User login
FDA okays difelikefalin for dialysis-associated pruritus in patients with CKD
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
‘Countdown to zero’: Endocrine disruptors and worldwide sperm counts
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Parental smoking linked to more adult RA in women
Childhood exposure to parental smoking appears to greatly boost the risk of confirmed cases of rheumatoid arthritis in adult women, although the overall rate is small, a new study reports. The findings, published Aug. 18, 2021, in Arthritis & Rheumatology, follows other evidence that early second-hand smoke exposure can trigger lifelong damage to the immune system.
“We estimated that there is 75% increased risk of adult seropositive RA due to the direct impact of childhood parental smoking,” said study lead author and Brigham & Women’s Hospital epidemiologist Kazuki Yoshida, MD, ScD, referring to an adjusted analysis conducted in the study. “Passive smoking is likely harmful throughout an individual’s life course regarding rheumatoid arthritis but potentially more harmful during the childhood period.”
The researchers launched the study to fill an evidence gap, Dr. Yoshida said in an interview. “Active smoking is a well-established risk factor for RA. However, studies on passive smoking’s impact on RA are sparse, and few studies had a well-characterized cohort of participants with comprehensive data of passive smoking during life course – in utero exposure, childhood exposure, adult exposure – and chart review–adjudicated RA outcomes.”
The study authors retrospectively tracked 90,923 subjects who joined the Nurses’ Health Study II in 1989 when they were aged 25-42. At the study’s start, the average age of subjects was 34.5, 93% were White, and 98% were premenopausal. Almost two-thirds had never smoked themselves, and 65% said their parents had smoked during their childhoods.
Of the subjects, the researchers found that 532 were identified as having RA over a median follow-up period of 27.7 years. Two-thirds of those cases (n = 352) were confirmed as seropositive by clinical testing.
The study linked maternal smoking during pregnancy to confirmed RA in adulthood via a confounder-adjusted analysis (hazard ratio, 1.25; 95% confidence interval, 1.03-1.52), but the connection vanished after researchers adjusted their statistics to reflect possible influences by later exposures to smoke.
After adjustment for confounders, the study linked childhood exposure to parental smoking to a 41% in increase in risk of confirmed adulthood RA (HR, 1.41; 95% CI, 1.08-1.83). A controlled direct effect analysis boosted the excess risk to 75% (HR, 1.75; 95% CI, 1.03-2.98).
This analysis reveals that “childhood parental smoking seems to be associated with adult rheumatoid arthritis beyond what is explained by the fact that childhood passive smoking can promote personal smoking uptake, a known risk factor for rheumatoid arthritis,” Dr. Yoshida said.
The overall rate of RA in the study population – roughly 0.6% – aligns with risk levels in the general population, he said. As a result, “the absolute risk increase may not be extremely high. But the concept that early life exposure may affect immunological health later in life is important.”
Potential pathophysiological mechanisms
Why might parental smoking boost the risk of RA? Exposure to secondhand smoke may irritate the lungs and cause abnormal proteins to form, Dr. Yoshida said. “The immune system produces antibodies in an attempt to attack such abnormal proteins. This immune reaction can spread to other body sites and attack normal tissues, including the joints.”
In addition, “smoking increases the risk of infections, which could in turn increase the risk of RA. Smoking is also known to result in epigenetic changes which could trigger RA in susceptible people,” University of California, San Francisco, autoimmune disease epidemiologist Milena A. Gianfrancesco, PhD, MPH, said in an interview. She cowrote a commentary accompanying the new study.
Other studies have linked smoking exposure to autoimmune disorders. Earlier this year, researchers who tracked 79,806 French women reported at the EULAR 2021 annual meeting that they found a link between exposure to second-hand smoking during childhood or adulthood and higher rates of RA.
Dr. Yoshida and colleagues noted their study’s limitations, including the inability to track cases of RA in subjects up to the age when they entered the nurses research project. Also, only one questionnaire over the entire period of the Nurses’ Health Study II asks subjects about whether they were exposed to secondhand smoke as adults.
The study also says nothing about whether a similar risk exists for males, and the nurse subjects are overwhelmingly White.
Still, Dr. Gianfrancesco praised the study and said it relies on extensive data and strong statistical methods. “The findings are important because they drive home the importance of reducing cigarette smoke exposure to reduce risk of disease,” she said. “They highlight the need to not only focus on one’s personal smoking habits, but also other sources of secondhand smoke exposure.”
She added that children with a family history of RA or other autoimmune diseases are especially vulnerable to the effects of secondhand smoke because they may be more susceptible to developing the diseases themselves. “Rheumatologists and other health care providers should be sure to discuss the risks of smoking with their patients, as well as the risk of secondhand smoke,” she said. “And parents should keep their children away from secondhand smoke in the home or other environments in which smoke is prevalent, such as the home of another caregiver or a workplace if the child accompanies their parent to work.”
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Institutes of Health. The study and commentary authors, including Dr. Yoshida and Dr. Gianfrancesco, reported having no relevant disclosures.
Childhood exposure to parental smoking appears to greatly boost the risk of confirmed cases of rheumatoid arthritis in adult women, although the overall rate is small, a new study reports. The findings, published Aug. 18, 2021, in Arthritis & Rheumatology, follows other evidence that early second-hand smoke exposure can trigger lifelong damage to the immune system.
“We estimated that there is 75% increased risk of adult seropositive RA due to the direct impact of childhood parental smoking,” said study lead author and Brigham & Women’s Hospital epidemiologist Kazuki Yoshida, MD, ScD, referring to an adjusted analysis conducted in the study. “Passive smoking is likely harmful throughout an individual’s life course regarding rheumatoid arthritis but potentially more harmful during the childhood period.”
The researchers launched the study to fill an evidence gap, Dr. Yoshida said in an interview. “Active smoking is a well-established risk factor for RA. However, studies on passive smoking’s impact on RA are sparse, and few studies had a well-characterized cohort of participants with comprehensive data of passive smoking during life course – in utero exposure, childhood exposure, adult exposure – and chart review–adjudicated RA outcomes.”
The study authors retrospectively tracked 90,923 subjects who joined the Nurses’ Health Study II in 1989 when they were aged 25-42. At the study’s start, the average age of subjects was 34.5, 93% were White, and 98% were premenopausal. Almost two-thirds had never smoked themselves, and 65% said their parents had smoked during their childhoods.
Of the subjects, the researchers found that 532 were identified as having RA over a median follow-up period of 27.7 years. Two-thirds of those cases (n = 352) were confirmed as seropositive by clinical testing.
The study linked maternal smoking during pregnancy to confirmed RA in adulthood via a confounder-adjusted analysis (hazard ratio, 1.25; 95% confidence interval, 1.03-1.52), but the connection vanished after researchers adjusted their statistics to reflect possible influences by later exposures to smoke.
After adjustment for confounders, the study linked childhood exposure to parental smoking to a 41% in increase in risk of confirmed adulthood RA (HR, 1.41; 95% CI, 1.08-1.83). A controlled direct effect analysis boosted the excess risk to 75% (HR, 1.75; 95% CI, 1.03-2.98).
This analysis reveals that “childhood parental smoking seems to be associated with adult rheumatoid arthritis beyond what is explained by the fact that childhood passive smoking can promote personal smoking uptake, a known risk factor for rheumatoid arthritis,” Dr. Yoshida said.
The overall rate of RA in the study population – roughly 0.6% – aligns with risk levels in the general population, he said. As a result, “the absolute risk increase may not be extremely high. But the concept that early life exposure may affect immunological health later in life is important.”
Potential pathophysiological mechanisms
Why might parental smoking boost the risk of RA? Exposure to secondhand smoke may irritate the lungs and cause abnormal proteins to form, Dr. Yoshida said. “The immune system produces antibodies in an attempt to attack such abnormal proteins. This immune reaction can spread to other body sites and attack normal tissues, including the joints.”
In addition, “smoking increases the risk of infections, which could in turn increase the risk of RA. Smoking is also known to result in epigenetic changes which could trigger RA in susceptible people,” University of California, San Francisco, autoimmune disease epidemiologist Milena A. Gianfrancesco, PhD, MPH, said in an interview. She cowrote a commentary accompanying the new study.
Other studies have linked smoking exposure to autoimmune disorders. Earlier this year, researchers who tracked 79,806 French women reported at the EULAR 2021 annual meeting that they found a link between exposure to second-hand smoking during childhood or adulthood and higher rates of RA.
Dr. Yoshida and colleagues noted their study’s limitations, including the inability to track cases of RA in subjects up to the age when they entered the nurses research project. Also, only one questionnaire over the entire period of the Nurses’ Health Study II asks subjects about whether they were exposed to secondhand smoke as adults.
The study also says nothing about whether a similar risk exists for males, and the nurse subjects are overwhelmingly White.
Still, Dr. Gianfrancesco praised the study and said it relies on extensive data and strong statistical methods. “The findings are important because they drive home the importance of reducing cigarette smoke exposure to reduce risk of disease,” she said. “They highlight the need to not only focus on one’s personal smoking habits, but also other sources of secondhand smoke exposure.”
She added that children with a family history of RA or other autoimmune diseases are especially vulnerable to the effects of secondhand smoke because they may be more susceptible to developing the diseases themselves. “Rheumatologists and other health care providers should be sure to discuss the risks of smoking with their patients, as well as the risk of secondhand smoke,” she said. “And parents should keep their children away from secondhand smoke in the home or other environments in which smoke is prevalent, such as the home of another caregiver or a workplace if the child accompanies their parent to work.”
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Institutes of Health. The study and commentary authors, including Dr. Yoshida and Dr. Gianfrancesco, reported having no relevant disclosures.
Childhood exposure to parental smoking appears to greatly boost the risk of confirmed cases of rheumatoid arthritis in adult women, although the overall rate is small, a new study reports. The findings, published Aug. 18, 2021, in Arthritis & Rheumatology, follows other evidence that early second-hand smoke exposure can trigger lifelong damage to the immune system.
