User login
Drug survival study looks at what lasts longest in RA, axSpA, PsA, and psoriasis
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Antiseptic as good as antibiotics for preventing recurrent UTI
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
Radioactive iodine shows no benefit in low-risk thyroid cancer
, suggesting these patients can be spared the previously common treatment.
The study’s take-home message for clinicians should be to “stop systematic radioiodine ablation administration in low-risk thyroid cancer patients,” lead author Sophie Leboulleux, MD, PhD, said in an interview.
The results were first reported at ENDO 2021 and have now been published in the New England Journal of Medicine by Dr. Leboulleux, of the department of nuclear medicine and endocrine oncology, Gustave Roussy Cancer Institute, Villejuif, France, and colleagues.
While American Thyroid Association (ATA) guidelines already indicate that radioiodine ablation is not routinely recommended after thyroidectomy for patients with low-risk thyroid cancer, the guidance is only a “weak recommendation,” supported by “low-quality evidence.”
However, the new findings should give that level of evidence a much-needed boost, said one expert. “I think the main contribution of this paper is to change the evidence level to ‘high quality,’ therefore making the recommendation ‘strong,’ rather than ‘weak,’ ” David S. Cooper, MD, said in an interview.
Dr. Cooper, professor of medicine and radiology at Johns Hopkins University, Baltimore, wrote an editorial that accompanies Dr. Leboulleux’s study.
The ability to safely spare patients the radioiodine ablation step after thyroidectomy has important benefits in terms of cost and convenience, Dr. Cooper stressed.
ESTIMABL2 trial
The new findings are from the prospective, randomized, phase 3 Essai Stimulation Ablation 2 (ESTIMABL2) trial, in which 730 patients at 35 centers in France with low-risk DTC scheduled to undergo thyroidectomy were enrolled between May 2013 and March 2017.
Patients were randomized to receive either postoperative radioiodine ablation (1.1 GBq) after injections of recombinant human thyrotropin (n = 363) or no postoperative radioiodine (n = 367).
Patients were a mean age of 52 years and 83% were women. About 96% had papillary tumors, and pathological tumor node (pTN) stages were mostly pT1b thyroid with a nodal status of N0 or Nx (81.1%). It is these patients in particular in whom retrospective studies of the use of radioiodine ablation have yielded inconsistent results, Dr. Leboulleux and colleagues noted. Hence, their decision to look at this prospectively.
Outcomes were based on the groups’ rates of events, defined as the presence of abnormal foci of radioiodine uptake on whole-body scanning that required treatment (in the radioiodine group only), abnormal findings on neck ultrasonography, or increased levels of thyroglobulin or thyroglobulin antibodies.
After a 3-year follow-up, the rates of having no events in both groups were very high – and nearly identical – at 95.6% among those receiving no radioiodine ablation and 95.9% in the radioiodine group, for a between-group difference of –0.3 percentage points, which met the criteria for noninferiority for the no-radioiodine group.
Likewise, the events that did occur were nearly equally split between the no-radioiodine group (16 events, 4.4%) and the radioiodine group (15 events, 4.1%).
Among patients who had events, subsequent treatments, including surgery, radioiodine administration, or both, were necessary for four patients in the no-radioiodine group and 10 in the radioiodine group, and additional treatments were not necessary for the other patients who experienced events.
There were no differences between those who did and did not experience events in terms of molecular alterations, and 50 of the tumors had BRAF mutations, with no significant differences between groups.
Of the adverse events that occurred in 30 patients, none were determined to be related to treatment, and there were no thyroid-related deaths.
The recurrence rates align with the rates observed overall with low-risk thyroid cancer, the authors noted.
“We observed that less than 5% of the patients in the two groups had events that included abnormal findings on whole-body scanning or neck ultrasonography or elevated levels of thyroglobulin or thyroglobulin antibodies during the first 3 years of follow-up,” they reported.
“This rate is concordant with the definition of low-risk thyroid cancer, and our trial showed that the risk of events was not higher in the absence of postoperative administration of radioiodine.”
Patients spared costs, work losses
Dr. Cooper elaborated on the advantages, for patients, of avoiding radioiodine ablation.
For one thing, the recombinant human TSH that is necessary to prepare for radioiodine therapy is very expensive, ranging from $2,000 to $3,000, with patients often having a copay, he explained.
“Patients usually have to take time off work, which is also an expense to society and to them if they don’t get paid for days that they don’t work,” Dr. Cooper added.
A possible study limitation is the question of whether 3 years is an ample follow-up period to detect events. However, Dr. Cooper said he considers the period to be sufficient.
“As the authors point out, most recurrences of thyroid cancer are detected within the first 3-5 years of initial treatment, so ... the 3-year window is still clinically relevant,” he said.
Regarding the study’s inclusion of centers only in France, Dr. Cooper added, “I do not think that this is a study limitation. There is nothing specific about the French population that would lead me to conclude that the results were not generalizable to all populations with low-risk papillary thyroid cancer.”
Some continue radioiodine use, but lobectomies add to decline
Despite the mounting evidence of the lack of benefit of radioiodine ablation in low-risk patients, some centers, particularly in Europe, continue the practice, which was standard in the treatment of DTC until relatively recently.
“[While] U.S. guidelines changed in 2015 in favor of no radioiodine in low-risk differentiated thyroid cancer patients, this study should help to change European guidelines,” Dr. Leboulleux said. “The results will help to change practice both in the U.S. and in Europe.”
In addition to awareness of guidelines and new evidence, another reason for the decline in radioiodine ablation for low-risk DTC is the increasing use of thyroid lobectomy, which does not involve the use of radioiodine ablation, rather than total thyroidectomy, Dr. Cooper noted.
“The [new] NEJM paper will hopefully decrease the inappropriate use of radioiodine in low-risk patients even further,” he concluded.
The study received support from the French Ministry of Health through a grant from the National Cancer Institute. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggesting these patients can be spared the previously common treatment.
The study’s take-home message for clinicians should be to “stop systematic radioiodine ablation administration in low-risk thyroid cancer patients,” lead author Sophie Leboulleux, MD, PhD, said in an interview.
The results were first reported at ENDO 2021 and have now been published in the New England Journal of Medicine by Dr. Leboulleux, of the department of nuclear medicine and endocrine oncology, Gustave Roussy Cancer Institute, Villejuif, France, and colleagues.
While American Thyroid Association (ATA) guidelines already indicate that radioiodine ablation is not routinely recommended after thyroidectomy for patients with low-risk thyroid cancer, the guidance is only a “weak recommendation,” supported by “low-quality evidence.”
However, the new findings should give that level of evidence a much-needed boost, said one expert. “I think the main contribution of this paper is to change the evidence level to ‘high quality,’ therefore making the recommendation ‘strong,’ rather than ‘weak,’ ” David S. Cooper, MD, said in an interview.
Dr. Cooper, professor of medicine and radiology at Johns Hopkins University, Baltimore, wrote an editorial that accompanies Dr. Leboulleux’s study.
The ability to safely spare patients the radioiodine ablation step after thyroidectomy has important benefits in terms of cost and convenience, Dr. Cooper stressed.
ESTIMABL2 trial
The new findings are from the prospective, randomized, phase 3 Essai Stimulation Ablation 2 (ESTIMABL2) trial, in which 730 patients at 35 centers in France with low-risk DTC scheduled to undergo thyroidectomy were enrolled between May 2013 and March 2017.
Patients were randomized to receive either postoperative radioiodine ablation (1.1 GBq) after injections of recombinant human thyrotropin (n = 363) or no postoperative radioiodine (n = 367).
Patients were a mean age of 52 years and 83% were women. About 96% had papillary tumors, and pathological tumor node (pTN) stages were mostly pT1b thyroid with a nodal status of N0 or Nx (81.1%). It is these patients in particular in whom retrospective studies of the use of radioiodine ablation have yielded inconsistent results, Dr. Leboulleux and colleagues noted. Hence, their decision to look at this prospectively.
Outcomes were based on the groups’ rates of events, defined as the presence of abnormal foci of radioiodine uptake on whole-body scanning that required treatment (in the radioiodine group only), abnormal findings on neck ultrasonography, or increased levels of thyroglobulin or thyroglobulin antibodies.
After a 3-year follow-up, the rates of having no events in both groups were very high – and nearly identical – at 95.6% among those receiving no radioiodine ablation and 95.9% in the radioiodine group, for a between-group difference of –0.3 percentage points, which met the criteria for noninferiority for the no-radioiodine group.
Likewise, the events that did occur were nearly equally split between the no-radioiodine group (16 events, 4.4%) and the radioiodine group (15 events, 4.1%).
Among patients who had events, subsequent treatments, including surgery, radioiodine administration, or both, were necessary for four patients in the no-radioiodine group and 10 in the radioiodine group, and additional treatments were not necessary for the other patients who experienced events.
There were no differences between those who did and did not experience events in terms of molecular alterations, and 50 of the tumors had BRAF mutations, with no significant differences between groups.
Of the adverse events that occurred in 30 patients, none were determined to be related to treatment, and there were no thyroid-related deaths.
The recurrence rates align with the rates observed overall with low-risk thyroid cancer, the authors noted.
“We observed that less than 5% of the patients in the two groups had events that included abnormal findings on whole-body scanning or neck ultrasonography or elevated levels of thyroglobulin or thyroglobulin antibodies during the first 3 years of follow-up,” they reported.
“This rate is concordant with the definition of low-risk thyroid cancer, and our trial showed that the risk of events was not higher in the absence of postoperative administration of radioiodine.”
Patients spared costs, work losses
Dr. Cooper elaborated on the advantages, for patients, of avoiding radioiodine ablation.
For one thing, the recombinant human TSH that is necessary to prepare for radioiodine therapy is very expensive, ranging from $2,000 to $3,000, with patients often having a copay, he explained.
“Patients usually have to take time off work, which is also an expense to society and to them if they don’t get paid for days that they don’t work,” Dr. Cooper added.
