportunity for setting the foundation for the requisite commitment so that, once things become difficult, they’re still able to maintain recovery,” Dr. Dakwar said.
‘Provocative findings’
In an accompanying editorial, Sanjay J. Mathew, MD, of the department of psychiatry and behavioral sciences at Baylor College of Medicine in Houston, and Rebecca B. Price, PhD, of the department of psychiatry at the University of Pittsburgh, noted that ketamine’s effects on abstinence “were robust” in this trial.
“It is also noteworthy that, in spite of recruiting from a population of patients with active and significant substance use history (a group that has routinely been excluded from ketamine trials in depression), no participant showed evidence of new drug-seeking behaviors,” Dr. Mathew and Dr. Price wrote.
“Overall, these findings are provocative and hypothesis generating but certainly not definitive because of the small sample size,” they add.
Other limitations cited include the short follow-up period and the fact that only half of the participants were available for a 6-month follow-up telephone interview. In addition, generalizability was limited because the population did not have additional medical or psychiatric illnesses or additional substance use disorders, the editorialists wrote.
Because of the limitations, the investigators “are appropriately circumspect about the immediate clinical implications of this small pilot study.”
Still, the results “affirm the potential of rational combinatorial approaches for a vexing medical and public health problem,” Dr. Mathew and Dr. Price concluded.
The study was funded by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute. The study authors and Dr. Price reported no relevant financial relationships. Dr Mathew reported serving as a consultant to or having received research support from several companies, including Alkermes, Allergan, Clexio Biosciences, and Janssen. The original article includes a full list of his disclosures.
A version of this article first appeared on Medscape.com.
More research suggests that a single infusion of ketamine combined with counseling may help alcohol-dependent patients curb their drinking.
In a pilot study of 40 participants, those who were randomly assigned to receive intravenous ketamine plus outpatient motivational enhancement therapy (MET) showed greater abstinence rates, longer time to relapse, and fewer heavy drinking days than did those who received MET plus midazolam.
The findings support a U.K. study published late last year showing that a single dose of intravenous ketamine plus therapy that focused on reactivating drinking-related “maladaptive reward memories” reduced drinking urges and alcohol intake more than just ketamine or a placebo infusion alone (Nat Commun. 2019 Nov 26;10[1]:5187).
of the New York State Psychiatric Institute, Columbia University, New York, said in an interview.
“It’s an important area of research to understand in order to make behavioral treatments more effective, and ketamine appears to have the properties to address those vulnerabilities,” Dr. Dakwar said.
The study was published in the American Journal of Psychiatry (2019 Dec 2. doi: 10.1176/appi.ajp.2019.19070684).
Real-world approach
Pathologic alcohol use is responsible for an estimated 3.8% of all deaths globally, yet current interventions for alcohol use disorder have limited efficacy, the researchers noted.
New treatments with innovative mechanisms would be valuable, they added.
Ketamine is a high-affinity N-methyl-d-aspartate receptor (NMDAR) antagonist.
Previously, research offered “promising results” with the use of ketamine for cocaine use disorder, including increased motivation to quit and decreased craving, Dr. Dakwar noted.
“Those results led us to think about how ketamine might be helpful for other substance use disorders, especially given the overlap in clinical vulnerabilities and epidemiology,” he said.
The study from the U.K. researchers was conducted in 90 patients with harmful drinking behavior but who had not been diagnosed with alcohol use disorder.
Dr. Dakwar noted that this was “a nontreatment study. None of the people there had alcohol use disorder; they were heavy drinkers. Also, the effects there were fairly modest.
“My interest was how to integrate ketamine into a clinical, real-world framework that could be helpful for people,” he added.
The study included 40 participants (52.5% women; 70.3% white; mean age, 53 years) with alcohol dependence whose average consumption was five drinks per day.
All entered a 5-week outpatient program of MET, which involved engaging in strategies to promote motivation and self-directed change.
During the program’s second week, the participants were randomly assigned to received a 52-minute IV infusion of ketamine 0.71 mg/kg (n = 17) or the benzodiazepine midazolam 0.025 mg/kg (n = 23).