“We estimated that there is 75% increased risk of adult seropositive RA due to the direct impact of childhood parental smoking,” said study lead author and Brigham & Women’s Hospital epidemiologist Kazuki Yoshida, MD, ScD, referring to an adjusted analysis conducted in the study. “Passive smoking is likely harmful throughout an individual’s life course regarding rheumatoid arthritis but potentially more harmful during the childhood period.”
The researchers launched the study to fill an evidence gap, Dr. Yoshida said in an interview. “Active smoking is a well-established risk factor for RA. However, studies on passive smoking’s impact on RA are sparse, and few studies had a well-characterized cohort of participants with comprehensive data of passive smoking during life course – in utero exposure, childhood exposure, adult exposure – and chart review–adjudicated RA outcomes.”
The study authors retrospectively tracked 90,923 subjects who joined the Nurses’ Health Study II in 1989 when they were aged 25-42. At the study’s start, the average age of subjects was 34.5, 93% were White, and 98% were premenopausal. Almost two-thirds had never smoked themselves, and 65% said their parents had smoked during their childhoods.
Of the subjects, the researchers found that 532 were identified as having RA over a median follow-up period of 27.7 years. Two-thirds of those cases (n = 352) were confirmed as seropositive by clinical testing.
The study linked maternal smoking during pregnancy to confirmed RA in adulthood via a confounder-adjusted analysis (hazard ratio, 1.25; 95% confidence interval, 1.03-1.52), but the connection vanished after researchers adjusted their statistics to reflect possible influences by later exposures to smoke.
After adjustment for confounders, the study linked childhood exposure to parental smoking to a 41% in increase in risk of confirmed adulthood RA (HR, 1.41; 95% CI, 1.08-1.83). A controlled direct effect analysis boosted the excess risk to 75% (HR, 1.75; 95% CI, 1.03-2.98).
This analysis reveals that “childhood parental smoking seems to be associated with adult rheumatoid arthritis beyond what is explained by the fact that childhood passive smoking can promote personal smoking uptake, a known risk factor for rheumatoid arthritis,” Dr. Yoshida said.
The overall rate of RA in the study population – roughly 0.6% – aligns with risk levels in the general population, he said. As a result, “the absolute risk increase may not be extremely high. But the concept that early life exposure may affect immunological health later in life is important.”
Potential pathophysiological mechanisms
Why might parental smoking boost the risk of RA? Exposure to secondhand smoke may irritate the lungs and cause abnormal proteins to form, Dr. Yoshida said. “The immune system produces antibodies in an attempt to attack such abnormal proteins. This immune reaction can spread to other body sites and attack normal tissues, including the joints.”
In addition, “smoking increases the risk of infections, which could in turn increase the risk of RA. Smoking is also known to result in epigenetic changes which could trigger RA in susceptible people,” University of California, San Francisco, autoimmune disease epidemiologist Milena A. Gianfrancesco, PhD, MPH, said in an interview. She cowrote a commentary accompanying the new study.
Other studies have linked smoking exposure to autoimmune disorders. Earlier this year, researchers who tracked 79,806 French women reported at the EULAR 2021 annual meeting that they found a link between exposure to second-hand smoking during childhood or adulthood and higher rates of RA.
Dr. Yoshida and colleagues noted their study’s limitations, including the inability to track cases of RA in subjects up to the age when they entered the nurses research project. Also, only one questionnaire over the entire period of the Nurses’ Health Study II asks subjects about whether they were exposed to secondhand smoke as adults.
The study also says nothing about whether a similar risk exists for males, and the nurse subjects are overwhelmingly White.
Still, Dr. Gianfrancesco praised the study and said it relies on extensive data and strong statistical methods. “The findings are important because they drive home the importance of reducing cigarette smoke exposure to reduce risk of disease,” she said. “They highlight the need to not only focus on one’s personal smoking habits, but also other sources of secondhand smoke exposure.”
She added that children with a family history of RA or other autoimmune diseases are especially vulnerable to the effects of secondhand smoke because they may be more susceptible to developing the diseases themselves. “Rheumatologists and other health care providers should be sure to discuss the risks of smoking with their patients, as well as the risk of secondhand smoke,” she said. “And parents should keep their children away from secondhand smoke in the home or other environments in which smoke is prevalent, such as the home of another caregiver or a workplace if the child accompanies their parent to work.”
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Institutes of Health. The study and commentary authors, including Dr. Yoshida and Dr. Gianfrancesco, reported having no relevant disclosures.
FROM ARTHRITIS & RHEUMATOLOGY
Tocilizumab shortage continues as pandemic wears on
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
Pfizer recalls four more lots of smoking cessation drug Chantix
Pfizer has recalled four more lots of the smoking cessation drug varenicline (Chantix), according to an Aug. 16 update on the U.S. Food and Drug Administration website.
In a new FDA MedWatch, the agency notes that these 0.5 mg/1 mg tablets are being recalled because of the presence of N-nitroso-varenicline, a nitrosamine impurity, at a level higher than Pfizer’s acceptable intake limit.
On July 2, the FDA reported that Pfizer had voluntarily recalled nine lots of the drug for this reason. As reported by this news organization, the company added three more lots to the recall a few weeks later.
In the update, the FDA noted that, although long-term ingestion of the impurity “may be associated with a theoretical potential increased cancer risk in humans,” there is no immediate risk in taking this medication. The agency added that no related adverse events (AEs) have been reported.
The four additional lots included in the newest recall are as follows:
- 00018522 (expiration date: August 2021).
- 00018523 (expiration date: August 2021).
- 00018739 (expiration date: August 2021).
- 00018740 (expiration date: August 2021).
The recalled lots were distributed in the United States and Puerto Rico from June 2019 to June 2021.
As before, the FDA noted that the benefits of stopping smoking “outweigh the theoretical potential cancer risk” from varenicline’s impurity.
It added that, although the impurities may increase risk for cancer if a high level of exposure continues over a long period, the drug is intended as a short-term treatment to aid in smoking cessation.
For now, clinicians should report any AEs from varenicline to the FDA’s MedWatch program, and patients taking this treatment should consult with their health care practitioner or pharmacy, the update notes.
A version of this article first appeared on Medscape.com.
Pfizer has recalled four more lots of the smoking cessation drug varenicline (Chantix), according to an Aug. 16 update on the U.S. Food and Drug Administration website.
In a new FDA MedWatch, the agency notes that these 0.5 mg/1 mg tablets are being recalled because of the presence of N-nitroso-varenicline, a nitrosamine impurity, at a level higher than Pfizer’s acceptable intake limit.
On July 2, the FDA reported that Pfizer had voluntarily recalled nine lots of the drug for this reason. As reported by this news organization, the company added three more lots to the recall a few weeks later.
In the update, the FDA noted that, although long-term ingestion of the impurity “may be associated with a theoretical potential increased cancer risk in humans,” there is no immediate risk in taking this medication. The agency added that no related adverse events (AEs) have been reported.
The four additional lots included in the newest recall are as follows:
- 00018522 (expiration date: August 2021).
- 00018523 (expiration date: August 2021).
- 00018739 (expiration date: August 2021).
- 00018740 (expiration date: August 2021).
The recalled lots were distributed in the United States and Puerto Rico from June 2019 to June 2021.
As before, the FDA noted that the benefits of stopping smoking “outweigh the theoretical potential cancer risk” from varenicline’s impurity.
It added that, although the impurities may increase risk for cancer if a high level of exposure continues over a long period, the drug is intended as a short-term treatment to aid in smoking cessation.
For now, clinicians should report any AEs from varenicline to the FDA’s MedWatch program, and patients taking this treatment should consult with their health care practitioner or pharmacy, the update notes.
A version of this article first appeared on Medscape.com.
Pfizer has recalled four more lots of the smoking cessation drug varenicline (Chantix), according to an Aug. 16 update on the U.S. Food and Drug Administration website.
In a new FDA MedWatch, the agency notes that these 0.5 mg/1 mg tablets are being recalled because of the presence of N-nitroso-varenicline, a nitrosamine impurity, at a level higher than Pfizer’s acceptable intake limit.
On July 2, the FDA reported that Pfizer had voluntarily recalled nine lots of the drug for this reason. As reported by this news organization, the company added three more lots to the recall a few weeks later.
In the update, the FDA noted that, although long-term ingestion of the impurity “may be associated with a theoretical potential increased cancer risk in humans,” there is no immediate risk in taking this medication. The agency added that no related adverse events (AEs) have been reported.
The four additional lots included in the newest recall are as follows:
- 00018522 (expiration date: August 2021).
- 00018523 (expiration date: August 2021).
- 00018739 (expiration date: August 2021).
- 00018740 (expiration date: August 2021).
The recalled lots were distributed in the United States and Puerto Rico from June 2019 to June 2021.
As before, the FDA noted that the benefits of stopping smoking “outweigh the theoretical potential cancer risk” from varenicline’s impurity.
It added that, although the impurities may increase risk for cancer if a high level of exposure continues over a long period, the drug is intended as a short-term treatment to aid in smoking cessation.
For now, clinicians should report any AEs from varenicline to the FDA’s MedWatch program, and patients taking this treatment should consult with their health care practitioner or pharmacy, the update notes.
A version of this article first appeared on Medscape.com.