A possible study limitation is the question of whether 3 years is an ample follow-up period to detect events. However, Dr. Cooper said he considers the period to be sufficient.
“As the authors point out, most recurrences of thyroid cancer are detected within the first 3-5 years of initial treatment, so ... the 3-year window is still clinically relevant,” he said.
Regarding the study’s inclusion of centers only in France, Dr. Cooper added, “I do not think that this is a study limitation. There is nothing specific about the French population that would lead me to conclude that the results were not generalizable to all populations with low-risk papillary thyroid cancer.”
Some continue radioiodine use, but lobectomies add to decline
Despite the mounting evidence of the lack of benefit of radioiodine ablation in low-risk patients, some centers, particularly in Europe, continue the practice, which was standard in the treatment of DTC until relatively recently.
“[While] U.S. guidelines changed in 2015 in favor of no radioiodine in low-risk differentiated thyroid cancer patients, this study should help to change European guidelines,” Dr. Leboulleux said. “The results will help to change practice both in the U.S. and in Europe.”
In addition to awareness of guidelines and new evidence, another reason for the decline in radioiodine ablation for low-risk DTC is the increasing use of thyroid lobectomy, which does not involve the use of radioiodine ablation, rather than total thyroidectomy, Dr. Cooper noted.
“The [new] NEJM paper will hopefully decrease the inappropriate use of radioiodine in low-risk patients even further,” he concluded.
The study received support from the French Ministry of Health through a grant from the National Cancer Institute. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggesting these patients can be spared the previously common treatment.
The study’s take-home message for clinicians should be to “stop systematic radioiodine ablation administration in low-risk thyroid cancer patients,” lead author Sophie Leboulleux, MD, PhD, said in an interview.
The results were first reported at ENDO 2021 and have now been published in the New England Journal of Medicine by Dr. Leboulleux, of the department of nuclear medicine and endocrine oncology, Gustave Roussy Cancer Institute, Villejuif, France, and colleagues.
While American Thyroid Association (ATA) guidelines already indicate that radioiodine ablation is not routinely recommended after thyroidectomy for patients with low-risk thyroid cancer, the guidance is only a “weak recommendation,” supported by “low-quality evidence.”
However, the new findings should give that level of evidence a much-needed boost, said one expert. “I think the main contribution of this paper is to change the evidence level to ‘high quality,’ therefore making the recommendation ‘strong,’ rather than ‘weak,’ ” David S. Cooper, MD, said in an interview.
Dr. Cooper, professor of medicine and radiology at Johns Hopkins University, Baltimore, wrote an editorial that accompanies Dr. Leboulleux’s study.
The ability to safely spare patients the radioiodine ablation step after thyroidectomy has important benefits in terms of cost and convenience, Dr. Cooper stressed.
ESTIMABL2 trial
The new findings are from the prospective, randomized, phase 3 Essai Stimulation Ablation 2 (ESTIMABL2) trial, in which 730 patients at 35 centers in France with low-risk DTC scheduled to undergo thyroidectomy were enrolled between May 2013 and March 2017.
Patients were randomized to receive either postoperative radioiodine ablation (1.1 GBq) after injections of recombinant human thyrotropin (n = 363) or no postoperative radioiodine (n = 367).
Patients were a mean age of 52 years and 83% were women. About 96% had papillary tumors, and pathological tumor node (pTN) stages were mostly pT1b thyroid with a nodal status of N0 or Nx (81.1%). It is these patients in particular in whom retrospective studies of the use of radioiodine ablation have yielded inconsistent results, Dr. Leboulleux and colleagues noted. Hence, their decision to look at this prospectively.
Outcomes were based on the groups’ rates of events, defined as the presence of abnormal foci of radioiodine uptake on whole-body scanning that required treatment (in the radioiodine group only), abnormal findings on neck ultrasonography, or increased levels of thyroglobulin or thyroglobulin antibodies.
After a 3-year follow-up, the rates of having no events in both groups were very high – and nearly identical – at 95.6% among those receiving no radioiodine ablation and 95.9% in the radioiodine group, for a between-group difference of –0.3 percentage points, which met the criteria for noninferiority for the no-radioiodine group.
Likewise, the events that did occur were nearly equally split between the no-radioiodine group (16 events, 4.4%) and the radioiodine group (15 events, 4.1%).
Among patients who had events, subsequent treatments, including surgery, radioiodine administration, or both, were necessary for four patients in the no-radioiodine group and 10 in the radioiodine group, and additional treatments were not necessary for the other patients who experienced events.
There were no differences between those who did and did not experience events in terms of molecular alterations, and 50 of the tumors had BRAF mutations, with no significant differences between groups.
Of the adverse events that occurred in 30 patients, none were determined to be related to treatment, and there were no thyroid-related deaths.
The recurrence rates align with the rates observed overall with low-risk thyroid cancer, the authors noted.
“We observed that less than 5% of the patients in the two groups had events that included abnormal findings on whole-body scanning or neck ultrasonography or elevated levels of thyroglobulin or thyroglobulin antibodies during the first 3 years of follow-up,” they reported.
“This rate is concordant with the definition of low-risk thyroid cancer, and our trial showed that the risk of events was not higher in the absence of postoperative administration of radioiodine.”
Patients spared costs, work losses
Dr. Cooper elaborated on the advantages, for patients, of avoiding radioiodine ablation.
For one thing, the recombinant human TSH that is necessary to prepare for radioiodine therapy is very expensive, ranging from $2,000 to $3,000, with patients often having a copay, he explained.
“Patients usually have to take time off work, which is also an expense to society and to them if they don’t get paid for days that they don’t work,” Dr. Cooper added.
A possible study limitation is the question of whether 3 years is an ample follow-up period to detect events. However, Dr. Cooper said he considers the period to be sufficient.
“As the authors point out, most recurrences of thyroid cancer are detected within the first 3-5 years of initial treatment, so ... the 3-year window is still clinically relevant,” he said.
Regarding the study’s inclusion of centers only in France, Dr. Cooper added, “I do not think that this is a study limitation. There is nothing specific about the French population that would lead me to conclude that the results were not generalizable to all populations with low-risk papillary thyroid cancer.”
Some continue radioiodine use, but lobectomies add to decline
Despite the mounting evidence of the lack of benefit of radioiodine ablation in low-risk patients, some centers, particularly in Europe, continue the practice, which was standard in the treatment of DTC until relatively recently.
“[While] U.S. guidelines changed in 2015 in favor of no radioiodine in low-risk differentiated thyroid cancer patients, this study should help to change European guidelines,” Dr. Leboulleux said. “The results will help to change practice both in the U.S. and in Europe.”
In addition to awareness of guidelines and new evidence, another reason for the decline in radioiodine ablation for low-risk DTC is the increasing use of thyroid lobectomy, which does not involve the use of radioiodine ablation, rather than total thyroidectomy, Dr. Cooper noted.
“The [new] NEJM paper will hopefully decrease the inappropriate use of radioiodine in low-risk patients even further,” he concluded.
The study received support from the French Ministry of Health through a grant from the National Cancer Institute. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Drug Overdose Suicide Rates: Down, But Also Up
Who is most at risk of suicide by drug overdose? Has that changed in recent years? Researchers at the National Institute on Drug Abuse analyzed data from 2001 to 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System to find out.
On the whole, they say, intentional overdose deaths have declined. But suicide rates increased in certain subgroups: young adults (aged 15-24 years), older adults (aged 75-84 years), and non-Hispanic Black women. Rates among women were “consistently higher” than those of men. The highest rates were observed in women aged 45 to 64 years.
Monday was the worst day, and the weekends had the lowest rates. The researchers say social factors, such as more social interactions on the weekend and reluctance about starting the workweek, could be factors.
Seasonally, the numbers ran true to the pattern seen in previous studies: The lowest rates occurred in December and highest in late spring and summer. Perhaps the “collective optimism” of the holiday season and social interactions exert protective effects against suicidality, the researchers suggest.
Factors also may include biological changes. In this study, the researchers found a positive linear relationship between daylength, which varies by latitude, and intentional overdose deaths for both sexes. Daylength is associated with mu opioid receptor (MOR) availability that might underlie seasonal variations in mood, they posit. MORs are the main target of opioid drugs; the researchers cite a study that found altered MOR expression in postmortem brains of suicide victims.
They note some limitations of their study, one being that, in 2019, 5% of overdose deaths had undetermined intent. Improving classifications of overdose deaths is needed, they say.
Moreover, the trends might have changed during the pandemic, as provisional mortality data indicate decreases in deaths by suicides, but also an approximate 30% increase in overall overdose deaths.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said Emily B. Einstein, PhD, chief of the National Institute on Drug Abuse’s Science Policy Branch and an author on the study. “The risk of intentional overdoses, and suicide risk in general, is not static. This is crucial for clinicians to keep in mind, as they may need to assess patients’ suicide risk frequently rather than at one point in time. It is also important for friends and family members of people who may be at an increased risk of suicide, and for those people themselves, so that they can be aware of the greatest periods of risk and seek help when needed.”
Sources: https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-people-black-women-despite-overall-downward-tren
Han B, Compton WM, Einstein EB, et al. Intentional drug overdose deaths in the United States. Am J Psychiatry. doi:10.1176/appi.ajp.2021.21060604
Who is most at risk of suicide by drug overdose? Has that changed in recent years? Researchers at the National Institute on Drug Abuse analyzed data from 2001 to 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System to find out.
On the whole, they say, intentional overdose deaths have declined. But suicide rates increased in certain subgroups: young adults (aged 15-24 years), older adults (aged 75-84 years), and non-Hispanic Black women. Rates among women were “consistently higher” than those of men. The highest rates were observed in women aged 45 to 64 years.
Monday was the worst day, and the weekends had the lowest rates. The researchers say social factors, such as more social interactions on the weekend and reluctance about starting the workweek, could be factors.