This ketamine dose was selected “because it was the highest dose tolerated by participants in preliminary studies,” the researchers reported.
“Midazolam was chosen as the active control because it alters consciousness without any known persistent ... effect on alcohol dependence,” they added.
The “timeline follow back method” was used to assess alcohol use after treatment. Abstinence was confirmed by measuring urine ethyl glucuronide levels with urine toxicology tests.
Other measures included use of a visual analogue scale, the Clinical Institute Withdrawal Assessment, and the modified Perceived Stress Scale.
Primary outcome met
Results showed that 47.1% of the ketamine group and 59.1% of the midazolam group used alcohol during the 21 days after treatment infusion; 17.6% and 40.9%, respectively, had a heavy drinking day.
For the primary outcome measure of alcohol abstinence, the “quadratic effect of time was significant” (P = .004), as was time-by-treatment interaction (P less than .001).
Although the model-estimated proportions of alcohol abstinence remained stable for the ketamine group for 21 days post infusion, the proportions decreased significantly for the control group.
The odds of having a heavy drinking day did not change significantly after treatment for the ketamine group (odds ratio, 0.98; P = .74) but increased significantly with each postinfusion day for the midazolam group (OR, 1.19; P less than .001).
For the ketamine group, time to relapse was also significantly longer (P = .04).
No significant differences were found between the groups in rates of withdrawal, craving, or stress sensitivity.
A new direction?
The most common adverse events after treatment were sedation, seen in 12 members of the midazolam group and in 8 members of the ketamine group, and headache, seen in four and six members, respectively.
Although two ketamine-group members experienced mild agitation for up to 1 hour post infusion, no incidents of persistent psychoactive effects were reported in either group.
No participants who received ketamine dropped out during the study period; among those who received midazolam, six dropped out.
“These preliminary data suggest new directions in integrated pharmacotherapy-behavioral treatments for alcohol use disorder,” the investigators wrote.
However, a larger patient population will be needed in future research in order to “replicate these promising results,” they added.
Dr. Dakwar noted that the time to first drink after treatment was comparable between the groups.
“But what was different in the ketamine group was that they didn’t continue drinking after that first drink. They didn’t initiate heavy drinking, they didn’t relapse, they were able to bounce back and stay with the program,” he said.
“It was surprising but still consistent with the central hypothesis that ketamine provides this opportunity for setting the foundation for the requisite commitment so that, once things become difficult, they’re still able to maintain recovery,” Dr. Dakwar said.
‘Provocative findings’
In an accompanying editorial, Sanjay J. Mathew, MD, of the department of psychiatry and behavioral sciences at Baylor College of Medicine in Houston, and Rebecca B. Price, PhD, of the department of psychiatry at the University of Pittsburgh, noted that ketamine’s effects on abstinence “were robust” in this trial.
“It is also noteworthy that, in spite of recruiting from a population of patients with active and significant substance use history (a group that has routinely been excluded from ketamine trials in depression), no participant showed evidence of new drug-seeking behaviors,” Dr. Mathew and Dr. Price wrote.
“Overall, these findings are provocative and hypothesis generating but certainly not definitive because of the small sample size,” they add.
Other limitations cited include the short follow-up period and the fact that only half of the participants were available for a 6-month follow-up telephone interview. In addition, generalizability was limited because the population did not have additional medical or psychiatric illnesses or additional substance use disorders, the editorialists wrote.
Because of the limitations, the investigators “are appropriately circumspect about the immediate clinical implications of this small pilot study.”
Still, the results “affirm the potential of rational combinatorial approaches for a vexing medical and public health problem,” Dr. Mathew and Dr. Price concluded.
The study was funded by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute. The study authors and Dr. Price reported no relevant financial relationships. Dr Mathew reported serving as a consultant to or having received research support from several companies, including Alkermes, Allergan, Clexio Biosciences, and Janssen. The original article includes a full list of his disclosures.
A version of this article first appeared on Medscape.com.