‘Reassuring’ findings for second-generation antipsychotics during pregnancy
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.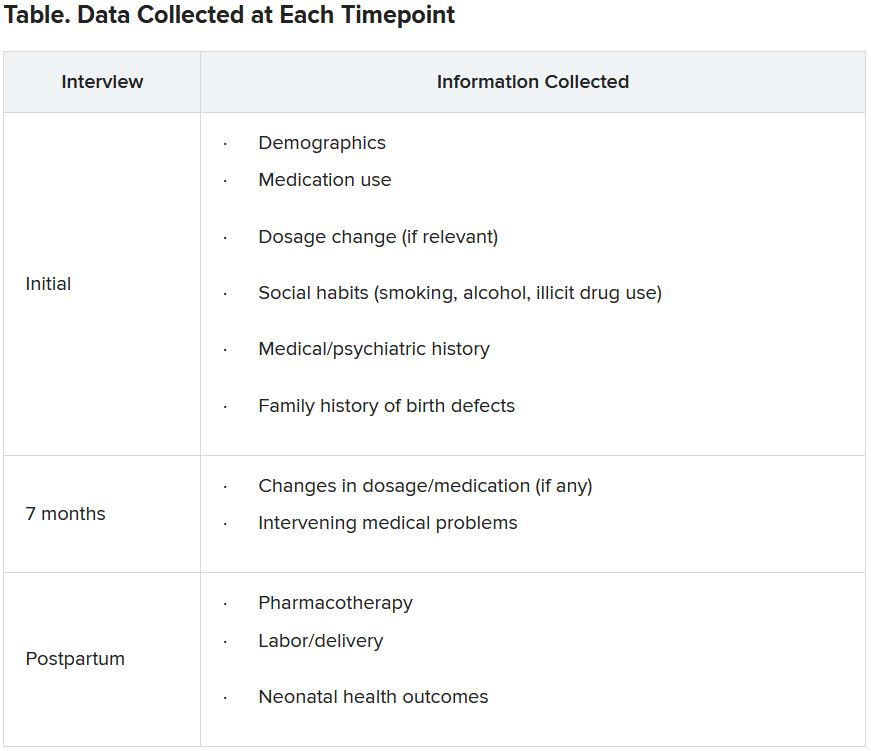
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘No justification’ for suicide warning on all antiseizure meds
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Half abandon metformin within a year of diabetes diagnosis
Nearly half of adults prescribed metformin after a new diagnosis of type 2 diabetes have stopped taking it by 1 year, new data show.
The findings, from a retrospective analysis of administrative data from Alberta, Canada, during 2012-2017, also show that the fall-off in metformin adherence was most dramatic during the first 30 days, and in most cases, there was no concomitant substitution of another glucose-lowering drug.
While the majority with newly diagnosed type 2 diabetes were prescribed metformin as first-line therapy, patients started on other agents incurred far higher medication and health care costs.
The data were recently published online in Diabetic Medicine by David J. T. Campbell, MD, PhD, of the University of Calgary (Alta.), and colleagues.
“We realized that even if someone is prescribed metformin that doesn’t mean they’re staying on metformin even for a year ... the drop-off rate is really quite abrupt,” Dr. Campbell said in an interview. Most who discontinued had A1c levels above 7.5%, so it wasn’t that they no longer needed glucose-lowering medication, he noted.
People don’t understand chronic use; meds don’t make you feel better
One reason for the discontinuations, he said, is that patients might not realize they need to keep taking the medication.
“When a physician is seeing a person with newly diagnosed diabetes, I think it’s important to remember that they might not know the implications of having a chronic condition. A lot of times we’re quick to prescribe metformin and forget about it. ... Physicians might write a script for 3 months and three refills and not see the patient again for a year ... We may need to keep a closer eye on these folks and have more regular follow-up, and make sure they’re getting early diabetes education.”
Side effects are an issue, but not for most. “Any clinician who prescribes metformin knows there are side effects, such as upset stomach, diarrhea, and nausea. But certainly, it’s not half [who experience these]. ... A lot of people just aren’t accepting of having to take it lifelong, especially since they probably don’t feel any better on it,” Dr. Campbell said.
James Flory, MD, an endocrinologist at Memorial Sloan Kettering Cancer Center, New York, said in an interview that about 25% of patients taking metformin experience gastrointestinal side effects.
Moreover, he noted that the drop-off in adherence is also seen with antihypertensive and lipid-lowering drugs that have fewer side effects than those of metformin. He pointed to a “striking example” of this, a 2011 randomized trial published in the New England Journal of Medicine, and as reported by this news organization, showing overall rates of adherence to these medications was around 50%, even among people who had already had a myocardial infarction.
“People really don’t want to be on these medications. ... They have an aversion to being medicalized and taking pills. If they’re not being pretty consistently prompted and reminded and urged to take them, I think people will find rationalizations, reasons for stopping. ... I think people want to handle things through lifestyle and not be on a drug,” noted Dr. Flory, who has published on the subject of metformin adherence.
“These drugs don’t make people feel better. None of them do. At best they don’t make you feel worse. You have to really believe in the chronic condition and believe that it’s hurting you and that you can’t handle it without the drugs to motivate you to keep taking them,” Dr. Flory explained.
Communication with the patient is key, he added.
“I don’t have empirical data to support this, but I feel it’s helpful to acknowledge the downsides to patients. I tell them to let me know [if they’re having side effects] and we’ll work on it. Don’t just stop taking the drug and never circle back.” At the same time, he added, “I think it’s important to emphasize metformin’s safety and effectiveness.”
For patients experiencing gastrointestinal side effects, options including switching to extended-release metformin or lowering the dose.
Also, while patients are typically advised to take metformin with food, some experience diarrhea when they do that and prefer to take it at bedtime than with dinner. “If that’s what works for people, that’s what they should do,” Dr. Flory advised.
“It doesn’t take a lot of time to emphasize to patients the safety and this level of flexibility and control they should be able to exercise over how much they take and when. These things should really help.”
Metformin usually prescribed, but not always taken
Dr. Campbell and colleagues analyzed 17,932 individuals with incident type 2 diabetes diagnosed between April 1, 2012, and March 31, 2017. Overall, 89% received metformin monotherapy as their initial diabetes prescription, 7.6% started metformin in combination with another glucose-lowering drug, and 3.3% were prescribed a nonmetformin diabetes medication. (Those prescribed insulin as their first diabetes medication were excluded.)
The most commonly coprescribed drugs with metformin were sulfonylureas (in 47%) and DPP-4 inhibitors (28%). Of those initiated with only nonmetformin medications, sulfonylureas were also the most common (53%) and dipeptidyl peptidase-4 (DPP-4) inhibitors second (21%).
The metformin prescribing rate of 89% reflects current guidelines, Dr. Campbell noted.
“In hypertension, clinicians weren’t really following the guidelines ... they were prescribing more expensive drugs than the guidelines say. ... We showed that in diabetes, contrary to hypertension, clinicians really are generally following the clinical practice guidelines. ... The vast majority who are started on metformin are started on monotherapy. That was reassuring to us. We’re not paying for a bunch of expensive drugs when metformin would do just as well,” he said.
However, the proportion who had been dispensed metformin to cover the prescribed number of days dropped by about 10% after 30 days, by a further 10% after 90 days, and yet again after 100 days, resulting in just 54% remaining on the drug by 1 year.
Factors associated with higher adherence included older age, presence of comorbidities, and highest versus lowest neighborhood income quintile.
Those who had been prescribed nonmetformin monotherapy had about twice the total health care costs of those initially prescribed metformin monotherapy. Higher health care costs were seen among patients who were younger, had lower incomes, had higher baseline A1c, had more comorbidities, and were men.
How will the newer type 2 diabetes drugs change prescribing?
Dr. Campbell noted that “a lot has changed since 2017. ... At least in Canada, the sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists were supposed to be reserved as second-line agents in patients with cardiovascular disease, but more and more they’re being thought of as first-line agents in high-risk patients.”
“I suspect as those guidelines are transmitted to primary care colleagues who are doing the bulk of the prescribing we’ll see more and more uptake of these agents.”
Indeed, Dr. Flory said, “The metformin data at this point are very dated and the body of trials showing health benefits for it is actually very weak compared to the big trials that have been done for the newer agents, to the point where you can imagine a consensus gradually forming where people start to recommend something other than metformin for nearly everybody with type 2 diabetes. The cost implications are just huge, and I think the safety implications as well.”
According to Dr. Flory, the SGLT2 inhibitors “are fundamentally not as safe as metformin. I think they’re very safe drugs – large good studies have established that – but if you’re going to give drugs to a large number of people who are pretty healthy at baseline the safety standards have to be pretty high.”
Just the elevated risk of euglycemic diabetic ketoacidosis alone is reason for pause, Dr. Flory said. “Even though it’s manageable ... metformin just doesn’t have a safety problem like that. I’m very comfortable prescribing SGLT2 inhibitors, but If I’m going to give a drug to a million people and have nothing go wrong with any of them, that would be metformin, not an SGLT2 [inhibitor].”
Dr. Campbell and colleagues will be conducting a follow-up of prescribing data through 2019, which will of course include the newer agents. They’ll also investigate reasons for drug discontinuation and outcomes of those who discontinue versus continue metformin.
Dr. Campbell has reported no relevant financial relationships. Dr. Flory consults for a legal firm on litigation related to insulin analog pricing issues, not for or pertaining to a specific company.
A version of this article first appeared on Medscape.com.
Nearly half of adults prescribed metformin after a new diagnosis of type 2 diabetes have stopped taking it by 1 year, new data show.
The findings, from a retrospective analysis of administrative data from Alberta, Canada, during 2012-2017, also show that the fall-off in metformin adherence was most dramatic during the first 30 days, and in most cases, there was no concomitant substitution of another glucose-lowering drug.
While the majority with newly diagnosed type 2 diabetes were prescribed metformin as first-line therapy, patients started on other agents incurred far higher medication and health care costs.
The data were recently published online in Diabetic Medicine by David J. T. Campbell, MD, PhD, of the University of Calgary (Alta.), and colleagues.
“We realized that even if someone is prescribed metformin that doesn’t mean they’re staying on metformin even for a year ... the drop-off rate is really quite abrupt,” Dr. Campbell said in an interview. Most who discontinued had A1c levels above 7.5%, so it wasn’t that they no longer needed glucose-lowering medication, he noted.
People don’t understand chronic use; meds don’t make you feel better
One reason for the discontinuations, he said, is that patients might not realize they need to keep taking the medication.
“When a physician is seeing a person with newly diagnosed diabetes, I think it’s important to remember that they might not know the implications of having a chronic condition. A lot of times we’re quick to prescribe metformin and forget about it. ... Physicians might write a script for 3 months and three refills and not see the patient again for a year ... We may need to keep a closer eye on these folks and have more regular follow-up, and make sure they’re getting early diabetes education.”