Seasonally, the numbers ran true to the pattern seen in previous studies: The lowest rates occurred in December and highest in late spring and summer. Perhaps the “collective optimism” of the holiday season and social interactions exert protective effects against suicidality, the researchers suggest.
Factors also may include biological changes. In this study, the researchers found a positive linear relationship between daylength, which varies by latitude, and intentional overdose deaths for both sexes. Daylength is associated with mu opioid receptor (MOR) availability that might underlie seasonal variations in mood, they posit. MORs are the main target of opioid drugs; the researchers cite a study that found altered MOR expression in postmortem brains of suicide victims.
They note some limitations of their study, one being that, in 2019, 5% of overdose deaths had undetermined intent. Improving classifications of overdose deaths is needed, they say.
Moreover, the trends might have changed during the pandemic, as provisional mortality data indicate decreases in deaths by suicides, but also an approximate 30% increase in overall overdose deaths.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said Emily B. Einstein, PhD, chief of the National Institute on Drug Abuse’s Science Policy Branch and an author on the study. “The risk of intentional overdoses, and suicide risk in general, is not static. This is crucial for clinicians to keep in mind, as they may need to assess patients’ suicide risk frequently rather than at one point in time. It is also important for friends and family members of people who may be at an increased risk of suicide, and for those people themselves, so that they can be aware of the greatest periods of risk and seek help when needed.”
Sources: https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-people-black-women-despite-overall-downward-tren
Han B, Compton WM, Einstein EB, et al. Intentional drug overdose deaths in the United States. Am J Psychiatry. doi:10.1176/appi.ajp.2021.21060604
Who is most at risk of suicide by drug overdose? Has that changed in recent years? Researchers at the National Institute on Drug Abuse analyzed data from 2001 to 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System to find out.
On the whole, they say, intentional overdose deaths have declined. But suicide rates increased in certain subgroups: young adults (aged 15-24 years), older adults (aged 75-84 years), and non-Hispanic Black women. Rates among women were “consistently higher” than those of men. The highest rates were observed in women aged 45 to 64 years.
Monday was the worst day, and the weekends had the lowest rates. The researchers say social factors, such as more social interactions on the weekend and reluctance about starting the workweek, could be factors.
Seasonally, the numbers ran true to the pattern seen in previous studies: The lowest rates occurred in December and highest in late spring and summer. Perhaps the “collective optimism” of the holiday season and social interactions exert protective effects against suicidality, the researchers suggest.
Factors also may include biological changes. In this study, the researchers found a positive linear relationship between daylength, which varies by latitude, and intentional overdose deaths for both sexes. Daylength is associated with mu opioid receptor (MOR) availability that might underlie seasonal variations in mood, they posit. MORs are the main target of opioid drugs; the researchers cite a study that found altered MOR expression in postmortem brains of suicide victims.
They note some limitations of their study, one being that, in 2019, 5% of overdose deaths had undetermined intent. Improving classifications of overdose deaths is needed, they say.
Moreover, the trends might have changed during the pandemic, as provisional mortality data indicate decreases in deaths by suicides, but also an approximate 30% increase in overall overdose deaths.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said Emily B. Einstein, PhD, chief of the National Institute on Drug Abuse’s Science Policy Branch and an author on the study. “The risk of intentional overdoses, and suicide risk in general, is not static. This is crucial for clinicians to keep in mind, as they may need to assess patients’ suicide risk frequently rather than at one point in time. It is also important for friends and family members of people who may be at an increased risk of suicide, and for those people themselves, so that they can be aware of the greatest periods of risk and seek help when needed.”
Sources: https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-people-black-women-despite-overall-downward-tren
Han B, Compton WM, Einstein EB, et al. Intentional drug overdose deaths in the United States. Am J Psychiatry. doi:10.1176/appi.ajp.2021.21060604
Clozapine interrupted: APA, others seek FDA forum on REMS
In a Feb. 14 letter, the groups asked the FDA to reconsider its new risk evaluation and mitigation strategy (REMS) for clozapine because of concerns it had the potential to cause abrupt discontinuation of the medication.
The groups cite an Institute for Safe Medication Practices (ISMP) report of a 40-year-old woman who was a long-time clozapine user, had a cardiac arrest, and died after she stopped taking the drug because her psychiatrist was unable to register for the updated version of the REMS.
“It is unacceptable for a REMS with unproven effectiveness at meeting its goal to carry risks of interruptions that can result in rehospitalization, acute exacerbation of psychosis, increased risk of suicide, and potentially fatal orthostatic hypotension/bradycardic syndromes associated with incorrect restarts,” the groups said in the letter.
“We feel certain that this case reported in the literature is not the only serious adverse outcome from the REMS and the transition,” they added.
The letter was signed by the American Psychiatric Association, the American Association for Community Psychiatry, the American Psychiatric Nurses Association, the College of Psychiatric and Neurologic Pharmacists, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, and the National Council for Mental Wellbeing.
Clozapine can decrease the neutrophil count, which can lead to severe neutropenia, serious infection, and death. Consequently, the FDA put additional safety measures in place governing clozapine prescribing.
In 2015, a centralized clozapine REMS replaced separate prescribing registries that the drug manufacturers maintained. There were technical issues with the 2015 start-up of that website, including data migration problems and long call wait times, the FDA said.
Subsequently, the drug’s manufacturers then decided to change the REMS platform, which created new issues that led to high call volume and long wait times for clinicians and pharmacists who were trying to enroll.
Maintaining access
In November 2021, the FDA announced it would put some aspects of a planned switch on hold. A month later, the agency made further modifications to its plan.
The FDA said it would exercise “enforcement discretion” to try to maintain access to clozapine amid hitches with the REMS transition efforts. The agency also said at the time that it would not object if pharmacists dispensed clozapine without the usual authorization. In addition, wholesalers could ship the drug to pharmacies and health care settings without confirming REMS enrollment.
The FDA also held two December meetings to allow various stakeholders to air concerns.
In their letter, the APA and other groups asked if the FDA intends to continue with accommodations, such as allowing pharmacies to order clozapine from wholesalers without restriction.
“We do not feel the issues are resolved,” the groups said.
A version of this article first appeared on Medscape.com.
In a Feb. 14 letter, the groups asked the FDA to reconsider its new risk evaluation and mitigation strategy (REMS) for clozapine because of concerns it had the potential to cause abrupt discontinuation of the medication.
The groups cite an Institute for Safe Medication Practices (ISMP) report of a 40-year-old woman who was a long-time clozapine user, had a cardiac arrest, and died after she stopped taking the drug because her psychiatrist was unable to register for the updated version of the REMS.
“It is unacceptable for a REMS with unproven effectiveness at meeting its goal to carry risks of interruptions that can result in rehospitalization, acute exacerbation of psychosis, increased risk of suicide, and potentially fatal orthostatic hypotension/bradycardic syndromes associated with incorrect restarts,” the groups said in the letter.
“We feel certain that this case reported in the literature is not the only serious adverse outcome from the REMS and the transition,” they added.
The letter was signed by the American Psychiatric Association, the American Association for Community Psychiatry, the American Psychiatric Nurses Association, the College of Psychiatric and Neurologic Pharmacists, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, and the National Council for Mental Wellbeing.
Clozapine can decrease the neutrophil count, which can lead to severe neutropenia, serious infection, and death. Consequently, the FDA put additional safety measures in place governing clozapine prescribing.
In 2015, a centralized clozapine REMS replaced separate prescribing registries that the drug manufacturers maintained. There were technical issues with the 2015 start-up of that website, including data migration problems and long call wait times, the FDA said.
Subsequently, the drug’s manufacturers then decided to change the REMS platform, which created new issues that led to high call volume and long wait times for clinicians and pharmacists who were trying to enroll.
Maintaining access
In November 2021, the FDA announced it would put some aspects of a planned switch on hold. A month later, the agency made further modifications to its plan.
The FDA said it would exercise “enforcement discretion” to try to maintain access to clozapine amid hitches with the REMS transition efforts. The agency also said at the time that it would not object if pharmacists dispensed clozapine without the usual authorization. In addition, wholesalers could ship the drug to pharmacies and health care settings without confirming REMS enrollment.
The FDA also held two December meetings to allow various stakeholders to air concerns.
In their letter, the APA and other groups asked if the FDA intends to continue with accommodations, such as allowing pharmacies to order clozapine from wholesalers without restriction.
“We do not feel the issues are resolved,” the groups said.
A version of this article first appeared on Medscape.com.
In a Feb. 14 letter, the groups asked the FDA to reconsider its new risk evaluation and mitigation strategy (REMS) for clozapine because of concerns it had the potential to cause abrupt discontinuation of the medication.
The groups cite an Institute for Safe Medication Practices (ISMP) report of a 40-year-old woman who was a long-time clozapine user, had a cardiac arrest, and died after she stopped taking the drug because her psychiatrist was unable to register for the updated version of the REMS.
“It is unacceptable for a REMS with unproven effectiveness at meeting its goal to carry risks of interruptions that can result in rehospitalization, acute exacerbation of psychosis, increased risk of suicide, and potentially fatal orthostatic hypotension/bradycardic syndromes associated with incorrect restarts,” the groups said in the letter.
“We feel certain that this case reported in the literature is not the only serious adverse outcome from the REMS and the transition,” they added.
The letter was signed by the American Psychiatric Association, the American Association for Community Psychiatry, the American Psychiatric Nurses Association, the College of Psychiatric and Neurologic Pharmacists, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, and the National Council for Mental Wellbeing.
Clozapine can decrease the neutrophil count, which can lead to severe neutropenia, serious infection, and death. Consequently, the FDA put additional safety measures in place governing clozapine prescribing.
In 2015, a centralized clozapine REMS replaced separate prescribing registries that the drug manufacturers maintained. There were technical issues with the 2015 start-up of that website, including data migration problems and long call wait times, the FDA said.
Subsequently, the drug’s manufacturers then decided to change the REMS platform, which created new issues that led to high call volume and long wait times for clinicians and pharmacists who were trying to enroll.