Side effects are an issue, but not for most. “Any clinician who prescribes metformin knows there are side effects, such as upset stomach, diarrhea, and nausea. But certainly, it’s not half [who experience these]. ... A lot of people just aren’t accepting of having to take it lifelong, especially since they probably don’t feel any better on it,” Dr. Campbell said.
James Flory, MD, an endocrinologist at Memorial Sloan Kettering Cancer Center, New York, said in an interview that about 25% of patients taking metformin experience gastrointestinal side effects.
Moreover, he noted that the drop-off in adherence is also seen with antihypertensive and lipid-lowering drugs that have fewer side effects than those of metformin. He pointed to a “striking example” of this, a 2011 randomized trial published in the New England Journal of Medicine, and as reported by this news organization, showing overall rates of adherence to these medications was around 50%, even among people who had already had a myocardial infarction.
“People really don’t want to be on these medications. ... They have an aversion to being medicalized and taking pills. If they’re not being pretty consistently prompted and reminded and urged to take them, I think people will find rationalizations, reasons for stopping. ... I think people want to handle things through lifestyle and not be on a drug,” noted Dr. Flory, who has published on the subject of metformin adherence.
“These drugs don’t make people feel better. None of them do. At best they don’t make you feel worse. You have to really believe in the chronic condition and believe that it’s hurting you and that you can’t handle it without the drugs to motivate you to keep taking them,” Dr. Flory explained.
Communication with the patient is key, he added.
“I don’t have empirical data to support this, but I feel it’s helpful to acknowledge the downsides to patients. I tell them to let me know [if they’re having side effects] and we’ll work on it. Don’t just stop taking the drug and never circle back.” At the same time, he added, “I think it’s important to emphasize metformin’s safety and effectiveness.”
For patients experiencing gastrointestinal side effects, options including switching to extended-release metformin or lowering the dose.
Also, while patients are typically advised to take metformin with food, some experience diarrhea when they do that and prefer to take it at bedtime than with dinner. “If that’s what works for people, that’s what they should do,” Dr. Flory advised.
“It doesn’t take a lot of time to emphasize to patients the safety and this level of flexibility and control they should be able to exercise over how much they take and when. These things should really help.”
Metformin usually prescribed, but not always taken
Dr. Campbell and colleagues analyzed 17,932 individuals with incident type 2 diabetes diagnosed between April 1, 2012, and March 31, 2017. Overall, 89% received metformin monotherapy as their initial diabetes prescription, 7.6% started metformin in combination with another glucose-lowering drug, and 3.3% were prescribed a nonmetformin diabetes medication. (Those prescribed insulin as their first diabetes medication were excluded.)
The most commonly coprescribed drugs with metformin were sulfonylureas (in 47%) and DPP-4 inhibitors (28%). Of those initiated with only nonmetformin medications, sulfonylureas were also the most common (53%) and dipeptidyl peptidase-4 (DPP-4) inhibitors second (21%).
The metformin prescribing rate of 89% reflects current guidelines, Dr. Campbell noted.
“In hypertension, clinicians weren’t really following the guidelines ... they were prescribing more expensive drugs than the guidelines say. ... We showed that in diabetes, contrary to hypertension, clinicians really are generally following the clinical practice guidelines. ... The vast majority who are started on metformin are started on monotherapy. That was reassuring to us. We’re not paying for a bunch of expensive drugs when metformin would do just as well,” he said.
However, the proportion who had been dispensed metformin to cover the prescribed number of days dropped by about 10% after 30 days, by a further 10% after 90 days, and yet again after 100 days, resulting in just 54% remaining on the drug by 1 year.
Factors associated with higher adherence included older age, presence of comorbidities, and highest versus lowest neighborhood income quintile.
Those who had been prescribed nonmetformin monotherapy had about twice the total health care costs of those initially prescribed metformin monotherapy. Higher health care costs were seen among patients who were younger, had lower incomes, had higher baseline A1c, had more comorbidities, and were men.
How will the newer type 2 diabetes drugs change prescribing?
Dr. Campbell noted that “a lot has changed since 2017. ... At least in Canada, the sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists were supposed to be reserved as second-line agents in patients with cardiovascular disease, but more and more they’re being thought of as first-line agents in high-risk patients.”
“I suspect as those guidelines are transmitted to primary care colleagues who are doing the bulk of the prescribing we’ll see more and more uptake of these agents.”
Indeed, Dr. Flory said, “The metformin data at this point are very dated and the body of trials showing health benefits for it is actually very weak compared to the big trials that have been done for the newer agents, to the point where you can imagine a consensus gradually forming where people start to recommend something other than metformin for nearly everybody with type 2 diabetes. The cost implications are just huge, and I think the safety implications as well.”
According to Dr. Flory, the SGLT2 inhibitors “are fundamentally not as safe as metformin. I think they’re very safe drugs – large good studies have established that – but if you’re going to give drugs to a large number of people who are pretty healthy at baseline the safety standards have to be pretty high.”
Just the elevated risk of euglycemic diabetic ketoacidosis alone is reason for pause, Dr. Flory said. “Even though it’s manageable ... metformin just doesn’t have a safety problem like that. I’m very comfortable prescribing SGLT2 inhibitors, but If I’m going to give a drug to a million people and have nothing go wrong with any of them, that would be metformin, not an SGLT2 [inhibitor].”
Dr. Campbell and colleagues will be conducting a follow-up of prescribing data through 2019, which will of course include the newer agents. They’ll also investigate reasons for drug discontinuation and outcomes of those who discontinue versus continue metformin.
Dr. Campbell has reported no relevant financial relationships. Dr. Flory consults for a legal firm on litigation related to insulin analog pricing issues, not for or pertaining to a specific company.
A version of this article first appeared on Medscape.com.
Nearly half of adults prescribed metformin after a new diagnosis of type 2 diabetes have stopped taking it by 1 year, new data show.
The findings, from a retrospective analysis of administrative data from Alberta, Canada, during 2012-2017, also show that the fall-off in metformin adherence was most dramatic during the first 30 days, and in most cases, there was no concomitant substitution of another glucose-lowering drug.
While the majority with newly diagnosed type 2 diabetes were prescribed metformin as first-line therapy, patients started on other agents incurred far higher medication and health care costs.
The data were recently published online in Diabetic Medicine by David J. T. Campbell, MD, PhD, of the University of Calgary (Alta.), and colleagues.
“We realized that even if someone is prescribed metformin that doesn’t mean they’re staying on metformin even for a year ... the drop-off rate is really quite abrupt,” Dr. Campbell said in an interview. Most who discontinued had A1c levels above 7.5%, so it wasn’t that they no longer needed glucose-lowering medication, he noted.
People don’t understand chronic use; meds don’t make you feel better
One reason for the discontinuations, he said, is that patients might not realize they need to keep taking the medication.
“When a physician is seeing a person with newly diagnosed diabetes, I think it’s important to remember that they might not know the implications of having a chronic condition. A lot of times we’re quick to prescribe metformin and forget about it. ... Physicians might write a script for 3 months and three refills and not see the patient again for a year ... We may need to keep a closer eye on these folks and have more regular follow-up, and make sure they’re getting early diabetes education.”
Side effects are an issue, but not for most. “Any clinician who prescribes metformin knows there are side effects, such as upset stomach, diarrhea, and nausea. But certainly, it’s not half [who experience these]. ... A lot of people just aren’t accepting of having to take it lifelong, especially since they probably don’t feel any better on it,” Dr. Campbell said.
James Flory, MD, an endocrinologist at Memorial Sloan Kettering Cancer Center, New York, said in an interview that about 25% of patients taking metformin experience gastrointestinal side effects.
Moreover, he noted that the drop-off in adherence is also seen with antihypertensive and lipid-lowering drugs that have fewer side effects than those of metformin. He pointed to a “striking example” of this, a 2011 randomized trial published in the New England Journal of Medicine, and as reported by this news organization, showing overall rates of adherence to these medications was around 50%, even among people who had already had a myocardial infarction.
“People really don’t want to be on these medications. ... They have an aversion to being medicalized and taking pills. If they’re not being pretty consistently prompted and reminded and urged to take them, I think people will find rationalizations, reasons for stopping. ... I think people want to handle things through lifestyle and not be on a drug,” noted Dr. Flory, who has published on the subject of metformin adherence.
“These drugs don’t make people feel better. None of them do. At best they don’t make you feel worse. You have to really believe in the chronic condition and believe that it’s hurting you and that you can’t handle it without the drugs to motivate you to keep taking them,” Dr. Flory explained.
Communication with the patient is key, he added.
“I don’t have empirical data to support this, but I feel it’s helpful to acknowledge the downsides to patients. I tell them to let me know [if they’re having side effects] and we’ll work on it. Don’t just stop taking the drug and never circle back.” At the same time, he added, “I think it’s important to emphasize metformin’s safety and effectiveness.”
For patients experiencing gastrointestinal side effects, options including switching to extended-release metformin or lowering the dose.
Also, while patients are typically advised to take metformin with food, some experience diarrhea when they do that and prefer to take it at bedtime than with dinner. “If that’s what works for people, that’s what they should do,” Dr. Flory advised.
“It doesn’t take a lot of time to emphasize to patients the safety and this level of flexibility and control they should be able to exercise over how much they take and when. These things should really help.”
Metformin usually prescribed, but not always taken
Dr. Campbell and colleagues analyzed 17,932 individuals with incident type 2 diabetes diagnosed between April 1, 2012, and March 31, 2017. Overall, 89% received metformin monotherapy as their initial diabetes prescription, 7.6% started metformin in combination with another glucose-lowering drug, and 3.3% were prescribed a nonmetformin diabetes medication. (Those prescribed insulin as their first diabetes medication were excluded.)
The most commonly coprescribed drugs with metformin were sulfonylureas (in 47%) and DPP-4 inhibitors (28%). Of those initiated with only nonmetformin medications, sulfonylureas were also the most common (53%) and dipeptidyl peptidase-4 (DPP-4) inhibitors second (21%).
The metformin prescribing rate of 89% reflects current guidelines, Dr. Campbell noted.
“In hypertension, clinicians weren’t really following the guidelines ... they were prescribing more expensive drugs than the guidelines say. ... We showed that in diabetes, contrary to hypertension, clinicians really are generally following the clinical practice guidelines. ... The vast majority who are started on metformin are started on monotherapy. That was reassuring to us. We’re not paying for a bunch of expensive drugs when metformin would do just as well,” he said.
However, the proportion who had been dispensed metformin to cover the prescribed number of days dropped by about 10% after 30 days, by a further 10% after 90 days, and yet again after 100 days, resulting in just 54% remaining on the drug by 1 year.
Factors associated with higher adherence included older age, presence of comorbidities, and highest versus lowest neighborhood income quintile.
Those who had been prescribed nonmetformin monotherapy had about twice the total health care costs of those initially prescribed metformin monotherapy. Higher health care costs were seen among patients who were younger, had lower incomes, had higher baseline A1c, had more comorbidities, and were men.
How will the newer type 2 diabetes drugs change prescribing?
Dr. Campbell noted that “a lot has changed since 2017. ... At least in Canada, the sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists were supposed to be reserved as second-line agents in patients with cardiovascular disease, but more and more they’re being thought of as first-line agents in high-risk patients.”
“I suspect as those guidelines are transmitted to primary care colleagues who are doing the bulk of the prescribing we’ll see more and more uptake of these agents.”
Indeed, Dr. Flory said, “The metformin data at this point are very dated and the body of trials showing health benefits for it is actually very weak compared to the big trials that have been done for the newer agents, to the point where you can imagine a consensus gradually forming where people start to recommend something other than metformin for nearly everybody with type 2 diabetes. The cost implications are just huge, and I think the safety implications as well.”
According to Dr. Flory, the SGLT2 inhibitors “are fundamentally not as safe as metformin. I think they’re very safe drugs – large good studies have established that – but if you’re going to give drugs to a large number of people who are pretty healthy at baseline the safety standards have to be pretty high.”
Just the elevated risk of euglycemic diabetic ketoacidosis alone is reason for pause, Dr. Flory said. “Even though it’s manageable ... metformin just doesn’t have a safety problem like that. I’m very comfortable prescribing SGLT2 inhibitors, but If I’m going to give a drug to a million people and have nothing go wrong with any of them, that would be metformin, not an SGLT2 [inhibitor].”
Dr. Campbell and colleagues will be conducting a follow-up of prescribing data through 2019, which will of course include the newer agents. They’ll also investigate reasons for drug discontinuation and outcomes of those who discontinue versus continue metformin.
Dr. Campbell has reported no relevant financial relationships. Dr. Flory consults for a legal firm on litigation related to insulin analog pricing issues, not for or pertaining to a specific company.
A version of this article first appeared on Medscape.com.
Bone drugs for prostate cancer may result in survival benefit
Results from a retrospective study show that the addition of bone resorption inhibitors (BRIs), including zoledronic acid and denosumab (Xgeva), to abiraterone plus prednisolone was associated with significantly longer overall survival (OS). The median OS was increased by nearly 9 months among recipients, compared with men who didn’t receive these drugs in this setting.
The findings were published online July 22, 2021, in JAMA Network Open.
All men with prostate cancer should receive BRIs “as the disease reaches the castration resistance with bone metastases stage, as recommended by the international guidelines,” lead author Edoardo Francini, MD, PhD, of the University of Florence (Italy) said in a comment.
While there is no evidence that BRIs – when used alone – may improve survival in metastatic castration-resistant prostate cancer (mCRPC) with bone involvement, there has been a “suggestion” of a survival benefit with BRIs when combined with other anticancer therapies in this setting, say the authors.
So Dr. Francini and a team of international coinvestigators looked at the medical records of men with mCRPC and bone metastases treated at eight institutions in Canada, Europe, and the United States and focused on patients who received abiraterone acetate (with prednisone) because it is the most common first-line therapy in this setting.
Patients were classified by receipt versus nonreceipt of concomitant BRIs and subclassified by volume of disease (high or low volume).
There were two cohorts in the study population: 529 men (71.0%) who received abiraterone alone and 216 men (29.0%) who received abiraterone plus BRIs. The median follow-up was 23.5 months.
Patients in the BRI cohort experienced significantly longer OS compared with those in the abiraterone alone cohort (31.8 vs. 23.0 months; hazard ratio, 0.65; P < .001).
Notably, the OS benefit in the BRI cohort was greater for patients with high-volume versus low-volume disease (33.6 vs. 19.7 months; HR, 0.51; P < .001).
Dr. Francini hopes the new study results can effect change. “Hopefully, clinicians will be more inclined to use bone resorption inhibitors in combination with abiraterone acetate plus prednisone as soon as the disease reaches the castration-resistance with bone metastases stage, as recommended by the international guidelines.”
Importance of bone-targeted drugs
“This study highlights the importance of bone-targeted therapy in current practice for men with mCRPC and bone metastases,” Samuel Takvorian, MD, and Naomi Haas, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
But the study also reveals that work needs to be done to get clinicians to prescribe BRIs, they said, and that clinical pathways and behavioral “nudges” could help promote adoption.
Most (71%) of the men in this study did not get bone protective drug therapy, they pointed out, even though they were being treated at major hospital systems.
So, why aren’t more men receiving BRIs?
“I think this is less likely due to poor communication from professional societies (the guidelines are clear) and more likely due to bone health being low on the list of priorities for these patients and clinician uncertainty and/or lack of appreciation of the clinical benefit of these agents,” Dr. Takvorian said in an interview.
“When prostate cancer progresses to the castration-resistant phase, clinicians (and patients) rightfully are focused on the next cancer-directed therapy. However, this may be at the expense of supportive care, like bone agents, which often gets short shrift,” he added.
As would be expected, the men who were taking BRIs had a significantly shorter time to first skeletal-related events (SREs), compared with those who were not (32.4 vs. 42.7 months; HR, 1.27; P = .04), and the risk of a first SRE was more than double in the subgroup with low-volume disease (HR, 2.29; P < .001).
“These SREs collectively represent a clinically meaningful outcome that is often measured in clinical trials,” the editorialists observed. In the current study, SREs were comprised of pathological fractures, spinal cord compression, or the need for surgery or radiotherapy to bone.
“Up to one-half of men with mCRPC, the advanced and often fatal stage of disease, experience SREs, which are associated with considerable morbidity, decreased survival, and increased health care utilization and costs,” they wrote.
Costly vs. inexpensive BRI
The study found no difference in the OS benefit between the different BRIs used, that is, between that seen with zoledronic acid versus denosumab.
The editorialists suggested that this finding is important, even though it “must be considered preliminary given the limitations of a retrospective study.” These results add “to data suggesting that these agents are comparably beneficial; thus, decisions between them should focus on clinical factors, such as kidney function, patient preference, and cost.”
The two agents differ mechanistically, they added, with zoledronic acid preferentially inhibiting osteoclast proliferation and denosumab inhibiting an important factor in osteoclast maturation.
In terms of having differentiating characteristics, the editorialists say that zoledronic acid is “more often associated with acute phase reactions and required monitoring of kidney function” while “denosumab conferred a higher risk of hypocalcemia.” Rates of osteonecrosis of the jaw are comparable.
International guidelines endorse the use of either agent for the treatment of men with mCRPC. But “some argue that the marginal benefit of denosumab must be weighed against its dramatically higher cost (the annual cost of zoledronic acid is approximately $140 vs. $29,000 for denosumab),” the editorialists said.
The dramatically higher cost of denosumab versus zoledronic acid has also been noted by other oncologists treating patients with other cancers, including multiple myeloma.
In addition to drug costs, there is another issue at stake: the prescribing oncologist is reimbursed by Medicare Part D at 6% for whichever drug is chosen, which represents a “financial conflict” for oncologists, said Vincent Rajkumar, MD, professor of medicine and a hematologist/oncologist at the Mayo Clinic, Rochester, Minn.
There is also a difference in how the drugs are administered, which may influence patient preference, the myeloma experts noted. Zoledronic acid is given intravenously every 3 months and requires a 15-minute infusion at a center, while denosumab needs to be given more frequently (every month) but is administered by subcutaneous injection.
Dr. Francini reported receiving grants from Roche Italia and personal fees for research travel from Janssen-Cilag outside the submitted work. A number of other authors disclosed financial ties to Janssen or Amgen, makers of abiraterone and denosumab, respectively. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a retrospective study show that the addition of bone resorption inhibitors (BRIs), including zoledronic acid and denosumab (Xgeva), to abiraterone plus prednisolone was associated with significantly longer overall survival (OS). The median OS was increased by nearly 9 months among recipients, compared with men who didn’t receive these drugs in this setting.
The findings were published online July 22, 2021, in JAMA Network Open.
All men with prostate cancer should receive BRIs “as the disease reaches the castration resistance with bone metastases stage, as recommended by the international guidelines,” lead author Edoardo Francini, MD, PhD, of the University of Florence (Italy) said in a comment.
While there is no evidence that BRIs – when used alone – may improve survival in metastatic castration-resistant prostate cancer (mCRPC) with bone involvement, there has been a “suggestion” of a survival benefit with BRIs when combined with other anticancer therapies in this setting, say the authors.
So Dr. Francini and a team of international coinvestigators looked at the medical records of men with mCRPC and bone metastases treated at eight institutions in Canada, Europe, and the United States and focused on patients who received abiraterone acetate (with prednisone) because it is the most common first-line therapy in this setting.
Patients were classified by receipt versus nonreceipt of concomitant BRIs and subclassified by volume of disease (high or low volume).
There were two cohorts in the study population: 529 men (71.0%) who received abiraterone alone and 216 men (29.0%) who received abiraterone plus BRIs. The median follow-up was 23.5 months.
Patients in the BRI cohort experienced significantly longer OS compared with those in the abiraterone alone cohort (31.8 vs. 23.0 months; hazard ratio, 0.65; P < .001).