Maintaining access
In November 2021, the FDA announced it would put some aspects of a planned switch on hold. A month later, the agency made further modifications to its plan.
The FDA said it would exercise “enforcement discretion” to try to maintain access to clozapine amid hitches with the REMS transition efforts. The agency also said at the time that it would not object if pharmacists dispensed clozapine without the usual authorization. In addition, wholesalers could ship the drug to pharmacies and health care settings without confirming REMS enrollment.
The FDA also held two December meetings to allow various stakeholders to air concerns.
In their letter, the APA and other groups asked if the FDA intends to continue with accommodations, such as allowing pharmacies to order clozapine from wholesalers without restriction.
“We do not feel the issues are resolved,” the groups said.
A version of this article first appeared on Medscape.com.
Mental Health Pharmacists: Increasing Necessary Mental Health Service Delivery
The COVID-19 pandemic has significantly impacted mental health. Adolescents, adults, and health care professionals (HCPs) report worsening mental health outcomes since the pandemic.1-3 Anxiety rates have tripled, depression quadrupled, and substance and alcohol use also have increased.3 The World Health Organization (WHO) reported that during the COVID-19 pandemic, 93% of countries worldwide documented disruptions to mental health services.4 HCP shortages, worsened by the pandemic, have resulted in a mental health crisis. What can we do?
Over the past 20 years, pharmacists have assumed a more significant role in managing patients’ mental health conditions through multidisciplinary team engagement. Pharmacists’ training includes optimizing pharmacotherapy, identifying and managing adverse effects (AEs), improving medication adherence, and reducing unnecessary health care costs.5 Pharmacists have assumed pivotal roles in mental health management, including but not limited to screening, drug selection, medication management, and decision-making support for patients and HCPs. Pharmacist-provided services have led to improved medication therapy outcomes and patient satisfaction.6
According to the 2012 National Alliance on Mental Illness national survey, > 50% of patients treated for a mental health condition report having a strong relationship with their pharmacist.7 The US Department of Veterans Affairs (VA) has led the charge, engaging pharmacists in patient-oriented mental health care,including those specific to accessing mental health care (eg, fear of stigmatization).8 After obtaining a 4-year PharmD degree, psychiatric pharmacists receive additional postgraduate residency training (2 years) focused on direct patient care and then are eligible for board certification. There are about 2000 board-certified psychiatric pharmacists in the United States. Qualified psychiatric pharmacists, especially those in underresourced states, have increased the number of available patient-oriented mental health services.7 However, to continue expanding and improving access to care, we need more HCPs and pharmacists.
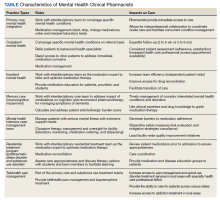
Mental health clinical pharmacy specialists (CPSs) within the VA work in a variety of settings, including but not limited to, the inpatient psychiatric unit; residential programs for posttraumatic stress disorder (PTSD) and substance misuse; as part of the Mental Health Intensive Case Management (MHICM) team; and in pain, telehealth, and other outpatient clinics. The VA’s mental health CPSs operate under an independent scope of practice (SOP) and manage a variety of mental health disorders. The SOP also allows pharmacists to independently manage medications for psychiatric conditions, request laboratory tests, and change therapy as needed based on patient response. The Table describes pharmacist-reported roles in a single VA facility in various mental health practice sites (eg, inpatient, outpatient, substance misuse). Pharmacist involvement in medication management with the interdisciplinary team improved symptoms, medication adherence, and reduced AEs for conditions such as depression.9
Within the VA, the outpatient mental health pharmacist works collaboratively with psychiatrists and HCPs to manage common psychiatric conditions on the phone and in person. VA pharmacists also are involved in the monitoring of patients on second-generation antipsychotics. Pharmacists assist with metabolic monitoring and assessing patients for movements disorders, using standardized rating scales. Pharmacists can manage complex psychiatric patients in collaboration with psychiatrists by providing medication management, laboratory test monitoring, medication counseling, and HCP referrals.
Pharmacists’ expertise is used in diverse ways in the VHA. At one facility, pharmacists functioned as interim prescribers when the facility experienced a turnover in behavioral health professionals. Pharmacists’ involvement decreased inappropriate use of psychiatric emergency services.10 VA pharmacists who manage patients’ mental health needs in primary care help achieve symptom improvement and medication adherence as well as lower referral rates for specialty mental health services.9 Pharmacist-managed electronic consult service provided a costs savings of about $40,000 a year.11
Pharmacists have shown that they can expand their roles. Pharmacists are versatile HCPs, currently working and collaborating with other HCPs in various settings to provide mental health services. Health care systems need to continue to use and expand the number of pharmacists. Including pharmacists in the primary and specialty care teams can increase access to care and improve health outcomes during the pandemic and beyond. The American Association of Colleges of Pharmacy in partnership with the American Medical Association established a resource to support and guide institutions interested in embedding pharmacists into different clinical sites.12 Opportunities for increased services by pharmacists can lead to improved outcomes, timely patient care, appropriate use of psychiatric medications and services, and cost savings.
Acknowledgments
We acknowledge the following Boise Veterans Affairs pharmacists: Paul Black, PharmD; Josh Gerving, PharmD; Kristin Helmboldt, PharmD; Samantha Patton, PharmD; Heather Walser, PharmD; and Andrea Winterswyk, PharmD, for contributing information about their practice roles and impact on patient care.
1. Panchal N, Kamal R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed February 8, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use
2. How the pandemic has impacted teen mental health. National poll on children’s health. Published December 21, 2020. Accessed February 8, 2022. https://mottpoll.org/reports/how-pandemic-has-impacted-teen-mental-health
3. Substance Abuse and Mental Health Services Administration. A preliminary look at the mental health and substance use-related effects of the COVID-19 pandemic. Published May 2021. Accessed February 8, 2022. https://www.samhsa.gov/sites/default/files/dtac/mental-health-substance-use-effects-covid-pandemic-srb.pdf
4. World Health Organization. News release. COVID-19 disrupting mental health services in most countries, WHO survey. Published October 5, 2020. Accessed February 9, 2022. https://www.who.int/news/item/05-10-2020-covid-19-disrupting-mental-health-services-in-most-countries-who-survey
5. Avalere Health LLC. Exploring pharmacists’ role in a changing healthcare environment. Published May 2014. Accessed February 9, 2022. https://www.nacds.org/pdfs/comm/2014/pharmacist-role.pdf.
6. Silvia R. Collaborative treatment of depression by a psychiatric pharmacist integrated within a community health center primary care clinic. J Pharm Practice. 2016;29(3):270-341. doi:10.1177/0897190016645328
7. Caley C, Stimmel G. Characterizing the relationship between individuals with mental health conditions and community pharmacists. Published 2012. Accessed February 9, 2022. https://www.nami.org/About-NAMI/Publications-Reports/Survey-Reports/nami-cpnp-survey-report2012.pdf
8. Bovin MJ, Koenig CJ, Zamora KA, et al. Veterans’ experiences initiating VA-based mental health care. Psychol Serv. 2019;16(4):612-620. doi:10.1037/ser0000233
9. Herbert C, Winkler H. Impact of a clinical pharmacist–managed clinic in primary care mental health integration at a Veterans Affairs health system. Ment Health Clin. 2018;8(3):105-109. doi:10.9740/mhc.2018.05.105
10. Gibu M, Clark J, Gold J. Mental health pharmacists as interim prescribers. Ment Health Clin. 2018;7(3):111-115. doi:10.9740/mhc.2017.05.111
11. Herbert C, Winkler H, Moore TA. Outcomes of mental health pharmacist-managed electronic consults at a Veterans Affairs health care system. Ment Health Clin. 2018;7(3):131-136. doi:10.9740/mhc.2017.05.131
12. AACP. Embedding pharmacists into the practice. Accessed February 9, 2022. https://edhub.ama-assn.org/steps-forward/module/2702554
The COVID-19 pandemic has significantly impacted mental health. Adolescents, adults, and health care professionals (HCPs) report worsening mental health outcomes since the pandemic.1-3 Anxiety rates have tripled, depression quadrupled, and substance and alcohol use also have increased.3 The World Health Organization (WHO) reported that during the COVID-19 pandemic, 93% of countries worldwide documented disruptions to mental health services.4 HCP shortages, worsened by the pandemic, have resulted in a mental health crisis. What can we do?
Over the past 20 years, pharmacists have assumed a more significant role in managing patients’ mental health conditions through multidisciplinary team engagement. Pharmacists’ training includes optimizing pharmacotherapy, identifying and managing adverse effects (AEs), improving medication adherence, and reducing unnecessary health care costs.5 Pharmacists have assumed pivotal roles in mental health management, including but not limited to screening, drug selection, medication management, and decision-making support for patients and HCPs. Pharmacist-provided services have led to improved medication therapy outcomes and patient satisfaction.6
According to the 2012 National Alliance on Mental Illness national survey, > 50% of patients treated for a mental health condition report having a strong relationship with their pharmacist.7 The US Department of Veterans Affairs (VA) has led the charge, engaging pharmacists in patient-oriented mental health care,including those specific to accessing mental health care (eg, fear of stigmatization).8 After obtaining a 4-year PharmD degree, psychiatric pharmacists receive additional postgraduate residency training (2 years) focused on direct patient care and then are eligible for board certification. There are about 2000 board-certified psychiatric pharmacists in the United States. Qualified psychiatric pharmacists, especially those in underresourced states, have increased the number of available patient-oriented mental health services.7 However, to continue expanding and improving access to care, we need more HCPs and pharmacists.