Notably, the OS benefit in the BRI cohort was greater for patients with high-volume versus low-volume disease (33.6 vs. 19.7 months; HR, 0.51; P < .001).
Dr. Francini hopes the new study results can effect change. “Hopefully, clinicians will be more inclined to use bone resorption inhibitors in combination with abiraterone acetate plus prednisone as soon as the disease reaches the castration-resistance with bone metastases stage, as recommended by the international guidelines.”
Importance of bone-targeted drugs
“This study highlights the importance of bone-targeted therapy in current practice for men with mCRPC and bone metastases,” Samuel Takvorian, MD, and Naomi Haas, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
But the study also reveals that work needs to be done to get clinicians to prescribe BRIs, they said, and that clinical pathways and behavioral “nudges” could help promote adoption.
Most (71%) of the men in this study did not get bone protective drug therapy, they pointed out, even though they were being treated at major hospital systems.
So, why aren’t more men receiving BRIs?
“I think this is less likely due to poor communication from professional societies (the guidelines are clear) and more likely due to bone health being low on the list of priorities for these patients and clinician uncertainty and/or lack of appreciation of the clinical benefit of these agents,” Dr. Takvorian said in an interview.
“When prostate cancer progresses to the castration-resistant phase, clinicians (and patients) rightfully are focused on the next cancer-directed therapy. However, this may be at the expense of supportive care, like bone agents, which often gets short shrift,” he added.
As would be expected, the men who were taking BRIs had a significantly shorter time to first skeletal-related events (SREs), compared with those who were not (32.4 vs. 42.7 months; HR, 1.27; P = .04), and the risk of a first SRE was more than double in the subgroup with low-volume disease (HR, 2.29; P < .001).
“These SREs collectively represent a clinically meaningful outcome that is often measured in clinical trials,” the editorialists observed. In the current study, SREs were comprised of pathological fractures, spinal cord compression, or the need for surgery or radiotherapy to bone.
“Up to one-half of men with mCRPC, the advanced and often fatal stage of disease, experience SREs, which are associated with considerable morbidity, decreased survival, and increased health care utilization and costs,” they wrote.
Costly vs. inexpensive BRI
The study found no difference in the OS benefit between the different BRIs used, that is, between that seen with zoledronic acid versus denosumab.
The editorialists suggested that this finding is important, even though it “must be considered preliminary given the limitations of a retrospective study.” These results add “to data suggesting that these agents are comparably beneficial; thus, decisions between them should focus on clinical factors, such as kidney function, patient preference, and cost.”
The two agents differ mechanistically, they added, with zoledronic acid preferentially inhibiting osteoclast proliferation and denosumab inhibiting an important factor in osteoclast maturation.
In terms of having differentiating characteristics, the editorialists say that zoledronic acid is “more often associated with acute phase reactions and required monitoring of kidney function” while “denosumab conferred a higher risk of hypocalcemia.” Rates of osteonecrosis of the jaw are comparable.
International guidelines endorse the use of either agent for the treatment of men with mCRPC. But “some argue that the marginal benefit of denosumab must be weighed against its dramatically higher cost (the annual cost of zoledronic acid is approximately $140 vs. $29,000 for denosumab),” the editorialists said.
The dramatically higher cost of denosumab versus zoledronic acid has also been noted by other oncologists treating patients with other cancers, including multiple myeloma.
In addition to drug costs, there is another issue at stake: the prescribing oncologist is reimbursed by Medicare Part D at 6% for whichever drug is chosen, which represents a “financial conflict” for oncologists, said Vincent Rajkumar, MD, professor of medicine and a hematologist/oncologist at the Mayo Clinic, Rochester, Minn.
There is also a difference in how the drugs are administered, which may influence patient preference, the myeloma experts noted. Zoledronic acid is given intravenously every 3 months and requires a 15-minute infusion at a center, while denosumab needs to be given more frequently (every month) but is administered by subcutaneous injection.
Dr. Francini reported receiving grants from Roche Italia and personal fees for research travel from Janssen-Cilag outside the submitted work. A number of other authors disclosed financial ties to Janssen or Amgen, makers of abiraterone and denosumab, respectively. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a retrospective study show that the addition of bone resorption inhibitors (BRIs), including zoledronic acid and denosumab (Xgeva), to abiraterone plus prednisolone was associated with significantly longer overall survival (OS). The median OS was increased by nearly 9 months among recipients, compared with men who didn’t receive these drugs in this setting.
The findings were published online July 22, 2021, in JAMA Network Open.
All men with prostate cancer should receive BRIs “as the disease reaches the castration resistance with bone metastases stage, as recommended by the international guidelines,” lead author Edoardo Francini, MD, PhD, of the University of Florence (Italy) said in a comment.
While there is no evidence that BRIs – when used alone – may improve survival in metastatic castration-resistant prostate cancer (mCRPC) with bone involvement, there has been a “suggestion” of a survival benefit with BRIs when combined with other anticancer therapies in this setting, say the authors.
So Dr. Francini and a team of international coinvestigators looked at the medical records of men with mCRPC and bone metastases treated at eight institutions in Canada, Europe, and the United States and focused on patients who received abiraterone acetate (with prednisone) because it is the most common first-line therapy in this setting.
Patients were classified by receipt versus nonreceipt of concomitant BRIs and subclassified by volume of disease (high or low volume).
There were two cohorts in the study population: 529 men (71.0%) who received abiraterone alone and 216 men (29.0%) who received abiraterone plus BRIs. The median follow-up was 23.5 months.
Patients in the BRI cohort experienced significantly longer OS compared with those in the abiraterone alone cohort (31.8 vs. 23.0 months; hazard ratio, 0.65; P < .001).
Notably, the OS benefit in the BRI cohort was greater for patients with high-volume versus low-volume disease (33.6 vs. 19.7 months; HR, 0.51; P < .001).
Dr. Francini hopes the new study results can effect change. “Hopefully, clinicians will be more inclined to use bone resorption inhibitors in combination with abiraterone acetate plus prednisone as soon as the disease reaches the castration-resistance with bone metastases stage, as recommended by the international guidelines.”
Importance of bone-targeted drugs
“This study highlights the importance of bone-targeted therapy in current practice for men with mCRPC and bone metastases,” Samuel Takvorian, MD, and Naomi Haas, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
But the study also reveals that work needs to be done to get clinicians to prescribe BRIs, they said, and that clinical pathways and behavioral “nudges” could help promote adoption.
Most (71%) of the men in this study did not get bone protective drug therapy, they pointed out, even though they were being treated at major hospital systems.
So, why aren’t more men receiving BRIs?
“I think this is less likely due to poor communication from professional societies (the guidelines are clear) and more likely due to bone health being low on the list of priorities for these patients and clinician uncertainty and/or lack of appreciation of the clinical benefit of these agents,” Dr. Takvorian said in an interview.
“When prostate cancer progresses to the castration-resistant phase, clinicians (and patients) rightfully are focused on the next cancer-directed therapy. However, this may be at the expense of supportive care, like bone agents, which often gets short shrift,” he added.
As would be expected, the men who were taking BRIs had a significantly shorter time to first skeletal-related events (SREs), compared with those who were not (32.4 vs. 42.7 months; HR, 1.27; P = .04), and the risk of a first SRE was more than double in the subgroup with low-volume disease (HR, 2.29; P < .001).
“These SREs collectively represent a clinically meaningful outcome that is often measured in clinical trials,” the editorialists observed. In the current study, SREs were comprised of pathological fractures, spinal cord compression, or the need for surgery or radiotherapy to bone.
“Up to one-half of men with mCRPC, the advanced and often fatal stage of disease, experience SREs, which are associated with considerable morbidity, decreased survival, and increased health care utilization and costs,” they wrote.
Costly vs. inexpensive BRI
The study found no difference in the OS benefit between the different BRIs used, that is, between that seen with zoledronic acid versus denosumab.
The editorialists suggested that this finding is important, even though it “must be considered preliminary given the limitations of a retrospective study.” These results add “to data suggesting that these agents are comparably beneficial; thus, decisions between them should focus on clinical factors, such as kidney function, patient preference, and cost.”
The two agents differ mechanistically, they added, with zoledronic acid preferentially inhibiting osteoclast proliferation and denosumab inhibiting an important factor in osteoclast maturation.
In terms of having differentiating characteristics, the editorialists say that zoledronic acid is “more often associated with acute phase reactions and required monitoring of kidney function” while “denosumab conferred a higher risk of hypocalcemia.” Rates of osteonecrosis of the jaw are comparable.
International guidelines endorse the use of either agent for the treatment of men with mCRPC. But “some argue that the marginal benefit of denosumab must be weighed against its dramatically higher cost (the annual cost of zoledronic acid is approximately $140 vs. $29,000 for denosumab),” the editorialists said.
The dramatically higher cost of denosumab versus zoledronic acid has also been noted by other oncologists treating patients with other cancers, including multiple myeloma.
In addition to drug costs, there is another issue at stake: the prescribing oncologist is reimbursed by Medicare Part D at 6% for whichever drug is chosen, which represents a “financial conflict” for oncologists, said Vincent Rajkumar, MD, professor of medicine and a hematologist/oncologist at the Mayo Clinic, Rochester, Minn.
There is also a difference in how the drugs are administered, which may influence patient preference, the myeloma experts noted. Zoledronic acid is given intravenously every 3 months and requires a 15-minute infusion at a center, while denosumab needs to be given more frequently (every month) but is administered by subcutaneous injection.
Dr. Francini reported receiving grants from Roche Italia and personal fees for research travel from Janssen-Cilag outside the submitted work. A number of other authors disclosed financial ties to Janssen or Amgen, makers of abiraterone and denosumab, respectively. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diabetes drug’s new weight-loss indication fuels cost-benefit debate
The long list of side effects that follow ads for newer expensive drugs to treat type 2 diabetes sometimes include an unusual warning: They might cause weight loss. That side effect is one that many people – especially those with type 2 diabetes, which is associated with obesity – may desperately want.
So it’s no surprise that some of the same drugs are being reformulated and renamed by manufacturers as a new obesity treatment. No longer limited to the crowded field of treatments for type 2 diabetes, which affects about 10% of Americans, they join the far smaller number of drugs for obesity, which affects 42% of Americans and is ready to be mined for profit.
One that recently hit the market – winning Food and Drug Administration approval in June – is Novo Nordisk’s Wegovy (semaglutide), a higher-dose version of the company’s injectable diabetes drug, Ozempic.
Ozempic’s peppy ads suggest that people who use it might lose weight, but also include a disclaimer: that it “is not a weight-loss drug.” Now – with a new name – it is. And clinical trials showed using it leads to significant weight loss for many patients.
“People who go on this medication lose more weight than with any drug we’ve seen, ever,” said Fatima Cody Stanford, MD, MPH, an obesity medicine specialist at Massachusetts General Hospital and Harvard Medical School, both in Boston, who was not involved with any of the clinical trials.
But that leaves employers and insurers in the uncomfortable position of deciding if it’s worth it.
Wegovy’s monthly wholesale price tag – set at $1,349 – is about 58% more than Ozempic’s, although, the company pointed out, the drug’s injector pens contain more than twice as much of the active ingredient. Studies so far show that patients may need to take it indefinitely to maintain weight loss, translating to a tab that could top $323,000 over 20 years at the current price. Weight-loss treatments are not universally covered by insurance policies.
The arrival of this new class of weight-loss drugs – one from Lilly may soon follow – has created a thicket of issues for those who will pay for them. The decision is complicated by many unknowables concerning their long-term use and whether competition might eventually lower the price.
“The metric we try to use is value,” said James Gelfand, senior vice president for health policy at the ERISA Industry Committee, which represents large, self-insured employers. “If we pay for this drug, how much is this going to cost and how much value will it provide to the beneficiaries?”
Weight-loss treatments have had a lackluster past in this regard, with only modest results. Many employers and insurers likely remember Fen-Phen, a combination of fenfluramine and dexfenfluramine that was pulled from the market in the late 1990s for causing heart valve problems.
New drugs like Wegovy, more effective but also pricier than previous weight-loss treatments, will add more fuel to that debate.
Past treatments were shown to prompt weight loss in the range of 5%-10% of body weight. But many had relatively serious or unpleasant side effects.
Wegovy, however, helped patients lose an average of 15% of their body weight over 68 weeks in the main clinical trial that led to its approval. A comparison group that got a placebo injection lost an average of 2.5% over the same period. On the high end, nearly a third of patients in the treatment group lost 20% or more. Both groups had counseling on diet and exercise.
Side effects, generally considered mild, included nausea, diarrhea, vomiting, and constipation. A few patients developed pancreatitis, a serious inflammation of the pancreas. Like the diabetes medication, the drug carries a warning about a potential risk of a type of thyroid cancer.
Weight loss in those taking Wegovy puts it close to the 20%-25% losses seen with bariatric surgery, said Stanford, and well above the 3%-4% seen with diet and other lifestyle changes alone.
Participants also saw reductions in their waistlines and improvements in their blood pressure and blood sugar levels, which may mean they won’t develop diabetes, said Sean Wharton, MD, an internal medicine specialist and adjunct professor at York University in Toronto who was among the coauthors of the report outlining the results of the first clinical trial on Wegovy.
Since weight loss is known to reduce the risk of heart attack, high blood pressure and diabetes, might the new drug type be worth it?
Covering such treatment would be a sea change for Medicare, which specifically bars coverage for obesity medications or drugs for “anorexia, weight loss, or weight gain,” although it does pay for bariatric surgery. Pharmaceutical companies, patient advocates, and some medical professionals are backing proposed federal legislation to allow coverage. But the legislation, the Treat and Reduce Obesity Act, has not made progress despite being reintroduced every year since 2012, and sponsors are now asking federal officials instead to rewrite existing rules.
Private insurers will have to consider a cost-benefit analysis of adding Wegovy to their list of covered treatments, either broadly or with limits. Obesity was first recognized as a disease by the American Medical Association, easing the path for insurance coverage, in 2013.
“Employers are going to have a bit of a challenge” deciding whether to add the benefit to insurance offerings, said Steve Pearson, founder and president of the Institute for Clinical and Economic Review, which provides cost-benefit analyses of medical treatments but has not yet looked at Wegovy.
The trade-offs are embodied in patients like Phylander Pannell, a 49-year-old Largo, Md., woman who said she lost 65 pounds in a clinical trial of Wegovy. That study gave the drug to all participants for the first 20 weeks, then randomly assigned patients to get either the drug or a placebo for the next 48 weeks to determine what happens when the medication is stopped. Only after the trial ended did she find out she was in the treatment group the entire time.
Her weight fell slowly at first, then ramped up, eventually bringing her 190-pound frame down to about 125. Pains in her joints eased; she felt better all around.
“I definitely feel the drug was it for me,” said Ms. Pannell, who also followed the trial’s guidance on diet and exercise.
The study found that both groups lost weight in the initial 20 weeks, but those who continued to get the drug lost an additional average of 7.9% of their body weight. Those who got a placebo gained back nearly 7%.
After the trial ended, and the COVID-19 pandemic hit, Ms. Pannell regained some weight and is now at 155. She is eager to get back on the medication and hopes her job-based insurance will cover it.
Many employers do cover obesity drugs. For example, about 40% of private employer plans include Novo Nordisk’s once-daily injection called Saxenda on their health plans, said Michael Bachner, Novo Nordisk’s director of media relations.
He said the $1,349-a-month wholesale acquisition price of Wegovy was determined by making it equivalent to that of Saxenda, which is less effective.
Still, that is more than the $851 monthly wholesale price of Ozempic. But, he pointed out, the recommended dosage of Wegovy is more than twice that of Ozempic. Four milligrams come in the Ozempic injector pens for the month, while Wegovy has 9.6.
“There’s more drug in the pen,” Mr. Bachner said. “That drives the price up.”
He added: “This is not a 20-year-old drug that we now have a new indication for and are pricing it higher. It’s a whole different clinical program,” which required new trials.
Now scientists, employers, physicians, and patients will have to decide whether the new drugs are worth it.
Earlier estimates – some commissioned by Novo Nordisk – of the potential cost of adding an obesity drug benefit to Medicare showed an overall reduction in spending when better health from the resulting weight loss was factored in.
Still, those earlier estimates considered much less expensive drugs, including a range of generic and branded drugs costing as little as $7 a month to more than $300, a small fraction of Wegovy’s cost.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The long list of side effects that follow ads for newer expensive drugs to treat type 2 diabetes sometimes include an unusual warning: They might cause weight loss. That side effect is one that many people – especially those with type 2 diabetes, which is associated with obesity – may desperately want.
So it’s no surprise that some of the same drugs are being reformulated and renamed by manufacturers as a new obesity treatment. No longer limited to the crowded field of treatments for type 2 diabetes, which affects about 10% of Americans, they join the far smaller number of drugs for obesity, which affects 42% of Americans and is ready to be mined for profit.
One that recently hit the market – winning Food and Drug Administration approval in June – is Novo Nordisk’s Wegovy (semaglutide), a higher-dose version of the company’s injectable diabetes drug, Ozempic.
Ozempic’s peppy ads suggest that people who use it might lose weight, but also include a disclaimer: that it “is not a weight-loss drug.” Now – with a new name – it is. And clinical trials showed using it leads to significant weight loss for many patients.
“People who go on this medication lose more weight than with any drug we’ve seen, ever,” said Fatima Cody Stanford, MD, MPH, an obesity medicine specialist at Massachusetts General Hospital and Harvard Medical School, both in Boston, who was not involved with any of the clinical trials.
But that leaves employers and insurers in the uncomfortable position of deciding if it’s worth it.
Wegovy’s monthly wholesale price tag – set at $1,349 – is about 58% more than Ozempic’s, although, the company pointed out, the drug’s injector pens contain more than twice as much of the active ingredient. Studies so far show that patients may need to take it indefinitely to maintain weight loss, translating to a tab that could top $323,000 over 20 years at the current price. Weight-loss treatments are not universally covered by insurance policies.
The arrival of this new class of weight-loss drugs – one from Lilly may soon follow – has created a thicket of issues for those who will pay for them. The decision is complicated by many unknowables concerning their long-term use and whether competition might eventually lower the price.
“The metric we try to use is value,” said James Gelfand, senior vice president for health policy at the ERISA Industry Committee, which represents large, self-insured employers. “If we pay for this drug, how much is this going to cost and how much value will it provide to the beneficiaries?”
Weight-loss treatments have had a lackluster past in this regard, with only modest results. Many employers and insurers likely remember Fen-Phen, a combination of fenfluramine and dexfenfluramine that was pulled from the market in the late 1990s for causing heart valve problems.
New drugs like Wegovy, more effective but also pricier than previous weight-loss treatments, will add more fuel to that debate.
Past treatments were shown to prompt weight loss in the range of 5%-10% of body weight. But many had relatively serious or unpleasant side effects.
Wegovy, however, helped patients lose an average of 15% of their body weight over 68 weeks in the main clinical trial that led to its approval. A comparison group that got a placebo injection lost an average of 2.5% over the same period. On the high end, nearly a third of patients in the treatment group lost 20% or more. Both groups had counseling on diet and exercise.
Side effects, generally considered mild, included nausea, diarrhea, vomiting, and constipation. A few patients developed pancreatitis, a serious inflammation of the pancreas. Like the diabetes medication, the drug carries a warning about a potential risk of a type of thyroid cancer.
Weight loss in those taking Wegovy puts it close to the 20%-25% losses seen with bariatric surgery, said Stanford, and well above the 3%-4% seen with diet and other lifestyle changes alone.
Participants also saw reductions in their waistlines and improvements in their blood pressure and blood sugar levels, which may mean they won’t develop diabetes, said Sean Wharton, MD, an internal medicine specialist and adjunct professor at York University in Toronto who was among the coauthors of the report outlining the results of the first clinical trial on Wegovy.