Mental health clinical pharmacy specialists (CPSs) within the VA work in a variety of settings, including but not limited to, the inpatient psychiatric unit; residential programs for posttraumatic stress disorder (PTSD) and substance misuse; as part of the Mental Health Intensive Case Management (MHICM) team; and in pain, telehealth, and other outpatient clinics. The VA’s mental health CPSs operate under an independent scope of practice (SOP) and manage a variety of mental health disorders. The SOP also allows pharmacists to independently manage medications for psychiatric conditions, request laboratory tests, and change therapy as needed based on patient response. The Table describes pharmacist-reported roles in a single VA facility in various mental health practice sites (eg, inpatient, outpatient, substance misuse). Pharmacist involvement in medication management with the interdisciplinary team improved symptoms, medication adherence, and reduced AEs for conditions such as depression.9
Within the VA, the outpatient mental health pharmacist works collaboratively with psychiatrists and HCPs to manage common psychiatric conditions on the phone and in person. VA pharmacists also are involved in the monitoring of patients on second-generation antipsychotics. Pharmacists assist with metabolic monitoring and assessing patients for movements disorders, using standardized rating scales. Pharmacists can manage complex psychiatric patients in collaboration with psychiatrists by providing medication management, laboratory test monitoring, medication counseling, and HCP referrals.
Pharmacists’ expertise is used in diverse ways in the VHA. At one facility, pharmacists functioned as interim prescribers when the facility experienced a turnover in behavioral health professionals. Pharmacists’ involvement decreased inappropriate use of psychiatric emergency services.10 VA pharmacists who manage patients’ mental health needs in primary care help achieve symptom improvement and medication adherence as well as lower referral rates for specialty mental health services.9 Pharmacist-managed electronic consult service provided a costs savings of about $40,000 a year.11
Pharmacists have shown that they can expand their roles. Pharmacists are versatile HCPs, currently working and collaborating with other HCPs in various settings to provide mental health services. Health care systems need to continue to use and expand the number of pharmacists. Including pharmacists in the primary and specialty care teams can increase access to care and improve health outcomes during the pandemic and beyond. The American Association of Colleges of Pharmacy in partnership with the American Medical Association established a resource to support and guide institutions interested in embedding pharmacists into different clinical sites.12 Opportunities for increased services by pharmacists can lead to improved outcomes, timely patient care, appropriate use of psychiatric medications and services, and cost savings.
Acknowledgments
We acknowledge the following Boise Veterans Affairs pharmacists: Paul Black, PharmD; Josh Gerving, PharmD; Kristin Helmboldt, PharmD; Samantha Patton, PharmD; Heather Walser, PharmD; and Andrea Winterswyk, PharmD, for contributing information about their practice roles and impact on patient care.
The COVID-19 pandemic has significantly impacted mental health. Adolescents, adults, and health care professionals (HCPs) report worsening mental health outcomes since the pandemic.1-3 Anxiety rates have tripled, depression quadrupled, and substance and alcohol use also have increased.3 The World Health Organization (WHO) reported that during the COVID-19 pandemic, 93% of countries worldwide documented disruptions to mental health services.4 HCP shortages, worsened by the pandemic, have resulted in a mental health crisis. What can we do?
Over the past 20 years, pharmacists have assumed a more significant role in managing patients’ mental health conditions through multidisciplinary team engagement. Pharmacists’ training includes optimizing pharmacotherapy, identifying and managing adverse effects (AEs), improving medication adherence, and reducing unnecessary health care costs.5 Pharmacists have assumed pivotal roles in mental health management, including but not limited to screening, drug selection, medication management, and decision-making support for patients and HCPs. Pharmacist-provided services have led to improved medication therapy outcomes and patient satisfaction.6
According to the 2012 National Alliance on Mental Illness national survey, > 50% of patients treated for a mental health condition report having a strong relationship with their pharmacist.7 The US Department of Veterans Affairs (VA) has led the charge, engaging pharmacists in patient-oriented mental health care,including those specific to accessing mental health care (eg, fear of stigmatization).8 After obtaining a 4-year PharmD degree, psychiatric pharmacists receive additional postgraduate residency training (2 years) focused on direct patient care and then are eligible for board certification. There are about 2000 board-certified psychiatric pharmacists in the United States. Qualified psychiatric pharmacists, especially those in underresourced states, have increased the number of available patient-oriented mental health services.7 However, to continue expanding and improving access to care, we need more HCPs and pharmacists.
Mental health clinical pharmacy specialists (CPSs) within the VA work in a variety of settings, including but not limited to, the inpatient psychiatric unit; residential programs for posttraumatic stress disorder (PTSD) and substance misuse; as part of the Mental Health Intensive Case Management (MHICM) team; and in pain, telehealth, and other outpatient clinics. The VA’s mental health CPSs operate under an independent scope of practice (SOP) and manage a variety of mental health disorders. The SOP also allows pharmacists to independently manage medications for psychiatric conditions, request laboratory tests, and change therapy as needed based on patient response. The Table describes pharmacist-reported roles in a single VA facility in various mental health practice sites (eg, inpatient, outpatient, substance misuse). Pharmacist involvement in medication management with the interdisciplinary team improved symptoms, medication adherence, and reduced AEs for conditions such as depression.9
Within the VA, the outpatient mental health pharmacist works collaboratively with psychiatrists and HCPs to manage common psychiatric conditions on the phone and in person. VA pharmacists also are involved in the monitoring of patients on second-generation antipsychotics. Pharmacists assist with metabolic monitoring and assessing patients for movements disorders, using standardized rating scales. Pharmacists can manage complex psychiatric patients in collaboration with psychiatrists by providing medication management, laboratory test monitoring, medication counseling, and HCP referrals.
Pharmacists’ expertise is used in diverse ways in the VHA. At one facility, pharmacists functioned as interim prescribers when the facility experienced a turnover in behavioral health professionals. Pharmacists’ involvement decreased inappropriate use of psychiatric emergency services.10 VA pharmacists who manage patients’ mental health needs in primary care help achieve symptom improvement and medication adherence as well as lower referral rates for specialty mental health services.9 Pharmacist-managed electronic consult service provided a costs savings of about $40,000 a year.11
Pharmacists have shown that they can expand their roles. Pharmacists are versatile HCPs, currently working and collaborating with other HCPs in various settings to provide mental health services. Health care systems need to continue to use and expand the number of pharmacists. Including pharmacists in the primary and specialty care teams can increase access to care and improve health outcomes during the pandemic and beyond. The American Association of Colleges of Pharmacy in partnership with the American Medical Association established a resource to support and guide institutions interested in embedding pharmacists into different clinical sites.12 Opportunities for increased services by pharmacists can lead to improved outcomes, timely patient care, appropriate use of psychiatric medications and services, and cost savings.
Acknowledgments
We acknowledge the following Boise Veterans Affairs pharmacists: Paul Black, PharmD; Josh Gerving, PharmD; Kristin Helmboldt, PharmD; Samantha Patton, PharmD; Heather Walser, PharmD; and Andrea Winterswyk, PharmD, for contributing information about their practice roles and impact on patient care.
1. Panchal N, Kamal R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed February 8, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use
2. How the pandemic has impacted teen mental health. National poll on children’s health. Published December 21, 2020. Accessed February 8, 2022. https://mottpoll.org/reports/how-pandemic-has-impacted-teen-mental-health
3. Substance Abuse and Mental Health Services Administration. A preliminary look at the mental health and substance use-related effects of the COVID-19 pandemic. Published May 2021. Accessed February 8, 2022. https://www.samhsa.gov/sites/default/files/dtac/mental-health-substance-use-effects-covid-pandemic-srb.pdf
4. World Health Organization. News release. COVID-19 disrupting mental health services in most countries, WHO survey. Published October 5, 2020. Accessed February 9, 2022. https://www.who.int/news/item/05-10-2020-covid-19-disrupting-mental-health-services-in-most-countries-who-survey
5. Avalere Health LLC. Exploring pharmacists’ role in a changing healthcare environment. Published May 2014. Accessed February 9, 2022. https://www.nacds.org/pdfs/comm/2014/pharmacist-role.pdf.
6. Silvia R. Collaborative treatment of depression by a psychiatric pharmacist integrated within a community health center primary care clinic. J Pharm Practice. 2016;29(3):270-341. doi:10.1177/0897190016645328
7. Caley C, Stimmel G. Characterizing the relationship between individuals with mental health conditions and community pharmacists. Published 2012. Accessed February 9, 2022. https://www.nami.org/About-NAMI/Publications-Reports/Survey-Reports/nami-cpnp-survey-report2012.pdf
8. Bovin MJ, Koenig CJ, Zamora KA, et al. Veterans’ experiences initiating VA-based mental health care. Psychol Serv. 2019;16(4):612-620. doi:10.1037/ser0000233
9. Herbert C, Winkler H. Impact of a clinical pharmacist–managed clinic in primary care mental health integration at a Veterans Affairs health system. Ment Health Clin. 2018;8(3):105-109. doi:10.9740/mhc.2018.05.105
10. Gibu M, Clark J, Gold J. Mental health pharmacists as interim prescribers. Ment Health Clin. 2018;7(3):111-115. doi:10.9740/mhc.2017.05.111
11. Herbert C, Winkler H, Moore TA. Outcomes of mental health pharmacist-managed electronic consults at a Veterans Affairs health care system. Ment Health Clin. 2018;7(3):131-136. doi:10.9740/mhc.2017.05.131
12. AACP. Embedding pharmacists into the practice. Accessed February 9, 2022. https://edhub.ama-assn.org/steps-forward/module/2702554
1. Panchal N, Kamal R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed February 8, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use
2. How the pandemic has impacted teen mental health. National poll on children’s health. Published December 21, 2020. Accessed February 8, 2022. https://mottpoll.org/reports/how-pandemic-has-impacted-teen-mental-health
3. Substance Abuse and Mental Health Services Administration. A preliminary look at the mental health and substance use-related effects of the COVID-19 pandemic. Published May 2021. Accessed February 8, 2022. https://www.samhsa.gov/sites/default/files/dtac/mental-health-substance-use-effects-covid-pandemic-srb.pdf
4. World Health Organization. News release. COVID-19 disrupting mental health services in most countries, WHO survey. Published October 5, 2020. Accessed February 9, 2022. https://www.who.int/news/item/05-10-2020-covid-19-disrupting-mental-health-services-in-most-countries-who-survey
5. Avalere Health LLC. Exploring pharmacists’ role in a changing healthcare environment. Published May 2014. Accessed February 9, 2022. https://www.nacds.org/pdfs/comm/2014/pharmacist-role.pdf.
6. Silvia R. Collaborative treatment of depression by a psychiatric pharmacist integrated within a community health center primary care clinic. J Pharm Practice. 2016;29(3):270-341. doi:10.1177/0897190016645328
7. Caley C, Stimmel G. Characterizing the relationship between individuals with mental health conditions and community pharmacists. Published 2012. Accessed February 9, 2022. https://www.nami.org/About-NAMI/Publications-Reports/Survey-Reports/nami-cpnp-survey-report2012.pdf
8. Bovin MJ, Koenig CJ, Zamora KA, et al. Veterans’ experiences initiating VA-based mental health care. Psychol Serv. 2019;16(4):612-620. doi:10.1037/ser0000233
9. Herbert C, Winkler H. Impact of a clinical pharmacist–managed clinic in primary care mental health integration at a Veterans Affairs health system. Ment Health Clin. 2018;8(3):105-109. doi:10.9740/mhc.2018.05.105
10. Gibu M, Clark J, Gold J. Mental health pharmacists as interim prescribers. Ment Health Clin. 2018;7(3):111-115. doi:10.9740/mhc.2017.05.111
11. Herbert C, Winkler H, Moore TA. Outcomes of mental health pharmacist-managed electronic consults at a Veterans Affairs health care system. Ment Health Clin. 2018;7(3):131-136. doi:10.9740/mhc.2017.05.131
12. AACP. Embedding pharmacists into the practice. Accessed February 9, 2022. https://edhub.ama-assn.org/steps-forward/module/2702554
Analysis questions tocilizumab in ventilated COVID patients
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Needle-free epinephrine products could be available in 2023
Longstanding anxiety around use of epinephrine autoinjectors has prompted research into alternative delivery routes for this life-saving medication. Several companies presented posters on their needle-free epinephrine products at the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
Intranasal formulations are under development at ARS Pharmaceuticals (San Diego) and Bryn Pharma (Raleigh, N.C.). And Aquestive Therapeutics (Warren, N.J.) is working on a sublingual film that delivers epinephrine prodrug when applied under the tongue.
Epinephrine is essential for stopping life-threatening allergic reactions, yet patients often don’t carry their autoinjectors and many hesitate to use them. “It’s needle phobia,” said ARS Pharmaceuticals CEO Richard Lowenthal in an interview with this news organization. “They’re afraid to use it. They don’t like to inject their children, so they hesitate.”
Both nasal sprays reached maximal plasma concentration in 20-30 minutes. ARS Pharmaceuticals compared its intranasal product (Neffy 1 mg) against manual intramuscular injection (0.3 mg) and two autoinjectors (EpiPen 0.3 mg and Symjepi 0.3 mg) by analyzing data from multiple randomized crossover Phase 1 studies examining pharmacokinetics and pharmacodynamics in 175 healthy adults. In this integrated analysis, EpiPen was fastest (20 minutes) at reaching maximal concentration (Tmax), followed by Symjepi and Neffy (both 30 minutes) and epinephrine 0.3 mg IM (45 minutes). In a human factors analysis, ARS Pharmaceuticals reported that untrained participants were able to administer the Neffy spray to themselves or another participant safely and effectively during a simulated emergency scenario.
Bryn Pharma compared pharmacokinetics of its nasal spray product (BRYN-NDS1C 6.6 mg) when self-administered or administered by trained professionals and found comparable profiles for each. Tmax values were also similar: 21.63 minutes (trained professional) and 19.82 minutes (self-administered).
Aquestive Therapeutics is developing a postage stamp-sized product (AQST-109) that delivers epinephrine and begins dissolving when placed under the tongue. No water or swallowing is required for administration, and its packaging is thinner and smaller than a credit card, according to CEO Keith Kendall.
Its analysis showed that the epinephrine reaches maximum plasma concentration in about 15 minutes, with a Tmax range narrower than that of the EpiPen. “The results showed dosing with AQST-109 resulted in PK concentration and Tmax values comparable to published data from autoinjectors,” said John Oppenheimer, MD, of Rutgers University School of Medicine, in a prerecorded poster summary.
Aquestive aims to move forward to the manufacture of registration batches and a pivotal pharmacokinetic study in the second half of 2022. Mr. Lowenthal said ARS Pharmaceuticals is hoping for approval and launch of its nasal spray by summer 2023.
“Having a non-needle delivery device would help many people overcome that fear and hopefully increase use in anaphylaxis,” said David Stukus, MD, an allergist-immunologist and professor of clinical pediatrics at Nationwide Children’s Hospital, Columbus, who was not involved with any of the studies on EpiPen alternatives. And “it’s not just food allergy – anaphylaxis can occur from venom stings, medications, or idiopathic causes.”
Mr. Lowenthal is the CEO of ARS Pharmaceuticals. Mr. Kendall is CEO of Aquestive Therapeutics. Dr. Oppenheimer is a consultant for Aquestive, GSK, Amgen, Sanofi, and Aimmune and sits on Aquestive’s advisory board. Dr. Stukus is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Longstanding anxiety around use of epinephrine autoinjectors has prompted research into alternative delivery routes for this life-saving medication. Several companies presented posters on their needle-free epinephrine products at the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
Intranasal formulations are under development at ARS Pharmaceuticals (San Diego) and Bryn Pharma (Raleigh, N.C.). And Aquestive Therapeutics (Warren, N.J.) is working on a sublingual film that delivers epinephrine prodrug when applied under the tongue.
Epinephrine is essential for stopping life-threatening allergic reactions, yet patients often don’t carry their autoinjectors and many hesitate to use them. “It’s needle phobia,” said ARS Pharmaceuticals CEO Richard Lowenthal in an interview with this news organization. “They’re afraid to use it. They don’t like to inject their children, so they hesitate.”
Both nasal sprays reached maximal plasma concentration in 20-30 minutes. ARS Pharmaceuticals compared its intranasal product (Neffy 1 mg) against manual intramuscular injection (0.3 mg) and two autoinjectors (EpiPen 0.3 mg and Symjepi 0.3 mg) by analyzing data from multiple randomized crossover Phase 1 studies examining pharmacokinetics and pharmacodynamics in 175 healthy adults. In this integrated analysis, EpiPen was fastest (20 minutes) at reaching maximal concentration (Tmax), followed by Symjepi and Neffy (both 30 minutes) and epinephrine 0.3 mg IM (45 minutes). In a human factors analysis, ARS Pharmaceuticals reported that untrained participants were able to administer the Neffy spray to themselves or another participant safely and effectively during a simulated emergency scenario.
Bryn Pharma compared pharmacokinetics of its nasal spray product (BRYN-NDS1C 6.6 mg) when self-administered or administered by trained professionals and found comparable profiles for each. Tmax values were also similar: 21.63 minutes (trained professional) and 19.82 minutes (self-administered).
Aquestive Therapeutics is developing a postage stamp-sized product (AQST-109) that delivers epinephrine and begins dissolving when placed under the tongue. No water or swallowing is required for administration, and its packaging is thinner and smaller than a credit card, according to CEO Keith Kendall.
Its analysis showed that the epinephrine reaches maximum plasma concentration in about 15 minutes, with a Tmax range narrower than that of the EpiPen. “The results showed dosing with AQST-109 resulted in PK concentration and Tmax values comparable to published data from autoinjectors,” said John Oppenheimer, MD, of Rutgers University School of Medicine, in a prerecorded poster summary.
Aquestive aims to move forward to the manufacture of registration batches and a pivotal pharmacokinetic study in the second half of 2022. Mr. Lowenthal said ARS Pharmaceuticals is hoping for approval and launch of its nasal spray by summer 2023.
“Having a non-needle delivery device would help many people overcome that fear and hopefully increase use in anaphylaxis,” said David Stukus, MD, an allergist-immunologist and professor of clinical pediatrics at Nationwide Children’s Hospital, Columbus, who was not involved with any of the studies on EpiPen alternatives. And “it’s not just food allergy – anaphylaxis can occur from venom stings, medications, or idiopathic causes.”
Mr. Lowenthal is the CEO of ARS Pharmaceuticals. Mr. Kendall is CEO of Aquestive Therapeutics. Dr. Oppenheimer is a consultant for Aquestive, GSK, Amgen, Sanofi, and Aimmune and sits on Aquestive’s advisory board. Dr. Stukus is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Longstanding anxiety around use of epinephrine autoinjectors has prompted research into alternative delivery routes for this life-saving medication. Several companies presented posters on their needle-free epinephrine products at the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
Intranasal formulations are under development at ARS Pharmaceuticals (San Diego) and Bryn Pharma (Raleigh, N.C.). And Aquestive Therapeutics (Warren, N.J.) is working on a sublingual film that delivers epinephrine prodrug when applied under the tongue.
Epinephrine is essential for stopping life-threatening allergic reactions, yet patients often don’t carry their autoinjectors and many hesitate to use them. “It’s needle phobia,” said ARS Pharmaceuticals CEO Richard Lowenthal in an interview with this news organization. “They’re afraid to use it. They don’t like to inject their children, so they hesitate.”
Both nasal sprays reached maximal plasma concentration in 20-30 minutes. ARS Pharmaceuticals compared its intranasal product (Neffy 1 mg) against manual intramuscular injection (0.3 mg) and two autoinjectors (EpiPen 0.3 mg and Symjepi 0.3 mg) by analyzing data from multiple randomized crossover Phase 1 studies examining pharmacokinetics and pharmacodynamics in 175 healthy adults. In this integrated analysis, EpiPen was fastest (20 minutes) at reaching maximal concentration (Tmax), followed by Symjepi and Neffy (both 30 minutes) and epinephrine 0.3 mg IM (45 minutes). In a human factors analysis, ARS Pharmaceuticals reported that untrained participants were able to administer the Neffy spray to themselves or another participant safely and effectively during a simulated emergency scenario.