Since weight loss is known to reduce the risk of heart attack, high blood pressure and diabetes, might the new drug type be worth it?
Covering such treatment would be a sea change for Medicare, which specifically bars coverage for obesity medications or drugs for “anorexia, weight loss, or weight gain,” although it does pay for bariatric surgery. Pharmaceutical companies, patient advocates, and some medical professionals are backing proposed federal legislation to allow coverage. But the legislation, the Treat and Reduce Obesity Act, has not made progress despite being reintroduced every year since 2012, and sponsors are now asking federal officials instead to rewrite existing rules.
Private insurers will have to consider a cost-benefit analysis of adding Wegovy to their list of covered treatments, either broadly or with limits. Obesity was first recognized as a disease by the American Medical Association, easing the path for insurance coverage, in 2013.
“Employers are going to have a bit of a challenge” deciding whether to add the benefit to insurance offerings, said Steve Pearson, founder and president of the Institute for Clinical and Economic Review, which provides cost-benefit analyses of medical treatments but has not yet looked at Wegovy.
The trade-offs are embodied in patients like Phylander Pannell, a 49-year-old Largo, Md., woman who said she lost 65 pounds in a clinical trial of Wegovy. That study gave the drug to all participants for the first 20 weeks, then randomly assigned patients to get either the drug or a placebo for the next 48 weeks to determine what happens when the medication is stopped. Only after the trial ended did she find out she was in the treatment group the entire time.
Her weight fell slowly at first, then ramped up, eventually bringing her 190-pound frame down to about 125. Pains in her joints eased; she felt better all around.
“I definitely feel the drug was it for me,” said Ms. Pannell, who also followed the trial’s guidance on diet and exercise.
The study found that both groups lost weight in the initial 20 weeks, but those who continued to get the drug lost an additional average of 7.9% of their body weight. Those who got a placebo gained back nearly 7%.
After the trial ended, and the COVID-19 pandemic hit, Ms. Pannell regained some weight and is now at 155. She is eager to get back on the medication and hopes her job-based insurance will cover it.
Many employers do cover obesity drugs. For example, about 40% of private employer plans include Novo Nordisk’s once-daily injection called Saxenda on their health plans, said Michael Bachner, Novo Nordisk’s director of media relations.
He said the $1,349-a-month wholesale acquisition price of Wegovy was determined by making it equivalent to that of Saxenda, which is less effective.
Still, that is more than the $851 monthly wholesale price of Ozempic. But, he pointed out, the recommended dosage of Wegovy is more than twice that of Ozempic. Four milligrams come in the Ozempic injector pens for the month, while Wegovy has 9.6.
“There’s more drug in the pen,” Mr. Bachner said. “That drives the price up.”
He added: “This is not a 20-year-old drug that we now have a new indication for and are pricing it higher. It’s a whole different clinical program,” which required new trials.
Now scientists, employers, physicians, and patients will have to decide whether the new drugs are worth it.
Earlier estimates – some commissioned by Novo Nordisk – of the potential cost of adding an obesity drug benefit to Medicare showed an overall reduction in spending when better health from the resulting weight loss was factored in.
Still, those earlier estimates considered much less expensive drugs, including a range of generic and branded drugs costing as little as $7 a month to more than $300, a small fraction of Wegovy’s cost.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The long list of side effects that follow ads for newer expensive drugs to treat type 2 diabetes sometimes include an unusual warning: They might cause weight loss. That side effect is one that many people – especially those with type 2 diabetes, which is associated with obesity – may desperately want.
So it’s no surprise that some of the same drugs are being reformulated and renamed by manufacturers as a new obesity treatment. No longer limited to the crowded field of treatments for type 2 diabetes, which affects about 10% of Americans, they join the far smaller number of drugs for obesity, which affects 42% of Americans and is ready to be mined for profit.
One that recently hit the market – winning Food and Drug Administration approval in June – is Novo Nordisk’s Wegovy (semaglutide), a higher-dose version of the company’s injectable diabetes drug, Ozempic.
Ozempic’s peppy ads suggest that people who use it might lose weight, but also include a disclaimer: that it “is not a weight-loss drug.” Now – with a new name – it is. And clinical trials showed using it leads to significant weight loss for many patients.
“People who go on this medication lose more weight than with any drug we’ve seen, ever,” said Fatima Cody Stanford, MD, MPH, an obesity medicine specialist at Massachusetts General Hospital and Harvard Medical School, both in Boston, who was not involved with any of the clinical trials.
But that leaves employers and insurers in the uncomfortable position of deciding if it’s worth it.
Wegovy’s monthly wholesale price tag – set at $1,349 – is about 58% more than Ozempic’s, although, the company pointed out, the drug’s injector pens contain more than twice as much of the active ingredient. Studies so far show that patients may need to take it indefinitely to maintain weight loss, translating to a tab that could top $323,000 over 20 years at the current price. Weight-loss treatments are not universally covered by insurance policies.
The arrival of this new class of weight-loss drugs – one from Lilly may soon follow – has created a thicket of issues for those who will pay for them. The decision is complicated by many unknowables concerning their long-term use and whether competition might eventually lower the price.
“The metric we try to use is value,” said James Gelfand, senior vice president for health policy at the ERISA Industry Committee, which represents large, self-insured employers. “If we pay for this drug, how much is this going to cost and how much value will it provide to the beneficiaries?”
Weight-loss treatments have had a lackluster past in this regard, with only modest results. Many employers and insurers likely remember Fen-Phen, a combination of fenfluramine and dexfenfluramine that was pulled from the market in the late 1990s for causing heart valve problems.
New drugs like Wegovy, more effective but also pricier than previous weight-loss treatments, will add more fuel to that debate.
Past treatments were shown to prompt weight loss in the range of 5%-10% of body weight. But many had relatively serious or unpleasant side effects.
Wegovy, however, helped patients lose an average of 15% of their body weight over 68 weeks in the main clinical trial that led to its approval. A comparison group that got a placebo injection lost an average of 2.5% over the same period. On the high end, nearly a third of patients in the treatment group lost 20% or more. Both groups had counseling on diet and exercise.
Side effects, generally considered mild, included nausea, diarrhea, vomiting, and constipation. A few patients developed pancreatitis, a serious inflammation of the pancreas. Like the diabetes medication, the drug carries a warning about a potential risk of a type of thyroid cancer.
Weight loss in those taking Wegovy puts it close to the 20%-25% losses seen with bariatric surgery, said Stanford, and well above the 3%-4% seen with diet and other lifestyle changes alone.
Participants also saw reductions in their waistlines and improvements in their blood pressure and blood sugar levels, which may mean they won’t develop diabetes, said Sean Wharton, MD, an internal medicine specialist and adjunct professor at York University in Toronto who was among the coauthors of the report outlining the results of the first clinical trial on Wegovy.
Since weight loss is known to reduce the risk of heart attack, high blood pressure and diabetes, might the new drug type be worth it?
Covering such treatment would be a sea change for Medicare, which specifically bars coverage for obesity medications or drugs for “anorexia, weight loss, or weight gain,” although it does pay for bariatric surgery. Pharmaceutical companies, patient advocates, and some medical professionals are backing proposed federal legislation to allow coverage. But the legislation, the Treat and Reduce Obesity Act, has not made progress despite being reintroduced every year since 2012, and sponsors are now asking federal officials instead to rewrite existing rules.
Private insurers will have to consider a cost-benefit analysis of adding Wegovy to their list of covered treatments, either broadly or with limits. Obesity was first recognized as a disease by the American Medical Association, easing the path for insurance coverage, in 2013.
“Employers are going to have a bit of a challenge” deciding whether to add the benefit to insurance offerings, said Steve Pearson, founder and president of the Institute for Clinical and Economic Review, which provides cost-benefit analyses of medical treatments but has not yet looked at Wegovy.
The trade-offs are embodied in patients like Phylander Pannell, a 49-year-old Largo, Md., woman who said she lost 65 pounds in a clinical trial of Wegovy. That study gave the drug to all participants for the first 20 weeks, then randomly assigned patients to get either the drug or a placebo for the next 48 weeks to determine what happens when the medication is stopped. Only after the trial ended did she find out she was in the treatment group the entire time.
Her weight fell slowly at first, then ramped up, eventually bringing her 190-pound frame down to about 125. Pains in her joints eased; she felt better all around.
“I definitely feel the drug was it for me,” said Ms. Pannell, who also followed the trial’s guidance on diet and exercise.
The study found that both groups lost weight in the initial 20 weeks, but those who continued to get the drug lost an additional average of 7.9% of their body weight. Those who got a placebo gained back nearly 7%.
After the trial ended, and the COVID-19 pandemic hit, Ms. Pannell regained some weight and is now at 155. She is eager to get back on the medication and hopes her job-based insurance will cover it.
Many employers do cover obesity drugs. For example, about 40% of private employer plans include Novo Nordisk’s once-daily injection called Saxenda on their health plans, said Michael Bachner, Novo Nordisk’s director of media relations.
He said the $1,349-a-month wholesale acquisition price of Wegovy was determined by making it equivalent to that of Saxenda, which is less effective.
Still, that is more than the $851 monthly wholesale price of Ozempic. But, he pointed out, the recommended dosage of Wegovy is more than twice that of Ozempic. Four milligrams come in the Ozempic injector pens for the month, while Wegovy has 9.6.
“There’s more drug in the pen,” Mr. Bachner said. “That drives the price up.”
He added: “This is not a 20-year-old drug that we now have a new indication for and are pricing it higher. It’s a whole different clinical program,” which required new trials.
Now scientists, employers, physicians, and patients will have to decide whether the new drugs are worth it.
Earlier estimates – some commissioned by Novo Nordisk – of the potential cost of adding an obesity drug benefit to Medicare showed an overall reduction in spending when better health from the resulting weight loss was factored in.
Still, those earlier estimates considered much less expensive drugs, including a range of generic and branded drugs costing as little as $7 a month to more than $300, a small fraction of Wegovy’s cost.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.