Bryn Pharma compared pharmacokinetics of its nasal spray product (BRYN-NDS1C 6.6 mg) when self-administered or administered by trained professionals and found comparable profiles for each. Tmax values were also similar: 21.63 minutes (trained professional) and 19.82 minutes (self-administered).
Aquestive Therapeutics is developing a postage stamp-sized product (AQST-109) that delivers epinephrine and begins dissolving when placed under the tongue. No water or swallowing is required for administration, and its packaging is thinner and smaller than a credit card, according to CEO Keith Kendall.
Its analysis showed that the epinephrine reaches maximum plasma concentration in about 15 minutes, with a Tmax range narrower than that of the EpiPen. “The results showed dosing with AQST-109 resulted in PK concentration and Tmax values comparable to published data from autoinjectors,” said John Oppenheimer, MD, of Rutgers University School of Medicine, in a prerecorded poster summary.
Aquestive aims to move forward to the manufacture of registration batches and a pivotal pharmacokinetic study in the second half of 2022. Mr. Lowenthal said ARS Pharmaceuticals is hoping for approval and launch of its nasal spray by summer 2023.
“Having a non-needle delivery device would help many people overcome that fear and hopefully increase use in anaphylaxis,” said David Stukus, MD, an allergist-immunologist and professor of clinical pediatrics at Nationwide Children’s Hospital, Columbus, who was not involved with any of the studies on EpiPen alternatives. And “it’s not just food allergy – anaphylaxis can occur from venom stings, medications, or idiopathic causes.”
Mr. Lowenthal is the CEO of ARS Pharmaceuticals. Mr. Kendall is CEO of Aquestive Therapeutics. Dr. Oppenheimer is a consultant for Aquestive, GSK, Amgen, Sanofi, and Aimmune and sits on Aquestive’s advisory board. Dr. Stukus is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
FROM AAAAI
FDA approves new CAR T-cell treatment for multiple myeloma
A new treatment option for patients with refractory/relapsed multiple myeloma who have already tried four or more therapies has been approved by the U.S. Food and Drug Administration.
There are already two other therapies on the market that target BCMA – another CAR T cell, idecabtagene vicleucel (Abecma), which was approved by the FDA in March 2021, and a drug conjugate, belantamab mafodotin (Blenrep), which was approved in August 2020.
The approval of cilta-cel was based on clinical data from the CARTITUDE-1 study, which were initially presented in December 2020 at the annual meeting of the American Society of Hematology, as reported at the time by this news organization.
The trial involved 97 patients with relapsed/refractory multiple myeloma who had already received a median of six previous treatments (range, three to 18), including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
“The treatment journey for the majority of patients living with multiple myeloma is a relentless cycle of remission and relapse, with fewer patients achieving a deep response as they progress through later lines of therapy,” commented Sundar Jagannath, MBBS, professor of medicine, hematology, and medical oncology at Mount Sinai, who was a principal investigator on the pivotal study.
“That is why I have been really excited about the results from the CARTITUDE-1 study, which has demonstrated that cilta-cel can provide deep and durable responses and long-term treatment-free intervals, even in this heavily pretreated multiple myeloma patient population,” he said.
“Today’s approval of Carvykti helps address a great unmet need for these patients,” he commented in a press release from the manufacturer.
Like other CAR T-cell therapies, ciltacabtagene autoleucel is a one-time treatment. It involves collecting blood from the patient, extracting T cells, genetically engineering them, then transfusing them back to the patient, who in the meantime has undergone conditioning.
The results from CARTITUDE-1 show that this one-time treatment resulted in deep and durable responses.
The overall response rate was 98%, and the majority of patients (78%) achieved a stringent complete response, in which physicians are unable to observe any signs or symptoms of disease via imaging or other tests after treatment.
At a median of 18 months’ follow-up, the median duration of response was 21.8 months.
“The responses in the CARTITUDE-1 study showed durability over time and resulted in the majority of heavily pretreated patients achieving deep responses after 18-month follow-up,” commented Mr. Jagannath.
“The approval of cilta-cel provides physicians an immunotherapy treatment option that offers patients an opportunity to be free from anti-myeloma therapies for a period of time,” he added.
As with other CAR T-cell therapies, there were serious side effects, and these products are available only through restricted programs under a risk evaluation and mitigation strategy.
The product information for Cartykti includes a boxed warning that mentions cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, parkinsonism, Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome, and prolonged and/or recurrent cytopenias.
The most common adverse reactions (reported in greater than or equal to 20% of patients) are pyrexia, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections–pathogens unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
A version of this article first appeared on Medscape.com.
A new treatment option for patients with refractory/relapsed multiple myeloma who have already tried four or more therapies has been approved by the U.S. Food and Drug Administration.
There are already two other therapies on the market that target BCMA – another CAR T cell, idecabtagene vicleucel (Abecma), which was approved by the FDA in March 2021, and a drug conjugate, belantamab mafodotin (Blenrep), which was approved in August 2020.
The approval of cilta-cel was based on clinical data from the CARTITUDE-1 study, which were initially presented in December 2020 at the annual meeting of the American Society of Hematology, as reported at the time by this news organization.
The trial involved 97 patients with relapsed/refractory multiple myeloma who had already received a median of six previous treatments (range, three to 18), including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
“The treatment journey for the majority of patients living with multiple myeloma is a relentless cycle of remission and relapse, with fewer patients achieving a deep response as they progress through later lines of therapy,” commented Sundar Jagannath, MBBS, professor of medicine, hematology, and medical oncology at Mount Sinai, who was a principal investigator on the pivotal study.
“That is why I have been really excited about the results from the CARTITUDE-1 study, which has demonstrated that cilta-cel can provide deep and durable responses and long-term treatment-free intervals, even in this heavily pretreated multiple myeloma patient population,” he said.
“Today’s approval of Carvykti helps address a great unmet need for these patients,” he commented in a press release from the manufacturer.
Like other CAR T-cell therapies, ciltacabtagene autoleucel is a one-time treatment. It involves collecting blood from the patient, extracting T cells, genetically engineering them, then transfusing them back to the patient, who in the meantime has undergone conditioning.
The results from CARTITUDE-1 show that this one-time treatment resulted in deep and durable responses.
The overall response rate was 98%, and the majority of patients (78%) achieved a stringent complete response, in which physicians are unable to observe any signs or symptoms of disease via imaging or other tests after treatment.
At a median of 18 months’ follow-up, the median duration of response was 21.8 months.
“The responses in the CARTITUDE-1 study showed durability over time and resulted in the majority of heavily pretreated patients achieving deep responses after 18-month follow-up,” commented Mr. Jagannath.
“The approval of cilta-cel provides physicians an immunotherapy treatment option that offers patients an opportunity to be free from anti-myeloma therapies for a period of time,” he added.
As with other CAR T-cell therapies, there were serious side effects, and these products are available only through restricted programs under a risk evaluation and mitigation strategy.
The product information for Cartykti includes a boxed warning that mentions cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, parkinsonism, Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome, and prolonged and/or recurrent cytopenias.
The most common adverse reactions (reported in greater than or equal to 20% of patients) are pyrexia, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections–pathogens unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
A version of this article first appeared on Medscape.com.
A new treatment option for patients with refractory/relapsed multiple myeloma who have already tried four or more therapies has been approved by the U.S. Food and Drug Administration.
There are already two other therapies on the market that target BCMA – another CAR T cell, idecabtagene vicleucel (Abecma), which was approved by the FDA in March 2021, and a drug conjugate, belantamab mafodotin (Blenrep), which was approved in August 2020.
The approval of cilta-cel was based on clinical data from the CARTITUDE-1 study, which were initially presented in December 2020 at the annual meeting of the American Society of Hematology, as reported at the time by this news organization.
The trial involved 97 patients with relapsed/refractory multiple myeloma who had already received a median of six previous treatments (range, three to 18), including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
“The treatment journey for the majority of patients living with multiple myeloma is a relentless cycle of remission and relapse, with fewer patients achieving a deep response as they progress through later lines of therapy,” commented Sundar Jagannath, MBBS, professor of medicine, hematology, and medical oncology at Mount Sinai, who was a principal investigator on the pivotal study.
“That is why I have been really excited about the results from the CARTITUDE-1 study, which has demonstrated that cilta-cel can provide deep and durable responses and long-term treatment-free intervals, even in this heavily pretreated multiple myeloma patient population,” he said.
“Today’s approval of Carvykti helps address a great unmet need for these patients,” he commented in a press release from the manufacturer.
Like other CAR T-cell therapies, ciltacabtagene autoleucel is a one-time treatment. It involves collecting blood from the patient, extracting T cells, genetically engineering them, then transfusing them back to the patient, who in the meantime has undergone conditioning.
The results from CARTITUDE-1 show that this one-time treatment resulted in deep and durable responses.
The overall response rate was 98%, and the majority of patients (78%) achieved a stringent complete response, in which physicians are unable to observe any signs or symptoms of disease via imaging or other tests after treatment.
At a median of 18 months’ follow-up, the median duration of response was 21.8 months.
“The responses in the CARTITUDE-1 study showed durability over time and resulted in the majority of heavily pretreated patients achieving deep responses after 18-month follow-up,” commented Mr. Jagannath.
“The approval of cilta-cel provides physicians an immunotherapy treatment option that offers patients an opportunity to be free from anti-myeloma therapies for a period of time,” he added.
As with other CAR T-cell therapies, there were serious side effects, and these products are available only through restricted programs under a risk evaluation and mitigation strategy.
The product information for Cartykti includes a boxed warning that mentions cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, parkinsonism, Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome, and prolonged and/or recurrent cytopenias.
The most common adverse reactions (reported in greater than or equal to 20% of patients) are pyrexia, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections–pathogens unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
A version of this article first appeared on Medscape.com.
Excess sodium in soluble acetaminophen tied to CVD risk, death
a large observational study of more than 300,000 adults suggests.
“Numerous studies have reported that high sodium intake is associated with increased risks of cardiovascular disease,” Yuqing Zhang, DSc, with Massachusetts General Hospital and Harvard Medical School, Boston, told this news organization. “Given that the pain relief effect of non–sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen, clinicians may prescribe non–sodium-containing acetaminophen to their patients to minimize the risk of CVD and mortality,” Dr. Zhang said.
The study was published online Feb. 24 in the European Heart Journal.
‘Compelling results’
Dr. Zhang and colleagues note that the effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively.
Therefore, the intake of maximum daily dose (4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of more than 3 g of sodium, a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (2 g/day).
“This hidden extra sodium intake is often overlooked,” Dr. Zhang told this news organization.
Using data from the Health Improvement Network, a U.K. primary care database, the researchers examined 4,532 patients with hypertension taking sodium-containing acetaminophen and compared them with 146,866 patients with hypertension taking non–sodium-containing acetaminophen (tablet, capsule, or oral suspension formulations).
After 1 year, the risk of incident CVD (myocardial infarction, stroke, and heart failure) was 5.6% in those taking sodium-containing acetaminophen, compared with 4.6% in those taking non–sodium-containing acetaminophen (average weighted hazard ratio, 1.59; 95% confidence interval, 1.32-1.92).
A separate analysis of normotensive patients taking sodium-containing acetaminophen (n = 5,351) or non–sodium-containing acetaminophen (n = 141,948) gave similar results.
The 1-year risk of incident CVD was 4.4% in those taking sodium-containing acetaminophen vs. 3.7% among those taking non–sodium-containing acetaminophen (average weighted HR, 1.45; 95% CI, 1.18-1.79).
There was also evidence of a dose-response relationship.
In those with hypertension, CVD risk increased by roughly one-quarter (odds ratio, 1.26) for those with one prescription of sodium-containing acetaminophen and by nearly one half (OR, 1.45) for those with five or more prescriptions of sodium-containing acetaminophen. Similar findings were observed among adults without hypertension.
Mortality at 1 year was also higher in those taking sodium-containing acetaminophen than non–sodium-containing acetaminophen, in patients with hypertension (7.6% vs. 6.1%) and without hypertension (7.3% vs. 5.9%).
“The results are compelling,” write the authors of an editorial published with the study.
“The direct message from this study is clear – there are likely to be millions of people worldwide taking paracetamol on a daily basis in a ‘fast-acting’ effervescent or soluble formulation who are increasing their risks of cardiovascular disease and premature death,” say Aletta Schutte, PhD, and Bruce Neal, MBChB, PhD, of the George Institute for Global Health, Sydney.
“The weight of the evidence makes ongoing inaction on sodium-containing medications untenable. The widespread use of effervescent medication in the general population, and the enormous doses of sodium that can be consumed inadvertently by unsuspecting consumers requires urgent action,” Dr. Schutte and Dr. Neal say.
The study was supported by the National Natural Science Foundation of China, the National Key Research and Development Project, the Project Program of National Clinical Research Center for Geriatric Disorders, the Key Research and Development Program of Hunan Province, and the Science and Technology Program of Hunan Province. Dr. Zhang, Dr. Schutte, and Dr. Neal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a large observational study of more than 300,000 adults suggests.
“Numerous studies have reported that high sodium intake is associated with increased risks of cardiovascular disease,” Yuqing Zhang, DSc, with Massachusetts General Hospital and Harvard Medical School, Boston, told this news organization. “Given that the pain relief effect of non–sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen, clinicians may prescribe non–sodium-containing acetaminophen to their patients to minimize the risk of CVD and mortality,” Dr. Zhang said.
The study was published online Feb. 24 in the European Heart Journal.
‘Compelling results’
Dr. Zhang and colleagues note that the effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively.
Therefore, the intake of maximum daily dose (4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of more than 3 g of sodium, a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (2 g/day).
“This hidden extra sodium intake is often overlooked,” Dr. Zhang told this news organization.
Using data from the Health Improvement Network, a U.K. primary care database, the researchers examined 4,532 patients with hypertension taking sodium-containing acetaminophen and compared them with 146,866 patients with hypertension taking non–sodium-containing acetaminophen (tablet, capsule, or oral suspension formulations).
After 1 year, the risk of incident CVD (myocardial infarction, stroke, and heart failure) was 5.6% in those taking sodium-containing acetaminophen, compared with 4.6% in those taking non–sodium-containing acetaminophen (average weighted hazard ratio, 1.59; 95% confidence interval, 1.32-1.92).
A separate analysis of normotensive patients taking sodium-containing acetaminophen (n = 5,351) or non–sodium-containing acetaminophen (n = 141,948) gave similar results.
The 1-year risk of incident CVD was 4.4% in those taking sodium-containing acetaminophen vs. 3.7% among those taking non–sodium-containing acetaminophen (average weighted HR, 1.45; 95% CI, 1.18-1.79).
There was also evidence of a dose-response relationship.
In those with hypertension, CVD risk increased by roughly one-quarter (odds ratio, 1.26) for those with one prescription of sodium-containing acetaminophen and by nearly one half (OR, 1.45) for those with five or more prescriptions of sodium-containing acetaminophen. Similar findings were observed among adults without hypertension.
Mortality at 1 year was also higher in those taking sodium-containing acetaminophen than non–sodium-containing acetaminophen, in patients with hypertension (7.6% vs. 6.1%) and without hypertension (7.3% vs. 5.9%).
“The results are compelling,” write the authors of an editorial published with the study.
“The direct message from this study is clear – there are likely to be millions of people worldwide taking paracetamol on a daily basis in a ‘fast-acting’ effervescent or soluble formulation who are increasing their risks of cardiovascular disease and premature death,” say Aletta Schutte, PhD, and Bruce Neal, MBChB, PhD, of the George Institute for Global Health, Sydney.
“The weight of the evidence makes ongoing inaction on sodium-containing medications untenable. The widespread use of effervescent medication in the general population, and the enormous doses of sodium that can be consumed inadvertently by unsuspecting consumers requires urgent action,” Dr. Schutte and Dr. Neal say.
The study was supported by the National Natural Science Foundation of China, the National Key Research and Development Project, the Project Program of National Clinical Research Center for Geriatric Disorders, the Key Research and Development Program of Hunan Province, and the Science and Technology Program of Hunan Province. Dr. Zhang, Dr. Schutte, and Dr. Neal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a large observational study of more than 300,000 adults suggests.
“Numerous studies have reported that high sodium intake is associated with increased risks of cardiovascular disease,” Yuqing Zhang, DSc, with Massachusetts General Hospital and Harvard Medical School, Boston, told this news organization. “Given that the pain relief effect of non–sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen, clinicians may prescribe non–sodium-containing acetaminophen to their patients to minimize the risk of CVD and mortality,” Dr. Zhang said.
The study was published online Feb. 24 in the European Heart Journal.
‘Compelling results’
Dr. Zhang and colleagues note that the effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively.
Therefore, the intake of maximum daily dose (4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of more than 3 g of sodium, a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (2 g/day).
“This hidden extra sodium intake is often overlooked,” Dr. Zhang told this news organization.
Using data from the Health Improvement Network, a U.K. primary care database, the researchers examined 4,532 patients with hypertension taking sodium-containing acetaminophen and compared them with 146,866 patients with hypertension taking non–sodium-containing acetaminophen (tablet, capsule, or oral suspension formulations).
After 1 year, the risk of incident CVD (myocardial infarction, stroke, and heart failure) was 5.6% in those taking sodium-containing acetaminophen, compared with 4.6% in those taking non–sodium-containing acetaminophen (average weighted hazard ratio, 1.59; 95% confidence interval, 1.32-1.92).
A separate analysis of normotensive patients taking sodium-containing acetaminophen (n = 5,351) or non–sodium-containing acetaminophen (n = 141,948) gave similar results.
The 1-year risk of incident CVD was 4.4% in those taking sodium-containing acetaminophen vs. 3.7% among those taking non–sodium-containing acetaminophen (average weighted HR, 1.45; 95% CI, 1.18-1.79).
There was also evidence of a dose-response relationship.
In those with hypertension, CVD risk increased by roughly one-quarter (odds ratio, 1.26) for those with one prescription of sodium-containing acetaminophen and by nearly one half (OR, 1.45) for those with five or more prescriptions of sodium-containing acetaminophen. Similar findings were observed among adults without hypertension.
Mortality at 1 year was also higher in those taking sodium-containing acetaminophen than non–sodium-containing acetaminophen, in patients with hypertension (7.6% vs. 6.1%) and without hypertension (7.3% vs. 5.9%).
“The results are compelling,” write the authors of an editorial published with the study.
“The direct message from this study is clear – there are likely to be millions of people worldwide taking paracetamol on a daily basis in a ‘fast-acting’ effervescent or soluble formulation who are increasing their risks of cardiovascular disease and premature death,” say Aletta Schutte, PhD, and Bruce Neal, MBChB, PhD, of the George Institute for Global Health, Sydney.
“The weight of the evidence makes ongoing inaction on sodium-containing medications untenable. The widespread use of effervescent medication in the general population, and the enormous doses of sodium that can be consumed inadvertently by unsuspecting consumers requires urgent action,” Dr. Schutte and Dr. Neal say.
The study was supported by the National Natural Science Foundation of China, the National Key Research and Development Project, the Project Program of National Clinical Research Center for Geriatric Disorders, the Key Research and Development Program of Hunan Province, and the Science and Technology Program of Hunan Province. Dr. Zhang, Dr. Schutte, and Dr. Neal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL